Abstract
The EFSA Panel on Plant Protection Products and their Residues was requested to establish health‐based reference values for groundwater metabolites (LM2, LM3, LM4, LM5 and LM6) of the active substance terbuthylazine based on the available evidence, unless the evidence was considered insufficient to do so. The request was accepted under the explicit circumstance that the reassessment would be made according to a different methodology than the routine methodology currently applied for the assessment of metabolites in groundwater. While for metabolites LM2, LM4 and LM5, it was concluded that the reference values for terbuthylazine are applicable, substance‐specific reference values could not be derived for metabolites LM3 and LM6. The applied threshold of toxicological concern (TTC) approach has shown that metabolites LM3 and LM6 are of potential concern for consumer health, since at least one representative groundwater leaching scenario results in exposure above the relevant threshold. Moreover, other sources of exposure to LM3 and LM6 could not be excluded with certainty. It is therefore recommended to address the specific toxicities of metabolites LM3 and LM6.
Keywords: plant protection products, terbuthylazine, groundwater metabolites, residue definition
Summary
Terbuthylazine was approved in accordance with Regulation (EC) No 1107/2009 by Commission Implementing Regulation (EU) No 820/2011 and listed in Part B of the Annex to Commission Implementing Regulation (EU) No 540/2011 as regards the list of approved substances under Regulation (EC) No 1107/2009. European Food Safety Authority (EFSA) previously finalised a conclusion on this active substance on 10 January 2011 (EFSA, 2011). It was a specific provision of the approval that the applicant was requested to submit to the European Commission further studies to confirm (among other things) a groundwater exposure assessment for the metabolites attributed the codes LM1, LM2, LM3, LM4, LM5 and LM6.
EFSA published a technical report in light of the confirmatory data (EFSA, 2016). With regard to the groundwater metabolites, none of them was considered toxicologically relevant on the basis of the available data and of the agreed classification of terbuthylazine at European Union (EU) level (Opinion of the Committee for Risk Assessment adopted in June 2015).
The rapporteur Member State (RMS), the United Kingdom, proposed that the reference values of terbuthylazine could be applied to the metabolites occurring in groundwater in concentrations of more than 0.75 μg/L (LM2, LM3, LM4, LM5 and LM6) and that based on these values, the exposure of consumers would be acceptable. EFSA, on the other hand, considered that further toxicological data would be needed to establish metabolite‐specific reference values to be used in the consumer risk assessment (taking into account exposure from drinking water abstracted from groundwater).
Following the continued disagreement between EFSA and the RMS, the Commission asked the Panel on Plant Protection Products and their Residues to establish health‐based reference values for the LM metabolites (LM2, LM3, LM4, LM5 and LM6) based on the available evidence, unless the evidence was considered insufficient to do so.
The Panel based its assessment on the documents and information submitted to EFSA during the peer review of the risk assessment of the active substance terbuthylazine. Additionally, information from public scientific literature has been taken into consideration. For this assessment, the methodologies and approaches described in the Guidance document on residue definition (EFSA PPR Panel, 2016) were applied.
Two groups of metabolites were considered in the current assessment: (a) the characterised group of metabolites including MT1, MT13 and MT14, and (b) the group of metabolites LM2, LM3, LM4, LM5 and LM6, for which a conclusion on toxicological reference values could not be drawn during the peer review.
Metabolites LM2 and LM4 were considered sufficiently similar to MT13 and MT14, for which there are experimental data available showing equal or lower toxicity compared with terbuthylazine and for which the acceptable daily intake (ADI) of terbuthylazine was considered applicable. LM2 and LM4 were also considered sufficiently similar to major rat metabolites (> 10%) measured in absorption, distribution, metabolism and excretion (ADME) studies conducted with terbuthylazine. Finally, although of low reliability, experimental toxicity data on LM2 and LM4 are available (90‐day rat study) from a cyanazine evaluation, showing no effects up to 1,000 mg/kg body weight (bw) per day.
Based upon all the available information, it was concluded that for the carboxylic acid metabolites LM2 and LM4, the ADI of the parent substance terbuthylazine (0.004 mg/kg bw per day) can be applied with confidence.
Based on the available data, a reliable conclusion on substance‐specific reference values for LM3 cannot be derived. If other sources of exposure to LM3 (except by soil degradation of terbuthylazine and subsequent groundwater contamination) could be excluded or quantified with certainty (not part of the current mandate), robust exposure estimates could be made for metabolite LM3 and compared with the applicable threshold of toxicological concern (TTC).
Taking into account all the available information on LM5 and applying read‐across principles, it was concluded that LM5 can be considered as sufficiently similar to MT14, for which reference values of terbuthylazine are applicable. It can therefore be reliably assumed that LM5 toxicity is covered by the reference values derived for terbuthylazine (0.004 mg/kg bw per day).
Based on the available data, a reliable conclusion on substance‐specific reference values for LM6 cannot be derived. If other sources of exposure to LM6 (except by soil degradation of terbuthylazine and subsequent groundwater contamination) could be excluded or quantified with certainty (not part of the current mandate), robust exposure estimates could be made for metabolite LM6 and compared with the applicable TTC value.
Under the assumption of there being no oral co‐exposure from additional sources and considering the WHO default values for drinking water consumption data for infants, children and adults (WHO, 2017), the calculated residue concentrations of metabolites LM3 and LM6 in groundwater abstracted for drinking water purposes (individually or combined) result in individual and combined exposures below the relevant TTC for infants of 0.5 μg/kg bw per day (Cramer class III with an additional uncertainty factor of 3) and for children and adults (Cramer class III) in at least one out of eight scenarios.
Considering the peak water consumption data for infants as published in EFSA Scientific Committee (2017) and the calculated residue concentrations of metabolites LM3 and LM6 in groundwater abstracted for drinking water purposes, an exceedance of the relevant TTC (Cramer class III with additional uncertainty factor of 3) is indicated for the combined exposure to LM3 and LM6 in all eight scenarios, while individual exposures to LM3 or LM6 are below the threshold in at least two scenarios.
Although particular exposure scenarios were identified without indications for consumer health risks, the TTC assessment has also demonstrated that metabolites LM3 and LM6 may exceed critical exposure levels under certain conditions. It is therefore recommended to address the specific toxicities of metabolites LM3 and LM6.
Several uncertainties having a potential impact on this assessment were identified across the different steps and molecules.
1. Introduction
1.1. Background and terms of reference as provided by the requestor
Terbuthylazine is an active substance covered by the third stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC1 concerning the placing of plant protection products on the market.
Terbuthylazine was approved in accordance with Regulation (EC) No 1107/20092 by Commission Implementing Regulation (EU) No 820/20113 and listed in Part B of the Annex to Commission Implementing Regulation (EU) No 540/20114 as regards the list of approved substances under Regulation (EC) No 1107/2009. EFSA previously finalised a Conclusion on this active substance on 10 January 2011 (EFSA, 2011).
It was a specific provision of the approval that the applicant was requested to submit to the European Commission further studies to confirm (among other things):
a groundwater exposure assessment for the metabolites attributed the codes LM1, LM2, LM3, LM4, LM5 and LM6;
the relevance of the metabolites MT1 (N‐tert‐butyl‐6‐chloro‐1,3,5‐triazine‐2,4‐diamine), MT 13 (4‐(tert‐butylamino)‐6‐(ethylamino)‐1,3,5‐triazin‐2‐ol or 6‐hydroxy‐N 2‐ethyl‐N 4‐tert‐butyl‐1,3,5‐triazine‐2,4‐diamine), MT14 (4‐amino‐6‐(tert‐butylamino)‐1,3,5‐triazin‐2‐ol or N‐tert‐butyl‐6‐hydroxy‐1,3,5‐triazine‐2,4‐diamine), and of the unidentified metabolites LM1, LM2, LM3, LM4, LM5 and LM6 with respect to cancer, if terbuthylazine is classified under Regulation (EC) No 1272/20085 as ‘suspected of causing cancer’.
In accordance with the specific provision, the applicant, Syngenta Crop Protection AG and Sipcam Oxon Italia SpA (previously Oxon Italia SpA), submitted an updated dossier in June 2012, which was evaluated by the designated rapporteur Member State (RMS), the United Kingdom, in the form of an addendum to the draft assessment report (United Kingdom, 2015). That assessment, in compliance with Guidance Document SANCO 5634/2009 (European Commission, 2013), was circulated to the applicant, the other Member States and EFSA for comments, all of which were collated in the technical report published on 20 January 2016 (EFSA, 2016). The requirements for confirmatory data related to the relevance of metabolites in groundwater (European Commission, 2003) if terbuthylazine were to be classified as carcinogenic (first bullet point) became obsolete because the Risk Assessment Committee of the European Chemicals Agency (ECHA) confirmed in its Opinion of 5 June 2015 (ECHA, 2015) that terbuthylazine should not be classified with respect to cancer. However, it was concluded that more information on the repeat‐dose toxicity for the groundwater metabolites LM2, LM3, LM4, LM5 and LM6 was needed in order to conclude on the relevant reference values to be used for the consumer risk assessment, which is triggered for those compounds being predicted to occur in groundwater in concentrations of more than 0.75 μg/L for the representative uses being assessed (European Commission, 2003).
