Abstract
Purpose:
Alcohol consumption is an established breast cancer risk factor, though further research is needed to advance our understanding of the mechanism underlying the association. We used global metabolomics profiling to identify serum metabolites and metabolic pathways that could potentially mediate the alcohol-breast cancer association.
Methods:
A cross-sectional analysis of reported alcohol consumption and serum metabolite concentrations was conducted among 211 healthy women 25-29 years old who participated in the Dietary Intervention Study in Children 2006 Follow-Up Study (DISC06). Alcohol-metabolite associations were evaluated using multivariable linear mixed effects regression.
Results:
Alcohol was significantly (FDR p<0.05) associated with several serum metabolites after adjustment for diet composition and other potential confounders. The amino acid sarcosine, the omega-3 fatty acid eicosapentaenoate (EPA), and the steroid 4-androsten-3beta,17beta-diol monosulfate were positively associated with alcohol intake, while the gamma-tocopherol metabolite gamma-carboxyethyl hydroxychroman (CEHC) was inversely associated. Positive associations of alcohol with 2-methylcitrate and 4-androsten-3beta,17beta-diol disulfate were borderline significant (FDR p<0.10). Metabolite set enrichment analysis identified steroids and the glycine pathway as having more members associated with alcohol consumption than expected by chance.
Conclusions:
Most of the metabolites associated with alcohol in the current analysis participate in pathways hypothesized to mediate the alcohol-breast cancer association including hormonal, one-carbon metabolism and oxidative stress pathways, but they could also affect risk via alternative pathways. Independent replication of alcohol-metabolite associations and prospective evaluation of confirmed associations with breast cancer risk are needed.
Keywords: Alcohol, serum metabolite, androgen, sarcosine, eicosapentaenoate
Introduction
Approximately 250,000 US women are diagnosed and 40,000 die of breast cancer annually.[5] Yet, the cause of breast cancer remains largely unknown, and of the known risk factors, few are modifiable. Alcohol consumption, an established potentially modifiable breast cancer risk factor, increases risk by approximately 7-10 percent/drink/day across all levels of intake.[3, 13, 15, 73] Risk does not differ by type of alcohol consumed – wine, beer or spirits,[3, 13, 45, 73] suggesting that it is ethanol, per se, and not another constituent that is responsible.
Alcohol-associated increased breast cancer risk is generally thought to be due to altered hormone or one-carbon metabolism, increased oxidative stress, or mutagenesis by acetaldehyde.[27] Alcohol stimulates the hypothalamic-pituitary-adrenal axis leading to increased secretion of androgens that are positively associated with breast cancer risk in both pre- and postmenopausal women.[28, 77] Alcohol also is well-known to inhibit the absorption and metabolism of key nutrients involved in one-carbon metabolism[34, 52, 83] that are critical for maintenance of DNA integrity and epigenetic regulation of gene expression.[6, 88] Ethanol is rapidly metabolized to acetaldehyde, which can lead to redox changes that result in a prooxidant state.[81] Acetaldehyde interacts with many cellular constituents including DNA and proteins and is an established potent mutagen and carcinogen.[71]
Given the plethora of alcohol’s adverse effects, metabolomics, which simultaneously quantifies levels of small molecules in multiple metabolic pathways, is ideally suited for advancing our understanding of mechanisms of alcohol’s effects on breast cancer risk. Though several studies on metabolic effects of alcohol have been performed,[33, 35, 41, 87, 91] most do not account for dietary composition, which is reported to differ between drinkers and non-drinkers.[14, 55, 65, 72] If in the causal pathway, these dietary differences could potentially mediate alcohol-metabolite associations. Alternatively, diet could confound or have no effect on these associations.
We evaluated associations of reported alcohol intake with serum metabolites in a cross-sectional analysis of women who participated in the Dietary Intervention Study in Children 2006 Follow-Up Study (DISC06). As part of this study women reported usual alcohol intake and diet was ascertained by three non-consecutive 24-hour dietary recalls. We estimated alcohol-metabolite associations both with and without adjustment for diet to obtain unbiased estimates and to assess potential for confounding. For alcohol-metabolite associations that changed following adjustment for diet, we also conducted mediation analysis.
Materials and Methods
Design
DISC was a multicenter randomized controlled clinical trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI) to test the safety and efficacy of a dietary intervention to reduce serum low-density lipoprotein cholesterol (LDL-C) in children with elevated LDL-C. The trial’s design and results have been described.[19, 21, 23–25, 57, 58, 78] Briefly, between 1988 and 1990, 663 healthy, pre-pubertal, 8-10 year old children, including 301 girls, with elevated LDL-C were recruited into DISC at six clinical centers1 and randomized to a behavioral dietary intervention or usual care control group. Planned intervention continued until 1997 when the mean age of participants was 16.7 years. In 2006-2008 when participants were 25 to 29 years old, the DISC06 follow-up study was conducted to evaluate the longer-term effects of the diet intervention on biomarkers associated with breast cancer risk in DISC female participants. Assent was obtained from DISC participants and informed consent was obtained from their parents/guardians prior to randomization. Informed consent was obtained from participants prior to the DISC06 follow-up visit. The original DISC protocol was approved by institutional review boards at all participating clinical centers and the data coordinating center.2 The DISC06 protocol was also approved by the institutional review board at the Fox Chase Cancer Center.
Participants
Eligibility criteria for girls in the original DISC were: 1)8 - 10 years old; 2) serum LDL-C in the 80th to 98th percentiles;[82] 3) no major illness or medication that could affect blood lipids or growth; 4) height ≥ 5th percentile and weight-for-height in the 5th to 90th percentile;[85] 5) Tanner stage 1 for breast and pubic hair;[76] and 6) normal psychosocial and cognitive development.[1] Girls were excluded if they or family members were following a low-fat diet, a parent had a history of early heart disease or the family planned to move. DISC participants were recruited through schools, health maintenance organizations, and pediatric practices.
All female DISC participants were invited to participate in DISC06 and 260 (86.4 percent) of the 301 females originally randomized took part. Women who were pregnant or breastfeeding at or within 12 weeks before the visit (n=30) or did not have a blood sample for metabolomics assays (n=3), were not fasting at blood donation (n=4), did not complete diet recalls (n=11) or had an implausible dietary intake (kcal=13,049/day; n=1) were not eligible for the current analysis, leaving 211 participants.
Data Collection
For the DISC06 follow-up study, each female participant attended a single visit at a DISC clinic between 2006 and 2008. Visits were scheduled to take place within 14 days of onset of next menses whenever possible. All data for a participant were collected on the same day except 24-hour dietary recalls, which were collected over two weeks following the visit. Data were collected by staff masked to treatment assignment. A centralized data collection training session was held before initiation of data collection.
Frequency of usual alcohol intake from wine, beer and spirits was ascertained by questionnaire. Otherwise, dietary data were ascertained via three nonconsecutive 24-hour dietary recalls collected by trained interviewers on two weekdays and one weekend day. Data from the three recalls were averaged to estimate usual nutrient intakes using the University of Minnesota’s Nutrition Data System for Research. Participants also completed questionnaires on demographics; medical, reproductive and menstrual histories; medications; smoking; and family history of breast cancer. Leisure physical activity was assessed using the Modifiable Activity Questionnaire.[51] Participants completed menstrual cycle calendars for up to 6 weeks following clinic visits until the start of their next menses.
Blood Sampling
Blood was collected in the morning after an overnight fast by venipuncture using standard procedures. After standing at room temperature for 45 minutes to allow complete clotting, blood was centrifuged and serum was separated and pipetted in 0.5 mL aliquots into cryovials that were stored at −80° C.
Metabolomics Assays
Global metabolomic profiling was performed by Metabolon (Durham, NC). Participants’ samples were randomly ordered and 10 percent blind quality control (QC) samples were integrated throughout to monitor laboratory performance. Additionally, a pooled matrix sample served as a technical replicate throughout analyses, extracted water samples served as process blanks, and a cocktail of QC standards spiked into every sample allowed for instrument performance monitoring and aided chromatographic alignment.
Samples were prepared using the automated MicroLab StAR system (Hamilton Co.). Proteins were precipitated with methanol under vigorous shaking for two minutes followed by centrifugation. The resulting extract was divided into five fractions for analysis by four ultra-high performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS) based methods with one sample reserved for backup. Samples were placed briefly on a TurboVap (Zymark) to remove organic solvents. The sample extracts were stored overnight under nitrogen before preparation for analysis.
