Abstract
Background:
Positive affect is associated with resiliency and beneficial health outcomes, but little is known about associations between positive affect and health-related quality of life (HRQOL) in Huntington’s disease (HD).
Objective:
This longitudinal study determined the association between positive affect and several HRQOL outcomes in persons with HD. Functional status was examined as a moderator of the association between positive affect and HRQOL.
Methods:
Participants, with premanifest (i.e., genetically at risk but no clinical diagnosis, n = 50) and manifest HD (early-stage n = 171; late-stage n = 101), completed a measure of positive affect and well-being and several HRQOL measures at baseline, 12-, and 24-month follow-ups. UHDRS Functional Assessment scale indicated functional status.
Results:
Positive affect was associated with better HRQOL for persons with premanifest and manifest HD over the 24-month time frame. These associations were moderated by functional status. For persons with higher functional status, positive affect was associated with better HRQOL, including less depression, lower anxiety, less anger, better social role satisfaction, better executive functions, greater upper extremity function, less dyscontrol, and less concern with death and dying. For persons with lower functional status, positive affect was not associated with HRQOL.
Conclusions:
Positive affect predicted better self-reported HRQOL over a 24-month period in persons with premanifest and manifest HD, particularly when participnats had better functional status. Interventions to enhance positive affect in HD may have beneficial effects on HRQOL.
Keywords: Quality of life, positive affect, functional assessment, Huntington’s disease, longitudinal
INTRODUCTION
Positive affect is defined by pleasant feelings, such as happiness, contentment, and interest [1, 2]. Positive affect is associated with resiliency and beneficial health outcomes [3]. Greater positive affect predicts viral suppression in women with HIV [4], lower risk for the common cold [5], decreased stroke incidence in older adults [6], and lower risk of mortality [7, 8]. Frederickson’s broaden-and-build theory of positive emotions suggests that positive affect achieves these beneficial outcomes via two pathways: 1) serving to buffer the harmful effects of stress, and 2) promoting the accumulation and availability of psychosocial resources that foster healthy behaviors [9].
Positive affect might be an area of preserved function in some persons with Huntington’s disease (HD) and could serve as a psychological resource and/or a target for therapeutic intervention. The benefits of positive affect in persons with HD, however, are not known. Greater attention to positive affect is warranted because HD is a progressive, debilitating, and ultimately fatal genetic disorder with onset in midlife [10]. Age of symptom onset is partially determined by genetic factors, but also likely influenced by environmental, emotional, and/or bevhaioral factors [11]. HD is characterized by a triad of psychiatric, motor, and cognitive symptoms that—cumulatively and over time—have profound and detrimental impacts on daily functioning. Indeed, in online support groups, persons with HD and those in their social networks report considerable stress and anxiety [12]. Neuropsychiatric features characterized by depressive symptoms, apathy, and irritability are common at all stages of the HD disease continuum, from premanifest (i.e., genetically at risk but showing no or only mild symptoms) through late stage HD [13, 14].
Positive affect and health outcomes
Positive affect is associated with indicators of good health, as well as adaptive coping with adverse conditions [15, 16]. However, the links between positive affect and health outcomes can be complex. In a prospective study of women with HIV, high positive affect was associated with a greater likelihood of undetectable viral load at follow-up [4]. The association between positive affect and reduced viral load, however, diminished in the context of stress. That is, the benefits of positive affect were limited by the presence of a negative affect state.
In HD, a greater understanding of positive affect and its associations with health-related quality of life (HRQOL) outcomes (i.e., mental, physical and social well-being [17]) could be useful in maximizing HRQOL and quality living. If positive affect, in the context of participants with different disease burden, is associated with better HRQOL, there may be a number of ways to preserve or enhance HRQOL in HD by increasing positive affect. For example, evidence-based interventions to strengthen positive affect (e.g., acceptance-based therapies, meditation, positive affect skill intervention [18-21]) might be useful for persons with HD to promote better HRQOL.
The current study
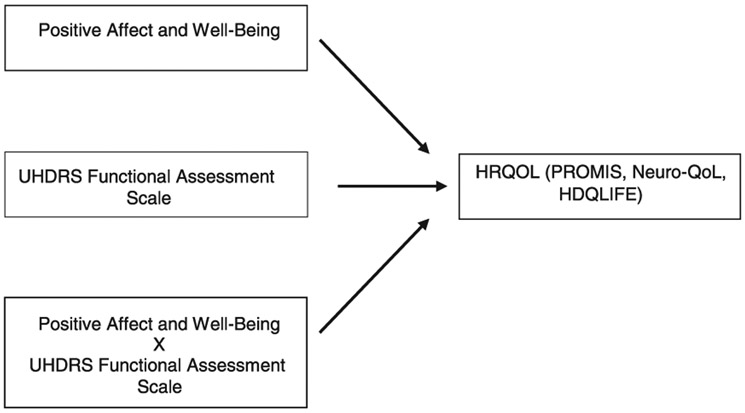
In the current longitudinal study, positive affect and a number of dimensions of HRQOL were measured in persons with HD over a 24-month period. Participants ranged from premanifest to late-stage HD. Disease severity was indicated by functional impairment, which is a good marker of HD severity and is associated with poorer HRQOL in other neurodegenerative diseases [22, 23]. The primary aims were to determine (1) if positive affect was associated with HRQOL in HD [24] and (2) if these associations were moderated by functional status (Fig. 1).
Fig. 1.
Moderation Diagram. Displays the moderation diagram of the primary analyses. Positive Affect and Well-Being is moderated by Functional Assessment; greater impairment weakens the relationship between Positive Affect and HRQOL.
