Abstract
Background:
Effectiveness of colorectal cancer (CRC) screening with fecal immunochemical stool blood tests (FIT) requires high rates of colonoscopy follow up for abnormal FIT, and use of high quality tests. We characterized colonoscopy referral and completion among patients with abnormal FIT, and type of FIT implemented in a sample of Southern California Federally Qualified Health Centers (FQHCs).
Methods:
FQHCs in San Diego, Imperial, and Los Angeles counties were invited to define a cohort of ≥150 consecutive abnormal FIT patients 2015–2016 and provide data on gender, insurance status, diagnostic colonoscopy referral and completion within six months of abnormal FIT, and type (brand) of FIT implemented. Primary outcomes were proportion with colonoscopy referral and completion for all patients at each FQHC and in aggregate.
Results:
Eight FQHCs provided data for 1,229 patients with abnormal FIT; 46% were male and 20% uninsured. Among abnormal FIT patients, 89% (n=1,091/1,229; 95%CI: 0.87–0.91) had colonoscopy referral and 44% (n=539/1,229; 95%CI: 0.41–0.47) had colonoscopy completion. Across FQHCs, range for colonoscopy referral was 73–96% and completion was 18–57%. Six of eight (75%) FQHCs reported FIT brands with limited data to support effectiveness.
Conclusions:
In a sample of Southern California FQHCs, diagnostic colonoscopy completion after abnormal FIT was substantially below the nationally recommended benchmark to achieve 80% completion, and use of FIT brands with limited data to support effectiveness was high. These findings suggest a need for policies and multi-level interventions to promote diagnostic colonoscopy among individuals with abnormal FIT, and use of higher quality FITs.
Keywords: abnormal FIT, colorectal cancer, screening, Hispanic, Federally Qualified Health Center
Precis:
In a survey of 8 Federally Qualified Health Centers, among 1,229 patients with an abnormal fecal immunochemical test for colorectal cancer screening, 89% had referral, but just 44% completed diagnostic colonoscopy, with range of completion ranging 18 to 57% across health centers. The findings suggest a major need for policies and multi-level interventions to promote diagnostic colonoscopy among patients with abnormal colorectal cancer screening tests.
Background and Significance
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States (1). Screening for CRC can reduce cancer incidence and mortality, but is underutilized. The national screening rate in 2015 was 62.6% (2). Screening rates are particularly low for racial/ethnic minorities (61.8% for Blacks , 49.9% for Hispanics and 54.3% for American Indian/Alaska Natives) (2), those covered by Medicaid (47%) and the uninsured (24.8%) (3). Federally Qualified Health Centers (FQHCs), the largest providers of care to under and uninsured individuals across the nation, are in a unique position to improve screening rates among disadvantaged, under and uninsured groups with low screening rates. Since 2012, FQHCs have been required to publically report rates of CRC screening as part of the Health and Human Services required Universal Data Set (UDS: https://bphc.hrsa.gov/uds/datacenter.), and many, with support from groups such as the NCCRT, have focused on improving screening rates. The most current UDS data from 2017 report an aggregate rate of CRC screening of 42% across FQHCs nationally.
For many FQHCs, CRC screening efforts focus on promoting uptake of the fecal immunochemical test (FIT), because of its low cost, convenience, and accessibility. Beyond ensuring test uptake, FQHCs implementing FIT-based screening face two additional key challenges to reducing the burden of CRC for their populations. First, effectiveness of FIT-based screening depends on high rates of diagnostic colonoscopy follow up after abnormal FIT. Between one in 11 and one in 28 individuals with an abnormal FIT have CRC, and between one in three and one in seven individuals have advanced neoplasia (4–8). As such, low rates of colonoscopy follow up after abnormal tests can blunt effectiveness of FIT-based programs. Compared to healthcare settings with integrated resources for specialty care, FQHCs face unique challenges to identify and coordinate care with outside specialty colonoscopy providers that function in separate healthcare systems. Few prior studies have evaluated rates of colonoscopy follow up in FQHC settings, and most were either single center or included just a few centers (9, 10). Second, effectiveness of FIT based programs depends on the brand of FIT selected for implementation. In its last major evidence review of CRC screening the United States Preventive Services Task force (USPSTF) concluded that sensitivity to detect CRC and high-risk adenomas varies widely across stool-based tests that have been approved by the Food and Drug Administration (FDA). Though many brands of FIT were evaluated, the USPSTF evidence review identified only two FIT brands [OC-Light and OC FIT-CHEK (marketed as OC-Sensor Diana, OC-Micro, and OC-Auto)] with adequate data to support high sensitivity and specificity. It is unclear which FITs or other stool tests FQHCs are choosing, and how often tests with low sensitivity/specificity are used.
