Abstract
Background:
Temporary mechanical circulatory support (MCS) devices provide hemodynamic assistance for shock refractory to pharmacologic treatment. Most registries have focused on single devices or specific etiologies of shock, limiting data regarding overall practice patterns with temporary MCS in cardiac intensive care units (CICUs).
Methods and Results:
The Critical Care Cardiology Trials Network (CCCTN) is a multicenter network of tertiary CICUs in North America. Between 9/2017 and 9/2018, each center (n=16) contributed a 2-month ‘snapshot’ of consecutive medical CICU admissions. Of the 270 admissions using temporary MCS, 33% had acute myocardial infarction (AMI)-related cardiogenic shock (CS), 31% had CS not related to AMI, 11% had mixed shock, and 22% had an indication other than shock. Among all 585 admissions with CS or mixed shock, 34% used temporary MCS during the CICU stay with substantial variation between centers (range: 17%-50%). The most common temporary MCS devices were intra-aortic balloon pumps (IABP) (70%), Impella (16%) and veno-arterial extracorporeal membrane oxygenation (VA-ECMO) (11%), though IABP use also varied between centers (range: 40%-100%). Patients managed with IABP vs. other forms of MCS (advanced MCS) had lower SOFA scores and less severe metabolic derangements. Illness severity was similar at high- vs. low-MCS-utilizing centers and at centers with more advanced MCS use.
Conclusions:
There is wide variation in the use of temporary MCS among patients with shock in tertiary CICUs. While hospital-level variation in temporary MCS device selection is not explained by differences in illness severity, patient-level variation appears to be related, at least in part, to illness severity.
Introduction
The epidemiology of shock in contemporary cardiac intensive care units (CICUs) is highly varied.1, 2 Cardiogenic shock (CS), a life-threatening state of low cardiac output with systemic hypoperfusion, accounts for the majority of shock cases in CICUs but is increasingly related to causes other than acute myocardial infarction (AMI), including but not limited to acute on chronic heart failure, valvular heart disease, and myocarditis.1, 3 Mixed shock, with a hemodynamic profile characterized by the low or borderline cardiac output and elevated ventricular filling pressures expected in CS, and the low systemic vascular resistance expected in distributive shock, is the second most common form of shock in contemporary CICUs.1, 3 Although early revascularization has improved outcomes in patients with AMI-related CS (AMICS), in-hospital mortality among patients with AMICS, CS not related to AMI, and mixed shock remains high (30–40%).1, 3-7
For patients with shock refractory to pharmacologic treatment, temporary mechanical circulatory support (MCS) devices, placed either percutaneously or surgically, can provide additional hemodynamic support. These devices may be used as a bridge to ventricular recovery, to a durable form of MCS, to heart transplantation, or to a decision, including withdrawal of life-sustaining therapies.3 The most readily available form of temporary MCS is intra-aortic balloon pump (IABP) counterpulsation; however, the IABP-SHOCK II trial demonstrated that IABP counterpulsation does not reduce mortality in patients with AMICS.8 Other forms of temporary MCS provide more robust hemodynamic support, but it is unclear whether they improve outcomes when used as therapeutic adjuncts in the treatment of CS or mixed shock.9 Furthermore, optimal patient and device selection, as well as timing of device placement, remain uncertain.3
Most registries have focused on single devices or specific etiologies of CS (e.g., AMICS), limiting data regarding overall practice patterns for temporary MCS use in CICUs. The objective of this analysis was therefore to investigate the patterns of temporary MCS use in contemporary tertiary care CICUs using a well characterized cohort from a multi-institutional clinical registry.
Methods
Study Population
The Critical Care Cardiology Trials Network (CCCTN) is an investigator-initiated collaborative research network of American Heart Association (AHA) Level 1 CICUs10 located in the United States and Canada. The detailed structure, composition, and data collection for the CCCTN Registry have been previously described.2 Scientific oversight of the CCCTN is conducted by its academic Executive and Steering Committees, and the data are coordinated by the TIMI Study Group (Boston, MA). During the first enrollment period, 16 participating centers contributed clinical data on all consecutive medical admissions to the CICU over a two-month time period anytime between September 2017 and September 2018. The CCCTN Registry protocol and waiver of informed consent were approved by the institutional review committees at each of the participating centers. We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for further discussions.
