Abstract
Aim:
Implantology has been widely accepted as the mainstay treatment for rehabilitating complete and partial edentulism. However, it is associated with some failures and complications, the most concerning being neurosensory disturbance. Although neurosensory disturbance has been extensively studied, the incidence and cause remains largely variable. Thus, the aim of this systematic review and meta-analysis was to evaluate the incidence, distribution, and recovery rate of neurosensory disturbance.
Settings and Design:
This systematic review was conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement. A structured literature review was conducted using the following databases: PubMed, Science Direct, Cochrane, Ovid, and Google Scholar for reports related to neurosensory disturbance experienced after implant placement in the mandible.
Statistical Analysis Used:
Incidence and recovery rate for 100 person-years was calculated using the Poisson regression model. The risk difference of incidence between anterior and posterior implants was calculated with a random effects model.
Results:
Electronic database search yielded 1589 articles; a total of nine articles were selected for the meta-analysis. The risk of neurosensory disturbance was estimated at 13.50/100 person-years (95% confidence interval (CI): 10.98–16.03), with a greater risk with anteriorly placed implants: −0.02 (95% CI: −0.21–0.16) (P = 0.05). The overall recovery rate was estimated at 51.30/100 person-years (95% CI: 31.2–71.4).
Conclusions:
Within the limitations of the study, it can be concluded that mandibular implant placement is associated with a considerable risk of neurosensory disturbance. A large proportion of these patients present with spontaneous recovery; however, clinicians must take necessary precautions to avoid such complications. More randomized controlled trials are required to quantify the effect of factors leading to altered sensation during implant placement.
Keywords: Implant surgery, incidence, neurosensory disturbance, paresthesia
INTRODUCTION
Implantology has revolutionized the field of prosthodontics since its inception. It has been widely accepted as the treatment modality of choice for the rehabilitation of completely and partially edentulous patients. Although it is practiced extensively, it is not always successful, nor is it devoid of complications. One of the most common complications associated with implant placement, especially in the mandible, is the injury to the inferior alveolar, lingual, and mental nerves.[1] Nerve injury is unpredictable and often occurs regardless of accurate presurgical planning to avoid encroaching vital structures found adjacent to the region of interest. Hemorrhage and infection can occur as a result of nerve damage and is often associated with sensory disturbance.[2] The Subcommittee on Taxonomy of the International Association for the Study of Pain 1986, classified sensory disturbances into anesthesia, paresthesia, and dysesthesia, and patients who experience these report with great discomfort and compromised quality of life.[3] Sensory disturbance is transient or persistent,[4] and the severity of nerve injury dictates its recovery.[5] One study reported a 0%–13% incidence of altered nerve sensation when placing implants in an atrophic mandible,[6] while another reported 3%–14% of transient paresthesia and 4% permanent paresthesia.[7] The reported incidence of neurosensory disturbance varies among different authors and the risk of nerve damage seems greater clinically than previously documented, indicating that the data have not been adequately assessed. Thus, the Population, Intervention, Control, and Outcomes (PICOS) question was formulated as follows: What is the incidence, duration, and recovery rate of neurosensory disturbance that results from mandibular implant surgery?
MATERIALS AND METHODS
This systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines.
Eligibility criteria
Inclusion criteria
Prospective studies that reported the total number of patients who received implants and the number that reported altered sensation
The onset and time of recovery of neurosensory disturbance must be reported
Articles published from 1990 to 2019
English articles.
Exclusion criteria
Studies that reported on nerve transposition
Case reports
Cross-sectional studies
Literature reviews
In-vitro studies and finite element analysis
Animal studies.
The focus question was formulated as per the PICOS format:
Population: Patients who received implants in the mandible and complained of neurosensory disturbance
Intervention: Implant placement in the mandible
Comparison: Transient versus persistent neurosensory disturbance and incidence of neurosensory disturbance in posterior versus anterior mandibular implants
Outcome 1: Incidence of neurosensory disturbance that occurs in mandibular implants
Outcome 2: Incidence of spontaneous recovery of neurosensory damage
Study design: Prospective clinical trials.
The PICOS question thus formulated was: What is the incidence, duration, and recovery rate of neurosensory disturbance that results from mandibular implant surgery?
Information sources
Literature published in the years 1990–2019 was sought in the following databases: PubMed, Science Direct, Cochrane, Ovid, and Google Scholar. The search for gray literature was carried out in OpenGrey database. Only English articles were included.
Research strategy
The following keywords were used to develop the search strategy:
Mandibular nerve, Inferior alveolar nerve, dental implant, nerve injury, altered sensation, sensory disturbance, hyperalgesia, paresthesia, anesthesia, and dysesthesia.
