Abstract
Introduction: White spot lesions (WSLs) occurring after orthodontic treatment lead to patient dissatisfaction and aesthetic problems. The role of calcium-phosphate demineralization systems and the Er:YAG laser in the treatment of these lesions has recently been taken into account. This study aimed to investigate the effect of the Er:YAG laser and MI Paste Plus on the treatment of WSLs.
Methods: A total of 65 premolars extracted due to orthodontic treatment were studied in this research. To create enamel lesions, the teeth were placed in a demineralizing solution. The teeth were then randomly divided into five groups (n=13) as follows: first group, control; second group, saliva; third group, MI Paste Plus; fourth group, Er:YAG laser; and fifth group, MI Paste Plus together with the Er:YAG laser. The teeth were kept in artificial saliva between treatment processes. Artificial saliva was replaced daily with fresh artificial saliva. The teeth were sectioned longitudinally by a disc from the middle of the exposed enamel and each section was mounted in polyester resin. The surface of the samples was serially polished and the microhardness of the teeth was measured at depths of 0, 50, 100, and 150 µm.
Results: The microhardness was significantly higher in the fifth group than other groups at depths of 50 and 150 µm (P <0.005). Using the laser or MI Paste Plus alone did not significantly increase the microhardness.
Conclusions: The combined application of the Er:YAG laser and MI Paste Plus is effective in the treatment of WSLs.
Keywords: Laser, Remineralization, Dental caries
Introduction
Despite the attempts made to inform the patients and encourage them to observe oral hygiene, White spot lesions (WSLs) due to fixed orthodontic appliances have still remained a major clinical problem and have increased since the advent of bonded brackets.1 Various studies have reported the prevalence of WSLs in orthodontic patients. Gorelick reported a prevalence rate of 50% for these lesions,2 while newer studies have reported a prevalence rate of 73-95%.3 These lesions are usually seen at the buccal surface of the teeth around the brackets, especially at the gingival area.2
Many efforts have been made to use different methods to prevent these lesions, and issues such as the improvement of appliances, bonding, fluoride application, and improve oral care have been emphasized.4 The use of topical fluoride is a helpful method for the prevention of these lesions, not for their treatment.5 The calcium-phosphate remineralization systems, whose effects are based on the increased natural capacity of saliva remineralization, have recently been considered for the treatment of these lesions. One of these materials is casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), a nano-complex derived from milk casein that significantly increases salivary calcium and phosphate levels. It prevents demineralization and increases remineralization or by elevating the buffering effect of saliva and protects the teeth against acidogenic crises by providing a large source of calcium and phosphate inside the plaque.5 Products such as MI and GC contain ACP. ACP has unstable calcium and phosphate salts. As the salts mix with saliva, they release calcium and phosphate ions. In an oral environment, ACP is transformed into a more stable, insoluble crystalline phase (e.g. hydroxyapatite). In vitro and in vivo studies have shown a reduction in WSLs as a result of the topical use of these materials. However, further studies are needed to prove their role in calcium-based remineralization systems.6
Studies have recently explored the effect of the erbium laser on the morphologic and chemical structure of enamel and its role in decreasing the microorganisms. For this reason, this type of laser is used in low energy to make chemical and structural changes to the enamel surface. This laser with a wavelength of 2.94 µm is located in the mid-infrared region and is used for the treatment of hard tissues. It is the first type of laser approved by the U.S. FDA for the treatment of hard tissues.7 This study aimed to investigate the efficacy of the Er:YAG laser and MI Paste Plus in the treatment of WSLs and compare the combined application of them with each one alone.
