Abstract
Following a request from the European Commission, the EFSA Panel on Plant Health performed a risk assessment of the entry of Pantoea stewartii subsp. stewartii on maize seed imported by the EU from the USA. This pest is a Gram‐negative bacterium which causes Stewart's vascular wilt and leaf blight of maize (including sweet corn), a disease responsible for serious crop losses throughout the world. The following scenarios were considered: scenario A0 (current practice), scenario A1 (US request for modification of EU conditions for derogation), and scenario A2 (EU conditions for derogation). Results from the quantitative seed pathway model presented here show that, despite the low rates of plant‐to‐seed and seed‐to‐seedling transmission that have been reported in the literature for Stewart's wilt, given the amount of traded seed, and in the case of voluntary (i.e. not mandatory) inspections of seed production fields at the origin (i.e. scenario A0), the frequency of introducing the disease is in the order of magnitude of some hundred introductions per year (median number). The EU conditions for derogation would lead to a decrease in the likelihood of entry compared to scenarios A0 (about 10,000 times fewer introductions) and A1 (about 2,000 times fewer introductions). This protective effect is mainly due to the requirement that only genotypes resistant to Stewart's wilt are traded, with the additional field inspection (two instead of one per season) providing additional reassurance. The Panel also concluded that seed lot inspections, as currently carried out (e.g. with a sample of 400 seeds) are not likely to lead to a relevant reduction in the level of infected imported maize seed, given the low prevalence of Stewart's wilt at the origin. If, however, there is aggregation in infection among consignments, inspection would work towards identifying the highly infected consignments. Recently, outbreaks of Stewart's wilt have occurred in Italy (Emilia Romagna, Friuli, Lombardy and Veneto). A review is provided of the available information to assess the possible role of seed imports in these outbreaks.
Keywords: bacterial plant pathogens, pathway model, pest prevalence, phytosanitary measures, quantitative risk assessment, seed lot sampling, transmission rate, uncertainty
Summary
Following a request from the European Commission, the European Food Safety Authority (EFSA) Plant Health Panel performed a risk assessment of the entry of Pantoea stewartii subsp. stewartii (hereafter P. s. subsp. stewartii) on maize seed imported by the European Union (EU) from the USA. This pest is a Gram‐negative bacterium which causes Stewart's vascular wilt and leaf blight of maize (including sweet corn), a disease responsible for serious crop losses throughout the world.
EFSA was requested to assess whether the information provided by the USA is sufficient to consider, whether the schema of field inspections suggested by the USA would provide a level of protection against the introduction of E. stewartii (the pest previously belonged to the genus Erwinia) via seeds of Zea mays which is equivalent to the one stipulated in Annex IVA, section I, point 52 of Council Directive 2000/29/EC. Following the EFSA pest categorisation, the current opinion addresses the likelihood of entry of P. s. subsp. stewartii, while Council Directive 2000/29/EC refers to the old name.
The Panel interpreted the Terms of Reference (ToR) as a request to perform a partial risk assessment focusing on the likelihood of entry of P. s. subsp. stewartii on the pathway of maize seed imported by the EU from the USA. The likelihood of establishment, spread and impacts was thus not addressed.
The pathogen is currently regulated in Council Directive 2000/29/EC (Annex IIAI) as a harmful organism whose introduction and spread in the EU is banned if present on seeds of Zea mays. Annex IVAI requires for import of maize seeds into the EU an official statement that:
-
a)
the seeds originate in areas known to be free from the pathogen; or
-
b)
a representative sample of the seeds has been tested and found free from the pathogen.
The current practice is elaborated in a scenario A0. Under this scenario, field inspections at the origin are voluntary, not mandatory.
For derogation to the USA to be granted, the European Commission (Letter to the USA of 22 June 2016, see section 1.2.3) proposed that 4 conditions would need to be met to achieve the expected level of protection in the EU:
-
a)
maize seeds have been produced in fields which have been registered and inspected by APHIS;
-
b)
those inspections have been carried out both early in the growing season and during late season, and it has been concluded that P. s. subsp. stewartii is absent in the inspected field and the adjacent fields are situated in areas where the prevalence of P. s. subsp. stewartii is known to be low;
-
c)
they are produced from resistant seed varieties;
-
d)
the fields of production are subject to appropriate seed treatments against vectors of the pest.
The Panel interpreted this requirement as a combination of Risk Reduction Options (RROs) and thus a single scenario (see Section 2.2.3). This is scenario A2.
USDA APHIS requested that derogation would be granted under a selection of the RROs proposed by EU (Letter of the USA to the European Commission of 30 October 2017, see section 1.2.3). To summarise:
maize seeds have been produced in fields which have been registered and inspected by APHIS;
those inspections have been carried out once; and
the fields of production are subject to appropriate seed treatments against vectors of the pest.
The Panel developed a single scenario to assess the risk under the options specified in the USA request for derogation (see Section 2.2.3). This is scenario A1, intermediate in its requirements between scenarios A0 and A2.
Furthermore, to disentangle the effect of restricting trade to resistant genotypes from the effect of an additional field inspection (these two RROs in A2 are both absent in A1), two sub‐scenarios were distinguished. These are called scenario A2a (conditions (a), (c) and (d) of EU: resistant seed and only one inspection) and A2b (conditions (a), (b) and (d) of EU: both resistant and susceptible seed, and two inspections). These two sub‐scenarios (A2a and A2b) are distinct from scenario A2.
The only pathway of entry considered was the import by the EU of maize seed (including sweet corn) for sowing from the USA. This pathway was modelled by estimating the number of infected seedlings growing each year within the EU out of infected seeds imported from the US. The calculation took into account:
the number of maize seeds imported from the USA to the EU,
the proportion of systemically infected mother plants in the USA maize fields producing these seeds,
the proportion of infected seeds on maize plants with systemic infection with P. s. subsp. stewartii (using two different distributions for the proportion of infected seed produced on infected plants of resistant and susceptible genotypes and taking into account whether imported seed has been produced on resistant or susceptible mother plants),
the effect of an additional late season inspection in USA fields,
the effect of sampling and testing seed (in the USA or at the EU border) on the number of infected seeds entering,
and the probability of transmission of the pathogen from the seed to the seedling in the EU.
Scenario A0 results in an order of magnitude of some hundred introductions per year (median number; 50%‐uncertainty interval between about hundred and about thousand), whereas the median number of introductions under scenarios A1 and A2b is in the order of some tens per year (50%‐uncertainty interval between about ten and about hundred). For scenarios A2a and A2, the median number of infected maize seedlings growing in the EU per year, deriving from the import of infected seed from the USA, is close to 0.1, i.e. one every ten years (50%‐uncertainty interval between about 0.01 and about 0.4).
The ratio of the mean number of infected maize seedlings growing in the EU per year deriving from the import of infected seed from the USA for scenario A0 by the mean number for scenario A1 is about 5. In other words, the expected number of infected seedlings is about five times larger under current practice than under the USA derogation request scenario. This difference is mainly due to the effectiveness of a mandatory field inspection under A1.
The ratio of the mean number for scenario A1 by the mean number for scenario A2 is about 2,000. In other words, the expected number of infected seedlings is about 2,000 times larger under the USA derogation request scenario than under the EU conditions for derogation.
This reduction is mainly due to the requirement of restricting trade to resistant maize genotypes; scenario A2a with a requirement for resistant seed but with only one inspection (as in A1) reduced the number of infected seedlings by a factor of about 1,000, whereas scenario A2b which requires two field inspections but does not require import of resistant seed only resulted in a reduction as compared to A1 by a factor of 2.
Combining the requirement of a second field inspection with a restriction of trade to resistant genotypes (scenario A2) provides the highest level of protection among the examined scenarios.
Based on the sensitivity analysis, for all scenarios, one important factor responsible for the uncertainty in the assessment is the prevalence of Stewart's wilt at the origin. In all scenarios, the seed to seedling transmission rate is also a variable explaining much of the variation of the output variable. In the scenario A2, also the proportion of infected seed on systemically infected mother plants for resistant varieties is a key uncertainty.
The quantitative model presented here shows that, despite the low prevalence of the disease in the country of origin, and the low rates of plant‐to‐seed and seed‐to‐seedling transmission that have been reported in the literature for Stewart's wilt, given the amount of traded seed, and in the case of voluntary (i.e. not mandatory) inspections of seed production fields (i.e. under current conditions), the frequency of introducing the disease is in the order of magnitude of some hundred introductions per year (median number). This frequency has been regarded as negligible in the literature (e.g. by Michener et al., 2002; Esker and Nutter, 2003; Pataky, 2004), but has (to the best of our knowledge) not been quantified with a pathway model before.
The likelihood of entry depends not just on the prevalence at origin and the plant to seed and seed to plant transmission rates, but also on the yearly flow of imported seed. With an average seed import in the order of 5,200 tonnes per year (Table 9), and an average seed weight of 0.35 g (see Section 3.2.2.3), the expected number of imported seeds per year is in the order of 15 billion. The size of the seed trade more than compensates for the low probability of transmission per seed.
Table 9.
Maize seed for sowing EU import from the USA (2013–2017) (in tonnes). Sum of the categories: simple hybrid maize seed for sowing; maize seed for sowing (excl. hybrids); three‐cross hybrid maize seed for sowing; and hybrid maize seed for sowing (excl. three‐cross and simple hybrid seed). Source: EUROSTAT, accessed December 2018
| Year | 2013 | 2014 | 2015 | 2016 | 2017 | Average |
|---|---|---|---|---|---|---|
| Imported maize seed (tonnes) | 6,890 | 6,730 | 5,720 | 2,040 | 4,650 | ~ 5,200 |
A further outcome of the model developed here is that the likelihood of introducing P. s. subsp. stewartii in the EU by importing maize seed from the USA is expected to be higher under the A0 scenario than under the A1 scenario. This is because the estimated prevalence of the disease at the origin is much higher under current conditions, due to the voluntary nature of the field inspection protocol, which led the Panel to estimate disease prevalence based on recent estimations of maize yield losses attributed to Stewart's wilt in the US. However, the comparison between scenario A0 and scenario A1 is affected by uncertainty on the effectiveness of seed lot testing (see Appendix A).
The Panel concluded that the answer to the question in the mandate from the European Commission (‘whether the schema of field inspections suggested by the USA would provide a level of protection against the introduction of E. stewartii via seeds of Zea mays which is equivalent to the one stipulated in Annex IVA, section I, point 52 of Council Directive 2000/29/EC’) is positive, given that the likelihood of entry under current practice, with voluntary field inspections at the origin (A0), is expected to be higher than under a scenario with mandatory field inspections at the origin (A1). However, if there is substantial variation in the proportion of infected seed among consignments, and if seed sample size is increased sufficiently (e.g. to 1,000 to 4,000 seeds), seed lot inspection in scenario A0 could lead to a relevant reduction in the level of infected imported maize seed. The Panel could not assess this accurately due to insufficient information on the variation among consignments in the level of infection.
However, the EU conditions for derogation would lead to a noticeable decrease in the likelihood of entry compared to the USA request for derogation. This protective effect is mainly due to the requirement that only genotypes resistant to Stewart's wilt are traded. The additional field inspection (two instead of one per season) provides additional reassurance.
Scenario A0 requires testing a representative sample to demonstrate absence of P. s. subsp. stewartii from the seed. The recommended sample size (both in the USA and by EPPO) is 400 seeds. Such a sample size is not suited to detect the pathogen if it occurs at levels of prevalence (such as one infected seed in 1,000,000 to one infected seed in 100,000,000 seeds) that can be anticipated if other RROs such as seed treatment and field inspections are carried out in the country of origin, unless there is high variability in the infection level among consignments.
For the scenario A0, the median number of infected maize seedlings emerging in the EU due to the import of maize seed from the USA is about 400 per year (98%‐uncertainty interval between about 6 and about 12,000). In the absence of vectors, and with infected plants occurring in ordinary production fields, many of these introductions can go unnoticed. Thus, under current conditions a few noticed outbreaks of Stewart's wilt can be expected in the EU each year due to import of maize seed from the USA. This is consistent with the recent observations from Italy.
Recently, outbreaks of P. s. subsp. stewartii have occurred in Italy. The Panel reviewed the available information to assess the possible role of seed imports in these outbreaks. Four north‐Italian regions, where maize is the most frequently cultivated crop, carried out surveys starting from 2014 to detect P. s. subsp. stewartii outbreaks. These regions are Emilia Romagna, Lombardy and Veneto (fields for production of maize seed for sowing), as well as Friuli Venezia Giulia (fields for production of grain or silage).
The pathogen was reported in August 2015 in Emilia Romagna (province of Parma), in June 2017 in Friuli (provinces of Pordenone and Udine), in June 2017 in Veneto (provinces of Venice and Vicenza), in May 2018 again in Emilia Romagna (province of Bologna), and in July 2018 in Lombardy (province of Cremona). The origin of the sown seed is unknown by the Panel in all cases.
In Friuli, the pathogen was detected again in 2018, with a double number of positive samples (42) compared to 2017. The data from Friuli suggest that the pathogen is able to overwinter in the region. This implies that not all the recent outbreaks from Italy have to originate from the import of infected maize seed: it could be that some of these outbreaks are the consequence of the pathogen overwintering after having been introduced in a previous year.
Given that the recent decline in the prevalence of Stewart's wilt in the USA can be attributed (at least in part, if not largely) to the widespread use of neonicotinoids, the ban of the use of these compounds in EU agriculture may lead to an increased likelihood of Stewart's wilt outbreaks, other things being equal. This is true at least for outbreaks that cannot be attributed to seed transmission.
The requirement of importing seed from maize varieties resistant to Stewart's wilt would not only contribute to lowering the likelihood of introducing the pathogen, but is also expected to reduce its potential spread and impacts, although this aspect was not studied quantitatively by the Panel.
The Panel also highlights that the impacts of Stewarts's wilt in the USA are higher in growing seasons following mild winters. This implies that, should the pest establish and spread in the EU, impacts might worsen in the coming decades due to ongoing climate warming.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. Annex IVA, section I, point 52 establishes special requirements for the import of seeds of Zea mays L. into the EU.
The United States of America (USA) made a first request to the Commission for examining a possible derogation from the requirements set out under point 52 and to accept alternative provisions for the import of seeds of corn and maize1 from the USA. This request was accompanied by a dossier regarding the certification of maize and corn seeds and a pest risk assessment concerning Erwinia stewartii (Smith) Dye.2
As requested, additional information has been submitted afterwards concerning the equivalency of procedures, namely the testing requirements as presented by the US, the relevance of latent infections, as well as data about the prevalence of Stewart's wilt (the disease caused by E. stewartii in Zea mays) and its development over the last years. On 21 June 2018, EFSA adopted a pest characterisation of E. stewartii (under the name of its senior synonym Pantoea stewartii).3
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29 of Regulation (EC) No 178/2002, to provide a scientific opinion. In particular, EFSA is requested to assess whether the information provided by the USA is sufficient to consider, whether the schema of field inspections suggested by the USA would provide a level of protection against the introduction of E. stewartii via seeds of Zea mays which is equivalent to the one stipulated in Annex IVA, section I, point 52 of Council Directive 2000/29/EC.
In this assessment, EFSA shall take into account the available scientific information, and in particular the scientific and technical information provided by the USA in the different steps of the process. EFSA is requested to put emphasis on the information submitted more recently, and in particular on the information submitted in October 2017, which is not referred to in the EFSA pest characterisation.
1.2. Interpretation of the Terms of Reference
1.2.1. Pest categorisation
Pantoea stewartii subsp. stewartii (hereafter P. s. subsp. stewartii) is a Gram‐negative bacterium which causes Stewart's vascular wilt and leaf blight of maize (including sweet corn), a disease responsible for serious crop losses throughout the world (Pepper, 1967; EPPO, 1997; Roper, 2011; CABI, 2019a). The bacterium (previously known as Erwinia stewartii) can be transmitted by infected seed, but in the USA the pest is largely dependent on insect vectors, mainly the corn flea beetle (Chaetocnema pulicaria), which is not known to be present in the EU. Of the other known North American vectors, Delia platura (seedcorn maggot or bean seed fly; CABI, 2019b) is reported as widespread in the EU (EFSA PLH Panel, 2018b).
The EFSA Panel on Plant Health (hereafter Panel) has recently published a pest categorisation on this bacterium (EFSA PLH Panel, 2018b), which concluded that the pest meets the criteria for consideration as Union quarantine pest. The reader is referred to that document for information on the identity, biology, detection and identification, establishment, spread and impacts of the pest. Information provided in the pest categorisation is not repeated here, unless required for the purposes of this risk assessment.
Over 60 countries place quarantine restrictions on maize seed to prevent the introduction of P. s. subsp. stewartii (Pataky, 2004; Chaky et al., 2013). For example, the pathogen has been considered as a quarantine pest in Brazil since 1995, in China since 1988, in Israel since 2009, in New Zealand since 2000, in Russia since 2014, in South Africa since 2001, in Turkey since 2007 and by the European and Mediterranean Plant Protection Organisation (EPPO) since 1975 (EPPO, 2019).
1.2.2. Interpretation of the Terms of Reference
The Panel interpreted the Terms of Reference (ToR) as a request to perform a partial risk assessment focusing on the likelihood of entry of P. s. subsp. stewartii on the pathway of maize seed imported by the EU from the USA. The likelihood of establishment, spread and impacts was thus not addressed.
The pathogen is currently regulated in Council Directive 2000/29/EC (Annex IIAI) as a harmful organism whose introduction and spread in the EU is banned if present on seeds of Zea mays. Annex IVAI requires for import of maize seeds into the EU an official statement that:
-
a)
the seeds originate in areas known to be free from P. s subsp. stewartii (Smith) Dye; or
-
b)
a representative sample of the seeds has been tested and found free from P. s subsp. stewartii (Smith) Dye in this test.
The implementation (‘current practice’) of Council Directive 2000/29/EC requirements regarding import of US maize seed to the EU in relation to mitigating the risk of introducing P. s subsp. stewartii is elaborated in a scenario A0.
For derogation to the USA to be granted, the European Commission (Letter to the USA of 22 June 2016, see section 1.2.3) proposed that four conditions would need to be met to achieve the expected level of protection in the EU:
-
a)
maize seeds have been produced in fields, which have been registered and inspected by APHIS;
-
b)
those inspections have been carried out both early in the growing season and during late season, and it has been concluded that E. stewartii is absent in the inspected field and the adjacent fields are situated in areas where the prevalence of E. stewartii is known to be low;
-
c)
they are produced from resistant seed varieties; The Panel has interpreted this as a requirement that the seed has been produced on resistant female plants, irrespective of whether the resulting seed is produced by selfing on an inbred line (in which case the seed genotype is also resistant) or the resulting seed is produced by cross‐pollination with a male genotype that is different from the female genotype, in which case the seed genotype is different from that of the mother.
