Abstract
The EFSA Panel on Food Additives and Flavourings was requested to evaluate 39 flavouring substances assigned to the Flavouring Group Evaluation 71 (FGE.71), using the Procedure in Commission Regulation (EC) No 1565/2000. Nine substances have already been considered in FGE.71 [FL‐no: 08.054, 08.073, 08.123, 09.037, 09.156, 09.157, 05.158, 09.235, 09.239]. The remaining 30 substances [FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841] have been cleared with respect to genotoxicity in FGE.200Rev1 and they are considered in this revision. The substances were evaluated through a stepwise approach that integrates information on the structure–activity relationships, intake from current uses, toxicological threshold of concern (TTC), and available data on metabolism and toxicity. The Panel concluded that none of the 39 substances gives rise to safety concerns at their levels of dietary intake, estimated on the basis of the ‘Maximised Survey‐derived Daily Intake’ (MSDI) approach. Besides the safety assessment of the flavouring substances, the specifications for the materials of commerce have also been considered and found adequate, except for [FL‐no: 08.073 and 09.235]. For these two substances, data on the composition of the stereoisomeric mixture should be requested. Normal and maximum use levels should be provided for nine flavouring substances [FL‐no: 08.054, 08.073, 08.123, 09.037, 09.156, 09.157, 05.158, 09.235, 09.239]. For two flavouring substances [FL‐no: 02.020 and 05.076], the ‘modified Theoretical Added Maximum Daily Intake’ (mTAMDI) estimates are above the TTC for their structural class I. Therefore, additional information on uses and use levels should be provided for these eleven substances in order to finalise their evaluation.
Keywords: flavourings, αβ‐unsaturated carbonyls and precursors, FGE.71, JECFA
1. Introduction
The revision of this Flavouring Group Evaluation (FGE) concerns the inclusion of 30 α,β‐unsaturated carbonyl substances (or precursors thereof; [FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841]), which have been evaluated with respect to genotoxicity in FGE.200Rev1. According to the Mandate and Terms of Reference from this FGE, when for a flavouring substance the concern for genotoxicity is ruled out, the European Food Safety Authority (EFSA) proceeds to the full evaluation of these flavouring substances, taking into account the requirements of Commission Regulation (EC) No 1565/20001 and of Regulation (EU) No 1334/2008.2 The mandate and the Terms of Reference for FGE.200Rev1 are cited below.
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background to mandate from FGE.200Rev1 (M‐2018‐0041)
The use flavourings is regulated under Regulation (EC) No 1334/20082 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/2012.3 The list includes a number of flavouring substances for which the safety evaluation should be completed in accordance with Commission Regulation (EC) No 1565/2000.1
In February 2011, the EFSA Panel had evaluated a first dossier submitted by Industry in response to the requested data for representative substances in FGE. 200. These data were not considered adequate to alleviate the genotoxicity concern for the substance in subgroup 1.1.1 and the Panel recommended at that time ‘to perform in vivo dietary Comet assays (in drinking water or in feed, not by gavage) for the three linear representatives of subgroup 1.1.1 [FL‐no: 05.073, 05.058 and 05.060]’.
Additional data was submitted in February and June 2013 by Industry related to one representative substance of subgroup 1.1.1, hex‐2(trans)‐enal [FL‐no: 05.073] and two other substances of the group.
On 21 May 2014 the EFSA CEF Panel adopted an opinion on this Flavouring Group Evaluation 200 (FGE.200). The Panel confirmed the need for an in vivo Comet assay performed in duodenum and liver for hex‐2(trans)‐enal [FL‐no: 05.073]. For the two representative substances of subgroup 1.1.1 (nona‐2(trans), 6(cis)‐dienal [FL‐no: 05.058] and oct‐2‐enal [FL‐no: 05.060]), a combined in vivo Comet assay and micronucleus assay would be required and that evidence of bone marrow exposure should be provided.
New data concerning the three representative substances of this group addressing the EFSA opinion have been submitted during 2017. The data also included updated poundage and use levels concerning these substances.
The list of the substances referred to in this letter is included in Annex II.4
1.1.2. Terms of Reference of Mandate from FGE.200Rev1 (M‐2018‐0041)
The European Commission requests the European Food Safety Authority (EFSA) to evaluate the new information submitted and, depending on the outcome, proceed to full evaluation of the substances in this group in accordance with Commission Regulation (EC) No 1565/2000. In accordance with the usual practice by the CEF Panel,5 the first step (assessment of the genotoxicity) should be completed within nine months. An additional 9 months if necessary is also established for the second step (evaluation through the CEF Procedure).
In case the genotoxic potential cannot be ruled out or the procedure cannot be applied in the first step, EFSA is asked to quantify the exposure.
1.2. Interpretation of the Terms of Reference
Flavouring substances [FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841] were first allocated to FGE.200Rev1 for evaluation with respect to genotoxicity. Based on the new genotoxicity data submitted, the Panel concluded that these 30 flavouring substances do not give rise to concern with respect to genotoxicity and can accordingly be evaluated through the Procedure in the present revision 1 of FGE.71 (FGE.71Rev1), in accordance with Commission Regulation (EC) No 1565/2000.
The above‐mentioned flavouring substances belong to a group of structurally related substances which had been evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in the past (JECFA, 2005a, 2008a). Other substances in this group have already been considered by EFSA in FGE.71 (EFSA CEF Panel, 2010). For substances already evaluated by JECFA, a full evaluation is not required but EFSA should consider whether the JECFA evaluation can be agreed to or not. If not, EFSA should carry out a full evaluation of such substances (for further explanations see Appendix A).
In addition, since the publication of FGE.71, data on EU production volumes have been provided by industry for the following four flavouring substances [FL‐no: 08.073, 08.123, 09.157 and 09.239] and therefore their safety evaluation through the Procedure can also be finalised in the current revision.
1.2.1. History of the evaluation of the substances in FGE.71
The FGE.71 includes linear aliphatic α,β‐unsaturated aldehydes, acids and related alcohols, acetals and esters, which have been evaluated before by JECFA in a group of 37 substances at their 63rd meeting (JECFA, 2005a).
Twenty‐three substances are α,β‐unsaturated aldehydes, or precursors, thereof considered by the Panel to be of concern for genotoxicity. They have been considered, together with other α,β‐unsaturated aldehydes and precursors, in FGE.200 (EFSA CEF Panel, 2014) for which a final conclusion on genotoxicity could not be reached and additional data were requested. Five JECFA‐evaluated substances (JECFA numbers 1370, 1371, 1379, 1380 and 1382) were not in the Register6 and were not further considered in FGE.71. Therefore, FGE.71 only dealt with nine α,β‐unsaturated acids or esters ([FL‐no: 08.054, 09.239, 09.235, 09.158, 09.157, 09.156, 09.037, 08.123, 08.073]).
The EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) concluded that these nine flavouring substances are structurally related to the group of branched‐ and straight‐chain unsaturated carboxylic acids and esters of these and straight‐chain aliphatic saturated alcohols evaluated by EFSA in the Flavouring Group Evaluation 05, Revision 2 (FGE.05Rev2).
The CEF Panel agreed with the way the application of the Procedure has been performed by JECFA for all nine substances in the group of aliphatic α,β‐unsaturated acids and related esters. However, for five substances, the Panel had reservations (no European production volumes available for [FL‐no: 08.073, 08.123, 09.157 and 09.239] preventing them to be evaluated using the Procedure, and missing data on stereoisomerism for [FL‐no: 08.073 and 09.235]). For the remaining four substances [FL‐no: 08.054, 09.156, 09.158 and 09.037], the Panel agreed with the JECFA conclusion ‘No safety concern at estimated levels of intake as flavouring substances’, based on the ‘Maximised Survey‐derived Daily Intake’ (MSDI) approach.
For all nine substances evaluated through the Procedure, use levels were needed to calculate the ‘modified Theoretical Added Maximum Daily Intake’ (mTAMDI) estimates in order to identify those flavouring substances that need more refined exposure assessment and to finalise the evaluation.
From the substances considered in the present revision 1 of FGE.71 (FGE.71Rev.1), 23 flavouring substances [FL‐no: 02.112, 02.156, 02.210, 02.020, 02.050,02.090, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 09.276, 09.277, 09.394, 09.395, 09.396, 09.398, 09.399] were evaluated by JECFA at its 63rd meeting (JECFA, 2005a) and 10 of these substances [FL‐no: 02.050, 02.112, 02.156, 02.210, 09.276, 09.277, 09.395, 09.396, 09.398, 09.399] were re‐evaluated by JECFA at its 69th meeting (JECFA, 2008a). These 23 candidate substances were evaluated by EFSA in FGE.200Rev1 (EFSA FAF Panel, 2018), where it was concluded that for these substances a concern for genotoxicity could be ruled out. Therefore, they could be evaluated through the Procedure.
In addition, FGE.71Rev1 also deals with seven flavouring substances [FL‐no: 02.137, 05.179, 09.303, 09.385, 09.397, 09.678 and 09.841] evaluated by JECFA at its 69th meeting (JECFA 2008a). By expert judgement, they have been included in FGE.71Rev1 on the basis of their structural similarities with the substances considered in this group. These flavouring substances were also considered of no genotoxic concern in FGE.200Rev1 (EFSA FAF Panel, 2018). Therefore, they can be evaluated through the Procedure.
Together with the nine substances that were already considered in FGE.71, the current revision comprises 39 substances. The four flavouring substances, for which the evaluation was finalised in FGE.71, will not be further discussed. The missing EU production volumes and/or information on stereoisomeric composition for five flavouring substances [FL‐no: 08.073, 08.123, 09.157, 09.235 and 09.239], considered in the previous revision (FGE.71), have been provided by industry (Documentation provided to EFSA nr: 3). This information will be included and considered in this revision (FGE.71Rev1).
Nevertheless, for the sake of completion the information for all 39 substances is maintained in the various tables in this FGE.
| FGE | Adopted by EFSA | Link | No. of Substances |
|---|---|---|---|
| FGE.71 | 25 November 2009 | https://www.efsa.europa.eu/efsajournal/pub/1205 | 9 |
| FGE.71Rev1 | 14 November 2019 | https://www.efsa.europa.eu/efsajournal/pub/5924 | 39 |
FGE: Flavouring Group Evaluation.
2. Data and methodologies
2.1. Data
The present opinion is based on the data presented in the Table 1.
Table 1.
Data considered in the current revision 1 of FGE.71
| FL‐no | Chemical name | Data provided for the current revision 1 of FGE.71 | Appendix (Table nr) and relevant section of the opinion | Documentation provided to EFSA nr/Reference |
|---|---|---|---|---|
| 02.020 | Hex‐2‐en‐1‐ol |
Specifications, EU poundage data (MSDI), use levels (mTAMDI), ADME data |
Appendix C (Table C.1 and C.4) Sections 3.3.1. |
Documentation provided to EFSA nr: 1, 2, 5 |
| 02.050 | Pent‐2‐en‐1‐ol | |||
| 02.090 | Non‐2(trans)‐en‐1‐ol | |||
| 02.112 | Non‐2(cis)‐en‐1‐ol | |||
| 02.137 | Dec‐2‐en‐1‐ol | |||
| 02.156 | Hex‐2(cis)‐en‐1‐ol | |||
| 02.210 | Undec‐2‐en‐1‐ol | |||
| 05.037 | 2‐Dodecenal | |||
| 05.060 | Oct‐2‐enal | |||
| 05.070 | 2‐Heptenal | |||
| 05.073 | Hex‐2(trans)‐enal |
Specifications, EU poundage data (MSDI), use levels (mTAMDI), ADME, toxicity data |
Appendix C (Table C.1 and C.4) Sections 3.3.1. |
Documentation provided to EFSA nr: 1, 2, 5. Gaunt et al., 1971; Ping et al., 2003; Stout et al., 2008 |
| 05.076 | Dec‐2‐enal |
Specifications, EU poundage data (MSDI), use levels (mTAMDI), ADME data |
Appendix C (Table C.1 and C.4) Sections 3.3.1. |
Documentation provided to EFSA nr: 1, 2, 5 |
| 05.078 | Tridec‐2‐enal | |||
| 05.102 | Pent‐2‐enal | |||
| 05.109 | 2‐Undecenal | |||
| 05.150 | Hept‐2(trans)‐enal | |||
| 05.171 | Non‐2‐enal | |||
| 05.179 | Tetradec‐2‐enal | |||
| 09.276 | Oct‐2‐enyl acetate | |||
| 09.277 | Oct‐2(trans)‐enyl butyrate | |||
| 09.303 | Hept‐2‐enyl isovalerate | |||
| 09.385 | Hept‐2‐enyl acetate | |||
| 09.394 | E‐Hex‐2‐enyl acetate | |||
| 09.395 | E‐Hex‐2‐enyl propionate | |||
| 09.396 | Hex‐2‐enyl butyrate | |||
| 09.397 | Hex‐2‐enyl formate | |||
| 09.398 | Hex‐(2E)‐enyl hexanoate | |||
| 09.399 | (2E)‐Hexenyl isovalerate | |||
| 09.678 | Pent‐2‐enyl hexanoate | |||
| 09.841 | 2‐Hexenyl octanoate | |||
| 08.123 | trans‐2‐Heptenoic acid | EU poundage data (MSDI) | Appendix C (Table C.4) | Documentation provided to EFSA nr: 4 |
| 09.157 | Ethyl 2‐nonynoate | |||
| 09.239 | Methyl 2‐undecanoate | |||
| 08.073 | Dec‐2‐enoic acid |
Specifications EU poundage data (MSDI) |
Documentation provided to EFSA nr: 4 | |
| 09.235 | Butyl dec‐2‐enoate | Specifications | Appendix B (Table B.1) | Documentation provided to EFSA nr: 4 |
FL‐no: FLAVIS number; FLAVIS: Flavour Information System (database); MSDI: Maximised Survey‐derived Daily Intake; mTAMDI: modified Theoretical Added Maximum Daily Intake; ADME: absorption, distribution, metabolism, and excretion.
In addition, the following data have been used in FGE.71Rev1:
-
2013;
JECFA specifications for the 30 candidate flavouring substances [FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841] (JECFA, 2005b, 2008b).
-
2013;
63rd and 69th JECFA reports (JECFA, 2005a, 2008a) and 63rd JECFA toxicology monograph (JECFA, 2006).
-
2013;
Genotoxicity data evaluated in FGE.200 (EFSA CEF Panel, 2014) and FGE.200Rev1 (EFSA FAF Panel, 2018).
-
2013;
EFSA scientific opinion on FGE.71 (EFSA CEF Panel, 2010).
-
2013;
EFSA scientific opinion on FGE.05Rev3 (EFSA FAF Panel, 2019a).
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee. The assessment strategy applied for the evaluation programme of flavouring substances, as laid down in Commission Regulation (EC) No 1565/2000, is based on the Opinion on a Programme for the Evaluation of Flavouring substances of the Scientific Committee on Food (SCF, 1999).
2.2.1. Procedure for the safety evaluation of flavouring substances
The approach for safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/2000, named the ‘Procedure’, is described in Appendix A.
2.2.2. Approach used for the calculation of exposure
The approach used for calculation of the intake of the flavouring substances is described in Appendix A (see point ‘a) Intake’) and in Appendix C (Section C.2 ‘mTAMDI calculation’).
Table C.2.
Estimated amount of flavourable foods, beverages, and exceptions assumed to be consumed per person per day (SCF, 1995)
| Class of product category | Intake estimate (g/day) |
|---|---|
| Beverages (non‐alcoholic) | 324.0 |
| Foods | 133.4 |
| Exception a: Candy, confectionery | 27.0 |
| Exception b: Condiments, seasonings | 20.0 |
| Exception c: Alcoholic beverages | 20.0 |
| Exception d: Soups, savouries | 20.0 |
| Exception e: Others, e.g. chewing gum | e.g. 2.0 (chewing gum) |
SCF: Scientific Committee on Food.
3. Assessment
3.1. Specifications
JECFA status
The JECFA specifications are available for all 39 flavouring substances [FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 08.054, 08.123, 08.073, 09.239, 09.235, 09.158, 09.157, 09.156, 09.037, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841] considered in the present opinion (FGE.71Rev1) (JECFA, 2005b; JECFA, 2008b).
EFSA considerations
Table 2 shows the chemical structures of the candidate substances which are considered in this revision of FGE.71 (FGE.71Rev1).
Table 2.
Flavouring substances under evaluation in FGE.71Rev1
| [FL‐no] | UL chemical name | Structural formula | Structural classa |
|---|---|---|---|
| 02.020 | Hex‐2‐en‐1‐ol |