In the technical report it was indicated that EFSA and the RMS had different views on the assessment of the groundwater metabolites. The RMS proposed that the reference values of the parent compound terbuthylazine could be applied to the metabolites occurring in groundwater in concentrations of more than 0.75 μg/L (LM2, LM3, LM4, LM5 and LM6) and that based on these values, the exposure of consumers would be acceptable. EFSA, on the other hand, considered that further toxicological data were required to establish substance‐specific reference values for metabolites to be used in the consumer risk assessment (taking into account exposure from drinking water abstracted from groundwater). The RMS believed that as conservative assumptions are used in the assessments, in respect of both the levels of metabolites predicted to occur in groundwater and comparison with the relatively low acceptable daily intake (ADI) for terbuthylazine, an acceptable risk to consumers was demonstrated. It was therefore considered that further animal studies to support the setting of substance‐specific reference values for metabolites were not justified.
Given this divergence in opinion, the European Commission requested EFSA to organise a peer review of the RMS's evaluation of the confirmatory data on whether the available data are sufficient to conclude on whether exposure to groundwater metabolites at concentrations of more than 0.75 μg/L would pose an unacceptable risk to consumers through consumption of drinking water. On the basis of this peer review, EFSA finalised its conclusion in May 2017 (EFSA, 2017a). It was concluded that the toxicological data on groundwater metabolites LM2, LM3, LM4, LM5 and LM6 were insufficient to determine toxicological reference values, and consequently that the consumer risk assessment could not be finalised.
In September 2018 the Commission asked the Panel on Plant Protection Products and their Residues (PPR Panel) to establish health‐based reference values for the LM metabolites (LM2, LM3, LM4, LM5 and LM6) based on the available evidence, unless the evidence was not considered sufficient to do so.
The Panel was also requested to specify any uncertainties associated with the values derived and how such uncertainties could be further addressed in the future.
More specifically, the Commission asked the PPR Panel to:
assess the available evidence;
consider the data gaps identified in the EFSA conclusion and whether they could be addressed in light of the current state of the art on read‐across and other alternative in silico and non‐testing methods and tools;
specify any uncertainties and how these could be further addressed; for example, in case additional safety factors are required;
based on these assessments, to establish health‐based reference values for the LM metabolites (LM2, LM3, LM4, LM5 and LM6) of terbuthylazine.
The deadline for providing the Scientific Opinion is 22 May 2019.
Exclusion of the evaluation for LM1 in the Scientific Opinion of the PPR Panel was not based upon scientific grounds but upon the fact that the request for health‐based reference values for this metabolite was not included in the Commission's mandate.
1.2. Interpretation of the terms of reference
The European Food Safety Authority (EFSA) PPR Panel will develop a Scientific Opinion on the setting of health‐based reference values for metabolites of the active substance terbuthylazine. It interpreted the terms of reference as follows:
Based on the available evidence, the PPR Panel should establish health‐based reference values for the LM metabolites (LM2, LM3, LM4, LM5 and LM6) of terbuthylazine, unless the evidence is considered insufficient to do so.
In the event that reference values could not be established by the Panel based on the available evidence, with regard to the need to carry out a consumer risk assessment of the exposure of these metabolites in drinking water, other methodologies than health‐based reference values could be investigated as well (e.g. the threshold of toxicological concern (TTC) approach).
The Panel will specify any uncertainties associated with the values derived and how these could be further addressed, for example, whether additional safety factors are required.
In the present case, the mandate was accepted under the explicit circumstance that the reassessment would be made according to a different methodology than the one specified for metabolites in groundwater (European Commission, 2003), i.e. using the Guidance of the EFSA PPR Panel (2016), which is applicable to the type of assessment requested.
Furthermore, if the Panel establishes reference values for LM metabolites, EFSA is requested to update the risk assessment for consumers taking into account exposure to the LM metabolites using the reference values established by the Panel in accordance with this mandate. This activity is requested under Article 31 of Regulation (EC) No 178/20026 with a deadline of 3 months from the time the Opinion of the PPR Panel is published in the EFSA Journal.
2. Data and methodologies
2.1. Data
The Panel based its assessment on the following documents and information submitted to EFSA during the peer review of the risk assessment of the active substance terbuthylazine, which were made available to the Panel:
The draft assessment report and its addenda prepared under Council Directive 91/414/EEC and Commission Regulation (EC) No 33/20087 (United Kingdom, 2007, 2010a,b, 2015).
The conclusion on the peer review of the pesticide risk assessment of the active substance terbuthylazine (EFSA, 2011).
The outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for terbuthylazine in light of confirmatory data (EFSA, 2016).
The peer review of the pesticide risk assessment for the active substance terbuthylazine in light of confirmatory data submitted (EFSA, 2017a,b).
Additionally, information from public scientific literature (retrieved mostly from PubMed and ToxNet) has been taken into consideration. All citations can be found in the References.
2.2. Methodologies
For this assessment, the Guidance document on residue definition (EFSA PPR Panel, 2016) and approaches described therein were applied.
3. Assessment
Two groups of metabolites were considered in the current assessment: (a) the characterised group of metabolites including MT1, MT13 and MT14; and (b) the group of metabolites LM2, LM3, LM4, LM5 and LM6, for which a conclusion on toxicological reference values could not be reached during the peer review (Table 1).
Table 1.
Chemical and toxicological data of terbuthylazine and metabolites MT1, MT13, MT14
| Structural formula |
|
|
|
|
|---|---|---|---|---|
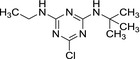
| Code/trivial name | Terbuthylazine |
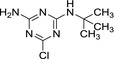
MT1 desethyl‐terbuthylazine |
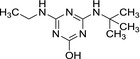
MT13 hydroxy‐terbuthylazine 2‐hydroxy‐terbuthylazine |
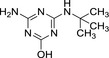
MT14 desethyl‐hydroxy‐terbuthylazine desethyl‐2‐hydroxy‐terbuthylazine |
| Chemical names | N 2‐tert‐butyl‐6‐chloro‐N 4‐ethyl‐1,3,5‐triazine‐2,4‐diamine | N‐tert‐butyl‐6‐chloro‐1,3,5‐triazine‐2,4‐diamine | 4‐(tert‐butylamino)‐6‐(ethylamino)‐1,3,5‐triazin‐2‐ol | 4‐amino‐6‐(tert‐butylamino)‐1,3,5‐triazin‐2‐ol |
| SMILES notation | Clc1nc(NCC)nc(NC(C)(C)C)n1 | Nc1nc(NC(C)(C)C)nc(Cl)n1 | Oc1nc(NCC)nc(NC(C)(C)C)n1 | Nc1nc(NC(C)(C)C)nc(O)n1 |
| InChI Key | FZXISNSWEXTPMF‐UHFFFAOYSA‐N | LMKQNTMFZLAJDV‐UHFFFAOYSA‐N | OYTCZOJKXCTBHG‐UHFFFAOYSA‐N | NUISVCFZNCYUIM‐UHFFFAOYSA‐N |
| Herbicidal activity | Yes | Yes | No | No |
| Acute oral LD 50 (rats), mg/kg bw | 1,000–1,590 |
1st study: 236 2nd study: 300–500 |
> 2,000 | > 2,000 |
| NOAEL in 90‐day study in rats (mg/kg bw per day) | 2.1 | No NOAEL in males (study predates any OECD Guideline) | 3.4 | 10.3 |
| Observed effects at LOAEL of 90‐day studies in rats | Reduced body weight and food consumption | Reduced body weight gain and total white blood cells | Changes in haematology and clinical chemistry parameters | Increased mortality and water consumption, changes in haematology, clinical chemistry and urinalysis parameters and increased kidney weight, renal (histo)pathology secondary to chronic renal failure |
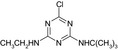
3.1. N‐tert‐butyl‐6‐chloro‐1,3,5‐triazine‐2,4‐diamine (MT1), 4‐(tert‐butylamino)‐6‐(ethylamino)‐1,3,5‐triazin‐2‐ol (MT13) and 4‐amino‐6‐(tert‐butylamino)‐1,3,5‐triazin‐2‐ol (MT14)
In the EFSA Conclusion (EFSA, 2011), it was concluded that the reference values of terbuthylazine are applicable to the metabolites desethyl‐terbuthylazine (MT1), hydroxy‐terbuthylazine (MT13) and desethyl‐hydroxy‐terbuthylazine (MT14).
3.2. LM2, LM3, LM4, LM5, LM6
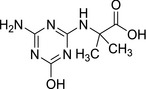
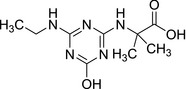
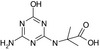
3.2.1. N‐(4‐amino‐6‐hydroxy‐1,3,5‐triazin‐2‐yl)‐2‐methylalanine (LM2) and N‐[4‐(ethylamino)‐6‐hydroxy‐1,3,5‐triazin‐2‐yl]‐2‐methylalanine (LM4)
|
|
| LM2 | LM4 |
| SMILES: CC(C)(Nc1nc(N)nc(O)n1)C(O)=O | SMILES: CCNc1nc(O)nc(NC(C)(C)C(O)=O)n1 |
| InChI Key: QKOJUBFULGSCQS‐UHFFFAOYSA‐N | InChI Key: AXUUYNNVNOSTRP‐UHFFFAOYSA‐N |
The transformations which lead to the formation of LM2 and LM4 are oxidative dechlorination (replacement of the Cl atom with the −OH group), terbuthylhydroxylation which leads to the formation of the carboxylic group and N‐dealkylation for LM2. LM2 and LM4 were not identified as metabolites in rat metabolism investigations with terbuthylazine and information on general toxicity studies was not available for the peer review.
