Abstract
In this opinion, the EFSA Panel on Food Additives and Flavourings (FAF Panel) was requested by the European Commission to carry out a scientific evaluation of an extended one‐generation reproductive toxicity study (EOGRTS) to determine whether it would allow reconsideration of the temporary group acceptable daily intake (ADI) for sorbic acid (E 200) and potassium sorbate (E 202), established by the Panel on Food Additives and Nutrient Sources added to Food (ANS Panel) in 2015. From the EOGTRS, the FAF Panel identified a lower confidence limit of the benchmark dose (BMDL) of 1,110 mg sorbic acid/kg body weight (bw) per day. By applying a default uncertainty factor of 100, the Panel established a group ADI expressed as 11 mg sorbic acid/kg bw per day for sorbic acid (E 200) and its potassium salt (E 202). In addition, European Commission asked EFSA to review a report on the ‘Stability of sorbic acid (E 200) and its potassium salt (E 202) during food processing and storage’ provided by industry. No new information was provided in this report, and therefore, in this opinion, there was no re‐assessment of the EFSA ANS opinion conclusions from 2015 regarding the stability of sorbates in food.
Keywords: sorbic acid, E200, potassium sorbate, E202, EOGRTS, BMDL, BMR
Summary
In this opinion, the EFSA Panel on Food Additives and Flavourings (FAF Panel) was requested to carry out a scientific evaluation of an extended one‐generation reproductive toxicity study (EOGRTS) to determine whether it would allow reconsideration of the temporary group acceptable daily intake (ADI) for sorbic acid (E 200) and potassium sorbate (E 202), established by the Panel on Food Additives and Nutrient Sources added to Food (ANS Panel) in 2015.
The FAF Panel was also asked to review a report on the ‘Stability of sorbic acid (E 200) and its potassium salt (E 202) during food processing and storage’ provided by industry. In this report, the available published studies on the stability, and possible degradation products, of sorbic acid and potassium sorbate in model food systems and in foods during storage were summarised. The Panel considered that no new relevant information was provided on the occurrence and toxicity of breakdown and reaction products of sorbic acid and its potassium salt in food under realistic conditions of processing and storage, as compared to the information evaluated in the previous opinion (EFSA ANS Panel, 2015) and therefore did not re‐assess previous conclusions of the 2015 ANS Panel opinion.
The EOGRTS was performed in compliance with Good Laboratory Practice (GLP). Sorbic acid was administered to CD/Crl:CD rats at doses of 0, 15,000, 30,000 and 60,000 mg/kg diet (approximately equal to 0, 1,000, 2,000 and 4,000 mg sorbic acid/kg body weight (bw) per day) following Organisation for Economic Co‐operation and Development (OECD, 1998) TG 443 regarding cohorts 1A and 1B (for testing reproductive toxicity); cohorts 2 (neurotoxicity) and 3 (immunotoxicity) were not included in the study.
In summary, the FAF Panel noted that various effects were observed in the study:
in reproductive parameters (decreased weights of ovaries and uterus, including cervix and oviduct of F1 females of cohort 1B) at the high‐dose group;
decreases in body weight gain in the parental F0 females of the mid‐ and high‐dose groups during gestation and lactation and in the F1A males of mid‐ and high‐dose group. No decreases were observed in F1A and B (until pairing) any dose level. Body weights were decreased in the high‐dose F1 females of cohort B during gestation and lactation;
decreases in food intake of F0 and F1 generations in the mid‐ and high‐dose groups;
increases in plasma cholesterol levels in the parental F0 females of the mid‐ and high‐dose groups and in the F1A males and females of the high‐dose group;
decreases in F2 pup body weight gain were observed in the mid‐ and high‐dose groups and in F1 offspring in the high‐dose group of both sexes.
increases in liver weight in F0 and F1A male animals and decreases in mean absolute and relative weight of ovaries and uterus, including cervix and oviduct in F1B females at the highest dose tested.
In the present dietary EOGRTS, the effect on anogenital distance in pups observed in a two‐generation reproductive toxicity study assessed previously (EFSA ANS Panel, 2015) was not confirmed and therefore not considered relevant for the risk assessment. The delay in functional development in F1 pups of the high‐dose group (3,000 mg/kg bw per day administered by gavage), also observed previously, was not tested in the present EOGRTS. Finally, neurodevelopmental effects [reduced reflexes for the auditory startle reflex (in both F1 males and females) and for the mid‐air righting reflex (in females)] were previously observed at the highest dose of 3,000 mg/kg bw per day in the two‐generation reproductive toxicity study. In the current EOGRTS, cohorts 2 (for testing neurotoxicity) and 3 (for testing immunotoxicity) were not included, and therefore, these endpoints were not tested.
The FAF Panel considered decreases in body weight gains in the pups of the F2 generation, as the most biologically relevant endpoint in order to establish the reference point and performed benchmark dose (BMD) analysis (annex I). The Panel selected the value of 1,110 mg sorbic acid/kg bw per day derived as the lower confidence limit of the benchmark dose (BMDL) from the data on body weight gains in pups from the F2 generations of both sexes on days 1–21. The omission of cohorts 2 and 3 of the EOGRTS was considered not to compromise the outcome of the assessment since the neurodevelopmental effects of the earlier study were observed only at dose levels higher than the BMDL.
The BMDL of 1,110 mg sorbic acid/kg bw per day was used to allocate a new group ADI for sorbic acid and its potassium salt. By applying a default uncertainty factor of 100, the Panel established a group ADI expressed as 11 mg sorbic acid/kg bw per day for sorbic acid (E 200) and its potassium salt (E 202).
The FAF Panel considered that the most realistic approach using reported use levels and analytical data was in the non‐brand‐loyal scenario as already stated in the previous opinion (EFSA ANS Panel, 2015). In the non‐brand loyal scenario, the highest estimates of the exposure resulting from the use of sorbic acid (E 200), potassium sorbate (E 202) including calcium sorbate (E 203) as calculated by the ANS Panel (EFSA ANS Panel, 2015) were 1.7 mg sorbic acid‐sorbates/kg bw per day at the mean and 3.7 mg sorbic acid‐sorbates/kg bw per day at the highest (95th) levels for children (3–9 years old).
The uncertainties identified previously (EFSA ANS Panel, 2015) would generally lead to an overestimate of the real exposure to sorbic acid–sorbates (E 200, E 202 and E 203) as food additives.
Calcium sorbate (E 203) has been withdrawn from the lists of food additives (Commission Regulation (EU) 2018/98 of 22 January 2018). The Panel noted that there is uncertainty on to which extent the possible replacement of calcium sorbate (E 203) by sorbic acid or potassium sorbate will affect the calculations of the exposure assessment. However, the Panel considered that this uncertainty will not change significantly the previously calculated estimates and the conclusion that they are an overestimation of the real exposure to sorbic acid (E 200) and its potassium salt (E 202).
1. Introduction
In this opinion, the European Food Safety Authority (EFSA) Panel on Food Additives and Flavourings (FAF Panel) was requested by the European Commission to carry out a scientific evaluation of a new extended one‐generation reproductive toxicity study (EOGRTS) to determine whether it would allow reconsideration of the temporary group acceptable daily intake (ADI) for sorbic acid (E 200) and potassium sorbate (E 202), established by the Panel on Food Additives and Nutrient Sources added to Food (ANS Panel) in 2015. The EOGRTS has been performed following the conclusions and recommendations of the Scientific Opinion on the re‐evaluation of sorbic acid (E 200), potassium sorbate (E 202) and calcium sorbate (E 203) as food additives by the ANS Panel of the EFSA published in 2015 (EFSA ANS Panel, 2015).
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/2008 on food additives.1 Only food additives that are included in the Union list, in particular in Annex II to that regulation, may be placed on the market and used in foods under the conditions of use specified therein. Moreover, food additives shall comply with the specifications as referred to in Article 14 of that Regulation and laid down in Commission Regulation (EU) No 231/20122.
Sorbic acid (E 200) and potassium sorbate (E 202) are authorised for use as a food additives in the Union. Since sorbic acid (E 200) and potassium sorbate (E 202) were permitted in the Union before 20 January 2009, they belong to the group of food additives which are subject to a new risk assessment by the European Food Safety Authority (EFSA), according to Commission Regulation (EU) No 257/20103, and in line with the provisions of Regulation (EC) No 1333/2008.
