Abstract
Laparoscopic myomectomy (LM) is becoming increasingly common in the management of uterine myomas and is usually offered regardless of the number, location, and size of the myomas. It has a generally low rate of periprocedural complications and is preferred to laparotomy for several reasons that are not limited to decreased length of hospital stay, number of sutures, smaller incisions, and decreased pain. However, blood loss during LM remains a challenge. To be able to stratify patients and provide better management after LM, it is crucial to identify these predictors of blood loss. Therefore, the aim of this review was to identify the risk factors for periprocedural blood loss after laparoscopic uterine myomectomy. According to our data synthesis, age, body mass index, and phase of the menstrual cycle do not seem to affect the blood loss during LM. Conversely, size and number of myomas, as well as operative time, was directly related to the increase of blood loss.
Keywords: Hemoglobin drop, laparoscopy, myomectomy, systematic review
INTRODUCTION
Uterine fibroids are reported to be the common benign tumor of the uterus, with an incidence exceeding 70-80% among women.[1] Although the etiology of myomas is still debated, accumulating evidence suggests that epigenetic changes may play a key role.[2] Myomas are generally found incidentally on medical imaging or less commonly due to symptoms, and can significantly grow in selective condition, such as during pregnancy.[3,4,5]
The majority of myomas are small and asymptomatic, but some might cause symptoms interfering with patients’ lives and warranting therapy.[6] In addition, in the case of submucous myomas, hysteroscopy represents a useful tool to confirm the diagnosis and plan the most appropriate management.[7,8] One of the first-line approaches is represented by hormonal treatments;[9] although, gynecological surgery represents a feasible option benign diseases,[10,11,12,13] such as myomas.
In this scenario, laparoscopic myomectomy (LM) is becoming increasingly common in the management of uterine myomas[14] and is usually offered regardless of the number, location, and size of the myomas.[15,16,17] It has a generally low rate of periprocedural complications and is preferred to laparotomy for several reasons that are not limited to decreased length of hospital stay, number of sutures, smaller incisions, and decreased pain.[15,18] However, blood loss during LM remains a challenge.[16,18] In a series of 500 or more LM, the rate of hemorrhage or blood transfusion varied widely from 0.1% to 6% with an average intraoperative blood loss of 80–248 ml (range 20–1000 ml).[19]
Both patient and myoma characteristics may influence the risk of bleeding. Several studies have tried to address this issue, aiming at identifying risk factors for peri-surgical blood loss.[20,21,22,23,24,25,26,27] However, controversy exists and those significant risk factors are yet to be identified.
To be able to stratify patients and provide better management after LM, it is crucial to identify these predictors of blood loss. Therefore, the aim of this review is to identify the risk factors for peri-procedural blood loss after laparoscopic uterine myomectomy.
METHODS
This is a systematic review and meta-analysis of all randomized controlled trials investigating hemoglobin drop in women undergoing LM. The review was reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement and the Cochrane Reviewers’ Handbook. Outcomes were defined, screened, selected, and reported following the recommendations of the Core Outcome Sets in Women's and Newborn Health initiative.
Search strategy
Ovid Medline (from January 1946 to May 2018), Embase (from January 1947 to May 2018), and Cochrane CENTRAL (Issue 6) were searched to identify relevant studies with no limitation on the year of publication or language. The search strategy included the following keywords and subject headings: risk factors, bleeding, and LM.
Titles and/or abstracts of studies retrieved using the search strategy, and those from additional sources were screened independently by two review authors to identify studies that potentially meet the inclusion criteria outlined above. The full text of these potentially eligible studies was retrieved and independently assessed for eligibility by other two review team members. Any disagreement between them over the eligibility of particular studies was resolved through discussion with a third external collaborator. All potentially relevant papers were retrieved and assessed in details and had their reference lists manually screened to identify any other relevant papers. Appendix 1 shows the detailed search strategy.
