Abstract
The EFSA Panel on Food Additives and Flavourings (FAF) provides a scientific opinion on the safety of Monk fruit extract proposed for use as a new food additive in different food categories. Monk fruit extracts are prepared by water extraction of the fruits of Siraitia grosvenorii. Cucurbitane glycosides, mogrosides, are the main components of the S. grosvenorii fruit and mogroside V is the main mogroside in the Monk fruit extract. Mogroside V is absorbed to some extent and is systemically bioavailable. Monk fruit extract containing 25% and 55% mogroside V were negative in the bacterial reverse mutation assay and did not induce structural and/or numerical chromosomal damage. However, the Panel noted that the in vitro toxicity studies including study with metabolic activation were not sufficiently informative to evaluate the genotoxic potential of the metabolites generated after microbial metabolism, including the aglycone. The effects on the testis observed in a 90‐day study with monk fruit extract‐52% mogroside V cannot be dismissed and the adversity of these effects cannot be ruled out. No effects on parental, reproductive or development toxicity were observed in a reproductive and developmental screening study in rats. For male animals, the time of exposure did not cover the full length of spermatogenesis and, therefore, a longer term study at higher doses would be needed to clarify the effects on testes observed in the 90‐day study. No maternal and developmental toxicity was observed. Considering the systemic availability of mogroside V, the effects observed in the rat subchronic study and following the principles of EFSA Guidance on food additives evaluation, data from chronic/carcinogenicity toxicity testing would have been warranted. Exposure to mogroside V was calculated based on the proposed use levels. The Panel concluded that toxicity database on Monk fruit extract is insufficient to conclude on the safety of the use of Monk fruit extract as a food additive.
Keywords: monk fruit extract, Luo Han Guo extract, mogroside V
Summary
Following a request from the European Commission to EFSA, the EFSA Panel on Food Additives and Flavourings (FAF) was asked to provide a scientific opinion on the safety of use of Monk fruit extract as a food additive in different food categories, in accordance with Regulation (EC) No 1331/2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings.
Monk fruit extracts are prepared by water extraction of the fruit of Siraitia grosvenorii. Cucurbitane glycosides are main components of S. grosvenorii fruit. Most of them taste sweet, so they are collectively called mogrosides. Mogrosides are present in the fresh fruit (0.55–0.65%). Mogroside V being the main mogroside in the Monk fruit extract.
Information on the composition of Monk fruit extracts with contents of mogroside V of 25, 40, 45, 50 and 55% has been provided. Monk fruit extract‐25% is the least refined product after water extraction, treatment with pectinase, concentration and the use of a resin column, while monk fruit extracts richer in mogroside V are obtained after additional purification steps, starting with the dissolution of the intermediate product in water, to remove impurities at the resin level via adsorption and elution steps. The Panel made some recommendations for the proposed specifications.
Mogroside V is absorbed to some extent and is systemically bioavailable.
Monk fruit extract containing 25% and 55% mogroside V were negative in the bacterial reverse mutation assay performed with Salmonella Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 both with the plate incorporation and the pre‐incubation method, in the presence and in the absence of metabolic activation. The two extracts did not induce structural and/or numerical chromosomal damage in an in vitro micronucleus assay in cultured human lymphocytes.
However, the Panel noted that the in vitro toxicity studies including study with S9 were not sufficiently informative to evaluate the genotoxic potential of the metabolites generated after microbial metabolism, including the aglycone.
In the 90‐day dietary rat study with monk fruit extract‐52%, mogroside V effect on the liver and testis weight was observed. Whether the liver effects could be interpreted as an adaptive response and were not considered to be adverse, the decrease in testis weight (absolute and relative to brain) was associated with histopathological findings and therefore the adversity of these effects cannot be ruled out by the Panel.
No effects on parental, reproductive or development toxicity were observed in a reproductive and developmental screening study at 1,200 mg mogroside V/kg body weight (bw) per day in rats. The Panel noted that for male animals the time of exposure did not cover the full length of spermatogenesis and, therefore, considered that a longer‐term study at higher doses would be needed to clarify the effects on testes observed in the 90‐day study.
In a prenatal developmental toxicity study, no maternal and developmental toxicity was found at 1,000 mg mogroside V/kg bw per day.
Considering the systemic availability of mogroside V, the effects observed in the rat 90‐day study and following the principles of the Guidance for the submission of food additives (EFSA ANS Panel, 2012), data from chronic/carcinogenicity toxicity testing would have been warranted.
To assess the dietary exposure to Monk fruit extract, the exposure was calculated based on the proposed use levels. In the general population, the exposure estimates ranged at the mean from 0.6 to 36.4 mg mogroside V/kg bw per day and at the p95 from 2.5 to 71.5 mg/kg bw per day. The Panel noted that the estimated long‐term exposures are very likely conservative, as this scenario assumes that all foods and beverages listed under the Annex II to Regulation No 1333/2008 would contain monk fruit extract at the proposed use levels.
The Panel concluded that toxicity database on Monk fruit extract is insufficient to conclude on the safety of the use of Monk fruit extract as a food additive.
1. Introduction
The present opinion deals with the re‐evaluation of the safety of Monk fruit/Luo han guo extract for use as sweetener in different categories of food.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/2008 on food additives.1 Only food additives that are included in the Union list, in particular in Annex II to that regulation, may be placed on the market and used as in foods under the conditions of use specified therein.
An application has been introduced for the authorisation of the use of monk fruit extract/Luo han guo (LHG) extract as sweetener in several food categories.
Monk fruit is the fruit of the plant Luo Han Guo (LHG), Siraitia grosvenorii (formerly Momordica grosvenorii Swingle). The plant is a perennial vine of the Cucurbitaceae family which produces a round green fruit. The chemical structures of the sweetening components naturally found in the fruit belong to the triterpenoid group of chemicals and are named mogrosides. These are present at 0.55–0.65% in the fresh fruit, and about 2.5% in the dried fruit of S. grosvenorii.
The monk fruit extract is obtained from the fruit through a process involving water extraction, filtration and selective concentration of the sweetening components. The primary sweetening component of monk fruit extract, with a content of 1.5–2% in the dried fruit, is mogroside V (CAS 88901‐36‐4), which is around 250 times as sweet as sucrose. The monk fruit extract may have different concentrations of mogroside V content (25%, 45% and 55%), varying according to the manufacturing process.
1.1.2. Terms of Reference
In accordance with Article 29(1)(a) of Regulation (EC) 178/2002 the European Commission asks the European Food Safety Authority to perform a risk assessment and to provide a scientific opinion on the safety of use of monk fruit/Luo han guo (LHG) extract as a food additive in different categories of food, in accordance with Regulation (EC) No 1331/2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings.2
1.2. Information on existing authorisations and evaluations
Monk fruit extract/LHG Siraitia grosvenorii Swingle was tabled on the agenda of the JECFA meeting (2016) and since no data were submitted, its discussion was removed from the agenda (JECFA, 2016).
Momordica grosvenorii fruit juice and fruit extract are authorised as a humectant and solvent, and antioxidant, respectively, in cosmetics in the EU (CosIng database3).
According to the applicant, the 3rd supplements to the 7th edition of the United States Pharmacopoeia (USP) Convention's Food Chemical Codex (FCC) published a monograph for monk fruit extract.
Furthermore, the applicant provided information on authorised uses and other evaluations in other countries (the United States, Canada, Japan, China, Australia, New Zealand (FSANZ, 2018; Documentation provided to EFSA No. 1)).
2. Data and methodologies
2.1. Data
The applicant has submitted a dossier in support of its application for the authorisation of monk fruit extract as a new food additive for the proposed uses in several food categories (Documentation provided to EFSA No. 1 and 2) and additional information provided during the assessment process in response to a following request by EFSA (Documentation provided to EFSA No. 3, 4, 5).
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance of the Scientific Committee on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009a) and following the relevant existing Guidances from the EFSA Scientific Committee.
The current ‘Guidance for submission for food additive evaluations’ (EFSA ANS Panel, 2012) and the Guidance on the ‘Safety assessment of botanicals and botanical preparations’ (EFSA Scientific Committee, 2009b) have been followed by the FAF Panel for authorisation of the new food additive Monk fruit extract.
When in animal studies, the test substance was administered in the feed or in drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake is calculated by the Panel using the relevant default values. In case of rodents the values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) are applied. In the case of other animal species, the default values by JECFA (2000) are used. In these cases, the dose was expressed as ‘equivalent to mg/kg bw per day’.
Dietary exposure to monk fruit extract from the proposed use as a food additive was estimated by combining the food consumption data available within the Comprehensive Database with the proposed use levels provided by the applicant. Uncertainties in the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
Monk fruit extracts are prepared by water extraction of the fruits of S. grosvenorii. The extracts obtained that after decolourisation and purification contain different amounts of the main component, mogroside V.
The plant Lo Han Guo (LHG, monk fruit), S. grosvenorii, is a perennial vine in the Cucurbitaceae family. According to the applicant, monk fruit is widely consumed in China and Japan.
Scientific name of the plant: Siraitia grosvenorii (Swingle) C. Jeffrey ex A. M. Lu & Zhi Y. Zhang (The Plant List, online).
Botanical synonyms are Momordica grosvenorii Swingle and Thladiantha grosvenorii (Swingle) C. Jeffrey.
Common names: Luo Han Guo, Lo Han Kuo, Arhat fruit, Fructus Momordicae, Momordicae Grosvenori Fructus.
Plant Family: Cucurbitaceae.
