Abstract
The Scientific Committee confirms that the Threshold of Toxicological Concern (TTC) is a pragmatic screening and prioritisation tool for use in food safety assessment. This Guidance provides clear step‐by‐step instructions for use of the TTC approach. The inclusion and exclusion criteria are defined and the use of the TTC decision tree is explained. The approach can be used when the chemical structure of the substance is known, there are limited chemical‐specific toxicity data and the exposure can be estimated. The TTC approach should not be used for substances for which EU food/feed legislation requires the submission of toxicity data or when sufficient data are available for a risk assessment or if the substance under consideration falls into one of the exclusion categories. For substances that have the potential to be DNA‐reactive mutagens and/or carcinogens based on the weight of evidence, the relevant TTC value is 0.0025 μg/kg body weight (bw) per day. For organophosphates or carbamates, the relevant TTC value is 0.3 μg/kg bw per day. All other substances are grouped according to the Cramer classification. The TTC values for Cramer Classes I, II and III are 30 μg/kg bw per day, 9 μg/kg bw per day and 1.5 μg/kg bw per day, respectively. For substances with exposures below the TTC values, the probability that they would cause adverse health effects is low. If the estimated exposure to a substance is higher than the relevant TTC value, a non‐TTC approach is required to reach a conclusion on potential adverse health effects.
Keywords: Threshold of toxicological concern, risk assessment, Cramer classification scheme
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1661/full
1. Introduction
The threshold of toxicological concern (TTC) approach is a pragmatic, scientifically valid methodology to assess the safety of substances of unknown toxicity found in food (EFSA and WHO, 2016a). It has been developed to screen and prioritise the risk assessment of substances when the chemical structure of the substance is known and where human oral exposure can be estimated to be relatively low. The TTC approach is used when there are limited chemical‐specific toxicity data and can be used for substances with or without structural alerts for genotoxicity and for cancer and non‐cancer endpoints.
The TTC approach should not be used for substances for which the European Union (EU) food/feed legislation requires the submission of toxicity data. Furthermore, when sufficient data are available for a risk assessment, these data should be used and not the TTC approach.
TTC values are numbers that describe generic human chronic exposure thresholds that have been established by grouping experimental toxicity data from animal bioassays. TTC values are derived by applying a probabilistic methodology such that the chance of adverse effects at exposures below these threshold values is considered to be low (Kroes et al., 2004).
This Guidance has been developed to provide practical help in the appropriate use of the TTC approach taking into account updated scientific information, new methodologies and recommendations from the EFSA and WHO report (2016a).
1.1. Background and Terms of Reference as provided by EFSA
The TTC approach is a screening and prioritization tool for the risk assessment of chemicals when hazard data are incomplete and human exposure can be estimated. In 2012, the Scientific Committee published a Scientific Opinion on the TTC approach (EFSA Scientific Committee, 2012b). In 2013, WHO and EFSA initiated a project to provide recommendations to improve the existing TTC approach and update/revise the methodology. A call for data and a review of publicly available information led to development of a background paper by WHO that was discussed at an expert EFSA/WHO workshop in December 2014 (EFSA and WHO, 2016a). The key topics of discussion at that workshop related to the Cramer classification scheme and its underlying concepts, and to the TTC values and decision tree. In the expert workshop, it was concluded that the TTC approach is based on scientific risk assessment principles and is fit for purpose as a screening tool to assess low‐dose chemical exposures and to identify those chemicals for which further data are necessary to assess the human health risk. The expert group made recommendations to improve and expand the TTC concept and update the methodology, considering the latest science and available toxicological databases. Following the workshop, the conclusions and recommendations were published for consultation, and responses to the consultation were addressed by the expert group prior to publication of the final workshop report (EFSA and WHO, 2016b).
The conclusions and recommendations of the expert group related to the following topics:
The Cramer classification scheme
Consideration of metabolism in the TTC values
The TTC domain of applicability
The TTC approach and value for genotoxic substances
TTC values for non‐DNA reactive carcinogens and non‐cancer endpoints
The points of departure and available databases
Chemical categories excluded from the TTC approach
Specific TTC values
Combined exposure to multiple chemicals and from multiple sources
Acute and other less than lifetime exposures
Potentially sensitive life‐stages
A revised TTC decision tree
Terms of Reference
To update the 2012 EFSA Scientific Opinion on exploring options for providing advice on possible human health risks based on the concept of Threshold of Toxicological Concern (TTC) by preparing a guidance document on the use of the TTC approach in food safety. The Guidance should take into consideration particular recommendations from the EFSA/WHO workshop (i.e. Cramer classification scheme, the exclusion of chemical categories and the TTC Decision Tree), as well as the latest scientific developments in the field. The Guidance will be subject to a public consultation prior to adoption by the EFSA Scientific Committee.
1.2. Approach taken to develop this Guidance
In this document, the TTC approach is summarised and updated. The 2012 EFSA Opinion remains available as a comprehensive review of the methodology but guidance on how to apply the TTC approach within EFSA is developed here. The Guidance covers only the application of the TTC approach to human exposure via the oral route; it does not address the applicability of the TTC approach to target animal species or ecotoxicological risk assessments. The recommendation from the EFSA/WHO workshop to combine existing databases is not addressed in this Guidance. EFSA is aware of ongoing efforts to review existing databases elsewhere. However, EFSA is of the opinion that in order to do this, an international agreement on the format and curation of all existing databases is required.
The Guidance also takes into account the literature on the TTC approach that has been published since the EFSA/WHO report (EFSA and WHO, 2016a). The period covered was January 2012 to November 2017 and the searches were performed in Web of Science.1 No search limits for document type or language were used, and the search strings were'threshold’ and ‘toxicological concern’ (topic). The number of hits was 262. Following application of the exclusion criteria (when TTC only appeared as a keyword and was not further used or described in the title or abstract or was mentioned only as a general method for risk assessment with no further description or analysis), the number of papers selected for further evaluation was 70.
1.3. Audience and degree of obligation
This Guidance is aimed specifically at all those contributing to EFSA chemical risk assessments but is broadly applicable for general use of the TTC approach. When using the TTC approach within EFSA, the application of this Guidance is mandatory.
2. The Cramer classification scheme
2.1. Development of the Cramer classification scheme
The application of the TTC concept utilises the classification scheme2 which was originally proposed by Cramer, Ford and Hall (Cramer et al., 1978) as a priority‐setting tool and as a means of making expert judgements in food chemical risk assessment more transparent and reproducible. These authors drew upon their experience in classifying food flavouring substances (Oser and Hall, 1977) and in evaluating pesticides and industrial chemicals. The criteria they proposed for the three structural classes are shown in Table 1.
Table 1.
