Abstract
Background
The risk of Cushing syndrome (CS) patients experiencing a thrombotic event (TE) is significantly higher (odds ratio; OR 18%) than that of the general population. However, there are currently no anticoagulation guidelines.
Methods
A retrospective, single-center, longitudinal study of patients undergoing all types of treatment—surgical (pituitary, unilateral, and bilateral adrenalectomy) and medical treatment—was undertaken. TEs were recorded at any point up until last patient follow-up; myocardial infarction (MI), deep venous thrombosis (DVT), and pulmonary embolism (PE) or stroke. Patients’ doses and complications of anticoagulation were recorded.
Results
Included were 208 patients; a total of 165 (79.3%) were women, and mean age at presentation was 44 ± 14.7 years. Thirty-nine (18.2%) patients had a TE; extremity DVT (38%), cerebrovascular accident (27%), MI (21%), and PE (14%). Of 56 TEs, 27 (48%) were arterial and 29 (52%) were venous. Patients who underwent bilateral adrenalectomy (BLA) had an odds ratio of 3.74 (95% CI 1.69-8.27) of developing a TE. Of patients with TEs, 40.5% experienced the event within the first 60 days after surgery. Baseline 24-hour urinary free cortisol levels did not differ in patients with or without TE after BLA. Of 197 patients who underwent surgery, 50 (25.38%) received anticoagulation after surgery, with 2% having bleeding complications.
Conclusions
The risk of TEs in patients with CS was approximately 20%. Many patients had more than 1 event, with higher risk 30 to 60 days postoperatively. The optimal prophylactic anticoagulation duration is unknown, but most likely needs to continue up to 60 days postoperatively, particularly after BLA.
Keywords: Cushing syndrome, Cushing disease, hypercoagulability, transsphenoidal surgery, bilateral adrenalectomy, thrombotic events, anticoagulation
Cushing syndrome (CS) is associated with a 2- to 4-fold higher mortality rate compared to the general population when not appropriately treated (1-4), largely because of an increase in cardiovascular disease and infection (1, 5-7). Hypercoagulability has been described both in patients with CS and in patients on glucocorticoid (GC) treatment (8); however, an underlying mechanism has yet to be determined. Coagulation factors VIII, IX, and XI, and von Willebrand factor were shown to be increased indirectly in some studies that compared healthy controls to endogenous hypercortisolemic patients, representing activation of the coagulation pathway (9-13). Patients with CS also have a significant increase in fast-activating plasminogen activator inhibitor 1, impairing the fibrinolytic system and thereby increasing coagulation risk (10, 14). The presence of inflammation in patients with CS, enhanced oxidative stress, and platelet activation may also have a pathogenic role (15, 16). Increased inflammatory and procoagulant activity, and decreased fibrinolytic factors, lead to an elevated risk both of venous and arterial thromboembolism in patients with CS.
The risk of a thrombotic event (TE) in patients with CS has been reported to be up to 10 times higher than that of the general population (17, 18). A systematic review found TE risk to be 1.9% to 2.5% in the absence of surgical intervention, corresponding to an incidence of 2.5 to 3.1 per 1000 person-years (18). In a recent meta-analysis, we reported that the calculated odds ratio (OR) of venous thromboembolism events (VTEs) in patients with CS was 17.82 compared to that of the general population (19). Furthermore, a multicenter cohort study reported that the risk of postoperative VTE was significantly higher in patients with Cushing disease (CD) compared to patients undergoing pituitary surgery for a nonfunctioning pituitary adenoma, suggesting that the high risk for postoperative VTE in patients with CS is cortisol mediated (17), with similarly reported results in smaller series (18, 20-22).
A thromboprophylaxis protocol is well established for orthopedic surgeries, which are considered high VTE risk (TE up to 40% to 60% of patients if not on prophylaxis, [23, 24]), but there are currently no guidelines for hypercortisolemic patients undergoing transsphenoidal surgery (TSS), unilateral adrenalectomy (UA), or bilateral adrenalectomy (BLA) (25). Thus, the outcomes for patients with CS, with or without perioperative anticoagulation treatment, remain unclear. Here we evaluate the risk both of arterial TEs and VTEs and thromboembolic events in patients with CS and postoperative complications associated with prophylactic anticoagulation after TSS or BLA.
1. Methods
We conducted an institutional review board–approved, retrospective, longitudinal study among 208 patients with CS seen at Oregon Health & Science University Pituitary Center (July 2006 to August 2018), with a waiver of informed consent. We included patients undergoing all types of treatment—surgery (pituitary, unilateral, and BLA)—as well as patients on medical treatment. Exclusion criteria included pediatric patients, incomplete data, and patients with severe illness. A single entry was removed as an extreme data outlier. Two patients with ectopic Cushing syndrome (ECS) were excluded because their primary neuroendocrine tumor was very advanced with significant tumor burden and they died before follow-up in clinic. Two other patients were excluded from the time to TE analysis because of unknown TE dates. The following TEs were recorded at any point up until their last follow-up: myocardial infarction (MI), deep venous thrombosis (DVT), and pulmonary embolism (PE) or stroke.
Diagnosis of CS was based on elevated 24-hour urinary free cortisol (UFC), elevated midnight salivary cortisol levels, and cortisol concentrations greater than 1.8 μg/dL after a 1 mg dexamethasone suppression test or a 2-day low-dose (2 mg/d) dexamethasone suppression test. Cortisol cutoff used for dexamethasone–corticotropin-releasing hormone (CRH) test was greater than 2.4 μg/dL.
