Abstract
The conclusions of EFSA following the peer review of the initial risk assessment carried out by the competent authority of the co‐rapporteur Member State the Czech Republic for the pesticide active substance sulfoxaflor are reported. The context of the peer review was that requested by the European Commission following the submission and evaluation of confirmatory ecotoxicology data. The conclusions were reached on the basis of the evaluation of the representative uses of sulfoxaflor as an insecticide on fruiting vegetables (field and greenhouse application), cucurbits (field and greenhouse application), spring and winter cereals (field application) and cotton (field application). The reliable endpoints concluded as being appropriate for use in regulatory risk assessment, derived from the available studies and literature in the dossier peer reviewed, are presented. For the field and non‐permanent structure greenhouses, a high risk to honeybees and bumble bees was identified related to some pertinent scenarios (treated crop scenario except after flowering period, weed scenario, field margin scenario). A low risk was concluded for honeybees, bumble bees and solitary bees in case of permanent structure greenhouse provided the low exposure in such scenarios.
Keywords: sulfoxaflor, peer review, bees, confirmatory data, risk assessment, pesticide, insecticide
Summary
Sulfoxaflor was approved in accordance with Regulation (EC) No 1107/2009, on 18 August 2015 by Commission Implementing Regulation (EU) No 2015/1295, amending the Annex to Commission Implementing Regulation (EU) No 540/2011. It was a specific provision of the approval that the applicant was required to submit to the European Commission further studies on:
-
a)
the risk to honey bees via the different routes of exposure, in particular nectar, pollen, guttation fluid and dust;
-
b)
risk to honey bees foraging in nectar or pollen in succeeding crops and flowering weeds;
-
c)
the risk to pollinators other than honey bees;
-
d)
the risk to bee brood.
by 18 August 2017.
In accordance with the specific provision, the applicant, Dow AgroSciences, submitted an updated dossier to the rapporteur Member State (RMS) Ireland, in August 2017. For the standard risk assessment, the recommendations of EFSA Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees) were applied by the applicant. The updated dossier was evaluated by the designated co‐rapporteur Member State (co‐RMS), the Czech Republic, on behalf of the RMS Ireland, in the form of an addendum to the draft assessment report. In compliance with guidance document SANCO 5634/2009‐rev. 6.1 (European Commission, 2013), the co‐RMS distributed the addendum to Member States, the applicant and the European Food Safety Authority (EFSA) for comments on 12 March 2018. The co‐RMS collated all comments in the format of a reporting table, which was submitted to EFSA on 2 July 2018. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table and finalised the related technical report on 27 July 2018.
Following consideration of the technical report, the European Commission requested EFSA to adopt conclusions on all the points where disagreements have been identified in the technical report and, where necessary, to organise a consultation of experts. The Commission also requested EFSA to take into consideration a recent publication related to the reproductive success of bumblebees as a result of sulfoxaflor exposure (Siviter et al., 2018).
The risk assessment for bees presented in the EFSA conclusion of 2014 has been amended considering the newly available laboratory and higher tier studies. Following the recommendations of the Pesticide Peer Review Meeting 133 (EFSA 2015), the co‐RMS evaluated the higher tier studies in light of the issues raised in EFSA (2013). It is noted that the tier 1 risk assessment according to (European Commission, 2002) remains unchanged compared to the previous conclusions reached during the peer review of the risk assessment of sulfoxaflor in 2014. The assessment of the higher tier studies was made using the latest state of knowledge on the topic, without diverging from the recommendations of the European Commission (2002). The risk assessment included some novel refinement steps on which divergent views were expressed by Member States during the commenting phase. Different opinions were also expressed in relation to the interpretation and the use of the available higher tier studies and as regards the consideration of risk mitigation measures for the use of sulfoxaflor. Following the discussions in the peer review meeting, it was concluded that for the field and non‐permanent structure greenhouses, a high risk to honeybees and bumble bees was identified related to some pertinent scenarios (treated crop scenario except after the flowering period, weed scenario, field margin scenario). Furthermore, the chronic risk assessments for bumble bees (adult and larvae) and solitary bees (acute and chronic) could not be finalised.
The representative uses on fruiting vegetables and cucurbits include uses in greenhouse. A low risk was concluded for honeybees, bumble bees and solitary bees in case of permanent structure greenhouse provided the low exposure in such situations.