Structural classes for chemicals proposed in the Cramer scheme (Cramer et al., 1978)
| Class I | Substances with simple chemical structures and for which efficient modes of metabolism exist, suggesting a low order of oral toxicity. This class would include normal constituents of the body (excluding hormones); simply‐branched, acyclic aliphatic hydrocarbons; common carbohydrates; common terpenes; substances that are sulfonate or sulfamate salts, without any free primary amines |
| Class II | Substances which possess structures that are less innocuous than Class I substances, but do not contain structural features suggestive of toxicity like those substances in Class III. This class would include common components of food; substances containing no functional groups other than alcohol, aldehyde, side‐chain ketone, acid, ester, or sodium, potassium or calcium sulfonate or sulfamate, or acyclic acetal or ketal and are either a monocycloalkanone or a bicyclic substance with or without a ring ketone |
| Class III | Substances with chemical structures that permit no strong initial presumption of safety or may even suggest significant toxicity or have reactive functional groups. This class would include structures that contain elements other than carbon, hydrogen, oxygen, nitrogen or divalent sulfur; certain benzene derivatives; certain heterocyclic substances; aliphatic substances containing more than three types of functional groups |
Cramer et al. (1978) based their classification on a series of 33 questions. These were mostly related to chemical structure, but also to natural occurrence in food and in the body. The set of 33 questions were intended to be a compromise between discrimination (into the three classes) and complexity (of the questions and their ordering). The logic of the sequential questions was based on the knowledge on toxicity available at the time and on how chemical structures are metabolised by mammalian metabolic pathways. The Scientific Committee concurs with the EFSA Scientific Committee (2012b) that the application of the Cramer classification scheme in the TTC approach is conservative and therefore protective of human health.
Cramer et al. (1978) predicted that the majority of substances would fall into either Class I or Class III, and that is indeed borne out by the database established by Munro et al. (1996) and by subsequent experience with the TTC approach. Cramer et al. (1978) tested the validity of their classification scheme by classifying 81 chemicals (used as food additives, drugs, industrial chemicals or pesticides), on which toxicity data from short‐term or chronic studies were available, into the three structural classes and by tabulating the NOAELs.3 There was overlap in the range of magnitudes of the NOAELs between the three structural classes, but it was clear that the NOAELs of Class I substances were generally higher than those of Class III, with those of Class II being in between.
2.2. Computer‐based implementation of the Cramer classification
Following a recommendation made in a workshop (Patlewicz et al., 2007), the Joint Research Centre (JRC) commissioned the development of a Toxtree rule base to facilitate the consistent application of the Cramer scheme. Toxtree is freely downloadable from the JRC website4 and from Sourceforge.5
Toxtree (current version v3.1.0, May 2018)6 includes both the original Cramer rule base with the 33 structural rules and an extended rule base with five additional rules which were introduced to overcome misclassification (in Class I or Class II) of several substances with low NOAELs. In both versions of the Cramer rule base, two predefined ‘look‐up’ lists of normal body constituents (around 100 substances) and common food components are used (more than 400 substances).
Cramer rule bases (original and extended) have also been implemented in Organisation for Economic Co‐operation and Development (OECD) QSAR Toolbox7 (current version v4.2, February 2018). The software manual mentions that the current versions of the Cramer rule bases implemented are comparable with those in Toxtree v.2.6.6.
It should be noted that the computer‐based implementation of the Cramer classification scheme in Toxtree and the OECD QSAR Toolbox has inevitably involved some decisions by the programmers, such as the chemically based interpretation of the original rules, and the establishment of predefined ‘look‐up lists’ of normal body constituents and common food components. Therefore, the use of different software tools and also their application by individual experts might lead to different classifications (Bhatia et al., 2015; Roberts et al., 2015), and therefore, the process used should be clearly documented. Both software platforms provide a decision tool for classification and list the rules that lead to the classification of the chemical. This allows for assessment of the classification as part of the weight of evidence.
3. The TTC approach
The original concept of the TTC approach and the databases that support that concept have been reviewed many times and will not be reiterated here (Munro et al., 1996, 2008; Cheeseman et al., 1999; Gold et al., 1999; Barlow et al., 2001; Kroes et al., 2004, 2007; SCCP, 2008; Brown et al., 2010; Boobis et al., 2017). As the validity of the TTC values is critically dependent on the quality of the databases used to derive them, a critical evaluation of the existing databases was performed and is detailed extensively in the EFSA Opinion (EFSA Scientific Committee, 2012b).
A TTC value was calculated from the distribution of NOAELs for each of the three Cramer structural classes, using a database of 613 chemicals with 2,941 NOAELs (Munro et al., 1996). This represented a broad range of chemicals: industrial, food, environmental, agricultural, pharmaceuticals and consumer product chemicals likely to be found commercially and with good supporting toxicological data, yielding 137, 28 and 448 chemicals in Cramer Classes I, II and III, respectively. For each of the 613 chemicals, the most conservative NOAEL was selected, based on the most sensitive species, sex and endpoint. Subchronic NOAELs were divided by a factor of three to extrapolate to a chronic NOAEL. The EFSA Scientific Committee recommended a factor of two for extrapolating from subchronic to chronic study duration in rodents (EFSA Scientific Committee, 2012a), which means that the factor of three used by Munro et al. (1996) is more conservative. The 5th percentile NOAEL (in mg/kg body weight (bw) per day) was calculated for each structural class and this was converted to the intake for a 60‐kg person following the application of an uncertainty factor to calculate the TTC value. A 100‐fold uncertainty factor was used, which is the default factor used for establishing health‐based guidance values for chemicals using toxicity data from animal studies. This procedure resulted in TTC values of 30, 9.0 and 1.5 μg/kg bw per day for Cramer Classes I, II and III, respectively (Table 2).
Table 2.
TTC values – classification of substances
| Classification | TTC value in μg/person per day | TTC value in μg/kg bw per daya |
|---|---|---|
| Potential DNA‐reactive mutagens and/or carcinogens | 0.15 | 0.0025 |
| Organophosphates and carbamates | 18 | 0.3 |
| Cramer Class III | 90 | 1.5 |
| Cramer Class II | 540 | 9.0 |
| Cramer Class I | 1,800 | 30 |
TTC: Threshold of Toxicological Concern; bw: body weight.
Note that there is no conflict with EFSA's recent recommendation to use a default value of 70 kg, when appropriate, for adult body weight (EFSA Scientific Committee, 2012a). In the case of the TTC approach, a body weight value of 60 kg was used by Munro et al. (1996) to derive the generic human exposure threshold values. Therefore, to convert these values back from a per person basis to a body weight basis, 60 kg must also be used.
In 2012, the Scientific Committee recommended that substances that would be classified in Cramer Class II under the Cramer classification should be treated as if they were Cramer Class III substances (EFSA Scientific Committee, 2012b). The rationale was that Cramer Class II was based on very few substances. However, the subsequent EFSA and WHO workshop recommended that Cramer Class II continue to be used and applied to the TTC approach for the time being (EFSA and WHO, 2016a).
