Abstract
The Panel on Food Additives and Flavourings (FAF) provided a scientific opinion re‐evaluating the safety of benzyl alcohol (E 1519) when used as a food additive. The Panel considered that adequate exposure and toxicity data were available. Benzyl alcohol (E 1519) is authorised as a food additive in the EU in accordance with Annex III to Regulation (EC) No 1333/2008. The Panel considered benzyl alcohol of low acute toxicity with no concern with respect to genotoxicity and carcinogenicity and established an acceptable daily intake (ADI) of 4 mg/kg body weight (bw) per day based on a no observable adverse effect level (NOAEL) of 400 mg/kg bw per day from the carcinogenicity study in rats. The mean and high exposure estimates in the refined exposure scenarios were maximally 0.27 and 0.81 mg/kg bw per day in toddlers, respectively. The exposure estimates to benzyl alcohol (E 1519) were below the ADI of 4 mg/kg bw per day in all population groups. The Panel noted that also the exposure in the regulatory maximum level exposure assessment scenario is below the ADI in all population groups. The Panel concluded that the exposure to benzyl alcohol (E 1519) does not raise a safety concern at the reported uses and use levels.
Keywords: benzyl alcohol, E 1519, food additive, safety, risk assessment, dietary exposure, acceptable daily intake
Summary
The present opinion deals with the re‐evaluation of benzyl alcohol (E 1519) when used as a food additive.
Benzyl alcohol (E 1519) is authorised as a food additive in the European Union (EU) in accordance with Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in Commission Regulation (EU) No 231/2012.
Benzyl alcohol was evaluated by JECFA in 1980 (JECFA, 1980) and concluded a group acceptable daily intake (ADI) (for benzoic acid together with benzyl alcohol) of 0–5 mg/kg body weight (bw), subsequently agreed by the Scientific Committee for Food (SCF) in 2002.
Benzyl alcohol is also included in the EU Union list (Commission Implementing Regulation (EU) No 872/2012) of authorised food flavourings substance with FL‐no: 02.010, following its evaluation by the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) in 2008 in flavouring group evaluation 54 (FGE.54Rev1). In 2012, the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) prepared a scientific opinion on safety and efficacy of benzyl alcohols (including benzyl alcohol) when used as flavouring in all animal species (EFSA FEEDAP Panel, 2012).
The European Medicines Agency (EMA) has registered the use of benzyl alcohol as an excipient in medical products.
In humans and animal species, benzyl alcohol is metabolised to benzoic acid which reacts with glycine in the liver and the resulting hippuric acid is excreted quickly in urine.
The Panel noted that benzyl alcohol was of low acute toxicity.
Oral short‐term or subchronic studies with benzyl alcohol showed neurotoxicity manifested as lethargy in mice (from 500 mg/kg per day) and lethargy or staggering (from 800 mg/kg bw per day) in rats.
Benzyl alcohol was tested with negative results in the base set of genotoxicity tests and the Panel concluded that benzyl alcohol does not raise safety concern with respect to genotoxicity.
Benzyl alcohol is not carcinogenic in mice and rats up to 200 and 400 mg/kg bw per day, respectively, which were highest doses tested. The no observable adverse effect level (NOAEL) in the carcinogenicity study in rats was 400 mg/kg bw per day.
The Panel noted that reproductive studies were not available and that two developmental screening studies in mice were of limited design.
Considering the available data set, the Panel established an ADI of 4 mg benzyl alcohol/kg bw per day based on a NOAEL of 400 mg/kg bw per day from the carcinogenicity study in rats, based on no effect in the highest dose tested.
The dietary exposure to benzyl alcohol (E 1519) from its use as a food additive was calculated based on (1) maximum permitted levels (MPLs) set out in the EU legislation (defined as the regulatory maximum level exposure assessment scenario) and (2) the reported use levels (defined as the refined exposure assessment scenario).
The mean and high exposure estimates in the refined exposure scenarios were maximally 0.27 and 0.81 mg/kg bw per day in toddlers, respectively. Considering the food categories for which the presence of benzyl alcohol (E 1519) can be due to carry over via its authorisation according to Annex III to Reg (EC) No 1333/2008, the exposure estimates to benzyl alcohol (E 1519) were below the ADI of 4 mg/kg bw per day in all population groups. The Panel noted that also the exposure in the regulatory maximum level exposure assessment scenario is below the ADI in all population groups.
In conclusion, based on the toxicological database available, the Panel established an ADI of 4 mg/kg bw per day for benzyl alcohol (E 1519) and concluded that the exposure to benzyl alcohol (E 1519) does not raise a safety concern at the reported uses and use levels.
The Panel recommend that the European Commission considers introducing the maximum levels of chlorinated organic compounds as impurities in the specifications for purity of E 1519 in EU Regulation 231/2012.
1. Introduction
1.1.
The present opinion deals with the re‐evaluation of benzyl alcohol (E 1519) when used as a food additive.
1.2. Background and Terms of Reference as provided by the European Commission
1.2.1. Background
Regulation (EC) No 1333/20081 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/2010.2 This Regulation also foresees that food additives are re‐evaluated whenever necessary in the light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report “Food additives in Europe 20004” submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.2.2. Terms of Reference
The Commission asks the European Food Safety Authority to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.3. Information on existing authorisations and evaluations
Benzyl alcohol (E 1519) is authorised as a food additive in the EU in accordance with Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20125.
In the EU, Benzyl alcohol (E 1519) has been evaluated by the Scientific Committee for Food (SCF) in 2002 (SCF, 2002) who concluded that ‘the metabolism of benzyl alcohol to benzoic acid by man is well established. In special studies, biochemical effects have been investigated. The toxicity has been studied extensively, including acute, short‐term and long‐term toxicity, carcinogenicity, genotoxicity and developmental toxicity’. The Committee considers that the studies on carcinogenicity in rats and mice did not show compound‐related adverse effects at dose levels up to 200 mg/kg body weight (bw) in the mouse and up to 400 mg/kg in the rat, in both cases the highest dose levels tested. Data from subchronic studies on rats and/or mice show no observable adverse effect levels (NOAELs) of 400 mg/kg bw or more. Taking into account the toxicity data and the fact that benzyl alcohol is metabolised via benzaldehyde to benzoic acid, the Committee confirms the inclusion of benzyl alcohol in the group acceptable daily intake (ADI) of 0–5 mg/kg bw for benzoic acid and benzoates, as agreed in the SCF opinion of 1981. It should be noted that the total intake of benzyl alcohol and benzoic acid can result from different sources including the use of additives and flavourings as well as the natural occurrence in food. It is therefore possible that in some instances the intake of these substances may exceed the group ADI. Better data are required on use/residual levels following use of benzyl alcohol as carrier solvent in different food categories against the background of overall exposure in order to facilitate a more precise intake assessment.
Benzyl alcohol was evaluated by JECFA in 1980 (JECFA, 1980) and concluded a group ADI (for benzoic acid together with benzyl alcohol) of 0–5 mg/kg bw. In 1997, JECFA re‐evaluated benzyl alcohol. JECFA concluded, based on long‐term studies in rats and mice, that it is not carcinogenic. It was not mutagenic in the Ames test. A four‐generation study in rats showed no effect on growth, fertility, lactation or survival. A group ADI of 0–5 mg per kg of body weight expressed as benzoic acid was confirmed.
In 2016, the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) delivered a scientific opinion re‐evaluating benzoic acid (E 210), sodium benzoate (E 211), potassium benzoate (E 212) and calcium benzoate (E 213) when used as food additives and derived a group ADI of 5 mg/kg bw per day (expressed as benzoic acid) in accordance with the ADI previously derived by SCF and JECFA.
Benzyl alcohol is also included in the EU Union list (EU Reg 872/2012) of authorised food flavourings substance with FL‐no: 02.010. Following its evaluation by the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) in 2009 in flavouring group evaluation 54 (FGE.54Rev1), the Panel considered that benzyl alcohol was expected to be metabolised into innocuous products (EFSA CEF Panel, 2009). In 2012, the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) prepared a scientific opinion on safety and efficacy of benzyl alcohols (including benzyl alcohol) when used as flavouring in all animal species (EFSA FEEDAP Panel, 2012).
European Medicines Agency (EMA) has registered the use of benzyl alcohol as an excipient in medical products (EMA, 2017).
Benzyl alcohol is registered under the REACH Regulation 1907/20066 (ECHA, online).
Benzyl alcohol (PM Ref. 13150) is included in the Union list of authorised substances that may be intentionally used in the manufacture of plastic layers in plastic materials and articles (Annex I to Commission Regulation (EU) No 10/20117). Furthermore, benzyl alcohol is permitted as an antioxidant in cosmetic products (European Commission database‐CosIng8). Benzyl alcohol is included in the European Union Register9 of feed additives as a flavouring substance in feeding stuffs (Regulation (EC) No 1831/200310).
2. Data and methodologies
2.1. Data
The Panel on Food Additives and Flavourings (FAF) was not provided with a newly submitted dossier. The Panel based its assessment on information submitted to EFSA following the public call for data,11 information from previous evaluations and additional available literature up to 17 May 2019. Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based; however, these were not always available to the Panel.