Background
Sulfoxaflor was approved in accordance with Regulation (EC) No 1107/20091, on 18 August 2015 by Commission Implementing Regulation (EU) No 2015/12952, amending the Annex to Commission Implementing Regulation (EU) No 540/20113. The European Food Safety Authority (EFSA) previously finalised a Conclusion on this active substance on 12 May 2014 (EFSA, 2014) and a Technical Report on 27 July 2018 (EFSA, 2018d).
-
a)
the risk to honey bees via the different routes of exposure, in particular nectar, pollen, guttation fluid and dust;
-
b)
risk to honey bees foraging in nectar or pollen in succeeding crops and flowering weeds;
-
c)
the risk to pollinators other than honey bees;
-
d)
the risk to bee brood.
by 18 August 2017.
In accordance with the specific provision, the applicant, Dow AgroSciences, submitted an updated dossier to the rapporteur Member State (RMS) Ireland, in August 2017. For the standard risk assessment, the recommendations of EFSA Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees) (EFSA, 2013) were applied by the applicant. The updated dossier was evaluated by the designated co‐rapporteur Member State (co‐RMS), the Czech Republic, on behalf of Ireland, in the form of an addendum to the draft assessment report (Czech Republic, 2018). In compliance with European Commission (2013), the co‐RMS distributed the addendum to Member States, the applicant and EFSA for comments on 12 March 2018. The co‐RMS collated all comments in the format of a reporting table, which was submitted to EFSA on 2 July 2018. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table and finalised the related technical report on 27 July 2018 (EFSA, 2018d).
Following consideration of the technical report, the European Commission requested EFSA to adopt conclusions on all the points where disagreements have been identified in the technical report and, where necessary, to organise a consultation of experts. The Commission also requested EFSA to take into consideration a recent publication related to the reproductive success of bumblebees as a result of sulfoxaflor exposure (Siviter et al., 2018).
The addendum and the reporting table were discussed at the Pesticides Peer Review Meeting on ecotoxicology (PPR 188) in November 2018. Details of the issues discussed, together with the outcome of these discussions were recorded in the meeting report.
A final consultation on the conclusions arising from the peer review took place with Member States via a written procedure in February 2019.
The conclusions laid down in this report were reached on the basis of the peer review of the co‐RMS's evaluation of the confirmatory data submitted in relation to ecotoxicology data. A key supporting document to this conclusion is the peer review report, which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review. The peer review report (EFSA, 2019) comprises the following documents, in which all views expressed during the course of the peer review, including minority views, can be found:
the evaluation table (20 February 2019);
the report of the scientific consultation with the Member State experts;
the comments received on the draft EFSA conclusion.
Given the importance of the addendum to the draft assessment report (Czech Republic, 2018) and the peer review report, these documents are considered as background documents to this conclusion.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the European Union (EU) for which the applicant has not demonstrated to have regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Sulfoxaflor is the ISO common name for [methyl(oxo){1‐[6‐(trifluoromethyl)‐3‐pyridyl]ethyl}‐λ6‐sulfanylidene]cyanamide (IUPAC).
The representative formulated products for the evaluation were ‘GF‐2626’, an aqueous suspension concentrate (SC) containing 120 g/L sulfoxaflor (11.3% w/w) and ‘GF‐2372’, a water‐dispersible granule (WG) containing 500 g/kg sulfoxaflor. The representative uses evaluated comprise applications by foliar spraying to control sap feeding insects on fruiting vegetables, cucurbits, spring and winter cereals and cotton.
Conclusions of the evaluation
The following studies on confirmatory data have been submitted by the applicant under this evaluation: additional laboratory studies and higher tier studies investigating the exposure and the effects of sulfoxaflor on bees (honeybee and bumble bee).
Both (European Commission, 2002) and (EFSA, 2013) were used for the risk assessment, as both assessments were presented by the applicant. Several aspects of the confirmatory data were discussed with Member States experts at the Peer review Expert Meeting on Ecotoxicology (PPR 188) in November 2018.
The risk via dust drift was not assessed as this route of exposure is not relevant for the representative uses considered.
The representative uses on fruiting vegetables and cucurbits include uses in greenhouse. A low risk was concluded for honeybees, bumble bees and solitary bees when sulfoxaflor is used in permanent structure greenhouse, provided the low exposure for such situations.
The risk assessments presented below consider the use of sulfoxaflor in the field (fruiting vegetables, cucurbits, cotton, cereals) or in greenhouses with non‐permanent structures (fruiting vegetables and cucurbits).
