Abstract
Sleep is a universal phenomenon occurring in all species studied thus far. Sleep loss results in adverse physiological effects at both the organismal and cellular levels suggesting an adaptive role for sleep in the maintenance of overall health. This review examines the bidirectional relationship between sleep and cellular stress. Cellular stress in this review refers to a shift in cellular homeostasis in response to an external stressor. Studies that illustrate the fact that sleep loss induces cellular stress and those that provide evidence that cellular stress in turn promotes sleep will be discussed.
Keywords: Unfolded protein response, ER stress, inflammation, immune, DNA repair, NFκB, Epidermal Growth Factor
Introduction
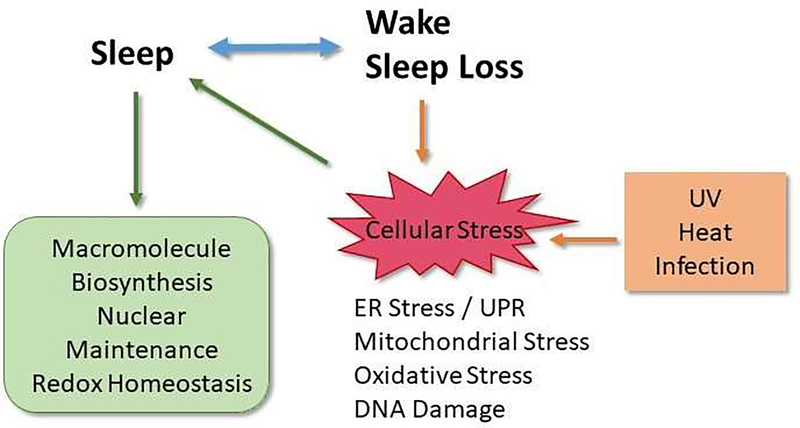
While sleep is a ubiquitous physiological process that is universal and a crucial aspect of overall health, the exact functions of sleep remain elusive. However, it is known that sleep loss or disruption results in adverse effects at both the organismal and cellular level suggesting an adaptive role for sleep in maintaining overall organismal and cellular health. Sleep loss or disruption has been reported to induce cellular stress [1, 2]. The term “cellular stress” broadly encompasses a wide range of molecular changes that cells undergo in response to environmental stressors, including extremes of temperature, exposure to ultraviolet (UV) radiation, toxins, infection, and mechanical damage. The term ‘stress’ is broadly used and often refers to psychological stress and may include stress due to activation of the hypothalamic-pituitary-adrenal axis and increased cortisol. This review will focus solely on those pathways activated in cellular compartments: the endoplasmic reticulum (ER), mitochondria, nucleus and cytoplasm in response to internal or external stimuli, and how these pathways may impact sleep under a number of circumstances, including acute sleep loss in addition to the environmental stressors mentioned above. As discussed below, numerous studies have shown that acute stress often leads to a recovery period characterized by an increase in sleep. Thus, induction of cellular stress activates pathways that facilitate induction of sleep as an adaptive response (Figure 1)
Figure 1:
Schematic illustrating the relationship between sleep and cellular stress responses
Sleep disruption, ER Stress and the Unfolded Protein Response (UPR)
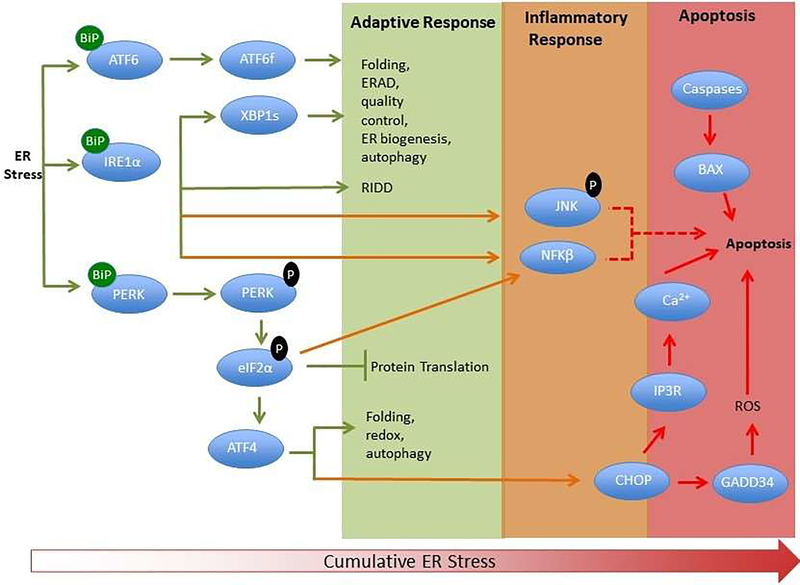
Several lines of evidence indicate that sleep is a period of intense macromolecular biosynthetic activity with increases in protein synthesis [3–5], gene expression within the cholesterol synthesis pathway, and heme production [3]. Thus, behavioral immobility and reduced sensory input during sleep may favor behavioral-state-dependent processes important for the maintenance of cellular integrity in neurons. Consistent with that idea these same processes are downregulated with prolonged waking or sleep deprivation [3]. Critically all of these biosynthetic processes occur largely in the ER. Prolonged waking or sleep loss/ sleep disruption is associated with an increase in the activity of homeostatic pathways that are involved in curtailing cellular stress, including ER and mitochondrial stress. ER stress leads to the induction of the unfolded protein response (UPR) which is a coordinated cascade of several pathways [6, 7] designed to restore protein homeostasis (Figure 2).
Figure 2:
Simplified scheme showing the adaptive, inflammatory and apoptotic phases of the UPR. The three ER stress sensors (PERK, IRE1 and ATF6) are activated upon dissociation from BiP initiating signaling events that increase protein-folding capacity and reduce protein load on the ER. Phosphorylation of eukaryotic Initiation Factor 2α (eIF2α) by PERK inhibits protein translation, while IRE1 activation leads to splicing of xbp1 and chaperone production as well as regulated IRE1-dependent decay (RIDD). Both ATF6 and IRE1 contribute to ER associated degradation (ERAD) of misfolded proteins.
These transcriptional and translational outputs tend to re-establish protein-folding homeostasis in the ER and promote cell survival. JNK activation downstream of IRE1 contributes to inflammatory signaling. Activated IRE1 acting on downstream factors activates JNK and caspases. ATF4 dependent transcription in mammals leads to increases in C/ebp homologous binding protein (CHOP), a pro-apoptotic transcription factor. CHOP and JNK also promote the translocation of Bax (BCl-2 associated X protein) to the mitochondria where it facilitates the release of cytochrome c required for caspase activation. ER specific caspases are thought to directly induce cell death. Translation attenuation leads to NFκB entry into the nucleus and transcription of inflammatory genes. Calcium release from the ER, exacerbated protein synthesis, and ROS production influences the induction of apoptosis.
The UPR plays a fundamental role in the maintenance of cellular homeostasis and thus is central to normal physiology. Specifically, it is an adaptive response that restores protein homeostasis through the activation of transmembrane factors, Inositol Requiring Element 1 (IRE1), Protein Kinase R Endoplasmic Reticulum Kinase (PERK), and Activating Factor 6 (ATF6), which act to initiate misfolded protein degradation, inhibit the production of proteins through translational inhibition, and increase protein chaperone [8] and membrane phospholipid syntheses [9, 10]. In normal un-stressed conditions, the major ER chaperone and key regulator of the UPR, Binding Immunoglobulin Protein/Glucose Regulated Protein 78 (BiP/GRP78) is bound to IRE1, PERK and ATF6 to maintain an inactive state. In response to a stressful stimulus, release of BiP/GRP78 to refold misfolded proteins activates these sensors initiating the UPR. Chronic ER stress and prolonged activation of the UPR activates inflammatory signaling through Jun N-terminal kinase (JNK) and Nuclear Factor κB (NFκB) [11, 12]. Unresolved ER stress results in apoptosis through calcium release from the ER and uptake by the mitochondria and activation of caspases ([11, 13];Figure 2). Several lines of evidence indicate that sleep loss activates the UPR, indicating that cellular stress is an inherent part of sleep loss. Early studies showed that BiP/GRP78 is upregulated in the brains of mice [2, 3, 14], rats [1], birds [15] and Drosophila melanogaster fruit flies [16, 17] in response to prolonged wakefulness, indicating a highly conserved response mechanism. Activation of both the PERK and IRE1 pathways with sleep loss has been described in mouse [2], rat [1], and Drosophila brains [18, 19]. Other UPR-specific transcripts upregulated by sleep loss include DNA-J, a co-chaperone of BiP, X-Box Binding Protein-1 (XBP-1), a transcription factor downstream of IRE1 responsible for the transcription of chaperones among other proteins, calreticulin, caspase-9, ATF4 and ATF6 [3]. Interestingly, PERK and IRE1 are also activated in socially isolated animals as these animals exhibit reduced sleep and more wakefulness [19]. Increasing sleep in socially isolated flies using a genetic approach reduces ER stress. Conversely, reducing sleep in socially enriched animals induces ER stress, indicated by activated PERK and IRE1 pathways. [19]. These findings clearly illustrate that cellular stress is sleep state-dependent.