Food consumption data used to estimate the dietary exposure to benzyl alcohol (E 1519) were derived from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database12).
The Mintel's Global New Products Database (GNPD) was checked to identify the uses of benzyl alcohol (E 1519) in food and beverage products and food supplements. The Mintel's GNPD is an online database that contains the compulsory ingredient information present on the label of numerous products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The FAF Panel assessed the safety of benzyl alcohol (E 1519) as food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the Scientific Committee on Food (SCF, 2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012a,b,c) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases the daily intake is expressed as equivalent.
Dietary exposure to benzyl alcohol (E 1519) from its use as a food additive was estimated combining food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum levels according to Annex III to Regulation (EC) No 1333/2008.13 Different scenarios were used to calculate exposure (see Section 3.4.1). Uncertainties on the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
Benzyl alcohol (E 1519)
Benzyl alcohol (E 1519) has the chemical structure shown in Figure 1, the chemical formula C7H8O and a molecular weight of 108.14 g/mol. The substance is described as a colourless, clear liquid with a faint, aromatic odour and is characterised by the following physico‐chemical properties: soluble in water, ethanol and ether; refractive index ([n]D 20), 1.538–1.541; specific gravity (25°C/25°C), 1.042–1.047; distillation range, not less than 95% v/v distils between 202 and 208°C. Synonyms: phenylcarbinol; phenylmethyl alcohol; benzenemethanol; alfa‐hydroxytoluene.
Figure 1.
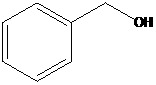
Structure of benzyl alcohol (E 1519)
3.1.2. Specifications
The specifications for benzyl alcohol (E 1519) as defined in the Commission Regulation (EU) No 231/2012 and by JECFA (2009) are listed in Table 1.
Table 1.
Specifications for benzyl alcohol (E 1519) according to Commission Regulation (EU) No 231/2012 and JECFA (2009)
| Commission Regulation (EU) No 231/2012 | JECFA (2009) | |
|---|---|---|
| Synonyms | Phenylcarbinol; phenylmethyl alcohol; benzenemethanol; alfa‐hydroxytoluene | Phenylcarbinol; phenylmethyl alcohol; benzenemethanol; alfa‐hydroxytoluene; INS No 1519 |
| Definition | EINECS (EC) No: — | CAS No: 100‐51‐6 |
| Chemical names: benzyl alcohol; phenylmethanol | Chemical names: benzyl alcohol; phenylmethanol | |
| Chemical formula: C7H8O | Chemical formula: C7H8O | |
| — | Structural formula: see Figure 1 | |
| Molecular weight (g/mol): 108.14 | Formula weight (g/mol): 108.14 | |
| Assay: not less than 98.0% | Assay: not less than 98.0% | |
| Description | Colourless, clear liquid with a faint, aromatic odour | Colourless, clear liquid with a faint, aromatic odour |
| Functional uses | — | Flavouring agent, carrier |
| Identification | Solubility: soluble in water, ethanol and ether | Solubility: soluble in water, ethanol and ether |
| Refractive index [n]D 20: 1.538–1.541 | Refractive index [n]D 20: 1.538–1.541 | |
| Specific gravity (25°C/25°C): 1.042–1.047 | Specific gravity (25°C/25°C): 1.042–1.047 | |
| Test for peroxides: passes testc | Test for peroxides: passes testa,c | |
| Distillation range: not less than 95% v/v distils between 202 and 208°C | Distillation range: not less than 95% v/v distils between 202 and 208°Ca | |
| — | Infrared absorption: passes testb | |
| Purity | Acid value: not more than 0.5 | Acid value: not more than 0.5 |
| Aldehydes: not more than 0.2 % v/v (as benzaldehyde) | Aldehydes: not more than 0.2 % v/v (as benzaldehyde) | |
| Lead: not more than 2 mg/kg | Lead: not more than 2 mg/kg | |
| — | Chlorinated organic compounds: passes testc |
In the JECFA data sheet, these specifications come under ‘Purity’.
A reference infrared spectrum is reported in the JECFA data sheet.
The specific test is directly available from the JECFA data sheet.
As food additive, benzyl alcohol must have a purity equal to or greater than 98.0%, an acid value not higher that 0.5, a content of aldehydes and lead not greater than 0.2% v/v (as benzaldehyde) and 2 mg/kg, respectively, and a very low level of peroxides. In the regulation, no reference to the present of chlorinated organic impurities is reported.
The Panel also noted that the EINECS (EC) identifier is missing in the Commission Regulation (EU) No 231/2012. The EC No 202‐859‐9 is allocated to benzyl alcohol (EC inventory, online) that corresponds to the CAS No 100‐51‐6 (SciFinder, software).
3.1.3. Manufacturing process
Large‐scale production of benzyl alcohol is achieved by the reaction of sodium or potassium carbonate with benzyl chloride (Nair, 2001). Benzyl chloride is in general manufactured by the thermal or photochemical chlorination of toluene at 65–100°C (Seper, 2004), lower temperatures favouring the increase of ring‐chlorinated by‐products. As the chlorination reaction is sensitive to metallic impurities, the presence of the latter is avoided; the crude benzyl chloride is purged of dissolved hydrogen chloride, neutralised with alkali and distilled.
3.1.4. Methods of analysis in food
Methods to detect benzyl alcohol in food and beverages at the μg/L or μg/kg level are available. There is a vast number of scientific publications dealing with the detection of benzyl alcohol and other volatile organic compounds (VOCs) in various food matrices, wines, spirits and beverages (Antón‐Díaz et al., 2016; Balcerek et al., 2017; Bellincontro et al., 2016; Cacho et al., 2013; Yang et al., 2012, Ganss et al., 2011; Rodríguez‐Bencomo et al., 2011; Villanova and Martínez, 2007). The methods, often reaching limits of detection (LODs) in the μg/L or μg/kg range, make inter alia use of liquid–liquid extraction (LLE), solid‐phase extraction (SPE), solid‐phase micro‐extraction (SPME), dynamic headspace (DHS), headspace solid‐phase micro‐extraction (HS‐SPME), ultrasonic solvent extraction (USE), hydrodistillation (HD), CO2‐based supercritical fluid extraction (SFE) and micro simultaneous steam distillation‐solvent extraction (MSDE) (Kuś and Jerković, 2018). The analysis is normally carried out by high‐resolution gas chromatography equipped with a flame ionisation detector (GC‐FID) or coupled to a mass spectrometer (GC‐MS). An array of well‐established tools is available to corroborate qualitative and/or quantitative results. These tools include the use of isotope‐labelled or other internal standards (IS). The extraction solvents generally used are common volatile compounds (e.g. pentane, dichloromethane, diethyl ether, ethyl acetate or mixtures thereof). The utilisation of Freon 11 (trichlorofluoromethane) is also reported (Forcén et al., 1992).
Detection of benzyl alcohol frequently concerns its volatile unbound form only, although a quite large number of publications also deal with the assessment of the total amount of benzyl alcohol present as both unbound and glycoside‐ and/or phosphate‐bound forms. The latter are subject to enzymatic hydrolysis (Garcia et al., 2011; Osorio et al., 2002; Ruiz‐García et al., 2014; Vilanova et al., 2012) or chemical hydrolysis (Rodríguez‐Bencomo et al., 2011) to obtain the free alcohol.
3.1.5. Stability of the substance, and reaction and fate in food
In the work of Sudareva and Chubarova (2006), aqueous solutions of benzyl alcohol – deprived of trace metal ions – were used to investigate the oxidative reaction
Chemical Formula:
as function of: storage time (up to 189 days), in darkness or light; the initial concentration of the chemical; water purified with two different procedures; solutions saturated or not with argon. As the work was not designed to investigate the kinetics of benzyl alcohol reactions, no quantitative relationships to predict solution composition changes with time were provided.
Solutions contained from 0.005 to 2.09 mg benzyl alcohol/mL; the presence of benzyl alcohol, benzaldehyde and benzoic acid in these solutions was tested by liquid chromatography (LC) and spectrophotometry. The authors concluded that, at room temperature, benzyl alcohol underwent oxidation even when its aqueous solutions were stored in darkness and under careful conditions to prevent exposure to air oxygen, i.e. saturated with argon in sealed‐off ampoules.
As reported in the preceding Section 3.1.4, benzyl alcohol can be found in food matrices, wines and beverages in the free form as well as in the bound form (glycoside‐ and/or phosphate‐bound), which can represent quite a large fraction of the total amount of the benzyl alcohol present.
A moderate antioxidant activity was assigned to benzyl alcohol (Lee and Shibamoto, 2000, 2001aa,b).
3.2. Authorised uses and use levels
Benzyl alcohol (E 1519) is authorised as a food additive in food flavourings according to Annex III, Part 4 to Regulation (EC) No 1333/200814, as amended, with a maximum level of 100 mg/L in final food of liqueurs, aromatised wines, aromatised wine‐based drinks and aromatised wine‐products cocktails, and with a maximum level of 250 mg/kg in confectionery, including chocolate and fine bakery wares (from all sources in foodstuffs as consumed or as reconstituted according to instruction of the manufacturer).