MATERIALS AND METHODS
Participants
Data for the current analyses included a subset of participants from the HDQLIFE study [25]; participants were included in this study if they had a Unified Huntington Disease Rating Scale (UHDRS) Functional Assessment (FA) score. The HDQLIFE study aimed to examine HRQOL across three time points (baseline, 12-, and 24-months). Recruitment included established HD clinics, existing research HD research studies, HD support groups, HD specialized nursing home units, the National Research Roster for Huntington’s Disease, online medical record data capture systems [26], and articles/advertisements in HD-specific newsletters and websites. To be eligible for the study, participants were required to have a positive gene mutation test and/or a clinical diagnosis of HD [25], be at least 18 years of age, and able to read and comprehend English, and be capable of providing written informed consent. Informed consent was obtained from all individual participants included in the study. The study was approved by the Institutional Review Boards at all participating institutions. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Self-reported health-related quality of life measures
Several HRQOL measures from the Patient Reported Outcomes Measurement Information System (PROMIS; [27]) and the Quality of Life in Neurological Disorders (Neuro-QoL; [28]) were administered as part of the larger study protocol [25]. This included the PROMIS measures of Depression, Anxiety and Anger, and the Neuro-QoL measures of Positive Affect and Well-being (PAW), Satisfaction with the Ability to Participate in Social Roles and Activities (Satisfaction with Roles and Activites), Ability to Participate in Social Roles and Activities (Participate in Roles and Activities), Upper Extremity Function, Lower Extremity Function, Stigma, Emotional/Behavioral Dyscontrol, and Applied Cognition-Executive Function. All PROMIS and Neuro-QoL measures are reported on a T-score metric with a mean of 50 (SD = 10) where higher scores indicate more of that domain being measured (i.e., higher Stigma scores indicate more stigma, higher PAW scores mean more positive affect and well-being).
In addition, several HD-specific HRQOL measures were administered from the Huntington Disease Quality of Life (HDQLIFE) measurement system [25, 29, 30]. Specifically, we administered HDQLIFE Chorea, Speech Difficulties, Swallowing Difficulties, Concern with Death and Dying, and Meaning and Purpose. Again, these measures are scored on a T-score metric with a mean of 50 (SD = 10), with higher scores indicating more of that domain being measured (i.e., higher Meaning and Purpose scores indicate better sense of purpose).
Clinician-administered measure of functioning
The UHDRS is a clinician-rated tool used to measure motor functioning, cognition, overall functioning, and independence [31]. For the purposes of analyzing the interaction between PAW and functional status on health-related life quality, the UHDRS FA scale was used to measure functional status. The FA scale is a 25-item clinician rated measure of participant’s ability to perform daily activities (e.g., dressing themselves, operating a motor vehicle, managing finances without help). Higher scores reflect better capabilities.
The UHDRS Motor Scale evaluates multiple aspects of motor abnormality and requires the rater to indicate their overall level of confidence for whether or not the participant has unequivocal signs of HD; participants with a confidence rating of ≥ 99% were required for inclusion in the manifest HD group.
The Total Functional Capacity (TFC), which assesses a participant’s ability to work, manage finances, do chores, live independently, and perform activities of daily living, was used to determine HD stage for those with manifest HD. TFC scores range from 0 to 13, with higher scores indicating better ability. For manifest participants, early-stage was defined as TFC scores of 7–13 and late-stage was defined as TFC scores of 0–6 [32].
Statistical analyses
Analyses for the current study were conducted using SAS 9.4. A one-way analysis of variance (ANOVA) was used to assess whether our three staging groups differed in levels of PAW at baseline. In addition, Pearson correlations were calculated at baseline to determine the association between PAW and HRQOL outcome measures (PROMIS Depression, Anxiety, and Anger; Neuro-QoL Satisfaction with Roles and Activites, Ability to Participate in Roles and Activities, Upper Extremity Function, Lower Extremity Function, Stigma, Emotional/Behavioral Dyscontrol, and Executive Function; HDQLIFE Chorea, Speech Difficulties, Swallowing Difficulties, Concern with Death and Dying, and Meaning and Purpose). Next, fifteen linear mixed models (LMM) with time points nested within each participant were used to determine if FA moderated the association between PAW and the HRQOL outcomes over time. Each LMM was conducted using restricted maximum likelihood (REML) estimation method with a compound symmetry covariance structure and Kenward-Roger degrees of freedom method. Each model contained two main fixed effects, PAW and FA, and an interaction term between them, to examine the significance of the slope change between the two continuous variables. Simple (conditional) slope estimates were tested for significance at varying levels of FA to determine at which point the slope of PAW became insignificant [33]. In addition, each LMM included time as a covariate and controlled for disease stage, gender and race.The maximum likelihood method allows for participants with missing assessments to be utilized to determine means for a single time-point. Since change over time was not the primary focus of the current study, participants were included in the analyses so long as they had complete baseline data. Type III partial eta-squared (ηp2) effect sizes were calculated to estimate the amount of variance accounted for by the predictor variables; effect sizes for ηp2 are classified as small (ηp2 = 0.01), moderate (ηp2 = 0.06), and large (ηp2 = 0.14) [34].
RESULTS
We examined data for 322 participants with HD (n = 50 premanifest, n = 171 early-stage manifest, n = 101 late-stage manifest). For the longitudinal analyses, 322 (100%) participants had data for their baseline visit, 221 (68.6%; n = 30 premanifest, n = 121 early-stage HD, n = 70 late-stage HD) provided 12-month data, and 177 (55.0%; n = 25 premanifest, n = 75 early-stage HD, n = 77 late-stage HD) provided 24-month data. Thus, there were 720 observations available for the LMMs.
Premanifest participants were significantly younger than the two manifest groups, F[2,319] = 16.8, p < 0.0001 (Table 1). Premanifest participants also had more years of education than either of the two manifest groups, F[2,309] = 5.31, p < 0.0001. Overall, the sample majority was female, white, and married. There were significant group differences in race (Fisher’s Exact = 0.0032); the late-stage participants included more African Americans than the other two groups. There were no significant differences between the three HD groups with regard to gender, race, or marital status.
Table 1.
