Abstract
The novel object recognition (NOR) task has emerged as a popular method for testing the neurobiology of nonspatial memory in rodents. This task exploits the natural tendency of rodents to explore novel items and depending on the amount of time that rodents spend exploring the presented objects, inferences about memory can be established. Despite its wide use, the underlying neural circuitry and mechanisms supporting NOR have not been clearly defined. In particular, considerable debate has focused on whether the hippocampus plays a significant role in the object memory that is encoded, consolidated and then retrieved during discrete stages of the NOR task. Here we analyzed the results of all published reports in which the role of the rodent hippocampus in object memory was inferred from performance in the task with restricted parameters. We note that the remarkable variability in NOR methods across studies complicates the ability to draw meaningful conclusions from the work. Focusing on twelve reports in which a minimum criterion of sample session object exploration was imposed, we find that temporary or permanent lesion of the hippocampus consistently disrupts object memory when a delay of 10 min or greater is imposed between the sample and test sessions. We discuss the significance of a delay-dependent role of the hippocampus in NOR within the framework of the medial temporal lobe. We assert that standardization of the NOR protocol is essential for obtaining reliable data that can then be compared across studies to build consensus as to the specific contribution of the rodent hippocampus to object memory.
Keywords: lesion, memory, hippocampus, inactivation, object recognition, rodents
1. Introduction
We can all recall a time when walking down a crowded corridor, we happen upon a person who looks familiar. While we are confident that we have encountered this person before, we are unable to remember how or when we previously met. It is only through the information gathered during interactive conversation that we are able to recall who this person is and where we encountered them for the first time. This uncomfortable, yet common, scenario depicts our ability to subjectively recall previous information through distinct memory processes.
Memory can be divided into two distinct categories, declarative and non-declarative forms. Declarative memory, or explicit memory, is the ability to recall personal history, facts and events, and is dependent on the interconnected structures of the medial temporal lobe. Recognition, a subtype of declarative memory, reflects that of people, objects, and experiences. Clearly, the example stated above illustrates the two forms of recognition memory that are commonly experienced during a test of information retrieval, that is, familiarity and recollection. Familiarity is the immediate feeling that an event, individual, or item was previously encountered. This experience, referred to as ‘knowing’, does not involve the conscious recollection of details from the prior experience. For example, “I know I have seen that person (or item) before; I just don’t remember where or when”. Recollection, or ‘remembering’ on the other hand, involves a slower process whereby full attention to the present stimuli (if any) induces an intended or conscious recall of the contextual details of the prior event or experience – that is, specific information as to where and when the original experience occurred [1, 2]. For example, “I remember you. We met at the 2012 Society for Neuroscience meeting; our posters were next to one another on the second day of that conference, and you commented on how well I coordinated my outfit with the color scheme of my poster”. Originally defined by Tulving [3], the remember/know distinction is considered by many to reflect separate underlying behavioral processes of recognition memory. Although the processes of recollection and familiarity are distinct in the manner that they are experienced, it remains unclear whether different neurobiological mechanisms support them. Dual-process models of recognition memory state that recollection and familiarity are functionally separate systems [4–8]. Studies of human amnesiacs have revealed selective impairment of recollection, while sparing familiarity, and numerous functional imaging studies have identified that the separate processes are associated with region-specific activation patterns. These findings are largely considered support for the view that familiarity and recollection utilize different underlying systems [9–11]. On the other hand, single-process models view the two declarative memory forms as a part of one distinct category of recognition memory [7, 10, 12]. Here, memories are represented along a scale that ranges from weak to strong. Studies have demonstrated that these two processes have a significant structural commonality that would point to a single process model. Similar structural activation is observed with both familiarity and recollection [11]. Regardless of how these forms of memory are thought to function, the fundamental concepts derived from the distinction between familiarity and recollection are useful for improving understanding of recognition memory mechanisms in both humans and laboratory animals.
The medial temporal lobe is organized in a manner that supports memory. Various subregions have been identified as the structures critical in supporting memory in a variety of species [13]. The perirhinal cortex, parahippocampal cortex, and entorhinal cortex are anatomical structures identified as components of the “what” and “where” streams of experience-dependent sensory inputs that converge within the hippocampus. Traditionally, it is believed that the “what” information is conveyed through the perirhinal cortex, while the “where” information is transmitted through the parahippocampal and entorhinal cortices. It is only in the hippocampus that the “what” and “where” information is associated [1]. However, in recent years, debate over whether the hippocampus is directly involved in encoding memories of the “what” information has increased. Similarly, many studies claim that familiarity is structurally distinct from that of recollection, with familiarity attributed to the perirhinal cortex and recollection to the hippocampus [14]. Nevertheless, it is apparent that during recollection, it is the “what” and “where” associations that are being recovered.
In general, memories are formed and stabilized through three distinct processes. Encoding refers to the initial acquisition of the memory. Then, through phases of consolidation, the memory is preserved and stored for later recall. Finally, retrieval is the process by which the previously stored memories are reactivated. Many different tasks have been developed to investigate the neural basis of memory and its distinct stages. However, it is important to note that all methodologies have limitations, which should be considered when analyzing outcomes. Human recognition memory is commonly tested in the visual paired comparisons task [15, see review 16], while a modified version of the task has been implemented for rodents [17]. Functional imaging studies, in humans, have identified patterns of region-specific neural activation associated with recollection and familiarity; however, animal models enable investigation of the neurobiological circuitry and cellular mechanisms of recognition memory, which are not possible in humans.
2. Novel Object Recognition
2.1. Task Procedures and Behavior Quantification
Implicit to the animal model approach is the necessity that the behavioral constructs that are modeled in rodents match to a large extent, human recognition memory. To this end, the spontaneous novel object recognition (NOR) task has emerged as the most popular test for assessing a rodent’s ability to recognize a previously presented stimulus [18]. Describing the task as such is misleading since it is not theoretically possible to recognize a novel object since recognition reflects prior exposure. While some have begun to adopt the more accurate phrase, spontaneous object recognition (SOR), most investigators continue to use novel object recognition or NOR in referring to the task. For the purposes of this review, we will refer to the aforementioned task as NOR; however, we assert that this designation does not adequately describe the object recognition memory that it can be inferred from it. Regardless, the NOR task has become the hallmark method used in assessing non-spatial object memory in rodents. Although there is considerable variability across labs in the NOR procedures used, most conduct the test in a familiar square or rectangular high-walled arena lacking polarizing spatial cues (see schematic in Fig 1 for a depiction of the most commonly applied variation of the NOR task). In an effort to further reduce contextual and spatial information, a Y-maze arena has been used in several influential studies [19–22]. Although this novel design reduces contextual information, reports using square or rectangular arenas limit spatial cues by minimizing all visual, textural, and odor stimuli. During what is referred to as the training or sample session, the rodent explores two identical novel objects encountered in a familiar arena. Object memory encoding is operationally defined as occurring during the sample session. Upon completion of the sample session the animal is removed from the arena for some specified amount of time (i.e., retention delay), during which the object memory is consolidated. For the subsequent test session, the rodent is returned to the same arena, which now contains an exact replica of the familiar object and a novel object, as a test of object memory retrieval. Rodents are self-motivated to spontaneously approach items and explore using multiple senses. Object exploration behavior is easily quantifiable and allows for the study of episodic-like memories in rodents. Rodents exhibit a natural proclivity to explore novel, non-threatening objects, and therefore, during the test session rodents exhibit a preference for exploring the novel object significantly more than the familiar one. Thus, sample object memory strength is inferred from the preference of the rodent to explore the novel object over the familiar object during the test session. Object memory is quantified by computing discrimination measures from scores of the amount of time during the test session that each animal explores the respective objects. Preference for the novel object, demonstrated by an increase in exploration time for that item, indicates that a memory trace for the familiar object was properly encoded, consolidated and then retrieved to guide the rodent’s behavior during the test session [23–28]. There are two quantitative measures that are commonly used in assessing test session exploration performance. The novel object preference ratio is determined by dividing the total object exploration time from the exploration time of the novel object. A value above 0.5 suggests preference for the novel object, while a value below 0.5 is indicative of familiar object preference. Chance performance is represented by a preference ratio of 0.5 [28]. Alternatively, the discrimination ratio is calculated by determining the difference in exploration time between the novel and familiar objects and dividing it by the total object exploration time. Discrimination ratio ranges from−1 to +1, with negative scores indicating preference for the familiar object and positive scores signifying preference for the novel object. Chance performance, or null preference, is represented by a score of 0 [23–26]. By factoring in total object exploration time, discrimination ratio has emerged as the preferred measure of object memory in studies of NOR. Regardless of the measurement strategy employed, deciphering object memory is dependent on the rodent exploring each presented object, in both the sample and test sessions, for at least a minimal amount of time.