The LC/MS platform was based on a Water ACQUITY UPLC and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with an electrospray ionization source and Orbitrap mass analyzer operated at 35,000 mass resolution. After drying sample extracts were reconstituted in solvents compatible with each of the four analytical methods. The first two methods used reverse phase UPLC-MS/MS with positive ion mode electrospray ionization. One chromatographically optimized for more hydrophilic compounds by gradient eluting the extract from a C18 column using water and methanol with 0.05% perfluoropentanoic acid and 0.1% formic acid. Whereas the other chromatographically optimized for more hydrophobic compounds by gradient eluting the extract from the same C18 column using methanol, acetonitrile, and water with 0.05% perfluoropentanoic acid and 0.01% formic acid. The third method used reverse phase UPLC-MS/MS with negative ion mode electrospray ionization. The basic extracts were gradient eluted from a separate dedicated C18 column using methanol and water with 6.5mM ammonium bicarbonate at pH 8. The fourth method also used negative ionization following gradient elution from a HILIC column using water and acetonitrile with 10mM ammonium formate at pH 10.8. The MS analysis alternated between MS and data-dependent MSn scans using dynamic exclusion. The scan range varied slightly between methods but covered 70-1000 m/z.
Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. Biochemical identifications were based on three criteria: retention index, accurate mass match to the library, and the MS/MS forward and reverse scores between the experimental data and authentic standards. At the time DISC06 samples were assayed, more than 3300 commercially available purified standard compounds had been acquired and characterized.
Proprietary visualization and interpretation software were used to confirm the consistency of peak identification among samples. Peaks were quantified using area-under-the-curve.
Statistical Analysis
A total of 705 named metabolites were semi-quantified as relative peak intensity. Metabolites with ≥30 percent of values less than the limit of detection or with coefficients of variation ≥25 percent calculated from masked quality control samples were dropped, leaving 449 metabolites for analysis. For metabolites with <30 percent of values below the limit of detection, undetected values were imputed at the lowest observed value multiplied by 0.75. Metabolites were transformed to the natural log scale and extreme values were winsorized using the median absolute deviation.[54]
The association of alcohol consumption with diet composition was assessed by the R2 from a linear regression model including alcohol intake (drinks/day) as the dependent variable and energy (kcal/day), fat (percent kcal/day) and carbohydrate (percent kcal/day) intakes as independent variables. Analyses also were performed replacing total fat in models with fat subtypes – saturated, polyunsaturated and monounsaturated fatty acids. Because the adjusted R2 from this model was slightly smaller than the adjusted R2 from the more parsimonious model and because associations of serum metabolites with alcohol were similar when total fat or fat subtypes were included in models, only results from simpler models including total fat are reported.
Univariate associations of individual metabolites with reported alcohol intake were evaluated using simple linear regression including each metabolite as a dependent variable and alcohol consumption as an independent continuous variable (drinks/week). Multivariable linear mixed effects regression was then used to adjust for a set of potential confounders identified a priori. Initial multivariable models included clinic as a random effect while body mass index (BMI; kg/m2), race, education, smoking, hormonal contraceptive use, parity, physical activity, menstrual cycle phase, time of blood collection and DISC treatment group were included as fixed effects. Multivariable models were then refit also including energy, fat and carbohydrate intakes to adjust for diet composition. Model details are included as a footnote to Table 2. Percentage differences in serum metabolite concentrations associated with a one drink/week increase in reported alcohol consumption were estimated from models as Δ% = (exp(β) – 1) x 100. We applied the false discovery rate (FDR) to control for multiple comparisons.[9]
Table 2.
Percent Change in Serum Metabolite Concentrations Associated with an Increase of One Alcoholic Beverage per Week for Metabolites Associated with Alcohol in a Multivariable Model at FDR p<0.10
| Unadjusteda | Multivariable Adjusted Excluding Dietb | Multivariable Adjusted Including Dietc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolite | % Change/Drink/Wk | Pvalue | FDR Pvalue | % Change/Drink/Wk | Pvalue | FDR Pvalue | % Change/Drink/Wk | Pvalue | FDR Pvalue |
| Amino Acid | |||||||||
| Glycine, Serine and Threonine Metabolism | |||||||||
| sarcosine | 3.86 | 4.05E-05 | 0.005 | 4.52 | 8.40E-06 | 0.001 | 3.89 | 3.98E-04 | 0.045 |
| Lysine Metabolism | |||||||||
| 3-methylglutarylcarnitine | −2.25 | 1.73E-02 | 0.152 | −2.58 | 3.34E-03 | 0.094 | −2.04 | 3.30E-02 | 0.463 |
| Carbohydrate | |||||||||
| Pentose Metabolism | |||||||||
| arabitol/xylitol | 0.88 | 2.05E-04 | 0.013 | 0.85 | 8.46E-04 | 0.032 | 0.82 | 2.67E-03 | 0.133 |
| Energy | |||||||||
| TCA Cycle | |||||||||
| 2-methylcitrate/homocitrate | 1.04 | 5.66E-04 | 0.020 | 1.22 | 2.31E-04 | 0.013 | 1.16 | 8.93E-04 | 0.067 |
| Lipid | |||||||||
| Medium Chain Fatty Acids | |||||||||
| 10-undecenoate (11:1n1) | 1.84 | 5.14E-05 | 0.005 | 1.65 | 4.91E-04 | 0.022 | 1.47 | 3.73E-03 | 0.159 |
| Polyunsaturated Fatty Acid (n3 and n6) | |||||||||
| eicosapentaenoate (EPA; 20:5n3) | 2.60 | 9.98E-06 | 0.001 | 2.76 | 2.27E-05 | 0.003 | 2.58 | 2.76E-04 | 0.045 |
| Inositol Metabolism | |||||||||
| myo-inositol | 0.79 | 2.92E-04 | 0.013 | 0.77 | 9.84E-04 | 0.034 | 0.50 | 4.63E-02 | 0.520 |
| Phospholipid Metabolism | |||||||||
| 1-palmitoyl-2-oleoyl-GPC (16:0/18:1) | 0.99 | 9.39E-05 | 0.007 | 0.78 | 5.28E-04 | 0.022 | 0.52 | 2.72E-02 | 0.459 |
| Steroid | |||||||||
| 4-androsten-3beta,17beta-diol monosulfate (2) | 5.34 | 1.21E-08 | <0.001 | 4.37 | 2.47E-06 | 0.001 | 3.78 | 1.64E-04 | 0.045 |
| 4-androsten-3beta,17beta-diol disulfate | 4.46 | 4.15E-07 | <0.001 | 4.18 | 4.89E-06 | 0.001 | 3.36 | 5.97E-04 | 0.054 |
| 4-androsten-3beta,17beta-diol monosulfate (1) | 1.97 | 1.35E-02 | 0.140 | 2.50 | 1.07E-03 | 0.034 | 2.01 | 1.51E-02 | 0.340 |
| etiocholanolone glucuronide | 2.86 | 1.60E-03 | 0.036 | 3.48 | 2.20E-04 | 0.013 | 3.23 | 1.71E-03 | 0.110 |
| Nucleotide | |||||||||
| 3-ureidopropionate | 1.04 | 2.41E-03 | 0.049 | 1.05 | 3.19E-03 | 0.094 | 0.96 | 1.41E-02 | 0.333 |
| Cofactors and Vitamins | |||||||||
| Tocopherol Metabolism | |||||||||
| gamma-CEHC | −2.33 | 2.36E-04 | 0.013 | −2.54 | 4.24E-05 | 0.004 | −2.40 | 3.94E-04 | 0.045 |
| Vitamin A Metabolism | |||||||||
| retinol (Vitamin A) | 1.13 | 8.76E-04 | 0.026 | 1.09 | 2.76E-04 | 0.014 | 0.84 | 9.16E-03 | 0.280 |
| Xenobiotic | |||||||||
| Food Component/Plant | |||||||||
| piperine | 3.73 | 3.90E-03 | 0.063 | 4.99 | 1.25E-04 | 0.009 | 4.04 | 3.88E-03 | 0.159 |
Results from simple linear regression of individual metabolite concentrations on alcohol intake (drinks/wk).
Results from linear mixed effects regression of individual metabolite concentrations on alcohol intake (drinks/wk) adjusted for clinic as a random effect and BMI (kg/m2;continuous), race (white/non-white), college graduate (yes/no), current smoker (no/yes≥5 cigarettes per day/yes >5 cigarettes per day), current hormonal contraceptive use (none/combined oral contraceptive pill/other), parous (yes/no), physical activity (MET·hr·wk−1;continuous), menstrual cycle phase (luteal/follicular/missing), time of blood collection (7-8am/9am/10am/11am-2pm), and DISC treatment group (intervention/usual care) as fixed effects.
Results from linear mixed effects regression of individual metabolite concentrations on alcohol intake (drinks/wk) adjusted as for b above plus energy (kcal/day; continuous), fat (%kcal/day; continuous) and carbohydrate (%kcal/day; continuous) as fixed effects.
To evaluate the potential influence of extreme values on observed associations, analyses were repeated after winsorizing reported alcohol intake using the median absolute deviation.[54]. We also conducted additional analyses restricted to non-smokers.