EFSA completed the re‐evaluation of sorbic acid (E 200) and potassium sorbate (E 202) as food additives and published a Scientific Opinion in June 20151. In that opinion, EFSA concluded that the available dataset on reproductive and developmental toxicity gives a reason to revise the group ADI of 25 mg/kg bw per day allocated by the Scientific Committee for Food (SCF) in 1996. EFSA considered that the no observed adverse effect level (NOAEL) of 300 mg sorbic acid/kg bw per day from a two‐generation reproductive toxicity study in rats can be used to allocate a temporary group ADI for sorbic acid and its potassium salt. By applying an uncertainty factor of 100, EFSA established a new temporary group ADI, expressed as 3 mg sorbic acid/kg bw per day, for sorbic acid (E 200) and potassium sorbate (E 202). The Panel considered that an extended one‐generation reproductive toxicity study in rats including the second generation by diet is needed to reconsider the temporary group ADI.
Therefore, the European Commission published on 10 June 2016 a call for data4 addressing the recommendations made by EFSA in the Scientific Opinion on the re‐evaluation of sorbic acid (E 200), potassium sorbate (E 202) and calcium sorbate (E 203) as food additives, which led to the submission on 3 May 2018 of new data of reproductive toxicity for sorbic acid (E 200) and potassium sorbate (E 202) by a consortium of business operators. In addition, the Commission also received a report on the “Stability of sorbic acid (E 200) and its potassium salt (E 202) during food processing and storage”, an area for which EFSA recommended additional research in the re‐evaluation scientific opinion.
Consequently, the European Commission has decided to consult EFSA of this matter.
1.1.2. Terms of Reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/20025, the European Commission requests the European Food Safety Authority (EFSA) to provide a scientific opinion in relation to a new reproductive toxicity study for sorbic acid (E 200) and potassium sorbate (E 202). In particular, EFSA is requested to carry out a scientific evaluation of this study to determine whether it would allow EFSA to reconsider the temporary group ADI for sorbic acid (E 200) and potassium sorbate (E 202) established by EFSA in 2015. In addition, EFSA is requested to review a report on the “Stability of sorbic acid (E 200) and its potassium salt (E 202) during food processing and storage”.
1.1.3. Interpretation of Terms of Reference
In the opinion of the ANS Panel on the re‐evaluation of sorbic acid (E 200), potassium sorbate (E 202) and calcium sorbate (E 203) as food additives (EFSA ANS Panel, 2015) the Panel recommended:
genotoxicity studies on calcium sorbate need to be performed in order to consider including calcium sorbate in the group ADI;
an extended one‐generation reproductive toxicity study in rats including the second generation by diet needs to be performed in order to reconsider the temporary group ADI;
if divalent transition metals are used as catalysts in the manufacturing process of sorbic acid, maximum residual levels of divalent transition metals should be included in the EC specifications for sorbic acid (E 200);
the maximum limits for the impurities of toxic elements (lead, mercury and arsenic) in the EC specification for sorbic acid and its salts (E 200, E 202, E 203) should be revised in order to ascertain that sorbic acid–sorbates (E 200, E 202, E 203) as food additives will not be a significant source of exposure to those toxic elements in food;
future research be performed on the occurrence of breakdown and reaction products of possible toxicological concern under realistic conditions of food processing and storage, especially when sorbic acid, potassium sorbate or calcium sorbate is used in parallel with ascorbic acid in the presence of iron salts or with nitrites.
In accordance with the outcome of the step 26 of the call for data on sorbic acid–sorbates (E 200‐E 203)4 of the European Commission (EC) approach for the follow‐up of EFSA's scientific opinions on the re‐evaluation of the safety of permitted food additives for which some concerns have been identified7 no business operator has committed to generating the requested genotoxicity data for calcium sorbate and therefore this substance cannot be further assessed. In line with the Commission Regulation (EU) 2018/98 of 22 January 2018 the entry for food additive E 203 calcium sorbate has been deleted in the Annexes II and III to Regulation (EC) No 1333/2008 and in the Annex to Regulation (EU) No 231/2012.
An extended one‐generation reproductive toxicity study (EOGRTS) in rats was provided to EFSA and has been assessed in this opinion by the current EFSA Panel on Food Additives and Flavourings (FAF Panel) in order to reconsider the temporary group ADI set by the EFSA ANS Panel (2015) (EFSA ANS Panel, 2015).
The EC did not request further assessment on the maximum residual levels for divalent transition metals and impurities of toxic elements. Therefore, the EFSA FAF Panel did not evaluate the specifications of sorbic acid (E 200) and potassium sorbate (E 202).
EFSA was provided with a report on the ‘Stability of sorbic acid (E 200) and its potassium salt (E 202) during food processing and storage’ which has been evaluated by the EFSA FAF Panel in the present opinion.
The safety of potassium resulting from the use of potassium sorbate is not the focus of this opinion.
1.2. Summary of the previous EFSA re‐evaluation of sorbic acid (E 200) and potassium sorbate (E 202) as food additives (EFSA ANS Panel, 2015)
Sorbic acid (E 200) and potassium sorbate (E 202) [together with calcium sorbate (E 203)] had previously been authorised as food additives in accordance to Annex II and Annex III of Regulation (EC) No 1333/2008 following evaluations by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1974 and by the Scientific Committee on Food (SCF) in 1996. These bodies had allocated a group ADI of 25 mg/kg body weight (bw) per day expressed as sorbic acid, covering sorbic acid, sodium sorbate, potassium sorbate and calcium sorbate (JECFA, 1974). This ADI was based on a no observed adverse effect level (NOAEL) of 5% in the diet of rats in a long‐term study, equivalent to 2,500 mg/kg bw per day.
The ANS Panel considered that data available on the absorption, distribution, metabolism and excretion of sorbic acid showed that it is absorbed and mainly excreted as expired carbon dioxide. Potassium sorbate might dissociate into its constituents – potassium and sorbate ions – in the small intestine. Accordingly, sorbate from potassium sorbate should be bioavailable and absorbed in the same manner as from sorbic acid. The potassium ions are expected to enter normal homoeostatic processes and are not expected to have an impact on the toxicity of the salts. Thus, the properties of the cations were not discussed further.
Short‐term and subchronic toxicity studies performed in rats and mice did not show any adverse effects of sorbic acid at the concentrations tested (up to 9,200 mg/kg bw per day in rats). Sorbic acid and potassium sorbate were tested in in vitro and in vivo genotoxicity assays. Overall, the Panel considered that the database was sufficiently robust and that there was no evidence of genotoxic activity for sorbic acid or potassium sorbate.
Five long‐term/carcinogenicity toxicity studies performed before 1976 were available; no more recent studies were identified in the literature at the time.
The Panel revisited all the relevant original reports and publications, except the Lang et al. (1967) study used to allocate the ADI by JECFA (1974), which was not available. A Good Laboratory Practice (GLP) compliant two‐generation reproductive toxicity study was performed in CD/Crl:CD rats in accordance with the Organisation for Economic Co‐operation and Development (OECD) Guideline 416 (EFSA ANS Panel, 2015). In this study with doses of 0, 300, 1,000 or 3,000 mg/kg bw per day administered by gavage, adverse effects in pups were described. The effects included a decrease in anogenital distance in male F2 pups in the mid‐ and high‐dose groups. In the highest dose tested, decreased mean litter weight, delay in milestones of physical development and a delay in functional development in F1 pups auditory startle reflex (in both F1 males and females) and the mid‐air righting reflex (in F1 females) were observed. No other effects on fertility, sex ratio or reproductive organs were observed (EFSA ANS Panel, 2015). In a developmental toxicity study performed in rabbits in accordance with OECD Guideline 414 and GLP at doses of 0, 300, 1,000 or 3,000 mg sorbic acid/kg bw per day by gavage from day 6 to day 29 of gestation, maternal and foetal toxicity were observed in the mid‐ and high‐dose groups. The Panel considered that the maternal NOAEL was 300 mg sorbic acid/kg bw per day and the NOAEL for the foetuses was also 300 mg sorbic acid/kg bw per day. The Panel concluded that the data set on reproductive and developmental toxicity at the time gave a reason to revise the group ADI of 25 mg/kg bw per day set by the SCF in 1996. By applying an uncertainty factor of 100, the Panel established a new temporary group ADI expressed as 3 mg sorbic acid/kg bw per day for sorbic acid (E 200) and potassium sorbate (E 202).
