Summary:
Objective.
Laryngeal endoscopy with stroboscopy, a critical component of the assessment of voice disorders, is rarely used as a treatment outcome measure in the scientific literature. We hypothesized that this is because of the lack of a widely used standardized, validated, and reliable method to assess and report laryngeal anatomy and physiology, and undertook a systematic literature review to determine the extent of the inconsistencies of the parameters and scales used in voice treatment outcome studies.
Study Design.
Systematic literature review.
Methods.
We searched PubMed, Ovid, and Cochrane for studies where laryngeal endoscopy with stroboscopy was used as a treatment outcome measure with search terms representing “stroboscopy” and “treatment” guided by Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement standards.
Results.
In the 62 included articles, we identified 141 terms representing 49 different parameters, which were further classified into 20 broad categories. The six most common parameters were magnitude of glottal gap, mucosal wave amplitude, location or shape of glottal gap, regularity of vibration, phase symmetry, and presence and size of specific lesions. Parameters were assessed on scales ranging from binary to 100 points. The number of scales used for each parameter varied from 1 to 24, with an average of four different scales per parameter.
Conclusions.
There is a lack of agreement in the scientific literature regarding which parameters should be assessed to measure voice treatment outcomes and which terms and scales should be used for each parameter. This greatly diminishes comparison and clinical implementation of the results of treatment outcomes research in voice disorders. We highlight a previously published tool and recommend it for future use in research and clinical settings.
Keywords: Voice, Stroboscopy, Rating, Outcomes, Standardization
INTRODUCTION
Despite being the only direct means of assessing the anatomic and physiological impairments underlying diminished voice quality, laryngeal endoscopy with stroboscopy (LES) is rarely used as a treatment outcome measure in research articles.1 This is in direct contrast to the use of LES in the clinical care of patients with voice disorders. Voice clinicians frequently rely on LES to determine the underlying pathophysiology of a voice disorder and guide treatment recommendations.2 It is well established that type of lesion and specific aspects of vocal fold vibration (eg, mucosal wave amplitude) cannot be determined via other commonly used types of assessment, such as perceptual judgment of voice quality or acoustic analysis, and that LES is imperative for the accurate assessment of voice disorders.
One reason for the rarity of the use of LES as a treatment outcome measure may be the lack of a broadly used standardized, validated, and reliable method to assess laryngeal anatomy and physiology. Thus, in preparation for a clinical trial of voice therapy techniques, one must either determine what is of interest based on the study aims or conduct a time-consuming review of the literature to understand what laryngeal parameters are commonly being assessed and reported. Once the parameters for assessment have been determined, the next decision is what scale to use for the judgment of these features. Once again, the determination is made either with or without the benefit of prior literature. These methods are highly likely to lead to inconsistencies across studies with different features being judged from LES and different scales used to report the features, causing the results of studies to be incomparable and limiting their usefulness for application to clinical practice and progression of the field.
With evidence-based practice, clinicians are instructed to use the scientific evidence as one means of information when determining clinical practice; however, if the scientific literature is not routinely demonstrating the use of LES as a treatment outcome measure, there is a significant disconnect between daily clinical practice and the assessment of voice disorders for research. This disconnect reduces clinicians’ ability to determine if they are getting the same effects as those reported in the scientific literature. Furthermore, one cannot expect clinicians in dispersed clinics and hospitals to use common language to report LES results if this does not occur in the literature. The lack of a broadly used clinical protocol to assess laryngeal anatomy and physiology, like the Voice-Vibratory Assessment With Laryngeal Imaging (VALI),3 reduces clinical efficiency and compromises patient care. It is critically important to have standardized commonly used definitions for laryngeal parameters and scales used to judge such parameters, as frequently the clinician conducting and interpreting the LES is different from the treating clinician.
The impetus for this systematic literature review was to assess which parameters and scales are most commonly included, determine the extent of the inconsistencies of the laryngeal parameters studied in voice treatment outcome studies, report on the variety of scales used to rate the parameters, and assess which parameters measured statistically significant improvements. The goal of this review is to call attention to a progress-limiting problem in the field and to provide a summary of the current state of the field with regard to the use of laryngeal parameters from LES as treatment outcome measures.
METHOD
Search strategy
Studies where LES was used as an outcome measure for the treatment of a voice disorder were identified from a search in three computerized journal databases (PubMed, Ovid, and Cochrane). The search terms used were “laryngostroboscopy,” “stroboscopy,” “strobovideolaryngoscopy,” “strobolaryngoscopy,” “videostroboscopy,” and “videolaryngostroboscopy.” Each of the search terms was combined with “treatment.” Specifically, the PubMed search was ((laryngostroboscopy OR stroboscopy OR strobovideolaryngoscopy OR strobolaryngoscopy OR videostroboscopy OR videolaryngostroboscopy) AND treatment). A first search was conducted on November 21, 2013, and a second search was conducted on October 30, 2015. All studies published from database inception (PubMed and Ovid electronic 1946, Cochrane 1993) to our last search on October 30, 2015 were reviewed for eligibility. Authors were not contacted. Unpublished reports were not considered for this review. Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement standards were used to conduct this review.4
Inclusion criteria
Articles were considered eligible for the review if they met the following inclusion criteria: English language; original article; human study; reported judgments of identical laryngeal parameters reported both pre and post treatment; and aggregate data reported for five or more participants. Duplicate results were deleted. Studies containing only objective assessment measures or only descriptive (without a scale) assessments were excluded. Articles in the first search were reviewed first by one reviewer (either KG or HB), and then a second reviewer (either KG or HB) assessed each study identified by the first reviewer for inclusion criteria. The articles found through the second search were reviewed by one reviewer (MD) and then validated by a second reviewer (HB) to assess eligibility.
Assessment of evidence
Five data elements were extracted by MD, HB, and KG from each of the eligible articles included in this review: (1) the parameters assessed, (2) the scales used, (3) if a previously published rating protocol was used, (4) whether treatment differences were reported, and (5) which parameters resulted in statistically significant changes.
Data synthesis
A meta-analysis of the results was not possible because of the heterogeneity of the results. Thus, the following analysis of the literature is descriptive in nature.
RESULTS
Search results
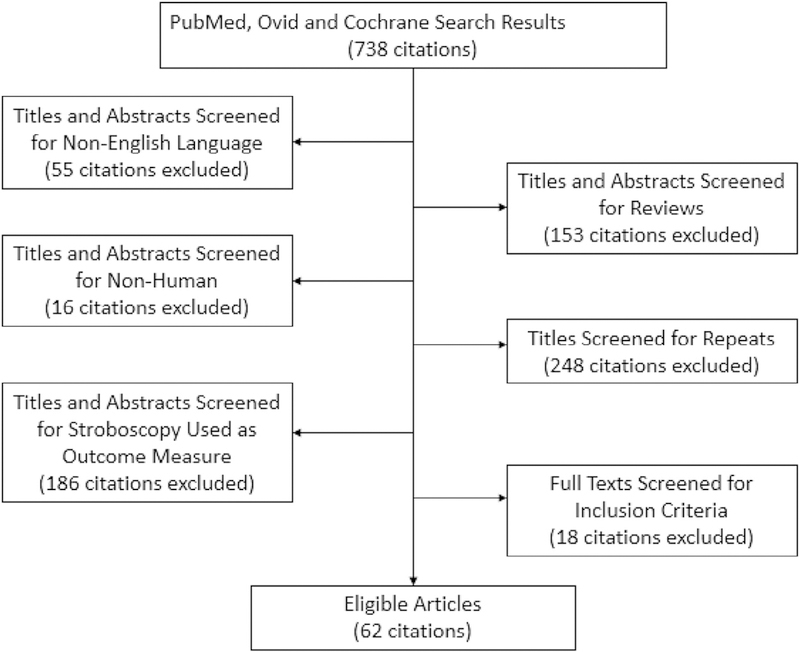
Eighty articles were submitted to full-text screening for critical evaluation. Sixty-two (77%) of these studies used rating scales to report attributes of vocal fold anatomy or physiology.5–66 Eighteen (23%) articles were eliminated because they provided descriptions of the parameters without using a rating scale or used only objective measurement (eg, glottal gap in millimeters). See Figure 1 for further details.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart.4
Study characteristics
The characteristics of the included studies are reported in Table 1, and include sample size, voice disorder(s) of the participants, and intervention(s) received. Sample size ranged from 5 to 474 participants, with an average of 52 participants (standard deviation = 82; median = 27) recruited for the studies.
TABLE 1.
Study Characteristics
| Author and Year | Total Sample Size | Voice Disorder(s) | Intervention Group(s) |
|---|---|---|---|
| (Beaver et al, 2003)5 | N=49 | G1: Diagnosis of laryngopharyngeal reflux disease (LPDR) (n = 49) | G1: High-dose proton pump inhibitor (PPI) therapy |
| G2: No signs of LPDR (n = 10) | G2: No intervention | ||
| (Chhetri and Berke, 2011)6 | N = 5 | G1: Unilateral or bilateral vocal fold scarring | G1: Injection of cultured autologous fibroblast in the lamina propria |
| (DeJonckere et al, 2003)7 | N=45 | G1: Various kinds of organic benign voice pathologies (Reinke edema, polyp, chronic laryngitis, muscle tension dysphonia, intracordal cyst, sulcus, or scar, nodules, unilateral vocal fold paralysis) | G1: Phonosurgery (always combined with voice therapy), voice therapy alone, or antireflux medication (with voice therapy when indicated) |
| (Demirhan et al, 2015)8 | N=12 | G1: Vocal fold polyps | G1: Quantum molecular resonance-assisted phonomicrosurgery |
| (Dixon, 1999)9 | N=34 | G1: Persistent intermittent dysphonia and positive history for delayed food allergy (n = 10) | G1–G3: Provocation or neutralization skin testing (injection of a neutralizing dose of antigen) |
| G2: Non-allergic subjects (controls) (n = 12) | |||
| G3: Allergic patients with negative skin test (controls) (n = 12) | |||
| (Djukic et al, 2014)10 | N=112 | G1: Vocal fold lesion with mild, moderate, or severe dysplasia | Laryngomicroscopy and different types of endoscopic cordectomy (types I–III) |
| (Fass et al, 2010)11 | N=41 | G1–G2: Diagnosis of laryngopharyngeal reflux disease (LPR) | G1: Esomeprazole 20 mg twice daily |
| G2: Placebo | |||
| (Finck and Lefebvre, 2005)12 | N=11 | G1: Benign vocal fold lesion requiring microsurgery (submucosal fibrosis, intracordal open cyst, intracordal organized hematoma, Reinke edema, localized vergeture, sulcus, mucous cyst) | G1: Microsurgical procedure and implantation of esterified hyaluronic acid (EHA) in Reinke space |
| (Finck et al, 2010)13 | N=83 | G1–G2: Benign vocal fold lesion requiring microsurgery (edema, mucous cyst, polyp, nodules, sulcus, scar, subepithelial fibrosis, vergetures, open cyst, pseudocyst) | G1: Microsurgical procedure and implantation of (EHA) in Reinke space G2: Microsurgical procedure without implantation of (EHA) in Reinke space |
| (Halawa et al, 2014)14 | N=97 | G1: Vocal nodules | G1: Voice therapy |
| (Halderman et al, 2014)15 | N=64 | G1: Vocal fold paralysis or glottic insufficiency | G1: Injection laryngoplasty with Restylane |
| (Hallen et al, 2001)16 | N=14 | G1: Insufficient closure of the vocal folds (vocal fold palsy, n = 6; or bowed vocal folds, n = 8) | G1: DiHA (dextranomer molecules in a 1% hyaluronan solution) injections |
| (Hamdan et al, 2015)17 | N = 9 | G1: Vocal fold paralysis or hypoadduction | G1: Transnasal fiberoptic injection laryngoplasty |
| (Hillel et al, 2013)18 | N=17 | G1–G2: Early laryngeal carcinoma | G1: Type II cordectomy (subligamentous); (n = 13) |
| G2: Type I cordectomy (subepithelial); (n = 4) | |||
| (Iloabachie et al, 2007)19 | N=14 | G1: Laryngeal cancer (T2 or T3 lesion) | G1: Radiation, with or without chemotherapy |
| (Jensen and Rasmussen, 2013)20 | N=82 | G1: Benign lesions of the vocal folds (polyps, cysts, nodules, edema) | G1: Phonosurgery (with or without postsurgery voice therapy) |
| (Karpenko et al, 2003)21 | N=10 | G1: Unilateral vocal fold paralysis | G1: Injection laryngoplasty with Cymetra |
| (Keilmann et al, 2011)22 | N=16 | G1: T1 or T2 tumor of the vocal cords | G1: Cordectomy-laser excision of the tumor (combined with voice therapy for all patients except one) |
| (Kodama et al, 2015)23 | N=33 | G1: Unilateral vocal fold paralysis | G1: Refined nerve-muscle pedicle flap implantation combined with arytenoid adduction |
| (Lam et al, 2010)24 | N=82 | G1–G2: LPR | G1: Rabeprazole (20 mg, twice daily) (n = 42) |
| G2: Placebo (n = 40) | |||
| (Lan et al, 2010)25 | N=20 | G1: Vocal fold polyp | G1: Transoral laryngeal surgery under flexible laryngovideostroboscopy (FLVS) |
| (Law et al, 2012)26 | N=12 | G1: Vocal nodules, vocal edema, hyperfunctional voice disorders | G1: Group voice therapy |
| (Lee et al, 2014)27 | N=22 | G1: Arytenoid dislocation | G1: Direct laryngoscopy and closed reduction of the dislocated arytenoid, with adjunct injection laryngoplasty or botulinum toxin administration in selected cases. |
| (Lee et al, 2007)28 | N=15 | G1: Unilateral vocal fold paralysis | G1: Ansa to recurrent laryngeal nerve reinnervation technique |
| (Li et al, 2011)29 | N=25 | G1–G3: Unilateral vocal fold paralysis | G1: Type I thyroplasty using a Silastic implant, combined with arytenoid adduction (n = 10) |
| G2: Type I thyroplasty only, using Gore-Tex (n = 15) | |||
| G3: Type I thyroplasty only, using a Silastic implant (n = 20) | |||
| (Li et al, 2014)30 | N=349 | G1–G4: UVFP By age: G1: <30 years old (n = 26) G2: 30–44 y (n = 165) G3: 45–59 y (n = 133) G4: ≥60 y (n = 25) |
G1–G4: Ansa cervicalis-recurrent laryngeal nerve anastomosis (laryngeal reinnervation) |
