Abstract
The AGRI committee of the European Parliament requested EFSA to assess the welfare of rabbits farmed in different production systems, including organic production, and to update its 2005 scientific opinion about the health and welfare of rabbits kept for meat production. Considering reproducing does, kits and growing rabbits, this scientific opinion focusses on six different housing systems, namely conventional cages, structurally enriched cages, elevated pens, floor pens, outdoor/partially outdoor systems and organic systems. To compare the level of welfare in the different housing systems and rabbit categories, welfare impact scores for 20 welfare consequences identified from the literature were calculated, taking their occurrence, duration and severity into account. Based on the overall welfare impact score (sum of scores for the single welfare consequences), obtained via a 2‐step expert knowledge elicitation process, the welfare of reproducing does is likely (certainty 66–90%) to be lower in conventional cages compared to the five other housing systems. In addition, it is likely to extremely likely (certainty 66–99%) that the welfare of kits is lower in outdoor systems compared to the other systems and that the welfare is higher in elevated pens than in the other systems. Finally, it is likely to extremely likely (certainty 66–99%) that the welfare of growing rabbits is lower in conventional cages compared to the other systems and that the welfare is higher in elevated pens than in the other systems. Ranking of the welfare consequences allowed an analysis of the main welfare consequences within each system and rabbit category. It was concluded that for reproducing does, as well as growing rabbits, welfare consequences related to behavioural restrictions were more prominent in conventional cages, elevated pens and enriched cages, whereas those related to health problems were more important in floor pens, outdoor and organic systems. Housing in organic rabbit farming is diverse, which can result in different welfare consequences, but the overall welfare impact scores suggest that welfare in organic systems is generally good.
Keywords: animal welfare, rabbit, reproducing doe, housing system, organic farming
Summary
Council Directive 98/58/EC lays down the minimum standards for the protection of farm animals, including rabbits. Beyond this Directive, there is no specific legislation for protecting the welfare of rabbits used for farming purposes at the European Union (EU) level. Therefore, the AGRI committee of the European Parliament requested the European Food Safety Authority (EFSA) to update its 2005 scientific opinion about the health and welfare of rabbits in Europe kept for meat production. The mandate also requested an assessment of the welfare of rabbits farmed in different production systems, including organic production systems, by considering the impact of all aspects related to housing, rearing and nutrition on rabbit welfare.
To respond to the mandate, at the end of 2018, EFSA set up a working group of European experts on different aspects of rabbit welfare including health. Rabbit farming takes place mainly in five member states of the EU: France, Hungary, Italy, Portugal and Spain. Both between and within countries the practices used for farming rabbits vary widely. To represent some of this variability, this scientific opinion focussed its assessment on six different housing systems: (1) conventional cages, (2) structurally enriched cages, (3) elevated pens, (4) floor pens, (5) outdoor/partially outdoor systems and (6) organic systems.
The assessment considered three animal categories: (i) reproducing does (from first kindling till culling); (ii) kits (from birth to weaning) and (iii) growing rabbits (from weaning to slaughter age).
A literature review of the available scientific evidence on the welfare of farmed rabbits identified 20 welfare consequences. Comparison of the level of welfare in the different housing systems was based on the calculation of an overall welfare impact score, taking into account the occurrence, duration and severity of the 20 welfare consequences. However, such data could not be fully retrieved from the literature, as comprehensive publications on farmed rabbit welfare are scarce and they rarely include quantitative information on these parameters. Therefore, an expert knowledge elicitation (EKE) process was implemented by EFSA to fill the gap and increase the validity of the qualitative knowledge found in literature. A 2‐step EKE process was thus used:
a survey was sent to 122 rabbit experts in the EU out of 135 which had expressed their interest in such a survey. The respondents (n = 88) estimated occurrence and duration of the 20 welfare consequences separately for the three rabbit categories in one or two of the six housing systems each, resulting in a total of 125 completed surveys. Occurrence referred to the proportion of all rabbits of a given category that are impaired by the stated welfare consequence at least once over their lifetime in this production stage (scale 0–1). Duration referred to the cumulative proportion of time that an average individual rabbit's welfare is impaired by the consequence in its lifetime in this production stage (scale 0–1).
an EKE workshop involving eight external experts and three hearing experts was carried out to assess the severity of the welfare consequences. Severity was defined as the level of distress and suffering (scale 0–10 with 10 as the score expressing maximum distress) that is caused by a given related welfare consequence.
The values for occurrence, duration and severity obtained from the EKE survey and workshop were used to derive welfare impact scores for each welfare consequence, and these were summed to give an overall welfare impact score for each system, with a higher score indicative of poorer welfare. The overall impact welfare score was used to derive the conclusions related to the welfare comparison among systems.
The results show that it is likely (certainty 66–90% based on probabilistic analysis of expert opinion), that the welfare of reproducing does is lower in conventional cages (median overall impact score: 3.2 with 90% probability interval of 1.8–5.4) compared to the five other housing systems (medians ranging between 1.8 [90% probability interval 1.0–3.3] and 2.3 [90% probability interval 1.2–4.0]). However, among the other systems no distinction can be made regarding the welfare impact on does.
In addition, it is likely to extremely likely (certainty 66–99%) that the welfare of kits is lower in outdoor systems (median overall impact score: 2.6 with 90% probability interval of 1.8–3.7) compared to the other systems and that the kit welfare is higher in elevated pens (median overall impact score: 1.0 with 90% probability interval of 0.4–1.9) than in the four other systems (medians ranging between 1.3 [90% probability interval 0.5–2.4] and 1.6 [90% probability interval 0.8–2.9]). However, no distinction can be made among the conventional cages, enriched cages, floor pens and organic systems regarding the welfare impact on kits.
Finally, it is likely to extremely likely (certainty 66–99%) that the welfare of growing rabbits is lower in conventional cages (median overall impact score: 3.5 with 90% probability interval of 2.1–5.9) but higher in elevated pens (median impact score: 1.0 with 90% probability interval of 0.5–2.0) compared to the other systems (medians ranging between 1.2 [90% probability interval 0.7–2.1] and 2.6 [90% probability interval 1.4–4.7]). However, no distinction can be made among the enriched cages, floor pens, organic systems and outdoor systems regarding the welfare impact on growing rabbits.
Additional tables present the top 5 welfare consequences for each system and for each animal category and allowed an analysis of the main welfare consequences within each system.
The outcomes of the assessment also highlighted possible welfare consequences in different rabbit categories. For instance, for reproducing does, restriction of movement gave the highest welfare impact scores and this welfare consequence (impact score: 0.87) together with lack of possibility for gnawing behaviour and hunger, made the greatest contribution to the higher impact score in conventional cages. For kits, heat stress gave the highest welfare impact scores (impact score: 0.45) and this welfare consequence, together with neonatal disorders and cold stress, made the greatest contribution to the higher impact score in outdoor systems. For growing rabbits, restriction of movement gave the highest welfare impact scores (impact score: 1.29). This welfare consequence, together with inability to perform gnawing behaviour and resting problems, made the greatest contribution to the higher impact score in conventional cages. Recommendations to address each of these welfare consequences are given in the opinion.
It was also concluded that for reproducing does, as well as growing rabbits, welfare consequences related to behavioural restrictions were more prominent in conventional cages, elevated pens and enriched cages, whereas those related to health problems occurred more often in floor pens, outdoor and organic systems.
Housing in organic rabbit farming is diverse, for example either movable cages or individual paddocks can be used for does. Therefore, organic rabbit farming can – according to the systems used ‐ result in different welfare consequences. Nevertheless, welfare impact scores given by experts suggest that welfare in organic systems is generally good.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The Coordinators of the AGRI committee endorsed a request for two scientific opinions by the European Food Safety Authority (EFSA) on the health and welfare of rabbits kept for meat production in Europe. This request is submitted in accordance with Article 29 of Regulation 178/2002 on “laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety”, which provides that the European Parliament may request the Authority to issue a scientific opinion on matters falling within the Authority's mission.
The request is introduced taking into account the worldwide importance of rabbit farming for meat production, including in the EU where rabbits are the second most farmed species in terms of numbers. Council Directive 98/58/EC1 lays down the minimum standards for the protection of animals kept for farming purposes, including rabbits, but there is no species‐specific legislation protecting the welfare of farmed rabbits in the EU. The European Animal Welfare Strategy 2012–2015 recommended that existing legislation should be fully implemented before introducing more legislation. However, the developmesnt of guides to best practice should be encouraged. Meanwhile, international non‐governmental organisations, stakeholders and consumer association have raised serious concerns regarding the poor welfare, high stress levels and high mortality and morbidity rates of rabbits farmed in Europe. Other concerns relate to the electrical stunning of rabbits often not rendering the animals fully unconscious and thus leading to pain, stress and suffering.
On 14 March 2017, Parliament adopted a resolution on minimum standards for the protection of farm rabbits, on the basis of a report initiated by the AGRI committee (2016/2077(INI) – rapporteur Stefan Eck). The AGRI Committee had proposed, among others, that the setting of minimum standards for the protection of farm rabbits could be assisted by an independent scientific opinion from EFSA.
In 2005 and 2006, EFSA published scientific opinions on (i) the impact of housing and husbandry systems on the health and welfare of farmed domestic rabbits2 and (ii) welfare aspects of the main systems of stunning and killing of farmed deer, goats, rabbits, ostriches, ducks and geese,3 respectively. More scientific studies on rabbit health and welfare became available in recent years. Hence, there is a need to update the EFSA assessments with view to the latest available scientific evidence.
The AGRI committee, therefore, considers it opportune for the Parliament to request EFSA to update its scientific opinions on different aspects of health and welfare of rabbits kept for meat production in Europe.
In particular, two scientific opinions should be developed addressing the following Terms of Reference (ToRs):
Scientific opinion on health and welfare of rabbit farmed in different production systems, including organic production systems. This will include all aspects related to housing, rearing and nutrition and the effects thereof on rabbit health, welfare and behaviour. Interactions between the different areas will also be addressed.
Scientific opinion on stunning and killing methods for rabbits. This will include the indication of the most suitable method for stunning and killing of rabbits, including indicators to assess unconsciousness and death of the animals.
This scientific opinion relates to health and welfare of rabbit farmed in different production systems.
1.2. Interpretation of the Terms of Reference
The EFSA Scientific Opinion EFSA‐Q‐2004‐023 (EFSA, 2005) served as basis for this opinion. This means that, starting from the state of the art in 2005, scientific literature published since 2005 was primarily considered.
Only rabbits bred and reared for meat production are considered in this opinion, and not those kept for other commercial purposes such as fur, or for research purposes or as pets. However, scientific literature, e.g. from laboratory or pet rabbits may also be referred to, provided that findings are applicable to farmed rabbits. While several animal categories can be distinguished in rabbit farming according to age, sex and reproductive stage (breeding does, breeding bucks, non‐conceiving adult does, young females/males for breeding, kits, growing rabbits), this opinion focuses on breeding does, kits and growing rabbits. These animal categories are by far the largest in terms of animal numbers, and findings partly also apply to the animal categories not specifically addressed due to similar features of the housing systems they are usually kept in and similar biological requirements (e.g. growing rabbits vs. young females/males for breeding).
The ToR request an opinion on health and welfare of rabbits farmed in different production systems. Animal production systems are characterised by complex interactions of many different components such as housing, feeding, breeding and health management. Housing systems are easiest to identify and describe, and so this opinion centres around six different housing systems, ranging from systems frequently found in current intensive rabbit production to alternative systems, including some still in the implementation phase for future adoption and organic farming. The possible interaction effects with other factors are taken into account by describing the management routines (including e.g. breeds/strains, ventilation systems, feeding, reproductive management used) most commonly found associated with the respective housing systems, and by separately considering each of these factors in the risk analyses for each of the different welfare consequences.
To address the ToR, this opinion progresses through a series of stages:
a description of the range of rabbit production systems in current use or under development, including organic systems;
the identification of the possible health‐ and behaviour‐related welfare consequences which might arise from differences in rabbit production systems;
a comparison of six housing systems in terms of their effects on these welfare consequences for the animals; scores for each of the welfare consequences were combined to produce an overall welfare impact score. Also, the five welfare consequences ranking highest in each system were identified;
a review of the hazards for these welfare consequences, including other aspects of production management that can be influential.
2. Data and methodologies
2.1. Methodologies
2.1.1. Approach
This opinion focuses on a range of welfare consequences originating from different housing systems in which various management practices are considered.
The target animal populations are breeding does, kits and growing rabbits kept in six different housing systems (conventional cages, structurally enriched cages, elevated pens (indoor parks), floor pens (indoor parks), outdoor/partially outdoor systems and organic systems). Animal categories are described in Section 3.2.2 and a description of the housing systems is provided in Section 3.3.5.
The working group identified 21 possible welfare consequences for farmed rabbits (see Sections 3.4.2 and 3.6). Two of these welfare consequences (metabolic disorders and pain) were considered to be mainly the result of others and therefore not assessed independently. One welfare consequence (thermal stress) was subdivided into heat stress and cold stress; therefore in total, 20 welfare consequences were subsequently used in the analysis and comparison of housing systems.
To compare the level of rabbit welfare in different housing systems, the occurrence, duration and severity of these 20 welfare consequences were assessed ‐ as suggested in the EFSA guidance on Risk Assessment for animal welfare (EFSA AHAW Panel, 2012) – and combined in an overall welfare assessment score.
In this project, for the generation of data on occurrence, duration and severity of welfare consequences, a 2‐step expert knowledge elicitation (EKE) process was used (see Section 2.1.2):
a technical workshop (EKE – Sheffield method) where a formal exercise of EKE was carried out regarding the severity of the welfare consequences for rabbits. Details are given in Section 2.1.2.1.
a survey was carried out to obtain judgements on the occurrence and duration of the 20 welfare consequences for rabbits in the six different housing systems. Details are given in Section 2.1.2.2.
Hazards for each welfare consequence were investigated by review of the scientific literature. These hazards can be linked to the management practices most commonly associated with the respective production systems and can be grouped into major categories: housing features, ambient conditions, genetics, nutrition and feeding, biosecurity, management of reproduction and others (EFSA AHAW Panel, 2012). The review focussed primarily on literature published since 2005, to update the previous EFSA Opinion. Each welfare consequence is described in Section 3.6 together with the identification and description of its main associated hazards.
A schematic representation of the conceptual model for the development of the scientific opinion is presented in Figure 1, where the various elements needed for the assessment are indicated as well as the activities necessary to retrieve information. Uncertainty analysis is performed to give decision‐makers a clear picture of the scientific uncertainties affecting each assessment (see Section 2.1.4).
Figure 1.
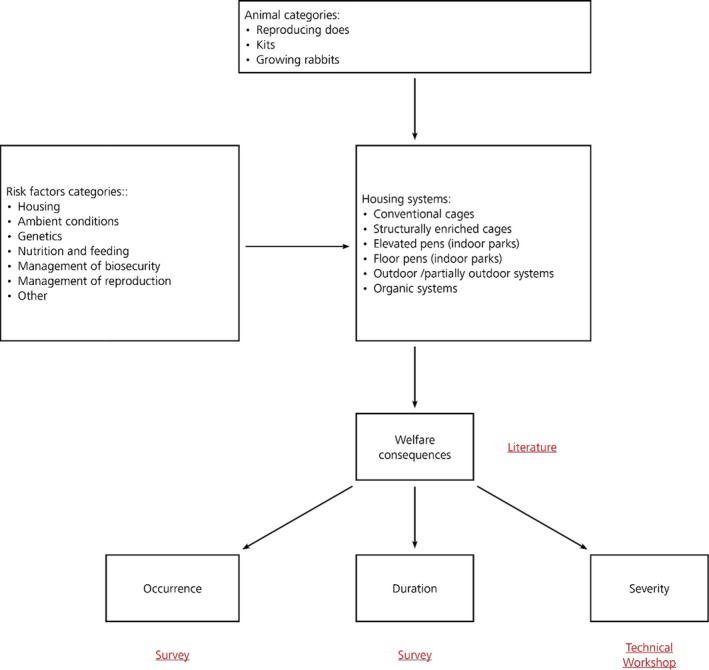
Conceptual model for the development of the opinion, including activities necessary to retrieve the information and type of uncertainty assessment
2.1.2. Expert knowledge elicitation (EKE)
Owing to the scarcity of scientific literature from which to derive data for quantitative risk assessment related to welfare consequences in rabbit production, an EKE was employed. A formal EKE is a systematic, documented and reviewable process to retrieve expert judgements from a group of experts, often in the form of a probability distribution.
The EFSA Guidance on EKE (EFSA 2014) provides detailed protocols for obtaining expert judgement in the areas covered by EFSA's food safety remit.
Several EKE methods exist to elicit expert judgement. In this opinion, two methods were employed:
-
–
Sheffield method: is designed to be employed to elicit the knowledge of a group of experts in a face‐to‐face elicitation workshop, with the result being a distribution representing the aggregated judgements of the experts.
-
–
Survey: is essentially a method that has the advantage of providing a quick feedback from the involved experts. The group judgement is obtained by aggregating the different judgements obtained from the survey responses. This method was used to derive estimates for the occurrence and duration of the welfare consequences.
2.1.2.1. Technical workshop (EKE Sheffield method)
A technical workshop was held following the Sheffield method for EKE to estimate the severity of the 20 welfare consequences for rabbits through expert judgement (see Section 2.1.1). The 20 welfare consequences were divided into behaviour related and health related, as shown in Table 1. The workshop was held on 1–2 April 2019. Eight external experts were selected based on the expertise needed for the exercise. The required expertise was related to the practical experience with evaluation of different dimensions of health and welfare of rabbits across the six housing systems. Additionally, three members of the working group with relevant expertise participated in the exercise.
Table 1.
List of 20 welfare consequences grouped in behaviour‐ and health‐related welfare consequences
| Behaviour‐related welfare consequences | Health‐related welfare consequences |
|---|---|
| Restriction of movement | Prolonged hunger |
| Resting problem | Prolonged thirst |
| Inability to express maternal behaviour | Pododermatitis |
| Inability to express positive social behaviour | Locomotory disorders |
| Inability to express gnawing behaviour | Skin lesions |
| Occurrence of abnormal behaviour | Respiratory disorders |
| Fear | Gastrointestinal disorders |
| Skin disorders | |
| Reproductive disorders | |
| Mastitis | |
| Neonatal disorders | |
| Heat stress | |
| Cold stress |
The mandate as well as the EKE principles were presented to the experts by the elicitor. After this, there was clarification of the definitions of the welfare consequences that had to be judged on their severity by the experts.
The severity of a welfare consequence was assessed as univocal; that is, based on the experience of the animal and independent of the housing system. Only direct consequences for animal welfare were considered. For instance, mastitis in a doe indirectly affects the welfare of kits – but it was not considered as a consequence for kits.
The EKE was divided into two exercises:
The experts ranked the welfare consequences that were related to behaviour according to the severity that would be experienced by the rabbit doe relative to each other, and then repeated this process for the consequences related to health. Following viewing and discussion of the initial rankings, experts had the possibility to revise their opinion.
Based on the previous relative rankings, experts merged the two sets of welfare consequences and scored them on a severity scale from 0 to 10. In this scale, score 0 corresponds to no distress and suffering at all or corresponds to those welfare consequences that might not be relevant for a certain animal category, such as neonatal disorders for the reproducing doe or mastitis for kits. Score 10 indicates the maximum level of suffering of a rabbit that can be imagined by the experts.
These two steps were then repeated to score the severity of the welfare consequences for kits and growing rabbits.
2.1.2.2. Survey
A survey was carried out to estimate the occurrence and duration of the 20 welfare consequences for rabbits (see Section 3.5.2) through expert judgement. One hundred and fifty‐four experts, mostly from the European Union (EU), with competence in different areas of rabbit production – including researchers, official veterinarians, farm consultants and industry technicians – were contacted to inform them about the survey and to indicate their competence about the six housing systems (conventional cages, structurally enriched cages, elevated pens, floor pens, outdoor/partially outdoor systems, organic systems).
One hundred and thirty‐five experts expressed their interest in completing the survey. To ensure feasibility of the survey, it was decided to request completion for a maximum of two housing systems per respondent. Some experts indicated experience only related to one of the systems for which a high number of respondents were expected and therefore were not involved further in the survey (9 experts for conventional cages and 4 for enriched cages). Therefore, the survey was sent to a total of 122 experts.
Aiming at an adequate distribution of responses among the systems, the two surveys per respondent were distributed to the experts according to an algorithm that combined the need to cover the systems for which fewer experts were available (i.e. organic and outdoor systems), then the second least abundant (i.e. floor pens, elevated pens) and finally the remainders while also ensuring as equal as possible distribution among Member States. The resulting distribution, i.e. the number of surveys requested per each system is indicated in Table 2 (aiming at a total number of 180 surveys).
Table 2.
Numbers of surveys requested and number of surveys received for each of the six different housing systems
| Conventional cages | Enriched cages | Elevated pens | Floor pens | Outdoor/partially indoor | Organic systems | Total | |
|---|---|---|---|---|---|---|---|
| Number of surveys requested for this systemb | 37 | 31 | 34 | 22 | 30 | 26 | 180 |
| Number of responses received | 38a | 20 | 28 | 13 | 15 | 11 | 125 |
One expert who had initially been allocated to another system also provided information for this housing system.
Since the experts were expected to respond to two surveys, the total number of surveys does not equal the total number of experts (total number of experts = 122).
One hundred and twenty‐five answers were retrieved from 88 experts, out of which 51 experts completed 1 survey only, and 37 experts completed 2 surveys (total of 125 surveys completed).
The survey was in English but instructions were given – if necessary – in Italian, Spanish, French and Hungarian. The survey consisted of three parts. In the first part, there were a few questions about expertise and experience.
In the second part, experts were asked to assess the occurrence of 20 potential welfare consequences for reproducing does, growing rabbits and kits in up to two out of six farming housing systems. Occurrence here refers to the proportion (scale 0–1) of all rabbits of a given category (e.g. kits) who are impaired by the stated welfare consequence at least once over their lifetime in this production stage (e.g. as a kit).
For this purpose, respondents were first asked whether the welfare consequence in question is considered relevant, i.e. whether it would be expected to occur in the given rabbit category. Only if this question was answered with ‘yes’, were the respondents asked to provide estimates for the occurrence.
In the third part, experts were asked to assess the lifetime duration of the same 20 consequences for the same rabbit categories and the same housing systems. Lifetime duration was defined as the proportion (scale 0–1) of the total lifetime in that production stage that an individual rabbit's welfare is impaired (i.e. for kits between birth and weaning, for reproducing does from first kindling to cull, for growing rabbits from weaning to slaughter).
For occurrence and lifetime duration, the experts were asked to indicate the likely range of their estimates, from ‘lowest’ to ‘highest’ as well as providing a ‘best estimate’. For example, they were asked to estimate the ‘lowest’ proportion of rabbits that might be impaired by a certain welfare consequence, the ‘highest’ proportion that might be affected, and the ‘best estimate’ of the proportion likely affected.
For occurrence, in addition to these three values (lowest, highest and best estimates), experts were also asked for a fourth value, namely, to express their confidence that the true value of the criterion (in the example above, the proportion of rabbits affected by a certain welfare consequence) falls within the range given.
Throughout the survey, the respondents were asked to assume that systems are managed according to good practices.
Adjustment of data set and processing of data
Exclusion of responses (plausibility check)
The exclusion of responses was based on two plausibility checks:
-
1
Experts considering welfare consequences relevant for a certain animal category even though they logically cannot occur (e.g. mastitis in kits). In total, 10 possible non‐logical combinations were identified in the survey (Table 3). Experts that provided more than five non‐logical answers in one survey were excluded, as it was then assumed that the expert had misunderstood the questions. As a result, one expert who had completed the survey for two housing systems was excluded (out of 125 answers, 1.6%).
Table 3.
Possible illogical combinations of rabbit categories and welfare consequences
| Rabbit category | Welfare consequence |
|---|---|
| Kits | Occurrence of abnormal behaviours |
| Inability to express maternal behaviour | |
| Mastitis | |
| Pododermatitis | |
| Reproductive disorders | |
| Reproducing does | Neo‐natal disorders |
| Growing rabbits | Inability to express maternal behaviour |
| Mastitis | |
| Reproductive disorders | |
| Neo‐natal disorders |
-
2
Responses from experts who stated that none of the welfare consequences could be found in a given housing system and type of rabbit (i.e. not relevant according to question on occurrence) were excluded for this housing system and type of rabbit. In total, 13 out of 375 (3.5%) rabbit category/housing system combinations (received from responses of 9 experts) were excluded using this criterion.
In total 5% of the answers were excluded following the plausibility check.
Calculation of occurrence while correcting for ‘not relevant’ responses
Based on the remaining data set, the percentage of ‘not relevant’ responses (see above) was calculated per welfare consequence, housing system and rabbit category. Median occurrence estimates (on a scale of 0–1) were then corrected by the proportion of answers ‘relevant’, assuming that combinations judged as ‘not relevant’ have a zero occurrence. This procedure was based on the assumption that the respondents giving a ‘not relevant’ response deemed the welfare consequence as absent or its occurrence as negligible.
Calculation of welfare impact scores
For the lifetime duration of welfare consequences (scale 0–1), the median provided for the different welfare consequences was calculated across housing systems per rabbit category (i.e. the same duration applies to all housing systems for a given rabbit category). This was based on the assumption that lifetime duration of the different consequences would not substantially differ among housing systems.
Following EFSA's ‘Risk Assessment in Animal Welfare’ approach (EFSA AHAW Panel, 2012), a welfare impact score was calculated using the product of the severity scores for each welfare consequence per animal category (see Section 3.5.1) and occurrence and lifetime duration estimates (see Section 3.5.2). This was, however, only done for welfare consequences for which at least 20% of the respondents considered it ‘relevant’, i.e. for which occurrence estimates larger than 0% had been provided. The impact score of the welfare consequences for which less than 20% of the respondents had considered ‘relevant’ was set to 0.
Comparison across housing systems
The product scores were also used to calculate a cumulative welfare impact score by summing up the scores for all welfare consequences per rabbit type and housing system.
2.1.3. Literature review
A literature search about welfare consequences for rabbits and related hazards was conducted on Web of Science and Pub Med. Detailed information on the literature search performed is provided in Appendix A. A separate literature search was carried out for each welfare topic (health and disease, behaviour). The search focused on the description of the main effects observed in the animals at the moment they experience the welfare consequence. In addition, detailed information about hazards potentially leading to the welfare consequences was also retrieved. In this context, a hazard is defined as any aspect of the environment of the animal in relation to housing, management and animal genetic selection, which might have the potential to cause poor welfare.
2.1.4. Uncertainty assessment
To substitute widely lacking comprehensive scientific data on occurrence or duration of welfare consequences in farmed rabbits, expert knowledge elicitation through a survey was deemed the most appropriate approach. The results obtained from the experts should not be overemphasised due to the limitations of such an approach, but the results should also not be undervalued as they constitute the best available information.
Qualitative uncertainty assessment through appraisal of the scientific literature and the working group experts’ knowledge was used regarding the selection and description of housing systems, welfare consequences and hazards.
Housing systems for rabbits are variable. The working group discussed the systems and concluded that some can be defined more specifically than others. As the number of systems included in the survey was limited, some generalisation had to be accepted. This was especially the case for organic and outdoor housing systems, which represent largely variable housing systems that however fulfil some overarching general characteristics (e.g. access to pasture in organic farming).
As regards the selection and definition of welfare consequences for rabbits, the working group experts followed the Welfare Quality framework of welfare principles and criteria (Blokhuis et al., 2010). There is however the risk of false negatives in the selection of consequences, namely the risk of missing potential welfare consequences apart from the selected ones. For example, one of the experts participating in the EKE on severity of the welfare consequences suggested to also include the inability of does to retreat from the kits (after the kits start leaving the nest) as an additional welfare consequence. At this stage of the survey this could however not be considered anymore.
Similarly, the extent differed to which scientific information on the welfare relevance (i.e. validity of the assumed welfare impact) as well as on the hazards potentially leading to the welfare consequences was available. Again, regarding the latter there is a chance of false negatives and false positives, as the literature may not be sufficiently comprehensive and therefore important hazards may have not (yet) been described, or that hazards mentioned by the experts are less valid than expected.
For the survey, substantial efforts were undertaken to involve a balanced set of relevant experts in rabbit farming in Europe from different stakeholder groups (e.g. industry, research, veterinarians) and it is not very likely that other experts would have agreed to participate using a different approach for identification and selection of experts. Response rate was, however, lower than expected. Uncertainty may additionally have increased as fewer industry stakeholders than intended responded.
Quantitative approaches were applied regarding the uncertainty around the EKE assessment of occurrence, duration and severity of welfare consequences for rabbits. Uncertainty involved in estimating the occurrence and duration (as obtained from the survey) was quantified using the median and the 90% confidence interval around the median. Regarding the assessment of severity of the different welfare consequences (physical EKE meeting), the median and the range among the expert panel was used.
For the overall welfare impact score (product of occurrence, duration and severity), a simulation was carried out using the uncertainty distributions for the individual parameters. The simulation refers to a 10,000 iteration process of taking one value of each distribution of ‘Severity’, ‘Occurrence’ and ‘Duration’ for each welfare consequence and each housing system. For each iteration, the overall welfare score for each housing system was calculated as a sum of products (of severity × occurrence × duration). This results in an overall distribution for each housing system from which the median value is taken. These distributions are used for the assessment of the uncertainty around the median values of the overall scores.
Therefore, a simple double‐uniform distribution (see Figure 2) respecting the median and the 5th and 95th percentiles was fitted and used for the propagation of uncertainty.
Figure 2.
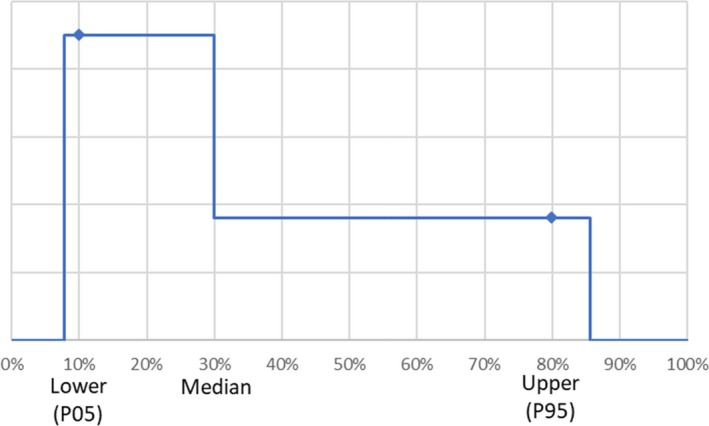
Fitted distribution used for the propagation of uncertainty of the overall welfare impact scores
For the resulting overall welfare impact score, the median was estimated. The 90% uncertainty range was calculated (P05, P95) to describe the precision of the median of overall welfare impact scores, i.e. if there was a true score, it would lie within this interval with 90% certainty.
3. Assessment
3.1. Rabbit production in Europe
The EU is the second largest meat‐rabbit producer in the world, after China. The Union holds 93% of the world's imports and exports, of which Germany, Belgium and Portugal are the main importing countries. Spain, Hungary, France and Belgium are the major exporting countries within the EU27 (Cullere and dalle Zotte, 2018). Professional rabbit farming for commercial rabbit meat production is concentrated in Spain, France and Italy (83% of EU production: ES: 48.5 million rabbits, FR: 29 million rabbits and IT: 24.5 million rabbits). There are also commercial rabbit farms in Germany, The Netherlands, Poland, Hungary, Belgium, Portugal and Greece. Together these countries produce 14% of the rabbit meat in Europe. However, there has been a decline in commercial rabbit farms in the EU in the past 20 years (−70% in NL and BE, −20% in HU) because of the decline in rabbit meat consumption of the European population.
According to the overview report of the European Commission (European Commission, 2017) the EU27 farm 180 million rabbits for meat annually, of which 66% (119 million) originate from conventional farms and are slaughtered for human consumption in approved slaughterhouses, and 34% (61 million) are reared, sold and consumed via back‐yard farms, direct and local sales.
As already defined by EFSA (2005), conventional rabbit farms are mainly based on family labour, with the number of reproducing does, representing the scale of the farm, varying from hundreds to thousands. A farm size of about 600 reproducing does should guarantee the economic sustainability of a single farmer (full time). Rabbit farms have become highly professional and technically advanced.
There are approximately 161,000 backyard farms and 4,500 commercial rabbit farms in the EU. Roughly, rabbit farming can be divided into conventional production systems used in large‐scale farming and niche production systems, such as floor pens, organic and outdoor farming. The non‐conventional systems are small in number (e.g. organic farming, with around 50 farms in France and a few examples in other countries) and diverse in nature (European Commission, 2017).
The EU directive for the protection of animals kept for farming purposes (Council Directive 98/58/EC of 20 July 1998) obliges the farmer to take care of the rabbits with the help of a veterinarian where relevant, but there is no specific legislation at the EU Level for rabbit housing. Some member states have developed national legislation or recommendations (Hungary, 1998; The Netherlands, 2006; Belgium, 2014; Germany, 2014; Italian Ministry of Health, 2014, 2019). There are also countries that have few commercial farms on their territories, such as Austria, Sweden or Finland.
Related to organic production, since 2018 rabbit production has been included into the EU organic farming regulation (EC Reg 2018/848) and this will come into effect in 2021. Specific rules for implementation are currently under discussion. At the moment, some (organic or not) alternative rabbit production is based on national production protocols, e.g. Label Rouge in France4 and organic rabbit farming in Italy.5
3.2. Production cycle of meat rabbits and animal categories
3.2.1. Life production cycle
Rabbit males are only ready for breeding use when they reach a constant daily spermatozoa production by 7 months of age (Castellini et al., 2017). The breeding lifetime of a male kept for reproduction in a conventional farm averages 2 years (Egea et al., 2000).
Under farming conditions, females of the genotypes most commonly used are usually inseminated at about 18 weeks of age, at a live weight (3.4–3.6 kg) corresponding to 80–85% adult weight. Then, the length of the reproductive career may vary with genotype, reproductive rhythm, feeding regimes and sanitary status, but the average culling age in reproducing does is 15 months and 6 parturitions (Rosell and de la Fuente, 2009a,b).
The slaughter age for growing rabbits varies greatly among the producing Member States (MS), but also within the same MS, depending on the consumer preference and local markets (Table 4). In fact, consumers in Spain, Portugal and the south of Italy prefer lighter and hence younger rabbits (live weight ca. 2.2 kg), whereas the consumers in central Europe and in North Italy demand heavier and older rabbits (live weight ≥ 2.6 kg). The slaughter age is around 63–77 days for light carcasses, but it is usually higher for heavy carcasses and it could even exceed 85 days in the case of backyard systems, as well as in alternative (organic or not organic) systems using local breeds and based on the use of fresh forages or on grazing in outdoor systems (90 days in the French Label rouge system).
Table 4.
Main features of European production of meat rabbits in conventional systems
| Producing Country | Slaughter age | Slaughter weight |
|---|---|---|
| Hungary | 75–77 | 2.5 kg |
| Italy |
65 days 75 days 85 days |
2.2 kg (light) 2.5 kg (standard) 3.0 kg (heavy) |
| France | 69–75 days | 2.4 kg |
| Spain | 63–65 days | 2.2 kg |
3.2.2. Animal categories
Based on the production cycle described in Section 3.3.4.2, different categories of rabbits are present, even contemporarily, in the farm, as detailed in Table 5.
Table 5.
Rabbit categories
| Categorya | Definition |
|---|---|
| Kits | From birth to weaning |
| Growing rabbits | From weaning to slaughter age |
| Young females for breeding | From selection (as a breeder) till first service |
| Young males for breeding | From selection (as a breeder) till appropriate age for mating or semen collection |
| Breeding bucks | From first mating/semen collection to culling |
| Non‐conceiving does | Non‐pregnant does after weaning of their litters till the next successful service |
| Reproducing does | From first kindling till culling – depending on the moment of the production cycle, this may include pregnant, lactating and lactating pregnant does |
For this opinion, the animal categories in bold have been selected as target populations for the survey.
All these rabbit categories usually coexist in a farm in relation to the hazards that they are exposed to and the occurrence of certain welfare consequences; similarities can be found among some categories (e.g. growing rabbits and male or female young breeding rabbits), which makes it possible to divide commercially farmed rabbits into three major categories: kits, growing rabbits and reproducing does. For the scope of the survey and in the discussion throughout this opinion it was therefore agreed to limit the number of target populations to 3, i.e. kits, growing rabbits, and reproducing does.
3.3. Rabbit production systems
3.3.1. Introduction
Rabbit production is commonly based on a continuous and closed cycle, with all stages simultaneously present on the same farm, and it can be operated under different systems that are a combination of several factors/aspects (Figure 3). These include different building types with different equipment (ventilation system, lighting, feed distribution and drinking pipeline), in which different biosecurity measures may be applied to different animal genetics, housed with different systems and subjected to different management of reproduction, rearing, and feeding (Lebas, 2000; Cerolini et al., 2008; Lavazza et al., 2009; Italian Ministry of Health, 2019). All these factors, as well as their different combinations, may affect animal health and welfare to a varying extent.
Figure 3.
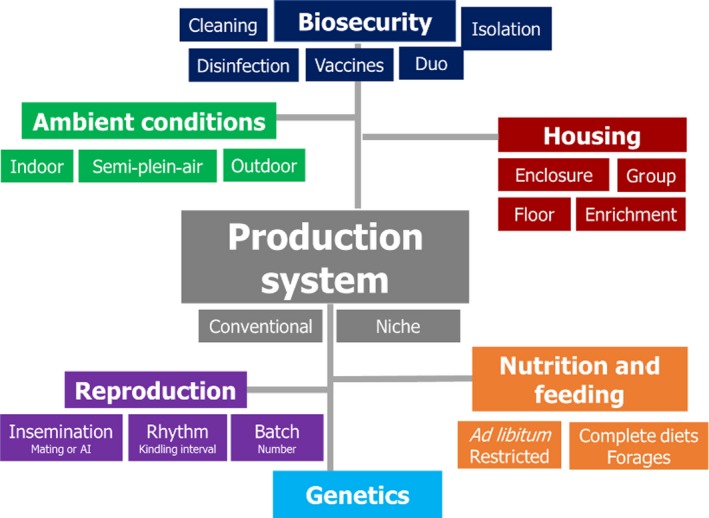
Production factors within conventional and niche production systems for rabbit farms
A variety of housing systems are used for rabbit farming. These range from conventional barren bicellular cages to alternative pen systems (commonly called ‘parks’), recently introduced in some European Countries and required by the Belgian legislation (Belgium, 2014). Some management practices might be more frequently associated to one or another housing system and thus provide different hazards for health and welfare.
Despite not being fully exhaustive, the following chapters aim to address the main production factors within a rabbit production system, which may affect welfare and health to different extents
3.3.2. Genetic lines
Most of the industrial production comes from commercial crossbred rabbits (also called ‘hybrids’) based on the crossing of lines from pure breeds selected by genetic suppliers, e.g. Hypharm‐Eurolap, Hycole, in France and Italy; in Spain: Universidad Politécnica de Valencia (UPV) and Institut of Agrifood Reasearch and Technology (IRTA), additionally to French lines; Zika in Germany; Martini in Italy. Some other commercial rabbit breeds are also available, e.g. SIKA in Slovenia and Pannon White in Hungary. The dam lines are usually based on New Zealand White and Californian medium‐size breeds; the sire lines are usually based on heavy breeds. Among heavy breeds, most are based on the Flemish Giant, which has the highest adult body weight. Native breeds are mostly bred in small farms, backyard and hobby production.
During the last decades, in rabbits as in other meat species, the genetic selection has been mainly focused to improve traits linked to the increase of growth rate and amount of muscle mass (Gondret et al., 2005; Hernández et al., 2006), as well as the number of offspring and milk production in females. This may have had some collateral negative effects on robustness, which is defined as the capacity to maintain good production levels, keeping all body functions at the highest performance, in many different environmental/housing conditions and in different production systems of farmed animals; breed or line is a predisposing hazard to some diseases (Sánchez et al., 2012; Rosell and de la Fuente, 2018).