The primary analysis population for the present study includes all admissions in the two-month “snap-shot” except for routine post-cardiac surgical patients and overflow from general medical ICUs. One center did not have any shock cases during the two-month “snap-shot” so did not contribute to the present analysis. In addition, several centers collected data on all consecutive admissions, including outside the two-month “snap-shot”. For several analyses in the present report, we utilize a full analysis population, which includes the primary analysis population as well as patients enrolled outside the two-month “snap-shot” (Supplemental Methods).
Temporary forms of MCS included intra-aortic balloon pump (IABP) counterpulsation, Impella percutaneous ventricular assist systems (2.5, CP, 5.0, RP), TandemHeart percutaneous ventricular assist systems, and veno-arterial extracorporeal membrane oxygenation (VA-ECMO). Centrally cannulated surgical devices (e.g., CentriMag) and durable left ventricular assist systems (LVAS) were not considered forms of temporary MCS for the purpose of this analysis; however, patients were included in this analysis if they had any form of temporary MCS prior to undergoing implantation of either of these surgical MCS systems. All temporary MCS device placements requiring transfer out of the CICU (e.g., to the cardiac surgical ICU) were included.
Data Collection
For all patients managed with temporary MCS, we collected the indication for support (e.g., shock vs. high-risk procedural support), pharmacologic support prior to temporary MCS, device type, and access site. A hierarchical classification scheme was used for patients who received multiple devices (VA-ECMO > Impella or TandemHeart > IABP). Patients who were managed with biventricular support (n=7) were classified according to the form of left ventricular support. Shock was defined as sustained hemodynamic impairment (i.e., systolic blood pressure [SBP] <90 mmHg or the need for inotropic or vasopressor support) with evidence of end-organ hypoperfusion.1 The etiology of shock was classified by the site investigator as cardiogenic, distributive, hypovolemic, mixed, or other/uncertain according to prespecified definitions. The “mixed” category included patients for whom both cardiogenic and distributive shock were determined to contribute (Supplemental Methods).1 All temporary MCS devices other than IABP were considered “advanced MCS.”
Data Analysis
For categorical variables, data are reported as counts and percentages with absolute 95% confidence intervals (CI) where relevant. For continuous variables, data are reported as medians with 25th and 75th percentiles unless otherwise specified.
The proportion of cardiogenic and mixed shock patients managed with temporary MCS and the proportions of IABP vs. advanced MCS were calculated for each site. Sites were then classified according to: (1) tertiles of the proportion of patients with cardiogenic or mixed shock who received temporary MCS for hemodynamic support (“high-” vs “low-MCS-utilizing sites”); and (2) tertiles of the proportion of patients who received IABP only as the form of temporary MCS. IABP-SHOCK II scores and SOFA scores were calculated for each tertile. Sensitivity analyses were performed restricting to patients with CS only (i.e., excluding mixed shock patients).
Among patients with cardiogenic or mixed shock in the primary analysis population, baseline characteristics, presenting features, and ICU resource utilization are summarized according to whether patients were managed without temporary MCS, with IABP only, or with advanced MCS. Differences in clinical variables between patients treated with advanced MCS and those treated with IABP only were evaluated with the Pearson chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables.
CICU and in-hospital mortality rates were calculated in all patients who received temporary MCS for cardiogenic or mixed shock. The associations between clinical variables and in-hospital mortality rates were evaluated using univariable logistic regression in the full analysis population. In addition, we compared mortality between patients who underwent temporary MCS device placement within vs. after 24 hours of admission in the full analysis population, adjusting for age, SOFA score, presenting lactate, number of inotropes/vasopressors, and preceding cardiac arrest.
Statistical significance was assessed at a nominal alpha level of 0.05. All reported p-values were two-sided. All statistical computations were performed with SAS System V9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Temporary mechanical circulatory support use
Among all 3049 CICU admissions in the primary analysis population, 270 were managed with temporary MCS during the CICU admission. Of those, 33% had AMICS, 31% had CS not related to AMI, 11% had mixed shock, 3% had other or uncertain shock, and 22% had an indication other than shock (e.g., severe valvular disease, refractory ischemia, peri-procedural support).
Among 585 patients with cardiogenic or mixed shock in the primary analysis population, 202 (34%) patients were managed with temporary MCS. The most commonly used temporary MCS devices for cardiogenic or mixed shock were IABP counterpulsation (70%), Impella (16%), and VA-ECMO (11%). The majority (79%) of cardiogenic or mixed shock patients managed with temporary MCS received only one type of device.