To further explore associations of metabolites associated with alcohol (FDR p<0.10) in diet adjusted models, analyses were repeated including alcohol as a factor with five levels.
For metabolites significantly associated with alcohol in continuous multivariable models (FDR p<0.05), we conducted mediation analysis if after adjustment for diet, the alcohol effect size decreased by at least 20 percent and was no longer significant (FDR p≥0.10). Analyses were performed using the model-based approach as implemented in R package mediation.[80] Mediation was evaluated separately for dietary fat and carbohydrates using models similar to those described above but including only fixed effects and using bootstrap variances.
Metabolite Set Enrichment Analysis (MSEA) with a one-sided Fisher’s exact test was used to identify over-represented classes of metabolites associated with reported alcohol intake.[67] Metabolites associated with alcohol at p<0.10 in diet adjusted models were considered related. Metabolite classes were defined a priori by Metabolon.
To quantify the variance of participants’ metabolite levels relative to assay variance, we divided the variance of participants’ analytical samples by the total variance, estimated as the sum of the variances of analytical samples plus 23 masked, replicate quality control aliquots from a single pool.
All tests of statistical significance were two-sided except where otherwise stated. All analyses were conducted using SAS 9.4 and R 3.5 statistical software.
Results
Participant characteristics at the DISC06 clinic visit are shown in Table 1. Ninety percent of women were white with a mean age of 27.2 ±1.1 years. Their mean BMI was 25.5 ±5.5 kg/m2; 24 percent were overweight and 19 percent were obese.[10] At the time of the visit, 26 percent of participants smoked cigarettes and 55 percent used hormonal contraceptives. By design, most (82 percent) were in the luteal phase of their menstrual cycles. All participants fasted overnight and 91 percent of blood collections took place before 11am. Reported alcohol intake ranged from 0 to 40 drinks per week with a median of 3.0 (IQR (interquartile range) = 0.9 – 6.0) drinks per week. Participants’ mean energy intake was 1735 ±487 kcal/day, with 17 percent of energy provided by protein, 50 percent by carbohydrates and 31 percent by fat. The distribution of participant characteristics across categories of alcohol intake is included as Supplemental Table 1.
Table 1.
Participant Characteristics (N=211)
| Continuous Variables | Mean | SD |
|---|---|---|
| Age, y | 27.22 | 1.06 |
| BMI (kg/m2) | 25.53 | 5.46 |
| Alcohol (drinks/wk) | 4.93 | 6.33 |
| Moderate-intense physical activity (MET·hr·wk 1) | 24.61 | 21.68 |
| Diet | ||
| Energy (kcal/day) | 1735 | 487 |
| Protein (%kcal) | 16.90 | 4.02 |
| Carbohydrate (%kcal) | 50.23 | 9.00 |
| Fat (%kcal) | 31.46 | 7.47 |
| Categorical Variables | N | % |
| Race | ||
| White | 190 | 90.05 |
| Other | 21 | 9.95 |
| Education | ||
| High school | 23 | 10.90 |
| Some college | 47 | 22.27 |
| Bachelor’s degree | 106 | 50.24 |
| Graduate school | 35 | 16.59 |
| Current Smoker | ||
| No | 156 | 73.93 |
| Yes, <5 cigarettes/day | 23 | 10.90 |
| Yes, ≥5 cigarettes/day | 32 | 15.17 |
| Full-Term Pregnancies | ||
| 0 | 156 | 73.93 |
| 1 | 32 | 15.17 |
| 2+ | 23 | 10.90 |
| Current Hormonal Contraceptive Use | ||
| None | 100 | 47.39 |
| Combined oral contraceptive pill | 88 | 41.71 |
| Other | 23 | 10.90 |
| Menstrual Cycle Phase at Blood Collection | ||
| Luteal | 172 | 81.52 |
| Follicular | 27 | 12.80 |
| Unknown | 12 | 5.69 |
| Time of Day at Blood Collection | ||
| 7-8am | 37 | 17.54 |
| 9am | 108 | 51.18 |
| 10am | 47 | 22.27 |
| 11am-2pm | 19 | 9.00 |
| DISC Treatment Group | ||
| Intervention | 107 | 50.71 |
| Usual care | 104 | 49.29 |
Diet macronutrient composition was strongly and significantly associated with alcohol intake. The adjusted R2 from a linear model that regressed reported alcohol intake (drinks/wk) on energy (kcal/day), fat and carbohydrate (percent kcal/day) intakes was 0.152 (p<0.0001). Though alcohol was not significantly associated with energy intake (β=0.0002, p=0.08), fat (β= −0.5014, p<0.0001) and carbohydrate (β= −0.4374 p<0.0001) intakes were independently significantly inversely associated with alcohol.
Table 2 presents associations of reported alcohol intake with metabolites that were associated in multivariable adjusted analysis with or without inclusion of diet. Associations of alcohol with all metabolites evaluated along with an estimate of the contribution of participant variance to total metabolite variance are included as Supplemental Table 2. Adjustment for diet composition generally attenuated alcohol-metabolite associations. Prior to adjustment for diet, 14 metabolites were significantly associated with alcohol consumption after adjustment for multiple comparisons (FDR p<0.05), while after adjustment for diet four metabolites were significantly associated with alcohol and two were borderline significant (FDR p<0.10).
Figure 1 graphically summarizes diet adjusted alcohol-metabolite associations by metabolite superclass. After adjusting for multiple comparisons, the amino acid sarcosine increased significantly by 3.89 percent/drink/wk (FDR p=0.045), whereas the tricarboxylic acid (TCA) cycle metabolite 2-methylcitrate increased slightly by 1.16 percent/drink/wk and was borderline significant (FDR p=0.067). Adjusted for multiple comparisons several lipids also increased in association with alcohol; the omega-3 fatty acid eicosapentaenoate (EPA) increased significantly by 2.58 percent/drink/wk (FDR p=0.045) and the steroids 4-androsten-3beta,17beta-diol mono- and disulfate increased by 3.78 percent /drink/wk (FDR p=0.045) and 3.36 percent/drink/wk (FDR p=0.054), respectively. In contrast, the gamma-tocopherol metabolite gamma-carboxyethyl hydroxychroman (gamma-CEHC) decreased significantly by 2.40 percent/drink/wk (FDR p=0.045). The association of alcohol with serum EPA was not materially changed by further adjustment for dietary EPA (Δ = 2.36 percent/drink/wk; p=6.08e−4), and its association with serum gamma-CEHC was unchanged by further adjustment for dietary gamma tocopherol from food and supplements (Δ = −2.39 percent/drink/wk; p=4.35 e−4). Results of the alcohol - gamma-CEHC association also were unchanged by adjustment for alpha-tocopherol from food and/or supplements or total vitamin E from food and/or supplements (data not shown).
Figure 1.
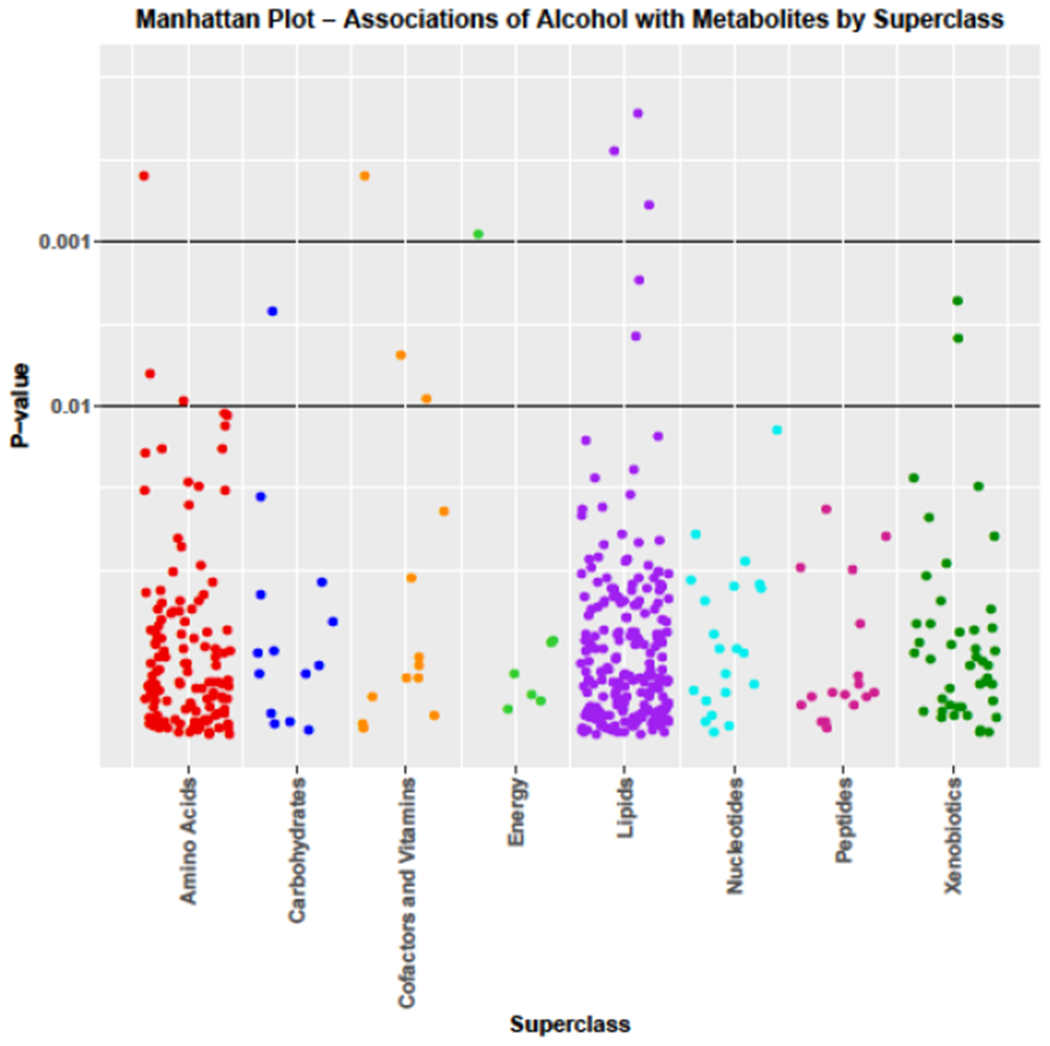
Manhattan plot associations of alcohol with metabolites by superclass
Winsorizing alcohol intake recoded ≥23 to 21.8 drinks per week. Effect sizes tended to be larger after winsorizing because of the truncated distribution of alcohol intakes. However, p-values generally were similar to those shown in Table 2, indicating results were not due to influential outliers (data not shown). Restricting analyses to non-smokers also did not change our main results materially (data not shown).