Selection for reproduction durability has efficacy in delaying senescence and these genetic lines have a lower sensitivity to external environmental factors, being likely mediated by higher body mass and energy supply (Pascual et al., 2013).
3.3.3. Provision of feed and water
In conventional farms, feed distribution can be manual or automatic, whereas in niche systems it is usually manual. In indoor and in semi‐plein‐air (semi‐outdoor) systems, drinking is usually guaranteed by automatic distribution and nipple systems, whereas under outdoor conditions, suitable supplementary devices are necessary to assure water availability across all seasons. The water origin may be different, water main or well, and accordingly the chemical and microbiological quality of water may vary and thus should be regularly checked; finally, different cleaning and disinfection procedures may be adopted for the drinking systems (tanks, pipelines and drinkers).
Under most farming conditions, complete pelleted diets are used, and feeding is intended to cover the rabbits’ physiological and nutritional requirements to assure their health and their productive performance (de Blas and Mateos, 2010; Maertens, 2010; Xiccato and Trocino, 2010; Gidenne et al., 2017a,b). The nutritional requirements depend on animal genetics, conditions for housing, management of reproduction and rearing/growing, as well as their combinations. Some dietary components, e.g. fibre fractions, also play a special role in the control of digestive diseases of the growing rabbit (Gidenne et al., 2010, 2015; Trocino et al., 2014).
Regarding breeding females, feeding is usually ad libitum. They usually receive a unique mixed diet formulated to meet the requirements of both the doe, or both the doe and kits, in one feeder. When kits begin to consume solid feed (around 17–21 days of age) they may consume the feed specifically formulated to satisfy the high lactation requirements during the first part of lactation. During the second part of the lactation (24–35 days post AI), the kits’ may consume a feed more adapted to their digestive physiology (Xiccato et al., 2008; de Blas and Mateos, 2010). Feed restriction is not used for reproducing females. Nevertheless, young females selected for breeding may be restricted during their growth, using quantitative or qualitative restriction to avoid excessive fattening, especially when a later age is selected for the first insemination.
Regarding growing rabbits, the feeding programmes may be different and may use more diets to closely match the specific requirements for each growth stage or may use fewer diets (even only one). Feeding may be ad libitum or restricted. In France, using a 42‐day cycle and slaughtering at 10–11 weeks, quantitative feed restriction (15–30% reduction from ad libitum) is usually applied in 95% of conventional farms during the first weeks after weaning, followed by a period of weak restriction or free intake, to reduce post‐weaning digestive disorders and to improve the feed efficiency (Gidenne et al., 2017a,b). In the other producing countries, the use of quantitative feed restriction is a less common practice. Table 6 summarises the most common feeding programmes adopted in conventional farms for the different categories of rabbits (Maertens, 2010).
Table 6.
Example of feeding scheme for conventional rabbit meat production (modified from (Maertens, 2010)
| Rabbit category | Quantity | Diet |
|---|---|---|
| Males | ||
| Young (until 18 weeks) | Ad libitum | Growing rabbits |
| Adult | Restricted (40 g/kg live weight) | Growing rabbits/specific diet for males |
| Young does | ||
| Early mating (15–16 weeks) | Ad libitum | Growing rabbits |
| Late mating (17–20 weeks) | Restricted (40 g/kg live weight, followed by a 4‐day flushing before insemination) | Growing rabbits or specific rearing diet |
| Does | ||
| Late gestation | Ad libitum | Lactation |
| Lactating | Ad libitum | |
| Kits < 3 weeks | Lactation | |
| Kits > 3 weeks | Weaners | |
| In pre‐gestation cages | Restricted (40 g/kg live weight), but ad libitum 4 days prior to insemination | Growing rabbits |
| Growing rabbits | ||
| 4–6/7 weeks | Restricted, 0.75 of ad libitum | Growing rabbits |
| 6/7–10/11 weeks | Ad libitum | Growing rabbits/finishing |
In outdoor or organic systems, supplementation with fresh forage or hay or access to grazing, besides the distribution of compound diets (pellets or whole grains) may be used. In organic systems, basic requirements according to EU Reg 2018/848 include access to pasture whenever conditions allow for it.
3.3.4. Management
3.3.4.1. Biosecurity
Within conventional rabbits farms, the biosecurity programmes are largely based on a series of provisions, requirements, rules, facilities and operational practices, all aimed: not only (1) to ‘isolate’ the farm environment from outside and thus to exclude the accidental introduction of disease‐causing organisms into the farm, but also (2) to reduce pathogen spread and damage resulting from infectious agents already present in the farm.
The setup of biosecurity programmes has to consider all the different aspects of farming, i.e. management, structural requirements, cleaning and disinfection, isolation (i.e. control of people, animals and vehicles movements) and other biosafety measures, preventive treatments and direct prophylaxis actions (Lavazza et al., 2009; Italian Ministry of Health, 2019). Moreover, the differences existing between production systems may condition the applicability and influence the efficacy of such biosecurity programmes.
The closed cycle production system of rabbit reproductive does not permit the adoption of complete all in/all out procedures and corresponding cleaning and disinfection procedures (Huneau‐Salaün et al., 2015). Therefore, the application of specific biosecurity measures is strongly recommended. This can be complemented by other measures of both direct (sanitary) and indirect (metaphylaxis/immunoprophylaxis) prevention (EFSA, 2005; Lavazza et al., 2009). In particular, infirmary and quarantine procedures, i.e. dedicated areas for ill animals and for entering animals, respectively, should be present and used in rabbit farms.
Specific vaccination programmes include those necessary for primary viral infectious diseases of lagomorphs such as myxomatosis and rabbit haemorrhagic disease (Rosell et al., 2019). This is defined in each area according to the epidemiological situation (EFSA, 2005; Italian Ministry of Health, 2019).
In some niche systems, certain specific biosecurity measures are impossible to realise. For instance, isolation from wildlife is difficult in systems with outdoor access.
3.3.4.2. Reproduction
Conventional farms mostly use artificial insemination (AI), which permits farmers to organise and schedule all the related operations inside the farm in a cyclic manner. Semen may be obtained from specialised farms/centres or from males reared and kept in the same farm, which implies that males may be absent or present in the farm. Usually, the doe is inseminated with 0.5 ml of fresh diluted semen (1:5 to 1:15) and immediately afterwards is subjected to an intramuscular injection of Gonadotropin releasing hormone (GnRH) synthetic analogue to induce ovulation. Natural mating is used only on small farms with few does as it is labour‐intensive and time consuming, because it requires frequent movement of the animals between cages. Pregnancy lasts 30–31 days. It is diagnosed by abdominal palpation at 13–17 days.
The timing of AI after kindling determines the reproductive rhythm and the interval between two consecutive kindlings. Rabbit does are receptive and may be inseminated immediately after parturition. Nevertheless, under conventional conditions the most common reproductive rhythms are based on AI at 11–12 days or 17–18 days post‐partum, which means an interval of 42 days or 49 days between two kindlings. An example of this reproductive rhythm is presented in Figure 4. Longer reproductive rhythms, with AI later than 25 days post‐partum, are also applied.
Figure 4.
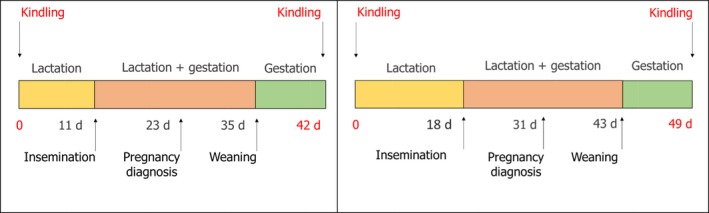
Main operations and physiological state of the doe under reproductive rhythms with 6‐ and 7‐week intervals, commonly used in conventional rabbit farms (kindling to kindling interval 42 days and 49 days, respectively)
At kindling, cross fostering and litter standardisation are usually applied when the does are healthy. The rabbit doe can give birth to 1–20 kits, but she can successfully nurse 8–10 kits. Thus, within 1–2 days after parturition, cross fostering is applied to standardise litter size and kits’ weight within the same litter. The litter size nursed varies from 8 to 10 according to the doe parity and genetics. After 2–3 days, some farmers close the entrance of the nest and allow the does to nurse their kits only once per day until 14–15 days after parturition, i.e. controlled lactation. Thereafter, and until litter weaning, the does and their kits are free to move in and out of the nest.
The timing of weaning, i.e. separation of kits from their doe, depends on the reproductive rhythm. In does mated immediately after parturition, kits are weaned at 23–25 days of age because the doe is going to give birth to the next litter within a few days, as for wild does. In rabbit farms, weaning age varies from 30 to 35 days of age.
To schedule operations in the farm, rabbit does may be managed as a single group (batch), or more groups subjected to the same operations on the same day. In a single batch system, all rabbit does of a barn are inseminated on the same day according to the reproductive rhythm, they will give birth on the same day, kits will be weaned on the same day and sold at the slaughtering age and weight at the same time. In the two‐batch system, two groups of does are present in the same barn, while the timing of the operations on the two batches is equal to half of the interval between two kindlings.
In some niche systems, such as those producing under the French label ‘Label Rouge’, the batch system can be used, as in conventional farms. However, in outdoor or organic farming, natural mating is used, since in the organic systems hormonal treatment for the reproduction control is forbidden.
3.3.5. Housing
3.3.5.1. Ambient conditions
Facilities for rearing rabbits may be placed indoors or outdoors. In conventional indoor systems, buildings are made of different materials (e.g. concrete, plastic) and might contain different equipment to control environmental conditions by automated fan ventilation, heating and cooling systems.
Under semi‐plein‐air and open‐air systems, environmental (micro)climate and light schedule are subject to seasonal changes. In semi‐plein‐air systems, the buildings have a roof, but lateral walls are only partial and openings are not completely closable. In open‐air systems, the buildings only comprise a roof to protect animals kept in cages. During summer, trees serve to alleviate heat stress, but above 30°C, artificial ventilation is necessary. In indoor farms, a controlled 16L:8D (16 h light:8 h dark) lighting schedule with automatic lighting (light bulbs, fluorescent light, LED), and even half‐hour crepuscular transition, may be used to control the seasonal effect on reproductive performance of breeding rabbits. A minimum intensity of 20 lux is usually provided.
In organic systems, reproducing animals and young kits might be housed indoors (according to climatic conditions), whereas growing rabbits are usually housed outdoors in movable cages or paddocks. Outdoor systems may be very different, but usually do not contain equipment to control environmental conditions.
3.3.5.2. Housing systems
Especially with view to the housing systems, farms can be distinguished into conventional farms (including conventional cages, enriched cages and elevated pens) and niche systems (including floor pens, outdoor and organic systems).
Conventional farms
In the large majority of conventional farms, replacement does before breeding, and inseminated but not pregnant does, are housed in small cages for a brief period (5–8 weeks) before entering the batch production management system. Then, reproducing does are housed individually with their offspring.
In some farms, the doe always remains in the same cage after the litter weaning, to give birth for the next litter, whereas weaned rabbits are moved into the growing enclosures. In other farms, at weaning, the doe is moved to a clean and disinfected enclosure, whereas the litter remains in the cage where they were born until slaughtering (all‐in all‐out system, using dual purpose cages).
Conventional wire cages are used for housing of both young females for breeding and reproducing does with their litters.
More recently, in some countries an increasing number of new or renovated farms have started to use structurally enriched cages, the so‐called ‘welfare cage’, i.e. larger cages equipped with elevated platforms and plastic footrests, and sometimes other internal enrichment objects.
Moreover, a few farms use alternative systems based on elevated pens, commonly called parks, in which does are normally kept individually but may be kept in groups for some periods (part‐time housing) by removing wire walls between single modules of a pen. Such group systems are still being further developed in terms of housing design and management (e.g. re‐grouping strategies).
Housing for growing rabbits may vary greatly among countries and within countries. In most European countries dual‐purpose conventional cages are common and the use of structurally enriched cages is increasing, in which small groups of rabbits are reared (4–5 rabbits). A few conventional farms have recently started to use elevated pens for group‐housing of growing rabbits in larger groups (32–36 rabbits). Bicellular cages are still used, usually in older units. An overview of the different sizes of the housing systems available in conventional farms for housing the different categories of rabbits is presented in the Table 7.
Table 7.
Sizes of the housing systems available in conventional farms for housing the different categories of rabbits
| Width (cm) | Length (cm) | Height (cm) | Total available surface (cm2) | |
|---|---|---|---|---|
| CONVENTIONAL FARMS | ||||
| Conventional cages | ||||
| Bicellular cages for growing rabbits | 25.4 | 44 | 28 | 1,200 |
|
Young or non‐pregnant female Growing rabbits |
38 | 43.5–66 | 28–41 | 1,650–2,510 |
| Basic standard models for reproducing does with litters or for growing rabbits (dual purpose cage) | 38 | 87–102 | 32–39 | 3,300–3,900 |
| Wider versions for reproducing does with litters or for growing rabbits (dual purpose cage) | 46 | 95–102 | 35 | 4,370–4,700 |
| Structurally enriched cages for reproducing does and litters or for growing rabbits (dual purpose) | ||||
| Enriched cages with wire‐mesh platform (width 20 cm) | 38–46 | 95–102 | 60–65 | 4,370–5,600 |
| Enriched cages with plastic‐mesh platform | 46–52.5 | 102 | 65–80 | 5,600–6,400 |
| Alternative elevated pen (park) systems for reproducing does with their litters (4 does) or for growing rabbits (32) (dual purpose) | ||||
| Pen/park with plastic‐mesh platform (width 20–25 cm) | 180–200 | 80–102 | Open top |
Total: 18,000–25,400 Per doe: 4,500–6,350 Per growing rabbit: 563–800 |
| Niche systems | ||||
| Outdoor systems | No standards available for housing enclosures | |||
| Organic systems | No standards available for housing enclosures | |||
Different types of floor are used for both reproducing does and growing rabbits. The most common is wire mesh, frequently paired with a plastic footrest pad (usually 25 × 36 cm with space between slats equal to 1.6 × 7 cm). Plastic slatted floors may also be used. Some niche systems also use a concrete floor covered by litter, combinations of solid and slatted floor, or animals may be kept directly on the ground as in outdoor movable cages (wire floor) or in open‐air enclosures.
Almost all housing solutions presented in Table 7 may contain some enrichment made of different materials. Platforms may be added to increase animal activity (e.g. jumping) and provide escape options, isolation possibilities or shelters. Platforms may be made of wire mesh, slatted plastic floors, or a solid surface of different materials. They may be differently positioned within the enclosure. Hiding places represented by pipes, boxes or walls to provide visual isolation may be included and these may be made of plastic or metal. Finally, gnawing sticks (wood, compressed hay or other materials) may be offered to rabbits.
In conventional farms, the stocking density of growing rabbits in terms of animals reared/m2 and kg final live weight/m2 differ according to each national regulation or national guidelines (when available) in respect to each housing system and to the slaughter age. Nevertheless, farmers adapt the stocking density used in their farm according to quality of (micro)climate (e.g. building, equipment), genetic lines, conditions for housing (structures in which animals are kept), management of reproduction, management of growing, feeding, and biosecurity measures.
The drawings below provide examples of typical conventional cages (Figures 5 and 6), enriched cages (Figure 7), elevated pens (Figure 8).
Figure 5.
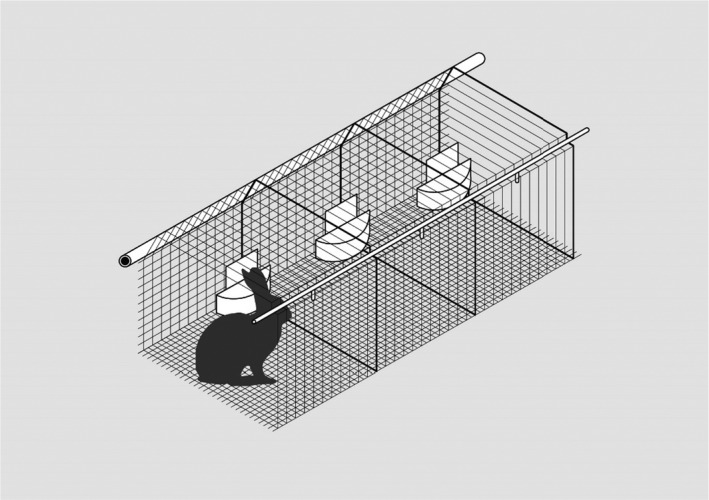
Conventional cages. Example of a bicellular conventional cage: this cage is used for housing of 1–2 growing rabbits from weaning until the end of fattening. It is made of wire mesh and it is equipped with a feeder and a nipple drinker
Figure 6.
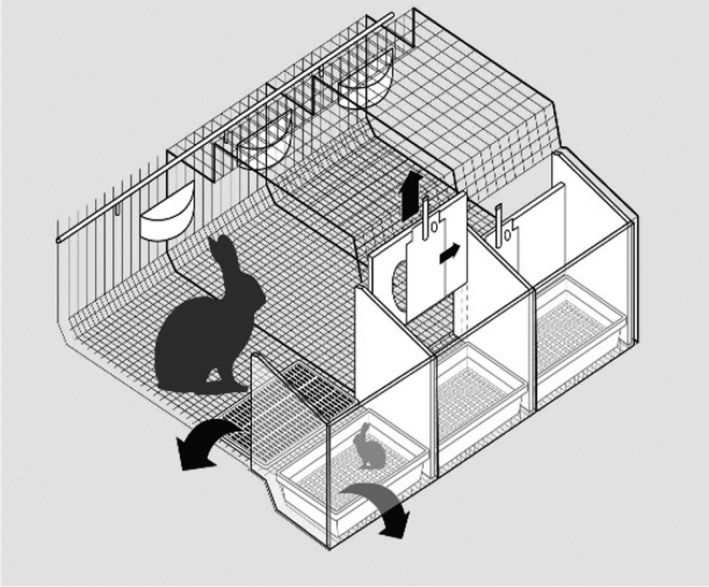
Conventional cages. Example of a dual‐purpose conventional cage: this cage is used for individual housing of the reproducing doe from a few days before kindling until the end of lactation with its litter and then, after removal of the nest box, for housing of growing rabbits. It is made of wire mesh and it is equipped with a feeder and a nipple drinker. A plastic footrest can be also used that could be removed or not during rearing of growing rabbits (see arrow on the plastic footrest of the left cage)
Figure 7.
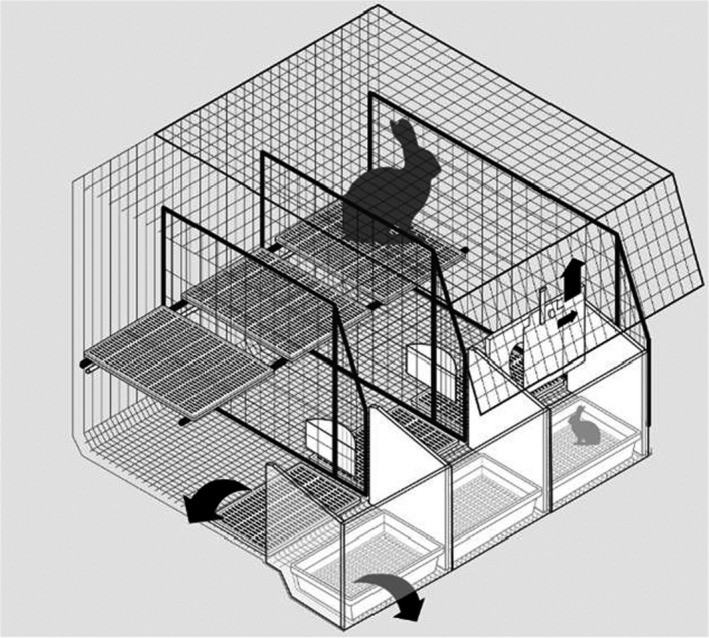
Enriched cage. Example of a dual‐purpose enriched cage: this cage is larger and higher than conventional dual‐purpose cages. It is used for individual housing of the reproducing doe from a few days before kindling until the end of lactation with its litter and then, after removal of some items, for housing of growing rabbits. It is made of wire mesh and it is equipped with a feeder and a nipple drinker. It always includes a platform with wire mesh or plastic slats flooring. A plastic footrest is also used that could be removed during rearing of growing rabbits (see arrow on the plastic footrest of the left cage)
Figure 8.
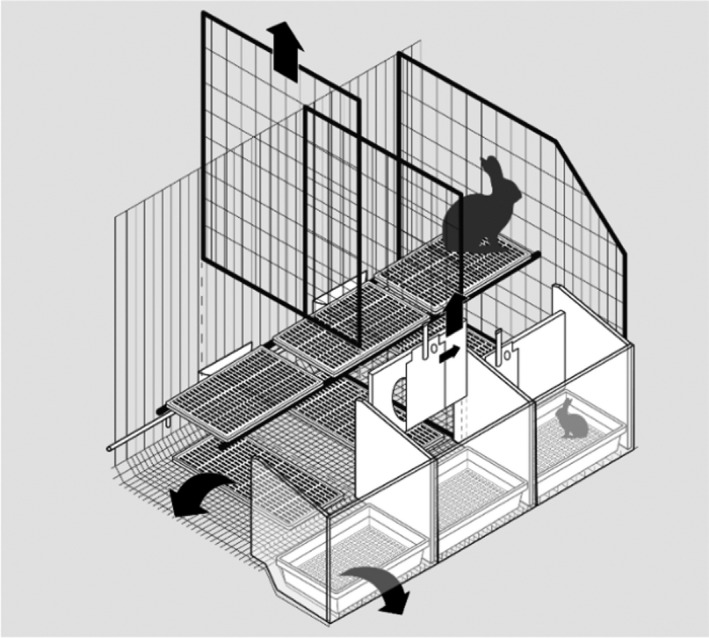
Elevated pen. Example of a dual‐purpose elevated pens (also called parks): this system comprises single modules that can be connected together. The single module is larger than enriched dual‐purpose cages and it is open‐top. The single module is used for individual housing of the reproducing doe from a few days before kindling until the end of lactation with its litter and then, after removal of some items and after joining the single modules, for group‐housing of growing rabbits. Walls are wire mesh, whereas flooring could be made of wire mesh or plastic slats. The single module is equipped with feeders and nipple drinkers. It always includes a platform with wire mesh or plastic slatted flooring. A plastic footrest is used if plastic flooring is not available
In the drawing (Figure 6), the cage on the right is equipped for the reproducing doe and its litter with a removable plastic nest containing the litter in the front; the nest area is separated from the rest of the cage by a removable wall with a sliding door. The door of the nest can be closed for controlled lactation during the first 1–2 weeks after kindling, as shown in the central cage. Then, the wall between the nest and the rest of the cage (see arrow on the nest wall of the central cage) and the nest box (see arrow on the nest box of the left cage) are removed to obtain a unique space in which growing rabbits will remain after separation of the doe, as shown in the left cage.
In the drawing (Figure 7), the right cage is equipped for the reproducing doe and its litter with a removable plastic nest containing the litter in the front; the nest area is separated from the rest of the cage by a removable wall with a sliding door. The door of the nest can be closed for controlled lactation during the first 1–2 weeks after kindling. Then, the wall between the nest and the rest of the cage (see arrow on the nest wall of the right cage) and the nest box (see arrow on the nest box of the left cage) are removed to provide a unique space in which growing rabbits will remain after separation of the doe, as shown in the left cage.
In the drawing (Figure 8), the right module is equipped for the reproducing doe and its litter with a removable plastic nest containing the litter in the front; the nest area is separated from the rest of the cage by a removable wall with a sliding door. The door of the nest can be closed for controlled lactation during the first 1–2 weeks after kindling. Then, the wall between the nest and the rest of the cage (see arrow on the nest wall of the central module) and the nest box (see arrow on the nest of the left module) are removed to provide a unique space. After separation of the doe, the walls between single modules are removed (see arrow on the wire mesh wall between the central and the left module) to form a pen/park for group housing of growing rabbits. Usually, four modules are joined to form one pen/park for growing rabbits.
Single modules could be joined also for part‐time group housing of reproducing does, which is not yet widely implemented in commercial farms.
Niche production systems
In niche production systems, a variety of solutions exist to house reproducing does and growing rabbits. They can be based on open‐air enclosures or underground systems that combine wire cages and underground confined spaces (Figure 8a) as well as hutches (Finzi and Mariani, 2011).
These systems are usually for individual housing of reproducing does with their litter and for collective housing of growing rabbits. Moreover, in Switzerland, small farms exist that use indoor deep litter parks, floor pens, for group housing of reproducing does or growing rabbits (Figure 9). A total of about 3,600 reproducing does are kept in this system in Switzerland in small farms (calculated average farm size: 64 does per farm; i.e. 56 farms) (Ruchti et al., 2018).
Figure 9.
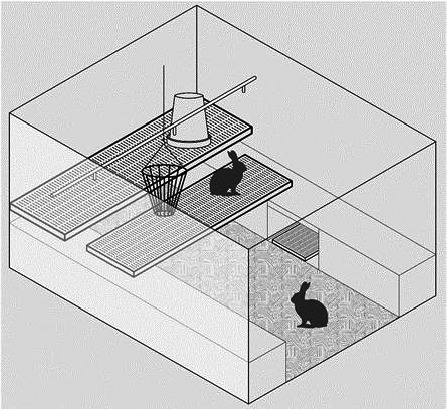
Example of a floor pen. This system is a niche system that uses indoor deep litter parks with plastic platforms for group housing of reproducing does or growing rabbits; males may be also present. No standards are available but, as a rule, they are large open‐top pens based on solid floors with litter. The example in the picture uses solid walls between pens, two platforms with plastic slatted flooring, and closed nest boxes provided with plastic footrests. Nipple drinkers are used for automatic water distribution, whereas a circular feeder to be filled manually is used for the diet provision. The pens are also equipped with a rack to provide hay for feeding and gnawing
Other niche systems use different fixed (cages, hutches, paddocks) or movable housing systems (usually cages) which may give access to outdoor areas and pasture, here referred as outdoor systems (Figure 10).
Figure 10.
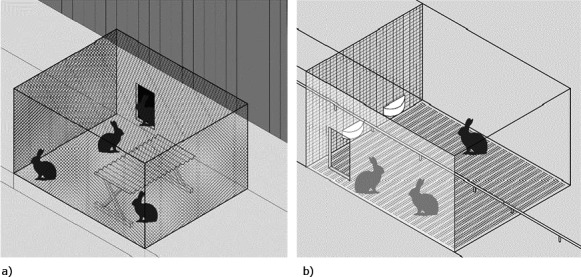
(a and b) Example of an outdoor housing system, which also belongs to the niche systems. No standards are available but, as a rule, animals have the possibility of accessing an outdoor area, which is not necessarily pasture. The example in the drawing is used in the French Label Rouge housing of growing rabbits. Rabbits are reared in groups from weaning onwards and they can access an outside area. This area is protected from wild animals with mesh. It includes a solid floor, and it can be equipped with a shelter as well as a rack to provide hay (Outside, Figure 10a). An opening in the wall permits the movement of the animals between the outside and the inside of the system where large pens with wire mesh walls are present (Inside, Figure 10b). These pens have plastic slatted flooring and are equipped with an automatic nipple drinker for water distribution as well as feeders for feed provision
As regards organic production, basic requirements according to EU Reg 2018/848 include access to pasture whenever conditions allow for it, group housing, access to a covered shelter including dark hiding place, a raised platform and nesting material for all nursing does. Implementation of these requirements will come into force from 2021. No further common standards in EU for housing of organic reproducing does with litters or growing rabbits have been specifically set until now.
Except for organic farming, there are no standards regarding size of housing for other niche production systems, and no data are available about stocking density in terms of animals reared/m2 and kg final live weight/m2. Nevertheless, these differ according to several factors, i.e. animal genetics, conditions for housing (including building, equipment and structures in which animals are kept), management of reproduction, management of growing/rearing, feeding, and biosecurity measures.
The drawings below provide examples of niche systems, i.e. floor pens (Figure 9), outdoor systems (Figure 10) and organic systems (Figure 11).
Figure 11.
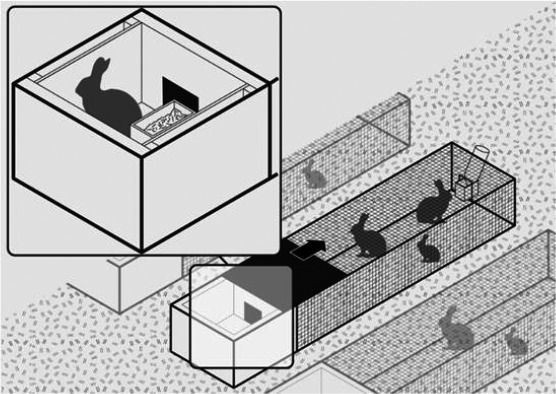
Example of an organic housing system. This system is a niche system. No standards are available but, as a rule, these systems should satisfy basic requirements according to EU Reg 2018/848. The example in the drawing is based on movable cages used for reproducing does kept individually with their litters, or growing rabbits kept in groups. The movable cage is made of wire‐mesh; it permits foraging on pasture. It includes a sheltered area with solid walls, which serves as a nest box in the case of reproducing does or functional resting/refuge area for growing rabbits. In this sheltered area, a feeder providing compound diets and/or hay is included as well as materials for nest construction in the case of the reproducing does. The movable cages are also equipped with drinkers for manual provision of water
3.3.6. Housing systems for the survey
To assess the occurrence of the most important welfare problems in farmed rabbits in Europe, an EKE web‐based survey was designed. Because of the complexity of production systems, with many interacting components as indicated in the previous sections, it was decided to elicit answers based on six different housing systems, which can be clearly defined and recognised, provided that all other production factors and conditions are managed according to good practice. The systems selected for the survey, were defined using the following descriptions.
Conventional cages
Wire cages with plastic footrests are used for housing both young females and reproducing does with their litters. Cages are equipped only with a feeder, a drinker and a nest area. They can be dual purpose cages (doe and its litter or growing rabbits in small groups).
Bicellular wire cages are also used to house two growing rabbits only.
Structurally enriched cages
Cages equipped with elevated platforms and plastic footrests. These cages have greater floor area and height than conventional cages. These are usually dual‐purpose cages (doe and its litter or growing rabbits in small groups).
Elevated pens
Open top larger elevated pens with slatted floors and platforms are used for housing growing rabbits in large groups (usually 32–36 rabbits).
Open top elevated pens for does are equipped as structurally enriched cages (modules) and linked together so that does may be grouped during some periods (part‐time housing) by removing wire walls between single modules of a pen.
Floor pens
Open top larger pens with totally or partially solid floor with bedding material, usually straw. These provide group housing for does or growing rabbits.
Outdoor/partially outdoor systems
Not‐organic fixed or movable housing with access to an outdoor area.
Organic systems
Any system currently certified as organic by national legislation, which encompasses more than just housing system. Regulations generally include a covered shelter including a dark hiding place, access to an outdoor area, preferably on pasture, sufficiently large, clean, comfortable and dry area for resting, solid materials, not slatted floor, straw bedding, use of organic feed, rearing system based on grazing, 60% raw forage produced on‐farm, use of robust breeds, no antibiotics, no hormones.
3.4. Describing rabbit welfare
3.4.1. Animal‐based measures
Animal‐based measures (ABM) can be used to assess the welfare state of individual animals (EFSA AHAW Panel, 2012). While validated assessment schemes exist for many farmed species, none has yet been fully validated for farmed rabbits. Measures have been validated for pain assessment in laboratory rabbits, based on changes in behaviour, facial expressions, and body temperature (Leach et al., 2009; Farnworth (Farnworth et al., 2011; Keating et al., 2012). These could also be utilised for farmed rabbits. Behavioural changes include reduced feeding and drinking, tight huddle posture (sitting with their back arched and fore and hind limbs drawn in tightly), locomotory changes including shuffle (walking at a very slow pace) and partial hop movements (forward extension of forelimbs as if to hop, without movement of hind limbs) (Farnworth et al., 2011). General grooming is also reduced, although sites of injury may receive increased grooming (Farnworth et al., 2011). EFSA (2005) noted that although rabbits are normally silent animals, they may squeal loudly if in severe pain or distress; they may also grind the teeth in cases of more chronic pain. More recently, the use of facial expression as an indicator of pain has been validated in rabbits (Keating et al., 2012). The Rabbit Grimace Scale assesses five different facial action units (orbital tightening, cheek flattening, nose shape, whisker position, and ear position) to create an overall score that increases when rabbits experience pain.
At the European level, a COST Action ‘Multi‐facetted research in rabbits: a model to develop a healthy and safe production in respect with animal welfare’ identified key welfare indicators in the assessment of rabbit housing (Hoy, 2009). This identified the main welfare indicators for rabbits to be:
Mortality: no or low (unavoidable) mortality;
Morbidity: pathologies (‘internal diseases’, infectious factorial diseases); injuries – the morbidity should be low and unavoidable;
Physiology: hormone levels, heart rate variation, immune reactions – the physiological parameters should be in the species‐specific standard;
Behaviour: ethogram, reaction to behavioural tests – species‐specific behaviour;
Performance (production): growth, feed conversion, fertility rate – the performance should be on a high level
Reduced performance is often used as an indirect measure of poor welfare, because of the known adverse effects of stress physiology on feed intake, growth and reproductive functions. Hoy (2018) proposed an integrative parameter, the Kit Index (KI), to characterise the complex fertility situation on a rabbit farm. KI is the product of the kindling rate (KR: the percentage of kindling per number of inseminated does) and the average litter size (LS), and represents the number of total or live‐born kits per 100 inseminated does. It can be used to compare groups of does, batches over time, or farms as part of an indirect welfare evaluation. However, it must be borne in mind that influences other than stress can affect reproduction and other performance measures. Furthermore, good performance in terms of production does not necessarily equal high welfare levels.
A more comprehensive welfare assessment protocol for farmed rabbits, based on the Welfare Quality® framework (Blokhuis et al., 2010), was proposed but not validated (de Jong et al., 2011). As documented in Table 8, appropriate measures for both does and breeding rabbits, based on scientific literature and expert opinion are summarised. Where possible, ABMs, like health and behaviour, are used but where these are not available then resource‐based measures are proposed. It is suggested that measurements on growing rabbits should be made shortly before the time of slaughter, and for reproducing does at the time of AI and at the end of the lactation period.
Table 8.
Animal‐based measures proposed for welfare assessment in farmed rabbits (de Jong et al., 2011). A number of resource‐based measures are also included in the protocol
| Does | Growing rabbits | |
|---|---|---|
| Good feeding | ||
| Absence of prolonged hunger | Body condition score | Body condition score |
| Absence of prolonged thirst | Resource‐based measures | Resource‐based measures |
| Good housing | ||
| Comfort around resting | Fully stretched lying in the pen or at the elevated platform or shelter | Fully stretched lying in the pen or at the elevated platform or shelter |
| Simultaneous resting in group housing | Simultaneous Resting In Group Housing | |
| Thermal comfort | Respiration rate | Respiration rate |
| Red ears | Red ears | |
| Ease of movement | Hopping (number of consecutive hops), jumping, turning, running | Hopping (number of consecutive hops), jumping, turning, running |
| Number of lame rabbits | Number of lame rabbits | |
| Good health | ||
| Absence of injuries | Skin injuries/wounds | Skin injuries/wounds |
| Pododermatitis | Number of toes and ear damage | |
| Trichophagy | ||
| Absence of diseases | Percentage mortality and selection (replacement) | Percentage mortality and selection (replacement) |
| Clinical scoring of rabbits, Consisting of symptoms listed | Clinical scoring of rabbits, Consisting of symptoms listed | |
| Technical performance | Technical performance | |
| Absence of pain induced by management procedures | Which mutilations are used (for identification) | Which mutilations are used (for identification) |
| Presence of tissue growth when using ear marks | Presence of tissue growth when using ear marks | |
| Appropriate behaviour | ||
| Expression of social behaviour | Scoring of injuries and wounds | Scoring of injuries and wounds |
| Scoring social behaviour | Scoring social behaviour | |
| Expression of other behaviours | Abnormal behaviours | Abnormal behaviours |
| Coat condition | Coat condition | |
| Kit mortality | ||
| Good human–animal relationship | Human approach test | Human approach test |
| Positive emotional state | Fear for novel objects | Fear for novel objects |
| Description of behaviour of a group | Description of behaviour of a group | |
| Hopping behaviour in young rabbits | Hopping behaviour in young rabbits | |
More detailed versions of the Welfare Quality protocol for does, bucks and kits, and separate protocols for growing rabbits and for finishing rabbits at the slaughterhouse have been proposed by IRTA (Dalmau et al., undated, a,b). These include details of sampling methods, scoring systems for each measure and threshold criteria for welfare assessment.
In France, the EBENE project to develop a welfare assessment protocol for the French rabbit industry has proposed measures for does and for growing rabbits, again based on the principles and criteria grid established in the Welfare Quality® project, and is carrying out work to validate these measures (Bignon et al., 2017).
In Italian Ministry of Health (2019) has produced, as an annex to rabbit farming guidelines, a list of measures to be collected in rabbit farms for assessing animal welfare. In addition to resource‐based measures, ABMs include mortality, mastitis, pododermatitis, skin lesions, coat hygiene, and body condition score. Cerioli et al. (2011) had previously defined a scoring system for health, productive, environmental and management parameters in commercial rabbit farms. However, some of the proposed measures were invasive in nature (involving nasal, vaginal or rectal smears) and others were performed by necropsy. The Italian Ministry have therefore funded a new national project to develop a protocol for assessing rabbit welfare in different types of housing systems in Italy. This project, with assessment based on the Welfare Quality protocol, is currently ongoing and preliminary results from 12 farms have been published (Zomeño et al., 2019). In addition to resource‐based measurements, a range of animal‐based measurements are made. In a sample of 75 does, taken at random and covering all parts of the farm, in the week before weaning the following measurements are taken: weight; body condition using a five‐point scale (0–4); health status, evaluating the presence of clinical signs related to respiratory pathologies (nasal and/or ocular secretion) or digestive (diarrhoea), and the presence and severity of dermatophytosis, mastitis, ulcerative pododermatitis and other injuries. In their litters, measurements made are total weight, number of rabbits and health status (respiratory and/or digestive pathologies, dermatophytosis and other injuries). In a sample of 100 growing rabbits, taken during the week prior to slaughter, the same measurements as for does are made of weight and health status. In addition, the tonic immobility test (in 30 rabbits) and the open‐field test (in another 30 rabbits) are carried out.
3.4.2. Definition of welfare consequences relevant to farmed rabbits
Based on the measures used in existing welfare assessment schemes, and on the Working Group (WG) members’ knowledge of rabbit biology, a list of possible welfare consequences was compiled.
A welfare consequence is the change in welfare that results from the effect of a hazard or factors influencing welfare. For the survey, the following definitions for different welfare consequences were used. It is important to highlight that some welfare outcomes may be difficult to assess in rabbits, since, as a prey species, they have evolved to show minimal external signs of pain or stress.
The following list includes welfare consequences that were considered most concerning by the WG experts, to allow the questionnaire to focus on these fewer consequences.
Prolonged hunger
Definition: The animal has been unable to get enough feed to meet its maintenance requirements for energy, proteins or specific nutrients. This has resulted in failure to grow, loss of body condition such that, palpating the lumbar spine, the bones are prominent and easy to feel. Alternatively, feed intake is less than 50% of ad libitum intake for more than 1 day.
Prolonged thirst:
Definition: The animal has been unable to get enough water to satisfy its daily needs, resulting in dehydration.
Pododermatitis:
Definition: Pododermatitis with ulceration of the hocks. Hyperkeratosis and alopecia have already progressed to signs of ulceration and, in the worst cases, to crusts from bloody wound secretion, deep ulceration, and degeneration of the surrounding tissues.
Locomotory disorders (other than due to pododermatitis)
Definition: The animal has impaired locomotion as a result of, e.g. bone fractures, muscle damage or neurological disorders.
