Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of astaxanthin‐dimethyldisuccinate (ATX‐DMDS) for salmonids, crustaceans and other fish. The applicant has provided evidence that ATX‐DMDS currently on the market complies with the conditions of authorisation for salmon and trout. ATX and ATX‐DMDS are safe for salmonids, crustaceans and fish up to 100 mg ATX/kg complete diet, corresponding to 138 mg ATX‐DMDS/kg. The FEEDAP Panel re‐assessed the toxicological profile of ATX based on data already considered in 2014, the literature review performed by the applicant and the data available in the context of an EFSA public call for data on ATX. The acceptable daily intake (ADI) of 0.2 mg astaxanthin/kg body weight (bw) per day obtained by applying an uncertainty factor of 200 to a lowest observed adverse effect level (LOAEL) of 40 mg/kg bw per day for the increased incidence of multinucleated hepatocytes observed in a 2‐year carcinogenicity study replaces the one of 0.034 mg/kg bw established by the FEEDAP Panel in 2014. The use of ATX‐DMDS in the nutrition of salmonids, other fish and crustaceans up to the maximum permitted dietary level is of no concern for the safety of the consumer. No dermal or ocular risk for the users is likely to occur under practical conditions. In the absence of inhalation toxicology study, the Panel is not in the position to establish the inhalation toxicity of the additive. The use of synthetic ATX‐DMDS does not pose a significant additional risk to the environment compared with natural astaxanthin. ATX‐DMDS is efficacious in colouring the flesh of salmonids and other fish. ATX‐DMDS is an effective pigment for crustaceans at the proposed conditions of use.
Keywords: sensory additive, colourant, astaxanthin dimethyldisuccinate, salmonids, other fish, crustaceans
Summary
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of astaxanthin‐dimethyldisuccinate (ATX‐DMDS, Carophyll® Stay‐Pink 10%‐CWS) for salmonids, crustaceans and other fish.
The applicant has provided evidence that ATX‐DMDS currently on the market complies with the conditions of authorisation for salmon and trout.
Orally administrated ATX‐DMDS is hydrolysed and converted to free ATX in the intestine of fish, then absorbed, metabolised and distributed in the same manner as free ATX.
ATX and ATX‐DMDS are safe for salmonids up to 100 mg ATX/kg complete diet corresponding to 138 mg ATX‐DMDS/kg. This conclusion is extrapolated to other fish. ATX at a maximum concentration of 100 mg/kg complete feed is safe for crustaceans with a margin of safety of at least 8. This conclusion covers also ATX from ATX‐DMDS and therefore the safety of 138 mg ATX‐DMDS/kg complete feed. The FEEDAP Panel has no reservation to the deletion of the provision that limits the age of use of ATX/ATX‐DMDS in salmon and trout.
The FEEDAP Panel re‐assessed the toxicological profile of ATX based on data already considered in 2014, the literature review performed by the applicant and the data available in the context of an EFSA public call for data on ATX. ATX is neither mutagenic nor carcinogenic. The FEEDAP Panel established an acceptable daily intake (ADI) of 0.2 mg astaxanthin/kg body weight (bw) per day by applying an uncertainty factor of 200 to a lowest observed adverse effect level (LOAEL) of 40 mg/kg bw per day for the increased incidence of multinucleated hepatocytes observed in a 2‐year carcinogenicity study. This ADI replaces the one of 0.034 mg/kg bw established by the FEEDAP Panel in 2014. The use of ATX‐DMDS in the nutrition of salmonids, other fish and crustaceans up to the maximum permitted dietary level is of no concern for the safety of the consumer.
The FEEDAP Panel concludes that there is no new evidence that would lead the Panel to reconsider its previous conclusions on the safety for the user. No dermal or ocular risk for the users is likely to occur under practical conditions. In the absence of inhalation toxicology study, the Panel is not in the position to establish the inhalation toxicity of the additive. Due to the susceptibility of the active substance to oxidation, the additive will be placed in the market only in the form of preparations. The FEEDAP Panel recognises that once authorised, multiple formulations of the additive can be placed in the market, and consequently, not all preparations can be directly tested for user safety.
The use of synthetic ATX‐DMDS does not pose a significant additional risk to the environment compared with natural astaxanthin.
ATX‐DMDS is efficacious in colouring the flesh of salmonids and other fish. ATX‐DMDS is an effective pigment for crustaceans at the proposed conditions of use.
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7; Article 13(3) of that Regulation lays down that if the holder of an authorisation proposes changing the terms of the authorisation by submitting an application to the Commission, accompanied by the relevant data supporting the request for the change, the Authority shall transmit its opinion on the proposal to the Commission and the Member States; Article 14(1) of that Regulation lays down that an application for renewal shall be sent to the Commission at the latest one year before the expiry date of the authorisation.
The European Commission received a request from DSM Nutritional Products2 for an authorisation of a new use, modification of the authorisation and renewal of the authorisation of the product astaxanthin‐dimethyldisuccinate (Carophyll® Stay‐Pink 10%‐CWS), when used as a feed additive for salmonids, crustaceans and other fish (category: sensory additives; functional group: a) colourants: ii substances which, when fed to animals, add colours to food of animal origin).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive), under Article 13(3) (modification of the authorisation of a feed additive) and under Article 14(1) (renewal of the authorisation). The particulars and documents in support of the application were considered valid by EFSA as of 19 October 2017.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product astaxanthin‐dimethyldisuccinate (Carophyll® Stay‐Pink 10%‐CWS), when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
Astaxanthin‐dimethyldisuccinate (ATX‐DMDS), the additive under assessment, is authorised for use in salmon and trout.3
Synthetic astaxanthin (E 161j) is authorised for fish, crustaceans and ornamental fish.4 Astaxanthin from natural origin is also authorised in the European Union (EU) for salmon and trout: astaxanthin‐rich Phaffia rhodozyma (ATCC SD‐5340)5 and red carotenoid‐rich Paracoccus carotinifaciens.6
ATX‐DMDS, the additive under assessment, was first evaluated by the FEEDAP Panel in 2007 (EFSA, 2007a).
Since 2004, the FEEDAP Panel issued several scientific opinions on the safety and efficacy of astaxanthin which include: the environmental impact of astaxanthin‐rich Phaffia rhodozyma (ATCC 74219) (EFSA, 2004), the safety of use of astaxanthin in animal nutrition (EFSA, 2005); the safety and efficacy of astaxanthin‐rich Phaffia rhodozyma (ATCC SD‐5340) for salmon and trout (EFSA, 2006) and the safety and efficacy of red carotenoid‐rich bacterium Paracoccus carotinifaciens as a feed additive for salmon and trout (EFSA, 2007b; EFSA FEEDAP Panel, 2010). The most recent ones deal with the re‐evaluation of synthetic astaxanthin for salmonids, crustaceans, ornamental fish, other fish and ornamental birds under Regulation (EC) No 1831/2003 (EFSA FEEDAP Panel, 2014a,b).
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier7 in support of the authorisation request for the use of ATX‐DMDS (Carophyll® Stay‐Pink 10%‐CWS) as a feed additive.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ elicitation knowledge, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the ATX‐DMDS in animal feed. The Executive Summary of the EURL report can be found in Annex A.8
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of astaxanthin‐dimethyldisuccinate (Carophyll® Stay‐Pink 10%‐CWS) is in line with the principles laid down in Regulation (EC) No 429/2008 and the relevant guidance documents: Guidance for the preparation of dossiers for sensory additives (EFSA FEEDAP Panel, 2012),9 Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008), Guidance on the renewal of the authorisation of feed additives (EFSA FEEDAP Panel, 2013), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012) and Technical Guidance: Extrapolation of data from major species to minor species regarding the assessment of additives for use in animal nutrition (EFSA, 2008).
The FEEDAP Panel performed the benchmark dose (BMD) analysis following the approach described in the Guidance on the use of the benchmark dose approach in risk assessment (EFSA Scientific Committee, 2017).
3. Assessment
Astaxanthin‐dimethyldisuccinate (Carophyll® Stay‐Pink 10%‐CWS), the additive under assessment, is authorised for salmon and trout as a sensory additive (functional group: colourant) at a maximum content of 138 mg ATX‐DMDS/kg complete feed.3
The applicant asks for (i) the renewal of the current authorisation, (ii) the modification of the current authorisation by deleting the provision ‘Use permitted from the age of 6 months onwards or from 50 g weight onwards’ and (iii) the authorisation of a new use of the additive for crustaceans and other fish.
3.1. Characterisation
3.1.1. Characterisation of the active substance
Astaxanthin‐dimethyldisuccinate (C50H64O10, (3R,3′R)‐(±)‐3,3′‐bis(4‐methoxy‐1,4‐dioxobutoxy)‐β,β‐carotene‐4,4′‐dione), CAS number 578006‐46‐9, is derived by esterification of synthetic ATX. The applicant states that no change in the manufacturing process have been introduced since the FEEDAP Panel made its assessment in 2007 (EFSA, 2007a,b) (Figure 1).
Figure 1.
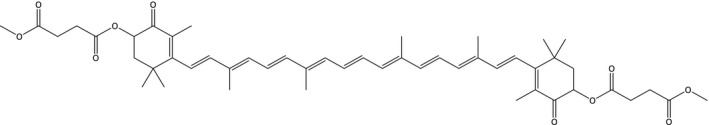
Structural formula of astaxanthin‐dimethyldisuccinate
ATX‐DMDS contains by specification not less than 96% ATX‐DMDS (all‐E, 9‐Z and 13‐Z isomers) and not more than 4% other carotenoids. Batch to batch consistency was confirmed by analysis of five batches (2015), all complying with the specifications: ■■■■■■■■■■■■■■■
3.1.2. Characterisation of the additive
ATX‐DMDS is sensitive to oxidation, light and temperature; therefore, it is necessary to produce a stabilised preparation for its use in premixtures and in feedingstuffs. CAROPHYLL® Stay‐Pink 10%‐CWS is one example of such a preparation. It contains 138 g ATX‐DMDS/kg (100 g ATX equivalent/kg), 512 g lignosulfonate, 180 g maize starch, 70 g dextrin yellow, 50 g beeswax and 50 g dl‐alpha‐tocopherol/kg. Certificate of analysis from three production batches (2018) showed product consistency with values of ATX equivalent content between 113 and 114 g/kg.11
The residual solvents concentrations measured in three batches of the formulated additive were ■■■■■■■■■■ These results are in compliance with the International Cooperation on Harmonisation of Technical requirements for Registration of Veterinary Medicinal Products (VICH) thresholds (VICH guidance GL18).13
Three batches of the formulated product CAROPHYLL® Stay‐Pink 10%‐CWS was analysed for arsenic, cadmium, lead and mercury, with all values below 0.1 mg/kg.14
Triphenylphosphine oxide (TPPO, a carry‐over impurity of ATX synthesis) could not be found in five batches of the crystalline ATX‐DMDS.15 , 10 This is in compliance with former opinions of the FEEDAP Panel (EFSA, 2007a; EFSA FEEDAP Panel, 2014a,b) and the current authorisation for ATX‐DMDS with the maximum level of 100 mg TPPO/kg additive.
3.1.3. Physical state of the product
Three batches of the newly formulated CAROPHYLL® Stay‐Pink 10%‐CWS were analysed for particle size distribution by laser diffraction and for dusting potential by Heubach test.16 The particle size distribution showed that all particles had a diameter < 600 μm and no particles < 100 μm were found. The mean dusting potential was 0.95 g/m3 ■■■■■. The measured ATX‐DMDS in the dust was 9.7 g/kg on average ■■■■■, the active substance in the air is calculated to be 9.2 mg ATX‐DMDS/m3.
3.1.4. Stability and homogeneity
The composition of the formulated product is different from the one assessed in 2007 (EFSA, 2007a) and the applicant provided new stability and homogeneity data.
3.1.4.1. Shelf‐life of the additive
The stability of three commercial batches of CAROPHYLL® Stay‐Pink 10%‐CWS kept in aluminium bottles was tested in two different conditions: at 15°C and at 40°C/75% relative humidity (RH). AXT‐DMDS concentration was measured at regular time intervals. Recoveries after 36 months at 15°C and after 6 months at 40°C/75% RH were in the range of 97.2–98.1% and 95.2–96.1, respectively.17
3.1.4.2. Stability in premixtures and feeding stuffs
Three batches of CAROPHYLL® Stay‐Pink 10%‐CWS were incorporated into a vitamin premixture at a level of 10 g ATX‐equivalent/kg premix. Samples were stored for 6 months at 25°C. The mean recoveries of ATX‐DMDS were in the range of 93.9–96.7% and 92.9–95.3% after 3 and 6 months, respectively.18
The stability of CAROPHYLL® Stay‐Pink 10%‐CWS (three batches) was studied in an extruded feed for trout, during processing and during subsequent storage for three months at 25°C. The target content of ATX‐DMDS was 40 mg ATX‐equivalent/kg feed. Recovery after processing was 95% and a monthly loss of 5.4% was calculated for a 3 months storage period.18
3.1.4.3. Homogeneity
The homogeneous distribution of ATX‐DMDS in three batches of trout feed (mash19 and extruded) each with target concentration of 40 mg ATX‐equivalent/kg feed was examined in three samples of each feed. The coefficient of variation obtained varied between 3.2–6.8% (mash feed) and between 0.7–1.5% (extruded feed).18
3.1.5. Conditions of use
The additive is currently authorised as a sensory additive, (functional group: colourant) for salmon and trout up to a maximum content of 138 mg ATX‐DMDS/kg complete feed (corresponding to 100 mg ATX equivalent/kg feed). The applicant proposes to keep the same conditions.
In addition, the applicant is proposing the extension of use to crustaceans and other fish with the same maximum content.
The modification of the current authorisation by deleting the provision ‘Use permitted from the age of 6 months onwards or from 50 g weight onwards’ is also requested.
3.2. Safety
3.2.1. Absorption, distribution, metabolism, excretion and residues
No new information has been provided on absorption, distribution, metabolism, excretion (ADME) and reference to previously adopted opinions have been made (EFSA, 2005, 2007a; EFSA FEEDAP Panel, 2014a).
The applicant provided information on residues of ATX in the new target species (crustaceans).
3.2.1.1. Absorption, distribution, metabolism and excretion
The FEEDAP Panel assessed the ADME of ATX‐DMDS in salmonids in its opinion in 2007 (EFSA, 2007a) and concluded that ‘Orally administered ATX‐DMDS from C®SP is hydrolysed and converted to free ATX in the intestine of fish, then absorbed, metabolised and distributed in the same manner as free ATX’.
The ADME of free ATX has been extensively evaluated by the FEEDAP Panel in 2005 and 2014 (EFSA, 2005; EFSA FEEDAP Panel, 2014a) and can be summarised as follows: (i) ATX apparent absorption varies mostly between 50% and 70% and is determined by several factors such as target species, dietary lipid levels and ATX stereochemistry, the all‐E isomer being absorbed more efficiently than the Z isomers (ii) ATX is metabolised in fish through oxidative and reductive pathways. However, no oxidation occurs in «sea bream type» of fish like salmonids. A double step reduction at the 4 and 4′‐oxo groups initiates a metabolic process leading to idoxanthin then to adonixanthin and zeaxanthin. ATX has been shown to be a vitamin A precursor for fish, which implies the cleavage of the polyene chain at C15,C15 (iii) after repeated administration of ATX, the pigment deposited in the flesh of trout and Chinook salmon (Oncorhynchus tshawytscha) is predominantly ATX (about 95%); in Arctic char (Salvelinus alpinus), idoxanthin is also deposited in the flesh (20–35%), the corresponding figures for the skin being 85% ATX and 10% idoxanthin, both esterified.