Honeybees: Lower tier risk assessment (tier 1)
Sufficient acute toxicity data on honeybees were available for the active substance and the representative formulations. The risk assessment conducted according to European Commission (2002) indicated a high acute contact and acute oral risk to honeybees.
In addition to the acute data, appropriate chronic data were available for adult honeybees and larvae with the active substance. These data, together with the relevant acute data, were used in the tier 1 risk assessment for honeybees according to EFSA (2013). The calculations indicated a high risk for the majority of the scenarios. The details are included in Table 1.
Table 1.
Tier‐1 risk assessment to honeybees for the field uses and for the non‐permanent structure greenhouse uses
| Risk issue/scenario | Fruiting vegetables and cucurbits (BBCH 20–89) GF‐2626 | Cotton (BBCH 20–89) GF‐2372 | Winter and spring cereals (BBCH 40–89) GF‐2372 |
|---|---|---|---|
| Contact risk | |||
| Treated crop | High risk for application when the crop flowers, otherwise low risk | High risk for application when the crop flowers, otherwise low risk | High risk for application when the crop flowers, otherwise low risk |
| Weeds | High risk at BBCH < 50, low risk at BBCH ≥ 50 | High risk at BBCH < 50, low risk at BBCH ≥ 50 | Low risk |
| Field margin | Low risk | Low risk | Low risk |
| Oral dietary risk | |||
| Treated crop | High risk except application after the flowering | High risk except application after the flowering | High risk except application after the flowering |
| Weeds | High risk | High risk | High risk |
| Field margin | High risk | High risk | High risk |
| Adjacent crop | Low risk | Low risk | Low risk |
| Succeeding crop | High risk | High risk | High risk |
| Risk from water consumption | |||
| Guttation water | High risk | High risk | High risk |
| Puddle water | A reliable risk assessment was not available (data gap) | A reliable risk assessment was not available (data gap) | A reliable risk assessment was not available (data gap) |
| Surface water | Low risk | Low risk | Low risk |
It is noted that sublethal endpoints that could be used in tier 1 risk assessments were not available. However, sublethal effects were investigated and reported in pertinent higher tier studies (see further below).
Honeybees: Higher tier risk assessment (tier 2 and tier 3) ‐ contact risk and risk via pollen and nectar
Treated crop scenario
Exposure refinement
A number of residue data were available from four different crops for tier 2 risk assessments. These data indicated high initial residue levels in pollen and nectar; some of them were even higher than the default residue levels considered in tier 1 risk assessments (when expressed as residue per unit dose (RUD)). The time for residue decline from pollen and nectar could also be estimated from the available residue data. The co‐RMS has proposed to refine the time‐weighted average (TWA) value using these estimations. However, considering the uncertainties identified for these estimations (i.e. DT50 derivation), the experts at the meeting agreed that the information on the residue decline is not suitable to be used in a quantitative way.4 Nevertheless, it was acknowledged that the available data indicate a rather fast dissipation from pollen and nectar and this information can be taken into consideration qualitatively.
Effects refinement (lethal and sublethal effects)
A new semi‐field study and a colony feeder study were available.
In the semi‐field study, an evening spray application was performed on blooming phacelia. Regarding the study methodology, several shortcomings were noted and the observations on brood development were considered as unreliable.5 A statistically significant elevated adult mortality was observed on the first day after the application. Also, a clear pattern for sublethal effects (lower foraging activity, impaired locomotor activity) was observed which was attributed to the treatment with sulfoxaflor. As for the mortality, the sublethal effects were only short‐lived (mainly observed in the day after the evening application). No significant impact on other parameters including the colony strengths was identified. Overall, the study was considered to have limited statistical power being sensitive for demonstrating negligible effects. However, the co‐RMS indicated that semi‐field studies have usually less replications than those in this study (six replications).
In the colony feeder study, five concentrations were selected and for each concentration, five colonies were tested. A 200 mL spiked sugar solution per day was offered to each of the free‐flying colonies for 10 consecutive days. The experts at the meeting6 noted some shortcomings in the study and the reliability of the results of the brood development and of the overwintering was questioned. Nevertheless, the experts agreed that a no observed effect concentration (NOEC) of 0.5 mg/kg (nominal) based on the colony strength and the mortality in front of the hive can be derived from this study7.
