Abstract
The Panel on Food Additives and Flavourings of the European Food Safety Authority was requested to evaluate the genotoxic potential of 5 flavouring substances in Flavouring Group Evaluation 210 Revision 3 (FGE.210Rev3). In FGE.210, the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids concluded that the genotoxic potential could not be ruled out for any of the flavouring substances. In FGE.210Rev1, the concern for genotoxic potential has been ruled out for eight substances [FL‐no: 02.105, 07.007, 07.009, 07.011, 07.036, 07.088, 07.091 and 07.170]. In FGE.210 Rev2, the concern for genotoxic potential has been ruled out for allyl α‐ionone [FL‐no: 07.061]. In the present revision of FGE 210 (FGE.210Rev3), additional in vitro and in vivo data on the representative substance α‐damascone [FL‐no: 07.134] are evaluated. To investigate equivocal and positive results observed in in vitro micronucleus studies, an in vivo combined micronucleus (bone marrow) and comet assay (liver and duodenum) was performed. α‐Damascone did not induce micronuclei in bone marrow and no primary DNA damage in duodenum; however, an increase in primary DNA damage was observed in liver. This positive result was attributed by the applicant to a high level of peroxides in the sample tested. Therefore, the comet assay was repeated with a new sample of α‐damascone, confirming the negative results observed in duodenum, but equivocal results were observed in liver. Two additional in vivo comet assays in liver were performed in order to clarify the potential impact of peroxides on the obtained results from the genotoxicity testing. However, the materials studied in these tests were not suitable to establish the potential role of peroxides in the genotoxicity of α‐damascone. The Panel concluded that the concern for genotoxicity cannot be ruled out for α‐damascone [FL‐no: 07.134] and the four structurally related substances [FL‐no: 07.130, 07.225, 07.226 and 07.231].
Keywords: α,β‐unsaturated alicyclic ketones; flavouring substances; safety evaluation; FGE.210; subgroup 2.4; FGE.19
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The use of flavourings is regulated under Regulation (EC) No 1334/20081 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation an evaluation and approval are required for flavouring substances.
The Union List of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20122. The list contains a number of flavouring substances for which the safety evaluation should be completed in accordance with Commission Regulation (EC) No 1565/20003.
In 2013 additional genotoxicity data were submitted on a number of substances from the Flavouring Group FGE.210 including alpha‐damascone [FL‐no: 07.134]. On 30 January 2014 the EFSA CEF Panel adopted an opinion on this Flavouring Group Evaluation 210 Rev.1 (FGE.210Rev1) and concluded that for the substance alpha‐damascone [FL‐no: 07.134] and the four structurally related substances [FL‐no: 07.130, 07.225, 07.226 and 07.231] the submitted data could not rule out the concern with respect to genotoxicity and additional data were requested.
In its further revision of this FGE group (FGE.210 Rev.2) of 10 July 2015 when examining additional information on other substances of this group, the Panel reinstated this request.
On 23 February 2016 the Industry submitted additional genotoxicity studies on alpha‐damascone [FL‐no: 07.134], as specified in the enclosures.
1.1.1. Terms of Reference
The European Commission requests the European Food Safety Authority (EFSA) to evaluate this new information and, depending on the outcome, proceed to the full evaluation on alpha‐damascone [FL‐no: 07.134] and four structurally related substances [FL‐no: 07.130, 07.225, 07.226 and 07.231] in accordance with Commission Regulation (EC) No 1565/2000, within nine months.
2. Data and methodologies
2.1. History of the evaluation of FGE.19 substances
Flavouring Group Evaluation 19 (FGE.19) contains 360 flavouring substances from the European Union (EU) Register being α,β‐unsaturated aldehydes or ketones and precursors which could give rise to such carbonyl substances via hydrolysis and/or oxidation (EFSA, 2008a).
The α,β‐unsaturated aldehyde and ketone structures are structural alerts for genotoxicity (EFSA, 2008a). The Panel noted that there were limited genotoxicity data on these flavouring substances, but that positive genotoxicity studies were identified for some substances in the group.
The α,β‐unsaturated carbonyls were subdivided into subgroups on the basis of structural similarity (EFSA, 2008a). In an attempt to decide which of the substances could go through the Procedure, a (quantitative) structure–activity relationship ((Q)SAR) prediction of the genotoxicity of these substances was undertaken considering a number of models that were available at that time (DEREKfW, TOPKAT, DTU‐NFI‐MultiCASE Models and ISS‐Local Models; Gry et al., 2007).
The Panel noted that, for most of these models, internal and external validation has been performed, but considered that the outcome of these validations was not always extensive enough to appreciate the validity of the predictions of these models for these α,β‐unsaturated carbonyls. Therefore, the Panel considered it inappropriate to totally rely on (Q)SAR predictions at this point in time and decided not to take substances through the procedure based on negative (Q)SAR predictions only.
The Panel took note of the (Q)SAR predictions by using two ISS Local Models (Benigni and Netzeva, 2007a,b) and four DTU‐NFI MultiCASE Models (Gry et al., 2007; Nikolov et al., 2007) and the fact that there are available data on genotoxicity, in vitro and in vivo, as well as data on carcinogenicity for several substances. Based on these data, the Panel decided that 15 subgroups (1.1.1, 1.2.1, 1.2.2, 1.2.3, 2.1, 2.2, 2.3, 2.5, 3.2, 4.3, 4.5, 4.6, 5.1, 5.2 and 5.3) (EFSA, 2008b) could not be evaluated through the Procedure due to concern with respect to genotoxicity. Corresponding to these subgroups, 15 FGEs were established: FGE.200, 204, 205, 206, 207, 208, 209, 211, 215, 219, 221, 222, 223, 224 and 225.
For 11 subgroups, the Panel decided, based on the available genotoxicity data and (Q)SAR predictions, that a further scrutiny of the data should take place before requesting additional data from the Flavouring Industry on genotoxicity. These subgroups were evaluated in FGE.201, 202, 203, 210, 212, 213, 214, 216, 217, 218 and 220. For the substances in FGE.202, 214 and 218, it was concluded that a genotoxic potential could be ruled out, and accordingly, these substances were evaluated using the Procedure. For all or some of the substances in the remaining FGEs, FGE.201, 203, 210, 212, 213, 216, 217 and 220, the genotoxic potential could not be ruled out.
To ease the data retrieval of the large number of structurally related α,β‐unsaturated substances in the different subgroups for which additional data are requested, EFSA worked out a list of representative substances for each subgroup (EFSA, 2008c). In selecting the representative substances, expert judgement was applied. In each subgroup, the representative substances were selected taking into account chain length, chain branching, lipophilicity and additional functional groups. Likewise an EFSA genotoxicity expert group has worked out a test strategy to be followed in the data retrieval for these substances (EFSA, 2008b).
The Flavouring Industry has been requested to submit additional genotoxicity data according to the list of representative substances and test strategy for each subgroup.
The Flavouring Industry has now submitted additional data and the present FGE concerns the evaluation of these data requested on genotoxicity.
2.2. Presentation of the substances in flavouring group evaluation 210
2.2.1. Description
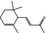
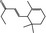
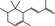
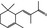
The FGE.210 concerns 14 substances, corresponding to subgroup 2.4 of FGE.19 (see Appendix A, Table A.1). Thirteen of these substances are α,β‐unsaturated alicyclic ketones [FL‐no: 07.007, 07.009, 07.011, 07.036, 07.061, 07.088, 07.091, 07.130, 07.134, 07.170, 07.225, 07.226 and 07.231] and one is a precursor for such ketones [FL‐no: 02.105]. One of the substances has a terminal double bond (allyl α‐ionone [FL‐no: 07.061]) and one is an epoxide (β‐ionone epoxide [FL‐no: 07.170]).
Table A.1.
Summary of Specification for the Substances in the Flavouring Group Evaluation 210Rev2 (JECFA, 1998, 2000, 2001, 2002, 2003, 2005, 2014)
| FL‐no JECFA‐no | EU Register name | Structural formula | FEMA no CoE no CAS no | Phys.form Mol.formula Mol.weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. Indexd Spec.gravitye | EFSA comment |
|---|---|---|---|---|---|---|---|---|
|
02.105 391 |
4‐(2,6,6‐Trimethyl‐2‐cyclohexenyl)but‐3‐en‐2‐ol |
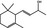
|
3624 25312‐34‐9 |
Liquid C13H22O 194.32 |
127 (20 hPa) IR 99% |
1.488–1.492 0.917–0.924 |
||
|
07.007 388 |
α‐Ionone |
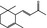
|
2594 141 127‐41‐3 |
Liquid C13H20O 192.30 |
Insoluble 1 mL in 3 mL 70% alcohol |
237 IR 85% |
1.497–1.502 0.927–0.933 |
|
|
07.009 398 |
Methyl‐α‐ionone |
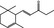
|
2711 143 7779‐30‐8 |
Liquid C14H22O 206.33 |
238 IR 90% |
1.498–1.503 0.921–0.930 |
||
|
07.011 403 |
4‐(2,5,6,6‐Tetramethyl‐2‐cyclohexenyl)‐3‐buten‐2‐one |
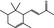
|
2597 145 79‐69‐6 |
Liquid C14H22O 206.33 |
1 mL in 4 mL 70% alcohol |
110–112 (4 hPa) IR 98% |
1.497–1.503 0.932–0.939 |
|
|
07.036 404 |
α‐Isomethyl ionone |
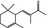
|
2714 169 127‐51‐5 |
Liquid C14H22O 206.33 |
238 IR 85% |
1.498–1.503 0.925–0.934 |
||
|
07.061 401 |
Allyl α‐ionone |
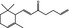
|
2033 2040 79‐78‐7 |
Liquid C16H24O 232.37 |
Insoluble 1 mL in 1 mL 90% alcohol |
265 IR 88% |
1.502–1.507 0.926–0.935 |
|
|
07.088 400 |
Methyl‐δ‐ionone |
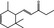
|
2713 11852 7784‐98‐7 |
Liquid C14H22O 206.33 |
Insoluble |
232 IR 95% |
1.493–1.499 0.931–0.938 |
|
|
07.091 390 |
γ‐Ionone |
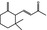
|
3175 79‐76‐5 |
Liquid C13H20O 192.30 |
125 (13 hPa) NMR MS 95% |
1.496–1.502 (25°) 0.932–0.935 (20°) |
||
|
07.130 386 |
δ‐Damascone |
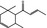
|
3622 57378‐68‐4 |
Liquid C13H20O 192.30 |
1 mL in 10 mL 95% alcohol |
82 (3 hPa) IR 96.5% |
1.485–1.502 0.920–0.940 |
|
|
07.134 385 |
α‐Damascone |
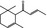
|
3659 11053 43052‐87‐5 |
Liquid C13H20O 192.30 |
1 mL in 10 mL 95% alcohol |
90–100 IR 99% |
1.492–1.499 0.928–0.938 |
Peroxides < 200 μg O2/g Other constituents < 0.03% (including epoxides and polyconjugated diones) |
|
07.170 1571 |
β‐Ionone epoxidef |
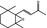
|
4144 11202 23267‐57‐4 |
Solid C13H20O2 208.30 |
Insoluble Soluble |
48 NMR MS 95% |
n.a. n.a. |
|
| 07.225 | cis‐1‐(2,6,6‐Trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one |
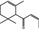
|
23726‐94‐5 |
Liquid C13H20O 192.3 |
Insoluble Soluble |
MS 92% |
1.492–1.499 0.928–0.938 |
|
|
07.226 2188 |
trans‐1‐(2,6,6‐Trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one |
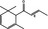
|
4088 ‐ 24720‐09‐0 |
Liquid C13H20O 192.30 |
Freely soluble |
54 (0.1 hPa) IR, MS 95% |
1.493–1.499 0.937–0.943 |
|
| 07.231 | α‐Damascenonef |
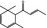
|
35044‐63‐4 |
Liquid C13H18O 190.28 |
Practically insoluble or insoluble Freely soluble |
51 (0.1 hPa) MS 95% |
1.502–1.508 1.015–1.021 |
n.a.: not applicable.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
Stereoisomeric composition not specified.