Cucurbitane glycosides are main components of S. grosvenorii fruit. Most of them taste sweet, they are collectively called mogrosides. Mogrosides are present in the fresh fruit (0.55–0.65%), mogroside V being the main component. Flavonoids, phenolic acids, anthraquinones, alkaloids, sterols, aliphatic acids and other compounds have been isolated from the fruit or leaf of S. grosvenorii. Polysaccharides are also important constituents of S. grosvenorii (Zheng et al., 2009; Zhang et al., 2012; Li et al., 2014; Zhou et al., 2016).
Mogrosides consist of various glycosylated compounds of mogrol (aglycone). The sweetening components in the fruit extract include penta‐ tetra‐ and tri‐glucose conjugate mogrosides. The chemical structure of mogroside V, the major component of S. grosvenorii, is given in Figure 1.
Figure 1.
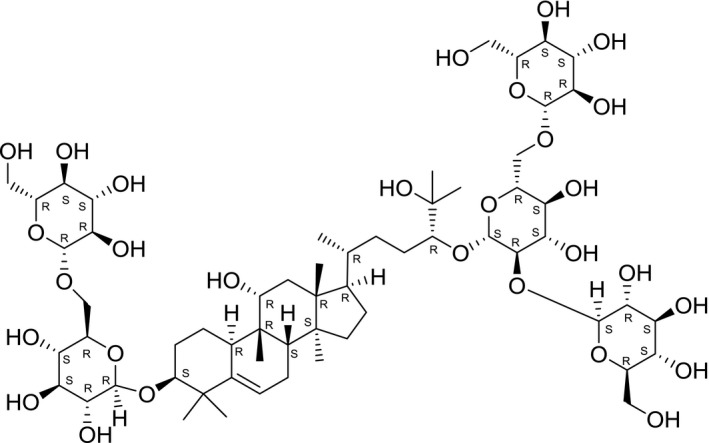
Chemical structure of mogroside V [(3β,9β,10α,11α,24R)‐3‐[(6‐O‐β‐d‐Glucopyranosyl‐β‐d‐glucopyranosyl)oxy]‐11,25‐dihydroxy‐9‐methyl‐19‐norlanost‐5‐en‐24‐yl O‐β‐d‐glucopyranosyl‐(1→2)‐O‐[β‐d‐glucopyranosyl‐(1→6)]‐β‐d‐glucopyranoside] (SciFinder,5 software)
Monk fruit extract is a mixture of naturally occurring compounds and has no Chemical Abstracts Service (CAS) Registry Number. CAS numbers do exist for individual mogrosides: mogroside V, the principal component, is CAS No 88901‐36‐4; 11‐oxo‐mogroside V CAS No 126105‐11‐1; siamenoside I CAS No 126105‐12‐2; grosmomoside I CAS No 916593‐21‐0. See Appendix A for their chemical structures.
The applicant provided information on the composition of Monk fruit extracts with contents of mogroside V of 25, 40, 45, 50 and 55%. The certificate of analysis was provided by the applicant as part of the dossier (‘Documentation provided to EFSA’ No 1, 2 and 3). The analyses were carried out on five batches of each extract of monk fruit. The information is reported in Table 1.
Table 1.
Compositions of Monk fruit extracts as provided by the applicant (Documentation provided to EFSA No. 3)
| Monk fruit extract with mogroside V 25% | Monk fruit extract with mogroside V 40% | Monk fruit extract with mogroside V 45% | Monk fruit extract with mogroside V 50% | Monk fruit extract with mogroside V 55% | |
|---|---|---|---|---|---|
| Mogroside V (%) | 25.96–26.53 | 40.95–41.95 | 46.11–46.82 | 51.03–51.81 | 55.96–56.68 |
| 11‐Oxo‐mogroside V (%) | 4.98–5.24 | 6.36–6.56 | 8.52–8.72 | 9.36–9.68 | 9.87–10.11 |
| Siamenoside I (%) | 3.09–3.61 | 1.24–4.54 | 4.47–4.69 | 0.43–4.71 | 3.24–3.79 |
| Grosmomoside I (%) | 2.73–3.21 | 3.35–3.72 | 4.72–4.91 | 4.94–5.34 | 5.57–5.85 |
| Mogroside IIIE (%) | 0.04–0.08 | 0.04–0.09 | 0.06–0.14 | 0.04–0.27 | 0.26–0.45 |
| Flavones (%) | 1.74–2.38 | 0.73–0.87 | 0.65–0.71 | 0.55–0.71 | 0.42–0.57 |
| Other saponins (%) | 12.35–13.11 | 8.20–9.43 | 7.18–7.62 | 5.14–5.64 | 4.18–4.66 |
| Other polyphenols (%) | 4.30–4.72 | 3.67–3.86 | 3.59–3.73 | 2.54–2.68 | 2.39–2.48 |
| Protein (%) | 30.98–32.95 | 23.84–24.73 | 17.17–17.54 | 13.62–14.81 | 10.97–11.63 |
| Total fat (%) | 0.82–0.92 | 0.52–0.69 | 0.49–0.59 | 0.38–0.49 | 0.22–0.39 |
| Saccharides | 3.08–3.61a | 0.67–0.88b | 0.71–0.79b | 0.63–0.70b | 0.51–0.61b |
| Dietary fibre (%) | 1.95–2.14 | 0.84–0.95 | 0.81–0.91 | 0.68–0.73 | 0.53–0.59 |
| Sodium (%) | 0.033–0.042 | 0.170–0.176 | 0.156–0.163 | 0.288–0.356 | 0.376–0.405 |
| Calcium (%) | 0.043–0.045 | 0.037–0.038 | 0.044–0.052 | 0.004–0.009 | 0.007–0.009 |
| Iron (%) | 0.0007–0.0009 | 0.0004–0.0006 | 0.0003–0.0008 | 0.0006–0.0009 | 0.0006–0.0009 |
| Potassium (%) | 0.445–0.449 | 0.015–0.017 | 0.026–0.036 | 0.028–0.047 | 0.039–0.046 |
| Loss on drying (%) | 3.47–4.21 | 3.25–0.61 | 3.15–0.47 | 3.11–3.44 | 3.16–3.38 |
Sucrose, glucose and fructose.
Glucose.
3.1.2. Specifications
The applicant provided specifications for Monk fruit extracts containing 25, 40, 45, 50 and 55% of mogroside V (‘Documentation provided to EFSA’ No. 3) (Table 2). Analytical results for five non‐consecutives batches for each extract have been provided to show that each extract complies with the proposed specifications; however not all the parameters proposed in the specification were analysed for all the batches.
Table 2.
Proposed specifications for each Monk fruit extracts as provided by the applicant (Documentation provided to EFSA No. 3)
| Monk fruit extract with mogroside V 25% | Monk fruit extract with mogroside V 40% | Monk fruit extract with mogroside V 45% | Monk fruit extract with mogroside V 50% | Monk fruit extract with mogroside V 55% | |
|---|---|---|---|---|---|
| Definition | Monk fruit extract is prepared by extraction of the fruit of Siraitia grosvenorii using a food approved solvent system then preliminary purification of the extract by employing resin adsorption purification systems to yield a primary of Siraitia grosvenorii extract. Extracts may then be decolourised and purified | ||||
| Trivial name | Mogroside V | ||||
| Chemical name | (3β,9β,10α,11α,24R)‐3‐[(6‐O‐β‐d‐glucopyranosyl‐β‐d‐glucopyranosyl)oxy]‐11,25‐dihydroxy‐9‐ methyl‐19‐norlanost‐5‐en‐24‐yl O‐β‐d‐glucopyranosyl‐(1→2)‐O‐[β‐d‐glucopyranosyl‐(1→6)]‐β‐d‐glucopyranoside | ||||
| CAS Number | 88901‐36‐4 | ||||
| EINECS | 695‐005‐3 | ||||
| Chemical formula | C60H102O29 | ||||
| Molecular weight | 1287.5 | ||||
| Description | Light yellow powder with characteristic odour and taste | Light yellow to off white powder with characteristic odour and taste | Almond white powder with characteristic odour and taste | Almond white powder with characteristic odour and taste | Off white powder with characteristic odour and taste |
| Identification/assay | |||||
| Mogroside V (%) | 25–27 | 40–42 | 45–47 | 50–52 | 55–57 |
| Loose density (g/mL) | > 0.2 | > 0.2 | > 0.2 | > 0.2 | > 0.2 |
| Tapped density (g/mL) | > 0.3 | > 0.3 | > 0.3 | > 0.3 | > 0.3 |
| Specific rotation | –25 to (–30) | –20 to (–25) | –18 to (–22) | –16 to (–20) | –14 to (–18) |
| Melting range | 198–202°C | 198–202°C | 198–202°C | 198–202°C | 198–202°C |
| Solubility in water | Freely soluble | Freely soluble | Freely soluble | Freely soluble | Freely soluble |
| Purity | |||||
| 11‐Oxo‐mogroside V (%) | 4–6 | 5–7 | 7–9 | 8–10 | 8.5–10.5 |
| Siamenoside I (%) | 2–5 | 3.5 | 3–5 | 3–5 | 2–4 |
| Grosmomoside I (%) | 2–4 | 2.4 | 3–5 | 4–6 | 4–6 |
| Mogroside IIIE (%) | 0.02–0.5 | 0.02–0.5 | 0.02–0.5 | 0.02–0.5 | 0.02–0.5 |
| Other saponin (%) | 10–15 | 5–10 | 4–8 | 3–6 | 2–5 |
| Flavone (%) | 1–3 | 0.5–1.5 | 0.4–1.0 | 0.2–0.8 | 0.1–0.6 |
| Other polyphenols (%) | < 5 | < 4 | < 4 | < 3 | < 3 |
| Protein (%) | 29–36 | 18–25 | 13–18 | 10–15 | 5–12 |
| Saccharides (%) | 3–6(a) | < 1a | < 1a | < 1a | < 1a |
| Dietary fibre (%) | < 5 | < 1.5 | < 1.5 | < 1.5 | < 1.5 |
| Total fat (%) | < 1 | < 1 | < 1 | < 1 | < 1 |
| Sodium (%) | < 0.25 | < 0.3 | < 0.4 | < 0.5 | < 0.5 |
| Calcium (%) | < 0.05 | < 0.05 | < 0.06 | < 0.03 | < 0.03 |
| Iron (%) | < 0.002 | < 0.001 | < 0.005 | < 0.005 | < 0.005 |
| Potassium (%) | < 0.5 | < 0.03 | < 0.05 | < 0.05 | < 0.05 |
| Loss of drying (%) | < 5 | < 5 | < 5 | < 5 | < 5 |
| Ash | < 5 | < 5 | < 5 | < 5 | < 5 |
| Arsenic (mg/kg) | < 1 | < 1 | < 1 | < 1 | < 1 |
| Lead (mg/kg) | < 1 | < 1 | < 1 | < 1 | < 1 |
| Cadmium (mg/kg) | < 0.1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 |
| Mercury (mg/kg) | < 0.1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 |
| Methanol (mg/kg) | < 50 | < 50 | < 50 | < 50 | < 50 |
| Ethanol (mg/kg) | < 500 | < 500 | < 500 | < 500 | < 500 |
| Pesticides | Complies to EP | Complies to EP | Complies to EP | Complies to EP | Complies to EP |
| PAH4 (μg/kg) | < 50 | < 50 | < 50 | < 50 | < 50 |
| Benzo(a) pyrene (μg/kg) | < 10 | < 10 | < 10 | < 10 | < 10 |
| Microbiological criteria | |||||
| Total aerobic plate count (cfu/g) | < 3,000 | < 3,000 | < 3,000 | < 3,000 | < 3,000 |
| Total yeast and mould (cfu/g) | < 300 | < 300 | < 300 | < 300 | < 300 |
| Bile‐tolerant Gram‐negative bacteria (cfu/g) | < 100 | < 100 | < 100 | < 100 | < 100 |
| Escherichia coli | Absence/g | Absence/g | Absence/g | Absence/g | Absence/g |
| Pseudomonas aeruginosa | Absence/g | Absence/g | Absence/g | Absence/g | Absence/g |
| Staphylococcus aereus | Absence/g | Absence/g | Absence/g | Absence/g | Absence/g |
| Salmonella | Absence/g | Absence/g | Absence/g | Absence/g | Absence/g |