Currently, FQHCs are not required to report colonoscopy completion rates after abnormal FIT, or type of FIT implemented. Understanding current rates of follow up, and whether high quality FITs are being implemented, could inform policies and research focused on optimizing outcomes of CRC screening, particularly for underserved populations. Accordingly, our goal was to characterize rates of diagnostic colonoscopy referral after abnormal FIT, rates of completion of diagnostic colonoscopy after positive FIT, and brand of FITs used across a sample of FQHCs in Southern California.
Methods
Study Design and Population
This was a cross-sectional study conducted between January 2015 and June 2016 at eight FQHCs in San Diego, Imperial, and Los Angeles counties of California.
Recruitment of FQHCs
Three study investigators (SG, JN, FPM) sent a letter (Appendix A) to quality improvement (QI) personnel in FQHCs in the San Diego, Imperial, and Los Angeles counties. FQHCs were identified via targeted recruitment, and American Cancer Society workshops designed to train local FQHCs about CRC screening strategies. The FQHCs contacted were informed of the importance of diagnostic colonoscopy after abnormal FIT and invited to participate in a study to understand rates of colonoscopy completion after abnormal FIT in Southern California. Each clinic was offered a $400 honorarium for their participation.
Data Collection
We provided each FQHC with a brief questionnaire, data collection form, and detailed instructions on how to define a cohort of patients with abnormal FIT (Appendix A). We requested each FQHC create a patient cohort including patients with an abnormal FIT during 6-month block periods between January 2015 and June 2016. Depending on the size of the population served by each FQHC, data were collected in one to three block periods to achieve a sample of at least 150 patients with abnormal FIT per site. Each clinic used the data collection form to perform either a manual chart review or electronic medical record query to abstract patient-level data for gender, medical insurance status, presence of a colonoscopy referral after abnormal FIT, and documentation of a completed colonoscopy after abnormal FIT. No further data were requested in an effort to maximize participation by FQHCs. In the questionnaire, FQHCs provided summative information about patient demographics, brand of FIT kit used in their clinics, and colonoscopy referral rates. Colonoscopy completion rates were determined from information in the data collection form. These aggregated, de-identified data were sent to the study PI at University of California San Diego (SG). The study was reviewed by the UC San Diego Human Research Protection Program and deemed exempt from further review due to use of de-identified aggregate data for analyses.
FQHC Health Resources and Services Administration (HRSA) Data
To gain details about the clinics represented in our sample, we collected demographic data on the FQHC sites from HRSA. These data (2015) are collected and made publically available (https://bphc.hrsa.gov/uds/datacenter). We merged clinic-level data with the primary data collected as part of this study.
Statistical Analyses
Outcomes
The primary outcome was the proportion of individuals with abnormal FIT with documented colonoscopy within six months of the test result. Secondary outcomes of interest were the proportion of patients with a colonoscopy referral within six months of the abnormal FIT, and brands of FIT used at the FQHCs.
Sample size
We estimated (a priori) that we would need a minimum of 150 patient participants to achieve 90% confidence that our calculated rate for diagnostic colonoscopy after positive FIT reflected the true population rate. This was based on the assumption that any given health center has approximately 300 abnormal test results over a 12-month time period.
Analyses
Descriptive statistics were used to characterize the study overall and by clinic study population and variation in FIT kit brand. We also calculated rates for colonoscopy referral and colonoscopy completion after abnormal FIT for each FQHC and across all FQHCs. We computed 95% confidence intervals estimates for all proportions.