Twelve of the substances in the present FGE have been evaluated by JECFA, a summary of their current evaluation status by JECFA is given in Appendix B, Table B.1 (JECFA, 1999, 2006, 2014).
Table B.1.
Summary of safety evaluation applying the procedure (based on intakes calculated by the MSDI approach)
| FL‐no JECFA‐no\ | EU Register name | Structural formula | MSDIa (μg/capita per day) | Classb Evaluation procedure pathc | Outcome on the named compoundd , e | EFSA comments |
|---|---|---|---|---|---|---|
|
02.105 391 |
4‐(2,6,6‐Trimethyl‐2‐cyclohexenyl)but‐3‐en‐2‐ol |
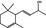
|
0.61 0.06 |
Class I A3: Intake below threshold |
d |
Evaluated in FGE.210Rev1, genotoxicity concern can be ruled out Evaluated by JECFA before 2000 |
|
07.007 388 |
α‐Ionone |
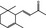
|
270 150 |
Class I A3: Intake below threshold |
d |
Evaluated in FGE.210Rev1, genotoxicity concern can be ruled out Evaluated by JECFA before 2000 |
|
07.009 398 |
Methyl‐ α‐ionone |
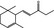
|
86 7 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.210Rev1, genotoxicity concern can be ruled out Evaluated by JECFA before 2000 |
|
07.011 403 |
4‐(2,5,6,6‐Tetramethyl‐2‐cyclohexenyl)‐3‐buten‐2‐one |
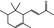
|
7.7 3 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.210Rev1, genotoxicity concern can be ruled out Evaluated by JECFA before 2000 |
|
07.036 404 |
α‐Isomethyl ionone |
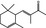
|
4.7 1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.210Rev1, genotoxicity concern can be ruled out Evaluated by JECFA before 2000 |
|
07.061 401 |
Allyl α‐ionone |
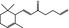
|
30 25 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.210Rev2, genotoxicity concern can be ruled out Evaluated by JECFA before 2000 |
|
07.088 400 |
Methyl‐ δ‐ionone |
|
0.37 1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.210Rev1, genotoxicity concern can be ruled out Evaluated by JECFA before 2000 |
|
07.091 390 |
γ‐Ionone |
|
0.012 15 |
Class I B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.210Rev1, genotoxicity concern can be ruled out Evaluated by JECFA before 2000 |
|
07.130 386 |
δ‐Damascone |
|
0.31f 0.6 |
Class I B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.210Rev3, genotoxicity concern could not be ruled out Evaluated by JECFA before 2000 |
|
07.170 1571 |
β‐Ionone epoxide |
|
0.073 0.1 |
Class III A3: Intake below threshold |
d | Evaluated in FGE.210Rev1, genotoxicity concern can be ruled out |
|
07.134 385 |
α‐Damascone |
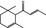
|
7.97f 0.4 |
Class I B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.210Rev3, genotoxicity concern could not be ruled out |
| 07.225 | cis‐1‐(2,6,6‐Trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one |
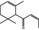
|
1.35f |
Class I No evaluation |
Evaluated in FGE.210Rev3, genotoxicity concern could not be ruled out | |
|
07.226 2188 |
tr‐1‐(2,6,6‐Trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one |
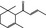
|
0.27f |
Class I B3: Intake below threshold, B4: no adequate NOAEL exists B5: intake greater than 1.5 μg/day |
e | Evaluated in FGE.210Rev3, genotoxicity concern could not be ruled out |
| 07.231 | α‐Damascenone |
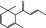
|
0.07f |
Class I No evaluation |
Evaluated in FGE.210Rev3, genotoxicity concern could not be ruled out |
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9 / (0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
MSDI value calculated based on updated EU poundage data (from EFFA Poundage Survey covering year 2015) submitted by EFFA (EFFA, 2019).
As the α,β‐unsaturated ketone structure is considered as structural alert for genotoxicity (EFSA, 2008a), the available data on genotoxic or carcinogenic activity for the 13 α,β‐unsaturated ketones [FL‐no: 07.007, 07.009, 07.011, 07.036, 07.061, 07.088, 07.091, 07.130, 07.134, 07.170, 07.225, 07.226 and 07.231] and a precursor for such ketones [FL‐no: 02.105] are considered in this FGE. The representative substances for the flavouring substances in subgroup 2.4 of FGE.19 are shown in Table 1 (EFSA, 2008c).
Table 1.
Representative substances for subgroup 2.4 of FGE.19 (EFSA, 2008c)
| Subgroup | FL‐no | Register name for representatives | Structural formula |
|---|---|---|---|
| 2.4a | 07.007 | α‐Ionone |
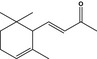
|
| 07.061 | Allyl α‐ionone |
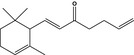
|
|
| 07.170 | β‐Ionone epoxide |
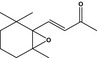
|
|
| 2.4b | 07.134 | α‐Damascone |
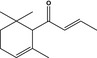
|
2.2.2. Specifications
Specifications of the flavouring substances in this FGE are presented in Appendix A, Table A.1 (JECFA, 1998, 2000, 2001, 2002, 2003, 2005, 2014).
2.3. History of the evaluation of the substances belonging to FGE.210
In FGE.210 (EFSA, 2009), EFSA considered 13 flavouring substances corresponding to subgroup 2.4 of FGE.19. Twelve of these substances are α,β‐unsaturated alicyclic ketones [FL‐no: 07.007, 07.009, 07.011, 07.036, 07.061, 07.088, 07.091, 07.130, 07.134, 07.170, 07.226 and 07.231] and one is a precursor for such ketones [FL‐no: 02.105]. One of the substances has a terminal double bond [FL‐no: 07.061] and one [FL‐no: 07.170] is an epoxide. The genotoxicity concern with respect to the 13 α,β‐unsaturated alicyclic ketones and precursors could not be ruled out based on the genotoxicity data and the (Q)SAR predictions available (Appendix C, Table C.1). The Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) Panel therefore concluded that additional data on genotoxicity on substances representative for this subgroup should be provided according to the Genotoxicity Test Strategy for Substances Belonging to Subgroups of FGE.19 (EFSA, 2008b, 2009).
Table C.1.
QSAR predictions on mutagenicity in five models for 10 ketones from subgroup 2.4
| FL‐no JECFA‐no | EU Register name | Structural formulaa | ISS Local Model Ames Test TA100b | MultiCASE Ames testc | MultiCASE Mouse lymphoma testd | MultiCASE Chromosomal aberration test in CHOe | MultiCASE Chromosomal aberration test in CHLf |
|---|---|---|---|---|---|---|---|
|
07.007 388 |
α‐Ionone |
|
NEG | NEG | NEG | NEG | EQU |
|
07.009 398 |
Methyl‐ α‐ionone |
|
NEG | NEG | OD | NEG | EQU |
|
07.011 403 |
4‐(2,5,6,6‐Tetramethyl‐2‐cyclohexenyl)‐3‐buten‐2‐one |
|
NEG | NEG | OD | NEG | EQU |
|
07.036 404 |
α‐Isomethyl ionone |
|
NEG | NEG | NEG | NEG | NEG |
|
07.061 401 |
Allyl α‐ionone |
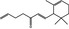
|
NEG | NEG | NEG | NEG | EQU |
|
07.088 400 |
Methyl‐ δ‐ionone |
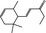
|
NEG | NEG | OD | OD | EQU |
|
07.091 390 |
γ‐Ionone |
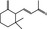
|
NEG | NEG | NEG | NEG | EQU |
|
07.130 386 |
δ‐Damascone |
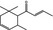
|
NEG | NEG | NEG | NEG | EQU |
|
07.134 385 |
α‐Damascone |
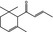
|
NEG | NEG | OD | NEG | OD |
| 07.231 | α‐Damascenone |
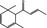
|
NEG | NEG | OD | OD | OD |
| 07.170 | β‐Ionone epoxide |
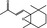
|
NYA | NEG | OD | OD | OD |
|
07.226 2188 |
tr‐1‐(2,6,6‐Trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one |
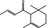
|
NYA | NEG | NEG | NEG | OD |
OD: out of applicability domain: not matching the range of conditions where a reliable prediction can be obtained in this model. These conditions may be physicochemical, structural, biological, etc; EQU: equivocal; NEG: negative; NYA: not yet assessed; POS: positive.
Structure group 2.4: α,β‐unsaturated ketones.
Local model on aldehydes and ketones, Ames TA100.
MultiCase Ames test.
MultiCase Mouse Lymphoma test.
MultiCase Chromosomal aberration in CHO.
MultiCase Chromosomal aberration in CHL.
In FGE.210Rev1 (EFSA CEF Panel, 2014), additional data submitted by Industry for the substances α‐ionone [FL‐no: 07.007], allyl α‐ionone [FL‐no: 07.061], δ‐damascone [FL‐no: 07.130], β‐ionone epoxide [FL‐no: 07.170] (IOFI, 2013a) and α‐damascone [FL‐no: 07.134] (IOFI, 2013b) were evaluated. In FGE.210Rev1, one additional substance was included in subgroup 2.4, cis‐1‐(2,6,6‐trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one [FL‐no: 07.225]. Based on the new data, the CEF Panel concluded that the genotoxicity concern for α‐ionone [FL‐no: 07.007] and six structurally related substances [FL‐no: 02.105, 07.009, 07.011, 07.036, 07.088 and 07.091] and for β‐ionone epoxide [FL‐no: 07.170] could be ruled out. These eight substances can accordingly be evaluated using the Procedure. For allyl α‐ionone [FL‐no: 07.061] and for α‐damascone [FL‐no: 07.134] and the four structurally related substances [FL‐no: 07.130, 07.225, 07.226 and 07.231], the newly submitted data could not rule out the concern with respect to genotoxicity and additional data were requested. The Flavouring Industry submitted additional genotoxicity data for allyl α‐ionone [FL‐no: 07.061], and based on these new data, in FGE.210Rev2 (EFSA CEF Panel, 2015), the CEF Panel concluded that the genotoxicity concern for allyl α‐ionone [FL‐no: 07.061] could be ruled out.
| FGE | Adopted by EFSA | Link | No. of Substances |
|---|---|---|---|
| FGE.210 | 29 January 2009 | http://www.efsa.europa.eu/en/efsajournal/pub/1030.htm | 13 |
| FGE.210Rev1 | 30 January 2014 | http://www.efsa.europa.eu/en/efsajournal/pub/3587.htm | 14 |
| FGE.210Rev2 | 24 June 2015 | http://www.efsa.europa.eu/en/efsajournal/pub/4172.htm | 14 |
| FGE.210Rev3 | 28 March 2019 | http://www.efsa.europa.eu/en/efsajournal/pub/5676.htm | 14 |
The present revision of FGE.210, FGE.210Rev3, concerns the evaluation of additional data submitted for α‐damascone [FL‐no: 07.134]: an in vitro micronucleus (MN) study (Covance, 2014), a combined in vivo MN assay and comet assay (Covance, 2016) and two in vivo comet assays in liver (BioReliance, 2018a,b). These data are considered to cover also the genotoxicity concern for the four structurally related substances [FL‐no: 07.130, 07.225, 07.226 and 07.231].