Sleep and Cellular Stress, a Bidirectional Interaction
While it is evident that sleep loss or disruption leads to ER stress, the relationship between sleep and ER stress is bidirectional. Relieving ER stress has been shown to result in improved sleep in aged animals [18]. Specifically, the use of chemical chaperones to reduce ER stress and supplement low BiP/GRP78 expression in aged Drosophila was successful in consolidating fragmented sleep. Sleep disturbances and disruption are features of many neurodegenerative diseases and often precede the motor or cognitive deficits observed in Parkinson’s and Alzheimer’s disease respectively [20, 21], and a growing body of work has shown that ER stress may contribute to sleep/wake disruption [18, 22] and disease progression [23, 24]. For example, ER lipid defects in neuropeptidergic neurons contribute to circadian rhythm and sleep pattern disruptions in Drosophila parkin and pink1 models of Parkinson’s disease [25]. Specifically, excess ER-mitochondrial contacts caused abnormal lipid trafficking that depleted phosphatidylserine from the ER disrupting the production of neuropeptide-containing vesicles. Feeding parkin and pink1 mutants phosphatidylserine rescued neuropeptidergic vesicle production and acutely restored normal sleep patterns [25]. These studies illustrate that sleep quality is dependent on protein and lipid homeostasis. Recent work has indicated a reciprocal relationship between the circadian clock and ER stress [26–28]. These findings suggest that clock function may also contribute to sleep quality through cellular stress-dependent mechanisms.
Sleep Loss, DNA Damage, and Nuclear Maintenance
There is growing evidence that sleep plays a role in nuclear maintenance. A variety of nuclear processes, including genome stability, transcription, DNA repair, chromosome segregation, and condensation constitute nuclear maintenance. Prolonged wakefulness has been associated with DNA double-strand breaks (DSBs) in mice and fruit flies. Interestingly, DSBs caused by sleep deprivation or gamma irradiation were reversed or repaired in animals that showed recovery sleep [29]. The causes of DSBs that occur with prolonged wakefulness are diverse and include reactive oxygen species (ROS; see below), neuronal activation, and enzyme activity.
Exposure to UV radiation, which is known to lead to DNA damage, increased sleep in mice [30] and Caenorhabditis elegans [31]. The mice became sleepy during the UV exposure, but only when this exposure was applied during the dark phase of a light: dark cycle, when the mice are normally active. In nematodes, the effect on sleep was dose-dependent and was surprisingly independent of ER stress mechanisms, suggesting a distinct cellular stress response that involved DNA repair.
More recently, it has been demonstrated in zebrafish that chromosome dynamics are affected by behavioral state [32]. Chromosome dynamics refers to chromosome movements and structural genomic arrangements that underlie vital cellular processes, including epigenetics, genomic stability, transcription, cell cycle, and DNA replication and repair [32, 33] Time-lapse imaging of telomere markers in zebrafish showed that chromosome dynamics increased by approximately two-fold during nighttime sleep in several brain regions [32]. In contrast, sleep deprivation decreased chromosome dynamics while recovery sleep restored the increase in chromosome dynamics [32].
