Abstract
Policies have been put in place internationally to reduce the overuse of certain medications that have a high risk of harm, such as sedative-hypnotic drugs for insomnia or opioids for chronic non-cancer pain. We explore and compare the outcomes of policies aimed at deprescribing sedative-hypnotic medication in community-dwelling older adults. Prescription monitoring policies led to the highest rate of discontinuation but triggered inappropriate substitutions. Financial deterrents through insurance scheme delistings increased patient out-of-pocket spending and had minimal impact. Pay-for-performance incentives to prescribers proved ineffective. Rescheduling alprazolam to a controlled substance raised the street drug price of the drug and shifted use to other benzodiazepines, causing similar rates of overdose deaths. Driving safety policies and jurisdiction-wide educational campaigns promoting non-drug alternatives appear most promising for achieving intended outcomes and avoiding unintended harms. Sustainable change should be supported with direct-to-patient education and improved access to non-drug therapy, with an emphasis on evaluating both intended and unintended consequences of any deprescribing-oriented policy.
Abstract
Des politiques ont été adoptées, un peu partout au monde, afin de réduire la surutilisation de certains médicaments qui présentent un risque élevé pour la santé, tels que les sédatifs hypnotiques pour traiter l'insomnie ou les opioïdes pour la douleur chronique non cancéreuse. Nous étudions et comparons les résultats des politiques qui visent la déprescription des sédatifs hypnotiques chez les personnes âgées vivant dans la communauté. Les politiques de surveillance des prescriptions ont mené aux plus hauts taux d'abandon, mais elles ont aussi donné lieu à des solutions inappropriées. Les moyens de dissuasion financière – par révision des listes d'assurance – ont poussé les patients à défrayer davantage les coûts à même leur poche et ont eu des impacts minimes. Les incitatifs de type « rémunération au rendement » visant les prescripteurs se sont montrés inefficaces. Le remplacement de l'alprazolam par une substance contrôlée a fait augmenter le prix du médicament sur le marché clandestin et a déplacé l'usage vers d'autres benzodiazépines, ce qui a causé des taux similaires de mortalité par surdose. Les politiques de sécurité et les campagnes de sensibilisation nationales, qui favorisent le recours à des choix non médicamenteux, s'avèrent les plus prometteuses pour atteindre les résultats escomptés et pour éviter les effets néfastes. Pour un changement durable, il faut offrir une éducation directement aux patients et assurer un meilleur accès aux thérapies non médicamenteuses, en mettant l'accent sur l'évaluation des répercussions souhaitées ou non de toute politique orientée vers la déprescription.
Background
Discontinuing potentially inappropriate medications is a foundational element of the broader de-adoption movement, which aims to use evidence-based health policy as one of several strategies to promote safe and appropriate prescribing (Baicker and Chandra 2017; Tannenbaum et al. 2017). National health and professional organizations have drawn attention to the overuse of antibiotics (Dar et al. 2016), opioids (Barnett et al. 2017; Califf et al. 2016), antipsychotics (Choosing Wisely 2018a; Desveaux et al. 2015), sedative-hypnotics (Bachhuber et al. 2016; Budnitz et al. 2011; Choosing Wisely 2018b; Hampton et al. 2014; Lembke et al. 2018) and polypharmacy (Mangin et al. 2018; Scott et al. 2015; Scottish Government 2018). Policies have been put in place to reduce the overuse of medications but have produced mixed outcomes. Careful assessment of the policy mechanisms that have failed is needed to avoid future implementation of pharmaceutical policies that may be ineffective or counterproductive, burdening health providers, consuming taxpayer resources and inconveniencing patients (Larochelle et al. 2015).
Sedative-hypnotic drugs, especially benzodiazepine receptor agonists such as alprazolam, diazepam, lorazepam, zolpidem and zopicolone, have been identified as a priority area for deprescribing among older adults (Tannenbaum et al. 2017). Benzodiazepines and Z-drugs are among the most frequently prescribed potentially inappropriate medications, especially for older women (Bachhuber et al. 2016; Brett et al. 2018; Tannenbaum et al. 2017). The harms associated with their short- and long-term use are well documented (Glass et al. 2005) and include falls and fractures (Donnelly et al. 2017; Woolcott et al. 2009), cognitive impairment (Billioti de Gage et al. 2012; Tannenbaum et al. 2012), automobile accidents (Hansen et al. 2015) and higher mortality alone and in combination with opioids (Kripke 2016; Lembke et al. 2018; Sun et al. 2017). There is a substantive body of clinical research assessing interventions to reduce benzodiazepine and Z-drug use that are amenable to implementation (Martin et al. 2018; Reeve et al. 2017; Smith and Tett 2010; Tannenbaum et al. 2014). Policy initiatives in support of clinical interventions should be informed by previous assessment of effectiveness and unintended harm (Kollen et al. 2012; Rat et al. 2014). Here we examine and compare the outcomes of different jurisdictional-wide policies on the reduction of benzodiazepine and Z-drug use among community-dwelling adults.