The median SOFA score in patients receiving temporary MCS for cardiogenic or mixed shock was 9 (IQR, 5–12), and 30% had a preceding cardiac arrest of which 37% underwent targeted temperature management. At the time of temporary MCS placement, 94% of these patients were receiving inotropes or vasopressors (median = 2 agents) and 22% were on ≥3 agents. Sixty-six percent of cardiogenic or mixed shock patients managed with temporary MCS were mechanically ventilated and 21% required renal replacement therapy. Excluding patients with temporary MCS placed at an outside facility (n=45), 72% of patients receiving temporary MCS for cardiogenic or mixed shock underwent device placement within 24 hours of CICU admission.
Variation in clinical practice patterns between CCCTN centers
There was substantial variation between centers in the proportion of patients with cardiogenic or mixed shock who received temporary MCS, ranging from 17% - 50% (Figure 1). As a percentage of total temporary MCS use, the use of IABP alone also varied substantially between centers, ranging from 40% to 100% (Figure 1). There was a moderately strong correlation between the overall proportion of cardiogenic or mixed shock patients receiving temporary MCS use and the proportion using advanced support vs. IABP at a given center (Spearman’s correlation coefficient = 0.53; p=0.04). The wide variation in the frequency and type of temporary MCS use was also observed in a sensitivity analysis restricted to patients with CS only (i.e., excluding mixed shock) (Supplemental Figure 1).
Figure 1. Proportion of cardiogenic and mixed shock patients managed with temporary mechanical circulatory support (MCS) by site.
IABP indicates intra-aortic balloon pump; MCS, mechanical circulatory support.
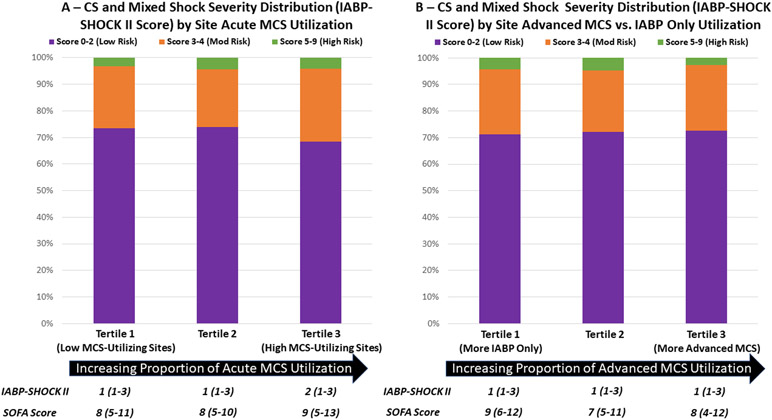
Although CS and mixed shock volumes were lowest at “low-MCS-utilizing sites” (12.5 cases/month vs. 22.6 cases/month at medium- and high-MCS-utilizing sites), the indices of shock severity (e.g., SOFA score, IABP-SHOCK II score) among cardiogenic and mixed shock patients at high-MCS-utilizing sites were similar to those at low-MCS-utilizing sites, suggesting that variability in MCS utilization was not driven by differences in overall risk profile of patients at each center. Furthermore, cardiogenic and mixed shock patients at sites where a higher proportion received advanced MCS had similar indices of shock severity as patients at sites where a higher proportion received IABP only (Figure 2). A sensitivity analysis restricted to patients with CS demonstrated similar patterns of shock severity across tertiles of temporary MCS utilization and tertiles of IABP only selection (Supplemental Figure 2).
Figure 2. Cardiogenic and mixed shock severity distribution by tertile of site temporary mechanical circulatory support (MCS) utilization and tertile of advanced MCS utilization.
Shock severity is categorized according to established cutpoints of IABP-SHOCK II score (0-2 points = low risk; 3-4 points = moderate risk; 5-9 points = high risk). The Y axis represents the total number of patients receiving temporary MCS. In Panel A, along the x axis, sites are classified into tertiles according to the proportion of patients with cardiogenic or mixed shock receiving temporary MCS. In Panel B, along the x axis, sites are classified into tertiles according to the proportion of patients receiving advanced MCS vs. IABP only among those receiving temporary MCS. CS indicates cardiogenic shock; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; mod, moderate.