No information on ADME of ATX in crustaceans has been provided. In the dossier, the applicant assumes that orally administrated ATX‐DMDS is hydrolysed and converted to free astaxanthin and succinate in the intestine of crustaceans before or during absorption, but no evidence on this has been provided. The literature searches performed by the applicant (see below Sections 3.2.2.1 and 3.3.2.1) did not reveal any information specific to the metabolism of ATX in crustaceans, but only data on ATX deposition.
3.2.1.2. Residues
No new data for ATX residues in salmonids and other fish were supplied by the applicant, which made reference to the former FEEDAP Panel conclusions as follows (EFSA, 2005; EFSA FEEDAP Panel, 2014a): ‘(i) a dose‐related increase in ATX in the flesh of trout and salmon was observed with graded ATX levels in the diet. Since absorption capacity is limited, a plateau is reached in Atlantic salmon at about 10 mg ATX/kg flesh and in trout at a higher level of about 20–25 mg ATX/kg flesh, (ii) the composition of carotenoids deposited in the flesh reflects that of the dietary prey organisms or added carotenoids in terms of ATX stereoisomers; all‐E isomers are deposited mainly in flesh, whereas Z isomers are preferentially stored in the liver and kidney’.
Two published studies provided for the present assessment (see Section 3.2.2.2) some data on ATX deposition in shrimp muscle.20 In the first one (Paibulkichakul et al., 2008), groups of shrimps were fed for 120 days a feed supplemented with 46 or 281 mg ATX/kg, respectively (analytical values). At the end of the study (number of animals and time after the last feeding not indicated), the shrimps were killed and ATX measured in the muscle. ATX contents amounted to about (evaluated from a graph) 2 mg and 4.5 mg/kg muscle, respectively. In the second study (Niu et al., 2014), shrimps were fed for 74 days a complete feed supplemented with 100 mg ATX/kg. Six shrimps were randomly collected 2 hours after the last feeding and dissected. ATX content in muscle amounted to 3.1 ± 0.04 mg/kg.
3.2.2. Safety for the target species
3.2.2.1. Safety for salmonids and other fish
In 2007, the FEEDAP Panel concluded that ATX‐DMDS from C®SP is safe for salmon and trout (EFSA, 2007a) at the highest authorised ATX level in feed.
The applicant performed a structured literature search for the period 2013 up to April 2017 aiming to demonstrate that, in the light of the current knowledge, the additive and its active principle ATX remain safe for the target species (salmon and trout).21 For ATX‐DMDS, no one of the documents retrieved by the structured literature database searches (12 hits found) was considered relevant. For ATX two published papers out of 64 hits were considered relevant (Rama and Manjabhat, 2014; Brizio et al., 2013).
Concerning the publication by Brizio et al. (2013), the FEEDAP Panel noted that the dietary level of ATX (75 mg/kg feed) in rainbow trout was below the currently authorised maximum level. Consequently, this study was not considered relevant for the assessment of target animal safety of ATX.
Rama and Manjabhat (2014) fed fingerling carps (Cyprinus carpio) diets with 100 and 200 mg ATX+ATX‐ester/kg extracted from shrimp exoskeleton for 14 weeks. The aim of the study was to evaluate a potential protective effect of these carotenoids against ammonia induced stress. After a single sublethal dose of ammonia, total antioxidant status (TAS), activities of superoxide dismutase (SOD) and catalase (CAT) as well as aspartate transaminase (AST) and alanine transaminase (ALT) were measured in plasma, liver, kidney and gills. TAS in tissues was reduced after exposure to ammonia. However, TAS was still higher in tissues from fish fed ATX+ATX‐ester compared with control tissues. ALT and AST increased after ammonia exposure in all samples of all groups; however, feeding ATX+ATX‐ester resulted in lower activities and prevented tissue damage by lipid peroxidation in the tissues. However, no performance parameters and viability/gross pathology of carps were reported in this study.
Overall, there were no recent studies (published between 2013 and April 2017) which would indicate concerns on the safety of ATX‐DMDS or ATX for salmonids and other fish at the highest dose currently authorised. Therefore, the FEEDAP Panel confirms its previous conclusion that ATX and ATX‐DMDS are safe for salmonids up to 100 mg ATX and 138 mg ATX‐DMDS/kg complete diet. This conclusion is extrapolated to other fish at the same dose.
The applicant requested to delete the provision ‘Use permitted from the age of 6 months onwards or from 50 g weight onwards’. In 2005, the Panel concluded that ‘Considering safety of astaxanthin for the target animal and fish physiology there is no serious reason to restrict the use of astaxanthin to a particular developmental stage’ (EFSA, 2005). Therefore, the FEEDAP Panel has no reservation to the deletion of the provision that limits the age of use of ATX/ATX‐DMDS.
3.2.2.2. Safety for crustaceans
To support safety of the additive for crustaceans, the applicant performed a structured literature search covering the period 2007–201822 to provide information on the safety of ATX‐DMDS, including ATX ester(s), synthetic ATX, and esterified ATX from Haematococcus pluvialis.
The outcome of the literature search, that retrieved 25 papers were relevant for the safety assessment, is reported in Appendix A and Table A.1. The FEEDAP Panel assessed the relevant hits and considered those in which crustaceans were fed with overdoses of AXT (between 200 and 1,600 mg/kg feed). In the absence of specific papers with ATX‐DMDS, the FEEDAP Panel assumes that the results of studies performed with ATX can be used for the evaluation of the safety of ATX‐DMDS in crustaceans.
Table A.1.
Summary of the design of 25 studies with ATX in crustaceans (if not specified, ATX is synthetic ATX)
| Author(s) | Crustacean | Test item (level in mg ATX/kg) | Duration (days) endpoints | Conclusions of the authors |
|---|---|---|---|---|
| Chien and Shiau (2005) | Kuruma prawn (Marsupenaeus japonicus) | ATX from H. pluvialis, esterified (0, 50, 100) |
63 days Growth, survival |
The survival rate of prawns treated with astaxanthin (50 and 100 mg synthetic or algal) was significantly higher (51% vs. 37%) No differences in final body weight or on weight gain were observed, although the weight gain was numerically higher in the astaxanthin‐fed prawns |
| Chithambaran and Ayaril (2018) | Indian white shrimp, (Fenneropenaeus indicus) | ATX (0, 250) |
45 days Colour, shell quality, taste and black spot formation |
Synthetic astaxanthin is a safe feed additive to improve colour in F. indicus |
| Chuchird et al. (2015) | Pacific white shrimp (Litopenaeus vannamei) | ATX (0, 50) |
90 days Growth, survival, immunologic parameters, tolerance to Vibrio infection |
ATX (50 mg/kg diet) can be used as a growth promoter in uninfected Pacific white shrimp, while astaxanthin + formic acid can enhance the survival rate of Vibrio parahaemolyticus‐infected shrimp |
| Daly et al. (2013) | Juvenile red king crabs (Paralithodes camtschaticus) | ATX from H. pluvialis, esterified (0, 380) |
56 days Survival, growth, shell colouration |
Astaxanthin (380 mg/kg) resulted in significantly higher survival, larger carapace width and darker colouration |
| Diaz et al. (2014) | Postlarvae of Pleoticus muelleri | ATX (0, 100, 300) |
30 days 96‐h LC50 of nitrite, activity to quench DPPH |
Astaxanthin acts as a protector of nitrite stress in P. muelleri |
| Flores et al. (2007) | Pacific white shrimp (Litopenaeus vannamei) | ATX (0, 40, 80, 150) |
42 days Growth, survival, moult frequency, osmoregulation |
Astaxanthin at 80 mg/kg improves growth, survival, moult frequency, osmoregulatory capacity and selected metabolic and haematological variables |
| Han et al. (2018) | Juvenile swimming crab, (Portunus trituberculatus) | ATX (0, 30, 60, 90, 120) |
56 days Growth, shell pigmentation, antioxidant function |
Based on the improved coloration, increase in nutritional value and antioxidant status, the authors suggested that diet containing 30–60 mg astaxanthin/kg feed was optimal for this crab species |
| Huang et al. (2008) | Giant tiger shrimp (Penaeus monodon) | ATX from H. pluvialis, esterified (0, 50, 100) |
25 days Growth, reproduction |
Supplementation of 50 mg algal esterified astaxanthin/kg increased significantly the proportion of spawns, spawning rate, absolute fecundity (331 × 103) and egg production. Also, the survival was numerically increased (80% vs. 60%) |
| Ju et al. (2011) | Pacific white shrimp (Litopenaeus vannamei) | ATX, ATX from H. pluvialis, esterified (0, 25, 50, 75, 100, 150) |
56 days Growth, survival, pigmentation |
No negative effects were observed with 150 mg ATX/kg diet on shrimp growth (final body weight, growth rate, feed conversion ratio) or survival |
| Liu et al. (2018) | Pacific white shrimp (Litopenaeus vannamei) | ATX from H. pluvialis, esterified (23, 46, 69) |
35 days Growth, survival |
The suggested appropriate level of algal esterified astaxanthin in diets was approximately 46 mg astaxanthin/kg |
| Long et al. (2017) | Chinese mitten crab (Eriocheir sinensis) | ATX from H. pluvialis, esterified (0, 29, 44, 83) |
60 days Coloration, ovarian development, antioxidative capacity |
The redness of ovaries and carapace as well as the contents of total carotenoid and astaxanthin in ovaries, hepatopancreas and carapace increased significantly with increasing ATX supplementation. The suggested appropriate level of algal esterified astaxanthin in feed is approximately 60 mg/kg |
| Niu et al. (2009) | Pacific white shrimp (Litopenaeus vannamei) | ATX (0, 100, 200, 400) |
30 days Growth, survival, stress tolerance |
Growth, survival and stress tolerance were significantly improved by astaxanthin levels of 100, 200 or 400 mg/kg |
| Niu et al. (2012) | Giant tiger prawn (Penaeus monodon) | ATX (0, 100, 200) |
74 days Growth, survival, pigmenting efficacy |
Astaxanthin (100 and 200 mg/kg) improves growth performance and survival at both dietary levels |
| Niu et al. (2014) | Giant tiger prawn (Penaeus monodon) | ATX (0, 100) |
74 days Growth, health status, defence ability to air exposure |
Astaxanthin (100 mg/kg) was better than β‐carotene (250 mg/kg) in improving growth performance, health status and defence ability to air exposure |
| Paibulkichakul et al. (2008) | Giant tiger prawn (Penaeus monodon) | ATX (100, 500) |
120 days Growth, reproduction |
Dietary supplementation of diets with 12% total lipids and at least 280 mg astaxanthin/kg feed will significantly improve maturation and spawning success |
| Pei et al. (2009) | Pacific white shrimp (Litopenaeus vannamei) | ATX from H. pluvialis, esterified (0, 20, 40, 60, 80, 100) |
49 days Survival, growth, antioxidant capability |
Based on survival rate, specific growth rate and antioxidant capability, supplementation of 80 mg astaxanthin/kg feed was most effective |
| Tizkar et al. (2014) | Juvenile prawn (Macrobrachium nipponense) | ATX (0, 50, 100, 150) |
70 days Resistance to thermal shock, shock by ammonia and reduced oxygen |
Higher levels of astaxanthin in the body under oxygen reduction stress can be beneficial for prawns |
| Wade et al. (2015) | Giant tiger prawn (Penaeus monodon) | ATX (0, 25, 50, 100) |
42 days Pigmentation efficacy |
Total dietary carotenoid intake of between 25 and 50 mg astaxanthin/kg diet is required for normal shrimp growth and health in P. monodon. Whole body carotenoids become depleted in shrimp without dietary supplementation of 50 mg astaxanthin/kg diet |
| Wade et al. (2017a) | Giant tiger prawn (Penaeus monodon) | ATX (0, 25, 50, 100) |
42 days Pigmentation efficacy |
Total dietary carotenoid intake of between 25 and 50 mg astaxanthin/kg diet is required for normal shrimp growth and health in P. monodon. Whole body carotenoids become depleted in shrimp without dietary supplementation of 50 mg astaxanthin/kg diet |
| Wang et al. (2018a) | Larval and postlarval kuruma shrimp (Marsupenaeus japonicus) | ATX (0, 50, 100, 200, 400, 800) |
8 days (larval), 30 days (post‐larval) Survival, stress resistance |
The optimal levels of astaxanthin for growth and stress resistance were 169 mg/kg and 82 mg/kg diet, respectively, for larvae, and 109 mg/kg and 178 mg/kg diet, respectively, for post‐larvae |
| Wang et al. (2018b) | Juvenile kuruma shrimp (Marsupenaeus japonicus) | ATX (0, 200, 400, 800, 1200, 1600) |
56 days Growth, survival, stress resistance, immune response |
The optimal level for growth, immune responses, and pigmentation of juvenile kuruma shrimp were approximately 400 mg astaxanthin/kg diet |
| Wang et al. (2018c) | Chinese mitten crab (Eriocheir sinensis) | ATX (0, 68) |
28 days Chronic high pH stress, pigmentation |
Supplementation of astaxanthin in the diet did not only alleviate oxidative damage (by chronic high pH stress), but also improved crab body colour |
| Xie et al. (2018) | Pacific white shrimp (Litopenaeus vannamei) | ATX from H. pluvialis, esterified (0, 50, 100, 200, 400) |
25 days Growth, survival, immune response, stress tolerance |
Dietary supplementation of H. pluvialis increases the survival and stress tolerance of post‐larval white shrimp, and also increases the antioxidative ability and immune capacity of shrimp. The optimal supplementation level of ATX from H. pluvialis is about 100–200 mg/kg diet |
| Yamada et al. (1990) | Prawn (Penaeus japonicas) | ATX (0, 50, 100, 200, 400) |
56 days Survival, growth, feed efficiency |
No negative impact on survival, growth and feed to gain ratio was seen up to the highest dietary ATX concentration of 400 mg/kg |
| Zhang et al. (2013) | Pacific white shrimp (Litopenaeus vannamei) | ATX (0, 25, 50, 75, 100, 125, 150) |
56 days Growth, total antioxidant status |
125 and 150 mg ATX/kg feed improve final body wet weight, weight gain, specific growth rate, feed to gain ratio and total antioxidant status |
LC50: lethal concentration, 50%; DPPH: 2,2‐diphenyl‐2‐picrylhydrazyl radical.