Methods
This study was performed under the precepts of the World Medical Association’s Declaration of Helsinki, as adopted in 2013 in Brazil. In this study, 65 premolars extracted due to orthodontic treatment were studied. The teeth were irrigated with normal saline and kept in 0.1% thymol solution. The premolars included in the study had normal anatomy and lacked WSL, crack, and caries under dental chair light. The teeth were placed in a demineralizing solution containing CaCl2 = 2.2 mM, NaH2 PO4 = 2.2 mM, lactic acid = 0.05 M, and fluoride = 0. 2 ppm, and the solution was mixed with 50% NaOH until reaching the pH=4.5. The solution was changed daily and washed with artificial saliva after 96 hours.7 The teeth were then randomly divided into 5 groups (n=13) as follows: group 1, control; group 2, saliva; group 3, MI Paste Plus; group 4, Er:YAG laser; and group 5, MI Paste Plus together with the Er:YAG laser.
All dental surfaces except one 4×4 window in the center of the buccal area as the treatment site were covered with two layers of nail polish. The teeth in group one after the demineralization process were sectioned longitudinally by a disc from the middle of the exposed enamel and each section was mounted in polyester resin and prepared for the microhardness test as explained below. Group two was kept in artificial saliva for 2 months, and saliva was exchanged daily. In group three, MI Paste Plus was applied to the site by a cotton swab and was washed with normal saline after 5 minutes. Group 4 was merely exposed to laser irradiation and group five received MI Paste Plus after laser irradiation. Groups 3, 4, and 5 underwent their treatment procedures at the beginning of the process, one month later, and 2 months after the start of the treatment. The teeth were kept in artificial saliva between treatment processes and were washed with an electric toothbrush and Crest toothpaste. Saliva was exchanged daily. This process was performed equally in all groups. The laser in groups 4 and 5 was done by an Er: YAG laser device (Fontona–1210 Ljubijana, Slovenia) with a wavelength of 2940 nm. A spot size of 0.9 mm and an R14 handpiece were used. The laser was operated at a pulse mode (long pulse) at a distance of 5–7 mm perpendicular to the surface. The laser was used at 100 mJ and 10 Hz for 5 seconds with air and water cooling spray, and the average output power was 1 W.
After this process, the teeth were sectioned longitudinally by a disc from the middle of the exposed enamel and each section was mounted in polyester resin. Then, since the surface of the samples had to be polished to perform the microhardness test, the surface of the samples was serially polished by Soflex discs (3M, ESPE, St, Paul, USA) from higher to lower roughness under the cooling water flow. In the second stage, the samples were polished by silicon carbide paper from the roughness of 6000 to the softest carbide paper on circulating plates under the cooling water flow. The microhardness of the profile was then measured by a microhardness tester (FM-700 series Future-Tech, Kawasaki, Japan) and Vickers diamond indenter under a force of 50 gr for 5 seconds at depths of 0, 50, 100, and 150 µm from the external border of enamel (Figure 1). To increase the accuracy of the procedure, microhardness was measured in each depth at three points and the mean number was reported. After analyzing the microhardness of samples by a microhardness tester, the figures obtained for each sample were recorded and fed into a computer for analysis.
Figure 1.

A View of the Effect of the Microhardness Test Indenter on the Enamel.
Data Analysis
Data were fed into SPSS-17 software. To analyze the normality of data, Kolmogorov-Smirnov and Shapiro-Wilk tests were used. If data were not normally distributed, the Kruskal-Wallis test was run to analyze the mean differences. Then, the Mann-Whitney test was used for pair comparisons. We used Bonferroni correction for pair comparisons; therefore, according to the results of Bonferroni correction, the level of significance for pair comparisons was considered as P < 0.005.
Results
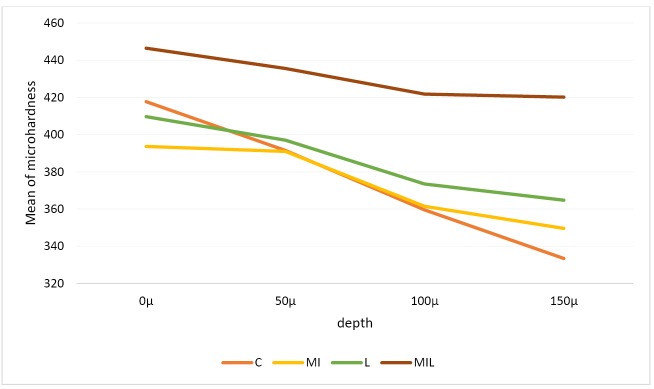
The means and standard deviations of microhardness of different study groups at various depths are presented in Table 1 and Figure 2 (group 1: control, group 2: saliva, group 3: MI Paste Plus, group 4: Er:YAG laser, group 5: Er:YAG laser plus MI Paste Plus).