The search strategy thus developed in PubMed was as follows: (((((Mandibular nerve) OR Inferior alveolar nerve) OR trigeminal nerve)) AND dental implants) AND (((((((hyperalgesia) OR paresthesia) OR nerve injury) OR altered sensation) OR sensory disturbance) OR dysesthesia) OR anesthesia).
Data collection
Data were collected by one author and filled into predefined forms that included the following items: author, year, number of patients who underwent implant placement, number that reported with altered sensation, nature of altered sensation, number of patients who experienced recovery, time of recovery, region of implant placement, and distance from the nerve. A second author checked the information collected.
Statistical analysis
The incidence of neurosensory disturbance and recovery for 100 person-years was calculated from each study with a Poisson regression model. A random effect model was used to calculate the pooled incidence rates and their 95% confidence intervals (CIs). Heterogeneity between the studies was evaluated by I2 statistics, and I2 > 50% or P < 0.05 indicated statistically significant heterogeneity.
Risk-of-bias assessment
Risk assessment for the nonrandomized studies was done using the “A Cochrane Risk of Bias Assessment Tool: for Nonrandomized Studies of Interventions (ACROBAT-NRSI), Version 1.0.0 (riskofbiastools.info), dated September 22, 2014. Risk-of-bias assessment for the randomized controlled trials (RCTs) was done using the “Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2) dated October 9, 2018.”
The data extracted were stratified and tabulated chronologically. Data synthesis was based on the evidence tables, and a descriptive summary was produced to enumerate information related to the various characteristics.
RESULTS
Published literature pertaining to the current review was accessed through Medline, Science Direct, Google Scholar, Cochrane, Ovid, and OpenGrey, and a total of 1589 articles were obtained. After removing duplicates, 1276 records remained. Articles were eliminated after screening titles for relevance and 53 were retained. Abstract and full-text screening resulted in the elimination of 32 records. At this juncture, the inclusion criteria were applied to assess the eligibility of the records obtained and eventually, a total of nine studies were selected for this systematic review and meta-analysis [Tables 1 and 2].
Table 1.
PRISMA flowchart
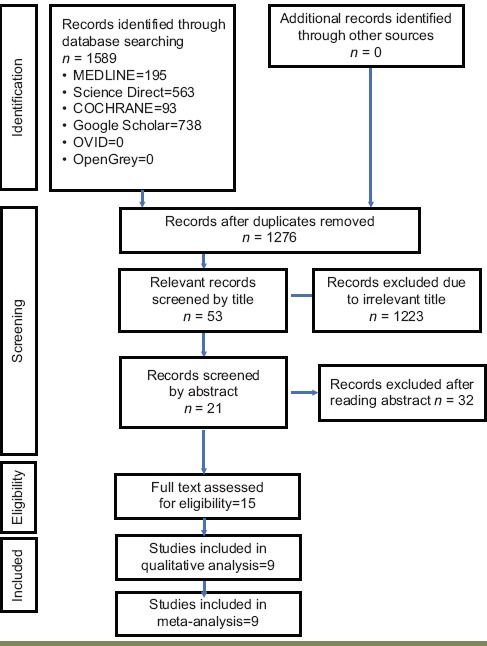
Table 2.
Excluded studies
| Studies | Reasons for exclusion |
|---|---|
| Batenburg (1994) | No patients reported sensory disturbance |
| Burnstein (2008) | No patients reported sensory disturbance |
| Dannan (2004) | A retrospective study (does not fulfill the inclusion criteria) |
| Delgado (2018) | A retrospective study (does not fulfill the inclusion criteria) |
| Ellies (1992) | A retrospective study (does not fulfill the inclusion criteria) |
| Givol (2013) | All the patients in the sample group have neurosensory disturbance |
| Juodzbalys (2011) | All the patients in the sample group have neurosensory disturbance |
| Kim (2013) | All the patients in the sample group have neurosensory disturbance |
| Kutuk (2013) | A retrospective study |
| Scarano (2017) | A retrospective study |
| van der Meij (2005) | A retrospective study |
| Visser (2004) | No patients reported sensory disturbance |
Study characteristics
The nine studies chosen for data extraction were all prospective studies [Table 3], with seven nonrandomized clinical studies and two randomized clinical trials. All the nine studies were included in the meta-analysis. The risk of bias is conducted for nonrandomized and randomized with separate tools as described in [Table 4 and 5]. Risk-of-bias assessment for the nonrandomized trials was done using the Risk of Bias in Nonrandomized Studies of Interventions, and all the studies presented with a low risk of bias [Table 4]. The RoB 2 was used for the two randomized trials and both studies presented with some concerns with regard to the risk of bias [Table 5].
Table 3.