In the study of Chithambaran and Ayaril (2018), Indian white shrimp (Fenneropenaeus indicus) fed with synthetic ATX (0 and 250 mg/kg feed for 45 days) did not show significant differences in loose shell/soft shell and black spot formation; Daly et al. (2013) reported significantly higher survival, larger carapace width and darker colouration in Juvenile red king crabs (Paralithodes camtschaticus) fed 380 mg ATX/kg feed (from H. pluvialis, esterified) for 56 days; in the study of Diaz et al. (2014), it is concluded that ATX acts as a protector of nitrite stress based on the results of the treatment of post‐larvae of Pleoticus muelleri with 0, 100, 300 mg ATX/kg feed for 30 days; Niu et al. (2009) examined the effect of ATX supplementation (0, 100, 200 or 400 mg/kg feed) for 30 days on growth, survival and stress tolerance of post‐larval shrimp (Litopenaeus vannamei). Survival, weight gain and final body weight were significantly higher in the groups fed diets supplemented with 100, 200 or 400 mg/kg than in the control group. The same author (Niu et al., 2012) reported that ATX given at 100 and 200 mg/kg to Giant tiger prawn (Penaeus monodon) for 74 days improves growth performance and survival at both dietary levels. Dietary supplementation of diets with 12% total lipids and at least 280 mg astaxanthin/kg feed significantly improve maturation and spawning success in Giant tiger prawn (Penaeus monodon) fed with 100 and 500 mg/kg ATX for 120 days; in Wang et al. (2018a) studied the effect on survival and stress resistance of ATX (0, 200, 400, 800, 1,200, 1,600 mg/kg feed) given to juvenile kuruma shrimp (Marsupenaeus japonicus) for 8 days at larval stage and 30 days at post‐larval. Supplementation with 100 and 200 mg/kg ATX yielded significantly higher final body weight, body weight gain and specific growth rate as compared to control group. The same author (Wang et al., 2018b) evaluated the effect on growth, survival, stress resistance, immune response in juvenile kuruma shrimp (Marsupenaeus japonicus) given ATX (0, 200, 400, 800, 1,200, 1,600 mg/kg) for 56 days. Animals fed diets supplemented with ATX showed a better growth performance and immune response compared to the control group; in the study of Xie et al. (2018) an increase in the survival, stress tolerance and antioxidative ability and immune capacity of pacific white shrimp (Litopenaeus vannamei) were observed in animals fed ATX from H. pluvialis, esterified (0, 50, 100, 200, 400 mg/kg) for 25 days. Yamada et al. (1990) fed prawns (Penaeus japonicus) diets supplemented with 0, 50, 100, 200 or 400 mg ATX/kg diet for eight weeks. No negative effects observed on weight gain, survival, daily feed intake, per cent gain or feed to gain ratio were observed in the prawns fed 400 mg/kg compared with the control or other treatment groups.
Overall, in all these studies, no findings which could be interpreted as adverse were reported. The absence of adverse effects is confirmed by a review of Wade et al. (2017) (review of several papers on carotenoid utilisation and function in crustacean aquaculture, published between 1990 and 2017). The authors concluded that ATX is safe for several crustaceans at up to 810 mg ATX/kg diet.
It is therefore concluded that ATX is tolerated by crustaceans at levels which are more than 8 times higher the maximum dietary concentration applied (100 mg/kg feed).
3.2.2.3. Conclusions on safety for the target species
ATX‐DMDS remains safe for salmonids up to 138 mg ATX‐DMDS/kg complete diet corresponding to 100 mg ATX/kg. This conclusion is extrapolated to other fish.
ATX at a maximum concentration of 100 mg/kg complete feed is safe for crustaceans with a margin of safety of at least 8. This conclusion covers also ATX from ATX‐DMDS and therefore the safety of 138 mg ATX‐DMDS/kg complete feed.
The FEEDAP Panel has no reservation to the deletion of the provision that limits the age of use of ATX/ATX‐DMDS.
3.2.3. Overview of the available toxicological dataset
The available toxicological data consists of (i) the previous assessments performed by the FEEDAP Panel (EFSA, 2005, 2007a; EFSA FEEDAP Panel, 2014a), (ii) a structured literature search performed by the applicant to demonstrate that, in the light of the current knowledge, the additive and its active substance remains safe for the consumer, and (iii) the data submitted to EFSA in the context of a public call for data on ATX,23 if relevant for the current safety assessment.24
3.2.3.1. Overview of the previous assessments performed by the FEEDAP Panel
The toxicological profile of synthetic ATX has been reviewed by the FEEDAP Panel in 2005, 2007 and 2014 (EFSA, 2005, 2007a; EFSA FEEDAP Panel, 2014a). An overview of the data assessed in the most recent opinion by the FEEDAP Panel (2014a) is given below.
ATX was negative in a bacterial reverse mutation assay in Salmonella Typhimurium strains TA1535, TA1537, TA98 and TA100 and in Escherichia coli strain WP2uvrA and in two cytogenetic assays conducted in cultured peripheral human lymphocytes, namely a micronucleus test and a chromosomal aberrations assay. On this basis the substance is considered non genotoxic.
In rats and dogs, 13‐week toxicological studies showed no treatment‐related adverse effects at any of the highest dose tested, with a no observed adverse effect level (NOAEL) of 750 mg ATX/kg body weight (bw) per day identified for the rat study and an NOAEL of 158 mg ATX/kg bw per day for the dog study.
One‐year chronic toxicity studies were also performed in rats and dogs. In the rat study, there was an increased incidence of centrilobular hepatocellular hypertrophy in female rats given doses of 250 mg ATX/kg bw per day or greater, but not in males given up to 1,000 mg ATX/kg bw per day or in the females given 125 mg ATX/kg bw per day. A NOAEL was not identified for this study as there was a statistically significant and dose‐related increase in serum cholesterol concentrations in female (but not male) rats at all tested dose levels. In the 1‐year dog study, the only adverse effect seen was a decreased rate of body weight gain which was associated with a decreased feed intake, and the NOAEL was 104 mg ATX/kg bw per day.
Carcinogenicity studies were performed using mice and rats.
ATX was not carcinogenic in mice and the only adverse effects seen were an increased plasma cholesterol concentration at the highest dose level of 1,400 ATX/kg bw per day and a reduced body weight gain at doses of 300 mg ATX/kg bw per day, with the NOAEL for the mouse study being 140 mg ATX/kg bw per day.
In the rat carcinogenicity study, increased incidences of liver hypertrophy and hepatocellular adenomas were seen in the females and of centrilobular hepatocellular vacuolation in both sexes. Incidences of malignant tumours and of benign tumours other than hepatocellular adenomas were not affected by treatment with ATX. At the time of the evaluation in 2014, the critical reference value identified by the FEEDAP Panel for the rat carcinogenicity study was a BMD lower confidence limit for a 10% extra risk (BMDL10) of 3.4 mg ATX/kg bw per day for liver hypertrophy in females (EFSA FEEDAP Panel, 2014a).
Reproduction toxicity was investigated in a one‐generation and a two‐generation study in rats, and in developmental toxicity studies in rats and rabbits. In the one‐generation study, there was decreased bodyweight gain in treated males and increased pup mortality during the lactation period in the group given 400 mg ATX/kg bw per day, with a NOAEL of 100 mg ATX/kg bw per day. The same NOAEL was identified for the two‐generation study: doses of 250 mg ATX/kg bw per day or greater caused decreases in body weight gain and feed intake in F1 pups, and 800 mg ATX/kg bw per day caused retarded pup growth during the lactation period. No treatment‐related adverse effects were seen in the developmental toxicity studies in rats and in rabbits, with the NOAELs being the highest doses tested: 1,000 mg ATX/kg bw per day and 400 mg ATX/kg bw per day, respectively.
3.2.3.2. Overview of the structured literature search performed by the applicant
The applicant performed a structured literature search covering the period 2013–April 2017 aiming to demonstrate that, in the light of the current knowledge, the additive and its active principle ATX remains safe for the consumer.25 For ATX‐DMDS, five papers retrieved by the structured literature database searches were considered relevant (Appendix A). For ATX, 45 relevant documents out of 134 hits were considered relevant (Appendix A).
No new studies on repeated dose toxicity of ATX‐DMDS were identified in the literature search. An article (Vega et al., 2015) put into the public domain describes details of a 13‐week toxicity study of ATX that had already been evaluated by FEEDAP (EFSA FEEDAP Panel, 2014a). Some new repeat‐dose toxicity studies performed with other forms of ATX other than ATX‐DMDS were found (Buesen et al., 2015; Tago et al., 2014; Katsumata et al., 2014; Lin et al., 2017; Stewart et al., 2008; Takahashi et al., 2005). The FEEDAP Panel checked these papers and noted that they do not affect the latest FEEDAP assessment since they were conducted with natural form of ATX or the lowest NOAEL identified is higher than the one established for sub‐chronic studies in the 2014 opinion.
No new studies of the possible carcinogenicity of ATX‐DMDS were identified in the literature search. A discussion of mechanisms of anti‐cancer effects of ATX was reported in a review article by Ranga Rao et al. (2014). Edwards et al. (2016) made a critical review of the genotoxicity and carcinogenicity data on ATX evaluated by the FEEDAP Panel in 2014.
No new reproduction or developmental studies of ATX‐DMDS were identified in the literature search. Vega et al. (2015) put into the public domain a report of a study of the developmental toxicity of ATX in rats that has been previously evaluated by FEEDAP (EFSA FEEDAP Panel, 2014a). Schneider et al. (2016) reported the results of a rabbit developmental toxicity study of [3S,3′S]‐ATX, finding no adverse effects up to the maximum dose tested of 400 mg/kg bw per day.
No new genotoxicity studies of ATX‐DMDS were identified in the literature search. In a paper of Tago et al. (2014), genotoxicity studies of a new ATX‐rich extract of Phaffia rhodozyma were reported to give negative results in a bacterial reverse mutation test and a mouse bone marrow micronucleus test.
No new human safety studies of ATX‐DMDS had been identified in the literature search. Several human studies using other forms of ATX were provided by the applicant and were also submitted to EFSA as a result of EFSA public call for data (for an overview see chapter ‘Other available information’).
3.2.3.3. Other available information to EFSA (public call for data)
Following the public call for data relevant to the safety assessment of ATX in the framework of Regulation (EU) 2283/201526, the following was submitted: in vitro studies on cell toxicity and microsomal enzyme induction and about 90 human intervention studies with repeated intakes of ATX as food supplements. A short overview of the information provided is given below.
In vitro studies on cell toxicity and microsomal enzyme induction
Primary cultures of female human, rat and murine hepatocytes were incubated 48 or 96 hrs at different concentrations (0.1 μM, 0.5 μM, 4 μM and 10 μM) of a racemic mix of three ATX isomers ((3‐R,3ʹ‐R), (3‐R,3ʹ‐S) and (3‐S,3ʹ‐S) to investigate induction of CYP1A1, CYP1A2, CYP1B1, CYP2B6 and CYP3A4 and effects on transcript levels of CYP genes induced by nuclear receptors other than aryl hydrocarbon receptor (AHR) (i.e. PXR/CAR (pregnane X receptor/constitutive androstane receptor)). Concentrations of 10 μM benzo‐a‐pyrene (BaP) or rifampicin (Rif) were used at as positive controls as these are known inducers for cytochrome P‐450 through AHR and PXR, respectively. In rat cells, induction of Cyp1a1 and Cyp1b1 was observed while Cyp2b1 (rat orthologue of CYP2B6) and Cyp3a2 (rat orthologue of CYP3A4) were not affected. In human cells, CYP1A1, CYP1A2 and CYP1B1 were not induced while non‐statistically significant induction of CYP2B6 was observed. With murine cells, Cyp1a1, Cyp1A2 and Cyp3a11 (murine orthologue of human CYP3A4) were induced while no induction of Cyp1b1 and Cyp2b10 (murine orthologue of human CYP3A4) was induced.
Based on a normalised comparison of CYP1A1, CYP1A2 and CYP1B1 through the three species, the authors concluded that ATX is a weak PXR inducer in human and mouse and that activation of AHR is occurring in rats but not in humans.
In addition, cell viability was evaluated by quantifying the mRNA of the cells. No significant cytotoxicity was observed with ATX, while RNA levels were reduced > 50% in BaP exposed rat and human hepatocytes after 96 h exposure.
The FEEDAP Panel notes that because of the scant reporting of methodology and, in particular, the scant and inconsistent reporting of results (in many instances numerical values are missing and/or statistical significance cannot be verified), interpretation and validation of the results are limited and will not be considered as relevant for the current assessment.
Human studies
Following the public call for data, about 90 human intervention studies were provided with repeated intakes of ATX as food supplements. In 40 of these studies, dosages from 8 mg up to 45 mg ATX were administered per day. The study durations varied from 3 weeks up to 1 year. There was high variability in study quality (i.e. from uncontrolled one‐arm trials to randomised controlled trials) and the study populations (e.g. healthy adults, obese subjects, athletes, elderly subjects with age‐related forgetfulness, immunosuppressed subjects, patients with functional dyspepsia, subjects with ‘fatigue’, adults with type 2 diabetes, rheumatoid arthritis, atopic dermatitis, dyslipidaemia or at risk of metabolic syndrome). Even though these human studies were primarily designed to investigate putative beneficial effects of ATX intake, many studies also assessed some safety‐related endpoints such as anthropometrics (body mass and composition), blood pressure, blood lipids, clinical chemistry (including liver enzymes), haematology (full blood counts), eye pressure, oxidation markers and adverse events. The Panel notes that no changes were found in the studied safety‐related parameters and that no adverse events related to the consumption of ATX were reported. The Panel notes, however, the inherent limitations of such human studies (which had not been designed for safety) for their use in the safety assessment.
Outcome of the overview of the available toxicological dataset
None one of the documents considered above included new toxicological evidence which were not already considered before. However, the publication of Edwards et al. (2016) has been given consideration by the FEEDAP Panel as it made a critical review of the two‐year carcinogenicity study in rat already assessed by the FEEDAP Panel in 2014. The critical reference value identified by the FEEDAP Panel from that study was a BMDL10 of 3.4 mg ATX/kg bw per day for liver hypertrophy in females (EFSA FEEDAP Panel, 2014a). The FEEDAP Panel re‐considered in great detail the 2‐year carcinogenicity study taking into account the end‐points of hepatic effect and retained necessary to update the assessment done in 2014.
3.2.4. Update of the assessment of the oral toxicity studies and carcinogenicity studies already assessed by EFSA in 2014
One‐year chronic toxicity studies of a commercial water‐soluble beadlet formulation containing ATX were performed in HanIbm Wistar rats27 and Beagle dogs.28 The studies were Good Laboratory Practice (GLP) compliant and broadly conformed to OECD Test Guideline 452, although the number of dogs in each group was less than the recommended amount (at least 20 per group).
A beadlet formulation, containing 8.7% ATX, was incorporated into the diets of groups of 26 rats of each sex and adjusted weekly (weeks 1–41 and 43–53 for females and weeks 1–28 for males) not exceeding 23% (males) or 20.5% (females) of the diet. The intended dosages of ATX given to the groups of rats were 0 (untreated control), 0 (placebo control), 125, 250, 500 and 1,000 mg/kg bw per day. The mean ATX intake over 53 weeks was 116, 230, 472 and 940 mg/kg bw per day in males and 126, 251, 503 and 1,004 mg/kg bw per day in females. No unscheduled deaths occurred and there were no clinical signs of toxicity. Body weight gain and feed intake were decreased in all treatment groups and in the placebo control, compared with the untreated control, but there was no association with the dosage of ATX. Ophthalmoscopy revealed no treatment‐related effects. Haematology showed no consistent effects, but there were some occasional changes in the red blood cell parameters (mainly reductions in mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC)) in the males and females in the highest dose group and in females in the mid‐dose group. Significantly increased cholesterol levels (compared to placebo) were found in females at all ATX doses and in males receiving 250–1,000 mg/kg bw per day. Significantly higher bilirubin levels were found in females treated with 500–1,000 mg ATX/kg bw per day but not in males. Urinalysis showed a slightly increased specific gravity in rats given 250 mg/kg bw per day or more and increased phosphate in urinary sediment from males receiving the highest dose. No treatment‐related gross pathology was seen at autopsy. Low weights of some organs relative to body weight were seen at 250 mg/kg bw per day or greater; the organs affected were the spleen, adrenals, ovaries, liver, heart and brain in females, and the spleen, kidneys and adrenals in males. Histopathology revealed no treatment‐related effects on organs other than the liver. The latter are summarised in Table 1. There was an increased incidence of yellow‐brown pigment accumulation in hepatocytes and macrophages in ATX‐treated rats (more pronounced in females) than in either control group, but there was no dose–response relationship. Pigment accumulation may be associated with the deposition of ATX or its metabolites in hepatocytes and macrophages. In treated females, there was a dose‐related increased incidence of centrilobular hepatocellular hypertrophy at doses of 250 (seven rats), 500 (11 rats) and 1,000 mg/kg bw per day (13 rats) (not found at the low dose (125 mg/kg bw per day) or in either control group), and there were dose‐related raised incidences of inflammatory cell foci and multinucleated and/or cytomegalic hepatocytes in treated females which were significant for the top‐dose group.
