Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver a scientific opinion on the extension of use of calcium l‐methylfolate to be used as a source of folate added for nutritional purposes to infant and follow‐on formula, baby food and processed cereal‐based food pursuant to Regulation (EU) 609/2013. In 2004, EFSA assessed the use of calcium l‐methylfolate as a source of folate in foods for particular nutritional uses, food supplements and foods intended for the general population. The new alternative synthetic step proposed to produce the nutrient source, using platinum as a catalyst, did not raise any safety concern and the production process was found to consistently yield a product in line with the proposed specifications. Based on the studies assessed in the previous evaluation, it was concluded that calcium l‐methylfolate is non‐genotoxic and that subchronic and embryotoxicity/teratogenicity studies in rats did not reveal any adverse effects up to the highest doses tested. The Panel considered that no additional toxicological studies are required on the nutrient source. The intervention study in healthy infants provided by the applicant did not indicate differences in growth and tolerance parameters in infants who consumed either an infant formula supplemented with calcium l‐methylfolate or with folic acid, and did not raise concerns regarding safety or tolerability of the infant formula with the proposed nutrient source. The study also provided further supporting evidence for the bioavailability of calcium l‐methylfolate. The Panel considers that calcium l‐methylfolate is a source from which folate is bioavailable and concludes that calcium l‐methylfolate is safe under the proposed uses and use levels for infants and young children.
Keywords: calcium l‐methylfolate, nutrient source, folate, folic acid, safety, bioavailability, infants, young children, extension of use
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
Background
The European Union legislation lists nutritional substances that may be used for nutritional purposes in certain categories of foods as sources of certain nutrients.
The Commission has received a request for the evaluation of calcium L‐methylfolate as a source of folate added for nutritional purposes to infant and follow‐on formula, baby food and processed cereal‐based food. The relevant Union legislative measure is:
Regulation (EU) No 609/2013 of the European Parliament and of the Council on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/20091.
Terms of Reference
In accordance with Article 29 (1) (a) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority to provide a scientific opinion, based on its consideration of the safety and bioavailability of calcium L‐methylfolate as a source of folate added for nutritional purposes to infant formula, follow‐on formula, baby food and processed cereal‐based food.
1.2. Information on existing evaluations and authorisations
Calcium l‐methylfolate is an authorised form of folate in food for special medical purposes and total diet replacement for weight control as per Regulation (EU) No 609/2013. It is also authorised for use in food supplements and for addition to foods as per Regulation (EC) No 1925/2006.
In 2004, the European Food Safety Authority (EFSA) issued an opinion on calcium l‐methylfolate (EFSA AFC Panel, 2004) concluding that its use as a source of folate in foods for particular nutritional uses, food supplements and foods intended for the general population, at level of 1 mg/adult person/day does not raise safety concerns in line with the tolerable upper level (UL) for folic acid for adults defined by the Scientific Committee on Food (2000).
Additionally, in 2013 EFSA issued another opinion on (6S)‐5‐methyltetrahydrofolic acid (5‐MTHF), glucosamine salt as an alternative source of folate added for nutritional purposes to food supplements. The Panel concluded that the proposed use levels (up to 1.8 mg/day, which equates to 1 mg 5‐MTHF and 0.8 mg glucosamine) are not of safety concern (EFSA ANS Panel, 2013).
In 2014, EFSA provided an opinion on the dietary reference values for folate (EFSA NDA Panel, 2014). For infants aged 7–11 months an adequate intake (AI) for folate was set at 80 μg dietary folate equivalents (DFE)/day but no UL could be defined. For children aged 1–3 years, the average requirement (AR) and the population reference intake (PRI) were set at 90 and 120 μg DFE/day respectively, and the UL of 200 μg/day for synthetic folic acid previously defined by the SCF (2000) was confirmed.
2. Data and methodologies
2.1. Data
The safety assessment of calcium l‐methylfolate as a new source of folate for use in infant and follow‐on formula, baby food and processed cereal‐based food is based on data supplied in the application and information submitted by the applicant following an EFSA request for supplementary information.
2.2. Methodologies
The evaluation of the safety and bioavailability of calcium l‐methylfolate was conducted in line with the principles contained in the ‘Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources’ (EFSA ANS Panel, 2018).
3. Assessment
3.1. Introduction
This opinion deals with the safety and bioavailability of calcium l‐methylfolate as a new source of folate added for nutritional purposes to infant and follow‐on formula, baby food and processed cereal‐based food.
The nutrient source (NS) calcium l‐methylfolate is produced by chemical synthesis starting from folic acid as described in Section 3.3.
3.2. Identity of the nutrient source
The NS is calcium l‐methylfolate, commercialised by the applicant as Metafolin®. The chemical name according to the IUPAC nomenclature is calcium;(2S,6S)‐2‐[[4‐[(2‐amino‐5‐methyl‐4‐oxo‐3,6,7,8‐tetrahydropteridin‐6‐yl)methylamino]benzoyl]amino]pentanedioate.
Two CAS registry numbers are available for the NS: 129025‐21‐4 for calcium salt with an unspecified ratio of l‐methylfolate and Ca2+, and 151533‐22‐1 for calcium salt with a specified 1:1 ratio of l‐methylfolate and Ca2+.
Synonyms, trade names and abbreviations commonly used for calcium l‐methylfolate include: calcium l‐5‐methyltetrahydrofolate; l‐methylfolate, calcium; l‐5‐methyltetrahydrofolic acid, calcium salt [l‐5‐MTHF‐Ca]; (6S)‐5‐methyltetrahydrofolic acid, calcium salt [(6S)‐5‐MTHF‐Ca]; (6S)‐5‐methyl‐5,6,7,8‐tetrahydropteroyl‐l‐glutamic acid, calcium salt; and Metafolin®.
Calcium l‐methylfolate has a molecular weight of 497.5 g/mol; its molecular formula is C20H23CaN7O6 and its structural formula is given in Figure 1 below.
Figure 1.
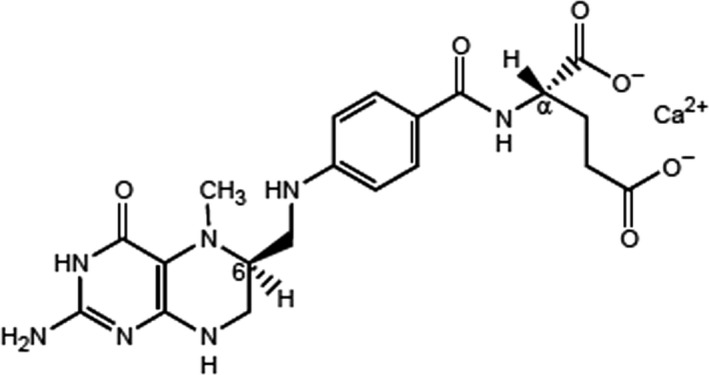
Structural formula of calcium l‐methylfolate
Calcium l‐methylfolate has two chiral carbon atoms: the α‐C atom in the l‐glutamic acid moiety and the C‐atom in position 6 of the pteroyl moiety.
