Abstract
Objective:
Describe relationships between self-reported personal demographics or familial characteristics and psychosocial outcomes (PROMIS® Global Health, Impact of Event Scale-R (pancreatic cancer risk related distress), cancer risk perception, and cancer worry) in participants with inherited or familial pancreatic cancer risk.
Methods:
A multi-site cross-sectional survey including high risk adults with no personal history of pancreatic cancer. All variables were summarized with descriptive statistics. T-test, Chi-Square/Fisher’s exact test were used to assess univariate associations and backward model selection was used in multivariable analysis.
Results:
Respondents (N=132) reported moderate to high frequency of cancer worry and 59.3% perceived a 50% or more perceived lifetime risk for pancreatic cancer, which far exceeds typical objective risk estimates. Cancer worry was associated with female gender (p=0.03) and distress (p=0.05). Higher risk perceptions were associated with having a high-school education or less (p=0.001), higher distress (p=0.02) and cancer worry (p=0.008) and family cancer death experience (p=0.02). Higher distress was associated with experience as a caregiver to a seriously ill family member in the past 5 years (p=0.006).
Conclusions:
Individuals with inherited or familial pancreatic cancer risk experience cancer worry, distress, and have increased risk perceptions, specifically in the period following caring for a loved one with cancer. Routine evaluation of distress in this setting, as well as the development of supportive care resources, will help support patients living with risk for pancreatic cancer.
Keywords: cancer, oncology, pancreatic cancer risk, patient reported outcomes, psychosocial
Background
Pancreatic cancer will be the second leading cause of cancer-related deaths by 2030 largely in part to the aging of the population and the limitations of current early detection modalities and curative treatments.1 Compared to other solid tumors, pancreatic cancer has the lowest 5-year survival rate,2 and if pancreatic cancer is stage IA when diagnosed, an individual has a 5-year survival rate of 14%4. For those with stage III and IV pancreatic cancer, the survival rate is only 1–3%.3 As there are few signs and symptoms, most patients are diagnosed at a late stage.
Individuals at hereditary or familial risk for pancreatic cancer are currently identified through cancer risk assessment, genetic counseling and testing. Those with risk related to familial or hereditary factors face up to a 3.5–40% lifetime chance of developing pancreatic cancer compared to the general population risk of 1.5%.3, 4 Specifically, the highest risk group includes those with known genetic syndromes of which pancreatic cancer is a component, Hereditary Breast and Ovarian Cancer syndrome (HBOC), Lynch syndrome, or more rarely Peutz Jeghers syndrome and Familial atypical multiple mole-melanoma syndrome (FAMMM). Most commonly, individuals are identified as having familial pancreatic cancer risk due to having 2 or more relatives on the same side of the family affected by pancreatic cancer.4, 5
Individuals living with inherited risk for pancreatic cancer are recommended a novel type of pancreatic cancer surveillance not provided to the general population. There has been limited research on the psychosocial impact of living with pancreatic cancer risk. Konings et al.19 report findings from a multicenter prospective observational study that evaluated high risk participant experiences and reports of distress related to undergoing surveillance as part of the Dutch pancreatic cancer surveillance study. A subset of the sample of 140 individuals who completed the assessment reported clinically significant distress (5–7%) and cancer worry. Overall, 21% of the sample reported sustained moderate to elevated distress.19 The same research team reports in a similar study that 33% of individuals undergoing pancreatic cancer surveillance experienced cancer worry, especially in those with a family history of early onset pancreatic cancer.20 Two other prospective studies have been completed in a sample of unaffected individuals with high pancreatic cancer risk. In these two studies, distress, cancer worry and risk perception were measured 3 months21 and 1 year following genetic counseling and screening.22 Overall no significant increases in outcomes were found over time in these studies, though it was reported that in 35 men and 60 women, 22.9% and 19.9% respectively scored above the cutoff for clinical distress.21 Distress occurred specifically in participants who were younger,21, 22 had a strong family history,21 and had baseline distress.21, 22
Preliminary qualitative work found that family experiences shape the individual’s perceptions and experiences of cancer risk.6 However, unlike other common cancers such as breast or colon, stories of survivorship rarely exist with pancreatic cancer, and thus family members often live within a context of uncertainty and fear. In these exploratory interviews with healthy individuals, we found that family cancer experience, specifically grief related to familial cancer deaths, was an important characteristic that impacted how a participant viewed their own risk for pancreatic cancer.