Baseline descriptive statistics for demographic, disease, and HRQOL variables
| Variable | Premanifest-HD (N = 50) |
Early-HD (N = 171) |
Late-HD (N = 101) |
Combined Sample (N = 322) |
|---|---|---|---|---|
| Age at Baseline (Years)* | ||||
| M (SD) | 43.4 (11.3) | 51.6 (12.6) | 55.5 (11.7) | 51.6 (12.7) |
| Gender (%) | ||||
| Female | 54.0 | 52.0 | 58.4 | 54.4 |
| Male | 46.0 | 48.0 | 41.6 | 45.6 |
| Race (%)* | ||||
| White | 96.0 | 95.9 | 92.1 | 94.7 |
| African American | 0.0 | 1.2 | 7.9 | 3.1 |
| Other | 2.0 | 2.9 | 0.0 | 1.9 |
| Unknown | 2.0 | 0.0 | 0.0 | 0.3 |
| Ethnicity (%) | ||||
| Not Hispanic or Latino | 96.0 | 93.0 | 97.0 | 94.7 |
| Hispanic or Latino | 2.0 | 4.1 | 1.0 | 2.8 |
| Not Provided | 2.0 | 2.9 | 2.0 | 2.5 |
| Education (# of years)* | ||||
| M (SD) | 15.7 (2.9) | 14.6 (2.7) | 14.2 (2.5) | 14.7 (2.7) |
| Marital Status (%) | ||||
| Single, Never Married | 10.0 | 16.4 | 10.9 | 13.7 |
| Married | 72.0 | 51.5 | 62.4 | 58.1 |
| Separated/Divorced | 16.0 | 24.6 | 22.8 | 22.7 |
| Widowed | 0.0 | 3.5 | 4.0 | 3.1 |
| Living with Partner | 2.0 | 4.1 | 0.0 | 2.5 |
| CAG Repeats* | ||||
| M (SD) | 41.9 (2.3) | 43.2 (4.0) | 44.8 (7.2) | 43.3 (4.7) |
| UHDRS Functional Assessment* | ||||
| M(SD) | 24.5 (1.1) | 22.0 (2.6) | 11.8 (5.5) | 19.2 (6.3) |
| Neuro-QoL Positive Affect and Well-Being | ||||
| M(SD) | 55.1 (8.7) | 54.9 (8.5) | 54.3 (8.5) | 54.8 (8.5) |
| PROMIS Depression | ||||
| M(SD) | 49.4 (10.0) | 51.3 (10.8) | 51.3 (11.1) | 51.0 (10.8) |
| PROMIS Anxiety | ||||
| M(SD) | 52.8 (9.7) | 53.4 (10.1) | 54.1 (11.4) | 53.5 (10.4) |
| PROMIS Anger | ||||
| M(SD) | 48.3 (12.1) | 48.4 (12.3) | 47.1 (12.8) | 48.0 (12.4) |
| Neuro-QoL Satisfaction with Social Roles and Activities* | ||||
| M(SD) | 50.5 (8.2) | 48.3 (7.9) | 44.2 (8.1) | 47.4 (8.3) |
| Neuro-QoL Ability to Participate in Social Roles and Activities* | ||||
| M(SD) | 50.0 (8.3) | 47.1 (7.9) | 42.8 (8.0) | 46.3 (8.3) |
| Neuro-QoL Executive Function* | ||||
| M(SD) | 44.4 (10.3) | 38.3 (8.9) | 28.6 (7.8) | 36.3 (10.4) |
| Neuro-QoL Stigma* | ||||
| M(SD) | 46.1 (7.7) | 51.7 (8.0) | 53.3 (9.7) | 51.3 (8.8) |
| Neuro-QoL Upper Extremities Function* | ||||
| M(SD) | 49.9 (7.7) | 43.0 (9.0) | 32.4 (7.3) | 40.8 (10.3) |
| Neuro-QoL Lower Extremities Function* | ||||
| M(SD) | 53.6 (8.3) | 46.7 (8.8) | 38.0 (8.1) | 45.1 (10.0) |
| Neuro-QoL Emotional/Behavioral Dyscontrol | ||||
| M(SD) | 46.2 (11.1) | 47.4 (10.4) | 46.9 (11.6) | 47.0 (10.9) |
| HDQLIFE Chorea* | ||||
| M(SD) | 42.9 (6.8) | 52.9 (7.3) | 57.4 (7.3) | 52.8 (8.6) |
| HDQLIFE Speech Difficulties* | ||||
| M(SD) | 45.3 (6.8) | 50.7 (7.6) | 55.2 (7.8) | 51.3 (8.2) |
| HDQLIFE Swallowing Difficulties* | ||||
| M(SD) | 46.1 (7.2) | 51.6 (8.1) | 56 (7.6) | 52.1 (8.5) |
| HDQLIFE Concern w/ Death and Dying | ||||
| M(SD) | 50.0 (9.1) | 50.6 (9.6) | 49.8 (11.0) | 50.3 (10.0) |
| HDQLIFE Meaning and Purpose | ||||
| M(SD) | 49.0 (10.0) | 50.3 (9.7) | 48.7 (8.3) | 49.6 (9.4) |
HD, Huntington’s disease.
Variable differs between the 3 groups (p < 0.05).
Average PAW score was 54.8 (SD = 8.5) at baseline, which is half a standard deviation better than individuals in the normative population. The three HD groups did not significantly differ in PAW, F[2,319] = 0.26, p = 0.76. Pearson correlations indicated that higher PAW was associated with better HRQOL on all measures at baseline (Table 2).
Table 2.
Pearson Correlation (r) between Positive Affect and Well-Being and HRQOL outcomes
| Correlation (r) | |
|---|---|
| PROMIS | |
| Depression | −0.59 |
| Anxiety | −0.48 |
| Anger | −0.45 |
| Neuro-QoL | |
| Satisfaction w/ Social Roles and Activities* | 0.43 |
| Ability to Participate in Social Roles and Activities* | 0.39 |
| Executive Function* | 0.30 |
| Stigma | −0.47 |
| Upper Extremities Function* | 0.15 |
| Lower Extremities Function* | 0.19 |
| Emotional/Behavioral Dyscontrol | −0.45 |
| HDQLIFE | |
| Chorea | −0.22 |
| Speech Difficulties | −0.15 |
| Swallowing Difficulties | −0.16 |
| Concern w/ Death and Dying | −0.45 |
| Meaning and Purpose* | 0.63 |
All p-values<0.01; correlations calculated at baseline.