Benzyl alcohol (E 1519) is not authorised to be added directly to food according to Annex II to Regulation (EC) No 1333/2008 on food additives.
According to Annex I of EU Regulation 1334/2008 (i.e. the EU Union List of flavouring substances Commission Implementing Regulation (EU) 872/2012), benzyl alcohol is authorised as a chemically defined flavourings substance (FL‐no: 02.010) without restrictions.
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of benzyl alcohol (E 1519)
Most food additives in the EU are authorised at a specific maximum permitted level (MPL). However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call15 for occurrence data (use level and/or analytical data) on benzyl alcohol (E 1519). In response to this public call, updated information on the actual levels of benzyl alcohol (E 1519) in foods was made available to EFSA by industry (levels resulting from carry over). No analytical data on the concentration of benzyl alcohol (E 1519) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with 13 use levels of benzyl alcohol (E 1519) in foods belonging to four food categories. These levels were provided by FoodDrinkEurope (FDE) and Intersnack. These use levels referred to the use of benzyl alcohol (E 1519) in confectionery, including chocolate and fine bakery wares according to Annex III to Regulation (EC) No 1333/2008. One use level was excluded from further evaluation due to unclear description of the food product.
Appendix B provides an overview of the use levels of benzyl alcohol (E 1519) in foods as reported by industry.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database that monitors new introductions of packaged goods in the market worldwide. It contains information of over 2.5 million food and beverage products of which more than 900,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently it contains data from 25 out of its 28 Member States and Norway.16
For the purpose of this Scientific Opinion, Mintel's GNPD17 was used for checking the labelling of food and beverages products and food supplements for benzyl alcohol (E 1519) within the EU's food market as the database contains the compulsory ingredient information on the label.
According to Mintel's GNPD, benzyl alcohol (E 1519) was labelled on only one product belonging to the food subcategory of Mintel's GNPD ‘Baking ingredients & mixes’.
The Panel noted that this information does not fully cover the actual use of benzyl alcohol (E 1519), because food additives authorised according to Annex III without a function as a food additive in the final product do not need to be labelled.18
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). Consumption surveys added in the Comprehensive Database in 2015 were also taken into account in this assessment.19
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from infants, toddlers, children, adolescents, adults and the elderly were used in the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 2).
Table 2.
Population groups considered for the exposure estimates of benzyl alcohol (E 1519)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Netherlands, Sweden, UK |
The term ‘toddlers’ in the Comprehensive Database corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in Comprehensive Database, respectively.
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D to perform an exposure assessment. In practise, FoodEx codes were matched to the FCS food categories.
Food categories considered in the exposure assessment of benzyl alcohol (E 1519)
As described in Section 3.2, benzyl alcohol (E 1519) is only authorised as a food additive in food flavourings without a function as a food additive in the final product according to Annex III to Regulation No 1333/2008. It is authorised in liqueurs, aromatised wines, aromatised wine‐based drinks and aromatised wine‐products cocktails, and in confectionery, including chocolate and fine bakery wares.
For the purpose of this assessment, relevant food categories of Annex II of Regulation (EC) No 1333/2008, part D were identified and assigned to the corresponding alcoholic‐based beverages and confectionary food products as defined in Annex III.
The food categories in which the use of benzyl alcohol (E 1519) is authorised were selected from the nomenclature of the Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
In the regulatory maximum level exposure scenario, the food categories taken into account were (in ascending order of the FCS codes):
05.1 Cocoa and Chocolate products as covered by Directive 2000/36/EC,
05.2 Other confectionery including breath freshening microsweets,
05.3 Chewing gum,
05.4 Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4,
07.2 Fine bakery wares,
14.2.6 Spirit drinks as defined in Reg (EC) No 110/2008, only liqueur,
14.2.7.1 Aromatised wines.
Some food categories or their restrictions/exceptions are not referenced in the Comprehensive Database and could therefore not be taken into account in the present estimate. This was the case for two food categories (Appendix C) and may have resulted in an underestimation of the exposure. The food categories that were not taken into account were:
Aromatised wine‐based drinks;
Aromatised wine‐product cocktails.
For the refined scenario, three additional food categories (FC 05.4 Decorations, coatings and fillings, FC 14.2.6 Spirit drinks, only liqueur and 14.2.7.1 Aromatised wines) were not taken into account because no concentration data were provided for these food categories to EFSA (Appendix C).
Overall, seven food categories were included in the regulatory maximum level exposure scenario, and four food categories were included in the refined scenarios (Appendix C).
3.4. Exposure estimates
3.4.1. Exposure to benzyl alcohol (E 1519) from its use as a food additive
The Panel estimated the chronic dietary exposure to benzyl alcohol (E 1519) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to benzyl alcohol (E 1519) was calculated by multiplying concentrations of benzyl alcohol (E 1519) per food category (Appendix B) with their respective consumption amount per kilogram body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only 1 day per subject were excluded as they are considered as not adequate to assess repeated exposure.
The exposure was calculated for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 2). Based on these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain was not estimated.
The exposure assessment to benzyl alcohol (E 1519) was carried out by the FAF Panel based on two different sets of concentration data: (1) MPLs as set down in the EU legislation (defined as the regulatory maximum level exposure assessment scenario); and (2) reported use levels (defined as the refined exposure assessment scenario). These two scenarios are discussed in detail below.
Regulatory maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario was based on the MPLs as set in Annex III to Regulation (EC) No 1333/2008.
The Panel considers the exposure estimates derived following this scenario as the most conservative since it is assumed that the population will be exposed to the food additive present in food at the MPL.
Refined exposure assessment scenario
The refined exposure assessment scenario is based on use levels reported by food industry. This exposure scenario can consider only food categories for which these data were available to the Panel.
Based on the available data set, the Panel calculated two refined exposure estimates for benzyl alcohol (E 1519) based on two model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long term to benzyl alcohol (E 1519) present at the maximum reported use level for one food category. This exposure estimate is calculated as follows:
-
–
Combining consumption levels of the main contributing food category at the individual level with the maximum of the reported use levels.
-
–
Combining consumption levels of the remaining food categories with the mean of the typical reported use levels for the remaining food categories.
-
–
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long term to benzyl alcohol (E 1519) present at the mean reported use levels in food. This exposure estimate is calculated using the mean of the typical reported use levels for all food categories.
Appendix B summarises the use levels of benzyl alcohol (E 1519) used in the regulatory maximum level exposure assessment scenario and refined exposure scenarios.
Dietary exposure to benzyl alcohol (E 1519)
Table 3 summarises the estimated exposure to benzyl alcohol (E 1519) from its use as a food additive in six population groups according to the different exposure scenarios. Detailed results per population group and survey are presented in Appendix C.
Table 3.
Summary of dietary exposure to benzyl alcohol (E 1519) from its use as a food additive in the regulatory maximum level exposure assessment scenario and in the refined exposure assessment scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Regulatory maximum level exposure assessment scenario | ||||||
| • Mean | < 0.01–0.34 | 0.05–0.98 | 0.21–1.04 | 0.13–0.59 | 0.05–0.37 | 0.05–0.33 |
| • 95th percentile | < 0.01–1.43 | 0.22–2.34 | 0.63–2.53 | 0.38–1.40 | 0.20–1.04 | 0.15–0.89 |
| Refined exposure assessment scenario | ||||||
| Brand‐loyal scenario | ||||||
| • Mean | < 0.01–0.03 | 0.01–0.27 | 0.05–0.26 | 0.04–0.18 | 0.01–0.05 | < 0.01–0.03 |
| • 95th percentile | < 0.01–0.12 | 0.05–0.81 | 0.21–0.67 | 0.15–0.47 | 0.05–0.20 | 0.02–0.11 |
| Non‐brand‐loyal scenario | ||||||
| • Mean | < 0.01–0.03 | 0.01–0.14 | 0.03–0.17 | 0.03–0.12 | 0.01–0.04 | < 0.01–0.02 |
| • 95th percentile | < 0.01–0.10 | 0.04–0.38 | 0.14–0.53 | 0.11–0.37 | 0.04–0.13 | 0.02–0.09 |
In the regulatory maximum level exposure assessment scenario, mean exposure to benzyl alcohol (E 1519) from its use as a food additive ranged from < 0.01 mg/kg bw per day in infants to 1.04 mg/kg bw per day in children. The 95th percentile of exposure to benzyl alcohol (E 1519) ranged from < 0.01 mg/kg bw per day in infants to 2.53 mg/kg bw per day in children.
In the refined brand‐loyal exposure scenario, the mean exposure to benzyl alcohol (E 1519) from its use as a food additive ranged from < 0.01 mg/kg bw per day in infants and the elderly to 0.27 mg/kg bw per day in toddlers. The high exposure to benzyl alcohol (E 1519) ranged from < 0.01 mg/kg bw per day in infants to 0.81 mg/kg bw per day in toddlers. Corresponding estimates for the non‐brand‐loyal scenario were < 0.01 mg/kg bw per day in infants and the elderly and 0.17 mg/kg bw per day in children for the mean exposure and < 0.01 mg/kg bw per day in infants to 0.53 mg/kg bw per day in children for the 95th percentile of exposure.