CD was diagnosed by the presence of a pituitary adenoma and/or positive central-to-peripheral gradient on inferior petrosal sinus sampling (IPSS). Adrenal Cushing syndrome (ACS) was diagnosed in the presence of suppressed adrenocorticotropic hormone (ACTH) levels, or lack of response of ACTH to the dexamethasone–corticotropin-releasing hormone stimulation test and imaging confirming the presence of an adrenal mass. ECS was diagnosed based on elevated ACTH levels and gradient-negative inferior petrosal sinus sampling (suggestive of a nonpituitary ACTH production origin).
A mean of 3 × 24-hour UFC in patients who had BLA was calculated from the last 3 UFC obtained within the 6 months prior to BLA. Samples were sometimes analyzed in different laboratories; furthermore, because of the long duration of the study, methods changed, including immunoassay and liquid chromatography–mass spectrometry. Thus, we standardized the UFC levels by calculating the ratio of the absolute UFC level to the upper limit of normal range (ULN).
We evaluated all patients who received anticoagulation in the immediate postoperative period and up to 3 months postsurgery. Doses and complications for anticoagulation were assessed and recorded for each patient.
A. Statistical Analysis
Statistical analysis was performed using SAS 9.4 and included descriptive statistics, analysis of variance, and Kruskal-Wallis, chi-square, and t tests, and simple linear regression analysis. Descriptive data are presented as ± SD. Significance was considered as P < .05.
2. Results
A. Demographics of Patients With Cushing Syndrome by Etiology
Of 208 patients included, 165 (79.3%) were women, with a mean age at presentation of 44 (± 14.7) years. All patients were overweight or obese with a mean body mass index (BMI) of 33.9 kg/m2 (± 8.4), 147 (70.7%) had hypertension and 87 (41.8%) had diabetes mellitus (Table 1).
Table 1.
Demographic and Baseline Characteristics Data
| Characteristic | All Patients (n = 208) |
|---|---|
| Age at presentation, y ± SD | 44.4 ± 14.7 |
| Female, n, % | 165, 79.3 |
| Male, n, % | 43, 20.7 |
| Body mass index ± SD | 33.9 ± 8.4 |
| Smoker, n, % | 69, 33.5 |
| Hypertension, n, % | 147, 70.7 |
| Diabetes mellitus, n, % | 87, 41.8 |
| On estrogen, n, % | 42, 25.4 of women |
| On testosterone replacement, n, % | 23, 51.1 of men |
| Cushing disease, n, % | 168, 89.4 |
| Adrenal Cushing syndrome, n, % | 14, 6.73 |
| Ectopic Cushing, n, % | 8, 3.85 |
| Transsphenoidal surgery | 152 (73%) |
| Transsphenoidal surgery + bilateral adrenalectomy | 32 (15.38%) |
| Transsphenoidal surgery + unilateral adrenalectomy | 4 (1.92%) |
| Unilateral adrenalectomy | 5 (2.4%) |
| Bilateral adrenalectomy | 4 (1.9%) |
| Medication only | 8 (3.85%) |
| No therapy | 3 (1.44%) |
B. Type of Treatment for Cushing Syndrome
The majority of individuals with CD were treated with TSS only. However, after failed TSS, BLA was more common than UA. Five patients underwent only a UA and 4 patients only a BLA. Pituitary radiation was used as adjunctive treatment for tumor growth, treatment failure, or Nelson syndrome. Medical therapy was used when the patient was a poor surgical candidate or refused surgery. Of the 14 patients diagnosed with ACS, 3 underwent BLA, 4 underwent UA, 4 underwent TSS followed by UA, and 3 were treated medically. Of the 8 patients with ECS, 3 had initial surgery for the ectopic source and then other therapies, 3 were treated with medical therapy, 1 underwent UA, 3 underwent BLA, and 1 opted for no therapy (Table 1).
C. Types of Thrombotic Events
Thirty-nine (18.2%) patients had a TE; however, there were a total of 56 TEs because 12 patients had more than 1 TE. The most common events were extremity DVT (32%), cerebrovascular accident (27%), followed by MI (21%) and PE (14%). The extremity TEs found in this study were 3 (5%) bilateral lower extremity (LE) DVTs, 11 (20%) unilateral LE DVTs, 1 (2%) bilateral upper extremity (UE) DVT, and 6 (11%) unilateral UE DVTs. Of the 56 TEs found in our study, 27 (48%) cases were arterial and 29 (52%) cases were venous.
Sex, age, BMI, smoking status, estrogen/testosterone supplementation, diabetes mellitus, and hypertension were not found to significantly increase the risk of developing a TE (Table 2). However, all 23 patients who had a UE DVT had a peripherally inserted central catheter (PICC); therefore, patients with a PICC line are 46% more likely to develop a UE DVT.
Table 2.
