Introduction
Sertraline, a selective serotonin reuptake inhibitor (SSRI), is commonly prescribed to treat several psychiatric disorders including major depressive disorder, panic, generalized and social anxiety disorders as well as obsessive-compulsive disorder (OCD) [1]. It has similar efficacy to other SSRIs and is considered to cause fewer side effects than some antidepressants, such as tricyclic antidepressants [2, 3]. However, sertraline-induced adverse events such as reversible hepatic injury and Stevens Johnson Syndrome/toxic epidermal necrolysis (SJS/TEN) have been reported [4, 5]. Sertraline is a secondary amine with two chiral centers [6–9]. The potent cis-(1S,4S) enantiomer is used as an antidepressant in either a tablet or an oral solution [1, 7, 10], although the (1R,4R) enantiomer also inhibits serotonin reuptake [10].
Pharmacokinetics
Sertraline is slowly absorbed, with peak concentrations (Cmax) occurring at 4–10 hours following ingestion, and has a half-life of 24–32 hours in children, adolescents and adults [11–20]. The therapeutic concentration range for sertraline has not been well established, but has been proposed to be 10–150 ng/mL [21]. Concentrations below 10 ng/mL are proposed to have reduced efficacy, whereas concentrations above 150 ng/mL may increase the risk of toxicity. Chronopharmacokinetic studies suggest that the time of day that sertraline is administered does not influence the pharmacokinetic parameters [22]. Taking sertraline with food can increase the peak concentration (Cmax) by 25%, but plasma concentrations and area under the curve (AUC) between fasted and nonfasted groups 12 hours post-dose are comparable [22].
Desmethylsertraline, the active metabolite of sertraline, has a half-life of 56–120 hours and reaches peak plasma concentrations 8–10 hours following administration [11, 23–25]. Serum desmethylsertraline and sertraline concentrations are correlated, although desmethylsertraline concentrations are generally higher than sertraline concentrations [2, 26].
There is a large interindividual variability in plasma concentrations between patients taking the same dose of sertraline, which may be partially due to variable expression of CYP3A4/5, discussed in the Metabolism section below [2, 6] and genetic variation in CYP2C19, discussed in the Pharmacogenomics section [27]. This variability contributes to the weak relationship between the dose and plasma sertraline concentrations [15, 26]. Although some studies have found a relationship between serum concentrations of sertraline and the magnitude of clinical effect on patients [28, 29], other research has failed to replicate this association [23, 30].
Pharmacokinetics in special patient populations
Age may influence sertraline pharmacokinetics with elderly patients having increased serum concentrations and concentration/dose (C/D) ratios of sertraline and desmethylsertraline, compared to younger patients [26, 27]. However, not all studies have observed significant relationships between age and plasma sertraline concentrations [23, 25, 30, 31]. Relative to adults, children (age 6–12 years) and adolescents (13–17 years) have reduced AUCs and Cmax, when plasma sertraline concentrations were adjusted for weight. However, Cmax and AUC vary considerably in pediatric pharmacokinetic studies of sertraline and some suggest that pharmacokinetic differences observed between children and adolescents are attributable to differences in body weight rather than age [19].
Patients with cirrhosis have a significantly increased exposure to sertraline compared to patients with no hepatic impairment. This results from an increased Cmax, half-life and AUC of sertraline [32, 33] and reduced doses are recommended in patients with liver impairment [1]. Sertraline AUC and Cmax are also increased in patients who have undergone roux-en-Y gastric bypass, suggesting that the absorption of sertraline is affected by the procedure [34]. Renal impairment does not significantly affect sertraline pharmacokinetics [35].
In pregnant women, sertraline metabolism may be increased, which results in the potential need for dose increases during pregnancy. [36, 37]. Due to their high lipophilicity, sertraline (log Poct = 4.30) and desmethylsertraline can be detected in breast milk and in the plasma of breastfed infants of sertraline-treated mothers. However, these levels rarely exceeded 8ng/ml, with desmethylsertraline detected at slightly higher concentrations and more consistently across infants than sertraline [38–46]. Sertraline has a negligible effect on serotonin transport in breastfed infants [42].
Transport, metabolism and excretion
Sertraline is highly protein-bound in the blood (98%) and binds to human serum albumin with a high affinity via hydrophobic interactions and hydrogen bonding [1, 47]. In an in vitro model of the blood-brain barrier using rodent cells, lipophilic sertraline molecules easily crossed the barrier [48]. However, the pharmacokinetics of sertraline vary considerably across species and caution must be used in extrapolating results of studies in lower animals to humans[49]. Additionally, both sertraline and desmethylsertraline are substrates of the transporter p-glycoprotein, encoded by ABCB1, and bind with high affinity [50]. Sertraline can also inhibit p-glycoprotein, but this inhibition is not thought to have clinical effects [51, 52].
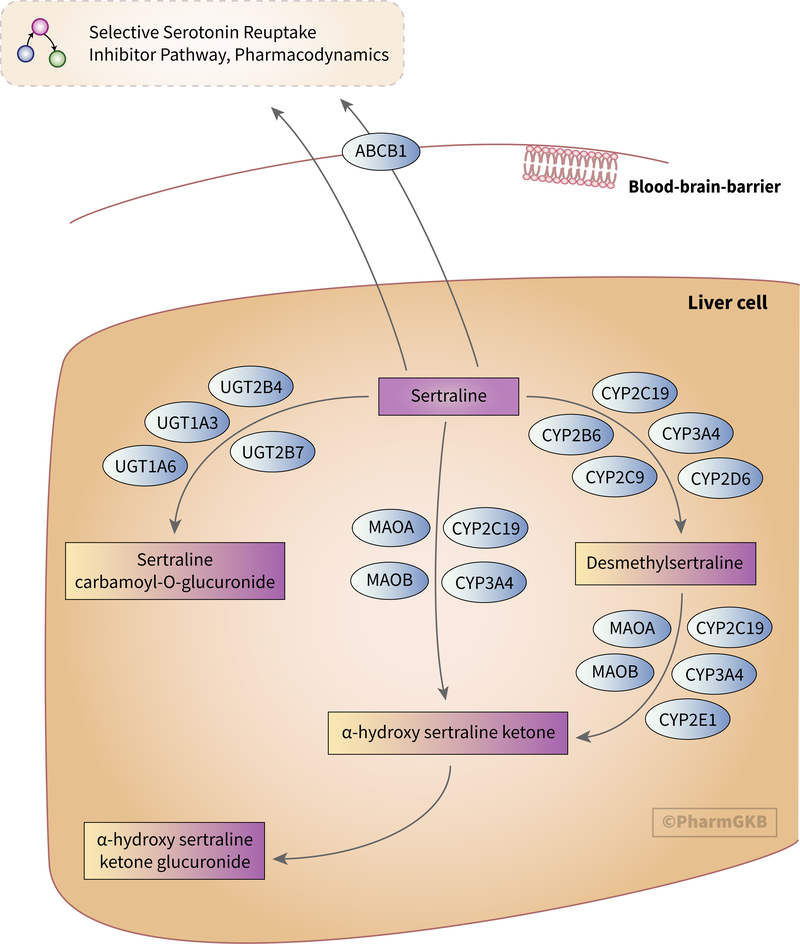
The main route of sertraline clearance is hepatic metabolism, as shown in Figure 1 [6, 53]. The vast majority of research concerning sertraline metabolism has focused on the demethylation of sertraline to form desmethylsertraline, the only active sertraline metabolite [54]. This reaction is mediated by multiple cytochrome P450 (CYP) enzymes [6, 55].
Figure 1.
Stylized diagram showing sertraline metabolism in the liver. A fully clickable version of this figure can be found at https://www.pharmgkb.org/pathway/PA166181117.
