Abstract
Introduction: Diabetes mellitus (DM) is a common disease with a highly significant burden among the Saudi population. This study aimed to investigate the effects of adding either magnetic or laser therapy to medications in patients with diabetic peripheral neuropathy (DPN).
Methods: Seventy-one medically controlled diabetic patients were randomly assigned to 1) Magnetic group: 26 patients were exposed to magnetic therapy for 20 minutes/session, 2 sessions/week, for 3 months 2) Laser group: 25 patients were exposed to laser therapy with intensity 5.7 J/cm2 for 30 minutes/session, 2 times/week, for 3 months. 3) Drug group: 20 patients received only the regular medications for diabetic control and pain analgesia. Pain and neuropathy were assessed by the visual analog scale (VAS) and the Toronto Clinical Neuropath Scoring System (TRCNSS). Conduction velocities and amplitudes of peroneal and sural nerves were measured by electromyography.
Results: The results showed significant increases in conduction velocities and amplitudes in both magnetic and laser groups in parallel with significant reductions in TRCNSS. Non-significant changes were obtained only after using only medications (P >0.05). The mean values of VAS reduced significantly in the three groups. The least significant differences showed significant changes among the three groups, whereas non-significant differences were obtained between both magnetic and laser groups.
Conclusion: There were non-significant differences between both magnetic and laser therapy groups. Addition of either magnetic or laser therapy to medications could bring extra positive benefits to patients with DPN. Both magnetic and laser therapy can be applied with medications for the treatment of patients with DPN.
Keywords: Neuropathic pain, Laser therapy, Conduction velocity
Introduction
Diabetes mellitus (DM) is a common disease which is accompanied by a highly significant social and economic burden.1 Diabetes has high prevalence and progressive rates among the Saudi population. It is strongly associated with both microvascular and macrovascular complications such as retinopathy, neuropathy, and cardiovascular diseases.2 Peripheral neuropathy is the most common complication of diabetic patients.3 Diabetic peripheral neuropathy (DPN) is commonly associated with a high rate of patients’ mortality and morbidity.4 The prevalence and progression of DPN increase in parallel with the chronicity of diabetes, poor glycemic control and pre-existing cardiovascular risk factors.3 DPN is usually characterized by increases in pain severity, impairments in tactile and proprioceptive sensation, vibration sense, and improper postural control.5 Distal symmetric polyneuropathy is the commonest form of diabetic neuropathy.6
Nevertheless, diagnostic methods of neuropathy are diverse. The highest sensitive and objective method is the conduction velocity test (CVT).6 Diagnosis of DPN is confirmed when the symptoms and signs of peripheral nerve dysfunctions are present after the exclusion of all other causes.3 Comprehensive evaluation of DPN also requires careful examination of the lower extremity with validated clinical tests such as light touch, vibration, and the Toronto Clinical Neuropathy Scoring System (TRCNSS) which is one of the most sensitive clinical scales to detect early neuropathies.7
To date, although one-third of diabetic patients are affected with neuropathic pain, its treatment is still very complex.6 The focus of management is usually on disease modifications and the relief of symptomatic pain. Up till now, no specific treatment has been able to completely prevent or reverse the progression of DPN.6 As hyperglycemia is the essential contributing factor in DPN, proper glycemic control is the main therapeutic target for its treatment.8 Neuropathy happened because of reductions in the nerve blood flow and increases in nerve hypoxia, thus vasodilating drugs as prostaglandin analogues are usually beneficial in the treatment of neuropathic pain.4,9 Although many drugs have been used for the treatment of DPN, their roles are still unconfirmed.10 The administration of analgesics as tricyclic anti-depressants and topical agents may be effective but with limited success and unsatisfactory results.11 However, around half of the patients with DPN have shown adequate symptomatic pain relief in association with frequent side effects such as drowsiness, lethargy and unsteadiness.11 In addition, these analgesics do not stop or delay the progression of the underlying pathological neuropathic changes.12
Physical therapy can actively relieve the signs and symptoms of DPN. Active exercises have had many benefits for diabetic patients.1,3,5,8 At the same time, there are limitations for many diabetic patients to practice physical exercise, which enforce us to seek other alternative therapeutic modalities for diabetic neuropathic pain.1 Magnetic and laser therapy are recent interventions that may enhance the treatment of DPN.13,14
Pulsed electro-magnetic therapy (PEMT) was applied mostly to animals in experimental studies.15 The application of PEMT for 12 consecutive days for 30 minutes relieved neuropathic pain and increased nerve conduction velocity.16 In general, PEMT is considered safe and supplemental therapy for DPN.17 Unfortunately, there are still contradictions about the benefits of magnetic therapy despite its wide uses on diabetic patients.15,16,18 The application of PEMT for 20 minutes/session for 3 weeks resulted in a non-significant improvement in pain intensity and sleep conditions of patients with DPN.18 The properly used parameters for the clinical application of PEMT are also still controversial.14 Its limited uses on human beings aroused our enthusiasm for increasing applied studies into the effects of magnetic therapy on human beings.15
Low-level laser therapy (LLLT) is generally and safely applied to many patients with co-morbid diseases.19,20 There is an unambiguous piece of evidence that LLLT with different wavelengths has different effects on the cellular level.21 It was previously applied for 1 minute/site on 4 para-vertebral points in the lumbosacral spine, 3 points on the ischial region, and 2 points on the dorsum of the foot.21 Also, LLLT has been applied on 6 para-spinal points (L4-S1) to irradiate along the output of the right and left sciatic nerve.22 Despite a wide range of LLLT uses in a clinical setting, there is non-sufficient evidence to confirm its effects on neuropathic pain in humans.23 Some authors suggested its usage as a new therapeutic modality for patients with DPN.24 and others proved that LLLT has been effective in the repair of nerve damage.25 However, objective clinical studies have failed to detect the exact benefits of laser therapy on DPN.26 Furthermore, up till now, there have been controversies over the effects and proper parameters of laser application techniques that can be used for the treatment of DPN to prevent unnecessary sufferings and to reduce direct and indirect costs to those patients and their families.20 There is still a need for more randomized controlled trials on physical therapy advanced modalities for the treatment of patients with DPN.23 Therefore, the objective of the current study was to investigate the effects of adding either magnetic or laser therapy to drug therapy in patients with DPN and to compare them.
