Abstract
An increase in confirmed human salmonellosis cases in the EU after 2014 triggered investigation of contributory factors and control options in poultry production. Reconsideration of the five current target serovars for breeding hens showed that there is justification for retaining Salmonella Enteritidis, Salmonella Typhimurium (including monophasic variants) and Salmonella Infantis, while Salmonella Virchow and Salmonella Hadar could be replaced by Salmonella Kentucky and either Salmonella Heidelberg, Salmonella Thompson or a variable serovar in national prevalence targets. However, a target that incorporates all serovars is expected to be more effective as the most relevant serovars in breeding flocks vary between Member State (MS) and over time. Achievement of a 1% target for the current target serovars in laying hen flocks is estimated to be reduced by 254,400 CrI95[98,540; 602,700] compared to the situation in 2016. This translates to a reduction of 53.4% CrI95[39.1; 65.7] considering the layer‐associated human salmonellosis true cases and 6.2% considering the overall human salmonellosis true cases in the 23 MSs included in attribution modelling. A review of risk factors for Salmonella in laying hens revealed that overall evidence points to a lower occurrence in non‐cage compared to cage systems. A conclusion on the effect of outdoor access or impact of the shift from conventional to enriched cages could not be reached. A similar review for broiler chickens concluded that the evidence that outdoor access affects the occurrence of Salmonella is inconclusive. There is conclusive evidence that an increased stocking density, larger farms and stress result in increased occurrence, persistence and spread of Salmonella in laying hen flocks. Based on scientific evidence, an impact of Salmonella control programmes, apart from general hygiene procedures, on the prevalence of Campylobacter in broiler flocks at the holding and on broiler meat at the end of the slaughter process is not expected.
Keywords: poultry, Salmonella, target, attribution, risk factor, welfare, Campylobacter
Summary
Following a request from the European Commission, the Scientific Panel on Biological Hazards (BIOHAZ) was asked to provide a scientific opinion on Salmonella control in poultry flocks and its public health impact.
In particular, the European Food Safety Authority (EFSA) was requested in Term of Reference 1 (ToR 1) to estimate the public health impact if the target serotypes in flocks of breeding hens of Gallus gallus (Salmonella Enteritidis, Salmonella Typhimurium, including monophasic S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐), Salmonella Hadar, Salmonella Virchow and Salmonella Infantis) are changed, while maintaining the current Union target (1%), testing scheme and trade restrictions. The target serotypes were to be defined based on their public health significance taking into account the criteria described in Annex III of Regulation (EC) No 2160/2003: (a) the most frequent Salmonella serovars associated with human salmonellosis; (b) the route of infection; (c) whether any serovar shows a rapid and recent ability to spread and cause disease in humans and/or animals; and (d) whether any serovar shows increased virulence, e.g. regarding invasiveness or resistance to relevant therapies for human infections. The scenarios to be assessed were (a) a new top five of serotypes and (b) all serotypes. The estimated impact on the reported prevalence in flocks of broilers and layers was to be evaluated and, if possible, also on the reported human salmonellosis cases through the poultry meat and egg production chains. This ToR was answered by literature review supplemented by descriptive analysis of serovar distributions in breeding, laying and broiler flocks, and humans based on data from 2014 to 2016. The connection with the impact on human health was also considered through ToR 2, which provided an indication of the relative contribution of the laying hen and broiler reservoirs to human salmonellosis cases, taking underestimation (combining underreporting and under‐ascertainment) into account. Unfortunately, it was not possible to assess the impact of proposed changes of target serovars in breeding flocks on laying hen or broiler populations or on human salmonellosis cases as there are multiple sources of these serovars other than breeding flocks and the impact of breeding flocks depends on the individual strains involved and control actions in the food chain. Therefore, the ‘impact’ was interpreted as the possibility for achieving a relevant reduction in the considered serovars in laying hen and broiler flocks. There is justification, based on their occurrence in populations of G. gallus and in humans for retaining S. Enteritidis, S. Typhimurium (including monophasic variants) and S. Infantis in the target for breeding flocks. S. Kentucky was proposed as the fourth serovar as it has recently spread among broiler populations in several European Union (EU) Member States (MSs), and because many strains are resistant to multiple antimicrobials, including critically important fluoroquinolones. For the fifth serovar, several options are proposed depending on Salmonella control priorities at the EU and MS level. S. Heidelberg (based on data showing increased potential for vertical transmission and resistance to multiple antimicrobials, including extended‐spectrum cephalosporins) could be considered to prevent it becoming established in the EU, in the way that has occurred in the American continent. S. Thompson could be considered, based on its occurrence in breeding flocks and dissemination in a small number of MSs or a variable fifth serovar in MS‐specific national prevalence targets could be proposed, based on the national situation and knowledge of potentially emerging serovars in other countries. On the other hand, a target that incorporates all serovars is expected to be more effective as the most relevant serovars in breeding flocks vary between MS and over time. It would be more effective in reducing the dissemination of all serovars, including newly emerging strains with ‘epidemic potential’ and the re‐emergence of previously specified target serovars. In order to provide more definitive evidence of a link between breeding flocks and human infections, use of whole genome sequencing (WGS) for comparing isolates from poultry breeding flocks with those in commercial generations of birds and in humans is recommended. In addition, the reporting of all individual Salmonella serovars in poultry flocks would facilitate source attribution and epidemiological studies.
The second ToR was to estimate the public health impact if the target set for adult flocks of laying hens of G. gallus is reduced from 2 to 1% for the current target serotypes (S. Enteritidis and S. Typhimurium, including monophasic variants), maintaining the current testing scheme and trade restrictions. The impact was to be expressed as a relative reduction of reported human salmonellosis cases and taking into account the data from the last reporting year (i.e. 2016). For this assessment, a ‘Salmonella source attribution model (SAM)’, based on the microbial subtyping attribution approach was used. The model included the laying hen, pig, broiler and turkey reservoirs and included 23 MSs and 28 serovars. The baseline model was compared with the scenario in which the prevalence of the current target serotypes was set at 1% (or less) in layers. The prevalence was kept as reported if it was already below 1%. In this scenario, the number of layer‐associated human salmonellosis true cases (465,200 CrI95[212,100; 979,800]) (i.e. accounting for under‐ascertainment and underreporting) was estimated to be reduced by 53.38% CrI95[39.11; 65.69] compared to the situation 2016. This corresponds to an estimated reduction of 254,400 cases CrI95 [98,540; 602,700]. This reduction would translate into a 6.2% reduction of the overall 4.08 million CrI95 [2.22; 7.39] human salmonellosis estimated true cases. It was recommended to review the SAM and its conclusions as new data emerges as the Salmonella situation in the EU is dynamic in terms of the foodstuff‐associated risks and the serovars of most importance. To reduce the data limitations in such modelling, it was also recommended to report all individual Salmonella serovars in poultry flocks to facilitate source attribution and epidemiological studies and to obtain more recent and comparable data on Salmonella in cattle and pigs or pork. In addition, it was recommended to investigate the potential and reasons for under‐detection of Salmonella, particularly S. Enteritidis, in flocks of laying hens and the reasons for failure to control S. Enteritidis in countries where it appears regularly in chicken breeding flocks, laying hens or broilers. Also, field investigations on the effectiveness of administration of Salmonella vaccination programmes used in laying flocks, and their protective effect, are proposed.
In ToR 3, the risk factors for the occurrence of Salmonella in laying hens for which targets have been set were to be evaluated, in relation to the farming methods based on monitoring data and a review of literature, and in particular with the view to assess if the ban on unenriched cages had an effect on such occurrence. To answer this ToR, the presence of Salmonella in laying hen flocks housed in different farming systems was compared using data supplied by European Economic Area (EEA) countries following a request from EFSA. In addition, a literature review on the influence of housing and management systems as well as biosecurity measures on Salmonella occurrence in laying hens was performed, focussing on recent studies in the EU and equivalent high‐income countries. Unfortunately, there are no field studies that specifically address the impact of the transition from conventional to enriched cage systems on the occurrence of Salmonella in laying flocks. It was concluded that conflicting evidence is found in the literature and MS data on the occurrence of Salmonella in laying hens when raised in cage systems compared to non‐cage systems. Overall, evidence points to a lower occurrence in non‐cage systems compared to cage systems. Whether this is linked to the housing system as such or whether it is caused by the associated change of furniture, break in the historical infection cycle or the reduced stocking density, is unclear. The evidence that outdoor access or conventional vs enriched cage systems affect Salmonella occurrence in laying hens at the EU level is inconclusive.
In ToR 4, the risk factors for the occurrence of Salmonella, based on monitoring data and literature, were to be reviewed in relation to the type of farming for broiler chickens and in relation to other animal welfare indicators for broilers and laying hens. To answer ToR 4, the Salmonella presence in broiler flocks by housing system with or without outdoor access and by stocking density and age at slaughter was compared by means of a literature review and additionally provided data from EEA countries. Similarly, the literature review was used to assess risk factors relating to management and housing systems and biosecurity practices, with the same focus as described for ToR 3. In addition to this, literature on other welfare indicators in laying hen and broiler flocks was examined. The evidence that outdoor access affects the occurrence of Salmonella in broiler flocks is inconclusive. There is conclusive evidence that an increased stocking density, larger farms and stress‐inducing conditions result in increased occurrence, persistence and spread of Salmonella in laying hen flocks. For broiler flocks, the limited evidence available shows that stress, stocking density and increasing the number of flocks per farm increases Salmonella susceptibility or infection rate. There is no data evaluating the link between welfare indicators and Salmonella occurrence in broilers. It was recommended to record in future monitoring programs the housing type of laying hen and broiler flocks to enable assessment of its impact on Salmonella occurrence in these flocks.
ToR 5 was to indicate if there is scientific evidence on a possible negative or positive impact of Salmonella control programmes on the prevalence of Campylobacter in broiler flocks at the holding and on broiler meat at the end of the slaughter process. As the effect of Salmonella control programmes on the prevalence of Campylobacter has never been explicitly studied, the approach taken to answer ToR 5 was to describe the co‐colonisation of Salmonella and Campylobacter in broiler flocks and individual birds and the presence of Salmonella and Campylobacter on broiler meat at the slaughterhouse level, using EU data and literature review. These sources of information were investigated to identify possible positive or negative associations between the organisms. In addition, literature was consulted to gather information about the epidemiology of Campylobacter in broiler flocks compared and contrasted with Salmonella. Unfortunately, field studies are lacking that have specifically investigated the impact of Salmonella control programmes on the occurrence of Campylobacter in broiler flocks. It was concluded that overall evidence points towards no association (negative or positive) between the occurrence of Salmonella and Campylobacter in broiler flocks or on broiler meat at the end of the slaughter line. The epidemiological and biological differences between the two organisms result in a greater likelihood of introduction of Campylobacter into broiler flocks, at any stage of the life of the birds, if there are lapses in biosecurity standards, than for Salmonella. In addition, successful Salmonella control has involved actions taken at the breeding flock level, such as culling and vaccination, which are not applicable to Campylobacter. Based on scientific evidence on the differences between Salmonella and Campylobacter epidemiology and patterns of colonisation, an impact of Salmonella control programmes, apart from general hygiene procedures, on the prevalence of Campylobacter in broiler flocks at the holding and on broiler meat at the end of the slaughter process is not expected. Reporting of the results of samples that contain combinations of organisms, and not just reporting the prevalence and concentrations of each organism separately, in any infection study that includes more than one organism, was recommended.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
In accordance with Regulation (EC) No 2160/20031 targets have been set for reduction of this comes from the mandate Salmonella in flocks of breeding hens, laying hens, broilers, breeding turkeys and fattening turkeys by the adoption of several implementing Regulations.2 The targets are set on two serotypes (Salmonella Enteritidis and Salmonella Typhimurium, including monophasic S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐), except for breeding hens for which the target includes also Salmonella Hadar, Salmonella Virchow and Salmonella Infantis. In order to achieve the targets, Member States (MSs) have introduced Salmonella control programmes in these poultry populations. In addition, a number of trade restrictions have been introduced in case these populations were still infected with S. Enteritidis or S. Typhimurium. An overview of measures is provided in Table 1.
Table 1.
An overview of measures in case poultry populations were still infected with Salmonella Enteritidis
| Population | Target serotypes | Maximum % remaining positive | Trade restrictionsa |
|---|---|---|---|
| Adult breeding hens (Gallus gallus) | S. Enteritidis, S. Typhimurium, S. Hadar, S. Virchow and S. Infantis | 1% | Destruction or safe disposal of (hatching) eggs and birds (Annex II C of Regulation (EC) No 2160/2003) |
| Adult laying hens (Gallus gallus) | S. Enteritidis, S. Typhimurium | 2% | Destruction or safe disposal of hens birds, marketing of eggs as class B (only for heat treated egg products) (Annex II D of Regulation (EC) No 2160/2003) |
| Broilers (Gallus gallus) | 1% | Absence in 25 g of fresh meat (Point 1.28 of Annex I to Regulation (EC) No 2073/2005b) | |
| Adult breeding turkeys (Meleagris gallopavo) | 1% | Destruction or safe disposal of (hatching) eggs and birds (Annex II C of Regulation (EC) No 2160/2003) | |
| Fattening turkeys (Meleagris gallopavo) | 1% | Absence in 25 g of fresh meat (Point 1.28 of Annex I to Regulation (EC) No 2073/2005) |
Only in case of detection of S. Enteritidis or S. Typhimurium.
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs (text with EEA relevance). OJ L 338, 22.12.2005, p. 1–26.
Regulation (EC) No 2160/2003 and the implementing Regulations also lay down testing schemes (frequency of sampling, sampling protocol, laboratory analysis, reporting requirements) for each of these populations. The outcome of the testing is reported to EFSA within the frame of the zoonoses monitoring Directive 2003/99/EC3. EFSA examines and publishes the data in the annual EFSA/ECDC EU summary reports on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks.4 These summary reports clearly illustrate the success of the Salmonella control programmes e.g. by the reporting of more than 200,000 reported confirmed human salmonellosis cases per year in 15 MSs in the first years of the millennium and less than 100,000 in 28 MSs in 2012–2015. In 2014 and 2015, the number of human confirmed salmonellosis cases in the EU seems to increase again slightly (see Figure 1).
Figure 1.
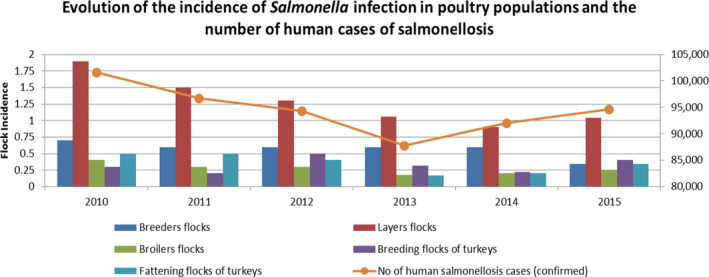
Evaluation of the incidence of Salmonella infection in poultry populations and the number of reported human cases of salmonellosis
The five serotypes of the target set in breeding hens, may no longer be the most relevant ones based on the most frequently reported ones in humans and/or in flocks of breeding hens. Furthermore, most MSs achieved the target set for flocks of laying hens in 2015 and 2016 and a more ambitious target could be considered. However, before considering a revision of targets in breeding and laying hens, the potential impact on reported human salmonellosis cases should be estimated.
During the 2004–2007 period, EU‐wide baseline surveys (BLS) were carried out to estimate the prevalence of Salmonella in flocks of laying hens, broilers and turkeys. The data were also used by EFSA to evaluate potential risk factors.5 It is relevant to review these risk factors based on more recent monitoring data, in particular since housing conditions have been changed as a consequence of animal welfare rules, both for laying hens6 and broilers.7
Finally, despite the obligation to introduce biosecurity measures in broiler flocks for control of Salmonella in accordance with Regulation (EC) No 2160/2003, and while such flocks are considered as the main reservoir of Campylobacter,8 no reduction of human campylobacteriosis is reported in recent years.
Terms of Reference
EFSA is asked to provide a scientific opinion on Salmonella control in poultry flocks and its public health impact. In particular, EFSA is requested:
-
1
To estimate the public health impact if the target serotypes in flocks of breeding hens of Gallus gallus are changed, maintaining the current Union target (1%), testing scheme and trade restrictions unchanged. The target serotypes should be defined by EFSA based on their public health significance taking into account the criteria described in Annex III of Regulation (EC) No 2160/2003). Following scenarios should be assessed:
a new top 5 of serotypes and
all serotypes. The impact of these scenarios should be estimated on the reported prevalence in flocks of broilers and layers (tested in accordance with Commission Regulations (EU) No 200/20129 and No 517/201110). If data allow, the impact should also be estimated on the reported human salmonellosis cases through the poultry meat and egg production chains.
-
2
To estimate the public health impact expressed as relative reduction of reported human salmonellosis cases if the target set for adult flocks of laying hens of Gallus gallus is reduced from 2 to 1% for the current target serotypes (Salmonella Enteritidis and Salmonella Typhimurium including the monophasic strains), maintaining the current testing scheme and trade restrictions unchanged and taking into account the data from the last reporting year (i.e. 2016).
-
3
To review the risk factors for the occurrence of Salmonella in laying hens for which targets have been set, in relation to the farming methods11 based on monitoring data and a review of literature, and in particular with the view to see if the ban on unenriched cages had an effect on such occurrence.
-
4
To review the risk factors for the occurrence of Salmonella based on monitoring data and a review of literature
-
5
To indicate if there is scientific evidence on a possible negative or positive impact of Salmonella control programmes on the prevalence of Campylobacter in broiler flocks at the holding and on broiler meat at the end of the slaughter process.
1.2. Interpretation of the Terms of Reference
In Term of Reference (ToR 1), ‘all serotypes’ refers to zoonotic serovars of Salmonella. ‘Breeder’ flocks, unless specified otherwise, refers to the combination of all types of breeding flocks, i.e. elite, grandparent and parent. For the second criterion of the ‘public health significance’, that is the route of infection (i.e. the presence of the serovar in relevant animal populations and feed). Feed is not considered in detail in the assessment as the focus is on the impact of breeding flocks, rather than the multiple other routes of infection that could be possible for broiler and laying hen flocks. The impact of targets largely depends on the sensitivity of detection of flock infections and the effectiveness of control actions, and therefore ‘impact’ is interpreted as the possibility for achieving a relevant reduction in the considered serovars in laying hen and broiler flocks. Decisions on monitoring and control programmes are considered to be risk management activities and are not included in the assessment.
For ToR 2, underestimation (combining underreporting and under‐ascertainment) was taken into account to estimate the relative reduction in the true number of salmonellosis cases.
In the scientific opinion, serovar was used as synonym of serotype.
1.3. Additional information
1.3.1. Additional background information
1.3.1.1. Previous scientific opinions of the BIOHAZ Panel
In April 2008, the European Commission requested EFSA to assess the public health impact of the setting of a permanent target for the prevalence of Salmonella in flocks in certain poultry populations (G. gallus). This resulted in the publication of three scientific opinions from the Panel on Biological Hazards (BIOHAZ Panel). The first one, published in April 2009, dealt with the impact of setting a new target for the reduction of the Salmonella prevalence in breeding hen flocks of G. gallus (EFSA, 2009). A second and third scientific opinion, published, respectively, in April 2010 and July 2011, provided an estimation of the public health impact of setting new targets for the reduction of Salmonella in laying hen (EFSA BIOHAZ Panel, 2010a) and broiler flocks (EFSA BIOHAZ Panel, 2011a). In April 2012, following an additional request by the European Commission in June 2010, a fourth scientific opinion was published dealing with the assessment of the public health impact of setting new targets for the reduction of Salmonella in breeding and fattening turkey flocks (EFSA BIOHAZ Panel, 2012). An overview of these risk assessments and the subsequent follow‐up by the European Commission are provided in Messens et al. (2013).
In 2008, the European Commission requested EFSA to assess the extent to which meat derived from broilers contributes to human campylobacteriosis at the EU level. It was concluded that the handling, preparation and consumption of broiler meat may account for 20% to 30% of human cases of campylobacteriosis, while 50–80% may be attributed to the chicken reservoir as a whole. The conclusions must be interpreted with care as data for source attribution was limited (EFSA BIOHAZ Panel, 2010b). In 2009, the European Commission asked EFSA to identify and rank the possible control options within the broiler meat production chain taking into account the expected efficiency in reducing human campylobacteriosis and to propose potential performance objectives and/or targets at different stages of the food chain in order to obtain, e.g. 50% and 90% reductions of the prevalence of human campylobacteriosis in the EU caused by broiler meat consumption or cross‐contamination (EFSA BIOHAZ Panel, 2011b).
A EU‐wide BLS on Campylobacter in broiler batches and on Campylobacter and Salmonella on broiler carcasses was carried out in 2008. A total of 10,132 broiler batches were sampled from 561 slaughterhouses in 26 EU MSs and 2 countries not belonging to the EU. From each randomly selected batch, the caecal contents of 10 slaughtered broilers were collected, pooled and examined for Campylobacter. From the same batch one carcass was collected after chilling and the neck skin together with the breast skin was examined for the presence of Campylobacter and Salmonella, in addition to the determination of the Campylobacter counts. EFSA analysed the results of this BLS, in particular to estimate the prevalence of Campylobacter in broiler flocks and the prevalence of Campylobacter and Salmonella on broiler carcasses in MSs and at the EU level (EFSA, 2010a); and to assess quantitatively the risk factors for Campylobacter in broiler flocks and Campylobacter (EFSA, 2010b) and Salmonella on broiler carcasses based on the information collected (EFSA, 2010b).
1.3.1.2. Legal background
According to Regulation (EC) No 2160/2003 and its following amendments, MS have to set up Salmonella National Control Programmes (NCP) aimed at reducing the prevalence of Salmonella serovars, which are considered relevant for public health, in certain animal populations. Currently, prevalence targets have been defined for breeding flocks of G. gallus, laying hens, broilers and breeding and fattening turkeys and correspond to the maximum annual percentage of flocks remaining positive for relevant serovars (S. Enteritidis and S. Typhimurium, including its monophasic variants, except for breeding flocks of G. gallus, where S. Infantis, S. Virchow and S. Hadar are considered to be relevant as well). In particular, the prevalence target is equal to 1% or less for breeding flocks of G. gallus. In breeding flocks of G. gallus, 2016 was the tenth year in which MS were obliged to implement a Salmonella NCP. These NCPs are based on Regulation (EC) No 200/201014 and the prevalence target (1% or less) was set for all commercial‐scale adult breeding flocks, during the production period, comprising at least 250 birds (however, MS with fewer than 100 breeding flocks would attain the target if only one adult breeding flock remained positive). The NCP are set up in individual MS to achieve the EU prevalence targets in these animal populations at the primary production level. NCP have to be approved by the European Commission, which evaluates the compliance of the programmes with the relevant EU legislation. The results of the programmes have to be reported by the MS to the EC and EFSA as part of the annual EU zoonoses monitoring (Boelaert et al., 2016; EFSA and ECDC, 2017d).
The minimum requirements for systems of production for the various egg farming methods is provided in Appendix A. Eggs can be produced from ‘enriched cages’ (otherwise known as ‘furnished cages’ or ‘colony cages’) and from alternative systems producing ‘barn eggs’, ‘free‐range eggs’ and ‘organic eggs’. Rearing in unenriched cages (or battery cages, referred to a ‘conventional cages’ in this scientific opinion) has been prohibited since 2012. Currently, there are no derogations regarding the implementation of the laying hens directive. The minimum requirements for systems of production for the fattening poultry can be found in Appendix B. In order to indicate types of farming of fattening poultry following terms may appear on the labelling: extensive indoor (barn‐reared), free range, traditional free range, free range – total freedom, ‘organic’.
1.3.1.3. Description of the poultry production chain
In 2016, 15.6 million tonnes (tonnes carcass weight) of poultry meat were produced in the EU, mainly broilers (ca 83%), turkeys (ca 14%) and ducks (ca 3%) (AVEC, 2018). For the egg line, the total production in the EU was approximately 7.15 million tonnes, representing 6.41 million tonnes of eggs produced per year for human consumption.15
Poultry meat or egg production (Figure 2) is based on selection of male and female breeding lines based on precise genetic criteria, such as productivity, quality of products and resistance to disease. The resulting chicks are reared in tiered grandparent and parent breeding stages, ultimately giving rise to chicks intended for fattening for poultry meat, and pullets for production of table eggs. Spent hens are also often slaughtered for human consumption, although in most countries the majority of such meat is used for heat‐treated products rather than as fresh meat.
Figure 2.
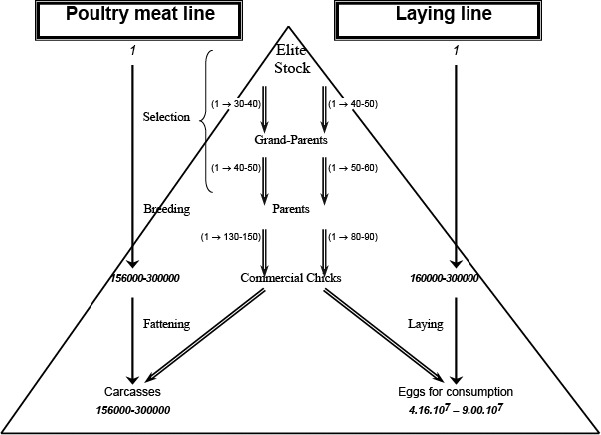
Simplified structure of poultry production (Modified from EFSA (2009))
Different genetic lines of birds are used for meat and egg producing flocks of chickens. There are also different genetic lines of birds for conventional and free‐range or organic production systems. Figure 2 shows how poultry production is structured between different types of breeding flocks and commercial (production) flocks. Theoretically, based on data from 2004, every Elite female could be the origin of up to 280,000 broilers or 300,000 laying hens producing up to 9.0 x 107 table eggs (Hunton, 1993; EFSA, 2004). In view of the continuous genetic improvement of birds to increase productivity, the current multiplication figures are likely to be considerably increased. More than 70% of the European production of hatching eggs in 2016 occurred in the United Kingdom, Poland, France, the Netherlands, Germany, Belgium and Spain.14
Continuous genetic selection is carried out in primary breeding or elite flocks to achieve ongoing progress in terms of performance characteristics of these valuable birds. These flocks are normally kept under conditions of extremely high biosecurity and in the case of chickens, normally in regions where there is a low prevalence of Salmonella and a low risk of other avian diseases.
Primary breeding of chickens is managed by a small number of companies globally. Consequently, there is considerable international trade in fertilised hatching eggs and day‐old chicks and grand‐parents and parents are distributed worldwide to be hatched and reared in many other countries.
Countries such as France, Spain, the Netherlands, Greece and the United Kingdom report a considerable number of movements (both import and export) of both breeding and production chicks while the other EU MSs report much less trade (EUROSTAT). There are control measures in place specified in regulatory instruments for the trade in poultry and hatching eggs.
France, Germany and Italy are the largest table egg producers, followed by Spain, the United Kingdom and the Netherlands, producing more than 70% of the 6.413 million eggs produced for human consumption per year in the EU.15 The distribution of laying hens by production system in the EU in 2016 was as follows: 55.5% were held in enriched cages, while the alternative systems producing barn eggs, free‐range eggs and organic eggs accounted for 25.6%, 14.1% and 4.6%, respectively.
Poland, the United Kingdom, Germany, Spain, France, the Netherlands and Italy were the top‐7 producers of broiler meat in the EU. These countries produced almost three quarters of the 12,326,000 tonnes carcass weight produced. There is no legal reporting obligation for MS under Regulation (EC) No 543/200812 on poultry meat marketing standards. In France, alternative production accounted for 25% of the broilers’ controlled slaughtering. In the United Kingdom, about 20% originates from ‘alternative production’, including indoor production of slow‐growing meat birds.
1.3.2. Approach to answer the ToR
The approach taken to answer ToR 1 was to provide an update of the literature review in the previous scientific opinion of the BIOHAZ Panel on the impact of setting a new target for the reduction of the Salmonella prevalence in breeding hen flocks of G. gallus (EFSA, 2009), supplemented by descriptive analysis of serovar distributions on breeding, laying and broiler flocks based on data from 2014 to 2016. The connection with the impact on human health was achieved through a descriptive analysis of serovar distributions and through ToR 2, which provided an indication of the relative contribution of the laying hen and broiler reservoirs to human salmonellosis cases, taking underestimation into account.
The approach taken to answer ToR 2 was to use a ‘Salmonella source attribution model (SAM)’, based on the microbial subtyping attribution approach using various animal/food sources of Salmonella (e.g. layers, broilers, turkeys and pigs), similar to the previous scientific opinions of the BIOHAZ Panel (EFSA BIOHAZ Panel, 2011a, 2012). The most recent 2016 data, when available, was used.
To answer ToR 3, the presence/prevalence of the regulated Salmonella serovars and Salmonella in laying hen flocks housed in different farming systems was compared, as far as data requested from the MS permitted. In addition, a review of the published literature on the influence of housing and management systems as well as biosecurity measures on Salmonella occurrence in laying hens and broilers was performed. This literature review was mainly focussed on studies in Europe and equivalent high‐income countries, but relevant studies from other global regions were included if relevant production systems, management and housing practices applied. The review was also focussed on recent studies to provide maximum comparability of the described housing and management systems with the current situation.
To answer ToR 4, the presence/prevalence of Salmonella in broiler flocks by housing system with or without outdoor access and by stocking density and age at slaughter was compared by means of a literature review and additionally provided data from European Economic Area (EEA) countries. Similarly, the literature review was used to assess risk factors relating to management and housing systems and biosecurity practices with the same focus as described for ToR 3. In addition to this, literature on other welfare indicators in laying hen and broiler flocks was examined.
The approach taken to answer ToR 5 was to describe the co‐colonisation of Salmonella and Campylobacter in broiler flocks and individual birds and the presence of Salmonella and Campylobacter on broiler meat at the slaughterhouse level using data provided by EEA countries and literature review. These sources of information were investigated in order to identify possible positive or negative associations between the organisms. In addition, literature was consulted to gather information about the epidemiology of Campylobacter in broiler flocks compared and contrasted with Salmonella. This included a consideration of similarities and differences in the biology, survival, infection/host response characteristics and colonising doses of the organisms that are relevant to different sources of infection or contamination and the ability of biosecurity to minimise infection at the farm level, including the role of compliance with required biosecurity standards.
2. Data and methodologies
2.1. Data
2.1.1. ToR 1
2.1.1.1. Occurrence of Salmonella in poultry populations
Salmonella testing (as required under the Directive 2003/99/EC) is carried out by each EU MS and reported to EFSA in the framework of the EU annual zoonoses monitoring (EFSA and ECDC, 2017d). As such, the following data in breeding (all types) and production flocks (adult productive laying hens and broilers) by number of reports, prevalence and serovar using data of 2014–2016 was used in the analysis. Only data from the NCPs were used, thus originating from adult birds and broilers before slaughter:
Salmonella serovar data from each year between 2014 and 2016 for each MS as well as summary data for the whole EU for breeders (if possible described according to the stage of breeding – elite, grandparent, parents and whether broiler or layer breeders);
Salmonella serovar data from each year between 2014 and 2016 for each MS as well as summary data for the whole EU for laying hens;
Salmonella serovar data from each year between 2014 and 2016 for each MS as well as summary data for the whole EU for broiler flocks.
Years 2014–2016 were selected due to the better data quality compared with earlier years and the unavailability of 2017 data in time for consideration in this scientific opinion. A 3‐year period was considered because of the limited amount of data from breeding flocks in any single year and because infection in a breeding flock may only be detected in progeny in subsequent years, after amplification in hatcheries.
Validated data were extracted from the EFSA zoonoses database on 20 April 2018. EU MSs for which data were missing or had a large proportion (~30%) of ‘unspecified’ serovars were contacted to request these data. As a consequence of this request, additional data on Salmonella serovars isolated between 2014 and 2016 from breeding, broiler and laying flocks were obtained from Italy, Poland and Spain. Malta also provided 2014 data (reported 2015–2016 data sufficiently included information on other serovars isolated). From France, only the additional serovar data from layer flocks in 2016 could be used. Due to the large poultry industry in France and number of Salmonella isolations, the broiler flock data from the 2017 submission by France was also used as this provided some additional information on non‐target serovars. Some changes have been applied to the reporting of the serovars to harmonise the nomenclature.16
2.1.1.2. Human salmonellosis
Data on reported cases of human salmonellosis in the EU by MSs and the related serovar distribution for both sporadic cases and food‐borne outbreaks in 2016 were used. Human cases of non‐typhoidal salmonellosis are reported by EU MSs and EEA countries in accordance with Decision No 1082/2013 on serious cross‐border threats to health, repealing Decision No 2119/98/EC. The cases are reported annually to The European Surveillance System (TESSy) in accordance with the EU case definition for salmonellosis. Data on Salmonella from 2014 to 2016 were extracted on 14 March 2018 from TESSy for all EU MSs, except for Croatia and Poland. For those two countries, case‐based data were first time available in 2017 (extracted on 20 August 2018). Only confirmed cases as defined in the EU case definition were used for analyses. Whether cases were either ‘domestic’, ‘EU travel’, ‘travel outside EU’ or ‘unknown’ was differentiated. Based on the reporting of the official national notification data from each EU/EEA MSs to TESSy, the European Centre for Disease Prevention and Control (ECDC) calculates notification rates and age standardised rate per 100,000 population by country and at the EU and EU/EEA level. The notification rate is the closest estimate to a population‐based incidence rate in the EU/EEA and the best available harmonised data at the EU level. In this scientific opinion, the wording ‘notification rate’ is used when references are made to the published EU‐wide data originating from TESSy. The human data are published annually in the EU summary reports and are available in the interactive Surveillance Atlas on the ECDC website. In addition, annual epidemiological reports are published on the ECDC website and a seroincidence calculator tool17 is available to estimate the frequency of exposure to Salmonella. Similarly, as above, some changes have been applied to the reporting of serovars.18 Compared to the poultry data sets, EU MSs had less ‘unspecified’ serovars so no further data were requested. These data were used to guide the consideration of serovars that were subjected to descriptive analysis.
In addition, the outcome in terms of mortality (death/alive) and hospitalisation status (yes/no) for a selection of serovars was extracted considering the period 2014–2016 for ‘all salmonellosis cases’ and ‘salmonellosis cases acquired in the EU (domestic and EU travel) only’.
Data on food‐borne outbreaks caused by Salmonella from 2014 to 2016 were extracted on 7 February 2018 from the EFSA zoonoses database. A selection was made for ‘strong evidence’ outbreaks as for these outbreaks more detailed information is collected than for ‘weak evidence’ food‐borne outbreaks, including food vehicle and its origin, nature of evidence linking the outbreak cases to the food vehicle, extent of the outbreak, place of exposure, place of origin of the problem and contributory factors. The technical specifications for harmonised reporting of food‐borne outbreaks through the EU reporting system, in accordance with the above mentioned EU Zoonoses Directive can be found in EFSA (2014).
2.1.1.3. Literature data
Literature searches for ToR 1 were carried out in Scopus and Google Scholar beginning from 2008 and using a broad range of exploratory search terms relating to occurrence of Salmonella and breeding chickens, integrated chicken production, hatcheries, vertical transmission, egg transmission, virulence, antimicrobial resistance, epidemic potential for Salmonella in general. Specific searches were also carried out for any data relating to named serovars occurring most frequently in humans and those that have spread rapidly in poultry populations in recent years. Monthly searches on ‘Salmonella’ and on ‘antimicrobial/antibiotic resistance’ were maintained during the time span of the working group and relevant new data were included in the scientific opinion. From the monthly searches, a small number of additional references were obtained in relation to antimicrobial resistance or virulence characteristics of Salmonella serovars, and this information was included in the scientific opinion.
2.1.2. ToR 2
The following data are key data employed for building the SAM.
2.1.2.1. Occurrence of Salmonella in food‐producing animal populations
The Salmonella monitoring data (prevalence data in food‐producing animal populations) for the years 2014–2016 were considered as reported by the MS in the framework of the EU annual zoonoses monitoring (EFSA and ECDC, 2017d):
Laying hens: from the official NCPs, adult flocks (flocks in production);
Broilers: from the official NCPs, flocks before slaughter;
Fattening turkeys: from the official NCPs, flocks before slaughter; breeding turkeys were also included to increase the data relating to the turkey reservoir and because these are also a relatively important source of turkey meat;
Pigs: from bacteriological monitoring programmes carried out in the MSs and also from the Salmonella monitoring data related to the Process Hygiene Criterion;
Cattle: from bacteriological monitoring programmes carried out in the MSs.
Validated data were extracted from the EFSA zoonoses database on 30 January 2018. Specific requests to zoonoses network representatives from the MS were made to complement these data in case of a large proportion (~30%) of ‘unspecified’ serovars in the data set. Only sparse data on Salmonella occurrence including Salmonella serovars in cattle herds, beef products or dairy products were available from MSs. For this reason, the cattle reservoir was not included in the model, which was also the case for previous EU‐level models.
The selection criteria for the prevalence and serovar data are shown in Table 2. For two MSs (Luxembourg and Romania), no relevant data representing the pig reservoir was available. For a single MS (Lithuania), no data on any source was available.
Table 2.
Selection criteria for prevalence and serovar data
| Prevalence data | 1st choice | 2nd choice | 3rd choice | 4th choice |
|---|---|---|---|---|
| Laying hens | Official NCPs (2016) | NA | NA | NA |
| Broilers | Official NCPs (2016) | NA | NA | NA |
| Fattening turkeys | Official NCPs (2016) | NA | NA | NA |
| Pigs | EU‐wide BLS (All MSs except MT and HR) | Bacteriological monitoring programmes (2016: MT) | Average prevalence as found in the BLS (HR) | NA |
| Serovar data | ||||
| Laying hens | Official NCPs (2016) | MS requesta (FR, ES, IT, NL, PL) | AMR data (DE) | NA |
| Broilers | Official NCPs (2016, 2017: FR) | MS requesta (ES, IT, NL, PL) | AMR data (DE) | NA |
| Fattening turkeys | Official NCPs (2016, 2017: FR) | MS requesta (ES, IT, PL) | AMR data (DE) | NA |
| Pigs | Bacteriological monitoring programmes (2016: MT) | AMR data (2014–2016) (BE, BG, CZ, DE, DK, EE, FI, HR, IE, IT, NL, SE, SI) | MS requesta (2014–2016) (ES, FR, PL, UK) | EU‐wide BLS (AT, CY, EL, HU, LV, PT, SK) |
AMR: antimicrobial resistance; BLS: baseline survey; NA: not applicable; NCP: National Control Programme.
Country abbreviations: AT: Austria; BE: Belgium; BG: Bulgaria; CZ: Czech Republic; CY: Cyprus; DE: Germany; DK: Denmark; EE: Estonia; ES: Spain; FI: Finland; FR: France; EL: Greece; HR: Croatia; HU: Hungary; IE: Ireland; IT: Italy; LV: Latvia; MT: Malta; NL: Netherlands; PL: Poland; PT: Portugal; SE: Sweden; SI: Slovenia; SK: Slovakia; UK: United Kingdom.
EU MSs for which data were missing or had a large proportion (> 30%) of ‘unspecified’ serovars were contacted to request these data.
2.1.2.2. Human salmonellosis
The total number of reported Salmonella cases in humans includes sporadic, travel inside EU, travel outside of EU and outbreak‐related infections and was obtained from ECDC (see Section 2.1.1.2). Human data reported for 2016 were included in the model for all MSs except Croatia and Poland, where 2017 data were used, as case‐based data were not available for 2016.
As the attribution model considers the EU, those cases resulting from travel inside EU were regarded as ‘domestic’, meaning that they would be allocated to a food‐animal source within the EU. Travel cases outside EU were not allocated to a food‐animal source, but to a travel category. For cases with unknown travel history, an extra number of travellers were estimated based on the proportion of travellers out of total per serovar and MS, and the remainder of the unknowns were assumed to be domestically acquired. If information on travel was unknown for all cases per serovar, cases were assumed to be domestically acquired. To estimate the number of sporadic cases, the number of outbreak‐related cases per serovar and MS were subtracted from the total number of domestically acquired cases, except for one case per outbreak, which was assumed to be sporadic, meaning that one outbreak contributed with one sporadic case.
To account for differences in underestimation (i.e. the combined underreporting and under‐ascertainment) between MSs (Gibbons et al., 2014), the true number of cases was estimated using the multiplication factors published by Havelaar et al. (2013) and used in the two previous Salmonella attribution models used to evaluate the target setting for broilers and turkeys (EFSA BIOHAZ Panel, 2011a, 2012). As no multiplication factor was available for Croatia, a multiplication factor of 60 was assumed, which is around the same level as other Eastern EU MSs. Multiplication factors were included as probability distributions to account for uncertainty around their true value.
2.1.2.3. Consumption of different types of foods
Production data and import/export data were used to calculate an approximation for the consumption of the different types of food with different origin for each MS in 2016. The production data were derived from AVEC (2017) for broiler and turkey meat (1,000 tonnes carcass weight), from the slaughtering in slaughterhouses ‐ annual data (apro_mt_pann19) database of the Statistical Office of the EU (EUROSTAT) for pigs (1,000 tonnes pig meat) and CIRCAB for consumption eggs (tonnes). Import and export data20 of 2016 were extracted from EUROSTAT (EU Trade Since 1988 By CN8 (DS‐016890)) for broiler meat,21 turkey meat,22 pork meat23 and eggs.24
The amount available for consumption was calculated by [production – export + import] for each MS. The amount available for consumption produced in a MS was calculated by [production – export]. In some instances, this resulted in negative production values, i.e. the amount exported was larger than the amount produced within the country. To ensure that MSs would still have nationally produced food available in their own country, it was assumed that imported products could also be re‐exported. This assumption was made for turkey meat for four MSs (Denmark, Estonia, Ireland and Latvia).
2.1.3. ToR 3 and 4
2.1.3.1. Literature data
The strategy for conducting the literature searches is provided in Appendix C. The search aimed to retrieve information on welfare aspects or housing systems and risk factors (e.g. biosecurity) in relation to Salmonella occurrence in laying hen production (i.e. laying hen flocks, breeder flocks or hen's eggs) and broiler production (i.e. broiler or breeding flocks) (see Tables C.1 and C.2, Appendix C). Information was collected on the country, the type of study (i.e. experimental and/or field study), the evaluation of the risk/protective factors (i.e. qualitatively and/or quantitatively), and the risk/protective factor(s) in relation to the farming system investigated and the animal welfare indictors described. The search was conducted on 6 February 2018.
Table C.1.
Details of search strings used for literature searches – search strings for Web of Science and CABI
| Set number | Search |
|---|---|
| 4 | #3 AND #2 AND #1 |
| 3 |
TOPIC: (cage OR cages OR aviary OR aviaries OR barn OR barns OR pen OR pens OR outdoor OR indoor OR “free range” OR house* OR “housing” OR “rearing” OR “stocking” OR farm* OR farming OR husbandr* OR crowding OR stress OR welfare OR (risk NEAR/4 factor*) OR (protective NEAR/4 factor*) OR “social environment”) DocType = All document types Language = All languages Time span = 2010–2018 |
| 2 |
TOPIC: (salmonella OR “S Enterica” OR “S Enteritidis” OR “S Typhimurium” OR “S Hadar” OR “S Virchow” OR “S Infantis”) DocType = All document types Language = All languages Time span = 2010–2018 |
| 1 |
TOPIC: (chicken OR chickens OR hen OR hens OR broiler* OR pullet* OR “Gallus gallus” OR “Gallus domesticus” OR “G gallus” OR “G domesticus” OR ((“layer” OR “layers”) NEAR/4 (farm* OR environment* OR production OR flock* OR hous*)) DocType = All document types Language = All languages Time span = 2010–2018 |
Table C.2.
Details of search strings used for literature searches – search strings for PubMed
| Set number | Search |
|---|---|
| 4 | #3 AND #2 AND #1 |
| 3 |
“animal husbandry” [Mesh:NoExp] OR “animal welfare” [Mesh] OR “farms” [Mesh] OR “risk factors” [Mesh] OR cage [Tiab] OR cages [Tiab] OR aviary [Tiab] OR aviaries [Tiab] OR barn [Tiab] OR barns [Tiab] OR pen [Tiab] OR pens [Tiab] OR outdoor [Tiab] OR indoor [Tiab] OR “free range” [Tiab] OR house* [Tiab] OR “housing” [Tiab] OR “rearing” [Tiab] OR “stocking” [Tiab] OR farm* [Tiab] OR farming [Tiab] OR husbandry [Tiab] OR crowding [Tiab] OR stress [Tiab] OR welfare [Tiab] OR (risk [Tiab] AND factor*[Tiab]) OR (protective [Tiab] AND factor*[Tiab]) OR “social environment” [Tiab] DocType = All document types Language = All languages Time span = 2010–2018 |
| 2 |
“salmonella” [Mesh] OR salmonella [Tiab] OR “S Enterica” [Tiab] OR “S Enteritidis” [Tiab] OR “S Typhimurium” [Tiab] OR “S Hadar” [Tiab] OR “S Virchow” [Tiab] OR “S Infantis” [Tiab] DocType = All document types Language = All languages Time span = 2010–2018 |
| 1 |
“Chickens” [Mesh] OR chicken [Tiab] OR chickens [Tiab] OR hen [Tiab] OR hens [Tiab] OR broiler* [Tiab] OR pullet* [Tiab] OR “Gallus gallus” [Tiab] OR “Gallus domesticus” [Tiab] OR “G gallus” [Tiab] OR “G domesticus” [Tiab] OR ((“layer” [Tiab] OR “layers” [Tiab]) AND (farm* [Tiab] OR environment* [Tiab] OR production [Tiab] OR flock* [Tiab] OR hous*[Tiab])) DocType = All document types Language = All languages Time span = 2010–2018 |
The risk/protective factor categories in relation to the farming system of laying hen production or breeder flocks listed were: outdoor access, cage systems (if yes: describe type of cage (free text)), alternative systems, group size, stocking density, genetic, rearing of pullets in the same place as layers, type of litter, biosecurity, and other diseases. The risk/protective factor categories in relation to the farming system of broiler production or breeder flocks listed were: outdoor access, group size, stocking density, genetic, farm hatching, existence of enrichment (such as perches, windows, nest boxes), type of litter, factors relating to biosecurity measures (e.g. disinfection, pest control), and occurrence of other diseases. The animal welfare indicators listed were: stress (other than heat stress), heat stress, activity/behaviour, body status (e.g. foot pad dermatitis (FPD)), other diseases, other: free text.
Factors relating to study design, such as control of confounding, potential for bias and applicability to current husbandry conditions in the EU were taken into account when drawing conclusions from the literature data.
2.1.3.2. Data from EEA countries
As monitoring data have no information on farming methods or flock size, data on the occurrence of the regulated Salmonella serovars (S. Enteritidis and S. Typhimurium, including monophasic S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐) in laying hen production flocks or broiler flocks in different housing systems in 2016 and 2017 were requested through EFSA's Microbiological Risk Assessment (MRA) Network.
Both data requests for laying hen production flocks and broiler flocks gathered data according to the:
Country
Reference
Year of sampling: 2016, 2017, 2016 and 2017
Salmonella: the regulated Salmonella serovars (S. Enteritidis and S. Typhimurium, including monophasic S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐), S. Enteritidis, S. Typhimurium, or monophasic S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐
Number of flocks tested
Number of positive flocks
Number of samples taken per flock
Sample type: faecal/boot swabs, caecal material or unknown.
In addition, the request for laying hen production flocks requested the following data:
-
Housing system related
-
–
System_level1: cage or non‐cage
-
–
System_level2:
-
–
In case of cage at System_level1: conventional cage, enriched or furnished cage, Kleingruppenhaltungen25 or unknown
-
–
In case of non‐cage at System_level1: single tier barn, multitier barn or unknown
-
–
-
–
System_level3: no outdoor access, outdoor access or unknown
-
–
System_level4: in case of cage at System_level1: without wintergarden,26 with wintergarden or unknown
-
–
Flock size (birds per house) when available
Number of laying hen flocks at holding when available.
In addition, the request for broiler flocks requested the following data:
-
Housing system related
-
–
System_level1: barn (multitier) or floor
-
–
System_level2: no outdoor access, outdoor access
-
–
Stocking density and unit of stocking density when available
Age at slaughter when available.
2.1.4. ToR 5
2.1.4.1. Literature data
The strategy for conducting the literature searches is also provided in Appendix C. The search aimed to retrieve information on the co‐colonisation or co‐detection of Salmonella and Campylobacter in poultry at the farm and slaughterhouse level (see Tables C.5 and C.6, Appendix C). The search was conducted on 6 February 2018.
Table C.5.
Details of search strings used for literature searches – search strings for Web of Science and CABI
| Set number | Search |
|---|---|
| 4 | #3 AND #2 AND #1 |
| 3 |
TOPIC: (campylobacter OR “C jejuni” OR “C coli”) DocType = All document types Language = All languages Time span = 2000–2018 |
| 2 |
TOPIC: (salmonella OR “S Enterica” OR “S Enteritidis” OR “S Typhimurium” OR “S Hadar” OR “S Virchow” OR “S Infantis”) DocType = All document types Language = All languages Time span = 2000–2018 |
| 1 |
TOPIC: (chicken OR chickens OR hen OR hens OR broiler* OR pullet* OR “Gallus gallus” OR “Gallus domesticus” OR “G gallus” OR “G domesticus” OR ((“layer” OR “layers”) NEAR/4 (farm* OR environment* OR production OR flock* OR hous*)) DocType = All document types Language = All languages Time span = 2000–2018 |
Table C.6.
Details of search strings used for literature searches ‐ search strings for PubMed
| Set number | Search |
|---|---|
| 4 | #3 AND #2 AND #1 |
| 3 |
“Chickens” [Mesh] OR chicken [Tiab] OR chickens [Tiab] OR hen [Tiab] OR hens [Tiab] OR broiler* [Tiab] OR pullet* [Tiab] OR “Gallus gallus” [Tiab] OR “Gallus domesticus” [Tiab] OR “G gallus” [Tiab] OR “G domesticus” [Tiab] OR ((“layer” [Tiab] OR “layers” [Tiab]) AND (farm* [Tiab] OR environment* [Tiab] OR production [Tiab] OR flock* [Tiab] OR hous*[Tiab])) DocType = All document types Language = All languages Time span = 2000–2018 |
| 2 |
“salmonella” [Mesh] OR salmonella [Tiab] OR “S Enterica” [Tiab] OR “S Enteritidis” [Tiab] OR “S Typhimurium” [Tiab] OR “S Hadar” [Tiab] OR “S Virchow” [Tiab] OR “S Infantis” [Tiab] DocType = All document types Language = All languages Time span = 2000–2018 |
| 1 |
“Chickens” [Mesh] OR chicken [Tiab] OR chickens [Tiab] OR hen [Tiab] OR hens [Tiab] OR broiler* [Tiab] OR pullet* [Tiab] OR “Gallus gallus” [Tiab] OR “Gallus domesticus” [Tiab] OR “G gallus” [Tiab] OR “G domesticus” [Tiab] OR ((“layer” [Tiab] OR “layers” [Tiab]) AND (farm* [Tiab] OR environment* [Tiab] OR production [Tiab] OR flock* [Tiab] OR hous*[Tiab])) DocType = All document types Language = All languages Time span = 2000–2018 |
In addition, separate broad monthly searches in Scopus using the search terms ‘Salmonella’, and ‘Campylobacter’ for ToR 5 were in place for the term of this mandate in order to identify any new relevant references since the original search was carried out. The papers remaining after screening were examined in detail and data on co‐colonisation or co‐contamination of carcasses at the abattoir by Salmonella and Campylobacter, plus key conclusions were tabulated. If studies reported a statistical analysis to detect possible positive or negative correlations between the occurrences of the two organisms, this was reported. No additional papers on co‐colonisation of Salmonella and Campylobacter were identified by the monthly searches, but a small number of papers on the immune response to infection and the potential for Campylobacter to enhance the invasion of other organisms beyond the intestinal tract were summarised. In addition, targeted searches were carried out in Scopus and Google Scholar to identify factors relating to differences in the epidemiology and biology of Salmonella and Campylobacter.
2.1.4.2. Data from EEA countries
EU data on the occurrence of Salmonella or Campylobacter for any broiler flocks and broiler batches at the farm or slaughterhouse level (e.g. neck skin or caecal samples) that had been tested for both pathogens from 2010 onwards were requested through EFSA's MRA Network.
The request for laying hen production flocks gathered data according to the:
Country
Reference
Year of sampling: 2010, 2011, 2012, 2013, 2014, 2015, 2016 or 2017
Sampling level: broiler flocks or broiler batches (at slaughterhouse)
Sample type: faecal/boot swabs (only for broiler flocks at sampling level); caecal material (for broiler flocks and Broiler batches at sampling level); neck/skin of carcass (only for broiler batches at sampling level)
Number of batches/flocks tested for both organisms
Number of batches/flocks positive for Salmonella only
Number of batches/flocks positive for Campylobacter only
Number of batches/flocks POSITIVE for Salmonella AND Campylobacter
Number of batches/flocks NEGATIVE for Salmonella AND Campylobacter.
2.2. Methodologies
2.2.1. ToR 1
It is not possible to directly model or quantify the impact of particular Salmonella serovars in breeding flocks on commercial generations of laying hens or broilers, or on humans directly from the Salmonella monitoring data reported to EFSA as part of MS's surveillance. This is because of the aggregated nature of the data and because of lack of data on flock sizes and ages, variations in the efficiency of detection of infected flocks, particularly for laying hens in cage systems, effect of control measures by MS after identifying positive breeding flocks, transmission rates and relative contribution of other routes of infection, such as feed, which may act as a common source for multiple food animal sectors. In addition, many MSs did not report the type of breeding flock from which Salmonella has been isolated, i.e. whether it was from a broiler or layer poultry line and from which level within the production pyramid (see Figure 2). As a consequence of this, the serovar data at the breeding flock level was aggregated across all production types. ToR 1 was therefore addressed by a combination of literature review and a descriptive analysis of the available surveillance data.
A critical literature review based on that provided in the previous EFSA scientific opinion relating to Salmonella targets in breeding flocks (EFSA, 2009) was updated to focus on evidence for vertical and pseudo‐vertical (hatchery contamination of egg origin) transmission of Salmonella. In addition, updates were provided on scientific literature relating to the range of Salmonella serovars that have been shown to be more capable of vertical transmission, have higher level of virulence for humans or ability to spread in poultry populations or pose a greater threat because of resistance to critically important antimicrobials. Literature searches used a wide range of exploratory search terms relating to these topics.
The descriptive analyses are based on the serovar data (including monophasic variants as a separate serovar in the case of S. Typhimurium) detected in breeding flocks, production flocks (layers, broilers) and humans within EU MSs. There were no data that enabled quantification of the impact of particular Salmonella serovars identified in breeding flocks on human cases and this depends on the timing of identification of infection and control actions in the breeding stages as well as hatchery and production holding practices and the propensity of individual strains to result in transient or resident hatchery contamination or to cause infection of the ovary and oviduct in breeding birds leading to vertical transmission. Factors relating to the infectivity and virulence for humans of each strain involved would also influence the burden of disease and data were also lacking in this respect. It was therefore only possible to address this part of ToR 1 qualitatively.
-
1
Consideration of the five current target serovars
Using the data sets described in Section 2.1.1, the distribution of the current five target serovars for breeding flocks of G. gallus (S. Enteritidis, S. Typhimurium (including monophasic S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐), S. Infantis, S. Hadar and S. Virchow) was obtained and plotted for 2014, 2015, 2016 for all EU MSs and for the EU overall. This allowed for an evaluation of the importance of the current target serovars in the different poultry populations and in humans. The distribution of the five target serovars was also plotted for layer flocks, broiler flocks and humans, years 2014–2016.
-
2
Consideration of an alternative five target serovars
Potential serovars for inclusion were identified in one of four ways: (1) being among the most commonly reported 20 serovars reported in humans as acquired in the EU during 2014–2016; (2) being linked to an outbreak of Salmonella attributed to eggs / egg products or broiler meat and products thereof between 2014 and 2016; (3) serovars identified by the source attribution (see Section 2.2.2; ToR 2) as serovars reported in humans that are attributed to broilers/layers; and (4) serovars identified by the literature review carried out within ToR 1, with special attention given to those serovars that can be vertically transmitted. For each serovar the frequency of isolations of the serovar in both the official monitoring data and the additional data acquired from some EU MSs was considered. Combining this descriptive analysis with the literature review, each serovar was assessed against four specific criteria (listed in Regulation (EC) No 2160/2003, Annex III) as summarised in Section 3.1.1 to ascertain whether a serovar could be considered to be relevant for inclusion within an alternative target for chicken breeding flocks. A Red‐Amber‐Green (RAG) score was assigned to each criterion for each serovar on the basis of the amount of evidence available. In particular, a red score was assigned when: (a) the serovar had been frequently reported (> 10% of EU cases) in humans or accounted for > 10% of human cases attributed to layers/broilers; (b) there was substantial evidence of transmission through the poultry industry; (c) there was substantial evidence of rapid and recent ability of the serovar to spread in poultry and cause disease in humans; and (d) there was substantial evidence of increased virulence (e.g. invasiveness or antimicrobial resistance). The score was amber when: (a) the serovar had only been occasionally reported in humans (1–10% of EU cases) or accounted for 1–10% of human cases attributed to layers/broilers; (b) there was some evidence of transmission through the poultry industry; (c) there was some evidence of rapid and recent ability to spread in poultry and cause disease in humans; and (d) there was some evidence of increased virulence (e.g. invasiveness or antimicrobial resistance). A green score was given when: (a) the serovar had been rarely reported in humans (< 1% of EU cases) or accounted for < 1% of human cases attributed to layers/broilers; (b) there was limited/no evidence of transmission through the poultry industry; (c) there was limited/no evidence of rapid and recent ability to spread and cause disease; and (d) there was limited/no evidence of increased virulence (e.g. invasiveness or antimicrobial resistance). Due to the uncertainty surrounding the prevalence of non‐target serovars in the breeding, broiler and layer flocks these data were not quantitatively used to assign a RAG status by percentage thresholds (as was done for the human data). However, these data informed the second criterion ‘transmission through the poultry industry’ by providing an indication of how widespread the serovar is within the EU and whether disseminated throughout the EU or clustered within a few MSs. As a consequence of the uncertainty regarding assessment of potential virulence of serovars expressed in terms of mortality (death/alive) and hospitalisation status (yes/no), the RAG status for criterion 4 prioritised information from scientific literature on the occurrence, or lack of reported evidence, of more severe infections associated with specific serovars. From this, an overall RAG status was assigned for each serovar based on an expert evaluation of the impact of the different criteria.
-
3
Consideration of inclusion of ‘all serovars’ as a possible target
The prevalence of Salmonella in breeding flocks was provided for all EU MSs (2014–2016) and compared to the prevalence for the five current target serovars. This provided an indication of which MSs would be likely to exceed a target of 1%. A consideration of the advantages and disadvantages of using all serovars as a target in breeding flocks was produced.
2.2.2. ToR 2
The EFSA Source Attribution Model (EFSA_SAM) was used. This model can be found at https://zenodo.org/record/57132#.W9cXYVVKiUk. The model included the data described in Section 2.1.2. Sources of uncertainty accounted for in the model included uncertainty around the underestimation/multiplication factors for estimating the true number of human salmonellosis cases, and uncertainty around factors associated with differences between serovars and food sources in their ability give rise to human infections. Parameters describing uncertainty were included as probability distributions in the model. Uncertainties that are not quantified in the model are given in Table F.2, Appendix F. The estimated changes to human incidence rates are therefore based on the MSs included in the model. More information on the model, including its principles, assumptions and uncertainties, can be found in Hald et al. (2012a) and Hald and Lund (2012).
Table F.2.
