Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver an opinion on phenylcapsaicin as a novel food (NF) pursuant to Regulation (EU) 2015/2283. Phenylcapsaicin is a chemically synthesised analogue of capsaicin intended to be marketed in food supplements and in foods for special medical purposes to the general population above the age of 11 years old at a maximum level of 2.5 mg/day. The highest intake of the NF is 2.5 mg/day which corresponds to 36 μg/kg body weight (bw) per day for adults, and 58 μg/kg bw per day for adolescents (10–14 years). The Panel considers that there is no concern with respect to genotoxicity of the NF. The reference point derived based on a 13‐week rat study was the lowest of the model averaged BMDL 20 values of 37.2 mg/kg bw per day in females for increased plasma alanine aminotransferase (ALAT) levels. The Panel concludes that the NF, phenylcapsaicin, is safe under the proposed uses and use levels.
Keywords: phenylcapsaicin, novel food, ingredient, safety
Summary
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver a scientific opinion on phenylcapsaicin as a novel food (NF) pursuant to Regulation (EU) 2015/2283. The assessment of the safety of this NF, which follows the methodology set out in the EFSA Guidance on the preparation and presentation of an application for authorisation of a NF1 in the context of Regulation (EU) 2015/2283 and in the Commission Implementing Regulation (EU) 2017/2469, is based on the data supplied in the application and information submitted by the applicant following EFSA's requests for supplementary information and additional data identified by the Panel.
The NF which is the subject of the application is phenylcapsaicin (> 98%), a chemically synthesised analogue of capsaicin. The NF is intended to be marketed in food supplements and in foods for special medical purposes to the general population above the age of 11 years old at a maximum level of 2.5 mg/day. The highest intake of the NF is 2.5 mg/day which corresponds to 36 μg/kg body weight (bw) per day for adults (considering an average bodyweight of 70 kg) and 58 μg/kg bw per day for adolescents (10–14 years) (considering an average bodyweight of 43.4 kg).
The information provided on composition, specifications, production process and stability of the NF does not raise safety concerns. The Panel considers that phenylcapsaicin does not have a nutritionally relevant role in the diet and that the consumption of the NF is not nutritionally disadvantageous.
The Panel considers that there is no concern with respect to genotoxicity of the NF. The applicant provided a 90‐day study where there were several changes related to effects in the gastrointestinal tract and the liver. The Panel considers both the effects observed as critical effects related to the compound. The reference point (RP) derived based on the critical effects of phenylcapsaicin lowest of the model averaged BMDL20 values for females for increased plasma alanine aminotransferase (ALAT) levels was 37.2 mg/kg bw per day (females).
The Panel considers that based on the proposed conditions of use, the margin of exposure between the RP and the maximal exposure to the NF of 1,033 for adults and 643 for adolescents are sufficient.
The Panel concludes that the NF, phenylcapsaicin, is safe under the proposed uses and use levels.
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
On 7 February 2018, the company aXichem AB, submitted a request to the European Commission in accordance with Article 10 of Regulation (EU) No 2015/2283 to place on the European Union (EU) market phenylcapsaicin as a novel food.
In accordance with Article 10(3) of Regulation (EU) 2015/2283, the European Commission asks the European Food Safety Authority to provide a scientific opinion by carrying out the assessment for phenylcapsaicin as a novel food ingredient.
2. Data and methodologies
2.1. Data
The safety assessment of this novel food (NF) is based on data supplied in the application and information submitted by the applicant following EFSA's requests for supplementary information.
During the assessment, the Panel identified additional data which were not included in the application: Paulsen et al. (2018).
Administrative and scientific requirements for NF applications referred to in Article 10 of Regulation (EU) 2015/2283 are listed in the Commission Implementing Regulation (EU) 2017/24692.
A common and structured format on the presentation of NF applications is described in the EFSA guidance on the preparation and presentation of a NF application.1 As indicated in this guidance, it is the duty of the applicant to provide all of the available (proprietary, confidential and published) scientific data, including both data in favour and not in favour to supporting the safety of the proposed NF.
This NF application includes a request for protection of proprietary data in accordance with Article 26 of Regulation (EU) 2015/2283. Data claimed to be proprietary by the applicant include: Donath (2016, unpublished), Feng et al. (2012a, unpublished), Feng et al. (2012b, unpublished), Schreib (2015, unpublished), Stiller (2016, unpublished) and Yang and Dong (2015, unpublished). The Panel considers that it could not have reached the conclusion on the safety of the NF under the proposed conditions of use without all these studies provided by the applicant.
2.2. Methodologies
The assessment follows the methodology set out in the EFSA guidance on NF applications and the principles described in the relevant existing guidance documents from the EFSA Scientific Committee. The legal provisions for the assessment are laid down in Article 11 of Regulation (EU) 2015/2283 and in Article 7 of the Commission Implementing Regulation (EU) 2017/2469.
This assessment concerns only risk that might be associated with consumption of the NF under the proposed conditions of use, and is not an assessment of the efficacy of phenylcapsaicin with regard to any claimed benefit.
3. Assessment
3.1. Introduction
The NF which is the subject of the application is phenylcapsaicin (> 98%), a chemically synthesised analogue of capsaicinoids that occur naturally in plants of the Capsicum genus. According to article 3 of the Regulation (EU) 2015/2283, the NF falls under category (i), i.e. food with a new or intentionally modified molecular structure, where that structure was not used as, or in, a food within the Union before 15 May 1997. The NF is intended to be marketed in food supplements and in foods for special medical purposes for the general population above the age of 11 years old at a maximum level of 2.5 mg/day.
3.2. Identity of the NF
The NF is chemically synthesised phenylcapsaicin (> 98%). Phenylcapsaicin has a molecular weight of 337.41 Da, solubility in water of 0.00193 g/L, a partition coefficient (n‐octanol/water) of 2.34. The pH is 6.12 at 25°C and it has a density of 1.152 g/cm3 at 20°C. The structure of phenylcapsaicin (N‐[(4‐hydroxy‐3‐methoxyphenyl)methyl]‐7‐phenylhept‐6‐ynamide, C22H23NO3, CAS no: 848127‐67‐3) is shown in Figure 1. Compared to the naturally occurring capsaicin, phenylcapsaicin presents a phenylethynyl group on the acyl chain.
Figure 1.
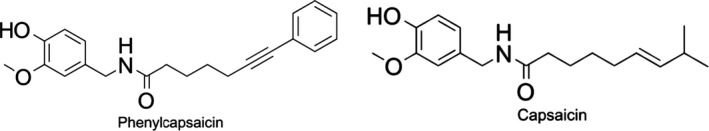
Structure of phenylcapsaicin and the structurally related capsaicin
To characterise the identity of the NF, the applicant provided a series of analyses on phenylcapsaicin including: high‐performance liquid chromatography (HPLC), electrospray ionisation mass spectrometry (ESI‐MS), nuclear magnetic resonance (NMR), infrared (IR) spectroscopy and ultraviolet (UV) spectroscopy.
3.3. Production process
The NF is produced by a two‐step chemical synthesis under controlled conditions. The first step is the production of the acetylenic acid intermediate through the reaction of phenyl acetylene with a carboxylic acid derivative. The second step comprises several reactions of the acetylenic acid intermediate with vanillylamine derivative to produce the final product. Phenylcapsaicin will be formulated with cellulose, fats and/or oil prior to use to a final concentration of 1–1.5%. The applicant provided detailed information on key parameters and critical control points of the production process.
The Panel considers that the production process is sufficiently described and does not raise safety concerns.