| (Li et al, 2014)31 | N=349 | G1–G3: UVFP By denervation duration: G1: 6–12 mo (n = 172) G2: 12–24 mo (n = 108) G3: >24 mo (n = 69) |
G1–G3: Ansa cervicalis-recurrent laryngeal nerve anastomosis (laryngeal reinnervation) |
| (Maia et al, 2012)32 | N=46 | G1: Vocal complaint group (presence of larynx lesions-nodules, cyst, polyps) (n = 23) | G1–G2: Pitched blowing exercise (10 repetitions) |
| G2: Without vocal complaint control group (n = 23) | |||
| (Malik et al, 2014)33 | N=40 | G1–G2: UVFP | G1: Type I thyroplasty using Silastic implant (n = 20) |
| G2: Type I thyroplasty using titanium vocal fold medializing implant (TVFMI) (n = 20) | |||
| (Maronian et al, 2003)34 | N = 9 (7 patients were available for post-treatment videostroboscopy) | G1: UVFP | G1: Recurrent laryngeal nerve reinnervation |
| (Milstein et al, 2005)35 | N = 20 (data missing for 1 participant) | G1: UVFP | G1: Cymetra injection laryngoplasty |
| (Monini et al, 2006)36 | N=60 | G1: Laryngopharyngeal reflux (LPR)-symptomatic and asymptomatic (n = 49) G2: LPR (symptomatic) (n = 11) |
G1: Omeprazole G2: Immunostimulating vaccine (JO7AX lyophilized lisatum bacterium 5) |
| (Morgan et al, 2007)37 | N=19 | G1–G2: UVFP | G1: Injection (calcium hydroxylapatite or micronized acellular dermis) (n = 10) |
| G2: Total medialization (with or without arytenoid adduction) (n = 9) | |||
| (Mortensen and Woo, 2006)38 | N=34 | G1: postoperative scar or polyp, nodule, cyst, fibrovascular lesions or granuloma, or granulation of the larynx | G1: Steroid injections |
| (Nakagawa et al, 2012)39 | N=132 | G1: Vocal fold polyp | G1: Conservative treatment (voice therapy, medication, or both) |
| (Nam et al, 2013)40 | N=50 | G1–G2: Lower-pitched voice after thyroidectomy | G1: Vocal function exercise |
| G1: Gliding group | G2: Vocal function exercise and indirect voice therapy | ||
| G2: Non-gliding group | |||
| (Paniello et al, 2008)41 | N=21 | G1: Vocal fold scarring | G1: Minithyrotomy procedure |
| (Portnoy et al, 2014)42 | N=35 | G1: LPR | G1: Proton pump inhibitors |
| (Rihkanen et al, 2004)43 | N = 14 (pre-operative videostroboscopy was available for 12 participants) | G1: UVFP | G1: Vocal fold augmentation by injection laryngoplasty (autologous fascia augmentation) |
| (Rontal and Rontal, 2003)44 | N = 18 (post-intervention results available for 10 participants) | G1: UVFP | G1: Permanent medialization using injections of botulinum toxin and Gelfoam |
| (Sanuki et al, 2015)45 | N=12 | G1: UVFP | G1: Laryngeal reinnervation via refined nerve-muscle pedicle flap implantation combined with arytenoid adduction |
| (Schindler et al, 2013)46 | N=65 | G1: Reinke edema (n = 23) | G1–G3: Voice therapy |
| G2: Vocal fold cysts (n = 22) | |||
| G3: Gelatinous polyps (n = 20) | |||
| (Schneider et al, 2003)47 | N=14 | G1: Recurrent laryngeal nerve paralysis because of cardiothoracic surgery | G1: Medialization thyroplasty using the titanium implant (TVFMI) |
| (Schneider et al, 2003)48 | N=28 | G1: UVFP | G1: Medialization thyroplasty using the titanium implant (TVFMI) |
| (Shoffel-Havakuk et al, 2014)49 | N=46 | G1: Lesion limited to the posterior glottis (inflammatory granulation tissue, cysts, carcinoma in situ, invasive squamous cell carcinoma) | G1: Conservative management or surgical intervention |
| (Silbergleit et al, 2013)50 | N = 8 | G1: Early stage laryngeal cancer (Tis-T1N0M0) | G1: Photofrin-mediated photodynamic therapy |
| (Smith et al, 1995)51 | N=22 | G1–G2: Parkinson disease | G1: Intensive therapy (focused on vocal and respiratory effort) (n = 13) |
| G2: Intensive therapy (focused on respiratory effort only) (n = 9) | |||
| (Steward et al, 2004)52 | N=21 | G1–G2: Laryngopharyngeal reflux | G1: Proton pump inhibitor (Rabeprazole sodium) and instructions for lifestyle modification (n = 21) |
| G2: Placebo and instructions for lifestyle modification (n = 21) | |||
| (Storck et al, 2007)53 | N=26 | G1: UVFP | G1: Medialization thyroplasty using a hydroxyapatite implant |
| (Storck et al, 2010)54 | N=26 | G1–G2: UVFP | G1: Medialization thyroplasty using a hydroxyapatite implant (VoCoM) (n = 11) |
| G2: Medialization thyroplasty using a titanium implant (TVFMI) (n = 15) | |||
| (Su et al, 2005)55 | N = 15 (2 patients were excluded from analysis) | G1: Early glottis carcinoma-vocal fold deficit caused by previous laser cordectomy | G1: Laryngoplasty with bipedicled strap muscle transposition |
| (Su et al, 2005)56 | N=30 | G1: UVFP | G1: Arytenoid adduction for vocal fold medialization, using a paramedian approach (with or without strap muscle transposition) |
| (Tsunoda et al, 2005)57 | N=10 | G1: Sulcus vocalis | G1: Autologous transplant of fascia into the vocal fold (ATFV) |
| (van Gogh et al, 2006)58 | N=23 | G1–G2: Have received treatment for early glottic carcinoma | G1: Voice therapy (n = 12) |
| G2: Control group (n = 11) | |||
| (Wang et al, 2013)59 | N=36 | G1–G2: Unilateral hemorrhagic vocal polyp | G1: Potassium titanyl phosphate (KTP) laser-assisted polypectomy (n = 20) |
| G2: KTP laser without endoscopic removal of the polyp (n = 16) | |||
| (Wang et al, 2013)60 | N=30 | G1: Vocal nodules (n = 13) G2: Vocal polyps (n = 17) |
G1–G2: transnasal endoscopic steroid injection (TESI) (with voice therapy) |
| (Wang et al, 2012)61 | N=20 | G1: UVFP | G1: Ansa cervicalis main branch-to-RLN anastomosis |
| (Wang et al, 2011)62 | N=474 | G1: UVFP caused by thyroid surgery (n = 237) | G1: Ansa cervicalis main branch-to-RLN anastomosis |
| G2: Control group of healthy subjects (N = 237) | G2: No intervention | ||
| (Wang et al, 2011)63 | N=56 | G1: UVFP | G1: Contralateral ansa cervicalis-to-RLN anastomosis |
| (Woo et al, 2013)64 | N=64 | G1–G2: UVFP | G1: Injection laryngoplasty through the cricothyroid (n = 30) |
| G2: Injection laryngoplasty through the thyrohyoid (n = 34) | |||
| (Yilmaz, 2012)65 | N=44 | G1: Sulcus vocalis (unilateral or bilateral) | G1: Excision of sulcus, primary suture of epithelial defect, and medialization laryngoplasty |
| (Yilmaz and Sozen, 2012)66 | N=40 | G1–G2: Vocal fold polyp, cyst, or Reinke edema | G1: Microsuture after lesion removal (n = 20) G2: No microsuture after lesion removal (n = 20) |
Abbreviations: G1, group 1; G2, group 2; N, total sample size.
The voice disorders that were the most frequently studied were vocal fold paralysis (25 articles, 40%) and organic benign lesions (20 articles, 32%). Laryngeal dysplasia or cancer was studied in seven articles (11%), and laryngopharyngeal reflux was studied in six articles (10%). Dysphonia related to food allergies, arytenoid dislocation, post-thyroidectomy dysphonia, and Parkinsondisease related dysphonia were each the pathology of interest in a single study.
Three main categories of interventions emerged from the included articles. The most common intervention category was surgery or injection (40 articles, 64%), followed by voice therapy (seven articles, 11%), and pharmaceutical intervention (six articles, 10%). Six articles (10%) included a combination of voice therapy with another intervention method. Three articles (5%) used a different type of intervention: injection of a neutralizing dose of antigen for food allergy,9 radiation therapy with or without chemotherapy,19 and Photofrin-mediated photodynamic therapy.50
Parameters
Number of terms and parameters
From the 62 studies that used a rating scale, 20 broad categories of parameters were identified by authors MD and HB: amplitude of vibration, hyperfunction or ventricular folds, arytenoids, edema, erythema, glottal closure or gap, granuloma, regularity or periodicity, phase closure, mucosal wave, phase symmetry, mucus, vibratory behavior, lesions (non-granulomatous), pachydermia, vertical height, vocal fold edge, vocal fold (other), stenosis or web, and recovery. Eleven of these categories were further divided into two to five subcategories, for a total of 49 parameters. For example, the category hyperfunction or ventricular folds was subdivided into four parameters: anteroposterior compression, medial compression, general hyperfunction, and symmetry of ventricular folds. In Table 3, each parameter contains the exact terms used by the authors to describe the parameter assessed, along with the associated scale. In total, 141 different terms were used in the 62 articles.
TABLE 3.
Parameters and Scales
| Parameter (Categories) | Parameters (Terms Used) | Scales | |
|---|---|---|---|
| Amplitude of vibration | Amplitude of vibration | Amplitude | ■ 1 = normal; 2 = decreased; 3 = increased ■ Normal; mild; moderate; severe ■ Improved or not improved ■ 0 normal; 1 reduced; 2 absent |
| Amplitude of vibration | ■ Normal if noted at 5, increased if above 5, and decreased below on a 10 cm scale | ||
| Amplitude left or right | ■ 1 = normal; 2 = slightly decreased; 3 = moderately decreased; 4 = severely decreased; 5 = no visible movement ■ VAS-amplitude is considered normal if noted at half the continuum, increased to the right and decreased to the left |
||
| Amplitude of vibration tumor side/nontumor side | ■ 5-point equally appearing interval scale-0 = normal function; 5 = most abnormal finding | ||
| Hyperfunction or ventricular folds | Anteroposterior compression | Anteroposterior hyperfunction | ■ 1 = no shortening of glottal length compared with resting, nonphonating state; 2 = glottal length shortened by 25%; 3 = glottal length shortened by 50%; 4 = glottal length shortened by 75%; 5 = arytenoids touch laryngeal surface of epiglottis, obscuring glottal view. |
| Medial compression | Hyperadduction of false vocal folds False-fold compression hyperfunction |
■ Present or absent ■ 1 = no false fold overclosure, laryngeal ventricles easily seen; 2 = one or both false folds obscure laryngeal ventricles; 3 = one or both false folds obscure ventricles and a portion of the true vocal folds; 4 = one or both false folds obscure the entire true vocal folds but are not touching; 5 = false folds touch and cover the entire glottis. |
|
| Ventricular folds movement | ■ 1 = normal; 4 = fully compressed | ||
| General hyperfunction | Presence of muscle tension dysphonia | ■ No or yes | |
| Degree of compression with phonation | ■ 0 = none; 1 = mild; 2 = moderate | ||
| Supraglottic activity | ■ 1 = normal to 5 = most abnormal | ||
| Hyperfunction | ■ 1 = not present; 2 = sometimes present; 3 = always present | ||
| Supraglottic effort | ■ 0 = none; 1 = mild; 2 = moderate; 3 = severe | ||
| Larynx vestibule involvement | ■ Unchanged; improved or worsened | ||
| Symmetry of ventricular folds | Ventricular folds—symmetry of movement | ■ R > L; L > R; L = R | |
| Arytenoids | Arytenoid movement | Arytenoid movement | ■ 1 = normal; 3 = poor ■ 0 = none; 1 = minimal; 2 = normal |
| Symmetry of arytenoid movement | Arytenoid symmetry | ■ R > L; L > R; L = R | |
| Position symmetry (during voicing) | Decrease of symmetry of the arytenoids and vocal folds | ■ No or yes | |
| Arytenoid position during speech | ■ 2 = normal; 1 = mildly abnormal; 0 = moderately abnormal | ||
| Position symmetry (during respiration) | Arytenoid position during quiet respiration | ■ 2 = normal; 1 = mildly abnormal; 0 = moderately abnormal | |
| Arytenoid position | Arytenoid position | ■ 0 = normal; 1 = mildly tilted forward; 2 = moderately tilted forward; 3 = severely tilted forward | |
| Edema | Vocal fold edema | Asymmetric localized edema on glottic edge | ■ 0 = normal; 1+ = mild; 2+ = moderate; 3+ = severe |
| Edema of vocal folds | ■ 0 = not present to 3 = most severe | ||
| ■ Mild; moderate; severe; polypoid | |||
| ■ Normal; mild; moderate | |||
| ■ Present or absent | |||
| True vocal cord edema | ■ Scored as none to severe; 0 to 4 on Likert scale | ||
| Vocal edema | ■ Present or absent | ||
| Diffuse edema | Diffuse laryngeal edema | ■ Mild; moderate; severe; obstructing | |
| Swelling | ■ 0 = normal; 1+ = mild; 2+ = moderate; 3+ = severe | ||
| Ventricular obliteration | ■ Partial or complete | ||
| Posterior glottic edema | Edema of posterior supraglottis Posterior glottic edema |
■ 0 = not present to 3 = most severe ■ Present or absent |
|
| Subglottic edema | Edema of subglottis | ■ 0 = not present to 3 = most severe | |
| Subglottic edema | ■ Present or absent | ||
| ■ No or yes | |||
| Arytenoid edema | Edema of the arytenoid | ■ Present or absent | |
| ■ Scored as none to severe; 0–4 on Likert scale | |||
| Erythema | Supraglottis erythema | Erythema of posterior supraglottis | ■ 0 = not present to 3 = most severe |
| Subglottis erythema | Erythema of subglottis | ■ 0 = not present to 3 = most severe | |
| Arytenoid erythema | Erythema of the arytenoid | ■ Present or absent | |
| ■ Scored as none to severe; 0–4 on Likert scale | |||
| Vocal fold erythema | Erythema of vocal folds Partial or total vocal fold erythema True vocal cord erythema |
■ 0 = not present to 3 = most severe ■ Present or absent ■ Scored as none to severe; 0–4 on Likert scale |
|
| Diffuse erythema | Erythema or hyperemia | ■ Arytenoids only or diffuse | |
| Reaction or inflammation | ■ Presence or absence | ||
| Glottal closure or gap | Magnitude of glottal gap | Closure | ■ 0 = a wide opening; 1 = intermediate closure; 2 = complete closure ■ Improved or not improved |
| Degree of glottic closure | ■ 0 = no deviance; 1 = slight deviance; 2 = deviance | ||
| Glottal closure | ■ Visual analog scales of 100 mm: a score of 0 meaning “normal, no deviance,” and a score of 100 “extremely deviant” | ||
| ■ 0 = no deviance to 3 = severe deviance | |||
| ■ Complete or incomplete | |||
| ■ 0 = complete; 1 = slightly incomplete; 2 = moderately incomplete; 3 = severely incomplete | |||
| ■ 0 = none; 1 = slight; 2 = one-third gap between both vocal folds; 3 = two-thirds gap; 4 = an open glottis during phonation | |||