Sorbic acid is an unsaturated aliphatic straight‐chain monocarboxylic fatty acid, 2,4‐hexadienoic acid of which the reaction with the carboxyl group yields salts and esters. The conjugated double bond makes it susceptible to nucleophilic attack, with sulfite ion and amines among the nucleophiles that can react with sorbic acid (Khandelwal and Wedzicha, 1990). Therefore, sorbic acid and sorbates can react with different food constituents. The Panel noted that the main compounds resulting from degradation of sorbic acid are carbonyl compounds, including crotonaldehyde, malondialdehyde, acrolein, formic acid, malonic acid, fumaraldehydic acid, 2‐methyl‐5 acetyl furan and others (Thakur et al., 1994).
The Panel noted that potential reaction products that may result from the interaction of sorbic acid with nitrites and with ascorbic acid in the presence of iron salts were demonstrated to be mutagenic in vitro and that there are certain food categories for which the use of these food additives (sorbic acid with ascorbic acid in the presence of iron salts or sorbic acid with nitrites) is permitted in parallel. However, the formation of these reaction products has been shown under optimal experimental conditions in an aqueous environment only, and may not be formed to any major extent in food matrices. The Panel also noted that the major reaction products resulting from the interaction of sorbic acid with different amines (e.g. methylamine, ethylamine, propylamine, butylamine and benzylamine) were not mutagenic in the bacterial reverse mutation assay.
Exposure assessments to sorbic acid–sorbates (E 200, E 202, E 203) were carried out by the ANS Panel based on (1) maximum permitted levels (MPLs) set out in the European Union (EU) legislation (defined as the regulatory maximum level exposure assessment scenario) and (2) usage or analytical data (defined as the refined exposure assessment scenario). Using the regulatory maximum level exposure assessment scenario, the Panel noted that the exposure estimates of sorbic acid–sorbates (E 200, E 202, E 203) exceeded the temporary group ADI of 3 mg/kg bw per day for all population groups at the mean and high levels. The main contributing food categories to the total mean exposure estimates for children, adolescents and adults in this scenario were bread and rolls, fine bakery wares and flavoured drinks. For the elderly, the main contributing food categories were bread and rolls and fine bakery wares, while, for toddlers, the main contributing food categories were bread and rolls, fine bakery wares and processed cheese. From the refined estimated exposure scenario using only reported use levels, the Panel noted that the refined brand‐loyal and non‐brand‐loyal exposure estimates exceeded the temporary group ADI of 3 mg/kg bw per day for all population groups at the mean and high levels. The main contributing food categories for all groups were bread and rolls and fine bakery wares. From the refined estimated exposure scenario using reported use levels and analytical data, the Panel noted that, for the refined brand‐loyal exposure estimate, all population groups exceeded the temporary group ADI of 3 mg/kg bw per day at the mean and high levels (95th percentile), whilst, for the non‐brand‐loyal scenario, the temporary group ADI was exceeded in only toddler and children population groups in one country. The main contributing food categories for all groups were bread and rolls and fine bakery wares in the brand‐loyal scenario and bread and rolls, fine bakery wares, flavoured drinks and sauces in the non‐brand‐loyal scenario. The Panel noted that the most realistic approach using reported use levels and analytical data in the non‐brand‐loyal scenario did not exceed the temporary group ADI in any population group at the mean or in adolescents, adults and the elderly at the high level, except in the toddler and children population groups in one country. The Panel noted that, in these estimates, the main food contributors were bread and rolls, fine bakery wares and flavoured drinks.
The ANS Panel previously considered that the database on reproductive and developmental toxicity available at the time gave a reason to revise the group ADI of 25 mg sorbic acid/kg bw per day set by the SCF in 1996 to a temporary group ADI of 3 mg sorbic acid/kg bw per day. The ANS Panel had noted that there appeared to be a difference between the results obtained from studies performed by gavage and the earlier reproductive toxicity study which was a diet study. Accordingly, the Panel considered that an EOGRTS in rats including the second generation by diet was needed to reconsider the temporary group ADI.
In addition, the Panel recommended future research on the occurrence of breakdown and reaction products of possible toxicological concern under realistic conditions of food processing and storage – especially when sorbic acid, potassium sorbate or calcium sorbate is used in parallel with ascorbic acid in the presence of iron salts or with nitrites (EFSA ANS Panel, 2015).
2. Data and Methodologies
2.1. Data
In accordance with the current European Commission approach7 for the follow‐up of EFSA's scientific opinions on the re‐evaluation of the safety of permitted food additives for which some concerns have been identified, a specific call for data has been published by the European Commission on DG SANTE's website4 on food additives asking for additional (missing) information to be provided by interested parties to the European Commission.
The FAF Panel based its assessment on the data submitted to European Commission and forwarded to EFSA following the European Commission public call for data and on information from previous evaluations.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
3. Assessment
3.1. Identity of the substances
3.1.1. Sorbic acid
Sorbic acid (E 200) has the chemical name (2E,4E)‐hexa‐2,4‐dienoic acid. The molecular formula is C6H8O2 and the molecular weight is 112.12 g/mol. The Chemical Abstracts Service (CAS) registry number is 110‐44‐1 and the European Inventory of Existing Commercial chemical Substances (EINECS) number is 203‐768‐7.
Synonyms include trans,trans‐2,4‐hexadienoic acid, 2,4‐hexadienoic acid (E,E), (E,E)‐1,3‐pentadiene‐1‐carboxylic acid (SciFinder® software).
The structural formula is shown in Figure 1.
Figure 1.
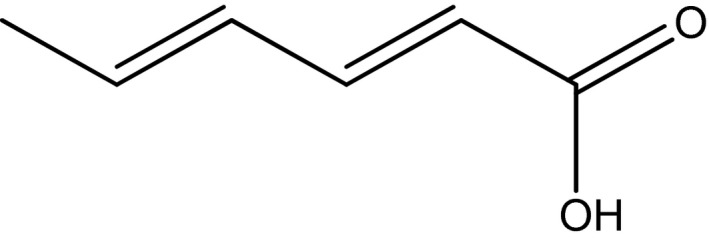
Structural formula of sorbic acid
Sorbic acid is a white free‐flowing powder or takes the form of colourless needles. It is slightly soluble in water and soluble in ethanol. The melting point is between 133 and 135 °C (Commission Regulation (EU) No 231/20128). The pK a is 4.76.
3.1.2. Potassium sorbate
Potassium sorbate (E 202) has the chemical name potassium (2E,4E)‐hexa‐2,4‐dienoate. The molecular formula is C6H7O2K and the molecular weight is 150.22 g/mol. The CAS registry number is 24634‐61‐5 and the EINECS number is 246‐376‐1.
Synonyms include 2,4‐hexadienoic acid potassium salt, (E,E); and sorbic acid, potassium salt (SciFinder software).
The structural formula is shown in Figure 2.
Figure 2.
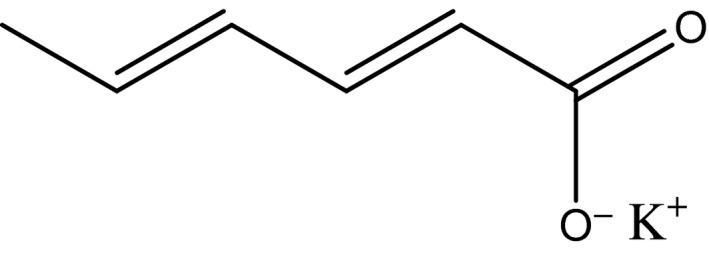
Structural formula of potassium sorbate
Potassium sorbate is a white crystalline powder. Potassium sorbate is freely soluble in water and soluble in ethanol (JECFA, 2006; Commission Regulation (EU) No 231/2012).
3.2. Stability of sorbic acid (E 200) and its potassium salt (E 202), and their reaction and fate in food
The Panel reviewed a report on the ‘Stability of sorbic acid (E 200) and its potassium salt (E 202) during food processing and storage’ (Documentation provided to EFSA No 2). In this report, the available published studies on the stability, and possible degradation products, of sorbic acid and potassium sorbate in model food systems and in foods during storage were summarised.