3.4. Compositional data
The applicant provided results from batch‐to‐batch analyses of five different production lots of the NF and the methods used for the analyses (Table 1). In these batches, the NF had a content of phenylcapsaicin > 98%, a water content ≤ 0.2%, a total content of residual solvents < 0.6%, and a content of process‐related by‐products < 1%. All investigated batches met the specifications (see Section 3.5) regarding microbiological contaminants and heavy metals.
Table 1.
Batch‐to‐batch analyses of five batches of the NF
| Parameter (unit) | Batch number | ||||
|---|---|---|---|---|---|
| Batch 1 | Batch 2 | Batch 3 | Batch 4 | Batch 5 | |
| Assay (HPLC, %) | 98.4 | 98.34 | 98.29 | 98.11 | 98.17 |
| Moisture (%) | 0.07 | 0.15 | 0.09 | 0.2 | 0.11 |
| Total synthesis related by‐products (%) | 0.58 | 0.47 | 0.44 | 0.53 | 0.65 |
| N,N‐dimethyl formamide (mg/kg) | 13 | 11 | 12 | 9 | 17 |
| Dichloromethane (mg/kg) | 19 | 72 | 26 | 4 | 8 |
| Dimethoxyethane (mg/kg) | 2 | 5 | 6 | 3 | 7 |
| Ethyl acetate (mg/kg) | 1900 | 1900 | 2002 | 2100 | 2008 |
| Other solvents (%) | 0.25 | 0.18 | 0.15 | 0.29 | 0.21 |
| Microbiological parameters | |||||
| Total plate count (CFU/g) | < 10 | < 10 | < 10 | < 10 | < 10 |
| Yeast and mould (CFU/g) | < 10 | < 10 | < 10 | < 10 | < 10 |
| Coliforms (CFU/g) | < 10 | < 10 | < 10 | < 10 | < 10 |
| Escherichia coli (Presence–Absence/10 g) | ND | ND | ND | ND | ND |
| Salmonella (Presence–Absence/10 g) | ND | ND | ND | ND | ND |
| Heavy metals | |||||
| Lead (mg/kg) | ND | ND | ND | ND | ND |
| Cadmium (mg/kg) | ND | ND | ND | ND | ND |
| Mercury (mg/kg) | 0.0024 | 0.006 | 0.007 | 0.0038 | 0.0056 |
| Arsenic (mg/kg) | 0.08 | 0.05 | 0.11 | 0.12 | 0.1 |
HPLC: high‐performance liquid chromatography; CFU: colony forming units; ND: not detected.
The Panel considers that the information provided on the composition of the NF is sufficient and does not raise safety concerns.
3.4.1. Stability
The applicant provided two studies on the stability of the NF, where five batches of the NF were stored tightly sealed and in the dark. One study was performed under ambient conditions (25 ± 2°C, 60 ± 3% relative humidity) for up to 24 months, and the other under accelerated conditions (40 ± 2°C; 75 ± 2% relative humidity) up to 6 months.
Upon storage under ambient conditions, there was an average decrease of phenylcapsaicin of 0.29% (0.38–0.22%) after 24 months. Storage under accelerated conditions resulted in phenylcapsaicin decreases of 0.20% (0.31–0.08%) after 3 months and 0.36% (0.49–0.24%) after 6 months.
The Panel considered that the data provided gave sufficient information with respect to the stability of the NF.
3.5. Specifications
The specifications of the NF as provided by the applicant are shown in Table 2.
Table 2.
Product specifications for phenylcapsaicin
| Parameter | Specification limit | Method |
|---|---|---|
| Purity (%) | > 98 | HPLC In‐house method |
| Moisture (%) | < 0.5 | USP 921 |
| Total synthesis related production by‐products (%) | < 1 | HPLC In house method |
| N,N‐dimethyl formamide (mg/kg) | ≤ 880 | USP 467 |
| Dichloromethane (mg/kg) | ≤ 600 | USP 467 |
| Dimethoxyethane (mg/kg) | ≤ 100 | USP 467 |
| Ethyl acetate (mg/kg) | ≤ 5,000 | USP 467 |
| Other solvents (%) | ≤ 0.5 | USP 467 |
| Microbiological parameters | ||
| Total plate count (CFU/g) | < 10 | USP 61 |
| Yeast and mould (CFU/g) | < 10 | USP 61 |
| Coliforms (CFU/g) | < 10 | USP 61 |
| Escherichia coli (Presence–Absence/10 g) | Absence | USP 62 |
| Salmonella (Presence–Absence/10 g) | Absence | USP 62 |
| Heavy metals | ||
| Lead (mg/kg) | < 1 | USP 231 |
| Cadmium (mg/kg) | < 1 | USP 231 |
| Mercury (mg/kg) | < 0.1 | USP 231 |
| Arsenic (mg/kg) | < 1 | USP 231 |
| Lead (mg/kg) | < 1 | USP 231 |
HPLC: high‐performance liquid chromatography; CFU: colony forming units; USP: United States Pharmacopeia.
The Panel considers that the information provided on the specification and the batch‐to‐batch variability of the NF is sufficient and does not raise safety concerns.
3.6. History of use of the NF and/or of its source
3.6.1. History of use of the source
The NF is a novel compound produced by chemical synthesis.
3.6.2. History of use of the NF
The chemically synthesised phenylcapsaicin has no existing history of use as food in the EU. However, capsaicinoids have a history of use causing the sharply burning taste of the fruits of many varieties of the genus Capsicum (e.g. chillies and peppers) (BfR, 2011).
In 2002, the Scientific Committee on Food (SCF) evaluated capsaicin as a flavouring substance or in ingredients with flavouring properties. They concluded that the data available at that time was not sufficient to establish a safe exposure level for capsaicinoids in food. The SCF also gave a rough estimate of the maximum daily intake of capsaicinoids from mild chillies and paprika in Europe of 1.5 mg/day (SCF, 2002).
Risks related to acute exposure to capsaicin were evaluated by the Federal Institute for Risk Assessment (BfR) of Germany in 2011 (BfR, 2011). In their report, the BfR described observations of undesirable effects (such as irritation of mucous membranes, nausea, vomiting and hypertension) in individuals with excessive high consumption; however, the ingested dose of capsaicinoids was unknown in these cases. In this assessment, it was also considered that consumption of one meal containing capsaicin causing hotness which can be tolerated by adults would correspond to an intake of capsaicin up to 5 mg/kg bw (BfR, 2011). The BfR also highlighted that children appeared to be particularly sensitive.
In 2012, EFSA evaluated the safety of the related capsinoid dihydrocapsiate as a NF pursuant to regulation EC 258/97. In that application, chemical synthesised dihydrocapsiate was intended as a NF ingredient for incorporation into foods of various categories at concentration levels varying from 8 to 2,050 mg/kg. Dihydrocapsiate was considered safe under the proposed conditions of use, with the 97.5th percentile intake estimate of 34 mg/day for adults and elderly and calculations based on body weights resulted in the highest intakes being 1.3 mg/kg bw per day for preschool children (EFSA NDA Panel, 2012).
3.7. Proposed uses and use levels and anticipated intake
3.7.1. Target population
The applicant intends to market the NF to the general population above the age of 11 years.
3.7.2. Proposed uses and use levels
The applicant intends to market the NF as a food supplement and in foods for special medical purposes at levels of 2.5 mg/day. The NF from food supplements and foods for special medical purposes are not meant to be consumed concomitantly.
