Abstract
The present opinion deals with the assessment of the data provided by interested business operators in support of an amendment of the EU specifications for titanium dioxide (E 171) with respect to the inclusion of additional parameters related to its particle size distribution. Titanium dioxide which is used as a food additive E 171 in food undergoes no surface treatment and is not coated. It consists of anatase or rutile generally containing small amounts of the other phase (rutile or anatase, < 2% m/m) and it may also contain small quantities (< 0.5%) of constituent particle growth and crystal phase control agents (alumina, sodium or potassium in combination with phosphate). Particle size analyses, by TEM, SEM, XDC or DC, have been carried out on five commercial brands of anatase E 171 and one of rutile E 171 manufactured by the only three EU manufacturers that, according to information submitted by interested business operators, produce food‐grade titanium dioxide. Interested business operators proposed to introduce in the EU specifications for E 171 a specification of more than 100 nm for median Feret min diameter and less than 50% of the number of constituent particles below 100 nm; measured by EM in both cases. The Panel, after reviewing the data, concluded that a specification of more than 100 nm for median minimal external dimension, equivalent to less than 50% of the number of constituent particles with a median minimal external dimension below 100 nm, should be inserted in the current EU specifications. The Panel considered that the conclusions made, and the uncertainties identified, in the previous EFSA assessments on E 171 remain valid. The Panel reiterates the need for the further research as recommended in the previous opinions in order to decrease the level of uncertainty and acknowledged that additional studies with characterised E 171 are being carried out by interested business operators.
Keywords: Titanium dioxide, E 171, food additive, particle size, specifications
Summary
The re‐evaluation of titanium dioxide as a food additive (E 171) was completed by the European Food Safety Authority (EFSA) in 2016 (EFSA ANS Panel, 2016). The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS Panel) noted that there were no set limits for the particle size of titanium dioxide (E 171) in its European Union (EU) specifications (Commission Regulation (EU) No 231/20121) and recommended, therefore, at the time, that the characterisation of the particle size in the food additive E 171 should be added to the existing specifications. A similar recommendation for a precise physico‐chemical characterisation of titanium dioxide used as a food additive in order to assess its safety is also reiterated in the ANSES opinion (ANSES, 2019).
The data gaps and uncertainties identified by the ANS Panel required a follow‐up by the European Commission by means of a subsequent call for additional data.
The present opinion deals with the assessment of the data provided by interested business operators in support of an amendment of the EU specifications for titanium dioxide (E 171) with respect to the inclusion of additional parameters related to its particle size distribution.
The Panel noted that according to interested business operators, titanium dioxide used as a food additive E 171 in food undergoes no surface treatment and is not coated. In contrast, titanium dioxide used for non‐food applications (e.g. in medicines or cosmetics) can have different physicochemical properties. The current assessment is based on information submitted only for titanium dioxide (E 171) to be used in food. It consists on anatase or rutile generally containing small amounts of the other phase (rutile or anatase, < 2% m/m) and it may also contain small quantities (< 0.5%) of constituent particle growth and crystal phase control agents (alumina, sodium or potassium in combination with phosphate).
The Panel noted that the parameters related to the number‐based size distribution of titanium dioxide (E 171) proposed by interested business operators for the specifications are based on the characterisation of six E 171 products manufactured by three EU manufacturers that, according to information submitted by interested business operators, are the only ones that produce food‐grade titanium dioxide. No other interested business operators submitted data regarding the particle size distribution following the call for data by the EC.
Data on five different commercial brands of anatase E 171 and one rutile E 171 analysed by transmission electron microscopy (TEM), scanning electron microscopy (SEM), scanning transmission electron microscopy (STEM), X‐ray disc centrifuge (XDC) or disc centrifuge (DC) were submitted by interested business operators.
The average median Feret min diameter of the constituent particles obtained by three laboratories using SEM was reported, for the five brands of anatase, to range between 104 and 166 nm and the percentage of particles by number < 100 nm ranges from 11.4 to 45.6%. For the rutile sample the median Feret min diameter was 151 nm and the percentage of particles by number < 100 nm was 5.4%.
Interested business operators proposed to introduce in the EU specifications for E 171 a specification of more than 100 nm for median Feret min diameter by number and less than 50% for the percentage of constituent particles below 100 nm; in both cases measured by EM.
The Panel noted that the lower limit for the median Feret min diameter proposed by interested business operators is close to the smallest value measured by one laboratory in one brand of E 171 (101 nm) that according to industry is on the EU market.
The specifications of E 171 based on the median value of the number‐based distribution of the minimal external dimension (> 100 nm), and on the percentage of particles with a minimal external dimension lower than 100 nm (< 50%) are equivalent: a median value of the minimal external dimension larger than 100 nm implies that the percentage of particles with a minimal external dimension larger than 100 nm is larger than 50%.
The Panel concluded, after reviewing the available data, that a specification of more than 100 nm, measured by EM and taking into account measurement uncertainty, for median minimal external dimension, equivalent to less than 50% for the percentage of constituent particles by number with a median minimal external dimension below 100 nm, should be inserted in the current EU specifications.
Based on the proposed change in the specifications, revisiting the toxicological database on titanium dioxide (E 171) as a food additive should consequently be conducted in line with the data requirements specified in the EFSA Guidance on nanotechnology (EFSA Scientific Committee, 2018).
The Panel acknowledges that ‘Potassium aluminium silicate, based pearlescent pigments’, defined under the INS number 176 by the Joint FAO/WHO Expert Committee on Food Additives (JECFA), are not defined under the food additive E 171 and, therefore, titanium dioxide used for the preparation of ‘pearlescent pigments’ is not considered in this assessment.
The Panel considered the EFSA ANS Panel opinions (2016, 2018) in the light of the current characterisation of the particle size distribution of titanium dioxide (E 171) and concluded that the conclusions made, and the uncertainties identified, in the previous EFSA assessments of the food additive E 171 remain valid. In particular, the characterisation of titanium dioxide (E 171) does not provide a reason to revise the conclusion on genotoxicity of titanium dioxide (E 171) previously drawn by the ANS Panel. The Panel reiterated the need for the further research as recommended in the previous opinions in order to decrease the level of uncertainty and acknowledged that additional studies with characterised E 171 are being carried out by interested business operators.
1. Introduction
The re‐evaluation of titanium dioxide as a food additive (E 171) was completed by the European Food Safety Authority (EFSA) in 2016 (EFSA ANS Panel, 2016). The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS Panel) noted that there were no set limits for the particle size of titanium dioxide (E 171) in its European Union (EU) specifications (Commission Regulation (EU) No 231/20121) and recommended, therefore, at the time, that the characterisation of the particle size in the food additive E 171 should be added to the existing specifications. A similar recommendation for a precise physico‐chemical characterisation of titanium dioxide used as a food additive in order to assess its safety is also reiterated in the ANSES opinion (ANSES, 2019).
The data gaps and uncertainties identified by the ANS Panel required a follow‐up by the European Commission by means of a subsequent call for additional data.2
The present opinion deals with the assessment of the data provided by interested business operators in support of an amendment of the EU specifications for titanium dioxide (E 171) with respect to the inclusion of additional parameters related to its particle size distribution.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/2008 on food additives.3 Only food additives that are included in the Union list, in particular in Annex II to that Regulation, may be placed on the market and used in foods under the conditions of use specified therein. Moreover, food additives shall comply with the specifications as referred to in Article 14 of that Regulation and laid down in the Commission Regulation (EU) No 231/2012.
Titanium dioxide (E 171) is authorized for use as a food additive in the Union (food colour). Since titanium dioxide (E 171) was permitted in the Union before 20 January 2009, this substance belongs to the group of food additives which are subject to a new risk assessment by the European Food Safety Authority (EFSA) according to Regulation (EU) No 257/20104, and in line with the provisions of Regulation (EC) No 1333/2008.
