Abstract
Preeclampsia is a hypertensive disorder of pregnancy that causes significant acute and long-term risk to the mother and the baby. The multifaceted maternal syndrome is driven by overproduction of circulating anti-angiogenic factors, widespread inflammation, and endothelial dysfunction. NF-κB is a transcription factor that plays a central role in the inflammatory response. Its activity is increased in the preeclamptic placenta, and it promotes the systemic endothelial dysfunction present in preeclampsia. There is an acute need for new therapeutics targeted to the causative pathways of preeclampsia. Our group has developed a drug delivery system based on the bioengineered protein Elastin-like polypeptide (ELP) that is capable of stabilizing therapeutics in the maternal circulation and preventing their placental transfer. Here we utilized the ELP carrier system to deliver a peptide known to inhibit the NF-κB pathway. This polypeptide, containing a cell penetrating peptide and an NF-κB inhibitory peptide derived from the p50 nuclear localization sequence (abbreviated SynB1-ELP-p50i), blocked NF-κB activation and prevented TNF-α - induced endothelin production in vitro. Fusion of the p50i peptide to the SynB1-ELP carrier slowed its plasma clearance and prevented its placental transfer in pregnant rats, resulting in increased deposition in the maternal kidney, liver, and placenta relative to the free peptide. When administered in a rat model of placental ischemia, SynB1-ELP-p50i partially ameliorated placental ischemia – induced hypertension and reduced placental TNF-α levels with no signs of toxicity. These data support the continued development of ELP-delivered NF-κB inhibitors as maternally sequestered anti-inflammatory agents for preeclampsia therapy.
Keywords: Preeclampsia, Elastin-like Polypeptide, Nuclear Factor kappa B, Drug Delivery, Pregnancy, Inflammation, Peptide
Graphical abstract
Introduction.
The hypertensive pregnancy disorder preeclampsia (PE) affects approximately 3-5% of all pregnancies in the United States 1. A major limitation in the management of preeclampsia is the lack of available therapeutics 2. Pregnancy-safe antihypertensives are used to combat dangerously high blood pressure with limited success, corticosteroids are used in some cases to promote fetal lung development and improve maternal liver and platelet function, and magnesium sulfate is administered for seizure prophylaxis 3. However, the ultimate treatment is induction of delivery, a strategy that depends on fetal viability and often results in pre-term delivery. Importantly, there are no currently approved medications targeted to the molecular pathways that are driving the maternal syndrome. The initiating etiology of preeclampsia has yet to be elucidated, but abnormal placentation is believed to play a key role in the development of the maternal syndrome 4,5. The abnormal placentation leads to a decreased blood supply to the developing fetal- placental unit, and in response, the placenta secretes a number of factors into the maternal circulation, ultimately driving the disorder by causing maternal endothelial dysfunction 4. Included in the list of secreted factors are pro-inflammatory cytokines such as TNFα, IL-6, and IL-8 6. Preclinical studies have targeted individual cytokines using clinically available or experimental biologics with mixed success 7-9, but likely due to the number of factors involved in the PE syndrome, targeting a single inflammatory cytokine has not made a significant clinical impact. We propose an alternative strategy to target the inflammatory response more generally by inhibiting one of its master mediators.
The transcription factor Nuclear factor- kappa B (NF-κB) is a hub in the inflammatory signaling cascade 10. NF-κB activation is downstream of IL-1 receptor, TNF-α receptor, and toll-like receptor signaling (among others), all of which have been implicated in PE 11-13. Also, NF-κB controls the transcription of many pro-inflammatory cytokines, and NF-κB activation has been implicated in PE pathophysiology. NF-κB expression levels and activation have been demonstrated in both the placenta and the systemic vasculature in women with preeclampsia 14,15, and this activation is implicated in trophoblast apoptosis 16 and systemic endothelial dysfunction 17. One strategy to target NF-κB is to use peptide inhibitors targeted to the NF-κB activation cascade (reviewed in 18). NF-κB activation is mediated by ubiquitylation and degradation of its endogenous inhibitor I-κB and subsequent phosphorylation of the NF-κB heterodimer 10. This heterodimer, often composed of p50 and p65 subunits, contains a nuclear localization sequence (NLS) that becomes exposed following I-κB degradation, mediating nuclear import of the transcription factor. A peptide mimic of the p50 NLS which we refer to as a p50 inhibitory peptide (p50i), when fused to a cell penetrating peptide, has been shown to function as a competitive inhibitor of NF-κB nuclear import 19. Therefore, we propose that this peptide, among others targeted to the NF-κB activation cascade 18, could represent a promising therapeutic to treat PE 20.
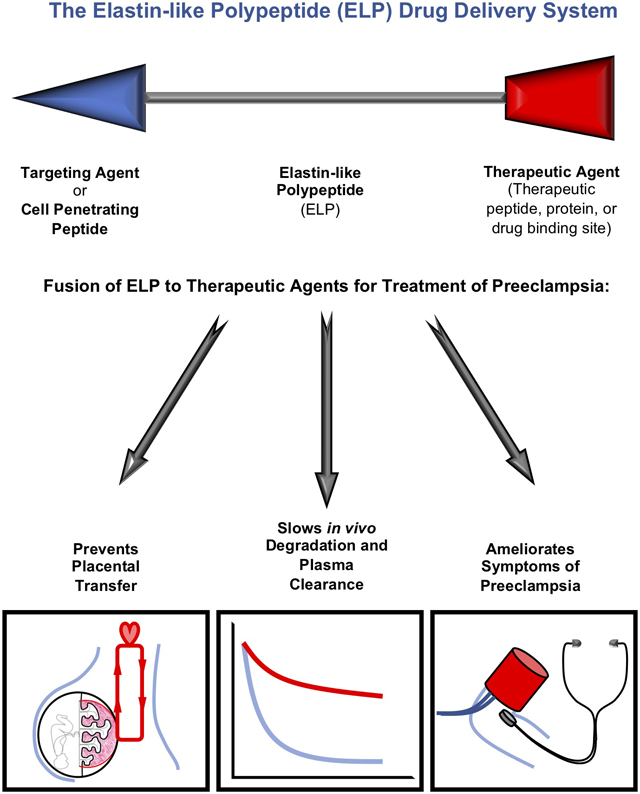
A major limitation of peptide therapeutics is their short plasma half-life and susceptibility to degradation in vivo. Additionally, in pregnancy, the small size of peptide therapeutics makes them susceptible to placental transfer, creating unknown risks to the developing fetus. In order to overcome these pharmacokinetic limitations and to achieve maternal sequestration, we fused the p50i peptide to Elastin-like polypeptide (ELP) 20. ELP is a synthetic protein built by repeating a five amino-acid motif 21, and it is amenable to modification for use as a drug carrier. Peptides or therapeutic proteins can be fused in-frame at the N- and C- termini to create chimeric therapeutic proteins 22-26, and the repetitive ELP domain can be made larger or smaller to control pharmacokinetics and biodistribution 27,28. We previously showed that ELP does not cross the placental barrier and is a promising carrier for improving the pharmacokinetics and achieving maternal sequestration of therapeutics for drug delivery during pregnancy 28,29. For delivery of the NF-κB inhibitory peptide, we are utilizing a construct that contains an N-terminal cell penetrating peptide called SynB1 30, a central ~60 kDa ELP domain, and an in-frame fusion of the p50i peptide 19 at the C-terminus (abbreviated SynB1-ELP-p50i, originally developed as an anti-cancer agent 31,32). Here we tested the ability of SynB1-ELP-p50i to block the NF-κB pathway in vitro and to interfere with the PE syndrome in vivo using a rat model of placental ischemia.