3.7.3. Anticipated intake of the NF
At the proposed uses and use levels (2.5 mg/day), the intake of the NF could be up to 36 μg/kg bw per day for a 70‐kg adult and up to 58 μg/kg bw per day for adolescents (10–14 years) considering an average bodyweight of 43.4 kg (EFSA Scientific Committee, 2012).
3.7.4. Combined intake from the NF and other sources
Phenylcapsaicin is not naturally present in foods, and there will thus not be a combined intake from the NF and other sources.
The Panel notes that phenylcapsaicin acts via the transient receptor potential vanilloid subfamily 1 (TRPV1) receptor as other capsaicinoids do. The intended intake of phenylcapsaicin of 2.5 mg/day may add to the intake of capsaicinoids from foods if consumed simultaneously. The estimated maximum daily intake of capsaicinoids from mild chillies and paprika in Europe is 1.5 mg/day (SCF, 2002). This is low compared to the 5 mg/kg bw which may be consumed by an adult with one meal (BfR, 2011).
3.7.5. Estimate of exposure to undesirable substances
With the proposed conditions of use and specifications of the residual process‐related by‐products, the Panel considers the exposure to undesirable substances does not raise safety concerns.
3.7.6. Precautions and restrictions of use
No additional precautions of use were addressed by the applicant.
3.8. Absorption, distribution, metabolism and excretion (ADME)
The applicant provided two in vivo ADME studies performed in parallel, one with phenylcapsaicin and one with capsaicin (Feng et al., 2012a,b). The studies were neither performed according to OECD test guideline 417 nor compliant with good laboratory practice (GLP). Male Sprague–Dawley (SD) rats received a single oral dose of 50 mg/kg of the 14C radiolabelled test material. Bile, plasma, blood and tissue samples were collected at 0.5, 2 and 24 h post‐dosing, and urine and faeces samples were collected up to 7 days post dosing. Radioactivity was determined in blood, plasma, liver, brain, stomach, small intestine, kidney, spleen, lung, heart, testes, skeletal muscle and fat. Metabolites were identified in plasma, urine and faeces. In addition, radioactivity and metabolites in bile were determined in bile duct‐cannulated rats up to 24 h after oral application (Appendix A).
Both, phenylcapsaicin and capsaicin were rapidly absorbed at similar rates reaching maximum levels in the blood 0.5 h post‐dosing. The radioactivity was distributed to various tissues, and was most abundant in the small intestine, stomach and liver within 0.5 h post‐dosing. An increase in radioactivity was found in fat from 0.5 to 24 h post‐dosing.
Numerous metabolites were identified. The dominant metabolic pathway for capsaicin was glucuronidation, whereas it was oxygenation and glucuronidation for phenylcapsaicin. Of the labelled 14C‐phenylcapsaicin and 14C‐capsaicin, 72% and 47% were identified in the bile within 24 h post‐oral dose to rats, with approximately 2% and 0.4% as the intact parent compound.
The compounds were excreted through both urine and faeces with a majority occurring in the first 24 h after oral dosing. A higher amount of radioactivity was excreted in the faeces after application of phenylcapsaicin compared to capsaicin. The recovery rates of phenylcapsaicin (54.1%) and capsaicin (47.6%) in excreta and carcass were similar within 7 days post dosing. The lack of full recovery in faeces, urine and carcass was attributed by the authors of the study to transfer of 14C from the radiolabelled carbonyl group to acetyl coenzyme after cleavage of the amide bond. This could lead to incorporation into endogenous substances, e.g. in fat or to biotransformation to 14CO2 and other 14C labelled volatile organic compounds that are expired. Radioactivity in expired air was, however, not measured.
Overall, the Panel notes that phenylcapsaicin derived radioactivity was rapidly absorbed, widely distributed in various tissues, metabolised by the liver, excreted into the bile and eliminated mainly via faeces.
3.9. Nutritional information
The Panel considers that phenylcapsaicin does not have a nutritionally relevant role in the diet and is not nutritionally disadvantageous under the proposed conditions of use.
3.10. Toxicological information
The applicant provided a bacterial reverse mutation test, an in vitro mammalian micronucleus test and a subchronic toxicity study. After a request from the Panel, the applicant also provided an unpublished study (Yang and Dong, 2015) cited by Paulsen et al. (2018).
3.10.1. Genotoxicity
In a bacterial reverse mutation test compliant with OECD 471 and GLP with Salmonella Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102, with and without metabolic activation (Schreib, 2015 unpublished; Paulsen et al., 2018), phenylcapsaicin was non‐mutagenic (Appendix B). In an in vitro mammalian cell micronucleus test with human lymphocytes compliant with OECD 487 and GLP with and without metabolic activation (Donath, 2016 unpublished; Paulsen et al., 2018), phenylcapsaicin was not clastogenic or aneugenic (Appendix B). The Panel considers that taking into account the results of the tests performed, there are no concerns regarding genotoxicity of the NF.
3.10.2. Subchronic toxicity
The applicant provided a subchronic 90‐day repeat‐dose oral toxicity study in compliance with OECD TG 408 and GLP in which groups of Wistar rats (15/sex per group in control and high‐dose group and 10/sex per group in mid‐ and low‐dose groups) were administered the NF by gavage at dose levels of 0, 30, 100 or 250 mg/kg bw per day (Stiller, 2016; unpublished; Paulsen et al., 2018).
In this study, several targets systems were affected after administration of phenylcapsaicin to rats (Appendix C). Effects were observed in the gastrointestinal tract, such as salivation and diarrhoea, inflammation in the glandular stomach, forestomach and caecum, which can be attributed to the irritating properties of the compound. The haematological effects (decreases in erythrocytes and reticulocytes) may be secondary to bleeding and sustained inflammation in the gastrointestinal tract. Reduced spleen weights, depletion of lymphocytes in spleen and lymph nodes as well as reduced cellularity in the bone marrow point to the haematopoetic system as another target. Furthermore, phenylcapsaicin affects the liver as can be concluded from increases in liver enzyme levels (alanine aminotransferase (ALAT) and alkaline phosphatase (AP), increases in prothrombin time, decreased levels of glucose, cholesterol, bilirubin and bile acids, increased liver weights and effects in histopathological examinations, which included diffuse hepatocellular hypertrophy, periportal vacuolation, eosinophilic cytoplasmic inclusions and focal necrosis.
Most effects were dose related, and not reversed in the recovery group or even more pronounced (e.g. white blood cell (WBC) counts, cholesterol). In contrast, for some liver enzymes (aspartate aminotransferase (ASAT), ALAT and AP), levels in the male recovery group were even lower than in the controls. Male rats were generally more susceptible than female rats.
The authors of the study considered 100 mg/kg bw per day as the no observable adverse effect level (NOAEL) for systemic toxicity based on degenerative changes in the liver and 30 mg/kg bw per day as the NOAEL for local effects due to irritating effects in the stomach.
The Panel noted that some effects were statistically significant already at the lowest dose of 30 mg/kg bw per day and therefore evaluated these effects. Upon request by EFSA, the applicant provided a benchmark dose (BMD) assessment and some considerations on the evaluation of the effects. The BMD assessment followed the EFSA Guidance on the BMD approach (EFSA Scientific Committee, 2017), using the EFSA web‐tool for BMD analysis, which is based on PROAST software (Appendix D). In addition, EFSA performed a BMD analysis of the effects on bodyweight and the erosions and ulcers in the stomach applying the same methodology.
A dose‐dependent decrease in bodyweight was observed in rats of both sexes, with no significant changes in the food consumption observed in the rats. This can be considered an adverse effect, and the lowest of the model averaged BMDL05 for body weight is 77.4 mg/kg bw per day (males).