Section 2.4 reports the same information that was presented in FGE.210. Section 2.5 reports the data evaluated by the Panel in FGE.210Rev1 and Section 2.6 reports the evaluation of the data for allyl α‐ionone [FL‐no: 07.061] in FGE.210Rev2. The new data to be evaluated in the present revision of FGE.210 (FGE.210Rev3) are presented in Section 3.
2.4. Data evaluated by the Panel in FGE.2104
2.4.1. (Q)SAR predictions
The CEF Panel has also taken into consideration the outcome of the predictions from five selected (Q)SAR models (Benigni & Netzeva, 2007a; Gry et al., 2007; Nikolov et al., 2007) on the ketones [FL‐no: 07.007, 07.009, 07.011, 07.036, 07.061, 07.088, 07.091, 07.130, 07.134, 07.170, 07.226 and 07.231].
In Appendix C, Table C.1, the outcomes of the (Q)SAR predictions for possible genotoxic activity in five in vitro (Q)SAR models (ISS‐Local Model‐Ames test, DTU‐NFI MultiCASE‐Ames test, ‐Chromosomal aberration test (Chinese hamster ovary (CHO)), ‐Chromosomal aberration test (Chinese hamster Lung (CHL)) and ‐mouse lymphoma test) are presented.
For all substances, the (Q)SAR models predict negative results in tests for gene mutations, with the restriction that about half of the substance predictions are out of domain for the mouse lymphoma assay. It is noted that predictions for chromosomal aberrations (CA) are diverging in the sense that, for CA in CHO cells, the predictions are invariably negative (three are out of domain), while for the same endpoint in another but very similar cell type (CHL cells), only for one substance a negative response was predicted. For most of the remaining substances, the predictions in the CA (CHL) test were equivocal, and for four substances, the predictions were out of domain (Appendix C, Table C.1).
2.4.2. Genotoxicity studies
In subgroup 2.4, there are two in vitro studies on α‐ionone [FL‐no: 07.007], one in vitro study on methyl‐α‐ionone [FL‐no: 07.009] and one in vitro study on methyl‐δ‐ionone [FL‐no: 07.088]. Only one in vivo study for methyl‐α‐ionone [FL‐no: 07.009] is available for this subgroup.
Study validation and results are presented in Appendix D, Tables D.1 and D.2.
Table D.1.
Genotoxicity data (in vitro)
| Chemical name [FL‐no] | Test system | Test object | Concentration | Reported result | Reference | Commentsc |
|---|---|---|---|---|---|---|
| α‐Ionone [07.007] | Chromosomal aberration | Chinese hamster B241 cell line | 25 nmol/L | Positivea | Kasamaki et al. (1982) | Limited validity (limited documentation; results for only one test concentration reported; long incubation period of 24 h; unusual cell line) |
| Reverse mutation | S. typhimurium TA98, TA100 | 0.01–50 μg/plate | Negativea | Kasamaki et al. (1982) | Limited validity (insufficiently reported; only two strains) | |
| Rec assay | B. subtilis H17 & M45 | 19 mg/disc | Negativeb | Oda et al. (1978) | Insufficient validity. This bacterial DNA‐repair test system is of low predictive value for genotoxicity | |
| Methyl‐α‐ionone [07.009] | Reverse mutation | S. typhimurium TA1535, TA1537, TA1538, TA98, TA100 | 5 concentrations up to cytotoxicity or max 3,600 μg/plate | Negativea | Wild et al. (1983) | Limited validity (no TA102 or E. coli) |
| Methyl‐δ‐ionone [07.088] | Reverse mutation | S. typhimurium TA1535, TA1537, TA1538, TA98, TA100 | 5 concentrations up to cytotoxicity or max 3,600 μg/plate | Negativea | Wild et al. (1983) | Limited validity (no TA102 or E. coli) |
With and without metabolic activation.
Activation status unknown.
- Valid.
- Limited validity (e.g. if certain aspects are not in accordance with OECD Guidelines or current standards and/or limited documentation).
- Insufficient validity (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards and/or inappropriate test system).
- Validity cannot be evaluated (e.g. insufficient documentation, short abstract only, too little experimental details provided).
Table D.2.
Genotoxicity data (in vivo)
| Chemical name [FL‐no] | Test system | Test object | Route | Dose | Reported result | Reference | Commentsa |
|---|---|---|---|---|---|---|---|
| Methyl α‐ionone [07.009] | Micronucleus formation | NMRI mice, male and female, bone marrow | I.P. | 825–2063 mg/kg bw | Negative | Wild et al. (1983) | Limited validity (only analysis at one time point; no PCE/NCE ratio reported) |
| Sex‐linked recessive lethals | Drosophila melanogaster | Feed | 20 mM | Negative | Limited validity (limited reporting, test system considered of limited relevance) |
- Valid.
- Limited validity (e.g. if certain aspects are not in accordance with OECD Guidelines or current standards and/or limited documentation).
- Insufficient validity (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards and/or inappropriate test system).
- Validity cannot be evaluated (e.g. insufficient documentation, short abstract only, too little experimental details provided).
The available in vitro bacterial gene mutation studies with limited validities do not indicate a concern for the tested substances from this group. One of the in vitro tests (Rec assay) is a system which has limited predictive validity for genotoxicity. An in vivo test with limited validity produced a negative result for gene mutations in Drosophila melanogaster. A limited in vitro test for structural chromosomal damage produced a positive response with α‐ionone, but a limited in vivo mammalian test for the same endpoint with α‐ionone gave a negative outcome.
2.4.3. Carcinogenicity studies
No carcinogenicity studies are available for the substances in subgroup 2.4.
2.4.4. Conclusion on genotoxicity and carcinogenicity
The data ((Q)SAR and testing data) are not sufficient to rule out a concern for genotoxicity for these substances in subgroup 2.4.
2.4.5. Conclusion based on the data available to the Panel in FGE.210
The CEF Panel concluded that a genotoxic potential of the 13 α,β‐unsaturated alicyclic ketones and precursors in the present FGE.210 [FL‐no: 02.105, 07.007, 07.009, 07.011, 07.036, 07.061, 07.088, 07.091, 07.130, 07.134, 07.170, 07.226 and 07.231] could not be ruled out based on the data available. Accordingly these 13 substances cannot be evaluated through the Procedure, presently. Additional data on genotoxicity for the representative substances of this subgroup should be provided according to the Genotoxicity Test Strategy for substances belonging to Subgroups of FGE.19 (EFSA, 2008b).
2.5. Additional genotoxicity data evaluated by the CEF Panel in FGE.210Rev15
2.5.1. Presentation of the additional data
The revision 1 of FGE.210 (FGE.210Rev1) concerns the evaluation of additional data submitted by Industry for the representative substances α‐ionone [FL‐no: 07.007], allyl α‐ionone [FL‐no: 07.061], β‐ionone epoxide [FL‐no: 07.170] and α‐damascone [FL‐no: 07.134] for subgroup 2.4 (EFSA CEF Panel, 2014). Furthermore, data on genotoxicity of δ‐damascone [FL‐no: 07.130] have been submitted.
In response to the EFSA request in FGE.210 (EFSA, 2009) for additional genotoxicity data for subgroup 2.4, the Flavouring Industry (IOFI, 2013a,b) has submitted genotoxicity data as reported in Table 2.
Table 2.
Studies evaluated in FGE.210Rev1
| Substance/study type | Bacterial mutation | Mouse lymphoma tk gene mutations | In vitro micronucleus | In vivo micronucleus |
|---|---|---|---|---|
| α‐Ionone [FL‐no: 07.007] | Bowen (2011) | Lloyd (2013b) | Krsmanovic and Huston (2006) | |
| Allyl α‐ionone [FL‐no: 07.061] | Ballantyne, (2011), Wild et al. (1983) | Lloyd (2013a) | ||
| δ‐Damascone [FL‐no: 07.130] | Shinya (2006) | |||
| α‐Damascone [FL‐no: 07.134] | Haddouk (2001) | Lloyd (2012), Lloyd (2013c), Whitwell (2012) | ||
| β‐Ionone epoxide [FL‐no: 07.170] | Jones and Wilson (1988), Kringstad (2005) | Flanders (2006) |
2.5.2. In vitro data
2.5.2.1. Bacterial reverse mutation assay
α‐Ionone [FL‐no: 07.007]
An Ames assay was conducted in Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 to assess the mutagenicity of α‐ionone, both in the absence and in the presence of metabolic activation by S9‐mix (from livers of rats induced with Aroclor 1254), in three separate experiments (Bowen, 2011). This study was performed following good laboratory practice (GLP) recommendations and according to OECD Guideline 471 (OECD, 1997a). An initial experiment was carried out both in the absence and presence of S9‐mix activation in all five strains, using 0.3, 1.6, 8, 40, 200, 1,000 and 5,000 μg of α‐ionone/plate, plus negative (solvent) and positive controls. Evidence of toxicity was observed at 1,000 and/or 5,000 μg/plate across all strains in the absence and presence of S9‐mix with the exception of TA100 in which no clear evidence of toxicity, in the presence of S9‐mix, was observed at 5,000 μg/plate (Appendix E, Table E.1).
Table E.1.