Study Data and Methods
Search strategy
A search was conducted for published articles evaluating the impact of regional or regulatory jurisdiction-wide policies aimed at limiting sedative-hypnotic use among community-dwelling older adults using Medline, Embase and PsychINFO from inception to January 2017, with search terms related to drug class policy and age and restricted to the English language. For drug class, we limited the MeSH terms and keywords specific to benzodiazepines and specific Z-drugs (eszopiclone, zopiclone, zaleplon and zolpidem). The policy concept was split into two related and required component terms. First, we included terms concerning “legislation,” “policy,” “public policy,” “health policy,” “program(s),” “patient education,” “campaign(s)” and “practice guidelines” to broadly identify studies of policies. Second, we included terms suggestive of an intended outcome related to discontinuation or deprescribing of inappropriate or unnecessary medications: “deprescriptions,” “inappropriate prescribing,” “drug utilization,” “drug prescriptions,” “withdraw*,” “discontinu*,” “appropriate*,” “optim*” and “prescri*.” To ensure that the policy targeted individuals aged 65 years and older, we included the search terms “aged” and “over 65.” Through hand-searching of references, we identified additional papers and grey literature sources. We limited our results to studies published in English. Figure 1 details the results of the search and the subsequent screening process.
Figure 1.
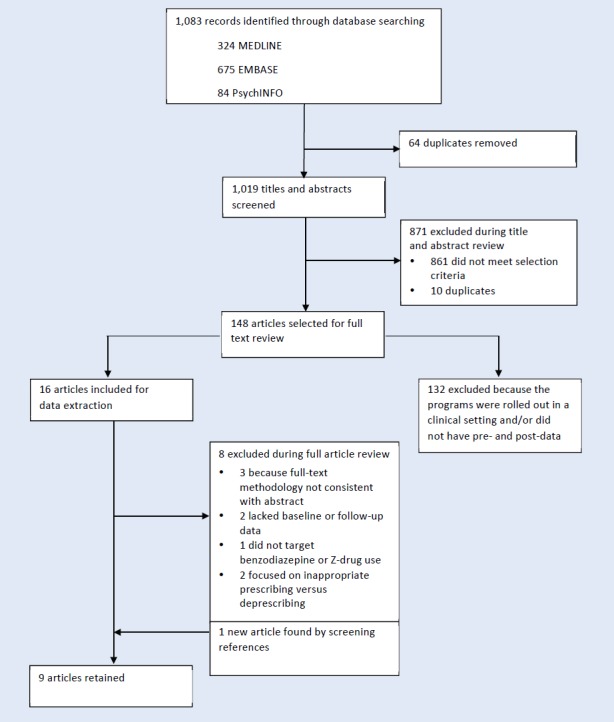
Flowchart of study identification, screening, eligibility and inclusion
Article screening and selection
Article titles and abstracts were each screened independently by two reviewers for policies or programs that targeted a reduction in benzodiazepine and Z-drug use among older adults. Article selection criteria included applicability of a jurisdictional-level policy to reduce the use of benzodiazepines and Z-drugs, clear documentation of pre- and post-policy population rates of sedative-hypnotic use to evaluate the policy's effectiveness and inclusion of community-dwelling individuals over the age of 65. Exclusion criteria included long-term care settings, programs that were rolled out in clinical settings, individuals solely under the age of 65 and baseline and follow-up data not available in the publication or via follow-up with the author directly. Article selection was completed by one investigator and reviewed by a second investigator. Disagreements were addressed through dialogue until agreement was reached among the authors.
Data extraction
Data were extracted from full-text articles by two members of the investigator team using a data extraction form, which included fields for the nature of the policy intervention, the relevant contexts, the time frame and the baseline and follow-up change metrics in drug use.
Outcomes
The primary outcome was the comparative effectiveness of each policy on reductions in population-based rates of benzodiazepine and Z-drug prescriptions. Absolute prescription change was calculated by comparing rates of sedative-hypnotic use pre- and post-policy implementation. The relative effectiveness of each policy was recorded as the relative change in prescription rates (absolute difference in post- vs. pre-policy rate/pre-policy rate). Although the unintended consequences of each policy were of interest, unintended outcomes were not always documented in the article or related articles and therefore were considered as a secondary outcome. We hand-searched the literature for each policy that was identified to ensure that, where possible, we captured the published unintended outcomes.
Study Results
The search returned 1,083 titles and abstracts. We excluded 74 duplicates and retained 148 full articles for review. One article was added from an external source. Of these, nine independent studies evaluating seven different policies for reducing the use of benzodiazepine and Z-drug medications in community-dwelling adults were included in the final analysis.
