Abstract
The European Commission asked EFSA for a Scientific Opinion: to revise the state of knowledge about the differences between the chronic wasting disease (CWD) strains found in North America (NA) and Europe and within Europe; to review new scientific evidence on the zoonotic potential of CWD and to provide recommendations to address the potential risks and to identify risk factors for the spread of CWD in the European Union. Full characterisation of European isolates is being pursued, whereas most NA CWD isolates have not been characterised in this way. The differing surveillance programmes in these continents result in biases in the types of cases that can be detected. Preliminary data support the contention that the CWD strains identified in Europe and NA are different and suggest the presence of strain diversity in European cervids. Current data do not allow any conclusion on the implications of strain diversity on transmissibility, pathogenesis or prevalence. Available data do not allow any conclusion on the zoonotic potential of NA or European CWD isolates. The risk of CWD to humans through consumption of meat cannot be directly assessed. At individual level, consumers of meat, meat products and offal derived from CWD‐infected cervids will be exposed to the CWD agent(s). Measures to reduce human dietary exposure could be applied, but exclusion from the food chain of whole carcasses of infected animals would be required to eliminate exposure. Based on NA experiences, all the risk factors identified for the spread of CWD may be associated with animals accumulating infectivity in both the peripheral tissues and the central nervous system. A subset of risk factors is relevant for infected animals without involvement of peripheral tissues. All the risk factors should be taken into account due to the potential co‐localisation of animals presenting with different disease phenotypes.
Keywords: chronic, wasting, cervids, strain, risk, zoonotic
Summary
In 2018, the European Food Safety Authority (EFSA) was asked by the European Commission to deliver a Scientific Opinion on three Terms of Reference (ToRs) related to chronic wasting disease (CWD): (ToR1) to revise the state of knowledge, considering new scientific data, about the differences: a) between the strains found in different species in North America (NA) and in Europe and b) between the strains found so far in moose, reindeer and red deer in Europe, with the main emphasis on transmissibility (transmission paths), pathogenicity and prevalence of the different strains and susceptibility of the different species/genotypes; (ToR2) to revise the new scientific evidence on the zoonotic potential of CWD, to assess the risk of transmission to humans through the consumption of fresh meat, meat products and offal of cervids and to provide recommendations on possible additional control measures to address the risks identified; and (ToR3) to identify risk factors that can facilitate the spread of CWD in the European Union given the current situation of the disease.
To source the relevant data, the extensive literature searches used in the previous recent EFSA Scientific Opinions on CWD (EFSA BIOHAZ Panel, 2017, 2018) were updated. In addition, research groups known to be conducting bioassay transmission studies and molecular/biochemical studies (protein misfolding cyclic amplification (PMCA), real‐time quaking‐induced conversion (RT‐QuIC), PrPres typing, conformational stability, proteinase K (PK) resistance, etc.) to characterise and the transmissibility of CWD field isolates from cases confirmed in Norway/Finland and in NA were asked to provide additional pre‐publication data. Regarding risk factors, the strength of the evidence for the causal role of each factor was appraised, based on study design and a score‐based ranking from the strongest evidence (intervention studies) to the weakest (theoretical biological plausibility) was applied.
Full characterisation of European isolates is being pursued through the collection of data on host species spectrum and genotype, clinical presentation, histopathology, immunopathology, tissue distribution, pathogenesis, the biochemical properties of the PrPSc, and bioassay through experimental transmission to a wide range of rodent models, whereas most NA CWD isolates have not been fully characterised in this way. However, preliminary data support the contention that the CWD strains identified to date in Europe and NA are different and suggest the presence of strain diversity in the European cervid population. The origin(s) of CWD in Europe remains unknown, and while it is clear that the disease identified in the reindeer in Nordfjella is contagious, the nature of the prion disease in the other species (the European moose and red deer) is still to be established.
NA CWD has been transmitted experimentally to cattle and sheep, but with incomplete attack rates. The species barrier appears higher for pigs, although challenged animals can support low‐level prion amplification. Experimental transmission to transgenic (tg) mice and other rodent models shows some difference in the host ranges of different isolates but, particularly for the European isolates, many bioassays are still ongoing and data are not yet available. The number of strains, the strain diversity, the prevalence and the potential host range of disease in both NA and Europe CWD may all be underestimated.
In vitro studies suggest that CWD isolates derived from experimentally challenged reindeer, and elk with a specific PRNP polymorphism (132 MM) would present an intermediate potential of conversion of human PrP to disease‐associated PrPSc. Some studies have shown that exposure to some NA CWD isolates can result in the conversion of human PrP in vitro and that some NA CWD isolates can transmit disease efficiently to squirrel monkeys. However, in vivo studies performed with humanised mice and macaques are considered to be the most pertinent models of human susceptibility and there is conflicting evidence on the transmissibility of NA CWD isolates in these models.
Epidemiological studies suffer from many methodological limitations and logistic constraints and some of them are still ongoing in NA but, until now, there is no epidemiological evidence of NA CWD causing disease in humans. The risk to humans through consumption of meat, meat products and offal derived from CWD‐infected cervids cannot be directly assessed. At individual level, consumers of meat, meat products and offal derived from CWD‐infected cervids will be exposed to the CWD agent(s). At the population level, the probability of exposure via consumption of venison depends on the prevalence of CWD agent(s) in each of the species that are consumed (reindeer, moose, red deer), which is not known. Preliminary testing of animals intended for human consumption with removal of any carcases that test positive, or the removal of high‐risk tissues from cervids intended for human consumption, or the combination of these measures, would reduce the probability of dietary exposure of humans to the CWD agent(s). The prohibition of harvesting/hunting susceptible species in infected premises/areas could also be considered as a preventive measure.
Current EU legislation requires a 3‐year monitoring programme for CWD from 1 January 2018 to 31 December 2020 to be implemented in six Member States (MSs) that have a wild and/or farmed and/or semi‐domesticated population of moose and/or reindeer: Estonia, Finland, Latvia, Lithuania, Poland and Sweden. In 2018, the six MS tested a total 5,110 cervids, of which 4,674 (91.5%) were wild animals, mostly roe deer and red deer, and 436 (8.5%) were captive, farmed or semi‐domesticated, with more than half of them being semi‐domesticated reindeer tested in Finland. Over 59% of all cervids tested were from healthy hunted/slaughtered fit for human consumption animals, whose probability of disease is lower than that of sick animals, road kills or fallen stock. Up to 20 September 2019, 28 cases have been reported in Europe: 19 wild reindeer, 4 moose and one red deer in Norway, one moose in Finland and three moose in Sweden.
Using data from the NA CWD experience, 13 groups of risk factors have been identified based on their biological plausibility to spread CWD. Some of these are supported by epidemiological evidence from NA CWD studies with variable strength of evidence after applying the score‐based ranking, while others remain hypothetical:
Natural movement of live wild deer from infected areas,
Man‐mediated movement of live farmed/free‐ranging deer from infected areas,
Failure to separate live farmed and free‐ranging deer,
High deer density,
Species‐specific social organisation,
Sex‐related behaviours,
Natural or man‐mediated animal aggregation,
Consumption of forage grown on contaminated soil,
Fallen stock or inappropriate disposal of carcasses and slaughter by‐products,
Movement of other animals (working dogs, scavengers, predators),
Transfer of inanimate vehicles of contamination (fomites),
Environmental persistence of prions,
Host genetics.
All the identified risk factors may contribute to the spread of the disease when it is associated with the accumulation of infectivity in peripheral tissues, a host phenotype that is compatible with a contagious disease. A subset of risk factors (1, 2, 6, 8, 9, 10, 11, 12 and 13) is relevant to cases of disease that do not involve peripheral accumulation of infectivity and are therefore less contagious or non‐contagious and may contribute to the spread of the disease mainly via environmental contamination following death. Some risk factors are man‐mediated and are considered preventable. Their management could contribute to the decrease in the theoretical risk of spread of CWD. The potential co‐localisation of cervid species and disease phenotypes mean that all the identified groups of risk factors should be taken into account when considering interventions.
It is recommended: (1) to document PRNP gene polymorphisms (nature, distribution and frequency) in European cervid populations; (2) to collate data to fully characterise European CWD isolates and collect data on new cases that may arise in Europe, together with NA positive control material; (3) to collect data on tissue distribution in naturally infected animals and, ideally, through experimental pathogenesis studies in cervid species; (4) and to maximise the effectiveness of the current compulsory surveillance by aiming at testing primarily animals in the risk groups and increasing sample size per primary sampling unit (PSU) (up to 30 at risk animals).
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor 1
1.1.1. Epidemiological situation
Until March 2016 when the disease was detected in Norway, CWD was believed to be absent of Europe, while it is widespread in the cervid population in USA and Canada. Since March 2016,2 23 cases of CWD have been detected in Norway (19 in reindeer (all in the same wild reindeer area of Nordfjella), three in wild moose and one wild red deer).3
Over that period 42.000 samples from wild, semi‐domesticated and farmed cervids have been examined for CWD in Norway. The competent authority in Norway culled the entire population of wild deer in Nordfjella in an attempt to eradicate CWD from that region.
The first case of CWD has been confirmed in the EU, in Finland in a moose on 7 March 2018. Finland announced the immediate suspension of intra‐trade movements and export of live cervids from Finland. No other cases have been reported since this date.4
1.1.2. Surveillance and control
Surveillance, control and eradication of CWD fall into the scope of Regulation (EC) No 999/2001 (the TSE Regulation). Its article 6(1) provides that each Member State is to carry out an annual monitoring based on active and passive surveillance, i.e. submission to TSE testing of animals whose neurological or behavioral disorders are compatible with a TSE, where an epidemiological investigation excluded the possibility of another infection. No eradication measure for CWD are laid down in the TSE Regulation, which implies that it is for the Member State to decide which measure to apply in case of detection of CWD in a farmed cervid.
Based on the part I of the EFSA Scientific Opinion of December 2016 on CWD in cervids and in order to estimate the prevalence and geographical spread of CWD, the Commission adopted a three‐year surveillance programme, starting in 2018, for CWD in cervids, in Estonia, Finland, Latvia, Lithuania, Poland and Sweden, as those countries have a reindeer and/or a moose population.
In the part II of its Scientific Opinion on CWD of December 2017, EFSA indicated that the finding of the first case of red deer in Norway meant that the surveillance in those countries did not cover geographically all the Member States in which red deer are present. It recommended thus that specific sampling and testing for CWD is incorporated into any general wildlife surveillance programmes.
1.1.3. Diagnostic tests
Testing for CWD should be done on both the brain and lymphoid tissues to maximise the diagnostic sensitivity, using rapid tests as well as confirmatory tests (Western Blotting and Immunohistochemistry). The rapid tests currently available and used in cervids have not been subject to a full standardised and laboratory assessment for use in cervids (they have been fully validated for the testing of brain from cattle and sheep in accordance with the OIE recommendations). Tests must be used in the assumption that extrapolation from other species in a different geographical location will provide satisfactory results.
1.1.4. Public health risk
The part I of the EFSA opinion (2016) highlighted that there is no evidence of an absolute species barrier between CWD‐affected cervids and humans. However, from the epidemiological investigations carried out till that moment, no association has been made between the occurrence of sCJD in humans and exposure to CWD.
The part II of the EFSA opinion (2017) stressed that, since the tissue distribution of infectivity in CWD infected cervids extends beyond the central nervous system (CNS) and lymphoid tissues into other edible tissues, exclusion from the food chain of the whole carcass of any infected animal would be required to ensure that human exposure is eliminated.
In April 2017, the scientific community was informed of the preliminary results of an ongoing Canadian research project on macaques fed with CWD infected meat. The results were presented at the international prion conference in May 2017 and showed for the first time that CWD prions could, after experimental transmission by the oral route, give rise to TSE in Cynomolgus macaques (Macaca fascicularis). The monkeys developed the disease after 5 to 6 years. The EFSA opinions did not take into account this research project as it was (and is) not yet published.
On the other hand, a study developed by the Laboratory of Persistent Viral Diseases, of the National Institute of Allergy and Infectious Diseases of Montana (USA), published on 25 April 2018,5 concluded that CWD is not transmissible to Cynomolgus macaques (CM). This study presents new data for seven CWD‐inoculated CM euthanised from 11–13 years post CWD‐inoculation and eight additional un‐inoculated control CM. New and archival CM tissues were screened for prion infection using the ultrasensitive RTQuIC assay, immunohistochemistry and immunoblot. In this study, there was no clinical, pathological or biochemical evidence suggesting that CWD was transmitted from cervids to CM. This study makes reference to the above‐mentioned ongoing experiment in Canada attempting transmission of CWD to CM. Although their results have not yet been published, oral presentations and abstracts have stated that they may have seen positive transmission of CWD to some CM. IHC staining of PrP in spinal cord of CWD inoculated CM was the main disease specific feature reported by the Canadians.
However, in the study developed by the Laboratory of Persistent Viral Diseases, similar PrP staining by IHC in spinal cord were seen in both un‐inoculated and CWD‐inoculated CM. Therefore, they did not regard the data on this observation to be evidence for CWD infection of CM.
1.1.5. New studies, investigations and evidences on the strains, pathogenicity, transmissibility and zoonotic aspects of CWD
Since the publication of EFSA's Scientific Opinion on CWD (I and II) several investigations have progressed. Preliminary results in rodent models indicate that the reindeer, moose and red deer are affected with different stains. The observed prevalence in Norwegian moose is lower than that in reindeer. It is still unknown if CWD poses a risk to human health. However, determining this risk is critical. Different studies have reached contradictory conclusions as regards the zoonotic potential as explained above.
EFSA is requested to provide a Scientific Opinion on the following questions on CWD in cervids:
ToR1: Revision of the state of knowledge, considering new scientific data, about the differences between the strains found in different species in North America and in Europe and between the strains found so far in moose, reindeer and red deer in Europe; with the main emphasis on transmissibility (transmission paths), pathogenicity and prevalence of the different strains and susceptibility of the different species/genotypes.
ToR2: To revise the new scientific evidence on the zoonotic potential of CWD; to assess the risk of transmission to humans through the consumption of meat and meat products of cervids and to provide recommendations on possible additional control measures to address the risks identified.
ToR3: Identify risk factors that can facilitate the spread of CWD in the European Union given the current situation of the disease.
1.2. Interpretation of the Terms of Reference (if appropriate)
The working group (WG) asked the European Commission for further clarification about the emphasis to be given to ‘transmissibility (transmission paths)’ and ‘pathogenicity’, as these terms in the context of the above ToR are not unequivocal.
The European Commission replied as follows:
In relation to transmissibility, considering that ToR2 refers to the zoonotic potential, the intention is to focus on the ability of a strain to transmit among cervids under field conditions. However, the absolute ability of cross‐species transmission can also be explored if there is scientific information available in this regard. The European Commission agreed that the transmission paths are actually covered by ToR3 and can be obviated in ToR1.
Concerning the term pathogenicity, it should be indeed understood as pathogenesis (progression of the infection within the individual host and the distribution of infectivity in tissues). Thus, the European Commission agreed to make reference to ‘pathogenesis’ instead of ‘pathogenicity’ in the Scientific Opinion. The term pathogenesis is hereinafter used in the Scientific Opinion.
Chronic wasting disease in this Opinion is defined as any transmissible spongiform encephalopathies (TSE) identified in cervids regardless the geographical location and its variability in terms of pathogenesis, transmissibility, host range and prevalence.
Most of the data required to address the ToRs are not in the public domain because they have not yet been published or they are not complete, as many of these studies are still ongoing. It must be highlighted that some of the conclusions in this Scientific Opinion have been drawn taking advantage of data shared with the WG in confidence by researchers undertaking these studies; therefore, full citations and references cannot always be provided.
2. Data and methodologies
2.1. Published data
Data have been sourced via different literature searches. The extensive literature searches used in the previous recent EFSA Scientific Opinions on chronic wasting disease (CWD) (EFSA BIOHAZ Panel, 2017, 2018) have been updated.
The search string of the literature search of in vivo transmission studies of TSE in animal models exploring the zoonotic potential of CWD used in the first Scientific Opinion on CWD (EFSA BIOHAZ Panel, 2017) was updated for the period 1 January 2016 until 30 September 2018, as follows: (BSE OR TSE OR scrapie OR CWD OR *CJD OR Nor98 OR Nor‐98 OR spongiform encephalopa* OR ‘chronic wasting disease’ OR ‘creutzfeldt‐jakob’ OR ‘creutzfeldt jakob’ OR prion OR prp*) AND (transmissible OR transmission OR transmitted OR transgenic OR barrier OR passage* OR tg OR humanised OR humanised). These terms were searched in the titles of the scientific publications. The search was conducted in the following databases: ISI Web of Knowledge; CAB Abstracts; Current Contents; FSTA; Journal Citation Reports and Web of Science. The search was restricted to the English language. In total, 127 references were retrieved and screened for studies of interest. A pair of reviewers conducted the screening: each reviewer independently screened the title and abstracts. Discrepancies were discussed by the two reviewers until a final shortlist of references was agreed. A subset of 16 relevant references was selected and considered in this assessment by reviewing the full papers.
An additional and more targeted literature search of the transmission studies exploring the zoonotic potential using bioassays was applied by the WG member responsible for this section, using the following search strings: ‘CWD AND prion AND human AND species barrier’ (25 references); ‘CWD AND prion AND human AND primates’ (102 references); ‘CWD AND prion AND human AND transgenic mice’ (29 references). These terms were searched in the titles of the scientific publications. The search was conducted in the PubMed NCBI database. In total, 100 unique references were retrieved and were screened for studies of interest by a single reviewer. A subset of 23 relevant studies was considered in this assessment.
To carry out the identification of the risk factors (Section 3.6), preliminarily, the search string of the literature search for exploring the zoonotic potential of CWD used in the first Scientific Opinion on CWD (EFSA BIOHAZ Panel, 2017) was updated for the period 1 January 2016 until 31 January 2019, as follows: (‘chronic wasting disease’ OR CWD OR wasting OR TSE* OR BSE OR scrapie OR PrP* OR PRNP OR prion*) AND (surveillance OR prevalence OR incidence OR epidem* OR introduc* OR spread OR risk OR ‘public health’ OR zoono*) AND (deer* OR cervid* OR moose* OR elk* OR reindeer*). In total, 11 references were retrieved and screened by a single reviewer for studies of interest and four references were added to the available collection and reviewed in full. In addition, risk assessment reports on the epidemiology, risk analysis, surveillance and control of CWD were reviewed.
The shortlist of references extracted from the literature reviews, relevant references including recent review papers and non‐peer‐review documents known by the experts and in the public domain, in particular the qualitative risk assessments on CWD produced by VKM (Norway), Defra (UK) and the New York State Interagency CWD Risk Minimisation Plan (NYSDEC), were also used and the references within these documents cross‐checked against the search string output to identify, through snowballing, any additional references of interest including review papers, book chapters, non‐peer reviewed papers and any relevant papers published since the literature reviews were conducted.
The abstract book from the Prion conference 2019 was also screened for any new data relevant to any section of the Opinion.
2.2. Unpublished Data
Research groups that are known to be conducting bioassay transmission studies to characterise and assess the transmissibility of CWD field isolates from cases confirmed in Norway/Finland and in NA were asked to provide additional data. In total, 17 research groups from France, Italy, Spain, UK, Germany, the Netherlands, Canada and USA were approached.
Data providers were asked specifically for permission to use the confidential information they have provided from their ongoing studies (such as incomplete bioassays and sub‐passages) to contribute to anonymised statements on aggregated data.
For research groups conducting bioassays, standardised data were requested on the identity (including host species, gender and age) and the geographical origin of each isolate and the bioassay model(s) in which transmission studies were ongoing. Information was also requested on emerging results, e.g. if the first passage was completed and if there is any evidence of transmission observed, are sub‐passages being undertaken? If so, which models are being used and is there any data yet available? Specific information was also requested on any planned or completed publications on these specific isolates and/or publications about the transmission outcomes of the present studies.
All research groups that were known to have conducted molecular/biochemical studies (protein misfolding cyclic amplification (PMCA), real‐time quaking‐induced conversion (RT‐QuIC), PrPres typing, conformational stability, PK resistance, etc.) to characterise CWD field isolates from cases confirmed in Norway and Finland, and in NA, were also asked to provide published and pre‐publication data/updates on individual isolates. In total, 12 research groups from France, Italy, Spain, UK, Norway, Sweden, Canada and USA were approached and asked if they would provide data on the identity and geographical origin of each isolate, and on the methods, substrates and positive controls used for the studies and any preliminary outcomes. Specific information was also requested on any planned or completed publications on these specific isolates and/or publications about the outcomes of the present studies.
It was acknowledged that some data may be already in the public domain but additional data, not recoverable from published literature, would be of paramount importance to ascertain differences between/within European and NA strains, should they exist, in order to address the ToRs as fully as possible. It was emphasised in the request that the WG was not only interested in the bioassays performed with the Scandinavian samples but also considered it important to collect data on the results of bioassays performed with NA CWD samples to enable comparison with European isolates and to identify any potential strain differences.
2.3. Surveillance data
According to Part I.A, Chapter B.I Annex III of Regulation (EC) 999/2001, the information to be presented by all the Member State (MS) in their annual report should include animals other than bovine, ovine and caprine and the number of samples and confirmed TSE cases per species. Specific reporting requirements apply to the MS covered by the 3‐year CWD monitoring programme referred to in Part III.A of Chapter A of this Annex, for which the annual report for the years 2018, 2019 and 2020 shall include:
-
The number of cervid samples submitted for testing, by target group according to the following criteria:
-
—
primary Sampling Unit (PSU) identifier,
-
—
species,
-
—
management system: farmed, captive, wild or semi‐domesticated,
-
—
target group: for farmed and captive cervids: (i) fallen/culled farmed or captive cervids, defined as farmed or captive cervids found dead on the enclosed territory in which they are kept, during transport or at slaughterhouse, as well as farmed or captive cervids killed for health/age reasons; (ii) clinical/sick farmed or captive cervids, defined as farmed or captive cervids showing abnormal behavioural signs and/or locomotor disturbances and/or as being generally in poor condition; (iii) slaughtered farmed cervids which have been declared unfit for human consumption; (iv) slaughtered farmed cervids considered fit for human consumption if a Member State identifies fewer than 3 000 farmed and captive cervids from the groups (i) to (iii). For wild and semi‐domesticated cervids: (i) fallen/culled wild or semi‐domesticated cervids, defined as cervids found dead in the wild as well as semi‐domesticated cervids found dead or killed for health/age reasons; (ii) road‐ or predator‐injured or killed cervids, defined as wild or semi‐domesticated cervids hit by road vehicles, by trains or attacked by predators; (iii) clinical/sick wild and semi‐domesticated cervids, defined as wild and semi‐domesticated cervids which are observed as showing abnormal behavioural signs and/or locomotor disturbances and/or as being generally in poor health condition; (iv) wild hunted cervids and slaughtered semi‐domesticated cervids which have been declared unfit for human consumption; (v) hunted wild game and slaughtered semi‐domesticated cervids considered fit for human consumption if a Member State identifies fewer than 3 000 wild and semi‐domesticated cervids from the groups (i) to (iv).
-
—
sex,
-
—
The results of the rapid and confirmatory tests (number of positives and negatives) and, where applicable, of further isolate characterisation investigations, the tissue sampled, and the rapid test and confirmatory technique used.
The geographical location, including the country of origin if not the same as the reporting Member State, of positive cases of TSE.
The genotype and species of each cervid found positive for TSE.
Where tested, the genotype of cervids tested and found negative for TSE.
EFSA collects and collates data on the animals tested for TSE by the 28 MS and four additional non‐MS, which since 2018 have been Iceland, Norway, Switzerland and North Macedonia. Surveillance data from Europe in 2016, 2017 and 2018 have been extracted from the above‐mentioned annual reports submitted by the MS and stored in EFSA's data warehouse and presented in tabular format.
3. Assessment
3.1. Summary of the knowledge about CWD strains and their pathogenesis
Due to the incomplete understanding of the nature and molecular characteristics of prion strains, ‘strain typing’ currently relies on characterising the disease phenotype in the host, using a range of approaches including clinical presentation, histopathology and immunopathology, the biochemical properties of the PrPSc and bioassay through experimental transmission to well‐established rodent models. These represent the only approaches available for the identification of prion strains, but they all have their own intrinsic limits, which impact on the final relevance of the results they provide and, on the nomenclature, used to describe the strain(s) identified within any TSE isolate.
It is beyond the scope of this opinion to exhaustively describe such methods, but more detailed reviews of these techniques can be found elsewhere (Bruce, 2003; Boyle et al., 2017) and a summary of some key points is provided in Appendix A, for ease of reference.
3.1.1. North America
Seminal transmission studies of NA CWD isolates in cervid‐PrP‐expressing transgenic (tg) mice (Browning et al., 2004; LaFauci et al., 2006) indicated the possibility of CWD strain variation.
Since then, there have been further transmission studies of NA CWD isolates to cervid‐ and non‐cervid‐PrP expressing animals supporting the contention that several prion strains are responsible for the CWD cases observed in NA (Tamguney et al., 2006, 2009a; Johnson et al., 2011; Sigurdson et al., 2011; Crowell et al., 2015; Duque‐Velásquez et al., 2015; Triscott et al., 2015; Herbst et al., 2017; Bian et al., 2019). However, due to a lack of consistency between the studies (i.e. no use of common reference isolates/strains) and differences in methodological approaches (i.e. different animal models), comparing and/or merging data from individual studies remains difficult.
Beyond these limitations, two studies originating from the same research group have provided valuable insights into the diversity of CWD strains in NA (Angers et al., 2010; Telling, 2011). The inoculation of a relatively large panel of isolates from various species and geographic locations in NA into transgenic mice overexpressing cervid PrP, indicated the presence of at least two CWD prion strains, referred to as CWD1 and CWD2, that circulate either independently or as a strain mixture. These results were consistent with transmission studies carried out using other models and CWD isolates (Tamguney et al., 2006; Di et al., 2013). Despite consistent differences in the incubation time and neuropathological profiles in cervid mice, the PrPres western blot (WB) banding patterns in the brains of mice infected by either CWD1 or CWD2 were indistinguishable from one another (Angers et al., 2010).
While the existence of at least two CWD strains in North America should be considered as an established fact, it is unlikely that the strain typing work carried out so far has provided a comprehensive or definitive picture of the diversity of CWD strains that are circulating in NA cervid populations.
CWD pathogenesis has been investigated using both naturally exposed and experimentally challenged animals and a full description of these studies can be found in a previous opinion (EFSA BIOHAZ Panel, 2017).
In summary, natural exposure apparently occurs by the oral route, through direct contact between individuals or via a contaminated environment (Moore et al., 2016a). Pathogenesis and abnormal PrP distribution in CWD are very similar to that reported in classical scrapie in small ruminants with susceptible genotypes (EFSA BIOHAZ Panel, 2014).
