Abstract
The conclusions of the EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, Norway, and co‐rapporteur Member State, the Netherlands, for the pesticide active substance benfluralin are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012. The conclusions were reached on the basis of the evaluation of the representative uses of benfluralin as a herbicide on chicory and lettuce. The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: benfluralin, peer review, risk assessment, pesticide, herbicide
Summary
Commission Implementing Regulation (EU) No 844/2012 (hereinafter referred to as ‘the Regulation’) lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012 as amended by Commission Implementing Regulation (EU) 2016/183. Benfluralin is one of the active substances listed in Regulation (EU) No 2016/183.
In accordance with Article 1 of the Regulation, the rapporteur Member State (RMS), Norway, and co‐rapporteur Member State (co‐RMS), the Netherlands, received an application from Gowan Crop Protection Limited and Finchimica S.p.A for the renewal of approval of the active substance benfluralin. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicants, the co‐RMS (the Netherlands), the European Commission and the European Food Safety Authority (EFSA) about the admissibility.
The RMS provided its initial evaluation of the dossier on benfluralin in the renewal assessment report (RAR), which was received by EFSA on 28 August 2017. In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicants, Gowan Crop Protection Limited and Finchimica S.p.A, for comments on 1 December 2017. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 5 February 2018.
Following consideration of the comments received on the RAR, it was concluded that additional information should be requested from the applicants, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
In accordance with Article 13(1) of the Regulation, EFSA should adopt a conclusion on whether benfluralin can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council.
The conclusions laid down in this report were reached on the basis of the evaluation of the representative uses of benfluralin as a herbicide on chicory and lettuce, as proposed by the applicants. Full details of the representative uses can be found in Appendix A of this report.
The uses of benfluralin according to the representative uses proposed at EU level result in a sufficient herbicidal efficacy against the target weeds.
In the area of identity, physical chemical properties and analytical methods data gaps were identified for an updated technical specification, for additional validation data concerning some data generation analytical methods, additional information to address the extraction efficiency in plant matrices and for a monitoring method of benfluralin metabolites in surface water.
Regarding the mammalian toxicology area, data gaps were identified for the assessment of the toxicological relevance of the individual impurities present in the technical specification and to establish a residue definition for body fluids and tissues relevant to human biomonitoring. Other data gaps led to issues not finalised, such as the lack of validated analytical methods for the developmental toxicity study in rabbits that questioned the reliability of this key study used to derive the acute reference dose (ARfD) and the acute acceptable operator exposure level (AAOEL), and the lack of identification and assessment of the toxicological relevance of two peaks in the in vitro interspecies comparative metabolism study that were significantly increased in human tissue compared to the other species tested. A critical area of concern was identified since the technical specification is not supported by the toxicological assessment, including the level of a genotoxic impurity that is high compared to the levels tested.
In the residue section, data gaps were identified for the elucidation of the metabolic pathway in primary and rotational crops; therefore, the proposed residue definition for risk assessment is provisional. Stability of residues in chicory roots during the storage was also not demonstrated as well as data on residue levels in pollen and honeybee products were not provided. The consumer risk assessment is provisional pending on the final decision on the residue definition for plants; the chronic consumer exposure intake accounted for max 0.1% of the acceptable daily intake (ADI) (ES, diet) for lettuce and the acute intake (international estimated short‐term intake (IESTI)) accounted for 0.1% of the ARfD for witloof (NL, diet).
The data available on environmental fate and behaviour are sufficient to carry out the required environmental exposure assessments at EU level for the representative uses. The potential for groundwater exposure above the parametric drinking water limit of 0.1 μg/L consequent to the representative uses was assessed as low for benfluralin and its soil metabolite B12 in geoclimatic situations represented by all the pertinent FOCUS groundwater scenarios.
The long‐term risk assessment for birds and mammals including the risk from secondary poisoning to earthworm‐eating birds and mammals is unresolved (critical area of concern). A high risk was identified for aquatic organisms (critical area of concern). Data gaps were identified for a second algae study and a study with a rooted macrophyte. A valid bioconcentration factor (BCF) study with fish is required for finalisation of the PBT assessment. A data gap was also identified for a chronic study with adult bees and honeybee larvae and to address all routes of exposure of honeybees.
From the available evidence, it could not be excluded that benfluralin might be considered a persistent (P) bioaccumulative (B) and toxic (T) or PBT substance. However, the assessment could not be finalised with regard to the B criterion.
Background
Commission Implementing Regulation (EU) No 844/20121 (hereinafter referred to as ‘the Regulation’) lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/20092. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of up to 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3).
In accordance with Article 1 of the Regulation, the RMS, Norway, and co‐RMS, the Netherlands, received an application from Gowan Crop Protection Limited and Finchimica S.p.A for the renewal of approval of the active substance benfluralin. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicants, the co‐RMS (the Netherlands), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on benfluralin in the RAR, which was received by EFSA on 28 August 2017 (Norway, 2017).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicants, Gowan Crop Protection Limited and Finchimica S.p.A, for consultation and comments on 1 December 2017. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 5 February 2018. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. In addition, the applicants were invited to respond to the comments received. The comments and the applicants’ response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicants in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA and the RMS on 22 March 2018. On the basis of the comments received, the applicants’ response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicants, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in October–November 2018.
In addition, a targeted written consultation with Member States took place in July–August 2019 subsequent to the completion of the peer review of the updated endocrine assessment conducted by EFSA in line with the new scientific criteria for the determination of endocrine disrupting properties, as laid down in Commission Regulation (EU) 2018/6053.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative uses of benfluralin as a herbicide on chicory and lettuce, as proposed by the applicants. A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
In addition, a key supporting document to this conclusion is the peer review report (EFSA, 2019a), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (23 March 2018)
the evaluation tables (26 November 2018 and 22 August 2019);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the EFSA addendum on endocrine assessment;
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Norway, 2018), as well as the peer review report and the EFSA addendum on endocrine assessment (EFSA, 2019b), all these documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
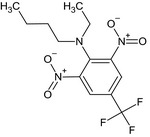
Benfluralin is the ISO common name for N‐butyl‐N‐ethyl‐α,α,α‐trifluoro‐2,6‐dinitro‐p‐toluidine (IUPAC).
The representative formulated product for the evaluation was ‘Bonalan (EF‐1533)’, an emulsifiable concentrate (EC) containing 180 g/L benfluralin.
The representative uses evaluated for ‘Bonalan (EF‐1533)’ as a herbicide were spray applications followed by mechanical incorporation in soil against annual weeds and seedlings of some perennial weeds in chicory and lettuce. Full details of the Good Agricultural Practices (GAPs) can be found in the list of end points in Appendix A.
Data were submitted to conclude that the uses of benfluralin according to the representative uses proposed at EU level result in a sufficient herbicidal efficacy against the target weeds following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: SANCO/3029/99‐rev. 4 (European Commission, 2000a), SANCO/3030/99‐rev. 4 (European Commission, 2000b), SANCO/825/00‐rev. 8.1 (European Commission, 2010) and SANCO/10597/2003‐rev. 10.1 (European Commission, 2012a).
The proposed minimum purity of the technical material is 960 g/kg. A new reference specification was proposed based on batch data from industrial scale production. The RMS and EFSA considered the specification proposed by the applicant inappropriate. The peer review suggested to remove the non‐significant impurities and to base the proposed specification on the batch data and the content of the impurities in the batches used for (eco)toxicological studies (data gap). Ethyl‐butyl‐nitrosamine (EBNA) was considered as a relevant impurity. The experts agreed that the technical specification should be revised and limited to a level of 0.085 mg/kg for the impurity EBNA (see Section 2). A FAO specification is not available.
The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity, physical, chemical and technical properties of benfluralin or the representative formulation. The main data regarding the identity of benfluralin and its physical and chemical properties are given in Appendix A.