Comparative effectiveness
The comparative effectiveness of each policy on a change in sedative-hypnotic drug use is shown in Table 1 (available at www.longwoods.com/content/25857). Prescriber monitoring was most effective during a two-year follow-up of New York State's benzodiazepine triplicate prescribing policy for seniors (McNutt et al. 1994; Wagner et al. 2003). The policy required physicians to obtain, pay for and transmit one of three copies of a serialized prescription form to state health authorities for surveillance for any senior receiving a benzodiazepine prescription. A 35% reduction in the number of seniors receiving monthly benzodiazepine prescriptions was reported after the first year post-policy, and a cumulative 53%–58% decrease was recorded over two years (McNutt et al. 1994; Wagner et al. 2003). Following a successful state-wide intervention involving prescriber monitoring and education in 2007, in 2014, Australia made a national regulatory change that involved the rescheduling of alprazolam from a “schedule 4” designation, like other benzodiazepines, to a “schedule 8” controlled substance designation, which led to its inclusion as a monitored drug (Schaffer et al. 2016). As with the New York triplicate prescription policy, this regulatory change introduced surveillance by state and territory health authorities for any patient prescribed alprazolam. In New South Wales, a 28% reduction in alprazolam use was achieved among 65–79-year-olds one year post-policy implementation and 39% for those 80 years and older.
Denmark implemented a number of public awareness campaigns designed to reduce the use of sedative-hypnotics beginning in 2003, and in 2008, the Danish National Board of Health introduced a driver's license restriction policy for seniors. The driving license policy diminished the use of long-acting and short-acting benzodiazepines and Z-drugs by 54% and 35%, respectively, over five years (Eriksen and Bjerrum 2015). Physicians were required to report patients taking a benzodiazepine or Z-drug based on duration of use and drug half-life guidelines. Driving safety regulations included that all long-acting benzodiazepine users have their driver's license revoked, short-acting benzodiazepine users have a one-year limited renewal imposed with mandatory cognitive testing, new users refrain from driving for four weeks and episodic users not drive the morning after ingestion.
An Australian regional awareness campaign aimed to promote non-pharmacological therapy for insomnia. The multifaceted campaign included healthcare provider engagement and education, public education and the development and distribution of patient education materials relating to non-drug alternatives for the treatment of insomnia. A 19% reduction in benzodiazepine use was observed and sustained over a two-year period (Dollman et al. 2005).
The effect of delisting benzodiazepines from public insurance programs varied by country and by specific policy. The Netherlands experienced an 11%–14% reduction over a two-year period (Hoebert et al. 2012; Kollen et al. 2012) and a one-year 5% reduction was observed in the US (Chen et al. 2008). France's financial incentive program for physicians was the least effective, with a 1.4% increase in the number of patients initiating benzodiazepines over the course of one year (Rat et al. 2014).
Unintended consequences
Two follow-up studies examined the consequences of the national rescheduling of alprazolam in Australia (Deacon et al. 2016; Lloyd et al. 2017). The first reported an overall reduction in alprazolam and total benzodiazepine use among a small sample of patients enrolled in an opioid substitution program (Deacon et al. 2016). The street drug price of alprazolam doubled from $5 to $10 per two-milligram tablet over the 12-month period. The second report examined coroner records to assess a change in overdose due to alprazolam and other benzodiazepines (Lloyd et al. 2017). Although overdose deaths involving alprazolam declined, there was a steady increase in any overdose death where a benzodiazepine contributed, suggesting that limiting access to individual benzodiazepines might not impact on overall benzodiazepine-related mortality. Similarly, the US Medicare Part D restriction of reimbursement policy for benzodiazepines in the US led to high rates of substitution with zolpidem, which was still covered under the policy program, and a significant increase in out-of-pocket spending for benzodiazepines (Chen et al. 2008; Chen and Kreling 2014). A parallel rise occurred in prescriptions for other classes of sedative-hypnotics such as antipsychotics (Briesacher et al. 2010). There was an increase in fracture rates observed among patients admitted to nursing homes, presumably because of substitutions with these other sedatives (Briesacher et al. 2010).
The New York State triplicate prescribing policy also had unintended outcomes (Fisher et al. 2012). Within one year of the policy, prescriptions for barbiturates, meprobamate and other medications climbed, in contrast to the rest of the US, where their use was trending downward (Weintraub et al. 1991). Greater reductions in sedative-hypnotics were observed among women and individuals who were black and living in urban or low-income areas (Fisher et al. 2012). Data also suggest that the policy detrimentally affected some clinically vulnerable populations (e.g., persons with chronic psychiatric and neurological disorders) (Fisher et al. 2012).