Higher scores indicate better outcomes.
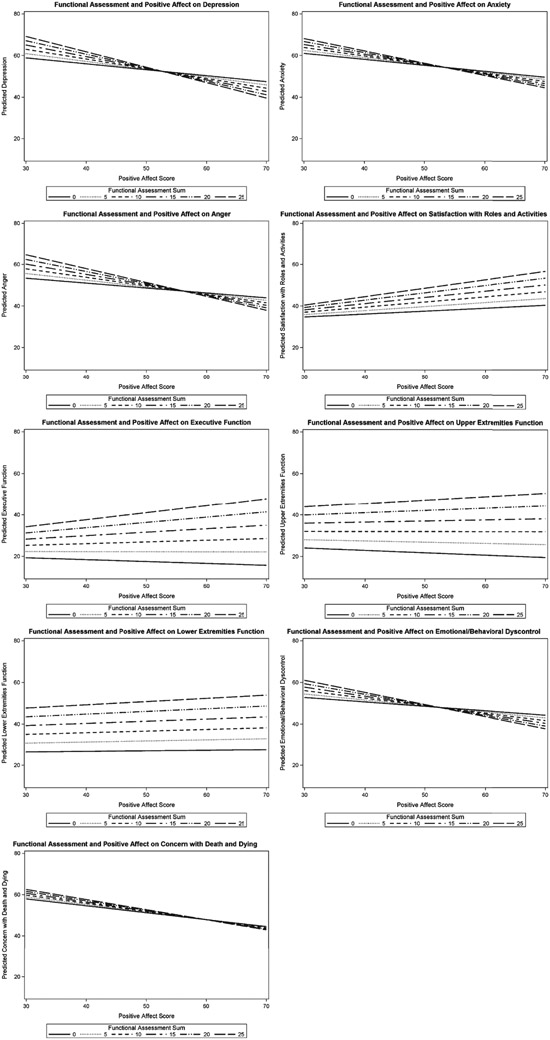
Figure 1 illustrates the path diagram for the moderation analyses. There were significant interactions between FA and PAW for PROMIS Depression (t[532] = −2.99; p = 0.003), PROMIS Anxiety (t[550] = −1.99; p = 0.047), and PROMIS Anger (t[666] = −2.55; p = 0.01) (Table 3). Additionally, FA moderated the effect of PAW on Neuro-QoL Satisfaction with Roles and Activites (t[645] =2.22; p = 0.03), Neuro-QoL Executive Function (t[546] = 2.02; p = 0.04), Neuro-QoL Upper Extremities Functioning (t[671] = 3.05; p = 0.002), Neuro-QoL Lower Extremities Functioning (t[676] = 2.06; p = 0.04), Neuro-QoL Emotional/Behavioral Dyscontrol (t[662] = −2.21; p = 0.03), and HDQLIFE Concern with Death and Dying (t[658] = −2.9; p = 0.01). For each of the significant interactions, the effect size was small. These interactions indicate that for individuals with higher functioning, greater PAW is significantly associated with better HRQOL. However, participants with lower functional status showed a weaker association between PAW and HRQOL (Fig. 2).
Table 3.
Linear regression predicting health related quality of life outcomes
| Functional Assessment | |||||
|---|---|---|---|---|---|
| Coefficient | SE | ηp2 | t-value (df) | p-value | |
| PROMIS | |||||
| Depression | |||||
| Assessment (Change per year) | −0.98 | 0.44 | 0.01 | −2.25 (331) | 0.0254 |
| FA | 1.22 | 0.41 | 0.02 | 3.00 (534) | 0.0029 |
| Positive Affect and Well-being | −0.24 | 0.15 | 0.01 | −1.65 (527) | 0.1001 |
| Interaction (FA * PAW) | −0.02 | 0.01 | 0.02 | −2.99 (533) | 0.0029 |
| Anxiety | |||||
| Assessment (Change per year) | −1.45 | 0.43 | 0.01 | −3.41 (316) | 0.0007 |
| FA | 0.80 | 0.42 | 0.01 | 1.90 (551) | 0.0579 |
| Positive Affect and Well-being | −0.25 | 0.15 | 0.00 | −1.68 (544) | 0.0944 |
| Interaction (FA * PAW) | −0.02 | 0.01 | 0.01 | −1.99 (550) | 0.0472 |
| Anger | |||||
| Assessment (Change per year) | 0.19 | 0.40 | 0.00 | 0.48 (442) | 0.6324 |
| FA | 1.09 | 0.47 | 0.01 | 2.34 (668) | 0.0194 |
| Positive Affect and Well-being | −0.15 | 0.17 | 0.00 | −0.92 (665) | 0.3571 |
| Interaction (FA * PAW) | −0.02 | 0.01 | 0.01 | −2.55 (666) | 0.0109 |
| Neuro-QoL | |||||
| Satisfaction w/ Social Roles and Activities* | |||||
| Assessment (Change per year) | 0.08 | 0.26 | 0.00 | 0.31 (440) | 0.7570 |
| FA | −0.25 | 0.30 | 0.00 | −0.83 (648) | 0.4064 |
| Positive Affect and Well-being | 0.11 | 0.11 | 0.00 | 1.05 (643) | 0.2947 |
| Interaction (FA * PAW) | 0.01 | 0.01 | 0.01 | 2.22 (645) | 0.0268 |
| Ability to Participate in Social Roles and Activities* | |||||
| Assessment (Change per year) | 0.11 | 0.31 | 0.00 | 0.35 (477) | 0.7258 |
| FA | −0.05 | 0.32 | 0.00 | −0.16 (619) | 0.8696 |
| Positive Affect and Well-being | 0.13 | 0.11 | 0.00 | 1.16 (620) | 0.2471 |
| Interaction (FA * PAW) | 0.01 | 0.01 | 0.00 | 1.38 (616) | 0.1687 |
| Executive Function* | |||||
| Assessment (Change per year) | 0.60 | 0.39 | 0.00 | 1.53 (327) | 0.1260 |
| FA | −0.05 | 0.38 | 0.00 | −0.13 (546) | 0.8977 |
| Positive Affect and Well-being | −0.02 | 0.14 | 0.00 | −0.14 (541) | 0.8925 |
| Interaction (FA * PAW) | 0.01 | 0.01 | 0.01 | 2.02 (546) | 0.0440 |
| Stigma | |||||
| Assessment (Change per year) | −0.50 | 0.27 | 0.00 | −1.85 (445) | 0.0653 |
| FA | −0.06 | 0.32 | 0.00 | −0.20 (667) | 0.8399 |
| Positive Affect and Well-being | −0.30 | 0.11 | 0.01 | −2.64 (665) | 0.0086 |