|
I |
| 02.050 | Pent‐2‐en‐1‐ol |

|
I |
| 02.090 | Non‐2(trans)‐en‐1‐ol |

|
I |
| 02.112 | Non‐2(cis)‐en‐1‐ol |
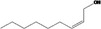
|
I |
| 02.137 | Dec‐2‐en‐1‐ol |

|
I |
| 02.156 | Hex‐2(cis)‐en‐1‐ol |
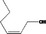
|
I |
| 02.210 | Undec‐2‐en‐1‐ol |
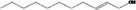
|
I |
| 05.037 | 2‐Dodecenal |
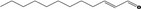
|
I |
| 05.060 | Oct‐2‐enal |
|
I |
| 05.070 | 2‐Heptenal |

|
I |
| 05.073 | Hex‐2(trans)‐enal |

|
I |
| 05.076 | Dec‐2‐enal |

|
I |
| 05.078 | Tridec‐2‐enal |

|
I |
| 05.102 | Pent‐2‐enal |

|
I |
| 05.109 | 2‐Undecenal |

|
I |
| 05.150 | Hept‐2(trans)‐enal |

|
I |
| 05.171 | Non‐2‐enal |

|
I |
| 05.179 | Tetradec‐2‐enal |

|
I |
| 09.276 | Oct‐2‐enyl acetate |
|
I |
| 09.277 | Oct‐2(trans)‐enyl butyrate |