Patient perceptions and physician receptivity in response to the administrative burden associated with prescription monitoring and other policies programs were analyzed (Fisher et al. 2012; Rodriguez 1991). A survey was conducted of 302 physicians and 103 patients from a Hispanic community in New York State two years after implementation of the benzodiazepine triplicate prescription policy (Rodriguez 1991). Forty-nine per cent of physicians reported that the policy affected their prescribing patterns, with 86% citing the increased administrative burden as the main reason for diminishing prescriptions, and 78% expressing opposition to prescription monitoring. Sixty-eight per cent of patients surveyed were dissatisfied with the triplicate prescription policy owing to the need for government documentation, breach of confidentiality and increased costs because of additional physician visits. In Denmark, sharing of information occurred between government departments during the driver's license restriction policy but was framed in the context of new public driving safety legislation implemented in 2007 that targeted prescribed medications together with illicit substances that can impair driving (Steentoft et al. 2010).
Limitations
Many of the reports relating to policy interventions are observational and retrospective, and only a small number purposively assessed unintended outcomes. In some cases, publication of the primary outcome and unintended outcomes was completed separately. We hand searched the literature to ensure that, where possible, we could present the published unintended outcomes. Our search did not find any negative outcomes from the drivers' license policy in Denmark nor the educational campaign in Australia; it is possible that negative consequences occurred but remain undocumented. Furthermore, unintended outcomes may have occurred at a clinical level and these would not have been captured. We limited our review to published peer-reviewed papers and did not scan government websites or the grey literature for other policies that were implemented. As policies do not have fixed evaluation periods and are situated within different contexts, it is difficult to compare the effectiveness of each policy, even with a generic calculation such as the relative reduction in use. We chose to study pharmaceutical policy for community-dwelling older adults. Policies that have been applied to reduce benzodiazepine use in acute or long-term care settings were not included, and the results of this analysis may not be generalizable to other contexts.
Other potentially effective solutions may exist that were not captured in this review. For instance, we did not find any jurisdictional-wide interventions that examined the effectiveness of policies to restrict pharmaceutical industry-based product promotion, for example via direct-to-consumer advertising (Becker and Midoun 2016). Policies that aim to reduce the promotion of prescription drugs to prescribers and directly to patients require further investigation (Gaffney and Lexchin 2018; Gardner et al. 2003). Nor did we find any assessments of jurisdiction-wide deprescribing initiatives that occurred at the patient level, rather than at the drug level. Medication reviews and deprescribing frameworks that address the patient's goals of care should be applied more broadly to patients with multimorbidity and polypharmacy in the clinical setting (Scott et al. 2015). Patient-specific interventions to deprescribe demonstrate a significant reduction in mortality (Page et al. 2016).
Discussion
The benefits of deprescribing policies may be diminished by a host of unanticipated consequences. Prescription monitoring policies such as the New York triplicate prescription policy led to the highest rates of discontinuation, but substitution with other inappropriate medication classes and the emergence of inequities undermined the intended effect. Financial deterrents through insurance scheme delistings were only minimally impactful and increased patient out-of-pocket spending. Financial incentivization in the form of a pay-for-performance supplement to prescribers was ineffective. Rescheduling alprazolam to a controlled substance that requires monitoring raised the street drug price of the drug and shifted use to other medications from the same class that caused similar rates of overdose deaths. Denmark's driver license policy and Australia's regional educational campaign on non-drug alternatives for treating insomnia were the only two policies that did not report unintended harms.
Extrapolation of the findings from policies targeting sedative-hypnotics to other drug classes such as opioids – given the current opioid abuse epidemic – would be useful, but is challenging to do. Prescription monitoring to reduce opioid prescribing has been shown to be modestly effective in some but not all jurisdictions in the US, particularly when it is part of a mandated program and covers the full range of prescription opioids (Barnett et al. 2017). Similar to the Australian alprazolam policy, however, substitution with other medications from the same class and a rise in illicit access and street drug costs can occur when a single opioid drug is targeted for restriction (Larochelle et al. 2015). Intensive and inclusive educational awareness programs that target the public, patients and health professionals to encourage use of non-drug substitutes appear to be moderately effective, at least for reducing sedative-hypnotics in the short term. The long-term sustainability and unforeseen consequences of time-limited education and awareness campaigns targeting sedative-hypnotics, opioids or other medication classes have yet to be fully elucidated (Califf et al. 2016). Widespread availability of opioids, while emphasizing alternative approaches and tools for the treatment of chronic pain, emerges as a promising approach associated with a substantially lower population-wide usage of opioids in Japan (Fischer et al. 2016).
Because opioids are implicated in three-quarters of benzodiazepine-related overdose deaths (Jones and McAninch 2015), parallel educational policies should be entertained and examined for sedatives and opioids. The EMPOWER study, that distributed direct-to-patient educational material on the harms of benzodiazepines, easy-to-follow tapering protocols and suggestions for non-drug alternates, yielded a 27% termination in chronic benzodiazepine use at the six-month follow-up versus 5% in the treatment as usual group (Tannenbaum et al. 2014). Additional benefit occurs with the combined use of patient education and pharmacist-initiated distribution of evidence-based pharmaceutical opinions to physicians to support patients to deprescribe and adopt non-drug therapies for symptom relief (Martin et al. 2018). Opportunities exist to encourage dialogue and legislation around drugs and driving safety when entertaining policies to reduce motor vehicle accidents. Patient-oriented policies have been proposed in the context of brain health and dementia strategies (Daiello and Tannenbaum 2018). The Canadian Deprescribing Network serves as a national hub in Canada for the promotion of evidence-informed initiatives and partnerships with policy makers, healthcare providers and the general public to reduce medication-related harm and improve access to non-drug therapies (Tannenbaum et al. 2017).