Different studies on the stability of sorbates have been carried out in aqueous model systems (Hayatsu et al., 1975; Tanaka et al., 1976; Arya, 1980; Namiki et al., 1980; Osawa et al., 1980; Saxby et al., 1982; Hartman, 1983; Seow and Cheah, 1985a,b; Gerschenson et al., 1986; Obanu and Ledward, 1986; Arya and Thakur, 1988; Wedzicha and Brook, 1989; Khandelwal and Wedzicha, 1990; Ledward, 1990; Jung et al., 1992; Campos and Gerschenson, 1996; Campos et al., 1997; Ferrand et al., 1998a,b, 2000a,b,c; Castro et al., 2002; Kitano et al., 2002; Perez‐Prior et al., 2005, 2009; Gliemmo et al., 2008; Lopes et al., 2012), foods, such as meat products (Campos et al., 1995), soybean cheese (Torres et al., 1989), orange squash product (Vidyasagar and Arya, 1983), juices and fish paste (Vidyasagar and Arya,1984) and intermediate moisture meat (Webster et al., 1986; Campos et al., 1995).
The stability of sorbic acid and sorbates in food depend on different factors such as pH, water activity, microbiological concentration, composition (organic acids, proteins, other additives such as ascorbic acid in the presence of iron salts, etc.), storage temperature and packaging (Thomas and Delves‐Broughton, 2014).
Most of the studies carried out in foods refer to the effectivity of sorbates as antimicrobials.
The Panel noted that:
although losses of sorbic acid in different conditions were reported in the literature, information on the amounts of potential reaction and degradation products of sorbic acid and its salts in foods was scarce and mainly referred to model systems;
model systems may not be representative of typical food processing or storage conditions,
the reaction medium is more complex in food and pro‐oxidant compounds or other food components enhancing the degradation of sorbic acid in different conditions could be present in food;
there are food categories in which sorbates are authorised together with ascorbic acid and iron (olives and food supplements) and there was no study on the sorbic acid degradation products formed during processing and storage in real food matrices where sorbic acid, ascorbic acid and iron are present.
Regarding the reaction with nitrites, it is stated in the report that the co‐existence of those two food additives should be considered rare; however, the Panel noted that sorbates are permitted in foods where nitrites are also authorised to be added. According to the Mintel's GNPD, for example, potassium sorbate and sodium nitrite were labelled in 710 products on foods belonging to the food categories Meals, Processed fish and meat, Snacks, Savoury Spreads, Side Dishes, Dairy, Soup and Dessert in the last 3 years. 88% of foods labelled with potassium sorbate and sodium nitrite belonged to the first three food categories.
No analytical results on measurement of the reaction products expected to be formed after this reaction have been submitted.
Overall, the Panel noted that, in the review prepared by industry, no new information was provided, and therefore, there was no re‐assessment of the conclusions of the previous EFSA opinion (EFSA ANS Panel, 2015).
3.3. Exposure assessment
The estimates of the exposure resulting from the use of sorbic acid (E 200), potassium sorbate (E 202) including calcium sorbate (E 203) as calculated by the ANS Panel (EFSA ANS Panel, 2015) are presented in Table 1.
Table 1.
Summary of anticipated exposure to sorbic acid–sorbates (E 200, E 202, E 203) in five population groups from their use as food additives using the regulatory maximum level exposure assessment scenario and refined exposure assessment scenarios (minimum to maximum across the dietary surveys in mg/kg bw per day)
| Toddlers | Children | Adolescents | Adults | The elderly | |
|---|---|---|---|---|---|
| (12–35 months) | (3–9 years) | (10–17 years) | (18–64 years) | (≥ 65 years) | |
| Regulatory maximum level exposure assessment scenario | |||||
| Mean | 7.7–23.7 | 10.1–19.9 | 4.7–11.5 | 5.0–8.9 | 5.0–7.1 |
| High level | 20.7–33.9 | 20.0–38.7 | 10.1–25.1 | 9.9–16.3 | 9.6–12.8 |
| Refined estimated exposure scenario using only reported use levels | |||||
| Brand‐loyal scenario | |||||
| Mean | 6.6–13.7 | 4.4–15.3 | 2.8–8.4 | 3.6–6.0 | 3.9–4.6 |
| High level | 16.0–27.7 | 11.0–30.8 | 6.3–19.0 | 7.2–11.1 | 7.8–9.2 |
| Non‐brand‐loyal scenario | |||||
| Mean | 5.2–10.6 | 2.5–11.3 | 2.0–6.3 | 2.6–4.5 | 2.8–3.4 |
| High level | 11.7–20.4 | 6.1–23.1 | 4.7–12.4 | 5.4–8.4 | 5.7–6.6 |
| Refined estimated exposure scenario using reported use levels and analytical data a | |||||
| Brand‐loyal scenario | |||||
| Mean | 5.6–11.9 | 5.4–13.0 | 2.5–6.8 | 3.2–4.9 | 3.7–4.6 |
| High level | 15.2–23.4 | 11.3–26.5 | 5.6–14.8 | 5.9–10.0 | 7.1–8.2 |
| Non‐brand‐loyal scenario | |||||
| Mean | 0.7–1.8 | 0.9–1.7 | 0.4–1.1 | 0.3–0.8 | 0.3–0.6 |
| High level | 2.2–3.1 | 1.8–3.7 | 0.9–2.4 | 0.7–1.7 | 0.6–1.2 |
This scenario included three food categories for which the direct addition of sorbic acid is not authorised according to Annex II to Regulation (EC) No 1333/2008; however, the use of sorbic acid may result in its presence in these food categories because of carry‐over.
If both reported use levels and analytical results were available for the same food category, the most reliable value was used. It should be noted that, when using the analytical results in the present exposure estimates, the degradation process and loss of sorbic acid‐sorbates caused by food processing were implicitly taken into account.
The main food contributors in these estimates were bread and rolls, fine bakery wares and flavoured drinks.
The ANS Panel concluded that the uncertainties identified would generally lead to an overestimation of the real exposure to sorbic acid–sorbates (E 200, E 202 and E 203) as food additives (EFSA ANS Panel, 2015).
Calcium sorbate (E 203) has since been withdrawn from the lists of food additives (Commission Regulation (EU) 2018/98 of 22 January 2018). The FAF Panel noted the uncertainty regarding the extent of the possible replacement of calcium sorbate (E 203) by sorbic acid or potassium sorbate on the calculations of the exposure assessment. The usage levels used in the previous exposure assessment did not refer specifically to calcium sorbate (E 203) (EFSA ANS Panel, 2015). Therefore, the FAF Panel considered that this uncertainty will not change substantially the previously calculated estimates and the conclusion that these are an overestimation of the real exposure to sorbic acid (E 200) and its potassium salt (E 202).
The FAF Panel considered that the approach using reported use levels and analytical data in a non‐brand‐loyal scenario is the most realistic, as it has already been stated in the previous opinion (EFSA ANS Panel, 2015).
In the non‐brand loyal scenario, the highest estimates of the exposure resulting from the use of sorbic acid (E 200), potassium sorbate (E 202) including calcium sorbate (E 203) as calculated by the ANS Panel (EFSA ANS Panel, 2015) were 1.7 mg sorbic acid‐sorbates/kg bw per day at the mean and 3.7 mg sorbic acid‐sorbates/kg bw per day at the highest (95th) levels for children (3–9 years old).
3.4. Biological and Toxicological data
In its previous evaluation, the Panel identified a possible reproductive toxicity potential of sorbic acid and sorbates in a two‐generation reproductive toxicity study performed in CD/Crl:CD rats (n = 30 and 25 per sex per group in F0 and F1, respectively) in accordance with OECD Guideline 416 and GLP. In this study with doses of 0, 300, 1,000 or 3,000 mg/kg bw per day administered by gavage, adverse effects in pups were described. The effects included a decrease in anogenital distance in male F2 pups in the mid‐ and high‐dose groups. In the highest dose tested, decreased mean litter weight, delay in milestones of physical development and a delay in functional development in F1 pups (auditory startle reflex (in both F1 males and females) and the mid‐air righting reflex (in F1 females) were observed. Considering the aforementioned observations, the Panel concluded that, by gavage, the NOAEL for developmental toxicity was 300 mg sorbic acid/kg bw per day. No other effects on fertility, sex ratio or reproductive organs were observed (EFSA ANS Panel, 2015). In order to appropriately evaluate the effects noted in the two‐generation reproductive toxicity study and to possibly reconsider the temporary group ADI, the Panel recommended that an EOGRTS in rats including the second generation by diet would have to be performed.