|
I |
| 09.303 | Hept‐2‐enyl isovalerate |

|
I |
| 09.385 | Hept‐2‐enyl acetate |

|
I |
| 09.394 | E‐Hex‐2‐enyl acetate |
|
I |
| 09.395 | E‐Hex‐2‐enyl propionate |
|
I |
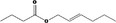
| 09.396 | Hex‐2‐enyl butyrate |
|
I |
| 09.397 | Hex‐2‐enyl formate |
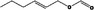
|
I |
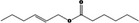
| 09.398 | Hex‐(2E)‐enyl hexanoate |
|
I |
| 09.399 | (2E)‐Hexenyl isovalerate |
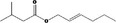
|
I |
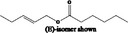
| 09.678 | Pent‐2‐enyl hexanoate |
|
I |
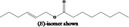
| 09.841 | 2‐Hexenyl octanoate |
|
I |
FL‐no: FLAVIS number; FLAVIS: Flavour Information System (database); FGE: Flavouring Group Evaluation.
According to OECD (Q)SAR Toolbox (version 4.3).
The 30 newly included flavouring substances (Table 2) can exist as geometrical stereoisomers due to the presence of a double bond (α,β unsaturation). For 13 flavouring substances ([FL‐no: 02.020, 02.050, 02.137, 02.210, 02.102, 05.102, 09.276, 09.303, 09.385, 09.396, 09.397, 09.678 and 09.841]), the chemical name in the Union list (UL) should be changed to reflect the stereochemistry (see ‘EFSA comments’ column in Table B.1 – Appendix B). Additionally, for four substances [FL‐no: 02.020, 02.050, 02.137, 02.210 and 05.102], the CAS number in the UL should be changed, as indicated in Table B.1 – Appendix B, according to the updated specifications provided by industry (Documentation provided to EFSA nr: 1).
Table B.1.
Summary table on specifications data for flavouring substances in FGE.71Rev1
| Information included in the EU Union list Regulation No. (EU) 1334/2008 as amended | Most recent available specifications dataa | EFSA Comments | |||||
|---|---|---|---|---|---|---|---|
| FL‐no JECFA‐no FEMA no CoE no CAS no | Chemical name | Purity of the named compound | Phys. form Mol. formula Mol. weight | Solubilityc Solubility in ethanold | Boiling point, °Ce Melting point, °C ID test Assay minimum (isomers distribution and SCh) | Refrac. Indexf Spec. gravityg | |
|
02.020 1354 2562 69 2305‐21‐7 |
Hex‐2‐en‐1‐ol | b |
Liquid C6H12O 100.16 |
Very slightly soluble Soluble |
158–160 – IR 95% (2E)‐isomer |
1.437–1.442 0.836–0.841 |
The chemical name should be changed to Hex‐(2E)‐en‐1‐ol and the CAS number to 928‐95‐0, according to the specifications provided (Documentation provided to EFSA nr: 1) |
|
02.050 1793 – 665 20273‐24‐9 |
Pent‐2‐en‐1‐ol | b |
Liquid C5H10O 86.13 |
Slightly soluble Soluble |
141 – MS 95% (2Z)‐isomer |
1.427–1.433 0.844–0.850 |
The chemical name should be changed to Pent‐(2Z)‐en‐1‐ol and the CAS number to 1576‐95‐0, according to the specifications provided (Documentation provided to EFSA nr: 1) |
|
02.090 1365 3379 10292 31502‐14‐4 |
Non‐2(trans)‐en‐1‐ol | b |
Liquid C9H18O 142.23 |
Insoluble Soluble |
105 (16 hPa) – IR 95% (2E)‐isomer |
1.444–1.448 0.835–0.845 |
|
|
02.112 1369 3720 10292 41453‐56‐9 |
Non‐2(cis)‐en‐1‐ol | b |
Liquid C9H18O 142.23 |
Slightly soluble Soluble |
96 (13 hPa) – NMR 96% (2Z)‐isomer |
1.447–1.453 0.841–0.847 |
|
|
02.137 1794 – 11750 22104‐80‐9 |
Dec‐2‐en‐1‐ol | b |
Liquid C10H20O 156.27 |
Slightly soluble Freely soluble |
117 (19 hPa) – MS 95% (2E)‐isomer |
1.446–1.452 0.842–0.848 |
The chemical name should be changed to Dec‐(2E)‐en‐1‐ol and CAS number to 18409‐18‐2, according to the specifications provided (Documentation provided to EFSA nr: 1) |
|
02.156 1374 3924 69 928‐94‐9 |
Hex‐2(cis)‐en‐1‐ol | At least 92%; secondary component 3‐4% hex‐2(trans)‐en‐1‐ol |
Liquid C6H12O 100.16 |
Insoluble Soluble |
65 (0.7 hPa) – NMR 95% (2Z)‐isomer (SC: 3–5% (2E)‐Hexen‐1‐ol) |
1.437–1.445 0.845–0.853 |
The purity requirement for the named compound [FL‐no: 02.156] and the percentage of the secondary component (2E)‐Hexen‐1‐ol should be updated in accordance with the specifications provided (Documentation provided to EFSA: 1) |
|
02.210 1384 4068 – 37617‐03‐1 |
Undec‐2‐en‐1‐ol | b |
Liquid C11H22O 170.30 |
Insoluble Soluble |
100–102 (3 hPa) – IR 92–95% (2E)‐isomer (3–4% (2Z)‐isomer) |
1.447–1.453 0.838–0.848 |
The chemical name should be changed to undec‐(2E)‐en‐1‐ol and the CAS number to 75039‐84‐8, according to the specifications provided (Documentation provided to EFSA nr: 1) |
|
05.037 1350 2402 124 4826‐62‐4 |
2‐Dodecenal | At least 93%; secondary component 3‐4% 2‐dodecenoic acid |
Liquid C12H22O 182.31 |
Insoluble Soluble |
272 – IR 93–95% (2E)‐isomer (2–3% (2Z)‐isomer and SC: 3–4% 2‐dodecenoic acid) |
1.452–1.458 0.839–0.849 |
According to the specifications provided (Documentation provided to EFSA nr: 1), this entry is synonymous with dodec‐2(trans)‐enal [FL‐no: 05.144], evaluated in FGE.05Rev3 |
|
05.060 1363 3215 663 2363‐89‐5 |
Oct‐2‐enal | At least 92%; secondary components 3‐4% 2‐octenoic acid and ethyl octanoate |
Liquid C8H14O 126.20 |
Slightly soluble Soluble |
84–86 (25 hPa) – IR 92–95% (2E)‐isomer (3–4% (2Z)‐isomer and SC: 3–4% 2‐octenoic acid, ethyl octanoate) |
1.449–1.455 0.835–0.845 |
According to the specifications provided (Documentation provided to EFSA nr: 1), this entry is synonymous with trans‐2‐octenal [FL‐no: 05.190], evaluated in FGE.05Rev3 |
|
05.070 1360 3165 730 2463‐63‐0 |
2‐Heptenal | b |
Liquid C7H12O 112.17 |
Practically insoluble or insoluble Freely soluble |
166 – IR MS 95% (2E)‐isomer |
1.428–1.434 0.857–0.863 |
According to the specifications provided (Documentation provided to EFSA nr: 1), this entry is synonymous with Hept‐2(trans)‐enal [FL‐no: 05.150], also evaluated in FGE.71Rev1 |
|
05.073 1353 2560 748 6728‐26‐3 |
Hex‐2(trans)‐enal | At least 92%; secondary component 3‐4% 2‐hexenoic acid |
Liquid C6 H10 O 98.14 |
Very slightly soluble Soluble |
47 (17 mm Hg) – NMR MS 95% (2E)‐isomer (SC: 3–4% 2‐hexenoic acid) |
1.443–1.449 0.841–0.848 |
The purity requirement for the named compound [FL‐no: 05.073] should be updated in accordance with the specifications provided (Documentation provided to EFSA: 1) |
|
05.076 1349 2366 2009 3913‐71‐1 |
Dec‐2‐enal | At least 92%; secondary components 3‐4% 2‐decenoic acid |
Liquid C10H18O 154.25 |
Insoluble Soluble |
229 – IR 92–95% (2E)‐isomer (3–4% (2Z)‐isomer and SC: 3–4% 2‐decenoic acid) |
1.452–1.458 0.836–0.846 |
According to the specifications provided, this entry is synonymous with trans‐2‐decenal [FL‐no: 05.191], evaluated in FGE.05Rev3 |
|
05.078 1359 3082 2011 7774‐82‐5 |
Tridec‐2‐enal | At least 92%; secondary components 3‐4% 2‐tridecenoic acid |
Liquid C13H24O 196.33 |
Insoluble Soluble |
115–118 (13 hPa) – IR 92–95% (2E)‐isomer (3–4% (2Z)‐isomer and SC: 3–4% 2‐tridecenoic acid) |
1.455–1.461 0.842–0.862 |
According to the specifications provided (Documentation provided to EFSA nr: 1), this entry is synonymous with trans‐2‐tridecenal [FL‐no: 05.195], evaluated in FGE.05Rev3 |
|
05.102 1364 3218 10375 764‐39‐6 |
Pent‐2‐enal | b |
Liquid C5H8O 84.11 |
Insoluble Soluble |
124 – NMR 95% (2E)‐isomer |
1.440–1.447 (21°) 0.850‐0.856 (21°) |
The chemical name should be changed to Pent‐(2E)‐enal and the CAS number to 1576‐87‐0, according to the specifications provided (Documentation provided to EFSA nr: 1) |
|
05.109 1366 3423 11827 2463‐77‐6 |
2‐Undecenal | b |
Liquid C11H20O 168.27 |
Insoluble Soluble |
115 (13 hPa) – NMR 94–95% (2E)‐isomer (1–2% (2Z)‐isomer) |
1.452–1.459 0.837–0.847 |
According to the specifications provided (Documentation provided to EFSA nr: 1), this entry is synonymous with undec‐2(trans)‐enal [FL‐no: 05.184], evaluated in FGE.05Rev3 |
|
05.150 1360 3165 730 18829‐55‐5 |
Hept‐2(trans)‐enal | b |
Liquid C7H12O 112.17 |
Insoluble Soluble |
165–167 – IR 95% (2E)‐isomer |
1.428–1.434 0.857–0.863 |
|
|
05.171 1362 3213 733 2463‐53‐8 |
Non‐2‐enal | At least 92%; secondary component 3‐4% 2‐nonenoic acid |
Liquid C9H16O 140.22 |
Insoluble Soluble |
88–90 (16 hPa) – IR 92–95% (2E)‐isomer (3–4% (2Z)‐isomer and SC: 3–4% 2‐nonenoic acid) |
1.454–1.460 0.855–0.865 |
According to the specifications provided (Documentation provided to EFSA nr: 1), this entry is synonymous with trans‐2‐nonenal [FL‐no: 05.072], evaluated in FGE.05Rev3 |
|
05.179 1803 4209 – 51534‐36‐2 |
e‐Tetradec‐2‐enal | b |
Solid C14H26O 210.36 |
Insoluble Soluble |
88 (0.3 hPa) 35 MS 95% (2E)‐isomer |
1.455–1.562 n.a |
|
|
08.054 1361 3169 11777 13419‐69‐7 |
Hex‐2(trans)‐enoic acid | b |
Solid C6H10O2 114.14 |
Slightly soluble Soluble |
n.a. 33–37 NMR 97% |
n.a. n.a. |
|
|
08.073 1372 3913 10087 3913‐85‐7 |
Dec‐2‐enoic acid | b |
Liquid C10H18O2 170.25 |
n.a. Soluble |
161–162 (20 hPa) – IR NMR MS 97% (sum of e and (Z) isomers) |
1.456–1.466 0.923–0.933 |
Mixture of (Z)‐ and (E)‐isomers (Documentation provided to EFSA nr. 4). Composition of stereoisomeric mixture to be specified |
|
08.123 1373 3920 – 10352‐88‐2 |
trans‐2‐Heptenoic acid | b |
Liquid C7H12O2 128.18 |
n.a. Soluble |
224–228 – IR NMR MS 97% |
1.447–1.157 0.968–0.978 |
|
|
09.037 1351 2418 245 140‐88‐5 |
Ethyl acrylate | b |
Liquid C5H8O2 100.12 |
Slightly soluble Soluble |
99–101 – IR 97% |
1.403–1.409 0.916–0.919 |
|
|
09.156 1356 2726 479 111‐80‐8 |
Methyl 2‐nonynoate | b |
Liquid C10H16O2 168.24 |
Insoluble Soluble |
121–122 (26 hPa) – NMR 97% |
1.445–1.451 0.913–0.916 |
|
|
09.157 1352 2448 480 10031‐92‐2 |
Ethyl 2‐nonynoate | b |
Liquid C11H18O2 182.26 |
Insoluble Soluble |
226–227 – NMR 96% |
1.450–1.456 0.901–0.907 |
|
|
09.158 1357 2729 481 111‐12‐6 |
Methyl 2‐octynoate | b |
Liquid C9H14O2 154.21 |
Insoluble Soluble |
215–217 – IR 95% |
1.443–1.449 0.919–0.924 |
|
|
09.235 1348 2194 2100 7492‐45‐7 |
Butyl dec‐2‐enoate | b |
Liquid C14H26O2 226.36 |
Insoluble Soluble |
119–120 (26 hPa) – NMR 98% (sum of (E) and (Z) isomers) |
1.444–1.451 0.877–0.883 |
Mixture of (Z)‐ and (E)‐isomers (Documentation provided to EFSA nr. 4). Composition of stereoisomeric mixture to be specified |
|
09.239 1358 2751 2111 10522‐18‐6 |
Methyl 2‐undecynoate | b |
Liquid C12H20O2 196.29 |
Insoluble Soluble |
230 – NMR 97% |
1.443–1.449 0.915–0.921 (20°) |
|
|
09.276 1367 3516 11906 3913‐80‐2 |
Oct‐2‐enyl acetate | b |
C10H18O2 170.25 |
Insoluble Soluble |
88–89 – IR NMR MS 95% (2E)‐isomer |
1.430–1.436 0.894–0.900 |
The chemical name should be changed to Oct‐(2E)‐enyl acetate, in accordance with the CAS number and the specifications provided (Documentation provided to EFSA nr: 1) |
|
09.277 1368 3517 11907 84642‐60‐4 |
Oct‐2(trans)‐enyl butyrate | b |
Liquid C12H22O2 198.30 |
Insoluble Soluble |
112–113 (10 hPa) – IR NMR MS 95% (2E)‐isomer |
1.433–1.439 0.890–0.896 |
|
|
09.303 1799 4126 10664 253596‐70‐2 |
Hept‐2‐enyl isovalerate | b |
Liquid C12H22O2 198.30 |
Insoluble Soluble |
262–263 – NMR 90–95% (2E)‐isomer (5–6% (2Z)‐isomer) |
1.443–1.449 0.868–0.873 |
The chemical name should be changed to Hept‐(2E)‐enyl isovalerate and the CAS number to 94109‐97‐4 (Documentation provided to EFSA nr: 1) |
|
09.385 1798 4125 10661 16939‐73‐4 |
Hept‐2‐enyl acetate | b |
Liquid C9H16O2 156.22 |
Practically insoluble to insoluble Freely soluble |
192–193 – MS 95% (2E)‐isomer |
1.428–1.434 0.889–0.895 |
The chemical name should be changed to Hept‐2(E)‐enyl acetate, in accordance with the CAS number and the specifications provided (Documentation provided to EFSA nr: 1) |
|
09.394 1355 2564 643 2497‐18‐9 |
E‐Hex‐2‐enyl acetate | At least 90%; secondary component 5‐6% (Z)‐2‐hexenyl acetate |
Liquid C8H14O2 142.20 |
Very slightly soluble Soluble |
165–166 – IR 95% (2E‐isomer) (5% (Z)‐2‐Hexenyl acetate) |
1.424–1.430 0.890–0.897 |
The purity requirement for the named compound [FL‐no: 09.394] should be updated in accordance with the specifications provided (Documentation provided to EFSA: 1) |
|
09.395 1378 3932 11830 53398‐80‐4 |
E‐Hex‐2‐enyl propionate | b |
Liquid C9H16O2 156.23 |
Insoluble Soluble |
91 (26 hPa) – NMR 95% (2E)‐isomer |
1.426–1.433 0.885–0.895 |
|
|
09.396 1375 3926 – 53398‐83‐7 |
Hex‐2‐enyl butyrate | b |
C10H16O2 170.25 |
Insoluble Soluble |
190 – NMR 95% (2E)‐isomer |
1.429–1.435 0.882–0.888 |
The chemical name should be changed to Hex‐(2E)‐enyl butyrate, in accordance with the CAS number and the specifications provided (Documentation provided to EFSA nr: 1) |
|
09.397 1376 3927 11858 53398‐78‐0 |
Hex‐2‐enyl formate | b |
C7H12O2 128.18 |
Insoluble Soluble |
75 – NMR 95% (2E)‐isomer |
1.420–1.424 0.915–0.925 |
The chemical name should be changed to Hex‐(2E)‐enyl formate, in accordance with the CAS number and the specifications provided (Documentation provided to EFSA nr: 1) |
|
09.398 1381 3983 – 53398‐86‐0 |
Hex‐(2E)‐enyl hexanoate | At least 93%; secondary components 2‐3% hexanoic acid and 2‐3% 2‐hexenol |
C12H22O2 198.31 |
Insoluble Soluble |
125 – IR 95% (2E)‐isomer (SC: 2–3% Hexanoic acid; 2–3% 2‐Hexenol) |
1.432–1.446 0.875–0.885 |
The purity requirement for the named compound [FL‐no: 09.398] should be updated in accordance with the specifications provided (Documentation provided to EFSA: 1) |
|
09.399 1377 3930 – 68698‐59‐9 |
(2E)‐Hexenyl isovalerate | b |
Liquid C11H20O2 184.28 |
Insoluble Soluble |
105 (26 hPa) – NMR 95% (2E)‐isomer |
1.425–1.435 0.875–0.885 |
|
|
09.678 1795 4191 – 74298‐89‐8 |
Pent‐2‐enyl hexanoate | b |
Liquid C11H20O2 184.28 |
Insoluble Soluble |
240–241 – MS 95% (2Z)‐isomer |
1.425–1.435 0.885–0.895 |
The chemical name should be changed to Pent‐(2Z)‐enyl hexanoate, in accordance with the CAS number and the specifications provided (Documentation provided to EFSA nr: 1) |
|
09.841 1796 4135 – 85554‐72‐9 |
2‐Hexenyl octanoate | b |