The clinical symptom ‘moving of the bedding’ was observed in both sexes, which can be interpreted as a sign of discomfort. This finding also occurred in the same intensity in the recovery group. Similarly, salivation was also observed in the recovery group, indicating that in addition to irritating effects of the NF, the expectation of treatment per se likely triggers responses in the animals treated with the test material. The effects were therefore not used for deriving a reference point.
One female rat developed at 30 mg/kg bw per day slight (grade 1) erosion/ulcer of the glandular stomach. At higher dose levels, the number of animals affected and severity of effects increased, and also other effects in the stomach occurred. Therefore, this finding reflects first signs of irritation in the stomach. These effects are also relevant for humans, as for capsaicin an increase in discharge of parietal cells, pepsin secretion and potassium exfoliation (desquamation) of stomach cells was found after ingestion of 1.5 g capsaicin dissolved in 100 mL water, the NOAEL was 0.5 g in this study (Myers et al., 1987). In the BMD analysis of the findings on erosions and ulcers in the stomach, the lowest of the model averaged BMDL10 was 38.7 mg/kg bw per day (females).
For other endpoints of liver toxicity, such as hepatocellular hypertrophy and periportal vacuolation as well as relative liver weight there was a clear dose response and the BMD analyses resulted in BMD values with narrow BMD confidence intervals. The lower confidence limit of the benchmark dose (BMDL) values for these data are in close agreement with each other; the lowest of the model averaged BMDL05 values for relative liver weights is 55.0 mg/kg bw per day (females) and the lowest of the model averaged BMDL10 values for hepatocellular hypertrophy is 56.4 mg/kg bw per day (males). Cholesterol levels were reduced by 30% in male rats and 19% in female rats, (statistically significant in males) at the low dose of 30 mg/kg bw per day. There was a reduction of total bilirubin levels (20% in male rats, 22% in female rats, statistically significant in female rats). The Panel notes that decreased bilirubin levels indicate enzyme induction in the liver and may be considered as a non‐adverse effect. At higher dose levels, there are additional effects on the liver, i.e. at 100 mg/kg bw per day, there is an increase in liver weight, hypertrophy and vacuolation in liver cells. The increase in prothrombin time at this dose level may also point to liver toxicity. Thus, there is a dose‐related continuum of effects in the liver. There was a statistically significant dose‐related increase in plasma ALAT levels in females at the highest dose. An increase in ALAT concentrations in the blood can be considered an adverse effect. The benchmark response (BMR) of 20% was suggested by the EFSA Scientific Committee et al. (2017) for liver enzymes. The lowest of the model averaged BMDL20 values for ALAT was 37.2 mg/kg bw per day (Females), and was used as the reference point (RP) for the NF.
3.10.3. Human studies
No human studies were available assessing the safety of the NF.
3.10.4. Other studies
Contact of the skin or the mucous membranes with capsaicin causes a sensation of heat, burning and hyperaemia. This effect is mediated by binding to ‘transient receptor potential vanilloid subfamily 1’ (TRPV1) in specific nerve fibres (C‐fibres) that transport calcium into the cells and subsequent release of various mediators, e.g. Substance P.
To investigate, whether phenylcapsaicin acts similarly as capsaicin, an in vitro study was performed by the applicant (Yang and Dong, 2015) and provided upon request from EFSA. In this study, the uptake of fluorescent calcium via the TRPV1 channel was investigated in HEK293 cells stably expressing the human TRPV1 channel. The EC50 (half maximal effective concentration) of phenylcapsaicin for stimulating Ca uptake was 57.8 nM, while for capsaicin it was 26.4 nM, indicating that phenylcapsaicin was about half as active as capsaicin.
The Panel notes that this study indicates that phenylcapsaicin acts as a capsaicin analogue.
3.11. Allergenicity
The Panel considers that the NF is a well‐defined mixture with a high content of phenylcapsaicin > 98% produced from non‐protein chemical sources and that it is not expected to have allergenic potential.
4. Discussion
The NF which is the subject of the application is phenylcapsaicin (> 98%) a chemically synthesised analogue of capsaicin. The highest intake of the NF is 2.5 mg/day which corresponds to 36 μg/kg bw per day for adults (considering an average bodyweight of 70 kg) and 58 μg/kg bw per day for adolescents (10–14 years) (considering an average bodyweight of 43.4 kg).
The information provided on composition, specifications, production process and stability of the NF does not raise safety concerns. The Panel considers that phenylcapsaicin does not have a nutritionally relevant role in the diet and that the consumption of the NF is not nutritionally disadvantageous under the proposed conditions of use.
The Panel considers that there is no concern with respect to genotoxicity of the NF. The applicant provided a 90‐day study where there were several changes related to effects in the gastrointestinal tract and the liver. The Panel considers both the effects observed as critical effects related to the compound. The RP derived based on the critical effects of phenylcapsaicin lowest of the model averaged BMDL20 values for the increase in plasma ALAT levels was 37.2 mg/kg bw per day (females).
The Panel considers that based on the proposed conditions of use, the margin of exposure between the RP and the maximal exposure to the NF of 1,033 for adults and 643 for adolescents are sufficient.
5. Conclusions
The Panel concludes that the NF is safe under the proposed uses and use levels.
The Panel could not have reached the conclusion on the safety of the NF under the proposed conditions of use without the data claimed as proprietary by the applicant, Donath (2016, unpublished), Feng et al. (2012a, unpublished), Feng et al. (2012b, unpublished), Schreib (2015, unpublished), Stiller (2016, unpublished) and Yang and Dong (2015, unpublished).
Steps taken by EFSA
Letter from the European Commission to the European Food Safety Authority with the request for a scientific opinion on the safety of phenylcapsaicin. Ref. Ares(2018)4394500, dated 27/08/2018.
On 28 August 2018, EFSA received a valid application from the European Commission on phenylcapsaicin as NF, which was submitted by aXichem AB, and the scientific evaluation procedure started.
On 20 November 2018, 7 February 2019 and 11 February 2019 EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
On 29 January 2019 and twice on 11 February 2019 additional information was provided by the applicant and the scientific evaluation was restarted.
During its meeting on 15 05 2019, the NDA Panel, having evaluated the data, adopted a scientific opinion on the safety of phenylcapsaicin as a NF pursuant to Regulation (EU) 2015/2283.
Abbreviations
- ADME
absorption, distribution, metabolism and excretion
- ALAT
alanine aminotransferase
- AP
alkaline phosphatase
- ASAT
aspartate aminotransferase
- BfR
German Federal Institute for Risk Assessment
- BMD
benchmark dose
- BMDL
lower confidence limit of the benchmark dose
- BMDU
upper confidence limit of the benchmark dose
- BMR
benchmark response
- bw
body weight
- CAS
Chemical Abstracts Service
- CFU
colony forming units
- EC50
half maximal effective concentration
- ESI‐MS
electrospray ionisation mass spectrometry
- GLP
good laboratory practice
- HPLC
high‐performance liquid chromatography
- IR
infrared
- NOAEL
no observable adverse effect level
- NF
novel food
- NMR
nuclear magnetic resonance
- OECD
Organisation for Economic Co‐operation and Development
- RP
Reference point
- TRPV1
transient receptor potential vanilloid subfamily 1
- USP
United States Pharmacopeia
- UV
ultraviolet
- WBC
white blood cell count
Appendix A – Comparisons of ADME properties in rats between capsaicin and phenylcapsaicin
1.