Challenges exist when comparing pharmaceutical policies to reduce medication overuse both within and across drug classes. To evaluate the magnitude of effect of each policy on medication reduction, methodological considerations include the use of different metrics and length of follow-up periods for each policy evaluation, the inability to evaluate policies that are not recorded in the medical literature, the lack of adequate explanation of contextual and cultural factors impacting the policy and widely different indications and determinants of use. Evidence-based policy also requires a hard look at downstream consequences, assessing trade-offs between specific goals (Baicker and Chandra 2017). Although prescriptions plummeted with prescriber monitoring and medication rescheduling policies, substitutions with equally and more harmful medications increased and the demand for street drug availability rose, leading to higher rates of morbidity and mortality. Direct and indirect costs are rarely ascertained in the real-world setting, though economic simulations suggest that access to and coverage of non-drug therapies may prove cost-effective, particularly for older adults with insomnia (Morgan et al. 2016; Tannenbaum et al. 2015).
The effort to generate policies that are effective at reducing the overuse of particular prescription drugs, that do not lead to pernicious consequences and that are acceptable to stakeholders requires close attention to political and health system contexts. Success will depend on the design, drug class, implementation details and the program particulars for each jurisdiction. Health promotion educational interventions that target specific patients may be the safest and most reasonable solution.
Acknowledgements
This work was funded by the Canadian Deprescribing Network through a Partnership for Health System Improvement Grant from the Canadian Institutes of Health Research (201410PHE-PHE-337814-96399).
Contributor Information
James Shaw, Assistant Professor, Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON.
Andrea L. Murphy, Associate Professor, College of Pharmacy, Dalhousie University, Halifax, NS.
Justin P. Turner, Graduate Student, Centre de Recherche Institut, Universitaire de Gériatrie de Montréal, Montreal, QC.
David M. Gardner, Professor, Department of Psychiatry, Dalhousie University, Halifax, NS.
James L. Silvius, Co-Director, Canadian Deprescribing Network; Associate Professor, Department of Medicine, University of Calgary, Calgary, AB.
Zachary Bouck, Biostatistician, Women's College Hospital Institute for Health System Solutions and Virtual Care, Toronto, ON.
Dara Gordon, Research Coordinator, Women's College Hospital Institute for Health System Solutions and Virtual Care, Toronto, ON.
Cara Tannenbaum, Co-Director, Canadian Deprescribing Network; Professor, Faculties of Medicine and Pharmacy, Université de Montréal, Montreal, QC.
References
- Bachhuber M.A., Hennessy S., Cunningham C.O., Starrels J.L. 2016. “Increasing Benzodiazepine Prescriptions and Overdose Mortality in the United States, 1996–2013.” American Journal of Public Health 106(4): 686–88. 10.2105/AJPH.2016.303061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baicker K., Chandra A. 2017. “Evidence-Based Health Policy.” The New England Journal of Medicine 377(25): 2413–15. 10.1056/NEJMp1709816. [DOI] [PubMed] [Google Scholar]
- Barnett M.L., Gray J., Zink A., Jena A.B. 2017. “Coupling Policymaking with Evaluation – The Case of the Opioid Crisis.” The New England Journal of Medicine 377(24): 2306–09. 10.1056/NEJMp1710014. [DOI] [PubMed] [Google Scholar]
- Becker S.J., Midoun M.M. 2016. “Effects of Direct-to-Consumer Advertising on Patient Prescription Requests and Physician Prescribing: A Systematic Review of Psychiatry-Relevant Studies.” The Journal of Clinical Psychiatry 77(10): e1293–300. 10.4088/JCP.15r10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billioti de Gage S., Bégaud B., Bazin F., Verdoux H., Dartigues J.-F., Pérès K. et al. 2012. “Benzodiazepine Use and Risk of Dementia: Prospective Population Based Study.” British Medical Journal 345; e6231. 10.1136/bmj.e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett J., Maust D.T., Bouck Z., Ignacio R.V., Mecredy G., Kerr E.A. et al. 2018. “Benzodiazepine Use in Older Adults in the United States, Ontario, and Australia from 2010 to 2016.” Journal of the American Geriatrics Society 66(6): 1180–85. 10.1111/jgs.