-
d)
the fields of production are subject to appropriate seed treatments against vectors of the pest.
The Panel interpreted this requirement as a combination of RROs and thus a single scenario (but see Section 2.2.3). This is scenario A2.
USDA APHIS requested that derogation would be granted under a selection of the RROs proposed by the EU (Letter of the USA to the European Commission of 30 October 2017, see section 1.2.3). To summarise (see Table 14 for an overview):
maize seeds have been produced in fields, which have been registered and inspected by APHIS;
those inspections have been carried out once; and:
the fields of production are subject to appropriate seed treatments against vectors of the pest.
Table 14.
Visualisation of the scenarios
| Parameter ↓ | Scenario → | A0 | A1 | A2a | A2b | A2 |
|---|---|---|---|---|---|---|
| Prevalence at the origin (p1) | Based on yield loss | ✓ | ||||
| Based on 1 inspection | ✓ | ✓ | ||||
| Based on 2 inspections | ✓ | ✓ | ||||
| Seed trade volume | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Plant to seed transmission (p2) | Mix of genotypes | ✓ | ✓ | ✓ | ||
| Resistant genotypes | ✓ | ✓ | ||||
| Seed sampling | In the USA (p3) | ✓ | ||||
| At the EU border (p4) | ||||||
| Seed to seedling transmission (p5) | ✓ | ✓ | ✓ | ✓ | ✓ | |
The Panel developed a single scenario to assess the likelihood of entry under the options specified in the USA request for derogation (see Section 2.2.3). This is scenario A1, intermediate in its requirements between scenarios A0 and A2.
To sum up, the following three main scenarios were considered:
A0: current practice
A1: USA proposal for derogation requirements
A2: EU proposal for derogation requirements.
Furthermore, to disentangle the effect of restricting trade to resistant genotypes from the effect of an additional field inspection, two sub‐scenarios were distinguished. These are called scenario A2a (conditions (a), (c) and (d) of EU: resistant seed and only one inspection) and A2b (conditions (a), (b) and (d) of EU: both resistant and susceptible seed, and two inspections). These two scenarios are distinct from scenario A2.
1.2.3. Additional information
The following additional information was included in the mandate received by the Panel:
Block CC, Hill JH and McGee DC, 1998. Seed transmission of Pantoea stewartii in field and sweet corn. Plant Disease, 82, 775–780.
Block CC, Hill JH and McGee DC, 1999. Relationship between late‐season severity of Stewart's bacterial wilt and seed infection in maize. Plant Disease, 83, 527–530.
A pest risk analysis on the risk of introducing Erwinia stewartii in maize seed, by J. Pataky and R. Ikin, drafted for the International Seed Federation, dated February 2003, 79 pp.
A draft proposal by the EU for the export certification of maize seed to the EU (a proposal to use phytosanitary field inspection for the detection and exclusion of Erwinia stewartii (Smith) Dye (syn.: Pantoea stewartii—Stewart's Wilt)), dated 2 May 2014, 14 pp.
Letter of the USA to the European Commission of 12 May 2014 regarding the export of USA corn seed imported into the EU (request for derogation from Annex IV, Part A, Section I, No. 52 of Council Directive 2000/29/EC).
Letter of the USA to the European Commission of 31 July 2015 in response to the European Commission letter of 27 January 2015 regarding the importation into the EU of USA seed corn and the USA request for derogation from Annex IV, Part A, Section I, No. 52 of Council Directive 2000/29/EC.
Letter of the European Commission to the USA of 13 October 2015 (reply to the request for a derogation from point 52 of Annex IV, Part A, Section I of Council Directive 2000/29/EC for the export to the EU of seeds of Zea mays L. originating in the USA).
Letter of the European Commission to the USA of 22 June 2016 on possible EU derogation on USA corn seeds.
Letter of the USA to the European Commission of 29 July 2016 in response to the European Commission letter of 22 June 2016 regarding the European Commission's proposed derogation for USA corn seed under Annex IV, Part A, Section I, No. 52 of Council Directive 2000/29/EC for seed corn certification.
A report on the analysis and status of Stewart's wilt caused by P. s. subsp. stewartii in USA corn seed for exportation into the EU (United States Department of Agriculture (USDA), Animal and Plant Health Inspection Service (APHIS), Raleigh, NC), dated 11 Sep 2017, 18 pp.
Letter of the USA to the European Commission of 30 October 2017 (in response to the European Commission letter of 22 June 2016 regarding a proposed derogation from Annex IV, Part A, Section I, No. 52 of Council Directive 2000/29/EC, for the EU import conditions of corn seed from the USA).
2. Data and methodologies
2.1. Data
A literature search on P. s. subsp. stewartii was conducted at the beginning of the risk assessment (December 2018) in the ISI Web of Science bibliographic database, using the scientific name of the pathogen and the common name of the disease (Stewart's wilt) as search terms, to retrieve relevant information and data appeared since the publication of the EFSA pest categorisation on this pathogen (EFSA PLH Panel, 2018b). Relevant papers were reviewed and further references and information were obtained from experts, as well as from citations within the references and grey literature.
Information on the pest distribution was retrieved from the EPPO Global Database (EPPO, 2019). Stewart's wilt is endemic to the USA and is now reported in Africa, North, Central and South America, Asia, Italy, Slovenia and Ukraine. In Ukraine, the pest was first reported in 2014 on maize on an area of approximately 100 ha. The total infected area (2018) is estimated at about 3,500 ha, in various regions of the country. However, the correct identification of the pathogen causing the reported outbreaks in Ukraine has been called into question (J.D. Janse, Dutch Plant Protection Service, pers. comm., March 2019). In the EU, Stewart's wilt was reported from Italy (2015–2018) with a restricted distribution (Friuli, Emilia‐Romagna, Veneto, Lombardy, under eradication). There were occasional outbreaks in the past in EU Member States (MS) due to import of infected seed (then eradicated) (EFSA PLH Panel, 2018b).
Data on interceptions and outbreaks of the pest within the risk assessment area were obtained from the Europhyt database. Between 1999 and September 2019, there were 15 records of interception of P. s. subsp. stewartii in the Europhyt database (code: ERWIST), all on Zea mays. Nine interceptions were made in 1999 originating from Hungary (7) and Romania (2) before they joined the EU. These interceptions were reported by Austria (3), France (2), Germany (3) and the Netherlands (1). One interception was made in 2005 (origin: Turkey; destination: Germany), one in 2008 (origin: USA; destination: Germany), one in 2013 (origin: Poland; destination: Italy) and three in 2017 (all originating from Mexico, with France (2) and Germany (1) as destination) (EFSA PLH Panel, 2018b).
For this opinion, the following additional data were searched:
Data on the prevalence of P. s. subsp. stewartii in the USA. Information was provided by the USA (report dated 2017, see section 1.2.3, with an additional clarification from USDA APHIS (dated 22 May 2019) that the field inspections described in the report ‘are voluntary; however, some inspections may have been carried out as required for official phytosanitary inspections’). In addition, data were obtained from EPPO (2019) and the literature.
Data on the EU import of maize seed from the USA. These data were obtained in December 2018 from EUROSTAT (Statistical Office of the European Communities) for the period 2013–2017. Additional data for 2017 and 2018 were kindly provided by USDA APHIS.
Data on the transmission rate of the pathogen from infected maize plants to seed and from infected seed to seedlings. Information published in the literature was provided by the USA (Block et al., 1998, 1999; Michener et al., 2002).
Data on the effectiveness of RROs for this pathogen (see Section 2.2.2.4).
2.2. Methodologies
The Panel performed this risk assessment following the Panel's guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018a).
Entry via trade in imported maize seed from the USA was assessed using pathway modelling in @Risk (https://www.palisade.com/risk/default.asp).
Expert elicitation was used to estimate model input numbers for each sub‐step of the pathway model.
2.2.1. Specification of the scenarios
The following scenarios were considered:
Scenario A0 (current practice),
Scenario A1 (US request for modification of EU conditions for derogation), and
Scenario A2 (EU conditions for derogation).
2.2.2. Conceptual model and definitions
2.2.2.1. Definition of the pathway
The only pathway of entry considered in the model was the import by the EU of maize seed for sowing from the USA.
2.2.2.2. Conceptual model
The entry pathway was modelled by estimating the number of infected seedlings growing each year within the EU from infected seeds imported from the US. The calculation took into account:
the number of maize seeds imported from the USA to the EU,
the proportion of systemically infected mother plants in the USA maize fields producing these seeds,
the proportion of infected seeds on maize plants with systemic infection with P. s. subsp. stewartii (using two different distributions for resistant and susceptible genotypes and taking into account whether imported seed has been produced on resistant or susceptible mother plants),
the effect of an additional late season inspection in USA fields,
the effect of sampling and testing seed (in the USA or at the EU border) on the number of infected seeds entering,
and the probability of transmission of the pathogen from the seed to the seedling.
2.2.2.3. Formal model
The model is a multiplication formula:
where the meaning and the units of the variables are defined in Table 1.
Table 1.
Definitions of the variables used in the entry model
| Variable name | Description | Units | |
|---|---|---|---|
|
|
Number of maize seedlings infected by P. s. subsp. stewartii in the EU due to import of maize seed from the USA per year | Number per year | |
|
|
Amount of traded maize seed for sowing from the USA to the EU per year | tonnes (1,000 kg) per year | |
| 106 | Conversion factor | Grams tonnes−1 | |
| seedw | Average maize seed weight | Grams | |
| p1 | Prevalence of Stewart's wilt in USA fields for production of seed for sowing (proportion of systemically infected maize plants) | Proportion | |
| pr | Proportion of the traded seed that is produced on resistant mother plants | Proportion | |
| p2r | Proportion of infected seed on systemically infected maize plants for resistant genotypes | Proportion | |
| p2s | Proportion of infected seed on systemically infected maize plants for susceptible genotypes | Proportion | |
| p3 | Effectiveness of seed sampling (proportion of remaining infected seeds after sampling of seed lots, in the USA) | Proportion | |
| p4 | Effectiveness of seed sampling (proportion of remaining infected seeds after sampling of seed lots, at the EU border) | Proportion | |
| p5 | Probability of transfer from infected maize seed to seedling | Proportion |
The model has nine parameters whose quantiles were estimated based on data and expert judgement, following EFSA guidance on expert knowledge elicitation and uncertainty (EFSA, 2014, 2019). In short: experts elicit five quantiles for each parameter (1, 25, 50, 75 and 99%) and a theoretical probability distribution is then fitted to these quantiles for each parameter, using least squares in @Risk.
The pathway model was run using Monte Carlo, by repeatedly (10,000 times) drawing random realisations out of the elicited distributions for the nine parameters and calculating the resulting 10,000 values of the outcome variable: the number of infected maize seedlings growing in the EU as a result of import of infected seed from the US. This calculation was made under different scenarios for regulation (see Section 2.2.3). The model was implemented in @Risk (see Supplementary Information – Annex A).
2.2.2.4. Potential risk reducing options
The following RROs were considered for the present risk assessment:
RRO1 – pest free area
Based on information provided by USDA APHIS, only two States in the USA are officially free of Stewart's wilt (Colorado and Washington). As these two States are not within the main maize growing area (the mid‐west), to simplify, pest free area was not considered further as an RRO in the model development.
RRO2 – representative seed sample found free of the pest
Statistically, a representative sample is a sample that truthfully represents the population; i.e. it is unbiased. The sample should have been drawn avoiding selection biases. This requires that the sample has been drawn using proper randomization procedures that guarantee representativeness. The first and foremost approaches to guarantee an unbiased sample are random sampling and stratified random sampling.
The EU plant health legislation does not mention the size of the sample for testing for the presence of Stewart's wilt in imported maize seed lots. The EPPO standard for P. s. subsp. stewartii mentions as usually recommended sample size 400 seeds (EPPO, 2016), in agreement with the USA seed testing approach (Pataky and Ikin, 2003).
RRO3 – pest free place of production (one inspection)
One inspection is advocated as sufficient by USDA APHIS in their request for derogation. USDA APHIS provided a dossier describing in detail how field inspections should be carried out (see Section 1.2.3). However, USDA APHIS clarified upon the Panel's request that these field inspections are not mandatory, but voluntary (see Section 2.1). The Panel applied formulas of Bourhis et al. (2019) to calculate the efficacy of single or dual field inspections.
RRO4 – pest free place of production (two inspections, one early and one later in the season)
A pest free place of production can be assured by appropriate management and surveillance of the field and its surroundings. Early inspections in maize are suited to detect the early phase of Stewart's wilt due to infection of plants in the seedling stage. Later inspections can be useful to detect Stewart's wilt due to later infection. Later inspection is especially effective if disease incidence increases over the season (Bourhis et al., 2019). Inspections early in the season are reported to be easier because plants are smaller and seedling wilt is easily identified (F. Finelli, Phytosanitary Service Emilia Romagna, Italy, personal communication, December 2018). The leaf blight phase occurs when plants are infected after the seedling stage, mainly due to pest transmission by insect vectors (Pataky, 2004).
In his classic reference book on seed pathology, Neergard (1977) (Vol. 1, p. 542) states that, in field inspections of seed crops, ‘a minimum of two inspections is needed’.
RRO5 – resistant varieties
Resistance to Stewart's wilt reduces the occurrence of systemic infections and the transmission from infected maize plants to seed (Block et al., 1998).
According to Bradley et al. (2017), ‘growing resistant varieties is the best means of managing Stewart's wilt’. The same authors also write that ‘although sweet corn and popcorn varieties tend to be more susceptible to Stewart's wilt than field (dent) corn, there may still be susceptible field corn inbreds and varieties on the market’.
RRO6 – seed treatment against vectors
Bradley et al. (2017) write that ‘insecticide seed treatments can kill corn flea beetles before the bacteria are transmitted to corn plants’.
Welty (2019) states that ‘tests done at the University of Illinois when seed treatments were under development showed that incidence of Stewart's wilt in susceptible varieties was reduced by about 70% by commercial seed treatment, and severity of symptoms was also reduced’. The same author concludes that ‘seed treatments are thus not products that alone will control corn flea beetle and Stewarts wilt’.
Seed treatment against vectors was considered in the model in all scenarios to affect the estimated prevalence at the origin and was thus not included as an additional RRO.
2.2.2.5. Ecological factors and conditions in the chosen scenarios
The risk assessment was performed under current ecological factors and conditions for the maize growing areas of the EU (risk assessment area) and USA (country of origin).
2.2.2.6. Temporal and spatial scales
The risk assessment area was the EU territory.
The temporal horizon considered for the risk assessment was 5 years (2020–2024). This temporal horizon is relevant inasmuch as it delimits the scope of the parameter elicitations done by the Panel. Entry was considered as a separate process for each year. No time‐cumulative processes were accounted for in the model (as would be the case in a spread model).
2.2.3. Summary of the different scenarios
Scenario A0: current practice
The current legislation (Annex IV, Part A, Section I, No. 52 of Council Directive 2000/29/EC) provides two options for import of maize seed for sowing:
-
a)
the seeds originate in areas known to be free from P. s subsp. stewartii (Smith) Dye;
-
b)
a representative sample of the seeds has been tested and found free from P. s subsp. stewartii (Smith) Dye in this test.
In the USA, only two States are considered free of Stewart's wilt (i.e. Colorado and Washington State) and these represent only a small proportion of the USA production of maize seed imported by the EU. This option was therefore not further considered. All other maize seed is imported under the option of demonstration of pest freedom by conducting a test on a representative sample of the seed. This was elaborated in scenario A0.
Scenario A1: USA request for modification of EU conditions for derogation
This scenario did not list additional RROs compared to the A0 scenario because all the proposed options are already in place under the current legislation (Annex IV, Part A, Section I, No. 52 of Council Directive 2000/29/EC).
Nevertheless, the prevalence of Stewart's wilt at the origin was estimated in scenario A1 assuming mandatory inspections of the fields for production of maize seed for sowing for export, while in scenario A0 such inspections were considered voluntary.
Scenario A2: EU conditions for derogation
The EU conditions for import require that:
-
a)
maize seeds have been produced in fields, which have been registered and inspected by APHIS;
-
b)
those inspections have been carried out both early in the growing season and during late season, and it has been concluded that P. s subsp. stewartii is absent in the inspected field and the adjacent fields are situated in areas where the prevalence of P. s subsp. stewartii is known to be low;
-
c)
they are produced from resistant seed varieties; The Panel interpreted this as a requirement that the seed has been produced on resistant mother plants, irrespective of whether the resulting seed is produced by selfing on an inbred line (in which case the seed genotype is also resistant) or the resulting seed is produced by cross‐pollination with a father genotype that is different from the mother genotype, in which case the seed genotype is different from that of the mother and its resistance level is not considered.
-
d)
the fields of production are subject to appropriate seed treatments against vectors of the pest.
The assessment in this case focused on the effectiveness of two inspections rather than one as in the USA proposal to demonstrate absence of the pathogen in the production field (pest free place of production; RRO4), and the effectiveness of producing seed only on resistant mother plants (RRO5).
As in scenario A1, the estimation of the prevalence of Stewart's wilt at the origin was performed assuming mandatory inspections of the fields for production of maize seed for sowing for export.
Two distinct sub‐scenarios were developed to disentangle the contribution to risk reduction of two RROs:
restricting trade to resistant genotypes, and
an additional field inspection.
This resulted in two additional sub‐scenarios: A2a without the second inspection, but with the other RROs, and A2b without the requirement for resistant seed, but with the other RROs. Scenarios A2a and A2b are distinct from scenario A2.
3. Entry assessment
This section has five main subsections. The first four subsections describe the assessment of entry of P. s. subsp. stewartii with maize seed from the USA, starting from the background information (3.1) that was used for the expert elicitation of the parameter distributions (see Section 3.2.2) of the entry risk assessment model (Section 3.2), the main uncertainties affecting the entry assessment (Section 3.3) and the conclusions of the assessment (Section 3.4). The fifth subsection (Section 3.5) provides an overview of the current status of the pathogen in northern Italy in order to assess from another perspective the importance of entry.