In vitro genotoxicity data evaluated in FGE.210Rev1
| Chemical name [FL‐no] | Test system in vitro | Test object | Concentrations of substance and test conditions | Result | Reference | Comments |
|---|---|---|---|---|---|---|
| α‐Ionone [07.007] | Reverse mutation | S. typhimurium TA98, TA100, TA1535, TA1537 and TA102 |
Negative Negative |
Bowen (2011) | Toxicity was observed at 1,000 and/or 5,000 μg/plate across all strains in the absence and presence of S9‐mix; no clear evidence of toxicity in TA100 in the presence of S9‐mix. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix | |
|
S. typhimurium TA98 TA100 |
Negative Negative |
Evidence of toxicity was observed at the highest three or four concentrations across all strains in the absence and presence of S9‐mix. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix | ||||
|
S. typhimurium TA98 TA1535, TA1537 and TA102 |
Negative Negative |
Evidence of toxicity was observed at the highest three or four concentrations across all strains in the absence or presence of S9‐mix. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix | ||||
|
S. typhimurium TA1535 TA102 TA1537 |
39.1–2,500 μg/platea , c or b , d |
Negative | Evidence of toxicity was observed at the highest three or four concentrations across all strains in the absence or presence of S9‐mix. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix | |||
| Micronucleus induction | Human peripheral blood lymphocytes |
Negative Negative |
Lloyd (2013b) | The MNBN cell frequencies in all treated cultures fell within the normal range. The study does not comply with OECD Guideline 487; therefore, it has limited validity | ||
| Allyl α‐ionone [07.061] | Reverse mutation | S. typhimurium TA102 | 1.6–5,000 μg/platea , c or b , c | Negative | Ballantyne (2011) | No evidence of toxicity was observed at any concentration. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix |
| 51.2–5,000 μg/platea , c or b , d | Negative | No evidence of toxicity was observed at any concentration. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix | ||||
| Reverse mutation | S. typhimurium TA1535, TA100, TA1537, TA1538, TA98 | Five concentrations up to cytotoxicity or max 3,600 μg/platea , c | Negative | Wild et al. (1983) | Limited validity (no TA102 or E. coli) | |
| Micronucleus induction | Human peripheral blood lymphocytes |
Negative Negative |
Lloyd (2013a) | The MNBN cell frequencies in all treated cultures fell within the normal range. The study does not comply with OECD Guideline 487; therefore, it has limited validity | ||
|
δ‐Damascone [07.130] |
Reverse mutation | S. typhimurium TA98, TA100, TA1535 and TA1537 | 4.9–5,000 μg/plated , e | Negative | Shinya (2006) | Evidence of toxicity was observed at the top three or four concentrations tested. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix |
| S. typhimurium TA98, TA100, TA1535 and TA1537 | Negative | Evidence of toxicity was observed at the top concentration in all strains in the absence of S9‐mix and at 156 μg/plate or above in the presence of S9‐mix. The study complies with current recommendations for upper concentration limit inclusion. The study included three replicate plates per concentration and was GLP compliant | ||||
| E. coli WP2 uvrA | 4.9–5,000 μg/platee , d | Negative | Evidence of toxicity was observed at the top three or four concentrations tested. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix | |||
| E. coli WP2 uvrA | Negative | Evidence of toxicity was observed at the highest concentration in the presence of S9‐mix. The study complies with current recommendations for upper concentration limit inclusion. The study included three replicate plates per concentration and was GLP compliant | ||||
|
α‐Damascone [07.134] |
Reverse mutation | S. typhimurium TA98 and TA100 | 10–5,000 μg/platee , c | Negative | Haddouk (2001) | In TA98, slight to marked toxicity was observed at concentrations ≥ 100 μg/plate or 500 μg/plate with and without S9‐mix, respectively. In TA100, toxicity was observed at concentrations ≥ 500 μg/plate with and without S9‐mix |
| S. typhimurium TA1537, TA98 |
Negative Negative |
Slight toxicity was observed in all strains. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix. | ||||
|
S. typhimurium TA100 and TA1535 |
Negative Negative |
Slight toxicity was observed in all strains. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix. | ||||
| S. typhimurium TA1535, TA1537, TA98 and TA100 | 15.6–250 μg/platea , c | Negative | Slight toxicity was observed in all strains. No statistically significant increase in revertant numbers was seen at any concentration. | |||
| E. coli WP2uvrA | 10–5,000 μg/platee , c | Negative | Slight toxicity was observed at 2,500 μg/plate and above without S9‐mix. | |||
| E. coli WP2 uvrA |
Negative Negative |
Slight toxicity was observed only at the highest concentration tested without S9‐mix. No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix | ||||
| Micronucleus induction | Human peripheral blood lymphocytes |
Negative Weakly positive Negative |
Lloyd (2012) | Weakly positive result was obtained only in the 3 + 21 h treatment in the presence of S9‐mix. Study design complies with OECD Guideline 487 | ||
| Positive | Whitwell (2012) | Follow‐up study to explore different methods of mixing and sample preparation to overcome the challenges in inconsistent cytotoxicity that results in difficulties in choosing concentrations for scoring of micronucleated binucleate cells. Experiment conducted only for 3 + 21 h in the presence of S9‐mix | ||||
| Positive at high toxic concentrations only | Lloyd (2013c) | Positive results were obtained only at high toxic concentrations in both test conditions. Study is robust and complies with GLP | ||||
| β‐Ionone epoxide [07.170] | Reverse mutation | S. typhimurium TA98, TA100, TA1535 and TA1537 | 5–500 μg/platee , c | Negative | Jones and Wilson (1988) | No statistically significant increase in revertant numbers was seen at any concentration, either in the presence or absence of S9‐mix |
| S. typhimurium TA97a, TA98, TA100, TA1535 |
Negative Negative |
Kringstad (2005) | Evidence of toxicity was observed at the highest concentration in strain TA97a in the absence of S9‐mix and in TA100 in the absence and presence of S9‐mix. The study, therefore, complies with current recommendations for upper concentration limit inclusion. The study included three replicate plates per concentration and was GLP compliant | |||
| E. coli WP2uvrA |
Negative Negative |
|||||
| tk Mutation induction | Mouse Lymphoma L5178Y TK +/− 3.7.2c cells |
Negative Negative |
Flanders (2006) | A preliminary range‐finder assay was conducted to establish maximum concentrations. Top concentrations in each arm of the study induced 77, 85 and 80% reductions in relative total growth. The study, therefore, complies with current recommendations |
Without S9 metabolic activation.
With S9 metabolic activation.
Plate incorporation method.
Pre‐incubation with S9 method.
With and without S9 metabolic activation.
4‐h treatment.
24‐h treatment.
3‐h treatment with 21‐h recovery.
24‐h treatment with 0‐h recovery.
Standard treatment in larger than typical vessel.
In a second experiment, the concentrations were changed lowering to 2,500 μg/plate for all strains and conditions with the exception of TA98 in the presence of S9‐mix and for TA100 in the presence and absence of S9‐mix. In this second experiment, the concentration intervals were narrowed, covering the ranges 156.3–5,000 μg/plate or 78.1–2,500 μg/plate in order to better detect possible concentration‐dependent mutation. In addition, a pre‐incubation step with S9‐mix activation treatment was added to increase the chance of detecting a positive response. In this experiment, evidence of toxicity ranging from a diminution of the background bacterial lawn and/or a reduction in revertant numbers to a complete killing of the test bacteria was observed at 1,250 μg/plate and above for strain TA98 in the presence of S9‐mix, at 625 μg/plate in strains TA98 in the absence of S9‐mix and TA100 with and without S9‐mix. Toxicity was observed at 312.5 μg/plate and above in all remaining strains.
The third experiment was conducted using strains TA1535 and TA102 in the absence and presence of S9‐mix activation and strain TA1537 in the presence of S9 activation. The maximum test concentration was 2,500 μg/plate for TA1535 while was further reduced for TA102 (± S9) and for TA1537 to 1,250 μg/plate. In addition, more narrow concentration intervals were used, covering either 39.06–2,500 μg/plate or 19.53–1,250 μg/plate. Evidence of toxicity was observed at the highest three or four concentrations across all strains in the absence or presence of S9‐mix.
In all three experiments, no statistically significant increases in revertant numbers were observed at any concentration, in any of the strains, either in the presence or absence of S9‐mix activation.
It was concluded that α‐ionone did not induce mutations in five strains of S. typhimurium, when tested under the conditions of this study.
Allyl α‐ionone [FL‐no: 07.061]
An Ames assay was conducted in S. typhimurium strain TA102 to assess the mutagenicity of allyl α‐ionone, both in the absence and in the presence of metabolic activation by S9‐mix (from livers of rats induced with Aroclor 1254), in two separate experiments (Ballantyne, 2011). The study was performed following GLP principles and according to the OECD Guideline 471 (OECD, 1997a), except that only TA102 was used (Appendix E, Table E.1). An initial experiment was carried out both in the absence and presence of S9‐mix activation in the TA102 strain, using 1.6, 8, 40, 200, 1,000 and 5,000 μg of allyl α‐ionone/plate plus vehicle and positive controls. In the second experiment, the highest concentration was retained, but more narrow concentration intervals were used, starting at 51.2 μg/plate (51.2, 128, 320, 800, 2,000 and 5,000 μg/plate). The standard plate incorporation assay was used in the first experiment and a pre‐incubation step with S9‐mix activation treatment was added in the second experiment to increase the chance of detecting a positive response. No evidence of toxicity was observed under any of the conditions tested.
In both experiments, no statistically significant increases in revertant numbers were observed at any concentration in strain TA102, either in the presence or absence of S9‐mix activation.
It was concluded that allyl α‐ionone did not induce mutation in the histidine‐requiring S. typhimurium strain TA102 when tested under the conditions of this study. The authors justified to test only TA102 strain because this study was intended to be complementary to a previous study from (Wild et al., 1983) where data on the other strains were provided.
δ‐Damascone [FL‐no: 07.130]
A modified Ames assay using the pre‐incubation method was conducted in S. typhimurium strains TA98, TA100, TA1535 and TA1537 and Escherichia coli WP2uvrA to assess the mutagenicity of δ‐damascone (purity: 93.8%), both in the absence and in the presence of metabolic activation by S9‐mix (from livers of rats induced with Aroclor 1254), in three separate experiments (Shinya, 2006). The assay was performed according to OECD Guideline 471 (OECD, 1997a) and according to GLP principles (Appendix E, Table E.1).
An initial experiment was carried out both in the absence and presence of S9‐mix activation in all five strains at 4.9, 19.5, 78.1, 313, 1,250 and 5,000 μg of δ‐damascone/plate, plus negative (solvent) and positive controls. In the absence of S9‐mix, toxicity (decrease of bacterial growth and/or of revertants) was reported at 78.1 μg/plate and above and in the presence of S9‐mix, toxicity was reported at 313 μg/plate and above. In the second experiment with tighter ranges of concentrations to reflect the toxicity observed in the previous experiment, δ‐damascone was incubated with all five tester strains in the absence of S9‐mix (2.4, 4.9, 9.8, 19.5, 39.1 or 78.1 μg of δ‐damascone/plate) and in the presence of S9‐mix (9.8, 19.5, 39.1, 78.1, 156 and 313 μg of δ‐damascone/plate). Toxicity was observed in the absence of S9‐mix at top concentration and in the presence of S9‐mix at 156 μg/plate and above. In the third experiment, the same conditions as described for the second experiment were used. In all three experiments, there were no significant increases in the number of revertants in the absence or presence of S9‐mix. It was concluded that δ‐damascone did not induce mutations in four strains of S. typhimurium or E. coli WP2uvrA under the conditions employed (Shinya, 2006).