Materials and Methods
Subjects
Nineteen type-II diabetic patients with peripheral neuropathy were enrolled in this study after screening by specialized physician (Figure 1). The patients’ demographic data, including age, sex, the duration of diabetic history and beginning of neuropathy, the body mass index, fasting blood glucose (FBG), and the used medications are shown in Table 1.
Figure 1.
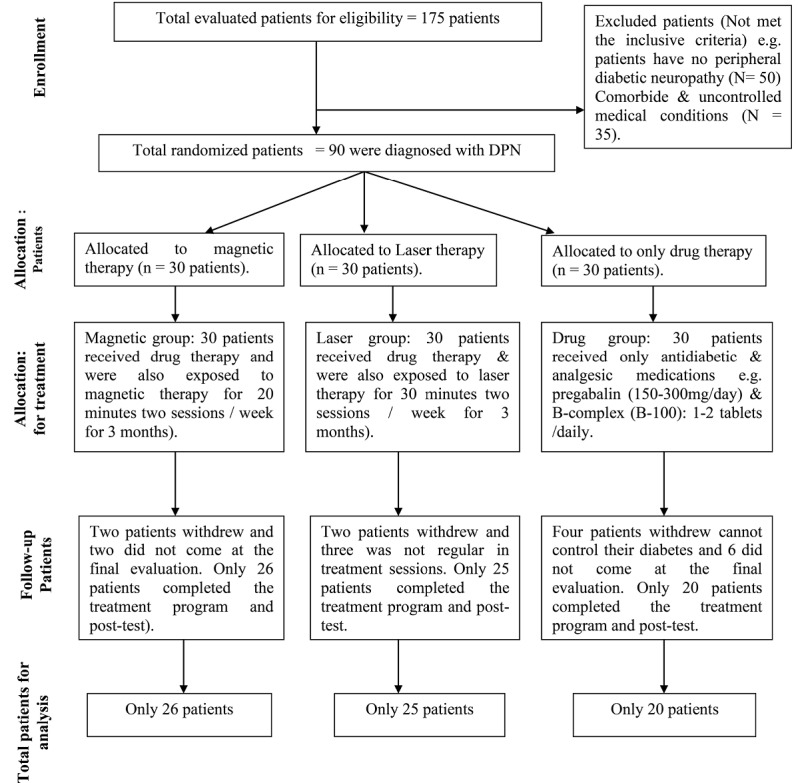
The Flow Diagram of Patients› Recruitment.
Table 1. Demographic Data of Recruited Patients .
| Variables | Magnetic Group | Laser Group | Drug Group | F Value | P Value |
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Age (y) | 60.31 ± 9.82 | 58.88 ± 12.15 | 54.05 ±10.69 | 1.971 | 0.147 |
| BMI, kg/ m2 | 27.32 ± 1.75 | 28.17 ± 1.97 | 27.79 ± 1.46 | 1.493 | 0.232 |
| Sex, No. (%) | 0.209 | 0.812 | |||
| Males | 18 (69.3) | 16 (64) | 12 -- (60) | ||
| Females | 8 (30.7) | 9 (36) | 8 -- (40) | ||
| Duration of incidence of diabetes (y) | 16.31 ±7.61 | 18.8 ±7.58 | 13.65 ± 6.71 | 2.726 | 0.073 |
| Duration of beginning of diabetic neuropathy (y) | 4.11 ±2.55 | 5.0 ±2.71 | 3.25 ± 2.07 | 2.767 | 0.070 |
| Pre-FBG, mg/dL | 142.38 ±6.8 | 140.68 ±6.77 | 141.5 ± 7.01 | 0.395 | 0.675 |
| in-FBG, mg/dL | 136.38 ±5.32 | 136.08 ±4.99 | 135.85 ±5.16 | 0.044 | 0.957 |
| Post-FBG, mg/dL | 136.5 ±5.46 | 136.2 ±5.17 | 136.6 ±5.19 | 0.062 | 0.940 |
| Oral drugs, No. (%) | 21 (80.8) | 21 (84) | 16 (80) | 0.142 | 0.931 |
| Insulin, No. (%) | 5 (19.2) | 4 (16) | 4 (20) |
BMI: body mass index; FBG, fasting blood glucose; in-FBG mg/dL: fasting blood glucose after 6 weeks.