The co‐RMS in their risk assessment proposed to compare the residue levels from the residue trials with the NOEC of 0.5 mg/kg from the colony feeder study. For this comparison, not only the residue levels found in pollen and nectar from the tested crops, but also the residue levels from comb matrices (pollen and nectar from comb, worker jelly, larva, pupa) were taken into consideration. This comparison between concentrations was discussed during the experts’ meeting but it was not considered to be appropriate since it did not reflect on the actual dose consumed by the bees. In addition, the agreed NOEC did not cover potential effects on brood development. Furthermore, the clear temporal effects that were seen in the semi‐field study (with realistic application rate) should also be taken into consideration. Overall, it was considered that a low risk could not be demonstrated by these higher tier studies. A high risk was concluded for the situations when sulfoxaflor is used in flowering stage of the representative crops (regardless if it was an evening application).
In the treated crop scenario, the co‐RMS has suggested that a low risk could be concluded for the situations when sulfoxaflor is used at least 5 days before the flowering period starts. For this conclusion, several aspects including semi‐field trials submitted for the previous peer‐review (EFSA, 2014) were considered (in these trials preflowering applications were applied). However, several shortcomings were identified in the previous peer‐review regarding these semi‐field trials. Overall, it was agreed that the studies and assessment that had been presented were not sufficient to demonstrate that a risk to honeybees can be excluded for applications made before the flowering period. The experts expressed the opinion that it is reasonable likely that the risk identified for the treated crop may be mitigated by restricting applications to a certain number of days before flowering. However, robust data were not available to quantify such value.
Weed scenario
A refined risk assessment has not been presented for the weed scenario. However, previous experts’ discussions and conclusions on the relevance of the weed scenario were quoted (EFSA, 2016a,b). On this basis, a low risk regarding the representative use in cereals was concluded. For vegetables and cotton previous assessments were not available, therefore, a low risk for these representative uses could not be concluded. Nevertheless, it is noted that long‐term exposure of bees may not be expected considering the available information on the residue decline (see above). The co‐RMS has proposed that some risk mitigation measures could be applied (i.e. label proposals: ‘Do not apply when flowering weeds are present/Remove weeds before flowering.’). Concluding on the appropriateness, feasibility and practicability of such mitigation measures is outside the scope of this assessment. Nevertheless, situations where flowering weeds are not present in the field would result in a low risk to bees.
Field margin scenario
A refined risk assessment has not been presented for the field margin scenario. Therefore, a low risk for the situations when honeybees are foraging on field margin vegetation was not demonstrated. Nevertheless, it is noted that long‐term exposure of bees may not be expected considering the available information on the residue decline (see above). It is further noted that based on the first tier risk assessments, it is reasonable likely that the risk identified for this scenario could be mitigated by spray drift reducing measures (i.e. tier 1 exposure toxicity ratio (ETR) values were breached by about 30%).
Succeeding crop scenario
A higher tier residue study simulating the exposure of the following crops via contaminated soil was available (summer oilseed rape as test crop at four sites in Germany). No residues were detected (limit of detection (LOD) 0.0003 mg/kg) in pollen and nectar of the test crops in this study. Considering this and that sulfoxaflor has a relatively short half‐life in soil (EFSA, 2014), a low risk to bees for the succeeding crop scenario was concluded by the experts at the meeting.8
Honeybees: Risk from exposure via consumption of guttation water
As regards guttation, a higher tier field study was available on summer oilseed rape as test crop over sprayed by sulfoxaflor (48 g/ha) at early crop stage (BBCH 11–12). Exposure and potential effects of honeybee colonies were studied. Relatively frequent guttation occurred, but practically no bees were observed collecting guttation liquid. Indeed, no apparent effects on the honeybee colonies located at the vicinity of the test field were observed. The study was supported with residue measurements of different bee relevant matrices. These data confirmed the low exposure of the colonies via this route of exposure. Some shortcomings of the study were also noted (i.e. low number of test colonies used, unknown spatial–temporal representativeness of the study, only the interim report was available).
Overall, considering the information above, the properties of the active substance and the representative Good Agricultural Practices (GAPs) of sulfoxaflor, as well as previous experts’ discussions and conclusions for guttation (EFSA, 2016a,b, 2018a,b,c), a low relevance for this route of exposure was concluded, hence the risk from guttation was considered as low for the representative uses.