Key research aims addressed in this exploratory paper are relationships between participant self-reported personal or familial characteristics (age, gender, highest level of education, marital status, personal and family history of cancer and cancer-related death, experience as a family caregiver, and social support) and psychosocial outcomes (global health, pancreatic cancer risk related distress, cancer risk perception and cancer worry) in participants at high risk for inherited or familial pancreatic cancer. The study was part of a larger study that aimed to describe beliefs, perceptions, emotions and behaviors or behavioral intent (reported separately) in individuals with inherited or familial pancreatic cancer risk. Participants utilization of surveillance and other health behavior modifications will be presented elsewhere.
Methods
Study Design
The study applied a multi-site descriptive, cross-sectional design utilizing mailed and web-based survey method.7 Measures included for this report were identified through the integration of theoretical frameworks and preliminary research, specifically by an ecological model of health,8 which guided our demographic measures, measures of social support, and global health measures, and the Health Belief Model, which guided our measurement of risk perception and health behavior factors (reported elsewhere),9 the Theory of Genetic Vulnerability, which posits that family experience, worry, and distress play a role in understanding cancer risk10 and preliminary qualitative research, which identified the role of being a family caregiver as an important part of understanding individual pancreatic cancer risk.6 The DFCI Institutional Review Board approved the study protocol.
Sample and Data Collection
Participants were recruited through two large academic medical centers in the Northeastern United States from January 2016 through January 2017. Eligible participants were adults without a diagnosis of pancreatic cancer who had been evaluated by a genetic counselor and determined to have hereditary or familial pancreatic cancer risk and were offered pancreatic surveillance by a physician. Participants were recruited through a mailed method that included an information sheet with consent and a survey. The information sheet contained a link to the web-based survey. A returned paper or web-based survey was considered implied informed consent. Potentially eligible participants received a nominal monetary gift ($5) at time of initial recruitment. The survey responses were reviewed by the study team which comprised of clinicians from each recruitment site.
Measures
Race, ethnicity and age are routinely collected within the clinic and were extracted from the medical record in order to reduce participant survey burden. Self-reported demographic items included personal cancer history, gender, marital status, highest level of education, household income, experience caring for a person with pancreatic cancer, experience caring for a person dying of pancreatic cancer, and experience taking care of others with cancer. Self-reported patient psychosocial experiences were measured using validated measures and with items derived from previous qualitative research conducted by the study team.6
Cancer-related worry was measured by the adapted Lerman Breast Cancer Worry Scale11 which includes 3 items measuring frequency and impact of worry. The scale has acceptable psychometric properties, with Cronbach’s alpha score previously reported as 0.77.12 Responses are provided on a scale of 1 (not at all) to 5 (almost all the time) for the first item and 1 (not at all) to 4 (a lot) for the other items. For item 1, the top 2 categories were condensed (often/almost all the time) to transform the response scale to a 1–4 scale. The items were summated into a total score. One additional item generated from preliminary qualitative research6 was included which asked “When do you tend to worry about pancreatic cancer?” and participants could check all that apply (specified in table 3).
Table 3:
Univariate analysis of associations between personal and familial characteristics and psychosocial outcomes
| IES-R Total M† (SD)‡ | Global Health: Mental M (SD) | Global Health: Physical M (SD) | |
|---|---|---|---|
| Have you had cancer? | |||
| No | 8.29 (12.79) | 49.73(10.12) | 54.42(8.06) |
| Yes | 11.31 (16.16) | 49.46(11.30) | 52.06(8.56) |
| Gender | |||
| Male | 5.96 (7.81) | 51.63(9.75) | 53.56(6.88) |
| Female | 11.37 (16.58) | 48.57(11.03) | 53.21(9.03) |
| Age | |||
| <50 | 9.25 (14.25) | 47.27(12.64) | 52.54(8.75) |
| ≥50 | 9.94 (14.71) | 50.52(9.69) | 53.55(8.24) |
| Marital States | |||
| Married/Partnered | 8.53 (13.18) | 51.75(10.27) | 54.69(7.76) |
| All other | 12.27 (17.27) | 44.11(9.87) | 49.95(8.98) |
| Highest Level of Education | |||
| High school degree or lower | 17.22(15.81) | 38.60(10.58) | 45.81(9.72) |
| Some college or higher | 8.99(14.27) | 50.51(10.21) | 53.84(8.07) |
| Have you taken care of a very ill parent or close family member? | |||
| No | 7.48 (10.44) | 49.06(10.55) | 49.93(8.93) |
| Yes | 10.81 (16.04) | 49.88(10.77) | 54.78(7.68) |
| How long ago did you provide this care? | |||
| ≤5 years | 15.0 (19.09) | 50.5(10.02) | 55.72(7.08) |
| ≥6 years | 5.51 (9.21) | 49.93(10.87) | 54.30(7.80) |
| Have you lost a family member to cancer? | |||
| No | 6.67 (11.24) | 54.21(7.02) | 54.88(7.75) |
| Yes | 9.90 (14.69) | 49.33(10.79) | 53.20(8.41) |
| How long ago did you lose a family member to pancreatic cancer? | |||
| ≤5 years | 10.71 (16.34) | 48.21(10.93) | 53.17(8.02) |
| ≥6 years | 9.15 (13.25) | 50.25(10.73) | 53.21(8.87) |
Mean;
Standard Deviation
Note: text in bold was statistically significant at p</=.05 in univariate analysis; Household income was not included due to amount of missing data attributed to a response item “prefer not to answer”.