Characteristics of patients receiving advanced MCS versus IABP
On an individual patient level, there were significant differences between patients who received advanced MCS vs. those who received IABP only. Patients receiving advanced MCS, as compared with IABP, were younger, had higher median SOFA scores and higher presenting lactates, and were managed with more ICU therapies, including renal replacement therapy, mechanical ventilation, invasive hemodynamic monitoring, and inotropes/vasopressors (all p<0.05) (Table 1). However, in a multivariable model for predicting selection of advanced MCS vs. IABP, study center alone accounted for approximately 90% of the explanatory value of the model (Supplemental Table 1).
Table 1.
Baseline characteristics, presenting features, and ICU resource utilization of patients receiving no MCS, IABP only, or advanced temporary MCS for management of cardiogenic or mixed shock.
| No MCS, % (n) |
IABP Only, % (n) |
Advanced MCS, % (n) |
|
|---|---|---|---|
| N=383 | N = 142 | N = 56 | |
| Demographics | |||
| Age, median (IQR), years | 65 (54 – 74) | 64 (54 – 74) | 63 (54 – 71) |
| Female sex | 36.8 (141) | 36.6 (52) | 26.8 (15) |
| Non-white race | 35.7 (172) | 25.2 (47) | 18.0 (15) |
| BMI, median (IQR), kg/m2 | 27.9 (23.5 -32.6) | 28.1 (24.5 – 33.0) | 28.4 (26.3 – 32.3) |
| Comorbidities | |||
| Current smoker | 13.8 (52) | 18.4 (26) | 26.8 (15) |
| Diabetes mellitus | 38.4 (147) | 48.6 (69) | 39.3 (22) |
| Hypertension | 58.7 (225) | 60.6 (86) | 53.6 (30) |
| Coronary artery disease | 43.1 (165) | 45.1 (64) | 48.2 (27) |
| Cerebrovascular disease | 7.8 (30) | 10.6 (15) | 10.7 (6) |
| Peripheral artery disease | 9.9 (38) | 14.1 (20) | 7.1 (4) |
| Prior heart failure | 59.0 (226) | 35.2 (50) | 41.1 (23) |
| Severe valvular disease | 17.0 (65) | 9.2 (13) | 12.5 (7) |
| Pulmonary hypertension | 7.3 (28) | 2.1 (3) | 7.1 (4) |
| Chronic kidney disease | 36.6 (140) | 29.6 (42) | 17.9 (10) |
| On dialysis | 20.0 (28) | 26.2 (11) | 20.0 (2) |
| Significant pulmonary disease | 17.5 (67) | 9.9 (14) | 12.5 (7) |
| Significant liver disease | 5.0 (19) | 4.9 (7) | 3.6 (2) |
| Clinical features/illness severity | |||
| SOFA score | 8 (5 – 11) | 8 (5 – 12) | 10 (7 – 13) |
| Preceding cardiac arrest | 22.5 (86) | 27.0 (40) | 39.7 (23) |
| Serum lactate (mmol/L) | 3.2 (1.9 – 6.3) | 3.6 (2.0 – 7.4) | 6.7 (2.9 – 11.6) |
| Management of shock prior to MCS | |||
| No. of inotropes/vasopressors | |||
| 0 agents | 6.1 (23) | 6.9 (9) | 3.7 (2) |
| 1 agent | 49.1 (186) | 30.0 (39) | 20.4 (11) |
| 2 agents | 30.1 (114) | 38.5 (50) | 31.5 (17) |
| 3 agents | 9.0 (34) | 19.2 (25) | 27.8 (15) |
| 4+ agents | 5.8 (22) | 5.4 (7) | 16.7 (9) |
| ICU resource utilization | |||
| Days of ICU care, median (IQR) | 4.0 (2.1 – 7.8) | 5.8 (2.9 – 10.3) | 5.5 (2.0 – 12.8) |
| Mechanical ventilation | 45.2 (173) | 59.5 (88) | 81.0 (47) |
| Renal replacement therapy | 15.4 (59) | 18.2 (27) | 29.3 (17) |
| Pulmonary artery catheter | 29.8 (114) | 48.6 (69) | 46.4 (26) |
Advanced temporary MCS includes Impella percutaneous ventricular assist systems (2.5, 5.0, CP), TandemHeart percutaneous left ventricular assist systems, and veno-arterial extracorporeal membrane oxygenation (VA-ECMO). Categorical variables are shown as percentages with counts in parentheses. Continuous variables are shown as medians with interquartile ranges. IABP indicates intra-aortic balloon pump; ICU, intensive care unit; IQR, interquartile range; MCS, mechanical circulatory support; No., number; SOFA, Sequential Organ Failure Assessment.