EFSA completed the re‐evaluation of titanium dioxide (E 171) as a food additive (E 171) and published a scientific opinion on 14 September 2016.5 In that opinion, EFSA noted that there are no set limits for the particle size of titanium dioxide in the EU specifications for E 171. Moreover, EFSA recommended that a full characterization of the particle size in the food additive E 171 should be included among the specifications. That full characterization should include the particle size distribution, together with the determination and quantification of any nanoparticulate material.
Therefore, the European Commission published on 30 January 2017 a call for data addressing the recommendations made by EFSA in the Scientific Opinion on the re‐evaluation of titanium dioxide (E 171) as a food additive. This call for data led to the submission by interested business operators on 29 June 2018 of new data on particle size and particle size distribution for titanium dioxide (E 171), including a proposal for the specifications of E 171 with respect to particle size and particle size distribution. Consequently, the European Commission has decided to consult EFSA on this matter.
1.1.2. Term of Reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/2002,6 the European Commission (EC) asks the European Food Safety Authority (EFSA) to provide a scientific opinion to confirm that the analytical data provided by interested business operators adequately support the proposed amendment of the specifications of the food additive titanium dioxide (E 171), with respect to the inclusion of additional parameters related to its particle size.
2. Data and methodologies
2.1. Data
The present evaluation is based on the data submitted (Documentation provided to EFSA n. 1) and additional information provided during the assessment process by interested business operators in response to a following request by EFSA (Documentation provided to EFSA n. 2, 3 and 5).
2.2. Methodologies
The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidance from the EFSA Scientific Committee.
Terms and definitions in this document are as described by the European Commission's Joint Research Centre (Rauscher et al., 2019).
3. Assessment
3.1. Data submitted
According to interested business operators (Documentation provided to EFSA n. 1 and 2), titanium dioxide which is used as a food additive E 171 in food undergoes no surface treatment and is not coated. It may contain small quantities (< 0.5%) of constituent particle growth and crystal phase control agents (alumina, sodium or potassium in combination with phosphate) that are added prior to the calcination process.
According to interested business operators (Documentation provided to EFSA n. 1 and 2), titanium dioxide (E 171) used for non‐food applications, such as pharmaceuticals and particularly cosmetics and sunscreens, may have significant surface coatings of alumina and silica to reduce photoactivity, and organic surface treatments such as coating with dimethicone or caprylylsilanes to render titanium dioxide hydrophobic. These materials are beyond the scope of this opinion.
Particle size analyses have been carried out on five commercial brands of anatase E 171 (which have no added surface treatment or coating) and reported by interested business operators to EFSA. Interested business operators claimed that these brands are representative for the food additive E 171, used in food applications, produced by the only three EU manufacturers that, according to information submitted by interested business operators, are the only ones that produce food‐grade titanium dioxide. Titanium dioxide anatase generally contains small amounts of rutile phase crystals (as separate monophasic crystals) typically < 2% m/m (the limit of variation that has been found due to production variability). The content of rutile in the five analysed samples ranged from 0.1 to 0.9% m/m (Documentation provided to EFSA n. 1 and 2).
Similarly, titanium dioxide rutile generally contains small amounts of anatase phase crystals (as separate monophasic crystals) typically < 2% m/m (the limit of variation that has been found due to production variability). An additional particle size analysis has been carried out on a sample of rutile E 171 (not coated) containing 0.4% m/m of anatase (Documentation provided to EFSA n. 3 and 5).
The data submitted ‘do not consider composite particles such as titanium dioxide coated mica which may claim to be mixtures of E 171 with other components’. These materials are beyond the scope of this opinion.
Preliminary screening and unpublished data from the European Commission's Joint Research Centre (EC‐JRC) were included in the documentation submitted by interested business operators (Documentation provided to EFSA n. 2.)
The data submitted by interested business operators were generated on five different commercial brands of anatase E 171 and one rutile E 171 by different analytical methodologies, i.e. transmission electron microscopy (TEM), scanning electron microscopy (SEM), scanning transmission electron microscopy (STEM), X‐ray disc centrifuge (XDC) or disc centrifuge (DC).
3.1.1. Electron microscopy
Electron microscopy analyses are suitable for deriving number‐based measurements of (constituent) particle properties.
All five anatase samples were analysed by five different laboratories, by SEM, TEM or STEM.
Similar results were obtained for the analysis of the five samples by SEM by three of the laboratories despite the sample preparation techniques and protocols for assessment of the images were different (Appendix A).
On the other hand, divergent results were obtained by the other two laboratories that analysed the same samples of titanium dioxide anatase by SEM, TEM or STEM. Methodological problems (point counting, astigmatism) resulting in strongly biased results from these laboratories were reported. These data were not considered further by interested business operators when proposing the specifications for the food additive.
The rutile sample was analysed by a laboratory using SEM. The results were compared with data for a similar sample submitted to EFSA during the re‐evaluation of titanium dioxide (E 171) (EFSA ANS Panel, 2016); results of the mean diameter were in the same range for that sample.
Anatase samples
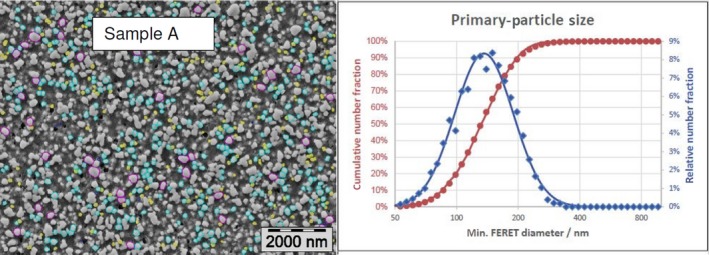
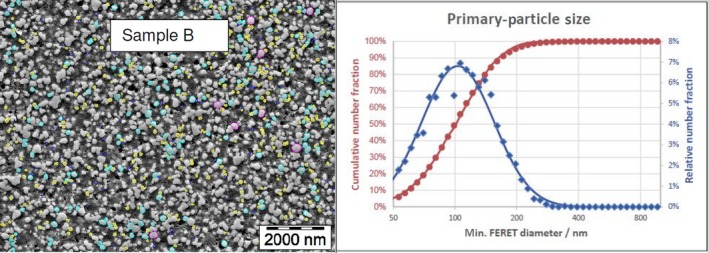
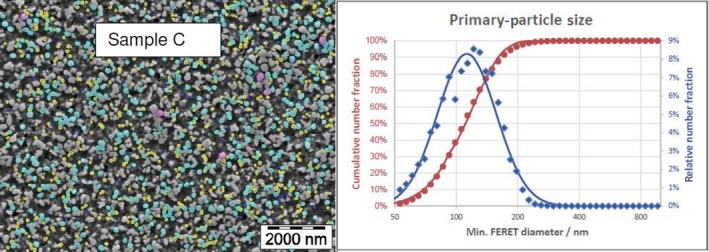
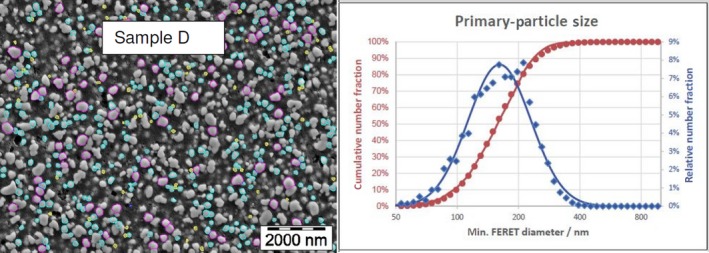
The average median Feret min diameter of the constituent particles (x50 Feret min) obtained by the three laboratories using SEM was reported to range between 104 and 166 nm and the percentage of the number of particles < 100 nm ranges from 11.4 to 45.6%. The 95% confidence interval (CI) of the average of the three laboratories was on average 3 nm for the median size and 1.8% for the percentage of nanoparticles by number across the five samples. The results are presented in Table 1.