Data gaps were identified for validation data for methods used for the generation of pre‐approval data required for the risk assessment in the areas of (eco)toxicology. Methods of analysis are available for the determination of the active substance in the technical material and the representative formulation. A HPLC‐MS/MS method is available for the determination of the relevant impurity in the technical material and Bonalan (EF‐1533) with a limit of quantification (LOQ) of 0.01 mg/kg.
The QuEChERS method based on HPLC‐MS/MS is applicable for the determination of benfluralin in food and feed of plant origin with an LOQ of 0.01 mg/kg in all commodity groups; however, additional information is required to address the extraction efficiency in plant matrices (data gap). There are no maximum residue levels (MRLs) proposed for the representative uses in animal commodities, as a consequence there is no need for monitoring method in the food and feed of animal origin (see Section 3). Liquid chromatography with tandem mass spectrometry (LC‐MS/MS) methods exist for the determination of benfluralin in soil and air with LOQs of 0.01 mg/kg and 0.15 μg/m3, respectively.
The residue definition for monitoring in drinking water was defined as benfluralin, while in surface water as benfluralin and metabolites propyl‐benzimidazole (371R) and methyl‐benzimidazole (372R). An appropriate GC‐MS method exists for monitoring benfluralin in surface, ground and drinking water with an LOQ of 0.05 μg/L; however, a data gap was identified for a monitoring method for the other compounds of the residue definition in surface water.
An LC‐MS/MS enforcement method exists for the determination of benfluralin residues in body fluids and tissues with LOQs of 0.05 mg/L and 0.1 mg/kg in urine and muscle, respectively. However, the residue definition for monitoring in body fluids and tissues is still open (see Section 2); as a consequence, a data gap might be identified for a corresponding method of analysis.
2. Mammalian toxicity
The following guidance documents were followed in the production of this conclusion: SANCO/221/2000‐rev. 10‐final (European Commission, 2003), SANCO/10597/2003‐rev. 10.1 (European Commission, 2012a), Guidance on dermal absorption (EFSA PPR Panel, 2012), Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products (EFSA, 2014b) and Guidance on the Application of the CLP Criteria (ECHA, 2017a).
Benfluralin was discussed at the Pesticides Peer Review Expert's Meeting 182 in September 2018 and at the Pesticide Peer Review Meeting 05 (joint Mammalian toxicology–Ecotoxicology meeting) in May 2019.
The technical specifications, either the current or the newly proposed technical specification, are not supported by the toxicological assessment; in addition, considering the impurity of known toxicological concern (EBNA) that has been tested up to 0.085 mg/kg in genotoxicity studies, this maximum limit should apply for this impurity in the technical specification (both issues triggering a critical area of concern). The toxicological relevance of other impurities has to be clarified; it was not possible to conclude on the genotoxic potential of other impurities present in the technical specification based on the predictions provided by the quantitative structure–activity relationship (QSAR) analysis (data gap). The analytical methods used in the toxicological studies were considered fit‐for‐purpose for some studies, such as the 2‐year and two‐generation reproductive toxicity studies in rat that were used to derive the acceptable daily intake (ADI) and the acceptable operator exposure level (AOEL), but not for the short‐term toxicity studies in rats and dogs, for the carcinogenicity study in mice, and for the developmental toxicity studies in rats and rabbits (data gap). This was considered an issue that could not be finalised since the developmental toxicity study in rabbit was used to derive the acute reference dose (ARfD) and the acute acceptable operator exposure level (AAOEL).
The oral absorption of benfluralin is limited (around 20% oral absorption was used to derive the AOEL and AAOEL). The active substance is widely distributed, showing some affinity to fat, extensively metabolised and rapidly eliminated, mainly via faeces. Considering the high number of metabolites retrieved in urine, a data gap has been established for the applicant to propose a residue definition for body fluids and tissues (blood and urine) relevant to human biomonitoring of the substance. Another data gap was identified since the majority of the experts considered that Peak 3 and Peak 7 of the in vitro metabolism study should be characterised and their toxicological relevance assessed because they were significantly higher in human samples than in the other four species tested (rat, mouse, dog and rabbit). Therefore, the need for further tests and risk assessment to unique human metabolites could not be finalised.
In the acute toxicity studies, benfluralin presented a low acute toxicity profile when administered by the oral, dermal or inhalation routes. Considering the hepatic and pulmonary congestion observed in two males and one female which died during exposure to 2.16 mg benfluralin/L air in the acute toxicity study by inhalation, it was noted that benfluralin may meet the criteria for STOT‐SE 2 classification4; currently, there is no harmonised classification for benfluralin according to the CLP Regulation.5 The active substance was found to be irritating to skin and eyes (in accordance with the classification criteria for Skin Irrit. 2, H315 ‘Causes skin irritation’ and Eye Irrit. 2, H319 ‘Causes serious eye irritation’); additionally, the substance presented skin sensitisation potential that may meet the classification criteria for Skin Sens 1, H317 ‘May cause an allergic skin reaction’. No phototoxic potential is attributed to benfluralin.
Following short‐ and long‐term administration in rat, dog and mouse, benfluralin showed target organ toxicity in the liver. In rats, red blood cells and kidneys were affected upon short‐term exposure as well as the thyroid upon long‐term exposure. Red blood cells were also affected in dogs. Liver and thyroid tumours were observed in rats and liver carcinomas in mice in the long‐term toxicity and carcinogenicity studies. The human non‐relevance of these tumours was not clearly demonstrated and therefore classification criteria may be met as Carc. 2 H351 ‘Suspected of causing cancer’, as suggested in the previous peer review (EFSA, 2008b). The relevant short‐term no‐observed adverse effect level (NOAEL) is 17 mg/kg body weight (bw) per day from the 90‐day study in rats and the relevant long‐term NOAEL is 0.5 mg/kg bw per day, from the 2‐year rat study. Based on genotoxicity studies containing a maximum level of 0.085 mg/kg of the genotoxic impurity EBNA, benfluralin was concluded not to possess genotoxic potential; however, it should be noted that at the level currently specified in the technical material as proposed by the applicant (0.3 mg/kg) and at the level specified in the current reference specification (0.1 mg/kg), a genotoxic potential cannot be excluded. Since no phototoxic potential was attributed to the substance, it is also considered unlikely to be photogenotoxic.
In a two‐generation reproductive toxicity study in rats, reduced body weight and increased pup mortality were observed at parental toxic doses (reduced body weight gain, increased liver and kidney weight associated with histopathological changes) with a parental and offspring's NOAEL of 5.5 mg/kg bw per day. In the developmental toxicity studies in rats and rabbits, developmental variations were seen at maternal toxic doses (early reduction of body weight gain) for which the critical NOAEL was 50 mg/kg bw per day based on maternal toxicity in the rabbit developmental toxicity study. There was no evidence for neurotoxicity or immunotoxicity potential in the overall data package.
Regarding the assessment of the endocrine disrupting (ED) potential of benfluralin according to the ECHA/EFSA guidance (2018), the T‐modality was sufficiently investigated and no adversity was observed. Therefore, based on the available evidence, benfluralin does not meet the ED criteria for the T‐modality. Regarding the oestrogen, androgen and steroidogenesis (EAS) modalities, the data set for adversity was not sufficiently investigated, and based on the available evidence, no EAS mediated adversity was observed. Regarding endocrine activity, the data set was sufficiently investigated and no effects were observed. Therefore, based on the available evidence, benfluralin does not meet the ED criteria for the EAS‐modalities. According to point 3.6.5 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605, it can be concluded that, for human health, benfluralin is not an endocrine disruptor based on the weight of available evidence.6
The potential groundwater metabolite 2,6‐dinitro‐4‐(trifluoromethyl)phenol (B12) was tested for gene mutation in bacteria and mammalian cells and presented negative results; chromosome aberrations and aneugenic potential were not investigated. For the representative uses, a groundwater assessment was not triggered for this metabolite (see Section 4). It is, however, noted that for other uses (than the representative uses), pending on the levels of metabolite B12 expected to occur in groundwater according to environmental fate and behaviour models, additional testing, including an assessment of its carcinogenic potential and genotoxicity testing (chromosome aberration and aneugenicity) may be required.