Methods.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Production, Fluorescent Labeling, and in vitro evaluation of Polypeptides.
SynB1-ELP-p50i and SynB1-ELP were produced recombinantly in E. coli. Plasmids encoding each protein were produced in the lab of Drazen Raucher, previously described in 31,32. Recombinant proteins were purified using inverse transition cycling 33 and fluorescently labeled on a unique cysteine residue 34. For control studies using the p50i peptide without ELP conjugation, the free peptide and an N-terminal rhodamine-conjugated form of the peptide were chemically synthesized by Genscript. Polypeptide cellular uptake, cytotoxicity, and in vitro activity were evaluated as described in the Supplemental Methods.
Animal Use.
All protocols were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee and were in agreement with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals. Timed pregnant Sprague Dawley rats (Charles River) were received on gestational day 11 (GD 11). Animals were maintained on a 12:12 hour light-dark cycle, at constant 23°C temperature, and given food and water ad libitum.
Assessment of Pharmacokinetics, Biodistribution, and Placental Transfer.
Rats were administered rhodamine-labeled SynB1-ELP-p50i or the free p50i peptide by bolus intravenous injection on GD 14 (n = 4). Plasma pharmacokinetics were determined by direct fluorescence measurement of plasma samples collected after injection and fitting to a two-compartment pharmacokinetic model. Maternal organs, fetal blood, and amniotic fluid were collected at sacrifice 4 h after injection, and maternal biodistribution and placental transfer of SynB1-ELP-p50i or free p50i were assessed by ex vivo whole organ imaging 35 and direct fluorescence measurement of maternal and fetal plasma. Details are provided in the Supplemental Methods.
Assessment of Therapeutic Efficacy and Anti-Inflammatory Activity.
The ability of SynB1-ELP-p50i and control agents lacking the ELP carrier or the p50i peptide to ameliorate hypertension or reduce markers of inflammation was tested in the reduced uterine perfusion pressure (RUPP) model 36. The RUPP or sham surgery was performed on GD 14, and test agents (SynB1-ELP-p50i, SynB1-ELP, free p50i, or saline) were administered via continuous infusion with intraperitoneal minipumps from GD 14 to GD 19 (n = 10-12, see Supplemental Methods for details). Mean arterial pressure was measured on GD 19 in conscious rats via an arterial catheter, and rats were euthanized, maternal organ and fetal weights were collected, and tissues were preserved by freezing or in RNA Later for analysis. Details are described in the Supplemental Methods.
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism 8 software. A two-way ANOVA was used to analyze the biodistribution studies, and a post-hoc Sidak multiple comparison was used to compare therapeutic levels within each organ. Two-way ANOVAs with factors for protein treatment and sham or RUPP surgery were used to analyze each outcome in the efficacy studies. Post-hoc Tukey’s multiple comparisons of all pairwise groups were used. For analysis of the efficacy data, all treatment groups (Saline, SynB1-ELP, p50i, SynB1-ELP-p50i (50 mg/kg/d dose), and SynB1-ELP-p50i (75 mg/kg/d dose)) were included in the multiple comparisons. Statistical significance was determined by an adjusted p value < 0.05 in multiple comparisons.
Results.
SynB1-ELP-p50i enters cells and blocks NF-κB Activation in vitro.
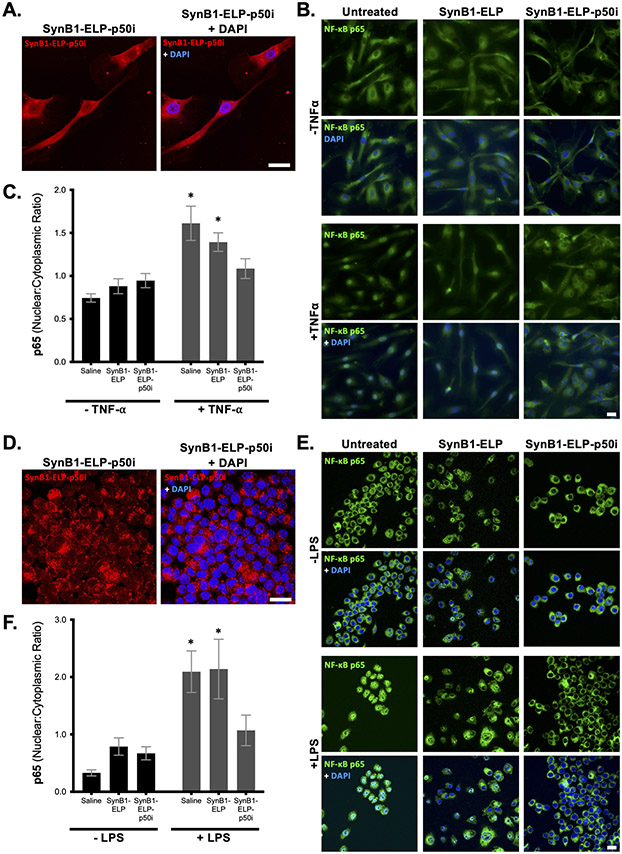
The p50i peptide functions as a competitive inhibitor of nuclear localization after NF-κB activation 19. Therefore, in order to reach its target, the p50i peptide must be delivered to the cell cytoplasm. To determine intracellular localization, endothelial cells (HUVECs) were treated with rhodamine-labeled SynB1-ELP-p50i, and localization of the polypeptide was determined 24 hours later by confocal microscopy. As shown in Figure 1A, SynB1-ELP-p50i was internalized by the endothelial cells in vitro and localized predominantly to the cell cytoplasm, with a lower concentration reaching the nucleoplasm.
Figure 1.