Associations of alcohol intake modeled as a factor with 5 levels and metabolites associated in continuous diet adjusted models at FDR p<0.10 are shown in Table 3. Women who consumed ≥10 drinks/week had serum sarcosine and 4-androsten-3beta,17beta-diol disulfate levels that were more than twice as high as those who consumed <1 drink/week. Their 4-androsten-3beta,17beta-diol monosulfate levels also were almost twice as high. EPA and 2-methylcitrate also were 60 and 20 percent higher, respectively, among the heaviest compared to lightest drinkers, whereas gamma-CEHC was 41 percent lower. Among drinkers serum metabolite levels changed monotonically with increasing alcohol intake except for 2-methycitrate, which was associated only at ≥10 drinks/week.
Table 3.
Multivariable Diet Adjusted Percent Change in Metabolite Concentrations for Categories of Alcohol Consumptiona
| Drinks per Week |
|||||
|---|---|---|---|---|---|
| 0 | >0 and <1 | ≥1 and <5 | ≥5 and <10 | ≥10 and ≤40 | |
| N | 17 | 41 | 81 | 46 | 26 |
| Sarcosine | |||||
| % change | −5.44 | ref | −1.90 | 21.49 | 138.02 |
| 95% CIb | −39.36, 47.43 | −27.23, 32.24 | −14.97, 73.57 | 55.52, 264.29 | |
| Pvalue | 0.815 | 0.905 | 0.312 | <0.001 | |
| Eicosapentaenoate | |||||
| % change | −1.56 | ref | 10.46 | 23.52 | 59.87 |
| 95% CI | −26.80, 32.39 | −9.50, 34.80 | −2.64, 56.71 | 20.36, 112.36 | |
| Pvalue | 0.921 | 0.355 | 0.101 | 0.002 | |
| 4-Androsten-3beta,17beta-diol monosulfate | |||||
| % change | −8.43 | ref | 6.01 | 50.68 | 97.88 |
| 95% CI | −38.58, 36.53 | −18.96, 38.66 | 9.32, 107.67 | 34.96, 190.15 | |
| Pvalue | 0.682 | 0.687 | 0.019 | 0.001 | |
| 4-Androsten-3beta,17beta-diol disulfate | |||||
| % change | 10.58 | ref | 3.13 | 53.52 | 123.64 |
| 95% CI | −26.31, 65.92 | −21.50, 35.48 | 10.82, 112.68 | 51.60, 229.93 | |
| Pvalue | 0.646 | 0.834 | 0.015 | <0.001 | |
| Gamma-CEHC | |||||
| % change | −13.26 | ref | −27.84 | −33.63 | −41.50 |
| 95% CI | −34.91, 14.89 | −40.75, −13.19 | −47.44, −17.04 | −55.72, −23.68 | |
| Pvalue | 0.355 | 0.002 | 0.001 | <0.001 | |
| 2-Methylcitrate | |||||
| % change | −4.85 | ref | −7.58 | −3.25 | 19.51 |
| 95% CI | −17.84, 9.56 | −16.03, 1.90 | −13.96, 8.41 | 4.09, 37.14 | |
| Pvalue | 0.522 | 0.133 | 0.595 | 0.017 | |
Results from linear mixed effects regression of individual metabolite concentrations on alcohol intake modeled as a factor with 5 levels and adjusted as described in Table 2 footnote c.
CI=confidence interval
Of the 14 metabolites significantly associated with alcohol at FDR p<0.05 in multivariable models before adjustment for diet, eight had an FDR p≥0.10 after adjustment, and alcohol effect sizes decreased by 20 percent or more for three of these. Results of mediation analysis shown in Table 4 suggest that dietary fat significantly (p=0.002) mediated 33 percent of the association of alcohol with the phospholipid 1-palmitoyl-2-oleoyl-GPC, whereas both fat and carbohydrate contributed to mediation of the association of alcohol with the lipid myo-inositol. However, small absolute values of the sensitivity parameter ρ when the average causal mediation effect equals 0 indicate results are sensitive to possible uncontrolled confounding.[40]
Table 4.
Mediation of Alcohol - Metabolite Associations by Dietary Fat and Carbohydrate Intakes
| Dietary Fat | Dietary Carbohydrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Proportion Mediated | ACMEa | Sensitivity | Proportion Mediated | ACMEa | Sensitivity | |||
| Metabolite | Proportion | 95% CIb | p-value | ρc | Proportion | 95% CIb | p-value | ρc |
| Lipids | ||||||||
| myo-inositol | 0.29 | 0.03 - 0.72 | 0.03 | −0.2 | 0.33 | 0.06 - 0.79 | 0.02 | −0.2 |
| 1-palmitoyl-2-oleoyl-GPC (16:0/18:1) | 0.33 | 0.11 - 0.88 | 0.002 | −0.2 | 0.29 | 0.02 - 0.87 | 0.04 | −0.2 |
| Cofactors and Vitamins | ||||||||
| retinol | 0.15 | −0.08 - 0.50 | 0.19 | −0.1 | 0.20 | −0.04 - 0.61 | 0.09 | −0.1 |
ACME = average causal mediation effects
CI = confidence interval
ρ at ACME=0
Metabolite Set Enrichment Analysis (MSEA) identified two classes of metabolites, steroids and glycine pathway metabolites as having more members associated with alcohol consumption in diet adjusted analysis than expected by chance. Seven of 19 steroid metabolites including 4-androsten-3beta,17beta-diol mono- and disulfate, 5-alpha-androstan-3beta,17beta-diol disulfate, etiocholanolone glucuronide, pregnendiol disulfate and pregnanediol-3-glucuronide were associated with alcohol (p=0.007). Similarly, 4 of 10 glycine pathway metabolites including sarcosine, N-acetylglycine, dimethylglycine and threonine were associated (p=0.03).
Discussion
Alcohol consumption was strongly associated with serum metabolites across a broad range of metabolite classes in this cross-sectional study of young adult women. Accounting for multiple comparisons, 14 metabolites were significantly associated with alcohol before diet adjustment, while after adjustment four remained significant and two were borderline significant. Results are compatible with alcohol-induced alterations in hormonal, one-carbon metabolism and oxidative stress pathways, all of which are implicated in breast cancer etiology.
Diets of drinkers and non-drinkers differ.[14, 55, 65, 72] In an analysis from NHANESIII, alcohol consumption was significantly positively associated with total energy intake but inversely associated with energy derived from protein, fat and particularly carbohydrate, suggesting that energy from alcohol replaces that from other macronutrients.[55] Even so, most studies of metabolomic profiles associated with alcohol do not adjust for diet or adjust only for energy intake. In our analysis eight metabolites that were significantly associated with alcohol before adjustment for diet composition were no longer associated after adjustment. With adjustment, alcohol effect sizes decreased by 20 percent or more for three of these metabolites, which appeared to be due to a combination of confounding and mediation.