No individual CYP enzyme is thought to mediate more than 25–35% of sertraline’s metabolism to desmethylsertraline [55, 56]. At high in vitro sertraline concentrations, metabolism is primarily driven by CYP2C9, CYP3A4 and CYP2C19, with CYP2D6 and CYP2B6 making minor contributions [31, 55, 57, 58]. However, at lower concentrations, CYP2D6 and CYP2B6 play a more prominent role in desmethylsertraline formation, while CYP3A4 has a smaller role [56, 58, 59].
In vivo, inhibition of CYP3A4 increases serum sertraline concentrations [60, 61] while possible CYP3A4 induction by carbamazepine decreases sertraline concentrations [62, 63]. Inhibition of CYP2C19 (e.g. by the proton pump inhibitor esomeprazole) increases sertraline C/D ratios. Readers should note that other proton pump inhibitors (e.g., omeprazole, lansoprazole and pantoprazole) do not appear to affect sertraline pharmacokinetics [64].
In addition to metabolism by CYP enzymes, sertraline and desmethylsertraline can be deaminated to α-hydroxy sertraline ketone by CYP3A4, CYP2C19, monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B) [58]. Desmethylsertraline can also be deaminated by CYP2E1 [58]. α-hydroxy sertraline ketone is subsequently glucuronidated [65]. Additionally, sertraline can be glucuronidated via a carbamic acid intermediate to sertraline carbamoyl-O-glucuronide by UGT1A3, UGT1A6, UGT2B4 and UGT2B7 [58, 66].
Only low concentrations of unchanged sertraline can be recovered from urine, indicating that sertraline is extensively metabolized to its major excretory metabolite, α-hydroxy sertraline, prior to excretion [53, 58].
Drug-drug interactions involving sertraline
The fact that sertraline interacts with a number of CYP enzymes means that the metabolism of other drugs can be affected by the presence of sertraline. Sertraline reduces the clearance and potentiates the effects of imipramine and desipramine, due to inhibition of CYP2D6 [67–71]. In the case of imipramine, it is thought that inhibition of CYP2D6 by sertraline may increase the conversion of imipramine to desipramine [71]. In vitro data indicate that sertraline also moderately inhibits the CYP2D6-mediated metabolism of timolol, sparteine and propafenone [72–74]. However, other work has found sertraline to only be a weak CYP2D6 inhibitor [75–81], concurring with the findings of the US Food and Drug Administration (FDA) (see Table 3–2 at https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers), or to have no significant inhibitory effect at all [82, 83]. Some in vivo data suggest that weak CYP2D6 inhibition (AUC fold-change 1.25–2) occurs after achievement of steady-state concentrations while insignificant inhibitory effects are typically seen prior to steady state [67, 70, 71, 77].
Research on the inhibitory effect of sertraline on CYP2C9 indicates that the degree of inhibition may be substrate specific. Sertraline is a moderate inhibitor of phenytoin hydroxylation in vitro and can increase plasma phenytoin concentrations in patients [84–86]. However, sertraline only has a weak inhibitory effect on the metabolism of warfarin and tolbutamide [87–89].
Two studies have investigated sertraline’s ability to inhibit CYP2C19. One study found that sertraline could inhibit S-mephenytoin 4’hydroxylation by 73.3% [90] while Wang et al. determined that sertraline was a weak inhibitor of CYP2C19 [91]. The inhibitory potency of sertraline may be allele-specific: it inhibits the CYP2C19*8, *9, *13, *14, *16, and *19 alleles to a lesser degree than the *1 allele, but more strongly inhibits the *11 allele than the *1 allele by assessment of IC50 values [91]. The mechanism which underlies these allele-specific inhibitory effects of sertraline is currently unclear.
Sertraline may act as a CYP3A4/5 inhibitor [76, 92–94], although several studies have failed to observe this [95–101]. Interestingly, in one study, sertraline increased the cytotoxicity of carbamazepine by increasing CYP3A4-mediated production of reactive carbamazepine metabolites [102]. Additionally, desmethylsertraline may inhibit CYP3A4/5 but to a lesser extent than sertraline [92]. Sertraline does not inhibit CYP1A2 [78, 95, 96, 103] but may act as a CYP1A2 inducer [104]. There is some evidence that sertraline inhibits CYP2B6 and impacts the pharmacokinetics of efavirenz and methadone, which are both CYP2B6 substrates [105–107]. This inhibition is reduced when the increased-function CYP2B6*4 allele or decreased-function CYP2B6*6 allele are present [105].
In addition to inhibiting cytochrome P450 enzymes, sertraline interacts with several other enzymes and transporters [108–110]. Cases of serotonin syndrome in patients taking sertraline and a concomitant serotonergic drug, such as tramadol or clozapine, have been reported and the potential for sertraline to contribute to serotonin syndrome is acknowledged on the drug label [1, 111–113]. However, such cases are extremely rare, and questions have been raised as to whether prescribing actions should be changed to account for the low possibility of a patient developing serotonin syndrome. A retrospective study of 19,017 patients prescribed both an SSRI and a triptan, a combination considered by the FDA to potentially increase the risk of serotonin syndrome, found only seven cases of serotonin syndrome [114].
Pharmacodynamics
Sertraline is prescribed for its property as an inhibitor of the serotonin transporter SLC6A4. The pharmacodynamics of SSRIs have been covered in the PharmGKB Selective Serotonin Reuptake Inhibitor Pathway, Pharmacodynamics, which can be viewed at https://www.pharmgkb.org/pathway/PA161749006 [115]. Briefly, inhibition of SLC6A4 by sertraline is thought to potentiate the synaptic effects of serotonin and enhance signaling by the serotonin receptors. However, sertraline also inhibits transport of sodium, norepinephrine reuptake, dopamine reuptake as well as glutamate and GABA cycling in rodent and human neurons [10, 116, 117].
Although desmethylsertraline is an active metabolite of sertraline, it has no significant effect on serotonin reuptake [118] as its potency as a serotonin reuptake inhibitor is approximately 20-fold less than that of sertraline [10, 119]. As such, desmethylsertraline is not considered to exert a notable clinical effect in sertraline-treated patients. Similar to sertraline, desmethylsertraline weakly inhibits the norepinephrine transporter [119].
Pharmacogenomics
CYP2C19 variants appear to have the greatest impact on sertraline pharmacokinetics. Patients who are CYP2C19 poor metabolizers (PMs), or those carrying the *2 or *3 no function alleles, have a significantly slower rate of desmethylsertraline formation compared to CYP2C19 normal metabolizers (NMs) and consequently have an increased exposure to the pharmacologically active parent compound [20, 27, 57, 120]. Sertraline dose extrapolations based on pharmacokinetic parameters among CYP2C19 phenotypes suggest a 50% dose reduction may be needed for poor metabolizers [121]. A recent study found that the number of CYP2C19 no function alleles influenced the rate of titration of sertraline in a retrospective cohort of children and adolescents with anxiety and depressive disorders [122]. While the relationship between CYP2C19 increased or no function alleles and sertraline exposure and response is still being elucidated [123, 124], the increased function CYP2C19*17 allele has not been observed to greatly affect sertraline plasma concentrations [27].
Several studies have found no significant effect of CYP2D6 and CYP2C9 alleles on sertraline pharmacokinetics or patient response [11, 57, 124] and research into the effect of genetic variants on SSRIs did not find a significant impact of CYP2D6*4 on sertraline dose and tolerability [125]. However, analysis of a single patient with the CYP2D6 ultrarapid metabolizer (UM) phenotype and a single patient with the CYP2D6 PM phenotype found a reduction in sertraline plasma concentrations in both patients compared to the median concentrations across the cohort [120]. Given that these observations were made in individual patients, the influence of confounding factors, such as treatment adherence, cannot be excluded. This is particularly true in the case of the CYP2D6 PM patient as they presented with side effects at the first visit and had a subsequent change in diagnoses. Despite its unclear contribution to sertraline metabolism, the *6 and *9 alleles of CYP2B6 have been shown to decrease the rate of desmethylsertraline formation in one study [123].