Table A.1.
| Capsaicin | Phenylcapsaicin | |
|---|---|---|
| Dose level | 50 mg/kg including 100 p,Ci/kg | 50 mg/kg including 100 p,Ci/kg |
| Cumulative radioactive dose in urine within 7 days (%) | 15.26 | 3.25 |
| Cumulative radioactive dose in faeces within 7 days (%) | 32.26 | 50.86 |
| Recovery in urine and faeces within 7 days | 47.61 | 54.11 |
| Radioactivity in carcass after 7 days | No data | 4.2% |
| Cumulative radioactive dose in bile (%) within 24 h | 47.06 | 72.29 |
| Percent parent in bile of the dose (%) within 4 h | 1.99 | 0.36 |
| Tmax in tissues and blood, plasma | 0.5 h | 0.5 h |
| Tissues with highest radioactivity after 0.5 h (% of dose) |
Small intestine 8.5% Stomach 3.9% Liver ˃ 0.7%* |
Small intestine 14.3% Stomach 1.6% Liver ˃ 1.8%* |
| Total number of metabolites | 50 | 27 |
| Metabolic pathway | Hydrolysis, deamination, hydrogenation, dehydrogenation, oxygenation, conjugation with glutathione, demethylation, glucuronidation and sulfation | Hydrolysis, deamination, dehydrogenation, glucuronidation, oxygenation, conjugation with glutathione, demethylation and sulfation |
| Major metabolic pathway and metabolites |
M42 (31.8% of the radioactive dose in bile), glucuronidation |
M18 (61.97% of the radioactive dose in bile), oxygenation and glucuronidation |

|
|
|
| Blood to plasma ratio (0.5–24 h) | 0.61–1.17 | 0.55–0.88 |
ADME: absorption, distribution, metabolism and excretion.
Note: Tissues collected: liver, brain, stomach, small intestine, kidney, spleen, lung, heart, testes, skeletal muscle and fat.
Only piece of liver was cut and used as a sample.
Appendix B – Genotoxicity studies of Phenylcapsaicin
1.
Table B1.
Results of Schreib (2015) unpublished full study report and Donath (2016) unpublished full study report
| Study | Bacterial reverse mutation test assay | In vitro mammalian Cell micronucleus assay |
|---|---|---|
| Title of the study report |
Schreib (2015), unpublished full study report (confidential) Paulsen et al. (2018), published report |
Donath, 2016;. unpublished full study report (confidential) Paulsen et al., 2018 published report |
| Method | OECD 471 | OECD 487 |
| Tested system | Salmonella Typhimurium strains TA98, TA100, TA102, TA1535 and TA1537 | Human lymphocytes |
| Test material | NF Batch: 20141201 (98.8% phenylcapsaicin) | NF Batch: 20141201 (98.8% phenylcapsaicin) |
| Dose/concentration |
Two independent experiments (plate incorporation (experiment 1) and pre‐incubation test (experiment 2)). Eight concentrations of phenylcapsaicin were used for each bacterial strain in triplicate with and without the presence of metabolic activation system (S9 mix). 3.16, 10.0, 31.6, 100, 316, 1,000, 2,500 and 5,000 μg/plate |
Dose selected based on the results of the cytotoxicity measurements and the precipitation study of the substance. Short‐term exposure (incubated for 4 h)
|
| Authors conclusions |
No increases in revertant colony numbers of any of the strains tested occurred in the presence or absence of metabolic activation in both experiments. There was precipitation of the test item in all strains at a concentration of 5,000 μg/plate. Toxicity was observed at concentrations of 316 μg/plate and above in the absence of S9. In the presence of S9 at 1,000 μg/plate and above, toxicity was observed in the preincubation test with S9, and 2,500 μg/plate and above in the plate incorporation test |
There was no statistically significant enhancement of micronucleated cells observed in the experiments. Phenylcapsaicin was not clastogenic or aneugenic in the in vitro mammalian cell micronucleus test. Cytostasis was close to the limit of 55 ± 5% in all experiments, 58, 57 and 60% at highest dose tested |
Appendix C – Results of oral dose 90‐day study
1.
Table C.1.
Summary of the 90‐day study by Stiller (2016) and the significant findings in the study
| Title of the study report | 90‐day repeated‐dose oral toxicity study in Wistar rats with phenylcapsaicin including a 28‐day recovery period (Stiller, 2016) |
| Tested system | Groups of 6‐week‐old adult Wistar rats |
| Test material | The NF Batch: 20141201 (98.8% phenylcapsaicin) |
| Dose/concentration (route of administration) |
The NF (suspended in polyethylene glycol 400) was prepared daily and administered to the rats by oral gavage at doses levels of: Control (15 animals/sex): 0 (vehicle‐only) Low dose (10 animals/sex): 30 mg/kg bw per day Mid dose (10 animals/sex): 100 mg/kg bw per day High dose (10 animals/sex): 250 mg/kg bw per day |
| Statistical analysis |
Results of the body weight, food consumption, parameters of haematology, blood coagulation and clinical biochemistry and absolute and relative organ weights were analysed using one‐way ANOVA and a post hoc Dunnett's test Statistical comparisons of data acquired during the recovery period were performed with a Student's t‐test |
| Method | OECD 408 |
| Parameter | Sex | Control 0 mg/kg bw per day | Low dose 30 mg/kg bw per day | Mid dose 100 mg/kg bw per day | High dose 250 mg/kg bw per day | Control 0 mg/kg bw per day + Recovery | High dose 250 mg/kg bw per day + Recovery |
|---|---|---|---|---|---|---|---|
| Clinical observations | |||||||
| Animals with signs of toxicity (frequency) | M | 55.7 | 117.3 | 165.9** | 203.5** | NA | 198.2** |
| F | 55.1 | 103.5 | 164.7** | 205.1** | NA | 191.2** | |
| Diarrhoea (frequency) | M | 55.2 | 60.7 | 57.9 | 67.3** | NA | 65.4** |
| F | 55.1 | 52.5 | 54.6 | 62.0** | NA | 66.6** | |
| Salivation (frequency) | M | 0 | 4.8 | 42.3** | 59.9** | NA | 60.0** |
| F | 0.0 | 6.1 | 45.1** | 58.2** | NA | 58.6** | |
| Piloerection (frequency) | M | 0 | 0 | 0 | 0.9 | NA | 0.4** |
| F | 0.0 | 0.0 | 0.1 | 3.5 | NA | 0.0 | |
| Musculoskeletal system wasp waist (frequency) | M | 0 | 0 | 0 | 0 | NA | 0.4** |
| F | 0.0 | 0.0 | 0.1 | 1.6 | NA | 0.0 | |
|
Spontaneous Activity Reduced (frequency) |
M | 0 | 0 | 0 | 3.9** | NA | 4.0** |
| F | 0.0 | 0.0 | 0.1 | 2.5** | NA | 4.0** | |
| Moving the bedding (frequency) | M | 0 | 51.8 | 65.7** | 68.9** | NA | 68.0** |