3.1. Background information
3.1.1. Pest prevalence at the origin (p1)
The Panel used two lines of reasoning to estimate the proportion of infected mother plants in production fields in the USA.
First, the Panel estimated current prevalence and yield losses due to Stewart's wilt in USA maize on the basis of information available in the literature (Mueller et al., 2016) and online (https://cropprotectionnetwork.org/library/). This estimation was used for scenario A0.
Second, the Panel made a theoretical calculation of the level of freedom of the disease that may be reached by inspecting production fields, using inspection procedures prescribed by the USA National Seed Health System as provided by USDA APHIS by letter of 11 Sep 2017. This estimation was used for scenarios A1, A2a, A2b and A2, where the field inspections were assumed to be mandatory.
3.1.1.1. Current prevalence of Stewart's wilt in the USA
Stewart's wilt has been reported as endemic in the mid‐Atlantic USA states, the Ohio River Valley and the southern portion of the Corn Belt (Pataky, 2004).
The pest is reported by EPPO (2019) to occur (in all cases without details) in most USA States (Figure 1): Alabama, Arkansas, California, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Dakota, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Vermont, Virginia, West Virginia and Wisconsin. There are two USA states (Idaho and Washington) where the pest is reported by EPPO (2019) as absent (no longer present, in both cases based on information dated 1967). According to USDA APHIS, Colorado and Washington are free of P. s. subsp. stewartii.
Figure 1.
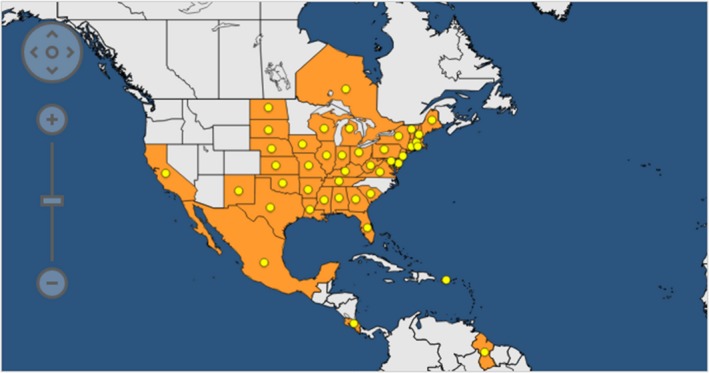
Distribution of Pantoea stewartii subsp. stewartii in North America (countries in Central and South America are also shown in the map) (from EPPO, 2019)
The distribution reported by EPPO partly overlaps with the one reported by a review of widely prevalent bacteria in the USA (Figure 2), which reports P. s. subsp. stewartii in the above‐mentioned States, with addition of Oregon and Wyoming (but not in Alabama, Arkansas, Florida, Louisiana, Maine, New Mexico, North Dakota, South Carolina, South Dakota, Tennessee, Texas, Vermont and West Virginia).
Figure 2.
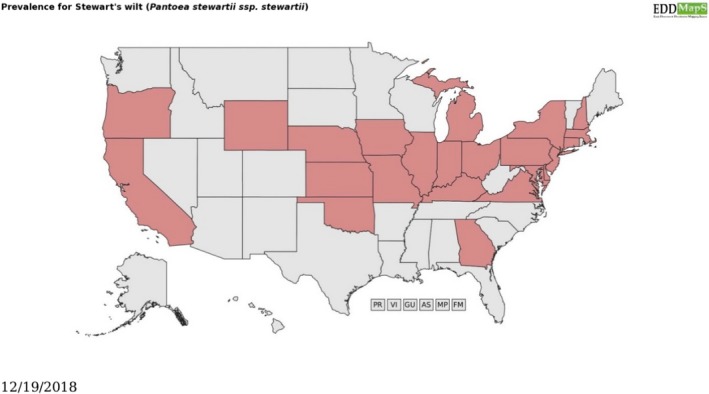
Distribution of Pantoea stewartii subsp. stewartii in the USA, according to prevalentbacteria.org. Reprinted courtesy of Bugwood.org. Available online [accessed December 2018]: https://www.prevalentbacteria.org/subject.cfm?id=11123
In turn, the distribution of Figure 2 overlaps well with the historical distribution range (and the area with the highest levels of damage) of the pest (Figure 3, from Pataky, 2004).
Figure 3.
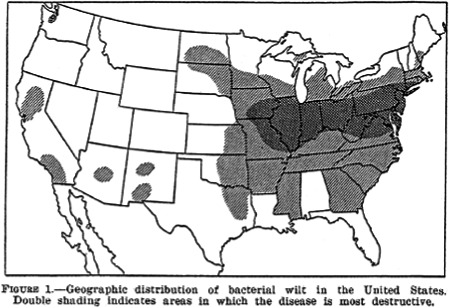
Historical distribution range of Stewart's wilt in the USA (from Pataky, 2004; original source: Elliott, 1941 – copyright free). Pataky writes that ‘the current geographic distribution of Stewart's wilt in the USA is similar to the distribution reported in the early 1940s’
Stewart's wilt is reported to have declined in prevalence (number of fields in which the pathogen is reported to be present) in the USA in recent years due to the use of resistant varieties and the widespread use of neonicotinoid seed treatment. Neonicotinoids reduced the population abundance of the corn flea beetle vector (C. pulicaria), in which P. s. subsp. stewartii overwinters (Chaky et al., 2013; Bradley et al., 2017; Welty, 2019). However, there are still concerns in many USA states that Stewart's wilt will affect sweet corn, as suggested by the yearly maps of Stewart's wilt risk for New York State and surroundings (2010–2018) shown in Figure 4.
Figure 4.
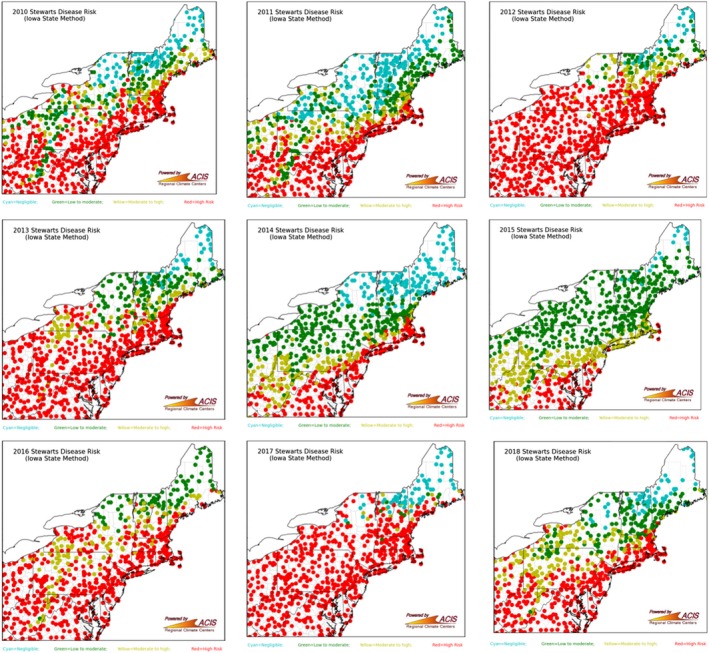
Maps of Stewart's wilt risk in New York State and surrounding regions (2010‐2018), based on the Iowa State University Model. The model predicts the prevalence of Stewart's wilt based on average temperatures for the months of December, January and February. Warm winter temperatures suggest that survival of large corn flea beetle populations is likely. Therefore, a high disease prevalence (red dots on map) is predicted if the mean monthly temperatures for December, January and February are each above 24°F (−4.4°C). If two of the three months average above 24°F, the risk is moderate to high (yellow dots on map). If one of the three months averages above 24°F, the risk is low to moderate (green dots on map). If all three months average below 24°F, survival of the beetle is unlikely and the risk of disease is negligible. With kind permission of the Network for Environment and Weather Applications and the Northeast Regional Climate Center, both at Cornell University, USA. Available online at: http://newa.cornell.edu/index.php?page=sweet-corn
The yearly maps of Stewart's wilt risk for New York State and surroundings (Figure 4) are consistent with the estimated maize losses due to Stewart's wilt in the USA and Ontario, Canada, from 2012 to 2017 (Table 2). Both disease risk and maize losses were higher in 2012 and 2017 compared to 2014 and 2015.
Table 2.
Estimated maize loss (in tonnes) due to Stewart's wilt in the USA and Ontario, Canada (2012–2015, from Mueller et al., 2016; 2016–2017, from: https://cropprotectionnetwork.org/library/)
| Year | Estimated maize loss due to Stewart's wilt (tonnes) |
|---|---|
| 2012 | 286,000 |
| 2013 | 2,830 |
| 2014 | 530 |
| 2015 | 1,100 |
| 2016 | 0 |
| 2017 | 597,500 |
The estimated USA and Ontario yearly maize losses due to Stewart's wilt were then compared with the estimated USA yearly maize production, so as to obtain a proportion of losses due to this disease per year (Table 3). This proportion is essentially the same whether or not considering maize production in Ontario (estimated assuming that Canada produces about 3% of USA maize production, and that Ontario produces about 60% of Canada maize production), because USA maize production dwarfs Ontario maize production.
Table 3.
Estimated USA maize production (with and without Ontario) in comparison with the estimated maize loss due to Stewart's wilt (USA and Ontario), for the period 2012–2017
| Year | Estimated USA maize production | 3% × 60% of USA production (Ontario production) | Estimated maize loss due to Stewart's wilt (USA & Ontario) | Percentage losses without Ontario | Percentage losses with Ontario | |
|---|---|---|---|---|---|---|
| (billion bushels) | (tonnes) | (tonnes) | (tonnes) | (%) | (%) | |
| 2012 | 10.8 | 274,320,000 | 4,937,760 | 286,000 | 0.10 | 0.10 |
| 2013 | 13.9 | 353,060,000 | 6,355,080 | 2,830 | 0.0008 | 0.0008 |
| 2014 | 14.2 | 360,680,000 | 6,492,240 | 530 | 0.0001 | 0.0001 |
| 2015 | 13.6 | 345,440,000 | 6,217,920 | 1,100 | 0.0003 | 0.0003 |
| 2016 | 15.1 | 383,540,000 | 6,903,720 | 0 | 0 | 0 |
| 2017 | 14.6 | 370,840,000 | 6,675,120 | 597,500 | 0.16 | 0.16 |
Also, in the Mid‐West, Stewart's wilt is reported as having ‘become less prevalent in recent years in Kentucky and surrounding states’, but ‘the disease still occurs occasionally’ (Bradley et al., 2017). A compilation of the corn flea beetle index at Ohio locations, which is used to predict severity of Stewart's wilt on sweet corn, shows that winters were generally not favourable to the vector between 2007 and 2011, as well as in 2014 and 2015, but mild winters conducive to Stewart's wilt losses occurred in 2012, 2016 and 2017 (Anon, 2018).
Additional evidence to assess prevalence of Stewart's wilt at the origin was obtained from the field inspection scheme provided by USDA APHIS.
3.1.1.2. Theoretical effectiveness of field inspections to guarantee absence of the disease in production fields
Field inspection of seed crops aims to eliminate infected plants and start control efforts to ensure that seed produced is free of the pathogen. Calculations were made to assess the level of disease freedom that could be ensured by field inspections that are conducted according to official procedures of USDA APHIS.
USDA provided us with a protocol for sampling for assessing crops for seed health. The protocol describes the number of passes that are made through a maize field for seed production depending on the size of the field (Table 4). The protocol is described in an annex to a report on the analysis and status of Stewart's wilt caused by P. s. subsp. stewartii in USA corn seed for exportation into the EU (United States Department of Agriculture (USDA), Animal and Plant Health Inspection Service (APHIS), Raleigh, NC), dated 11 Sep 2017, 18 pp (see Section 1.2.3).
Table 4.
Passes made by an inspector in a seed crop according to a standard protocol of the USA National Seed Health System
| Area of the field (acres) | Prescribed number of passes | Passes per acre | Passes per hectare | |
|---|---|---|---|---|
| From | To | |||
| 0 | 1 | 6 | 6 | 15 |
| 1 | 5 | 9 | 1.80 | 4.50 |
| 5 | 10 | 11 | 1.10 | 2.75 |
| 10 | 20 | 13 | 0.65 | 1.625 |
| 20 | 50 | 17 | 0.34 | 0.85 |
| 50 | 100 | 20 | 0.20 | 0.50 |
| 100 | 200 | 24 | 0.12 | 0.30 |
| 200 | 500 | 30 | 0.06 | 0.15 |
| 500 | 1,000 | 36 | 0.036 | 0.09 |
| 1,000 | 2,000 | 42 | 0.021 | 0.0525 |
The protocol does not define the length of a pass. We therefore considered three cases: square fields, rectangular fields (1:10 length:width) inspected with passes over the short side and rectangular fields with passes made over the long side. For each case, an estimate was made of the number of plants that an inspector will see. We make calculations assuming 100% sensitivity of inspections, which represents the best situation.
Assuming a density of 6 plants per m2 and a row distance of 60 cm, there are 1/0.36 = 2.78 plants per m row length. Inspectors can inspect plants at both sides, so they can see 2 × 2.78 = 5.56 plants per m transect. Then for the three cases we can estimate the distance travelled and the number of plants that the inspector has seen. If the inspector has seen n plants, and one or more is infected, the field is no longer suitable for seed production. If the inspector has seen n plants, and zero plants have been found infected, we can estimate an upper limit for the proportion of infected plants, p.
Assume that the number of infected plants found during the sample follows a binomial distribution. Then, the number of infected plants, k is a stochastic variable with distribution:
Suppose the inspector finds 0 infected plants. The probability of this event is given by:
Then an α confidence limit for the true proportion of infected plants in the population given the negative sample of size n can be calculated by solving The result is:
| (1) |
An 1−α upper limit can also be calculated using Bayes’ rule (Bourhis et al., 2019). The result is:
| (2) |
Table 5 shows that with a sample of 10,000 plants, a 5% upper confidence limit for the true proportion of infected plants in the field is 3 infected plants per 10,000.
Table 5.
Number of sampled maize plants in fields for production of maize seed for sowing and 5% upper confidence limit for the true proportion of plants infected by Stewart's wilt
The Panel combined the information on the number of passes per seed production field with the upper limits for the proportion of infected plants given zero infected plants being found during an inspection. In the table below, estimates are made of the row length of a single pass through a field. The total row length covered is calculated, and this is translated into an approximate number of plants inspected. This number of plants is then translated into quantile estimates for the true proportion of infected plants in those fields in which no infected plants have been found. The calculations acknowledge the fact that the production regions of seed are not considered disease‐free in an absolute sense, but effectively disease‐free to the extent that the disease occurs at levels that are below the detection threshold. It is statistically impossible to state that a field is disease‐free unless all plants are inspected with perfect sensitivity, and even then, the claim only holds for that surveyed snapshot in time. In Tables 6–8, the Panel calculated the levels of disease that are compatible with absence of disease findings in the fields that pass inspection.
Table 6.
Length of a single pass through an inspected field under three cases for the ratio of length to width of the field
| Classes of field size for making field inspections (USDA‐APHIS) | Row length (m) of a single pass for three length: width ratios of a rectangular field | ||||||
|---|---|---|---|---|---|---|---|
| From | To | Mean field area (acres) | Mean field area (ha) | Number of passes through the field | 1:1 | 10:1 | 1:10 |
| 0 | 1 | 0.5 | 0.2 | 6 | 45 | 141 | 14 |
| 1 | 5 | 3 | 1.2 | 9 | 110 | 346 | 35 |
| 5 | 10 | 7.5 | 3 | 11 | 173 | 548 | 55 |
| 10 | 20 | 15 | 6 | 13 | 245 | 775 | 77 |
| 20 | 50 | 35 | 14 | 17 | 374 | 1,183 | 118 |
| 50 | 100 | 75 | 30 | 20 | 548 | 1,732 | 173 |
| 100 | 200 | 150 | 60 | 24 | 775 | 2,449 | 245 |
| 200 | 500 | 350 | 140 | 30 | 1,183 | 3,742 | 374 |
| 500 | 1,000 | 750 | 300 | 36 | 1,732 | 5,477 | 548 |
| 1,000 | 2,000 | 1,500 | 600 | 42 | 2,449 | 7,746 | 775 |
Table 8.
Quantiles of p assuming square fields, and probabilities of 0.01, 0.25, 0.5, 0.75 and 0.99. The table lists the number of infected plants per 10,000 plants
| Field size (acres) | Number of plants inspected | Quantiles of p based on finding no infected plants in a field | |||||
|---|---|---|---|---|---|---|---|
| From | To | 1% | 25% | 50% | 75% | 99% | |
| 0 | 1 | 1,492 | 0.0674 | 1.928 | 4.65 | 9.29 | 30.87 |
| 1 | 5 | 5,482 | 0.0183 | 0.525 | 1.26 | 2.53 | 8.40 |
| 5 | 10 | 10,593 | 0.0095 | 0.272 | 0.65 | 1.31 | 4.35 |
| 10 | 20 | 17,705 | 0.0057 | 0.162 | 0.39 | 0.78 | 2.60 |
| 20 | 50 | 35,366 | 0.0028 | 0.081 | 0.20 | 0.39 | 1.30 |
| 50 | 100 | 60,907 | 0.0017 | 0.047 | 0.11 | 0.23 | 0.76 |
| 100 | 200 | 103,362 | 0.0010 | 0.028 | 0.07 | 0.13 | 0.45 |
| 200 | 500 | 197,360 | 0.0005 | 0.015 | 0.04 | 0.07 | 0.23 |
| 500 | 1,000 | 346,687 | 0.0003 | 0.008 | 0.02 | 0.04 | 0.13 |
| 1,000 | 2,000 | 572,005 | 0.0002 | 0.005 | 0.01 | 0.02 | 0.08 |
Table 7.