| ■ 0 = normal; 1 = mild; 2 = moderate; 3 = severe abnormality Glottic closure ■ Complete at 5 and diminished if noted between 0 and 5, on a 10 cm scale. | |||
| ■ Complete at the right end, absent at the left end, on a VAS | |||
| ■ 1 = complete; 2 = incomplete; 3 = inconsistent | |||
| ■ 0 = complete; 1 = slightly incomplete; 2 = moderately incomplete; 3 = fully incomplete | |||
| ■ Unchanged; improved; worsened | |||
| ■ Present or absent | |||
| Glottal gap | ■ Absent or present | ||
| ■ 0 = severe; 1 = moderate; 2 = mild; 3 = absent | |||
| ■ GR—gap remains or none | |||
| Glottic gap | ■ Presence or absence | ||
| Size of glottal gap | ■ <2 mm or >2 mm | ||
| Degree of glottal incompetence | ■ A 5-point scale was used: 1 = slight, folds just not touching; 3 = moderate, −50% of the length of the folds not touching with 1- to 2-mm gap; 5 = severe, no vocal fold contact throughout the length of the folds and a large (3- to 4-ram) glottal gap | ||
| Vocal gap size | ■ Disappeared; improved or no change | ||
| Presence of slit | ■ Unchanged; improved or worsened | ||
| Glottal incompetence | ■ Present or absent | ||
| Glottal competency | ■ 1 = normal to 5 = most abnormal | ||
| Glottal insufficiency | ■ 1 = complete closure; 2 = mildly incomplete; 3 = moderately incomplete; 4 = severely incomplete | ||
| Glottic closure (insufficiency) | ■ 0 = none; 1 = mild; 3 = moderate; 5 = pronounced | ||
| Glottic occlusion | ■ 1 = sufficient or 2 = insufficient | ||
| Location or shape of glottal gap | Closure at the midmembranous vocal fold Glottic closure of the membranous part of the vocal folds |
■ 100 mm VAS—0 = no gap to 100 = large gap ■ Complete; incomplete (anterior or posterior) or totally missing |
|
| Closure in the respiratory (cartilaginous) glottis | ■ 100 mm VAS—0 = no gap and 100 = large gap | ||
| Closure just anterior to the vocal processes | ■ 100 mm VAS—0 = no gap and 100 = large gap | ||
| Glottic gap at the middle and posterior aspects of the vocal folds | ■ 0 = none; 1 = mild; 2 = moderate | ||
| Degree of glottal closure | ■ None, anterior one-third, anterior two-thirds, and complete | ||
| Glottal closure | ■ 1 = complete closure all along the vocal folds; 2 = indication of incomplete closure of the cartilaginous part; 3 = triangular incomplete closure reaching anterior to the vocal processes; 4 = triangular incomplete closure of the posterior third of the folds; 5 = incomplete closure of the posterior two-thirds of the folds; 6 = incomplete closure all along the folds; A = spindle-shaped incomplete closure, closure at the vocal processes; B = spindle-shaped incomplete closure at the posterior third of the membranous folds, closure at the vocal processes; C = spindle-shaped incomplete closure at the anterior third of the folds, closure at the vocal processes; D = spindle-shaped incomplete closure at the posterior and the anterior thirds of the folds, closure at the vocal processes and at the middle of the membranous portion (“hourglass”) ■ No closure; anterior third; anterior two-thirds; complete ■ 0 = normal; 1 = anterior gap; 2 = posterior gap; 3 = hourglass; 4 = no contact |
||
| Glottic closure | ■ Pictoral | ||
| Glottal gap | ■ 0 = no gap; 1 = small gap between the membranous vocal folds; 2 = glottal gap about one-third of the portion between the membranous v.f.; 3 = glottal gap about two-thirds of the portion between the membranous v.f.; 4 = gap along the whole glottis | ||
| Location of glottal gap | ■ Anteroposterior; posterior; anterior; bowing | ||
| Relative size of glottal gap | ■ 0 = no gap during phonation; 1 = minimal gap; 2 = small gap extending up to one third of the anterior membranous vocal folds; 3 = moderate gap extending up to two-thirds of the posterior membranous vocal folds; 4 = complete glottal incompetence with no observable contact between the vocal folds | ||
| Glottal configuration | ■ Complete; bowing; hourglass; anterior chink; posterior chink; incomplete | ||
| Granuloma | Granuloma formation | Contact granuloma | ■ Present or absent |
| Granuloma formation | ■ Presence or absent | ||
| Granuloma or granulation | ■ Present or absent ■ Noted if present |
||
| Regularity/Periodicity | Regularity of vibration | Decrease of regularity | ■ No or yes |
| Regularity | ■ 0 = no deviance to 3 = severe deviance ■ 0 = periodical and symmetrical; 1 = periodical but asymmetrical; 2 = not periodical ■ 0 = no deviance; 1 = slight deviance; 2 = deviance ■ 0 = normal; 1 = mildly irregular; 2 = moderately irregular; 3 = severely irregular |
||
| Regularity of vibration | ■ Visual analog scales of 100 mm: a score of 0 meaning “normal, no deviance,” and a score of 100 “extremely deviant” Periodicity ■ 1 = regular; 2 = irregular successive vibrations | ||
| ■ Normal; mild; moderate; severe | |||
| ■ 1 = regular to 4 = always irregular | |||
| Regularity of vocal fold vibration | ■ 0 = normal; 1 = mildly abnormal; 2 = moderately abnormal | ||
| ■ Absent or visible | |||
| Phase closure | Phase closure ratio | Duration of closure | ■ Predominately closed; half closed and half open; predominately open; always open |
| Phase closure | ■ 1 = open phase predominates to 5 = closed phase predominates | ||
| Closure pattern of vocal folds | ■ 1 = predominantly closed; 2 = closed or open; 3 = predominantly open; 4 = always open | ||
| Phase closure (ratio not specified) | Closure patterns | ■ Normal; mild; moderate; severe | |
| Mucosal wave | Mucosal wave presence or amplitude | Mucosal wave | ■ 1 = absent; 2 = limited to the most medial edge of the vocal folds; 3 = present laterally up to one-fourth of the width of the vocal folds; 4 = present up to but less than one-half the width of the vocal folds; 5 = present at more than one-half the width of the vocal folds (normal) |
| ■ 1 = normal with 30%–50% lateral travel; 2 = increased with lateral travel >50%; 3 = decreased with lateral travel <30% | |||
| ■ Normal if noted at 10 on a 10-cm scale. Every value between 10 and 0 is considered a decreased wave. A wave noted 0 is a completely absent wave. | |||
| ■ Normal or absent or abnormal | |||
| ■ 1 = normal; 2 = small reduction; 3 = moderate reduction; 4 = severely reduced or absent | |||
| ■ 0 = absent; 1 = severely reduced; 2 = mildly reduces; 3 = intact | |||
| ■ Presence or absence | |||
| ■ NNN—normal or near-normal; IBR—improved but reduced | |||
| ■ Present to absent; 0–4 on Likert scale | |||
| ■ Absent or visible | |||
| Glottic wave movement | ■ 0 = no wave; 1 = decreased; 2 = full wave | ||
| Mucosal wave amplitude | ■ Preserved or not | ||
| ■ 0 = normal; 1 = mild; 2 = moderate; 3 = severe abnormality | |||
| ■ 0 = normal; 1 = mild; 2 = moderate; 3 = severe | |||
| Mucosal wave pattern (operated side) | ■ 0 = present, 2 = not present | ||
| Mucosal wave left or right | ■ 1 = normal to 5 = absent | ||
| ■ Scale is unipolar (normal at the right end, completely absent at the left end, VAS) | |||
| Mucosal wave quality | Mucosal wave | ■ 0 = no deviance to 3 = severe deviance | |
| ■ Improved or not improved | |||
| ■ 0 = no deviance; 1 = slight deviance; 2 = deviance | |||
| Mucosal wave characteristics | ■ 1 = normal to 5 = most abnormal | ||
| Quality of mucosal wave | ■ Visual analog scales of 100 mm: a score of 0 meaning “normal, no deviance,” and a score of 100 “extremely deviant” | ||
| Mucosal wave pattern reduction (contralateral side) | ■ 0 = none; 1 = mild; 2 = severe; 3 = complete | ||
| Mucosal wave tumor side or nontumor side | ■ A five-point equally appearing interval scale. Zero represented normal function and five represented the most abnormal finding. | ||
| Mucosal wave amplitude symmetry | Mucosal wave amplitude Amplitude |
■ Symmetric or asymmetric ■ 0 = symmetrical; 1 = smaller than the healthy side; 2 = mucosal traveling wave is not discernible |
|
| Mucosal wave symmetry (not specified) | Mucosal wave symmetry | ■ 0 = normal; 1 = mild; 2 = moderate; 3 = severe abnormality ■ 0 normal; 1 mild irregularity; 2 moderate irregularity; 3 always irregular ■ Severely asymmetrical or normal |
|
| Mucosal wave periodicity | Mucosal wave periodicity | ■ 0 = normal; 1 = mild; 2 = moderate; 3 = severe abnormality ■ 0 = normal; 1 = mild irregularity; 2 = moderate irregularity; 3 = always irregular ■ Severely aperiodic or normal |
|
| Phase symmetry | Phase symmetry | Phase symmetry | ■ 1 = regular to 4 = always irregular ■ 0 = normal; 1 = mildly asymmetrical; 2 = moderately asymmetrical; 3 = severely asymmetrical ■ 1 = symmetrical or 2 = asymmetrical ■ Symmetric or asymmetric ■ 0 = no deviance; 1 = slight deviance; 2 = deviance ■ Improved or not improved |
| Symmetry | ■ 0 = no deviance to 3 = severe deviance ■ Normal; mild; moderate; severe ■ Visual analog scales of 100 mm: a score of 0 meaning “normal, no deviance,” and a score of 100 “extremely deviant” |
||
| Phase synchrony | ■ Stays in synchrony during all cycles, in most of the cycles or is out of synchrony | ||
| Mucus | Presence of mucus | Increased mucus on vocal folds | ■ 0 = normal; 1+ = mild; 2+ = moderate; 3+ = severe |
| Laryngeal mucus | ■ No or yes | ||
| Thick endolaryngeal mucus or other | ■ Present or absent | ||
| Vibratory behavior | Presence and extent of vibration | Vibratory patterns | ■ Improved or not improved |
| Vibratory behavior left or right | ■ 1 = always fully present to 5 = complete absence always | ||
| Return of vocal fold function | ■ Yes or no | ||
| Fold vibration | ■ Present or absent | ||
| Vibratory behavior | ■ Improved or not improved | ||
| Presence and extent of nonvibrating portions | Nonvibratory segments Nonvibrating portion of the vocal folds tumor side or nontumor side |
■ 1 = presence or 2 = absence of nonvibratory segment ■ A five-point equally appearing interval scale. Zero represented normal function and five represented the most abnormal finding. |
|
| Inability to sustain waveform | ■ 0 = normal; 1+ = mild; 2+ = moderate; and 3+ = severe | ||
| Location of nonvibrating portions | Location of nonvibratory segments | ■ Anterior; middle; posterior third of the true vocal fold | |
| Level of phonation | Level of phonation | ■ True vocal cord; false vocal cord; supraglottic phonation | |
| Lesions (non-granulomatous) | General lesions (presence and size) | Lesion presence Lesion size |
■ Presence or absence ■ Improvement or nonimprovement ■ 1 = complete resolution of the vocal lesions; 2 = much improvement (>50% of lesion size reduction); 3 = some improvement (<50% of lesion size reduction); 4 = no change |
| Specific lesions (presence and size) | Polyp size Polyps Posterior glottic lesion Ulceration Scarring |
■ Complete remission; shrinkage; no change in size; enlargement ■ Present or absent ■ Present or absent ■ Noted if present ■ Present or absent |
|
| Morphological changes (tissue hyperplasia or cicatrisation) | ■ 0 = none; 1 = mild; 3 = moderate; 5 = severe | ||
| Leukoplakia | ■ Present or absent | ||
| Nodules or prenodules | ■ Present or absent ■ Noted if present |
||
| Vascular injection | ■ 0 = normal; 1+ = mild; 2+ = moderate; 3+ = severe | ||
| Pachydermia | Pachydermia | Pachydermia | ■ Scored as none to severe; 0–4 on Likert scale |
| Posterior pachydermia | ■ Present or absent | ||
| Posterior commissure hypertrophy | ■ Mild; moderate; severe; obstructing | ||
| Vertical height | Vertical height difference | Vertical height difference Vertical height difference between the vocal folds |
■ Yes or no ■ 0 = severe and 100 = none (VAS) |
| Vertical level of approximation | ■ Improved or not improved ■ 1 = equal; 2 = right lower; 3 = left lower |
||
| Vocal fold edge | Straightness | Vocal fold edge | ■ 0 = straight; 1 = mildly bowed; 2 = moderately bowed; 3 = severely bowed |
| Vocal fold edge of paralyzed side | ■ 0 = straight; 1 = mildly bowing; 2 = moderately bowing; 3 = severely bowing | ||
| Degree of vocal fold bowing | ■ 1 = straight vocal fold edge; 2 = mildly bowed; 3 = moderately bowed; 4 = severely bowed | ||
| Muscle atrophy | ■ Present or absent | ||
| Roughness | Vocal fold edge left or right | ■ 1 = smooth to 5 = rough irregular | |
| Glottic edge irregularity | ■ 0 = normal; 1+ = mild; 2+ = moderate; 3+ = severe | ||
| Not specified | Vocal fold edge | ■ Improved or not improved | |
| Vocal fold edge tumor side/nontumor side | ■ 5-point equally appearing interval scale (0 = normal function; 5 = most abnormal finding) | ||
| Vocal fold (other) | Vocal fold position | Vocal fold position | ■ 0 = midline; 1 = paramedian; 2 = intermediate; 3 = lateral |
| ■ 1 = lateral; 2 = paramedian; 3 = near-midline | |||
| Vocal fold tension | Vocal fold tension | ■ Normal; mild; moderate | |
| Vocal fold size | Muscle bulk asymmetry | ■ Present or absent | |
| Stenosis/web | Presence of stenosis or web | Stenosis Web (may be anterior microweb) |
■ Noted if present ■ Present or absent |
| Recovery | Recovery | Recovery | ■ 1 = complete recovery (normal mucosal wave propagation with symmetric phase and amplitude); 2 = much improvement (normal mucosal wave propagation with minimal asymmetric phase or amplitude); 3 = some improvement (presence of phase asymmetry or decreased amplitude, but the mucosal wave was improved compared with baseline); 4 = no change or worse |
Abbreviations: L, left; R, right; VAS, visual analog scale; v.f., vocal fold.
Terms were classified into the different categories by authors MD and HB. For example, the different terms hyperadduction of false vocal folds, false-fold compression hyperfunction, and ventricular fold movement were judged to refer to the same parameter—medial compression—in the broader category of hyperfunction of the ventricular folds. These judgments were made by the authors to facilitate the interpretation of the results. On average, studies used four terms with a range from 1 to 14. Table 3 provides the full list of terminology and parameters from the included studies.