Pancreatic cancer risk specific distress was measured with the Impact of Event Scale-Revised (IES-R).13 The IES-R is a 22-item scale that measures perceptions of event specific distress in the past 7 days, in this case pancreatic cancer risk. The scale consists of a total score and three subscale scores: intrusion, avoidance and hyperarousal. Responses to items are scaled 0–4, and total scores can range 0–88 with a higher score indicating greater distress. Previous literature has reported acceptable psychometric properties of this measure used in samples with hereditary cancer risk (Cronbach’s alphas= 0.84–0.91).14
The risk perception measure described by Levy et al. was used to evaluate perceptions of lifetime chance of developing pancreatic cancer on a scale of 0% to 100% in increments of 10%.15 Risk perceptions were dichotomized for analysis into two categories, 0%−40% and ≥ 50% to reflect the objective understanding that the maximum level of pancreatic cancer risk presented to at risk individuals rarely exceeds 40% and therefore a 50% or higher perception would indicate an over estimate
Patient Reported Outcome Measurement Information System (PROMIS ®) measures were used to asses social support and global health.16 Social support was measured by the 18 item PROMIS®: Emotional, Informational, and Instrumental Social Support Scales-short for.17 Responses were provided on a scale of 1 (never) to 5 (always) and total scores ranged from 6–30 for each domain with a higher score indicating higher social support. Overall health was measured using the 10 item PROMIS®: Global Health Scale,17 which rates self-reported overall physical and mental health as well as symptoms of emotional problems, fatigue, and pain over the past 7 days. Two subscales scores were created for 1) physical and 2) mental health with 4 items in each. The remaining two items related to overall quality of life and satisfaction with social roles were scored separately. Items are scored on a scale 5 (Excellent) to 1 (Poor) with higher score indicating better global health. Pain and fatigue are scored on a 1–10 scale and recategorized to a 1–5 scale for analysis and are included in the Physical Health subscale. Overall physical and mental health summary scores were converted into T-score based on PROMIS® scoring manual.18
Statistics Analysis
The study aim was exploratory and therefore no power analysis was conducted. Descriptive statistics were used to summarize baseline personal and family characteristics and outcome measures. Assumptions for normality were evaluated. Univariate associations between psychosocial measures (cancer worry, risk perception, distress and global health) and personal factors (Table 3) were assessed. At-test with equal or unequal variance (when appropriate) was used for continuous variables and Chi-square test/Fisher’s exact test were used for categorical variables. Backward model selection was used for multivariable analysis, all variables that were assessed in univariate analysis (table 3) were included in the initial model. Logistic regression was used for dichotomized perceived risk (0%−40% vs. ≥ 50%) and linear regression was used for continuous outcomes. Variables with p≤0.1 were retained. Possible two-way interactions were evaluated among the remaining variables and interactions with p≤0.2 were retained due to exploratory nature.
Results
186 participants were recruited and 133 returned a survey (response rate 71.5%) with analyzable data available for 132 participants. There were no observed or statistically significant differences in between the two recruitment sites. Table 1 describes the participants’ personal and familial characteristics. Of note, female gender was significantly associated with having had cancer (p=0.007) and having taken care of a very ill parent or family member (p=0.05). Having taken care of a very ill parent or family member was also associated with higher education (p=0.01). The overall mean age at time of enrollment was 58.5 (SD=11.9) and 92 of the 132 participants reported engaging in pancreatic screening. Health behavior information, including screening, will be reported in more detail elsewhere. Participants reported moderate/high levels of social support, with a median instrumental support score of 28 (range 6–30); informational support score of 27.5 (range 11–30); and emotional support score of 29 (range 6–30). Cancer Worry and Risk Perception Responses are summarized in Table 2. The Cancer Worry and Impact of Event-Revised scales were associated with risk perception. Higher risk perception (vs. low risk perception) was associated with higher cancer worry (8.66 vs. 7.37; p=0.008) and higher distress (11.62 vs. 15.81; p=0.02). Table 3 presents outcomes from univariate analysis to explore the relationship between personal and familial characteristics and the IES-R and Global Health outcomes. Below we will summarize how personal and familial factors are associated with each of the key psychosocial outcome measures.