| Interaction (FA * PAW) | −0.004 | 0.01 | 0.00 | −0.64 (666) | 0.5201 |
| Upper Extremities* | |||||
| Assessment (Change per year) | −0.84 | 0.24 | 0.01 | −3.53 (428) | 0.0005 |
| FA | −0.06 | 0.30 | 0.00 | −0.21 (670) | 0.8323 |
| Positive Affect and Well-being | −0.26 | 0.11 | 0.01 | −2.33 (701) | 0.0201 |
| Interaction (FA * PAW) | 0.02 | 0.01 | 0.02 | 3.05 (671) | 0.0023 |
| Lower Extremities* | |||||
| Assessment (Change per year) | −0.35 | 0.25 | 0.00 | −1.39 (438) | 0.1646 |
| FA | 0.12 | 0.31 | 0.00 | 0.38 (675) | 0.7068 |
| Positive Affect and Well-being | −0.10 | 0.11 | 0.01 | −0.90 (677) | 0.3694 |
| Interaction (FA * PAW) | 0.01 | 0.01 | 0.01 | 2.06 (676) | 0.0393 |
| Emotional/Behavioral Dyscontrol | |||||
| Assessment (Change per year) | −0.12 | 0.36 | 0.00 | −0.35 (449) | 0.7293 |
| FA | 0.78 | 0.42 | 0.01 | 1.87 (663) | 0.0623 |
| Positive Affect and Well-being | −0.18 | 0.15 | 0.01 | −1.22 (660) | 0.2243 |
| Interaction (FA * PAW) | −0.02 | 0.01 | 0.01 | −2.21 (662) | 0.0273 |
| HDQLIFE | |||||
| Chorea | |||||
| Assessment (Change per year) | 0.19 | 0.21 | 0.00 | 0.91 (423) | 0.3621 |
| FA | −0.24 | 0.26 | 0.01 | −0.90 (667) | 0.3691 |
| Positive Affect and Well-being | −0.04 | 0.09 | 0.01 | −0.47 (672) | 0.6406 |
| Interaction (FA * PAW) | −0.004 | 0.005 | 0.00 | −0.79 (668) | 0.4288 |
| Speech Difficulties | |||||
| Assessment (Change per year) | −0.06 | 0.24 | 0.00 | −0.25 (446) | 0.8060 |
| FA | −0.44 | 0.30 | 0.00 | −1.50 (672) | 0.1340 |
| Positive Affect and Well-being | −0.07 | 0.11 | 0.00 | −0.70 (674) | 0.4811 |
| Interaction (FA * PAW) | 0.001 | 0.01 | 0.00 | 0.05 (673) | 0.9628 |
| Swallowing Difficulties | |||||
| Assessment (Change per year) | 0.53 | 0.24 | 0.00 | 2.21 (431) | 0.0276 |
| FA | −0.39 | 0.31 | 0.00 | −1.28 (666) | 0.2027 |
| Positive Affect and Well-being | −0.06 | 0.11 | 0.00 | −0.51 (670) | 0.6087 |
| Interaction (FA * PAW) | −0.001 | 0.01 | 0.00 | −0.04 (667) | 0.9707 |
| Concern w/ Death and Dying | |||||
| Assessment (Change per year) | 0.32 | 0.32 | 0.00 | 0.98 (449) | 0.3257 |
| FA | 1.05 | 0.38 | 0.02 | 2.78 (661) | 0.0056 |
| Positive Affect and Well-being | −0.17 | 0.13 | 0.00 | −1.24 (654) | 0.2145 |
| Interaction (FA * PAW) | −0.02 | 0.01 | 0.02 | −2.49 (658) | 0.0129 |
| Meaning and Purpose* | |||||
| Assessment (Change per year) | −0.41 | 0.28 | 0.00 | −1.43 (468) | 0.1524 |
| FA | −0.40 | 0.32 | 0.01 | −1.25 (658) | 0.2113 |
| Positive Affect and Well-being | 0.47 | 0.12 | 0.02 | 4.05 (652) | <.0001 |
| Interaction (FA * PAW) | 0.01 | 0.01 | 0.01 | 1.69 (653) | 0.0922 |
FA, Functional Assessment; PAW, Positive Affect and Well-Being; (df), degrees of freedom; all models control for gender and race.
Higher Scores indicate better outcomes.
Fig. 2.
Interactions between Functional Assessment and Positive Affect on HRQOL. Scores shows the slope of Positive Affect and Well-Being at various levels of functioning. Refer to Table 4 for the value of functioning where the slope of Positive Affect and Well-Being becomes insignificant.
For models with significant interaction effects between PAW and FA, cut-points were identified for FA to determine when PAW no longer exhibited a potentially protective association with HRQOL (i.e., when the slope of PAW was no longer significant, Table 4). For example, when FA is below 2, the association between PAW and depression becomes insignificant; thus, having high PAW is not associated with less depression when functioning drops to 1 or less.
Table 4.