Calculation of quantiles for the true proportion of infected plants in fields that are cleared upon inspection
| Field area (acres) | Number of plants inspected (# passes × length of a pass × 5.56 plants/m transect) | pupper for 3 length:width ratios | |||||
|---|---|---|---|---|---|---|---|
| From | To | 1:1 | 10:1 | 1:10 | 1:1 | 10:1 | 1:10 |
| 0 | 1 | 1,492 | 4,718 | 472 | 0.00046 | 0.00015 | 0.00147 |
| 1 | 5 | 5,482 | 17,334 | 1,733 | 0.00013 | 0.00004 | 0.00040 |
| 5 | 10 | 10,593 | 33,499 | 3,350 | 0.00007 | 0.00002 | 0.00021 |
| 10 | 20 | 17,705 | 55,988 | 5,599 | 0.00004 | 0.00001 | 0.00012 |
| 20 | 50 | 35,366 | 111,838 | 11,184 | 0.00002 | 0.00001 | 0.00006 |
| 50 | 100 | 60,907 | 192,604 | 19,260 | 0.00001 | 0.00000 | 0.00004 |
| 100 | 200 | 103,362 | 326,860 | 32,686 | 0.00001 | 0.00000 | 0.00002 |
| 200 | 500 | 197,360 | 624,108 | 62,411 | 0.00000 | 0.00000 | 0.00001 |
| 500 | 1,000 | 346,687 | 1,096,321 | 109,632 | 0.00000 | 0.00000 | 0.00001 |
| 1,000 | 2,000 | 572,005 | 1,808,838 | 180,884 | 0.00000 | 0.00000 | 0.00000 |
Previous tables provide an upper limit for the proportion of infected plants, given no infected plants were found in a sample of size n. Table 8 presents five quantiles of p, using an equation similar to Equation (1):
| (3) |
Here, Qc(p) is a quantile that characterises the distribution of the proportion of infected plants, p. The quantiles are calculated for cumulative probabilities C of 0.01, 0.25, 0.5, 0.75 and 0.99 (Table 8). These quantiles were directly fed into the distribution fitting software that is used for parameterising the risk model.
From Equation (2), we would calculate a C quantile with:
| (4) |
With results very similar to Equation (3). Note that for a 95% probability (C = 0.95), Equation 4 yields the rule of 3 (ISPM 31; Anon, 2008):
| (5) |
for large enough n.
Bourhis et al. (2019) developed formulas for the case of multiple inspections in a field. They considered an epidemic that increased in incidence by a factor λ between inspections. If the incidence is p at the first inspection, it would be λ × p at the second inspection. Thus, the disease would be easier to find at the second inspection, making the second inspection more efficient than the first (other things being equal). The quantiles for p with two inspections are calculated with:
| (6) |
where C is a cumulative probability of p, n1 and n2 are the sample sizes at the first and second inspection, p is incidence at the first inspection and λ is the factor by which incidence has increased from the first to the second inspection.
Liu (2010) investigated an epidemic of P. s. subsp. stewartii in maize in a field experiment in Iowa in 2007. Half the plots were treated with a neonicotinoid seed treatment (Cruiser; Thiamethoxam) while the other half were untreated. Incidence increased by a factor 2–4 from day 160–190 to day 230. Judging from the graphs (Figure 5), seed treatment with Cruiser appears to affect the rate of spread, but Liu (2010) reports that the difference in rate of increase was not significant.
Figure 5.
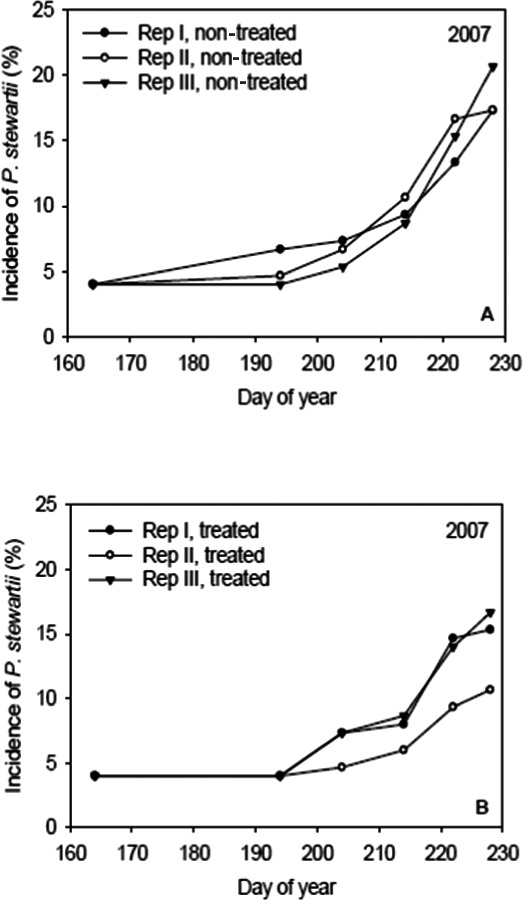
Development of an epidemic of Stewart's wilt in seed‐treated vs. non‐seed‐treated maize fields (from Liu, 2010; courtesy of Plant Pathology Commons, http://network.bepress.com/about/life-sciences/plant-sciences/plant-pathology/)
The above reasoning applies if field inspections are mandatory in maize fields for the production of seed for export. This is the case in scenarios A1, A2a, A2b and A2, but not in scenario A0. USDA APHIS informed us that inspections are currently voluntary, therefore for the A0 scenario only the data on the estimated yield losses attributed to Stewart's wilt were used (Table 3).
3.1.2. Seed trade flow (Ntrade)
Data on yearly maize seed for sowing EU import from the USA (2013–2017) were extracted from EUROSTAT (Table 9). The major exporter of maize seed for sowing to the EU is Turkey (average of 9,580 tonnes over the same period), followed by the USA. The USA accounted for an average of 28% of EU maize seed for sowing import. Further details on countries reporting presence of P. s. subsp. stewartii and exporting maize seed to the EU are given in the pest categorisation for P. s. subsp. stewartii (EFSA PLH Panel, 2018b).
Data were also kindly provided on request by USDA APHIS (tonnes) for 2017 and 2018 (Table 10).
Table 10.
Maize seed for sowing EU import from the USA (2017–2018) (in tonnes). Source: USDA APHIS, March 2019
| Year | Number of shipments | Total of all maize seed (tonnes) | Total of sweet corn seed (tonnes) |
|---|---|---|---|
| 2017 | 2,789 | 11,439 | 1,252 |
| 2018 | 2,334 | 9,512 | 944 |
These data were used by the Panel to assess the trade flow, as well as the proportion of sweet corn seed in the trade. Sweet corn has generally lower levels of resistance to P. s. subsp. stewartii than dent corn (maize other than sweet corn), and poses therefore a greater risk for entry of the pathogen.
To put these numbers in perspective, with an average maize seed for sowing import in the order of 5,200 tonnes per year (Table 9), and an average seed weight of 0.35 g (see Section 3.2.2.3), the expected number of imported seeds is in the order of 15 billion per year.
3.1.3. Plant to seed transmission rate (p2)
The rate of transmission from infected mother plants to seed is reported by Pataky and Ikin (2003) as follows:
For resistant varieties: < 0.03%
For moderately resistant varieties: < 0.3%
For susceptible varieties: 10%.
These numbers are based on Block et al. (1998), who studied plant to seed transmission of P. s. subsp. stewartii by assays of more than 76,000 plants in greenhouse and field grow‐out trials. Fourteen P. s. subsp. stewartii–infected seed lots were obtained from two dent corn inbreds and two sweet corn cultivars that were inoculated with the pathogen. Four additional seed lots were obtained from naturally infected inbreds. Percentages of infected kernels ranged from 0.8% to 72%.
3.1.4. Proportion of resistant seed (pr)
The parameter pr was assessed based on the following assumptions:
The EU import of maize seed for sowing from the USA in 2017–2018 was about 90% dent corn and 10% sweet corn (information provided by USDA APHIS; Table 11).
Dent corn was assumed to consist of about 90% resistant genotypes and 10% susceptible genotypes. As mentioned in the USDA APHIS letter to the European Commission of July 2016, ‘high resistance to E. stewartii in U.S. corn seed varieties is generally acknowledged and observed by the U.S. Seed Industry’.
Sweet corn was assumed to consist of about 80% susceptible genotypes and 20% resistant genotypes.
Table 11.
Approximate composition (%) of the trade in terms of dent corn and sweet corn, and resistant varieties and susceptible varieties
| Resistant | Susceptible | Total | |
|---|---|---|---|
| Dent corn | 81 | 9 | 90 |
| Sweet corn | 2 | 8 | 10 |
| Total | 83 | 17 | 100 |
Therefore, overall, the percentage of resistant maize import was assumed to be 83% (composed of 81% dent corn and 2% sweet corn) and the proportion of susceptible maize import was assumed to be 17% (composed of 9% dent corn and 8% sweet corn).
3.1.5. Effectiveness of seed lot sampling (in the USA (p3) or at the EU border (p4))
The sample size recommended by a USA National Seed Health System technical panel reviewing seed health tests for P. s. subsp. stewartii is 400 seeds (Pataky and Ikin, 2003). Likewise, the EPPO standard for P. s. subsp. stewartii mentions 400 seeds as the usually recommended sample size, corresponding to a 95% probability of detecting an infection level of 1% (EPPO, 2016).
The effectiveness of sampling seed lots for P. s. subsp. stewartii depends on the sensitivity of the test and the number of seeds taken for testing. Assuming 100% sensitivity (i.e. an infected seed is found with 100% chance if it is in the sample), the formulas 1–5 for field inspection apply (see Section 3.1.1.2).
For a sample size of 400, if no infected seeds are found, the rule of 3 (Equation (5)) indicates that a 95% upper limit for the proportion infected seed is 3/400 = slightly less than 1%. To put this number in perspective: if a seed test is carried out, and no infected seeds are found, and we plant a hectare of maize (60,000 plants) using the seed lot from which the sample was drawn, we can be 95% confident that not more than 3/400 × 60,000 = 450 infected seeds would be sown on this hectare. Evidently, this is not providing the level of protection that is required.
The Panel inquired among EU MS and Switzerland about their practices of seed testing for P. s. subsp. stewartii in maize imports:
The Netherlands (which has a large import) does not conduct tests for P. s. subsp. stewartii (R. Potting, Nederlandse Voedsel‐ en Warenautoriteit, pers. comm., March 2019).
France tests 10% of the seed lots for P. s. subsp. stewartii. If an infected seed lot is intercepted, then all subsequent seed lots from that country are examined. If for 6 months there are no interceptions from that country, the inspections are done again on 10% of the seed lots (A. Chatry, Ministère de l'Agriculture et de l'Alimentation, France, pers. comm., March 2019).
Italy does not conduct tests in the laboratory on imported maize seed lots for the presence of P. s. subsp. stewartii (L. Campus, Servizio fitosanitario centrale, Italy, pers. comm., May 2019).
Romania (the major grower of maize in the EU) tested all imported maize seed lots for the presence of P. s. subsp. stewartii, but imported very few maize seed lots from the USA in recent years (Paulina Gabor, Autoritatea Nationala Fitosanitara, Romania, pers. comm., March 2019).
Switzerland has a protocol to test 1,000 seeds, but has had no import of maize seed for sowing in the last years from countries with reported presence of P. s. subsp. stewartii (P. Kupferschmied, Swiss Plant Protection Service, pers. comm., March 2019).
Given the lack of testing in some main importers of maize seeds in the EU, and the inefficacy of testing due to the low sample size, testing at EU borders for the presence of P. s. subsp. stewartii was not considered. Testing a representative sample of seeds is considered in the USA, and may be used to assess the suitability of seed for exporting when the disease has been found in the production field of the seed. Block et al. (1999) wrote ‘Seed lots that fail a Stewart's wilt field inspection can be certified if they pass certain laboratory tests, typically a seedling grow‐out or a bulk‐seed enzyme‐linked immuno‐sorbent assay (ELISA) […]. Experience in the Iowa State University Seed Health Testing laboratory has shown that in most fields where the incidence or severity of Stewart's wilt was low, and the fields thus failed the field inspection, the pathogen was not detected by ELISA in the harvested seed’. That procedure may be justified in the sense that the rate of transmission of the pathogen to the seed is low if a variety is resistant and infection occurs late in the season. On the other hand, the sample size used for seed testing does not ensure an acceptable level of protection. It has been previously recognized that a sample size of 400 seeds may easily miss the pathogen even if it occurs in seed lots (Pataky and Ikin, 2003).
The actual proportions of infected seeds that we are interested in are much smaller, in the order of 1 in 100,000 or 1 in 1,000,000. With the large flows of seeds, these small proportions still result in biologically relevant numbers of infected seedlings emerging in the EU.
In Figure 6, the distribution for p1, i.e. the number of infected plants at the origin, is compared to the probability of detecting infected seed using a sample size of 400 seeds (p3), 1,000 seeds and 4,000 seeds. The distribution for the proportion of infected seeds produced at the origin will be even lower than p1, given that only a proportion of seed produced by infected plants will be infected by P. s. subsp. stewartii, further emphasising the futility of testing seeds with a sample size of 400 seeds, or even 1,000 and 4,000 seeds, unless there is high variability in the infection level among consignments (an issue on which the Panel was not able to retrieve information).
Figure 6.
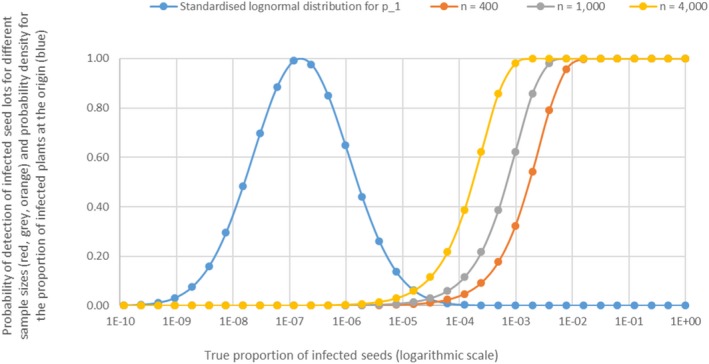
Probability distribution for the proportion of infected plants at the origin (p1) (blue) and probability of detection of infection when a sample of 400 (red), 1,000 (grey) and 4,000 (orange) seeds is tested using standard procedures, assuming 100% sensitivity of the test (p3)
Variation in infection level among consignments can increase the effectiveness of seed lot sampling (Appendix A), but given the lack of information on variation in P. s. subsp. stewartii infection level among consignments of maize seed, this issue was not included in the risk assessment model.
3.1.6. Seed to seedling transmission rate (p5)
The seed to plant transmission of P. s. subsp. stewartii was studied by Block et al. (1998). Four seed lots (AN1, PN1, PN2 and LN1) were collected from naturally infected maize fields, all grown in 1992:
Seed lot AN1 was harvested from a small field plot of inbred A632Ht from plants showing symptoms of systemic infection at harvest;
Seed lots PN1 and PN2 were harvested from a single commercial seed production field: PN1 was collected from plants that were infected but not prematurely killed, PN2 was harvested from plants that were infected and prematurely killed by Stewart's disease;
Seed lot LN1 originated from a commercial seed production field of inbred LH204.
Infected seeds were detected by ELISA of individual kernels. At least 100 kernels were sampled per seed lot; 100 additional kernels were assayed when lots contained less than 20% infected kernels, to increase the accuracy of the test.
A stem‐printing technique was used to evaluate seed to seedling transmission of P. s. subsp. stewartii at growth stages V2–V3. As reported by Block et al. (1998), ‘each stem was cut off 2–3 cm above the soil line, and the base of the stem was pinched to express sap. The cut cross‐section of each stem was then gently pressed onto agar media to leave a stem imprint containing plant sap on the agar’.
Grow‐out tests were carried out in a greenhouse in flats containing a pasteurised soil mix to determine the transmission of the bacterium from the seed to the seedling. The transmission of the bacterium from seeds to seedlings was calculated as the ratio of the number of infected seedlings to the number of potentially infected seedlings (i.e. the number of seeds multiplied by the proportion infected seeds determined for the same batch of seeds) (Table 12).
Table 12.
Summary of the results of Block et al. (1998) on seed to seedling transmission
| Lots | Tested kernels | Percentage of infected seeds | Probability of transmission from seed to seedlinga | Maximum estimated probability of transmission from seed to seedling (95% CLb) |
|---|---|---|---|---|
| AN1 | 200 | 10 ± 3.5 | 0.00000 | 0.00159 |
| LN1 | 200 | 3.5 ± 2.1 | 0.00000 | 0.03261 |
| PN1 | 200 | 9.0 ± 3.3 | 0.00137 | 0.00648 |
| PN2 | 100 | 35.0 ± 7.8 | 0.00000 | 0.00162 |
| Overall | 700 | – | 0.00022 | 0.00104 |
Total number of actual PS positive plants/total number of potential PS positive plants for the seed lot.
Upper one‐sided confidence limit.
Based on these results, the rate of transmission from seed to seedling was estimated at 0.022%; the expected proportion of P. s. subsp. stewartii–infected seedlings growing from infected seed would be 0.00022, or about two cases per 10,000 plants. At 62,500 plants per hectare, there would be an average of 13.75 infected plants per ha if all 62,500 seeds that are sown are infected seeds.
Michener et al. (2002) studied in detail the role of genotype susceptibility in plant to seed transmission. They rated Stewart's wilt and shared hybrids in resistant, moderately resistant and susceptible, based on the score. Hybrids with ratings less than 3 were classified as resistant. Hybrids with ratings between 3 and 4.5 were classified as moderately resistant. Hybrids with ratings greater than 4.5 were classified as susceptible. The impact of genotype susceptibility on plant to seed transmission is summarised in Table 13.
Table 13.
Summary of findings by Michener et al. (2002) on the seed to seedling transmission of Stewart's wilt
| Resistant | Moderately resistant | Susceptible | Year | |
|---|---|---|---|---|
| Infected kernels (%) | 0.024 | 0.19 | 11.58 | 1998 |
| 0.0007 | 0.07 | 7.80 | 1999 | |
| 0.0123 | 0.13 | 9.69 | Average |
3.2. Assessment
3.2.1. Scenario recapitulation
The following scenarios (visualised in Table 14) were considered:
Scenario A0 (current practice), based on the implementation of Annex IV, Part A, Section I, No. 52 of Council Directive 2000/29/EC:
Seed trade volume × prevalence at origin (based on reported yield losses) × plant to seed transmission rate (for a mixture of resistant and susceptible varieties) × effectiveness of sampling (representative seed sample found free of the pest in the US) × seed to seedling transmission rate.
Scenario A1 (US request for modification of EU conditions for derogation):
Seed trade volume × prevalence at origin (based on one mandatory inspection) × plant to seed transmission rate (for a mixture of resistant and susceptible varieties) × seed to seedling transmission rate.
Scenario A2 (EU conditions for derogation):
Seed trade volume × prevalence at origin (based on two mandatory inspections) × plant to seed transmission rate (for seed of resistant varieties) × seed to seedlings transmission rate.