Table 1:
Participant demographic and family characteristics (n=132)
| N | % | |
|---|---|---|
| Gender | ||
| Male | 42 | 31.8 |
| Female | 89 | 67.4 |
| Race | ||
| White | 121 | 91.7 |
| Marital Status | ||
| Married/Partnered | 94 | 71.2 |
| Highest Level of Education | ||
| High School or Less | 10 | 7.6 |
| Some college or more | 121 | 91.7 |
| Household Income | ||
| < $50,000 | 20 | 15.2 |
| $50,000-$99,999 | 26 | 19.7 |
| > $100,000 | 59 | 44.7 |
| Have you ever had cancer? | ||
| No | 68 | 51.5 |
| Yes | 64 | 48.6 |
| Have you taken care of a very ill parent or close family member? | ||
| No | 44 | 33.3 |
| Yes | 88 | 66.7 |
| If yes, how long ago? | ||
| ≤5 years | 46 | 52.3 |
| ≥6 years | 40 | 45.5 |
| Was this illness pancreatic cancer? | ||
| No | 20 | 22.7 |
| Yes | 44 | 33.3 |
| How many family members lost to pancreatic cancer | ||
| 0 | 2 | 1.5 |
| 1 | 44 | 33.3 |
| 2 | 50 | 37.9 |
| 3 | 13 | 9.8 |
| 4 | 10 | 7.6 |
| 5 or more | 3 | 2.3 |
| How many family members lost to a cancer other than pancreatic cancer | ||
| 0 | 25 | 18.9 |
| 1 | 24 | 18.2 |
| 2 | 15 | 11.4 |
| 3 | 15 | 11.4 |
| 4 | 14 | 10.6 |
| 5 or more | 16 | 11.9 |
| How long ago was your last experience with losing a family member to pancreatic cancer? | ||
| ≤5 years | 58 | 43.9 |
| ≥6 years | 66 | 50 |
Table 2:
Pancreatic cancer risk perception and cancer worry
| N | % | |
|---|---|---|
| Perceived lifetime chance of pancreatic cancer | ||
| 0% | 2 | 1.5 |
| 10% | 20 | 15.2 |
| 20% | 11 | 8.3 |
| 30% | 14 | 10.6 |
| 40% | 4 | 3.0 |
| 50% | 38 | 28.8 |
| 60% | 14 | 10.6 |
| 70% | 8 | 6.1 |
| 80% | 10 | 7.6 |
| 90% | 1 | .8 |
| 100% | 0 | 0 |
| How often do you worry about getting pancreatic cancer? | ||
| Not at all/rarely | 54 | 40.9 |
| Sometimes | 55 | 41.7 |
| Often/almost all the time | 21 | 15.9 |
| How much does your worry affect your mood? | ||
| Not at all/rarely | 104 | 78.8 |
| Sometimes | 17 | 12.9 |
| Often/almost all the time | 8 | 6.1 |
| How much does your worry affect your ability to perform your daily activities? | ||
| Not at all/rarely | 120 | 90.9 |
| Sometimes | 8 | 6.1 |
| Often/almost all the time | 3 | 2.3 |
| When do you tend to worry about pancreatic cancer? | ||
| When I am at the doctor/When I am sick | 46 | 34.8 |
| When I go for screening/waiting for my screening results | 75 | 56.8 |
| When I am with family | 7 | 5.3 |
| When I think about loved ones who have had pancreatic cancer | 76 | 57.6 |
| During the holidays | 11 | 8.3 |
| On my birthday | 40 | 30.3 |
| On the birthday of my loved ones who have died of pancreatic cancer | 40 | 30.3 |
| I worry all the time about pancreatic cancer | 6 | 4.5 |