The ADI of benfluralin is 0.005 mg/kg bw per day7 based on the NOAEL of 0.5 mg/kg bw per day for liver toxicity in the 2‐year rat study, applying an uncertainty factor (UF) of 100. The AOEL is 0.011 mg/kg bw per day8 based on the offspring NOAEL of 5.5 mg/kg bw per day for reduced body weight and increased pup mortality from the two‐generation reproductive toxicity study in rat, applying an UF of 100 and correction for the limited oral absorption of 20% (overall factor of 500). The ARfD is 0.5 mg/kg bw9 based on the maternal NOAEL of 50 mg/kg bw per day for the early reduction in body weight gain in the developmental toxicity study in rabbit, UF of 100 applied. The AAOEL is 0.1 mg/kg bw on the same basis as the ARfD and correcting for the oral absorption of 20% (overall factor of 500).
Regarding the representative formulation, Bonalan (EF‐1533), an emulsifiable concentrate formulation containing 180 g/L benfluralin, dermal absorption was established at 2% for the concentrated formulation and 11% for a spray dilution of 2.7 g/L based on the triple pack approach (rat in vivo and comparative in vitro dermal absorption study on human and rat skin). For the representative uses of pre‐sowing chicory and lettuce or pre‐planting lettuce applications followed by mechanical incorporation into soil, operators have to use personal protective equipment (PPE), such as workwear, gloves during mixing, loading and application and respiratory protective equipment (RPE) to ensure that the AOEL and AAOEL are not exceeded according to the EFSA calculator (EFSA, 2014b). Workers are not expected to re‐enter treated fields. The estimated bystander exposure does not exceed the AAOEL according to the EFSA calculator. According to the EFSA calculator estimates provided in the revised RAR, the AOEL is exceeded for both adults and children residents; this exceedance is mainly driven by re‐entry into treated fields. Residential exposure during re‐entry is not considered relevant for the type of application on bare soils. Accordingly, it is concluded that resident adults’ exposure does not exceed the AOEL. Regarding children residents, exceedance is also driven by spray drift exposure. It is noted that mitigation measures may be applied in addition to the ones proposed by the RMS in the revised RAR, such as restricting applications to those using drift reducing technology and requiring minimum spray volume of 400 L/ha (while the GAP refers 200–400 L/ha).10 On that basis, the resident child exposure via all relevant pathways is less than 50% of the AOEL.
3. Residues
The assessment in the residue section is based on the OECD guidance document on overview of residue chemistry studies (OECD, 2009), the OECD publication on MRL calculations (OECD, 2011), the European Commission guideline document on MRL setting (European Commission, 2016) and the Joint Meeting on Pesticide Residues (JMPR) recommendations on livestock burden calculations (JMPR, 2004, 2007).
Benfluralin was discussed at the Pesticides Peer Review Experts’ Meeting 184 in September 2018.
The metabolism of benfluralin in primary crops was investigated in leafy crops (lettuces) and pulses and oilseeds (alfalfa, peanuts) following soil application and in cereals (wheat) following foliar application (BBCH 20–21 or 29). The dose rate for soil application was ca. 3N rate when compared to the representative use on lettuce, and 1N rate via foliar application when compared to the authorised use on cereals. Benfluralin was identified in lettuces at a low level (1.3% total radioactive residues (TRRs)); the major part of the radioactive residues was recovered as organo‐soluble and aqueous fractions (46% and 16% TRRs respectively), whilst up to 47% TRR remained unextracted of which 17.7% TRR was found to be incorporated into the natural constituents of the plants. A similar pattern was reported for alfalfa and peanuts with the extracted fractions consisting of numerous unidentified metabolites, individually accounting for less than 3% TRRs. However, during the expert meeting, it was noted that a metabolism study with benfluralin in peanuts and alfalfa, with higher rate of metabolites identification, is available in the public domain.11 Therefore, and to support a more complete elaboration of the metabolic pathway in plants, the experts agreed that this metabolism study on peanuts and alfalfa indicating the presence of potentially relevant metabolites should be assessed, i.e. analysis of the proposed structures of the identified metabolites, their toxicological relevance and whether the study can address the metabolism of benfluralin in the pulses and oilseeds crop group (data gap).
In the wheat metabolism study following foliar application, benfluralin was found only in wheat forage (57% TRRs) and hay (2% TRRs), while in the extracted residue radioactivity of straw and grains (up to 57% TRRs and 82% TRRs, respectively), no further metabolites’ identification occurred. A metabolism study in root crops to support the use in chicory roots was not provided. Therefore, and pending upon the outcome of the identified data gap for the detailed assessment of the pulses and oilseeds metabolism study from the public domain, if it turns out that the identified metabolites are relevant (i.e. toxicologically), additional metabolism studies compliant with the current guidelines and representative for the use on lettuce and chicory roots will also have to be provided. Currently, for the representative uses following soil application, the presence of benfluralin was only shown in leafy crops at limited level (1.3% TRRs).
In view of the low rate of characterisation and identification of the radioactive residues in food and feed items in the available metabolism studies and also pending upon the outcome of the further assessment of the metabolism study in pulses and oilseeds from the public domain, the risk assessment residue definition is provisionally proposed as benfluralin. For monitoring, the residue definition is derived by default as benfluralin.
The confined rotational crop studies in cereals (wheat, maize), leafy crops (cabbage), pulses and oilseeds (soyabean) and root crops (sugar beet) showed significant deficiencies in terms of the metabolites’ identification and characterisation, similar to what was observed in primary crops. In addition, the dose rate was at 0.88N when compared to the annual dose rate of application for the representative uses. Therefore, a rotational crop metabolism study on leafy crops, cereal small grains and root crops and conducted according to the current guidelines at the appropriate dose rate of application and covering all plant back intervals is needed (data gap). From the available information, the elucidation of the metabolic pattern was not possible, and therefore, all the information either from the public domain and/or from assessment of similar dinitro‐trifluoromethyl‐aniline pesticides (i.e. trifluralin) may be used by the applicant for the evaluation of the identified metabolites, their toxicological relevance and to depict the metabolic pattern of benfluralin also in rotational crops. Meanwhile, the same residue definitions as for primary crops are applicable to rotational crops on a provisional basis. Pending upon a complete elucidation of the metabolic pathway of benfluralin in rotational crops, additional rotational crop field trials addressing the magnitude of the relevant compounds might also be triggered.
Sufficient number of residue trials for lettuces and chicory roots supported by validated analytical methods were available. For witloof, although only two trials out of four are independent, considering that the residue levels are below the LOQ, the limited number of trials is considered acceptable. Only the trials on lettuces were supported by storage stability data. Although the metabolic pattern is not fully elucidated, provisional MRLs at the level of LOQ of 0.01* mg/kg are proposed for lettuces and witloof since they are at the default level. For chicory roots, besides the non‐elucidated metabolic pattern, the stability of residues in high starch content commodities was also not demonstrated (data gap), hence no MRL has been proposed.
The nature of benfluralin residues under standard hydrolysis conditions was not investigated and based on the residue level in chicory roots (< 0.01 mg/kg) and considering also that lettuce is consumed raw, this would not be triggered. However, since the chicory trials were not supported by storage stability data and the metabolic pattern is also not elucidated in root crops, the need to address the nature of benfluralin residues at processing will need to be reconsidered pending upon the outcome of the requested outstanding data.
Livestock and fish studies are not triggered since the representative and authorised uses are not feed items. Nevertheless, metabolism studies were provided in ruminants and poultries at dosing levels of 10 and 15 mg/kg feed dry matter (DM), respectively. However, several deficiencies were noted such as the dosing period of 3 days instead of 5 for ruminants, no information on the storage conditions of samples was provided, while the TRRs identification occurred at limited level only for some poultry matrices. Most of the radioactivity remained either non‐extracted (up to 68% TRRs in ruminants liver) or extracted (50% TRRs and 17% TRRs in milk and fat, respectively) without further characterisation and identification. In poultries, benfluralin was identified only in poultry skin (up to 34% TRRs) and in eggs (up to 4% TRRs), while in liver and muscle (i.e. 80% TRRs), the majority of radioactive residues was recovered in organo‐soluble fraction without further identification. Based on the available data, a metabolic pattern could not be depicted for livestock and therefore no reliable residue definition for risk assessment could be proposed. For monitoring, the residue definition is derived by default as benfluralin.