Main food categories contributing to exposure to benzyl alcohol (E 1519)
In the regulatory maximum level exposure assessment scenario, the main contributing food category to the total mean exposure estimates for all population groups was fine bakery wares (see Appendix D for more details). The main contributing food category in the brand‐loyal and non‐brand‐loyal scenario was cocoa and chocolate products for all population groups. (see Appendix D for more details).
Appendix D can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.5876
The Panel considered that the refined exposure assessment approach resulted in more realistic long‐term exposure estimates compared to the regulatory maximum level exposure assessment scenario.
Benzyl alcohol (E 1519) is used as flavour enhancer and influences the organoleptic properties of the final food. Furthermore, the main contributing food category in the refined scenarios was cocoa and chocolate products, for which brand loyalty to a specific food product(s) can occur. For these reasons, the Panel considered the brand‐loyal scenario as the most appropriate scenario for risk characterisation.
Uncertainty analysis
Uncertainties in the exposure assessment of benzyl alcohol (E 1519) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 4.
Table 4.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Methodology used to estimate high percentiles (95th) long‐term (chronic) exposure based on data from food consumption surveys covering only a few days | + |
| Correspondence of reported use levels to the food items in the Comprehensive Database: uncertainties to which types of food the levels refer | +/– |
| Uncertainty in possible national differences in use levels of food categories | +/– |
| Use levels:
• use levels considered applicable to all foods within the entire food category • unclear representativeness of foods on the EU market |
+ +/− |
| Food categories used in the maximum regulatory exposure assessment scenario: exclusion of food categories due to missing FoodEx linkage (n = 2/9 total number of food categories) | – |
|
Food categories used in the refined exposure assessment: Two food categories were excluded due to missing FoodEx linkage and three food categories were excluded due to missing use levels (n = 5/9 total number of food categories) The four remaining food categories out of all authorised food categories (n = 9), corresponded to 0.7–97% of the amount (g of foods by body weight) of food consumption documented in the Comprehensive Database |
– |
|
Regulatory maximum level exposure assessment scenario: – exposure calculations based on the MPL according to Annex III to Regulation (EC) No 1333/2008 |
+ |
|
Refined exposure assessment scenarios: – exposure calculations based on the maximum or mean levels (reported use from industry) |
+/– |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Overall the Panel considered that the uncertainties identified resulted in an overestimation of the exposure to benzyl alcohol (E 1519) as a food additive in European countries considered in the Comprehensive Database for the regulatory maximum level exposure scenario and for the refined exposure scenario. This was mainly due to the assumption that all foods within a relevant food category were assumed to contain the additive at the MPL or a level equal to the maximum or typical use level.
3.4.2. Other sources
Benzyl alcohol is also a naturally occurring substance, for example, wines, grapes, sour cherry, cider, mushrooms, chestnuts, almonds and clove at concentrations up to 3 mg/kg. In wine and tomato, benzyl alcohol is present either free or bound in the form of glycosides, and it can be released from the glycosides via a further fermentation or enzymatic activity (Diéguez et al., 2003; Marlatt et al., 2004; Ganss et al., 2011).
3.5. Biological and Toxicological data
3.5.1. Absorption, distribution, metabolism and excretion
The text below summarises common knowledge on the subject and was extracted from reports of JECFA (JECFA 1996, 2015) and the Scientific Committee on Food (SCF 2002) and the reviews by Nair et al. (2001), Adams et al. (2005), Belsito et al. (2012), Scognamiglio et al. (2012), Api et al. (2015), Gry et al. (2015).
In humans and various animal species, benzyl alcohol is metabolised to benzoic acid which reacts with glycine in the liver. The resulting hippuric acid is excreted quickly in urine and 75–100% of the dose is excreted within 6 h. Biotransformation of benzoic acid to hippuric acid follows saturable or Michaelis–Menten kinetics in humans (Kubota and Ishizaki, 1991) and the availability of glycine is the rate limiting step. When glycine is depleted, free benzoic acid maybe excreted unchanged or as the glucuronic acid conjugate. Even with saturation of hippuric acid formation, clearance of compounds in the benzyl group is relatively rapid in most laboratory animal species and in humans.
The octanol/water partition coefficient of benzyl alcohol (log[POW] = 1.1) and the relatively high solubility in water (40 g/L at 20°C) indicate a low potential for bioaccumulation (Api et al., 2015; EFSA, 2012; OECD, 2001).
3.5.2. Acute toxicity
Acute oral toxicity (LD50) reported for benzyl alcohol was 1,580 mg/kg bw in mice (Jenner et al., 1964 cited in Belsito et al. 2012), ranged from 1,230 to 3,100 mg/kg bw in rats (Graham and Kuizenga, 1945; Smyth et al., 1951; Jenner et al., 1964; Bar and Griepentrog, 1967; Koch et al., 1993; Nishimura et al., 1994) and was 1,040 mg/kg bw in rabbits (Graham and Kuizenga, 1945).
The Panel noted that benzyl alcohol was of low acute toxicity.
3.5.3. Short‐term and subchronic toxicity
Mice
B6C3F1 mice (5/sex per group) received by gavage 0, 125, 250, 500, 1,000 and 2,000 mg benzyl alcohol/lkg bw per day in corn oil for 5 day per week during 16 days (NTP, 1989). All mice from 2,000 mg/kg bw per day group and 1 male and 2 females from 1,000 mg/kg bw per day group died before the scheduled termination. Lethargy and rough hair coats were seen in males from 500 mg/kg bw per day group or higher and in females from the two highest dose groups. At necropsy, blood in urinary bladder was observed in two highest groups. There were no treatment‐related microscopic findings in treated animals.
B6C3F1 mice (10/sex per group) received by gavage 0, 50, 100, 200, 400 or 800 mg/kg bw benzyl alcohol daily by gavage 5 days per week for a period of 13 weeks (NTP, 1989). Although the deaths occurred in all treatment groups, they were except one attributed by the authors of the study to the gavage error. High‐dose females were lethargic during the first 2 weeks of the study. The terminal body weights of females from 400 and 800 mg/kg bw per day groups were 5 and 8% lower as compared to controls. The Panel considered the decrease in body weight as non‐adverse because of the low magnitude of the change. There were no treatment‐related microscopic findings at any of the doses.
Rats
F344 rats (5/sex per group) received by gavage 0, 125, 250, 500, 1,000 and 2,000 mg benzyl alcohol/lkg bw per day in corn oil for 5 day per week during 16 days (NTP, 1989). All rats from 2,000 mg/kg bw per day group and two males and three females from 1,000 mg/kg bw per day group died before the scheduled termination. Lethargy was seen at two highest doses in animals of both sexes. Rough hair coats were observed at 500 and 1,000 mg/kg bw per day in males and at 250 and 500 mg/kg bw per day in females. The terminal body weights of males from 1,000 mg/kg bw per day groups was 18% lower as compared to controls. Rats from groups receiving doses of 1,000 or 2,000 mg/kg per day had blood around the mouth and nose, subcutaneous haemorrhages, and blood in the urinary and gastrointestinal tracts. There were no treatment‐related microscopic findings at any of the doses.
F344 rats (10/sex per group) received by gavage 0, 50, 100, 200, 400 or 800 mg/kg bw benzyl alcohol 5 days per week for a period of 13 weeks (NTP, 1989). Eight males and two females at 800 mg/kg bw, one female at 400 mg/kg bw, one male at 200 mg/kg bw and one female in the control group died prior to the scheduled termination. Some of these deaths were attributed by the authors to gavage error. Rats of each sex in the highest dose group manifested neurotoxicity in form of staggering, laboured breathing and lethargy. Furthermore, five of males from this group had blood around nose and mouth after 8 weeks of treatment. The body weight at the end of treatment was 7% and 5% lower in males and females from in the highest dose group as compared to controls. The Panel considered the decrease in body weight as non‐adverse because of the low magnitude of the change. The treatment‐related microscopic changes were recorded only in the highest dose group: necrosis of the dentate gyrus of the hippocampus (9/9 males and 7/7 females), skeletal muscle necrosis (5/10 males), thymic congestion, haemorrhage and atrophy (8/10 males).
Overall, oral short‐term or subchronic treatment with benzyl alcohol resulted in neurotoxicity manifested as lethargy in mice (from 500 mg/kg per day) and lethargy or staggering (from 800 mg/kg bw per day) in rats. No clinical chemistry and haematology parameters were examined in these studies.
3.5.4. Genotoxicity
In vitro
A number of studies demonstrated that benzyl alcohol is not mutagenic in the bacterial reverse mutation assay with Salmonella Typhimurium TA92, TA94, TA98, TA100, TA1535, TA1537 and TA1538 with and without metabolic activation (NTP TR343, 1989; (Ishidate et al., 1984) (Rogan et al., 1986)). Equivocal results were obtained with the benzyl alcohol in the Rec DNA repair assay using Bacillus subtilis strains H17 and M45 (Oda et al., 1978; Kuroda et al., 1984; Yoo, 1986). In the SOS/umu test with Salmonella Typhimurium TA1535/pSK1002 benzyl alcohol did not induce positive response (Yasunaga et al., 2004).