| I do not worry about pancreatic cancer at specific times, it happens randomly when I do not expect to worry | 41 | 31.3 |
| I do not worry about pancreatic cancer | 10 | 7.6 |
Cancer Worry and Risk Perception (Table 2)
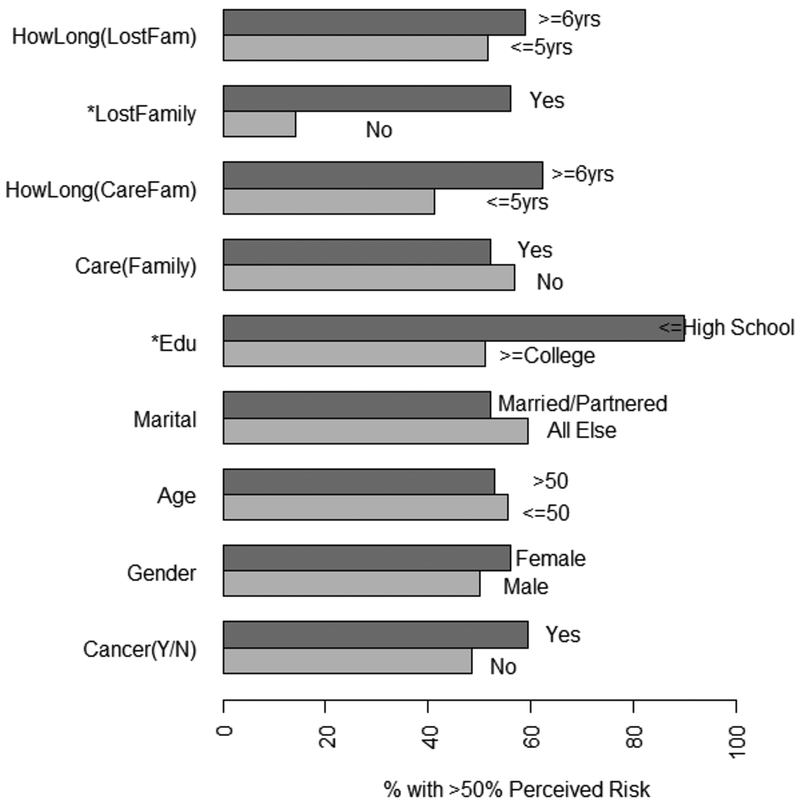
The overall median cancer worry scale score was 8 (range 4–16), indicating moderate cancer worry, which was significantly associated with gender, with females (mean=8.46) reporting higher worry than males (mean=7.39) (p=0.03). The percentage of participants who responded to each level of risk (0 through 100%) are reported in table 2. The percent of individuals who report ≥ 50% lifetime risk perception for pancreatic cancer based on personal or familial characteristics are described in Figure 1. Univariate analysis of risk perception as a continuous variable identified a statistically significant association between higher risk perception and having a high school education or less (n=10; p=0.002).
Figure 1: % of cases with ≥50% perceived risk.
*Lost family member and low education (edu) (p=0.01) (p-0.02) were significantly associated with high percentage of >50% risk perception.
Pancreatic Cancer Risk Related Distress (Table 3)
The mean IES subscale scores were 3.6 (SD=5.7) for intrusion, 4.2 (SD=5.8) for avoidance and 1.8 (SD+4.1) for hyper-arousal. Univariate associations were found between the total score (p=0.006), intrusion (p=0.01), avoidance (p=0.004) and hyper-arousal subscales (p=0.03) and the length of time since being a caregiver for a very ill parent or family member, with those having provided care less than or equal to 5 years ago having higher levels of distress. Additionally, female gender was associated with significantly higher total score (p=0.05) and hyper-arousal subscale scores (p=0.01).
Global Health
The mean score for PROMIS physical health was 53.28 (SD=8.4) and mental health was 49.6 (10.7) indicating high overall global health. Associations based on personal and familial variables are included in Table 4. Those who were married reported higher global mental (p=0.0002) and physical (p=0.004) health, as well as higher overall quality of life (p=0.004) and role satisfaction (p=0.0003). Lower global health scores were associated with having a high school or less education (n=10), and these participants had significantly lower global mental (p=0.0006) and physical (p=0.008) health, quality of life (0=0.006) and role satisfaction (p=<.001) compared to all others. Lower physical health was associated with having had taken care of a very ill parent or loved one (p=.002) and, though not significant, a trend towards lower quality of life (p=0.08) was identified.