| F | 0 | 44.8* | 64.4** | 68.0** | NA | 62.0* | |
| Bodyweight | |||||||
| Day 91 (g) | M | 356.53 | 351.30 | 347.60 | 322.80 | – | 329.80 |
| F | 209.33 | 206.22 | 205.44 | 194.56 | – | 210.50 | |
| Day 119 (g) | M | – | – | – | – | 379,00 | 366.25 |
| F | – | – | – | – | 210,00 | 207.25 | |
| Haematology | |||||||
| RBC (1012/L) | M | 9.15 | 9.14 | 8.86 | 8.72* | 9.27 | 8.71 |
| F | 8.26 | 8.00 | 8.08 | 7.58 | 7.99 | 7.83 | |
| WBC (109/L) | M | 4.23 | 4.80 | 3.40 | 3.69 | 4.81 | 2.90* |
| F | 3.34 | 2.90 | 2.17 | 3.64 | 2.05 | 1.38 | |
| MCV (fL) | M | 52.36 | 52.35 | 52.73 | 54.03* | 52.22 | 54.45 |
| F | 55.08 | 53.49 | 54.39 | 54.94 | 54.82 | 55.33 | |
| Monocytes (%) | M | 2.35 | 1.89 | 2.75 | 2.22 | 3.44 | 1.53 |
| F | 2.08 | 1.89 | 2.57 | 3.79* | 2.52 | 2.80 | |
| Reticulocytes (%) | M | 1.99 | 1.70 | 1.46 | 1.07** | 1.35 | 1.70* |
| F | 2.57 | 2.37 | 1.88 | 1.60 | 1.58 | 1.34 | |
| Prothrombin Time (seconds) | M | 22.30 | 23.68 | 25.76** | 24.75* | 22.92 | 23.43 |
| F | 24.09 | 25.80 | 25.74 | 24.83 | 23.88 | 25.83* | |
| Clinical biochemistry | |||||||
| Total bilirubin (μmol/L) | M | 2.21 | 1.77 | 1.39** | 1.78 | 2.32 | 1.13** |
| F | 2.64 | 2.07* | 1.99* | 2.11 | 2.36 | 1.90 | |
| Total bile acids (μmol/L) | M | 69.52 | 45.49 | 34.59* | 34.13* | 48.55 | 25.58 |
| F | 66.06 | 54.36 | 55.41 | 67.15 | 39.78 | 31.90 | |
| Total cholesterol (mmol/L) | M | 1.74 | 1.25* | 1.02** | 1.08* | 1.66 | 0.65*** |
| F | 1.27 | 1.03 | 1.08 | 1.33 | 1.08 | 1.32 | |
| Glucose (mmol/L) | M | 7.32 | 6.66 | 4.77* | 4.53* | 11.93 | 5.16** |
| F | 5.22 | 4.85 | 4.50 | 6.48 | 6.71 | 7.30 | |
| Alanine aminotransferase (U/L) | M | 35.54 | 31.23 | 32.58 | 44.59 | 39.30 | 15.48*** |
| F | 34.58 | 26.48 | 35.90 | 59.83* | 25.84 | 38.03 | |
| Aspartate aminotransferase (U/L) | M | 106.80 | 88.50 | 91.62 | 91.60 | 90.80 | 29.63** |
| F | 86.93 | 79.90 | 85.34 | 101.85 | 59.30 | 85.13 | |
| Alkaline phosphatase (U/L) | M | 103.15 | 112.62 | 113.50 | 123.31 | 115.31 | 33.10** |
| F | 54.68 | 72.13 | 74.84 | 100.48* | 57.27 | 67.46 | |
| Urea (mmol/L) | M | 9.06 | 9.49 | 9.38 | 9.58 | 5.26 | 2.23*** |
| F | 10.23 | 10.01 | 9.82 | 9.58 | 5.26 | 4.75 | |
| Sodium (mmol/L) | M | 131.00 | 125.50 | 121.20 | 125.70 | 125.60 | 57.67** |
| F | 128.70 | 133.44 | 130.44 | 141.38 | 97.00 | 113.75 | |
| Potassium (mmol/L) | M | 4.13 | 3.71 | 3.71 | 3.89 | 3.78 | 1.60*** |
| F | 3.91 | 3.55 | 3.43 | 4.11 | 2.45 | 2.97 | |
| Organ weights | |||||||
| Liver (mean weight (g)) | M | 8.5285 | 8.7392 | 8.8405 | 11.3357**** | 9.3246 | 9.2301 |
| F | 5.3444 | 5.4581 | 5.9997 | 7.2987**** | 5.5875 | 6.0377 | |
| Liver weight (relative to body weight %) | M | 2.4232 | 2.4856 | 2.5398 | 3.5107 | 2.5543 | 2.8617 |
| F | 2.6058 | 2.6478 | 2.9255 | 3.7866 | 2.5819 | 2.8658 | |
| Spleen (mean weight (g)) | M | 0.7841 | 0.7419 | 0.6560 | 0.6200**** | 0.6453 | 0.7266 |
| F | 0.5240 | 0.5003 | 0.5230 | 0.4525**** | 0.5042 | 0.4993 | |
| Histopathology | |||||||
| Liver | |||||||
| Hepatocellular hypertrophy, diffuse (incidence/mean severity) | M | 0 | 0 | 3/1.0 | 9/1.0 | 0 | 0 |
| F | 0 | 0 | 0 | 7/1.0 | 0 | 0 | |
| Vacuolation (fatty change), periportal (incidence/mean severity) | M | 0 | 0 | 1/1.0 | 4/1.5 | 0 | 0 |
| F | 2/1.0 | 1/1.0 | 0 | 3/1.7 | 1/1.0 | 1/1.0 | |
| Eosinophilic cytoplasmic inclusions, periportal hepatocytes (incidence/mean severity) | M | 0 | 0 | 0 | 1/1.0 | 0 | 0 |
| F | 0 | 0 | 0 | 0 | 0 | 0 | |
| Focal necrosis (incidence/mean severity) | M | 0 | 0 | 0 | 0 | 0 | 0 |
| F | 0 | 0 | 0 | 1/1.0 | 0 | 0 | |
| Stomach | |||||||
| Forestomach | |||||||
| Hyperkeratosis, diffuse (incidence/mean severity) | M | 0 | 0 | 0 | 10/1.5 | 0 | 0 |
| F | 0 | 0 | 0 | 8/1.3 | 0 | 0 | |
| Squamous hyperplasia (incidence/mean severity) | M | 0 | 0 | 0 | 10/1.0 | 0 | 0 |
| F | 0 | 0 | 0 | 8/1.0 | 0 | 0 | |
| Oedema, submucosa (incidence/mean severity) | M | 0 | 0 | 0 | 3/2.0 | 0 | 0 |
| F | 0 | 0 | 0 | 3/1.0 | 0 | 0 | |
| Inflammation, submucosa (incidence/mean severity) | M | 0 | 0 | 0 | 0 | 0 | 0 |
| F | 0 | 0 | 0 | 1/1.0 | 0 | 0 | |
| Glandular stomach | |||||||
| Erosion/Ulcer (incidence/mean severity) | M | 0 | 0 | 1/1.0 | 3/1.7 | 0 | 1/1.0 |
| F | 0 | 1/1.0 | 1/2.0 | 5/2.4 | 0 | 1/1.0 | |
| Inflammation (incidence/mean severity) | M | 0 | 0 | 1/1.0 | 3/1.7 | 0 | 1/1.0 |
| F | 0 | 0 | 2/1.0 | 7/1.6 | 0 | 0 | |
| Cecum | |||||||
| Congestion, lamina propria (incidence/mean severity) | M | 0 | 0 | 2/1.0 | 8/1.0 | 0 | 0 |
| F | 0 | 0 | 0 | 2/1.0 | 0 | 0 | |
| Increased basophilia, surface epithelial cells (incidence/mean severity) | M | 0 | 0 | 3/1.0 | 9/1.0 | 0 | 0 |
| F | 0 | 0 | 0 | 5/1.0 | 0 | 0 | |
| Increased apoptosis, surface epithelial cells (incidence/mean severity) | M | 0 | 0 | 0 | 2/1.0 | 0 | 0 |
| F | 0 | 0 | 0 | 0 | 0 | 0 | |
| Others | |||||||
| Bone marrow (sternum) – decreased cellularity (decreased cell density) (incidence/mean severity) | M | 0 | 0 | 0 | 5/2.0 | 0 | 1/1.0 |
| F | 0 | 0 | 0 | 6/1.8 | 0 | 1/1.0 | |
| Spleen – lymphoid depletion (incidence/mean severity) | M | 0 | 0 | NE | 0 | NE | NE |
| F | 0 | 0 | 0 | 1/1.0 | 0 | 0 | |
| Thymus – atrophy (incidence/mean severity) | M | 9/1.2 | 10/1.3 | 9/1.2 | 10/1.7 | 5/1.2 | 5/1.0 |
| F | 10/1.3 | 9/1.4 | 9.1.4 | 8/2.3 | 5/1.4 | 4/1.5 | |
| Lymph node – axillary – lymphoid depletion (incidence/mean severity) | M | 0 | NE | NE | 0 | NE | NE |
| F | 0 | 0 | 0 | 1/1.0 | 0 | 0 | |
| Lymph node – mesenteric – lymphoid depletion (incidence/mean severity) | M | 0 | NE | NE | 0 | NE | NE |
| F | 0 | 0 | 0 | 1/1.0 | 0 | 0 | |
| Adrenal glands – cortical hypertrophy, diffuse (incidence/mean severity) | M | 0 | 0 | 0 | 2/1.1 | 0 | 0 |
| F | 0 | 0 | 0 | 5/1.0 | 0 | 0 |
bw: body weight; ANOVA: analysis of variance; RBC: red blood cell; WBC: white blood cell; MCV: mean corpuscular volume; C: control group; F: female; HD: high‐dose group; LD: low‐dose group; M: male; MD: mid‐dose group; NA: not applicable; NE: not examined, U: enzyme activity.