The SNPs rs1045642, rs2032583, rs2032582, rs2235040, rs2235015, and rs9282564 in ABCB1 are associated with side effects and time to remission in patients taking sertraline [126]. However, the mechanism behind this observation is still unclear and is complicated by a study which found no effect of rs1045642 on sertraline clearance [18].
Patients that are homozygous for the 5HTTLPR long allele in SLC6A4 have shown an improved response to sertraline compared to patients with one or two copies of the short allele [122, 127, 128]. However, not all studies have observed this effect [129, 130]. In addition, one study found no association between a variable number tandem repeat (VNTR) in SLC6A4 and sertraline response [130]. An association between the deletion allele of rs1799752 in ACE and improved sertraline response has also been reported [131]. The deletion allele has been linked to increased plasma levels of angiotensin-converting enzyme (ACE) [132] and may affect the response of the renin angiotensin system to antidepressant therapy.
The GG genotype of rs45476395 in GNB3 has been associated with an improved response to sertraline in patients with major depressive disorder (MDD) [133]. However, the same study did not find an association between rs5441 in GNB3 and response to sertraline. As GNB3 encodes the G protein β3 subunit, the authors suggest that variants in this gene may affect signaling cascades involved in MDD or in the physiological response to sertraline.
In a study of Australian and Malaysian patients, Hong Ng et al. noted an association between a patient’s ethnic background and their sertraline plasma levels and treatment response. However, the authors only analyzed associations between ethnicity and sertraline pharmacokinetics and response and did not attempt to determine which pharmacogenetic variants may subtend these differences [134].
There are limited data on the pharmacogenetics underlying sertraline-related side effects. HLA-A*33:01 has been identified as a risk factor for sertraline drug-induced liver injury [135], but there is currently no information on which HLA alleles are involved in the onset of sertraline-induced SJS/TEN. A study of the HTR2A variants rs6311 and rs6313 did not find an association between either SNP and sertraline-induced nausea and vomiting [136].
Pharmacogenomics of antidepressants
Sertraline has been included in a number of studies investigating the pharmacogenetics of antidepressants more generally. The A allele of rs2235040 and the C allele of rs2032583, both in ABCB1, have been associated with increased adverse effects, serotonergic side effects (e.g., diarrhea or nausea) and SSRI-related insomnia [137]. However, another study failed to find an association between rs2032583 and SSRI side effects in addition to finding no association between rs2032583 or rs2235015 and SSRI dose/plasma concentrations [138]. Similarly, variants in ABCB6 and ABCG1 have not been associated with SSRI response [139].
Other research into genetic influences on antidepressant response have implicated a haplotype of rs6311, rs6313 and rs1928040 in HTR2A, rs6295 in HTR1A and rs495794 and rs153560 in REEP5 with response to antidepressants including sertraline [140–142]. Specifically, in one retrospective pediatric study of 249 sertraline-treated patients, HTR2A rs6313 and rs7997012 were associated with the dose at the time of response [122]. HTR1A and HTR2A encode serotonin receptor proteins, while REEP5 encodes an accessory protein.
Clinical implementation
As a result of this research, clinical guidelines have been published concerning sertraline use in CYP2C19 PMs [143–145]. These include the Clinical Pharmacogenetic Implementation Consortium (CPIC) guideline which recommends that, due to the risk of increased side effects, clinicians treating CYP2C19 PMs may consider reducing the starting dose by 50% before titrating to response or selecting an alternative antidepressant not predominantly metabolized by CYP2C19 [144]. The Royal Dutch Pharmacists Association - Pharmacogenetics Working Group (DPWG) also recommends using lower doses in patients who are CYP2C19 PMs [145]. Both of these guidelines have been annotated on the PharmGKB website. Several institutions have implemented the CPIC and DPWG guidelines into clinical care for routine use or research projects [146–148].
Conclusion
While the in vivo metabolism of sertraline in humans has not been fully investigated, variants in a number of genes involved in the sertraline pharmacokinetics and pharmacodynamics appear to affect its metabolism and clinical response. Current clinical guidelines focus on the possible effect of CYP2C19 variants on sertraline but further research could provide evidence to justify the inclusion of variants in genes involved in drug transport and response in future guidelines.
Acknowledgements
The authors thank Katrin Sangkuhl and Ethan Poweleit for critical review of the manuscript. This work is supported by the NIH/NIGMS grant GM61374.
Conflict of interest: JKH is a paid advisor for Quest Diagnostics and has received research support from OneOme. LBR has received research support from BTG International, Ltd. JRS has received research support from the National Institutes of Health, Allergan Pharmaceuticals, Neuronetics and has received material support from and provided consultation to Myriad Genetics. RBA is a stockholder in Personalis Inc. and 23andMe, and a paid advisor for Youscript.
References
- 1.Pfizer, Zoloft Prescribing Information. Revised December 2016. [Google Scholar]
- 2.Gupta RN and Dziurdzy SA, Therapeutic monitoring of sertraline. Clin Chem, 1994. 40(3): p. 498–9. [PubMed] [Google Scholar]
- 3.Cipriani A, La Ferla T, Furukawa TA, Signoretti A, Nakagawa A, Churchill R, et al. , Sertraline versus other antidepressive agents for depression. Cochrane Database Syst Rev, 2010(4): p. Cd006117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabak F, Gunduz F, Tahan V, Tabak O, and Ozaras R, Sertraline hepatotoxicity: report of a case and review of the literature. Dig Dis Sci, 2009. 54(7): p. 1589–91. [DOI] [PubMed] [Google Scholar]
- 5.Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. , Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol, 2008. 128(1): p. 35–44. [DOI] [PubMed] [Google Scholar]
- 6.Hiemke C and Hartter S, Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther, 2000. 85(1): p. 11–28. [DOI] [PubMed] [Google Scholar]
- 7.Baumann P, Clinical pharmacokinetics of citalopram and other selective serotonergic reuptake inhibitors (SSRI). Int Clin Psychopharmacol, 1992. 6 Suppl 5: p. 13–20. [PubMed] [Google Scholar]
- 8.Cheng H, He B, Zhang Q, and Tu Y, Chiral separation of sertraline with microemulsion electrokinetic chromatography on a polymer/beta-cyclodextrin assembling molecular film modified capillary. Anal Sci, 2010. 26(10): p. 1087–92. [DOI] [PubMed] [Google Scholar]