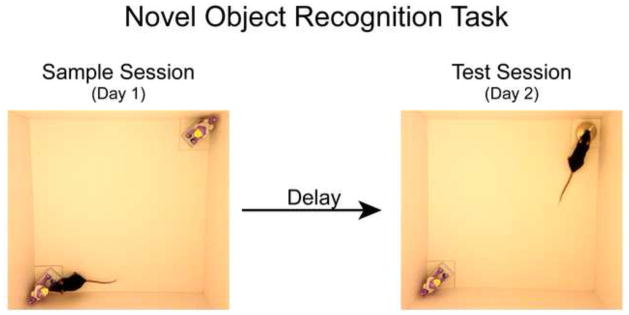
Figure 1. The novel object recognition (NOR) task.
The typical NOR protocol consists of a sample session (left) and a test session (right) conducted within a familiar cylindrical, rectangular, or y-shaped arena. During the sample session, the rodent is placed into the familiar arena to freely explore two presented novel objects for a specified amount of time. Upon completion of the sample session, the rodent is removed from the arena and is most often returned to its home cage. After a predetermined delay, the rodent is returned to the familiar arena for a time-bound test session in which one of the sample objects is replaced with a novel object. Task performance is assessed by analyzing the differences in time spent exploring both test session objects. These photographs depict an example of the arena and the objects our lab has used to test object memory in mice [34]. Although our objects are placed into opposite corners of the arena; others have placed the objects into the center of the arena. Our typical sample session concludes once the mouse has accumulated 38 s of exploration on one object, or 30 s of exploration of both objects; our sample session exploration criterion. During the test session presented on Day 2, the arena includes a copy of the familiar object from the sample session and a novel object. The relative positions of the novel and familiar objects during the test session are counterbalanced to eliminate spatial bias in the task.
2.2. Advantages of NOR Task
The NOR task offers advantages over other object memory tests for assessing recognition memory because it does not require any external motivation, reward or punishment [29], such as that required by the delayed non-match to sample task, in which a reward is administered when the subject correctly chooses a novel item over one that is familiar. Additionally, while rodents tend to require extensive training to accurately perform the delayed non-match to sample task [30], no training other than arena habituation is necessary to elicit object exploratory behavior in the NOR task. Finally, the NOR task procedures do not generate stressful conditions, while offering a robust test of nonspatial memory in rodents [31, 32].
2.3. Variability in Sample Session Exploration Criteria
During the sample session, the rodent is placed into the familiar arena that typically contains two identical novel objects, which the animal is then permitted to freely explore. The duration of the sample session tends to vary considerably across published papers (from 2 – 15 min). Many studies have implemented a procedure in which the sample session is terminated once the rodent accumulates a specific criterion amount of object exploration. Rodents that do not meet this sample session criterion are excluded from further analyses. Although there is considerable variability with respect to this criterion (at least 20 – 30 s of total sample object exploration), it has been a common practice to allot mice a longer exploration criterion over rat counterparts. On average, mice are required to explore the sample objects for about 38 s while rats are generally allotted around 30 s of total object exploration (see Table 1). However, this may be a factor that contributes to differences in experimental outcomes, given that the amount of time spent exploring an object may be directly proportional to the strength and detail of the memory formed. Imposing a criterion on sample object exploration is advantageous because all of the subjects, regardless of time in the arena, are matched for the amount of object exploration or degree of object training experienced. In fact, we have previously reported preliminary data demonstrating that the amount of object exploration during the sample session directly relates to the recruitment of different brain structures [33]; these results will be discussed in a later section. Therefore, it is extremely important that each experimental parameter, like exploration criteria, be explicitly considered when designing a specific task, as varying results are bound to occur. For those studies in which a fixed length sample session was imposed without the requirement of a sample object exploration criterion, one can only assume that the subjects had sufficiently explored the objects. Nevertheless, during the sample session of the NOR task, the animal is expected to devote equal time to exploring each of the presented objects since they should all be novel. During the test session, one of the familiar objects, from training, is replaced with a novel item. The presented familiar item is typically an exact replica of those explored during the sample session. However, in some cases the test session familiar object is one of the previously explored objects chosen at random for test session exploration. It is expected that rodents will preferentially explore the novel object, if the memory of the familiar object was successfully encoded, consolidated and then retrieved. Failure to exhibit novel object preference during the test session would be expected if object exploration during the sample session was minimal; a memory that was never encoded cannot be retrieved. Furthermore, weak novel object preference during the test session is widely considered to be indicative of impaired object memory; however, it could also reflect insufficient exploration of sample objects, which would go unnoticed if a sample session object exploration criterion was not required. Alternatively, the weak preference for the novel object can be interpreted as the retrieval of a weak memory for the sample objects. Although without sufficient information about the amount of object exploration during the sample session, test session performance is difficult to assess. Therefore, we contend that imposing a sample session object exploration criterion is essential for properly interpreting test session behavior.
Table 1.
Index of the experimental details and results from a subset of published NOR studies testing the involvement of the rodent hippocampus and which a sample session exploration criterion was imposed.
| Species & Strain | Technique | Structure | Treatment | Lesion size/Time of Drug Administration | Arena | Criterion | Delay | Detailed Results | Reference | Summary of Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Rat Long-Evans | inactivation | HPC (dorsal) | NMDA antag, APV (4 sites) | pre-sample | square 0.6m × 0.6m | 30-s sample | 5-m | not diff from sham | [74] | not impaired |
| Rat Long-Evans | inactivation | HPC (dorsal) | NMDA antag, APV (4 sites) | pre-sample | square 0.6m × 0.6m | 30-s sample | 3-h | sig diff from sham | [74] | impaired |
| Mouse C57BL/6J | inactivation | HPC (dorsal) | muscimol | pre-sample | square 0.4m × 0.4m | 30-s sample | 24-h | sig diff from sham | [34] | impaired |
| Mouse C57BL/6J | inactivation | HPC (dorsal) | muscimol | post-sample | square 0.4m × 0.4m | 30-s sample | 24-h | sig diff from sham | [34] | impaired |
| Mouse C57BL/6J | inactivation | HPC (dorsal) | muscimol | pre-, post-sample & pretest | square 0.4m × 0.4m | 30-s sample | 24-h | sig diff from sham | [34] | impaired |
| Mouse C57BL/6J | inactivation | HPC (dorsal) | anisomycin | post-sample 0h and 2h | square 0.4m × 0.4m | 30-s sample | 24-h | sig diff from sham | [34] | impaired |
| Mouse C57BL/6J | inactivation | HPC (dorsal) | anisomycin | post-sample 2h only (control study) | square 0.4m × 0.4m | 30-s sample | 24-h | not sig diff from sham | [34] | not impaired |
| Mouse C57BL/6J | inactivation | HPC (dorsal) | muscimol | pre-test (control study) | novel context for test (square 0.2m × 0.2m) | 30-s sample | 24-h | not sig diff from sham - controls fail to prefer novel obj | [34] | not impaired |
| Mouse C57BL/6J | inactivation | HPC (dorsal) | Lidocaine (0.5 μl of 4%/side) | pre-sample | square 0.4m × 0.4m | 38-s sample | 5-m | not sig diff from sham | [35] | not impaired |