Following experimental challenge by the oral route, initial entry of the agent occurs through the gut‐associated lymphoid tissue (GALT) with rapid involvement of the lymphoreticular system (LRS) and passage to the enteric nervous system (ENS). The CWD agent(s) then spreads to the CNS via autonomic nervous structures (Sigurdson et al., 1999, 2002; Fox et al., 2006). Early prion infectivity/seeding activity has also been observed in the blood during the preclinical phase of the incubation period (Mathiason et al., 2006; Kramm et al., 2017).
Race et al. (2007) concluded that the involvement of the LRS seems to vary between deer and elk, with less abnormal PrP deposition in the lymphoid tissues of elk compared with deer. It is unclear at this stage whether such variations are the consequence of differences in CWD strains with differing lymphotropism and/or host PrP gene polymorphisms, or both.
A limiting factor for the understanding of the diversity of natural disease is that surveillance has not been consistently applied in NA, with some regions undertaking initial screening of lymphoid tissues only. This means that animals in which PrP accumulation is largely, or completely, limited to the CNS (as is the case for the European moose and red deer) would not be detected or investigated.
The possibility of such cases having occurred undetected is supported by the recent report of the first confirmed case of CWD in a farmed red deer in the province of Quebec in Canada in 2018, in a region where no cases of CWD had been confirmed before. The index case was a 15‐month‐old male, clinically healthy at slaughter. The initial enzyme‐linked immunosorbent assay (ELISA) test was positive in the brain stem but negative in the lymph node, even though a low level of abnormal PrP was subsequently detected by immunohistochemistry (IHC) in a low number of follicles in the lymph node. Following the cull of the herd, over 1700 red deer were tested and an additional 10 cases of CWD were identified in females ranging in age from 18 to 28 months. All cases were positive in the obex by ELISA, IHCand WB, although the extent of abnormal PrP deposition detected by IHC was somewhat variable. A higher degree of variability in abnormal PrP accumulation was found in lymphoid tissues, with no detection by IHC in the tonsils of several cases (Walther et al., 2019). This presentation of the first outbreak of red deer in NA resembles that of a disease that is transmitted animal‐to‐animal, facilitated by the high density of animals, leading to high contact rates and local environmental contamination that favour horizontal transmission of disease, as opposed to the situation in wild populations.
In animals incubating CWD, abnormal PrP and/or infectivity has been demonstrated in placenta, saliva, faeces and urine which are all likely to contribute to inter‐individual transmission but also to the general contamination of the environment (Mathiason et al., 2006; Tamguney et al., 2009a; Haley and Hoover, 2015; Plummer et al., 2017).
Abnormal PrP and/or prion seeding activity and/or prion infectivity has been detected in a large number of tissues (see Table 1 in EFSA BIOHAZ Panel, 2017), in particular in those commonly consumed as venison (heart, skeletal muscles, tongue, liver, kidneys) or used as dietary supplements (antler velvet) (Sigurdson et al., 2001; Angers et al., 2006, 2009; Mitchell et al., 2012). Unfortunately, in most instances, it is difficult, or not possible, to derive a quantitative estimate from the published data (Section 3.4.2) of the amount of prion/seeding activity in these tissues.
Table 1.
Summary of the state of research on experimental transmission models of CWD isolates in North America and Europe
| Species modelled | CWD isolates | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | USA | Canada | Norway | Finland | |||||||||||
| Rodent models | Elk | Mule deer | WT deer | Moose | Elk | Mule deer | WT deer | Moose | Red deer (exp) | Reindeer (exp) | Moose | Red deer | Reindeer | Moose | |
| Mouse | conventional mice | Y/N | Y/N | Y | N | Y/N | ong/N | Y | Y | ||||||
| Mouse | tg‐mousePrP | Y | Y | ong | Y | ong | Y | ||||||||
| Hamster | hamsters | ong | ong | ong | |||||||||||
| Hamster | tg‐hamsterPrP | Y | ong | N | ong | ||||||||||
| Bank vole | bank voles | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |||
| Deer | tg‐cervidPrP(all variants) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ong | Y | ong | |
| Bovine | tg‐bovinePrP | N | Y/N | Y/N | Y | Y/N | Y | ong/N | ong | ong | ong | ||||
| Ovine | tg‐sheepPrP(all variants) | Y | ong | ong/N | N | Y | N | Y/N | ong | ong | ong | ||||
| Porcine | tg‐porcinePrP | ong/N | ong/N | ong | ong | ong | |||||||||
| Human | tg‐humanPrP (all variants) | N | N | ong/N | N | ong/N | N | N | N | ong | ong | ong | |||
| Vulture | tg‐vulturePrP | ong | ong | ong | |||||||||||
RED: ongoing or no transmission; GREEN: successful transmission; AMBER: mixed results. ong: ongoing.
‘Y’: completed or ongoing experiments show there is evidence of positive transmission in at least a subset of recipient rodents, independently on the attack rate and incubation time.
‘N’: completed bioassays show there is no evidence of positive transmission in any of the recipient rodents.
‘ong’: ongoing experiments, but there is still no evidence of positive transmission in at least one of the recipient rodents.
Some results are expressed with a double symbol, i.e. ‘Y/N’ or ‘ong/N’, meaning that different results have been obtained with different CWD isolates from the same species and country, or that different outcomes have been obtained with the same CWD isolate after inoculation in different rodent lines gathered in each row.
3.1.2. Europe
Due to their recent identification, the characterisation of the EU CWD cases remains incomplete. The amount of data related to the pathogenesis of the prion disease(s) identified in these animals is extremely limited as they are all field cases and most of the samples are autolysed.
In Norwegian reindeer, the presence of PrPSc in certain lymphoid organs (including in cases in which no abnormal PrP is detected in the CNS) suggests a pathogenesis similar to that of most NA cervids. However, because of the potential impact of CWD strain and/or PrP genetics on the pathogenesis of prion disease, the final distribution and level of prion infectivity in tissues of incubating and affected animals could significantly differ between EU and NA cervids. The PrPres WB patterns obtained from Norwegian reindeer were similar to those observed in an American elk CWD sample and Canadian CWD isolates including reindeer orally inoculated with NA CWD material. The immunohistochemical (IHC) distribution of PrPSc in the obex, the cerebellum and the lymph nodes was also similar to that of Canadian reindeer orally challenged with CWD (Mitchell et al., 2012; Benestad et al., 2016; Moore et al., 2016a).
Investigations carried out on three of the four positive moose cases (Alces alces) identified in Norway, for which lymphoid tissues were available, revealed the presence of detectable PrPSc in the brain but not in lymphoid tissues. Immunohistochemistry revealed that the Norwegian moose shared a common neuropathological phenotype (mainly intraneuronal staining) that clearly differed from that observed in both Norwegian reindeer and in CWD‐infected NA cervids. Moreover, WB revealed a PrPres banding pattern that clearly differed from CWD cases reported so far in NA cervids and in Norwegian reindeer (Pirisinu et al., 2018). The absence of detectable abnormal PrP in the lymphoid tissues of the Norwegian, Swedish (Gavier‐Widen, 2019) and Finnish moose (Korpenfelt, 2019) and the red deer (Vikøren et al., 2019), in which prion disease has been confirmed, clearly supports this contention and also suggests that in the EU, distribution patterns and level of accumulation of prions in tissues might significantly vary according to the prion strains and/or cervid species. Any extrapolation from the studies reviewed and summarised in EFSA's Scientific Opinion (EFSA BIOHAZ Panel, 2017) to cases arising in the European context should therefore be undertaken with caution.
There are no published data related to the molecular characterisation of the Norwegian red deer isolate, but personal communication from Norway (Benestad, 2019a) indicates that this isolate has a WB pattern that is different from both the Norwegian moose and reindeer and from CWD from North America. It does have some similarities with bovine spongiform encephalopathy (BSE), but the presence of BSE has been formally ruled out as a possibility by the EURL Strain Typing Expert Group (STEG) by sPMCA methods aiming at identifying the BSE strain in an isolate (Gough et al., 2014). As for the Norwegian CWD moose, no PrPSc was detectable in the lymph nodes and tonsils of this red deer (Vikøren et al., 2019).
Another feature of the European moose and red deer CWD cases is their advanced age, all between 13 and 16 years for the moose and 16 years for the red deer, as opposed to the CWD‐affected Norwegian reindeer that were aged between 1.5 and 8 years.
Transmission experiments to strain type the Norwegian CWD isolates are still ongoing, and therefore, definitive results are not yet available. Characterisation of the isolates from the four cases recently identified in elderly moose in Sweden and Finland is ongoing (see Section 3.5).
The origin(s) of CWD in Europe remain unknown and while it is clear that the disease identified in the reindeer in Nordfjella is contagious, the nature of the prion disease in the other species (the European moose and red deer) remains to be established.
3.1.3. Concluding remarks
A lack of consistency in methodologies makes comparison of data from different individual studies difficult. Although the strain typing work carried out so far has not provided comprehensive data on the CWD strains circulating in NA cervid populations, at least two CWD prion strains (CWD1 and CWD2) are responsible for disease in North America.
The pathogenesis of NA CWD typically starts with the accumulation of abnormal PrP in the lymphoid tissues, with subsequent involvement of the CNS. The extent of involvement of the LRS seems to vary between deer and elk. However, the first NA red deer case was positive in the brainstem and negative in lymphoid tissue with the primary ELISA screening test, although low levels of PrP accumulation were subsequently detected in lymphoid tissue.
NA surveillance has relied, in some regions, on the primary screening of lymphoid tissues only. Animals in which PrP accumulation is largely, or completely, limited to the CNS may not be detected by this method.
Disease pathogenesis in Norwegian reindeer is similar to most NA CWD cases. However, the Norwegian moose share a common neuropathological phenotype that differs from that observed in both Norwegian reindeer and in NA CWD cases. The Norwegian red deer isolate has a Western Blot pattern that is different from both the Norwegian moose and reindeer and from NA CWD.
The laboratory characterisation of European cervid TSE cases remains incomplete.
3.2. Transmissibility of CWD across species barriers
The transmission of prions between species is limited by the ‘transmission barrier’ (EFSA BIOHAZ Panel, 2015). The host factor that has been shown to play a very key role in the overall susceptibility to TSE is the amino acid sequence of the host PrP and its associated species‐specific polymorphisms. Early studies suggested that the cross‐species barrier resides essentially in the differences of the PrP primary structure between the host and donor species (EFSA Panel on Biological Hazards (BIOHAZ), 2011). Even single amino acid divergences may therefore have a major impact on transmission barriers. Besides species‐related primary structural incompatibilities between PrPSc and PrPC expressed in the new host, strain properties of the infectious agent have significant additional influence on the outcome of interspecies prion transmissions, as exemplified by the extensive host range properties of BSE. Therefore, even for prions deriving from the same species, the host range may vary according to the prion strain, implying that different CWD strains might have different host ranges and different potential for transmitting to livestock species and to man. In contrast, putative differences in the CWD host range between NA and Europe might be an indication of different CWD strains.
Animal species known to be susceptible to infection by CWD have been identified through the outcomes of surveillance activities and field research studies. Surveillance systems and field studies are challenging to design and to implement for wild species and have inherent limitations on the species that could be effectively targeted. Therefore, our knowledge of the current host range of CWD in NA and EU is limited to the species targeted by the surveillance systems in place.
The host range of CWD can be experimentally modelled through transmission studies in the species of interest. In these studies, CWD isolates from different cervid species and geographical origin are inoculated by the oral or the intracerebral (IC) routes into the species of interest and the challenged animals are monitored for the development of CWD. The oral route of inoculation is seen as the best proxy for assessing the susceptibility of a species under field conditions, as CWD is acquired by the oral route and peripheral host factors are known to have a significant impact on the outcome of TSE infections once exposure occurs (EFSA BIOHAZ Panel, 2015). Economical, ethical and practical issues, however, limit the feasibility of large‐scale transmission experiments in several species known to be potentially exposed to CWD.
The recognised importance of PrP primary structure in controlling prion transmissibility across species barriers paved the way for the development of transgenic (tg) mouse models expressing heterologous PrP sequences (Telling, 2011). Several tg mouse lines expressing PrP and polymorphic variants from cervids, small ruminants, cattle, pig, hamster, vole, human and several other species have been developed (Telling, 2011). Tg mice expressing the PrP of the species of interest can therefore be used as a surrogate recipient species in IC challenges aimed at modelling interspecies prion transmissions. Such tg mouse models cannot always be considered as an accurate proxy for the natural host, so a failure to transmit needs to be interpreted with caution (for a review, see EFSA BIOHAZ Panel, 2015). However, studies in tg mouse models allow the investigation of the permeability of a transmission barrier at the molecular level, i.e. in terms of donor PrPSc/recipient PrPC interactions in a physiological environment, which is a prerequisite for prion transmission to occur.
3.2.1. North American isolates
3.2.1.1. Natural hosts
Naturally infected species in North America include all the indigenous wild cervid species: white‐tailed deer, mule deer, moose and elk/wapiti. Natural CWD infection has been also detected in captive red deer in the United States (Schwabenlander et al., 2013) and Canada (Walther et al., 2019) and in a farmed reindeer in Illinois.6 Other cervid species are susceptible to CWD following experimental challenge. These include muntjac and fallow deer. Muntjac deer were susceptible to oral challenge with a NA CWD source and have been proposed as a suitable experimental species for modelling natural transmission routes of CWD (Nalls et al., 2013). Finally, fallow deer were susceptible to IC inoculation with CWD from white‐tailed deer and mule deer (Hamir et al., 2011).
Whether the natural host range of CWD in North America extends beyond the family Cervidae is currently unclear and no natural infections have been reported so far in wildlife species with substantial overlapping geographical range and which could play a role in the spread of CWD, such as predators and scavengers.
3.2.1.2. Non‐cervid domestic species
The remarkably high rate of natural CWD transmission in the ongoing NA epidemics raises the question of the risk to livestock grazing on CWD‐contaminated shared rangeland and subsequently developing a novel CWD‐related prion disease. This issue has been investigated by transmitting CWD via experimental challenge to cattle, sheep and pigs and to tg mouse lines expressing the relevant species PrP.
For cattle challenged with CWD, PrPSc was detected in approximately 40% of intracerebrally inoculated animals (Hamir et al., 2005, 2006a, 2007). Tg mice expressing bovine PrP have also been challenged with CWD and while published studies have negative outcomes (Tamguney et al., 2009b), unpublished data provided for the purposes of this Opinion indicate that some transmission of individual isolates to bovinised mice is possible (Table 1).
In small ruminant recipients, a low rate of transmission was reported between 35 and 72 months post‐infection (mpi) in ARQ/ARQ and ARQ/VRQ sheep intracerebrally challenged with mule deer CWD (Hamir et al., 2006b), while two out of two ARQ/ARQ sheep intracerebrally inoculated with elk CWD developed clinical disease after 28 mpi (Madsen‐Bouterse et al., 2016). However, tg mice expressing ARQ sheep PrP were resistant (Tamguney et al., 2006) and tg mice expressing the VRQ PrP allele were poorly susceptible to clinical disease (Beringue et al., 2012; Madsen‐Bouterse et al., 2016). In contrast, tg mice expressing VRQ sheep PrP challenged with CWD have resulted in highly efficient, life‐long asymptomatic replication of these prions in the spleen tissue (Beringue et al., 2012).
A recent study investigated the potential for swine to serve as hosts of the CWD agent(s) by intracerebral or oral challenge of crossbred piglets (Moore et al., 2016b, 2017). Pigs sacrificed at 6 mpi, approximately the age at which pigs reach market weight, were clinically healthy and negative by diagnostic tests, although low‐level CWD agent replication could be detected in the CNS by bioassay in tg cervinised mice. Among pigs that were incubated for up to 73 mpi, some gave diagnostic evidence of CWD replication in the brain between 42 and 72 mpi. Importantly, this was observed also in one orally challenged pig at 64 mpi and the presence of low‐level CWD replication was confirmed by mouse bioassay. The authors of this study argued that pigs can support low‐level amplification of CWD prions, although the species barrier to CWD infection is relatively high and that the detection of infectivity in orally inoculated pigs with a mouse bioassay raises the possibility that naturally exposed pigs could act as a reservoir of CWD infectivity.
3.2.1.3. Other species
Studies have demonstrated that the CWD agent(s) can be transmitted by the IC route in several species of rodents, such as voles (Subfamily Arvicolinae), deer mice (Peromyscus maniculatus), mice and hamsters (Subfamily Cricetinae). The susceptibility was, however, variable, being high in voles and deer mice but lower in mice and hamsters (Raymond et al., 2007; Heisey et al., 2010; Kurt et al., 2011; Di et al., 2013; Lee et al., 2013). Mink (subfamily Mustelinae) (Harrington et al., 2008), ferrets (Mustela putorius) (Bartz et al., 1998; Sigurdson et al., 2008) and cats (Mathiason et al., 2013) were susceptible to IC challenge with NA CWD sources, while CWD transmitted poorly to raccoons (Procyon lotor) by the IC route (Moore et al., 2019).
3.2.2. European isolates
The host range of CWD in Europe has been much less investigated so far, due to its recent identification. Among the cervid species involved in the CWD epidemics in North America, only some species (such as moose and reindeer) inhabit Europe; mule deer, white‐tailed deer and elk/wapiti are American cervid species, although a few populations of white‐tailed deer have been introduced into Europe. Others cervids that mainly inhabit Europe are red deer and roe deer. After the first detection in a reindeer in Norway in 2016 (Benestad et al., 2016), CWD has been detected in wild reindeer, moose and one red deer in Norway (Mysterud and Edmunds, 2019), in a moose in Finland in March 2018 and in three moose in Sweden in March, May and September 2019. CWD has not been detected so far in wild roe deer, fallow deer or white‐tailed deer nor in any farmed cervid species. However, for fallow deer and white‐tailed deer, the number of animals tested by the surveillance systems is still very low.
The potential host range of European CWD strains is under investigation by bioassay experiments in a range of model species; most of these studies are ongoing and there are no published data available so far. Data from the experiments that are known to be ongoing in different laboratories have been gathered for the purposes of this Opinion. Overall, reindeer CWD, moose CWD and red deer CWD brain isolates (and LRS isolates from some selected cases) are being tested for transmissibility in mice, hamsters, bank voles and in a range of tg mouse lines expressing PrP sequences from: cervids (Q226 or E226 deer PrP variants), small ruminants (ARQ, VRQ, AHQ and ARR PrP polymorphic variants), cattle, pig, vulture and human (M129 and V129 PrP polymorphic variants) (see Section 3.3.1). Importantly, in most of these animal models, the transmissibility of European CWD isolates will be directly comparable with the outcome of similar (published or ongoing) experiments with CWD isolates from North America.
While most of these studies are still ongoing, some experiments with CWD isolates from Europe have already produced evidence of transmission in some recipient species (Table 1). These include bank voles, conventional laboratory mice and tg mice expressing cervid PrP, sheep PrP and mouse PrP. The same rodent models are also susceptible to NA CWD isolates and will therefore allow comparative strain typing of NA and European CWD strains in due course. There is no strong evidence so far for rodent models being widely susceptible to NA isolates but not to European isolates or vice versa.
Table 1 summarises more than 500 ongoing, published or unpublished primary transmission experiments of NA or European CWD isolates from different cervids into various rodent models, which have been gathered following the requests described in Section 2.2. The CWD isolates are grouped according to geographical origin and cervid species, with each column summarising the results obtained with one or more CWD isolates from a given species and country. Rodent models are grouped according to the PrP species expressed. Some species have polymorphic PrP sequences, so more than one PrP sequence per species has been modelled. In these cases, each row summarises the data obtained with more than one PrP variant of a given species. Therefore, conventional mice include wt mice expressing PRNP a or PRNP b mouse PrP variants; bank voles include two genetic lines with different amino acids at codon 109 (Bv109M and Bv109I); tg‐cervidPrP mice include mouse lines expressing several cervid PrP variants (the deer wt Q226, the elk wt E226, the WTD variant S96, the elk variant M132); tg‐sheepPrP mice include mouse lines expressing the ARQ, VRQ, AHQ or ARR small ruminant PrP variants; finally, tg‐humanPrP mice include mouse lines expressing M or V at the human PrP polymorphic codon 129. Therefore, each box in the Table 1 summarises the outcome of bioassay experiments with one or more CWD isolates (from the same species and origin) in one or more recipient rodent models (expressing PrP from a single given species).
Most of the studies conducted by molecular/biochemical methods are still ongoing. The preliminary data obtained by molecular/biochemical methods were difficult to summarise and will not be reported in the present Opinion. This was mainly due to lack of detail in the results obtained by direct PrPSc analyses (PrPres typing, conformational stability, proteinase K resistance, which are intended to investigate CWD strains) and to the different methodological approaches employed in amplification assays (PMCA and RT‐QuIC). The information gathered by this activity shows that experiments aimed at modelling the species barrier for NA or European CWD isolates into different animal species, including humans, are underway in different laboratories and will be of help for understanding the potential host range of CWD strains.
3.2.3. Impact of the PRNP gene on transmissibility
Polymorphisms in the PRNP gene are known to influence susceptibility/resistance to prion disease in both small ruminants and humans (for recent review, see EFSA BIOHAZ Panel 2014, EFSA BIOHAZ Panel, 2017; Diack et al., 2014). Effects of host PRNP polymorphisms on CWD susceptibility/resistance have also been described in a number of cervid species (reviewed in EFSA BIOHAZ Panel, 2017, 2018).
However, deer and elk wild‐type PrP primary structures are equivalent, except at residue 226, which is glutamate in elk and glutamine in deer. The effect of this difference on CWD pathogenesis has been recently investigated using a gene‐targeting approach in which the mouse PrP coding sequence was replaced with elk or deer PrP. The results obtained following experimental challenge with deer and elk CWD inocula from NA showed that the resulting GtE226 and GtQ226 mice had distinct kinetics of disease onset, with incubation times shorter in GtE226 than in GtQ226 mice, indicating that amino acid differences at PrP residue 226 dictate the selection and propagation of divergent strains in deer and elk with CWD. As prion strain properties largely dictate host range potential, these findings suggest that prion strains from elk and deer might pose distinct risks to sympatric species or humans exposed to CWD (Bian et al., 2019).
The most common cervid species in Europe (moose, red deer, reindeer and roe deer) share the same PrP primary structure, i.e. Q226. However, red deer PrP is polymorphic at residue 226 and can therefore code for either Q226 or E226. Interestingly, CWD cases detected so far in four Norwegian moose, the first Swedish moose and one Norwegian reindeer are all homozygous for Q226 (Benestad, 2019b,c,d), but the CWD case in red deer is instead homozygous for E226 (Vikøren et al., 2019). The impact of these differences in PrP genotype on the transmissibility and strain properties of European CWD isolates is currently under investigation using GtE226 and GtQ226 mice (Bian et al., 2019). PRNP genotypes of the other reindeer from Norway and of the other moose cases in Sweden and Finland are not in the public domain.
Data on the transmissibility of CWD in species with different PrP sequences obtained by in vivo or in vitro modelling allow the investigation of the structural basis of the transmission barriers for CWD. This in turn could provide hints for predicting, to some extent, the susceptibility of non‐cervid species to CWD. Taken together, studies with CWD isolates from NA suggest that the 165–175 sequence similarity between cervid and host PrP is one important factor governing the susceptibility of different species to CWD (reviewed by Kurt et al., 2016). In particular, polymorphisms at N/S170 in the recipient species might be important for susceptibility, with species that have N170 being more susceptible than those with S170 (Kurt et al., 2016). However, this must not be seen as an absolute rule, as species having S170 in their PrP, such as squirrel monkeys, have also been reported to be susceptible to CWD. It is however pertinent to note that all livestock species and humans have PrP sequences with S170, so they should not be considered among the species with supposedly high susceptibility to NA CWD isolates. Ongoing experiments in rodent models seem to indicate a similar trend for European CWD isolates, as rodent models, apparently more susceptible to European CWD isolates such as bank voles and tg mice expressing deer PrP, are N170.
Little information is currently known about the genetics of either wild or farmed cervid populations in Europe. A recent published study of several deer species (mostly in Great Britain) reported that red deer showed the most PRNP gene variation, with polymorphisms at codons 98, 168, 226 and 247 and marked variability in genotype frequencies in different regions. Other deer species showed less variation, with roe and fallow deer having identical PRNP gene sequences in all the animals sampled. Based on comparison with PRNP sequences of NA cervids affected by CWD and limited experimental challenge data, the authors conclude that a high proportion of wild deer in Great Britain may be susceptible to CWD (Robinson et al., 2019). A similar conclusion was reached by a previous study of 715 genotyped cervids (red deer, roe deer and chamois) from the UK and Italy (Peletto et al., 2009).
3.2.4. Concluding remarks
The transmission of prions between species is limited by the ‘transmission barrier’ and the amino acid sequence of the host PrP plays a very key role in the overall susceptibility to TSE. Even for prions deriving from the same species, the host range may vary according to the prion strain, implying that different CWD strains might have different host ranges and different potential for transmitting to livestock species and to humans.
Whether the natural host range of CWD in NA extends beyond the family Cervidae is currently unclear and no natural infections have been reported so far in other wildlife species (e.g. predators and scavengers) with overlapping geographical ranges.
NA CWD has been transmitted experimentally to cattle and sheep, but with incomplete attack rates. The species barrier appears higher for pigs, although challenged animals can support low‐level prion amplification.
Experimental transmission to tg mice and other rodent models shows some difference in the host ranges of different isolates but, particularly for the European isolates, many bioassays are still ongoing and data are not yet available.
The number of strains, the strain diversity, the prevalence and the potential host range of disease in both NA and Europe CWD may be underestimated.
3.3. Transmissibility to humans: the zoonotic potential
The zoonotic potential of CWD has been addressed by several research groups through in vitro and in vivo approaches. However, it must be underlined that, at the time of writing this Opinion, the corresponding publications report results only based on CWD isolates derived from naturally or experimentally infected cervids from NA and none with isolates from Europe.
3.3.1. In vitro conversion of human PrP
Several approaches developed for converting PrP in vitro have been proposed to model the species barrier (EFSA BIOHAZ Panel, 2015). These techniques are based on the ability of infected samples (here derived from animals) to convert normal (here human) PrP (PrPc) in vitro under different experimental conditions. For these studies, the PrP substrate submitted to conversion can be either recombinant PrP or a brain homogenate. The latter source not only has the advantage of being able to present the natural diversity of PrP isoforms, but other components (e.g. nucleic acids, proteins, lipids) are also present with their potential influence on the reactions of conversion (e.g. co‐factors, inhibitors).