Inclusion criteria: Patients (male and female) with painful DPN lasted > 6 months. Their age ranged from 35 to 70 years. Patients have sensory abnormalities, burning pain with paresthesia in both lower extremities,4,27 impaired sensory and/or motor conduction velocity (MCV) in a minimum one nerve of the lower limbs.13 All patients were medically controlled. The usage of analgesic medications such as anti-depressants and anticonvulsants could be received without any change for at least 4 weeks prior to recruitment and during the study.4,6 A consent form was signed by all the patients prior to their participation. They were informed that the collected data would be published.
Exclusion criteria: The patients were excluded if they had DPN from other causes than diabetes, lack of blood sugar control, vascular insufficiency, other neurological impairments, uncontrolled medical conditions (e.g. tumors and thyroid dysfunctions), pregnancy, metallic implantation, drug abuse, and a TRNCSS total score <5.4,7,8,27,28
Treatment Equipment
1- BEMER mattress: BEMER International AG (Liechtenstein) mattress product was used for producing magnetic therapy. The maximum average flux density (intensity) of BEMER on its highest output level is 150 microtesla (μT). The B. BOX Professional control units had 3 pre-stored programs with different intensity and treatment times.29
2- The Laser Scanner device (ASA Com. srl, Arcugnano, Italy): It emits a mixed light of both the He-Ne continuous wavelength of 850 nanometers (nm) and the pulsed infrared laser with the wavelength 905 nm. The device had maximum power of 10 watt (W). The output density of the device is calibrated at each applied program and selected frequency.4
Procedures
Assessment Procedure
The following measures were employed for all recruited patients by a blinded investigator.
a. Fasting Blood Glucose
A venous fasting blood sample was drawn from the anti-cubital vein to detect the blood glucose level (pre-intervention, after 6 weeks and at the end of treatment) for all patients.28
b. Electrophysiological Test
MCV of the peroneal nerve and sensory conduction velocity (SCV) of the sural nerve in addition its amplitudes were measured by a neuro-consultant.22,28 The patients with conduction velocity from 33-48 meter/second were recruited as confirmed DPN.16,21 All measures were performed by using (NicoletTM EDX- Viking version 20 software Germany) at a controlled temperature of 25°C.27,28
1. Peroneal nerve conduction velocity: It was investigated by putting standard surface electrodes. The distal stimulation was applied at 8 cm near to the active pickup electrode, just lateral to the Tibialis Anterior Tendon. The ground electrode was put at mid-calf. The proximal stimulation was applied just below the head of the fibula while the recording electrode was applied over the Extensor Digitorum Brevis to get a supra-maximal stimulus.4,22,27
2. Sural nerve conduction velocity : It was investigated by putting an active pick-up electrode posterior and below the lateral malleolus. The reference electrode was put at 3 cm distal to the active electrode and the ground electrode was put between the cathode of the stimulator and the active pickup electrode. Stimulation was applied slightly lateral to the midline in the lower third of the posterior aspects of the leg with the cathode distally about 17 cm from the active electrode (10-14 cm).4,8,22,27
c. Neuropathy Severity
It was also evaluated by the TRCNSS which is an objective method to detect the presence and intensity of DPN. Total scores ranged from 0 (normal) to 19 points (maximum).7
d. Pain
Itwas measured by VAS which is a valid instrument for the determination of perceived pain. Every patient was asked to point to his or her pain level ranged from 0 (no pain) to 10 (maximal pain).4,27
e. Vibration Sense
It was evaluated by using a 128-Hz tuning fork from the bony prominence of the big toe.5
f. Deep Tendon Reflexes
The ankle and knee reflexes were evaluated from relaxed sitting position.22
Treatment Procedure
Only 90 patients were randomly and equally assigned to 3 treatment groups by an independent blinded research assistant who opened sealed envelopes that contained a computer-generated randomization card (Figure 1).
1. Magnetic group: Twenty-six patients received drug therapy and they were exposed to magnetic therapy by a BEMER mattress (the density of the magnetic field was between 35 and 50 μT for 20 minutes/session, 2 sessions/week, for 3 months. The BEMER mattress was applied from a relaxed supine lying position. After connecting the appliance to the electrical supply, the action of PEMT was adjusted below the affected parts from the lower back to the feet. The program 3 was applied to achieve the proper treatment for 20 minutes.29
2. Laser group: Twenty-five patients received drug therapy and they were exposed to the infrared laser (850 nm) in the continuous wave mode with the total power of 5.7 J/cm2 for 30 minutes /session, 2 times per week, for 3 months.4,22,27 Both the plantar surface of the feet and the lumbosacral area were treated by laser therapy from a comfortable prone lying position . The distance between the laser head and the treated area of every patient was adjusted at 30 cm. The X-Y dimensions of the lumbo-sacral area were marked by 4 points, one on the lumbar 2, one on the sacral 1, and 2 points laterally to the spine by about 2 cm. The plantar surface and the lumbosacral areas were cleaned with alcohol (95%). Both areas were exposed to laser therapy through a sweeping computerized scan at an angle of 30° ± 15°. The power density was 6.3 mW/cm2 and irradiation time was set to 90 sec/cm2 to achieve the total dose of 5.7 J/cm2 through 15 min/site/session. Every patient and the physical therapist wore protective glasses for protection from the laser beam.4,22,27
3. Drug group: Twenty patients received only their regular drug therapy in the form of anti-diabetic drugs (either oral or insulin) and analgesic medications for peripheral neuropathic pain, including pregabalin (150 mg/d-300 mg/d) and B-complex multivitamin (B-100), one tablet once or twice daily.9,14,22
Statistical Analysis
All measured variables with their output data were analyzed by using SPSS software (version 23.0) in the form of descriptive and inferential analysis. A repeated measures ANOVA was used to evaluate the therapeutic effects within each group and among 3 groups. ANOVA and Pearson’s chi-square were used to compare demographic data of all groups. Statistical significance was measured at a P value < 0.05 with a confidence interval of 95%.