Kroes et al. (2004) explored whether particular neurotoxicants should be considered as a separate class. They noted that the 5th percentile NOAEL for organophosphates was lower, by around an order of magnitude, than the corresponding 5th percentile NOAEL for other neurotoxicants. The other neurotoxicants resulted in a plot comparable to the Cramer Class III substances examined by Munro et al. (1996). By applying an uncertainty factor of 100 to the 5th percentile NOAEL for organophosphates, Kroes et al. (2004) derived a human exposure threshold of 0.3 μg/kg bw per day (18 μg/person per day) (EFSA Scientific Committee, 2012b). The Scientific Committee conducted a further analysis of organophosphates and carbamates. It recommended that a TTC value of 0.3 μg/kg bw per day (18 μg/person per day) (Table 2) should be used for both these groups of substances rather than the value of 1.5 μg/kg bw per day used for other substances in structural Class III. The rationale and validity of this value is discussed in detail by the EFSA Scientific Committee (2012b).
For potentially genotoxic substances, Kroes et al. (2004) derived a TTC value of 0.0025 μg/kg bw per day (0.15 μg/person per day) from the Carcinogenic Potency Database (Cheeseman et al., 1999; Gold et al., 1999) (Table 2). The rationale and validity of this value is discussed in detail by the EFSA Scientific Committee (2012b). Recently, Boobis et al. (2017) reviewed the origin of the TTC values for genotoxic or carcinogenic substances and recommended an approach for updating the database on the basis of current knowledge, including mode of action (i.e. DNA reactivity). In this Guidance, EFSA has updated the term ‘genotoxic substances’ to ‘potential DNA‐reactive mutagens and/or carcinogens’, in recognition of the importance of mode of action.
The Scientific Committee agrees with these TTC values. The Scientific Committee notes the recommendations from the EFSA and WHO, 2016a report that a review of the existing non‐cancer databases is needed. In the light of the review by Boobis et al. (2017), this should also include relevant cancer databases. This requires an international agreement on the format and curation of all existing databases. The Scientific Committee is aware of the ongoing CEFIC‐LRI‐sponsored project8 to generate a curated and quality‐controlled database on genotoxic and non‐genotoxic carcinogens.
To facilitate the application of the TTC approach, Kroes et al. (2004) proposed a decision tree which has since been modified. The TTC decision tree presented in this Guidance (Figure 1) is based on the EFSA and WHO (2016a) version.
Figure 1.
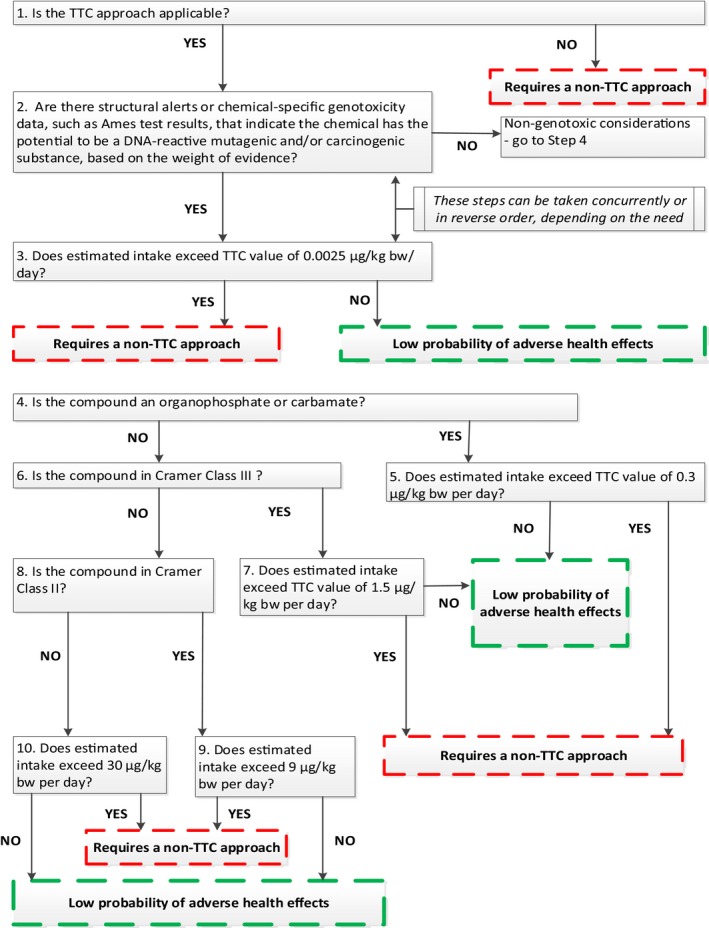
The TTC decision tree (intended for use only in conjunction with the guidance provided in Section 4)
The TTC approach is currently used by several international and European bodies (e.g. Joint FAO/WHO Expert Committee on Food Additives, EFSA, European Chemicals Agency, European Medicines Agency, the non‐food Scientific Committees of the European Commission). Adaptation of the TTC concept has been considered with respect to other routes of human exposure such as inhalation (Drew and Frangos, 2007; Carthew et al., 2009; Escher et al., 2010; Barle et al., 2016; Schuurmann et al., 2016; Tluczkiewicz et al., 2016; Chebekoue and Krishnan, 2017) and dermal exposure (Safford, 2008; Safford et al., 2011; Williams et al., 2016). Similar principles to those underlying the TTC approach have also been considered for use in screening chemicals for effects on environmental species (De Wolf et al., 2005; Belanger et al., 2015).
Within EFSA, examples of the use of the TTC approach include the evaluation of:
Impurities, metabolites and degradation products of food additives (EFSA ANS Panel, 2012)8
Pharmacologically active substances present in food of animal origin (EFSA CONTAM Panel, 2018)
Some metabolites and degradation products of plant protection products in the context of residue definition for risk assessment (EFSA PPR Panel, 2016)
The derivation of ‘maximum acceptable feed concentrations’ for flavouring additives based on default values for feed consumption (EFSA FEEDAP Panel, 2017)
The development of the criteria for the safety evaluation of mechanical processes to produce recycled poly(ethylene terephthalate) (PET) intended to be used for manufacture of materials and articles in contact with food (EFSA CEF Panel, 2011).10
3.1. Consideration of TTC values for less‐than‐lifetime exposure
Exposure to substances in food or feed will generally be of a chronic nature, and the TTC values are calculated based on, or extrapolated to, chronic exposure. However, there may be situations where a short‐term or intermittent exposure period may be considered, such as incidents or the presence of a substance during a time‐limited production period. The TTC approach is applicable in these situations. If exposure exceeds the relevant TTC value, case‐by‐case considerations should be applied.
Some authors have proposed that higher TTC values should be established for short‐term and less‐than‐lifetime exposures in the area of pharmaceutical impurities (European Medicines Agency, 2006; Muller et al., 2006), cosmetics (Kroes et al., 2007) and trace chemicals with structural alerts for genotoxicity (Felter et al., 2009, 2011). Less‐than‐lifetime exposure was also considered at the EFSA/WHO expert workshop, which recommended that such TTC values would require development of a database for acute or other less‐than‐lifetime toxicity (EFSA and WHO, 2016a).