Multivariable Analysis
Female gender (est. 0.94; p=.06) and high school education or less (est. −1.74; p=0.06) were retained in the model associated with higher cancer worry. No significant two-way interactions were identified. The multivariable model with risk perception as both a continuous and categorical variable had similar findings as reported in univariate analysis. The outcome of distress (IES-R) was modeled two ways, first including the total sample (n=124). Female gender (est. 5.42; p=0.05) and having some college education (est. −8.25; p=0.09) were retained. Distress was also modeled including only those who responded that they had cared for an ill family member (n=83). Having some college education (est.17.61; p=0.10), female gender (est. 7.23; p=0.06) and having been a caregiver less than 5 years ago (est. 8.7; p=0.008) were retained. A marginally significant interaction effect (p=.07) was identified between female gender and time elapsed since providing care, indicating that females who had provided care ≤ 5 years ago had higher IES scores compared to all others. Lower global mental health was associated with age less than 50 (est. −3.20; p=0.1), being unmarried, and high school education or less (est. −10.28, p=0.0007). Lower physical health was associated with having had cancer (est. −2.10, p=0.11) and being unmarried (est. −5.12, p=0.0008). An interaction effect was identified between having taken care of an ill family member and having a high school education or less (n=3), with those individuals reporting lower physical global health scores.
Discussion
In a study of individuals without a diagnosis of pancreatic cancer who had been identified as having hereditary or familial risk, we identified that overall participants had mild pancreatic cancer risk specific distress, high levels of risk perception and moderate cancer worry. Experience as a caregiver for a person with cancer in the past 5 years was associated with the individual’s self-reported risk perception and psychosocial outcomes, especially in females. Results from our study and previous literature suggest that assessment of distress in the setting of cancer genetics, as is recommended in all of cancer care, might help identify individuals in need for additional supportive care.23 Routine and systematic assessment of psychosocial concerns in the context of hereditary cancer care has been found to improve communication about psychosocial issues between patient and provider24 and would be an important addition to standard hereditary cancer care.
In this study, we found that most participants subjectively report a risk for pancreatic cancer that exceeds a 50% lifetime risk, which is not reflective of objective risks associated with familial or hereditary factors. The discordance between objective and subjective risk has been previously identified within the domain of hereditary cancer, predominately in hereditary breast and ovarian cancer.25, 26 Outside of breast cancer, one study focused solely on patient experiences with genetic counseling related to pancreatic cancer and found that genetic testing was perceived as helpful and that risk perceptions were high, with perceptions of lifetime risk being on average 50.8 on a scale of 0–100.27 Within the current context of multigene panel testing where inconclusive results are common, there are even more challenges with subjective interpretation of objective cancer risk.28 Risks related to hereditary or familial pancreatic cancer vary widely based on personal or family history3, 4 and are often difficult to characterize by clinicians; however, rarely does risk exceed 30%. The challenge patients have interpreting risk within this context is reflected in the risk perceptions reported by the study sample.
A potential contributor to risk perception is family experience with cancer and cancer death. Witnessing loved ones die from a disease they themselves are known to be at risk for plays a role in how individuals with hereditary or familiar cancer risk perceive that risk. The subjective experience of cancer in a family often varies based on the type of diagnosis and the severity of the disease. In our sample, approximately one-third of participants with high risk for cancer reported having been a caregiver themselves. In literature pertaining to HBOC, stories of survivorship, empowerment and cure often shape the discussion about breast and ovarian cancer in a family. Women discuss prevention, early detection and successful treatment because those outcomes have been modeled within the family. Even in the absence of survivorship, in the context of breast and ovarian cancer women share that they are “changing their family story” with risk reduction and early detection options.29 These survivorship stories rarely exist within families with pancreatic cancer6; therefore, the severity of the disease and lack of prevention recommendations shape how the individual views risk. Evidence is still emerging related to the effectiveness of early detection or prevention for pancreatic cancer and it is yet to be decided if current surveillance recommendations are effective. Thus, individuals are left with knowledge of a disease that may never occur and a high chance of death if it does, with minimal available evidence to support early detection or prevention. There has been some literature discussing the caregiving experience of first-degree relatives in the context of pancreatic cancer,30, 31 however, implications for family risk or the experience of a family member being a caregiver within a hereditary or familial risk setting were not discussed. The diagnosis of pancreatic cancer is severe, moves quickly, and often places a heavy burden on both patient and caregiver, and therefore the focus is often not the potentially at-risk family member.32
It is intuitive to learn from our study that individuals who cared for a family member with cancer or experienced a cancer death in the family would have higher levels of distress. These findings were significant especially in those of female gender, for those who have experienced caregiving within the past 5 years. Often family members seek genetic counseling and testing after a loved one is diagnosed with cancer and familial cancer care begins during or in the immediate period following a loved one being diagnosed with cancer or dying of cancer. The family cancer experience is often a catalyst for encouraging high risk family members to seek genetics cancer care. Knowing that the caregiving and death experience will play a role in how the patient understands and experiences cancer risk provides an opportunity within both a clinical and research context to offer support to individuals who may be at risk for poorer outcomes up-front. The role of being a “pre-vivor” in the context of caregiving has not been widely studied, especially in pancreatic cancer. As genetics in cancer care expands and individuals with a variety of cancer risks are identified, psychosocial support should be tailored to the specific disease and to the individuals needs based not only on cancer risk but also on experiences in the family.