Data extraction sheet
| Name | Study design | Total number of patients | Loading | Bone quality | Imaging modality | Implant dimensions | Safety margin | Method of evaluation | Patients with altered sensations | Nature of sensory alteration | Region placement of the implants with disturbance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wismeijer (1997) | Prospective; RCT | 102 | Delayed loading | Symphyseal height of 16.06 (average) | NA | NA | 3 mm | Hopkins Symptoms Checklist | 11 | Numbness (4); prickly (2); hypersensitive (5) | Anterior (9) Posterior (2) |
| Walton (2000) | Prospective; RCT | 70 | Delayed loading | Normal-severe resorption | OPG | NA | NA | Von Frey Hairs/touch sensation | 17 | Numbness and tingling of the lower lip | Anterior |
| Vazquez (2007) | Prospective; RCT | 1527 | NR | NR | OPG | ≥12 mm | 2 mm | Two-point discrimination, Weinstein and pinprick test | 2 | Paresthesia of the lower lip (1), altered sensation of the lip and chin | Posterior |
| Steenberg (1990) | Prospective | 91 | Delayed loading | Enough bone to accommodate 7 mm and 3.75 mm; Zarb and Bolender Grade 3 and 4 | Long cone radiograph | 7 mm × 3.5 mm | NR | NR | 16 | Paresthesia of the lower lip | NR |
| Higuchi (1995) | Prospective | 117 | NR | Enough bone to accommodate 7 mm implant; Grade 3 and 4 | Long cone radiograph | Minimum of 7 mm | Pin prick detection, two point discrimination, directional brush stroke response and thermal testing | 16 | Paresthesia of the lower lip | NR | |
| Bartling (1999) | Prospective | 94 | NR | NR | CT and OPG | NR | 2 mm above (OPG) and 1 mm (CT) | Two-point discrimination | 8 | Midline of the lip and chin (3), complete anesthesia (1) | Anterior (1) posterior (3) both (4) |
| Abarca (2005) | Prospective | 58 | Immediate loading | NR | NR | NR | NR | Hopkins Symptoms Checklist; two-point perception | 19 | Numbness (9), cutting, beating itching (2), gingiva (6), inferior lip (4), chin (4) | Anterior |
| Boven (2014) | Prospective | 40 | Delayed loading | Extremely atrophic mandible. Illiac crest graft (12 mm at the infraforaminal region) | OPG | 4.1 mm × 10 mm | NR | NR | 11 | Sensory disturbance | Anterior |
| Bormann (2010) | Prospective | 13 | Delayed loading | Atrophied posterior mandible (underwent segmental mandibular osteotomy) | OPG and DVT | NR | NR | NR | 5 | Hypoesthesia | Posterior |
RCT: Randomized controlled trial, NA: Not available, CT: Computed tomography, NR: Not relevant, OPG: Orthopantomograph, DVT: Deep-vein thrombosis
Table 4.
Risk of Bias in Nonrandomized Studies of Interventions-I
| Study name | Confounding bias | Selection bias | Measuring interventions | Departures from interventions | Missing data | Measuring outcomes | Reporting bias | Overall bias |
|---|---|---|---|---|---|---|---|---|
| Steenberg (1990) | Low | Low | Low | Low | Low | Low | Low | Low |
| Higuchi (1995) | Low | Low | Low | Low | Low | Low | Low | Low |
| Bartling (1999) | Low | Low | Low | Low | Low | Low | Low | Low |
| Abarca (2005) | Low | Low | Low | Low | Low | Low | Low | Low |
| Vazquez 2007 | Low | Low | Low | Low | Low | Low | Low | Low |
| Boven (2014) | Low | Low | Low | Low | Low | Low | Low | Low |
| Bormann (2010) | Low | Low | Low | Low | Low | Low | Low | Low |
Table 5.
Revised Cochrane Risk of Bias Tool for Randomized Trials (Rob 2)
| Study | Randomization | Deviations from intended interventions | Missing data | Measurement of outcome | Selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|
| Wismeijer (1997) | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Walton (2000) | High | Some concerns | Low | Some concerns | Low | Some concerns |
Patient and implant characteristics
The studies included reported a total of 2112 patients who underwent implant placement in the mandibular ridge. Orthopantomography (OPG),[8,9,10,11,12] long cone radiographs,[1,13] and conventional tomography[9] were the most commonly employed diagnostic imaging techniques for treatment planning. Implants were placed in mandibular bone that were normal[8] and severely resorbed.[11] Two studies selected patients who had adequate mandibular ridge dimensions to accommodate implants of 7 mm (Zarb and Bolender Class 3 and 4).[1,13] The margin of safety from the mandibular nerve for implant placement in relation to the mandibular ridge was reported in only few studies, namely, 2 mm[10] and 3 mm.[14] Bartling et al. considered a safety margin of 2 mm from the mandibular nerve as evaluated in an OPG and 1 mm as evaluated in a computed tomography (CT).[9] Some studies reported implant placement in both anterior and posterior mandibular regions. After implant placement, a total of 105 (4.9%) patients complained of neurosensory disturbance. The nature of altered sensation reported was as follows: numbness (30); prickly (2); hypersensitive (5); paresthesia of the lower lip (37) and chin (1); complete anesthesia (1); cutting, beating, and itching (2); tingling of gingiva (6); and hypoesthesia (5) [Table 2].