SynB1-ELP-p50i blocks NF-κB activation. A and D. SynB1-ELP-p50i (red, 10 μM) localizes diffusely to the cytoplasm and, to a lesser extent, the nucleoplasm in HUVECs (A) and RAW264.7 (D) after 24 hours incubation. B, C, E, F. HUVECs (B, quantified in C) or RAW264.7 macrophages (E, quantified in F) were pre-treated with SynB1-ELP-p50i (50 μM) or a control protein lacking the NF-κB inhibitory peptide (SynB1-ELP, 50 μM). 24 hours after protein exposure, cells were stimulated with TNF-α (HUVECs, 20 ng/mL) or LPS (RAW264.7, 10 μg/mL), and NF-κB activation was assessed by immunostaining for p65 intracellular localization (green) overlaid with DAPI nuclear stain (blue). Scale bar = 20 μm. * Statistically significant difference between unstimulated and stimulated (two-way ANOVA with post-hoc Sidak multiple comparison (n = 40 cells per sample).
To test the function of the SynB1-ELP-p50i polypeptide, an in vitro assay of NF-κB activation was used 37. HUVECs pre-treated with SynB1-ELP-p50i or the control protein lacking the NF-κB inhibitory peptide (SynB1-ELP) were subsequently stimulated with TNF-α or saline control. NF-κB was assessed by immunostaining for p65 intracellular localization. TNF-α stimulation led to rapid re-localization of NF-κB into the nucleus (Figure 1B, left panel), indicating its activation. Pretreatment of the cells with SynB1-ELP-p50i, but not the SynB1-ELP control polypeptide, completely blocked this nuclear translocation of NF-κB (Figure 1B, middle and right panels). Quantitation of the ratio of nuclear:cytoplasmic fluorescence intensity confirmed the ability of SynB1-ELP-p50i to block NF-κB nuclear import (Figure 1C). A similar experiment was performed in macrophages. RAW 264.7 cells were treated with labeled SynB1-ELP-p50i, and the protein localized predominantly in the cell cytoplasm with weak nucleoplasmic staining after 24 hours (Figure 1D). To assess NF-κB activity in the macrophage cell line, the cells were stimulated with lipopolysaccharide (LPS), which binds to toll-like receptors and signals through the NF-κB pathway 38, after pretreatment with SynB1-ELP-p50i or SynB1-ELP for 24 hours. LPS led to rapid activation of NF-κB as assessed by its nuclear localization (Figure 1E, left panel). SynB1-ELP-p50i, but not SynB1-ELP, was able to prevent LPS-induced nuclear localization of NF-κB (Figure 1E, middle and right panels, quantified in Figure 1F) in macrophages.
Importantly, SynB1-ELP-p50i exhibited these NF-κB inhibitory effects without causing cytotoxicity or interfering with cell proliferation. Continuous exposure to SynB1-ELP or SynB1-ELP-p50i for 72 hours at concentrations up to 50 μM had no effect on the proliferation of endothelial, trophoblast, macrophage, or T cells, all potential cellular targets of NF-κB inhibition (Figure S1).
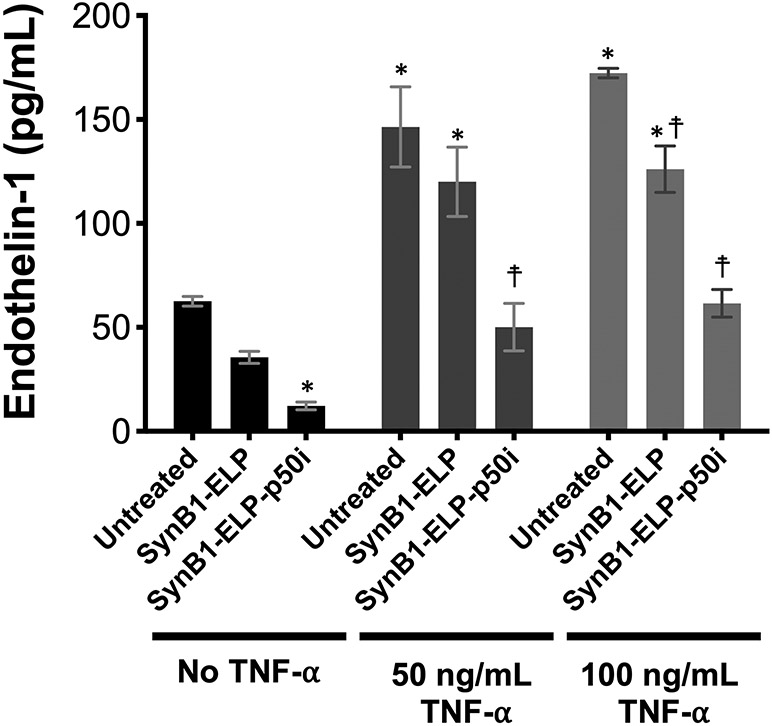
SynB1-ELP-p50i Reduces TNF-α Release by Endothelial Cells.
When endothelial cells are stimulated with the pro-inflammatory cytokine TNF-α, one of their responses is to secrete the vasoactive peptide endothelin-1 (ET-1) 39. To assess the ability of SynB1-ELP-p50i to inhibit this pathway, HUVECs were stimulated with an intermediate or high dose of TNF-α 24 hours after treatment with SynB1-ELP-p50i or SynB1-ELP. TNF-α induced ET-1 secretion into the culture media three-fold (Figure 2). Pre-treatment of cells with SynB1-ELP-p50i prior to TNF-α exposure completely blocked the heightened ET-1 release, even at very high TNF-α doses. Furthermore, SynB1-ELP-p50i lowered basal ET-1 production by HUVECs without TNF-α stimulation. The control protein, SynB1-ELP, also had a small but statistically significant effect on ET-1 release, which is possibly related to effects on the cells’ import/export pathways due to endocytosis of large amounts of the protein.
Figure 2.
SynB1-ELP-p50i prevents TNF-α induced endothelin-1 release. HUVECs pre-treated with SynB1-ELP-p50i (50 μM) were stimulated with TNF- α, and endothelin-1 release into the cell culture media was quantified by ELISA. * Statistically different from untreated, unstimulated cells; ☨, statistically different from untreated cells within each stimulation group (2-way ANOVA with post-hoc Tukey’s multiple comparison).
Fusion of the p50i Peptide to the ELP Carrier Extends its Plasma Half-life and Prevents it from Crossing into the Fetal Circulation.