Potential sources of uncertainty identified in the source attribution analysis (ToR 2) and the impact that these uncertainties could have on the absolute and relative reduction of human salmonellosis cases under the scenario in which the prevalence of the current target serotypes (S. Enteritidis and S. Typhimurium including monophasic strains) is set at 1% (or less) in layers and compared to the baseline model
| Input/parameter/ model structure | Source of uncertainty | How uncertainty has been addressed | Direction of the effect on the absolute reduction of human salmonellosis cases attributed to the laying hen reservoir (−/+)a | Direction of the effect on the relative reduction of human salmonellosis cases attributed to the laying hen reservoir (−/+)a | |
|---|---|---|---|---|---|
| Model | Model selection |
The principle behind the applied model is that there are strong links between certain subtypes (here: serovars) and one or more animal reservoirs. Food products are seen as vehicles, and it assumed that the subtype distribution in a particular food source is similar to the one found in the reservoir from which it was contaminated. The model cannot distinguish between transmission pathways within the same reservoir, e.g. between eggs and meat from spent laying hens. However, the proportion of human infections from the latter source is assessed to be minor compared to the proportion from eggs and egg products due to differences in the amount consumed and in preparation and cooking procedures. The ‘effect’ on the reduction (absolute or relative) that the uncertainty may result in refers to the final results of the model, i.e. the reduction observed going from the baseline situation to the scenario. This means that the anticipated reduction in the number of egg‐associated cases would be less than estimated if some cases originate from other pathways (e.g. direct contact, meat of spent hens, environmental contamination, raw meat pet food etc.). However, it can be argued that since the prevalence is measured at flock level, a prevalence reduction would probably also have an effect on the other pathways relating to the reservoir. If eggs are overestimated as a source, the reduction in egg‐associated cases would in reality be less (‐) than estimated by the model which uses egg consumption data as one of the parameters. At least in absolute numbers. In relative numbers, it is less straightforward to assess, as a reduction in flock prevalence presumably would also have an effect on the other transmission pathways from the layer reservoir. |
− | − | |
| Model | Model structure | By using this SAM, it is assumed that the number of sporadic and domestic (as acquired in EU) human cases can be explained as a function of exposure: number of cases per MS, source and subtype = amount of source available for consumption per MS × prevalence per subtype, source and MS. This approach involves uncertainties that cannot be measured directly such as differences between subtypes (serovars), countries and food sources, which are likely to influence the number of human cases | Uncertainty is addressed by estimating two factors accounting for these potential differences. Namely, the food‐source dependent factor, which account for differences between food sources in their ability to give rise to human infections. As these may vary by country, one factor is estimated per food source and MS. Similarly, there are differences between the subtypes (serovars) in their ability to survive and infect humans. This uncertainty is described by including a subtype‐depending factor, which is assumed not to vary across countries. All factors are included a priori as uniform distributions, which are to most uninformed distribution one can, meaning that the model itself has few assumptions | −/+a | −/+a |
| Data | Compared to previous EU‐level attribution models, this model included fewer MSs. The MSs not included were Bulgaria, Cyprus, Lithuania, Luxembourg and Romania, where the latter four were included in the TT‐SAM (Hald et al., 2012a). Cyprus and Luxembourg are small countries contributing only with a minor fraction to all Salmonella cases in the EU. Romania and Lithuania had around 1,000 reported cases each in 2016, meaning that around 410,000 human true cases are ‘missing’ in the model, when accounting for underestimation. Export of eggs and eggs products from these countries including Bulgaria is according to the obtained trade data very limited. In addition, all these countries met the Salmonella target in layer flocks in 2016. Eggs produced in these MSs are, therefore, assessed only to contribute a very small number of Salmonella cases in other EU MSs | Uncertainty not possible to address in the model, as the MSs could not be included due to lack of data | + | No effect | |
| Data | The model included 28 of 944 serovars reported in 2016 and 2017 (Poland and Croatia). The 28 serovars corresponded to 65,087 (85%) of all 76,284 reported human cases. The serovars included individually and therefore available for attribution, were selected based on their occurrence in human domestic/EU cases and their occurrence in the four included animal reservoir. The remaining 916 serovars were grouped into ‘Others serovars’ and human cases belonging to this group were assigned to an unknown source category. The majority of these cases are expected to be linked with other sources than the four included in the model and the consequence of not counting them is assessed to be minimal. As an example, S. Dublin, which is known to be highly linked to the cattle reservoir, was not included as an individual serovar | + | + | ||
| Data | Salmonella occurrence in animal reservoirs | The principle behind the SAM assumes that the data included represent all the important sources of human salmonellosis, but food sources like beef, dairy products, imported food (from outside EU) and fruits and vegetables are not included, although they are known to act as vehicles for Salmonella. From a previous study based on outbreak data, fruits and vegetables were estimated to contribute 1.2–2.6% to the burden of human salmonellosis in the EU in 2007–2009 (Pires et al., 2011). Foodstuffs imported from outside the EU are not included in the model unless they resulted in outbreaks reported in the EU Summary Report in 2016. | For potential non‐animal food sources, it is emphasised that the SAM attributes human cases to the animal reservoir. This means that human infections caused by fruit and vegetables contaminated with faeces from an animal reservoir would be attributed to this reservoir, if produced in the EU. For some type of risk management decisions (relating to control in primary production), this may be appropriate, whereas for other decisions (relating to control in later stages of the food chain), alternative attribution approaches may need to be explored. | +b | +b |
| Data | Salmonella occurrence in pigs | Prevalence data from the BLS in slaughter pigs were used for all MS included in the model, except Malta. These data are more than 10 years old and may not reflect the situation in 2016, but are the best data available. | −/+c | −/+c | |
| Data | Sporadic human salmonellosis | There are differences in the level of reporting of human food‐borne infections in the EU MSs reflecting both differences in the methodologies used as well as the degree of reporting of human salmonellosis. Underestimation (i.e. the combined underreporting and under‐ascertainment) was accounted for in the SAM, but the estimation of the multiplication factors is based on Swedish traveller's data, which in itself involves some degree of uncertainty by assuming that the incidence rate among travellers returning from a particular country is the same as the overall incidence rate in the country's native population (Havelaar et al., 2013). In addition, the estimates are almost 10 years old and reporting may have change considerably in many MSs. Initiatives to obtain more recent data is therefore recommended | Underestimation was accounted for in the model by including the multiplication factors as probability distribution. | −/+ | −/+ |
| Data | Level of subtyping detail | The data limitation linked to the reporting of Salmonella data in the animal/food reservoirs with limited serotyping information (e.g. for animal reservoir data some MSs only report the targeted serovars individually and aggregate the rest in ‘others’) made it necessary to use other data sources. These uncertainties as well as the lack of further discriminatory subtyping information for the common serovars (S. Enteritidis and S. Typhimurium incl. the monophasic variants) may have resulted in attribution of some human cases to the wrong source. For instance, further typing of S. Enteritidis would very likely have resulted in a better distinction between the pig, broiler and laying hen reservoir in MSs where S. Enteritidis is widely prevalent in two or more of these sources, and it is considered extremely likely that this would have reduced the number of S. Enteritidis infections from the pig reservoir, and increased the number from the laying‐hen reservoir | A comparison with a specific model for Denmark, which uses more complete, detailed and specific data, was conducted to evaluate potential discrepancies at the MS level. The estimates for broilers and layers agreed well, whereas this was not the case for the pig reservoir. Here the comparison indicated that the role of the pig reservoir may in general be underestimated, but due to the low‐quality data on the pig reservoir and the lack of further subtyping data for the common serovars (S. Enteritidis, S. Typhimurium and monophasic Typhimurium), it is not possible to assess the contribution from of pigs/pork more accurately at this point. | −d | −d |
| Data | Salmonella subtypes distributions | For some sources/reservoirs, AMR monitoring data was used for estimating the serovar distribution. These data may not represent the situation at farm/reservoir level, as it is possible that resident contamination of complex slaughter equipment may contribute to Salmonella serovars found on carcass samples taken for AMR monitoring (Corry et al., 2002; Barbut, 2015). It has, for instance, been noted in the United Kingdom that Salmonella serovars that have not been found, or are rare in the NCP monitoring data for broiler flocks, such as S. Kentucky or S. Indiana are predominant in AMR monitoring samples. | By including the before mentioned subtype‐dependent factor, such differences are accounted for. For instance, if certain subtypes are predominant in e.g. slaughter‐level samples, but this is not reflected in the human cases, the model will allocate a relatively low subtype‐dependent factor. The SAM will not estimate more cases to these serovar. | −/+ (Minimal impact) | −/+ (Minimal impact) |
| Data | Production | The same data source has not been used to obtain information on the production of the different animal foods as the EUROSTAT production data for table eggs were insufficient and for poultry meat is not reported by species. The other data sources collect data using different methodologies, which may have resulted in amounts of food available for consumption that is not representing the same level of aggregation for each of the animal food sources. For instance, using the weight of slaughtered carcasses for pigs as an approximation for the amount available for consumption, may have resulted in an overestimation as compared to the other sources, as whole pig carcasses are obviously not consumed. | As long as the methods used for estimating the amount available for consumption is the same within a food source, the food‐source dependent factor will to some extent account for any methodological differences between food sources. | −/+ (Minimal impact) | −/+ (Minimal impact) |
| Data | Trade data | If some MSs systematically report more or less of a food source to be exported from their country than what is in reality exported, the model results will be impacted resulting in more or less cases in MSs importing from that country. | −/+ (Impact unknown) | −/+ (Impact unknown) |
AMR: antimicrobial resistance; BLS: baseline survey; NCP: national control programme; SAM: source attribution model.
+ means that the (real) outcome/effect is possibly overestimated; ‐ means that the (real) outcome/effect is possibly underestimated.
Omitting the cattle reservoir from the model may have the consequence that a proportion of human cases were wrongly attributed to reservoirs with similar serovar distributions. If more cases on this account were attributed to the laying hen reservoir, it may have resulted in a higher reduction of the estimated number of both absolute and relative human cases in the scenario analyses than what would be occurring in reality. Overall, it is considered unlikely that the lack of available data for other food sources bias the model result significantly, as long as it is kept in mind that SAM only attributes domestic and sporadic cases to EU animal reservoirs.
If the true prevalence figures are very different from the ones used, the number of attributed human cases to the pig reservoir would likewise be incorrect, which can also influence the number of cases attributed to the laying hen reservoir. However, the direction of the effect cannot be assessed.
If the number of S. Enteritidis infections from laying‐hens in reality is higher, the expected reduction in the absolute and relative number cases when reducing the target to 1% would be higher.
The model included the following 23 MSs: Austria, Belgium, Croatia, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Malta, Poland, Portugal, Slovakia, Slovenia, Spain, Sweden, the Netherlands and the United Kingdom. Five MSs were excluded from the analysis because of lack of sufficient data: Bulgaria (human data), Cyprus (human data), Lithuania (all food source data), Luxembourg (pig data) and Romania (pig data).
Based on an assessment of the most prevalent serovars in humans that were not primarily related to travel outside EU, 28 serovars (S. Enteritidis, S. Typhimurium, monophasic S. Typhimurium, S. Agona, S. Anatum, S. Bovismorbificans, S. Braenderup, S. Brandenburg, S. Derby, S. Goldcoast, S. Hadar, S. Havana, S. Indiana, S. Infantis, S. Java, S. Kentucky, S. Livingstone, S. London, S. Mbandaka, S. Montevideo, S. Napoli, S. Newport, S. Rissen, S. Saintpaul, S. Senftenberg, S. Tennessee, S. Virchow and S. Weltevreden) were included individually in the model. If not reported as monophasic S. Typhimurium, the monophasic variants of S. Typhimurium were grouped into this category using the antigenic formula, where the following were considered to be monophasic S. Typhimurium: S. 1,4,[5],12:i:‐, S. 1,4,5,12:i:‐, S. 1,4,12:i:‐, S. 4,[5],12:i:‐, S. 4,5,12:i:‐ and S. 4,12:i:‐. The remaining serovars (> 900) were grouped in an ‘Other serovar’ category. Cases belonging to this category were per definition allocated to an ‘unknown source’ category.
The results of the baseline model were compared with the scenario in which the prevalence of the current target serotypes (S. Enteritidis and S. Typhimurium including monophasic variants) was set at 1% (or less) in layers, assuming all MS would comply with this requirement. The prevalence for laying flocks was kept as reported to EFSA, if it was already below 1%.
2.2.3. ToR 3
Although many countries (n = 19) provided data on the occurrence of the regulated Salmonella serovars in laying hen production flocks in different housing systems, some important limitations were observed. First, some countries provided data on the positive flocks only and not on negative flocks. Secondly, some countries specified which Salmonella was tested for (e.g. S. Enteritidis) whereas others did not provide this information or indicated ‘the regulated serovars’, in which case the serovar of Salmonella was not known. Also, there was a very variable level of detail in the provided data regarding the description of the characteristics of the housing systems, although the information on ‘cage’ vs ‘non‐cage’ was always provided. Moreover, the cage systems always referred to enriched cages as the data originates from 2016/2017 when the rearing in conventional cage was prohibited in the EU. Yet, for the non‐cage systems additional information such as ‘outdoor access’ vs no ‘outdoor access’ and presence of a ‘winter garden’ was missing in the majority of the provided data and could therefore not be analysed. As a result of these data issues, a thorough data selection step was first performed. As the majority of the data was on S. Enteritidis, it was decided to work with this data only (from 13 countries). From these results, it was obvious that in a number of countries (Belgium, Latvia, Lithuania, Portugal, Poland, Slovenia and the United Kingdom) typically a combination of a small number of sampled flocks and a high prevalence of S. Enteritidis was provided. It is likely that for these countries only the data on the positive flocks was provided. As this would have distorted the analysis it was decided to exclude these countries. The final data set that was used comprised data from six MS.
2.2.4. ToR 4
Data on the occurrence of the regulated Salmonella serovars in broiler flocks in different housing systems in 2016 and 2017 were provided by 17 countries, all EU MSs. This included data on S. Enteritidis, S. Typhimurium and monophasic S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐ and non‐specified Salmonella. As the different serotypes may differ substantially in their epidemiology it was decided to split the data set in three based on the reported serovars. If the serovar was not reported, the data were not included.
2.2.5. ToR 5
Eleven countries provided data on the occurrence of Salmonella or Campylobacter for broiler flocks and broiler batches at the farm or slaughterhouse level that had been tested for both pathogens. Of those 11 countries, 8 provided useful data. In some cases, the same procedure was not used for sampling Salmonella and Campylobacter but it was considered justified to include those data when the sampling would represent the status of the live animal (e.g. swabs at farm and caeca at slaughter represent faecal/caecal (intestinal) material). Fisher's Exact Test for Count Data in R software R version 3.3.2 (2016‐10‐31) was used to investigate if the proportion of Campylobacter‐positive flocks/batches is independent from their Salmonella status. The confidence level was set at 95% and the alternative hypothesis was two‐sided.
2.2.6. Uncertainty
EFSA's Scientific Committee has developed a guidance document on how to characterise, document and explain all types of uncertainty arising in EFSA's scientific assessments. The document (EFSA Scientific Committee, 2018) provides a framework and principles for uncertainty analysis, with the flexibility for assessors to select different methods to suit the needs of each assessment. Attention was given to identifying sources of uncertainty and their potential impact on the outcome of the assessment (for ToR 2 only) (see Table F.2, Appendix F). Uncertainty relating to ToRs 1, 3, 4 and 5 was addressed descriptively (Table F.1, Appendix F).
Table F.1.
Examples of the major sources of uncertainty in the assessment of ToR 1, 3‐5
| Assessment components | Sources of uncertainty | Types of uncertainty | Potential impact of the uncertainty | |
|---|---|---|---|---|
| ToR | Assessment inputs | |||
| ToR 1 | Literature data – transmission of Salmonella from breeding flocks to progeny | The data relate to experimental studies using high inoculation doses of single isolates of Salmonella serovars. Data is in some cases conflicting, probably because of different strains, lines of birds, challenge and housing conditions | Extrapolation uncertainty | Extrapolation from experimental challenge studies to full scale poultry breeding and production could be misleading, especially if based on limited studies. The cumulative findings of challenge studies support a greater ability of S. Enteritidis, and S. Heidelberg and, to a lesser extent, certain strains of S. Typhimurium to transmit vertically via internal contamination of eggs. Most of this work has been done in the USA, using American strains, so it is not certain that the same ability would apply to strains of the same serovars circulating in Europe, but cumulative laboratory and field investigations support the special ability of S. Enteritidis to transmit vertically, but there is less evidence for other serovars |
| Literature data ‐ field studies on the distribution of Salmonella within poultry integrations | Many studies from a wide range of countries describe the occurrence of the same Salmonella serovars in breeding flocks and their progeny, and this applies to a wide range of serovars. It is assumed that the breeding flocks are responsible for dissemination of infection within the breeding pyramid | Ambiguity (incomplete information) | Although in many cases breeding flocks would be responsible for further dissemination of various Salmonella serovars, particularly via external contamination of eggs and consequent introduction of Salmonella into hatcheries, where it can multiply and become resident, it is also possible that breeding flocks and progeny may be exposed to the same source of contaminated feed. There is sufficient evidence from field investigations to suggest that most hatchery contamination is likely to originate from breeding flocks, but in some cases it may also be introduced by contaminated equipment or hatchery workers and there is little published definitive evidence. Although the same serovars may be found at different levels of poultry breeding and production, few studies have carried out sensitive molecular typing of isolates to show that the same strains are involved. Cumulative study findings and experience suggest that breeding flocks can be the source of a wide range of serovars, but this is highly variable according to the strain and hygiene measures at the farm and hatchery, and some studies have reported no apparent dissemination of certain strains of S. Typhimurium from broiler breeders to commercial broiler flocks | |
| Lack of detailed knowledge of the transmissibility of reported Salmonella strains and the control actions applied to positive flocks at breeder and progeny level, as well as the size of the flocks | Ambiguity (incomplete information) | Implementation of prevalence targets may not always prevent dissemination of Salmonella from breeding flocks to commercial generations of chickens if effective action is not taken to avoid this and action taken to limit dissemination at an early stage by removing eggs from infected flocks from the hatchery. Some strains of various serovars may not transmit vertically or do not colonise hatcheries, or if they do persist in hatcheries do not persist in chicks at detectable levels to the age of sampling. It is therefore difficult to assess the actual impact of prevalence targets at breeder level. If infected, a large breeding flock would be likely to have a much larger impact than a small one and large layer or broiler flocks would present more public health risk, but larger scale breeding and production may be controlled better in some countries. If infected progeny is culled on‐farm, as in some countries, the impact of Salmonella transmitted from breeding flocks is minimised | ||
| Sensitivity of the sampling programmes | Ambiguity (incomplete information) | Sampling, sample handling and test methods are often applied in a suboptimal way, as demonstrated by an up to 10‐fold higher detection rate of infected flocks by official sampling compared with operator sampling in many countries for breeding, laying and broiler flocks. In particular, certain types of laying hen flocks (e.g. large enriched cage systems, multitier aviary houses or extensive outdoor units with multiple small mobile housing) can be difficult to sample in a representative way. There is also a strong incentive to obtain negative results in laying hen flocks because of the economic consequences of egg restrictions or in broiler flocks because of the cost and inconvenience of on‐farm culling or slaughter under special hygiene precautions. In general, breeding flocks are likely to be better sampled because of the frequency of testing and the fear of losing trade and reputational damage if infected progeny are identified by customers. This uncertainty makes accurate estimation of the true prevalence of Salmonella in the breeding and production pyramid very difficult | ||
| Incomplete reporting of Salmonella serovars from poultry monitoring programmes, with a bias towards reporting of regulated serovars | Ambiguity (incomplete information) | A large proportion of Salmonella reports have not been further investigated to determine their serovar, e.g. only the target Salmonella serovars are reported by some MSs. In some cases data do exist but is not reported because of resource limitations in MSs. EU MSs for which data were missing or had a large proportion (~ 30%) of ‘unspecified’ serovars were contacted to request these data. As a consequence of this request, additional data on Salmonella serovars isolated between 2014 and 2016 from breeding, broiler and laying flocks were obtained from some MSs. Still, the absence of data for these MSs does not necessarily indicate the absence of a particular serovar. This limits the representativeness and conclusions that can be drawn from reported data for the many MSs that do type all of their Salmonella isolates as there can be a large proportion of ‘Salmonella unspecified’ which cannot be compared across breeding and production sectors or with human data | ||
| Lack of epidemiological data to definitively link a Salmonella serovar found in breeding flocks in a country with the same serovar found in layer or broiler flocks | Ambiguity (incomplete information) | Due to the aggregated nature of the data, the presence of a particular serovar in a broiler or layer flock cannot be directly attributed to the detected presence in a breeder flock without epidemiological data linking the establishments and the confirmation that, genetically, the isolates are the same. Even with such evidence, there is still a strong possibility that infection may have resulted from exposure to a common source, e.g. contaminated feed from a feed mill with resident Salmonella. This is currently the situation in the United Kingdom with S. 13, 23:i:‐ (a monophasic variant of S. Idikan) which has become established in several feed mills and hatcheries, leading to infection of both broiler breeding flocks and broiler flocks even though this serovar would not be expected to be vertically transmitted. It is therefore not possible to assess the relative contribution of different infection routes | ||
| Non‐inclusion of 2017 Salmonella monitoring data | Ambiguity (incomplete information) | If Salmonella was detected in a breeder flock in 2016, transmission to a broiler/layer flock might not be detected until the following year | ||
| Breeding flock type not specified in the official monitoring | Ambiguity (incomplete information) | Many MSs do not provide the type of breeding flock, e.g. stage (elite, grandparent, parent) and sector (broiler or egg production line). Therefore, all of the breeding flock data was aggregated for the descriptive analysis and it was not possible to consider alternative serovars in the different breeding sectors (broiler/egg line) | ||
| Lack of information on hospitalisation status and outcome in terms of mortality from human monitoring data | Ambiguity (incomplete information) | Hospitalisation and outcome in terms of mortality resulting from a Salmonella infection acquired in the EU is used to ascertain the virulence of the 28 different Salmonella serovars considered in Table E.1 in Appendix E. However, for hospitalisation, overall, this information is not available for 39.7% of cases. Similarly, for outcome in terms of mortality, overall, this information is not available for 15.4% of cases. There is also potential bias associated with different levels of reporting in different countries, with many only reporting the most serious cases or those associated with outbreaks. As the serovar distribution varies between countries, particularly in relation to outbreaks, the data may not provide an accurate representation of the situation at the EU level. Mortality levels for Salmonella are low and often associated with other predisposing health conditions, so an apparent high mortality rate for an individual serovar is likely to be misleading as this is based on a very small number of cases | ||
| ToR 3 | Evaluation of literature data for the assessment of the impact of housing conditions for layers on Salmonella occurrence | Use of experimental models | Extrapolation uncertainty | Some studies have used experimental models. The artificial infection of hens with high doses of Salmonella is not representative of the situation in the field and the spread of infection in different housing systems |
| Stocking densities used in studies are not used in farms in EU and/or pecking and scratching area missing in enriched cage studies | Extrapolation uncertainty | Some experimental studies comparing conventional cages and enriched cages consider stocking densities different from the ones usually present in farms (minimum required by the regulation) or lack of pecking and scratching area in enriched cages thus introducing additional confounders and decreasing their external validity. | ||
| Size and selection of sample | Extrapolation uncertainty | Some field studies have used very small sample sizes (farm and individual) which decreases the accuracy of the results. Sampling strategy is not always described which could lead to biased samples by intentional or unintentional targeting | ||
| Diversity in sample matrix and laboratory analysis | Extrapolation uncertainty | There is a large diversity in sample matrix and laboratory analyses conducted to assess/determine the Salmonella status in a bird/farm, thus making comparison of results between studies difficult | ||
| Conflicting outcomes | Ambiguity (incomplete information) | The fact that some studies indicate more Salmonella occurrence in cages vs non‐cages, some other studies indicate the reverse and finally some studies do not find differences leads to uncertainty on the results linked to ambiguity in the conclusions | ||
| Missing data within studies | Ambiguity (incomplete information) | Most of the studies do not report the OR or RR of the risk/protective factors | ||
| Older studies | Extrapolation uncertainty | Many publications are old and do not reflect current housing systems, particularly the use of enriched cages | ||
| Lack of welfare data | Ambiguity (incomplete information) | Data on the correlation between welfare of animals and Salmonella occurrence are lacking | ||
| Evaluation of MS data for the assessment of the impact of housing conditions for layers on Salmonella occurrence | Lack of sufficiently detailed information on housing systems | Ambiguity (incomplete information) | The results of the statistical analysis on the effect of the housing system on the occurrence of Salmonella in laying hen flocks should be interpreted with caution as the data were not collected though a study that was specifically designed for this purpose | |
| ToR 4 | Evaluation of literature data for the assessment of the impact of housing conditions for broilers on Salmonella occurrence | Use of experimental models | Extrapolation uncertainty | Some studies have used experimental models. The artificial infection of hens with high doses of Salmonella is not representative of the situation in the field and the spread of infection in different housing systems |
| Conflicting outcomes | Ambiguity (incomplete information) | The fact that some studies indicate more Salmonella occurrence in conventional vs non‐conventional systems, some other studies indicate the reverse and finally some studies do not show a difference, leads to uncertainty on the results link to ambiguity in conclusions | ||
| Size and selection of sample | Extrapolation uncertainty | Some field studies have used very small sample sizes (farm and individual) which decrease the precision of the results. Sampling strategy is not always described, which could lead to biased samples by intentional or unintentional targeting. | ||
| Diversity in sample matrix and laboratory analysis | Extrapolation uncertainty | There is a large diversity in sample matrix and laboratory analysis conducted to assess/determine the Salmonella status in a bird/farm, thus making comparison of results between studies difficult | ||
| Missing data within studies | Ambiguity (incomplete information) | Most of the studies do not report the OR or RR of the risk/protective factors | ||
| Older studies | Extrapolation uncertainty | Some publications are old (before 2000) and do not reflect the large scale broiler housing systems that are currently used in most countries | ||
| Lack of welfare data | Ambiguity (incomplete information) | Data on the correlation between welfare indicators of animals and Salmonella occurrence are lacking | ||
| Evaluation of MS data for the assessment of the impact of housing conditions for broilers on Salmonella occurrence | Lack of sufficiently detailed information on housing systems | Ambiguity (incomplete information) | The results of the statistical analysis on the effect of the housing system on the occurrence of Salmonella in broiler flocks should be interpreted with caution as the data were not collected through a study that was specifically designed for this purpose | |
| ToR 5 | Evaluation of literature data on co‐colonisation of broilers/poultry by Salmonella and Campylobacter | Most publications do not report on the data on both organisms in the same sample, instead the prevalence and load of each is reported separately. Of those publications that do report this, only two report a positive association and two report no association. The studies are often carried out according to different study designs with different sampling times, types of samples and test methods. | Ambiguity (incomplete information) | No study has reported a negative association, so this is unlikely, and evidence of a positive association is limited, although possibly suggested by a small number of studies that suggest the presence of Campylobacter may sometimes alter gut conditions in a way that may enhance the colonisation, persistence and extra‐intestinal invasion of birds by other organisms, including Salmonella. It is not possible to determine whether co‐colonisation occurs more often than expected on the basis of randomness and if it does, the reason for this is not known. It could be due to ‘external’ factors, e.g. the route of infection is common for both and is influenced by management practices (e.g. bad hygiene), or due to some synergy in infection dynamics in the birds’ intestinal tract |
| Analysis of data from EU MS on co‐colonisation by Salmonella and Campylobacter | Data was received from very few countries and is dominated by data from a single MS which reported low prevalence of both organisms. | Ambiguity (incomplete information) | The suggestion of a possible positive association between Campylobacter and Salmonella is likely to be subject to bias due to a limited number of sources of data and low prevalence of Campylobacter in one MS. | |
| Evaluation of literature data and analysis of data from EU MS on co‐contamination of broiler carcasses/meat by Salmonella and Campylobacter | There is considerable opportunity for cross‐contamination of broiler carcasses at slaughter by both organisms | Ambiguity (incomplete information) | There is potential for cross‐contamination at slaughter. The level of cross‐contamination in terms of the contribution of the slaughter process itself is not known, but is influenced by the level of infection (within‐flock prevalence and bacterial load) of the slaughter batches being processed | |
OR: odds ratio; RR: relative risk.
3. Assessment
3.1. Public health impact of a new Salmonella target in flocks of breeding hens of Gallus gallus
3.1.1. Salmonella serovars of public health significance
Criteria for Salmonella monitoring have been laid down in Regulation (EC) No 2160/2003. Annex II lists the minimum requirements that food business operators have to respect in relation to having samples taken and analysed for the control of Salmonella in different animal species and categories. As far as flocks of G. gallus, turkeys and pigs are concerned, the Regulation requires all Salmonella serovars ‘with public health significance’ to be monitored at various production stages. Annex III of this Regulation defines the specific criteria to be adopted to determine Salmonella serovars with public health significance to which community targets will apply:
the most frequent Salmonella serovars associated with human salmonellosis on this basis of data collected through EU surveillance system;
the route of infection (i.e. the presence of the serovar in relevant animal populations and feed);
whether any serovar shows a rapid and recent ability to spread and cause disease in humans and/or animals; and
whether any serovar shows increased virulence for instance, as regards invasiveness or resistance to relevant therapies for human infections.
The assessment therefore focuses on these aspects of the epidemiology of the considered serovars.
3.1.1.1. Salmonella serovars in human salmonellosis
Human sporadic cases of salmonellosis in the EU
In 2016, 96,039 salmonellosis cases were reported by 28 MS, with 94,530 confirmed cases resulting in an EU notification rate of 20.4 cases per 100,000. As in the previous year, the highest notification rates were reported by the Czech Republic and Slovakia (≥ 97.7 per 100,000), while the lowest rates were reported by Greece, Italy, Ireland and Portugal (≤ 6.8 per 100,000) (EFSA and ECDC, 2017d).
There was a statistically significant (p < 0.05) decreasing trend for the number of reported cases of human salmonellosis in the EU/EEA in 2008–2016; however, at the EU level, there was no significant change over the last 5 years (2012–2016) (Figure 3). The same observation was made using incidence rates. In those last 5 years, in four MS there was a decreasing trend, while a statistically significant increasing trend was observed in seven MS (EFSA and ECDC, 2017d).
Figure 3.
Trend in reported confirmed human cases of non‐typhoidal salmonellosis in the EU/EEA, by month, 2012–2016
- Source: Austria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Lithuania, Luxembourg, Latvia, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, and United Kingdom. Belgium, Bulgaria and Croatia did not report data to the level of detail required for the analysis.
Due to differences in national surveillance systems, notification rates are not directly comparable between MSs. Several attempts have been made to estimate the level of underestimation (combining underreporting and under‐ascertainment) for Salmonella infections in different MSs. The multiplication factor for underestimation was estimated at 57.5 CI95[9.0; 172] in 27 EU MSs, and ranged from 0.4 times the number of reported cases for Finland to more than 2,000 times for Portugal (Havelaar et al., 2013).
In 2016, information on Salmonella serovars was available for 73.4% of reported cases (69,336 of the total 94,530 reported cases) from 25 MS (all except Bulgaria, Croatia and Poland) (see Tables D.1 and D.2 in Appendix D). Data includes all cases reported with serovar information regardless the importation/travel status.
Table D.1.
Distribution of confirmed sporadic salmonellosis cases in humans by serovar (the 20 most frequent serovars, TESSy data, 2010–2016)
| 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % |
| Enteritidis | 32,994 | 47.6 | Enteritidis | 31,971 | 44.4 | Enteritidis | 32,447 | 44.5 | Enteritidis | 28,460 | 39.4 | Enteritidis | 32,389 | 41.1 | Enteritidis | 34,285 | 44.4 | Enteritidis | 36,436 | 44.3 |
| Typhimurium | 9,691 | 14.0 | Typhimurium | 11,900 | 16.5 | Typhimurium | 12,740 | 17.5 | Typhimurium | 14,727 | 20.4 | Typhimurium | 17,783 | 22.6 | Typhimurium | 19,569 | 25.4 | Typhimurium | 21,184 | 25.8 |
| Monophasic Typhimurium 1,4,[5],12:i:‐ | 5,697 | 8.2 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 5,786 | 8.0 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 5,774 | 7.9 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 6,310 | 8.7 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 5,785 | 7.3 | Monophasic Typhimurium 1.4.[5].12:i:‐ | 3,638 | 4.7 | Infantis | 1,779 | 2.2 |
| Infantis | 1,641 | 2.4 | Infantis | 1,647 | 2.3 | Infantis | 1,829 | 2.5 | Infantis | 2,205 | 3.1 | Infantis | 1,914 | 2.4 | Infantis | 1,672 | 2.2 | Monophasic Typhimurium 1.4.[5].12:i:‐ | 1,545 | 1.9 |
| Newport | 732 | 1.1 | Stanley | 784 | 1.1 | Derby | 752 | 1.0 | Derby | 817 | 1.1 | Thompson | 1,074 | 1.4 | Newport | 766 | 1.0 | Newport | 834 | 1.0 |
| Derby | 615 | 0.9 | Newport | 728 | 1.0 | Newport | 726 | 1.0 | Stanley | 751 | 1.0 | Stanley | 902 | 1.1 | Derby | 703 | 0.9 | Kentucky | 780 | 0.9 |
| Kentucky | 552 | 0.8 | Derby | 693 | 1.0 | Stanley | 716 | 1.0 | Newport | 682 | 0.9 | Derby | 725 | 0.9 | Kentucky | 555 | 0.7 | Virchow | 686 | 0.8 |
| Stanley | 497 | 0.7 | Kentucky | 533 | 0.7 | Kentucky | 581 | 0.8 | Kentucky | 631 | 0.9 | Newport | 712 | 0.9 | Poona | 547 | 0.7 | Derby | 666 | 0.8 |
| Virchow | 497 | 0.7 | Virchow | 503 | 0.7 | Virchow | 491 | 0.7 | Agona | 559 | 0.8 | Panama | 693 | 0.9 | Virchow | 464 | 0.6 | Java | 505 | 0.6 |
| Saintpaul | 443 | 0.6 | Java | 420 | 0.6 | Bovismorbificans | 433 | 0.6 | Virchow | 556 | 0.8 | Kentucky | 615 | 0.8 | Agona | 453 | 0.6 | Mbandaka | 471 | 0.6 |
| Agona | 436 | 0.6 | Agona | 388 | 0.5 | Java | 370 | 0.5 | Muenchen | 443 | 0.6 | Virchow | 507 | 0.6 | Stanley | 390 | 0.5 | Agona | 445 | 0.5 |
| Java | 402 | 0.6 | Bovismorbificans | 381 | 0.5 | Agona | 365 | 0.5 | Napoli | 427 | 0.6 | Braenderup | 441 | 0.6 | Bovismorbificans | 379 | 0.5 | Bareilly | 434 | 0.5 |
| Bovismorbificans | 387 | 0.6 | Napoli | 366 | 0.5 | Muenchen | 360 | 0.5 | Bovismorbificans | 409 | 0.6 | Agona | 434 | 0.6 | Montevideo | 358 | 0.5 | Bovismorbificans | 408 | 0.5 |
| Braenderup | 377 | 0.5 | Oranienburg | 309 | 0.4 | Saintpaul | 356 | 0.5 | Saintpaul | 382 | 0.5 | Java | 410 | 0.5 | Oranienburg | 352 | 0.5 | Saintpaul | 390 | 0.5 |
| Panama | 306 | 0.4 | Chester | 287 | 0.4 | Napoli | 327 | 0.4 | Montevideo | 372 | 0.5 | Bovismorbificans | 404 | 0.5 | Saintpaul | 337 | 0.4 | Stanley | 385 | 0.5 |
| Napoli | 295 | 0.4 | Saintpaul | 282 | 0.4 | Brandenburg | 291 | 0.4 | Panama | 341 | 0.5 | Napoli | 359 | 0.5 | Java | 333 | 0.4 | Braenderup | 378 | 0.5 |
| Chester | 284 | 0.4 | Panama | 275 | 0.4 | Chester | 289 | 0.4 | Java | 318 | 0.4 | Saintpaul | 337 | 0.4 | Napoli | 309 | 0.4 | Hadar | 351 | 0.4 |
| Hadar | 272 | 0.4 | Thompson | 255 | 0.4 | Hadar | 281 | 0.4 | Brandenburg | 285 | 0.4 | Oranienburg | 302 | 0.4 | Braenderup | 269 | 0.3 | Corvallis | 315 | 0.4 |
| Bareilly | 259 | 0.4 | Hadar | 251 | 0.3 | Braenderup | 269 | 0.4 | Oranienburg | 264 | 0.4 | Brandenburg | 300 | 0.4 | Hadar | 268 | 0.3 | Panama | 304 | 0.4 |
| Dublin | 232 | 0.3 | Braenderup | 238 | 0.3 | Oranienburg | 254 | 0.3 | Hada | 260 | 0.4 | Hadar | 292 | 0.4 | Brandenburg | 254 | 0.3 | Montevideo | 280 | 0.3 |
| Other | 12,727 | 18.4 | Other | 13,956 | 19.4 | Other | 13,222 | 18.1 | Other | 13,021 | 18.0 | Other | 12,454 | 15.8 | Other | 11,288 | 14.6 | Other | 13,652 | 16.6 |
| Total | 69,336 | 91.7 | Total | 71,953 | 93.7 | Total | 72,873 | 94.1 | Total | 72,220 | 92.6 | Total | 78,832 | 92.8 | Total | 77,189 | 90.5 | Total | 82,228 | 93.4 |
| Unknown serovar | 6,303 | 8.3 | Unknown serovar | 4,863 | 6.3 | Unknown serovar | 4,530 | 5.9 | Unknown serovar | 5,798 | 7.4 | Unknown serovar | 6,076 | 7.2 | Unknown serovar | 8,121 | 9.5 | Unknown serovar | 5,767 | 6.6 |
N: number of cases; TESSy: The European Surveillance System.
Source: TESSy. Country abbreviations: AT: Austria; BE: Belgium; CZ: Czech Republic; DE: Germany; DK: Denmark; EE: Estonia; EL: Greece; ES: Spain; FI: Finland; FR: France; HU: Hungary; IE: Ireland; IT: Italy; LT: Lithuania; LU: Luxembourg; LV: Latvia; MT: Malta; NL: Netherlands; PT: Portugal; RO: Romania; SE: Sweden; SI: Slovenia; SK: Slovakia; UK: United Kingdom.
Table D.2.
Distribution of confirmed salmonellosis cases in humans acquired outside the EU by serovar (the 20 most frequent serovars, TESSy data, 2010–2016)
| 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % |
| Enteritidis | 1,487 | 30.0 | Enteritidis | 1,773 | 30.9 | Enteritidis | 1,810 | 35.3 | Enteritidis | 2,213 | 39.9 | Enteritidis | 1,813 | 34.6 | Enteritidis | 1,747 | 37.1 | Enteritidis | 2,192 | 37.3 |
| Typhimurium | 403 | 8.1 | Typhimurium | 505 | 8.8 | Typhimurium | 468 | 9.1 | Typhimurium | 505 | 9.1 | Typhimurium | 599 | 11.4 | Typhimurium | 463 | 9.8 | Typhimurium | 563 | 9.6 |
| Stanley | 186 | 3.8 | Kentucky | 170 | 3.0 | Kentucky | 168 | 3.3 | Stanley | 187 | 3.4 | Stanley | 191 | 3.6 | Stanley | 159 | 3.4 | Virchow | 224 | 3.8 |
| Kentucky | 178 | 3.6 | Stanley | 139 | 2.4 | Stanley | 138 | 2.7 | Virchow | 180 | 3.2 | Virchow | 156 | 3.0 | Kentucky | 135 | 2.9 | Kentucky | 213 | 3.6 |
| Monophasic Typhimurium 1,4,[5],12:i:‐ | 151 | 3.0 | Virchow | 137 | 2.4 | Virchow | 131 | 2.6 | Kentucky | 156 | 2.8 | Infantis | 138 | 2.6 | Virchow | 132 | 2.8 | Stanley | 195 | 3.3 |
| Newport | 131 | 2.6 | Newport | 121 | 2.1 | Newport | 128 | 2.5 | Infantis | 140 | 2.5 | Kentucky | 132 | 2.5 | Infantis | 114 | 2.4 | Newport | 146 | 2.5 |
| Virchow | 109 | 2.2 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 116 | 2.0 | Infantis | 112 | 2.2 | Newport | 109 | 2.0 | Newport | 121 | 2.3 | Newport | 95 | 2.0 | Infantis | 118 | 2.0 |
| Agona | 107 | 2.2 | Infantis | 88 | 1.5 | Corvallis | 97 | 1.9 | Corvallis | 86 | 1.6 | Braenderup | 92 | 1.8 | Agona | 94 | 2.0 | Braenderup | 115 | 2.0 |
| Java | 106 | 2.1 | Agona | 83 | 1.4 | Braenderup | 76 | 1.5 | Agona | 85 | 1.5 | Corvallis | 88 | 1.7 | Corvallis | 83 | 1.8 | Corvallis | 96 | 1.6 |
| Chester | 91 | 1.8 | Java | 70 | 1.2 | Java | 76 | 1.5 | Java | 84 | 1.5 | Agona | 78 | 1.5 | Oranienburg | 74 | 1.6 | Hadar | 78 | 1.3 |
| Braenderup | 89 | 1.8 | Chester | 67 | 1.2 | Saintpaul | 70 | 1.4 | Braenderup | 67 | 1.2 | Java | 77 | 1.5 | Braenderup | 73 | 1.5 | Agona | 77 | 1.3 |
| Infantis | 86 | 1.7 | Corvallis | 66 | 1.1 | Hadar | 67 | 1.3 | Saintpaul | 67 | 1.2 | Mbandaka | 61 | 1.2 | Bareilly | 66 | 1.4 | Java | 73 | 1.2 |
| Saintpaul | 82 | 1.7 | Hadar | 61 | 1.1 | Agona | 59 | 1.2 | Hadar | 57 | 1.0 | Bareilly | 61 | 1.2 | Mbandaka | 65 | 1.4 | Saintpaul | 69 | 1.2 |
| Hadar | 68 | 1.4 | Braenderup | 58 | 1.0 | Mbandaka | 55 | 1.1 | Oranienburg | 55 | 1.0 | Oranienburg | 60 | 1.1 | Java | 59 | 1.3 | Senftenberg | 62 | 1.1 |
| Corvallis | 63 | 1.3 | Saintpaul | 55 | 1.0 | Bareilly | 52 | 1.0 | Rissen | 55 | 1.0 | Saintpaul | 59 | 1.1 | Hadar | 52 | 1.1 | Bareilly | 56 | 1.0 |
| Weltevreden | 59 | 1.2 | Bareilly | 54 | 0.9 | Weltevreden | 46 | 0.9 | Weltevreden | 49 | 0.9 | Hadar | 58 | 1.1 | Saintpaul | 47 | 1.0 | Mbandaka | 56 | 1.0 |
| Bareilly | 56 | 1.1 | Weltevreden | 52 | 0.9 | Oranienburg | 39 | 0.8 | Mbandaka | 48 | 0.9 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 50 | 1.0 | Weltevreden | 45 | 1.0 | Oranienburg | 52 | 0.9 |
| Mbandaka | 37 | 0.7 | Mbandaka | 37 | 0.6 | Chester | 39 | 0.8 | Bareilly | 44 | 0.8 | Heidelberg | 40 | 0.8 | Heidelberg | 39 | 0.8 | Haifa | 46 | 0.8 |
| Poona | 37 | 0.7 | Montevideo | 33 | 0.6 | Heidelberg | 34 | 0.7 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 44 | 0.8 | Rissen | 37 | 0.7 | Haifa | 39 | 0.8 | Anatum | 45 | 0.8 |
| Heidelberg | 33 | 0.7 | Oranienburg | 30 | 0.5 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 34 | 0.7 | Schwarzengrund | 38 | 0.7 | Abony | 37 | 0.7 | Rissen | 38 | 0.8 | Heidelberg | 40 | 0.7 |
| Other | 1,398 | 28.2 | Other | 2,030 | 35.3 | Other | 1,426 | 27.8 | Other | 1,272 | 23.0 | Other | 1,288 | 24.6 | Other | 1,111 | 23.6 | Other | 1,362 | 23.2 |
| Total | 4,957 | 75.5 | Total | 5,745 | 83.2 | Total | 5,125 | 81.4 | Total | 5,541 | 74.4 | Total | 5,236 | 72.4 | Total | 4,715 | 64.1 | Total | 5,878 | 73.2 |
| Unknown serovar | 1,610 | 24.5 | Unknown serovar | 1,158 | 16.8 | Unknown serovar | 1,170 | 18.6 | Unknown serovar | 1,904 | 25.6 | Unknown serovar | 2,001 | 27.6 | Unknown serovar | 2,645 | 35.9 | Unknown serovar | 2,147 | 26.8 |
N: number of cases; TESSy: The European Surveillance System.
Source: TESSy. Country abbreviations: AT: Austria; BE: Belgium; CZ: Czech Republic; DE: Germany; DK: Denmark; EE: Estonia; EL: Greece; ES: Spain; FI: Finland; FR: France; HU: Hungary; IE: Ireland; IT: Italy; LT: Lithuania; LU: Luxembourg; LV: Latvia; MT: Malta; NL: Netherlands; PT: Portugal; RO: Romania; SE: Sweden; SI: Slovenia; SK: Slovakia; UK: United Kingdom.
The proportion of domestic vs travel‐associated cases varied markedly between countries, but most of the salmonellosis cases were infected in the EU (53.3% cases acquired in EU, 6.8% travel outside EU and 39.9% of unknown origin). Among 8,337 travel‐associated cases with known information on probable country of infection, 79.0% of the cases were related to travel outside the EU and 21.0% to travel within the EU (EFSA and ECDC, 2017d).
To estimate the impact of the Salmonella infections acquired in the EU, case‐based serovar data reported to TESSy were analysed for domestic and travel‐associated cases when the probable country of infection was a MS (cases acquired in EU; Table 3). Information on Salmonella serovars with importation (domestic vs travel) data was available for 52,679 cases and in addition, information of the country of infection for travel‐acquired cases was available for 6,427 cases (88.8% of all travel related cases) for all 20 MS reporting such cases. Thereby the estimation of the most frequent serovars obtained in EU was based on data on 51,866 cases from 24 MS. This represents 74.8% of cases with known serovar data (69,336 cases) and 54.9% of all 94,530 reported cases in 2016. The majority (96.8%) of the cases were acquired in the reporting country.
Table 3.
Distribution of confirmed salmonellosis cases in humans acquired in the EU (domestic and EU travel) by serovar (the 20 most frequent serovars). TESSy data, 2010–2016
| 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % | Serovar | N | % |
| Enteritidis | 26,779 | 57.1 | Enteritidis | 25,788 | 54.7 | Enteritidis | 25,474 | 54.6 | Enteritidis | 21,621 | 48.0 | Enteritidis | 24,828 | 50.9 | Enteritidis | 26,609 | 52.7 | Enteritidis | 27,039 | 51.6 |
| Typhimurium | 6,719 | 14.3 | Typhimurium | 7,971 | 16.9 | Typhimurium | 8,625 | 18.5 | Typhimurium | 9,981 | 22.2 | Typhimurium | 10,635 | 21.8 | Typhimurium | 12,402 | 24.6 | Typhimurium | 13,498 | 25.8 |
| Monophasic Typhimurium 1,4,[5],12:i:‐ | 2,088 | 4.5 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 2,303 | 4.9 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 1,775 | 3.8 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 1,823 | 4.0 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 1,577 | 3.2 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 1,208 | 2.4 | Infantis | 1,231 | 2.4 |
| Infantis | 1,098 | 2.3 | Infantis | 1,137 | 2.4 | Infantis | 1,163 | 2.5 | Infantis | 1,540 | 3.4 | Infantis | 1,238 | 2.5 | Infantis | 1,130 | 2.2 | Monophasic Typhimurium 1,4,[5],12:i:‐ | 372 | 0.7 |
| Derby | 372 | 0.8 | Stanley | 450 | 1.0 | Derby | 447 | 1.0 | Derby | 453 | 1.0 | Thompson | 938 | 1.9 | Newport | 375 | 0.7 | Derby | 294 | 0.6 |
| Newport | 316 | 0.7 | Derby | 338 | 0.7 | Stanley | 420 | 0.9 | Stanley | 404 | 0.9 | Stanley | 508 | 1.0 | Poona | 356 | 0.7 | Newport | 271 | 0.5 |
| Virchow | 257 | 0.5 | Newport | 278 | 0.6 | Bovismorbificans | 309 | 0.7 | Agona | 319 | 0.7 | Panama | 400 | 0.8 | Derby | 355 | 0.7 | Bovismorbificans | 256 | 0.5 |
| Bovismorbificans | 242 | 0.5 | Bovismorbificans | 259 | 0.5 | Newport | 276 | 0.6 | Muenchen | 278 | 0.6 | Derby | 327 | 0.7 | Bovismorbificans | 202 | 0.4 | Virchow | 208 | 0.4 |
| Stanley | 181 | 0.4 | Virchow | 203 | 0.4 | Muenchen | 262 | 0.6 | Newport | 255 | 0.6 | Newport | 268 | 0.5 | Agona | 199 | 0.4 | Kentucky | 206 | 0.4 |
| Kentucky | 181 | 0.4 | Oranienburg | 182 | 0.4 | Virchow | 195 | 0.4 | Bovismorbificans | 232 | 0.5 | Bovismorbificans | 249 | 0.5 | Java | 166 | 0.3 | Java | 199 | 0.4 |
| Agona | 172 | 0.4 | Java | 179 | 0.4 | Brandenburg | 179 | 0.4 | Virchow | 191 | 0.4 | Braenderup | 204 | 0.4 | Virchow | 164 | 0.3 | Agona | 192 | 0.4 |
| Saintpaul | 148 | 0.3 | Agona | 162 | 0.3 | Agona | 164 | 0.4 | Kentucky | 188 | 0.4 | Agona | 191 | 0.4 | Oranienburg | 157 | 0.3 | Bareilly | 181 | 0.3 |
| Braenderup | 142 | 0.3 | Kentucky | 154 | 0.3 | Java | 139 | 0.3 | Montevideo | 186 | 0.4 | Java | 181 | 0.4 | Goldcoast | 154 | 0.3 | Saintpaul | 128 | 0.2 |
| Java | 128 | 0.3 | Thompson | 149 | 0.3 | Kentucky | 135 | 0.3 | Brandenburg | 154 | 0.3 | Brandenburg | 165 | 0.3 | Kentucky | 151 | 0.3 | Braenderup | 121 | 0.2 |
| Thompson | 124 | 0.3 | Saintpaul | 105 | 0.2 | Saintpaul | 127 | 0.3 | Thompson | 140 | 0.3 | Kentucky | 163 | 0.3 | Montevideo | 141 | 0.3 | Brandenburg | 120 | 0.2 |
| Brandenburg | 108 | 0.2 | Mikawasima | 101 | 0.2 | Montevideo | 117 | 0.3 | Saintpaul | 133 | 0.3 | Virchow | 161 | 0.3 | Brandenburg | 139 | 0.3 | Montevideo | 116 | 0.2 |
| Bareilly | 106 | 0.2 | Kottbus | 99 | 0.2 | Goldcoast | 114 | 0.2 | Napoli | 113 | 0.3 | Goldcoast | 144 | 0.3 | Thompson | 123 | 0.2 | Hadar | 115 | 0.2 |
| Napoli | 97 | 0.2 | Livingstone | 97 | 0.2 | Indiana | 105 | 0.2 | Goldcoast | 112 | 0.2 | Saintpaul | 142 | 0.3 | Saintpaul | 117 | 0.2 | Poona | 106 | 0.2 |
| Hadar | 97 | 0.2 | Bareilly | 96 | 0.2 | Enterica | 104 | 0.2 | Java | 106 | 0.2 | Groupc | 118 | 0.2 | Stanley | 102 | 0.2 | London | 100 | 0.2 |
| Mikawasima | 95 | 0.2 | Montevideo | 95 | 0.2 | Oranienburg | 94 | 0.2 | Oranienburg | 98 | 0.2 | Muenchen | 114 | 0.2 | Hadar | 92 | 0.2 | Mbandaka | 101 | 0.2 |
| Other | 7,459 | 15.9 | Other | 7,003 | 14.9 | Other | 6,474 | 13.9 | Other | 6,818 | 15.1 | Other | 6,570 | 13.5 | Other | 6,280 | 12.4 | Other | 7,355 | 14.0 |
| Total | 46,909 | 93.0 | Total | 47,149 | 95.1 | Total | 46,698 | 95.4 | Total | 45,039 | 94.9 | Total | 48,822 | 95.0 | Total | 50,456 | 94.1 | Total | 52,377 | 97.6 |
| Unknown serovar | 3,509 | 7.0 | Unknown serovar | 2,439 | 4.9 | Unknown serovar | 2,247 | 4.6 | Unknown serovar | 2,430 | 5.1 | Unknown serovar | 2,589 | 5.0 | Unknown serovar | 3,189 | 5.9 | Unknown serovar | 1,265 | 2.4 |
Source: TESSy. Country abbreviations: AT: Austria; BE: Belgium; CZ: Czech Republic; DE: Germany; DK: Denmark; EE: Estonia; EL: Greece; ES: Spain; FI: Finland; FR: France; HU: Hungary; IE: Ireland; IT: Italy; LT: Lithuania; LU: Luxembourg; LV: Latvia; MT: Malta; NL: Netherlands; PT: Portugal; RO: Romania; SE: Sweden; SI: Slovenia; SK: Slovakia; UK: United Kingdom.
More than half (57.1%) of the reported cases were infected by S. Enteritidis (Table 3). Together with S. Typhimurium, including monophasic S. Typhimurium 1,4,[5],12:i:‐, these three serovars represented 75.9% of the reported human cases with known serovar and country of origin of infection in 2016. The proportion of S. Enteritidis continued to increase in 2016 compared with 2014 and 2015, although this was not a statistically significant trend. The proportion of S. Typhimurium (excluding the monophasic variants) decreased significantly in 2016, while its monophasic variant strains (S. 1,4,[5],12:i:‐), and S. Infantis remained at similar levels to 2015 and 2014.
The cases of the top five serovars were mostly acquired in the EU in 2016: S. Enteritidis (94.7%), S. Typhimurium (93.8%), monophasic S. Typhimurium (93.3%), S. Infantis (92.4%) and S. Derby (95.6%), whereas S. Newport, the fifth most commonly reported serovar, included 36.6% of cases associated with travel outside the EU. The highest proportion of travel‐associated cases was reported for S. Stanley (52.9%) and S. Kentucky (53.2%).
There was a statistically significant (p < 0.01) decreasing trend for S. Enteritidis acquired in the EU from 2008 until 2012, but from 2012 to 2016 the number of reported cases stabilised and no statistically significant change was observed (EFSA and ECDC, 2017d).
Reported food‐borne salmonellosis outbreaks
Data from outbreaks is particularly valuable as this can often be linked either epidemiologically or microbiologically with particular sources. A total of 3,637 food‐borne outbreaks (FBO) caused by Salmonella at EU level (2014–2016) were reported with 29,250 human cases. Of these, 756 were strong‐evidence FBOs with 10,995 human cases. The vehicle was known for 753 of these strong‐evidence outbreaks with in total 10,858 cases; it was ‘broiler meat (Gallus gallus) and products thereof’ in 46 outbreaks (550 cases), ‘eggs and egg products’ in 296 outbreaks (3,547 cases), ‘meat and meat products’ in 32 outbreaks (566 cases), ‘other, mixed or unspecified poultry meat and products thereof’ in 10 outbreaks (44 cases) and ‘mixed food’ in 65 outbreaks (1,143 cases). An overview of the Salmonella serovars involved in the FBOs caused by ‘egg and egg products’ and ‘broiler meat (Gallus gallus) and products thereof’ is shown in Table 4. S. Enteritidis is by far the most important serovar in terms of the number of outbreaks and outbreak cases relating to both eggs and egg products and broiler meat.
Table 4.
Salmonella serovars involved in the strong‐evidence food‐borne outbreaks in the EU caused by Salmonella in egg and egg products and in broiler meat (Gallus gallus) and products thereof (2014–2016) as reported in the zoonoses database on occurrence of strong‐evidence food‐borne outbreaks
| Serovar | Number of outbreaks according to attributed source | Number of outbreak cases according to attributed source | ||||
|---|---|---|---|---|---|---|
| Eggs and egg products | Broiler meat and products thereof | Total | Eggs and egg products | Broiler meat and products thereof | Total | |
| S. Enteritidis | 218 (92.0%) | 28 (84.8%) | 246 (91.1%) | 3,007 (95.3%) | 400 (84.7%) | 3,407 (93.9%) |
| S. Typhimurium | 14 (5.9%) | 3 (9.1%) | 17 6.3%) | 92 (2.9%) | 36 (7.6%) | 128 (3.5%) |
| S. Senftenberg | 0 (0%) | 1 (3.0%) | 1 (0.4%) | 0 (0%) | 34 (7.2%) | 34 (0.9%) |
| S. 9,12:‐:‐ | 1 (0.4%) | 0 (0%) | 1 (0.4%) | 21 (0.7%) | 0 (0%) | 21 (0.6%) |
| S. Typhimurium, monophasic | 1 (0.4%) | 0 (0%) | 1 (0.4%) | 17 (0.5%) | 0 (0%) | 17 (0.5%) |
| S. Virchow | 1 (0.4%) | 0 (0%) | 1 (0.4%) | 9 (0.3%) | 0 (0%) | 9 (0.2%) |
| S. Infantis | 1 (0.4%) | 0 (0%) | 1 (0.4%) | 5 (0.2%) | 0 (0%) | 5 (0.1%) |
| S. group B | 1 (0.4%) | 0 (0%) | 1 (0.4%) | 4 (0.1%) | 0 (0%) | 4 (0.1%) |
| S. Goldcoast | 0 (0%) | 1 (3.0%) | 1 (0.4%) | 0 (0%) | 2 (0.4%) | 2 (0.1%) |
| Total Typed | 237 | 33 | 270 | 3,155 | 472 | 3,591 |
| Salmonella spp., unspecified | 43 | 4 | 47 | 315 | 24 | 339 |
| S. enterica subsp. enterica | 2 | 0 | 2 | 16 | 0 | 16 |
| Not typeable | 5 | 0 | 5 | 15 | 0 | 15 |
| Blank | 9 | 9 | 18 | 46 | 54 | 100 |
| Grand Total | 296 | 46 | 342 | 3,547 | 550 | 4,097 |
The vehicle was ‘pig meat and products thereof’ in 55 outbreaks (1,041 cases), ‘bovine meat and products thereof’ in 17 outbreaks (132 cases) and ‘turkey meat and products thereof’ in 5 outbreaks (297 cases).
S. Enteritidis has been causing large and ongoing outbreaks associated with international trade in table eggs. Eggs are more frequently associated with outbreaks, many of which are diffuse outbreaks that may appear to be sporadic cases unless whole genome sequencing (WGS) is carried out on isolates to demonstrate links to a common source of eggs (Dallman et al., 2018). Eggs are more frequently associated with outbreaks than poultry meat because of the occurrence of internal contamination due to vertical transmission, common use of raw pooled eggs as an ingredient or glaze for various food products and the possibility for temperature abuse. Eggs are not required to be refrigerated at retail and are not individually marked with use‐by dates in most countries (Howard et al., 2012; EFSA BIOHAZ Panel, 2014). Climatic factors and consumer preferences can also influence the proportion of Salmonella cases linked to meat products, if these are more likely to be eaten raw or lightly cooked. Standards of kitchen hygiene also impact the degree of cross‐contamination of cooked and ready to eat foodstuffs (EFSA BIOHAZ Panel, 2011a).
There appears to be a seasonal trend towards increased infection in laying hens as well as humans in warmer parts of the year (Powell et al., 2018). Improved monitoring, use of WGS to definitively link outbreak isolates from diverse sources and good co‐operation between competent authorities in different MS have facilitated more rapid identification and control of outbreaks such as recent examples involving international trade in table eggs (EFSA and ECDC, 2017c; Hormansdorfer et al., 2017). Five EFSA reports on multicountry outbreaks linked to eggs have been published since 2006, all involving S. Enteritidis and international trade in table eggs originating from Germany in 2014 or Poland, with cases ongoing since 2016 (ECDC and EFSA, 2014a; EFSA and ECDC, 2016). Based on the current evidence, the source of infection of these outbreaks is likely to be at the level of the laying hen farms (EFSA and ECDC, 2017b). WGS and epidemiological evidence suggests that there is persistent contamination of laying hen premises in Poland (EFSA and ECDC, 2017a).27 A focus on improved detection and control of S. Enteritidis in all poultry populations, particularly early and sensitive detection in laying hens, is therefore needed to supplement the proposed targets for breeding flocks.
3.1.1.2. The route of infection (i.e. the presence of the serovar in relevant animal populations and feed)
Several factors (e.g. feed (Hald et al., 2012b) and other sources such as, replacement animals, humans, domestic, wild and feral animals and birds, insects, contaminated equipment or water) (Chousalkar et al., 2018)) can introduce infection into a poultry unit and salmonellas can further spread within and between holdings through movements of people, vehicles and equipment (Cerda‐Cuellar et al., 2018; Velhner et al., 2018). After introduction, infection may be perpetuated in poultry houses, often without detection (Davies et al., 2001).
Infected chicks may acquire Salmonella infections via ‘vertical’ transmission within the egg from the parent breeder flock, by ‘pseudo‐vertical’ transmission from externally contaminated eggs or via ‘horizontal’ transmission from pre‐existing or resident contamination including cross‐contamination in the hatchery (Sivaramalingam et al., 2013; Gaucher et al., 2017). These transmission routes are described in more detail in EFSA (2009).
The chicken breeding pyramid (Figure 2) has provided a means of maintaining Salmonella‐free replacement birds for parent breeding flocks since the international dissemination of S. Enteritidis in the 1970/1980s (Ward et al., 2000), but at parent level control of infection has not always been so robust and there have been outbreaks of S. Enteritidis associated with international trade in broiler hatching eggs (Lawes et al., 2016).
It is not always possible to distinguish infections originating from hatchery and other horizontal contamination routes from those transmitted from breeding flocks and vertical transmission, but trace‐back exercises (McLlroy et al., 1989; Liljebjelke et al., 2005; Crabb et al., 2018), risk factor analyses (Chriel et al., 1999; Skov et al., 1999) and temporal correlations (Van Der Fels‐Klerx et al., 2008) have demonstrated that breeding flocks can often be identified as the original source. In Canada, hatchery‐associated Salmonella contamination has been suggested, on the basis of Expert Knowledge Elicitation (EKE) used for risk modelling, to account for 4% of human infections due to poultry meat and 8% due to eggs, leading to a conclusion that stronger regulatory control at hatchery level would be beneficial for public health (Gaucher et al., 2017; Racicot et al., 2018).
Some zoonotic serovars are more commonly found to contaminate hatching and table eggs internally, especially certain strains of S. Enteritidis (Campioni et al., 2018; Gast et al., 2018; Ricke et al., 2018; Wang et al., 2018b). This serovar is more consistently traced from breeders to broilers and broiler meat than other serovars (Byrd et al., 1998; Kim et al., 2007) and has been shown to be able to invade both epithelial and lymphoid cells in the reproductive tract of chickens, and thus evade the immune system of some birds (Legesse and Garesu, 2017) as well as being able to establish a super‐shedder intestinal carriage state in a minority of birds (Menanteau et al., 2018; Eade et al., 2019). However, Salmonella serovars other than S. Enteritidis trace‐backs to breeders have been documented, including S. Typhimurium, S. Infantis (Crabb et al., 2018), S. Heidelberg (Nakao et al., 2018), S. Kentucky and S. Senftenberg (Byrd et al., 1998; Chriel et al., 1999; Liljebjelke et al., 2005; Kim et al., 2007; Moffatt et al., 2016). By contrast, hatchery contamination originating from faecal contamination of hatching eggs leading to broiler flock infection can involve a wide range of serovars (Bailey et al., 2001).
In oral and intravenous inoculation models, S. Heidelberg (a major serovar involved in egg and poultry meat contamination in the USA) and certain S. Typhimurium strains appeared to colonise the chicken reproductive organs as efficiently as S. Enteritidis, despite being recovered less frequently from egg contents (Gast et al., 2005, 2007; Gantois et al., 2008). In Australia, certain S. Typhimurium strains have been strongly associated with laying hen flocks and egg‐borne food poisoning outbreaks, and show invasive properties in tissue culture assays (Chousalkar et al., 2018) (McWhorter et al., 2018; Moffatt et al., 2016). It is not known if similar strains exist in Europe, but in the EU S. Typhimurium is much more frequently associated with pig, ruminant and environmental reservoirs than with chickens (Carson and Davies, 2018; Gavin et al., 2018; MacDonald et al., 2018).
Chicken egg‐associated serovars of S. Enteritidis, S. Typhimurium and S. Heidelberg also survived in egg albumen better than S. Hadar and S. Virchow. A genetic locus identified in S. Enteritidis (yafD) appears to confer an enhanced phenotype for survival in albumen, which was transferable to a S. Typhimurium strain (Lu et al., 2003). Other factors proposed to be important for intra‐egg survival and access to the yolk (permitting multiplication to high numbers) include storage conditions (EFSA BIOHAZ Panel, 2014), stress response genes, high molecular mass lipopolysaccharide and surface appendages such as curli, type I fimbriae and flagella (Guard‐Petter, 2001; Cogan et al., 2004; De Buck et al., 2004; Guard‐Bouldin et al., 2004; Quan et al., 2018; Xiaojie et al., 2019).
In the USA, S. Braenderup has been intermittently reported and is associated with outbreaks of Salmonella associated with eggs and newly hatched chicks, suggesting that some strains of this serovar may also have an ability to transmit vertically (Nakao et al., 2015; Ricke, 2017; CDC, 2018).
A variety of serovars other than those considered to have vertical transmission abilities can be found in breeding flocks, hatcheries and broiler flocks. Breeding flocks and broiler flocks may share the same source of contaminated feed, so finding the same serovar in both populations does not necessarily indicate transmission from breeders (Corry et al., 2002).
As an example, in a multistate study of four integrated broiler companies in the USA (Bailey et al., 2001) examined samples from hatcher basket liners through to slaughter. Of 36 serovars isolated, 12 were found on processed carcasses, and nine of these were also found on hatcher basket liners. S. Thompson was common in liners, on farms and on broiler carcasses. Other serovars found on hatcher basket liners and carcasses were: S. Brandenburg, S. Infantis, S. Kentucky, S. Mbandaka, S. Montevideo, S. Senftenberg, S. Typhimurium and a monophasic variant of S. Typhimurium; S. 4,5,12:i–. Most of these are also common feed contaminants.
Fewer data are available in the case of laying hens, but transmission of S. Enteritidis PT6 from a layer breeding company to commercial laying flocks via the hatchery was demonstrated in the United Kingdom by field investigations and molecular typing (Davies et al., 2003). The international dissemination of S. Enteritidis during the 1980s is thought to have been promoted by international trade in breeding chickens before monitoring programmes were in place (Evans et al., 1999), with evidence of S. Enteritidis infection of primary breeding flocks, possibly originally acquired from feed, occurring sporadically in various parts of the world (Obrien, 1990; Nakamura et al., 1993; Edel, 1994).
In conclusion, a variety of serovars other than S. Enteritidis and S. Typhimurium and the other currently regulated serovars in breeding flocks may be associated with pseudo‐vertical transmission, resulting in wider dissemination of infection via day‐old chicks among production flocks (Namata et al., 2009), including by means of omphalitis in day old chicks traded internationally (Saad et al., 2017). However, vertical transmission seems to be largely associated with particular strains of S. Enteritidis, so control of this serovar in breeding flocks and laying hens, including sensitive sampling and detection methods, remains a major priority in the EU (Kangas et al., 2007; Carrique‐Mas and Davies, 2008; Mahe et al., 2008). The impact of improved controls on S. Enteritidis in breeding flocks on human infections in the United Kingdom was smaller than the effect of vaccination of laying hens, demonstrating the importance of the laying hen reservoir (O'Brien, 2013; Lane et al., 2014).