Bumble bees: Lower tier risk assessment (tier 1)
Only acute contact and acute oral toxicity data were available for bumble bees and a data gap was set for chronic data (adults and larvae). These data demonstrated that sulfoxaflor has a comparable or lower toxicity to bumble bees than for honeybees. The screening assessment according to EFSA (2013) indicated a low contact risk and a high acute oral risk. Tier 1 risk assessments resulted in a low risk for the field margin and the adjacent crop scenarios for all the representative uses. However, the calculations resulted in a high risk for the other tier 1 scenarios (except for the treated crop scenario after the flowering period).
Bumble bees: Higher tier risk assessment – risk via pollen and nectar (tier 3)
Treated crop scenario
A greenhouse study was available where an evening spray application was performed on flowering tomato. The introduced bumble bee colonies were closed into their hives for more than a whole day after the spray application in order to decrease their potential exposure. No apparent effects were recorded on the foraging activity of the bumble bee colonies or on other parameters. However, the experts agreed that this study was not suitable for the risk assessment of wild (i.e. non‐managed) bumble bees.9 Therefore, a low risk to bumble bees could not be demonstrated.
A relevant, recently published colony‐feeder study from the open literature was also considered (Siviter et al., 2018), and a detailed discussion took place during the Peer review Experts’ Meeting.10 Some drawbacks regarding the methodology were noted (e.g. the exact formulation was unknown, colonies from wild caught queens were used, high disease/parasite infection rates were recorded in this queen population, several observations of the test colonies were performed at different time slots). Nevertheless, three parameters (endpoints) measured in this study were considered as directly relevant for the risk assessments for bumble bees and are explained below.
For the parameter of the number of worker bees (considered as a reliable endpoint with minor restrictions), the study authors concluded an effect. However, the experts at the meeting considered that the results did not indicate a treatment related effect given that the numbers were similar in both the control and treatment.
As regards to the male production (considered as a reliable endpoint with minor restrictions), the experts noted a high variability and no clear pattern of effects. Some significant differences from the control were observed only when the colonies were already in decline. However, the experts expressed their view that the pattern of production should also be accounted for. On the other hand, it was also acknowledged that for a more robust conclusion a detailed analysis of the raw data would be needed.
The parameter for the queen (gyne) production was not considered to be reliable.
Some other parameters (less relevant for the risk assessments) were also discussed, but it was considered that those do not indicate a clear treatment related effect.
Overall, the experts agreed that when considering the relevant and reliable endpoints, there were no clear treatment related effects observed in this study. However, the experts also expressed the view that the study cannot be used to demonstrate lack of effects on bumble bees for the reasons discussed above. Therefore, this study does not change the outcome of the lower tier risk assessments.
Weed scenario
No refined risk assessment has been presented for the weed scenario. However, based on assessments for the weed scenario under the risk assessments for honeybees above, a low risk was concluded for the representative use on cereals. A low risk for the representative uses on vegetables and cotton could not be concluded. For further considerations for the weed scenario, refer to the weed scenario section under the risk assessments for honeybees.
Succeeding crop scenario
Based on the assessments for the succeeding crop scenario under the risk assessments for honeybees above, a low risk was concluded for this scenario.
Risk assessment for solitary bees
No data and risk assessments were available for solitary bees. Therefore, a low risk for solitary bees could not be concluded for field uses and for uses in non‐permanent structure greenhouses (data gap).
Risk assessments for metabolites
Acute oral toxicity data for honeybees were available for pertinent plant metabolites and for metabolites occurring in soil and water. These laboratory tests indicated lower toxicity to bees from the metabolites compared with sulfoxaflor. The available risk assessments using these data (considering both the European Commission (2002) and EFSA (2013)) indicated a low acute risk to bees of these metabolites. Chronic data for honeybees (on adults and on larva) and data on non‐Apis bees were not available, however it is acknowledged that this was not part of the confirmatory data requirements.
Data gaps
This is a list of data gaps identified in the focussed peer review process of confirmatory data. Data gaps identified in the previously finalised EFSA conclusion on the active substance (EFSA, 2014) that were not part of the focussed peer review process of confirmatory data remain unchanged.
Reliable risk assessments for the risk via consumption of contaminated puddle water (relevant for all field uses and uses in non‐permanent structure greenhouses).
Chronic data (adult and larvae) and related risk assessments for bumble bees (relevant for all field uses and uses in non‐permanent structure greenhouses).
Data and risk assessments for solitary bees (relevant for all field uses and uses in non‐permanent structure greenhouses).