Oxidative Stress and Sleep Homeostasis
Oxidative stress occurs when the generation of reactive oxygen species (ROS) by various cellular processes exceeds its antioxidant capacity. The source of ROS may derive from mitochondrial respiration, ER stress, or through enzymatic synthesis as in the case of nitric oxide (NO; reviewed in [34]). Early studies showed that NO and various NO synthase isoforms fluctuated with spontaneous sleep and wakefulness, which may vary depending on the brain structure measured [35, 36]. Other investigators found that NO production increased with sleep deprivation and was required in basal forebrain for promoting recovery sleep [37, 38]. However, another group was unable to detect an induction of ROS, lipid peroxidation, or any sign of oxidative stress in brains or peripheral tissues from sleep deprived rats [39]. This latter study indicates that oxidative stress or the production of ROS by prolonged wakefulness is site-specific and cannot be detected across the brain as a whole. In support of this hypothesis, more recent work has shown ROS-dependent neuronal damage in the locus coeruleus in response to sleep deprivation [40].
Recent studies in Drosophila have also implicated ROS as a contributing factor to sleep homeostasis, which is defined as a balance between waking, sleep, and sleep need [41, 42]. In particular, Hill et al [43] found that numerous short-sleeping mutants were susceptible to oxidative stress. Increasing sleep through genetic or pharmacological methods reduced susceptibility to oxidative stress. Predictably, pan-neuronal over-expression of the antioxidant enzymes, either superoxide dismutase or catalase, suppressed daily sleep in flies. Recent work showed that a specific underlying mechanism for this effect involved the Kvβ subunit (hyperkinetic; Hk) of the potassium ion channel, shaker [44]. Earlier work showed that daily sleep was reduced in both shaker [45] and Hk mutants [46]. The recent work by Kempf and colleagues demonstrated that Hk is a redox sensor that increases activity in response to ROS production. Specifically, it binds to nicotinamide adenine dinucleotide phosphate (NADPH), and converts to an NADP+ form when production of ROS is elevated. The presence of NADP+ is associated with a slowing of inactivation of IA, thereby maintaining activity of sleep-promoting neurons that project to the dorsal fan-shaped body [47]. Together, these findings support the idea that mitochondrial metabolism contributes to, and may underlie, a sleep homeostatic mechanism.
Sustained Cellular Stress, Inflammatory Cascades and the Immune Response
The UPR is an adaptive response but during persistent ER stress, as is expected to occur with chronic sleep disruption/disease, the adaptive UPR wanes, and a secondary inflammatory process to promote cell survival is induced [48, 49]. An example of this process is immune challenge, such as bacterial infection, which produces a massive demand on protein synthetic pathways that are necessary for fighting the infection. As an infection persists, the unresolved cellular stress leads to a prolonged inflammatory process. These cellular responses correlate with observations that infection produces an acute sleep response in rabbits [50], flies [51], and humans [52, 53], which is consistent with findings discussed above that activated components of the UPR promote sleep. Likewise, later stages of infection show increased fragmented sleep and reduced sleep quality [53, 54], and is also consistent with the notion that dysregulated ER stress may underlie sleep disturbance.
The connection between sleep and the immune response has been investigated for over forty years and has been reviewed in detail elsewhere [55–57]. It is now known that components of the innate immune system affect sleep. For example, proinflammatory cytokines such as TNFα (tumor necrosis factor α) and IL-1 (interleukin- 1) promote sleep, and interestingly, prolonged inflammation and ensuing sleep disturbance are also characterized by high expression of the same cytokines and other immune factors [53, 58]. While effects of immune components on sleep have been characterized to some degree, the function of sleep in the immune response is not as well understood. Whether sleep promotes survival of infection has been addressed to a limited extent in mammals [59]. In flies, increasing sleep through a genetic approach increased survival of infection [60]. Mechanically sleep depriving flies before or after inoculation also produced a surprising increase in survival of infection, but this correlated with a strong recovery sleep after the deprivation period [61]. NFκB transcription factors, Relish and DIF (Dorsal Immunity Factor), both contribute to post-infection recovery sleep and survival of infection in flies [61]. Moreover, Relish is necessary for post-infection sleep: null mutants do not show post-infection sleep and rapidly succumb to infection [51]. The role of NFκB transcription factors in mammalian sleep has been addressed to a limited extent, and to our knowledge, only one study evaluated sleep in mice lacking one of the NFκB genes, p50 [62]. Similar to Relish mutants, p50 mutant animals did not show an increase in sleep normally induced by LPS. NFκB transcription factors are central to innate immunity in mammals and flies, and target cytokines as well as antimicrobial peptides. Recently, a forward genetic screen for sleep promoting molecules identified nemuri, a novel brain-expressed antimicrobial peptide, that not only strongly promoted sleep but also survival of infection [63]. This latter finding indicates that nemuri, and possibly other antimicrobial peptides, is an important molecular link between sleep and immune function. Thus, more sleep is associated with better survival of infection, but the mechanism by which this occurs remains poorly understood and will be an important topic for future investigation.