3.1.1.3. Changes in the ability of different Salmonella serovars to spread and cause disease in humans
The main source of infection for humans is the consumption of contaminated foods of animal origin, including eggs and poultry meat. Since the implementation of harmonised Salmonella control programmes in Europe, beginning in 2007 with chicken breeding flocks, the most commonly detected poultry‐associated serovar, S. Enteritidis, declined substantially till 2013, then began to increase in both people and laying hens (Poirier et al., 2008; Messens et al., 2013; Hugas and Beloeil, 2014; EFSA and ECDC, 2017d; De Cesare, 2018). This trend has also been accompanied by some expansion of certain clones within previously less common serovars, e.g. S. Infantis, S. Stanley, S. Kentucky and S. Heidelberg (Campos et al., 2018).
In the USA and Canada, multidrug resistant (MDR) S. Newport emerged in cattle after routine use of ceftiofur and other cephalosporins and subsequently spread to other species, including turkeys and humans and resulting in environmental contamination (Poppe et al., 1995; Iwamoto et al., 2017; Crim et al., 2018). Similar strains have not been reported in food animals in the EU.
S. Kentucky ST198 with resistance to multiple antimicrobials including ciprofloxacin is the latest clone to show rapid international dissemination both in humans and in the food chain in the EU and more widely (Le Hello et al., 2011; Le Hello et al., 2013; Rauch et al., 2018). This clone was observed in a high proportion of isolates from humans, broilers and fattening turkeys (EFSA and ECDC, 2018). The spread of ST198 in broiler production in the EU has led to France including S. Kentucky among the target regulated serovars for poultry in 2016.28
Monophasic S. 1,4,[5],12:i:‐ variants of S. Typhimurium have increased dramatically in pigs in recent years (Hauser et al., 2010), but are considered to be opportunist infections of poultry flocks rather than becoming persistent.
The potential for the emergence of new strains, such as S. Infantis which has arisen in poultry in Hungary, many other European countries and Israel (Nogrady et al., 2008; Gal‐Mor et al., 2010; Hauser et al., 2012; Wilk et al., 2017) is present in a situation where mobile virulence and antimicrobial or heavy metal resistance genes and phage incursions may lead to the selection and emergence of new strains with enhanced epidemiological fitness (Litrup et al., 2010; Figueiredo et al., 2018; Worley et al., 2018). Seven MS (Austria, Belgium, Estonia, France, the Netherlands, Slovenia and Spain) reported MDR S. Infantis isolates in humans in 2016 with the highest proportions in Austria (69%) and Slovenia (64%) (EFSA and ECDC, 2018). In the USA, a recent increase in S. Infantis and some other serovars is thought to be associated with increased regulatory focus on a limited range of serovars in poultry, thus potentially creating a niche for expansion of others (Marder et al., 2018).
A large S. Stanley outbreak involving related strains of MDR organisms occurred between 2011 and 2013 in the EU, affecting 10 countries (ECDC and EFSA, 2014b). Although this was linked to infected turkey flocks and meat, some infected broiler flocks were reported and similar strains were identified from diverse sources, including poultry feed. S. Stanley still occurs, but less commonly in food animals and humans after its initial emergence in the EU in Poland, followed by limited spread to poultry in other countries and there have not been further large outbreaks (EFSA and ECDC, 2017d; Skarzynska et al., 2017; Huang et al., 2019). This serves as an example of how there can be rapid spread of specific Salmonella strains, which may originate from countries outside Europe, through poultry breeding and production chains when there is international trade in eggs and meat (Pastore et al., 2008; Wasyl et al., 2013; ECDC and EFSA, 2014b; Kinross et al., 2014). The occurrence of such outbreaks relating to non‐regulated serovars also prompted calls for improved legislative control of emerging potentially ‘epidemic’ Salmonella serovars other than those currently targeted in the EU (Springer et al., 2014).
3.1.1.4. Increased virulence or resistance to relevant therapies for human salmonellosis
Certain serovars of Salmonella have been consistently reported to be associated with excess mortality and serious clinical disease in humans. These include S. Enteritidis, S. Typhimurium, S. Choleraesuis (only associated with pigs), S. Dublin (largely associated with cattle), S. Virchow, S. Heidelberg and S. Infantis (Helms et al., 2003; Wollin, 2007; Silva et al., 2017; Karacan Sever and Akan, 2018) and new MDR variants of these with greater health impact often emerge and spread internationally (Luk‐in et al., 2018; Parisi et al., 2018).
Table D.3 in Appendix D shows human case hospitalisation and mortality rates of those cases acquired in the EU (domestic and EU travel) between 2014 and 2016 for the reported serovars considered in Section 3.1.2.3 for possible inclusion in a revised serovar selection for breeding chicken flocks. The reported hospitalisation rate for Salmonella infections acquired in the EU ranges from 23.8% for S. Havana to 57.6% for S. Heidelberg, but confidence limits are wide for less common serovars and there is a large potential bias associated with variations in reporting practices in different countries, with some only reporting the most serious cases and a high proportion of cases lacking hospitalisation or mortality data. The completeness of this data appears to vary by serovar. There is also potential bias associated with the variable distribution of some serovars in different countries with different reporting practices and the influence of outbreaks on completeness of serotyping and reporting. Uncertainty is even greater in the case of mortality data, since fatal cases are uncommon. Reported mortality ranges from 0% for several serovars to 2.6% for S. Havana, for which there were only 63 reported cases and only one of the cases with mortality data provided died. As a consequence of the uncertainty regarding assessment of potential virulence of serovars based on monitoring data, the RAG status for this criterion prioritised information from scientific literature on the occurrence, or lack of reported evidence, of more severe infections associated with specific serovars.
Table D.3.
Outcome in terms of mortality (death/alive) and hospitalisation status (yes/no) of the salmonellosis cases acquired in the EU (domestic and EU travel) for a selection of Salmonella serovars, TESSy data, 2014–2016)
| Serotype | Hospitalisation status | Outcome in terms of mortality | Total (N) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hospitalised (N) | Not hospitalised (N) | Unknown (N) | % hospitalised CI95 a | Alive (N) | Death (N) | Unknown (N) | % death CI95 b | ||
| Enteritidis | 17,833 | 41,047 | 19,161 | 30.3 [29.9–30.7] | 71,691 | 110 | 6,240 | 0.153 [0.126–0.185] | 78,041 |
| Typhimurium | 2,358 | 3,487 | 17,470 | 40.3 [39.1–41.6] | 17,775 | 35 | 5,505 | 0.197 [0.137–0.273] | 23,315 |
| Monophasic Typhimurium 1,4,[5],12:i:‐ | 896 | 1,768 | 3,502 | 33.6 [31.8–35.5] | 2,978 | 15 | 3,173 | 0.501 [0.281–0.825] | 6,166 |
| Infantis | 654 | 1,338 | 1,406 | 32.8 [30.8–34.9] | 2,874 | 6 | 518 | 0.208 [0.076–0.453] | 3,398 |
| Derby | 131 | 188 | 838 | 41.1 [35.6–46.7] | 841 | 1 | 315 | 0.119 [0.003–0.660] | 1,157 |
| Stanley | 221 | 420 | 410 | 34.5 [30.8–38.3] | 805 | 3 | 243 | 0.371 [0.077–1.08] | 1,051 |
| Newport | 110 | 189 | 571 | 36.8 [31.3–42.5] | 550 | 3 | 317 | 0.543 [0.112–1.58] | 870 |
| Bovismorbificans | 165 | 182 | 463 | 47.6 [42.2–53.0] | 630 | 1 | 179 | 0.158 [0.004–0.880] | 810 |
| Virchow | 101 | 182 | 372 | 35.7 [30.1–41.6] | 496 | 3 | 156 | 0.601 [0.124–1.75] | 655 |
| Agona | 38 | 86 | 374 | 30.6 [22.7–39.6] | 338 | 0 | 160 | 0 [0–1.09] | 498 |
| Kentucky | 63 | 104 | 303 | 37.7 [30.4–45.5] | 278 | 2 | 190 | 0.714 [0.087–2.56] | 470 |
| Java | 64 | 138 | 244 | 31.7 [25.3–38.6] | 288 | 0 | 158 | 0 [0–2.326] | 446 |
| Muenchen | 24 | 36 | 363 | 40.0 [27.6–53.5] | 306 | 2 | 115 | 0.649 [0.079–2.33] | 423 |
| Brandenburg | 56 | 101 | 224 | 35.7 [28.2–43.7] | 243 | 0 | 138 | 0 [0–1.507] | 381 |
| Saintpaul | 39 | 126 | 215 | 23.6 [17.4–30.9] | 243 | 0 | 137 | 0 [0–1.507] | 380 |
| Oranienburg | 56 | 45 | 258 | 55.4 [45.2–65.3] | 198 | 1 | 160 | 0.503 [0.013–2.77] | 359 |
| Thompson | 56 | 146 | 154 | 27.7 [21.7–34.4] | 264 | 1 | 91 | 0.377 [0.010–2.08] | 356 |
| Braenderup | 35 | 40 | 228 | 46.7 [35.1–58.6] | 189 | 2 | 112 | 1.05 [0.127–3.73] | 303 |
| Montevideo | 26 | 46 | 216 | 36.1 [25.1–48.3] | 184 | 1 | 103 | 0.541 [0.014–2.98] | 288 |
| Goldcoast | 29 | 31 | 221 | 48.3 [35.2–61.6] | 217 | 0 | 64 | 0 [0–1.686] | 281 |
| Hadar | 31 | 48 | 182 | 39.2 [28.4–50.9] | 164 | 0 | 97 | 0 [0–2.224] | 261 |
| Napoli | 11 | 15 | 227 | 42.3 [23.4–63.1] | 51 | 0 | 202 | 0 [0–6.978] | 253 |
| Mbandaka | 24 | 46 | 150 | 34.3 [23.3–46.6] | 158 | 1 | 61 | 0.628 [0.016–3.454] | 220 |
| Livingstone | 11 | 22 | 169 | 33.3 [18.0–51.8] | 128 | 0 | 74 | 0 [0–2.841] | 202 |
| Senftenberg | 27 | 37 | 96 | 42.2 [29.9–55.2] | 123 | 0 | 37 | 0 [0–2.955] | 160 |
| Weltevreden | 13 | 14 | 97 | 48.1 [28.7–68.1] | 87 | 0 | 37 | 0 [0–4.151] | 124 |
| Heidelberg | 19 | 14 | 88 | 57.6 [39.2–74.5] | 55 | 0 | 66 | 0 [0–6.487] | 121 |
| Havana | 5 | 16 | 42 | 23.8 [8.2–47.2] | 39 | 1 | 23 | 2.50 [0.063–13.16] | 63 |
| Other | 2,264 | 2,463 | 14,977 | 47.9 [46.5–49.3] | 14,149 | 35 | 5,520 | 0.247 [0.172–0.343] | 19,704 |
| Total | 25,360 | 52,375 | 63,021 | 32.6 [32.3–33.0] | 116,342 | 223 | 24,191 | 0.191 [0.167–0.218] | 140,756 |
TESSy: The European Surveillance System.
The percentages have been calculated by serovar considering those cases with known hospitalisation status.
The percentages have been calculated by serovar considering those cases with known outcome in terms of mortality.
More routine use of WGS would allow a focus on high‐risk strains within serovars that have emerged in other parts of Europe or globally, since epidemic spread of a specific serovar follows the emergence of particular strains with enhanced dissemination potential, while the majority of strains within a serovar may be of more limited relevance (Kenney, 2018; Worley et al., 2018; Chiou et al., 2019; Yokoyama et al., 2019; Zhang et al., 2019).
Some studies have suggested a greater number of virulence genes in S. Enteritidis and S. Typhimurium than in other serovars, but panels of strains used are not fully representative of those circulating currently, and recent data from ECDC suggests the reported hospitalisation (30.3%) and mortality rate (0.153%) for S. Enteritidis in 2014–2016 was not greater than the mean for all serovars, although this may be influenced by increased detection and reporting of milder cases that are associated with outbreaks. It is recommended that larger studies using WGS linking epidemiology, phylogeny and virulotyping are performed, using Salmonella isolates from the harmonised monitoring of poultry and from human disease in the EU, to facilitate attribution studies and identify trends associated with virulence and stress‐response genes (M'Ikanatha et al., 2010; Bertelloni et al., 2017; Allard et al., 2018; Pornsukarom et al., 2018). Further definition of the genes that promote enhanced colonisation of the reproductive tract in chickens is also required (Szmolka et al., 2018).
Emerging threats such as resistance of microorganisms to extended‐spectrum cephalosporins (ESCs), carbapenems, fluoroquinolones (FQs) and colistin are considered to be among the highest public health priorities within the EU (Florez‐Cuadrado et al., 2018; Stella et al., 2018). Two of the most important antimicrobial classes for treatment of human salmonellosis are FQs, such as ciprofloxacin, and ESCs, such as ceftriaxone. There is conflicting evidence regarding the magnitude of the threat presented by AMR in Salmonella in terms of the potential for treatment failure and severity of disease, but some studies have suggested more severe illness associated with resistant strains (Helms et al., 2002; Angulo et al., 2004; Martin et al., 2004; Hald et al., 2007; Jones et al., 2008; de Curraize et al., 2017).
It has been postulated that antimicrobial resistance might be partly responsible for the emergence of certain strains with enhanced virulence in food animal populations (Nde et al., 2006; Singer and Hofacre, 2006; Nde and Logue, 2008). Use of preventive therapeutic antimicrobials may have contributed to selection of resistant strains of Salmonella or mobilisation of plasmids, including hybrid antimicrobial resistance (AMR/virulence plasmids) (Silva et al., 2017; Touzain et al., 2018), from other intestinal organisms (Chander et al., 2008).
Initial emergence of a MDR Salmonella strain following plasmid transfer may be followed by clonal expansion and further dissemination nationally or internationally (Cao et al., 2018b). The serovars most frequently showing MDR in the EU are S. Typhimurium, S. Paratyphi B variant Java (S. Java), S. Hadar, S. Virchow, S. Heidelberg, S. Newport and certain clones of S. Infantis (Aviv et al., 2014; EFSA and ECDC, 2018; McDermott et al., 2018).
Resistance to third‐generation ESCs in Salmonella has emerged more recently, predominantly in S. Newport, S. Typhimurium and S. Heidelberg, in the USA, Canada and South America initially (Salmon and Watts, 2000; Gray et al., 2004; Poppe et al., 2005; Poppe et al., 2006; Taylor et al., 2008; Sjolund‐Karlsson et al., 2010) but increasingly also in the EU and globally (Pan et al., 2018b). These strains typically possess resistance genes on transferable multidrug resistance plasmids or transposons that can move between different Salmonella serovars and E. coli or other commensal flora (Allen and Poppe, 2002).
Large outbreaks of S. Heidelberg have occurred on the American continent which have involved highly multiple resistant strains associated with egg, broiler and turkey meat production that also demonstrate increased tolerance to biocides (Antony et al., 2018; Stefani et al., 2018). The occurrence of high severity food‐borne disease outbreaks caused by S. Heidelberg has increased in the USA in recent years, and contaminated chicken caused infections in 634 patients in 29 US states and Puerto Rico, with a 38% being hospitalised. Approximately 14% of S. Heidelberg isolates were from human bloodstream infections with resistance to one or more antimicrobial agents, including third‐generation cephalosporins, and being associated with increased risk for invasive disease. S. Heidelberg is responsible for 7% of human deaths due to non‐typhoidal Salmonella in the USA, the second most frequent serovar causing mortality after S. Typhimurium. The prevalence of MDR in S. Heidelberg increased 2.6‐fold since 2004, associated with transferrable genomic elements encoding resistance genes (Nakao et al., 2015; Bearson et al., 2017; Deblais et al., 2018), but control efforts in poultry flocks and meat production facilities have been followed by some reduction in human cases (Green et al., 2018). There is trade between the USA and the EU in grandparent layer breeding chicks, and S. Heidelberg (but not the MDR epidemic strains) has been detected in such chicks on arrival in quarantine. Use of antibiotic egg or chick injection that is used to minimise transport‐related infections in countries outside the EU may interfere with detection of infection in imported chicks or hatching eggs from third countries (Dutil et al., 2010).
Importation of poultry meat has been suggested as a possible means of the introduction of Salmonella serovars infrequently reported in Europe (Mueller et al., 2018). S. Heidelberg and S. Minnesota, both with multidrug‐resistant profiles, including to ESCs and/or FQs, were identified in meat imported from outside the EU into Portugal. All but one isolate carried bla CMY‐2, located on two epidemic plasmids, IncA/C or IncI1. S. Heidelberg was associated with five pulsed field gel electrophoresis (PFGE) types, including one similar to a previously reported American epidemic clone (Campos et al., 2018). MDR S. Heidelberg has also been found in humans and poultry in the Netherlands and Ireland, with the source being identified as imported poultry meat from Brazil (Liakopoulos et al., 2016). The Netherlands is an important source of broiler hatching eggs for several other countries so occurrence of MDR S. Heidelberg there is a concern for the EU as a whole. Various Salmonella serovars can also be found in day‐old parent breeder chicks traded outside the EU (Osman et al., 2010) or imported into the EU (Nogrady et al., 2012) and plasmid‐mediated transferrable FQ resistance is increasing reported in some countries (Karp et al., 2018; Ma et al., 2018).
S. Infantis producing VIM‐I carbapenemase was found in broilers, as well as pigs, during an anonymised survey in Germany during 2011 (Fischer et al., 2013a), but there does not appear to have been any increase in detection of such resistant strains in EU food animals since then, even though a NDM‐1 carbapenemase‐producing S. Corvalis isolate was obtained from a wild black kite around the same time (Fischer et al., 2013b; Fernandez et al., 2018). Reporting of miscellaneous Salmonella isolates with important AMR is summarised in EFSA and ECDC (2018). More recently a cephalosporin resistant strain of S. Infantis has emerged in the broiler industry and people in Italy (Franco et al., 2015) and may disseminate further if not effectively controlled at an early stage. Some of the Italian strains also show reduced susceptibility to FQs (EFSA and ECDC, 2018) and/or transferable colistin resistance due to the mcr‐1 gene (Carfora et al., 2018).
3.1.2. Impact of a new Salmonella target in flocks of breeding hens of Gallus gallus
3.1.2.1. Monitoring of Salmonella in flocks of Gallus gallus
During 2007–2016, 3 MS (out of 26) reported a 0% prevalence for target serovars in their breeding G. gallus flocks. The estimated EU flock prevalence of the target Salmonella serovars in breeding flocks decreased from 1.12% CI95[0.77; 1.62] in 2007 to 0.32% CI95[0.20; 0.49] in 2016. The 2007–2016 trend for the estimated EU flock prevalence of S. Enteritidis of breeding flocks was very similar to the one for the target serovars. The estimated EU Salmonella flock prevalence decreased from 2.7% CI95[1.8; 4] in 2012 to 1.3% CI95[0.92; 2.02] in 2014 and then it remained stable over time.
Figure 4 shows the overall EU prevalence of Salmonella target and non‐target serovars in the various poultry populations, EU, 2007–2016. There is similarity in the occurrence of regulated serovars (dominated by S. Enteritidis) in breeding flocks and laying hens over time, but no apparent correlation with broiler flocks, for which feed and hatchery contamination by a variety of serovars is a more common source. No relationship is apparent between the collective occurrence of non‐target serovars in breeding and commercial generations.
Figure 4.
Overall EU prevalence of Salmonella target serovars (top) and Salmonella non‐target serovars (bottom) in various poultry populations, EU, 2007–2016
Reported monitoring figures for chicken breeding flocks in 2016 (Table 5) show that all but two MS achieved the current target of 1% flock prevalence for the five target serovars, but 13 MS exceeded this level for all serovars combined. Across the EU, 32% of Salmonella reported were S. Enteritidis; 16% S. Typhimurium and 6% S. Infantis. However less than 1% of Salmonella serovars reported in breeding flocks were S. Hadar or S. Virchow.
Table 5.
Salmonella in breeding flocks of Gallus gallus during the production period (all types of breeding flocks, flock‐based data) in countries running control programmes in accordance with Regulation (EC) No 2160/2003, 2016
| Country | Tested flocks | Salmonella positive flocks | Percentage positive flocksa for | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella | Five target serovars | S. Enteritidis | S. Typhimurium | S. Infantis | S. Virchow | S. Hadar | Other than target | |||
| Austria | 146 | 2 | 1.37 | 0 | 0 | 0 | 0 | 0 | 0 | 1.37 |
| Belgium | 565 | 16 | 2.83 | 0.35 | 0.35 | 0 | 0 | 0 | 0 | 2.48 |
| Bulgaria | 222 | 4 | 1.8 | 0.45 | 0 | 0 | 0.45 | 0 | 0 | 1.35 |
| Croatia | 109 | 2 | 1.83 | 0 | 0 | 0 | 0 | 0 | 0 | 1.83 |
| Cyprus | 33 | 4 | 12.12 | 0 | 0 | 0 | 0 | 0 | 0 | 12.12 |
| Czech Republic | 673 | 2 | 0.3 | 0.3 | 0.3 | 0 | 0 | 0 | 0 | 0 |
| Denmark | 202 | 3 | 1.49 | 0 | 0 | 0 | 0 | 0 | 0 | 1.49 |
| Estonia | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | 163 | 1 | 0.61 | 0.61 | 0 | 0.61 | 0 | 0 | 0 | 0 |
| France | 2,057 | 12 | 0.58 | 0.58 | 0.29 | 0.24 | 0.05 | 0 | 0 | 0 |
| Germany | 868 | 11 | 1.27 | 0.46 | 0 | 0.35 | 0.12 | 0 | 0 | 0.81 |
| Greece | 242 | 4 | 1.65 | 1.24 | 0.83 | 0 | 0.41 | 0 | 0 | 0.41 |
| Hungary | 654 | 6 | 0.92 | 0.92 | 0.31 | 0.46 | 0.15 | 0 | 0 | 0 |
| Ireland | 182 | 4 | 2.2 | 0 | 0 | 0 | 0 | 0 | 0 | 2.2 |
| Italy | 1,194 | 11 | 0.92 | 0 | 0 | 0 | 0 | 0 | 0 | 0.92 |
| Latvia | 31 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| The Netherlands | 1,669 | 10 | 0.6 | 0.54 | 0.54 | 0 | 0 | 0 | 0 | 0.06 |
| Poland | 1,927 | 46 | 2.39 | 1.45 | 1.35 | 0.1 | 0 | 0 | 0 | 0.99 |
| Portugal | 537 | 1 | 0.19 | 0 | 0 | 0 | 0 | 0 | 0 | 0.19 |
| Romania | 377 | 13 | 3.45 | 0.8 | 0 | 0 | 0.8 | 0 | 0 | 2.65 |
| Slovakia | 110 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slovenia | 131 | 1 | 0.76 | 0 | 0 | 0 | 0 | 0 | 0 | 0.76 |
| Spain | 1819 | 47 | 2.58 | 0.44 | 0 | 0.38 | 0.05 | 0.05 | 0.05 | 2.75 |
| Sweden | 159 | 1 | 0.63 | 0.63 | 0 | 0.63 | 0 | 0 | 0 | 0 |
| United Kingdom | 1,396 | 27 | 1.93 | 0.14 | 0 | 0.14 | 0 | 0 | 0 | 1.79 |
| Total (MSs) | 15,477 | 228 | 1.47 | 0.54 | 0.32 | 0.16 | 0.06 | < 0.01 | < 0.01 | 1.02 |
When the prevalence is > 1%, the figure is shown in bold font.
Table E.1 in Appendix E shows the distribution of Salmonella serovars in flocks of breeders, layers and broilers of G. gallus during the production period at the EU level and for confirmed human salmonellosis cases acquired in the EU in 2014–2016, based on the MS reporting. S. Enteritidis predominated in breeder (24.8%) and laying hen (43.7%) sectors and was third most common (8.8%) in broilers, confirming the importance of including this serovar in the target. S. Mbandaka, was the second most common (9.8%) in breeders and broilers (9.7%), and also occurred in laying hens (ranked fifth; 4.0%). S. Typhimurium was ranked third (9.4%) in breeders, second (9.0%) in layers and fifth (4.7%) in broilers. S. Senftenberg ranked fifth (5.5%) in breeding flocks, was ranked as ninth in laying hens (1.8%) and was ranked eighth (2.6%) in broilers. S. Infantis ranked fifth (8.0%) in breeding flocks, fourth (7.9%) in laying hens and first (42.5%) in broilers. Monophasic S. Typhimurium ranked ninth (2.7%) in breeding flocks, but was much less common in laying hen flocks and broilers (both 1.8%). Neither of the currently regulated serovars, S. Virchow and S. Hadar, were reported among the top 15 in breeding flocks, laying hens or broilers.
Table E.1.
Distribution of Salmonella serovars in breeders, layers and broilers of Gallus gallus during the production period (flock‐based data) in countries running control programmes in accordance with Regulation (EC) No 2160/2003, 2014–2016
| Breeding flocks | N | % | Broiler flocks | N | % | Laying hen flocks | N | % | Human cases acquired in the EU | N | % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enteritidis | 121 | 24.80 | Infantis | 5,666 | 42.46 | Enteritidis | 965 | 43.68 | Enteritidis | 78,041 | 61.00 |
| Mbandaka | 48 | 9.84 | Mbandaka | 1,295 | 9.71 | Typhimurium | 199 | 9.01 | Typhimurium | 23,315 | 18.22 |
| Typhimurium | 46 | 9.43 | Enteritidis | 1,176 | 8.81 | Kentucky | 191 | 8.65 | Typhimurium, monophasic | 6,166 | 4.82 |
| Infantis | 39 | 7.99 | Livingstone | 779 | 5.84 | Infantis | 175 | 7.92 | Infantis | 3,398 | 2.66 |
| Senftenberg | 27 | 5.53 | Typhimurium | 627 | 4.70 | Mbandaka | 89 | 4.03 | Derby | 1,157 | 0.90 |
| Mikawasima | 15 | 3.07 | Thompson | 546 | 4.09 | Agona | 51 | 2.31 | Stanley | 1,051 | 0.82 |
| Rissen | 14 | 2.87 | Kedougou | 379 | 2.84 | Typhimurium, monophasic | 40 | 1.81 | Newport | 870 | 0.68 |
| Kentucky | 14 | 2.87 | Senftenberg | 352 | 2.64 | Montevideo | 40 | 1.81 | Bovismorbificans | 810 | 0.63 |
| Typhimurium, monophasic | 13 | 2.66 | Tennessee | 259 | 1.94 | Senftenberg | 39 | 1.77 | Virchow | 655 | 0.51 |
| Livingstone | 12 | 2.46 | 1,13,23:i:‐ | 238 | 1.78 | Ohio | 37 | 1.67 | Agona | 498 | 0.39 |
| Ohio | 10 | 2.05 | Kentucky | 236 | 1.77 | Livingstone | 28 | 1.27 | Kentucky | 470 | 0.37 |
| Taksony | 10 | 2.05 | Typhimurium, monophasic | 234 | 1.75 | Newport | 27 | 1.22 | Java | 446 | 0.35 |
| Derby | 9 | 1.84 | Cerro | 232 | 1.74 | Kottbus | 25 | 1.13 | Muenchen | 423 | 0.33 |
| Toulon | 9 | 1.84 | Montevideo | 148 | 1.11 | Thompson | 22 | 1.00 | Brandenburg | 381 | 0.30 |
| Liverpool | 9 | 1.84 | Agona | 129 | 0.97 | Braenderup | 20 | 0.91 | Saintpaul | 380 | 0.30 |
| Worthington | 8 | 1.64 | Ohio | 104 | 0.78 | Hadar | 18 | 0.81 | Oranienburg | 359 | 0.28 |
| Virchow | 6 | 1.23 | Muenster | 91 | 0.68 | Corvallis | 17 | 0.77 | Thompson | 356 | 0.28 |
| Altona | 6 | 1.23 | Havana | 90 | 0.67 | Agama | 13 | 0.59 | Braenderup | 303 | 0.24 |
| Kedougou | 6 | 1.23 | Liverpool | 85 | 0.64 | Virchow | 13 | 0.59 | Montevideo | 288 | 0.23 |
| Agona | 5 | 1.02 | Kottbus | 62 | 0.46 | Tennessee | 11 | 0.50 | Goldcoast | 281 | 0.22 |
| Amsterdam | 5 | 1.02 | Derby | 51 | 0.38 | Cerro | 11 | 0.50 | Mikawasima | 280 | 0.22 |
| 1,13,23:i:‐ | 4 | 0.82 | Indiana | 43 | 0.32 | Anatum | 11 | 0.50 | Bareilly | 270 | 0.21 |
| Hadar | 4 | 0.82 | Coeln | 42 | 0.31 | Havana | 9 | 0.41 | Hadar | 261 | 0.20 |
| Mishmarhaemek | 3 | 0.61 | Taksony | 37 | 0.28 | Rissen | 9 | 0.41 | Rissen | 255 | 0.20 |
| Idikan | 3 | 0.61 | Java | 36 | 0.27 | Idikan | 9 | 0.41 | Napoli | 253 | 0.20 |
| Jerusalem | 3 | 0.61 | Anatum | 32 | 0.24 | Kedougou | 9 | 0.41 | Chester | 237 | 0.19 |
| Bredeney | 3 | 0.61 | Newport | 29 | 0.22 | Dublin | 8 | 0.36 | Kottbus | 237 | 0.19 |
| Llandoff | 3 | 0.61 | Gaminara | 24 | 0.18 | Blockley | 8 | 0.36 | Coeln | 225 | 0.18 |
| Muenster | 2 | 0.41 | Stanley | 21 | 0.16 | Albany | 6 | 0.27 | Dublin | 221 | 0.17 |
| Kottbus | 2 | 0.41 | Bredeney | 20 | 0.15 | Coeln | 6 | 0.27 | Mbandaka | 220 | 0.17 |
| Newport | 2 | 0.41 | Orion | 18 | 0.13 | Offa | 5 | 0.23 | Ohio | 215 | 0.17 |
| Poona | 2 | 0.41 | Chester | 18 | 0.13 | Derby | 4 | 0.18 | Livingstone | 203 | 0.16 |
| Montevideo | 2 | 0.41 | Give | 18 | 0.13 | Indiana | 4 | 0.18 | Indiana | 201 | 0.16 |
| Meleagridis | 2 | 0.41 | Saintpaul | 13 | 0.10 | Lexington | 4 | 0.18 | Panama | 176 | 0.14 |
| Other | 21 | 4.30 | Dublin | 13 | 0.10 | Bovismorbificans | 4 | 0.18 | London | 173 | 0.14 |
| Total | 488 | 100 | Minnesota | 12 | 0.09 | Muenster | 4 | 0.18 | Senftenberg | 160 | 0.13 |
| Salmonella spp., unspecified | 245 | Virchow | 11 | 0.08 | Mishmarhaemek | 4 | 0.18 | Poona | 158 | 0.12 | |
| Grand Total | 733 | Rissen | 11 | 0.08 | Brandenburg | 3 | 0.14 | Bredeney | 145 | 0.11 | |
| Hadar | 9 | 0.07 | Joal | 3 | 0.14 | Tennessee | 144 | 0.11 | |||
| Goldcoast | 9 | 0.07 | Gloucester | 3 | 0.14 | Litchfield | 137 | 0.11 | |||
| Idikan | 8 | 0.06 | Paratyphi B | 3 | 0.14 | Abony | 134 | 0.10 | |||
| Llandoff | 8 | 0.06 | Llandoff | 3 | 0.14 | Weltevreden | 125 | 0.10 | |||
| Mikawasima | 8 | 0.06 | Nottingham | 2 | 0.09 | Heidelberg | 121 | 0.09 | |||
| Nyborg | 7 | 0.05 | Stanleyville | 2 | 0.09 | Anatum | 108 | 0.08 | |||
| Abony | 6 | 0.04 | Elomrane | 2 | 0.09 | Corvallis | 107 | 0.08 | |||
| Reading | 6 | 0.04 | Kingston | 2 | 0.09 | Give | 100 | 0.08 | |||
| Albany | 5 | 0.04 | Tshiongwe | 2 | 0.09 | Arizonae | 93 | 0.07 | |||
| Haifa | 5 | 0.04 | Glostrup | 2 | 0.09 | Kedougou | 91 | 0.07 | |||
| Duesseldorf | 5 | 0.04 | Orion | 2 | 0.09 | Schwarzengrund | 86 | 0.07 | |||
| Braenderup | 5 | 0.04 | Meleagridis | 2 | 0.09 | Stanleyville | 85 | 0.07 | |||
| Glostrup | 4 | 0.03 | Schwarzengrund | 2 | 0.09 | Agama | 79 | 0.06 | |||
| Duisburg | 4 | 0.03 | Dabou | 2 | 0.09 | Javiana | 78 | 0.06 | |||
| Haardt | 4 | 0.03 | Essen | 2 | 0.09 | Manhattan | 75 | 0.06 | |||
| Bareilly | 4 | 0.03 | Muenchen | 2 | 0.09 | Sandiego | 71 | 0.06 | |||
| Brandenburg | 3 | 0.02 | Java | 2 | 0.09 | Blockley | 69 | 0.05 | |||
| II 42:b:e,n,x,z15 | 3 | 0.02 | Bredeney | 2 | 0.09 | Havana | 63 | 0.05 | |||
| Agama | 3 | 0.02 | Cubana | 2 | 0.09 | Muenster | 61 | 0.05 | |||
| Schwarzengrund | 3 | 0.02 | Other | 29 | 1.31 | Pomona | 61 | 0.05 | |||
| 1,4,12:d:‐ | 3 | 0.02 | Total | 2,209 | 100 | Haifa | 60 | 0.05 | |||
| Meleagridis | 3 | 0.02 | Salmonella spp., unspecified | 1,114 | Choleraesuis | 57 | 0.04 | ||||
| Stanleyville | 2 | 0.01 | Grand Total | 3,323 | Reading | 45 | 0.04 | ||||
| Bovismorbificans | 2 | 0.01 | Hvittingfoss | 44 | 0.03 | ||||||
| Madelia | 2 | 0.01 | Durham | 42 | 0.03 | ||||||
| Butantan | 2 | 0.01 | Telelkebir | 42 | 0.03 | ||||||
| Lille | 2 | 0.01 | Veneziana | 39 | 0.03 | ||||||
| Epinay | 2 | 0.01 | Monschaui | 39 | 0.03 | ||||||
| Szentes | 2 | 0.01 | Richmond | 38 | 0.03 | ||||||
| Mishmarhaemek | 2 | 0.01 | Cotham | 38 | 0.03 | ||||||
| Istanbul | 2 | 0.01 | Bardo | 38 | 0.03 | ||||||
| Putten | 2 | 0.01 | Umbilo | 36 | 0.03 | ||||||
| Other | 32 | 0.24 | Oslo | 33 | 0.03 | ||||||
| Total | 13,344 | 100 | Agbeni | 32 | 0.03 | ||||||
| Salmonella spp., unspecified | 11,188 | Stourbridge | 32 | 0.03 | |||||||
| Grand Total | 24,532 | Isangi | 32 | 0.03 | |||||||
| Uganda | 31 | 0.02 | |||||||||
| Szentes | 31 | 0.02 | |||||||||
| Cerro | 29 | 0.02 | |||||||||
| Minnesota | 29 | 0.02 | |||||||||
| Albany | 28 | 0.02 | |||||||||
| Altona | 28 | 0.02 | |||||||||
| Kenya | 27 | 0.02 | |||||||||
| Gloucester | 27 | 0.02 | |||||||||
| Schleissheim | 25 | 0.02 | |||||||||
| Eastbourne | 24 | 0.02 | |||||||||
| Potsdam | 24 | 0.02 | |||||||||
| Adelaide | 24 | 0.02 | |||||||||
| Urbana | 24 | 0.02 | |||||||||
| Singapore | 22 | 0.02 | |||||||||
| Ndolo | 22 | 0.02 | |||||||||
| Kambole | 22 | 0.02 | |||||||||
| Ajiobo | 21 | 0.02 | |||||||||
| Gaminara | 21 | 0.02 | |||||||||
| Other | 1,405 | 1.10 | |||||||||
| Total | 127,937 | 100 | |||||||||
| Salmonella spp., unspecified | 12,819 | ||||||||||
| Grand Total | 140,756 |
N = number of cases.
3.1.2.2. Review of the current target serovars in breeding and production chicken populations and humans
Figure 5 shows the proportion of reported Salmonella that are the current target five serovars in breeding flocks for each EU MS between 2014 and 2016. In order to compare the prevalence of the five target serovars across the different poultry populations and humans, Figure 6 provides this information for breeders, broilers, layers and humans in the EU. For broilers and layers, this figure includes both official monitoring data as well as any MS data in response to the data requests. This particularly relates to S. Infantis, S. Hadar and S. Virchow for which there is no mandatory reporting for broilers and layers as they are not target serovars and may be grouped as part of the unspecified serovars for reporting purposes). As reporting of the five target serovars is mandatory for breeding flocks, additional data were not needed.
Figure 5.
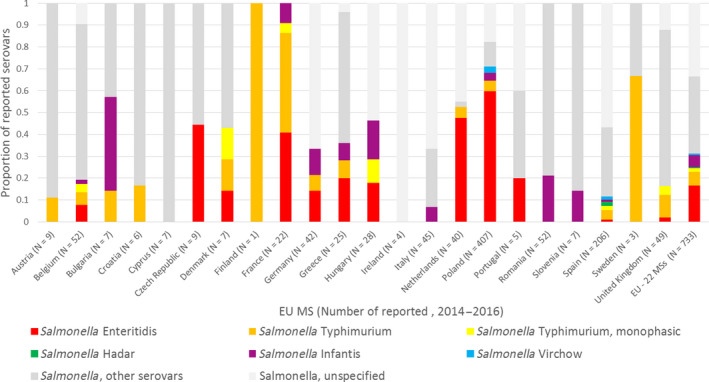
Distribution of the five target Salmonella serovars, other Salmonella serovars and unspecified Salmonella serovars in breeding flocks of Gallus gallus in countries running control programmes in accordance with Regulation (EC) No 2160/2003, 2014–2016
Figure 6.
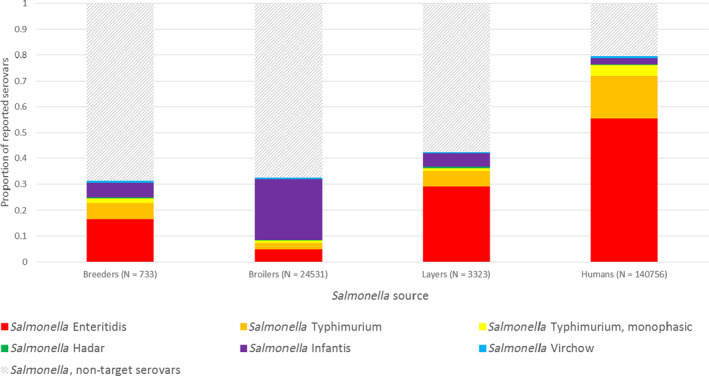
Distribution of the five target Salmonella serovars and other or unspecified Salmonella serovars reported in breeding, broiler and layer flocks and in humans in the EU (domestically acquired and EU travel only), 2014–2016
From Figure 5, it is difficult to make robust conclusions due to the low sample sizes for many MSs; however, at EU level it is clear that S. Hadar and S. Virchow are rarely reported in breeding flocks. Indeed, across all of the MSs reporting to EFSA during 2014–2016, 4 (0.5%) out of 733 isolations were S. Hadar (all from Spain) and 6 (0.8%) out of 733 isolations were S. Virchow (3 from Poland; 3 from Spain). S. Enteritidis, S. Typhimurium (incl. monophasic strains) and S. Infantis accounted for 219 of the 733 (~ 30%) of Salmonella detections across all EU MSs.
A wide range of other serovars were present in the three populations, but the data available did not allow a relationship between breeding flocks and the other populations to be determined. In breeding flocks, 35% of reported Salmonella were ‘other’ serovars (i.e. not the target 5). Across the EU MSs, between 2014 and 2016, 54 different serovars were reported from breeding flocks, of which 35 serovars were reported less than five times within this 3‐year time period. Finally, a significant proportion of Salmonella reports were unspecified regarding the serovar involved; across all EU MSs 33% of Salmonella serovars were unspecified, with variability between MSs.
The human data includes domestically acquired cases and EU travel cases only. From Figure 6, it is concluded that S. Enteritidis, S. Typhimurium (incl. monophasic strains) and S. Infantis, which are regularly found in breeding flocks, are also reported in broiler flocks, layer flocks and human salmonellosis cases (31.4%, 41.4% and 78.8%, respectively). However, S. Hadar and S. Virchow are only infrequently reported in the progeny of breeding flocks. From broilers (N = 24,531 flocks), there were only 9 isolations of S. Hadar and 11 isolations of S. Virchow in the official monitoring data. Including the additional data, provided by some MSs on request, led to identification of 48 isolations of S. Hadar and 160 isolations of S. Virchow. From layers (N = 3,323 flocks), there were only 18 isolations of S. Hadar and 13 isolations of S. Virchow in the official monitoring data; including the additional data provided by some MSs identified 29 isolations of S. Hadar and 27 isolations of S. Virchow. As mentioned previously, some MSs did not report the non‐target serovars identified on poultry farms. This is particularly frequent for broiler flocks where 44.4% of Salmonella were unspecified (2014–2016). In some countries over 90% of broiler flock isolations reported provided no serovar information in 2014–2016 (e.g. from Germany, Hungary, the Netherlands and Spain).
In humans, the number of reported cases of S. Hadar and S. Virchow was also low: 261 reports of S. Hadar were submitted and 655 reports of S. Virchow, from a total of 140,756 sporadic cases occurring in the EU (domestically acquired or from travel within the EU). There was a single small outbreak (9 cases) of S. Virchow within this time period, which was linked to eggs.
From this descriptive analysis, it can be concluded that there is a case for reconsidering the 5 target serovars in breeding flocks due to the low frequency of S. Hadar and S. Virchow reported in all sources (breeder, broiler, layer flocks and humans), although it is not possible to assess the impact of such changes because there are multiple sources of these serovars other than breeding flocks and the impact of breeding flocks depends on the individual strains involved and control actions in the food chain. For example, as shown in Table E.2 in Appendix E, including S. Kentucky in a target for breeding flocks could only provide a maximum reduction of 0.53% of reported broiler flock cases and 5.75% of positive laying hen flocks, assuming that breeding flocks are responsible for all infections in commercial broiler and laying flocks, that there are no other sources of sporadic or persistent infections and that application of a target would be 100% effective. None of these assumptions are realistic. Similarly, it is not possible to assess the impact of proposed changes of target serovars on human cases, but for serovars that are ranked below S. Infantis, the maximum reduction for any individual serovar, assuming that all human cases would be abolished (which is impossible to achieve) by adding a serovar to the breeding flock target, would be less than 1% of human cases. In Section 3.1.2.3, alternative serovars for inclusion as a target serovar are considered.
Table E.2.
Suitability of the 28 selected Salmonella serovars for inclusion within an alternative target for breeding flocks of Gallus gallus based on the four specific criteria to be adopted to determine Salmonella serovars with public health significance to which community targets will apply (Annex III of Regulation (EC) No 2160/2003, 2014–2016
| Salmonella serovar | Association with human salmonellosis based on reported sporadic cases acquired in the EU (% of 140,756 cases); reported number of outbreaks/outbreak cases caused by Salmonella in egg and egg products and in broiler meat (2014–2016); estimated % of human cases (from 1.48 million) attributable to broilers/layers in 2016 | Route of infection: Proportion of reported Salmonella in the EU and the number of reports in each EU MS (2014–2016)b | Recent/rapid ability to spread and cause disease | Increased virulence for human infections or resistance to critically important antimicrobials Including hospitalisation and mortality rates for reported infections acquired in the EU 2014–2016 | |||
|---|---|---|---|---|---|---|---|
| Number of times a particular MS reported that serovar between 2014 and 2016 from breeding flocks (N = 733) | Number of times a particular MS reported that serovar between 2014 and 2016 from broiler flocks (N = 24,531) | Number of times a particular MS reported that serovar between 2014 and 2016 from layer flocks (N = 3,323) | |||||
| Enteritidis |
55.44% of sporadic cases 218 outbreaks (3,007 cases) attributed to eggs and egg products 28 outbreaks (400 cases) attributed to broiler meat and products 89.7% of cases attributed to laying hens and broilers |
16.51% BE(4), CZ(4), DE(6), DK(1), EL(5), ES(2), FR(9), HU(5), NL(19), PL(64), PT(1), UK(1) |
4.79% AT(19), BE(15), BG(2), CZ(291), DE(31), DK(1), EL(1), ES(18), FR(290), HR(48), HU(32), MT(1), NL(15), PL(191), PT(29), RO(95), SI(1), SK(35), UK(59) |
29.04% AT(20), BE(23), BG(6), CZ(19), DE(124), DK(1), EE(2), EL(13), ES(62), FI(1), FR(140), HR(17), HU(43), IT(47), LV(2), MT(2), NL(110), PL(278), PT(17), RO(28), SK(3), SI(3), UK(4) |
S. Enteritidis emerged as a global epidemic in the 1980s and has persisted in most countries as the leading cause of human salmonellosis ever since in almost all countries |
High virulence is reported in the literature but analysis of recent human data from the EU does not suggest higher virulence than the mean for all serovars among recent infections Occasionally resistant to CIAs such as FQs in countries where historic usage in breeding flocks has been common, but most EU isolates are fully sensitive 30.3% of reported cases hospitalised and 0.15% of cases died |
|
|
S. Enteritidis is widely reported by EU MSs. PL reported the highest number of cases in breeding flocks (64), of which most were parent broiler‐breeders, and in layer flocks (278). They also reported the second highest number in broiler flocks (191). FR reported the highest number of positive broiler flocks (290) Vertical transmission via colonisation of the reproductive tract and internal egg contamination has resulted in S. Enteritidis becoming the most important serovar for transmission from breeding flocks and to humans via egg products (primarily) and also broiler meat. Several outbreaks in broiler flocks have resulted from infection of imported hatching eggs from within or outside the EU. The organism also survives well in the environment and readily infects rodents so is able to persist in poultry houses, particularly housing for laying hens | |||||||
| Typhimurium |
16.56% of sporadic cases 14 outbreaks (92 cases) attributed to eggs and egg products 3 outbreaks (36 cases) attributed to broiler meat and products 1.46% of cases attributed to laying hens and broilers |
6.28% AT(1), BE(3), BG(1), DE(3), DK(1), EL(2), ES(9), FI(1), FR(10), HR(1), NL(2), PL(5), SE(2), UK(5) |
2.56% AT(6), BE(44), BG(1), CY(1), CZ(4), DE(48), DK(8), EL(1), ES(86), FR(311), HR(12), HU(26), IE(5), IT(3), LU(1), MT(3), NL(18), PL(5), PT(5), RO(7), SE(16), SI(2), SK(2), UK(12) |
5.99% AT(12), BE(1), BG(1), DE(49), DK(1), EL(4), ES(18), FI(1), FR(59), HR(1), HU(7), IE(1), IT(22), NL(6), PL(1), PT(5), RO(4), SE(2), SI(2), UK(2) |
S. Typhimurium is the second most common serovar in humans in the EU and various strains have shown epidemic potential in food animal and human populations |
Many MDR strains, such DT104, have shown enhanced virulence in humans and animals in the arly years after their emergence and there is a general tendency towards multiple antimicrobial resistance, although resistance to CIAs is not common in most countries 40.3% of reported cases hospitalised and 0.20% of cases died |
|
|
Reported in many EU MSs, particularly in FR in parent broiler‐breeding and broiler flocks (10 and 311 reports, respectively). There were also 59 reports of S. Typhimurium in FR in layers and 49 reports in DE. ES (86), DE (48) and BE (44) also reported S. Typhimurium more frequently than other MS S. Typhimurium is a very diverse serovar containing host‐adapted strains associated with wild birds as well as strains with a wide host range. It is particularly common in pigs in most countries, but is also associated with ruminants, horses and companion animals. Some strains, e.g. DT104, have achieved epidemic proportions in previous years and every decade there has been emergence of a new epidemic type which also affects humans and can be acquired by poultry. Although vertical transmission of most strains is limited, the regular occurrence of S. Typhimurium in chicken flocks and its unpredictability in terms of further dissemination makes it a relevant target for control actions | |||||||
| Typhimurium monophasic |
4.38% of sporadic cases 1 outbreak (17 cases) attributed to eggs and egg products No reported outbreaks attributed to broiler meat and products 0.54% of cases attributed to laying hens and broilers |
1.77% BE(2), DK(1), ES(4), FR(1), HU(3), UK(2) |
0.95% BE(18), CY(1), DK(29), ES(25), FR(118), HR(2), HU(5), MT(9), NL(4), PT(10), UK(12) |
1.20% AT(2), DK(2), ES(10), FR(11), HR(2), HU(1), IT(9), MT(2), UK(1) |
S. Typhimurium monophasic strains emerged in pig populations in the mid‐2000s and have spread widely on a global basis since then, probably via the movement of infected breeding pigs. It is the third most common serovar in humans in the EU |
Most isolates have resistance to multiple antimicrobials and occasionally to CIAs such as cephalosporins or gentamicin, but most EU isolates are only resistant to ampicillin, sulfonamides, tetracyclines and streptomycin/spectinomycin 33.6% of reported cases hospitalised and 0.50% of cases died. This apparent higher mortality rate relates to only 15 reported cases in a 3‐year period |
|
|
Reported in poultry flocks in 13 MSs, most isolations reported by FR in broilers (118). The single isolation from FR came from a parent broiler‐breeding flock but with the data available it is not possible to conclude that the broiler cases are attributable to this case S. Typhimurium monophasic strains are primarily associated with pigs, but have regularly spread into ruminant and poultry populations, particularly in areas where there is a heavy density of pig production, especially outdoor pig breeding units. Dissemination via contamination of feed ingredients grown or stored near to pig farms is also likely. There is little evidence of dissemination from breeding to commercial poultry flocks, contamination of eggs or spread or persistence of the organism on chicken farms | |||||||
| Infantis |
2.41% of sporadic cases 1 outbreak (5 cases) attributed to eggs and egg products No reported outbreaks attributed to broiler meat and products 3.7% of cases attributed to laying hens and broilers |
5.32% BE(1), BG(3), DE(5), EL(2), ES(2), FR(2), HU(5), IT(3), PL(4), RO(11), SI(1) |
23.10% AT(244), BE(128), BG(4), CY(50), CZ(64), DK(14), EL(5), HR(197), IT(2535), MT(13), PL(65), RO(1752), SK(37), SI(553), UK(5) |
5.27% AT(8), BE(15), BG(2), CY(3), CZ(1), EL(14), ES(29), HR(5), IT(23), LV(4), MT(2), PL(44), PT(8), RO(15), SI(1), UK(1) |
There has been rapid spread of MDR Infantis across EU broiler production and occurrence of human cases in those countries that are most affected |
Some strains may show enhanced virulence. Many isolates have resistance to multiple antimicrobials including ESCs and colistin 32.8% of reported cases hospitalised and 0.21% of cases died |
|
|
Reported in broiler flocks in many MSs and especially in AT, BE, ES, IT, PL, RO and SI S. Infantis is often associated with broiler production as well as laying hens and other food animal sources. In recent years MDR strains that have originated in Eastern Europe have spread in broiler production in many EU countries. Once established these are very difficult to eliminate from broiler farms. S. Infantis is consistently the fourth most common serovar in humans, although the sources of infection are diverse and most isolates in most countries are not MDR broiler‐related strains | |||||||
| Derby |
0.82% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.08% of cases attributed to laying hens and broilers |
1.23% DK(1),IT(7), UK(1) ES(1) |
0.21% BE(20), CZ(3), DK(4), IE(2), MT(6), PT(1), UK(15) ES(6), IT(10), MT(2) ** |
0.12% BG(1), DK(1), PT(2) ES(2), FR(12) * , IT(6) |
Although S. Derby has been among the top 10 serovars in humans, the actual numbers of cases are relatively low, and showed a reduction in 2017 |
Virulence is considered to be low and resistance to critically important antimicrobials is unusual in the EU 41.1% of reported cases hospitalised, which contradicts literature reports of low virulence, but only 0.12% of cases died |
|
|
The breeding flock case of S. Derby in DK was in a parent breeding flock for the broiler production line in 2016. All the other incidents in breeding flocks were unspecified as to their flock type. All the other incidents of S. Derby in DK also occurred in 2016 The seven reports of S. Derby in breeding flocks in IT were reported in 2015. Using the additional data provided by IT S. Derby was also detected in broiler and layer flocks in 2014 (8 in broiler; 4 in layer) and 2015 (2 in broiler and 2 in layers) In the UK, the type of breeding flock was unspecified, but the detection of S. Derby occurred in 2014. Of the 15 detections of S. Derby in broilers, 14 occurred in 2016, but this was related to contamination of a hatchery that also hatched eggs from an infected turkey breeding flock and then became persistently contaminated In ES, the single isolation of S. Derby was from a breeding flock for the broiler production line in 2015. S. Derby was also detected in broiler flocks (3 reports in 2014, 1 in 2015, 2 in 2016) and layer flocks (1 report in 2014 and 1 in 2016) S. Derby is predominantly associated with pigs and, to a lesser extent, turkeys rather than with chickens, but appears to be able to persist in some hatcheries and poultry houses, despite regular cleaning and disinfection. Human cases mostly relate to consumption of undercooked pork products. No evidence of vertical transmission | |||||||
| Stanley |
0.75% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products |
0% |
0.09% AT(1), HR(20) IT(1) |
0% | S. Stanley showed a rapid emergence in turkey populations in some Eastern European countries in earlier years, but has since largely regressed |
Little evidence of enhanced virulence. Some isolates have resistance to multiple antimicrobials and occasionally to CIAs 34.5% of reported cases hospitalised and 0.37% of cases (3 people) died |
|
| S. Stanley could be considered as potentially relevant for enhanced control based on previous outbreaks in the EU, but these were mostly confined to a small number of countries and mainly related to turkey flocks/meat, and such outbreaks are no longer occurring. No evidence of involvement of chicken breeding flocks. No evidence of vertical transmission | |||||||
| Newport |
0.62% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.15% of cases attributed to laying hens and broilers |
0.27% CY(1), UK(1) ES(2), PL(3) |
0.12% DK(3), IE (1), PO(4), PT(4), RO(4), UK(13) ES(8), IT(6), PL(12) |
0.82% PO(8), RO(10), UK(7) ES(3), FR(1) * , IT(2), PL(8) |
S. Newport increased to the no. 4 position in people in 2017, but this was mainly related to a large outbreak caused by imported food of non‐animal origin |
Little evidence of enhanced virulence in EU strains. Some isolates have resistance to multiple antimicrobials and occasionally to CIAs such as FQs, but most EU isolates are fully sensitive. MDR strains with resistance to ESCs, associated mainly with cattle, which have emerged in the USA and Canada are not known to occur in food animals in the EU 36.8% of reported cases hospitalised and 0.54% of cases (3 people) died |
|
|
The UK case of S. Newport in breeders was in an unspecified adult flock in 2016; 10 of the 13 UK broiler cases occurred in 2016; for the layers, there was 2 in 2016 and 5 in 2014 CY did not report human cases to ECDC. No S. Newport was reported in broilers or layers. In ES, one of the isolations was in 2014, and one in 2016 (both for breeders for the broiler production line). Seven of the eight ES isolations of S. Newport in broilers were in 2015 (4) and 2016 (3). In layer flocks, there was a single isolation each year In PL all three isolations were from parent breeding flocks for broiler production line (2 in 2014 and 1 in 2015). The 12 isolations from broiler flocks were distributed across the years (5 in 2015, 3 in 2015 and 4 in 2016). In the layer flocks, all eight isolations occurred in 2016. S. Newport is a very genetically diverse serovar and outbreaks are often associated with FoNAO or exposure to reptiles. In the UK, it can be found in badgers, resulting in feed grain contamination, and so can be isolated occasionally from all animal species, but is particularly likely to be found in turkeys and cattle. No evidence of vertical transmission | |||||||
| Bovismorbificans |
0.58% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.01% of cases attributed to laying hens and broilers |
0% |
0.008% RO(1), EL(1) |
0.12% HR(4) IT(2) |
S. Bovismorbificans has caused some human outbreaks, mainly linked with contaminated pork products |
Some strains may show enhanced virulence. Many isolates have resistance to multiple antimicrobials 47.6% of reported cases hospitalised but only 0.16% of cases died |
|
| S. Bovismorbificans is more likely to be associated with pigs, ducks or turkeys than chickens. It is sometimes found in animal feed so could be transmitted to a variety of food animals | |||||||
| Virchow |
0.47% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.9% of cases attributed to laying hens and broilers |
0.82% PL(3), ES(3) |
0.04% BE(1), CY(4), IT(2), PL(1), PT(1), RO(2) ES(118), FR(38) * , IT(2), PL(3) |
0.39% AT(1), CY(7), PT(5) ES(7), FR(3) * , IT(1), PL(3) |
No evidence of recent rapid spread or unusual levels of disease |
Some strains may show enhanced virulence. Some isolates have resistance to multiple antimicrobials 35.7% of reported cases hospitalised and 0.60% of cases (3 people) died |
|
|
The PL cases in breeding flocks occurred in 2014 (2 reports) and 2015 (1 report). All detections were recorded as being from a parent flock for broiler production. The reports of S. Virchow from the official monitoring data and the data provided by PL differ (so both have been included for transparency) but it can be concluded that there was a low number of isolations in 2014–2016 in broiler and layer flocks In ES, all three cases in breeding flock for broiler production (level unknown): two reported in 2014 and one in 2016. In ES and FR, a larger number of isolations were reported laying hens in the additional data provided: 118 cases reported in ES (2014–2016) and 38 in FR (2017) No evidence of vertical transmission or chickens as a major source of human infections | |||||||
| Agona |
0.35% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.16% of cases attributed to laying hens and broilers |
0.68% AT(1), BG(1), UK(3) IT(2) |
0.53% AT(8), BE(28), HR(1), CZ(1), EL(1), MT(1), PT(2), RO(79), SL(2), UK(6) ES(13), IT(2), MT(1) |
1.53% AT(5), BE(2), CZ(1), EL(3), PO(1), RO(33), UK(6) ES(8), FR(4) * , IT(7), PL(2) |
Little evidence of significant spread despite common occurrence as a feed contaminant |
No evidence of enhanced virulence or relevant antimicrobial resistance 30.6% of reported cases hospitalised and 0% of cases died |
|
|
Large number of cases of S. Agona in BE (broilers) and RO (broilers and layers), but not reported in breeding flocks. There were also a large number of reports in FR in 2016 S. Agona is a common feed contaminant and human outbreaks have often originated from FoNAO. No evidence of vertical transmission | |||||||
| Kentucky |
0.33% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.29% of cases attributed to laying hens and broilers |
1.91% BG(1), PO(1), RO(1), ES(11) PL(1) |
0.96% AT(1), BE(1), CY(9), CZ(12), IE(8), MT(36), PT(1), RO(167), UK(1) ES(221), IT(52), MT(32), PL(1) |
5.75% CY(1), FR(1), EL(1), IT(176), MT(3), PO(4), RO(5) ES(18), FR(1) * , IT(183), MT(10), PL(4) |
Rapid emergence of ST198, which has rapidly spread in broiler populations in many EU countries |
Many isolates have resistance to multiple antimicrobials and/or very high MICs to ciprofloxacin 37.7% of reported cases hospitalised and 0.71% of cases (2 people) died |
|
|
S. Kentucky in layers occurring throughout 2014 (75 reports), 2015 (54 reports), 2016 (47 reports) in IT. However, it was not isolated in any Italian breeding flocks. Also high but decreasing number of S. Kentucky detections in RO in broilers: 2014 (179 reports), 2015 (652 reports), 2016 (36 reports). The isolation found in a breeding flock was in 2016 and the flock type was unspecified. In ES, there were 11 isolations of S. Kentucky in breeding flocks (7 in 2014; 4 in 2015), all of which were in breeding flocks for broiler production line. Using data subsequently provided by ES there were 221 isolations of S. Kentucky in breeding flocks (83 in 2014; 99 in 2015; 39 in 2016). It cannot be certain, but this could be considered as the possible dissemination of S. Kentucky from breeding flock(s) to broiler flocks via hatchery contamination S. Kentucky is primarily associated with chicken flocks among potential food animal sources for humans in the EU, but is also associated with foreign travel outside the EU | |||||||
| Java |
0.32% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.19% of cases attributed to laying hens and broilers |
0.14% NL(1) |
0.15% BE(36) |
0.06% AT(2) |
S. Java spread rapidly in broiler populations in earlier years but little obvious involvement of transmission from breeding flocks |
Virulence is considered to be low in most countries. Many isolates have resistance to multiple antimicrobials, including CIAs in some cases 31.7% of cases hospitalised and 0% of cases died |
|
| In BE, 26 detections of S. Java in broilers in 2014 and 10 in 2016. This serovar has been associated with persistent contamination of broiler farms in a small number of countries and has not spread to others. Human cases can originate from many sources, including reptiles | |||||||
| Muenchen |
0.3% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products |
0% |
0% ES(5), IT(13) |
0.06% AT(1), CY(1) ES(2), IT(4) |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence or AMR 40.0% of reported cases hospitalised and 0.65% of cases (2 people) died |
|
| Uncommon in poultry flocks, occasional contaminant of animal feed | |||||||
| Brandenburg |
0.27% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.01% of cases attributed to laying hens and broilers |
0% |
0.01% RO(2), UK(1) IT(1) |
0.09% PT(3) ES(1) |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence or AMR 35.7% of reported cases hospitalised and 0% of cases died |
|
| Uncommon in poultry flocks, occasional contaminant of animal feed | |||||||
| Saintpaul |
0.27% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.05% of cases attributed to laying hens and broilers |
0% |
0.05% CZ(1), PT(1), SL(11) IT(5), PL(1) |
0% FR(2) * |
No evidence of recent rapid spread or unusual levels of disease |
Some strains may have resistance to multiple antimicrobials 23.6% of cases hospitalised and 0% of cases died |
|
| More commonly associated with turkeys than chickens, though more common in broilers in a small number of countries. Human infections often linked with FoNAO | |||||||
| Oranienberg |
0.26% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products |
0% |
0.008% RO(1), EL(1) |
0.12% HR(4) IT(2) |
No evidence of recent rapid spread or unusual levels of disease | No evidence of special concerns regarding virulence or AMR in the literature, but 55.4% of reported cases hospitalised and 0.50% of cases (1 person) died | |
| S. Oranienberg has been largely associated with FoNAO from outside the EU, rather than food animals | |||||||
| Thompson |
0.25% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products |
0.14% AT(1) IT(6) |
2.23% AT(48),EL(5), IT(442), RO(50), UK(1) ES(10), IT(698) |
0.66% AT(7), HR(2), EL(12), SL(1) ES(7), FR(1) * , IT(16) |
Recent rapid spread in poultry populations is limited to certain countries |
Some strains may show enhanced virulence and have resistance to multiple antimicrobials 27.7% of reported cases hospitalised and 0.38% of cases died |
|
|
The reported S. Thompson in the breeding flock was in a parent breeder for eggs in AT in 2015. Broiler cases: 2014 (1 report), 2015 (16 reports), 2016 (31 reports). In layers 2014 (1 report), 2015 (3 reports), 2016 (3 reports) From the additional data received from IT, there were six reports of S. Thompson (5 in 2014, 1 in 2015), but breeding flock type (broiler or layer breeding flock) was unknown. There were 256 reported cases in 2014, 123 in 2015 and 319 in 2016 (the official data did not include the 2014 data) The 50 cases in RO broilers included 43 cases in 2015. It is likely that S. Thompson, which is recognised as being a common contaminant of broiler farms in many countries worldwide has been introduced into broiler production in a small number of countries, but there is no evidence of the ongoing involvement of broiler breeding flocks in most countries or of spreading across the EU in the way that S. Infantis or S. Kentucky has done. Human cases also originate from a wide variety of sources other than poultry meat, such as FoNAO and processed fish products. There could be a case for specific controls on breeding flocks in IT and AT | |||||||
| Braenderup |
0.22% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.85% of cases attributed to laying hens and broilers |
0% ES(3) |
0.02% SK(4), UK(1) ES(7), IT(8), PL(1) |
0.6% AT(1), CY(2), EL(17) ES(3), FR(1) * , IT(7), PL(1) |
No evidence of recent rapid spread or unusual levels of disease | No evidence of special concerns regarding virulence or AMR in the literature, but 46.7% of reported cases hospitalised and 1.1% of cases (2 people) died | |
|
In ES, there were three reports of the serovar S. Braenderup in breeding flocks for broiler production (2 in 2014; 1 in 2015). Of the seven isolations in broilers, six of them occurred in 2015 14 cases of S. Braenderup were detected in layers in EL in 2016. In the USA there is some evidence of vertical transmission and outbreaks associated with handling chicks | |||||||
| Montevideo |
0.2% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.12% of cases attributed to laying hens and broilers |
0.27% BE(1), CY(2) ES(3), FR(1) * |
0.60% AT(23), BE(2), CZ(9), EL(1), RO(21), SL(1), UK(91) ES(8), FR(257) * , IT(16) |
1.2% AT(19), BE(1), EL(8), PT(1), RO(4), SL(2), UK(5) ES(5), FR(13) * , IT(7), PL(1) |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence or AMR 36.1% of reported cases hospitalised and 0.54% of cases (1 person) died |
|
|
For the reports in breeding flocks, for both BE and CY, it is unknown whether the flock was for egg or meat production. For the BE reported case it was for a parent flock FR recorded the detection of S. Montevideo in hatching eggs from a breeding flock for broiler production line in 2016. In 2016 FR reported 231 isolations of S. Montevideo in broiler flocks The UK cases in broilers were spread across the 3 years as 2014 (40 cases), 2015 (20 cases), 2016 (31 cases) This serovar is a common contaminant of feed production facilities and hatcheries so is likely to be found at all stages of poultry production. It is also a common contaminant of FoNAO | |||||||
| Goldcoast |
0.2% of sporadic cases 1 outbreak (2 cases) attributed to broiler meat and products No reported outbreaks attributed to eggs and egg products 0.02% of cases attributed to laying hens and broilers |
0% ES(1) |
0.04% BE(1), PT(1), UK(7) ES(5) |
0% ES(2), IT(1) |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence or AMR 48.3% of reported cases hospitalised and 0% of cases died |
|
| Multiple sources including produce, uncommon in chickens | |||||||
| Hadar |
0.19% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.06% of cases attributed to laying hens and broilers |
0.55% ES(4) |
0.04% EL(3), IT(4), PL(1), UK(1) ES(24), FR(14) * , IT(5) |
0.54% CY(1), EL(9), PL(1), RO(7) ES(8), FR(1) * , IT(2) |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence. Some isolates have resistance to multiple antimicrobials 39.2% of cases hospitalised and 0% of cases died |
|
| The four reports of S. Hadar were in breeding flocks for broiler production (level unknown). There were two reports in 2014 and 1 report in 2015 and 2016. S. Hadar in poultry is known to be a relevant source of human infection in some countries, but there is little evidence of vertical transmission and the serovar is more common in ducks | |||||||
| Napoli |
0.18% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.69% of cases attributed to laying hens and broilers |
0% FR(3) * , IT(4) |
0% FR(77), IT(3) |
0% IT(2), ES(1) |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence or AMR 42.3% of reported cases hospitalised and 0% of cases died |
|
|
France reported 77 isolations of S. Napoli in 2017. In breeding flocks, the three reports were derived from broiler breeding flocks. IT also identified this serovar in broilers and layers and ES identified S. Napoli in layers This serovar is more likely to be associated with environmental contamination than poultry, with human cases often liked to FoNAO rather than meat or eggs. It has a limited distribution in poultry in the EU | |||||||
| Mbandaka |
0.16% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.15% of cases attributed to laying hens and broilers |
6.55% AT(1), BE(17), CZ(1), HR(3), IT(5), PL(11), RO(1), UK(9) ES(2), FR(1) * , IT(17), PL(3) |
5.28% AT(13), BE(21), CZ(18), HR(58), IE(4), IT(559), PL(20), PT(1), RO(34), SE(2), SI(1), UK(564) ES(31), FR(75) * , IT(633), MT(1), PL(31) |
2.68% AT(14), BE(9), PL(35), PT(9), RO(13), SE(1), SI(1), UK(7) ES(17), FR(12) * , IT(13), MT(2), PL(37) |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence or AMR. S. Mbandaka is considered to be relatively avirulent compared with most serovars 34.3% of reported cases hospitalised but 0.63% of cases (1 person) died |
|
|
Commonly reported serovar, especially in broiler flocks in IT and UK. PL, BE and UK have larger number of reports from breeding flocks. Ten of the cases in PL were from parent broiler‐breeding flocks. BE and UK provide no additional data on the type of breeding flock S. Mbandaka is a common feed contaminant also found in hatcheries so is detected on hatcher basket liners as well as on broiler carcasses. The feed route is very prominent for this serovar because of its occurrence in by‐products of the vegetable oil extraction process (e.g. soya bean and rape seed meal) and is commonly found in various food animal populations, particularly broilers and dairy cattle | |||||||
| Livingstone |
0.14% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.19% of cases attributed to laying hens and broilers |
1.64% BE(1), HR(1), EL(8), RO(2) FR(1) * , IT(2) |
3.18% AT(1), BE(46), HR(3), FI(1), EL(18), IT(663), MT(2), RO(25), UK(20) MT(1) |
0.84% BE(7), CY(4), EL(2), MT(3), RO(5), SE(3), UK(4) ES(9), FR(11) * , IT(6), MT(11), PL(1) |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence or AMR 33.3% of reported cases hospitalised and 0% of cases died |
|
|
No information is provided on the type of breeding flocks from which S. Livingstone was isolated in BE, HR, EL or RO. IT reported a large number of cases: 302 in 2014, 199 in 2015 and 162 in 2016. S. Livingstone often occurs as a hatchery contaminant so is likely to be occasionally found in poultry flocks during routine monitoring, but does not usually persist in flocks. Some apparent isolations from breeding flocks may be false positives resulting from sampling at the hatchery level that is carried out in many countries | |||||||
| Senftenberg |
0.11% of sporadic cases. No reported outbreaks (2012–2016) 1 outbreak (34 cases) attributed to broiler meat and products No reported outbreaks attributed to eggs and egg products 0.07% of cases attributed to laying hens and broilers |
3.68% AT(1), BE(5), BG(1), EL(1), RO(8), UK(11) ES(6), IT(2) |
1.43% AT(24), BE(6), CY(1), CZ(1), DK(2), EL(9), HR(9), MT(1), PL(7), PT(3), RO(194), SI(2), UK(93) ES(421), FR(97) * , IT(39), PL(7) |
1.17% AT(4), BE(5), CY(1), EE(1), EL(1), HR(12), PL(6), PT(5), RO(3), UK(1) ES(5), FR(4) * , IT(13), PL(10) |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence or AMR 42.2% of cases hospitalised and 0% of cases died |
|
|
Reports of S. Senftenberg in breeding flocks in the UK, RO and BE. Of the 11 UK cases, 9 were reported in 2016 but the breeder type is unspecified. Likewise, six of the eight Romanian reported cases were in 2014. In the additional data provided by ES, all of the cases (1 in 2014, 5 in 2015) were in broiler‐breeding flocks. 421 broiler flocks were reported to have infection with S. Senftenberg (101, 182, 138 in 2014, 2015 and 2016, respectively); the highest reporting within the EU. RO and UK also have higher levels across the 3 years of investigation and FR had a high level of reported cases in broiler flocks in 2017 S. Senftenberg is a common resident contaminant of hatcheries, which could result in false‐positive test results for breeding flocks that are monitored via hatchery samples. It is also a common contaminant of animal feed, so is likely to be found in all poultry populations because of this common exposure. In broiler flocks, although it may be found in young chicks, it is often undetectable by the time of slaughter | |||||||
| Weltevreden |
0.09% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.22% of cases attributed to laying hens and broilers |
0% | 0% |
0% FR(1) * |
No evidence of recent rapid spread or unusual levels of disease |
No evidence of special concerns regarding virulence or AMR 48.1% of reported cases hospitalised and 0% of cases died |
|
| This serovar is rare in animal populations in the EU and is usually associated with FoNAO or travel to Asian countries | |||||||
| Heidelberg |
0.09% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products |
0% IT(4) |
0.004% IE(1) IT(27) |
0% | Rapid spread in chickens and turkeys in the American continent. High potential for vertical transmission |
Some non‐EU strains show enhanced virulence. Many isolates have resistance to multiple antimicrobials including ESCs 57.6% of reported cases hospitalised but 0% of EU cases died |
|
|
S. Heidelberg was isolated in breeding flocks Italy in 2015 (3) and 2016 (1). No information was provided on the types of breeding flocks. In broilers in IT there were 16 reports in 2014, 5 in 2015 and 6 in 2016 Not currently common in the EU in humans or chicken populations. Some information suggests some strains found in Portugal, the Netherlands and Ireland after importation of Brazilian poultry meat are similar to strains that have emerged rapidly in the USA and Canada | |||||||
| Havana |
0.04% of sporadic cases No reported outbreaks attributed to eggs and egg products or broiler meat and products 0.16% of cases attributed to laying hens and broilers |
0.14% EL(1) ES(4) |
0.37% BE(2), EL(1), PT(71), RO(14), UK(2) ES(11), IT(45) |
0.27% AT(1), BE(5), PT(3) ES(5), FR(7) * , IT(5) |
No evidence of recent rapid spread or unusual levels of disease |
Some strains may show enhanced virulence in humans resulting in a higher than expected frequency of extra‐intestinal disease 23.8% of reported cases hospitalised but 2.5% of cases (1 person) died |
|
|
The reports of S. Havana in ES were from breeding farms for the broiler production line (1 in 2014, 1 in 2015 and 2 in 2016). Most of the broiler cases in ES were in 2014: 2014 (11); 2015 (4) and 2016 (1). In broilers Portugal reported 71 isolations of S. Havana. The majority of these (53) were in 2015 Not currently common in the EU in humans or chicken populations. Can occasionally be found in animal feed ingredients imported from outside the EU | |||||||
Note: A Red‐Amber‐Green (RAG) status has been assigned for each serovar to each criterion and overall (see column 1) on the basis of the amount of evidence availablea
RED: serovar is frequently reported (> 10% of EU cases) in humans or accounts for > 10% of human cases attributed to layers/broilers; substantial evidence of transmission through the poultry industry; substantial evidence of rapid and recent ability to spread in poultry and cause disease in humans; substantial evidence of increased virulence (e.g. invasiveness or antimicrobial resistance). AMBER: serovar is occasionally reported in humans (1–10% of EU cases) or accounts for 1–10% of human cases attributed to layers/broilers; some evidence of transmission through the poultry industry; some evidence of rapid and recent ability to spread in poultry and cause disease in humans; some evidence of increased virulence (e.g. invasiveness or antimicrobial resistance). GREEN: is rarely reported in humans (< 1% of EU cases) or accounts for < 1% of human cases attributed to layers/broilers; limited/no evidence of transmission through the poultry industry; limited/no evidence of rapid and recent ability to spread and cause disease; limited/no evidence of increased virulence (e.g. invasiveness or antimicrobial resistance).