Table 1.
Histopathological findings in the liver of rats (chronic toxicity study). Figures given are numbers of animals out of 26 rats per treatment and sex
| Astaxanthin intended (mg/kg bw) | 0 (control) | 0 (Placebo) | 125 | 250 | 500 | 1,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | |
| Yellow‐brown pigmentation hepatocytes | 0 | 3 | 0 | 2 | 1 | 23 | 2 | 25 | 10 | 26 | 10 | 23 |
| Yellow‐brown pigmentation macrophages | 0 | 3 | 1 | 2 | 0 | 16 | 0 | 20 | 4 | 24 | 2 | 22 |
| Hepatocellular hypertrophy | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 7 | 0 | 11 | 0 | 13 |
| Inflammatory foci | 3 | 4 | 9 | 9 | 4 | 3 | 4 | 4 | 3 | 7 | 5 | 11 |
| Vacuolation1 | 9 | 12 | 8 | 11 | 10 | 6 | 11 | 15 | 6 | 14 | 12 | 11 |
| Multinucleated cells/cytomegalic hepatocytes | 0 | 2 | 0 | 2 | 0 | 4 | 0 | 4 | 0 | 5 | 0 | 7 |
Periportal, diffuse and centrilobular hepatocytes.
It is concluded from these results that the major effect of ATX was on the liver of females. This was indicated by a higher occurrence of hepatocellular hypertrophy from 250 mg ATX/kg bw day onwards and a dose‐dependent increase in serum cholesterol, which was seen at all time measurements for each dose level for females. Although a NOAEL was not identified in this study for the effect on cholesterol, it is noted that there was no effect on this parameter at 40 mg ATX/kg bw per day in the two‐year carcinogenicity rat study. In males, hepatocellular hypertrophy was not increased by ATX and serum cholesterol was only higher (than the 0 control) at 1,000 mg ATX/kg bw per day.
One possible cause of the occurrence of hepatocellular hypertrophy could be the induction of drug metabolising enzymes (e.g. cytochrome P450 (Gradelet et al., 1997; Paolini et al., 2001). Such enzyme induction is generally regarded as an adaptive metabolic, non‐adverse process in the case that this condition is not accompanied by histopathological alterations indicative of liver toxicity (Hall et al., 2012). However, in the presence of histopathological hepatocellular changes indicative of liver toxicity, such as an increased incidence of (single) cell necrosis and multinucleated cells, hepatocellular hypertrophy might be an initial step in the development of hepatocellular tumours.
In another study, gelatine capsules filled with beadlets of a product containing 8.7% ATX were administered orally to groups of four dogs of each sex at initial dosages of 75, 300 and 1,200 mg/kg bw per day. Controls received empty capsules. After 5 months, the dosage of the highest dose group was increased to 2,500 mg/kg bw per day for the remainder of the 53‐week treatment period. The dosages of ATX received by the dogs were 0, 6.5, 24 and 104/218 mg/kg bw per day. Body weight gain was reduced in the highest dose group, with a small decrease in feed intake also occurring in this group. There were no effects on mortality, clinical signs or ophthalmoscopy. Haematology, blood biochemistry, urinalysis, gross pathology, organ weights and histopathology revealed no treatment‐related effects. The NOAEL for this study was 1,200 mg beadlets/kg bw per day, corresponding to 104 mg ATX/kg bw per day (218 mg ATX/kg bw per day for seven months).
Carcinogenicity studies of a beadlet formulation (8% ATX) were performed, conforming to OECD Test Guideline 451 and in accordance with the principles of GLP, using NMRI Ibm MORO strain mice29 and HanIbm Wistar strain rats.30
For 18 months, groups of 50 mice of each sex were fed a beadlet formulation containing 8% ATX at dietary levels equivalent to dosages of 0 (untreated control), 0 (placebo control), 140, 300, 650 and 1,400 mg ATX/kg bw per day. Mortality was quite high in all groups, ranging between 50% and 62% of the mice surviving until the end of the study. There was a significant reduction in body weight gain at dosages of 300 mg/kg bw per day or more over the last 6 months of the study. There were no treatment‐related adverse effects on clinical appearance, food consumption or haematology. The only blood biochemistry parameter affected was a slight increase (compared with the placebo controls) in plasma cholesterol concentrations in both sexes at the highest dose level. There were no treatment‐related increases in the incidences of any type of tumour and no neoplastic or non‐neoplastic lesions were revealed by microscopic examination of tissues. It is concluded that ATX was not carcinogenic in mice and that the NOAEL for this study was 140 mg/kg bw per day.
In another study, groups of 50 rats of each sex were fed a beadlet formulation containing 8% ATX at dietary levels equivalent to dosages of 0 (untreated control), 0 (placebo control), 40, 200 or 1,000 mg ATX/kg bw per day for 2 years. Satellite groups of 10 rats of each sex were treated for only 1 year, followed by an untreated recovery period of one year. Survival in the groups treated for 2 years was 76–88% in males and 56–82% in females (not dose related).
Feed consumption was unaffected by ATX exposure. Body weight gain of all animals with the beadlet formulation (with or without ATX) was reduced compared with the untreated controls. Body weight gain of females given ATX (significant at 200 and 1,000 mg/kg bw per day) was lower than in controls, and there was some recovery of body weight in the satellite groups during the recovery phase. There were no treatment‐related adverse effects on clinical signs. Haematology showed minor changes in some red blood cell parameters in the groups given 200 or 1,000 mg/kg bw per day for 2 years: reduced erythrocyte count and packed cell volume and increased MCH and MCHC. Some effects were seen on blood biochemistry parameters in the female groups given 1,000 mg/kg bw per day and only rarely on those given 200 mg/kg bw per day, including increased plasma levels of cholesterol (p ≤ 0.01), bilirubin (p ≤ 0.05), alkaline phosphatase (p ≤ 0.05), ALT and AST. No relevant haematological or biochemical changes were observed in the recover animals after the second year of the study without treatment. A few organ weight variations (e.g. of the heart, brain or spleen) in the placebo‐ or ATX‐treated groups were considered to be due to the lower body weights of treated groups than of the untreated controls. After the 2‐year treatment period, the treatment‐related non‐neoplastic changes were confined to the liver. Histopathological findings are summarised in Table 2. In female rats, increased incidences of hepatocellular vacuolation, hepatocellular hypertrophy and multinucleated hepatocytes at all dietary levels of ATX were observed, and there was also a significant increase in the incidence of hepatocellular adenomas at 200 and 1,000 mg ATX/kg bw per day. The number of females with hepatocellular adenomas in the negative control, placebo control and low‐dose, mid‐dose and high‐dose groups given ATX for 2 years was 2, 1, 5, 9 and 14, respectively. The increased incidences of hepatocellular adenomas in females were statistically significant at 200 and 1,000 mg/kg bw per day. In males, there were increased incidences of centrilobular vacuolation of hepatocytes at 200 and 1,000 mg/kg bw per day dose levels. No increased incidence of malignant tumours was observed and, apart from the liver adenomas in females, there was no increased incidence of benign tumours.
Table 2.
Histopathological findings in the liver of rats (carcinogenicity study). Figures given are numbers of animals out of 50 rats per treatment and sex (49 in the placebo group)
| Astaxanthin intended (mg/kg bw) | 0 (control) | 0 (Placebo) | 40 | 200 | 1,000 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | |
| Carcinoma | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 2 |
| Adenoma | 3 | 2 | 7 | 1 | 3 | 5 | 5 | 9 | 3 | 14 |
| Yellow‐brown pigmentation hepatocytes | 0 | 11 | 0 | 13 | 0 | 40 | 1 | 34 | 0 | 34 |
| Yellow‐brown pigmentation macrophages | 1 | 13 | 1 | 12 | 1 | 46 | 1 | 49 | 3 | 49 |
| Hepatocellular hypertrophy | 0 | 1 | 1 | 3 | 1 | 21 | 1 | 37 | 2 | 37 |
| Inflammatory foci | 6 | 5 | 8 | 7 | 1 | 7 | 1 | 17 | 5 | 17 |
| Vacuolation1 | 8 | 11 | 7 | 5 | 5 | 8 | 9 | 16 | 15 | 32 |
| Multinucleated cells/cytomegalic cells | 0 | 13 | 0 | 12 | 1 | 23 | 0 | 39 | 1 | 41 |
Periportal, diffuse and centrilobular.
Multinucleated hepatocytes were observed in about 13 control female rats and in 23, 39 and 41 rats treated with 40, 250 and 1,000 mg ATX/kg bw per day, respectively. In males, only one animal in each of the groups with 40 and 1,000 mg ATX/Kg bw per day showed multinucleated hepatocytes. The increased incidence of multinucleated hepatocytes in females can be considered a response to increased hepatic cell injury and cell deaths as observed by increased single‐cell necrosis at the ATX top dose and inflammatory foci at the intermediate and top doses (see Table 3). The FEEDAP Panel agrees with Buser et al. (2003) and Edwards et al. (2016) that the increased incidence of single cell necrosis, vacuolated hepatocytes and inflammatory cell foci reflect a hepatotoxic effect of the test compound, whereas the increased incidence of multinucleated hepatocytes may represent a regenerative process secondary to cell injury or cell death. Recurrent cell damage and repair may ultimately have caused the dose‐related increase hepatocellular adenomas in the female rats.
Table 3.
Chronic dietary exposure of consumers to ATX based on residue data in salmonids and crustaceans – Summary statistics across European dietary surveys
| Population class | Number of surveys | Highest exposure estimate (mg/kg bw per day) | % ADI1 |
|---|---|---|---|
| Infants | 6 | 0.0532 | 27 |
| Toddlers | 10 | 0.1459 | 73 |
| Other children | 18 | 0.1011 | 51 |
| Adolescents | 17 | 0.0709 | 35 |
| Adults | 17 | 0.0598 | 30 |
| Elderly | 14 | 0.0555 | 28 |
| Very elderly | 12 | 0.0417 | 21 |
ADI: 0.2 mg/kg bw.
The results of the satellite group (rats treated for 53 weeks followed by a 51‐week treatment‐free recovery period) showed no treatment‐related adverse effects. The FEEDAP Panel noted that histopathological examination of animals after 53 weeks was not performed in the carcinogenicity study, but the FEEDAP Panel considered the treatment‐related histopathological changes present in the liver of the female rats (hepatocellular hypertrophy and multinucleated/cytomegalic hepatocytes, see Table 2) in the chronic (53‐week) toxicity study performed with the same strain of rats as an acceptable surrogate.
As histopathological changes were seen in the livers of female rats at all tested doses of ATX (40 mg/kg bw per day or more), it was not possible to identify a NOAEL for this study.
3.2.5. Updated assessment of consumer safety
3.2.5.1. Determination of a safe concentration
The toxicity observed in the liver of female rats in the chronic (53 weeks) and carcinogenicity (104 weeks) studies is not reproduced in any other study, including the 90‐day study in rats at similar doses (EFSA FEEDAP Panel, 2014a,b). The FEEDAP Panel noted that while it is possible that the signs of hepatotoxicity present after 53 and 104 weeks of exposure to ATX may be unique to the species and/or strain, and thus not relevant to human risk assessment, it is considered that there is insufficient evidence to justify eliminating the liver findings observed in rats from the risk assessment and to consider them not relevant for humans.
Consequently, the FEEDAP Panel has taken the liver toxicity observed in the 2‐year study as the basis for the safety assessment of ATX. Since a NOAEL cannot be identified from the critical toxicity study, the evaluation has to rely on the lowest observed adverse effect level (LOAEL). As recommended in an opinion of the Scientific Committee of EFSA, it is preferable to use the BMD approach (EFSA Scientific Committee, 2017) instead of the NOAEL/LOAEL.
The Panel considered the incidence of multinucleated hepatocytes in female rats in the 2‐year rat study as the critical effect for risk assessment. The BMD approach was applied to analyse the dose‐response of the incidence of multinucleated hepatocytes, using model averaging and the default benchmark dose response (BMR) for quantal data of 10% extra risk (placebo control and three treated groups, details see Appendix B). The resulting BMD confidence interval was very wide (the ratio of the 95% upper bound limit (BMDU10) to the 95% lower bound limit (BMDL10) was about 500), indicating that there is a large uncertainty in the BMD estimate. This indicates that there is insufficient dose‐response information in this data set to use the BMDL10 as a reference point to establish an acceptable daily intake (ADI). It must be noted that the design of the study (three ATX doses only) was not optimal for the BMD approach and that the incidence of multinucleated hepatocytes in the mid and high dose groups was nearly identical, leading to large model uncertainty in the estimate of the BMD.
Since the application of the BMD approach showed a large uncertainty of the dose–response data, the FEEDAP Panel decided to use the LOAEL of 40 mg ATX/kg bw per day for the increased incidence of multinucleated hepatocytes as the point of departure to derive an updated ADI for ATX.
3.2.5.2. Acceptable daily intake
Applying an uncertainty factor (UF) of 100, with an additional UF of 2 (EFSA Scientific Committee, 2012), an ADI of 0.2 mg ATX/kg bw per day was established. The FEEDAP Panel considered an additional UF of 2 acceptable since the adverse hepatocellular changes were reversible and only observed in female rats and not in male rats, mice or dogs. The newly derived ADI replaces the one established by the FEEDAP Panel in 2014 (0.034 mg/kg bw) (EFSA FEEDAP Panel, 2014a).
3.2.5.3. Consumer exposure
In its opinion on the safety and efficacy of ATX (EFSA FEEDAP Panel, 2014a), the FEEDAP Panel calculated consumer exposure to ATX using residue data of 10 mg/kg from salmon and 25 mg/kg from trout. The current application is also for crustaceans (i.e. shrimp) in which the ATX deposition occurring mainly in the exoskeleton, which is not eaten. On the basis of the data now available, it can be assumed that ATX deposition in crustaceans (shrimp) muscle is less than half the amount deposited in salmon flesh (about 4 mg ATX/kg see Section 3.2.1.2).
The worst‐case chronic exposure of consumers to ATX residues in fish (including consumption of salmon and trout) and seafood (including crustaceans) is calculated following the methodology described in the Guidance on the safety of feed additives for consumers (EFSA FEEDAP Panel, 2017) (for further details see Appendix C and Table 1C). The residue value of 25 mg ATX/kg and 4 mg ATX/kg has been used for the calculation of consumer exposure to residues in fish (including salmon and trout) and seafood (including crustaceans), respectively.
The results showed that the highest chronic exposure was for the age class toddlers, with 0.15 mg/kg bw per day (Table 3). This exposure represents 73% of the ADI of 0.2 mg/kg bw.
3.2.5.4. Conclusions on safety for the consumer
The FEEDAP Panel re‐assessed the toxicological profile of ATX based on data already considered in 2014, the literature review performed by the applicant and the data available in the context of an EFSA public call for data on ATX.