Results
Statistical analysis was applied to seventy-one patients who completed this study (Figure 1) as nineteen patients dropped out.
Demographic Characteristics of Recruited Patients
The data were reported by using the percent. The Pearson chi-square showed non-significant differences in gender and used drugs among the 3 groups. The results of ANOVA proved non-significant differences in the mean values of age, body mass index (BMI), FBG, the duration of diabetes, and the beginning of neuropathy among 3 groups prior to the interventions. The baseline demographic data were similar among the 3 groups except for increases number of participated men than women (Table 1).
Rate of Adherence
The mean values of patients’ adherence of both magnetic and laser groups were 20.85 ± 1.78 and 21.32 ± 1.49, P value = 0.309, with the percent of 86.87% and 88.83% respectively.
Electro Diagnosis Parameters in the 3 Treatment Groups
The results of the repeated measures ANOVA showed measured conduction velocities of the peroneal and sural nerves and its amplitudes increased significantly after adding either magnetic or laser therapy to drug therapy (P < 0.05) , whereas non-significant changes were obtained only after usage of drug therapy(P > 0.05) (Table 2; Figures 2 & 3).
Table 2. Mean Values of Motor Conduction Velocities and Amplitudes of the Peroneal Nerve in the Treatment Groups .
| Treatment Groups | Mean | SD | Mean Square | F Value | P Value | |
| Magnetic group | ||||||
| MCV m/s | Pre | 43.02 | 3.37 | 15.84 | 13.354 | 0.001* |
| Post | 44.13 | 2.94 | ||||
| M-ampl mv | Pre | 1.28 | 0.55 | 2.862 | 31.008 | <0.001* |
| Post | 1.75 | 0.2 | ||||
| Laser group | ||||||
| MCV m/s | Pre | 42.51 | 3.15 | 161.31 | 13.453 | 0.001* |
| Post | 43.64 | 2.79 | ||||
| M-ampl mv | Pre | 1.21 | 0.68 | 3.645 | 17.963 | <0.001* |
| Post | 1.75 | 0.29 | ||||
| Drug group | ||||||
| MCV m/s | Pre | 40.28 | 4.3 | 0.342 | 0.356 | 0.558 |
| Post | 40.47 | 3.74 | ||||
| M-ampl mv | Pre | 1.24 | 0.56 | 0.025 | 0.175 | 0.68 |
| Post | 1.09 | 0.28 | ||||
MCV m/s: Motor condition velocity in meter per second.
M -ampl mv: Motor amplitude in millivolt.
* Significance P value < 0.05.
Figure 2.
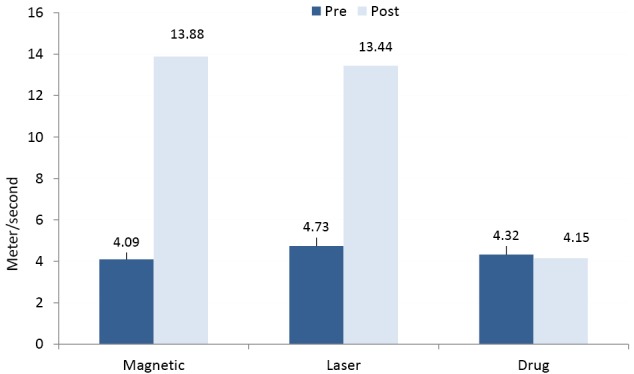
Mean Values of Sensory Conduction Velocity of the 3 Groups.
Figure 3.
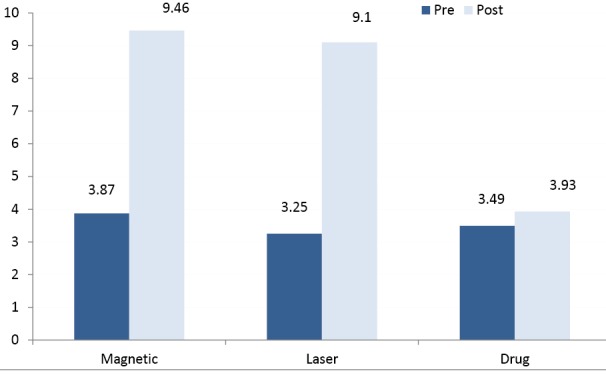
Mean Values of Sensory Amplitudes of the 3 Groups.
Toronto Clinical Neuropath Scoring System and VAS in Treatment Groups
The TRCNSS reduced significantly after adding either magnetic or laser therapy to drug therapy(P < 0.05), whereas non-significant changes were obtained only after usage of drug therapyP > 0.05) (Figure 4). The VAS reduced significantly after interventions in the 3 treatment groups(P < 0.05) (Figure 5).
Figure 4.