Estimating the overall incidence of neurosensory disturbance
The pooled incidence rate of the nine studies was 13.50/100 person-years (95% CI: 10.98–16.03). There was a very serious risk of inconsistency due to high heterogeneity (I2 = 99.8%) across the studies. The risk of imprecision was considered high due to the small sample size. No serious risk of bias, indirectness, or publication bias was detected. Overall, the certainty of evidence was considered to be moderate [Table 6].
Table 6.
Incidence of neurosensory disturbance
Risk of neurosensory disturbance in anterior versus posterior implants
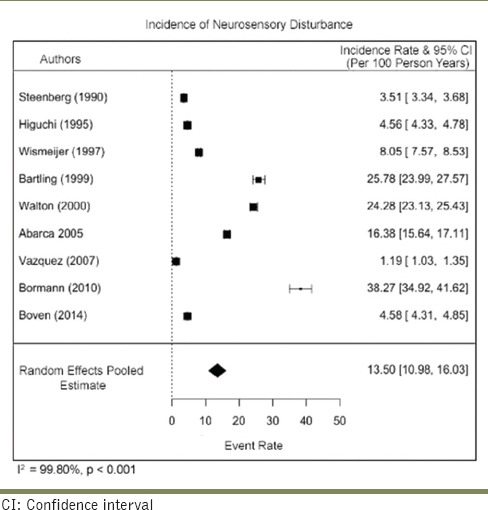
Two studies reported neurosensory disturbance with the placement of both anterior and posterior implants. The risk difference between the two implants from these studies was −0.02 (95% CI: −0.21–0.16), indicative of greater risk associated with the anteriorly placed implants. There was a serious risk of inconsistency due to the high heterogeneity among the studies (I2 = 70.942%) and very serious risk of imprecision due to the small sample size. The certainty of evidence was considered high [Table 7].
Table 7.
Neurosensory disturbance in anterior versus posterior
Estimating the recovery rate
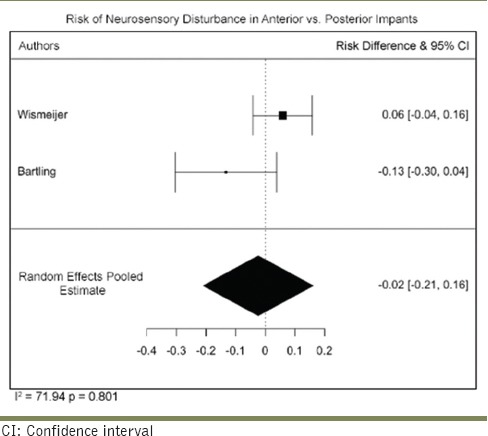
Only studies that had a follow-up of 1 year and above were included. Seven studies were found to fulfill this criterion and were subjected to meta-analysis. The pooled recovery rate was 51.30/100 person-years (95% CI: 31.2–71.4). There was a very serious risk of inconsistency due to high heterogeneity (I2 = 99.90%) across the studies. The risk of imprecision was considered high due to the small sample size. No serious risk of bias, indirectness, or publication bias was detected. Overall, the certainty of evidence was considered to be moderate [Table 8].
Table 8.
Recovery rate
Estimating short-term versus long-term recovery rates
Data were extracted to evaluate the time point of recovery and stratified based on the classification of Jalbout and Tabourian. Recovery that took place within 2–4 weeks was considered short term, and only two studies fulfilled the inclusion criteria. The short-term recovery rate was 46.96/100 person-years (95% CI: 10.77–104.69). There was a serious risk of inconsistency due to high heterogeneity (I2 = 98.28%) across the studies. The sample size was small along with a very wide CI, resulting in a serious risk of imprecision. No risk of bias, indirectness, or publication bias was detected. Overall, the certainty of evidence was graded as low. Three studies reported with patients who presented with intermediate-term recovery (between 5 weeks and 1 year). The pooled intermediate-term recovery rate was 10.97/100 person-years (95% CI: 3.51–18.43). There was a serious risk of inconsistency due to high heterogeneity (I2 = 99.77%) across the studies. The sample size was small along with a very wide CI, resulting in a serious risk of imprecision. No risk of bias, indirectness, or publication bias was detected. Overall, the certainty of evidence was moderate [Tables 9 and 10].
Table 9.