Conclusions
In conclusion, results from this study indicate that individuals with inherited or familial pancreatic cancer risk may experience cancer worry and have inflated risk perceptions, specifically after caring for a loved one with cancer or experiencing recent cancer death in the family. Lower education, being unmarried and female gender are associated with increased distress and cancer worry.
Limitations
The results of this study should be interpreted considering some limitations. Results are from one geographic area within an academic medical setting and may not be applicable to other locations. Respondents were predominately white and therefore the applicability of findings to individuals with other racial or ethnic backgrounds is limited. There were missing data related to household income, and therefore the associations of that variable could not be analyzed. Additionally, time since genetic counseling appointment and reason for attending a high-risk program were not collected, which could impact study findings. Findings related to education level should be considered carefully as they result from a very small number (n=10) of participants. Finally, results should not be interpreted as causal as the study was designed to permit causal inference.
Clinical and Research Implications
Future clinical initiatives should focus on routinely assessing distress within this setting and research initiatives should work towards developing supportive care resources for individuals living with risk for pancreatic cancer, specifically targeting those who have cared for loved ones with pancreatic cancer and experienced cancer-related death in the family. Considerable effort should be made to support individuals and their families in understanding the magnitude of cancer risk associated with hereditary or familial pancreatic cancer.
Acknowledgements
Funding provided by DFCI Mittleman Family Fund, KL2/Catalyst Medical Research Investigator Training award (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award KL2 TR001100). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. Additional funding for SS by U01 CA210170 (PI Goggins) and the DFCI Bowen-Chapman Family Research Fund for Pancreatic Cancer Prevention. We acknowledge the survey participants, Ruth Lederman in the Survey Methods Data Core, and Taylor Hendel for support.
Footnotes
Conflict of Interest
No conflicts of interest to report.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 3.Underhill ML, Germansky KA, Yurgelun MB. Advances in Hereditary Colorectal and Pancreatic Cancers. Clin Ther. 2016;38(7):1600–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am J Gastroenterol. 2015;110(2):223–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhen DB, Rabe KG, Gallinger S, Syngal S, Schwartz AG, Goggins MG, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med. 2015;17(7):569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Underhill M, Berry D, Dalton E, Schienda J, Syngal S. Patient experiences living with pancreatic cancer risk. Hered Cancer Clin Pract. 2015;13(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillman DA, Smyth JD, & Christian LM Internet, Mail, and Mixed Mode Surveys: The Tailored Design Method. 3rd Edition ed. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 8.Patrick K, Intille SS, Zabinski MF. An ecological framework for cancer communication: implications for research. J Med Internet Res. 2005;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champion VL, Skinner CS. The health belief model In: Glanz K, Rimer BK, Viswanath K (eds). Health behavior and health education: Theory, research, and practice. 4th ed. San Farncisco, CA: John Wiler & Sons; 2008;4:45–65. [Google Scholar]
- 10.Hamilton RJ, Bowers BJ. The theory of genetic vulnerability: a Roy model exemplar. Nurs Sci Q. 2007;20(3):254–64. [DOI] [PubMed] [Google Scholar]
- 11.Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. Psychological side effects of breast cancer screening. Health Psychol. 1991;10(4):259. [DOI] [PubMed] [Google Scholar]
- 12.Phillips KM, McGinty HL, Gonzalez BD, Jim HSL, Small BJ, Minton S, et al. Factors associated with breast cancer worry 3 years after completion of adjuvant treatment. Psychooncology. 2013;22(4):936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss DS. The Impact of Event Scale: Revised In: Wilson JP, Tang CS (eds) Cross-Cultural Assessment of Psychological Trauma and PTSD. International and Cultural Psychology Series. Boston, MA:Springer; 2007. [Google Scholar]
- 14.Thewes B, Meiser B, Hickie IB. Psychometric properties of the Impact of Event Scale amongst women at increased risk for hereditary breast cancer. Psychooncology. 2001;10(6):459–68. [DOI] [PubMed] [Google Scholar]
- 15.Levy AG, Shea J, Williams SV, Quistberg A, Armstrong K. Measuring perceptions of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1893–8. [DOI] [PubMed] [Google Scholar]
- 16.Cook KF, Jensen SE, Schalet BD, Beaumont JL, Amtmann D, Czajkowski S, et al. PROMIS(®) measures of pain, fatigue, negative affect, physical function, and social function demonstrate clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PROMIS. PROMIS Scoring Guide 2011. [Available from: https://www.assessmentcenter.net/documents/PROMIS%20Scoring%20Manual-%20CATs,%20Profiles,%20Short%20Forms.pdf.