UHDRS values not protected by Positive Affect and Well-Being
| Outcome | Functional Assessment Value where Positive Affect no longer provides any benefit |
|---|---|
| Depression | Below 2 |
| Anxiety | Below 2 |
| Anger | Below 5 |
| Satisfaction with Roles and Activities | Below 5 |
| Executive Function | Below 12 |
| Upper Extremities Function | Below 20 |
| Lower Extremities Function | Below 16 |
| Emotional/Behavioral Dyscontrol | Below 4 |
| Concern with Death and Dying | Below 4 |
Functional Assessment is scored on a scale of 0 to 25 with higher scores indicating better functioning.
DISCUSSION
Positive affect, as defined by pleasant feelings, predicted better self-reported HRQOL over a 24-month period in a large sample of persons with premanifest and manifest HD. The associations between positive affect and HRQOL often depended on participant’s functional status. For persons with higher functional status, positive affect was strongly associated with better HRQOL along a number of dimensions, including less depression, lower anxiety, less anger, better social role satisfaction, better executive functions, greater upper extremity function, less emotional/behavioral dyscontrol, and less concern with death and dying. Our longitudinal data give some confidence that, although the effect sizes were small, positive affect may be predictive of future HRQOL in persons with HD, particularly for persons with HD that have higher level functioning. For persons with lower functional status, positive affect was not associated with HRQOL.
Our results are similar to studies in other populations. For example, as mentioned earlier, in women with HIV, Wilson et al. (2017) found that high positive affect was prospectively associated with greater health outcomes (e.g., undetectable viral load). However, the association between positive affect and reduced viral load diminished in the context of a liability (e.g., high negative affect). That is, the benefits of positive affect were limited by greater negative affect. Likewise, our data suggest that the potentially adaptive links between positive affect and HRQOL in HD could be limited by functional status. There may be limitations on the beneficial mental health effects of positive affect. When persons are faced with a certain intensity of stressor (e.g., more severe functional limitations), positive affect confers diminishing returns and/or is a less potent protective factor. If so, theoretical models about the protective effects of PA should be modified to account for boundary effects of its protective actions. Our data suggest the limitations of PA to be associated with positive outcomes may vary by domain (Table 4).
Positive affect had associations with a broad range of HRQOL outcomes. Positive affect was associated with less depression, anxiety, and anger. These findings are consistent with data suggesting that strong negative affect states are inversely associated with positive affect [35-37]. Indeed, there were inverse links between positive affect and other adverse outcomes, including behavioral dyscontrol and concern with death the dying. Positive affect also was associated with the adaptive outcome of better upper extremity function, which also is consistent with a large corpus of data [38].
Positive affect is conceptualized as (1) a buffer against stress and (2) a means by which psychosocial resources are accrued [39]. Positive affect in persons with HD, operating via one or both of these pathways, may improve or maintain HRQOL over time. The association between positive affect and social role satisfaction is particularly pertinent to Frederickson’s Broaden and Build model of positive affect, confirming important links between positive affect and social role fulfillment and better function. Social partners are a major psychological resource by which the benefits of positive affect are accrued.
Implications
Interventions to enhance positive affect in HD may benefit HRQOL, at least when patients are higher functioning. Acceptance and meditation therapies hold promise to enhance positive emotions in HD and may have beneficial outcomes [4-8, 40]. Indeed, data suggest that persons with HD who are more accepting of their disorder also have greater well-being [41]. Whereas knowledge about non-pharmacologic therapies for persons with HD is limited [42, 43], persons with HD could respond positively to these interventions. Persons with HD have rich emotional experiences in laboratory paradigms that include positive as well as negative emotions [44], and there may be potential to capitalize on and enhance their positive emotions. Further, persons with cognitive impairments associated with other neurologic disorders have successfully engaged in psychotherapy [45]. Our data indicate that there were no significant differences in positive affect and well-being based on HD disease stage (i.e., premanifest, early-stage, late-stage). Thus, positive affect may not necessarily align with disease stage and may be a preserved resource for some persons that can be targeted in therapies.
Limitations
Our sample was primarily female, highly educated, and white, which may limit the generalizability of our findings. Individuals with lower education and of other races may experience different associations between positive affect, functional status, and HRQOL. We did not have cognitive status measures, which likely are an important predictor of life quality ifn persons with HD, and could not address their association with HRQOL. Additionally, the protocol for the study allowed for the participant to answer self-report measures up to two weeks following the functional status measure (i.e., functional assessment). Therefore, correlations between our measures may be less robust when broken down between those who completed self-report measures at the time of their in-person assessment and those who completed the measures afterward, though a change in functional status over two weeks would not be expected.
As noted earlier, for persons with lower functional status, positive affect was not associated with HRQOL. It is possible that the lack of significant association between positive affect and HRQOL was due, in part, to the fact that more severely impaired persons with HD provide self-report data of lesser reliability than persons who are higher functioning [46]. Thus, research that relies less exclusively on self-report will be an important step in this line of work.
Future directions
Future research should include a broader assessment of positive and negative affect in persons with HD, to provide a more comprehensive assessment of current affective state. Methods should capitalize on multiple assessments of affect and emotion that include self-report, observational, and physiological data. Further research on how affect predicts coping with functional status in HD could lead to preventative and therapeutic interventions to enhance HRQOL. Essential to this effort would be identification of the mechanisms that drive the associations between positive affect and HRQOL in higher functioning persons with HD, how negative affect may interfere with this association, and how this protective mechanism may fail in individuals with lower functioning.