Skin lesions and wounds:
Definition: The animal has physical damage to the skin or underlying tissues e.g. multiple scratches, open or scabbed wounds or abscesses to the body or ears. This may result from aggression.
Respiratory disorders:
Definition: The animal has impaired function of the lungs or airways seen as sneezing, nasal discharge (snuffles, observed also as wet spots on the paws), laboured breathing or chronic sneezing.
Gastroenteric disorders:
Definition: The animal has impaired function of the gastrointestinal tract resulting in inappetence, loss of weight, abnormal faeces consistency (diarrhoea, mucus excretion), hard consistency of the abdomen (suggesting caecal impaction). This may result from conditions including infectious, parasitic or toxigenic agents.
Reproductive disorders:
Definition: The animal has a disorder of the reproductive system resulting from physical injury or infection, seen as infertility, or pregnancy and kindling difficulties.
Skin disorders (other than pododermatitis or skin lesions):
Definition: Skin disorders including infections (e.g. dermatophytosis/ringworm, pseudomonosis, staphylococcosis, viral diseases), ectoparasites (e.g. mange), seen as abnormal conditions of the skin or coat or excessive rubbing and scratching, hair loss unrelated to nest building behaviour, inflamed scabs or exuding skin.
Thermal stress:
Definition: The animal is unable to maintain constant body temperature by behavioural adaptation alone.
This consequence was further subdivided for the survey into two sub‐categories which were considered to have different animal outcomes and hazards.
Heat stress: The rabbit shows increased respiration rate, higher temperature of ears and keeping ears spread open and away from the body.
Cold stress: Kits (first week of life) that are unable to return to the nest, become hypothermic and immobile.
Mastitis:
Definition: The animal has inflammation of at least one of the mammary glands, indicated by swelling or a hardened mass, abscess or penetrating wound of the mammary tissue.
Neonatal disorders (including starvation/mis‐mothering and cannibalism/exposure complex):
Definition: The newborn kit shows compromised functions, seen as weakness, which results in death or would lead to death without intervention.
Restriction of movement:
Definition: The rabbit is unable to perform three consecutive hops because of physical restraint or lack of space.
Resting problem:
Definition: The animal is unable to lie comfortably because of insufficient amount of space, space of inadequate quality (in terms of floor type, dryness and hygiene), or both. This results in an inability to lie fully stretched or in coat soiling.
Inability to express maternal behaviour:
Definition: Provision of unsuitable, or absence of, nest material which challenges doe nesting behaviour and kit survival at kindling, restlessness, repeated visits to the nest box, aggression towards the kits.
Inability to express positive social interactions:
Definition: The absence of social sniffing and grooming of conspecifics or of bodily contact with conspecifics especially during resting.
Inability to express gnawing behaviour:
Definition: The absence of suitable material for the expression of gnawing behaviour such as roughage or gnawing sticks
Occurrence of abnormal behaviours:
Definition: The animal shows non‐functional behaviours not normally exhibited by healthy animals in an unrestricted environment. These include hair pulling unrelated to nest building, ear chewing or stereotypic behaviours, such as repetitive chewing, nibbling and licking at the bars of the cages or prolonged scratching.
Fear:
Definition: The animal shows signs of fearfulness such as immobility, repeated escape attempts or increased vigilance.
Two further welfare consequences were identified but were not included in the survey because they were viewed as being primarily the secondary result of other welfare consequences which had already been included.
Metabolic disorders:
Definition: ‘Metabolic disorders’ of rabbits, are mainly related with the metabolism of ions/minerals (and hazards leading to e.g. hypokalaemia, hypocalcaemia and hypercalcaemia), or the energetic metabolism (e.g. ketosis).
Pain:
Definition: Pain can be defined as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage’ (IASP, 1979).
3.5. Results from EKE on occurrence, duration and severity of welfare consequences in six housing systems
3.5.1. Results of EKE formal exercise on severity
In a first exercise, the aim was to rank the welfare consequences relative to each other according to the severity that would be experienced by the rabbit doe. Figure 12 shows the relative ranking of behaviour‐ and health‐related welfare consequences for a rabbit doe from lower to higher severity. Welfare consequences that change in severity in the different animal categories (kits and growing rabbits) were judged separately in the following rounds. For this purpose, the welfare consequences were divided into behaviour‐ (including emotional states such as fear) and health‐related consequences (including consequences such as thermal stress, hunger and thirst). Results are reported in the figures below (Figures 13, 14 and 15 for does, kits and growing rabbits respectively) from lower severity (e.g. inability to express positive social behaviour) to higher severity (e.g. inability to express maternal behaviour), separately for behaviour‐ and health‐related consequences.
Figure 12.
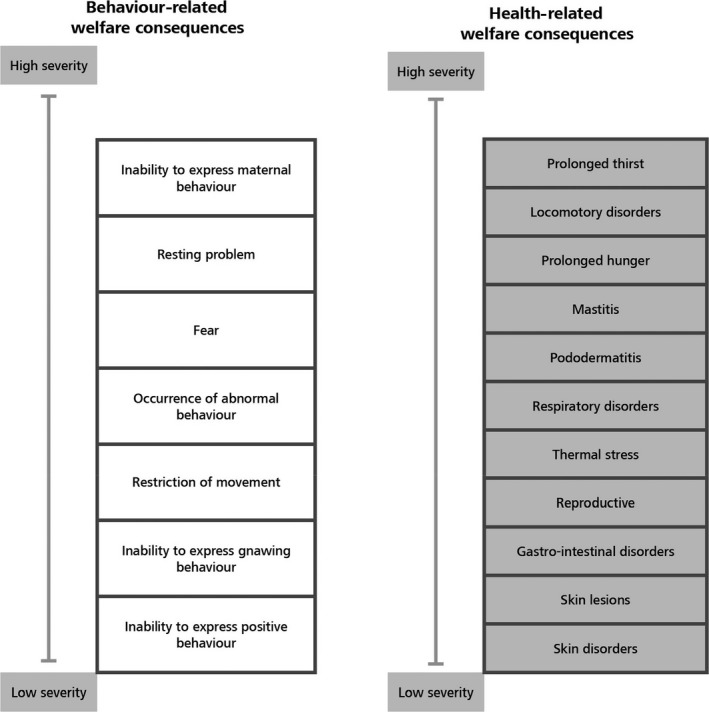
Relative ranking of behaviour‐ and health‐related welfare consequences for a rabbit doe from lower to higher severity
Figure 13.
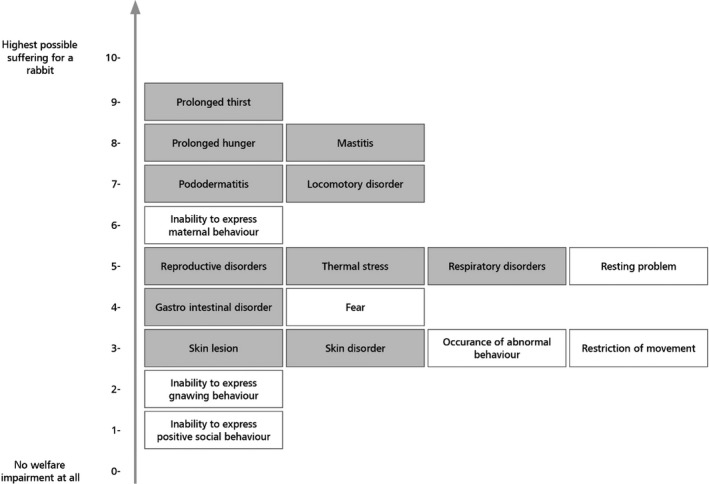
Severity scale for the welfare consequences relevant for does – in grey the health‐related welfare consequences, in white the behavioural welfare consequences
Figure 14.
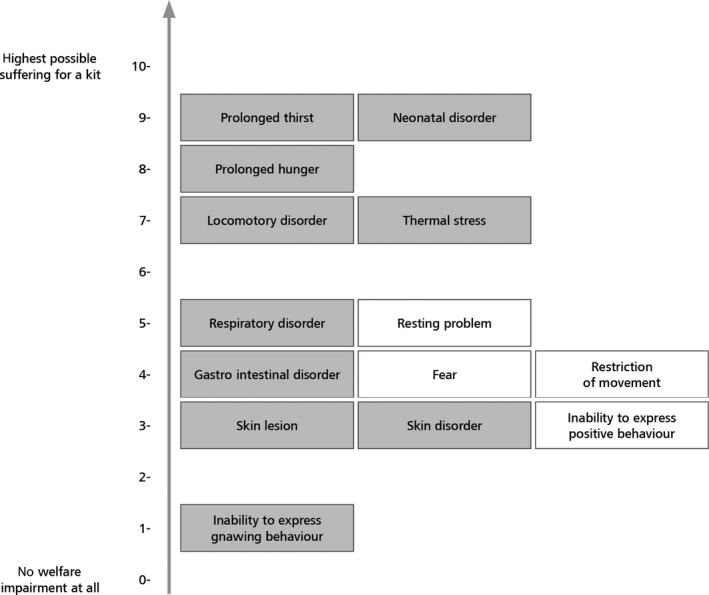
Severity scale for the welfare consequences relevant for kits – in grey the health‐related welfare consequences, in white the behavioural welfare consequences
Figure 15.
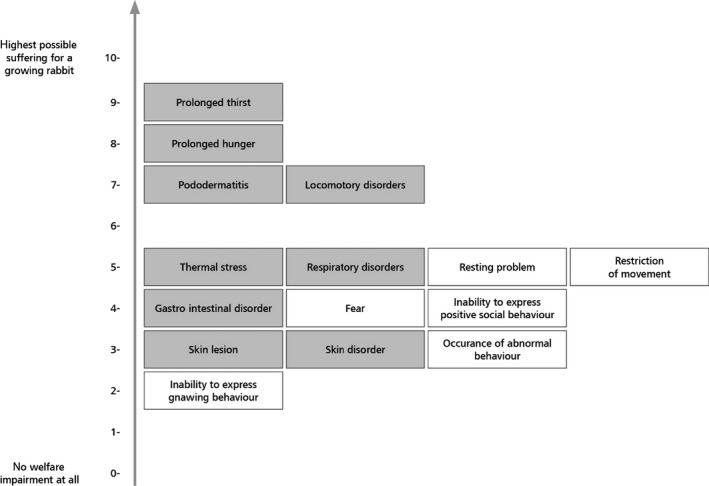
Severity scale for the welfare consequences relevant for growing rabbits – in grey the health‐related welfare consequences, in white the behavioural welfare consequences
For the behaviour‐related welfare consequences, the severity of the inability to perform positive social behaviour was scored the lowest as the experts concluded that, even though rabbits are gregarious animals, the motivation for a rabbit doe to search for social contact during pregnancy or after kindling would be relatively low. Also, there would be no physical or pathological consequences if the behaviour could not be performed. This, however, could be different for other age groups. In contrast, the motivation for maternal behaviour is high in rabbit does. As in most mammals, the mothering instinct and the connected behaviours are strong. Therefore, the welfare consequence ‘inability to express maternal behaviour’ was ranked highest in terms of severity for the rabbit doe.
For the health‐related welfare consequences, skin disorders were ranked lowest in severity relative to the other health‐related welfare consequences, whereas prolonged thirst was ranked highest.
In a second exercise, the experts, based on the previous relative rankings, merged the two sets of behaviour‐ and health‐related welfare consequences and scored them on a severity scale from 0 to 10. The experts agreed that the welfare consequence that is considered to reach this highest level of suffering for the rabbit (doe, kit, growing rabbit) would be a combination of different welfare consequences (such as experienced in myxomatosis). Therefore, it was decided to score the welfare consequence that was considered to have the highest severity with a maximum of 9.
The experts discussed possible common criteria to be used to compare health and behaviour related consequences. It was agreed that the common criteria to judge severity of the respective welfare consequences are as shown in Table 9.
Table 9.
Criteria for judgement of severity
| Criteria for judgement of severity | |
|---|---|
| Behaviour‐related welfare consequences | Health‐related welfare consequences |
|
|
|
|
|
|
In the next question, the experts were asked to judge if the severity of the welfare consequences differs among the three defined animal categories (rabbit does, growing rabbits and kits). It was agreed that only direct consequences for animal welfare should be considered. For instance, mastitis in a doe can affect the welfare of kits – but it will not be considered as a consequence for kits although it might contribute to other welfare consequences such as prolonged hunger.
The experts concluded that the following welfare consequences do not occur in kits: inability to perform maternal behaviour, reproductive disorders, mastitis, pododermatitis, occurrence of abnormal behaviour. Other consequences are instead expected to have a different severity for kits than for does (neonatal disorders, inability to perform gnawing behaviour, inability to perform positive social behaviour, restriction of movement, thermal stress). Similarly, for growing rabbits some welfare consequences do not occur (inability to perform maternal behaviour, reproductive disorders, mastitis, neonatal disorders) and others are considered to have a different severity (inability to perform positive social behaviour, restriction of movement).
A third elicitation exercise was then held to score the severity of the welfare consequences that are expected to have a different severity for kits and growing rabbits.
The severity scale resulting from the elicitations is reported in Table 10 below, where welfare consequences are ranked for rabbit does. For kits and growing rabbits, the scores for the welfare consequences for which the median score differs from those of rabbit does are highlighted in bold letters.
Table 10.
Severity of different welfare consequences experienced by rabbits as scored during the workshop (11 experts)
| Welfare consequence | Severity score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reproducing does | Kits | Growing rabbits | |||||||
| Median | Min | Max | Median | Min | Max | Median | Min | Max | |
| Neonatal disorders | na | 9 | 7 | 9 | na | ||||
| Inability to express positive social behaviour | 1 | 0 | 4 | 3 | 0 | 7 | 3 | 1 | 8 |
| Inability to express gnawing behaviour | 1 | 0 | 5 | 1 | 0 | 4 | 1 | 0 | 5 |
| Restriction of movement | 2 | 1 | 6 | 3 | 1 | 8 | 4 | 2 | 8 |
| Skin disorders | 3 | 1 | 4 | 3 | 1 | 4 | 3 | 1 | 4 |
| Occurrence of abnormal behaviour | 3 | 1 | 7 | na | 3 | 1 | 7 | ||
| Skin lesions | 3 | 2 | 5 | 3 | 2 | 5 | 3 | 2 | 5 |
| Gastrointestinal disorders | 4 | 3 | 6 | 4 | 3 | 6 | 4 | 3 | 6 |
| Fear | 5 | 2 | 7 | 5 | 2 | 7 | 5 | 2 | 7 |
| Reproductive disorders | 5 | 3 | 6 | na | na | ||||
| Resting problem | 6 | 2 | 8 | 6 | 2 | 8 | 6 | 2 | 8 |
| Respiratory disorders | 6 | 2 | 7 | 6 | 2 | 7 | 6 | 2 | 7 |
| Thermal stress | 5 | 5 | 7 | 7 | 6 | 9 | 5 | 5 | 7 |
| Inability to express maternal behaviour | 7 | 2 | 9 | na | na | ||||
| Pododermatitis | 7 | 4 | 8 | na | 7 | 4 | 8 | ||
| Mastitis | 7 | 5 | 8 | na | na | ||||
| Prolonged hunger | 8 | 7 | 8 | 8 | 7 | 8 | 8 | 7 | 8 |
| Locomotory disorders | 9 | 7 | 9 | 9 | 7 | 9 | 9 | 7 | 9 |
| Prolonged thirst a | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
na: not applicable.
Maximum severity score of all the selected welfare consequences.
3.5.2. Results of survey on occurrence and duration
The occurrence of each welfare consequence in the different housing systems and the overall duration are shown for each animal category in Tables 11–13. The occurrences described in the tables are given according to the housing system as provided by experts; duration is the median across all system of the estimate provided by the experts per each housing system.
Table 11.
Median estimates for occurrence and duration of welfare consequences in reproducing does (occurrence depending on the housing system, while duration is assumed to be similar in different housing systems)
| REPRODUCING DOES | |||||||
|---|---|---|---|---|---|---|---|
| Welfare consequence | Occurrencea | Durationa , b | |||||
| Conventional cages | Elevated pens | Enriched cages | Floor pens | Organic systems | Outdoor system | (All systems) | |
| Prolonged hunger | 6.3% | 2.8% | 8.0% | 12.6% | 2.1% | 7.3% | 44.0% |
| Prolonged thirst | 7.1% | 0.1% | 8.7% | 9.4% | 1.1% | 6.4% | 25.0 |
| Resting problem | 14.8% | 6.2% | 5.0% | 11.4% | 9.2% | 7.5% | 25.0 |
| Heat stress | 16.1% | 2.8% | 10.5% | 19.6% | 20.5% | 20.2% | 19.0% |
| Cold stress | 6.3% | 0.2% | 1.8% | 1.5% | 0.5% | 7.2% | 18.0% |
| Restriction of movement | 54.7% | 58.9% | 57.9% | 1.7% | 30.2% | 3.0% | 70.0% |
| Pododermatitis | 8.0% | 2.7% | 2.6% | 6.6% | 1.6% | 10.8% | 15.2% |
| Locomotory disorders | 2.1% | 1.4% | 0.9% | 0.9% | 0.0% | 3.1% | 12.5% |
| Skin lesions and wounds | 1.6% | 12.7% | 0.5% | 14.9% | 4.7% | 15.1% | 20.0% |
| Skin disorders | 2.9% | 1.1% | 1.9% | 7.1% | 6.5% | 5.6% | 30.0 |
| Respiratory disorders | 6.3% | 3.5% | 1.2% | 4.1% | 3.7% | 7.1% | 25.0% |
| Gastroenteric disorders | 3.0% | 2.8% | 0.0% | 5.9% | 3.3% | 10.4% | 10.5% |
| Reproductive disorders | 3.1% | 4.3% | 2.8% | 18.7% | 24.9% | 5.0% | 12.5% |
| Mastitis | 4.0% | 3.7% | 2.8% | 7.1% | 2.2% | 5.7% | 19.5% |
| Occurrence of abnormal behaviours | 5.7% | 2.5% | 0.8% | 12.1% | 2.1% | 6.4% | 20.5% |
| Fear | 3.4% | 4.5% | 0.6% | 5.9% | 6.4% | 13.7% | 10.0% |
| Inability to express maternal behaviour | 1.7% | 2.3% | 0.6% | 8.7% | 3.1% | 9.1% | 18.5% |
| Inability to express positive social interactions | 33.4% | 14.6% | 10.9% | 7.9% | 6.2% | 6.5% | 50.0% |
| Inability to express gnawing behaviour | 59.6% | 16.7% | 10.0% | 0.0% | 8.3% | 6.0% | 60.0% |
For better readability, percentages are provided here. However, please note that for the calculation of the welfare impact scores proportions were used.
Duration: Lifetime duration was defined as the proportion of the total lifetime in that production stage that an individual rabbit's welfare is impaired.
Table 13.
Median estimates for occurrence and duration of welfare consequences in growing rabbits (occurrence depending on the housing system, while duration is assumed to be similar in different housing systems)
| GROWING RABBITS | |||||||
|---|---|---|---|---|---|---|---|
| Welfare consequence | Occurrencea | Durationa , b | |||||
| Conventional cages | Elevated pens | Enriched cages | Floor pens | Organic systems | Outdoor system | (All systems) | |
| Prolonged hunger | 6.4% | 1.5% | 5.1% | 12.6% | 0.7% | 9.2% | 36.2% |
| Prolonged thirst | 7.1% | 0.1% | 9.2% | 1.2% | 2.3% | 3.8% | 22.5% |
| Resting problem | 16.8% | 7.0% | 5.8% | 9.6% | 9.9% | 8.8% | 36.0% |
| Heat stress | 11.9% | 2.5% | 0.0% | 10.7% | 16.0% | 17.4% | 11.5% |
| Cold stress | 6.2% | 0.2% | 1.9% | 1.4% | 0.5% | 9.4% | 18.0 |
| Restriction of movement | 50.8% | 5.8% | 52.9% | 0.0% | 3.0% | 5.1% | 51.0% |
| Pododermatitis | 2.1% | 0.3% | 0.4% | 3.2% | 2.5% | 5.8% | 10.0 |
| Locomotory disorders | 1.5% | 2.9% | 1.4% | 4.8% | 0.0% | 0.8% | 10.0% |
| Skin lesions and wounds | 4.4% | 5.5% | 2.3% | 17.0% | 5.2% | 28.3% | 17.5% |
| Skin disorders | 5.1% | 15.9% | 26.9% | 20.9% | 6.1% | 4.9% | 50.0% |
| Respiratory disorders | 3.4% | 1.7% | 0.9% | 2.5% | 11.2% | 21.8% | 20.0% |
| Gastroenteric disorders | 8.5% | 8.8% | 7.0% | 24.7% | 12.8% | 28.3% | 25.0% |
| Occurrence of abnormal behaviours | 3.7% | 1.7% | 1.8% | 13.6% | 1.5% | 1.4% | 25.0% |
| Fear | 2.6% | 6.8% | 3.1% | 11.0% | 13.0% | 31.0% | 20.0% |
| Inability to express positive social interactions | 15.2% | 3.7% | 2.1% | 7.3% | 0.0% | 4.3% | 43.7% |
| Inability to express gnawing behaviour | 59.5% | 10.9% | 10.7% | 5.0% | 8.3% | 11.2% | 60.0% |
For better readability, percentages are provided here. However, please note that for the calculation of the welfare impact scores proportions were used.
Duration: Lifetime duration was defined as the proportion of the total lifetime in that production stage that an individual rabbit's welfare is impaired.
Table 12.
Median estimates for occurrence and duration of welfare consequences in kits (occurrence depending on the housing system, while duration is assumed to be similar in different housing systems)
| KITS | |||||||
|---|---|---|---|---|---|---|---|
| Welfare consequence | Occurrencea | Durationa , b | |||||
| Conventional cages | Elevated pens | Enriched cages | Floor pens | Organic systems | Outdoor system | (All systems) | |
| Prolonged hunger | 4.9% | 5.0% | 4.7% | 10.6% | 7.9% | 11.3% | 35.0% |
| Prolonged thirst | 9.7% | 3.3% | 8.9% | 8.3% | 0.0% | 2.3% | 30.0% |
| Resting problem | 6.9% | 3.7% | 3.2% | 5.9% | 1.0% | 5.3% | 32.5% |
| Heat stress | 5.5% | 1.0% | 9.8% | 1.5% | 15.8% | 41.4% | 15.0% |
| Cold stress | 4.9% | 0.0% | 0.0% | 6.8% | 20.3% | 26.7% | 15.0% |
| Restriction of movement | 13.6% | 2.8% | 42.5% | 0.0% | 0.0% | 4.8% | 15.0% |
| Locomotory disorders | 0.8% | 0.4% | 0.6% | 2.3% | 0.3% | 4.4% | 3.0% |
| Skin lesions and wounds | 0.9% | 2.9% | 0.6% | 8.0% | 0.8% | 16.5 | 15.0% |
| Skin disorders | 3.1% | 2.9% | 15.1% | 0.9% | 1.5% | 3.5% | 34.5% |
| Respiratory disorders | 1.3% | 1.2% | 3.3% | 7.5% | 3.0% | 22.7% | 17.5% |
| Gastroenteric disorders | 2.7% | 2.4% | 2.2% | 9.9% | 12.8% | 33.8% | 17.5% |
| Neonatal disorders | 3.3% | 4.6% | 0.8% | 12.3% | 6.5% | 23.8% | 13.5% |
| Fear | 2.3% | 4.3% | 5.0% | 3.7% | 8.6% | 6.7% | 25.0% |
| Inability to express positive social interactions | 3.4% | 4.2% | 0.0% | 0.0% | 0.0% | 7.5% | 40.0% |
| Inability to express gnawing behaviour | 29.7% | 37.6% | 44.4% | 0.0% | 22.2% | 8.3% | 80.0% |
For better readability, percentages are provided here. However, please note that for the calculation of the welfare impact scores proportions were used.
Duration: Lifetime duration was defined as the proportion of the total lifetime in that production stage that an individual rabbit's welfare is impaired.
These data were then used, together with the severity score, in the calculation of an overall welfare impact score and the identification of the most important welfare consequences in each housing system.
3.5.3. Overall welfare impact score
Tables 14, 16 and 18 first summarise for each rabbit category the overall welfare impact score for each housing system. For this purpose, the values for each welfare consequence were calculated as the product of occurrence (range 0–1, i.e. proportion of population affected at least once during that stage), duration (range 0–1, i.e. proportion of total time in that stage) and severity (range 0–9). These products were then summed for all relevant welfare consequences to give the overall welfare impact score. Higher values indicate poorer welfare with a maximum possible value of 9 multiplied by the number of different welfare consequences. However, this would represent the hypothetical extreme situation in which 100% of all animals experience the maximum welfare detriment for all consequences for the whole of their life time.
Table 14.
Median overall welfare score and 90% probability intervals for reproducing does in the different housing systems
| Does | Conventional cages | Elevated pens | Enriched cages | Floor pens | Organic systems | Outdoor systems |
|---|---|---|---|---|---|---|
| Median Score | 3.2 | 2.0 | 2.1 | 2.3 | 1.8 | 2.1 |
| P05 | 1.8 | 1.1 | 1.0 | 1.2 | 1.0 | 1.2 |
| P95 | 5.4 | 3.9 | 4.1 | 4.0 | 3.3 | 3.3 |
Table 16.
Median overall welfare score and 90% probability intervals for kits in the different housing systems
| Kits | Conventional cages | Elevated pens | Enriched cages | Floor pens | Organic systems | Outdoor systems |
|---|---|---|---|---|---|---|
| Median score | 1.3 | 1.0 | 1.5 | 1.6 | 1.4 | 2.6 |
| P05 | 0.5 | 0.4 | 0.7 | 0.8 | 0.8 | 1.8 |
| P95 | 2.4 | 1.9 | 2.8 | 2.9 | 2.5 | 3.7 |
Table 18.
Median overall welfare score and 90% probability intervals for growing rabbits in the different housing systems
| Conventional cages | Elevated pens | Enriched cages | Floor pens | Organic systems | Outdoor systems | |
|---|---|---|---|---|---|---|
| Median score | 3.5 | 1.0 | 2.6 | 2.0 | 1.2 | 2.6 |
| P05 | 2.1 | 0.5 | 1.4 | 1.0 | 0.7 | 1.6 |
| P95 | 5.9 | 2.0 | 4.7 | 3.3 | 2.1 | 3.8 |
As an example, more related to real life situations, if 10% of does experienced a really severe welfare problem for a short period of time e.g. severity 8 for 2 days (0.5% of their lifetime), this contributes 0.1 × 0.005 × 8 = 0.004 to the welfare impact score for that system. Furthermore if 50% of does experience a less severe welfare problem for a long period of time e.g. severity 3 for 200 days (55% of their lifetime), this contributes 0.5 × 0.55 × 3 = 0.825 to the welfare impact score. Therefore, if both of these occur in the same system, they would add together to give 0.004 + 0.825 = 0.829.
Additionally, the results of the simulation for the overall welfare impact score are provided for each rabbit category, indicating how often a certain housing system would be expected to actually perform lower or higher in terms of overall welfare (Tables 15, 17 and 19). For example, the simulation for conventional cages for does (first column in Table 15) revealed that in more than 75% of the runs welfare was lower in conventional cages compared to the other 5 systems (i.e. conventional cages obtained a higher welfare impact score).
Table 15.
Results of the simulation for reproducing does, where the percentage indicates how often a certain housing system achieved a lower or higher overall welfare impact score compared to the other systems
| Does | System has lower welfare (higher welfare impact score) | ||||||
|---|---|---|---|---|---|---|---|
| Conventional cages | Elevated pens | Enriched cages | Floor pens | Organic systems | Outdoor systems | ||
| System has higher welfare (lower welfare impact score) | Conventional cages | – | 20% | 21% | 25% | 13% | 17% |
| Elevated pens | 80% | – | 49% | 59% | 40% | 51% | |
| Enriched cages | 79% | 51% | – | 58% | 42% | 51% | |
| Floor pens | 75% | 41% | 42% | – | 32% | 41% | |
| Organic systems | 87% | 60% | 58% | 68% | – | 61% | |
| Outdoor systems | 83% | 49% | 49% | 59% | 39% | – | |
Table 17.
Results of the simulation for reproducing does, where the percentage indicates how often a certain housing system achieved a lower or higher overall welfare impact score compared to the other systems
| Kits | System has higher welfare score (lower welfare) | ||||||
|---|---|---|---|---|---|---|---|
| Conventional cages | Elevated pens | Enriched cages | Floor pens | Organic systems | Outdoor systems | ||
| System has lower welfare score (higher welfare) | Conventional cages | – | 33% | 60% | 65% | 57% | 94% |
| Elevated pens | 67% | – | 75% | 80% | 75% | 98% | |
| Enriched cages | 40% | 25% | – | 54% | 46% | 89% | |
| Floor pens | 35% | 20% | 46% | – | 41% | 87% | |
| Organic systems | 43% | 25% | 54% | 59% | – | 93% | |
| Outdoor systems | 6% | 2% | 11% | 13% | 7% | – | |
Table 19.
Results of the simulation for growing rabbits, where the percentage indicates how often a certain housing system achieved a lower or higher overall welfare impact score compared to the other systems
| Growing rabbits | System has higher welfare score (lower welfare) | ||||||
|---|---|---|---|---|---|---|---|
| Conventional cages | Elevated pens | Enriched cages | Floor pens | Organic systems | Outdoor systems | ||
| System has lower welfare score (higher welfare) | Conventional cages | – | 1% | 26% | 10% | 1% | 22% |
| Elevated pens | 99% | – | 96% | 88% | 64% | 97% | |
| Enriched cages | 74% | 4% | – | 29% | 6% | 49% | |
| Floor pens | 90% | 12% | 71% | – | 17% | 73% | |
| Organic systems | 99% | 36% | 94% | 83% | – | 96% | |
| Outdoor systems | 78% | 3% | 51% | 27% | 4% | – | |
Based on these tables, statements which translate the percentages from the tables into degrees of certainty were added to the conclusions of the opinion (for example, a percentage of 75% falls into the range for ‘likely’ (66–90%) used in the probability scale of the EFSA uncertainty assessment guidance (EFSA, 2019).
The welfare of reproducing does is lower in conventional cages (median overall impact score: 3.2 with 90% probability interval of 1.8–5.4) compared to the five other housing systems (medians of 1.8 [90% probability interval 1.0–3.3] to 2.3 [90% probability interval 1.2–4.0]). However, among the other systems, no distinction can be made regarding the welfare impact on does.
The welfare of kits is lower in outdoor systems (median overall impact score: 2.6 with 90% probability interval of 1.8–3.7) compared to the other systems and the welfare is higher in elevated pens (median overall impact score: 1.0 with 90% probability interval of 0.4–1.9) than in the four other systems (medians of 1.3 [90% probability interval 0.5–2.4] to 1.6 [90% probability interval 0.8–2.9]). However, no distinction can be made among the conventional cages, enriched cages, floor pens and organic systems regarding the welfare impact on kits.
The welfare of growing rabbits is lower in conventional cages (median overall impact score: 3.5 with 90% probability interval of 2.1–5.9) compared to the other systems and the welfare is higher in elevated pens (median impact score: 1.0 with 90% probability interval of 0.5–2.0) than in the other systems (medians of 1.2 [90% probability interval 0.7–2.1] to 2.6 [90% probability interval 1.4–4.7]). However, no distinction can be made among the enriched cages, floor pens, organic systems and outdoor systems regarding the welfare impact on growing rabbits.
3.5.4. Highest ranking welfare consequences for each animal category and housing system
To identify the most critical welfare issues in each housing system, the different welfare consequences were ranked according to their welfare score (occurrence * duration* severity, with a theoretical maximum score of 10). Tables 20, 21–22 show the top 5 ranked consequences for each animal category.
Table 20.
Top 5 welfare consequences for reproducing does per system (from probabilistic values as above)
| Conventional | Elevated pens | Enriched cages | Floor | Organic | Outdoor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score |
| 1 | Restriction of movement | 0.87 | Restriction of movement | 0.92 | Restriction of movement | 0.90 | Prolonged hunger | 0.36 | Restriction of movement | 0.45 | Heat stress | 0.18 |
| 2 | Inability to express gnawing behaviour | 0.40 | Inability to express gnawing behaviour | 0.11 | Heat Stress | 0.09 | Heat stress | 0.18 | Heat stress | 0.19 | Pododermatitis | 0.11 |
| 3 | Resting problem | 0.21 | Skin lesion | 0.09 | Resting Problems | 0.05 | Resting Problem | 0.15 | Reproductive Disorders | 0.15 | Resting problem | 0.11 |
| 4 | Inability to express positive social behaviour | 0.15 | Resting problem | 0.08 | Inability to express gnawing behaviour | 0.04 | Reproductive disease | 0.11 | Resting problem | 0.12 | Skin lesion | 0.10 |
| 5 | Heat stress | 0.15 | Inability to express positive social behaviour | 0.08 | Inability to express positive social behaviour | 0.04 | Skin lesion | 0.10 | Skin disorder | 0.05 | Respiratory disorders | 0.09 |
Table 21.
Top 5 welfare consequences for kits per system (from probabilistic values as above)
| Conventional | Elevated pens | Enriched cages | Floor | Organic | Outdoor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score |
| 1 | Inability to express gnawing behaviour | 0.22 | Inability to express gnawing behaviour | 0.30 | Inability to express gnawing behaviour | 0.36 | Prolonged hunger | 0.31 | Prolonged hunger | 0.23 | Heat stress | 0.45 |
| 2 | Resting problem | 0.08 | Prolonged hunger | 0.14 | Restriction of Movement | 0.24 | Prolonged thirst | 0.18 | Cold stress | 0.21 | Prolonged hunger | 0.32 |
| 3 | Heat stress | 0.07 | Neonatal disorder | 0.06 | Skin Disorder | 0.13 | Neonatal disorder | 0.16 | Heat stress | 0.19 | Neonatal disorder | 0.29 |
| 4 | Prolonged hunger | 0.06 | Fear | 0.03 | Heat stress | 0.04 | Gastro intestinal disorder | 0.09 | Gastrointestinal disorder | 0.09 | Cold stress | 0.28 |
| 5 | Restriction of movement | 0.05 | Skin disorder | 0.03 | Respiratory disorder | 0.04 | Cold stress | 0.07 | Neonatal disorders | 0.09 | Gastrointestinal disorder | 0.25 |
Table 22.
Top 5 welfare consequences for growing rabbits per system (from probabilistic values as above)
| Conventional | Elevated pens | Enriched cages | Floor | Organic | Outdoor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score | Welfare consequence | Welfare score |
| 1 | Restriction of Movement | 1.29 | Skin disorder | 0.17 | Restriction of movement | 1.34 | Gastrointestinal disorder | 0.25 | Resting problem | 0.16 | Gastrointestinal disorder | 0.30 |
| 2 | Inability to express gnawing behaviour | 0.41 | Resting problem | 0.10 | Skin disorder | 0.31 | Skin disorder | 0.24 | Gastrointestinal disorder | 0.14 | Respiratory disorder | 0.25 |
| 3 | Resting problem | 0.32 | Gastrointestinal disorder | 0.09 | Gastrointestinal disorder | 0.08 | Prolonged hunger | 0.23 | Respiratory disorders | 0.12 | Fear | 0.25 |
| 4 | Inability to express positive social behaviour | 0.17 | Inability to express gnawing behaviour | 0.06 | Inability to express gnawing behaviour | 0.05 | Resting Problem | 0.17 | Heat stress | 0.11 | Prolonged hunger | 0.20 |
| 5 | Prolonged hunger | 0.14 | Fear | 0.06 | Resting problem | 0.04 | Skin lesion | 0.10 | Fear | 0.11 | Resting Problem | 0.15 |
The results for does (Table 20) show that resting problems are a main welfare concern in all six systems, heat stress in five systems, restriction of movement in four systems and gnawing and skin lesions in three systems. The median values of the welfare scores ranged from a minimum of 0.04 to a maximum of 0.92. Welfare scores were markedly higher for restriction of movement in most systems than for other welfare consequences.
The score for restriction of movement is similar across the three more conventional systems (conventional cages, elevated pens and enriched cages). Although one might expect that the score for restriction of movement might be higher in conventional cages that in the remaining systems, the results of the survey show similar values. This might be due to the fact that the definition of ‘restriction of movement’ was to perform three consecutive hops. This may explain why the welfare scores are similar in these three systems, even though the surface area is different.
For the niche systems that offer the possibility for the rabbit to perform three consecutive hops, the restriction of movement is also in the top 5 welfare consequences.
Gnawing is the second most important welfare consequence in conventional cages and, while also appearing in the top 5 for elevated pens and enriched cages, the values for the latter two are lower.
In comparison, heat stress is one of the most important welfare consequences for enriched cages, floor pens, organic and outdoor systems. For the three latter systems, resting problems are in the same range as in conventional cages, organic and outdoor systems.
Hunger appears as a high ranked welfare consequence only in the floor pen system, where it has a relatively high welfare score.
Social behaviour appears in the top 5 welfare consequences in the conventional cages and enriched cages but has a relatively low welfare score in all cases because of its relatively low severity score.
Pododermatitis is highlighted only in the outdoor system. Other health problems (reproductive and respiratory disorders) also appear in the top 5 only in the niche systems with relatively low welfare scores.
While skin lesions also occur in the top 5 for three of the systems, the welfare scores are again relatively low.
The results for kits (Table 21) show that hunger is a main welfare concern in five systems, neonatal disorders and heat stress in four systems and gnawing and cold stress in three systems. The median values of the welfare scores ranged from a minimum of 0.03 to a maximum of 0.45. Welfare consequences were scored more highly in the outdoor system but overall no welfare consequence had a consistently higher welfare score than others across all systems.
Hunger was absent from the top 5 welfare consequences for enriched cages only. This was another unexpected result.
Neonatal disorders were in the top 5 welfare consequences in the niche systems (floor pens, outdoor and organic systems). This may stem from greater concerns about maternal health, environmental control and kit management practices. This, however, does not explain the occurrence of neonatal disorder in the top 5 for elevated pens.
Gnawing in kits is similarly highly ranked across the three more conventional systems (conventional cages, elevated pens and enriched cages). Occurrence of inability to gnaw as a main welfare consequence for kits is unexpected as there is no literature description about this topic in such young rabbits. Similarly, restriction of movement, which was reported as a main welfare consequence in two systems, is unexpected for kits.
Thermal stress was a main welfare consequence in all systems, with heat stress affecting all systems except elevated pens and floor pens. This may be explained by the fact that these systems are generally in use in regions with lower temperature in the summer months. Cold stress was an issue in the organic and outdoor systems, where it could be explained by reduced environmental control associated with outdoor access, although this does not explain the result in the floor pens.
Gastroenteric disorders were in the top 5 in floor pens, organic and outdoor systems, likely due to the greater exposure to pathogens and environmental stressors in these systems.
The results for growing rabbits (Table 22) show that resting problems are a main welfare concern in all six systems, gastroenteric disorders in five systems and skin disorders, hunger, fear and gnawing in three systems. The median values of the welfare scores ranged from a minimum 0.04 to a maximum of 1.34. Welfare scores were markedly higher for restriction of movement where this welfare consequence is ranked in the top 5.
Although gastroenteric disorders did not enter the top 5 welfare consequences for the conventional cages, a similar score was recorded in this system (0.09) as in the other ones.
The inability to gnaw was a main consequence for conventional cages, elevated pens and enriched cages, which is associated with the absence of gnawing material in these systems (enriched cages and elevated pens do not necessarily include gnawing material, whereas gnawing material is very uncommon in conventional cages).
The score for restriction of movement is similar and high for conventional cages and enriched cages. The lack of difference between these two systems is partly explained by the fact that the same duration was used for all systems. Thus, although conventional cages restrict growing rabbits for a greater proportion of the rearing phase as these are smaller than enriched cages, this would not be reflected in the welfare scores.
Resting problems are featured in the top 5 for all systems, but with the highest score for conventional cages, intermediate score for niche systems and the lowest score for elevated pens and enriched cages. Multiple hazards might be involved in differences between systems, including available surface area, and floor characteristics and cleanliness, and social interactions.