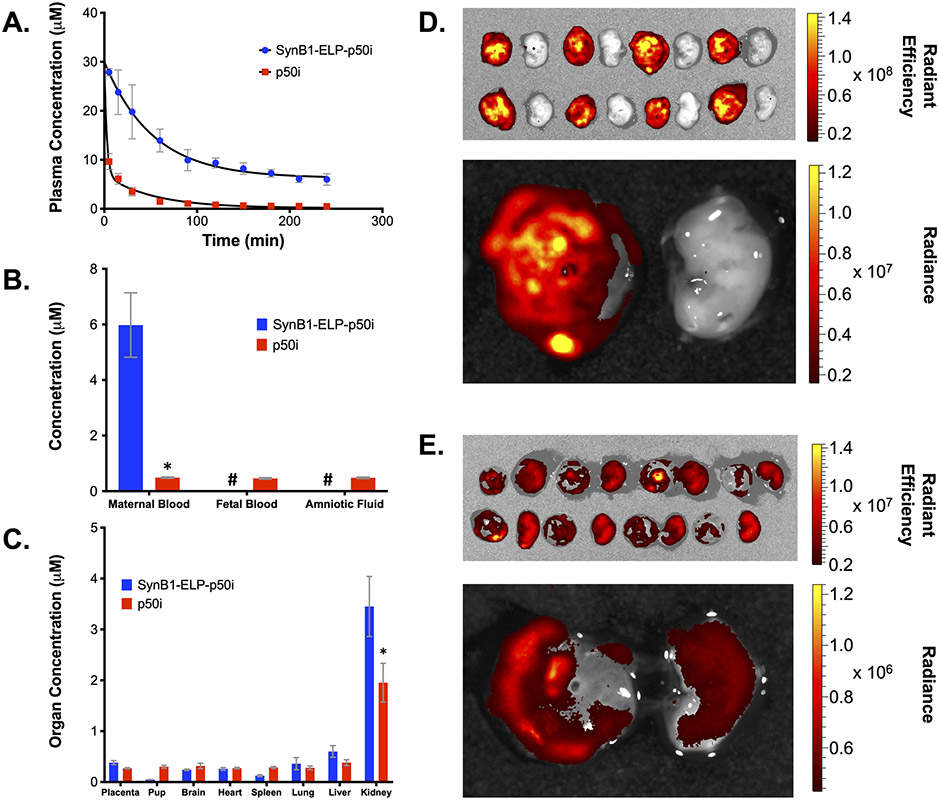
A major limitation of peptide therapeutics is their rapid plasma clearance. In order to determine if fusion to the SynB1-ELP carrier affected the pharmacokinetics and biodistribution of the p50i peptide, equimolar doses of rhodamine labeled SynB1-ELP-p50i or rhodamine-labeled free p50i peptide were administered to timed pregnant rats on GD 14 by bolus intravenous injection. As shown in Figure 3A, the free p50i peptide cleared from plasma very rapidly, with over 90% of the injected dose eliminated within 60 minutes (distribution phase half-life of 12.2 minutes, terminal half-life 53 minutes). SynB1-ELP-p50i, in contrast, cleared much more slowly, showing a distribution phase half-life of approximately 30 minutes and a terminal half-life of over 14 hours. Terminal maternal and fetal blood samples and amniotic fluid samples further demonstrated the effects of fusion to the ELP-based carrier (Figure 3B). Maternal blood levels 4 hours after injection were 6.0 ± 1.2 μM for the SynB1-ELP-p50i polypeptide and 0.49 ± 0.03 μM for the p50i peptide. p50i peptide levels were identical in maternal and fetal blood, demonstrating free passage of the peptide across the placental barrier. SynB1-ELP-p50i was undetectable in the fetal blood or in amniotic fluid, demonstrating that fusion of the peptide to the ELP carrier effectively restricted it to the maternal circulation.
Figure 3.
Biodistribution of SynB1-ELP-p50i and the free p50i peptide. A. Plasma pharmacokinetics after administration of equivalent doses of the free p50i peptide or the SynB1-ELP-delivered p50i peptide. B. Levels of free and SynB1-ELP-delivered peptide in maternal blood, fetal blood, and amniotic fluid four hours after bolus intravenous administration. C. Biodistribution of free and SynB1-ELP-delivered p50i peptide four hours after administration. D and E. Representative ex vivo tissue imaging of placentae and associated pups four hours after bolus intravenous administration of (D.) SynB1-ELP-p50i or (E.) free p50i peptide. Image intensity was adjusted independently between D. and E. to allow visualization of signal in both treatment groups, and scale bars represent the raw intensity values for each agent. * Statistically significant difference between SynB1-ELP-p50i and free p50i levels in each organ were assessed using a two-way ANOVA with post-hoc Sidak multiple comparison (n = 4). # Levels below detection limit.
Ex vivo imaging of maternal organs at harvest revealed that both SynB1-ELP-p50i and p50i accumulated to the highest levels in the maternal kidneys, and SynB1-ELP-p50i kidney levels were higher than p50i kidney levels (3.45 ± 0.49 μM vs. 1.95 ± 0.38 μM, p < 0.0001) (Figure 3C). All other maternal organs had similar levels of SynB1-ELP-p50i, and, in contrast to free p50i, SynB1-ELP-p50i was barely detectable in the pups. Ex vivo imaging of placenta and pups further supported the maternal sequestration of SynB1-ELP-p50i. There was almost no visible signal of SynB1-ELP-p50i in the pups, while placental levels were high (Figure 3D). In contrast, the small p50i peptide freely crossed the placentae, and pup levels were equivalent to placental levels (Figure 3E).
SynB1-ELP-p50i Partially Ameliorates the Effects of Placental Ischemia.