| Coliforms (cfu/g) | < 10 | < 10 | < 10 | < 10 | < 10 |
| Sum aflatoxins B1, B2, G1, G2 (ppb) | < 4 | < 4 | < 4 | < 4 | < 4 |
| Aflatoxin B1 (μg/kg) | < 2 | < 2 | < 2 | < 2 | < 2 |
CAS: Chemical Abstracts Service; EINECS: European Inventory of Existing Commercial Substances; cfu: colony forming unit.
Sucrose, glucose and fructose.
Glucose.
According to the certificate of analysis (‘Documentation provided to EFSA’ No. 2), more than 95% of the particles pass through 80 mesh (177 μm). On the basis of the data provided by the applicant, the Panel has no indication that particles in the nano range are present in the commercial material.
The Panel considered that general specifications for Monk fruit extracts covering from 25% to 55% content of mogroside V could be proposed for its use as a food additive, indicating the minimum and maximum range for the specific rotation and the melting range and indicating ‘not more than’ (< x) values for the remaining parameters. The Panel noted that some of the parameters listed in Table 2 should not be necessary in order to characterise the Monk fruit extract, e.g. the content of loose density, tapped density, dietary fibre, total fat, sodium, calcium, iron potassium and loss of drying and limit for bile‐tolerant Gram‐negative bacteria. The presence of pesticides should comply with the EU regulation. Limits for PAH4 (ppb), benzo(a)pyrene and aflatoxin B1 (ppb) are included in the proposed specification. The Panel considered that these impurities need not be included in the specifications.
Regarding toxic elements, results for the analysis of arsenic, lead, cadmium and mercury were also reported (up to 0.076 mg/kg for arsenic, up to 0.058 mg/kg for lead, up to 0.0085 mg/kg for cadmium and 0.0031 mg/kg for mercury). Based on the analytical data, the Panel noted that lower levels of the maximum limits for arsenic, lead, cadmium and mercury could be considered in the proposed specifications since the results obtained in the five batches analysed were substantially lower than the proposed limits.
The Panel noted that some of the substances listed under purity in the proposed specifications correspond to components rather than impurities.
The Panel noted that the definition of monk fruit extract in the proposed specifications does not include the extraction solvent (water).
3.1.3. Manufacturing process
Monk fruit extracts are prepared by water extraction of the fruits of S. grosvenorii. The primary difference in the manufacture of the various Monk fruit extracts is associated with the purification steps resulting in different concentration of mogroside V content.
Monk fruit extract‐25% is the least refined product after water extraction, treatment with pectinase, concentration and the use of a resin column, while monk fruit extracts richer in mogroside V are obtained after additional purification steps, starting with the dissolution of the intermediate product in water, to remove impurities at the resin level via adsorption and elution steps.
The removal of saccharides in the monk fruit extracts occurs during the removal of impurities at the resin adsorption and elution steps. During the manufacturing process, Monk fruit‐25%, the concentrated liquid goes only through resin adsorption process that does not completely remove all saccharides, therefore it still contains 3–6% of saccharides, including glucose, fructose and sucrose. The manufacturing process for monk fruit extracts richer in mogroside V has a purification step through an additional resin adsorption process, as a result the saccharides content reduced to below 1%, and only containing glucose (Documentation provided to EFSA No. 3).
3.1.4. Methods of analysis in food
A description of a high‐performance liquid chromatography‐ultraviolet (HPLC‐UV) method for the determination of mogroside V in Monk fruit extracts was provided.
No information on a method of analysis of mogroside V in food was provided.
3.1.5. Stability of the substance, and reaction and fate in food
The stability of Monk fruit‐55% (content of mogroside V, appearance, taste and pH) has been investigated under accelerated conditions (37°C, relative humidity (RH) 75 ± 5%) for 3 months and until 12 months at (25°C, RH 60 ± 10%) observing a loss of mogroside V of less than 3% (Documentation provided to EFSA No. 1).
The content of mogroside V in Monk fruit‐25% stored for 36 months (25°C, RH 60 ± 10%) remain stable as well as when stored for 6 months at 40°C (RH 75 ± 5%) (Documentation provided to EFSA No. 1).
The stability of Monk fruit‐25%, Monk fruit‐40%, Monk fruit‐45%, Monk fruit‐50% (content of mogroside V, appearance, loss of drying and microbial) has been investigated under accelerated conditions (40°C, RH 75 ± 5%) for 6 months and until 36 months at (25°C, RH 60 ± 10%) observing that the content of mogroside V remains stable (Documentation provided to EFSA No. 3). The content of mogroside V is stable under different condition of pH 3–10.
The stability of mogroside V in Monk fruit‐25% was investigated for only 10 min at 100°C and for 5 min at 260°C and its concentration remained stable (Documentation provided to EFSA No. 1).
The thermal stability of Monk fruit extract (containing around 30% Mogroside V) has been investigated (changes of sweetness, absorbance, content of mogroside V and total mogroside) in a constant temperature oven (100–150°C) for 4 h and as 1% solution in boiling water (100°C) up to 8 h. The content of mogroside V remains stable in those conditions (Documentation provided to EFSA No. 3).
Mogroside V in Monk fruit‐25% is not stable at pH 1, but it is stable at pH 3, 6 and 12 for 4 weeks (stored at 2–8°C) (Documentation provided to EFSA No. 1).
The Panel noted that no stability data of monk fruit extract in food were provided; however, the stability data of monk fruit extract investigated in model systems are considered to represent scenarios to be expected in food.
3.2. Proposed uses and use levels
Through the current application, an authorisation is sought with regards to the food categories listed in Table 3.
Table 3.
Proposed uses and use levels for monk fruit extract (in mg/kg or mg/L) expressed as mogroside V (Documentation provided to EFSA No. 4 and 5)
| Food category number | Food category name | Restrictions/exception | Proposed use levels (mg mogroside V/L or mg mogroside V/kg as appropriate) |
|---|---|---|---|
| 01.4 | Flavoured fermented milk products including heat treated products | 1,000 | |
| 03 | Edible ices | 800 | |
| 04.2.2 | Fruit and vegetables in vinegar, oil or brine | 1,000 | |
| 04.2.3 | Canned or bottled fruits and vegetables | 1,000 | |
| 04.2.4.1 | Fruit and vegetable preparations, excluding compote | 1,000 | |
| 04.2.5 | Jams, jellies, and marmalades and similar products | 1,000 | |
| 05.2 | Other confectionery | 1,000 | |
| 05.3.2 | Chewing gum | Without added sugar | 10,000 |
| 06.3 | Breakfast cereals | 1,000 | |
| 09.2 | Processed fish and fishery products including molluscs and crustaceans | 600 | |
| 10.2 | Processed eggs and egg product | 1,000 | |
| 11.4 | Table‐top sweeteners | 3,200 | |
| 12.4 | Mustard | 350 | |
| 12.5 | Soups and broths | 110 | |
| 12.6 | Sauces | 350 | |
| 12.7 | Salads and savoury based sandwich spread | 350 | |
| 13.2 | Dietary foods for special medical purposes defined in EC Directive 1999/21 (excluding products from category 13.1.5) | 1,000 | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | 800 | |
| 14.1.4 | Flavoured drinks | 600 | |
| 14.2.1 | Beer and malt beverages | 600 | |
| 14.2.3 | Cider and perry | 600 | |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% alcohol | 600 | |
| 15.1 | Potato‐, cereal‐, flour‐ or starches‐based snacks | 500 | |
| 15.2 | Processed nuts | 500 | |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | 1,000 | |
| 17 | Food supplements as defined in Directive 2002/46/EC | 5,500 |
The panel noted that the applicant has submitted the information regarding 28 proposed uses and use levels for Monk fruit extract according to the food categories as presented in Annex II of Regulation (EC) No 1333/2008, part D (Table 3). The applicant provided only maximum use levels for monk fruit extract (in mg/kg or mg/L) expressed as mogroside V.