Table 1. The Mean and Standard Deviation of the Microhardness of Samples in Different Groups at Different Depths .
| Groups | 0 µ | 50 µ | 100 µ | 150 µ | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Saliva | 402.39 | 32.38 | 379.27 | 45.48 | 384.56 | 31.48 | 340.45 | 30.45 |
| Control | 417.69 | 20.15 | 391.51 | 49.63 | 359.54 | 43.29 | 333.45 | 40.43 |
| MI Paste Plus | 393.64 | 11.23 | 390.98 | 11.51 | 361.48 | 14.20 | 349.60 | 11.75 |
| Laser | 409.65 | 27.17 | 397.00 | 34.06 | 373.45 | 28.11 | 364.76 | 22.29 |
| Laser + MI Paste Plus | 446.44 | 30.50 | 435.53 | 21.86 | 421.78 | 25.07 | 420.15 | 31.71 |
| P value | 0.001 | <0.0001 | <0.0001 | <0.0001 | ||||
Figure 2.
The Comparison of the Microhardness of Different Groups at Different Depths.
The results of pair comparisons between the groups at different depths by the Mann-Whitney test showed group five had the highest microhardness at all depths. This difference was statistically significant with all other groups at depths of 50 and 150 µm (P < 0.005). Group 4 did not have a statistically significant difference with other groups (P < 0.005). None of the depths of the control and artificial saliva groups showed a statistically significant difference (Table 2).
Table 2. Pair Comparisons of the Groups at Different Depths .
| Groups | 0 µ | 50 µ | 100 µ | 150 µ |
| Saliva & control | 0.118 | 0.397 | 0.174 | 0.343 |
| Saliva & MI | 0.043 | 0.98 | 0.006 | 0.739 |
| Saliva & laser | 0.778 | 0.0369 | 0.521 | 0.022 |
| Saliva & MIL | 0.003 | <0.0001 | 0.013 | <0.0001 |
| Control & MI | <0.0001 | 0.555 | 0.959 | 0.048 |
| Control & laser | 0.441 | 0.798 | 0.521 | 0.033 |
| Control & MIL | 0.020 | 0.002 | <0.0001 | <0.0001 |
| MI & laser | 0.248 | 0.858 | 0.106 | 0.031 |
| MI & MIL | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Laser & MIL | 0.011 | 0.002 | <0.0001 | <0.0001 |
Note: According to the results of Bonferroni correction, the level of significance for pair comparisons was considered as P <0.005.
Discussion
The results of the current study showed the use of MI Paste Plus along with the Er:YAG laser was the most effective method for increasing the microhardness of WSLs. The only effective method for increasing the microhardness of these lesions was the simultaneous use of the Er:YAG laser and MI Paste Plus at depths of 50 and 150 µm. The application of the Er:YAG laser alone did not have statistically significant results (P < 0.005).