3.4.1. Reproductive and developmental toxicity
Extended one‐generation reproductive toxicity study in rats (documentation provided to EFSA No 1)
An EOGRTS compliant with GLP was performed in CD/Crl:CD rats following OECD TG 443 regarding cohorts 1A and 1B (reproductive toxicity). Cohorts 2 (neurotoxicity) and 3 (immunotoxicity) were not included without justification. Sorbic acid (in SDS VRF1 diet to provide the required concentrations) was administered at doses of 0, 15,000, 30,000 and 60,000 mg/kg diet expressed as sorbic acid to 25 animals/sex per group in the F0‐generation and to 20 animals/sex per group to cohort F1A and F1B. In Table 2, the mean minimum values of sorbic acid administered during the different study generations and periods are presented per sex. These values were calculated based on measured body weights and food intake. Test diets were prepared freshly every week and the homogeneity and stability of sorbic acid in these diet formulations were confirmed under the study conditions. The concentration of sorbic acid in the diets per dose group was the same during the entire study.
Table 2.
Sorbic acid dose levels (mg/kg bw per day) in the different study generations
| Dose group Generation/sex | 15,000 mg/kg diet (low‐dose) | 30,000 mg/kg diet (mid‐dose) | 60,000 mg/kg diet (high‐dose) |
|---|---|---|---|
| Parental males | 861 | 1,681 | 3,350 |
| Parental femalesa | 1,098 | 2,170 | 4,134 |
| F1 Cohort A males | 1,314 | 2,544 | 5,113 |
| F1 Cohort B males | 1,275 | 2,483 | 5,020 |
| F1 Cohort A females | 1,356 | 2,611 | 5,349 |
| F1 Cohort B femalesa | 1,124 | 2,120 | 4,151 |
Mean minimum value during prepairing treatment phase, gestation phase and lactation phase.
In all generations, neither test item‐related mortality nor effect on clinical signs in the animals and their pups were observed.
During the first week of administration of the test diets, the body weight gain was decreased in all F0 males (20, 23 and 26%, respectively, in the low‐, mid‐ and high‐dose group). During the 2‐week premating period, the body weight gain of the F0 females was decreased by 22% and 26% in the mid‐ and high‐dose groups, respectively. These decreases were accompanied by a decreased food intake of 7–10%. During the gestation and lactation periods, body weight gain decreases were observed in the mid‐ and high‐dose groups (gestation period, 5% and 7% and lactation period, 13% and 22% in the mid‐and high‐dose groups, respectively). During these periods, food consumption was also decreased (gestation period 7% in the mid‐ and high‐dose groups and during the lactation period 7 and 16% in the mid‐ and high‐dose groups, respectively).
Body weight gains of the F1 male animals of the mid‐ and high‐dose groups of cohort A and of cohort B were decreased (males 6%). No differences in body weight gains were noted for F1 females of cohort A and F1 males and females (until pairing) of cohort B. No effect on body weights of F1 males and females was observed apart from the high‐dose males of cohort A which showed decreased body weights (7%) and high‐dose F1 females of cohort B during gestation and lactation (6–7%). Total food consumption (weeks 0–9) for the F1 male animals of the mid‐ and high‐dose group was decreased when compared to controls (9% and 12%, respectively). Food consumption in the male and female F1 animals of cohort 1A was decreased in the mid‐dose by 6 and 10% and in the high‐dose with 13% for both sexes. Until pairing, food consumption in the male and female F1 animals of cohort 1B was decreased in the mid‐dose by 6% and 12% and in the high‐dose with 11% and 8%. During gestation, the food intake of the F1 animals of cohort B was decreased by 9% and 13% and during lactation by 4% and 11% for the mid‐ and high‐dose groups.
No relevant treatment‐related effects on haematology were observed in the parental animals of the F0‐generation and the F1‐animals of cohort A.
Clinical chemistry at termination revealed the following changes when compared to control: parental F0 females of the mid‐ and high dose showed significantly higher cholesterol levels (28% compared to the controls). In addition, in the high‐dose group of the F1 male animals of cohort 1A, a statistically significant increase in plasma cholesterol (30% compared to the controls) was observed, and in F1 females of this cohort group, the increase was 15% (not statistically significant). In F0 females of the high‐dose group and cohort F1A males of the mid‐ and high‐dose groups, a decrease in urinary potassium levels was observed. The authors stated that as these differences were minor and no related pathological findings were observed the changes should be considered as not adverse.
Up to the highest dose tested, no treatment‐related effects on oestrous cycles, sperm analyses, mating performance, fertility, gestation length and gestation index were observed in the animals of both generations.
The Panel noted that the variation of individual values in the thyroid hormone levels (TSH and T4) was broad and no histopathological findings and no changes in thyroid weight were observed. Although historical values for thyroid hormone levels were not included in the study report, this variation could be considered as random finding and not indicative for an adverse effect.
For the F1 and F2 offspring, general condition, litter size, offspring survival, sex ratio, anogenital distance on postnatal day (PND) 1 and nipple counts on PND 13 remained unaffected by parental treatment with sorbic acid. Mean body weight gain for both male and female F1 offspring in the high‐dose group from PND 4–7 and PND 14–21 was lower than in controls over the same period. Mean body weight of the F1 pups of the high‐dose group was statistically significantly decreased on PND 21 (9% in males and 8% in females compared to controls). No effect was observed on F2 pup weight on PND 1. Pup weight gain of the F2 male and female pups from PND 1–21 was lower in the mid‐ and high‐dose groups (mid‐dose group males and females, 8 and 7% and high‐dose group males and females 12 and 23%, respectively) than the controls. Mean F2 pup weight was decreased on PND 14 and 21 for the mid‐dose group by 6% in males and 5–6% in females. On PND 7, 14 and 21 for the high‐dose group, the mean F2 pup decrease was from 10% to 20% in males and 9% to 20% in females.
Timing to first oestrus cycle was comparable between the sorbic acid‐treated and control group in the F1 animals. Male sexual maturation (the day of age that males completed balano preputial separation) of the F1 animals was similar across all groups. In the F1 females of the high‐dose group, the vaginal opening was delayed in both cohorts (3 days in cohort A and 2 days in cohort B). The mean body weight of this group on completion of vaginal opening was slightly but significantly higher than controls for cohort A females.
At necropsy of the parental F0 animals, the only treatment‐related effect was an increased liver weight (approx. 9%) in the male animals. This increase in relative liver weight (approx. 9%) was also observed in the F1 males of cohort A. At necropsy on day 21 of lactation of F1 females of cohort B, the mean absolute and relative weight of ovaries (abs: 18%; rel. 15%) and uterus, including cervix and oviduct (abs. 30%, rel. 27%) of the high‐dose group were decreased (approx. 23%). No differences were observed in the organ weights of the F0 and F1 female animals of cohort A up to the highest dose tested. The authors stated that the other minor differences in the high‐dose group were rather attributed to differences in body weight than a direct effect of treatment. The Panel agreed with this opinion apart from the effects on liver, ovary and uterus (including cervix and oviduct) weights, which were considered treatment related.
Macroscopical and microscopical examination of organs of parental animals (F0, F1) and F1 and F2 offspring did not reveal treatment‐related effects. In the females of both generations, ovarian follicle and corpora lutea and sperm assessment up to the highest dose tested were comparable between the treated and the control groups. The Panel noted that the ovaries and the uterine tissues of the F1 females of cohort B which showed decreased weights were not examined histologically.
The authors did not consider the effect on pup weight as adverse. Their reasoning was that no effect on general condition was observed and the effect on pup weight and pup weight gain in the mid‐dose group was less than 10%. The Panel did not agree with this argumentation and because the changes were dose related, the reported decrease of 5% in pup body weight was considered as adverse.
3.5. Discussion
In this opinion, the FAF Panel was requested to carry out a scientific evaluation of an EOGRTS to determine whether it would allow reconsideration of the temporary group ADI for sorbic acid (E 200) and potassium sorbate (E 202), established by the ANS Panel in 2015.