| Mouse C57BL/6J | inactivation | HPC (dorsal) | Lidocaine (0.5 μl of 4%/side) | pre-sample | square 0.4m × 0.4m | 38-s sample | 24-h | sig diff from sham | [35] | impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Partial: 58% | square 0.9m × 0.9m | 30-s sample | 10-s | not diff from sham - results differ if looking at first minute of test or 30-s exp. | [75] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Partial: 58% | square 0.9m × 0.9m | 30-s sample | 1-m | not diff from sham | [75] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Partial: 58% | square 0.9m × 0.9m | 30-s sample | 10-m | chance | [75] | impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Partial: 58% | square 0.9m × 0.9m | 30-s sample | 1-h | chance | [75] | impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Partial: 58% | square 0.9m × 0.9m | 30-s sample | 24-h | chance | [75] | impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete: 98% | square 0.9m × 0.9m | 30-s sample | 10-s | not diff from sham | [75] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete: 98% | square 0.9m × 0.9m | 30-s sample | 1-m | not diff from sham | [75] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete: 98% | square 0.9m × 0.9m | 30-s sample | 10-m | not diff from sham | [75] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete: 98% | square 0.9m × 0.9m | 30-s sample | 1-h | not diff from sham | [75] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete: 98% | square 0.9m × 0.9m | 30-s sample | 24-h | chance | [75] | impaired |
| Rat Pigmented DA | lesion | HPC | NMDA | Partial: average 58% | rectangle 0.5m × 0.9m | 40-s sample | 5-m | not diff from sham - HPC: 58% (40–93%) | [76] | not impaired |
| Rat Pigmented DA | lesion | HPC | NMDA | Partial: average 58% | rectangle 0.5m × 0.9m | 40-s sample | 3-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | HPC | NMDA | Partial: average 58% | rectangle 0.5m × 0.9m | 40-s sample | 24-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | PRH+HPC contra | NMDA | PRH: average 85% HPC: average 54% | rectangle 0.5m × 0.9m | 40-s sample | 5-m | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | PRH+HPC contra | NMDA | PRH: average 85% HPC: average 54% | rectangle 0.5m × 0.9m | 40-s sample | 3-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | PRH+HPC contra | NMDA | PRH: average 85% HPC: average 54% | rectangle 0.5m × 0.9m | 40-s sample | 24-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | PRH+HPC ipsi | NMDA | PRH: average 86% HPC: average 61% | rectangle 0.5m × 0.9m | 40-s sample | 5-m | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | PRH+HPC ipsi | NMDA | PRH: average 86% HPC: average 61% | rectangle 0.5m × 0.9m | 40-s sample | 3-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | PRH+HPC ipsi | NMDA | PRH: average 86% HPC: average 61% | rectangle 0.5m × 0.9m | 40-s sample | 24-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | mPFC+ HPC contra | NMDA | mPFC: average 74% HPC: average 57% | rectangle 0.5m × 0.9m | 40-s sample | 5-m | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | mPFC+ HPC contra | NMDA | mPFC: average 74% HPC: average 57% | rectangle 0.5m × 0.9m | 40-s sample | 3-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | mPFC+ HPC contra | NMDA | mPFC: average 74% HPC: average 57% | rectangle 0.5m × 0.9m | 40-s sample | 24-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | mPFC+ HPC ipsi | NMDA | mPFC: average 69% HPC: average 54% | rectangle 0.5m × 0.9m | 40-s sample | 5-m | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | mPFC+ HPC ipsi | NMDA | mPFC: average 69% HPC: average 54% | rectangle 0.5m × 0.9m | 40-s sample | 3-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | mPFC+ HPC ipsi | NMDA | mPFC: average 69% HPC: average 54% | rectangle 0.5m × 0.9m | 40-s sample | 24-h | not diff from sham | [76] | not impaired |
| Rat Pigmented DA | lesion | HPC | Radiofrequency | Fornix Complete: ~80% | square 1.0m × 1.0m | 25-s sample | 15-m | not sig diff from sham - sig diff DR based on 1st min of test | [21] | not impaired |
| Rat Long-Evans | lesion | HPC | Ibotenic acid | Complete: 89.8% | square 0.9m × 0.9m | 30-s sample | 10-s | not sig diff from sham | [55] | not impaired |
| Rat Long-Evans | lesion | HPC | Ibotenic acid | Complete: 89.8% | square 0.9m × 0.9m | 30-s sample | 1-m | not sig diff from sham | [55] | not impaired |
| Rat Long-Evans | lesion | HPC | Ibotenic acid | Complete: 89.8% | square 0.9m × 0.9m | 30-s sample | 10-m | sig diff from sham | [55] | impaired |
| Rat Long-Evans | lesion | HPC | Ibotenic acid | Complete: 89.8% | square 0.9m × 0.9m | 30-s sample | 1-h | sig diff from sham | [55] | impaired |
| Rat Long-Evans | lesion | HPC | Ibotenic acid | Complete: 89.8% | square 0.9m × 0.9m | 30-s sample | 24-h | sig diff from sham | [55] | impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Complete: 71.2% | square 0.9m × 0.9m | 30-s sample | 10-s | not sig diff from sham | [55] | not impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Complete: 71.2% | square 0.9m × 0.9m | 30-s sample | 1-m | not sig diff from sham | [55] | not impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Complete: 71.2% | square 0.9m × 0.9m | 30-s sample | 10-m | sig diff from sham | [55] | impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Complete: 71.2% | square 0.9m × 0.9m | 30-s sample | 1-h | sig diff from sham | [55] | impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Complete: 71.2% | square 0.9m × 0.9m | 30-s sample | 24-h | sig diff from sham | [55] | impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Fornix Complete: 100% | square 0.9m × 0.9m | 30-s sample | 10-s | not sig diff from sham | [55] | not impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Fornix Complete: 100% | square 0.9m × 0.9m | 30-s sample | 1-m | not sig diff from sham | [55] | not impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Fornix Complete: 100% | square 0.9m × 0.9m | 30-s sample | 10-m | not sig diff from sham | [55] | not impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Fornix Complete: 100% | square 0.9m × 0.9m | 30-s sample | 1-h | not sig diff from sham | [55] | not impaired |
| Rat Long-Evans | lesion | HPC | Radiofrequency | Fornix Complete: 100% | square 0.9m × 0.9m | 30-s sample | 24-h | not sig diff from sham | [55] | not impaired |
| Rat Pigmented DA | lesion | PRH+HPC | NMDA+ Radiofrequency | Extensive | square 1m × 1m | 20-s (3 sessions) | 15-m | sig diff from sham - failed to discrim on all 3 sessions | [77] | impaired |
| Rat Pigmented DA | lesion | PRH+HPC | NMDA+ Radiofrequency | Extensive | square 1m × 1m | 40-s (3 sessions) | 15-m | sig diff from sham - failed to discrim on 1st and 3rd sessions | [77] | impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete | Y-shaped arena | 25-s sample (or 3 min) | 15-m | not sig diff from sham | [22] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete | Y-shaped arena | 25-s sample (or 3 min) | 1-h | not sig diff from sham | [22] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete | Y-shaped arena | 25-s sample (or 3 min) | 24-h | not sig diff from sham | [22] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete | Y-shaped arena | 25-s sample (or 3 min) | 48-h | not sig diff from sham | [22] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete: >70% | square 1m × 1m | 40-s sample | 2-m | not sig diff from sham - DR’s = sham 0.63; HPC 0.6 | [78] | not impaired |
| Rat Lister hooded | lesion | HPC | Ibotenic acid | Complete: 85–100% | cylinder 0.8m diameter | 15-s sample | 2-m | not sig diff from sham | [79] | not impaired |
| Rat Lister hooded | lesion | HPC | NMDA (pre-train) | Extensive loss DG,CA3-1 | Y-shaped arena | 25-s sample | 0-s | not sig diff from sham | [19] | not impaired |
| Rat Lister hooded | lesion | HPC | NMDA (pre-train) | Extensive loss DG,CA3-1 | Y-shaped arena | 25-s sample | 15-m | not sig diff from sham | [19] | not impaired |
| Rat Lister hooded | lesion | HPC | NMDA (pre-train) | Extensive loss DG,CA3-1 | Y-shaped arena | 25-s sample | 1-h | not sig diff from sham | [19] | not impaired |
| Rat Lister hooded | lesion | HPC | NMDA (pre-train) | Extensive loss DG,CA3-1 | Y-shaped arena | 25-s sample | 24-h | not sig diff from sham | [19] | not impaired |
Note.