Steroids as a class were significantly positively upregulated by alcohol. In particular, the mono- and disulfates of the testosterone precursor 4-androsten-3beta,17beta-diol were strongly and significantly positively associated with alcohol after adjustment for multiple comparisons. Two additional testosterone metabolites, 5-alpha-androstan-3beta,17beta-diol disulfate and etiocholanolone glucuronide, as well as several progesterone metabolites also were significantly positively associated with alcohol before, but not after, adjustment for multiple comparisons. Alcohol stimulates the hypothalamic-pituitary-adrenal axis leading to increased adrenal androgen production, and 4-androsten-3beta,17beta-diol was previously reported to be increased in association with alcohol.[33, 60, 91] Associations of testosterone with breast cancer risk are well established in both pre- and postmenopausal women.[28, 77] 4-Androsten-3beta,17beta-diol monosulfate also was significantly positively associated with postmenopausal breast cancer in a prospective study.[60] Thus, alcohol could potentially increase breast cancer risk by increasing circulating androgens.
To our knowledge this is the first report of significant alterations of the glycine pathway by alcohol. Sarcosine (methylglycine), an intermediate in this pathway, was strongly and significantly positively associated with alcohol intake in diet-adjusted models after accounting for multiple comparisons. N-acetylglycine and dimethylglycine also were positively associated while threonine was inversely associated with alcohol before, though not after, adjustment for multiple comparisons. We are not aware of any prior reports of an association of alcohol with serum sarcosine levels, but alcohol has been reported to increase sarcosine’s precursors choline and dimethyglycine under some conditions.[35, 62] The glycine pathway fuels one-carbon metabolism,[4] and approximately 60 percent of methyl groups required for methylation of homocysteine to methionine in one-carbon metabolism are contributed by betaine,[59] a precursor of dimethylglycine. Thus, in addition to established mechanisms,[83] alcohol could potentially inhibit one-carbon metabolism by interfering with the glycine pathway. Sarcosine is metabolized to glycine, an integral component of glutathione, which is the primary cellular antioxidant,[4] and elevated sarcosine in association with alcohol could alternatively be related to oxidative stress. We are not aware of any reports of an association of sarcosine with breast cancer risk, but breast tumor expression of sarcosine-related proteins has been reported.[11, 49, 89]
We observed higher levels of 2-methylcitrate associated with alcohol consumption. Though circulating citrate was reduced in association with alcohol consumption in two metabolomics studies,[35, 87] to our knowledge there have been no prior reports of associations with 2-methylcitrate. 2-Methylcitrate is formed by the condensation of propionyl CoA with oxaloacetate in a reaction that is catalyzed by citrate synthase and is more common to bacteria and fungi than humans.[2] In humans propionyl CoA is primarily metabolized via the vitamin B12 dependent methylmalonyl pathway,[20] though under certain circumstances, such as vitamin B12 deficiency, it can be metabolized via citrate synthase to 2-methylcitrate.[2] Alcohol decreases circulating vitamin B12,[53] and 2-methylcitrate levels are reportedly elevated in vitamin B12 deficiency.[37, 43] Even so, the magnitude of the association of alcohol with 2-methylcitrate in our study was small and should be interpreted cautiously.
In the current analysis gamma-CEHC, a gamma-tocopherol metabolite, was significantly inversely associated with alcohol intake, and the association was unchanged by adjustment for dietary gamma-tocopherol. Alcohol is rapidly oxidized to acetaldehyde, which leads to oxidative stress as a consequence of increased free radical generation and depletion of antioxidant defenses.[16] Tocopherols are one of the primary defense systems acting by preventing the generation of free radicals.[44, 84] Gamma-tocopherol also is anti-inflammatory, and its CEHC derivative acts as an antioxidant and anti-inflammatory as well.[38, 42] Plasma tocopherols generally are not associated[30, 31, 60] or are positively associated with alcohol consumption in cross sectional studies.[86, 92] In a controlled feeding study, consumption of two alcoholic beverages per day increased serum alpha-tocopherol but gamma-tocopherol was unchanged.[36] Gamma-tocopherol has been shown to inhibit development of estrogen dependent mammary tumors in animal models.[74] However, in most prospective epidemiologic studies gamma-tocopherol is not significantly associated with breast cancer,[7, 26, 29, 68, 75] with the exception of one that observed a positive association.[46] In a recent prospective metabolomics study,[60] serum gamma-CEHC glucuronide also was significantly positively associated with postmenopausal breast cancer risk. Thus, even though the biology of gamma-CEHC suggests our observed lower serum levels with alcohol consumption could potentially increase breast cancer risk, this is not supported by empirical evidence. In a small reproducibility study, the intraclass correlation coefficient for serum gamma-CEHC measured in samples collected twice, approximately one year apart, from 15 premenopausal women was 0.27 (unpublished data), suggesting poor reproducibility over time, which could contribute to inconsistent findings.
EPA was significantly positively associated with alcohol consumption in the current analysis, and the association was not altered by adjustment for EPA intake from food and supplements. EPA was previously positively associated with moderate alcohol consumption.[17, 18] Though these studies suggest the association may be specific to wine,[17, 18] we only ascertained usual total alcohol intake. EPA is an omega-3 fatty acid plentiful in fish, a component of the Mediterranean diet associated with decreased cancer risk and mortality at several sites including the breast.[69] Though plasma EPA levels generally are not associated with breast cancer risk,[8, 12, 61, 66] most prospective studies support a protective effect of dietary EPA or marine omega-3 fatty acids for breast cancer.[8, 50, 70, 90] Increased serum EPA may counter some of alcohol’s adverse health effects while contributing to some of its beneficial effects when consumed in moderation.
Our study had several strengths. The sample size was reasonably large and participants, who were all women, reported a wide range of usual alcohol intake. Data on potential confounders were ascertained concurrently with alcohol intake. Usual diet was assessed by three nonconsecutive 24-hour recalls, the gold standard for population research.[79] Participants fasted overnight prior to donating blood, which was processed following a standardized protocol, and metabolomics assays were performed by Metabolon, a leader in the field. There also were some limitations. All participants had elevated serum LDL-C at baseline[82] and met several additional eligibility criteria, which could limit generalizability of findings. Even so, at the DISC06 follow-up visit only 16 (7.6%) had elevated serum LDL-C according to current guidelines[32] and one was taking cholesterol lowering medication. Usual alcohol intake was self-reported, which could have led to misclassification of exposure, since people tend to under-report intake,[56] but if misclassification occurred it would likely have been non-differential. Metabolomics assays were performed using a single blood sample from each participant. Even though we adjusted for menstrual cycle phase as well as time of day of blood collection in addition to multiple potential confounders in analysis, other sources of variation could have inflated variances and diminished power. The Metabolon platform does not include estrogens, steroid hormones that are associated with breast cancer risk[28, 47] and previously have been associated with alcohol consumption in both pre- and postmenopausal women.[22, 39, 48, 63, 64] Finally, the cross-sectional study design limits our ability to infer causality.
In summary, the current analysis identified several novel metabolites associated with alcohol consumption. Most participate in pathways hypothesized to mediate the alcohol-breast cancer association including hormonal, one-carbon metabolism and oxidative stress pathways, but they could also affect risk via alternative pathways. Independent replication of alcohol-metabolite associations and prospective evaluation of confirmed associations with breast cancer risk are needed.
Supplementary Material
Acknowledgements:
We thank all DISC06 participants.
Funding: This work was supported by the National Institutes of Health (R01CA104670, P30CA134274) and the Maryland Department of Health’s Cigarette Restitution Fund Program. The sponsors had no role in the study design, analysis, collection and interpretation of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Children’s Hospital, New Orleans, LA; Johns Hopkins Hospital, Baltimore, MD, Kaiser Permanente Center for Health Research, Portland, OR; University of Medicine and Dentistry of New Jersey, Newark, NJ; Northwestern University Medical School, Chicago, IL; University of Iowa Hospital and Clinics, Iowa City, IA.
Maryland Medical Research Institute, Baltimore, MD
Disclosures: The authors declare no potential conflicts of interest.