A provisional consumer risk assessment using the EFSA Pesticide Residues Intake model (PRIMo) rev.2 was conducted for lettuces and witloof only. The chronic and acute dietary intakes were all below the ADI and ARfD for all considered European consumer groups, with the theoretical maximum daily intake (TMDI) accounting for max 0.1% of the ADI (ES adult, lettuce) and the maximum international estimated short‐term intake (IESTI) accounting for 0.1% of the ARfD for witloof (NL, diet). Although the consumer exposure intake is not significant due to the level of residues in lettuces, the consumer risk assessment is not finalised since the metabolic pattern in primary and rotational crops could not be elucidated and the proposed risk assessment residue definition in plants has to be regarded as provisional.
It is noted that in the framework of the current peer review for the renewal of the approval of benfluralin, the derivation of an ARfD was considered necessary (see Section 2). Thus, acute consumer intake calculations associated with the MRLs derived during the Art 12 review under Regulation (EC) No 396/2005 (EFSA, 2013a) were performed; the IESTI accounted for 3% of the ARfD for melon (BE, diet). However, this should be considered also provisional pending on the outcome of the final agreed risk assessment residue definition for plants.
Although lettuce and chicory are harvested before flowering and they are not expected to be visited by bees for pollen collection, the metabolic pattern in rotational crops has not been elucidated. Benfluralin is a persistent compound and its uptake by the following crops growing in rotation cannot be excluded. Therefore, data addressing the requirement on the residue levels analysed according to the risk assessment residue definition in pollen and honeybee products covering rotational crops need to be submitted to complete the consumer risk assessment (data gap).
4. Environmental fate and behaviour
Benfluralin was discussed at the Pesticides Peer Review Experts’ Teleconference 192 in September 2018.
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, benfluralin exhibited moderate to high persistence, forming numerous minor metabolites, including metabolite 2,6‐dinitro‐4‐(trifluoromethyl)phenol (B12), which exhibited low to medium persistence in soil. Metabolite B12 was further considered (as a groundwater metabolite) due to the toxicological properties of the parent (regarding at least carcinogenicity) and the chemical structure (of potential concern) of the metabolite (refer to Section 2). Mineralisation of the phenyl ring 14C radiolabel to carbon dioxide accounted for 2–17% AR after 120–125 days. The formation of unextractable residues (not extracted by acetonitrile/water) for this radiolabel accounted for 23–63% AR after 112–125 days. Based on the information available, it can be concluded that the degradation rate of metabolite B12 in soil is pH dependent.12 Under anaerobic soil incubations, benfluralin was rapidly degraded and also a different metabolic pathway was observed when compared to the aerobic conditions. The major degradation products formed were benfluralin diamine (B36, 23.2% AR) and ethyl‐propyl‐benzimidazole (U6#1 or 379R, 25% AR). Anaerobic conditions are unlikely to occur for the proposed uses of benfluralin in pre‐emergence on chicory and lettuce. However, Member States might need to perform the environmental risk assessment of the two major anaerobic metabolites for uses of benfluralin on other crops than the representative uses considered to address situations where prolonged soil anaerobic conditions are prevalent. Information on the photochemical transformation of benfluralin on soil was not available. However, soil photolysis is not considered a significant degradation mechanism for benfluralin for the representative uses assessed, which involve soil incorporation.
Benfluralin may be considered immobile in soil, while metabolite 2,6‐dinitro‐4‐(trifluoromethyl)phenol (B12) exhibited very high to high mobility in soil. It was concluded that the adsorption of benfluralin and metabolite B12 was not pH dependent. In satisfactory field dissipation studies carried out at two sites in Northern France and one in Belgium in two different years (spray application to the soil surface on bare soil plots in spring) benfluralin exhibited moderate to medium persistence. Sample analyses were only carried out for the parent benfluralin. Field study DegT50 values were derived following normalisation to FOCUS reference conditions (20°C and pF2 soil moisture) following the EFSA (2014a) DegT50 guidance. The field data endpoints were not combined with laboratory values to derive modelling endpoints.
In laboratory incubations in dark aerobic natural sediment water systems, benfluralin dissipated from the water phase mainly via volatilisation (up to 64% AR after 100 days) and partitioning to the sediment. One major metabolite was observed in the sediment (benfluralin diamine, B36, max 8.7% AR after 2 days). The unextractable sediment fraction accounted for 26–31% AR at study end (100 days). Mineralisation accounted for only 1.7–2.5% AR at the end of the study. The rate of decline in the whole water/sediment system for benfluralin and the sediment metabolite benfluralin diamine B36 was calculated based on biphasic kinetic models by applying the correction procedure recommended for the volatilisation losses by the FOCUS kinetics guidance (2006). Benfluralin is rapidly photolytically degraded in aqueous media forming the major metabolites desalkyl benfluralin diamine (358R; max 14.1% AR), propyl‐benzimidazole (371R; max. 15.4% AR), methyl‐benzimidazole (372R; max 19.8% AR) and ethyl‐propyl‐benzimidazole (379R; max 15.1% AR).
The necessary surface water and sediment exposure assessments (predicted environmental concentration (PEC) calculations) were carried out for benfluralin, the sediment metabolite B36 and the aqueous photolysis metabolites 371R, 372R, 358R and 379R. For the active substance benfluralin, appropriate step 3 (FOCUS, 2001) calculations were available.13 Step 4 simulations were provided using the SWAN tool (version 4.0.1) to include different mitigation options. Additionally, the contribution of deposition after volatilisation was taken into account by incorporating results from the EVA 3.0 rev 2e tool into the SWAN tool. However, for most of the scenarios, the combinations of risk mitigation measures based on 10 or 20 meter spray drift buffers and 75% or 90% drift reducing equipment resulted in more than a 95% reduction in exposure concentration, which is higher than the allowed maximum spray drift reduction that is proposed in the FOCUS Landscape guidance. Therefore, Step 4 PECsw values calculated based on these risk mitigation measures are not considered valid.
The concentrations of the four aqueous photolysis metabolites were calculated using Step 1–3 values for benfluralin, corrected for the maximum observed % in the aqueous photolysis studies and the molecular weight ratio of the metabolite and benfluralin. PEC calculations for the sediment metabolite benfluralin diamine B36 were not available.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (European Commission, 2014a) scenarios and the models PEARL 4.4.4, PELMO 5.5.3 and MACRO 5.5.4.13 The potential for groundwater exposure from the representative uses by benfluralin and metabolite 2,6‐dinitro‐4‐(trifluoromethyl)phenol (B12) above the parametric drinking water limit of 0.1 μg/L was concluded to be low in geoclimatic situations that are represented by all the relevant FOCUS groundwater scenarios. Member States, however, may wish to consider that for other uses (than the representative uses assessed in this conclusion), the groundwater exposure for metabolite B12 might trigger additional toxicological testing, including genotoxicity testing (see Section 2).
The applicant provided appropriate information to address the effect of water treatment processes on the nature of the residues that might be present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water. The conclusion of this consideration was that neither benfluralin nor any of its degradation products that trigger assessment (2,6‐dinitro‐4‐(trifluoromethyl)phenol (B12), desalkyl benfluralin diamine (358R), propyl‐benzimidazole (371R), methyl‐benzimidazole (372R), ethyl‐propyl‐benzimidazole (379R) and benfluralin diamine (B36)) would be expected to undergo any substantial transformation due to oxidation at the disinfection stage of usual water treatment processes.