The percentage given for each flock type is based on the 2014–2016 official monitoring data only. However, details of which MSs had reported the serovar are provided (and the number of reports between 2014 and 2016), using both data reported to EFSA and additional data provided by MSs (identified by the use of italics). The data sources were kept separate as the additional data provided by the MS did not always correspond with the data officially reported to EFSA. In some cases, additional information is also given from the raw data set providing specific years and number of cases the serovar was reported for a particular MS and included in the ‘Comments’ column.
AMR: antimicrobial resistance; CIA: clinically important antimicrobial; ECDC: European Centre for Disease Prevention and Control; ESC: extended‐spectrum cephalosporins; FoNAO: foods of non‐animal origin; FQ: fluoroquinolones; MDR: multidrug‐resistant; MIC: minimum inhibitory concentration; MS: Member State; DT: definitive phage type.
Country abbreviations: AT: Austria; BE: Belgium; BG: Bulgaria; CZ: the Czech Republic; CY: Cyprus; DE: Germany; DK: Denmark; EE: Estonia; EL: Greece; ES: Spain; FI: Finland; FR: France; HR: Croatia; HU: Hungary; IE: Ireland; IT: Italy; LT: Lithuania; LU: Luxembourg; LV: Latvia; MT: Malta; NL: the Netherlands; PL: Poland; PT: Portugal; RO: Romania; SE: Sweden; SI: Slovenia; SK: Slovakia; UK: the United Kingdom.
*FR: Table includes 2016 layer, 2016 breeding or 2017 broiler data only; **MT: 2014 data (2015, 2016 included in the EFSA official reporting).
3.1.2.3. Alternative serovars of public health significance in top‐5 target of breeding flocks
Table 3 shows the most common serovars for human salmonellosis cases acquired in the EU (domestically acquired and EU travel in 2016). In 2014–2016, S. Enteritidis, S. Typhimurium (including monophasic strains) and S. Infantis accounted for a high proportion of reported serovars: 78.2%, 78.9%, and 79.4% in 2016, 2015 and 2014, respectively. Other serovars individually accounted for 1% or less. S. Hadar is no longer in the top 20 human serovars; it was ranked as the 24th most common serovar for EU‐acquired infections during this time period. Salmonella Derby was among the top 5 most prevalent serovars in human cases acquired in the EU in 2016, but declined in 2017 to eighth position, behind S. Newport, S. Agona and S. Kentucky (EFSA and ECDC, 2017d). In the EU the main reservoir host is pigs (Zheng et al., 2017a) with which this serovar is considered to be host‐associated, and to a lesser extent, turkeys in some countries, due to previous international trade in breeding turkeys and subsequent persistence in the commercial breeding and production pyramid. In the United Kingdom, S. Derby has recently spread to broilers following contamination by association with infection in turkey breeders and has persisted on some broiler farms where it has been introduced (APHA, 2018). S. Derby is considered to be less virulent than many serovars and lacks certain genes associated with invasiveness (Litrup et al., 2010; Hauser et al., 2011; Card et al., 2016), but large numbers of cases occur in countries with high consumption of uncooked or lightly cooked pig meat and outside the EU strains with MDR and an extended host range are reported (Xu et al., 2017; Cao et al., 2018b), reflecting the occurrence of MDR in many bacterial species in response to liberal antimicrobial use in food animals. S. Newport is also among the most common Salmonella serovars acquired in the EU, but this serovar is very diverse genetically (Zheng et al., 2017b) and many strains are not considered to have a primary reservoir in food animals, being frequently found in wildlife, reptile and amphibian pets and foods of non‐animal origin (McLauchlin et al., 2018; Pan et al., 2018a; Teplitski and de Moraes, 2018). The MDR S. Newport clones that are common in the USA and Canada (Campbell et al., 2018; Cao et al., 2018a) are not known to be resident in the EU (Espie et al., 2005; Horton et al., 2016) and despite the MDR profile, appear to be less virulent than S. Typhimurium (Parisi et al., 2018). It is therefore not considered appropriate to specify S. Derby or S. Newport in a target for breeding flocks, and the ranking of these serovars has already changed in 2017. In the case of S. Newport, the ranking increased due to human cases resulting from importation of contaminated foods of non‐animal origin from outside the EU in one MS (EFSA and ECDC, 2018).
Table 6 provides the basis for selection of the serovars considered as alternative target serovars while Table E.2 in Appendix E considers the suitability of 28 Salmonella serovars to be target serovars. In addition to the most 20 common human serovars, the table also includes serovars linked to outbreaks of Salmonella attributed to eggs/egg products or broiler meat and products thereof between 2014 and 2016, serovars identified by the SAM (ToR 2) as serovars contributing > 0.1% of human cases that are attributed to broilers/layers (see Appendix E) and serovars identified by the literature review carried out within Sections 3.1.1.2, 3.1.1.3, 3.1.1.4). S. Hadar, a current target serovar in breeding flocks, was not identified in any of these sources but was included in Table E.2 in Appendix E for completeness as it is included in the current target. S. Heidelberg is also included as it was identified in the literature review as having an enhanced capability for vertical transmission and high virulence.
Table 6.
Basis for the selection of the Salmonella serovars to be considered as alternative target serovars of breeding hen flocks
| Source of serovar identification | Salmonella serovars selected as an alternative target serovar |
|---|---|
| Top 20 serovars infecting humans in the EU (sporadic cases) | S. Enteritidis, S. Typhimurium, S. Typhimurium monophasic variants, S. Infantis, S. Derby, S. Stanley, S. Newport, S. Bovismorbificans, S. Virchow, S. Agona, S. Kentucky, S. Java, S. Muenchen, S. Brandenburg, S. Saintpaul, S. Oranienberg, S. Thompson, S. Braenderup, S. Montevideo, S. Goldcoast |
| Additional serovars associated with outbreaks attributed to broilers/eggs | S. Senftenberg |
| Additional serovars identified by the source attribution model (ToR 2) | S. Napoli, S. Weltevreden, S. Livingstone, S. Havana, S. Mbandaka |
| Additional serovar identified by the literature review | S. Heidelberg |
| Additional serovar for completeness (current target serovar) | S. Hadar |
The prevalence of each of the current and potential alternative target serovars in breeding, broiler and layer flocks was reviewed and included in Table E.2 in Appendix E. The percentage is given for each flock type based on the official monitoring data only. Details of which MSs had reported the serovar are provided as well as, in some cases, additional information providing specific years and number of cases the serovar was reported for a particular MS. This aims to ascertain whether there is any likelihood of a serovar that has been detected in a broiler / layer flock could have originated from a breeding flock. However due to the aggregated nature of the data, this can only be observational and cannot indicate any causal link between breeding and production flocks (see Section 2.1.1). Reviewing the data provided in Table E.2 in Appendix E it can be seen that, other than S. Enteritidis, S. Typhimurium and S. Infantis, the serovars that are more frequently observed in humans are not commonly found in breeding flocks of G. gallus, so the impact of introducing additional serovar targets is considered to be limited unless there is substantial emergence of a serovar that is not subject to regulatory control. S. Infantis, for example, has largely spread via environmental contamination within broiler production rather than through the breeding pyramid (Karacan Sever and Akan, 2018). However, it is still considered important to include S. Infantis in targets for breeding flocks because of the frequency of its occurrence in poultry populations and humans.
For some serovars found in humans, there is a high frequency of detection in broiler/layer flocks in some MS (e.g. S. Thompson in broilers in Italy; S. Kentucky in broilers in Spain) and a small number of reports from breeding flocks. There are also reports from broiler/layer flocks in relevant numbers (e.g. S. Kentucky in broilers in Romania and in both broilers and layers in Italy); however, there is no record of important levels of detection of the common human serovars, other than S. Enteritidis, in breeding flocks. Therefore, along with the difficulty of being unable to epidemiologically link detection of Salmonella between breeders, production flocks and humans, it is not straightforward to discern which other serovars should replace (if at all) S. Hadar and S. Virchow within the target 5.
For each of the serovars considered in Table E.2 in Appendix E, the four specific criteria provided were used to determine Salmonella serovars with public health significance (see Section 3.1.1). A Red‐Amber‐Green (RAG) score is assigned to each criterion for each serovar on the basis of the amount of evidence available. From the specified criteria, an overall RAG status is assigned for each serovar, based on an expert evaluation of the impact of the different criteria.
Therefore, after reviewing the available evidence, S. Kentucky is identified as a potential alternative serovar for inclusion in a breeding flock target. S. Kentucky has spread among broiler populations in several EU MS in recent years, and many strains are resistant to multiple antimicrobials and exhibit high level resistance to critically important FQ antimicrobials.
Although not currently common in the EU, S. Heidelberg has shown a high propensity for vertical transmission and resistance to ESCs in the American continent and some incursion into Europe via imported poultry meat has been reported (see Section 3.1.1.4). This serovar could therefore be considered for inclusion in a new top 5 to help prevent its dissemination within the EU, but it could be more likely that a different serovar could be introduced from third countries bordering on Eastern Europe than introduction of an important strain from a different continent (Jansen et al., 2019).
From this descriptive analysis it can be concluded that S. Enteritidis, S. Typhimurium (including monophasic strains), S. Infantis, S. Kentucky, and possibly S. Heidelberg or S. Thompson could be relevant for consideration within a new selection of five target serovars, based on its occurrence in breeding flocks, laying flocks and broiler production in some MS, plus enhanced virulence of some strains. All of the other considered serovars were assigned a green RAG status overall and therefore not considered as an alternative target Salmonella serovar in breeding flocks.
A further alternative could be a more proactive selective approach to control of Salmonella in the breeding and production pyramid via inclusion of a variable fifth serovar in MS‐specific national prevalence targets for breeding flocks if serovars of particular concern arise in breeding flocks and progeny in that country, or are considered to be a threat, based on occurrence and epidemiology in other regions. For example, France has included S. Kentucky in flock prevalence targets on the basis of its rapid emergence and dissemination in poultry and humans in countries bordering Europe and in some other EU MS.
3.1.2.4. Consideration of ‘all serovars’ as a target for breeding flocks
In the mandate for this scientific opinion, the possibility of considering all serovars in flocks of breeding hens as target serovars was requested. Collectively many other serovars are likely to be transmitted from breeding flocks to hatcheries and thence to progeny, at least intermittently, and may lead to long‐term or permanent contamination of hatcheries or poultry farms, therefore there is a case for considering a target that includes all serovars.
It is assumed, since the mandate specifies that control measures would remain unchanged, that only the detection of S. Enteritidis and S. Typhimurium in a breeding flock will result in the mandatory cull of the flock and extensive cleaning and disinfection in all MS, which would limit the impact of the target if infected flocks remain in production, although in many MS there would be a cull if any of the target serovars were found and in some MS, or in grandparent flocks and above, breeding flocks would be culled if any Salmonella serovar was confirmed.
To consider the impact of a 1% target for Salmonella in breeding flocks on EU MSs, the EU data were analysed. Presented in Figure 7 is both the prevalence, for each MS, for both the five target serovars (the current scenario) and also the prevalence of all Salmonella in 2014, 2015 and 2016. From this descriptive analysis, it can be seen that under the current five serovar target six MSs failed the target in breeding flocks at some point in the previous 3 years: Belgium (2014), Bulgaria (2015), Denmark (2014), Greece (2014, 2016), Poland (2014, 2015, 2016) and Romania (2014). However, if there had been an ‘all Salmonella’ serovar target, 18 MSs would have failed the target at some point during the 3 years; only the Czech Republic, Estonia, Finland, France, Portugal and Sweden did not exceed the 1% target in this time frame. Since, apart from S. Enteritidis, Salmonella serovars that occur in broiler and laying hen flocks predominantly originate from feed or environmental contamination rather than from breeding flocks, it is not possible to assess the impact of an ‘all Salmonella’ target on either production flocks or human cases.
Figure 7.
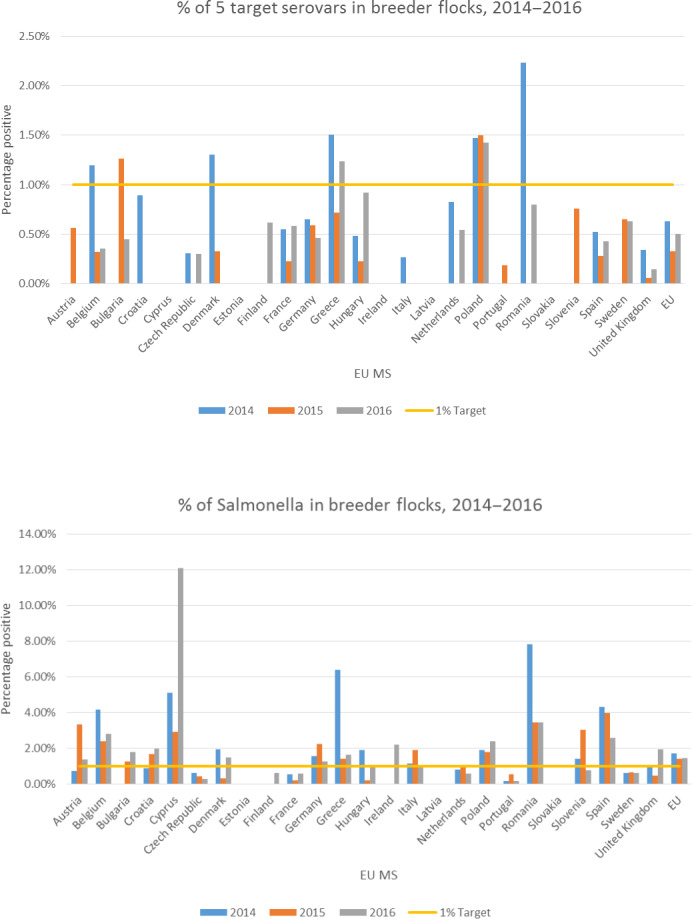
Percentage of breeding flocks in EU MSs that are positive for the 5 target Salmonella serovars (top) and for Salmonella (bottom) in 2014, 2015 and 2016
Advantages of an ‘all serovar’ target
A target that incorporates all serovars could be more effective than one for selected serovars as the most relevant serovars in breeding flocks vary between MS and over time. A target for all serovars would help focus greater attention on any serovar that occurs in breeding flocks, which should help minimise further dissemination and reduce the occurrence of resident contamination in hatcheries and dissemination of Salmonella via international trade in hatching eggs. A target that includes all serovars would be more effective in reducing the risk of introduction of newly emerging or re‐emerging strains with ‘epidemic potential’, especially if control measures are taken to deal effectively with those serovars or strains that have already been shown to present a serious risk to poultry and human populations in other MS or outside the EU. An ‘all serovar’ target would also focus more attention on the feed industry, because of fears of introduction of Salmonella to flocks via breeder feed. A large proportion of Salmonella infections in chickens originate from contaminated oil seed residue‐based feed ingredients or recontamination of heat‐treated feed during cooling or fat coating stages. The same feed mills are usually used for producing breeder feed and feed for other food animals, so improved feed hygiene would have wider benefits (Jones, 2011).
Disadvantages of an ‘all serovar’ target
Many MS may not be able to achieve a 1% prevalence target for all serovars, whereas most MS can meet the current target (Figure 7). A dual target could therefore be considered for the previously mentioned high priority serovars and for all serovars. There is also not a clear relationship between the occurrence of many serovars in breeding flocks and commercial generations or humans, since sources of Salmonella for both populations are diverse. This could result in unnecessary control action and loss of sources of food if more breeding flocks are culled. Still, culling is the most effective option for breeding flocks that are infected with strains of Salmonella serovars that are being transmitted to progeny and could be recommended for infections involving the top five named serovars. Other containment methods such as improved egg hygiene, segregated hatching, competitive exclusion treatment and improved terminal hygiene following depopulation of infected flocks could be applied for other serovars that are considered less relevant.
3.1.3. Concluding remarks and recommendations
Although vertical transmission of Salmonella within the contents of eggs resulting from transfer from the ovary or oviduct during the formation of eggs is an uncommon event, it can occur and infection that can be subsequently spread between hatchlings further amplifies the level of Salmonella. Certain zoonotic Salmonella serovars, particularly S. Enteritidis and S. Heidelberg (in the American continent), have been shown to be disseminated by vertical transmission, but this can be strain‐dependent. Vertical transmission also increases the public health risk in relation to table eggs.
Most Salmonella serovars can transmit from breeding flocks to progeny via faecal contamination of the shells of hatching eggs, which can result in cross‐contamination of various sources of chicks during the hatching process, either on a short‐term basis, or as a result of establishment of longer term resident contamination within some hatcheries.
It is not possible to assess the impact of proposed changes of target serovars in breeding flocks, either on laying hen or broiler populations. There are multiple sources of these serovars other than breeding flocks and the impact of breeding flocks depends on the individual strains involved and control actions in the food chain. It is also not possible to assess the impact of proposed changes of target serovars on human salmonellosis cases for the same reasons; for example, control of S. Enteritidis in breeding flocks in the United Kingdom had a small impact on human cases compared to vaccination of laying hens.
There is justification for retaining S. Enteritidis, S. Typhimurium (including monophasic variants) and S. Infantis in the target for breeding flocks considering their occurrence in breeding hen, laying hen and broiler flocks, as well as in humans. S. Infantis can readily acquire resistance to critically important antimicrobials, be disseminated within the poultry industry and is difficult to eliminate from broiler farms. It is not clear whether the inclusion of serovars other than S. Enteritidis would have limited their dissemination, and S. Infantis, which has been included in breeding flock targets since 2007, has still emerged and spread in broiler flocks across the EU since then.
Apart from these three serovars, the predominant Salmonella serovars in humans are subject to change on an annual basis, so a new top 5 serovar target list based on human prevalence could become out of date quite rapidly, as has occurred for the current target serovars, S. Virchow and S. Hadar.
S. Kentucky could be proposed for inclusion in a breeding flock target as the fourth serovar as it has spread among broiler populations in several EU MS in recent years, and many strains are resistant to multiple antimicrobials and exhibit high level resistance to critically important FQ antimicrobials.
-
For the fifth serovar, there are several options depending on Salmonella control priorities at the EU and MS level
-
–
S. Heidelberg could be included on a precautionary basis to prevent dissemination of epidemic strains in the EU. Although not currently common in the EU, this serovar has shown a high propensity for vertical transmission and resistance to ESCs in the American continent.
-
–
S. Thompson could be proposed as an option for enhanced control in the EU, based on its occurrence in breeding flocks and dissemination in a small number of MS, plus enhanced virulence of some strains.
-
–
A further alternative could be a more proactive selective approach to control of Salmonella in the breeding and production pyramid via inclusion of a variable fifth serovar in MS‐specific national prevalence targets in breeding flocks if serovars of particular concern arise in breeding flocks and progeny in that country, or are considered to be a threat, based on occurrence and epidemiology in other regions.
-
–
A target that incorporates all serovars could be more effective than one for selected serovars as the most relevant serovars in breeding flocks vary between MS and over time. A target for all serovars would help focus attention on any serovar that occurs in breeding flocks, which should help minimise further dissemination. Such a target would be more effective in reducing the dissemination of all serovars, including newly emerging or re‐emerging strains with ‘epidemic potential’, especially if measures are taken to effectively control those serovars or strains that have already been shown to present a serious risk to poultry and human populations in other MS or outside the EU, and would place greater pressure on poultry feed producers to minimise Salmonella contamination. It would also avoid the possibility of reversion if previously specified target serovars were to re‐emerge in breeding flocks.
In 2016, 13 MSs exceeded the prevalence target of 1% for all serovars. A dual prevalence target for all serovars and for the selected high priority serovars could be proposed.
A focus on improved detection and control of S. Enteritidis in all poultry populations, particularly early and sensitive detection in laying hens, could support the proposed targets for breeding flocks.
It is recommended to investigate in detail the reasons for failure to control S. Enteritidis in countries where it appears regularly in chicken breeding flocks, laying hens or broilers, especially if current or proposed targets are exceeded.
It is also recommended to use WGS to compare isolates from poultry breeding flocks with those in commercial generations of birds and in humans in order to provide more definitive evidence of a link between breeding flocks, commercial generations and human infections. This could be done via the EU Reference Laboratories (EURL) and network of Salmonella National Reference Laboratories (NRLs), as well as the joint ECDC/EFSA database for animal and food Salmonella isolates from 2019 when WGS data will be available, using stored isolates and sequence data from the MS NCPs.
Reporting of all individual Salmonella serovars in poultry flocks would facilitate source attribution and epidemiological studies.
3.2. Public health impact of a new Salmonella target in adult flocks of laying hens of Gallus gallus
3.2.1. Monitoring of Salmonella in laying hen flocks
In laying hens flocks, 2016 was the ninth year in which MS were obliged to implement a Salmonella NCP. According to Regulation (EC) No 517/2011, targeted serovars are S. Enteritidis and S. Typhimurium, including its monophasic variant. The prevalence target (which depends on the prevalence of the preceding year and was equal in 2016 to 2% or less for all MS except for Poland where it was 2.56% or less) was set for all commercial‐scale adult laying hen flocks in the production period. However, MS with fewer than 50 flocks of adult laying hens would attain the target if only one adult flock remained positive.
During 2008–2016, no MS reported a 0% prevalence for target serovars in these flocks. The estimated EU flock prevalence of the target Salmonella serovars in laying hens was 3.7% CI95[2.6; 5.3] in 2008 and decreased to 0.8% CI95[0.54; 1.2] in 2014. From 2014 onwards, it increased to 0.9% CI95[0.62; 1.3] in 2015 and to 1.3% CI95[0.86; 1.9] in 2016. The 2016 prevalence was higher than 2014 at the limits of significance (p = 0.056). The decreasing EU‐level flock prevalence of target Salmonella serovars in laying hens reported since the implementation in 2008 of NCPs, has thus been reversed into an increasing trend during the last two years. The exclusion of Poland did not change this EU trend, and during 2015–2016, seven MS reported an increased target Salmonella serovars flock prevalence in laying hens. The trend for estimated EU S. Enteritidis flock prevalence in laying hens during 2008–2016 was similar to the trend described for the target serovars and notably increased since 2014. This recent increase involved several MS and was more pronounced in some of them.
Reported monitoring figures for flocks of laying hens in 2016 (see Figure 8) show that seven MS exceeded the 2016 flock prevalence targets for S. Enteritidis and S. Typhimurium, while during 2015 only one MS, Poland, did not meet it. However, 15 MS exceeded 1% during 2016, including some of the major egg producing and exporting countries.
Figure 8.
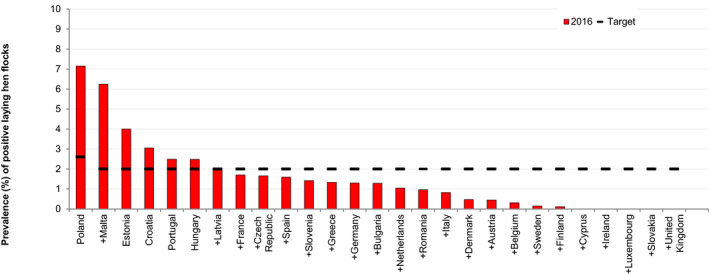
Prevalence of laying hen flocks of Gallus gallus during the production period positive for S. Enteritidis or S. Typhimurium (including the monophasic variants) and targets for MS, 2016
3.2.2. Baseline source attribution model
The SAM included the following 23 MSs: Austria, Belgium, Croatia, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Malta, Poland, Portugal, Slovakia, Slovenia, Spain, Sweden, the Netherlands and the United Kingdom. Five MSs were excluded from the analysis because of lack of sufficient data as explained in Section 2.2.2.
The results of the baseline model are presented in Table 7. As explained above, the baseline model applied to the extent possible reported monitoring (prevalence) data and human surveillance data from 2016, except for slaughter pigs, where for most countries the BLS data from 2006 to 2007 were used. The results indicate that 11.7% (with 95% credibility interval according to Bayesian modelling CrI95[5.7; 22.2]) of the true human salmonellosis cases (i.e. estimated number of cases when accounting for underestimation) in the EU were attributed to the laying‐hen reservoir. This corresponds to 465,200 CrI95[212,100; 979,800] human cases in 2016.
Table 7.
Estimated number and percentage (%) of human salmonellosis cases in EU attributable to the four main animal reservoirs included in the baseline model
| Animal reservoir | Estimated number of human casesa | Percentage of human cases | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | 2.5% | 97.5% | Mean | Median | 2.5% | 97.5% | |
| Broilers | 1,018,000 | 878,600 | 404,600 | 2,465,000 | 24.9 | 23.6 | 11.1 | 45.7 |
| Layers | 465,200 | 420,600 | 212,100 | 979,800 | 11.7 | 10.9 | 5.7 | 22.2 |
| Pigs | 1,718,000 | 1,534,000 | 753,600 | 3,781,000 | 41.5 | 41.5 | 23.1 | 59.7 |
| Turkeys | 306,600 | 282,600 | 144,400 | 610,300 | 7.5 | 7.5 | 4.7 | 10.6 |
| Total cases b | 4,083,000 | 3,838,000 | 2,220,000 | 7,390,000 | ||||
Accounting for underreporting.
Includes unknown and travel (accounting for a mean of 575,200 cases i.e. 14.1%).
For the other animal‐food sources included in the model, the attribution estimates were that 41.5% CrI95[23.1–59.7], 24.9% CrI95[11.1; 45.7] and 7.5% CrI95[4.7; 10.6] of the estimated number of human salmonellosis cases could be attributed to the pig, broiler and turkey reservoir, respectively. In total, 14.1% of human cases could not be attributed to any of the included sources. A proportion of these were reported as known travel‐related. The estimated number of true human salmonellosis cases in the EU in 2016 was 4.08 million CrI95[2.22; 7.39]. The outbreak figures in Section 3.1.1.1, give a much higher attribution to ‘eggs and egg products’ thereof’, being involved in 70.6% of the outbreaks and 63.7% of the cases when comparing with ‘broiler meat (Gallus gallus) and products thereof’, ‘pig meat and products thereof’, ‘turkey meat and products thereof’ and ‘bovine meat and products thereof’. These were involved in 11.0%, 13.1%, 1.2%, and 4.1% of the outbreaks and 9.9%, 18.7%, 5.3% and 2.4% of the outbreak cases, respectively.
In 2010, the estimated number of true human salmonellosis cases was 5.41 million CrI95[3.03; 9.51] (EFSA BIOHAZ Panel, 2011a, 2012). It should be noted though that two model outcomes are not directly comparable as fewer MSs are included in the current model and therefore the estimated number of cases is expected to be lower (both models excluded Bulgaria, while the 2010 model included Cyprus, Lithuania, Luxembourg and Romania, whereas the present model did not, but it did include Croatia and Malta, while the 2010 model did not).
At the EU population level, the baseline model estimated the pig reservoir to be the most important source of human infections, followed by the broiler and laying hen reservoir. However, when looking at the relative risk between laying hens/eggs and the other three sources weighted by the tonne of meat/eggs available for consumption, this picture changes, indicating that the risk of infection for the individual consumer is highest when consuming turkey meat followed by the consumption of broiler meat, whereas the risk for consuming pig meat was lower and almost the same as for eggs/meat from spent hens (i.e. the laying hen reservoir) (Table 8). The relative risks can be interpreted as the probability of acquiring salmonellosis for the individual consumer when consuming e.g. 100 g of turkey meat is almost two times higher than when eating 100 g of shell eggs.
Table 8.
Relative risk of human salmonellosis in EU per kg meat/eggs eaten attributable to the four main animal reservoirs included in the baseline model
| Broilers | Layers | Pigs | Turkeys | |
|---|---|---|---|---|
| Available for consumption (× 1,000 tonnes) | 11,382 | 6,264 | 22,676 | 2,107 |
| Cases per tonne | 0.0894 | 0.0743 | 0.0758 | 0.1455 |
| Risk relative to laying hens/eggsa | 1.20 | 1 | 1.02 | 1.96 |
The risk ratios can be interpreted as the risk of salmonellosis for the individual consumer when consuming e.g. 100 grams of turkey meat is two times higher than when eating 100 grams of eggs.
The results presented in Table E.3 in Appendix E show the distribution of the estimated laying hen‐associated cases by serovar. Around 95.4% of the laying hen‐associated human salmonellosis cases were caused by the currently regulated serovars, and S. Enteritidis is still by far the most important serovar, representing 93.3% of human cases. Table E.4 in Appendix E provides this information for all the reservoirs included in the model. Most (40.9%) of the estimated 2.19 million human cases involving S. Enteritidis could be attributed to the broiler reservoir, followed by 33.2% attributed to pigs, 19.8% to layers and 6.1% to turkeys. For S. Typhimurium and its monophasic variant, the majority (87.2% of 0.48 million human cases and 91.5% of 0.45 million human cases, respectively) could attributed to the pig reservoir. Only 1.67% and 0.46%, respectively, could be attributed to the laying hen reservoir.
Table E.3.
Number of human salmonellosis true cases (i.e. accounting for under‐ascertainment and underreporting) by the 28 serovars included in the model and originating from the laying hen reservoir as estimated by the source attribution model (SAM) (baseline model)
| Serovar | % of totala | Mean | Median | 95% CrI |
|---|---|---|---|---|
| ENTERITIDIS | 93.3 | 433,800 | 390,100 | [194,500; 932,200] |
| BRAENDERUP | 2.09 | 9,703 | 6,629 | [1,242; 36,820] |
| TYPHIMURIUM | 1.74 | 8,087 | 7,269 | [3,346; 17,680] |
| NAPOLI | 0.641 | 2,983 | 2,377 | [689.7; 8,887] |
| MONOPHASIC TYPHIMURIUM | 0.441 | 2,052 | 1,866 | [882.7; 4,335] |
| INFANTIS | 0.357 | 1,663 | 1,491 | [703.6; 3,646] |
| AGONA | 0.209 | 973.5 | 815.5 | [301.6; 2,598] |
| KENTUCKY | 0.201 | 935.3 | 778.8 | [290; 2,504] |
| VIRCHOW | 0.148 | 689.3 | 505.8 | [141.5; 2,364] |
| HAVANA | 0.134 | 622.2 | 515.6 | [175; 1,703] |
| NEWPORT | 0.114 | 529.2 | 469.9 | [206.6; 1,195] |
| MONTEVIDEO | 0.100 | 467.4 | 377.8 | [146.7; 1,331] |
| HADAR | 0.0965 | 446.8 | 349.1 | [103; 1,376] |
| LIVINGSTONE | 0.0958 | 445.5 | 385.4 | [167; 1,082] |
| TENNESSEE | 0.0944 | 438.9 | 372 | [151.4; 1,120] |
| ANATUM | 0.0766 | 356.2 | 278.8 | [94.76; 1,082] |
| INDIANA | 0.0451 | 209.9 | 162.7 | [50.85; 654.2] |
| MBANDAKA | 0.0357 | 166.1 | 149 | [68.21; 365.1] |
| BRANDENBURG | 0.0352 | 163.9 | 107.7 | [18.76; 653] |
| BOVISMORBIFICANS | 0.0287 | 133.5 | 67.82 | [2.187; 659] |
| GOLDCOAST | 0.0180 | 83.72 | 57.46 | [9.974; 314.5] |
| DERBY | 0.0130 | 60.37 | 52.42 | [20.16; 146.8] |
| SENFTENBERG | 0.0121 | 56.36 | 50.35 | [22.59; 125] |
| RISSEN | 0.0102 | 47.23 | 40.25 | [14.94; 120.7] |
| LONDON | 0.00700 | 32.56 | 23.2 | [4.641; 115.5] |
| SAINTPAUL | 0.00656 | 30.52 | 24.97 | [7.587; 86.17] |
| JAVA | 0.0000631 | 0.2937 | 0.1962 | [0.03122; 1.127] |
| WELTEVREDEN | 0 | 0 | 0 | [0; 0] |
CrI: credibility interval.
Based on the mean value.
Table E.4.
Number of human salmonellosis true cases (i.e. accounting for under‐ascertainment and underreporting) by serovar and animal reservoir as estimated by the source attribution model (SAM)
| Serovar | Broilers | Layers | Pigs | Turkeys | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| ENTERITIDIS | 896,900 | 760,300 | [312,000; 2,300,000] | 433,800 | 390,100 | [194,500; 932,200] | 727,900 | 635,000 | [283,000; 1,730,000] | 134,300 | 120,900 | [59,270; 287,700] |
| AGONA | 1,352 | 1,253 | [661.5; 2,617] | 973.5 | 815.5 | [301.6; 2,598] | 4,458 | 3,432 | [1,102; 13,880] | 14,450 | 12,160 | [5,084; 37,470] |
| ANATUM | 1,007 | 913.9 | [451.2; 2,114] | 356.2 | 278.8 | [94.76; 1,082] | 2,882 | 2,136 | [556.7; 9,629] | 410.6 | 295.5 | [60.6; 1,453] |
| BOVISMORBIFICANS | 0 | 0 | [0; 0] | 133.5 | 67.82 | [2.187; 659] | 35,440 | 30,170 | [12,620; 89,210] | 1,820 | 1,665 | [835.9; 3,731] |
| BRAENDERUP | 2,849 | 2,523 | [1,063; 6,545] | 9,703 | 6,629 | [1,242; 36,820] | 0 | 0 | [0; 0] | 0 | 0 | [0; 0] |
| BRANDENBURG | 0 | 0 | [0; 0] | 163.9 | 107.7 | [18.76; 653] | 17,050 | 14,260 | [6,257; 44,450] | 1,210 | 1,041 | [413.7; 3,005] |
| DERBY | 1,113 | 957.4 | [431.7; 2,723] | 60.37 | 52.42 | [20.16; 146.8] | 40,610 | 36,340 | [17,720; 88,870] | 4,831 | 4,408 | [2,212; 9,952] |
| GOLDCOAST | 246.7 | 217.7 | [100.6; 565.1] | 83.72 | 57.46 | [9.974; 314.5] | 7,336 | 6,584 | [3,249; 15,960] | 0 | 0 | [0; 0] |
| HADAR | 486.7 | 423.5 | [176.3; 1,174] | 446.8 | 349.1 | [103; 1,376] | 0 | 0 | [0; 0] | 9,371 | 7,386 | [2,622; 27,860] |
| HAVANA | 1,808 | 1,473 | [541.4; 5,088] | 622.2 | 515.6 | [175; 1,703] | 1,403 | 1,016 | [239; 4,854] | 0 | 0 | [0; 0] |
| INDIANA | 715.1 | 628.6 | [296.2; 1,648] | 209.9 | 162.7 | [50.85; 654.2] | 0 | 0 | [0; 0] | 2,278 | 2,008 | [888.5; 5,219] |
| INFANTIS | 53,300 | 49,000 | [27,020; 105,000] | 1,663 | 1,491 | [703.6; 3,646] | 2,990 | 2,645 | [1,305; 6,756] | 10,200 | 9,396 | [4,832; 20,320] |
| JAVA | 2,869 | 2,635 | [1,384; 5,712] | 0.2937 | 0.1962 | [0.03122; 1.127] | 0 | 0 | [0; 0] | 16.17 | 14.91 | [7.738; 32.05] |
| KENTUCKY | 3,365 | 2,732 | [1,038; 9,458] | 935.3 | 778.8 | [290; 2,504] | 5.017 | 2.958 | [0.1969; 22.23] | 14,760 | 12,260 | [4,971; 39,510] |
| LIVINGSTONE | 2,414 | 2,074 | [921; 5,940] | 445.5 | 385.4 | [167; 1,082] | 341.7 | 308.4 | [153.2; 727.3] | 108.1 | 92.75 | [36.22; 272.2] |
| LONDON | 38.05 | 29.4 | [9.327; 117.8] | 32.56 | 23.2 | [4.641; 115.5] | 6,408 | 5,845 | [2,989; 13,080] | 182.2 | 157.3 | [65.18; 447.5] |
| MBANDAKA | 2,058 | 1,859 | [902.2; 4,391] | 166.1 | 149 | [68.21; 365.1] | 163.7 | 141.6 | [62.19; 393.6] | 137.7 | 119.8 | [52.2; 330.7] |
| MONOTYPH | 5,949 | 5,430 | [2,856; 12,150] | 2,052 | 1,866 | [882.7; 4,335] | 411,400 | 367,400 | [181,100; 900,700] | 300,00 | 25,800 | [10,870; 74,280] |
| MONTEVIDEO | 1,341 | 1,204 | [614.2; 2,910] | 467.4 | 377.8 | [146.7; 1,331] | 975.8 | 734.6 | [209.4; 3,214] | 503.4 | 441.1 | [195; 1,182] |
| NAPOLI | 7,203 | 6,554 | [3,381; 14,860] | 2,983 | 2,377 | [689.7; 8,887] | 0 | 0 | [0; 0] | 0 | 0 | [0; 0] |
| NEWPORT | 1,647 | 1,364 | [543.5; 4,443] | 529.2 | 469.9 | [206.6; 1,195] | 5,335 | 4,025 | [1,216; 17,220] | 21,330 | 19,510 | [10,320; 42,880] |
| RISSEN | 54.63 | 47.8 | [23.21; 126.8] | 47.23 | 40.25 | [14.94; 120.7] | 31,110 | 26,290 | [10,480; 80,160] | 0 | 0 | [0; 0] |
| SAINTPAUL | 749.2 | 611.6 | [258; 2,066] | 30.52 | 24.97 | [7.587; 86.17] | 0 | 0 | [0; 0] | 13,050 | 11,820 | 5,746; 27,580] |
| SENFTENBERG | 946.6 | 818.6 | [358.4; 2,297] | 56.36 | 50.35 | [22.59; 125] | 180.6 | 147.9 | [58.14; 500.5] | 2,928 | 2,596 | [1,140; 6,702] |
| TENNESSEE | 538.7 | 466.9 | [216.3; 1,294] | 438.9 | 372 | [151.4; 1,120] | 208.2 | 187 | [87.76; 451.8] | 0 | 0 | [0; 0] |
| TYPHIMURIUM | 13,540 | 12,590 | [7,063; 25,630] | 8,087 | 7,269 | [3,346; 17,680] | 421,400 | 382,600 | [197,100; 872,300] | 40,420 | 36,160 | [16,510; 89,240] |
| VIRCHOW | 12,630 | 10,800 | [5,042; 31,170] | 689.3 | 505.8 | [141.5; 2,364] | 365.6 | 320 | [156.6; 848.2] | 4,333 | 3,165 | [760.3; 14,930] |
| WELTEVREDEN | 3,247 | 2,881 | [1,382; 7,283] | 0 | 0 | [0; 0] | 0 | 0 | [0; 0] | 0 | 0 | [0; 0] |
CrI: credibility interval.
The higher attribution to the broiler reservoir, especially for S. Enteritidis cases, was unexpected but can be explained by particularly high reported prevalence in two MSs (the Czech Republic and Poland) that contributed substantially to the number of broiler‐related S. Enteritidis cases. Therefore, an additional model was run excluding these two countries, which resulted in a decline in the mean number of broiler‐related S. Enteritidis cases from 896,900 broiler‐related cases to 130,000 cases. Similarly, the exclusion of these two MSs from the model would result in a relative risk of broiler meat consumption compared to egg consumption of 0.39 (broiler‐related cases per tonne: 0.0259; egg‐related cases per tonne: 0.0662).
The high attribution of S. Enteritidis cases to the pig reservoir has also been observed in previous studies (Hald et al., 2012a). In this study, around 662,000 (91%) of the 728,000 pork‐associated S. Enteritidis cases could be explained by four MSs (Spain, Portugal, Greece and Hungary), which were also the countries having the highest prevalence of S. Enteritidis in pigs ranging from 1.15% to 1.52%). In comparison, the average prevalence in the other 19 MSs was 0.2%. Salmonella Enteritidis was also found in laying‐hen flocks in the same four MSs with prevalence ranging from 0.95% to 4.00%, and it is considered that the model, because of lack of further subtyping information, was unable to distinguish sufficiently between the pig and laying hen reservoir, resulting in more cases than expected being attributed to the pig reservoir. In addition, the serovar data used for pigs in three of these MSs (Greece, Hungary and Portugal) were from the BLS, and therefore likely to not represent the situation in 2016.
As mentioned in Section 2.1.2.1, data from the cattle reservoir were insufficient to allow for its inclusion in the model. The consequence of this may be that a proportion of human cases were wrongly attributed to reservoirs with similar serovar distributions. If more cases were attributed to the laying‐hen reservoir because of this, it may have resulted in a higher reduction of the estimated number of both absolute and relative human cases in the scenario analyses than what would occur in reality. Overall, it is considered unlikely that the lack of available data from other food sources biases the model result significantly, as long as it is kept in mind that SAM only attributes domestic and sporadic cases to EU animal reservoirs.
3.2.3. Impact of new Salmonella target in laying hen flocks
The results of the baseline model were compared with the scenario in which the combined prevalence of the current target serotypes (S. Enteritidis and S. Typhimurium including monophasic strains) in laying hen flocks was set at 1%. The prevalence was kept as reported if it was already below 1%. It should be noted that the estimated changes to human incidence rates only apply to the MSs included in the model.
Table 9 provides the overall summary statistics for each output and scenario. The scenario results in an estimated reduction in the number of layer‐associated human salmonellosis cases of 53.38% CrI95[39.11; 65.69] compared to the situation in 2016. In absolute numbers, this corresponds to an estimated reduction of 254,400 cases CrI95[98,540; 602,700] out of the total 4.08 million human salmonellosis number of estimated true cases, considering the 23 MSs included in the model. This reduction would translate into a 6.2% reduction of the 4.08 million CrI95[2.22; 7.39] human salmonellosis estimated true cases.
Table 9.
Estimated number and percentage (%) of human salmonellosis cases in EU originating from the laying hen reservoir under the scenario where the prevalence of the current target serovars (S. Enteritidis and S. Typhimurium including monophasic strains) was set at a maximum of 1% (or less if the prevalence was already below 1%)
| Mean | Median | 2.5% | 97.5% | |
|---|---|---|---|---|
| Estimated number of human cases (baseline) | 465,200 | 420,600 | 212,100 | 979,800 |
| Estimated number of human cases (scenario) | 210,800 | 195,400 | 104,000 | 407,200 |
| Absolute difference baseline vs scenario | 254,400 | 222,100 | 98,540 | 602,700 |
| Percentage difference baseline vs scenario | 53.38 | 53.75 | 39.11 | 65.69 |
3.2.4. Model validation, assumptions and data uncertainty
Results of the goodness of fit ratio (i.e. the reported divided by the estimated number of cases per MS) showed that the model fit was satisfactory for the vast majority of the countries. Poor fit was in particular observed for countries with estimated low underreporting factors (Finland and Sweden). However, since these MS contributed less than 3,000 true cases to the total figure, their impact was considered minimal, but the results do suggest revisiting the estimated underreporting factors. The goodness of fit ratios for the Czech Republic and Poland were both very close to 1 (0.997 and 0.993, respectively) and could not help explain the estimated high number of broiler‐related cases in these MSs.
The attribution of human Salmonella infections to food‐animal sources in the EU on the basis of available data implied a number of assumptions:
All major food sources of human salmonellosis in EU are included in the model;
The sampling schemes and data collection of the EU harmonised monitoring programs in broilers, laying hens and turkeys, and the BLS of slaughter pigs generate prevalence data that are representative of the included animal reservoirs and MSs;
The serovar distribution data used for each animal reservoir are representative of the included animal reservoirs and MSs;
The food‐borne outbreak reporting system captures all large Salmonella outbreaks with around the same detection sensitivity in each MSs;
The TESSy generates data that are representative of the occurrence of human salmonellosis in each MSs as well as the serovar distribution;
If travel information was not recorded, or was reported as ‘Unknown’, and it was not possible to estimate an additional number of travellers based on reported travel data, the human Salmonella infection was assumed to be acquired in the country where it was reported;
The EUROSTAT production and trade data reflects the true flow of food in EU and food imported into a country is generally also consumed in that country, unless the amount produced in a country is less than the amount exported; in such cases re‐exportation of imported food was assumed;
The ability of a Salmonella serovar to cause infection is a characteristic of the serovar and independent of time period and country of isolation;
Food preparation practices and consumption patterns influence the estimated ability of a food source to act as a vehicle for infection, and so the source‐dependent factors may vary from country to country.
Due to data limitations and data uncertainty, it is obvious that some of the above assumptions can be questioned. It is not possible to quantify the effect of these assumptions and data uncertainties may have on the model results, but the most important data issues identified have been included in Table F.2 in Appendix F.
A similar model to the SAM is applied annually to report on the contribution of different sources to human salmonellosis cases in Denmark (de Knegt et al., 2016). The data sources used in 2016 were quite different, particular for the pig reservoir and for imported food, where prevalence and serovar data were obtained from extensive monitoring of pork carcasses at Danish slaughterhouses and from monitoring of imported meat at importers’ premises. In addition, the Danish model used multiple locus variable number tandem repeat analysis (MLVA) typing and resistance profiling to further distinguish strains within S. Typhimurium, monophasic Typhimurium and S. Enteritidis. Despite these differences, it was considered useful to compare the results from the Danish model with the Danish results from the EU model. For broilers and layers, the two models agreed well (broilers: 29% DK model and 31% EU model; laying hens: 12% DK model and 10% EU model). In contrast, the proportion of attributed cases from pigs/pork was considerably larger according to the DK model than the EU model (57% vs 22%), whereas the opposite was the case for turkeys (2% vs 37%). It is well recognised in Denmark that pork is a major source of domestic human salmonellosis, particularly S. Typhimurium and monophasic Typhimurium. The lack of further subtyping data, (especially for S. Typhimurium, monophasic Typhimurium and S. Enteritidis) in the EU data that would allow for better distinction between reservoirs with similar serovar distribution, as well as the low‐quality EU data on the pig reservoir are assessed to be the main reasons for the observed discrepancies between the DK and EU model.
Despite the relatively limited estimated contribution of the laying hen reservoir calculated by the model, effective control of S. Enteritidis in laying hens is essential because of the important role of eggs in human infections and outbreaks. If the contribution of the laying hen reservoir to human cases has been underestimated, for example because of failure to identify infected laying flocks resulting in a substantially lower reported prevalence than is actually the case, as has been shown in recent outbreak investigations (EFSA and ECDC, 2017a; ECDC, 2018), and studies cited below, the potential impact of a lower prevalence target would be greater if it leads to improved control of S. Enteritidis. A stricter target for laying hens is expected to be exceeded when S. Enteritidis‐infected flocks remain in production for heat‐treated eggs, as most laying farms comprise multiple age flocks and if infected hens remain, there is an increased probability of transmission to other flocks on the holding (Dewaele et al., 2012). The true prevalence and impact of national targets is difficult to assess because of a low detection rate in flocks using limited numbers of faecal samples. This mean that some infected flocks may only be detected months after introduction of infection, or in some cases may not be detected at all (Riemann et al., 1998; Van Hoorebeke et al., 2009; Arnold et al., 2010; Arnold et al., 2011; Arnold et al., 2014a,b). Culling of infected laying hen flocks, followed by effective decontamination of housing, is therefore a more effective control measure than heat treatment of eggs.
It is also possible that changes in vaccination programmes used in the EU for laying‐hen flocks against S. Enteritidis, now that vaccination for S. Enteritidis is no longer obligatory under EU regulations in any MS as the 10% flock prevalence is not exceeded, may play a role in the increasing occurrence of S. Enteritidis. For instance, certain live vaccines are more difficult to administer effectively via long complex drinker lines in large laying houses, because of limited stability once reconstituted (Davies and Carrique‐Mas, 2010a) so may provide more limited protection. Similarly, complacency in implementation of biosecurity measures such as pest control and terminal hygiene between flock cycles may have followed the initial success of the Salmonella control programmes leading to a greater risk of introduction or recurrence of infection. It is therefore recommended that further investigation of these aspects is carried out, including gathering more detailed information on the diverse vaccination practices in different EU MS.
3.2.5. Concluding remarks and recommendations
The estimated number of all human salmonellosis true cases (i.e. accounting for under‐ascertainment and underreporting) in the 23 EU Ms included in the Salmonella SAM in 2016 was 4.08 million CrI95[2.22; 7.39].
11.7% CrI95[5.7; 22.2] of the cases were attributed to the laying hen reservoir. This is estimated to correspond to around 465,200 human true cases CrI95[212,100; 979,800] in 2016.
For the other Salmonella sources included, the SAM estimated that 41.5% CrI95[23.1–59.7], 24.9% CrI95[11.1; 45.7] and 7.5% CrI95[4.7; 10.6] of the estimated number of human salmonellosis true cases could be attributed to the pig, broiler and turkey reservoirs, respectively. Around 14% of human cases could not be attributed to any of the included sources. A proportion of these were reported as known travel‐related.
The SAM estimated that per tonne of food available for consumption, table eggs were associated with a similar risk as pig meat (0.07 and 0.08 cases per tonne, respectively). The risks associated with broiler meat and in especially turkey meat were higher (0.09 and 0.15 cases per tonne, respectively).
Around 95.4% of the layer‐associated human salmonellosis true cases were caused by the currently regulated serovars. Most (40.9%) of the estimated 2.19 million human cases of S. Enteritidis could be attributed to the broiler reservoir, followed by 33.2% attributed to pigs, 19.8% to layers and 6.1% to turkeys.
For S. Typhimurium and its monophasic variant, the majority (87.2% of 0.48 million human cases and 91.5% of 0.45 million human cases, respectively) could be attributed to the pig reservoir. Only 1.67% and 0.46%, respectively, could be attributed to the laying hen reservoir.
These conclusions are based on analysis of retrospectively collected data. The Salmonella situation in the EU is, however, dynamic, and ‐ the food animal reservoir‐ associated risks and the relative importance of the different serovars are expected to change over time and data quality may improve. Therefore, it is important to review the model and its conclusions as new information emerges.
In 2016, 7 MS exceeded the 2016 2% flock prevalence targets in laying hen flocks for S. Enteritidis and S. Typhimurium including the monophasic strain. In total, 15 MS exceeded 1% during 2016, including some of the major egg producing and exporting countries.
If a 1% target of the EU control programme of Salmonella in laying hen flocks would be met in the 23 EU MSs, the number of layer‐associated human salmonellosis true cases is estimated to be reduced by 53.38% CrI95[39.11; 65.69] compared to the situation in 2016.
In absolute numbers, this corresponds to an estimated reduction of 254,400 true cases CrI95[98,540; 602,700] associated with the laying‐hen reservoir. This reduction would translate in a 6.2% reduction of the overall 4.08 CrI95[2.22; 7.39] million human salmonellosis estimated true cases.
Investigation of the potential and reasons for under‐detection of Salmonella, particularly S. Enteritidis, in flocks of laying hens and field investigations on the effectiveness of administration of Salmonella vaccination programmes used in laying flocks and their protective effect are recommended.
An EU‐wide survey of Salmonella in cattle or beef would help to investigate the role of the cattle reservoir as a source of human infections and in pigs or pork to obtain more recent and comparable data in order to reduce the uncertainty on the role of the pig reservoir as a source of human infections.
3.3. Risk factors for the occurrence of Salmonella in laying hens in relation to the farming methods and other animal welfare indicators
The risk/protective factor(s) in relation to the farming systems for laying hen production or breeding flocks considered, based on the available evidence, were: outdoor access, cage systems, alternative systems, age of the production system and previous Salmonella infection, farm size, group size and stocking density, the presence of rodents, and cleaning and disinfection. The animal welfare indicators considered were: stress (other than heat stress), heat stress, moulting, activity/behaviour, body status (e.g. FPD) and other diseases.
The assessment was based on both literature review, mainly focussed on studies in the EU and equivalent high‐income countries, on the occurrence of Salmonella in laying hen flocks housed in different farming systems, and based on the data requested from the MS. More information on housing systems is provided in Appendix A and elsewhere.29
3.3.1. Literature data
Table C.3 in Appendix C provides an overview of the studies dealing with the occurrence of Salmonella in laying hens in relation to the farming methods and other animal welfare indicators.
Table C.3.