The conversion of human recombinant PrPc (recPrPc) triggered by NA CWD isolates is very limited, if any, in comparison with the conversion induced by other prion sources reputed to be efficiently infectious for humans. Therefore, in the presence of sodium dodecyl sulfate (SDS), human recPrPc was efficiently seeded by sporadic Creutzfeldt–Jakob disease (sCJD) or BSE purified infectious samples, but not by scrapie or white‐tailed deer CWD samples (Luers et al., 2013). Conversely, the RT‐QuiC technique of PrP amplification was efficient with eight different isolates derived from CWD‐infected white‐tailed deer, compared with BSE, which fails to convert human recPrP with this technique (Davenport et al., 2015). In the presence of guanidine, elk and mule deer PrPSc are able to induce a limited conversion of methionine homozygous at codon 129 (MM) human recPrPc (these CWD seed sources converted homologous cervid recPrPc with a 15‐fold higher conversion rate) but did not convert valine homozygous at codon 129 (VV) human recPrPc (Raymond et al., 2000). Similar levels of conversion of human recPrPc were obtained with scrapie‐infected ovine PrPSc, whereas BSE‐infected bovine PrPSc was slightly more efficient at converting human recPrPc (conversion rate ninefold less efficient than with homologous PrPc).
The conversion of human PrPc by CWD seeds was more efficient when normal brain homogenates were used as the source of normal PrP. Amplification studies based on the PMCA technique have demonstrated that the efficiency of in vitro conversion of human PrP is highly influenced by the PrP polymorphisms of the human recipient, PrP polymorphisms of the cervid donor and the origin of the isolates (Barria et al., 2018). In cervids, the polymorphism at codon 132 (methionine or leucine) plays an important role in CWD susceptibility (Hamir et al., 2006c), as does the polymorphism at codon 129 (methionine or valine) for humans with regard to susceptibility to CJD. With elk CWD samples, ‘only the homologues methionine homozygous seed‐substrate reactions could readily convert the human PrP’ (132 MM elk sample on 129 MM human PrP) whereas other combinations were less efficient (Barria et al., 2018). However, the rate of conversion of human PrPc by elk CWD samples remains lower than the conversion observed with BSE samples, but higher than the conversion induced by L‐BSE, H‐BSE or scrapie (Barria et al., 2014). Also, efficient PMCA conversion and amplification of human PrPc of the three human genotypes (MM, MV and VV) were observed with infectious materials derived from two reindeer experimentally infected with CWD (Barria et al., 2018). Conversely, PMCA amplification was very limited with white‐tailed deer (Barria et al., 2018) and mule deer CWD samples (Barria et al., 2011). In the latter, the species barrier was broken through serial amplifications of mule deer CWD on a homologous substrate, suggesting that, after serial intraspecific passages, the zoonotic potential of CWD (if any) might increase. Other parameters (like the low pH of the lumen of the stomach) might influence susceptibility to CWD (Li et al., 2007): the rate of conversion of human PrPc by CWD‐infected elk brain in the presence of guanidine is highly enhanced (reaching similar levels of conversion to those obtained for cervid PrP) in acidic conditions but not in neutral conditions. Table 2 describes the main in vitro studies exploring the zoonotic potential of CWD by assessing the conversion of human PrP in the presence of CWD seeding material.
Table 2.
Summary of the in vitro studies aimed at exploring the human species barrier to CWD
| Model | Description of the results | Reference |
|---|---|---|
| Cell‐free conversion assay | Very low levels of human recombinant PrP is converted by deer CWD PrPsc WTDrPsc), less than by bovine BSE PrP (bo‐PrPbse) | Raymond et al. (2000) |
| In vitro conversion of GdnHCl‐treated PrP c | Elk CWD converts human PrP, more easily than bovine, sheep or mouse PrP (brain homogenate) | Li et al. (2007) |
| PMCA | Conversion of human PrP enhanced by preliminary PMCA amplification cycles on cervid PrP | Barria et al. (2011) |
| SDS‐based fibrillation assay | No human recombinant PrP conversion by wdPrPsc, while converted by bo‐PrPbse | Luers et al. (2013) |
| huPrP and 293F cells expressing human PrP/PMCA | Conversion of human PrP less efficient than that obtained with BSE prion, 129 VV < 129 MM | Barria et al. (2014) |
| RT‐QuiC | Very efficient conversion of recombination human PrP by CWD samples (better than BSE) | Davenport et al. (2014) |
| PMCA | Efficient conversion of human PrP by CWD from elk and experimentally challenged reindeer, depending on human genotype, cervid genotype and cervid species | Barria et al. (2018) |
Taken together, these in vitro studies suggest that, within the limits of the ruminant isolates that were used in them,
BSE (established zoonotic potential) presents the highest ability to convert human PrP;
CWD isolates derived from (132 MM) elk and experimentally challenged reindeer would present an intermediate potential for conversion of human PrP;
other sources would be less efficient at converting human PrP. This includes CWD isolates derived from deer, atypical H‐BSE and L‐BSE strains or scrapie isolates, even if the zoonotic potential of atypical BSE and scrapie is supported by experimental evidence of transmission in tg humanised mice (Beringue et al., 2008a; Cassard et al., 2014) and non‐human primates (Gajdusek, 1972; Gibbs and Gajdusek, 1973; Comoy et al., 2008, 2015; Ono et al., 2011).
3.3.2. In vivo studies of zoonotic potential of CWD isolates
Transgenic mice expressing human PrP and non‐human primates are the two classes of in vivo experimental models used to evaluate the zoonotic risk of animal prions for humans.
3.3.2.1. Transgenic humanised mouse models
Several tg humanised mice lineages based on key polymorphisms at codon 129 of the human PRNP gene (six lineages of MM, one lineage of MV and two lineages of VV) expressing onefold to 16‐fold the physiological levels of PrPc have been exposed to NA CWD isolates derived from elk, mule deer or white‐tailed deer in the context of six independent experiments. No clinical, histopathological or biochemical evidence of neurological prion disease has been observed in any of these mice, even after extended incubation periods. According to these results, there is a high transmission barrier to humans for CWD. Nevertheless, this conclusion should be modulated by the fact that no blind secondary mice‐to‐mice transmission was performed in any of these studies. Indeed, this approach in tg humanised mice resulted in the conclusion that some zoonotic potential for scrapie could not be ruled out (Cassard et al., 2014).
Table 3 describes the main in vivo studies exploring the transmissibility of CWD to humanised transgenic models and non‐human primate species as proxies for the zoonotic potential of CWD.
Table 3.
Summary of the in vivo studies exploring the transmissibility of CWD to humanised transgenic models and non‐human primate species
| Model | Description | Result | Reference |
|---|---|---|---|
| tg40 (MM, 1x)tg1 (MM, 2x) | No transmission from elk CWD | − | Kong et al. (2005) |
| Tg440 (MM, 2x) | No transmission from 4 elk, 2 mule deer (MD) and 2 WTD isolates | − | Tamguney et al. (2006) |
| tg35 (MM, 2x)tg45 (MM, 4x)tg152 (VV, 6x) | No transmission from MD CWD | − | Sandberg et al. (2010) |
| tgHu MM (1x)tgHu MV (1x)tgHu VV (1x) | No transmission | − | Wilson et al. (2012) |
| tg40 (MM, 1x) | No transmission of CWD, except in mice expressing chimeric human PrP (expressing 4 elk amino acids (see Section 3.3.2.2)) | − (+ in chimeric) | Kurt et al. (2015) |
| tg66 (MM, 8‐16x)tgRM (MM, 2‐4x) | Clinical suspicion but no IHC or immunoblot confirmation. Faint positive RT‐QuIC reactions | +/− | Race et al. (2019) |
| tg66 (MM, 8‐16x) | No transmission | − | Mitchell et al. (2011) |
| tgRM (MM, 2‐4x) | No transmission | − | Cervenakova et al. (2014) |
| Squirrel monkey | IC transmission from MD CWD | + | Marsh et al. (2005) |
| Squirrel monkey cynomolgus macaque | IC and oral transmission to squirrel monkey, no IC or oral transmission to macaques after 6 years | + Squirrel,− macaque | Race et al. (2009) |
| Squirrel monkey cynomolgus macaque | IC and oral transmission to squirrel monkey, accelerated transmission after secondary passage. No IC or oral transmission to macaques after 10 years | + Squirrel,− macaque | Race et al. (2014) |
| Squirrel monkey cynomolgus macaque | IC and oral transmission to squirrel monkey, no IC or oral transmission to macaques even after 13 years | + Squirrel,− macaque | Race et al. (2018) |
| Cynomolgus macaque | No IC transmission to macaques after 7 years | − | Comoy et al. (2015) |
| Cynomolgus macaque | No transmission | − | Schmaedicke (2012) |
| Cynomolgus macaque | Wasting and mild neurological signs in IC and orally challenged macaques. | + with clinical signs not pathognomonic for TSE | Schatzl et al. (2018) |
+: transmission; −: no transmission; +/−: inconclusive.
Nevertheless, Race et al. (2019) recently published a study performed with two lineages of humanised mice that provided intriguing results. They exposed tgRM and tg66 mice, which overexpress MM human PrP at levels two‐ to fourfold and 8‐ to 16‐fold higher than the physiological levels, respectively, to different CWD sources. These two models harbour the highest levels of PrPc expression among all those that have been tested in these studies. Among these mice, 7% (3/45) tgRM and 30% (15/52) tg66 mice fitted the ‘criteria as prion disease suspects’ that the authors considered to be relevant and that correspond to ‘signs of wasting, weakness, neurologic disease and behavioural changes’ (Race et al., 2019). Extensive analyses of the brains of these animals did not provide any lesional, biochemical or immunohistochemical element that could confirm the diagnosis of prion disease and the authors expressed some reservations about the relevance of those observations because of the advanced age of these animals (> 500 days of incubation). In parallel, the authors analysed the brains of all the inoculated animals using RT‐QuIC. Four tg66 mice exposed to CWD samples were identified with repeated positive reactions (nine other exposed animals showed initial, positive reactions that were not reproducible). The authors wonder whether ‘the seeding activity detected in these mice may represent a low level of CWD agent, suggesting a possible transfer of CWD infection’, or ‘these results might be due to false‐positive reactions or residual CWD inoculum’. Unfortunately, the authors did not indicate whether the animals that exhibited a positive RT‐QuIC reaction were those that were clinically suspect.
It must be noted that the authors did not test the spleens of these animals. Indeed, during Prion 2015 (Fort Collins) and Prion 2019 (Edmonton) conferences, Kong et al. reported in oral presentations the partial transmission at subclinical levels of CWD to humanised mice (different transgenic constructions), with splenic involvement (Comoy et al., 2019a). This situation is reminiscent of observations made in humanised mice exposed to variant Creutzfeldt‐Jakob disease (vCJD) prion strain (Beringue et al., 2008b). In a similar way, the study of Race et al. (2018) missed the analysis of the spinal cords of these CWD‐exposed humanised mice. Indeed, unexpected disease phenotypes selectively affecting spinal cord without brain involvement have been observed in mice and macaques under non‐optimal conditions of experimental transmission (Comoy et al., 2017).
3.3.2.2. Chimeric mouse models
Humanised mice that were highly susceptible to CWD were obtained by modifying four amino acids (positions 166, 168, 170 and 174) in red in Table 4 at the level of the α2–β2 loop of the human PrP to perfectly match that of the elk PrP (Kurt et al., 2015). These observations suggest that this part of the prion protein constitutes a substantial structural barrier for CWD transmission to humans. The influence of this protein loop on species barrier might be different for BSE (transmissible to humans) and scrapie (zoonotic potential under question), as cattle and ovine PrP share the same amino acid sequence at the level of the α2–β2 loop that only differ for amino acids 166 and 168 from the human PrP sequence.
Table 4.
Comparison of the wild‐type PrP sequences of different species (only differences from the sequence of elk PrP are shown) (Schatzl et al., 1995; Li et al., 2007; Kurt et al., 2015). The reference numbers of equivalent amino acids positions (AA) differ among species due to a difference of octapeptides numbers and/or insertion/deletion of AA at the N‐terminal part of the PrP. The letters refer to the standard designation for amino acids and the box denotes the section of the PrP corresponding to the α2–β2 loop
| 2 | 3 | 4 | 8 | 10 | 17 | 21 | 98 | 100 | 103 | 111 | 115 | 141 | 146 | 158 | 162 | 169 | 171 | 173 | 177 | 185 | 187 | 189 | 206 | 208 | 210 | 222 | 223 | 226 | 233 | 235 | 236 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elk | V | K | S | S | I | M | V | T | S | N | N | V | L | N | Y | N | V | Q | N | T | I | V | Q | I | M | E | Q | R | E | A | V | I |
| Deer/Moose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Q | ‐ | ‐ | ‐ |
| Sheep | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | S | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | S | N | ‐ | ‐ | ‐ | ‐ | I | ‐ | ‐ | ‐ | Q | ‐ | ‐ | ‐ |
| 2 | 3 | 4 | 6 | 8 | 15 | 19 | 95 | 97 | 100 | 108 | 112 | 138 | 143 | 155 | 159 | 166 | 168 | 170 | 174 | 182 | 184 | 186 | 203 | 205 | 207 | 219 | 220 | 223 | 230 | 232 | 233 | |
| Man | A | N | L | C | M | T | L | ‐ | ‐ | ‐ | ‐ | M | I | S | H | ‐ | M | E | S | N | ‐ | I | ‐ | V | ‐ | ‐ | E | ‐ | Q | S | M | V |
| Cynomolgus macaque | A | N | L | C | M | T | L | ‐ | N | H | S | M | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | S | N | ‐ | I | ‐ | V | ‐ | ‐ | E | K | Q | S | M | V |
| 2 | 3 | 4 | 6 | 8 | 15 | 19 | 102 | 104 | 107 | 115 | 119 | 145 | 150 | 162 | 166 | 173 | 175 | 177 | 181 | 189 | 191 | 193 | 210 | 212 | 214 | 226 | 227 | 230 | 237 | 239 | 240 | |
| Saimiri | A | N | L | C | M | T | L | ‐ | N | ‐ | ‐ | M | ‐ | ‐ | ‐ | S | ‐ | ‐ | S | N | V | I | ‐ | V | ‐ | ‐ | E | K | Q | S | M | V |
| 2 | 3 | 4 | 8 | 10 | 17 | 21 | 106 | 108 | 111 | 119 | 123 | 149 | 154 | 166 | 170 | 177 | 179 | 181 | 185 | 193 | 195 | 197 | 214 | 216 | 218 | 230 | 231 | 234 | 241 | 243 | 244 | |
| Bos taurus | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | G | ‐ | ‐ | ‐ | ‐ | S | H | ‐ | ‐ | ‐ | S | N | ‐ | ‐ | E | ‐ | ‐ | K | ‐ | ‐ | Q | ‐ | ‐ | ‐ |
The experiments performed in non‐human primate (NHP) models modulate the influence of this α2‐β2 loop on the species barrier towards CWD. Squirrel monkeys (subfamily Saimirinae) and CM (Macaca fascicularis) exhibit different susceptibilities to CWD (Race et al., 2009, 2014), whereas they harbour the same amino acids in the α2–β2 loop (i.e. they differ from human at positions 166 and 168 and from cervids at positions 170 and 174).
3.3.2.3. Non‐human primate models
In New World NHP squirrel monkeys, CWD from both MD and elk‐derived isolates is transmissible through the IC route with incubation periods similar to those obtained with BSE (Marsh et al., 2005; Williams et al., 2007; Race et al., 2009; Piccardo et al., 2012) suggesting a similar susceptibility of squirrel monkeys to these two animal prions. CWD isolates derived from WTD also transmitted disease, but with longer incubation periods despite a higher infectious titre in the inocula. All these cervid isolates also transmitted CWD to squirrel monkeys after exposure through the oral route (Table 5). After secondary transmission, the incubation periods for CWD decreased (Race et al., 2014): all the features of a classical prion disease, namely specific lesions and accumulation of abnormal PrP, reproducible lesional profile and adaptation of the prion strain to the new host, are found in squirrel monkeys after exposure to CWD.
Table 5.
Incubation periods of infected squirrel monkeys after exposure to different prion isolates through the intracerebral route
| Quantity of inoculum | BSE(Piccardo et al., 2012) | vCJD(Williams et al., 2007) | Mule deer CWD(Race et al., 2009;Marsh et al., 2005) | Elk CWD(Race et al., 2009) | WTD CWD(Race et al., 2009) |
|---|---|---|---|---|---|
| 40 mg | – | – | 31–34 mpi | – | – |
| 15 mg | 29–38 mpi | – | – | – | – |
| 10 mg | – | 21–30 mpi | – | – | – |
| 2 mg | – | – | 36–46 mpi | 33–53 mpi | 50–75 mpi |
| 1.5 mg | 38–46 mpi | – | – | – | – |
| 0.1 mg | – | 29–41 mpi | – | – | – |
mpi: months post‐inoculation.
The situation in CM is very different. After intracerebral (six animals) and oral exposure (eight animals) to the same inoculum as those that have transmitted CWD to squirrel monkeys, no clinical, lesional or biochemical evidence of prion disease was identified in macaque recipients (Race et al., 2009, 2014, 2018) and extensive RT‐QuIC analyses of their brain did not provide any evidence of seeding. However, it must be underlined that the incubation periods of macaques after primary exposure to animal prions strains through the intracerebral route are classically extended, with BSE developing up to 93 mpi (~ 8 years) and scrapie proving transmissible 118 mpi (~ 10 years) (Comoy et al., 2015). In this study performed at the Rocky Mountain Laboratories (RML) (USA), it can therefore be concluded that four of the six IC‐inoculated macaques have been kept under surveillance for too short a period (less than 95 months) to draw any definitive conclusion (Table 6).
Table 6.
Duration of surveillance periods of cynomolgus macaques exposed to different prion isolates
| Route and quantity of inoculum | BSE | vCJD | Scrapie(Comoy et al., 2015) | MD CWD(Race et al., 2018) | Elk CWD(Race et al., 2018) | WTD CWD(Race et al., 2018) |
|---|---|---|---|---|---|---|
| IC route, 40/50 mg | 31–49 mpi (6/6)(Montag et al., 2013) | 25–44 mpi (5/5) (Herzog et al., 2005) | 118 mpi (1/1) | – | – | – |
| IC route, 5 mg | – | – | – | 48–160 mpi (0/2) | 95–161 mpi (0/2) | 79–88 mpi (0/2) |
| IC route, 0.5 mg | 47–93 mpi (3/4)(Comoy et al., 2015) | – | – | – | – | – |
| Clinical outcome | 9/10 BSE | 5/5 vCJD | 1/1 Scrapie | AggressionEnd of study | SeizuresEnd of study | DiabetesWasting |
| Oral route, 4/5 g | 52–63 mpi (6/7)(Lasmezas et al., 2005; Holznagel et al., 2013) | – | – | 97‐106‐142 mpi (0/3) | 131‐149‐157 mpi (0/3) | 106–160 mpi (0/2) |
| Clinical outcome | 6/7 BSE | – | – | TremorDiabetesAnorexia | DiabetesHaemorrhoidsAbdominal pain | DiabetesEnd of study |
IC: intracerebral; CWD: chronic wasting disease; MD: mule deer; WTD: white‐tailed deer.
mpi: months post‐inoculation. (X/Y): attack rate; number of positive animals out of the number of exposed animals.
Another study (Canadian), described in the background of this mandate and based on exposure of CM to CWD, is still in progress and not yet published. However, intriguing/interim observations have been the subject of several oral presentations. Eighteen 4‐year‐old female macaques were exposed through intracerebral, oral, intravenous routes and skin scarification to different isolates derived from CWD‐infected animals (MD, WTD and elk sources, experimentally or naturally exposed, from USA and Canadian origins). Among these animals, 10 animals were subjected to intense/extensive diagnostic testing. Animals were sacrificed, or died from unrelated causes, less than 90 months after exposure (two animals IC exposed to elk CWD, four animals IC exposed to CWD‐infected WTD, one animal orally exposed to brain samples derived from CWD‐infected WTD and three animals exposed to muscles derived from CWD‐infected WTD and MD). Among these, four (54–81 months of incubation) exhibited behavioural/neurological changes and/or alterations of the general status including anxiety, apathy, ataxia, tremor and wasting. Three of them were diagnosed as diabetic via elevated blood glucose and glycated haemoglobin (HbA1c) levels. It is notable that in the previously mentioned RML study (Race et al., 2018), nine of the 14 macaques were euthanised because they exhibited similar signs associated with neurological manifestations (aggression, seizure or tremor) or metabolic problems (anorexia, wasting, four diabetes cases). Spontaneous diabetes is described in the CM, but it classically occurs in 1–2% of captive monkeys (Clarkson et al., 1985), often in obese animals that represent less than 20% of the general population (Bauer et al., 2010). A correlation between the onset of obesity and diabetes and the infection with prions (BSE in their case) has been described by other authors (Strom et al., 2014).
The animals included in the Canadian study were tested for the presence of specific hallmarks of prion disease. Throughout oral presentations in congresses and personal communications with WG members, the authors mentioned that they observed an increased PrP staining evidenced by IHC in the substantia gelatinosa of suspected animals (i.e. the upper part of the posterior horns of their spinal cord) and brain areas (Comoy et al., 2019b). They consider that this labelling is reminiscent of what has been observed and described in primates orally challenged with BSE and that did not develop classical BSE but lesions and atypical PrP accumulation confined to the lumbar cord (Holznagel et al., 2013). However, this labelling is considered by several authors to be physiological as it was observed in the spinal cords of control animals in the RML study (Race et al., 2018) and has been observed in the spinal cord of normal animals of other species (Simmons et al., 2011).
Moreover, the authors of the Canadian study reported that abnormal PrPres in WB and positive RT‐QuiC reactions were obtained with lumbar cord samples from some suspected macaques.
These two studies provide apparently contradictory results that may be explained by several factors. Indeed, the RML study exposed older individuals (mean 7.2 years, range 5.2–11.4 years except two animals that were 3–4 years old), in contrast to the Canadian study where animals were 4 years old at exposure, as it is customary. Moreover, the sources of inocula were different, and the animals were challenged with larger amounts of inocula in the Canadian study (10 mg vs 5 mg for IC route, 10 g vs 1 g for oral route, oral dosing with 5 kg of muscle per animal in the Canadian study). Definitive conclusions and publication of these studies are now required in order to have more detailed information and more accurate comparisons, including bioassay results in tg mice and bank voles.
It can be concluded from these two studies that after incubation periods of between 5 and 13 years, no classical prion disease with neurological manifestations, spongiform changes and accumulation of abnormal PrP is seen in CM following challenge with CWD. However, it must be remembered that under non‐optimal conditions of exposure, two independent studies have reported that BSE/vCJD infection in macaques and mice might lead to the expression of an atypical disease that does not fit the current criteria of prion diseases (Holznagel et al., 2013; Comoy et al., 2017). The observations provided by both the RML and Canadian studies raise the possibility of a similar situation after exposure of primates to CWD.
3.3.3. Concluding remarks
CWD isolates from NA have shown variable ability to convert human PrP in in vitro studies. In vivo studies have also produced contradictory results, precluding any definitive conclusion about the ability of CWD to cross the human species barrier.
Extrapolation from the NA situation with regard to zoonotic potential might lead to either an underestimation or an overestimation of the zoonotic potential of the already identified European isolates, which have been shown to be distinct from NA CWD and from each other, based on the biochemical and biological characteristics that have been defined to date.
3.4. The risk to humans from CWD
As described in the EFSA Scientific Opinion on the review of a scientific publication on the zoonotic potential of ovine scrapie prions (EFSA BIOHAZ Panel, 2015), the link between human and animal TSE cases can be addressed ‘directly by conducting molecular/biological comparison to determine the biological and biochemical similarities of TSE isolates from two different species. It can also be addressed through epidemiological studies that could set hypotheses for putative risk factors via descriptive observational studies or via analytical studies aimed at detecting significant associations between exposure (risk factors) and outcome (health event of interest)’.
Previous sections summarise the biological and biochemical characteristics of the different CWD isolates in Europe and NA. There is a lack of knowledge about the ability of the CWD agent(s) to cross the species barrier and to infect humans. No data are available either on other factors that could determine the uptake of the infectious agent by a new host (humans), such as the amount of agent present, the age of the host at exposure and the possible potentiating effects of intercurrent disease or injury of the host. These and many other factors have been speculated to affect the success of infection following exposure, but the precise roles and interdependence (if any) of these factors are not clear. It is therefore not possible to perform a risk assessment of CWD in humans.
3.4.1. Epidemiological studies
The caveats associated with using epidemiological studies to investigate the association between human and animals TSE have all been previously highlighted (EFSA BIOHAZ Panel, 2015). These include the reliability of disease occurrence estimates from surveillance data and the inability to control for the effects of potential confounding factors, such as CWD diversity, host genetics, lag time (potential incubation period of human TSE), ecological fallacy and exposure misclassification.
A systematic review of the current evidence on the transmissibility of CWD prions to humans has been published recently (Waddell et al., 2018), using the criteria set‐up through the Grading of Recommendations Assessment, Development and Evaluation (GRADE) (Schünemann et al., 2013). Three types of studies were assessed: epidemiological studies, in vitro and in vivo experiments. The GRADE of the quality of evidence for the five epidemiological studies assessed suggested that future evidence may be inconsistent with the conclusions of the currently available studies. For the two studies on macaques (see Section 3.3.2), GRADE indicated that there is limited confidence that the results and estimates presented will not change with future research (mainly due to the limited number of studies and observations available). GRADE of the seven studies on humanised transgenic mice, which provided no evidence to support the possibility of transmission of CWD prions to humans, indicated that there is some confidence the overall conclusions of this research will not change with future research. The authors concluded that ‘future discovery of CWD transmission to humans cannot be entirely ruled out on the basis of current studies, particularly in the light of possible decades‐long incubation periods for CWD prions in humans and recommended to exercise caution when handling potentially contaminated material and explore CWD management opportunities’.
Among the more recent studies not included in the systematic review by Waddell et al. (2018), Abrams et al. (2018) have investigated the rates of human prion disease in States of the USA with and without CWD, to examine the possibility of undetermined zoonotic transmission. Although final results of this study have not been published yet, the authors concluded that ‘while higher prion disease mortality rates in certain categories of states with CWD in free‐ranging cervids were noted, additional stratified analyses did not reveal markedly elevated rates for potentially sensitive subgroups that would be suggestive of zoonotic transmission. Unknown confounding factors or other biases may explain state‐ by‐state differences in prion disease mortality’.
Maddox et al. (2019) conducted a retrospective cohort study of 1,546 residents in 15 counties of the State of Wisconsin in which CWD has been confirmed among harvested free‐ranging deer. By linking three databases, the study aimed to evaluate causes of mortality in individuals potentially exposed to CWD. The results of the study have not been published yet but preliminary results identified individuals that consumed CWD‐positive deer but with no matches in the National Prion Disease Pathology Surveillance Center (NPDPSC) neuropathology database. Due to the long incubation period, should transmission to humans occur, many years of vital status tracking are needed.