Two studies of the in vitro mammalian cell gene mutation assay with mouse L5178Y/TK+/− lymphoma cells were performed with benzyl alcohol. In both studies, it was negative in the presence of metabolic activation, whereas without metabolic activation, an increase in mutant frequency was observed at 4,500 μg/mL with a relative total growth of 20% (NTP, 1989). The Panel noted that such increase was reported at a concentration above the maximum level recommended by the current OECD test guideline and in the presence of excessive cytotoxicity. Anyway, the mutation frequency did not exceed the Global Evaluation Factor indicated in OECD TG 490; therefore, the Panel considered the results of this study as negative.
In the two studies of the vitro chromosomal aberrations assays, benzyl alcohol at high concentration (4,000 μg/mL) induced increase in chromosomal aberrations in Chinese hamster ovary cells in the presence but not in the absence of metabolic activation (NTP, 1989). A negative result of a chromosomal aberration assay was reported in Chinese hamster fibroblast cells exposed to up to 1 mg/mL benzyl alcohol without metabolic activation (Ishidate et al., 1984). The results of the induction of sister chromatid exchanges in CHO cells were equivocal (NTP, 1989). A more recent in vitro micronucleus assay that was performed according to OECD TG 487 with Chinese hamster lung cells and TK6 human lymphoblastoid cells gave negative results at the maximum test concentration of 10 mM (1,081 μg/mL) recommended in the guideline as the limit concentration (Fowler et al., 2012).
In rat hepatocytes, the induction of DNA damage by benzyl alcohol (1 and 10 mM) was determined with the alkaline elution assay. DNA damage was detected at 10 mM; however, it was associated with cytotoxicity and the author concluded that the result is negative (Storer et al., 1996). In the in vitro comet assay with isolated blood cells exposed to 1–50 mM benzyl alcohol induced statistically significant increase in DNA damage at 25 and 50 mM (Demir et al., 2010). However, the exposure conditions are not described and cytotoxicity was not determined; thus, the observed DNA damage might be due to the cytotoxicity; moreover, this test does not belong to the tests recommended by the SC for the assessment of genotoxicity (EFSA, 2011a,b).
In vivo
In a mouse micronucleus assay, benzyl alcohol was tested at doses 50, 100, 200 mg/kg administered by i.p. injection. The animals were sacrificed 24 h after the administration of the tested compound and the micronuclei were determined in bone marrow polychromatic erythrocytes. The test results were negative at all doses tested (Hayashi et al., 1988).
In summary, benzyl alcohol was tested with negative results in the base set of genotoxicity tests recommended by EFSA (Ames and in vitro micronucleus). Equivocal or weakly positive result were reported in some other in vitro tests, but only at high doses, above the current maximum recommended dose level (10 mM or 2,000 μg/mL, whichever is lower; OECD 2016). Negative results were obtained in an in vivo micronucleus assay by i.p. administration. Overall, the Panel concluded that benzyl alcohol does not raise concern with respect to genotoxicity.
3.5.5. Chronic toxicity and carcinogenicity
No chronic toxicity studies were available.
Mice
In a 2‐year carcinogenicity study, benzyl alcohol (purity 99%) was given by gavage to groups of 50 B6C3F1 mice of each sex, 8–9 weeks of age, at a dose of 0, 100, 200 mg/kg bw per day in corn oil on 5 days per week for 103 weeks (NTP, 1989). The mean body weights of treated and control mice were comparable throughout the study. The survival of control females was significantly lower than that of mice of the high‐dose group after week 74, but no other differences in survival were observed. No significant treatment‐related effects were noted at gross necropsy or histopathological examination. A dose‐related negative trend was observed in the incidence of Harderian gland adenomas in male mice (8/50, in controls; 3/50, in low‐dose group; 2/50, in high dose group). An increased incidence of adrenal cortex adenomas was noted in high‐dose male mice (0/48; 0/44; 3/48), was within the historical control range and not considered compound related. The NOAEL in this study was 200 mg/kg bw per day, the highest dose tested.
Rats
Groups of 100 F344/N rats (50 each sex) were dosed by gavage with 0, 200 or 400 mg/kg bw per day benzyl alcohol in corn oil, 5 days per week for 103 weeks (NTP, 1989). Administration of benzyl alcohol did not affect survival in male rats, but reduced survival of female rats by half (70% of controls; 34% of low‐dose females and 34% of high‐dose females). Most of these accidental deaths were considered related to gavage errors. No effect on body weight gain was observed. No apparent compound‐related non‐neoplastic lesions were observed. A dose‐related negative trend in incidence of anterior pituitary neoplasms was seen in female rats (vehicle control, 29/509; low dose, 17/47; high dose, 9/49). Epithelial hyperplasia of the forestomach was noted in 4/50 high‐dose male rats, whereas it was not seen in controls or low‐dose rats. The Panel noted that effect on forestomach is a local irritation due to high concentration when administered by gavage. The NOAEL was 400 mg/kg bw per day, the highest dose tested.
Overall, benzyl alcohol is not carcinogenic in mice and rats up to 200 and 400 mg/kg bw per day, respectively, the highest doses tested.
3.5.6. Reproductive and developmental toxicity
Reproductive toxicity studies
No studies available.
Developmental toxicity studies
Mice
Fifty female CD‐1 mice were administered 550 mg/kg bw per day benzyl alcohol by gavage on days 6–15 of gestation. A control group of 50 mice received corn oil only. Body weight, clinical observations and mortality were recorded daily throughout treatment and up to 3 days postpartum. All parameters tested, including gestation index, average number of live pups/litter, postnatal survival and pup body weight, were statistically similar for the treated and control animals (York et al., 1986).
In a preliminary screening test, benzyl alcohol was administered by gavage to CD1 mice (50 females) at 750 mg/kg bw per day from gestational days 6–13 (Hardin et al., 1987; RIFM, 1986). Only 62% of the dams survived, and they showed a decrease in body weight. There were no changes in reproductive or gestation indices. Statistically significant reduction in litter weight, birth weight and weight gain of pups was also reported. It was reported in a review of this study that ‘clinical signs of maternal toxicity were observed, including hunched posture, tremors, inactivity, prostration, hypothermia, ataxia, dyspnoea, swollen or cyanotic abdomen, and piloerection’ (York et al., 1986).
In summary, no reproductive toxicity studies were available for benzyl alcohol. The study design of the two developmental screening studies in mice was limited. In both studies, both only one dose of benzyl alcohol was tested. Benzyl alcohol was administered from gestational day (GD) 6–15 or GD 6–13 and dams were allowed to litter and necropsied on PND 3. No fetal pathology was performed. The study of York et al. (1986) showed no effects at 550 mg/kg bw per day and in the study of Hardin et al. (1987), 38% mortality of the dams and effects on pup weight were observed at 750 mg/kg bw per day.
3.5.7. Hypersensitivity, allergenicity and food intolerance
Case reports of adverse reactions to benzyl alcohol that may point at hypersensitivity reactions after injection have been reported (Barbaud (1995), Shmunes (1984), Wilson et al. (1986), Turvey et al. (2004), Grant et al. (1982).
As reviewed by Api et al. (2015), based on the structure of the molecule benzyl alcohol is not predicted to be directly reactive to proteins, while in in chemico experimental studies, little to no reactivity to cysteine‐based peptides has been reported. Benzyl alcohol has been reported to be both positive and negative in guinea pig test (GPMT) methods. Additionally, benzyl alcohol has been evaluated in the murine local lymph node assay (LLNA), and was reported to have an EC3 value of > 50%. The dermal sensitisation potential of benzyl alcohol has also been evaluated in the Human Repeated Insult Patch Test (HRIPT) and the human maximisation test. Based on the available data, benzyl alcohol is considered to be a weak skin sensitiser.
Even if benzyl alcohol possesses some sensitising capacity, the intestinal immune system has a regulatory mechanism in place that induces oral tolerance to ingested low molecular compounds (Gutting et al., 2003; Wambre and Jeong, 2018). Low molecular weight compounds will therefore not readily induce hypersensitivity reactions after oral ingestion. In addition, no allergic reactions after ingestion of benzyl alcohol after ingestion have been reported in the population.
For this reason, whereas allergic reactions to benzyl alcohol after ingestion cannot be ruled out, the Panel considers the likelihood of such reactions to occur as low.
3.5.8. Other studies
The Panel is aware of cases of ‘gasping syndrome’ in pre‐term new‐born infants after intravenous administration of benzyl alcohol as an excipient of pharmaceutical product (add references). However, the use of this compound has been discontinued and this opinion does not consider this effect because benzyl alcohol (E 1519) is not authorised in food for infants below 16 weeks of age (https://www.cdc.gov/mmwr/preview/mmwrhtml/00001109.htm).
3.6. Discussion
Benzyl alcohol (E 1519) is authorised as a food additive in the EU in accordance with Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012[Link].
In the EU, benzyl alcohol (E 1519) has been evaluated by the Scientific Committee for Food (SCF) in 2002 (SCF, 2002) who concluded that ‘the metabolism of benzyl alcohol to benzoic acid by man is well established. In special studies, biochemical effects have been investigated. The toxicity has been studied extensively, including acute, short‐term and long‐term toxicity, carcinogenicity, genotoxicity and developmental toxicity’.