The assessment in relation to the persistence (P) criteria was discussed at the Pesticides Peer Review Teleconference 192. Based on the available information, benfluralin may be considered by risk managers to fulfil the persistence criterion in relation to its persistent, bioaccumulative and toxic (PBT) properties according to point 3.7.2 of Annex II of Regulation (EC) 1107/2009. This regards soil transformation rate in three out of the six available investigations (half‐life12°C = 58.3–423 days, soil trigger 120 days) normalised14 to a temperature of 12°C (this being the reference temperature in the ECHA guidance (2014, 2017b). The trigger of 120 days in soil is exceeded even at 20°C with soil half‐life 20°C = 27.3–198 days in six soils, with just a single soil being above the trigger. It should be noted that the slowest degradation rates were obtained from the study where benfluralin was not incorporated into the soil resulting in volatilisation losses and, therefore, slower rates of transformation than expected from the proposed use. In field dissipation studies (four trials), the longest non‐normalised soil dissipation DT50 values are below the lowest P criteria of 120 days with the worst‐case single first‐order (SFO) DegT50 of 63.7 days. The relevant environmental condition is indicated as being 12°C when criteria in the relevant ECHA Guidance are followed (ECHA, 2014, 2017b). A pertinent working document (European Commission, 2012b) did not provide definitive information on what would be considered relevant environmental conditions, just making reference to the fact that the conditions of the available investigations should be reported (which has been done here), that half‐lives should not be aggregated and that a weight of evidence approach considering both laboratory and field studies should be used.
Regarding the aquatic environmental compartment, in the available OECD 309 aquatic mineralisation study no degradation of benfluralin was observed during the test period of 17 days; however, it was mainly evaporated from the water due to its high vapour pressure and Henry's Law constant. Therefore, it can be concluded that benfluralin is not expected to be persistent in the aquatic compartment.
The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix A of this conclusion.
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002a,b), SETAC (2001), EFSA (2009), EFSA PPR Panel (2013) and EFSA (2013b).
Benfluralin was discussed at the Pesticides Peer Review Experts’ Meeting 183 in September 2018 and at the Pesticide Peer Review Meeting 05 (joint Mammalian toxicology – Ecotoxicology meeting) in May 2019.
The acute risk of benfluralin to birds and mammals was assessed as low. The first tier long‐term risk assessment and the risk assessment for earthworm‐eating birds and mammals indicated a high risk. Refinements of the risk assessment were proposed by the applicant.
The endpoints from three long‐term (reproduction) studies with bobwhite quail (two studies) and with mallard duck (one study) were discussed in the Pesticides Peer Review meeting. The experts agreed on an endpoint of 6.7 mg a.s./kg bw per day for the bird long‐term risk assessment. The refinement of (PT)15 and (PD)16 values was not accepted by the experts as the choice of focal species was not sufficiently supported and the long‐term risk to birds remains unresolved.
The refined long‐term endpoint for mammals suggested by the applicant was rejected by the experts. It was agreed that the endpoint (NOAEL 5.5 mg/kg bw per day), which was also confirmed by the mammalian toxicology experts, should be used in the long‐term risk assessment. The refinement based on PT and PD for wood mouse was not accepted because the choice of wood mouse as a focal species was not sufficiently supported by data.
The risk to earthworm‐eating birds and mammals from secondary poisoning was refined with earthworm residue trials (residue per unit dose (RUD) refinement). However, there were a number of uncertainties identified by the experts e.g. only one study available limiting the representativeness at EU level, the soil content of organic carbon (OC) was not considered worst case, the earthworm extraction method was not considered appropriate. Those shortcomings led to rejection of the proposed refinement. Overall, the experts concluded that the standard RUD values cannot be overruled by the earthworm field study.
No reliable bioconcentration factor (BCF, see data gap below in the paragraph on aquatic organisms) was available for the risk assessment of fish‐eating birds and mammals. The experts agreed as a first option to back‐calculate the BCF value which would result in a high risk for fish‐eating birds and mammals. The BCF in fish would need to exceed 27763 and 30208 in order to breach the trigger of 5 for fish‐eating mammals and fish‐eating birds, respectively. This BCF is unrealistically high and therefore the risk to fish‐eating birds and mammals from secondary poisoning is likely to be low based on expert judgement.
The risk for birds and mammals from exposure to contaminated drinking water was assessed as low.
Overall, the long‐term risk assessment for birds and mammals and the risk to earthworm‐eating birds and mammals from secondary poisoning is unresolved, leading to a critical area of concern.
Benfluralin is highly toxic to all groups of aquatic organisms with the chronic toxicity to fish driving the aquatic risk assessment. The endpoint from the early‐life‐stage study was discussed by the experts. It was agreed to use the lower confidence limit of the LC10 (1.3 μg a.s./L),as calculated by the RMS, in the risk assessment.17 A second early‐life‐stage study with modified exposure was submitted by the applicant which resulted in a higher no observed effect concentration (NOEC) (12 μg a.s./L) than in the previous study. However, this study was not accepted for the risk assessment since the exposure started too late (as late as 9 days after hatching).
All FOCUS step 3 PECsw scenarios exceeded the regulatory acceptable concentration (RAC) of 0.134 μg a.s./L, indicating a high risk. More than 95% reduction of PECSW concentrations would be necessary in all step 3 scenarios. However, in the EFSA Aquatic Guidance Document (EFSA PPR Panel, 2013), it is stated that it is not likely that the FOCUS step 3 PECs can be reduced by more than 95% by drift reducing measures. Only for some of the scenarios, the proposed risk mitigation was sufficient (see Section 4). The risk to aquatic organisms is therefore identified as a critical area of concern.
The applicant proposed to refine the risk assessment by using time‐weighted average (twa) PECs. However, since the chronic endpoint for fish is based on mortality, it is not considered appropriate to apply a twa PEC.
No valid study with a second algae species was available. Therefore, a data gap was identified for a study with a second algae species with the active substance as this is a requirement for active substances with herbicidal activity. The experts identified a data gap for a study with a rooted monocotyledonous macrophyte because of the mode of action which is focused on the roots and because monocotyledons were consistently more sensitive in tests with terrestrial plants. In addition, the high Koc value of benfluralin leads to rapid partitioning to the sediment.
Acute endpoints for metabolite 358R were available for fish, daphnids and a chronic endpoint for algae. The acute risk for metabolite 358R was assessed as low. A high chronic risk was indicated based on the assumption of 10 times greater toxicity to fish, daphnids and aquatic plants compared to benfluralin. However, the chronic risk of metabolite 358R is considered to be covered by the risk assessment for benfluralin. The risk assessment for metabolites 371R, 372R and 379R was conducted based on the assumption of 10 times greater toxicity than the parent compound benfluralin. The risk for metabolite 379R was assessed as being covered by the risk assessment for benfluralin. A high risk could not be excluded for metabolites 371R and 372R. The provided QSAR estimates were considered very uncertain for long‐term assessment, and therefore, further data are needed to address the risk from metabolites 371R and 372R (data gap). No PECsed values were available for the sediment metabolite B36. The risk to metabolite B36 was assessed as low assuming a 10 times greater toxicity than the parent to sediment‐dwelling organisms (RAC of 830 μg a.s./kg sediment for B36) and assuming the same amount of metabolite in sediment as the parent (maximum FOCUS step 3 PECsed of 3.559 μg/kg, see also Section 4).
Acute oral and contact studies with honeybees and benfluralin and the representative formulation Bonalan (EF‐1533) were available and the acute risk was assessed as low according to EFSA, 2013b. The acute oral and contact hazard quotient (HQ) values calculated according to SANCO (European Commission, 2002a) are < 44 (oral) and < 14 (contact), indicating a low acute risk to honeybees from oral and contact exposure. No chronic studies with adult honeybees or honeybee larvae were submitted by the applicant (data gap). The risk from other routes of exposure (contaminated water and metabolites) is currently not addressed (data gap).