One of the most important concerns of dentists is the incidence and development of WSLs.8 Numerous methods have been proposed for the prevention and treatment of these lesions. The calcium-phosphate remineralization systems, whose effects are based on the increased natural capacity of saliva remineralization, have recently been considered for the treatment of these lesions. One of these methods is the use of CPP-ACP paste, which is derived from milk. The fluoride-free compound of this material is MI Paste and its fluoride compound is MI Paste Plus. These products are used to induce supersaturated amorphous calcium phosphate in dental plaque.9,10 It has been shown that CPP-ACP causes calcium phosphate deposition in the dental plaque around the teeth.11 After consuming sugar products and foodstuffs, which is followed by the occurrence of acidic conditions, this material leads to supersaturated minerals, limits the demineralization of teeth, and contributes to the remineralization of WSLs.11 MI Paste Plus contains CPP-ACP and fluoride 900 ppm. It is believed that adding fluoride ion to this material increases its effect on the remineralization of enamel lesions compared to CPP-ACP or sodium fluoride alone.12-14
In this study, the only effective method for increasing the microhardness of these lesions was the simultaneous use of the Er:YAG laser and MI Paste Plus at depths of 50 and 150 µm. The application of the Er:YAG laser alone did not increase the enamel microhardness. The better results observed in group 5 may be due to the synergistic effect of laser and MI Paste plus. The laser appears to increase the penetration and effectiveness of the MI Paste plus. Various theories have been proposed to explain the effects of lasers on the tooth structure. Mechanisms such as connection due to melting and strength of enamel crystals, the reduction of water and enamel carbonate, an increase in hydroxyl ions, and changes in the structure of enamel proteins are theories that explain the increased resistance of teeth against acid in laser irradiation.15-17 High-power lasers such as Er: YAG lasers can induce cracks in the enamel. It can also roughen the enamel surface, which can make the tooth more susceptible to decay. These effects seem to be greater in demineralized enamel.18-20 The results of the fourth group appear to be related to the detrimental effects of laser use alone.
Previous studies have generally used high-power lasers such as Er:YAG and CO2 to prevent the occurrence of these lesions.16,21-25 Few studies, however, have used these lasers for the sake of treatment.24,26,27 In the study of Bevilacqua et al and some similar ones, the use of erbium lasers to prevent caries has been reported to be effective.28-30 Yet, some other studies have not reported such preventive effects for erbium lasers.18,19,31 The present study made use of the Er:YAG laser with 100 MJ energy, 10 Hz frequency, and 1 W power along with MI Paste Plus to analyze their treatment effects.
The microhardness test is generally used to measure the remineralizing effect of materials.32 The Vickers microhardness test indirectly measures the tooth remineralization via measuring the surface harness changes due to demineralization and remineralization.33 In this study, the Vickers microhardness test was sued after performing the treatment procedures. Many other studies have also used this test to measure the remineralization changes.26,27,31,34
The findings of the present study were in contrast with those of Heravi et al indicating that the use of the erbium laser alone and in combination with CPP-ACPF was not effective in the treatment of WSLs.26 These differing results may be due to the difference in various parameters of lasers used such as pulse energy, pulse duration, energy density, radiation time, and use of or not use of air or water for cooling during laser irradiation. It should be noted that most previous studies have investigated the anti-caries effects of erbium lasers on the prevention of WSLs. Moreover, these studies have used lasers in combination with materials other than MI Paste Plus.28,35,36
The use of MI Paste Plus seems not to have desirable clinical effects in these conditions. Robertson et al37 and Bailey et al38 used materials containing CPP-ACP and CPP-ACPF (MI Paste Plus) for the prevention and treatment of WSLs respectively. They stated that the use of these products yielded good results. They used these products for one to three months with a higher frequency. However, the present study used MI Paste Plus three times, each time for five minutes. The difference in results may be due to the less use of these products compared to the previous studies.37-40 Yet, some studies have found no significant difference between these materials and the saliva group, whether used for prevention or treatment, which seems to be due to short-term use of these materials followed by daily irrigation.11,21,41
Since the duration of use of the material in this study was increased by five minutes each time, no significant difference was observed compared to the control group. The results of this study were in line with those of Hervai et al,26 indicating no significant difference between MI Paste Plus and the control group in the treatment of WSLs. Only some in vivo studies have reported the positive effects of materials containing CPP-ACP used daily for one to three months for treatment purposes.5,38,39 The increased frequency in in vitro studies can yield better results. Further studies are needed to prove this.
Conclusion
The results of this study showed that the simultaneous use of MI Paste Plus and the Er:YAG laser had optimal results regarding the microhardness measurement for the treatment of WSLs. It seems that the simultaneous use of MI Paste Plus and the Er:YAG laser has synergistic effects. The use of laser singly was not effective. It seems that the use of MI Paste Plus alone is not effective in the treatment of WSLs, but it is more effective when it is used with the Er:YAG laser.