Study selection criteria and outcome measures
Articles were included if they: (1) were retrospective or prospective cohorts, case–control and clinical studies; (2) evaluated adult female patients presenting for LM; (3) reported periprocedural blood loss (as defined by each study); and (4) analyzed predictors for blood loss. We excluded: (1) studies not reporting on blood loss; (2) case reports or cross-sectional studies (lack of causality); and (3) no analysis of risk factors for bleeding. The primary outcome was blood loss, as defined by each study.
Data extraction
Data extracted from each study included author's name and year of publication, study design and location, number of participants, study period, characteristics of the total study population, randomization and blinding methods if applicable, definition of blood loss and risk factors. A standardized, prepiloted form was used to extract data from the included studies for the assessment of study quality and evidence synthesis. Two authors independently extracted data from studies about study features and included populations. Potential discrepancies were solved through a discussion with a third external collaborator.
Quality assessment
All studies that were included were cohort studies. Risk of bias assessment was done using the Newcastle Ottawa scale, which is an eight-item instrument consisting of three subscales, totaling nine points, represented by stars.[28] These subscales address: Selection of cohorts (four-item), comparability of cohorts (one item), and the assessment of outcome (three-item). Studies with more than six points were considered of good quality.
Data analysis
The articles were organized into subgroups based on the definition of blood loss. A summary of each study was recorded in Microsoft Word before summarizing all studies in one table to facilitate comparisons between the studies. Preliminary conclusions were drawn. Articles were then re-examined to further explore and verify the findings. Finally, a narrative synthesis was produced in relation to key findings.
RESULTS
Figure 1 shows the flow diagram of the selection process of the trials to be included. Fourteen studies were identified through the electronic database search, of which eight were conducted between 2009 and 2017 were included.
Figure 1.
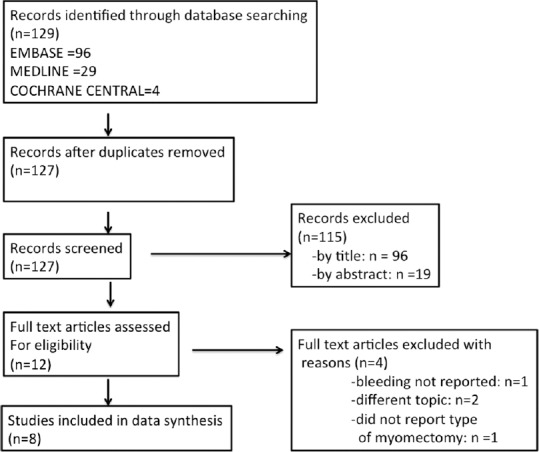
Flow diagram of the search process
Included study characteristics and quality evaluation
Table 1 shows a summary of the characteristics of the included studies and quality appraisal. Eight articles are included in the review.[20,21,22,23,24,25,26,27] All studies were published in English, and the majority of the studies were done in the USA (n = 5). All studies were cohorts, 7 of which were retrospective.
Table 1.
Characteristics of the included studies listed by the study quality and year
| Author, year | Type | Duration | Location | Patient (n) | Age, years | Bleeding definition | Risk factors | Quality score |
|---|---|---|---|---|---|---|---|---|
| Catanzarite, 2016 | Retrospective | 2005-2012 | USA | 1017 | NR | Transfusion requirement | Single (operative time) | 7 |
| Clark, 2017 | Retrospective | 2007-2012 | USA | 268 | NR | Intra-operative EBL | Single (menstrual cycle) | 6 |
| Saccardi, 2014 | Prospective | 2000-2012 | Italy | 444 | Mean 36.7±6.4 | Intra-operative EBL | Multiple | 6 |
| Walid, 2009 | Retrospective | 1999-2009 | USA | 41 | NR | Intra-operative EBL | Single (myoma size) | 6 |
| George, 2009 | Retrospective | 2005-2008 | USA | 77 | Median=37 (26-50) | Intra-operative EBL | Single (BMI) | 6 |
| Watrowski, 2017 | Retrospective | 2010-2015 | Germany | 150 | Median=37 (33-42) | Intra-operative EBL, pre- and postoperative Hb level | Multiple | 5 |
| Gingold, 2017 | Retrospective | 2011-2016 | USA | 140 | NR | Transfusion requirement within 1 week of surgery | Multiple | 5 |
| Cho, 2011 | Retrospective | 2008-2009 | South Korea | 167 | NR | Hb change more than 2 units | Multiple | 4 |
BMI: Body mass index, NR: Not reported, EBL: Estimated blood loss, Hb: Hemoglobin
Out of eight studies, five were of good quality, the remaining 3 were of poor quality mainly due to lack of comparability.