Risk Factors for Thrombosis: Demographic, Clinical Data, and Treatment Modalities of Adult Patients With Cushing Syndrome Subdivided by Patients With TEs and Without TEs
| Characteristic | Patients With TE (n = 39) | Patients Without TE (n = 167) | P | OR | 95%CI |
|---|---|---|---|---|---|
| Age at time of event, y ± SD | 49 ± 13.5 | 43 ± 13.5 | |||
| Female, n, % | 30, 81 | 133, 78.7 | .69 | 0.83 | 0.34-2.05 |
| Male, n, % | 7, 19 | 36, 21 | .69 | 0.83 | 0.34-2.05 |
| Body mass index ± SD | 33.3 ± 7.5 | 34 ± 8.6 | .69 | 0.99 | 0.95-1.03 |
| Smoker, n, % | 14, 37.8 | 55, 32.5 | .57 | 1.35 | 0.65-2.79 |
| Hypertension, n, % | 28, 75.6 | 117, 69.2 | .38 | 1.43 | 0.63-3.24 |
| Diabetes mellitus, n, % | 15, 40.5 | 71, 42 | .77 | 0.90 | 0.44-1.85 |
| Estrogena, n, % | 3, 7.14 | 39, 92.86 | .62 | b | b |
| Testosteronea, n, % | 3, 13.1 | 17, 73.9 | .59 | b | b |
| Cushing disease, n, % | 35, 18.8 | 151, 81.2 | .005 | 1.04 | 0.33-3.27 |
| Adrenal Cushing syndrome, n, % | 2, 14.3 | 12, 85.7 | .19 | b | b |
| Ectopic Cushing, n, % | 2, 25 | 6, 75 | .21 | b | b |
| TSS, n, % | 36, 19.15 | 152, 80.85 | .35 | 2.01 | 0.44-9.10 |
| BLA, n, % | 14, 38.88 | 22, 61.11 | .001 | 3.74 | 1.69-8.27 |
| Pituitary radiation, n, % | 5, 21.74 | 18, 78.26 | .65 | 1.27 | 0.44-3.67 |
Abbreviations: BLA, bilateral adrenalectomy; OR, odds ratio; TE, thrombotic event; TSS, transsphenoidal surgery.
aAnalysis comparing individuals who were taking estrogen and testosterone supplementation at the time of TE compared to patients without TE who were ever treated with estrogen or testosterone.
bSample size was too small to calculate statistical significance.
From the patients who had a TE, the majority underwent only TSS. However, one-third of patients who underwent BLA alone or TSS + BLA had a TE. We found that 14 (38.8%) of 36 patients who underwent a BLA had a TE, with a calculated OR of 3.74 (95% CI 1.69-8.27). We did not find an increased risk of TE in patients who underwent TSS + UA, UA alone, medical therapy, or those that did not want to pursue therapy (Table 3).
Table 3.
Thrombotic Events in Different Therapeutic Groups
| Therapy | Thromboembolic Events | ||
|---|---|---|---|
| No | Yes | Total | |
| BLA only, n | 1 | 3 | 4 |
| % of all patients | 0.48 | 1.44 | 1.92 |
| % of patient with BLA | 25 | 75 | 100 |
| % of patients w/wo TE | 0.59 | 7.69 | |
| Medication only, n | 8 | 0 | 8 |
| % of all patients | 3.86 | 0 | 3.85 |
| % of patients with medication only | 100 | 0 | 100 |
| % of all patients w/wo TE | 4.73 | 0 | |
| No therapy, n | 3 | 0 | 3 |
| % of all patients | 1.44 | 0 | 1.44 |
| % of patient with no therapy | 100 | 0 | 100 |
| % of all patients w/wo TE | 1.78 | 0 | |
| TSS + BLA, n | 21 | 11 | 32 |
| % of all patients | 10.1 | 5.29 | 15.38 |
| % of patients with TSS + BLA | 65.63 | 34.38 | 100 |
| % of all patients w/wo TE | 12.43 | 28.21 | |
| TSS + UA, n | 4 | 0 | 4 |
| % of all patients | 1.92 | 0 | 1.92 |
| % of patients with TSS + UA | 100 | 0 | 100 |
| % of all patients w/wo TE | 2.37 | 0 | |
| TSS only, n | 127 | 25 | 152 |
| % of all patients | 61.06 | 12.02 | 73.08 |
| % of patients with TSS only | 83.55 | 16.45 | 100 |
| % of all patients w/wo TE | 75.15 | 65.1 | |
| UA only, n | 5 | 0 | 5 |
| % of all patients | 2.4 | 0 | 2.4 |
| % of patients with UA only | 100 | 0 | 100 |
| % of all patients w/wo TE | 2.96 | 0 | |
| Total, n | 169 | 39 | 207 |
| % of all patients | 81.25 | 18.75 | 100 |
| % of all patients w/wo TE | 100 | 100 |
Thrombotic event rates subdivided into different treatment modalities in patients with Cushing syndrome.
Abbreviations: BLA, bilateral adrenalectomy; TE, thrombotic event; TSS, transsphenoidal surgery; UA, unilateral adrenalectomy; w/wo, with or without.