Results
FQHC Sample
Of the 20 health centers invited, eight FQHCs agreed to participate in the study and contributed data for 1,229 patients (Figure 1). Among non-participants, eight FQHCs cited lack of resources (funding and staff time) as the main barriers to participation. The remaining FQHCs did not respond to the invitation in a timely manner. The number of patients seen annually in each participating FQHC ranged from 55,000 to 135,000 individuals based on UDS data (Table 1). As is shown in Table 1, nearly all patients served by the eight FQHCs were at or below 200% of the Federal Poverty Level. The proportion of uninsured ranged from 20 to 45%, while the proportion covered by MediCal (Medicaid) ranged from 34 to 72%. Across all clinics, range of patients who were identified as Hispanic/Latino was 52 to 94%. UDS CRC screening rates across the 8 FQHCs during the study timeframe ranged from 28% to 49% (mean=42%).
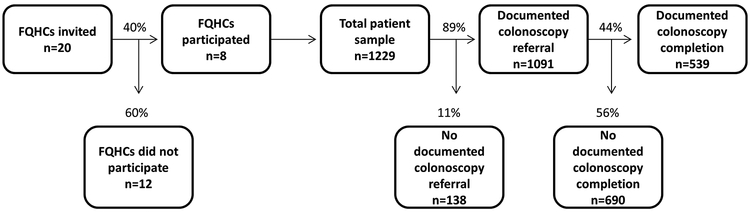
Figure 1: Study flow and overall summary of colonoscopy referral and completion in a sample of Federally Qualified Health Centers in San Diego, Imperial and Los Angeles Counties of California; Jan 2015-June 2016; N= 1229.
The process for sample selection, and overall summary rate of colonoscopy referral and completion at included Federally Qualified Health Centers is depicted.
Table 1:
Demographic characteristics of individuals served by included Federally Qualified Health Centers in San Diego, Imperial and Los Angeles Counties of California 2015 (n=8)
| FQHC 1 | FQHC 2 | FQHC 3 | FQHC 4 | FQHC 5 | FQHC 6 | FQHC 7 | FQHC 8 | |
|---|---|---|---|---|---|---|---|---|
| Total Patients Served | 89,662 | 134,788 | 55,465 | 64,834 | 23,119 | 17,214 | 19,415 | 85,860 |
| Adult (18 – 64) | 55% | 70% | 63% | 64% | 68% | 89% | 64% | 53% |
| Older Adults (≥ 65) | 9% | 5% | 4% | 5% | 5% | 6% | 5% | 3% |
| Patients ≤ 200% of poverty | 97% | 97% | 98% | 98% | 97% | 97% | 99% | 98% |
| Patients ≤ 100% of poverty | 79% | 81% | 79% | 80% | 75% | 73% | 90% | 82% |
| Insurance Status | ||||||||
| Uninsured | 31% | 30% | 31% | 20% | 22% | 39% | 45% | 28% |
| Medicaid/CHIP | 57% | 62% | 58% | 72% | 69% | 34% | 47% | 66% |
| Medicare | 8% | 4% | 2% | 6% | 5% | 3% | 3% | 3% |
| Other Third Party | 4% | 4% | 8% | 2% | 5% | 24% | 4% | 4% |
| Ethnicity | ||||||||
| Hispanic/Latino | 81% | 59% | 62% | 53% | 57% | 52% | 94% | 75% |
| Non-Hispanic/ Latino | 19% | 41% | 38% | 47% | 43% | 48% | 6% | 25% |
| Race* | ||||||||
| White | 46% | 77% | 91% | 90% | 85% | 77% | 95% | 89% |
| Black/African American | 28% | 12% | 3% | 3% | 11% | 15% | 4% | 9% |
| Asian | 21% | 5% | 2% | 4% | 4% | 8% | 0. 1% | 2% |
| American Indian/Alaska Native | 2% | 1% | 0. 2% | 0. 4% | 0. 2% | 0. 2% | 0. 0% | 0. 4% |
| Native Hawaiian / Other Pacific Islander | 2% | 1% | 1% | 0% | 0% | 0% | 0% | 0. 2% |
| More than one race | 1% | 4% | 3% | 3% | 0. 2% | 0. 2% | 1% | 0. 1% |
| Patients age 50–75 years Up-to-Date with colorectal cancer screening | 43% | 34% | 28% | 46% | 43% | 45% | 45% | 49% |
Source: Publically reported United States Health and Human Services data at https://bphc.hrsa.gov/uds/datacenter; Percentages have been rounded to the nearest numbers;
due to rounding, percentages do not sum to 100% for all clinics
Study Population
The eight participating FQHCs contributed data for 1,229 individuals with abnormal FIT (Table 2). Each FQHC contributed between 75 and 251 patients. Males comprised 46% of the sample; the range for gender was 34% to 54% across sites. Twenty percent (n=244) were uninsured, ranging from 11% to 40% across sites.