Values for the HD recovery group are compared to values for the C recovery group, when such data are available. otherwise, values are compared to values for the C group.
significantly different from control (p < 0.05).
significantly different from control (p < 0.01).
significantly different from control (p < 0.001).
significantly different from control (p = not reported).
Appendix D – Benchmark dose modelling reports
Benchmark dose modelling of continuous data
Data Description
The endpoint analysed was plasma alanine aminotransferase levels, summary data for the endpoint is found in Table D.1. The BMD analysis was performed using individual data, and therefore slightly different results may be obtained using the summary data in the analysis.
Table D.1.
Summary data of Alanine aminotransferase (Stiller, 2016)
| Dose | ALAT (U/L) | SD | n | Sex |
|---|---|---|---|---|
| C | 34.58 | 10.99 | 10 | F |
| LD | 26.48 | 8.32 | 8 | F |
| MD | 35.90 | 10.63 | 9 | F |
| HD | 59.83 | 35.89 | 8 | F |
| C | 35.54 | 8.88 | 10 | M |
| LD | 31.23 | 9.07 | 10 | M |
| MD | 32.58 | 7.27 | 10 | M |
| HD | 44.59 | 19.78 | 10 | M |
Software used
Results are obtained using the EFSA web‐tool for BMD analysis, which uses the R‐package PROAST, version 66.35, for the underlying calculations.
Selection of the BMR
The benchmark response (BMR) used is a 20% change in mean response compared to the controls. The benchmark dose (BMD) is the dose corresponding with the BMR of interest.
A 90% confidence interval around the BMD will be estimated; the lower bound is reported by BMDL and the upper bound by BMDU.
Results
Fitted Models
Table D.3.
List of fitted BMD models
| Model | Converged | Loglik | npar | AIC |
|---|---|---|---|---|
| full model | Yes | −32.32 | 9 | 82.64 |
| full‐v | Yes | −31.43 | 10 | 82.86 |
| null model | Yes | −41.22 | 2 | 86.44 |
| null model‐a | Yes | −41.20 | 3 | 88.40 |
| Expon. m3‐ | Yes | −35.58 | 4 | 79.16 |
| Expon. m3‐a | Yes | −35.54 | 5 | 81.08 |
| Expon. m3‐b | Yes | −34.42 | 5 | 78.84 |
| Expon. m3‐ab | Yes | −34.16 | 6 | 80.32 |
| Expon. m5‐ | Yes | −35.50 | 5 | 81.00 |
| Expon. m5‐a | Yes | −35.46 | 6 | 82.92 |
| Expon. m5‐b | Yes | −34.33 | 6 | 80.66 |
| Expon. m5‐ab | Yes | −33.86 | 7 | 81.72 |
| Hill m3‐ | Yes | −35.58 | 4 | 79.16 |
| Hill m3‐a | Yes | −35.54 | 5 | 81.08 |
| Hill m3‐b | Yes | −34.42 | 5 | 78.84 |
| Hill m3‐ab | Yes | −34.16 | 6 | 80.32 |
| Hill m5‐ | Yes | −35.50 | 5 | 81.00 |
| Hill m5‐a | Yes | −35.47 | 6 | 82.94 |
| Hill m5‐b | Yes | −34.33 | 6 | 80.66 |
| Hill m5‐ab | Yes | −33.86 | 7 | 81.72 |
| Inv.Expon. m3‐ | Yes | −35.53 | 4 | 79.06 |
| Inv.Expon. m3‐a | Yes | −35.49 | 5 | 80.98 |
| Inv.Expon. m3‐b | Yes | −34.35 | 5 | 78.70 |
| Inv.Expon. m3‐ab | Yes | −34.03 | 6 | 80.06 |
| Inv.Expon. m5‐ | Yes | −35.49 | 5 | 80.98 |
| Inv.Expon. m5‐a | Yes | −35.46 | 6 | 82.92 |
| Inv.Expon. m5‐b | Yes | −34.31 | 6 | 80.62 |
| Inv.Expon. m5‐ab | Yes | −33.84 | 7 | 81.68 |
| LN m3‐ | Yes | −35.55 | 4 | 79.10 |
| LN m3‐a | Yes | −35.51 | 5 | 81.02 |
| LN m3‐b | Yes | −34.38 | 5 | 78.76 |
| LN m3‐ab | Yes | −34.09 | 6 | 80.18 |
| LN m5‐ | Yes | −35.49 | 5 | 80.98 |
| LN m5‐a | Yes | −35.46 | 6 | 82.92 |
| LN m5‐b | Yes | −34.31 | 6 | 80.62 |
| LN m5‐ab | Yes | −33.84 | 7 | 81.68 |
Estimated Model Parameters
Table D.4.
Estimated model parameters for the BMD models
| EXP | HILL | INVEXP | LOGN | |
|---|---|---|---|---|
| Estimate for var‐ | 0.1466 | 0.1466 | 0.1463 | 0.1465 |
| Estimate for a‐ | 30.67 | 30.66 | 30.62 | 30.64 |
| Estimate for CED‐F | 149.7 | 149.5 | 136.2 | 141.8 |
| Estimate for CED‐M | 210.9 | 210.8 | 206.4 | 208.2 |
| Estimate for d‐ | 2.112 | 2.115 | 0.3659 | 0.6916 |
Weights for Model Averaging
Table D.5.
Weights for the models used in the BMD model averaging
| EXP | HILL | INVEXP | LOGN |
|---|---|---|---|
| 0.24 | 0.24 | 0.26 | 0.25 |
Final BMD Values
Confidence intervals for the BMD are based on 200 bootstrap data sets.