Overview of studies dealing with the occurrence of Salmonella in laying hens (production flocks or hen's eggs) in relation to the farming methods and other animal welfare indicators
| Reference | Country | Welfare aspects or housing systems considered | Which risk/protective factor(s) in relation to the farming system have been evaluated? | Which animal welfare indicators are described? | Other risk factors considered |
|---|---|---|---|---|---|
| Field studies | |||||
| Adesiyun et al. (2014) | Trinidad and Tobago | YES | Cage systems; Alternative systems; Farm size | None | YES |
| Carrique‐Mas et al. (2009) | United Kingdom | YES | Cage systems (step cages/cage‐scraper/cage belt); Biosecurity | None | YES |
| Chemaly et al. (2009) | France | YES | Group size; Rearing of pullets in the same place as layers; Biosecurity | None | YES |
| Davies and Breslin (2001) | United Kingdom | YES | Cage systems; Alternative systems; Vaccination | None | YES |
| Davies and Breslin (2003) | United Kingdom | YES | Cage systems (conventional cage); Alternative systems; Biosecurity, Cleaning and disinfection; Presence of rats and mice | None | YES |
| Galis et al. (2012) | Romania | YES | Outdoor access; Cage systems; Alternative systems | None | NO |
| Garber et al. (2003) | USA | YES | Alternative systems; Biosecurity; Cleaning and disinfection, Moulting | None | YES |
| Geue and Schluter (1998) | Germany | YES | Cage systems; Alternative systems | None | YES |
| Green et al. (2009) | USA | YES | Cage systems (high rise/manure belt); Stocking density | Heat stress | YES |
| Huneau‐Salaun et al. (2007) | France | YES | Cage systems; Alternative systems | None | YES |
| Huneau‐Salaun et al. (2009) | France | YES | Cage systems; Biosecurity; farm size | None | YES |
| Jones et al. (2012) | USA | YES | Outdoor access, Cage systems (CC) | None | NO |
| Jones et al. (2015) | USA | YES | Cage systems (CC, enriched cage); Alternative systems | None | NO |
| Jones et al. (2016) | USA | YES | Cage systems; Alternative systems | None | NO |
| Kaufmann‐Bart and Hoop (2009) | Switzerland | YES | Alternative systems | None | NO |
| Lassnig et al. (2011) | Austria | YES | Outdoor access, Cage systems; Alternative systems | None | NO |
| Lee et al. (2013) | Republic of Korea | YES | Outdoor access; Cage systems | None | NO |
| Matsumoto et al. (2001) | Japan | YES | Presence of windows in stable | None | YES |
| Methner et al. (2006) | Germany | YES | Outdoor access, cage systems (alternative systems) | None | NO |
| Mollenhorst et al. (2005) | Netherlands | YES | Outdoor access; Cage systems; Group size; flock of different age | None | YES |
| Much et al. (2007) | Austria | YES | Cage systems (traditional, barn), Alternative systems | None | YES |
| Namata et al. (2008) | Belgium | YES | Outdoor access, cage systems (traditional cages, barn) | None | YES |
| Nistor et al. (2015) | Romania | YES | Cage systems; Alternative systems | None | YES |
| Ovelhey et al. (2009) | Germany | YES | Outdoor access; vaccination; farm size | None | YES |
| Pieskus et al. (2008b) | Lithuania | YES | Cage systems (aviary and enriched cages) | None | NO |
| Proietti et al. (2001) | Italy | YES | Outdoor access; Cage systems (traditional) | None | NO |
| Proietti et al. (2004) | Italy | YES | Cage systems (traditional) | None | NO |
| Sasaki et al. (2012) | Japan | YES | Outdoor access | None | NO |
| Schaar et al. (1997) | Germany | YES | Cage systems; Alternative systems | None | YES |
| Schulz et al. (2009) | Germany | YES | Outdoor access; Cage systems (conventional); Alternative systems | None | YES |
| Snow et al. (2010) | United Kingdom | YES | Outdoor access, Cage systems (traditional/barn); Alternative systems; Biosecurity; Vaccination; Farm size | None | YES |
| Sunagawa et al. (1997) | Japan | YES | Cage systems; Presence of windows in stable | None | YES |
| Tamba et al. (2000) | Italy | YES | Cage systems (traditional), vaccination; farm size | None | YES |
| Van Der Zijpp et al. (2006) | Netherlands | YES | Cage systems; alternative systems; type of litter | Activity/behaviour, mortality | NO |
| Emous and Fiks‐van Niekerk (2004) | Netherlands | YES | Alternative systems | Mortality | NO |
| Van Hoorebeke et al. (2010a) | Belgium | YES | Cage systems, Alternative systems, flock size, age of hens, age of infrastructure | None | YES |
| Van Hoorebeke et al. (2010b) | Belgium | YES | Outdoor access, Cage systems (conventional/aviary); Alternative systems; Biosecurity; farm size | None | YES |
| Wierup et al. (2017) | Sweden | YES | Outdoor access | None | NO |
| Experimental studies | |||||
| De Vylder et al. (2009) | Belgium | YES | Cage systems (conventional, enriched, aviary) | None | NO |
| de Vylder et al. (2011) | Belgium | YES | Cage systems; Alternative systems; Group size | None | NO |
| Gast et al. (2013) | USA | YES | Cage systems (CC, enriched cage) | None | NO |
| Gast et al. (2014a) | USA | YES | Cage systems (CC, enriched cage) | None | NO |
| Gast et al. (2014b) | USA | YES | Cage systems (CC, enriched cage) | None | NO |
| Gast et al. (2015) | USA | YES | Cage systems; Alternative systems | None | NO |
| Gast et al. (2017b) | USA | YES | Cage systems (enriched cages); Stocking density | None | YES |
| Gast et al. (2017a) | USA | YES | Cage systems (enriched cage); Stocking density | None | YES |
| Gast et al. (2017c) | USA | YES | Cage systems (enriched cage); Stocking density | None | YES |
| Hannah et al. (2011) | USA | YES | Cage systems; Alternative systems | None | NO |
| Holt and Porter (1992) | USA | YES | Moulting | None | YES |
| Holt (1995) | USA | YES | Moulting | None | YES |
| Holt et al. (1998) | USA | YES | Moulting | None | YES |
| Koelkebeck et al. (1987) | USA | YES | Cage systems; Stocking density | Stress (not heat stress); Activity/behaviour | YES |
| Kubena et al. (2005) | USA | YES | Moulting | None | NO |
| Murase et al. (2006) | Japan | YES | Moulting | None | NO |
| Nordentoft et al. (2011) | Denmark | YES | Cage systems; Alternative systems | None | NO |
| Parisi et al. (2015) | USA | YES | Outdoor access; Cage systems (CC); Alternative systems | None | NO |
| Posadas Hernandez et al. (2005) | Mexico | YES | Outdoor access, cage systems (traditional) | Stress (not heat stress) | NO |
| Thomas et al. (2011) | Netherlands | YES | Group size | None | NO |
| Zongo et al. (2015) | France | YES | Cage systems (enriched cage); Group size | None | NO |
The papers have been screened for internal and external validity and some limiting factors have been identified:
Limited sample size on both farm and individual sample level;
Existence of confounding factors not (properly) addressed in some studies;
Diversity in sample matrix and laboratory analysis conducted;
Non‐recent data in studies.
Most of the studies do not report on the odds ratio (OR) or relative risk (RR) of the risk/protective factors and therefore a meta‐analysis was not conducted. Whenever available, this information is provided in the summary below.
3.3.1.1. Impact of cage vs non‐cage farming (alternative systems)
Results from studies investigating the effect of housing systems/farming methods on Salmonella prevalence/transmissions/persistence in laying hen populations are very diverse and sometimes conflicting. This could be due to differences in the methodology used and the populations being studied in different countries. The literature found can be divided into three groups: (1) studies that concluded that the occurrence of Salmonella in cage systems was higher than in alternative systems; (2) studies that concluded occurrence of Salmonella was lower in cage systems, and (3) studies that do not show any difference between cage and alternative systems.
Occurrence of Salmonella in cage systems was higher than in alternative systems
The majority of studies that compared cage to non‐cage systems in the EU found a higher Salmonella prevalence in laying hen flocks housed in conventional cages compared to alternative systems. These studies were mostly carried out before the ban on conventional cages in 2012. Small battery cages have been replaced by larger cages holding bigger groups of birds in which enrichment by installation of a nest boxes pecking and scratching area, claw shorteners and perches was provided. It has been speculated that replacement of conventional cages by enriched cages may have played a part in the initial reduction of S. Enteritidis in laying hens from 2009, when restrictions on sales of fresh eggs from infected flocks were introduced (ACMSF, 2016).
A higher occurrence of Salmonella in cage systems was observed in studies from multiple countries as cited by Holt et al. (2011), more specifically including in Germany (Methner et al., 2006), the United Kingdom (Snow et al., 2010; Wales et al., 2010), France (Mahe et al., 2008), and Belgium (Namata et al., 2008).
Furthermore, a retrospective epidemiological study in Denmark (Molbak et al., 2002) found that consumption of eggs from conventional cages was associated with human salmonellosis, whereas no association with eggs from free‐range or organic production was found.
Surveys in Austria (Lassnig et al., 2011) and France (Huneau‐Salaun et al., 2007), conducted in the framework of the EU‐wide BLS on Salmonella in laying flocks, found higher occurrence of Salmonella contamination in cage systems compared to alternative systems. In the latter study, it was hypothesised that better cleaning practices in non‐cage houses had a protective effect against Salmonella persistence. A later study from the same research group (Huneau‐Salaun et al., 2009) reported results from 519 flocks studied between October 2004 and September 2005. The Salmonella status of the flocks was assessed from five faeces and two dust samples analysed using a classical bacteriological method. At least one Salmonella‐positive sample was found in 93 flocks. The prevalence was significantly higher in caged flocks than in on‐floor flocks (30.9% in caged flocks vs 7.9% in on‐floor flocks; p < 0.001); the non‐adjusted OR associated with cage housing compared to on‐floor housing was 35.1 CI95[12.2; 101.1] (p < 0.001).
Several other studies also used the data collected as part of the BLS. In these, a significant difference between the housing systems was often observed, and cages were found to be a risk factor compared to non‐cage systems, as was also found in the overall analysis of the BLS (EFSA, 2007). Among the related studies, Snow et al. (2010) collected data from 380 laying hen holdings to investigate risk factors for Salmonella at farm level. Using a multivariable logistic model weighted to account for the survey design, non‐caged systems (OR 0.14 CI95[0.04; 0.49], p = 0.002), vaccination (OR 0.08 CI95[0.02; 0.38], p = 0.001), the use of a non‐company feed source (i.e. from independent feed mill) (OR 0.11 CI95[0.03; 0.47], p = 0.003), running the site as all‐in/all‐out (OR 0.06 CI95[0.01; 0.24], p < 0.001) and the presence of cats and dogs on the farm (OR 0.14 CI95[0.04; 0.50], p = 0.002) were associated with a reduced risk of being positive for S. Enteritidis. A similar study was conducted in Germany (Ovelhey et al., 2009). Based on the results of the univariable logistic regression model, the highest risk of Salmonella was found in cage systems, but confounding may have existed, as the authors could not separate the effect of different risk factors such as holding or flock size, and residual confounding was also deemed likely because of factors not considered in the BLS.
Similarly, Belgian data from the 2005 BLS (Namata et al., 2008) showed that flocks reared in cages were more likely to be positive compared to flocks reared in barns and free‐range systems. Moreover, controlling for other variables, rearing layer flocks in cages was still a significant risk factor whereas flock age, flock size and the season of sampling became borderline significant and Salmonella vaccination status was non‐significant.
In the Austrian BLS (Much et al., 2007), 96 cage and 241 non‐cage (72 barn, 100 free‐range standard and 69 free‐range organic) flocks were sampled. Salmonella was found in 34.4% of the cage flocks but only in 7.9% of the non‐cage flocks (more specifically in 15.3%, 7.0%, and 1.4% of the barn, free‐range standard and free‐range organic flocks, respectively).
Persistence of Salmonella laying hen houses in the United Kingdom was analysed by the use of survival analysis methodology applied to retrospective data relating to 264 reported Salmonella cases in 152 flocks of laying hens that occurred between 1998 and 2007 (Carrique‐Mas et al., 2009). For cases involving S. Enteritidis, both the level of rodent infestation of the houses and the housing system were positively associated with longer duration of detectable infection, especially in houses with deep manure pits, where high levels of mice or rats were harboured, compared to free‐range or barn houses that used deep litter and/or slatted flooring or cage systems that had manure belts instead of droppings pits.
Within the EU project Safehouse (Schulz et al., 2009), 92 German laying hens flocks (27 in conventional cages, 23 in floor‐raised systems, 25 in free‐range systems and 17 in organic free‐range systems) were intensively sampled for Salmonella between May 2007 and June 2008. Information on risk factors was collected through a questionnaire. In total, 30% of the flocks were positive for Salmonella. Lower occurrence was found in the alternative housing systems compared to conventional cages (conventional cages vs floor‐raised system OR = 9.73 CI95[2.73; 43.11], conventional cages vs free‐range OR = 8.82 CI95[2.45; 39.29], conventional cages vs organic free range OR = 13.32 CI95[2.89; 107.56]).
In a US field study, the prevalence of Salmonella colonisation of individual 77 week‐old laying hens was significantly higher (13.33%) in a conventional cage system than in an aviary (3.3%) or enriched (colony) cage system (5%) (120 hens per system) (Jones et al., 2016).
Occurrence of Salmonella in cage systems was lower than in alternative systems
Conversely, other studies found a lower occurrence of Salmonella in conventional cage systems compared to alternative systems, as cited by Holt et al. (2011), more specifically in the USA (Kinde et al., 1996), Germany (Schaar et al., 1997), and the Netherlands (Mollenhorst et al., 2005).
A more recent (2015) USA study compared the prevalence of Salmonella and other microorganisms in environmental swabs and eggshell pools from commercial farms using conventional cages, enriched cages, and aviary systems. The environmental swabs revealed that all farms were contaminated with Salmonella. Higher proportions of Salmonella‐positive swabs were obtained for samples collected from manure scrapers and the forage area from aviary systems and lower proportions in samples from the enriched colony cage system wire (16%) and nest box (16%) swabs. Nevertheless, there was no difference in the frequency of Salmonella contamination of eggshells, which remained low (0–8%), regardless of the housing system (Jones et al., 2015).
In a study on packed table eggs in Romanian supermarkets, Galis et al. (2012) detected Salmonella in the egg's albumen in 33% of the organic eggs, 28% of the free‐range eggs, 25% of the aviary‐obtained eggs and 14% of the cage production eggs. Thus, eggs from non‐cage systems were found to be more often contaminated than those from cage systems, and eggs from the outdoor systems were most often contaminated.
An experimental study on bird‐to‐bird transmission of Salmonella in layers showed a trend (but non‐significant) towards a higher risk of transmission of S. Enteritidis for birds housed in the aviary and floor systems compared with the conventional and enriched cage systems (de Vylder et al., 2011). Moreover, in this study significantly more internally contaminated eggs were laid by hens kept in an aviary compared with birds in the cage systems and the floor system. Differences in reproduction ratios were attributed to housing system‐specific characteristics such as hygienic status, air quality, and large group housing that would result in more intensive contact between birds and with litter and droppings in aviary/floor systems, thus increasing the risk of contact with faecally contaminated materials.
Mollenhorst et al. (2005) collected questionnaires on risk factors from farms submitted for serological investigation to detect S. Enteritidis; analysing information from about 1,900 flocks. The data set contained information on S. Enteritidis status, month and year of sampling, housing system (i.e., conventional cage with wet and dry manure, deep litter and aviary, with and without outdoor run), number of hens, vaccination against S. Enteritidis, and the presence of hens of different ages in the same house or on the farm. On farms with all hens of the same age, a flock kept in a cage system with wet manure had a significantly (OR = 0.26, p < 0.01) lower chance of infection with S. Enteritidis compared with a cage system with dry manure. For a farm with all hens of the same age, a deep litter system also had a significantly (OR = 0.47, p < 0.05) lower chance of infection with S. Enteritidis compared with a cage system with dry manure. The difference between a deep litter system and a cage system with wet manure was not significant. On a farm with hens of different ages, however, a deep litter system had a significantly (OR = 2.09 and OR = 2.91, p < 0.01) for cages with dry and wet manure) higher chance of infection with S. Enteritidis compared with both types of cage systems. An outdoor run increased the chance of infection with S. Enteritidis significantly (OR = 2.14, p < 0.05) for farms with all hens of the same age. On farms with hens of different ages, no effect of an outdoor run could be found. A higher number of hens increased the chance of infection with S. Enteritidis of a flock significantly (OR = 1.02, p < 0.01).
Housing system did not influence the occurrence of Salmonella
Finally, some studies did not observe a difference between the housing systems, as cited by Holt et al. (2011).
An exploratory field study in Belgium found Salmonella in 20.7% (6 out of 29) laying hen farms (of which 8 were conventional cage units, 10 floor‐raised, 8 free‐range, and 3 organic farms) using faecal and dust samples. Using multivariate logistic regression with the Salmonella status of the flock as an outcome variable, previous Salmonella contamination on the farm and the age of the production system were identified as risk factors for the presence of Salmonella in laying hens (p < 0.05), while the housing system did not have a significant influence on Salmonella prevalence (Van Hoorebeke et al., 2010a).
Non‐significant results were also reported in a small study in Lithuania (8 farms) where the prevalence of Salmonella in laying hens housed in an aviary system (n = 2) was similar to cages (n = 6) (Pieskus et al., 2008b). However, the absence of significant differences could be due to the limited discriminatory power of these studies.
In an experimental study by De Vylder et al. (2009), shedding and colonisation of layers housed in 3 different housing systems (conventional cage, furnished cage, and aviary) were measured at regular time points post‐inoculation with S. Enteritidis. The results did not show an increased risk for alternative housing systems compared with the conventional cage system.
An experimental study by Hannah et al. (2011) on horizontal transmission in conventional cages (0.06 m2/hen), slatted floor systems (0.6 m2/hen) and floor systems with shavings (0.6 m2/hen) again showed no impact of the housing system on the frequency of horizontal transmission from S. Enteritidis or S. Typhimurium‐inoculated hens to pen mates. Still, the recovery of Salmonella from the non‐challenged hens infected by contact was lower when they were housed in cages (15%), intermediate for hens on slats (20%), and highest for hens on shavings (38%). Results could be explained by the housing system effect in addition to the positive impact of a lower stocking density on the immune system.
3.3.1.2. Outdoor access and organic production as a factor
In the Austrian BLS on Salmonella in laying hens (2004–2006), the ranking of Salmonella prevalence went from cages to barns, to standard free‐range and to organic free‐range housing systems (with 34.4%, 15.3%, 7.0% and 1.4%, respectively). These results suggest that outdoor access and the possible contact of eggs with faeces or litter was not a risk factor for Salmonella infection (Lassnig et al., 2011).
In Sweden, the occurrence of Salmonella between 2007 and 2015 in broilers and laying hens in outdoor and indoor production was evaluated. There was no indication that the Salmonella exposure in outdoor poultry production was higher than in indoor production. The annual incidence of Salmonella‐infected flocks in outdoor production remained at a very low level, similar to that observed for indoor production (Wierup et al., 2017).
In the Netherlands in 2004, a field study on the sustainability of different housing systems was performed (Van Der Zijpp et al., 2006) which collected data from 16 farms with conventional cages, 15 with a deep litter system without outdoor run, 17 with a deep litter system with outdoor run and 13 with an aviary system with outdoor run. There was only one S. Enteritidis‐infected flock in this study, which resulted in no significant differences among housing systems.
An experimental study in the USA found no Salmonella on the shells of eggs produced in conventional cages vs 2.36% on eggs from free‐range systems. This suggests that the greater physical access to the eggs by the hens in free‐range systems may lead to a higher probability of contamination if Salmonella is present in the barn (Parisi et al., 2015). In contrast, a survey conducted in the same country found no difference in the level of environmental and egg (content/shell) Salmonella contamination over time in two sister flocks using conventional cage and free‐range housing systems. The small sample size (one flock per treatment), however, limits the external validity of these findings (Jones et al., 2012).
In a Mexican paper by Posadas Hernandez et al. (2005), an experimental study on laying hens was reported. Three hundred and sixty, 24‐week‐old, laying hens were used. The birds were randomly assigned into two treatments of 180 birds each. Treatments included 4 replicates of 45 birds distributed as follows: treatment 1, birds housed in conventional cages; and treatment 2, housed in pens with access to green areas. S. Enteritidis, isolated from internal organs, showed no statistical difference between treatments. Similar results were obtained in a field observational study in the US (Green et al., 2009), involving 3 types of laying‐hen houses (4 houses for each type), namely, high‐rise, manure‐belt, and cage‐free floor‐raised, which were monitored for air temperature, relative humidity (RH), CO2, and atmospheric ammonia under winter and summer conditions in Iowa. No statistical difference in Salmonella prevalence was identified, but non‐caged houses were only found to be negative for Salmonella during winter time.
3.3.1.3. Impact of the type of cage: conventional vs enriched cages
The mandate requests an exploration of whether the gradual change from conventional cages to enriched cages could have been associated with the initial reduction of Salmonella prevalence in laying hens that occurred from 2009 until 2013.
Pieskus et al. (2008b) found that the prevalence of Salmonella in laying hens reared in enriched cages was not significantly different (26.8%) than those reared in conventional cages (33.3%) in a small field study in Lithuania.
An experimental study by Gast et al. (2014b) showed no difference in the prevalence of S. Enteritidis in eggs laid by experimentally infected laying hens housed in conventional cages (3.97%) and enriched cages (3.58%). However, extrapolation of these results should be carried out with care given that (i) an artificial infection challenge may not be the best way to detect the effect of the type of cage since all hens were infected experimentally; and (ii) the enriched cages in this study were composed of nests and perches but not pecking and scratching areas, which are compulsory and more likely to retain faeces and disseminate Salmonella in enriched cages.
Another experimental study looking into bird‐to‐bird transmission of S. Enteritidis (Gast et al., 2014a) did not find any difference in the frequency of horizontal transmission between animals housed in conventional and enriched cages. Still, enriched cages did not have pecking and scratching areas, which can accumulate faeces and eventually increase the risk of horizontal transmission of Enterobacteriaceae, and thus extrapolation of findings should be made carefully.
In a third experimental study by the same group (Gast et al., 2015) on the persistence of Salmonella shedding after an infection it was found that faecal shedding of S. Enteritidis was detected for up to 8 weeks post‐inoculation in hens housed in enriched cages and 10 weeks in hens housed in conventional cages. For both trials combined, the frequency of positive faecal cultures was significantly (p < 0.05) greater for conventional cages than for enriched colony cages at 1 week (84.7 vs 71.5%), 2 weeks (54.2 vs 31.3%), 3 weeks (21.5 vs 7.6%), and 4 weeks (9.7 vs 2.8%) post‐inoculation.
Another experimental study, using laying hens inoculated with S. Enteritidis, showed a higher diversity in the microbiota of hens housed in enriched cages or aviaries than in conventional cages (due to contact with faeces). However, this did not translate into differences between these groups in the colonisation and shedding patterns of Salmonella (Nordentoft et al., 2011).
In a USA field study, the within‐flock prevalence of Salmonella colonisation of 77 week‐old laying hens removed from commercial farms was significantly higher in conventional cage (13.33%) systems than in aviaries (3.3%) or enriched (colony) cage systems (5%) (Jones et al., 2016).
In the United Kingdom, conversion of conventional cages to enriched colony cages was associated with a marked improvement in control of persistent S. Enteritidis. This was because laying houses were totally cleared for refurbishment, which takes several weeks rather than the normal few days turnaround period. During this time all existing cages and feeding and egg‐collection equipment is removed from the houses allowing total cleaning and elimination of rodent and litter beetle populations. The original cage houses were predominantly based on deep pit manure removal systems, which provided perfect harbourage for breeding rodent populations, litter beetles and flies in the droppings pits beneath the birds. The new colony cage houses used the whole house space for cages, from which manure is regularly removed (usually at least twice a week) by belts beneath the cages. This eliminated the harbourage for farm pests and there has been no evidence of persistent Salmonella infections in such colony cage houses (Carrique‐Mas et al., 2009; Davies and Carrique‐Mas, 2010b; ACMSF, 2016; Martelli et al., 2017; Chousalkar et al., 2018). The new cages are much more complex than the old conventional cages and more difficult to clean, so effective cleaning and disinfection is difficult and this has been associated with increased red mite problems, but no evidence of increased occurrence of Salmonella over time has been found so far. There has been a gradually reducing incidence of S. Enteritidis in laying hen flocks in the United Kingdom since 2012, when all cages would have been converted to colony systems, and since 2015, all S. Enteritidis cases and the majority of S. Typhimurium incidents have occurred in free‐range flocks (APHA, 2018), demonstrating that there may be an increased environmental risk associated with outdoor access and that maintaining effective biosecurity may be easier in enclosed housing once resident S. Enteritidis contamination has been eliminated.
3.3.1.4. Farm size, group size and stocking density
The French BLS on Salmonella prevalence in flocks of laying hens found a higher Salmonella contamination rate in larger flocks (> 10,000 laying hens), which could be due to the presence of animals of different ages in the farm (Huneau‐Salaun et al., 2007), an already described risk factor for Salmonella (Van Hoorebeke et al., 2012). In Trinidad and Tobago, Grenada and St Lucia, (Adesiyun et al., 2014) found similar results, with the farm size being the only risk factor significantly associated (p = 0.031) with the prevalence of Salmonella. The prevalence was established through pooled data from layers, feed, and environmental sampling: 77.8% of large (> 10,000 layers, n = 9) farms were positive for this pathogen compared with 33.3 and 26.1% of medium (5,000–10,000 layers, n = 3) and small (< 5,000 layers, n = 23) farms, respectively.
Large holdings (≥ 30,000 birds) were observed to have higher odds of Salmonella occurrence (OR = 4.79 CI95[1.22; 18.78]; p = 0.025) in the United Kingdom BLS compared to smallest holdings < 3,000 birds) (Snow et al., 2010).
In another French study (Huneau‐Salaun et al., 2009), in caged flocks the risk of Salmonella contamination increased when the size of the poultry‐house exceeded 20,000 laying hens (OR = 6.02; CI95[1.8; 19.8]; p = 0.003).
In a French study, Chemaly et al. (2009), conducted in conjunction with the EU‐wide BLS, significant correlations were found between the Salmonella status of eggshells and a farm size of more than 30,000 birds. In this study 4,200 eggs collected from 28 positive flocks were tested.
Also, the highest risk for Salmonella contamination appeared to be in farms with a higher number of birds (> 30,000 hens) in a study by Ovelhey et al. (2009).
A USA experimental infection study was undertaken on laying hens housed in colony cages enriched with perching and nesting areas and in conventional cages at two different stocking densities. After S. Heidelberg infection, the overall frequency of positive faecal cultures was significantly (p < 0.05) greater from either conventional (51.0%) or enriched colony cages (46.5%) at high stocking density (648 cm2/bird) than from enriched colony cages at low stocking density (33.3%) (973 cm2/bird). No significant differences in S. Typhimurium faecal isolation were identified between housing groups. This demonstrates that stocking density can affect intestinal colonisation and faecal shedding in laying hens for some (but not necessarily all) Salmonella serovars or strains (Gast et al., 2017b). The invasion of the internal organs was not significantly influenced in this study by the housing systems (Gast et al., 2017c). In a comparable study with S. Enteritidis, it was again found that stocking density can affect S. Enteritidis intestinal colonisation and faecal shedding in laying hens and that lower shedding was observed in enriched colony cages compared to conventional ones (Gast et al., 2017a).
An experimental study on the colonisation of organs post‐inoculation by S. Enteritidis, detected Salmonella at significantly higher frequencies in several different sample types from hens in conventional cages compared to enriched cages (with half the stocking density, enclosed nest, perches and pecking and scratching area) (Gast et al., 2013). The samples included livers (96.9% vs 75.0%), spleens (93.8% vs 53.1%), ovaries (25.0% vs 10.4%), and oviducts (19.8% vs 2.1%). These results indicate that differences in housing systems for egg‐laying flocks can affect the susceptibility of hens to colonisation of internal organs by S. Enteritidis. This could be due to stocking density, group size, cage enrichment, or a combination of various factors, providing better living conditions for the birds and hence a higher resistance to pathogen colonisation. High stocking densities can lead to stress and increase contact between animals and faeces, and therefore increase Salmonella transmission (Van Hoorebeke et al., 2012).
In a modelling exercise relating to cage size, the basic reproduction number (R0 of an infectious agent; defined as the average number of secondary infections produced by an infected individual) has been shown to increase with increasing cage length, which is related to a higher number of birds in close contact (Zongo et al., 2015).
3.3.1.5. Age of the production system and previous Salmonella infections
In the study of Van Hoorebeke et al. (2010a), described earlier, the age of the production system (OR = 1.35 CI95[1.01; 1.81], p = 0.04)) as well as previous Salmonella infections (OR = 77 CI95[1.68; 3,596], p = 0.03) were found to be significant risk factors for Salmonella infection in a multivariable analysis.
3.3.1.6. Presence of rodents
The number of rodents trapped was found to be a significant risk factor for Salmonella infection of laying hens in several studies (Davies and Breslin, 2003; Garber et al., 2003) and clearance of rodents was identified as the most important factor in elimination of Salmonella from persistently infected laying farms in the United Kingdom (Davies and Carrique‐Mas, 2010b).
3.3.1.7. Cleaning and disinfection
Cleaning and disinfecting houses between flocks has been associated with a reduced risk of persistence of Salmonella in the USA (Garber et al., 2003).
Significant residual contamination remained on the surfaces of buildings and equipment in the barn and especially the cage layer houses after cleaning and disinfection in a study in the United Kingdom (Davies and Breslin, 2003). Large enriched cage houses can be difficult to clean due to complex fixed cage fittings and limited drainage, and often only dry cleaning, followed by fogging with disinfectant, is carried out (ACMSF, 2016).
3.3.1.8. Impact of stress on Salmonella spread and persistence
Several studies have shown that stress induced by restrictive housing conditions, high stocking density, induced moulting and high temperatures can induce immunosuppression that promotes spread and persistence of Salmonella within a flock (Holt and Porter, 1992; Holt, 1995; Holt et al., 1998; Garber et al., 2003; Kubena et al., 2005; Sasaki et al., 2012; Van Hoorebeke et al., 2012).
A study (Sasaki et al., 2012) performed to determine the baseline Salmonella prevalence and identify associated risk factors in laying‐hen farms in Japan found a significantly higher prevalence of Salmonella in windowless farms (50%, n = 101) than in open houses (28%, n = 299). In a comparable study, Salmonella was isolated from four (80%) of five farms with windowless hen houses whereas only one serotype of Salmonella was isolated from 1 (6.7%) of 15 farms with open hen houses (Matsumoto et al., 2001). In another Japanese study in a slaughterhouse, samples from spent hens were tested. Salmonella was isolated from the birds of ten laying‐hen farms; all of these hens were raised in houses without windows and with automatic feeders; factors linked with stressful rearing conditions and management practices (e.g. no access to natural daylight; use of forced moulting). No isolations of Salmonella were obtained from birds raised in houses with windows (Sunagawa et al., 1997). This may be associated with induced moulting that is exclusively performed in windowless houses and in older flocks. Murase et al. (2006) used an enzyme‐linked immunosorbent assay (ELISA) test to detect Salmonella antibodies in chicken eggs, comparing two naturally infected flocks moulted by feed withdrawal, and two moulted through the administration of a wheat bran diet, concluding that that a less severe moult initiated through the administration of a wheat bran diet can reduce the risk of Salmonella infection in a commercial egg‐producing setting. This result supports other evidence that stress induced by moulting procedures such as feed withdrawal can increase susceptibility to Salmonella.
3.3.2. Salmonella prevalence according to distribution of housing system
3.3.2.1. Official monitoring data
Figure 9 shows a reducing prevalence of Salmonella, both for total Salmonella and target serovars (based on the official monitoring), in EU MS as the national proportion of non‐cage flocks or free‐range flocks increases. Several factors could be implicated in this observation, such as the size of farm, number of flocks per farm, number of birds per flock and historic association of persistent S. Enteritidis with cage systems, if contamination was not eliminated during conversion of laying houses from conventional to enriched cages. In addition, country–related factors that could be linked (e.g. climate, economy, and attention to animal welfare) may influence this finding.
Figure 9.
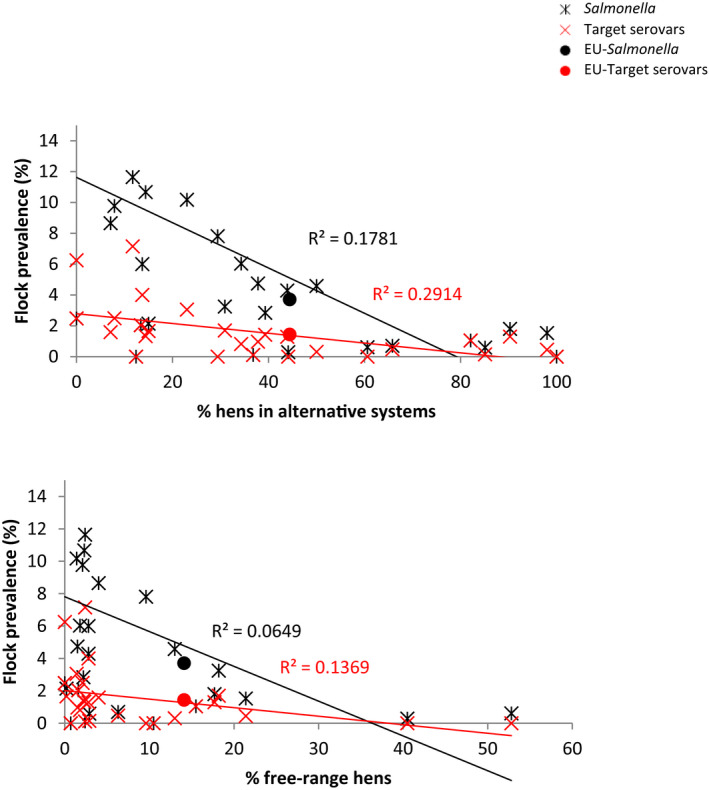
Prevalence of Salmonella and Salmonella target serovars in laying hen production flocks by fraction of laying hens held in alternative systems (top) and free‐range systems (bottom) by MS and in the EU‐25 (without Hungary, Lithuania, and Malta), 2016
3.3.2.2. MS data
The descriptive results of the prevalence of S. Enteritidis in laying hen flocks per MS and housing system in 2016–2017 are detailed in Table 10. It should be highlighted there is a need for caution in the interpretation of the results based on these MS data as they were not collected according to any scientific study design but within a monitoring framework, with varying levels of reported detail by MS.
Table 10.
Salmonella Enteritidis flock level prevalence in laying hen production flocks per country and housing system, 2016–2017
| Housing system | Country | Mean prevalence (%) | N |
|---|---|---|---|
| Cage | Czech Republic | 1.52 | 592 |
| Italy | 0.63 | 2521 | |
| the Netherlands | 4.50 | 222 | |
| Slovakia | 1.86 | 215 | |
| Total | 1.10 | 3,550 | |
| Non‐cage | Austria | 0.52 | 4,657 |
| Czech Republic | 1.41 | 213 | |
| Denmark | 0.46 | 217 | |
| Italy | 0.69 | 3,784 | |
| the Netherlands | 0.68 | 13,626 | |
| Total | 0.63 | 21,123 |
N: number of flocks tested.
Based on a multivariate mixed model analysis of the data, taking country (six countries from Table 10) and housing system into account, it was found that there are significant differences between countries (p < 0.001) and housing systems (p < 0.001). Based on the same analysis considering the three countries that provided data for cage and non‐cage systems (i.e. the Czech Republic, Italy and the Netherlands), it can be concluded that there are still significant differences between countries (p < 0.001) and housing systems (p < 0.001) with a lower level of S. Enteritidis in the non‐cage systems. This was in particular obvious for the Netherlands with 4.5% of flocks being S. Enteritidis‐positive flocks in cage systems compared to 0.68% in non‐cage systems. For the Czech Republic and Italy, the prevalence in cage and non‐cage systems is similar.
The lowest flock prevalence of S. Enteritidis in 2016 and 2017 in the six included countries is observed in the non‐cage systems (see Figure 10).
Figure 10.
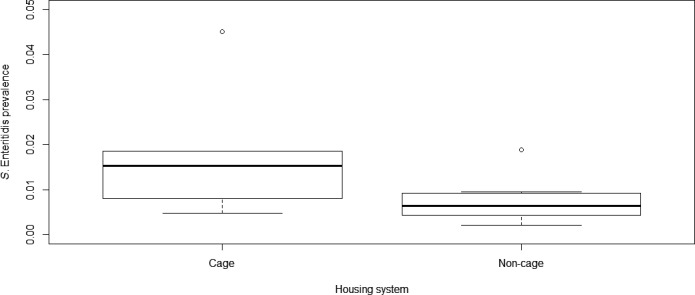
Salmonella Enteritidis flock level prevalence in laying hen production flocks by housing system, based on data provided by six countries (Austria, the Czech Republic, Denmark, Italy, the Netherlands, Slovakia)
Based on these limited data, it can be concluded that S. Enteritidis apparently occurs less frequently in laying hens housed in non‐cage systems in comparison to laying hens housed in enriched cages. For the future, it would be desirable to collect more data on the housing type used as part of the monitoring data reported to EFSA as this will facilitate better analyses based on more data.
3.3.3. Concluding remarks and recommendations
Conflicting evidence is found in the literature and MS data on the occurrence of Salmonella in laying hens when raised in cage systems compared to non‐cage systems. Overall, evidence points to a lower occurrence in non‐cage systems compared to cage systems (hosting large flocks and with a structure which is often difficult to clean and disinfect). Whether this is linked to the housing system as such or whether it is caused by the associated change of furniture, break in the historical infection cycle or reduced stocking density, is unclear.
The limited evidence in the literature that outdoor access affects Salmonella occurrence in laying hens at the EU level is inconclusive.
No studies have specifically investigated the impact of the shift from conventional to enriched cages longitudinally, so it is not possible to conclude on the impact of this change on the occurrence of Salmonella at the EU level.
The literature provides conclusive evidence that an increased stocking density, larger farms and stress‐inducing conditions result in increased presence, persistence and spread of Salmonella in laying hen flocks.
It is recommended that future monitoring programs record the housing type of laying hen flocks to enable assessment of its impact on Salmonella occurrence in these flocks.
3.4. Risk factors for the occurrence of Salmonella in broiler chickens in relation to the type of farming methods and other animal welfare indicators
The risk/protective factors in relation to the farming system of broiler production or breeding flocks considered, based on the available evidence, were: outdoor access, group (flock size/farm) size, stocking density, existence of enrichment, previous Salmonella infection, type of litter, biosecurity. The animal welfare indicators considered were: stress (apart from heat stress), heat stress, activity/behaviour, body status (e.g. FPD and other diseases).
The assessment was based both on literature review to assess risk factors relating to management and housing systems and biosecurity practices and based on the data requested from the MS.
More information on housing systems is provided in Appendix B and elsewhere.30
3.4.1. Literature data
Table C.4 in Appendix C provides an overview of studies dealing with the occurrence of Salmonella in broiler chickens in relation to the farming methods and other animal welfare indicators.
Table C.4.
Overview of studies dealing with the occurrence of Salmonella in broiler chickens in relation to the farming methods and other animal welfare indicators
| Reference | Population | Country | Welfare aspects or housing systems considered | Which risk/protective factor(s) in relation to the farming system have been evaluated? | Which animal welfare indicators are described? | Other risk factors |
|---|---|---|---|---|---|---|
| Field study | ||||||
| Alali et al. (2010) | Production flocks | USA | YES | Outdoor access; Group size; Stocking density; Organic | None | NO |
| Alebachew and Mekonnen (2013) | Production flocks | Ethiopia | YES | Outdoor access | None | NO |
| Le Bouquin et al. (2010) | Production flocks | France | YES | Outdoor access, Organic | None | YES |
| Chriel et al. (1999) | Production flocks | Denmark | YES | Group size; Type of litter; Previous infections | None | NO |
| Esteban et al. (2008) | Production flocks | Spain | YES | Outdoor access | None | NO |
| Franz et al. (2012) | Production flocks | the Netherlands | YES | Group size | None | YES |
| Henken et al. (1992) | Breeding flocks | the Netherlands | YES | Biosecurity; Feeding | None | YES |
| Jacobs‐Reitsma et al. (1994) | Production flocks | the Netherlands | YES | Biosecurity; Feeding | None | YES |
| Opara et al. (1992) | Production flocks | USA | YES | Type of litter | None | YES |
| Pieskus et al. (2008a) | Production flocks | Italy | YES | Organic | None | NO |
| Proietti et al. (2007) | Production flocks | Italy | YES | Organic | None | NO |
| Rose et al. (1999) | Production flocks | France | YES | Biosecurity, Feeding; Previous infections | None | YES |
| Rossa et al. (2013) | Production flocks | Brazil | YES | Outdoor access | None | NO |
| Sapkota et al. (2014) | Production flocks | USA | YES | Outdoor access; Organic | None | NO |
| Siemon et al. (2007) | Production flocks | USA | YES | Outdoor access; Pasture poultry farms | None | NO |
| Skov et al. (1999) | Production flocks | Denmark | YES | Group size; Stocking density; Genetic; Biosecurity | None | YES |
| Snow et al. (2008) | Production flocks | United Kingdom | YES | Outdoor access; group size; organic | None | NO |
| Thakur et al. (2013) | Production flocks | USA | YES | Outdoor access | None | NO |
| Tuyttens et al. (2005) | Production flocks | Belgium | YES | Outdoor access; Group size; Stocking density | None | NO |
| Tuyttens et al. (2008) | Production flocks | Belgium | YES | Outdoor access | None | NO |
| Van Overbeke et al. (2006) | Production flocks | Belgium | YES | Organic | None | NO |
| Volkel et al. (2011) | Production flocks | Germany | YES | Type of litter | None | NO |
| Wierup et al. (2017) | Production flocks | Sweden | YES | Outdoor access | None | NO |
| Experimental study | ||||||
| Corrier et al. (1992) | Production flocks | USA | YES | Type of litter | None | YES |
| Fries et al. (1994) | Production flocks | Germany | YES | Type of floor | None | YES |
| Gomes et al. (2014) | Production flocks | Brazil | YES | Stocking density | Stress (not heat stress) | NO |
| Gustafson and Kobland (1984) | Production flocks | USA | YES | Type of litter | None | YES |
| Lund et al. (2003) | Production flocks | USA | YES | Organic; Feeding | None | YES |
| Santos et al. (2008) | Production flocks | USA | YES | Type of litter, feeding | None | YES |
| Soliman et al. (2009) | Production flocks | USA | YES | There were no such factors evaluated | Heat stress, food withdrawal | NO |
| Waldroup et al. (1992) | Production flocks | USA | YES | Stocking density | None | YES |
The papers have been screened for internal and external validity and some limiting factors have been identified:
Limited sample size on both farm and individual sample level;
Existence of confounding factors not (properly) addressed in some studies;
Diversity in sample matrix and laboratory analysis conducted;
Non‐recent data in studies.
Generally, less literature data are available on the effect of housing systems on the prevalence of Salmonella in broilers in comparison to layers.
3.4.1.1. Effect of outdoor access and organic production
As in layers, some conflicting results are found in the literature.
Alali et al. (2010) found a lower Salmonella prevalence in faecal samples from organic farms (5.6%) compared to conventional farms (38.8%). This was demonstrated through a cross‐sectional study by sampling one house per farm at three organic and four conventional broiler farms from the same company in North Carolina.
Esteban et al. (2008) investigated 60 flocks of ‘traditional free‐range’ chicken from 34 farms in the Basque Country (Northern Spain). A low Salmonella flock prevalence of 1.7% CI95[0.0; 4.0] was found and the authors considered such prevalence lower than literature data on conventional flocks. In a study in the USA on 31 broiler farms no significant difference in Salmonella prevalence between housing systems was found with 33% pasture and 47% conventional poultry farms being positive (p = 0.49). On an individual sample level, flocks reared conventionally had higher prevalence than in pasture (Siemon et al., 2007).
In other studies, no statistically significant differences were obtained, even if in some cases the prevalence in organic farms appeared to be lower than in conventional ones. Pieskus et al. (2008a) described a study performed in 4 different countries (Italy, Germany, Lithuania and the Netherlands), sampling conventional and organic broiler flocks (Italy: 11 organic, 10 conventional; the Netherlands: 18 conventional, 108 organic; Lithuania and Germany: 27 and 22 conventional, respectively, no organic flocks sampled). Different sampling schemes were applied in different countries, and therefore insufficient power was available in the study. Proietti et al. (2007) compared the presence of Salmonella in two organic and two conventional broiler farms located in central Italy, also with inconclusive results due to limited number of farms included. Van Overbeke et al. (2006) compared 9 organic and 11 conventional farms belonging to the same integrated company, and located in the southern part of Belgium. No significant differences were found in Salmonella presence at any sampling point during rearing (overshoes) and at the slaughterhouse (gastrointestinal tracts). Also in a study by Lund et al. (2003) no difference in Salmonella occurrence between free‐range and pastured pen (free‐range unit with mobile housing) was found.
Also in Sweden, no evidence was found in a study conducted between 2007 and 2015, that Salmonella prevalence in the small number of free‐range flocks included in the study was greater than for conventional housing systems. The finding that 0.16% CI95[0.09; 0.2] of indoor flocks and 0% CI95[0; 2] of outdoor flocks tested positive for Salmonella was not statistically significant and was even less relevant after exclusion of flocks that were likely to have been infected via vertical transmission or from a contaminated hatchery. Despite the limitations of the study such field data is limited and this investigation was still considered to provide useful information (Wierup et al., 2017).
In the analysis of data from the French BLS (Le Bouquin et al., 2010), flock size and the type of housing system did not significantly affect the Salmonella prevalence of broiler flocks, which was 8.6% CI95[5.7; 11.5] overall. Detection of Salmonella at clearance of flocks was increased when neighbouring farmers helped with chick placement and reduced when mobile equipment was dismantled for cleaning, when drinking water was acidified and if there were specific disposal bins for dead birds. A similar study on the data from the BLS in the United Kingdom (Snow et al., 2008), on 382 holdings, did not demonstrate any significant risk factor, apart from a slight significance relating to the number of birds in the holding. This finding was mirrored by the results of the overall BLS, which also failed to identify statistically significant differences between production systems, and only identified factors relating to the number of flock cycles per year and the season when samples were taken as statistically significant (EFSA, 2007).
In the USA, a field study found a significantly higher prevalence of Salmonella in poultry litter samples from newly organic compared to conventional farms, but the small sample size requires cautious interpretation (5/5 vs 2/5 positive, respectively, (Sapkota et al., 2014)).
Santos et al. (2008) demonstrated, through an experimental study comparing different feeding regimes and different housing systems, a lower rate of caecal contamination and shedding of Salmonella by broilers raised on litter, compared with caged animals.
Van Hoorebeke et al. (2012) reported that ‘it is recognized that both conventionally reared and free range broilers can be colonized with Salmonella (McCrea et al., 2006; EFSA and ECDC, 2009). Data from United Kingdom retail surveys of chicken have shown that Salmonella can be isolated from standard, free range and organic chicken meat (FSA, 2003; CLASSP, 2007). Various risk factors have been identified for Salmonella infection on broiler farms including farm management factors, biosecurity, flock size, age of chickens and season (Le Bouquin et al., 2010a; Namata et al., 2009).’
3.4.1.2. Effect of the flock size/farm size and stocking density
A national Salmonella and Campylobacter monitoring programme for the broiler supply chain in the Netherlands found an increase of Salmonella prevalence with increasing flock size (Franz et al., 2012), whereas the study by Chriel et al. (1999) showed no effect of flock size on S. Typhimurium contamination in Danish broiler flocks.
In another longitudinal retrospective survey in Denmark, it was shown that farms with five barns were significantly associated with S. Typhimurium infection in broiler flocks (OR = 2.5) (Skov et al., 1999). Authors have not been able to explain this finding biologically, and considering the small number of farms having more than five houses, it has been difficult to investigate if special problems can be related to having more than five houses on a farm.
A Brazilian team (Gomes et al., 2014) studied the effect of overcrowding stress in broiler chicken by comparing chicken reared at 10/m2 vs 16/m2 in an experimental setting and focussing on serum corticosterone levels, the relative weight of the bursa of Fabricius, plasma IgA and IgG levels, intestinal integrity, macrophage activity and experimental S. Enteritidis invasion. They showed a deleterious impact of overcrowding on performance parameters, inducing enteritis and decreasing macrophage activity and the relative Fabricius bursa weight in broiler chickens. The data strengthen the hypothesis that avoidance of overcrowding stress in chicken are relevant factors for the maintenance of intestinal integrity, performance and decreased susceptibility to Salmonella infection. Environmental enrichment can help reduce stress levels in broilers (Baxter et al., 2018).
3.4.1.3. Effect of the type of litter
A study comparing the impact of the type of litter on the detection of Salmonella through boots swabs over time was performed on one farm. In a limited sample set collected over time in a single broiler house, wood shavings bedding showed the highest Salmonella prevalence (3/6 positive samples), followed by peat (2/6) and corn silage (1/6), while no positive chopped straw samples were collected in the study. These results need to be confirmed in a wider study (Volkel et al., 2011). Chriel et al. (1999) did not demonstrate any effect of the quality of bedding (dry, wet, hard) on S. Typhimurium contamination in Danish broiler flocks.
3.4.1.4. Previous Salmonella infection
In a French field study on 86 broiler flocks, litter swabs and dust samples were analysed for the presence of Salmonella. Using a multivariable logistic regression analysis, it was demonstrated that Salmonella contamination inside of the house before placing day‐old chicks (OR = 18.4 CI90[4.1; 82.5] and the Salmonella infection of incoming day‐old chicks (OR = 11.0 CI90[3.2; 38.6]) were significantly related to Salmonella contamination of the flock at the end of the rearing period (Rose et al., 1999).
3.4.1.5. Effect of stress
As in layers, stress has been shown to increase Salmonella shedding by broilers. Quinteiro‐Filho et al. (2012) observed that heat stress led to reduced daily live‐weight gain and suppressed feed intake. Feed conversion efficiency was also reduced when heat stress was combined with S. Enteritidis infection. An increase in Salmonella counts in the spleens of the stressed and Salmonella‐infected chickens was also found. However, a study on the effect of heat stress on S. Typhimurium definitive phage type (DT) 104 and S. Infantis of Traub‐Dargatz et al. (2006) did not identify any effect of heat stress of the shedding of these strains.
Soliman et al. (2009) studied the effect of environmental stress on S. Enteritidis colonisation in broilers. Withdrawal of feed or exposure to high temperature for 24 h led to increased attachment of S. Enteritidis to the mucosa of birds in a post‐mortem in‐vitro ileal loop assay (9.05 log10 vs 7.59 log10 S. Enteritidis/g of ileal tissue; p = 0.0006). Increased mucosal attachment also occurred when birds had been subjected to 30°C room temperature for 24 h (8.77 log10 vs 8.50 log10 S. Enteritidis /g of ileum; p = 0.01) compared with birds held at 23°C.
3.4.1.6. Relation between animal welfare indicators and food borne diseases
Up to now there is a lack of data showing any link between welfare indicators and Salmonella in broilers, in part due to the fact that the prevalence of Salmonella is low.
For example, a recent study from Alpigiani et al. (2017) at farm and slaughterhouse level, studied the potential relationship between welfare indicators and food‐borne pathogen prevalence/incidence levels. Salmonella was very low in prevalence, therefore only Campylobacter results were presented in the paper. Flocks with more than 25% animals with severe footpad dermatitis lesions were predicted to be Campylobacter positive, whereas flocks in which fewer than 13 individuals had arthritis were predicted to be Campylobacter negative. Similar factors may apply for Salmonella, as footpad lesions are linked with wet litter, which can also increase transmission of Salmonella in broiler flocks (Dunlop et al., 2016; Opengart et al., 2018).
3.4.2. Data from EEA countries
The goal of the data analysis was to assess the effect of the housing system on the occurrence of Salmonella in broiler flocks using data from 2016 to 2017. In all countries the broilers were housed on ‘floor‐raised’ systems, therefore the comparison was focused on ‘outdoor access’. As in Section 3.3.2, it should be highlighted that there is a need for caution in the interpretation of the results based on these MS data as they were not collected according to any scientific study design.
3.4.2.1. Salmonella Enteritidis
The results for S. Enteritidis are shown in Table G.1 in Appendix G. Only Austria provided data on flocks raised with and without outdoor access. In Austria, one positive flock was found in 3,761 tested flocks without outdoor access (flock prevalence = 0.03%) whereas in flocks with outdoor access four flocks tested positive out of 2,421 flocks tested (flock prevalence = 0.16%). This resulted in a significant difference (p < 0.001). The biological importance of this difference is uncertain given the very low number of positive flocks.
Table G.1.
Broiler flocks testeda for S. Enteritidis per country and their distribution with regard to outdoor access, 2016–2017
| Country | No outdoor access | Outdoor access | Unknownb | Total |
|---|---|---|---|---|
| Austria | 1/3,761 | 4/2,421 | NA | 5/6,182 |
| Belgium | 12/16 | NA | 1/1 | 13/17 |
| Czech Republic | 123/9,598 | NA | NA | 123/9,598 |
| Denmark | NA | NA | 2/4,290 | 2/4,290 |
| Italy | NA | NA | 7/23,203 | 7/23,203 |
| Lithuania | 1/1 | NA | NA | 1/1 |
| the Netherlands | 21/34,643 | NA | NA | 21/34,643 |
| Poland | 102/163,098 | NA | NA | 102/163,098 |
| Portugal | 15/254 | NA | NA | 15/254 |
| Slovakia | NA | NA | 23/5,076 | 23/5,076 |
| Slovenia | 1/2 | NA | NA | 1/2 |
| Total | 276/211,373 | 1/2,421 | 33/32,570 | 313/246,364 |
NA: not applicable as no such flocks were tested.
Number of positive flocks/number of tested flocks.
Not known whether the birds had outdoor access.
3.4.2.2. Salmonella Typhimurium
The results for S. Typhimurium are provided in Table G.2 in Appendix G. Only Sweden provided data on flocks raised with and without outdoor access. Based on the Swedish results, 10 positive flocks were found in 8,852 tested flocks without outdoor access (flock prevalence = 0.09%) whereas in flocks with outdoor access zero positive flocks were found out of 384 tested flocks (flock prevalence = 0.0%). Based on this, it cannot be concluded whether the prevalence is affected by the housing system.
Table G.2.
Broiler flocks testeda for S. Typhimurium per country and their distribution with regard to outdoor access, 2016–2017
| Country | No outdoor access | Outdoor access | Unknownb | Total |
|---|---|---|---|---|
| Austria | 5/3,583 | NA | NA | 5/3,583 |
| Belgium | 9/16 | NA | NA | 9/16 |
| Czech Republic | 5/9,598 | NA | NA | 5/9,598 |
| Denmark | NA | NA | 1/3,606 | 1/3,606 |
| Italy | NA | NA | 1/20,705 | 1/20,705 |
| Netherlands | 47/34,643 | NA | NA | 47/34,643 |
| Poland | 3/81,549 | NA | NA | 3/81,549 |
| Portugal | 6/36 | NA | NA | 6/36 |
| Slovakia | NA | NA | 2/2,199 | 2/2,199 |
| Sweden | 10/8,852 | 0/384 | NA | 10/9,236 |
| Total | 80/138,277 | 384 | 4/26,510 | 89/165,171 |
NA: not applicable as no such flocks were tested.
Number of positive flocks/number of tested flocks.
Not known whether the birds had outdoor access.
3.4.2.3. Monophasic S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐
The results for monophasic S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐ are provided in Table G.3 in Appendix G. Based on these results it can be observed that data on flocks raised with and without outdoor access and results on the monophasic variant were provided only by Belgium. As in the layer data set, the data provided by Belgium only considers positive flocks and therefore does not allow conclusions to be made on the housing type.
Table G.3.
Broiler flocks testeda for S. Typhimurium with the antigenic formula 1,4,[5],12:i:‐ per country and their distribution with regard to outdoor access, 2016–2017
| Country | No outdoor access | Outdoor access | Unknownb | Total |
|---|---|---|---|---|
| Belgium | 4/10 | 1/1 | 1 | 5/11 |
| Czech Republic | 2/4,838 | NA | NA | 2/4,838 |
| Denmark | NA | NA | 21/7,896 | 21/7,896 |
| Italy | NA | NA | NA | 1/23,203 |
| Lithuania | 1/1 | NA | NA | 1/1 |
| Netherlands | 13/34,643 | NA | NA | 13/34,643 |
| Portugal | 10/168 | NA | NA | 10/168 |
| Spain | NA | 7/1,393 | NA | 7/1,393 |
| Total | 30/39,660 | 8/1,394 | 31,099 | 72,153 |
NA: not applicable as no such flocks were tested.
Number of positive flocks/number of tested flocks.
Not known whether the birds had outdoor access.
Overall, it can be concluded that for the occurrence of Salmonella in broilers no clear effect of outdoor access can be observed. For the future it would be desirable to collect more data on the housing type used as part of the monitoring data as this would enable better analysis based on more data.
3.4.3. Concluding remarks
The impact of the housing system on the occurrence of Salmonella in broilers was investigated in only a limited number of studies that mostly did not report significant differences. Therefore, the evidence that outdoor access affects the occurrence of Salmonella in broiler flocks is inconclusive.
There is a lack of data evaluating the impact of flock/farm size and stocking density on Salmonella occurrence in broilers. However, the limited evidence available shows that stress, stocking density and increasing the number of flocks per farm increases Salmonella susceptibility or infection rate.
Up to now there is no data evaluating the link between welfare indicators and Salmonella occurrence in broilers.
It is recommended that future monitoring programs record the housing type of broiler flocks to enable assessment of its impact on Salmonella occurrence in these flocks.
3.5. Impact of Salmonella control programmes on the prevalence of Campylobacter in broiler flocks and on broiler meat
In total, 38 records were considered in the assessment, but on further examination of the full papers, 13 papers contained data on concurrent occurrence of Salmonella and Campylobacter in the same flock and 7 considered co‐contamination at slaughter, so only these papers were taken into account. Despite not meeting the set criteria given in Appendix C, two extra records were included (Jacobs‐Reitsma et al., 1994; Rouxel et al., 2018) because of their relevance to the topic.
3.5.1. Differential characteristics of Salmonella and Campylobacter
Table 11 details numerous differences in the biology, epidemiology and immunology between Salmonella and Campylobacter that can influence the persistence and dissemination of microorganisms and thereby the infection pathways and impact of control programmes. Salmonella is a robust organism that can survive for long periods, sometime years, outside the host and can multiply in warm moist environmental niches, whereas Campylobacter is much more fragile and fastidious, being unable to survive for long periods outside a living host, to multiply outside the gut or to resist desiccation or oxidative stress. Still, less susceptible variants of Campylobacter may be linked with AMR and survive better along the food chain (Ugarte‐Ruiz et al., 2018) and survival may be underestimated because of relatively insensitive detection methods compared to Salmonella and the ability to enter a viable but non‐culturable (VBNC) state, although VBNC cells appear to show reduced infectivity (Sibanda et al., 2018). Survivability impacts the main infection routes for the organism such that transmission via feed contamination, vertical transmission or hatchery contamination are currently considered to be uncommon infection pathways, whereas these are the most common sources of infection for Salmonella in broiler flocks (Shreeve et al., 2002; Alter et al., 2011; Garcia‐Sanchez et al., 2018).
Table 11.
Differences in the biology, epidemiology and immunology between Salmonella and Campylobacter
| Characteristic | Salmonella | Campylobacter | References |
|---|---|---|---|
| Survival outside the gut | Good – up to several years in dry shady conditions | Poor, especially in dry conditions. Can form biofilms that may prolong survival in moist conditions or enter a VBNC state | Trachoo et al. (2002); Davies (2005); Murphy et al. (2006); Bronowski et al. (2014) |
| Multiplication outside the host | Common, especially in feed mills and hatcheries and warm, moist environmental areas. Can grow aerobically and anaerobically | Very unlikely to occur outside the laboratory. Usually requires microaerobic environment so normally unable to grow in air | Silva et al. (2011); Wales and Davies (2013) |
| Multiplication temperature range | 7–45°C | > 30°C, 41–42°C is optimal; sensitive to high temperatures so easily killed in heat‐treated feed | Fehihaber and Krueger (1998); Park (2002) |
| Susceptibility to freezing | Reduction in numbers during freeze‐thaw cycles but generally resistant to freezing | More susceptible than coliform indicator organisms, e.g. a 2 log reduction can be achieved in broiler meat by freezing | Olson et al. (1981); Georgsson et al. (2006); EFSA BIOHAZ Panel (2011b) |
| Occurrence in wild animals | Sporadic, but more common in reptiles, badgers and some species of wild birds | Very common, so also common in the environment | Wahlstrom et al. (2003); Wilson et al. (2003); Briones et al. (2004); Hilbert et al. (2012) |
| Dissemination by vertical transmission in eggs or by hatchery contamination | Vertical transmission likely for some strains within certain serovars and persistent hatchery contamination is common due to multiplication in incubators and waste/wash areas | Unlikely; would not survive normal hatchery conditions or within egg contents | Callicott et al. (2006); Howard et al. (2012); Crabb et al. (2018) |
| Dissemination via contaminated feed | Persistent contamination of feed storage and production facilities, including multiplication within cooling systems, is common | Very unlikely due to poor desiccation survival | Jones (2011); Ge et al. (2013); Magossi et al. (2018) |
| Survival in broiler house between crops | Common, due to good environmental survival and relatively high tolerance for disinfectants | Unlikely unless poor cleaning and disinfection standards apply | Evans and Sayers (2000); Rose et al. (2000) |
| Colonisation of broilers (natural) | The colonising dose can be low for chicks within the first 2–3 days of life, then progressively increases with age as the chick immune system and gut flora matures. Most infections occur within the first week of life, peak at 2–3 weeks then start to regress, often not being readily detected by the time of slaughter | Most infections occur after a lag phase of 2–3 weeks, thought to be related to maternal immunity, with the flock being much more likely to be infected with high levels of Campylobacter by the time of slaughter than earlier in life. Dose very low throughout the life of a broiler resulting in a much higher flock prevalence than for Salmonella, especially in summer | Gradel et al. (2002); Newell and Fearnley (2003); Van Immerseel et al. (2004); Berghaus et al. (2013); Vidal et al. (2014); Awad et al. (2018) |
| Colonisation of broilers (experimental) | Colonisation can be achieved with a concentration in the feed as low as 0.1–0.3 CFU/g feed for day‐old chicks, but is age‐dependent and the within‐flock prevalence and duration of shedding is dose dependent, with at least 100–1,000 CFU needed to reliably establish infection in older. Colonised within 6 h, oesophagus, small intestine and caeca. Clearance by 20 h except in caeca (7 log10 CFU/g at 48 h). High level in liver (6 log10 CFU/g at 48 h) and low level in spleen | Infection can be established in broilers with inoculation doses of 20–90 CFU. Colonised within 6 h, oesophagus, small intestine and caeca but colonisation of caeca was over 10‐fold higher than in oesophagus and small intestine and reached 11 log10 CFU/g at 48 h. Low levels in liver and spleen | Ruiz‐Palacios et al. (1981); Hinton (1988); Gast and Beard (1989); Cawthraw et al. (1996); Heres et al. (2004); Meade et al. (2009); Awad et al. (2018) |
| Within‐flock prevalence | Very variable from 1–50%; typically much lower at the time of slaughter | Considered to be likely to be 100% birds within a few days of introduction of infection, persisting to normal broiler slaughter age | Limawongpranee et al. (1999); Newell and Fearnley (2003) |
| Mean count in caeca | Usually < 2 log10 CFU/g | > 5 log10 CFU/g | Berghaus et al. (2013) |
| Impact of farm biosecurity | Infection due to staff or visitors entering houses is rare as long as reasonable precautions are applied to footwear. Infection within one broiler house can normally be prevented from spreading to others by normal precautions | Entry of personnel into broiler houses is thought to be the main means of introduction and spread. Compliance with excellent biosecurity standards must be much higher than for Salmonella, probably because of the low colonising dose, and high numbers of organisms shed by infected animals. In some countries flies are also considered to be an important means of introduction of infection during summer months and these are not impacted by normal biosecurity measures | Gibbens et al. (2001); Hald et al. (2004); Davies (2005); Newell et al. (2011); Bahrndorff et al. (2013); Battersby et al. (2016); CAMCON (2016); Sommer et al. (2016); Meerburg and Schoelitsz (2018) |
| Role of farm vectors | Rodents and wild birds most important | Cattle, rodents and flies have been considered to be most important, but more recent molecular genetic evidence suggests this may be over‐estimated in comparison with environmental contamination originating from other poultry flocks | Meerburg and Kijlstra (2007); Agunos et al. (2014) |
| Role of contaminated water | Little evidence of water as an important route or survival within protozoa | Several studies report non‐municipal water or untreated water to increase risk. Survival within protozoa may be a factor | Kapperud et al. (1993); Pearson et al. (1993); Snelling et al. (2005); Alali et al. (2010); Riquelme et al. (2016) |
| Seasonality | Little evidence of seasonality but heat stress may influence shedding and extra‐intestinal infection in infected birds | Large increase in risk in summer months in many countries and reports of very effective control by good biosecurity in some countries apart from during the summer | Jacobs‐Reitsma et al. (1994); Ravel et al. (2010); Quinteiro‐Filho et al. (2012); Allain et al. (2014) |
| Colonisation site | Mainly colonises subepithelial gut‐associated lymphoid tissue with intermittent egress to the gut lumen, especially in the caecum | Mainly in the caeca, large intestine and cloaca and is generally restricted to the intestinal mucous layer in the crypts of the intestinal epithelium This is a highly specialised environment and C. jejuni has evolved specialised strategies that allow it retain motility within mucus and utilise local nutrients | Desmidt et al. (1997); Park (2002) |
| Impact of thinning | Very limited evidence of an increased rate of infection at slaughter because of the higher infectious dose for Salmonella at slaughter age and slow rate of spread in older birds | Major impact on flock infection, with very few flocks remaining clear of Campylobacter after thinning, except in low prevalence countries or seasons | Van Der Fels‐Klerx et al. (2008); EFSA (2010b); Koolman et al. (2014) |
| Proportions of innate cells (heterophils, monocytes) | Significant variation in numbers as response to infection | No variation in numbers as response to infection | Meade et al. (2009); Shaughnessy et al. (2009) |
| Gene expression (CD4/5, chemokine, cytokine, AMP gene) | Increase in response to S. Typhimurium infection | Transient, if any, increase and in general no significant change in response to C. jejuni infection | Meade et al. (2009); Shaughnessy et al. (2009) |
VBNC: viable but non‐culturable; CFU: colony forming units.