ATX is neither genotoxic nor carcinogenic.
The FEEDAP Panel established an ADI of 0.2 mg ATX/kg bw per day by applying an uncertainty factor of 200 to a LOAEL of 40 mg/kg bw per day for the increased incidence of multinucleated hepatocytes observed in a 2‐year carcinogenicity study. This ADI replaces the one established by the FEEDAP Panel of 0.034 mg/kg bw (EFSA FEEDAP Panel, 2014a).
The use of ATX‐DMDS in the nutrition of salmonids, other fish and crustaceans up to the maximum permitted dietary level of 138 mg ATX‐DMDS/kg complete feed is of no concern for the safety of the consumer.
3.2.6. Safety for the user
3.2.6.1. Effects on eyes and skin
In its opinion on the safety and efficacy of ATX‐DMDS for salmon and trout (EFSA, 2007a), the FEEDAP Panel concluded that ‘no dermal or ocular risk for the users of CAROPHYLL® Stay‐Pink is likely to occur under practical conditions’. No new study has been submitted. The applicant performed two literature searches; one specifically on ATX‐DMDS31 and another one on ATX and ATX‐esters.32 The searches did not reveal any new information that would require modification of the earlier conclusions made by the FEEDAP Panel.
3.2.6.2. Effects on the respiratory system
In 2007, the Panel was not in the position to establish the inhalation toxicity of the additive in absence of an acute inhalation study. The applicant did not perform a study. No information could be retrieved from the literature in relation to respiratory toxicity of ATX‐DMDS31 nor ATX/ATX‐esters.32
3.2.6.3. Inhalation exposure
The applicant provided new data on particle size distribution and dusting potential for the formulation assessed in the current dossier (see Section 3.1.3). The newly submitted data showed that all particles had a diameter < 600 μm; no particles < 100 μm were found. The mean dusting potential was 0.95 g/m3 (single values: 0.94, 0.90, 1.00 g/m3). The measured ATX‐DMS in the dust is 9.7 g/kg on average (single values: 10.2, 9.7, 9.2), the active substance in the air is calculated to be 9.2 mg ATX‐DMDS/m3.
The potential exposure of users by handling the additive to inhaled ATX‐DMDS was calculated according to the Technical Guidance on User safety (EFSA FEEDAP Panel, 2012) and reported in Appendix X. From dusting potential and ATX‐DMDS content of the dust, the ATX‐DMDS concentration in the inhaled air could be calculated as 10 mg/m3, resulting in inhalation exposure of 1.1 mg ATX‐DMDS from Carophyll® Stay‐Pink 10%‐CWS per person during an 8‐h working day. The Panel notes that the ADI for a 70 kg person will be 14 mg ATX per day.
Conclusions on user safety
The FEEDAP Panel concludes that there is no new evidence that would lead the Panel to reconsider its previous conclusions on the safety for the user. No dermal or ocular risk for the users is likely to occur under practical conditions. In the absence of inhalation toxicology study, the Panel is not in the position to establish the inhalation toxicity of the additive.
Due to the susceptibility of the active substance to oxidation, the additive will be placed in the market only in the form of preparations. The FEEDAP Panel recognises that once authorised, multiple formulations of the additive can be placed in the market, and consequently, not all preparations can be directly tested for user safety.
3.2.6.4. Safety for the environment
In its opinions on synthetic ATX (EFSA FEEDAP Panel 2014a), the FEEDAP Panel concluded that: ‘the use of synthetic ATX (100 mg ATX/kg fish feed) does not pose a significant additional risk to the environment compared with natural astaxanthin.’ Since ATX‐DMDS is metabolised to ATX in fish, and excreted mainly in this form, the potential environmental risks are predominantly from ATX, the above conclusions apply to ATX‐DMDS.
The applicant performed a structured literature search for the period 2013 up to April 2017 aiming to demonstrate that, in the light of the current knowledge, the additive and its active principle ATX remain safe for the environment.33 For ATX‐DMDS and ATX, none of the documents retrieved by the structured literature database searches identified new data requiring consideration in the current opinion, therefore the above conclusions are reiterated for the current assessment.
3.3. Efficacy
3.3.1. Efficacy for salmon and trout
The present application for renewal of the authorisation does not include a proposal for amending or supplementing the conditions of the original authorisation that would have an impact on the efficacy of the additive for salmon and trout. Therefore, there is no need for assessing the efficacy of the additive in the context of the renewal of the authorisation for these two target species.
3.3.2. Efficacy for crustaceans and other fish
The applicant applied for an extension of use of the additive in all fish and proposed a new use in crustaceans. To support efficacy of the additive for crustaceans and other fish, the applicant performed a structured literature search covering the period 2007–201834 to provide information on the efficacy of ATX‐DMDS, including ATX ester(s), synthetic ATX, and esterified ATX from H. pluvialis. No papers were found for the efficacy of ATX‐DMDS in pigmentation of crustaceans and other fish. Fourteen and six relevant papers for the efficacy of free and esterified ATX, respectively, in the pigmentation of crustaceans and other fish were found (Appendix A). In the absence of specific papers with ATX‐DMDS, the FEEDAP Panel assumes that the results of studies performed with ATX can be used for the evaluation of the efficacy of ATX‐DMDS in crustaceans and other fish.
3.3.2.1. Crustaceans
Most studies found in the literature search for ATX in crustaceans were already identified in the search for tolerance (Appendix A, Table A.1). The FEEDAP Panel re‐assessed them considering the relevant results in support of the efficacy of ATX in crustaceans. The main results are summarised in Table 4.
Table 4.
Summary of efficacy studies with ATX in crustaceans
| Author(s) | Crustacean | Test item | Levels (mg ATX/kg) | Duration (days) | Endpoints | Results/conclusions |
|---|---|---|---|---|---|---|
| Chithambaran and Ayaril (2018) | Indian white shrimp, (Fenneropenaeus indicus) | ATX | 0, 250 | 45 | Colour of fresh and cooked shrimp by panel experts | Colour of shrimp (fresh and cooked) was significantly increased by ATX treatment |
| Daly et al. (2013) | Juvenile red king crabs (Paralithodes camtschaticus) | ATX from Haematococcus pluvialis, esterified | 0, 380 | 56 | Shell colouration | ATX significantly increased colour saturation (amount of hue) in treated animals as compared to controls (48.7 vs. 46.1), and reduced both colour hue (shade of colour, 20.8 vs. 27.2°) and brightness values (light vs. dark). Colour was quantified from digital photographs |
| Han et al. (2018) | Juvenile swimming crab, (Portunus trituberculatus) | ATX | 0, 30, 60, 90, 120 | 56 | Shell pigmentation | ATX supplementation significantly enhanced the redness (a*) of cooked crabs in a dose dependent manner. ATX concentrations of the whole body, shell and hepatopancreas showed a linear increase with increasing astaxanthin supplementation |
| Ju et al. (2011) | Pacific white shrimp (Litopenaeus vannamei) | ATX, ATX from H. pluvialis, esterified | 0, 25, 50, 75, 100, 150 | 56 | Measurement of colour of body and tail | Addition of ATX (above 50 mg/kg) resulted in significant (p < 0.05) increase in redness values in whole body and tail muscle. ATX content in shrimp tail muscle was significantly correlated with the level of dietary astaxanthin |
| Long et al. (2017) | Chinese mitten crab (Eriocheir sinensis) | ATX from H. pluvialis, esterified | 0, 29, 44, 83 | 60 | ATX tissue levels, tissue coloration | The redness (a*) of ovaries and carapace as well as the contents of total carotenoid and astaxanthin in ovaries, hepatopancreas and carapace increased significantly (p < 0.05) with increasing ATX supplementation |
| Niu et al. (2012) | Giant tiger prawn (Penaeus monodon) | ATX | 0, 100, 200 | 74 | ATX tissue retention | Dietary ATX was retained in whole body, muscle and carapace |
| Niu et al. (2014) | Giant tiger prawn (Penaeus monodon) | ATX | 0, 100 | 74 | ATX tissue retention | Apparent digestibility of ATX was high (> 90%). Tissue retention of ATX was further improved by dietary cholesterol. Astaxanthin (100 mg/kg) was better than β‐carotene (250 mg/kg) as dietary pigment, measured with the color scores (SalmonFan™) |
| Wade et al. (2015) | Giant tiger prawn (Penaeus monodon) | ATX | 0, 25, 50, 100 | 42 | Pigmentation efficacy | Dietary astaxanthin supplementation (25–100 mg/kg) can both improve pigmentation of animals exposed to black substrates, and prevent the negative effects of exposure to white substrates. Average RGB colour was used to evaluate pigmentation |
| Wade et al. (2017a) | Giant tiger prawn (Penaeus monodon) | ATX | 0, 25, 50, 100 | 42 | Pigmentation efficacy | Shrimp fed astaxanthin‐free diets had significantly reduced colour (and growth) than those fed diets supplemented with ATX. Average RGB colour was used to evaluate pigmentation |
| Wang et al. (2018b) | Juvenile kuruma shrimp (Marsupenaeus japonicus) | ATX | 0, 200, 400, 800, 1,200, 1,600 | 56 | Tissue deposition, pigmentation | The ATX content of whole shrimp increased with increasing ATX supplementation levels. The authors suggested that the optimal level of ATX for pigmentation to enhance the performance of juvenile kuruma shrimp is approximately 400 mg astaxanthin/kg diet |
| Wang et al. (2018c) | Chinese mitten crab (Eriocheir sinensis) | ATX | 0, 68 | 28 | Carapace pigmentation | The ATX was measured in the carapace of control and treated animals. The results indicated that ATX concentration was significantly higher (p < 0.05) in the treated animals and improved crab body colour |
| Yamada et al. (1990) | Prawn (Penaeus japonicas) | ATX | 0, 50, 100, 200, 400 | 56 | Tissue deposition | Total carotenoid and astaxanthin ester concentrations in tissues increased with increasing dietary astaxanthin level up to 200 mg/kg. Dietary astaxanthin was incorporated into body tissues at a higher rate than ß‐carotene or canthaxanthin |
| Zhang et al. (2013) | Pacific white shrimp (Litopenaeus vannamei) | ATX | 0, 25, 50, 75, 100, 125, 150 | 56 | ATX deposition in shell | Dietary ATX was significantly (p < 0.05) retained in the shell of Pacific white shrimp fed astaxanthin levels of 25–150 mg/kg for 56 days as compared to controls. Highest effect already reached at 50 mg/kg |
Overall, all studies show that ATX is an effective pigment for crustaceans. Nine studies demonstrate this effect by colouring body or exoskeleton, five by ATX deposition in the muscle (in five study the effects were seen at 100 mg ATX or lower). In addition, in a review of Wade et al. (2017), in which publications from 1990 to 2017 has been considered, it is concluded that ATX is efficient in pigmenting several crustaceans with optimal pigmentation at levels of 50–380 mg ATX/kg diet depending on the species.
3.3.2.2. Other fish
The FEEDAP Panel assessed the publications found by the literature search on other fish and summarised the relevant information in Table 5. The six relevant studies were published between 2014 and 2018. The studies showed that addition of ATX to the diets at levels ranging between 37.5 (not clear if this was a positive effect) and 350 mg/kg significantly increased the coloration (redness) of fish flesh or skin.
Table 5.
Summary of efficacy studies with ATX in other fish
| Author(s) | Other fish | ATX (mg/kg feed) | Days | Results |
|---|---|---|---|---|
| Gopan et al. (2018) | Striped catfish (Pangasianodon hypophthalmus) | 0, 150, 300 | 45 | The fillet colour exhibited significantly higher (p < 0.05) intensity of redness (a*) in the groups fed astaxanthin |
| Grassi et al. (2016) | Tilapia (Oreochromis niloticus) | 0, 350 | 80 | The redness (a*) of tilapia fillets as well as carotenoid content of the flesh was significantly enhanced (p < 0.05) when fish were fed ATX |
| Pham et al. (2014) | Juvenile olive flounder, (Paralichthys olivaceus) | 0, 100, 200 | 56 | Total carotenoids in dorsal muscle, skin and whole body of fish fed ATX increased significantly as compared to controls. Also, skin redness values (a*) were significantly higher as compared to controls |
| Yi et al. (2014) | Large yellow croaker (Larimichthys crocea) | 0, 37.5, 75 | 63 | The results suggested that overall ATX was effective in improving skin colour as compared to controls. Redness (a*) values of the ventral skin tended to increase during the experiment, while this phenomena did not appear on the dorsal skin |
| Yi et al. (2015) | Large yellow croaker (Larimichthys crocea) | 0, 75 | 56 | Redness (a*) values of ventral skin and yellowness (b*) values of ventral and dorsal skin was significantly higher by ATX treatment |
| Yi et al. (2018) | Large yellow croaker (Larimichthys crocea) | 25, 50 | 70 | Redness of ventral skin was significantly improved by 50 mg ATX compared to 25 mg/kg |
3.3.2.3. Conclusions on efficacy
Since the conditions of authorisation of the additive remain unchanged for salmon and trout, there is no need for assessing the efficacy of the additive for these target species. Therefore, it is concluded that ATX‐DMDS is efficacious in colouring the flesh of salmonids.
Based on the new data submitted, it is concluded that ATX‐DMDS is effective in colouring the flesh and skin of other fish and the body or exoskeleton of crustaceans at the proposed conditions of use.
4. Conclusions
The applicant has provided evidence that ATX‐dimethyldisuccinate currently on the market complies with the conditions of authorisation for salmon and trout.
Orally administrated ATX‐DMDS is hydrolysed and converted to free ATX in the intestine of fish, then absorbed, metabolised and distributed in the same manner as free ATX.
ATX and ATX‐DMDS are safe for salmonids up to 100 mg ATX/kg complete diet corresponding to 138 mg ATX‐DMDS/kg. This conclusion is extrapolated to other fish. ATX at a maximum concentration of 100 mg/kg complete feed is safe for crustaceans with a margin of safety of at least 8. This conclusion covers also ATX from ATX‐DMDS and therefore the safety of 138 mg ATX‐DMDS/kg complete feed. The FEEDAP Panel has no reservation to the deletion of the provision that limits the age of use of ATX/ATX‐DMDS in salmon and trout.
The FEEDAP Panel re‐assessed the toxicological profile of ATX based on data already considered in 2014, the literature review performed by the applicant and the data available in the context of an EFSA public call for data on ATX. ATX is neither mutagenic nor carcinogenic. The FEEDAP Panel established an ADI of 0.2 mg astaxanthin/kg bw per day by applying an uncertainty factor of 200 to a LOAEL of 40 mg/kg bw per day for the increased incidence of multinucleated hepatocytes observed in a 2‐year carcinogenicity study. This ADI replaces the one of 0.034 mg/kg bw established by the FEEDAP Panel in 2014. The use of ATX‐DMDS in the nutrition of salmonids, other fish and crustaceans up to the maximum permitted dietary level is of no concern for the safety of the consumer.
The FEEDAP Panel concludes that there is no new evidence that would lead the Panel to reconsider its previous conclusions on the safety for the user. No dermal or ocular risk for the users is likely to occur under practical conditions. In the absence of inhalation toxicology study, the Panel is not in the position to establish the inhalation toxicity of the additive. Due to the susceptibility of the active substance to oxidation, the additive will be placed in the market only in the form of preparations. The FEEDAP Panel recognises that once authorised, multiple formulations of the additive can be placed in the market, and consequently, not all preparations can be directly tested for user safety.