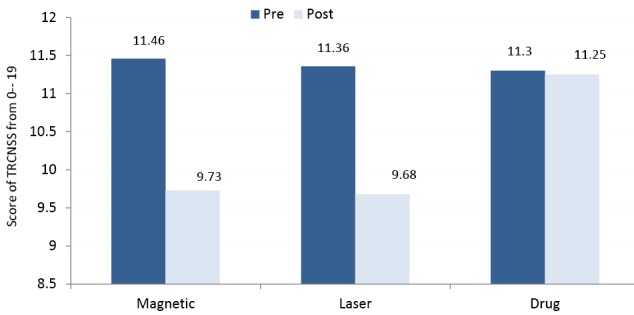
Mean Values of the Toronto Clinical Neuropath Scoring System of the 3 Groups.
Figure 5.
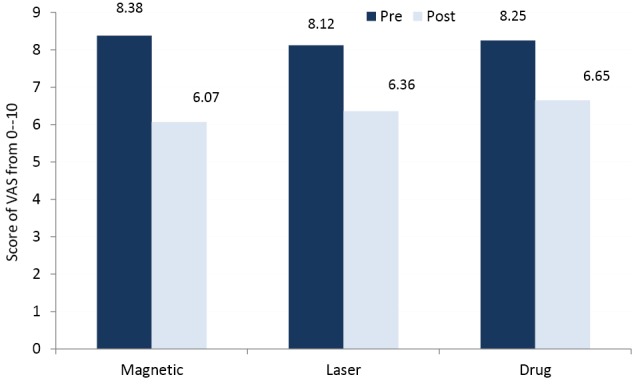
Mean Values of the Visual Analog Scale of the 3 Groups.
Comparison Among the 3 Treatment Groups
The repeated measures ANOVA showed significant differences in the mean values of MCV, SCV, amplitudes and TRCNSS among the 3 groups (P < 0.05). The comparison of the least significant difference (LSD) by the post hoc test proved that there were significant differences in the mean values of measured conduction velocities, amplitudes, and TRCNSS between the magnetic and drug groups. Also, there were significant differences between the laser and drug groups (P < 0.05), whereas non-significant differences were obtained between the magnetic and laser groups (P > 0.05) (Table 3). In addition, the post hoc test proved that there were non-significant differences in the mean values of VAS among the 3 groups after the interventions (P > 0.05) (Table 3).
Table 3. Post Hoc Test Comparison Among the 3 Treatment Groups .
| Variables | Groups | Mean Differences | Standard Error | 95% CI | P Value | |
| Upper | Lower | |||||
| MCV m/s | Magnetic & drug | 3.2 | 0.97 | 5.15 | 1.25 | 0.002* |
| Laser & drug | 2.69 | 0.98 | 4.66 | 0.73 | 0.008* | |
| Magnetic & laser | 0.59 | 0.92 | 2.34 | -1.33 | 0.586 | |
| M-ampl mv | Magnetic & drug | 0.25 | 0.12 | 0.48 | 0.01 | 0.030* |
| Laser & drug | 0.27 | 0.11 | 0.51 | 0.04 | 0.023* | |
| Magnetic & laser | -0.01 | 0.11 | 0.27 | 0.26 | 0.892 | |
| SCV m/s | Magnetic & drug | 4.58 | 2.06 | 8.71 | 0.46 | 0.030* |
| Laser & drug | 4.68 | 2.08 | 8.84 | 0.51 | 0.028* | |
| Magnetic & laser | -0.09 | 1.95 | 3.79 | -3.98 | 0.961 | |
| S-ampl µv | Magnetic & drug | 2.45 | 1.15 | 4.65 | 0.26 | 0.029* |
| Laser & drug | 2.46 | 1.11 | 4.68 | 0.24 | 0.030* | |
| Magnetic & laser | -0.01 | 1.04 | 2.06 | -2.07 | 0.993 | |
| TRCNSS | Magnetic & drug | -0.68 | 0.14 | -0.39 | -0.96 | 0.000* |
| Laser & drug | - 0.75 | 0.14 | -0. 47 | - 1.04 | 0.000* | |
| Magnetic & laser | 0.07 | 0.13 | 0.34 | - 0.19 | 0.571 | |
| VAS | Magnetic & drug | -0.22 | 0.16 | 0.09 | -0.53 | 0.169 |
| Laser & drug | -0.21 | 0.16 | 0.11 | -0.53 | 0.191 | |
| Magnetic & laser | -0.01 | 0.15 | 0.29 | -0.31 | 0.951 | |
MCV m/s, Motor condition velocity in meter per second; M-ampl mv, Motor amplitude in millivolt; SCV m/s, Sensory conduction velocity in meter/second; S-ampl µv, Sensory amplitudes in microvolt; VAS, visual analog scale; TRCNSS, Toronto Clinical Neuropath Scoring System.
* Significance P value < 0.05.