Secreted factors induced by Cellular Stress
Our discussion has so far covered intracellular events in response to stressful stimuli, including sleep deprivation, UV radiation, and bacterial infection. UPR and DNA repair mechanisms are activated by these processes and subsequently promote or facilitate sleep. However, while these cellular events are ubiquitous across cell types, sleep and wakefulness in invertebrate and vertebrate species is controlled by coordinated interactions among specific brain structures [64, 65]. In most cases, distinct neuronal circuits respond to peripheral signals as a means for the whole animal to engage in an adaptive behavioral response to stress. Thus, questions that remain are what cell types are responsive to these stimuli, and what secretable factor do they produce to control sleep as an adaptive behavior? As discussed in the previous section, nemuri is one such example [63].
The nematode C. elegans increases quiescent behavior in response to multiple types of stress including bacterial toxins, osmotic shock, heat shock [66], and UV radiation as mentioned above [31]. In response to stress, the ALA neuron is activated by the epidermal growth factor (EGF; LET-23 in worms) receptor, and subsequently releases Flp-13 peptides to promote quiescence [67, 68]. Although EGFR signaling promotes daily sleep in flies [69], its role in heat or other stress-induced sleep has not been investigated. However, it is interesting to note that the ligand for EGFR in flies, spitz (spi), is processed and activated by the rhomboid (rho) serine protease, and negatively regulated by the pseudoprotease, iRhom. In Drosophila, iRhom is an endoplasmic reticulum protein that is expressed predominantly in neurons. It counteracts rhomboid activity by targeting ligands for proteasomal degradation, and thereby reduces EGFR signaling. Thus knockdown of rho reduces processing of the SPI peptide and decreases daily sleep in flies [69]. Conversely, iRhom null mutants showed increased sleep relative to controls, and this phenotype was rescued by RNAi knockdown spi or EGFR specifically in neurons [70]. This finding indicates that iRhom is part of the ER quality control machinery and is important for maintaining wakefulness by suppressing EGFR-dependent signaling.
Flp-13 is similar to the Drosophila Phe-Met-Arg-Phe-amide (FMRFa) and vertebrate neuropeptide VF (NPVF) peptides. In flies, mutants of the FMRFa peptide and its receptor, FR, showed decreases in both heat-stress and infection-induced sleep [71]. Although the relationship between EGF and FMRFa signaling in flies has not yet been established, NPVF was also shown to mediate EGF signals to promote sleep in zebrafish [72]. Genetic analysis of a human cohort also showed that variants of EGF alleles associated with effects on sleep [72]. Together these findings indicate that EGF-FMRF/NPVF signaling derives from the ER quality control machinery and represent an important mechanism of sleep that is shared across species from worms to humans.
Concluding Remarks
Sleep is an important part of a recovery process from acute stress or illness. A growing body of work has demonstrated that enhancing sleep promotes recovery and survival, and that an adaptive cellular response underlies this process. EGF-dependent signaling is clearly an important sleep promoting factor that may derive from ER stress, but the cell types involved have not been well characterized for some species. Moreover, other secreted factors that are direct targets of the UPR are also expected to contribute to daily and stress-induced sleep, possibly through distinct brain circuitry.
Acknowledgements
We would like to thank Jennifer Hafycz for assistance with figures.