Ethical Considerations
Ethical approval was obtained from the research ethics committee of shahid sadoughi university of medical sciences (IR.SSU.REC.1396.197).
Conflict of Interests
The authors declare no conflict of interest.
Please cite this article as follows: Yassaei S, Motallaei MN. The effect of the Er:YAG laser and MI paste plus on the treatment of white spot lesions. J Lasers Med Sci. 2020;11(1):50-55. doi:10.15171/jlms.2020.09.
References
- 1.Verran J, Sandoval G, Allen NS, Edge M, Stratton J. Variables affecting the antibacterial properties of nano and pigmentary titania particles in suspension. Dyes Pigments. 2007;73(3):298–304. doi: 10.1016/j.dyepig.2006.01.003. [DOI] [Google Scholar]
- 2.Tufekci E, Dixon JS, Gunsolley JC, Lindauer SJ. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 2011;81(2):206–10. doi: 10.2319/051710-262.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behnan SM, Arruda AO, González-Cabezas C, Sohn W, Peters MC. In-vitro evaluation of various treatments to prevent demineralization next to orthodontic brackets. Am J Orthod Dentofacial Orthop. 2010;138(6):712 .e1–7. doi: 10.1016/j.ajodo.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz A. A report on the NIH consensus development conference on diagnosis and management of dental caries throughout life. J Dental Res. 2004;83:C15–17. doi: 10.1177/154405910408301s03. [DOI] [PubMed] [Google Scholar]
- 5.Bröchner A, Christensen C, Kristensen B, Tranæus S, Karlsson L, Sonnesen L. et al. Treatment of post-orthodontic white spot lesions with casein phosphopeptide-stabilised amorphous calcium phosphate. Clin Oral Investig. 2011;15(3):369–73. doi: 10.1007/s00784-010-0401-2. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds EC. Calcium phosphate‐based remineralization systems: scientific evidence? Aust Dent J. 2008;53(3):268–73. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 7.Lata S, Varghese NQ, Varughese JM. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation. J Conserv Dent. 2010;13(1):42–6. doi: 10.4103/0972-0707.62634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dean JA, Avery DR, McDonald RE. McDonald and Avery’s Dentistry for the Child and Adolescent. 9th ed. Boston: Mosby; 2011. 10.1016/C2009-0-48382-X. [DOI]
- 9.Rose RK. Effects of an anticariogenic casein phosphopeptide on calcium diffusion in streptococcal model dental plaques. Arch Oral Biol. 2000;45(7):569–75. doi: 10.1016/s0003-9969(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 10.Rose RK. Binding characteristics of Streptococcus mutans for calcium and casein phosphopeptide. Caries Res. 2000;34(5):427–31. doi: 10.1159/000016618. [DOI] [PubMed] [Google Scholar]
- 11.Pulido MT, Wefel JS, Hernandez MM, Denehy GE, Guzman-Armstrong S, Chalmers JM. et al. The inhibitory effect of MI paste, fluoride and a combination of both on the progression of artificial caries-like lesions in enamel. Oper Dent. 2008;33(5):550–5. doi: 10.2341/07-136. [DOI] [PubMed] [Google Scholar]
- 12.Shetty S, Hegde MN, Bopanna TP. Enamel remineralization assessment after treatment with three different remineralizing agents using surface microhardness: An in vitro study. J Conserv Dent. 2014;17(1):49–52. doi: 10.4103/0972-0707.124136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds EC. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008;42(2):88–97. doi: 10.1159/000113161. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan N, Kavitha M, Loganathan SC. Comparison of the remineralization potential of CPP-ACP and CPP-ACP with 900 ppm fluoride on eroded human enamel: An in situ study. Arch Oral Biol. 2010;55(7):541–4. doi: 10.1016/j.archoralbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Hsu CY. Laser-induced compositional changes on enamel: a FT-Raman study. J Dent. 2007;35(3):226–30. doi: 10.1016/j.jdent.