The potential variability of phenotypes of prion disease in humans and the potential for association with a zoonotic strain of CWD is an increasing area of concern in CWD‐endemic regions given the impossibility of knowing what the phenotype of human CWD prions would present with or whether they would be distinct from sporadic CJD cases. One example of a study looking at this aspect is the current analysis of the molecular diversity of CJD cases in patients who resided in Alberta and Saskatchewan (Canada) at their time of death (Myskiw et al., 2019). In a similar study, frozen brain tissues from all available sCJD cases archived in the NPDPSC from a US State with a high incidence of CWD are being analysed, looking for unusual patterns, characteristics and/or distribution of PrPSc in comparison with sCJD samples from States that have not detected CWD (Liu et al. (2019)).
3.4.2. Exposure assessment
There are two distinct routes of potential exposure of humans to the CWD agent(s):
Handling and manipulation by professionals (including industrial workers), hunters, consumers, etc., of dead bodies, carcasses, meat cuts, animal by‐products including risk material and meat, meat products and offal infected with the CWD agent(s).
Consumption of meat, meat products and offal from animals infected with the CWD agent(s).
The assessment of CWD risk to humans, generically defined as the probability of transmission to humans through the handling and/or consumption of meat and meat products from cervids is currently impossible, but could hypothetically be defined at two levels:
At the individual level, as the probability that at least one human is exposed to the CWD agent, acquiring the infection and developing either a known, an emergent or a new TSE.
At the population level, as the number of new human TSE cases in a defined population (incidence).
Considering each of these routes in turn, there is no evidence of anyone having acquired a human prion disease by handling and butchering infected animals of any species. However, the exposure of humans via direct contact (wounds, skin lacerations) while handling this type of material cannot be ruled out. Exposure to infected meat at household level may occur when consumers handle purchased raw meat, meat products or offal or, for home‐slaughtered or hunted cervids when carcasses are butchered and dressed. The frequency of exposure through handling of fresh meat, meat products or offal will be different between professionals and consumers. However, ToR3 only requires the consideration of the risk of transmission to humans through the consumption of cervid meat and meat products.
The most likely source of exposure and the only one considered in this Scientific Opinion, is through the consumption of cervid meat, meat products and offal sourced from infected animals. Two events must occur for a human to be exposed: the animal must be infected with a TSE in which abnormal PrP/infection is present in edible peripheral tissues, mainly the musculoskeletal (including blood) and lymphatic systems and the consumer must have access to meat, meat products or offal from such animals in which the abnormal prion has not been inactivated by physicochemical or any other processing.
There are no studies in the scientific literature aimed to derive the amount of CWD infectivity potentially present in different tissues of susceptible species. Several studies describe the presence/absence of detectable PrP in a range of tissues (see table 1 in EFSA BIOHAZ Panel, 2017). A single study (Gavin et al., 2019) has attempted to estimate, via mathematical modelling, the titre of infectivity that would pass, through abattoir killed or field dressed deer, into the human food chain and the environment. This study assumed that infectivity in red deer infected with CWD would be peripherally distributed and with a pathogenesis similar to classical scrapie. The model estimated that the total infectivity in a red deer carcass would be approximately 83,000 mouse IC log ID50 units, compared with around 22,000 in a scrapie‐infected sheep carcass, mainly due to the size difference between sheep and red deer. The model estimated that around 13% (11,000 mouse IC log ID50 units) of the total infectivity would enter the food chain through either the farmed or wild route.
For the probability of exposure to the CWD agent(s):
At the individual level, consumers of meat, meat products and offal derived from CWD‐infected cervids will be exposed to the CWD agent(s) and, to a lesser extent, via the handling of infected material with strain(s) causing peripheral tissue distribution of infectivity in CWD‐infected cervids. As stated in a previous EFSA Opinion on CWD (EFSA BIOHAZ Panel, 2018), even if specific tissues harbouring most of the infectivity were removed from the food chain, therefore reducing the human dietary exposure to infectivity, consumers would still be exposed to the CWD agent(s) via consumption of meat and meat products.
At the population level, not only it is important to consider the strain(s) of agent in susceptible cervid species that are usually consumed (reindeer, moose, red deer), but also the prevalence of each CWD strain in each of these species. This would provide an overall estimation of the probability of exposure of EU citizens to the CWD agents/strains. However, there is no conclusive knowledge about the range of agents/strains of CWD in the European cervid population, their tissue distribution in infected animals or their prevalence in each/any of the populations. Therefore, the overall exposure of humans to CWD in the EU cannot be estimated.
3.4.3. Recommendations on possible additional control measures to address the risks identified
Recommendations that would reduce/eliminate human exposure to CWD all relate to the reduction or removal of infected tissues from the food chain by:
Systematic testing: Only allowing human consumption of meat, meat products and offal sourced from animals that have been tested negative for CWD.
Targeted measures: Prohibition of harvesting/hunting susceptible species or the introduction of compulsory testing of animals before human consumption in/from declared infected premises/areas (e.g. a farm, or a surveillance PSU (Section 3.6), region, country, etc.).
Systematic removal of high‐risk tissues from all cervids intended for human consumption with no requirement for testing.
3.4.4. Concluding remarks
Due to uncertainties and lack of data, it is not possible to conduct a risk assessment of CWD in humans and to directly quantify the human risk at either individual or population levels. The risk to humans through consumption of meat, meat products and offal derived from CWD‐infected cervids cannot be directly assessed; only the probability of exposure to the CWD agent(s).
Lack of comprehensive data on both the tissue distribution of infectivity in European cases and the prevalence of the CWD gent in each of the species, increases the uncertainty around human exposure and related risk factors.
The available epidemiological studies have not shown any evidence of association between human TSE and NA CWD. However, these studies suffer from many methodological and logistic constraints.
A recent systematic review concluded that the long incubation periods in human TSE mean that current studies do not definitely exclude any potential for CWD to transmit to humans.
Even if the specific tissues harbouring most of the infectivity were removed from the food chain, therefore reducing the human dietary exposure to infectivity, consumers would still be exposed to the CWD agent(s) via consumption of meat and meat products from infected animals.
Exclusion from the food chain of the whole carcass of any infected animal would be required to eliminate human dietary exposure. This could be facilitated by the preliminary testing of animals intended for consumption and/or the prohibition of harvesting or hunting susceptible species in infected premises/areas.
3.5. Situation of CWD in Europe: latest results
In 2018, Norway ‘embarked in an unprecedented eradication campaign of CWD in Nordfjella area where all the cases of CWD in reindeer have been found so far. As a result, between 10 August 2017 and 14 May 2018, 582 reindeer were culled during the normal hunting seasons, 1,399 reindeer were shot dead by professional marksmen. An additional 43 reindeer were found dead due to natural causes, making the total of the wild reindeer population removed by the eradication programme 2,024 wild reindeer’ (Mysterud and Rolandsen, 2018).
A recent study analysing the data of the Nordfjella area estimated the apparent CWD prevalence at 1.2% of adults in the infected population of wild reindeer, while the estimated true prevalence was 1.6% (Viljugrein et al., 2019).
The results of the surveillance conducted in cervids for TSE in the European countries have been summarised in the Tables 7 and 8. Before 2018, European countries had tested cervid populations for TSE in a very heterogeneous and inconsistent way (for a review of the EU surveillance before 2016 see EFSA BIOHAZ Panel, 2017, 2018), with Romania accounting for more than 90% of all cervids tested in the EU in the last 3 years.
Table 7.
Number of tested cervids for TSE in the EU and European Economic Area (EEA) reporting countries for the period 2016–2018. In parentheses, the number of CWD cases
| 2016 | Wild deer | Semi‐domesticated/farmed deer | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | A | B | C | D | E | F | G | H | Subtotal | A | B | C | D | E | F | G | H | Subtotal | Total |
| AT | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| EE | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| FI | 0 | 5 | 26 | 7 | 12 | 0 | 0 | 0 | 50 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 56 |
| HU | 0 | 0 | 0 | 6 | 0 | 1 | 1 | 1 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| IT | 0 | 0 | 0 | 49 | 0 | 24 | 1 | 0 | 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 74 |
| RO | 0 | 0 | 0 | 0 | 0 | 459 | 7 | 2,034 | 2,500 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2,500 |
| SE | 0 | 0 | 60 | 7 | 0 | 3 | 0 | 0 | 70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 70 |
| Total EU | 0 | 5 | 87 | 69 | 12 | 488 | 9 | 2,035 | 2,705 | 6 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 7 | 2,712 |
| NO | 842 (4)a | 0 | 4,403 (2)a | 473 | 0 | 2,451 | 0 | 88 | 8,257 (6) | 1,739 | 0 | 0 | 0 | 0 | 143 | 0 | 0 | 1,882 | 10,139 (6) |
| IS | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14 |
| Total EFTA | 855 (4) | 0 | 4,403 (2) | 473 | 0 | 2,451 | 0 | 0 | 8,270 (6) | 1,740 | 0 | 0 | 0 | 0 | 143 | 0 | 0 | 1,883 | 10,153 (6) |
| Total | 855 (4) | 5 | 4,490 (2) | 542 | 12 | 2,939 | 9 | 2,123 | 10,975 (6) | 1,746 | 0 | 0 | 0 | 0 | 144 | 0 | 0 | 1,890 | 12,865 (6) |
Four out of 842 tested wild Eurasian tundra reindeer were found to be positive as well as 2 out of 4,403 tested wild moose. Source of cases: http://apps.vetinst.no/skrantesykestatistikk/NO/#omrade
A: Eurasian tundra reindeer (Rangifer tarandus tarandus); B: Finnish (Eurasian) forest reindeer (Rangifer tarandus fennicus); C: Moose (or Eurasian/European elk) (Alces alces alces); D: Roe deer (Capreolus capreolus); E: White‐tailed deer (Odocoileus virginianus); F: Red deer (Cervus elaphus); G: Fallow deer (Dama dama); H: Other or Unknown.
Only countries that reported tested cervids are included in the table.
AT: Austria; EE: Estonia; FI: Finland; HU: Hungary; IT: Italy; RO: Romania; SE: Sweden; NO: Norway; IS: Iceland.
Table 8.
Number of tested cervids in the EU and reporting countries by country, management system, species and target group in 2018
| Country/Management system/Species | Wild deer | Semi‐domesticated/farmed deer | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | Subtotal | A | B | C | D | E | F | G | H | Subtotal | Total | |||
| Estonia | SU | 1 | 1 | 2 | 2 | ||||||||||||||||
| RK | 5 | 66 | 71 | 71 | |||||||||||||||||
| FC | 9 | 22 | 1 | 32 | 1 | 4 | 5 | 37 | |||||||||||||
| HSNHC | 2 | 6 | 8 | 8 | |||||||||||||||||
| HSHC | 67 | 32 | 99 | 99 | |||||||||||||||||
| Total | 84 | 127 | 0 | 1 | 0 | 0 | 212 | 1 | 4 | 5 | 217 | ||||||||||
| Finland | RK | 6 | 48 | 39 | 17 | 110 | 128 | 128 | 238 | ||||||||||||
| FC | 6 | 75 | 21 | 26 | 128 | 164 | 4 | 2 | 6 | 176 | 304 | ||||||||||
| HSNHC | 16 | 16 | 4 | 4 | 20 | ||||||||||||||||
| HSHC | 99 | 1 | 1 | 101 | 0 | 101 | |||||||||||||||
| Total | 12 | 238 | 61 | 44 | 0 | 0 | 0 | 355 | 296 | 4 | 2 | 6 | 0 | 0 | 0 | 308 | 663 | ||||
| Lithuania | RK | 8 | 110 | 1 | 23 | 142 | 0 | 142 | |||||||||||||
| FC | 6 | 85 | 9 | 100 | 7 | 2 | 9 | 109 | |||||||||||||
| HSNHC | 23 | 1 | 24 | 3 | 3 | 27 | |||||||||||||||
| HSHC | 105 | 1,050 | 355 | 1,510 | 3 | 19 | 25 | 47 | 1,557 | ||||||||||||
| Total | 119 | 1,268 | 1 | 388 | 1,776 | 0 | 3 | 19 | 0 | 35 | 0 | 2 | 59 | 1,835 | |||||||
| Latvia | SU | 1 | 2 | 5 | 8 | 8 | |||||||||||||||
| RK | 4 | 16 | 3 | 23 | 23 | ||||||||||||||||
| FC | 7 | 15 | 8 | 30 | 30 | ||||||||||||||||
| HSHC | 227 | 477 | 269 | 973 | 20 | 20 | 993 | ||||||||||||||
| Total | 239 | 510 | 0 | 285 | 0 | 0 | 1,034 | 20 | 20 | 1,054 | |||||||||||
| Poland | SU | 8 | 1 | 9 | 9 | ||||||||||||||||
| RK | 38 | 657 | 60 | 755 | 755 | ||||||||||||||||
| FC | 4 | 72 | 11 | 87 | 3 | 3 | 90 | ||||||||||||||
| HSNHC | 2 | 1 | 3 | 5 | 5 | 8 | |||||||||||||||
| HSHC | 136 | 128 | 264 | 15 | 15 | 279 | |||||||||||||||
| Total | 42 | 875 | 0 | 201 | 0 | 0 | 1,118 | 0 | 0 | 0 | 0 | 23 | 0 | 0 | 23 | 1,141 | |||||
| Sweden | SU | 9 | 3 | 2 | 14 | 1 | 1 | 15 | |||||||||||||
| RK | 8 | 8 | 6 | 6 | 14 | ||||||||||||||||
| FC | 123 | 12 | 4 | 139 | 5 | 5 | 10 | 149 | |||||||||||||
| HSNHC | 6 | 2 | 8 | 4 | 4 | 12 | |||||||||||||||
| HSHC | 10 | 10 | 0 | 10 | |||||||||||||||||
| Total | 156 | 15 | 0 | 8 | 0 | 0 | 179 | 15 | 1 | 0 | 0 | 5 | 0 | 0 | 21 | 200 | |||||
SUS: clinical suspect animals; RK: Road/predator killed; FC: Fallen/culled; HSNHC: Hunted/slaughtered not fit for human consumption; HSHC: Hunted/slaughtered fit for human consumption;
A: Eurasian tundra reindeer (Rangifer tarandus tarandus); B: Finnish (Eurasian) forest reindeer (Rangifer tarandus fennicus); C: Moose (or Eurasian/European elk) (Alces alces alces); D: Roe deer (Capreolus capreolus); E: White‐tailed deer (Odocoileus virginianus); F: Red deer (Cervus elaphus); G: Fallow deer (Dama dama); H: Other or Unknown.
Only countries that reported tested cervids are included in the table.
Following the confirmation of the first case of CWD in Europe, the European Commission through Annex III of Regulation (EC) No. 999/2001, amended by Commission Regulation (EU) 2017/1972, enforced the implementation of a 3‐year monitoring programme for CWD from 1 January 2018 to 31 December 2020 in the six MS that have a wild and/or farmed and/or semi‐domesticated population of moose and/or reindeer: Estonia, Finland, Latvia, Lithuania, Poland and Sweden.
The programme requires the identification of geographically based PSU, which shall cover all territories in which cervid populations are present, for farmed and captive cervids and for wild and semi‐domesticated cervids. The sampling strategy is two‐stage: sample all PSU if the identified PSU list contains less than 100, or a random sample of 100 PSU if the identified PSU list contains more than 100. Within each selected PSU, a target sample of 30 risk animals is required.7 If an MS identifies fewer than 3,000 risk animals either among wild/semi‐domesticated or farmed/captive cervids, it may extend the monitoring to hunted or slaughtered cervids declared fit for human consumption with the objective of approaching a total number of 3,000 wild and semi‐domesticated cervids tested at the national level over the 3‐year period that is relevant to it.
According to the Regulation, the risk animals include:
-
For farmed and captive cervids:
-
–
fallen/culled farmed or captive cervids, defined as farmed or captive cervids found dead on the enclosed territory in which they are kept, during transport or at slaughterhouse, as well as farmed or captive cervids killed for health/age reasons;
-
–
clinical/sick farmed or captive cervids, defined as farmed or captive cervids showing abnormal behavioural signs and/or locomotor disturbances and/or as being generally in poor condition;
-
–
slaughtered farmed cervids which have been declared unfit for human consumption.
-
–
-
For wild and semi‐domesticated cervids:
-
–
fallen/culled wild or semi‐domesticated cervids, defined as cervids found dead in the wild as well as semi‐domesticated cervids found dead or killed for health/age reasons;
-
–
road‐ or predator‐injured or killed cervids, defined as wild or semi‐domesticated cervids hit by road vehicles, by trains or attacked by predators;
-
–
clinical/sick wild and semi‐domesticated cervids, defined as wild and semi‐domesticated cervids which are observed as showing abnormal behavioural signs and/or locomotor disturbances and/or as being generally in poor health condition;
-
–
wild hunted cervids and slaughtered semi‐domesticated cervids which have been declared unfit for human consumption.
-
–
The total number of tested cervids in Europe in 2018 (as reported to EFSA) was 41,322, with 80% of these (33,037) tested by Norway where seven new cases (six in reindeer and one in a moose) were confirmed.
The remaining 20% were tested by EU MSs, apart from 100 reindeer tested by Iceland. Twelve MSs reported tested cervids, ranging from four each by Austria and Hungary, to Romania, the MS with the largest throughput in the EU, which tested 2,387, 75% of them wild roe deer.
In 2018, the six MS conducting mandatory surveillance tested a total 5,110 cervids, of which 4,674 (91.5%) were wild animals, mostly roe deer and red deer and 436 (8.5%) were captive, farmed or semi‐domesticated, with more than half of them being semi‐domesticated reindeer tested in Finland (Table 7).
The most common target group was the ‘hunted/slaughtered fit for human consumption’ (HSHC) that is not a ‘risk animals’ category and that group accounted for 59.5% of all cervids tested by the six MS. The rest were risk animals in the different target groups (SUS: clinical suspect animals; RK: Road/predator killed; FC: fallen/culled; HSNHC: hunted/slaughtered not fit for human consumption). There is a huge variability between countries in the proportion of cervids tested in the HSHC, ranging from 5% tested by Sweden to 94.2% by Latvia or 84.8% by Lithuania (Table 8).
Up to 20 September 2019, 28 cases have been reported in Europe: 19 wild reindeer, four moose and one red deer in Norway and since the mandatory surveillance started in 2018 one moose in Finland and three moose in Sweden.
The first Finnish case was reported in March 2018 as a 15‐year‐old moose that was found dead in Kuhmo (Kainuu region), in Easter Finland, near the border with the Russian Federation. It was positive in brain using Bio‐Rad and IDEXX ELISA rapid tests and negative in the tested lymph nodes (Korpenfelt, 2019).
The first Swedish case was reported on 26 March 2019 as a 16‐year‐old female, euthanised because it was emaciated and showed circling and abnormal behaviour in the area of Arjeplog, municipality of Norrbotten, north of Sweden, ~ 400 km from the closest area where Norwegian cases in moose had been identified and ~ 950 km from where the Finnish moose case was found. It was positive using Bio‐Rad TeSeE ELISA, Bio‐Rad TeSeE WB and in immunohistochemistry in the brainstem and negative using Bio‐Rad TeSeE ELISA and IHC in the retropharyngeal lymph node (Gavier‐Widen, 2019). Preliminary results show a similar presentation to that of the CWD observed in moose in Norway and in Finland, but further analyses are needed to confirm possible similarities.
The second Swedish moose, also a 16‐year‐old female that was emaciated and showing behavioural changes, was found in Arvidsjaur, 70 km from the first case, in late May 2019. It was positive using Bio‐Rad TeSeE ELISA and Bio‐Rad TeSeE WB in the brainstem and negative using Bio‐Rad TeSeE ELISA in the retropharyngeal lymph node (Gavier‐Widen, 2019). The third Swedish moose was an at least 9‐year‐old female, shot during the normal hunting season in the same area as the two previous cases and without any signs of illness.8
It is premature to make any assessment of the effectiveness of the monitoring programme in the six selected countries. It is important to remember that the aims of the proposed surveillance system are twofold: (1) to detect disease in countries where CWD has not yet been detected, using a predefined design prevalence based on previous known occurrence in newly infected areas in NA; and (2) to estimate prevalence in areas where disease has been detected (EFSA BIOHAZ Panel, 2017).
The logistical constraints of implementing such a programme at national level cannot be underestimated, hence the slow start of some of the countries during the first year of implementation. However, the detection of three cases already in two of the six countries at early stages of the programme leads to two immediate and preliminary conclusions: (1) it is of paramount importance to conduct active surveillance for CWD to ascertain the actual distribution of the disease in susceptible species of European cervids; and (2) the distribution and prevalence of CWD in Europe may be underestimated as the three species known to be susceptible to CWD in Europe, namely reindeer, moose and red deer, only accounted for 5.8%, 17.3% and 19% of all cervids tested in the six countries in 2018, with one case in moose found during that period and only four of every 10 cervids tested so far have been from the high‐risk target groups.
For example, in a large country with more than 100 PSU defined, assuming a perfect random sampling of PSU and risk animals within the PSU and the same surveillance results as in Finland in 2018, the prevalence of CWD in the subpopulation of moose in the fallen/culled risk category would be 1/176 = 0.57% (95% CI: 0.01–3.1%). There are no surveillance data for Sweden in 2019 with which to make similar estimations.
| 2017 | Wild deer | Semi‐domesticated/farmed deer | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | A | B | C | D | E | F | G | H | Subtotal | A | B | C | D | E | F | G | H | Subtotal | Total |
| BE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 3 |
| DK | 0 | 0 | 0 | 27 | 0 | 3 | 2 | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| ES | 0 | 0 | 0 | 15 | 0 | 38 | 6 | 0 | 59 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 59 |
| FI | 0 | 13 | 47 | 12 | 20 | 0 | 0 | 0 | 92 | 16 | 0 | 1 | 0 | 0 | 0 | 1 | 4 | 22 | 114 |
| HU | 0 | 0 | 0 | 21 | 0 | 2 | 1 | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 |
| IT | 1 | 0 | 0 | 362 | 0 | 131 | 16 | 0 | 510 | 13 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 18 | 528 |
| LV | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| RO | 0 | 0 | 0 | 2,069 | 0 | 508 | 34 | 26 | 2,637 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 3 | 7 | 2,644 |
| SE | 21 | 0 | 134 | 11 | 0 | 4 | 6 | 0 | 176 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 176 |
| UK | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Total EU | 22 | 13 | 181 | 2,519 | 20 | 687 | 65 | 27 | 3,534 | 30 | 0 | 1 | 0 | 0 | 5 | 6 | 9 | 51 | 3,585 |
| IS | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 54 |
| NO | 2,931 (9) | 0 | 5,464 (1) | 1,970 | 0 | 3,641 (1) | 0 | 350 | 14,356 (11) | 10,925 | 0 | 3 | 0 | 0 | 431 | 21 | 0 | 11,380 | 25,736 (11) |
| Total EFTA | 2,985 (9) | 0 | 5,464 (1) | 1,970 | 0 | 3,641 (1) | 0 | 350 | 14,410 (11) | 10,925 | 0 | 3 | 0 | 0 | 431 | 21 | 0 | 11,380 | 25,790 (11) |
| Total | 3,007 (9) | 13 | 5,645 (1) | 4,489 | 20 | 4,328 (1) | 65 | 377 | 17,944 (11) | 10,955 | 0 | 4 | 0 | 0 | 436 | 27 | 9 | 11,431 | 29,375 (11) |
In parentheses, number of cases.
A: Eurasian tundra reindeer (Rangifer tarandus tarandus); B: Finnish (Eurasian) forest reindeer (Rangifer tarandus fennicus); C: Moose (or Eurasian/European elk) (Alces alces alces); D: Roe deer (Capreolus capreolus); E: White‐tailed deer (Odocoileus virginianus); F: Red deer (Cervus elaphus); G: Fallow deer (Dama dama); H: Other or Unknown.
Only countries that reported tested cervids are included in the table.
BE: Belgium; DK: Denmark; ES: Spain; FI: Finland; HU: Hungary; IT: Italy; LV: Latvia; RO: Romania; SE: Sweden; UK: United Kingdom; IS: Iceland; NO: Norway.
| 2018 | Wild deer | Semi‐domesticated/farmed deer | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | A | B | C | D | E | F | G | H | Subtotal | A | B | C | D | E | F | G | H | Subtotal | Total |
| AT | 0 | 4 | 4 | 4 | |||||||||||||||
| DK | 47 | 3 | 8 | 58 | 0 | 58 | |||||||||||||
| EE | 84 | 127 | 1 | 212 | 1 | 4 | 5 | 217 | |||||||||||
| ES | 5 | 32 | 37 | 0 | 37 | ||||||||||||||
| FI | 12 | 238 (1) | 61 | 44 | 355 (1) | 296 | 4 | 2 | 6 | 308 | 663 (1) | ||||||||
| HU | 1 | 3 | 4 | 0 | 4 | ||||||||||||||
| IT | 446 | 96 | 20 | 562 | 6 | 3 | 3 | 11 | 23 | 585 | |||||||||
| LT | 119 | 1,268 | 1 | 388 | 1,776 | 3 | 19 | 35 | 2 | 59 | 1,835 | ||||||||
| LV | 239 | 510 | 285 | 1,034 | 20 | 20 | 1,054 | ||||||||||||
| PL | 42 | 875 | 2011 | 1,118 | 23 | 23 | 1,141 | ||||||||||||
| RO | 1,796 | 526 | 15 | 10 | 2,347 | 26 | 14 | 40 | 2,387 | ||||||||||
| SE | 156 | 15 | 8 | 179 | 15 | 1 | 5 | 21 | 200 | ||||||||||
| Total EU | 0 | 12 | 878 (1) | 5,151 | 45 | 1,5433 | 43 | 10 | 7,682 (1) | 311 | 6 | 8 | 51 | 6 | 108 | 11 | 2 | 503 | 8,185 (1) |
| IS | 1 | 1 | 99 | 99 | 100 | ||||||||||||||
| NO | 3,624 (6) | 6,643 (1) | 2,106 | 7,762 | 4 | 164 | 20,303 (7) | 12,043 | 1 | 639 | 42 | 9 | 12,734 | 33,037 (7) | |||||
| Total EFTA | 3,625 (6) | 0 | 6,643 (1) | 2,106 | 0 | 7,762 | 4 | 164 | 20,304 (7) | 12,142 | 0 | 1 | 0 | 0 | 639 | 42 | 9 | 12,833 | 33,137 (7) |
| Total | 3,625 (6) | 12 | 7,521 (2) | 7,257 | 45 | 9,3055 | 47 | 174 | 27,986 (8) | 12,453 | 6 | 9 | 51 | 6 | 747 | 53 | 11 | 13,336 | 41,322 (8) |
Shaded in grey, MS conducting mandatory surveillance from 2018. In parentheses, number of cases.
A: Eurasian tundra reindeer (Rangifer tarandus tarandus); B: Finnish (Eurasian) forest reindeer (Rangifer tarandus fennicus); C: Moose (or Eurasian/European elk) (Alces alces alces); D: Roe deer (Capreolus capreolus); E: White‐tailed deer (Odocoileus virginianus); F: Red deer (Cervus elaphus); G: Fallow deer (Dama dama); H: Other or Unknown.