Discussion
DPN is a common significant source of diabetic patients’ distress.6 Although one-third of diabetic patients are affected with neuropathic pain, its treatment is still very complex.3 The drugs of diabetic control and neuropathic pain remain the standard line of treatment because the good control of FBG can postpone or delay the onset of neuropathy and its associated symptoms.9,10,12 Thus all participated patients primarily and regularly received the needed drugs.6,9,12,22
The current results revealed that adding magnetic therapy to drug therapy significantly increased the mean values of measured conduction velocities and amplitudes in parallel with a significant reduction in the mean values of TRCNSS. These benefits of PEMT are supported by a study by Lei et al.15 They found that PEMT restores nerve abnormalities in treated rats with induced DPN. Also, some authors reported that PEMT has direct therapeutic effects on injured nerves and it has a hypoglycemic effect which may explain the relief of DPN.15,30 The underlying mechanisms for these positive effects of PEMT may be due to the fact that it modifies peripheral nerve functions, increases microvascular blood flow,13,30 accelerates nerve conduction velocity,17 and increases the compound action potential of peripheral nerves.31 Moreover, PEMT has an anti-inflammatory effect accompanied by pain relief. It reverses the damage of the peripheral neuropathy and finally restores normal conduction velocity.16 In contrast to the current results,14,18 they did not recommend the usage of PEMT for the treatment of DPN because they failed to demonstrate any significant positive effects on those patients with DPN. Also, other authors found that PEMF did not induce any significant reduction in neuropathic pain.32,33 These contradictions between the previous studies and the current results may be due to the differences in applied parameters, the qualifications of magnetic equipment, and the used techniques of PEMF.
The results of the current study also revealed significant improvements in conduction velocities, amplitudes and TRCNSS in patients with DPN after treating with LLLT in combination with drug therapy. These results were supported by some recent studies.4,22,24,25,27 They have reported that LLLT is a clinically safe therapy for DPN.24,25 Some authors stated that LLLT was effective in nerve protection and repair with different damage degrees.4,22,24,27 Other authors have postulated that LLLT can effectively reduce DPN, increase MCV, SCV, neural amplitudes, and increase microcirculation in patients with DPN.3,22,27 The underlying mechanisms of these induced improvements by LLLT may be due to the fact that it improves tissue perfusion and microcirculation of the ischemic area, has an anti-inflammatory effect on the site of injury through the reduction of prostaglandin synthesis, increases lymphatic flow, decreases local edema, and improves neurological functions.19,34 Laser therapy can also allow higher neural metabolism and increase myelination and axon regeneration.35 It increases adenosine triphosphate synthesis and both serotonin and endorphins secretions.22 The combined effects of all these various mechanisms can justify the induced positive effects of LLLT on the pathogenesis of DPN.24
In contrast to the current results, Bril et al14 recommended that LLLT cannot be used as a treatment modality for DPN. Also, Zinman et al26 did not support its usage in DPN because they did not find any sufficient evidence for the usage of LLLT in painful DPN. The different laser responses might be due to the differences in the intervention time adopted in the 2 studies.21 They found LILT has non-significant influences on MCV and SCV values in patients with painful DPN. The LLLT parameters used in the current study are completely different from those applied in a study by Peric and Cvetkovic.21 They applied laser therapy for one minute/site on nine points, while in the current study the target areas were the plantar surface of the feet and the lumbo-sacral area. Each one was exposed for 15 minutes 2 times per week for 3 months. In addition, the patients received laser therapy only without proper medical control in Peric and Cvetkovic,21 whereas in the current study, the patients received laser therapy combined with proper analgesic medications. The proper parameters of laser application are still controversial in the literature, and consequently high scientific rigor is needed to define the optimum LLLT protocol which is specific in the treatment of DPN.20 There are numerous factors can influence the effects of LLLT such as the type of laser, radiation characteristics, the type of underlying pathology, and treatment regimens such as specific type of laser radiation can have different results on different pathologies.24 Consequently, more studies may be needed prior to generalizing these results.36,37
This study also revealed a significant reduction in the VAS score in the 3 treated groups with non-significant differences among them. It means that proper medical treatment alone or in addition to magnetic or laser interventions can achieve satisfactory analgesic effects for patients with DPN.4,11,27 Thus analgesic medications are still the most commonly used option to manage DPN. These drugs can achieve the analgesic effects for neuropathic pain with wide variability depending upon the used drugs and the extent of underlying neuropathic pathology changes.11,23 In addition, in the long run, the required analgesic doses of those patients who will be exposed to magnetic or laser therapy will be less because of extra positive changes in underlying neuropathic pathology. The TRCNSS reduced significantly after adding either magnetic or laser therapy to drug therapy, but non-significant reductions were obtained in those patients who received only drug therapy. This may be due to the fact that TRCNSS includes a pain measure in addition to other parameters such as touch sensation, tendon reflexes and vibration sense which improved significantly in those patients exposed to both magnetic and laser therapy because of the achievements of other physiological advantages in addition to the effectiveness of drug therapy.
Unfortunately, the results of the current study did not find any significant differences between the effects of adding either magnetic therapy or laser therapy to drug therapy in patients with DPN. To the best of investigators’ knowledge, no previous study compared both magnetic therapy and laser therapy combined with analgesic medications.
Conclusion
It was concluded that the addition of either magnetic or laser therapy to analgesic medications could bring extra positive therapeutic benefits to patients with DPN. There were non-significant differences between the effect of both magnetic and laser therapy on DPN. Both magnetic and laser therapy can be applied in combination with analgesic medications as therapeutic modalities for the treatment of patients with DPN.
Limitations
Small sample size, evaluation with measurements taken only before and after treatment without long-term follow-up.
Recommendations
Further studies are needed to compare the effects of different intensities of laser and magnetic on DPN using a larger sample size for a longer time and with long-term follow up.