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briesacher B.A., Soumerai S.B., Field T.S., Fouayzi H., Gurwitz J.H. 2010. “Medicare Part D's Exclusion of Benzodiazepines and Fracture Risk in Nursing Homes.” Archives of Internal Medicine 170(8): 693–98. 10.1001/archinternmed.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnitz D.S., Lovegrove M.C., Shehab N., Richards C.L. 2011. “Emergency Hospitalizations for Adverse Drug Events in Older Americans.” The New England Journal of Medicine 365(21): 2002–12. 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- Califf R.M., Woodcock J., Ostroff S. 2016. “A Proactive Response to Prescription Opioid Abuse.” The New England Journal of Medicine 374(15): 1480–85. 10.1056/NEJMsr1601307. [DOI] [PubMed] [Google Scholar]
- Chen H., Nwangwu A., Aparasu R., Essien E., Sun S., Lee K. 2008. “The Impact of Medicare Part D on Psychotropic Utilization and Financial Burden for Community-Based Seniors.” Psychiatric Services 59(10): 1191–97. 10.1176/appi.ps.59.10.1191. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Kreling D.H. 2014. “The Effect of the Medicare Part D Benzodiazepine Exclusion on the Utilization Patterns of Benzodiazepines and Substitute Medications.” Research in Social and Administrative Pharmacy 10(2): 438–47. 10.1016/j.sapharm.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Choosing Wisely A.G.S. 2018a. Don't Routinely Use Antipsychotics as First Choice to Treat Behavioral and Psychological Symptoms of Dementia. Retrieved May 22, 2018. <http://www.choosingwisely.org/clinician-lists/american-psychiatric-association-antipsychotics-in-patients-with-dementia/>.
- Choosing Wisely A.G.S. 2018b. Don't Use Benzodiazepines or Other Sedative-Hypnotics in Older Adults as First Choice for Insomnia, Agitation or Delirium. Retrieved May 22, 2018. <http://www.choosingwisely.org/clinician-lists/american-geriatrics-society-benzodiazepines-sedative-hypnotics-for-insomnia-in-older-adults/>.
- Daiello L.A., Tannenbaum C. 2018. “Patient-Oriented Policies to Reduce the Harmful Effects of Medication on Seniors' Brain Function.” Public Policy Aging Report 28(4): 124–28. 10.1177/2042098618794165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar O.A., Hasan R., Schlundt J., Harbarth S., Caleo G., Dar F.K. et al. 2016. “Exploring the Evidence Base for National and Regional Policy Interventions to Combat Resistance.” Lancet 387(10015): 285–95. 10.1016/S0140-6736(15)00520-6. [DOI] [PubMed] [Google Scholar]
- Deacon R.M., Nielsen S., Leung S., Rivas G., Cubitt T., Monds L.A. et al. 2016. “Alprazolam Use and Related Harm Among Opioid Substitution Treatment Clients–12 Months Follow Up after Regulatory Rescheduling.” International Journal of Drug Policy 36: 104–11. 10.1016/j.drugpo.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Desveaux L., Gomes T., Tadrous M., Jeffs L., Taljaard M., Rogers J. et al. 2015. “Appropriate Prescribing in Nursing Homes Demonstration Project (APDP) Study Protocol: Pragmatic, Cluster-Randomized Trial and Mixed Methods Process Evaluation of an Ontario Policy-Maker Initiative to Improve Appropriate Prescribing Of Antipsychotics.” Implementation Science 11(1): 45. 10.1186/s13012-016-0410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollman W.B., Leblanc V.T., Stevens L., O'Connor P.J., Roughead E.E., Gilbert A.L. 2005. “Achieving a Sustained Reduction in Benzodiazepine Use Through Implementation of an Area-Wide Multi-Strategic Approach.” Journal of Clinical Pharmacy and Therapeutics 30(5): 425–32. 10.1111/j.1365-2710.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- Donnelly K., Bracchi R., Hewitt J., Routledge P.A., Carter B. 2017. “Benzodiazepines, Z-drugs and the Risk of Hip Fracture: A Systematic Review and Meta-Analysis.” PLoS One 12(4): e0174730. 10.1371/journal.pone.0174730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen S.I., Bjerrum L. 2015. “Reducing Prescriptions of Long-Acting Benzodiazepine Drugs in Denmark: A Descriptive Analysis of Nationwide Prescriptions during a 10-Year Period.” Basic & Clinical Pharmacology and Toxicology 116(6): 499–502. 10.1111/bcpt.12347. [DOI] [PubMed] [Google Scholar]