References
- 1.Achenbach TM, Edelbrook C (1983) Manual for the Child Behavior Checklist. University of Vermont, Burlington (VT) [Google Scholar]
- 2.Al Dhahouri N, Langhans C, Al Hammadi Z, JG O, Hoffmann G, Al-Jasmi F, Al-Dirbashi O (2018) Quantification of methylcitrate in dried urine spots by liquid chromatography tandem mass spectrometry for the diagnosis of propionic and methylmalonic acidemias. Clin Chim Acta 487:41–45 [DOI] [PubMed] [Google Scholar]
- 3.Allen N, Beral V, Casabonne D, Kan S, Reeves G, Brown A, Green J, Collaborators oboMWS (2009) Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst 101:296–305 [DOI] [PubMed] [Google Scholar]
- 4.Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G (2014) Serine and glycine metabolism in cancer. Trends Biochem Sci 39:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society (2017) Cancer Facts & Figures 2017. [Google Scholar]
- 6.Anderson O, Sant K, Dolinoy D (2012) Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem 23:853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakker M, Peeters P, Klaasen V, Bueno-de-Mesquita H, Jansen E, Ros M, Travier N, Olsen A, Tjønneland A, Overvad K, Rinaldi S, Romieu I, Brennan P, Boutron-Ruault M, Perquier F, Cadeau C, Boeing H, Aleksandrova K, Kaaks R, Kühn T, Trichopoulou A, Lagiou P, Trichopoulos D, Vineis P, Krogh V, Panico S, Masala G, Tumino R, Weiderpass E, Skeie G, Lund E, Quirós J, Ardanaz E, Navarro C, Amiano P, Sánchez M, Buckland G, Ericson U, Sonestedt E, Johansson M, Sund M, Travis R, Key T, Khaw K, Wareham N, Riboli E, van Gils C (2016) Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 103:454–464 [DOI] [PubMed] [Google Scholar]
- 8.Bassett J, Hodge A, English D, MacInnis R, Giles G (2016) Plasma phospholipids fatty acids, dietary fatty acids, and breast cancer risk. Cancer Causes Control 27:759–773 [DOI] [PubMed] [Google Scholar]
- 9.Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Statist Soc Ser B 1:289–300 [Google Scholar]
- 10.Centers for Disease Control and Prevention (2017) Healthy Weight. [Google Scholar]
- 11.Cha Y, Jung W, Cho N, Koo J (2015) Expression of sarcosine metabolism-related proteins in invasive lobular carcinoma: comparison to invasive ductal carcinoma. Yonsei Med J 56:598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chajès V, Assi N, Biessy C, Ferrari P, Rinaldi S, Slimani N, Lenoir G, Baglietto L, His M, Boutron-Ruault M, Trichopoulou A, Lagiou P, Katsoulis M, Kaaks R, Kühn T, Panico S, Pala V, Masala G, Bueno-de-Mesquita H, Peeters P, van Gils C, Hjartåker A, Standahl Olsen K, Borgund Barnung R, Barricarte A, Redondo-Sanchez D, Menéndez V, Amiano P, Wennberg M, Key T, Khaw K, Merritt M, Riboli E, Gunter M, Romieu I (2017) A prospective evaluation of plasma phospholipid fatty acids and breast cancer risk in the EPIC study. Ann Oncol 28:2836–2842 [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Rosner B, Hankinson S, Colditz G, Willett W (2011) Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 306:1884–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colditz G, Giovannucci E, Rimm E, Stampfer M, Rosner B, Speizer F, Gordis E, Willett W (1991) Alcohol intake in relation to diet and obesity in women and men. Am J Clin Nutr 54:49–55 [DOI] [PubMed] [Google Scholar]
- 15.Collaborative Group on Hormonal Factors in Breast Cancer (2002) Alcohol, tobacco and breast cancer - collaborative reanalysis of individual data from 53 epidemiologic studies, including 58515 women with breast cancer and 95067 women without the disease. Br J Cancer 87:1234–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das S, Vasudevan D (2007) Alcohol-induced oxidative stress. Life Sci 81:177–187 [DOI] [PubMed] [Google Scholar]
- 17.de Lorgeril M, Salen P, Martin J, Boucher F, de Leiris J (2008) Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: a fish-like effect of moderate wine drinking. Am Heart J 155:175–181 [DOI] [PubMed] [Google Scholar]
- 18.di Giuseppe R, de Lorgeril M, Salen P, Laporte F, Di Castelnuovo A, Krogh V, Siani A, Arnout J, Cappuccio F, van Dongen M, Donati M, de Gaetano G, Iacoviello L, European Collaborative Group of the IMMIDIET Project (2009) Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am J Clin Nutr 89:354–362 [DOI] [PubMed] [Google Scholar]
- 19.DISC Collaborative Research Group (1993) Dietary intervention study in children (DISC) with elevated low-density-lipoprotein cholesterol. Design and baseline characteristics. Ann Epidemiol 3:393–402. [DOI] [PubMed] [Google Scholar]
- 20.Dolan S, Wijaya A, Geddis S, Spring D, Silva-Rocha R, Welch M (2018) Loving the poison: methylcitrate and bacterial pathogenesis. Microbiology 164:251–259 [DOI] [PubMed] [Google Scholar]
- 21.Dorgan J, Liu L, Barton B, Deshmukh S, Snetselaar L, Van Horn L, Stevens V, Robson A, Lasser N, Himes J, Shepherd J, Pourfarzib R, Pettee Gabriel K, Kriska A, Kwiterovich P (2011) Adolescent diet and metabolic syndrome in young women: results of the Dietary Intervention Study in Children (DISC) Follow-Up Study. J Clin Endocrinol Metab 96:E1999–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorgan JF, Baer DJ, Albert PS, Judd JT, Brown ED, Corle DK, Campbell WS, Hartman TJ, Tejpar AA, Clevidence BA, Giffen CA, Chandler DW, Stanczyk FZ, Taylor PR (2001) Serum hormones and the alcohol-breast cancer association in postmenopausal women. J Natl Cancer Inst 93:710–715 [DOI] [PubMed] [Google Scholar]
- 23.Dorgan JF, Hunsberger SA, McMahon RP, Kwiterovich PO Jr., Lauer RM, Van Horn L, Lasser NL, Stevens VJ, Friedman LA, Yanovski JA, Greenhut SF, Chandler DW, Franklin FA, Barton BA, Buckman DW, Snetselaar LG, Patterson BH, Schatzkin A, Taylor PR (2003) Diet and sex hormones in girls: findings from a randomized controlled clinical trial. J Natl Cancer Inst 95:132–141 [DOI] [PubMed] [Google Scholar]
- 24.Dorgan JF, McMahon RP, Friedman LA, Van Horn L, Snetselaar LG, Kwiterovich PO Jr., Lauer RM, Lasser NL, Stevens VJ, Robson A, Cooper SF, Chandler DW, Franklin FA, Barton BA, Patterson BH, Taylor PR, Schatzkin A (2006) Diet and sex hormones in boys: findings from the dietary intervention study in children. J Clin Endocrinol Metab 91:3992–3996 [DOI] [PubMed] [Google Scholar]
- 25.Dorgan JF, Liu L, Klifa C, Hylton N, Shepherd JA, Stanczyk FZ, Snetselaar LG, Van Horn L, Stevens VJ, Robson A, Kwiterovich PO Jr., Lasser NL H JH, Pettee Gabriel K, Kriska A, Ruder EH, Fang CY, Barton BA (2010) Adolescent diet and subsequent serum hormones, breast density, and bone mineral density in young women: results of the Dietary Intervention Study in Children. Cancer Epidemiol Biomarkers Prev 19:1545–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorjgochoo T, Gao Y, Chow W, Shu X, Li H, Yang G, Cai Q, Rothman N, Cai H, Franke A, Zheng W, Dai Q (2009) Plasma carotenoids, tocopherols, retinol and breast cancer risk: results from the Shanghai Women Health Study (SWHS). Breast Cancer Res Treat 117:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumitrescu R, Shields P (2005) The etiology of alcohol-induced breast cancer. Alcohol 35:213–225 [DOI] [PubMed] [Google Scholar]
- 28.Hormones Endogenous and Breast Cancer Collaborative Group, Key T, Appleby P, Reeves G, Travis R, Alberg A, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton L, Dorgan J, Dossus L, Dowsett M, Eliassen A, Fortner R, Hankinson S, Helzlsouer K, Hoff man-Bolton J, Comstock G, Kaaks R, Kahle L, Muti P, Overvad K, Peeters P, Riboli E, Rinaldi S, Rollison D, Stanczyk F, Trichopoulos D, Tworoger S, Vineis P (2013) Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 14:1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epplein M, Shvetsov Y, Wilkens L, Franke A, Cooney R, Le Marchand L, Henderson B, Kolonel L, Goodman M (2009) Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the Multiethnic Cohort Study: a nested case-control study. Breast Cancer Res 11:R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faure H, Preziosi P, Roussel A, Bertrais S, Galan P, Hercberg S, Favier A (2006) Factors influencing blood concentration of retinol, alpha-tocopherol, vitamin C, and beta-carotene in the French participants of the SU.VI.MAX trial. Eur J Clin Nutr 60:706–717 [DOI] [PubMed] [Google Scholar]