The highest structural similarity (around 80%, Dice coefficient) was estimated by OECD QSAR Toolbox ver. 4.2 between LM2 and MT14 and between LM4 and MT13 (see Appendix C). However, it is generally considered that the simple similarity measure may not be enough to fully justify a read‐across prediction as it does not recognise small changes in structure which can potentially lead to significant change in the reactivity (Schultz et al., 2015, 2019). Therefore, further analyses at organic functional group levels were performed.
Dechlorination in cases of chlorotriazines has been demonstrated to change the toxicity spectrum of metabolites and usually decreases the observed toxicity when compared with chlorine‐preserving metabolic processes (Stoker et al., 2002, 2013; Laws et al., 2003). These observations were confirmed by the experimental data provided for MT13 and MT14, where at least higher toxicity than for terbuthylazine was not observed (EFSA, 2011).
Exocyclic nitrogen in an N‐alkyl group without or with N‐dealkylation could undergo N‐oxidation leading to the formation of reactive metabolites (imines) and intermediates resulting ultimately in glutathione conjugation or potentially in tissue injuries, genotoxicity and carcinogenicity (Macherey and Dansette, 2008; LeBlanc and Sleno, 2011; Parkinson et al., 2018). However, it should be noted that experimental genotoxicity studies provided for LM2 and LM4 did not show a concern.
Regarding the formation of carboxylic groups, there is no general rule to conclude that carboxylic acids, as polar structures, are per se less toxic molecules than their precursors are, although their elimination from the body is promoted. It is known from pharmaceuticals, which are frequently formulated as carboxylic acids that the formation of acyl glucuronides by glucuronidation of a carboxylic acid group can lead to increased toxicity of molecules by enhancing chemical reactivity and consequently leading to covalent binding with macromolecules (Lassila et al., 2015; Van Vleet et al., 2015; Smith et al., 2018). As regards chlorotriazine's toxicity, no public information is known to the Panel which suggests a potentially higher toxicity of acyl glucuronides.
Although LM2 and LM4 were not identified in the rat metabolism in the draft assessment report on terbuthylazine, it is stated there that the metabolism of terbuthylazine in the rat following oral administration was found to be extensive and proceeded via two major pathways: (a) hydroxylation of the t‐butyl moiety with further oxidation or conjugation; or (b) oxidative cleavage of the amino‐ethyl bond followed by further oxidation or conjugation (see Appendix B). In absorption, distribution, metabolism and excretion (ADME) studies of both notifiers for terbuthylazine (Syngenta Crop Protection AG and Sipcam Oxon Italia SpA), other carboxylic acid metabolites were detected. For example, metabolite 3U (halogenated and de‐alkylated carboxylic acid metabolite) in Syngenta's studies was detected at levels above 10% in rat urine, in both sexes and after single and repeated dose administration. In the Oxon ADME study, the same halogenated and de‐alkylated carboxylic acid called M5 was the major urine metabolite identified in the urine of male and female rats > 30% of the administered low dose. Therefore, metabolite 3U (=M5) as a major rat metabolite is considered to be covered by the toxicological profile of the parent. It is presumed that still halogenated carboxylic acid derivatives of terbuthylazine (as 3U (=M5)) would have at least the same, if not higher toxicity, than dehalogenated carboxylic acid metabolites (LM2 and LM4).
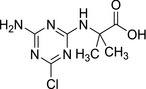
|
| Metabolite 3U (= metabolite M5) |
| Chemical name: 2‐((4‐amino‐6‐chloro‐1,3,5‐triazin‐2‐yl)amino)‐2‐methylpropanoic acid |
| SMILES: CC(C(O)=O)(C)NC1=NC(N)=NC(Cl)=N1 |
| InChI Key: GOVSIGWGHYIRFN‐UHFFFAOYSA‐N |
In addition, information from public literature showed that general toxicity studies with LM2 and LM4 are available for another chlorotriazine compound, cyanazine (CEPA, 1997). Cyanazine was not included in Annex I of Directive 91/414/EEC (Commission Regulation (EC) No 2076/20028 of 20 November 2002), but a risk assessment evaluation of cyanazine performed by the Californian Environmental Protection Agency (EPA) from 1997 is publicly available.
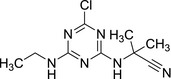
|
| Cyanazine |
| Chemical name: 2‐((4‐chloro‐6‐(ethylamino)‐1,3,5‐triazin‐2‐yl)amino)‐2‐methylpropanenitrile |
| SMILES: CCNC1=NC(NC(C)(C#N)C)=NC(Cl)=N1 |
| InChI Key: MZZBPDKVEFVLFF‐UHFFFAOYSA‐N |
Metabolites LM2 and LM4 were identified in the rat metabolic pathway after cyanazine administration. The codes used in the California EPA document are SD 31,224 (= LM2) and SD 31,223 (= LM4).
Regarding acute toxicity, LD50 of > 2,000 mg/kg body weight (bw) and 789 mg/kg bw were reported for LM2 and LM4, respectively.
As regards subchronic toxicity studies (90‐day rat, Sprague‐Dawley CEF strain), conducted with each of these two metabolites, it is reported that adverse effects were not observed at doses of up to 10,000 ppm (approximately 1,000 mg/kg bw per day, according to EFSA Scientific Committee, 2012a) for neither of these compounds. In the publication of Walker et al. (1974) (cited by California EPA, where LM2 = DW 4,394 and LM4 = DW 4,385), some additional data on the parameters measured (general health and behaviour, food intake, body and major organ weights, ‘haematological, clinical chemical and urine values’) were reported. A detailed assessment of these studies is not available. As these studies predate any OECD Guidelines and very limited parameters were measured, these studies can only be taken into account as supporting studies of uncertain, and therefore low, reliability.
Conclusion
LM2 and LM4 were considered sufficiently similar to MT14 and MT13, respectively, for which there is experimental data available showing equal or lower toxicity compared with terbuthylazine, and therefore, the ADI of terbuthylazine was considered applicable to them. LM2 and LM4 were also considered sufficiently similar to a major rat metabolite (3U or M5) measured in ADME studies conducted with terbuthylazine. Finally, although of low reliability, experimental data on LM2 and LM4 are available (90‐day rat studies) from a cyanazine evaluation, showing no effects up to 1,000 mg/kg bw per day.
Considering all the available information, it is concluded that for the carboxylic acid metabolites LM2 and LM4, the ADI of the parent substance terbuthylazine (0.004 mg/kg bw per day) can be applied with confidence.
3.2.2. 2,6‐dihydroxy‐7,7‐dimethyl‐7,8‐dihydroimidazo[1,2‐a][1,3,5]triazin‐4(6H)‐one (LM3)
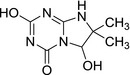
|
| LM3 |
| SMILES: O=C1N=C(O)N=C2N1C(O)C(C)(C)N2 |
| InChI Key: SDFNUIRNRULFGI‐UHFFFAOYSA‐N |
LM3 is an internally fused triazine imidazole ring containing a hydroxyl group (after N‐de‐ethylation and oxidative deamination) and a new keto group on the triazine ring. It was negative in genotoxicity testing (in vitro testing battery, EFSA, 2016). General toxicity studies were not available for LM3. LM3 was not identified as a metabolite in rat metabolism investigations with terbuthylazine, nor were any compounds with a sufficiently similar structure.
There is no evidence that LM3 is of higher, the same or lower toxicity than the parent terbuthylazine molecule. Therefore, a reliable conclusion on substance‐specific reference values for LM3 cannot be made.
If robust exposure estimates including other potential sources of LM3, in addition to terbuthylazine degradation in soil (leading to groundwater contamination) could be made, the TTC approach outlined in the EFSA Guidance document on residue definition (EFSA PPR Panel, 2016) could be applied.
According to the OECD QSAR Toolbox ver. 4.2 evaluation, LM3 is allocated to Cramer class III substances for which a chronic exposure value of 1.5 μg/kg bw per day is applicable (EFSA Scientific Committee, 2012b). If the sole source of exposure to LM3 was proven to be via drinking water, 100% of the exposure to LM3, instead of 20% (WHO, 2017), could be allocated to water consumption.
During the current assessment, an in‐depth evaluation of other sources of exposure to LM3 or its precursors (besides the exposure via degradation of terbuthylazine in soil leading to groundwater contamination) was not performed. It is noted that general transformations of triazines, used as pesticides but also as biocides (e.g. terbutryn), are very similar and may result in precursor molecules in the environment like 2‐hydroxy‐terbutryn (syn. TP‐212, TB‐OH, GS 23158; identical to terbuthylazine metabolite MT13) or desethyl‐2‐hydroxy‐terbutryn (syn. TP‐184, TB‐OH‐DesE; identical to terbuthylazine metabolite MT14) which could potentially form LM3.
Conclusion
Based on the available data, a reliable conclusion on substance‐specific reference values for LM3 cannot be derived. If other sources of exposure to LM3 (except by soil degradation of terbuthylazine and subsequent groundwater contamination) could be excluded or quantified with certainty (which is not part of the current mandate), robust exposure estimates could be made for metabolite LM3 and compared with the applicable TTC value.