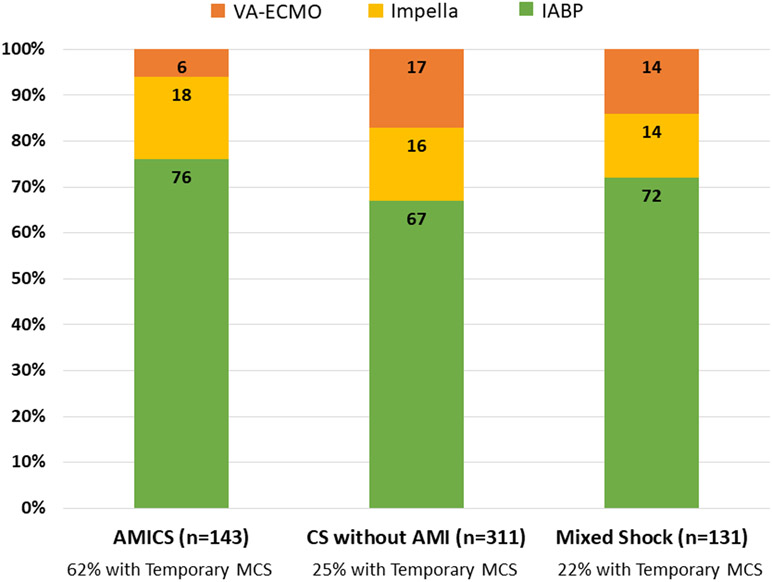
Although more patients with AMICS were treated with temporary MCS as compared with patients with CS without AMI and mixed shock, the pattern of specific device selection was similar between patients with AMICS, CS without AMI, and mixed shock (Figure 3).
Figure 3. Frequency of specific device types by shock type.
Proportions of patients receiving each of the most common types of temporary MCS devices according to the type of shock. A hierarchical classification scheme was used for patients who received multiple devices (VA-ECMO > Impella > IABP). AMI indicates acute myocardial infarction; AMICS, acute myocardial infarction-related cardiogenic. Shock; CS, cardiogenic shock; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
Mortality in patients receiving temporary mechanical circulatory support for shock
Among all patients receiving temporary MCS for cardiogenic or mixed shock in the primary analysis population (n=202), the CICU and in-hospital mortality rates were 34% (95% CI, 27% - 41%) and 40% (95% CI, 33% - 47%), respectively. Clinical variables associated with increased risk of in-hospital mortality in the full analysis population included preceding cardiac arrest, higher SOFA score, higher serum lactate, higher number of inotropes or vasopressors prior to temporary MCS placement, mechanical ventilation, and renal replacement therapy (Table 2). For example, in-hospital mortality for patients receiving temporary MCS for cardiogenic or mixed shock who had a preceding cardiac arrest (n=60) was 60.0%, as compared with 31.7% in those without a preceding cardiac arrest.
Table 2.
Univariable risk of in-hospital mortality in patients receiving temporary MCS for shock (full analysis population).
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Preceding cardiac arrest | 2.67 (1.67 – 4.26) | <0.001 |
| SOFA score (per 1 point) | 1.22 (1.15 – 1.29) | <0.001 |
| Highest serum lactate (per mmol/L) | 1.23 (1.14 – 1.34) | <0.001 |
| Mechanical ventilation | 3.56 (2.11 – 6.02) | <0.001 |
| Renal replacement therapy | 5.59 (3.09 – 10.1) | <0.001 |
| No. of inotropes/vasopressors (referent: 0 agents) | ||
| 1 agent | 3.24 (0.68 – 15.36) | 0.139 |
| 2 agents | 4.01 (0.87 – 18.50) | 0.075 |
| 3 agents | 12.60 (2.60 – 61.12) | 0.002 |
| 4+ agents | 19.73 (3.85 – 101.04) | <0.001 |
CI indicates confidence interval; MCS, mechanical circulatory support; No., number; SOFA, sequential organ failure assessment.