The risk to other non‐target arthropod species Typhlodoromus pyri, Poecilus cupreus and Chrysoperla carnea was assessed as low (LR50s greater than the application rate). However, the leaf‐dwelling arthropod Aphidius rhopalosiphi was much more sensitive. The refined risk assessment based on extended laboratory studies resulted in a high in‐field risk. The off‐field risk was assessed as low. The product is applied pre‐planting; therefore, the overall in‐field risk to leaf‐dwelling arthropods was considered to be low.
The risk to non‐target soil meso‐ and macrofauna, soil microorganisms and non‐target plants was assessed as low.
The study with activated sludge was evaluated as not valid. However, the majority of the experts were of the opinion that a high risk to biological methods of sewage treatment is unlikely since (despite being invalid) the available study gives some indication that even at an unrealistically high concentration (1,000 mg a.s./L) no effects above 50% were observed and exposure in reality will most likely be negligible for field uses of benfluralin.
With regard to the assessment of the endocrine disrupting potential of benfluralin according to the ECHA and EFSA et al. (2018), as discussed in Section 2, benfluralin is not an endocrine disruptor in humans through EATS‐modalities. This conclusion also applies to wild mammals as non‐target organisms.
For non‐target organisms other than mammals, for the T‐modality, a level 3 test according to OECD Test Guideline (TG) 231 (Amphibian Metamorphosis Assay) was available. No effects in all the measured parameters were observed. Considering the lack of endocrine activity, T‐mediated adversity is not expected.
For the E, A and S modalities, a Fish Short‐Term Reproduction Assay (FSTRA) (OECD TG 229) was available. Furthermore, a level 4 study according to OECD TG 210 (ELS) on fish and three reproductive toxicity studies on birds (OECD TG 206) were available and considered in the overall weight of evidence. In the available FSTRA, only limited effects were observed at the highest tested concentration. However, the study is considered reliable with restrictions due to a number of shortcomings: (i) parameters were analysed only for two concentrations (0.33 and 3.14 μg/L18) since the highest concentration (36.5 μg/L) was excluded due to the high level of mortality (38% in both sexes); (ii) the performance of the solvent control was poor in particular for some parameters (i.e. vitellogenin (VTG) in males and fecundity).
In the available early‐life‐stage study (ELS), evidence of systemic toxicity (10% mortality) in fish larvae was observed at 5 μg/L. Considering that this concentration (5 μg/L) is very close to the highest concentration in the FSTRA where all the parameters were measured (3.14 μg/L), no endocrine‐mediated adversity and/or endocrine activity is expected in the absence of systemic toxicity.
Based on the available data and assessment, it is concluded that benfluralin is not an endocrine disruptor for non‐target organisms through EATS‐modalities according to point 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.6
Benfluralin is considered to fulfil the toxic (T) criterion in relation to its PBT properties. The long‐term endpoint (lower confidence limit of the LC10) for fish relevant for the risk assessment is 0.00134 mg/L and the long‐term NOEC based on length is 0.0019 mg a.s./L (T criterion is ≤ 0.01 mg/L). The study to determine the BCF in fish was evaluated as not valid by the experts since the validity criteria were not met and the feeding regime and the lipid content were not reported. A data gap was identified for a new BCF study with fish. Given that also the P criterion may be fulfilled (see Section 4), it is essential that a valid BCF study is submitted in order to finalise the PBT evaluation.
6. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1, 2, 3, 4)
Table 1.
Soil
| Compound (name and/or code) | Persistencea | Ecotoxicology |
|---|---|---|
| benfluralin |
Moderate to high persistence Single first‐order DT50 31.7–198 days (20°C pF 2 soil moisture) Northern European field dissipation studies single first‐order and biphasic DT50 31.5–63.7 days (DT90 115–349 days) |
The risk to non‐target soil meso‐ and macrofauna, soil microorganisms was assessed as low |
The descriptors (in words) used here are unrelated to the ‘P’ assessment comparison to ‘P’ triggers in PBT, vPvB and POP hazard cut‐offs.
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| benfluralin |
Immobile KFoc 10,736–14,400 (3 soils) (KFoc 53,345 mL/g in a volcanic soil) |
No | Yes | Yes |
| 2,6‐dinitro‐4‐(trifluoromethyl)phenol (B12)b |
Very high to high mobility KFoc 22.8–50.1 mL/g |
No | No data | Negative gene mutation in bacteria and mammalian cells), further data are not required for the representative uses since the assessment is not triggered |
FOCUS scenarios or relevant lysimeter.
Minor soil metabolite that was further considered (as a groundwater metabolite) due to the toxicological properties of the parent (regarding at least carcinogenicity) and the chemical structure (of potential concern) of the metabolite.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| benfluralin | High risk to aquatic organisms for all FOCUS step 3 scenarios, mitigation comparable to a 10 m no‐spray buffer zone and 90% spray drift reduction is not sufficient as a risk mitigation for most scenarios |
| benfluralin diamine (B36) (sediment) | Low risk to sediment‐dwelling organisms |
| propyl‐benzimidazole (371R) (aqueous photolysis) | High risk to aquatic organisms based on the assumption of 10 times greater toxicity than the parent, data gap identified |
| methyl‐benzimidazole (372R) (aqueous photolysis) | High risk to aquatic organisms based on the assumption of 10 times greater toxicity than the parent, data gap identified |
| desalkyl benfluralin diamine (358R) (aqueous photolysis) | A high risk is indicated based on the assumption of 10 times greater toxicity than the parent. However, the risk is considered to be addressed with the risk assessment for benfluralin |
| Ethyl‐propyl‐benzimidazole (379R) (aqueous photolysis) | A high risk is indicated based on the assumption of 10 times greater toxicity than the parent. However, the risk is considered to be addressed with the risk assessment for benfluralin |
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| benfluralin | Rat LC50 inhalation > 2.16 mg/L air/4h (dust, nose only), classification as STOT SE 2 proposed by the peer review |
7. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of Regulation (EC) No 1107/2009 concerning information on potentially harmful effects).
Revised technical specification to remove the non‐significant impurities and to consider the level of the relevant impurity EBNA (max 0.085 mg/kg) (relevant for all representative uses evaluated; see Sections 1 and 2).
Validation data of analytical methods used for the generation of pre‐approval data for the mammalian toxicology assessment (short‐term toxicity studies in rats and dogs, carcinogenicity study in mice and developmental toxicity studies in rat and rabbit) and for all ecotoxicology studies for which the validation is missing (relevant for all representative uses evaluated; see Sections 1, 2, 5).
Additional information to address the extraction efficiency in plant matrices (relevant for all representative uses evaluated; see Section 1).
Monitoring method for propyl‐benzimidazole (371R) and methyl‐benzimidazole (372R) in surface water (relevant for all representative uses evaluated; see Section 1).
Assessment of the toxicological relevance of the individual impurities present in the technical specification in comparison with the toxicological profile of benfluralin (relevant for all representative uses evaluated, see Section 2).
Identification and assessment of the toxicological relevance of Peak 3 and Peak 7 in the in vitro interspecies comparative metabolism study (relevant for all representative uses evaluated, see Section 2).
Establishment of a residue definition for body fluids and tissues (blood and urine) relevant to human biomonitoring of the substance (relevant for all representative uses evaluated, see Section 2).
Detailed assessment of the metabolism study on peanuts and alfalfa from the public domain (i.e. Fate of Benefin in soils, Plants, Artificial Rumen Fluid, and the Ruminant Animal, Tomasz Golab et al., Journal of Agricultural Food Chemistry, 18, 1970) should be provided, i.e. analysis of the proposed structures of the identified metabolites, their toxicological relevance and whether this study can address the metabolism of benfluralin in primary crops (relevant for all representative uses evaluated; see Section 3).
Rotational crop metabolism study on leafy crops, cereal small grains and root crops and conducted according to the current guidelines at the appropriate dose rate of application and covering all plant back intervals is needed for the elucidation of the metabolic pattern of benfluralin in succeeding crops. Pending upon a complete elucidation of the metabolic pathway of benfluralin in rotational crops, additional rotational crop field trials addressing the magnitude of the relevant compounds might also be triggered (relevant for all representative uses evaluated; see Section 3).