α‐Damascone [FL‐no: 07.134]
Ames assays were conducted in S. typhimurium strains TA1535, TA1537, TA98 and TA100 and E. coli WP2uvrA to assess the mutagenicity of α‐damascone (purity: 96.9%), both in the absence and in the presence of metabolic activation by S9‐mix (from livers of rats induced with Aroclor 1254), in two separate experiments (Haddouk, 2001). The assay was performed according to OECD Guideline 471 (OECD, 1997a) and according to GLP principles (see Appendix E, Table E.1).
An initial experiment to assess toxicity was carried out both in the absence and presence of S9‐mix activation in the tester strains, using 10, 100, 500, 1,000, 2,500 and 5,000 μg of α‐damascone/plate in strains TA98, TA100 and WP2 uvrA, plus vehicle and positive controls. Concentration levels greater than or equal to 2,500 μg/plate showed evidence of an emulsion on the plates. In TA98, slight to marked toxicity was observed at concentrations greater than or equal to 100 μg/plate or 500 μg/plate in the absence and presence of S9‐mix, respectively. In TA100, toxicity was observed at concentrations greater than or equal to 500 μg/plate in the absence and presence of S9‐mix. In E. coli WP2 uvrA, slight toxicity was observed at 2,500 μg/plate and above without S9‐mix but not with S9‐mix. Based on the preliminary toxicity test, a standard Ames test using the plate incorporation method was conducted using 31.2, 62.5, 125, 250 and 500 μg of α‐damascone/plate for strains TA1535 and TA100 and 7.8, 15.6, 31.2, 62.5 and 125 μg of α‐damascone/plate for strains TA1537 and TA98 in the absence and presence of S9‐mix. Additionally α‐damascone (312.5, 625, 1250, 2,500 and 5,000 μg/plate) was tested in E. coli WP2 uvrA for reverse mutation in the absence and presence of S9‐mix. In the second experiment, α‐damascone was tested in all S. typhimurium tester strains in the absence of S9‐mix at the following concentrations: 15.6, 31.2, 62.5, 125 and 250 μg/plate. In the second experiment, the tests run in the presence of S9‐mix were performed with the pre‐incubation (modified Ames) method at concentrations of 31.2, 62.5, 125, 250 and 500 μg of α‐damascone/plate for strains TA1535 and TA100 and at concentrations of 15.6, 31.2, 62.5, 125 and 250 μg of α‐damascone/plate for strains TA1537 and TA98. Additionally, α‐damascone (312.5, 625, 1,250, 2,500 and 5,000 μg/plate) was tested in E. coli WP2 uvrA for reverse mutation in the absence of S9‐mix (with the plate incorporation method) and in the presence of S9‐mix (with the pre‐incubation method). Slight evidence of toxicity was observed under the conditions tested through thinning of the background bacterial lawn and/or a decrease in revertant count in Salmonella strains. In the E. coli strain, a slight toxicity was observed only at 5,000 μg/plate in the absence of S9‐mix.
In both experiments, no statistically significant increases in revertant numbers were observed at any concentration in any of the strains, either in the presence or absence of S9‐mix activation.
It was concluded that α‐damascone did not show mutagenic activity towards S. typhimurium or E. coli in the bacterial reverse mutation test (Haddouk, 2001). The Panel agreed with the conclusion of the author.
β‐Ionone epoxide [FL‐no: 07.170]
β‐Ionone epoxide was tested for mutagenicity in an Ames test including four strains of S. typhimurium (TA98, TA100, TA1535 and TA1537) at five concentrations (5, 15, 50, 150, 500 μg/plate) in the absence and in the presence of metabolic activation (S9‐mix at two different concentrations, 3% and 10%) (Jones and Wilson, 1988). The study was performed under GLP and mainly compliant with OECD Guideline 471 (OECD, 1997a), except that only four strains were used (Appendix E, Table E.1). Two independent experiments were performed and the top concentration was selected at 500 μg/plate based on toxicity in a prior range‐finding test. At the concentration tested, no significant toxicity was observed and no substantial increases in mutation were observed in all strains tested and in the presence or absence of S9‐mix.
A more recently reported Ames study on β‐ionone epoxide included four strains of S. typhimurium (TA97a, TA98, TA100, TA1535) plus one strain of E. coli (WP2‐uvrA‐) (Kringstad, 2005). Following a range‐finding assay, β‐ionone was tested in three replicates at 501, 1,582 and 5,000 μg/plate in the absence of S9‐mix metabolic activation and at 158, 501 and 1,582 μg/plate in the presence of metabolic activation, in a single experiment using the plate incorporation method. The top concentration (5,000 μg/plate) induced significant toxicity in strain TA97a in the absence of S9‐mix and also reduced the background lawn in strain TA100 in the presence and absence of S9‐mix, and therefore, the study complies with current recommendations for the choice of concentration. There was no evidence of mutagenicity. Since there are some deviations from the OECD Guideline 471 (OECD, 1997a) (only three concentrations of chemical were tested, in some cases only two concentrations could be analysed due to an excessive level of cytotoxicity and only a single experiment was performed), the test is considered of limited validity.
2.5.2.2. Mouse lymphoma thymidine kinase gene mutation assay
β‐Ionone epoxide [FL‐no: 07.170]
An assay for induction of tk mutations in mouse lymphoma cells (L5178Y T/K +/− 3.7.2c) was conducted on β‐ionone‐epoxide (Flanders, 2006). It included 4 h treatment in the absence and presence of S9‐mix and a 24‐h treatment in the absence of S9‐mix. The concentrations were selected based on a preliminary toxicity test. The test groups included single replicates at eight concentrations ranging from 200 to 900 μg/mL in the 4 h treatment arm and from 4.1 to 520 μg/mL in the 24 h treatment arm. The maximum concentration was limited by toxicity. The substance did not induce biologically or statistically significant increases in mutant frequency, and therefore, it was considered non‐mutagenic in this assay. The study is compliant with OECD Guideline 476 (OECD, 1997c) (Appendix E, Table E.1).
2.5.2.3. In vitro micronucleus assays
α‐Ionone [FL‐no: 07.007]
α‐Ionone was evaluated in an in vitro MN assay in human peripheral blood lymphocytes for its ability to induce chromosomal damage or aneuploidy in the presence and absence of rat S9‐mix fraction (Appendix E, Table E.1). Information about the method used to induce lymphocyte cell division and the duration of the induction before the treatment was not provided. Cells were treated for 24 h with 14 concentrations in a range from 15 to 120 μg/mL of α‐ionone in the absence of S9‐mix. In the presence of S9‐mix, cells were exposed for 3 h followed by 21 h recovery with 15 concentrations in a range from 30 to 200 μg/mL. Based on the toxicity induced by α‐ionone, three concentrations were selected for MN assessment. In the absence of S9‐mix, cells were treated with 40, 50 and 65 μg/mL, while in the presence of S9‐mix, the concentrations selected were 160, 170 and 180 μg/mL. The highest concentrations induced 51% and 56% reduction of index (RI) in the absence and presence of S9‐mix, respectively. MN assessment was performed in a single experiment with duplicates and a total of 1,000 binucleate cells per replicate were scored. No assay with 3 h treatment + 21 h recovery in the absence of S9‐mix was performed as recommended by OECD Guideline 487 (OECD, 2010). Treatment of cells with α‐ionone for 3 h with a 21‐h recovery period in the presence of S9‐mix or for 24 h with no recovery period in the absence of S9‐mix showed no increase in the frequency of micronucleated binucleate (MNBN) cells at any concentration when compared to both concurrent and historical controls. It was concluded that α‐ionone did not induce MN up to the limit of toxicity when assayed in cultured human peripheral lymphocytes under the described exposure conditions (Lloyd, 2013b).
Due to the deviation from the OECD Guideline 487 (OECD, 2010), the study is considered of limited validity.
Allyl α‐ionone [FL‐no: 07.061]
Allyl α‐ionone (purity of 88.5%) was evaluated in an in vitro MN assay in human peripheral blood lymphocytes (Appendix E, Table E.1). Information about the method used to induce lymphocytes cell division and the duration of the induction before the treatment was not provided. Cells were treated for 24 h with 14 concentrations in a range from 5 to 50 μg/mL of allyl α‐ionone in the absence of S9‐mix. In the presence of S9‐mix cells were exposed for 3 h followed by 21 h recovery with 15 concentrations in a range from 25 to 200 μg/mL. Based on the toxicity induced by α‐ionone, three concentrations were selected for MN assessment. In the absence of S9‐mix (24 h treatment), cells were treated with 25, 33, 36 and 38 μg/mL, while in the presence of S9‐mix (3 h treatment), the concentrations selected were 110, 140, 150 and 160 μg/mL. The highest concentrations induced 54% and 63% reduction of RI in the absence and presence of S9‐mix, respectively. MN assessment was performed in a single experiment with duplicates and a total of 2,000 binucleate cells per replicate were scored in the experiment performed in the absence of S9‐mix, while 1,000 binucleated cells were scored in the presence of S9‐mix. No assay with 3‐h treatment + 21‐h recovery in the absence of S9‐mix was performed as recommended by OECD Guideline 487 (OECD, 2010).
Treatment of cells with allyl α‐ionone for 3 h with a 21‐h recovery period in the presence of S9‐mix or for 24 h with no recovery period in the absence of S9‐mix showed no increase in the frequency of MNBN cells at any concentration when compared to both concurrent and historical controls. It was concluded that allyl α‐ionone did not induce MN at concentration up to the limit of toxicity when assayed in cultured human peripheral lymphocytes in the described exposure conditions (Lloyd, 2013a).
Due to the deviation from the OECD Guideline 487 (OECD, 2010), the study is considered of limited validity.
α‐Damascone [FL‐no: 07.134]
Three in vitro MN experiments have been performed in human peripheral blood lymphocytes to determine whether α‐damascone is able to induce chromosomal damage or aneuploidy in the presence and absence of rat S9 fraction as an in vitro metabolising system (Appendix E, Table E.1).
In all three experiments, cells were stimulated for 48 h with phytohaemagglutinin (PHA) to produce exponentially growing cells.