- Fischer B., Rehm J., Tyndall M. 2016. “Effective Canadian Policy to Reduce Harms from Prescription Opioids: Learning from Past Failures.” Canadian Medical Association Journal 188(17–18): 1240–44. 10.1503/cmaj.160356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Sanyal C., Frail D., Sketris I. 2012. “The Intended and Unintended Consequences of Benzodiazepine Monitoring Programmes: A Review of the Literature.” Journal of Clinical Pharmacy and Therapeutics 37(1): 7–21. 10.1111/j.1365-2710.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- Gaffney A., Lexchin J. 2018. “US–Canadian Pharmaceutical Policy Reform Working Group. Healing an Ailing Pharmaceutical System: Prescription for Reform for United States and Canada.” The BMJ 361:k1039. 10.1136/bmj.k1039. [DOI] [PubMed] [Google Scholar]
- Gardner D.M., Mintzes B., Ostry A. 2003. “Direct-to-Consumer Advertising of Prescription Drugs in Canada: Permission by Default?” Canadian Medical Association Journal 169: 425–27. [PMC free article] [PubMed] [Google Scholar]
- Glass J., Lanctot K.L., Herrmann N., Sproule B.A., Busto U.E. 2005. “Sedative Hypnotics in Older People with Insomnia: Meta-Analysis of Risks and Benefits.” The BMJ 331(7526): 1169. 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton L.M., Daubresse M., Chang H.Y., Alexander G.C., Budnitz D.S. 2014. “Emergency Department Visits by Adults for Psychiatric Medication Adverse Events.” JAMA Psychiatry 71(9): 1006–14. 10.1001/jamapsychiatry.2014.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R.N., Boudreau D.M., Ebel B.E., Grossman D.C., Sullivan S.D. 2015. “Sedative Hypnotic Medication use and the Risk of Motor Vehicle Crash.” American Journal of Public Health 105(8): e64–e69. 10.2105/AJPH.2015.302723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebert J.M., Souverein P.C., Mantel-Teeuwisse A.K., Leufkens H.G., van Dijk L. 2012. “Reimbursement Restriction and Moderate Decrease in Benzodiazepine Use in General Practice.” The Annals of Family Medicine 10(1): 42–49. 10.1370/afm.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., McAninch J.K. 2015. “Emergency Department Visits and Overdose Deaths From Combined Use of Opioids and Benzodiazepines.” American Journal of Preventive Medicine 49(4): 493–501. 10.1016/j.amepre.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Kollen B.J., van der Veen W.J., Groenhof F., Donker G.A., van der Meer K. 2012. “Discontinuation of Reimbursement of Benzodiazepines in the Netherlands: Does it Make a Difference?” BMC Family Practice 13(1): 111. 10.1186/1471-2296-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke D.F. 2016. “Mortality Risk of Hypnotics: Strengths and Limits of Evidence.” Drug Safety 39(2): 93–107. 10.1007/s40264-015-0362-0. [DOI] [PubMed] [Google Scholar]
- Larochelle M.R., Zhang F., Ross-Degnan D., Wharam J.F. 2015. “Rates of Opioid Dispensing and Overdose After Introduction of Abuse-Deterrent Extended-Release Oxycodone and Withdrawal of Propoxyphene.” JAMA Internal Medicine 175(6): 978–87. 10.1001/jamainternmed.2015.0914. [DOI] [PubMed] [Google Scholar]
- Lembke A., Papac J., Humphreys K. 2018. “Our Other Prescription Drug Problem.” New England Journal of Medicine 378(8): 693–95. 10.1056/NEJMp1715050. [DOI] [PubMed] [Google Scholar]
- Lloyd B., Dwyer J., Bugeja L., Jamieson A. 2017. “Alprazolam in Fatal Overdose Following Regulatory Rescheduling: A Response to Deacon et al.” International Journal of Drug Policy 39: 138–39. 10.1016/j.drugpo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Mangin D., Bahat G., Golomb B.A., Mallery L.H., Moorhouse P., Onder G. et al. 2018. “International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): Position Statement and 10 Recommendations for Action.” Drugs Aging 35(7): 575–87. 10.1007/s40266-018-0554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Tamblyn R., Benedetti A., Ahmed S., Tannenbaum C. 2018. “Effect of a Pharmacist-Led Educational Intervention on Inappropriate Medication Prescriptions in Older Adults: The D-PRESCRIBE Randomized Clinical Trial.” JAMA 320(18):1889–98. 10.1001/jama.2018.16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt L.A., Coles F.B., McAuliffe T., Baird S., Morse D.L., Strogatz D.S. et al. 1994. “Impact of Regulation on Benzodiazepine Prescribing to a Low Income Elderly Population, New York State.” Journal of Clinical Epidemiology 47(6): 613–25. 10.1016/0895-4356(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Morgan S.G., Hunt J., Rioux J., Proulx J., Weymann D., Tannenbaum C. 2016. “Frequency and Cost of Potentially Inappropriate Prescribing for Older Adults: A Cross-Sectional Study.” CMAJ Open 4(2): E346–51. 