According to the specifications provided, the stereoisomer configuration of calcium l‐methylfolate is (6S, αS). In fact, the α‐C atom in the l‐glutamic acid moiety originates from the starting material folic acid and its configuration (αS or L) and remains unchanged during the chemical synthesis used by the applicant. The (6S, αS) and (6R, αS) diastereoisomers obtained in the first step of the chemical synthesis are separated afterwards by crystallisation and further purification steps to isolate the (6S, αS) isomer only, which corresponds to the natural form of folate.
The particle size of the manufactured calcium l‐methylfolate, subject of this opinion, has been measured by laser diffraction and electron microscopy. The latter indicated the presence of few small‐sized particles (including particles in the nanoscale), which however, based on the water solubility of the nutrient source in its formulation, the Panel consider that they are likely to readily and entirely dissolve.
The manufactured calcium l‐methylfolate has been characterised by the applicant by means of ultraviolet‐visible (UV‐Vis) spectroscopy, infrared (IR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry, X‐ray diffraction and elemental analysis. Upon request of the Panel the applicant also provided a chiral high‐performance liquid chromatography (HPLC) analysis of the novel food which demonstrated that the manufactured calcium l‐methylfolate corresponds to the natural diastereoisomer (6S, αS).
3.3. Production process
The nutrient source is produced according to Good Manufacturing Practice (GMP) principles. Calcium l‐methylfolate is produced by a three‐step chemical synthesis starting from folic acid.
The first synthetic step is a reduction of the folic acid to produce a mixture of (6S, αS)‐ and (6R, αS)‐tetrahydrofolic acid benzenesulfonate. For this first synthetic step two alternatives exist and are used: a catalytic hydrogenation or a reduction with sodium borohydride. The pure (6S, αS)‐diastereoisomer is then isolated by crystallisation with benzenesulfonic acid.
In the second step, the first isolated intermediate, (6S, αS)‐tetrahydrofolic acid benzenesulfonate, is then methylated in the 5‐position by condensation with formaldehyde and subsequent reduction with sodium borohydride to form (6S, αS)‐5‐methyltetrahydrofolic acid.
The third and last synthetic step is the crystallisation of the second isolated intermediate – (6S, αS)‐5‐methyltetrahydrofolic acid – as calcium salt to produce the final product (6S, αS)‐5‐methyltetrahydrofolic acid, calcium salt (calcium l‐methylfolate).
According to the applicant, the manufacturing process is the same as the one assessed by EFSA in the opinion of 2004, apart from the first synthetic step where a catalytic hydrogenation, using platinum as catalyst, can be used as alternative to the sodium borohydride reduction step assessed in the previous opinion (both techniques can be used in the production process). Then, the second and third synthetic steps are equivalent to those previously assessed.
The Panel assessed the new alternative first synthetic step (namely, the hydrogenation with platinum catalyst) and the experimental analytical data provided by the applicant to demonstrate the equivalent profiles of the production batches obtained by the two alternative synthetic steps. In particular, the Panel evaluated: (i) the content of the residual platinum catalyst, (ii) the overall impurity profile including the compliance with the same product specifications and (iii) the stability of the different production batches as further described below.
The applicant provided the concentration of platinum for four different batches produced by hydrogenation with platinum as first synthetic step. The results indicated that the NS had a measured concentration of platinum ranging from < 0.1 to 0.14 mg/kg.
With regard to the overall impurity profile and the compliance with the same product specifications for the production batches obtained by the two alternatives for the first synthetic step, the applicant reported analytical data for both manufacturing methods (i.e. hydrogenation or reduction). The Panel notes that both manufacturing methods lead to a final product with quality and impurity profiles within the specification limits provided by the applicant.
Finally, in relation to the stability of the final product, the Panel considers that no influence on the stability is expected using the two alternatives as first synthetic step (catalytic hydrogenation or reduction with borohydride). Considering the quality and impurity profiles reported for the first intermediates obtained by these two synthetic alternatives and the fact that the last two synthetic steps are equivalent, the Panel considers that the physical properties of the final product are not influenced by the manufacturing process applied. This is also confirmed by the stability results of the product obtained with the catalytic hydrogenation step provided by the applicant.
The Panel considers that the production process is sufficiently described and does not raise safety concerns. Further considerations on the safety of the residual amounts of platinum are reported in Section 3.7.5 (‘Estimate of exposure to undesirable substances’).
3.4. Compositional data
In order to confirm that the manufacturing process is reproducible and adequate to yield on a commercial scale a product with the required characteristics, the applicant provided a certificate of analysis for three independent batches of the NS produced by the reduction step with sodium borohydride (see Table 1).
Table 1.
Batch‐to‐batch analysis of the NS
| Parameter | Batch number | ||
|---|---|---|---|
| LMCG045801 | LMCG046801 | LMCM048001 | |
| Calcium l‐methylfolatea (6S, αS‐diastereoisomer) | 101.0% | 100.6% | 100.5% |
| Water | 13.0% | 12.1% | 12.3% |
| Total calciuma | 8.0% | 8.0% | 8.0% |
| Calcium d‐methylfolate (6R, αS‐diastereoisomer) | 0.6% | 0.5% | 0.5% |
| Other folates and related substances | 1.08% | 1.10% | 0.91% |
| Lead | < LOQ (1 mg/kg) | < LOQ (1 mg/kg) | < LOQ (1 mg/kg) |
| Total viable aerobic counts | < LOQ (10 CFU/g) | < LOQ (10 CFU/g) | < LOQ (10 CFU/g) |
CFU: colony forming unit; LOQ: limit of quantification.
Dry basis.
Information was provided on the accreditation of the laboratories that conducted the analyses presented in the application.
The applicant reported the different impurities identified in the final commercial batches of calcium l‐methylfolate, both in terms of residual solvents, inorganic and other potential elemental impurities, organic impurities derived from the chemical synthesis and microbial impurities.
The Panel notes that the compositional data and the impurity profile is consistent for the three commercial batches analysed and in line with the specifications provided to EFSA in 2004, and with the current manufacturer specifications, as confirmed by the certificate of analysis provided for these three commercial batches of the NS.
The Panel further notes that the applicant reported a list of potential organic impurities such as the residual starting material, by‐products and degradation products obtained during the chemical synthesis of calcium l‐methylfolate and identified them by HPLC. The analytical data reported by the applicant consistently confirmed the presence of the non‐natural d‐5‐methylfolate diastereoisomer at a concentration below 1% and the overall concentration of ‘other folates and related substances’ below 2.5% in line with the purity of calcium l‐methylfolate previously assessed by EFSA (EFSA AFC Panel, 2004) and the specifications from JECFA (2005).