Short-term recovery
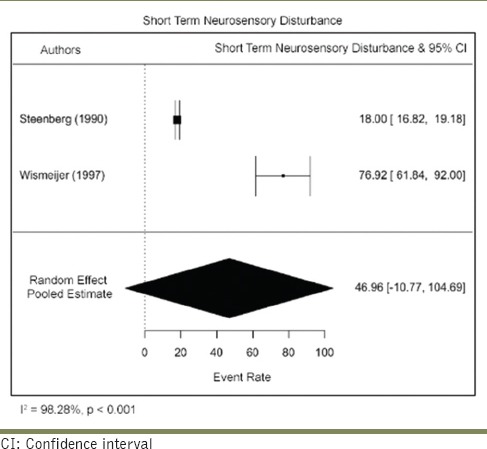
Table 10.
Intermediate-term recovery
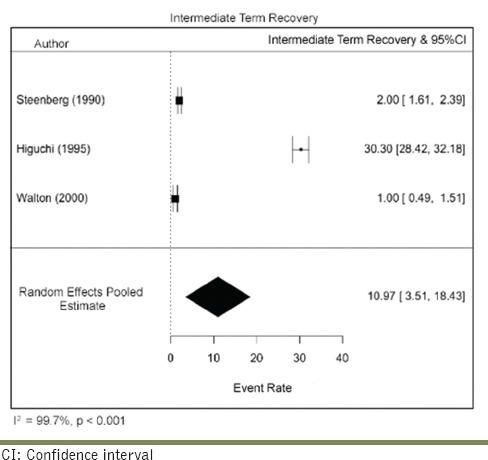
Estimating the rate of persistant neurosensory disturbance
Only studies that had a follow-up of 1 year or more were included.
The rate of persistant neurosensory disturbance rate was 18.67/100 person-years (95% CI: 14.54–22.79). There was a very serious risk of inconsistency due to high heterogeneity (I2 = 99.6%) across the studies. The risk of imprecision was considered high due to the small sample size. No serious risk of bias, indirectness, or publication bias was detected. Overall, the certainty of evidence was considered to be moderate [Table 11]. The evidence thus obtained with each parameter was subjected to the Gradepro assessment for certainty [Table 12].
Table 11.
Persistant neurosensory disturbance
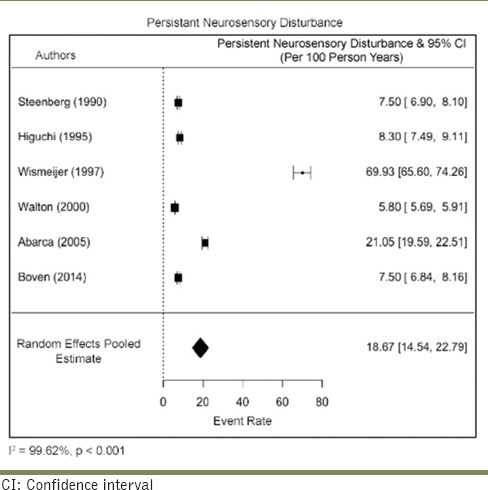
Table 12.
Gradepro assessment
| Question: What is the incidence of neurosensory disturbance in patients with mandibular implants | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Certainty assessment | Effect | Certainty | Importance | |||||||
| Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Number of events | Number of individuals | Rate (95% CI) | |||
| Incidence of NSD | |||||||||||
| 9 | Observational studies | Not serious | Very seriousa | Not serious | Seriousb | Strong association dose-response gradient | 105 | 2112 | Event rate 13.50/100 person-year(s) (10.98-16.03) | ⨁⨁⨁◯ Moderate | Critical |
| Rate of recovery | |||||||||||
| 7 | Observational studies | Not serious | Very seriousc | Not serious | Seriousb | Strong association dose-response gradient | 60 | 95 | Event rate 51.30/100 person-year(s) (31.21-71.4) | ⨁⨁⨁◯ Moderate | Critical |
| Incidence of permanent NSD | |||||||||||
| 6 | Observational studies | Not serious | Very seriousd | Not serious | Seriousb | Strong association dose-response gradient | 34 | 90 | Event rate 18.67/100 person-year(s) (14.54-22.79) | ⨁⨁⨁◯ Moderate | Important |
| Short-term recovery rate | |||||||||||
| 2 | Observational studies | Not serious | Very seriouse | Not serious | Seriousb | Strong association dose-response gradient | 10 | 11 | Event rate 46.96/100 person-year(s) (−10.77-104.69) | ⨁⨁⨁◯ Moderate | Important |
| Long-term recovery rate | |||||||||||
| 3 | Observational studies | Not serious | Very seriousf | Not serious | Seriousb | Strong association dose-response gradient | 26 | 36 | Event rate 10.97/100 person-year(s) (3.51-18.43) | ⨁⨁◯◯ Low | Important |
| Risk of NSD in anterior versus posterior implants | |||||||||||
| 2 | Observational studies | Not serious | Seriousg | Not serious | Very seriousb | Very strong association dose-response gradient | 15 | 177 | Event rate −0.02/1 person-year(s) (−0.21-0.16) | ⨁⨁⨁⨁ High | Critical |
⨁◯The certaintly of evidence is graded as very low to high with upto four crosshairs. One crosshair representing one point to mean very low while four crosshairs represent high certainty of evidence aThe lack of overlap in the CIs of the studies included and the high estimated heterogeneity (I2=99.8%) result in a high risk of inconsistency, bSmall sample size resulting in a serious risk for imprecision, cThe lack of overlap in the CIs of the studies included and the high estimated heterogeneity (I2=99.90%) result in a high risk of inconsistency, dThe lack of CI overlap in the studies included and the high estimated heterogeneity (I2=99.77%) result in a high risk of inconsistency, eThe lack of CI overlap in the studies included and the high estimated heterogeneity (I2=98.28%) result in a high risk of inconsistency, fThe lack of CI overlap in the included studies and the high estimated heterogeneity (I2=99.77%) result in a serious risk of inconsistency, gThe lack of CI overlap in the included studies and the high estimated heterogeneity (I2=71.94%) result in a serious risk of inconsistency. NSD: Neurosensory disturbance, CIs: Confidence intervals
DISCUSSION
The inferior alveolar nerve (64.4%) and the lower lingual nerve (28.8%)[15] are the most commonly injured nerves during dental procedures, and mandibular implant placement is one of the biggest causes. Almost 73% of dentists who placed implants reported that their patients complained of some form of neurosensory disturbance.[2] The reported incidence of altered sensation ranges from 6.5% to 40% and varies between different publications. This variability has been attributed to different biological factors; however, the cause and occurrence of neurosensory disturbance remains an enigma to most clinicians. One needs to acknowledge the risk of nerve damage, take necessary precautions to avoid it, and must educate the patients prior to implant procedures to reduce liability. This systematic review was thus developed to estimate the incidence of neurosensory disturbance during mandibular implant placement, its recovery, and the risks of persistent neurosensory disturbance to help clinicians develop a protocol for management in the event of its occurrence.