Benzyl alcohol was evaluated by JECFA in 1980 (JECFA, 1980) and concluded a group ADI (for benzoic acid together with benzyl alcohol) of 0–5 mg/kg bw, subsequently agreed by the SCF in 2002.
In 2016, EFSA ANS Panel re‐evaluated the safety of benzoic acid and benzoates (E210‐231) and established a group ADI of 5 mg/kg bw per day (expressed a benzoic acid). The re‐evaluation did not include but excluding benzyl‐alcohol E 1519.
In humans and animal species benzyl alcohol is metabolised to benzoic acid which reacts with glycine in the liver and the resulting hippuric acid is excreted quickly in urine.
The Panel noted that benzyl alcohol was of low acute toxicity.
Oral short‐term or subchronic studies with benzyl alcohol showed neurotoxicity manifested as lethargy in mice (from 500 mg/kg per day) and lethargy or staggering (from 800 mg/kg bw per day) in rats.
Benzyl alcohol was tested with negative results in the base set of genotoxicity tests and the Panel concluded that benzyl alcohol does not raise safety concern with respect to genotoxicity.
Benzyl alcohol is not carcinogenic in mice and rats up to 200 and 400 mg/kg bw per day, respectively, which were highest doses tested. The NOAEL in the carcinogenicity study in rats was 400 mg/kg bw per day.
The Panel noted that reproductive studies were not available and that two developmental screening studies in mice were of limited design.
Although some case reports of adverse reactions to benzyl alcohol after injection have been reported, the Panel noted that no allergic reactions after ingestion of benzyl alcohol have been reported in the population. For this reason, whereas allergic reactions to benzyl alcohol after ingestion cannot be ruled out, the Panel considers the likelihood of such reactions to occur as low.
Considering the available data set, the Panel established an ADI of 4 mg benzyl alcohol/kg bw per day based on a NOAEL of 400 mg/kg bw per day from the carcinogenicity study in rats, based on no effect in the highest dose tested.
The dietary exposure to benzyl alcohol (E 1519) from its use as a food additive, the exposure was calculated based on (1) MPLs set out in the EU legislation (defined as the regulatory maximum level exposure assessment scenario) and (2) the reported use levels (defined as the refined exposure assessment scenario). Dietary exposure through this latter scenario was assessed using reported use levels for food categories for which benzyl alcohol (E 1519) is authorised (Annex III to Regulation No 1333/2008).
Using the available data, the Panel calculated exposure estimates based on different assumptions as described in Section 3.4. The Panel considered that the refined exposure assessment approach resulted in more realistic long‐term exposure estimates compared to the regulatory maximum level exposure assessment scenario. However, also the refined exposure estimates resulted in overestimates of the exposure to benzyl alcohol (E 1519) from its use as a food additive according to Annex III to Regulation (EC) No 1333/2008 (Section 3.4.1).
Because benzyl alcohol (E 1519) is authorised only according to Annex III, the information about its use from Mintel GNPD does not provide information about its actual use in food. Food additives authorised according to Annex III without a function as a food additive in the final product do not need to be labelled18.
The mean and high exposure estimates in the refined exposure scenarios were maximally 0.27 and 0.81 mg/kg bw per day in toddlers, respectively (Table 3). Considering the food categories for which the presence of benzyl alcohol (E 1519) can be due to carry over via its authorisation according to Annex III to Reg (EC) No 1333/2008, the exposure estimates to benzyl alcohol (E 1519) were below the ADI of 4 mg/kg bw per day in all population groups. The Panel noted that also the exposure in the regulatory maximum level exposure assessment scenario is well below the ADI in all population groups.
The Panel also noted that the refined exposure estimates are based on information provided on the reported level of use of food additive benzyl alcohol (E 1519). If actual practice changes, this refined estimate may no longer be representative and should be updated.
4. Conclusions
Based on the toxicological database available, the Panel established the ADI of 4 mg/kg bw per day for benzyl alcohol (E 1519) and concluded that the exposure to benzyl alcohol (E 1519) does not raise a safety concern at the reported uses and use levels.
5. Recommendations
The Panel recommend that:
The European Commission considers introducing the maximum levels of chlorinated organic compounds as impurities in the specifications for purity of E 1519 in EU Regulation 231/2012.
Documentation provided to EFSA
FDE (FoodDrinkEurope), 2017. Data on use levels of benzyl alcohol (E 1519) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 29 November 2017.
Intersnack, 2017. Data on use levels of benzyl alcohol (E 1519) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 29 November 2017.
Abbreviations
- ADI
Acceptable Daily Intake
- bw
Body Weight
- CAS
Chemical Abstract Service
- DHS
dynamic headspace
- ECHA
European Chemicals Agency
- EINECS
European Inventory of Existing Commercial chemical Substances
- EMA
European Medicines Agency
- F1
First generation pups
- F2
Second generation pups
- FAF
Food Additives and Flavourings
- GC‐FID
gas chromatography equipped with a flame ionization detector
- GC‐MS
gas chromatography coupled to a mass spectrometer
- GD
Gestation Days
- GNPD
Global New Products Database
- HD
hydrodistillation
- HRIPT
Human Repeated Insult Patch Test
- HS‐SPME
headspace solid‐phase micro‐extraction
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LLE
liquid–liquid extraction
- LLNA
local lymph node assay
- LOD
limit of detection
- MPLs
Maximum Permitted Levels
- MSDE
micro simultaneous steam distillation‐solvent extraction
- NOAEL
No Observable Adverse Effect Level
- NTP
National Toxicology Program
- OECD
Organisation for Economic Co‐operation and Development
- P1
First generation adults
- P2
Second generation adults
- P3
Third generation adults
- REACH
Registration, Evaluation, Authorisation and restriction of Chemicals
- SCF
Scientific Committee for Food
- SFE
supercritical fluid extraction
- SPE
solid‐phase extraction
- SPME
solid‐phase micro‐extraction
- TemaNord
Nordic Council of Ministers
- USE
ultrasonic solvent extraction
- VOCs
volatile organic compounds
- WHO
World Health Organization
Appendix A – Summary of reported use levels (mg/kg) of benzyl‐alcohol (E 1519) provided by industry
Appendix B – Concentration levels of benzyl‐alcohol (E 1519) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Appendix C – Summary of total estimated exposure of benzyl‐alcohol (E 1519) from its use as a food additive for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix D – Main food categories contributing to exposure to benzyl‐alcohol (E 1519) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
1.
Appendixes Appendix A – Summary of reported use levels (mg/kg) of benzyl‐alcohol (E 1519) provided by industry, Appendix B – Concentration levels of benzyl‐alcohol (E 1519) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)–Appendix A – Summary of reported use levels (mg/kg) of benzyl‐alcohol (E 1519) provided by industry, Appendix B – Concentration levels of benzyl‐alcohol (E 1519) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate), Appendix C – Summary of total estimated exposure of benzyl‐alcohol (E 1519) from its use as a food additive for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day), Appendix D – Main food categories contributing to exposure to benzyl‐alcohol (E 1519) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure) can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2019.5876
Supporting information
Summary of reported use levels (mg/kg) of benzyl‐alcohol (E 1519) provided by industry
Concentration levels of benzyl‐alcohol (E 1519) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of benzyl‐alcohol (E 1519) from its use as a food additive for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to benzylalcohol (E 1519) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Fürst P, Gürtler R, Gundert‐Remy U, Husøy T, Mennes W, Moldeus P, Oskarsson A, Shah R, Waalkens‐Berendsen I, Wölfle D, Boon P, Crebelli R, Di Domenico A, Filipič M, Mortensen A, Van Loveren H, Woutersen R, Gergelova P, Giarola A, Lodi F and Frutos Fernandez MJ, 2019. Scientific Opinion on the re‐evaluation of benzyl alcohol (E 1519) as food additive. EFSA Journal 2019;17(10):5876, 25 pp. 10.2903/j.efsa.2019.5876
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00590
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus, Agneta Oskarsson, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle and Maged Younes.
Acknowledgements: The Panel wishes to thank: Dimitrios Chrysafidis for the preparatory work on this scientific output. The FAF Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 26 September 2019
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord 2002, 560.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). OJ L 396, 30.12.2006.
Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. OJ L 12, 15.1.2011, p. 1–89.
Available online: http://ec.europa.eu/consumers/cosmetics/cosing/index.cfm?fuseaction=search.simple
Available online: http://ec.europa.eu/food/food/animalnutrition/feedadditives/comm_register_feed_additives_1831-03.pdf
Regulation (EC) No 1831/2003 of the European Parliament and the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, 29–43.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published: 22 November 2009. Available from: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Available online: http://www.efsa.europa.eu/en/food-consumption/comprehensive-database
Commission Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
Missing Cyprus, Luxembourg and Malta.
http://www.gnpd.com/sinatra/home/ accessed on 17/5/2019.
Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. OJ L 304/18, 22.11.2011, p. 46.