The applicant provided also analytical data for several consecutive batches produced using both alternatives for the first synthetic step (hydrogenation or reduction) showing that all batches have quality and impurity profiles within the provided specification limits.
The Panel considers that the information provided on the composition and reported impurity profile of calcium l‐methylfolate is sufficient and does not raise safety concerns.
3.4.1. Stability
The applicant performed stability tests with fourteen independent batches of the NS. The tests were carried out at refrigerated conditions and room temperature (25°C, 60% RH) for up to 24 months. The batches were analysed for compliance with specifications for appearance, water content and diastereomeric purity.
The applicant also provided information on the stability in powdered infant formula and follow‐on formula, showing that the folate concentration was stable over the 18‐month period tested. Moreover, the applicant also tested the stability in prepared liquid infant formula showing no loss of folate during the preparation process of the liquid infant formula.
The Panel notes that the appearance of the tested batches did not change and complied with the proposed specifications for all tested parameters (HPLC assay of related compounds, water content, diastereomeric purity) for all time periods and for all samples stored at both temperatures.
The Panel considers that the data provided sufficient information with respect to the stability of the NS for a period of 18 months.
3.5. Specifications
The specifications of the NS as proposed by the applicant are reported in Table 2.
Table 2.
Specifications of the NS
| Description: the NS is a purified, white to yellow or beige powder that is produced by chemical synthesis | ||
|---|---|---|
| Parameter | Amount | Method of analysis |
| Calcium l‐methylfolate (6S, αS‐diastereoisomer) | ≥ 95.0% (Dry basis) | HPLC |
| Water | 6.0–17.0% | Karl Fischer method |
| Total calcium | 7.0–8.5% (Dry basis) | Titration |
| Calcium d‐methylfolate (6R, αS‐diastereoisomer) | ≤ 1.0% | HPLC |
| Other folates and related substances | ≤ 2.5% | HPLC |
| Heavy metals and specified elemental impurities | ||
| Lead | ≤ 1 mg/kg | ICP‐MS |
| Cadmium | ≤ 0.5 mg/kg | ICP‐MS |
| Mercury | ≤ 1.0 mg/kg | ICP‐MS |
| Arsenic | ≤ 1.5 mg/kg | ICP‐MS |
| Platinum | ≤ 2 mg/kg | ICP‐MS |
| Boron | ≤ 10 mg/kg | ICP‐OES |
| Microbiological | ||
| TAMC | ≤ 100 CFU/g | Membrane filter technique |
| TYMC | ≤ 100 CFU/g | Membrane filter technique |
CFU: colony forming unit; HPLC: high performance liquid chromatography; ICP‐MS: inductively coupled plasma mass spectrometry; ICP‐OES: inductively coupled plasma optical emission spectroscopy; TAMC: total aerobic microbial count; TYMC: total yeast and mould count.
The Panel notes that the certificates of analysis provided for three representative commercial batches of the NS (Table 1) are compliant with the specifications indicated in the table above and reported for this application.
The Panel considers that the information provided on the specifications of the NS is sufficient and does not raise safety concerns.
3.6. History of use of the NS
l‐Methylfolate is the predominant natural form of folates found in food (Friedrich, 1987) and breast milk (Page et al., 2017) and the major form in human plasma (Pfeiffer et al., 2015).
Following the earlier assessment of calcium l‐methylfolate (EFSA AFC Panel, 2004), the NS was authorised on the EU market as source of folate in food for special medical purposes and total diet replacement for weight control and in food supplements and fortified foods.
Calcium l‐methylfolate has a generally recognised as safe (GRAS) status in the USA, where it is also listed as a new dietary ingredient for use in dietary supplements.
l‐5‐Methyltetrahydrofolate, calcium salt is registered as new dietary ingredient in Canada in the Natural Health Products Ingredients Database and l‐5‐methyltetrahydrofolate calcium salt is listed as a permitted form of folic acid for the fortification of specific foods in Australia and New Zealand.
3.7. Proposed uses and use levels and anticipated intake
Calcium l‐methylfolate is intended to be used as a source of folate in infant formula, follow‐on formula, processed cereal‐based food and baby food. Calcium l ‐methylfolate is intended as an alternative to folic acid, currently the only authorised form of folate added to infant formula, follow‐on formula, processed cereal‐based food and baby food.
3.7.1. Target population
The target population proposed by the applicant is infants (< 12 months) and young children (12–< 36 months).
3.7.2. Proposed uses and maximum use levels
The applicant proposes to use calcium l‐methylfolate to meet the compositional requirements for folate in infant formula and follow‐on formula and in processed cereal‐based food and baby food.
Infant formula and follow‐on formula are regulated to contain folate at concentrations of 3.6–11.4 μg DFE/100 kJ or 15–47.6 μg DFE/100 kcal, where 1 μg DFE corresponds to 1 μg of food folate and to 0.6 μg of folic acid2 (see Section 3.8). Based on this, the amount of folic acid needed to meet the compositional requirements for folate in infant formula and follow‐on formula corresponds to 2.2–6.8 μg/100 kJ or 9.0–28.6 μg/100 kcal.
In the case of processed cereal‐based food and baby food for infants and young children, no minimum compositional requirements are set for folic acid,3 but these products are regulated to contain a maximum level of folic acid of 50 μg/100 kcal.
3.7.3. Anticipated intake of the nutrient source
The EFSA AFC Panel concluded that folic acid and calcium l‐methylfolate have similar bioavailability at equimolar doses of supplementation (EFSA AFC Panel, 2004). Therefore, the amount of calcium l‐methylfolate to be added to meet the compositional requirements for folate can be calculated on the basis of the respective molecular weights of calcium l‐methylfolate (497.5 g/mol) and folic acid (441.4 g/mol).
In the case of infant formula and follow‐on formula, the compositional requirements for folate would be met by 2.5–7.7 μg/100 kJ or 10.1–32.2 μg/100 kcal of calcium l‐methylfolate. By applying the energy density value of 280 kJ/100 mL (67 kcal/100 mL) for formula for infants below 16 weeks of age (EFSA Scientific Committee, 2017), infant formula may contain calcium l‐methylfolate up to 21.6 μg/100 mL.
In the case of processed cereal‐based food and baby food for infants and young children, the maximum limit of folic acid permitted is 50 μg/100 kcal, which would correspond to 56 μg calcium l‐methylfolate/100 kcal.
Upon request of EFSA, the applicant provided a refined exposure assessment for the nutrient source in the target population groups.
Infants below 16 weeks of age
The applicant estimated the exposure for a scenario based on the 95th percentile (P95) of formula intake per kg body weight for different age ranges in line with (EFSA Scientific Committee, 2017). Using the average body weights of different age ranges and their infant formula consumption at the P95 level as described in (EFSA Scientific Committee, 2017), the applicant estimated the intakes of folic acid and calcium l‐methylfolate corresponding to their occurrence at the maximum permitted amount of total folate (Table 3).
Table 3.