The use of synthetic ATX‐DMDS does not pose a significant additional risk to the environment compared with natural astaxanthin.
ATX‐DMDS is efficacious in colouring the flesh of salmonids and other fish. ATX‐DMDS is an effective pigment for crustaceans at the proposed conditions of use.
Documentation provided to EFSA and Chronology
| Date | Event |
|---|---|
| 18/05/2017 | Dossier received by EFSA |
| 16/06/2017 | Reception mandate from the European Commission |
| 19/10/2017 | Application validated by EFSA – Start of the scientific assessment |
| 19/01/2018 | Comments received from Member States |
| 16/02/2018 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 27/04/2018 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issues: Characterisation, Safety for the target species, Efficacy |
| 31/07/2018 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 13/11/2019 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism, excretion
- ATCC
American Type Culture Collection
- ATX
astaxanthin
- ATX‐DMDS
astaxanthin‐dimethyldisuccinate
- BMD
benchmark dose
- BMDL10
benchmark dose lower confidence limit for a 10% extra risk
- BMR
benchmark dose response
- CAS
Chemical Abstracts Service
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- GLP
Good laboratory practice
- HPLC‐UV/VIS
high‐performance liquid chromatography coupled with UV/VIS detection
- HRP
highest reliable percentile
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- LC50
lethal concentration, 50%
- LOAEL
lowest observed adverse effect level
- LOQ
limit of quantification
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- RAC
raw agricultural commodities
- UF
uncertainty factor
- VICH
Registration of Veterinary Medicinal Products
Appendix A – List of references retrieved from the literature search provided by the applicant
1.
Safety for the target species
Brizio P, Benedetto A, Righetti M, Prearo M, Gasco L, Squadrone S and Abete MC, 2013. Astaxanthin and canthaxanthin (xanthophyll) as supplements in rainbow trout diet: in vivo assessment of residual levels and contributions to human health. Journal of Agricultural and Food Chemistry, 61, 10954–10959.
Chien Y‐H and Shiau W‐C, 2005. The effects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn, Marsupenaeus japonicus Bate. Journal of Experimental Marine Biology and Ecology, 318, 201–211.
Chithambaran S and Ayaril NK, 2018. Effect of synthetic astaxanthin, Lucantin on colour and physical quality of Indian white prawn, Fenneropenaeus indicus. Current Science, 114, 2558–2560.
Chuchird N, Rorkwiree P and Rairat T, 2015. Effect of dietary formic acid and astaxanthin on the survival and growth of Pacific white shrimp (Litopenaeus vannamei) and their resistance to Vibrio parahaemolyticus. SpringerPlus, 4, art. no. 440, 12 p.
Daly B, Swingle JS and Eckert GL, 2013. Dietary astaxanthin supplementation for hatchery‐cultured red king crab, Paralithodes camtschaticus, juveniles. Aquaculture Nutrition, 19, 312–320.
Díaz AC, Velurtas SM, Espino ML and Fenucci JL, 2014. Effect of dietary astaxanthin on free radical scavenging capacity and nitrite stress tolerance of postlarvae shrimp, Pleoticus muelleri. Journal of Agricultural and Food Chemistry, 62, 12326–12331.
Flores M, Díaz F, Medina R, Re AD and Licea A, 2007. Physiological, metabolic and haematological responses in white shrimp Litopenaeus vannamei (Boone) juveniles fed diets supplemented with astaxanthin acclimated to low‐salinity water. Aquaculture Research, 38, 740–747.
Han T, Li X, Wang J, Wang C, Yang M and Zheng P, 2018. Effects of dietary astaxanthin (AX) supplementation on pigmentation, antioxidant capacity and nutritional value of swimming crab, Portunus trituberculatus. Aquaculture, 490, 169–177.
Huang J‐H, Jiang S‐G, Lin H‐Z, Zhou F‐L and Ye L, 2008. Effects of dietary highly unsaturated fatty acids and astaxanthin on the fecundity and lipid content of pond‐reared Penaeus monodon (Fabricius) broodstock. Aquaculture Research, 39, 240–251.
Ju ZY, Deng D‐F, Dominy WG and Forster IP, 2011. Pigmentation of Pacific White Shrimp, Litopenaeus vannamei, by Dietary Astaxanthin Extracted from Haematococcus pluvialis. Journal of the World Aquaculture Society, 42, 633–644.
Liu X, Wang B, Li Y, Wang L and Liu J, 2018. Effects of dietary botanical and synthetic astaxanthin on E/Z and R/S isomer composition, growth performance, and antioxidant capacity of white shrimp, Litopenaeus vannamei, in the nursery phase. Invertebrate Survival Journal, 15, 131–140.
Long X, Wu X, Zhao L, Liu J and Cheng Y, 2017. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab, Eriocheir sinensis. Aquaculture, 473, 545–553.
Niu J, Tian L‐X, Liu Y‐J, Yang H‐J, Ye C‐X, Gao W and Mai K‐S, 2009. Effect of dietary astaxanthin on growth, survival, and stress tolerance of postlarval shrimp, Litopenaeus vannamei. Journal of the World Aquaculture Society, 40, 795–802.
Niu J, Li C‐H, Liu Y‐J, Tian L‐X, Chen X, Huang Z and Lin H‐Z, 2012. Dietary values of astaxanthin and canthaxanthin in Penaeus monodon in the presence and absence of cholesterol supplementation: effect on growth, nutrient digestibility and tissue carotenoid composition. British Journal of Nutrition, 108, 80–91.
Niu J, Wen H, Li C‐H, Liu Y‐J, Tian L‐X, Chen X, Huang Z and Lin H‐Z, 2014. Comparison effect of dietary astaxanthin and β‐carotene in the presence and absence of cholesterol supplementation on growth performance, antioxidant capacity and gene expression of Penaeus monodon under normoxia and hypoxia condition. Aquaculture, 422–423, 8–17.
Paibulkichakul C, Piyatiratitivorakul S, Sorgeloos P and Menasveta P, 2008. Improved maturation of pond‐reared, black tiger shrimp (Penaeus monodon) using fish oil and astaxanthin feed supplements. Aquaculture, 282, 83–89.
Pei S, Guan Y and Ma Y, 2009. Effect of dietary supplementation of astaxanthin on growth, survival and antioxidant capacity of pacific white shrimp (Litopenaeus vannamei) Shuichan Kexue, 28, 126–129.
Rama S and Manjabhat SN, 2014. Protective effect of shrimp carotenoids against ammonia stress in common carp, Cyprinus carpio. Ecotoxicology and Environmental Safety, 107, 207–213.
Tizkar B, Seidavi A, Ponce‐Palafox JT and Pourashoori P, 2014. The effect of astaxanthin on resistance of juvenile prawns Macrobrachium nipponense (Decapoda: Palaemonidae) to physical and chemical stress. Revista de Biologia Tropical, 62, 1331–1342.
Wade NM, Budd A, Irvin S and Glencross BD, 2015. The combined effects of diet, environment and genetics on pigmentation in the Giant Tiger Prawn, Penaeus monodon. Aquaculture, 449, 78–86.
Wade NM, Cheers S, Bourne N, Irvin S, Blyth D and Glencross BD, 2017. Dietary astaxanthin levels affect colour, growth, carotenoid digestibility and the accumulation of specific carotenoid esters in the Giant Tiger Shrimp, Penaeus monodon. Aquaculture Research, 48, 395–406.
Wang W, Ishikawa M, Koshio S, Yokoyama S, Dawood MAO and Zhang Y, 2018a. Effects of dietary astaxanthin supplementation on survival, growth and stress resistance in larval and post‐larval kuruma shrimp, Marsupenaeus japonicus. Aquaculture Research, 49, 2225–2232.
Wang W, Ishikawa M, Koshio S, Yokoyama S, Sakhawat Hossain M and Moss AS, 2018b. Effects of dietary astaxanthin supplementation on juvenile kuruma shrimp, Marsupenaeus japonicus. Aquaculture, 491, 197–204.
Wang Z, Cai C‐F, Cao X‐M, Zhu J‐M, He J, Wu P and Ye Y‐T, 2018c. Supplementation of dietary astaxanthin alleviated oxidative damage induced by chronic high pH stress, and enhanced carapace astaxanthin concentration of Chinese mitten crab Eriocheir sinensis. Aquaculture, 483, 230–237.
Xie S, Fang W, Wei D, Liu Y, Yin P, Niu J and Tian L, 2018. Dietary supplementation of Haematococcus pluvialis improved the immune capacity and low salinity tolerance ability of post‐larval white shrimp, Litopenaeus vannamei. Fish and Shellfish Immunology, 80, 452–457.
Zhang J, Liu Y‐J, Tian L‐X, Yang H‐J, Liang G‐Y, Yue Y‐R and Xu D‐H, 2013. Effects of dietary astaxanthin on growth, antioxidant capacity and gene expression in Pacific white shrimp Litopenaeus vannamei. Aquaculture Nutrition, 19, 917–927.
Toxicology and Consumer exposure and User safety
Andersen L, Holck S, Kupcinskas L, Kiudelis G, Jonaitis L, Janciauskas D, Permin H and Wadstrom T, 2007. Gastric inflammatory markers and interleukins in patients with functional dyspepsia treated with astaxanthin. FEMS Immunology and Medical Microbiology, 50, 244e248.
Beilstein P and Vogel J, 2010. Local lymph node assay (LLNA) in mice with Carophyll® Pink 10%‐CWS, Harlan CCR Study Number 1359700, DSM RDR Number 00008252.
Buesen R, Schulte S, Strauss V, Treumann S, Becker M, Groters S, Carvalho S and van Ravenzwaay B, 2015. Safety assessment of [3S,3′S]‐astaxanthin ‐ sub‐chronic toxicity study in rats. Food Chemical and Toxicology, 81, 129e136.
Chen J‐T and Kotani K, 2016. Astaxanthin as a potential protector of liver function: a review. Journal of Clinical and Medical Research, 8, 701–704.
Coombes JS, Sharman JE and Fassett RG, 2016. Astaxanthin has no effect on arterial stiffness, oxidative stress, or inflammation in renal transplant recipients: a randomized controlled trial (the XANTHIN trial). American Journal of Clinical Nutrition, 103, 283–289.
Edwards JA, Bellion P, Beilstein P, Ruembeli R and Schierle, 2016. Review of genotoxicity and rat carcinogenicity investigations with astaxanthin. Regulatory toxicology and pharmacology (RTP). 73, 819–828.
EFSA (European Food Safety Authority), 2007. Safety and efficacy of CAROPHYLL® Stay‐Pink (astaxanthin dimethyldisuccinate) as feed additive for salmon and trout, Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed. EFSA Journal 2007;5(11):574, 25 pp. https://doi.org/10.2903/j.efsa.2007.574
EFSA (European Food Safety Authority), 2014a. Scientific opinion on the safety and efficacy of astaxanthin (CAROPHYLL® Pink synthetic 10% CWS) for salmonids and ornamental fish, EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). EFSA Journal 2014;12(6):3725, 33 pp. https://doi.org/10.2903/j.efsa.2014.3725. CODEN: EJFOA6; ISSN: 1831‐4732.
EFSA (European Food Safety Authority), 2014b. Scientific opinion on the safety and efficacy of synthetic astaxanthin as feed additive for salmon and trout, other fish, ornamental fish, crustaceans and ornamental birds. EFSA panel on additives and products or substances used in animal feed. EFSA Journal 2014;12(6):3724, 35 pp. https://doi.org/10.2903/j.efsa.2014.3724. CODEN: EJFOA6; ISSN: 1831‐4732.
EFSA (European Food Safety Authority), 2014c. Scientific opinion on the safety of astaxanthin‐rich ingredients (AstaREAL A1010 and AstaREAL L10) as novel food ingredients. EFSA Journal 2014;12(7):3757, 35 pp. https://doi.org/10.2903/j.efsa.2014.3757
FDA (Food and Drug Administration), 2009. U.S. Food and Drug Administration, Federal Register, 74, 57248–57251. CODEN: FEREAC. ISSN: 0097‐6326.
Fuji Chemical Industry Co., Ltd, 2009. Notification of GRAS Determination for Haematococcus pluvialis Extract Characterized by Component Astaxanthin Esters (of Common Edible Fatty Acid). Filed by U.S. FDA as GRAS Notice GRN No. 294. Available online: http://www.accessdata.fda.gov/scripts/fcn/gras_notices/grn0294.pdf
Hummler H and McKinney B, 1982. Embryotoxicity study in rabbits with oral administration of astaxanthin, Ro 11‐3741/000. Segment II‐teratological study. F. Hoffmann–La Roche, Basle (CH). Research Report B 0046303 of 07‐Dec‐1982.
Iwamoto T, Hosoda K, Hirano R, Kurata H, Matsumoto A, Miki W, Kamiyama M, Itakura H, Yamamoto S and Kondo K, 2000. Inhibition of low‐density lipoprotein oxidation by astaxanthin, Journal of Atherosclerosis and Thrombosis, 7, 216–222.
Katsumata T, Ishibashi T and Kyle D, 2014. A sub‐chronic toxicity evaluation of a natural astaxanthin‐rich carotenoid extract of Paracoccus carotinifaciens in rats, Toxicology Reports, 1, 582–588. CODEN: TROEF9; ISSN: 2214‐7500.
Kim JH, Chang MJ, Choi HD, Youn Y‐K, Kim JT, Oh JM and Shin WG, 2011. Protective effects of haematococcus astaxanthin on oxidative stress in healthy smokers. Journal of Medicinal Food, 14, 1469e1475.
Kupcinskas L, Lafolie P, Lignell A, Kiudelis G, Jonaitis L, Adamonis K, Andersen LP and Wadstrom T, 2008. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: a prospective, randomized, double blind, and placebo controlled study. Phytomedicine, 15(6e7), 391e399.
Nakagawa K, Kiko T, Miyazawa T, Burdeos GC, Kimura F, Satoh A and Miyazawa T, 2011. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. British Journal of Nutrition, 105, 1563e1571.
Ranga Rao A, Siew Moi P, Sarada R and Gokare AR, 2014. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Marine Drugs, 12, 128–152.
Schneider S, Mellert W, Schulte S and van Ravenzwaay B, 2016. A developmental toxicity study of 3S, 30S‐Astaxanthin in New Zealand white rabbits. Food and Chemical Toxicology, 90, 95e101.
Showalter LA, Weinman SA, Østerlie M and Lockwood SF, 2004. Plasma appearance and tissue accumulation of non‐esterified, free astaxanthin in C57BLy6 mice after oral dosing of a disodium disuccinate diester of astaxanthin (Heptax), Comparative Biochemistry and Physiology, Toxicology & Pharmacology, 137, 227–236.
Spiller GA and Dewell A, 2003. Safety of an astaxanthin‐rich Haematococcus pluvialis algal extract: a randomised clinical trial. Journal of Medicinal Food, 6, 51–56.
Stewart J, Lignell A, Pettersson A, Elfving E and Soni MG, 2008. Safety assessment of astaxanthin‐rich microalgae biomass: acute and subchronic toxicity studies in rats Food and Chemical Toxicology, 46, 3030–3036.
Tago Y, Fujii T, Wada J, Kato M, Wei M, Wanibuchi H and Kitano M, 2014. Genotoxicity and subacute toxicity studies of a new astaxanthin containing Phaffia rhodozyma extract. Journal of Toxicological Sciences, 39, 373–382. CODEN: JTSCDR; ISSN: 0388‐1350.