- 9.Budau M, Hancu G, Rusu A, Carcu-Dobrin M, and Muntean DL, Chirality of Modern Antidepressants: An Overview. Adv Pharm Bull, 2017. 7(4): p. 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koe BK, Weissman A, Welch WM, and Browne RG, Sertraline, 1S,4S-N-methyl-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthylamine, a new uptake inhibitor with selectivity for serotonin. J Pharmacol Exp Ther, 1983. 226(3): p. 686–700. [PubMed] [Google Scholar]
- 11.Hamelin BA, Turgeon J, Vallee F, Belanger PM, Paquet F, and LeBel M, The disposition of fluoxetine but not sertraline is altered in poor metabolizers of debrisoquin. Clin Pharmacol Ther, 1996. 60(5): p. 512–21. [DOI] [PubMed] [Google Scholar]
- 12.Axelson DA, Perel JM, Birmaher B, Rudolph GR, Nuss S, Bridge J, et al. , Sertraline pharmacokinetics and dynamics in adolescents. J Am Acad Child Adolesc Psychiatry, 2002. 41(9): p. 1037–44. [DOI] [PubMed] [Google Scholar]
- 13.Tassaneeyakul W, Kanchanawat S, Gaysonsiri D, Vannaprasath S, Paupairoj P, Kittiwattanagul K, et al. , Comparative bioavailability of two sertraline tablet formulations after single-dose administration in healthy Thai volunteers. Int J Clin Pharmacol Ther, 2008. 46(3): p. 151–6. [DOI] [PubMed] [Google Scholar]
- 14.Saletu B, Grunberger J, and Linzmayer L, On central effects of serotonin re-uptake inhibitors: quantitative EEG and psychometric studies with sertraline and zimelidine. J Neural Transm, 1986. 67(3–4): p. 241–66. [DOI] [PubMed] [Google Scholar]
- 15.Park MK, Shin KH, Kim KP, Kim TE, Yoon SH, Cho JY, et al. , Open label, three period, single sequence, study of 5, 25, 50 mg sertraline pharmacokinetics in healthy male Korean volunteers. Int J Clin Pharmacol Ther, 2011. 49(11): p. 672–8. [DOI] [PubMed] [Google Scholar]
- 16.He L, Feng F, and Wu J, Determination of sertraline in human plasma by high-performance liquid chromatography-electrospray ionization mass spectrometry and method validation. J Chromatogr Sci, 2005. 43(10): p. 532–5. [DOI] [PubMed] [Google Scholar]
- 17.Fouda HG, Ronfeld RA, and Weidler DJ, Gas chromatographic-mass spectrometric analysis and preliminary human pharmacokinetics of sertraline, a new antidepressant drug. J Chromatogr, 1987. 417(1): p. 197–202. [DOI] [PubMed] [Google Scholar]
- 18.Saiz-Rodriguez M, Belmonte C, Roman M, Ochoa D, Jiang-Zheng C, Koller D, et al. , Effect of ABCB1 C3435T Polymorphism on Pharmacokinetics of Antipsychotics and Antidepressants. Basic Clin Pharmacol Toxicol, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Alderman J, Wolkow R, Chung M, and Johnston HF, Sertraline treatment of children and adolescents with obsessive-compulsive disorder or depression: pharmacokinetics, tolerability, and efficacy. J Am Acad Child Adolesc Psychiatry, 1998. 37(4): p. 386–94. [DOI] [PubMed] [Google Scholar]
- 20.Wang JH, Liu ZQ, Wang W, Chen XP, Shu Y, He N, et al. , Pharmacokinetics of sertraline in relation to genetic polymorphism of CYP2C19. Clin Pharmacol Ther, 2001. 70(1): p. 42–7. [DOI] [PubMed] [Google Scholar]
- 21.Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, et al. , AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry: Update 2011. Pharmacopsychiatry, 2011. 44(6): p. 195–235. [DOI] [PubMed] [Google Scholar]
- 22.Ronfeld RA, Wilner KD, and Baris BA, Sertraline. Chronopharmacokinetics and the effect of coadministration with food. Clin Pharmacokinet, 1997. 32 Suppl 1: p. 50–5. [DOI] [PubMed] [Google Scholar]
- 23.Reis M, Aberg-Wistedt A, Agren H, Hoglund P, Akerblad AC, and Bengtsson F, Serum disposition of sertraline, N-desmethylsertraline and paroxetine: a pharmacokinetic evaluation of repeated drug concentration measurements during 6 months of treatment for major depression. Hum Psychopharmacol, 2004. 19(5): p. 283–91. [DOI] [PubMed] [Google Scholar]
- 24.Patel BN, Sharma N, Sanyal M, and Shrivastav PS, Analysis of second-generation antidepressant drug, sertraline and its active metabolite, N-desmethyl sertraline in human plasma by a sensitive and selective liquid chromatography-tandem mass spectrometry method. J Chromatogr B Analyt Technol Biomed Life Sci, 2009. 877(3): p. 221–9. [DOI] [PubMed] [Google Scholar]
- 25.Ronfeld RA, Tremaine LM, and Wilner KD, Pharmacokinetics of sertraline and its N-demethyl metabolite in elderly and young male and female volunteers. Clin Pharmacokinet, 1997. 32 Suppl 1: p. 22–30. [DOI] [PubMed] [Google Scholar]
- 26.Lundmark J, Reis M, and Bengtsson F, Therapeutic drug monitoring of sertraline: variability factors as displayed in a clinical setting. Ther Drug Monit, 2000. 22(4): p. 446–54. [DOI] [PubMed] [Google Scholar]
- 27.Rudberg I, Hermann M, Refsum H, and Molden E, Serum concentrations of sertraline and N-desmethyl sertraline in relation to CYP2C19 genotype in psychiatric patients. Eur J Clin Pharmacol, 2008. 64(12): p. 1181–8. [DOI] [PubMed] [Google Scholar]
- 28.Lundmark J, Bengtsson F, Nordin C, Reis M, and Walinder J, Therapeutic drug monitoring of selective serotonin reuptake inhibitors influences clinical dosing strategies and reduces drug costs in depressed elderly patients. Acta Psychiatr Scand, 2000. 101(5): p. 354–9. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V, Garcia V, and Loewenstein D. Plasma levels of sertraline and the clinical response (abstract). 1996. European Neuropsychopharmocology. [Google Scholar]
- 30.Taurines R, Burger R, Wewetzer C, Pfuhlmann B, Mehler-Wex C, Gerlach M, et al. , The relation between dosage, serum concentrations, and clinical outcome in children and adolescents treated with sertraline: a naturalistic study. Ther Drug Monit, 2013. 35(1): p. 84–91. [DOI] [PubMed] [Google Scholar]
- 31.Harvey A, Preskorn S, Lane R, and Wilner K. Sertraline and P450A¾ (abstract). 1996. European Neuropsychopharmocology. [Google Scholar]
- 32.Wilner KD, Everson G, Foulds GH, Hansen RA, Shrestra R, McKinley C, et al. Multiple dose pharmacokinetics of sertraline in subjects with varying degrees of hepatic impairment (abstract). 1996. European Neuropsychopharmacology. [Google Scholar]
- 33.Demolis JL, Angebaud P, Grange JD, Coates P, Funck-Brentano C, and Jaillon P, Influence of liver cirrhosis on sertraline pharmacokinetics. Br J Clin Pharmacol, 1996. 42(3): p. 394–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roerig JL, Steffen K, Zimmerman C, Mitchell JE, Crosby RD, and Cao L, Preliminary comparison of sertraline levels in postbariatric surgery patients versus matched nonsurgical cohort. Surg Obes Relat Dis, 2012. 8(1): p. 62–6. [DOI] [PubMed] [Google Scholar]
- 35.Wilner KD, Baris BA, Foulds GH, Anziano RJ, Berl T, and Ziegler MG. Multiple dose pharmacokinetics of sertraline in subjects with varying degrees of renal impairment (abstract). 1996. European Neuropsychpharmocology. [Google Scholar]