The occurrence of hunger and skin disorders in the top 5 for some systems but not others cannot be explained by inherent differences between the systems.
The occurrence of fear in the top 5 for organic and outdoor systems may result from exposure to outdoor stimuli. The fact that fear appears in the top 5 score in elevated pens has no obvious explanation.
Respiratory disorders were in the top 5 for systems with reduced environmental control (outdoor and organic systems).
3.6. Results from literature on welfare consequences and associated hazards
To assist in the interpretation of the welfare scores in the different systems, and to identify aspects of production systems other than housing which might impact on rabbit welfare, a literature review was carried out on each welfare consequence. This began with a review of information in the 2005 EFSA Opinion on rabbit welfare, and then considered new literature published since this time (see Appendix A). Seventy‐five publications from the literature search were considered relevant. They were complemented by additional publications mainly from the proceedings of the World Rabbit Science congresses held in the last 10 years but also by original language, other than English, publications. At the end of each welfare consequence review there is a table indicating the main hazards and their degree of scientific support (Tables 23–50). Hazards written in bold are scientifically proven by more than one source. Hazards written in normal text were found in only one paper. Hazards indicated in italic have been mentioned by the experts invited to the technical hearing meeting based on their personal experience but could not be proven by literature. The term hazard is used in a general sense to indicate anything which might have the potential to cause a welfare consequence, rather than in a strict epidemiological sense.
Table 23.
Voluntary feed intake and energy requirements according to the rabbit categoriesa
| Young rabbits | Pregnant does | Lactating does | Non‐reproducing does and bucks | |
|---|---|---|---|---|
| Feed intake (g/day per kg LW 0.75 ) | 65 | 75 | 105 | 60 |
| Digestible energy for maintenance (kJ/day per kg LW 0.75 ) | 430 | 430 | 430 | 400 |
| Digestible energy for growth (kJ/day per kg LW 0.75 ) | 500 | |||
| Digestible energy for lactation (250 g milk produced, kJ/day per kg LW 0.75 ) | 750 |
Table 50.
Hazards related to pain. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Group housing, injuries from aggression, injuries arising from poor flooring or cage equipment |
| Ambient condition | No hazard identified |
| Genetics | No hazard identified |
| Nutrition and feeding | Mismanaged feeding causing enteric disease |
| Management of biosecurity | Health conditions (respiratory, enteric and reproductive diseases, pododermatitis) |
| Management of reproduction | Poor AI technique, injection |
| Other |
Identification procedures – tattooing, ear tagging Poor handling of animals (cage changes, transfer in boxes for slaughter trucks) |
3.6.1. Prolonged hunger
The feeding should cover all the needs of the animal, such as metabolic maintenance, locomotion, growth, gestation or milk production. In conventional systems, rabbits are most commonly housed in controlled environment buildings to maximise the efficiency of feed use by ensuring that they are kept, at all times, under thermo‐neutral conditions. Thus, their energy requirements to produce heat for thermoregulation is reduced, in comparison with rabbits kept outdoors.
A properly balanced diet and water supplied in adequate amounts should avoid physical and psychological suffering from hunger and thirst. However, ‘prolonged hunger’ may arise from absence of sufficient feed in subsistence systems, malfunction of the feeding systems, the deliberate prolonged restriction of feed for growing rabbits over specific periods, and the possibility of nutrient specific hungers arising from imbalances between the diet supplied and the metabolic needs of the animal. Furthermore, if a low water intake is observed (for any reason), this may lead to a low feed intake and thus to chronic hunger.
In all systems, the nutritional needs vary greatly according to the physiological state of the rabbit (age, lactation period, etc.). As an example, in Table 23 the voluntary feed intake and need for energy are summarised for several categories of rabbit kept in a conventional system. Moreover, these needs also vary according to the genetic background of the animal. In conventional rabbit farming, high‐performance (growth, milking) lines are used and, for example, the requirements for lactation depend on the litter size, since milk production is positively correlated to litter size (Maertens et al., 2006).
In conventional systems, feed is usually given ad libitum to the animals, and feeding systems can be highly automated. Logically, the animals should therefore never experience hunger through prolonged absence of feed. Although breakdown of the automated supply systems can occur and cause temporary situations of feed deprivation, daily checks by farmers normally guarantee immediate intervention. Risks arise where feed and water provision to the animals does not ensure a reliable supply or where automated systems are not regularly checked and maintained and have no alarm systems to alert staff in case of breakdown.
Where feed is restricted, the ability of all animals to access a fair share may be compromised by inadequate width of feeders. In badly designed accommodation with inadequate feeding space, low ranking animals could be unable to access adequate feed and could be subject to aggression from other more dominant rabbits (Dalmau et al., 2015). However, in good farming conditions, a dominant rabbit usually does not express very different feeding behaviour compared to a dominated one (Le Normand et al., 2013).
For the reproducing doe, the production level can challenge its metabolic homeostasis, and the live‐weight and body condition may be reduced. Situations of ‘under‐nutrition’ or ‘nutritional hunger’ can arise transiently when a doe nurses a large litter (> 10 kits) and when her voluntary feed intake does not cover the needs for maintenance and milk production, especially in the first 1–2 productive cycles before does reach their full ingestion capacity (Xiccato and Trocino, 2010). Loss of body condition can also occur in ‘hot environments’, where voluntary feed intake is reduced. Moreover, in intensive rabbit farming, high‐prolificacy lines are used and, even when the feeds are correctly formulated, the doe may lose weight and body condition during several days (around the lactation peak), since the need for milk production is positively correlated to the litter size. After the lactation period, with correct feeding, the doe recovers her weight; if not this could compromise rebreeding. However, a feed which is not adequately balanced to meet the doe's physiological needs could lead to metabolic disorders (specific hungers) and even to severe pathology or sudden death (at the kindling period or start of lactation).
Some situations of chronic hunger may arise in kits before weaning, when the milking capacity of the doe is insufficient (poor body condition, pathology, poor maternal behaviour). This situation is particularly hard to manage for young rabbits before three weeks of age, since they are not themselves able to access solid feed (especially before two weeks old) in sufficient quantity.
Rabbits may also be temporarily restrict‐fed for specific purposes in the production cycle. Two main purposes are relevant for a temporary feed restriction strategy: after the weaning for the young growing rabbit, and for the young females for breeding (between 12 and 17 weeks of age). For the young growing rabbit, feed restriction strategies are currently used in most of the French rabbit farms to reduce the risk of digestive troubles after weaning and improve the resistance against ERE (epizootic rabbit enteropathy). The intake level during feed restriction programs usually ranges from 70% to 90% of the ad libitum daily intake, and the duration of the restriction period ranges between 1 and 4 weeks (Gidenne et al., 2012a). Thus, under such a restriction strategy, the young rabbit experiences transient hunger, every day for 5–8 h, and the growth is impaired in proportion to the restriction intensity. Nevertheless, and contrary to other species, even with a restriction of 25%, welfare detriment of the growing rabbit could not be demonstrated by Martignon et al. (2011), since stereotypic behaviour or aggressive behaviour were not detected. On the other hand, Dalmau et al. (2015) observed that rabbits with 25% restriction showed some competition for feed and drink, with signs of agonistic behaviour such as biting, displacement and animals jumping on top of each other. However, this competition did not impact the growth of the animal and body weigh homogeneity within a cage, suggesting that all animals could consume similar quantities of feed. Similarly, Pinheiro et al. (2012) observed that limiting access to feed to 10 h/day seemed beneficial to rabbits because it did not impair growth and improved feed efficiency, although some behaviours were modified. Besides, no ‘competitive’ behaviour to access the feeder and no increase in injury were observed compared to the control group, and variability of growth was similar to the control (Gidenne et al., 2012b). It was hypothesised that, since the rabbit has a natural eating behaviour with numerous meals in a day (n = 20 to 30, so a small stomach capacity), one rabbit in a group cannot express ‘dominant’ behaviour for the feed. Furthermore, a restricted growing rabbit drinks more compared to the control (Boisot et al., 2004). In return for a daily ‘transient’ hunger, a higher health status is observed and thus a higher welfare.
A potential situation of chronic hunger could be found for restrict‐fed growing rabbit females destined for breeding since, when fed ad libitum between 12 and 17 weeks of age, the growing female could be over‐fattened, thus leading to fertility disorders (Rebollar et al., 2011; Naturil‐Alfonso et al., 2017). This situation is encountered in some farms that choose to restrict the level of feed offered, rather than to give a specific ‘low density’ feed (such as a high‐fibre diet). Giving a high‐fibre feed increases the feeding time, and a greater dietary bulk promotes satiety (Gidenne et al., 2012a), as found in pigs and poultry (Meunier‐Salaün et al., 2001; Nielsen et al., 2011). Since rabbits in this category are housed individually, there is no competition for the feed. Furthermore, the presence of a foraging substrate in the form of bedding or daily provision of a foraging material, such as chopped straw, compressed fodder or wood shavings, allows the animals to express foraging behaviour in a relatively natural way, and reduces the risk of hunger and development of abnormal stereotyped behaviour patterns. In outdoor niche systems, chronic hunger should be encountered more rarely, provided the pasture (or hay supply) is accessible.
In conclusion, the occurrence of prolonged hunger appears to be infrequent under current conventional rabbit farming. However, transitory hunger is possible for the young weaned rabbit subjected to a feed restriction strategy, although this is compensated by a lower risk of digestive pathologies. There is also a risk of transitory hunger for the feed restricted young rabbit female (during 5 weeks before the first reproductive cycle), unless ameliorated by use of a low energy diet.
Table 24 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 24.
Hazards related to prolonged hunger. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing |
Badly designed or managed (soiled) feeder leading to reduced access or increased competition for feed Poorly designed or managed drinker leading to low water intake |
| Ambient condition |
Low (< 10°C) or high (> 30°) ambient temperature High humidity leading to a poor quality of the feeds |
| Genetics | High prolificacy lines |
| Nutrition and feeding |
Breakdown of automatic feeding systems Underfeeding due to too high feed restriction Badly formulated feeds, inadequate for nutritional requirements |
| Management of biosecurity | Unhygienic feed through soiling |
| Management of reproduction | Intensive reproduction cycle length (35–38 days) |
| Other | No hazard identified |
3.6.2. Prolonged thirst
Feed and water intake are closely related (Gidenne et al., 2012a). The ratio of water/feed intake ranges usually around 1.8 for the growing rabbit but reaches 2.1 in the milking doe. It increases in hot climatic conditions, to over 3.1 for a 30°C air temperature (De Blas and Wiseman, 2010). In addition, a rabbit reduces its water intake when the temperature of the water is too low or too high (< 10°C or > 25°C; Remois et al., 1999); the comfortable temperature is around 20°C. Another possibility giving rise to insufficient drinking is an inadequate drinking system (position of the drinker or bad nipple, etc.), sometimes observed with backyard rabbit farming.
For indoor conventional systems, the main hazard for prolonged thirst is a malfunction of the drinking system. Problems are more likely where automated systems are used but are not regularly maintained (checked and cleaned), and have no alarm system to alert staff in case of breakdown. In outdoor systems, hazards are linked to environmental temperatures which are either too high, increasing the need to provide supplementary/fresh water, or too low, when water may freeze.
In addition, the quality of the drinking water can be a hazard. For example, water which is too saline is consumed less, and may lead to insufficient drinking and chronic thirst.
One possibility to give rise to transitory thirst corresponds to a strategy of feed restriction through a deliberate limitation of the time of access to drinking water (Foubert et al., 2008; El Maghraby, 2011; Bovera et al., 2013). Farmers, who do not have a system to quantitatively restrict the feed intake, may use a drinking restriction to induce feed restriction. For this purpose, the access to water may be limited to 2–3 h per day to reach a 15–30% feed reduction. As a consequence, the water to feed ratio is reduced from 1:74 for rabbits fed ad libitum to 1:54 for those receiving water during only 1.5–4 h/day (Foubert et al., 2008). Apart from a prolonged thirst, such deprivation of water may lead to metabolic disturbances and kidney dysfunction, particularly in a hot season or climate.
In conventional systems, drinking behaviour is usually observed through the feed intake behaviour, and the quality of the drinking water is particularly checked. The drinking behaviour of the milking doe is particularly monitored to avoid any disturbance in the milk production and thus in the health of the young. Therefore, dehydration is almost never observed in a conventional system. In outdoor systems, when the rabbit has access to fresh pasture or plants (roots etc.), the water needs are greatly reduced and thus the drinking behaviour may be less frequent. When the temperature falls below zero, drinking water freezes and this could lead to prolonged thirst without intervention by the farmers.
Table 25 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 25.
Hazards related to prolonged thirst. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those on italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Badly designed drinker leading to competition for water access |
| Ambient condition | Low (< 10°C) or high (> 30°) ambient temperature |
| Genetics | No hazard identified |
| Nutrition and feeding |
Breakdown of automatic watering systems Poor quality of the water |
| Management of biosecurity | No hazard identified |
| Management of reproduction | No hazard identified |
| Other | No hazard identified |
3.6.3. Pododermatitis
Sore hocks or ulcerative pododermatitis is a multifactorial disease, which often involves Staphylococcus aureus as an opportunistic infectious agent (EFSA, 2005). It can affect the plantar surface of the foot and more frequently the heel of the hind foot. It can affect both front and rear limbs and be monolateral or bilateral. In ulcerative pododermatitis, hyperkeratosis and alopecia are associated firstly to scabs from clear wound secretion and beginning ulceration; then to scabs from bloody wound secretion and ulceration; and, in the worst cases, to crusts from bloody wound secretion, deep ulceration, and degeneration of the surrounding tissues (Dreschen and Schlenden‐Böbbis, 1996).
These conditions are painful for the animal, which may reduce movement and adopt postures and abnormal behaviour to alleviate pain (EFSA, 2005).
To our knowledge, ulcerative pododermatitis has never been described in growing rabbits kept on the wire mesh floor of conventional cages or on litter floors. However, Masthoff and Hoy (2019) reported that 25.3% of growing rabbits showed injuries on hind limbs, 10.4% of rabbits had moderate lesions and 3.1% had severe lesions, when kept on a plastic floor with maximum slat width of 11 mm and a degree of perforation of the raised level of 15%, designed in accordance with the latest German animal protection regulation. This percentage of injuries was significantly higher compared to values (0.7–7.2% of injured rabbits) recorded on other types of plastic slatted floor with different slot (5–12 mm) and slat width (10–13 mm) and different degrees of perforation both at the floor and platform level (50–75%).
Ulcerative pododermatitis mainly affects adult breeding rabbits, both females and males; its occurrence increases with animal age and may vary with genetic lines (Rosell et al., 2000), being more frequent in heavy weight rabbit breeds. In a large data set from 16 conventional farms in Spain, sore hocks accounted for 0.3% of the median monthly cumulative incidence of culling (Rosell and de la Fuente, 2009a).
The main data on the prevalence of ulcerative pododermatitis in reproducing does in different studies and under different conditions are summarised in Table 26.
Table 26.
Prevalence of ulcerative pododermatitis in reproducing does (modified from Szendro et al., 2019)
| Author | Period/age | Animal category/production system | Prevalence (% of does) |
|---|---|---|---|
| Rosell and De La Fuente (2013) | Several ages | 105,009 does in conventional farms (2001–2012) |
Ulcerative pododermatitis 5% ± 0.3 (with footrest) 14% ± 0.3 (without footrest) |
| Rommers and de Jong (2011) | At the 5th kindling | 250 does in 5 conventional farms (50 per farm) |
Non–ulcerative and ulcerative pododermatitis 19% (with footrest) 87% (without footrest) |
| Rosell and de la Fuente (2009b) | Until the 5th kindling | 224 does in 1 conventional farm |
Accumulated incidence of ulcerative pododermatitis: 15% (with footrest) 72% (without footrest) Curative effect of footrest in 81% of affected does |
| Miko et al. (2014) | At the 5th kindling | 108 does |
Ulcerative pododermatitis: 48% in wire cages without footrest 0% in wire cages with footrest 5% in pens with wire mesh platform and footrest 0% in pens with plastic platform |
| Buijs et al. (2014) | At the 5th kindling | 72 does |
Ulcerative pododermatitis: 0% in all groups Small (29% of all does) and large (11%) hyperkeratosis area and cracked callus (1.4%): 65% in wire pens with plastic mats 5% in plastic slatted pens 68% in wire floor cages with plastic mats |
| Ruchti et al. (2018) | Several ages | 1,090 does (= 30% of total Switzerland group‐housed does) in 17 farms that used floor pens with litter (June‐September 2016) |
Ulcerative pododermatitis: 25% Hyperkeratosis and scaling: 68% |
Based on literature, the main hazards are flooring (type and integrity) and its hygiene (cleanliness and presence of faecal residuals) (EFSA, 2005). In particular, altered (abrasive, rusty, corroded or broken) wire mesh could favour micro‐traumatic lesions which can initiate, after bacterial contamination, the development of sore hocks. Nevertheless, regarding wire diameter, the increase from 2 to 3 mm is not effective in reducing sore hocks (de Jong et al., 2008). The use of a plastic mat over the wire mesh of the cage floor has been shown to reduce ulcerative pododermatitis both in the field (Rosell and de la Fuente, 2009a,b; Rommers and de Jong, 2011; Rosell and De La Fuente, 2013) and under controlled conditions (Miko et al., 2014). On the other hand, when comparing different types of flooring and housing (wire mesh floor in individual cages equipped with plastic mat vs. wire mesh equipped with plastic mats in collective systems vs. fully slatted plastic floor in collective systems), Buijs et al. (2014) did not observe any ulcerative pododermatitis in any doe after four reproducing cycles. Nevertheless, in the same study, the use of fully plastic floors reduced plantar hyperkeratosis (hair loss and callus formation which are expected to be not painful), compared to the other two systems. In Switzerland, under alternative systems based on collective housing in parks with litter on a solid floor or on plastic slats combined with plastic platforms, 25% of the rabbits displayed ulcerative pododermatitis on at least one hind leg (Ruchti et al., 2018). In these systems, non‐ulcerative (hyperkeratosis, alopecia and scaling) and ulcerative pododermatitis were present in 40% of nulliparous and primiparous does (Ruchti et al., 2018). In these systems, the relative humidity inside the barns, body weight, number of kindlings, age, and claw length were identified as the most important hazards (Ruchti et al., 2019). In contrast, recordings in housing systems that used wire mesh or plastic floors showed no sign of hyperkeratosis in nulliparous and primiparous does (Rommers and de Jong, 2011; Miko et al., 2014).
According to EFSA (2005), the production system as such is not considered a hazard for pododermatitis, whereas enabling hazards of the disease are related with type of floors and their degree of hygiene/faecal contamination. Based on literature published after 2005, hazards for pododermatitis can be summarised as shown in Table 27.
Table 27.
Hazards related to Pododermatitis. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing |
Floor type (wire mesh flooring without either plastic mats or plastic platforms) Floor bedding (litter on either full floors or on plastic slatted floors) Any damaged equipment Restriction of movement |
| Ambient condition | Ambient humidity in the case of litter floors |
| Genetics | Heavy strains |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity |
Proper cleaning and disinfection procedures Absence of the duo system |
| Management of reproduction | No hazard identified |
| Other |
Poor body condition‐ feeding Presence of mycosis (growing rabbits) |
3.6.4. Locomotory disorders (other than due to pododermatitis)
The most frequent disorders are torticollis and related loss of stability. Torticollis (wry neck, head tilt) in rabbits is due to infection by Pasteurella multocida, among others, with otitis (Coudert et al., 2006) and, occasionally, infection by Encephalitozoon cuniculi, and central nervous system alterations (Künzel and Fisher, 2018). Other clinical signs of encephalitozoonosis can be diagnosed in young rabbits, before weaning; however, wry neck is observed in older rabbits. The sporadic existence of splay leg in runt rabbits during the growing period might be related to the infection due to E. cuniculi. Farmed rabbits can have traumas, such as broken back (in adults) and broken legs (seen in adults, kits and weaned rabbits). There is an increased risk of traumas (i.e. broken back) which are related to management when rough handling occurs. Young rabbits can be caught in the wire mesh if housing design is not optimal (e.g. big holes in the mesh, mobile footrest that can hurt the limb of a kit when sliding). These injuries cause acute pain, and culling is the final solution.
Hazards for respiratory disorders causing otitis and torticollis are discussed in Section 3.6.10. Hazards for E. cuniculi are summarised below (Table 28).
Table 28.
Hazards related to locomotory disorders. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Inadequate flooring, distance between floor and wall, old feeder (sharp edges) |
| Ambient condition | Cold weather compromising the immune system |
| Genetics | No hazard identified |
| Nutrition and feeding |
Dietary imbalances Inadequate oligo‐elements, vitamins |
| Management of biosecurity |
Poorly controlled environmental conditions (increasing risk of infections). Lack of hygienic measures after the sale of a batch of young rabbits, to decrease contamination by E. cuniculi Inadequate control of suppliers of semen and young does Absence of control/quarantine for new batches of animals |
| Management of reproduction | No hazard identified |
| Other | Inadequate handling, fear (sudden noise) x rapid movements |
3.6.5. Skin lesions and wounds
Animals may present minor and limited skin abrasions (< 1 cm); extended skin abrasions (> 1 cm); extended lesions (> 1 cm); deeper, extended (> 1 cm), and open lesions (ulcers with bloody exudate); as well as abscesses to the body or ears which may be associated to more or less serious traumas (Andrist et al., 2013). Skin lesions and wounds, such as multiple scratches, open or scabbed wounds or abscesses to the body or ears, may cause pain and chronic fear to rabbits, as well as compromise their health and thus their welfare.
Lesions and wounds can result from inadequate housing equipment or aggression or chewing (see Section 3.6.15). No literature is available describing the housing equipment conditions which can cause skin lesions and wounds. Logically, any damaged equipment that could come into contact with animals (e.g. broken, corroded or rusty wire mesh) or any sharp element (e.g. from a damaged feeder or nest or an unsuitable platform inside the cage) are likely to cause such lesions to animals of all categories. Trauma and microtrauma are then easily and frequently contaminated by opportunistic bacteria (e.g. Staphylococcus spp.). Lesions from aggression can occur among growing rabbits or reproducing animals when kept in groups, and in kits because of aggression from reproducing does.
Growing rabbits
Rabbit social behaviour starts at the beginning of their life when their survival is promoted by the increased thermal efficiency provided by the sibling presence (EFSA, 2005). Thereafter, in the wild, rabbit dispersal occurs before sexual maturity, with almost 100% of young males and 50% of young females leaving the original group (EFSA, 2005). Thus, under farming conditions, damaging aggression among growing rabbits kept in groups usually appears when animals are approaching sexual maturity, i.e. after 9–10 weeks of age depending on breed and genotype. Moreover, the steepness of hierarchy in growing rabbits has been found to be positively related to the number of wounds (Vervaecke et al., 2010).
Based on previous literature on behaviour and aggression, EFSA (2005) recommended to keep a group size for growing rabbits limited to 7–9 animals, preferably retaining the same litter group. In collective pen/park systems with large group sizes (> 10 rabbits/pen), the risk of aggression among animals and the risk of distress as well as injury rates are increased (Szendrö et al., 2010; Szendro et al., 2015; Princz et al., 2009), particularly at later ages and when approaching sexual maturity (Lambertini et al., 2005; Szendro and Dalle Zotte, 2011; Trocino et al., 2015).
As regards stocking density, EFSA (2005) established 16 rabbits/m2 (i.e. 40 kg slaughter weight/m2 at 2.5 kg slaughter weight) as a ‘safe’ stocking density from the perspective of both rabbit welfare and performance, based mostly on literature on cage housing. Later studies on alternative pen housing systems and larger group sizes have not identified any benefit of further reductions of stocking density (Szendrő et al., 2010; Trocino et al., 2013). On the other hand, at 11–12 weeks of age, Trocino et al. (2015) observed a higher percentage of growing rabbits with scratches and lesions due to aggression in pens with 16 animals/m2 compared with the pens with 12 rabbits/m2 (26.2% vs. 8.2%, respectively; p ≤ 0.001) (Table 29). However, the increase of group size from 20 to 27 animals per pen (to increase stocking density from 12 to 16 animals/m2) could have contributed to the higher aggression in pens with the higher stocking density, together with developing sexual maturity and reduced available functional surface during the last week of the trial with increasing animal body size under the specific conditions of the study, i.e. heavy live weight (2.6–2.8 kg) and rather late slaughtering (> 75 days of age) (Table 29).
Table 29.
Prevalence of growing rabbits showing skin lesions and wounds under different housing and management conditions
| Authors | Housing system (surface) | Age (w = weeks; d = days) | Stocking density (rabbits/m2) | Group size (no rabbits) | Injured animals (%) |
|---|---|---|---|---|---|
| Szendrö et al. (2009) |
Small cage (0.12 m2) Large cage (0.50 m2) Small pen (0.86 m2) Large pen (1.72 m2) |
9 vs. 10 vs. 11 w | 3.5% vs. 6.1% vs. 10.4% | ||
| 12 vs. 16 | 23.8% vs. 2.7% | ||||
| 2 vs. 6–8 vs. 10–13 vs. 20–26 | 0.0% vs. 7.1% vs. 8.7% vs. 17.4% | ||||
| Princz et al. (2009) |
Small cage (0.122 m2) Open top pens (0.86 m2) |
11 w | 16 | 2 vs. 13 | 5.97% vs. 12.1% in cages vs. pens |
| Szendro et al. (2015) |
Small cage (0.19 m2) Large pen (0.95 m2) |
12 w | 15 | 3 vs. 14 | 0.0% vs. 34.4% in cages vs. pens |
| Trocino et al. (2015) | Open‐top pens (1.68 m2) | 12 vs. 16 | 20 and 27 | 8.2% v. 26.2% a | |
| 76 v. 83 d | 15.0% v. 22.0% (0.10) | ||||
| 11.3% v. 25.8% for females v. males(a) mixed groups | |||||
| Bozicovich et al. (2016) | Cage (0.48 m2) | 77 d | 6 | 58% vs. 25% vs. 79% male group vs. female group vs. mixed group(a) |
Prevalence was calculated basing on 24 animals per group (i.e. 6 rabbits × 4 cages × experimental group).
Aggression, and thus skin lesions and wounds, also depend on the sex of the animals. In mixed groups of growing rabbits, the rate of injured animals averaged 11.3% for females and 25.8% for males (p ≤ 0.001) (Trocino et al., 2015). Whether this result depended on major aggressiveness of the females towards the males, or major aggressiveness among the males approaching sexual maturity, is not clear. Nevertheless, Bozicovich et al. (2016) also observed a higher number of injured rabbits in cages with only males or mixed‐sex compared to females (Table 29).
Reproducing does
In the wild, or in large near‐to‐nature enclosures, domestic rabbits live in stable matrilineal family groups of 2–9 does, 1–3 adult bucks, their offspring and, eventually, some sub‐adult satellite males (EFSA, 2005). Nevertheless, does with small kits tend to be separated from other adults (EFSA, 2005).
Under laboratory conditions, group housing of adult animals (either males or females) usually results in serious injuries and is considered to have deleterious effects on their wellbeing (DiVincenti and Jr, 2016). Under farming conditions, EFSA (2005) stated that insufficient knowledge and technology was available at that time to recommend implementation on farms of the group‐housing of reproducing does. Indeed, later literature confirmed that continuous group housing of reproducing does usually results in very high rates of aggression among females and competition for nesting areas, which impairs animal welfare in terms of frequency and degree of injuries among reproducing does, as well as towards kits (Andrist et al., 2013; Szendro et al., 2013) (Table 30).
Table 30.
Prevalence of injured does in collective housing systems for reproducing does with different management systems (modified from Szendro et al., 2019)
| Reference | Group characteristics | Prevalence |
|---|---|---|
| Mirabito et al. (2005) | 4 does/pen, during rearing | 32% |
| Rommers et al. (2006) | 8 does/pen | 16.8–21.0% |
| Andrist et al. (2013) | Swiss farms with different systems (generally 8 does/group) | 33% (9% severe) |
| Buijs et al. (2015) | 4 does/pen regrouped 18 days after kindling | 91.7% and 75.0% of does in pens with plastic floor and wire net, respectively |
| Buijs et al. (2016) | 4 and 8 does per pen regrouped 18 days after kindling |
28.4% score 0 31.3% score 1 33.7% score 2 6.6% score 3 |
| Zomeno et al., 2018 | 4 does/pen regrouped 2 day after kindling | 34%, 47%, 13%, 13% and 10% at 3, 10, 17, 24 and 32 day after regrouping |
| Rommers and de Greef, 2018 | 5 does/pen regrouped 23 day after kindling | 34% and 53% at 4 days after regrouping and at weaning, respectively |
Furthermore, even in ‘part‐time’ housing systems, in which reproducing does are kept in a group during some periods and individually in others, aggression, fighting and presence of injured rabbits (46–66%) after each re‐grouping remain unsolved problems, as shown by several studies (Andrist et al., 2012, 2014; Rommers et al., 2014a; Buijs et al., 2015; Machado et al., 2016; Maertens and Buijs, 2016) (Table 30).
Different strategies (platform, plastic pipe, hiding place, straw, territory, dark corridor, group stability, regrouped into home or new pen; sprayed odours) have been tested without huge success to reduce aggression at re‐grouping (Graf et al., 2011; Rommers and de Jong, 2011; Rommers et al., 2013, 2014a; Buijs and Tuyttens, 2015). A combined system with four individual modules and a common area has also been tested: 18 days after kindling the entrances of the individual modules were opened and a 21‐day group‐housing period started, but the prevalence of injured rabbits was higher than 50%; it reached the highest peak on day 2 and remained high for many days in some pens (Gerencsér et al., 2018).
The time of group formation (first days after kindling, early or late lactation) may affect the aggression level among does (Zomeno et al., 2017, 2018). Does in late lactation may be less stressed, since more time has passed after kindling and the presence of the kits out of nest boxes may positively modulate female to female aggression (Zomeno et al., 2017). Nevertheless, Rommers and de Greef (2018) found that the percentage of injured does in a part‐time system with 5 does increased from 34% measured 4 days after group formation (23 days of lactation) to 53% at litter weaning (36 days) (Table 30).
The little available information also shows a negative effect of the increase of group size from 2 to 4 or from 4 to 8 does on aggression rates among does (Buijs et al., 2016; Zomeno et al., 2017).
In EFSA (2005), skin lesions due to aggression were not analysed separately as a specific welfare consequence, but they were indirectly treated when evaluating hazards for aggression and related wounds. In the case of lactating females, keeping animals in pairs was defined as a serious problem because of aggression and was not recommended. In the case of growing rabbits, group size higher than 7–9 rabbits was considered to increase the risk of aggression and wound rates as well as the risk of disease spread.
Based on literature published after 2005 and presented above, hazards for skin lesions and wounds in growing rabbits kept in groups are given in Table 31:
Table 31.
Hazards related to skin lesions in growing rabbits. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing |
Collective housing (increased group size) Stocking density (> 40 kg/m2) The combination of collective housing with large group size and at high stocking density with late slaughter age Damaged equipment – see does |
| Ambient condition | Photoperiod (inducing sexual activity) |
| Genetics | lines with early onset of sexual activity |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity | No hazard identified |
| Management of reproduction | No hazard identified |
| Other |
Late slaughter age (> 10–11 weeks) (developing sexual maturity and reduced available functional surface) Absence of enrichment (gnawing stick) Group social‐management (no removal of biting rabbits) |
Based on literature published after 2005 and presented above, hazards for skin lesions in reproducing does are shown in Table 32:
Table 32.
Hazards related to skin lesions in reproducing does. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing |
Group housing (continuous or part‐time) Group size Any damaged equipment, structure of the housing (fleeing into shelter), position of drinker, time of mixing the groups‐ stability of groups |
| Ambient condition | No hazard identified |
| Genetics | Some breeds/lines are more aggressive than others |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity | Hygiene of injections |
| Management of reproduction | Time of regrouping |
| Other |
Physiological state of the doe at the time of group formation Iatrogenic |
3.6.6. Respiratory disorders
Respiratory disorders are most relevant in breeding rabbits, with welfare consequences that include pain and death. Dyspnoea is one of the symptoms of respiratory disease and is considered as painful. It also causes metabolic disorders such as respiratory acidosis (EFSA, 2005). Pneumonia was reported as the most common cause of death in a study of 505 farms in Spain and Portugal visited during 2006–2014; monthly mortality risks were 0.70 % (0.64–0.76) in does, and 0.88% (0.56–1.20) in bucks (Rosell and de la Fuente, 2016a). Disorders of the upper respiratory tract are clinically evaluated through presence of rhinitis (snuffles). Sporadically some cases of atrophic rhinitis can be seen. From the sinus, pathogens can diffuse and cause septicaemia or otitis, metritis and subcutaneous abscesses, which is relevant also in finishing rabbits (Coudert et al., 2006). The mean prevalence of rhinitis in Spanish and Portuguese farms studied from 2001 to 2017 was 18.7 (CI95% [18.1–19.3]) (Rosell and de la Fuente, 2018). Pasteurella multocida is the main etiologic agent (García‐Alvarez et al., 2015; Massacci et al., 2018), but there are also several opportunistic pathogens (Deeb and DiGiacomo, 2000). Lastly, an intercurrent disorder such as myxomatosis can be a causal factor because the myxoma virus is immunosuppressive (Bertagnoli and Marchandeau, 2015), and enables diffusion of P. multocida or S. aureus, causing productive rhinitis, dyspnea, or death.
Since EFSA (2005), little new information exists for respiratory disease risks. Hazards for rhinitis can be divided into 2 main groups: predisposing hazards were linked with the line or breed of rabbits, and age, whereas enabling hazards were mainly related with the incorrect combination of the ambient temperature, humidity and air speed (Calvet et al., 2011; da Borso et al., 2016). A genetic component to resistance to Pasteurella has been identified (Gunia et al., 2015, 2018; Shrestha et al., 2019); however, currently commercial lines are not characterised for this trait.
Table 33 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 33.
Hazards related to respiratory disorders. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | High stocking density in growing rabbits |
| Ambient condition | Temperature, air speed, humidity or sudden change in any of these. Air quality including ammonia and dust levels |
| Genetics | High fertility lines‐ (reduced thoracic space), genetic predisposition to Pasteurella |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity |
Control of new breeders entering to the farm Delayed culling of affected animals |
| Management of reproduction | No hazard identified |
| Other | No hazard identified |
3.6.7. Gastroenteric disorders
Digestive troubles are responsible for welfare impairment in the growing rabbit, that can range from slight troubles (transitory low feed intake, light diarrhoea) to acute painful ones (no feed intake, weight losses, acute diarrhoea or caecal impaction, intestinal inflammation, gastric or intestinal dilatation or swelling, mucus excretion, etc.). The young rabbit is particularly susceptible to gastroenteric disorders, and more particularly around the period of weaning. Diarrhoea can sometimes be present in kits during the pre‐weaning period (3rd–4th week of age) or more rarely in adults, where it generally represents the ultimate consequence of another affliction. In conventional rabbit farming, digestive disorders are the main cause of morbidity and mortality for the growing rabbit (from 3 weeks of age and after). Two main causes of intestinal pathologies can be differentiated (Marlier et al., 2003; Licois, 2004; Agnoletti, 2012): parasites and bacteria.
Intestinal pathologies clearly prevailed over all other health problems in rabbit farms in Spain, France, Italy and Portugal (Boucher and Leplat, 2005; Grilli et al., 2006), without showing seasonal variations (Rosell and de la Fuente, 2009a), with similar reports from France. Mortality levels from digestive disorders typically range from 5% to 15%, depending on the sanitary strategy of the farm (cleaning and hygiene, metaphylactic procedures, etc.). The morbidity from digestive disorders is much more difficult to estimate in conventional rabbit farms, since it is characterised by transient growth depression and poor feed conversion, but often induces important economic losses.
Hazards for digestive disorders include poor prophylaxis procedures (including housing, cleaning, etc.). However, the feeding strategy can contribute to prevention of digestive troubles of the growing rabbit. Two main options are available: use of high fibre diets (Gidenne et al., 2010), and use of feed restriction strategies (Gidenne et al., 2012a). Some preventive measures which are more acceptable to the consumer (i.e. no antibiotics) are on the market to combat sub‐clinical enteric diseases, such as the use of prebiotics and probiotics (mainly live yeast) or phytotherapeutic products, but their real contribution to reduce the prevalence of digestive disorders is questionable. Studies which are still in progress focus on genetic resistance to enteric diseases (Gunia et al., 2015, 2018) or on the microbiota of the young rabbit, and on factors that can contribute to the maintenance of the gut microbiota equilibrium and digestive immunity (Combes et al., 2013, 2017; Arrazuria et al., 2018).
In conclusion, digestive troubles are probably one of the main hazards for welfare impairment in rabbit farming, both in terms of prevalence and pain.
Table 34 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 34.
Hazards related to gastroenteric disorders. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Restricted space, high stocking density, unsuitable floor type (solid floor impairing hygiene), lack of roughage, stress |
| Ambient condition | Too high air speed and low temperature |
| Genetics | Commercial lines with less resistance to digestive diseases |
| Nutrition and feeding |
Unbalanced diet, contamination of feed and water, insufficient quantity of milk for kits Excessive feed intake after weaning |
| Management of biosecurity | Poor hygiene of housing equipment, poor removal of faeces (cage and nest, feeder), lack of control of insects and rodents (transmission of Salmonella ) |
| Management of reproduction | Early weaning, intensive reproductive cycle |
| Other | Inadequate use of antibiotics for early treatment |
3.6.8. Skin disorders (other than pododermatitis or skin lesions)
Ringworm is the most important skin disorder because it is zoonotic and also affects many rabbits, especially weaners and growers. Ringworm causes pruritus and provokes rabbits to scratching, and subsequent infections with Staphylococcus spp.
There were affected rabbits in 50% of 1,100 farms visited in Spain from 1985 to 1999 (Rosell et al., 2000); prevalence of ringworm within a positive farm is variable. There are asymptomatic rabbit carriers of dermatophytes (Vangeel et al., 2000), but there are also farms free of dermatophytes (Ramos and Payá, cited by Rosell opus cit.). Rubbing against the feeder enables the infection with opportunistic fungi, such Alternaria spp., and provokes skin areas with alopecia or with hypotrichosis (Ramos and Payá, cited by Rosell opus cit.).
Among types of mange, the sarcoptic form is most painful; however, it is rare in comparison with the psoroptic form (Sant and Rowland, 2009). During 2001–2017, the observed prevalence of psoroptic mange on 531 farms visited in Spain and Portugal and among 144,455 examined lactating does was 2.44% (CI95% [2.15–2.73]) (Rosell and de la Fuente, 2018). There are also manges due to less pathogenic‐opportunistic mites, causing skin alterations, e.g. in the shoulder due to primary lesions after subcutaneous injections on this site, and scratching. Staphylococcosis causes painful dermatitis in newborn, growers and breeding rabbits (Corpa et al., 2009).
Pseudomonosis is a common skin lesion in some farms, mainly during summer. The enabling hazard is the contact with the drinker in two main body areas: lumbar back and throat region. When the origin is an obstruction in the drinker, all the individual housing of a breeding rabbit can be wet and Pseudomonas infection affects extensive areas. Welfare consequences are pain, or death in severe‐chronic cases.
Viral infections also cause skin lesions. Myxomatosis is the most common, due to natural infection or interference with the immune system in vaccinated rabbits. Skin papilloma might rarely be seen in adults; e.g. in the ear. Concerning fibromatosis, kits are more sensitive (Bertagnoli and Marchandeau, 2015). In farmed meat rabbits, non‐viral skin tumours are scarce, due to the age of breeding rabbits; older animals, such pet rabbits are more affected (Van Praag et al., 2010).
Behavioural dermatopathies (Tynes, 2013) are relevant in some farms, particularly barbering within litters or with neighbours. Lastly, skin disorders such as inherited alopecia might rarely be observed in young rabbits. Hypotrichosis in kits can be seen after primary intestinal lesions on some affected farms (Vela et al., 2010).