Frequency of parameters
The parameters most frequently reported were magnitude of glottal gap (31 articles, 50%); mucosal wave presence or amplitude (17 articles, 27%); location or shape of glottal gap (15 articles, 24%); regularity of vibration (12 articles, 19%); phase symmetry (11 articles, 18%); and presence and size of specific lesions (11 articles, 18%).
Use of previously published protocols
Of the articles that used a rating scale, 18 (29%) adapted their rating parameters and scales from a previously published protocol. The protocols used were the Reflux Finding Score from Belafsky et al,67 used in three studies11,24,42; adaptations from Hirano and Bless’ stroboscopic assessment,68–70 used in four studies14,18,54,58; Ott et al scale,71 used in one study22; Lee et al scale,72 used in one study27; Lorenz et al scale,73 used in two studies30,31; the Stroboscopy Research Instrument (SRI),74 used in one study37; Yumoto et al scale,75 used in one study45; Yun et al scale,76 used in one study46; and Sodersten and Lindestad scale,77 used in two studies.47,48 Two studies62,63 used a combination of the Lorenz et al scale73 and the SRI74 protocol. The articles that used published protocols can be found in Table 2.
TABLE 2.
Study Results
| Author and Year | Parameters | Scales | Difference Reported (Pre to Post Treatment) | Parameters Significantly Improved (From Pre to Post Treatment) | Use of Previously Published Scale |
|---|---|---|---|---|---|
| (Beaver et al, 2003)5 | 1. Edema of posterior supraglottis 2. Edema of vocal folds 3. Edema of subglottis 4. Erythema of posterior supraglottis 5. Erythema of vocal folds 6. Erythema of subglottis 7. Leukoplakia 8. Nodules or prenodules 9. Polyp(s) 10. Posterior pachydermia 11. Web 12. Contact granuloma |
1–6: 0 = not present to 3 = most severe 7–12: present or absent |
When comparing index scores of laryngopharyngeal reflux disease patients before and after treatment, there was a significant decrease in scores of parameters 1–6 after 6 weeks of treatment. ** Still digital photographs of the abducted larynx were analyzed. |
1–6 | No |
| (Chhetri and Berke, 2011)6 | 1. Mucosal wave | 1: 1 = absent; 2 = limited to the most medial edge of the vocal folds; 3 = present laterally up to one-fourth of the width of the vocal folds; 4 = present up to but less than one-half the width of the vocal folds; 5 = present at more than one-half the width of the vocal folds (normal) | The mucosal wave grade was significantly improved after autologous fibroblast injection treatment. | 1 | No |
| (DeJonckere et al, 2003)7 | 1. Glottal closure 2. Regularity of vibration 3. Symmetry 4. Quality of mucosal wave |
1–4: VAS of 100 mm—a score of 0 meaning “normal, no deviance,” and a score of 100 “extremely deviant” | At group level, the overall effects for each parameter showed a significant improvement following treatment (phonosurgery, voice therapy, or antireflux medication). | 1–4 | No |
| (Demirhan et al, 2015)8 | 1. Glottal closure 2. Regularity 3. Mucosal wave 4. Symmetry |
1–4: 0 = no deviance to 3 = severe deviance | Results show a decrease in the mean ratings for all four parameters after quantum molecular resonance-assisted phonomicrosurgery. | N/A | No |
| (Dixon, 1999)9 | 1. Vascular injection 2. Swelling 3. Glottic edge irregularity 4. Asymmetric localized edema on glottic edge 5. Increased mucus on vocal folds 6. Inability to sustain waveform |
1–6: 0 = normal; 1+ = mild; 2+ = moderate; and 3+ = severe | Results show improvement in all parameters after injection of a neutralizing dose of antigen. | N/A | No |
| (Djukic et al, 2014)10 | 1. Glottic occlusion 2. Symmetry phase 3. Periodicity 4. Amplitude 5. Mucosal wave 6. Nonvibratory segment |
1: 1 = sufficient; 2 = insufficient 2: 1 = symmetrical; 2 = asymmetrical 3: 1 = regular; 2 = irregular successive vibrations 4: 1 = normal; 2 = decreased; 3 = increased 5: 1 = normal with 30%–50% lateral travel; 2 = increased with lateral travel >50%; 3 = decreased with lateral travel <30% 6: 1 = presence; 2 = absence of nonvibratory segment |
Parameters 2–5 were significantly improved in the mild and moderate groups, and parameters 3–6 were significantly improved in the severe group after surgery (laryngomicroscopy and different types of endoscopic cordectomy). | 2–5 (groups: mild and moderate) 3–6 (group: severe) |
No |
| (Fass et al, 2010)11 | 1. Subglottic edema 2. Granuloma or granulation 3. Thick endolaryngeal mucus or other 4. Ventricular obliteration 5. Erythema or hyperemia 6. Vocal cord edema 7. Diffuse laryngeal edema 8. Posterior commissure hypertrophy |
1–3: Present or absent 4: Partial or complete 5: Arytenoids only or diffuse 6: Mild; moderate; severe; polypoid 7–8: Mild; moderate; severe; obstructing |
No significant difference between the baseline RFS and the post-treatment scores for the esomeprazole group or the placebo group. **Still-frame images of open and closed vocal fold positions were analyzed. |
None | Yes (Belafsky et al, 2001)67 Reflux Finding Score |
| (Finck and Lefebvre, 2005)12 | 1. Amplitude of vibration 2. Mucosal wave 3. Glottic closure Descriptions of changes were provided for presence of inflammation and vocal fold appearance (symmetry and presence of deformation) |
1: 10 cm scale—normal if noted at 5, increased if above 5, and decreased if below 2: 10 cm scale—normal at the right end, completely absent at the left end 3: 5 cm scale—closure is considered complete at 5 and diminished if noted between 0 and 5 |
Description of changes, positive for majority, neutral for a couple, and negative for a few, after the microsurgical procedure and the implantation of esterified hyaluronic acid in Reinke space. | N/A | No |
| (Finck et al, 2010)13 | 1. Left amplitude 2. Right amplitude 3. Left mucosal wave 4. Right mucosal wave 5. Glottic closure |
1–2: VAS—amplitude is considered normal if noted at half the continuum, increased to the right and decreased to the left 3–4: VAS—mucosal wave is normal at the right end and completely absent at the left end. 5: VAS—closure is complete at the right end, absent at the left end |
A significant postoperative increase in glottic closure, right amplitude and mucosal wave, and left amplitude and mucosal wave was observed. There was no difference between the two groups regarding the impact of the microsurgery, except for the left mucosal wave, which increased more in the implanted group. | 1–5 | No |
| (Halawa et al, 2014)14 | 1. Glottal closure 2. Fold vibration 3. Mucosal wave 4. Phase symmetry |
1: Complete or incomplete 2: Present or absent 3: Normal or absent or abnormal 4: Symmetric or asymmetric |
According to the overall evaluation of perceptual, laryngostroboscopic, and subjective measures, the patients were classified into two groups: clinical improvement (75 patients, 77.3%) and no considerable clinical improvement (22 patients, 22.7%). | N/A | Yes. Adapted from Hirano and Bless, 199370 Stroboscopic Assessment |
| (Halderman et al, 2014)15 | 1. Reaction or inflammation 2. Scarring 3. Granuloma formation 4. Mucosal wave amplitude |
1–3: Present or absent 4: Preserved or not |
None of the postinjection examinations revealed foreign body reaction or inflammation at the site of injection. Mucosal wave amplitude appeared preserved without evidence of loss of mucosal pliability. No scarring or granulomatous changes were noted. | N/A | No |
| (Hallen et al, 2001)16 | 1. Glottic wave movement 2. Closure |
1: 0 = no wave; 1 = decreased; 2 = full wave 2: 0 = a wide opening; 1 = intermediate closure; 2 = complete closure |
All patients with unilateral palsy had improved stroboscopic status regarding both the wave and the closure after injection. In patients with spindle-shaped vocal fold closure pattern (n = 8), the glottic wave improved in all but two patients and the glottic closure improved in five patients. | N/A | No |
| (Hamdan et al, 2015)17 | 1. Glottal gap 2. Size of glottal gap 3. Location of glottal gap |
1: Present or absent 2: <2 mm or >2 mm 3: Anteroposterior; posterior; anterior; bowing |
All subjects had a glottal gap preoperatively. The gap was closed completely in 66.66% of the patients and reduced to <2 mm in 33.33% of the patients. | N/A | No |
| (Hillel et al, 2013)18 | 1. Glottic closure 2. Mucosal wave |
1: 1 = complete; 2 = incomplete; 3 = inconsistent 2: 1 = normal; 2 = small reduction; 3 = moderate reduction; 4 = severely reduced or absent |
The scores for mucosal wave ratings decreased significantly (P = 0.008) from the preoperative time point to the postoperative time point, as did the glottic closure ratings (P = 0.005) from the preoperative time point to the postoperative time point. | 1,2 | Yes: adapted from Bless et al, 198768 Videostroboscopic Evaluation of the Larynx |
| (Iloabachie et al, 2007)19 | 1. Return of vocal cord function | 1: Yes or no | Nine patients had return of vocal fold function and five patients did not have return of vocal cord function. | N/A | No |
| (Jensen and Rasmussen, 2013)20 | 1. Lesion presence | 1: Present or absent | All vocal fold lesions—except one with a polyp in the anterior commissure requiring a reoperation—were completely removed. | N/A | No |
| (Karpenko et al, 2003)21 | 1. Supraglottic activity 2. Mucosal wave characteristics* 3. Glottal competency* |
1–3: 1 = normal to 5 = most abnormal *The values for mucosal wave characteristics and glottal competency were combined to give a composite score for which 10 is most abnormal |
Combined scores of mucosal wave characteristics and glottal competency improved from pre to post injection. These results proved to be statistically significant (P < 0.02). The score of perceived supraglottic activity decreased after the operation but the difference was not statistically significant (P > 0.30). | 2–3 (values combined) | No |
| (Keilmann et al, 2011)22 | 1. Morphological changes (tissue hyperplasia or cicatrisation) 2. Glottic closure (insufficiency) 3. Mucosal wave pattern reduction (contralateral side) 4. Mucosal wave pattern (operated side) 5. Level of phonation |
1: 0 = none; 1 = mild; 3 = moderate; 5 = severe 2: 0 = none; 1 = mild; 3 = moderate; 5 = pronounced 3: 0 = none; 1 = mild; 2 = severe; 3 = complete 4: 0 = present; 2 = not present 5: true vocal cord; false vocal cord; supraglottic phonation |
The mean value of the score seemed to demonstrate a slight tendency of gradual improvement, but the time effect could not be proven statistically. Patients with more extended resections demonstrated less favorable results compared to those after limited resections. |
None | Yes (Ott et al, 1992)71 Clinical assessment of the post treatment changes of the glottis |
| (Kodama et al, 2015)23 | 1. Regularity 2. Amplitude 3. Glottal gap |
1: 0 = periodical and symmetrical; 1 = periodical but asymmetrical; 2 = not periodical 2: 0 = symmetrical; 1 = smaller than the healthy side; 2 = mucosal traveling wave is not discernible 3: 0 = no gap; 1 = small gap between the membranous vocal folds; 2 = glottal gap about one-third of the portion between the membranous v.f.; 3 = glottal gap about two-thirds of the portion between the membranous v.f.; 4 = gap along the whole glottis |
Results show significant improvements for all parameters from baseline to 1, 3, 6, 12, and 24 months post surgery (P < 0.01). The parameter for regularity 24 months after surgery showed significant improvement compared with those at 1, 3, and 6 months after surgery. Amplitude at 24 months post surgery improved significantly compared with measurements at 3 and 6 months. The glottal gap at 24 months after surgery improved significantly compared with the results at 3 months follow-up. |
1–3 | No |
| (Lam et al, 2010)24 | 1. Subglottic edema 2. Granuloma or granulation 3. Thick endolaryngeal mucus or other 4. Ventricular obliteration 5. Erythema or hyperemia 6. Vocal cord edema 7. Diffuse laryngeal edema 8. Posterior commissure hypertrophy |
1–3: Present or absent 4: Partial or complete 5: Arytenoids only or diffuse 6: Mild; moderate; severe; polypoid 7–8: Mild; moderate; severe; obstructing |
Within the rabeprazole group, the total RFS score obtained at week 12 was significantly lower than baseline (P = 0.002). The patients had significant improvement in vocal cord edema (P = 0.001), diffuse laryngeal edema (P = 0.006), and posterior commissure hypertrophy (P = 0.003). The total RFS score obtained at week 18 was significantly lower than baseline (P = 0.0001). They showed improved vocal cord edema (P = 0.0001) and diffuse laryngeal edema (P = 0.001). Significant improvements were also found in the placebo group. The total RFS score was not significantly different between the rabeprazole and placebo groups at weeks 6, 12, and 18. | 6–8 and RFS total score. | Yes (Belafsky et al, 2001)67 Reflux Finding Score |
| (Lan et al, 2010)25 | 1. Degree of glottic closure 2. Regularity 3. Phase symmetry 4. Mucosal wave |
1–4: 0 = no deviance; 1 = slight deviance; 2 = deviance | At 3 months postoperatively, significant improvements in the degree of glottic closure (P < 0.001), regularity (P = 0.046), phase symmetry (P = 0.008), and mucosal wave (P = 0.008) were noted when compared with preoperative status. | 1–4 | No |
| (Law et al, 2012)26 | 1. Vocal fold nodules 2. Vocal edema |
1–2: Present or absent | There were no statistically significant changes in laryngeal pathology preand post-voice therapy. | None | No |
| (Lee et al, 2014)27 | 1. Glottal gap 2. Mucosal wave |
1: 0 = severe; 1 = moderate; 2 = mild; 3 = absent 2: 0 = absent; 1 = severely reduced; 2 = mildly reduces; 3 = intact |
Significant improvements of glottal gap and mucosal wave were found at 1 and 6 months post-surgery when compared to baseline (P < 0.05). | 1–2 | Yes, adapted from Lee et al, 201072 |
| (Lee et al, 2007)28 | 1. Glottic closure 2. Supraglottic effort 3. Arytenoid position 4. Vocal fold edge 5. Vertical height difference 6. Vocal fold position |
1: 0 = complete; 1 = slightly incomplete; 2 = moderately incomplete; 3 = fully incomplete 2: 0 = none; 1 = mild; 2 = moderate; 3 = severe 3: 0 = normal; 1 = mildly tilted forward; 2 = moderately tilted forward; 3 = severely tilted forward 4: 0 = straight; 1 = mildly bowed; 2 = moderately bowed; 3 = severely bowed 5: yes or no 6: 0 = midline; 1 = paramedian; 2 = intermediate; 3 = lateral |
All investigated data were either the same or improved postoperatively compared with the preoperative values. | N/A | No |
| (Li et al, 2011)29 | 1. Closure at the midmembranous vocal fold 2. Closure just anterior to the vocal processes 3. Closure in the respiratory glottis 4. Vertical height difference between the vocal folds |
1–3: 100-mm VAS—0 = no gap and 100 = large gap 4: 100-mm VAS; 0 = severe and 100 = none |
A significant improvement in the postoperative scores was found for all groups for all parameters (P < 0.05), with the exception of closure in the respiratory glottis and vertical height difference, which did not significantly improve in G3 (thyroplasty only, with a Silastic implant). No significant difference in postoperative scores was found between the groups, for any parameter. |
G1: 1–4 G2: 1–4 G3: 1–2 All groups combined: 1–4 |
No |
| (Li et al, 2014)30 | 1. Glottal closure | 1: 0 = complete; 1 = slightly incomplete; 2 = moderately incomplete; 3 = severely incomplete | The postoperative stroboscopic findings were significantly improved in total sample and within each group compared with the corresponding preoperative findings (P < 0.01). | 1 | Yes, adapted from Lorenz et al, 200873 |
| (Li et al, 2014)31 | 1. Glottal closure | 1: 0 = complete; 1 = slightly incomplete; 2 = moderately incomplete; 3 = severely incomplete | The postoperative stroboscopic findings were significantly improved in total sample and within each group compared with the corresponding preoperative findings (P < 0.01). | 1 | Yes, adapted from Lorenz et al, 200873 |
| (Maia et al, 2012)32 | 1. Glottic closure 2. Presence of slit 3. Larynx vestibule involvement |