Table 2:
Demographic characteristics of individuals with abnormal FIT at Federally Qualified Health Centers in San Diego, Imperial and Los Angeles Counties of California; Jan 2015-June 2016; N= 1229
| Health Centers | Male n (%) | Uninsured n (%) |
|---|---|---|
| Overall N=1229 | 559 (46%) | 244 (20%) |
| FQHC 1 (n=150) | 81 (54%) | 16 (11%) |
| FQHC 2 (n=150) | 73 (49%) | 35 (23%) |
| FQHC 3 (n=188) | 89 (47%) | 27 (14%) |
| FQHC 4 (n=251) | 128 (51%) | 28 (11%) |
| FQHC 5 (n=150) | 63 (42%) | 32 (21%) |
| FQHC 6 (n=75) | 26 (35%) | 30 (40%) |
| FQHC 7 (n=150) | 51 (34%) | 35 (23%) |
| FQHC 8 (n=115) | 48 (42%) | 41 (36%) |
Source: Survey of health center utilized for study
Referral for Diagnostic Colonoscopy and Colonoscopy Completion After Abnormal FIT
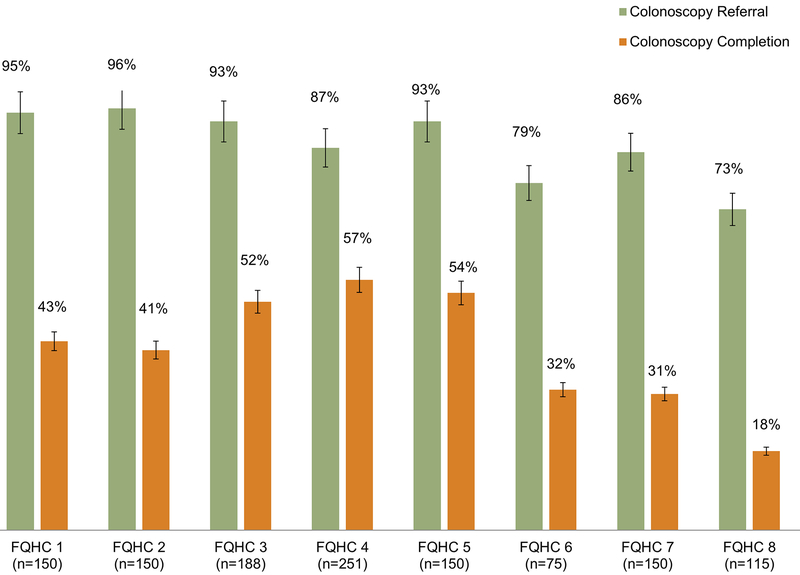
Among 1,229 patients with abnormal FIT, 89% (n=1,091/1,229 95% CI: 0.87–0.91) had documented colonoscopy referral and 44% (n=539/1,229 95% CI: 0.41–0.47) had documentation of colonoscopy completion (Figure 1). Across FQHCs, the range of colonoscopy referral was 73–96%, and the range of colonoscopy completion was 18–57% (Figure 2).
Figure 2: Diagnostic colonoscopy referral and completion rates after abnormal FIT across a sample of 8 Federally Qualified Health Centers in San Diego, Imperial and Los Angeles Counties of California; Jan 2015-June 2016; N= 1229.
Proportion of patients with abnormal FIT with colonoscopy referral and colonoscopy completion are depicted for each included FQHC. FIT, fecal immunochemical test; FQHC, Federally Qualified Health Center.
FIT Brand
There were several brands of FIT kits represented in the eight FQHCs: Hemosure (n=3), Polymedco-OC Auto (n=2), and both Polymedco-OC Auto and Insure (n=3). Clinics offering more than one test did so to accommodate insurance coverage. Among these tests, only Polymedco-OC Auto is recognized by USPSTF as having sufficient evidence to support use. Thus, six out of eight (75%) FQHCs were utilizing FITs with questionable data to support effectiveness.