The main hazards for skin diseases relate to the presence and spread of the causal agents. Thus, prevalence of ringworm in commercial farms depends on biosecurity measures (Cafarchia et al., 2010) and attention to treatment. The risk of manges is also associated to introduction of infected young breeders. Pseudomonosis risks arise from poor design of housing and disposition of drinkers, which must be evaluated in relation to the body size of rabbits.
Table 35 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 35.
Hazards related to skin disorders. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Situation of the drinkers in relation to the body size of the breeding rabbits. Wood in the enclosure |
| Ambient condition | High temperature and humidity enable the diffusion of dermatophytes |
| Genetics | No hazard identified |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity |
Poor control of new breeders entering to the farm Poor control of birds and rodents |
| Management of reproduction | No hazard identified |
| Other | Ear tags‐ wound as entrance for pathogens |
3.6.9. Reproductive disorders
Infertility is the main reproductive disorder and is mostly due to inflammation of the genital tract (Boiti, 2004). Infectious metritis as well as dystocia and uterine torsion are considered painful, with the latter conditions usually leading to death (Rosell and de la Fuente, 2016b).
Post‐mortem examinations, performed during farm visits on 2,065 female rabbits found dead and 368 moribund‐euthanised does, revealed 24% of cases with disorders of the reproductive system. Monthly mortality risk (MMR) was estimated to be 0.21% (CI95% [0.18–0.24]) for metritis, pyometra, or foetal mummification, 0.20% (CI95% [0.18–0.22]) for obstetric problems such as uterine torsion, and 0.10% (CI95% [0.08–0.12]) for uterine prolapse (Rosell and de la Fuente, 2016a,b).
Reproductive disorders might also be a consequence of concurrent health disorders of the doe which have welfare implications, such as gastroenteric disorders and therefore enteric pain. For example, abortion has been associated with several other health disorders. Of 68 does necropsied with the outcome ‘abortion’, 28% had pneumonia, 20% metritis, 19% digestive disorders, 14% septicaemia, 5.4% ketosis, and 13% miscellaneous disorders (Rosell and de la Fuente, 2016a,b). In the study of Boucher et al. (2001), involving 77 does with abortion from 32 farms, the main causes were infections with P. Mycoplasma spp.
Hazards for reproductive disorders include lack or quality of water, poor energy provision in the feed (Trocino and Xiccato, 2006; Rosell and de la Fuente, 2016b), poor body condition and health disorders of the doe (de Jong et al., 2011). The main (indirect) hazards for reproductive disorders which are secondary to respiratory and gastroenteric disturbances relate to housing (mainly poor ventilation related to humidity and temperature).
Table 36 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 36.
Hazards related to reproductive disorders. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | No hazard identified |
| Ambient condition | Unsuitable temperature/airspeed |
| Genetics | No hazard identified |
| Nutrition and feeding | Lack of food, poor body condition |
| Management of biosecurity | Concurrent diseases (e.g. digestive disorders) |
| Management of reproduction | Inappropriate insemination technique |
| Other | No hazard identified |
3.6.10. Mastitis
In rabbit does, mastitis is usually associated with S. aureus (which is also commonly involved in ulcerative pododermatitis). Both high‐virulent and low‐virulent strains of S. aureus can cause mastitis and lead to similar types of lesions (Viana et al., 2011) although immune titres may differ (Guerrero et al., 2015). Pasteurella multocida and Enterobacteriaceae also contribute (Rosell and de la Fuente, 2018). Mastitis can be lethal to the affected doe, as well as to her litter if she refuses to nurse it due to the pain caused by the condition. Affected does may also refuse to mate. Mastitis is the most important gross pathological cause of culling in adult rabbit does (Corpa et al., 2009; Viana et al., 2011) and is associated with a lowered body condition score (Sánchez et al., 2012). It can occur at any time during lactation or during the dry period (Viana et al., 2011) and does that recover from mastitis are often re‐infected in later lactations (Corpa et al., 2009).
Mastitis occurs in an acute and a chronic form. Acute mastitis (or ‘blue breast’) results in one or more warm, reddened and swollen mammary gland which can turn bluish/purple/black in a later stage. It can cause mortality within hours of infection or may lead to chronic mammary gland changes after recovery (Corpa et al., 2009; Rosell and de la Fuente, 2018). Because the litter is considered an infection risk, kits would normally then be euthanised rather than cross‐fostered. Chronic (or purulent or suppurative) mastitis manifests as the thickening of the skin, development of a hardened mass, formation of abscesses of 1–12 cm in diameter that can develop into chronic lesions, or a combination of these (Viana et al., 2011). Rabbits with chronic mastitis can be lethargic and unwilling to nurse their young. Although acute mastitis more commonly leads to mortality in the first week of lactation (Rosell and de la Fuente, 2016a,b), chronic mastitis prevalence increases between week 1 and 5 of lactation (Rosell and de la Fuente, 2018).
A long running study on conventional farms in Spain and Portugal (Sánchez et al., 2012; Rosell and de la Fuente, 2016b; Rosell and de la Fuente, 2018) has provided most recent evidence on mastitis. It indicated an average prevalence of 4% (range 0–36%) for clinical mastitis in lactating does. Although no decrease in mastitis prevalence was detected throughout the 17‐year study period, this is markedly lower than estimates from earlier studies in France and Spain during the 1980s. Recent data from other EU countries are lacking. Chronic forms were most often detected, which is expected as acute mastitis usually leads to rapid mortality and is thus unlikely to be detected during routine visits.
EFSA (2005) describes the contamination of farms with S. aureus (and to a lesser extent Pasteurella multocida) as a main hazard for mastitis. Poorly designed floors and dirty or rusty materials contribute by enabling infection. Additional environmental (cold, draughts, damp) and managerial (not vaccinating against S. aureus) hazards were mentioned. Furthermore, the risk of mastitis is reported to increase proportionally with the age of the does.
Certain genetic lines have a higher risk of mastitis (prevalences ranging from 1% in the best line to 7% in the worst, Rosell and de la Fuente, 2018). Using multiple batches (i.e. separate groups of does that kindle at a different time) and not moving does to a cleaned and disinfected room prior to kindling are also a hazards for mastitis. Although previous research (EFSA, 2005) indicates that cold is a hazard, Spanish/Portuguese data (Rosell and de la Fuente, 2018) indicate that mastitis is more prevalent in spring than in winter (or in other seasons). An extensive breeding rhythm (insemination 32–56 days after kindling) decreased mastitis prevalence. Again, in contrast to older data (EFSA, 2005), no effect of age on mastitis risk was found in a large‐scale study on conventional farms (Sánchez et al., 2012). Teat lesions caused by suckling kits also increase the risk of mastitis (Rosell and de la Fuente, 2018).
Table 37 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 37.
Hazards related to mastitis. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Poorly designed floors and dirty or rusty materials |
| Ambient condition | Inadequate climate (cold, damp, draughts) |
| Genetics | Use of certain genetic lines |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity |
Contamination with S. aureus Use of multiple batches Cross‐contamination through cross‐fostering |
| Management of reproduction |
Not moving does to clean disinfected rooms prior to kindling Intensive breeding rhythm (insemination < 31 days after kindling) |
| Other |
Season (increased in spring) Age (according to older, but not to recent literature) Later stages of lactation Lesions of the teats (by kits) |
3.6.11. Neonatal disorders (including starvation/mis‐mothering and cannibalism/exposure complex)
During the first few days of life, survival of kits requires an adequate environment, i.e. a well‐built nest in a separate section of the mother's living environment. Poor care, leading to hypothermia, starvation, dehydration, and infanticide are all serious welfare issues (EFSA, 2005).
During the nesting phase, low individual birth weight is a hazard for mortality from hypothermia, injuries, starvation and weakness. A minimal birth weight, under which the survival chance of kits is low, has been suggested in several publications: Argente et al. (1999): 50 g; Drummond et al. (2000): 43 g; Coureaud et al. (2007): 48 g; Martínez‐Paredes et al. (2018): 45 g. The birth weight of kits depends on several factors, one of which is the genetic background. With increasing litter size at birth, the heterogeneity of kits’ weight within a litter increases and the average kit weight decreases (Lenoir et al., 2012). However, the homogeneity of kits’ weight within a litter can be successfully improved with selection (Bolet et al., 2007; Garreau et al., 2008a,b).
Cannibalism occurs when the mother, after swallowing the placenta, also devours part of the kits (Hafez et al., 1966a,b; EFSA, 2005). González‐Redondo and Zamora‐Lozano (2008) found, in cage‐bred wild rabbits, that in 13.3% of litters either all kits (10.2%) or some kits (3.1%) were cannibalised. Leone‐Singer and Hoop (2003) observed a considerably lower incidence of cannibalism (0.5% of kits) in meat rabbits bred at Swiss farms. In the study of González‐Redondo and Zamora‐Lozano (2008), a close connection was revealed between inadequate nesting behaviour of does and the occurrence of cannibalism. When wild rabbit does did not introduce hair or straw into the nest box, a higher rate of cannibalism occurred.
Under farm conditions, litters are standardised (with cross‐fostering) within 1–3 days after parturition to equalise litter size and, as much as possible, to give the same weight of kits within a litter. Without this standardisation, smaller and weaker kits die because stronger and heavier kits suckle a higher share of milk during the short daily suckling events. During the first days of lactation, farmers have to take special care about kits and regularly (daily, when possible after the controlled nursing event) monitor if there are kits without milk intake. In case the kits have not been nursed adequately, the doe has to be forced to nurse, or the kits have to be cross‐fostered. Failure of prompt problem detection and alleviation is therefore a hazard for kit mortality.
It has been stated in EFSA (2005), that neonatal mortality is higher when does are kept in groups than when they are singly caged. In individual housing, infanticide of the doe's own kits can occur, but she cannot injure, hurt or cannibalise the kits of another doe (Table 38).
Table 38.
Mortality of kits in collective housing systems for reproducing does with different management systems (modified from Szendro et al., 2019)
| Author | Housing system | Individual | Group |
|---|---|---|---|
| Mirabito et al. (2005) | 4 does/pen | 9.7% | 17.7% |
| Rommers et al. (2006) | 8 does/pen, natural mating | 5.2–8.8% | 12.8% |
| Rommers et al. (2006) | 8 does/pen, AI | 7.4% | 10.1% |
| Szendro et al. (2013) | 4 does and 1 buck/pen | 14.0–15.2% | 38.5% |
| Hoy and Matics (2016) | 4 does/pen, combination of individual cage with common area | – | 18.1% |
Table 39 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 39.
Hazards related to neonatal disorders
| Hazard category | Hazard |
|---|---|
| Housing | Group housing of does |
| Ambient condition | Low temperature |
| Genetics | Low birth weight (high litter size) |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity | No hazard identified |
| Management of reproduction |
Poor care, handling of litters Lack of cross‐fostering |
| Other | Inadequate nesting behaviour, lack of nest material, does at first parturition, poor health of does |
3.6.12. Thermal stress
One of the most important climatic components that influences the welfare of farmed animals is the ambient temperature. When rabbits are kept in conventional indoor systems, the ambient temperature is often controlled and this can influence the air velocity, the relative humidity, the dust level and other atmospheric conditions. Under good farming practices, cooling and heating systems are applied to maintain appropriate environmental temperatures and relative humidity during hot and cold seasons. The temperature within buildings is normally maintained between 15°C and 25°C. When rabbits are kept outdoors, they must be protected as far as possible from thermal discomfort (EFSA, 2005) and direct exposure to environmental factors (direct sun, wind, rain, etc.).
Since there is a close relationship between the ambient temperature and humidity, the relative humidity has to be taken into consideration when measuring the severity of thermal discomfort (e.g. heat stress). For this reason, application of a ‘Temperature‐humidity index’ (THI) was proposed by LPHSI (1990), which was modified and adopted for rabbits by Marai et al. (2001) as follows:
THI = dboC – [(0.31 – 0.31 RH) (dboC – 14.4)]
where dboC dry bulb temperature in degrees Celsius and RH = relative humidity.
A value for THI below 27.8 was taken to signify an absence of heat stress, while a value in excess of 28.9 was considered to represent severe heat stress. Based on practical experience and data in literature (Verga et al., 2007), the optimal ambient temperature ranges are shown in Table 40.
Table 40.
Optimal ambient temperatures (and relative humidity) for indoor housing of rabbits (Verga et al., 2007)
| Ambient temperature (oC) | Relative humidity (%) | |
|---|---|---|
| Reproducing does | 16–21 | 60–70 |
| Breeding males (bucks) | 12–16 | 60 |
| Growing rabbits | 15–20 | 60–70 |
| (early weaned rabbits) a | (20–22)a |
EFSA (2005).
Rabbits are very sensitive to high temperature, since they are fur animals and have limited ability to eliminate excess body heat. Heat stress induces physiological changes, e.g. reduction in feed intake, disturbances in water, protein, energy and mineral balances, enzymatic reactions, hormonal secretions and blood metabolites (Marai and Rashwan, 2004) Johnson, 1980; Habeeb et al., 1992; Kasa and Thwaites, 1992; Wittroff et al., 1998). When the temperature rises above 30°C, the feed intake of rabbits decreases, which can decrease the performance of rabbit does and growing rabbits. According to Lebas et al. (1986), rabbits could no longer regulate their internal temperature above 35°C and heat prostration set in, while at 40°C considerable panting and salivation occurred. The average lethal body temperature was considered to be 42.8°C. Some diseases (e.g. dermatomycoses) are directly related with environmental factors such as high temperature and humidity, or temperature changes (EFSA, 2005).
Outside the range of optimal ambient temperature, behavioural changes can be observed in rabbits. During the first 10–12 postnatal days, the kits have only a limited capacity for independent thermoregulation. They huddle together, covering themselves with the nest material by crawling under it (Hudson and Distel, 1982; Muciño et al., 2008). In case of 30 min at 20.0°C, the rectal temperature of kits drops from 37.7o to 32.7°C (Cardasis and Sinclair, 1972). After the nest phase, when the body of rabbits is covered by hair, to a certain extent they are able to adapt to thermal changes with behavioural responses. When the ambient temperature is below 10°C, rabbits curl up to minimise their body's surface area, the ear temperature is lower, the ear pinnae are folded to remove the internal surface from contact with air and rabbits drop the ears to bring them closer to the body (Marai and Rashwan, 2004).
When the ambient temperature increases above 30°C, rabbits show a significantly higher respiration rate, they stretch out to lose heat by radiation and convection, stretch ear pinnae and spread them far from the body to expose the surface to the surroundings, while the ear temperature increases (Marai and Rashwan, 2004). Rafel et al. (2012) observed that, when housed under 20°C, reproducing does spent 15–25% of time in prostration (lying in a stretched out position, ventrally, laterally or dorsally) while rabbits under heat stress spent more time prostrated (45–54%), with a peak during the warmest hours. Moreover, when the temperature changes according to circadian cycles, animals are able to predict the pattern of temperature and increase some important activities (e.g. grooming) in the colder period, which will be not performed during the warmer hours. In free choice observations, the time spent by growing rabbits on different floor types (wire mesh, plastic mesh or straw litter) was influenced by the ambient temperature (Gerencsér et al., 2014).
In conclusion, rabbit kits are mainly affected by cold stress in case of disturbances in maternal behaviour (e.g. kindling out of the nest, inadequate nest quality, being outside the nest). This can cause hypothermia and death of kits. Rabbits after weaning age are mostly affected by heat stress, especially in Mediterranean countries or in hot summer periods. In buildings, the ambient temperature and other atmospheric conditions are controlled to a certain extent, whereas this is not possible in outdoor systems.
Table 41 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 41.
Hazards related to thermal stress. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Outdoor systems, high stocking density |
| Ambient condition |
Climate – malfunction of ventilation, cooling and heating system season |
| Genetics | Angora rabbits |
| Nutrition and feeding | Inadequate water provision, low milk supply |
| Management of biosecurity | No hazard identified |
| Management of reproduction | Inadequate nest quality |
| Other | Age (kits – first week) |
3.6.13. Restriction of movement
Rabbits move by hopping, jumping and running; in the wild, they cover distances of several hundred metres per day (Vastrade Françoise, 1987). The length of a hop has been reported to be up to 70 cm, depending on body size. Stride length can even be longer, depending on the speed of moving. High speed movements mainly occur during locomotor play, which is more frequently seen in young rabbits, as well as during social encounters and escape (EFSA, 2005).
While little is known about the rabbits’ actual needs to move under farming conditions, based on rabbit behaviour under semi‐natural conditions (Lehmann, 1989; Stauffacher, 1992), organisations for animal welfare have proposed that rabbits should be able to perform at least three consecutive hops and this has also been recommended by the Council of Europe for the housing of rabbits used for experimental purposes (Council of European Union, 2006). In general, locomotory activity under farming conditions appears to be low; running and rearing was observed in less than 1% of the scans in several studies (Buijs et al., 2011, 2015; Trocino et al., 2014, 2019). Restriction of movement may, however, lead to thwarted motivation and thus induce negative affective states such as frustration.
More recent studies in does have shown that active behaviours (sitting, standing, moving considered together) increased when cage size of individually housed young does increased from 1,150 cm² to 3,420 cm² (plus platform of 875 cm²) (Bignon et al., 2012). Does also showed more walking and running when housed in colony cages compared to individual cages (Mugnai et al., 2009). Reproducing does have been observed to hop or run for a lower time in smaller individual systems (0.11–0.25% of total observation time; Buijs et al., 2015) compared to larger part‐time group systems (0.17–1.41% of total observation time at 12 days after group formation, Buijs et al., 2015; 2% of total observation time at 4 days after group formation, Rommers et al., 2014a,b). In these studies, however, the space effect cannot be disentangled from the group effect.
In growing rabbits, several studies have shown that locomotor activity is lower in cages compared to pen systems, mainly due to a lack of space. The total surface area available seems to be the most important factor for locomotor activities (EFSA, 2005) and this has been confirmed in more recent studies. Comparing bicellular cages (2 animals per cage) and collective systems with either 9 animals per cage (Trocino et al., 2013) or 20–54 animals per pen (Trocino et al., 2014), the animals in the small bicellular cages spent less time moving and/or running. Similar results were revealed when comparing bicellular cages with pens with 13 rabbits/0.86 m² (Princz et al., 2008b). Providing free access from a wire pen (10 rabbits per m²) to a grassland paddock resulted in higher motor activities than in rabbits kept in bicellular cages (Mugnai et al., 2014). However, there was also an interaction with breed, as the more extensive Leprino of Viterbo breed showed more aptitude to movement than standard New Zealand White rabbits.
At constant stocking densities (14–15 rabbits per m²; 6 rabbits in small cages, 10 rabbits in small pens, 50 rabbits in large pens), the percentage of animals performing locomotor activities (moving, walking, running, jumping) in small pens was significantly lower than in either cages or large pens. However, the percentage of animals performing at least one jump or one run was significantly higher in rabbits in large pens compared with rabbits in the other two housing systems (Postollec et al., 2006). Even when, in an earlier study, total locomotion was not significantly different between pens (1.6 m2; 24 animals) and cages (0.4 m2; 6 animals), the mean number of double, triple and quadruple hops was higher in pens than in cages at 6 weeks of age and at 9 weeks of age, quadruple hops tended to be more frequent in pens (Martrenchar et al., 2001). The lack of effects on locomotion in older animals was interpreted as resulting from a lack of space with increasing age and thus size of the animals so that they were not able to perform longer hops anymore. This was also supported by the observation that walking over other animals increased with age. To our knowledge, this is the only paper that focused on the characterisation of the number of hops in rabbits kept under farming conditions.
When group sizes are kept constant but space allowance is increased, no effect on locomotor behaviour has been found (Buijs et al., 2012): 8 animals per group, cage size 100 cm × 40–160 cm; (Stewart and Suckow, 2016): 10 animals per group, 0.46 vs. 0.51 m²). This finding suggests that factors other than the mere space availability (e.g. social dynamics with increasing group size) affect locomotor activity.
Increasing space by installing an elevated platform in group cages for growing rabbits may promote jumping. However, hiding places and straw bedding did not affect locomotion in group housed does (Rommers et al., 2014a). Matics et al. (2018) found no difference in productive traits of growing rabbits with and without access to a platform, and, although not directly observing behaviour, concluded from this result that the platform did not substantially alter the locomotor behaviour.
Apart from effects on behaviour, the possibility to perform locomotor activity may also have physiological consequences. In growing rabbits, a low space allowance, and therefore a presumed lack of possibilities to exercise, reduces bone thickness (diameter) but not strength of the tibiofibula (Buijs et al., 2012): constant group size at different cage sizes) or bone moment of inertia in tibia and femur (Combes et al., 2009): increased cage and group sizes resulting in constant stocking density). Similarly, rabbits kept in collective pens (stocking density 12–18 animals per m²) had a thicker femur compared to animals kept in bicellular cages, but rabbits kept at 16 animals per m² had a higher femur resistance to fracture than those kept at 12 per m² (Xiccato et al., 2013a,b). Lack of possibility for full rearing (vigilance posture) due to low cage height has also been associated with deformations of the vertebral column and osteoporosis in does (Drescher and Loeffler, 1996). The latter study, however, has been criticised for confounding with dietary deficits.
Together with a lack of effect on locomotor behaviour, glucocorticoid metabolite concentrations in rabbit faeces were not affected by cage size (Buijs et al., 2011); dimensions see above). However, El‐Tarabany et al. (2019) found altered concentrations of serum cortisol and neurotransmitters with changes in stocking density (0.06–0.14 m² per rabbit). Animals kept at the highest stocking density had the highest cortisol and the lowest dopamine, brain serotonin and GABA levels, while brain acetylcholinesterase levels remained unchanged; behavioural data were, however, not recorded in the latter study.
Table 42 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 42.
Hazards related to restriction of movement. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Low total space available, dimensions (e.g. less possibilities in squared cages/pens compared to rectangular ones), structural elements (e.g. elevated platforms), floor properties |
| Ambient condition | No hazard identified |
| Genetics | No hazard identified |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity | No hazard identified |
| Management of reproduction | No hazard identified |
| Other | Small group size (at common stocking densities) |
3.6.14. Resting problem
Rabbits rest for 12–18 h a day. During resting, they adopt a crouched position (lying alert) or lying postures characterised by stretching of the hind‐legs and of the body, including full lateral lying on the side, that are thought to be associated with relaxation (EFSA, 2005). Lying stretched out also supports thermoregulation in terms of dissipating excess heat (Rafel et al., 2012). Animals which are not able to adopt relaxed postures, or are forced to lie on inadequate or dirty surfaces, can suffer from physical discomfort (e.g. cold stress, lesions, pain) as well as an impaired affective state. Increased levels of self‐grooming in response to soiling suggest that soiling causes discomfort (Dal Bosco et al., 2002).
Floor properties are an important determinant of resting behaviour. Rabbits prefer a wire floor over straw littered areas, especially for lying (Morisse et al., 1999). According to the authors, the cleanliness and dryness of the wire is the most plausible explanation for this finding. However, when given the choice between wire floors and other floor types, such as plastic foot mats, plastic grids, plastic slats or galvanised steel bars, which provide more support for the feet, fattening rabbits and breeding rabbits showed a clear preference especially for plastic‐mesh floors (Matics et al., 2003; Princz et al., 2008a; Gerencsér et al., 2012; Alfonso‐Carrillo et al., 2014a,b). Furthermore, one study revealed a significant avoidance of wire mesh flooring (Gerencsér et al., 2012). However, the size of the openings of plastic floors may affect the animals’ cleanliness. Soiling of cages and animals, as well as coccidial oocyte burden, were significantly higher on floors with 12 mm circular holes as compared with 10 mm slats (Tillmann et al., 2019). In a recent study comparing pens with straw‐bedded concrete floor and pens with slatted plastic panels (60 rabbits per pen, 9.6 animals/m², additional plastic slatted platforms in both treatments), less rabbits had a soiled fur when reared on straw, and parasitic burden did not differ (Windschnurer et al., 2019).
Factors affecting rabbits’ postures have been investigated less well. In growing rabbits, an increase in stocking density resulted in an increase in sternal lying (Buijs et al., 2011), which requires less space than lateral lying (Giersberg et al., 2015). At a live weight of 2.5 kg, stretched lying positions required between 593 and 621 cm² per animal, the latter almost equalling (97%) the space allowance at a stocking density of 16 animals per m² (Giersberg et al., 2015). In terms of floor type, Trocino et al. (2018) found that growing rabbits housed on wooden slats rested more in the crouched position (41.4 vs. 35.5% of the observed time) and showed less allogrooming than those housed in plastic grid pens. Finally, higher temperatures (constant 18°C vs. 20.1°C together with a THI of 23.6–28.2 for 7 h) led to more resting behaviour and adoption of a prostrated lying posture in female and male breeding animals (Dalmau et al., 2014).
Table 43 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 43.
Hazards related to resting problems. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Floor type, space allowance |
| Ambient condition | High temperatures |
| Genetics | No hazard identified |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity | No hazard identified |
| Management of reproduction | No hazard identified |
| Other | Dirty surfaces |
3.6.15. Inability to express maternal behaviour
Maternal behaviour of rabbits consists of three main components: nest building, kindling and nursing. In farm conditions, boxes or separable parts of the housing system are provided in which the doe kindles. In these nesting places, wood shavings, hay or other materials are provided for the doe to mix with the fur from her body as maternal nest building. The doe generally kindles in the early morning, nurses the litter during or immediately after kindling, and then nurses the kits only once, or exceptionally twice, a day thereafter. The doe seldom removes extraneous tissue from the nest after kindling and does not retrieve those kits that may leave the nest by hanging onto a nipple after nursing or by other means (González‐Mariscal et al., 2007).
Nest building and kindling:
Under conventional farm conditions, the following disturbances can be occasionally observed in nest building behaviour: poor nest quality because of inadequate amount of nesting material or hair, or soiling of the nest by the doe with urine or faeces. These challenge kits’ welfare in terms of thermal stress and survival. Some does give birth or place the kits outside the nest, which results in hypothermia and death of neonates without intervention. Infanticide is also considered as a behavioural disturbance of does (see Section 3.6.14).
According to EFSA (2005), for ability to express maternal behaviour, good farming practice means to provide an adequately sized nest box and suitable material for nest building.
No published literature has been found concerning the optimal dimensions of the nest box or nest area but, based on farm data in practice, maternal behaviour is fully expressed with commercial nests measuring at least 25 × 35 cm. According to Schlolaut et al. (2013), in a nest box with a permanently open top or entrance the doe is exposed to light while building the nest (as well as the kits after birth), which might be one reason for behavioural disturbances in the doe leading to failures in nest building, giving birth and placing the kits outside the nest, or to infanticide. However, kindling mainly occurs during the dark period of the day (Rashwan et al., 2003; cited in EFSA, 2005).
Concerning nesting materials, Blumetto et al. (2010) found that does have a strong preference for straw rather than wood shavings as nest material. However, de ‐Oliveira et al. (2017) observed that wood shavings, Tifton hay and newspapers cut into strips may be used for nest bedding. as there were no negative effects on nest‐building behaviour or performance. Farkas et al. (2018) concluded that rabbit does showed the following preference for nest materials: Lignocel® (fine fibre material for pet animals made of wood) > straw > hay > wood shavings. Based on literature, although rabbit does may have different preferences for the nesting materials mentioned above, they can express their normal maternal behaviour whatever the nesting material. On the other hand, there is a lack of scientific information on the effect of type of nesting material on the mortality of kits.
Both in wild (González‐Redondo and Zamora‐Lozano, 2008) and domesticated rabbits (Leone‐Singer and Hoop, 2003) infanticide can be observed. The aggression toward kits is more serious problem in group housing of rabbit does (see Section 3.6.5).
Nursing:
In general, both wild and domesticated rabbit does nurse their kits once a day (Hoy and Selzer 2002). González‐Mariscal et al. (2013) examined the expression of nursing behaviour in case of different litter sizes and concluded that there is a threshold (5 or more kits) of suckling stimulation which influences the nursing rhythm.
Another important factor is that the nest entrance can be closed by the doe's own activity or by management measures. The limited access to the nest for the doe in rabbit farming may more closely resemble what happens in nature, and limiting the doe's access to her kits has been proven to reduce mortality and injury to the kits (Verga et al., 1986; Arveux, 1994; Hudson et al., 1996; Verga, 1997; EFSA, 2005). Coureaud et al. (1998) examined the different nursing methods and found that controlled nursing was more favourable during the first 3–5 days after parturition, whereas free nursing was advantageous later on in terms of kit mortality. In contrast, Szendrö et al. (1999) found free nursing more favourable in the first 7 days of lactation. Some abnormal maternal behaviours were observed in does by Baumann et al. (2005) when the nest entrance was continuously open, such as excessive nest contacts and stereotyped nest plugging behaviour When the entrance of the nest box was visually closed with a metal cat‐flap (with free access to the nest), the does showed half as many potentially disturbing nest contacts than in the case of an open entrance. The mortality of kits between 16 and 35 days was higher when the metal cat‐flap was used (2.9% vs. 0%), and the cause of death was identified as weakness of the kits.
Rommers et al. (2012) found less frequent nest box visits by the doe in group housing systems than in individual housing and concluded that it can cause reduced weaning weight of kits. Moreover, group housed does spent longer time in the empty nest boxes during the last two weeks of lactation, which might have served as a resting place or a place to hide and withdraw from group mates.
Different biostimulation methods have been experimentally tested (Eiben et al., 2007) in which different nest closing periods and methods were applied some days prior to insemination for improving the reproductive performance of does. These interventions can disturb the circadian periodicity of nursing events (Matics et al., 2004b).
In recent years, elevated platforms in rabbit housing systems have been tested as environmental enrichment, as they provide opportunities for movement (jumping up and down) (Maertens et al., 2011). They also offer does the possibility of escaping from their kits once the kits leave the nest box and disturb the doe with persistent nursing attempts (Mirabito et al., 1999). Some authors found that does spend more time on the platform when kits begin to leave the nest box (14–16 days of age), but then spend more time on the bottom level of the cage when 3‐week‐old kits are able to jump up onto the platform (Mirabito et al., 1999, 2004; Miko et al., 2014).
Table 44 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 44.
Hazards related to the inability to perform maternal behaviour. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Artificially closed nest entrance, inability of doe to close the nest, enforced proximity to the nest, inadequate nesting place (position), group housing of does, absence of platform |
| Ambient condition | No hazard identified |
| Genetics | No hazard identified |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity | No hazard identified |
| Management of reproduction | Temporary change of nursing methods as ‘biostimulation’ |
| Other | Inadequate nest material, presence of mastitis |
3.6.16. Inability to express positive social interactions
The rabbits’ behaviour has not been markedly changed by domestication and domestic rabbits show behaviours typical of wild rabbits (EFSA, 2005; Trocino and Xiccato, 2006). European wild rabbits live in groups, which provide the possibility to express positive social interactions (bodily contact, social sniffing, social grooming), while aggressive behaviour, fighting and infanticide are also well‐known among wild rabbits. Group housing of breeding females allows them to develop social interactions, which can be both positive (allogrooming) and negative (aggression). Grouping growing rabbits is important, as they are social animals and show diversity of social interactions. In EFSA (2005), based on available studies until 2005 and practical experience, a group size of 7–9 is suggested for growing rabbits, preferably retaining litter groups.
According to EFSA (2005), social isolation can have consequences on animal welfare as this does not meet the behavioural needs of rabbits and restriction of their behaviour may cause mental distress (suffering) involving feelings such as boredom and frustration. Contact‐making (neutral) behaviours such as sniffing the nose, body or anogenital region of another rabbit may occur at any time (Lehmann, 1991). Contact‐promoting (tolerant) behaviours such as lying against each other and mutual grooming are restricted to resting periods. Rabbits are typically in body‐contact with at least one other animal for about 50% of resting time. Does with small kits tend to be separate from other adults (Stauffacher, 1988; cited in EFSA, 2005).
Under laboratory conditions, in individually housed laboratory rabbits Gunn and Morton (1995) recorded 1–3 stereotype behavioural events per hour (e.g. hair‐chew, chew or lick objects, head‐sway, paw and nose slide, head‐corner). Chu et al. (2004) compared the behaviour of four individually and eight paired housed laboratory rabbits from 10 to 30 weeks of age. They observed an increase (from 0.25% to 1.77%) in the proportion of time spent in abnormal behaviours (digging, floor chewing, bar biting) in the individually housed group, while such behaviours occurred in an unchanged proportion of time (0.95%) in the case of paired housed rabbits. However, the presence of abnormal behaviour in pair housing suggests that it is not sufficient to eliminate these behavioural patterns. Moreover, paired rabbits showed more locomotory behaviour (2.71%) than individually housed ones (0.70%).
Pet rabbits are also suggested not to be housed individually in order to avoid behavioural problems and ensure them social contacts with conspecifics (Crowell‐Davis, 2007).
In conventional farms, kits are kept with their mother until weaning; then growing rabbits are kept in pairs or groups of different sizes during growth; reproducing does are alone in their cage only for one or two weeks within a reproduction cycle, since they share the space with their kits. Only young future‐ breeding and non‐pregnant does are housed individually on a longer period (4–8 weeks). This housing can restrict some natural behaviour (allogroming), as it prevents direct social interactions, but it allows olfactory, acoustic and visual contact and neighbour rabbits can have social contacts through the barred walls of the cages (sniffing, licking, removing hair) (Alfonso‐Carrillo et al., 2014a,b).
Although the best productive performance can be achieved in individual housing of growing rabbits, in practice, growing rabbits (from weaning to slaughtering) are most commonly housed in groups of 2–6 animals in cages (Verga et al., 2007), or in larger groups in pens, which allows social contact among rabbits. In case of free choice among different sized cages, early weaned growing rabbits (until 6 weeks of age) preferred to huddle together in the smallest cage during the resting period, occasionally reaching an extremely high stocking density (> 100 rabbits/m2) (Matics et al., 2004a).
In growing rabbits, concerning different housing systems, Princz et al. (2008b) observed more frequent social behaviour with increasing space allowance. Buijs et al. (2011) could not confirm these findings, but they observed significantly less social contact, cage manipulation behaviour and lateral lying in enriched than in unenriched cages. Bozicovich et al. (2016) found that environmental enrichment (with a wooden stick) decreased the number of positive social interactions (making contact, rubbing, licking and sniffing) among growing rabbits, but the occurrence of stereotyped behaviours (licking or gnawing cage bars, scratching the cage floor insistently) was unaffected. Moreover, the incidence of the above mentioned positive social interactions was higher in mixed‐gender than in same sex (only males or only females) groups. Trocino et al. (2013) examined the fear level and behavioural patterns of growing rabbits housed individually, in pairs or in collective cages (9 rabbits/pen). Although the housing system did not have an effect on the main activities (resting, feeding), individually and paired housed rabbits spent less time allogrooming (0.27%) than rabbits in collective cages (1.44%). Stereotypic behaviours were not observed in any of the housing systems.
To ensure greater possibility for positive social interactions, several studies have been made with group and semi‐group (part time group) housing of rabbits. Up to the present time, no acceptable solution has been found to avoid negative social interactions among rabbits in such housing (Andrist et al., 2012, 2013, 2014; Szendro and McNitt, 2012; Rommers and de Greef, 2018; Rommers and de Jong, 2011, Rommers et al., 2013, 2014a,b; Buijs et al., 2015, 2016). The studies showed that negative social interactions overcome positive social ones (see also Section 3.6.8).
Mugnai et al. (2009) compared the production and behaviour of does in individual housing and group pens for four does, where half of the does were trained to go into their own nest and the others were not trained. They observed more allogrooming (0.86% vs. 0.20%) and fewer attacks (0.60% vs. 1.29%) and dominance behaviours (0.39% vs. 0.63%) in trained does. Rabbits housed in colonies spent more time lying down with stretched legs, whereas in single‐caged does crouching was the most performed static behaviour. In individual housing, fewer moving activities and more stereotypic ones (e.g. biting the cage bars) were observed. However, production performance of grouped does was significantly lower than that of individually housed ones.
When four rabbit does were housed together in a pen with four individual cages and a common area (a commercial individual electronic nest box recognition system was used, only allowing a doe to have access to her own nest box), in each replicate at least one doe did not use the common area whereas the other three does used it for very different percentages of time (Hoy and Matics, 2016).
In conclusion, in farm practice, breeding males, future breeding animals, non‐pregnant does and, temporarily, the reproducing does are housed individually, which restricts the expression of some positive social interactions but avoids negative interactions (e.g. aggression and injuries). Individual housing and restricted social interactions may cause problems more commonly in laboratory and pet rabbits, where the individual housing period is longer and social and visual contacts may also be prevented.
Table 45 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 45.
Hazards related to the inability to express positive social behaviour. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | Social isolation, individual housing Restricted space allowance |
| Ambient condition | No hazard identified |
| Genetics | No hazard identified |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity | No hazard identified |
| Management of reproduction | No hazard identified |
| Other | No hazard identified |
3.6.17. Inability to express gnawing behaviour
Effects of foraging materials (which can be gnawed) on prolonged hunger and digestive disorders are described in Sections 3.6.1 and 3.6.11, respectively. Here, we focus on effects on behaviour and indicators of stress.
Gnawing material is considered to be an important type of environmental enrichment for rabbits, as it allows them to perform a species‐specific behaviour to a fuller extent (Baumans, 2005). As described in the section on abnormal behaviour (Section 3.6.15), one of the main effects of a lack of suitable materials for the expression of gnawing behaviour is a redirection of gnawing to the cage or conspecifics. This section discusses other effects of the absence of gnawing materials, attraction towards gnawing materials, as well as preferences for different types of gnawing materials. No studies on the rabbit's motivation to access gnawing materials were identified (e.g. willingness‐to‐pay or other types of motivation testing). This is an important research gap, as a main consequence of the absence of gnawing material is a redirection towards cage components and conspecifics and it is unknown if, and to what extent, this actually satisfies the rabbit's motivation to gnaw. EFSA (2005) described a lack of information on how gnawing materials affect rabbit welfare under farm conditions and most knowledge was therefore extrapolated from a laboratory animal context (leading to inconsistencies in age, social grouping, feeding, reproductive status or several of these factors when compared to conventional rabbit farming). However, several studies on gnawing materials carried out under conditions representative of conventional farm practice were discussed. Wooden sticks were reported to increase feeding behaviour and caecotrophy (Luzi et al., 2003), or jumping and smelling the environment (Verga et al., 2004). However, such effects were unique to the respective studies and effects in the opposite direction were even observed (Jordan et al., 2004). Straw was provided in only one study, in which it was found to decrease feeding and locomotion (Postollec et al., 2002). Newer studies have also described effects of the absence of gnawing materials on a variety of behaviours in farmed rabbits, but again there is little consistency in which behaviour was affected (apart from effects on abnormal behaviour discussed in Section 3.6.15). One study indicated that wooden sticks increased locomotion in group housed growing rabbits (Princz et al., 2008b), but no such effect was observed for vertical wooden boards (Buijs et al., 2011) which instead increased lateral lying. A study on individual housed growing rabbits’ reports that stick increase feeding, drinking and sniffing (Hesham and Nasr, 2017). Another study found no differences at all in the behavioural repertoire of individually housed sub‐adult bucks with and without gnawing sticks (Jordan et al., 2008), and similar results have been described for individually housed reproducing does (Rommers et al., 2014b). Providing straw to group housed reproducing does led them to spend 1% of their time interacting with the straw, but this did not affect their other behaviours (Rommers et al., 2014a). Cardboard and rubber chewing materials increased chewing and reduced sitting in individually housed laboratory rabbits, but left behaviours like locomotion and lying unaffected (Poggiagliolmi et al., 2011). There are some indications that gnawing materials can improve welfare by decreasing stress levels in group housed growing rabbits. Vertical wooden boards reduced glucocorticoid metabolite levels (Buijs et al., 2011), without affecting fluctuating asymmetry (which is suggested to result from increased stress during physical development, Buijs et al., 2012). The combination of gnawing sticks, a platform, wooden hiding box and lowered density decreased fluctuating asymmetry and improved early weight gain of growing rabbits (Tuyttens et al., 2005). One study indicated that groups of growing rabbits reached a higher slaughter weight when they had access to gnawing materials (Princz et al., 2009), but no evidence of increased growth throughout rearing was found by others (Tuyttens et al., 2005; Buijs et al., 2011). Group housed male growing rabbits provided with gnawing sticks had heavier brains than those without such enrichment (Bozicovich et al., 2016), although at present it is not clear what such a difference indicates. Gnawing sticks were also found to decrease cortisol levels in individually housed growing rabbits (Hesham and Nasr, 2017).