Estimated intake of folic acid and calcium l‐methylfolate from infant formula by 1‐ to 16‐week‐old infants based on the P95 of formula consumption and maximum permitted total folate levels
| Age (days) | Average body weighta (kg) | P95 formula intakeb | Folic acid intakec (μg/day) | Calcium l‐methylfolate intaked (μg/day) | |
|---|---|---|---|---|---|
| (mL/kg bw per day) | (mL/day) | ||||
| Boys | |||||
| 8–13 | 3.5 | 254 | 889 | 169 | 192 |
| 14–27 | 4.0 | 261 | 1,044 | 198 | 226 |
| 28–41 | 4.6 | 251 | 1,155 | 219 | 250 |
| 42–55 | 5.1 | 238 | 1,214 | 231 | 262 |
| 56–83 | 5.7 | 211 | 1,203 | 229 | 260 |
| 84–111 | 6.5 | 195 | 1,268 | 241 | 274 |
| Girls | |||||
| 8–13 | 3.3 | 251 | 828 | 157 | 179 |
| 14–27 | 3.7 | 257 | 951 | 181 | 205 |
| 28–41 | 4.2 | 253 | 1,063 | 202 | 230 |
| 42–55 | 4.7 | 233 | 1,095 | 208 | 237 |
| 56–83 | 5.3 | 212 | 1,124 | 214 | 243 |
| 84–111 | 6.0 | 195 | 1,170 | 222 | 253 |
Based on WHO Child Growth Standards (https://www.who.int/childgrowth/standards/en/).
Data for formula intake in units of mg/kg body weight (bw)/day based on (EFSA SC, 2017).
Concentration in formula 0.190 μg/mL.
Calculated from folic acid intake.
At the highest permitted level of total folate in infant formula, exclusively formula‐fed infants at the P95 of formula consumption could ingest up to 241 μg/day of folic acid assuming a scenario in which total folate is present exclusively as synthetic folic acid.
Assuming a scenario where calcium l‐methylfolate would replace entirely other sources of folates in infant formula, infants below 16 weeks of age may be exposed to up to 274 μg/day of calcium l‐methylfolate. This intake corresponds to an intake of calcium of 22 μg/day.
Infants above 16 weeks of age and young children
The applicant performed a market survey for follow‐on formula, processed cereal‐based food and baby food in order to gather typical (average) and maximum levels of folates contained in these products. The results are reported in Table 4 expressed as folic acid (i.e. assuming that folates are present exclusively as synthetic folic acid).
Table 4.
Typical and maximum concentrations of folic acid from a market survey for the food categories proposed by the applicant
| Food category | Folic acid (mg/kg) | |
|---|---|---|
| Typical | Maximum | |
| Infant formulae, powder | 0.96 | 1.52 |
| Infant formulae, liquid | 0.12 | 0.19 |
| Follow‐on formulae, powder | 1.04 | 1.52 |
| Follow‐on formulae, liquid | 0.13 | 0.19 |
| Ready‐to‐eat meal for infants and young children | 0 | 0.36 |
| Processed cereal‐based food for infants and young children | 0.08 | 0.30 |
The applicant used these data to perform an exposure assessment of synthetic folates and to simulate the substitution of calcium l‐methylfolate for existing folate formulations. The exposure assessment was based on the summary statistics from the EFSA Comprehensive European Consumption Database, using the CEDEM model (Tennant, 2016) and using the brand‐loyal consumer scenario. The results of this assessment indicated that the highest 95th percentile exposure level in infants and young children was 220 and 140 μg/day of folic acid, respectively.
EFSA performed a detailed assessment for a refined estimate of the anticipated daily intake of folic acid at the maximum proposed concentrations in the different food categories, using individual data from EU dietary surveys (EFSA, 2011). The lowest and highest mean and 95th percentile estimated daily intake of folic acid (on a μg/day basis), among the EU dietary surveys, are presented in Table 5.
Table 5.
Lowest and highest mean and 95th percentile anticipated daily intake of folic acid among the EU dietary surveys for the food categories proposed by the applicant
| Mean exposure lowest (μg/day) | Mean exposure highest (μg/day) | P95 exposure lowest (μg/day) | P95 exposure highest (μg/day) | |
|---|---|---|---|---|
| Infants (< 12 months) | 28 | 109 | 131 | 223 |
| Young children (12–< 36 months) | 3 | 38 | 10 | 187 |
Assuming a scenario where calcium l‐methylfolate would replace entirely other sources of folates in the food categories proposed up to the maximum concentrations described in Table 4, the estimated range of intake of calcium l‐methylfolate for the 95th percentile of the population (calculated from the tabulated intake of folic acid) would be 148–251 μg/day for infants and 11–211 μg/day for young children. This intake corresponds to an intake of calcium of 20 and 17 μg/day for infants and young children, respectively.
Since the NS is intended to replace existing folic acid products in infant formula, follow‐on formula, and cereal‐based food and other baby food to supply folate at identical fortification levels, the overall intake of synthetic folate is not expected to be affected in these population groups.
3.7.4. Combined intake from the nutrient source and other sources
Naturally occurring folates are found in a wide variety of foods that could be fed to infants and young children (EFSA NDA Panel, 2014). Dark green leafy vegetables, legumes, orange and grapefruit (juice), peanuts and almonds are among the principle sources of folate in the diet. Offal such as liver and kidney are particularly high in folate. Baker's yeast is another rich source of folate. Potatoes and dairy products are not considered rich sources of naturally occurring folate but may contribute to folate intake when consumed in relatively large quantities. In European countries with a voluntary folic acid food fortification policy in place, contributions to folic acid intake from the diet may also come from fortified foods, such as breakfast cereals and fat spreads.
Limited information is available on folate intake from the background diet in infants and young children. The EFSA opinion on dietary reference values for folate (EFSA NDA Panel, 2014) reports results on dietary intake of folate for one national survey for infants (0.5 to < 1 year) reporting median DFE intake of 70 μg/day, and two national surveys for young children (1 to < 4 years) reporting median DFE intakes ranging from 111 to 128 μg/day.
3.7.5. Estimate of exposure to undesirable substances
The possible presence of platinum impurities was calculated based on the maximum exposure to calcium l‐methylfolate as a folate source as calculated in Section 3.7.3 and based on the proposed specification limit for platinum in the final product, i.e. 2 mg/kg. From this calculation, the maximum exposure to platinum would be 0.55 ng/day for infants below 16 weeks of age, 0.50 ng/day for infants above 16 weeks of age and 0.42 ng/day for young children.
The European Medicines Agency defined a permitted daily exposure (PDE) for platinum from drug substances (EMA, 2016). The PDE defined for oral exposure to platinum is 108 μg/day when calculated for a 50 kg adult.
The Panel considers that the values reported above for platinum exposure through the NS are of no toxicological concern for the target population groups.