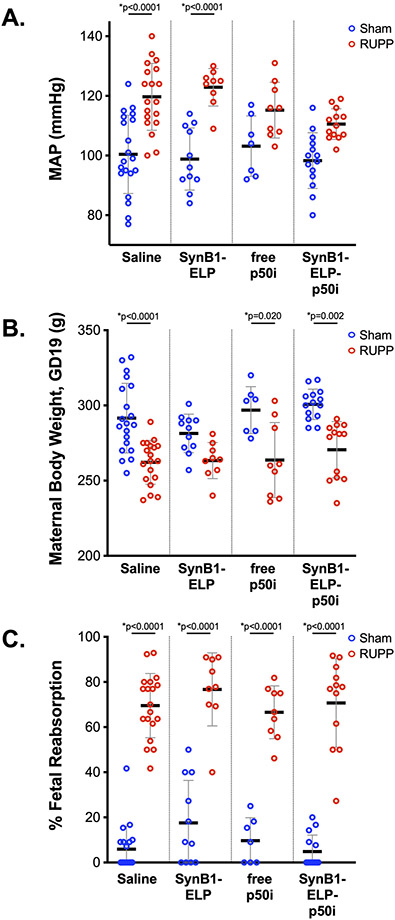
After establishing the clear pharmacokinetic benefits and maternal sequestration achieved by fusing the p50i peptide to the SynB1-ELP carrier, we tested the ability of SynB1-ELP-p50i to alter the maternal preeclampsia syndrome using a model of placental ischemia 40. Rats subjected to placental ischemia or sham control surgery on GD 14 were administered SynB1-ELP-p50i at a dose of 50 mg/kg/d (or an equimolar dose of free peptide or the SynB1-ELP control protein, or saline) via continuous intraperitoneal infusion. Continuous infusion was used in order to directly compare the efficacy of free peptide to the ELP-fused peptide for preventing placental ischemia-induced hypertension since our pharmacokinetic work clearly showed that IV injection of the free peptide is not a viable treatment option. The hallmark of the placental ischemia model is a rise in maternal blood pressure. As show in Figure 4A, rats subjected to the reduced uterine perfusion pressure (RUPP) placental ischemia procedure had a mean arterial pressure (MAP) that was 20 mmHg higher on GD 19 compared to sham operated animals (100 ± 13 vs. 120 ± 11, p < 0.0001). A similar rise in MAP of over 20 mmHg was seen in placental ischemia animals treated with the control SynB1-ELP polypeptide (99 ± 10 vs. 123 ± 6, p < 0.0001). In contrast, rats treated with SynB1-ELP-p50i had a smaller rise in MAP of 12 mmHg that did not reach statistical significance (98 ± 9 vs. 111 ± 5, p = 0.071). While SynB1-ELP-p50i partially ameliorated the increase in blood pressure in response to placental ischemia, the trend for decreasing blood pressure when comparing RUPP operated saline treated rats with RUPP operated SynB1-ELP-p50i treated rats did not reach statistical significance due to the large number of multiple comparisons in this multi-group study (120 ± 11 vs. 111 ± 5, p = 0.296). Continuous infusion of the free p50i peptide showed similar mean pressure increases in response to placental ischemia as SynB1-ELP-p50i treated rats (103 ± 10 vs. 115 ± 9, p = 0.378), and the increase in MAP due to placental ischemia was not statistically significant. It is likely that continuous infusion via minipump negated much of the pharmacokinetic advantage achieved by fusion p50i to the SynB1-ELP carrier, thus the free peptide functioned with a similar efficacy to SynB1-ELP-p50i in this assay. These data indicate that the partial amelioration of the placental-ischemia-induced blood pressure increase is due to the p50i moiety. We also increased the dose of SynB1-ELP-p50i to 75 mg/kg/d in a separate cohort of rats. The higher dose performed in a similar manner to the 50 mg/kg/d dose, blunting the placental ischemia – induced blood pressure rise but not fully preventing it (92 ± 12 vs. 112 ± 10, p < 0.0001, Table 1).
Figure 4.
SynB1-ELP-p50i partially prevents placental ischemia – induced increases in mean arterial pressure. MAP (A.), maternal body weight (B.), and fetal reabsorption rate (C.) were measured on GD19 in rats exposed to the RUPP procedure to induce placental ischemia or to sham surgery. Rats were administered saline, the control protein SynB1-ELP, a non-ELP conjugated p50i peptide, or the SynB1-ELP-p50i protein at equal doses. * Statistically significant differences between sham and RUPP groups as assessed by two-way ANOVA with post-hoc Tukey’s multiple comparisons.
Table 1.
Maternal Characteristics of Polypeptide – Treated Rats in the Placental Ischemia Model.
| Metrics | Sham Saline |
RUPP Saline |
Sham SynB1 -ELP (50 mg/kg/ d) |
RUPP SynB1 -ELP (50 mg/kg /d) |
Sham Free p50i (2 mg/k g/d) |
RUPP Free p50i (2 mg/kg/ d) |
Sham SynB1 -ELP- p50i (50 mg/kg /d) |
RUPP SynB1 -ELP- p50i (50 mg/kg /d) |
Sham SynB1 -ELP- p50i (75 mg/kg /d) |
RUPP SynB1 -ELP- p50i (75 mg/kg /d) |
|---|---|---|---|---|---|---|---|---|---|---|
| Maternal Body Weight (g) | 291 ± 23 | 262 ± 15* | 281 ± 13 | 263 ± 12 | 297 ± 16 | 264 ± 25* | 301 ± 10 | 271 ± 19* | 293 ± 21 | 266 ± 22* |
| Maternal MAP (mmHg) | 100 ± 13 | 120 ± 11* | 99 ± 10 | 123 ± 6* | 103 ± 10 | 115 ± 9 | 98 ± 9 | 111 ± 5 | 92 ± 12 | 112 ± 10* |
| Maternal Heart Weight (g) | 0.77 ± 0.10 | 0.82 ± 0.10 | 0.71 ± 0.04 | 0.72 ± 0.08 | 0.79 ± 0.04 | 0.76 ± 0.05 | 0.75 ± 0.08 | 0.80 ± 0.08 | 0.77 ± 0.12 | 0.86 ± 0.11 |
| Maternal Kidney Weight (g) | 0.81 ± 0.09 | 0.85 ± 0.09 | 0.76 ± 0.08 | 0.84 ± 0.08 | 0.81 ± 0.07 | 0.81 ± 0.10 | 0.77 ± 0.09 | 0.82 ± 0.1 | 0.80 ± 0.08 | 0.87 ± 0.1* |
| Total Litter Size (GD14, pre-surgery) | 11.3 ± 1.8 | 11.8 ± 1.5 | 10.7 ± 0.9 | 11.1 ± 1.5 | 11.3 ± 1.0 | 11.7 ± 1.2 | 11.4 ± 1.6 | 12.0 ± 1.5 | 10.7 ± 1.5 | 12.1 ± 1.8 |
| Reabsorption Rate (%) | 5.9 ± 10.1 | 69.5 ± 14.2* | 17.5 ± 18.8 | 76.7 ± 16.2* | 9.7 ± 10.2 | 66.6 ± 11.7* | 4.8 ± 7.3 | 70.8 ± 18.9* | 12.6 ± 20.1 | 68.3 ± 18.4* |
| Placental Weight (g) | 0.62 ± 0.05 | 0.59 ± 0.07 | 0.59 ± 0.09 | 0.53 ± 0.09 | 0.63 ± 0.04 | 0.53 ± 0.07 | 0.55 ± 0.06 | 0.55 ± 0.12 | 0.62 ± 0.06 | 0.60 ± 0.10 |
| Fetal Weight (g) | 2.56 ± 0.20 | 2.43 ± 0.20 | 2.51 ± 0.29 | 2.24 ± 0.28 | 2.57 ± 0.32 | 2.22 ± 0.28 | 2.47 ± 0.15 | 2.33 ± 0.20 | 2.55 ± 0.19 | 2.46 ± 0.25 |
| Placental Efficiency | 4.15 ± 0.40 | 4.16 ± 0.59 | 4.27 ± 0.51 | 4.32 ± 0.76 | 4.10 ± 0.32 | 4.20 ± 0.55 | 4.54 ± 0.45 | 4.35 ± 0.78 | 4.14 ± 0.26 | 4.17 ± 0.61 |
Mean ± SD are shown.