10.9778/cmajo.20150131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A.T., Clifford R.M., Potter K., Schwartz D., Etherton-Beer C.D. 2016. “The Feasibility and Effect of Deprescribing in Older Adults on Mortality and Health: A Systematic Review and Meta-Analysis.” British Journal of Clinical Pharmacology 82(3): 583–623. 10.1111/bcp.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rat C., Penhouet G., Gaultier A., Chaslerie A., Pivette J., Nguyen J.M., Victorri-Vigneau C. 2014. “Did the New French Pay-for-Performance System Modify Benzodiazepine Prescribing Practices?” BMC Health Services Research 14: 301. 10.1186/1472-6963-14-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve E., Ong M., Wu A., Jansen J., Petrovic M., Gnjidic D. 2017. “A Systematic Review of Interventions to Deprescribe Benzodiazepines and Other Hypnotics among Older People.” European Journal of Clinical Pharmacology 73(8): 927–35. 10.1007/s00228-017-2257-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez R.F. 1991. “The Impact of the New York Triplicate Prescription Program on the Hispanic Community.” New York State Journal of Medicine 91(11 Suppl): 24S–27S. [PubMed] [Google Scholar]
- Schaffer A.L., Buckley N.A., Cairns R., Pearson S.-A. 2016. “Interrupted Time Series Analysis of the Effect of Rescheduling Alprazolam in Australia: Taking Control of Prescription Drug Use.” JAMA Internal Medicine 176(8): 1223–25. 10.1001/jamainternmed.2016.2992. [DOI] [PubMed] [Google Scholar]
- Scott I.A., Hilmer S.N., Reeve E., Potter K., Le Couteur D., Rigby D. et al. 2015. “Reducing Inappropriate Polypharmacy: The Process of Deprescribing.” JAMA Internal Medicine 175(5): 827–34. 10.1001/jamainternmed.2015.0324. [DOI] [PubMed] [Google Scholar]
- Smith A.J., Tett S.E. 2010. “Improving the Use of Benzodiazepines – Is it Possible? A Non-Systematic Review of Interventions Tried in the Last 20 Years.” BMC Health Services Research 10: 321. 10.1186/1472-6963-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steentoft A., Simonsen K.W., Linnet K. 2010. “The Frequency of Drugs Among Danish Drivers Before and After the Introduction of Fixed Concentration Limits.” Traffic injury prevention 11(4): 329–33. 10.1080/15389581003792783. [DOI] [PubMed] [Google Scholar]
- Sun E.C., Dixit A., Humphreys K., Darnall B.D., Baker L.C., Mackey S. 2017. “Association Between Concurrent Use of Prescription Opioids and Benzodiazepines and Overdose: Retrospective Analysis.” BMJ 356: j760. 10.1136/bmj.j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C., Diaby V., Singh D., Perreault S., Luc M., Vasiliadis H.M. 2015. “Sedative-Hypnotic Medicines and Falls in Community-Dwelling Older Adults: A Cost-Effectiveness (Decision-Tree) Analysis from a US Medicare Perspective.” Drugs Aging 32(4): 305–14. 10.1007/s40266-015-0251-3. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C., Farrell B., Shaw J., Morgan S., Trimble J., Currie J. et al. 2017. “An Ecological Approach to Reducing Potentially Inappropriate Medication Use: Canadian Deprescribing Network.” Canadian Journal on Aging 36(1): 97–107. 10.1017/S0714980816000702. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C., Martin P., Tamblyn R., Benedetti A., Ahmed S. 2014. “Reduction of Inappropriate Benzodiazepine Prescriptions Among Older Adults Through Direct Patient Education: The EMPOWER Cluster Randomized Trial.” JAMA Internal Medicine 174(6): 890–98. 10.1001/jamainternmed.2014.949. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C., Paquette A., Hilmer S., Holroyd-Leduc J., Carnahan R. 2012. “A Systematic Review of Amnestic and Non-Amnestic Mild Cognitive Impairment Induced by Anticholinergic, Antihistamine, GABAergic and Opioid Drugs.” Drugs Aging 29(8): 639–58. 10.2165/11633250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Wagner A.K., Soumerai S.B., Zhang F., Mah C., Simoni-Wastila L., Cosler L. et al. 2003. “Effects of State Surveillance on New Post-Hospitalization Benzodiazepine Use.” International Journal for Quality in Health Care 15(5): 423–31. 10.1093/intqhc/mzg064. [DOI] [PubMed] [Google Scholar]
- Weintraub M., Singh S., Byrne L., Maharaj K., Guttmacher L. 1991. “Consequences of the 1989 New York State Triplicate Benzodiazepine Prescription Regulations.” JAMA 266(17): 2392–97. 10.1001/jama.1991.03470170080028. [PubMed] [Google Scholar]
- Woolcott J.C., Richardson K.J., Wiens M.O., Patel B., Marin J., Khan K.M. et al. 2009. “Meta-Analysis of the Impact of 9 Medication Classes on Falls in Elderly Persons.” Archives of Internal Medicine 169(21): 1952–60. 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]