3.8. Absorption, distribution, metabolism and excretion (ADME)
Folate in food is mainly in polyglutamate forms. Before absorption, glutamate residues must be removed from the side chain by conjugases to form folate monoglutamate. Conjugase action may be inhibited by food factors and impaired at acid pH. The bioavailability of ingested folate monoglutamates is therefore significantly greater than that of folate polyglutamate where 1 μg of food folate corresponds to 0.6 μg of folic acid (see Section 3.7.2).
Polyglutamate folate forms are largely hydrolysed in the gut lumen and reduced and formylated or methylated in the enterocytes. Reduced and formylated or methylated forms of folate are transferred faster from the intestine to the circulation than is folic acid (Herbert, 1998; Halstead, 1998; Darcy‐Villon et al., 1988).
In its previous evaluation, EFSA assessed the bioavailability of calcium l‐methylfolate (EFSA AFC Panel, 2004). In aqueous media, calcium l‐methylfolate was found to readily dissociate into Ca and l‐methylfolate ions; l‐methylfolate is subsequently absorbed and enters directly the circulation. The studies assessed in the previous evaluation demonstrate that the bioavailability of l‐methylfolate and folic acid administered to healthy women are comparable (Prinz‐Langenohl et al., 2003, Venn et al., 2002). Based on the information assessed, the EFSA AFC Panel concluded that the bioavailability of calcium l‐methylfolate is similar to that of folic acid. Literature data indicates that the short‐term bioavailability of l‐methylfolate and folic acid are equivalent also when assessed in men (Pentieva et al., 2004).
Since l‐methylfolate is the predominant natural form of folate in foods (Friedrich, 1987) and in breast milk (Page et al., 2017), the Panel considers that the bioavailability of l‐methylfolate is expected to be comparable to that of folic acid also in infants and young children. Further evidence on the bioavailability of the NS in infants is provided in Section 3.10.1.
3.9. Nutritional information
Folate belongs to the group of B‐vitamins and is the generic name for a number of compounds having a similar activity to folic acid, which is essential for the synthesis of ribo‐ and deoxyribonucleic acids (RNA and DNA) and consequently for cell division and tissue growth, methylation reactions and amino acid metabolism (EFSA NDA Panel, 2014). Calcium l‐methylfolate is intended to be used as an alternative to folic acid in infant formula and follow‐on formula and in processed cereal‐based food and baby food (see Section 3.7).
Assuming a scenario where calcium l‐methylfolate would replace entirely other sources of folate in the proposed food categories at the maximum proposed use levels (Section 3.7.3), the highest intake levels of l‐methylfolate and calcium in infants below 16 weeks of age would be 254 and 22 μg/day. Under the same assumptions, the estimated highest intake of l‐methylfolate and calcium for the 95th percentile of the population would be 231 and 20 μg/day for infants and 194 and 17 μg/day for young children.
These amounts provide folate intakes that are above the AI for folate of 80 μg DFE/day for infants aged 7–11 months and the PRI for folate of 120 μg DFE/day for children aged 1–3 years (EFSA NDA Panel, 2014). No UL was set for 5‐methyltetrahydrofolate. With regard to synthetic folic acid, the UL is 200 μg/day for children aged 1–3 years, while no UL is available for infants.
The maximum intake of calcium resulting from the intake assessment is negligible both compared to the DRV for infants aged 7–11 months (280 mg/day; EFSA NDA Panel, 2015) and to the maximum amount of calcium permitted in infant and follow‐on formula (33.5 mg/100 kJ; Commission Delegated Regulation (EU) 2016/127).
The Panel considers that, taking into account the composition of the NS and the proposed conditions of use, consumption of calcium l‐methylfolate is not nutritionally disadvantageous.
3.10. Toxicological information
No new toxicological data were provided by the applicant in the context of the current application.
The Panel notes that the AFC Panel evaluated a battery of toxicological studies submitted to substantiate the safety of calcium l‐methylfolate when used in food for particular nutritional uses, food supplements and foods intended for the general population (EFSA AFC Panel, 2004). All these studies were performed in accordance with OECD guidelines (Niederberger et al., 2019). The studies are listed in Table 6.
Table 6.
List of genotoxicity and toxicity studies performed with calcium l‐methylfolate assessed in (EFSA AFC Panel, 2004)
| Test substance | Reference as in (EFSA AFC Panel, 2004) | Type of study | Tested population | Dose |
|---|---|---|---|---|
| Calcium l‐methylfolate* | Utesch ( 1999) | Bacterial reverse mutation test (Ames test) (OECD TG 471) | Salmonella Typhimurium TA98, TA100, TA102, TA1535 and TA1537 and Escherichia coli strain WP2 uvrA (pKM101) | Up to 5,000 μg/plate (absence and presence of S9 mix) |
| Utesch ( 2000a) | Mouse lymphoma thymidine kinase gene mutation assay (OECD TG 476) | Mouse lymphoma | Up to 5,000 μg/plate (absence and presence of S9 mix) | |
| Howe (2002) | Unscheduled DNA Synthesis (UDS) test (OECD TG 486) | Male Wistar rats | 800 and 2,000 mg/kg bw | |
| Utesch ( 2000bb) | In vivo micronucleus test (OECD TG 474) | Male Wistar rats | 2,000 mg/kg bw by gavage | |
| Heusener and Eberstein ( 1998) | Acute oral toxicity study | 7‐to 8‐week‐old rats (3 rats/sex) | 2,000 mg/kg bw by gavage | |
| Hamann et al. ( 2001) | 90‐day repeated dose oral toxicity study (OECD TG 408) |
Wistar rats For the 90‐day period (10/sex per group) For the 4‐week recovery period (5/sex per group) |
90‐day period: 0, 25, 100 and 400 mg/kg bw per day by gavage Recovery period: 0, 400 mg/kg bw per day by gavage |
|
| Schubert and Jacobs ( 2003) | Embryotoxicity/teratogenicity study (OECD TG 414) |
4 groups of pregnant Wistar rats (25 rats/group) |
0, 100, 300 and 1,000 mg/kg bw from day 5 to 19 of pregnancy |
Purity of the product in line with the specification in the current dossier.
Based on those studies the AFC Panel noted that calcium l‐methylfolate is non‐genotoxic and that subchronic and embryotoxicity/teratogenicity studies in rats did not reveal any adverse effects up to the highest doses tested.
Taking into account the evidence provided by the studies mentioned above and the earlier assessment of the AFC Panel, the Panel considers that no additional toxicological studies are required on the NS.