Timing of temporary mechanical circulatory support placement in patients with shock
Patients who received temporary MCS within 24 hours of CICU admission were more likely to have had a cardiac arrest prior to CICU admission, and had worse indices of illness severity, including higher SOFA scores and higher serum lactate levels (Supplemental Table 2). However, after adjusting for age, SOFA score, serum lactate, number of inotropes/vasopressors, and preceding cardiac arrest, shock patients who received temporary MCS within 24 hours of CICU admission had lower CICU mortality, as compared to those who received temporary MCS after 24 hours of CICU admission (adjusted OR 0.44, 95% CI 0.21–0.95; p=0.04).
Discussion
In this analysis of temporary MCS use in the CCCTN Registry, we found that approximately one-third of patients received temporary MCS for the management of cardiogenic or mixed shock, with the most common form of support being IABP counterpulsation. We also demonstrated that there was wide variation between centers in both the proportion of patients with cardiogenic or mixed shock who received temporary MCS devices and the proportion of patients who received advanced MCS versus IABP alone. Further, while hospital-level variation in temporary MCS use was not explained by differences in illness severity, patient-level variation in the selection of advanced MCS versus IABP was clearly associated with illness severity. We also observed an association between the timing of MCS placement and mortality that may or may not be causally related. These data underscore that the factors that inform a clinician’s decision to use a particular form of temporary MCS are nuanced and highlight the critical need for randomized comparisons of therapeutic strategies involving temporary MCS devices for the treatment of patients with cardiogenic and mixed shock.
IABP versus advanced MCS for the treatment of shock
The use of IABPs in the United States has been steadily declining over the last decade,11, 12 accelerated in part by evidence from clinical trials that IABPs do not decrease mortality among patients with AMICS.8 In parallel, other forms of temporary MCS that provide more robust hemodynamic support, including Impella, TandemHeart, and VA-ECMO, have gained traction as alternative therapeutic options for the treatment of severe, refractory shock. Despite these shifts, multiple studies,11, 13 including the present analysis with data from 2017–2018, have demonstrated that IABPs remain the most commonly used form of temporary MCS for the treatment of AMICS, even in contemporary tertiary CICUs. This analysis extends these findings by showing that IABP is also the most common form of support in patients with CS without AMI and mixed shock. Importantly, these data include patients with temporary MCS placed at outside facilities; therefore, while the results provide an accurate picture of temporary MCS use in contemporary CICUs, they do not reflect only clinical decision-making at the CCCTN centers.
Consistent with data from administrative and disease-based registries,11, 13 our analysis also highlights the wide variation in clinical practice patterns of temporary MCS device utilization for the treatment of shock. Given the comprehensive assessment of shock severity and ICU resource utilization provided by our dataset, this analysis suggests that differences in MCS utilization at a center level are not explained by differences in the severity of shock presentations between centers. Nevertheless, at an individual patient level, patients treated with advanced MCS, as compared with IABP, tend to have more severe shock, as reflected by higher median SOFA scores, higher presenting lactates, and higher inotrope/vasopressor requirements at the time of device implantation. Taken together, these data suggest that in contemporary CICU practice, clinicians are selecting devices capable of providing more robust hemodynamic support for patients with more severe presentations but that the specific thresholds for doing so are highly variable.
Implications for Practice and Future Research
In recent years, professional societies have developed expert consensus statements supporting criteria for temporary MCS device selection for patients with cardiogenic shock that are based in part on shock severity.14, 15 Therefore, the pattern of device selection observed in this analysis may reflect efforts within the field to titrate the level of support to the patient’s clinical and hemodynamic needs. At the same time, the substantial between-center variation highlights the distinct interpretations of what constitutes “severe” shock, which is essential for defining when these resource-intensive and potentially risky technologies might be applied.16
This analysis provides several additional insights that may inform the development of future clinical trials of temporary MCS utilization in the treatment of shock. First, we identified important indicators of increased risk for in-hospital mortality in patients receiving temporary MCS for shock, namely higher SOFA scores, preceding cardiac arrest, and higher number of inotropes or vasopressors prior to temporary MCS placement. Since the efficacy of temporary MCS devices for the treatment of shock will need to be evaluated using a common shock definition with stratification based on clinical severity, these factors should be considered in the development of trial eligibility criteria and rigorously compared with other criteria for classifying shock severity.