Scenario A2a (EU conditions for derogation but with only one inspection = USA conditions for derogation but with resistant seed only):
Seed trade volume × prevalence at origin (based on one mandatory inspection) × plant to seed transmission rate (for seed of resistant varieties) × seed to seedlings transmission rate.
Scenario A2b (EU conditions for derogation but with both resistant and susceptible seed = USA conditions for derogation but with two inspections):
Seed trade volume × prevalence at origin (based on two mandatory inspections) × plant to seed transmission rate (for seed of resistant and susceptible varieties) × seed to seedlings transmission rate.
The effectiveness of requiring seed of resistant varieties was considered to affect the proportion of infected seed on infected mother plants. Calculations were done with and without this factor, so as to gauge its role in comparison with requiring two mandatory field inspections (Table 15).
Table 15.
Summary of the differences between scenarios A1, A2a, A2b and A2
| Import of resistant and susceptible genotypes | Import of resistant genotypes only | |
|---|---|---|
| One field inspection at the origin | A1 | A2a |
| Two field inspections at the origin | A2b | A2 |
3.2.2. Definition of the variables and elicitation of their distribution
3.2.2.1. Prevalence at the origin
The proportion of systemically infected mother plants in the USA (prevalence at the origin, p1) is defined in Table 16.
Table 16.
Definition of prevalence at the origin (p1)
| Parameter name | Definition | Sources |
|---|---|---|
| p1 | Proportion of systemically infected mother plants in the USA territory | Information on prevalence of P. s. subsp. stewartii in the USA; methodology for sampling in seed production fields |
The elicited distribution of the prevalence at the origin is reported in Table 17 and Figures 7, 8, 9.
Table 17.
Proportion of systemically infected mother plants in the USA (p1)
| Scenario | 1% | 25% | Median | 75% | 99% |
|---|---|---|---|---|---|
| A0 | 0.0002 | 0.00044 | 0.00088 | 0.00176 | 0.004 |
| A1 and A2a | 0.0000028 | 0.000081 | 0.0002 | 0.00039 | 0.00013 |
| A2b and A2 | 0.0000014 | 0.0000405 | 0.0001 | 0.000195 | 0.000065 |
Figure 7.
Fitted distribution for prevalence at the origin (p1) under scenario A0
Figure 8.
Fitted distribution for prevalence at the origin (p1) under scenarios A1 and A2a (one mandatory field inspection at the origin)
Figure 9.
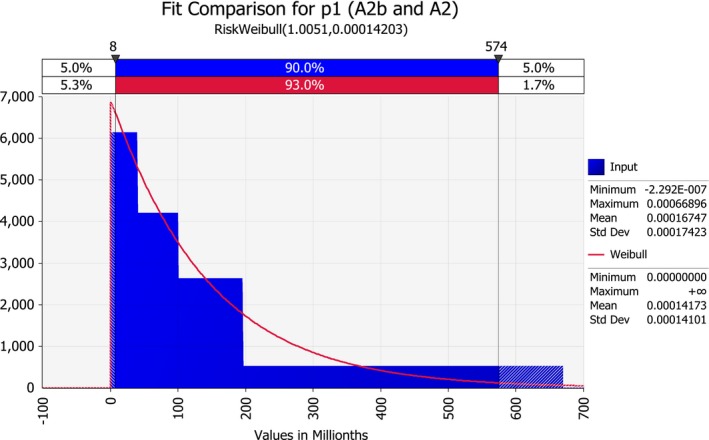
Fitted distribution for prevalence at the origin (p1) under scenarios A2b and A2 (two mandatory field inspections at the origin)
Justification: based on Table 3, the Panel calculated the average proportion yield loss of maize due to Stewart's wilt as 0.044%, i.e. an average ratio of 4.4 in 10,000. This proportion is related to the proportion of infected plants, but the relationship is not known. In an extreme assumption, we could take that 4.4 plants out of 10,000 are infected with total yield loss for these plants and no compensation by neighbours. As the plants have no yield, no infected seeds would be produced in the field. It could also be assumed that 8.8 plants are infected out of 10,000, with 50% yield loss for these plants, implying heavy systemic infection of those 8.8 plants, and a high likelihood of plant to seed transmission (Block et al., 1998). Likewise, a multiplier of three or four, or even up to 20 could be assumed, based on the assumed percentage loss per plant, respectively, 33, 25 or 5%. These numbers would be highly speculative, and the Panel used a multiplier of two to calculate the average incidence of Stewart's wilt from the yield loss and used substantial bounds of uncertainty around this number (scenario A0) (Table 17). Block et al. (1998) suggested a 15–25% threshold of severity for Stewart's wilt on leaves before bacteria are detected in seed.
With a rigorous inspection, these prevalence values could be decreased, affecting especially the higher quantiles. When considering a single inspection for a field size of 20–50 acres, more than 35,000 plants would be inspected. As a result, the expected level of prevalence is unlikely to be more than 1 in 10,000 plants. Based on Table 8 for a field size of 20–50 acres, the Panel used incidence values for fields with a single field inspection (scenarios A1 and A2a) as shown in Table 17.
When two inspections are carried out (scenarios A2b and A2), these values can likely be further decreased, but this depends on the sample size and the change in disease prevalence between the two inspections. With later inspection, going through the crop is substantially more difficult, and therefore it will be difficult to rigorously maintain the sampling protocol of NSHS. An increase in disease incidence would make the second inspection more efficient than the first; however, a lower sample size would decrease the efficiency. The Panel assumed equal efficiency for a first and second sample, but due to the extra sampling effort, the expected prevalence is further decreased. The Panel applied Equation (5) with λ set to 1 and n1 = n2.
3.2.2.2. Seed trade volume
Seed trade volume is defined in Table 18.
Table 18.
Definition of seed trade volume
| Parameter name | Definition | Sources |
|---|---|---|
| Ntrade | Number of maize seeds for sowing imported yearly (2020‐2024) from the USA to the EU | Eurostat information (2013–2017) and information from USDA (2017 and 2018) |
The elicited distribution of the trade volume of maize seed for sowing imported by the EU from the USA yearly is reported in Table 19 and Figure 10. In the model calculations, the distribution for the average (and not the yearly realisation) was used.
Table 19.
Elicitation of maize seed flow (metric tonnes (= 1,000 kg) per year)
| Quantile | 1% | 25% | Median | 75% | 99% |
|---|---|---|---|---|---|
| Yearly realisation | 1,600 | 6,000 | 9,000 | 11,500 | 14,000 |
| Average | 4,300 | 7,500 | 9,000 | 10,000 | 11,500 |
Figure 10.
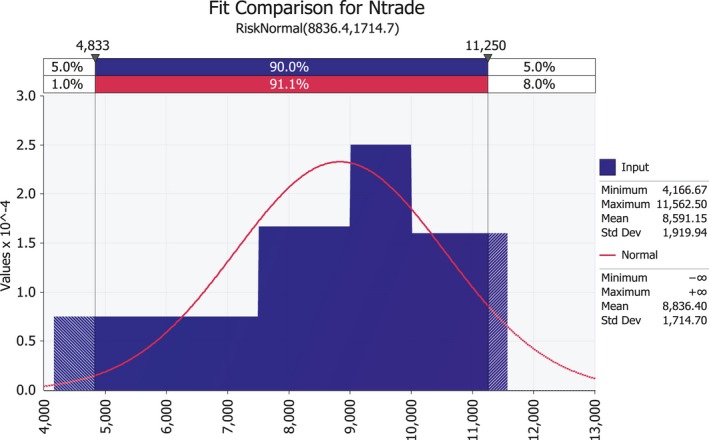
Fitted distribution for seed trade volume (all scenarios)
Justification: for the upper and lower boundaries of the yearly realisation, 20% was added resp. removed from the larger and lower numbers available from Eurostat (see Section 3.1.1.2) and USDA APHIS data. The 1% quantile (1,600) was obtained by removing 20% from 2040 (import in 2016). The 99% quantile (14,000) was obtained by adding 20% to 11439.
The median was obtained by averaging the average of EUROSTAT data (2013–2017) and the average of USDA APHIS data (2017 and 2018).
In the simulations, the average elicited numbers and not the yearly realisation were used.
3.2.2.3. Seed weight
The elicited distribution of the average weight of maize seed for sowing imported by the EU from the USA is reported in Table 20 and Figure 11.
Table 20.
Elicitation of seed weight (grams per seed)
| 1% | 25% | Median | 75% | 99% |
|---|---|---|---|---|
| 0.25 | 0.31 | 0.35 | 0.37 | 0.40 |
Figure 11.
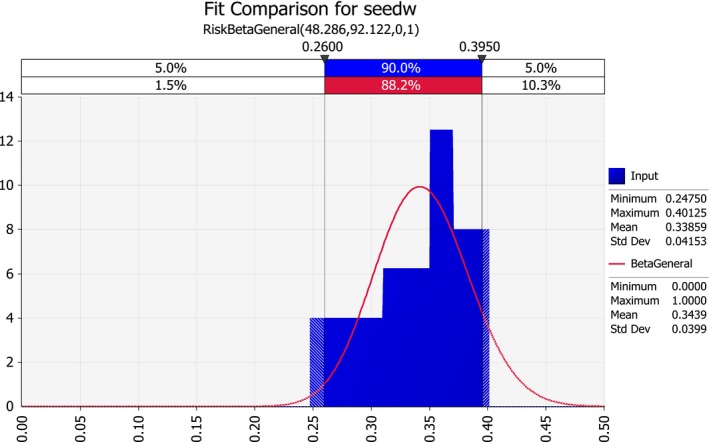
Fitted distribution for seed weight (all scenarios)
Justification: to elicit this distribution, the Panel checked online information from seed suppliers reporting maize seed weights for a range of commercial varieties (e.g. Caussade Semences, https://www.caussade-semences.it/download/CAUSS_CatPRIM_18_WEB.pdf; data for 2018).
3.2.2.4. Plant to seed transmission rate
The transmission rate from infected mother plants to seed is defined in Table 21. This parameter was assessed separately for susceptible vs. resistant varieties.
Table 21.
Definition of the transmission rate from plants to seed (p2)
| Parameter name | Definition | Sources |
|---|---|---|
| p2s and p2r | Proportion of infected seed on systemically infected mother plants (for susceptible and for resistant genotypes) | Block et al. (1998) |
The elicited distribution of the transmission rate from plants to seed (for susceptible genotypes) is reported in Table 22 and Figure 12.
Table 22.
Proportion of infected seed on susceptible mother plants (p2s)
| 1% | 25% | Median | 75% | 99% |
|---|---|---|---|---|
| 0.025 | 0.07 | 0.10 | 0.15 | 0.25 |
Figure 12.
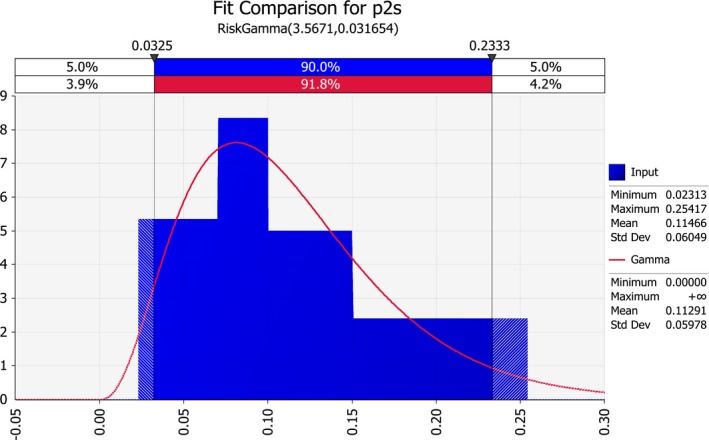
Fitted distribution for the proportion of infected seed on susceptible mother plants (scenarios A0, A1 and A2b)
Justification: the median was based on the average of about 10% from the Michener et al. (2002) experimental study. A similar average is reported by Block et al. (1998) for natural infection. A transmission rate of 10% is also reported in the Pataky and Ikin (2003) risk analysis.
The data provided by Block et al. (1998) for artificial infections were not considered here because not reflecting conditions in the field.
A transmission rate as high as 30% has been reported (Mezzalama, 2012), but a 99% quantile of 25% was chosen because the elicitation is an average across all susceptible genotypes.
The lowest reported transmission rate by Block et al. (1998) for natural infection is 3.5%, but a value of 2.5% was chosen for the 1% quantile because the available evidence only comes from two studies, which could have methodological limitations and may not apply to currently available maize genotypes.
The elicited distribution of the transmission rate from plants to seed (for resistant genotypes) is reported in Table 23 and Figure 13.
Table 23.
Proportion of infected seed on resistant mother plants (p2r)
| 1% | 25% | Median | 75% | 99% |
|---|---|---|---|---|
| 0.000003 | 0.000007 | 0.00012 | 0.00024 | 0.001 |
Figure 13.
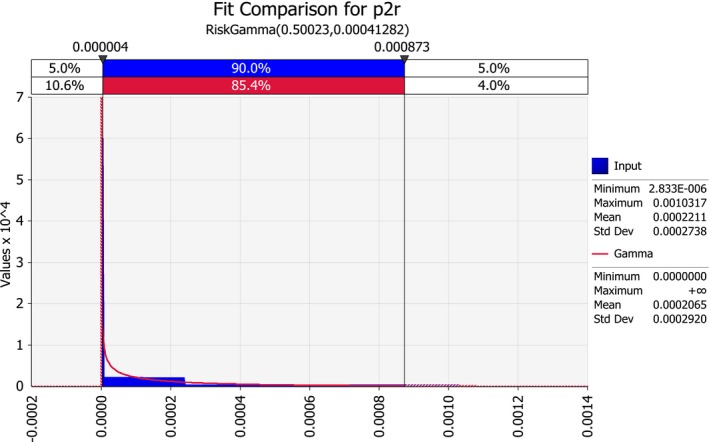
Fitted distribution for the proportion of infected seed on resistant mother plants (all scenarios)
Justification: Michener et al. (2002) reported an average for resistant genotypes of 0.0007% (1999) and 0.024% (1998). The corresponding proportions are: 0.000007 and 0.00024, which were used for the 25% and 75% percentiles. The median was chosen in between (i.e. averaging) these two numbers. The 99% and 1% percentiles were chosen in the light of the lack of available evidence, to reflect the uncertainty in this estimation.
3.2.2.5. Proportion of resistant seed
The proportion of resistant seed is defined in Table 24.
Table 24.
Proportion of seed resistant to Stewart's wilt in maize seed for sowing imported by the EU from the USA
| Parameter name | Definition | Sources |
|---|---|---|
| pr | Proportion of seed resistant to Stewart's wilt in maize seed for sowing imported by the EU from the USA | Pers. comm. USDA APHIS; Pal et al. (2019) |
The elicited distribution of the proportion of resistant seed in the trade is reported in Table 25 and Figure 14.
Table 25.
Proportion of resistant seed in the trade (pr)
| 1% | 25% | Median | 75% | 99% |
|---|---|---|---|---|
| 0.01 | 0.73 | 0.83 | 0.88 | 0.97 |
Figure 14.
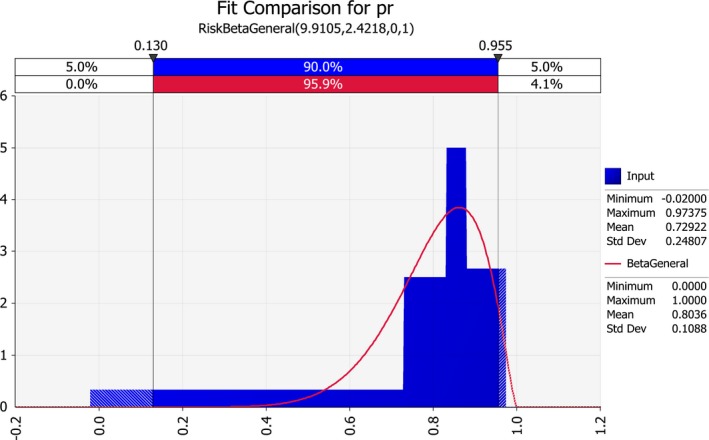
Fitted distribution for the proportion of resistant seed in the trade (scenarios with a mix of resistant and susceptible genotypes, i.e. A0, A1 and A2b)
Justification: the median quantile was estimated by assuming that 10% of trade is made of sweet corn (information kindly made available by USDA APHIS for 2017 and 2018), which was assumed to be only 20% of the times resistant to Stewart's wilt (see Section 3.1.4). For the 90% of trade comprising dent corn, it was assumed that seed is resistant to Stewart's wilt 90% of the times. Dent (field) maize is described as ‘generally less susceptible than sweet corn to the wilt phase, and although some inbred lines are susceptible, commercial dent corn hybrids are rarely susceptible’ (Pal et al., 2019).
The lower quantile (1%) was chosen taking into account the lack of evidence on the levels of susceptibility/resistance to Stewart's wilt in maize imported by the EU from the US. Note that because the fitted distribution for these quantiles is right‐skewed, there is only a 1% probability that pr is lower than 0.48 in the simulations.
3.2.2.6. Effectiveness of seed lot sampling
The effectiveness of testing seed lots (in the USA or at the EU border) is defined in Table 26.
Table 26.
Effectiveness of seed lot sampling (in the USA (p3) or at the EU border (p4))
| Parameter name | Definition | Sources |
|---|---|---|
| p3 and p4 | Proportional reduction of the inflow of infected seed into the EU as a result of seed lot testing (in the USA or at the EU border) | Separate calculations on efficacy of sampling and detection of P. s. subsp. stewartii in seed lots |
The elicited distribution of the effectiveness of seed lot sampling (in the USA) is reported in Table 27 and Figure 15.
Table 27.
Probability of interception based on seed lot testing (in the USA) (p3)
| 1% | 25% | Median | 75% | 99% |
|---|---|---|---|---|
| 0.0001 | 0.0003 | 0.001 | 0.003 | 0.01 |
Figure 15.