- 19.Konings ICAW, Sidharta GN, Harinck F, Aalfs CM, Poley J-W, Kieffer JM, et al. Repeated participation in pancreatic cancer surveillance by high-risk individuals imposes low psychological burden. Psychooncology. 2015:n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 20.Konings ICAW, Harinck F, Kuenen MA, Sidharta GN, Kieffer, Aalfs CM, et al. Factors associated with cancer worries in individuals participating in annual pancreatic cancer surveillance. Fam Cancer. 2016:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maheu C, Vodermaier A, Rothenmund H, Gallinger S, Ardiles P, Semotiuk K, et al. Pancreatic cancer risk counselling and screening: impact on perceived risk and psychological functioning. Fam Cancer. 2010;9(4):617–24. [DOI] [PubMed] [Google Scholar]
- 22.Hart SL, Torbit LA, Crangle CJ, Esplen MJ, Holter S, Semotiuk K, et al. Moderators of cancer-related distress and worry after a pancreatic cancer genetic counseling and screening intervention. Psychooncology. 2012;21(12):1324–30. [DOI] [PubMed] [Google Scholar]
- 23.Hirschberg AM, Chan-Smutko G, Pirl WF. Psychiatric implications of cancer genetic testing. Cancer. 2015;121(3):341–60. [DOI] [PubMed] [Google Scholar]
- 24.Eijzenga W, Aaronson NK, Hahn DEE, Sidharta GN, Kolk LEvd, Velthuizen ME, et al. Effect of Routine Assessment of Specific Psychosocial Problems on Personalized Communication, Counselors’ Awareness, and Distress Levels in Cancer Genetic Counseling Practice: A Randomized Controlled Trial. J Clin Oncol. 2014;32(27):2998–3004. [DOI] [PubMed] [Google Scholar]
- 25.Caruso A, Vigna C, Marozzo B, Sega FM, Sperduti I, Cognetti F, et al. Subjective versus objective risk in genetic counseling for hereditary breast and/or ovarian cancers. J Exp Clin Cancer Res. 2009;28(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rantala J, Platten U, Lindgren G, Nilsson B, Arver B, Lindblom A, et al. Risk perception after genetic counseling in patients with increased risk of cancer. Hered Cancer Clin Pract. 2009;7(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axilbund JE, Brune KA, Canto MI, Brehon BC, Wroblewski LD, Griffin CA. Patient Perspective on the Value of Genetic Counselling for Familial Pancreas Cancer. Hered Cancer Clin Pract. 2005;3(3):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanoch Y, Miron-Shatz T, Rolison JJ, Ozanne E. Understanding of BRCA1/2 genetic tests results: the importance of objective and subjective numeracy. Psychooncology. 2014;23(10):1142–8. [DOI] [PubMed] [Google Scholar]
- 29.Underhill ML, Lally RM, Kiviniemi MT, Murekeyisoni C, Dickerson SS. Living My Family’s Story: Identifying the Lived Experience in Healthy Women at Risk for Hereditary Breast Cancer. Cancer Nurs. 2012;35(6):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrin K, Bowen DJ, Alfano CM, Bennett R. Adjusting to pancreatic cancer: Perspectives from first-degree relatives. Palliat Support Care. 2009;7(3):281–8. [DOI] [PubMed] [Google Scholar]
- 31.Sherman DW, McGuire DB, Free D, Cheon JY. A pilot study of the experience of family caregivers of patients with advanced pancreatic cancer using a mixed methods approach. J Pain Symptom Manage. 2014;48(3):385–99. e2. [DOI] [PubMed] [Google Scholar]
- 32.Petrin K, Bowen DJ, Alfano CM, Bennett R. Adjusting to pancreatic cancer: perspectives from first-degree relatives. Palliat Support Care. 2009;7(3):281–8. [DOI] [PubMed] [Google Scholar]