Overall, the studies included 2304 patients. The most commonly used way to determine bleeding in most studies was a subjective assessment of estimated blood loss (EBL) by the surgeon or anesthesiologist intraoperatively. Two studies also compared hemoglobin level pre and post operatively[22,26] with one study defining blood loss as a drop in more than two units in hemoglobin level.[26] Transfusion requirement was evaluated in two studies.[21,27]
Mean EBL was 184.1 ± 233.5 ml and 261 ± 159 ml in two studies[22,24] One study reported a median EBL of 100 ml (range 10–700)[24] and another reported a range of 20–1200 ml.[23]
In the two studies that reported transfusion as a primary endpoint, a mean of 2.57 units ± 1.27 was transfused[21] and 3.4% of the patients undergoing LM received a blood transfusion.[27]
Risk factors
Half of the studies evaluated multiple risk factors,[21,22,24,26] while the other half focused on a single risk factor (body mass index [BMI], myoma size, menstrual cycle, and operative time).[20,23,25,27]
Patient factors
No relationship between mean age and blood loss was found.[21,22,26] BMI was evaluated in two studies and found no variation in blood loss or blood transfusion in relation to that factor.[21,25] The phase of the menstrual cycle was assessed in one study with no difference in the amount of estimated intra-operative blood loss by each phase.[20]
Lesion characteristics
Myoma size was evaluated as a risk factor in several studies, and the finding of increased blood loss with size was consistent across the studies.[22,23,24,26] Two studies used regression analysis to determine the relation between EBL and myoma size, and both found that myoma diameter was a predictor of the amount of blood lost, with an increase of 2.51 ml of blood lost[23] and 0.292 drop in hemoglobin unit[22] for every 1 mm increase in myoma size. One study found that myomas >6.5 cm were associated with a hemoglobin drop >2 g/dl.[26] In another study, myomas were clustered myomas by size and type and found that intramural myomas with a size between 8 and 12 cm had a higher amount of blood loss compared to sub-serosal myomas of the same size (median blood loss = 275 vs. 200, P < 0.05). In the same study, both intramural and subserosal myomas were associated with an increased amount of blood loss if their size was >12 cm.[24] Conversely, one study found no impact of the localization on the amount of blood lost.[22]
In addition, the impact of the number of myomas was also evaluated. ≥5 myomas were found to be significantly associated with blood transfusion requirements within 1 week of the myomectomy[21] and a hemoglobin drop of more than 2 units g/dl was found when there were ≥3 myomas.[26]
Procedural details
Three studies evaluated the effect of the duration of the operation on blood loss, and all three found that increased operative time was a predictor for increased bleeding.[22,26,27] When comparing blood transfusion, one study found that a surgery duration ≥240 min had a 4.8 times risk compared to <240 min (95% confidence interval (2.27–10.11)), P < 0.001.[27] An operation time longer than 92 min was independently associated with more than two units drop in hemoglobin in another study.[26] Regression analysis in the third study showed that there was a 0.395 ml increase in EBL for every 1 surgical minute.[22]
Only one study evaluated operator factors and found no difference in bleeding according to the operator's surgical volume.[22]
DISCUSSION
Gynecological surgery, especially using minimally invasive approach, represent the gold standard treatment for the management of benign diseases.[29,30,31,32,33,34,35]
Adequate care of patients undergoing LM requires adequate knowledge of the risk factors for complications, of which the most common is bleeding. When excessive, this bleeding can lead to conversion to abdominal hysterectomy in 0.37%–2.7% of cases.[18,36]
This review evaluated predictors of bleeding after LM for uterine fibroids. It is noteworthy because it reflects the lack of literature on this issue despite the common occurrence of bleeding.