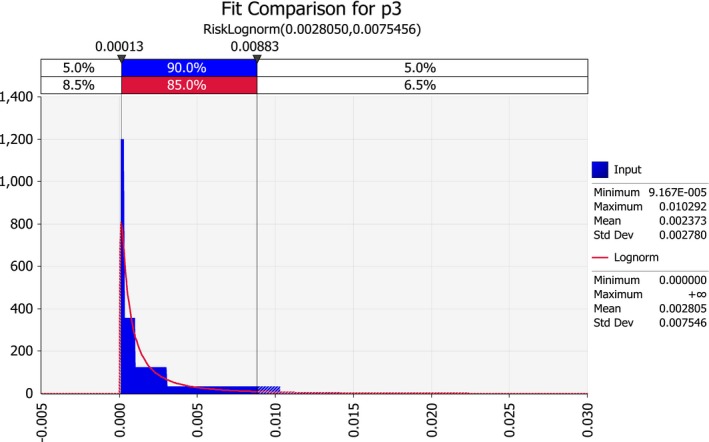
Fitted distribution for the probability of interception based on seed lot testing in the USA (scenario A0)
Justification: the quantiles were based on the probability of detecting infection if 400 seeds are tested (EPPO, 2016) depending upon the actual proportion of infected seeds in the seed lots, assuming the test has 100% sensitivity, assuming the prevalence at the origin estimated with p1, and applying this probability across a range of consignment sizes. The median was considered to be 10 times lower than the upper boundary.
If trade consists of seeds of resistant varieties, then this probability of interception goes further down, but this is not considered here to simplify.
If seed lot testing was performed systematically at the EU border, the same elicitation quantiles would also apply to p4 (probability of interception based on seed lot testing at the EU border). However, not all EU MS test for the presence of Stewart's wilt in imported maize seed lots from the US. Therefore, to simplify, and also given the low expected effectiveness of this measure, the Panel decided not to include in the models p4 (see Section 3.1.5).
3.2.2.7. Seed to seedling transmission rate
The transmission from infected seed to seedling is defined in Table 28.
Table 28.
Definition of transmission from seed to seedling (p5)
| Parameter name | Definition | Sources |
|---|---|---|
| p5 | Probability of transmission of P. s. subsp. stewartii from an infected seed to the seedling in the EU | Block et al. (1998) |
The elicited distribution for the probability of transmission from infected seed to seedling is reported in Table 29 and Figure 16.
Table 29.
Probability of transmission from seed to seedling (p5)
| 1% | 25% | Median | 75% | 99% |
|---|---|---|---|---|
| 0.00003 | 0.0001 | 0.00022 | 0.0005 | 0.0014 |
Figure 16.
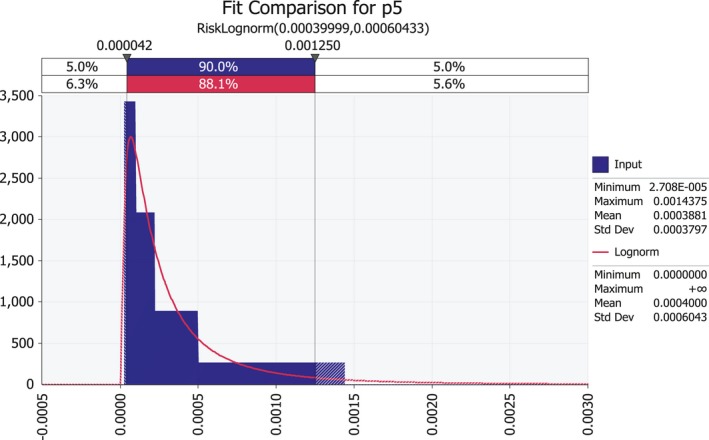
Fitted distribution for the probability of transmission from seed to seedling (all scenarios)
Justification: the quantiles were based on the data reported by Block et al. (1998) (see Section 3.1.6). Infected seed is reported to be produced by naturally infected plants at a rate of 0.022% (mean from infected kernels, which was used for the median quantile of the elicited distribution).
The upper quantile was based on the maximum estimated seed to seedling transmission rate (0.00137) for seed lot PN1 (Block et al., 1998).
The lower quantile was based on the seed to seedling transmission rate from seed produced on naturally infected plants (0.0029%; 1 of 34,924 plants) (Block et al., 1998), by taking into account the uncertainty inherent in this kind of transmission rate studies.
To simplify, the Panel did not consider the probability of transmission from seed to seedling to be different for susceptible vs. resistant varieties. Block et al. (1998) stated that there is no evidence for a different seed to seedling transmission rate in dent versus sweet corn.
3.2.3. Assessment results
Table 30 shows the outcome of the model calculations for Ninf (the number of infected seedlings growing each year out of infected USA seeds from import into the EU territory) for the considered scenarios. The results are visualised in Figures 17–21.
Table 30.
Outcome of the model calculation for the response variable (the number of infected seedlings growing each year out of infected USA seeds from import into the EU territory) under the five considered scenarios, using 10,000 simulation runs
| Scenario | Mean | SD | SE | 1% | 25% | Median | 75% | 99% |
|---|---|---|---|---|---|---|---|---|
| A0 | 1,140 | 2,895 | 28.9 | 6.4 | 128 | 383 | 1,062 | 11,662 |
| A1 | 269 | 756 | 7.6 | 0.57 | 23 | 77 | 238 | 2,903 |
| A2b | 133 | 395 | 3.9 | 0.31 | 11 | 39 | 117 | 1,422 |
| A2a | 0.61 | 2.48 | 0.02 | 0.00001 | 0.01 | 0.07 | 0.36 | 8.8 |
| A2 | 0.29 | 1.09 | 0.01 | 0.000006 | 0.01 | 0.04 | 0.17 | 4.0 |
Figure 17.
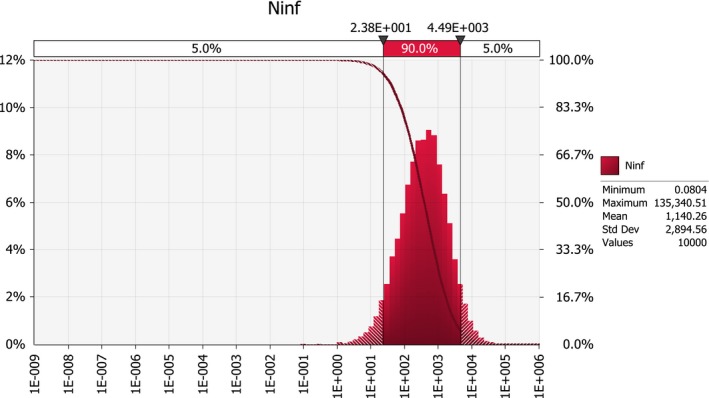
Outcome of the model simulations for scenario A0 (relative frequency and cumulative descending probability; x‐axis on a log scale; same scale as in Figures 18–21). The number of infected maize seedlings growing in the EU due to import of USA seed per year is between about 24 and about 4,490 with a 90% probability
Figure 21.
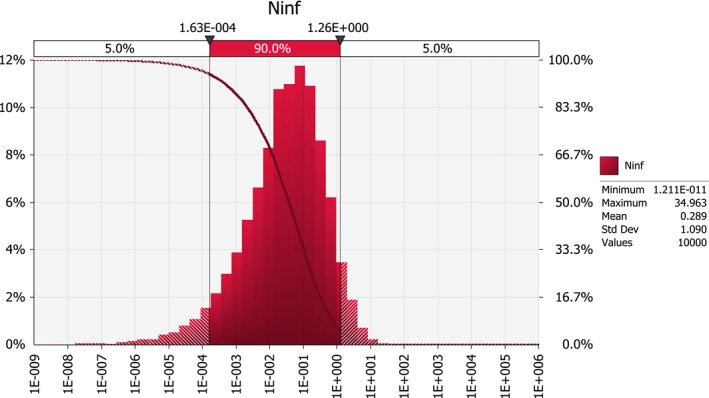
Outcome of the model simulations for scenario A2 (relative frequency and cumulative descending probability; x‐axis on a log scale; same scale as in Figures 17–20). The number of infected maize seedlings growing in the EU due to import of USA seed per year is between 0.0002 and about 1.3 with a 90% probability
Figure 18.
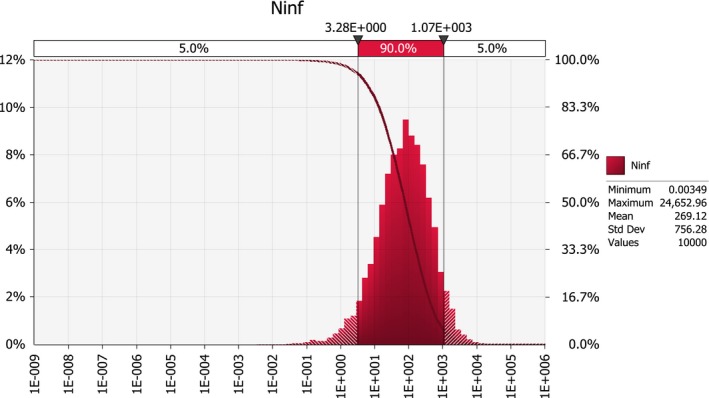
Outcome of the model simulations for scenario A1 (relative frequency and cumulative descending probability; x‐axis on a log scale; same scale as in Figures 17, 19, 20, 21, 19–21). The number of infected maize seedlings growing in the EU due to import of USA seed per year is between about 3.3 and about 1,070 with a 90% probability
Figure 19.
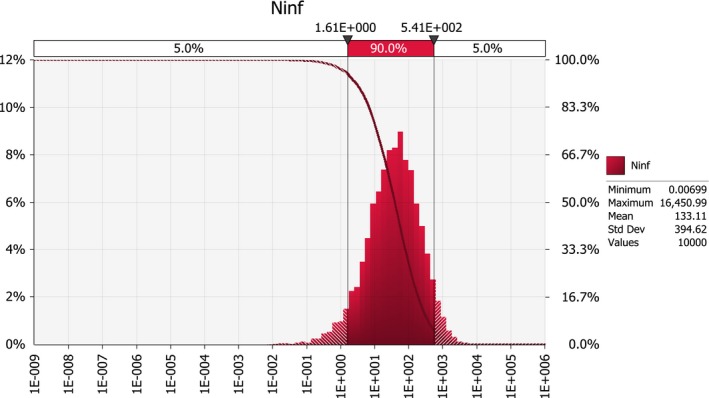
Outcome of the model simulations for scenario A2b (relative frequency and cumulative descending probability; x‐axis on a log scale; same scale as in Figures 17, 18, 20 and 21). The number of infected maize seedlings growing in the EU due to import of USA seed per year is between about 1.6 and about 540 with a 90% probability
Figure 20.
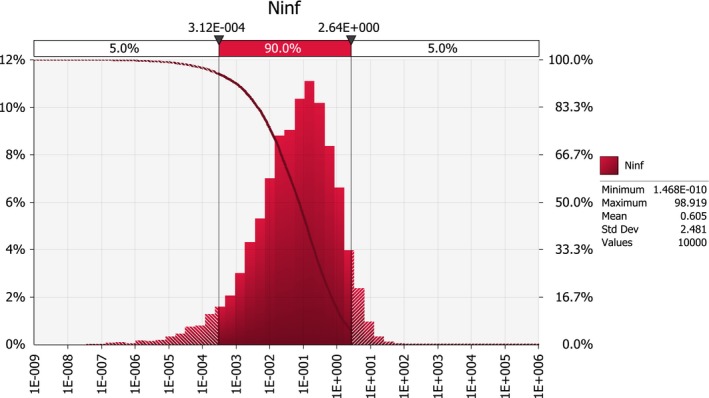
Outcome of the model simulations for scenario A2a (relative frequency and cumulative descending probability; x‐axis on a log scale; same scale as in Figures 17–19, 21). The number of infected maize seedlings growing in the EU due to import of USA seed per year is between 0.0003 and about 2.6 with a 90% probability
Scenario A0 results in an order of magnitude of some hundred introductions per year (median number), whereas the median number of entries under scenarios A1 and A2b is in the order of some tens per year. For scenarios A2a and A2, the median number of infected maize seedlings growing in the EU per year deriving from the import of infected seed from the USA is close to 0.1, i.e. one every ten years. Table 30 provides the uncertainty intervals for these estimates.
The ratio of the mean number of infected maize seedlings growing in the EU per year deriving from the import of infected seed from the USA for scenario A0 by the mean number for scenario A1 is about five. In other words, the expected number of infected seedlings is about five times larger under current practice than under the USA derogation request scenario. This implies that a requirement for field inspection, even while scrapping the option of seed testing, reduces the likelihood of entry by a factor of about five. It also substantiates the assertion of the USA that the prevalence of the disease is low; had the prevalence of the disease under current conditions been higher, then field inspection would have had greater effect.
The ratio of the mean number of entries for scenario A1 by the mean number of entries for scenario A2 is about 2,000. In other words, the expected number of infected seedlings is about 2,000 times larger under the USA derogation request scenario than under the EU conditions for derogation.
This reduction is mainly due to the requirement of restricting trade to resistant maize genotypes; scenario A2a with a requirement for resistant seed but with only one inspection (as in A1) reduced the number of infected seedlings by a factor of about 1,000, whereas scenario A2b which requires two field inspections but does not require import of resistant seed only resulted in a reduction by a factor of 2 compared to scenario A1.
Combining the requirement of a second field inspection with a restriction of trade to resistant genotypes (scenario A2) provides the highest level of protection among the examined scenarios.
3.3. Uncertainties affecting the assessment of entry
Based on the sensitivity analysis, for all scenarios one important factor responsible for the uncertainty in the assessment is the prevalence of Stewart's wilt at the origin (p1). In all scenarios, the seed to seedling transmission rate (p5) is also a variable explaining much of the variation of the output variable. In the scenarios A2 and A2a, also p2r (proportion of infected seed on systemically infected mother plants for resistant varieties) is a key uncertainty.
Nevertheless, even if the variables considered in the assessment model were not ranked among the first variables contributing to the uncertainty of the outcome, the estimation of the distribution for these variables was also uncertain, due to the limited nature of the available data.
From a biological point of view, various uncertainties regarding P. s. subsp. stewartii have been listed by the Panel in the previous pest categorisation (EFSA PLH Panel, 2018b). These uncertainties include:
the host range of P. s. subsp. stewartii: some of the papers recently published describe ‘P. stewartii’ as pathogenic to new hosts, but do not provide sufficient evidence to determine whether the isolated bacteria belong to the subspecies P. s. subsp. stewartii and cause Stewart's vascular wilt and leaf blight of maize.
the virulence of the strains of P. s. subsp. stewartii found in the EU.
the level of resistance available in EU maize germplasm collections, in commercial maize and sweet corn varieties grown in the EU and in the lines for hybrid creation.
the capacity of insects present in the EU and neighbouring countries to carry and disseminate P. s. subsp. stewartii.
whether papers related to Stewart's wilt refer to sweet corn varieties or maize (other than sweet corn) varieties.
Furthermore, there is an uncertainty regarding which species of Pantoea has been associated with symptomatic maize in Ukraine and whether this is P. s. subsp. stewartii (the pathogen causing Stewart's wilt) or not.
We know that, currently (scenario A0), the USDA APHIS field inspection protocol is voluntary, not mandatory. For scenarios A1, A2a, A2b and A2, there is uncertainty about the effectiveness of the USA field protocol for sampling maize fields for seed production for export, given the low prevalence of the disease at the origin.
The Panel considered the likelihood of entry with seed for breeding purposes. Such seed could be produced on susceptible parents. The Panel found no information on the size of this flow, how often susceptible parents are used and the location of production of such seed. Therefore, the Panel refrained from quantitative assessment for this particular pathway of entry.
Seed lot testing could lead to a relevant reduction in the import of infected maize seed if there is high variability in the infection level among consignments. There appears to be no information available about this variability (Appendix A).
Seed testing is not an effective measure for mitigating the likelihood of entry via seed for sowing if there is limited variability among consignments, given the low level of prevalence of the pathogen at the origin (see Figure 6). There could be specific instances in which seed testing is still advisable, for instance when seed is produced for breeding purposes from susceptible parents in locations where Stewart's wilt is endemic. Even in this case, field inspection after sowing the seed in the EU is needed to ascertain pest freedom because the efficacy of seed testing is constrained by sample size (number of seeds), especially for small seed lots.
3.4. Conclusion on the assessment of entry for the different scenarios
The quantitative model presented here shows that, despite the low prevalence of the disease in the country of origin, and the low rates of plant‐to‐seed and seed‐to‐seedling transmission that have been reported in the literature for Stewart's wilt, given the amount of traded seed, and in the case of voluntary (i.e. not mandatory) inspections of seed production fields (i.e. under current conditions), the frequency of introducing the pathogen is in the order of magnitude of some hundred introductions per year (median number). The likelihood of entry of P. s. subsp. stewartii has been regarded as negligible in the literature (e.g. by Michener et al., 2002; Esker and Nutter, 2003; Pataky, 2004), but has (to the best of our knowledge) not been quantified with a pathway model before. The likelihood of entry depends not just on the prevalence at origin and plant to seed and seed to plant transmission rates, but also on the yearly flow of imported seed. With an average seed import in the order of 5,200 tonnes per year (Table 9), and an average seed weight of about 0.35 g (see Section 3.2.2.3), the expected number of imported seeds is in the order of 15 billion per year. The size of the seed trade more than compensates for the low probability of transmission per seed.
A further outcome of the model developed here is that the likelihood of introducing P. s. subsp. stewartii in the EU by importing maize seed from the USA is expected to be higher under the A0 scenario (current practice) than under the A1 scenario (USA request for modification of EU conditions for derogation). This is because the estimated prevalence of the disease at the origin is much higher under current conditions, due to the voluntary nature of the field inspection protocol, which led the Panel to estimate disease prevalence based on recent estimations of maize yield losses attributed to Stewart's wilt.
Thus, the Panel concludes that the answer to the question in the mandate from the European Commission (‘whether the schema of field inspections suggested by the USA would provide a level of protection against the introduction of E. stewartii via seeds of Zea mays which is equivalent to the one stipulated in Annex IVA, section I, point 52 of Council Directive 2000/29/EC’) is positive, given that the likelihood of entry under current practice, with voluntary field inspections at the origin (A0), is expected to be higher than under a scenario with mandatory field inspections at the origin (A1). However, if there is substantial variation in the proportion of infected seed among consignments, and if seed sample size is increased sufficiently (e.g. to 1,000 to 4,000 seeds), seed lot inspection could lead to a relevant reduction in the level of infected imported maize seed. The Panel could not assess this accurately due to insufficient information on the variation among consignments in the level of infection.
However, the EU conditions for derogation would lead to a noticeable decrease in the likelihood of entry compared to the USA request for derogation. This protective effect is mainly due to the requirement that only genotypes resistant to Stewart's wilt are traded. The additional field inspection (two instead of one per season) provides additional reassurance.