Further data to address the chronic risk to honeybees and the acute and chronic risk to non‐Apis bees from pertinent metabolites (relevant for all field uses and uses in non‐permanent structure greenhouses, however it is acknowledged that this was not part of the confirmatory data requirements).
Concerns
1. Issues that could not be finalised
An issue is listed as an issue that could not be finalised where there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the Uniform Principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/201111, and where the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
The chronic risk assessments (adult and larvae) for bumble bees could not be finalised.
The acute and chronic risk assessments for solitary bees could not be finalised in the absence of any data and risk assessments.
2. Critical areas of concern
An issue is listed as a critical area of concern where there is enough information available to perform an assessment for the representative uses in line with the Uniform Principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and where this assessment does not permit to conclude that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern where the assessment at a higher tier level could not be finalised due to lack of information, and where the assessment performed at the lower tier level does not permit to conclude that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
No critical area of concern identified based on the representative uses evaluated.
3. Overview of the concerns identified for each representative use considered (Table 2)
Table 2.
Overview of concerns
| Representative use | Fruiting vegetable, cucurbits Field and non‐permanent structure greenhouses | Fruiting vegetable, cucurbits Permanent structure greenhouses | Cotton | Spring and winter cereals | |
|---|---|---|---|---|---|
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | Xa , b , c | Xa , b , c | Xa , c | |
| Assessment not finalised | X1,2 | X1,2 | X1,2 | ||
The superscript numbers relate to the numbered points indicated in the Concerns Section.
Risk identified for the treated crop scenario before and during the flowering period for honeybees and bumble bees.
Risk identified for the weed scenario for honeybees and bumble bees, based on tier 1.
Risk identified for the field margin scenario for honeybees, based on tier 1.
Abbreviations
- DT50
period required for 50% dissipation (define method of estimation)
- EEC
European Economic Community
- ETR
exposure toxicity ratio
- GAP
Good Agricultural Practice
- InChiKey
International Chemical Identifier Key
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- LOD
limit of detection
- NOEC
no observed effect concentration
- RMS
rapporteur Member State
- RUD
residue per unit dose
- SC
suspension concentrate
- SMILES
simplified molecular‐input line‐entry system
- TWA
time‐weighted average
- WG
water‐dispersible granule
Appendix A – List of endpoints for the active substance and the representative formulation
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2019.5633
Appendix B – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
| Sulfoxaflor |
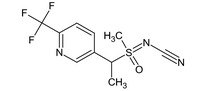
[methyl(oxo){1‐[6‐(trifluoromethyl)‐3‐pyridyl]ethyl}‐λ6‐sulfanylidene]cyanamide FC(F)(F)c1ccc(cn1)C(C)S(C)(=O)=NC#N ZVQOOHYFBIDMTQ‐UHFFFAOYSA‐N |
|
| X11719474 |
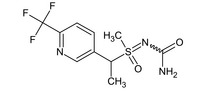
1‐[(RS)‐methyl(oxido){(1RS)‐1‐[6‐(trifluoromethyl)‐3‐pyridinyl]ethyl}‐λ6‐sulfanylidene]urea FC(F)(F)c1ccc(cn1)C(C)S(C)(=O)=NC(N)=O YLQFVPNHUKREEW‐UHFFFAOYSA‐N |
|
| X11721061 |
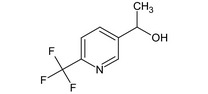
(1RS)‐1‐[6‐(trifluoromethyl)‐3‐pyridinyl]ethanol FC(F)(F)c1ccc(cn1)C(C)O JGVSFNXTWYOUFV‐UHFFFAOYSA‐N |
|
| X11519540 |
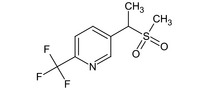
5‐[(1RS)‐1‐(methylsulfonyl)ethyl]‐2‐(trifluoromethyl)pyridine FC(F)(F)c1ccc(cn1)C(C)S(C)(=O)=O HTDSUIIOMMBPMN‐UHFFFAOYSA‐N |
|
| X11579457 |
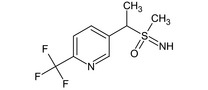
5‐[(1RS)‐1‐(S‐methylsulfonimidoyl)ethyl]‐2‐(trifluoromethyl)pyridine FC(F)(F)c1ccc(cn1)C(C)S(C)(=N)=O SDEFOCWEOKCRJR‐UHFFFAOYSA‐N |
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2017.2.1 ACD/Labs 2017 Release (File version N40E41, Build 96719, 6 September 2017).