3.2. Developments
Several initiatives have confirmed the original TTC values set by Munro et al. (1996) using additional data sources (e.g. (Pinalli et al., 2011; Tluczkiewicz et al., 2011; Laufersweiler et al., 2012; Leeman et al., 2014; Feigenbaum et al., 2015; Zarn et al., 2015; Yang et al., 2017; Baken et al., 2018). Within the framework of COSMOS,11 a collaborative EU seventh framework project that was conducted over the period 2011–2015, one task force considered approaches to developing TTC values for cosmetic‐related substances (Yang et al., 2017). The TTC values derived in these studies were generally in agreement with those of Munro et al. (1996).
Additional work addressed the derivation of internal TTC values as a more accurate approach that would also allow for a route‐to‐route extrapolation (Partosch et al., 2015). In that approach, NOAEL values for each chemical in the three Cramer classes as described by Munro were multiplied by their own bioavailability. The Scientific Committee is also aware of an ongoing project entitled ‘The Expanded Decision Tree (EDT) Project’ by the US Food and Drug Administration (FDA). More than 18,000 scientific studies were reviewed to determine the influence of species, strain, sex and target organ on toxicity (Tim Adams and Szabina Stice, US FDA, personal communication). These studies provided NOAELs for approximately 2,000 substances that will be organised according to their structure, metabolic fate and toxic potential. Publications on the concept and approach are expected in the near future. In addition, the FDA is in the process of developing EDT software.
3.3. Substances currently not suitable for the TTC approach
As outlined in the Opinion of the Scientific Committee on the TTC approach (EFSA Scientific Committee, 2012b) and reiterated in Section 1, the TTC approach should not be used for substances for which EU food/feed legislation requires the submission of toxicity data. Furthermore, when data are available that allow for a risk assessment, these data should be used and not the TTC approach.
It is necessary to consider whether the substance under consideration belongs to one of the categories of substances for which it is not appropriate to apply the TTC approach. Several categories for exclusion have been identified by Cramer et al. (1978), Kroes et al. (2004), the EFSA Scientific Committee (2012b), and EFSA and WHO (2016a). The TTC approach should be limited to the evaluation of structure(s) that is represented by the chemicals in the database used to derive the respective TTC value. Structures that are outside the chemical space represented by the substances in the database are therefore outside the domain of applicability. Furthermore, some substances with special properties were also excluded. The rationale for these exclusions from the TTC approach can be found in the publications by Cramer et al. (1978), Kroes et al. (2004) and EFSA (EFSA Scientific Committee, 2012b). For the current list of exclusions, see Section 4.1.
However, the Scientific Committee has made modifications to the exclusion list presented in EFSA Scientific Committee (2012b). Hydrazines are no longer excluded from the TTC approach because only 4% of them (2 out of 57 hydrazines) exceed a cancer risk of 1 in 106 at an intake of 0.0025 μg/kg bw (i.e. the TTC value for potential DNA‐reactive mutagens and/or carcinogens).
The 2014 EFSA/WHO workshop recommended excluding organosilicon substances from the TTC approach because they are not represented in the toxicity database of Munro et al. (1996) (EFSA and WHO, 2016a). The Scientific Committee concludes, therefore, that they should also be excluded from the TTC approach.
3.4. Applicability of the TTC approach to chemical mixtures
For mixtures of fully defined chemical composition, a tiered approach is recommended beginning with the assumption of dose addition (EFSA Scientific Committee, 2012a; EFSA and WHO, 2016a), in line with the EFSA Guidance on risk assessment of combined exposure to multiple chemicals (EFSA Scientific Committee, 2019).
In general, the TTC requires knowledge of the structure of the chemical substance(s) under consideration. EFSA and WHO (2016a) recommended that in the case of mixtures that are not fully defined, the application of the TTC approach may be acceptable if sufficient information or analysis is available to confirm that the mixture does not contain substances from the exclusion categories. In this case, the unknown components could be treated as potentially DNA reactive and the TTC value of 0.0025 μg/kg bw would apply to the sum of these (mixture) components. If it were determined that there are no concerns for DNA reactivity and the mixture does not contain organophosphates or carbamates, the mixture may be placed directly in Cramer Class III. Use of the lowest applicable TTC value to the sum of the components in a mixture is a conservative approach if some components are of lower toxicity.
The applicability of the TTC approach as a tool for the evaluation of mixtures depends on the nature and the level of characterisation of the mixture and should, therefore, be considered on a case–by‐case basis.
3.5. Applicability of the TTC values for infants and children
In general, the TTC approach is applicable to the whole population. However, when exposure in infants below the age of 16 weeks is in the region of the relevant TTC, special considerations apply, as outlined in the guidance on the risk assessment of substances present in food intended for infants under 16 weeks of age (EFSA Scientific Committee, 2017). Potential differences between infants or children and adults in dietary exposure and susceptibility to chemicals are also addressed in the Scientific Opinion on pesticides in food for infants and young children (EFSA PPR Panel, 2018). These documents should be followed. Infants and children have a higher food intake per kilogram body weight than adults, and also have other dietary habits and food preferences, and therefore, it is important to take these into consideration when making exposure estimates for the TTC approach. In addition, infants and children are considered to be more sensitive to some toxicological insults than adults (e.g. the metabolic capacity and the renal function is two‐ to threefold lower in infants under the age of 16 weeks than in adults).
3.6. Genotoxicity prediction tools
In applying the TTC approach, it is necessary to assess the potential for DNA‐reactive mutagenicity or carcinogenicity often based on few or no experimental data. Evidence may come from read across from structurally similar chemicals, use of structural alerts or (Q)SAR models. Modelling of genotoxicity is one of the most extensively developed fields in computational toxicology (Serafimova et al., 2010; Worth et al., 2010, 2013; Mombelli et al., 2016; Patlewicz and Fitzpatrick, 2016). This has been facilitated by our understanding of the underlying biological mechanisms, well established experimental protocols, and availability of a large amount of experimental data in the public domain. Some of the software packages implementing these models are freely available (e.g. Toxtree, T.E.S.T, VEGA, LAZAR).
Prediction of DNA reactivity should not be based on the use of a single model alone. In order to optimise sensitivity/specificity when using prediction tools, it is recommended that at least two independent (Q)SAR models are applied which are suitable for the structure under consideration to maximise the sensitivity and specificity of the prediction (EFSA PPR Panel, 2016). The independence of the models is based on different training sets or algorithms (e.g. knowledge‐based and statistically based models) used for developing the models (EFSA PPR Panel, 2016). Each prediction should be evaluated, based on expert judgement, for relevance and reliability following internationally agreed standards (ECHA, 2008, 2016; OECD, 2014). Particular caution has to be taken for substances that are ‘out of domain’ of the model and for which a reliable prediction is not possible. The same applies when the reported confidence score is low.
3.7. Exposure
It is essential for the application of the TTC approach to have fit‐for‐purpose estimates of dietary exposure at the upper end of the distribution. These should be calculated using the methods commonly applied for dietary exposure assessment; for example, high percentile food consumption (e.g. 95th percentile) and average measured chemical concentration values to estimate chronic dietary exposure for high consumers. It is also important to consider exposure in specific population subgroups; for example, infants and children for whom dietary exposure is often higher when expressed on a bodyweight basis. In certain situations, it might be necessary to consider acute exposure (24 h or less), using high percentile concentration values as well as high percentile food consumption. If there are insufficient data to calculate a high percentile, then the maximum reported level could be used in order to be conservative. In the absence of TTC values for acute exposure, the chronic TTC values should be applied, which is conservative for acute exposure.