The current practice (scenario A0) has a requirement for testing a representative sample to demonstrate absence of P. s. subsp. stewartii from the seed. The recommended sample size is 400 seeds (Pataky and Ikin, 2003; EPPO, 2016). Such a sample size is not suited to detect the pathogen at the levels of prevalence (e.g. one infected seed in 1,000,000 to one infected seed in 100,000,000 seeds) that can be anticipated if other RROs such as seed treatment and field inspections are carried out in the country of origin, unless there is high variability in the infection level among consignments.
Increasing the sample size of seed testing to thousands of seeds or more (while not improving the effectiveness of seed sampling, given the low prevalence of the pathogen at the origin (see Figure 6), again unless there is high variability in the infection level among consignments) would not be a realistic option, especially in the case of seed from inbred lines, because inbred lines produce a limited amount of seeds. The alternative requirement in Council Directive 2000/29/EC of producing the seed in a pest free area does not appear to be realistic for the USA, given that only two USA States are officially free from P.s. subsp. stewartii (see Section 3.1.1).
For the scenario A0, the median number of infected maize seedlings emerging in the EU due to the import of maize seed from the USA is estimated to be about 400 per year. In the absence of vectors, and with infected plants occurring in ordinary production fields, many of these introductions can go unnoticed. Thus, under current practice, a few noticed outbreaks of Stewart's wilt can be expected in the EU each year due to import of maize seed from the US. This is consistent with the recent observations from Italy (see Section 3.1.5).
In addition, the Panel notes that:
Not all EU import of maize seed for sowing from countries with reported presence of Stewart's wilt originates from the USA. For example, the EU also imports maize seed from Argentina and Mexico (with three recent interceptions on maize seed originating from Mexico).
To simplify, the Panel did not take into account that maize breeders in the USA are increasingly using winter nurseries in the Southern Hemisphere to accelerate breeding and seed production (Zaworski, 2016). This practice can facilitate the spread and increase the virulence of bacterial maize pathogens, as shown for the recent emergence of Xanthomonas vasicola pv. vasculorum (the causal agent of bacterial leaf streak on maize, which was first described in South Africa) in the USA and South America (Perez‐Quintero et al., 2019).
The data from Friuli in Italy suggest that the pathogen is able to overwinter in that region. This implies that not all the recent outbreaks from Italy have to originate directly from the import of infected maize seed: it could be that some of these outbreaks are the consequence of the pathogen overwintering after having been introduced in a previous year.
3.5. The situation in Italy (recent outbreaks)
Recently, outbreaks of P. s. subsp. stewartii have occurred in Italy. Here we review the available information to assess the possible role of seed imports in these outbreaks. With the exception of Table 31 (which summarises the reports of P. s. subsp. stewartii in EUROPHYT) and the data published in Alessandrini et al. (2017) and Bernardinelli et al. (2018), the information reported in this section was shared by the Italian working group on Stewart's wilt, with kind permission to include it here.
Table 31.
Summary of the reports of P. s. subsp. stewartii from Italy in EUROPHYT. There is an additional EUROPHYT finding from Slovenia (at the border with Friuli Venezia Giulia) from November 2018
| Reference | Date of detection | Region | Number of municipalities (Province) | Surface (ha) | Product destination | Severity | Phytosanitary measures |
|---|---|---|---|---|---|---|---|
| IT/SFC/2015/HH687 | August 2015 | Emilia Romagna | 1 (Parma) | 18 | – | Few symptomatic plants | – |
| IT/06/2017/02 | June 2017 | Friuli Venezia Giulia | 13 (Pordenone, Udine) | ~ 7 | Forage | – | – |
| IT/05/2017/01a | June 2017 | Veneto | 2 (Venice, Vicenza) | 59 | Seeds | < 1% | Change of destination (feed) |
| IT/08/2018/5b | May 2018 | Emilia Romagna | 2 (Bologna) | 2 | Seeds (Pioneer), parentals France | Low | Change of destination |
| IT/03/2018/4 | July 2018 | Lombardy | 1 (Cremona) | 35 | Seeds | 8 plants | Change of destination |
A control on parental plants was carried out, which resulted negative.
The Ministry decided that seed companies should analyse the parent lines before sowing. The analysis was done by Eurofins (France).
3.5.1. Field surveys
Four north‐Italian regions, where maize is the most frequently cultivated crop, carried out surveys starting from 2014 to detect P. s. subsp. stewartii outbreaks. These regions are Emilia Romagna, Lombardy and Veneto (fields for production of maize seed for sowing; Table 32), as well as Friuli Venezia Giulia (fields other than for production of maize seed for sowing; Table 33).
Table 32.
Surveys for the detection of P. s. subsp. stewartii in North Italian regions (2014–2018) in fields for production of seed for sowing. The area of fields for the production of maize seed for sowing in these regions varies and is generally in the order of 10 ha
| 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|
| Emilia Romagna | |||||
| N surveyed hectares | 835 | 840 | ~ 1,000 | 970 | 950 |
| N collected samples | 4 | 2 | 15 | 29 | 33 |
| N positive samples | 0 | 2 | 1 | 0 | 2 |
| Veneto | |||||
| N surveyed hectares | 930 | – | 743 | 106 | 122 |
| N collected samples | 0 | – | 0 | 11 | 10 |
| N positive samples | 0 | – | 0 | 3 | 6 |
| Lombardy | |||||
| N surveyed hectares | 2,654 | 1,852 | 1,446 | 1,102 | 984 |
| N collected samples | 0 | 0 | 0 | 0 | 11 |
| N positive samples | 0 | 0 | 0 | 0 | 8 |
Table 33.
Surveys for the detection of Stewart's wilt in Friuli Venezia Giulia (Italy) (2017–2018) in maize fields other than for the production of seed for sowing (grain or silage). Some fields were repeatedly sampled; therefore, positive samples do not necessarily correspond to different fields. In several cases, symptomatic plants were found close to each other, e.g. adjacent plants on the same row, or plants in different rows but very close to each other
| Friuli Venezia Giulia | 2017 | 2018 |
|---|---|---|
| Surveyed hectares | 134 | 139 |
| Collected samples | 168 | 187 |
| Positive samples | 21 | 42 |
In addition, the Ministry of Agricultural Policies set up an Italian working group on Stewart's wilt that shared a national program for the management of the disease in 2017 and 2018 in fields destined to seed production, with the following steps:
survey of at least 100 ha of maize destined to seed production in 3 regions (Lombardy, Veneto and Emilia Romagna);
sharing of a detection sheet with data on survey protocol, date and sample management;
use of pest traps for monitoring the known vector (C. pulicaria) in fields where the occurrence of Stewart's wilt is suspected;
destruction or change of destination (feed) for fields with confirmed occurrence of P. s. subsp. stewartii.
3.5.1.1. Emilia Romagna
The phytosanitary service of Emilia Romagna has carried out surveys to detect the occurrence of P. s. subsp. stewartii over the last years. Maize crops intended for seed production are geo‐referenced and surveyed in June, when the plants are 50–70 cm high, in growth stage 5–6 true leaves. The field sampling is carried out along the field diagonal in a staggered pattern across rows. If suspected symptoms of Stewart's wilt are detected, 5–10 symptomatic leaves are collected and samples are analysed to detect the bacterium. The applied diagnostic protocol follows EPPO (2016). In brief, collected symptomatic leaves are plated on agar media where bacterial colonies are visible after 3 days. Suspect colonies are identified with molecular tools and pathogenicity tests are performed on sweet corn plants.
In 2014, 835 ha of maize for seed production were surveyed, 4 samples were analysed and none was positive.
In 2015, 840 ha of maize for seed production were surveyed, 3 samples were analysed and one was positive, from the Province of Parma.
In 2016, around 1,000 ha of maize for seed production were surveyed, 15 samples were analysed and one was positive, from the Province of Bologna.
Since 2017, because of the outbreaks reported in 2015 and 2016 in two different fields in Emilia Romagna region, the bacterium has been included in the surveys co‐funded by European Commission (Alessandrini et al., 2017).
According to the Italian working group on Stewart's wilt, 970 and 950 ha of maize for seed production were surveyed in 2017 and 2018, respectively. In the same years, 29 and 33 samples were analysed and 0 and 2 samples were confirmed as P. s. subsp. stewartii, both from the province of Bologna.
3.5.1.2. Veneto
In Veneto, field surveys in maize crops for seed production started in 2014 and 930 ha were considered. In 2015, no survey was carried out, while in 2016 743 ha were monitored. No suspect samples were collected in those 3 years.
In 2017 and 2018, 106 and 122 ha were surveyed, respectively, and 11 and 10 suspect samples were collected. 3 positive samples were reported in 2017 and 6 in 2018.
3.5.1.3. Lombardy
In Lombardy, field surveys in maize crops for seed production started in 2014; 2,654, 1,852 and 1,446 ha were monitored in the 3‐year period 2014–2016, respectively. No suspect samples were collected in the 3 years.
In 2017 and 2018, 1,102 and 984 ha were surveyed, respectively, and 0 and 11 suspect samples were collected. 8 positive samples were reported in 2018.
3.5.1.4. Friuli Venezia Giulia
In 2017, for the first time, field surveys were performed in Friuli Venezia Giulia under the program mentioned before, co‐funded by the European Commission (Reg. UE. 652/2014).
The whole maize producing area was considered in the survey, but the selected maize crops were not for seed production, but for grain or silage. Inspections were carried out randomly, in agreement as much as possible with the criterium of optimal sampling point distribution and representativeness of the studied area. Seed origin and destination of the crop products were not considered.
Maize crops were surveyed walking along the diagonals of the fields or following the rows (depending on the plant growth stage and field size) looking for the characteristic symptoms caused by P. s. subsp. stewartii on the leaves. In case of suspect symptoms, 5–10 symptomatic leaves were collected, or whole seedlings in the case of particularly small plants, delivered to the laboratory in charge, and analysed according to the diagnostic protocol, with some modifications. In particular, DNA was extracted from leaves (not defined by the EPPO protocol) following a protocol, based on cetyl trimethylammonium bromide (CTAB), implemented according to UNI EN ISO 21571:2013 and Thapa et al. (2012). Pathogenicity tests were not performed. Some fields were repeatedly sampled; therefore, each positive sample does not necessarily correspond to a different field.
In 2017, 135 ha of maize, around 0.25% of the maize growing area, were monitored. A total of 361 visual inspections were carried out, with 202 of them showing suspect symptoms (Bernardinelli et al., 2018). 168 samples were collected, 45 samples resulted positive at real time polymerase chain reaction (RT‐PCR), and 21 were confirmed by plating. Positive samples were associated with yellow/green longitudinal strips along vessels and stunted plants. A few plant deaths were also noticed. Symptoms were commonly observed in small patches consisting of a few plants, usually close to each other and located at the border of the fields.
In 2018, 140 ha of maize were surveyed; some fields were sampled in the same areas as those infected in the previous year and some others randomly. On the whole, 187 samples were collected, with 42 samples resulting positive, as confirmed by plating (Table 33).
Infected plants can originate from infected seeds, but the various origins of the maize seeds used in the regions, as well as the origin of seeds from countries where the disease is not reported, make it doubtful that all findings of Stewart's wilt are directly due to the import of infected seed. The endemic presence of the disease in this region cannot be excluded because (i) the 2017 survey was the first survey, (ii) the incidence of infected plants is very low and (iii) it is not associated to economic damage.
The commonly reported location of infected plants at the border of fields could be related to the occurrence of P. s. subsp. stewartii in other host plants in the field margin or adjacent fields (Pataky and Ikin, 2003; EPPO, 2016; Alessandrini et al., 2017).
3.5.1.5. Insect sampling
In some of the Italian fields where symptoms of Stewart's wilt were found, some insects were also caught and some were positive to PCR screening (one insect belonged to the genus Phyllotreta – this genus is similar to the main vector in the country of origin C. pulicaria ‐ as well as some specimens of Halyomorpha halys). Other species such as Diabrotica virgifera virgifera were never found positive.
3.5.2. Conclusions on the situation in Italy
The data from northern Italy indicate rather frequent findings in recent years of P. s. subsp. stewartii in Friuli Venezia Giulia and sporadic findings in other provinces. Notifications in Emilia‐Romagna appear related to breeding activities and could be related to international movement of seed, but the more numerous notifications in Friuli Venezia Giulia indicate instead a locally endemic status of the pathogen with potentially a vector and winter reservoir of the pathogen.
Further studies in Friuli Venezia Giulia are needed to analyse the disease life cycle and the availability of vectors in this region. The Panel has not been able to obtain clarity on the origin of the seed used for sowing the fields with reports of Stewart's wilt in any of the regions.
4. Conclusions
The assessment answered positively the question in the mandate from the European Commission about ‘whether the schema of field inspections suggested by the USA would provide a level of protection against the introduction of E. stewartii via seeds of Zea mays which is equivalent to the one stipulated in Annex IVA, section I, point 52 of Council Directive 2000/29/EC’.
However, the assessment showed that under current practice, the level of protection against the introduction of P. s. subsp. stewartii may lead to the introduction of the disease, with a number of potential outbreaks per year as observed over the last years in Italy. The likelihood of entry depends not just on the prevalence at origin and the plant to seed and seed to plant transmission rates, but also on the yearly flow of imported seed. With an average seed import in the order of 5,200 tonnes per year (Table 9), and an average seed weight of 0.35 g (see Section 3.2.2.3), the expected number of imported seeds per year is in the order of 15 billion. The size of the seed trade more than compensates for the low probability of transmission per seed.
The likelihood of entry of the pathogen under the conditions of the USA request for derogation would be lower than under current practice (due to the mandatory, instead of voluntary, nature of the current field inspections at the origin), but it would be higher than under the EU conditions for a derogation (because the latter scenario would require an additional inspection per season and it would restrict trade to maize seed of genotypes resistant to Stewart's wilt).
The assessment also suggests that seed lot inspections, as currently carried out (e.g. with a sample of 400 seeds) are not likely to lead to a relevant reduction in the level of infected imported maize seed, given the low prevalence of Stewart's wilt at the origin, unless there is high variability in the infection level among consignments. This result is consistent with a study of viroid‐infected tomato and capsicum seed shipments to Australia (Constable et al., 2019), which showed that when infected lots are largely composed of uninfected seeds and only a few infected seeds (i.e. something like one infected seed in 16,000 healthy seeds), then the number of seeds per lot that need to be sampled in order to have a realistic chance of detecting a pathogen is much higher than 400.
The Panel has not been able to quantify how much seed is being shipped in small shipments and derives from susceptible parents in the country of origin for breeding purposes. Such consignment could pose a risk of entry that can possibly be mitigated by seed testing.
The Panel thinks it would be useful to conduct a global survey to characterise the molecular diversity of P. s. subsp. stewartii so as to pinpoint likely pathways of past introductions (invasion history) of the pathogen to regions where it is not endemic, as recently done for other plant pests (e.g. Anoplophora glabripennis: Javal et al., 2019; Cryphonectria parasitica: Demené et al., 2019; Phytophthora cinnamomi: Socorro Serrano et al., 2019; Pieris rapae: Ryan et al., 2019; Seiridium cardinale: Della Rocca et al., 2019). Furthermore, it would be useful to clarify the origin of the seed associated with current outbreaks in Italy and Slovenia. Finally, the identity of the pathogen causing bacterial symptoms on maize in the Ukraine warrants further investigation.
The Panel also notes in passing that, given that the recent decline in the prevalence of Stewart's wilt in the USA can be attributed (at least in part, if not largely) to the use of neonicotinoids, the ban of the use of these compounds in EU agriculture may lead to an increased likelihood of Stewart's wilt outbreaks, other things being equal. This is true at least for outbreaks that are unlikely to be attributed only to seed transmission, like some of those reported for Friuli Venezia Giulia.
The requirement of importing seed from maize varieties resistant to Stewart's wilt would not only contribute to lowering the likelihood of introducing the pathogen, but is also expected to reduce its potential spread and thus impacts, although this aspect was not studied quantitatively by the Panel.
The Panel also highlights that the impacts of Stewarts's wilt in the USA are higher in growing seasons following mild winters. This implies that, should the pest establish and spread in the EU, impacts might worsen in the coming decades due to ongoing climate warming.
Documentation provided to EFSA
See Section 1.2.3.
Abbreviations
- A0
Scenario reflecting current practice
- A1
Scenario reflecting the USA request for derogation
- A2
Scenario reflecting the EU conditions for derogation
- A2a
As in scenario A2, but without a second inspection
- A2b
As in scenario A2, but without the restriction of trade to resistant genotypes
- ELISA
Enzyme‐linked immuno‐sorbent assay
- EPPO
European and Mediterranean Plant Protection Organization
- IPPC
International Plant Protection Convention
- MS
Member State
- Ninf
Number of maize seedlings infected by P. s. subsp. stewartii growing in the EU due to import of USA seed per year
- Ntrade
Quantity of maize seed imported by the EU from the USA
- p1
Prevalence at the origin
- p2r
Probability of transmission from infected plant to seed for resistant genotypes
- p2s
Probability of transmission from infected plant to seed for susceptible genotypes
- p3
Probability of interception due to seed lot sampling in the USA
- p4
Probability of interception due to seed lot sampling at the EU border
- p5
Probability of transmission from infected seed to seedling
- pr
Proportion of resistant genotypes in the seed trade
- PLH
EFSA Panel on Plant Health
- RRO
Risk reduction option
- ToR
Terms of Reference
Appendix A – Variability in infection level among consignments and effectiveness of seed lot testing
1.
The pathway model described in Section 2.2.2 accounts mathematically for uncertainty in the parameters contained in the model, but the model does not include the effect of variability among consignments in the proportion of infected seeds on the probability of pathogen detection by seed testing. In this Appendix, the Panel developed a separate model to study the effect of variability among consignments.
With this additional model, three cases (Tables A.1–A.3) were analysed. The first case only includes uncertainty in the proportion of infected mother plants in the country of origin and the plant to seed transmission rate. The second and third cases include Monte Carlo methods to generate variation among consignments in the proportion of infected seeds, while keeping the overall average level of infection equal to the first case. In principle, the more the infected seeds are concentrated in few consignments, the greater is the efficacy of seed testing as a methodology to remove the more severely infected consignments from the trade flow. Variability was included using the beta distribution, and the model was programmed in the R programming language (see Supplementary Information – Annex B).
Table A.1.