ACD/ChemSketch 2017.2.1 ACD/Labs 2017 Release (File version C40H41, Build 99535, 14 February 2018).
Supporting information
List of endpoints for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Abdourahime H, Arena M, Auteri D, Barmaz S, Ctverackova L, De Lentdecker C, Ippolito A, Kardassi D, Messinetti S, Molnar T, Saari KE, Sharp R, Streissl F, Sturma J, Szentes C, Tiramani M, Vagenende B, Van Dijk J and Villamar‐Bouza L, 2019. Conclusion on the peer review of the pesticide risk assessment for the active substance sulfoxaflor in light of confirmatory data submitted. EFSA Journal 2019;17(3):5633, 14 pp. 10.2903/j.efsa.2019.5633
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00709
Approved: 26 February 2019
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) 2015/1295 of 27 July 2015 approving the active substance sulfoxaflor, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011.OJ L 199, 29.7.2015, p. 8–11.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p.1–186.
Refer to experts’ consultation 1 in the Report of Pesticides Peer Review experts’ meetings 188 (EFSA, 2019).
Refer to experts’ consultation 2 in the Report of Pesticides Peer Review experts’ meetings 188 (EFSA, 2019).
Refer to experts’ consultation 3 in the Report of Pesticides Peer Review experts’ meetings 188 (EFSA, 2019).
Refer to experts’ consultation 4 in the Report of Pesticides Peer Review experts’ meetings 188 (EFSA, 2019).
Refer to experts’ consultation 5 in the Report of Pesticides Peer Review experts’ meetings 188 (EFSA, 2019).
Refer to experts’ consultation 6 in the Report of Pesticides Peer Review experts’ meetings 188 (EFSA, 2019).
Refer to experts’ consultation 7 in the Report of Pesticides Peer Review experts’ meetings 188 (EFSA, 2019).
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Czech Republic , 2018. Revised Addendum to the assessment report on sulfoxaflor in light of confirmatory data, October 2018, as revised in December 2018.
- EFSA (European Food Safety Authority), 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp., 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Conclusion on the peer review of the pesticide risk assessment of the active substance sulfoxaflor. EFSA Journal 2014;12(5):3692, 170 pp. 10.2903/j.efsa.2014.3692 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Technical report on the outcome of the pesticides peer review meeting on general recurring issues in ecotoxicology. EFSA Supporting Publication 2015;12(12):EN‐924. 62 pp. 10.2903/sp.efsa.2015.en-924 [DOI]
- EFSA (European Food Safety Authority), 2016a. Conclusion on the peer review of the pesticide risk assessment for the active substance clothianidin in light of confirmatory data submitted. EFSA Journal 2016;14(11):4606, 34 pp. 10.2903/j.efsa.2016.4606 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016b. Conclusion on the peer review of the pesticide risk assessment for the active substance imidacloprid in light of confirmatory data submitted. EFSA Journal 2016;14(11):4607, 39 pp. 10.2903/j.efsa.2016.4607 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2018a. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance clothianidin considering the uses as seed treatments and granules. EFSA Journal 2018;16(2):5177, 86 pp. 10.2903/j.efsa.2018.5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018b. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance imidacloprid considering the uses as seed treatments and granules. EFSA Journal 2018;16(2):5178, 113 pp. 10.2903/j.efsa.2018.5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018c. Conclusions on the peer review of the pesticide risk assessment for bees for the active substance thiamethoxam considering the uses as seed treatments and granules. EFSA Journal 2018;16(2):5179, 59 pp. 10.2903/j.efsa.2018.5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018d. Technical report on the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for sulfoxaflor in light of confirmatory data. EFSA supporting publication 2018:15(9):EN‐1474, 73 pp. 10.2903/sp.efsa.2018.en-1474 [DOI]
- EFSA (European Food Safety Authority), 2019. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment for the active substance sulfoxaflor in light of confirmatory data submitted. Available online: http://www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- European Commission , 2002. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002
- European Commission , 2013. Guidance document on the procedures for submission and assessment of confirmatory information following approval of an active substance in accordance with Regulation (EC) No 1107/2009. SANCO 5634/2009‐rev. 6.1.
- Siviter H, Brown JF and Leadbeater E, 2018. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature, 561, 109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of endpoints for the active substance and the representative formulation