D. Timing of Thrombotic Events
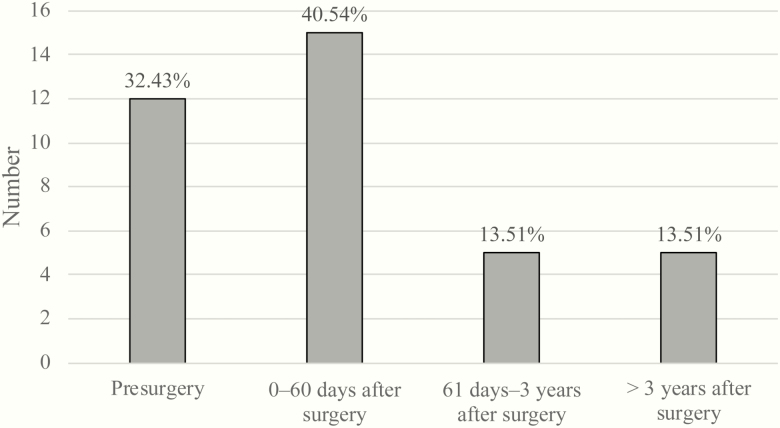
Of all patients evaluated in this study, 39 had a TE. As previously indicated, 2 patients were excluded from this particular analysis because the date of their TE was unknown. We found that 15 (40.5%) patients had a TE within the first 60 days after surgery, 5 (13.51%) patients between the first 61 days and 3 years after surgery, and 5 (13.51%) more than 3 years after surgery. We also found that 12 (32.43%) patients had a TE before surgery (Fig. 1). When analyzed within each surgical therapy, almost the same number of patients had a TE before undergoing TSS as within the first 60 days after surgery.
Figure 1.
Interval from surgery to thrombotic event (TE). Incidence of TEs subdivided into groups based on the time period when patients experienced a TE.
This highest incidence of TE within the first 60 days after surgery prevailed when we examined a subgroup of patients depending on whether they underwent TSS only, TSS and BLA, or BLA only. There was a clear trend that the highest TE incidence occurred during the first 60 days after TSS and BLA, and BLA alone.
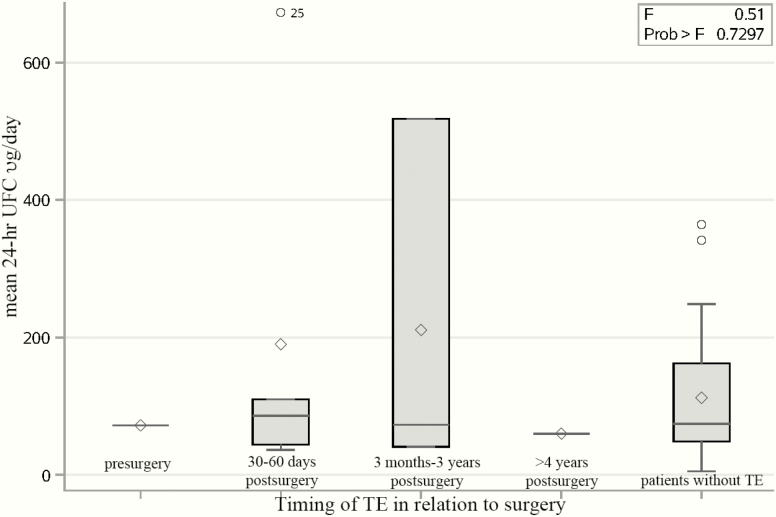
For patients who had a BLA, there was no statistically significant difference in the mean 3 × 24-hour UFC levels (expressed as the ratio UFC:ULN) prior to BLA between patients with and without TE (5.3 vs 1.3, respectively, P = .129), albeit with possible limitations related to different UFC methods. Concomitantly, there was no significant correlation between mean 24-hour UFC levels and the timing of TE after 1 patient, with a 24-hour UFC of 1900 µg/day, was removed from the analysis as an outlier.
There was no significant relationship between mean 24-hour UFC at the time of TE in patients who underwent BLA (Fig. 2) or in the timing of TE with respect to BLA surgery.
Figure 2.
Mean 24-hour urinary free cortisol (UFC) in relation to timing of thrombotic events (TEs) in patients undergoing bilateral adrenalectomy (BLA). The distribution analysis of mean 24-h UFC (µg/d) in patients who did not have a TE and those who did have a TE in different time periods in relation to the date of BLA. ◊ = data mean, ○ = data outliers, Prob > F = P value for the effect of the classification variable on the response. Small F, with a big P value indicates not significantly different.
E. Length of Stay for Surgery
The overall longer length of stay (LOS) in patients who underwent TSS and had a TE in the immediate postoperative period was 5.8 ± 3.1 days compared to patients who did not have a TE of 4.4 ± 3.3 days. Additionally, patients who underwent a BLA who had a TE in the immediate postoperative period had a longer LOS (7.5 ± 0.5 days) when compared to those who did not have a TE (3.9 ± 2.0 days). However, it is important to note that there were only 4 patients with a TE in the immediate postoperative period in the TSS group and only 2 patients with a TE in the BLA group, thus limiting data interpretation.
F. Anticoagulation Risks
A total of 197 patients underwent surgery, either TSS or BLA, of whom 50 (25.3%) received anticoagulation after surgery and only 1 (2%) developed complications. Per hospital protocol, all our patients are placed on compression stockings and undergo mobilization the day of surgery. There were 19 (9.6%) patients who received prophylactic anticoagulation before surgery with no reported complications during surgery or after. Nine (4.6%) patients were started on anticoagulation with a therapeutic dose of warfarin during the first 2 days after surgery; 8 of the patients taking warfarin underwent TSS and 1 underwent BLA. Concomitantly, in the immediate postoperative period, 5 (2.5%) patients received a therapeutic dose of enoxaparin and 42 (24.3%) were on prophylactic doses. Of all the patients anticoagulated with enoxaparin, 17 (36.1%) underwent adrenalectomy and 30 (63.8%) TSS; only 1 patient developed a complication after being treated with dual warfarin and enoxaparin therapy for bilateral LE DVTs after TSS. This patient sustained an intraventricular hemorrhage and developed hemoptysis. Anticoagulation was held temporarily and the patient did not develop any further complications. No other patients developed bleeding or complications at the site or surgery associated with anticoagulation. At the time of the TE event, 5 patients (12.8% of those who had a TE) were prophylactically anticoagulated with enoxaparin.