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
References
- Adams TB, Cohen SM, Doull J, Feron VJ, Goodman JI, Marnett LJ, Munro IC, Portoghese PS, Smith RL, Waddell WJ and Wagner BM, 2005. The FEMA GRAS assessment of benzyl derivatives used as flavor ingredients. Food and Chemical Toxicology, 43, 1207–1240. 10.1016/j.fct.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Antón‐Díaz MJ, Suárez Valles B, Mangas‐Alonso JJ, Fernández‐García O and Picinelli‐Lobo A, 2016. Impact of different techniques involving contact with lees on the volatile composition of cider. Food Chemistry, 190, 1116–1122. [DOI] [PubMed] [Google Scholar]
- Api AM, Belsito D, Bhatia S, Bruze M, Calow P, Dagli ML, Dekant W, Fryer AD, Kromidas L, La Cava S, Lalko JF, Lapczynski A, Liebler DC, Politano VT, Ritacco G, Salvito D, Schultz TW, Shen J, Sipes IG, Wall B and Wilcox DK, 2015. RIFM fragrance ingredient safety assessment, Benzyl alcohol, CAS Registry Number 100‐51‐6. Food and Chemical Toxicology, 84, 1–14. [DOI] [PubMed] [Google Scholar]
- Balcerek M, Pielech‐Przybylska K, Dziekońska‐Kubczak U, Patelski P and Strąk E, 2017. Changes in the chemical composition of plum distillate during maturation with oak chips under different conditions. Food Technology and Biotechnology, 55, 333–359. 10.17113/ftb.55.03.17.5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar VF and Griepentrog F, 1967. Die Situation in der gesundheitlichen Beurteilung Aromatisierungsmittel fur Lebensmittel (where we stand concerning the evaluation of flavoring substances from the viewpoint of health). Medizinische Ernahrung, 8, 244–251. [Google Scholar]
- Barbaud A, 1995. Place of excipients in drug‐related allergy. Clinical Reviews in Allergy and Immunology, 13, 253–263. [DOI] [PubMed] [Google Scholar]
- Bellincontro A, Matarese F, D'Onofrio C, Accordini D, Tosi E and Mencarelli F, 2016. Management of postharvest grape withering to optimise the aroma of the final wine: a case study on Amarone. Food Chemistry, 213, 378–387. [DOI] [PubMed] [Google Scholar]
- Belsito D, Bickers D, Bruze M, Calow P, Dagli ML, Fryer AD, Greim H, Miyachi Y, Saurat JH and Sipes IG, 2012. A toxicological and dermatological assessment of aryl alkyl alcohol simple acid ester derivatives when used as fragrance ingredients. Food and Chemical Toxicology, 50, S269–S313. [DOI] [PubMed] [Google Scholar]
- Cacho J, Moncayo L, Carlos Palma J, Ferreira V and Culleré L, 2013. Comparison of the aromatic profile of three aromatic varieties of Peruvian pisco (Albilla,Muscat and Torontel) by chemical analysis and gas chromatography–olfactometry. Flavour Fragr. J., 28, 340–352. [Google Scholar]
- Coralia VG, Quek SY, Stevenson RJ and Winz RA, 2011. Characterization of the Bound Volatile Extract from Baby Kiwi (Actinidia arguta). Journal of Agriculture and Food Chemistry, 59, 8358–8365. [DOI] [PubMed] [Google Scholar]
- Demir E, Kocaoğlu S and Kaya B, 2010. Assessment of genotoxic effects of benzyl derivatives by the comet assay. Food and Chemical Toxicology, 48, 1239–1242. [DOI] [PubMed] [Google Scholar]
- Diéguez SC, Lois LC, Gomez EF and de la Peña MLG, 2003. Aromatic composition of the Vitis vinifera grape Albariñ o. Lebensm.‐Wiss. u.‐Technol., 36, 585–590. [Google Scholar]
- ECHA , online. https://echa.europa.eu/it/substance-information/-/substanceinfo/100.002.600
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2009. SCIENTIFIC OPINION Flavouring Group Evaluation 54, Revision 1 (FGE.54Rev1): consideration of benzyl derivatives evaluated by JECFA (57th meeting) structurally related to benzyl alcohols, benzaldehydes, a related acetal, benzoic acids and related esters evaluated by EFSA in FGE.20Rev.1. EFSA Journal 2009:7(5);1025, 73 pp. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.1025 [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Scientific Opinion on the safety and efficacy of benzyl alcohols, aldehydes, acids, esters and acetals (chemical group 23) when used as flavourings for all animal species. EFSA Journal 2012;10(7):2785, 30 pp. 10.2903/j.efsa.2012.2785 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Scientific Opinion on Exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA Journal 2012;10(7):2750, 103 pp. 10.2903/j.efsa.2012.2750 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012c. Statement on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA Journal 2012;10(3):2578, 5 pp. 10.2903/j.efsa.2012.2578 [DOI] [Google Scholar]
- EMA (European Medicine Agency), 2017. Benzyl alcohol and benzoic acid group used as excipients. Available online: https://www.ema.europa.eu/en/documents/report/benzyl-alcohol-benzoic-acid-group-used-excipients-report-published-support-questions-answers-benzyl/chmp/508188/2013-t_en.pdf
- Forcen M, 1992. Contribution of gas chromatographic and conventional data to the characterisation of majorcan musts and wines by means of pattern recognition techniques. Journal of the Science of Food and Agriculture, 60, 229–238. [Google Scholar]
- Fowler P, Smith K, Young J, Jeffrey L, Kirkland D, Pfuhler S and Carmichael P, 2012. Reduction of misleading (“false”) positive results in mammalian cell genotoxicity assays. I. Choice of cell type. Mutation Research, 742, 11–25. [DOI] [PubMed] [Google Scholar]
- Ganss S, Kirsch F, Winterhalter P, Fischer U and Schmarr HG, 2011. Aroma changes due to second fermentation and glycosylated precursors in chardonnay and riesling sparkling wines. Journal of Agriculture and Food Chemistry, 59, 2524–2533. [DOI] [PubMed] [Google Scholar]
- Graham BE and Kuizenga MH, 1945. Toxicity studies on benzyl benzoate and related benzyl compounds. Journal Pharmacology Experimental Therapeutics, 84, 358–362. [PubMed] [Google Scholar]
- Grant JA, Bilodeau PA, Guernsey BG and Gardner FH, 1982. Unsuspected benzyl alcohol hypersensitivity. New England Journal of Medicine, 306, 108. [DOI] [PubMed] [Google Scholar]
- Gry J, Renwick AG and Sipes IG, 2015. Safety evaluation of certain food additives and contaminants. Benzyl derivatives. WHO Food Additives Series: 48. http://www.inchem.org/documents/jecfa/jecmono/v48je14.htm
- Gutting BW, Bouzahzah F, Kong PL, Updyke LW, Amacher DE and Craft J, 2003. Oxazolone and diclofenac‐induced popliteal lymph node assay reactions are attenuated in mice orally pretreated with the respective compound: potential role for the induction of regulatory mechanisms following enteric administration. Toxicology and Applied Pharmacology, 189, 120–133. [DOI] [PubMed] [Google Scholar]
- Hardin BD, Schuler RL, Burg JR, Booth GM, Hazelden KP, MacKenzie KM, Piccirillo VJ and Smith KN, 1987. Evaluation of 60 chemicals in a preliminary developmental toxicity test. Teratogenesis, Carcinogenesis and Mutagenesis, 7, 29–48. http://www.alegesanatos.ro/dbimg/files/Benzyl%20Alcohol%20-%20Benzoic%20Acid.pdf [DOI] [PubMed] [Google Scholar]
- Hayashi M, Kishi M, Sofuni T and Ishidate M, 1988. Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food and Chemical Toxicology, 26, 487–500, (as cited in JECFA, 1996). [DOI] [PubMed] [Google Scholar]
- Ishidate M, Sofuni J, Yoshikawa T, Hayashi K, Nohmi M, Sawada T and Matsuoka MA, 1984. Primary mutagenicity screening of food additives currently used in Japan. Food and Chemical Toxicology, 22, 623–636. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1980. Evaluation of certain food additives (Twenty‐third repord of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series, No. 648. World Health Organization, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996. WHO Food Additives Series 37, Toxicological evaluation of certain food additives, Prepared by the forty‐sixth meeting of the Joint FAO/WHO Expert Committee on Food Additives, World Health Organization, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2009. http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/detail/en/c/276/
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2015. Safety Evaluation of Certain Food Additives and Contaminants: Prepared by the Eightieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) World Health Organization (WHO), Geneva, Switzerland (2015) WHO Food Additives Series 71. 142 pp.