3.3. Exposure data
3.3.1. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). Consumption surveys added in the Comprehensive database in 2015 were also taken into account in this assessment.4
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the Comprehensive Database includes the currently best available source of food consumption data across Europe.
Food consumption data from infants, toddlers, children, adolescents, adults and the elderly were used for the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 4).
Table 4.
Population groups considered for the exposure estimates of monk fruit extract
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Sweden, UK |
The term ‘toddlers’ in the Comprehensive Database (EFSA, 2011a) corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in Comprehensive Database (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system was linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure assessment. In practice, the FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of monk fruit extract
The food categories for which use levels are proposed for monk fruit extract were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Monk fruit extract is requested to be added in FC 13.2 and 13.3. Food items belonging to these food categories, consumed by children, adolescents, adults and the elderly, may be very diverse and, in addition, the Comprehensive Database has only very limited information on their consumption. Therefore, eating occasions belonging to these food categories were reclassified under food categories in accordance with their main ingredient.
3.3.2. Exposure to monk fruit extract from its proposed use as a food additive
The applicant has provided an estimate of the exposure to monk fruit extract based on the output obtained using the Food Additives Intake Model (FAIM) tool (version 1) (‘Documentation provided to EFSA’ No. 1).
The Panel decided not to use the estimate exposure generated from the FAIM tool version 1 and provided by the applicant but calculated the exposure estimates by selecting foods at the most detailed level. Thus, the Panel estimated the chronic dietary exposure to monk fruit extract for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to monk fruit extract was calculated by multiplying the proposed use levels per food category (Appendix C) with their respective consumption amount per kilogram body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only 1 day per subject were excluded as they are considered as not adequate to assess repeated exposure.
The exposure was estimated in this way for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 4). Based on these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size (EFSA, 2011a). Therefore, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain was not estimated in the present assessment.
This scenario does not consider the exposure to monk fruit extract through the consumption of food supplements. As exposure via food supplements may deviate largely from the one via food, and that the number of food supplement consumers may be low depending on populations and surveys, an additional scenario was calculated in order to reflect additional exposure to food additives from food supplements. This additional exposure scenario was estimated considering the consumers only of food supplements, assumed to be exposed to the monk fruit extract present at the proposed use levels.
As FC 17 does not consider food supplements for infants and toddlers as defined in the legislation, the exposure to monk fruit extract from food supplements was not estimated for these two population groups.
Dietary exposure to monk fruit extract
Table 5 summarises the estimated exposure to monk fruit extract in six population groups (Table 4) at the maximum proposed use levels. Detailed results per population group and survey are presented in Appendix D.
Table 5.
Estimate exposure to monk fruit extract (mg/kg bw per day, expressed as mogroside V) from its proposed use as a food additive
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Proposed use level exposure assessment scenario | ||||||
| • Mean | 0.8–5.9 | 4.5–36.4 | 5.2–31.1 | 3.4–20.0 | 1.5–9.7 | 0.6–4.5 |
| • 95th percentile | 6.7–21.3 | 23.2–71.5 | 14.8–60.5 | 9.7–42.5 | 4.9–25.4 | 2.5–11.4 |
bw: body weight.
The mean exposure to monk fruit extract ranged from 0.6 mg/kg bw per day in the elderly to 36.4 mg/kg bw per day in toddlers. The 95th percentile of exposure to monk fruit extract ranged from 2.5 mg/kg bw per day in the elderly to 71.5 mg/kg bw per day in toddlers.
For the food supplements consumers only scenario, mean exposure monk fruit extract ranged from between 0.9 mg/kg bw per day in the elderly to 45.8 mg/kg bw per day in children The 95th percentile of exposure to monk fruit extract ranged from 7.0 mg/kg bw per day in adults to 55.4 mg/kg bw per day in children.
Main food categories contributing to exposure to monk fruit extract using the proposed use levels
The main contributing food categories to the total mean exposure estimates for infants were flavoured fermented milk products, processed fruits and vegetables, breakfast cereals and flavoured drinks. For toddlers, the main contributing food categories were flavoured fermented milk products and flavoured drinks. For children and adolescents, the main contributing food categories were other confectionery including breath freshening microsweets and flavoured drinks; while, for adults, the main contributing food categories were flavoured drinks and alcoholic beverages; for the elderly, they were flavoured drinks and processed fruits and vegetables (Appendix E).
Uncertainty analysis
Uncertainties in the exposure assessment of monk fruit extract have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 6.
Table 6.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Eating occasions belonging to FC 13.2 and 13.3 (reclassified under food categories in accordance with their main ingredient) | +/– |
| Methodology used to estimate high percentiles (95th) long‐term (chronic) exposure based on data from food consumption surveys covering only a few days | + |
| Correspondence of proposed use levels to the food items in the EFSA Comprehensive Database: uncertainties to which types of food the levels refer | +/– |
Concentration data:
|
+ |
Proposed use level exposure assessment scenarios:
|
+ |
FC: food category.
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Monk fruit extract is requested to be authorised in 28 different uses, corresponding to 28 different food categories. For all food categories taken into account, all foods belongings to the FC, were assumed that these will contain monk fruit extract at the maximum proposed use level. Therefore, overall, the Panel considered that the uncertainties identified resulted in an overestimation of the exposure to monk fruit extract (expressed as mogroside V) in European countries considered in the EFSA Comprehensive Database at the maximum proposed use levels.
3.4. Biological and toxicological data
3.4.1. Absorption, distribution, metabolism and excretion
3.4.1.1.
In vitro
Biotransformation of mogroside V and of mogroside III in the human intestine has been investigated in vitro.
Xu et al. (2015) incubated mogroside V under anaerobic conditions with the intestinal microbiota of a healthy Chinese man, and analyzed the biotransformation products by HPLC in tandem with an electrospray ionisation ion‐trap time‐of‐flight multistage mass spectrometer (HPLC‐ESI‐IT‐TOF‐MS). Along with unchanged mogroside V, mainly siamenoside I, mogroside IVE, mogroside IIIE, mogroside IIIA1, and mogroside IIA were detected. These are all metabolites formed by stepwise deglycosylation of the parent compound.
Additional supporting study on mogroside III was provided in the application dossier (Yang et al., 2007). Considering that mogroside III is structural similar to mogroside V, having only two sugar moieties less than mogroside V, the Panel considered read‐across approach to be used from a study on mogroside III for the assessment of absorption, distribution, metabolism, excretion (ADME) for mogroside V.
In the study by Yang et al. (2007), mogroside III, was incubated with crude enzymes of human intestinal microbiota under anaerobic conditions at 37°C. Yang et al. found that mogroside III was converted to mogroside IIA1 and mogrol (the aglycone) by successive deglycosylation at C‐3 of the glucosyl group and C‐24 of the gentiobiosyl group.
In vivo
Three in vivo studies on the metabolic fate and disposition of mogroside V in rats were provided (Documentation provided to EFSA No. 3) or identified in the scientific literature. Although differing in design and scope of analysis, in vivo studies summarised in Appendix F provide evidence of systemic bioavailability of mogroside V in rats.
Murata et al. (2010) studied the triterpenoid contents in the small intestine, portal blood, faeces and urine of Wistar rats given orally 1 mL of S. grosvenorii glycoside solution (117 mg/mL) with a mogroside V content of 72% w/w, i.e. a single dose of 65.5 μmol mogroside V. The small intestinal contents and portal blood were collected at 2 h after administration; faeces were collected at 24 h after administration, and all samples analysed by liquid chromatography coupled with mass spectrometry (LC–MS). At 2 h, mogroside V was the main component in the small intestine, followed by siamenoside I, mogroside IV and mogroside III, and low levels of other mogrosides (IIE, IE) or mogrol. In portal blood, the only metabolites found at 2 h in low levels were mogrol and mogroside IE (present as glucuronides/sulfates conjugates). In urine (sampling time not given), no triterpenoids were detected, either in free or conjugated form. Upon analysis of faeces, the excreted metabolites were mostly mogrol, mogroside IIA and mogroside IE, along with other identifiable triterpenoids, showing that mogroside V is mostly degraded by digestive enzymes and intestinal microbiota. The total amount of mogrosides and mogrol in the faeces was about 40 μmol, which corresponds to 61% of administered mogroside V dose. The remaining 39% balance of the administered dose was not accounted for in this study.