Most of the studies included in this systematic review were non-RCTs, therefore the results must be viewed with some caution. All the studies were obtained from the electronic database search, and both assessors selected the articles together simultaneously. This was done to save the time of re-evaluating the articles in the event of disagreement.
The pooled incidence rate of neurosensory disturbance was 13.50/100 person-years (95% CI: 10.98–16.03). Nerve damage can occur during all the phases of implant placement such as administration of local anesthetic, incision, elevation of the flap, drilling, implant placement, or soft-tissue swelling after surgery.[2] Although Choi et al. considered direct contact of the implant with the nerve as the primary cause for neurosensory disturbance, 10.1% of the affected patients in their study presented with no contact.[16] Hirsh and Branemasrk have attributed (a) direct mechanical damage, (b) pressure on nerve/blood vessel, and (c) formation of a hemangioma/osteoma as the possible causes for nerve injury.[17] The average density of the bone surrounding the mandibular canal is not sufficient to resist the implant drill and one could potentially avoid the risk of nerve damage by accurately determining the bone mass around the canal with a CT.[18] The implant dimensions reported were not greater than 10 mm and the studies reported a safety margin that ranged between 2 and 3 mm.
The association for the study of pain has stratified altered sensation under three subdivisions, namely, paresthesia, anesthesia, and dysesthesia.[17] The most common symptoms reported in the studies involved were numbness and paresthesia (that involved cutting and beating and prickly sensation) involving the lip and chin.
The risk difference between anteriorly and posteriorly placed implants was estimated at − 0.02 (95% CI: −0.21–0.16), indicating that anteriorly placed implants pose a greater risk of nerve damage than posteriorly placed ones. This is inconsistent with the theory that posteriorly placed implants are associated with a greater risk as they are more susceptible to the twist drills dropping into the trabecular spaces and positioning implants deeper than originally planned.[19] Sammartino et al. also suggested that low mandibular cortical bone density seen in the posterior mandible when compared to the anterior regions, is associated with increased nerve pressure.[20] The evidence estimated by the current meta-analysis was, however, statistically insignificant (P > 0.05).
The estimated pooled recovery rate was 51.30/100 person-years (95% CI: 31.21–71.40). The nerve is surrounded by several extraneural tissues (epineurium, perineurium, endoneurium, and mesoneurium) and injury to any of these will result in neurosensory alterations. When a nerve undergoes injury of significant magnitude, the endoneurial capillaries undergo damage and result in a conduction block due to intrafascicular edema and in these cases, normal function returns within 1–2 weeks. Sometimes, increased pressure could result in segmental demyelination which typically recovers within 1 month.[21] Although it was originally considered that only patients with the perineurium layer intact could potentially recover from nerve damage, a study by Na et al. reported recovery in half of the patients who presented with implant intrusion.[22]
Neurosensory alterations may be classified according to Jalbout and Tabourian as (1) neuropraxia (mild injury in which feeling is reversed within 4 weeks postsurgery); (2) axonotmesis (nerve compression, structure remains intact, and signs of feeling return 5–11 weeks postsurgery and continue to improve in the next 10 months); and (3) neurotmesis (disruption of the nerve with poor prognosis for return of feeling).[23] The duration of recovery in the meta-analysis was stratified based on the above classification, and the estimated rate of recovery was as follows: early recovery (within 1 month) was 46.96/100 person-years and intermediate recovery (5th week–11 months) was 10.97/100 person-years.