Second, our analysis suggests that patients who received temporary MCS earlier in their CICU course were more likely to survive to CICU discharge after adjusting for differences in illness severity. Importantly, since the comparison between early versus late initiation of temporary MCS is not randomized, these results may be confounded and should be interpreted with caution. Nevertheless, they raise a hypothesis that is worth testing that earlier initiation of temporary MCS device therapy may improve survival of shock treated in the CICU.
Strengths and limitations
This analysis has several strengths. First, our study population was derived from a well-characterized, multicenter clinical registry, which allowed us to comprehensively profile the clinical characteristics of these patients. Second, because the cohort includes all consecutive medical CICU admissions, our findings extend to a broader population of patients with cardiogenic and mixed shock than do studies based on disease-specific or device-specific registries.
This analysis also has several limitations. First, invasive hemodynamic assessments were not required for shock classification, nor were they captured in the CCCTN registry. This limitation is relevant to our analysis since decisions about type and timing of temporary MCS placement may be based on initial hemodynamics. Second, since the CCCTN registry was focused on medical CICU admissions, while our data are expected to be reflective of the vast majority of patients with CS, these data do not include shock patients with temporary MCS who were managed exclusively in cardiac surgical ICUs, which may influence the estimates of hospital-level MCS utilization, particularly VA-ECMO. Mitigating this limitation, all patients who were admitted to the CICU during their hospital course but who required transfer out of the CICU for temporary MCS device placement (e.g., transfers to the cardiac surgical ICU for VA-ECMO) were still captured in this dataset. Therefore, our data accurately reflect decision-making regarding MCS in the medical CICU. Third, because the two-month “snapshot” is not required to be simultaneous across centers and all dates are anonymized in the CCCTN registry, we are not able to directly analyze the possibility of an influence of seasonal variation on the underlying etiology of cardiogenic shock or on temporary MCS use. Further, because most centers did not collect information throughout the full year, we are not able to confirm whether the two-month “snapshot” is a representative sample for all centers. Fourth, since CCCTN centers are predominantly urban, tertiary care medical centers, these data likely do not reflect the clinical practice patterns of smaller community hospitals. Finally, all outcome comparisons in this analysis are non-randomized and are therefore subject to confounding despite attempts to adjust for differences in disease severity using multivariable regression. For this reason, comparisons of outcomes between cardiogenic and mixed shock patients who were managed without temporary MCS vs. IABP only vs. advanced MCS were deferred in the absence of sufficient sample size to support robust propensity-adjusted modeling.
Conclusions
There is wide variation in the proportion of patients with shock who receive temporary MCS devices in tertiary CICUs in North America. Despite the lack of evidence supporting IABP use for shock in patients with AMICS, IABPs remain the most frequently used temporary MCS devices. Hospital-level variation in temporary MCS utilization may not be explained by center-based differences in the severity of illness. These data highlight the critical need for randomized comparisons of therapeutic strategies involving temporary MCS devices for the treatment of patients with cardiogenic and mixed shock and illustrate opportunities for standardization of care.
Supplementary Material
What is New?
In the Critical Care Cardiology Trials Network Registry of advanced cardiac intensive care units (CICUs) in North America, there was wide variation in the proportion of patients with cardiogenic or mixed shock who were managed with temporary mechanical circulatory support.
Despite the evidence against intra-aortic balloon pump (IABP) use for treatment of patients with acute myocardial infarction-related cardiogenic shock, in these data from 2017–2018, IABPs remained the most frequently used temporary mechanical circulatory support devices, even in contemporary tertiary CICUs.
Hospital-level variation in temporary mechanical circulatory support utilization may not be explained by center-based differences in the severity of illness.
What are the Clinical Implications?
The factors that inform a clinician’s decision to use a particular form of temporary MCS are nuanced but appear to be heavily influenced by local practice patterns.
There is a critical need for randomized comparisons of therapeutic strategies involving temporary mechanical circulatory support devices for the treatment of patients with cardiogenic and mixed shock.
Acknowledgments
Sources of Funding
Dr. Berg is supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL007604). Dr. Solomon receives research support from the National Institutes of Health Clinical Center intramural research funds.
Non-standard Abbreviations and Acronyms
- AMI
acute myocardial infarction
- AMICS
acute myocardial infarction-related cardiogenic shock
- CCCTN
Critical Care Cardiology Trials Network
- CICU
cardiac intensive care unit
- CS
cardiogenic shock
- IABP
intra-aortic balloon pump
- MCS
mechanical circulatory support
- SOFA
Sequential Organ Failure Assessment
- VA-ECMO
veno-arterial extracorporeal membrane oxygenation
Footnotes
Disclosures
The authors report no disclosures related to the submitted work.