For a more complete review of rodent object recognition memory studies in which the role of medial temporal lobe structures has been tested, please see supplementary table S1. Abbreviations: HPC, hippocampus; antag, antagonist; diff, different; sig, significant; obj, object; PRH, perirhinal cortex; contra, contralateral; ipsi, ipsilateral; mPFC, medial prefrontal cortex; discrim, discriminate; exp, exploration; DR, discrimination ratio. Statistical analyses revealed no significant effect of lesion treatment, arena characteristics, or rodent strain, on hippocampal involvement in the NOR task.
Given that object exploration is spontaneous, and variable from experiment to experiment and across labs, we suggest that it is important to determine whether the observed mean discrimination ratio or preference ratio score of each respective group of rodents differs significantly from chance. For example, it is expected that untreated, or sham lesioned rodents, should demonstrate significant novel object preference, during the test session, for the reasons described above. This analysis provides a good determinant of a failure of memory retrieval vs. retrieval of a weak memory for the sample objects. Moreover, in interpreting an observed lack of significant difference in discrimination ratios between sham and hippocampal lesioned rodents, one should consider whether performance of both groups is above chance (i.e., by comparing each group’s discrimination ratio scores to chance). Our review of the literature reveals that this additional analysis of performance compared to chance is not always pursued, and thereby one’s view of a particular result might be limited. For example, one might report a significant difference in mean discrimination ratio between two groups of rats (e.g., sham and lesion of region A) and interpret the result as the lesion leading to a failure of object recognition memory. However, if subsequent analyses against chance were to reveal that the mean discrimination ratios of both groups were significantly above chance, then one would have to have to consider that the lesion of region A merely attenuated object recognition memory, or that another region (e.g., region B) may also contribute to task performance. As this scenario suggests, analysis of test session performance against chance can provide a more thorough appreciation of a manipulation’s influence on object memory in the NOR task.
2.4. Protocol Modifications
To date, there have been numerous reports in which permanent or temporary manipulations are made to discrete brain structures in order to define the exact neural substrates of object memory. The results of these studies have been largely inconclusive, and one goal of the present review is to determine similarities and differences within the studies to bring about some clarity to the role of the rodent hippocampus in object memory.
Although the NOR task has been widely applied to the neurobiological study of memory, and in particular, to the analysis of the neural circuitry that subserves object memory, there appears to be little consistency in task procedures. There are vast differences in the characteristics of the objects used, the type of arena, lesion technique, lesion size, degree of lesion specificity, duration of the delay imposed and sample session training criterion (i.e., whether one was imposed or not, and if so, then the specific criterion also varies). In addition, the effects of rodent hippocampal lesions on object recognition memory could be influenced by the species used. Typically mice are allotted a longer sample session exploration time [34, 35] over their rat counterparts; however, this difference in required object exploration could contribute to a stronger vs. weaker memory formed. The significant number of variations across NOR protocols represents a considerable challenge to any effort to compare and contrast studies of object memory based on NOR performance. Clearly, any change in the protocol, whether minor or major, can influence the results. This problem was potently demonstrated in studies in which an identical set of behaviors were assessed in several strains of inbred mice simultaneously in three distinct geographic locations, with all other experimental features (i.e., experimenters, arenas, objects, exploration parameters and mouse strains) held consistent. Inconclusive findings are evidence that even when standardizations to the protocols are attempted, slight differences are still possible, which can lead to even greater alterations in data outcomes and interpretations [36]. Similarly, changes to experimental protocols that lead to differences in result outcome have been demonstrated with other behavioral tasks. It was found that monkeys with hippocampal lesions show a more profound impairment in a visual paired-comparisons task over the delayed nonmatching-to-sample test [37]. This finding was attributed to the fact that the encoding time used for the former task was longer than that of the latter. However, it was later discovered that in addition to the encoding times being different, the object stimuli also varied in terms of shape, color, size, brightness and texture. In turn, the reported findings may be confounded by the differences in procedure, leading to inconclusive data [37]. Regardless of the behavioral task employed, experimental parameters require consistency to elicit reliable and definitive findings. The NOR test may have significant advantages; however, it is the uniqueness and modifications of each experimental design, from lab to lab that make it hard to evaluate the growing amount of data.
Therefore, this review will stress the need for consistency among NOR protocol parameters to ensure accurate findings. We are not asserting that there is one “correct” method in which NOR should be performed, we are primarily attempting to demonstrate that the vast contradictory findings in the literature may be merely due to variations in NOR task parameters employed. Specifically, we will highlight two specific parameters that when varied seem to elicit different structural involvement in the rodent brain.
3. Effects of Permanent vs. Temporary Lesion of the Rodent Hippocampus
3.1. Effects by Size of Permanent Lesion
The NOR task has been applied extensively to decipher the differential contributions of rodent medial temporal lobe structures to object recognition memory. Permanent lesions have been traditionally used to investigate the role of a brain structure in a given behavior, or to establish brain region-behavioral relationships. The functions impaired, or the differences in behavior observed between sham and lesioned rodents, are then interpreted as those dependent upon the damaged or absent region. To take this position is to assume that all other brain regions are intact and functioning normally in the absence of the damaged region - even those regions deafferented from the lesioned structure. However, as others have argued in the past, this lesion followed by test approach is best suited for studying the degree of compensation the CNS can achieve after the lesion, or simply, what the brain-damaged rodent can learn and remember. The large majority of published studies have compared the NOR performance of rodents after sham or permanent brain lesions. The present review focuses solely on the effects of permanent or temporary lesions of the hippocampus on object memory in the NOR task. The permanent lesion studies tend to follow a common plan in which the anterograde effects of hippocampal lesions are examined on object memory. Specifically, rodents receive a partial or complete lesion of the hippocampus and after a recovery period of ~14 days, are habituated to the testing arena. During the subsequent sample and test sessions, hippocampal-lesioned rodents generally exhibit object exploration behavior consistent with that of sham-lesioned rodents. However, the majority of studies (72% of experiments in multi-study papers) find that novel object preference is spared in the hippocampal-lesioned rodents during the test session, while the remaining 28% of experiments report impaired novel object preference during the test session (see supplementary table S1). It has been suggested that this discrepancy in lesion outcome depends upon the relative completeness of the hippocampal lesion. Further, a few studies have examined retrograde effects of hippocampal lesions on object memory, by presenting the sample session, and then following some interval, lesions are made to the hippocampus of a subset of the rodents. Differences in test session performance between the sham and lesioned groups are interpreted as evidence that consolidation or retrieval of object memory is or is not dependent upon the hippocampus. Of the six studies that have examined retrograde effects of hippocampal lesions on NOR, two find that the hippocampal lesion impair retrieval of object memory encoded before the lesion. Such results provide support for the view that the hippocampus participates in the encoding and consolidation of object memory; the post-training lesion likely disrupts ongoing memory consolidation processes or interferes with the object memory retrieval. In one case [38], rats were found to exhibit impaired novel object preference when the hippocampus was lesioned post-training, an effect consistent with the view that object memory depends upon the hippocampus. However, these rats were later presented with a sample session of new objects and exhibited intact object memory during the subsequent test session. These results are consistent with our contention that object memory normally depends upon the hippocampus [34], and Gaskin et al. [38] interpreted their observed lack of lesion effects on anterograde object memory as evidence that when the hippocampus is not available, then other extrahippocampal regions compensate for the lost structure and support the ability of the animals to discriminate the objects. We completely agree with this interpretation. However, we contest that the lesion-induced compensatory role for extrahippocampal structures to support object memory likely also explains the spared object memory performance of hippocampal-lesioned rodents observed in so many experiments (see supplementary table S1). It is unclear why such an interpretation is largely ignored – our analyses of the permanent lesion of rodent hippocampus/NOR literature found remarkably little attention given to the possibility that spared hippocampal tissue or lesion-induced plasticity or covert pathology could influence the behavior expressed by rodents with permanent hippocampal lesions. Aggleton and colleagues have provided some of the most compelling evidence that permanent lesions of the rat hippocampus causes a marked decrease in immediate early gene expression in the retrosplenial cortex [39–41]. Such pathology is considered ‘covert’ since the authors found no detectable cell loss in the retrosplenial cortex. Similar covert pathology in retrosplenial cortex has also been reported after lesions of the anterior thalamic nuclei [42, 43]. Thus, it is imperative that interpretations of behaviors spared or impaired in rodents after permanent hippocampal lesions account for possible covert or overt pathology.