Obviously, not providing suitable gnawing materials is the main hazard for an inability to express gnawing behaviour. Therefore, knowledge on which gnawing materials are perceived as most suitable by rabbits is of importance. EFSA (2005) stated that, although wooden sticks are the most common form of enrichment, individually housed 12‐week‐old laboratory rabbits were reported to interact most with hay, less with pressed grass cubes and the least with wooden sticks (Lidfors, 1997). The low interest that older rabbits show in wooden sticks was confirmed in a newer study on reproducing does under conventional conditions, which interacted six times more with straw and three times more with compressed wooden blocks than with sticks of pinewood (Rommers et al., 2014a). When does had access to straw as well as pinewood sticks, the pinewood was barely touched. Although this indicates that wooden sticks may not be an optimal enrichment for rabbits, growing rabbits did prefer wooden sticks over PVC tubes (Bozicovich et al., 2016), and spent 6–8% more time in cages with sticks than in those without when they could move freely between such cages (Princz et al., 2008a).
Apart from not providing the right kind of gnawing material, lack of easy access could also be considered a hazard. Therefore, incorrect positioning of gnawing material, high stocking densities or social tension hindering movement towards the enrichment, and competition are potential additional hazards for the inability to express gnawing behaviour. Little is known about this, although growing rabbits consumed more of their gnawing stick if it was floor mounted rather than ceiling mounted (Marin et al., 2018) and when lactating does spent more time on their platform later in lactation (presumably to avoid their kits) they also used the enrichment mounted above the platform more often (Rommers et al., 2014b). Since younger rabbits gnaw more than older ones (Katsarou et al., 2011), the impact of a lack of suitable material may be greater for them.
Table 46 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 46.
Hazards related to the inability to perform gnawing behaviour. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing |
Absence of suitable materials to gnaw Lack of easy access to suitable materials to gnaw |
| Ambient condition | No hazard identified |
| Genetics | No hazard identified |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity | No hazard identified |
| Management of reproduction | No hazard identified |
| Other | No hazard identified |
3.6.18. Occurrence of abnormal behaviours:
Chewing and gnawing are part of the normal behavioural repertoire of the rabbit (EFSA, 2005; Trocino and Xiccato, 2006). However, when farmed rabbits chew or scratch on parts of the cage or on other rabbits such behaviour is usually classified as abnormal behaviour or behaviour redirected to an abnormal target (Princz et al., 2008b; Verga et al., 2007). Group‐housed growing rabbits and individually housed does spend 2–4% of their time chewing or scratching cage parts (Princz et al., 2008b; Buijs et al., 2011; Rommers et al., 2014b).
Cage chewing is much more common in younger laboratory rabbits than older ones (2 vs. 6 months, Katsarou et al., 2011) and can thus be expected to be more common in growing rabbits than in breeding animals. Over‐grooming (i.e. excessive self‐grooming) is sometimes also included as abnormal (Bozicovich et al., 2016; Stewart and Suckow, 2016). However, this poses some difficulty as a certain level of grooming is normal and even necessary, and there is no established level at which grooming would be considered excessive. Grouped growing rabbits and individually housed does spend 15–20% and 11–13% of their time grooming, respectively (Princz et al., 2008b; Buijs et al., 2011, 2015; Rommers et al., 2014b). Information on clinical evidence of over‐grooming is lacking for farmed rabbits, but was rare (1%) in pet rabbits (Mullan and Main, 2006). Like cage gnawing, self‐grooming is more common in younger laboratory rabbits than in older ones (Katsarou et al., 2011). Behaviours like faeces eating (excluding caecotroph eating or consumption of the mother's faeces by kits), head swaying, nose sliding, excessive thumping, keeping the head in the corner, sitting in a hunched posture and sham‐chewing are also mentioned as examples of abnormal behaviour in laboratory rabbits (Stewart and Suckow, 2016), but these are not commonly observed in farmed rabbits.
EFSA (2005) describes the lack of gnawing material as a main hazard for abnormal behaviour. In laboratory rabbits, hay was more effective in reducing abnormal behaviour than grass cubes, wooden sticks, or a hiding box (Lidfors, 1997). However, wooden sticks were still effective enough to cause a significant reduction in abnormal behaviour in individually housed (Hesham and Nasr, 2017) and group housed growing rabbits (Luzi et al., 2003; Verga et al., 2004), although not in all studies (Jordan et al., 2004). Individual housing was also mentioned as a hazard for stereotypic behaviour in lactating and non‐lactating lactating does (EFSA, 2005). Group size (apart from individual housing) and cage height were reported not to affect abnormal behaviour in growing rabbits (EFSA, 2005), although there is some new evidence to the contrary (see below).
Most new research supports the effectiveness of gnawing materials to reduce abnormal behaviours such as stereotypic biting, chewing or licking of the cage in growing rabbits (Verga et al., 2007) and wood is most commonly provided for this purpose. Provision of wood to group housed growing rabbits can reduce cage chewing (Verga et al., 2007; Buijs et al., 2011), non‐aggressive social contact (Buijs et al., 2011) and skin and ear lesions (Bozicovich et al., 2016; Princz et al., 2008b, 2009).
Gnawing material can reduce abnormal behaviour but does not eliminate it fully because gnawing/scratching are intrinsically motivated exploratory behaviours for the rabbit. Growing rabbits spend 1–2% less of their total time budget on cage gnawing/scratching when enrichment is present than without (corresponding with a 33–50% reduction in the time spent on such behaviour, Buijs et al., 2011; Princz et al., 2008b; Bozicovich et al., 2016). The effectiveness of enrichment in reducing abnormal behaviour may depend on the number of cage or pen mates. In contrast to the aforementioned effects on group housed growing rabbits, enrichment provision did not affect abnormal behaviour in individually housed rabbits (sub‐adult males: Jordan et al., 2008, adult males: Poggiagliolmi et al., 2011, adult females: Maertens et al., 2013; Rommers et al., 2014b). In line with the suggestion that enrichment may be more effective in larger groups, Princz et al. (2009) reported higher stick consumption per rabbit in larger groups of growing rabbits (2 vs. 13) and suggested that social facilitation may lead to greater use of such enrichment. As most studies on individual housing were conducted with adult animals, it is also possible that these reflect that enrichment is less effective in older animals. In line with this, enrichment did not affect abnormal behaviour in adult group housed rabbit does (Rommers et al., 2014a).
The effects of space allowance and group composition on abnormal behaviour have been less studied and their interpretation is less clear. Studies on the effect of space allowance on abnormal behaviour in growing rabbits have provided contradictory results (Morisse and Maurice, 1997; Princz et al., 2008b; Jekkel et al., 2010; Buijs et al., 2011). Group housed does are reported to groom themselves less than individually housed ones (Mugnai et al., 2009; Buijs et al., 2015). However, reduced grooming in grouped does may be due to unrest in newly formed groups rather than abnormal over‐grooming in individually housed does, as grooming increases later after group formation (Buijs et al., 2015). Mixed‐sex groups were shown to spend less time manipulating cage parts than same‐sex groups. However, non‐aggressive social interactions (a category that may include abnormal and damaging interactions as well as positive interactions, Buijs et al., 2011) were more common in same‐sex groups (Bozicovich et al., 2016). Social interactions are described in more detail in chapter 3.6.18.
Stewart and Suckow (2016) found no effect of a small difference in cage height (36–38 vs. 40 cm) on an extensive list of abnormal behaviours in individually housed laboratory rabbits. However, grouped growing rabbits in even lower cages (20 cm) had more ear lesions than those in cages ≥ 30 cm, some of which may have resulted from gnawing (Princz et al., 2008a).
When does do not have free access to the litter they are nursing, this can cause them to scratch the nest entrance or the floor in front of it for prolonged periods, in what is assumed to be an attempt to open and close the nest. In does that could smell, but not access, their litter (as would be common when controlled nursing is applied on farm) bouts of such behaviour continued throughout the day, with the exception of the hour after the does had been allowed to nurse their litter (Baumann et al., 2005). Nursing behaviour is discussed in more detail in Section 3.6.17. Feed restrictions can increase bar biting in does (Martinez‐Paredes et al., 2015).
Table 47 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 47.
Hazards that could lead to the occurrence of abnormal behaviour. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing |
Individual housing Very low cages (20 cm) Lack of gnawable materials (e.g. roughage, wood), especially in (larger) groups |
| Ambient condition | No hazard identified |
| Genetics | No hazard identified |
| Nutrition and feeding | Feed restriction |
| Management of biosecurity | No hazard identified |
| Management of reproduction | Disturbance of maternal behaviour |
| Other | Sub‐adult animals |
3.6.19. Fear
Fear has been defined as ‘a feeling which occurs when there is perceived to be actual danger or a high risk of danger’; it can produce changes in behaviour, physiology and in the brain (Broom and Fraser, 2015). Excessive fear may cause chronic stress, which affects animal welfare and health (Forkman et al., 2007). Moreover, excessive fear may cause serious trauma and injuries during handling when animals struggle and are difficult to handle.
In free living conditions, European rabbits differentially increased two different forms of vigilance behaviour in social and anti‐predator contexts. Two forms of vigilance of different intensity, i.e. subtle and overt could be distinguished. The frequencies of both forms of vigilance displayed by the rabbits differ significantly in occurrence, duration and distribution over time, e.g. the presence of conspecifics in close proximity affects the display of subtle but not overt vigilance, which was associated with predator presence (Monclùs and Rödel, 2008). The alert and anti‐predator responses of the domestic rabbits consist of ‘look‐out’ positions, foot‐thumping as an alarm signal, rearing on their hind legs, running at high speed for shelter and ‘freezing’.
Thus, rabbits not only react to fear or threat by ‘fight‐or‐flight’ response, but also assume motionless postures in tonic immobility (also defined as immobilisation catonia or death feigning) (Giannico et al., 2014). Under different conditions (farmed, lab or pet rabbits), rabbits exposed to different possible threats (noises, presence of man or unknown operators, introduction of new animals) have been observed running away into a hiding place or into a corner of the cage with their head, or freezing, or attacking with teeth and claws (Mullan and Main, 2006; Crowell‐Davis, 2007; Verga et al., 2007). Measurements of fear levels in rabbits have been based on changes in behaviour or occurrence of some behaviours, reactivity tests as well as physiological indicators (EFSA, 2005; Verga et al., 2007; Verwer et al., 2009; Buijs and Tuyttens, 2015; Trocino et al., 2018). No measurable (numerical) thresholds have been given to identify and determine with certainty what are unacceptable fear levels with regard to animal welfare and health.
Fear can be elicited by different occasional stimuli or even by defective management and housing conditions, which can affect animal response and welfare to a different extent depending on the frequency of occurrence, duration, and severity of the threat.
EFSA (2005) recognised every practice producing a negative experience of rabbits towards humans as a hazard and recommended to adopt the following measures: a progressive approach to kits; quiet and slow movements during handling and catching; rabbits must never be picked up or held by ears; rabbits should be caught with a minimum of chasing. To improve human‐rabbit relationship and to reduce fearful reaction, the regular daily handling of lactating kits recommended by EFSA (2005) has been confirmed to be useful by later studies (Csatadi et al., 2005; Verga et al., 2007; Verwer et al., 2009; Zucca et al., 2012). Indeed, kit exposure to only human smell reduces fear towards man and improves their welfare (Dúcs et al., 2009). Under farming conditions, staff are present daily in proximity to the rabbits. They handle kits at the time of kindling, litter standardisation and litter control during lactation; then, at weaning, litters or does may be moved from one cage to another (depending on the farm management). Thereafter, growing rabbits are not usually touched by the staff until slaughter, when they will be taken out and loaded in baskets and into the transport cages. During the reproductive cycle, reproducing does are usually handled at the time of artificial insemination (if used), at the time of pregnancy palpation and at the time of any medical interventions (e.g. vaccination, treatment).
In addition, strange situations can be a hazard for fear. Young rabbits should be given appropriate experience of management practices (e.g. particular feeding and watering systems) and environmental conditions (e.g. natural light, litter, ventilation fans and other sounds and noises) to enable them to adapt to the husbandry systems they will encounter later in life.
Evidence is available that social isolation may negatively affect fear reactions in rabbits and is explained by the importance of social vigilance in natural conditions. Under farming conditions, growing rabbits reared in individual cages showed a higher level of fear towards humans compared to rabbits kept in bicellular and collective cages, as they were more sensitive to the immobility test (Trocino et al., 2013). Negative reactions towards humans (more aggression) (d'Ovidio et al., 2016) or towards a new environment (Schepers et al., 2009) have also been found in single–caged pet rabbits compared to those caged with mates.
Under farming conditions, farmers report that some lactating does may occasionally show aggressive behaviours towards them when approaching the cages. Nevertheless, territoriality, rather than fear towards humans, could explain this behaviour as reported for pet rabbits (Crowell‐Davis, 2007).
Under farming conditions, an inadequate social environment associated with overcrowding and group housing may affect animal fear and stress levels, due to competition and aggression (Verga et al., 2007). In the case of growing rabbits kept in small groups (8), fear response to a human in the tonic immobility test was not affected by the stocking density (Trocino et al., 2004). In collective pens with large group size (20–27), a fearful reaction towards a new environment was measured in the open field test. At the pre‐slaughter age, the highest latency to enter the arena was observed in rabbits kept in pens with 16 rabbits/m2 compared to those at 12 animals/m2, (with group size decreasing from 27 to 20 rabbits/pen). However, this difference was not associated to differences in corticosterone levels in hair or faeces (p > 0.05) (Trocino et al., 2018). The higher incidence of injured rabbits (26.2% vs. 8.2%, respectively; p < 0.001; Trocino et al., 2015), and the higher related aggression in the larger group at the end of the trial could have accounted for their fearful reaction in the new environment and, especially, for the low motivation the rabbits had to reinstate contact with conspecifics (Forkman et al., 2007; Buijs and Tuyttens, 2015).
In the case of reproducing does, to our knowledge, no data are available on fear responses of the animals kept under different farming conditions. In the case of continuous or part‐time group housing, the high aggression level and related skin injuries (see Section 3.6.8) might be expected to induce a chronic fear level, especially in low ranking does. In fact, Szendro et al. (2013) found a higher faecal corticosterone level in does housed collectively (4 does and 1 buck/pen) than those kept individually (175 nmol/g vs. 54–61 nmol/g). Direct observations under experimental conditions indicated that injured does spent most time motionless, in a location in the enclosure which guaranteed visual isolation from other rabbits, and reduced feed intake (Trocino, unpublished data).
Unsuitable environmental/housing conditions also induce fear and stress levels in rabbits. The use of a wooden slatted floor with too large a space between the slats (3 cm) challenged animal comfort and movement, and negatively affected rabbit response towards humans or objects during different tests when compared to animals kept in the pens with a plastic grid, especially when young (Trocino et al., 2018). The corticosterone levels in the hair confirmed that rabbits reared on the wooden slatted floor had a higher stress level compared to those reared on the plastic grid. Nevertheless, in this case, reaction towards man during the tonic immobility test did not change. Rabbits housed on a straw‐bedded wire floor exhibited more fearful behaviours (i.e. standing still) in the open field test and were more fearful towards man compared to rabbits kept on other floors (plastic slat and wire‐mesh) (Trocino et al., 2008).
Finally, in conventional systems, where rabbits are housed in closed barns, no stimulus for predation fear should be present under good farming practice. Nevertheless, in semi‐outdoor or outdoor systems, regardless of the specific housing enclosure (fixed or moveable cages, underground systems, garenne, hutches), rabbits may be exposed to predator challenges (from both birds and carnivores). D'Agata et al. (2009) observed more escape attempts and digging and less exploratory biting behaviour during the open field test in rabbits in colony wire cages kept outdoor under a shelter compared to indoor. Even the odour from predator proximity may elicit a fear response in rabbits, which are macrosmatic animals (EFSA, 2005). Monclús et al. (2006) found that the simulated presence of a predator (fox odour) in the enclosure significantly increased faecal corticosterone metabolite concentrations, with males experiencing a higher increase than females.
In conclusion, the hazards for fear responses in rabbits are shown in Table 48.
Table 48.
Hazards related to fear. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing |
Social isolation (greater impact in absence of olfactory and visual contacts) Aggression among animals in any housing or management condition (see hazards under Section 3.6.8) Flooring, which does not permit safe movements or impairs comfort (coat soiling) |
| Ambient condition |
Semi‐plein air and outdoor systems (exposure to predator odours) Exposure to sudden and unknown sounds |
| Genetics | More nervous breeds/lines |
| Nutrition and feeding | No hazard identified |
| Management of biosecurity | Entrance of wild/other animals (cats) |
| Management of reproduction | No hazard identified |
| Other | Reduced contact with human presence and odour. Rough handling |
3.6.20. Metabolic disorders (not included in the survey)
Metabolic disorders are often secondary to other welfare consequences such as gastrointestinal disorders (Licois, 2010). Several different types of metabolic disorder can be considered.
Hypocalcaemias probably affect rabbits but their prevalence has not been evaluated in conventional farms. Hypercalcaemia is a main consequence of vitamin D toxicosis, characterised by calcification of soft tissues (Rosell et al., 2012). Ketosis is a metabolic disease that results from impaired metabolism of carbohydrates and volatile fatty acids (Brownlow et al., 2017). From the study of 2,237 emergency visits performed during 1997–2007 on 660 conventional farms, only one outbreak of pregnancy toxaemia with many affected does was observed (Rosell and de la Fuente, 2009a). This disorder (correctly defined as hepatic lipidosis) might affect some rabbit does with subclinical status. It can also affect does during the postpartum period (Greene, 1937) and exceptionally males, with clinical signs, including death. In a study performed on 490 doe farms, with a median size of 769 does, from 2006 to 2014, Rosell and de la Fuente (2016a) necropsied 140 does with lesions compatible with pregnancy toxaemia/ketosis, and a monthly mortality risk/MMR of 0.16% (CI95% [0.13–0.19]); in addition, diagnosed calcinosis was very low (MMR = 0.001%).
Risk of metabolic disorders is increased by obesity during the rearing of young does (Martínez‐Paredes et al., 2018). Extremes of body condition represent a hazard in adults (de la Fuente and Rosell, 2012) and failure to carry out body condition examination frequently increases risk because a doe in production changes very quickly, and sometimes is on the border between normal physiology and pathology (Lebas, 2000). Inappropriate levels of vitamins A, D and E increase the risk of calcinosis.
Table 49 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
Table 49.
Hazards related to metabolic disorders. Hazards written in bold are scientifically proven by more than one source, those in normal text were found in only one paper, those in italic have been mentioned by the experts invited to the technical hearing meeting
| Hazard category | Hazard |
|---|---|
| Housing | No hazard apart from ambient conditions |
| Ambient condition | Heat stress and decreased food consumption |
| Genetics | High producing does are specially predisposed |
| Nutrition and feeding |
Restricted feeding prior to parturition Obesity (predisposing pregnancy toxaemia) Inappropriate levels of vitamins A, D and E (calcinosis) |
| Management of biosecurity | No hazard identified |
| Management of reproduction | Failure to cull non‐pregnant does at first service |
| Other | No hazard identified |
3.6.21. Pain (not included in the survey)
EFSA (2005) identified various causes in conventional rabbit production of acute and chronic pain, generally identified by behavioural and postural changes. These causes included a number of clinical disorders of the respiratory system and alimentary tract, such as epizootic rabbit enteropathy (ERE, or mucoid enteropathy), and other clinical conditions (such as mastitis, pyoderma) of staphylococcosis. They also highlighted pododermatitis (sore hocks, often caused by wire flooring; Castellini et al., 2003) as a cause of pain which is more severe in the case of deep unresolving infection. Many subsequent studies have reinforced the painfulness of this condition (Rosell and de la Fuente, 2009b; Rommers and de Jong, 2011; Buijs et al., 2014; Mancinelli et al., 2014). Finally, EFSA (2005) noted that poorly administered injections are painful.
The more recent review of Dorning and Harris (2017) further identified that high levels of aggression and injury in group‐housed does in current systems are a significant source of pain.
Unlike many other farmed livestock species, few management procedures likely to cause pain are routinely performed in rabbit production. The main management procedure which is likely to be painful is the application of means of individual identification by ear tattooing or tagging, which is commonly applied to all reproducing does on the farm. This has been shown to cause pain, as indicated by significantly greater struggling behaviour and vocalisation, greater facial expression scores of pain, higher peak heart rate, as well as higher systolic and mean arterial blood pressure compared to sham treated controls (Keating et al., 2012). However, serum corticosterone responses did not differ between sham and tattoo treatments, probably as a result of the handling stress experienced by all animals. Behavioural changes were of relatively short duration, with no pain behaviours identifiable by 1 h post‐treatment.
In addition to the poorly administered injections identified by EFSA (2005), other hazards for causing pain identified during the EKE include careless handling and the unskilled application of AI procedures.
Table 50 summarises the information on the main hazards for this welfare consequence and their degree of scientific support.
3.7. Synthesis of findings on different production systems
In this section, information obtained from the EKE process is brought together with findings from the literature review to provide an overview of each of the housing systems. First, the conclusions on the overall welfare score and the highest ranked problem areas for welfare in each animal category are summarised. It should be noted that the top 5 ranked welfare consequences do not always have high welfare impact scores, and so the absolute score values are also given for context. Findings from the literature review are used to highlight the likely reasons for the important welfare consequences seen in each housing system, how they might be influenced by other aspects of the production system, and hence how they might be prevented or alleviated.
3.7.1. Conventional cages
For does and growing rabbits, the conventional cages numerically have the highest (i.e. worst) overall welfare impact score (3.2 for does, followed by the next highest system with a score of 2.3; 3.6 for growing rabbits, followed by the next highest system with a score of 2.6), whereas for kits conventional cages have the second lowest (i.e. second best; 1.3, with the best system scoring 0.9). For all animal categories restriction of movement, inability to gnaw and resting problems featured in the top 5 welfare consequences for conventional cages. Social behaviour, heat stress and hunger were each in the top five for two out of three animal categories (social behaviour for does and growing rabbits, heat stress for does and kits, and hunger for kits and growing rabbits).
Restriction of movement is scored high for growing rabbits (score 1.3) and does (score 0.9). This is mainly due to limited total space allowance and lower cage height which is an inherent characteristic of conventional cages. However, for growing rabbits, the stocking density also contributes, and this effect can be ameliorated by decreasing the number of growers per cage. The reason for restriction of movement for kits in conventional cages is less clear, as these prefer to remain tightly grouped in the nest box for most of their life in this stage. However, the score is relatively low compared to the other animal categories. It may result from kits’ difficulty to move around on the wire floor of the main cage as soon as they leave the nest box (including returning to the nest). Although floor type and nest access could be improved within the conventional cage system, the improvements made by such changes could have trade‐offs (e.g. less space between the wires of the floor would lead to increased cage soiling as more faeces will remain on the floor).
Inability to gnaw could be solved relatively easily by adding gnawing materials to conventional cages. As inability to gnaw was identified as a problem in all three animal categories (score 0.4 for does, 0.2 for kits and 0.4 for growing rabbits), material supplied in such a way that it is accessible for all three categories (e.g. in reach of small kits as well) is likely to improve welfare. The identification of inability to gnaw as the most important welfare consequence for kits in conventional cages was not expected as there is no published literature on kits’ motivation for gnawing or use of gnawing materials. Additionally, considering the level of maturity at birth the duration estimates for kits appear to be very high.
Resting problems in conventional cages may result from different factors in the different animal categories. In does (score 0.2) and growing rabbits (score 0.3), it may be a result of the wire floor (which preference tests suggests may be experienced as uncomfortable even if partially covered with a foot rest). Addition of a solid or plastic‐slatted platform could be considered, although many conventional cages would not be high enough to allow this, and the benefits should be weighed against a possible risk of soiling. Soiling may occasionally already contribute to resting problems in does and growing rabbits if faeces accumulate on the wire or footrests due to an inappropriate width between wires/slats. In addition, high stocking densities constrain resting behaviour for growing rabbits kept in conventional cages, and total space allowance may do so for reproducing does (a problem that is likely to increase at high temperatures, which increase the reproducing does motivation to adopt a prostrated lying posture). Each of these factors are unlikely to result in resting problems in kits (score 0.1). Instead, in kits resting problems may occur due to soiled or inappropriately built nests.
For the doe, the inability to show positive social behaviour (score 0.2) could be related to the period between weaning and the following parturition in which the doe is kept alone. Inability to have social interaction directed at other adult animals is an inherent characteristic of the conventional cage system. It cannot be altered without switching to a different housing system. It is surprising that inability to express social behaviour also came up as a top 5 welfare consequence for growing rabbits in conventional cages, with a similar score (0.2) to that given for does, as these are almost always housed in groups. However, limited space allowance may constrain social behaviour in growing rabbits. In addition to greater space allowance, lack of enrichment and mixed‐gender groups are associated with more social interaction. This can both mean that the motivation for social interactions is higher in these situations, or that the situations allow for a higher expression of it. As such, it is unknown if changes in these variables would improve growing rabbits’ ability to express positive social behaviour in conventional cages.
Heat stress was indicated as a main welfare consequence for does in conventional cages (score 0.2), suggesting that, in the experts’ opinion, current climatisation (e.g. house construction and ventilation systems) could be insufficient to prevent too high ambient temperatures in practice. More surprisingly, the experts considered heat stress as a main welfare consequence for kits in conventional cages (score 0.1). In contrast, the existing literature highlights the risk of cold stress for kits rather than heat stress. Thus, heat stress in kits may require further research.
Hunger was a main consequence for kits (score 0.1) and growing rabbits (score 0.1) in conventional cages. These are not inherent to the system but may be reduced by avoiding technical failure of feeding systems (growing rabbits) and improving doe behaviour and health (e.g. failure or inability to suckle or produce enough milk).
3.7.2. Elevated pens
For does, elevated pens were assigned the second lowest cumulative welfare impact score (2.0), while for both kits (1.0) and growing rabbits (1.0) it obtained the lowest score. The score for does was similar to the one elicited for enriched cages and outdoor systems and only slightly higher than the one for organic systems. Regarding the top 5 welfare consequences for does, restriction of movement (0.9) contributes by far most to the overall impact score, followed by the relatively less important consequences inability of gnawing, skin lesions, resting problems and inability to perform positive social behaviour (mean 0.1). In kits, the experts judged the inability to gnaw as the most important welfare consequence (0.3), followed by prolonged hunger (0.1) and neonatal disorders, fear and skin disorders (0.06–0.03). Skin disorders (0.2) was the welfare consequence contributing most to the overall welfare impact score for growing rabbits, while resting problems, gastroenteric disorders, inability to gnaw and fear were judged to be of relatively less importance (≤ 0.1).
If elevated pens are not used for group housing of does, alleviating restriction of movement (here: does) requires fundamental changes to the housing system in terms of total space allowance and dimensions of the cages. However, a platform and suitable gnawing material can be provided in existing systems.
Increased space allowance together with appropriate floor quality (e.g. plastic flooring, correct size of slats/openings) may also reduce soiling and improve resting behaviour. Group housing of does is also the most important reason for skin lesions in does due to aggressive interactions between the adult animals. If elevated pens are used for individual housing, the does are however prevented from performing social behaviours between weaning and the next kindling. Gastrointestinal disorders in kits may be associated with the health of the mother and with unbalanced diets. Prolonged hunger in kits and neonatal disorders may result from inadequate nesting behaviour and poor maternal care. Such conditions may more frequently be found in group housing of does, for which elevated pens may be used. This may also explain why neonatal disorders have been found among the top 5 welfare consequences in this housing system only. Reduced contact with humans and/or rough handling can contribute to fear as well as to a high level of aggression towards humans.
If elevated pens are used for growing rabbits, biosecurity procedures to avoid introduction of pathogens, climate control to maintain moderate air temperature and relative humidity as well as positioning of the drinkers so that wetting of the fur and thereby transmission of Pseudomonas infections is prevented, are measures to reduce the occurrence of skin disorders. Regarding resting problems, gastrointestinal disorders, inability to gnaw and fear, the same measures as outlined above apply.
3.7.3. Enriched cages
With respect to the different housing systems, enriched cages got the second highest overall welfare impact score for growing rabbits (2.6) and intermediate welfare impact scores for reproducing does (2.1; the 4th highest) and for kits (2.6; the 3rd highest). Restriction of movement for each of the animal categories (does 0.9; kits 0.2; growing rabbits 1.3), skin disorders for kits (0.1) and growing rabbits (0.3) were considered as major welfare consequences in this system. Heat stress for does (0.1) and kits (< 0.1), gastrointestinal disorders for growing rabbits (< 0.1), resting problems for does (< 0.1) and growing rabbits (< 0.1), inability to express social behaviour for does (< 0.1), inability to express gnawing behaviour for growing rabbits (< 0.1), respiratory disorders for kits (0.04) were also ranked in the top 5 welfare consequences for this system with relative lower welfare scores. Although the occurrence of resting problems as estimated by the experts was actually the lowest in the enriched cages compared to all other systems, the data suggest that within the system it would constitute a further area for improvement.
Welfare impact scores for restriction of movement were similar for does and growing rabbits but higher for kits, compared to conventional cages, however, the floor space is increased in enriched cages and rabbits have the possibility to jump. Restriction of movement cannot be solved without significant change of the system. The risk of skin disorders can be decreased with management of biosecurity. Inability to express gnawing behaviour in enriched cages is strange because these cage types are in general equipped with gnawing sticks or other suitable materials for gnaw. Risk of resting problems can be decreased with adequate cage size and floor material. To avoid heat stress, a proper cooling and ventilation system should be used in the buildings. The main hazards of gastrointestinal disorders are unbalanced diet and early weaning.
3.7.4. Floor pens
Floor pens were the housing system with the second highest overall welfare impact score for does (2.3) after conventional cages, the second highest welfare score for kits (1.6) after outdoor systems, and an overall welfare score for growing rabbits (2.0) which was intermediate among the housing systems. This score for does resulted from a higher score for hunger (0.4) than seen in other systems, with heat stress (0.2), resting problems (0.2), reproductive disorders (0.1) and skin lesions (0.1) also making a significant contribution of similar magnitude. For kits, this housing system again had the highest score for hunger (0.3), with thirst (0.2) and neonatal disorders (0.2) also making a significant contribution. Gastrointestinal disorders (0.1) and cold stress (< 0.1) also featured in the top 5 welfare problems, but made a relatively lower contribution. For growing rabbits, gastrointestinal disorders (0.3), skin diseases (0.2) and hunger (0.2) featured highest, with resting problems (0.2) and skin lesions (0.1) also making a significant contribution. In general, physical and health problems featured more highly than behavioural problems. The occurrence of pododermatitis in does, which literature review suggests might be a problem in this system, was scored by the experts as higher than for elevated pens, enriched cages and organic systems but lower than in conventional cages and outdoor systems.
Many of these problems may reflect the hygiene challenges of floor pens, with the presence of soiled bedding contributing to gastroenteric, reproductive and skin infections, as well as to hypothermia and impaired resting. Soiling of the feed and drinkers could also impair intake of food and water. The insulating properties of bedding could also contribute to heat stress in hot weather. The other factor influencing resting problems and skin lesions in does, and neonatal disorders in kits, might be the fact that floor pens are generally group‐housing systems. Management recommendations to improve welfare in this system therefore relate to the quality and quantity of bedding material provided, and to ensure good design of feeders and drinkers. Provision of adequate space per animal and restricted group size would improve resting behaviour reduce risk of aggression and skin lesions, while good controlled ventilation systems to minimise ambient temperature extremes would aid thermal comfort.
3.7.5. Outdoor systems
Outdoor housing systems include several types with a variety of housing (made of different materials), which provide different degrees of protection against the weather. The overall welfare impact score for these systems was intermediate for does among the six housing systems, but was higher for the other animal categories. For does, the welfare score (2.1) was the third across the six, for growing rabbits the welfare score (2.6) was placed second, but it was the worst of all systems for kits (2.6). With regard to health and welfare outcomes, heat stress was the highest scoring welfare consequence and among the top 5 for does (0.2) and kits (0.5). Gastrointestinal disorders featured also in the top 5 welfare consequences, placed first for growers (0.3), and fifth for kits (0.3). The results also highlighted hunger in kits (0.3) and growing rabbits (0.2). Lastly, a common welfare consequence was resting problems that affects does (0.1) and growers (0.2).
The main hazards relevant to rabbits housed outdoors are related to climate conditions and the difficulty to implement biosecurity measures. Therefore, improving housing to provide better protection would be an important measure to reduce climatic impact and fear. Besides, investment in fans, trees and humidifiers is perhaps required. There are several alternatives to improve health and mitigate welfare consequences. Training of the farm staff is important in addition to the time and effort invested in the care and observation of the rabbits (e.g. behaviour, clinical signs, feed and water consumption), checking of the kits in the nests, especially when temperatures are < 12° or > 30°C. Lastly, for resting problems, increasing the surface area for does or decreasing the density for growers in the resting area together with good hygienic conditions can be useful solutions. The diversity of outdoor systems means that solutions to the important hazards need to be tailored to each set of circumstances.
3.7.6. Organic systems
For does, the overall welfare impact score (1.8) is the lowest score among all housing systems. Similarly, for the growing rabbit, this score (1.2) has a low range compared to the other systems. The median of the overall welfare impact score (1.4) is similar to many other systems but unexpectedly much lower than the score for outdoor systems (2.6). Restriction of movement is the highest welfare consequence for does (score = 0.5) followed by heat stress, reproductive disorders and resting problems (around 0.2). Hunger, heat and cold stress are the three most important welfare consequences for kits (score around 0.2), followed by gastrointestinal and neonatal disorders (0.1). For growing rabbits, the top 5 welfare consequences are at a similar level (median around 0.1) and are: resting problems, gastrointestinal disorders, heat stress and fear.
Modification of the housing system can reduce the problems of movement restriction for does, particularly when climatic condition do not allow an outdoor access. For instance, the sheltered part of a movable cage or in a paddock could be enlarged but this will probably make it heavier and thus difficult to move. The types and cleanliness of the floor could be improved to make the lying surface more comfortable. Another option is to use an individual paddock for doe housing, as already observed in some farms.
Heat and cold stress are dependent on the climatic area and housing system. The relatively lower score than for resting problems suggests this is generally well managed by the farmers and can be improved by using items to provide shade in summer and by improving the insulation.
Gastrointestinal disorders in growing rabbits could be reduced by using a strict management of housing hygiene combined with a good feeding strategy and controlled with a daily visit to the animals looking at their health. As for outdoor systems, the diversity of organic systems means that solutions to the important hazards need to be tailored to each set of circumstances.
4. Conclusions
An overview of each of the housing systems is provided in Section 3.7 where findings from the literature review are used to highlight the likely reasons for the important welfare consequences seen in each housing system, how they might be influenced by other aspects of the production system, and hence how they might be prevented or alleviated.
The values for occurrence, duration and severity obtained from the EKE survey and workshop were used to derive a welfare impact score for each welfare consequence, that were summed to give an overall welfare impact score for each system with a higher score indicative of poorer welfare. The welfare impact scores obtained from the EKE process showed a reduced level of certainty and differences must therefore be interpreted with caution.
Conclusions about comparison among systems
-
1
Production and housing systems are highly diverse but, compared to the niche systems, the conventional systems are more uniform.
-
2
Generally, objective data are lacking on the welfare consequences occurring in different production systems and expert opinion about the occurrence and relative severity of different welfare consequences is highly variable.
-
3
Schemes to evaluate welfare outcomes ‐ e.g. through the use of ABMs – exist for rabbits but have not been widely used or validated.
-
4
It is likely (certainty 66–90% from probabilistic analysis based on expert opinion), that the welfare of reproducing does is lower in conventional cages compared to the other housing systems. However, no distinction can be made among the other housing systems regarding the welfare impact.
-
5
It is likely to extremely likely (certainty 66–99%), that the welfare of kits is lower in outdoor systems compared to the other housing systems and that the welfare is higher in elevated pens than in the four other systems. However, no distinction can be made among the conventional cages, enriched cages, floor pens and organic systems regarding the welfare impact.
-
6
The median welfare impact scores for growing rabbits were more diverse than those observed for reproducing does. It is likely to extremely likely (certainty 66–99%), that the welfare of growing rabbits is lower in conventional cages compared to the other housing systems and that the welfare is higher in elevated pens than in the other systems. However, no distinction can be made among the enriched cages, floor pens, organic systems and outdoor systems regarding the welfare impact.
Conclusions about main welfare consequences in different rabbit categories
-
7
For reproducing does, restriction of movement obtained the highest welfare impact scores. In addition, the lack of possibility for gnawing behaviour and hunger, contributed substantially to the higher impact score in conventional cages (see conclusion no. 4).
-
8
For kits, heat stress obtained the highest welfare impact scores and this welfare consequence, together with neonatal disorders and cold stress contributed substantially to the higher impact score in outdoor systems (see conclusion no. 5). However, in outdoor systems, the inability to perform gnawing behaviour is not in the top five welfare consequence even though it appears in all other systems.
-
9
For growing rabbits, restriction of movement gave the highest welfare impact scores. This welfare consequence, together with inability to perform gnawing behaviour and resting problems, made the greatest contribution to the higher impact score in conventional cages (see conclusion no. 6).
-
10
For reproducing does as well as growing rabbits, welfare consequences related to behavioural restrictions were more prominent in conventional cages, elevated pens and enriched cages, whereas those related to health problems appeared more often in the top five welfare consequences of ‘niche’ housing systems (i.e. floor pens, outdoor, organic systems).
Conclusions about organic systems
Apart from housing conditions, organic standards also include requirements related to e.g. feeding and health management. Housing in organic rabbit farming is diverse and complex and may consist, for example in does, of either movable cages or individual paddocks that can be used. Therefore, whether one or the other is used this can result in different welfare consequences.
-
11
Welfare impact scores given by experts suggest that welfare in organic systems is generally good.
-
12
Welfare consequences relate especially to the outdoor housing. Extreme temperatures can cause heat or cold stress, and movement restriction if access to pasture is restricted. Fear may result from perceived exposure to predators. Health problems may result from exposure to thermal stress and limitations on biosecurity measures.
-
13
The diversity of organic systems means that solutions to the important hazards need to be tailored to each set of circumstances.
-
14
Identified hazards suggest that shelter should be insulated to mitigate the effect of climatic extremes, barriers against predators (foxes, dogs, prey birds, etc.) should be checked carefully, and a thorough prophylactic program should be observed to limit the welfare consequences associated to health problems.
General conclusions about welfare of farmed rabbits also including feedback on the EFSA, 2005 Opinion.
-
15
The EFSA 2005 conclusions covered the general provisions regarding welfare in all systems. The Panel agrees with these in the light of new evidence reviewed, except for the following (the chapter number from EFSA, 2005 is also indicated):
EFSA (2005, chapter 3.5.1.3) highlighted hygienic hazards linked to the use of enrichment material. However, it is now concluded that, while certain types of enrichment give the possibility of hygienic hazards, other types of enrichment, such as gnawing sticks, have not given reported problems. However, there is insufficient knowledge on this.
EFSA, 2005, chapter 3.5.1.4. For reproducing animals, beyond the use of floor mats (floor rests), plastic slatted floors have now also been confirmed to reduce pododermatitis. These can also guarantee hygiene, comparable to wire mesh, provided that their design ensures passage of faeces.
EFSA, 2005, chapter 3.7.4. Dam‐litter separation as a bio‐stimulation method for oestrus synchronisation is no longer recommended due to adverse welfare consequence for the kits.