A recently published systematic review on the complications of LM reported an estimated intra-operative blood loss of 84–1200 ml, with increased risk of bleeding with myoma number and size.[37] Similar results were found in this review. Myoma characteristics were the most commonly evaluated, such as number of myomas with increased blood loss if more than 3.[21,26] Most importantly, there was a linear increase in blood loss with the size of myoma,[22,23] with Cho et al. and Saccardi et al. in two other studies reporting a significant blood loss if the lesions were more than 6.5 cm and more than 8 cm.[24,26] This could be due to a bigger myometrial incision with bigger myomas leading to increased bleeding. The effect of the type of myoma was only reported in two studies with inconsistent findings. On the multivariate analysis, Watrowski et al. found no effect of localization of the myoma on amount of blood loss,[22] while as Saccardi et al. were clustered location with the size and found that intramural myoma with a size between 8 and 12 cm were associated with a greater risk of bleeding than subserosal myomas of the same size.[24]
Procedural factors may also be associated with increased blood loss, namely procedure duration. It is well established that a shorter surgical duration is associated with decreased blood loss. This was reported in several studies evaluating modifications of myomectomy such as temporary uterine artery occlusion.[38,39] A consistent result was found among studies in this review. Operator related factors were only assessed in one study and found no effect on blood loss.[22] However, surgical expertise is also reflected by the duration of the surgery. In addition, it should be taken into account that the administration of the medical therapies, such as gonadotropin-releasing hormone-analogs[40,41] and ulipristal acetate,[42,43] to reduce myoma (s) size before the procedure and intra-operative blood loss. In particular, it was recently found that a 3-month treatment with ulipristal acetate before laparoscopy for large uterine myomas decreases intra-operative blood loss, hemoglobin drop, postoperative blood transfusion, and length of surgery.[44]
Three factors related to the patients were identified in the literature. These were age, BMI, and menstrual cycle and were not found to be associated with increased bleeding.[21,22,26] As was recently found,[45] intraoperative blood loss volume and other important factors such as operative time and myoma retrieval time do not seem to be influenced by the use of power morcellator during the procedure.
This review has several limitations, mainly pertaining to the methodology of the studies included. Some of the studies were of poor quality and threatening the internal validity of their findings. Furthermore, all of the studies were observational, and hence, causality is less established. Several studies had a small number of patients raising the possibility of being underpowered. Moreover, the definition of bleeding and blood loss in those studies was not homogenous and did not reflect the severity of bleeding. Since there was a lack of classification of the bleeding, risk factors could not be stratified according to those classes, and hence, subgroup data analysis could not be performed.