Liquid C14H26O2 226.36 |
Insoluble Soluble |
308–309 – MS 95% (2E)‐isomer |
1.448–1.453 0.881–0.887 |
The chemical name should be changed to (2E)‐Hexenyl octanoate, in accordance with the CAS number and the specifications provided (Documentation provided to EFSA nr: 1) |
FL‐no: FLAVIS number; FLAVIS: Flavour Information System (database); JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; ID: Identity; IR: infrared spectroscopy; MS: mass spectrometry; NMR: nuclear magnetic resonance.
At least 95% unless otherwise specified.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
SC: Secondary components
The purity requirements for flavouring substances [FL‐no: 02.156, 05.073, 09.394 and 09.398] should be updated according to the specifications provided by industry (Documentation provided to EFSA nr: 1).
According to the new specifications provided, the flavouring substances [FL‐no: 05.037, 05.060, 05.070, 05.076, 05.078, 05.109 and 05.171] are synonymous with [FL‐no: 05.144, 05.190, 05.150, 05.191, 05.195, 05.184 and 05.072] which have been evaluated in FGE.05Rev3 (EFSA FAF Panel, 2019a) and one substance ([FL‐no: 05.150]) in the current revision of this FGE (FGE.71Rev1).
Industry informed that two flavouring substances ([FL‐no: 08.073 and 09.235], for which EFSA requested in FGE.71 to clarify the stereochemistry, are mixtures of E and Z stereoisomers (Documentation provided to EFSA nr: 4). However, the Panel considered this information not adequate and requests quantitative figures of the stereoisomers in these mixtures (see ‘EFSA comments’ column in Table B.1 – Appendix B).
The Panel considered that the available specifications for the remaining flavouring substances are adequate.
The most recent specifications data for all 39 substances in FGE.71Rev1 are summarised in Table B.1 – Appendix B.
3.2. Estimation of intake
JECFA status
For 35 flavouring substances [FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 08.054, 09.235, 09.158, 09.156, 09.037, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841], evaluated through the JECFA Procedure, annual production data are available for the EU (JECFA, 2005a, 2008a).
For the remaining four flavouring substances [FL‐no: 08.123, 08.073, 09.157, 09.239], production figures are only available for the USA.
EFSA considerations
Updated EU production figures for 30 flavouring substances [FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841] have been submitted by industry (Documentation provided to EFSA nr: 2). Additionally, for four flavouring substances [FL‐no: 08.123, 08.073, 09.157, 09.239], considered in the previous revision of this FGE (FGE.71), EU production volumes have been provided (Documentation provided to EFSA nr: 4) and therefore the EU MSDI values can now be calculated. The MSDI values range from 0.012 to 2,800 μg/capita per day (see Table C.4 – Appendix C).
Table C.4.
Estimated intakes based on the MSDI approach and the mTAMDI approach for the substances in FGE.71Rev1
| FL‐no | EU Union List name | MSDI – EUa (μg/capita per day) | MSDI – USAb (μg/capita per day) | mTAMDIc (μg/person per day) | Structural class | Threshold of concern (μg/person per day) |
|---|---|---|---|---|---|---|
| 02.020 | Hex‐2‐en‐1‐ol | 650 | 291 | 5,900 | Class I | 1,800 |
| 02.050 | Pent‐2‐en‐1‐ol | 2.4 | ND | 1,700 | Class I | 1,800 |
| 02.090 | Non‐2(trans)‐en‐1‐ol | 0.016 | 0.03 | 1,700 | Class I | 1,800 |
| 02.112 | Non‐2(cis)‐en‐1‐ol | 0.012 | 2 | 1,700 | Class I | 1,800 |
| 02.137 | Dec‐2‐en‐1‐ol | 0.012 | ND | 1,700 | Class I | 1,800 |
| 02.156 | Hex‐2(cis)‐en‐1‐ol | 0.012 | 10 | 1,700 | Class I | 1,800 |
| 02.210 | Undec‐2‐en‐1‐ol | 0.012 | 1 | 1,700 | Class I | 1,800 |
| 05.037 | 2‐Dodecenal | 1.2 | 2 | 1,100 | Class I | 1,800 |
| 05.060 | Oct‐2‐enal | 0.84 | 0.9 | 900 | Class I | 1,800 |
| 05.070 | 2‐Heptenal | 8.2 | 30 | 510 | Class I | 1,800 |
| 05.073 | Hex‐2(trans)‐enal | 2800 | 409 | 1,400 | Class I | 1,800 |
| 05.076 | Dec‐2‐enal | 13 | 6 | 5,900 | Class I | 1,800 |
| 05.078 | Tridec‐2‐enal | 0.97 | 0.7 | 270 | Class I | 1,800 |
| 05.102 | Pent‐2‐enal | 0.37 | 0.1 | 1,500 | Class I | 1,800 |
| 05.109 | 2‐Undecenal | 0.65 | 0.4 | 1,700 | Class I | 1,800 |
| 05.150 | Hept‐2(trans)‐enal | 16 | 30 | 1,700 | Class I | 1,800 |
| 05.171 | Non‐2‐enal | 9.9 | 0.4 | 1,700 | Class I | 1,800 |
| 05.179 | Tetradec‐2‐enal | 0.012 | 0.07 | 1,700 | Class I | 1,800 |
| 08.054 | Hex‐2(trans)‐enoic acid | 16 | 36 | Class I | 1,800 | |
| 08.073 | Dec‐2‐enoic acid | 0.012 | 4 | Class I | 1,800 | |
| 08.123 | trans‐2‐Heptenoic acid | 4.7 | 4 | Class I | 1,800 | |
| 09.037 | Ethyl acrylate | 1.3 | 0.7 | Class I | 1,800 | |
| 09.156 | Methyl 2‐nonynoate | 1.9 | 21 | Class I | 1,800 | |
| 09.157 | Ethyl 2‐nonynoate | 1.1 | 0.9 | Class I | 1,800 | |
| 09.158 | Methyl 2‐octynoate | 18 | 38 | Class I | 1,800 | |
| 09.235 | Butyl dec‐2‐enoate | 0.01 | 0.3 | Class I | 1,800 | |
| 09.239 | Methyl 2‐undecynoate | 0.012 | 0.04 | Class I | 1,800 | |
| 09.276 | Oct‐2‐enyl acetate | 0.028 | 0.7 | 1,700 | Class I | 1,800 |
| 09.277 | Oct‐2(trans)‐enyl butyrate | 0.15 | 0.7 | 1,700 | Class I | 1,800 |
| 09.303 | Hept‐2‐enyl isovalerate | 0.012 | 5 | 1,700 | Class I | 1,800 |
| 09.385 | Hept‐2‐enyl acetate | 0.012 | 0.01 | 1,700 | Class I | 1,800 |
| 09.394 | E‐Hex‐2‐enyl acetate | 270 | 56 | 1,700 | Class I | 1,800 |
| 09.395 | E‐Hex‐2‐enyl propionate | 0.078 | 4 | 1,700 | Class I | 1,800 |
| 09.396 | Hex‐2‐enyl butyrate | 5.6 | 4 | 1,700 | Class I | 1,800 |
| 09.397 | Hex‐2‐enyl formate | 0.012 | 7 | 1,700 | Class I | 1,800 |
| 09.398 | Hex‐(2E)‐enyl hexanoate | 1.4 | 0.09 | 1,700 | Class I | 1,800 |
| 09.399 | (2E)‐Hexenyl isovalerate | 1.4 | 4 | 1,700 | Class I | 1,800 |
| 09.678 | Pent‐2‐enyl hexanoate | 0.012 | ND | 1,700 | Class I | 1,800 |
| 09.841 | 2‐Hexenyl octanoate | 0.012 | ND | 1,700 | Class I | 1,800 |
FL‐no: FLAVIS number; FLAVIS: Flavour Information System (database); FGE: Flavouring Group Evaluation; MSDI: Maximised Survey‐derived Daily Intake; mTAMDI: modified Theoretical Added Maximum Daily Intake; NOAEL: no observed adverse effect level.
Based on EU production figures by JECFA (2005a and 2008a) and submitted by industry (Documentation provided to EFSA nr: 2).
Based on use levels submitted by industry (Documentation provided to EFSA nr: 1).
For the 30 newly allocated flavouring substances [FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841], normal and maximum use levels have been submitted by industry (Documentation provided to EFSA nr: 1) and mTAMDI values can be calculated.
The mTAMDI intake estimates for 28 flavouring substances, for which use levels were provided (see above), were below the threshold of concern for their structural class (I). For two flavouring substances [FL‐no: 02.020 and 05.076], the mTAMDI intake estimates were above the threshold of concern for their structural class (I). For these two substances, more reliable data on use levels should be provided in order to refine the exposure assessment and to finalise their safety evaluation.
No normal and maximum use levels have been provided for the nine flavouring substances ([FL‐no: 08.054, 08.073, 08.123, 09.037, 09.156, 09.157, 05.158, 09.235 and 09.239]) previously considered in FGE.71.
The MSDI figures and mTAMDI intake estimates for the 39 flavouring substances in FGE.71Rev1 are shown in Table C.4 – Appendix C.
3.3. Biological and toxicological data
3.3.1. ADME data
According to JECFA (63rd and 69th meeting), all the 30 flavouring substances additionally considered in the present revision (FGE.71Rev1) are expected to be metabolised to innocuous products through normal fatty acid metabolism, including β‐oxidation and citric acid cycle, which finally leads to their total oxidation. In addition to the oxidative metabolism, also conjugation with glutathione (GSH) has been described. The relevant data are available in the 63rd and 69th JECFA toxicology monograph (JECFA, 2006, 2009) and in FGE.200Rev1 (EFSA FAF Panel, 2018). Based on this information, JECFA concluded that these flavouring substances, which are subject of this revision of FGE.71, can be evaluated along the A‐side of the Procedure (see Appendix A).
In addition, in the literature, two publications were found regarding a physiologically based in silico model for detoxification of the candidate substance trans‐2‐hexenal [FL‐no: 05.073] (Kiwamoto et al., 2012; Kiwamoto et al., 2013). A physiologically based in silico model for the rat was developed for trans‐2‐hexenal [FL‐no: 05.073] to examine the time‐ and dose‐dependent detoxification. The model was evaluated against in vivo data from the literature. A rapid detoxification, mainly by conjugation with GSH, was revealed at an exposure of 0.04 mg/kg bw, estimated to correspond to the daily human dietary intake for this substance. This estimate is in concordance with the MSDI and mTAMDI estimates for this substance (i.e. 2,800 and 1,400 μg/person per day or 0.05 and 0.02 mg/kg bw per day).
EFSA consideration
Based on the information provided by JECFA and taking into account the outcome of the evaluation of the genotoxicity which also includes potential DNA binding, as described in Section 3.3.2, the Panel agrees with JECFA and considers that these flavouring substances would be expected to be biotransformed into innocuous metabolites.
The data above mentioned are available in FGE.200Rev1 (EFSA FAF Panel, 2018).
3.3.2. Genotoxicity data
This revision involves the inclusion of 30 flavouring substances [FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841]), for which in FGE.19 a concern for genotoxicity had been identified based on the presence of a structural alert (i.e. α,β‐unsaturated carbonyl or precursor for that), preventing their evaluation through the Procedure (see also Appendix A). Because of this, these 30 flavouring substances needed further attention in FGE.200.
The genotoxicity, which also includes the potential DNA binding of these flavouring substances, has been assessed in FGE.200 (EFSA CEF Panel, 2014) and FGE.200Rev.1 (EFSA FAF Panel, 2018). Based on the genotoxicity data submitted, the Panel ruled out genotoxicity concerns for these flavouring substances.
Therefore, it is concluded that all 30 flavouring substances can be evaluated through the Procedure in the current revision 1 of FGE.71.
3.3.3. Toxicological data
3.3.3.1. Repeated dose toxicity studies
In the 63rd JECFA toxicology monograph (JECFA, 2006), detailed descriptions on short‐term toxicity studies with some of the flavouring substances belonging to FGE.71 ([FL‐no: 09.037 and 05.073] are available. In particular for flavouring substance [FL‐no: 05.073], under evaluation in this revision, a 13‐week toxicity study is available (Gaunt et al., 1971) from which a no observed adverse effect level (NOAEL) could be derived. A 28‐day study in rats following gavage administration of [FL‐no: 05.073] (Stout et al., 2008) was also reported in the 69th JECFA safety evaluation of flavouring agents (JECFA, 2008a). Additionally, for trans‐2‐hexenal [FL‐no: 05.073], a study related to cardiotoxicity in mice following trans‐2‐hexenal exposure was also considered (Ping et al., 2003).
A 14‐week NTP (National Toxicology Program) study in rats and mice on a structurally related substance (NTP, 2003), i.e. hexa‐2(trans),4(trans)‐dienal [05.057]) was evaluated in FGE.70Rev1 (EFSA FAF Panel, 2019b).
The toxicity studies on the candidate substance [FL‐no: 05.073] are shortly described below.
All the toxicological studies are summarised in Table E.1 – Appendix E.
Table E.1.
Acute, subacute, subchronic and chronic toxicity studies considered in FGE.71Rev1. The supporting substance is listed in brackets
| UL chemical name [FL‐no] | Species; Sex No./Group | Route | Dose levels (mg/kg bw perday) if not specified | Duration | NO(A)EL (mg/kg bw/day) | Reference | Comments |
|---|---|---|---|---|---|---|---|
| Hex‐2(trans)‐enal [FL‐no: 05.073] |
Rats; Male, Female 5/sex per group |
Stomach tube and intraperitoneal (i.p) | Not specified | 14 days |
LD50: 780 (males) and 1,130 (females) – by stomach tube. LD50: 200 (males) and 180 (females) – by i.p. |
Gaunt et al. (1971) | |
|
Mice; Male, Female 10/sex per group |
LD50: 1,750 (males) and 1,550 (females)‐ by stomach tube. LD50: 100 (males) and 160 (females) – by i.p. |
||||||
| Rats; female pairs | Diet | 0 (control diet) and 260, 640, 1600 or 4000 mg/kg feed | 8 days | – | Palatability test. No difference in consumption of the control diet and the lowest test concentration diet. The intake of feed decreased with increasing concentrations of [FL‐no: 05.073] in the feed | ||
|
Rats; Male, Female 15/sex per group |
Diet |
0, 18, 45, 110 and 257 in males 0, 21, 52, 131 and 304 in females |
13 weeks | 257 (males) and 304 (females) | NOAEL is highest dose tested | ||
| Rabbits; 10/female per group | Gavage | 0 and 200 | 13 weeks | 200 | Decreased haemoglobin concentration and increased absolute and relative stomach weight. These findings were associated with high ulceration and haemorrhage in the gastric mucose. The Panel considered the effects to be due to local irritant effects of the tested substance | ||
| Rats | Gavage | 0, 10, 30 and 100 | 4 weeks | – | Stout et al. (2008) | Forestomach hyperplasia was the predominant lesion and probably the cause of the observed decrease in body weight gain, observed at the highest dose | |