1–3: Unchanged, improved or worsened comparing both images | The evaluation revealed improvements in glottic closure and lower larynx vestibule activation in post exercise moment in both groups (P < 0.001). There were no changes regarding the presence of a glottic slit. | 1,3 | No |
| (Malik et al, 2014)33 | 1. Glottic gap 2. Mucosal wave |
1–2: Presence or absence | Closure of the glottic gap was achieved in all cases except for one participant, who underwent medialization with the Silastic implant. Mucosal wave was absent in all cases preoperatively. After the surgery, it was present in 14 of 20 patients in G1 and in 16 of 20 patients in G2. | N/A | No |
| (Maronian et al, 2003)34 | 1. Vocal fold position 2. Degree of compression with phonation 3. Glottic gap 4. Muscle atrophy 5. Muscle bulk asymmetry 6. Arytenoid position during quiet respiration 7. Arytenoid position during speech 8. Arytenoid movement |
1: 1 = lateral; 2 = paramedian; 3 = near-midline 2–3: 0 = none; 1 = mild; 2 = moderate; 4–5: Present or absent 6–7: 2 = normal; 1 = mildly abnormal; 0 = moderately abnormal 8: 0 = none; 1 = minimal; 2 = normal |
Improvements were found in all parameters for at least 2 of 7 participants. 100% of the participants improved regarding glottic gap and muscle atrophy. | N/A | No |
| (Milstein et al, 2005)35 | 1. Glottic insufficiency 2. Degree of vocal fold bowing |
1: 1 = complete closure; 2 = mildly incomplete; 3 = moderately incomplete; 4 = severely incomplete 2: 1 = straight vocal fold edge; 2 = mildly bowed; 3 = moderately bowed; 4 = severely bowed |
Mean scores of glottal closure and degree of vocal fold bowing improved from pre to post intervention (P < 0.0001). | 1–2 | No |
| (Monini et al., 2006)36 | 1. Edema of the arytenoid 2. Erythema of the arytenoid 3. Partial or total vocal fold erythema 4. Vocal fold edema 5. Posterior glottic edema |
1–5: Present or absent | In G1, laryngeal features were improved in 93% of the participants. In G2, improvements were observed in 54.5% of the participants and pre-post difference was also significant (P = 0.025). | N/A | No |
| (Morgan et al, 2007)37 | 1. Symmetry 2. Amplitude 3. Periodicity 4. Closure patterns 5. Location of nonvibratory segments 6. Duration of closure |
1–4: Normal; mild; moderate; severe 5: Anterior; middle; posterior third of the true vocal fold 6: Predominately closed; half closed and half open; predominately open; or always open *Ratings were then converted to a 100-point scale (0 = normal; 100 = most abnormal) |
Pre- to post-changes within each intervention group were not assessed for statistical significance. Pre and post scores for SRI improved in both groups from pre to post treatment, but the statistical significance of these improvements was not assessed. | N/A | Yes adapted from Rosen, 200574 Stroboscopy Research Instrument |
| (Mortensen and Woo, 2006)38 | 1. Vocal fold edge 2. Vertical level of approximation 3. Amplitude 4. Mucosal wave 5. Vibratory behavior 6. Phase symmetry 7. Closure |
1–7: Improved or not improved | Significant improvement was observed for the following parameters: vocal fold edge, amplitude, wave, and vibratory behavior (P = 0.01). | 1, 3, 4, 5 | No |
| (Nakagawa et al, 2012)39 | 1. Polyp size | 1: complete remission; shrinkage; no change in size; enlargement | 41.7% of the patients showed complete remission of the polyps following conservative treatment. Lesion shrinkage was observed in 29.1% of the patients. | N/A | No |
| (Nam et al, 2013)40 | 1. Vocal fold edema 2. Vocal fold tension 3. Subglottic edema 4. Laryngeal mucus 5. Decrease of symmetry of the arytenoids and vocal folds 6. Decrease of regularity 7. Presence of muscle tension dysphonia |
1–2: Normal; mild; moderate 3–7: Yes or no |
In the non-gliding group, the parameters were not improved following direct voice therapy only. However, an important improvement was observed after indirect voice therapy followed by vocal function exercise (a higher percentage of patients improved). | N/A | No |
| (Paniello et al, 2008)41 | 1. Mucosal wave 2. Glottal gap |
1: NNN—normal or near-normal; IBR—improved but reduced 2: Gap remains or none |
Mucosal wave was improved in 18 of the 21 patients, and glottal gap was improved in 19 of the 21 patients. | N/A | No |
| (Portnoy et al, 2014)42 | 1. Subglottic edema 2. Granuloma of granulation 3. Thick endolaryngeal mucus/other 4. Ventricular obliteration 5. Erythema/hyperemia 6. Vocal cord edema 7. Diffuse laryngeal edema 8. Posterior commissure hypertrophy |
1–3: Present or absent 4: Partial or complete 5: Arytenoids only or diffuse 6: Mild; moderate; severe; polypoid 7–8: Mild; moderate; severe; obstructing |
A small but significant decrease in mean RFS scores was found on the super high dose regimen. The average change in RFS scores (from standard to super high dose) was 1.45 ± 2.9 (P = 0.0007). | Yes (average RFS score) | Yes (Belafsky et al, 2001)67 Reflux Finding Score |
| (Rihkanen et al, 2004)43 | 1. Glottic closure of the membranous part of the vocal folds 2. Mucosal wave amplitude 3. Phase synchrony |
1: Complete; incomplete (anterior or posterior); totally missing 2: Symmetric or asymmetric 3: Staying in synchrony during all cycles; in most of the cycles; out of synchrony. |
The number of patients with complete or partial vocal fold closure, good phase synchrony, and mucosal wave amplitude was increased following injection laryngoplasty. The difference with preintervention findings was significant for vocal fold closure and phase synchrony (P < 0.01). | 1,3 | No |
| (Rontal and Rontal, 2003)44 | 1. Glottic closure | 1: Present or absent (as signaled as a return of mucosal wave) | Of the remaining 10 participants, nine had an effective vocal fold approximation after the procedure, allowing glottic closure. | N/A | No |
| (Sanuki et al, 2015)45 | 1. Glottal closure 2. Regularity of vocal fold vibration 3. Amplitude |
1: 0 = none; 1 = slight; 2 = one-third gap between both vocal folds; 3 = two-thirds gap; 4 = an open glottis during phonation 2: 0 = normal; 1 = mildly abnormal; 2 = moderately abnormal 3: 0 = normal; 1 = reduced; 2 = absent |
Glottal closure (P = 0.0050), regularity of the vocal fold vibration (P = 0.0067), and amplitude of vocal fold vibration (P = 0.0048) were significantly improved after laryngeal reinnervation when compared with the preoperative values. | 1–3 | Yes adapted from Yumoto et al, 200675 |
| (Schindler et al, 2013)46 | 1. Lesion size | 1: Improvement or no improvement | Improvement in the lesion size was observed in 11 of the 65 participants after 10 weeks of voice therapy. However, none of the lesions disappeared completely after therapy. | N/A | Yes (Yun et al, 2007)76 |
| (Schneider et al, 2003)47 | 1. Glottal closure 2. Vibratory patterns |
1: 1 = Complete closure all along the vocal folds; 2 = indication of incomplete closure of the cartilaginous part; 3 = triangular incomplete closure reaching anterior to the vocal processes; 4 = triangular incomplete closure of the posterior third of the folds; 5 = incomplete closure of the posterior two-thirds of the folds; 6 = incomplete closure all along the folds; A = spindle-shaped incomplete closure, closure at the vocal processes; B = spindle-shaped incomplete closure at the posterior third of the membranous folds, closure at the vocal processes; C = spindle-shaped incomplete closure at the anterior third of the folds, closure at the vocal processes; D = spindle-shaped incomplete closure at the posterior and the anterior thirds of the folds, closure at the vocal processes and at the middle of the membranous portion (“hourglass”) 2: Improved or not improved |
Following surgery, a complete closure was noted in six subjects, three had an almost complete closure with a minor gap in the cartilaginous part, four had a triangular incomplete closure reaching anterior to the vocal processes, and one had a triangular incomplete closure of the posterior third of the folds. The vibration patterns of the vocal folds were improved in all participants. | N/A | Yes adapted from Sodersten and Lindestad, 199077 |
| (Schneider et al, 2003)48 | 1. Glottal closure 2. Vibratory patterns |
1: See above (Schneider et al, 2003)47 2: Improved or not improved |
Glottal closure during phonation, as well as vibratory patterns, was improved in all patients. | N/A | Yes adapted from Sodersten and Lindestad, 199077 |
| (Shoffel-Havakuk et al, 2014)49 | 1. Posterior glottic lesion | 1: Present or absent | Treatment failure occurred in three cases, in which the granuloma reoccurred or persisted during the follow-up period. 91% of the interventions were successful. | N/A | No |
| (Silbergleit et al, 2013)50 | 1. Vocal fold edge tumor side 2. Vocal fold edge nontumor side 3. Amplitude of vibration tumor side 4. Amplitude of vibration nontumor side 5. Mucosal wave tumor side 6. Mucosal wave nontumor side 7. Nonvibrating portion of the vocal folds tumor side 8. Nonvibrating portion of the vocal folds nontumor side |
1–8: 5-point equally appearing interval scale—0 represents normal function and 5 represents the most abnormal finding. | Amplitude of vibration and mucosal wave on the tumor side were significantly improved (P = 0.004; P = 0.003) at time 5 (20 weeks or more follow-up), when compared with baseline. When compared with the first post-procedure time point, significant improvements were found on the tumor side for amplitude (P = 0.001), nonvibrating portion (P = 0.001), and mucosal wave (P = 0.001) at the end of follow-up. On the nontumor side, significant improvements were found for mucosal wave (P = 0.013) and nonvibration portion (P = 0.008). |
3,5,6,7,8 | No |
| (Smith et al, 1995)51 | 1. Glottal configuration 2. Degree of glottal incompetence 3. False-fold compression hyperfunction 4. Anterior-posterior hyperfunction |
1: Complete; bowing; hourglass; anterior chink; posterior chink; incomplete 2: A 5-point scale was used: 1 = slight, folds just not touching; 3 = moderate, −50% of the length of the folds not touching with 1- to 2-mm gap; 5 = severe, no vocal fold contact throughout the length of the folds and a large (3–4 mm) glottal gap 3: 1 = no false fold overclosure, laryngeal ventricles easily seen; 2 = one or both false folds obscure laryngeal ventricles; 3 = one or both false folds obscure ventricles and a portion of the true vocal folds; 4 = one or both false folds obscure the entire true vocal folds but are not touching; 5 = false folds touch and cover the entire glottis 4: 1 = no shortening of glottal length compared with resting, nonphonating state; 2 = glottal length shortened by 25%; 3 = glottal length shortened by 50%; 4 = glottal length shortened by 75%; 5 = arytenoids touch laryngeal surface of epiglottis, obscuring glottal view |
At a loud phonation effort, there was no significant difference between the pre- and post-treatment outcomes in both groups. The authors reported a trend for improvement to normal glottal configuration after therapy in the vocal and respiratory therapy group, and a significant difference in change between the groups for degree of glottal incompetence (P < 0.01). | None | No |
| (Steward et al, 2004)52 | 1. True vocal fold edema 2. True vocal fold erythema 3. Arytenoid edema 4. Arytenoid erythema 5. Pachydermia 6. Granuloma 7. Stenosis 8. Ulceration 9. Nodules |
1–5: Scored as none to severe; 0 to 4 on Likert scale 6–9: Noted if present |
In both groups, a score reduction was noted at post-treatment when compared to baseline. The change did not reach statistical significance. | None | No |
| (Storck et al, 2007)53 | 1. Glottal closure 2. Mucosal wave |
1: No closure; anterior third; anterior two-thirds; complete 2: Improved or not improved |
Before surgery, 88% of the patients had a total glottal gap during phonation. After surgery, a complete glottal closure was observed in 77% of patients, and a closure of the anterior two thirds in six (23%) patients. Mucosal wave movements improved in all patients. | N/A | No |
| (Storck et al, 2010)54 | 1. Degree of glottal closure 2. Mucosal wave 3. Symmetry of mucosal waves 4. Periodicity of mucosal waves |
1: No; anterior one-third; anterior two-thirds, complete 2: Absent or visible 3: Severely asymmetrical or normal 4: Severally aperiodic or normal |
Improvements in glottal closure, symmetry of vocal fold vibration, mucosal wave, and presence of periodic vocal fold vibrations improved in most of the patients from both groups. The rate of complete glottal closure was higher in G2 (titanium implant) | N/A | Yes adapted from Hirano, 198169 |
| (Su et al, 2005)55 | 1. Relative size of the glottal gap | 1: 0 = no gap during phonation; 1 = minimal gap; 2 = small gap extending up to one-third of the anterior membranous vocal folds; 3 = moderate gap extending up to two-thirds of the posterior membranous vocal folds; 4 = complete glottal incompetence with no observable contact between the vocal folds | Glottal closure improved in 12 of the 13 patients after the intervention. Postoperatively, complete or nearcomplete glottal closure was observed in six patients, and partial closure in six patients. One patient had persistent complete glottal incompetence after surgery. | 1 | No |
| (Su et al, 2005)56 | 1. Relative size of the glottal gap | See above (Su et al, 2005)55 | A significant improvement in postoperative glottal closure at the horizontal and vertical planes was found when compared with baseline. After the surgery, complete or nearly complete glottal closure was obtained in 52% of the participants. Incomplete closure was observed in 14 participants, and none of the patients had a persistent complete glottal incompetence. | 1 | No |
| (Tsunoda et al, 2005)57 | 1. Glottal incompetence 2. Mucosal wave 3. Hyperadduction of false vocal folds |
1–3: Present or absent | Before the intervention, all patients had glottal incompetence and hyperadduction of the false vocal folds, and none had satisfactory mucosal waves. Six months after the transplantations, satisfactory glottal closure and mucosal wave were obtained in all but the oldest participant. Hyperadduction of the false vocal folds disappeared in seven cases. One year after the ATFV, satisfactory glottal closure, excellent mucosal wave, and no hyperadduction were observed for all subjects. | N/A | No |
| (van Gogh et al, 2006)58 | 1. Vocal fold edge R/L 2. Glottic closure 3. Phase closure 4. Vertical level of approximation 5. Amplitude R/L 6. Mucosal wave R/L 7. Vibratory behavior R/L 8. Phase symmetry 9. Periodicity 10. Ventricular folds—symmetry of movement 11. Arytenoid symmetry 12. Ventricular folds movement 13. Arytenoid movement 14. Hyperfunction |
1: 1 = smooth; 5 = rough irregular 2: Pictoral 3: 1 = open phase predominates; 5 = closed phase predominates 4: 1 = equal; 2 = right lower; 3 = left lower 5: 1 = normal; 2 = slightly decreased; 3 = moderately decreased; 4 = severely decreased; 5 = no visible movement 6: 1 = normal to 5 = absent 7: 1 = always fully present to 5 = complete absence always 8–9: 1 = regular to 4 = always irregular 10–11: R > L; L > R; equal 12: 1 = normal to 4 = full compress 13: 1 = normal to 3 = poor 14: 1 = not present; 2 = sometimes present; 3 = always present |
Following voice therapy, no parameter was significantly changed when compared with baseline, except for the left vocal fold edge, which became more irregular (P = 0.034) than before treatment. | None (1 was significantly worsened) | Yes (Hirano and Bless, 1993)70 |
| (Wang et al, 2013)59 | 1. Recovery | 1: 1 = complete recovery (normal mucosal wave propagation with symmetric phase and amplitude); 2 = much improvement (normal mucosal wave propagation with minimal asymmetric phase or amplitude); 3 = some improvement (presence of phase asymmetry or decreased amplitude, but the mucosal wave was improved compared with baseline); 4 = no change or worse | After 6 weeks, examination in the polypectomy group showed complete recovery in 14 patients (70%) and much improvement of vocal fold vibration in 5 (25%). In the KTP laser treatment only, results at 6 weeks showed complete recovery in 6 patients (38%), much improvement in 6 (38%), some improvement in 2 (12%), and no improvement in 2 (12%). | N/A | No |