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Ahrari F, Poosti M, Motahari P. Enamel resistance to demineralization following Er:YAG laser etching for bonding orthodontic brackets. Dent Res J (Isfahan) 2012;9(4):472–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Moosavi H, Ghorbanzadeh S, Ahrari F. Structural and morphological changes in human dentin after ablative and subablative Er:YAG Laser Irradiation. J Lasers Med Sci. 2016;7(2):86–91. doi: 10.15171/jlms.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apel C, Birker L, Meister J, Weiss C, Gutknecht N. The caries-preventive potential of subablative Er:YAG and Er:YSGG laser radiation in an intraoral model: a pilot study. Photomed Laser Surg. 2004;22(4):312–7. doi: 10.1089/pho.2004.22.312. [DOI] [PubMed] [Google Scholar]
- 19.Apel C, Meister J, Schmitt N, Gräber HG, Gutknecht N. Calcium solubility of dental enamel following sub-ablative Er:YAG and Er:YSGG laser irradiation in vitro. Lasers Surg Med. 2002;30(5):337–41. doi: 10.1002/lsm.10058. [DOI] [PubMed] [Google Scholar]
- 20.Yassaei S, Aghili H, Joshan N. Effects of removing adhesive from tooth surfaces by Er:YAG laser and a composite bur on enamel surface roughness and pulp chamber temperature. Dent Res J (Isfahan) 2015;12(3):254–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Souza-Gabriel AE, Turssi CP, Colucci V, Tenuta LM, Serra MC, Corona SA. In situ study of the anticariogenic potential of fluoride varnish combined with CO2 laser on enamel. Arch Oral Biol. 2015;60(6):804–10. doi: 10.1016/j.archoralbio.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Stangler LP, Romano FL, Shirozaki MU, Galo R, Afonso AM, Borsatto MC. et al. Microhardness of enamel adjacent to orthodontic brackets after CO2 laser irradiation and fluoride application. Braz Dent J. 2013;24(5):508–12. doi: 10.1590/0103-6440201302292. [DOI] [PubMed] [Google Scholar]
- 23.Bedini R, Manzon L, Fratto G, Pecci R. Microhardness and morphological changes induced by Nd:Yag laser on dental enamel: an in vitro study. Ann Ist Super Sanita. 2010;46(2):168–72. doi: 10.4415/ANN_10_02_10. [DOI] [PubMed] [Google Scholar]
- 24.Esteves-Oliveira M, Pasaporti C, Heussen N, Eduardo CP, Lampert F, Apel C. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J Dent. 2011;39(6):414–21. doi: 10.1016/j.jdent.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Yassaei S, Shahraki N, Aghili H, Davari A. Combined effects of Er: YAG laser and casein phosphopeptide-amorphous calcium phosphate on the inhibition of enamel demineralization: An in vitro study. Dent Res J (Isfahan) 2014;11(2):193–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Heravi F, Ahrari F, Mahdavi M, Basafa S. Comparative evaluation of the effect of Er: YAG laser and low level laser irradiation combined with CPP-ACPF cream on treatment of enamel caries. J Clin Exp Dent. 2014;6(2):e121–6. doi: 10.4317/jced.51309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khamverdi Z, Kordestani M, Panahandeh N, Naderi F, Kasraei S. Influence of CO2 laser irradiation and CPPACP paste application on demineralized enamel microhardness. J Lasers Med Sci. 2018;9(2):144–148. doi: 10.15171/jlms.2018.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bevilácqua FM, Zezell DM, Magnani R, da Ana PA, Eduardo Cde P. Fluoride uptake and acid resistance of enamel irradiated with Er:YAG laser. Lasers Med Sci. 2008;23(2):141–7. doi: 10.1007/s10103-007-0466-6. [DOI] [PubMed] [Google Scholar]