| Mice | Gavage | 0, 0.1, 1, 10 and 50 mg/kg bw per week | 4 weeks | – | Ping et al. (2003) | A non‐dose‐related effect on cardiac function; condensed nuclei in the heart, not reproduced in other studies at even higher doses | |
| (Hexa‐2(trans),4(trans)‐dienal [05.057]) | Rats; Male and Female, 10/sex per group | Gavage | 0, 7.5, 15, 30, 60 and 120 in corn oil 5 days per week | 14 weeks | 60 | NTP (2003) | Based on the magnitude of the observed effect (body weight changes) |
| Mice Male and Female, 10/sex per group | Gavage | 0, 7.5, 15, 30, 60 and 120 in corn oil 5 days per week | 120 | No effects on body weight. Minimal to moderate hyperplasia of forestomach in both rats and mice at 120 mg/kg bw per day, probably due to local irritant effect of test substance | |||
|
Rats; Male, Female 50/sex per group |
Gavage | 0 (controls), 22.5, 45, or 90 in corn oil 5 days per week | 2 years | Effects on forestomach – not applicable to the use of flavourings. | NTP (2003) | Increased incidence of hyperplasia, squamous cell papillomas and squamous cell carcinoma of the forestomach. The Panel considered the effects to be due to local irritant effects of the tested substance | |
|
Mice; Male, Female 50/sex per group |
Gavage | 0 (controls), 30, 60, or 120, in corn oil 5 days per week | 2 years | Effects on forestomach – not applicable to the use of flavourings. Squamous cell carcinoma of the tongue observed in two mice of the high dose group. | NTP (2003) | Increased incidence of hyperplasia, squamous cell papillomas and squamous cell carcinoma of the forestomach. The Panel considered the effects to be due to local irritant effects of the tested substance |
FL‐no: FLAVIS number; FLAVIS: Flavour Information System (database); FGE: Flavouring Group Evaluation; bw: body weight; NOAEL: no observed adverse effect level; LD50: lethal dose, median.
3.3.3.2. Acute and subacute toxicity studies on trans‐2‐hexenal [FL‐no: 05.073]
Trans‐2‐hexenal [FL‐no: 05.073] (95% minimum purity) was tested in mice (10 males and 10 females) and rats (5 males and 5 females) for acute toxicity after a single dose given both by intraperitoneal (i.p.) injection and by stomach tube. Surviving animals were observed for 14 days after administration of the single dose. LD50 for rats were 780 mg/kg (males) and 1,130 mg/kg (females) and for mice 1,750 mg/kg (males) and 1,550 mg/kg (females) when given by stomach tube. LD50 for rats were 200 mg/kg (males) and 180 mg/kg (females) and for mice 100 mg/kg (males) and 160 mg/kg (females) when administered intraperitoneally. Subsequently, a palatability test was performed over an 8‐day period where pairs of female rats were offered two diets simultaneously – a basal (control) diet and a diet spiked with either 260, 640, 1,600 or 4,000 mg trans‐2‐hexenal/kg feed. Feed consumption was decreased at 640 mg/kg feed and above (Gaunt et al., 1971).
Trans‐2‐hexenal (purity 98%) in corn oil was tested in rats at single gavage doses of 0, 50, 200 and 500 mg/kg bw (Stout et al., 2008). Decreased body weights and necroulcerative lesions with inflammation in the forestomach were reported at the two highest doses. At 50 mg/kg bw, the damage was minimal. Stout et al. (2008) also administered trans‐2‐hexenal to 4–5 rats per dose group by oral gavage for 5 days or 5 days per week during 4 weeks at doses of 0, 10, 30 and 100 mg/kg bw per day. Hyperplasia of the forestomach was the main effect and reported in increasing incidence and severity in animals administered 10, 30 and 100 mg/kg bw per day.
With respect to the acute and subacute toxicity studies, the Panel concluded that due to the gavage administration of an irritating substance, these toxicological studies are not suitable for risk assessment of flavouring substances under evaluation in this FGE, including derivation of a NOAEL.
Effects on heart muscle tissue and function were studied by Ping et al. (2003) after single weekly gavage administration of trans‐2‐hexenal to mice in doses of 0, 0.1, 1, 10 and 50 mg/kg bw per week for 4 weeks. The source and purity of the substance were not reported. According to the authors, the four gavage treatments with trans‐2‐hexenal induced some condensed nuclei in the heart and changes that were indicative of impaired left ventricular contractile function. However, the Panel noted the lack of dose response in the effects on cardiac function. In addition, no histopathological findings in heart tissue were found in subchronic feeding studies with higher doses of this substance in rats (see below). The Panel observed that the study design and the reporting were of poor quality. Therefore, the Panel considered this study not reliable.
3.3.3.3. Subchronic toxicity study on 2‐trans‐hexenal [FL‐no: 05.073]
Rats
In a study by Gaunt et al. (1971), groups of 15 male and 15 female rats (CFE strain), were fed diets containing 0 (control), 260, 640, 1,600 or 4,000 mg trans‐2‐hexenal/kg feed (corresponding to a mean intake of 0, 18, 45, 110 and 257 mg/kg bw per day for males and 0, 21, 52, 131 and 304 mg/kg bw per day for females as calculated from data on body weight and food consumption by the author) of trans‐2‐hexenal for 13 weeks. Body weights for individual animals and food consumption per cage were recorded weekly. Specific gravity and volume were determined in urine after water deprivation and after an oral water load. Collected urine sampled during a 6 hours period was analysed for protein, glucose, bile salts, ketones, blood, microscopic constituents and aspartate transaminase (AST) concentration.
Blood for haematology (haemoglobin concentrations, packed cell volumes, erythrocyte counts, reticulocytes, total leucocytes and various types of leucocytes) was collected at week 6 from eight males and eight females from groups fed 0, 1,600 and 4,000 mg/kg, and at autopsy at week 13 from all treated animals (serum analysis of urea content and alanine transaminase (ALT) and AST). At autopsy, gross lesions were recorded and brain, pituitary, thyroid, heart, liver, spleen, adrenal glands, kidneys and gonads were sampled and weighed. For control and high‐dose animals, samples of weighed organs as well as lymph nodes, thymus, urinary bladder, stomach, duodenum, ileum, colon, caecum, rectum, pancreas, uterus and skeletal muscle from control and highest treated groups were examined by histopathology.
No abnormalities in clinical observations were seen and no differences in feed consumption were reported between groups fed control or test diets except at the high dose, where the feed intake was statistically lower (p < 0.01) both for males and females.
In males, there was a slight but statistically significant decrease in haemoglobin concentration at six weeks (4,000 mg/kg diet) and at 13 weeks (1,600 mg/kg diet). There was also a statistically significant decrease in red blood cell counts in some dose groups in males, although not dose‐dependent. The Panel considered the haematological effects to be spurious and not treatment‐related.
The only parameter in the urine analysis which showed statistically significant results was a lower specific gravity in high‐dose males compared to controls under condition of dehydration. However, there were no indications of renal dysfunction based on other urinary function parameters or from histopathology of the kidney.
There were no dose‐related effects on organ weights or treatment‐related effects on histopathology, although statistically significant increased ovary weights were observed in all treated female rats (approximately 20–30%). However, no histological abnormalities were seen in the ovaries and the effects on ovary weights were not confirmed in a supplementary study in female rats fed 4,000 mg/kg of trans‐2‐hexenal in the diet. Neither there were any effects on the number of corpora lutea or on the oestrus cycle in the supplementary study.
Rabbits
In order to address the findings of increased ovary weights in the 13 weeks study, a limited study was set up in rabbits. Groups of ten female rabbits were given daily doses of 0 (control) or 200 mg/kg bw of trans‐2‐hexenal via oral intubation for 13 weeks. The animals were weighed weekly. At autopsy, blood was collected for haematology and the brain, heart, liver, spleen, kidneys, stomach, small intestine, ovaries, uterus, pituitary and adrenal glands were weighed. The same tissues as listed in the 13 weeks rat study were sampled and prepared for histopathology. A statistically significant decrease in haemoglobin concentration was observed in the rabbits administered trans‐2‐hexenal as well as an increase in absolute and relative stomach weight. The latter findings were associated with signs of ulceration and haemorrhage in the gastric mucosa of the dosed animals and were, according to the author, probably due to high local concentrations of the test compound resulting from oral intubation and the irritant nature of the test compound. The Panel agrees with the explanation. No changes in the weight or microscopic appearance of the ovaries, uterus or endocrine organs were observed compared to controls.
EFSA consideration
Overall, the Panel noted that no dose‐related adverse effects were revealed in the 90‐day rat toxicity study by Gaunt et al. (1971), where trans‐2‐hexenal [FL‐no: 05.073] was administered in the diet. With respect to the subchronic toxicity study by Gaunt, the Panel considered the NOAEL of this study to be the highest dose tested, corresponding to 257 mg/kg bw per day in males and 304 mg/kg bw per day in females, which is supported by the absence of systemic toxicity in a 13 weeks toxicity study in rabbits, given 200 trans‐2‐hexenal mg/kg bw per day by gavage.
3.4. Application of the procedure
Application of the Procedure to aliphatic, α,β‐unsaturated linear aldehydes, acids and related alcohols, acetals and esters by JECFA (JECFA, 2005a ; JECFA, 2008a )
In the respective meeting reports where the 30 additional flavouring substances included in this revision of FGE.71 are discussed ([FL‐no: 02.020, 02.050, 02.090, 02.112, 02.137, 02.156, 02.210, 05.037, 05.060, 05.070, 05.073, 05.076, 05.078, 05.102, 05.109, 05.150, 05.171, 05.179, 09.276, 09.277, 09.303, 09.385, 09.394, 09.395, 09.396, 09.397, 09.398, 09.399, 09.678 and 09.841]), JECFA allocated all these flavouring substances to structural class I using the decision tree approach presented by Cramer et al. (Cramer et al., 1978).
JECFA concluded for all 30 candidate flavouring substances that these can be anticipated to be metabolised to innocuous products (step 2) and the intakes (MSDIs) for all substances are below the threshold of concern for their structural class I (i.e. 1,800 μg/person per day) (step A3).
In conclusion, JECFA evaluated all the 30 candidate substances as to be of no safety concern at the estimated levels of intake as flavouring substances based on the MSDI approach.
The JECFA safety evaluations of the flavouring substances are summarised in Table D.1 – Appendix D.
Table D.1.
Summary of Safety Evaluations performed by JECFA (JECFA, 2005a, 2008a) and EFSA conclusions on flavouring substances in FGE.71 and FGE.71Rev1
| JECFA conclusions | EFSA conclusions | |||
|---|---|---|---|---|
| FL‐no JECFA‐no | EU Union List chemical name | Structural formula | Classa Evaluation procedure pathb Outcome on the named compound based on the MSDIc approach | Procedural path if different from JECFA, Conclusion based on the MSDId approach on the named compound and on the material of commerce |
|
02.020 1354 |
Hex‐2‐en‐1‐ol |
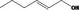
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to Hex‐(2E)‐en‐1‐ol and CAS number to 928‐95‐0. Concluded in FGE.71Rev1 |
|
02.050 1793 |
Pent‐2‐en‐1‐ol |
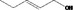
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to Pent‐(2Z)‐en‐1‐ol and CAS number to 1576‐95‐0. Concluded in FGE.71Rev1 |
|
02.090 1365 |
Non‐2(trans)‐en‐1‐ol |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71Rev1 |
|
02.112 1369 |
Non‐2(cis)‐en‐1‐ol |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71Rev1 |
|
02.137 1794 |
Dec‐2‐en‐1‐ol |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to Dec‐(2E)‐en‐1‐ol and CAS number to 18409‐18‐2. Concluded in FGE.71Rev1 |
|
02.156 1374 |
Hex‐2(cis)‐en‐1‐ol |
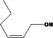
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The purity requirement for the named compound [FL‐no: 02.156] and the percentage of the secondary component (2E)‐hexen‐1‐ol should be updated (see Table B.1 – Appendix B). Concluded in FGE.71Rev1 |
|
02.210 1384 |
Undec‐2‐en‐1‐ol |
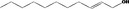
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to undec‐(2E)‐en‐1‐ol and CAS number to 75039‐84‐8 Concluded in FGE.71Rev1 |
|
05.037 1350 |
2‐Dodecenal |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Synonymous with dodec‐2(trans)‐enal [FL‐no: 05.144], evaluated in FGE.05Rev3. Concluded in FGE.71Rev1 |
|
05.060 1363 |
Oct‐2‐enal |
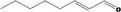
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Synonymous with trans‐2‐octenal [FL‐no: 05.190], evaluated in FGE.05Rev3 Concluded in FGE.71Rev1 |
|
05.070 1360 |
2‐Heptenal |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Synonymous with Hept‐2(trans)‐enal [FL‐no: 05.150], also evaluated in FGE.71Rev1 Concluded in FGE.71Rev1 |
|
05.073 1353 |
Hex‐2(trans)‐enal |