The timing of infection is also very different, with introduction of Salmonella into a flock being much more likely during the first week of life, but for Campylobacter there is usually a ‘lag phase’ of 2–3 weeks. If Salmonella is not present in feed, day‐old chick deliveries or the internal environment of broiler houses, it is normally possible to exclude its entry by means of basic precautions such as disinfectant boot dips of boot changes, but with Campylobacter this appears to be much more difficult, and any lapse in implementation of high biosecurity and farm hygiene standards is much more likely to lead to introduction of infection from the external environment, either from the direct farm environment or from other farms visited by personnel such as field managers and auditors on the same day (Sibanda et al., 2018). This is likely to be related to a lower number of organisms needed to infect broiler flocks than for Salmonella and the lack of age‐related reduced susceptibility. Poultry flocks infected with Campylobacter also shed huge numbers of organisms; with flock prevalence increasing rapidly to approach 100% within days of the first bird becoming infected and with Campylobacter loads of between 6 and 9 logs CFU/g of caecal contents or fresh faeces. This makes it very difficult to avoid spreading infection between different flocks on a broiler farm once one flock has become infected or to prevent spread of infection by thinning personnel and equipment, which have very close contact with birds on a daily basis (Thornton, 2010; Higham et al., 2018; Wales et al., 2018).
The seasonality of Campylobacter is also different from Salmonella, which usually shows more limited seasonality, especially in colder climates. In the Nordic countries where there is very good control of Salmonella through a high level of compliance with best biosecurity standards, Campylobacter also occurs at very low levels for most of the year, becoming a rare event in winter months, but is much more difficult to control in summer, resulting in a high flock prevalence even though Salmonella remains rare (Kuhn et al., 2018).
There is also evidence suggesting different colonisation mechanisms, colonisation sites in the gut and many host immune responses relating to infection with the two organisms in different hosts (Iglesias‐Torrens et al., 2018; Korolik, 2018), although a proportion of host‐response genes may be common to both infections (Calenge and Beaumont, 2012; Psifidi et al., 2016). According to Shaughnessy et al. (2009), the innate regulation of immune responses differentiates the avian intestinal colonisation between Salmonella and Campylobacter, the latter being able to modulate and possibly evade host immune mechanisms. Meade et al. (2009) demonstrated the difference in colonisation patterns were due to the innate immune system (Toll‐Like Receptors and avian B‐defensin gene expression), which responds differently to Salmonella and Campylobacter infection.
These factors suggest that interaction between the two organisms in the competitive way that applies to more closely related organisms is unlikely (Yang et al., 2018), although the inflammation and intestinal damage associated with some Campylobacter infections may enhance the establishment and tissue dissemination of other organisms (Awad et al., 2018) and this has also been shown in mice (Wang et al., 2018a). This observation is also supported by the ability to readily infect chickens with both organisms in laboratory in‐vivo infection models (Heres et al., 2003; Meade et al., 2009; Rouxel et al., 2018; Wilson et al., 2018).
3.5.2. Presence of Salmonella vs Campylobacter in broiler flocks
3.5.2.1. Literature data
Studies on co‐colonisation
Despite increased biosecurity and farm hygiene standards that have been associated with a reduction in Salmonella prevalence in broiler flocks in the EU (Figure 4), a statistically increasing trend in reported Campylobacter infection of humans has occurred at the EU level since 2008, although this has stabilised in recent years (EFSA and ECDC, 2017d). In some countries where regular monitoring for Campylobacter is carried out, there has also been no improvement at broiler flock level (Lawes et al., 2012; Vidal et al., 2014). In order to investigate the possible relationships between Salmonella and Campylobacter prevalence, publications reporting co‐colonisation by both organisms were reviewed for a possible negative or positive association between the organisms. Although many studies have produced data on the occurrence and bacterial load of Salmonella and Campylobacter resulting from natural or artificial infections and concurrent persistence of both organisms has been shown in experimental models (Cox et al., 2018), a relatively small number of studies have considered both organisms in the same naturally infected birds or flocks and even fewer have specifically reported the data on co‐colonisation of individual birds or flocks by both organisms and have usually only reported the total proportion positive for either Campylobacter or Salmonella. Therefore, co‐colonisation studies in poultry other than broilers were also included. Other studies have reported such high prevalence of both organisms that co‐colonisation must have been present, but is not explicitly stated in the publications (e.g. Line (2002); Cox et al. (2003)). No negative associations have been reported from any studies. Two large studies that did provide data and conducted analysis on concurrent infection reported no positive or negative statistical association between the organisms (Wedderkopp et al., 2001; Rasschaert et al., 2007), whereas in a third study a positive statistical correlation was found between Campylobacter and Salmonella colonisation (Jacobs‐Reitsma et al., 1994). This may have been influenced by the lower standards of broiler farm biosecurity and terminal hygiene that applied in the early 1990s, which could have increased the likelihood of occurrence of both organisms. A similar positive statistical correlation was more recently reported by (Franz et al., 2012), but no data were provided. Another recent finding, from an experimental trial of co‐infected birds by Salmonella and Campylobacter, indicated that co‐colonisation by both microorganisms did not affect negatively the mean counts (log CFU/g) in caeca of each but seemed to enhance Salmonella mean counts by 1 log in birds co‐infected with Campylobacter compared to birds infected only by Salmonella (Rouxel et al., 2018). If these suggestions of enhanced colonisation and invasion of other pathogens in some batches of birds that are infected with Campylobacter are correct, this could pose an increased likelihood of higher levels of Salmonella being present in birds at slaughter and therefore the higher level of biosecurity needed to control Campylobacter could have additional benefits in reducing the risk of dual infections. A summary of the findings of various studies is tabulated in Table C.7 in Appendix C.
Table C.7.
Literature data on the occurrence of Salmonella and Campylobacter in poultry at farm/group level
| Population | Infection | Sample type | Additional information | Prevalence | Mean load (log10 CFU/g) | Text extract, incl. statistics used and risk and protective factors, if relevant | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Salmonella | Campylobacter | Salmonella | Campylobacter | ||||||
| Turkeys | Natural | Caecal material | Period of 16 days | 28.8% of 125 birds | 87.2% of 125 birds | NS | NS | In each of 5 AGP trial groups, both organisms persisted for the whole of the trial period and although results from individual birds are not presented, the high prevalence of Campylobacter means that at least some of the birds must have been colonised by both organisms | Cox et al. (2003) |
| Broilers | Experimental | Caecal material | Control birds in a litter acidification experiment at 4 weeks considered | 30.0% of 40 birds | 100.0% of 40 birds | 0.48 | 5.72 | In a trial of the effect of litter acidification, intervention and control birds infected by contact with contaminated litter were sampled periodically. Although data for individual birds is not recorded, at the end of the study all birds were infected with Campylobacter and 30% with Salmonella, showing colonisation by both organisms | Line (2002) |
| Broilers | Natural | Cloacal material and boot swabs |
Cloacal swabs from live birds at slaughter (pool 10) tested for Campylobacter and boot socks at farm used for Salmonella: 8,911 flocks |
5.5% of 8,911 flocks 2.2% of 8,911 flocks with both organisms |
42.5% of 8,911 flocks 2.2% of 8,911 flocks both organisms |
NS | NS | In national surveillance programmes of broiler flocks carried out in Denmark during 1998 and 1999, 89,110 samples for Campylobacter; ten per flock, were taken at 10 different abattoirs, and 44,550 samples for Salmonella were taken from the same flocks in the broiler houses. No seasonal variation was identified, unlike for Campylobacter where there was a pronounced summer peak. 198 of 8,911 flocks (2.2%) were positive for both Campylobacter and Salmonella, and 4,863 (54.6%) flocks were negative for both. There was no significant correlation of flock colonization with Campylobacter and Salmonella (OR = 1.04 CI95[0.86; 1.26] | Wedderkopp et al. (2001) |
| Broilers | Experimental | Caecal material | Control birds considered in competitive exclusion experiment | 90.0% of 30 birds | 100.0% of 30 birds | 4.2 | 7.3 | In a trial of different competitive exclusion formulations for protection against experimental challenge, all control birds remained positive for Campylobacter after one week and almost all for Salmonella, demonstrating co‐colonisation in most of the birds and all 3 batches of 10 birds | Stern et al. (2001) |
| Turkeys | Natural | Caecal droppings | Two flocks sampled periodically |
31.1% of 30 birds (toms) 17.7% of 30 birds (hens) |
80% of 30 birds (toms) 70% of 30 birds (hens) |
NS | NS | Overall, in a study of 2 naturally infected turkey flocks, toms were 80% positive for Campylobacter and 31.1% positive for Salmonella; hens were 70% positive for Campylobacter and 17.7% positive for Salmonella. Both sampled flocks remained positive for both organisms for at least 15 weeks, although no results from individual birds for both organisms were reported | Cox et al. (2000) |
| Broilers | Experimental | Caecal material | Control group, considered in feed additive experiment using 50 chickens per group (10 samples taken at 6 weeks | 50% of 10 samples | 90% of 10 samples | NS | NS | No data on coinfection in individual birds but high prevalence means some birds must have had both and all study groups had both organisms for the 6‐week study period | Borta et al. (2011) |
| Turkeys | Natural | Caecal material | 33 days old birds considered | 98% of 50 birds | 90% of 50 birds | NS | NS | Most birds infected so most will have been co‐colonised | Thornton (2010) |
| Broilers | Experimental | Cloacal swabs (%) and caecal content (log10 CFU/g) | Effect of fermented liquid feed on C. jejuni and S. Enteritidis colonisation in broiler chickens Enumeration of individual birds at 4,19,47 and 61 weeks (control and inoculated birds) | From 13 to 100% depending on the groups (dry feed and fermented liquid feed; n = 6 or 8 birds) | From 0 to 100% depending on the groups (dry feed and fermented liquid feed; n = 6, 8 or 10 birds) |
6.8–8.2 depending on the groups (dry feed and fermented liquid feed; n = 6 or 8 birds) |
7.6–9.0 depending on the groups (dry feed and fermented liquid feed; n = 10 birds) |
Birds can be colonised by Campylobacter and Salmonella concurrently without impacting the results of colonisation when the birds are infected by one of the pathogens: ‘no significant effect of co‐inoculation was found’ | Heres et al. (2003) |
| Turkeys (breeder) | Experimental | Pooled faecal droppings | Routes of transmission of Salmonella and Campylobacter in breeder turkeys | From 52 to 70% depending on the groups (control and infected toms and hens; n = 184) | NS | NS |
2–7 depending on the groups (control and infected toms and hens; n = 200) |
No analysis for co‐colonisation. The routes of transmission as a function of time for each pathogen are developed. Both were able to colonise without impacting each other | Crespo et al. (2016) |
| Laying hens (male chicks) | Experimental | Caecal material | Investigating the effect of co‐infected birds by Salmonella and Campylobacter Enumeration: individual birds at 5, 13, 19 days (15 birds per group) | NS | NS | 7.9 (day 5), 6.8 (day 13), 6.7 (day 19) | 8.2 (day 5), 7.4 (day 13), 8.7 (day 19) | At day 5, no significant differences in mean counts in the caecal material were found between the groups (mean count 8 log10 CFU/mL) inoculated by Salmonella and Campylobacter alone compared to the group inoculated by both. At day 13, in the group inoculated by both, the counts of Salmonella and Campylobacter were around one log higher than those obtained from birds inoculated by one of each separately. At day 19, only the group inoculated by Salmonella was still significantly different (one log lower) compared to the group inoculated by both. In conclusion, co‐colonisation of birds by Salmonella and Campylobacter did not affect the mean counts of each negatively but it seemed that Salmonella would be able to grow better when birds are infected by Campylobacter | Rouxel et al. (2018)a |
| Broilers | Experimental | Caecal samples | Immune gene expression and intestinal responses between Salmonella and Campylobacter. Results 48 h post‐challenge considered | NS | NS | 7 | 11 | This article explains one of the reasons why the two organisms are able to grow together without negative impact due to differences in immune responses from animals infected by Salmonella and Campylobacter | Shaughnessy et al. (2009) |
| Broilers | Experimental trials | Oesophagus, small intestine, caeca, liver and spleen samples | Comparative in vivo infection models yield insights on early host immune response to Campylobacter in chickens. Results 48 h post‐challenge considered | NS | NS | Clearance (oesophagus); clearance (small intestine); 7 (caeca); < 5 (liver, only one bird); < 5 (spleen, only one bird) | 9 (oesophagus); 9 (small intestine); 11 (caeca); 6 (liver, only 3 birds); 6 (spleen, only 3 birds) | Animals were inoculated separately by Salmonella and Campylobacter. Considered together, microbiological, cellular and gene expression profiles indicate that the innate immune system responds differently to Salmonella and to Campylobacter infection. Furthermore, reduction in the expression of AMPs may play a role in the persistence of high level colonisation of the host by Campylobacter. ‘This study provided novel evidence that the chicken host immune system can differentiate between and differentially respond to Salmonella and Campylobacter infection. Being asymptomatic the identification of an AMP expression signature may shed light not only on the pathogenesis of infection but also the mechanisms associated with commensal tolerance in the chicken’ | Meade et al. (2009)b |
| Broilers | Natural | Farm: 2 pooled samples of fresh faecal droppings (Salmonella); 1 pooled sample of 5 swabs of fresh faecal droppings (Campylobacter)Slaughterhouse: 30 caecal swabs pooled in 1 sample – 25 g of neck skin (both) | Farm and SH characteristics affecting the occurrence of Salmonella and Campylobacter: data set collected between 2002 and 2005 (n = 59,495 at farm departure and slaughterhouse arrival; n = 73,233 at slaughterhouse departure) | NS | NS | NS | NS | Positive, association between Salmonella and Campylobacter at 2 sampling points: departure from farms and arrival at slaughter | Franz et al. (2012) |
| Broilers | Natural | Caecal contents (pool of 25 samples per flock at slaughter) | Commercial broiler flocks by culturing 25 pooled caecal samples per flock at slaughter | 27% (49/181) flocks | 82% (153/187) flocks | NS | NS | 187 Dutch broiler flocks were screened in 1992/3 for Campylobacter and Salmonella carriage. Campylobacter were isolated from caecal samples from 82% and Salmonella was isolated from 27% broiler flocks. A positive statistical correlation was found between Campylobacter and Salmonella occurrence at flock level | Jacobs‐Reitsma et al. (1994)b |
| Broilers; laying hens and breeders | Natural | Crop, duodenum and caeca | Concurrent colonisation with Campylobacter and Salmonella and assessment of sampling site at slaughter |
13% (7/56) broiler flocks of which 3/56 colonised by both pathogens 65% (13/20) laying and breeding hen flocks of which 13/20 colonised by both pathogens |
73% (41/56) broilers 100% (20/20) laying and breeding hen flocks |
NS | NS |
Little to no association between Campylobacter and Salmonella occurrence in broiler flocks (Cramers’ V test) Since all flocks were colonised with Campylobacter, it was not possible to determine an association between Campylobacter and Salmonella occurrence in laying and breeding hen flocks |
Rasschaert et al. (2007) |
AGP: antimicrobial growth promoter; AMP: antimicrobial peptide; NS: not specified.
This study, presented at the International Salmonella and Salmonellosis conference, Sep 2018, was added to the articles from the literature search because of its relevance to the topic.
This study cited by a reference from the literature search was added to the selected articles because of its relevance to the topic.
In the EU‐wide BLS of Campylobacter in caeca and Salmonella and Campylobacter on carcasses, Salmonella‐contamination results on the broiler carcasses were not significantly associated with Campylobacter status of the flocks. There was also no evidence of association between Campylobacter and Salmonella contamination based on broiler carcass samples. The prevalence of Campylobacter on the Salmonella‐positive and ‐negative samples was nearly 70%. Unfortunately testing for Salmonella in caeca, which would have provided a more meaningful comparison to study co‐colonisation, was not carried out (EFSA, 2010b).
In addition, comparison of the prevalence of the EU Campylobacter survey results of 2008 with the Salmonella BLS of broiler flocks in 2006 (Figure 11) suggests that some MS that have low prevalence of Salmonella may also have lower Campylobacter prevalence in flocks. The 2008 data in Figure 11 provides a correlation value of 0.46 (p = 0.09), suggesting a weak correlation overall and that the null hypothesis that there is no correlation cannot be rejected. In Figure 11, countries such as Sweden, Finland and Denmark which are known to have very high biosecurity standards, have low levels of both Salmonella and Campylobacter in the BLSs and in monitoring data. However, other countries, such as France and the United Kingdom, have been able to control Salmonella much more effectively than Campylobacter and factors relating to climatic or production system differences may also be involved (Trompette et al., 2018; Wales et al., 2018).
Figure 11.
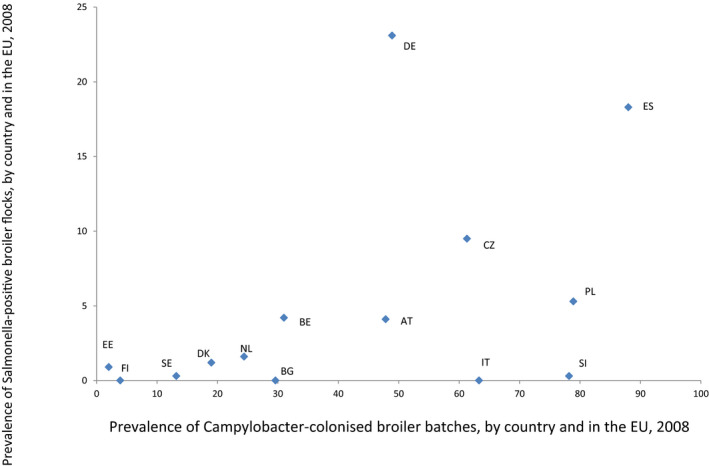
Comparison of the Campylobacter prevalence of broiler batches and Salmonella prevalence of broiler flocks in EU Members States as derived from the EU‐wide baseline surveys (in 2008 for Campylobacter and in 2006 for Salmonella)
- Country abbreviations: AT: Austria; BE: Belgium; BG: Bulgaria; CZ: Czech Republic; DE: Germany; DK: Denmark; EE: Estonia; ES: Spain; FI: Finland; IT: Italy; NL: Netherlands; PL: Poland; SE: Sweden; SI: Slovenia.
These observations are supported by several studies that have demonstrated the importance of good compliance with high biosecurity standards when entering broiler houses for effective control of Campylobacter (Humphrey et al., 1993; Berndtson et al., 1996; Rosenquist et al., 2009; Hansson et al., 2010; EFSA BIOHAZ Panel, 2011b; Battersby et al., 2016; CAMCON, 2016; Van Wagenberg et al., 2016; Higham et al., 2018). The epidemiological and biological differences between the two organisms result in a greater likelihood of introduction of Campylobacter into broiler flocks, at any stage of the life of the birds, if there are lapses in biosecurity standards than for Salmonella. In addition, successful Salmonella control has involved actions taken at breeding flock level, such as culling and vaccination, which are not applicable to Campylobacter.
3.5.2.2. Data from EEA countries
The data request on the occurrence of Salmonella or Campylobacter for any broiler flocks and broiler batches at the farm or slaughterhouse level (e.g. neck skin or caecal samples) resulted in potentially useful data provided by three countries on the co‐occurrence of Salmonella and Campylobacter of broiler flocks at the farm level. The data, consisting of test results from 4,914 (Iceland); 1,386 (Spain) and 247 (Latvia) flocks, are summarised in Table 12.
Table 12.
Co‐occurrence of Salmonella or Campylobacter of broiler flocks at the farm level based on data from three countries, 2010–2017
| Spain | Status | Campylobacter b | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Salmonella a | Positive | 29 | 16 | 45 |
| Negative | 773 | 568 | 1,341 | |
| Total | 802 | 584 | 1,386 | |
| Iceland | Status | Campylobacter c | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Salmonella c | Positive | 0 | 14 | 14 |
| Negative | 178 | 4,722 | 4,900 | |
| Total | 178 | 4,736 | 4,914 | |
| Latvia | Status | Campylobacter d | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Salmonella d | Positive | 0 | 0 | 0 |
| Negative | 141 | 106 | 247 | |
| Total | 141 | 106 | 247 | |
broilers holding tested using as sampling type: swabs.
broilers batches tested using as sampling type: caecum.
Sample type: faecal/boot swabs.
Sample type: caecal material.
For Spain, the flock positivity for Campylobacter was not significantly (p > 0.05) influenced by the Salmonella flock status (1.33; CI95[0.69–2.65]). Latvia and Iceland had no flocks being positive for both pathogens and low flock positivity for Salmonella (Latvia had no flock positive for Salmonella, while Iceland had only 14 flocks positive for Salmonella).
Limited evidence was obtained on whether Salmonella and Campylobacter positivity was found in the same flocks more or less often than expected if they were independent infections, at least partly because few flocks had a Salmonella‐positive status. Because of the limited quality and representativeness of the data, it was not possible to conclude on any possible association between the organisms.
3.5.3. Presence of Salmonella vs Campylobacter on broiler meat
3.5.3.1. Literature data
At the slaughterhouse level, similar findings have been reported for studies carried out on broiler meat (Table C.8 in Appendix C). In this case, the data are more difficult to relate to the flock infection status. Cross‐contamination between slaughter batches at the abattoir occurs commonly, but in situations where there is a low flock prevalence of either Salmonella or Campylobacter carcass contamination is most likely to originate from the flock itself rather than the slaughter process (Kagambega et al., 2018).
Table C.8.
Data from published literature on the occurrence of Salmonella and Campylobacter in poultry at the slaughterhouse
| Population | Infection | Sample type | Additional information | Prevalence | Mean load (log10 CFU/g) | Text Extract | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Salmonella | Campylobacter | Salmonella | Campylobacter | ||||||
| Broiler | Experimental | Carcass rinse of broiler carcasses | Carcasses of control birds in a litter acidification experiment at 4 weeks considered | 50.0% of 40 carcasses | 77.5% of 40 carcasses | 0.78 | 2.20 | In a trial of the effect of litter acidification, intervention and control birds infected by contact with contaminated litter were sampled periodically. Although data for individual birds is not recorded, at 4 weeks slaughtered a high prevalence of birds was found positive for Campylobacter and Salmonella, showing contamination of some carcasses by both organisms | Line (2002) |
| Broiler | Natural | Carcass rinse of broiler carcasses | Comparison of conventional and organic production | 19% of 299 carcasses; 65% of 20 organic broiler farms | 95% of 299 carcasses; 95% of 20 organic broiler farms | NS | NS | Salmonella was found on carcasses from 20 organic broiler farms in a USA study. Campylobacter was found on most carcasses from 19 of 20 farms. This is comparable to rates seen on conventional farms. No data on co‐occurrence but high prevalence means that some birds and most farms are likely to be co‐infected, but cross‐contamination of carcasses not taken into account | Bender (2010) |
| Broiler | Natural | Carcass rinse | Prevalence and risk factors for Salmonella and Campylobacter on carcasses | 21.2% of 2,414 carcasses from 82 lots | 35.8% of 2,414 carcasses from 82 lots | NS | NS | No impact of co‐occurrence is described. The prevalence of each pathogen and potential risk factors associated to its presence on carcasses described | Arsenault et al. (2007) |
| Broiler | Natural | Carcass rinse | Baseline study in abattoirs (Alberta) | 37.5% of 1,295 carcasses | 75% of 1,234 carcasses | NS | 0.85–1.94 depending on the size of the abattoirs | No impact of co‐occurrence is described | Bohaychuk et al. (2009) |
| Broiler | Natural | Neck skins | Campylobacter and Salmonella correlation on carcasses | 7.5% of 425 batches | 87.5% of 425 batches | NS | 2.39 | No significant correlation (p = 0.269) between the presence of Campylobacter and Salmonella on carcasses (Spearman coefficient = 0.054). The Campylobacter load did not vary (p = 0.268) according to the Salmonella contamination status of the carcasses | Hue et al. (2011) |
| Broiler | Natural | Environmental farm samples (swabs, faecal, litter); carcass rinses | Enumeration of Salmonella and Campylobacter at farm and processing plant samples |
90.9% of 55 flocks (F) 94.5% of carcass samples from 48 flocks (PP) |
63.6% of 55 flocks (F) 87.3% of carcass samples from 48 flocks (PP) |
0.19–1.14 depending on sampling types | 0.90–2.05 depending on sampling types | Significant relationships between F and PP samples for both pathogens (qualitative and quantitative). No indication about relationships between Salmonella and Campylobacter. Behaviour of both pathogens within the farms or the PP while describing the differences among them | Berghaus et al. (2013) |
| Broiler | Natural | Carcass rinse | Correlation between concentrations of 2 species of bacteria with censored microbial testing data | 45.8% of 3,164 carcasses | 70.9% of 3,164 carcasses | NS | NS | A weak positive correlation between Salmonella and Campylobacter prevalence but not significant. Both pathogens were found in 33 % of samples. Parameter estimates from a model (no real data of bacterial counts). | Williams and Ebel (2014) |
| Broiler | Natural |
Farm: 2 pooled samples of fresh faecal droppings (Salmonella); 1 pooled sample of 5 swabs of fresh faecal droppings (Campylobacter) SH: 30 caecal swabs pooled in 1 sample – 25 g of neck skin (both) |
Farm and SH characteristics affecting the occurrence of Salmonella and Campylobacter: data set collected between 2002 and 2005 (n = 59, 495 at farm departure and slaughterhouse arrival; n = 73, 233 at slaughterhouse departure) |
NS | NS | NS | NS |
Positive, significant (p ≤ 0.005) association between Salmonella and Campylobacter at 2 sampling points: departure from farms and arrival at slaughter |
Franz et al. (2012) |
NS: not stated; F: farm; PP: processing plant.
Several studies report associations between the contamination of farm samples and processing plant samples for Salmonella and Campylobacter (Arsenault et al., 2007; Hue et al., 2011; Franz et al., 2012; Berghaus et al., 2013). Very few studies are available on the co‐occurrence of both microorganisms on broiler meat. An experimental study of the effect of litter acidification on carriage of Salmonella and Campylobacter by broiler chicks and contamination of their carcases did not report any association between the organisms (Line, 2002). In another study (Hue et al., 2011), the authors reported the data collected from 425 samples of carcasses analysed for Salmonella and Campylobacter and found no significant (p = 0.269) correlation between both microorganisms, and the counts of Campylobacter were not influenced (p = 0.268) by the presence or absence of Salmonella on carcasses. Franz et al. (2012) studied the occurrence of Salmonella and Campylobacter in the broiler supply chain. They considered three different sampling points, the farm (one pair of shoe covers, fresh droppings), arrival at the slaughterhouse (caecal swabs) and the end of the slaughter line (skins of breast caps). While they found a significant positive correlation between both microorganisms at the two first sampling points (farm (p = 0.008), arrival at the slaughterhouse (p = 0.018)), they did not observe any significant correlation at the final sampling point; slaughterhouse departure. Williams and Ebel (2014) reported a study based on a data set of 3,164 observations (400 ml carcass rinses) used in a model to estimate the correlations between generic E. coli, Salmonella and Campylobacter. The authors found a weak positive correlation between Campylobacter and Salmonella but this was not statistically significant. These studies were not able to show a correlation between the two organisms on broiler carcasses, despite the possibility for cross‐contamination at slaughter and were therefore not suggestive of any impact of Salmonella controls carried out at farm level.
3.5.3.2. MS data
The data request on the occurrence of Salmonella or Campylobacter for any broiler flocks and broiler batches at the farm or slaughterhouse level (e.g. neck skin or caecal samples) resulted in useful data provided by five countries on the co‐occurrence of Salmonella and Campylobacter of broiler batches at the slaughterhouse level. The data, consisting of test results from 1,081 (the Czech Republic); 202 (Estonia); 138 (Slovenia); 253 (Romania); and 11 (Luxembourg) broiler batches, are summarised in Table 13.
Table 13.
Co‐occurrence of Salmonella or Campylobacter of broiler batches at the slaughterhouse level based on data from five countries, 2010–2017
| The Czech Republic | Status | Campylobacter a | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Salmonella a | Positive | 121 | 109 | 230 |
| Negative | 546 | 305 | 851 | |
| Total | 667 | 414 | 1,081 | |
| Slovenia | Status | Campylobacter b | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Salmonella b | Positive | 11 | 2 | 13 |
| Negative | 112 | 13 | 125 | |
| Total | 123 | 15 | 138 | |
| Romania | Status | Campylobacter c | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Salmonella c | Positive | 14 | 14 | 28 |
| Negative | 91 | 134 | 225 | |
| Total | 105 | 148 | 253 | |
| Estonia | Status | Campylobacter d | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Salmonella d | Positive | 0 | 0 | 0 |
| Negative | 15 | 187 | 202 | |
| Total | 15 | 187 | 202 | |
| Luxembourg | Status | Campylobacter (f) | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Salmonella (f) | Positive | 0 | 0 | 0 |
| Negative | 11 | 0 | 11 | |
| Total | 11 | 0 | 11 | |
No info on sample type.
Sample type: neck/skin of carcass.
Sample type: neck/skin of carcass.
Sample type: neck/skin of carcass.
Sample type: neck/skin of carcass.
Broiler batch positivity by Campylobacter is not significantly (p > 0.05) associated with the Salmonella batch status using the data from Slovenia (OR = 0.64; CI95[0.12–6.58]) and Romania (OR = 1.48; CI95[0.62–3.50]). Sampling of the neck skin of carcasses was used in these studies. The data from the Czech Republic seem to indicate that Salmonella positive batches are less likely to be Campylobacter positive (OR = 0.62; CI95[0.46–0.84] (p = 0.001695). However, the sample type is unknown in this study. Estonia and Luxembourg did not detect Salmonella in the flocks sampled.
Hence, no evidence was found that the occurrence of Salmonella and Campylobacter on broiler batches at the slaughterhouse was correlated among these observations. The apparent negative correlation found in samples from the Czech Republic is biologically implausible, and may relate to differences in sample types, which were not reported.
3.5.4. Concluding remarks and recommendations
Salmonella and Campylobacter have very different biological characteristics, epidemiology and modes of infection and host immune response, occupying different intestinal niches, so a relevant influence of one organism on infection patterns of the other is not expected.
There is limited experimental evidence that a prior Campylobacter infection may result in a greater colonisation and extra‐intestinal invasion by other organisms, which may include Salmonella.
Very few studies considering both Salmonella and Campylobacter in broiler flocks or on broiler meat provided data on the prevalence of both organisms together in the samples.
Overall evidence points towards no association (negative or positive) between the occurrence of Salmonella and Campylobacter in broiler flocks or on broiler meat at the end of the slaughter line.
The epidemiological and biological differences between the two organisms result in a greater likelihood of introduction of Campylobacter into broiler flocks, at any stage of the life of the birds, if there are lapses in biosecurity standards, than for Salmonella. In addition, successful Salmonella control has involved actions taken at breeding flock level, such as culling and vaccination, which are not applicable to Campylobacter.
No longitudinal studies are available to directly assess the impact of Salmonella control programmes on the Campylobacter prevalence. Based on scientific evidence on the differences between Salmonella and Campylobacter epidemiology and patterns of colonisation, an impact of Salmonella control programmes, apart from general hygiene procedures, on the prevalence of Campylobacter in broiler flocks at the holding and on broiler meat at the end of the slaughter process is not expected.
It is recommended that any infection study that includes more than one organism should report the results of samples that contain combinations of organisms and not just report the prevalence and concentrations of each organism separately.
4. Conclusions
ToR 1: To estimate the public health impact if the target serotypes in flocks of breeding hens of Gallus gallus are changed, maintaining the current Union target (1%), testing scheme and trade restrictions unchanged. The target serotypes should be defined by EFSA based on their public health significance taking into account the criteria described in Annex III of Regulation (EC) No 2160/2003). Following scenarios should be assessed: (a) a new top 5 of serotypes and (b) all serotypes. The impact of these scenarios should be estimated on the reported prevalence in flocks of broilers and layers (tested in accordance with Commission Regulations (EU) No 200/2012 and No 517/2011). If data allow, the impact should also be estimated on the reported human salmonellosis cases through the poultry meat and egg production chains.
It is not possible to assess the impact of proposed changes of target serovars in breeding flocks either on laying hen or broiler populations. There are multiple sources of these serovars other than breeding flocks and the impact of breeding flocks depends on the individual strains involved and control actions in the food chain. It is also not possible to assess the impact of proposed changes of target serovars on human salmonellosis cases for the same reasons.
There is justification for retaining S. Enteritidis, S. Typhimurium (including monophasic variants) and S. Infantis in the target for breeding flocks considering their occurrence in breeding hen, laying hen and broiler flocks, as well as in humans.
Apart from these three serovars, the predominant Salmonella serovars in humans are subject to change on an annual basis, so a new top 5 serovar target list based on human prevalence could become out of date quite rapidly, as has occurred for the current target serovars S. Virchow and S. Hadar.
S. Kentucky could be proposed for inclusion in a breeding flock target as the fourth serovar as it has spread among broiler populations in several EU MS in recent years, and many strains are resistant to multiple antimicrobials and exhibit high level resistance to critically important FQ antimicrobials.
-
For the fifth serovar, there are several options depending on Salmonella control priorities at the EU and MS level
-
–
S. Heidelberg could be included on a precautionary basis to prevent dissemination of epidemic strains in the EU. Although not currently common in the EU, this serovar has shown a high propensity for vertical transmission and resistance to extended‐spectrum cephalosporins in the American continent.
-
–
S. Thompson could be proposed as an option for enhanced control in the EU, based on its occurrence in breeding flocks and dissemination in a small number of MS, plus enhanced virulence of some strains.
-
–
A further alternative could be a more proactive selective approach to control of Salmonella in the breeding and production pyramid via inclusion of a variable fifth serovar in MS‐specific national prevalence targets in breeding flocks if serovars of particular concern arise in breeding flocks and progeny in that country, or are considered to be a threat, based on occurrence and epidemiology in other regions.
-
–
A target that incorporates all serovars is expected to be more effective than one for selected serovars as the most relevant serovars in breeding flocks vary between MS and over time. It would be more effective in reducing the dissemination of all serovars, including newly emerging strains with ‘epidemic potential’ and the re‐emergence of previously specified target serovars.
ToR 2: To estimate the public health impact expressed as relative reduction of reported human salmonellosis cases if the target set for adult flocks of laying hens of Gallus gallus is reduced from 2 to 1% for the current target serotypes ( Salmonella Enteritidis and Salmonella Typhimurium including the monophasic strain), maintaining the current testing scheme and trade restrictions unchanged and taking into account the data from the last reporting year (i.e. 2016)
If a 1% target of the EU control programme of Salmonella for the current target serovars in laying hen flocks would be met in the 23 EU MSs included in the SAM, the number of human salmonellosis true cases (i.e. accounting for under‐ascertainment and underreporting) is estimated to be reduced by 254,400 CrI95[98,540; 602,700] compared to the situation in 2016. This corresponds to a reduction of 53.38% CrI95[39.11; 65.69] considering the layer‐associated true cases and 6.2% considering the overall human salmonellosis estimated true cases.
ToR 3: To review the risk factors for the occurrence of Salmonella in laying hens for which targets have been set, in relation to the farming methods based on monitoring data and a review of literature, and in particular with the view to see if the ban on unenriched cages had an effect on such occurrence
There are no field studies that specifically address the impact of the transition from conventional to enriched cage systems on the occurrence of Salmonella in laying flocks.
Conflicting evidence is found in the literature and MS data on the occurrence of Salmonella in laying hens when raised in cage systems compared to non‐cage systems. Overall, evidence points to a lower occurrence in non‐cage systems compared to cage systems. Whether this is linked to the housing system as such or whether it is caused by the associated change of furniture, break in the historical infection cycle or reduced stocking density, is unclear.
The evidence that outdoor access or conventional vs enriched cage systems affect Salmonella occurrence in laying hens at the EU level is inconclusive.
ToR 4: To review the risk factors for the occurrence of Salmonella based on monitoring data and a review of literature (a) in broiler chickens, in relation to the type of farming and (b) in broilers and laying hens in relation to other animal welfare indicators
The evidence that outdoor access affects the occurrence of Salmonella in broiler flocks is inconclusive.
There is conclusive evidence that an increased stocking density, larger farms and stress‐inducing conditions result in increased occurrence, persistence and spread of Salmonella in laying hen flocks.
For broiler flocks, the limited evidence available shows that stress, stocking density and increasing the number of flocks per farm increases Salmonella susceptibility or infection rate.
There is no data evaluating links between welfare indicators and Salmonella occurrence in broilers.
ToR 5 ‐ To indicate if there is scientific evidence on a possible negative or positive impact of Salmonella control programmes on the prevalence of Campylobacter in broiler flocks at the holding and on broiler meat at the end of the slaughter process
There are no field studies that have specifically investigated the impact of Salmonella control programmes on the occurrence of Campylobacter in broiler flocks.
Overall evidence points towards no association (negative or positive) between the occurrence of Salmonella and Campylobacter in broiler flocks or on broiler meat at the end of the slaughter line.
The epidemiological and biological differences between the two organisms result in a greater likelihood of introduction of Campylobacter into broiler flocks, at any stage of the life of the birds, if there are lapses in biosecurity standards, than for Salmonella. In addition, successful Salmonella control has involved actions taken at breeding flock level, such as culling and vaccination, which are not applicable to Campylobacter.
Considering the differences between Salmonella and Campylobacter epidemiology and patterns of colonisation, an impact of Salmonella control programmes, apart from general hygiene procedures, on the prevalence of Campylobacter in broiler flocks at the holding and on broiler meat at the end of the slaughter process is not expected.
5. Recommendations
To use WGS for comparing isolates from poultry breeding flocks with those in commercial generations of birds and in humans in order to provide more definitive evidence of a link between breeding flocks, commercial generations and human infections.
To investigate the potential and reasons for under‐detection of Salmonella, particularly S. Enteritidis, in flocks of laying hens and to conduct field investigations on the effectiveness of administration of Salmonella vaccination programmes used in laying flocks, and their protective effect.
To investigate in detail the reasons for failure to control S. Enteritidis in countries where it appears regularly in chicken breeding flocks, laying hens or broilers, especially if current or proposed targets are exceeded.
To report all individual Salmonella serovars in poultry flocks to facilitate source attribution and epidemiological studies.
To review the SAM and its conclusions as new data emerges as the Salmonella situation in the EU is dynamic in terms of the foodstuff‐associated risks and the serovars of most importance.
To perform an EU‐wide survey of Salmonella in cattle and beef to investigate the role of the cattle reservoir as a source of human infections and in pigs or pork to obtain more recent and comparable data in order to reduce the uncertainty on the role of the pig reservoir as a source of human infections.
To record in future monitoring programs the housing type of laying hen and broiler flocks to enable assessment of its impact on Salmonella occurrence in these flocks.
To report the results of samples that contain combinations of organisms, and not just report the prevalence and concentrations of each organism separately, in any infection study that includes more than one organism.
Abbreviations
- AGP
antimicrobial growth promoter
- AMR
antimicrobial resistance
- BLS
baseline survey
- CFU
colony‐forming units
- CI
confidence interval
- CIA
clinically important antimicrobial
- CrI
credibility interval
- DT
definitive phage type
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- ELISA
enzyme‐linked immunosorbent assay
- EKE
Expert Knowledge Elicitation
- ESC
extended‐spectrum cephalosporin
- EUROSTAT
Statistical Office of the EU
- FBO
food‐borne outbreaks
- FoNAO
foods of non‐animal origin
- FPD
foot pad dermatitis
- FQ
fluoroquinolones
- MDR
multidrug‐resistant
- MIC
minimum inhibitory concentration
- MLVA
multiple locus variable number tandem repeat analysis
- MRA
Microbiological Risk Assessment
- MS
Member State
- NCP
National Control Programme
- NS
Not stated
- OR
Odds ratio
- PFGE
pulsed field gel electrophoresis
- PT
phage type
- RAG
Red‐Amber‐Green
- RH
relative humidity
- RR
relative risks
- SAM
source attribution model
- TESSy
The European Surveillance System
- ToR
Terms of Reference
- VBNC
viable but non‐culturable
- WGS
Whole Genome Sequencing
Appendix A – Minimum requirements for systems of production for the various egg farming methods
1.
Caged systems
According to Regulation (EC) No 589/2008, ‘eggs from unenriched caged hens’ must be produced in systems of production which satisfy at least the conditions specified in Article 5 of Directive 1999/74/EC. Rearing in unenriched cages has been prohibited since 2012:
at least 550 cm2/hen of cage area, measured in a horizontal plane, which may be used without restriction, in particular not including non‐waste deflection plates liable to restrict the area available, must be provided for each laying hen;
a feed trough which may be used without restriction must be provided. Its length must be at least 10 cm multiplied by the number of hens in the cage;
unless nipple drinkers or drinking cups are provided, each cage must have a continuous drinking channel of the same length as the feed trough mentioned in point 2. Where drinking points are plumbed in, at least two nipple drinkers or two cups must be within reach of each cage;
cages must be at least 40 cm high over at least 65% of the cage area and not less than 35 cm at any point;
floors of cages must be constructed so as to support adequately each of the forward‐facing claws of each foot. Floor slope must not exceed 14% or 8%. In the case of floors using other than rectangular wire mesh, MSs may permit steeper slopes;
cages shall be fitted with suitable claw‐shortening devices.
According to Commission Regulation (EC) No 589/2008, ‘eggs from enriched caged hens’ must be produced in systems of production which satisfy at least the conditions specified in Article 6 of Directive 1999/74/EC:
-
1
laying hens must have:
at least 750 cm2 of cage area/hen, 600 cm2 of which shall be usable; the height of the cage other than that above the usable area shall be at least 20 cm at every point and no cage shall have a total area that is less than 2,000 cm2;
a nest;
litter such that pecking and scratching are possible;
appropriate perches allowing at least 15 cm/hen;
-
2
a feed trough which may be used without restriction must be provided. Its length must be at least 12 cm multiplied by the number of hens in the cage;
-
3
each cage must have a drinking system appropriate to the size of the group; where nipple drinkers are provided, at least two nipple drinkers or two cups must be within the reach of each hen;
-
4
to facilitate inspection, installation and depopulation of hens there must be a minimum aisle width of 90 cm between tiers of cages and a space of at least 35 cm must be allowed between the floor of the building and the bottom tier of cages;
-
5
cages must be fitted with suitable claw‐shortening devices.
Alternative systems
According to Commission Regulation (EC) No 589/2008, ‘barn eggs’ must be produced in systems of production which satisfy at least the conditions specified in Article 4 of Directive 1999/74/EC. This Article relates to alternative systems and specifies that:
-
All systems must be equipped in such a way that all laying hens have:
either linear feeders providing at least 10 cm/bird or circular feeders providing at least 4 cm per bird;
either continuous drinking troughs providing 2.5 cm/hen or circular drinking troughs providing 1 cm/hen.
In addition, where nipple drinkers or cups are used, there shall be at least one nipple drinker or cup for every 10 hens. Where drinking points are plumbed in, at least two cups or two nipple drinkers shall be within reach of each hen;
at least one nest for every seven hens. If group nests are used, there must be at least 1 m2 of nest space for a maximum of 120 hens;
adequate perches, without sharp edges and providing at least 15 cm/hen. Perches must not be mounted above the litter and the horizontal distance between perches must be at least 30 cm and the horizontal distance between the perch and the wall must be at least 20 cm;
at least 250 cm2 of littered area per hen, the litter occupying at least one third of the ground surface.
The floors of installations must be constructed so as to support adequately each of the forward‐facing claws of each foot.
-
In addition to the provisions laid down in points 1 and 2,
-
e.
if systems of rearing are used where the laying hens can move freely between different levels,
there shall be no more than four levels;
the headroom between the levels must be at least 45 cm;
the drinking and feeding facilities must be distributed in such a way as to provide equal access for all hens;
the levels must be so arranged as to prevent droppings falling on the levels below.
-
f.
If laying hens have access to open runs:
there must be several pop‐holes giving direct access to the outer area, at least 35 cm high and 40 cm wide and extending along the entire length of the building; in any case, a total opening of 2 m must be available per group of 1,000 hens;
open runs must be of an area appropriate to the stocking density and to the nature of the ground, in order to prevent any contamination and equipped with shelter from inclement weather and predators and, if necessary, appropriate drinking troughs.
-
e.
The stocking density must not exceed nine laying hens/m2 usable area. However, where the usable area corresponds to the available ground surface, MSs may, until 31 December 2011, authorise a stocking density of 12 hens/m2 of available area for those establishments applying this system on 3 August 1999.
According to Commission Regulation (EC) No 589/2008, ‘free‐range eggs’ must be produced in systems of production which satisfy at least the conditions specified in Article 4 of Council Directive 1999/74/EC. In particular, for free‐range systems the following conditions must be satisfied:
hens must have continuous daytime access to open‐air runs. However, this requirement does not prevent a producer from restricting access for a limited period of time in the morning hours in accordance with usual good farming practice, including good animal husbandry practice. In case of other restrictions, including veterinary restrictions, adopted under Community law to protect public and animal health, having the effect of restricting access of hens to open‐air runs, eggs may continue to be marketed as ‘free‐range eggs’ for the duration of the restriction, but under no circumstances for more than 12 weeks;
open‐air runs to which hens have access must be mainly covered with vegetation and not be used for other purposes except for orchards, woodland and livestock grazing if the latter is authorised by the competent authorities;
the maximum stocking density of open‐air runs must not be greater than 2,500 hens/ha of ground available to the hens or 1 hen/4 m2 at all times. However, where at least 10 m2/hen is available and where rotation is practised and hens are given even access to the whole area over the flock's life, each paddock used must at any time assure at least 2,5 m2/hen;
open‐air runs must not extend beyond a radius of 150 m from the nearest pop‐hole of the building. However, an extension of up to 350 m from the nearest pop‐hole of the building is permissible provided that a sufficient number of shelters as referred to in Article 4(1)(3)(b)(ii) of Directive 1999/74/EC are evenly distributed throughout the whole open‐air run with at least four shelters per hectare.
According to Council Regulation (EC) No 1804/199931, ‘organic eggs’ must be produced in systems of production which satisfy at least the following
Poultry must be reared in open‐range conditions and cannot be kept in cages.
-
Buildings for all poultry must meet the following minimum conditions:
-
✔
at least one third shall be solid, that is, not of slatted or of grid construction, and covered with a litter material such as straw, wood shavings, sand or turf;
-
✔
in poultry houses for laying hens, a sufficiently large part of the floor area available to the hens must be available for the collection of bird droppings;
-
✔
they must have perches of a size and number commensurate with the size of the group and of the birds as laid down in Annex VIII:
-
–
regarding the indoor area (1) the stocking density of may not exceed 6 laying hens per m2 usable area; (2) 18 cm perch per animal; (3) 8 laying hens per nest or in case of a common nest 120 cm2/bird;
-
–
regarding the outdoor area: each laying hen must at least have 4 m2 of open run area provided that the limit of 170 kg of N/ha/year is not exceeded;
-
–
-
✔
they must have exit/entry pop‐holes of a size adequate for the birds, and these pop‐holes must have a combined length of at least 4 m/100 m2 area of the house available to the birds;
-
✔
each poultry house must not contain more than 3,000 laying hens;
-
✔
the total usable area of poultry houses for meat production on any single production unit, must not exceed 1,600 m2.
-
✔
In the case of laying hens natural light may be supplemented by artificial means to provide a maximum of 16 h light/day with a continuous nocturnal rest period without artificial light of at least 8 h.
Poultry must have access to an open‐air run whenever the weather conditions permit and, whenever possible, must have such access for at least one third of their life. These open‐air runs must be mainly covered with vegetation be provided with protective facilities, and permit animals to have easy access to adequate numbers of drinking and feeding troughs.
For health reasons, buildings must be emptied of livestock between each batch of poultry reared. The buildings and fittings are to be cleaned and disinfected during this time. In addition, when the rearing of each batch of poultry has been completed, runs must be left empty to allow vegetation to grow back, and for health reasons. MSs will establish the period in which runs must be empty and they will communicate their decision to the Commission and the other MSs. These requirements shall not apply to small numbers of poultry which are not kept in runs and which are free to roam, throughout the day.
Appendix B – Minimum requirements for systems of production for the fattening poultry
1.
According to Annex V of Commission Regulation (EC) No 543/2008,13 in order to indicate types of farming of fattening poultry with the exception of organic or biological farming, no other terms except those set out hereunder may appear on the labelling.
Extensive indoor (barn‐reared) may only be used where:
the stocking rate per m2 floor space does not exceed 15 birds but not more than 25 kg live weight;
the birds are slaughtered at 56 days or later.
Free range may only be used where:
the indoor stocking rate per m2 floor space does not exceed 13 birds but not more than 27.5 kg live weight;
the birds are slaughtered at 56 days or later;
the birds have had during at least half their lifetime continuous daytime access to open‐air runs comprising an area mainly covered by vegetation of not less than 1 m2;
the feed formula used in the fattening stage contains at least 70% of cereals;
the poultry house is provided with pop‐holes of a combined length at least equal to 4 m/100 m2 surface of the house.
Traditional free range may only be used where:
the indoor stocking rate per m2 floor space does not exceed 12 birds but not more than 25 kg live weight however, in the case of mobile houses not exceeding 150 m2 floor space and which remain open at night, the stocking rate may be increased to 20, but not more than 40 kg live weight/m2;
the total usable area of poultry houses at any single production site does not exceed 1,600 m2;
each poultry house does not contain more than 4,800 chickens;
the poultry house is provided with pop‐holes of a combined length at least equal to 4 m/100 m2 surface of the house;
there is continuous daytime access to open‐air runs at least as from the age of 6 weeks;
open‐air runs comprise an area mainly covered by vegetation amounting to at least 2 m2/chicken;
the birds fattened are of a strain recognised as being slow growing;
the feed formula used in the fattening stage contains at least 70% of cereals;
the birds are slaughtered at 81 days or later;
finition in claustration does not exceed for chickens after 90 days of age: 15 days.
The use of free range – total freedom shall require conformity with the criteria set out under traditional free range above, except that the birds shall have continuous daytime access to open‐air runs of unlimited area.
According to Council Regulation (EC) No 1804/1999, ‘organic’ must be produced in systems of production which satisfy at least the following:
The birds are slaughtered at 81 days or later.
Poultry must be reared in open‐range conditions and cannot be kept in cages.
-
Buildings for all poultry must meet the following minimum conditions:
-
✔
at least one third shall be solid, that is, not of slatted or of grid construction, and covered with a litter material such as straw, wood shavings, sand or turf;
-
✔
they must have perches of a size and number commensurate with the size of the group and of the birds as laid down in Annex VIII:
-
–
in the case of fixed housing:
-
–
the stocking density of the indoor area may not exceed 10 animals/m2 usable area with a maximum of 21 kg live weight/m2
-
–
each animal must have 4 m2 of outdoor area provided that the limit of 170 kg of N/ha/year is not exceeded
-
–
-
–
in the case of mobile housing:
-
–
the stocking density of the indoor area may not exceed 16 animals/m2 usable area with a maximum of 30 kg live weight/m2
-
–
each animal must have 2.5 m2 of outdoor area provided that the limit of 170 kg of N/ha per year is not exceeded
-
–
-
–
-
✔
they must have exit/entry pop‐holes of a size adequate for the birds, and these pop‐holes must have a combined length of at least 4 m/100 m2 area of the house available to the birds;
-
✔
each poultry house must not contain more than 4,800 chickens;
-
✔
the total usable area of poultry houses for meat production on any single production unit, must not exceed 1,600 m2.
-
✔
Poultry must have access to an open‐air run whenever the weather conditions permit and, whenever possible, must have such access for at least one third of their life. These open‐air runs must be mainly covered with vegetation be provided with protective facilities, and permit animals to have easy access to adequate numbers of drinking and feeding troughs.
For health reasons, buildings must be emptied of livestock between each batch of poultry reared. The buildings and fittings are to be cleaned and disinfected during this time. In addition, when the rearing of each batch of poultry has been completed, runs must be left empty to allow vegetation to grow back, and for health reasons. MSs will establish the period in which runs must be empty and they will communicate their decision to the Commission and the other MSs. These requirements shall not apply to small numbers of poultry which are not kept in runs and which are free to roam, throughout the day.
Appendix C – Search strategies for literature searches and overview of literature for ToR 3–5
C.1. ToR 3 and 4
Literature searches were conducted on 6 February 2018 in the Web of Science™ Core Collection (1975–present), CABI: CAB Abstracts® (1910–present) and PubMed. The search strategies are reported in Tables C.1 and C.2. Searches retrieved 1,786 (Web of Science), 1,721 (CABI) and 1,148 (PubMed) hits.
Three EndNote X7 files were created, containing the outputs from the three searches, including all indexed fields per hit (e.g. title, authors, abstract). Files were combined and duplicate records removed yielding 3,066 records after de‐duplication. The EndNote file was transferred into DistillerSR® Web‐Based Systematic Review Software (Evidence Partners, Ottawa, Canada) for the selection procedure.
The records were screened for relevance to the review question (ToR 3 and 4) in three steps:
Step 1: title screening of 3,066 records to exclude obviously irrelevant records
Step 2: screening of title and abstract of 546 remaining records by answering to specific questions: Q1: Is Salmonella investigated?; Q2: The presence of Salmonella in relevant population; Q3: Welfare aspects or housing systems considered in relation to Salmonella occurrence; Q4: Other risk factors (e.g. biosecurity) considered in relation to Salmonella occurrence? The answers to the first three questions needed to be positive or unclear to proceed to the next step.
Step 3: screening of remaining 171 records at full text based on records characteristics (i.e. availability of full text, primary research study and language).
Step 4: screening of remaining records at full text by answering to specific questions: Q1: Is Salmonella investigated?; Q2: The presence of Salmonella in relevant population (i.e. laying hen production flocks or hen's eggs, laying hen breeder flocks, broiler production flocks, broiler breeder flocks); Q3: Welfare aspects or housing systems considered in relation to Salmonella occurrence?; Q4: Other risk factors (e.g. biosecurity) considered in relation to Salmonella occurrence?; Q5: Country; Q6: Type of study (i.e. experimental, field study, experimental and field study); Q7: How have the risk/protective factors been evaluated (i.e. qualitatively, quantitatively, qualitatively and quantitatively); Q8: Which risk/protective factor(s) in relation to farming system have been investigated?; Q9: Which animal welfare indicators are described?
The risk/protective factor(s) in relation to the farming system of laying hen production or breeder flocks listed were: outdoor access, cage systems; if yes: describe type of cage (free text), alternative systems, group size, stocking density, genetic, rearing of pullets in the same place as layers, type of litter, biosecurity, other diseases. Those factor(s) in relation to the farming system of broiler production or breeder flocks listed were: outdoor access, group size, stocking density, genetic, farm hatching, existence of enrichment, type of litter, biosecurity, and other diseases. The animal welfare indicators listed were: stress (not heat stress), heat stress, activity/behaviour, body status (e.g. FPD), other diseases, other: free text. An overview of the studies obtained is given in Tables C.3 and C.4.
C.2. ToR 5
Literature searches were conducted on 6 February 2018 in the Web of Science™ Core Collection (1975–present), CABI: CAB Abstracts® (1910–present) and PubMed. The search strategies are reported in Tables C.5 and C.6. Searches retrieved 1,316 (Web of Science), 360 (CABI) and 331 (PubMed) hits.
Three EndNote X7 files were created, containing the outputs from the three searches, including all indexed fields per hit (e.g. title, authors, abstract). Files were combined and duplicate records removed yielding 1,533 records after de‐duplication. The EndNote file was transferred into DistillerSR® Web‐Based Systematic Review Software (Evidence Partners, Ottawa, Canada) for the selection procedure.
The records were screened for relevance to the review question (ToR 5) in three steps:
Step 1: title screening of 1,533 records to exclude obviously irrelevant records: 452 remained.
Step 2: screening of title and abstracts of 452 remaining records by answering to three specific questions: Q1: Occurrence of Salmonella and Campylobacter; Q2: The presence in poultry (i.e. poultry at farm (consider broiler, laying hen, turkey, and ducks), poultry at slaughterhouse); Q3: Co‐infection (co‐colonisation) or co‐detection in samples, animals. The answers to these questions needed to be positive or unclear to proceed to the next step.
Step 3: screening of remaining 199 records at full text based on records characteristics (i.e. availability of full text, primary research study and language).
Step 4: screening of remaining 199 records at full‐text level by answering to the same specific questions as for step 2. The answers to these questions needed to be positive for inclusion.
An overview of the studies obtained is given in Tables C.7 (farm) and 21 (slaughterhouse).
Appendix D – Additional tables on the distribution of confirmed salmonellosis cases in humans by serovar
1.
Appendix E – Salmonella serovars in various animal populations based on reported data and model estimates
1.
Appendix F – Uncertainty
1.
Appendix G – MS data used to assess the effect of the housing system on the Salmonella occurrence in broiler flocks, 2016–2017
1.
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Dewulf J, Hald T, Michel V, N Taina, Ricci A, Snary E, Boelaert F, Messens W and Davies R, 2019. Scientific Opinion on the Salmonella control in poultry flocks and its public health impact. EFSA Journal 2019;17(2):5596, 155 pp. 10.2903/j.efsa.2019.5596
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00692
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Kostas Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Acknowledgements: The BIOHAZ Panel wishes to thank the following for the support provided to this scientific output: the AHAW Panel: Julio Alvarez, Dominique Bicout, Paolo Calistri, Klaus Depner, Julian Ashley Drewe, Bruno Garin‐Bastuji, Jose Luis Gonzales Rojas, Christian Gortázar, Virginie Michel, Miguel Angel Miranda, Helen Roberts, Liisa Sihvonen, Hans Spoolder, Karl Stahl, Arvo Viltrop, and Christoph Winckler. The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Amendment: An editorial correction was carried out that does not materially affect the contents or outcome of this scientific output. On page 27, section 3.1.1.2, percentages of hatchery associated Salmonella contamination in Canada have been changed from 15.5% of human infections due to poultry meat and 20% due to eggs, to 4% and 8%, respectively. To avoid confusion, the older version has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.
Adopted: 16 January 2019
Amended: 8 April 2019
Notes
Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of Salmonella and other specified food‐borne zoonotic agents. OJ L 325, 12.12.2003, p. 1–15.
The latest targets can be found in Regulation (EU) No 200/2010 (breeding hens), Regulation (EC) No 517/2011 (laying hens), Regulation (EU) No 200/2012 (broilers) and Regulation (EU) No 1190/2012 (turkeys).
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC (OJ L 325, 12.12.2003, p. 31).
The latest published version contains 2015 data (EFSA Journal 2016;14(12):4634).
Report of the Task Force on Zoonoses Data Collection on the Analysis of the baseline survey on the prevalence of Salmonella in holdings of laying hens flocks of Gallus gallus, The EFSA Journal (2007), 97; Report of the Task Force on Zoonoses Data Collection on the Analysis of the baseline survey on the prevalence of Salmonella in holdings of broiler flocks of Gallus gallus, Part B, The EFSA Journal (2007), 101, 1–86; Report of the Task Force on Zoonoses Data Collection on the Analysis of the baseline survey on the prevalence of Salmonella in turkey flocks, Part B, The EFSA Journal (2008), 198, 1–224.
From 1 January 2012, due to the application of Directive 1999/74/EC laying down minimum standards for the protection of laying hens, the use of unenriched cages systems was banned in the EU for all holdings. Council Directive 1999/74/EC of 19 July 1999 laying down minimum standards for the protection of laying hens. OJ L 203, 3.8.1999, p. 1–53.
From 30 June 2010, due to the application of Directive 2007/42/EC laying down minimum rules for the protection of chicken kept for meat production, animal welfare standards were applicable to most broiler chickens farms in the EU. This Directive also introduces the principle of animal‐based indicators to be monitored in slaughterhouses.
EFSA Scientific Opinion on the risk of human campylobacteriosis linked to broiler meat (EFSA Journal 2010: 8(1): 1437).
Commission Regulation (EU) No 200/2012 of 8 March 2012 concerning a Union target for the reduction of Salmonella enteritidis and Salmonella typhimurium in flocks of broilers, as provided for in Regulation (EC) No 2160/2003 of the European Parliament and of the Council Text with EEA relevance. OJ L 71, 9.3.2012, p. 31–36.
Commission Regulation (EU) No 517/2011 of 25 May 2011 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of certain Salmonella serotypes in laying hens of Gallus gallus and amending Regulation (EC) No 2160/2003 and Commission Regulation (EU) No 200/2010 (text with EEA relevance). OJ L 138, 26.5.2011, p. 45–51
Farming methods for laying hens are defined in the EU by Commission Regulation (EC) No 589/2008 of 23 June 2008 laying down detailed rules for implementing Council Regulation (EC) No 1234/2007 as regards marketing standards for eggs. OJ L 163, 24.6.2008, p. 1–6.
Types of farming, besides organic farming, are defined in EU by Commission Regulation (EC) No 543/2008 of 16 June 2008 laying down detailed rules for the application of Council Regulation (EC) No 1234/2007 as regards the marketing standards for poultry meat. OJ L 157, 17.6.2008, p. 1‐46.