To further clarify its biotransformation process and fate in vivo, Xu et al. (2015) studied the metabolism of mogroside V (> 98% purity) in Sprague–Dawley rats that received oral doses of 50 mg/kg bw per day on three consecutive days (after an adaptation period and 2 days of collecting ‘blank’ samples). Analysis of triterpenoids in urine, blood, faeces and several organs was performed by HPLC‐ESI‐IT‐TOF‐MS. This technique allowed to identify 77 distinct metabolites, eight of which were identified by comparison with pure standards, i.e. siamenoside I, mogrosides IVE, IIIE, IIIA1, IIE, IIA2, 11‐oxomogroside IIE, and mogrol, all products of deglycosylation of mogroside V to varying degrees. The remaining metabolites were tentatively identified by mass spectrometry data as products of successive hydroxylation, dehydrogenation, methylation or isomerisation reactions of the above mentioned as well as metabolites of the aglycone mogrol with varying degrees of hydroxylation and dehydrogenation. Many of these metabolites were found in the faeces, predominantly mogrol metabolites and mogroside IIA and IE1. The pattern of analytes differs between various biospecimen: mogroside V was mainly excreted in urine, whereas its metabolites were mainly excreted in faeces. Mogroside V and mogroside IIE were found at low levels in blood plasma in this study. Also distribution in rat organs was studied: seven bioactive metabolites of mogroside V were identified, among which mogroside IIE was abundant in four organs (heart, liver, spleen and lung) collected from rats treated with mogroside V, suggesting that it may contribute to the bioactivity of mogroside V in vivo. Moreover, the 39% mass deficiency noted in the former study by Murata et al. (2010) appears to be close to the proportion of metabolites found by Xu et al. (2015) in the rat organs. After repeated dosing, mogroside V itself was also found throughout all analysed organs, in blood plasma and in urine. Thus, the study by Xu et al. (2015) indicates absorption of mogroside V and systemic bioavailability along with its extensive conversion by digestive enzymes and the intestinal microbiota of rats.
Luo et al. (2016a,b) studied the kinetics of mogroside V in male Sprague–Dawley rats that were given a single dose of either 2 mg/kg bw by i.v. or 5 mg/kg bw by oral administration in dosing solution. The authors of this study focused on the analysis of mogroside V and mogrol in blood plasma collected at different time intervals post‐dosing by an in‐house validated LC–MS/MS method. The analyte levels determined in blood plasma samples served to construct concentration‐time profiles for mogroside V and its metabolite mogrol for characterisation of kinetic parameters. Mogroside V was rapidly deglycosylated and metabolised to mogrol: after i.v. injection the elimination half‐life value was estimated to be 0.33 and 1.53 h for mogroside V and mogrol, respectively. After oral dosing, only mogrol was found in plasma (other metabolites were not determined). The oral absolute bioavailability of mogroside V was estimated to be 8.73 ± 1.46% and the elimination half‐life of mogrol was 2.46 ± 0.19 h in rat (Luo et al. 2016).
Overall, the Panel considered that mogroside V and its metabolites are absorbed to some extent and are systemic bioavailable.
3.4.2. Genotoxicity
Bacterial reverse mutation assay
Monk fruit extract with mogroside V 25% (Go‐Luo®‐25) and with mogroside V 55% (Go‐Luo®‐55) were tested for the ability to induce gene mutations in the S. Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102, in compliance with OECD guideline 471 and with the principles of Good Laboratory Practice (GLP) (Documentation provided to EFSA No. 2).
Each extract was analysed in two separate experiments, with the plate incorporation method and the pre‐incubation method, respectively. Five concentrations were tested, up to a maximum of 5,000 μg/plate, with and without metabolic activation.
No precipitation of the test item and no toxic effects and was observed in any experimental condition. No biologically relevant increases in revertant colony numbers of any of the five tester strains were observed following treatment with both monk fruit extracts at any concentration level, neither in the presence nor absence of metabolic activation. The positive controls performed as expected.
In vitro micronucleus assay in human lymphocytes
The clastogenic and aneugenic potential of Monk fruit extract‐25% and Monk fruit extract‐55% was tested in a micronucleus assay in human lymphocytes in vitro, in compliance with OECD guideline 487 and with the principles of Good Laboratory Practise (Documentation provided to EFSA No. 2).
The following concentrations were evaluated for micronuclei frequencies: Experiment I, short‐term exposure (4 h), without and with metabolic activation: 2,000, 3,000 and 5,000 μg/mL. Experiment II, long‐term exposure (44 h), only without metabolic activation: 1,000, 2,500 and 5,000 μg/mL.
No precipitate of the test item and no cytotoxic effect was noted in any concentration group.
After the short‐term exposure without metabolic activation (experiment I) a statistically significant enhancement (p = 0.0034) of cells with micronuclei was noted with Monk fruit extract‐25% at the lowest evaluated concentration of 2,000 μg/mL. However, the frequency of micronucleated cells was within the historical control limits of the negative control and the enhancement was not confirmed at higher test concentrations, therefore the finding was regarded as not biologically relevant. No statistically significant increase of cells with micronuclei was noted in any other experimental condition.
The positive control chemicals induced distinct and statistically significant increases of the micronucleus frequency, demonstrating the validity of the assay.
Overall, the Panel considered that Monk fruit extract‐25% and Monk fruit extract‐55% were negative in the bacterial reverse mutation assay performed with S. Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 both with the plate incorporation and the pre‐incubation method, in the presence and in the absence of metabolic activation. The two extracts did not induce structural and/or numerical chromosomal damage in an in vitro micronucleus assay in cultured human lymphocytes.
However, the Panel noted that the in vitro toxicity studies including study with S9 were not sufficiently informative to evaluate the genotoxic potential of the metabolites that may be generated after microbial metabolism, including the aglycone.
3.4.3. Short term and sub‐chronic toxicity
Rats
A 28‐day toxicity study in Hsd:SD rats was performed administering Monk fruit extract with 39% mogroside V (Marone et al., 2008). Animals were randomly assigned to four groups of 10 per each sex and fed 0, 10,000, 30,000 or 100,000 mg/kg of Monk fruit extract‐39% in their diet (equal to 0, 733, 2,096 and 7,071 mg/kg bw per day for males and 0, 743, 2,147 and 7,478 mg/kg bw per day for females). The study was performed according to OECD TG 407 and was in compliance with GLP principles. Significantly reduced body weights and body weight gains in both sexes in the high dose group associated with reduction in food consumption – possibly due to poor palatability of the diet – were noted during several time points. Slight, statistically significant (p < 0.05) haematological changes included the following: increase in haemoglobin and haematocrit and decrease in white blood cells and lymphocyte at high dose in males), increase in mean red blood cell haemoglobin concentration at mid dose in females and decreased prothrombin time at mid‐ and high dose in females. These effects were considered not adverse. Liver‐to‐body weight and liver‐to‐brain weight ratios were increased in males of the high‐dose group and dose dependently in all treated females (up to > 30%). Related biochemical changes were slightly reduced levels of total bilirubin and dose dependently increased values of total proteins in males and females. However, no increases in liver enzyme activities or histopathological hepatic changes were observed. The no‐observed‐adverse‐effect level (NOAEL) for Monk fruit extract‐39% was determined to be 7,478 mg/kg bw per day, the highest dose tested, corresponding to 2,916 mg mogroside V/kg bw per day.
In a 13‐week toxicity study Wistar Hannover (GALAS) rats were fed with Monk fruit extract (Jin et al. (2007); the mogroside V content in the extract was not specified. Rats were divided into 5 groups of 8 per each sex and administered a diet containing 0%, 0.04%, 0.2% 1% or 5% of Monk fruit extract (equal to 0, 20, 90, 530 and 2,520 mg/kg bw per day and 0, 30, 130, 650 and 3,200 mg/kg bw per day in males and females, respectively). There were no mortalities, changes in body weights, food and water consumption (except in females, possibly due to spillage). Some haematological parameters (increase in stab cells and monocytes in males) and clinical chemical parameters (a statistical increase in total cholesterol compared to concomitant controls and a decrease in inorganic phosphate levels in high dose females) were changed. Statistically small effects on organ weights, i.e. increases in relative liver weight in the high‐dose males and in absolute and relative pituitary gland weights in females were observed. However, no changes were found in histopathological examinations. The NOAEL was determined to be 3,200 mg Monk fruit extract/kg bw per day, the highest dose tested, however the content of mogroside V is unknown.
In a 90‐day oral GLP toxicity study rats were fed with Monk fruit extract‐52% mogroside V (Go‐Luo™) (Documentation provided to EFSA No. 6). Sprague–Dawley rats (20 animals/sex per group) for the main study groups, plus an additional 10 animals/sex per group for dietary control and high‐dose recovery groups, were fed with 0 (control), 12,500, 25,000 or 50,000 ppm Monk fruit extract‐52% (equal to 766, 1,542 or 3,124 mg/kg bw per day and 901, 1,773 or 3,752 mg/kg bw per day in males and females, respectively) in the diet. Four male rats (one from each of the four dose groups) were found dead or had to be euthanised because of accidental trauma. None of these deaths were considered to be treatment‐related because of the sporadic nature and the absence of any similar pathology in the terminal animals. Ophthalmological examination revealed no abnormalities at the end of the dosing and recovery periods. There were no statistically significant differences in body weight or food consumption during the dosing and recovery periods. Haematological results showed a slight increase in mean platelet volume at mid‐ and high‐dose groups of both sexes; slightly increased mean corpuscular haemoglobin and slightly decreased red cell distribution in the high‐dose males; and slightly increased circulating lymphocyte counts in the mid‐ and high‐dose groups females. However, all these parameters were within historical control variation. A decrease in prothrombin time was observed in treated females which was not found at the end of recovery period. A decrease in triglyceride values were measured in both sexes at all doses and of total bilirubin was measured in females at all doses. However, this latter change was minor and not observed consistently in other dietary studies.
In females, dose‐dependent increase in absolute and relative liver weights (1.2–1.3‐fold vs. controls) was observed. No changes were observed at the end of the recovery period and based on the reversible nature and the absence of histological observations; these findings in liver were considered by authors adaptive rather than adverse. The Panel agreed with this consideration.