3.2.3. 6‐(tert‐butylamino)‐1,3,5‐triazine‐2,4‐diol (LM5)
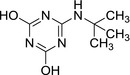
|
| LM5 |
| SMILES: OC1=NC(O)=NC(NC(C)(C)C)=N1 |
| InChI Key: RMGNIWIYFYKTDC‐UHFFFAOYSA‐N |
LM5 is an oxidatively de‐ethylated, deaminated and dehalogenated (−OH group replaces chlorine) derivative of the parent compound terbuthylazine. It was negative in genotoxicity testing (in vitro testing battery, EFSA, 2016). LM5 was not identified as a metabolite in rat metabolism investigations and no other metabolite with double hydroxylation on the ring was identified in rats in vivo. General toxicity studies were not available for this molecule.
OECD QSAR Toolbox ver. 4.2 estimated the highest similarity between LM5 and MT14 (85%, Dice coefficient, see Appendix C).
Since it is generally considered that a simple similarity measure does not always reflect changes in reactivity and itself may not be enough to fully justify a read‐across prediction (Schultz et al., 2015, 2019), further analyses at organic functional group levels were performed.
It is noted that the characterised metabolite MT14 (Desethyl‐hydroxy‐terbuthylazine) contains an amine group instead of a second −OH group on the triazine ring which is not expected to significantly change the chemical reactivity. This is exemplified by the common metabolic transformation of amino groups to ‐OH groups by CYPs or amine oxidases often leading to improved detoxification and decreased activity (Parkinson et al., 2018).
In a 90‐day rat study with MT14, an no observed adverse effect level (NOAEL) of 10.3 mg/kg bw per day was set, which is slightly higher than the NOAEL from a 90‐day rat study with terbuthylazine (2.1 mg/kg bw per day). Although it appears that chlorinated and dechlorinated derivatives of terbuthylazine have different target organs, it was concluded in the peer review that the ADI of terbuthylazine (0.004 mg/kg bw per day) should also be applied to MT14 and an MT14‐specific reference value was not derived.
Conclusion
Taking into account all the available information on LM5 and applying read‐across principles, it was concluded that LM5 can be considered as sufficiently similar to MT14 in order to reliably assume that its toxicity is also covered by reference values derived for the parent substance terbuthylazine (0.004 mg/kg bw per day).
3.2.4. 4‐(tert‐butylamino)‐6‐hydroxy‐1‐methyl‐1,3,5‐triazin‐2(1H)‐one (LM6)
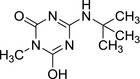
|
| LM6 |
| SMILES: O=C1N(C)C(O)=NC(NC(C)(C)C)=N1 |
| InChI Key: SKWILWLBILDCEB‐UHFFFAOYSA‐N |
LM6 is a N‐de‐ethylated and oxidatively deaminated, dehalogenated and N‐methylated derivative of terbuthylazine. It was concluded negative in genotoxicity testing (in vitro testing battery and one in vivo assay, EFSA, 2011). Neither LM6 nor a sufficiently similar structure was identified as metabolite in rat metabolism investigations and general toxicity studies with LM6 were not available.
There is no evidence that LM6 is of higher, the same or lower toxicity than the parent molecule terbuthylazine. Therefore, a reliable conclusion on reference values for LM6 cannot be made.
If it can be shown with confidence that terbuthylazine degradation in soil (leading to groundwater contamination) is the only source of exposure to LM6 (via drinking water), the TTC approach outlined in the EFSA Guidance document on residue definition (EFSA PPR Panel, 2016) could be applied.
According to the OECD QSAR Toolbox ver. 4.2 evaluation (see Appendix C), LM6 is allocated to Cramer class III substances for which a chronic exposure value of 1.5 μg/kg bw per day is applicable (EFSA Scientific Committee, 2012b). If the sole source of exposure to LM6 is proven to be via drinking water, 100% of the exposure to LM6, instead of 20% (WHO, 2017), could be allocated to water consumption.
During the current assessment, an in‐depth evaluation of other sources of exposure to LM6 or its precursors (besides the exposure via degradation of terbuthylazine in soil leading to groundwater contamination) was not performed. It is noted that general transformations of triazines used as pesticides but also as biocides (e.g. terbutryn) are very similar and may result in precursor molecules in the environment like 2‐hydroxy‐terbutryn (syn. TP‐212, TB‐OH, GS 23158; identical to terbuthylazine metabolite MT13) or desethyl‐2‐hydroxy‐terbutryn (syn. TP‐184, TB‐OH‐DesE; identical to terbuthylazine metabolite MT14) which could potentially form LM6.
Conclusion
Based on the available data, a reliable conclusion on substance‐specific reference values for LM6 cannot be derived. If other sources of exposure to LM6 (except by soil degradation of terbuthylazine and subsequent groundwater contamination) could be excluded or quantified with certainty (which is not part of the current mandate), robust exposure estimates could be made for metabolite LM6 and compared with the applicable TTC value.
4. Exposure calculations for LM3 and LM6
To evaluate the potential of the lysimeter metabolites LM3 and LM6 to cause a human health concern, the TTC approach is followed on an as yet provisional basis, i.e. under the assumption of no existing oral co‐exposure of these metabolites from additional sources following, for example, biocidal or pesticidal uses of related chlorinated or non‐chlorinated triazines.
Drinking water consumption
The consumption of drinking water is based on default values for representative consumer groups of bottle‐fed infants (150 mL/kg bw per day), children (100 mL/kg bw per day) and adults (33 mL/kg bw per day) (WHO, 2017).
Additionally, more critical consumption values for infants were derived from EFSA Scientific Committee (2017), where a peak consumption of 260 mL/kg bw per day of infant formula was calculated for infants of 14–27 days of age, based on a 7:8 weight ratio of water to the final product. Accordingly, a peak water consumption of 227 mL/kg bw per day is considered for the exposure estimate of drinking water from groundwater sources.
Residue input values
The groundwater exposure assessments for metabolites LM3 and LM6 were carried out using Forum for the Co‐ordination of Pesticide Fate Models and their Use (FOCUS) (2009) scenarios with the PEARL 4.4.4 model and published for the individual metabolites and good agricultural practices in Appendix A of the EFSA conclusion (EFSA, 2017a) and presented in Table 2. Metabolite levels are not converted into parent equivalents.
Table 2.
PECGW for metabolites LM3 and LM6 at 1 m soil depth (μg/L) for the relevant FOCUS scenarios (Appendix A of the EFSA conclusion; EFSA, 2017a)
| Application to maize at 750 g/ha | Application to maize at 850 g/ha | |||
|---|---|---|---|---|
| Scenario | LM3 | LM6 | LM3 | LM6 |
| Châteaudun | 2.10 | 3.97 | 2.39 | 4.49 |
| Hamburg | 3.40 | 4.72 | 3.86 | 5.32 |
| Kremsmünster | 1.74 | 2.78 | 1.97 | 3.13 |
| Okehampton | 1.64 | 1.80 | 1.86 | 2.02 |
| Piacenza | 1.88 | 4.54 | 1.66 | 5.14 |
| Porto | 0.93 | 1.67 | 1.06 | 1.88 |
| Sevilla | 0.54 | 3.71 | 0.62 | 4.21 |
| Thiva | 1.88 | 8.00 | 2.15 | 9.06 |
Exposure and TTC assessment
Calculated human exposure to metabolites LM3 and LM6 via drinking water is presented in Table 3 and Table 4 based on the WHO default assumptions (infant, child and adult; WHO, 2017) and additionally for infants with peak consumption data at day 14–27 of life (EFSA Scientific Committee, 2017).
Table 3.
Human exposure for FOCUS scenario concentrations of groundwater metabolites LM3 and LM6 in maize following application of 750 g as/ha
| Application to maize at 750 g as/ha | |||||
|---|---|---|---|---|---|
| LM3 | |||||
| PECGW scenario | PECGW a | Infant day 14–27b | Infant 5 kg bwc | Child 10 kg bwc | Adult 60 kg bwc |
| μg/L | μg/kg bw per day | ||||
| Châteaudun | 2.10 | 0.477 | 0.315 | 0.210 | 0.070 |
| Hamburg | 3.40 | 0.772 | 0.510 | 0.340 | 0.113 |
| Kremsmünster | 1.74 | 0.395 | 0.261 | 0.174 | 0.058 |
| Okehampton | 1.64 | 0.372 | 0.246 | 0.164 | 0.055 |
| Piacenza | 1.88 | 0.427 | 0.282 | 0.188 | 0.063 |
| Porto | 0.93 | 0.211 | 0.140 | 0.093 | 0.031 |
| Sevilla | 0.54 | 0.123 | 0.081 | 0.054 | 0.018 |
| Thiva | 1.88 | 0.427 | 0.282 | 0.188 | 0.063 |
| LM6 | |||||
| PEC GW scenario | PEC GW a |
Infant day 14–27 b |
Infant 5 kg bw c |
Child 10 kg bw c |
Adult 60 kg bw c |
| μg/L | μg/kg bw per day | ||||
| Châteaudun | 3.97 | 0.901 | 0.596 | 0.397 | 0.132 |
| Hamburg | 4.72 | 1.071 | 0.708 | 0.472 | 0.157 |
| Kremsmünster | 2.78 | 0.631 | 0.417 | 0.278 | 0.093 |
| Okehampton | 1.80 | 0.409 | 0.270 | 0.180 | 0.060 |
| Piacenza | 4.54 | 1.031 | 0.681 | 0.454 | 0.151 |
| Porto | 1.67 | 0.379 | 0.251 | 0.167 | 0.056 |
| Sevilla | 3.71 | 0.842 | 0.557 | 0.371 | 0.124 |
| Thiva | 8.00 | 1.816 | 1.200 | 0.800 | 0.267 |
PEARL model 4.4.4 (EFSA, 2017a).