The rate of persistent neurosensory disturbance was 18.67/100 person-years (95% CI: 14.54–2.79). The chances of recovery are directly related to the extent of injury, and diagnostic tests are necessary to evaluate the region of altered sensation and to decide management options. The primary treatment options for neurosensory disturbance include administration of steroids, cryotherapy, acupuncture, low-level laser therapy, and, in extreme cases, surgical intervention that entails direct anastomosis, autogenous nerve grafts, and alloplastic grafts. When patients complain of paresthesia, it is important to monitor them every 2–3 weeks to evaluate the extent of nerve repair. Typically, recovery ought to occur in 2–3 months. The prognosis for recovery becomes questionable beyond 3 months and patients should be referred to a specialist and surgical intervention must be considered before Wallerian degeneration takes place resulting in chronic neuropathies.[2] Despite this estimate, several studies have reported complete recovery even after 2–3 years.[13,24]
Limitations
This study failed to take into consideration the various biologic and systemic factors that would render one at a greater risk for neurosensory disturbance. The incidence is also considered to be more pronounced in women. As the distribution of altered sensation among men and women was not reported in any of the studies, we were unable to evaluate whether this claim was in fact true. More systematic reviews with RCTs are necessary to help evaluate the role these factors play, to help clinicians take necessary precautions
None of the studies described the altered sensation experienced by the patient, as those with and without pain. Although most patients report that the benefits of implant treatment outweigh the discomfort of altered sensory response, neuropathic pain disorders would considerably affect the patient's quality of life as opposed to those who experience no pain. Further studies must be assessed to help clinicians preemptively plan to avoid and develop a protocol to manage patients in the event of such occurrences
All the studies used different methods to evaluate the change in sensory perception. These psychophysical tests do not reach the ideal threshold in the oral cavity as they were not originally designed for the said purpose. Thus, results obtained from these tests may not always translate to reality.[25] It was also difficult to stratify altered sensation according to intensity and nature
As only one study undertook an immediate loading protocol, the effect of loading on the incidence of neurosensory disturbance could not be evaluated with the present meta-analysis. To the best of our knowledge, no research is available to compare the effect of loading on neurosensory disturbance
The damage to the underlying nerve can occur at any stage of implant placement. It is almost impossible to predict the cause for nerve damage and consequently neurosensory disturbance, to thereby claim that one procedure poses a greater risk than the other.
CONCLUSIONS
Patients who undergo mandibular implant placement present with a considerably high risk of nerve damage and consequently sensory disturbance. Clinicians must take all necessary precautions to avoid such complications and educate the patient about the risks
A significant proportion of these patients will undergo spontaneous recovery and must be monitored regularly. Upon no signs of recovery after 3 months, the clinician must explore surgical management to prevent further long-standing damage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.van Steenberghe D, Lekholm U, Bolender C, Folmer T, Henry P, Herrmann I, et al. Applicability of osseointegrated oral implants in the rehabilitation of partial edentulism: A prospective multicenter study on 558 fixtures. Int J Oral Maxillofac Implants. 1990;5:272–81. [PubMed] [Google Scholar]
- 2.Misch CE, Resnik R. Mandibular nerve neurosensory impairment after dental implant surgery: Management and protocol. Implant Dent. 2010;19:378–86. doi: 10.1097/ID.0b013e3181effa92. [DOI] [PubMed] [Google Scholar]
- 3.Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the international association for the study of pain, subcommittee on taxonomy. Pain Suppl. 1986;3:S1–226. [PubMed] [Google Scholar]
- 4.Merrill RG. Prevention, treatment, and prognosis for nerve injury related to the difficult impaction. Dent Clin North Am. 1979;23:471–88. [PubMed] [Google Scholar]