The notion that neural circuitry undergoes reorganization after peripheral or central damage has been fundamental to the explanation for partial or complete recovery of motor and cognitive functions after stroke or traumatic brain injury [44–46], and reorganization of the human sensory and motor cortices is a well accepted consequence of practiced use [47, 48]. The phenomenon of phantom limb pain is considered to be one of the most compelling (and accepted) models of deafferentation-induced plasticity or reorganization of the somatotopic body surface map in human primary somatosensory cortex [49–52]. In each of these conditions, experience-dependent reorganization of neural circuitry enables recovery of function or new abilities. One could apply this notion to the spared object memory observed in hippocampal lesioned rodents. That is, spared object memory reflects the emerging significant participation of extrahippocampal regions, as the ‘phantom hippocampus’.
The availability of excitotoxins [53] and higher resolution stereotaxic manipulators [54] have greatly improved the specificity and accuracy of the permanent lesion technique. However, this approach is not appropriate for testing specific time-dependent processes, and the differential role of given brain structures in distinct stages of memory processing. Therefore, understanding the anatomical basis of the discrete steps in memory encoding, consolidation and retrieval cannot be sufficiently addressed with a permanent lesion. Further, as discussed above, potential compensatory mechanisms can govern the restructuring of the memory circuit in the post-lesion animal. Newly developed temporary pharmacological and genetic inactivation techniques allow for more precise investigation of distinct stages of memory and the brain structures involved.
3.2. Effects of Temporary Pharmacological Inactivation of the Hippocampus
There has been a steady increase in the application of neuropharmacological and genetic tools, which do not impose permanent structural changes in hippocampal circuitry, to investigate influences on distinct object memory processes (see Fig 2). The NOR task permits a clear operational definition of memory encoding, consolidation and retrieval due to its typical sample, then delay, then test sequence of a given trial. For example, administration of a drug, with a short onset of effect, before the sample session would enable one to examine effects of that treatment on the encoding of object memory; administration immediately following the sample session would test the treatment’s effect on consolidation of object memory; and, administration immediately before the test session would test the treatment’s effect on the retrieval of object memory. In addition, unintentional effects of experimental manipulations on task performance can be distinguished from those on learning and memory. For example, if a given treatment increases anxiety as a side effect, thigmotaxis and other mobility impairing or enhancing responses can be quantified; if the treatment affects attention or motivation one can assess the time required for the subjects to acquire some criterion amount of object exploration (although this measure is not always taken into consideration and could affect the interpretation of findings, as previously stated). Clearly, when determining the treatment or technique that will be employed in a study, it is important to consider a variety of analytical measures to ensure that the treatment or method is not causing any unintended effects that can alter the interpretation of the data.
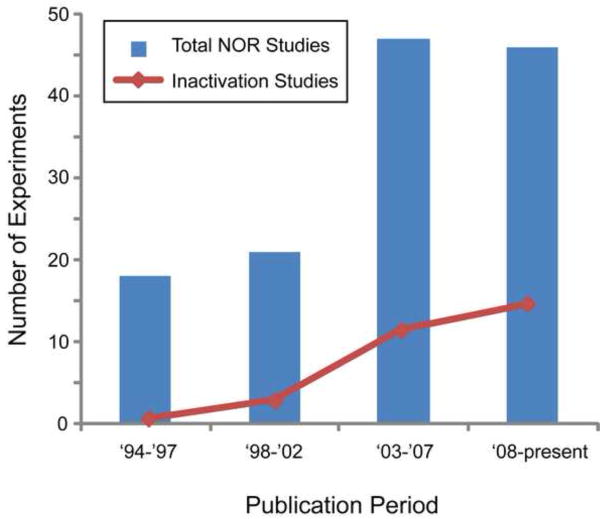
Figure 2. Publication rate of permanent or temporary hippocampal lesion experiments in which a sample session exploration criterion was imposed.
The graph depicts the number of experiments testing the involvement of the hippocampus appearing within multi-experiment peer-reviewed object recognition reports published over the last 20 years (bars) and the specific number of hippocampal inactivation experiments (line). The time bins are defined as 3 to 5-year intervals. It is noteworthy that the publication rates of experiments that utilize the temporary inactivation technique have increased considerable in the last decade.
Many studies have utilized the permanent lesion technique to test the role of the rodent hippocampus in NOR, and most report that non-spatial object memory is spared. Such results are then interpreted as evidence that object memory is independent of the rodent hippocampus. However, the previously stated drawbacks of the permanent lesion method are likely to have contributed to these findings (e.g., lesion-induced compensation). Therefore, it is possible that this strategy of permanently silencing a brain region is not adequate for addressing the underlying function of a structure, but rather, it is beneficial for studying the structures needed to support memory when the structures that normally support it are missing. On the other hand, temporary pharmacological inactivation techniques have emerged as an alternative method that may be better suited for understanding the roles of specific brain regions. Drug administration directly into a discrete brain structure can transiently inactivate the area of interest. For example, a variety of drugs are available to inactivate the hippocampus via blockade of excitatory neurotransmission or increasing inhibitory neurotransmission. The transient nature of these pharmacological approaches circumvents some of the issues that arise from permanent lesion. However, the temporary inactivation technique is not without limitations. Specifically, intracranial drug administration is thought to lack regional specificity, although the same can be said about lesions [55]. Many studies have used intracranial muscimol, a GABAA agonist, as the inactivating pharmacological agent, because it can impair the function of a structure without affecting fibers of passage [56, 57]. The precise distribution of muscimol or other drugs after local infusion within the CNS appears to depend on a number of factors such as lipid solubility, presence of fiber tracts, etc. [58, 59]. The development of a fluorophor-conjugated muscimol has aided in relating observed behavioral effects to the drug’s distribution within and beyond its intended target [34, 56, 60].
Taken collectively, the large majority of published results indicate that temporary inactivation of the rodent hippocampus impairs object recognition memory (see Table 1). Our lab reported that bilateral intrahippocampal microinfusion of lidocaine before the sample session impaired novel object preference in mice during a test session 24 h later, but spared novel object preference during a test session 5 min after the sample session [28]. These results were interpreted as support for the view that the hippocampus is critical for the processes of object recognition memory encoding and consolidation. However, an influence of intrahippocampal lidocaine on neural activity in fibers of passage cannot be ruled out in interpreting these results. We recently reported the impairing effect that muscimol microinfusion into the CA1 region of the dorsal hippocampus has on the encoding, consolidation and retrieval of object memory in mice [25]. Regardless of whether the treatment was administered prior to the sample session, immediately following the sample session, or before the test session, the mice that received intrahippocampal muscimol, exhibited significantly lower discrimination ratios compared to controls. It is also important to note that the behavior of the muscimol-treated or anisomycin-treated (0 and 2 hr post sample) groups were not significantly different from chance performance [34]. These results confirm our earlier conclusion based on our lidocaine results and support those of others [61, 62]. Furthermore, we reported that fluorophor-conjugated muscimol impaired retrieval of object memory in mice after bilateral intrahippocampal infusion, a behavioral effect due to the drug diffusing within, but not beyond, the CA1 region of the dorsal hippocampus [34].
Our finding that object memory processes depend on the dorsal hippocampus is in conflict with the spared object memory reported in rodents after permanent hippocampal lesion. It is noteworthy that inactivation of the hippocampus also impaired a form of object memory encoded explicitly independent of the arena context using a modified NOR protocol in which mice explored the same sample objects for three sessions (1/day) with each session in a novel context [34]. Thus, temporary inactivation studies reveal that the rodent hippocampus is necessary for distinct stages of object memory, and for an object memory independent of context. In this manner we have reduced the relevance of spatial and contextual cues that some have argued define the hippocampal involvement in the NOR task. Therefore, we have created task parameters that mirror those of Winters and Bussey’s enclosed Y-maze [19, 20], yet we find that the hippocampus is involved in the object aspects of the memory, while they report that only the perirhinal cortex is involved in object recognition memory. Certainly a clear picture of the neural substrates of object memory is difficult to determine from the conflicting findings that have arisen from the various methodologies applied to the question of the hippocampal role in object memory.