Table 1.
SEM results of the analysis of samples A‐E
| Sample | Laboratory | x50 Feret min (nm) | % nanoparticles by number < 100 nm | Particles counted | Median size CI (0.05)a , b (nm) | % nanoparticles CI (0.05)a , b |
|---|---|---|---|---|---|---|
| A | Lab 1 | 137 | 18.1 | 4,396 | ||
| Lab 2 | 140 | 18 | 703 | |||
| Lab 3 | 138 | 19 | 317 | |||
| Average | 138 | 18.4 | 1 | 0.5 | ||
| B | Lab 1 | 105 | 46.1 | 4,868 | ||
| Lab 2 | 101 | 49.6 | 1,214 | |||
| Lab 3 | 108 | 41 | 305 | |||
| Average | 105 | 45.6 | 3 | 4 | ||
| C | Lab 1 | 115 | 35.7 | 18,155 | ||
| Lab 2 | 110 | 37 | 616 | |||
| Lab 3 | 113 | 36 | 306 | |||
| Average | 113 | 36.2 | 2 | 0.6 | ||
| D | Lab 1 | 163 | 10.3 | 4,617 | ||
| Lab 2 | 173 | 10 | 847 | |||
| Lab 3 | 162 | 14 | 315 | |||
| Average | 166 | 11.4 | 5 | 2.1 | ||
| E | Lab 1 | 106 | 44.1 | 8,583 | ||
| Lab 2 | 102 | 47 | 766 | |||
| Lab 3 | 105 | 44 | 384 | |||
| Average | 104 | 45.0 | 2 | 1.6 |
x50 Feret min: median Feret min diameter of the constituent particles; CI: confidence interval.
Z × SD/(N^0.5).
CI calculated for 95% confidence (Z = 1.96) for N = 3 laboratories.
The same samples were the subject of a preliminary screening test by the EC‐JRC using TEM. The JRC results show a higher median size (105–187 nm) and a lower percentage of nanoparticles (5–42%), being four times lower for one sample.
Where several batches were analysed, the range of number median size (x50 Feret min) Feret min diameter was reported to be around ±10% of the mean, and the range of percentage of particles < 100 nm was also reported to be around ±10%. The difference between particle size of the different brands, some produced by the same manufacturer, are much larger than the production variability, batch‐to‐batch, of a single brand.
Rutile sample
The median Feret min diameter was 151 nm and the percentage of the number of particles < 100 nm was 5.4%. The same sample was the subject of a preliminary screening test by the JRC using TEM. The median size was slightly higher (165 nm) and the percentage of the number of particles < 100 nm was equal to 4%.
3.1.2. Direct volume or mass‐based methods – disc centrifuge
Anatase samples
The five E 171 samples were analysed by XDC by one laboratory and by DC by two laboratories to provide data on mass or volume‐based size and percentage of nanoparticles.
According to the interested business operators (Documentation provided to EFSA n. 1 and 2), the results from such direct volume or mass‐based methods are heavily dependent upon the method and energy of dispersion; there is no ISO Standard or regulation in place which covers the determination of the particle size of titanium dioxide as produced or applied in food or any other application. A dispersion energy of 240 J/mL was applied for the analysis.
The results of the five samples showed a range of median volume particle size between 260 and 380 nm) and the percentage of particles < 100 nm by volume ranges from 0.0% to 1.52%. The CIs between the three types of measurement for the five samples was on average only 6 nm for the median size and 0.2% to the percentage of nanoparticles, respectively.
An additional analysis by XDC using the NIST 1200‐3 protocol (50 W for 15 min, corresponding to 250 kWh/m3) (equivalent7 to 900 J/mL)) developed to find an optimum dispersion of P258 (Taurozzi et al., 2012) was also carried out. The median volume particle size ranged between 241 and 308 nm) and the percentage of particles < 100 nm by volume ranges from 0% to 2% The results are not greatly different to those measured at 67 kWh/m3 (equivalent8 to 240 J/mL) as reported above (260–380 nm), for the median volume particle size and 0% to 1% for the percentage of particles < 100 nm by volume. The results are presented in Table 2.
Table 2.
XDC/DC results of the analysis of samples A–E
| Sample | Laboratory | Instrument | x50 volume (nm) | % nanoparticles by volume < 100 nm | PSD volume |
|---|---|---|---|---|---|
| A | Lab 1 | DC | 270 | 0.86 | 1.47 |
| Lab 2 | DC | 280 | 1.12 | 1.54 | |
| Lab 2 | XDC | 280 | 0.47 | 1.39 | |
| B | Lab 1 | DC | 260 | 1.32 | 1.45 |
| Lab 2 | DC | 270 | 1.20 | 1.50 | |
| Lab 2 | XDC | 260 | 0.77 | 1.38 | |
| C | Lab 1 | DC | 270 | 1.07 | 1.42 |
| Lab 2 | DC | 270 | 0.97 | 1.44 | |
| Lab 2 | XDC | 260 | 0.33 | 1.35 | |
| D | Lab 1 | DC | 350 | 0.24 | 1.42 |
| Lab 2 | DC | 380 | 0.19 | 1.41 | |
| Lab 2 | XDC | 360 | 0.00 | 1.36 | |
| E | Lab 1 | DC | 290 | 1.52 | 1.59 |
| Lab 2 | DC | 300 | 1.52 | 1.65 | |
| Lab 2 | XDC | 300 | 1.00 | 1.53 |
XDC: X‐ray disc centrifuge; DC: disc centrifuge; x50 volume: median volume particle size; PSD: population standard deviation.
Rutile sample
The rutile sample was analysed only by DC resulting in a median volume particle size of 310 nm and a percentage of particles < 100 nm by volume of 0.5%.
3.1.3. Surface area
Specific surface area (SSA) was calculated using a method based upon ISO 9227. The values were considered for the estimation of the volume diameter (volume diameter = 6/(SSA × skeletal density)) (Documentation provided to EFSA n. 2), assuming that the constituent particles are spherical.
Anatase samples
The calculated volume diameters ranged from 150 to 260 nm, being smaller than the range of volume diameters measured by DC but larger than the number‐based diameters measured by EM.
Rutile sample
The calculated volume diameter was 160 nm.
3.1.4. Overall results
The distributions of the particle sizes for the different brands were available for the assessment (Appendix A).
The summary of the data submitted is presented in Table 3.
Table 3.
Summary results for anatase E 171
| XDC | DC | SEM | TEM | ||
|---|---|---|---|---|---|
| 40W, 5 min | NIST 1200‐3 50W, 15 min | 40W, 5 min | Averagea | JRCb | |
| Min x50 Feret min (nm) | 104 | 105 | |||
| Max x50 Feret min (nm) | 166 | 187 | |||
| Min x50 volume (nm) | 260 | 228 | 260 | ||
| Max x50 volume (nm) | 360 | 292 | 380 | ||
| Min % nanoparticles by number < 100 nm | 11.4 | 5 | |||
| Max % nanoparticles by number < 100 nm | 45.6 | 42 | |||
| Min % nanoparticles by volume < 100 nm | 0 | 0 | 0.19 | ||
| Max % nanoparticles by volume < 100 nm | 1 | 2 | 1.52 | ||
XDC: X‐ray disc centrifuge; DC: disc centrifuge; SEM: scanning electron microscopy; TEM: transmission electron microscopy; x50 Feret min: average median Feret min diameter of the constituent particles; x50 volume: median volume particle size.
Average calculated from the results of three laboratories.
Preliminary screening test by the EC‐JRC.