3. Discussion
TEs contribute to high mortality rates in CS (26, 27, 4), with PE accounting for 11%, ischemic cardiac disease for 19%, and stroke for 17% of deaths (26). Standardized mortality ratio decreases in patients with CS who are successfully treated but does not return to that of the normal population (1, 2, 4, 5).
Our study is to date the largest single-center study to analyze both arterial and venous TE. Despite inherent retrospective study limitations, we were able to determine a high (~18%) prevalence of all TE in patients with CS. Notably, 12.8% of these patients were prophylactically anticoagulated with enoxaparin at the time of the event, confirming the high risk of hypercoagulability.
Interestingly, we did not find any statistically significant correlation between TE and UFC levels, sex, age, BMI, smoking, diabetes mellitus, hypertension, or estrogen/testosterone replacement. However, there was a slightly higher trend of TE in patients with hypertension and those who smoked. Similar to data from a recent meta-analysis (19), patients with CS in our center were more often women with a mean age of 44 years. Other studies have also demonstrated a lack of correlation between UFC levels and severity of CS comorbidities (28). Studies examining the relationship between metabolic syndrome and the risk of VTE remain controversial (29, 30]. Kastelan et al found no significant association between UFC and increased procoagulant factors in CS (31). However, Koutroumpi and colleagues demonstrated a negative correlation between UFC levels and partial thromboplastin time, suggesting that severe hypercortisolism may result in elevated factor VIII (20). However, the clinical manifestations of CS do not always correlate with hypercortisolism severity (32), which could explain why we did not find a correlation between degree of biochemical hypercortisolism and TE. In a large, multicenter European study, preoperative medical treatment of hypercortisolism also did not decrease VTE risks (33).
Data on arterial TE are scarce. A population study found that patients with CS were at increased risk of acute MI (hazard ratio; HR 3.7, 95% CI 2.4-5.5) and stroke (HR 2.0, 95% CI 1.3-3.2) compared with the general population (4).
Our study found an overall TE rate of 18%, of which 52% were VTE. Without VTE prophylaxis, VTE incidence in medical and general surgical hospitalized patients is 10% to 40% (23). Coelho et al reviewed 13 studies with 1356 patients with CS (1080 with CD) and observed a VTE incidence rate close to what we report here, 8.9%, 53% of which were related to surgery (34). Furthermore, Dekkers and colleagues found, when compared to the general population, an HR of 2.6 (95% CI 1.5-4.7) of VTE in CS (4). Use of a PICC line clearly increased risk of UE DVT in our group, and use has been discontinued in patients with CS over the last decade.
Timelines of TE from CS diagnosis and/or in relation to surgery are also intriguing (4, 35). We found that there is an increased overall risk of TE 3 years before diagnosis and is highest during the 2 months following BLA or TSS. This risk was significantly higher in patients undergoing BLA (OR 3.47l; CI 1.55-7.78). Babic et al found that adrenalectomy for CS significantly increases risk of VTE vs patients having adrenalectomy for other etiologies (2.6% [n = 8/310] vs 0.9% [n = 37/3907]; P = .007) (36). We cannot exclude postoperative iatrogenic hypercortisolemia as a contributor to VTE in our study; however, the majority of patients were tapered down to a physiologic replacement dose with hydrocortisone by 1 month.
Based on these results, we suggest that patients undergoing BLA for CS be anticoagulated up to 30 to 60 days postoperatively if there are no contraindications.
Although it is well recognized that patients with CS are at increased risk of VTE events, especially in the postoperative setting, use of thromboprophylaxis is neither routine nor standardized (34, 37). Few studies have examined the risk of VTE in CS in patients receiving thromboprophylaxis for surgery. In our study, prophylactic anticoagulation 1 week preoperatively and up to 28 days postoperatively was reserved for high-risk patients (as deemed by our multidisciplinary team based on previous history of TE, significantly elevated UFC, and other known risks factors for TE not related to CS). Of all the patients in this study, 10% were anticoagulated preoperatively and 25.3% postoperatively. Only 1 patient (2%) developed an intraventricular hemorrhage after being started on warfarin and enoxaparin for bilateral LE DVT. However, no patients who had prophylactic anticoagulation developed any complications, demonstrating the relative safety of anticoagulation in these patients.
Boscaro and colleagues retrospectively examined 307 patients with CS (66% with CD) in 2 groups. The first group did not receive postoperative thromboprophylaxis (75 patients) and the second (232 patients) received unfractionated heparin for at least 2 weeks and warfarin for at least 4 months after surgery. Twenty percent of patients in the first group developed VTE, as compared to only 6% in the second group who received thromboprophylaxis. Sixty-two percent of events occurred in the first 3 months after surgery (38). In a recent retrospective study focused on patients with CD, 78 patients who underwent TSS were analyzed in 2 groups. The first group received fractionated heparin for a maximum of 14 days after surgery, and the second group was treated with subcutaneous enoxaparin for 30 days plus graduated elastic stockings until mobilization. Three VTE events were reported in the nonanticoagulated group, all within 30 days of surgery, compared to no VTE in the group that was anticoagulated (39). However, in our study 5 (12%) patients developed a TE event while on prophylactic anticoagulation with enoxaparin. Smith et al discussed the possible use of aspirin to decrease risk of TE (40) and suggested that possible DVT risk reduction in their center was due to use of aspirin from day 1 postoperatively for 6 weeks, as implemented by the senior surgeon. The role of aspirin has not been directly studied in patients with CS; however, serum- and GC-regulated kinase 1 (SGK1) is a powerful regulator of a key mediator of the store-operated Ca2+ entry required for platelet activity (41). Also, oxidative stress–induced platelet activation via the thromboxane pathway is significantly higher in CS and demonstrated by increased thromboxane B2 levels (6, 15).