In conclusion, despite there are research gaps, this review identified the most commonly reported risk factors for blood loss in LM. This can help gynecologic surgeons to identify patients at increased risk of bleeding. While as myoma size cannot be modified, factors influencing operation duration can be and measures targeting that can be taken into account to minimize blood loss.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDIX 1
Ovid MEDLINE(R) In-Process and Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present
Search done: June 14, 2018
| Search terms | Results | |
|---|---|---|
| 1 | exp Risk Factors/or risk factor*.mp. | 975,119 |
| 2 | anemia or anaemia).mp. | 179,118 |
| 3 | exp ANEMIA/ | 152,368 |
| 4 | hemoglobin drop.mp. | 307 |
| 5 | h?emorrhage.mp. | 274,578 |
| 6 | exp HEMORRHAGE/or exp POSTOPERATIVE HEMORRHAGE/ | 303,421 |
| 7 | exp Blood Loss, Surgical/or blood loss*.mp. | 51,627 |
| 8 | bleed*.mp. | 182,778 |
| 9 | Or/2-8 | 1,156,045 |
| 10 | exp LAPAROSCOPY/or laparoscopy.mp. | 99,549 |
| 11 | minimally invasive procedure*.mp. or exp Minimally Invasive Surgical Procedures/ | 463,138 |
| 12 | myomectomy.mp | 3072 |
| 13 | 10 or 11 | 474,096 |
| 14 | 12 and 13 | 1287 |
| 15 | 1 and 9 and 14 | 29 |
Embase Classic + Embase 1947 to 2018 June 14
June 14, 2018
| Search terms | Results | |
|---|---|---|
| 1 | exp Risk Factors/or risk factor*.mp. | 1,151,716 |
| 2 | (anemia or anaemia).mp. | 350,639 |
| 3 | exp anemia/ | 371,296 |
| 4 | blood loss*.mp. or exp postoperative hemorrhage/or exp operative blood loss/ | 107,186 |
| 5 | h?emorrhage.mp. or exp bleeding/ | 920,230 |
| 6 | exp Blood Loss, Surgical/ | 15,478 |
| 7 | Hemoglobin drop | 718 |
| 8 | exp bleeding/or bleed*.mp. | 951,769 |
| 9 | postoperative complications.mp. or exp postoperative complication/ | 644,999 |
| 10 | exp laparoscopic surgery/or exp laparoscopy/or laparoscop*.mp. | 207,978 |
| 11 | exp minimally invasive surgery/or exp minimally invasive procedure/or minimally invasive procedure*.mp. | 50,149 |
| 12 | myomectomy.mp. or exp myomectomy/ | 7281 |
| 13 | Or/2-9 | 1,876,860 |
| 14 | 10 and 12 | 3117 |
| 15 | 11 and 12 | 423 |
| 16 | 14 or 15 | 3248 |
| 17 | 1 and 13 and 16 | 96 |
Cochrane Central Register of Controlled Trials: Issue 6 of 12, June 2018–June 14, 2018
| Search terms | Results | |
|---|---|---|
| 1 | risk factor: ti, ab, kw | 30,850 |
| 2 | MeSH descriptor: [Risk Factors] explode all trees | 26,223 |
| 3 | anemia or anaemia: ti, ab, kw | 15,310 |
| 4 | MeSH descriptor: [Anemia] explode all trees | 4907 |
| 5 | Bleed*: ti, ab, kw | 29,905 |
| 6 | MeSH descriptor: [Hemorrhage] explode all trees | 13,809 |
| 7 | hemorrhage: ti, ab, kw | 21,154 |
| 8 | blood loss: ti, ab, kw | 21,729 |
| 9 | MeSH descriptor: [Postoperative Hemorrhage] explode all trees | 1238 |
| 10 | MeSH descriptor: [Blood Loss, Surgical] explode all trees | 2660 |
| 11 | postoperative complication: ti, ab, kw | 18,449 |
| 12 | MeSH descriptor: [Postoperative Complications] explode all trees | 37,882 |
| 13 | #1 or #2 | 52,679 |
| 14 | #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 | 138,403 |
| 15 | laparoscopy: ti, ab, kw | 7205 |
| 16 | MeSH descriptor: [Laparoscopy] explode all trees | 6421 |
| 17 | minimally invasive procedure: ti, ab, kw | 1809 |
| 18 | MeSH descriptor: [Uterine Myomectomy] explode all trees | 53 |
| 19 | myomectomy: ti, ab, kw | 552 |
| 20 | #15 or #16 or #17 | 10,031 |
| 21 | #18 or #19 | 552 |
| 22 | #21 and #20 | 156 |
| 23 | #22 and #13 and #14 | 4 |
REFERENCES