Funding Support : NIGMS (R01GM123783) to Naidoo and Williams
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.*.Cirelli C, Gutierrez CM, and Tononi G, Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron, 2004. 41(1): p. 35–43. [DOI] [PubMed] [Google Scholar]
- 2.Naidoo N, et al. , Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem, 2005. 92(5): p. 1150–7. [DOI] [PubMed] [Google Scholar]
- 3.**.Mackiewicz M, et al. , Macromolecule biosynthesis: a key function of sleep. Physiol Genomics, 2007. 31(3): p. 441–57. [DOI] [PubMed] [Google Scholar]
- 4.Ramm P and Smith CT, Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiology and Behavior, 1990. 48(5): p. 749–53. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi H, et al. , Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. European Journal of Neuroscience, 1997. 9(2): p. 271–9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang K and Kaufman RJ, From endoplasmic-reticulum stress to the inflammatory response. Nature, 2008. 454(7203): p. 455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naidoo N, Cellular stress/the unfolded protein response: relevance to sleep and sleep disorders. Sleep Med Rev, 2009. 13(3): p. 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.*.Brown MK and Naidoo N, The endoplasmic reticulum stress response in aging and age-related diseases. Front Physiol, 2012. 3: p. 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sriburi R, et al. , Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem, 2007. 282(10): p. 7024–34. [DOI] [PubMed] [Google Scholar]
- 10.Fagone P and Jackowski S, Membrane phospholipid synthesis and endoplasmic reticulum function. Journal of Lipid Research, 2009. 50 Suppl: p. S311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hetz C and Papa FR, The Unfolded Protein Response and Cell Fate Control. Molecular Cell, 2018. 69(2): p. 169–181. [DOI] [PubMed] [Google Scholar]
- 12.Urano F, et al. , Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science, 2000. 287(5453): p. 664–6. [DOI] [PubMed] [Google Scholar]
- 13.Szegezdi E, et al. , Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Reports, 2006. 7(9): p. 880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.**.Naidoo N, et al. , Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. Journal of Neuroscience, 2008. 28(26): p. 6539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones S, et al. , Molecular correlates of sleep and wakefulness in the brain of the white-crowned sparrow. J Neurochem, 2008. 105(1): p. 46–62. [DOI] [PubMed] [Google Scholar]
- 16.Shaw PJ, et al. , Correlates of Sleep and Waking in Drosophila melanogaster. Science, 2000. 287: p. 1834–1837. [DOI] [PubMed] [Google Scholar]
- 17.Naidoo N, et al. , A role for the molecular chaperone protein BiP/GRP78 in Drosophila sleep homeostasis. Sleep, 2007. 30(5): p. 557–65. [DOI] [PubMed] [Google Scholar]
- 18.Brown MK, et al. , Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis. Neurobiology of Aging, 2014. 35(6): p. 1431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MK, Strus E, and Naidoo N, Reduced Sleep during Social Isolation leads to Cellular Stress and induction of the Unfolded Protein Response (UPR). Sleep, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.**.Musiek ES, Xiong DD, and Holtzman DM, Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Experimental and Molecular Medicine, 2015. 47: p. e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.*.Zhu Y, et al. , Chronic Sleep Disruption Advances the Temporal Progression of Tauopathy in P301S Mutant Mice. Journal of Neuroscience, 2018. 38(48): p. 10255–10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naidoo N, et al. , Endoplasmic reticulum stress in wake-active neurons progresses with aging. Aging Cell, 2011. 10(4): p. 640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.**.Hafycz JM and Naidoo NN, Sleep, Aging, and Cellular Health: Aged-Related Changes in Sleep and Protein Homeostasis Converge in Neurodegenerative Diseases. Front Aging Neurosci, 2019. 11: p. 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.**.Hetz C and Saxena S, ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol, 2017. 13(8): p. 477–491. [DOI] [PubMed] [Google Scholar]