|
Class I A3: Intake below threshold No safety concern |
Class I A3: Intake above the threshold A4: The substance or its metabolites are not endogenous A5: Adequate NOAEL exists No safety concern The purity requirement for the named compound [FL‐no: 05.073] should be updated (see Table B.1 – Appendix B) Concluded in FGE.71Rev1 |
|
05.076 1349 |
Dec‐2‐enal |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Synonymous with trans‐2‐decenal [FL‐no: 05.191], evaluated in FGE.05Rev3 Concluded in FGE.71Rev1 |
|
05.078 1359 |
Tridec‐2‐enal |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Synonymous with trans‐2‐tridecenal [FL‐no: 05.195], evaluated in FGE.05Rev3 Concluded in FGE.71Rev1 |
|
05.102 1364 |
Pent‐2‐enal |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to Pent‐(2E)‐enal and CAS number to 1576‐87‐0 Concluded in FGE.71Rev1 |
|
05.109 1366 |
2‐Undecenal |
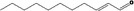
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Synonymous with undec‐2(trans)‐enal [FL‐no: 05.184], evaluated in FGE.05Rev3 Concluded in FGE.71Rev1 |
|
05.150 1360 |
Hept‐2(trans)‐enal |
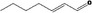
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71Rev1 |
|
05.171 1362 |
Non‐2‐enal |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Synonymous with trans‐2‐nonenal [FL‐no: 05.072], evaluated in FGE.05Rev3 Concluded in FGE.71Rev1 |
|
05.179 1803 |
(E)‐Tetradec‐2‐enal |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71Rev1 |
|
08.054 1361 |
Hex‐2(trans)‐enoic acid |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71 |
|
08.073 1372 |
Dec‐2‐enoic acid |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Currently not applicable to the material of commerce pending further information on stereochemistry (see ‘EFSA comments’ in Table B.1 in Appendix B) Concluded in FGE.71 |
|
08.123 1373 |
trans‐2‐Heptenoic acid |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71 |
|
09.037 1351 |
Ethyl acrylate |
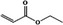
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71 |
|
09.156 1356 |
Methyl 2‐nonynoate |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71 |
|
09.157 1352 |
Ethyl 2‐nonynoate |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71 |
|
09.158 1357 |
Methyl 2‐octynoate |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71 |
|
09.235 1348 |
Butyl dec‐2‐enoate |
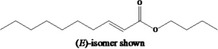
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Currently not applicable to the material of commerce pending further information on stereochemistry (see ‘EFSA comments’ in Table B.1 in Appendix B.1) Concluded in FGE.71 |
|
09.239 1358 |
Methyl 2‐undecynoate |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71 |
|
09.276 1367 |
Oct‐2‐enyl acetate |
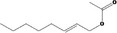
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to Oct‐(2E)‐enyl acetate Concluded in FGE.71Rev1 |
|
09.277 1368 |
Oct‐2(trans)‐enyl butyrate |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.71Rev1 |
|
09.303 1799 |
Hept‐2‐enyl isovalerate |
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to Hept‐(2E)‐enyl isovalerate and the CAS number to 94109‐97‐4 Concluded in FGE.71Rev1 |
|
09.385 1798 |
Hept‐2‐enyl acetate |
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to Hept‐(2E)‐enyl acetate Concluded in FGE.71Rev1 |
|
09.394 1355 |
E‐Hex‐2‐enyl acetate |
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The purity requirement for the named compound [FL‐no: 09.394] should be updated (see Table B.1 – Appendix B) Concluded in FGE.71Rev1 |
|
09.395 1378 |
E‐Hex‐2‐enyl propionate |
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71Rev1 |
|
09.396 1375 |
Hex‐2‐enyl butyrate |
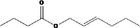
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to Hex‐(2E)‐enyl butyrate Concluded in FGE.71Rev1 |
|
09.397 1376 |
Hex‐2‐enyl formate |
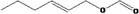
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to Hex‐(2E)‐enyl formate Concluded in FGE.71Rev1 |
|
09.398 1381 |
Hex‐(2E)‐enyl hexanoate |
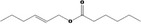
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The purity requirement for the named compound [FL‐no: 09.398] should be updated (see Table B.1 – Appendix B) Concluded in FGE.71Rev1 |
|
09.399 1377 |
(2E)‐Hexenyl isovalerate |
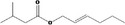
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake Concluded in FGE.71Rev1 |
|
09.678 1795 |
Pent‐2‐enyl hexanoate |
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to pent‐(2Z)‐enyl hexanoate Concluded in FGE.71Rev1 |
|
09.841 1796 |
2‐Hexenyl octanoate |
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake The chemical name should be changed to (2E)‐hexenyl octanoate Concluded in FGE.71Rev1 |
FL‐no: FLAVIS number; FLAVIS: Flavour Information System (database); FGE: Flavouring Group Evaluation; MSDI: Maximised Survey‐derived Daily Intake; mTAMDI: modified Theoretical Added Maximum Daily Intake.
Thresholds of concern: Class I = 1800 μg/person/day, Class II = 540 μg/person/day, Class III = 90 μg/person/day.
Procedure path A: substances can be predicted to be metabolised to innocuous products. Procedure path B: substances cannot.
EU MSDI: Amount added to food as flavour in (kg/year) × 109/(0.1 × population in Europe (= 375 × 106) × 0.6 × 365) = μg/capita/day.
Refer to Appendix C (Table C.4) for MSDI values considered by EFSA based on EU production figures submitted by industry (documentation provided to EFSA nr: 2 and 4).
EFSA considerations
The FAF Panel agrees with JECFA with respect to the allocation of the candidate flavouring substances to Cramer class I.
In line with JECFA, the Panel considers all the 30 newly included flavouring substances to be expected to be metabolised to innocuous products (step 2) and accordingly to evaluate these substances along the A‐side of the Procedure. The same conclusion was also reached for four flavouring substances [FL‐no: 08.073, 08.123, 09.157 and 09.239], considered in FGE.71 (EFSA CEF Panel, 2010), for which the assessment could not be finalised due to lacking information on exposure.
The estimated daily intake, based on MSDI approach, of all flavouring substances is below the threshold of concern for their structural class I (step A3), except for [FL‐no: 05.073]. The MSDI value, based on updated EU poundage data, for trans‐2‐hexenal [FL‐no: 05.073] is above the threshold of concern for structural class I (2,800 μg/person per day vs. 1800 μg/person per day).
The Panel considers that the available NOAEL for trans‐2‐hexenal [FL‐no: 05.073] (i.e. 257 mg/kg bw per day in male rats and 304 mg/kg bw per day in female rats, Gaunt et al., 1971) provides an adequate margin of safety (> 5,000).
Therefore, the Panel agrees with the evaluation for 29 flavouring substances as performed by JECFA, i.e. the substances are expected to be metabolised to innocuous products (step 2) and the estimated daily intake, based on MSDI approach, is below the threshold of concern for their structural class I (step A3). The Panel deviates from JECFA in the evaluation of flavouring substance [FL‐no: 05.073]. The Panel concludes [FL‐no: 05.073] at step A5 of the Procedure scheme, i.e. the substance is not endogenous (step A4) and a NOAEL for the candidate substance, which provides an adequate margin of safety under conditions of intended use, exists (step A5).
For the four flavouring substances [FL‐no: 08.073, 08.123, 09.157 and 09.239], already considered in FGE.71, EU production volumes became available after publication of the FGE.71 (EFSA CEF Panel, 2010). The MSDI exposure estimates for these four substances range from 0.012 to 4 μg/capita per day and they are all below the threshold of concern for their structural class I. At step A3 of the Procedure it can be concluded that these substances do not raise a safety concern under their intended conditions of use.
Overall in this revision 1 of FGE.71, the Panel evaluates all 30 additional candidate substances and four substances, for which the assessment could not be finished in FGE.71, as of no safety concern at the estimated levels of intake as flavouring substances based on MSDI approach.
The stepwise evaluations of the 34 substances are summarised in Table D.1 – Appendix D.
4. Discussion
This revision 1 of FGE.71 comprises in total 39 substances, 9 of which had already been considered in FGE.71. The additional 30 flavouring substances have been included in this revision, following an extensive evaluation in FGE.200Rev1 of their possible genotoxic potential due to a structural alert for genotoxicity (i.e. α,β unsaturated carbonyl compounds or precursors for that). Five of the previously considered substances in FGE.71 have been reconsidered because of additional information.
Based on absence of genotoxic potential in vivo, consideration of structural class, metabolism and toxicological data and the MSDI exposure estimates, the FAF Panel concludes that the flavouring substances considered in this revision of FGE.71 (FGE.71Rev1) do not raise a safety concern at step A3 and one substance (trans‐2‐hexenal [FL‐no: 05.073]) at step A5 of the Procedure scheme, for which an available NOAEL provides an adequate margin of safety.
For all 30 newly added flavouring substances considered in this FGE.71Rev1, normal and maximum use levels have been provided, from which mTAMDI exposure estimates have been calculated. For all these newly added substances, the mTAMDI values are below the threshold of concern for their structural class I, with the exception of two flavouring substances [FL‐no: 02.020 and 05.076] which have the mTAMDI values above the threshold of concern for their structural class (I). For these two substances, more detailed information on uses and use levels is necessary to refine the exposure assessment and to finalise the evaluation. For the previously considered (in FGE.71) nine substances [FL‐no: 08.054, 08.073, 08.123, 09.037, 09.156, 09.157, 05.158, 09.235 and 09.239], no normal or maximum use levels have been provided. For these nine substances, use levels are needed to calculate the mTAMDIs in order to identify those flavouring substances that need more refined exposure assessment and to finalise their evaluation.
In order to determine whether the conclusion for the 39 JECFA‐evaluated substances can be applied to the materials of commerce, it is necessary to consider the available specifications. Adequate specifications, including complete purity criteria and identity data, are available for 37 JECFA‐evaluated substances. For two substances [FL‐no: 08.073 and 09.235], the information on the composition of the stereoisomeric mixtures is incomplete. According to the new specifications provided, the flavouring substances [FL‐no: 05.037, 05.060, 05.070, 05.076, 05.078, 05.109 and 05.171] are synonymous with [FL‐no: 05.144, 05.190, 05.150, 05.191, 05.195, 05.184 and 05.072] which have been evaluated in FGE.05Rev3 and one substance ([FL‐no: 05.150]) in the current revision of FGE.71.
5. Conclusions
For 37 flavouring substances in FGE.71Rev1, the Panel agrees with JECFA conclusions ‘No safety concern at estimated levels of intake as flavouring substances’ based on the MSDI approach. For the remaining two flavouring substances [FL‐no: 08.073 and 09.235], the Panel has reservations as there is incomplete information on their chemical identity (composition of the stereoisomeric mixtures is lacking). For the previously considered (in FGE.71) nine substances [FL‐no: 08.054, 08.073, 08.123, 09.037, 09.156, 09.157, 05.158, 09.235 and 09.239], no normal or maximum use levels have been provided. For two flavouring substances [FL‐no: 02.020 and 05.076], the mTAMDI estimates are above the TTC for their structural class I. Therefore, additional information on uses and use levels should be provided for these eleven substances in order to finalise their evaluation.
6. Recommendations
The Panel recommends the European Commission to consider:
to request normal and maximum use levels for [FL‐no: 08.054, 08.073, 08.123, 09.037, 09.156, 09.157, 05.158, 09.235 and 09.239];
to request more reliable data on uses and use levels for [FL‐no: 02.020 and 05.076], as the mTAMDI exposure estimates are above the threshold of concern for their structural class I; When the above data are received, the assessment for these flavouring substances should be updated accordingly and expanded if necessary (i.e. request of additional toxicology data);
to change the chemical names in the Union List for 13 flavouring substances ([FL‐no: 02.020, 02.050, 02.137, 02.210, 02.102, 05.102, 09.276, 09.303, 09.385, 09.396, 09.397, 09.678 and 09.841]) to reflect the stereochemistry (see Table B.1 – Appendix B);
to change the CAS number in the Union List for five substances [FL‐no: 02.020, 02.050, 02.137, 02.210 and 05.102], as indicated in Table B.1 – Appendix B, according to the updated specifications provided;
to update the purity requirements for flavouring substances [FL‐no: 02.156, 05.073, 09.394 and 09.398] as indicated in Table B.1 – Appendix B, according to the updated specifications provided;
to delete seven flavouring substances from the Union List because [FL‐no: 05.037, 05.060, 05.070, 05.076, 05.078, 05.109 and 05.171] are synonymous with [FL‐no: 05.144, 05.190, 05.150, 05.191, 05.195, 05.184 and 05.072]. It is further recommended to request information from industry which of these synonymous substances should be deleted. For the substances that will remain in the Union List, adequate specifications and data on uses and normal and maximum use levels should be provided, because the available information on these substances is not fully consistent;