3.3. Variability in Intersession Delay and Sample Session Object Exploration Criteria
The relative difficulty, or memory demand, of the NOR task can be adjusted by increasing or decreasing the delay imposed between the sample and test sessions. In this way, NOR has been used to study short-term and long-term object recognition memory, as well as the rate of object memory degradation. With sufficiently long delays, the memory of the familiar item becomes progressively more vulnerable to decay, and, in this case, during the test session the animal will explore the familiar object and the novel object to an equal extent. It is this delay variable that can be widely manipulated to achieve vastly different results. The same holds true for sample session exploration criterion. Changes made to increase or decrease the time required to explore sample session objects can affect test session performance. Central to this review is our contention that the differential sensitivity of rodent object memory to hippocampal compromise when delays of varying length are imposed between sample and test sessions reveals a temporal specificity for hippocampal involvement in object memory. Additionally, our analysis of previously reported data indicates that the largest inconsistencies in results of hippocampal manipulation are found amongst the training criterion imposed during the sample session. Based on our analysis of the published reports, we suggest that the large variability of behavioral findings may be related to differences in the inter-session delay and the sample session object exploration criterion. However, it is important to note that even when efforts are made to reduce variability across testing procedures, contradictory findings may still arise [36]. Due to the large number of published studies, and the sizable variability between each study parameter, determining the neural structures that are needed for object recognition memory has been widely debated. As stated above, the predominant view is that the perirhinal cortex is responsible for rodent object memory, while a few other groups have argued for the critical contribution of the hippocampus. However, there is no indication in the literature that both structures are needed. This seeming contradiction is hard to reconcile with the current published studies, given all of the variations in task procedures that have been imposed. It is likely that this type of episodic memory depends upon both the perirhinal cortex and hippocampus, with each structure holding a specific unique contribution, irrespective of context.
The present review focuses on those reports in which the role of the mouse or rat hippocampus on object memory was tested in the NOR task using traditional lesions (i.e., electrolytic, radiofrequency or excitotoxic means) or functional inactivations (i.e., local microinfusion of lidocaine, muscimol, or AMPA receptor antagonist). For an extensive review of the NOR task and its application to the neurobiological study of memory, see review by Dere et al. [63]. A more complete list of rodent object recognition experimental findings can be found in the supplementary table (S1). Limiting our scope to the subset of studies that imposed a specific sample session exploration criterion, we analyzed 12 peer-reviewed reports – most of which were multi-experiment papers. Noticeable trends in the patterns of results can be gleaned from Table 1, which summarizes a number of experiment features and the results of the 132 experiments contained within those selected reports.
Over the last 20 years there has been a marked escalation in the total number of NOR publications, with the largest increase over the last decade. It is interesting to note that the number of studies using the temporary inactivation approach has progressively and consistently increased in recent years (see Fig 2). Thus, the steady increase in total publications likely reflects a converging acceptance of the NOR task as a method for assessing object memory and the interest in whether object memory is hippocampal-dependent. The increase in the number of temporary inactivation papers may reflect the previously mentioned advantages of this technique over permanent lesions. Given the aforementioned variations in NOR task procedures used by distinct labs to meet experimental requirements, we assert that it is difficult to draw overall conclusions about these techniques for assessing the neural basis of object memory. Therefore, no distinct differences or trends could be made based on the specific lesion technique, species of rodent studied, or on the type or extent of the lesion. However, interesting developments were evident in terms of sample session object exploration criteria and intersession delay (Table 1). Across all experiments analyzed, in which a training criteria was imposed (63 experiments in total) and regardless of technique used to impair hippocampal function, object memory was never found to be impaired when a delay of less than 10 min was imposed between the sample and test sessions. However, when the delay was extended to 10 min or greater, the results of 25 of the 37 permanent hippocampal lesion experiments indicated sparing of object memory, while 12 indicated significant impairment. The reasons for this considerable discrepancy in the data are not clear.
A clearer picture emerges when one considers those studies in which temporary inactivation was used to test the role of the rodent hippocampus in object memory using the NOR task. Specifically, 6 of the 8 published experiments found a significant impairment in object memory when a sample session exploration criteria and a delay greater than 10 min were both imposed. The two remaining studies had been designed as control experiments, and as such the functional inactivation method used was not anticipated to yield an impairment of object memory. More specifically, these control experiments were designed to demonstrate that in situations that are not expected to impair the hippocampus, hippocampal inactivation would not elicit changes in overall behavior. First, when mice were administered intrahippocampal anisomycin, a protein synthesis inhibitor, 2 h after the sample session, it was expected that enough time had passed for protein translation to occur, such that object memory consolidation would not be affected. Thus, the study was designed as a control measure ensuring that the impairment found when anisomycin was given immediately and 2 hr post sample was truly a result of protein synthesis inhibition during a critical consolidation time window. The second control study was performed to confirm that mice explore familiar objects more when placed in a novel environment, as had been established in rats [64]. Mice explored two sample objects in the same arena for 10 min over 3 consecutive days. The test session was then conducted in a novel context. Indeed, as expected in the novel context both intrahippocampal vehicle and muscimol treated mice explored the familiar and novel objects equally. In contrast, when the 3 sample sessions were each presented in different contexts, the intrahippocampal vehicle mice would show preference for the novel object during the test session, while the muscimol mice did not [34].
In light of the motivation for these control studies, it is reasonable to assert that 100% of the hippocampal inactivation experiments found that object memory was impaired when a sample exploration criterion was imposed and a delay of 10 min or greater was defined between sample and test. This pattern of results suggests that the involvement of the rodent hippocampus in object memory depends upon the interval between training and testing. Given that the hippocampus is not informed a priori as to the retention interval to be imposed (i.e., that it will be less than or greater than 10 min), it is more parsimonious to suggest that the delay-dependent involvement of the hippocampus reflects a delay in the hippocampus receiving and processing object information from the sample session, or that sufficient training or exploration of the sample objects is required before the hippocampus is engaged. Our interpretation of the discrepancy in results of the inactivation experiments with those of permanent lesion experiments is multi-faceted. It is clear from both permanent and temporary lesion experiments that the hippocampus is not required if the delay between sample and test is less than 10 min. In this case we infer that the test session is presented before the hippocampus has received the object information, or the episodic memory of the sample session object exploration has been encoded by the hippocampus. It is possible that for a short time, extrahippocampal structures (e.g., the perirhinal cortex) are responsible for the temporary maintenance of the object memory. This explanation fits the observed lack of behavioral impairment when the intersession delay was less than 10 min. However, when longer delays are imposed, our analysis reveals that hippocampal inactivation is more likely to impair object memory, while permanent hippocampal lesions are not. This inconsistency is possibly due to the aforementioned drawbacks of the permanent lesion technique.
As a corollary, the other trend that emerged from our analysis was the notion that imposing a sample session object exploration criterion affected the experimental outcome after hippocampal inactivation or lesion. Based on the studies examined, when analyzing delay, exploration times during the sample session seem to be important in determining if the test session would reveal behavioral impairments. Since there does not appear to be any consistency within the literature as to a standard and sufficient training criterion, the interpretation of these data is much more difficult. It is reasonable to state that less time spent exploring objects (e.g., less than 20 – 30 s of total object exploration) would lead to a weaker object memory encoded, since only basic information about the items is learned in a short time frame, and the opposite would hold true for longer exploration times (e.g., greater than 20 – 30 s of total object exploration) [65]. The more information and details that can be acquired about an item, the stronger that memory is expected to be. This notion lends support to the theory that the perirhinal cortex is involved in object familiarity while the hippocampus is needed for recollection of the object experience or event. As previously mentioned, we recently reported preliminary evidence that object memory reflecting a low level of sample object exploration is vulnerable to inactivation of the mouse perirhinal cortex, but not hippocampus [33]. Specifically, mice that were required to accumulate a very short sample session object exploration criterion demonstrated weak object memory during the test session, 24 h later. However, if the perirhinal cortex were temporarily inactivated immediately after acquiring this shortened sample session criterion, then these mice were impaired in the test session. Conversely, if the infusion was directed into the dorsal hippocampus, then the mice performed equivalently to controls in the test session, indicating retrieval of a weak object memory. Alternatively, if the mice were required to accumulate a large sample session object exploration criterion, perirhinal cortex inactivation post-sample did not impair test session performance, while direct infusion into dorsal hippocampus elicited significant impairments [33]. Our interpretation is that imposing a low sample session object exploration criterion probably yields an object memory that is weak, with fewer details being encoded and retained. The perirhinal cortex would most likely support memory of objects explored for a brief or limited amount of time, or in the absence of robust contextual cues [20]. Conversely, object memories that reflect significantly greater time spent exploring the sample session objects to reach a higher training criterion are more vulnerable to inactivation of the hippocampus than the perirhinal cortex. This result implies that stronger, more deeply encoded object memory is more likely to be supported by the hippocampus. This theory reasonably justifies the seeming discrepancy in the literature regarding the contributions of the hippocampus and perirhinal cortex to object memory.