The estimates of exposure for substances to which the TTC approach is applied should, ideally, take into account not only exposure via the diet but also any systemic exposure resulting from non‐oral routes and sources. However, this is often difficult to achieve in practice due to a lack of data. If this is the case, it adds further uncertainty to the estimates of exposure, which should be described (see also the EFSA Guidance on uncertainty assessment (EFSA Scientific Committee, 2018)).
4. Guidance
The TTC approach is a pragmatic, scientifically valid methodology to assess the safety of substances of unknown toxicity found in food and the environment. From a scientific perspective, the TTC approach could, in principle, be applied to any substances with known structure and that do not belong to the chemical exclusion categories, for which oral exposures can be estimated and toxicity data are sparse. In the EU, there are legislative requirements to submit toxicity data in several areas (e.g. the technically active substances in pesticides, food and feed additives, etc.). Therefore, the TTC approach should not be used for substances for which EU food/feed legislation requires the submission of toxicity data.
For EFSA's work in the area of food and feed, the TTC approach is recommended as a useful screening tool. It can be used either for setting priorities for the data needed to enable a chemical‐specific risk assessment or for deciding whether exposure is so low that adverse health effects are unlikely. In which case, the substance has a low priority for risk assessment.
This Guidance uses the TTC decision tree in Figure 1, which is based on the EFSA and WHO (2016a) version.
Sections 4.1 and 4.2 give guidance on what considerations are needed before applying the TTC decision tree and Section 4.3 describes the application of the TTC decision tree.
4.1. Initial considerations
Before applying the TTC decision tree:
Perform a literature search for toxicity data for the substance under consideration (or a structural analogue) and decide whether there are sufficient data available for a substance‐specific risk assessment (including read‐across considerations). If the substance is a member of a group that has well‐established toxicity data, the TTC approach is not applicable.
Check whether the substance under consideration falls under any EU food/feed legislation which requires submission of toxicity data. If so, the TTC approach is not applicable.
Check whether the substance under consideration falls into one of the current exclusion categories (see Section 3.3). If so, the TTC approach is not applicable. The exclusion categories are:
Substances which are not represented in the database or are outside the domain of applicability:
Inorganic substances
Proteins
Nanomaterials
Radioactive substances
Organosilicon substances
Metals in elemental, ionic or organic form. However, in the case of organic salts, where the counter ion is an essential metal (e.g. sodium), the Scientific Committee recommends that the TTC approach could be applied to the organic ion.
Substances with special properties:
High potency carcinogens: aflatoxin‐like, azoxy‐ or N‐nitroso substances and benzidines
Steroids
Substances with a potential for bioaccumulation (see EFSA Scientific Committee 2012b, Section 4.4.2.4) This includes substances like polyhalogenated‐dibenzodioxins, ‐dibenzofurans and ‐biphenyls.
4.2. Exposure considerations
Estimate chronic exposure using the methods commonly applied for dietary exposure assessments and take the resulting exposure at the upper end of the distribution. It is also important to consider exposure in specific population subgroups; for example, infants and children for whom dietary exposure is often higher when expressed on a bodyweight basis. Where the structure of the substance indicates a potential for acute toxicity, it might be necessary to consider acute exposure (24 h or less), using high percentile concentration values as well as high percentile food consumption. If there are insufficient data to calculate a high percentile, then the maximum reported level could be used in order to be conservative.
Decide what the exposure duration will be. If less than chronic exposure does not exceed the relevant TTC value, there is a low probability of an adverse health effect. If the relevant TTC value is exceeded, expert judgement is necessary to consider whether a non‐TTC approach is required.
4.3. Applying the TTC decision tree
Step 1: Check whether the TTC approach is applicable (see Section 4.1).
If the TTC approach is applicable proceed either to Step 2 or Step 3.
Step 2: Decide whether the substance raises concern for potential DNA‐reactive mutagenicity or carcinogenicity. The decision should not be based on a single piece of evidence. Evidence may come from experimental data, read across from structurally similar chemicals, use of structural alerts or (Q)SAR models. A ‘weight of evidence’ approach should be followed, based on an expert judgement of all available information (see Section 3.6). If the weight of evidence does not indicate that the substance has the potential for DNA‐reactive mutagenicity and/or carcinogenicity, proceed to Step 4. Otherwise proceed to Step 3.
Step 3: If the estimated exposure is below the TTC value for DNA‐reactive mutagenic or carcinogenic substances of 0.0025 μg/kg bw per day, it can be concluded that there is a low probability of adverse health effects.
If Step 2 is considered first and a concern regarding DNA reactivity was identified together with an estimated exposure higher than this TTC value, then a non‐TTC approach (e.g. substance‐specific risk assessment) is required in order to reach a conclusion on potential adverse health effects.12
If Step 3 is considered before Step 2 and the estimated exposure is higher than the TTC value for DNA‐reactive mutagenic or carcinogenic substances, go to Step 2.
Steps 4/5: If the substance is an organophosphate or carbamate (Step 4) and the estimated exposure is below the TTC value of 0.3 μg/kg bw per day (Step 5), it can be concluded that there is a low probability of adverse health effects. If the estimated exposure is higher than this TTC value, a non‐TTC approach (e.g. substance‐specific risk assessment) is required in order to reach a conclusion on potential adverse health effects.
If the substance is not an organophosphate or carbamate, proceed to Step 6.
Steps 6/7: Identify the appropriate Cramer class of the substance (see Sections 2.1 and 2.2). If the substance belongs to Cramer Class III (Step 6) and the estimated exposure is below the TTC value of 1.5 μg/kg bw per day (Step 7), it can be concluded that there is a low probability of adverse health effects. If the estimated exposure is higher than this TTC value, a non‐TTC approach (e.g. substance‐specific risk assessment) is required in order to reach a conclusion on potential adverse health effects.
If the substance does not belong to Cramer Class III, proceed to Step 8.
Steps 8/9: If the substance belongs to Cramer Class II (Step 8) and the estimated exposure is below the TTC value of 9 μg/kg bw per day (Step 9), it can be concluded that there is a low probability of adverse health effects. If the estimated exposure is higher than this TTC value, a non‐TTC approach (e.g. substance‐specific risk assessment) is required in order to reach a conclusion on potential adverse health effects.
If the substance does not belong to Cramer Class II, proceed to Step 10.
Step 10: The substance belongs to Cramer Class I. If the estimated exposure is below the TTC value of 30 μg/kg bw per day, it can be concluded that there is a low probability of adverse health effects. If the estimated exposure is higher than this TTC value, a non‐TTC approach (e.g. substance‐specific risk assessment) is required in order to reach a conclusion on potential adverse health effects.
In general, the TTC approach is applicable to the whole population. However, when exposure in infants below the age of 16 weeks is in the region of the relevant TTC, special considerations apply (EFSA Scientific Committee, 2017) (see Section 3.5).