- 36.Sit DK, Perel JM, Helsel JC, and Wisner KL, Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry, 2008. 69(4): p. 652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman MP, Nolan PE Jr., Davis MF, Anthony M, Fried K, Fankhauser M, et al. , Pharmacokinetics of sertraline across pregnancy and postpartum. J Clin Psychopharmacol, 2008. 28(6): p. 646–53. [DOI] [PubMed] [Google Scholar]
- 38.Deak K, Takacs-Novak K, Tihanyi K, and Noszal B, Physico-chemical profiling of antidepressive sertraline: solubility, ionisation, lipophilicity. Med Chem, 2006. 2(4): p. 385–9. [DOI] [PubMed] [Google Scholar]
- 39.Stowe ZN, Owens MJ, Landry JC, Kilts CD, Ely T, Llewellyn A, et al. , Sertraline and desmethylsertraline in human breast milk and nursing infants. Am J Psychiatry, 1997. 154(9): p. 1255–60. [DOI] [PubMed] [Google Scholar]
- 40.Weisskopf E, Panchaud A, Nguyen KA, Grosjean D, Hascoet JM, Csajka C, et al. , Simultaneous determination of selective serotonin reuptake inhibitors and their main metabolites in human breast milk by liquid chromatography-electrospray mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 2017. 1057: p. 101–109. [DOI] [PubMed] [Google Scholar]
- 41.Dodd S, Stocky A, Buist A, Burrows GD, and Norman TR, Sertraline analysis in the plasma of breast-fed infants. Aust N Z J Psychiatry, 2001. 35(4): p. 545–6. [DOI] [PubMed] [Google Scholar]
- 42.Epperson N, Czarkowski KA, Ward-O’Brien D, Weiss E, Gueorguieva R, Jatlow P, et al. , Maternal sertraline treatment and serotonin transport in breast-feeding mother-infant pairs. Am J Psychiatry, 2001. 158(10): p. 1631–7. [DOI] [PubMed] [Google Scholar]
- 43.Hendrick V, Fukuchi A, Altshuler L, Widawski M, Wertheimer A, and Brunhuber MV, Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry, 2001. 179: p. 163–6. [DOI] [PubMed] [Google Scholar]
- 44.Kristensen JH, Ilett KF, Dusci LJ, Hackett LP, Yapp P, Wojnar-Horton RE, et al. , Distribution and excretion of sertraline and N-desmethylsertraline in human milk. Br J Clin Pharmacol, 1998. 45(5): p. 453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mammen OK, Perel JM, Rudolph G, Foglia JP, and Wheeler SB, Sertraline and norsertraline levels in three breastfed infants. J Clin Psychiatry, 1997. 58(3): p. 100–3. [DOI] [PubMed] [Google Scholar]
- 46.Wisner KL, Perel JM, and Blumer J, Serum sertraline and N-desmethylsertraline levels in breast-feeding mother-infant pairs. Am J Psychiatry, 1998. 155(5): p. 690–2. [DOI] [PubMed] [Google Scholar]
- 47.Shahlaei M, Rahimi B, Nowroozi A, Ashrafi-Kooshk MR, Sadrjavadi K, and Khodarahmi R, Exploring binding properties of sertraline with human serum albumin: Combination of spectroscopic and molecular modeling studies. Chem Biol Interact, 2015. 242: p. 235–46. [DOI] [PubMed] [Google Scholar]
- 48.Booth R and Kim H, Permeability analysis of neuroactive drugs through a dynamic microfluidic in vitro blood-brain barrier model. Ann Biomed Eng, 2014. 42(12): p. 2379–91. [DOI] [PubMed] [Google Scholar]
- 49.Tremaine LM, Welch WM, and Ronfeld RA, Metabolism and disposition of the 5-hydroxytryptamine uptake blocker sertraline in the rat and dog. Drug Metab Dispos, 1989. 17(5): p. 542–50. [PubMed] [Google Scholar]
- 50.Wang JS, Zhu HJ, Gibson BB, Markowitz JS, Donovan JL, and DeVane CL, Sertraline and its metabolite desmethylsertraline, but not bupropion or its three major metabolites, have high affinity for P-glycoprotein. Biol Pharm Bull, 2008. 31(2): p. 231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss J, Dormann SM, Martin-Facklam M, Kerpen CJ, Ketabi-Kiyanvash N, and Haefeli WE, Inhibition of P-glycoprotein by newer antidepressants. J Pharmacol Exp Ther, 2003. 305(1): p. 197–204. [DOI] [PubMed] [Google Scholar]
- 52.Kapoor A, Iqbal M, Petropoulos S, Ho HL, Gibb W, and Matthews SG, Effects of sertraline and fluoxetine on p-glycoprotein at barrier sites: in vivo and in vitro approaches. PLoS One, 2013. 8(2): p. e56525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levine B, Jenkins AJ, and Smialek JE, Distribution of sertraline in postmortem cases. J Anal Toxicol, 1994. 18(5): p. 272–4. [DOI] [PubMed] [Google Scholar]
- 54.DeVane CL, Metabolism and pharmacokinetics of selective serotonin reuptake inhibitors. Cell Mol Neurobiol, 1999. 19(4): p. 443–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenblatt DJ, von Moltke LL, Harmatz JS, and Shader RI, Human cytochromes mediating sertraline biotransformation: seeking attribution. J Clin Psychopharmacol, 1999. 19(6): p. 489–93. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi K, Ishizuka T, Shimada N, Yoshimura Y, Kamijima K, and Chiba K, Sertraline N-demethylation is catalyzed by multiple isoforms of human cytochrome P-450 in vitro. Drug Metab Dispos, 1999. 27(7): p. 763–6. [PubMed] [Google Scholar]
- 57.Xu ZH, Wang W, Zhao XJ, Huang SL, Zhu B, He N, et al. , Evidence for involvement of polymorphic CYP2C19 and 2C9 in the N-demethylation of sertraline in human liver microsomes. Br J Clin Pharmacol, 1999. 48(3): p. 416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obach RS, Cox LM, and Tremaine LM, Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos, 2005. 33(2): p. 262–70. [DOI] [PubMed] [Google Scholar]
- 59.Palacharla RC, Nirogi R, Uthukam V, Manoharan A, Ponnamaneni RK, and Kalaikadhiban I, Quantitative in vitro phenotyping and prediction of drug interaction potential of CYP2B6 substrates as victims. Xenobiotica, 2018. 48(7): p. 663–675. [DOI] [PubMed] [Google Scholar]
- 60.Lee AJ, Chan WK, Harralson AF, Buffum J, and Bui BC, The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther, 1999. 21(11): p. 1890–9. [DOI] [PubMed] [Google Scholar]
- 61.Ueda N, Yoshimura R, Umene-Nakano W, Ikenouchi-Sugita A, Hori H, Hayashi K, et al. , Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry, 2009. 10(4 Pt 3): p. 832–5. [DOI] [PubMed] [Google Scholar]
- 62.Pihlsgard M and Eliasson E, Significant reduction of sertraline plasma levels by carbamazepine and phenytoin. Eur J Clin Pharmacol, 2002. 57(12): p. 915–6. [DOI] [PubMed] [Google Scholar]
- 63.Khan A, Shad MU, and Preskorn SH, Lack of sertraline efficacy probably due to an interaction with carbamazepine. J Clin Psychiatry, 2000. 61(7): p. 526–7. [DOI] [PubMed] [Google Scholar]
- 64.Gjestad C, Westin AA, Skogvoll E, and Spigset O, Effect of proton pump inhibitors on the serum concentrations of the selective serotonin reuptake inhibitors citalopram, escitalopram, and sertraline. Ther Drug Monit, 2015. 37(1): p. 90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacQueen G, Born L, and Steiner M, The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev, 2001. 7(1): p. 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tremaine LM, Stroh JG, and Ronfeld RA, Characterization of a carbamic acid ester glucuronide of the secondary amine sertraline. Drug Metab Dispos, 1989. 17(1): p. 58–63. [PubMed] [Google Scholar]