- 25.*.Valadas JS, et al. , ER Lipid Defects in Neuropeptidergic Neurons Impair Sleep Patterns in Parkinson’s Disease. Neuron, 2018. 98(6): p. 1155–1169 e6. [DOI] [PubMed] [Google Scholar]
- 26.Gao L, et al. , ER stress activation impairs the expression of circadian clock and clock-controlled genes in NIH3T3 cells via an ATF4-dependent mechanism. Cellular Signalling, 2019. 57: p. 89–101. [DOI] [PubMed] [Google Scholar]
- 27.Bu Y, et al. , A PERK-miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival. Nat Cell Biol, 2018. 20(1): p. 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan G, et al. , Clock mediates liver senescence by controlling ER stress. Aging (Albany NY), 2017. 9(12): p. 2647–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellesi M, et al. , Contribution of sleep to the repair of neuronal DNA double-strand breaks: evidence from flies and mice. Sci Rep, 2016. 6: p. 36804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Oosterhout F, et al. , Ultraviolet light provides a major input to non-image-forming light detection in mice. Curr Biol, 2012. 22(15): p. 1397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeBardeleben HK, et al. , Stress-Induced Sleep After Exposure to Ultraviolet Light Is Promoted by p53 in Caenorhabditis elegans. Genetics, 2017. 207(2): p. 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.**.Zada D, et al. , Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat Commun, 2019. 10(1): p. 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauer MH and Gasser SM, Chromatin and nucleosome dynamics in DNA damage and repair. Genes Dev, 2017. 31(22): p. 2204–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J and Veasey S, Making sense of oxidative stress in obstructive sleep apnea: mediator or distracter? Front Neurol, 2012. 3: p. 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cespuglio R, et al. , [Voltametric detection of cerebral NO in rats. Variations of the signal throughout the sleep-wakefulness cycle]. C R Acad Sci III, 1996. 319(3): p. 191–200. [PubMed] [Google Scholar]
- 36.Williams JA, Vincent SR, and Reiner PB, Nitric oxide production in rat thalamus changes with behavioral state, local depolarization, and brainstem stimulation. J Neurosci, 1997. 17(1): p. 420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalinchuk AV, et al. , Nitric oxide production in the basal forebrain is required for recovery sleep. J Neurochem, 2006. 99(2): p. 483–98. [DOI] [PubMed] [Google Scholar]
- 38.Kalinchuk AV, et al. , Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J Neurosci, 2010. 30(40): p. 13254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopalakrishnan A, Ji LL, and Cirelli C, Sleep deprivation and cellular responses to oxidative stress. Sleep, 2004. 27(1): p. 27–35. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, et al. , Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. J Neurosci, 2014. 34(12): p. 4418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allada R, Cirelli C, and Sehgal A, Molecular Mechanisms of Sleep Homeostasis in Flies and Mammals. Cold Spring Harb Perspect Biol, 2017. 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vyazovskiy VV, et al. , Sleep homeostasis, habits and habituation. Current Opinion in Neurobiology, 2017. 44: p. 202–211. [DOI] [PubMed] [Google Scholar]
- 43.Hill VM, et al. , A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol, 2018. 16(7): p. e2005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.**.Kempf A, et al. , A potassium channel beta-subunit couples mitochondrial electron transport to sleep. Nature, 2019. 568(7751): p. 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirelli C, et al. , Reduced sleep in Drosophila Shaker mutants. Nature, 2005. 434(7037): p. 1087–92. [DOI] [PubMed] [Google Scholar]
- 46.Bushey D, et al. , Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J Neurosci, 2007. 27(20): p. 5384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pimentel D, et al. , Operation of a homeostatic sleep switch. Nature, 2016. 536(7616): p. 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitamura M, Biphasic, bidirectional regulation of NF-kappaB by endoplasmic reticulum stress. Antioxid Redox Signal, 2009. 11(9): p. 2353–64. [DOI] [PubMed] [Google Scholar]