| (Wang et al, 2013)60 | 1. Lesion size | 1: 1 = complete resolution of the vocal lesions; 2 = much improvement (>50% of lesion size reduction); 3 = some improvement (<50% of lesion size reduction); 4 = no change | Three months after treatment, vocal fold lesions were completely resolved in 10 subjects and much improved in 19 subjects. There was no significant difference in outcomes between the nodules and the polyps groups. | N/A | No |
| (Wang et al, 2012)61 | 1. Closure pattern of vocal folds | 1: 1 = predominantly closed; 2 = closed or open; 3 = predominantly open; 4 = always open | The pattern of glottic closure was “predominantly open” before the procedure and significantly improved to a “half-open and halfclosed” pattern 3 months post intervention (P < 0.001). | 1 | No |
| (Wang et al, 2011)62 | 1. Glottal closure 2. Vocal fold position 3. Vocal fold edge of paralyzed side 4. Phase symmetry 5. Regularity |
1: 0 = complete; 1 = slightly incomplete; 2 = moderately incomplete; 3 = severely incomplete 2: 0 = midline; 1 = paramedian; 2 = intermediate; 3 = lateral 3: 0 = straight; 1 = mildly bowing; 2 = moderately bowing; 3 = severely bowing 4: 0 = normal; 1 = mildly asymmetrical; 2 = moderately asymmetrical; 3 = severely asymmetrical 5: 0 = normal; 1 = mildly irregular; 2 = moderately irregular; 3 = severely irregular |
Glottal closure, vocal fold position, vocal fold edge, phase symmetry, and regularity were significantly improved after the surgery when compared with baseline (P < 0.001). | 1–5 | Yes, adapted from Lorenz et al, 200873; Rosen, 200574 |
| (Wang et al, 2011)63 | 1. Glottal closure 2. Vocal fold position 3. Vocal fold edge of paralyzed side 4. Phase symmetry 5. Regularity |
See above (Wang et al, 2011)62 | Glottal closure, vocal fold position, vocal fold edge, phase symmetry, and regularity were significantly improved postoperatively (P < 0.001). | 1–5 | Yes, adapted from Lorenz et al, 200873; Rosen, 200574 |
| (Woo et al, 2013)64 | 1. Vocal gap size | 1: Disappeared; improved; no change | The vocal gap disappeared in all patients at 1 month post-intervention. However, at 3 months, a vocal gap was observed in some of the patients in G1 (cricothyroid) group but not in G2 (thyrohyoid). | N/A | No |
| (Yilmaz, 2012)65 | 1. Glottal closure 2. Mucosal wave amplitude 3. Mucosal wave symmetry 4. Mucosal wave periodicity |
1: 0 = normal; 1 = anterior gap; 2 = posterior gap; 3 = hourglass; 4 = no contact 2: 0 = normal; 1 = mild; 2 = moderate; 3 = severe 3–4: 0 = normal; 1 = mild irregularity; 2 = moderate irregularity; 3 = always irregular |
Glottal closure and mucosal wave amplitude improved significantly after the procedure (P < 0.001; P = 0.002). Mucosal wave symmetry and mucosal wave periodicity did not improve significantly following the surgery. | 1–2 | No |
| (Yilmaz and Sozen, 2012)66 | 1. Mucosal wave amplitude 2. Mucosal wave symmetry 3. Mucosal wave periodicity 4. Glottal closure |
1–4: 0 = normal; 1 = mild; 2 = moderate; 3 = severe abnormality | At the end of the 4-week study, all patients had a normal videolaryngostroboscopy (VLS). | N/A | No |
Notes: when possible, direct quotes from the articles were used to report the pre to post treatment changes.
Abbreviations: G1, group 1; G2, group 2; G3, group 3; N/A, not available; RFS, Reflux Finding Score; SRI, Stroboscopy Research Instrument; VAS, visual analog scale; v.f., vocal fold.
Scales
Thirty-six (73%) of the 49 parameters were reported using more than one scale. These scales varied from dichotomous ratings presence or absence to 100-point visual analog scales. The number of scales was, as expected, greatest for the most frequently reported features: magnitude of glottal gap was reported using 24 scales; mucosal wave presence or amplitude was reported using 17 scales; location or shape of glottal gap was reported using 11 scales; regularity of vibration was reported using 11 scales; and phase symmetry was reported using 10 scales. The number of scales used for each parameter varied from 1 to 24, with an average of four different scales per parameter. A list of the parameters, terms, and scales can be found in Table 3.
Treatment differences and statistical significance
Statistical significance of the pre to post-treatment change in LES parameters was assessed and reported in 33 (53%) of the included studies. Of those, 27 articles found a statistically significant improvement following treatment for at least one of the LES parameters investigated, and six articles reported no change. One of those studies also found a negative effect of treatment on one of the assessed parameters (vocal fold edge).58 The percentage of times a parameter showed a significant post-intervention change was calculated for parameters on which statistical significance was assessed in at least five articles. Parameters that most commonly revealed statistically significant differences from pre treatment to post treatment were regularity of vibration (significant change found 88% of the time); phase symmetry (85%); mucosal wave quality (83%); amplitude of vibration (83%); location or shape of glottal gap (80%); mucosal wave presence or amplitude (75%); and magnitude of glottal gap (72%). A significant change for vocal fold edema was found only 40% of the time, and no significant change was found for specific lesions (size and presence) in any of the five articles where that parameter was assessed.
DISCUSSION
The present review provides information on the different LES parameters and scales used across studies to measure laryngeal changes following treatment. The results confirm the lack of a broadly accepted standardized rating form and reveal a wide variety of laryngeal parameters, terms for laryngeal parameters, and scales used to rate those parameters in the literature. The incompatibility of the terms, definitions, and scales used across treatment outcome research has implications for the methodological rigor of any single study and greatly hinders the ability to compare outcomes across studies, with negative impacts on clinical practice as well as research progress. The results of the review are discussed below.
Why do so few articles use LES as a treatment outcome measure?
One key element of information provided through the literature search is that voice treatment studies do not commonly use LES to measure pre to post-intervention changes. Even though an initial stroboscopic evaluation is usually performed on the participants before their inclusion in the study, results from the visual examination of the larynx are rarely included in the outcome measures. A recent literature review78 on behavioral voice therapy effectiveness revealed that, of the 15 included randomized controlled trials, only three included data from LES in their outcome measures, making it the least popular assessment. In the present review, LES was also rarely used to measure changes related to behavioral voice therapy. Only 11% of the included articles involved voice therapy by a speech-language pathologist (SLP), whereas almost two-thirds of the articles comprised a surgical or injection intervention. One reason for this unequal distribution could be related to the fact that otolaryngologists often have more access to stroboscopy equipment than SLPs, making it more realistic for them to use LES to measure treatment effects. This difference in use, if due to access, may signify a lack of research collaboration between SLPs and otolaryngologists.
The overall scarcity of LES as a treatment outcome measure in voice research could also be related to the equivocal definitions of the LES parameters and the unclear link between changes in these parameters and clinically meaningful improvements. Even though the clinical value of LES for diagnosing voice disorders and defining therapy goals has previously been demonstrated,2,79,80 progress is still needed to gain a better understanding of how specific LES findings translate into vocal symptoms or behaviors and how these can be beneficially modified through treatment. Without a thorough understanding of these relationships, interpreting data obtained with LES is challenging, and this potentially detracts researchers from using it as an outcome measure. Hopefully, the development of a standardized nomenclature for LES will encourage researchers to adopt LES as a treatment outcome measure to help establish correlations between LES parameters and vocal symptoms and behaviors.
Parameters: What is used?
Parameters most frequently reported were magnitude of glottal gap, location or shape of glottal gap, mucosal wave amplitude, regularity or periodicity of vibration, phase symmetry, and specific lesions—all present in more than 15% of the included studies. Most of these parameters significantly improved at least 70% of the time from pre to post-treatment measures, except for the specific lesions parameter, which did not show a significant improvement in any of the five articles that reported this parameter. Although this could possibly be explained by the lack of validity of the measure and scales used, it may also be that lesions are more difficult to change than other laryngeal parameters.
Parameters: The issue of reliability
Reliability is a factor of primary importance to ensure the value of findings related to LES ratings. Despite their seemingly good ability to detect treatment-related changes, most of the frequently reported parameters have been associated with reliability concerns in previous studies. The reliability issues in LES ratings have been recently described in a literature review by Bonilha et al.1 Results of the review revealed that reliability analysis results for LES ratings were reported in approximately 10% of the articles, with at least half of these results indicating poor inter- or intra-rater reliability.1 Results from Bonilha et al’s review also indicated that, overall, functional (temporally dependent) parameters were less reliable than anatomic (stationary dependent) parameters.1 Mucosal wave, regularity of vibration, and phase symmetry, identified as some of the most frequently reported parameters, are all temporally dependent because they involve a movement pattern, contrary to static, geometric parameters that merely involve the shape of a structure.81,82 The challenge of rating temporally dependent parameters has also been raised by Poburka and Bless82 in their work to improve the reliability of stroboscopy ratings. They found that amplitude, mucosal wave, phase closure, and regularity were the features most likely to cause a disagreement between expert raters. When comparing experienced and student raters with expert raters, vocal fold edge and amplitude of excursion measures consistently had the highest correlations, whereas phase closure and regularity—two dynamic parameters—had the lowest correlations. In a later study, Poburka81 tested an improved Stroboscopy Examination Rating Form (SERF) on the constructs of agreement and reliability using three judges who commonly use stroboscopy clinically. He provided agreement data for exact agreement (three judges agreed) and consensus agreement (two out of three judges agreed). Consensus agreement was above 80% for all features except mucosal wave, edge-straightness left, phase closure, and phase symmetry. The author pointed out that overall, the parameters with the lowest reliability across all trials of inter-rater agreement and intra-rater reliability were phase closure, phase symmetry, and phase regularity. In a study by Rosen,74 another stroboscopy rating tool was tested, and four features did not show adequate reliability when assessed by raters with high intra-rater reliability: closure pattern, periodicity (right-side), amplitude (right), and nonvibratory (right). These features also had inadequate interclass correlations, which could be explained, in part, by low variability of some of these parameters. Finally, a recent article by Poburka et al3 revealed that some parameters were more difficult to rate than others, leading to a “could not rate” answer from more than one rater. Parameters most associated with this answer were mucosal wave, nonvibratory portion, amplitude, phase closure, symmetry, and regularity. Interestingly, these represent temporally dependent parameters also associated with poor reliability in the previously mentioned studies and are also among the most frequently reported parameters in the studies included in this review.
Terms for parameters and scales
One reason for the abovementioned reliability issues might reside in the general ambiguity that surrounds LES parameters and their definitions. The fact that 49 different parameters were used across the included studies, each associated with up to 15 different terms and 24 different scales, is indicative of a serious lack of agreement in the field regarding (1) which parameters should be assessed to measure voice treatment outcomes and (2) what terms should be used to define the parameters without ambiguity. This lack of agreement is likely to cause confusion among raters and should be compensated for by clear instructions and definitions for each parameter and each level of the scales used. However, the literature review recently conducted by Bonilha et al1 revealed that out of 80 articles using LES to measure changes following treatment, only two reported attempts to improve rater reliability by either providing a 1-hour training session52 or by offering clear anatomic definitions for each parameter and using feature-specific scales.51 The same problem applies to the majority of the articles included in the present review—there was no mention of instructions offered to the raters, despite the use of ambiguous terms to describe the LES parameters. For instance, the broad category mucosal wave contains numerous examples of inexplicit terms, such as mucosal wave characteristics, quality of mucosal wave, or mucosal wave. In these cases, it is not obvious what the rater should be evaluating: is the term referring to mucosal wave amplitude, mucosal wave amplitude symmetry, or mucosal wave presence? In some situations, the associated scale provides supplemental information on what is being assessed, such as in the following example in which it becomes clear that the mucosal wave amplitude is the feature of interest: 1 = absent; 2 = limited to the most medial edge of the vocal folds; 3 = present laterally up to one-fourth of the width of the vocal folds; 4 = present up to but less than one-half the width of the vocal folds; and 5 = present at more than one-half the width of the vocal folds (normal).6 Conversely, some scales, such as 1 (normal) to 5 (abnormal), do not clarify the vagueness of the terms employed to label the LES parameters.21
Scales
The scales used to judge and report laryngeal anatomy and physiology must consider the number of increments of the feature that a trained clinician can reliably differentiate. For example, phase closure has been rated on a three-level scale (predominately open, equally open and closed, predominately closed) and a 100-level scale (percentage of the cycle that is open). Yet we have no knowledge of whether a trained clinician can differentiate three levels, 100 levels, or somewhere in between. If we ask a clinician to differentiate three levels when they can truly differentiate five levels, we lose available, needed precision of the measure important for detecting treatment effects. Conversely, if we ask a clinician to differentiate 100 levels when they can truly differentiate only five levels, the clinician is not able to be so precise and will be reliable only to +/− 10 levels, creating inaccuracy in the measure. Scales not aligned with clinicians’ perceptual ability will increase measurement error. Furthermore, the use of different scales for the same parameter hinders the readers’ ability to compare results across studies, which has direct implications for evidence-based practice.