The risk of VTE may remain elevated for many years after surgery (4), even after remission from CS. Casonato and colleagues demonstrated that in 20 patients with CS, factor VIII and von Willebrand factor levels decreased, starting at 3 months postoperatively and normalized 1 year after surgical cure (42). Likewise, Manetti et al demonstrated significant improvements in procoagulant markers 1 year following successful surgery (43). Kastelan et al also reported that 6 months after surgical remission, some but not all procoagulant markers declined to levels comparable with that in the control population. Although factor VIII and plasminogen activator inhibitor 1 tended to normalize, there was no significant difference in these levels compared to the ones before surgery (31). Together, these data suggest that a minimal period of sustained remission may be required to reverse the hypercoagulable state in CS, and that despite normalization of cortisol levels, persistence of other factors may play a role in residual VTE risk (4, 37), which could explain why 27% of our patients developed a TE years after their BLA. Although we did not find a correlation between TE and degree of UFC elevation preoperatively, in patients with adrenal insufficiency after CS remission, supraphysiological GC replacement could theoretically also be a culprit as described in patients without CS (8).
A survey of Pituitary Society members (44) showed that awareness regarding hypercoagulability in CD increased 4-fold in 2 years, and routine VTE prophylaxis increased from 50% to 75% perioperatively. However, prophylactic treatment for hypercoagulability was not universally administered in many centers despite published data on possible benefits. Low-molecular-weight heparin was the preferred agent used for VTE prophylaxis in this survey (44), and most of the responders used it for just approximately 2 weeks postoperatively. Zilio et al identified in a small study 6 VTE independent risk factors: age 69 years or older, reduced mobility, acute severe infections, previous cardiovascular events, midnight plasma cortisol level greater than 3.15 ULN, and decreased activated partial thromboplastin time (45). However, we were not able to identify any clear-cut risk factors, nor were any identified in a large meta-analysis (19).
Thromboprophylaxis in patients with CS appears to be safe. Similar to previously reported results, the overall risk of bleeding in our study was low (38, 39). Data suggest that thromboprophylaxis be used routinely in patients undergoing surgery for CS, but there is presently no consensus on the choice and duration of thromboprophylaxis administration (25, 34, 37). In patients with gliomas (which have an even higher risk of VTE), recent guidelines suggest VTE prophylaxis with low-molecular-weight heparin be started within 24 hours postoperatively to decrease risk of hemorrhage (46). A similar approach could be considered in patients with CS. Though not used in our patients, a role for aspirin postoperatively remains to be studied.
Our results suggest that thromboprophylaxis needs to be extended up to 60 days after surgery for most patients, especially after BLA. However, the exact duration and patient selection is yet to be established and requires further research (17, 37, 39).
Study limitations include the retrospective design, relatively small sample size, and reporting of symptomatic events because not all patients with the exception of those who underwent BLA were screened for TE. Establishing a relationship between etiology of hypercortisolism and TE was not possible given the small number of patients with ACS and ECS. We were unable to determine whether there was a higher risk of TE in patients on estrogen or testosterone supplementation because the sample size was too small. Concomitantly, when subdividing patients into different treatment options, there was a lack of power analysis. Several patients had 24-hour UFC performed in different laboratories before their referral to our center. Throughout the time period during which this study took place, the methods used to measure free cortisol have changed, but we have attempted to correct for this by using UFC UNL standardization. Strengths included a large number of patients for a single-center study, all being managed uniformly in a tertiary referral, multidisciplinary pituitary center.
4. Conclusion
An increased awareness of thromboembolism in CS is extremely important. We show that patients with CS are at a higher risk of thromboembolism for approximately 30 to 60 days during the postoperative period. This risk is higher in patients undergoing BLA. Interestingly, the degree of hypercortisolemia or other known risk factors for TE did not correlate with risk of TE. We also found that prophylactic anticoagulation, although administered in just one-quarter of our patients, did not increase the risk of bleeding or surgical complications in patients undergoing TSS or adrenalectomy. The precise duration of prophylactic anticoagulation is unknown, but most likely should continue up to 60 days after surgery, particularly after BLA, the time frame in which patients are at a higher risk for TEs. However, further prospective investigations are required to raise awareness of the hypercoagulability risks and to optimize anticoagulant protocols along with individualized preoperative assessments of thromboembolism and bleeding risk in patients with CS.
Acknowledgments
Financial Support: The authors received no financial support for the research, authorship, and/or publication of this article.