Ethical Considerations
All procedures were approved by the Ethics Research Committee of the Institutional Review Board of Imam Abdualrahman Bin Faisal University (IRB-2014-04-061.No. 2014171). Also, This study was registered in ClinicalTrials.gov (Identifier: NCT03049605).
Conflict of Interests
The authors declare no conflict of interest.
Funding
This research is a grant project. It was financially supported by the Dean of Scientific Research of Imam Abdulrahman Bin Faisal University, KSA.
Acknowledgement
The authors thank the Dean of Scientific Research of Imam Abdulrahman Bin Faisal University for financial support, Dr Turki Abualait, the chairman of the department; Mahmoud Elsayed Shanab, Mr Anas Alqarni, Belal Elsayed Shanb, and Ms Manar Al Masoud (researcher assistants), and all participants for their cooperation and valuable efforts through conducting this research.
Please cite this article as follows: Shanb AA, Youssef EF, Al Baker WI, Al-Khamis FA, Hassan A, Jatoi NA. The efficacy of adding electromagnetic therapy or laser therapy to medications in patients with diabetic peripheral neuropathy. J Lasers Med Sci. 2020;11(1):20- 25. doi:10.15171/jlms.2020.05.
References
- 1.Maronesi CT, Cecagno-Zanini SC, de Oliveira LZ, Bavaresco SS, Leguisamo CP. Physical exercise in patients with diabetic neuropathy: systematic review and meta-analysis of randomized clinical trials. Fisioter Pesqui. 2016;23(2):216–23. doi: 10.1590/1809-2950/14649323022016. [DOI] [Google Scholar]
- 2.Alqurashi KA, Aljabri KS, Bokhari SA. Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med. 2011;31(1):19–23. doi: 10.4103/0256-4947.75773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad I, Hussain E, Singla D, Verma S, Ali K. Balance training in diabetic peripheral neuropathy: A Narrative Review. JSM Diabetol Manag. 2017;2(1):1002. [Google Scholar]
- 4.Yamany AA, Sayed HM. Effect of low level laser therapy on neurovascular function of diabetic peripheral neuropathy. J Adv Res. 2012;3(1):21–8. doi: 10.1016/j.jare.2011.02.009. [DOI] [Google Scholar]
- 5.Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev. 2012;49(2):333–38. doi: 10.1682/jrrd.2010.10.0197. [DOI] [PubMed] [Google Scholar]
- 6.Kaku M, Vinik A, Simpson DM. Pathways in the diagnosis and management of diabetic polyneuropathy. Curr Diab Rep. 2015;15(6):609. doi: 10.1007/s11892-015-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic poly-neuropathy. Diabetes Care. 2002;25(11):2048–52. doi: 10.2337/diacare.25.11.2048. [DOI] [PubMed] [Google Scholar]
- 8.Dixit S, Maiya AG, Shastry BA. Effect of aerobic exercise on peripheral nerve functions of population with diabetic peripheral neuropathy in type 2 diabetes: A single blind, parallel group randomized controlled trial. J Diabetes Complications. 2014;28(3):332–9. doi: 10.1016/j.jdiacomp.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Parminder K, Kushwash AS, Ravinderpal K. Current therapeutic strategy in diabetic neuropathy. Int Res J Pharm. 2012;3(3):22–9. [Google Scholar]
- 10.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;13(6):1–60. doi: 10.1002/14651858.CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. Br Med J. 2007;335:(14). doi: 10.2147/IJGM.S64419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin RH, O’Connor AB, Backonja M, Farrar MF, Finnerup NB, Jensen TS. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain. 2007;132(3):237–51. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Conti M, Peretti E, Cazzetta G, Galimberti G, Vermigli C, Pola R. et al. Frequency-modulated electromagnetic neural stimulation enhances cutaneous microvascular flow in Patients with diabetic neuropathy. J Diabetes Complications. 2009;23(1):46–48. doi: 10.1016/j.jdiacomp.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D. et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–65. doi: 10.1212/WNL.0b013e3182166ebe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei T, Jing D, Xie K, Jiang M, Li F, Cai J. Therapeutic effects of 15 Hz pulsed electromagnetic field on diabetic peripheral neuropathy in streptozotocin-treated Rats. PLoS One. 2013;8(4):e61414. doi: 10.1371/journal.pone.0061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graak V, Chaudhary S, Bal BS, Sandhu JS. Evaluation of the efficacy of pulsed electromagnetic field in the management of patients with diabetic poly-neuropathy. Int J Diabetes Dev Ctries. 2009;29(2):56–61. doi: 10.4103/0973-3930.53121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filimban WA, El-Fiky AAR, Helal OF, Abdelaal AA. Effect of magnetic therapy on balance deficits in patients with diabetic polyneuropathy: Randomized Controlled Trial. Jokull Journal. 2015;65(3):187–96. [Google Scholar]