A first experiment (Lloyd, 2012) was performed using standard conditions. Human peripheral blood lymphocytes where treated with α‐damascone (purity 98.3%) for 3 h (followed by 21 h recovery) with 9, 16, 18 or 22 μg/mL and 12, 18, 20, 21 or 22 μg/mL of α‐damascone in the absence and presence of S9‐mix, respectively. The levels of cytotoxicity (reduction in replication index (RI)) at the top concentrations were 55% and 56%, respectively. In a parallel assay, cells were treated for 24 h with 5, 7, 9 and 10 μg/mL of α‐damascone in the absence of S9‐mix with no recovery period. The top concentration induced 57% cytotoxicity. Levels of cytotoxicity were achieved at the top concentrations used in all parts of the study and are acceptable. There were two replicate cultures per treatment and 1,000 binucleate cells per replicate were scored for MN. The study design complies with OECD Guideline 487 and follows GLP principles. Treatment of cells with α‐damascone for 3 h with a 21‐h recovery period in the absence of S9‐mix or for 24 h with no recovery period in the absence of S9‐mix showed no increase in the frequency of MNBN cells at any concentration when compared to both concurrent and historical controls. Treatment of cells with α‐damascone for 3 + 21 h in the presence of S9‐mix resulted in frequencies of MNBN cells that were significantly higher (p < 0.001) when compared to concurrent controls at the two highest test concentrations with 1.40% and 1.70% MNBN at 21 and 22 μg/mL, compared to 0.25% MNBN in the concurrent control. It is noted that although the frequencies of MNBN cells exceed the 95th percentile of the historical controls (0.1–1.2% MNBN), they are still within the normal range when considering extreme limits (0–2.0% MNBN). An additional reading on 1,000 BN cells scored per replicate confirmed the statistically significant increase with 1.03 and 1.0% MNBN at 21 and 22 μg/mL; however, no additional reading was performed in the controls and coding slides. It was concluded that α‐damascone showed weak induction of MN when assayed in cultured human peripheral lymphocytes for 3 + 21 h in the presence of S9‐mix while in the absence of S9‐mix, no induction of MN was observed when tested up to toxic concentrations for 3 + 21 h and 24 + 0 h (Lloyd, 2012).
Since this study (Lloyd, 2012) showed a variable toxicity profile in the treatment for 3 h with a 21‐h recovery period in the presence of S9‐mix, a follow‐up in vitro MN assay was performed (Whitwell, 2012). α‐Damascone was tested on human lymphocyte cultures using different methods of addition/mixing the test substance to the treatment medium, in order to assess and compare the cytotoxicity. A high variability in cytotoxicity was observed and it was concluded that where α‐damascone is prepared for 100% medium replacement and treated in a large volume vessel with vigorous mixing, a smoother and steeper toxicity curve is obtained as compared to using a standard method of addition to the test system. The MN assay was performed using three different methods of adding/mixing α‐damascone to the treatment medium: standard treatment in larger volume vessel (experiment 1), standard treatment in standard vessel (experiment 2) and 100 % medium replacement in a larger volume vessel (experiment 3). The following concentrations were tested: 7.5–14 μg/mL (experiments 1 and 3), 14–20 μg/mL (experiment 2). Data indicated a positive induction of MNBN cells for at least one concentration for each experiment with a concentration‐dependent effect.
Repetition of the experiments under slightly different conditions (with respect to the volume of vessel, mixing conditions and medium replacement) resulted in similar induction of MN. The author of the repeated study concluded that α‐damascone did not induce consistent and biologically relevant increases in the frequency of MN in cultured human peripheral blood lymphocytes, when tested for 3 + 21 h in the presence of S9‐mix and for 24 + 0 h in the absence of S9‐mix (Lloyd, 2013c).
The CEF Panel noted that statistically significant increases of MNBN cells were observed at concentrations that are above the limits of cytotoxicity recommended by the guideline and that the increases were higher than the 95th percentile of the historical control, but that the effects were observed only at the high concentrations at a cytotoxicity level higher than 55%. Under these conditions, the CEF Panel concluded that α‐damascone presents, in this study, an equivocal effect in the in vitro MN test.
The results of in vitro MN studies are summarised in Appendix E, Table E.1.
2.5.3. In Vivo data
2.5.3.1. Bone marrow micronucleus assay
α‐Ionone [FL‐no: 07.007]
α‐Ionone was tested in a mouse bone marrow MN assay (Krsmanovic and Huston, 2006). An initial extensive range‐finding test established a maximum tolerated dose (MTD) of 1,200 mg/kg. Animals were dosed by a single intraperitoneal injection, either with vehicle or with α‐ionone at 300, 600 or 1,200 mg/kg. Groups of five male and five female mice from all treatment levels were sacrificed 24 h after dosing, and additional five mice of each sex from top dose and vehicle control groups were also sacrificed at 48 h after dosing (Appendix E, Table E.2).
Table E.2.
In vivo genotoxicity data evaluated in FGE.210Rev1
| Chemical name [FL‐no] | Test system | Test object | Route | Dose | Reported result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| α‐Ionone [07.009] | Micronucleus formation | Male and female mice | Gavage | 300, 600 and 1,200 mg/kg bw per day | Negative | Krsmanovic and Huston (2006) | Complies with draft OECD Guideline 474. Evidence of bone marrow toxicity as evidenced by reductions in polychromatic erythrocytes observed at 24 h after dosing and in a satellite group at the top dose 48 h after dosing |
Two thousand Polychromatic (PCE) and normochromatic (NCE) erythrocytes per animal were scored for MN. Reductions of 7–18% in PCE were observed in treated males and females at 24 h after dosing; a reduction of 21% in PCE was observed in males at the top dose at 48 h after dosing, indicating bone marrow toxicity. Systemic availability was confirmed by additional clinical signs in treated animals. There were no statistically or biologically significant increases in MN frequency in treated animals. The study is compliant with OECD Guideline 474 (OECD, 1997b) and is sufficiently robust to contribute to the evaluation of clastogenic or aneugenic potential of α‐ionone.
2.5.4. Conclusion based on the new data available to the Panel in FGE.210Rev1
In the first evaluation of the available data on α,β‐unsaturated alicyclic ketones and precursors in FGE.210 (subgroup 2.4 of FGE.19), it was concluded that additional data should be provided for the proper consideration of the genotoxic potential of these substances (EFSA, 2009).
New in vitro data have been submitted for five substances of FGE.19 subgroup 2.4 (FGE.210), four representatives (α‐ionone [FL‐no: 07.007], α‐damascone [FL‐no: 07.134], allyl α‐ionone [FL‐no: 07.061], β‐ionone epoxide [FL‐no: 07.170]), as requested, and one other substance, δ‐damascone [FL‐no: 07.130]. Furthermore, new in vivo data have been submitted for the representative α‐ionone [FL‐no: 07.007].
α‐Ionone [FL‐no: 07.007] did not induce gene mutation in S. typhimurium nor structural or numerical CA when tested with human peripheral lymphocytes. The latter study is of limited validity due to deviation to the OECD Guideline 487 (OECD, 2010) and it is not a GLP study. However, α‐ionone was tested in an in vivo mouse bone marrow MN assay in which no statistically significant increase in the frequency of micronucleated cells was observed. There was an indication for bone marrow exposure; thus, the result is considered reliable.
The new data submitted for β‐ionone epoxide [FL‐no: 07.170] included two in vitro studies in bacteria and mammalian cells. β‐Ionone epoxide did not induce any significant increase in bacterial mutation when evaluated in five different S. typhimurium strains and an E. coli strain, either in the presence or absence of S9 metabolic activation in two independent studies. β‐Ionone epoxide also did not increase mutation frequencies when tested in a tk mutation assay using mouse lymphoma cells either in the presence or absence of S9 metabolic activation. No in vitro assay for chromosomal aberration is available, but the mouse lymphoma assay is a test that is able to detect the chemical potential to induce structural chromosomal aberrations. The lack of an in vitro MN assay is not consistent with the current EFSA guideline (EFSA Scientific Committee, 2011), it is however consistent with the genotoxicity test strategy for substances belonging to subgroups of FGE.19 (EFSA, 2008a) applicable at the time when the scientific opinion on FGE.210 was adopted (EFSA, 2009). The Panel concluded that the data submitted for β‐ionone epoxide are sufficient in the light of data available for structurally related substances.
Therefore, the CEF Panel concluded that based on the current data on the representative substances α‐ionone [FL‐no: 07.007] and β‐ionone epoxide [FL‐no: 07.170], the concern with respect to genotoxicity could be ruled out for these two substances [FL‐no: 07.007 and 07.170] as well as for the six substances structurally related to ionones [FL‐no: 02.105, 07.009, 07.011, 07.036, 07.088 and 07.091]. Accordingly, these eight substances can be evaluated through the Procedure.
Allyl α‐ionone [FL‐no: 07.061] is, due to a terminal double bond, not considered sufficiently structurally related to the other ionones. For allyl α‐ionone [FL‐no: 07.061], two in vitro studies were submitted, a bacterial reverse mutation assay and a MN assay in human peripheral blood lymphocytes. The bacterial mutation assay has been performed only in TA102 strain of S. typhimurium, in the presence and absence of S9 metabolic activation, where no indication of mutation has been observed. A study was previously performed in the other four strains of S. typhimurium and there was no indication of mutation after treatment with allyl α‐ionone. Allyl α‐ionone did not induce chromosomal damage or aneuploidy when tested with human peripheral blood lymphocytes in the absence and presence of S9 metabolic activation. This study is of limited validity due to deviations from the OECD Guideline 487 (OECD, 2010). In fact, the treatment of cells for 3 h (with 21 h recovery) in the absence of S9‐mix was not performed. Therefore, an in vitro MN assay with treatment for 3 h (with 21 h recovery) in the absence of S9‐mix should be performed.
α‐Damascone [FL‐no: 07.134] did not induce any significant increase in bacterial mutation frequency when evaluated in four histidine‐requiring strains (TA98, TA100, TA1535 and TA1537) of S. typhimurium and E. coli WP2uvrA in the presence and absence of metabolic activation.
α‐Damascone did induce statistically significant chromosomal damage or aneuploidy, when tested in the in vitro MN test with human peripheral lymphocytes in the absence and presence of S9 metabolic activation. However, the results with α‐damascone were difficult to interpret due to the difficulty in assessing the cytotoxicity of the test substance to the peripheral blood human lymphocytes. The CEF Panel concluded that the study result was equivocal.
The current data available for α‐damascone [FL‐no: 07.134] cannot be used to exclude a genotoxicity concern, and accordingly, the CEF Panel requests additional data for this substance in order to conclude on the genotoxicity of this substance and the four structurally related substances [FL‐no: 07.130, 07.225, 07.226 and 07.231].
Overall, the CEF Panel concluded that the concern for genotoxicity is ruled out for eight of the substances [FL‐no: 02.105, 07.007, 07.009, 07.011, 07.036, 07.088, 07.091 and 07.170]. These eight substances can accordingly be evaluated using the Procedure. For allyl α‐ionone [FL‐no: 07.061] and for α‐damascone [FL‐no: 07.134] and the four structurally related substances [FL‐no: 07.130, 07.225, 07.226 and 07.231], the new submitted data could not rule out the Panel concern with respect to genotoxicity and additional data are requested.
2.6. Additional genotoxicity data evaluated by the CEF Panel in FGE.210Rev26
The present revision of FGE.210 (FGE.210Rev2), concerns the evaluation of additional data submitted by Industry for the substance, allyl α‐ionone [FL‐no: 07.061], from subgroup 2.4 (EFSA, 2008a), as requested by the Panel in FGE.210Rev1.
The Industry has tested allyl α‐ionone [FL‐no: 07.061] in an in vitro MN assay performed on human peripheral blood lymphocytes treated for 3 h (plus 21 h recovery) in the absence of metabolic activation (Lloyd, 2014). This test completes the in vitro MN assay (Lloyd, 2013a), evaluated in FGE.210Rev1, where allyl α‐ionone [FL‐no: 07.061] was tested only for 3 + 21 h in the presence of a rat liver metabolising system (S9‐mix) and for 24 h in the absence of S9‐mix. In the previous screening study (Lloyd, 2013a), the MN assay showed that MNBN cells frequencies were similar to those observed in concurrent vehicle controls at all concentrations analysed, no statistically significant differences were observed.