For example, if there is correlation between animal welfare indicators monitored in farms or in slaughterhouses and the risk of contamination (see I Alpigiani, C Bacci, LJ Keeling, MD Salman, F Brindani, S Pongolini, PL Hitchens and S Bonardi. 2016, The associations between animal‐based welfare measures and the presence of indicators of food safety in finishing pigs, Universities Federation for Animal Welfare, Animal Welfare 2016, 25: 355‐363, ISSN 0962‐7286).
Commission Regulation (EU) No 200/2010 of 10 March 2010 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of Salmonella serotypes in adult breeding flocks of Gallus gallus. OJ L 61, 11.3.2010, p. 1–9.
More specifically, ‘S. 13,23:i:‐’ has been revised to ‘S. 1,13,23:i:‐’, while ‘S. I 4,12:d:‐’ and ‘S. I 4,12:d:‐’ have been revised to ‘S. 1,4,12:d:‐’. The following reporting has been revised to ‘Salmonella spp., unspecified’: ‘Salmonella’, ‘Salmonella other serovars’, ‘Salmonella not typeable’, ‘S. enterica’, ‘S. Enterica, unspecified’, ‘S. enterica subsp. enterica rough’, ‘S. enterica, subspecies diarizonae’, ‘S. enterica, subspecies enterica’, ‘Salmonella enterica, subspecies salamae’, ‘S. enterica, unspecified O:3,10’, ‘S. enterica, unspecified O:21’, ‘S. group C1’, ‘S. group O:7’, ‘S. I, group O:1,3,19’, ‘S. I, group O:7’, ‘S. I, group O:30’, ‘S. I, group O:41’, ‘S. I 4,12:‐:‐‘, ‘S. I 6,7:‐:‐‘, ‘S. I 6,7:‐:1,5’, ‘S. II, group O:4’, ‘S. 1,4,5,12:‐:1,2’, ‘S. 1,4,[5],12:‐:1,2’, ‘S. 1,4,[5],12:‐:‐’, ‘S. 3,19:‐:‐’, ‘S. 4,12:l,v:‐’, ‘S. 6,7:‐:e,n,z15’, ‘S. 6,7:b:‐’, ‘S. 6,7:r:‐’, ‘S. 6,8:z10:‐’, ‘S. 9,46:‐:‐’, ‘S. 13,23:‐:‐’. Monophasic variants of S. Typhimurium have been reported by MS using several different designations, generally as the generic term ‘monophasic S. Typhimurium’ or by using the antigenic formula with different levels of details in terms of antigens investigated and identified. The isolates belonging to the group of monophasic variants of S. Typhimurium and reported by MS with different designations (S. Typhimurium monophasic, S. 1,4,[5],12:i:‐, S. 1,4,5,12:i:‐, S. 1,4,12:i:‐, S. 4,[5],12:i:‐, S. 4,5,12:i:‐ and S. 4,12:i:‐) were merged into the same group and named ‘S. Typhimurium, monophasic’.
Following reporting has been revised to ‘Salmonella spp., unspecified’: ‘ENTERICA’, ‘Group11’, ‘Group13’, ‘Group3.10’, ‘Group4’, Group6.14’, ‘Group60’, ‘Group61’, ‘Group7’, ‘Group8’, ‘Group9’, ‘GroupA’, ‘GroupB’, ‘GroupC’, ‘GroupC1’, ‘GroupC2’, ‘GroupD’, ‘GroupD1’, ‘GroupD2’, ‘GroupE’, ‘GroupE1’, ‘GroupE4’, ‘GroupF’, GroupG, ‘GroupH’, ‘GroupI’, ‘GroupJ’, ‘GroupK’, ‘GroupM’, ‘GroupO’, ‘GroupR’, ‘GroupS’, ‘GroupT’, ‘GroupX’, ‘GroupZ’, ‘OTHER’, ‘SUBSPI’, ‘SUBSPII’, ‘SubspIIIa’, ‘SubspIIIb’, ‘SubspIV’, ‘SubspV’, ‘SubspVI’. Also ‘S. choleraesuis var. Decatur’ and ‘S. choleraesuis var. Kunzendorf’ have been grouped as ‘S. Choleraesuis’, ‘S. anatum var. 15+’ has been changed to ‘S. Anatum’ and ‘S. weltevreden var. 15+’ has been revised to ‘S. Weltevreden’. ‘S. monophasic Typhimurium 1.4.[5].12:I:‐‘ has been named ‘S. Typhimurium, monophasic’.
Aggregate of codes 020711;01/01/1996;31/12/2500;LIBEN;0;FRESH OR CHILLED FOWLS OF THE SPECIES GALLUS DOMESTICUS, 020712;01/01/1996;31/12/2500;LIBEN;0;FROZEN FOWLS OF THE SPECIES GALLUS DOMESTICUS, 020713;01/01/1996;31/12/2500;LIBEN;0;FRESH OR CHILLED CUTS AND EDIBLE OFFAL OF FOWLS OF THE SPECIES GALLUS DOMESTICUS and 020714;01/01/1996;31/12/2500;LIBEN;0;FROZEN CUTS AND EDIBLE OFFAL OF FOWLS OF THE SPECIES GALLUS DOMESTICUS.
Aggregate of codes 020724;01/01/1996;31/12/2500;LIBEN;0;FRESH OR CHILLED TURKEYS OF THE SPECIES DOMESTICUS, 020725;01/01/1996;31/12/2500;LIBEN;0;FROZEN TURKEYS OF THE SPECIES DOMESTICUS, 020726;01/01/1996;31/12/2500;LIBEN;0;FRESH OR CHILLED CUTS AND EDIBLE OFFAL OF TURKEYS OF THE SPECIES DOMESTICUS and 020727;01/01/1996;31/12/2500;LIBEN;0;FROZEN CUTS AND EDIBLE OFFAL OF TURKEYS OF THE SPECIES DOMESTICUS.
Code 0203;01/01/1988;31/12/2500;LIBEN;0;MEAT OF SWINE.
Code 04079010 – POULTRY EGGS, IN SHELL, PRESERVED OR COOKED.
German colony system with group production similar to the enriched cage.
A run area attached to a barn that allows birds additional space to dust‐bath in litter and experience natural light.
JORF no 0194 du 24 août 2018 texte no 50. Arrêté du 1er août 2018 relatif à la surveillance et à la lutte contre les infections à Salmonella dans les troupeaux de l'espèce Gallus gallus en filière ponte d’œufs de consommation; NOR: AGRG1734200A; ELI: https://www.legifrance.gouv.fr/eli/arrete/2018/8/1/AGRG1734200A/jo/texte
Council Regulation (EC) No 1804/1999 of 19 July 1999 supplementing Regulation (EEC) No 2092/91 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs to include livestock production. OJ L 222, 24.8.1999, p. 1–28.
References
- ACMSF (Advisory Committee on the Microbiological Safety of Food), 2016.Ad Hoc Group on Eggs An update on the microbiological risk from shell eggs and their products. 181 pp. https://acmsf.food.gov.uk/sites/default/files/acmsf-egg-reportv1.pdf
- Adesiyun A, Webb L, Musai L, Louison B, Joseph G, Stewart‐Johnson A, Samlal S and Rodrigo S, 2014. Survey of Salmonella contamination in chicken layer farms in three Caribbean countries. Journal of Food Protection, 77, 1471–1480. 10.4315/0362-028x.jfp-14-021 [DOI] [PubMed] [Google Scholar]
- Agunos A, Waddell L, Leger D and Taboada E, 2014. A Systematic Review Characterizing On‐Farm Sources of Campylobacter spp. for Broiler Chickens. PLoS ONE, 9, e104905. 10.1371/journal.pone.0104905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alali WQ, Siddhartha T, Berghaus RD, Martin MP and Gebreyes WA, 2010. Prevalence and distribution of Salmonella in organic and conventional broiler poultry farms. Foodborne Pathogens and Disease, 7, 1363–1371. [DOI] [PubMed] [Google Scholar]
- Alebachew K and Mekonnen A, 2013. A survey on Salmonella infection among chicken flocks in Jimma Town, Ethiopia. World Applied Sciences Journal, 21, 1415–1420. [Google Scholar]
- Allain V, Chemaly M, Laisney MJ, Rouxel S, Quesne S and Le Bouquin S, 2014. Prevalence of and risk factors for Campylobacter colonisation in broiler flocks at the end of the rearing period in France. British Poultry Science, 55, 452–459. 10.1080/00071668.2014.941788 [DOI] [PubMed] [Google Scholar]
- Allard MW, Bell R, Ferreira CM, Gonzalez‐Escalona N, Hoffmann M, Muruvanda T, Ottesen A, Ramachandran P, Reed E, Sharma S, Stevens E, Timme R, Zheng J and Brown EW, 2018. Genomics of foodborne pathogens for microbial food safety. Current Opinion in Biotechnology, 49, 224–229. 10.1016/j.copbio.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Allen KJ and Poppe C, 2002. Occurrence and characterization of resistance to extended‐spectrum cephalosporins mediated by beta‐lactamase CMY‐2 in Salmonella isolated from food‐producing animals in Canada. Canadian Journal of Veterinary Research‐Revue Canadienne De Recherche Veterinaire, 66, 137–144. [PMC free article] [PubMed] [Google Scholar]
- Alpigiani I, Abrahantes JC, Michel V, Huneau‐Salaun A, Chemaly M, Keeling LJ, Gervelmeyer A, Bacci C, Brindani F, Bonardi S and Berthe F, 2017. Associations between animal welfare indicators and Campylobacter spp. in broiler chickens under commercial settings: A case study. Preventive Veterinary Medicine, 147, 186–193. 10.1016/j.preveimed.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Alter T, Weber RM, Hamedy A and Glunder G, 2011. Carry‐over of thermophilic Campylobacter spp. between sequential and adjacent poultry flocks. Veterinary Microbiology, 147, 90–95. 10.1016/j.vetmic.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Angulo FJ, Nargund VN and Chiller TC, 2004. Evidence of an association between use of anti‐microbial agents in food animals and anti‐microbial resistance among bacteria isolated from humans and the human health consequences of such resistance. Journal of Veterinary Medicine Series B‐Infectious Diseases and Veterinary Public Health, 51, 374–379. 10.1111/j.1439-0450.2004.00789.x [DOI] [PubMed] [Google Scholar]
- Antony L, Behr M, Sockett D, Miskimins D, Aulik N, Christopher‐Hennings J, Nelson E, Allard MW and Scaria J, 2018. Genome divergence and increased virulence of outbreak associated Salmonella enterica subspecies enterica serovar Heidelberg. Gut Pathogens, 10, 53. 10.1186/s13099-018-0279-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- APHA (Animal Plant and Health Agency), 2018. Salmonella in livestock production in Great Britain, 2017.
- Arnold ME, Carrique‐Mas JJ and Davies RH, 2010. Sensitivity of environmental sampling methods for detecting Salmonella Enteritidis in commercial laying flocks relative to the within‐flock prevalence. Epidemiology and Infection, 138, 330–339. 10.1017/s0950268809990598 [DOI] [PubMed] [Google Scholar]
- Arnold ME, Carrique‐Mas JJ, McLaren I and Davies RH, 2011. A comparison of pooled and individual bird sampling for detection of Salmonella in commercial egg laying flocks. Preventive Veterinary Medicine, 99, 176–184. 10.1016/j.prevetmed.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Arnold ME, Martelli F, McLaren I and Davies RH, 2014a. Estimation of the sensitivity of environmental sampling for detection of Salmonella in commercial layer flocks post‐introduction of national control programmes. Epidemiology and Infection, 142, 1061–1069. 10.1017/s0950268813002173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold ME, Martelli F, McLaren I and Davies RH, 2014b. Estimation of the rate of egg contamination from Salmonella‐infected chickens. Zoonoses Public Health, 61, 18–27. 10.1111/zph.12038 [DOI] [PubMed] [Google Scholar]
- Arsenault J, Letellier A, Quessy S and Boulianne M, 2007. Prevalence and risk factors for Salmonella and Campylobacter spp. carcass contamination in broiler chickens slaughtered in Quebec, Canada. Journal of Food Protection, 70, 1820–1828. [DOI] [PubMed] [Google Scholar]
- AVEC (Association of Poultry Processors and Poultry Trade in the EU Countries), 2017. Annual report. 52 pp.
- AVEC (Association of Poultry Processors and Poultry Trade in the EU Countries), 2018. Annual report. 38 pp.
- Aviv G, Tsyba K, Steck N, Salmon‐Divon M, Cornelius A, Rahav G, Grassl GA and Gal‐Mor O, 2014. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environmental Microbiology, 16, 977–994. 10.1111/1462-2920.12351 [DOI] [PubMed] [Google Scholar]
- Awad WA, Hess C and Hess M, 2018. Re‐thinking the chicken‐Campylobacter jejuni interaction: a review. Avian Pathology, 47, 352–363. 10.1080/03079457.2018.1475724 [DOI] [PubMed] [Google Scholar]
- Bahrndorff S, Rangstrup‐Christensen L, Nordentoft S and Hald B, 2013. Foodborne disease prevention and broiler chickens with reduced Campylobacter infection. Emerging Infectious Diseases, 19, 425–430. 10.3201/eid1903.111593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JS, Stern NJ, Fedorka‐Cray P, Craven SE, Cox NA, Cosby DE, Ladely S and Musgrove MT, 2001. Sources and movement of Salmonella through integrated poultry operations: A multistate epidemiological investigation. Journal of Food Protection, 64, 1690–1697. Available online: 10.4315/0362-028x-64.11.1690 [DOI] [PubMed] [Google Scholar]
- Barbut S, 2015.The Science of Poultry and Meat Processing. http://hdl.handle.net/10214/9300
- Battersby T, Whyte P and Bolton D, 2016. Protecting broilers against Campylobacter infection by preventing direct contact between farm staff and broilers. Food Control, 69, 346–351. 10.1016/j.foodcont.2016.04.053 [DOI] [Google Scholar]
- Baxter M, Bailie CL and O'Connell NE, 2018.Play behaviour, fear responses and activity levels in commercial broiler chickens provided with preferred environmental enrichments. Animal: An International Journal of Animal Bioscience, 13, 171–179. 10.1017/s1751731118001118 [DOI] [PubMed] [Google Scholar]
- Bearson BL, Bearson SMD, Looft T, Cai G and Shippy DC, 2017. Characterization of a multidrug‐resistant Salmonella enterica serovar Heidelberg outbreak strain in commercial turkeys: colonization, transmission, and host transcriptional response. Frontiers in Veterinary Science, 4, 156. 10.3389/fvets.2017.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender J, 2010. Organic foods: are they safer? Ontario Veterinary Medical Association 2010 Conference Proceedings. Toronto, Canada, January 28‐30, 2010, 185–188.
- Berghaus RD, Thayer SG, Law BF, Mild RM, Hofacre CL and Singer RS, 2013. Enumeration of Salmonella and Campylobacter spp. in environmental farm samples and processing plant carcass rinses from commercial broiler chicken flocks. Applied and Environmental Microbiology, 79, 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndtson E, Emanuelson U, Engvall A and DainelssonTham ML, 1996. A 1‐year epidemiological study of campylobacters in 18 Swedish chicken farms. Preventive Veterinary Medicine, 26, 167–185. 10.1016/0167-5877(95)01008-4 [DOI] [Google Scholar]
- Bertelloni F, Tosi G, Massi P, Fiorentini L, Parigi M, Cerri D and Ebani VV, 2017. Some pathogenic characters of paratyphoid Salmonella enterica strains isolated from poultry. Asian Pacific Journal of Tropical Medicine, 10, 1161–1166. 10.1016/j.apjtm.2017.10.023 [DOI] [PubMed] [Google Scholar]
- Boelaert F, Amore G, Van der Stede Y and Hugas M, 2016. EU‐wide monitoring of biological hazards along the food chain: achievements, challenges and EFSA vision for the future. Current Opinion in Food Science, 12, 52–62. 10.1016/j.cofs.2016.08.004 [DOI] [Google Scholar]
- Bohaychuk VM, Checkley SL, Gensler GE and Barrios PR, 2009. Microbiological baseline study of poultry slaughtered in provincially inspected abattoirs in Alberta, Canada. Canadian Veterinary Journal‐Revue Veterinaire Canadienne, 50, 173–178. [PMC free article] [PubMed] [Google Scholar]
- Borta ND, Pop IM, Carp‐Carare M and Obada MD, 2011. Highlighting of some commensal bacteria, potentially pathogenic, from broiler chicken cecum in which feed were used feed additives. Lucrari Stiintifice ‐ Universitatea de Stiinte Agricole si Medicina Veterinara, Seria Zootehnie, 56, 163–166. [Google Scholar]
- Briones V, Tellez S, Goyache J, Ballesteros C, Lanzarot MD, Dominguez L and Fernandez‐Garayzabal JF, 2004. Salmonella diversity associated with wild reptiles and amphibians in Spain. Environmental Microbiology, 6, 868–871. 10.1111/j.1462-2920.2004.00631.x [DOI] [PubMed] [Google Scholar]
- Bronowski C, James CE and Winstanley C, 2014. Role of environmental survival in transmission of Campylobacter jejuni . Fems Microbiology Letters, 356, 8–19. 10.1111/1574-6968.12488 [DOI] [PubMed] [Google Scholar]
- Byrd JA, Corrier DE, Deloach JR, Nisbet DJ and Stanker LH, 1998. Horizontal transmission of Salmonella typhimurium in broiler chicks. Journal of Applied Poultry Research, 7, 75–80. 10.1093/japr/7.1.75 [DOI] [Google Scholar]
- Calenge F and Beaumont C, 2012. Toward integrative genomics study of genetic resistance to Salmonella and Campylobacter intestinal colonization in fowl. Frontiers in Genetics, 3, 261‐Article No.: 261. 10.3389/fgene.2012.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott KA, Fridriksdottir V, Reiersen J, Lowman R, Bisaillon J‐R, Gunnarsson E, Berndtson E, Hiett KL, Needleman DS and Stern NJ, 2006. Lack of evidence for vertical transmission of Campylobacter spp. in chickens. Applied and Environmental Microbiology, 72, 5794–5798. 10.1128/aem.02991-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMCON (Campylobacter control – novel approaches in primary poultry production), 2016. CAMCON Report Summary. https://cordis.europa.eu/result/rcn/176026_en.html
- Campbell D, Tagg K, Bicknese A, McCullough A, Chen J, Karp BE and Folster JP, 2018. Identification and characterization of Salmonella enterica serotype Newport isolates with decreased susceptibility to ciprofloxacin in the United States. Antimicrobial Agents and Chemotherapy, 62, e00653–18. 10.1128/aac.00653-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni F, Cao G, Kastanis G, Janies DA, Morato Bergamini AM, Rodrigues DdP, Stones R, Brown E, Allard MW and Falcao JP, 2018. Changing of the genomic pattern of Salmonella Enteritidis strains isolated in Brazil over a 48 year‐period revealed by Whole Genome SNP analyses. Scientific Reports, 8. 10.1038/s41598-018-28844-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J, Mourao J, Silveira L, Saraiva M, Correia CB, Macas AP, Peixe L and Antunes P, 2018. Imported poultry meat as a source of extended‐spectrum cephalosporin‐resistant CMY‐2‐producing Salmonella Heidelberg and Salmonella Minnesota in the European Union, 2014‐2015. International Journal of Antimicrobial Agents, 51, 151–154. 10.1016/j.ijantimicag.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Cao G, Allard M, Hoffmann M, Muruvanda T, Luo Y, Payne J, Meng K, Zhao S, McDermott P, Brown E and Meng J, 2018a. Sequence Analysis of IncA/C and IncI1 Plasmids Isolated from Multidrug‐Resistant Salmonella Newport Using Single‐Molecule Real‐Time Sequencing. Foodborne Pathogens and Disease, 15, 361–371. 10.1089/fpd.2017.2385 [DOI] [PubMed] [Google Scholar]
- Cao Y, Shen Y, Cheng L, Zhang X, Wang C, Wang Y, Zhou X, Chao G and Wu Y, 2018b. Combination of multilocus sequence typing and pulsed‐field gel electrophoresis reveals an association of molecular clonality with the emergence of extensive‐drug resistance (XDR) in Salmonella . Microbiological Research, 207, 170–176. 10.1016/j.micres.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Card R, Vaughan K, Bagnall M, Spiropoulos J, Cooley W, Strickland T, Davies R and Anjum MF, 2016. Virulence Characterisation of Salmonella enterica Isolates of Differing Antimicrobial Resistance Recovered from UK Livestock and Imported Meat Samples. Frontiers in Microbiology, 7. 10.3389/fmicb.2016.00640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfora V, Alba P, Leekitcharoenphon P, Ballaro D, Cordaro G, Di Matteo P, Donati V, Ianzano A, Iurescia M, Stravino F, Tagliaferri T, Battisti A and Franco A, 2018. Colistin Resistance Mediated by mcr‐1 in ESBL‐Producing, Multidrug Resistant Salmonella Infantis in Broiler Chicken Industry, Italy (2016‐2017). Frontiers in Microbiology, 9. 10.3389/fmicb.2018.01880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrique‐Mas JJ and Davies RH, 2008. Sampling and bacteriological detection of Salmonella in poultry and poultry premises: a review. Revue Scientifique Et Technique‐Office International Des Epizooties, 27, 665–677. 10.20506/rst.27.3.1829 [DOI] [PubMed] [Google Scholar]
- Carrique‐Mas JJ, Breslin M, Snow L, McLaren I, Sayers AR and Davies RH, 2009. Persistence and clearance of different Salmonella serovars in buildings housing laying hens. Epidemiology and Infection, 137, 837–846. 10.1017/s0950268808001568 [DOI] [PubMed] [Google Scholar]
- Carson A and Davies R, 2018. Salmonellosis in sheep. Veterinary Record, 183, 539–539. 10.1136/vr.k4569 [DOI] [PubMed] [Google Scholar]
- Cawthraw SA, Wassenaar TM, Ayling R and Newell DG, 1996. Increased colonization potential of Campylobacter jejuni strain 81116 after passage through chickens and its implication on the rate of transmission within flocks. Epidemiology and Infection, 117, 213–215. 10.1017/s0950268800001333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention), 2018. Multistate Outbreak of Salmonella Braenderup Infections Linked to Rose Acre Farms Shell Eggs (Final Update). https://www.cdc.gov/salmonella/braenderup-04-18/index.html
- Cerda‐Cuellar M, More E, Ayats T, Aguilera M, Munoz‐Gonzalez S, Antilles N, Ryan PG and Gonzalez‐Solis J, 2018. Do humans spread zoonotic enteric bacteria in Antarctica? The Science of the Total Environment, 654, 190–196. 10.1016/j.scitotenv.2018.10.272 [DOI] [PubMed] [Google Scholar]
- Chander Y, Gupta SC, Kumar K, Goyall SM and Murray H, 2008. Antibiotic use and the prevalence of antibiotic resistant bacteria on turkey farms. Journal of the Science of Food and Agriculture, 88, 714–719. 10.1002/jsfa.3141 [DOI] [Google Scholar]
- Chemaly M, Huneau‐Salaun A, Labbe A, Houdayer C, Petetin I and Fravalo P, 2009. Isolation of Salmonella enterica in laying‐hen flocks and assessment of eggshell contamination in France. Journal of Food Protection, 72, 2071–2077. 10.4315/0362-028x-72.10.2071 [DOI] [PubMed] [Google Scholar]
- Chiou C‐S, Hong Y‐P, Liao Y‐S, Wang Y‐W, Tu Y‐H, Chen B‐H and Chen Y‐S, 2019. New Multidrug‐Resistant Salmonella enterica Serovar Anatum Clone, Taiwan, 2015‐2017. Emerging Infectious Diseases, 25, 144–147. 10.3201/eid2501.181103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousalkar K, Gast R, Martelli F and Pande V, 2018. Review of egg‐related salmonellosis and reduction strategies in United States, Australia, United Kingdom and New Zealand. Critical Reviews in Microbiology, 44, 290–303. 10.1080/1040841x.2017.1368998 [DOI] [PubMed] [Google Scholar]
- Chriel M, Stryhn H and Dauphin G, 1999. Generalised linear mixed models analysis of risk factors for contamination of Danish broiler flocks with Salmonella typhimurium . Preventive Veterinary Medicine, 40, 1–17. [DOI] [PubMed] [Google Scholar]
- CLASSP (LACORS/HPA Coordinated Local Authority Sentinel Surveillance of Pathogens), 2007. Two Year Surveillance Report 1 November 2004 to 31 October 2006. LACORS/HPA Coordinated Local Authority Sentinel Surveillance of Pathogens. http://www.lacors.gov.uk/LACORS/ContentDetails.aspx?id=17579
- Cogan TA, Jorgensen F, Lappin‐Scott HM, Benson CE, Woodward MJ and Humphrey TJ, 2004. Flagella and curli fimbriae are important for the growth of Salmonella enterica serovars in hen eggs. Microbiology, 150, 1063–1071. 10.1099/mic.0.26791-0 [DOI] [PubMed] [Google Scholar]
- Corrier DE, Hinton A Jr, Hargis B and DeLoach JR, 1992. Effect of used litter from floor pens of adult broilers on Salmonella colonization of broiler chicks. Avian Diseases, 36, 897–902. [PubMed] [Google Scholar]
- Corry JE, Allen VM, Hudson WR, Breslin MF and Davies RH, 2002. Sources of Salmonella on broiler carcasses during transportation and processing: modes of contamination and methods of control. Journal of Applied Microbiology, 92, 424–432. [DOI] [PubMed] [Google Scholar]
- Cox NA, Stern NJ, Craven SE, Berrang ME and Musgrove MT, 2000. Prevalence of Campylobacter and Salmonella in the cecal droppings of turkeys during production. Journal of Applied Poultry Research, 9, 542–545. [Google Scholar]
- Cox NA, Craven SE, Musgrove MT, Berrang ME and Stern NJ, 2003. Effect of sub‐therapeutic levels of antimicrobials in feed on the intestinal carriage of Campylobacter and Salmonella in turkeys. Journal of Applied Poultry Research, 12, 32–36. [Google Scholar]
- Cox NA, Cosby DE, McLendon BL, Wilson JL, Hofacre CL, Berrang ME and Hinton A Jr, 2018. Persistence of Campylobacter and Salmonella in the Ceca, Spleen and Liver/gallbladder of Inoculated Broilers. International Journal of Poultry Science, 17, 374–377. 10.3923/ijps.2018.374.377 [DOI] [Google Scholar]
- Crabb HK, Allen JL, Devlin JM, Firestone SM, Wilks CR and Gilkerson JR, 2018. Salmonella spp. transmission in a vertically integrated poultry operation: Clustering and diversity analysis using phenotyping (serotyping, phage typing) and genotyping (MLVA). PLoS ONE, 13, e0201031. 10.1371/journal.pone.0201031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo MD, Kathariou S, Grimes JL, Cox NA, Buhr RJ, Frye JG, Miller WG, Jackson CR and Smith DP, 2016. Routes of transmission of Salmonella and Campylobacter in breeder turkeys. Journal of Applied Poultry Research, 25, 591–609. [Google Scholar]
- Crim SM, Chai SJ, Karp BE, Judd MC, Reynolds J, Swanson KC, Nisler A, McCullough A and Gould LH, 2018. Salmonella enterica serotype Newport Infections in the United States, 2004‐2013: Increased Incidence Investigated Through Four Surveillance Systems. Foodborne Pathogens and Disease, 15, 612–620. 10.1089/fpd.2018.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCurraize C , Amoureux L, Bador J, Chapuis A, Siebor E, Clement C, Sauge J, Aho‐Glele LS and Neuwirth C, 2017. “Does the Salmonella Genomic Island 1 (SGI1) confer invasiveness properties to human isolates?”. BMC Infectious Diseases, 17. 10.1186/s12879-017-2847-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman T, Ashton P, Schafer U, Jironkin A, Painset A, Shaaban S, Hartman H, Myers R, Underwood A, Jenkins C and Grant K, 2018. SnapperDB: a database solution for routine sequencing analysis of bacterial isolates. Bioinformatics, 34, 3028–3029. 10.1093/bioinformatics/bty212 [DOI] [PubMed] [Google Scholar]
- Davies RH, 2005. Pathogen populations on poultry farms. Ed Mead GC, pp. 101–152. Food safety control in the poultry industry. ISBN: 978‐1‐85573‐954‐3\1‐85573‐954‐2
- Davies R and Breslin M, 2001. Environmental contamination and detection of Salmonella enterica serovar enteritidis in laying flocks. Veterinary Record, 149, 699–704. [PubMed] [Google Scholar]
- Davies R and Breslin M, 2003. Observations on Salmonella contamination of commercial laying farms before and after cleaning and disinfection. Veterinary Record, 152, 283–287. 10.1136/vr.152.10.283 [DOI] [PubMed] [Google Scholar]
- Davies R and Carrique‐Mas JJ, 2010a. Investigation of the efficiency of delivery of Salmonella vaccines in laying hens. Proceedings of the I3S International Symposium Salmonella and Salmonellosis, St Malo, France, p. 411–415.
- Davies R and Carrique‐Mas JJ, 2010b. Clearance of Salmonella from laying flocks after eradication of rodents from laying houses. Proceedings of the I3S International Symposium Salmonella and Salmonellosis, St Malo, France, p. 28–30.
- Davies R, Breslin M, Corry JEL, Hudson W and Allen VM, 2001. Observations on the distribution and control of Salmonella species in two integrated broiler companies. Veterinary Record, 149, 227–232. 10.1136/vr.149.8.227 [DOI] [PubMed] [Google Scholar]
- Davies R, Liebana E and Breslin M, 2003. Investigation of the distribution and control of Salmonella enterica serovar Enteritidis PT6 in layer breeding and egg production. Avian Pathology, 32, 225–237. 10.1080/0307945031000097822 [DOI] [PubMed] [Google Scholar]
- De Buck J, Van Immerseel F, Haesebrouck F and Ducatelle R, 2004. Effect of type 1 fimbriae of Salmonella enterica serotype Enteritidis on bacteraemia and reproductive tract infection in laying hens. Avian Pathology, 33, 314–320. 10.1080/0307945042000220561 [DOI] [PubMed] [Google Scholar]
- De Cesare A, 2018. Salmonella in Foods: A Reemerging Problem. Advances in Food and Nutrition Research, 86, 137–179. 10.1016/bs.afnr.2018.02.007 [DOI] [PubMed] [Google Scholar]
- De Vylder J, Van Hoorebeke S, Ducatelle R, Pasmans F, Haesebrouck F, Dewulf J and Van Immerseel F, 2009. Effect of the housing system on shedding and colonization of gut and internal organs of laying hens with Salmonella Enteritidis. Poultry Science, 88, 2491–2495. 10.3382/ps.2009-00203 [DOI] [PubMed] [Google Scholar]
- Deblais L, Lorentz B, Scaria J, Nagaraja KV, Nisar M, Lauer D, Voss S and Rajashekara G, 2018. Comparative Genomic Studies of Salmonella Heidelberg Isolated From Chicken‐ and Turkey‐Associated Farm Environmental Samples. Frontiers in Microbiology, 9. 10.3389/fmicb.2018.01841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmidt M, Ducatelle R and Haesebrouck F, 1997. Pathogenesis of Salmonella enteritidis phage type four after experimental infection of young chickens. Veterinary Microbiology, 56, 99–109. 10.1016/s0378-1135(96)01350-8 [DOI] [PubMed] [Google Scholar]
- Dewaele I, Van Meirhaeghe H, Rasschaert G, Vanrobaeys M, De Graef E, Herman L, Ducatelle R, Heyndrickx M and De Reu K, 2012. Persistent Salmonella Enteritidis environmental contamination on layer farms in the context of an implemented national control program with obligatory vaccination. Poultry Science, 91, 282–291. 10.3382/ps.2011-01673 [DOI] [PubMed] [Google Scholar]
- Dunlop MW, Moss AF, Groves PJ, Wilkinson SJ, Stuetz RM and Selle PH, 2016. The multidimensional causal factors of ‘wet litter’ in chicken‐meat production. Science of the Total Environment, 562, 766–776. 10.1016/j.scitotenv.2016.03.147 [DOI] [PubMed] [Google Scholar]
- Dutil L, Irwin R, Finley R, Lai King N, Avery B, Boerlin P, Bourgault AM, Cole L, Daignault D, Desruisseau A, Demczuk W, Hoang L, Horsman GB, Ismail J, Jamieson F, Maki A, Pacagnella A and Pillai DR, 2010. Ceftiofur resistance in Salmonella enterica Serovar Heidelberg from chicken meat and humans, Canada. Emerging Infectious Diseases, 16, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Knegt LV, Pires SM, Lofstrom C, Sorensen G, Pedersen K, Torpdahl M, Nielsen EM and Hald T, 2016. Application of Molecular Typing Results in Source Attribution Models: The Case of Multiple Locus Variable Number Tandem Repeat Analysis (MLVA) of Salmonella Isolates Obtained from Integrated Surveillance in Denmark. Risk Analysis, 36, 571–588. 10.1111/risa.12483 [DOI] [PubMed] [Google Scholar]
- de Vylder J, Dewulf J, Sv Hoorebeke, Pasmans F, Haesebrouck F, Ducatelle R and Fv Immerseel, 2011. Horizontal transmission of Salmonella Enteritidis in groups of experimentally infected laying hens housed in different housing systems. Poultry Science, 90, 1391–1396. 10.3382/ps.2010-00944 [DOI] [PubMed] [Google Scholar]
- Eade CR, Bogomolnaya L, Hung CC, Betteken MI, Adams LG, Andrews‐Polymenis H and Altier C, 2019. Salmonella Pathogenicity Island 1 Is Expressed in the Chicken Intestine and Promotes Bacterial Proliferation. Infection and Immunity, 87, e00503–e00518. 10.1128/iai.00503-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2018. Epidemiological update: Multi‐country outbreak of Salmonella Enteritidis infections linked to Polish eggs. https://ecdc.europa.eu/en/news-events/epidemiological-update-multi-country-outbreak-salmonella-enteritidis-infections-linked
- ECDC and EFSA (European Centre for Disease Prevention and Control and European Food Safety Authority), 2014a. Multi‐country outbreak of Salmonella Enteritidis infections associated with consumption of eggs from Germany. EFSA supporting publication 2014:EN‐646, 10 pp. 10.2903/sp.efsa.2014.en-646 [DOI] [Google Scholar]
- ECDC and EFSA (European Centre for Disease Prevention and Control and European Food Safety Authority), 2014b. Multi‐country outbreak of Salmonella Stanley infections ‐ Third update. EFSA supporting publication 2014:EN‐592, 8 pp. 10.2903/sp.efsa.2014.en-592 [DOI] [Google Scholar]
- Edel W, 1994. Salmonella enteritidis eradication programme in poultry breeder flocks in The Netherlands. International Journal of Food Microbiology, 21, 171–178. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on Biological hazards on a request from the Commission related to the use of antimicrobials for the control of Salmonella in poultry. EFSA Journal 2004;2(12):115, 76 pp. 10.2903/j.efsa.2004.115 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Report of the Task Force on Zoonoses Data Collection on the Analysis of the baseline study on the prevalence of Salmonella in holdings of laying hen flocks of Gallus gallus . EFSA Journal 2007;5(2):97r, 85 pp. 10.2903/j.efsa.2007.97r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Scientific Opinion of the Panel on Biological hazards on a quantitative estimation of the impact of setting a new target for the reduction of Salmonella in breeding hens of Gallus gallus . EFSA Journal 2009;7(4):1036, 68 pp. 10.2903/j.efsa.2009.1036 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008 – Part A: Campylobacter and Salmonella prevalence estimates. EFSA Journal 2010;8(3):1503, 100 pp. 10.2903/j.efsa.2010.1503 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses, in the EU, 2008 – Part B: Analysis of factors associated with Campylobacter colonisation of broiler batches and with Campylobacter contamination of broiler carcasses; and investigation of the culture method diagnostic characteristics used to analyse broiler carcass samples. EFSA Journal 2010:8(8):1522, 132 pp. 10.2903/j.efsa.2010.1522 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Update of the technical specifications for harmonised reporting of food‐borne outbreaks through the European Union reporting system in accordance with Directive 2003/99/EC. EFSA Journal 2014;12(3):3598, 25 pp. 10.2903/j.efsa.2014.3598 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010a. Scientific Opinion on a quantitative estimate of the public health impact of setting a new target for the reduction of Salmonella in laying hens. EFSA Journal 2010;8(4):1546, 86 pp. 10.2903/j.efsa.2010.1546 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010b. Scientific Opinion on Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA Journal 2010;8(1):1437, 89 pp. 10.2903/j.efsa.2010.1437 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011a. Scientific Opinion on a quantitative estimation of the public health impact of setting a new target for the reduction of Salmonella in broilers. EFSA Journal 2011;9(7):2106, 94 pp. 10.2903/j.efsa.2011.2106 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011b. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA Journal 2011;9(4):2105, 141 pp. 10.2903/j.efsa.2011.2105 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2012. Scientific Opinion on an estimation of the public health impact of setting a new target for the reduction of Salmonella in turkeys. EFSA Journal 2012;10(4):2616, 89 pp. 10.2903/j.efsa.2012.2616 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014. Scientific Opinion on the public health risks of table eggs due to deterioration and development of pathogens. EFSA Journal 2014;12(7):3782, 147 pp. 10.2903/j.efsa.2014.3782 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and The European Centre for Disease Prevention and Control), 2009. The Community Summary Report on Trends and Sources of Zoonoses and Zoonotic Agents in the European Union in 2007. EFSA Journal 2009;7(1):223r, 313 pp. 10.2903/j.efsa.2009.223r [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016. Multi‐country outbreak of Salmonella Enteritidis phage type 8, MLVA type 2‐9‐7‐3‐2 and 2‐9‐6‐3‐2 infections. EFSA supporting publication 2016:EN‐1110, 20 pp. 10.2903/sp.efsa.2016.en-1110 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017a. Multi‐country outbreak of Salmonella Enteritidis infections linked to Polish eggs. EFSA supporting publication 2017:EN‐1353. 21 pp. 10.2903/sp.efsa.2017.en-1353 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017b. Multi‐country outbreak of Salmonella Enteritidis phage type 8, MLVA profile 2‐9‐7‐3‐2 and 2‐9‐6‐3‐2 infections. EFSA supporting publication 2017:EN‐1188, 22 pp. 10.2903/sp.efsa.2017.en-1188 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and The European Centre for Disease Prevention and Control), 2017c. Multi‐country outbreak of Salmonella Enteritidis infections linked to Polish eggs. EFSA supporting publication 2017:EN‐1353. 21 pp. 10.2903/sp.efsa.2017.en-1353 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017d. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal 2017;15(12):5077, 228 pp. 10.2903/j.efsa.2017.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA Journal 2018;16(2):5182, 270 pp. 10.2903/j.efsa.2018.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emous RAv and Fiks‐van Niekerk TGCM, 2004. Higher mortality in free‐range aviary houses. World Poultry, 20, 26–27. [Google Scholar]
- Espie E, De Valk H, Vaillant V, Quelquejeu N, Le Querrec F and Weill FX, 2005. An outbreak of multidrug‐resistant Salmonella enterica serotype Newport infections linked to the consumption of imported horse meat in France. Epidemiology and Infection, 133, 373–376. 10.1017/s0950268804003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JI, Oporto B, Aduriz G, Juste RA and Hurtado A, 2008. A survey of food‐borne pathogens in free‐range poultry farms. International Journal of Food Microbiology, 123, 177–182. 10.1016/j.ijfoodmicro.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Evans SJ and Sayers AR, 2000. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Preventive Veterinary Medicine, 46, 209–223. 10.1016/s0167-5877(00)00143-4 [DOI] [PubMed] [Google Scholar]
- Evans SJ, Davies RH and Wray C, 1999. Epidemiology of Salmonella enterica serovar enteritidis infection in British poultry flocks. Salmonella Enterica Serovar Enteritidis in Humans and Animals: Epidemiology, Pathogenesis and Control, 313–323. [Google Scholar]
- Fehihaber K and Krueger G, 1998. The study of Salmonella enteritidis growth kinetics using rapid automated bacterial impedance technique. Journal of Applied Microbiology, 84, 945–949. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Guerra B and Rodicio MR, 2018. Resistance to Carbapenems in Non‐Typhoidal Salmonella enterica Serovars from Humans, Animals and Food. Veterinary Sciences, 5, 40. 10.3390/vetsci5020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo R, Card RM, Nunez‐Garcia J, Mendonca N, da Silva GJ and Anjum MF, 2018. Multidrug‐Resistant Salmonella enterica Isolated from Food Animal and Foodstuff May Also Be Less Susceptible to Heavy Metals. Foodborne Pathogens and Disease. 10.1089/fpd.2017.2418 [DOI] [PubMed] [Google Scholar]
- Fischer J, Rodriguez I, Schmoger S, Friese A, Roesler U, Helmuth R and Guerra B, 2013a. Salmonella enterica subsp. enterica producing VIM‐1 carbapenemase isolated from livestock farms. Journal of Antimicrobial Chemotherapy, 68, 478–480. 10.1093/jac/dks393 [DOI] [PubMed] [Google Scholar]
- Fischer J, Schmoger S, Jahn S, Helmuth R and Guerra B, 2013b. NDM‐1 carbapenemase‐producing Salmonella enterica subsp. enterica serovar Corvallis isolated from a wild bird in Germany. Journal of Antimicrobial Chemotherapy, 68, 2954–2956. 10.1093/jac/dkt260 [DOI] [PubMed] [Google Scholar]
- Florez‐Cuadrado D, Moreno MA, Ugarte‐Ruiz M and Dominguez L, 2018. Antimicrobial Resistance in the Food Chain in the European Union. Advances in Food and Nutrition Research, 86, 115–136. 10.1016/bs.afnr.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Franco A, Leekitcharoenphon P, Feltrin F, Alba P, Cordaro G, Iurescia M, Tolli R, D'Incau M, Staffolani M, Di Giannatale E, Hendriksen RS and Battisti A, 2015. Emergence of a Clonal Lineage of Multidrug‐Resistant ESBL‐Producing Salmonella Infantis Transmitted from Broilers and Broiler Meat to Humans in Italy between 2011 and 2014. PLoS ONE, 10, e0144802. 10.1371/journal.pone.0144802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz E, van der Fels‐Klerx HJ, Thissen J and van Asselt ED, 2012. Farm and slaughterhouse characteristics affecting the occurrence of Salmonella and Campylobacter in the broiler supply chain. Poultry Science, 91, 2376–2381. [DOI] [PubMed] [Google Scholar]
- Fries R, Neumann‐Fuhrmann D and Grashorn M, 1994. Salmonella in broiler intestines in relation to flooring system. DTW. Deutsche Tierarztliche Wochenschrift, 101, 391–393. [PubMed] [Google Scholar]
- FSA (Food Standards Agency), 2003. UK‐wide survey of Salmonella and Campylobacter contamination of fresh and frozen chicken on retail sale. https://www.food.gov.uk/research/foodborne-diseases/uk-wide-survey-of-salmonella-and-campylobacter-contamination-of-fresh-and-frozen-chicken-on-retail-sale
- Galis AM, Van I and Thewis A, 2012. Organoleptic, chemical and microbiological quality of table eggs obtained in different exploitation systems for laying hens in Romania. Scientific Papers Series D. Animal Science, 55, 162–166. [Google Scholar]
- Gal‐Mor O, Valinsky L, Weinberger M, Guy S, Jaffe J, Schorr YI, Raisfeld A, Agmon V and Nissan I, 2010. Multidrug‐resistant Salmonella enterica serovar Infantis, Israel. Emerging Infectious Diseases, 16, 1754–1757. 10.3201/eid1611.100100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I, Ducatelle R, Pasmans F, Haesebrouck F and van Immerseel F, 2008. Salmonella enterica serovar Enteritidis genes induced during oviduct colonization and egg contamination in laying hens. Applied and Environmental Microbiology, 74, 6616–6622. 10.1128/aem.01087-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber L, Smeltzer M, Fedorka‐Cray P, Ladely S and Ferris K, 2003. Salmonella enterica serotype enteritidis in table egg layer house environments and in mice in U.S. layer houses and associated risk factors. Avian Diseases, 47, 134–142. 10.1637/0005-2086(2003)047[0134:seseit]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Garcia‐Sanchez L, Melero B and Rovira J, 2018. Campylobacter in the Food Chain. Advances in Food and Nutrition Research, 86, 215–252. 10.1016/bs.afnr.2018.04.005 [DOI] [PubMed] [Google Scholar]
- Gast RK and Beard CW, 1989. AGE‐RELATED‐CHANGES IN THE PERSISTENCE AND PATHOGENICITY OF SALMONELLA‐TYPHIMURIUM IN CHICKS. Poultry Science, 68, 1454–1460. 10.3382/ps.0681454 [DOI] [PubMed] [Google Scholar]
- Gast RK, Holt PS and Murase T, 2005. Penetration of Salmonella enteritidis and Salmonella heidelberg into egg yolks in an in vitro contamination model. Poultry Science, 84, 621–625. 10.1093/ps/84.4.621 [DOI] [PubMed] [Google Scholar]
- Gast RK, Guraya R, Guard‐Bouldin J, Holt PS and Moore RW, 2007. Colonization of specific regions of the reproductive tract and deposition at different locations inside eggs laid by hens infected with Salmonella Enteritidis or Salmonella Heidelberg. Avian Diseases, 51, 40–44. 10.1637/0005-2086(2007)051[0040:cosrot]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Gast RK, Guraya R, Jones DR and Anderson KE, 2013. Colonization of internal organs by Salmonella Enteritidis in experimentally infected laying hens housed in conventional or enriched cages. Poultry Science, 92, 468–473. 10.3382/ps.2012-02811 [DOI] [PubMed] [Google Scholar]
- Gast RK, Guraya R, Jones DR and Anderson KE, 2014a. Horizontal transmission of Salmonella Enteritidis in experimentally infected laying hens housed in conventional or enriched cages. Poultry Science, 93, 3145–3151. [DOI] [PubMed] [Google Scholar]
- Gast RK, Guraya R, Jones DR and Anderson KE, 2014b. Contamination of eggs by Salmonella Enteritidis in experimentally infected laying hens housed in conventional or enriched cages. Poultry Science, 93, 728–733. 10.3382/ps.2013-03641 [DOI] [PubMed] [Google Scholar]
- Gast RK, Guraya R, Jones DR and Anderson KE, 2015. Persistence of fecal shedding of Salmonella Enteritidis by experimentally infected laying hens housed in conventional or enriched cages. Poultry Science, 94, 1650–1656. 10.3382/ps/pev113 [DOI] [PubMed] [Google Scholar]
- Gast RK, Guraya R, Jones DR, Anderson KE and Karcher DM, 2017a. Frequency and duration of fecal shedding of Salmonella Enteritidis by experimentally infected laying hens housed in enriched colony cages at different stocking densities. Frontiers in Veterinary Science, 4, 47. 10.3389/fvets.2017.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast RK, Guraya R, Jones DR, Guard J, Anderson KE and Karcher DM, 2017b. Frequency and Duration of Fecal Shedding of Salmonella Serovars Heidelberg and Typhimurium by Experimentally Infected Laying Hens Housed in Enriched Colony Cages at Different Stocking Densities. Avian Diseases, 61, 366–371. 10.1637/11635-032517-RegR [DOI] [PubMed] [Google Scholar]
- Gast RK, Guraya R, Jones DR, Guard J, Anderson KE and Karcher DM, 2017c. Colonization of internal organs by Salmonella serovars Heidelberg and Typhimurium in experimentally infected laying hens housed in enriched colony cages at different stocking densities. Poultry Science, 96, 1402–1409. [DOI] [PubMed] [Google Scholar]
- Gast RK, Guard J, Guraya R and Locatelli A, 2018. Multiplication in Egg Yolk and Survival in Egg Albumen of Genetically and Phenotypically Characterized Salmonella Enteritidis Strains. Journal of Food Protection, 81, 876–880. 10.4315/0362-028x.jfp-17-484 [DOI] [PubMed] [Google Scholar]
- Gaucher M‐L, Trembley A, Quessy S, Leroux A, Ng S, Comeau G, Moreau P, Cereno T and Racicot M, 2017. Identification, selection and weighting of food safety risk factors to be considered for their inclusion in the Canadian Food Inspection Agency's Hatchery Risk Assessment model. Proceedings of the 4th International Congress on Pathogens at the Human‐Animal Interface (ICOPHAI): Environmental Changes and Impact on Global Health, Doha, Qatar Ritz Carlton, West Bay Lagoon.
- Gavin C, Simons RRL, Berriman ADC, Moorhouse D, Snary EL, Smith RP and Hill AA, 2018. A cost‐benefit assessment of Salmonella‐control strategies in pigs reared in the United Kingdom. Preventive Veterinary Medicine, 160, 54–62. 10.1016/j.prevetmed.2018.09.022 [DOI] [PubMed] [Google Scholar]
- Ge B, LaFon PC, Carter PJ, McDermott SD, Abbott J, Glenn A, Ayers SL, Friedman SL, Paige JC, Wagner DD, Zhao S, McDermott PF and Rasmussen MA, 2013. Retrospective Analysis of Salmonella, Campylobacter, Escherichia coli, and Enterococcus in Animal Feed Ingredients. Foodborne Pathogens and Disease, 10, 684–691. 10.1089/fpd.2012.1470 [DOI] [PubMed] [Google Scholar]
- Georgsson F, Porkelsson AE, Geirsdottir M, Reiersen J and Stern NJ, 2006. The influence of freezing and duration of storage on Campylobacter and indicator bacteria in broiler carcasses. Food Microbiology, 23, 677–683. 10.1016/j.fm.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Geue L and Schluter H, 1998. A Salmonella monitoring programme in egg production farms in Germany. Zentralbl Veterinarmed B, 45, 95–103. [DOI] [PubMed] [Google Scholar]
- Gibbens JC, Pascoe SJS, Evans SJ, Davies RH and Sayers AR, 2001. A trial of biosecurity as a means to control Campylobacter infection of broiler chickens. Preventive Veterinary Medicine, 48, 85–99. 10.1016/s0167-5877(00)00189-6 [DOI] [PubMed] [Google Scholar]
- Gibbons CL, Mangen M‐JJ, Plass D, Havelaar AH, Brooke RJ, Kramarz P, Peterson KL, Stuurman AL, Cassini A, Fevre EM, Kretzschmar MEE and Burden Communicable Dis Europe B, 2014.Measuring underreporting and under‐ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health, 14, 147. 10.1186/1471-2458-14-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AVS, Quinteiro Filho WM, Ribeiro A, Ferraz‐de‐Paula V, Pinheiro ML, Baskeville E, Akamine AT, Astolfi‐Ferreira CS, Ferreira AJP and Palermo Neto J, 2014. Overcrowding stress decreases macrophage activity and increases Salmonella Enteritidis invasion in broiler chickens. Avian Pathology, 43, 82–90. 10.1080/03079457.2013.874006 [DOI] [PubMed] [Google Scholar]
- Gradel KO, Andersen J and Madsen M, 2002. Comparisons of sampling procedures and time of sampling for the detection of Salmonella in Danish infected chicken flocks raised in floor systems. Acta Veterinaria Scandinavica, 43, 21–30. 10.1186/1751-0147-43-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JT, Hungerford LL, Fedorka‐Cray PJ and Headrick ML, 2004. Extended‐spectrum‐cephalosporin resistance in Salmonella enterica isolates of animal origin. Antimicrobial Agents and Chemotherapy, 48, 3179–3181. 10.1128/aac.48.8.3179-3181.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Wesley I, Trampel DW and Xin H, 2009. Air quality and bird health status in three types of commercial egg layer houses. Journal of Applied Poultry Research, 18, 605–621. 10.3382/japr.2007-00086 [DOI] [Google Scholar]
- Green A, Defibaugh‐Chavez S, Douris A, Vetter D, Atkinson R, Kissler B, Khroustalev A, Robertson K, Sharma Y, Becker K, Dessai U, Antoine N, Allen L, Holt K, Gieraltowski L, Wise M and Schwensohn C and Food Safety Inspection Serv F , 2018. Intensified Sampling in Response to a Salmonella Heidelberg Outbreak Associated with Multiple Establishments Within a Single Poultry Corporation. Foodborne Pathogens and Disease, 15, 153–160. 10.1089/fpd.2017.2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard‐Bouldin J, Gast RK, Humphrey TJ, Henzler DJ, Morales C and Coles K, 2004. Subpopulation characteristics of egg‐contaminating Salmonella enterica serovar enteritidis as defined by the lipopolysaccharide O chain. Applied and Environmental Microbiology, 70, 2756–2763. 10.1128/aem.70.5.2756-2763.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard‐Petter J, 2001. The chicken, the egg and Salmonella enteritidis . Environmental Microbiology, 3, 421–430. 10.1046/j.1462-2920.2001.00213.x [DOI] [PubMed] [Google Scholar]
- Gustafson RH and Kobland JD, 1984. Factors influencing Salmonella shedding in broiler chickens. The Journal of Hygiene, 92, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald T and Lund J, 2012. Development of a user‐friendly interface version of the Salmonella source attribution model. Supporting Publications 2012:EN‐318, 77 pp. [Google Scholar]
- Hald B, Skovgard H, Bang DD, Pedersen K, Dybdahl J, Jespersen JB and Madsen M, 2004. Flies and Campylobacter infection of broiler flocks. Emerging Infectious Diseases, 10, 1490–1492. 10.3201/eid1008.040129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald T, Wong DMALF and Aarestrup FM, 2007. The attribution of human infections with antimicrobial resistant Salmonella bacteria in Denmark to sources of animal origin. Foodborne Pathogens and Disease, 4, 313–326. 10.1089/fpd.2007.0002 [DOI] [PubMed] [Google Scholar]
- Hald T, Pires SM and Knegt L, 2012a. Development of a Salmonella source‐attribution model for evaluating targets in the turkey meat production. Supporting Publications 2012:EN‐259, 35 pp. [Google Scholar]
- Hald T, Wingstrand A, Pires SM, Vieira A, Coutinho Calado Domingues AR, Lundsby KL, Dalhoff Andersen V and Thrane C (Danmarks Tekniske Universitet (DTU)), 2012b. Assessment of the human‐health impact of Salmonella in animal feed. 75 pp. http://orbit.dtu.dk/files/51295782/Assessment_of_the_human_health_impact.pdf
- Hannah JF, Wilson JL, Cox NA, Richardson LJ, Cason JA, Bourassa DV and Buhr RJ, 2011. Horizontal transmission of Salmonella and Campylobacter among caged and cage‐free laying hens. Avian Diseases, 55, 580–587. 10.1637/9717-031511-Reg.1 [DOI] [PubMed] [Google Scholar]
- Hansson I, Engvall EO, Vagsholm I and Nyman A, 2010. Risk factors associated with the presence of Campylobacter‐positive broiler flocks in Sweden. Preventive Veterinary Medicine, 96, 114–121. 10.1016/j.prevetmed.2010.05.007 [DOI] [PubMed] [Google Scholar]
- Hauser E, Tietze E, Helmuth R, Junker E, Blank K, Prager R, Rabsch W, Appel B, Fruth A and Malorny B, 2010. Pork contaminated with Salmonella enterica serovar 4,[5],12:i:‐, an emerging health risk for humans. Applied and Environmental Microbiology, 76, 4601–4610. 10.1128/aem.02991-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser E, Hebner F, Tietze E, Helmuth R, Junker E, Prager R, Schroeter A, Rabsch W, Fruth A and Malorny B, 2011. Diversity of Salmonella enterica serovar Derby isolated from pig, pork and humans in Germany. International Journal of Food Microbiology, 151, 141–149. 10.1016/j.ijfoodmicro.2011.08.020 [DOI] [PubMed] [Google Scholar]
- Hauser E, Tietze E, Helmuth R, Junker E, Prager R, Schroeter A, Rabsch W, Fruth A, Toboldt A and Malorny B, 2012. Clonal Dissemination of Salmonella enterica Serovar Infantis in Germany. Foodborne Pathogens and Disease, 9, 352–360. 10.1089/fpd.2011.1038 [DOI] [PubMed] [Google Scholar]
- Havelaar AH, Ivarsson S, Loefdahl M and Nauta MJ, 2013. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiology and Infection, 141, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms M, Vastrup P, Gerner‐Smidt P and Molbak K, 2002. Excess mortality associated with antimicrobial drug‐resistant Salmonella typhimurium . Emerging Infectious Diseases, 8, 490–495. 10.3201/eid0805.010267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms M, Vastrup P, Gerner‐Smidt P and Molbak K, 2003. Short and long term mortality associated with foodborne bacterial gastrointestinal infections: registry based study. British Medical Journal, 326, 357–359. 10.1136/bmj.326.7385.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henken AM, Frankena K, Goelema JO, Graat EA and Noordhuizen JP, 1992. Multivariate epidemiological approach to salmonellosis in broiler breeder flocks. Poultry Science, 71, 838–843. [DOI] [PubMed] [Google Scholar]
- Heres L, Engel B, Van Knapen F, Wagenaar JA and Urlings BAP, 2003. Effect of fermented feed on the susceptibility for Campylobacter jejuni colonisation in broiler chickens with and without concurrent inoculation of Salmonella enteritidis. International Journal of Food Microbiology, 87, 75–86. [DOI] [PubMed] [Google Scholar]
- Heres L, Engel B, Urlings HAP, Wagenaar JA and van Knapen F, 2004. Effect of acidified feed on susceptibility of broiler to intestinal infection by Campylobacter and Salmonella . Veterinary Microbiology, 99, 259–267. 10.1016/j.vetmic.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Higham LE, Scott C, Akehurst K, Dring D, Parnham A, Waterman M and Bright A, 2018. Effects of financial incentives and cessation of thinning on prevalence of Campylobacter: a longitudinal monitoring study on commercial broiler farms in the UK. The Veterinary Record, 183, 595. 10.1136/vr.104823 [DOI] [PubMed] [Google Scholar]
- Hilbert F, Smulders FJM, Chopra‐Dewasthaly R and Paulsen P, 2012. Salmonella in the wildlife‐human interface. Food Research International, 45, 603–608. 10.1016/j.foodres.2011.08.015 [DOI] [Google Scholar]
- Hinton M, 1988. SALMONELLA INFECTION IN CHICKS FOLLOWING THE CONSUMPTION OF ARTIFICIALLY CONTAMINATED FEED. Epidemiology and Infection, 100, 247–256. 10.1017/s0950268800067388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PS, 1995. Horizontal transmission of Salmonella enteritidis in molted and unmolted laying chickens. Avian Diseases, 39, 239–249. [PubMed] [Google Scholar]
- Holt PS and Porter RE Jr, 1992. Effect of induced molting on the course of infection and transmission of Salmonella enteritidis in white Leghorn hens of different ages. Poultry Science, 71, 1842–1848. [DOI] [PubMed] [Google Scholar]
- Holt PS, Mitchell BW and Gast RK, 1998. Airborne horizontal transmission of Salmonella enteritidis in molted laying chickens. Avian Diseases, 42, 45–52. [PubMed] [Google Scholar]
- Holt PS, Davies RH, Dewulf J, Gast RK, Huwe JK, Jones DR, Waltman D and Willian KR, 2011. The impact of different housing systems on egg safety and quality. Poultry Science, 90, 251–262. 10.3382/ps.2010-00794 [DOI] [PubMed] [Google Scholar]
- Hormansdorfer S, Messelhausser U, Rampp A, Schonberger K, Dallman T, Allerberger F, Kornschober C, Sing A, Wallner P and Zapf A, 2017. Re‐evaluation of a 2014 multi‐country European outbreak of Salmonella Enteritidis phage type 14b using recent epidemiological and molecular data. Eurosurveillance Weekly, 22. 10.2807/1560-7917.ES.2017.22.50.17-00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RA, Card R, Randall LP and Teale CJ, 2016. Differentiation of UK endemic strains of Salmonella enterica serovar Newport from epidemic North American strains by PCR detection of a truncated bapA chromosomal gene. Research in Veterinary Science, 104, 113–116. 10.1016/j.rvsc.2015.12.008 [DOI] [PubMed] [Google Scholar]
- Howard ZR, O'Bryan CA, Crandall PG and Ricke SC, 2012. Salmonella Enteritidis in shell eggs: Current issues and prospects for control. Food Research International, 45, 755–764. 10.1016/j.foodres.2011.04.030 [DOI] [Google Scholar]
- Huang CC, Wang SH, Chin LT, Huang CL, Sun LT, Chiou CS, Tu PC and Chu C, 2019. Salmonella enterica serotype Typhimurium and S. Stanley Differ in Genomic Evolutionary Patterns and Early Immune Responses in Human THP‐1 cell line and CD14+ Monocytes. Comparative Immunology, Microbiology and Infectious Diseases, 63, 10–16. [DOI] [PubMed] [Google Scholar]
- Hue O, Allain V, Laisney MJ, Le Bouquin S, Lalande F, Petetin I, Rouxel S, Quesne S, Gloaguen PY, Picherot M, Santolini J, Bougeard S, Salvat G and Chemaly M, 2011. Campylobacter contamination of broiler caeca and carcasses at the slaughterhouse and correlation with Salmonella contamination. Food Microbiology, 28, 862–868. [DOI] [PubMed] [Google Scholar]
- Hugas M and Beloeil P, 2014. Controlling Salmonella along the food chain in the European Union ‐ progress over the last ten years. Eurosurveillance Weekly, 19. [DOI] [PubMed] [Google Scholar]
- Humphrey TJ, Henley A and Lanning DG, 1993. The Colonization of Broiler‐chickens with Campylobacter‐Jejuni ‐ Some Epidemiologic Investigations. Epidemiology and Infection, 110, 601–607. 10.1017/s0950268800051025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneau‐Salaun A, Chemaly M, Petetin I, Rouxel S, Lalande F and Bouquin Sl, 2007. Multidimensional descriptive analysis of farming hens contaminated with Salmonella spp.: Research hypotheses of risk factors Analyse descriptive multidimensionnelle des elevages de pondeuses contamines par Salmonella spp.: recherche d'hypotheses de facteurs de risque. Actes des 7emes Journees de la Recherche Avicole, Tours, France, 28 et 29 mars 2007, 505‐509.