5. Recommendations
There are generic issues noted in this Guidance, such as the need for improved methods to assess aggregate exposure to chemicals from multiple routes and sources and for improved tools to predict the bioaccumulation of substances that are not specific to the TTC approach. The following are the main TTC‐specific recommendations from the current Guidance, which should be carried out in the order given:
International agreement should be sought on the format and curation of all existing databases, including the inclusion and exclusion criteria to be used.
An overall non‐cancer database should be created by an international collaboration using these criteria.
A review of the existing cancer databases should be carried out through an international collaboration effort.
An assessment of the impact of these curated databases on the TTC values should be carried out through an international collaboration effort.
EFSA should review this Guidance if the TTC values change.
Glossary
- bw
body weight
- DNA‐reactive mutagen
A substance that acts directly on the DNA, thereby causing direct changes to the DNA that lead to a replication error
Abbreviations
- FDA
US Food and Drug Administration
- JRC
Joint Research Centre
- NOAEL
no‐observed‐adverse‐effect level
- OECD
Organisation for Economic Co‐operation and Development
- (Q)SAR
(Quantitative) Structure–Activity Relationship
- TTC
Threshold of Toxicological Concern
- WHO
World Health Organization
Suggested citation: EFSA Scientific Committee , More SJ, Bampidis V, Benford D, Bragard C, Halldorsson TI, Hernández‐Jerez AF, Hougaard BS, Koutsoumanis KP, Machera K, Naegeli H, Nielsen SS, Schlatter JR, Schrenk D, Silano V, Turck D, Younes M, Gundert‐Remy U, Kass GEN, Kleiner J, Rossi AM, Serafimova R, Reilly L and Wallace HM, 2019. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA Journal 2019;17(6):5708, 17 pp. 10.2903/j.efsa.2019.5708
Requestor: European Food Safety Authority
Question number: EFSA‐Q‐2017‐00468
Acknowledgements: The Scientific Committee wishes to thank the following for the support provided to this scientific output: Daniela Maurici (EFSA) and as hearing experts: Tim Adams, Beat J Brüschweiler, Sylvia E Escher and Szabina Stice.
Adopted: 24 April 2019
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1661/full
Notes
To avoid confusion between the Cramer classification scheme for the structural classes (originally referred to as decision tree by Cramer et al. (1978) and the TTC decision tree, the term decision tree is exclusively used in this Guidance to make reference to the TTC decision tree.
The term no‐observed‐adverse‐effect level (NOAEL) is used throughout this Guidance. It is noted that Munro et al. (1996) used the term NOEL with the same meaning.
It should be noted that the software platforms are being updated regularly and that attention should be paid to the version used and Cramer rule bases implemented.
Under the remit of the new Panel on Food Additives and Flavourings (FAF).
Under the remit of the new Panel on Food Contact Materials, Enzymes and Processing Aids (CEP).
A more refined approach for exposure assessment may be considered (EFSA Scientific Committee, 2012b). It is likely that there will be insufficient data for such refinement, and therefore exceedance of the TTC value generally indicates the need for chemical‐specific toxicity data.
References
- Baken KA, Sjerps RMA, Schriks M and van Wezel AP, 2018. Toxicological risk assessment and prioritization of drinking water relevant contaminants of emerging concern. Environment International, 118, 293–303. [DOI] [PubMed] [Google Scholar]
- Barle E, Winkler GC, Glowienke S, Elhajouji A, Nunic J and Martus H‐J, 2016. Setting Occupational Exposure Limits for Genotoxic Substances in the Pharmaceutical Industry. Toxicological Sciences, 151, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SM, Kozianowski G, Wurtzen G and Schlatter J, 2001. Threshold of toxicological concern for chemical substances present in the diet. Report of a workshop, 5–6 October 1999, Paris, France. Food and Chemical Toxicology, 39, 893–905. [DOI] [PubMed] [Google Scholar]
- Belanger SE, Sanderson H, Embry MR, Coady K, DeZwart D, Farr BA, Gutsell S, Halder M, Sternberg R and Wilson P, 2015. It is time to develop ecological thresholds of toxicological concern to assist environmental hazard assessment. Environmental Toxicology and Chemistry, 34, 2864–2869. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Schultz T, Roberts D, Shen J, Kromidas L and Api AM, 2015. Comparison of Cramer classification between Toxtree, the OECD QSAR Toolbox and expert judgment. Regulatory Toxicology and Pharmacology, 71, 52–62. [DOI] [PubMed] [Google Scholar]
- Boobis A, Brown P, Cronin MTD, Edwards J, Galli CL, Goodman J, Jacobs A, Kirkland D, Luijten M, Marsaux C, Martin M, Yang C and Hollnagel HM, 2017. Origin of the TTC values for compounds that are genotoxic and/or carcinogenic and an approach for their re‐evaluation. Critical Reviews in Toxicology, 47, 705–727. [DOI] [PubMed] [Google Scholar]
- Brown R, Carter J, Dewhurst I, Stephenson C and Tessier S, 2010. Applicability of thresholds of toxicological concern in the dietary risk assessment of metabolites, degradation and reaction products of pesticides. EFSA Supporting Publications, 7, 2397–8325. [Google Scholar]
- Carthew P, Clapp C and Gutsell S, 2009. Exposure based waiving: The application of the toxicological threshold of concern (TTC) to inhalation exposure for aerosol ingredients in consumer products. Food and Chemical Toxicology, 47, 1287–1295. [DOI] [PubMed] [Google Scholar]
- Chebekoue SF and Krishnan K, 2017. Derivation of Occupational Thresholds of Toxicological Concern for Systemically Acting Noncarcinogenic Organic Chemicals. Toxicological Sciences, 160, 47–56. [DOI] [PubMed] [Google Scholar]
- Cheeseman M, Machuga E and Bailey A, 1999. A tiered approach to threshold of regulation. Food and Chemical Toxicology, 37, 387–412. [DOI] [PubMed] [Google Scholar]
- Cramer GM, Ford RA and Hall RL, 1978. Estimation of toxic hazard–a decision tree approach. Food and Cosmetics Toxicology, 16, 255–276. [DOI] [PubMed] [Google Scholar]
- De Wolf W, Siebel‐Sauer A, Lecloux A, Koch V, Holt M, Feijtel T, Comber M and Boeije G, 2005. Mode of action and aquatic exposure thresholds of no concern. Environmental Toxicology and Chemistry, 24, 479–485. [DOI] [PubMed] [Google Scholar]