The FAF Panel was also asked to review a report on the ‘Stability of sorbic acid (E 200) and its potassium salt (E 202) during food processing and storage’ provided by industry. In this report, the available published studies on the stability, and possible degradation products, of sorbic acid and potassium sorbate in model food systems and in foods during storage were summarised. The Panel considered that no new relevant information was provided on the occurrence and toxicity of breakdown and reaction products of sorbic acid and its potassium salt in food under realistic conditions of processing and storage, as compared to the information evaluated in the previous opinion (EFSA ANS Panel, 2015) and therefore did not reassess previous conclusions of the 2015 ANS Panel opinion.
The EOGRTS was performed in compliance with GLP. Sorbic acid was administered to CD/Crl:CD rats at doses of 0, 15,000, 30,000 and 60,000 mg/kg diet (approximately equal to 0, 1,000, 2,000 and 4,000 mg sorbic acid/kg bw per day) following OECD TG 443 regarding cohorts 1A and 1B (for testing reproductive toxicity).
In summary, the FAF Panel noted that various effects were observed in the EOGRTS study:
in reproductive parameters (decreased weights of ovaries and uterus, including cervix and oviduct of F1 females of cohort 1B) at the high‐dose group;
decreases in body weight gain in the parental F0 females of the mid‐ and high dose groups during gestation and lactation and in the F1A males of mid‐ and high‐dose group. No decreases were observed in F1A and B (until pairing) any dose level. Body weights were decreased in the high‐dose F1 females of cohort B during gestation and lactation;
decreases in food intake of F0 and F1 generations in the mid‐ and high‐dose groups;
increases in plasma cholesterol levels in the parental F0 females of the mid‐ and high‐dose groups and in the F1A males and females of the high‐dose group;
decreases in F2 pup body weight gain were observed in the mid‐ and high‐dose groups and in F1 offspring in the high‐dose group of both sexes;
increases in liver weight in F0 and F1A male animals and decreases in mean absolute and relative weight of ovaries and uterus, including cervix and oviduct in F1B females at the highest dose tested.
In the present dietary EOGRTS, the effect on anogenital distance in pups observed in a two‐generation reproductive toxicity study assessed previously (EFSA ANS Panel, 2015) was not confirmed and therefore not considered relevant for the risk assessment. The delay, also observed previously, in functional development in F1 pups of the high‐dose group (3,000 mg/kg bw per day administered by gavage) was not tested in the present EOGRTS. Finally, neurodevelopmental effects [reduced reflexes for the auditory startle reflex (in both F1 males and females) and for the mid‐air righting reflex (in females)] were previously observed at the highest dose of 3,000 mg/kg bw per day in the two‐generation reproductive toxicity study.
In the current EOGRTS, cohorts 2 (for testing neurotoxicity) and 3 (for testing immunotoxicity) were not included, and therefore, these endpoints were not tested.
The FAF Panel considered decreases in body weight gains in the pups of the F2 generation, as the most biologically relevant endpoint in order to establish the reference point and performed benchmark dose (BMD) analysis (Annex I). The Panel selected the value of 1,110 mg sorbic acid/kg bw per day derived as the lower confidence limit of the benchmark dose (BMDL) from the data on body weight gains in pups from the F2 generations of both sexes on days 1–21. The omission of cohorts 2 of the EOGRTS was considered not to compromise the outcome of the assessment since the neurodevelopmental effects of the earlier study reported in the ANS Panel opinion (2015) were observed only at dose levels higher than the BMDL.
The BMDL of 1,110 mg sorbic acid/kg bw per day was used to allocate a new group ADI for sorbic acid and its potassium salt. By applying a default uncertainty factor of 100, the Panel established a group ADI expressed as 11 mg sorbic acid/kg bw per day for sorbic acid (E 200) and its potassium salt (E 202).
The FAF Panel considered that the most realistic approach using reported use levels and analytical data was in the non‐brand‐loyal scenario as already stated in the previous opinion (EFSA ANS Panel, 2015).
In the non‐brand loyal scenario, the highest estimates of the exposure resulting from the use of sorbic acid (E 200), potassium sorbate (E 202) including calcium sorbate (E 203) as calculated by the ANS Panel (EFSA ANS Panel, 2015) were 1.7 mg sorbic acid‐sorbates/kg bw per day at the mean and 3.7 mg sorbic acid‐sorbates/kg bw per day at the highest (95th) levels for children (3–9 years old).
The uncertainties identified previously (EFSA ANS Panel, 2015) would generally lead to an overestimate of the real exposure to sorbic acid–sorbates (E 200, E 202 and E 203) as food additives.
Calcium sorbate (E 203) has been withdrawn from the lists of food additives (Commission Regulation (EU) 2018/98 of 22 January 2018). The Panel noted that there is uncertainty on to which extent the possible replacement of calcium sorbate (E 203) by sorbic acid or potassium sorbate will affect the calculations of the exposure assessment. However, the Panel considered that this uncertainty will not change significantly the previously calculated estimates and the conclusion that they are an overestimation of the real exposure to sorbic acid (E 200) and its potassium salt (E 202).
3.6. Conclusions
The Panel changed the temporary group ADI of 3 mg sorbic acid/kg bw per day for sorbic acid (E 200) and its potassium salt (E 202) to a new group ADI of 11 mg sorbic acid/kg bw per day based on the findings from an EOGRTS provided as a follow‐up of the conclusions and recommendations of the previous EFSA Panel opinion (EFSA ANS Panel, 2015).
The Panel concluded that considering the most realistic approach for exposure of a non‐brand‐loyal scenario (EFSA ANS Panel, 2015), the newly established ADI would not be exceeded.
The Panel concluded that in the review on ‘Stability of sorbic acid (E200) and its potassium salt (E202) during food processing and storage’ prepared by industry, no new information was provided, and therefore, in this opinion, there was no re‐assessment of the EFSA ANS opinion conclusions from 2015 regarding the stability of sorbates in food.
Documentation provided to EFSA
Sorbic acid: Extended One‐Generation Reproductive Toxicity Study in the CD Rat by Dietary Administration. Celanese Europe B.V. Unpublished study report submitted to EFSA by EC on 23 October 2018.
Report on “Stability of sorbic acid (E 200) and its potassium salt (E 202) during food processing and storage”. Celanese Europe B.V. Review submitted to EFSA by EC on 23 October 2018.
Abbreviations
- ADI
Acceptable Daily Intake
- ANS
Scientific Panel on Food Additives and Nutrient Sources added to Food
- BMD
Benchmark Dose
- BMDL
Lower confidence limit of the benchmark dose
- BMDU
Upper confidence limit of the benchmark dose
- BMR
Benchmark Response
- bw
body weight
- CAS
Chemical Abstracts Service
- EINECS
European Inventory of Existing Commercial chemical Substances
- EOGRTS
extended one‐generation reproductive toxicity study
- FAO/WHO
Food and Agriculture Organization/World Health Organization
- GLP
Good Laboratory Practice
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- MPL
maximum permitted level
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PND
postnatal day
- SCF
EU Scientific Committee on Food
Annex A – BMD Report
1. Data Description
The ANS Panel had previously set a temporary group ADI for sorbates based on the no observed adverse effect level (NOAEL) of a two‐generation reproductive toxicity study performed in CD/Crl:CD rats (EFSA, 2015). The ANS Panel recommended an extended one‐generation reproductive toxicity study (EOGRTS) in rats including the second generation by diet in order to reconsider the temporary group ADI. The FAF Panel was provided with the EOGRTS (Documentation provided to EFSA No 1) and considered the reproductive and developmental effects for a BMD analysis. The only such an effect observed was in the body weight gain in F2 pups in both generations on postnatal day 1–21. Therefore, this effect was used as a response variable for a BMD analysis.
The data in the Table 2 reflect the averages and SDs of the litter averages (with n = number of litters per treatment group), thus taking implicit account of possible litter effects.
Table 2.
Data set (Documentation provided to EFSA, No 1, Table 106–108, pp 249–251)
| Dose levels to F1B females (mg/bw per day) | Body weight gains (g) of F2 pups on PND1–21 | SD | No of litters | Sex |
|---|---|---|---|---|
| 0 | 45.8 | 4.5 | 19 | F |
| 1124 | 43.6 | 4.5 | 19 | F |
| 2120 | 42.9 | 2.3 | 19 | F |
| 4151 | 37.2 | 3.8 | 19 | F |
| 0 | 47.2 | 4.7 | 19 | M |
| 1124 | 45.9 | 5.0 | 20 | M |
| 2120 | 43.8 | 3.2 | 19 | M |
| 4151 | 38.0 | 4.0 | 19 | M |
2. Selection of the BMR
The BMR (benchmark response) used is a 5% change in mean response compared to the controls. A reduction higher that 5% in body weight gain in pups can be considered adverse in the long term. The BMD (benchmark dose) is the dose corresponding with the BMR of interest.