Turkez H, Geyikoglu F, Yousef M and Mokhtar I, 2013. Beneficial effect of astaxanthin on 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin‐induced liver injury in rats, Toxicology and Industrial Health, 29, 591–599. CODEN: TIHEEC; ISSN: 0748‐2337.
Vega K, Edwards J and Beilstein P, 2015. Subchronic (13‐week) toxicity and prenatal developmental toxicity studies of dietary astaxanthin in rats, Regulatory Toxicology and Pharmacology, 73, 819e828.
Lin Y‐J, Lin J‐Y, Wang D‐S, Chen C‐H, Chiou M‐H, 2017. Regulatory Toxicology and Pharmcology, 87, 95–105.
Takahashi J, Tsukahara H and Minato S, 2005. Toxicological studies of Astaxanthin from Haematococcus pluvialis – Ames test, oral single dose and 90‐days subchronic toxicity studies in rats. Journal of Clinical Theraphy Medicine, 20.
Efficacy in other fish and crustaceans
Gopan A, Varghese T, Sahu NP, Lalappan S, Srivastava PP, Jain KK and Ande MP, 2018. Dietary Carotenoid Supplementation Improves Fillet Appearance, Antioxidant Status and Immuneresponses in Striped Catfish (Pangasianodon hypophthalmus) Nevertheless the Growth Performance. Journal of Fisheries and Aquatic Sciences, 18, 1303–1313.
Grassi TLM, do Espírito Santo EF, de Siqueira Marcos MT, Cavazzana JF, Oliveira DL, Bossolani ILC and Ponsano EHG, 2016. Bacterial pigment for Nile tilapia feeding. Aquaculture International, 24, 647–660.
Yi X, Shen H, Li J, Wei Z, Zhang W, Mai K and Shentu J, 2018. Effects of dietary vitamin E and astaxanthin on growth, skin colour and antioxidative capacity of large yellow croaker Larimichthys crocea. Aquaculture Nutrition, 24, 472–480.
Yi X, Xu W, Zhou H, Zhang Y, Luo Y, Zhang W and Mai K, 2014. Effects of dietary astaxanthin and xanthophylls on the growth and skin pigmentation of large yellow croaker Larimichthys croceus. Aquaculture, 433, 377–383.
Yi X‐W, Li J, Xu W, Zhang Y‐J, Zhou H‐H, Zhang W‐B, Mai K‐S and Smith AA, 2015. Effects of dietary xanthophylls/astaxanthin ratios on the growth and skin pigmentation of large yellow croaker Larimichthys crocea (Richardson, 1846). Journal of Applied Ichthyology, 31, 780–786.
Pham MA, Byun H‐G, Lee S‐M and Kim K‐D, 2014. Effects of dietary carotenoid source and level on growth, skin pigmentation, antioxidant activity and chemical composition of juvenile olive flounder Paralichthys olivaceus. Aquaculture, 431, 65–72.
de Carvalho CCCR and Caramujo MJ, 2017. Carotenoids in aquatic ecosystems and aquaculture: a colorful business with implications for human health. Frontiers in Marine Science, 4, article 93, pp. 14.
Yamada S, Tanaka Y, Sameshima M and Ito Y, 1990 Pigmentation of prawn (Penaeus japonicus) with carotenoids. I. Effect of dietary astaxanthin, β‐carotene, canthaxanthin on pigmentation. Aquaculture, 87, 323–330.
Ju ZY, Deng DF, Dominy WG and Forster IP, 2011. Pigmentation of Pacific white shrimp, Litopenaeus vannamei, by dietary astaxanthin extracted from Haematococcus pluvialis. Journal of the World Aquaculture Society, 42, 633–644.
Niu J, Li CH, Chen X, Huang Z, Lin HZ, Liu YJ and Tian LX, 2012. Dietary values of astaxanthin and canthaxanthin in Penaeus monodon in the presence and absence of cholesterol supplementation: effect on growth, nutrient digestibility and tissue carotenoid composition. British Journal of Nutrition, 108, 80–91.
Zhang J, Liu YJ, Tian LX, Yang HJ, Lian GY, Yue YR and Xu DH, 2013. Effects of dietary astaxanthin on growth, antioxidant capacity and gene expression in Pacific white shrimp Litopenaeus vannamei. Aquaculture Nutrition, 19, 917–927.
Daly B, Swingle JS and Eckert GL, 2013. Dietary astaxanthin supplementation for hatchery‐cultured red king crab, Paralithodes camtschaticus, juveniles. Aquaculture Nutrition, 19, 312–320.
Niu J, Li CH, Chen X, Huang Z, Lin HZ, Liu YJ, Tian LX and Wen H, 2014. Comparison effect of dietary astaxanthin and β‐carotene in the presence and absence of cholesterol supplementation on growth performance, antioxidant capacity and gene expression of Penaeus monodon under normoxia and hypoxia condition. Aquaculture, 422–423_8–17.
Wade NM, Budd A, Irvin S and Glencross BD, 2015. The combined effects of diet, environment and genetics on pigmentation in the Giant Tiger Prawn, Penaeus monodon. Aquaculture, 449, 78–86.
Wade NM, Cheers S, Bourne N, Glencross BD, Irvin S and Blyth D, 2017a. Dietary astaxanthin levels affect colour, growth, carotenoid digestibility and the accumulation of specific carotenoid esters in the Giant Tiger Shrimp, Penaeus monodon. Aquaculture Research, 48, 395–406.
Long X, Wu X, Zhao L, Liu J and Cheng Y, 2017. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab, Eriocheir sinensis. Aquaculture, 473, 545–553.
Chithambaran S and Ayaril NK, 2018. Effect of synthetic astaxanthin, Lucantin on colour and physical quality of Indian white prawn, Fenneropenaeus indicus. Current Science, 114, 2558–2560.
Wang W, Ishikawa M, Koshio S, Yokoyama S, Sakhawat Hossain M and Moss AS, 2018a. Effects of dietary astaxanthin supplementation on juvenile kuruma shrimp, Marsupenaeus japonicus. Aquaculture, 491, 197–204.
Wang Z, Cai CF, Cao XM, Zhu JM, He J, Wu P and Ye YT, 2018b. Supplementation of dietary astaxanthin alleviated oxidative damage induced by chronic high pH stress, and enhanced carapace astaxanthin concentration of Chinese mitten crab Eriocheir sinensis. Aquaculture, 483, 230–237.
Han T, Li X, Wang J, Wang C, Yang M and Zheng P, 2018. Effects of dietary astaxanthin (AX) supplementation on pigmentation, antioxidant capacity and nutritional value of swimming crab, Portunus trituberculatus. Aquaculture, 490, 169–177.
Wade NM, Gabaudan J and Glencross BD, 2017b. A review of carotenoid utilisation and function in crustacean aquaculture. Reviews in Aquaculture, 9, 141–156.
Appendix B – Benchmark dose analysis of the incidence of multinucleated hepatocytes in female rats in a 2‐year rat study (EFSA FEEDAP Panel, 2014a)
B.1. Data description
The endpoint to be analysed is: incidence of multinucleated hepatocytes.
Data used for analysis:
| Dose | Incidence of multinucleated hepatocytes | N |
|---|---|---|
| 0 | 12 | 49 |
| 40 | 23 | 50 |
| 200 | 39 | 51 |
| 1,000 | 41 | 50 |
B.2. Selection of the BMR
The BMR used is an extra risk of 10% compared to the controls.
The BMD is the dose corresponding with the BMR of interest.
A 90% confidence interval for the BMD will be estimated, with the lower and upper bound denoted BMDL and BMDU, respectively.
B.3. Software Used
Results are obtained using the EFSA web‐tool for BMD analysis, which uses the R‐package PROAST, version 67.0, for the underlying calculations.
B.4. Results
Response variable: multinucleated hepatocytes
Fitted Models:
| Model | No.par | loglik | AIC | Accepted | BMDL | BMDU | BMD | Conv |
|---|---|---|---|---|---|---|---|---|
| Null | 1 | −1.3551e+02 | 2.7302e+02 | NA | NA | NA | NA | |
| Full | 4 | −1.1169e+02 | 2.3138e+02 | NA | NA | NA | NA | |
| two.stage | 3 | −1.2016e+02 | 2.4632e+02 | No | NA | NA | 61.800 | Yes |
| log.logist | 3 | −1.1299e+02 | 2.3198e+02 | Yes | 1.17e‐01 | 16.10 | 3.240 | Yes |
| Weibull | 3 | −1.1345e+02 | 2.3290e+02 | Yes | 5.83e‐03 | 7.92 | 0.772 | Yes |
| log.prob | 3 | −1.1307e+02 | 2.3214e+02 | Yes | 1.45e‐01 | 18.00 | 3.720 | Yes |
| gamma | 3 | −1.1382e+02 | 2.3364e+02 | Yes | 4.35e‐05 | 5.46 | 0.148 | Yes |
| logistic | 2 | −1.2213e+02 | 2.4826e+02 | No | NA | NA | 106.000 | Yes |
| probit | 2 | −1.0000e+10 | 2.0000e+10 | No | NA | NA | NA | Yes |
| LVM: Expon. m3‐ | 3 | −1.1364e+02 | 2.3328e+02 | Yes | 1.54e‐01 | 4.42 | 0.283 | Yes |
| LVM: Hill m3‐ | 3 | −1.1353e+02 | 2.3306e+02 | Yes | 8.24e‐02 | 5.78 | 0.410 | Yes |
Estimated Model Parameters
two.stage
estimate for a‐ : 0.3907
estimate for BMD‐ : 61.78
estimate for c : 1e‐06
log.logist
estimate for a‐ : 0.2386
estimate for BMD‐ : 3.243
estimate for c : 0.6379
Weibull
estimate for a‐ : 0.2383
estimate for BMD‐ : 0.7722
estimate for c : 0.3828
log.prob
estimate for a‐ : 0.2398
estimate for BMD‐ : 3.724
estimate for c : 0.3855
gamma
estimate for a‐ : 0.2398
estimate for BMD‐ : 0.1483
estimate for cc : 0.2437
logistic
estimate for a‐ : ‐0.2483
estimate for BMD‐ : 106.3
probit
estimate for a‐ : ‐0.1517
estimate for BMD‐ : 110400000
EXP
estimate for a‐ : 1.193
estimate for CED‐ : 0.2827
estimate for d‐ : 0.25
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for a‐ : 1.196
estimate for CED‐ : 0.4104
estimate for d‐ : 0.2867
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
Weights for Model Averaging
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.24 | 0.15 | 0.23 | 0.11 | 0 | 0 | 0.13 | 0.14 |
B.5. Final BMD values
| Subgroup | BMDL | BMDU |
|---|---|---|
| 0.03 | 14.4 |
Confidence intervals for the BMD are based on 200 bootstrap data sets.
B.6. Visualisation
Appendix C – Calculation of consumer exposure with FACE model
1.
Methodology
As described in the Guidance on the safety of feed additives for consumers (EFSA FEEDAP Panel, 2017), consumption data of edible tissues and products as derived from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) will be used to assess exposure to residues from the use of feed additives in different EU countries, age classes35 and special population groups. For each EU country and age class, only the latest survey available in the Comprehensive Database will be used.
While the residue data reported for feed additives refer to organs and tissues (raw agricultural commodities (RAC)), the Comprehensive Database includes consumption data for foods as consumed. In order to match those consumption data with the available residue data for feed additives, the consumption data reported in the Comprehensive Database have been converted into RAC equivalents. For assessing the exposure to coccidiostats from their use in (non‐reproductive) poultry, the following list of commodities is considered: meat, fat, liver, other offals (including kidney).
Depending on the nature of the health‐based guidance derived, either a chronic or acute exposure assessment may be required.
For chronic exposure assessments, the total relevant residues will be combined for each individual with the average daily consumptions of the corresponding food commodities, and the resulting exposures per food will be summed in order to obtain total chronic exposure at individual level (standardised by using the individual body weight). The mean and the higher percentile (usually the 95th percentile) of the individual exposures will be subsequently calculated for each dietary survey (country) and each age class separately.
As opposed to the chronic exposure assessments, acute exposure calculation will be carried out for each RAC value separately. The higher percentile (usually the 95th percentile) exposures based on the consuming days only will be calculated for each food commodity, dietary survey and age class separately.
Detailed results on chronic exposure calculation
Table C.1.