3.10.1. Human data
The applicant provided the full study report of a monocentric, randomised, double‐blind, parallel‐group, controlled prospective intervention study on healthy term infants later published as (Troesch et al., 2019). The primary objective of the study was to show equivalence of an infant formula containing methyltetrahydrofolate (MTHF) compared to a standard infant formula containing folic acid on weight gain of infants receiving exclusively these infant formulas until age 16 weeks, starting in the first month of life. The source of MTHF in the test formula was calcium l‐methylfolate. Besides the primary objective, recumbent length, head circumference and energy intake estimated via a 3‐day dietary questionnaire, were assessed in both infant formula groups. Exploratory objectives of the study were to compare folate status of the infants among the different feeding groups and to assess the association of the distribution of the MTHFR C677T and A1289C genotypes with the folate status of the infants. Furthermore, the impact of the feeding regimen on safety parameters (blood chemistry, haematology and the occurrence of reported adverse events (AEs) and serious AEs (SAEs)) was assessed, as well as tolerability (stool consistency, stool colour and odour, reflux symptoms, presence or absence of colic, crying, sleeping behaviour) and acceptability of the formula.
The study groups were a control group fed an infant formula containing folic acid in line with the European legislation on infant formula; an intervention group fed an equimolar amount of MTHF instead of folic acid; and a reference group fed breast milk.
At the baseline visit (BV) and at visit 1 (age ~ 4 weeks), 2 (age ~ 8 weeks), 3 (age ~ 12 weeks) and 4 (age ~ 16 weeks) infants were examined and anthropometric data were collected. Blood samples were taken for red blood cell folate (RCF), folate metabolites (methyltetrahydrofolate (MTHF), acetamidobenzoylglutamate (apABG), para‐aminobenzoylglutamate (pABG), formyltetrahydrofolate fTHF), 4‐alpha‐hydroxy‐5‐methyltetrahydrofolate (hmTHF)), blood chemistry (creatinine, alanine aminotransferase (ALAT)) and haematology (haemoglobin, haematocrit, mean corpuscular volume) at BV and at visit 4. In addition, at visit 2, blood and breast milk samples were collected from mothers of infants in the reference group to measure folate status. Adverse events were documented throughout the entire study period.
The study was considered an equivalence trial based on the International Council of Harmonization (ICH) E9 Guideline on Statistical Principles for Clinical Trials (EMA, 1998). As weight gain was the primary objective, the study was powered to detect a difference in weight gain equal to 0.5 standard deviations in compliance with the recommendation laid down by the SCF (2003). To achieve the required power of 80% and the type I error rate of 2.5%, the calculated sample size was 84 infants per group. To account for possible attrition, 120 infants were enrolled in each of the study groups.
The modified intention‐to‐treat population (mITT) comprised all subjects who completed visit 1, as dropouts were not replaced, and consisted of 101, 99 and 115 infants in the control group, intervention group and reference group, respectively. Because of drop‐outs due to protocol deviation (visit window, insufficient aliquots, compliance, blood samples) the number of infants for the per protocol (PP) analyses were 83, 71 and 90 in the control group, intervention group and reference group, respectively.
The response to interventions was examined by a linear model where the confidence interval for the difference of the mean daily weight gain was based on the coefficient for the time‐treatment interaction. Treatment, time in days and the time‐treatment interaction were used as fixed effects, birth weight and sex were used as adjusting covariates and a random intercept and random slope (time) per subject was used. The primary analysis was done by sex and polymorphism subgroup for which the time‐treatment subgroup interaction was added to the model. The 95% CI for the three‐way interaction was used for testing. All endpoints were analysed descriptively in all three groups for both mITT and PP populations.
Based on the outcomes of the equivalence tests performed by the authors, equivalence between the intervention and control groups was demonstrated for weight gain and changes in head circumference. For length gain, equivalence could not be demonstrated probably owing to an insufficient sample size, although the length difference between the two groups at visit 4 was not statistically significant. Neither sub‐group analysis by sex nor by MTHFR C677T and A1289T genotypes revealed group specific effects of the treatment on weight gain. For adjusted means of daily increase in calorie intake, no statistically significant difference between the groups at visit 4 was found. No differences between the two formula groups regarding feeding‐related behaviour were found.
At the end of the study, the majority of markers for folate status did not differ statistically between the intervention group and the control group; however, the adjusted plasma folic acid concentration was found to be higher in the control group, presumably due to the fact that the intervention group received methyl‐folate and not folic acid, while the total red blood cell folate (RCF) concentration was higher in the intervention group (Table 7) indicating that the calcium l‐methylfolate might be more bioavailable than folic acid.
Table 7.
Folate markers at visit 4 in the PP population
| Control group | Intervention group | Reference (breastfeed) group | ||
|---|---|---|---|---|
| l‐5‐MTHF [nmol/L] | N | 79 | 68 | 89 |
| Mean | 52.70 | 55.34 | 32.95 | |
| SD | 18.97 | 18.05 | 17.59 | |
| Folic acid [nmol/L] | N | 74 | 23 | 27 |
| Median | 1.15 | 0.73 | 0.74 | |
| P25 | 0.92 | 0.60 | 0.62 | |
| P75 | 1.36 | 1.00 | 0.87 | |
| p | < 0.0001 | |||
| fTHF [nmol/L] | < LOQ | < LOQ | < LOQ | |
| hmTHF [nmol/L] | N | 79 | 68 | 89 |
| Mean | 5.77 | 6.57 | 4.00 | |
| SD | 3.04 | 3.48 | 2.69 | |
| pABG [nmol/L] | N | 79 | 68 | 89 |
| Mean | 19.39 | 20.12 | 15.76 | |
| SD | 9.39 | 9.49 | 10.83 | |
| apABG [nmol/L] | N | 79 | 68 | 89 |
| Mean | 0.74 | 0.72 | 0.62 | |
| SD | 0.18 | 0.17 | 0.28 | |
| Total RCF [nmol/L] | N | 80 | 70 | 88 |
| Mean | 839.40 | 906.99 | 484.18 | |
| SD | 142.41 | 192.81 | 212.98 | |
| p | < 0.02 |
apABG: acetamidobenzoylglutamate; pABG: para‐aminobenzoylglutamate; fTHF: formyltetrahydrofolate; hmTHF: 4‐alpha‐hydroxy‐5‐methyltetrahydrofolate; LOQ: limit of quantification; l‐5‐MTHF: l‐5‐methyltetrahydrofolate; N: number of subjects; p: p‐value [reported only when significant] (For MTHF: t‐test for equal variance; for folic acid, hmTHF, pABG, apABG: Mann‐Whitney‐U test; for RCF: t‐test for unequal variance; p < 0.05 is considered significant); RCF: red blood cell folate; SD: standard deviation.
No difference in the frequency of AEs was observed between the intervention and the control group, while there were slightly more AEs reported in the reference group. The authors concluded that the large majority of AEs was unrelated to the intervention and of the kind to be expected in a population of young infants. Only three AEs were judged to be related to the feeding regime: one in the reference group (poor growth), one in the intervention group (formula not tolerated well) and one in the control group (overweight at visit 4).
Regarding the safety parameters assessed (serum creatinine, alanine aminotransferase, haemoglobin, haematocrit and mean corpuscular volume) at visit 4 the majority of the mean values were within the normal range for this age group and the values comparable among groups.