- Huneau‐Salaun A, Chemaly M, Sl Bouquin, Lalande F, Petetin I, Rouxel S, Michel V, Fravalo P and Rose N, 2009. Risk factors for Salmonella enterica subsp. enterica contamination in 519 French laying hen flocks at the end of the laying period. Preventive Veterinary Medicine, 89, 51–58. 10.1016/j.prevetmed.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Hunton P, 1993. Genetics and breeding as they affect flock health. Ed Pattison M, pp. 1–22. The health of poultry. ISBN: 0‐582‐06579‐8
- Iglesias‐Torrens Y, Miro E, Guirado P, Llovet T, Munoz C, Cerda‐Cuellar M, Madrid C, Balsalobre C and Navarro F, 2018. Population Structure, Antimicrobial Resistance, and Virulence‐Associated Genes in Campylobacter jejuni Isolated From Three Ecological Niches: Gastroenteritis Patients, Broilers, and Wild Birds. Frontiers in Microbiology, 9. 10.3389/fmicb.2018.01676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M, Reynolds J, Karp BE, Tate H, Fedorka‐Cray PJ, Plumblee JR, Hoekstra RM, Whichard JM and Mahon BE, 2017. Ceftriaxone‐Resistant Nontyphoidal Salmonella from Humans, Retail Meats, and Food Animals in the United States, 1996‐2013. Foodborne Pathogens and Disease, 14, 74–83. 10.1089/fpd.2016.2180 [DOI] [PubMed] [Google Scholar]
- Jacobs‐Reitsma WF, Bolder NM and Mulder RW, 1994. Cecal Carriage of Campylobacter and Salmonella in dutch Broiler FLOCKS at Slaughter ‐ a One‐year Study. Poultry Science, 73, 1260–1266. 10.3382/ps.0731260 [DOI] [PubMed] [Google Scholar]
- Jansen W, Müller A, Grabowski N, Kehrenberg C, Muylkens B and Al Dahouk S, 2019. Foodborne diseases do not respect borders: Zoonotic pathogens and antimicrobial resistant bacteria in food products of animal origin illegally imported into the European Union. The Veterinary Journal, 244, 75–82. [DOI] [PubMed] [Google Scholar]
- Jones FT, 2011. A review of practical Salmonella control measures in animal feed. Journal of Applied Poultry Research, 20, 102–113. [Google Scholar]
- Jones TF, Ingram LA, Cieslak PR, Vugia DJ, Tobin‐D'Angelo M, Hurd S, Medus C, Cronquist A and Angulo FJ, 2008. Salmonellosis outcomes differ substantially by serotype. Journal of Infectious Diseases, 198, 109–114. 10.1086/588823 [DOI] [PubMed] [Google Scholar]
- Jones DR, Anderson KE and Guard JY, 2012. Prevalence of coliforms, Salmonella, Listeria, and Campylobacter associated with eggs and the environment of conventional cage and free‐range egg production. Poultry Science, 91, 1195–1202. 10.3382/ps.2011-01795 [DOI] [PubMed] [Google Scholar]
- Jones DR, Cox NA, Guard J, Fedorka‐Cray PJ, Buhr RJ, Gast RK, Abdo Z, Rigsby LL, Plumblee JR, Karcher DM, Robison CI, Blatchford RA and Makagon MM, 2015. Microbiological impact of three commercial laying hen housing systems. Poultry Science, 94, 544–551. 10.3382/ps/peu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DR, Guard J, Gast RK, Buhr RJ, Fedorka‐Cray PJ, Abdo Z, Plumblee JR, Bourassa DV, Cox NA, Rigsby LL, Robison CI, Regmi P and Karcher DM, 2016. Influence of commercial laying hen housing systems on the incidence and identification of Salmonella and Campylobacter . Poultry Science, 95, 1116–1124. 10.3382/ps/pew036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagambega A, Thibodeau A, Trinetta V, Soro DK, Sama FN, Bako E, Bouda CS, N'Diaye AW, Fravalo P and Barro N, 2018. Salmonella spp. and Campylobacter spp. in poultry feces and carcasses in Ouagadougou, Burkina Faso. Food Science & Nutrition, 6, 1601–1606. 10.1002/fsn3.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas S, Lyytikainen T, Peltola J, Ranta J and Maijala R, 2007. Costs of two alternative Salmonella control policies in Finnish broiler production. Acta Veterinaria Scandinavica, 49, 35. 10.1186/1751-0147-49-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G, Skjerve E, Vik L, Hauge K, Lysaker A, Aalmen I, Ostroff SM and Potter M, 1993. Epidemiologic Investigation of Risk‐Factors For Campylobacter Colonization in Norwegian Broiler Flocks. Epidemiology and Infection, 111, 245–255. 10.1017/s0950268800056958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karacan Sever N and Akan M, 2018. Molecular analysis of virulence genes of Salmonella Infantis isolated from chickens and turkeys. Microbial Pathogenesis, 126, 199–204. 10.1016/j.micpath.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Karp BE, Campbell D, Chen JC, Folster JP and Friedman CR, 2018. Plasmid‐mediated quinolone resistance in human non‐typhoidal Salmonella infections: An emerging public health problem in the United States. Zoonoses and Public Health, 65, 838–849. 10.1111/zph.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann‐Bart M and Hoop RK, 2009. Diseases in chicks and laying hens during the first 12 years after battery cages were banned in Switzerland. Veterinary Record, 164, 203–207. 10.1136/vr.164.7.203 [DOI] [PubMed] [Google Scholar]
- Kenney LJ, 2018. The role of acid stress in Salmonella pathogenesis. Current Opinion in Microbiology, 47, 45–51. 10.1016/j.mib.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Kim A, Lee YJ, Kang MS, Kwag SI and Cho JK, 2007. Dissemination and tracking of Salmonella spp. in integrated broiler operation. Journal of Veterinary Science, 8, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinde H, Read DH, Chin RP, Bickford AA, Walker RL, Ardans A, Breitmeyer RE, Willoughby D, Little HE, Kerr D and Gardner IA, 1996. Salmonella enteritidis, phage type 4 infection in a commercial layer flock in Southern California: Bacteriologic and epidemiologic findings. Avian Diseases, 40, 665–671. 10.2307/1592279 [DOI] [PubMed] [Google Scholar]
- Kinross P, vanAlphen L , Martinez Urtaza J, Struelens M, Takkinen J, Coulombier D, Makela P, Bertrand S, Mattheus W, Schmid D, Kanitz E, Rucker V, Krisztalovics K, Paszti J, Szogyenyi Z, Lancz Z, Rabsch W, Pfefferkorn B, Hiller P, Mooijman K and Gossner C, 2014. Multidisciplinary investigation of a multicountry outbreak of Salmonella Stanley infections associated with turkey meat in the European Union, August 2011 to January 2013. Eurosurveillance Weekly, 19, 20801. [DOI] [PubMed] [Google Scholar]
- Koelkebeck KW, Amoss MS Jr and Cain JR, 1987. Production, physiological, and behavioral responses of laying hens in different management environments. Poultry Science, 66, 397–407. [DOI] [PubMed] [Google Scholar]
- Koolman L, Whyte P and Bolton DJ, 2014. An investigation of broiler caecal Campylobacter counts at first and second thinning. Journal of Applied Microbiology, 117, 876–881. 10.1111/jam.12580 [DOI] [PubMed] [Google Scholar]
- Korolik V, 2018. The role of chemotaxis during Campylobacter jejuni colonisation and pathogenesis. Current Opinion in Microbiology, 47, 32–37. 10.1016/j.mib.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Kubena LF, Byrd JA, Moore RW, Ricke SC and Nisbet DJ, 2005. Effects of drinking water treatment on susceptibility of laying hens to Salmonella enteritidis during forced molt. Poultry Science, 84, 204–211. 10.1093/ps/84.2.204 [DOI] [PubMed] [Google Scholar]
- Kuhn KG, Nielsen EM, Molbak K and Ethelberg S, 2018. Epidemiology of campylobacteriosis in Denmark 2000‐2015. Zoonoses and Public Health, 65, 59–66. 10.1111/zph.12367 [DOI] [PubMed] [Google Scholar]
- Lane CR, LeBaigue S, Esan OB, Awofisyo AA, Adams NL, Fisher IS, Grant KA, Peters TM, Larkin L, Davies RH and Adak GK, 2014. Salmonella enterica serovar Enteritidis, England and Wales, 1945‐2011. Emerging Infectious Diseases, 20, 1097–1104. 10.3201/eid2007.121850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassnig H, Much P, Schliessnig H, Osterreicher E, Kostenzer K, Kornschober C and Kofer J, 2011. Baseline surveys on the prevalence of Salmonella spp. in Austrian poultry farms: an overview. Eds Kofer J and Schobesberger H, Tribun EU, Brno, Czech Republic, pp. ??
- Lawes JR, Vidal A, Clifton‐Hadley FA, Sayers R, Rodgers J, Snow L, Evans SJ and Powell LF, 2012. Investigation of prevalence and risk factors for Campylobacter in broiler flocks at slaughter: results from a UK survey. Epidemiology and Infection, 140, 1725–1737. 10.1017/s0950268812000982 [DOI] [PubMed] [Google Scholar]
- Lawes JL, Mueller‐Doblies D, Marco C, Petrovska L and Davies R, 2016. An outbreak of Salmonella Enteritidis in broiler flocks in GB in 2015. Proceedings of the I3S Conference, St.Malo, France, p. 36.
- Le Bouquin S, Allain V, Rouxel S, Petetin I, Picherot M, Michel V and Chemaly M, 2010. Prevalence and risk factors for Salmonella spp. contamination in French broiler‐chicken flocks at the end of the rearing period. Preventive Veterinary Medicine, 97, 245–251. 10.1016/j.prevetmed.2010.09.014 [DOI] [PubMed] [Google Scholar]
- Le Hello S, Hendriksen RS, Doublet B, Fisher I, Nielsen EM, Whichard JM, Bouchrif B, Fashae K, Granier SA, Jourdan‐Da Silva N, Cloeckaert A, Threlfall EJ, Angulo FJ, Aarestrup FM, Wain J and Weill F‐X, 2011. International spread of an epidemic population of Salmonella enterica serotype Kentucky ST198 resistant to ciprofloxacin. Journal of Infectious Diseases, 204, 675–684. 10.1093/infdis/jir409 [DOI] [PubMed] [Google Scholar]
- Le Hello S, Bekhit A, Granier SA, Barua H, Beutlich J, Zajac M, Munch S, Sintchenko V, Bouchrif B, Fashae K, Pinsard J‐L, Sontag L, Fabre L, Garnier M, Guibert V, Howard P, Hendriksen RS, Christensen JP, Biswas PK, Cloeckaert A, Rabsch W, Wasyl D, Doublet B and Weill F‐X, 2013. The global establishment of a highly‐fluoroquinolone resistant Salmonella enterica serotype Kentucky ST198 strain. Frontiers in Microbiology, 4. 10.3389/fmicb.2013.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Chon JW, Song KY, Hyeon JY, Moon JS and Seo KH, 2013. Prevalence, characterization, and antimicrobial susceptibility of Salmonella Gallinarum isolated from eggs produced in conventional or organic farms in South Korea. Poultry Science, 92, 2789–2797. 10.3382/ps.2013-03175 [DOI] [PubMed] [Google Scholar]
- Legesse D and Garesu M, 2017. Salmonella Enteritidis. Transovarial Transmission Mechanism in laying Chickens. Term paper 32 pp. https://www.grin.com/document/383938
- Liakopoulos A, Geurts Y, Dierikx CM, Brouwer MSM, Kant A, Wit B, Heymans R, van Pelt W and Mevius DJ, 2016. Extended‐Spectrum Cephalosporin‐Resistant Salmonella enterica serovar Heidelberg Strains, the Netherlands. Emerging Infectious Diseases, 22, 1257–1261. 10.3201/eid2207.151377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljebjelke KA, Hofacre CL, Liu T, White DG, Ayers S, Young S and Maurer JJ, 2005. Vertical and horizontal transmission of Salmonella within integrated broiler production system. Foodbourne Pathogens and Disease, 2, 90–102. 10.1089/fpd.2005.2.90 [DOI] [PubMed] [Google Scholar]
- Limawongpranee S, Hayashidani H, Okatani AT, Ono K, Hirota C, Kaneko K and Ogawa M, 1999. Prevalence and persistence of Salmonella in broiler chicken flocks. Journal of Veterinary Medical Science, 61, 255–259. 10.1292/jvms.61.255 [DOI] [PubMed] [Google Scholar]
- Line JE, 2002. Campylobacter and Salmonella populations associated with chickens raised on acidified litter. Poultry Science, 81, 1473–1477. [DOI] [PubMed] [Google Scholar]
- Litrup E, Torpdahl M, Malorny B, Huehn S, Christensen H and Nielsen EM, 2010. Association between phylogeny, virulence potential and serovars of Salmonella enterica . Infection Genetics and Evolution, 10, 1132–1139. 10.1016/j.meegid.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Lu SW, Killoran PB and Riley LW, 2003. Association of Salmonella enterica serovar enteritidis YafD with resistance to chicken egg albumen. Infection and Immunity, 71, 6734–6741. 10.1128/iai.71.12.6734-6741.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk‐in S, Chatsuwan T, Pulsrikarn C, Bangtrakulnonth A, Rirerm U and Kulwichit W, 2018. High prevalence of ceftriaxone resistance among invasive Salmonella enterica serotype Choleraesuis isolates in Thailand: The emergence and increase of CTX‐M‐55 in ciprofloxacin‐resistant S. Choleraesuis isolates. International Journal of Medical Microbiology, 308, 447–453. 10.1016/j.ijmm.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Lund M, Welch TK, Griswold K, Endres JB and Shepherd B, 2003. Occurrence of Campylobacter and Salmonella in broiler chickens raised in different production systems and fed organic and traditional feed. Food Protection Trends, 23, 252–256. [Google Scholar]
- Ma Y, Li M, Xu X, Fu Y, Xiong Z, Zhang L, Qu X, Zhang H, Wei Y, Zhan Z, Chen Z, Bai J, Liao M and Zhang J, 2018. High‐levels of resistance to quinolone and cephalosporin antibiotics in MDR‐ACSSuT Salmonella enterica serovar Enteritidis mainly isolated from patients and foods in Shanghai, China. International Journal of Food Microbiology, 286, 190–196. 10.1016/j.ijfoodmicro.2018.09.022 [DOI] [PubMed] [Google Scholar]
- MacDonald E, White R, Mexia R, Bruun T, Kapperud G, Brandal LT, Lange H, Nygard K and Vold L, 2018. The role of domestic reservoirs in domestically acquired Salmonella infections in Norway: epidemiology of salmonellosis, 2000‐2015, and results of a national prospective case‐control study, 2010‐2012. Epidemiology and Infection, 1–8. 10.1017/s0950268818002911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magossi G, Cernicchiaro N, Dritz S, Houser T, Woodworth J, Jones C and Trinetta V, 2018. Evaluation of Salmonella presence in selected United States feed mills. MicrobiologyOpen, e00711–e00711. 10.1002/mbo3.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe A, Bougeard S, Huneau‐Salaun A, Le Bouquin S, Petetin I, Rouxel S, Lalande F, Beloeil PA and Rose N, 2008. Bayesian estimation of flock‐level sensitivity of detection of Salmonella spp., Enteritidis and Typhimurium according to the sampling procedure in French laying‐hen houses. Preventive Veterinary Medicine, 84, 11–26. 10.1016/j.prevetmed.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Marder EP, Griffin PM, Cieslak PR, Dunn J, Hurd S, Jervis R, Lathrop S, Muse A, Ryan P, Smith K, Tobin‐D'Angelo M, Vugia DJ, Holt KG, Wolpert BJ, Tauxe R and Geissler AL, 2018. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food ‐ Foodborne Diseases Active Surveillance Network, 10 US Sites, 2006‐2017. Mmwr‐Morbidity and Mortality Weekly Report, 67, 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli F, Wales A and Davies R, 2017. Of Mice and Hens‐Tackling Salmonella in Table Egg Production in the United Kingdom and Europe. Eds Ricke SC and Gast RK, Academic Press Ltd‐Elsevier Science Ltd, London, pp. 3–23.
- Martin LJ, Fyfe D and Dore K, 2004. Increased burden of illness associated with antimicrobial‐resistant Salmonella enterica serotype Typhimurium infections. (vol 189, pg 377, 2004). Journal of Infectious Diseases, 190, 2198–2198. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Miyama M and Murakami S, 2001. Comparison of Salmonella isolation rates in different types of egg‐layer hen houses in Chiba, Japan. Avian Diseases, 45, 195–200. 10.2307/1593028 [DOI] [PubMed] [Google Scholar]
- McCrea BA, Tonooka KH, VanWorth C, Boggs CL, Atwill R and Schrader JS, 2006. Prevalence of Campylobacter and Salmonella species on farm, after transport, and at processing in specialty market poultry. Poultry Science, 85, 136–143. 10.1093/ps/85.1.136 [DOI] [PubMed] [Google Scholar]
- McDermott P, Zhao S and Tate H, 2018. Antimicrobial Resistance in Nontyphoidal Salmonella Microbiology Spectrum, 6. 10.1128/microbiolspec.arba-0014-2017 [DOI] [PMC free article] [PubMed]
- McLauchlin J, Aird H, Charlett A, Chattaway M, Elviss N, Hartman H, Jenkins C, Jorgensen F, Larkin L, Sadler‐Reeves L and Willis C, 2018. Imported edible leaves collected at retail sale in England during 2017 with an emphasis on betel and curry leaves: microbiological quality with respect to Salmonella, Shiga‐toxin‐producing E.coli (STEC) and levels of Escherichia coli. Journal of Applied Microbiology, 125, 1175–1185. 10.1111/jam.13931 [DOI] [PubMed] [Google Scholar]
- McLlroy SG, McCracken RM, Neill SD and Obrien JJ, 1989. Control, prevention and eradication of Salmonella enteritidis infection in broiler and broiler breeder flocks. Veterinary Record, 125, 545–548. [DOI] [PubMed] [Google Scholar]
- McWhorter AR, Phan G, Hocking H and Chousalkar KK, 2018. In vitro invasion capacity of Salmonella Typhimurium DT9 isolates sourced from humans and layer hen environments. Zoonoses and Public Health, 65, E259–E264. 10.1111/zph.12434 [DOI] [PubMed] [Google Scholar]
- Meade KG, Narciandi F, Cahalane S, Reiman C, Allan B and O'Farrelly C, 2009. Comparative in vivo infection models yield insights on early host immune response to Campylobacter in chickens. Immunogenetics, 61, 101–110. [DOI] [PubMed] [Google Scholar]
- Meerburg BG and Kijlstra A, 2007. Role of rodents in transmission of Salmonella and Campylobacter . Journal of the Science of Food and Agriculture, 87, 2774–2781. 10.1002/jsfa.3004 [DOI] [Google Scholar]
- Meerburg B and Schoelitsz B, 2018. Biosecurity: methods to reduce contact risks between vectors and livestock. In: Garros C, JBouyer J, Takken W and Smallegange R (eds.). Pests and vector‐borne diseases in the livestock industry. Wageningen Academic Publishers, Netherlands. pp. 453–464. [Google Scholar]
- Menanteau P, Kempf F, Trotereau J, Virlogeux‐Payant I, Gitton E, Dalifard J, Gabriel I, Rychlik I and Velge P, 2018. Role of systemic infection, cross contaminations and super‐shedders in Salmonella carrier state in chicken. Environmental Microbiology, 20, 3246–3260. 10.1111/1462-2920.14294 [DOI] [PubMed] [Google Scholar]
- Messens W, Vivas‐Alegre L, Bashir S, Amore G, Romero‐Barrios P and Hugas M, 2013. Estimating the public health impact of setting targets at the European level for the reduction of zoonotic Salmonella in certain poultry populations. International Journal of Environmental Research and Public Health, 10, 4836–4850. 10.3390/ijerph10104836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methner U, Diller R, Reiche R and Bohland K, 2006. Occurence of salmonellae in laying hens in different housing systems and conclusion for the control Zum Vorkommen von Salmonellen bei Legehennen in unterschiedlichen Haltungsformen und Schlussfolgerungen fur die Bekampfung. Berliner und Munchener Tierarztliche Wochenschrift, 119, 467–473. [PubMed] [Google Scholar]
- M'Ikanatha NM, Sandt CH, Localio AR, Tewari D, Rankin SC, Whichard JM, Altekruse SF, Lautenbach E, Folster JP, Russo A, Chiller TM, Reynolds SM and McDermott PF, 2010. Multidrug‐resistant Salmonella isolates from retail chicken meat compared with human clinical isolates. Foodbourne Pathogens and Disease, 7, 929–934. 10.1089/fpd.2009.0499 [DOI] [PubMed] [Google Scholar]
- Moffatt CRM, Musto J, Pingault N, Miller M, Stafford R, Gregory J, Polkinghorne BG and Kirk MD, 2016. Salmonella Typhimurium and Outbreaks of Egg‐Associated Disease in Australia, 2001 to 2011. Foodbourne Pathogens and Disease, 13, 379–385. 10.1089/fpd.2015.2110 [DOI] [PubMed] [Google Scholar]
- Molbak K, Gerner‐Smidt P and Wegener HC, 2002. Increasing quinolone resistance in Salmonella enterica serotype Enteritidis. Emerging Infectious Diseases, 8, 514–515. 10.3201/eid0805.010288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhorst H, Woudenbergh CJv, Bokkers EGM and Boer IJMd, 2005. Risk factors for Salmonella enteritidis infections in laying hens. Poultry Science, 84, 1308–1313. 10.1093/ps/84.8.1308 [DOI] [PubMed] [Google Scholar]
- Much P, Osterreicher E, Lassnig H, Kornschober C and Kofer J, 2007. Results of the EU‐wide baseline study on the prevalence of Salmonella spp. in holdings of laying hens in Austria. Archiv fur Lebensmittelhygiene, 58, 225–229. [Google Scholar]
- Mueller A, Jansen W, Grabowski NT and Kehrenberg C, 2018. Characterization of Salmonella enterica serovars recovered from meat products legally and illegally imported into the EU reveals the presence of multiresistant and AmpC‐producing isolates. Gut Pathogens, 10. 10.1186/s13099-018-0268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase T, Chiba K, Sato T, Otsuki K and Holt PS, 2006. Effects of different molting procedures on incidence of Salmonella infection in flocks of naturally contaminated laying hens in a commercial egg‐producing farm by detection of yolk antibodies to Salmonella in eggs. Journal of Food Protection, 69, 2883–2888. 10.4315/0362-028x-69.12.2883 [DOI] [PubMed] [Google Scholar]
- Murphy C, Carroll C and Jordan KN, 2006. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni . Journal of Applied Microbiology, 100, 623–632. 10.1111/j.1365-2672.2006.02903.x [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nagamine N, Norimatsu M, Suzuki S, Ohishi K, Kijima M, Tamura Y and Sato S, 1993. The ability of Salmonella enteritidis isolated from chicks imported from England to cause transovarian infection. Journal of Veterinary Medical Science, 55, 135–136. [DOI] [PubMed] [Google Scholar]
- Nakao JH, Pringle J, Jones RW, Nix BE, Borders J, Heseltine G, Gomez TM, McCluskey B, Roney CS, Brinson D, Erdman M, McDaniel A and Behravesh CB, 2015. ‘One Health’ investigation: outbreak of human Salmonella Braenderup infections traced to a mail‐order hatchery ‐ United States, 2012‐2013. Epidemiology and Infection, 143, 2178–2186. 10.1017/s0950268815000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao JH, Talkington D, Bopp CA, Besser J, Sanchez ML, Guarisco J, Davidson SL, Warner C, Mc IM, Group JP, Comstock N, Xavier K, Pinsent TS, Brown J, Douglas JM, Gomez GA, Garrett NM, Carleton HA, Tolar B and Wise ME, 2018. Unusually high illness severity and short incubation periods in two foodborne outbreaks of Salmonella Heidelberg infections with potential coincident Staphylococcus aureus intoxication. Epidemiology and Infection, 146, 19–27. 10.1017/S0950268817002655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namata H, Meroc E, Aerts M, Faes C, Abrahantes JC, Imberechts H and Mintiens K, 2008. Salmonella in Belgian laying hens: an identification of risk factors. Preventive Veterinary Medicine, 83, 323–336. 10.1016/j.prevetmed.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Namata H, Welby S, Aerts M, Faes C, Cortinas Abrahantes J, Imberechts H, Vermeersch K, Hooyberghs J, Meroc E and Mintiens K, 2009. Identification of risk factors for the prevalence and persistence of Salmonella in Belgian broiler chicken flocks. Preventive Veterinary Medicine, 90, 211–222. 10.1016/j.prevetmed.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Nde CW and Logue CM, 2008. Characterization of antimicrobial susceptibility and virulence genes of Salmonella serovars collected at a commercial turkey processing plant. Journal of Applied Microbiology, 104, 215–223. 10.1111/j.1365-2672.2007.03535.x [DOI] [PubMed] [Google Scholar]
- Nde CW, Sherwood JS, Doetkott C and Logue CM, 2006. Prevalence and molecular profiles of Salmonella collected at a commercial turkey processing plant. Journal of Food Protection, 69, 1794–1801. [DOI] [PubMed] [Google Scholar]
- Newell DG and Fearnley C, 2003. Sources of Campylobacter colonization in broiler chickens. Applied and Environmental Microbiology, 69, 4343–4351. 10.1128/aem.69.8.4343-4351.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell DG, Elvers KT, Dopfer D, Hansson I, Jones P, James S, Gittins J, Stern NJ, Davies R, Connerton I, Pearson D, Salvat G and Allen VM, 2011. Biosecurity‐based interventions and strategies to reduce Campylobacter spp. on poultry farms. Applied and Environmental Microbiology, 77, 8605–8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor LI, Duminica CG and Usturoi MG, 2015. Influence of rearing systems of laying hens on eggs microbiological indicators. Lucrari Stiintifice ‐ Universitatea de Stiinte Agricole si Medicina Veterinara, Seria Zootehnie, 63, 118–121. [Google Scholar]
- Nogrady N, Kardos G, Bistyak A, Turcsanyi I, Meszaros J, Galantai Z, Juhasz A, Samu R, Kaszanyitzky JE, Paszti J and Kiss I, 2008. Prevalence and characterization of Salmonella infantis isolates originating from different points of the broiler chicken‐human food chain in Hungary. International Journal of Food Microbiology, 127, 162–167. 10.1016/j.ijfoodmicro.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Nogrady N, Kiraly M, Davies R and Nagy B, 2012. Multidrug resistant clones of Salmonella Infantis of broiler origin in Europe. International Journal of Food Microbiology, 157, 108–112. 10.1016/j.ijfoodmicro.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Nordentoft S, Molbak L, Bjerrum L, Vylder Jd, Immerseel Fv and Pedersen K, 2011. The influence of the cage system and colonisation of Salmonella Enteritidis on the microbial gut flora of laying hens studied by T‐RFLP and 454 pyrosequencing. BMC Microbiology, 11, 187 (22 August 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrien JDP, 1990. Aspects of Salmonella enteritidis control in poultry. Worlds Poultry Science Journal, 46, 119–124. 10.1079/wps19900015 [DOI] [Google Scholar]
- O'Brien SJ, 2013. The “decline and fall” of nontyphoidal salmonella in the United kingdom. Clinical Infectious Diseases, 56, 705–710. 10.1093/cid/cis967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VM, Swaminathan B and Stadelman WJ, 1981. Reduction of numbers of Salmonella typhimurium on poultry parts by repeated freeze‐thaw treatments. Journal of Food Science, 46, 1323–1326. 10.1111/j.1365-2621.1981.tb04164.x [DOI] [PubMed] [Google Scholar]
- Opara OO, Carr LE, Russek‐Cohen E, Tate CR, Mallinson ET, Miller RG, Stewart LE, Johnston RW and Joseph SW, 1992. Correlation of water activity and other environmental conditions with repeated detection of Salmonella contamination on poultry farms. Avian Diseases, 36, 664–671. [PubMed] [Google Scholar]
- Opengart K, Bilgili SF, Warren GL, Baker KT, Moore JD and Dougherty S, 2018. Incidence, severity, and relationship of broiler footpad lesions and gait scores of market‐age broilers raised under commercial conditions in the southeastern United States. The Journal of Applied Poultry Research, 27, 424–432. 10.3382/japr/pfy002 [DOI] [Google Scholar]
- Osman KM, Yousef AM, Aly MM and Radwan MI, 2010. Salmonella spp. infection in imported 1‐day‐old chicks, ducklings, and turkey poults: a public health risk. Foodbourne Pathogens and Disease, 7, 383–390. 10.1089/fpd.2009.0358 [DOI] [PubMed] [Google Scholar]
- Ovelhey A, Kaesbohrer A, Schneider B, Beyerbach M and Kreienbrock L, 2009. Risk factors for Salmonella in flocks of laying hens in Germany. Eds Briese A, Clauss M, Springorum A and Hartung J, Tribun EU, Brno, Czech Republic, pp. 701–704.
- Pan H, Paudyal N, Li X, Fang W and Yue M, 2018a. Multiple Food‐Animal‐Borne Route in Transmission of Antibiotic‐Resistant Salmonella Newport to Humans. Frontiers in Microbiology, 9. 10.3389/fmicb.2018.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Paudyal N, Li X, Fang W and Yue M, 2018b. Multiple Food‐Animal‐Borne Route in Transmission of Antibiotic‐Resistant Salmonella Newport to Humans. Frontiers in Microbiology, 9, 23. 10.3389/fmicb.2018.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA, Northcutt JK, Smith DP, Steinberg EL and Dawson PL, 2015. Microbiological contamination of shell eggs produced in conventional and free‐range housing systems. Food Control, 47, 161–165. 10.1016/j.foodcont.2014.06.038 [DOI] [Google Scholar]
- Parisi A, Crump JA, Glass K, Howden BP, Furuya‐Kanamori L, Vilkins S, Gray DJ and Kirk MD, 2018. Health Outcomes from Multidrug‐Resistant Salmonella Infections in High‐Income Countries: A Systematic Review and Meta‐Analysis. Foodbourne Pathogens and Disease, 15, 428–436. 10.1089/fpd.2017.2403 [DOI] [PubMed] [Google Scholar]
- Park SF, 2002. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. International Journal of Food Microbiology, 74, 177–188. 10.1016/s0168-1605(01)00678-x [DOI] [PubMed] [Google Scholar]
- Pastore R, Schmid H, Altpeter E, Baumgartner A, Hachler H, Imhof R, Sudre P and Boubaker K, 2008. Outbreak of Salmonella serovar Stanley infections in Switzerland linked to locally produced soft cheese, September 2006 ‐ February 2007. Eurosurveillance Weekly, 13, 18979. [DOI] [PubMed] [Google Scholar]
- Pearson AD, Greenwood M, Healing TD, Rollins D, Shahamat M, Donaldson J and Colwell RR, 1993. Colonization of broiler‐chickens by waterborne Campylobacter jejuni . Applied and Environmental Microbiology, 59, 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieskus J, Franciosini MP, Proietti PC, Reich F, Kazeniauskas E, Butrimaite‐Ambrozeviciene C, Mauricas M and Bolder N, 2008a. Preliminary investigations on Salmonella spp. incidence in meat chicken farms in Italy, Germany, Lithuania and the Netherlands. International Journal of Poultry Science, 7, 813–817. [Google Scholar]
- Pieskus J, Kazeniauskas E, Butrimaite‐Ambrozeviciene C, Stanevicius Z and Mauricas M, 2008b. Salmonella incidence in broiler and laying hens with the different housing systems. Journal of Poultry Science, 45, 227–231. 10.2141/jpsa.45.227 [DOI] [Google Scholar]
- Pires S, deKnegt L and Hald T, 2011. Scientific/Technical report submitted to EFSA on an estimation of the relative contribution of different food and animal sources to human Salmonella infections in the European Union. Report to contract CT/EFSA/Zoonoses/2010/02. National Food Institute, Technical University of Denmark, 80 pp.
- Poirier E, Watier L, Espie E, Weill FX, De Valk H and Desenclos JC, 2008. Evaluation of the impact on human salmonellosis of control measures targeted to Salmonella Enteritidis and Typhimurium in poultry breeding using time‐series analysis and intervention models in France. Epidemiology and Infection, 136, 1217–1224. 10.1017/S0950268807009788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Kolar JJ, Demczuk WHB and Harris JE, 1995. Drug‐resistance and biochemical characteristics of Salmonella from turkeys. Canadian Journal of Veterinary Research‐Revue Canadienne De Recherche Veterinaire, 59, 241–248. [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Martin LC, Gyles CL, Reid‐Smith R, Boerlin P, McEwen SA, Prescott JF and Forward KR, 2005. Acquisition of resistance to extended‐spectrum Cephalosporins by Salmonella enterica subsp enterica serovar Newport and Escherichia coli in the turkey poult intestinal tract. Applied and Environmental Microbiology, 71, 1184–1192. 10.1128/aem.71.3.1184-1192.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Martin L, Muckle A, Archambault M, McEwen S and Weir E, 2006. Characterization of antimicrobial resistance of Salmonella Newport isolated from animals, the environment, and animal food products in Canada. Canadian Journal of Veterinary Research‐Revue Canadienne De Recherche Veterinaire, 70, 105–114. [PMC free article] [PubMed] [Google Scholar]
- Pornsukarom S, vanVliet AHM and Thakur S, 2018. Whole genome sequencing analysis of multiple Salmonella serovars provides insights into phylogenetic relatedness, antimicrobial resistance, and virulence markers across humans, food animals and agriculture environmental sources. Bmc Genomics, 19. 10.1186/s12864-018-5137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadas Hernandez E, Sanchez Ramirez E, Avila Gonzalez E, Tellez Isaias G and Salmeron Sosa F, 2005. Behaviour of certain productive characteristics, stress and resistance to Salmonella enteritidis in semi‐heavy poultry under two production systems. Veterinaria Mexico, 36, 205–215. [Google Scholar]
- Powell MR, Crim SM, Hoekstra RM, Williams MS and Gu W, 2018. Temporal patterns in principal Salmonella serotypes in the USA; 1996‐2014. Epidemiology and Infection, 146, 437–441. 10.1017/s0950268818000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti PC, Passamonti F and Asdrubali G, 2001. Egg‐type fowls on the ground and in batteries La gallina ovaiola a terra e in batteria. Rivista di Avicoltura, 70, 12–15. [Google Scholar]
- Proietti PC, Passamonti F and Asdrubali G, 2004. Research on some production and hygiene‐sanitary aspects of laying hens raised in floor pens and in cages Indagini su alcuni aspetti produttivi e igienico‐sanitari dell'allevamento della gallina ovaiola allevata a terra e in batteria. Large Animals Review, 10, 33–37. [Google Scholar]
- Proietti PC, Castellini C, Bosco Ad, Franciosini MP and Asdrubali G, 2007. Investigation on intestinal bacterial flora and Salmonella spp. presence in organic and conventional chickens. Italian Journal of Animal Science, 6, 305–308. [Google Scholar]
- Psifidi A, Fife M, Howell J, Matika O, vanDiemen PM , Kuo R, Smith J, Hocking PM, Salmon N, Jones MA, Hume DA, Banos G, Stevens MP and Kaiser P, 2016. The genomic architecture of resistance to Campylobacter jejuni intestinal colonisation in chickens. BMC Genomics, 17, 293. 10.1186/s12864-016-2612-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan G, Xia P, Zhao J, Zhu C, Meng X, Yang Y, Wang Y, Tian Y, Ding X and Zhu G, 2018. Fimbriae and related receptors for Salmonella Enteritidis. Microbial Pathogenesis. 10.1016/j.micpath.2018.10.025 [DOI] [PubMed] [Google Scholar]
- Quinteiro‐Filho WM, Gomes AVS, Pinheiro ML, Ribeiro A, Ferraz‐de‐Paula V, Astolfi‐Ferreira CS, Ferreira AJP and Palermo‐Neto J, 2012. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathology, 41, 421–427. 10.1080/03079457.2012.709315 [DOI] [PubMed] [Google Scholar]
- Racicot M, Leroux A, Ng S, Comeau G, Quessy S and Gaucher M‐L, 2018. The Canadian Food Inspection Agency Establishment‐Based Risk Assessment Model for Hatcheries: Principles and Algorithm. Proceedings of the European Symposium of the International Association for Food Protection, Stockholm, Sweden. 25–27 April 2018. https://iafp.confex.com/iafp/euro18/meetingapp.cgi/Paper/17993
- Rasschaert G, Houf K, Van Hende J and De Zutter L, 2007. Investigation of the concurrent colonization with Campylobacter and Salmonella in poultry flocks and assessment of the sampling site for status determination at slaughter. Veterinary Microbiology, 123, 104–109. [DOI] [PubMed] [Google Scholar]
- Rauch HE, Vosik D, Kariyawasam S, M'Ikanatha N and Shariat NW, 2018. Prevalence of Group I Salmonella Kentucky in domestic food animals from Pennsylvania and overlap with human clinical CRISPR sequence types. Zoonoses and Public Health, 65, 831–837. 10.1111/zph.12506 [DOI] [PubMed] [Google Scholar]
- Ravel A, Smolina E, Sargeant JM, Cook A, Marshall B, Fleury MD and Pollari F, 2010. Seasonality in Human Salmonellosis: Assessment of Human Activities and Chicken Contamination as Driving Factors. Foodbourne Pathogens and Disease, 7, 785–794. 10.1089/fpd.2009.0460 [DOI] [PubMed] [Google Scholar]
- Ricke SC, 2017. Insights and challenges of Salmonella infection of laying hens. Current Opinion in Food Science, 18, 43–49. 10.1016/j.cofs.2017.10.012 [DOI] [Google Scholar]
- Ricke SC, Dawoud TM, Shi Z, Kaldhone P and Kwon YM, 2018. Chapter 9 ‐ Foodborne Salmonella in Laying Hens and Egg Production. Food and Feed Safety Systems and Analysis.
- Riemann H, Himathongkham S, Willoughby D, Tarbell R and Breitmeyer R, 1998. A survey for Salmonella by drag swabbing manure riles in California egg ranches. Avian Diseases, 42, 67–71. 10.2307/1592577 [DOI] [PubMed] [Google Scholar]
- Riquelme S, Varas M, Valenzuela C, Velozo P, Chahin N, Aguilera P, Sabag A, Labra B, Alvarez SA, Chavez FP and Santiviago CA, 2016.Relevant Genes Linked to Virulence Are Required for Salmonella Typhimurium to Survive Intracellularly in the Social Amoeba Dictyostelium discoideum. Frontiers in Microbiology, 7, 1305. 10.3389/fmicb.2016.01305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N, Beaudeau F, Drouin P, Toux JY, Rose V and Colin P, 1999. Risk factors for Salmonella enterica subsp. enterica contamination in French broiler‐chicken flocks at the end of the rearing period. Preventive Veterinary Medicine, 39, 265–277. [DOI] [PubMed] [Google Scholar]
- Rose N, Beaudeau F, Drouin P, Toux JY, Rose V and Colin P, 2000. Risk factors for Salmonella persistence after cleansing and disinfection in French broiler‐chicken houses. Preventive Veterinary Medicine, 44, 9–20. 10.1016/s0167-5877(00)00100-8 [DOI] [PubMed] [Google Scholar]
- Rosenquist H, Boysen L, Galliano C, Nordentoft S, Ethelberg S and Borck B, 2009. Danish strategies to control Campylobacter in broilers and broiler meat: facts and effects. Epidemiology and Infection, 137, 1742–1750. 10.1017/s0950268809002684 [DOI] [PubMed] [Google Scholar]
- Rossa LS, Stahlke EvR, Diez DC, Weber SH, Stertz SC and Macedo REFd, 2013. Antimicrobial resistance and occurrence of indicator and pathogenic bacteria in organic and conventional chicken meat: comparative study Resistencia antimicrobiana e ocorrencia de micro‐organismos patogenicos e indicadores em frangos organicos e convencionais: estudo comparativo. Biotemas, 26, 211–220. [Google Scholar]
- Rouxel S, Le Gall F, Laisney M, Bohnert M, Fravalo P and Chemaly M, 2018. Investigating the effect of co‐infected birds by Salmonella and Campylobacter. Proceedings of the Proceedings of the International Symposium Salmonella and Salmonellosis, 8th edition. 24 ‐26 September, Saint‐Malo, France, p. 26.
- Ruiz‐Palacios GM, Escamilla E and Torres N, 1981. Experimental Campylobacter Diarrhea in Chickens. Infection and Immunity, 34, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZA, Nasef SA, Elhariri M, Elhelw R and Ezzeldeen N, 2017. Resistance patterns associated with bacterial pathogens causing omphalitis in baby chicks. Bioscience Research, 14, 845–851. [Google Scholar]
- Salmon SA and Watts JL, 2000. Minimum inhibitory concentration determinations for various antimicrobial agents against 1570 bacterial isolates from turkey poults. Avian Diseases, 44, 85–98. 10.2307/1592511 [DOI] [PubMed] [Google Scholar]
- Santos FBO, Sheldon BW, Santos AA Jr and Ferket PR, 2008. Influence of housing system, grain type, and particle size on Salmonella colonization and shedding of broilers fed triticale or corn‐soybean meal diets. Poultry Science, 87, 405–420. 10.3382/ps.2006-00417 [DOI] [PubMed] [Google Scholar]
- Sapkota AR, Kinney EL, George A, Hulet RM, Cruz‐Cano R, Schwab KJ, Zhang GY and Joseph SW, 2014. Lower prevalence of antibiotic‐resistant Salmonella on large‐scale US conventional poultry farms that transitioned to organic practices. Science of the Total Environment, 476, 387–392. 10.1016/j.scitotenv.2013.12.005 [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Murakami M, Maruyama N, Tsujiyama Y, Kusukawa M, Asai T and Yamada Y, 2012. Risk factors for Salmonella prevalence in laying‐hen farms in Japan. Epidemiology and Infection, 140, 982–990. 10.1017/s0950268811001506 [DOI] [PubMed] [Google Scholar]
- Schaar U, Kaleta EF and Baumbach B, 1997. Prevalence of Salmonella enteritidis and Salmonella typhimurium in laying hen flocks battery and on floor housing. Comparative studies using bacteriological and serological demonstration methods. Tierarztl Prax Ausg G Grosstiere Nutztiere, 25, 451–459. [PubMed] [Google Scholar]
- Schulz J, Luecking G, Dewulf J and Hartung J, 2009. Prevalence of Salmonella in German battery cages and alternative housing systems. Eds Briese A, Clauss M, Springorum A and Hartung J, Tribun EU, Brno, Czech Republic, pp. 697–700. Sustainable animal husbandry: prevention is better than cure, Volume 2. Proceedings of the 14th International Congress of the International Society for Animal Hygiene.
- Shaughnessy RG, Meade KG, Cahalane S, Allan B, Reiman C, Callanan JJ and O'Farrelly C, 2009. Innate immune gene expression differentiates the early avian intestinal response between Salmonella and Campylobacter . Veterinary Immunology and Immunopathology, 132, 191–198. [DOI] [PubMed] [Google Scholar]
- Shreeve JE, Toszeghy M, Ridley A and Newell DG, 2002. The carry‐over of Campylobacter isolates between sequential poultry flocks. Avian Diseases, 46, 378–385. 10.1637/0005-2086(2002)046[0378:tcooci]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Sibanda N, McKenna A, Richmond A, Ricke SC, Callaway T, Stratakos AC, Gundogdu O and Corcionivoschi N, 2018. A Review of the Effect of Management Practices on Campylobacter Prevalence in Poultry Farms. Frontiers in Microbiology, 9. 10.3389/fmicb.2018.02002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemon CE, Bahnson PB and Gebreyes WA, 2007. Comparative investigations of prevalence and antimicrobial resistance of Salmonella between pasture and conventionally reared poultry. Avian Diseases, 51, 112–117. 10.1637/0005-2086(2007)051[0112:ciopaa]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Silva J, Leite D, Fernandes M, Mena C, Gibbs PA and Teixeira P, 2011. Campylobacter spp. as a foodborne pathogen: a review. Frontiers in Microbiology, 2, 200‐Article 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C, Puente JL and Calva E, 2017. Salmonella virulence plasmid: pathogenesis and ecology. Pathogens and Disease, 75. 10.1093/femspd/ftx070 [DOI] [PubMed] [Google Scholar]
- Singer RS and Hofacre CL, 2006. Potential impacts of antibiotic use in poultry production. Avian Diseases, 50, 161–172. 10.1637/7569-033106r.1 [DOI] [PubMed] [Google Scholar]
- Sivaramalingam T, Pearl DL, McEwen SA, Ojkic D and Guerin MT, 2013. A temporal study of Salmonella serovars from fluff samples from poultry breeder hatcheries in Ontario between 1998 and 2008. Canadian Journal of Veterinary Research, 77, 12–23. [PMC free article] [PubMed] [Google Scholar]
- Sjolund‐Karlsson M, Rickert R, Matar C, Pecic G, Howie RL, Joyce K, Medalla F, Barzilay EJ and Whichard JM, 2010. Salmonella isolates with decreased susceptibility to extended‐spectrum cephalosporins in the United States. Foodborne Pathogens and Disease, 7, 1503–1509. 10.1089/fpd.2010.0607 [DOI] [PubMed] [Google Scholar]
- Skarzynska M, Hoszowski A, Zajac M, Lalak A, Samcik I, Kwit R and Wasyl D, 2017. Distribution of Salmonella serovars along the food chain in Poland, 2010‐2015. Journal of Veterinary Research, 61, 173–179. 10.1515/jvetres-2017-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov MN, Angen O, Chriel M, Olsen JE and Bisgaard M, 1999. Risk factors associated with Salmonella enterica serovar typhimurium infection in Danish broiler flocks. Poultry Science, 78, 848–854. [DOI] [PubMed] [Google Scholar]
- Snelling WJ, McKenna JP, Lecky DM and Dooley JSG, 2005. Survival of Campylobacter jejuni in waterborne protozoa. Applied and Environmental Microbiology, 71, 5560–5571. 10.1128/aem.71.9.5560-5571.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow LC, Davies RH, Christiansen KH, Carrique‐Mas JJ, Cook AJC, Teale CJ and Evans SJ, 2008. Survey of the prevalence of Salmonella on commercial broiler farms in the United Kingdom, 2005/06. Veterinary Record, 163, 649–654. 10.1136/vr.163.22.649 [DOI] [PubMed] [Google Scholar]
- Snow LC, Davies RH, Christiansen KH, Carrique‐Mas JJ, Cook AJC and Evans SJ, 2010. Investigation of risk factors for Salmonella on commercial egg‐laying farms in Great Britain, 2004‐2005. Veterinary Record, 166, 579–586. [DOI] [PubMed] [Google Scholar]
- Soliman ES, Taha E, Infante KD, Laboy K, Sobieh MA and Reddy PG, 2009. Stressors influence on Salmonella enterica serovar enteritidis colonization in broilers. American Journal of Animal and Veterinary Sciences, 4, 42–48. [Google Scholar]
- Sommer M, Borck Høg B, Larsen LS, Sørensen AIV, Williams N, Merga JY, Cerdà‐Cuéllar M, Urdaneta S, Dolz R, Wieczorek K, Osek J, David B, Hofshagen M, Jonsson M, Wagenaar JA, Bolder N and Rosenquist H, 2016. Analysis of farm specific risk factors for Campylobacter colonization of broilers in six European countries. Microbial Risk Analysis, 2–3, 16–26. [Google Scholar]
- Springer B, Allerberger F and Kornschober C, 2014. Letter to the editor: Salmonella Stanley outbreaks–a prompt to reevaluate existing food regulations. Eurosurveillance Weekly, 19, 20818. [DOI] [PubMed] [Google Scholar]
- Stefani LM, das Neves GB, Brisola MC, Crecencio RB, Pick EC and Araujo DN, 2018. Salmonella Heidelberg resistant to ceftiofur and disinfectants routinely used in poultry. Semina‐Ciencias Agrarias, 39, 1029–1035. 10.5433/1679-0359.2018v39n3p1029 [DOI] [Google Scholar]
- Stella P, Beloeil PA, Guerra B, Hugas M and Liebana E, 2018. The role of the European Food Safety Authority (EFSA) in the fight against antimicrobial resistance (AMR). Food Protection Trends, 38, 72–80. [Google Scholar]
- Stern NJ, Cox NA, Bailey JS, Berrang ME and Musgrove MT, 2001. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poultry Science, 80, 156–160. [DOI] [PubMed] [Google Scholar]
- Sunagawa H, Ikeda T, Takeshi K, Takada T, Tsukamoto K, Fujii M, Kurokawa M, Watabe K, Yamane Y and Ohta H, 1997. A survey of Salmonella enteritidis in spent hens and its relation to farming style in Hokkaido, Japan. International Journal of Food Microbiology, 38, 95–102. [DOI] [PubMed] [Google Scholar]
- Szmolka A, Matulova ME and Rychlik I, 2018. Impact of fliD and virulence plasmid pSEV on response of chicken embryo fibroblasts to Salmonella Enteritidis. Veterinary Immunology and Immunopathology, 196, 1–4. 10.1016/j.vetimm.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Tamba M, Massi P, Marzadori F, Tosi G and Paganelli G, 2000. A surveillance programme for Salmonella infection in poultry: first results Un piano di sorveglianza per le Salmonelle nel settore avicolo: risultati preliminari. Selezione Veterinaria, 587–599. [Google Scholar]
- Taylor NM, Davies RH, Ridley A, Clouting C, Wales AD and Clifton‐Hadley FA, 2008. A survey of fluoroquinolone resistance in Escherichia coli and thermophilic Campylobacter spp. on poultry and pig farms in Great Britain. Journal of Applied Microbiology, 105, 1421–1431. 10.1111/j.1365-2672.2008.03877.x [DOI] [PubMed] [Google Scholar]
- Teplitski M and de Moraes M, 2018. Of Mice and Men.. and Plants: Comparative Genomics of the Dual Lifestyles of Enteric Pathogens. Trends in Microbiology, 26, 748–754. 10.1016/j.tim.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Thakur S, Brake J, Keelara S, Zou M and Susick E, 2013. Farm and environmental distribution of Campylobacter and Salmonella in broiler flocks. Research in Veterinary Science, 94, 33–42. 10.1016/j.rvsc.2012.07.014 [DOI] [PubMed] [Google Scholar]
- Thomas E, Bouma A and Klinkenberg D, 2011. A comparison of transmission characteristics of Salmonella enterica serovar Enteritidis between pair‐housed and group‐housed laying hens. Veterinary Research, 42, 40(23 February 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton G, 2010. Dynamics of Salmonella, Campylobacter in poultry. WATT Poultry USA, 11, 24–25. [Google Scholar]
- Touzain F, Le Devendec L, deBoisseson C , Baron S, Jouy E, Perrin‐Guyomard A, Blanchard Y and Kempf I, 2018. Characterization of plasmids harboring blaCTX‐M and blaCMY genes in E. coli from French broilers. PLoS ONE, 13, e0188768. 10.1371/journal.pone.0188768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachoo N, Frank JF and Stern NJ, 2002. Survival of Campylobacter jejuni in biofilms isolated from chicken houses. Journal of Food Protection, 65, 1110–1116. 10.4315/0362-028x-65.7.1110 [DOI] [PubMed] [Google Scholar]
- Traub‐Dargatz JL, Ladely SR, Dargatz DA and Fedorka‐Cray PJ, 2006. Impact of Heat Stress on the Fecal Shedding Patterns of Salmonella enterica Typhimurium DT104 and Salmonella enterica Infantis by 5‐Week‐Old Male Broilers. Foodbourne Pathogens and Disease, 3, 178–183. 10.1089/fpd.2006.3.178 [DOI] [PubMed] [Google Scholar]
- Trompette M, Le Guilloux L, Souply L, Denis B, Tsouria A, Garrec H, Quentin V, Vaucel J, Locher C, Barjonet G, Marthelet P, Causse X, Poisson D, Nahon S, Joubrel‐Guyot C, Grasset D, Pouedras P, Renou C, Toyer A‐L, Boruchowicz A, Cattoen C, Heluwaert F, Bland S, Faroux R, Desroys V, Paupard T, Verhaeghe A, Correro MO, Pujol C, Picon M, Gallou J, Kaassi M, Touroult‐Jupin P, Arotcarena R, Villeneuve L, Payen J‐L, Libier L, Charpignon C, Rahma M, Manuardi AG, Jeanne A, Lahmek P, Condat B and Macaigne G, 2018. Increased incidence of Campylobacter enteritis and their quinolone resistance between 2010 and 2015: Results of a French national observatory conducted in 21 general hospitals (CHG). Clinics and Research in Hepatology and Gastroenterology. S22107401(18)30231–S22107401(18)30236. 10.1016/j.clinre.2018.10.015 [DOI] [PubMed] [Google Scholar]
- Tuyttens F, Heyndrickx M, Boeck Md, Moreels A, Av Nuffel, Ev Poucke, Ev Coillie, Sv Dongen and Lens L, 2005. Comparison of broiler chicken health and welfare in organic versus traditional production systems. Animal Science Papers and Reports, 23, 217–222. [Google Scholar]
- Tuyttens F, Heyndrickx M, Boeck Md, Moreels A, Av Nuffel, Ev Poucke, Ev Coillie, Sv Dongen and Lens L, 2008. Broiler chicken health, welfare and fluctuating asymmetry in organic versus conventional production systems. Livestock Science, 113, 123–132. 10.1016/j.livsci.2007.02.019 [DOI] [Google Scholar]
- Ugarte‐Ruiz M, Dominguez L, Corcionivoschi N, Wren BW, Dorrell N and Gundogdu O, 2018. Exploring the oxidative, antimicrobial and genomic properties of Campylobacter jejuni strains isolated from poultry. Research in Veterinary Science, 119, 170–175. 10.1016/j.rvsc.2018.06.016 [DOI] [PubMed] [Google Scholar]
- Van Der Fels‐Klerx HJ, Jacobs‐Reitsma WF, Rv Brakel, Van Der Voet H and Van Asselt ED, 2008. Prevalence of Salmonella in the broiler supply chain in The Netherlands. Journal of Food Protection, 71, 1974–1980. [DOI] [PubMed] [Google Scholar]
- Van Der Zijpp AJ, Mollenhorst H, Berentsen PBM and De Boer IJM, 2006. Alternatives for the battery cage system: a comparison of economic, environmental and societal performance. EPC 2006 ‐ 12th European Poultry Conference, Verona, Italy, 10‐14 September, 2006, paper 206.
- Van Hoorebeke S, Van Immerseel F, De Vylder J, Ducatelle R, Haesebrouck F, Pasmans F, De Kruif A and Dewulf J, 2009. Faecal sampling underestimates the actual prevalence of Salmonella in laying hen flocks. Zoonoses and Public Health, 56, 471–476. 10.1111/j.1863-2378.2008.01211.x [DOI] [PubMed] [Google Scholar]
- Van Hoorebeke S, Van Immerseel F, De Vylder J, Ducatelle R, Haesebrouck F, Pasmans F, De Kruif A and Dewulf J, 2010a. The age of production system and previous Salmonella infections on‐farm are risk factors for low‐level Salmonella infections in laying hen flocks. Poultry Science, 89, 1315–1319. [DOI] [PubMed] [Google Scholar]
- Van Hoorebeke S, Van Immerseel F, Schulz J, Hartung J, Harisberger M, Barco L, Ricci A, Theodoropoulos G, Xylouri E, Vylder Jd, Ducatelle R, Haesebrouck F, Pasmans F, De Kruif A and Dewulf J, 2010b. Determination of the within and between flock prevalence and identification of risk factors for Salmonella infections in laying hen flocks housed in conventional and alternative systems. Preventive Veterinary Medicine, 94, 94–100. 10.1016/j.prevetmed.2009.11.022 [DOI] [PubMed] [Google Scholar]
- Van Hoorebeke S, Dewulf J, Van Immerseel F and Jorgensen F, 2012. Production systems for laying hens and broilers and risk of human pathogens. Eds Sandilands V and Hocking P, Cabi, Wallingford, UK, pp. 77–96. Alternative systems for poultry: health, welfare and productivity.
- Van Immerseel F, De Buck J, Pasmans F, Bohez L, Boyen F, Haesebrouck F and Ducatelle R, 2004. Intermittent long‐term shedding and induction of carrier birds after infection of chickens early posthatch with a low or high dose of Salmonella Enteritidis. Poultry Science, 83, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Van Overbeke I, Duchateau L, De Zutter L, Albers G and Ducatelle R, 2006. A comparison survey of organic and conventional broiler chickens for infectious agents affecting health and food safety. Avian Diseases, 50, 196–200. 10.1637/7448-093005r.1 [DOI] [PubMed] [Google Scholar]
- Van Wagenberg CPA, Van Horne PLM, Sommer HM and Nauta MJ, 2016. Cost‐effectiveness of Campylobacter interventions on broiler farms in six European countries. Microbial Risk Analysis, 2–3, 53–62. [Google Scholar]
- Velhner M, Milanov D and Kozoderovic G, 2018. Salmonella spp. in poultry: a constant challenge and new insights. Journal of the Hellenic Veterinary Medical Society, 69, 899–910. 10.12681/jhvms.18012 [DOI] [Google Scholar]
- Vidal A, Davies R, Rodgers J, Ridley A and Clifton‐Hadley F, 2014. Epidemiology and Control of Campylobacter in Modern Broiler Production. In: Sheppard S (ed.). Campylobacter Ecology and Evolution. Caister Academic Press, UK. pp. 287–312. [Google Scholar]
- Volkel I, Schmitz C, Moors E, Gauly M and Czerny CP, 2011. Frequency of Salmonella detection in a broiler flock depending on different litter materials ‐ a field study. Berliner und Munchener Tierarztliche Wochenschrift, 124, 71–77. [PubMed] [Google Scholar]
- Wahlstrom H, Tysen E, Engvall EO, Brandstrom B, Eriksson E, Morner T and Vagsholm I, 2003. Survey of Campylobacter species, VTEC O157 and Salmonella species in Swedish wildlife. Veterinary Record, 153, 74–80. 10.1136/vr.153.3.74 [DOI] [PubMed] [Google Scholar]
- Waldroup AL, Skinner JT, Hierholzer RE, Kopek JM and Waldroup PW, 1992. Effects of bird density on Salmonella contamination of prechill carcasses. Poultry Science, 71, 844–849. [DOI] [PubMed] [Google Scholar]
- Wales A and Davies RH, 2013. Environmental Aspects of Salmonella. Barrow PA and Methner U (eds.)., pp 399–425. Salmonella in Domestic Animals, 2nd Edition.
- Wales AD, Carrique‐Mas JJ, Rankin M, Bell B, Thind BB and Davies RH, 2010. Review of the Carriage of Zoonotic Bacteria by Arthropods, with Special Reference to Salmonella in Mites, Flies and Litter Beetles. Zoonoses and Public Health, 57, 299–314. 10.1111/j.1863-2378.2008.01222.x [DOI] [PubMed] [Google Scholar]
- Wales AD, Vidal AB, Davies RH and Rodgers JD, 2018. Field interventions against colonization of broilers by Campylobacter . Comprehensive Reviews in Food Science and Food Safety, 18, 167–188. 10.1111/1541-4337.12397 [DOI] [PubMed] [Google Scholar]
- Wang G, He YF, Jin X, Zhou YH, Chen XH, Zhao JX, Zhang H and Chen W, 2018a. The Effect of Co‐infection of Food‐Borne Pathogenic Bacteria on the Progression of Campylobacter jejuni Infection in Mice. Frontiers in Microbiology, 9. 10.3389/fmicb.2018.01977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jia B, Xu X, Zhang L, Wei C, Ou H, Cui Y, Shi C and Shi X, 2018b. Comparative Genomic Analysis and Characterization of Two Salmonella enterica Serovar Enteritidis Isolates From Poultry With Notably Different Survival Abilities in Egg Whites. Frontiers in Microbiology, 9. 10.3389/fmicb.2018.02111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LR, Threlfall J, Smith HR and O'Brien SJ, 2000. Salmonella enteritidis epidemic. Science, 287, 1753–1754. [DOI] [PubMed] [Google Scholar]
- Wasyl D, Zając M, Domanska‐Blicharz K and Hoszowski A, 2013. “Ad hoc” study on Salmonella Stanley outbreak strains. Proceedings of the Proceedings of the International Symposium Salmonella and Salmonellosis, Saint‐Malo, France, 27‐29.
- Wedderkopp A, Gradel KO, Jorgensen JC and Madsen M, 2001. Pre‐harvest surveillance of Campylobacter and Salmonella in Danish broiler flocks: a 2‐year study. International Journal of Food Microbiology, 68, 53–59. [DOI] [PubMed] [Google Scholar]
- Wierup M, Wahlstrom H, Lahti E, Eriksson H, Jansson DS, Odelros A and Ernholm L, 2017. Occurrence of Salmonella spp.: a comparison between indoor and outdoor housing of broilers and laying hens. Acta Veterinaria Scandinavica, 59, (21 February 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk T, Szabo M, Szmolka A, Kiss J, Olasz F and Nagy B, 2017. Genome Sequences of Salmonella enterica subsp. enterica Serovar Infantis Strains from Hungary Representing Two Peak Incidence Periods in Three Decades. Genome Announcements, 5. 10.1128/genomeA.01735-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MS and Ebel ED, 2014. Estimating the correlation between concentrations of two species of bacteria with censored microbial testing data. International Journal of Food Microbiology, 175, 1–5. [DOI] [PubMed] [Google Scholar]
- Wilson JS, Hazel SM, Williams NJ, Phiri A, French NP and Hart CA, 2003. Nontyphoidal salmonellae in United Kingdom badgers: Prevalence and spatial distribution. Applied and Environmental Microbiology, 69, 4312–4315. 10.1128/aem.69.7.4312-4315.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KM, Bourassa DV, McLendon BL, Wilson JL and Buhr RJ, 2018. Impact of Skip‐a‐Day and Every‐Day Feeding Programs for Broiler Breeder Pullets on the Recovery of Salmonella and Campylobacter following challenge. Poultry Science, 97, 2775–2784. 10.3382/ps/pey150 [DOI] [PubMed] [Google Scholar]
- Wollin R, 2007. A study of invasiveness of different Salmonella serovars based on analysis of the Enter‐net database. Euro Surveillance, 12:pii=3275, Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3275 [DOI] [PubMed] [Google Scholar]
- Worley J, Meng J, Allard MW, Brown EW and Timme RE, 2018. Salmonella enterica Phylogeny Based on Whole‐Genome Sequencing Reveals Two New Clades and Novel Patterns of Horizontally Acquired Genetic Elements. MBio, 9. 10.1128/mBio.02303-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaojie Q, Shoukui H, Xiujuan Z, Xu C, Xiaozhen H, Yanyan W, Siyun W, Yan C, Chunlei S and Xianming S, 2019. Quantitative proteomics reveals the crucial role of YbgC for Salmonella enterica serovar Enteritidis survival in egg white. International Journal of Food Microbiology, 289, 115–126. 10.1016/j.ijfoodmicro.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Xu C, Ren X, Feng Z, Fu Y, Hong Y, Shen Z, Zhang L, Liao M, Xu X and Zhang J, 2017. Phenotypic Characteristics and Genetic Diversity of Salmonella enterica Serotype Derby Isolated from Human Patients and Foods of Animal Origin. Foodbourne Pathogens and Disease, 14, 593–599. 10.1089/fpd.2017.2278 [DOI] [PubMed] [Google Scholar]
- Yang Y, Tellez G, Latorre JD, Ray PM, Hernandez X, Hargis BM, Ricke SC and Kwon YM, 2018. Salmonella Excludes Salmonella in Poultry: Confirming an Old Paradigm Using Conventional and Barcode‐Tagging Approaches. Frontiers in Veterinary Science, 5, 101–101. 10.3389/fvets.2018.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama E, Torii Y, Shigemura H, Ishige T, Yanagimoto K, Uematsu K, Ando N and Murakami S, 2019. Isolation of Salmonella enterica serovar Agona strains and their similarities to strains derived from a clone caused a serovar shift in broilers. Journal of Infection and Chemotherapy, 25, 71–74. 10.1016/j.jiac.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Zhang S, Li S, Gu W, den Bakker H, Boxrud D, Taylor A, Roe C, Driebe E, Engelthaler DM, Allard M, Brown E, McDermott P, Zhao S, Bruce BB, Trees E, Fields PI and Deng X, 2019. Zoonotic Source Attribution of Salmonella enterica Serotype Typhimurium Using Genomic Surveillance Data, United States. Emerging Infectious Diseases, 25, 82–91. 10.3201/eid2501.180835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Hu Y, Li Q, Tao J, Cai Y, Wang Y, Li J, Zhou Z, Pan Z and Jiao X, 2017a. Subtyping Salmonella enterica serovar Derby with multilocus sequence typing (MLST) and clustered regularly interspaced short palindromic repeats (CRISPRs). Food Control, 73, 474–484. 10.1016/j.foodcont.2016.08.051 [DOI] [Google Scholar]
- Zheng J, Luo Y, Reed E, Bell R, Brown EW and Hoffmann M, 2017b. Whole‐Genome Comparative Analysis of Salmonella enterica Serovar Newport Strains Reveals Lineage‐Specific Divergence. Genome Biology and Evolution, 9, 1047–1050. 10.1093/gbe/evx065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zongo P, Ducrot A, Burie JB and Beaumont C, 2015. Prevalence of Salmonella in flocks housed in enriched cages. Epidemiology and Infection, 143, 1194–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]