- 67.Preskorn SH, Alderman J, Chung M, Harrison W, Messig M, and Harris S, Pharmacokinetics of desipramine coadministered with sertraline or fluoxetine. J Clin Psychopharmacol, 1994. 14(2): p. 90–8. [PubMed] [Google Scholar]
- 68.Lydiard RB, Anton RF, and Cunningham T, Interactions between sertraline and tricyclic antidepressants. Am J Psychiatry, 1993. 150(7): p. 1125–6. [DOI] [PubMed] [Google Scholar]
- 69.Barros J and Asnis G, An interaction of sertraline and desipramine. Am J Psychiatry, 1993. 150(11): p. 1751. [DOI] [PubMed] [Google Scholar]
- 70.Alderman J, Preskorn SH, Greenblatt DJ, Harrison W, Penenberg D, Allison J, et al. , Desipramine pharmacokinetics when coadministered with paroxetine or sertraline in extensive metabolizers. J Clin Psychopharmacol, 1997. 17(4): p. 284–91. [DOI] [PubMed] [Google Scholar]
- 71.Kurtz DL, Bergstrom RF, Goldberg MJ, and Cerimele BJ, The effect of sertraline on the pharmacokinetics of desipramine and imipramine. Clin Pharmacol Ther, 1997. 62(2): p. 145–56. [DOI] [PubMed] [Google Scholar]
- 72.Hemeryck A, De Vriendt C, and Belpaire FM, Effect of selective serotonin reuptake inhibitors on the oxidative metabolism of propafenone: in vitro studies using human liver microsomes. J Clin Psychopharmacol, 2000. 20(4): p. 428–34. [DOI] [PubMed] [Google Scholar]
- 73.Volotinen M, Korjamo T, Tolonen A, Turpeinen M, Pelkonen O, Hakkola J, et al. , Effects of selective serotonin reuptake inhibitors on timolol metabolism in human liver microsomes and cryo-preserved hepatocytes. Basic Clin Pharmacol Toxicol, 2010. 106(4): p. 302–9. [DOI] [PubMed] [Google Scholar]
- 74.Crewe HK, Lennard MS, Tucker GT, Woods FR, and Haddock RE, The effect of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol, 1992. 34(3): p. 262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sproule BA, Otton SV, Cheung SW, Zhong XH, Romach MK, and Sellers EM, CYP2D6 inhibition in patients treated with sertraline. J Clin Psychopharmacol, 1997. 17(2): p. 102–6. [DOI] [PubMed] [Google Scholar]
- 76.Spina E, D’Arrigo C, Migliardi G, Morgante L, Zoccali R, Ancione M, et al. , Plasma risperidone concentrations during combined treatment with sertraline. Ther Drug Monit, 2004. 26(4): p. 386–90. [DOI] [PubMed] [Google Scholar]
- 77.Preskorn SH, Greenblatt DJ, Flockhart D, Luo Y, Perloff ES, Harmatz JS, et al. , Comparison of duloxetine, escitalopram, and sertraline effects on cytochrome P450 2D6 function in healthy volunteers. J Clin Psychopharmacol, 2007. 27(1): p. 28–34. [DOI] [PubMed] [Google Scholar]
- 78.Ozdemir V, Naranjo CA, Herrmann N, Shulman RW, Sellers EM, Reed K, et al. , The extent and determinants of changes in CYP2D6 and CYP1A2 activities with therapeutic doses of sertraline. J Clin Psychopharmacol, 1998. 18(1): p. 55–61. [DOI] [PubMed] [Google Scholar]
- 79.Liston HL, DeVane CL, Boulton DW, Risch SC, Markowitz JS, and Goldman J, Differential time course of cytochrome P450 2D6 enzyme inhibition by fluoxetine, sertraline, and paroxetine in healthy volunteers. J Clin Psychopharmacol, 2002. 22(2): p. 169–73. [DOI] [PubMed] [Google Scholar]
- 80.Lam YW, Gaedigk A, Ereshefsky L, Alfaro CL, and Simpson J, CYP2D6 inhibition by selective serotonin reuptake inhibitors: analysis of achievable steady-state plasma concentrations and the effect of ultrarapid metabolism at CYP2D6. Pharmacotherapy, 2002. 22(8): p. 1001–6. [DOI] [PubMed] [Google Scholar]
- 81.Belpaire FM, Wijnant P, Temmerman A, Rasmussen BB, and Brosen K, The oxidative metabolism of metoprolol in human liver microsomes: inhibition by the selective serotonin reuptake inhibitors. Eur J Clin Pharmacol, 1998. 54(3): p. 261–4. [DOI] [PubMed] [Google Scholar]
- 82.Alfaro CL, Lam YW, Simpson J, and Ereshefsky L, CYP2D6 status of extensive metabolizers after multiple-dose fluoxetine, fluvoxamine, paroxetine, or sertraline. J Clin Psychopharmacol, 1999. 19(2): p. 155–63. [DOI] [PubMed] [Google Scholar]
- 83.Alfaro CL, Lam YW, Simpson J, and Ereshefsky L, CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: intraindividual variability and plasma concentration correlations. J Clin Pharmacol, 2000. 40(1): p. 58–66. [DOI] [PubMed] [Google Scholar]
- 84.Schmider J, Greenblatt DJ, von Moltke LL, Karsov D, and Shader RI, Inhibition of CYP2C9 by selective serotonin reuptake inhibitors in vitro: studies of phenytoin p-hydroxylation. Br J Clin Pharmacol, 1997. 44(5): p. 495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nelson MH, Birnbaum AK, and Remmel RP, Inhibition of phenytoin hydroxylation in human liver microsomes by several selective serotonin re-uptake inhibitors. Epilepsy Res, 2001. 44(1): p. 71–82. [DOI] [PubMed] [Google Scholar]
- 86.Haselberger MB, Freedman LS, and Tolbert S, Elevated serum phenytoin concentrations associated with coadministration of sertraline. J Clin Psychopharmacol, 1997. 17(2): p. 107–9. [DOI] [PubMed] [Google Scholar]
- 87.Tremaine LM, Wilner KD, and Preskorn SH, A study of the potential effect of sertraline on the pharmacokinetics and protein binding of tolbutamide. Clin Pharmacokinet, 1997. 32 Suppl 1: p. 31–6. [DOI] [PubMed] [Google Scholar]
- 88.Apseloff G, Wilner KD, Gerber N, and Tremaine LM, Effect of sertraline on protein binding of warfarin. Clin Pharmacokinet, 1997. 32 Suppl 1: p. 37–42. [DOI] [PubMed] [Google Scholar]
- 89.Hemeryck A, De Vriendt C, and Belpaire FM, Inhibition of CYP2C9 by selective serotonin reuptake inhibitors: in vitro studies with tolbutamide and (S)-warfarin using human liver microsomes. Eur J Clin Pharmacol, 1999. 54(12): p. 947–51. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi K, Yamamoto T, Chiba K, Tani M, Ishizaki T, and Kuroiwa Y, The effects of selective serotonin reuptake inhibitors and their metabolites on S-mephenytoin 4’-hydroxylase activity in human liver microsomes. Br J Clin Pharmacol, 1995. 40(5): p. 481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang H, Kim RA, Sun D, Gao Y, Wang H, Zhu J, et al. , Evaluation of the effects of 18 non-synonymous single-nucleotide polymorphisms of CYP450 2C19 on in vitro drug inhibition potential by a fluorescence-based high-throughput assay. Xenobiotica, 2011. 41(9): p. 826–35. [DOI] [PubMed] [Google Scholar]
- 92.Masubuchi Y and Kawaguchi Y, Time-dependent inhibition of CYP3A4 by sertraline, a selective serotonin reuptake inhibitor. Biopharm Drug Dispos, 2013. 34(8): p. 423–30. [DOI] [PubMed] [Google Scholar]
- 93.Ring BJ, Binkley SN, Roskos L, and Wrighton SA, Effect of fluoxetine, norfluoxetine, sertraline and desmethyl sertraline on human CYP3A catalyzed 1’-hydroxy midazolam formation in vitro. J Pharmacol Exp Ther, 1995. 275(3): p. 1131–5. [PubMed] [Google Scholar]