A 90% confidence interval around the BMD will be estimated, the lower bound is reported by BMDL and the upper bound by BMDU.
2.1.
2.1.1.
Software Used
Results are obtained using the EFSA web tool for BMD analysis, which uses the R‐package PROAST, version 65.7, for the underlying calculations.
Dose–response models
Default set of fitted models:
| Model | Number of parameters | Formula | |
|---|---|---|---|
| Null | 1 | y = a | |
| Full | No. of groups | y = group mean | |
| Exp model 3 | 3 | y = a · exp(bxd) | |
| Exp model 5 | 4 |
|
|
| Hill model 3 | 3 |
|
|
| Hill model 5 | 4 |
|
As a covariate is included in the analysis, these models will also be fitted assuming that some of the parameters [background response parameter (a), potency parameter (BMD) and/or variance (var)] depend on the subgroup defined by the covariate. Therefore, the number of parameters in each model might be larger than indicated in the table above.
3. Procedure for selection of BMDL
There were no deviations from the procedure described in the flow chart to obtain the final BMD confidence interval.
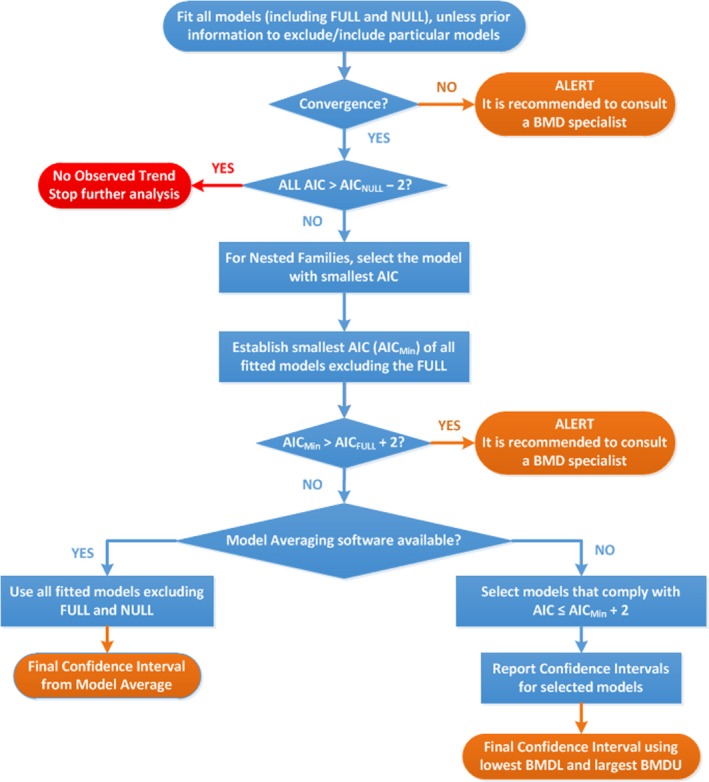
Flowchart for selection of BMDL
4. Results
4.1.
4.1.1.
Response variable: Body weight change in F2 pups
Fitted Models
| model | converged | loglik | npar | AIC |
|---|---|---|---|---|
| full | yes | 147.77 | 9 | −277.54 |
| full‐v | yes | 147.94 | 10 | −275.88 |
| null‐ | yes | 103.14 | 2 | −202.28 |
| null‐a | yes | 104.36 | 3 | −202.72 |
| Expon. m3‐ | yes | 145.03 | 4 | −282.06 |
| Expon. m3‐a | yes | 147.05 | 5 | −284.10 |
| Expon. m3‐b | yes | 145.58 | 5 | −281.16 |
| Expon. m3‐ab | yes | 147.15 | 6 | −282.30 |
| Expon. m5‐ | yes | 145.03 | 5 | −280.06 |
| Expon. m5‐a | yes | 147.05 | 6 | −282.10 |
| Expon. m5‐b | yes | 145.58 | 6 | −279.16 |
| Expon. m5‐ab | yes | 147.15 | 7 | −280.30 |
| Hill m3‐ | yes | 144.98 | 4 | −281.96 |
| Hill m3‐a | yes | 147.00 | 5 | −284.00 |
| Hill m3‐b | yes | 145.55 | 5 | −281.10 |
| Hill m3‐ab | yes | 147.11 | 6 | −282.22 |
| Hill m5‐ | yes | 144.98 | 5 | −279.96 |
| Hill m5‐a | yes | 147.00 | 6 | −282.00 |
| Hill m5‐b | yes | 145.55 | 6 | −279.10 |
| Hill m5‐ab | yes | 147.11 | 7 | −280.22 |
Estimated Model Parameters
EXP
estimate for var‐ : 0.008565
estimate for a‐F : 45.38
estimate for a‐M : 46.78
estimate for CED‐ : 1748
estimate for d‐ : 1.616
HILL
estimate for var‐ : 0.00857
estimate for a‐F : 45.36
estimate for a‐M : 46.76
estimate for CED‐ : 1761
estimate for d‐ : 1.72
Final BMD Values
| model | BMDL | BMDU | BMD |
|---|---|---|---|
| Expon. m3‐a | 1110 | 2530 | 1748 |
| Hill m3‐a | 1140 | 2530 | 1761 |
Lowest BMDL and highest BMDU Values
| subgroup | bmdl.lowest | bmdu.highest |
|---|---|---|
| all | 1110 | 2530 |
Visualisation
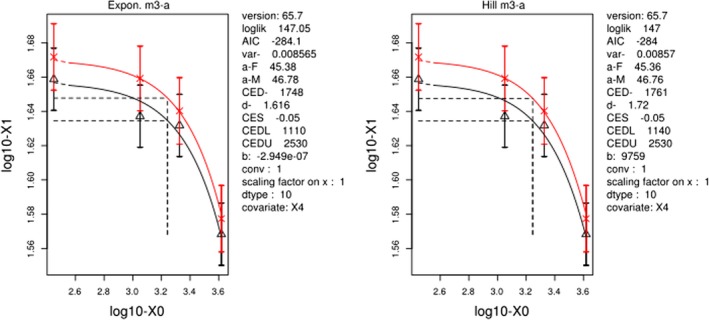
Plot for response body weight gains in F2 pups
5. Conclusions
The BMR used for the assessment has been set to 5% producing a lower bound (BMDL) of 1110 mg/kg bw per day. Separate analysis produced a lower bound (BMDL) of 882 for males mg/kg bw per day and 899 mg/kg bw per day for females. The analysis presented and the resulting confidence intervals concern the analysis with males and females as co‐variates.
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Gundert‐Remy U, Husøy T, Mennes W, Moldeus P, Oskarsson A, Shah R, Wölfle D, Lambré C, Christodoulidou A and Waalkens‐Berendsen I, 2019. Scientific Opinion on the follow‐up of the re‐evaluation of sorbic acid (E200) and potassium sorbate (E202) as food additives. EFSA Journal 2019;17(3):5625, 21 pp. 10.2903/j.efsa.2019.5625
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00137
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus, Agneta Oskarsson, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle and Maged Younes.
Acknowledgements: The FAF Panel wishes to thank EFSA staff member(s): José Cortinas Abrahantes, Dimitrios Chrysafidis and Petra Gelgelova for the support provided to this scientific output.
Adopted: 30 January 2019
Notes
OJ L 354, 31.12.2008, p. 16.
OJ L 83, 22.3.2012, p. 1.