Only countries that reported tested cervids are included in the table.
AT: Austria; DK: Denmark; EE: Estonia; ES: Spain; FI: Finland; HU: Hungary; IT: Italy; LT: Lithuania: LV: Latvia; PL: Poland; RO: Romania; SE: Sweden; IS: Iceland; NO: Norway.
3.5.1. Concluding remarks
A recent study analysing the data of the Nordfjella area estimated the apparent CWD prevalence at 1.2% of adults in the infected population of wild reindeer, while the estimated true prevalence was 1.6% (Viljugrein et al., 2019).
The current mandatory surveillance programme in six EU MS has led to the detection of the first cases of CWD in the European Union, in a moose in Finland and in three moose in Sweden.
Based on the available active and passive surveillance data from the EU as a whole, the overall situation of the disease is still unknown: CWD could be present in any of the other four MS under mandatory surveillance and/or in any of the other 22 MS not subject to mandatory surveillance. The available data on the prevalence of CWD in Sweden and Finland are still limited.
3.6. Risk factors for the spread of CWD in Europe
CWD in NA and Europe affects cervid species whose geographic ranges overlap with those of several other wild and domesticated species. Based on the experience gained in NA, CWD can be considered highly contagious, with infected individuals contributing to the spread of the disease through direct contact and/or through the shedding of the CWD agent(s) in the environment, where it remains infectious for a long time. Furthermore, the CWD agent(s) can be transported by other animal species (i.e. scavengers) or as a consequence of human activities, leading to a wider exposure of cervids and other potentially susceptible species to CWD. Therefore, among known prion diseases, CWD seems to have the widest potential species range, a notion which can have significant impact for understanding the biology of the CWD epidemics, for designing surveillance systems and control measures and for estimating the risks for food safety and human health.
As mentioned in Section 3.5, six EU MS are currently implementing mandatory surveillance. Due to the low throughput (data only available for 1 year of the 3‐year cycle), no conclusions can yet be drawn about the prevalence of CWD in Finland and Sweden or the presence of disease in the remaining four countries. The uncertainty about the presence of the disease in the rest of Europe is even higher.
Except for the recent data from Norway, any information on host susceptibility, transmission and spread of CWD is based on the situation in NA. Previous European risk assessments (Defra, 2016a,b, 2018) have assumed that the disease, if it occurred in Europe, would have most likely been imported from NA and would therefore behave similarly in European cervid populations. However, the data emerging from the characterisation of the recently identified European cervid isolates (see Section 3.3) indicate that these European isolates are molecularly and biologically distinct from those identified in NA.
Given the uncertainties associated with the actual geographical distribution of CWD in Europe and the possible transmissibility of the different strains (known and unknown) in Europe, the following description of potential risk factors for the spread of CWD in Europe assumes two hypothetical scenarios, drawing on knowledge of CWD and other TSE in domestic animals and without any reference to specific locations where the disease is/might be present.
3.6.1. Scenario development
The description of the current scientific evidence on the characteristics of European CWD isolates (see Section 3.1) has implications for their potential capacity to spread naturally within cervid populations. In particular, the presence/absence of detectable PrPSc outside the CNS is thought to be an indication of the probability of spread under field conditions. While CNS involvement is a consistent feature of all clinical TSE cases regardless of species, the presence or absence of detectable PrPSc in peripheral tissues is more of a continuum, with some phenotypes presenting early accumulations of PrPSc, while others only accumulate limited amounts, or none. These differences may be associated either with strain or host genotype, or a combination of the two (for recent review of the cervid context, see Section 3.2.2 of the EFSA BIOHAZ Panel, 2018). For example, most cases of classical scrapie and NA CWD have widespread peripheral tissue involvement, the extent of which is affected by genotype and both are contagious under field conditions. BSE, H‐ and L‐type BSE and atypical/Nor98 scrapie, conversely, have little or no detectable PrPSc in peripheral tissues (Benestad et al., 2003; Wells et al., 2005; Andreoletti et al., 2011; Stack et al., 2011; EFSA, 2014) and tend to present as individual cases within herds/flocks. (It is important to note that while the term ‘atypical’ is often applied to this latter phenotype, this does not mean that they necessarily share the same biochemical or biological properties, or zoonotic potential; see Appendix A.5) The NA elk, however, is in‐between, with a low amounts of abnormal PrP deposition (Race et al., 2007) associated with longer incubation periods (Moore et al., 2018) and low prevalence (Mysterud and Edmunds, 2019). These differences allow the description for developing hypothetical epidemiological scenarios of what can be considered representative of the extremes of this phenotypic spectrum:
A contagious form presenting with peripheral distribution of PrPSc/infectivity, similar to the well‐known presentation of CWD in NA and classical scrapie in small ruminants with susceptible PrP genotypes. It is assumed, for this scenario, that infected animals would be able to transmit disease horizontally under field conditions and that infection could also be spread via environmental contamination by either live animals (e.g. through saliva, urine, faeces) or carcass disintegration. Subsequent dissemination could occur as a result of human activity, fomites and/or scavengers or via the feed chain.
A non‐contagious form presenting little or no detectable involvement of peripheral tissues, akin to BSE in cattle or atypical/Nor98 scrapie in small ruminants. It is assumed, for this scenario, that infection would be less likely to transmit horizontally under field conditions and that environmental contamination by live animals would be minimal. Environmental contamination could still arise from the CNS of disintegrating carcasses and be spread via human activity, fomites and/or scavengers or via the feed chain.
Disease can spread by:
extending the geographic extent of a focus to a new area/region/country: mainly by natural migration of infected animals and by the translocation of infected cervids by humans;
once introduced into a herd or population, the local spread of the agent is associated with the rate of contacts between animals or the environmental contamination. Behavioural and social factors of the different susceptible and non‐susceptible species present explain the transmission rate of the disease and the consequent spread to non‐infected animals within the population of interest.
In both cases, the agent(s) must be released from the infected individual(s), hence allowing other non‐infected animals to be exposed resulting in further release or translocation and giving rise to:
a new individual being infected that, if it is of a susceptible species, will develop clinical disease and will be capable of shedding infectivity in excreta throughout most of the incubation period if the disease phenotype includes PrP accumulation in peripheral tissues
a subclinical or carrier animal, in which some abnormal PrP accumulates, but the animal does not develop clinical disease within its lifetime. Such animals may or may not shed infectivity in excreta, potentially over prolonged periods.
a passive carrier, in which infection does not establish itself and infected material passes through the gut and into the environment via faeces in a relatively short time post‐consumption (e.g. non‐susceptible species such as predators, scavengers and carrion birds)
The two working scenarios may or not reflect the ability and the extent to which any of them can contribute to the transmission and spread of the disease. These scenarios do not account either for the possibility of the biological properties of an isolate changing on passage through another host species (EFSA BIOHAZ Panel, 2015).
3.6.2. Identification of risk factors
In the context of this Opinion, a risk factor for the spread of CWD in Europe will be considered as any feature that increases the likelihood of a non‐infected animal becoming exposed and infected in areas where the disease has already been identified, or in areas not yet considered as infected.
The risk factors for the spread of prion disease have been widely described in the published literature and extensively reviewed in publications and published national risk assessments (most recently in UK and Norway). It is beyond the scope of this Opinion to undertake a further extensive literature review, particularly as it is likely that European and NA isolates differ from one another (see Sections 3.1 and 3.2) and may present with different, and as yet unknown, risks.
The table of risk factors below (Table 9) is a summary of all previously identified risks for the spread of prion disease assessed against the two hypothetical scenarios. The criteria applied for the inclusion of an ‘identified risk factor’ for either scenario are based on three components:
The biological plausibility of the release of the agent or of the exposure to the agent from an infected individual even if there is only a theoretical likelihood of occurrence (a theoretical risk factor).
A hypothesis raised from the field observation of the distribution of the disease based on descriptive epidemiological studies (a hypothetical risk factors).
The reporting of a risk factor as an output of analytical epidemiological studies in which the association between its exposure and the disease occurrence has been shown to be statistically significant (a confirmed risk factor).
Table 9.
Groups of risk factors according to the two working scenarios (I: contagious animal vs II: non‐contagious animal)
| Main risk factors: description | Relevant scenario | Biological explanation and associated risk factorsa | References | Type of studyb | Strength of evidencec | Preventable |
|---|---|---|---|---|---|---|
| 1. Natural movement of live wild deer from infected areas | I & II | Sub‐adults dispersal i.e. the sub‐adults (in particular male deer) that leave their maternal home range without returning may contribute to long‐distance spread of the disease i.e. the sub‐adults (in particular male deer) that leave their maternal home range without returning may contribute to long‐distance spread of the disease | VKM et al. (2018) | review | F | N |
| Dispersal of infected individuals is possible, yet the risk of CWD spread from the focus of infection will decline gradually with increasing distance, but there are no barriers to the spread over time (Joly et al., 2006; Cullingham et al., 2011a) | Joly et al. (2006) | cross‐s | D | |||
| Cullingham et al. (2011a) | c‐c | C | ||||
| Seasonal migration: movement of cervids over an annual cycle from low elevation winter ranges (range contraction leading to higher density) to higher elevation summer ranges (range expansion) (VKM, 2018) | VKM et al. (2018) | review | F | N | ||
| Regardless of the ultimate origin, much of the geographic ‘spread’ of CWD appears attributable to natural movements in some jurisdictions (Miller and Fischer, 2016) | Miller and Fischer (2016) | review | F | |||
| 2. Man‐mediated movement of live farmed/free‐ranging deer from infected areas | I & II | Trade between farms. Entry and spread of CWD in South Korea (Sohn et al., 2002; Kim et al., 2005) and Canada (Bollinger et al., 2004) occurred due to the import of farmed cervids | Sohn et al. (2002) | c‐report | E | Y |
| Kim et al. (2005) | c‐series | E | ||||
| Bollinger et al. (2004) | review | F | ||||
| Evidence of multiple contacts between cervid farms by transferring at least one deer has been described (Rorres et al., 2018) | Rorres et al. (2018) | other | F | |||
| Primary anthropogenic factor identified in the dissemination of CWD is human‐facilitated movement of live animals (Williams and Miller, 2003) | Williams and Miller (2003) | review | F | |||
| Significant risk factors for transmission of CWD on elk farms were introduction from an infected farm of trace‐in elk that died of CWD (RR = 13.5, 95% CI 2.0–91) or developed clinical signs of CWD (RR = 7.1, 95% CI 0.93–54) (Argue et al., 2007) | Argue et al. (2007) | cohort | B | |||
| Transfer, even at a great distance, of farmed animals could account more effectively for the introduction of the disease in areas previously considered free (NYSDEC, 2018) | NYSDEC (2018) | review | F | |||
| Translocating free‐ranging cervids from an infected source would also present a similar risk for spreading CWD (Miller and Fischer, 2016) | Miller and Fischer (2016) | review | F | Y | ||
| Illegal liberation to the wild in case of cervid facilities going out of business (NYSDEC, 2018) | NYSDEC (2018) | review | F | (Y) | ||
| Liberation after rehabilitation. Moving and concentrating wild deer in confinement at a rehabilitation facility could potentially spread disease to a group of wild deer that would be liberated back into the environment (NYSDEC, 2018) | NYSDEC (2018) | review | F | Y | ||
| Temporary movement of semi‐domesticated reindeer across borders for grazing, cultural and sport events (EFSA BIOHAZ Panel, 2017) | EFSA BIOHAZ Panel (2017) | review | F | Y | ||
| 3. Failure to separate live farmed and free‐ranging deer | I | No effective barriers that guarantee a strict, complete separation between farmed and free‐ranging animals living in the surrounding areas (Williams and Miller, 2003; Vercauteren et al., 2007) | Williams and Miller (2003) | review | F | Y |
| Vercauteren et al. (2007) | other | F | ||||
| Following the introduction of an infected and contagious animal, contact may occur involving free‐ranging or farmed animals (both combinations are possible). In NA, involvement of farmed animals seems to precede that of free‐ranging animals in the same areas (Miller and Fischer, 2016) | Miller and Fischer (2016) | review | F | |||
| ‘Hot spot and spark’ model, where hot spots represent new herd outbreaks whereas the sparks represent subsequent isolated cases detected in the neighbouring wild population (Nusser et al., 2008) | Nusser et al. (2008) | simul | E | |||
| 4. High deer density | I | The rate of contacts between groups increases with deer density (Habib et al., 2011) that may therefore play a role in the spread of the disease | Habib et al. (2011) | simul | E | (Y) |
| The risk of spreading the disease among young (< 2 years) white‐tail deer in central‐southern Wisconsin has been associated with both the frequency of the disease (prevalence) and the density of deer (Storm et al., 2013) | Storm et al. (2013) | simul | E | |||
| Selective removal of animals in concentrated areas where CWD is known to occur may reduce prevalence and transmission (WAFWA, 2017) | WAFWA (2017) | review | F | |||
| However not all researchers agree with the role of density (Jennelle et al., 2014; Mysterud and Edmunds, 2019), but incline towards a frequency‐dependent transmission | Jennelle et al. (2014) | simul | E | |||
| Mysterud and Edmunds (2019) | review | F | ||||
| 5. Species‐specific social organisation | I | Species‐specific social behaviour can be totally different: mule deer, wapiti, reindeer and fallow deer spend their life in large to very large groups; WTD and red deer live in small groups in summer, larger in winter; moose and roe deer are solitary or live in small groups. The variable gregarious nature of the species may affect the contact rate among individuals and therefore the probability of transmission (Mysterud and Edmunds, 2019) | Mysterud and Edmunds (2019) | review | F | N |
| Social groups: in white‐tailed deer, increased infection probability has been associated with closely related female deer that were spatially proximate. This observation was based on the genetic relatedness of the animals (full‐sibling, mother‐offspring) forming matrilineal social groups (Grear et al., 2010) | Grear et al. (2010) | cross‐s | D | N | ||
| Importance of social groups in CWD transmission was also suggested for young white‐tailed deer (Storm et al., 2013) | Storm et al. (2013) | simul | E | |||
| As per the factors that facilitate animal aggregation, the role of social organisation and interactions is consistent with a frequency‐dependent CWD transmission, in contrast with a density‐dependent transmission (Cullingham et al., 2011a; Magle et al., 2013) | Cullingham et al. (2011b) | c–c | C | |||
| Magle et al. (2013) | other | F | ||||
| 6. Sex‐related behaviours | I & II | Males tend to have more contacts with each other than females and this is reflected in higher levels of disease prevalence in males (Jennelle et al., 2014) | Jennelle et al. (2014) | simul | E | N |
| In a mule deer cohort, prevalence among adult males tested was about twice that of females of the same age (Miller et al., 2008; Jennelle et al., 2018); the same holds also for white‐tailed deer (Samuel and Storm, 2016) | Miller et al. (2008) | cohort | B | |||
| Jennelle et al. (2018) | cohort | B | ||||
| Samuel and Storm (2016) | cohort | B | ||||
| Males move more widely and interact with more groups than females (Koutnik, 1981). | Koutnik (1981) | other | F | |||
| Male–female and female–female direct contact is higher during the rut (autumn); male–male direct contact is higher in the pre‐rut period (summer) (Kjær et al., 2008; Mysterud and Edmunds, 2019) | Kjær et al. (2008) | other | F | |||
| Mysterud and Edmunds (2019) | review | F | ||||
| Males consume more food than females increasing the probability of being exposed to environmental contamination (Potapov et al., 2013) | Potapov et al. (2013) | simul | F | N | ||
| 7. Natural or man‐mediated animal aggregation | I | Concentrating any wild species has the potential to increase spread of infectious disease by direct animal‐to‐animal contact, aerosol transmission, ingestion of feed contaminated with fluids (i.e. saliva) from another infected animal, or contact with body fluids, such as urine or faeces (NYSDEC, 2018). Artificial aggregation of deer: rearing animals in captivity, using urine lures for hunting purposes, feeding during the winter season and placing mineral salt licks in the field even if they are intended for the domestic species (Miller and Williams, 2003; Sorensen et al., 2014; Plummer et al., 2018) | NYSDEC (2018) | review | F | Y |
| Miller and Williams (2003) | cohort | B | ||||
| Sorensen et al. (2014) | review | F | ||||
| Plummer et al. (2018) | other | F | ||||
| There are areas where deer gather seasonally and in winter the animals tend to concentrate and become sedentary. Local environmental characteristics (e.g. watering sites, riparian habitats) can increase the probability of aggregation of animals and therefore of transmission of the disease (Miller and Conner, 2005; Edmunds et al., 2018) | Miller and Conner (2005) | cross‐s | D | N | ||
| Edmunds et al. (2018) | cross‐s | D | ||||
| Association between landscape factors (e.g. deciduous forest cover and forest edge density) and higher rates of infection in certain areas of NA (Miller et al., 2004; Thompson et al., 2008; Storm et al., 2013; Lavelle et al., 2014; WAFWA, 2017; Mejía‐Salazar et al., 2018) | Miller et al. (2004) | int | A | N | ||
| Thompson et al. (2008) | other | F | ||||
| Storm et al. (2013) | simul | E | ||||
| Lavelle et al. (2014) | other | F | ||||
| WAFWA (2017) | review | F | ||||
| Mejía‐Salazar et al. (2018) | other | F | ||||
| 8. Consumption of forage grown on contaminated soil | I & II | Prions can persist on the leaf apparatus directly soiled with contaminated materials; plants, grown on contaminated soils, can absorb the prions from topsoil and transfer them to the leaves. By PMCA prions have been shown to bind tightly to roots and leaves when exposed to either brain homogenate or excreta (Pritzkow et al., 2015) | Pritzkow et al. (2015) | other | F | N |
| 1% increase in the clay‐sized particle content in soils within the approximate home range of an individual deer increased its odds of infection by up to 8.9% | Walter et al. (2011) | cross‐s | D | N | ||
| In northern Europe, the collection and marketing of potentially contaminated lichens is also widespread for the feeding of farmed deer (VKM, 2017; VKM et al., 2018) | VKM (VKM, 2017; VKM et al., 2018) | review | F | Y | ||
| 9. Fallen stock or inappropriate disposal of carcasses and slaughter by‐products | I & II | Environmental contamination is associated with infected carcasses of fallen stock in the field (Miller et al., 2004) i.e. animals dead for natural or traumatic causes (e.g. as a result of collisions with motor vehicles) | Miller et al. (2004) | int | A | (Y) |
| Environmental contamination is associated with hunted animals whose remains are not appropriately disposed. Carcass/leftovers of deer are often given to working dogs (moved for hunting or sledging competitions) or disposed of and left to decompose in the environment (Benestad et al., 2019e) | Benestad et al. (2019e) | other | F | Y | ||
| Import of the carcasses of hunted animals combined with subsequent inappropriate disposal of parts of them (e.g. heads) | Defra (2016a) | review | F | Y | ||
| Inappropriate disposal of slaughter waste in countries where the disease is present (Gillette et al., 2004) | Gillette et al. (2004) | review | F | Y | ||
| A common destination for cervid‐derived slaughterhouse waste is pet food: as a result, parts of the central nervous system or lymphatic tissue could enter the diet of pets and pass into their faeces (Nichols et al., 2015; Defra, 2016b) | Nichols et al. (2015) | other | F | Y | ||
| Defra (2016b) | review | F | ||||
| Deer urine as a commercial lure used by hunters/photographers could be sourced from captive populations with preclinical cases of infection and be transported via commercial platforms (Defra, 2016a,b) | Defra (2016a,b) | review | F | Y | ||
| Slaughterhouse waste to produce organic fertilisers (Defra, 2016b). ‘Despite a partial inactivation of the agent due to the preliminary rendering of the raw material, the import and subsequent application in the field might determine the environmental contamination of grazing areas (or their surrounding areas)’. Spread on pasture areas of fertilisers/compost (alone or associated with topsoil) obtained from contaminated slaughter waste (Defra, 2016b) | Defra (2016b) | review | F | Y | ||
| Import of ornamental plants accompanied by topsoil in which the mentioned fertilisers could be present (Defra, 2016b) | Defra (2016b) | review | F | Y | ||
| 10. Movement of other animals (working dogs, scavengers, predators) | I & II | Working dogs are moved for hunting or sledging competitions | Defra (2016a,b) | review | F | Y |
| Faeces of dogs accompanying hunters returning from infected areas/countries can serve as a vehicle for prions contributing to the spread of the infectious agent in the environment (Defra, 2016a,b) | ||||||
| Scavengers Various species of (raptors, corvids) birds or mammals that feed on animal carcasses can act as spreaders of the infection. It has been shown that carcasses abandoned in the field in an area of Wisconsin were a source of food for at least 14 species of mammals and 14 species of birds. Carcasses could persist in the field from 18 to 101 days depending on the season and year. The involvement of the birds also suggests that the infectious agent could be transferred at great distances from the infected carcass (Jennelle et al., 2009) | Jennelle et al. (2009) | other | F | N | ||
| Predators. Prion‐infected deer were much more likely to be killed by mountain lions than uninfected deer (Miller et al., 2008). The presence of prions and their infectious ability in cervinised transgenic mice have been demonstrated in the faeces of coyotes (Canis latrans) 3 days after they had fed on with infected deer carcasses (Nichols et al., 2015). Faeces of predators (in North America e.g. coyotes or pumas) can serve as a vehicle for prions contributing to the spread of the infectious agent in the environment. | Miller et al. (2008) | cohort | B | N | ||
| Nichols et al. (2015) | other | F | ||||
| 11. Transfer of inanimate vehicles of contamination (fomites) | I & II | Hunting clothing, boots or knives poorly cleaned and used in infected and areas, could help disseminate contaminated material (e.g. clods of soil attached to their boots) (Defra, 2016a,b) | Defra (2016a,b) | review | F | Y |
| During their activity, hunters have more opportunities than any other segment of the population for direct exposure to infected material (secretions, excreta, infected tissues) (Saunders et al., 2012). | Saunders et al. (2012) | review | F | |||
| Wood, rocks, plastic, glass, cement, stainless steel and aluminium can all bind, retain and release prions and therefore act efficiently as fomites and transmit disease. Not every strain had the same affinities. Effective transmission only required contact with the contaminated surfaces – nothing more invasive. Good binding to wood and rock have implications for environmental contamination in natural/wildlife settings (Pritzkow et al., 2018) | Pritzkow et al. (2018) | other | F | N | ||
| 12. Environmental persistence of prions | I & II | Prions, due to their resistance to degradation, can persist for years by binding to soil particles without losing their infectious potential. For instance, it has been shown that BSE infectivity survives burial for 5 years with only limited spread (Williams and Young, 1992; Miller and Williams, 2003; Miller et al., 2004; Johnson et al., 2007; Saunders et al., 2012; Somerville et al., 2019) | Williams and Young (1992) | review | F | N |
| Miller and Williams (2003) | cohort | B | ||||
| Miller et al. (2004) | int | A | ||||
| Johnson et al. (2007) | other | F | ||||
| Saunders et al. (2012) | review | F | ||||
| Somerville et al. (2019) | other | F | ||||
| Such a persistence allows an infected animal to cause secondary infections long after its death (Almberg et al., 2011). | Almberg et al. (2011) | simul | E | |||
| 13. Host genetics | I & II | Species‐specific polymorphisms in the PRNP gene (e.g. S225F in mule deer, G96S in white‐tailed deer, L132M in wapiti) appear to be associated with a relative protective effect, namely, longer incubation times and reduced peripheral distribution of the infectivity (Peletto et al., 2009; Robinson et al., 2012; Wik et al., 2012; EFSA BIOHAZ Panel, 2017) | EFSA BIOHAZ Panel (2017) | review | F | (Y) |
| Peletto et al. (2009) | other | F | ||||
| Wik et al. (2012) | other | F | ||||
| Robinson et al. (2012) | review | F | ||||
| Frequency of protective haplotypes in WTD varies significantly among infected and non‐infected areas in Illinois and Wisconsin (Brandt et al., 2018) | Brandt et al. (2018) | cross‐s | D |
Individual risk factors that are specific to the overarching groups are highlighted in bold.
Type of study: Intervention (int), cohort (cohort), case–control (c–c), cross‐sectional (cross‐s), case series (c‐series), case report (c‐report), mathematical simulation model (simul), literature review (review), non‐epidemiological studies (other).
Strength of evidence: ‘A’ strongest evidence; ‘F’ weakest evidence. Preventable: Y = Yes; (Y) = Yes but difficult or theoretical; N = No.
The studies included in Table 9 have been classified, based on their design (Rothman et al., 2008; Dohoo et al., 2009) into intervention vs observational studies. The latter are further distinguished into analytical studies that are designed to test aetiological hypotheses and quantify the effect of risk factors (i.e. cohort studies, case–control studies and cross‐sectional studies) vs descriptive studies (i.e. case reports and case series) that are considered as hypothesis‐screening studies. By combining descriptive data and assumptions, epidemiological hypotheses may also be supported (but not tested) by mathematical simulation models.
The biological plausibility of the involvement of a factor in the spread may be suggested by non‐epidemiological studies or qualitative risk assessments or reviews, so these were considered as sources able to suggest ‘theoretical risk factors’. Due to the uncertainties associated with both the unknown European epidemiological situation and the differences between involved strains, it is not possible to assess the epidemiological relevance of any of the listed risk factors even in the NA context, let alone in the European one.
However, the strength of the evidence for the causal role of each group of risk factors can be appraised. For this purpose, a score‐based ranking (as proposed by Dohoo et al., 2009) has been applied based on the different informative potential of the studies:
score = A: if the risk factor has been proposed through intervention studies (strongest evidence);
score = B: if through cohort studies;
score = C: if through case–control studies;
score = D: if through cross‐sectional studies;
score = E: if through descriptive studies (i.e. an epidemiologically screened hypothesis) or mathematical simulation models;
score = F: if through biologically plausible hypotheses alone, based on non‐epidemiological studies or qualitative risk assessments or reviews (weakest evidence).
Based on the above inclusion criteria, Table 9 shows a list of groups of potential risk factors for the spread of CWD in Europe within or between areas/regions/countries, with the indication of the studies used to source each risk factor from the literature and their score. Finally, all factors depending on, or affected, by human practices (with the exception of rearing cervids in captivity) and therefore possible targets for preventive measures, have been highlighted.
A general conceptual map (Figure 1) has been developed to show all the factors mentioned in the list and to summarise the relationships among them within theoretical risk pathways. This map also highlights potential targets for interventions that could be used as preventive measures against the spread of disease.
Figure 1.