EFSA, 2005, chapter 3.5.2.1. In agreement with EFSA 2005, continuous group housing systems for reproducing does cannot be recommended because of the decreased welfare associated to aggression and lesions among does and to kits. Subsequent studies have shown that part‐time group housing systems are also associated with high aggression among animals and high injury rates. Nevertheless, these systems may have potential, but existing knowledge is not sufficiently developed to recommend them for implementation on farms.
EFSA, 2005, chapter 3.9. While commercial genetic selection still incorporates litter size, growth rate and feed efficiency, there is now increasing emphasis on other traits including disease resistance. Genetics of body composition and stress resistance are also the subject of research.
-
16
Many production factors other than housing influence welfare consequences, including genetics, nutrition, and aspects of management such as biosecurity, reproduction and training of farm staff.
-
17
In general, there is a lack of information on many of the behavioural needs of rabbits.
The present size of conventional cages, enriched cages, elevated pens and organic systems (the latter only in case no access to outdoor area is provided) restricts movement according to EKE experts. However, knowledge on the space requirement which is necessary to acceptably meet the behavioural and physiological needs for all rabbit categories is still lacking. Therefore, it is not possible to recommend a minimum space requirement which gives acceptable welfare.
There is a lack of evidence on which gnawing materials best satisfy the rabbit's gnawing motivation.
The motivation for social contact in adult rabbits at each of the reproductive phases is insufficiently understood.
5. Recommendations
General recommendations
-
1
A systematic and large‐scale data collection exercise should be carried out to provide objective information on rabbit welfare in different housing and management systems in the EU.
-
2
To facilitate objective comparisons of rabbit welfare, adoption of a validated welfare assessment protocol suitable for on‐farm use should be standardised across the EU.
-
3
Because of the diversity of rabbit farming systems, defining general resource‐based standards is difficult. In the future, these should be complemented by use of ABMs.
-
4
Basic research should be carried out to better understand the behavioural needs of rabbits, and the provisions for these, which are necessary in farm conditions to ensure good welfare.
Recommendations regarding different housing systems
The following recommendations for specific housing systems are based on the welfare consequences highlighted as most important in the expert survey.
-
5
For conventional cages:
The main welfare consequences in conventional cages are directly related to the size of the cage (restriction of movement, resting and social behaviour). As such, it can be recommended to increase the size of these cages or to add structures that allow more efficient use of the cage (platforms). Effectively, this means a shift from conventional cages to enriched ones. Problems for growing rabbits can also be addressed by reducing stocking density.
In case of wire mesh flooring, plastic foot mats should be used. Cage floors (especially any plastic mats) should be cleaned regularly to avoid soiling and faeces accumulation.
Gnawing materials suitable for all production categories (does, growing rabbits, kits) should be supplied. Wooden sticks are one solution to achieve this, especially as conventional cages may lack the space necessary to add hay/straw racks.
Thermal stress, although not directly caused by the cage itself, should be minimised by appropriate building and ventilation design.
Transient hunger (< 6 h/day) may occur in fatteners submitted to a necessary feed restriction programme, and could be addressed by giving more frequent smaller meals. In kits, hunger may be addressed by avoiding factors that disrupt nursing behaviour. The correct functioning of drinkers and automatic feeders should be checked daily.
-
6
For enriched cages:
Restriction of movement for does in enriched cages cannot be solved without significant changes in the system. Restriction of movement for growing rabbits can be addressed by reducing stocking density.
Risk of skin disorders can be reduced by good management of biosecurity.
Gnawing materials suitable for all production categories (does, growing rabbits, kits) should be supplied.
The risk of resting problems should be decreased with adequate cage size and floor material. In case of wire mesh flooring, plastic foot mats should be used. Cage floors (especially plastic floors) should be cleaned regularly to avoid soiling and faeces accumulation.
Thermal stress, although not directly caused by the cage itself, should be minimised by appropriate building and ventilation design
Gastrointestinal disorders can be minimised by a balanced diet and appropriate weaning age.
-
7
For elevated pens:
Restriction of movement of does can be ameliorated by group housing but this can come with increased aggressive interactions, inadequate nesting behaviour and poor maternal care. Pens should be equipped with platforms to allow for vertical movements.
Resting problems for growing rabbits can be addressed by reducing stocking density. In addition, resting comfort can be improved by use of appropriate flooring material, such as plastic, and by good hygiene ensured by correct slat design.
Skin disorders in growing rabbits can be reduced by avoiding introduction and transmission of pathogens through good biosecurity procedures, climate control as well as positioning of the drinkers so that wetting of the fur is prevented.
Gnawing materials suitable for all production categories (does, growing rabbits, kits) should be supplied.
Gastrointestinal disorders in kits and growing rabbits can be minimised by a balanced diet and appropriate weaning age.
Fearfulness can be reduced by avoiding rough handling and situations contributing to aggression between rabbit does.
-
8
For floor pens:
Gastrointestinal disorders, skin disorders, reproductive disorders, neonatal disorders and resting problems can all be ameliorated by maintenance of hygiene through provision of an adequate quantity of suitable bedding and frequent removal of soiled bedding.
Prolonged hunger and thirst can be avoided if feeding and drinking facilities are designed to remain free of soiled bedding and regularly cleaned. For kits, the occurrence of prolonged hunger should be reduced by, firstly, a correct health status and feeding of the doe, and, secondly, by a correct design of the nestbox to only allow kits access to the main cage when sufficiently mature.
Skin lesions and resting problems can be reduced by reducing stocking density for growing rabbits, but may be an unavoidable consequence of group housing of does.
Thermal stress can be avoided by controlled ventilation systems, to minimise ambient temperature extremes. In hot weather, provision of an unbedded area of floor might be beneficial if well drained.
-
9
For outdoor systems:
Heat or cold stress for any rabbit category could be reduced by insulating the shelter or by adding shade in the outdoor area. Where possible, supplementary heaters, humidifiers or fans can be employed. For kits, correct management of the nest is important to reduce thermal stress.
Gastrointestinal disorders in growing rabbits could be reduced by using a strict management of housing hygiene combined with a good feeding strategy and a daily checking of the animals looking at their health.
Prolonged Hunger for kits and growing rabbits can be avoided by correct design, location and maintenance of feeding and drinking facilities, and regular checking of availability. For kits, the occurrence of prolonged hunger should be reduced by, firstly, a correct health status and feeding of the doe, and, secondly, by a correct design of the nestbox to only allow kits access to the main cage when sufficiently mature.
Resting problems for does and growing rabbits can be reduced by using items to provide shade in summer and by improving the insulation and hygiene of shelter.
-
10
For organic systems:
Restriction of movement may be reduced by enlarging the sheltered part of the housing during any period when outdoor access is difficult.
Heat or cold stress for any rabbit category could be reduced by insulating the shelter or by adding shade in the outdoor area.
Resting problems can be reduced by using items to provide shade in summer and by improving the insulation and hygiene of shelter.
Reproductive disorders in does, neonatal disorders in kits and gastrointestinal disorders in growing rabbits could be reduced by using a strict management of housing hygiene combined with a good feeding strategy and a daily checking of the animals looking at their health.
Fear in growing rabbits can be minimised by protections against potential predators (dogs, foxes, birds of prey, etc.), such as a robust electrified fence, a net top protection against birds of prey, and setting up hiding places in paddocks. Familiarity with people by regular visits from the farmer to the animals should also be beneficial.
For kits, the occurrence of prolonged hunger should be reduced by, firstly, a correct health status and feeding of the doe, and, secondly, by a correct design of the nestbox to only allow kits access to the main cage when sufficiently mature.
Abbreviations
- ABM
animal‐based measure
- AHAW
EFSA Panel on Animal Health and Welfare
- AI
artificial insemination
- EKE
expert knowledge elicitation
- ERE
epizootic rabbit enteropathy
- GnRH
Gonadotropin releasing hormone
- IRTA
Institut of Agrifood Reasearch and Technology
- KI
Kit Index
- KR
kindling rate
- LS
litter size
- LW
live weight
- MMR
monthly mortality risk
- MS
Member State
- THI
Temperature‐humidity index
- ToR
Terms of Reference
- UPV
Universidad Politécnica de Valencia
- WG
Working Group
Appendix A – Literature search
1.
As described in Section 2.1.3, a literature search was carried out to identify peer‐reviewed scientific evidence on the description of the welfare consequences for farmed rabbits. The results were successively screened and refined as described below.
Sources of information included in the search: Bibliographic database ‘Web of Science’.
Search string used in the bibliographic database:
The search string was designed to retrieve relevant documents to ‘animal welfare’ for ‘farming or rearing or production or breeding’ of ‘rabbits’. Restrictions were applied to the date of publication (considering only those records published after EFSA, 2005) and English language.
Date of the search: 19 December 2018
| Web of science search string | |
|---|---|
| Years 2004–2019 | |
| Category | |
| Search terms | Field searched |
| Rabbit* OR TS=lepor* OR TS=oryctolagus OR TS=“oryctolagus cuniculus” | Topic |
| AND | |
| Farm* OR rear* OR production OR wean* OR breed* | Topic |
| AND | |
| Welf* | Topic |
| Results: 235 | |
| Results after screening: 83 | |
The search yielded a total of 235 records that were exported to an EndNote library together with the relevant metadata (e.g. title, authors, abstract). Titles and abstracts were first screened to remove irrelevant publications (e.g. related to species, productive systems, and research purposes that were out of the scope of this opinion) and duplicates, and successively to identify their relevance to the topic. Full‐text publications were screened if title and abstract did not allow assessing the relevance of a paper. The screening was performed by one reviewer, with support by a second reviewer in cases of doubt; publications that were not considered relevant nor providing any additional value to address the question were also removed. Most papers related to research conducted on rabbits kept for experimental purposes and were therefore excluded. The screening led to 75 relevant records which are reported in Table A.1.
Table A.1.
List of relevant publications
| ID | Reference |
|---|---|
| 1 | Alfonso‐Carrillo et al. (2014a) |
| 2 | Alfonso‐Carrillo et al. (2014b) |
| 3 | EFSA (2005) |
| 4 | EFSA (2006) |
| 5 | Andrist et al. (2013) |
| 6 | Baumann et al. (2005) |
| 7 | Bovera et al. (2013) |
| 8 | Bozicovich et al. (2016) |
| 9 | Buijs et al. (2011) |
| 10 | Buijs et al. (2011) |
| 11 | Buijs et al. (2012) |
| 12 | Buijs et al. (2014) |
| 13 | Buijs et al. (2015) |
| 14 | Buijs et al. (2016) |
| 15 | Calvet et al. (2011) |
| 16 | Castellini et al. (2010) |
| 17 | Chu et al. (2004) |
| 18 | Combes et al. (2009) |
| 19 | Csatadi et al. (2005) |
| 20 | Cullere and dalle Zotte (2018) |
| 21 | D'Agata et al. (2009) |
| 22 | Dalmau et al. (2014) |
| 23 | Dalmau et al. (2015) |
| 24 | de la Fuente and Rosell (2012) |
| 25 | Dixon et al. (2010) |
| 26 | El‐Tarabany et al. (2019) |
| 27 | Garreau et al. (2008a,b) |
| 28 | Gidenne et al. (2012a) |
| 29 | Gidenne et al. (2012b) |
| 30 | Graf et al. (2011) |
| 31 | Gunia et al. (2018) |
| 32 | Hesham and Nasr (2017) |
| 33 | Hoy (2009) |
| 34 | Jekkel et al. (2010) |
| 35 | Jordan et al. (2008) |
| 36 | Katsarou et al. (2011) |
| 37 | Marai and Rashwan (2004) |
| 38 | Marin et al. (2018) |
| 39 | Martinez‐Paredes et al. (2015) |
| 40 | Masthoff and Hoy (2019) |
| 41 | Matics et al. (2018) |
| 42 | Miko et al. (2014) |
| 43 | Mugnai et al. (2009) |
| 44 | Mugnai et al. (2014) |
| 45 | Pascual et al. (2013) |
| 46 | Postollec et al. (2006) |
| 47 | Princz et al. (2008a) |
| 48 | Princz et al. (2008b) |
| 49 | Princz et al. (2009) |
| 50 | Rommers et al. (2006) |
| 51 | Rommers and de Jong (2011) |
| 52 | Rommers et al. (2012) |
| 53 | Rommers et al. (2014a,b) |
| 54 | Rommers and de Greef (2018) |
| 55 | Rosell et al. (2019) |
| 56 | Rosell and de la Fuente (2009a) |
| 57 | Rosell and de la Fuente (2009) |
| 58 | Rosell and de la Fuente (2016a,b) |
| 59 | Rosell and de la Fuente (2018) |
| 60 | Ruchti et al. (2018) |
| 61 | Ruchti et al. (2019) |
| 62 | Schlolaut et al. (2013) |
| 63 | Stewart and Suckow (2016) |
| 64 | Szendro and Dalle Zotte (2011) |
| 65 | Szendro and McNitt (2012) |
| 66 | Szendro et al. (2013) |
| 67 | Szendro et al. (2015) |
| 68 | Szendro et al. (2019) |
| 69 | Tillmann et al. (2019) |
| 70 | Trocino and Xiccato (2006) |
| 71 | Trocino et al. (2013) |
| 72 | Trocino et al. (2014) |
| 73 | Trocino et al. (2015) |
| 74 | Trocino et al. (2018) |
| 75 | Tuyttens et al. (2005) |
| 76 | Verga et al. (2007) |
| 77 | Vervaecke et al. (2010) |
| 78 | Windschnurer et al. (2019) |
| 79 | Xiccato et al. (2013a) |
| 80 | Xiccato et al. (2013b) |
| 81 | Zomeno et al. (2017) |
| 82 | Zomeno et al. (2018) |
| 83 | Zucca et al. (2012) |
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Saxmose Nielsen S, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Michel V, Miranda Chueca MÁ, Roberts HC, Sihvonen LH, Spoolder H, Stahl K, Velarde Calvo A, Viltrop A, Buijs S, Edwards S, Candiani D, Mosbach‐Schulz O, Van der Stede Y and Winckler C, 2020. Scientific Opinion on the health and welfare of rabbits farmed in different production systems. EFSA Journal 2020;18(1):5944, 96 pp. 10.2903/j.efsa.2020.5944
Requestor: European Parliament
Question number: EFSA‐Q‐2019‐00593
Panel members: Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Klaus Depner, Julian Ashley Drewe, Bruno Garin‐Bastuji, Jose Luis Gonzales Rojas, Christian Gortázar Schmidt, Miguel Ángel Miranda Chueca, Virginie Michel, Søren Saxmose Nielsen, Helen Clare Roberts, Liisa Helena Sihvonen, Hans Spoolder, Karl Stahl, Antonio Velarde Calvo, Arvo Viltrop and Christoph Winckler.
Acknowledgements: The AHAW Panel wishes to thank the following for the support provided to this scientific output: the hearing experts Thierry Gidenne, Zsolt Matics, Joan Rosell, Angela Trocino for their support to the development of the opinion; the trainee Marie Louise Schneider for her full support to the opinion, the ad‐interim staff Cristina Rapagná for her work on the citations (AHAW team, ALPHA unit, EFSA); all external respondents to the survey and the experts Tams Atkári, Lotti Bigler, Ricard Garriga Baraut, Antonio Lavazza, Laurence Nouvel, Zsolt Szendrö, Alessandro Ravagnani and Laura Warin for their participation in the technical workshop on severity for welfare consequences for rabbits.
Adopted: 21 November 2019
Notes
References
- Agnoletti F, 2012. Update on rabbit enteric diseases: despite improved diagnostic capacity, where does disease control and prevention stand? 10th World Rabbit Congress Sharm. El‐ Sheikh, –Egypt, 1113–1127.
- Alfonso‐Carrillo C, Garcia‐Rebollar P, De Bias C, Ibanez MA and Garcia‐Ruiz AI, 2014a. Effect of late weaning and use of alternative cages on performance of does, Suckling and Fattening Rabbits under Extensive reproductive management. Livestock Science, 167, 425–434. 10.1016/j.livsci.2014.05.018 [DOI] [Google Scholar]
- Alfonso‐Carrillo C, Martin E, De Blas C, Ibanez MA, Garcia‐Rebollar P and Garcia‐Ruiz AI, 2014b. Effect of cage type on the behaviour pattern of rabbit does at different physiological stages. World Rabbit Science, 22, 59–69. 10.4995/wrs.2014.1396 [DOI] [Google Scholar]
- Andrist CA, Bigler LM, Würbel H and Roth BA, 2012. Effects of group stability on aggression, stress and injuries in breeding rabbits. Applied Animal Behaviour Science, 142, 182–188. [Google Scholar]
- Andrist CA, van den Borne BHP, Bigler LM, Buchwalder T and Roth BA, 2013. Epidemiologic survey in Swiss group‐housed Breeding Rabbits: extent of lesions and potential risk factors. Preventive Veterinary Medicine, 108, 218–224. 10.1016/j.prevetmed.2012.07.015 [DOI] [PubMed] [Google Scholar]
- Andrist C, Bigler L, Würbel H and Roth B, 2014. Masking odour when regrouping rabbit does: effect on aggression, stress and lesions. Livestock Science, 170, 150–157. [Google Scholar]
- Argente M, Santacreu M, Climent A and Blasco A, 1999. Phenotypic and genetic parameters of birth weight and weaning weight of rabbits born from unilaterally ovariectomized and intact does. Livestock Production Science, 57, 159–167. [Google Scholar]
- Arrazuria R, Pérez V, Molina E, Juste RA and Khafipour E, 2018. Diet induced changes in the microbiota and cell composition of rabbit gut associated lymphoid tissue (GALT). Scientific Report, 8, 14103 10.1038/s41598-018-32484-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arveux P, 1994. L'allaitement controlé. Cuniculture, 21, 240–241. [Google Scholar]
- Baumann P, Oester H and Stauffacher M, 2005. Effects of temporary nest box removal on maternal behaviour and pup survival in caged rabbits (Oryctolagus cuniculus). Applied Animal Behaviour Science, 91, 167–178. [Google Scholar]
- Baumans V, 2005. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. ILAR Journal, 46, 162–170. [DOI] [PubMed] [Google Scholar]
- Belgium , 2014. Arrêté Royal relatif au bien‐être des lapins dans les élevages. Moniteur Belge [C − 2014/24303], 60861–60864.
- Bertagnoli S and Marchandeau S, 2015. La myxomatose. Revue Scientifique et Technique International Office of Epizootics, 34, 539–547. [PubMed] [Google Scholar]
- Bignon L, Bouchier M, Coutelet G, Galliot P, Souchet C and Fortun‐Lamothe L, 2012. Individual housing of young does in different sized cages: impact on welfare, economic costs and productive data. Proceedings 10th World Rabbit Congress, Sharm El‐ Sheikh, Egypt, 1045–1049.
- Bignon L, Mika A, Mindus C, Litt J, Souchet C, Bonnaud V, Picchiottino C, Warin L, Dennery G, Brame C, Guesdon V and Bouvarel I, 2017. Une méthode pratique et partagée d’évaluation du bien‐être en filières avicole et cunicole: EBENE. 12e Journées de la Recherche Avicole et Palmipèdes à Foie Gras, 5 & 6 avril 2017, Tours, France, 1015–1019.
- de Blas C and Mateos GG, 2010. Feed formulation. Nutrition of the rabbit J. (Eds). CAB International, 2.
- Blokhuis HJ, Veissier I, Miele M and Jones RB, 2010. The Welfare Quality® project and beyond: safeguarding animal well‐being. Acta Agricultura Scandinavia Section A, Animal Science, 60, 129–140. [Google Scholar]
- Blumetto O, Olivas I, Torres AG and Villagrá A, 2010. Use of straw and wood shavings as nest material in primiparous does. World Rabbit Science, 18, 237–242. [Google Scholar]
- Boisot P, Duperray J, Dugenetais X and Guyonvarch A, 2004. Interest of hydric restriction times of 2 and 3 hours per day to induce feed restriction in growing rabbits. Proceedings of the Proceedings of the 8th World Rabbit Congress, Puebla. Colegio de Postgraduados, Montecillo, Spain, 759–764.
- Boiti C, 2004. Underlying physiological mechanisms controlling the reproductive axis of rabbit does. Proceedings of the Proc. 8th World Rabbit Congress, September, 7–10.
- Bolet G, Devinoy E, Viràg G, Harsányi I and Bösze Z, 2007. Association between litter size and the K‐casein genotype in the INRA rabbit lines. World Rabbit Science, 15, 147–150. [Google Scholar]
- da Borso F, Chiumenti A, Mezzadri M and Teri F, 2016. Noxious gases in rabbit housing systems: effects of cross and longitudinal ventilation. Journal of Agricultural Engineering, 47, 222–229. [Google Scholar]
- Boucher S and Leplat A, 2005. De quoi meurent nos lapins. In Table Ronde: Effets des conduits post‐sevrage sur les performances et la santé des laperaux. 11e Journées de la Recherche Cunicole, 29‐30 November.
- Boucher S, Gracia E, Villa A, Fernández A, Nouaille L, Briffaud MA, Albizu I and Baselga R, 2001. Pathogens in the reproductive tract of farm rabbits. Veterinary Record, 149, 677–678. [DOI] [PubMed] [Google Scholar]
- Bovera F, Lestingi A, Piccolo G, Iannaccone F, Attia YA and Tateo A, 2013. Effects of water restriction on growth performance, feed nutrient digestibility, carcass and meat traits of rabbits. Animal, 7, 1600–1606. [DOI] [PubMed] [Google Scholar]
- Bozicovich TFM, Moura A, Fernandes S, Oliveira AA and Siqueira ERS, 2016. Effect of environmental enrichment and composition of the social group on the behavior, welfare, and relative brain weight of growing rabbits. Applied Animal Behaviour Science, 182, 72–79. [Google Scholar]
- Broom DM and Fraser AF, 2015. Domestic Animal Behaviour and Welfare, 5th Edition University of Cambridge Veterinary School, UK, 472 pp. [Google Scholar]
- Brownlow ML, Jung SH, Moore RJ, Bechmann N and Jankord R, 2017. Nutritional ketosis affects metabolism and behavior in Sprague‐Dawley rats in both control and chronic stress environments. Front Mol Neurosci, 10, 129 10.3389/fnmol.2017.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs S and Tuyttens FAM, 2015. Evaluating the effect of semi‐group housing of rabbit does on their offspring's fearfulness: can we use the open‐field test? Applied Animal Behaviour Science, 162, 58–66. [Google Scholar]
- Buijs S, Keeling LJ and Tuyttens FAM, 2011. Behaviour and use of space in fattening rabbits as influenced by cage size and enrichment. Applied Animal Behaviour Science, 134, 229–238. [Google Scholar]
- Buijs S, Van Poucke E, Van Dongen S, Lens L and Tuyttens FAM, 2012. Cage size and enrichment effects on the bone quality and fluctuating asymmetry of fattening rabbits. Journal of Animal Science, 90, 3568–3573. [DOI] [PubMed] [Google Scholar]
- Buijs S, Hermans K, Maertens L, Van Caelenberg A and Tuyttens FAM, 2014. Effects of semi‐group housing and floor type on pododermatitis, spinal deformation and bone quality in rabbit does. Animal, 8, 1728–1734. 10.1017/s1751731114001669 [DOI] [PubMed] [Google Scholar]
- Buijs S, Maertens L, Hermans K, Vangeyte J and Tuyttens FAM, 2015. Behaviour, wounds, weight loss and adrenal weight of rabbit does as affected by semi‐group housing. Applied Animal Behaviour Science, 172, 44–51. [Google Scholar]
- Buijs S, Vangeyte J and Tuyttens FA, 2016. Effects of communal rearing and group size on breeding rabbits’ post‐grouping behaviour and its relation to ano‐genital distance. Applied Animal Behaviour Science, 182, 53–60. [Google Scholar]
- Cafarchia C, Camarda A, Coccioli C, Figueredo LA, Circella E, Danesi P, Capelli G and Otranto D, 2010. Epidemiology and risk factors for dermatophytoses in rabbit farms. Medical Mycology, 48, 975–980. 10.3109/13693781003652620 [DOI] [PubMed] [Google Scholar]
- Calvet SM, Cambra‐Lopez F, Estelles and Torres AG, 2011. Characterisation of the indoor environment and gas emissions in rabbit farms. World Rabbit Science, 19, 49–61. 10.4995/wrs.2011.802 [DOI] [Google Scholar]
- Cardasis CA and Sinclair JC, 1972. The effects of ambient temperature on the fasted newborn rabbit. 1. Survival times, weight loss, body temperature and oxygen consumption. Biological of Neonates, 21, 330–346. [DOI] [PubMed] [Google Scholar]
- Castellini C, Dal Bosco A and Mugnai C, 2003. Comparison of different reproduction protocols for rabbit does: effect of litter size and mating interval. Livestock Production Science, 83, 131–139. [Google Scholar]
- Castellini C, Dal Bosco A, Arias‐Alvarez M, Lorenzo PL, Cardinali R and Rebollar PG, 2010. The main factors affecting the reproductive performance of rabbit does: a review. Animal Reproduction Sciences, 122, 174–182. [DOI] [PubMed] [Google Scholar]
- Castellini C, Dal Bosco A and Mattioli S, 2017. Nuove conoscenze e applicazioni nella riproduzione del coniglio. Il coniglio: storia ed evoluzione dell'allevamento in Italia e in Europa, 51–65. [Google Scholar]
- Cerioli M, Brivio R, Tittarelli C, Grilli G and Lavazza A, 2011. Identification of health and welfare parameters for rabbit production and definition of an evaluation score. Journal of Agriculture, Science and Technology, 1, 500–507. [Google Scholar]
- Cerolini S, Marzoni Fecia di Cossato M, Romboli I, S A and L Z, 2008. Avicoltura e Coniglicoltura. Point Veterinaire, Milan, Italy. [Google Scholar]
- Chu L, Garner JP and Mench JA, 2004. A behavioural comparison of New Zealand White rabbits (Oryctolagus cuniculus) housed individually or in pairs in conventional laboratory cages. Applied Animal Behaviour Science, 85, 121–139. [Google Scholar]
- Combes S, Postollec G, Cauquil L and Gidenne T, 2009. Influence of cage or pen housing on carcass traits and meat quality of rabbit. Animal, 4, 295–302. 10.1017/S1751731109991030 [DOI] [PubMed] [Google Scholar]
- Combes S, Fortune‐Lamothe L, Cauiquil L and Gidenne T, 2013. Engineering the rabbit digestive ecosystem to improve digestive health and efficacy. Animal, 7, 1–11. 10.1017/S1751731113001079 [DOI] [PubMed] [Google Scholar]
- Combes S, Ikken S, Gidenne T, Balmisse E, Aymard P and Travel A, 2017. Effect of solid intake stimulation of suckling rabbit in the nest on survival and growth performance. Conference paper. 68th EAAP annual meeting. Strasbourg, 15 June 2006 Cons 123.
- Corpa J, Hermans K and Haesebrouck F, 2009. Main pathologies associated with Staphylococcus aureus infections in rabbits: a review. World Rabbit Science, 17, 115–125. [Google Scholar]
- Coudert P, Rideaud P, Virag G and Cerrone A, 2006. Pasteurellosis in rabbits. Recent Advances in Rabbit Sciences, 147–162. [Google Scholar]
- Council of European Union , 2006. European convention for the protection of vertebrate animals used for experimental and other scientific purposes (ets no. 123) guidelines for accommodation and care of animals (article 5 of the convention).
- Council of European Union , 1998. Council Directive 98/58/EC of 20 July 1998 concerning the protection of animals kept for farming purposes (OJ L 221, 8.8.1998, p. 23). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:01998L0058-20030605
- Coureaud G, Schaal B, Orgeur P and Coudert P, 1998. Le contrôle de l'accès au nid chez la lapine: conséquences sur la mortalité des lapereaux. Proc. 7émes Journ. Rech. Cunicole, 245–249. [Google Scholar]
- Coureaud G, Fortun‐Lamothe L, Langlois D and Schaal B, 2007. The reactivity of neonatal rabbits to the mammary pheromone as a probe for viability. Animal, 1, 1026–1032. [DOI] [PubMed] [Google Scholar]
- Crowell‐Davis SL, 2007. Behavior problems in pet rabbits. Journal of Exotic Pet Medicine, 16, 38–44. 10.1053/j.jepm.2006.11.022 [DOI] [Google Scholar]
- Csatadi K, Kustos K, Eiben C, Bilko A and Altbacker V, 2005. Even minimal human contact linked to nursing reduces fear responses toward humans in rabbits. Applied Animal Behaviour Science, 95, 123–128. 10.1016/j.applanim.2005.05.002 [DOI] [Google Scholar]
- Cullere M and dalle Zotte A, 2018. Rabbit meat production and consumption: state of knowledge and future perspectives. Meat Science, 143, 137–146. [DOI] [PubMed] [Google Scholar]
- D'Agata M, Preziuso G, Russo C, Zotte AD, Mourvaki E and Paci G, 2009. Effect of an outdoor rearing system on the welfare, growth performance, carcass and meat quality of a slow‐growing rabbit population. Meat Science, 83, 691–696. 10.1016/j.meatsci.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Dal Bosco A, Castellini C and Mugnai C, 2002. Rearing rabbits on a wire net floor or straw litter: behaviour, growth and meat qualitative traits. Livestock Production Science, 75, 149–156. [Google Scholar]
- Dalmau A, Catanese B, Rafel O, Rodriguez P, Fuentes C, Llonch P, Mainau E, Velarde A, Ramón J, Taberner E, López‐Béjar M and Piles M, 2014. Effect of high temperatures on breeding rabbit behaviour. Animal Production Science, 55, 1207–1214. [Google Scholar]
- Dalmau A, Abdel‐Khalek AM, Ramon J, Piles M, Sanchez JP, Velarde A and Rafel O, 2015. Comparison of behaviour, performance and mortality in restricted and ad libitum‐fed growing rabbits. Animal, 9, 1172–1180. [DOI] [PubMed] [Google Scholar]
- Dalmau A, Pallisera J, Moles X, Xercavins A and Velarde A, undated a. Protocol for does, bucks and kid rabbits. IRTA.
- Dalmau A, Pallisera J, Moles X, Xercavins A and Velarde A, undated b. Protocol for growing rabbits. IRTA.
- De Blas C and Wiseman J, 2010. Nutrition of the rabbit. CABI.
- Deeb B and DiGiacomo RF, 2000. Respiratory diseases of rabbits. Veterinary Clinics of North America: Exotic Animal Practice, 3, 465–479. [DOI] [PubMed] [Google Scholar]
- DiVincenti L and Jr Rehrig, 2016. The Social Nature of European Rabbits (Oryctolagus cuniculus). Journal of the American Association for Laboratory Animal Science, 55, 729–736. [PMC free article] [PubMed] [Google Scholar]
- Dixon LM, Hardiman JR and Cooper JJ, 2010. The effects of spatial restriction on the behavior of rabbits (Oryctolagus cuniculus). Journal of Veterinary Behavior‐Clinical Applications and Research, 5, 302–308. [Google Scholar]
- Dorning J and Harris S, 2017. Farmed rabbits in commercial production systems. Available online: https://www.ciwf.org.uk/media/7430014/the-welfare-of-farmed-rabbits-in-commercial-production-systems-a-scientific-review-february-2017.pdf
- Dreschen B and Schlenden‐Böbbis I, 1996. Pathologic study of pododermatitis among heavy breeders on wire floors. Etude pathologique de la pododermatites chez les lapins reproducteurs de souche loured sur grillage. World Rabbit Science, 4, 143–148. [Google Scholar]
- Drescher B and Loeffler K, 1996. Skoliosen, Lordosen und Kyphosen bei Zuchtkaninchen. Tieraerztliche Praxis, 24, 292–300. [PubMed] [Google Scholar]
- Drummond H, Vázquez E, Sánchez‐Colón S, Martinez‐Gómez M and Hudson R, 2000. Competition for milk in the domestic rabbit: survivors benefit from littermate deaths. Ethology, 106, 511–526. [Google Scholar]
- Dúcs A, Bilkó A and Altbäcker V, 2009. Physical contact while handling is not necessary to reduce fearfulness in the rabbit. Applied Animal Behaviour Science, 121, 51–54. 10.1016/j.applanim.2009.07.005 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. The Impact of the current housing and husbandry systems on the health and welfare of farmed domestic rabbits. EFSA Journal 2005;267:1–31. 10.2903/j.efsa.2005.267 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Guidance on Expert Knowledge Elicitation in Food and Feed Safety Risk Assessment. EFSA Journal 2014;12(6): 278. 10.2903/j.efsa.2014.3734 https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Hart A, Maxim L, Siegrist M, Von Goetz N, da Cruz C, Merten C, Mosbach‐Schulz O, Lahaniatis M, Smith A and Hardy A, 2019. Guidance on Communication of Uncertainty in Scientific Assessments. EFSA Journal 2019;17(1):5520, 73 pp. 10.2903/j.efsa.2019.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2012. Guidance on Risk Assessment for Animal Welfare. EFSA Journal 2012;10(1):2513, https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2012.2513 [Google Scholar]
- Egea MD, Rosell JM and De la Fuente LF, 2000. Enfermedades de la reproducción: Machos e inseminación. Enfermedades del conejo., 2, 70. [Google Scholar]
- Eiben C, Tóbiás G, Kustos K, Gódor‐Surmann K, Sz Kotány, Gulyás B and Szira G, 2007. The change of nursing for oestrus induction (biostimulation): effect of contact between rabbit doe and its young. Livestock Science, 111, 193–203. 10.1016/j.livsci.2007.01.146 [DOI] [Google Scholar]
- El Maghraby M, 2011. Effect of restricted access to drinking water on growth, feed efficiency and carcass characteristics of fattening rabbits. Asian Journal of Animal Sciences, 5, 136–144. [Google Scholar]
- El‐Tarabany MS, Ahmed‐Farid OA and El‐Tarabany AA, 2019. Impact of space allowance on performance traits, brain neurotransmitters and blood antioxidant activity of New Zealand White rabbits. Preventive Veterinary Medicine, 163, 44–50. [DOI] [PubMed] [Google Scholar]
- European Commission , 2017. Overview report: commercial rabbit farming in the European Union.
- Farkas T, Szendrő Z, Matics Z, Radnai I, Nagy I and Gerencsér Z, 2018. Preference of rabbit does among different nest materials. World Rabbit Sciences, 26(1), 81–90. 10.4995/wrs.2018.7373 [DOI] [Google Scholar]
- Farnworth MJ, Walker JK, Schweizer KA, Chuang CL, Guild SJ, Barrett CJ, Leach MC and Waran NK, 2011. Potential behavioural indicators of post‐operative pain in male laboratory rabbits following abdominal surgery. Animal Welfare, 20, 225–237. [Google Scholar]
- Finzi A and Mariani G, 2011. L'allevamento ecologico del coniglio (Organic rabbit farming). Edagricole, Milano, Italy. 130 pp. ISBN 978‐88‐506‐5367‐6. [Google Scholar]
- Forkman B, Boissy A, Meunier‐Salaün MC, Canali E and Jones RB, 2007. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiology & Behavior, 92, 340–374. [DOI] [PubMed] [Google Scholar]
- Foubert C, Duperray J, Boisot P and Guyonvarch A, 2008. Effect of feed restriction with or without free access to drinking water on performance of growing rabbits in healthy or epizootic rabbit enteropathy conditions. 9th World Rabbit Congress, Verona, Italy, 667–672.
- de la Fuente LF and Rosell JM, 2012. Body weight and body condition of breeding rabbits in commercial units. Journal of Animal Science, 90, 3252–3258. 10.2527/jas.2011-4764 [DOI] [PubMed] [Google Scholar]
- García‐Alvarez AA, Chaves FB, Fernández AC, Sanz CC, Borobia MM and Cid D, 2015. An ST11 clone of Pasteurella multocida, widely spread among farmed rabbits in the Iberian Peninsula, demonstrates respiratory niche association. Infection, Genetics and Evolution, 34, 81–87. [DOI] [PubMed] [Google Scholar]
- Garreau H, Eady S, Hurtaud J and Legarra A, 2008a. Genetic parameters of production traits and resistance to digestive disorders in a commercial rabbit population, 9th Edition World Rabbit Congress. [Google Scholar]
- Garreau H, Brun JM, Theau‐Clement M and Bolet G, 2008b. Evolution of research axes at Inra for the genetic improvement of meat rabbits. Productions Animales, 21, 269–275. [Google Scholar]
- Gerencsér Z, Odermatt M, Radnai I, Mikó A, Zs M, Nagy I and Zs S, 2012. Examination of free choice of growing rabbits among different floor‐types.
- Gerencsér Z, Szendro K, Odermatt M, Radnai I and Nagy I, 2014. Effect of floor type on behavior and productive performance of growing rabbits. Livestock Science, 165, 114–119. [Google Scholar]
- Gerencsér ZS, Farkas TP, Szendrő Zs, Nagy I, Odermatt M, Radnai I, Kacsala L, Kasza R, Savanyó Zs and Matics Zs, 2018. Location preference, behaviour and performance of rabbit does in a pen system of combination of group and individual housing. 30th Hung. Rabbit Prod. Conf., Kaposvár, Hungary. Regulation on the protection of farm animals. Available online: http://www.gesetze-im-internet.de/tierschnutztv/
- Germany, 2014. Animal welfare act. http://www.gesetze-im-internet.de/tierschg/index.html
- Germany, 2017. Animal welfare act. http://www.gesetze-im-internet.de/tierschg/index.html
- Giannico AT, Lima L, Lange RR, Froes TR and Montiani‐Ferreira F, 2014. Proven cardiac changes during death‐feigning (tonic immobility) in rabbits (Oryctolagus cuniculus). Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, Behavioral Physiology, 200, 305–310. 10.1007/s00359-014-0884-4 [DOI] [PubMed] [Google Scholar]
- Gidenne T, García J, Lebas F and Licois D, 2010. 10 Nutrition and feeding strategy: interactions with pathology. CABI, 233–252. [Google Scholar]
- Gidenne T, Combes S and Fortun‐Lamothe L, 2012a. Feed intake limitation strategies for the growing rabbit: effect on feeding behaviour, welfare, performance, digestive physiology and health: a review. Animal, 6, 1407–1419. [DOI] [PubMed] [Google Scholar]
- Gidenne T, Fortun‐Lamothe L and Combes S, 2012b. Intake restriction for the young rabbit: new strategies to enhance its digestive health and feed efficiency. Inra Productions Animales, 25, 323–336. [Google Scholar]
- Gidenne T, Lebas F, Savietto D, Dorchies P, Duperray J, Davoust C and Lamothe L, 2015. Nutrition et alimentation. Le lapin. De la biologie à l’élevage, 152–196.