In the present study (Lloyd, 2014), the same batch of allyl α‐ionone (purity of 88.5%) was used as in the previous study (Lloyd, 2013a).
Whole blood cultures were established using blood from two healthy male volunteers. Cells were cultured for 48 h with PHA.
Allyl α‐ionone was diluted in dimethyl sulfoxide (DMSO) at a concentration of 5 mg/mL and tested in the MN assay at a maximum concentration of 50 μg/mL. This highest concentration was determined in a preliminary cytotoxicity range‐finder experiment.
Concurrent positive and negative (vehicle) controls were included in this study. Mitomycin C (MMC), at final concentration of 0.3 μg/mL, was employed as the clastogenic positive control chemical.
The human peripheral blood lymphocytes were treated for 3 h with 21 h recovery period in the absence of S9‐mix. At the end of the treatment period, cell culture medium was replaced with fresh culture medium for the recovery period. Cytochalasin‐B was added at 6 μg/mL per culture. All cultures were harvested 24 h after the initiation of treatment. Cytotoxicity was assessed by calculating the RI in test article‐treated cultures, relative to the concurrent vehicle control values. In the MN experiment, allyl α‐ionone was evaluated over 16 concentrations spanning a range from 2.5 to 50 μg/mL. Binucleate cells were analysed for MN in cultures treated at 12.5, 20, 25 and 27.5 μg/mL. The highest concentration analysed for MN, 27.5 μg/mL, induced 52% cytotoxicity. Treatment of cells with allyl α‐ionone in the absence of S9‐mix resulted in frequencies of MNBN cells, which were similar to and not statistically significant higher than those observed in concurrent vehicle controls for all concentrations analysed. The MNBN cell frequency of all allyl α‐ionone treated cultures fell within normal ranges.
Allyl α‐ionone did not induce MN in cultured human peripheral blood lymphocytes when tested up to the limit of cytotoxicity for 3 + 21 h in the absence of metabolic activation (Appendix F, Table F.1).
Table F.1.
Additional genotoxicity data (in vitro)
| Chemical name [FL‐no] | Test system in vitro | Test object | Concentrations of substance and test conditions | Result | Reference | Comments |
|---|---|---|---|---|---|---|
| Allyl α‐ionone [07.061] | Micronucleus induction | Human peripheral blood lymphocytes | 12.50, 20.00, 25.00 and 27.50 μg/mLa , b | Negative | Lloyd (2014) | The MNBN cell frequencies in all treated cultures fell within the normal range. This study completes the study by Lloyd (2013a), (Table E.1) and complies with OECD Guideline 487 |
Without S9 metabolic activation.
3‐h treatment with 21‐h recovery.
2.6.1. Conclusion based on the new data available to the Panel in FGE.210Rev2
Based on these new data, the CEF Panel concluded that the concern for genotoxicity of allyl α‐ionone [FL‐no: 07.061] can be ruled out. For α‐damascone [FL‐no: 07.134], no new data are available; therefore, the genotoxicity concern cannot be ruled out and additional data are still required. The same applies to the four structurally related substances [FL‐no: 07.130, 07.225, 07.226 and 07.231].
3. Assessment
3.1. Additional genotoxicity data evaluated by the Panel in FGE.210Rev3
The present revision of FGE.210 (FGE.210Rev3) concerns the evaluation of additional data submitted by Industry for the flavouring substance α‐damascone [FL‐no: 07.134] from subgroup 2.4 (EFSA, 2008a), as requested by the CEF Panel in FGE.210Rev1. The new data are an in vitro MN study (Covance, 2014), an in vivo combined MN and comet assay (Covance, 2016) and two in vivo comet assays in liver (BioReliance, 2018a,b) performed with α‐damascone (Table 3). These data are considered to cover also the genotoxicity evaluation for the four structurally related substances [FL‐no: 07.130, 07.225, 07.226 and 07.231]. The applicant submitted also a 14‐day toxicity/palatability study (Product Safety Labs, 2015) and a 90‐day toxicity study (Product Safety Labs, 2016). These studies are not evaluated in the present opinion, which focuses on the evaluation of genotoxicity data. However, the Panel considered these studies as supportive for the evaluation of the in vivo bone marrow MN assay (Covance, 2016) in particular for the assessment of systemic exposure to α‐damascone.
Table 3.
In vitro and in vivo studies evaluated in FGE.210Rev3
| Test substance | Additional data submitted | Reference |
|---|---|---|
| α‐damascone [FL‐no: 07.134] | In vitro micronucleus assay in human peripheral blood lymphocytes | Covance, 2014 |
| In vivo combined bone marrow micronucleus test and comet assay in duodenum and liver | Covance, 2016 | |
| In vivo comet assay in liver | BioReliance, 2018a | |
| In vivo comet assay in liver | BioReliance, 2018b |
Additional information was sought from the applicant during the assessment process in response to a request from EFSA sent on 8/11/2016, 9/2/2017, 29/6/2017, 8/2/2019 and was consequently provided (see Documentation provided to EFSA n.4, 5, 7, 10, 11, 12). Information requested is summarised below.
The in vitro MN study (Covance, 2014) was erroneously included in the package of data submitted for allyl α‐ionone [FL‐no: 07.061] that was evaluated in FGE.210Rev2. Therefore, the applicant was requested to submit this study under the correct dossier (EFSA letter dated 8/11/2016). The applicant submitted the in vitro MN study (Covance, 2014) under the dossier for α‐damascone on 21/11/2016 (see Documentation provided to EFSA n.7).
The applicant justified the inconsistent results observed in two in vivo comet assays in liver (Covance, 2016) by the high content of peroxides in the sample that showed positive results. Therefore, the Panel requested (EFSA letter dated 9/2/2017) to provide experimental evidence for the identity(ies) of the constituent(s) in the first sample tested in the in vivo comet assay; to provide evidence whether their formation was batch‐ or production‐specific or typical for the flavouring substance α‐damascone as such; to describe measures how their formation could be avoided under the normal conditions of storage production and use of α‐damascone as flavouring substance. Subsequent to the provision of the requested data on 30 May 2017 (EFFA, 2017, see Documentation provided to EFSA n.10), the Panel additionally requested to repeat the in vivo comet assay in liver with a freshly synthetised sample of α‐damascone (low peroxide value) and with a sample of α‐damascone with a peroxide value above 200, in order to compare if the genotoxic effect observed is due to the flavouring substance itself or to secondary components (EFSA letter dated 29/6/2017).
In reply to the EFSA letter dated 29/6/2017, on 7/12/2018, the applicant submitted two in vivo comet assay studies (EFFA, 2018, see Documentation provided to EFSA n.4, 5, 11 and Section 3.1.3).
On 28th February 2019, EFFA submitted clarifications on poundage data and use levels in reply to EFSA letter dated 8/2/2019 (EFFA, 2019, see Documentation provided to EFSA n.12).
3.1.1. In vitro Micronucleus assay
α‐Damascone [FL‐no: 07.134] (purity 99.1%) was tested in an in vitro MN assay, according to OECD guideline 487 (2010), using duplicate human lymphocyte cultures prepared from the pooled blood of two female donors in a single experiment (Covance, 2014). Treatments were performed both in the absence and presence of metabolic activation (S9‐mix) from Aroclor 1254‐induced rats. The test article was formulated in DMSO and the highest concentrations analysed in the MN experiment were determined following a preliminary cytotoxicity range‐finder experiment.
Treatments were conducted 48 h following mitogen stimulation by PHA with and without metabolic activation (Appendix G, Table G.1).
Table G.1.
Additional genotoxicity data (in vitro and in vivo) evaluated in FGE.210Rev3Additional genotoxicity data (in vitro)
| Chemical name [FL‐no] | Test system | Test object | Concentrations or doses of substance and test conditions | Result | Reference | Comments |
|---|---|---|---|---|---|---|
| α‐damascone [07.134] | In vitro micronucleus assay | Human peripheral blood lymphocytes |
8, 15, 22.5 and 25 μg/mLa 8, 12, 14 and 15 μg/mLb 5, 8, 9 and 10 μg/mLc |
Equivocal | Covance (2014) | Reliable without restrictions. Equivocal results observed in all treatment conditions |
| In vivo combined micronucleus and comet | Han Wistar rats | 125, 250 and 500 mg/kg bw | Covance (2016) | Reliable without restrictions | ||
| Bone marrow micronucleus assay | Negative | Evidence of bone marrow exposure from a 90‐day toxicity study in rats | ||||
| Comet assay in liver | Positive | In the first experiment, α‐damascone (stored without nitrogen protection) was positive in the comet assay in liver | ||||
| Comet assay in liver | Equivocal | The experiment was repeated with α‐damascone stored under nitrogen gas to prevent oxidation. In this second experiment, there were no statistically significant increases in tail intensity, but a statistically significant (p ≤ 0.05) linear trend was observed | ||||
| Comet assay in duodenum | Negative | α‐Damascone was negative in duodenum, independently on the storage conditions of the testing substance | ||||
| α‐damascone ‘fresh’ sample | Comet assay in liver | Sprague‐Dawley rats | 125, 250 or 500 mg/kg bw | Equivocal | BioReliance (2018a) | Reliable with restrictions. Deviations from GLP observed. One animal was considered as outlier, but no results were presented in the study report |
| α‐damascone ‘aged’ sample | Comet assay in liver | Sprague‐Dawley rats | 125, 250 or 500 mg/kg bw | Inconclusive | BioReliance (2018b) | Not reliable, with deviations from GLP. The mean tail intensity value of the negative control group (3.43 ± 1.46%) is outside the 95% confidence range of the historical negative controls (0.00–0.98%) |
3‐h treatment with 21‐h recovery, without S9 metabolic activation.
3‐h treatment with 21‐h recovery, with S9 metabolic activation.
24‐h treatment with 0‐h recovery.
α‐Damascone was tested at 8, 15, 22.5, 25 μg/mL (3 + 21 h without metabolic activation), at 8, 12, 14, 15 μg/mL (3 + 21 h with metabolic activation) and at 5, 8, 9, 10 μg/mL (24 + 0 h without metabolic activation).
The test article concentrations for MN analysis were selected by evaluating the effect of α‐damascone on the RI. Two thousand binucleate cells from each of the two cultures (4,000 per concentration) were analysed for MN.
Appropriate negative (vehicle) control cultures were included in the test system under each treatment condition. The proportion of MNBN cells in the vehicle cultures fell within the 95th percentile of the observed historical vehicle control ranges. MMC and noscapine (NOS) were employed as clastogenic and aneugenic positive control chemicals, respectively, in the absence of rat liver S9‐mix. Cyclophosphamide (CPA) was employed as a clastogenic positive control chemical in the presence of rat liver S9‐mix. All positive controls induced statistically significant increases in the proportion of cells with MN.