The results reported for the rutile sample fall in the range of the anatase samples. The percentage of particles < 100 nm by number in this sample is lower than in the anatase samples.
3.1.5. Specifications proposed by interested business operators
To derive a potential specification for titanium dioxide (E 171), the smallest median Feret min diameter and highest percentage of particles < 100 nm measured for the five samples of E 171 have been used as the basis. A range of +/−2.576 production standard deviations (i.e. 99% population) has been added to cover the normal production variation. According to interested business operators, when several batches were assessed by EM, the range of number median size was around +/−10% of the mean, the range of percentage of particles < 100 nm is also around +/−10%.
Interested business operators propose that the specification is only one‐sided, i.e. it only specifies a lower median size limit and an upper percentage limit of particles < 100 nm and that the upper size limit and lower %nanoparticles limit are only noted as typical values (Table 4). Interested business operators also indicated that for titanium dioxide (anatase) E 171 used in food, the typical range for the median diameter would be 100–250 nm, the percentage of particles < 100 nm between 5% and 50% by number containing less than 2% m/m of rutile.
Table 4.
Proposed specifications from interested business operators for number‐based size distribution measured by EM
| Constituent particle size for titanium dioxide (E 171) | Measured by electron microscopy (Feret min) |
|---|---|
| Median diameter by number (nm) | > 100 nm |
| Percentage of constituent particles below 100 nm by number (%) | < 50% |
EM: electron microscopy.
According to interested business operators, it is possible to include rutile E 171 into the proposed specification for anatase E 171 for particle size without any alterations.
3.2. Evaluation of the submitted data
The Panel noted that according to interested business operators (Documentation provided to EFSA n. 1 and 2), titanium dioxide used as a food additive E 171 undergoes no surface treatment and is not coated. In contrast, titanium dioxide used for non‐food applications (e.g. in medicines or cosmetics) can have different physicochemical properties. The current assessment is based on information submitted only for titanium dioxide (E 171) to be used in food.
The Panel noted that the parameters related to the number‐based size distribution of titanium dioxide (E 171) proposed by interested business operators for the specifications are based on the characterisation of six E 171 products manufactured by three EU manufacturers that, according to information submitted by interested business operators, are the only ones that produce food‐grade titanium dioxide. No other interested business operators submitted data regarding the particle size distribution following the call for data by the European Commission.9
Data on five different commercial brands of anatase E 171 and one rutile E 171 analysed by TEM, SEM, XDC or DC were submitted by interested business operators (see Section 3.1).
XDC and DC measurements provided information on the size, estimated as hydrodynamic diameter, of the particles of E 171, measuring a mixture of aggregates, agglomerates and isolated constituent particles, and on the percentage of particles, and on the particles below 100 nm, by mass or volume.
Mass and volume‐based methods, like XDC and DC, cannot distinguish between aggregates, agglomerates and constituent particles. The measured size is determined by the number of constituent particles per aggregate/agglomerate and by the secondary structure of the mix of aggregates and agglomerates. These can be complex and dynamic depending strongly on the dispersion conditions (pH, concentrations, salinity, etc.). Particularly the size of agglomerates can change. Standardisation of the conditions for E 171 particle size determination is problematic for the sample preparation and analysis for pristine E 171 and for E 171 present in food, and the analytical methods cannot differentiate E 171 particles from other particles. Therefore, the Panel recommends not to base specifications of E 171 on the (size) measurement of agglomerates/aggregates by applying mass and volume‐based methods.
Measurement by EM provides information on the minimal external dimension of the constituent particles of E 171, assessed as the Feret min diameter, and on the percentage, by number, of the constituent particles with a minimal external dimension below 100 nm (nanoparticles).
The minimal external dimension of the constituent particles of E 171 is a stable characteristic that does not change when E 171 is applied in a product. Therefore, the Panel recommends that the EU specification of E 171 should be based on the measurement of the minimal external dimension of the constituent particles of E 171 (as defined by Commission Recommendation 2011/696/EU10). EM analysis is the golden standard to measure the minimal external dimension of E 171 particles and can be applied to measure particles both in pristine E 171 and in E 171 extracted from food products (JRC, 2019 (Documentation provided to EFSA n. 7)).
EM measurement of the Feret min diameter directly assesses the minimal external of (constituent) particles. The Feret min diameter is often reported because this size parameter is simple to visualise and understand, and it enables the material to be assessed as to whether it is a nanomaterial according to the Commission Recommendation 2011/696/EU12. The measured Feret min diameter depends, however, on the relative orientation of the particle and the virtual calliper. The maximum inscribed circle diameter is an alternative size parameter estimating the minimal external dimension: it is the diameter of the largest circle that fits inside the virtual envelope of the boundaries of the particle on a 2D image (Rauscher et al., 2019). The optimal choice of the measurand depends on the shape of the measured particles. Rauscher et al. (2019) indicate which measurand should be used to assess the external dimensions of irregularly shaped particles depending on the particle shape.
The proposed values (Table 4) are based on the results of the measurement, by three laboratories, of six different marketed titanium dioxide (E 171) brands as a food additive. The ‘production variability’ was estimated to be in the order of ±10% and the measurement uncertainty, in the order of 2%, was estimated based on the variation between the measurements of three laboratories under repeatability conditions.
Measurement uncertainty should contain components such as: the calibration uncertainty, the intermediate precision uncertainty, the trueness uncertainty, and possible bias related to the sample preparation (Crouzier et al., 2019). The calibration uncertainty, the intermediate precision uncertainty and the trueness uncertainty were not taken in account by interested business operators. Typically, the expanded, combined measurement uncertainty ranges in the order of 10% for highly experienced analysts/staff that routinely perform these analyses, and higher for less‐experienced analysts/staff (Verleysen et al., 2019, (Documentation provided to EFSA n. 7)). The high variation observed from the analysis of two out of the five laboratories reported in the documentation provided by interested business operators underpins this, as are the results of interlaboratory comparisons and validation studies done during European Commission research projects such as NANoREG and NanoDefine (De Temmerman et al., 2014a,b; Verleysen et al., 2015, 2019 (Documentation provided to EFSA n. 7)).
The Panel noted that the approach to estimate the uncertainty of the proposed results underestimates the uncertainty which should, at least, contain the ‘within brand variation’ (‘production variability’) and a realistic estimation of the measurement uncertainty. The former is not taken in account when proposing the parameters for the specifications, and the measurement uncertainty is unrealistically low (in the order of 2%). Several components of the measurement uncertainty budget including the calibration uncertainty, the intermediate precision uncertainty, the trueness uncertainty, and possible bias related to the sample preparation are not considered in the proposed measurement uncertainty.
Methodological issues associated with the sample preparation applied by the three laboratories, from which the interested business operators derived the specifications, were observed. Due to the high degree of agglomeration of the particles, the particle size tends to be overestimated when using EM:
Identification of individual particles that are closely together is more difficult, particularly if a small particle is attached to a large particle
Multiple layers of particles result in a bias: there is a higher chance that large particles are measured than small particles, because larger particles block the smaller ones.
The Panel agreed that due to the methodological problems also identified by interested business operators in the protocols of the other two laboratories, their results should not be considered to establish parameters for titanium dioxide (E 171) specifications and they have not been reported in Section 3.1.
The Panel was informed that the EC‐JRC received six samples of E 171 from the interested business operators and carried out a preliminary screening analysis aiming at gathering information on the characteristics of food‐grade titanium dioxide and potentially include them in the JRC Nanomaterials repository (Documentation provided to EFSA n. 6). Hence, a statistical evaluation was not performed due to the limited analysis replicates. The EC‐JRC shared the results with the interested business operators indicating the limitation of the study in order to draw any conclusion based on the information given. The Panel noted that the charge of the TEM grids applied by the EC‐JRC in the preliminary screening analysis was not compatible with the charge of the particles which can result in biased results.