- Gidenne T, Fortun‐Lamothe L, Bannelier C, Molette C, Gilbert H, Chemit ML, Segura M, Benitez F, Richard F, Garreau H and Drouilhet L, 2017a. Direct and correlated responses to selection in two lines of rabbits selected for feed efficiency under ad libitum and restricted feeding: III. Digestion and excretion of nitrogen and minerals. Journal of Animal Science, 95, 1301–1312. [DOI] [PubMed] [Google Scholar]
- Gidenne T, Garreau H, Drouilhet L, Aubert C and Maertens L, 2017b. Improving feed efficiency in rabbit production, a review on nutritional, technico‐economical, genetic and environmental aspects. Animal Feed Science and Technology, 225. /j.anifeedsci.2017.01.016 [Google Scholar]
- Giersberg MF, Kemper N and Fels MJLS, 2015. Planimetric measurement of floor space covered by fattening rabbits and breeding does in different body positions and weight classes. Livestock Science, 177, 142–150. [Google Scholar]
- Gondret F, Larzul C, Combes S and De Rochambeau H, 2005. Carcass composition, bone mechanical properties, and meat quality traits in relation to growth rate in rabbits. Journal of Animal Science, 83, 1526–1535. [DOI] [PubMed] [Google Scholar]
- González‐Mariscal G, McNitt JI and Lukefahr SD, 2007. Maternal care of rabbits in the lab and on the farm: endocrine regulation of behavior and productivity. Hormonal Behavior, 52, 86–91. [DOI] [PubMed] [Google Scholar]
- González‐Mariscal G, Lemus AC, Vega‐Gonzalez A and Aguilar‐Roblero R, 2013. Litter size determines circadian periodicity of nursing in rabbits. Chronobiology International, 30, 711–718. 10.3109/07420528.2013.784769 [DOI] [PubMed] [Google Scholar]
- González‐Redondo P and Zamora‐Lozano M, 2008. Neonatal cannibalism in cage‐bred wild rabbits (Oryctolagus cuniculus). Archivos de Medicina Veterinaria, 40, 281–287. [Google Scholar]
- Graf S, Bigler L, Failing K, Wurbel H and Buchwalder T, 2011. Regrouping rabbit does in a familiar or novel pen: effects on agonistic behaviour, injuries and core body temperature. Applied Animal Behaviour Science, 135, 121–127. [Google Scholar]
- Greene HSN, 1937. Toxemia of pregnancy in the rabbit. I. Clinical manifestations and pathology. Journal of Experimental Medicine, 65, 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli G, Ferrazzi V, Agnoletti F, Piccirillo A, Pisoni AM and Gallazzi D, 2006. La patologia enterica nel coniglio da carne allevato in Italia. Rivista Zootenic Veterinary, 34, 51–56. [Google Scholar]
- Guerrero I, Ferrian S, Penades M, Garcia‐Quiros A, Pascual JJ, Selva L, Viana D and Corpa JM, 2015. Host responses associated with chronic staphylococcal mastitis in rabbits. Veterinary Journal, 204, 338–344. [DOI] [PubMed] [Google Scholar]
- Gunia M, David I, Hurtaud J, Maupin M, Gilbert H and Garreau H, 2015. Resistance to infectious diseases is a heritable trait in rabbits. Journal of Animal Sciences, 93, 5631–5638. 10.2527/jas.2015-9377 [DOI] [PubMed] [Google Scholar]
- Gunia M, David I, Hurtaud J, Maupin M, Gilbert H and Garreau H, 2018. Genetic parameters for resistance to non‐specific diseases and production traits measured in challenging and selection environments; application to a rabbit case. Frontiers in Genetics, 9, 10.3389/fgene.2018.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn D and Morton DB, 1995. Inventory of the behaviour of New Zealand White rabbits in laboratory cages. Applied Animal Behavorial Sciences, 45, 277–292. [Google Scholar]
- Habeeb AA, Marai IFM and Kamal TH, 1992. Heat stress. Farm Animals and the Environment, 27–47. [Google Scholar]
- Hafez ESE, Lindsay DR and Moustafa LA, 1966a. Some factors affecting nest building in the domestic rabbit. Zeitschrift fur Tierpsychologie, 23, 691–700. [DOI] [PubMed] [Google Scholar]
- Hafez ESE, Lindsay DR and Moustafa LA, 1966b. Some maternal factors affecting nest building in the domestic rabbit. Zeitschrift fur Tierpsychologie, 6, 691–700. [DOI] [PubMed] [Google Scholar]
- Hernández P, Ariño B, Grimal A and Blasco A, 2006. Comparison of carcass and meat characteristics of three rabbit lines selected for litter size or growth rate. Meat Science, 73, 645–650. [DOI] [PubMed] [Google Scholar]
- Hesham M and Nasr M, 2017. Growth performance, carcass traits, behaviour and welfare of New Zeland White rabbits housed in different enriched cages. Animal Production Science, 57, 1759–1766. 10.1071/ANI5865 [DOI] [Google Scholar]
- Hoy S, 2009. Rabbit housing with respect to animal welfare. Deutsche Tierarztliche Wochenschrift, 116, 97–100. [Google Scholar]
- Hoy S, 2018. A new parameter describing fertility in rabbits at the farm level: the kit index. Archives Animal Breeding, 61, 463–467. 10.5194/aab-61-463-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy S and Selzer D, 2002. Frequency and time of nursing in wild and domestic rabbits housed outdoors in free range. World Rabbit Sciences, 10, 77–84. [Google Scholar]
- Hoy S and Matics Z, 2016. Alternative housing systems for rabbit does (Invited paper). Proceedings 11th World Rabbit Congress. 637–651.
- Hudson R and Distel H, 1982. The pattern of behaviour of rabbit pups in the nest. Behaviour, 79, 255–271. Available online: https://brill.com/view/journals/beh/79/2-4/article-p255_11.xml [Google Scholar]
- Hudson R, Schaal B, Bilkò A and Altbäcker V, 1996. Just three minutes a day: the behaviour of young rabbits viewed in the context of limited maternal care. 6th World Rabbit Congress., 2, 395–403. [Google Scholar]
- Huneau‐Salaün A, Bougeard S, Balaine l, Eono F, le Bouquin S and Chauvin C, 2015. Husbandry Factors and health conditions influencing the productivity of French rabbit farms. World Rabbit Sci., 23, 27–37. 10.4995/wrs.2015.3076 [DOI] [Google Scholar]
- Hungary , 1998. Law No. XXVIII of 1998 on animal protection. http://extwprlegs1.fao.org/docs/pdf/hun15674.pdf
- IASP , 1979. Pain, 6, 250.
- Italian Ministry of Health , 2014. Attuazione della direttiva 2010/63/UE sulla protezione degli animali utilizzati a fini scientifici. Available online: http://extwprlegs1.fao.org/docs/pdf/ita153404.pdf
- Italian Ministry of Health , 2019. Linee di indirizzo per l'allevamento del coniglio. Prot. N°001620031/07/2014‐DGSAF‐COD_UO‐P.
- Jekkel G, Milisits G and Nagy I, 2010. Effect of alternative rearing methods on the behaviour and on the growth and slaughter traits of growing rabbits. Archiv Fur Tierzucht‐Archives of Animal Breeding, 53, 205–215. [Google Scholar]
- Johnson HD, 1980. Environmental management of cattle to minimize the stress of climatic change. International Biometeorology, 24, 14. [Google Scholar]
- de Jong IC, Reimert H and Rommers JM, 2008. Effect of floor type on footpad injuries in does: a pilot study., Congress, Verona, Italy. [Google Scholar]
- de Jong IC, Reuvekamp BF and Rommers JM, 2011. A welfare assessment protocol for commercially housed rabbits= Een protocol voor het meten van welzijn bij commercieel gehouden konijnen. Wageningen UR Livestock Research, 1570–8616. [Google Scholar]
- Jordan D, Varga A, Kermauner A, Gorjanc G and Stuhec I, 2004. Acta agriculturae Slovenica, 1, 73–79. [Google Scholar]
- Jordan D, Gorjanc G and Stuhec I, 2008. Effect of gnawing wood as environmental enrichment on behaviour of individually housed growing rabbits. Archiv Fur Geflugelkunde, 72, 181–187. [Google Scholar]
- Kasa IW and Thwaites CJ, 1992. Semen quality in bucks exposed to 34°C for 8 hours on either 1 or 5 days. Applied Rabbit Research, 15, 560–568. [Google Scholar]
- Katsarou A, Tsironi A, Serafetinidou M, Voyazaki C, Baumans V and Kostomitsopoulos N, 2011. Age ‐ related behaviour on individually caged rabbits. Journal of the Hellenic Veterinary Medical Society, 62, 21–28. [Google Scholar]
- Keating SC, Thomas AA, Flecknell PA and Leach MC, 2012. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PLoS ONE, 7, e44437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzel F and Fisher PG, 2018. Clinical signs, diagnosis, and treatment of Encephalitozoon cuniculi infection in rabbits. Exotic Animal Practice, 21, 69–82. [DOI] [PubMed] [Google Scholar]
- Lambertini L, Paci G, Morittu VM, Vignola G, Orlandi P, Zaghini G and Formigoni A, 2005. Consequences of behaviour on productive performances of rabbits reared in pens. Italian Journal of Animal Science, 4. [Google Scholar]
- Lavazza A, Cerioli M and Grilli G, 2009. Biosicurezza negli allevamenti cunicoli [Biosafety in rabbit breeding]. 74, 91–120. ISBN 978‐88‐902814‐8‐8. [Google Scholar]
- Le Normand B, Chatellier S and Couteau M, 2013. Comportement individuel de lapins en croissance, logés en cages collectives et rationnés. Premiers résultats. J. Rech. Cunicol., 15, 51–54. [Google Scholar]
- Leach MC, Allweiler S, Richardson C, Roughan JV, Narbe R and Flecknell PA, 2009. Behavioural effects of ovariohysterectomy and oral administration of meloxicam in laboratory housed rabbits. Research in Veterinary Science, 87, 336–347. [DOI] [PubMed] [Google Scholar]
- Lebas F, 2000. Biología (Biology). Enfermedades del Conejo (Diseases of the Rabbit), 1, 55–126. Available online: http://www.cuniculture.info/Docs/indexbiol.htm [Google Scholar]
- Lebas F, Coudert P, Rouvier R and De Rochambeau H, 1986. The Rabbit: husbandry, health, and production. Food and Agriculture organization of the United Nations, Rome. [Google Scholar]
- Lehmann M, 1989. Das Verhalten junger Hauskaninchen unter verschiedenen Umgebungsbedingungen – Beurteilung von Haltungssystemen sowie Entwicklung eines Haltungskonzeptes für Mastgruppen. Dissertation, Universität Bern.
- Lehmann M, 1991. Social behaviour in young domestic rabbit under semi‐natural conditions. Applied Animal Behavior Sciences, 32, 269–292. [Google Scholar]
- Lenoir G, Garreau H and Banville M, 2012. Estimation of genetic parameters and trends for birth weight criteria in HyCole d line. World Rabbit Science Association, 183–187. [Google Scholar]
- Leone‐Singer A and Hoop R, 2003. Untersuchung zur Säuglingsmortalität bei Mastkaninchen in der Schweiz. Tierheilk, 145, 329–335. [DOI] [PubMed] [Google Scholar]
- Licois D, 2004. Domestic rabbit enteropathies. Becerril, 8th World Rabbit Congress, Colegio de Postgraduados for WRSA, 385–403. Available online: http://www.dcam.upv.es/388wrc/
- Licois D, 2010. Pathologie d'origine bactérienne et parasitaire chez le Lapin: apports de la dernière décennie. Cuniculture Magazine, 37, 35–49. Available online: https://www.cuniculture.info/Docs/Magazine/Magazine2010/mag37-035.html [Google Scholar]
- Lidfors L, 1997. Behavioural effects of environmental enrichment for individually caged rabbits. Applied Animal Behavior Sciences, 52, 157–169. [Google Scholar]
- LPHSI , 1990. Livestock and poultry heat stress indices agriculture engineering technology guide. Clemson University, Clemson, SC, USA. [Google Scholar]
- Luzi F, Ferrante V, Heinzl E and Verga M, 2003. Effect of environmental enrichment on productive performance and welfare aspects in fattening rabbits. Italian Journal of Animal Science, 2(Suppl. 1), 438–440. [Google Scholar]
- Machado LC, Martínez‐Paredes E, Paragliola F and Cervera C, 2016. Performance of rabbit kits originating from collective and individual cages Proc.: 11th. World Rabbit Congress; pp. 699–702. [Google Scholar]
- Maertens L, 2010. 14 Feeding Systems for Intensive Production. Nutrition of the Rabbit, 253.
- Maertens L and Buijs S, 2016. Comparison of fattening performances housed in parks or enriched cages. Congress, Qingdao, China. [Google Scholar]
- Maertens L, Lebas F and Szendro Z, 2006. Rabbit milk: A review of quantity, quality and non‐dietary affecting factors. Proceedings of the World Rabbit Science.
- Maertens L, Rommers J and Jacquet M, 2011. Le logement des lapins en parcs, une alternative pour les cages classiques dans un système “duo”? 14èmes Journ. Rech. Cunicole, 85–88. [Google Scholar]
- Maertens L, Buijs S and Davoust C, 2013. Gnawing blocks as cage enrichment and dietary supplement for does and fatteners: intake, performance and behaviour. World Rabbit Science, 21, 185–192. [Google Scholar]
- Mancinelli E, Keeble E, Richardson J and Hedley J, 2014. Husbandry risk factors associated with hock pododermatitis in UK pet rabbits (Oryctolagus cuniculus). Vet. Rec., 174, 429 10.1136/vr.101830 [DOI] [PubMed] [Google Scholar]
- Marai IFM and Rashwan AA, 2004. Rabbits behavioural response to climatic and managerial conditions ‐ a review. Archiv Fur Tierzucht‐Archives of Animal Breeding, 47, 469–482. [Google Scholar]
- Marai IFM, Ayyat MS and Abd El‐Monem UM, 2001. Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Tropical Animal Health Production, 33, 457–462. [DOI] [PubMed] [Google Scholar]
- Marin C, Simarro‐Catala L and Villagra A, 2018. Technical note: assessment of best location of gnawing sticks in growing rabbit cages. World Rabbit Science, 26, 249–254. 10.4995/wrs.2018.7547 [DOI] [Google Scholar]
- Marlier D, Dewree R, Delleur V, Licois D, Lassence C, Poulipoulis A and Vindevogel H, 2003. A review of the major causes of digestive disorders in the European rabbit. Annales de Medecine Veterinaire, 147, 385–392. [Google Scholar]
- Martignon MH, Guinebretière M, Postollec G, Huonnic D, Boilletot E, Jourden A, Marie F, Burel C, Gidenne T and Michel V, 2011. Consequences of feed restriction on behaviour and welfare of growing rabbits. Rech. Cunicoles., 25–28. [Google Scholar]
- Martinez‐Paredes E, Rodenas L, Pascual JJ, Blas E, Brecchia G, Boiti C and Cervera C, 2015. Effects of rearing feeding programme on the young rabbit females’ behaviour and welfare indicators. World Rabbit Science, 23, 197–205. 10.4995/wrs.2015.3987 [DOI] [Google Scholar]
- Martínez‐Paredes E, Ródenas L, Pascual J and Savietto D, 2018. Early development and reproductive lifespan of rabbit females: implications of growth rate, rearing diet and body condition at first mating. Animal, 12, 2347–2355. [DOI] [PubMed] [Google Scholar]
- Martrenchar A, Boilletot E, Cotte JP and Morisse JP, 2001. Wire‐floor pens as an alternative to metallic cages in fattening rabbits: influence on some welfare traits. Animal Welfare, 10, 153–161. [Google Scholar]
- Massacci FR, Magistrali CF, Cucco L, Curcio L, Bano L, Mangili P, Scoccia E, Bisgaard M, Aalbæk B and Christensen H, 2018. Characterization of Pasteurella multocida involved in rabbit infections. Veterinary Microbiology, 213, 66–72. 10.1016/j.vetmic.2017.11.023 [DOI] [PubMed] [Google Scholar]
- Masthoff T and Hoy S, 2019. Investigations on the influence of floor design on dirtiness and foot pad lesions in growing rabbits. Animals, 9, 6 10.3390/ani9060354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matics Z, Szendrő Z, Radnai Edit Biró‐Németh I and Gyovai M, 2003. Examination of free choice of rabbits among different cage‐floors. Agriculturae Conspectus Scientificus, 68, 265–269. [Google Scholar]
- Matics Z, Zs Szendrő, Bessei W, Radnai I, Biró‐Németh E, Orova Z and Gyovai M, 2004a. The free choice of rabbits among identically and differently sized cages. Puebla, Mexico, World Rabbit Congress: pp. 1251–1256. [Google Scholar]
- Matics ZS, Szendrő Z, St Hoy, Nagy I, Radnai I, Biró‐Németh E and Gyovai M, 2004b. Effect of different management methods on the nursing behaviour of rabbits. World Rabbit Sciences, 12, 95–108. [Google Scholar]
- Matics Z T P, Farkas A, Dal Bosco Z, Szendro E, Filiou I, Nagy M, Odermatt G, Paci and Gerencser Z, 2018. Comparison of Pens without and with Multilevel Platforms for Growing Rabbits. Italian Journal of Animal Science, 17, 469–476. 10.1080/1828051x.2017.1363640 [DOI] [Google Scholar]
- Meunier‐Salaün M, Edwards S and Robert S, 2001. Effect of dietary fibre on the behaviour and health of the restricted fed sow. Animal Feed Science and Technology, 90, 53–69. [Google Scholar]
- Miko A, Matics Z, Gerencser M, Odermatt I, Radnai I, Nagy K, Szendro and Szendro S, 2014. Performance and Welfare of Rabbit Does in Various Caging Systems. Animal 8, 1146–1152. 10.1017/s1751731114001244 [DOI] [PubMed] [Google Scholar]
- Mirabito L, Buthon L, Cialdi G, Galliot P and Souchet C, 1999. Effet du logement des lapines en cages réhaussées avec plate‐forme : premiers resultats. 8èmes Journées de la Recherche Cunicole, 9, 67–70. [Google Scholar]
- Mirabito L, Galliot P and Souchet C, 2004. Effet de la surface disponible et de l'aménagement des cages sur les performances zootechniques et le comportement des lapines et des jeunes. Journée
- Mirabito L, Galliot P, Souchet C, Dumont F and Thomeret F, 2005. Logement collectif des lapines reproductrices: Conséquences zootechniques. 11émes Journées Recherche Cunicole, November 22‐23, 2011. Paris, France, 53–56.
- Monclùs R and Rödel H, 2008. Different forms of vigilance in response to the presence of predators and conspecifics in a group‐living mammal, the European Rabbit. Ethics, 114, 287–297. [Google Scholar]
- Monclús R, Rödel HG and Von Holst D, 2006. Fox odour increases vigilance in european rabbits: a study under semi‐natural conditions. Ethology, 112, 1186–1193. 10.1111/j.1439-0310.2006.01275.x [DOI] [Google Scholar]
- Morisse JP and Maurice R, 1997. Influence of stocking density or groupsize on behaviour of fattening rabbits kept under intensive conditions. Applied Animal Behavorial Sciences, 54, 351–357. [Google Scholar]
- Morisse J, Boilletot E and Martrenchar A, 1999. Preference testing in intensively kept meat production rabbits for straw on wire grid floor. Applied Animal Behaviour Science, 64, 71–80. [Google Scholar]
- Muciño E, Bautista A, Jiménez I, Martínez‐Gómez M and Hudson R, 2008. Differential development of body equilibrium among littermates in the newborn rabbit. Developmenatl Psychobiology, 51, 24–33. 10.1002/dev.20339 [DOI] [PubMed] [Google Scholar]
- Mugnai C, Dal Bosco A and Castellini C, 2009. Effect of different rearing systems and pre‐kindling handling on behaviour and performance of rabbit does. Applied Animal Behaviour Science, 118, 91–100. [Google Scholar]
- Mugnai C, Dal Bosco A, Cardinali R, Rebollar PG, Moscati L and Castellini C, 2014. Effect of pasture availability and genotype on welfare, immune function, performance and meat characteristics of growing rabbits. World Rabbit Science, 22, 29–39. [Google Scholar]
- Mullan SM and Main DCJ, 2006. Survey of the husbandry, health and welfare of 102 pet rabbits’. Veterinary Record, 159, 103–109. [DOI] [PubMed] [Google Scholar]
- Naturil‐Alfonso C, Marco‐Jimenez F, Pascual JJ and Vicente JS, 2017. Effect of restricted feeding under rearing on reproduction, body condition and blood metabolites of rabbit does selected for growth rate. World Rabbit Science, 25, 303–312. [Google Scholar]
- Nielsen BL, Thodberg K, Malmkvist J and Steenfeldt S, 2011. Proportion of insoluble fibre in the diet affects behaviour and hunger in broiler breeders growing at similar rates. Animal, 5, 1247–1258. [DOI] [PubMed] [Google Scholar]
- de ‐Oliveira MC, Lima S CO, Mesquita SA, Jessica SA, Gomes YS, Attia YA and Oliveira HC, 2017. Nesting materials for does: Effect on nest building and performance at first parturition. Revista Colombiana de Ciencias Pecuarias, 30, 308–315. 10.17533/udea.rccp.v30n4a06 [DOI] [Google Scholar]
- d'Ovidio D, Pierantoni L, Noviello E and Pirrone F, 2016. Sex differences in human‐directed social behavior in pet rabbits. Journal of Veterinary Behavior Clinical Applications and Research, 15 10.1016/j.jveb.2016.08.072 [DOI] [Google Scholar]
- Pascual JJ, Savietto D, Cervera C and Baselga M, 2013. Resources allocation in reproductive rabbit does: a review of feeding and genetic strategies for suitable performance. World Rabbit Science, 21, 123–144. [Google Scholar]
- Pinheiro V, Torres S, Monteiro D, Silva S and Mourão J, 2012. Growth performance and behaviour of growing rabbits subjected to feed restriction. World Rabbit Congress–September; pp. 3–6. [Google Scholar]
- Poggiagliolmi S, Crowell‐Davis SL, Alworth L and Harvey SB, 2011. Environmental enrichment of New Zealand White rabbits Living in laboratory cages. Journal of Veterinary Behavior: Clinical Applications and Research, 6, 343–350. 10.1016/j.jveb.2010.12.001 [DOI] [Google Scholar]
- Postollec G, Boilletot E, Maurice R and Michel V, 2002. Effets de l'enrichissement du milieu sur les performances zootechniques et le comportement des lapins d'engraissement élevés en groupe. Journée Nationale ITAVI sur l’élevage du lapin de chair, Nantes, 21 Novembre 2002, ITAVI Ed. [Google Scholar]
- Postollec G, Boilletot E, Maurice R and Michel V, 2006. The effect of housing system on the behaviour and growth parameters of fattening rabbits. Animal Welfare, 15, 105–111. [Google Scholar]
- Princz Z, Radnai I, Biro‐Nemeth E, Matics Z, Gerencser Z, Nagy I and Szendro Z, 2008a. Effect of cage height on the welfare of growing rabbits. Applied Animal Behaviour Science, 114, 284–295. [Google Scholar]
- Princz Z, Zotte AD, Radnai I, Biro‐Nemeth E, Matics Z, Gerencser Z, Nagy I and Szendro Z, 2008b. Behaviour of growing rabbits under various housing conditions. Applied Animal Behaviour Science, 111, 342–356. [Google Scholar]
- Princz Z, Zotte AD, Metzger S, Radnai I, Biro‐Nemeth E, Orova Z and Szendro Z, 2009. Response of fattening rabbits reared under different housing conditions. 1. Live performance and health status. Livestock Science, 121, 86–91. [Google Scholar]
- Rafel O, Catanese B, Rodriguez P, Fuentes C, Llonch P, Mainau E, Piles M, Velarde A, Ramon J and Lopez B, 2012. Effect of temperature on breeding rabbit behavior. Proceedings of the Proceedings 10th World Rabbit Congress. 1075–1079.
- Rashwan AA, Szendrő Zs, Zs Matics, Szalai A, Biró‐Németh E, Szendrő É and Nagy I, 2003. Effect of the time of insemination and the litter size on the gestation length of rabbits. World Rabbit Sciences, 11, 75–85. [Google Scholar]
- Rebollar PG, Pereda N, Schwarz BF, Millan P, Lorenzo PL and Nicodemus N, 2011. Effect of feed restriction or feeding high‐fibre diet during the rearing period on body composition, serum parameters and productive performance of rabbit does. Animal Feed Science and Technology, 163, 67–76. [Google Scholar]
- Remois G, Lafargue‐Hauret P and Sureault A, 1999. Effect of water temperature on the feed and water consumption of fattening rabbits. 1‐ Effect of the hot water. 2‐ Effect of the cold water. Cahiers Options Méditerranéennes, 41, 25–30 & 31–35. [Google Scholar]
- Rommers J and de Greef KH, 2018. Are combi parks just as useful as regular parks for fatteners for part‐time group housing of rabbit does? World Rabbit Science, 26, 299–305. [Google Scholar]
- Rommers J and de Jong I, 2011. Plastic mats prevent footpad injuries in rabbit does. World Rabbit Science, 19, 233–237. [Google Scholar]
- Rommers JM, Boiti C, De Jong I and Brecchia G, 2006. Performance and behaviour of rabbit does in a group‐housing system with natural mating or artificial insemination. Reproduction, Nutrition, Development, 46, 677–687. 10.1051/rnd:2006038 [DOI] [PubMed] [Google Scholar]
- Rommers JM, Kemp B, Houwers HW, Gunnink H and de Jong IC, 2012. Description of nestbox visits and suckling events in a group housing system for rabbit does as compared to individual cages. World Rabbit Science, 20, 231–240. 10.4995/wrs.2012.1231 [DOI] [Google Scholar]
- Rommers J, Gunnink H and de Jong I, 2013. Effect of different types of hiding places on aggression among rabbits does in a group‐housing system: a pilot study.
- Rommers JM, Reuvekamp BJF, Gunnink H and de Jong IC, 2014a. Effect of hiding places, straw and territory on aggression in group‐housed rabbit does. Applied Animal Behaviour Science, 157, 117–126. [Google Scholar]
- Rommers J, Bracke M, Reuvekamp B, Gunnink H and de Jong I, 2014b. Cage‐enrichment: rabbit does prefer straw or a compressed wooden block. World Rabbit Science, 22, 301–309. 10.4995/wrs.2014.1353 [DOI] [Google Scholar]
- Rosell JM and de la Fuente LF, 2009a. Culling and mortality in breeding rabbits. Preventive Veterinary Medicine, 88, 120–127. [DOI] [PubMed] [Google Scholar]
- Rosell JM and de la Fuente LF, 2009b. Effect of Footrests on the Incidence of Ulcerative Pododermatitis in Domestic Rabbit Does. Animal Welfare, 18, 199–204. [Google Scholar]
- Rosell JM and De La Fuente L, 2013. Assessing ulcerative pododermatitis of breeding rabbits. Animals, 3, 318–326. 10.3390/ani3020318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell J and de la Fuente L, 2016a. Causes of mortality in breeding rabbits. Preventive Veterinary Medicine, 127, 56–63. [DOI] [PubMed] [Google Scholar]
- Rosell J and de la Fuente L, 2016b. Infertility of female rabbits on commercial units. Proceedings of the 11th World Rabbit Congress, Qingdao, China, 15‐18.
- Rosell JM and de la Fuente LF, 2018. Mastitis on Rabbit Farms: Prevalence and Risk Factors. Animals, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell JM, Dronda MA and de la Fuente LF, 2000. Dermatología. Enfermedades del conejo, 19, 355–398. [Google Scholar]
- Rosell JM, Garriga R, Mrtinez J and Domingo M, 2012. Calcinosis in female rabbits. Proceedings of the 10th World Rabbit Congress. 1151–1154.
- Rosell JM, de la Fuente F, Parra F, Dalton KP, Badiola SJI, Pérez de Rosaz A, Badiola Díez JJ, de Luco FD, Casal J, Majò N, Casas J, Garriga R and Magariños FXM, 2019. Myxomatosis and Rabbit Haemorrhagic Disease: a 30‐year Study of the Occurrence on Commercial Farms in Spain. Animals, 9, 780 10.3390/ani9100780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchti S, Meier AR, Wurbel H, Kratzer G, Gebhardt‐Henrich SG and Hartnack S, 2018. Pododermatitis in Group Housed Rabbit Does in Switzerland‐Prevalence, Severity and Risk Factors. Preventive Veterinary Medicine, 158, 114–121. 10.1016/j.prevenned.2018.06.011 [DOI] [PubMed] [Google Scholar]
- Ruchti S, Kratzer G, Furrer R, Hartnack S, Wurbel H and Gebhardt‐Henrich SG, 2019. Progression and risk factors of pododermatitis in part‐time group housed rabbit does in Switzerland. Preventive Veterinary Medicine, 166, 56–64. 10.1016/j.prevetmed.2019.01.013 [DOI] [PubMed] [Google Scholar]
- Sánchez JP, de la Fuente LF and Rosell JM, 2012. Health and body condition of lactating females on rabbit farms. Journal of Animal Science, 90, 2353–2361. 10.2527/jas.2011-4065. https://www.ncbi.nlm.nih.gov/pubmed/22266998. [DOI] [PubMed] [Google Scholar]
- Sant R and Rowland M, 2009. Skin disease in rabbits. In Practice. 31, 233–239. [Google Scholar]
- Schepers F, Koene P and Beerda B, 2009. Welfare assessment in pet rabbits. Animal Welfare, 18. [Google Scholar]
- Schlolaut W, Hudson R and Rodel HG, 2013. Impact of rearing management on health in domestic rabbits: a review. World Rabbit Science, 21, 145–159. 10.4995/wrs.2013.1029 [DOI] [Google Scholar]
- Shrestha M, Garreau H, Balmisse E, Bed'Hom B, David I, Guitton E, Helloin E, Lenoir G, Maupin M, Robert R, Lantier F and Gunia M, 2019. Projet RELAPA (génomique pour la REsistance génétique des LApins à la PAsteurellose): paramètres génétiques.
- Stauffacher M, 1988. Entwicklung und ethologische Prüfung der tiergerechtheit einer bodenhaltung für hauskaninchen‐zuchtgruppen. Thesis, Univ, Bern. [Google Scholar]
- Stauffacher M, 1992. group housing and enrichment cages for breeding, fattening and laboratory rabbits. Animal Welfare, 1, 105–125. [Google Scholar]
- Stewart KL and Suckow MA, 2016. Effects of nominal differences in cage height and floor space on the wellbeing of rabbits. Journal of the American Association for Laboratory Animal Science, 55(2), 168–171. [PMC free article] [PubMed] [Google Scholar]
- Szendro Z and Dalle Zotte A, 2011. Effect of housing conditions on production and behaviour of growing meat rabbits: a review. Livestock Science, 137, 296–303. [Google Scholar]
- Szendro Z and McNitt JI, 2012. Housing of rabbit does: group and individual systems: a review. Livestock Science, 150, 1–3. 10.1016/j.livsci.2012.09.017 [DOI] [Google Scholar]
- Szendro Z, Miko A, Odermatt M, Gerencser Z, Radnai I, Dezsery B, Garai E, Nagy I, Ndrondro K and Matics Z, 2013. Comparison of performance and welfare of single‐caged and group‐housed rabbit does. Animal, 7, 463–468. [DOI] [PubMed] [Google Scholar]
- Szendro K, Szendro Z, Matics Z, Zotte AD, Odermatt M, Radnai I and Gerencser Z, 2015. Effect of genotype, housing system and hay supplementation on performance and ear lesions of growing rabbits. Livestock Science, 174, 105–112. 10.1016/j.livsci.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Szendro ZS, Trocino A, Hoy ST, Xiccato G, Villagra A and Maertens L, 2019. A review of recent research outcomes on the housing of farmed domestic rabbits: reproducing does. World Rabbit Science, 27, 1–14. 10.4995/wrs.2019.10599 [DOI] [Google Scholar]
- Szendrö Z, Jovánczai Z, Theau‐Clement M, Radnai I, Biró‐Németh E and Milisits G, 1999. The effect of doe‐litter separation on production performance in rabbit does and their kits. World Rabbit Sciences, 7, 165–169. [Google Scholar]
- Szendrö Z, Princz Z, Romvári R, Locsmándi L, Szabó A, Bázár G, Radnai I, Biró‐Németh E, Matics Z and Nagy I, 2010. Effect of group size and stocking density on productive, carcass, meat quality and aggression traits of growing rabbits. World Rabbit Science, 17, 153–162. 10.4995/wrs.2009.655 [DOI] [Google Scholar]
- The Netherlands , 2006. Regulations welfare standards Rabbits (PPE). Regulation of the Dutch Board for Poultry and Eggs dated 9 February 2006, establishing provisions relating to the welfare of rabbits used in rabbit husbandry. Available online: http://www.wrsadeutschland.de/uploads/media/Kaninchenverordnung_NL_2008.pdf
- Tillmann K, Windschnurer I, Gamper J, Hinney B, Rulicke T, Podesser BK, Troxler J and Plasenzotti R, 2019. Welfare assessment in rabbits raised for meat and laboratory purposes in enclosures with two floor types: perforated plastic with holes versus slats. Research in Veterinary Science, 122, 200–209. [DOI] [PubMed] [Google Scholar]
- Trocino A and Xiccato G, 2006. Animal welfare in reared rabbits: a review with emphasis on housing systems. World Rabbit Science, 14, 77–93. [Google Scholar]
- Trocino A, Xiccato G, Queaque PI and Sartori A, 2004. Group housing of growing rabbits: effect of stocking density and cage floor on performance, welfare, and meat quality. Proceedings of the 8th World Rabbit Congress. 1277‐1282. Available online: http://world-rabbit-science.com/WRSA-Proceedings/Congress-2004-Puebla/Papers/Welfare%20&%20Ethology/W-Trocino.pdf
- Trocino A, Xiccato G, Majolini D and Fragkiadakis M, 2008. Effect of cage floor and stocking density on growth performance and welfare of group‐housed rabbits, 9th Edition World Rabbit Congress. [Google Scholar]
- Trocino A, Majolini D, Tazzoli M, Filiou E and Xiccato G, 2013. Housing of growing rabbits in individual, bicellular and collective cages: fear level and behavioural patterns. Animal, 7, 633–639. [DOI] [PubMed] [Google Scholar]
- Trocino A, Filiou E, Tazzoli M, Bertotto D, Negrato E and Xiccato G, 2014. Behaviour and welfare of growing rabbits housed in cages and pens. Livestock Science, 167, 305–314. [Google Scholar]
- Trocino A, Filiou E, Tazzoli M, Birolo M, Zuffellato A and Xiccato G, 2015. Effects of floor type, stocking density, slaughter age and gender on productive and qualitative traits of rabbits reared in collective pens. Animal, 9, 855–861. [DOI] [PubMed] [Google Scholar]
- Trocino A, Filiou E, Zomeno C, Birolo M, Bertotto D and Xiccato G, 2018. Behaviour and reactivity of female and male rabbits housed in collective pens: effects of floor type and stocking density at different ages. World Rabbit Science, 26, 135–147. [Google Scholar]
- Trocino A, Zomeño C, Filiou E, Birolo M, White P and Xiccato G, 2019. The use of environmental enrichments affects performance and behavior of growing rabbits housed in collective pens. Animals (Basel), 9, 537 10.3390/ani9080537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyttens FAM, Maertens L, Van Poucke E, Van Nuffel A, Debeuckelaere S, Creve J and Lens L, 2005. Measuring fluctuating asymmetry in fattening rabbits: a valid indicator of performance and housing quality? Journal of Animal Science, 83, 2645–2652. [DOI] [PubMed] [Google Scholar]
- Tynes VV, 2013. Behavioral dermatopathies in small mammals, veterinary clinics of North America. Exotic Animal Practice, 16, 801–820. 10.1016/j.cvex.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Van Praag E, Maurer A and Saarony T, 2010. Non‐viral cutaneous tumors. Skin diseases of rabbits. Medirabbit.com, 11, 247–301. [Google Scholar]
- Vangeel I, Pasmans F, Vanrobaeys M, de Herdt P and Haesebrouck F, 2000. Prevalence of dermatophytes in asymptomatic guinea pigs and rabbits. Veterinary Record, 146, 440–441. [DOI] [PubMed] [Google Scholar]
- Vastrade Françoise M, 1987. Spacing behaviour of free‐ranging domestic rabbits, Oryctolagus cuniculus . Applied Animal Behaviour Science, 18, 185–195. [Google Scholar]
- Vela AI, Fernández A, Moreno B, Casamayor A, Chacón G, Villa A, Comenge J and Fernández‐Garayzábal JF, 2010. Isolation of Enterococcus hirae from suckling rabbits with diarrhoea. Veterinary Record, 167, 345–346. [DOI] [PubMed] [Google Scholar]
- Verga M, 1997. Troppo stress fa male ai conigli. Coniglicoltura, 34, 13–19. [Google Scholar]
- Verga M, Canali E, Pizzi F and Crimella C, 1986. Induced reactions in young rabbits of dams of different parity and reared on two different nursing schedules. Applied Animal Behavorial Sciences, 16, 285–293. [Google Scholar]
- Verga M, Zingarelli I, Heinzl E, Ferrante V, Martino PA and Luzi F, 2004. Effect of housing and environmental enrichment on performance and behaviour in fattening rabbits. Proceedings of the 8th World Rabbit Congress, Puebla, Mexico, 1283–1288.
- Verga M, Luzi F and Carenzi C, 2007. Effects of husbandry and management systems on physiology and behaviour of farmed and laboratory rabbits. Hormones and Behavior, 52, 122–129. 10.1016/j.yhbeh.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Vervaecke H, De Bonte L, Maertens L, Tuyttens F, Stevens JMG and Lips D, 2010. Development of Hierarchy and Rank Effects in Weaned Growing Rabbits (Oryctolagus cuniculus). World Rabbit Science, 18, 139–149. 10.4995/wrs.2010.8229 [DOI] [Google Scholar]
- Verwer CM, van Amerongen G, van den Bos R and Hendriksen CFM, 2009. Handling effects on body weight and behaviour of group‐housed male rabbits in a laboratory setting. Applied Animal Behavorial Sciences, 117(1–2), 93–102. 10.1016/j.applanim.2008.12.004 [DOI] [Google Scholar]
- Viana D, Selva L, Callanan JJ, Guerrero I, Ferrian S and Corpa JM, 2011. Strains of Staphylococcus aureus and pathology associated with chronic suppurative mastitis in rabbits. Veterinary Journal, 190, 403–407. [DOI] [PubMed] [Google Scholar]
- Windschnurer I, Waiblinger S, Hanslik S, Klang A, Smajlhodzic F, Lowenstein M and Niebuhr K, 2019. Effects of ground floor type on selected health‐parameters and weight of rabbits reared in group pens. Animals, 9, 5 10.3390/ani9050216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittroff EK, Heird CE, Rakes JM and Johnson ZB, 1998. Growth and reproduction of nutrient restricted rabbits in a heat stressed environment. Applied Rabbit Research, 11, 87–92. [Google Scholar]
- Xiccato G and Trocino A, 2010. Energy and protein metabolism and requirements. Nutrition of the Rabbit, 2, 83–118. [Google Scholar]
- Xiccato G, Trocino A, Carraro L, Fragkiadakis M and Majolini D, 2008. Digestible fibre to starch ratio and antibiotic treatment time in growing rabbits affected by epizootic rabbit enteropathy. 9 th World Rabbit Congress, Verona, Italy.
- Xiccato G, Trocino A, Filiou E, Majolini D, Tazzoli M and Zuffellato A, 2013a. Bicellular cage vs. collective pen housing for rabbits: Growth performance, carcass and meat quality. Livestock Science, 155, 407–414. [Google Scholar]
- Xiccato G, Trocino A, Majolini D, Tazzoli M and Zuffellato A, 2013b. Housing of growing rabbits in individual, bicellular and collective cages: growth performance, carcass traits and meat quality. Animal, 7(4), 627–632. 10.1017/s175173111200198x [DOI] [PubMed] [Google Scholar]
- Zomeno C, Birolo M, Zuffellato A, Xiccato G and Trocino A, 2017. Aggressiveness in group‐housed rabbit does: influence of group size and pen characteristics. Applied Animal Behaviour Science, 194, 79–85. [Google Scholar]
- Zomeno C, Birolo M, Gratta F, Zuffellato A, Xiccato G and Trocino A, 2018. Effects of group housing system, pen floor type, and lactation management on performance and behaviour in rabbit does. Applied Animal Behaviour Science, 203, 55–63. [Google Scholar]
- Zomeño C, Dalla Costa A, Trocino A, Santagiuliana M, Lavazza A, Dorigo F, Bonfanti L, Birolo M, Xiccato G and Di Martino G, 2019. Medidas para evaluar la salud y el bienestar de los conejos: resultados descriptivos de un ciclo productivo en granjas comerciales. ASESCU.
- Zucca D, Redaelli V, Marelli S, Bonazza V, Heinzl E, Verga M and Luzi F, 2012. Effect of handling in pre‐weaning rabbits. World Rabbit Sciences, 20, 97–101. 10.4995/wrs.2012.1083 [DOI] [Google Scholar]