The majority of the scales that were used in the studies were ordinal scales instead of interval scales. This means that the intervals between the levels of the scales may be inconsistent or unknown. In other words, ordinal ranks are discrete categories similar to nominal labels, with the difference that they are ranked on the basis of a specific characteristic.83 This has implications regarding the interpretation and validity of change scores: because ordinal values are not true quantities, they do not have arithmetic properties and are more appropriate for descriptive analyses than other forms of significance testing.83 An appropriate method for interpretation of change based on ordinal values is to analyse the results in terms of frequency counts83; for example, what percentage of participants changed from severe to moderate as a result of the intervention. Nominal values were also frequently used. Most of the nominal scales used in the reviewed studies were dichotomous, for example, improvement or no improvement; present or absent; yes or no; complete or incomplete.
Efforts to improve stroboscopy rating forms
The validity and reliability of LES as a treatment outcome measure is greatly dependent on the clinician’s interpretation of the findings. As discussed in the previous sections, reliability issues have been associated with many of the most commonly used LES parameters and this could be related to the unclear terms and definitions associated with the parameters, as well as to limited instructions and training offered to the raters. Efforts have been made in the past to overcome these limitations and to develop, disseminate, and implement a standardized, reliable, and valid protocol for the interpretation of laryngeal features assessed with LES. However, the field of voice, as reflected in the reviewed scientific literature, has mostly ignored those efforts. Only 29% of the reviewed articles reported using a previously published protocol. In most of these cases, the protocol was modified instead of being used as originally published, consequently altering a main purpose of using a protocol—to allow for comparison across different studies using the exact same scales and parameters. Protocols most commonly used were versions of the Bless et al68 rating form (n = 4) and the Reflux Finding Score67 (n = 3). Other important efforts to improve LES ratings, such as those described below, were not employed in the reviewed studies with the exception of the SRI74 used in two studies.
Efforts to improve the reliability of ratings from LES have been reported in four articles. Poburka and Bless82 devised and reported on the effectiveness of a multimedia computer-based method for LES rating training. The stated purpose of the project was to overcome known problems in inter- and intra-rater reliability of stroboscopic assessment by providing training involving written definitions, graphics, and video clips of laryngeal parameters. The parameters included were supraglottic activity, vocal fold edge, amplitude, mucosal wave, phase closure, phase symmetry, regularity, and glottal closure. Although training did not significantly improve reliability in experienced raters, it led to improved agreement between students and expert judges. After training, the students’ performance was comparable with that of experienced raters. It was hypothesized by the authors that example-based training helped the students develop an internal conceptualization of normal and abnormal features, a skill that didactic knowledge by itself cannot provide.82 Although rating skills seem to improve with experience, offering training to young clinicians could positively impact their reliability and compensate for a lack of experience.
Poburka81 developed a new LES rating form, the SERF, to resolve known flaws in LES ratings and improve inter- and intra-rater reliability. The features targeted for improvement were supraglottic activity, vocal fold edge, phase closure, phase symmetry, regularity, and glottic closure. The author sought to improve the reliability of the ratings of these features by modifying some of the instructions to enhance clarity and by using a graphical or pictorial type of rating as opposed to the numerical Likert-type scale typically used. However, the features of vocal fold edge smoothness, vocal fold edge straightness, phase closure, phase symmetry, and regularity were still rated on either four- or six-level ordinal scales. Although the author concluded that the rating scale did not completely solve the reliability problems, he did mention several benefits of its use that were acknowledged by the raters: faster rating times, less hesitation in making the ratings, and less confusion over left and right orientations on the form. Moreover, the study helped gather more information for further improvement of the rating form.
Rosen74 published a study entitled, “Stroboscopy as a Research Instrument: Development of a Perceptual Evaluation Tool,” with the objective of addressing the need for a LES protocol that provides reproducible and reliable results. He approached the problem of lack of standardization primarily from a research mindset; however, he discussed how this lack of standardization also inhibits education, communication, and advancement of the field as a whole. The first step of the project was to develop the tool based on a literature review and the author’s clinical knowledge, which led to the inclusion of six components: symmetry, amplitude, periodicity, identification of non-vibratory segments, duration of closure during vocal fold vibration, and closure pattern, all of which were rated on a scale ranging from three, four, or six points. Standardized instructions were given to each of the 18 reviewers of varying experience, but no training was employed. An interesting finding from this study is that experienced raters were not found to have higher interclass reliability results than inexperienced raters, and thus, experience in LES does not constitute an advantage when using the SRI. In the analysis of intra-rater reliability, Rosen found that the top 10 raters had a reliability of 0.81–0.99 for all features, and that the lowest intra-rater reliability reported was 0.43. Rosen concludes with acknowledgment of the need for further research and with two main suggestions to enhance reliability of LES ratings in research: (1) to include a variability check for each parameter before proceeding to ratings, and (2) to select judges with an intra-rater reliability of at least 0.80.
Despite the development of these forms and the efforts made to improve their reliability, there is still no standardized form widely used for LES ratings reported as outcomes measures in research. In addition, a new technology, high-speed videoendoscopy (HSV), is being increasingly used both in research and in clinical settings without a widely accepted standardized form for rating laryngeal parameters. Based on these considerations, Poburka et al3 refined a previously published protocol, the SERF, and adapted it for the rating of laryngeal parameters from both LES and HSV. They named the new rating form VALI.3 To improve the reliability of the ratings, they included four key elements to the forms and associated training: definitions, graphics, and rating instructions for each parameter, as well as video samples for training. Ten parameters were chosen for inclusion: amplitude, mucosal wave, nonvibratory portion, supraglottal activity, phase closure, symmetry, regularity, glottal closure, vertical level, and free-edge contour. In the HSV form, regularity was not included, and two supplemental parameters were added (glottal cycle periodicity and other voice vibratory patterns). The authors compared their reliability results with those of previous studies and noted stronger correlations, particularly for the parameters of phase closure and regularity. They hypothesized that the graphic illustrations and the specific training provided in the VALI helped elucidate the temporal aspect of these features, therefore facilitating the rating process and improving reliability. The VALI form is the result of many years of research pertaining to the development of a valid and reliable instrument for assessing laryngeal features. It contains half of the 20 categories of parameters that were identified in the present review, including the most frequent ones (glottal gap, mucosal wave, regularity of vibration, and phase symmetry). Most of the parameters that are not comprised in the form can be included in the nonvibratory observations section (such as lesions, edema, erythema, granuloma, mucus, pachydermia, and stenosis or web). When possible, the form uses ratio scales (with an absolute 0), which is the most appropriate scale for statistical comparisons and, therefore, for measuring change following treatment. Based on these considerations and on the improved reliability scores as reported by the authors, the VALI is a promising choice as a widely adoptable assessment form for stroboscopy ratings.
Clinical implications
The lack of a widely used, standardized, reliable, and valid rating system for LES impacts clinicians and patients in two main ways. First, one clinician cannot accurately interpret another clinician’s LES report if there is no common language (terminology, definitions, defined scales, etc) used. This results in, at best, the clinician accessing the recording of the examination and using valuable clinic time to rate it themselves. More often, a clinician will either repeat the examination because of lack of access to the original recording or, in the most likely scenario, will treat the patient based on their interpretation of the LES report because they do not have access to the LES equipment. Second, even when used by the same clinician, without a standardized, reliable, and valid scoring system, it is not possible to confidently assess treatment effects using LES as one cannot know if the variation in the scores is actually because of the patient improving. All of these situations result in a less than optimally efficient clinic indicating the potential to improve patient care and use of health-care resources if a widely used standardized, reliable, and valid protocol was adopted. Considering results from LES are judged by clinicians to be important for defining therapy goals, educating the patient and evaluating therapy outcomes,2 the lack of a widely used, standardized, reliable, and valid rating system has clear clinical implications.
Research implications
The results of this systematic review have implications for research in two ways: (1) methodological rigor of individual studies and (2) research progress in the field. The results of individual studies that do not use a rating system shown to be reliable and valid are questionable and may not be reproducible by other researchers using different rating systems. On a larger scale, research progress in the field is hindered by the lack of ability to compare research results across treatment studies that use different rating systems (parameters, scales, terms, definitions, etc). The lack of comparability across studies requires each new treatment study to be compared anew with all other viable treatments—an expensive and lengthy process—or to not fully vet the improvements to patient care made by that new treatment.
General comments
The field of voice has made largely unrecognized gains in creating standardized protocols for the assessment of laryngeal parameters from LES. Those gains are not reflected in the current literature. The adoption of one of the recently developed standardized protocol, such as the VALI,3 is likely to have a major impact on the field and positively affect clinicians, patients, and researchers. Other fields have created standardized broadly used protocols for similar clinical assessments. One notable example is in swallowing disorders; the Modified Barium Swallow Impairment Profile84 is an assessment protocol, scoring and reporting system, as well as a training method for the judgment of swallowing impairment from modified barium swallow studies. The implementation of this method has allowed the field of swallowing to make noteworthy progress, and a similar method may be the solution to the disparate use and reporting of laryngeal parameters from LES.
Limitations of the review
This review contains limitations that may have influenced our findings. First, it included only articles that were published in English. Moreover, it is possible that the search terms used did not detect all pertinent articles related to the topic. The review did not include a robust statistical analysis combining results of eligible studies, because the wide variety of parameters and scales used did preclude such an analysis. Regarding the interpretation of the results, it is possible that some of the terms related to the stroboscopic parameters were not classified in the categories they were intended to represent: the terms were classified based on the information available in the articles, and when no sufficient information was provided, assumptions were made by the authors of the review.
CONCLUSIONS
An assessment of the voice treatment outcome literature revealed an overwhelming lack of standardization in terminology, operational definitions, parameters, and associated scales. This lack of standardization has clear negative implications for patient management and research progress. There has been much effort devoted to the development of standardized protocols to evaluate laryngeal parameters from LES. To date, these protocols are not used in the treatment outcome literature. The leaders of the field and entities with power, namely journal editors and funding agencies, should rally behind the need for standardization to ultimately improve the efficacy and effectiveness of patient care.
Acknowledgments
This publication was supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH Grant Numbers KL2 RR029880 and KL2 TR000060.