References
- 1.Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird-Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis AP, Haleem A, Hollenberg SM, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Park JG, Phreaner N, Roswell RO, Schulman SP, Jeffrey Snell R, Solomon MA, Ternus B, Tymchak W, Vikram F and Morrow DA. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ Cardiovasc Qual Outcomes. 2019;12:e005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohula EA, Katz JN, van Diepen S, Alviar CL, Baird-Zars VM, Park JG, Barnett CF, Bhattal G, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis A, Granger CB, Hollenberg S, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Phreaner N, Roswell RO, Schulman SP, Snell RJ, Solomon MA, Ternus B, Tymchak W, Vikram F, Morrow DA and Critical Care Cardiology Trials N. Demographics, Care Patterns, and Outcomes of Patients Admitted to Cardiac Intensive Care Units: The Critical Care Cardiology Trials Network Prospective North American Multicenter Registry of Cardiac Critical Illness. JAMA Cardiol. 2019;4(9):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG, American Heart Association Council on Clinical C, Council on C, Stroke N, Council on Quality of C, Outcomes R and Mission L. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RJ, Spencer FA, Gore JM, Lessard D and Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119:1211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aissaoui N, Puymirat E, Tabone X, Charbonnier B, Schiele F, Lefevre T, Durand E, Blanchard D, Simon T, Cambou JP and Danchin N. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J. 2012;33:2535–43. [DOI] [PubMed] [Google Scholar]
- 6.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH and Fonarow GC. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D and Gore JM. Decade-Long Trends (2001–2011) in the Incidence and Hospital Death Rates Associated with the In-Hospital Development of Cardiogenic Shock after Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Bohm M, Ebelt H, Schneider S, Schuler G, Werdan K and Investigators I-SIT. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 9.Miller PE, Solomon MA and McAreavey D. Advanced Percutaneous Mechanical Circulatory Support Devices for Cardiogenic Shock. Crit Care Med. 2017;45:1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrow DA, Fang JC, Fintel DJ, Granger CB, Katz JN, Kushner FG, Kuvin JT, Lopez-Sendon J, McAreavey D, Nallamothu B, Page RL, 2nd, Parrillo JE, Peterson PN, Winkelman C, American Heart Association Council on Cardiopulmonary CCP, Resuscitation CoCCCoCN, Council on Quality of C and Outcomes R. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation. 2012;126:1408–28. [DOI] [PubMed] [Google Scholar]
- 11.Sandhu A, McCoy LA, Negi SI, Hameed I, Atri P, Al’Aref SJ, Curtis J, McNulty E, Anderson HV, Shroff A, Menegus M, Swaminathan RV, Gurm H, Messenger J, Wang T and Bradley SM. Use of mechanical circulatory support in patients undergoing percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circulation. 2015;132:1243–51. [DOI] [PubMed] [Google Scholar]
- 12.Strom JB, Zhao Y, Shen C, Chung M, Pinto DS, Popma JJ and Yeh RW. National trends, predictors of use, and in-hospital outcomes in mechanical circulatory support for cardiogenic shock. EuroIntervention. 2018;13:e2152–e2159. [DOI] [PubMed] [Google Scholar]
- 13.Strom JB, Zhao Y, Shen C, Chung M, Pinto DS, Popma JJ, Cohen DJ and Yeh RW. Hospital Variation in the Utilization of Short-Term Nondurable Mechanical Circulatory Support in Myocardial Infarction Complicated by Cardiogenic Shock. Circ Cardiovasc Interv. 2019;12:e007270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson TM, Ohman EM, O’Neill WW, Rab T, Cigarroa JE and Interventional Scientific Council of the American College of C. A Practical Approach to Mechanical Circulatory Support in Patients Undergoing Percutaneous Coronary Intervention: An Interventional Perspective. JACC Cardiovasc Interv. 2016;9:871–83. [DOI] [PubMed] [Google Scholar]
- 15.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U and Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40:2671–2683. [DOI] [PubMed] [Google Scholar]
- 16.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O’Neill W, Ornato JP, Stelling K, Thiele H, van Diepen S and Naidu SS. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.