The Panel notes that overall the study did not indicate differences in growth and tolerance parameters in infants who consumed either an infant formula supplemented with the NS or with folic acid at equimolar doses. Neither the adverse events nor the blood chemistry and haematology raised any concern regarding safety or tolerability of the infant formula with the NS.
The Panel notes that the NS is a bioavailable form of folate in the tested population group.
The Panel concludes that the available human data do not raise safety concerns.
3.11. Allergenicity
The Panel considers that, owing to the absence of protein, the NS is unlikely to trigger allergic reactions in the target population under the proposed conditions of use.
4. Discussion
This opinion deals with the safety and bioavailability of calcium l‐methylfolate as a new source of folate added for nutritional purposes to infant formula, follow‐on formula, baby food and processed cereal‐based food. In 2004 EFSA issued an opinion on calcium l‐methylfolate (EFSA AFC Panel, 2004) produced by the same manufacturer, concluding that its use as a source of folate in foods for particular nutritional uses, food supplements and foods intended for the general population, with a tolerable upper level of 1 mg/adult person per day, is not of concern from a safety point of view.
Calcium l‐methylfolate is produced by a three‐step chemical synthesis starting from folic acid. The manufacturing process is the same as the one assessed by EFSA in the opinion of 2004, apart from the first synthetic step where a catalytic hydrogenation, using platinum as a catalyst, can be used as alternative to the sodium borohydride reduction step assessed in the previous opinion (both options are possible). The second and third synthetic steps are equivalent to those previously assessed. Based on (i) the content of the residual platinum catalyst, (ii) the overall impurity profile including the compliance with the same product specifications and (iii) the stability of the different production batches produced with the catalytic hydrogenation, the Panel considers that the production process is sufficiently described and does not raise safety concerns.
Based on the proposed specification limit for platinum in the final product, the maximum exposure to platinum would be 0.55 ng/day for infants below 16 weeks of age, 0.50 ng/day for infants above 16 weeks of age and 0.42 ng/day for young children. In the light of the permitted oral daily exposure for platinum from drug substances defined by EMA for adults, i.e. 108 μg/day when calculated for a 50‐kg adult, the Panel considers that the exposure to platinum resulting from the intake of calcium‐l‐methylfolate is not of toxicological concern for the target population groups.
In the previous evaluation, the EFSA AFC Panel indicated that calcium l‐methylfolate readily dissociates into Ca and l‐methylfolate ions in aqueous media and that l‐methylfolate is subsequently absorbed and enters directly the circulation. The AFC Panel concluded that the bioavailability of calcium l‐methylfolate is similar to that of folic acid in adults. Since l‐methylfolate is the predominant natural form of folates in foods and in breast milk the Panel considers that the bioavailability of l‐methylfolate is expected to be comparable to that of folic acid also in infants and young children.
Based on the studies assessed in the previous evaluation, the EFSA AFC Panel concluded that calcium l‐methylfolate is non‐genotoxic and that subchronic and embryotoxicity/teratogenicity studies in rats did not reveal any adverse effects up to the highest doses tested. The Panel considers that no additional toxicological studies are required on the NS.
Having assessed the intervention study in healthy infants provided by the applicant, the Panel notes that overall the study did not indicate differences in growth and tolerance parameters in infants who consumed either an infant formula supplemented with the NS or with folic acid, and that no concerns were raised regarding safety or tolerability of the infant formula with the NS. The study also provided supporting evidence for the bioavailability of the NS.
5. Conclusions
The Panel considers that calcium l‐methylfolate is a source from which folate is bioavailable.
The Panel concludes that the NS, calcium l‐methylfolate, is safe under the proposed uses and use levels for infants and young children.
Steps taken by EFSA
Letter from the European Commission to the European Food Safety Authority with the request for a scientific opinion on the safety of calcium L‐methylfolate as a source of folate added for nutritional purposes to infant and follow‐on formula, baby food and processed‐cereal based food. Ref Ares(2018)5464074, dated 25 October 2018.
On 31 October 2018, a valid application on Calcium L‐methylfolate, which was submitted by DSM Nutritional Products France, was made available to EFSA by the European Commission and the scientific evaluation procedure was initiated.
On 18 July 2019, EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
On 9 September 2019, additional information was provided by the applicant and the scientific evaluation was restarted.
During its meeting on 27 November 2019, the NDA Panel, having evaluated the data, adopted a scientific opinion on the safety and bioavailability of Calcium L‐methylfolate as a NS pursuant to Regulation (EU) 609/2013.
Abbreviations
- ADME
absorption, distribution, metabolism and excretion
- AE
adverse event
- AI
adequate intake
- ALAT
alanine aminotransferase
- apABG
acetamidobenzoylglutamate
- AR
average requirement
- BV
baseline visit
- BW
body weight
- DFE
dietary folate equivalent
- DRV
dietary reference value
- GMP
good manufacturing practice
- GRAS
generally recognized as safe
- fTHF
formyltetrahydrofolate
- HACCP
hazard analysis critical control points
- hmTHF
4‐alpha‐hydroxy‐5‐methyltetrahydrofolate
- HPLC
high‐performance liquid chromatography
- IR
infrared
- LOQ
limit of quantification
- MTHF
methyltetrahydrofolic acid
- mITT
modified intention‐to‐treat
- NMR
nuclear magnetic resonance
- NOAEL
no observed adverse effect level
- NS
nutrient source
- pABG
para‐aminobenzoylglutamate
- PDE
permitted daily exposure
- PP
per protocol
- PRI
population reference intake
- RCF
red blood cell folate
- RH
relative humidity
- SAE
serious adverse event
- UL
tolerable upper level
- UV
ultraviolet
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Castenmiller J, De Henauw S, Hirsch‐Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Engel K‐H, Frenzel T, Heinonen M, Marchelli R, Neuhäuser‐Berthold M, Poulsen M, Sanz Y, Schlatter JR, van Loveren H, Bernasconi G, Germini A and Knutsen HK, 2020. Scientific Opinion on calcium l‐methylfolate as a source of folate added for nutritional purposes to infant and follow‐on formula, baby food and processed cereal‐based food. EFSA Journal 2020;18(1):5947, 17 pp. 10.2903/j.efsa.2020.5947
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00816
Panel members: Dominique Turck, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, John Kearney, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Adopted: 27 November 2019
Notes
OJ L 181, 29.6.2013, p. 35.
Commission Delegated Regulation (EU) 2016/127 of 25 September 2015, OJ L25, 2.2.2016, p. 1–29.
Commission Directive 2006/125/EC. OJ L 339, 6/12/2006 p. 16–35.