- 1.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Laganà AS, Vergara D, Favilli A, La Rosa VL, Tinelli A, Gerli S, et al. Epigenetic and genetic landscape of uterine leiomyomas: A current view over a common gynecological disease. Arch Gynecol Obstet. 2017;296:855–67. doi: 10.1007/s00404-017-4515-5. [DOI] [PubMed] [Google Scholar]
- 3.Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: Poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43:1789–804. doi: 10.1111/jog.13437. [DOI] [PubMed] [Google Scholar]
- 4.Levast F, Legendre G, Bouet PE, Sentilhes L. Management of uterine myomas during pregnancy. Gynecol Obstet Fertil. 2016;44:350–4. doi: 10.1016/j.gyobfe.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Vitale SG, Padula F, Gulino FA. Management of uterine fibroids in pregnancy: Recent trends. Curr Opin Obstet Gynecol. 2015;27:432–7. doi: 10.1097/GCO.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 6.Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: A national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1–20. doi: 10.1016/j.ajog.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babacan A, Gun I, Kizilaslan C, Ozden O, Muhcu M, Mungen E, et al. Comparison of transvaginal ultrasonography and hysteroscopy in the diagnosis of uterine pathologies. Int J Clin Exp Med. 2014;7:764–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Vitale SG, Sapia F, Rapisarda AMC, Valenti G, Santangelo F, Rossetti D, et al. Hysteroscopic morcellation of submucous myomas: A Systematic review. Biomed Res Int. 2017;2017:6848250. doi: 10.1155/2017/6848250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnez J, Arriagada P, Donnez O, Dolmans MM. Current management of myomas: The place of medical therapy with the advent of selective progesterone receptor modulators. Curr Opin Obstet Gynecol. 2015;27:422–31. doi: 10.1097/GCO.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 10.Tinelli A, Gasbarro N, Lupo P, Malvasi A, Tsin DA, Davila F, et al. Safe introduction of ancillary trocars. JSLS. 2012;16:276–9. doi: 10.4293/108680812X13427982376464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitale SG, Laganà AS, Noventa M, Giampaolino P, Zizolfi B, Butticè S, et al. Transvaginal bilateral sacrospinous fixation after second recurrence of vaginal vault prolapse: Efficacy and impact on quality of life and sexuality. Biomed Res Int. 2018;2018:5727165. doi: 10.1155/2018/5727165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahn DD, Mamik MM, Sanses TV, Matteson KA, Aschkenazi SO, Washington BB, et al. Venous thromboembolism prophylaxis in gynecologic surgery: A systematic review. Obstet Gynecol. 2011;118:1111–25. doi: 10.1097/AOG.0b013e318232a394. [DOI] [PubMed] [Google Scholar]
- 13.Alves J, Puga M, Fernandes R, Pinton A, Miranda I, Kovoor E, et al. Laparoscopic management of ureteral endometriosis and hydronephrosis associated with endometriosis. J Minim Invasive Gynecol. 2017;24:466–72. doi: 10.1016/j.jmig.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 14.D'Silva EC, Muda AM, Safiee AI, Ghazali WA. Five-year lapsed: Review of laparoscopic myomectomy versus open myomectomy in Putrajaya hospital. Gynecol Minim Invasive Ther. 2018;7:161–6. doi: 10.4103/GMIT.GMIT_38_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin C, Hu Y, Chen XC, Zheng FY, Lin F, Zhou K, et al. Laparoscopic versus open myomectomy – A meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14–21. doi: 10.1016/j.ejogrb.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Malzoni M, Sizzi O, Rossetti A, Imperato F. Laparoscopic myomectomy: A report of 982 procedures. Surg Technol Int. 2006;15:123–9. [PubMed] [Google Scholar]