In addition, an increase in relative adrenals weight at the high dose was observed in females. This effect was not seen in recovery group.
In males, there was an increase in relative kidneys weight at the high dose. Moreover, a decrease in testis weight (absolute and relative to brain) was observed at the high dose. These effects both on kidney and testis were not present in the recovery group.
In addition to the decrease in testes weight, an increase in tubular degeneration/atrophy at high‐dose was observed. There were no other histopathological observations in any other tissue samples or organs.
According to the authors, all changes were in the historical control ranges. Consequently, they identified a NOAEL of 3,752 mg/kg bw per day (corresponding to 1,951 mg mogroside V/kg bw per day), the highest dose tested, also based on the reversible nature and the absence of histological observations. However, the Panel considered the justification on historical control not sufficient to dismiss the observed effects on the testes and that the adversity of these effects cannot be ruled out.
Dog
In a combined 28‐ and 90‐day study toxicity study, dogs (3/sex per group) were given Monk fruit extract via gavage (Qin et al., 2006). The mogroside V content in the extract was not specified. The study was not in line with OECD test guidelines and only poorly reported. Two groups (3 animals/sex per group), LHG I (28 days) and LHG II (90 days), were given a dose of 3,000 mg/kg bw per day. There were no mortalities, clinical signs or changes in clinical chemistry parameters. During the study, there were only some minor differences in body weights. Some statistically significant changes were observed in haematology (RBC and WBC) and in urinalysis parameters (urine volume, creatinine) in either time points. The NOAEL for Monk fruit extract was determined to be 3,000 mg/kg bw per day, the only dose tested.
Overall, the Panel considered the 90‐day study in rats (Documentation provided to EFSA No. 6) with Monk fruit extract‐52% mogroside V the most relevant and robust study available because of the study design and the precise specification of the mogroside V content in the Monk fruit extract. Based on the results of this rat study, the Panel noted that the effects on the testis cannot be dismissed and the adversity of these effects cannot be ruled out.
3.4.4. Chronic toxicity and carcinogenicity
No data provided.
3.4.5. Reproductive and developmental toxicity
A reproductive and developmental screening study in Sprague–Dawley rats (n = 13/sex per group) by daily gavage with Monk fruit extract containing 30% mogroside V (w/w) was performed according to OECD TG 421 and in compliance with GLP (Documentation provided to EFSA No. 7). The parental animals were daily dosed by gavage with Monk fruit extract 0, 1,000, 2,000 and 4,000 mg/kg bw per day from 2 weeks prior to mating. Both parental males and females dosed at the 2,000 and 4,000 mg/kg bw were found to have sticky soft faeces from Day 4 onwards. There were no other remarkable differences in clinical observations between test item and vehicle control treated animals. No reproductive effects were observed in the parental male or female rat, or on F1 pups up to postnatal day 13. Treatment did not have any effect on development or on markers of sexual differentiation (sex ratio, anogenital distance, and nipple retention in males) or thyroid function in the F1 pups. The NOAEL of the study was 4,000 mg/kg bw per day (corresponding to 1,200 mg mogroside V/kg bw per day).
Monk fruit extract‐52% mogroside V (Go‐Luo™) was tested in a prenatal development toxicity study in Sprague–Dawley rats (Documentation provided to EFSA No. 8). The study was performed according the ICH Guideline for the design of embryo‐fetal toxicity studies and in compliance with GLP. Groups of mated females (n = 22) were dosed by gavage with vehicle (water), 100, 300 or 1,000 mg mogroside V/kg bw per day from gestation day (GD) 6 ‐17. A Caesarean section was performed on GD 20. No maternal or developmental effects were observed. The NOAEL of the study was 1,000 mg mogroside V/kg bw per day, the highest dose tested.
Overall, in Sprague–Dawley rats, no effects on parental, reproductive or development toxicity were observed in a reproductive and developmental screening study at 1,200 mg mogroside V/kg bw per day. The Panel noted that for male animals the time of exposure did not cover the full length of spermatogenesis. In a prenatal developmental toxicity study, no maternal and developmental toxicity was found at 1,000 mg mogroside V/kg bw per day.
3.4.6. Other information
The Panel noted that, according to the information reported in the EU Novel Food Catalogue, Siraitia was not used as a food or food ingredient before 15 May 1997 in the EU. There has been no assessment of monk fruit or monk fruit extract as a novel food or novel food ingredient.
One study investigated the effect of fresh Monk fruit extract containing 31% mogroside V on histamine‐induced nasal rubbing and skin scratching behaviour in mice. Monk fruit has been shown to inhibit the histamine‐release, possibly through inhibiting its release from mast cells (Hossen et al., 2005).
The Panel considered that potential allergenicity of Monk fruit extract cannot be ruled out taking into account the percentage of protein (up to 36%) in the extract. According to the applicant Monk fruit is widely consumed in China and Japan and no allergic reaction has been reported, however data supporting this statement was not provided.
3.4.7. Human studies
In a cross‐over design, the comparative effect of consumption of Monk fruit extract and sucrose on blood glucose level was investigated (Xu et al., 2005a). After fasting overnight, five healthy men and five healthy women aged 19–25 years consumed 200 mg/kg bw of Monk fruit extract dissolved in water. Their blood glucose levels were tested at 0, 15, 30, 60, 120 and 180 min after dosing. Three days later, the same 10 participants consumed 3,000 mg/kg bw of sucrose dissolved in water, again after an overnight fast, and blood samples were taken at the same time intervals. While ingestion of sucrose resulted in a 70% increase in blood glucose, level during the first 15 min, gradually decreasing to the starting level over 3 h, ingestion of Monk fruit concentrate had no effect on blood glucose.
In another cross‐over design, Monk fruit extract was tested to investigate the effects on blood levels of liver enzymes (Xu et al., 2005b). Six healthy males aged 19–25 years fasted overnight and then consumed 200 mg/kg bw of Monk fruit extract dissolved in water; 3 days later they consumed only water. On both days, blood samples were taken at 0, 1, 2, 3, and 6 h after administration. Five liver enzymes were analysed: alkaline phosphatase (ALP), y‐glutamyl‐transpeptidase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH). There was no statistically significant change in the blood level of any of these enzymes over time, nor any difference between enzyme levels after dosing with Monk fruit extract or with water.
Overall, studies in humans demonstrated that Monk fruit extract, following ingestion of a single 200 mg/kg bw per day dose, had no effect on blood glucose nor did it produce changes in liver enzymes (ALP, GGT, ALT, AST or LDH). No adverse effects were reported in these clinical studies. However, it should be noted that the duration of the studies is very short (single administration), the number of subjects tested was too low to draw conclusions on the safety of monk fruit extract in humans. In addition, the composition of the extract was not reported.
3.4.8. Discussion
The applicant provided specifications for Monk fruit extracts containing 25, 40, 45, 50 and 55% of mogroside V.
The Panel considered that general specifications for Monk fruit extracts covering from 25% to 55% content of mogroside V could be proposed for its use as a food additive, indicating the minimum and maximum range for the specific rotation and the melting range and indicating ‘not more than’ (< x) values for the remaining parameters. The Panel noted that some of the parameters listed in Table 2 should not be necessary in order to characterise the Monk fruit extract, e.g. the content of loose density, tapped density, dietary fibre, total fat, sodium, calcium, iron potassium and loss of drying and limit for bile‐tolerant Gram‐negative bacteria. The presence of pesticides should comply with the EU regulation. Limits for PAH4 (ppb), benzo(a)pyrene, and aflatoxin B1 (ppb) are included in the proposed specification. The Panel considered that these impurities need not be included in the specifications.
Based on the analytical data, the Panel noted that lower levels of the maximum limits for arsenic, lead, cadmium and mercury could be considered in the proposed specifications since the results obtained in the five batches analysed were substantially lower than the proposed limits.
The Panel noted that some of the substances listed under purity in the proposed specifications correspond to components rather than impurities.
The Panel noted that the definition of monk fruit extract in the proposed specifications does not include the extraction solvent (water).
The Panel evaluated three studies investigating the ADME of mogroside V in rats (Murata et al., 2010; Xu et al., 2015; Luo et al., 2016). Overall, the Panel considered that mogroside V is absorbed to some extent and is systemically bioavailable.
Monk fruit extract‐25% and Monk fruit extract‐55% were negative in the bacterial reverse mutation assay performed with S. Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 both with the plate incorporation and the pre‐incubation method, in the presence and in the absence of metabolic activation. The two extracts did not induce structural and/or numerical chromosomal damage in an in vitro micronucleus assay in cultured human lymphocytes.
However, the Panel noted that the in vitro toxicity studies including study with S9 were not sufficiently informative to evaluate the genotoxic potential of the metabolites generated after microbial metabolism, including the aglycone.
Monk fruit extract with different or not specified mogroside V concentrations has been tested in one 28‐day study and two 90‐day studies in rats. A combined 28‐ and 90‐day study in dog has been performed as well but was poorly reported and had a limited design.
Based on results from the dietary rat studies, the liver and testes were considered as the target organs of mogroside V in the 90‐day study with monk fruit extract‐52% mogroside V. The liver weight increase was considered to be reversible as this effect was not found in the recovery groups. In addition, no changes in liver enzymes activities or liver histopathology were observed. Therefore, the liver effects could be interpreted as an adaptive response and were not considered to be adverse. A decrease in testis weight (absolute and relative to brain) was observed at the high dose in the presence of histopathological findings at 1,951 mg mogroside V/kg bw per day. Therefore, Panel considered that the effects on the testis cannot be dismissed and the adversity of these effects cannot be ruled out.