Statically significant difference between Sham and RUPP operated rats with the same test agent treatment were assessed using a two-way ANOVA with post-hoc Tukey’s multiple comparison of all groups pair-wise.
As an initial assessment of toxicity, we also monitored daily body weights and fetal reabsorption of all rats in the study. GD 19 weights are shown in Figure 4B. Among the sham operated rats, there were no differences among the four treatment groups, indicating no overt toxicity from the test agents. RUPP operated rats were consistently lighter than sham operated rats due to the reduced fetal and placental mass, but again there were no differences among polypeptide treatment groups. Fetal reabsorption is an expected consequence of the placental ischemia surgery and provides a useful surrogate marker of the extent of blood flow restriction. In sham operated rats, there were no differences among treatment groups in the fetal reabsorption percentage, and all error bars crossed zero. This is an important observation, demonstrating that SynB1-ELP-p50i does not cause fetal demise (even at the higher 75 mg/kg/d dose, Table 1). RUPP operated rats all showed fetal reabsorption rates in the 65 – 75% range, demonstrating consistency in the model, and there were no differences among polypeptide treatment groups. Other maternal metrics, including heart weight, kidney weight, placental weight, and placental efficiency showed no significant differences among polypeptide treatment groups or in response to the RUPP procedure (Table 1 and Figure S2). Equivalent pre-treatment litter size demonstrated good randomization among all treatment groups (Table 1 and Figure S2A). The RUPP procedure is known to cause a reduction in pup weight 41, and this was observed in our study with trending reductions in fetal weight in all RUPP treatment groups (Table 1 and Figure S2F). Treatment with SynB1-ELP-p50i or the control agents was not able to rescue the fetal growth restriction, as expected due to the mechanical nature of the placental ischemia procedure.
SynB1-ELP-p50i Reduces Inflammation in Response to Placental Ischemia.
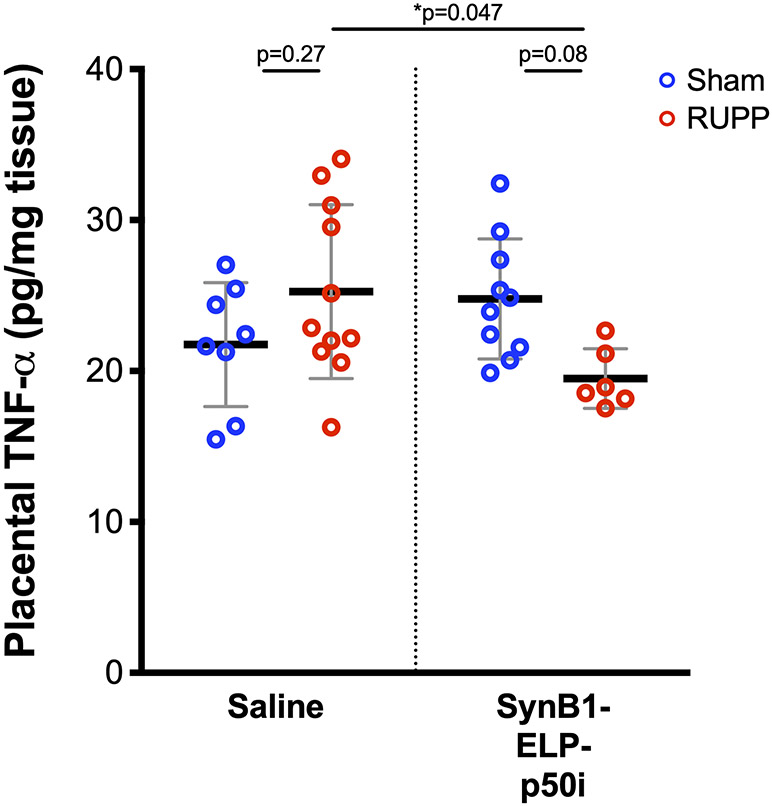
To determine whether SynB1-ELP-p50i modulated the inflammatory response in the dams, we measured several markers including placental TNF-α protein levels, placental Ccl2 mRNA levels, and placental IL-2 and IL-6 mRNA levels. Placental TNF-α trended higher in rats subjected to placental ischemia, though the difference did not reach statistical significance. There was, however, a significant reduction in placental TNF-α levels in RUPP rats treated with SynB1-ELP-p50i versus RUPP rats treated with saline (Figure 5). Placental Ccl2, IL-2, and IL-6 mRNA levels and aortic ET-1 mRNA were not affected by the RUPP surgery or by protein treatment (Figure S3). Resident T-cell populations were examined in placental tissue by flow cytometry to assess differences between sham-operated and RUPP animals and to determine if SynB1-ELP-p50i affected immune cell recruitment to the placenta. No differences were seen between sham and RUPP groups in helper T-cells, cytotoxic T-cells or naïve T-cells. Additionally, administration of SynB1-ELP-p50i showed no difference in the resident placental T-cell populations in either sham or RUPP treated animals (Figure S4).
Figure 5.
SynB1-ELP-p50i reduces placental TNF-α levels Placental TNF-α levels were significantly lower in RUPP rats treated with SynB1-ELP-p50i relative to saline-treated RUPP rats. * Statistically significant differences between groups as assessed by two-way ANOVA with post-hoc Tukey’s multiple comparisons.
Discussion.