- Drew R and Frangos J, 2007. The Concentration of No Toxicological Concern (CoNTC): A Risk Assessment Screening Tool for Air Toxics. Journal of Toxicology and Environmental Health, Part A, 70, 1584–1593. [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency), 2008. Guidance on information requirements and chemical safety assessment. Chapter R.5: Adaptation of information requirements. May 2008. European Chemicals Agency. Available online: https://guidance.echa.europa.eu/docs/guidance_document/information_requirements_r5_en.pdf?vers=20_08_08
- ECHA (European Chemicals Agency), 2016. Practical guide how to use and report (Q)SARs. ECHA, Helsinki, Finland. Available online: https://echa.europa.eu/documents/10162/13655/pg_report_qsars_en.pdf/407dff11-aa4a-4eef-a1ce-9300f8460099
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials E, Flavourings and Processing Aids), 2010. Guidance on the data required for the risk assessment of flavourings to be used in or on foods. EFSA Journal 2010;8(6):1623, 38 pp. 10.2093/j.efsa.2010.1623 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials E, Flavourings and Processing Aids), 2011. Scientific Opinion on the criteria to be used for safety evaluation of a mechanical recycling process to produce recycled PET intended to be used for manufacture of materials and articles in contact with food. EFSA Journal 2011;9(7):2184, 25 pp. 10.2903/j.efsa.2011.2184 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hogstrand C, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A‐C, Schwerdtle T, Vollmer G, Vleminckx C, Wallace H, Filipič M, Fürst P, O'Keeffe M, Penninks A, Van Leeuwen R, Baert K and Hoogenboom L, 2018. Update: methodological principles and scientific methods to be taken into account when establishing Reference Points for Action (RPAs) for non‐allowed pharmacologically active substances present in food of animal origin. EFSA Journal 2018;16(7):5332, 25 pp. 10.2903/j.efsa.2018.5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2016. Guidance on the establishment of the residue definition for dietary risk assessment. EFSA Journal 2016;14(12):4549, 129 pp. 10.2903/j.efsa.2016.4549 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), Ockleford C, Adriaanse P, Hougaard Bennekou S, Berny P, Brock T, Duquesne S, Grilli S, Hernandez‐Jerez AF, Klein M, Kuhl T, Laskowski R, Machera K, Pelkonen O, Pieper S, Smith R, Stemmer M, Sundh I, Teodorovic I, Tiktak A, Topping CJ, Gundert‐Remy U, Kersting M, Waalkens‐Berendsen I, Chiusolo A, Court Marques D, Dujardin B, Kass GEN, Mohimont L, Nougadere A, Reich H and Wolterink G, 2018. Scientific opinion on pesticides in foods for infants and young children. EFSA Journal 2018;16(6):5286, 75 pp. 10.2903/j.efsa.2018.5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3): 2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Scientific Opinion on Exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA Journal 2012;10(7), 2750, 103 pp. 10.2903/j.efsa.2012.2750 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Bresson J‐L, Dusemund B, Gundert‐Remy U, Kersting M, Lambre C, Penninks A, Tritscher A, Waalkens‐Berendsen I, Woutersen R, Arcella D, Court Marques D, Dorne J‐L, Kass GEN and Mortensen A, 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More SJ, Bampidis V, Benford D, Bennekou SH, Bragard C, Halldorsson TI, Hernandez‐Jerez AF, Koutsoumanis K, Naegeli H, Schlatter JR, Silano V, Nielsen SS, Schrenk D, Turck D, Younes M, Benfenati E, Castle L, Cedergreen N, Hardy A, Laskowski R, Leblanc JC, Kortenkamp A, Ragas A, Posthuma L, Svendsen C, Solecki R, Testai E, Dujardin B, Kass GEN, Manini P, Jeddi MZ, Dorne J‐LCM and Hogstrand C, 2019. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal 2019;17(3):5634, 77 pp. 10.2903/j.efsa.2019.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and WHO (European Food Safety Authority and World Health Organization), 2016a. Review of the Threshold of Toxicological Concern (TTC) approach and development of new TTC decision tree. EFSA Supporting Publications, EN‐1006, 50 pp.
- EFSA and WHO (European Food Safety Authority and World Health Organization), 2016b. Outcome of a public consultation on the conclusions and recommendations of the EFSA–WHO workshop on the Threshold of Toxicological Concern approach. EFSA Supporting Publications, EN‐1000, 71.
- Escher SE, Tluczkiewicz I, Batke M, Bitsch A, Melber C, Kroese ED, Buist HE and Mangelsdorf I, 2010. Evaluation of inhalation TTC values with the database RepDose. Regulatory Toxicology and Pharmacology, 58, 259–274. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (European Medicines Agency), 2006. Guideline on the Limits of Genotoxic Impurities London. Committee for Medicinal Products for Human Use (CPMP).
- Feigenbaum A, Pinalli R, Giannetto M and Barlow S, 2015. Reliability of the TTC approach: Learning from inclusion of pesticide active substances in the supporting database. Food and Chemical Toxicology, 75, 24–38. [DOI] [PubMed] [Google Scholar]
- Felter S, Lane RW, Latulippe ME, Craig Llewellyn G, Olin SS, Scimeca JA and Trautman TD, 2009. Refining the threshold of toxicological concern (TTC) for risk prioritization of trace chemicals in food. Food and Chemical Toxicology, 47, 2236–2245. [DOI] [PubMed] [Google Scholar]
- Felter SP, Conolly RB, Bercu JP, Bolger PM, Boobis AR, Bos PMJ, Carthew P, Doerrer NG, Goodman JI, Harrouk WA, Kirkland DJ, Lau SS, Llewellyn GC, Preston RJ, Schoeny R, Schnatter AR, Tritscher A, van Velsen F and Williams GM, 2011. A proposed framework for assessing risk from less‐than‐lifetime exposures to carcinogens. Critical Reviews in Toxicology, 41, 507–544. [DOI] [PubMed] [Google Scholar]
- Gold LS, Manley NB, Slone TH and Rohrbach L, 1999. Supplement to the Carcinogenic Potency Database (CPDB): results of animal bioassays published in the general literature in 1993 to 1994 and by the National Toxicology Program in 1995 to 1996. Environmental Health Perspectives, 107, 527–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, Schilter B, Schlatter J, van Schothorst F, Vos JG and Wurtzen G, 2004. Structure‐based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food and Chemical Toxicology, 42, 65–83. [DOI] [PubMed] [Google Scholar]
- Kroes R, Renwick AG, Feron V, Galli CL, Gibney M, Greim H, Guy RH, Lhuguenot JC and van de Sandt JJ, 2007. Application of the threshold of toxicological concern (TTC) to the safety evaluation of cosmetic ingredients. Food and Chemical Toxicology, 45, 2533–2562. [DOI] [PubMed] [Google Scholar]
- Laufersweiler MC, Gadagbui B, Baskerville‐Abraham IM, Maier A, Willis A, Scialli AR, Carr GJ, Felter SP, Blackburn K and Daston G, 2012. Correlation of chemical structure with reproductive and developmental toxicity as it relates to the use of the threshold of toxicological concern. Regulatory Toxicology and Pharmacology, 62, 160–182. [DOI] [PubMed] [Google Scholar]