to request data on the composition of the stereoisomeric mixture for [FL‐no: 08.073 and 09.235].
Documentation provided to EFSA
EFFA (European Flavour Association), 2019. EFFA Submission of additional information on isomeric composition of substances within FGE.71Rev1 (FGE.19 Subgroup 1.1.1) and refined use levels.
EFFA (European Flavour Association), 2018a. EFFA 2015 poundage information for 74 substances from FGE.19 subgroup 1.1.1 corresponding to FGE.200. Unpublished data submitted from EFFA to EFSA. Dated August 2018.
EFFA (European Flavour Association), 2017a. Use levels survey for 84 substances from FGE.200. Unpublished data submitted from EFFA to EFSA. Dated 31/07/17.
EFFA (European Flavour Association), 2010. EFFA Letter to EFSA on EFSA questions on FGE.71 (The EFSA Journal (2010), 8(2):1401). Dated 19/04/2010.
EFFA (European Flavour Association), 2017. Submission by the European Flavour Association to the European Food Safety Authority. Flavouring Group Evaluation 19 Subgroup 1.1.1(corresponding to FGE.200): Addendum to Flavouring Group Evaluation 19 Subgroup 1.1.1: 74 Flavouring Substances (Flavouring Substances) of the Chemical Group 3 (Annex I of 1565/2000/EC) Structurally Related to Straight‐Chain Aliphatic Acyclic alpha,beta‐Unsaturated Aldehydes, with or without Non Conjugated Double Bonds, Used as Flavouring Substances. 14 August 2017.
EFFA (European Flavour Association), 2002. Letter from EFFA to Dr. Joern Gry, Danish Veterinary and Food Administration. Dated 31 October 2002. Re.: Second group of questions. FLAVIS/8.26.
Abbreviations
- ADME
absorption, distribution, metabolism, and excretion
- ALT
alanine transaminase
- AST
aspartate transaminase
- BW
body weight
- CAS
Chemical Abstract Service
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CoE
Council of Europe
- EFFA
European Flavour Association
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS (FL)
Flavour Information System (database)
- GSH
glutathione
- ID
identity
- i.p.
intraperitoneal
- IR
infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- LD50
lethal dose, median
- MS
mass spectrometry
- MSDI
maximised survey‐derived daily intake
- mTAMDI
modified Theoretical Added Maximum Daily Intake
- NMR
nuclear magnetic resonance
- No
number
- NOAEL
No observed adverse effect level
- (Q)SAR
(quantitative) structure–activity relationship
- SCF
Scientific Committee on Food
- TTC
threshold of toxicological of concern
- WHO
World Health Organization
Appendix A – Procedure of the safety evaluation
1.
The approach for a safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/2000, named the ‘Procedure’, is shown in schematic form in Figure A.1. The Procedure is based on the Opinion of the Scientific Committee on Food expressed on 2 December 1999 (SCF, 1999), which is derived from the evaluation Procedure developed by the Joint FAO/WHO Expert Committee on Food Additives at its 44th, 46th and 49th meetings (JECFA, 1995, 1996, 1997, 1999), hereafter named the ‘JECFA Procedure’.7
Figure A.1.
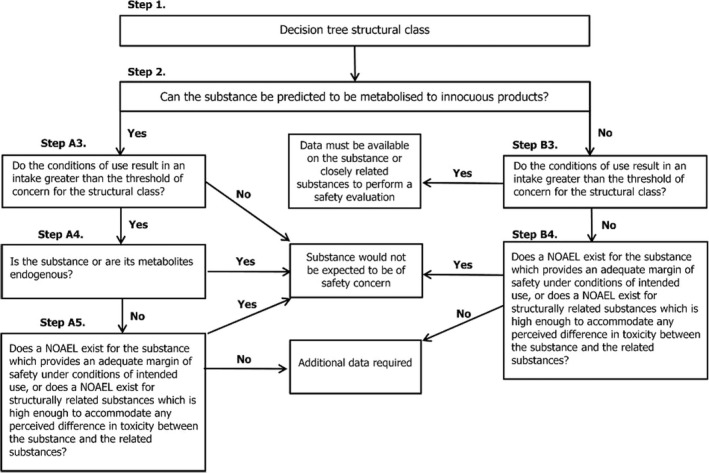
Procedure for the safety evaluation of chemically defined flavouring substances
The Procedure is a stepwise approach that integrates information on intake from current uses, structure‐activity relationships, metabolism and, when needed, toxicity. One of the key elements in the Procedure is the subdivision of flavourings into three structural classes (I, II and III) for which toxicological thresholds of concern (TTCs) (human exposure thresholds) have been specified. Exposures below these TTCs are not considered to present a safety concern.
Class I contains flavourings that have simple chemical structures and efficient modes of metabolism, which would suggest a low order of oral toxicity. Class II contains flavourings that have structural features that are less innocuous, but are not suggestive of toxicity. Class III comprises flavourings that have structural features that permit no strong initial presumption of safety, or may even suggest significant toxicity (Cramer et al., 1978). The TTCs for these structural classes of 1, 800, 540 or 90 μg/person per day, respectively, are derived from a large database containing data on subchronic and chronic animal studies (JECFA, 1996).
In step 1 of the Procedure, the flavourings are assigned to one of the structural classes. The further steps address the following questions:
Can the flavourings be predicted to be metabolised to innocuous8 products (step 2)?
Do their exposures exceed the TTC for the structural class (steps A3 and B3)?
Are the flavourings or their metabolites endogenous8 (step A4)?
Does a NOAEL exist on the flavourings or on structurally related substances (steps A5 and B4)?
In addition to the data provided for the flavouring substances to be evaluated (candidate substances), toxicological background information available for compounds structurally related to the candidate substances is considered (supporting substances), in order to assure that these data are consistent with the results obtained after application of the Procedure.
The Procedure is not to be applied to flavourings with existing unresolved problems of toxicity. Therefore, the right is reserved to use alternative approaches if data on specific flavourings warranted such actions (Figure A.1).
For the flavouring substances considered in this FGE, the EFSA Panel on Food additives and Flavourings compares the JECFA evaluation of structurally related substances with the result of a corresponding EFSA evaluation, focussing on specifications, intake estimations and toxicity data, especially genotoxicity data. The considerations by EFSA will conclude whether the flavouring substances are of no safety concern at their estimated levels of intake, whether additional data are required or whether certain substances should not be evaluated through the EFSA Procedure.
The following issues are of special importance:
-
a)
Intake
In its evaluation, the Panel as a default uses the ‘maximised survey‐derived daily intake’ (MSDI)9 approach to estimate the per capita intakes of the flavouring substances in Europe.
In its evaluation, the JECFA includes intake estimates based on the MSDI approach derived from both European and USA production figures. The highest of the two MSDI figures is used in the evaluation by the JECFA. It is noted that in several cases, only the MSDI figures from the USA were available, meaning that certain flavouring substances have been evaluated by the JECFA only on the basis of these figures. For substances in the Union List of flavouring substances10 for which this is the case, the Panel will need European Union (EU) production figures in order to finalise the evaluation.
When the Panel examined the information provided by the European Flavour Industry on the use levels in various foods, it appeared obvious that the MSDI approach in a number of cases would grossly underestimate the intake by regular consumers of products flavoured at the use level reported by the Industry, especially in those cases where the annual production values were reported to be small. In consequence, the Panel had reservations about the data on use and use levels provided and the intake estimates obtained by the MSDI approach. It is noted that the JECFA, at its 65th meeting, considered ‘how to improve the identification and assessment of flavouring agents, for which the MSDI estimates may be substantially lower than the dietary exposures that would be estimated from the anticipated average use levels in foods’ (JECFA, 2006).
In the absence of more accurate information that would enable the Panel to make a more realistic estimate of the intakes of the flavouring substances, the Panel has decided also to perform an estimate of the daily intakes per person using a modified Theoretical Added Maximum Daily Intake (mTAMDI) approach based on the normal use levels reported by Industry (EFSA, 2010).
As information on use levels for the flavouring substances has not been requested by the JECFA or has not otherwise been provided to the Panel, it is not possible to estimate the daily intakes using the mTAMDI approach for many of the substances evaluated by JECFA. The Panel will need information on use levels in order to finalise the evaluation.
-
b)
Threshold of 1.5 microgram/person over day (step B5) used by the JECFA
JECFA uses the threshold of concern of 1.5 μg/person per day as part of the evaluation procedure:
‘The Committee noted that this value was based on a risk analysis of known carcinogens which involved several conservative assumptions. The use of this value was supported by additional information on developmental toxicity, neurotoxicity and immunotoxicity. In the judgement of the Committee, flavouring substances for which insufficient data are available for them to be evaluated using earlier steps in the Procedure, but for which the intake would not exceed 1.5 microgram per person per day would not be expected to present a safety concern. The Committee recommended that the Procedure for the Safety Evaluation of Flavouring Agents used at the forty‐sixth meeting be amended to include the last step on the right‐hand side of the original procedure (“Do the condition of use result in an intake greater than 1.5 μg per day?”)’ (JECFA, 1999).
In line with the opinion expressed by the SCF (1999), the Panel does not make use of this threshold of 1.5 μg/person per day.
-
c)
Genotoxicity
As reflected in the opinion of the SCF (1999), the Panel has in its evaluation focussed on a possible genotoxic potential of the flavouring substances or of structurally related substances. Generally, substances for which the Panel has concluded that there is an indication of genotoxic potential in vitro, will not be evaluated using the EFSA Procedure until further genotoxicity data are provided. Substances for which a genotoxic potential in vivo has been concluded, will not be evaluated through the Procedure.
-
d)
Specifications
Regarding specifications, the evaluation by the Panel could lead to a different opinion than that of JECFA, since the Panel requests information on e.g. isomerism.
-
e)
Structural Relationship
In the consideration of the JECFA‐evaluated substances, the Panel will examine the structural relationship and metabolism features of the substances within the flavouring group and compare this with the corresponding Flavouring Group Evaluation (FGE).
Appendix B – Specifications
1.
Appendix C – Exposure estimates
1.
-
C.1
Normal and Maximum Use Levels
Table C.1.
Normal and maximum use levelsa (mg/kg) of JECFA‐evaluated flavouring substances in FGE.71Rev1 in food categories listed in Annex III of Reg. (EC) 1565/2000 (Documentation provided to EFSA nr: 1)
| FL‐no | Food Categories | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal use levels (mg/kg) Maximum use levels (mg/kg) | |||||||||||||||||||
| 01.0 | 02.0 | 03.0 | 04.1 | 04.2 | 05.0 | 05.3b | 06.0 | 07.0 | 08.0 | 09.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.1 | 14.2 | 15.0 | 16.0 | |
| 02.020 |
21.56 23.75 |
– – |
– – |
– – |
– – |
20.16 23.9 |
1.63 3.92 |
21.56 23.75 |
22.29 27.03 |
– – |
– – |
– – |
– – |
– – |
– – |
7.32 9.7 |
0 0 |
– – |
– – |
| 02.050 |
5.7 12 |
1.5 14.25 |
– – |
– |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 02.090 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 02.112 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 02.137 |
5.7 12 |
1.5 14.25 |
– – |
– |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 02.156 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 02.210 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 05.037 |
3.29 5.83 |
– – |
– – |
– – |
– – |
2.63 4.75 |
– – |
2.1 4.2 |
3.2 7.25 |
– – |
– – |
– – |
– – |
– – |
– – |
1.81 3.28 |
1 3 |
– – |
– – |
| 05.060 |
1.92 3.83 |
– – |
– – |
– – |
– – |
2.06 4.13 |
– – |
1.92 3.83 |
2.35 4.69 |
0.79 1.35 |
– – |
– – |
– – |
– – |
– – |
1.34 2.71 |
0 0 |
5 10 |
– – |
| 05.070 |
1.14 5.48 |
– – |
– – |
– – |
– – |
0.78 4.78 |
– – |
0.73 4.78 |
1.45 9.22 |
0.15 1.03 |
– – |
– – |
– – |
0.05 0.2 |
– – |
0.9 4.24 |
0 0 |
– – |
– – |
| 05.073 |
4.64 14.68 |
– – |
– – |
– – |
– – |
4.61 16.41 |
– – |
3.55 8.5 |
5 16.87 |
1 1 |
– – |
– – |
– – |
– – |
– – |
1.95 6.7 |
1 3 |
– – |
– – |
| 05.076 |
30 35.5 |
– – |
– – |
– – |
– – |
11.59 19.8 |
– – |
30 35.5 |
25.4 28.6 |
– – |
– – |
– – |
– – |
– – |
– – |
4.94 7.46 |
1 3 |
– – |
– – |
| 05.078 |
1.6 2.2 |
– – |
– – |
– – |
– – |
2 3.5 |
– – |
1.6 2.2 |
1.27 2.3 |
– – |
– – |
– – |
– – |
– – |
– – |
0.01 1 |
0 0 |
– – |
– – |
| 05.102 |
3.7 7.19 |
– – |
– – |
– – |
– – |
6.5 12.9 |
– – |
2.32 4.44 |
7.08 13.47 |
– – |
– – |
– – |
– – |
– – |
– – |
1.01 2.02 |
2 4 |
– – |
– – |
| 05.109 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 05.150 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 05.171 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 05.179 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.276 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.277 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.303 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.385 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.394 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.395 |
2 2 |
– – |
2 2 |
– – |
1.1 1.1 |
1.8 1.8 |
0.22 0.22 |
3.7 6.4 |
1.2 1.2 |
– – |
– – |
– – |
1 1 |
1.3 1.3 |
– – |
3.5 5.6 |
0 0 |
– – |
– – |
| 09.396 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.397 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.398 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.399 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.3 |