- 18.Wróbel MP, Szymborska-Kajanek A, Wystrychowski G, Biniszkiewicz T, Sieroń-Stołtny K, Sieroń A. et al. Impact of low frequency pulsed magnetic fields on pain intensity, quality of life and sleep disturbances in patients with painful diabetic poly-neuropathy. Diabetes Metab. 2008;34(4):349–54. doi: 10.1016/j.diabet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Alves AC, De Paula VR, Leal-Junior EC, Dos Santos SA, Ligeiro AP. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther. 2013;15(5):R116. doi: 10.1186/ar4296.000000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Andrade AL, Bossini PS, Parizotto NA. Use of low level laser therapy to control neuropathic pain: a systematic review. J Photochem Photobiol B. 2016;164:36–42. doi: 10.1016/j.jphotobiol.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Peric Z, Cvetkovic B. Electrophysiological evaluation of low intensity laser therapy in patients with diabetic polyneuropathy. Facta Universitatis, Series: Medicine and Biology. 2006;13(1):11–14. [Google Scholar]
- 22.Khamseh ME, Kazemikho M, Aghili R, Forough B, Lajevardi M, Dabaghian HF. Diabetic distal symmetric poly-neuropathy: Effect of low-intensity laser therapy. Lasers Med Sci. 2011;26(6):831–35. doi: 10.1007/s10103-011-0977-z. [DOI] [PubMed] [Google Scholar]
- 23.Akyuz G, Kenis O. Physical therapy modalities and rehabilitation techniques in the treatment of neuropathic pain. Int J Phys Med Rehabil. 2014;93(3):253–9. doi: 10.1097/PHM.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 24.Fallah A, Mirzaei A, Gutknecht N, Demneh AS. Clinical effectiveness of low-level laser treatment on peripheral somatosensory neuropathy. Lasers Med Sci. 2017;32(3):721–28. doi: 10.1007/s10103-016-2137-y. [DOI] [PubMed] [Google Scholar]
- 25.Shen CC, Yang YC, Liu BS. Effects of large-area irradiated laser phototherapy on peripheral nerve regeneration across a large gap in a biomaterial conduit. J Biomed Mater Res A. 2013;101:239–52. doi: 10.1002/jbm.a.34314. [DOI] [PubMed] [Google Scholar]
- 26.Zinman LH, Mylan Ngo F, Eduardo T, NweKT Gogov S, Vera Bril F. Low-intensity laser therapy for painful symptoms of diabetic sensorimotor poly-neuropathy A controlled trial. Diabetes Care. 2004;27(4):921–24. doi: 10.2337/diacare.27.4.921. [DOI] [PubMed] [Google Scholar]
- 27.Yamany AA, Bitesha K. Effect of 850 nm He-Ne laser therapy on nerve conduction and foot planter pressures distribution of painful diabetic neuropathy: a randomized controlled trial. J Nov Physiother. 2016;6(4):300. doi: 10.4172/2165-7025.1000300. [DOI] [Google Scholar]
- 28.Asad A, Hameed MA, Khan UA, Butt MU, Ahmed N, Nadeem A. Comparison of nerve conduction studies with diabetic neuropathy symptom score and diabetic neuropathy examination score in type-2 diabetics for detection of sensorimotor polyneuropathy. J Pak Med Assoc. 2009;59(9):594–98. [PubMed] [Google Scholar]
- 29.Gyulai F, Rába K, Baranyai I, Berkes E. Bender T BEMER therapy combined with physiotherapy in patients with musculoskeletal diseases: A randomized, controlled double blind follow-up pilot study. Evid Based Complement Alternat Med. 2015;2015:245742. doi: 10.1155/2015/245742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosi E, Conti M, Vermigli C, Cazzetta G, Peretti E. Effectiveness of frequency-modulated electromagnetic neural stimulation in the treatment of painful diabetic neuropathy. Diabetologia. 2005;48(5):817–23. doi: 10.1007/s00125-005-1734-2. [DOI] [PubMed] [Google Scholar]
- 31.Tasset I, Medina FJ, Jimena I, Aguera E, Gascon F, Feijóo M. Neuroprotective effects of extremely low-frequency electromagnetic fields on a Huntington’s disease rat model: Effects on neuropathic factors and neuronal density. Neuroscience. 2012;209:54–63. doi: 10.1016/j.neuroscience.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 32.Weintraub MI, Herrmann DN, Smith AG, Backonja MM, Cole SP. Pulsed electromagnetic fields to reduce diabetic neuropathic pain and stimulate neuronal repair: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:1102–9. doi: 10.1016/j.apmr.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Stein C, Eibel B, Sbruzzi G, Lago PD, Plentz RD. Electrical stimulation and electromagnetic field use in patients with diabetic neuropathy: systematic review and meta-analysis. Braz J Phys Ther. 2013;17(2):93–104. doi: 10.1590/S1413-35552012005000083. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh YL, Chou LW, Chang PL, Yang CC, Kao MJ. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1alpha (HIF-1alpha) J Comp Neurol. 2012;520(13):2903–16. doi: 10.1002/cne.23072. [DOI] [PubMed] [Google Scholar]
- 35.Barbosa RI1, Marcolino AM, de Jesus Guirro RR, Mazzer N, Barbieri CH, de Cássia Registro Fonseca M . Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci. 2010;25(3):423–30. doi: 10.1007/s10103-009-0750-8. [DOI] [PubMed] [Google Scholar]
- 36.Jang H, Lee H. Meta-analysis of pain relief effects by laser irradiation on joint areas. Photomed Laser Surg. 2012;30(8):405–17. doi: 10.1089/pho.2012.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR. Low level laser treatment of tendinopathy: A systematic review with meta-analysis. Photomed Laser Surg. 2010;28(1):3–16. doi: 10.1089/pho.2008.2470. [DOI] [PubMed] [Google Scholar]