Glossary
Abbreviations
- BLA
bilateral adrenalectomy
- CS
Cushing syndrome
- DVT
deep venous thrombosis
- GC
glucocorticoid
- TE
thrombotic event
- TSS
transsphenoidal surgery
Additional Information
Disclosure Summary: Dr Fleseriu has served as principal investigator with research funding to Oregon Health & Science University from Novartis, Millendo, and Strongbridge, and has received occasional scientific consulting fees from Novartis and Strongbridge. Maria Gabriela Suarez, Madeleine Stack, Jose Miguel Hinojosa-Amaya, Michael D. Mitchell, Elena Varlamov, Chris G. Yedinak, Justin S. Cetas, and Brett Sheppard have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab. 2011;96(3):632–642. [DOI] [PubMed] [Google Scholar]
- 2. Ntali G, Asimakopoulou A, Siamatras T, et al. Mortality in Cushing’s syndrome: systematic analysis of a large series with prolonged follow-up. Eur J Endocrinol. 2013;169(5):715–723. [DOI] [PubMed] [Google Scholar]
- 3. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593–5602. [DOI] [PubMed] [Google Scholar]
- 4. Dekkers OM, Horváth-Puhó E, Jørgensen JO, et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98(6):2277–2284. [DOI] [PubMed] [Google Scholar]
- 5. Yaneva M, Kalinov K, Zacharieva S. Mortality in Cushing’s syndrome: data from 386 patients from a single tertiary referral center. Eur J Endocrinol. 2013;169(5):621–627. [DOI] [PubMed] [Google Scholar]
- 6. Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611–629. [DOI] [PubMed] [Google Scholar]
- 7. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913–927. [DOI] [PubMed] [Google Scholar]
- 8. Johannesdottir SA, Horváth-Puhó E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743–752. [DOI] [PubMed] [Google Scholar]
- 9. Świątkowska-Stodulska R, Skibowska-Bielińska A, Wiśniewski P, Sworczak K. Activity of selected coagulation factors in overt and subclinical hypercortisolism. Endocr J. 2015;62(8):687–694. [DOI] [PubMed] [Google Scholar]
- 10. Fatti LM, Bottasso B, Invitti C, Coppola R, Cavagnini F, Mannucci PM. Markers of activation of coagulation and fibrinolysis in patients with Cushing’s syndrome. J Endocrinol Invest. 2000;23(3):145–150. [DOI] [PubMed] [Google Scholar]
- 11. Jenkins PV, Rawley O, Smith OP, O’Donnell JS. Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol. 2012;157(6):653–663. [DOI] [PubMed] [Google Scholar]
- 12. Cavusoglu E, Marmur JD, Hojjati MR, et al. Plasma interleukin-10 levels and adverse outcomes in acute coronary syndrome. Am J Med. 2011;124(8):724–730. [DOI] [PubMed] [Google Scholar]
- 13. Pecori Giraldi F, Ambrogio AG, Fatti LM, et al. Von Willebrand factor and fibrinolytic parameters during the desmopressin test in patients with Cushing’s disease. Br J Clin Pharmacol. 2011;71(1):132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Pas R, de Bruin C, Leebeek FW, et al. The hypercoagulable state in Cushing’s disease is associated with increased levels of procoagulant factors and impaired fibrinolysis, but is not reversible after short-term biochemical remission induced by medical therapy. J Clin Endocrinol Metab. 2012;97(4):1303–1310. [DOI] [PubMed] [Google Scholar]
- 15. Karamouzis I, Berardelli R, D’Angelo V, et al. Enhanced oxidative stress and platelet activation in patients with Cushing’s syndrome. Clin Endocrinol (Oxf). 2015;82(4):517–524. [DOI] [PubMed] [Google Scholar]
- 16. Greenhill C. Pituitary disease: inflammation in patients with Cushing disease. Nat Rev Endocrinol. 2016;12(12):687. [DOI] [PubMed] [Google Scholar]
- 17. Stuijver DJ, van Zaane B, Feelders RA, et al. Incidence of venous thromboembolism in patients with Cushing’s syndrome: a multicenter cohort study. J Clin Endocrinol Metab. 2011;96(11):3525–3532. [DOI] [PubMed] [Google Scholar]
- 18. Van Zaane B, Nur E, Squizzato A, et al. Hypercoagulable state in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. 2009;94(8):2743–2750. [DOI] [PubMed] [Google Scholar]
- 19. Wagner J, Langlois F, Lim DST, McCartney S, Fleseriu M. Hypercoagulability and risk of venous thromboembolic events in endogenous Cushing’s syndrome: a systematic meta-analysis. Front Endocrinol (Lausanne). 2018;9:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koutroumpi S, Daidone V, Sartori MT, et al. Venous thromboembolism in patients with Cushing’s syndrome: need of a careful investigation of the prothrombotic risk profile. Pituitary. 2013;16(2):175–181. [DOI] [PubMed] [Google Scholar]
- 21. Semple PL, Laws ER Jr. Complications in a contemporary series of patients who underwent transsphenoidal surgery for Cushing’s disease. J Neurosurg. 1999;91(2):175–179. [DOI] [PubMed] [Google Scholar]
- 22. Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF. Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf). 2002;56(4):541–551. [DOI] [PubMed] [Google Scholar]