- 31.Galan P, Viteri F, Bertrais S, Czernichow S, Faure H, Arnaud J, Ruffieux D, Chenal S, Arnault N, Favier A, Roussel A, Hercberg S (2005) Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr 59:1181–1190 [DOI] [PubMed] [Google Scholar]
- 32.Grundy S, Stone N, Bailey A, Beam C, Birtcher K, Blumenthal R, Braun L, de Ferranti S, Faiella-Tommasino J, Forman D, Goldberg R, Fleidenreich P, Hlatky M, Jones D, Lloyd-Jones D, Lopez-Pajares N, Ndumele C, Orringer C, Peralta C, Saseen J, Smith SJ, Sperling L, Virani S, Yeboah J (2019) 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary. Circulation 139:el046–1081 [DOI] [PubMed] [Google Scholar]
- 33.Guertin K, Moore S, Sampson J, Fluang W, Xiao Q, Stolzenberg-Solomon R, Sinha R, Cross A (2014) Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr 100:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flamid A, Wani N, Kaur J (2009. ) New perspectives on folate transport in relation to alcoholism-induced folate malabsorption-association with epigenome stability and cancer development. FEBSJ 276:2175–2191 [DOI] [PubMed] [Google Scholar]
- 35.Flarada S, Takebayashi T, Kurihara A, Akiyama M, Suzuki A, Flatakeyama Y, Sugiyama D, Kuwabara K, Takeuchi A, Okamura T, Nishiwaki Y, Tanaka T, Flirayama A, Sugimoto M, Soga T, Tomita M (2016) Metabolomic profiling reveals novel biomarkers of alcohol intake and alcohol-induced liver injury in community-dwelling men. Env Health Prev Med 21:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flartman TJ, Baer DJ, Graham LB, Stone WL, Gunter EW, Parker CE, Albert PS, Dorgan JF, Clevidence BA, Campbell WS, Tomer KB, Judd JT, Taylor PR (2005) Moderate alcohol consumption and levels of antioxidant vitamins and isoprostanes in postmenopausal women. Eur J Clin Nutr 59:161–168 [DOI] [PubMed] [Google Scholar]
- 37.Henning B, Tepel M, Riezler R, Naurath H (2001) Long-term effects of vitamin B(12), folate, and vitamin B(6) supplements in elderly people with normal serum vitamin B(12) concentrations. Gerontology 47:30–35 [DOI] [PubMed] [Google Scholar]
- 38.Hensley K, Benaksas E, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye Q, Stoddard M, Wallis G, Williamson K, West M, Wechter W, Floyd R (2004) New perspectives on vitamin E: gamm-tocopherol and carboxyethylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med 36:1–15 [DOI] [PubMed] [Google Scholar]
- 39.Hirko K, Spiegelman D, Willett W, Hankinson S, Eliassen A (2014) Alcohol consumption in relation to plasma sex hormones, prolactin, and sex hormone-binding globulin in premenopausal women. Cancer Epidemiol Biomarkers and Prev 23:2943–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imai K, Keele L, Yamamoto T (2010) Identification, inference and sensitivity analysis for causal mediation effects. Statistical Science 25:51–71 [Google Scholar]
- 41.Jaremek M, Yu Z, Mangino M, Mittelstrass K, Prehn C, Singmann P, Xu T, Dahmen N, Weinberger K, Suhre K, Peters A, Döring A, Hauner H, Adamski J, IIIig T, Spector T, Wang-Sattler R (2013) Alcohol-induced metabolomic differences in humans. Transl Psychiatry 3:e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Q, Christian S, Shigenaga M, Ames B (2001) gamma-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 74:714–722 [DOI] [PubMed] [Google Scholar]
- 43.Johnson M, Hausman D, Davey A, Poon L, Allen R, Stabler S, Georgia Centarian Study (2010) Vitamin B12 deficiency in African American and White octogenarians and centenarians in Georgia. J Nutr Health Aging 14:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ju J, Picinich S, Yang Z, Zhao Y, Suh N, Kong A, Yang C (2010) Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 31:533–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung S, Wang M, Anderson K, Baglietto L, Bergkvist L, Bernstein L, van den Brandt PA, Brinton L, Buring JE, Eliassen AH, Falk R, Gapstur SM, Giles GG, Goodman G, Hoffman-Bolton J, Horn-Ross PL, Inoue M, Kolonel LN, Krogh V, Lof M, Maas P, Miller AB, Neuhouser ML, Park Y, Robien K, Rohan TE, Scarmo S, Schouten LJ, Sieri S, Stevens VL, Tsugane S, Visvanathan K, Wilkens LR, Wolk A, Weiderpass E, Willett WC, Zeleniuch-Jacquotte A, Zhang SM, Zhang X, Ziegler RG, Smith-Warner SA (2016) Alcohol consumption and breast cancer risk by estrogen receptor status: in a pooled analysis of 20 studies. Int J Epidemiol 45:916–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kabat G, Kim M, Adams-Campbell L, Caan B, Chlebowski R, Neuhouser M, Shikany J, Rohan T, WHI Investigators (2009) Longitudinal study of serum carotenoid, retinol, and tocopherol concentrations in relation to breast cancer risk among postmenopausal women. Am J Clin Nutr 90:162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Key T, Appleby P, Barnes I, Reeves G (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94:606–616 [DOI] [PubMed] [Google Scholar]
- 48.Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, Rollison DE, Dorgan JF, Brinton LA, Overvad K, Kaaks R, Trichopoulou A, Clavel-Chapelon F, Panico S, Duell EJ, Peeters PH, Rinaldi S, Fentiman IS, Dowsett M, Manjer J, Lenner P, Hallmans G, Baglietto L, English DR, Giles GG, Hopper JL, Severi G, Morris HA, Hankinson SE, Tworoger SS, Koenig K, Zeleniuch-Jacquotte A, Arslan AA, Toniolo P, Shore RE, Krogh V, Micheli A, Berrino F, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Lui LY, Cummings SR, Gunter MJ, Rohan TE, Strickler HD (2011) Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer 105:709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim M, Jung W, Koo J (2015) Expression of sarcosine metabolism-related proteins in estrogen receptor negative breast cancer according to the androgen receptor and HER-2 status. Int J Clin Exp Pathol 8:7967–7977 [PMC free article] [PubMed] [Google Scholar]
- 50.Kiyabu G, Inoue M, Saito E, Abe S, Sawada N, Ishihara J, Iwasaki M, Yamaji T, Shimazu T, Sasazuki S, Shibuya K, Tsugane S, JPHC Study Group (2015) Fish, n - 3 polyunsaturated fatty acids and n - 6 polyunsaturated fatty acids intake and breast cancer risk: the Japan Public Health Center-based prospective study. Int J Cancer 137:2915–2926 [DOI] [PubMed] [Google Scholar]
- 51.Kriska AM (1997) Historical Leisure Activity Questionnaire. Med Sci Sport Exerc 29:S43–S45 [Google Scholar]
- 52.Kruman I, Fowler A (2014) Impaired one carbon metabolism and DNA methylation in alcohol toxicity. J Neurochem 129:770–780 [DOI] [PubMed] [Google Scholar]
- 53.Laufer EM, Hartman TJ, Baer DJ, Gunter EW, Dorgan JF, Campbell WS, Clevidence BA, Brown ED, Albanes D, Judd JT, Taylor PR (2004) Effects of moderate alcohol consumption on folate and vitamin B(12) status in postmenopausal women. Eur J Clin Nutr 58:1518–1524 [DOI] [PubMed] [Google Scholar]
- 54.Leys C, Ley C, Klein O, Bernard P, Licata L (2013) Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. JESP 49:764–766 [Google Scholar]
- 55.Liangpunsakul S (2010) Relationship between alcohol intake and dietary pattern: findings from NHANES III. World J Gastroenterol 16:4055–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson D, Naimi T, Brewer R, Roeber J (2010) US state alcohol sales compared to survey data, 1993–2006. Addiction 105:1589–1596 [DOI] [PubMed] [Google Scholar]
- 57.Obarzanek E, Hunsberger S, Van Horn L, Hartmuller V, Barton B, Stevens V, Kwiterovich PJ, Franklin F, Kimm S, Lasser N, Simons-Morton D, Lauer R (1997) Safety of a fat-reduced diet: the Dietary Intervention Study in Children (DISC). Pediatrics 100:51–59 [DOI] [PubMed] [Google Scholar]
- 58.Obarzanek E, Kimm S, Barton B, Van Horn L, Kwiterovich PJ, Simons-Morton D, Hunsberger S, Lasser N, Robson A, Franklin FJ, Lauer R, Stevens V, Friedman L, Dorgan J, Greenlick M (2001) Long-term safety and efficacy of a cholesterol-lowering diet in children with elevated low-density lipoprotein cholesterol: seven-year results of the Dietary Intervention Study in Children (DISC). Pediatrics 107:256–264 [DOI] [PubMed] [Google Scholar]
- 59.Pellanda H (2013) Betaine homocysteine methyltransferase (BHMT)-dependent remethylation pathway in human healthy and tumoral liver. Clin Chem Lab Med 51:617–621 [DOI] [PubMed] [Google Scholar]