Overall detection chance of P. s. ssp. stewartii when importing seed lots when accounting for the uncertainty in the elicited proportion of infected seeds, for three sample sizes (400, 1,000 and 4,000 seeds). 10,000 Monte Carlo draws were made to represent the variation in the proportion of infected seed, based on uncertainty about the proportion of infected mother plants in the country of origin and the rate of transmission to seed
| Import of seeds | Average level of infection | Sample size | ||
|---|---|---|---|---|
| n = 400 | n = 1,000 | n = 4,000 | ||
| Susceptible seeds only | 131 per million | 0.05 | 0.12 | 0.34 |
| Resistant seeds only | 0.24 per million | 0.00009 | 0.0002 | 0.0009 |
| Mixture of susceptible and resistant seeds (A0 scenario) | 27 per million | 0.01 | 0.03 | 0.09 |
Table A.3.
Detection chance of P. s. subsp. stewartii when importing seed lots when accounting for the uncertainty in the proportion of infected seeds elicited, for three sample sizes. One million Monte Carlo draws were made to represent the variation in the proportion of infected seed, based on uncertainty about the proportion of infected mother plants in the country of origin and the rate of transmission to seed. In addition, for this table, the variability in the proportion of infected seeds among consignments was simulated with a beta distribution. Here a beta distribution with an overdispersion parameter was used
| Import of seeds (A0 scenario) | Average level of infection | Sample size | ||
|---|---|---|---|---|
| n = 400 | n = 1,000 | n = 4,000 | ||
| Susceptible seeds only | 131 per million | 0.11 | 0.24 | 0.60 |
| Resistant seeds only | 0.24 per million | 0.008 | 0.02 | 0.08 |
| Mixture of susceptible and resistant seeds | 27 per million | 0.04 | 0.09 | 0.28 |
The three cases were studied in three situations regarding the susceptibility/resistance to Stewart's wilt of the maize parent plants:
seed produced on susceptible parents (‘susceptible seed’) only.
seed produced on resistant parents only (‘resistant seed’) only.
seed produced on both susceptible and resistant parents as described before.
In all three situations, the prevalence at the origin (p1) is estimated as in scenario A0 (see Section 3.2.2.1).
The proportion of infected seed in the three situations is:
p1 × p2s for seed produced on susceptible parents (‘susceptible seed’)
p1 × p2r for seed produced on resistant parents (‘resistant seed’)
for a mix of resistant and susceptible seed, as in scenario A0.
The probability of detection, given the proportion of infected seed in the trade p, is:
1 – (1 – p)n
where n is the sample size for seed testing (see Annex B).
Results for the case without variability among consignments is given in Table A.1.
Results in Table A.1 show that for a sample size of n = 400, and a mixture of susceptible and resistant seeds, the interception chance is about 0.01. When the sample size is increased, the interception chance also increases. The chance of interception falls short of reaching levels that could be considered representative of an effective test (e.g. greater than 80% chance of detection), but it should be kept in mind that in this case, variation among consignments is not accounted for.
Table A.2 gives results when assuming substantial variation among consignments in the proportion of infected seeds. This variation results in higher numbers of consignments with a sufficient number of infected seeds to be detectable in seed tests. With increasing sample sizes, the level of detection becomes also higher. However, the level of detection becomes lower if the trade would only consist of resistant seeds, for which the average level of infection is far lower than in the case of a mixture of susceptible and resistant seeds.
Table A.2.
Detection chance of P. s. subsp. stewartii when importing seed lots when accounting for the uncertainty in the proportion of infected seeds elicited, for three sample sizes. One million Monte Carlo draws were made to represent the variation in the proportion of infected seed, based on uncertainty about the proportion of infected mother plants in the country of origin and the rate of transmission to seed. In addition, for this table, the variability in the proportion of infected seeds among consignments was simulated with a beta distribution. Here a beta distribution with an overdispersion parameter θ = 500 was used
| Import of seeds | Average level of infection | Sample size | ||
|---|---|---|---|---|
| n = 400 | n = 1,000 | n = 4,000 | ||
| Susceptible seeds only | 131 per million | 0.49 | 0.71 | 0.92 |
| Resistant seeds only | 0.24 per million | 0.42 | 0.66 | 0.89 |
| Mixture of susceptible and resistant seeds (A0 scenario) | 27 per million | 0.46 | 0.68 | 0.90 |
Table A.3 gives results when assuming less variation among consignments in the proportion of infected seeds than assumed in the previous case of Table A.2. With less variation among consignments, the overall effectiveness of seed testing decreases.
Conclusion
The Panel was not able to obtain information on the actual variation among consignments in the proportion of infected seeds. New research and knowledge elicitation with inspectors would be required to obtain such information. Therefore, the above results can only be used to illustrate the principle that variation in the proportion of infected seed among consignments would increase the effectiveness of testing seed samples. Thus, if there is substantial variation among consignments in the proportion of infected seeds, seed testing could be an effective or moderately effective method to detect the infected lots between the majority of lots that are (relatively) pathogen free. If such variation is not present, seed testing is not an effective method to mitigate entry of infected seeds, due to the overall average low level of infection in the traded seed (see Section 3.1.5).
Annex A – @risk file for the calculations for the risk assessment of the entry of Pantoea stewartii subsp. stewartii on maize seed imported by the EU from the USA
1.
Annex A can be found in the online version of this output (‘Supporting information‘ section): https://doi.org/10.2903/j.efsa.2019.5851
Annex B – R code Pantoea stewartii subsp. stewartii seed lot sampling
1.
Annex B (R code for calculating the effectiveness of seed sampling as a measure to reduce or prevent entry of the pathogen in the EU with trade in maize seeds from the USA) can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2019.5851
Supporting information
©risk file for the calculations for the risk assessment of the entry of Pantoea stewartii subsp. stewartii on maize seed imported by the EU from the USA
R code Pantoea stewartii subsp. stewartii seed lot sampling
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Vicent Civera A, Yuen J, Zappalà L, Battilani P, Pautasso M and van der Werf W, 2019. Scientific Opinion on the risk assessment of the entry of Pantoea stewartii subsp. stewartii on maize seed imported by the EU from the USA. EFSA Journal 2019;17(10):5851, 49 pp. 10.2903/j.efsa.2019.5851
Requestor: European Commission
Question Number: EFSA‐Q‐2018‐00902
Panel members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen, Lucia Zappalà
Acknowledgments: The Panel thanks for the information provided to this scientific output: Olga Bashynska (Department of Phytosanitary Safety, Ukraine), Charles Block (National Seed Health System, USA), Lina Campus and Franco Finelli (Phytosanitary Service, Italy), Arnaud Chatry (Ministère de l'Agriculture et de l'Alimentation, France), Oene Dolstra (Wageningen University, the Netherlands), Paulina Gabor (Autoritatea Nationala Fitosanitara, Romania), Jaap Janse (formerly at the Dutch Plant Protection Service), Peter Kupferschmied (Swiss Plant Protection Service), David Makowski and Valérie Olivier (INRA, France), Matthew Messenger and Michael Perry (USDA APHIS, hearing experts), Monica Mezzalama (University of Turin, Italy), Andrew Mitchell (DEFRA, UK), Daren Mueller (Iowa State University, USA) and Primož Pajk (Ministry of Agriculture, Forestry and Food, Slovenia). Technical support was kindly provided by: José Cortinas Abrahantes, Valeria Ercolano, Olaf Mosbach Schulz (EFSA, AMU Unit) and Andrea Gennaro (EFSA, GMO Unit). The Panel acknowledges all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO; Figure 2: © Bugwood.org; Figure 4: © Cornell University, US; Figure 5: © Lu Liu, Iowa State University, US.
Adopted: 26 September 2019
Notes
In this risk assessment, the terms ‘maize’ and ‘corn’ are treated as synonymous.
Erwinia stewartii (Smith) Dye is a junior synonym for Pantoea stewartii (E.F. Smith, 1898). As E. stewartii is the name which is mentioned in Annex IVA to Directive 2000/29/EC, it is used also here.
EFSA Journal 2018;16(7):5356, 27 pp. https://doi.org/10.2903/j.efsa.2018.5356
References
- Alessandrini A, Babini AR, Barioni E, Gozzi R, Manzali D and Biccheri R, 2017. Avvizzimento del mais, rafforzata la vigilanza. Agricoltura, luglio‐agosto, 39–40. [Google Scholar]
- Anon , 2008. ISPM No. 31. Methodologies for sampling of consignments. International Standards for Phytosanitary Measures. Available online: https://www.ippc.int/static/media/files/publication/en/2016/11/ISPM_31_2008_Sampling_of_consignments_EN.pdf [Accessed: September 2019].
- Anon , 2018. Corn flea beetle index at Ohio locations, used to predict severity of Stewart's bacterial wilt disease on sweet corn. No publisher, 1 p. Available online: https://cpb-us-west-2-juc1ugur1qwqqqo4.stackpathdns.com/u.osu.edu/dist/1/8311/files/2016/02/Corn-Flea-BeetleCumul2018r-1rcl18l.pdf [Accessed: December 2018].
- Bernardinelli I, Malossini G, Benvenuto L, Zanolli P, Bianchi GL, Di Bernardo N, Lenardon A and Saro S, 2018. La batteriosi del mais: risultati del monitoraggio realizzato in Friuli Venezia Giulia nel 2017. Notiziario ERSAF, 1, 13–17. Available online: http://www.ersa.fvg.it/export/sites/ersa/aziende/in-formazione/notiziario/allegati/2018/1/La-batteriosi-del-mais.pdf [Accessed: September 2019]. [Google Scholar]
- Block CC, Hill JH and McGee DC, 1998. Seed transmission of Pantoea stewartii in field and sweet corn. Plant Disease, 82, 775–780. [DOI] [PubMed] [Google Scholar]
- Block CC, Hill JH and McGee DC, 1999. Relationship between late‐season severity of Stewart's bacterial wilt and seed infection in maize. Plant Disease, 83, 527–530. [DOI] [PubMed] [Google Scholar]
- Bourhis Y, Gottwald T and van den Bosch F, 2019. Translating surveillance data into incidence estimates. Philosophical Transactions of the Royal Society B, 374, 20180262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CA, Mehl K and Pfeufer E, 2017. Stewart's wilt of corn. Plant Pathology Fact Sheet, University of Kentucky, 3 pp. Available online: https://plantpathology.ca.uky.edu/files/ppfs-ag-c-04.pdf [Accessed: September 2019]
- CABI , 2019a. Bacterial wilt of maize. Plantwise Technical Factsheet. Available online: https://www.plantwise.org/KnowledgeBank/Datasheet.aspx?dsid=21939 [Accessed: September 2019].
- CABI , 2019b. Bean seed fly. Invasive Species Compendium. Available online: https://www.cabi.org/isc/datasheet/28168 [Accessed: September 2019].
- Chaky JL, Dolezal WE, Ruhl GE and Jesse L, 2013. A trend study of the confirmed incidence of Stewart's wilt of corn, Pantoea stewartii subsp. stewartii, in the U.S.A. (2001‐2012). Phytopathology, 103(9), 4–4. [Google Scholar]
- Constable F, Chambers G, Penrose L, Daly A, Mackie J, Davis K, Rodoni B and Gibbs M, 2019. Viroid‐infected tomato and capsicum seed shipments to Australia. Viruses, 11, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Rocca G, Danti R, Williams N, Eyre C and Garbelotto M, 2019. Molecular analyses indicate that both native and exotic pathogen populations serve as sources of novel outbreaks of Cypress Canker Disease. Biological Invasions, 21, 2919–2932. [Google Scholar]
- Demené A, Legrand L, Gouzy J, Debuchy R, Saint‐Jean G, Fabreguettes O and Dutech C, 2019. Whole‐genome sequencing reveals recent and frequent genetic recombination between clonal lineages of Cryphonectria parasitica in western Europe. Fungal Genetics and Biology, 130, 122–133. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Guidance on expert knowledge elicitation in food and feed safety risk assessment. EFSA Journal 2014;12(6):3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G 2018a. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Manceau C, Pautasso M and Caffier D, 2018b. Scientific opinion on the pest categorisation of Pantoea stewartii subsp. stewartii . EFSA Journal 2018;16(7):5356, 27 pp. 10.2903/j.efsa.2018.5356 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Hart A, Maxim L, Siegrist M, Von Goetz N, Da Cruz C, Merten C, Mosbach‐Schulz O, Lahaniatis M, Smith A and Hardy A, 2019. Guidance on communication of uncertainty in scientific assessments. EFSA Journal 2019;17(1):5520, 73 pp. 10.2903/j.efsa.2019.5520i [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott C, 1941. Bacterial wilt of corn. US Department of Agriculture, Farmer's Bulletin, No. 1878.
- EPPO (European and Mediterranean Plant Protection Organization), 1997. Data sheets on quarantine pests: Pantoea stewartii subsp. stewartii In: Smith IM, McNamara DG, Scott PR, Holderness M. (eds.). Quarantine Pests for Europe, 2nd Edition CABI/EPPO, Wallingford. 1425 pp. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2016. PM 7/60 (2) Pantoea stewartii subsp. stewartii . EPPO Bulletin, 46, 226–236. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2019. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: September 2019].
- Esker PD and Nutter FW, 2003. Temporal dynamics of corn flea beetle populations infested with Pantoea stewartii, causal agent of Stewart's disease of corn. Phytopathology, 93, 210–218. [DOI] [PubMed] [Google Scholar]
- Javal M, Lombaert E, Tsykun T, Courtin C, Kerdelhué C, Prospero S, Roques A and Roux G, 2019. Deciphering the worldwide invasion of the Asian long‐horned beetle: a recurrent invasion process from the native area together with a bridgehead effect. Molecular Ecology, 28, 951–967. [DOI] [PubMed] [Google Scholar]
- Liu L, 2010. Quantifying the aggressiveness, temporal and spatial spread of Pantoea stewartii in sweet corn. MSc thesis, Iowa State University, Iowa, US, 110 pp. Available online: https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=2733%26context=etd [Accessed: September 2019].
- Mezzalama M, 2012. Seed Health: Fostering the Safe Distribution of Maize and Wheat Seed: General Guidelines. 3rd Edition. CIMMYT, Mexico. Available online: https://cropgenebank.sgrp.cgiar.org/images/file/management/FosteringSafeDistributionMaizeWheatThirdEdition.pdf [Accessed: September 2019].
- Michener PM, Pataky JK and White DG, 2002. Transmission of Erwinia stewartii from plants to kernels and reactions of corn hybrids to Stewart's wilt. Plant Disease, 86, 167–172. [DOI] [PubMed] [Google Scholar]
- Mueller DS, Wise KA, Sisson AJ, Allen TW, Bergstrom GC, Bosley DB, Bradley CA, Broders KD, Byamukama E, Chilvers MI, Collins A, Faske TR, Friskop AJ, Heiniger RW, Hollier CA, Hooker DC, Isakelt T, Jackson‐Ziems TA, Jardine DJ, Kelly HM, Kinzer K, Koenning SR, Malvick DK, McMullen M, Meyer RF, Paul PA, Robertson AE, Roth GW, Smith DM, Tande CA, Tenuta AU, Vincelli P and Warner F, 2016. Corn yield loss estimates due to diseases in the United States and Ontario, Canada from 2012 to 2015. Plant Health Progress, 17, 211–222. [Google Scholar]
- Neergard P, 1977. Seed Pathology. Vols. 1 and 2. MacMillan, London. [Google Scholar]
- Pal N, Block CC and Gardner C, 2019. A real‐time PCR differentiating Pantoea stewartii subsp. stewartii from P. stewartii subsp. indologenes in corn seed. Plant Disease, 103, 1474–1486. [DOI] [PubMed] [Google Scholar]
- Pataky JK, 2004. Stewart's wilt of corn. The Plant Health Instructor. Available online: 10.1094/PHI-I-2004-0113-01 [Accessed: September 2019]. [DOI]
- Pataky J and Ikin R, 2003. The risk of introducing Erwinia stewartii in maize seed. International Seed Federation, Nyon, Switzerland, 79 pp.
- Pepper EH, 1967. Stewart's bacterial wilt of corn. Monograph No. 4. American Phytopathological Society, St. Paul, MN, USA, 37 pp.
- Perez‐Quintero AL, Ortiz‐Castro M, Wu G, Lang JM, Liu S, Chapman TA, Chang C, Ziegle J, Peng Z, White FF, Plazas MC, Leach JE and Broders K, 2019. Genomic acquisitions in emerging populations of Xanthomonas vasicola pv. vasculorum infecting corn in the U.S. and Argentina. ArXiv, 25 March 2019. Available online: 10.1101/587915 [Accessed: July 2019]. [DOI] [PubMed]
- Roper MC, 2011. Pantoea stewartii subsp. stewartii: lessons learned from a xylem‐dwelling pathogen of sweet corn. Molecular Plant Pathology, 12, 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SF, Lombaert E, Espeset A, Vila R, Talavera G, Dincă V, Doellman MM, Renshaw MA, Eng MW, Hornett EA, Li Y, Pfrender ME and Shoemaker D, 2019. Global invasion history of the agricultural pest butterfly Pieris rapae revealed with genomics and citizen science. Proceedings of the National Academy of Sciences, 116(40), 20015–20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socorro Serrano M, Osmundson T, Almaraz‐Sánchez A, Croucher PJ, Swiecki T, Alvarado D and Garbelotto M, 2019. A microsatellite analysis identifies global pathways of movement of Phytophthora cinnamomi and the likely sources of wildland infestations in California and Mexico. Phytopathology, 10, 1577–1593. [DOI] [PubMed] [Google Scholar]
- Thapa SP, Park DH, Wilson C, Hur JH and Lim CK, 2012. Multiplex PCR assay for the detection of Pantoea stewartii subsp. stewartii using species‐specific genetic markers. Australasian Plant Pathology, 41, 559–564. [Google Scholar]
- Welty C, 2019. Corn flea beetle & Stewart's wilt predictions for 2019. Vegnet Newsletter, Ohio State University, 3 March 2019. Available online: https://u.osu.edu/vegnetnews/2019/03/09/corn-flea-beetle-stewarts-wilt-predictions-for-sweet-corn-in-2019/ [Accessed: September 2019].
- Zaworski F, 2016. Winter Breeding Programs in South America. SeedWorld, 18 May 2016. Available online: https://seedworld.com/winter-breeding-programs-south-america/ [Accessed: September 2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
©risk file for the calculations for the risk assessment of the entry of Pantoea stewartii subsp. stewartii on maize seed imported by the EU from the USA
R code Pantoea stewartii subsp. stewartii seed lot sampling