OJ L 80, 26.3.2010, p. 19.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
References
- Arya SS, 1980. Stability of sorbic acid in aqueous solutions. Journal of Agricultural and Food Chemistry, 28, 1246–1249. [Google Scholar]
- Arya SS and Thakur BR, 1988. Degradation products of sorbic acid in aqueous solutions. Food Chemistry, 29, 41–49. [Google Scholar]
- Campos CA and Gerschenson LA, 1996. Effect of certain additives on sorbate stability. Food Research International, 29, 147–154. [Google Scholar]
- Campos CA, Alzamora SM and Gerschenson LN, 1995. Sorbic acid stability in meat products of reduced water activity. Meat Science, 41, 37–46. [DOI] [PubMed] [Google Scholar]
- Campos CA, Alzamora SM and Gerschenson LN, 1997. Sorbate destruction and non‐enzymatic browning in model aqueous systems. Food Science and Technology International, 3, 405–411. [Google Scholar]
- Castro M, Garro O, Campos CA and Gerschenson LN, 2002. Interactions between additives: its effect on sorbate stability and Z. bailli minimum inhibitory concentration in model aqueous systems resembling salad dressings. Food Science and Technology International, 8, 33–39. [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2015. Scientific Opinion on the re‐evaluation of sorbic acid (E 200), potassium sorbate (E 202) and calcium sorbate (E 203) as food additives. EFSA Journal 2015;13(6):4144, 28 pp. 10.2903/j.efsa.2015.4144 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- Ferrand C, Marc F, Fritsch P and de Saint‐Blanquat G, 1998a. Sorbic acid‐amine function interactions. Food Additives and Contaminants, 15, 487–493. [DOI] [PubMed] [Google Scholar]
- Ferrand C, Marc F, Fritsch P and De Saint‐Blanquat G, 1998b. Interactions of sorbic acid and sorbates with food amines: role of light, oxygen, temperature and the presence of glycerol in emulsifiers. Sciences des Aliments, 18, 603–616. [Google Scholar]
- Ferrand C, Marc F, Fritsch P, Cassand P and de Saint‐Blanquat G, 2000a. Mutagenicity and genotoxicity of sorbic acid‐amine reaction products. Food Additives and Contaminants, 17, 895–901. [DOI] [PubMed] [Google Scholar]
- Ferrand C, Marc F, Fritsch P, Cassand P and de Saint‐Blanquat G, 2000b. Chemical and toxicological studies of products resulting from sorbic acid and methylamine interaction in food conditions. Amino Acids, 18, 251–263. [DOI] [PubMed] [Google Scholar]
- Ferrand C, Marc F, Fritsch P and de Saint Blanquat G, 2000c. Influence of various parameters on the browning of potassium sorbate in the presence of amines. Food Additives and Contaminants, 17, 947–956. [DOI] [PubMed] [Google Scholar]
- Gerschenson LN, Alzamora SM and Chirife J, 1986. Stability of sorbic acid in model food systems of reduced water activity: sugar solutions. Journal of Food Science, 51, 1028–1031. [Google Scholar]
- Gliemmo MF, Calvino AM, Tamasi O, Gerschenson LN and Campos CA, 2008. Interactions between aspartame, glucose and xylitol in aqueous systems containing potassium sorbate. LWT ‐ Food Science and Technology, 41, 611–619. [Google Scholar]
- Hartman PE, 1983. Putative mutagens and carcinogens in foods. IV. Malonaldehyde (malondialdehyde). Environmental mutagenesis, 5, 603–607. [DOI] [PubMed] [Google Scholar]
- Hayatsu H, Chung KC, Kada T and Nikajima T, 1975. Generation of mutagenic compound(s) by a reaction between sorbic acid and nitrite. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 30, 417–419. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974. Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. Seventeenth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additive Series No 5. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Combined compendium of food additive specifications. Monograph 1.
- Jung R, Cojocel C, Muller W, Bottger D and Luck E, 1992. Evaluation of the genotoxic potential of sorbic acid and potassium sorbate. Food and Chemical Toxicology, 30, 1–7. [DOI] [PubMed] [Google Scholar]
- Khandelwal GD and Wedzicha BL, 1990. Nucleophilic reactions of sorbic acid. Food Additives and Contaminants, 7, 685–694. [DOI] [PubMed] [Google Scholar]
- Kitano K, Fukukawa T, Ohtsuji Y, Masuda T and Yamaguchi H, 2002. Mutagenicity and DNA damaging activity caused by decomposed products of potassium sorbate reacting with ascorbic acid in the presence of Fe salt. Food and Chemical Toxicology, 40, 1589–1594. [DOI] [PubMed] [Google Scholar]
- Lang K, et al, 1967. Unpublished report (as referred to by JECFA, 1974).
- Ledward DA, 1990. Stability of sorbic acid in intermediate moisture systems. Food Additives and Contaminants, 7, 677–683. [DOI] [PubMed] [Google Scholar]
- Lopes IC, Santos PVF, Diculescu VC, deAraujo MC and Oliveria‐Brett AM, 2012. Sorbic acid and its degradation products. Electrochemical Characterisation; Analytical Letters, 45, 408–417. [Google Scholar]
- Namiki M, Udaka S, Osawa T, Tsuji K and Kada T, 1980. Formation of mutagens by sorbic acid nitrite reaction: effects of reaction conditions on biological activities. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 73, 21–28. [DOI] [PubMed] [Google Scholar]
- Obanu ZA and Ledward DA, 1986. Reactivity of sorbate and glycerol in some model intermediate moisture systems. Food Chemistry, 21, 57–75. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 1998. OECD Guideline for the testing of chemicals, 443. Extended One‐Generation Reproductive Toxicity Study. Adopted on 28 July 2011.
- Osawa T, Ishibashi H, Namiki M and Kada T, 1980. Desmutagenic actions of ascorbic acid and cysteine on a new pyrole mutagen formed by the reaction between food additives; Sorbic acid and sodium nitrite. Biochemical and Biophysical Research Communications, 95, 835–841. [DOI] [PubMed] [Google Scholar]
- Perez‐Prior MT, Manso JA, Garcia‐Santos Mdel P, Calle E and Casado J, 2005. Alkylating potential of potassium sorbate. Journal of Agricultural and Food Chemistry, 53, 10244–10247. [DOI] [PubMed] [Google Scholar]
- Perez‐Prior MT, Gomez‐Bombarelli R, Gonzalez‐Perez M, Manso JA, Garcia‐Santos MP, Calle E and Casado J, 2009. Sorbate‐nitrite interactions: acetonitrile oxide as an alkylating agent. Chemical Research in Toxicology, 22, 1320–1324. [DOI] [PubMed] [Google Scholar]
- Saxby MJ, Stephens MA and Reid RG, 1982. Degradation of sorbic acid in model food systems. Food Chemistry, 9, 283–287. [Google Scholar]
- SCF (Scientific Committee for Food), 1996. Reports of the Scientific Committee for Food (Thirty‐fifth series). Opinion on sorbic acid and its calcium and potassium salts. Opinion expressed on 25 February 1994, 19–22.
- Seow CC and Cheah PB, 1985a. Kinetics of degradation of sorbic acid in aqueous glycerol systems. Food Chemistry, 17, 95–103. [Google Scholar]
- Seow CC and Cheah PB, 1985b. Reactivity of sorbic acid and glycerol in non‐enzymatic browning in liquid intermediate moisture model systems. Food Chemistry, 18, 71–80. [Google Scholar]
- Tanaka K, Chung KC, Hayatsu H and Kada T, 1976. Inhibition of nitrosamine formation in vitro by sorbic acid. Food and Cosmetics Toxicology, 16, 209–215. [DOI] [PubMed] [Google Scholar]
- Thakur BR, Singh RK and Arya SS, 1994. Chemistry of sorbates – a basic perspective. Food Reviews International, 10, 71–79. [Google Scholar]
- Thomas LV and Delves‐Broughton J, 2014. PRESERVATIVES|Permitted Preservatives–Sorbic Acid. 102–107.
- Torres JA, Jorge OB and Karel M, 1989. Sorbic acid stability during processing and storage of an intermediate moisture cheese analog. Journal of Food Processing and Preservation, 13, 409–415. [Google Scholar]
- Vidyasagar K and Arya SS, 1983. Stability of sorbic acid in orange squash. Journal of Agricultural and Food Chemistry, 31, 1262–1264. [Google Scholar]
- Vidyasagar K and Arya SS, 1984. Degradation of sorbic acid in fruit squashes and fish paste. Journal of Food Technology, 19, 447–454. [Google Scholar]
- Webster CEM, Allison SE, Adelakun IO, Obanu ZA and Ledward DA, 1986. Reactivity of sorbate and glycerol in intermediate moisture meat products. Food Chemistry, 21, 133–143. [Google Scholar]
- Wedzicha BL and Brook MA, 1989. Reaction of sorbic acid with nucleophiles: preliminary studies. Food Chemistry, 31, 29–40. [Google Scholar]