Discussion
In a sample of eight FQHCs that serve three Southern California counties, we found high rates of colonoscopy referral but low completion rates after abnormal FIT, and use of FIT brands with uncertain effectiveness for screening. Specifically, 1 in 2 individuals with abnormal FIT did not have evidence of colonoscopy completion within 6 months, despite colonoscopy referral rates approaching 90% in this study. The public health implications are significant, since up to 1 in 10 of the 1,229 individuals with an abnormal FIT who did not complete colonoscopy in this sample are expected to have undiagnosed CRC, and failure to complete colonoscopy is expected to result in a 2.4 fold increased risk of CRC mortality (4). In our sample, additionally, 75% of FQHCs reported using FIT brands not recognized by the USPSTF as having sufficient evidence to support use.
These results confirm and extend prior work examining colonoscopy follow up after abnormal stool-based screening (11–15). A retrospective study in 2016, also performed in a safety-net setting, found that only half of individuals with a positive FIT completed their colonoscopy within a year (15). Additional studies in similar settings have identified follow-up colonoscopy rates between 42 and 68% (14). In these cohorts, barriers to colonoscopy completion included increasing age, Hispanic ethnicity, female gender, and high comorbidity (11, 12, 14, 15). To our knowledge, our sample describing colonoscopy referral and completion rates includes the largest number of distinct FQHCs to date. In addition, our study provides the first analysis of FIT brands used in usual practice in this setting.
Our observation that many FQHCs use FIT brands of uncertain effectiveness requires further study. Use of suboptimal quality FITs may blunt impact of screening efforts, and also strain resources. For example, implementation of tests with suboptimal sensitivity may reduce cancer detection, while implementation of tests with suboptimal specificity may lead to higher rates of false positive tests, challenging diagnostic colonoscopy capacity and burdening patients with recommendations for potentially unnecessary evaluations.
Taken together with prior work, our results have several implications. From a policy standpoint, the proportion of patients with abnormal non-invasive tests completing diagnostic colonoscopy should be considered as a new, required quality metric. Low rates of diagnostic colonoscopy follow up have the potential to greatly compromise efforts to reduce CRC incidence and mortality, particularly because failure to complete diagnostic colonoscopy after abnormal FIT has been associated with increased risk for CRC death (4). A colonoscopy completion metric may be especially salient, given the expanding number of alternative tests to colonoscopy being made available in practice (though not universally implemented across practice settings), including the FIT-multi target DNA stool test, CT colonography, capsule colonoscopy, and the serum septin 9 blood test. A novel abnormal test follow up metric would likely be most powerful if required of both health systems (such as for FQHCs through inclusion as part of UDS reporting), and insurers (such as through Medicaid policies or inclusion as part of the Healthcare Effectiveness Data Information Set). From a clinical care and research standpoint, more work to understand reasons for colonoscopy non-completion, as well as to develop and implement interventions to improve colonoscopy follow up is needed. Potential reasons for failure to complete colonoscopy after stool-based testing include difficulty with bowel preparation, transportation, worry about exacerbating existing conditions, concerns about potential complications, and insurance status and language barriers (16–18). There is also evidence that organizational challenges in care coordination between FQHCs and outside specialty providers hinder care delivery for safety-net patients with cancer (19, 20). A limitation of prior work is a lack of data on patient reported reasons for failure to follow up.
Interventions to improve rates of colonoscopy follow up include use of patient navigators, provider reminders and/or performance data reports, automated referral to gastroenterology, and multicomponent QI efforts (21). However, very few interventions have been implemented specifically for follow up of abnormal stool blood tests in safety-net settings. Future research should seek to expand our knowledge of reasons for failure to follow up and develop optimized interventions for colonoscopy completion. The importance of the implementation and dissemination of such efforts within FQHCs is magnified by the fact that FQHCs serve disproportionately poor and underserved populations with the highest burden from CRC. Based on our findings that USPSTF recommended FIT brands is infrequent, additional studies should be conducted to verify effectiveness of FITs currently approved by the FDA. Further, a larger survey across multiple healthcare settings may be needed to understand the frequency with which FITs with limited evidence are in use. Education efforts should raise awareness about the differences between FIT brands among health systems and providers to support selection of tests with optimal screening test characteristics.