In view of the fact that the results of the analysis depend on the sample preparation protocol and in the absence of a validated protocol, information from the ‘Guidance on detection and identification of nano‐objects in complex matrices’ (CEN, 2018) can be used for the development of an approach for sample preparation and for measurement, to control the specification.
The Panel noted that the lower limit for the median diameter proposed by interested business operators is close to the smallest value measured by one laboratory in one brand of E 171 (101 nm) that according to industry is on the EU market.
The specifications of E 171 based on the median value of the number‐based distribution of the minimal external dimension of constituent particles (> 100 nm), and on the percentage of constituent particles with a minimal external dimension lower than 100 nm (< 50%) are equivalent: a median value of the minimal external dimension larger than 100 nm implies that the percentage of particles with a minimal external dimension larger than 100 nm is larger than 50%.
Referring to the median size value has the advantage that for method validation and evaluation, one can refer to reference materials (Linsinger, 2011; Stefaniak et al., 2013; Braun et al., 2011; Franks et al., 2012; Kestens and Roebben, 2014) with certified size values and uncertainties, allowing assessment of the trueness, the trueness uncertainty, and the calibration uncertainty of the size measurements.
Overall, the Panel agrees with the proposed median diameter (by number) of more than 100 nm to describe titanium dioxide used as a food additive E 171, based on the data submitted to EFSA (Documentation provided to EFSA 1, 2 and 3). This is in agreement with the published results of samples of E 171 analysed by TEM (Dudefoi et al., 2017). However, the Panel noted a sample of E 171 of unknown origin out of the five analysed samples by the EC‐JRC (2019 (Documentation provided to EFSA n. 7)) does not meet the proposed specifications for E 171.
The Panel was informed that the ongoing extended one‐generation reproductive toxicity (EOGRT) study, requested in the European Commission call for additional data,2 is being carried out with the brand of titanium dioxide (E 171) with the smallest median size and highest percentage of nanoparticle described in the documentation submitted to EFSA (Documentation provided to EFSA n. 5).
3.3. Titanium dioxide from other titanium dioxide‐containing food colours
The Panel noted that the Joint FAO/WHO Expert Committee on Food Additives (JECFA) established specifications for ‘potassium aluminium silicate, based pearlescent pigments’ under the INS number 176 and are described as potassium aluminium silicate (mica) coated with titanium dioxide or iron oxides or both of them (JECFA, 2013). The definition of ‘potassium aluminium silicate, based pearlescent pigments’ is not included in the definition of none of the authorised food additives in the EU (E 171, E 555 or E 172) according to Commission Regulation (EU) No 231/2012.
Analysis of samples named as ‘additive mixture of E 171 with E 555’ has shown that the material consists on mica platelets coated with titanium dioxide particles with a median particle size in the nano range (20–30 nm) (JRC, 2019 (Documentation provided to EFSA n. 7); Sciensano 2019 (Documentation provided to EFSA n. 8)).
The Panel noted that ‘potassium aluminium silicate, based pearlescent pigments’ (INS 176) as described by JECFA specifications (JECFA, 2013) is not a mixture of the food additives titanium dioxide (E 171) and potassium aluminium silicate (E 555).
According to interested business operators (Documentation provided to EFSA n. 4), titanium dioxide used in the marketed ‘pearlescent pigments’ is not produced by any of three EU manufacturers and titanium dioxide used for the synthesis of ‘pearlescent pigments’ is produced by a different manufacturing process. ‘Potassium aluminium silicate, based pearlescent pigments’ are not defined under E 171 and, therefore, titanium dioxide used for the preparation of ‘pearlescent pigments’ is not considered in this assessment.
4. Impact of the characterisation of the particle size distribution of titanium dioxide (E 171) on the previous EFSA's assessments on the food additive
In 2016, the EFSA ANS Panel re‐evaluated titanium dioxide (E 171) and concluded the following:
‘From the available data on absorption, distribution and excretion, the Panel concluded that:
the absorption of orally administered titanium dioxide is extremely low;
the bioavailability of titanium dioxide (measured either as particles or as titanium) is low;
the bioavailability measured as titanium appeared to be independent of particle size;
the vast majority of an oral dose of titanium dioxide is eliminated unchanged in the faeces;
a small amount (maximum of 0.1%) of orally ingested titanium dioxide was absorbed by the gut associated lymphoid tissue (GALT) and subsequently distributed to various organs and elimination rates from these organs were variable.
The Panel further concluded that there were significant and highly variable background levels of titanium in animals and humans, which presented challenges in the analysis at the low levels of titanium uptake reported and could complicate interpretation of the reported findings. The Panel concluded that, based on the available genotoxicity database and the Panel's evaluation of the data on absorption, distribution, and excretion of micro‐ and nanosized titanium dioxide particles, orally ingested titanium dioxide particles (micro‐ and nanosized) are unlikely to represent a genotoxic hazard in vivo.
The Panel noted that possible adverse effects in the reproductive system were identified in some studies conducted with material which was either non‐food‐grade or inadequately characterised nanomaterial (i.e. not E 171). There were no such indications in the available, albeit limited, database on reproductive endpoints for the food additive (E 171). The Panel was unable to reach a definitive conclusion on this endpoint due to the lack of an extended 90‐day study as in the Guidance for submission of food additives (EFSA ANS Panel, 2012) or a multigeneration or extended‐one generation reproduction toxicity EOGRT study with the food additive (E 171). Therefore, the Panel did not establish an ADI.
From a carcinogenicity study with titanium dioxide in mice and in rats, the Panel chose the lowest NOAEL reported which was 2,250 mg titanium dioxide/kg bw per day for males from the rat study, the highest dose tested in this species and sex.
The Panel considered that on the database currently available and the considerations on the absorption of titanium dioxide the margins of safety calculated from the NOAEL of 2,250 mg titanium dioxide/kg bw per day identified in the toxicological data available and exposure data obtained from the reported use/analytical levels of titanium dioxide (E 171) considered in this opinion would not be of concern.
The Panel concluded that once definitive and reliable data on the reproductive toxicity of E 171 were available, the full dataset would enable the Panel to establish a health‐based guidance value (ADI)’ (EFSA ANS Panel, 2016).
In 2018, the EFSA ANS Panel evaluated four studies concerning the potential adverse health effects of titanium dioxide and considered that:
‘the results of the Bettini et al. (2017) study did not provide enough justification for a new carcinogenicity study, but, should additional useful mechanistic information become available, this could be reconsidered in future;
the new in vitro findings in the study by Proquin et al. (2017) did not modify the conclusion on the genotoxicity of titanium dioxide as stated in the previous EFSA opinion (EFSA ANS Panel, 2016) on the safety of titanium dioxide (E 171) when used as a food additive;
the effects of engineered titanium dioxide nanoparticles reported by the Guo et al. (2017) study were of uncertain biological significance and therefore of limited relevance for the risk assessment of the food additive titanium dioxide (E 171);
there was significant uncertainty in the risk assessment performed by Heringa et al. (2016), which did not include a weight of evidence analysis of the whole database;
the four studies evaluated, highlighted some concerns but with uncertainties, therefore their relevance for the risk assessment was considered limited and further research would be needed to decrease the level of uncertainties.
Overall, three of the studies assessed in this opinion reported that titanium dioxide was able to induce various effects in in vitro and in vivo models. These studies may be useful for hazard identification of titanium dioxide. The Panel considered that the limited relevance of the protocols of these studies to the use of E 171 under realistic conditions in food hampered the use of the data in the risk assessment of the food additive E 171. In the fourth study by Heringa et al. (2016), numerous assumptions were made, which resulted in large uncertainty in their conclusion.