Water consumption of 0.227 L/kg bw per day is based on formula intake of 260 mL/kg bw per day and 1:8 milk powder (33 g/kg bw per day) per end product (weight basis); EFSA Scientific Committee (2017).
WHO (2017) assumed drinking water consumption of 0.75 L (infant), 1 L (child) and 2 L (adult).
Table 4.
Human exposure for FOCUS scenario concentrations of groundwater metabolites LM3 and LM6 in maize following application of 850 g as/ha
| Application to maize at 850 g as/ha | |||||
|---|---|---|---|---|---|
| LM3 | |||||
| PECGW scenario | PECGW a | Infant day 14–27b | Infant 5 kg bwc | Child 10 kg bwc | Adult 60 kg bwc |
| μg/L | μg/kg bw per day | ||||
| Châteaudun | 2.39 | 0.543 | 0.359 | 0.239 | 0.080 |
| Hamburg | 3.86 | 0.876 | 0.579 | 0.386 | 0.129 |
| Kremsmünster | 1.97 | 0.447 | 0.296 | 0.197 | 0.066 |
| Okehampton | 1.86 | 0.422 | 0.279 | 0.186 | 0.062 |
| Piacenza | 1.66 | 0.377 | 0.249 | 0.166 | 0.055 |
| Porto | 1.06 | 0.241 | 0.159 | 0.106 | 0.035 |
| Sevilla | 0.62 | 0.141 | 0.093 | 0.062 | 0.021 |
| Thiva | 2.15 | 0.488 | 0.323 | 0.215 | 0.072 |
| LM6 | |||||
| PEC GW scenario | PEC GW a |
Infant day 14–27 |
Infant 5 kg bw c |
Child 10 kg bw c |
Adult 60 kg bw c |
| μg/L | μg/kg bw per day | ||||
| Châteaudun | 4.49 | 1.019 | 0.674 | 0.449 | 0.150 |
| Hamburg | 5.32 | 1.208 | 0.798 | 0.532 | 0.177 |
| Kremsmünster | 3.13 | 0.711 | 0.470 | 0.313 | 0.104 |
| Okehampton | 2.02 | 0.459 | 0.303 | 0.202 | 0.067 |
| Piacenza | 5.14 | 1.167 | 0.771 | 0.514 | 0.171 |
| Porto | 1.88 | 0.427 | 0.282 | 0.188 | 0.063 |
| Sevilla | 4.21 | 0.956 | 0.632 | 0.421 | 0.140 |
| Thiva | 9.06 | 2.057 | 1.359 | 0.906 | 0.302 |
PEARL model 4.4.4 (EFSA, 2017a).
Water consumption of 0.227 L/kg bw per day is based on formula intake of 260 mL/kg bw per day and 1:8 milk powder (33 g/kg bw per day) per end product (weight basis); EFSA Scientific Committee (2017).
WHO (2017) assumed drinking water consumption of 0.75 L (infant), 1 L (child) and 2 L (adult).
In accordance with EFSA Scientific Committee (2017) and EFSA PPR Panel (2018), an additional uncertainty factor (UF) of 3 is considered necessary for the Cramer class III threshold to account for the non‐availability of substance‐specific data or justified predictions on metabolic elimination route (e.g. glucuronidation, sulfation) of LM3 and LM6 in infants below 16 weeks of age. If the elimination route involved in metabolism of LM3 and LM6 can be demonstrated with certainty and if this route is sufficiently developed at birth, this additional UF of 3 for infants < 16 weeks of age for these two metabolites could be reconsidered in the future.
Comparison of individual, as well as combined, exposure levels for metabolites LM3 and LM6 against the relevant thresholds (0.5 μg/kg bw per day for infants < 16 weeks, 1.5 μg/kg bw per day for all other consumer groups; TTC Cramer class III) is shown in Table 5 for the low (750 g as/ha) and in Table 6 for the high application rate (850 g as/ha).
Table 5.
TTC assessment for FOCUS scenario concentrations of groundwater metabolites LM3 and LM6 in maize following application of 750 g as/ha
| Application to maize at 750 g/ha | ||||
|---|---|---|---|---|
| LM3 | ||||
| PECGW scenario | Infant day 14–27a | Infant 5 kg bwb | Child 10 kg bwb | Adult 60 kg bwb |
| % TTC Cramer class III, UF 3c | % TTC Cramer class IIId | |||
| Châteaudun | 95 | 63 | 14 | 5 |
| Hamburg | 154 | 102 | 23 | 8 |
| Kremsmünster | 79 | 52 | 12 | 4 |
| Okehampton | 74 | 49 | 11 | 4 |
| Piacenza | 85 | 56 | 13 | 4 |
| Porto | 42 | 28 | 6 | 2 |
| Sevilla | 25 | 16 | 4 | 1 |
| Thiva | 85 | 56 | 13 | 4 |
| LM6 | ||||
| PEC GW scenario |
Infant day 14–27 a |
Infant 5 kg bw b |
Child 10 kg bw b |
Adult 60 kg bw b |
| % TTC Cramer class III, UF 3 c | % TTC Cramer class III d | |||
| Châteaudun | 180 | 119 | 26 | 9 |
| Hamburg | 214 | 142 | 31 | 10 |
| Kremsmünster | 126 | 83 | 19 | 6 |
| Okehampton | 82 | 54 | 12 | 4 |
| Piacenza | 206 | 136 | 30 | 10 |
| Porto | 76 | 50 | 11 | 4 |
| Sevilla | 168 | 111 | 25 | 8 |
| Thiva | 363 | 240 | 53 | 18 |
| Sum of LM3 and LM6 contributions to TTC e | ||||
| PEC GW scenario |
Infant day 14–27 a |
Infant 5 kg bw b |
Child 10 kg bw b |
Adult 60 kg bw b |
| % TTC Cramer class III, UF 3 c | % TTC Cramer class III d | |||
| Châteaudun | 276 | 182 | 40 | 13 |
| Hamburg | 369 | 244 | 54 | 18 |
| Kremsmünster | 205 | 136 | 30 | 10 |
| Okehampton | 156 | 103 | 23 | 8 |
| Piacenza | 291 | 193 | 43 | 14 |
| Porto | 118 | 78 | 17 | 6 |
| Sevilla | 193 | 128 | 28 | 9 |
| Thiva | 449 | 296 | 66 | 22 |
Infant, day 14–27 (EFSA Scientific Committee, 2017), 0.227 L/kg bw per day, based on 260 mL/kg bw per day formula intake and 1:8 milk powder (33 g/kg bw per day) per end product (weight basis).
WHO (2017) assumed drinking water consumption of 0.75 L (infant), 1 L (child) and 2 L (adult).
0.5 μg/kg bw per day.
1.5 μg/kg bw per day.
Based on unrounded values for LM3 and LM6 (EFSA Scientific Committee, 2012a).
Table 6.
TTC assessment for FOCUS scenario concentrations of groundwater metabolites LM3 and LM6 in maize following application of 850 g as/ha
| Application to maize at 850 g/ha | ||||
|---|---|---|---|---|
| LM3 | ||||
| PECGW scenario | Infant day 14–27a | Infant 5 kg bwb | Child 10 kg bwb | Adult 60 kg bwb |
| % TTC Cramer class III, UF 3c | % TTC Cramer class IIId | |||
| Châteaudun | 109 | 72 | 16 | 5 |
| Hamburg | 175 | 116 | 26 | 9 |
| Kremsmünster | 89 | 59 | 13 | 4 |
| Okehampton | 84 | 56 | 12 | 4 |
| Piacenza | 75 | 50 | 11 | 4 |
| Porto | 48 | 32 | 7 | 2 |
| Sevilla | 28 | 19 | 4 | 1 |
| Thiva | 98 | 65 | 14 | 5 |
| LM6 | ||||
| PEC GW scenario |
Infant day 14–27 a |
Infant 5 kg bw b |
Child 10 kg bw b |
Adult 60 kg bw b |
| % TTC Cramer class III, UF 3 c | % TTC Cramer class III d | |||
| Châteaudun | 204 | 135 | 30 | 10 |
| Hamburg | 242 | 160 | 35 | 12 |
| Kremsmünster | 142 | 94 | 21 | 7 |
| Okehampton | 92 | 61 | 13 | 4 |
| Piacenza | 233 | 154 | 34 | 11 |
| Porto | 85 | 56 | 13 | 4 |
| Sevilla | 191 | 126 | 28 | 9 |
| Thiva | 411 | 272 | 60 | 20 |
| Sum of LM3 and LM6 contributions to TTC e | ||||
| PEC GW scenario |
Infant day 14–27 a |
Infant 5 kg bw b |
Child 10 kg bw b |
Adult 60 kg bw b |
| % TTC Cramer class III, UF 3 c | % TTC Cramer class III d | |||
| Châteaudun | 312 | 206 | 46 | 15 |
| Hamburg | 417 | 275 | 61 | 20 |
| Kremsmünster | 232 | 153 | 34 | 11 |
| Okehampton | 176 | 116 | 26 | 9 |
| Piacenza | 309 | 204 | 45 | 15 |
| Porto | 133 | 88 | 20 | 7 |
| Sevilla | 219 | 145 | 32 | 11 |
| Thiva | 509 | 336 | 75 | 25 |
Infant, day 14–27 (EFSA Scientific Committee, 2017), 0.227 L/kg bw per day, based on 260 mL/kg bw per day formula intake and 1:8 milk powder (33 g/kg bw per day) per end product (weight basis).