Several limitations must be considered in interpreting our results. Because we aimed to evaluate FIT follow-up rates in FQHCs in Southern California, our findings may not be generalizable to non-FQHC settings or to FQHCs in other regions of the country. In addition, only 8 out of 20 FQHCs agreed to participate in the study and our findings may not be representative of all FQHCs in San Diego, Imperial, and Los Angeles counties. The FQHCs that did not participate commonly cited lack of budget/resources and time for data collection. Another limitation is that because only clinic level aggregate data were used, we cannot comment on individual barriers to colonoscopy completion. Colonoscopy exposure could have been under-ascertained due to factors such as use of a 6 month time window to ascertain exposure, or inconsistencies in return of reports from completed colonoscopies to the health centers. The 6 month time period was chosen as a practical measure and because 6 months has been recommended as the maximum allowable time to complete colonoscopy within which outcomes may not be affected.(22) To minimize burden of data collection on FQHCs and maximize likelihood of participation, only a limited scope of data were requested from health centers, precluding ability to conduct patient level analyses of predictors of colonoscopy completion. Because additional patient and system level data were not collected, we were unable to explore in depth systematic factors (e.g. referral processes) and barriers that may have contributed to low rates of follow up and variation in completion across health centers, or report on rates of CRC and advanced neoplasia detection. We defined high quality FIT brands as those recommended for use in 2016 by the USPSTF based on its evidence review. Since the 2016 publication, new comparative FIT data have been published, including one study suggesting that the InSure FIT brand has higher sensitivity than OC-FIT CHEK, albeit when InSure is used as a 2-sample test compared to OC-FIT CHEK as a 1-sample test (Appendix B) (23). In our data, three FQHCs reported using the InSure FIT, suggesting that up to three out of eight (instead of two out of eight) were using FITs with sufficient data to support use. Even taking into account this adjustment, use of a FIT brand with weak evidence was common (62.5% of FQHCs). These limitations may be considered in light of our ability to recruit the largest sample of FQHCs reported to assess diagnostic colonoscopy follow up rates, as well as our novel measurement of types of FIT implemented.
In summary, among a sample of FQHCs in Southern California, we found that fewer than 1 in 2 patients with abnormal FIT had evidence of colonoscopy completion, thus placing them at markedly increased risk for CRC mortality (4). Further, 6 out of 8 FQHCs were implementing FITs not endorsed by the USPSTF for population screening. To realize the full potential benefits of CRC screening across the population, health policies, research, and interventions are required to optimize high quality screening, including high rates of colonoscopy follow up after abnormal FIT, and implementation of high quality FITs.
Supplementary Material
Acknowledgement
We would like to thank all the FQHCs that have participated in this project. A special thanks to Giovanna Perez who contributed to the first phase of the project.
Funding support
This work was supported in part by Cooperative Agreement number 1U58DP003862‐01 from The Centers for Disease Control and Prevention (CDC), obtained through the California Department of Public Health (CDPH) and grants numbers U54CA132379 and U54CA132384 from the National Institute of Health (NIH), and grant number U54CA143931 from Charles Drew Univeristy (CDU)-UCLA Partnership to Eliminate Cancer Disparities Grant. The work was also supported by a Specialized Cancer Center Support Grant to the UC San Diego Moores Cancer Center (CA023100–32). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of CDC, CDPH, NIH, CDU, or the UCLA Jonsson Comprehensive Cancer Center.
Footnotes
Conflict of Interest Statement
The Authors have no relevant conflicts of interest to disclose.