- 23. Flevas DA, Megaloikonomos PD, Dimopoulos L, Mitsiokapa E, Koulouvaris P, Mavrogenis AF. Thromboembolism prophylaxis in orthopaedics: an update. EFORT Open Rev. 2018;3(4):136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386–391. [DOI] [PubMed] [Google Scholar]
- 25. Nieman LK, Biller BM, Findling JW, et al. ; Endocrine Society Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bolland MJ, Holdaway IM, Berkeley JE, et al. Mortality and morbidity in Cushing’s syndrome in New Zealand. Clin Endocrinol (Oxf). 2011;75(4):436–442. [DOI] [PubMed] [Google Scholar]
- 27. Lambert JK, Goldberg L, Fayngold S, Kostadinov J, Post KD, Geer EB. Predictors of mortality and long-term outcomes in treated Cushing’s disease: a study of 346 patients. J Clin Endocrinol Metab. 2013;98(3):1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petersenn S, Newell-Price J, Findling JW, et al. ; Pasireotide B2305 Study Group High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin Endocrinol (Oxf). 2014;80(2):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giordano R, Picu A, Marinazzo E, et al. Metabolic and cardiovascular outcomes in patients with Cushing’s syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol (Oxf). 2011;75(3):354–360. [DOI] [PubMed] [Google Scholar]
- 30. van Haalen FM, Broersen LH, Jorgensen JO, Pereira AM, Dekkers OM. Management of endocrine disease: mortality remains increased in Cushing’s disease despite biochemical remission: a systematic review and meta-analysis. Eur J Endocrinol. 2015;172(4):R143–R149. [DOI] [PubMed] [Google Scholar]
- 31. Kastelan D, Dusek T, Kraljevic I, Aganovic I. Hypercoagulable state in Cushing’s syndrome is reversible following remission. Clin Endocrinol (Oxf). 2013;78(1):102–106. [DOI] [PubMed] [Google Scholar]
- 32. Guarnotta V, Amato MC, Pivonello R, et al. The degree of urinary hypercortisolism is not correlated with the severity of Cushing’s syndrome. Endocrine. 2017;55(2):564–572. [DOI] [PubMed] [Google Scholar]
- 33. Valassi E, Franz H, Brue T, et al. ; ERCUSYN Study Group Preoperative medical treatment in Cushing’s syndrome: frequency of use and its impact on postoperative assessment: data from ERCUSYN. Eur J Endocrinol. 2018;178(4):399–409. [DOI] [PubMed] [Google Scholar]
- 34. Coelho MC, Santos CV, Vieira Neto L, Gadelha MR. Adverse effects of glucocorticoids: coagulopathy. Eur J Endocrinol. 2015;173(4):M11–M21. [DOI] [PubMed] [Google Scholar]
- 35. Small M, Lowe GD, Forbes CD, Thomson JA. Thromboembolic complications in Cushing’s syndrome. Clin Endocrinol (Oxf). 1983;19(4):503–511. [DOI] [PubMed] [Google Scholar]
- 36. Babic B, De Roulet A, Volpe A, Nilubol N. Is VTE prophylaxis necessary on discharge for patients undergoing adrenalectomy for Cushing syndrome? J Endocr Soc. 2019;3(2):304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Pas R, Leebeek FW, Hofland LJ, de Herder WW, Feelders RA. Hypercoagulability in Cushing’s syndrome: prevalence, pathogenesis and treatment. Clin Endocrinol (Oxf). 2013;78(4):481–488. [DOI] [PubMed] [Google Scholar]
- 38. Boscaro M, Sonino N, Scarda A, et al. Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing’s syndrome. J Clin Endocrinol Metab. 2002;87(8):3662–3666. [DOI] [PubMed] [Google Scholar]
- 39. Barbot M, Daidone V, Zilio M, et al. Perioperative thromboprophylaxis in Cushing’s disease: what we did and what we are doing? Pituitary. 2015;18(4):487–493. [DOI] [PubMed] [Google Scholar]
- 40. Smith TR, Habib A, Rosenow JM, et al. Defensive medicine in neurosurgery: does state-level liability risk matter? Neurosurgery. 2015;76(2):105–113; discussion 113. [DOI] [PubMed] [Google Scholar]
- 41. Lang F, Gawaz M, Borst O. The serum- & glucocorticoid-inducible kinase in the regulation of platelet function. Acta Physiol (Oxf). 2015;213(1):181–190. [DOI] [PubMed] [Google Scholar]
- 42. Casonato A, Pontara E, Boscaro M, et al. Abnormalities of von Willebrand factor are also part of the prothrombotic state of Cushing’s syndrome. Blood Coagul Fibrinolysis. 1999;10(3):145–151. [DOI] [PubMed] [Google Scholar]
- 43. Manetti L, Bogazzi F, Giovannetti C, et al. Changes in coagulation indexes and occurrence of venous thromboembolism in patients with Cushing’s syndrome: results from a prospective study before and after surgery. Eur J Endocrinol. 2010;163(5):783–791. [DOI] [PubMed] [Google Scholar]
- 44. Fleseriu MBB, Swearingen AGB, Melmed S.. Hypercoagulability in Cushing’s disease: a risk awareness and prophylaxis survey on behalf of the Pituitary Society. The Pituitary Society website. 2017. https://www.pituitarysociety.org/sites/all/pdfs/15_Pituitary_Congress_program.pdf. Accessed July 26, 2019.
- 45. Zilio M, Mazzai L, Sartori MT, et al. A venous thromboembolism risk assessment model for patients with Cushing’s syndrome. Endocrine. 2016;52(2):322–332. [DOI] [PubMed] [Google Scholar]
- 46. Pace A, Dirven L, Koekkoek JAF, et al. ; European Association of Neuro-Oncology Palliative Care Task Force European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18(6):e330–e340. [DOI] [PubMed] [Google Scholar]