Increased NF-κB activation has been demonstrated in preeclamptic women, with increases observed in both the vessels 15 and the placentae 17, possibly representing a promising therapeutic target. As an initial attempt to intervene in this pathway, we tested the ability of a peptide agent, known to be a competitive inhibitor of NF-κB nuclear localization 19, to modulate the PE syndrome in a rat model of placental ischemia. Fusion of the NF-κB inhibitory peptide to the ELP carrier had several advantages. We first showed that the fusion protein maintained NF-κB inhibitory activity, blocking both TNF-α and LPS – induced NF-κB nuclear localization and preventing TNF-α induced ET-1 production in vitro. This is an important observation, as it rules out the possibility that fusion of the small peptide to the large ELP domain may interfere with its ability to bind its molecular target. Fusion to the ELP carrier had significant advantages when the chimeric protein was administered in vivo. The free peptide was rapidly cleared from the maternal circulation, and it also crossed the placental barrier. However, when fused to the polypeptide carrier, the maternal half-life was extended significantly, and the chimeric protein was entirely maternally sequestered. Finally, we also determined the potential efficacy of SynB1-ELP-p50i using a rat model of placental ischemia. This model is a valuable one for evaluation of potential PE therapeutics because it reproduces many of the important molecular pathways known to drive the maternal syndrome of PE, exhibiting hypertension, endothelial dysfunction, systemic inflammation, angiogenic imbalance, and fetal growth restriction 36. As a first and most important observation, SynB1-ELP-p50i had no overt toxicity when used in pregnant rats. It had no effect on maternal weight gain throughout gestation and did not induce fetal loss. SynB1-ELP-p50i partially ameliorated the maternal hypertension induced by placental ischemia, and it reduced placental TNF-α levels relative to vehicle treated placental ischemic rats.
These results demonstrate the promise of this therapeutic strategy, though more work is needed to refine the overall treatment strategy and the agent itself. There is still some question as to the precise mechanism by which SynB1-ELP-P50i affects blood pressure. It is likely that there is a maternal vascular component as inflammation has been shown to directly affect vascular function 42,43. Perhaps coupled with this would be changes in renal blood flow and filtration rate, which are also suppressed in this model 44. SynB1-ELP-p50i only partially ameliorated the increase in blood pressure in response to placental ischemia. Increasing the dose of SynB1-ELP-p50i did not result in further blood pressure reduction, suggesting that the blood pressure increase in response to placental ischemia may not be solely due to inflammatory factors. Given this observation, our future plans include exploration of combination therapies, including combination of anti-inflammatory agents with other agents our lab is developing that target the anti-angiogenic pathways known to drive the PE syndrome 23,45. Also, we did not attempt in this study to reduce the in vivo dose to determine the lowest effective dose, so more work is needed on this front. Finally, though no overt fetal toxicity was seen, future work is needed to evaluate the health and development of offspring after SynB1-ELP-p50i treatment. Ongoing work in our lab is studying how therapy of the maternal syndrome with our developmental agents not only affects the acute maternal outcomes during the index pregnancy, but also how it affects the fetal growth, development, and cardiovascular risk factors in the offsprings’ later life.
Perspectives.
This study establishes the value of the ELP drug delivery system for stabilizing peptide therapeutics and for prevention of their placental transfer. More broadly, ELPs have the potential to be utilized for prevention of fetal drug exposure, increasing the safety of pharmacological therapy during pregnancy. The amenability of ELP to fusion with many types of therapeutic agents expands the applicability of this system to small molecule, peptide, and protein therapeutics used during pregnancy. This work also demonstrates that NF-κB is a potentially viable target for PE therapy, though possibly best achieved in combination with agents targeting other molecular pathways known to drive the PE maternal syndrome.
Supplementary Material
Novelty and Significance.
What Is New?
This work describes a new system for improving the safety of treatments used in pregnant women.
This system prevents the treatment from crossing the placenta, so the developing baby is not exposed to the drug
Furthermore, the drug delivery system is used to deliver a new type of therapeutic for the pregnancy-specific disorder preeclampsia
What Is Relevant?
The new therapeutic described in this work did not cross the placenta and was not found in the fetus in an animal model.
The new therapeutic partially alleviated several symptoms, including preventing a large rise in blood pressure, in an animal model of preeclampsia.
Summary.
This work describes the use of a drug delivery system to prevent placental transfer of a therapeutic agent. The molecule, a multi-component therapeutic protein, reduced inflammation and partially corrected the symptoms of preeclampsia in a preclinical animal model.
Acknowledgements.
We thank Drazen Raucher for SynB1-ELP and SynB1-ELP-p50i expression constructs and Rowshan Begum for assistance purifying polypeptides. IVIS imaging was performed using the UMMC Animal Imaging Core Facility, and flow cytometry was performed using the UMMC Flow Cytometry Core Facility.
Sources of Funding. This work was directly funded by NIH grant R01HL121527. ACE was supported by American Heart Associate Fellowship number 19PRE34430044.
Footnotes
Conflicts of Interest/Disclosures. GLB is the owner of Leflore Technologies, LLC, a private company working to translate the ELP drug delivery technology. GLB and EMG are inventors on patents related to the work described.
References.
- 1.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, Zamudio S, Illsley NP, Myatt L, Colvis C, Costantine MM, Haas DM, Sadovsky Y, Weiner C, Rytting E, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. American Journal of Obstetrics and Gynecology. 2016;215(1 Suppl):S1–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown CM, Garovic VD. Drug Treatment of Hypertension in Pregnancy. Drugs. 2014;74(3):283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates DO. Pre-eclampsia and the microcirculation: a novel explanation. Clinical Science (London, England: 1979). 2003;104(4):413–414. [DOI] [PubMed] [Google Scholar]
- 5.Pijnenborg R, Robertson WB, Brosens I, Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2(1):71–91. [DOI] [PubMed] [Google Scholar]
- 6.Rolfo A, Giuffrida D, Nuzzo AM, Pierobon D, Cardaropoli S, Piccoli E, Giovarelli M, Todros T. Pro-inflammatory profile of preeclamptic placental mesenchymal stromal cells: new insights into the etiopathogenesis of preeclampsia. PloS One. 2013;8(3):e59403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy SR, Lamarca BB, Parrish M, Cockrell K, Granger JP. Control of Soluble fms-like tyrosine-1 (sFlt-1) production in response to placental ischemia/hypoxia: Role of tumor necrosis factor-alpha (TNF-alpha). Am J Physiol Regul Integr Comp Physiol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Llurba E, Gris JM. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review. Clinical Reviews in Allergy & Immunology. 2017;53(1):40–53. [DOI] [PubMed] [Google Scholar]