- 60.Playdon M, Ziegler R, Sampson J, Stolzenberg-Solomon R, Thompson H, Irwin M, Mayne S, Hoover R, Moore S (2017) Nutritional metabolomics and breast cancer risk in a prospective study. Am J Clin Nutr 106:637–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pouchieu C, Chajès V, Laporte F, Kesse-Guyot E, Galan P, Hercberg S, Latino-Martel P, Touvier M (2014) Prospective associations between plasma saturated, monounsaturated and polyunsaturated fatty acids and overall and breast cancer risk - modulation by antioxidants: a nested case-control study. PLoS One 9:e90442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajdl D, Racek J, Trefil L, Stehlik P, Dobra J, Babuska V (2016) Effect of folic acid, betaine, vitamin B6, and vitamin B12 on homocysteine and dimethylglycine levels in middle-aged men drinking white wine. Nutrients 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reichman ME, Judd JT, Longcope C, Schatzkin A, Clevidence BA, Nair PP, Campbell WS, Taylor PR (1993) Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst 85:722–727. [DOI] [PubMed] [Google Scholar]
- 64.Rinaldi S, Peeters P, Bezemer I, Dossus L, Biessy C, Sacerdote C, Berrino F, Panico S, Palli D, Tumino R, Khaw K, Bingham S, Allen N, Key T, Jensen M, Overvad K, Olsen A, Tjonneland A, Amiano P, Ardanaz E, Agudo A, Martinez-Garcia C, Quiros J, Tormo M, Nagel G, Linseisen J, Boeing H, Schulz M, Grobbee D, Bueno-de-Mesquita H, Koliva M, Kyriazi G, Thrichooulou A, Boutron-Ruault M, Clavel-Chapelon F, Ferrari P, Slimani N, Saracci R, Riboli E, Kaaks R (2006) Relationship of alcohol intake and sex steroid concentrations in blood in pre- and post-menopausal women: the European Prospective Investigation into Cancer and Nutrition. Cancer Causes & Control 17 [DOI] [PubMed] [Google Scholar]
- 65.Ruidavets J, Bataille V, Dallongeville J, Simon C, Bingham A, Amouyel P, Arveiler D, Ducimetière P, Ferrieres J (2004) Alcohol intake and diet in France, the prominent role of lifestyle. Eur Heart J 25:1153–1162 [DOI] [PubMed] [Google Scholar]
- 66.Saadatian-Elahi M, Toniolo P, Ferrari P, Goudable J, Arslan A, Zeleniuch-Jacquotte A, Riboli E (2002) Serum fatty acids and risk of breast cancer in a nested case-control study of the New York University Women’s Health Study. Cancer Epidemiol Biomarkers Prev 11:1353–1360 [PubMed] [Google Scholar]
- 67.Sas K, Karnovsky A, Michailidis G, Subramaniam P (2015) Metabolomics and diabetes: analytical and computational approaches. Diabetes 64:718–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sato R, Helzlsouer K, Alberg A, Hoffman S, Norkus E, Comstock G (2002) Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 11:451–457 [PubMed] [Google Scholar]
- 69.Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G (2017) Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients 9:E1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sczaniecka A, Brasky T, Lampe J, Patterson R, White E (2012) Dietary intake of specific fatty acids and breast cancer risk among postmenopausal women in the VITAL cohort. Nutr Cancer 64:1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seitz H, Stickel F (2007) Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 7:599–612 [DOI] [PubMed] [Google Scholar]
- 72.Sieri S, Krogh V, Saieva C, Grobbee D, Bergmann M, Rohrmann S, Tjønneland A, Ferrari P, Chloptsios Y, Dilis V, Jenab M, Linseisen J, Wallstrüm P, Johansson I, Chirlaque M, Sanchez M, Niravong M, Clavel-Chapelon F, Welch A, Allen N, Bueno-de-Mesquita H, van der Schouw Y, Sacerdote C, Panico S, Parr C, Braaten T, Olsen A, Jensen M, Bingham S, Riboli E, Slimani N (2009) Alcohol consumption patterns, diet and body weight in 10 European countries. Eur J Clin Nutr 63:S81–100 [DOI] [PubMed] [Google Scholar]
- 73.Smith-Warner SA, Spiegelman D, Yaun SS, van den Brandt PA, Folsom AR, Goldbohm RA, Graham S, Holmberg L, Howe GR, Marshall JR, Miller AB, Potter JD, Speizer FE, Willett WC, Wolk A, Hunter DJ (1998) Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 279:535–540 [DOI] [PubMed] [Google Scholar]
- 74.Smolarek A, So J, Burgess B, Kong A, Reuhl K, Lin Y, WJ S, Li G, Lee M, Chen Y, Yang C, Suh N (2012) Dietary administration of delta- and gamma-tocopherol inhibits tumorigenesis in the animal model of estrogen receptor-positive, but not HER-2 breast cancer. Cancer Prev Res 5:1310–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tamimi R, Hankinson S, Campos H, Spiegelman D, Zhang S, Colditz G, Willett W, Hunter D (2005) Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am J Epidemiol 161:153–160 [DOI] [PubMed] [Google Scholar]
- 76.Tanner JM (1962) Growth at adolescence., 2nd edn Blackwell Scientific, Oxford (U.K.) [Google Scholar]
- 77.The Endogenous Hormones and Breast Collaborative Group (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94:606–616 [DOI] [PubMed] [Google Scholar]
- 78.The Writing Group for the DISC Collaborative Research Group (1995) Efficacy and safety of lowering dietary intake of fat and cholesterol in children with elevated low-density lipoprotein cholesterol. The Dietary Intervention Study in Children (DISC). JAMA 273:1429–1435. [DOI] [PubMed] [Google Scholar]
- 79.Thompson F, Kirkpatrick S, Subar A, Reedy J, Schap T, Wilson M, Krebs-Smith S (2015) The National Cancer Institute’s Dietary Assessment Primer: A resource for diet research. J Acad Nutr Diet 115:1986–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K (2014) mediation: R Package for Causal Mediation Analysis. J Stat Softw 59 [Google Scholar]
- 81.Triano E, Slusher L, Atkins T, Beneski J, Gestl S, Zolfaghari R, Polavarapu R, Frauenhoffer E, Weisz J (2003) Class I alcohol dehydrogenase is highly expressed in normal human mammary epithelium but not in invasive breast cancer: implications for breast carcinogenesis. Cancer Res 63:3092–3100 [PubMed] [Google Scholar]
- 82.Public Health Service US (1980) Lipid research clinics population studies data book, I: the prevalence study. U.S. Department of Health and Human Services, Public Health Service, Bethesda (MD) [Google Scholar]
- 83.Varela-Rey M, Woodhoo A, Martinez-Chantar M, Mato J, Lu S (2013) Alcohol, DNA methylation, and cancer. Alcohol Res 35:25–35 [PMC free article] [PubMed] [Google Scholar]
- 84.Wagner K, Kamal-Eldin A, Elmadfa I (2004) Gamma-tocopherol - an underestimated vitamin. Ann Nutr Metab 48:169–188 [DOI] [PubMed] [Google Scholar]
- 85.Weber L (1987) Bogalusa Heart Study height and weight percentiles.
- 86.Woodside J, Young I, Gilchrist S, Vioque J, Chakravarthy U, de Jong P, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling J, Fletcher A (2013) Factors associated with serum/plasma concentrations of vitamins A, C, E and carotenoids in older people throughout Europe: the EUREYE study. Eur J Nutr 52:1493–1501 [DOI] [PubMed] [Google Scholar]
- 87.Würtz P, Cook S, Wang Q, Tiainen M, Tynkkynen T, Kangas A, Soininen P, Laitinen J, Viikari J, Kähönen M, Lehtimäki T, Perola M, Blankenberg S, Zeller T, Männistö S, Salomaa V, Järvelin M, Raitakari O, Ala-Korpela M, Leon D (2016) Metabolic profiling of alcohol consumption in 9778 young adults. Int J Epidemiol 45:1493–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu X, Chen J (2009) One-carbon metabolism and breast cancer: an epidemiological perspective. J Genet Genomics 36:203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoon J, Kim D, Koo J (2014) Implications of differences in expression of sarcosine metabolism-related proteins according to the molecular subtype of breast cancer. J Transl Med 12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng JS, Hu XJ, Zhao YM, Yang J, Li D (2013) Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. Bmj 346:f3706. [DOI] [PubMed] [Google Scholar]
- 91.Zheng Y, Yu B, Alexander D, Steffen L, Nettleton J, Boerwinkle E (2014) Metabolomic patterns and alcohol consumption in African Americans in the Atherosclerosis Risk in Communities Study. Am J Clin Nutr 99:1470–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zou Y, Wang D, Sakano N, Sato Y, Iwanaga S, Taketa K, Kubo M, Takemoto K, Masatomi C, Inoue K, Ogino K (2014) Associations of serum retinol, α-tocopherol, and γ-tocopherol with biomarkers among healthy Japanese men. Int J Environ Res Public Health 11:1647–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