REFERENCES
- 1.Bonilha HS, Focht KL, Martin-Harris B. Rater methodology for stroboscopy: a systematic review. J Voice. 2015;29:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrman A. Common practices of voice therapists in the evaluation of patients. J Voice. 2005;19:454–469. [DOI] [PubMed] [Google Scholar]
- 3.Poburka BJ, Patel RR, Bless DM. Voice-Vibratory Assessment With Laryngeal Imaging (VALI) form: reliability of rating stroboscopy and high-speed videoendoscopy. J Voice. 2016;31:513. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaver ME, Stasney CR, Weitzel E, et al. Diagnosis of laryngopharyngeal reflux disease with digital imaging. Otolaryngol Head Neck Surg. 2003;128:103–108. [DOI] [PubMed] [Google Scholar]
- 6.Chhetri DK, Berke GS. Injection of cultured autologous fibroblasts for human vocal fold scars. Laryngoscope. 2011;121:785–792. [DOI] [PubMed] [Google Scholar]
- 7.DeJonckere PH, Crevier-Buchman L, Marie JP, et al. Implementation of the European Laryngological Society (ELS) basic protocol for assessing voice treatment effect. Rev Laryngol Otol Rhinol (Bord). 2003;124:279–283. [PubMed] [Google Scholar]
- 8.Demirhan E, Cukurova I, Arslan IB, et al. Quantum molecular resonance-assisted phonomicrosurgery: preliminary experience. Otolaryngol Head Neck Surg. 2015;152:189–192. [DOI] [PubMed] [Google Scholar]
- 9.Dixon HS. Dysphonia and delayed food allergy: a provocation/neutralization study with strobovideolaryngoscopy. Otolaryngol Head Neck Surg. 1999;121:418–429. [DOI] [PubMed] [Google Scholar]
- 10.Djukic V, Milovanovic J, Jotic AD, et al. Stroboscopy in detection of laryngeal dysplasia effectiveness and limitations. J Voice. 2014;28:262.e13–262.e21. [DOI] [PubMed] [Google Scholar]
- 11.Fass R, Noelck N, Willis MR, et al. The effect of esomeprazole 20 mg twice daily on acoustic and perception parameters of the voice in laryngopharyngeal reflux. Neurogastroenterol Motil. 2010;22:134–141, e44–5. [DOI] [PubMed] [Google Scholar]
- 12.Finck C, Lefebvre P. Implantation of esterified hyaluronic acid in microdissected Reinke’s space after vocal fold microsurgery: first clinical experiences. Laryngoscope. 2005;115:1841–1847. [DOI] [PubMed] [Google Scholar]
- 13.Finck CL, Harmegnies B, Remacle A, et al. Implantation of esterified hyaluronic acid in microdissected Reinke’s space after vocal fold microsurgery: short- and long-term results. J Voice. 2010;24:626–635. [DOI] [PubMed] [Google Scholar]
- 14.Halawa WE, Rodriguez Fernandez Freire A, Munoz IV, et al. Assessment of effectiveness of acoustic analysis of voice for monitoring the evolution of vocal nodules after vocal treatment. Eur Arch Otorhinolaryngol. 2014;271:749–756. [DOI] [PubMed] [Google Scholar]
- 15.Halderman AA, Bryson PC, Benninger MS, et al. Safety and length of benefit of restylane for office-based injection medialization-a retrospective review of one institution’s experience. J Voice. 2014;28:631–635. [DOI] [PubMed] [Google Scholar]
- 16.Hallen L, Testad P, Sederholm E, et al. DiHA (dextranomers in hyaluronan) injections for treatment of insufficient closure of the vocal folds: early clinical experiences. Laryngoscope. 2001;111:1063–1067. [DOI] [PubMed] [Google Scholar]
- 17.Hamdan AL, Ziade G, Jaffal H, et al. Transnasal injection laryngoplasty. Ann Otol Rhinol Laryngol. 2015;124:474–479. [DOI] [PubMed] [Google Scholar]
- 18.Hillel AT, Johns MM 3rd, Hapner ER, et al. Voice outcomes from subligamentous cordectomy for early glottic cancer. Ann Otol Rhinol Laryngol. 2013;122:190–196. [DOI] [PubMed] [Google Scholar]
- 19.Iloabachie K, Nathan CO, Ampil F, et al. Return of vocal cord movement: an independent predictor of response to nonsurgical management of laryngeal cancers. Laryngoscope. 2007;117:1925–1929. [DOI] [PubMed] [Google Scholar]
- 20.Jensen JB, Rasmussen N. Phonosurgery of vocal fold polyps, cysts and nodules is beneficial. Dan Med J. 2013;60:A4577. [PubMed] [Google Scholar]
- 21.Karpenko AN, Dworkin JP, Meleca RJ, et al. Cymetra injection for unilateral vocal fold paralysis. Ann Otol Rhinol Laryngol. 2003;112:927–934. [DOI] [PubMed] [Google Scholar]
- 22.Keilmann A, Napiontek U, Engel C, et al. Long-term functional outcome after unilateral cordectomy. ORL J Otorhinolaryngol Relat Spec. 2011;73:38–46. [DOI] [PubMed] [Google Scholar]
- 23.Kodama N, Sanuki T, Kumai Y, et al. Long-term vocal outcomes of refined nerve-muscle pedicle flap implantation combined with arytenoid adduction. Eur Arch Otorhinolaryngol. 2015;272:681–688. [DOI] [PubMed] [Google Scholar]
- 24.Lam PK, Ng ML, Cheung TK, et al. Rabeprazole is effective in treating laryngopharyngeal reflux in a randomized placebo-controlled trial. Clin Gastroenterol Hepatol. 2010;8:770–776. [DOI] [PubMed] [Google Scholar]
- 25.Lan MC, Hsu YB, Chang SY, et al. Office-based treatment of vocal fold polyp with flexible laryngosvideostroboscopic surgery. J Otolaryngol Head Neck Surg. 2010;39:90–95. [PubMed] [Google Scholar]
- 26.Law T, Lee KY, Ho FN, et al. The effectiveness of group voice therapy: a group climate perspective. J Voice. 2012;26:e41–e48. [DOI] [PubMed] [Google Scholar]
- 27.Lee SW, Park KN, Welham NV. Clinical features and surgical outcomes following closed reduction of arytenoid dislocation. JAMA Otolaryngol Head Neck Surg. 2014;140:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WT, Milstein C, Hicks D, et al. Results of ansa to recurrent laryngeal nerve reinnervation. Otolaryngol Head Neck Surg. 2007;136:450–454. [DOI] [PubMed] [Google Scholar]
- 29.Li AJ, Johns MM, Jackson-Menaldi C, et al. Glottic closure patterns: type I thyroplasty versus type I thyroplasty with arytenoid adduction. J Voice. 2011;25:259–264. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Chen D, Song X, et al. The effect of patient age on the success of laryngeal reinnervation. Eur Arch Otorhinolaryngol. 2014;271:3241–3247. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Chen S, Wang W, et al. Effect of duration of denervation on outcomes of ansa-recurrent laryngeal nerve reinnervation. Laryngoscope. 2014;124:1900–1905. [DOI] [PubMed] [Google Scholar]
- 32.Maia ME, Maia MO, Gama AC, et al. Immediate effects of the high-pitched blowing vocal exercise. Jornal da Sociedade Brasileira de Fonoaudiologia. 2012;24:1–6. [DOI] [PubMed] [Google Scholar]
- 33.Malik A, Ramalingam WV, Nilakantan A, et al. Comparison of the use of silastic with titanium prefabricated implant in type I thyroplasty. Braz J Otorhinolaryngol. 2014;80:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maronian N, Waugh P, Robinson L, et al. Electromyographic findings in recurrent laryngeal nerve reinnervation. Ann Otol Rhinol Laryngol. 2003;112:314–323. [DOI] [PubMed] [Google Scholar]
- 35.Milstein CF, Akst LM, Hicks MD, et al. Long-term effects of micronized Alloderm injection for unilateral vocal fold paralysis. Laryngoscope. 2005;115:1691–1696. [DOI] [PubMed] [Google Scholar]
- 36.Monini S, Di Stadio A, Vestri A, et al. Silent reflux: ex juvantibus criteria for diagnosis and treatment of laryngeal disorders. Acta Otolaryngol. 2006;126:866–871. [DOI] [PubMed] [Google Scholar]
- 37.Morgan JE, Zraick RI, Griffin AW, et al. Injection versus medialization laryngoplasty for the treatment of unilateral vocal fold paralysis. Laryngoscope. 2007;117:2068–2074. [DOI] [PubMed] [Google Scholar]
- 38.Mortensen M, Woo P. Office steroid injections of the larynx. Laryngoscope. 2006;116:1735–1739. [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa H, Miyamoto M, Kusuyama T, et al. Resolution of vocal fold polyps with conservative treatment. J Voice. 2012;26:e107–e110. [DOI] [PubMed] [Google Scholar]
- 40.Nam IC, Bae JS, Chae BJ, et al. Therapeutic approach to patients with a lower-pitched voice after thyroidectomy. World J Surg. 2013;37:1940–1950. [DOI] [PubMed] [Google Scholar]
- 41.Paniello RC, Sulica L, Khosla SM, et al. Clinical experience with Gray’s minithyrotomy procedure. Ann Otol Rhinol Laryngol. 2008;117:437–442. [DOI] [PubMed] [Google Scholar]
- 42.Portnoy JE, Gregory ND, Cerulli CE, et al. Efficacy of super high dose proton pump inhibitor administration in refractory laryngopharyngeal reflux: a pilot study. J Voice. 2014;28:369–377. [DOI] [PubMed] [Google Scholar]
- 43.Rihkanen H, Reijonen P, Lehikoinen-Soderlund S, et al. Videostroboscopic assessment of unilateral vocal fold paralysis after augmentation with autologous fascia. Eur Arch Otorhinolaryngol. 2004;261:177–183. [DOI] [PubMed] [Google Scholar]
- 44.Rontal E, Rontal M. Permanent medialization of the paralyzed vocal fold utilizing botulinum toxin and Gelfoam. J Voice. 2003;17:434–441. [DOI] [PubMed] [Google Scholar]
- 45.Sanuki T, Yumoto E, Nishimoto K, et al. Laryngeal reinnervation featuring refined nerve-muscle pedicle implantation evaluated via electromyography and use of coronal images. Otolaryngol Head Neck Surg. 2015;152:697–705. [DOI] [PubMed] [Google Scholar]
- 46.Schindler A, Mozzanica F, Maruzzi P, et al. Multidimensional assessment of vocal changes in benign vocal fold lesions after voice therapy. Auris Nasus Larynx. 2013;40:291–297. [DOI] [PubMed] [Google Scholar]
- 47.Schneider B, Bigenzahn W, End A, et al. External vocal fold medialization in patients with recurrent nerve paralysis following cardiothoracic surgery. Eur J Cardiothorac Surg. 2003;23:477–483. [DOI] [PubMed] [Google Scholar]
- 48.Schneider B, Denk DM Bigenzahn W. Acoustic assessment of the voice quality before and after medialization thyroplasty using the titanium vocal fold medialization implant (TVFMI). Otolaryngol Head Neck Surg. 2003;128:815–822. [DOI] [PubMed] [Google Scholar]
- 49.Shoffel-Havakuk H, Halperin D, Yosef L, et al. Lesions of the posterior glottis: clinical and pathologic considerations and treatment outcome. J Voice. 2014;28:263.e1–263.e8. [DOI] [PubMed] [Google Scholar]
- 50.Silbergleit AK, Somers ML, Schweitzer VG, et al. Vocal fold vibration after photofrin-mediated photodynamic therapy for treatment of early-stage laryngeal malignancies. J Voice. 2013;27:762–764. [DOI] [PubMed] [Google Scholar]
- 51.Smith ME, Ramig LO, Dromey C, et al. Intensive voice treatment in Parkinson disease: laryngostroboscopic findings. J Voice. 1995;9:453–459. [DOI] [PubMed] [Google Scholar]
- 52.Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131:342–350. [DOI] [PubMed] [Google Scholar]
- 53.Storck C, Brockmann M, Schnellmann E, et al. Functional outcome of vocal fold medialization thyroplasty with a hydroxyapatite implant. Laryngoscope. 2007;117:1118–1122. [DOI] [PubMed] [Google Scholar]
- 54.Storck C, Fischer C, Cecon M, et al. Hydroxyapatite versus titanium implant: comparison of the functional outcome after vocal fold medialization in unilateral recurrent nerve paralysis. Head Neck. 2010;32:1605–1612. [DOI] [PubMed] [Google Scholar]
- 55.Su CY, Chuang HC, Tsai SS, et al. Bipedicled strap muscle transposition for vocal fold deficit after laser cordectomy in early glottic cancer patients. Laryngoscope. 2005;115:528–533. [DOI] [PubMed] [Google Scholar]
- 56.Su CY, Tsai SS, Chuang HC, et al. Functional significance of arytenoid adduction with the suture attaching to cricoid cartilage versus to thyroid cartilage for unilateral paralytic dysphonia. Laryngoscope. 2005;115:1752–1759. [DOI] [PubMed] [Google Scholar]
- 57.Tsunoda K, Kondou K, Kaga K, et al. Autologous transplantation of fascia into the vocal fold: long-term result of type-1 transplantation and the future. Laryngoscope. 2005;115(12 Pt 2 suppl 108):1–10. [DOI] [PubMed] [Google Scholar]
- 58.van Gogh CD, Verdonck-de Leeuw IM, Boon-Kamma BA, et al. The efficacy of voice therapy in patients after treatment for early glottic carcinoma. Cancer. 2006;106:95–105. [DOI] [PubMed] [Google Scholar]
- 59.Wang CT, Huang TW, Liao LJ, et al. Office-based potassium titanyl phosphate laser-assisted endoscopic vocal polypectomy. JAMA Otolaryngol Head Neck Surg. 2013;139:610–616. [DOI] [PubMed] [Google Scholar]
- 60.Wang CT, Lai MS, Liao LJ, et al. Transnasal endoscopic steroid injection: a practical and effective alternative treatment for benign vocal fold disorders. Laryngoscope. 2013;123:1464–1468. [DOI] [PubMed] [Google Scholar]
- 61.Wang CT, Liao LJ, Huang TW, et al. Preliminary report of vocal fold augmentation with cross-linked porcine collagen. Otolaryngol Head Neck Surg. 2012;146:606–610. [DOI] [PubMed] [Google Scholar]
- 62.Wang W, Chen D, Chen S, et al. Laryngeal reinnervation using ansa cervicalis for thyroid surgery-related unilateral vocal fold paralysis: a long-term outcome analysis of 237 cases. PLoS ONE. 2011;6:e19128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W, Chen S, Chen D, et al. Contralateral ansa cervicalis-to-recurrent laryngeal nerve anastomosis for unilateral vocal fold paralysis: a long-term outcome analysis of 56 cases. Laryngoscope. 2011;121:1027–1034. [DOI] [PubMed] [Google Scholar]
- 64.Woo SH, Son YI, Lee SH, et al. Comparative analysis on the efficiency of the injection laryngoplasty technique using calcium hydroxyapatite (CaHA): the thyrohyoid approach versus the cricothyroid approach. J Voice. 2013;27:236–241. [DOI] [PubMed] [Google Scholar]
- 65.Yilmaz T. Sulcus vocalis: excision, primary suture and medialization laryngoplasty: personal experience with 44 cases. Eur Arch Otorhinolaryngol. 2012;269:2381–2389. [DOI] [PubMed] [Google Scholar]
- 66.Yilmaz T, Sozen T. Microsuture after benign vocal fold lesion removal: a randomized trial. Am J Otolaryngol. 2012;33:702–707. [DOI] [PubMed] [Google Scholar]
- 67.Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001;111:1313–1317. [DOI] [PubMed] [Google Scholar]
- 68.Bless DM, Hirano M, Feder RJ. Videostroboscopic evaluation of the larynx. Ear Nose Throat J. 1987;66:289–296. [PubMed] [Google Scholar]
- 69.Hirano M. In: Arnold G, Winckel F, Wyke BD, eds. Clinical Examination of Voice. Vienna: Springer-Verlag; 1981. [Google Scholar]
- 70.Hirano M, Bless D. Videostroboscopic Examination of the Larynx. San Diego: Singular Publishing Group Inc; 1993. [Google Scholar]
- 71.Ott S, Klingholz F, Willich N, et al. Assessing the quality of the speaking voice after therapy of T1 and T2 vocal cord cancers. Laryngorhinootologie. 1992;71:236–241. [DOI] [PubMed] [Google Scholar]
- 72.Lee SW, Kim JW, Koh YW, et al. Comparative analysis of efficiency of injection laryngoplasty technique for with or without neck treatment patients: a transcartilaginous approach versus the cricothyroid approach. Clin Exp Otorhinolaryngol. 2010;3:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorenz RR, Esclamado RM, Teker AM, et al. Ansa cervicalis-to-recurrent laryngeal nerve anastomosis for unilateral vocal fold paralysis: experience of a single institution. Ann Otol Rhinol Laryngol. 2008;117:40–45. [DOI] [PubMed] [Google Scholar]
- 74.Rosen CA. Stroboscopy as a research instrument: development of a perceptual evaluation tool. Laryngoscope. 2005;115:423–428. [DOI] [PubMed] [Google Scholar]
- 75.Yumoto E, Sanuki T, Kumai Y. Immediate recurrent laryngeal nerve reconstruction and vocal outcome. Laryngoscope. 2006;116:1657–1661. [DOI] [PubMed] [Google Scholar]
- 76.Yun YS, Kim MB, Son YI. The effect of vocal hygiene education for patients with vocal polyp. Otolaryngol Head Neck Surg. 2007;137:569–575. [DOI] [PubMed] [Google Scholar]
- 77.Sodersten M, Lindestad PA. Glottal closure and perceived breathiness during phonation in normally speaking subjects. J Speech Hear Res. 1990;33:601–611. [DOI] [PubMed] [Google Scholar]
- 78.Desjardins M, Halstead L, Cooke M, et al. A systematic review of voice therapy: what “Effectiveness” really implies. J Voice. 2017;31:392.e13–392.e32. [DOI] [PubMed] [Google Scholar]
- 79.Remacle M. The contribution of videostroboscopy in daily ENT practice. Acta Otorhinolaryngol Belg. 1996;50:265–281. [PubMed] [Google Scholar]
- 80.Sataloff RT, Spiegel JR, Hawkshaw MJ. Strobovideolaryngoscopy: results and clinical value. Ann Otol Rhinol Laryngol. 1991;100(9 pt 1):725–727. [DOI] [PubMed] [Google Scholar]
- 81.Poburka BJ. A new stroboscopy rating form. J Voice. 1999;13:403–413. [DOI] [PubMed] [Google Scholar]
- 82.Poburka BJ, Bless DM. A multi-media, computer-based method for stroboscopy rating training. J Voice. 1998;12:513–526. [DOI] [PubMed] [Google Scholar]
- 83.Watkins MP, Portney LG. Foundations of Clinical Research Applications to Practice Third ed In: JL Alexander. Pearson Prentice Hall; 2009. 892 p. [Google Scholar]
- 84.Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]