- 29.de Freitas PM, Rapozo-Hilo M, Eduardo Cde P, Featherstone JD. In vitro evaluation of erbium, chromium:yttrium-scandium-gallium-garnet laser-treated enamel demineralization. Lasers Med Sci. 2010;25(2):165–70. doi: 10.1007/s10103-008-0597-4. [DOI] [PubMed] [Google Scholar]
- 30.Geraldo-Martins VR, Lepri CP, Palma-Dibb RG. Influence of Er,Cr:YSGG laser irradiation on enamel caries prevention. Lasers Med Sci. 2013;28(1):33–9. doi: 10.1007/s10103-012-1056-9. [DOI] [PubMed] [Google Scholar]
- 31.Ahrari F, Mohammadipour HS, Hajimomenian L, Fallah-Rastegar A. The effect of diode laser irradiation associated with photoabsorbing agents containing remineralizing materials on microhardness, morphology and chemical structure of early enamel caries. J Clin Exp Dent. 2018;10(10):e955–e962. doi: 10.4317/jced.55059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uysal T, Amasyali M, Koyuturk AE, Ozcan S. Effects of different topical agents on enamel demineralization around orthodontic brackets: an in vivo and in vitro study. Aust Dent J. 2010;55(3):268–74. doi: 10.1111/j.1834-7819.2010.01233.x. [DOI] [PubMed] [Google Scholar]
- 33.Gutiérrez-Salazara MP, Reyes-Gasga J. Microhardness and chemical composition of human tooth. Mat Res. 2003;6(3):367–73. doi: 10.1590/S1516-14392003000300011. [DOI] [Google Scholar]
- 34.Farhadian N, Rezaei-Soufi L, Jamalian SF, Farhadian M, Tamasoki S, Malekshoar M. et al. Effect of CPP-ACP paste with and without CO2 laser irradiation on demineralized enamel microhardness and bracket shear bond strength. Dental Press J Orthod. 2017;22(4):53–60. doi: 10.1590/2177-6709.22.4.053-060.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Sant’anna GR, dos Santos EA, Soares LE, do Espírito Santo AM, Martin AA, Duarte DA. et al. Dental enamel irradiated with infrared diode laser and photoabsorbing cream: Part 1 -- FT-Raman Study. Photomed Laser Surg. 2009;27(3):499–507. doi: 10.1089/pho.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moslemi M, Fekrazad R, Tadayon N, Ghorbani M, Torabzadeh H, Shadkar MM. Effects of ER,Cr:YSGG laser irradiation and fluoride treatment on acid resistance of the enamel. Pediatr Dent. 2009;31(5):409–13. [PubMed] [Google Scholar]
- 37.Robertson MA, Kau CH, English JD, Lee RP, Powers J, Nguyen JT. MI Paste Plus to prevent demineralization in orthodontic patients: a prospective randomized controlled trial. Am J Orthod Dentofacial Orthop. 2011;140(5):660–8. doi: 10.1016/j.ajodo.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Bailey DL, Adams GG, Tsao CE, Hyslop A, Escobar K, Manton DJ. et al. Regression of post-orthodontic lesions by a remineralizing cream. J Dent Res. 2009;88(12):1148–53. doi: 10.1177/0022034509347168. [DOI] [PubMed] [Google Scholar]
- 39.Heravi F, Ahrari F, Tanbakuchi B. Effectiveness of MI Paste Plus and Remin Pro on remineralization and color improvement of postorthodontic white spot lesions. Dent Res J (Isfahan) 2018;15(2):95–103. doi: 10.4103/1735-3327.226532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebrahimi M, Mehrabkhani M, Ahrari F, Parisay I, Jahantigh M. The effects of three remineralizing agents on regression of white spot lesions in children: A two-week, single-blind, randomized clinical trial. J Clin Exp Dent. 2017;9(5):e641–e648. doi: 10.4317/jced.53582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souza-Gabriel AE, Colucci V, Turssi CP, Serra MC, Corona SA. Microhardness and SEM after CO(2) laser irradiation or fluoride treatment in human and bovine enamel. Microsc Res Tech. 2010;73(11):1030–5. doi: 10.1002/jemt.20827. [DOI] [PubMed] [Google Scholar]