- 49.Kitamura M, Control of NF-kappaB and inflammation by the unfolded protein response. Int Rev Immunol, 2011. 30(1): p. 4–15. [DOI] [PubMed] [Google Scholar]
- 50.Toth LA and Krueger JM, Alteration of sleep in rabbits by Staphylococcus aureus infection. Infect Immun, 1988. 56(7): p. 1785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.**.Kuo TH, et al. , Sleep triggered by an immune response in Drosophila is regulated by the circadian clock and requires the NFkappaB Relish. BMC Neurosci, 2010. 11: p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollmacher T, et al. , Influence of host defense activation on sleep in humans. Adv Neuroimmunol, 1995. 5(2): p. 155–69. [DOI] [PubMed] [Google Scholar]
- 53.Mullington J, et al. , Dose-dependent effects of endotoxin on human sleep. Am J Physiol Regul Integr Comp Physiol, 2000. 278(4): p. R947–55. [DOI] [PubMed] [Google Scholar]
- 54.Shirasu-Hiza MM, et al. , Interactions between circadian rhythm and immunity in Drosophila melanogaster. Curr Biol, 2007. 17(10): p. R353–5. [DOI] [PubMed] [Google Scholar]
- 55.Imeri L and Opp MR, How (and why) the immune system makes us sleep. Nat Rev Neurosci, 2009. 10(3): p. 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irwin MR, Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol, 2019. 19(11): p. 702–715. [DOI] [PubMed] [Google Scholar]
- 57.Williams JA, Chapter 23 - Sleep, Immunity, and Stress: Novel Insights From Drosophila, in Handbook of Behavioral Neuroscience, Dringenberg HC, Editor. 2019, Elsevier; p. 349–362. [Google Scholar]
- 58.Rockstrom MD, et al. , Tumor necrosis factor alpha in sleep regulation. Sleep Med Rev, 2018. 40: p. 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toth LA, Tolley EA, and Krueger JM, Sleep as a prognostic indicator during infectious disease in rabbits. Proc Soc Exp Biol Med, 1993. 203(2): p. 179–92. [DOI] [PubMed] [Google Scholar]
- 60.*.Kuo TH and Williams JA, Increased sleep promotes survival during a bacterial infection in Drosophila. Sleep, 2014. 37(6): p. 1077–86, 1086A-1086D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.*.Kuo TH and Williams JA, Acute sleep deprivation enhances post-infection sleep and promotes survival during bacterial infection in Drosophila. Sleep, 2014. 37(5): p. 859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jhaveri KA, et al. , Spontaneous, homeostatic, and inflammation-induced sleep in NF-{kappa}B p50 knockout mice. 10.1152/ajpregu.00262.2006 Am J Physiol Regul Integr Comp Physiol, 2006. 291(5): p. R1516–1526. [DOI] [PubMed] [Google Scholar]
- 63.**.Toda H, et al. , A sleep-inducing gene, nemuri, links sleep and immune function in Drosophila. Science, 2019. 363(6426): p. 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubowy C and Sehgal A, Circadian Rhythms and Sleep in Drosophila melanogaster. Genetics, 2017. 205(4): p. 1373–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joiner WJ, Unraveling the Evolutionary Determinants of Sleep. Curr Biol, 2016. 26(20): p. R1073–R1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.*.Hill AJ, et al. , Cellular Stress Induces a Protective Sleep-like State in C. elegans. Curr Biol, 2014. 24(20): p. 2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.*.Van Buskirk C and Sternberg PW, Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nat Neurosci, 2007. 10(10): p. 1300–7. [DOI] [PubMed] [Google Scholar]
- 68.Nelson MD, et al. , FMRFamide-like FLP-13 Neuropeptides Promote Quiescence following Heat Stress in Caenorhabditis elegans. Curr Biol, 2014. 24(20): p. 2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.*.Foltenyi K, Greenspan RJ, and Newport JW, Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nature Neuroscience, 2007. 10: p. 1160. [DOI] [PubMed] [Google Scholar]
- 70.*.Zettl M, et al. , Rhomboid family pseudoproteases use the ER quality control machinery to regulate intercellular signaling. Cell, 2011. 145(1): p. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lenz O, et al. , FMRFamide signaling promotes stress-induced sleep in Drosophila. Brain Behav Immun, 2015. 47: p. 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.**.Lee DA, et al. , Evolutionarily conserved regulation of sleep by epidermal growth factor receptor signaling. Science Advances, 2019. 5(11): p. eaax4249. [DOI] [PMC free article] [PubMed] [Google Scholar]