In rats, no effects on parental, reproductive or development toxicity were observed in a reproductive and developmental screening study at 1,200 mg mogroside V/kg bw per day. The Panel noted that for male animals the time of exposure did not cover the full length of spermatogenesis and, therefore, considered that a longer‐term study at higher doses would be needed to clarify the effects on testes observed in the 90‐day study.
In a prenatal developmental toxicity study, no maternal and developmental toxicity was found at 1,000 mg mogroside V/kg bw per day.
According to the applicant, taking into account the toxicity and genotoxicity studies submitted and, the fact, that monk fruit extract has been consumed for its sweetness for more than a century in many places, a safety threshold can be established and no further studies for a Tier 2 evaluation is deemed necessary (Documentation provided to EFSA No. 3).
Considering the systemic availability of mogroside V, the effects observed in the rat 90‐day study and following the principles of Guidance for the submission of food additives (EFSA ANS Panel, 2012) data from chronic/carcinogenicity toxicity testing would have been warranted. Therefore, justification provided by the applicant for waiving the Tier 2 toxicity testing was considered by the Panel as inadequate to address the uncertainties in the toxicological database.
To assess the dietary exposure to monk fruit extract, the exposure was calculated based on the proposed use levels. In the general population, the exposure estimates ranged at the mean from 0.6 to 36.4 mg mogroside V/kg bw per day and at the p95 from 2.5 to 71.5 mg/kg bw per day (Table 5). The Panel noted that the estimated long‐term exposures are very likely conservative, as this scenario assumes that all foods and beverages listed under the annex II to Regulation No 1333/2008 would contain monk fruit extract at the proposed use levels.
The Panel noted that according to the Mintel's GNPD,6 monk fruit extract (LHG extract) is labelled on a carbonated soft drink commercialised in the EU.
Based on the available toxicity database, the Panel could not establish a health based guidance value and, therefore, it was not possible to conclude on the safety of monk fruit extract as a food additive.
4. Conclusions
The Panel concluded that toxicity database on Monk fruit extract is insufficient to conclude on the safety of the use of monk fruit extract as a food additive.
Documentation provided to EFSA
Dossier ‘Mogroside V in Monk Fruit (Siraitia grosvenorii) juice powder extract (Go‐Luo™), standardized’. Submitted by Layn Europe SRL. First submission on 17th May 2017. Second submission on 28 September 2017.
Additional information on 27th September 2018. Submitted by Layn Natural Ingredients Corp in response to a request from EFSA.
Additional information on 5th June 2019. Submitted by Layn Natural Ingredients Corp in response to a request from EFSA.
Additional information on 6th August 2019. Submitted by Layn Natural Ingredients Corp in response to a request from EFSA.
Additional information on 19th September 2019. Submitted by Layn Natural Ingredients Corp in response to a request from EFSA.
Huntingdon Life Science Study No. 08‐2085. GO‐LUO™: A 90 DAY ORAL (DIETARY) TOXICITY STUDY IN RATS WITH A 28‐DAY RECOVERY PERIOD. Included in the first submission on 17th May 2017.
ICP Firefly Pty. Ltd. Study No. ICPQN1378. Reproductive/developmental toxicity screening test (based on OECD 421) of Luo Han Guo Glucoside 30 in Sprague Dawley rats. Additional information submitted on 5th June 2019.
Huntingdon Life Science Study No. 08‐4341. GO‐LUO™: Embryo‐fetal toxicity study in rats. Included in the first submission on 17th May 2017.
Abbreviations
- ADME
absorption, distribution, metabolism, excretion
- ANS
EFSA Panel on Food Additives and Nutrient Sources added to Food
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- bw
body weight
- CAS
Chemical Abstracts Service
- CFU
colony forming unit
- FAF
EFSA Panel on Food Additives and Flavourings
- FAIM
Food Additives Intake Model
- FAO
Food and Agriculture Organisation
- FCC
Food Chemical Codex
- FCS
food categorisation system
- GD
gestation day
- GGT
y‐glutamyl‐transpeptidase
- GNPD
Global New Products Database
- HPLC
high‐performance liquid chromatography
- ICH
International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC–MS
liquid chromatography–mass spectrometry
- LDH
lactate dehydrogenase
- LHG
Luo han guo
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PAH4
the sum of benzo[a]pyrene, chrysene, benz[a]anthracene and benzo[b]fluoranthene
- QS
quantum satis
- RBC
red blood cell
- RH
relative humidity
- USP
United States Pharmacopoeia
- UV
ultraviolet
- WBC
white blood cell
- WHO
World Health Organization
Appendix A – Chemical structure for minor mogrosides in Monk fruit extracts
1.
Figure A.1.
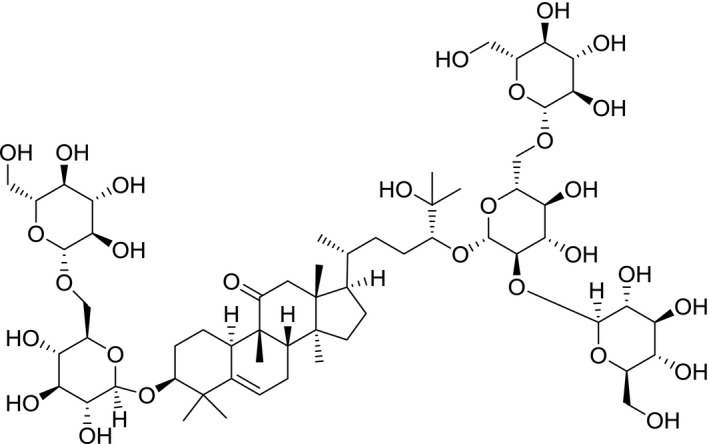
Chemical structure of 11‐oxo‐mogroside V (SciFinder4, software)
Figure A.2.
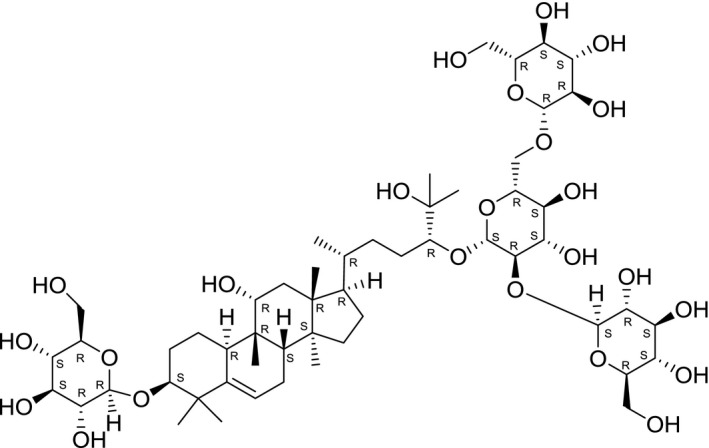
Chemical structure of siamenoside I (SciFinder, software)
Figure A.3.
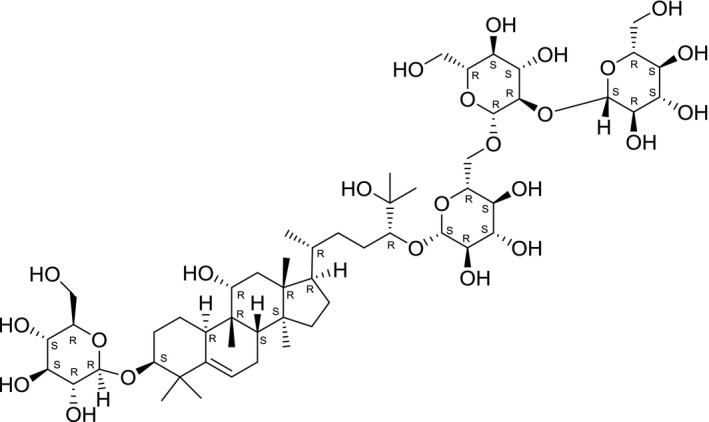
Chemical structure of grosmomiside I (SciFinder, software)
Appendix B – Proposed use levels as proposed by the applicant
Appendix C – Proposed use levels of monk fruit extract used in the refined exposure scenarios (mg Mogroside V/L or mg Mogroside V/kg as appropriate)
Appendix D – Summary of total estimated exposure to monk fruit extract using the proposed use level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix E – Main food categories contributing to exposure to monk fruit extract using the proposed level exposure assessment scenario (> 5% to the total mean exposure)
1.
Appendixes B–E can be found in the online version of this output (‘Supporting information’ section).