- 5.Hillerup S. Iatrogenic injury to the inferior alveolar nerve: Etiology, signs and symptoms, and observations on recovery. Int J Oral Maxillofac Surg. 2008;37:704–9. doi: 10.1016/j.ijom.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Burstein J, Mastin C, Le B. Avoiding injury to the inferior alveolar nerve by routine use of intraoperative radiographs during implant placement. J Oral Implantol. 2008;34:34–8. doi: 10.1563/1548-1336(2008)34[34:AITTIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Dannan A, Alkattan F, Jackowski J. Altered sensations of the inferior alveolar nerve after dental implant surgery: A retrospective study. Dentistry. 2013;13:S13–002. [Google Scholar]
- 8.Walton JN. Altered sensation associated with implants in the anterior mandible: A prospective study. J Prosthet Dent. 2000;83:443–9. doi: 10.1016/s0022-3913(00)70039-4. [DOI] [PubMed] [Google Scholar]
- 9.Bartling R, Freeman K, Kraut RA. The incidence of altered sensation of the mental nerve after mandibular implant placement. J Oral Maxillofac Surg. 1999;57:1408–12. doi: 10.1016/s0278-2391(99)90720-6. [DOI] [PubMed] [Google Scholar]
- 10.Vazquez L, Saulacic N, Belser U, Bernard JP. Efficacy of panoramic radiographs in the preoperative planning of posterior mandibular implants: A prospective clinical study of 1527 consecutively treated patients. Clin Oral Implants Res. 2008;19:81–5. doi: 10.1111/j.1600-0501.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 11.Boven GC, Meijer HJ, Vissink A, Raghoebar GM. Reconstruction of the extremely atrophied mandible with iliac crest onlay grafts followed by two endosteal implants: A retrospective study with long-term follow-up. Int J Oral Maxillofac Surg. 2014;43:626–32. doi: 10.1016/j.ijom.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Bormann KH, Suarez-Cunqueiro MM, von See C, Kokemüller H, Schumann P, Gellrich NC. Sandwich osteotomy for vertical and transversal augmentation of the posterior mandible. Int J Oral Maxillofac Surg. 2010;39:554–60. doi: 10.1016/j.ijom.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi KW, Folmer T, Kultje C. Implant survival rates in partially edentulous patients: A 3-year prospective multicenter study. J Oral Maxillofac Surg. 1995;53:264–8. doi: 10.1016/0278-2391(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 14.Wismeijer D, van Waas MA, Vermeeren JI, Kalk W. Patients’ perception of sensory disturbances of the mental nerve before and after implant surgery: A prospective study of 110 patients. Br J Oral Maxillofac Surg. 1997;35:254–9. doi: 10.1016/s0266-4356(97)90043-7. [DOI] [PubMed] [Google Scholar]
- 15.Tay AB, Zuniga JR. Clinical characteristics of trigeminal nerve injury referrals to a university centre. Int J Oral Maxillofac Surg. 2007;36:922–7. doi: 10.1016/j.ijom.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Choi YC, Cho ES, Merrill RL, Kim ST, Ahn HJ. Analysis of neurosensory dysfunction after dental implant surgery. J Oral Med Pain. 2014;39:133–9. [Google Scholar]
- 17.Merskey H, Bogduk N, editors. 2nd ed. Kyoto: International Association for the Study of Pain Task Force on Taxonomy, IASP Press IASP Council; 2007. Classification of Chronic Pain. [Google Scholar]
- 18.Başa O, Dilek OC. Assessment of the risk of perforation of the mandibular canal by implant drill using density and thickness parameters. Gerodontology. 2011;28:213–20. doi: 10.1111/j.1741-2358.2009.00362.x. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz MS, Rothman SL, Rhodes ML, Chafetz N. Computed tomography: Part I. Preoperative assessment of the mandible for endosseous implant surgery. Int J Oral Maxillofac Implants. 1987;2:137–41. [PubMed] [Google Scholar]
- 20.Sammartino G, Marenzi G, Citarella R, Ciccarelli R, Wang HL. Analysis of the occlusal stress transmitted to the inferior alveolar nerve by an osseointegrated threaded fixture. J Periodontol. 2008;79:1735–44. doi: 10.1902/jop.2008.080030. [DOI] [PubMed] [Google Scholar]
- 21.LaBanc JP. Trigeminal nerve injury: Diagnosis and management. Oral Maxillofac Clin North Am. 1992;4:285–96. [Google Scholar]
- 22.Na JY, Han SS, Jeon K, Choi YJ, Choi SH, Lee C. Prognosis in case of nerve disturbance after mandibular implant surgery in relation to computed tomography findings and symptoms. J Periodontal Implant Sci. 2019;49:127–35. doi: 10.5051/jpis.2019.49.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Academy of Implant Dentistry. Glossary of implant terms. J Oral Implantol. 1986;12:284–94. [PubMed] [Google Scholar]
- 24.Astrand P, Borg K, Gunne J, Olsson M. Combination of natural teeth and osseointegrated implants as prosthesis abutments: A 2-year longitudinal study. Int J Oral Maxillofac Implants. 1991;6:305–12. [PubMed] [Google Scholar]
- 25.Abarca M, van Steenberghe D, Malevez C, De Ridder J, Jacobs R. Neurosensory disturbances after immediate loading of implants in the anterior mandible: An initial questionnaire approach followed by a psychophysical assessment. Clin Oral Investig. 2006;10:269–77. doi: 10.1007/s00784-006-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