References
- 1.Force USPST, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–75. [DOI] [PubMed] [Google Scholar]
- 2.Kim Andrews; Rick Alteri; Afsaneh Barzi ea. Colorectal Cancer Facts & Figures. American Cancer Society 2017. [Google Scholar]
- 3.de Moor JS, Cohen RA, Shapiro JA, Nadel MR, Sabatino SA, Robin Yabroff K, et al. Colorectal cancer screening in the United States: Trends from 2008 to 2015 and variation by health insurance coverage. Prev Med. 2018;112:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152(5):1217–37 e3. [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, Yueh-Hsia Chiu S, Ching-Yuan Fann J, Chuang SL, et al. Association Between Colorectal Cancer Mortality and Gradient Fecal Hemoglobin Concentration in Colonoscopy Noncompliers. J Natl Cancer Inst. 2017;109(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–97. [DOI] [PubMed] [Google Scholar]
- 7.Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7(12):1272–8. [DOI] [PubMed] [Google Scholar]
- 8.Knudsen AB, Zauber AG, Rutter CM, Naber SK, Doria-Rose VP, Pabiniak C, et al. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. JAMA. 2016;315(23):2595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coronado GD, Schneider JL, Petrik A, Rivelli J, Taplin S, Green BB. Implementation successes and challenges in participating in a pragmatic study to improve colon cancer screening: perspectives of health center leaders. Transl Behav Med. 2017;7(3):557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khankari K, Eder M, Osborn CY, Makoul G, Clayman M, Skripkauskas S, et al. Improving colorectal cancer screening among the medically underserved: a pilot study within a federally qualified health center. J Gen Intern Med. 2007;22(10):1410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thamarasseril S, Bhuket T, Chan C, Liu B, Wong RJ. The Need for an Integrated Patient Navigation Pathway to Improve Access to Colonoscopy After Positive Fecal Immunochemical Testing: A Safety-Net Hospital Experience. J Community Health. 2017;42(3):551–7. [DOI] [PubMed] [Google Scholar]
- 12.Oluloro A, Petrik AF, Turner A, Kapka T, Rivelli J, Carney PA, et al. Timeliness of Colonoscopy After Abnormal Fecal Test Results in a Safety Net Practice. J Community Health. 2016;41(4):864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy AM, Kim JJ, Beaber EF, Zheng Y, Burnett-Hartman A, Chubak J, et al. Follow-Up of Abnormal Breast and Colorectal Cancer Screening by Race/Ethnicity. Am J Prev Med. 2016;51(4):507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin J, Halm EA, Tiro JA, Merchant Z, Balasubramanian BA, McCallister K, et al. Reasons for Lack of Diagnostic Colonoscopy After Positive Result on Fecal Immunochemical Test in a Safety-Net Health System. Am J Med. 2017;130(1):93 e1- e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Issaka RB, Singh MH, Oshima SM, Laleau VJ, Rachocki CD, Chen EH, et al. Inadequate Utilization of Diagnostic Colonoscopy Following Abnormal FIT Results in an Integrated Safety-Net System. Am J Gastroenterol. 2017;112(2):375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sultan S, Partin MR, Shah P, LeLaurin J, Freytes IM, Nightingale CL, et al. Barriers and facilitators associated with colonoscopy completion in individuals with multiple chronic conditions: a qualitative study. Patient Prefer Adherence. 2017;11:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayor J, Maniar S, Chan WW. Appointment-keeping behaviors and procedure day are associated with colonoscopy attendance in a patient navigator population. Prev Med. 2017;97:8–12. [DOI] [PubMed] [Google Scholar]
- 18.Lamanna A, Sheaffer H, Guerra C, Kochman M. Colorectal Cancer Screening Navigation for the Underserved: Experience of an Urban Program. Gastroenterol Hepatol (N Y). 2016;12(9):547–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Gutierrez J, Jhingan E, Angulo A, Jimenez R, Thompson B, Coronado GD. Cancer screening at a federally qualified health center: a qualitative study on organizational challenges in the era of the patient-centered medical home. J Immigr Minor Health. 2013;15(5):993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAlearney AS, Murray K, Sieck C, Lin JJ, Bellacera B, Bickell NA. The Challenge of Improving Breast Cancer Care Coordination in Safety-net Hospitals: Barriers, Facilitators, and Opportunities. Med Care. 2016;54(2):147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selby K, Baumgartner C, Levin TR, Doubeni CA, Zauber AG, Schottinger J, et al. Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests: A Systematic Review. Ann Intern Med. 2017;167(8):565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doubeni CA, Weinmann S, Adams K, Kamineni A, Buist DS, Ash AS, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med. 2013;158(5 Pt 1):312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro JA, Bobo JK, Church TR, Rex DK, Chovnick G, Thompson TD, et al. A Comparison of Fecal Immunochemical and High-Sensitivity Guaiac Tests for Colorectal Cancer Screening. Am J Gastroenterol. 2017;112(11):1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.