Storage stability studies in high starch content commodities are needed to validate the residue trials (relevant for the chicory roots uses; see Section 3).
Data on the level of the residues in pollen and honeybee products analysed according to the risk assessment residue definition and covering the rotational crops need to be submitted to complete the consumer risk assessment (relevant for all uses; see Section 3).
A second aquatic macrophyte (rooted, monocotyledonous plant) should be tested (relevant for all representative uses evaluated, see Section 5).
The risk from the aquatic metabolites 371R and 372R needs to be addressed further (relevant for all representative uses evaluated, see Section 5).
A second algae study is required for active substances with herbicidal mode of action (relevant for all representative uses evaluated, see Section 5).
A chronic study with adult bees and a study with honeybee larvae are required. All routes of exposure including contaminated water and metabolites should be addressed (relevant for all representative uses evaluated, see Section 5).
A BCF study with fish (relevant for all representative uses evaluated, see Section 5).
8. Particular conditions proposed to be taken into account to manage the risk(s) identified
Operators have to use personal protective equipment (PPE), such as workwear, gloves during mixing, loading and application and RPE to ensure that the AOEL and AAOEL are not exceeded according to the EFSA calculator (see Section 2).
To reduce resident's child exposure below the AOEL, there is the need for restricting applications to those using drift reducing technology and requiring minimum spray volume of 400 L/ha (see Section 2).
9. Concerns
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/201119 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
No validated analytical methods were reported for the developmental toxicity study in rabbits (key study in deriving the ARfD and AAOEL); therefore, the reliability of the study is questioned and the developmental toxicity endpoint could not be finalised (see Section 2).
The need for further tests and risk assessment to unique human metabolites (i.e. significantly increased in comparison with other tested species) could not be finalised whilst the identification and assessment of the toxicological relevance of Peak 3 and Peak 7 in the in vitro interspecies comparative metabolism study have not been provided (see Section 2).
Although the consumer exposure intake is not significant due to the level of residues in lettuces, the consumer risk assessment is not finalised since the metabolic pattern in primary and rotational crops could not be elucidated and the proposed residue definition for risk assessment has to be regarded as provisional (see Section 3).
Based on the available information, benfluralin may be considered to fulfil the persistence criterion in relation to its persistent, bioaccumulative and toxic (PBT) properties according to point 3.7.2 of Annex II of Regulation (EC) 1107/2009. The P criterion may be considered fulfilled for soil, although the evidence for this is from the results of just one of a number of available soil investigations at 20°C and of three soils when the half‐lives are normalised to 12°C (see Section 4). The T criterion is fulfilled (NOEC = 0.0019 mg a.s./L; the long‐term endpoint for risk assessment is based on the lower confidence limit LC10 for fish of 0.00134 mg/L). No valid BCF study with fish is available, and therefore, the B criterion cannot be evaluated at this stage (see Section 5).
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
-
5
The technical specification (either the current or the newly proposed one) is not supported by the toxicological assessment, including the level of a genotoxic impurity (EBNA) that is high compared to the levels tested; at the level currently specified in the technical material, a genotoxic potential cannot be excluded (see Section 2).
-
6
The long‐term risk to birds and mammals including the risk from secondary poisoning of earthworm‐eating birds and mammals (see Section 5).
-
7
The risk to aquatic organisms from benfluralin and metabolites 371R and 372R (see Section 5).
9.3. Overview of the concerns identified for each representative use considered (Table 5)
Table 5.
Overview of concerns
| Representative use | Chicory (chicon/endive production) industrial chicory (‘coffee’, fructose, inulin production) | Lettuce | |
|---|---|---|---|
| Operator risk | Risk identified | ||
| Assessment not finalised | |||
| Worker risk | Risk identified | ||
| Assessment not finalised | |||
| Resident/bystander risk | Risk identified | ||
| Assessment not finalised | |||
| Consumer risk | Risk identified | ||
| Assessment not finalised | X3 | X3 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X6,c | X6,c |
| Assessment not finalised | |||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | ||
| Assessment not finalised | |||
| Risk to aquatic organisms | Risk identified | X7 | X7 |
| Assessment not finalised | |||
| Groundwater exposure to active substance | Legal parametric value breached | ||
| Assessment not finalised | |||
| Groundwater exposure to metabolites | Legal parametric value breacheda | ||
| Parametric value of 10 μg/Lb breached | |||
| Assessment not finalised | |||
The superscript numbers relate to the numbered points indicated in Sections 9.1 and 9.2. Where there is no superscript number, see Sections 2, 3, 4, 5–6 for further information.
When the consideration for classification made in the context of this evaluation under Regulation (EC) No 1107/2009 is confirmed under Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission, 2003.
Based on first tier risk assessment.
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Abbreviations
- ε
decadic molar extinction coefficient
- a.s.
active substance
- AAOEL
acute acceptable operator exposure level
- ADI
acceptable daily intake
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- AR
androgen receptor
- ARfD
acute reference dose
- BCF
bioconcentration factor
- bw
body weight
- CL
confidence limits
- DM
dry matter
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EAS
oestrogen, androgen, and steroidogenesis modalities
- EATS
oestrogen, androgen, thyroid and steroidogenesis modalities
- EC
emulsifiable concentrate
- EC50
effective concentration
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- HPLC
high‐pressure liquid chromatography or high‐performance liquid chromatography
- HPLC‐MS
high‐pressure liquid chromatography–mass spectrometry
- HQ
hazard quotient
- IESTI
international estimated short‐term intake
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- iv
intravenous
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- KFoc
Freundlich organic carbon adsorption coefficient
- LC
liquid chromatography
- LC50
lethal concentration, median
- LC‐MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- LR50
lethal rate that causes 50% mortality
- mm
millimetre (also used for mean measured concentrations)
- mN
milli‐Newton
- MRL
maximum residue level
- NOAEL
no observed adverse effect level
- NOEC
no observed effect concentration
- OECD
Organisation for Economic Co‐operation and Development
- Pa
Pascal
- PD
proportion of different food types
- PEC
predicted environmental concentration
- PECair
predicted environmental concentration in air
- PECgw
predicted environmental concentration in groundwater
- PECsed
predicted environmental concentration in sediment
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- pF2
pF value of 2 (suction pressure that defines field capacity soil moisture)
- PPE
personal protective equipment
- PT
proportion of diet obtained in the treated area
- QSAR
quantitative structure–activity relationship
- RAC
regulatory acceptable concentration
- RAR
Renewal Assessment Report
- RPE
respiratory protective equipment
- RUD
residue per unit dose
- SFO
single first‐order
- SMILES
simplified molecular‐input line‐entry system
- STOT‐SE
specific target organ toxicity – single exposure
- t1/2
half‐life (define method of estimation)
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- twa
time‐weighted average
- UF
uncertainty factor
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2019.5842
Appendix B – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
| benfluralin |
N‐butyl‐N‐ethyl‐α,α,α‐trifluoro‐2,6‐dinitro‐p‐toluidine [O‐][N+](=O)c1cc(cc([N+]([O‐])=O)c1N(CCCC)CC)C(F)(F)F SMDHCQAYESWHAE‐UHFFFAOYSA‐N |
|
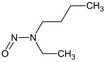
| ethyl‐butyl‐nitrosamine (EBNA) | N‐butyl‐N‐ethylnitrous amideCCN(CCCC)N=OZGMCNGHHUQZNIH‐UHFFFAOYSA‐N |
|
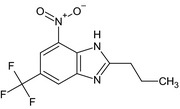
| propyl‐benzimidazole (371R) | 4‐nitro‐2‐propyl‐6‐(trifluoromethyl)‐1H‐benzimidazoleFC(F)(F)c1cc2nc(CCC)[NH]c2c(c1)[N+]([O‐])=OVBWGGQYTGJYAOC‐UHFFFAOYSA‐N |
|
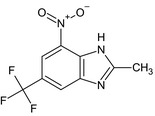
| methyl‐benzimidazole (372R) | 2‐methyl‐4‐nitro‐6‐(trifluoromethyl)‐1H‐benzimidazoleFC(F)(F)c1cc2nc(C) [NH]c2c(c1)[N+]([O‐])=OJVDICTFQLGBWOK‐UHFFFAOYSA‐N |
|
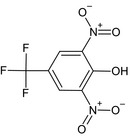
| 2,6‐dinitro‐4‐(trifluoromethyl)phenol (B12) | 2,6‐dinitro‐4‐(trifluoromethyl)phenolO=[N+]([O‐])c1cc(cc([N+]([O‐])=O)c1O)C(F)(F)FFXZGYEWQIGIFMC‐UHFFFAOYSA‐N |
|
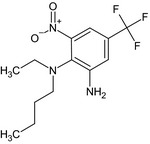
| benfluralin diamine (B36) |
N 2‐butyl‐N 2‐ethyl‐3‐nitro‐5‐(trifluoromethyl)‐1,2‐benzenediamine [O‐1][N+](=O)c1cc(cc(N)c1N(CCCC)CC)C(F)(F)F WCWGIPXTBMUTDI‐UHFFFAOYSA‐N |
|
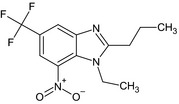
| ethyl‐propyl‐benzimidazole (U6#1) (379R) |
1‐ethyl‐7‐nitro‐2‐propyl‐5‐(trifluoromethyl)‐1H‐benzimidazole FC(F)(F)c1cc2nc(CCC)n(CC)c2c(c1)[N+]([O‐])=O VFHKPDWMZHEIDV‐UHFFFAOYSA‐N |
|
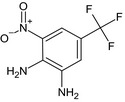
| desalkyl benfluralin diamine (358R) |
3‐nitro‐5‐(trifluoromethyl)‐1,2‐benzenediamine Nc1cc(cc([N+]([O‐])=O)c1N)C(F)(F)F WQRYZWVAWJMKPB‐UHFFFAOYSA‐N |
|
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2018.2.2 ACD/Labs 2018 Release (File version N50E41, Build 103230, 21 July 2018).