WHO (2017) assumed drinking water consumption of 0.75 L (infant), 1 L (child) and 2 L (adult).
0.5 μg/kg bw per day.
1.5 μg/kg bw per day.
Based on unrounded values for LM3 and LM6 (EFSA Scientific Committee, 2012a).
For LM3, exposure of infants, calculated according to the WHO consumption data (WHO, 2017), is below the TTC in seven out of eight scenarios for both application rates. Considering the peak infant consumption data (EFSA Scientific Committee, 2017), exposures are below the TTC in seven and six out of eight scenarios for the low and high application rate, respectively.
For LM6, exposure of infants, calculated according to the WHO consumption data (2017), is below the TTC in three out of eight scenarios for both application rates. Considering the peak infant consumption data (EFSA Scientific Committee, 2017), exposures are below the TTC in two out of eight scenarios for both application rates.
For infants, combined exposures of LM3 and LM6, calculated according to the WHO consumption data (2017), do not exceed the TTC in one out of eight scenarios for both application rates. Considering peak infant consumption data (EFSA Scientific Committee, 2017), exposures are above the TTC in all scenarios for both application rates.
Conclusion
Considering the WHO default values for drinking water consumption by infants, children and adults (WHO, 2017) and the calculated residue concentrations of metabolites LM3 and LM6 in groundwater abstracted for drinking water purposes, the individual and combined exposures are below the relevant TTC for infants of 0.5 μg/kg bw per day (Cramer class III with additional UF of 3) and for children and adults (Cramer class III) in at least one out of eight FOCUS groundwater scenarios.
Considering the peak water consumption data for infants as published in EFSA Scientific Committee (2017) and the calculated residue concentrations of metabolites LM3 and LM6 in groundwater abstracted for drinking water purposes, an exceedance of the relevant TTC (Cramer class III with additional UF of 3) is indicated for the combined exposure to LM3 and LM6 in all eight scenarios, while individual exposures to LM3 or LM6 are below the threshold in at least two scenarios.
The TTC assessment has shown that metabolites LM3 and LM6 are of potential concern for consumer health, since at least one representative groundwater leaching scenario results in exposure above the relevant threshold. It is therefore recommended to address the specific toxicities of metabolites LM3 and LM6.
5. Uncertainties
Several uncertainties having a potential impact on this assessment were noted across the different steps and molecules.
A number of uncertainties are linked to the experimental conditions applied for the characterisation of the metabolic and toxicological profile of the parent compound and of the metabolites.
Read‐across is applied as a tool to support the general toxicological assessment of metabolites and this is a potential source of uncertainty.
Read‐across to a single molecule carries a higher level of uncertainty than read‐across to analogues with similar toxicological properties.
The quality and reporting of old studies, especially if these predate any OECD Guidelines, is a limiting factor in toxicological assessment.
The use of the ADME and toxicity studies conducted in rodent species is a relevant source of uncertainty because of species and strain differences.
The use of TTC as a screening tool in the toxicological risk assessment of residues is considered a source of uncertainty, particularly because of the uncertainties linked to the exposure estimates.
Use of the additional UF of 3 for Cramer class III (TTC) for infants below 16 weeks of age is attributed to lacking data on infants’ metabolism, which is a source of uncertainty.
The potential toxicity of metabolites LM3 and LM6 is unknown and it cannot be excluded that they could share the toxicological profile of terbuthylazine. This would lead to uncertainty as regards combined human exposure estimates and exceedance of reference values of terbuthylazine.
Data on consumption of drinking water, both default values from WHO (2017) and the most critical consumption as derived from the EFSA Scientific Committee, 2017, is another source of uncertainty as it is not known how well these data reflect the actual consumption in different populations.
Conclusions
While for metabolites LM2, LM4 and LM5, by taking into account all available information, it can be concluded that reference values of the parent substance terbuthylazine (ADI of 0.004 mg/kg bw per day) can be applied, substance‐specific reference values could not be concluded for metabolites LM3 and LM6.
If other sources of exposure to LM3 and LM6 (except exposure by soil degradation of terbuthylazine and subsequent groundwater contamination) could be excluded or quantified with certainty (which is not part of the current mandate), robust exposure estimates could be made for metabolites LM3 and LM6 and compared with the applicable TTC value.
Under the assumption of there being no oral co‐exposure from additional sources and considering the WHO default values for drinking water consumption by infants, children and adults (WHO, 2017), the calculated residue concentrations of metabolites LM3 and LM6 in groundwater abstracted for drinking water purposes result in individual and combined exposures below the relevant TTC for infants of 0.5 μg/kg bw per day (Cramer class III with an additional UF of 3) and for children and adults (Cramer class III) in at least one out of eight scenarios.
Considering the peak water consumption data for infants as published in EFSA Scientific Committee (2017) and the calculated residue concentrations of metabolites LM3 and LM6 in groundwater abstracted for drinking water purposes, an exceedance of the relevant TTC (Cramer class III with additional UF of 3) is indicated for the combined exposure to LM3 and LM6 in all eight scenarios, while individual exposures to LM3 or LM6 are below the threshold in at least two scenarios.
Although particular exposure scenarios were identified without indications for consumer health risks, the TTC assessment has also demonstrated that metabolites LM3 and LM6 may exceed critical exposure levels under certain conditions. It is therefore recommended to address the specific toxicities of metabolites LM3 and LM6.
Several uncertainties having a potential impact on this assessment were noted across the different steps and molecules.
Abbreviations
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism, and excretion
- bw
body weight
- ECHA
European Chemicals Agency
- EPA
Environmental Protection Agency
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- LOAEL
lowest observed adverse effect level
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PEC
predicted environmental concentration
- PECGW
predicted environmental concentration in groundwater
- PPR Panel
EFSA Panel on Protection Products and their Residues
- QSAR
quantitative structure–activity relationship
- RMS
Rapporteur Member State
- SMILES
simplified molecular‐input line‐entry system
- TTC
threshold of toxicological concern
- UF
uncertainty factor
Appendix A – Metabolic map of chlorotriazines in mammals
1.
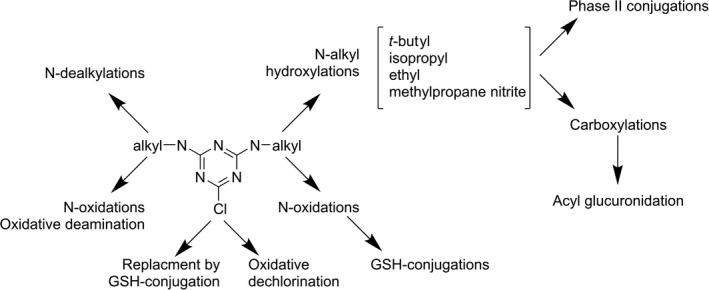
Chloro‐s‐triazines – terbuthylazine, atrazine, simazine, propazine and cyanazine – are structurally very similar herbicides, as they differ only for specific s‐triazine 4‐ and 6‐N‐alkyl substituents. A general metabolic map for chloro‐s‐triazines is shown above. In general, metabolism proceeds via a few common primary pathways – principally: (1) N‐dealkylation of different alkyl groups (ethyl, isopropyl, t‐butyl, methylpropanenitrile); (2) hydroxylation of N‐alkyl groups, and potential subsequent phase II conjugations or carboxylic acid formation; (3) N‐oxidations and oxidative deamination; and (4) dechlorination, which could be either oxidative, replacement of chlorine with a hydroxyl group, or as suggested in mammals, to proceed via non‐enzymatic glutathione trapping (LeBlanc and Sleno, 2011) and subsequent mercapturate formation (Ross et al., 2009; Joo et al., 2010). Also, N‐oxidations and imine formation at the N‐alkyl group and subsequent glutathione conjugation (or trapping) have been detected (LeBlanc and Sleno, 2011), suggesting the production of reactive metabolites. Because parent compounds differ with respect to only one or two alkyl groups, their removals by dealkylation often result in common metabolites for different s‐triazines. ‘Signature metabolites’ are only those that preserve groups typical for a single compound, i.e. t‐butyl group for terbuthylazine and methylpropanenitrile for cyanazine and even these compounds have common more distal metabolites (alkyl carboxylic acids). Metabolites in urine and bile (faeces) are generally the same as in vitro systems, but quantitative differences abound; for example, conjugates with glucuronic acid (UDPGA) or downstream mercapturates derived from glutathione conjugates are proportionally more represented in vivo.