Table D.6.
Final BMD values for plasma alanine aminotransferase (ALAT) levels
| Endpoint | Subgroup | BMDL | BMDU |
|---|---|---|---|
| ALAT | F | 37.2 | 235 |
| ALAT | M | 86.6 | 593 |
Visualisation
Figure D.1.
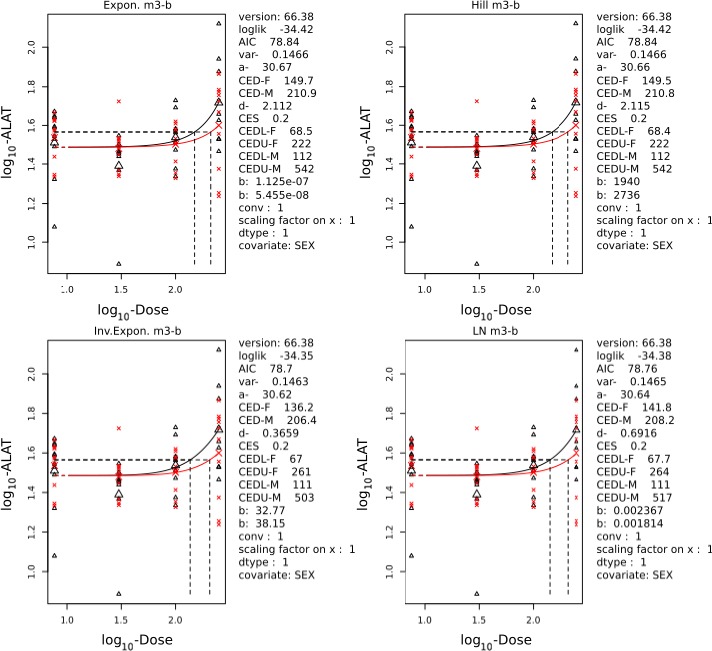
Visualisation of the individual BMD model curves
Figure D.2.
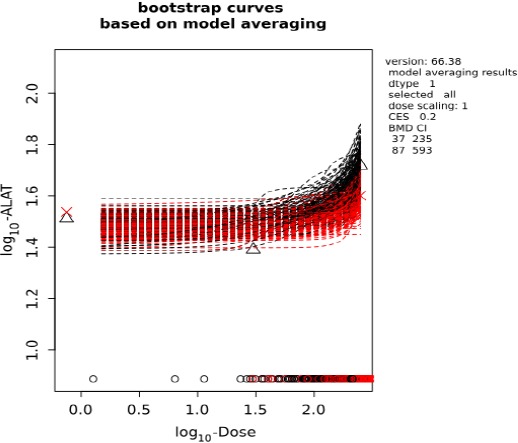
Visualisation of bootstrap curves based on BMD model averaging
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Castenmiller J, De Henauw S, Hirsch‐Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Engel KH, Frenzel T, Heinonen M, Marchelli R, Neuhäuser‐Berthold M, Pöting A, Poulsen M, Sanz Y, Schlatter JR, van Loveren H, Amundsen M and Knutsen HK, 2019. Scientific Opinion on the safety of phenylcapsaicin as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2019;17(6):5718, 24 pp. 10.2903/j.efsa.2019.5718
Requestor: European Commission following an application by aXichem AB
Question number: EFSA‐Q‐2018‐00280
Panel members: Dominique Turck, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, John Kearney, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Adopted: 15 May 2019
Notes
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), Turck D, Bresson J‐L, Burlingame B, Dean T, Fairweather‐Tait S, Heinonen M, Hirsch‐Ernst KI, Mangelsdorf I, McArdle H, Naska A, Neuhäuser‐Berthold M, Nowicka G, Pentieva K, Sanz Y, Siani A, Sjödin A, Stern M, Tomé D, Vinceti M, Willatts P, Engel K‐H, Marchelli R, Pöting A, Poulsen M, Salminen S, Schlatter J, Arcella D, Gelbmann W, de Sesmaisons‐Lecarré A, Verhagen H and van Loveren H, 2016. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA Journal 2016;14(11):4594, 24 pp. https://doi.org/10.2903/j.efsa.2016.4594
Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, pp. 64–71.
References
- BfR (Bundesinstitut für Risikobewertung), 2011. Too hot isn't healthy ‐ Foods with very high capsaicin concentrations can damage health. BfR Opinion No. 053/2011 of 18 October 2011. Available online: http://www.bfr.bund.de/cm/349/too-hot-isnt-healthy-foods-with-very-high-capsaicinconcentrations-can-damage-health.pdf
- Donath C, 2016. Dated: 17 May 2016. Eurofins BioPharma, Planegg, Germany. Study Title: In vitro mammalian micronucleus assay in human lymphocytes with phenylcapsaicin: Final: [Confidential]. Eurofins Munich Study No: 152391. Unpublished report
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2012. Scientific Opinion on Dihydrocapsiate. EFSA Journal 2012;10(7):2812, 28 pp. 10.2903/j.efsa.2012.2812 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W‐Y, Ma Z, Liu Y, Gao Z‐D, Li R‐X, Yuan W‐M and Li X‐C, 2012a. Dated: 22 December 2012. Wuxi Apptec Co. Ltd., Shanghai, China. Title: A mass balance, excretion and metabolism study of 14C‐labeled Phenyl‐capsaicin in male Sprague Dawley rats following single oral administration. Confidential. Wuxi Apptec Study No.: AM‐12J0720‐RMD. Unpublished report
- Feng W‐Y, Ma Z, Liu Y, Gao Z‐D, Li R‐X, Yuan W‐M and Li X‐C, 2012b. Dated: 22 December 2012. Wuxi Apptec Co. Ltd., Shanghai, China. Title: Tissue distribution and metabolism study of 14C labelled capsaicin in male Sprague Dawley rats following single oral administration. Confidential. Wuxi Apptec Study No.: AM‐12J0710‐RMD. Unpublished report
- Myers BM, Smith JL and Graham DY, 1987. Effect of red pepper and black pepper on the stomach. American Journal of Gastroenterology, 82, 211–214. [PubMed] [Google Scholar]
- Paulsen TR, Stiller S, Weber K, Donath C, Schreiband G and Jensen KH, 2018. A 90‐day toxicity and genotoxicity study with high‐purity phenylcapsaicin. Toxicology Research and Application. 10.1177/2397847318773060 [DOI] [Google Scholar]
- SCF (Scientific Committee on Food), 2002. Opinion of the Scientific Committee on Food on capsaicin (adopted on 28 February 2002. (SCF/CS/FLAV/FLAVOUR/8 ADD1 Final. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out120_en.pdf
- Schreib G, 2015. Dated: 09 November, 2015. Eurofins BioPharma, Planegg, Germany. Study Title: Reverse mutation assay using bacteria (Salmonella typhimurium) with phenylcapsaicin: Final: [Confidential]. Eurofins Munich Study No: 152390. Unpublished report
- Stiller S, 2016. Dated: 28 October 2016. Eurofins BioPharma, Planegg, Germany. Study Title: 90‐day repeated‐dose oral toxicity study in Wistar rats with phenylcapsaicin including a 28‐day recovery period: Final: [Confidential]. Eurofins Munich Study No: 152393. Unpublished report
- Yang C and Dong H, 2015. Effects of Capsaicin and Phenylcapsaicin on TRPV! Stably Expressed GEK293 cells using FLIPR (Study number AXIMED‐20151229) [Confidential], Unpublished report