- 17.Sinha R, Hegde A, Mahajan C, Dubey N, Sundaram M. Laparoscopic myomectomy: Do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292–300. doi: 10.1016/j.jmig.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Sizzi O, Rossetti A, Malzoni M, Minelli L, La Grotta F, Soranna L, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14:453–62. doi: 10.1016/j.jmig.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Paul GP, Naik SA, Madhu KN, Thomas T. Complications of laparoscopic myomectomy: A single surgeon's series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385–90. doi: 10.1111/j.1479-828X.2010.01191.x. [DOI] [PubMed] [Google Scholar]
- 20.Clark NV, Wang KC, Opoku-Anane J, Hill-Lydecker CI, Vitonis AF, Einarsson JI, et al. The menstrual cycle and blood loss during laparoscopic myomectomy. Acta Obstet Gynecol Scand. 2017;96:1446–52. doi: 10.1111/aogs.13240. [DOI] [PubMed] [Google Scholar]
- 21.Gingold J, Flyckt R. Identification of factors associated with laparoscopic myomectomy transfusion requirement. J Minim Invasive Gynecol. 2017;24:S175. [Google Scholar]
- 22.Watrowski R, Jäger C, Forster J. Predictors of postoperative hemoglobin drop after laparoscopic myomectomy. Wideochir Inne Tech Maloinwazyjne. 2017;12:81–7. doi: 10.5114/wiitm.2017.66515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walid MS, Heaton RL. Laparoscopic myomectomy: An intent-to-treat study. Arch Gynecol Obstet. 2010;281:645–9. doi: 10.1007/s00404-009-1154-5. [DOI] [PubMed] [Google Scholar]
- 24.Saccardi C, Gizzo S, Noventa M, Ancona E, Borghero A, Litta PS, et al. Limits and complications of laparoscopic myomectomy: Which are the best predictors? A large cohort single-center experience. Arch Gynecol Obstet. 2014;290:951–6. doi: 10.1007/s00404-014-3289-2. [DOI] [PubMed] [Google Scholar]
- 25.George A, Eisenstein D, Wegienka G. Analysis of the impact of body mass index on the surgical outcomes after robot-assisted laparoscopic myomectomy. J Minim Invasive Gynecol. 2009;16:730–3. doi: 10.1016/j.jmig.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Cho H. Vol. 8. London: United Kingdom Conference Publication; 2011. Prediction of Postoperative Anemia Following Laparoscopic Myomectomy. Gynecol Surgery Conference 20th Annu Congr ESGE; pp. S159–60. [Google Scholar]
- 27.Catanzarite T, Vieira B, Hackett N, Kim JY, Milad MP. Longer operative time during laparoscopic myomectomy is associated with increased 30-day complications and blood transfusion. J Gynecol Surg. 2016;32:11–8. [Google Scholar]
- 28.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle – Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. 2001. [Last accessed on 2019 Mar 01]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 29.Laganà AS, Vitale SG, Palmara V, Ban Frangež H, Triolo O. Transvaginal specimen removal in minimally invasive surgery: Feasibility and possible complications during the incision of the posterior vaginal wall. World J Urol. 2017;35:1155–6. doi: 10.1007/s00345-016-1955-7. [DOI] [PubMed] [Google Scholar]
- 30.Peri L, Musquera M, Vilaseca A, Garcia-Cruz E, Ribal MJ, Carrión A, et al. Perioperative outcome and female sexual function after laparoscopic transvaginal NOTES-assisted nephrectomy. World J Urol. 2015;33:2009–14. doi: 10.1007/s00345-015-1573-9. [DOI] [PubMed] [Google Scholar]
- 31.Laganà AS, Vitale SG, Trovato MA, Palmara VI, Rapisarda AM, Granese R, et al. Full-thickness excision versus shaving by laparoscopy for intestinal deep infiltrating endometriosis: Rationale and potential treatment options. Biomed Res Int. 2016;2016:3617179. doi: 10.1155/2016/3617179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Zhu D, Wu Q, Yu Y. Fertility outcomes after laparoscopic salpingectomy or salpingotomy for tubal ectopic pregnancy: A retrospective cohort study of 95 patients. Int J Surg. 2017;48:59–63. doi: 10.1016/j.ijsu.2017.09.058. [DOI] [PubMed] [Google Scholar]