- Jenner PM, Hagan EC, Taylor JM, Cook E and Fitzhugh OG, 1964. Food flavorings and compounds of related structure I. Acute oral toxicity. Food and Cosmetics Toxicology, 2, 327–343. [DOI] [PubMed] [Google Scholar]
- Koch HP, Hofeneder M and Bohne B, 1993. The yeast test: an alternative method for the testing of acute toxicity of drug substances and environmental chemicals. Methods and Findings in Experimental and Clinical Pharmacology, 15, 141–152. [PubMed] [Google Scholar]
- Kubota K and Ishizaki T, 1991. Dose‐dependent pharmacokinetics of benzoic acid following oral administration of sodium benzoate to humans. Eur J Clin Pharmacology, 41, 363–368. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Yoo YS and Ishibashi T, 1984. Antimutagenic activity of food additives. Mutation Research, 130, 369. [Google Scholar]
- Kuś PM and Jerković I, 2018. New Sample preparation method for honey volatiles fingerprinting based on dehydration homogeneous liquid⁻liquid extraction (DHLLE). Molecules, 23, pii: E1769. 10.3390/molecules23071769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KG and Shibamoto T, 2000. Antioxidant properties of aroma compounds isolated from soybeans and mung beans. Journal of Agriculture and Food Chemistry, 48, 4290–4293. [DOI] [PubMed] [Google Scholar]
- Lee KG and Shibamoto T, 2001a. Inhibition of malonaldehyde formation from blood plasma oxidation by aroma extracts and aroma components isolated from clove and eucalyptus. Food and Chemical Toxicology, 39, 1199–1204. [DOI] [PubMed] [Google Scholar]
- Lee KG and Shibamoto T, 2001b. Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry]. Food Chemistry, 74, 443–448. [Google Scholar]
- Marlatt C, Ho CT and Chien's M, 2002. Studies of aroma constituents bound as glycosides in tomato. Journal of Agriculture and Food Chemistry, 40, 249–252. [Google Scholar]
- Nair B , 2001. Final report on the safety assessment of Benzyl Alcohol, Benzoic acid, and Sodium Benzoate. International Journal of Toxicology, 20(Suppl.3), 23–50. 10.1080/10915810152630729 [DOI] [PubMed] [Google Scholar]
- Nishimura H, Saito S, Kishida F and Matsuo M, 1994. Analysis of acute toxicity (LD50‐value) of organic chemicals to mammals by solubility parameter (delta). 1. Acute oral toxicity to rats. Sangyo Igaku, 36, 314–323(Japanese Journal Industrial Health). [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicological Program), 1989. NTP Toxicology and Carcinogenesis Studies of Benzyl Alcohol (CAS No. 100‐51‐6) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser. 343, 1–158. [PubMed] [Google Scholar]
- Oda Y, Hamano Y, Inoue K, Yamamoto H, Niihara T and Kunita N, 1978. Mutagenicity of food flavours in bacteria (1st Report). Osaka‐furitsu Koshu Eisei Kenkyu Hokoku Shokuhin Eisei Hen, 9, 177–181. [Google Scholar]
- OECD , 2001. Available online: https://hpvchemicals.oecd.org/ui/handler.axd?id=aa89d225-a2a7-4ed5-b8d6-c06b5e30b45b
- OECD , 2016. Guidance Document on Revisions to OECD Genetic Toxicology Test Guidelines. Available online: https://www.oecd.org/chemicalsafety/testing/Genetic%20Toxicology%20Guidance%20Document%20Aug%2031%202015.pdf
- Osorio C, Duque C, Suμrez M, Salamanca LE and Urueæa F, 2002. Free, glycosidically bound, and phosphate bound flavor constituents of badea (Passiflora quadrangularis) fruit pulp. Journal of Separation Science, 25, 147–154. [Google Scholar]
- RIFM (Research Institute for Fragrance Materials, Inc), 1986. Screening of Priority Chemicals for Reproductive Hazards. RIFM report number 5943. RIFM, Woodcliff Lake, NJ, USA.
- Rodríguez‐Bencomo JJ, Cabrera‐Valido HM, Pérez‐Trujillo JP and Cacho J, 2011. Bound aroma compounds of Marmajuelo and Malvası′a grape varieties and their influence on the elaborated wines. European Food Research and Technology, 233, 413–426. [DOI] [PubMed] [Google Scholar]
- Rogan EG, Cavalieri EL, Walker BA, Balasubramanian R, Wislocki PG, Roth RW and Saugier RK, 1986. Mutagenicity of benzylic acetates, sulfates and bromides of polycyclic aromatic hydrocarbons. Chem. Biol. International Rep, 58, 253–275. [DOI] [PubMed] [Google Scholar]
- Ruiz‐García L, Hellín P, Flores P and Fenoll J, 2014. Prediction of Muscat aroma in table grape by analysis of rose oxide. Food Chemistry, 154, 151–157. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food), 2001. Guidance on submissions for food additive evaluations by the scientific committee on food. Opinion expressed on 11 July 2001. Available online: http://ec.europa.eu/food/fs/sc/scf/out98_en.pdf
- SCF (Scientific Committee on Food), 2002. Opinion of the Scientific Committee on Food on Benzyl alcohol. Opinon expressed on 24 September 2002. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/fs_food-improvement-agents_flavourings-out138.pdf
- Scognamiglio J, Jones L, Vitale D, Letizia CS and Api AM, 2012. Fragrance material review on benzyl alcohol. Food and Chemical Toxicology, 50 (Suppl 2), S140‐60. 10.1016/j.fct.2011.10.013. Epub 2011 Oct 19. [DOI] [PubMed] [Google Scholar]
- Seper KW, 2004. Chlorotoluenes, benzyl chloride, benzal chloride, and benzotrichloride In: Kirk‐Othmer Encyclopedia of Chemical Technology, fifth edition, Volume 6, pp. 323–337. Wiley‐Interscience, John Wiley & Sons, Inc. (Hoboken). [Google Scholar]
- Shmunes E, 1984. Allergic dermatitis to benzyl alcohol in an injectable solution. Archives of Dermatology, 120, 1200–1201. [PubMed] [Google Scholar]
- Smyth HF, Carpenter CP and Weil CS, 1951. Range finding toxicity data: List IV. Archives of Industrial Hygiene and Occupational Medicine, 4, 119–122. [PubMed] [Google Scholar]
- Storer RD, McKelvey TW, Kraynak AR, Elia MC, Barnum JE, Harmon LS, Nichols WW and DeLuca JG, 1996. Revalidation of the in vitro alkaline elution/rat hepatocyte assay for DNA damage: improved criteria forassessment of cytotoxicity and genotoxicity and results for 81 compounds. Mutation Research, 368, 59–101. [DOI] [PubMed] [Google Scholar]
- Sudareva NN and Chubarova EV, 2006. Time‐dependent conversion of benzyl alcohol to benzaldehyde and benzoic acid in aqueous solutions. Journal of Pharmaceutical and Biomedical Analysis, 41, 1380–1385. [DOI] [PubMed] [Google Scholar]
- TemaNord , 2002. Food Additives in Europe 2000 ‐ Status of safety assessments of food additives presently permitted in the EU, TemaNord 2002:560. Available online: https://www.researchgate.net/publication/263859718_Food_additives_in_Europe_2000_Status_of_safety_assessments_of_food_additives_presently_permitted_in_EU
- Turvey SE, Cronin B, Arnold AD, Twarog FJ, Dioun AF, 2004. Adverse reactions to vitamin B12 injections due to benzyl alcohol sensitivity: successful treatment with intranasal cyanocobalamin. Allergy, 59, 1023–1024. [DOI] [PubMed] [Google Scholar]
- Villanova and Martínez , 2007. First study of determination of aromatic compounds of red wine from Vitis vinifera CV. Castañal grown in Galicia (NW Spain). European Food Research and Technology, 224, 431–436. [Google Scholar]
- Vilanova M, Genisheva Z, Bescansa L, Masa A and Oliveira JM, 2012. Changes in free and bound fractions of aroma compounds of four Vitis vinifera cultivars at the last ripening stages. Phytochemistry, 74, 196–205. [DOI] [PubMed] [Google Scholar]
- Wambre E and Jeong D, 2018. Oral Tolerance Development and Maintenance. Immunol Allergy Clin North Am., 38, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. Environment Health Criteria 240. Available online: http://www.who.int/foodsafety/publications/chemical-food/en/
- Wilson JP, Solimando Jr DA and Edwards MS, 1986. Parenteral benzyl alcohol‐induced hypersensitivity reaction. Drug Intell Clin Pharm, 20, 689–691. [DOI] [PubMed] [Google Scholar]
- Yang J, Li D and Sun C, 2012. Simultaneous determination of eleven preservatives in foods using ultrasound‐assisted emulsification micro‐extraction coupled with gas chromatography‐mass spectrometry. Analytical Methods, 4, 3436. [Google Scholar]
- Yasunaga K, Kiyonari A, Oikawa T, Abe N and Yoshikawa K, 2004. Evaluation of the Salmonella umu test with 83 NTP chemicals. Environmental and Molecular Mutagenesis, 44, 329–345. [DOI] [PubMed] [Google Scholar]
- Yoo YS, 1986. Mutagenic and antimutagenic activities of flavoring agents used in foodstuffs. Journal Osaka City Medical Center, 34, 267–288 [Osaka‐shi Igakkai Zasshi]. [Google Scholar]
- York RG, Barnwell P and Bailes W, 1986. Final Report. Screening of priority chemicals for reproductive hazards. Environmental Health Research and Testing, Inc. No. ETOX‐1002.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of reported use levels (mg/kg) of benzyl‐alcohol (E 1519) provided by industry
Concentration levels of benzyl‐alcohol (E 1519) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of benzyl‐alcohol (E 1519) from its use as a food additive for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to benzylalcohol (E 1519) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)