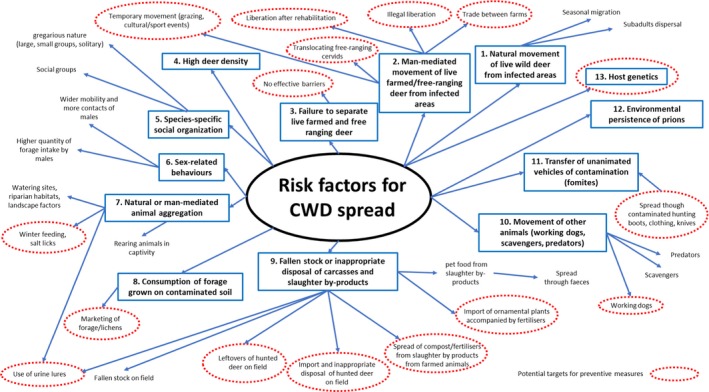
Conceptual map summarising the relationship among risk factors within risk pathways and potential targets for preventive measures
3.6.3. Concluding remarks
The identification of risk factors associated with the spread of the disease can only be based on the epidemiological and biological understanding of NA CWD outbreaks, although the biological characterisation of the European isolates indicates that they differ from the known NA strains.
The presence/absence of detectable PrPSc in the peripheral tissues as well as the CNS of affected animals can be used as an indicator of the probability of natural spread and animals assigned to different categories based on their pathogenesis. Both infected carriers and passive carriers are thought to be involved in the dissemination of the agent.
Two distinct, hypothetical working scenarios have been built on these categories: a contagious form (Scenario 1) affecting animals which can spread infectivity through direct contacts with other animals and/or contamination of the environment and a non‐contagious form (Scenario 2) that is transmitted through environmental contamination from infected carcasses or via human activity, scavengers or via the feed chain.
Thirteen main groups of risk factors have been identified based on their biological plausibility to spread CWD. Some of them are supported by epidemiological evidence from NA CWD.
The only risk factors for the spread of CWD affecting exclusively Scenario 1 refer to animal to animal contact which will increase through the aggregation of live animals, promoted, or not, by human activities.
All other risk factors refer to natural or man‐mediated movement or translocation of wild or farmed/free ranging animals and to environmental contamination and the subsequent exposure of animals to contaminated soil or vegetation. This can occur at the point of contamination (close to a fallen animal) or following translocation of contaminated materials by humans or scavengers. These risks will also vary depending on the social structures of different species and by habitat and the geography of specific regions. In wild populations, the co‐existence of different species makes it important to consider all the identified risk factors in both scenarios.
Risk reduction through intervention would be possible for some man‐mediated risk factors, namely activities leading to the translocation or aggregation of both wild and farmed live animals, as well as fallen stock management and appropriate disposal of carcasses and slaughter by‐products.
3.7. Uncertainty analysis
The sources of uncertainty associated with the available data have been summarised in tabular format (Table 10), describing the nature or cause of the uncertainty. The factors contributing to the list of uncertainties are mostly missing or incomplete data on disease prevalence, pathogenesis and isolate characterisation, affecting the entire Opinion and the answers to all of the ToRs.
Table 10.
Sources of uncertainty identified in the risk assessment
| Source or location of uncertainty | Nature or cause of uncertainty as described by the experts |
|---|---|
| Cervid surveillance approaches |
|
| Current strain typing approaches to identify reliably all the prion strains that might be present in a field TSE isolate. |
|
| Range and characteristics of CWD strains circulating in NA |
|
| Range, characteristics and prevalence of CWD strains circulating in Europe in susceptible cervid species |
|
| Ability of the CWD agent(s) to cross the human species barrier |
|
4. Answers to the Terms of Reference
4.1. Answer to ToR1
To revise the state of knowledge, considering new scientific data, about the differences between the strains found in different species in NA and in Europe and between the strains found so far in moose, reindeer and red deer in Europe; with the main emphasis on transmissibility (transmission paths), pathogenicity and prevalence of the different strains and susceptibility of the different species/genotypes.
Full characterisation of European isolates is being pursued through the collection of data on host species spectrum and genotype, clinical presentation, histopathology, immunopathology, tissue distribution, pathogenesis, the biochemical properties of the PrPSc and bioassay through experimental transmission to a wide range of rodent models, whereas most NA CWD isolates have not been fully characterised in this way. However, preliminary data support the contention that the CWD strains identified to date in Europe and NA are different and suggest the presence of strain diversity in the European cervid population.
The current spectrum of known host phenotypes may be biased due to differences in surveillance in NA and Europe. NA surveillance testing protocols mainly target peripheral lymphoid tissues, while European testing protocols focus on the central nervous system complemented, or not, with testing of peripheral lymphoid tissues. This may have prevented the detection of specific phenotypes and the identification of strains potentially common to both regions.
Current data do not allow any conclusion on the implications of strain diversity on transmissibility (including host susceptibility), pathogenesis and prevalence.
4.2. Answer to ToR2
To revise the new scientific evidence on the zoonotic potential of CWD; to assess the risk of transmission to humans through the consumption of meat and meat products of cervids and to provide recommendations on possible additional control measures to address the risks identified.
A number of studies have shown that exposure to some NA CWD isolates can result in the conversion of human PrP to disease‐specific PrPSc in vitro and that some NA CWD isolates efficiently transmit the disease to squirrel monkeys. However, in vivo studies performed with humanised mice and macaques are considered to be the most pertinent models of human susceptibility and there is conflicting evidence on the transmissibility of NA CWD isolates in these models.
Epidemiological studies suffer from many methodological limitations and logistic constraints and some of them are still ongoing in NA but, until now, there is no epidemiological evidence of NA CWD causing disease in humans.
At this stage, the available data do not allow any conclusion on the zoonotic potential of NA cervid CWD isolates and no data are currently available on the zoonotic potential of European cervid CWD isolates.
The risk to humans through consumption of meat, meat products and offal derived from CWD‐infected cervids cannot be directly assessed. At individual level, consumers of meat, meat products and offal derived from CWD‐infected cervids will be exposed to the CWD agent(s). At the population level, the probability of exposure via consumption of venison depends on the prevalence of CWD agent(s) in each of the species that are consumed (reindeer, moose, red deer), which is not known.
Testing cervids before human consumption or the removal of high‐risk tissues from cervids intended for human consumption, or the combination of these measures would reduce the probability of dietary exposure of humans to the CWD agent(s). The prohibition of harvesting/hunting susceptible species in infected premises/areas could also be considered as a preventive measure.
4.3. Answer to ToR3
To identify risk factors that can facilitate the spread of CWD in the European Union given the current situation of the disease.
Using data from the NA CWD experience, 13 groups of risk factors have been identified based on their biological plausibility to spread CWD. Some of these are supported by epidemiological evidence from NA CWD studies with variable strength of evidence, while others remain hypothetical: 1. Natural movement of live wild deer from infected areas. 2. Man‐mediated movement of live farmed/free‐ranging deer from infected areas. 3. Failure to separate live farmed and free‐ranging deer. 4. High deer density. 5. Species‐specific social organisation. 6. Sex‐related behaviours. 7. Natural or man‐mediated animal aggregation. 8. Consumption of forage grown on contaminated soil. 9. Fallen stock or inappropriate disposal of carcasses and slaughter by‐products. 10. Movement of other animals (working dogs, scavengers, predators). 11. Transfer of inanimate vehicles of contamination (fomites). 12. Environmental persistence of prions. 13. Host genetics.
All the identified risk factors may contribute to the spread of the disease when it is associated with the accumulation of infectivity in peripheral tissues, a host phenotype that is compatible with a contagious disease.
A subset of risk factors (1,2,6,8,9,10,11,12 and 13) are relevant to cases of disease that do not involve peripheral accumulation of infectivity and are therefore less contagious or non‐contagious and may contribute to the spread of the disease mainly via environmental contamination following death.
Some risk factors are man‐mediated and are considered preventable. Their management could contribute to a decrease in the theoretical risk of spread of CWD.
The potential co‐localisation of cervid species and disease phenotypes mean that all the identified groups of risk factors should be taken into account when considering interventions.
Recommendations
To document PRNP gene polymorphisms (nature, distribution and frequency) in European cervid populations and the impact of these genotypes on susceptibility to the different CWD strains that appear to be emerging in Europe.
To collate, when available, data on host species spectrum and genotype, clinical presentation, histopathology, immunopathology, tissue distribution, pathogenesis, the biochemical properties of the PrPSc and bioassay through experimental transmission to a wide range of rodent models of the European CWD isolates and to continue collecting such data on new cases that may arise in Europe, together with NA positive control material in order to make appropriate comparisons.
To collect data on tissue distribution in naturally infected animals and, ideally, through experimental pathogenesis studies in cervid species. However, as stated in the previous EFSA opinion (EFSA BIOHAZ Panel, 2018), ‘they are not easy to perform in non‐domesticated species. They would require the maintenance of experimentally infected individuals in high biosafety facilities for a long period of time and would raise practical and welfare issues for such animals’.
To maximise the effectiveness of the current compulsory monitoring programme by aiming to test primarily animals from the predefined risk groups and to meet the required sample size per PSU (30 animals) over the 3‐year period.
Glossary
- Isolate
Biological material that has been obtained through the sampling of an individual infected animal.
- Strain
A TSE strain is defined as an agent that has distinct and reproducible pathological, biochemical and molecular characteristics in the original host/s and which, when serially passaged through congenic or transgenic mouse lines, produces consistent characteristics of relative incubation period, spongiform lesion profile, molecular profile and immunopathology. Adapted from the definition of strain as in the ‘TSE strain characterisation in small ruminants. A technical handbook for national reference laboratories in the EU’: https://science.vla.gov.uk/tse-lab-net/documents/tse-oie-rl-handbook.pdf
- Transmissible
(of a disease or trait) able to be passed on from one person or organism to another,9 either by natural (i.e. contagious) or experimental or iatrogenic routes.
- Contagious
(of a disease) spread from one person or organism to another, typically by direct contact10 in natural conditions.
- Meat products
Processed products resulting from the processing of meat or from the further processing of such processed products, so that the cut surface shows that the product no longer has the characteristics of fresh meat (Regulation (EC) No 853/2004 of the European Parliament and the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs)
- Offal
Fresh meat other than that of the carcass, including viscera and blood (Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs)
- Fresh meat
Meat that has not undergone any preserving process other than chilling, freezing or quick‐freezing, including meat that is vacuum‐wrapped or wrapped in a controlled atmosphere (Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs).
Abbreviations
- BIOHAZ
EFSA Panel on Biological Hazards
- BSE
bovine spongiform encephalopathy
- CJD
Creutzfeldt–Jakob disease
- CM
cynomolgus macaques
- CNS
central nervous system
- CWD
chronic wasting disease
- Defra
Department of Environment, Food and Rural Affairs
- EEA
European Economic Area
- ELISA
enzyme‐linked immunosorbent assay
- ENS
enteric nervous system
- FC
Fallen/culled
- GALT
gut‐associated lymphoid tissue
- GdnHC
guanidine hydrochloride
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HbA1c
glycated haemoglobin
- HSHC
hunted/slaughtered fit for human consumption
- IC
intracerebrally
- ID50
infectious dose 50%
- IHC
immunohistochemistry
- LRS
lymphoreticular system
- MD
mule deer
- mpi
months post‐inoculation
- MS
Member State
- NA
North America
- NPDPSC
National Prion Disease Pathology Surveillance Center
- NYSDEC
New York State Department of Environmental Conservation
- PK
proteinase K
- PMCA
protein misfolding cyclic amplification
- PRNP
prion protein gene
- PrP
normal cellular prion protein
- PrPC
protease‐sensitive PRion Protein Cellular
- PrPCWD
disease‐associated (CWD‐associated) isoform of the host prion protein
- PrPres
protease‐resistant prion protein
- PrPSc
abnormal protease‐resistant isoform of prion protein
- PSU
primary sampling unit
- recPrPC
recombinant protease‐sensitive PRion Protein Cellular
- RK
road/predator killed
- RML
Rocky Mountains Laboratory
- RT‐QuIC
real‐time quaking‐induced conversion
- sCJD
sporadic Creutzfeldt–Jakob disease
- SDS
sodium dodecyl sulfate
- STEG
EURL Strain Typing Expert Group
- SUS
clinical suspect animals
- tg
transgenic
- TME
Transmissible mink encephalopathy
- ToR
Terms of Reference
- TSE
transmissible spongiform encephalopathies
- UK
United Kingdom
- USA
United States of America
- vCJD
variant Creutzfeldt‐Jakob disease
- VKM
Vitenskapskomiteen for mat og miljø [Norwegian Scientific Committee for Food and Environment]
- WB
western blot
- WG
working group
- wt
wild‐type
- WTD
white‐tailed deer
Appendix A – Biological diversity of TSE agents
A.1. Strains and strain typing
The biological diversity of prion agents was first observed in goats in 1961 by Pattison and Millson. Goats infected with scrapie developed different clinical phenotypes that were termed ‘scratching’ and ‘drowsy’ by the authors. These differences in symptomatology were preserved upon iterative passages in goats. This led to the conclusion that scrapie can be caused by different strains of transmissible agents (Pattison and Millson, 1961).
Historically, the appreciation of the potential diversity of transmissible spongiform encephalopathies (TSE) agents has relied on the characterisation of the disease phenotypes observed after serial transmission to conventional rodent models. After inoculation of a TSE isolate in a conventional rodent model, if transmission occurs, the incubation period will progressively shorten upon iterative passage before stabilising (generally after three or four passages). The same lesion profile (i.e. severity and distribution of spongiform degeneration in standardised brain regions) is observed in all the animals (if they are the same inbred rodent strain/model) inoculated with a stabilised TSE agent (Bruce, 2003).
Strikingly, the transmission of different TSE isolates to a same model can lead to different phenotypes (incubation periods and/or lesion profile). The phenotypes observed after passage of a TSE isolate in a panel of rodent models are operationally used to define and identify prion strains.
When two different isolates assayed in the same panel of rodent models result in different phenotypes, it can be concluded that the isolates contained different prion strains. Conversely when the same phenotypes are observed, the two original isolates are more likely to contain the same strain.
A.2. The limits of strain typing bioassay models
For more than three decades, TSE strain typing relied on the use of a panel of three wild‐type mouse lines: RIII, C57BL and VM. RIII and C57BL lines share the same PRNP (PrP gene) amino acid sequence (PRNP a) while VM mice (PRNP b) differ at codons 108 and 189 (Westaway et al., 1987a,b). Polymorphisms in this gene have been shown to have a significant effect on susceptibility/resistance to TSE in a range of species (see Section 3.2.3).
However, due to the transmission barrier phenomenon (EFSA BIOHAZ Panel, 2015), a large proportion of TSE isolates (from humans, small ruminants) were unable to propagate into these models. This limited the operational capacity to characterise prion strain diversity, particularly in field cases (Dickinson, 1976; Beringue et al., 2008c).
Moreover, the transmission of a TSE agent between host species can lead in certain instances to a radical evolution of the original agent's biological properties (strain mutation). It is therefore difficult to estimate how much the phenotype observed after passage of the mouse transmission barrier really reflects the diversity of the prion strains present into the original isolate (Beringue et al., 2008b).
These issues raised substantial concerns about the value of prion strain typing results obtained in conventional rodent models.
The introduction of bank voles as a model for prion strain typing partly resolved the issue. Indeed, in contrast with the mouse, bank voles are permissive to most prion isolates regardless of the donor's species (Nonno et al., 2006; Di Bari et al., 2008; Watts et al., 2014). However, the use of the bank voles in strain typing experiments does not solve the issue of the potential evolution of strain properties upon interspecies passage.
After identification of the PRNP gene, it was discovered that differences in amino acid sequence between host PrPC and donor PrPSc were the main driver for the transmission barrier. For example, the resistance of wild‐type mice to clinical disease induced by hamster scrapie is abrogated by transgenic expression of hamster PrPC in mice (Kimberlin and Walker, 1978; Scott et al., 1989).
Over the last 15–20 years, large numbers of tg mice expressing PrP from a wide variety of species have been generated (Groschup and Buschmann, 2008). tg mouse models have solved many of the issues caused by the transmission barrier and have been demonstrated as valuable tools for characterising the biological diversity of TSE agents (Beringue et al., 2008b; Thackray et al., 2011) and new ones continue to be developed.
However, the increasing diversity of the tg mouse lines used by different research groups makes it difficult to compare the strain typing results they obtained. Beyond this, the nature of the genomic construction used to generate transgenic PrP mice, which impacts on both the final expression levels and the pattern of PrP expression in the tissues, appears to have a selection effect on the strain(s) that might propagate following inoculation of a particular TSE isolate (Le Dur et al., 2017).
A.3. PrPSc properties and prion strain discrimination
Initially, the existence of prion strains in the context of the protein‐only hypothesis (Prusiner, 1991) was met with scepticism. A first indication of the molecular basis of prion strain diversity came from a study in which scrapie‐associated fibrils (SAF) were isolated and purified from animals infected with three different scrapie agents: ME7 and 139A in mice and 263K in hamster (Kascsak et al., 1985). Mouse ME7 and 139A SAF differed from hamster 263K in terms of morphology and sensitivity to PK digestion. Importantly, SAF co‐purified with infectivity in both animal systems; therefore, it was hypothesised that the different molecular properties characterising the different SAF may correlate with the biological and pathological differences which are found among these agents.
Subsequently, the isolation and characterisation of two biologically distinct strains of hamster‐adapted transmissible mink encephalopathy (TME), the ‘hyper’ (HY) and the ‘drowsy’ (DY), significantly improved the understanding of the molecular basis of prion strains. Indeed, purification and analysis of abnormal PrPSc from hamsters infected with the HY and the DY strains revealed differences in terms of PrPSc sedimentation in N‐lauroylsarcosine, sensitivity to PK digestion and electrophoretic mobility. Moreover, antigenic mapping of PrPSc with antibodies raised against different synthetic peptides showed a strain‐specific difference in immunoreactivity in the N‐terminus of the two PrPSc variants (Bessen and Marsh, 1992, 1994). Taken together, these observations indicated that PrPSc from the two agent strains, although originating from the same host, differ in composition, conformation or possibly both.
Several techniques have been developed to distinguish PrPSc variants at the biochemical level. Among these, electrophoretic mobility after PK digestion is the most commonly used method because it is easy to visualise while providing indirect evidence of conformational changes. The partial resistance of prions to proteolytic degradation lies within the C‐terminal region of the prion protein. However, the length of the PK‐resistant segment varies depending on the prion strain being analysed. This can be easily distinguished in WB. The prion protein has two putative glycosylation sites, allowing it to exist in di‐, mono‐ or unglycosylated forms. Differences in the proportions of PrPres glycoforms can also be used to differentiate prion strains. Other biochemical techniques used to differentiate between PrPSc variants rely on the extent of their proteolytic degradation or denaturation when exposed to increasing concentrations of PK or chaotropic agents (Safar, 1996; Safer and Prusiner, 1998; Uro‐Coste et al., 2008).
PrPSc biochemical properties (PrPres WB pattern, glycoprofile, resistance to digestion or denaturation by chaotropic agents) can enable the differentiation of prion strains. However, it is not uncommon that different prion strains remain indistinguishable on the basis of their PrPSc properties. Moreover, as PrPSc biochemical properties are dependent on both the prion strain and the host PrP amino acid sequence, these approaches are unsuitable for the direct comparison of isolates originating from hosts with different PrPC sequences.
A.4. Multiple strains in a single isolate
Intuitively, the occurrence of a prion disease is expected to be the consequence of the replication of a single prion strain in the host. However, the presence of several prion strains in a single natural TSE isolate has been observed occasionally (Dickinson, 1976; Angers et al., 2010; Mazza et al., 2010; Thackray et al., 2012). For instance, the transmission of NA CWD isolates to cervid PrP transgenic mice allowed the differentiation of two distinct prion strains (CWD1 and CWD2). The transmission of certain CWD isolates led to the propagation of either CWD1 and/or CWD2 in the reported mice (Angers et al., 2010). As the identification of a strain mixture within an individual isolate is highly dependent on the capacity of the strain typing approaches used to differentiate the original isolate's components, the frequency of the phenomenon remains difficult to establish.
The principle of the stable co‐propagation of strain mixtures (artificial mixture of rodent‐stabilised scrapie strains) was validated several decades ago (Bruce et al., 1991) and might explain the reported observations of strain mixtures in field isolates. The concept of a ‘prion cloud’ may also provide an explanation of the strain mixtures in natural field isolates. According to this concept, a prion strain naturally propagates in its host as a ‘cloud’ of PrPSc conformers (each corresponding to a different prion strain). The cloud is dominated by a stable PrPSc conformer that preferentially propagates in the particular host and is responsible for the observable prion strain phenotype. However, when the conditions of propagation (host, route of infection) are modified, one of the minor (but better fitted) PrPSc conformers present in the cloud may be selected, resulting in a shift in the observed prion phenotype (Collinge and Clarke, 2007).
The emergence of a new prion strain during iterative passages of a prion isolate in the same host species has occasionally been reported. For instance, the 87A scrapie strain, that was obtained by the biological cloning (end point titration) of a sheep scrapie isolate adapted to C57Bl6 mice, remained stable after multiple passages at a low dose (highly diluted brain homogenate – intracerebral route) in C57Bl6 mice. However, when a higher dose of the cloned 87A strain was transmitted by the same route in C57Bl6 mice, a different prion strain, named ME7, occasionally emerged (Dickinson and Outram, 1973; Bruce and Dickinson, 1987).
Under a scenario in which the prion cloud is valid, the value of strain typing approaches (bioassay and PrPSc biochemical characterisation) to describe the nature of the prions that are present in the isolates would be limited.
A.5. TSE nomenclature
There is no official nomenclature related to prion strains. When TSE were first identified, the disease nomenclature was based on its clinical presentation (e.g. scrapie in sheep and CWD in cervids). More recently, naturally occurring forms of disease were more likely to be named based on host species and pathognomonic lesions (e.g. bovine spongiform encephalopathy, feline spongiform encephalopathy).
In other instances, the name of the strain can be directly inspired by the species of origin and the phenotypic characteristics of the prion. This is the case for the different prions that have, so far, been identified in cattle. Besides the bovine spongiform encephalopathy (BSE) agent that was responsible for the major epidemic of prion disease observed in cattle population in Europe after 1986, two other strains named BSE‐H and BSE‐L (for bovine spongiform encephalopathy with high PrPres banding profile and low PrPres banding profile as observed on WB) were identified (Biacabe et al., 2004). This discovery resulted in the epidemic BSE strain being re‐named ‘classical BSE’ and the new strains are often grouped together as ‘atypical’ BSE, despite the two having distinct biochemical and biological properties. The overarching term ‘BSE’ therefore now represents strains with three distinct sets of properties, for which originally it referred to one.
When a new prion is identified (based on bioassay transmission and/or biochemical properties of the abnormal PrP), the authors reporting its existence usually define a name/acronym, but there is still no established naming convention to be followed.
In many instances, the strain acronym is inspired by the laboratory internal codification assigned to the samples (e.g. 22L, 263K for rodents scrapie strains), the code of the source isolate used to derive the strain (for instance CH1641 or PG127 for classical sheep scrapie) or to a geographical location (RML strain in conventional mice for Rocky Mountain Laboratory in USA, the place where the strain was biologically cloned). This leads to the possibility of the same strain being independently isolated in different laboratories and being inadvertently given more than one name. While the original name proposed by the authors reporting the characteristic of a new prion is usually retained, in certain instances some changes can occur.
For instance, in 1998, a new prion was identified in small ruminants in Norway. This prion displayed particular PrPres WB profile (multiband pattern) and was named by its discoverers as NOR98 (Benestad et al., 2003). Over the years, bioassay in rodent models confirmed that this phenotype corresponded to a new and unique TSE agent strain. During the next decade, the NOR98 prion strain was identified in all of the major small ruminants breeding countries across the world (Benestad et al., 2008). During this process, the term of atypical scrapie has progressively emerged and is now largely used to designate this prion strain. However, this scrapie strain was referred to as atypical because of its differences from ‘classical scrapie’ (which is in itself an umbrella term for ovine TSE) and is quite distinct biochemically and biologically from the atypical BSE strains mentioned earlier.
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordoňez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Skandamis P, Suffredini E, Andreoletti O, Benestad SL, Comoy E, Nonno R, da Silva Felicio T, Ortiz‐Pelaez A and Simmons MM, 2019. Scientific Opinion on the update on chronic wasting disease (CWD) III. EFSA Journal 2019;17(11):5863, 63 pp. 10.2903/j.efsa.2019.5863
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00763
Panel members: Kostas Koutsoumanis, Ana Allende, Avelino Alvarez‐Ordoñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion M. Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Acknowledgements: The EFSA Panel on Biological Hazards (BIOHAZ) wishes to acknowledge all European and North American researchers who provided data and information for this scientific output, and Maria Francesca Iulietto for her contributions to the draft.
Adopted: 26 September 2019
This article was originally published on the EFSA website www.efsa.europa.eu on 7 November 2019
Notes
This section is copied verbatim from the mandate received from the European Commission.
Date of the mandate: 20 September 2018.
The fourth case of CWD in a moose in Norway was detected in 2018 after the mandate had been submitted.
Three additional cases of CWD have been diagnosed in moose in Sweden in March, May and September 2019.
Commission Regulation 1972/2017 amending Commission Regulation (EU) No. 999/2001.
Adapted from the definition of the Oxford Dictionary: https://en.oxforddictionaries.com/definition/transmissible
Adapted from the definition of the Oxford Dictionary: https://en.oxforddictionaries.com/definition/contagious
References
- Abrams JY, Maddox RA, Schonberger LB, Person MK, Appleby BS and Belay ED, 2018. Human prion disease mortality rates by occurrence of chronic wasting disease in free‐ranging cervids, United States. Prion, 14, 182–183. [Google Scholar]
- Almberg ES, Cross PC, Johnson CJ, Heisey DM and Richards BJ, 2011. Modeling routes of chronic wasting disease transmission: environmental prion persistence promotes deer population decline and extinction. PLoS ONE, 6, e19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoletti O, Orge L, Benestad SL, Beringue V, Litaise C, Simon S, Le Dur A, Laude H, Simmons H, Lugan S, Corbiere F, Costes P, Morel N, Schelcher F and Lacroux C, 2011. Atypical/Nor98 scrapie infectivity in sheep peripheral tissues. PLoS Pathogens, 7, e1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA and Telling GC, 2006. Prions in skeletal muscles of deer with chronic wasting disease. Science, 311, 1117–1117. [DOI] [PubMed] [Google Scholar]
- Angers RC, Seward TS, Napier D, Green M, Hoover E, Spraker T, O'Rourke K, Balachandran A and Telling GC, 2009. Chronic wasting disease prions in elk antler velvet. Emerging Infectious Diseases, 15, 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers RC, Kang HE, Napier D, Browning S, Seward T, Mathiason C, Balachandran A, McKenzie D, Castilla J, Soto C, Jewell J, Graham C, Hoover EA and Telling GC, 2010. Prion Strain Mutation Determined by Prion Protein Conformational Compatibility and Primary Structure. Science, 328, 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue CK, Ribble C, Lees VW, McLane J and Balachandran A, 2007. Epidemiology of an outbreak of chronic wasting disease on elk farms in Saskatchewan. Canadian Veterinary Journal‐Revue Veterinaire Canadienne, 48, 1241–1248. [PMC free article] [PubMed] [Google Scholar]
- Barria MA, Telling GC, Gambetti P, Mastrianni JA and Soto C, 2011. Generation of a new form of human PrPSc in vitro by interspecies transmission from cervid prions. Journal of Biological Chemistry, 286, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria MA, Ironside JW and Head MW, 2014. Exploring the zoonotic potential of animal prion diseases In vivo and in vitro approaches. Prion, 8, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria MA, Libori A, Mitchell G and Head MW, 2018. Susceptibility of human prion protein to conversion by chronic wasting disease prions. Emerging Infectious Diseases, 24, 1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JC, Marsh RF, McKenzie DI and Aiken JM, 1998. The host range of chronic wasting disease is altered on passage in ferrets. Virology, 251, 297–301. [DOI] [PubMed] [Google Scholar]
- Bauer SA, Leslie KE, Pearl DL, Fournier J and Turner PV, 2010. Survey of Prevalence of Overweight Body Condition in Laboratory‐Housed Cynomolgus Macaques (Macaca fascicularis). Journal of the American Association for Laboratory Animal Science, 49, 407–414. [PMC free article] [PubMed] [Google Scholar]
- Benestad S, 2019a. sPMCA text for inclusion in the CWD opinion part III. Message to Sylvie Benestad. 18 July 2019.