- 33.Vitale SG, Laganà AS, Gulino FA, Tropea A, Tarda S. Prosthetic surgery versus native tissue repair of cystocele: Literature review. Updates Surg. 2016;68:325–9. doi: 10.1007/s13304-015-0343-y. [DOI] [PubMed] [Google Scholar]
- 34.Monti M, Schiavi MC, Colagiovanni V, Sciuga V, D’oria O, Cerone G, et al. Effectiveness, quality of life and sexual functions in women with anterior compartment prolapse treated by native tissue repair. Minerva Ginecol. 2019;71:18–24. doi: 10.23736/S0026-4784.18.04305-8. [DOI] [PubMed] [Google Scholar]
- 35.Vitale SG, Caruso S, Rapisarda AM, Valenti G, Rossetti D, Cianci S, et al. Biocompatible porcine dermis graft to treat severe cystocele: Impact on quality of life and sexuality. Arch Gynecol Obstet. 2016;293:125–31. doi: 10.1007/s00404-015-3820-0. [DOI] [PubMed] [Google Scholar]
- 36.Mallick R, Odejinmi F. Pushing the boundaries of laparoscopic myomectomy: A comparative analysis of peri-operative outcomes in 323 women undergoing laparoscopic myomectomy in a tertiary referral centre. Gynecol Surg. 2017;14:22. doi: 10.1186/s10397-017-1025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanos V, Berry KE, Frist M, Campo R, DeWilde RL. Prevention and management of complications in laparoscopic myomectomy. Biomed Res Int. 2018;2018:8250952. doi: 10.1155/2018/8250952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iavazzo C, Mamais I, Gkegkes ID. Use of misoprostol in myomectomy: A systematic review and meta-analysis. Arch Gynecol Obstet. 2015;292:1185–91. doi: 10.1007/s00404-015-3779-x. [DOI] [PubMed] [Google Scholar]
- 39.Raba G, Kotarski J, Szczupak K, Obloza B, Fudali-Walczak M. Uterus banding with the Osada method effectively reduces intraoperative blood loss during myomectomy. Minim Invasive Ther Allied Technol. 2016;25:43–7. doi: 10.3109/13645706.2015.1075558. [DOI] [PubMed] [Google Scholar]
- 40.Chang WC, Chu LH, Huang PS, Huang SC, Sheu BC. Comparison of laparoscopic myomectomy in large myomas with and without leuprolide acetate. J Minim Invasive Gynecol. 2015;22:992–6. doi: 10.1016/j.jmig.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 41.Palomba S, Pellicano M, Affinito P, Di Carlo C, Zullo F, Nappi C. Effectiveness of short-term administration of tibolone plus gonadotropin-releasing hormone analogue on the surgical outcome of laparoscopic myomectomy. Fertil Steril. 2001;75:429–33. doi: 10.1016/s0015-0282(00)01676-9. [DOI] [PubMed] [Google Scholar]
- 42.Ferrero S, Vellone VG, Barra F, Scala C. Ulipristal acetate before hysteroscopic and laparoscopic surgery for uterine myomas: Help or hindrance? Gynecol Obstet Invest. 2018:1–3. doi: 10.1159/000495347. doi: 10.1159/000495347. [DOI] [PubMed] [Google Scholar]
- 43.Ferrero S, Vellone VG, Barra F. Pharmacokinetic drug evaluation of ulipristal acetate for the treatment of uterine fibroids. Expert Opin Drug Metab Toxicol. 2018;14:107–16. doi: 10.1080/17425255.2018.1417389. [DOI] [PubMed] [Google Scholar]
- 44.Ferrero S, Alessandri F, Vellone VG, Venturini PL, Leone Roberti Maggiore U. Three-month treatment with ulipristal acetate prior to laparoscopic myomectomy of large uterine myomas: A retrospective study. Eur J Obstet Gynecol Reprod Biol. 2016;205:43–7. doi: 10.1016/j.ejogrb.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 45.Amemiya K, Adachi K, Sasamoto N, Yamamoto Y. Transumbilical extraction of 151-300-g myomas without morcellator versus conventional laparoscopic myomectomy with power morcellator. Gynecol Minim Invasive Ther. 2017;6:162–6. doi: 10.1016/j.gmit.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