More research exploring the possible effects observed in three of the four studies could address their applicability to the risk assessment of the food additive E 171 under realistic conditions of use.
Altogether, the Panel concluded that the outcome of the four studies did not merit re‐opening the existing opinion of EFSA related to the safety of titanium dioxide (E 171) as a food additive’ (EFSA ANS Panel, 2018).
The FAF Panel considered the EFSA ANS Panel (2016) in light of the current characterisation of the particle size distribution of titanium dioxide (E 171). The new specifications proposed are based on a better characterisation by interested business operators of the existing titanium dioxide (E 171) used in food applications using additional analytical methods.
The Panel is aware of ongoing studies using well‐characterised titanium dioxide (E 171), with the maximum percentage of nanoparticles according to the proposed specifications. These studies include an EOGRT study including additional parameters as requested in the previous opinions. In addition to this, a subchronic dietary (100‐day) study would be available soon with E 171 to address the tumour initiation/promotion potential as raised by Bettini et al. (2017); and a toxicokinetic study with E 171 to investigate the kinetics, tissues distribution and elimination/excretion mass balance will be initiated in 2019 (EFSA, 2019).
Overall, the Panel considers that the conclusions made, and the uncertainties identified, in the previous EFSA assessments of the food additive E 171 remain valid. In particular, the characterisation of E 171 does not provide a reason to revise the conclusion on genotoxicity of titanium dioxide (E 171) previously drawn by the ANS Panel (EFSA ANS Panel, 2016, 2018). The Panel reiterates the need for the further research as recommended in the previous opinions in order to decrease the level of uncertainty and acknowledged that additional studies with characterised E 171 are being carried out by interested business operators.
The Panel noted that the ongoing toxicity testing would decrease the level of uncertainty related to the safety assessment of titanium dioxide (E 171).
5. Conclusions
According to interested business operators, six different brands of titanium dioxide (E 171) containing different percentage of nanoparticles are used in food in the EU.
Based on the information provided by interested business operators related to E 171 on the EU market as a food additive, the Panel concluded that the following specification regarding the constituent particle size for titanium dioxide (E 171) as measured by EM and taking into account measurement uncertainty should be inserted in the current EU specifications.
| Constituent particle size for titanium dioxide (E 171) as a food additive | Measured by electron microscopy |
|---|---|
| Median minimal external dimension by number (nm) | More than 100 nma |
Equivalent to a percentage of constituent particles with a median minimal external dimension below 100 nm by number (%) less than 50%.
Based on the proposed change in the specifications, revisiting the toxicological database on titanium dioxide (E 171) as a food additive should consequently be conducted in line with the data requirements specified in the EFSA Guidance on nanotechnology (EFSA Scientific Committee, 2018).
6. Recommendation
In view of the additional information submitted by interested business operators, the Panel considers that the definition of the food additive E 171 should be revised in the current EU specifications for titanium dioxide (E 171) (Commission Regulation (EU) No 231/2012):
Titanium dioxide (E 171) as a food additive may contain small quantities (< 0.5%) of constituent particle growth and crystal phase control agents (alumina, sodium or potassium in combination with phosphate)
Titanium dioxide (E 171) as a food additive has no surface treatments or coatings.
Documentation provided to EFSA
CEFIC, 2018. Data on particle size and particle size distribution for titanium dioxide (E 171). Response by the Titanium Dioxide Manufacturers Association (TDMA) to the EFSA call for data published on 30th January 2017. June 2018. Submitted to EFSA by the EC, August 2018.
CEFIC, 2019a. Data on particle size and particle size distribution for titanium dioxide (E 171). Response by the Titanium Dioxide Manufacturers Association (TDMA) to the EFSA call for data published on 30th January 2017. Updated March 2019 following request for additional information from EFSA. Submitted to EFSA by CEFIC, April 2019
CEFIC, 2019b. Addendum to data on purity, particle size and particle size distribution for titanium dioxide (E 171) in rutile phase. Response by KRONOS INTERNATIONAL, In., to the EFSA call for data published on 30th January 2017. April 2019 following request for additional information from EFSA. Submitted to EFSA by CEFIC, April 2019
Technical hearing, 24 May 2019.
CEFIC, 2019c. Clarifications provided to points discussed at the Technical hearing (24 May 2019). Submitted to EFSA by CEFIC, 7th June 2019.
Personal communication from the EC‐JRC to the Working Group.
EC‐JRC (European Commission's Joint Research Centre), 2019. JRC communication to EFSA about an unpublished study on a multimethod approach for the characterization of pure and from confectionary extracted E 171 food grade titanium dioxide. 3rd June 2019.
Sciensano communication to EFSA on the unpublished results related to the project “Implementation and validation of an analytical methodology to assess the nanofriction in the food additives E 171, E 174 and E 175 with exposure analysis in the context of risk assessment”. https://www.sciensano.be/en/projects/implementation-and-validation-analyticalmethodology-assess-nanofraction-food-additives-E171-e174
Eveline Verleysen, Thorsten Wagner, Hans‐Gerd Lipinski, Ralf Kägi, Robert Koeber, Ana Boix‐Sanfeliu, Pieter‐Jan De Temmerman and Jan Mast, 2019. Evaluation of a TEM based approach for size measurement of particulate materials. Draft manuscript submitted for publication. Personal communication from Sciensano to EFSA.
Abbreviations
- ADI
acceptable daily intake
- ANS
EFSA Panel on Food Additives and Nutrient Sources added to Food
- ANSES
Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement e du travail/ French Agency for Food, Environmental and Occupational Health and Safety
- CEN
European Committee for Standardisation
- CI
confidence interval
- DC
disc centrifuge
- EC‐JRC
European Commission's Joint Research Centre
- EM
electron microscopy
- EOGRT
extended one‐generation reproductive toxicity
- FAF
EFSA Panel on Food Additives and Flavourings
- GALT
gut associated lymphoid tissue
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- NOAEL
no‐observed‐adverse‐effect‐level
- PSD
population standard deviation
- SEM
scanning electron microscopy
- SSA
specific surface area
- STEM
scanning transmission electron microscopy
- TEM
transmission electron microscopy
- WHO
World Health Organization
- XDC
X‐ray disc centrifuge
Appendix A – SEM diagrams and imagines for samples A–E
1.
Figure A.1.
Lab 1 SEM image and PSD diagram sample A
Figure A.2.
Lab 1 SEM image and PSD diagram sample B
Figure A.3.
Lab 1 SEM image and PSD diagram sample C
Figure A.4.
Lab 1 SEM image and PSD diagram sample D
Figure A.5.
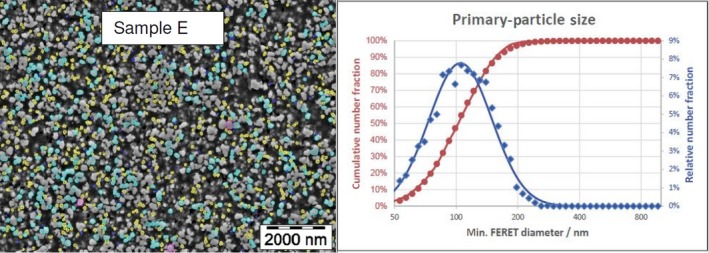
Lab 1 SEM image and PSD diagram sample E
Figure A.6.
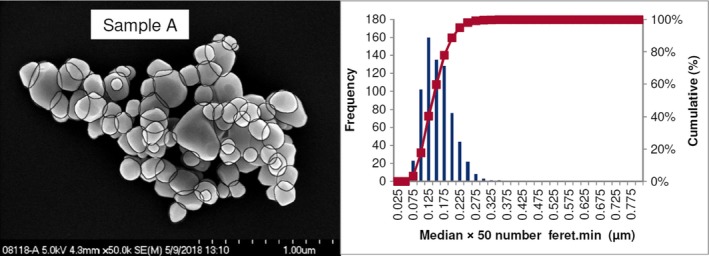
Lab 2 SEM image and PSD diagram sample A
Figure A.7.