Appendix F – Studies on the fate and biotransformation of mogroside V in vivo and in vitro
1.
| Study type | Species | Substance/purity | Dosing | Samples analysed | Analysis | Results | Conclusions | |
|---|---|---|---|---|---|---|---|---|
| Murata et al. (2010) | In vivo | Wistar rat | SG‐Gly powder with a MV content of 72% | Single oral dose of SG‐gly solution (117 mg/mL) with 65.5 μmol MV (about 300 mg/kg bw) | Small intestine (at 2 h), portal blood (at 2 h), faeces (at 24 h), and urine (?) |
LC–MS/MS for MV, siamenoside I, M‐IV, III, IIE, IIA, IE and MO In blood and urine after enzymatic hydrolysis of glucuronides/sulfates |
In small intestine: MV as major form, also metabolites present In portal blood: traces of M‐IE and MO; no MV detected In faeces: mostly MO, M‐IIA and IE; also other metabolites and some MV present In urine: triterpenoids not detected (no data shown) |
Total amount of analytes in faeces is about 40 μmol which corresponds to 61% of administered MV (65.5 μmol) As only traces or no SG‐triterpenoids were detected in blood or urine, the absorbed amount of SG‐gly and its metabolites was very low; the ingested M‐V is partly converted by intestinal bacterial enzymes and excreted without being absorbed |
| Xu et al. (2015) | In vivo | Sprague–Dawley rats | M‐V (> 98%) |
Oral in solution MV 50 mg/kg bw per day (on 3 days, after earlier collection of blank biosample) |
Faeces and urine collected on 3 days; then 1 h after last MV administration blood collected heart, liver, spleen, lungs, kidneys, stomach, small intestine |
HPLC‐ESI‐IT‐TOF‐MSn for MV and in toto 77 metabolites; eight identified with standards; others were tentatively identified by MS data; quantified by peak areas |
In plasma: M‐V and IIE and MO found in low levels In urine: mainly M‐V, also several of its metabolites In faeces: high levels of M‐IIA and IE1 plus MO metabolites In organs: M‐V found at low levels in all; most abundant metabolite is M‐IIE, in liver > heart > spleen> intestine > kidney > lung |
The pattern of analytes differs between various biospecimen: M‐V was mainly excreted in urine, whereas its metabolites were mainly excreted in faeces Many M‐V metabolites that were not detected in plasma were detected in various organs The study results indicate absorption of MV and systemic bioavailability along with its extensive conversion by digestive enzymes and the intestinal microflora of rats |
| Luo et al. (2016a,b) | In vivo | SD rats, male | M‐V (> 98.5%) | Single dose of 2.0 mg/kg bw by i.v. injection or 5.0 mg/kg bw by oral dosing | Blood sampling at various time points after i.v. or oral dosing (up to 6 h) | LC–MS/MS analysis of M‐V and MO (no other products monitored) | Concentration‐time profiles for M‐V and MO in plasma after i.v. and oral dosing; kinetic parameters: t1/2, Cmax, AUC, clearance, and others; oral bioavailability | After i.v. injection elimination half‐life was 0.33 and 1.53 h for M‐V and MO, respectively. After oral dosing, only MO was found in plasma. The oral absolute bioavailability of M‐V was estimated to be 8.73 ± 1.46% and the elimination half‐life of MO was 2.46 ± 0.19 h in rat |
M‐V: mogroside V; M‐IV: mogroside IV; M‐III: mogroside III; M‐IIE: mogroside IIE; M‐IIA: mogroside IIA; M‐IA: mogroside IA; MIE: mogroside IE; MO: mogrol; SG: Saraitia grosvenori Swingle; SG‐Gly: Saraitia grosvenori Swingle‐glycoside; LC–MS: liquid chromatography–mass spectrometry; HPLC: high‐performance liquid chromatography; HPLC‐ESI‐IT‐TOF‐MSn: HPLC in tandem with an electrospray ionisation ion‐trap time‐of‐flight multistage mass spectrometry.
Supporting information
Proposed use levels as proposed by the applicant
Proposed use levels of monk fruit extract used in the refined exposure scenarios (mg Mogroside V/L or mg Mogroside V/kg as appropriate)
Summary of total estimated exposure to monk fruit extract using the proposed use level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to monk fruit extract using the proposed level exposure assessment scenario (> 5% to the total mean exposure)
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Gundert‐Remy U, Husøy T, Mennes W, Moldeus P, Oskarsson A, Shah R, Waalkens‐Berendsen I, Wölfle D, Degen G, Herman L, Gott D, Leblanc J‐C, Giarola A, Rincon AM, Tard A and Castle L, 2019. Scientific Opinion on the safety of use of Monk fruit extract as a food additive in different food categories. EFSA Journal 2019;17(12):5921, 26 pp. 10.2903/j.efsa.2019.5921
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00527
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus, Agneta Oskarsson, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle and Maged Youne.
Note: The full opinion will be published in accordance with Article 12 of Regulation (EC) No 1331/2008 once decision on confidentiality will be received from the European Commission.
Acknowledgements: The FAF Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 13 November 2019
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December establishing a common authorisation procedure for food additives, food eznymes and food flavourings. OJ L 354, 31.12.2008.
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
SciFinder® the choice for chemistry research™.
Access on 17/10/2019.
References
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009a. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009; 7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009b. Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements, on request of EFSA. EFSA Journal 2009;7(9):1249, 19 pp. 10.2093/j.efsa.2009.1249 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- FSANZ (Food Standards Australia New Zealand), 2018. Approval report – Application A1129 Monk Fruit Extract1 as a Food Additive. Available online: http://www.foodstandards.gov.au/code/applications/Documents/A1129%20Approval%20report.pdf
- Hossen MA, Shinmei Y, Jiang S, Takubo M, Tsumuro T, Murata Y, Sugiura M and Kamei C, 2005. Effect of Lo Han Kuo (Siraitia grosvenori Swingle)on nasal rubbing and scratching behavior in ICR mice. Biological and Pharmaceutical Bulletin, 28, 238–241. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016. Eightieth meeting of the Joint FAO/WHO Expert Committee on Food Additives. Available online: https://apps.who.int/iris/bitstream/handle/10665/204410/9789240695405_eng.pdf;jsessionid=1911133043BB43074D35D581696132C5?sequence=1
- Jin M, Muguruma M, Moto M, Okamura M, Kashida Y and Mitsumori K, 2007. Thirteen‐week repeated dose toxicity of Siraitia grosvenori extract in Wistar Hannover (GALAS) rats. Food and Chemical Toxicology, 45, 1231–1237. [DOI] [PubMed] [Google Scholar]
- Li C, Lin LM, Sui F, Wang ZM, Huo HR, Dai L and Jiang TL, 2014. Chemistry and pharmacology of Siraitia grosvenorii: a review. Chinese Journal of Natural Medicines, 12, 89–102. [DOI] [PubMed] [Google Scholar]
- Luo Z, Shi H, Zhang K, Qin X, Guo Y and Ma X, 2016a. Liquid chromatography with tandem mass spectrometry method forthe simultaneous determination of multiple sweet mogrosides in the fruits of Siraitia grosvenorii and its marketed sweeteners. Journal of Separation Science, 39, 4124–4135. 10.1002/jssc.201600563 [DOI] [PubMed] [Google Scholar]
- Luo Z, Qiu F, Zhang K, Qin X, Guo Y, Shi H, Zhang L, Zhang Z and Ma X, 2016b. In vitro AMPK activating effect and in vivo pharmacokinetics of mogroside V, a cucurbitane‐type triterpenoid from Siraitia grosvenorii fruits. RSC Advances, 6, 7034–7041. [Google Scholar]
- Marone PA, Borzelleca JF, Merkel D, Heimbach JT and Kennepohl E, 2008. Twenty eight‐day dietary toxicity study of Luo Han fruit concentrate in Hsd:SD rats. Food and Chemical Toxicology, 46, 910–919. [DOI] [PubMed] [Google Scholar]
- Murata Y, Ogawa T, Suzuki YA, Yoshikawa S, Inui H, Sugiura M and Nakano Y, 2010. Digestion and absorption of Siraitia grosvenori triterpenoids in the rat. Bioscience, Biotechnology, and Biochemistry, 74, 673–676. [DOI] [PubMed] [Google Scholar]
- Qin X, Xiaojian S, Ronggan L, Yuxian W, Zhunian T, Shouji G and Heimbach J, 2006. Subchronic 90‐day oral (Gavage) toxicity study of a Luo Han Guo mogroside extract in dogs. Food and Chemical Toxicology, 44, 2106–2109. [DOI] [PubMed] [Google Scholar]
- The Plant List , online. Retrieved from http://www.theplantlist.org/tpl1.1/record/kew-2489947?ref=tpl1
- Xu Q, Liang R and Li L, 2005a. Experiment report: effect of luo han guo mogroside on human's blood sugar content. Unpublished report, August 19.
- Xu Q, Liang R and Li L, 2005b. Experiment report: effect of PureLo luo han guo mogroside on liver enzymes of human. Unpublished report, September 6.
- Xu F, Li DP, Huang ZC, Lu FL, Wang L, Huang YL, Wang RF, Liu GX, Shang MY and Cai SQ, 2015. Exploring in vitro, in vivo metabolism of mogroside V and distribution of its metabolites in rats by HPLC‐ESI‐IT‐TOF‐MS(n). Journal of Pharmaceutical and Biomedical Analysis, 115, 418–430. 10.1016/j.jpba.2015.07.024 [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang J and Xu W, 2007. Biotransformation of mogroside III by human intestinal bacteria. Journal of Peking University (Health Sciences), 39, 657–662. (abstract in English, article in Chinese). [PubMed] [Google Scholar]
- Zhang H, Yang H, Zhang M, Wang Y, Wang J, Yau L, Jiang Z and Hu P, 2012. Identification of flavonol and triterpene glycosides in Luo‐Han‐Guo extract using ultra‐high performance liquid chromatography/quadrupole time‐of‐flight mass spectrometry. Journal of Food Composition and Analysis, 25, 142–148. [Google Scholar]
- Zheng Y, Liu Z, Ebersole J and Huang CB, 2009. A new antibacterial compound from Luo Han Kuo fruit extract (Siraitia grosvenori). Journal of Asian natural products research, 11, 761–765. [DOI] [PubMed] [Google Scholar]
- Zhou G, Wang M, Li Y, Xu R and Li X, 2016. Comprehensive analysis of 61 characteristic constituents from Siraitiae fructus using ultrahigh‐pressure liquid chromatography with time‐of‐flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 125, 1–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proposed use levels as proposed by the applicant
Proposed use levels of monk fruit extract used in the refined exposure scenarios (mg Mogroside V/L or mg Mogroside V/kg as appropriate)
Summary of total estimated exposure to monk fruit extract using the proposed use level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to monk fruit extract using the proposed level exposure assessment scenario (> 5% to the total mean exposure)