3.4. A Model of the Contributions of Perirhinal Cortex and Hippocampus to NOR
Although the hippocampus and perirhinal cortex often function as two parts of an interacting memory system, their contributions are distinct and dissociable [10, 20, 66, 67]. Generally, it is the belief that the hippocampus and perirhinal cortex are functionally distinct based on the information they process. As previous stated we hold that the sample session exploration criterion, or more specifically the amount of object information acquired, that is the deciding factor in when the perirhinal cortex and hippocampus are playing their respective roles in object recognition memory. Therefore, we assert that it is not the categorical information that these regions process that separates them, but the strength of the memory formed. This gradient of memory strength between the two structures may account for some of the disagreements in the literature as to how these structures are involved in episodic memory. The theory that these memories can be formed along a continuum of memory strength lends itself to the notion that these two structures could be storing information based on a level of weak familiarity or strong recollection (for a schematic of the proposed theory, see Fig 3). As the theoretical model depicts, at the start of the sample session, object information will begin to “flow” into the perirhinal cortex. After a critical or threshold amount of object information is acquired, a process by which the information is “transferred” to the hippocampus commences. If this threshold is not reached, then the information will remain perirhinal cortex dependent as a weak object memory. Conversely, if the threshold is reached, then the information will become hippocampal dependent as a strong object memory.
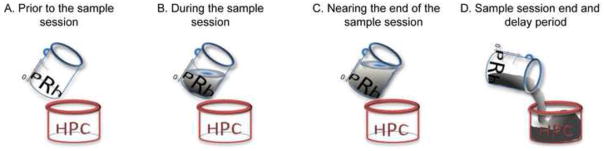
Figure 3. Qualitative model depicting how the perirhinal cortex and hippocampus contribute to the object recognition memory.
A. Prior to the start of the NOR sample session, both the perirhinal cortex and the hippocampus (labeled as PRh and HPC, respectively) lack sample object information. B. As object exploration commences during the sample session, the perirhinal cortex begins to accumulate with information. C. Until a minimum amount of sample object exploration has elapsed, the object information remains perirhinal cortex dependent. D. Once the critical threshold of object exploration is reached (perhaps 30/38 s of sample object exploration), a transfer of the object information to the hippocampus is initiated; this multi-synaptic transfer requires some time delay to be completed.
3.5. Alternative Explanations for Differences in Results and New Method Implementations
Although the interpretation of the emergent trends is plausible, there are other possible explanations for the inconsistencies in the effects of hippocampal manipulation on object memory. For example, an overwhelming majority of the studies we reviewed used rats, while very few have used mice. Thus, it is reasonable that some of the irregularities between studies could be attributable to species differences. However, this does not seem likely, as few other behavioral differences between species have been reported within these studies. Beyond the NOR task, there are reports demonstrating that relative to rats, mice are impaired in hippocampal-dependent place and matching-to-place learning in swimming pools [68, 69], and that mice demonstrate significantly more bouts of aggressive behavior to juvenile counterparts compared to rats [70]. It is equally or even more plausible that the trends we have identified in our analysis of the hippocampal involvement in NOR, are due to the inconsistencies and variations in the NOR protocol itself. As previously stated, there is no standard method by which the NOR task is conducted across labs. The overwhelmingly large number of task variables and manipulations make comparing studies extremely difficult. In turn, it is nearly impossible to gain definitive global insights about brain structures from all of the data that has been previously collected. A standardization of the NOR protocol is essential before one can equate findings across studies accurately. This is especially true for sample session object exploration criterions imposed. Given that this is the pivotal stage for which all subsequent results are based, consistency of training protocols is paramount to accurate data interpretation. As stated above, the sheer amount of information attained from the training session could be the deciding factor determining which brain structure is responsible for maintaining that information. Additionally, due to the number of disadvantages attributed to the permanent lesion and temporary inactivation approaches, other techniques are now being explored. The lack of specificity and the imposed duration of the lesion/inactivation are key shortcomings of current procedures. Optogenetics and DREADDs are two new techniques that use viral vectors to infect discrete types of neurons or brain regions with non-native channels or designer G-protein coupled receptors, which permit subsequent exquisite temporal and regional control over the activity of the infected neurons. Optogenetics permits near immediate and reversible silencing or activation of neurons in a given brain region [71, 72], features that make the technique well suited for defining the neuronal bases of discrete object memory processes. The DREADDS approach permits one to silence or activate a given brain region via systemic injection of a drug whose only target is the designer receptor [73]; by infecting all of the neurons in a given brain region, this strategy is particularly well suited to determining the behavioral roles of restricted brain regions of interest. These new methods prevail over the limitations and criticisms of the currently utilized techniques. It is the hope that with these new methods, investigating the roles of specific brain regions in the NOR task will provide clearer evidence as to how the hippocampus, specifically, is functionally involved in object memory.
4. Conclusions
In summary, the goal of our review was to evaluate the current literature regarding the role of the rodent hippocampus in object recognition memory as assessed with the NOR task. As discussed, this literature is divisive and replete with conflicting results. Our analysis of those 12 published reports that met our exclusion criteria and in which temporary or permanent lesions were employed, revealed remarkable differences in experimental outcomes depending on the method of hippocampal lesion used. That is, the majority of experiments find object recognition memory to be unimpaired in rodents with permanent lesions of the hippocampus, while a majority of experiments find object recognition memory processes to be impaired after temporary inactivation of the hippocampus. The permanent lesion approach has enjoyed a long history in behavioral neuroscience and is appropriate for developing rodent models of human amnesia. However, we caution that the marked differences in outcome of experiments using permanent vs. temporary hippocampal lesion should call into question the appropriateness of the permanent lesion method for testing the involvement of a given brain region in time-dependent behavioral processes. Moreover, that behavior displayed by rodents after permanent hippocampal lesion reflects compensation of neural circuitry that includes overt and covert pathology. In this light, we contend that spared object recognition memory so often reported in rodents after permanent lesions of the hippocampus is actually ‘phantom hippocampal-dependent object memory’; in the same way that phantom limb pain arises after amputation-induced reorganization of the human somatosensory cortex. The spared object recognition memory in the hippocampal-lesioned rodent arises from extrahippocampal structures due to reorganization of the medial temporal lobe circuit. Although temporary inactivation methods are better suited for defining the neural substrates of such time-dependent memory processes, the current techniques are not free of limitations. The on-going development of optogenetic and chemogenetic tools appears to hold strong promise as such approaches offer considerable advantages in terms of regional specificity and temporal control over more traditional functional inactivation methods. Our analysis also provides a theory that dictates two clear predictions as to the precise NOR test conditions that engage the rodent hippocampus. First, that the hippocampus is necessary for the retention of object recognition memory when a delay greater than 10 min is imposed between the NOR sample and test sessions. If a delay less than 10 min is imposed, then interrupting hippocampal function does not impair NOR performance; however, under these conditions the object memory is sensitive to perirhinal cortex inactivation. This theory suggests a partnership of sorts in which the perirhinal cortex and the hippocampus both participate in object memory processing. Second to this hypothesis is that the involvement of the hippocampus appears to depend upon the amount of time the rodents spend exploring the objects during the sample session. That is, a threshold amount of sample object exploration beyond ~30 s on each object appears to be a condition required to engage the hippocampus or, more specifically, to “move” neural control over the memory from the perirhinal cortex to the hippocampus. Interestingly, both predictions hold the assertion that neither the hippocampus nor perirhinal cortex is solely responsible for object memory as assessed by the NOR task. Instead, our interpretation is that episodic memory for objects explored during the NOR sample session emerges from interactions between the perirhinal cortex and the hippocampus. It will be of interest to test these predictions more thoroughly in order to understand the neural circuit that subserves object memory and to further identify the behavioral roles for the individual structures within the medial temporal lobe memory system. We propose that the key to further investigating the relationship between these structures, and the issues of memory strength, and familiarity vs. recollection, requires that the NOR protocol be standardized in order to enable meaningful interpretation across experiments. It is only in such a common-use framework that meta-analyses of the sort attempted here, might reveal the definitive roles of these structures in object memory.