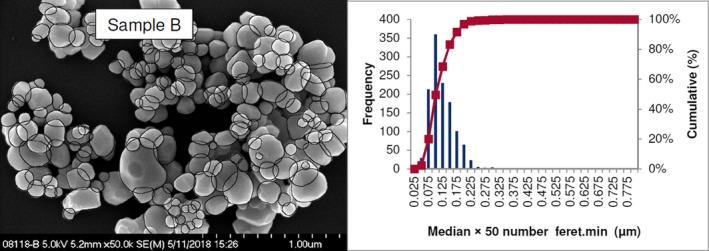
Lab 2 SEM image and PSD diagram sample B
Figure A.8.
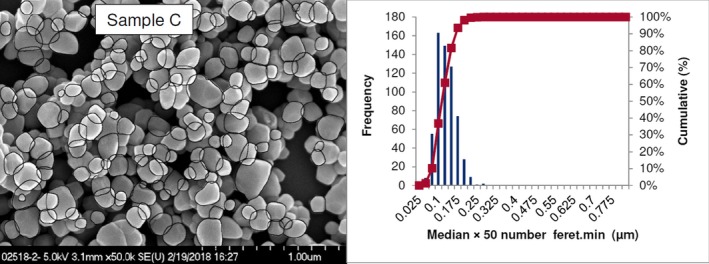
Lab 2 SEM image and PSD diagram sample C
Figure A.9.
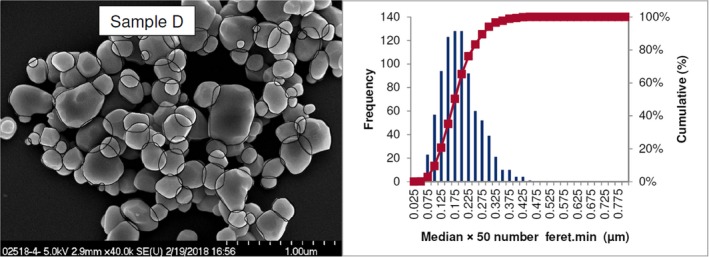
Lab 2 SEM image and PSD diagram sample D
Figure A.10.
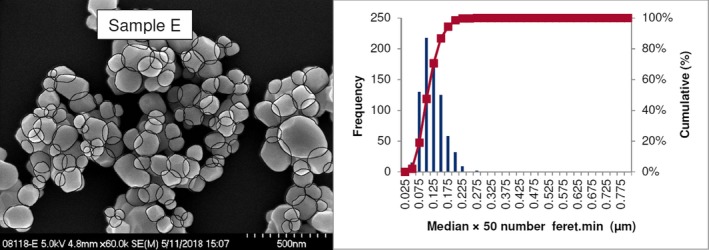
Lab 2 SEM image and PSD diagram sample E
Figure A.11.
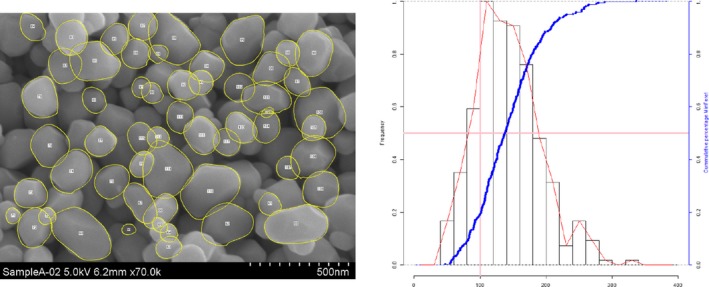
Lab 3 SEM image and PSD diagram sample A
Figure A.12.
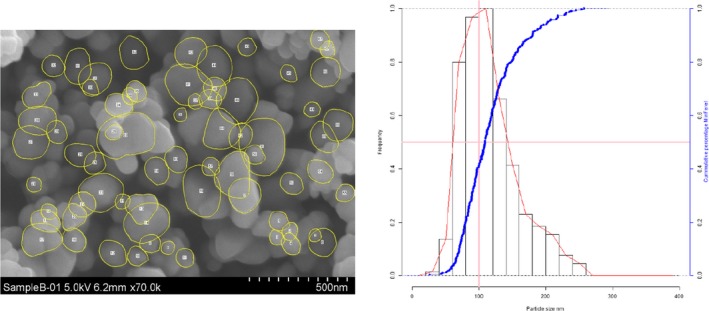
Lab 3 SEM image and PSD diagram sample B
Figure A.13.
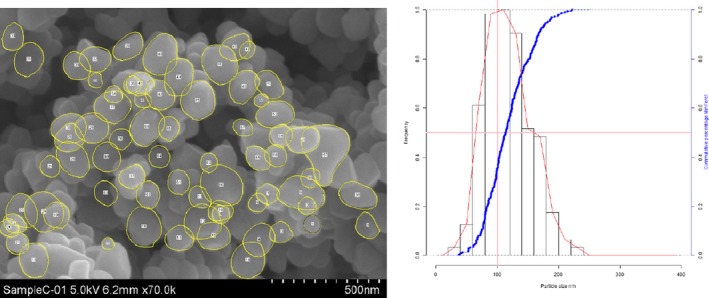
Lab 3 SEM image and PSD diagram sample C
Figure A.14.
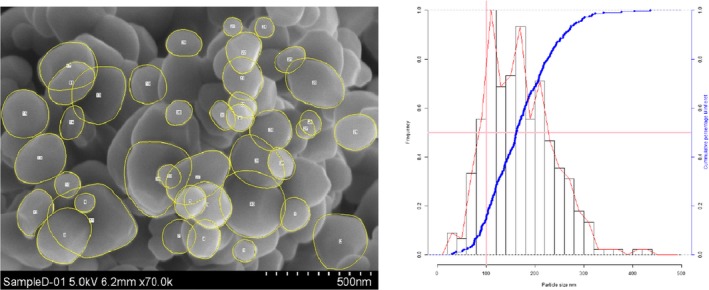
Lab 3 SEM image and PSD diagram sample D
Figure A.15.
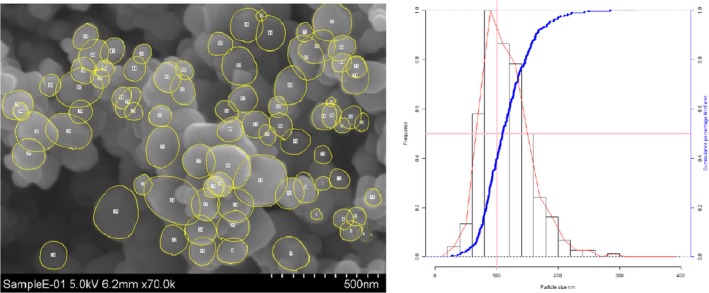
Lab 3 SEM image and PSD diagram sample E
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Gürtler R, Gundert‐Remy U, Husøy T, Mennes W, Agneta Oskarsson PM, Rainieri S, Shah R, Waalkens‐Berendsen I, Wölfle D, Gaffet E, Mast J, Peters R, Rincon AM and Fürst P, 2019. Scientific opinion on the proposed amendment of the EU specifications for titanium dioxide (E 171) with respect to the inclusion of additional parameters related to its particle size distribution. EFSA Journal 2019;17(7):5760, 23 pp. 10.2903/j.efsa.2019.5760
Requestor: European Commission
Question number(s): EFSA‐Q‐2018‐00625
Panel members: Maged Younes, Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus Agneta Oskarsson, Sandra Rainieri, Romina Shah, Ine Waalkens‐Berendsen and Detlef Wölfle.