- 9.Winship A, Menkhorst E, Van Sinderen M, Dimitriadis E. Interleukin 11 blockade during mid to late gestation does not affect maternal blood pressure, pregnancy viability or subsequent fertility in mice. Reproductive Biomedicine Online. 2018;36(3):250–258. [DOI] [PubMed] [Google Scholar]
- 10.Lee JI, Burckart GJ. Nuclear factor kappa B: important transcription factor and therapeutic target. Journal of Clinical Pharmacology. 1998;38(11):981–993. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Wilson R null, Wang SH, Zheng HZ, Walker JJ, McKillop JH. Tumour necrosis factor-alpha (TNF-alpha) gene polymorphism and expression in pre-eclampsia. Clinical and Experimental Immunology. 1996;104(1):154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalinderis M, Papanikolaou A, Kalinderi K, Ioannidou E, Giannoulis C, Karagiannis V, Tarlatzis BC. Elevated serum levels of interleukin-6, interleukin-1β and human chorionic gonadotropin in pre-eclampsia. American Journal of Reproductive Immunology (New York, N.Y.: 1989). 2011;66(6):468–475. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee P, Weaver LE, Doersch KM, Kopriva SE, Chiasson VL, Allen SJ, Narayanan AM, Young KJ, Jones KA, Kuehl TJ, Mitchell BM. Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS One. 2012;7:e41884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centlow M, Wingren C, Borrebaeck C, Brownstein MJ, Hansson SR. Differential gene expression analysis of placentas with increased vascular resistance and pre-eclampsia using whole-genome microarrays. J Pregnancy. 2011;2011:472354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaughan JE, Walsh SW. Activation of NF-κB in placentas of women with preeclampsia. Hypertension in Pregnancy. 2012;31(2):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aban M, Cinel L, Arslan M, Dilek U, Kaplanoglu M, Arpaci R, Dilek S. Expression of nuclear factor-kappa B and placental apoptosis in pregnancies complicated with intrauterine growth restriction and preeclampsia: an immunohistochemical study. Tohoku J Exp Med. 2004;204:195–202. [DOI] [PubMed] [Google Scholar]
- 17.Shah TJ, Walsh SW. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196:48 e1–8. [DOI] [PubMed] [Google Scholar]
- 18.Bidwell GL, Raucher D. Therapeutic peptides for cancer therapy. Part I - peptide inhibitors of signal transduction cascades. Expert Opin Drug Deliv. 2009;6:1033–47. [DOI] [PubMed] [Google Scholar]
- 19.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–8. [DOI] [PubMed] [Google Scholar]
- 20.Bidwell GL, George EM. Maternally sequestered therapeutic polypeptides - a new approach for the management of preeclampsia. Frontiers in Pharmacology. 2014;5:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urry DW, Parker TM, Reid MC, Gowda DC. Biocompatibility of the bioelastic materials, poly(GVGVP) and its gamma-irradiation cross-linked matrix - summary of generic biological test results. Bioact Compat Polym. 1991;6:263–282. [Google Scholar]
- 22.Bidwell GL, Raucher D. Cell penetrating elastin-like polypeptides for therapeutic peptide delivery. Adv Drug Deliv Rev. 2010;62:1486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logue OC, Mahdi F, Chapman H, George EM, Bidwell GL. A Maternally Sequestered, Biopolymer-Stabilized Vascular Endothelial Growth Factor (VEGF) Chimera for Treatment of Preeclampsia. Journal of the American Heart Association. 2017;6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, Masuda K, Setton LA. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: sustained release of a local antiinflammatory therapeutic. Arthritis Rheum. 2007;56:3650–61. [DOI] [PubMed] [Google Scholar]
- 25.Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. Injectable protease-operated depots of glucagon-like peptide-1 provide extended and tunable glucose control. Proc Natl Acad Sci U S A. 2013;110:2792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bidwell GL, Perkins E, Raucher D. A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth. Cancer Lett. 2012;319:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuna M, Mahdi F, Chade AR, Bidwell GL. Molecular Size Modulates Pharmacokinetics, Biodistribution, and Renal Deposition of the Drug Delivery Biopolymer Elastin-like Polypeptide. Scientific Reports. 2018;8(1):7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuna M, Waller JP, Logue OC, Bidwell GL. Polymer size affects biodistribution and placental accumulation of the drug delivery biopolymer elastin-like polypeptide in a rodent pregnancy model. Placenta. 2018;72–73:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George EM, Liu H, Robinson GG, Bidwell GL. A polypeptide drug carrier for maternal delivery and prevention of fetal exposure. Journal of Drug Targeting. 2014:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rousselle C, Clair P, Lefauconnier JM, Kaczorek M, Scherrmann JM, Temsamani J. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol Pharmacol. 2000;57:679–86. [DOI] [PubMed] [Google Scholar]
- 31.Thomas EH, Raucher D. Abstract 2659: A thermally targeted carrier of p50 NLS peptide inhibits cancer cell proliferation by preventing NFκB nuclear translocation. Cancer Research. 2010;70(8 Supplement):2659–2659. [Google Scholar]
- 32.Thomas EH. Optimizing the delivery of therapeutic peptides using elastin-like polypeptide. Master’s Thesis, University of Mississippi Medical Center; 2010. [Google Scholar]
- 33.George EM, Liu H, Robinson GG, Mahdi F, Perkins E, Bidwell GL. Growth factor purification and delivery systems (PADS) for therapeutic angiogenesis. Vascular Cell. 2015;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bidwell GL, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4:1076–85. [DOI] [PubMed] [Google Scholar]
- 35.McGowan JWD, Bidwell GL. The Use of Ex Vivo Whole-organ Imaging and Quantitative Tissue Histology to Determine the Bio-distribution of Fluorescently Labeled Molecules. Journal of Visualized Experiments: JoVE. 2016;(118). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. American Journal of Physiology. Heart and Circulatory Physiology. 2012;303(1):H1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowenthal JW, Ballard DW. Tumor necrosis factor a induces proteins that bind specifically to cB-like enhancer elements and regulate interleukin 2 receptor a-chain gene expression in primary human T lymphocytes. Proc. Natl. Acad Sci. USA. 1989:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annual Review of Biochemistry. 2002;71:635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. The American Journal of Physiology. 1992;262(4 Pt 1):C854–861. [DOI] [PubMed] [Google Scholar]
- 40.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37(4):1191–1195. [DOI] [PubMed] [Google Scholar]
- 41.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–62. [DOI] [PubMed] [Google Scholar]
- 42.Davis JR, Giardina JB, Green GM, Alexander BT, Granger JP, Khalil RA. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-alpha-induced hypertension in pregnant rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2002;282(2):R390–399. [DOI] [PubMed] [Google Scholar]
- 43.Giardina JB, Green GM, Cockrell KL, Granger JP, Khalil RA. TNF-alpha enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2002;283(1):R130–143. [DOI] [PubMed] [Google Scholar]
- 44.Llinás MT, Alexander BT, Seedek M, Abram SR, Crell A, Granger JP. Enhanced thromboxane synthesis during chronic reductions in uterine perfusion pressure in pregnant rats. American Journal of Hypertension. 2002;15(9):793–797. [DOI] [PubMed] [Google Scholar]
- 45.Eddy AC, Bidwell GL, George EM. Pro-angiogenic therapeutics for preeclampsia. Biology of Sex Differences. 2018;9(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.