- Leeman WR, Krul L and Houben GF, 2014. Reevaluation of the Munro dataset to derive more specific TTC thresholds. Regulatory Toxicology and Pharmacology, 69, 273–278. [DOI] [PubMed] [Google Scholar]
- Mombelli E, Raitano G and Benfenati E, 2016. In Silico Prediction of Chemically Induced Mutagenicity: How to Use QSAR Models and Interpret Their Results. Methods in Molecular Biology, 1425, 87–105. [DOI] [PubMed] [Google Scholar]
- Muller L, Mauthe RJ, Riley CM, Andino MM, Antonis DD, Beels C, DeGeorge J, De Knaep AG, Ellison D, Fagerland JA, Frank R, Fritschel B, Galloway S, Harpur E, Humfrey CD, Jacks AS, Jagota N, Mackinnon J, Mohan G, Ness DK, O'Donovan MR, Smith MD, Vudathala G and Yotti L, 2006. A rationale for determining, testing, and controlling specific impurities in pharmaceuticals that possess potential for genotoxicity. Regulatory Toxicology and Pharmacology, 44, 198–211. [DOI] [PubMed] [Google Scholar]
- Munro IC, Ford RA, Kennepohl E and Sprenger JG, 1996. Correlation of structural class with no‐observed‐effect levels: a proposal for establishing a threshold of concern. Food and Chemical Toxicology, 34, 829–867. [DOI] [PubMed] [Google Scholar]
- Munro IC, Renwick AG and Danielewska‐Nikiel B, 2008. The Threshold of Toxicological Concern (TTC) in risk assessment. Toxicology Letters, 180, 151–156. [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2014. Guidance Document on the Validation of (Quantitative) Structure Activity Relationship ((Q)SAR) Models. OECD Series on Testing and Assessment No. 69.ENV/JM/MONO(2007)2. OECD Publishing, Paris. Available online: http://www.oecd.org/env/guidance-document-on-the-validation-of-quantitative-structure-activity-relationship-q-sar-models-9789264085442-en.htm
- Oser BL and Hall RL, 1977. Criteria employed by the expert panel of FEMA for the GRAS evaluation of flavouring substances. Food and Cosmetics Toxicology, 15, 457–466. [DOI] [PubMed] [Google Scholar]
- Partosch F, Mielke H, Stahlmann R, Kleuser B, Barlow S and Gundert‐Remy U, 2015. Internal threshold of toxicological concern values: enabling route‐to‐route extrapolation. Archives of Toxicology, 89, 941–948. [DOI] [PubMed] [Google Scholar]
- Patlewicz G and Fitzpatrick JM, 2016. Current and Future Perspectives on the Development, Evaluation, and Application of in Silico Approaches for Predicting Toxicity. Chemical Research in Toxicology, 29, 438–451. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Gallegos Saliner A, Pavan M, Worth A, Benigni R, Aptula A, Bassan A, Bossa C, Falk‐Filipsson A and Gillet V, 2007. Chemical similarity and Threshold of Toxicological Concern (TTC) approaches. Report of an ECB Workshop held in Ispra.
- Pinalli R, Croera C, Theobald A and Feigenbaum A, 2011. Threshold of toxicological concern approach for the risk assessment of substances used for the manufacture of plastic food contact materials. Trends in Food Science & Technology, 22, 523–534. [Google Scholar]
- Roberts DW, Aptula A, Schultz TW, Shen J, Api AM, Bhatia S and Kromidas L, 2015. A practical guidance for Cramer class determination. Regulatory Toxicology and Pharmacology, 73, 971–984. [DOI] [PubMed] [Google Scholar]
- Safford RJ, 2008. The Dermal Sensitisation Threshold‐ a TTC approach for allergic contact dermatitis. Regulatory Toxicology and Pharmacology, 51, 195–200. [DOI] [PubMed] [Google Scholar]
- Safford RJ, Aptula AO and Gilmour N, 2011. Refinement of the Dermal Sensitisation Threshold (DST) approach using a larger dataset and incorporating mechanistic chemistry domains. Regulatory Toxicology and Pharmacology, 60, 218–224. [DOI] [PubMed] [Google Scholar]
- SCCP (Scientific Committee on Consumer Products), 2008. Opinion of the SCCNFP concerning basic criteria for the in vitro assessment of percutaneous absorption of cosmetic ingredients, SCCNFP/0750/03, updated October 2003, 20 October 2003.
- Schuurmann G, Ebert RU, Tluczkiewicz I, Escher SE and Kuhne R, 2016. Inhalation threshold of toxicological concern (TTC) ‐ Structural alerts discriminate high from low repeated‐dose inhalation toxicity. Environment International, 88, 123–132. [DOI] [PubMed] [Google Scholar]
- Serafimova R, Gatnik MF and Worth AP, 2010. Review of QSA R Models and Software Tools for Predicting Genotoxicity and Carcinogenicity. Publications Office of the European Union Luxembourg, 58, pp. [Google Scholar]
- Tluczkiewicz I, Buist HE, Martin MT, Mangelsdorf I and Escher SE, 2011. Improvement of the Cramer classification for oral exposure using the database TTC RepDose–a strategy description. Regulatory Toxicology and Pharmacology, 61, 340–350. [DOI] [PubMed] [Google Scholar]
- Tluczkiewicz I, Kuhne R, Ebert RU, Batke M, Schuurmann G, Mangelsdorf I and Escher SE, 2016. Inhalation TTC values: A new integrative grouping approach considering structural, toxicological and mechanistic features. Regulatory Toxicology and Pharmacology, 78, 8–23. [DOI] [PubMed] [Google Scholar]
- Williams FM, Rothe H, Barrett G, Chiodini A, Whyte J, Cronin MT, Monteiro‐Riviere NA, Plautz J, Roper C, Westerhout J, Yang C and Guy RH, 2016. Assessing the safety of cosmetic chemicals: Consideration of a flux decision tree to predict dermally delivered systemic dose for comparison with oral TTC (Threshold of Toxicological Concern). Regulatory Toxicology and Pharmacology, 76, 174–186. [DOI] [PubMed] [Google Scholar]
- Worth A, Lapenna S, Lo Piparo E, Mostrag‐Szlichtyng A and Serafimova R, 2010. The applicability of software tools for genotoxicity and carcinogenicity prediction: case studies relevant to the assessment of pesticides. JRC scientific and technical reports. EC Joint Research Centre Institute for Health and Consumer Protection, Ispra, 18–19. [Google Scholar]
- Worth A, Lapenna S and Serafimova R, 2013. QSAR and metabolic assessment tools in the assessment of genotoxicity. Methods in Molecular Biology, 930, 125–162. [DOI] [PubMed] [Google Scholar]
- Yang C, Barlow SM, Muldoon Jacobs KL, Vitcheva V, Boobis AR, Felter SP, Arvidson KB, Keller D, Cronin MTD, Enoch S, Worth A and Hollnagel HM, 2017. Thresholds of Toxicological Concern for cosmetics‐related substances: New database, thresholds, and enrichment of chemical space. Food and Chemical Toxicology, 109, 170–193. [DOI] [PubMed] [Google Scholar]
- Zarn JA, Hänggi E and Engeli BE, 2015. Impact of study design and database parameters on NOAEL distributions used for toxicological concern (TTC) values. Regulatory Toxicology and Pharmacology, 72, 491–500. [DOI] [PubMed] [Google Scholar]