ACD/ChemSketch 2018.2.2 ACD/Labs 2018 Release (File version C60H41, Build 106041, 07 December 2018).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Arena M, Auteri D, Brancato A, Bura L, Carrasco Cabrera L, Chaideftou E, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Ferreira L, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Pedersen R, Reich H, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Terron A, Tiramani M, Vagenende B and Villamar‐Bouza L, 2019. Conclusion on the peer review of the pesticide risk assessment of the active substance benfluralin. EFSA Journal 2019;17(11):5842, 26 pp. 10.2903/j.efsa.2019.5842
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00803
Update: This scientific output, approved on 23 September 2019, supersedes the previous output published on 28 July 2008 (EFSA, 2008b, 2012).
Acknowledgement: EFSA wishes to thank the rapporteur Member State, Norway, for the preparatory work on this scientific output.
Approved: 23 September 2019
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine disrupting properties. OJ L 101, 20.4.2018, p. 33–36.
It should be noted that harmonised classification and labelling are formally proposed and decided in accordance with Regulation (EC) No 1272/2008.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
See also the report from the Pesticide Peer Review Meeting 05 Mammalian toxicology – Ecotoxicology (joint session on ED) (7–8 May 2019) (EFSA, 2019a).
The AOEL was previously established at 0.05 mg/kg bw per day based on the NOAEL of 17 mg/kg bw per day from the 90‐day rat study, 30% correction for the limited oral absorption and UF of 100 (EFSA, 2008b; European Commission, 2008).
See Member States comments on the draft EFSA conclusion on benfluralin, comment 4 in the mammalian toxicity section (EFSA, 2019a).
Fate of Benefin in soils, Plants, Artificial Rumen Fluid, and the Ruminant Animal, Tomasz Golab et al., Journal of Agricultural Food Chemistry, 18, 1970.
See Data Requirement 4.7 of the Evaluation Table (EFSA, 2019a).
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008a) and Walker equation coefficient of 0.7
Using a Q10 of 2.58 according to EFSA (2008a).
Proportion of an animal's daily diet obtained in habitat treated with pesticide.
Composition of diet obtained from treated area.
for details refer to the report from the Pesticides Peer Review Experts’ Meeting 183, Expert consultation point 5.9 (EFSA, 2019a).
A dose spacing of 10 was selected in the study. The reported concentrations are based on mean measured concentrations. The nominal concentrations were 0.33, 3.3 and 33 μg/L.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
MRL is proposed at the level of LOQ.
References
- ECHA (European Chemicals Agency), 2014. Guidance on the information requirements and chemical safety assessment. Chapter R11: PBT/vPvB assessment. Version 2.0. November 2014.
- ECHA (European Chemicals Agency), 2017a. Guidance on the Application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0, July 2017. Reference: ECHA‐17‐G‐21‐EN; ISBN: 978‐92‐9020‐050‐5. Available online: https://echa.europa.eu/guidance-documents/guidance-on-clp
- ECHA (European Chemicals Agency), 2017b. Guidance on Information Requirements and Chemical Safety Assessment. Part C: PBT/vPvB assessment. Version 3.0, June 2017. 10.2823/139408 [DOI]
- ECHA and EFSA (European Chemicals Agency and European Food Safety Authority) with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311.echa-18-g-01-en [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32 pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Conclusion regarding the peer review of the pesticide risk assessment of the active substance benfluralin. EFSA Journal 2008;6(7):127r, 82 pp. 10.2903/j.efsa.2008.127r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Conclusion on the peer review of the pesticide risk assessment of confirmatory data submitted for the active substance benfluralin. EFSA Journal 2012;10(7):2800, 13 pp. 10.2800/j.efsa.2012.2800 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013a. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for benfluralin according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2013;11(6):3278, 33 pp. 10.2903/j.efsa.2013.3278 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013b. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55 pp. 10.2903/j.efsa.2014.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2019a. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance benfluralin. Available online: http://www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority), 2019b. EFSA addendum: Updated assessment on the endocrine disrupting properties of the active substance benfluralin in accordance with Commission Regulation (EU) 2018/605. 5 March 2019, updated in May 2019. Available online: http://www.efsa.europa.eu
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002.
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2008. Review report for the active substance benfluralin. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 20 May 2008 in view of the inclusion of benfluralin in Annex I of Council Directive 91/414/EEC. SANCO/122/08‐rev.2 20 May 2008.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2012a. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2012b. DG SANCO Working Document on “Evidence Needed to Identify POP, PBT and vPvB Properties for Pesticides”. 25.09.2012 – rev. 3.
- European Commission , 2014a. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2, May 2014.
- European Commission , 2014b. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- European Commission , 2016. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.2, September 2016.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.4, May 2015.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp., as updated by the Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, v. 1.1, December 2014.
- Golab T, Herberg RJ, Gramlich JV, Raun AP and Probst GW, 1970. Fate of benefin in soils, plants, artificial rumen fluid, and the ruminant animal. Journal of Agricultural and Food Chemistry, 18, 838–844. 10.1021/jf60171a028 [DOI] [PubMed] [Google Scholar]
- JMPR (Joint Meeting on Pesticide Residues), 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp.
- JMPR (Joint Meeting on Pesticide Residues), 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 18–27 September 2007, 164 pp.
- Norway , 2017. Renewal Assessment Report (RAR) on the active substance benfluralin prepared by the rapporteur Member State Norway, in the framework of Commission Implementing Regulation (EU) No 844/2012, August 2017. Available online: http://www.efsa.europa.eu
- Norway , 2018. Revised Renewal Assessment Report (RAR) on benfluralin prepared by the rapporteur Member State Norway in the framework of Commission Implementing Regulation (EU) No 844/2012, November 2018. Available online: http://www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- SETAC (Society of Environmental Toxicology and Chemistry), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