Acknowledgments: The Panel wishes to thank Josefa Barrero (EC‐JRC) and Stéphane Jomini (ANSES) for the support provided to this scientific output.
Adopted: 27 June 2019
Notes
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p 1.
Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/fs_food-improvement-agents_reeval_call_20170130_E171_data.pdf
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Calculated by the Panel.
AEROXIDE® TiO2 P25 is a fine‐particulate, pure titanium dioxide (unique combination of anatase and rutile crystal structure) with high specific surface area and marked aggregate and agglomerate structure suitable for many catalytic and photocatalytic applications. More information available online: https://corporate.evonik.com/en/products/search-products/pages/product-details.aspx?productId=43469
Commission Recommendation of 18 October 2011 on the definition of nanomaterial. OJ L 275, 20.10.2011, p. 38–40.
References
- ANSES , 2019. Avis de l'Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail relatif au risques liés à la ingestion de l'additif alimentaire E 171. Saisine n° 2019‐SA‐0036. Available online: https://www.anses.fr/fr/system/files/ERCA2019SA0036.pdf
- Bettini S, Boutet‐Robinet E, Cartier C, Coméra C, Gaultier E, Dupuy J, Naud N, Taché S, Grysan P, Reguer S, Thieriet N, Réfrégiers M, Thiaudière D, Cravedi J‐P, Carrière M, Audinot J‐N, Pierre FH, Guzylack‐Piriou L and Houdeau E, 2017. Food‐grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Scientific Reports, 7, 40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Franks K, Kestens V, Roebben G, Lamberty MA and Linsinger T, 2011. Certified Reference Material ERM®‐ FD100: Certification of Equivalent Spherical Diameters of Silica Nanoparticles in Water. Report EUR 25018 EN. 2011, Luxembourg: European Union. ISBN 978‐92‐79‐18676‐9 10.2787/33725. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/certification-equivalent-spherical-diameters-silica-nanoparticles-water-certified-reference [DOI]
- CEN (European Committee for Standarisation), 2018. Nanotechnologies ‐ Guidance on detection and identification of nano‐objects in complex matrices. ICS 07.120. CEN/TS 17273:2018. Available online: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:37705,508478&cs=1B363D2465349533EBD3CABA96180879D
- Crouzier L, Delvallee A, Allard A, Devoille L, Cucourtieux S and Feltin N, 2019. Methodology to evaluate the uncertainty associated with nanoparticles dimensional measurement by SEM. Sci. Technol. in press, Meas: 10.1088/1361-6501/ab1495 [DOI] [Google Scholar]
- De Temmerman P‐J, Lammertyn J, De Ketelaere B, Kestens V, Roebben G, Verleysen E and Mast J, 2014a. Measurement uncertainties of size, shape, and surface measurements using transmission electron microscopy of near‐monodisperse, near‐spherical nanoparticles. Journal of Nanoparticle Research, 16, 1–22. [Google Scholar]
- De Temmerman P‐J, Verleysen E, Lammertyn J and Mast J, 2014b. Size measurement uncertainties of near‐monodisperse, near‐spherical nanoparticles using transmission electron microscopy and particle‐tracking analysis. Journal of Nanoparticle Research, 16, 1–17. [Google Scholar]
- Dudefoi W, Moniz K, Allen‐Vercoe E, Ropers MH and Walker VK, 2017. Impact of food grade and nano‐TiO2 particles on a human intestinal. Community. Food and Chemical Toxicology, 106, 242–249. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety), 2019. EFSA statement on the review on the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA Journal 2019;17(5):5714, 2 pp. 10.2903/j.efsa.2019.5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2016. Scientific Opinion on the re‐evaluation of titanium dioxide (E 171) as a food additive. EFSA Journal 2016;14(9):4545, 83 pp. 10.2903/j.efsa.2016.4545 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Wright M, Lodi F, Rincon AM, Smeraldi C and Woutersen RA, 2018. Scientific Opinion on the evaluation of four new studies on the potential toxicity of titanium dioxide used as a food additive (E 171). EFSA Journal 2018;16(7):5366, 27 pp. 10.2903/j.efsa.2018.5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: General Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Chaudhry Q, Cubadda F, Gott D, Oomen A, Weigel S, Karamitrou M, Schoonjans R and Mortensen A, 2018. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA Journal 2018;16(7):5327, 95 pp. 10.2903/j.efsa.2018.5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks K, Braun A, Charoud‐Got J, Couteau O, Kestens V, Lamberty A, Linsinger TPJ and Roebben G, 2012. Certified Reference Material ERM®‐FD304: Certification of the Equivalent Spherical Diameters of Silica Nanoparticles in Aqueous Solution. EUR 24620 EN. 2012, Luxembourg: European Union. ISBN 978‐92‐79‐21867‐5 10.2787/53551. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC67374/reqno_jrc67374_erm-fd304_final%20report%20pdf%20version.pdf [DOI]
- Guo Z, Martucci N, Moreno‐Olivas F, Tako E and Mahler G, 2017. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact, 5, 70–82, janvier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringa MB, Geraets L, van Eijkeren JCH, Vandebriel RJ, de Jong W and Oomen AG, 2016. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology, 10. [DOI] [PubMed] [Google Scholar]
- JECFA (Join FAO/WHO Expert Committee on Food Additives), 2013. Potassium aluminium silicate‐based pearlescent pigments, Type 1. Monograph 14. Combined compendium of food additive specifications. Volume 4, Available from: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/result/en/
- Kestens V and Roebben G, 2014. The certification of equivalent diameters of a mixture of silica nanoparticles in aqueous solution: ERM‐ FD102 EUR 26656 EN. 2014, Luxembourg: European Union. ISBN 978‐92‐79‐38396‐0 10.2787/95996. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC90438/final%20report%20erm%20fd102%20jrc%2090438.pdf [DOI]
- Linsinger TP, 2011. Reference materials for measuring the size of nanoparticles. TrAC Trends in Analytical Chemistry, 30, 18–27. [Google Scholar]
- Proquin H, Rodríguez‐Ibarra C, Moonen C, Urrutia Ortega I, Briedé J, de Kok T, van Loveren H and Chirino Y, 2017. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: contribution of micro and nano‐sized fractions. Mutagenesis, 32, 139–149. 10.1093/mutage/gew051 [DOI] [PubMed] [Google Scholar]
- Rauscher H, Roebben G, Mech A, Gibson N, Kestens V, Linsinger TPJ and Riego Sintes J, 2019. An overview of concepts and terms used in the European Commission definition of nanomaterials. EUR 29647 EN European Commission, JRC, Ispra, 10.2760/459136 [DOI]
- Stefaniak AB, Hackley VA, Roebben G, Ehara K, Hankin S, Postek MT, Lynch I, Fu W‐E, Linsinger TPJ and Thüneman AF, 2013. Nanoscale reference materials for environmental, health and safety measurements: needs, gaps and opportunities. Nanotoxicology, 7, 1325–1337. Available online: https://shop.standards.ie/store/PreviewDoc.aspx?saleItemID=3125735 [DOI] [PubMed] [Google Scholar]
- Taurozzi JS, Hackley VA and Wiesner MR, 2012. Preparation of a nanoscale TiO2 aqueous dispersion for toxicological or environmental testing. Special Publication 1200‐3, National Institute of Standards and Technology, Gaithersburg, MD. Available online: https://nvlpubs.nist.gov/nistpubs/SpecialPublications/NIST.SP.1200-3.pdf
- Verleysen E, Van Doren E, Waegeneers N and De Temmerman P‐J, 2015. TEM and SP‐ICP‐MS analysis of the release of silver nanoparticles from decoration of pastry. Journal of Agriculture and Food Chemistry, 63, 3570–3578. [DOI] [PubMed] [Google Scholar]