Chronic dietary exposure per population class, country and survey (mg/kg bw per day) of consumers to ATX based on residue data in salmonids and crustaceans
| Population class | Survey's country | Number of subjects | HRP1 | HRP description |
|---|---|---|---|---|
| Infants | Bulgaria | 523 | 0.0000000000 | 95th |
| Infants | Germany | 142 | 0.0155248512 | 95th |
| Infants | Denmark | 799 | 0.0405240825 | 95th |
| Infants | Finland | 427 | 0.0190985410 | 95th |
| Infants | United Kingdom | 1,251 | 0.0531743012 | 95th |
| Infants | Italy | 9 | 0.0000000000 | 50th |
| Toddlers | Belgium | 36 | 0.0298611111 | 90th |
| Toddlers | Bulgaria | 428 | 0.0766392059 | 95th |
| Toddlers | Germany | 348 | 0.0361814174 | 95th |
| Toddlers | Denmark | 917 | 0.0366057530 | 95th |
| Toddlers | Spain | 17 | 0.0129870130 | 75th |
| Toddlers | Finland | 500 | 0.0681417605 | 95th |
| Toddlers | United Kingdom | 1,314 | 0.0613005729 | 95th |
| Toddlers | United Kingdom | 185 | 0.0597246868 | 95th |
| Toddlers | Italy | 36 | 0.1458837778 | 90th |
| Toddlers | Netherlands | 322 | 0.0346398509 | 95th |
| Other children | Austria | 128 | 0.0615094340 | 95th |
| Other children | Belgium | 625 | 0.0588333333 | 95th |
| Other children | Bulgaria | 433 | 0.0811546053 | 95th |
| Other children | Czech Republic | 389 | 0.0750000000 | 95th |
| Other children | Germany | 293 | 0.0322785384 | 95th |
| Other children | Germany | 835 | 0.0371206598 | 95th |
| Other children | Denmark | 298 | 0.0380734109 | 95th |
| Other children | Spain | 399 | 0.1011072324 | 95th |
| Other children | Spain | 156 | 0.0979132401 | 95th |
| Other children | Finland | 750 | 0.0580373769 | 95th |
| Other children | France | 482 | 0.0474931832 | 95th |
| Other children | United Kingdom | 651 | 0.0519004909 | 95th |
| Other children | Greece | 838 | 0.0646364623 | 95th |
| Other children | Italy | 193 | 0.0856311139 | 95th |
| Other children | Latvia | 187 | 0.0295986661 | 95th |
| Other children | Netherlands | 957 | 0.0366291264 | 95th |
| Other children | Netherlands | 447 | 0.0268332506 | 95th |
| Other children | Sweden | 1,473 | 0.0486234375 | 95th |
| Adolescents | Austria | 237 | 0.0372759305 | 95th |
| Adolescents | Belgium | 576 | 0.0297076956 | 95th |
| Adolescents | Cyprus | 303 | 0.0304693274 | 95th |
| Adolescents | Czech Republic | 298 | 0.0538077731 | 95th |
| Adolescents | Germany | 393 | 0.0325687569 | 95th |
| Adolescents | Germany | 1,011 | 0.0190614917 | 95th |
| Adolescents | Denmark | 377 | 0.0174084859 | 95th |
| Adolescents | Spain | 651 | 0.0655118074 | 95th |
| Adolescents | Spain | 209 | 0.0709087333 | 95th |
| Adolescents | Spain | 86 | 0.0415052334 | 95th |
| Adolescents | Finland | 306 | 0.0300830578 | 95th |
| Adolescents | France | 973 | 0.0280212456 | 95th |
| Adolescents | United Kingdom | 666 | 0.0261632069 | 95th |
| Adolescents | Italy | 247 | 0.0491515872 | 95th |
| Adolescents | Latvia | 453 | 0.0282051282 | 95th |
| Adolescents | Netherlands | 1,142 | 0.0207009549 | 95th |
| Adolescents | Sweden | 1,018 | 0.0322204380 | 95th |
| Adults | Austria | 308 | 0.0365060092 | 95th |
| Adults | Belgium | 1,292 | 0.0342266640 | 95th |
| Adults | Czech Republic | 1,666 | 0.0359333554 | 95th |
| Adults | Germany | 10,419 | 0.0319010756 | 95th |
| Adults | Denmark | 1,739 | 0.0168619360 | 95th |
| Adults | Spain | 981 | 0.0597684426 | 95th |
| Adults | Spain | 410 | 0.0578441892 | 95th |
| Adults | Finland | 1,295 | 0.0434783173 | 95th |
| Adults | France | 2,276 | 0.0263795883 | 95th |
| Adults | United Kingdom | 1,265 | 0.0288574379 | 95th |
| Adults | Hungary | 1,074 | 0.0225217491 | 95th |
| Adults | Ireland | 1,274 | 0.0287095221 | 95th |
| Adults | Italy | 2,313 | 0.0400171696 | 95th |
| Adults | Latvia | 1,271 | 0.0354377498 | 95th |
| Adults | Netherlands | 2,055 | 0.0282548759 | 95th |
| Adults | Romania | 1,254 | 0.0271502976 | 95th |
| Adults | Sweden | 1,430 | 0.0459766212 | 95th |
| Elderly | Austria | 67 | 0.0345729695 | 95th |
| Elderly | Belgium | 511 | 0.0358173077 | 95th |
| Elderly | Germany | 2,006 | 0.0366768662 | 95th |
| Elderly | Denmark | 274 | 0.0192436576 | 95th |
| Elderly | Finland | 413 | 0.0465412562 | 95th |
| Elderly | France | 264 | 0.0281298642 | 95th |
| Elderly | United Kingdom | 166 | 0.0316339246 | 95th |
| Elderly | Hungary | 206 | 0.0145209293 | 95th |
| Elderly | Ireland | 149 | 0.0338966568 | 95th |
| Elderly | Italy | 289 | 0.0431951120 | 95th |
| Elderly | Netherlands | 173 | 0.0403912914 | 95th |
| Elderly | Netherlands | 289 | 0.0356367293 | 95th |
| Elderly | Romania | 83 | 0.0335699176 | 95th |
| Elderly | Sweden | 295 | 0.0555303830 | 95th |
| Very elderly | Austria | 25 | 0.0000000000 | 75th |
| Very elderly | Belgium | 704 | 0.0375991717 | 95th |
| Very elderly | Germany | 490 | 0.0370098039 | 95th |
| Very elderly | Denmark | 12 | 0.0153862800 | 75th |
| Very elderly | France | 84 | 0.0282704768 | 95th |
| Very elderly | United Kingdom | 139 | 0.0333656481 | 95th |
| Very elderly | Hungary | 80 | 0.0131720430 | 95th |
| Very elderly | Ireland | 77 | 0.0291886235 | 95th |
| Very elderly | Italy | 228 | 0.0309811432 | 95th |
| Very elderly | Netherlands | 450 | 0.0362331101 | 95th |
| Very elderly | Romania | 45 | 0.0208333333 | 90th |
| Very elderly | Sweden | 72 | 0.0417399856 | 95th |
HRP: highest reliable percentile, i.e. the highest percentile that is considered statistically robust for combinations of dietary survey, age class and possibly raw primary commodity, considering that a minimum of 5, 12, 30 and 61 observations are respectively required to derive 50th, 75th and 90th and 95th percentile estimates. Estimates with less than 5 observations were not included in this table.
Appendix D – Estimation of user exposure to ATX‐DMDS from the additive Carophyll® Stay‐Pink 10%‐CWS
1.
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | ATX‐DMDS in the dust (mg/g) | 100 | Technical dossier | |
| b | Dusting potential (g/m3) | 1 | Technical dossier | |
| a × b | c | ATX‐DMDS in the air (mg/m3) | 10 | |
| d | No of premixture batches prepared/working day | 8 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012) | |
| e | Time of exposure per production of one batch (s) | 20 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012) | |
| d × e | f | Total duration of daily exposure/worker (s) | 160 | |
| g | Uncertainty factor | 2 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012) | |
| f × g | h | Refined total duration of daily exposure/worker (s) | 320 | |
| h/3 600 | i | Refined total duration of daily exposure (h) | 0.11 | |
| j | Inhaled air per hour (m3) | 1.25 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012) | |
| j × i | k | Inhaled air during exposure (m3) | 0.11 | |
| c × k | l | ATX‐DMDS inhaled during exposure per eight‐hour working day (mg) | 1.11 |
ATX‐DMDS: astaxanthin‐dimethyldisuccinate.
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Methods of Analysis for astaxanthin dimethyldisuccinate
1.
In the current application, authorisation is sought under articles 4(1), 13 and 14 for astaxanthin dimethyldisuccinate under the category/functional group (2 a) ‘sensory additives’/‘colourants’, according to the classification system of Annex I of Regulation (EC) No 1831/2003. Specifically, the feed additive is sought to be used for salmon, trout, crustaceans and other fish.
The feed additive, which is already authorised by Commission Regulation (EC) No 393/2008, is to be marketed as Carophyll® Stay‐Pink 10%‐CWS. This is a brown to violet‐red free flowing powder formulation, consisting of a minimum of 13.8% astaxanthin dimethyldisuccinate (equivalent to a minimum of 10% astaxanthin) and formulated in an organic matrix. In addition, maximum limits for triphenylphospine oxide (100 mg/kg) and dichloromethane (600 mg/kg) are specified for the feed additive.
The feed additive is intended to be incorporated directly into feedingstuffs or through premixtures with a proposed maximum astaxanthin dimethyldisuccinate content of 138 mg/kg feedingstuffs. If the feed additive is mixed with other sources of astaxanthin, the total maximum dose of astaxanthin equivalent is set to 100 mg/kg feedingstuffs.
For the quantification of the total astaxanthin dimethyldisuccinate in the feed additive the Applicant submitted a single‐laboratory validated and further verified method based on spectrophotometry at 486 nm wavelength. The following performance characteristics were reported: a precision (relative standard deviations for repeatability – RSDr and intermediate precision – RSDip) ranging from 0.3% to 1.2%; and a recovery rate (Rrec) ranging from 98% to 103%. Based on the acceptable performance characteristics available, the EURL recommends this method for official control.
For the quantification of the total astaxanthin dimethyldisuccinate in the feed additive, premixtures and feedingstuffs the Applicant submitted another single‐laboratory validated and further verified method based on normal phase high‐performance liquid chromatography coupled with UV/VIS detection (HPLC‐UV/VIS). The following performance characteristics were reported: a precision (RSDr and RSDip) ranging from 0.7% to 3.9%; Rrec ranging from 98% to 105%; and a limit of quantification (LOQ) ranging from 0.2 to 2 mg astaxanthin dimethyldisuccinate (expressed as astaxanthin equivalent)/kg feedingstuffs. Based on the acceptable performance characteristics presented, the EURL recommends this method for official control.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005, as last amended by Regulation (EU) 2015/1761) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Bories G, Brantom P, Renshaw D, Schlatter JR, Ackerl R, Holczknecht O, Steinkellner H, Vettori MV and Gropp J, 2019. Scientific Opinion on the safety and efficacy of astaxanthin‐dimethyldisuccinate (Carophyll® Stay‐Pink 10%‐CWS) for salmonids, crustaceans and other fish. EFSA Journal 2019;17(12):5920, 42 pp. 10.2903/j.efsa.2019.5920
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00499
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Maryline Kouba, Mojca Kos Durjava, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Acknowledgements: The Panel wishes to thank Bruno Dujardin, José Cortiñas Abrahantes, Jaume Galobart, Wolfgang Gelbmann and Paola Manini for the support provided to this scientific output.
Adopted: 13 November 2019
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
DSM Nutritional Products Ltd. (Switzerland), represented by DSM Nutritional Products Spz.o.o. (Poland), Tarczynska 113, 96–320, Mszczonow, Poland.
Commission Regulation (EC) No 393/2008 of 30 April 2008 concerning the authorisation of astaxanthin‐dimethyldisuccinate as a feed additive. OJ L 117, 1.5.2008, p. 20.
Commission Implementing Regulation (EU) 2015/1415 of 20 August 2015 concerning the authorisation of astaxanthin as a feed additive for fish, crustaceans and ornamental fish, OJ L 220, 21.8.2015, p. 7.
Commission Regulation (EC) No 828/2007 of 13 July 2007 concerning the permanent and provisional authorisation of certain additives in feedingstuffs. OJ L 184, 14.7.2007, p. 12.
Commission Regulation (EC) No 721/2008 of 25 July 2008 concerning the authorisation of a preparation of red carotenoid‐rich bacterium Paracoccus carotinifaciens as feed additives. OJ L 193, 26.7.2008, p. 23.
FEED dossier reference: FAD‐2017‐0029.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep-fad-2017-0029-astaxanthin-dimethyldisuccinate.pdf
EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. https://doi.org/10.2903/j.efsa.2012.2534
■■■■■
Technical dossier/Section II/Appendix 2‐9.
■■■■■
http://www.vichsec.org/guidelines/pharmaceuticals/pharma-quality/impurities.html VICH limits: methylene chloride < 600 mg/kg, pyridine < 200 mg/kg, methanol < 3,000 mg/kg).
Technical dossier/Section II.
Technical dossier/Section II/Appendix 2‐10 and 2‐33; LOD: 18 ppm.
Technical dossier/Section II/Appendix 2‐11.
Technical dossier/Section II/Appendix 2‐31.
Technical dossier/Section II/Appendix 2‐4.
Without added soy oil and fish oil, representing 82% of the extruded feed.
Technical dossier/Supplementary information July 2018/Annex S/S5 and S15.
Technical dossier/Section III/Appendix 3‐5 (Appendix 1 and 2). Databases searched: RTECS, CAS Databases, TOXCENTER and SCOPUS.
Technical dossier/Supplementary information July 2018/Annexes S.
Call for data relevant to the safety assessment of astaxanthin in the framework of Regulation (EU) 2015/2283 (http://www.efsa.europa.eu/en/consultations/call/180725).
The interested parties who shared their data have authorised EFSA to use the concerned data for the purpose of the present scientific opinion.
Technical dossier/Section III/Annex III_9 (Annex S031454 and Annex S031476). Data bases searched: STN/RTECS, Scopus, and ToxNet/ToxLine.
Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001.
FAD‐2010‐0080 – Technical dossier/Section III/Annex 10.
FAD‐2010‐0080 – Technical dossier/Section III/Annex 7.
FAD‐2010‐0080 – Technical dossier/Section III/Annex 12.
FAD‐2010‐0080 – Technical dossier/Section III/Annex 11.
Technical dossier/Section III/Annex II.9; Time span: 2007–2017.
Technical dossier/Section III/Annex II.9; Time span: 2014–2017.
Technical dossier/Section III/Appendix 3‐13 (Appendix 1 and 2). Databases searched: RTECS, CAS Databases, TOXCENTER and SCOPUS.
Technical dossier/Supplementary information July 2018/Annexes E.
Infants: < 12 months old, toddlers: ≥ 12 months to < 36 months old, other children: ≥ 36 months to < 10 years old, adolescents: ≥ 10 years to < 18 years old, adults: ≥ 18 years to < 65 years old, elderly: ≥ 65 years to < 75 years old, and very elderly: ≥ 75 years old.
References
- EFSA (European Food Safety Authority), 2004. Scientific Opinion on environmental impact of Astaxanthin‐rich Phaffia rhodozyma (Ecotone®) as feed additive in accordance with Council Directive 70/524/EEC. EFSA Journal 2004;2(4):43, 4 pp. 10.2903/j.efsa.2004.43 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Scientific Opinion on the request from the European Commission on the safety of use of colouring agents in animal nutrition. PART I. General principle and Astaxanthin. EFSA Journal 2005;3(12):291, 40 pp. 10.2903/j.efsa.2005.291 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Scientific Opinion on a request from the European Commission on the safety and efficacy of the product AQUASTA, an Astaxanthin‐rich Phaffia rhodozyma ATCC SD‐5340 for salmon and trout. EFSA Journal 2006;4(2):320, 19 pp. 10.2903/j.efsa.2006.320 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007a. Scientific Opinion on the safety and efficacy of CAROPHYLL® Stay‐Pink (astaxanthin dimethylsuccinate) as feed additive for salmon and trout. EFSA Journal 2007;5(11):574, 25 pp. 10.2903/j.efsa.2007.574 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007b. Scientific Opinion on the safety and efficacy of Panaferd‐AX (red carotenoid‐rich bacterium Paracoccus carotinifaciens as feed additive for salmon and trout. EFSA Journal 2007;5(10):546, 30 pp. 10.2903/j.efsa.2007.546 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2010. Scientific Opinion on modification of the terms of authorisation of a red carotenoid‐rich bacterium Paracoccus carotinifaciens (Panaferd‐AX) as feed additive for salmon and trout. EFSA Journal 2010;8(1):1428. 10.2903/j.efsa.2010.1428 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Technical guidance: tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (Panel on Additives and Products or Substances used in Animal Feed), 2013. Guidance on the renewal of the authorisation of feed additives. EFSA Journal 2013;11(10):3431, 8 pp. 10.2903/j.efsa.2013.3431 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014a. Scientific Opinion on the safety and efficacy of astaxanthin (CAROPHYLL® Pink 10% CWS) for salmonids and ornamental fish. EFSA Journal 2014;12(6):3725, 33 pp. 10.2903/j.efsa.2014.3725 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014b. Scientific Opinion on the safety and efficacy of synthetic astaxanthin as feed additive for salmon and trout, other fish, ornamental fish, crustaceans and ornamental birds. EFSA Journal 2014;12(6):3724, 35 pp. 10.2903/j.efsa.2014.3724 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Update: guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [Google Scholar]
- Gradelet S, Astorg P, Pineau T, Canivenc MC, Siess MH, Leclerc J and Lesca P, 1997. Ah receptor‐dependent CYP1A induction by two carotenoids, canthaxanthin and β‐apo‐8′‐carotenal with no affinity for the TCDD binding site. Biochemical Pharmacology, 54, 307–315. [DOI] [PubMed] [Google Scholar]
- Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knippel A, Kuttler K, Malarkey DE, Maronpot RR, Nishikawa A, Notte T, Schlte A, Strauss V and York MJ, 2012. Liver hypertrophy: a review of adaptive (adverse and non‐adverse) changes – conclusions from the 3rd International ESTP Expert Workshop. Toxicologic Pathology, 40, 971–994. [DOI] [PubMed] [Google Scholar]
- Paolini M, Antelli A, Pozzetti L, Spetlova D, Perocco P, Valgimigli L, Pedulli GF and Cantelli‐Forti G, 2001. Induction of cytochrome P450 enzymes and over‐generation of oxygen radicals in b‐carotene supplemented rats. Carcinogenesis, 22, 1483–1495. [DOI] [PubMed] [Google Scholar]