- 94.von Moltke LL, Greenblatt DJ, Cotreau-Bibbo MM, Harmatz JS, and Shader RI, Inhibitors of alprazolam metabolism in vitro: effect of serotonin-reuptake-inhibitor antidepressants, ketoconazole and quinidine. Br J Clin Pharmacol, 1994. 38(1): p. 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davies SJ, Mulsant BH, Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, et al. , The Impact of Sertraline Co-Administration on the Pharmacokinetics of Olanzapine: A Population Pharmacokinetic Analysis of the STOP-PD. Clin Pharmacokinet, 2015. 54(11): p. 1161–8. [DOI] [PubMed] [Google Scholar]
- 96.Davies SJ, Mulsant BH, Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, et al. , SSRI-antipsychotic combination in psychotic depression: sertraline pharmacokinetics in the presence of olanzapine, a brief report from the STOP-PD study. Hum Psychopharmacol, 2016. 31(3): p. 252–5. [DOI] [PubMed] [Google Scholar]
- 97.Desta Z, Soukhova N, and Flockhart DA, In vitro inhibition of pimozide N-dealkylation by selective serotonin reuptake inhibitors and azithromycin. J Clin Psychopharmacol, 2002. 22(2): p. 162–8. [DOI] [PubMed] [Google Scholar]
- 98.DeVane CL, Donovan JL, Liston HL, Markowitz JS, Cheng KT, Risch SC, et al. , Comparative CYP3A4 inhibitory effects of venlafaxine, fluoxetine, sertraline, and nefazodone in healthy volunteers. J Clin Psychopharmacol, 2004. 24(1): p. 4–10. [DOI] [PubMed] [Google Scholar]
- 99.Gardner MJ, Baris BA, Wilner KD, and Preskorn SH, Effect of sertraline on the pharmacokinetics and protein binding of diazepam in healthy volunteers. Clin Pharmacokinet, 1997. 32 Suppl 1: p. 43–9. [DOI] [PubMed] [Google Scholar]
- 100.Hassan PC, Sproule BA, Naranjo CA, and Herrmann N, Dose-response evaluation of the interaction between sertraline and alprazolam in vivo. J Clin Psychopharmacol, 2000. 20(2): p. 150–8. [DOI] [PubMed] [Google Scholar]
- 101.Preskorn SH, Greenblatt DJ, and Harvey AT, Lack of effect of sertraline on the pharmacokinetics of alprazolam. J Clin Psychopharmacol, 2000. 20(5): p. 585–6. [DOI] [PubMed] [Google Scholar]
- 102.Ghosh C, Hossain M, Spriggs A, Ghosh A, Grant GA, Marchi N, et al. , Sertraline-induced potentiation of the CYP3A4-dependent neurotoxicity of carbamazepine: an in vitro study. Epilepsia, 2015. 56(3): p. 439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weigmann H, Gerek S, Zeisig A, Muller M, Hartter S, and Hiemke C, Fluvoxamine but not sertraline inhibits the metabolism of olanzapine: evidence from a therapeutic drug monitoring service. Ther Drug Monit, 2001. 23(4): p. 410–3. [DOI] [PubMed] [Google Scholar]
- 104.Pinninti NR and de Leon J, Interaction of sertraline with clozapine. J Clin Psychopharmacol, 1997. 17(2): p. 119–20. [DOI] [PubMed] [Google Scholar]
- 105.Talakad JC, Kumar S, and Halpert JR, Decreased susceptibility of the cytochrome P450 2B6 variant K262R to inhibition by several clinically important drugs. Drug Metab Dispos, 2009. 37(3): p. 644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Melis V, Usach I, Gandia P, and Peris JE, Inhibition of Efavirenz Metabolism by Sertraline and Nortriptyline and Their Effect on Efavirenz Plasma Concentrations. Antimicrob Agents Chemother, 2016. 60(2): p. 1022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hamilton SP, Nunes EV, Janal M, and Weber L, The effect of sertraline on methadone plasma levels in methadone-maintenance patients. Am J Addict, 2000. 9(1): p. 63–9. [DOI] [PubMed] [Google Scholar]
- 108.Griffin LD and Mellon SH, Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A, 1999. 96(23): p. 13512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaufman KR and Gerner R, Lamotrigine toxicity secondary to sertraline. Seizure, 1998. 7(2): p. 163–5. [DOI] [PubMed] [Google Scholar]
- 110.Nielsen CU, Frolund S, Abdulhadi S, Sari H, Langthaler L, Nohr MK, et al. , Sertraline inhibits the transport of PAT1 substrates in vivo and in vitro. Br J Pharmacol, 2013. 170(5): p. 1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mason BJ and Blackburn KH, Possible serotonin syndrome associated with tramadol and sertraline coadministration. Ann Pharmacother, 1997. 31(2): p. 175–7. [DOI] [PubMed] [Google Scholar]
- 112.Mittino D, Mula M, and Monaco F, Serotonin syndrome associated with tramadol-sertraline coadministration. Clin Neuropharmacol, 2004. 27(3): p. 150–1. [DOI] [PubMed] [Google Scholar]
- 113.Srisuma S, Hoyte CO, Wongvisavakorn S, and Wanaukul W, Serotonin syndrome precipitated by sertraline and discontinuation of clozapine. Clin Toxicol (Phila), 2015. 53(8): p. 840–1. [DOI] [PubMed] [Google Scholar]
- 114.Orlova Y, Rizzoli P, and Loder E, Association of Coprescription of Triptan Antimigraine Drugs and Selective Serotonin Reuptake Inhibitor or Selective Norepinephrine Reuptake Inhibitor Antidepressants With Serotonin Syndrome. JAMA Neurol, 2018. 75(5): p. 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sangkuhl K, Klein TE, and Altman RB, Selective serotonin reuptake inhibitors pathway. Pharmacogenet Genomics, 2009. 19(11): p. 907–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Owens MJ, Morgan WN, Plott SJ, and Nemeroff CB, Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther, 1997. 283(3): p. 1305–22. [PubMed] [Google Scholar]
- 117.Aldana BI and Sitges M, Sertraline inhibits pre-synaptic Na(+) channel-mediated responses in hippocampus-isolated nerve endings. J Neurochem, 2012. 121(2): p. 197–205. [DOI] [PubMed] [Google Scholar]
- 118.Sprouse J, Clarke T, Reynolds L, Heym J, and Rollema H, Comparison of the effects of sertraline and its metabolite desmethylsertraline on blockade of central 5-HT reuptake in vivo. Neuropsychopharmacology, 1996. 14(4): p. 225–31. [DOI] [PubMed] [Google Scholar]
- 119.Fuller RW, Hemrick-Luecke SK, Littlefield ES, and Audia JE, Comparison of desmethylsertraline with sertraline as a monoamine uptake inhibitor in vivo. Prog Neuropsychopharmacol Biol Psychiatry, 1995. 19(1): p. 135–49. [DOI] [PubMed] [Google Scholar]
- 120.Grasmader K, Verwohlt PL, Rietschel M, Dragicevic A, Muller M, Hiemke C, et al. , Impact of polymorphisms of cytochrome-P450 isoenzymes 2C9, 2C19 and 2D6 on plasma concentrations and clinical effects of antidepressants in a naturalistic clinical setting. Eur J Clin Pharmacol, 2004. 60(5): p. 329–36. [DOI] [PubMed] [Google Scholar]
- 121.Stingl JC, Brockmoller J, and Viviani R, Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry, 2013. 18(3): p. 273–87. [DOI] [PubMed] [Google Scholar]
- 122.Poweleit EA, Aldrich SL, Martin LJ, Hahn D, Strawn JR, and Ramsey LB, Pharmacogenetics of Sertraline Tolerability and Response in Pediatric Anxiety and Depressive Disorders. J Child Adolesc Psychopharmacol, 2019. 29(5): p. 348–361. [DOI] [PubMed] [Google Scholar]