- Benestad S, 2019b. Q226 reindeer EFSA opinion. Message to Angel Ortiz Pelaez. 20 June 2019.
- Benestad S, 2019c. Moose 4. Message to Angel Ortiz Pelaez. 26 June 2019.
- Benestad S, 2019d. Fwd: Sv: En fråga om CWD‐älgarna, EFSA‐arbetsgrupp undrar, VB: Some Swedish CWD‐data. Message to Angel Ortiz Pelaez. 27 August 2019.
- Benestad SL, Sarradin P, Thu B, Schonheit J, Tranulis MA and Bratberg B, 2003. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Veterinary Record, 153, 202–208. [DOI] [PubMed] [Google Scholar]
- Benestad SL, Arsac JN, Goldmann W and Noremark M, 2008. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Veterinary Research, 39, 1–4. [DOI] [PubMed] [Google Scholar]
- Benestad SL, Mitchell G, Simmons M, Ytrehus B and Vikoren T, 2016. First case of chronic wasting disease in Europe in a Norwegian free‐ranging reindeer. Veterinary Research, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benestad S, 2019e. VS: Hjortedyr skrott. Message to Angel Ortiz Pelaez. 29 January 2019.
- Beringue V, Herzog L, Reine F, Le Dur A, Casalone C, Vilotte JL and Laude H, 2008a. Transmission of Atypical Bovine Prions to Mice Transgenic for Human Prion Protein. Emerging Infectious Diseases, 14, 1898–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringue V, Vilotte JL and Laude H, 2008b. Prion agent diversity and species barrier. Veterinary Research, 39, 47. [DOI] [PubMed] [Google Scholar]
- Beringue V, Le Dur A, Tixador P, Reine F, Lepourry I, Perret‐Liaudet A, Haik S, Vilotte JL, Fontés M and Laude H, 2008c. Prominent and persistent extraneural infection in human PrP transgenic mice infected with variant vCJD. PLoS ONE, 3, e1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringue V, Herzog L, Jaumain E, Reine F, Sibille P, Le Dur A, Vilotte JL and Laude H, 2012. Facilitated Cross‐Species Transmission of Prions in Extraneural Tissue. Science, 335, 472–475. [DOI] [PubMed] [Google Scholar]
- Bessen RA and Marsh RF, 1992. Biochemical and Physical‐Properties of the Prion Protein from 2 Strains of the Transmissible Mink Encephalopathy Agent. Journal of Virology, 66, 2096–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen RA and Marsh RF, 1994. Distinct Prp Properties Suggest the Molecular‐Basis of Strain Variation in Transmissible Mink Encephalopathy. Journal of Virology, 68, 7859–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biacabe AG, Laplanche JL, Ryder S and Baron T, 2004. Distinct molecular phenotypes in bovine prion diseases. Embo Reports, 5, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian JF, Christiansen JR, Moreno JA, Kane SJ, Khaychuk V, Gallegos J, Kim S and Telling GC, 2019. Primary structural differences at residue 226 of deer and elk PrP dictate selection of distinct CWD prion strains in gene‐targeted mice. Proceedings of the National Academy of Sciences of the United States of America, 116, 12478–12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger D, Caley D, Merrill D, Messier D, Miller W, Samuel D, Michael D and Vanopdenbosch D, 2004. Expert Scientific Panel on Chronic Wasting Disease. “Expert Scientific Panel on Chronic Wasting Disease”. Canadian Cooperative Wildlife Health. Centre: Newsletters and Publications. 19. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1018%26context=icwdmccwhcnews
- Boyle A, Hogan K, Manson JC and Diack AB, 2017. Strain typing of prion diseases using in vivo mouse models In: Lawson VA. (ed). Prions: Methods and Protocols. Humana Press, New York, USA: pp. 263–283. [DOI] [PubMed] [Google Scholar]
- Brandt AL, Green ML, Ishida Y, Roca AL, Novakofski J and Mateus‐Pinilla NE, 2018. Influence of the geographic distribution of prion protein gene sequence variation on patterns of chronic wasting disease spread in white‐tailed deer (Odocoileus virginianus). Prion, 12, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning SR, Mason GL, Seward T, Green M, Eliason GA, Mathiason C, Miller MW, Williams ES, Hoover E and Telling GC, 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. Journal of Virology, 78, 13345–13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce ME, 2003. TSE strain variation. British Medical Bulletin, 66, 99–108. [DOI] [PubMed] [Google Scholar]
- Bruce ME and Dickinson AG, 1987. Biological Evidence That Scrapie Agent Has an Independent Genome. Journal of General Virology, 68, 79–89. [DOI] [PubMed] [Google Scholar]
- Bruce ME, Mcconnell I, Fraser H and Dickinson AG, 1991. The Disease Characteristics of Different Strains of Scrapie in Sinc Congenic Mouse Lines – Implications for the Nature of the Agent and Host Control of Pathogenesis. Journal of General Virology, 72, 595–603. [DOI] [PubMed] [Google Scholar]
- Cassard H, Torres JM, Lacroux C, Douet JY, Benestad SL, Lantier F, Lugan S, Lantier I, Costes P, Aron N, Reine F, Herzog L, Espinosa JC, Beringue V and Andreoletti O, 2014. Evidence for zoonotic potential of ovine scrapie prions. Nature Communications, 5, 5821. [DOI] [PubMed] [Google Scholar]
- Cervenakova L, Sigurdson CJ, Piccardo P, Yakovleva O, Vasilyeva I, De Castro J, Saa P and Cervenak A, 2014. Successful transmission of chronic wasting disease (CWD) into mice over‐expressing bovine prion protein (TgSB3985). Prion, 8, 84. [Google Scholar]
- Clarkson TB, Koritnik DR, Weingand KW and Miller LC, 1985. Nonhuman Primate Models of Atherosclerosis – Potential for the Study of Diabetes‐Mellitus and Hyperinsulinemia. Metabolism‐Clinical and Experimental, 34, 51–59. [DOI] [PubMed] [Google Scholar]
- Collinge J and Clarke AR, 2007. A general model of prion strains and their pathogenicity. Science, 318, 930–936. [DOI] [PubMed] [Google Scholar]
- Comoy EE, Casalone C, Lescoutra‐Etchegaray N, Zanusso G, Freire S, Marce D, Auvre F, Ruchoux MM, Ferrari S, Monaco S, Sales N, Caramelli M, Leboulch P, Brown P, Lasmezas CI and Deslys JP, 2008. Atypical BSE (BASE) Transmitted from Asymptomatic Aging Cattle to a Primate. Plos, One, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoy E, Mikol J, Durand V, Luccantoni S, Correia E, Lescoutra N, Dehen C and Deslys JP, 2015. Transmission of prions to primates after extended silent incubation periods: Implications for BSE and scrapie risk assessment in human populations. Prion, 9, S3–S3. [Google Scholar]
- Comoy EE, Mikol J, Jaffre N, Lebon V, Levavasseur E, Streichenberger N, Sumian C, Perret‐Liaudet A, Eloit M, Andreoletti O, Haik S, Hantraye P and Deslys JP, 2017. Experimental transfusion of variant CJD‐infected blood reveals previously uncharacterised prion disorder in mice and macaque. Nature Communications, 8, 1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoy E, 2019a. Fwd: EFSA updated opinion on CWD in Europe. Message to Marion Simmons. 2 August 2019.
- Comoy E, 2019b. Re EFSA updated opinion on CWD in Europe. Message to Marion Simmons. 28 June 2019.
- Crowell J, Hughson A, Caughey B and Bessen RA, 2015. Host Determinants of Prion Strain Diversity Independent of Prion Protein Genotype. Journal of Virology, 89, 10427–10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullingham CI, Nakada SM, Merrill EH, Bollinger TK, Pybus MJ and Coltman DW, 2011a. Multiscale population genetic analysis of mule deer (Odocoileus hemionus hemionus) in western Canada sheds new light on the spread of chronic wasting disease. Canadian Journal of Zoology‐Revue canadienne de zoologie, 89, 134–147. [Google Scholar]
- Cullingham CI, Merrill EH, Pybus MJ, Bollinger TK, Wilson GA and Coltman DW, 2011b. Broad and fine‐scale genetic analysis of white‐tailed deer populations: estimating the relative risk of chronic wasting disease spread. Evolutionary Applications, 4, 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport KA, Henderson DM, Mathiason CK and Hoover EA, 2014. Modeling prion species barriers and the new host effect using RT‐ QuIC. Prion, 8, 36–37. [Google Scholar]
- Davenport KA, Henderson DM, Bian JF, Telling GC, Mathiason CK and Hoover EA, 2015. Insights into Chronic Wasting Disease and Bovine Spongiform Encephalopathy Species Barriers by Use of Real‐Time Conversion. Journal of Virology, 89, 9524–9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defra , 2016a. Qualitative Risk Assessment: Risk of chronic wasting disease being introduced into Great Britain. Available online: https://www.gov.uk/government/publications/qualitative-risk-assessment-risk-of-chronic-wasting-disease-being-introduced-into-great-britain
- Defra , 2016b. What is the risk of a cervid TSE being introduced from Norway into Great Britain? Qualitative Risk Assessment. September 2016. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/733407/DEFRA_QRA_TSE_in_cervids_June2018_v1.pdf
- Defra , 2018. What is the risk of a cervid TSE being introduced from Norway into Great Britain? Qualitative Risk Assessment. June 2018. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/733407/DEFRA_QRA_TSE_in_cervids_June2018_v1.pdf
- Di Bari MA, Chianini F, Vaccari G, Esposito E, Conte M, Eaton SL, Hamilton S, Finlayson J, Steele PJ, Dagleish MP, Reid HW, Bruce M, Jeffrey M, Agrimi U and Nonno R, 2008. The bank vole (Myodes glareolus) as a sensitive bioassay for sheep scrapie. Journal of General Virology, 89, 2975–2985. [DOI] [PubMed] [Google Scholar]
- Di Bari MA, Nonno R, Castilla J, D'Agostino C, Pirisinu L, Riccardi G, Conte M, Richt J, Kunkle R, Langeveld J and Vaccari G, 2013. Chronic wasting disease in bank voles: characterisation of the shortest incubation time model for prion diseases. PLoS Pathogens,9, e1003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diack AB, Head MW, McCutcheon S, Boyle A, Knight R, Ironside JW, Manson JC and Will RG, 2014. Variant CJD 18 years of research and surveillance. Prion, 8, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson AG, 1976. Scrapie in sheep and goats. Frontiers in Biology, 44, 209–241. [PubMed] [Google Scholar]
- Dickinson AG and Outram GW, 1973. Differences in access into the central nervous system of ME7 scrapie agent from two strains of mice. Journal of Comparative Pathology, 83, 13–18. [DOI] [PubMed] [Google Scholar]
- Dohoo IR, Martin SW and Stryhn H, 2009. Veterinary Epidemiologic Research. VER. Inc., Charlottetown, PE, Canada. [Google Scholar]
- Duque‐Velásquez C, Kim C, Herbst A, Daude N, Garza MC, Wille H, Aiken J and McKenzie D, 2015. Deer prion proteins modulate the emergence and adaptation of chronic wasting disease strains. Journal of Virology, 89, 12362–12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds DR, Albeke SE, Grogan RG, Lindzey FG, Legg DE, Cook WE, Schumaker BA, Kreeger TJ and Cornish TE, 2018. Chronic wasting disease influences activity and behavior in white‐tailed deer. Journal of Wildlife Management, 82, 138–154. [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Protocol for further laboratory investigations into the distribution of infectivity of Atypical BSE. EFSA Journal 2014;12(7):3798, 55 pp. 10.2903/j.efsa.2014.3798 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Joint Scientific Opinion on any possible epidemiological or molecular association between TSEs in animals and humans. EFSA Journal 2011;9(1):1945, 111 pp. 10.2903/j.efsa.2011.1945 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014. Scientific Opinion on the scrapie situation in the EU after 10 years of monitoring and control in sheep and goats. EFSA Journal 2014;12(7):3781, 155 pp. 10.2903/j.efsa.2014.3781 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2015. Scientific Opinion on a request for a review of a scientific publication concerning the zoonotic potential of ovine scrapie prions. EFSA Journal 2015;13(8):4197, 58 pp. 10.2903/j.efsa.2015.4197 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernandez Escamez PS, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Sanaa M, Skandamis P, Snary E, Speybroeck N, Kuile BT, Threlfall J, Wahlström H, Benestad S, Gavier‐Widen D, Miller MW, Ru G, Telling GC, Tryland M, Ortiz Pelaez A and Simmons M, 2017Scientific Opinion on chronic wasting disease (CWD) in cervids. EFSA Journal 2017;15(1):4667, 62 pp. 10.2903/j.efsa.2017.4667 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernandez Escamez PS, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Skandamis P, Snary E, Speybroeck N, Kuile BT, Threlfall J, Wahlström H, Benestad S, Gavier‐Widen D, Miller MW, Telling GC, Tryland M, Latronico F, Ortiz‐Pelaez A, Stella P and Simmons M, 2018. Scientific Opinion on chronic wasting disease (II). EFSA Journal 2018;16(1):5132, 59 pp. 10.2903/j.efsa.2018.5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KA, Jewell JE, Williams ES and Miller MW, 2006. Patterns of Prp(CWD) accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). Journal of General Virology, 87, 3451–3461. [DOI] [PubMed] [Google Scholar]
- Gavier‐Widen D, 2019. CWD data from Sweden. Message to Angel Ortiz Pelaez. 1 July 2019.
- Gajdusek D, 1972. Transmission of scrapie to the cynomolgus monkey (Macaca fascicularis). Nature, 236, 73. [DOI] [PubMed] [Google Scholar]
- Gavin C, Henderson D, Benestad SL, Simmons M and Adkin A, 2019. Estimating the amount of Chronic Wasting Disease infectivity passing through abattoirs and field slaughter. Preventive Veterinary Medicine, 166, 28–38. [DOI] [PubMed] [Google Scholar]
- Gibbs CJ and Gajdusek DC, 1973. Experimental subacute spongiform virus encephalopathies in primates and other laboratory animals. Science, 182, 67–68. [DOI] [PubMed] [Google Scholar]
- Gillette S, Dein J, Salman M, Richards B and Duarte P, 2004. Chronic wasting disease risk analysis workshop: an integrative approach. US Geological Survey., Available online: https://www.researchgate.net/profile/F_Dein/publication/228882368_Chronic_Wasting_Disease_Risk_Analysis_Workshop_An_Integrative_Approach/links/09e4150fecd0116272000000.pdf [Google Scholar]
- Gough KC, Bishop K and Maddison BC, 2014. Highly sensitive detection of small ruminant bovine spongiform encephalopathy within transmissible spongiform encephalopathy mixes by serial protein misfolding cyclic amplification. Journal of Clinical Microbiology, 52, 3863–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grear DA, Samuel MD, Scribner KT, Weckworth BV and Langenberg JA, 2010. Influence of genetic relatedness and spatial proximity on chronic wasting disease infection among female white‐tailed deer. Journal of Applied Ecology, 47, 532–540. [Google Scholar]
- Groschup MH and Buschmann A, 2008. Rodent models for prion diseases. Veterinary Research, 39. [DOI] [PubMed] [Google Scholar]
- Habib TJ, Merrill EH, Pybus MJ and Coltman DW, 2011. Modelling landscape effects on density‐contact rate relationships of deer in eastern Alberta: implications for chronic wasting disease. Ecological Modelling, 222, 2722–2732. [Google Scholar]
- Haley NJ and Hoover EA, 2015. Chronic Wasting Disease of Cervids: Current Knowledge and Future Perspectives. Annual Review of Animal Biosciences, 3, 305–325. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Kunkle RA, Richt JA, Miller JM, Cutlip RC and Jenny AL, 2005. Experimental transmission of sheep scrapie by intracerebral and oral routes to genetically susceptible Suffolk sheep in the United States. Journal of Veterinary Diagnostic Investigation, 17, 3–9. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Kunkle RA, Cutlip RC, Miller JM, Williams ES and Richt JA , 2006a. Transmission of chronic wasting disease of mule deer to Suffolk sheep following intracerebral inoculation. Journal of Veterinary Diagnostic Investigation, 18, 558–565. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Kunkle RA, Miller JM, Greenlee JJ and Richt JA, 2006b. Experimental second passage of chronic wasting disease (CWD mule (deer)) agent to cattle. Journal of Comparative Pathology, 134, 63–69. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Gidlewski T, Spraker TR, Miller JM, Creekmore L, Crocheck M, Cline T and O'Rourke KI, 2006c. Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. Journal of Veterinary Diagnostic Investigation, 18, 110–114. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Miller JM, Kunkle RA, Hall SM and Richt JA, 2007. Susceptibility of cattle to first‐passage intracerebral inoculation with chronic wasting disease agent from white‐tailed deer. Veterinary Pathology, 44, 487–493. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Greenlee JJ, Nicholson EM, Kunkle RA, Richt JA, Miller JM and Hall M, 2011. Experimental transmission of chronic wasting disease (CWD) from elk and white‐tailed deer to fallow deer by intracerebral route: Final report. Canadian Journal of Veterinary Research‐Revue Canadienne De Recherche Veterinaire, 75, 152–156. [PMC free article] [PubMed] [Google Scholar]
- Harrington RD, Baszler TV, O'Rourke KI, Schneider DA, Spraker TR, Liggitt HD and Knowles DP, 2008. A species barrier limits transmission of chronic wasting disease to mink (Mustela vison). Journal of General Virology, 89, 1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisey DM, Mickelsen NA, Schneider JR, Johnson CJ, Johnson CJ, Langenberg JA, Bochsler PN, Keane DP and Barr DJ, 2010. Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. Journal of Virology, 84, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst A, Velasquez CD, Triscott E, Aiken JM and McKenzie D, 2017. Chronic Wasting Disease Prion Strain Emergence and Host Range Expansion. Emerging Infectious Diseases, 23, 1598–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C, Riviere J, Lescoutra‐Etchegaray N, Charbonnier A, Leblanc V, Sales S, Deslys JP and Lasmezas CI, 2005. Prp(TSE) distribution in a primate model of variant, sporadic, and iatrogenic Creutzfeldt‐Jakob disease. Journal of Virology, 79, 14339–14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holznagel E, Yutzy B, Schulz‐Schaeffer W, Kruip C, Hahmann U, Bierke P, Torres JM, Kim YS, Thomzig A, Beekes M, Hunsmann G and Loewer J, 2013. Foodborne Transmission of Bovine Spongiform Encephalopathy to Nonhuman Primates. Emerging Infectious Diseases, 19, 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennelle CS, Samuel MD, Nolden CA and Berkley EA, 2009. Deer Carcass Decomposition and Potential Scavenger Exposure to Chronic Wasting Disease. Journal of Wildlife Management, 73, 655–662. [Google Scholar]
- Jennelle CS, Henaux V, Wasserberg G, Thiagarajan B, Rolley RE and Samuel MD, 2014. Transmission of Chronic Wasting Disease in Wisconsin White‐Tailed Deer: Implications for Disease Spread and Management. PLoS ONE, 9, e91043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennelle CS, Walsh DP, Samuel MD, Osnas EE, Rolley R, Langenberg J, Powers JG, Monello RJ, Demarest ED, Gubler R and Heisey DM, 2018. Applying a Bayesian weighted surveillance approach to detect chronic wasting disease in white‐tailed deer. Journal of Applied Ecology, 55, 2944–2953. [Google Scholar]
- Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D and Aiken JM, 2007. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathogens, 3, 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CJ, Herbst A, Duque‐Velasquez C, Vanderloo JP, Bochsler P, Chappell R and McKenzie D, 2011. Prion Protein Polymorphisms Affect Chronic Wasting Disease Progression. PLoS ONE, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly DO, Samuel MD, Langenberg JA, Blanchong JA, Batha CA, Rolley RE, Keane DP and Ribic CA, 2006. Spatial epidemiology of chronic wasting disease in Wisconsin white‐tailed deer. Journal of Wildlife Diseases, 42, 578–588. [DOI] [PubMed] [Google Scholar]
- Kascsak RJ, Rubenstein R, Merz PA, Carp RI, Wisniewski HM and Diringer H, 1985. Biochemical Differences among Scrapie‐Associated Fibrils Support the Biological Diversity of Scrapie Agents. Journal of General Virology, 66, 1715–1722. [DOI] [PubMed] [Google Scholar]
- Kim TY, Shon HJ, Joo YS, Mun UK, Kang KS and Lee YS, 2005. Additional cases of chronic wasting disease in imported deer in Korea. Journal of Veterinary Medical Science, 67, 753–759. [DOI] [PubMed] [Google Scholar]
- Kimberlin RH and Walker CA, 1978. Evidence That Transmission of One Source of Scrapie Agent to Hamsters Involves Separation of Agent Strains from a Mixture. Journal of General Virology, 39, 487–496. [DOI] [PubMed] [Google Scholar]
- Kjaer LJ, Schauber EM and Nielsen CK, 2008. Spatial and Temporal Analysis of Contact Rates in Female White‐Tailed Deer. Journal of Wildlife Management, 72, 1819–1825. [Google Scholar]
- Kong QZ, Huang SH, Zou WQ, Vanegas D, Wang ML, Wu D, Yuan J, Zheng MJ, Bai H, Deng HY, Chen K, Jenny AL, O'Rourke K, Belay ED, Schonberger LB, Petersen RB, Sy MS, Chen SG and Gambetti P, 2005. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. Journal of Neuroscience, 25, 7944–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpenfelt , 2019. VS: EFSA Scientific Opinion CWD. Message to Angel Ortiz Pelaez. 18 July 2019.