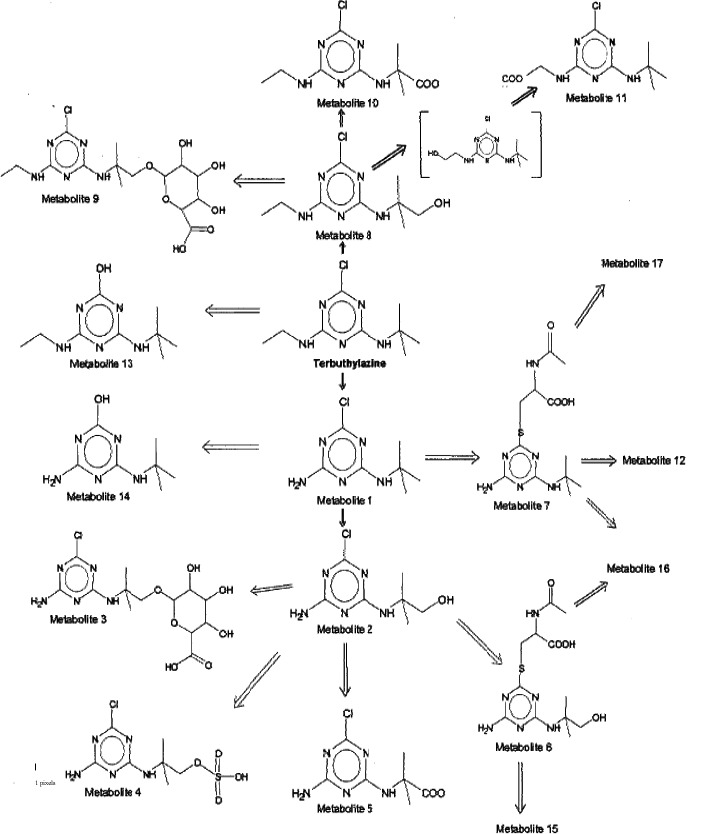
Appendix B – Metabolic pathway of terbuthylazine in rats
Studies owned by Syngenta Crop Protection AG
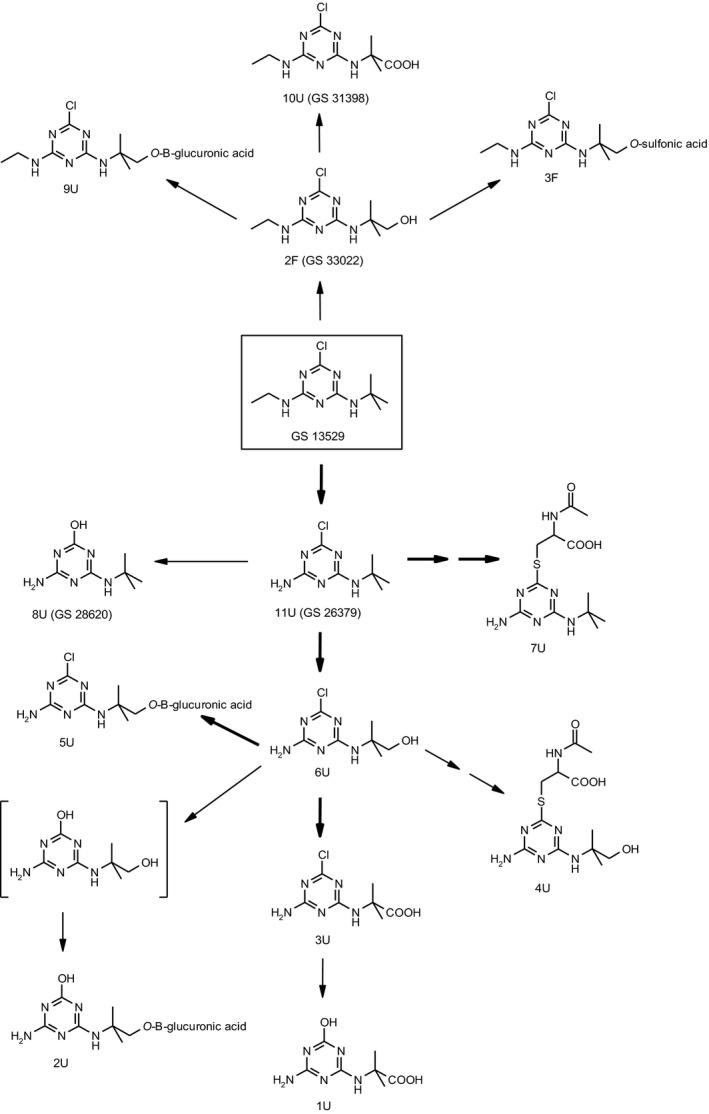
Studies owned by Sipcam Oxon Italia SpA
Appendix C – OECD QSAR Toolbox ver. 4.2 output for LM2, LM4 and LM5
LM2, terbuthylazine, MT1, MT13 and MT14
| Target substance | Analogue 1 | Analogue 2 | Analogue 3 | Analogue 4 | |
|---|---|---|---|---|---|
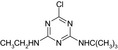
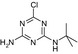
| Name | LM2 | Terbuthylazine | MT1 | MT13 | MT14 |
| CAS No | – | 5915‐41‐3 | 30125‐63‐4 | – | – |
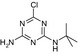
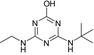
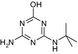
| SMILES | CC(C)(Nc1nc(N)nc(O)n1)C(O)=O | CCNc1nc(Cl)nc(NC(C)(C)C)n1 | CC(C)(C)Nc1nc(N)nc(Cl)n1 | CCNc1nc(O)nc(NC(C)(C)C)n1 | CC(C)(C)Nc1nc(N)nc(O)n1 |
| Structural formula |
|
|
|
|
|
| Molecular formula | C7H11N5O3 | C9H16ClN5 | C7H12ClN5 | C9H17N5O | C7H13N5O |
| Data for physico‐chemical parameters were available only for terbuthylazine, therefore excluded | |||||
| Data for human health parameters were available only for terbuthylazine, therefore excluded (LD50 of > 2,000 mg/kg bw for LM2 from cyanazine evaluation appears not to be included in OECD QSAR Toolbox ver. 4.2 dataset) | |||||
| Structure similarity* to LM2 | 100% | 40% | 64% | 53% | 79% |
Dice coefficient, atom centred fragments used as molecular features.
LM4, terbuthylazine, MT1, MT13 and MT14
| Target substance | Analogue 1 | Analogue 2 | Analogue 3 | Analogue 4 | |
|---|---|---|---|---|---|
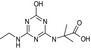
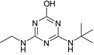
| Name | LM4 | Terbuthylazine | MT1 | MT13 | MT14 |
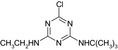
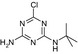
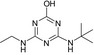
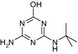
| CAS No | 36576‐44‐0 | 5915‐41‐3 | 30125‐63‐4 | – | – |
| SMILES | CCNc1nc(O)nc(NC(C)(C)C(O)=O)n1 | CCNc1nc(Cl)nc(NC(C)(C)C)n1 | CC(C)(C)Nc1nc(N)nc(Cl)n1 | CCNc1nc(O)nc(NC(C)(C)C)n1 | CC(C)(C)Nc1nc(N)nc(O)n1 |
| Structural formula |
|
|
|
|
|
| Molecular formula | C9H15N5O3 | C9H16ClN5 | C7H12ClN5 | C9H17N5O | C7H13N5O |
| Data for physico‐chemical parameters were available only for terbuthylazine, therefore excluded | |||||
| Human health data | LD50 = 790 mg/kg bw | LD50 = 1,850 mg/kg bw | – | – | – |
| Structure similarity* to LM4 | 100% | 67% | 40% | 81% | 53% |
Dice coefficient, atom centred fragments used as molecular features.
LM5, terbuthylazine, MT1, MT13 and MT14
| Target substance | Analogue 1 | Analogue 2 | Analogue 3 | Analogue 4 | |
|---|---|---|---|---|---|
| Name | LM5 | Terbuthylazine | MT1 | MT13 | MT14 |
| CAS No | – | 5915‐41‐3 | 30125‐63‐4 | – | – |
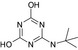
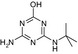
| SMILES | CC(C)(C)Nc1nc(O)nc(O)n1 | CCNc1nc(Cl)nc(NC(C)(C)C)n1 | CC(C)(C)Nc1nc(N)nc(Cl)n1 | CCNc1nc(O)nc(NC(C)(C)C)n1 | CC(C)(C)Nc1nc(N)nc(O)n1 |
| Structural formula |
|
|
|
|
|
| Molecular formula | C7H12N4O2 | C9H16ClN5 | C7H12ClN5 | C9H17N5O | C7H13N5O |
| Data for physico‐chemical parameters were available only for terbuthylazine, therefore excluded | |||||
| Data for human health parameters were available only for terbuthylazine, therefore excluded | |||||
| Structure similarity* to LM5 | 100% | 57% | 69% | 71% | 85% |
Dice coefficient, atom centred fragments used as molecular features.
Suggested citation: EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , Hernandez‐Jerez AF, Adriaanse P, Aldrich AP, Berny P, Duquesne S, Gimsing AL, Millet M, Pelkonen O, Pieper S, Tiktak A, Topping CJ, Tzoulaki I, Widenfalk A, Wolterink G, Kuhl T, Friel A, Istace F, Kardassi D, Lythgo C, Serafimova R and Coja T, 2019. Scientific Opinion on the setting of health‐based reference values for metabolites of the active substance terbuthylazine. EFSA Journal 2019;17(6):5712, 26 pp. 10.2903/j.efsa.2019.5712
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00950
Amendment: The herbicidal activity of the groundwater metabolite MT1 has been corrected in Table 1 since it was erroneously reported as ‘No’. To avoid confusion, the older version of the output has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.