References
- Darcy‐Villon B, Selhub J and Rosenberg IH, 1988. Analysis of sequential events in intestinal absorption of folylpolyglutamate. American Journal of Physiology, 255(3 Pt 1), G361‐6. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA AFC Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2004. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to Calcium l‐Methylfolate. EFSA Journal 2004;2(11);135, 20 pp. 10.2903/j.efsa.2004.135 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2013. Scientific Opinion on (6S)‐5‐methyltetrahydrofolic acid, glucosamine salt as a source of folate added for nutritional purposes to food supplements. EFSA Journal 2013;11(10):3358, 20 pp. 10.2903/j.efsa.2013.3358 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gundert‐Remy U, Kuhnle GG, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Di Domenico A, Fairweather‐Tait S, McArdle H, Smeraldi C and Gott D, 2018. Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources. EFSA Journal 2018;16(6):5294, 35 pp. 10.2903/j.efsa.2018.5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014. Scientific Opinion on Dietary Reference Values for folate. EFSA Journal 2014;12(11):3893, 59 pp. 10.2903/j.efsa.2014.3893 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific Opinion on Dietary Reference Values for calcium. EFSA Journal 2015;13(5):4101, 82 pp. 10.2903/j.efsa.2015.4101 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V,Solecki R, Turck D, Bresson J‐L, Dusemund B, Gundert‐Remy U, Kersting M, Lambré C, Penninks A, Tritscher A, Waalkens‐Berendsen I, Woutersen R, Arcella D, Court Marques D, Dorne J‐L, Kass GEN and Mortensen A, 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (European Medicines Agency), 1998. ICH Topic E 9. Statistical Principles for Clinical Trials. September 1998. CPMP/ICH/363/96. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-9-statistical-principles-clinical-trials-step-5_en.pdf
- EMA (European Medicines Agency), 2016. Committee for Human Medicinal Products. 2016. ICH guideline Q3D on elemental impurities. 25 July 2016. EMA/CHMP/ICH/353369/2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-13.pdf
- Friedrich W, 1987. Handbuch der Vitamine. Urban & Schwarzenberg. [Google Scholar]
- Halstead CH, 1998. Folylpoly‐y‐glutamate carboxypeptidase In: Barret A. and Woessner F. (eds.). Handbook of proteolytic enzymes. Academic Press, USA. 4094 pp. [Google Scholar]
- Hamann H‐J, Luetkemeier H, Knuppe C and Millar PM, 2001. Calcium‐L‐Mefolinate (L‐MTHF): 13‐week oral toxicity (gavage) study in Wistar rats. RCC Project 758316. Unpublished study report of RCC Ltd., Itingen (Switzerland) for Merck KgaA, Darmstadt (Germany).
- Herbert V, 1998. Folic acid In: Shils ME, Olson JA, Shike M. and Ross AC. (eds.). Modern Nutrition in Health and Disease, 9th Edition Williams & Wilkins, Philadelphia, Pennsylvania, USA: pp. 433–466. [Google Scholar]
- Heusener A and von Eberstein M, 1998. Calcium‐L‐Mefolinat ‐ Acute toxicity study in rats after oral administration (ATC method). Unpublished study report for Eprova AG, Schaffhausen (Switzerland).
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2005. Joint FAO/WHO Expert Committee on Food Additives (JECFA) specifications for Calcium L‐5‐Methyltetrahydrofolate. Prepared at the 65th meeting. Available online: http://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-090.pdf
- Niederberger KE, Dahms I, Broschard TH, Boehni R and Moser R, 2019. Safety evaluation of calcium L‐methylfolate. Toxicology Reports, 6, 1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R, Robichaud A, Arbuckle TE, Fraser WD and MacFarlane AJ, 2017. Total folate and unmetabolized folic acid in the breast milk of a cross‐section of Canadian women. The American Journal of Clinical Nutrition, 105, 1101–1109. 10.3945/ajcn.116.137968 [DOI] [PubMed] [Google Scholar]
- Pentieva K, McNulty H, Reichert R, Ward M, Strain JJ, McKillop DJ, McPartlin JM, Connolly E, Molloy A, Krämer K and Scott JM, 2004. The short‐term bioavailabilities of [6S]‐5‐methyltetrahydrofolate and folic acid are equivalent in men. The Journal of Nutrition, 134, 580–585. 10.1093/jn/134.3.580 [DOI] [PubMed] [Google Scholar]
- Pfeiffer CM, Sternberg MR, Fazili Z, Lacher DA, Zhang M, Johnson CL, Hamner HC, Bailey RL, Rader JI, Yamini S, Berry RJ and Yetley EA, 2015. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Survey 2011‐2. British Journal of Nutrition, 113, 1965–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz‐Langenohl R, Lamers Y, Moser R and Pietrzik K, 2003. Effect of folic acid preload on the bioequivalence of [6S]‐5‐methyltetrahydrofolate and folic acid in healthy volunteers. Journal of Inherited Metabolic Disease, 26(Suppl. 1), 124. [Google Scholar]
- SCF (Scientific Committee on Food), 2000. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of folate. SCF/CS/NUT/UPPLEV/18 Final, 9 pp.
- SCF (Scientific Committee on Food), 2003. Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow‐on Formulae. European Commission. Brussels, Belgium.
- Schubert Ch and Jacobs M, 2003. Art. 100461 (Metafolin)‐Prenatal developmental toxicity study after oral administration to rats. Unpublished study report of Merck KgaA, Darmstadt, Germany for Merck Eprova AG, Schaffhausen, Switzerland.
- Tennant DR, 2016. Comprehensive European dietary exposure model (CEDEM) for food additives. Food Additives & Contaminants: Part A, 33, 772–781. [DOI] [PubMed] [Google Scholar]
- Troesch B, Demmelmair J, Gimpfl M, Hecht C, Lakovic G, Roehle R, Sipka L, Trisic B, Vusurovic M, Schoop R, Zdjelar S and Koletzko B, MEFOLIN Study Group , 2019. Suitability and safety of L‐5‐methyltetrahydrofolate as a folate source in infant formula: A randomized‐controlled trial. PLoS One, 14(8), e0216790 10.1371/journal.pone.0216790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utesch D, 1999. Calcium‐L‐Mefolinat ‐ Bacterial mutagenicity assay, Salmonella typhimurium and Escherichia coli. Unpublished study report for Eprova AG, Schaffhausen (Switzerland).
- Utesch D, 2000a. Calcium‐L‐Mefolinat ‐ In vitro mammalian cell gene mutation test (L5178Y/TK+/‐). Unpublished study report for Merck KGaA, Darmstadt (Germany).
- Utesch D, 2000b. Calcium‐L‐Mefolinat ‐ Micronucleus test in male rats after oral administration. Unpublished study report for Merck KGaA, Darmstadt (Germany).
- Venn BJ, Green TJ, Moser R, McKenzie JE, Skeaff CM and Mann J, 2002. Increases in blood folate indices are similar in women of childbearing age supplemented with [6S]‐5‐methyltetrahydrofolate and folic acid. Journal of Nutrition, 132, 3353–3355. [DOI] [PubMed] [Google Scholar]