ACKNOWLEDGMENTS
We thank the University of Iowa, the Investigators and Coordinators of this study, the study participants, the National Research Roster for Huntington Disease Patients and Families, the Huntington Study Group, and the Huntington’s Disease Society of America. We acknowledge the assistance of Jeffrey D. Long, Hans J. Johnson, Jeremy H. Bockholt, and Roland Zschiegner. We also acknowledge Roger Albin, Kelvin Chou, and Henry Paulsen for the assistance with participant recruitment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. HDQLIFE Site Investigators and Coordinators: Noelle Carlozzi, Praveen Dayalu, Stephen Schilling, Amy Austin, Matthew Canter, Siera Goodnight, Jennifer Miner, Nicholas Migliore (University of Michigan, Ann Arbor, MI); Jane Paulsen, Nancy Downing, Isabella DeSoriano, Courtney Shadrick, Amanda Miller (University of Iowa, Iowa City, IA); Kimberly Quaid, Melissa Wesson (Indiana University, Indianapolis, IN); Christopher Ross, Gregory Churchill, Mary Jane Ong (Johns Hopkins University, Baltimore, MD); Susan Perlman, Brian Clemente, Aaron Fisher, Gloria Obialisi, Michael Rosco (University of California Los Angeles, Los Angeles, CA); Michael McCormack, Humberto Marin, Allison Dicke, Judy Rokeach (Rutgers University, Piscataway, NJ); Joel Perlmutter, Stacey Barton, Shineeka Smith (Washington University in St. Louis, St. Louis, MO); Martha Nance, Pat Ede (Struthers Parkinson’s Center); Stephen Rao, Anwar Ahmed, Michael Lengen, Lyla Mourany, Christine Reece, (Cleveland Clinic Foundation, Cleveland, OH); Michael Geschwind, Joseph Winer (University of California – San Francisco, San Francisco, CA), David Cella, Richard Gershon, Elizabeth Hahn, Jin-Shei Lai (Northwestern University, Chicago, IL).
Work on this manuscript was supported by the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (R01NS077946) and the National Center for Advancing Translational Sciences (UL1TR000433). In addition, a portion of this study sample was collected in conjunction with the Predict-HD study. The Predict-HD study was supported by the NIH, National Institute of Neurological Disorders and Stroke (R01NS040068),Center for Inherited Disease Research (provided supported for sample phenotyping), and the CHDI Foundation (award to the University of Iowa).
Footnotes
CONFLICT OF INTEREST
Drs. Ready, Boileau, Barton, Cella, and Fritz have no conflicts of interest to report. J.-S. Lai currently has research grants from the NIH; she declares no conflicts of interest. M.K. McCormack, M.K. currently has grants from the NJ Department of Health; he has no conflicts of interest to report. J.S. Paulsen currently has research grants funded by NIH and CHDI; she consults for CHDI, Stryker, and Wave Life Sciences. Dr. Paulsen has no conflicts of interest to report. N.E. Carlozzi is supported by grant funding from the NIH, Neilsen Foundation, Department of Defense, CHDI, and Goldfinch, LLC, as well as a contract from the Centers for Medicare & Medicaide Services (CMMS); she has served as a measurement consultant for Teva Pharmaceuticals. Dr. Carlozzi has no conflicts of interest to report.
REFERENCES
- [1].Isen AM. Positive affect In: Dalgleish T, Power MJ, Dalgleish T, Power MJ, editors. Handbook of cognition and emotion. New York, NY: John Wiley & Sons Ltd.; 1999. p. 521–39. [Google Scholar]
- [2].Watson D, Clark LA. The PANAS-X: Manual For The Positive And Negative Affect Schedule - Expanded Form. Iowa City, IA: The University Of Iowa; 1999. [Google Scholar]
- [3].Frederickson B The role of positive emotions in positive psychology. The Broaden-And-Build Theory of positive emotions. Am Psychol. 2001;56:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wilson T, Weedon J, Golub E, Young M, Cohen J, Cohen M, et al. Positive affect and its association with viral control among women with HIV infection. Health Psychol. 2017;36(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cohen SA, Doyle W, Turner R, Alper C, Skoner DP. Emotional style and susceptibility to the common cold. Psychosom Med. 2003;65:652–7. [DOI] [PubMed] [Google Scholar]
- [6].Oster G, Markides KS, Peek M, Goodwin J. The association between emotional wel-being and the incidence of stroke in older adults. Psychosom Med. 2001;63:210–5. [DOI] [PubMed] [Google Scholar]
- [7].Moskowitz J Positive affect predicts lower risk of AIDS mortality. Psychosom Med. 2003;65:620–6. [DOI] [PubMed] [Google Scholar]
- [8].Steptoe A, Wardle J. Positive affect measured using ecological momentary assessment and survival in older men and women. Proc Natl Acad Sci U S A. 2011;108:18244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Frederickson BL. Positive emotions broaden and build In: Devine P, Plant A, Editors. Advances in experimental social psychology, Vol 47 Burlington, VT: Academic Press; 2013. p. 1–53. [Google Scholar]
- [10].Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee J, Ramos EM, Lee J, Gillis T, Mysore JS, Hayden MR, et al. CAG repeat expansion in Huntington disease determines age at onset in a fully dominant fashion. Neurology. 2012;78:690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coulson NS, Buchanan H, Aubeeluck A. Social support in cyberspace: a content analysis of communication within a Huntington’s disease online support group. Patient Educ Couns. 2007;68(2):173–8. [DOI] [PubMed] [Google Scholar]
- [13].Martinez-Horta S, Perez-Perez J, Van Duijn E, Fernandez-Bobadilla R, Carceller M, Pagonabarraga J, et al. Neuropsychiatric symptoms are very common in premanifest and early stage Huntington’s disease. Parkinsonism Relat Disord. 2016;25:58–64. [DOI] [PubMed] [Google Scholar]
- [14].Paulsen JS, Ready RE, Hamilton JM, Mega MS, Cummings JL. Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry. 2001. September;71(3):310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Greenglass ER, Fiskenbaum L. Proactive coping, positive affect, and well-being: testing for meditation using path analysis. Eur Psychol. 2009;14(1):29–39. [Google Scholar]
- [16].Park SH, Sonty N. Positive affect mediates the relationship between pain-related coping efficacy and interference in social functioning. J Pain. 2010;11(12):1267–73. [DOI] [PubMed] [Google Scholar]
- [17].Cella DF. Measuring quality of life in palliative care. Semin Oncol. 1995. 04;22(2 Suppl 3):73–81. [PubMed] [Google Scholar]