Supplementary Material
Highlights.
Recent papers on the role of hippocampus in NOR are reviewed
Object recognition is a well accepted task for testing rodent nonspatial memory
Temporary and permanent hippocampal lesions inconsistently affect NOR performance
Differences in exploration criterion and delay confound interpretation of results
Need for the standardization of NOR procedures is stressed
Acknowledgments
This work was supported in part by NIMH MH086591 to RWS.
Footnotes
Author Contributions: S.J.C. and R.W.S. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yonelinas AP, Levy BJ. Dissociating familiarity from recollection in human recognition memory: different rates of forgetting over short retention intervals. Psychon Bull Rev. 2002;9:575–82. doi: 10.3758/bf03196315. [DOI] [PubMed] [Google Scholar]
- 3.Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–2. [Google Scholar]
- 4.Curran T, DeBuse C, Woroch B, Hirshman E. Combined pharmacological and electrophysiological dissociation of familiarity and recollection. J Neurosci. 2006;26:1979–85. doi: 10.1523/JNEUROSCI.5370-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110:611–46. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 6.Rugg MD, Yonelinas AP. Human recognition memory: a cognitive neuroscience perspective. Trends Cogn Sci. 2003;7:313–9. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 7.Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- 8.Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–8. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–44. discussion 44–89. [PubMed] [Google Scholar]
- 10.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannula DE, Ranganath C. The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–9. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psych Rev. 2007;114:152–76. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- 13.Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- 14.Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Fagan JF., 3rd Memory in the infant. J Exp Child Psychol. 1970;9:217–26. doi: 10.1016/0022-0965(70)90087-1. [DOI] [PubMed] [Google Scholar]
- 16.Burbacher TM, Grant KS. Measuring infant memory: Utility of the visual paired-comparison test paradigm for studies in developmental neurotoxicology. Neurotoxicol Teratol. 2012;34:473–80. doi: 10.1016/j.ntt.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244–54. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 19.Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–8. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–70. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 22.Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15:347–55. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- 23.Aggleton JP, Albasser MM, Aggleton DJ, Poirier GL, Pearce JM. Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behav Neurosci. 2010;124:55–68. doi: 10.1037/a0018320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–73. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen SJ, Munchow AH, Rios LM, Zhang G, Ásgeirsdóttir HN, Stackman RW., Jr The rodent hippocampus is essential for nonspatial object memory. Curr Biol. 2013;23:1685–90. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–60. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–26. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci. 2004;24:1948–53. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 32.Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, et al. Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry. 2014;19:811–22. doi: 10.1038/mp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen SJ, Munchow AH, Stackman RW., Jr Behavioral and molecular evidence that the rodent perirhinal cortex and dorsal hippocampus are essential in object recognition memory. Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2013. p. 772.05. [Google Scholar]
- 34.Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW., Jr The rodent hippocampus is essential for nonspatial object memory. Curr Biol. 2013;23:1685–90. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 37.Zeamer A, Meunier M, Bachevalier J. Stimulus similarity and encoding time influence incidental recognition memory in adult monkeys with selective hippocampal lesions. Learn Mem. 2011;18:170–80. doi: 10.1101/lm.2076811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaskin S, Tremblay A, Mumby DG. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus. 2003;13:962–9. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- 39.Aggleton JP. EPS Mid-Career Award 2006. Understanding anterograde amnesia: disconnections and hidden lesions. Q J Exp Psychol. 2008;61:1441–71. doi: 10.1080/17470210802215335. [DOI] [PubMed] [Google Scholar]
- 40.Albasser MM, Poirier GL, Warburton EC, Aggleton JP. Hippocampal lesions halve immediate-early gene protein counts in retrosplenial cortex: distal dysfunctions in a spatial memory system. Eur J Neurosci. 2007;26:1254–66. doi: 10.1111/j.1460-9568.2007.05753.x. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins TA, Amin E, Brown MW, Aggleton JP. Changes in immediate early gene expression in the rat brain after unilateral lesions of the hippocampus. Neuroscience. 2006;137:747–59. doi: 10.1016/j.neuroscience.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins TA, Dias R, Amin E, Aggleton JP. Changes in Fos expression in the rat brain after unilateral lesions of the anterior thalamic nuclei. Eur J Neurosci. 2002;16:1425–32. doi: 10.1046/j.1460-9568.2002.02211.x. [DOI] [PubMed] [Google Scholar]
- 43.Jenkins TA, Vann SD, Amin E, Aggleton JP. Anterior thalamic lesions stop immediate early gene activation in selective laminae of the retrosplenial cortex: evidence of covert pathology in rats? Eur J Neurosci. 2004;19:3291–304. doi: 10.1111/j.0953-816X.2004.03421.x. [DOI] [PubMed] [Google Scholar]
- 44.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–87. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 45.Kokotilo KJ, Eng JJ, Curt A. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. J Neurotrauma. 2009;26:2113–26. doi: 10.1089/neu.2008.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita T, Abe K. Potential Treatment Strategies for Enhancing Neuroplasticity and Regeneration After Ischemic Stroke. Future Neurol. 2012;7:279–85. [Google Scholar]
- 47.May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn Sci. 2011;15:475–82. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Thomas C, Baker CI. Teaching an adult brain new tricks: a critical review of evidence for training-dependent structural plasticity in humans. Neuroimage. 2013;73:225–36. doi: 10.1016/j.neuroimage.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 49.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–4. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 50.Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci. 2001;21:3609–18. doi: 10.1523/JNEUROSCI.21-10-03609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–60. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 52.Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121( Pt 9):1603–30. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 53.Jarrard LE. Use of excitotoxins to lesion the hippocampus: update. Hippocampus. 2002;12:405–14. doi: 10.1002/hipo.10054. [DOI] [PubMed] [Google Scholar]
- 54.Coffey KR, Barker DJ, Ma S, West MO. Building an open-source robotic stereotaxic instrument. J Vis Exp. 2013:e51006. doi: 10.3791/51006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–60. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. J Neurosci Methods. 2008;171:30–8. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chrobak JJ, Stackman RW, Walsh TJ. Intraseptal administration of muscimol produces dose-dependent memory impairments in the rat. Behav Neural Biol. 1989;52:357–69. doi: 10.1016/s0163-1047(89)90472-x. [DOI] [PubMed] [Google Scholar]
- 58.Arikan R, Blake NM, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods. 2002;118:51–7. doi: 10.1016/s0165-0270(02)00143-7. [DOI] [PubMed] [Google Scholar]
- 59.Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–4. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- 60.Stackman RW, Jr, Lora JC, Williams SB. Directional responding of C57BL/6J mice in the Morris water maze is influenced by visual and vestibular cues and is dependent on the anterior thalamic nuclei. J Neurosci. 2012;32:10211–25. doi: 10.1523/JNEUROSCI.4868-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Lima MN, Luft T, Roesler R, Schroder N. Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neurosci Lett. 2006;405:142–6. doi: 10.1016/j.neulet.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 62.Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, et al. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–71. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clark RE. Recognition memory: an old idea given new life. Curr Biol. 2013;23:R725–7. doi: 10.1016/j.cub.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 67.Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn Sci. 2011;15:210–7. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whishaw IQ, Tomie J. Of mice and mazes: similarities between mice and rats on dry land but not water mazes. Physiol Behav. 1996;60:1191–7. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]
- 69.Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11:3461–5. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- 70.Engelmann M, Hadicke J, Noack J. Testing declarative memory in laboratory rats and mice using the nonconditioned social discrimination procedure. Nat Protoc. 2011;6:1152–62. doi: 10.1038/nprot.2011.353. [DOI] [PubMed] [Google Scholar]
- 71.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16:805–15. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res. 2006;167:183–95. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–31. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ennaceur A, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res. 1997;88:181–93. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- 78.Good MA, Barnes P, Staal V, McGregor A, Honey RC. Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci. 2007;121:218–23. doi: 10.1037/0735-7044.121.1.218. [DOI] [PubMed] [Google Scholar]
- 79.Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–53. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.