Panel members: Antonio F Hernandez‐Jerez, Paulien Adriaanse, Annette Patrizia Aldrich, Philippe Berny, Sabine Duquesne, Anne Louise Gimsing, Maurice Millet, Olavi Pelkonen, Silvia Pieper, Aaldrik Tiktak, Christopher John Topping, Ioanna Tzoulaki, Anneli Widenfalk, Gerrit Wolterink and Tamara Coja.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Working group members Tamara Coja, Thomas Kuhl, Olavi Pelkonen and Anneli Widenfalk, and staff members Anja Friel, Frederique Istace, Dimitra Kardassi, Christopher Lythgo and Rositsa Serafimova.
Adopted: 4 April 2019
Amended: 30 August 2019
Notes
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 820/2011 of 16 August 2011 approving the active substance terbuthylazine, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011 and Commission Decision 2008/934/EC Text with EEA relevance. OJ L 209, 17.8.2011, p. 18–23.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Commission Regulation (EC) No 33/2008 of 17 January 2008 laying down detailed rules for the application of Council Directive 91/414/EEC as regards a regular and an accelerated procedure for the assessment of active substances which were part of the programme of work referred to in Article 8(2) of that Directive but have not been included into its Annex I (Text with EEA relevance). OJ L 15, 18.1.2008, p. 5–12.
Commission Regulation (EC) No 2076/2002 of 20 November 2002 extending the time period referred to in Article 8(2) of Council Directive 91/414/EEC and concerning the non‐inclusion of certain active substances in Annex I to that Directive and the withdrawal of authorisations for plant protection products containing these substances. OJ L 319, 23.11.2002, p. 3–11.
References
- California Environmental Protection Agency (CEPA) , 1997. Cyanazine (Bladex) Volume 1 Risk Characterization Document. Available online: http://www.cdpr.ca.gov/docs/risk/rcd/cyanazine.pdf
- ECHA (European Chemicals Agency), 2015. Committee for Risk Assessment (RAC) Opinion proposing harmonised classification and labelling at EU level of terbuthylazine. [CLH‐0‐0000001412‐86‐66/F]. Adopted on 5 June 2015.
- EFSA (European Food Safety Authority), 2011. Conclusion on the peer review of the pesticide risk assessment of the active substance terbuthylazine. EFSA Journal 2011;9(1):1969, 133 pp. 10.2903/j.efsa.2011.1969 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for terbuthylazine in light of confirmatory data. EFSA Supporting Publication 2016;13(1):EN‐919, 54 pp. 10.2903/sp.efsa.2016.en-919 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Conclusion on the peer review of the pesticide risk assessment for the active substance terbuthylazine in light of confirmatory data submitted. EFSA Journal 2017;15(6):4868, 20 pp. 10.2903/j.efsa.2017.4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance terbuthylazine. Available online: http://www.efsa.europa.eu
- EFSA Panel on Plant Protection Products and their Residues (PPR) , Ockleford C, Adriaanse P, Hougaard Bennekou S, Berny P, Brock T, Duquesne S, Grilli S, Hernandez‐Jerez AF, Klein M, Kuhl T, Laskowski R, Machera K, Pelkonen O, Pieper S, Smith R, Stemmer M, Sundh I, Teodorovic I, Tiktak A, Topping CJ, Gundert‐Remy U, Kersting M, Waalkens‐Berendsen I, Chiusolo A, Court Marques D, Dujardin B, Kass GEN, Mohimont L, Nougadere A, Reich H and Wolterink G, 2018. Scientific opinion on pesticides in foods for infants and young children. EFSA Journal 2018;16(6):5286, 75 pp. 10.2903/j.efsa.2018.5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2016. Guidance on the establishment of the residue definition for dietary risk assessment. EFSA Journal 2016;14(12):4549, 129 pp. 10.2903/j.efsa.2016.4549 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Scientific Opinion on Exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA Journal 2012;10(7):2750, 103 pp. 10.2903/j.efsa.2012.2750 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2013. Guidance document on the procedures for submission and assessment of confirmatory information following approval of an active substance in accordance with Regulation (EC) No 1107/2009. SANCO 5634/2009‐rev. 6.1
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2009. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 1, 604 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.0, January 2011.
- Joo H, Choi K and Hodgson E, 2010. Human metabolism of atrazine. Pesticide Biochemistry and Physiology, 98, 73–79. [Google Scholar]
- Lassila T, Hokkanen J, Aatsinki A‐A, Mattila S, Turperinen M and Tolonen A, 2015. Toxicity of carboxylic acid‐containing drugs: the role of acyl migration and CoA conjugation investigated. Chemical Research in Toxicology, 28, 2292–2303. [DOI] [PubMed] [Google Scholar]
- Laws SC, Ferrell JM, Stoker TE and Cooper RL, 2003. Pubertal development in female Wistar rats following exposure to propazine and atrazine biotransformation by‐products, diamino‐S‐chlorotriazine and hydroxyatrazine. Toxicological Sciences, 76, 190–200. [DOI] [PubMed] [Google Scholar]
- LeBlanc A and Sleno L, 2011. Atrazine metabolite screening in human microsomes: detection of novel reactive metabolites and glutathione adducts by LC‐MS. Chemical Research in Toxicology, 24, 329–339. 10.1021/tx200008f [DOI] [PubMed] [Google Scholar]
- Macherey A‐C and Dansette PM, 2008. Biotransformations leading to toxic metabolites: chemical aspects In: Wermuth C. (ed.). The Practice of Medicinal Chemistry. 3rd Edition Academic Press, Cambridge, MA, Chapter 33. [Google Scholar]
- Parkinson A, Ogilvie BW, Buckley DR, Kazmi F and Parkinson O, 2018. Biotransformation of Xenobiotics In: Klaassen CD. (ed.). Casarett & Doull's Toxicology. The Basic Science of Poisons. 9th Edition McGraw Hill Education, New York, pp. 193–398. [Google Scholar]
- Ross MK, Jones TL and Filipov NM, 2009. Disposition of the herbicide 2‐chloro‐4‐(ethylamino)‐6‐(isopropylamino)‐s‐triazine (Atrazine) and its major metabolites in mice: a liquid chromatography/mass spectrometry analysis of urine, plasma, and tissue levels. Drug Metabolism and Disposition, 37, 776–786. 10.1124/dmd.108.024927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz TW, Amcoff P, Berggren E, Gautier F, Klaric M, Knight DJ, Mahony C, Schwarz M, White A and Cronin MTD, 2015. A strategy for structuring and reporting a read‐across prediction of toxicity. Regulatory Toxicology and Pharmacology, 72, 586–601. [DOI] [PubMed] [Google Scholar]
- Schultz TW, Richarz A‐N and Cronin MTD, 2019. Assessing uncertainty in read‐across: Questions to evaluate toxicity predictions based on knowledge gained from case studies. Computational Toxicology, 9, 1–11. [Google Scholar]
- Smith DA, Hammond T and Baillie TA, 2018. Safety assessment of acyl glucuronides – a simplified paradigm. Drug Metabolism and Disposition, 46, 908–912. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Guidici DL, Laws SC and Cooper RL, 2002. The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicological Sciences, 67, 198–206. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Hallinger DR, Seely JC and Zorrilla LM, 2013. Evaluation of hydroxyatrazine in the endocrine disruptor screening and testing program's male and female pubertal protocols. Birth Defects Research Part B, Developmental and Reproductive Toxicology, 88, 428–435. [DOI] [PubMed] [Google Scholar]
- United Kingdom , 2007. Draft Assessment Report (DAR) on the active substance terbuthylazine prepared by the rapporteur Member State the United Kingdom in the framework of Directive 91/414/EEC, August 2007. Available online: http://www.efsa.europa.eu.
- United Kingdom , 2010a. Additional Report to the Draft Assessment Report on the active substance terbuthylazine prepared by the rapporteur Member State the United Kingdom in the framework of Commission Regulation (EC) No 33/2008, February 2010. Available online: http://www.efsa.europa.eu
- United Kingdom , 2010b. Final Addendum to the Additional Report on terbuthylazine, compiled by EFSA, October 2010. Available online: http://www.efsa.europa.eu
- United Kingdom , 2015. Addendum to the assessment report on terbuthylazine prepared by the rapporteur Member State the United Kingdom in light of confirmatory data, November 2015. Available online: http://www.efsa.europa.eu
- Van Vleet TR, Liu H, Lee A and Bloome EAG, 2015. Acyl glucuronide metabolites: Implications for drug safety assessment. Toxicology Letters, 272, 1–7. [DOI] [PubMed] [Google Scholar]
- Walker AIT, Brown VKH, Kodama JK, Thorpe E and Wilson AB, 1974. Toxicological studies with the 1,3,5‐triazine herbicide cyanazine. Pesticide Science, 5, 153–159. [Google Scholar]
- WHO (World Health Organization), 2017. WHO Guidelines for drinking‐water quality, 4th edition, incorporating the first addendum. WHO, Geneva: 631 pp. [PubMed] [Google Scholar]