- Koutnik DL, 1981. Sex‐Related Differences in the Seasonality of Agonistic Behavior in Mule Deer. Journal of Mammalogy, 62, 1–11. [Google Scholar]
- Kramm C, Pritzkow S, Lyon A, Nichols T, Morales R and Soto C, 2017. Detection of Prions in Blood of Cervids at the Asymptomatic Stage of Chronic Wasting Disease. Scientific Reports, 7, 17241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt TD, Seelig DM, Schneider JR, Johnson CJ, Telling GC, Heisey DM and Hoover EA, 2011. Alteration of the Chronic Wasting Disease Species Barrier by In Vitro Prion Amplification. Journal of Virology, 85, 8528–8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt TD, Jiang L, Fernandez‐Borges N, Bett C, Liu J, Yang T, Spraker TR, Castilla J, Eisenberg D, Kong QZ and Sigurdson CJ, 2015. Human prion protein sequence elements impede cross‐species chronic wasting disease transmission (vol 125, pg 1485, 2015). Journal of Clinical Investigation, 125, 2548–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt T, Jiang L, Alderson N, Liu J, Eisenberg D and Sigurdson C, 2016. The molecular basis for cross‐species prion transmission. FASEB Journal, 30 Suppl., Abstract Number, 814.7. [Google Scholar]
- LaFauci G, Carp RI, Meeker HC, Ye X, Kim JI, Natelli M, Cedeno M, Petersen RB, Kascsak R and Rubenstein R, 2006. Passage of chronic wasting disease prion into transgenic mice expressing Rocky Mountain elk (Cervus elaphus nelsoni) PrPC. Journal of General Virology, 87, 3773–3780. [DOI] [PubMed] [Google Scholar]
- Lasmezas CI, Comoy E, Hawkins S, Herzog C, Mouthon F, Konold T, Auvre F, Correia E, Lescoutra‐Etchegaray N, Sales N, Wells G, Brown P and Deslys JP, 2005. Risk of oral infection with bovine spongiform encephalopathy agent in primates. Lancet, 365, 781–783. [DOI] [PubMed] [Google Scholar]
- Lavelle MJ, Phillips GE, Fischer JW, Burke PW, Seward NW, Stahl RS, Nichols TA, Wunder BA and VerCauteren KC, 2014. Mineral licks: motivational factors for visitation and accompanying disease risk at communal use sites of elk and deer. Environmental Geochemistry and Health, 36, 1049–1061. [DOI] [PubMed] [Google Scholar]
- Le Dur A, Lai TL, Stinnakre MG, Laisne A, Chenais N, Rakotobe S, Passet B, Reine F, Soulier S, Herzog L, Tilly G, Rezaei H, Beringue V, Vilotte JL and Laude H, 2017. Divergent prion strain evolution driven by PrPC expression level in transgenic mice. Nature. Communications, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Sohn HJ, Kim MJ, Kim HJ, Park KJ, Lee WY, Yun EI, Tark DS, Choi YP, Cho IS and Balachandran A, 2013. Experimental Chronic Wasting Disease in Wild Type VM mice. Journal of Veterinary Medical Science, 75, 1107–1110. [DOI] [PubMed] [Google Scholar]
- Li L, Coulthart MB, Balachandran A, Chakrabartty A and Cashman NR, 2007. Species barriers for chronic wasting disease by in vitro conversion of prion protein. Biochemical and Biophysical Research Communications, 364, 796–800. [DOI] [PubMed] [Google Scholar]
- Liu YH, Camacho M, Zou WQ and Kong QZ, 2019. Screening and characterisation of unusual sCJD cases in a CWD endemic state in the USA. Prion, 13, 84–85. [Google Scholar]
- Luers L, Bannach O, Stohr J, Wordehoff MM, Wolff M, Nagel‐Steger L, Riesner D, Willbold D and Birkmann E, 2013. Seeded Fibrillation as Molecular Basis of the Species Barrier in Human Prion Diseases. PLoS ONE, 8, e72623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox RA, Person MK, Blevins JE, Abrams JY, Bryant BL, Appleby BS, Schonberger LB and Belay ED, 2019. Prion disease incidence, United States, 2003‐2016. Prion, 13, 39. [DOI] [PubMed] [Google Scholar]
- Madsen‐Bouterse SA, Schneider DA, Zhuang D, Dassanayake RP, Balachandran A, Mitchell GB and O'Rourke KI, 2016. Primary transmission of chronic wasting disease versus scrapie prions from small ruminants to transgenic mice expressing ovine or cervid prion protein. Journal of General Virology, 97, 2451–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magle SB, Samuel MD, Van Deelen TR, Robinson SJ and Mathews NE, 2013. Evaluating spatial overlap and relatedness of white‐tailed deer in a chronic wasting disease management zone. PLoS ONE, 8, e56568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh RF, Kincaid AE, Bessen RA and Bartz JC, 2005. Interspecies transmission of chronic wasting disease prions to squirrel monkeys (Saimiti sciureus). Journal of Virology, 79, 13794–13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes‐Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC and Hoover EA, 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science, 314, 133–136. [DOI] [PubMed] [Google Scholar]
- Mathiason CK, Nalls AV, Seelig DM, Kraft SL, Carnes K, Anderson KR, Hayes‐Klug J and Hoover EA, 2013. Susceptibility of Domestic Cats to Chronic Wasting Disease. Journal of Virology, 87, 1947–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M, Iulini B, Vaccari G, Acutis PL, Martucci F, Esposito E, Peletto S, Barocci S, Chiappini B, Corona C, Barbieri I, Caramelli M, Agrimi U, Casalone C and Nonno R, 2010. Co‐existence of classical scrapie and Nor98 in a sheep from an Italian outbreak. Research in Veterinary Science, 88, 478–485. [DOI] [PubMed] [Google Scholar]
- Mejía‐Salazar MF, Waldner CL, Ten Hwang Y and Bollinger TK, 2018. Use of environmental sites by mule deer: a proxy for relative risk of chronic wasting disease exposure and transmission. Ecosphere, 9, e02055. [Google Scholar]
- Miller MW and Conner MM, 2005. Epidemiology of chronic wasting disease in free‐ranging mule deer: Spatial, temporal, and demographic influences on observed prevalence patterns. Journal of Wildlife Diseases, 41, 275–290. [DOI] [PubMed] [Google Scholar]
- Miller MW and Fischer JR, 2016. The first five (or more) decades of chronic wasting disease: lessons for the five decades to come. Transactions of the North American Wildlife and Natural Resources Conference, 81, 1–12. [Google Scholar]
- Miller MW and Williams ES, 2003. Horizontal prion transmission in mule deer. Nature, 425, 35–36. [DOI] [PubMed] [Google Scholar]
- Miller MW, Williams ES, Hobbs NT and Wolfe LL, 2004. Environmental sources of prion transmission in mule deer. Emerging Infectious Diseases, 10, 1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Swanson HM, Wolfe LL, Quartarone FG, Huwer SL, Southwick CH and Lukacs PM, 2008. Lions and prions and deer demise. PLoS ONE, 3, e4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G, Shaffer P, Foster S, Ghazi D, Rendulich J, Yogasingam N, Hills B and Balachandran A, 2011. Transmission of Canadian CWD isolates to transgenic mice. Prion, 5, 106–107. [Google Scholar]
- Mitchell GB, Sigurdson CJ, O'Rourke KI, Algire J, Harrington NP, Walther I, Spraker TR and Balachandran A, 2012. Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS ONE, 7, e39055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag J, Schulz‐Schaeffer W, Schrod A, Hunsmann G and Motzkus D, 2013. Asynchronous Onset of Clinical Disease in BSE‐Infected Macaques. Emerging Infectious Diseases, 19, 1125–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Kunkle R, Greenlee MHW, Nicholson E, Richt J, Hamir A, Waters WR and Greenlee J, 2016a. Horizontal Transmission of Chronic Wasting Disease in Reindeer. Emerging Infectious Diseases, 22, 2142–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Kunkle RA, Smith JD, West‐Greenlee MH and Greenlee JJ, 2016b. Prion infectivity detected in swine challenged with chronic wasting disease via the intracerebral or oral route. Prion, 10, S117–S118. [Google Scholar]
- Moore SJ, Greenlee MHW, Kondru N, Manne S, Smith JD, Kunkle RA, Kanthasamy A and Greenlee JJ, 2017. Experimental transmission of the chronic wasting disease agent to swine after oral or intracranial inoculation. Journal of Virology, 91, pii: e00926–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Vrentas CE, Hwang S, Greenlee MHW, Nicholson EM and Greenlee JJ, 2018. Pathologic and biochemical characterisation of PrPSc from elk with PRNP polymorphisms at codon 132 after experimental infection with the chronic wasting disease agent. BMC Veterinary Research, 14, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Smith JD, Richt JA and Greenlee JJ, 2019. Raccoons accumulate PrPSc after intracranial inoculation of the agents of chronic wasting disease or transmissible mink encephalopathy but not atypical scrapie. Journal of Veterinary Diagnostic Investigation, 31, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myskiw J, Lamourieux L, Coulthart M, Sim V and Booth S, 2019. Strain profiles of CJD in venison consumers and non‐consumers from Alberta and Saskatchewan. Prion, 13, 69–69. [Google Scholar]
- Mysterud A and Edmunds DR, 2019. A review of chronic wasting disease in North America with implications for Europe. European Journal of Wildlife Research, 65, 1–26. [Google Scholar]
- Mysterud A and Rolandsen CM, 2018. A reindeer cull to prevent chronic wasting disease in Europe. Nature Ecology Evolution, 2, 1343–1345. [DOI] [PubMed] [Google Scholar]
- Nalls AV, McNulty E, Powers J, Seelig DM, Hoover C, Haley NJ, Hayes‐Klug J, Anderson K, Stewart P, Goldmann W, Hoover EA and Mathiason CK, 2013. Mother to Offspring Transmission of Chronic Wasting Disease in Reeves’ Muntjac Deer. PLoS ONE, 8, e71844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York State Department of Environmental Conservation (NYSDEC) , New York State Department of Agriculture and Markets, Cornell University College of Veterinary Medicine, 2018. New York State Interagency CWD Risk Minimization Plan. February 2018. Available online: https://www.dec.ny.gov/docs/wildlife_pdf/cwdpreventionplan2018.pdf
- Nichols TA, Fischer JW, Spraker TR, Kong QZ and VerCauteren KC, 2015. CWD prions remain infectious after passage through the digestive system of coyotes (Canis latrans). Prion, 9, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonno R, Di Bari MA, Cardone F, Vaccari G, Fazzi P, Dell'Omo G, Cartoni C, Ingrosso L, Boyle A, Galeno R, Sbriccoli M, Lipp HP, Bruce M, Pocchiari M and Agrimi U, 2006. Efficient transmission and characterisation of Creutzfeldt‐Jakob disease strains in bank voles. PLoS Pathogens, 2, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser SM, Clark WR, Otis DL and Huang L, 2008. Sampling considerations for disease surveillance in wildlife populations. Journal of Wildlife Management, 72, 52–60. [Google Scholar]
- Ono F, Tase N, Kurosawa A, Hiyaoka A, Ohyama A, Tezuka Y, Wada N, Sato Y, Tobiume M, Hagiwara K, Yamakawa Y, Terao K and Sata T, 2011. Atypical L‐Type Bovine Spongiform Encephalopathy (L‐BSE) Transmission to Cynomolgus Macaques, a Non‐Human Primate. Japanese Journal of Infectious Diseases, 64, 81–84. [PubMed] [Google Scholar]
- Pattison IH and Millson GC, 1961. Further experimental observations on scrapie. Journal of Comparative Patholoy, 71, 350–359. [DOI] [PubMed] [Google Scholar]
- Peletto S, Perucchini M, Acin C, Dagleish MP, Reid HW, Rasero R, Sacchi P, Stewart P, Caramelli M, Ferroglio E, Bozzetta E, Meloni D, Orusa R, Robetto S, Gennero S, Goldmann W and Acutis PL, 2009. Genetic variability of the prion protein gene (PRNP) in wild ruminants from Italy and Scotland. Journal of Veterinary Science, 10, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccardo P, Cervenak J, Yakovleva O, Gregori L, Pomeroy K, Cook A, Muhammad FS, Seuberlich T, Cervenakova L and Asher DM, 2012. Squirrel monkeys (Saimiri sciureus) Infected with the Agent of Bovine Spongiform Encephalopathy Develop Tau Pathology. Journal of Comparative Pathology, 147, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirisinu L, Tran L, Chiappini B, Vanni I, Di Bari MA, Vaccari G, Vikoren T, Madslien KI, Våge J, Spraker T, Mitchell G, Balachandran A, Baron T, Casalone C, Rolandsen CM, Roed KH, Agrimi U, Nonno R and Benestad SL, 2018. Novel Type of Chronic Wasting Disease Detected in Moose (Alces alces), Norway. Emerging Infectious Diseases, 24, 2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer IH, Wright SD, Johnson CJ, Pedersen JA and Samuel MD, 2017. Temporal patterns of chronic wasting disease prion excretion in three cervid species. Journal of General Virology, 98, 1932–1942. [DOI] [PubMed] [Google Scholar]
- Plummer IH, Johnson CJ, Chesney AR, Pedersen JA and Samuel MD, 2018. Mineral licks as environmental reservoirs of chronic wasting disease prions. PLoS ONE, 13, e0196745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapov A, Merrill E, Pybus M, Coltman D and Lewis MA, 2013. Chronic wasting disease: Possible transmission mechanisms in deer. Ecological Modelling, 250, 244–257. [Google Scholar]
- Pritzkow S, Morales R, Moda F, Khan U, Telling GC, Hoover E and Soto C, 2015. Grass Plants Bind, Retain, Uptake, and Transport Infectious Prions. Cell Reports, 11, 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritzkow S, Morales R, Lyon A, Concha‐Marambio L, Urayama A and Soto C, 2018. Efficient prion disease transmission through common environmental materials. Journal of Biological Chemistry, 293, 3363–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB, 1991. Molecular‐Biology and Transgenetics of Prions Causing CNS Degeneration in Humans and Animals. Sub‐Acute Spongiform Encephalopathies, 55, 59–82. [Google Scholar]
- Race BL, Meade‐White KD, Ward A, Jewellj J, Millert MW, Williams ES, Chesebro B and Race RE, 2007. Levels of abnormal prion protein in deer and elk with chronic wasting disease. Emerging Infectious Diseases, 13, 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race B, Meade‐White KD, Miller MW, Barbian KD, Rubenstein R, LaFauci G, Cervenakova L, Favara C, Gardner D, Long D, Parnell M, Striebel J, Priola SA, Ward A, Williams ES, Race R and Chesebro B, 2009. Susceptibilities of Nonhuman Primates to Chronic Wasting Disease. Emerging Infectious Diseases, 15, 1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race B, Meade‐White KD, Phillips K, Striebel J, Race R and Chesebro B, 2014. Chronic Wasting Disease Agents in Nonhuman Primates. Emerging Infectious Diseases, 20, 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race B, Williams K, Orru CD, Hughson AG, Lubke L and Chesebro B, 2018. Lack of Transmission of Chronic Wasting Disease to Cynomolgus Macaques. Journal of Virology, 92, e00550–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race B, Williams K and Chesebro B, 2019. Transmission studies of chronic wasting disease to transgenic mice overexpressing human prion protein using the RT‐QuIC assay. Veterinary Research, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond GJ, Bossers A, Raymond LD, O'Rourke KI, McHolland LE, Bryant PK, Miller MW, Williams ES, Smits M and Caughey B, 2000. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. Embo Journal, 19, 4425–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond GJ, Raymond LD, Meade‐White KD, Hughson AG, Favara C, Gardner D, Williams ES, Miller MW, Race RE and Caughey B, 2007. Transmission and adaptation of chronic wasting disease to hamsters and transgenic mice: evidence for strains. Journal of Virology, 81, 4305–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SJ, Samuel MD, O'Rourke KI and Johnson CJ, 2012. The role of genetics in chronic wasting disease of North American cervids. Prion, 6, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AL, Williamson H, Güere ME, Tharaldsen H, Baker K, Smith SL, Pérez‐Espona S, Krojerová‐Prokešová J, Pemberton JM, Goldmann W and Houston F, 2019. Variation in the prion protein gene (PRNP) sequence of wild deer in Great Britain and mainland Europe. Veterinary Research, 50, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorres C, Romano M, Miller JA, Mossey JM, Grubesic TH, Zellner DE and Smith G, 2018. Contact tracing for the control of infectious disease epidemics: Chronic Wasting Disease in deer farms. Epidemics, 23, 71–75. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S and Lash TL, 2008. Modern epidemiology. Wolters Kluwer Health/Lippincott Williams and Wilkins, Philadelphia. [Google Scholar]
- Safar J, 1996. The folding intermediate concept of prion protein formation and conformational links to infectivity. Prions Prions Prions, 207, 69–76. [DOI] [PubMed] [Google Scholar]
- Safer J and Prusiner SB, 1998. Molecular studies of prion diseases. Neuronal Degeneration and Regeneration: From Basic Mechanisms to Prospects for Therapy, 117, 421–434. [DOI] [PubMed] [Google Scholar]
- Samuel MD and Storm DJ, 2016. Chronic wasting disease in white‐tailed deer: infection, mortality, and implications for heterogeneous transmission. Ecology, 97, 3195–3205. [DOI] [PubMed] [Google Scholar]
- Sandberg MK, Al‐Doujaily H, Sigurdson CJ, Glatzel M, O'Malley C, Powell C, Asante EA, Linehan JM, Brandner S, Wadsworth JDF and Collinge J, 2010. Chronic wasting disease prions are not transmissible to transgenic mice overexpressing human prion protein. Journal of General Virology, 91, 2651–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders SE, Bartz JC and Bartelt‐Hunt SL, 2012. Soil‐mediated prion transmission: is local soil‐type a key determinant of prion disease incidence? Chemosphere, 87, 661–667. [DOI] [PubMed] [Google Scholar]
- Schatzl HM, Dacosta M, Taylor L, Cohen FE and Prusiner SB, 1995. Prion Protein Gene Variation among Primates. Journal of Molecular Biology, 245, 362–374. [DOI] [PubMed] [Google Scholar]
- Schatzl HM, Hannaoui S, Cheng Y‐C, Gilch S, Beekes M, Schulz‐Schaeffer W, Stahl‐Hennig C and Czub S, 2018. Oral transmission of CWD into cynomolgus macaques: signs of atypical disease, prion conversion and infectivity in macaques and bio‐assayed transgenic mice. Prion, 2018, 35–36. [Google Scholar]
- Schmaedicke AC, 2012. Estimating the risk of CWD transmission to humans‐An interim report of a comprehensive study in non‐human primates. Prion, 6, 67–68. [Google Scholar]
- Schünemann H, Brożek J, Guyatt G and Oxman A (eds.)., 2013. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Available online: https://gdt.gradepro.org/app/handbook/handbook.html
- Schwabenlander MD, Culhane MR, Hall SM, Goyal SM, Anderson PL, Carstensen M, Wells SJ, Slade WB and Armien AG, 2013. A case of chronic wasting disease in a captive red deer (Cervus elaphus). Journal of Veterinary Diagnostic Investigation, 25, 573–576. [DOI] [PubMed] [Google Scholar]
- Scott M, Foster D, Mirenda C, Serban D, Coufal F, Walchli M, Torchia M, Groth D, Carlson G, Dearmond SJ, Westaway D and Prusiner SB, 1989. Transgenic Mice Expressing Hamster Prion Protein Produce Species‐Specific Scrapie Infectivity and Amyloid Plaques. Cell, 59, 847–857. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O'Rourke KI and Hoover EA, 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). Journal of General Virology, 80, 2757–2764. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Spraker TR, Miller MW, Oesch B and Hoover EA, 2001. PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. Journal of General Virology, 82, 2327–2334. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Barillas‐Mury C, Miller MW, Oesch B, van Keulen LJM, Langeveld JPM and Hoover EA, 2002. PrPCWD lymphoid cell targets in early and advanced chronic wasting disease of mule deer. Journal of General Virology, 83, 2617–2628. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Mathiason CK, Perrott MR, Eliason GA, Spraker TR, Glatzel M, Manco G, Bartz JC, Miller MW and Hoover EA, 2008. Experimental chronic wasting disease (CWD) in the ferret. Journal of Comparative Pathology, 138, 189–196. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Joshi‐Barr S, Bett C, Winson O, Manco G, Schwarz P, Rulicke T, Nilsson KPR, Margalith I, Raeber A, Peretz D, Hornemann S, Wuthrich K and Aguzzi A, 2011. Spongiform encephalopathy in transgenic mice expressing a point mutation in the beta 2‐alpha 2 loop of the prion protein. Journal of Neuroscience, 31, 13840–13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MM, Spiropoulos J, Webb PR, Spencer YI, Czub S, Mueller R, Davis A, Arnold ME, Marsh S, Hawkins SAC, Cooper JA, Konold T and Wells GAH, 2011. Experimental Classical Bovine Spongiform Encephalopathy: Definition and Progression of Neural PrP Immunolabeling in Relation to Diagnosis and Disease Controls. Veterinary Pathology, 48, 948–963. [DOI] [PubMed] [Google Scholar]
- Sohn HJ, Kim JH, Choi KS, Nah JJ, Joo YS, Jean YH, Ahn SW, Kim OK, Kim DY and Balachandran A, 2002. A case of chronic wasting disease in an elk imported to Korea from Canada. Journal of Veterinary Medical Science, 64, 855–858. [DOI] [PubMed] [Google Scholar]
- Somerville RA, Fernie K, Smith A, Bishop K, Maddison B, Gough KC and Hunter N, 2019. BSE infectivity survives burial for five years with only limited spread. Archives of Virology, 164, 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen A, van Beest FM and Brook RK, 2014. Impacts of wildlife baiting and supplemental feeding on infectious disease transmission risk: a synthesis of knowledge. Preventive Veterinary Medicine, 113, 356–363. [DOI] [PubMed] [Google Scholar]
- Stack MJ, Moore SJ, Davis A, Webb PR, Bradshaw JM, Lee YH, Chaplin M, Focosi‐Snyman R, Thurston L, Spencer YI, Hawkins SAC, Arnold ME, Simmons MM and Wells GAH, 2011. Bovine Spongiform Encephalopathy: Investigation of Phenotypic Variation among Passive Surveillance Cases. Journal of Comparative Pathology, 144, 277–288. [DOI] [PubMed] [Google Scholar]
- Storm DJ, Samuel MD, Rolley RE, Shelton P, Keuler NS, Richards BJ and Van Deelen TR, 2013. Deer density and disease prevalence influence transmission of chronic wasting disease in white‐tailed deer. Ecosphere, 4, 1–14. [Google Scholar]
- Strom A, Yutzy B, Kruip C, Ooms M, Schloot NC, Roden M, Scott FW, Loewer J and Holznagel E, 2014. Foodborne transmission of bovine spongiform encephalopathy to non‐human primates results in preclinical rapid‐onset obesity. PLoS ONE, 9, e104343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamguney G, Giles K, Bouzamondo‐Bernstein E, Bosque PJ, Miller MW, Safar J, DeArmond SJ and Prusiner SB, 2006. Transmission of elk and deer prions to transgenic mice. Journal of Virology, 80, 9104–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamguney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ and Prusiner SB, 2009a. Asymptomatic deer excrete infectious prions in faeces. Nature, 461, 529–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamguney G, Miller MW, Giles K, Lemus A, Glidden DV, DeArmond SJ and Prusiner SB, 2009b. Transmission of scrapie and sheep‐passaged bovine spongiform encephalopathy prions to transgenic mice expressing elk prion protein. Journal of General Virology, 90, 1035–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling GC, 2011. Transgenic Mouse Models and Prion Strains. Prion Proteins, 305, 79–99. [DOI] [PubMed] [Google Scholar]
- Thackray AM, Hopkins L, Lockey R, Spiropoulos J and Bujdoso R, 2011. Emergence of multiple prion strains from single isolates of ovine scrapie. Journal of General Virology, 92, 1482–1491. [DOI] [PubMed] [Google Scholar]
- Thackray AM, Lockey R, Beck KE, Spiropoulos J and Bujdoso R, 2012. Evidence for Co‐infection of Ovine Prion Strains in Classical Scrapie Isolates. Journal of Comparative Pathology, 147, 316–329. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Samuel MD and Van Deelen TR, 2008. Alternative feeding strategies and potential disease transmission in Wisconsin white‐tailed deer. Journal of Wildlife Management, 72, 416–421. [Google Scholar]
- Triscott E, Duque‐Velasquez C, Aiken JM and McKenzie D, 2015. Chronic wasting disease (CWD) transmission into hamsters. Prion, 9, S86. [Google Scholar]
- Uro‐Coste E, Cassard H, Simon S, Lugan S, Bilheude JM, Perret‐Liaudet A, Ironside JW, Haik S, Basset‐Leobon C, Lacroux C, Peoch’ K, Streichenberger N, Langeveld J, Head MW, Grassi J, Hauw JJ, Schelcher F, Delisle MB and Andreoletti O, 2008. Beyond PrPres type 1/type 2 dichotomy in Creutzfeldt–Jakob disease. PLoS Pathogens, 4, e1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren KC, Lavelle MJ, Seward NW, Fischer JW and Phillips GE, 2007. Fence‐line contact between wild and farmed cervids in Colorado: potential for disease transmission. Journal of Wildlife Management, 71, 1594–1602. [Google Scholar]
- Vikøren T, Våge J, Madslien KI, Røed KH, Rolandsen CM, Tran L, Hopp P, Veiberg V, Heum M, Moldal T, Neves CGD, Handeland K, Ytrehus B, Kolbjørnsen Ø, Wisløff H, Terland R, Saure B, Dessen KM, Svendsen SG, Nordvik BS and Benestad SL, 2019. First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. Journal of Wildlife Disease, 55, 970–972. [PubMed] [Google Scholar]
- Viljugrein H, Hopp P, Benestad SL, Nilsen EB, Våge J, Tavornpanich S, Rolandsen CM, Strand O and Mysterud A, 2019. A method that accounts for differential detectability in mixed samples of long‐term infections with applications to the case of chronic wasting disease in cervids. Methods in Ecology and Evolution, 10, 134–145. [Google Scholar]
- VKM , 2017. CWD in Norway – a state of emergency for the future of cervids (Phase II). Opinion of the Panel on Biological Hazards. ISBN: 978‐82‐8259‐266‐6, Oslo, Norway. [Google Scholar]
- VKM , Ytrehus B, Grahek‐Ogden D, Strand O, Tranulis M, Mysterud A, Aspholm M, Jore S, Kapperud G, Møretrø T, Nesbakken T, Robertson L, Melby K and Skjerdal T, 2018. Factors that can contribute to spread of CWD – an update on the situation in Nordfjella, Norway. Opinion of the Panel on Biological Environment (VKM), Oslo, Norway. Available online: https://vkm.no/download/18.696229a71677d983532c0c11/1547126741061/CWD%20factors%20for%20spread%202018.pdf
- Waddell L, Greig J, Mascarenhas M, Otten A, Corrin T and Hierlihy K, 2018. Current evidence on the transmissibility of chronic wasting disease prions to humans – a systematic review. Transboundary and Emerging Diseases, 65, 37–49. [DOI] [PubMed] [Google Scholar]
- WAFWA (Western Association of Fish and Wildlife Agencies), 2017. Recommendations for adaptive management of chronic wasting disease in the west. WAFWA Wildlife Health Committee and Mule Deer Working Group. Edmonton, Alberta, Canada and Fort Collins, Colorado, USA.
- Walter WD, Walsh DP, Farnsworth ML, Winkelman DL and Miller MW, 2011. Soil clay content underlies prion infection odds. Nature. Communications, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther I, Staskevicius A, Soutyrine A and Mitchell G, 2019. Diagnosis of CWD in a herd of farmed red deer. Prion, 13, 59–60. [Google Scholar]
- Watts JC, Giles K, Patel S, Oehler A, DeArmond SJ and Prusiner SB, 2014. Evidence that bank vole PrP is a universal acceptor for prions. PLoS Pathogens, 10, e1003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GAH, Spiropoulos J, Hawkins SAC and Ryder SJ, 2005. Pathogenesis of experimental bovine spongiform encephalopathy: preclinical infectivity in tonsil and observations on the distribution of lingual tonsil in slaughtered cattle. Veterinary Record, 156, 401–407. [DOI] [PubMed] [Google Scholar]
- Westaway D, Goodman P, Mirenda C, Carlson G and Prusiner SB, 1987a. Nucleotide‐Sequence Analysis of Prn‐P Genes from Short and Long Scrapie Prion Incubation Period Mice. Annals of Neurology, 22, 157–157. [Google Scholar]
- Westaway D, Goodman PA, Mirenda CA, Mckinley MP, Carlson GA and Prusiner SB, 1987b. Distinct prion proteins in short and long scrapie incubation period mice. Cell, 51, 651–662. [DOI] [PubMed] [Google Scholar]
- Wik L, Mikko S, Klingeborn M, Steen M, Simonsson M and Linne T, 2012. Polymorphisms and variants in the prion protein sequence of European moose (Alces alces), reindeer (Rangifer tarandus), roe deer (Capreolus capreolus) and fallow deer (Dama dama) in Scandinavia. Prion, 6, 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ES and Miller MW, 2003. Transmissible spongiform encephalopathies in non‐domestic animals: origin, transmission and risk factors. Revue scientifique et technique de l'Office international des épizooties, 22, 145–156. [DOI] [PubMed] [Google Scholar]
- Williams ES and Young S, 1992. Spongiform encephalopathies in Cervidae. Revue Scientifique et Technique, 11, 551–567. [DOI] [PubMed] [Google Scholar]
- Williams L, Brown P, Ironside J, Gibson S, Will R, Ritchie D, Kreil TR and Abeel C, 2007. Clinical, neuropathological and immunohistochemical features of sporadic and variant forms of Creutzfeldt‐Jakob disease in the squirrel monkey (Saimiri sciureus). Journal of General Virology, 88, 688–695. [DOI] [PubMed] [Google Scholar]
- Wilson R, Plinston C, Hunter N, Casalone C, Corona C, Tagliavini F, Suardi S, Ruggerone M, Moda F, Graziano S, Sbriccoli M, Cardone F, Pocchiari M, Ingrosso L, Baron T, Richt J, Andreoletti O, Simmons M, Lockey R, Manson JC and Barron RM, 2012. Chronic wasting disease and atypical forms of bovine spongiform encephalopathy and scrapie are not transmissible to mice expressing wild‐type levels of human prion protein. Journal of General Virology, 93, 1624–1629. [DOI] [PubMed] [Google Scholar]