- 123.Yuce-Artun N, Baskak B, Ozel-Kizil ET, Ozdemir H, Uckun Z, Devrimci-Ozguven H, et al. , Influence of CYP2B6 and CYP2C19 polymorphisms on sertraline metabolism in major depression patients. Int J Clin Pharm, 2016. 38(2): p. 388–94. [DOI] [PubMed] [Google Scholar]
- 124.Brandl EJ, Tiwari AK, Zhou X, Deluce J, Kennedy JL, Muller DJ, et al. , Influence of CYP2D6 and CYP2C19 gene variants on antidepressant response in obsessive-compulsive disorder. Pharmacogenomics J, 2014. 14(2): p. 176–81. [DOI] [PubMed] [Google Scholar]
- 125.Bijl MJ, Visser LE, Hofman A, Vulto AG, van Gelder T, Stricker BH, et al. , Influence of the CYP2D6*4 polymorphism on dose, switching and discontinuation of antidepressants. Br J Clin Pharmacol, 2008. 65(4): p. 558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ray A, Tennakoon L, Keller J, Sarginson JE, Ryan HS, Murphy GM, et al. , ABCB1 (MDR1) predicts remission on P-gp substrates in chronic depression. Pharmacogenomics J, 2015. 15(4): p. 332–9. [DOI] [PubMed] [Google Scholar]
- 127.Mushtaq D, Ali A, Margoob MA, Murtaza I, and Andrade C, Association between serotonin transporter gene promoter-region polymorphism and 4- and 12-week treatment response to sertraline in posttraumatic stress disorder. J Affect Disord, 2012. 136(3): p. 955–62. [DOI] [PubMed] [Google Scholar]
- 128.Durham LK, Webb SM, Milos PM, Clary CM, and Seymour AB, The serotonin transporter polymorphism, 5HTTLPR, is associated with a faster response time to sertraline in an elderly population with major depressive disorder. Psychopharmacology (Berl), 2004. 174(4): p. 525–9. [DOI] [PubMed] [Google Scholar]
- 129.Ng CH, Easteal S, Tan S, Schweitzer I, Ho BK, and Aziz S, Serotonin transporter polymorphisms and clinical response to sertraline across ethnicities. Prog Neuropsychopharmacol Biol Psychiatry, 2006. 30(5): p. 953–7. [DOI] [PubMed] [Google Scholar]
- 130.Dogan O, Yuksel N, Ergun MA, Yilmaz A, Ilhan MN, Karslioglu HE, et al. , Serotonin transporter gene polymorphisms and sertraline response in major depression patients. Genet Test, 2008. 12(2): p. 225–31. [DOI] [PubMed] [Google Scholar]
- 131.Bahramali E, Firouzabadi N, Yavarian I, Shayesteh MR, Erfani N, Shoushtari AA, et al. , Influence of ACE gene on differential response to sertraline versus fluoxetine in patients with major depression: a randomized controlled trial. Eur J Clin Pharmacol, 2016. 72(9): p. 1059–64. [DOI] [PubMed] [Google Scholar]
- 132.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, and Soubrier F, An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest, 1990. 86(4): p. 1343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Firouzabadi D, Firouzabadi N, Kalani K, Zomorrodian K, and Tehrani ES, Response to sertraline is influenced by GNbeta3 gene G-350A variant in patients with major depressive disorder. Eur J Clin Pharmacol, 2019. 75(2): p. 189–194. [DOI] [PubMed] [Google Scholar]
- 134.Hong Ng C, Norman TR, Naing KO, Schweitzer I, Kong Wai Ho B, Fan A, et al. , A comparative study of sertraline dosages, plasma concentrations, efficacy and adverse reactions in Chinese versus Caucasian patients. Int Clin Psychopharmacol, 2006. 21(2): p. 87–92. [DOI] [PubMed] [Google Scholar]
- 135.Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M, et al. , Association of Liver Injury From Specific Drugs, or Groups of Drugs, With Polymorphisms in HLA and Other Genes in a Genome-Wide Association Study. Gastroenterology, 2017. 152(5): p. 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Demirbugen Oz M, Uckun Z, Yuce-Artun N, Baskak B, Ozdemir H, Kizil Ozel T, et al. , The relationship between the serotonin 2A receptor gene −1438A/G and 102T/C polymorphisms and citalopram/sertraline-induced nausea in major depressed patients. Hum Psychopharmacol, 2018. 33(5): p. e2673. [DOI] [PubMed] [Google Scholar]
- 137.de Klerk OL, Nolte IM, Bet PM, Bosker FJ, Snieder H, den Boer JA, et al. , ABCB1 gene variants influence tolerance to selective serotonin reuptake inhibitors in a large sample of Dutch cases with major depressive disorder. Pharmacogenomics J, 2013. 13(4): p. 349–53. [DOI] [PubMed] [Google Scholar]
- 138.Breitenstein B, Scheuer S, Bruckl TM, Meyer J, Ising M, Uhr M, et al. , Association of ABCB1 gene variants, plasma antidepressant concentration, and treatment response: Results from a randomized clinical study. J Psychiatr Res, 2016. 73: p. 86–95. [DOI] [PubMed] [Google Scholar]
- 139.Huang X, Yu T, Li X, Cao Y, Li X, Liu B, et al. , ABCB6, ABCB1 and ABCG1 genetic polymorphisms and antidepressant response of SSRIs in Chinese depressive patients. Pharmacogenomics, 2013. 14(14): p. 1723–30. [DOI] [PubMed] [Google Scholar]
- 140.Kishi T, Yoshimura R, Kitajima T, Okochi T, Okumura T, Tsunoka T, et al. , HTR2A is associated with SSRI response in major depressive disorder in a Japanese cohort. Neuromolecular Med, 2010. 12(3): p. 237–42. [DOI] [PubMed] [Google Scholar]
- 141.Yevtushenko OO, Oros MM, and Reynolds GP, Early response to selective serotonin reuptake inhibitors in panic disorder is associated with a functional 5-HT1A receptor gene polymorphism. J Affect Disord, 2010. 123(1–3): p. 308–11. [DOI] [PubMed] [Google Scholar]
- 142.Yang Z, Ma X, Wang Y, Wang J, Xiang B, Wu J, et al. , Association of APC and REEP5 gene polymorphisms with major depression disorder and treatment response to antidepressants in a Han Chinese population. Gen Hosp Psychiatry, 2012. 34(5): p. 571–7. [DOI] [PubMed] [Google Scholar]
- 143.de Leon J, Armstrong SC, and Cozza KL, Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics, 2006. 47(1): p. 75–85. [DOI] [PubMed] [Google Scholar]
- 144.Hicks JK, Bishop JR, Sangkuhl K, Muller DJ, Ji Y, Leckband SG, et al. , Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin Pharmacol Ther, 2015. 98(2): p. 127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Swen JJ, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee AH, Mulder H, et al. , Pharmacogenetics: from bench to byte--an update of guidelines. Clin Pharmacol Ther, 2011. 89(5): p. 662–73. [DOI] [PubMed] [Google Scholar]
- 146.Bousman C, Maruf AA, and Muller DJ, Towards the integration of pharmacogenetics in psychiatry: a minimum, evidence-based genetic testing panel. Curr Opin Psychiatry, 2019. 32(1): p. 7–15. [DOI] [PubMed] [Google Scholar]
- 147.Ramsey LB, Prows CA, Zhang K, Saldana SN, Sorter MT, Pestian JP, et al. , Implementation of Pharmacogenetics at Cincinnati Children’s Hospital Medical Center: Lessons Learned Over 14 Years of Personalizing Medicine. Clin Pharmacol Ther, 2019. 105(1): p. 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hicks JK, Shealy A, Schreiber A, Coleridge M, Noss R, Natowicz M, et al. , Patient Decisions to Receive Secondary Pharmacogenomic Findings and Development of a Multidisciplinary Practice Model to Integrate Results Into Patient Care. Clin Transl Sci, 2018. 11(1): p. 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]