- [18].Sears S, Kraus S. I think therefore i om: cognitive distortions and coping style as mediators for the effects of mindfulness meditation on anxiety, positive and negative affect, and hope. J Clin Psychol. 2009;65(6):561–73. [DOI] [PubMed] [Google Scholar]
- [19].Frederickson B, Cohn M, Coffey K, Pek J, Finkel S. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. J Pers Soc Psychol. 2008;95(5):1045–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheung EO, Cohn MA, Dunn LB, Melisko ME, Morgan S, Penedo FJ, et al. A randomized pilot trial of a positive affect skill intervention (lessons in linking affect and coping) for women with metastatic breast cancer. Psychooncology. 2017;26(12):2101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moskowitz JT. Coping interventions and the regulation of positive affect In: Folkman S, Editor. The Oxford Handbook Of Stress, Health, And Coping. Oxford; New York: Oxford University Press; 2011. [Google Scholar]
- [22].Giovannetti AM, Schiavolin S, Brenna G, Brambilla L, Confalonieri P, Cortese F, et al. Cognitive function alone is a poor predictor of health-related quality of life in employed patiest with MS: results from a cross-sectional study. Clin Neuropsychol. 2016;30(2):201–15. [DOI] [PubMed] [Google Scholar]
- [23].Logsdon RG, Gibbons LE, Mccurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. J Mental Health Aging. 1999;5(1):21–32. [Google Scholar]
- [24].Carlozzi NE, Tulsky DS. Identification Of health-related quality of life (HRQOL) issues relevant to individuals with Huntington disease. J Health Psychol. 2013;18:212–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Carlozzi NE, Schilling SG, Lai JS, Paulsen JS, Hahn EA, Perlmutter JS, et al. HDQLIFE: development and assessment of health-related quality of life in Huntington disease (HD). Qual Life Res. 2016. August 13;25(10):2441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hanauer DA, Mei Q, Law J, Khanna R, Zheng K. Supporting information retrieval from electronic health records: a report of University Of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). J Biomed Inform. 2015. June;55:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested in its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cella D, Nowinski C, Peterman A, Victorson D, Miller D, Lai J-S, et al. The Neurology Quality Of Life Measurement (Neuro-QOL) Initiative. Arch Phys Med Rehabil. 2011. October;92(10 Suppl):S28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Carlozzi NE, Downing NR, Mccormack MK, Schilling SG, Perlmutter JS, Hahn EA, et al. New measures to capture end of life concerns in Huntington disease: meaning and purpose and concern with death and dying from HDQLIFE (a patient-reported outcomes measurement system). Qual Life Res. 25(10):2403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carlozzi NE, Schilling SG, Lai JS, Perlmutter JS, Nance MA, Waljee JF, et al. HDQLIFE: the development of two new computer adaptive tests for use in Huntington disease, speech difficulties, and swallowing difficulties. Qual Life Res. 25:2417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Unified Huntington’s Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 1996;11:136–42. [DOI] [PubMed] [Google Scholar]
- [32].Shoulson I, Kurlan R, Rubin A, Goldblatt D, Behr J, Miller C, et al. Assessment of functional capacity in neurodegenerative movement disorders: Huntington’s disease as a prototype, quantification of neurologic deficit. In: Munsat T, Editor. Stoneham, MA: Butterworths; 1989. p. 271–83. [Google Scholar]
- [33].Aiken LS, West SG, Reno RR. Multiple regression: testing and interpreting interactions. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- [34].Cohen J Statistical power analysis for the behavioral sciences (2nd Edition). Hillsdale, NJ: L. Erlbaum; 1988. [Google Scholar]
- [35].Saboonchi F, Lundh L-G. Perfectionism, anger, somatic health, and positive affect. Pers Individ Dif. 2003;35:1585–99. [Google Scholar]
- [36].Cohen JN, Dryman MT, Morrison AS, Gilbert KE, Heimberg RG, Gruber J. Positive and negative affect as links between social anxiety and depression: predicting concurrent and prospective mood symptoms in unipolar and bipolar mood disorders. Behav Ther. 2017;48:820–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Eisner LR, Johnson SL, Carver CS. Positive affect regulation in anxiety disorders. J Anxiety Disord. 2009;23:645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cameron DS, Bertenshaw EJ, Sheeran P. The impact of positive affect on health cognitions and behaviours: a meta-analysis of the experimental evidence. Health Psychol Rev 2015;9:345–65. [DOI] [PubMed] [Google Scholar]
- [39].Frederickson B Positive emotions broaden and build. Adv Exp Soc Psychol. 2013a;47:1–53. [Google Scholar]
- [40].Frederickson B The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol. 2001;56:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Helder DI, Kaptein AA, Van Kempen GM, Weinman J, Van Houwelingen JC, Roos R. Living with Huntington’s disease: illness perceptions, coping mechanisms, and patients’ well-being. J Health Psychol. 2002;7(Part 4):449–32. [DOI] [PubMed] [Google Scholar]
- [42].Silver A Cognitive-behavioural therapy with a Huntington’s gene positive patient. Patient Educ Couns. 2003; 49(2):133–8. [DOI] [PubMed] [Google Scholar]
- [43].Van Bruggen-Rufi M, Vink A, Achterberg W, Roos R. Music therapy in Huntington’s disease: a protocol for a multi-centetr randomized controlled trial. BMC Psychol. 2016;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ille R, Holl A, Kapfhammer H, Reisinger K, Schafer A, Schienle A. Emotion recognition and experience in Huntington’s disease: is there a differential impairment? Psychiatry Res. 2011;188(3):377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gottberg K, Chruzander C, Backenroth G, Johansson S, Ahlstrom G, Ytterberg C. Individual face-to-face cognitive behavioural therapy in multiple sclerosis: a qualitative study. J Clin Psychol. 2016;72(7):651–62. [DOI] [PubMed] [Google Scholar]
- [46].Carlozzi NE, Schilling S, Kratz AL, Paulsen JS, Frank S, Stout JC. Understanding patient-reported outcome measures in Huntington disease: at what point is cognitive impairment related to poor measurement reliability? Qual Life Res. 2018;27(10):2541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]