1 2 |
2.5 4.5 |
– – |
| 09.678 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
| 09.841 |
5.7 12 |
1.5 14.25 |
– – |
– – |
5 5.03 |
5.5 14.46 |
6.02 20.87 |
4.8 11.55 |
5 17 |
0.9 2.98 |
– – |
– – |
– – |
2 5 |
– – |
2 4.43 |
1 2 |
2.5 4.5 |
– – |
FL‐no: FLAVIS number; FLAVIS: Flavour Information System (database); JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FGE: Flavouring Group Evaluation.
‘Normal use’ is defined as the average of reported usages and ‘maximum use’ is defined as the 95th percentile of reported usages (Documentation provided to EFSA n. 6)
Additional food category 05.3 (chewing‐gum as per Annex II part D of Reg. (EC) 1333/2008) for which EFFA submitted use levels. These have been considered in the calculation of mTAMDI.
-
C.2
mTAMDI calculations
The method for calculation of modified Theoretical Added Maximum Daily Intake (mTAMDI) values is based on the approach used by the SCF up to 1995 (SCF, 1995). The assumption is that a person may consume the amount of flavourable foods and beverages listed in Table C.2. These consumption estimates are then multiplied by the reported use levels in the different food categories and summed up.
The mTAMDI calculations are based on the normal use levels reported by Industry. The seven food categories used in the SCF TAMDI approach (SCF, 1995) correspond to the 18 food categories as outlined in Commission Regulation (EC) No 1565/2000 and reported by the Flavour Industry in the following way (see Table C.3):
Table C.3.
Distribution of the 18 food categories listed in Commission Regulation (EC) No 1565/2000 into the seven SCF food categories used for mTAMDI calculation (SCF, 1995)
| Food categories according to Commission Regulation 1565/2000 | Distribution of the seven SCF food categories | |||
|---|---|---|---|---|
| Key | Food category | Foods | Beverages | Exceptions |
| 01.0 | Dairy products, excluding products of category 02.0 | Foods | ||
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) | Foods | ||
| 03.0 | Edible ices, including sherbet and sorbet | Foods | ||
| 04.1 | Processed fruit | Foods | ||
| 04.2 | Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds | Foods | ||
| 05.0 | Confectionery | Exception a | ||
| 06.0 | Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery | Foods | ||
| 07.0 | Bakery wares | Foods | ||
| 08.0 | Meat and meat products, including poultry and game | Foods | ||
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | Foods | ||
| 10.0 | Eggs and egg products | Foods | ||
| 11.0 | Sweeteners, including honey | Exception a | ||
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. | Exception d | ||
| 13.0 | Foodstuffs intended for particular nutritional uses | Foods | ||
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products | Beverages | ||
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts | Exception c | ||
| 15.0 | Ready‐to‐eat savouries | Exception b | ||
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) ‐ foods that could not be placed in categories 01.0 ‐ 15.0 | Foods | ||
SCF: Scientific Committee on Food; mTAMDI: modified Theoretical Added Maximum Daily Intake.
Beverages (SCF, 1995) correspond to food category 14.1
Foods (SCF, 1995) correspond to the food categories 1, 2, 3, 4.1, 4.2, 6, 7, 8, 9, 10, 13, and/or 16
Exception a (SCF, 1995) corresponds to food category 5 and 11
Exception b (SCF, 1995) corresponds to food category 15
Exception c (SCF, 1995) corresponds to food category 14.2
Exception d (SCF, 1995) corresponds to food category 12
Exception e (SCF, 1995) corresponds to others, e.g. chewing gum.
Appendix D – Summary of safety evaluations
1.
Appendix E – Repeated dose toxicity and carcinogenicity studies
1.
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Gürtler R, Husøy T, Moldeus P, Oskarsson A, Shah R, Waalkens‐Berendsen I, Wölfle D, Benigni R, Bolognesi C, Chipman K, Cordelli E, Degen G, Marzin D, Svendsen C, Martino C and Mennes W, 2020. Scientific Opinion on Flavouring Group Evaluation 71 Revision 1 (FGE.71Rev1): consideration of aliphatic, linear, α,β‐unsaturated alcohols, aldehydes, carboxylic acids, and related esters evaluated by JECFA (63rd and 69th meeting) structurally related to flavouring substances evaluated in FGE.05Rev3. EFSA Journal 2020;18(1):5924, 44 pp. 10.2903/j.efsa.2020.5924
Requestor: European Commission
Question numbers: EFSA‐Q‐2018‐00892, EFSA‐Q‐2018‐00891, EFSA‐Q‐2018‐00890, EFSA‐Q‐2018‐00889, EFSA‐Q‐2018‐00888, EFSA‐Q‐2018‐00887, EFSA‐Q‐2018‐00886, EFSA‐Q‐2018‐00885, EFSA‐Q‐2018‐00884, EFSA‐Q‐2018‐00883, EFSA‐Q‐2018‐00882, EFSA‐Q‐2018‐00881, EFSA‐Q‐2018‐00880, EFSA‐Q‐2018‐00879, EFSA‐Q‐2018‐00878, EFSA‐Q‐2018‐00877, EFSA‐Q‐2018‐00876, EFSA‐Q‐2018‐00875, EFSA‐Q‐2018‐00874, EFSA‐Q‐2018‐00873, EFSA‐Q‐2018‐00872, EFSA‐Q‐2018‐00871, EFSA‐Q‐2018‐00870, EFSA‐Q‐2018‐00869, EFSA‐Q‐2018‐00868, EFSA‐Q‐2018‐00867, EFSA‐Q‐2018‐00866, EFSA‐Q‐2018‐00865 and EFSA‐Q‐2018‐00864
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus, Agneta Oskarsson, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle and Maged Younes.
Acknowledgements: The Panel wishes to thank the hearing experts: Vibe Beltoft, Karin Nørby and EFSA staff Giorgia Vianello for the support provided to this scientific output.
Adopted: 14 November 2019
Notes
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Annex II refers here to the annex of the mandate letter from the EC to EFSA related to FGE.200Rev1.
CEF Panel was responsible for the evaluation of flavouring substances at the time when the Mandate was received by EFSA from European Commission.
Commission Decision 1999/217/EC.
The FAF Panel is aware that a revised Procedure for the Safety Evaluation of Flavouring agents has been agreed by JECFA (JECFA, 2016). The EFSA Scientific Committee has developed a modified procedure for evaluation of substances based on the TTC approach (EFSA Scientific Committee, 2019). However, these developments have no impact on the present evaluation, which should follow the requirements as set out in Commission Regulation (EC) No 1565/2000.
Innocuous products: products that are known or readily predicted to be harmless to humans at the estimated intake of the flavouring agent (JECFA, 1997).
Endogenous substances: intermediary metabolites normally present in human tissues and fluids, whether free or conjugated; hormones and other substances with biochemical or physiological regulatory functions are not included. The estimated intake of a flavouring agent that is, or is metabolized to, an endogenous substance should be judged not to give rise to perturbations outside the physiological range (JECFA, 1997).
EU MSDI: Amount added to food as flavour in (kg/year) x 109/(0.1 × population in Europe (= 375 × 106) × 0.6 × 365) = µg/capita per day.
Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
References
- Cramer GM, Ford RA and Hall RL, 1978. Estimation of toxic hazard ‐ a decision tree approach. Food and Cosmetics Toxicology, 16, 255–276. [DOI] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2010. Scientific Opinion on Flavouring Group Evaluation 71 (FGE.71): Consideration of aliphatic, linear, alpha,beta‐unsaturated carboxylic acids and related esters evaluated by JECFA (63rd meeting) structurally related to esters of branched‐ and straight‐chain unsaturated carboxylic acids. Esters of these and straight‐chain aliphatic saturated alcohols evaluated by in FGE.05Rev2 (2009). EFSA Journal 2010;8(2):1401, 35 pp. 10.2903/j.efsa.2010.1401 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2014. Scientific Opinion on Flavouring Group Evaluation 200 (FGE.200): 74 α,β‐unsaturated aldehydes and precursors from subgroup 1.1.1 of FGE.19. EFSA Journal 2014;12(6):3709, 57 pp. 10.2903/j.efsa.2014.3709 [DOI] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), 2018. Scientific Opinion on Flavouring Group Evaluation 200, Revision 1 (FGE.200 Rev1): 74 a,b‐unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.1 of FGE.19. EFSA Journal 2018;16(10):5422, 60 pp. 10.2903/j.efsa.2018.5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), 2019a. Scientific Opinion on Flavouring Group Evaluation 05, Revision 3 (FGE.05Rev3): Branched‐ and straight‐chain unsaturated aldehydes, dienals, unsaturated and saturated carboxylic acids and related esters with saturated and unsaturated aliphatic alcohols and a phenylacetic acid related ester from chemical groups 1, 2, 3, 5 and 15. EFSA Journal 2019;17(8):5761, 69 pp. 10.2903/j.efsa.2019.5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), 2019b. Scientific Opinion on Flavouring Group Evaluation 70, Revision 1 (FGE.70 Rev1): consideration of aliphatic, linear, a,b‐unsaturated, di‐ and trienals and related alcohols, acids and esters evaluated by JECFA (61st‐68th‐69th meeting). EFSA Journal 2019;17(7):5749, 41 pp. 10.2903/j.efsa.2019.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA SC (EFSA Scientific Committee), 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA SC (EFSA Scientific Committee), 2019. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA Journal 2019;17(6):5708, 17 pp. 10.2903/j.efsa.2019.5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt IF, Colley J, Wright M, Creasey M, Grasso P and Gangolli SD, 1971. Acute and short‐term toxicity studies on trans‐2‐hexenal. Food and Cosmetics Toxicology, 9, 775–786. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1995. Evaluation of certain food additives and contaminants. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 859. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996. Toxicological evaluation of certain food additives. The forty‐fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives and contaminants.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Evaluation of certain food additives and contaminants. Forty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 6‐15 February 1996. WHO Technical Report Series, no. 868. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999Evaluation of certain food additives and contaminants. Forty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Rome, 17‐26 June 1997. WHO Technical Report Series, no. 884. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2005a. Evaluation of certain food additives. Sixty‐third report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 928. Geneva, 8‐17 June 2004.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2005b. Compendium of food additive specifications. Addendum 12. Joint FAO/WHO Expert Committee of Food Additives 63rd session. Rome, 8‐17 June 2004. FAO Food and Nutrition paper 52 Add. 12.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Safety evaluation of certain food additives and contaminants. Sixty‐third Meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 54. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2008a. Evaluation of certain food additives, sixty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives, WHO technical report series; no. 952, 2008, Rome, Italy.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2008b. Compendium of food additive specifications. Joint FAO/WHO Expert Committee of Food Additives. 69th meeting 2008. Rome, 2008. FAO JECFA Monograph 5.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2009. Safety evaluation of certain food additives and contaminants. Sixty‐ninth Meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 60. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016. Evaluation of certain food additives, eighty‐second report of the Joint FAO/WHO Expert Committee on Food Additives, WHO technical report series; no. 1000, 2016, Geneva, Switzerland.
- Kiwamoto R, Rietjens MCM and Punt A, 2012. A physiologically based in silico model for trans‐2‐hexenal detoxification and DNA adduct formation in rat. Chemical Research in Toxicology, 25, 2630–2641. [DOI] [PubMed] [Google Scholar]
- Kiwamoto R, Spenkelink A, Rietjens IMCM and Punt A, 2013. A physiologically based in silico model for trans‑2‑hexenal detoxification and DNA adduct formation in human including interindividual variation indicates efficient detoxification and a negligible genotoxicity risk. Archives of Toxicology, 87, 1725–1737. 10.1007/s00204-013-1091-8 [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 2003. Toxicology and carcinogenesis studies of 2,4‐hexadienal in F344/N rats and B6C3F1 mice (gavage studies). NTP TRS 509, NIH Publication No. 04–4443. Available online: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr509.pdf
- Ping P, Baines CP, Gu Y, Prabhu SD, Zhang J, Tsai LL, Cardwell E, Zong NC, Vondriska TM, Korge P, Bhatnagar A and Wang GW, 2003. Cardiac Toxic Effects of Trans‐2‐Hexenal Are Mediated by Induction of Cardiomyocyte Apoptotic Pathways. Cardiovascular Toxicology, 3, 341–351. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food), 1995. Scientific Committee for Food. First annual report on chemically defined flavouring substances. May 1995, 2nd draft prepared by the SCF Working Group on Flavouring Substances (Submitted by the SCF Secretariat, 17 May 1995). CS/FLAV/FL/140‐Rev2. Annex 6 to Document III/5611/95, European Commission, Directorate‐General III, Industry.
- SCF (Scientific Committee on Food), 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). SCF/CS/FLAV/TASK/11 Final 6/12/1999. Annex I the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- Stout MD, Bodes E, Schoonhoven R, Upton PB, Travlos GS and Swenberg JA, 2008. Toxicity, DNA Binding, and Cell Proliferation in Male F344 Rats following Short‐term Gavage Exposures to Trans‐2‐Hexenal. Toxicologic Pathology, 36, 232–246. [DOI] [PubMed] [Google Scholar]