Treatment of cells with α‐damascone for 3 + 21 h in the absence of S9‐mix resulted in frequencies of MNBN cells that were significantly higher (p ≤ 0.05) than the concurrent vehicle controls at the highest three concentrations analysed (15.00, 22.50 and 25.00 μg/mL, giving 16%, 44% and 59% reductions in RI, respectively). However, the mean MNBN cell frequencies of 0.58%, 0.53% and 0.60% at 15.00, 22.50 and 25.00 μg/mL, respectively, fell within the historical vehicle control range (95th percentile, 0.2–1.4%), as did the individual MNBN cell frequencies in both cultures at all concentrations analysed. The Panel considered the result of that experiment equivocal.
Treatment of cells for 3 + 21 h in the presence of S9‐mix resulted in frequencies of MNBN cells that were significantly higher (p ≤ 0.05) than the concurrent vehicle controls at two of the highest three concentrations analysed (12.00 and 15.00 μg/mL, giving 28% and 60% reductions in RI, respectively), but not at 14.00 μg/mL, giving 43% reduction in RI. The mean MNBN cell frequencies of 0.70% and 1.15% at 12.00 and 15.00 μg/mL, respectively, both fell within the normal range (95th percentile, 0.2–1.2%), as did the individual MNBN cell frequencies in both cultures at both concentrations. Under these conditions, this result is equivocal.
Treatment of cells for 24 + 0 h in the absence of S9‐mix resulted in frequencies of MNBN cells that were significantly higher (p ≤ 0.001) than the concurrent vehicle controls at the highest concentration analysed (10.00 μg/mL). The mean MNBN cell frequency of 1.05% at 10 μg/mL fell within the historical vehicle control range (0.2–1.2%), but the MNBN cell frequency in one of the replicates at this concentration (1.25%) was outside the normal range, and furthermore, this concentration gave 67% reduction in RI. At 9 μg/mL (46% reduction in RI), the mean MNBN cell frequency of 0.48% was within the normal range (95th percentile, 0.2–1.2%) and was not significantly different to the mean vehicle control MNBN cell frequency of 0.45%. The Panel considered the result of this experiment equivocal.
The authors of the study noted that treatment with α‐damascone resulted in statistically significant increases in MNBN cell frequency when tested up to the limit of toxicity for 3 + 21 h in the absence and presence of a rat liver metabolic activation system (S9‐mix) and in excess of the limit of toxicity for 24 + 0 h in the absence of S9‐mix. The authors of this study did not perform a trend test, however, the Panel performed a trend test (Cochran–Armitage test for trend7 ) that was statistically significant both in the absence of S9‐mix (p < 0.05) and in the presence of S9‐mix (p < 0.0001).
The Panel noted that for all treatment conditions of this study weak but statistically significant increases of MNBN cells were observed. In the treatment protocol of 3 + 21 h of recovery in the presence of metabolic activation, the highest MNBN cells frequency observed at 15 μg/mL is close to that observed with the positive control (CPA). The Panel concluded that α‐damascone induced a genotoxic effect, which is considered equivocal.
3.1.2. Combined in vivo micronucleus and comet assay
The genotoxic potential of α‐damascone (purity 99.1%) was assessed in vivo using a combined MN assay in bone marrow and comet assay in liver and duodenum in the same animals (Covance, 2016). These studies were performed with two α‐damascone samples: one had been stored between 15 and 25°C under protection of light, the other one had been stored under the same conditions, but in nitrogen atmosphere to prevent oxidation. The study was conducted in accordance with OECD TG 474 (OECD, 1997b), TG 489 (OECD, 2014) and GLP principles.
Groups of six male Han Wistar rats per dose group were administered three doses (125, 250 and 500 mg/kg body weight (bw)) of α‐damascone by oral gavage on three consecutive days (0, 24 and 45 h) and were sacrificed and sampled at 3 h after the last dose and 48 h after the initial dose. The highest dose tested is an estimate of the MTD determined in a range‐finding study (Covance, 2016). In animals treated with α‐damascone 500 mg/kg bw per day, piloerection, hunched posture and decreased activity were observed.
3.1.2.1. Micronucleus assay
In the MN assay, carried out only with α‐damascone stored without nitrogen, the MNPCE frequency in all dose groups was not statistically significantly different from the vehicle control group and values were within the historical vehicle control range (95th percentile, 0–0.4%). A slight decrease of %PCE was observed at the highest dose tested compared to the vehicle control (40.3% vs 50.8%); however, this was not considered sufficient to demonstrate bone marrow exposure in this assay. However, the applicant submitted a 90‐day toxicity study (Product Safety Labs, 2016), in which the same batch of α‐damascone (stored protected from light and under nitrogen) was tested in rats (through the diet) at doses up to 400 mg/kg bw per day. Findings in clinical pathology indicate that animals were systemically exposed to α‐damascone. In particular, the effects observed in haematology and histopathology (e.g. decrease in platelet, reticulocytes and in white blood cells counts correlated with histopathology findings of bone marrow hypocellularity and decreased spleen haematopoiesis) suggest that bone marrow was exposed to the testing substance. Considering that the same species and the same route of administration were used in both the 90‐day toxicity study and in the in vivo MN study and taking the recommendations from Scientific Committee into account (EFSA Scientific Committee, 2017), the Panel considered that the indications for bone marrow exposure observed in the toxicity study are valid also for the in vivo MN study. Therefore, the Panel concluded that α‐damascone did not induce MN in bone marrow under these testing conditions (Appendix G, Table G.1).
3.1.2.2. Comet assay
In the comet assay, no dose‐related increase in the frequency of clouds and cells with halos was observed in the liver or duodenum tissues, indicating the absence of excessive cytotoxicity, necrosis or apoptosis or mechanical damage during cell harvesting (Covance, 2016).
Comet assays in liver
The study was carried out with a sample of α‐damascone that had been protected from light and stored between 15 and 25°C, without nitrogen protection. No clinical signs of toxicity were observed with this sample of α‐damascone or positive control treatments; dose‐related decreases in body weight gain from days 1 to 3 were observed in animals treated with α‐damascone. Clinical chemistry analysis revealed a small increase in urea at all dose levels, a small decrease in creatinine at the high dose level and small decreases in calcium and glucose in animals of the mid‐ and high‐dose groups. No macroscopic findings were reported at any dose level. Histopathological evaluation of the liver showed a dose‐related decrease in glycogen vacuolation in all dose groups. Evidence of increased hepatocyte mitosis was observed in animals of the 125 mg/kg bw per day group and a single animal of the 500 mg/kg bw per day group which was considered an early indication of increased hepatocyte metabolism. Hepatocyte vacuolation was observed in animals in the 250 or 500 mg/kg bw per day groups and hepatocyte microvacuolation in animals of the top dose level. These signs of hepatotoxicity indicate liver exposure of the animals to α‐damascone following oral exposure.
Following treatment with α‐damascone, dose‐related increases in group mean % tail intensity and tail moment were observed in liver from animals of all test article‐treated groups. Assessment of % tail intensities revealed dose‐related increases in group mean values in the liver of 2.02 ± 0.81%, 2.87 ± 1.02% and 6.64 ± 1.99% in low‐, middle‐ and high‐dose levels, respectively, compared to 0.95 ± 0.56% in the concurrent control group. At 500 mg/kg bw per day the sevenfold increase in tail intensity was statistically significant (p ≤ 0.01). A positive linear trend (p ≤ 0.01) was reported. The effect observed at the high dose exceeded the historical negative control range (95% reference range: 0.08–5.08% tail intensity). These positive results were attributed by the study authors to the presence of peroxides in the tested α‐damascone (> 180 μg O2/g).
The study was repeated with another sample of α‐damascone (peroxides < 180 μg O2/g) which had been stored under the same conditions, but under nitrogen to prevent oxidation. In this experiment, a decrease in body weight gain from days 1 to 3 was observed only in animals of the 500 mg/kg bw group. No clinical chemistry analysis or histopathology was performed in this experiment.
Minor dose‐related increases in group mean % tail intensity and tail moment values were observed in liver sampled from animals treated with this α‐damascone sample. None of these increases were statistically significant; however, a statistically significant (p ≤ 0.05) linear trend was observed. All values for tail intensity observed fell within the 95% of the historical control reference range (0.08–5.08%). The Panel interpreted the results of this study with the nitrogen protected α‐damascone as equivocal (see Section 4 and Appendix G, Table G.1).
Comet assay in duodenum
Similar to the comet assay in liver, also the comet assay in duodenum was repeated using the same protocol with two different samples of α‐damascone.
In both experiments, group mean % tail intensity and tail moment values for all groups of animals treated with α‐damascone were comparable with the concurrent vehicle control and within the range of historical vehicle control.
The authors of the study concluded that there was no evidence of DNA damage induction in cells isolated from duodenum following oral administration of α‐damascone. The Panel agreed with this conclusion (see Section 4 and Appendix G, Table G.1).
3.1.3. In vivo comet assays with additional α‐damascone samples
The Panel considered that the results of the in vivo comet assay in the liver for two samples of α‐damascone (Covance, 2016) were inconsistent, with one sample providing a positive result and a second sample providing an equivocal result. Therefore, the Panel requested more information on the samples tested in order to verify if the inconsistent results obtained are due to the substance itself or to other factors such as reaction products, impurities or degradation products due to a poor stability.
Based on the data provided on purities and peroxides levels of commercially produced and stored α‐damascone samples (EFFA, 2017), the Panel requested to synthesise a new batch of α‐damascone and split this batch into two portions. One portion should be tested as soon as possible in an in vivo comet assay in liver. The second portion should be stored under conditions resulting in a peroxide value higher than 200 μg O2/g sample. Then, this material should be subjected to the in vivo comet assay in liver. The results from in vivo studies with these new α‐damascone samples would allow to discriminate if the genotoxic effect observed in the previous study (Covance, 2016) is due to α‐damascone or to oxidation products.
3.1.3.1. Preparation of the ‘fresh’ and ‘aged’ α‐damascone
The applicant did not synthesise a new batch as requested by the Panel, but used a batch already produced in July 2013. In the certificate of analysis for this material, no information on purity and peroxides concentration was reported. This starting material was split into two types of samples:
One sample termed as ‘fresh’ by the applicant was prepared by distillation to remove any trace of peroxides, but the Panel noted that peroxides were still present after distillation.
Another sample termed as ‘aged’ by the applicant was prepared taking the sample from the July 2013 stock material and adding some of the ‘fresh’ sample in order to obtain the desired concentration of peroxides. The ratio of the samples that were mixed and the concentrations of peroxides in the resulting ‘aged’ sample were not reported.
Both samples were stored under nitrogen at 4–5°C.
Certificates of analysis of these two samples (‘fresh’ and ‘aged’) were not included in the study reports, but provided as separate documents with no clear indications on when the analyses had been performed. In the study reports of the comet assays (BioReliance, 2018a,b), the peroxide values were stated to be about 61 μg O2/g (pretest) and 78 μg O2/g (post‐test). For the stock sample mixed with the distilled α‐damascone, the peroxide values were about 173 μg O2/g (pretest) and 219 μg O2/g (post‐test). A long delay between sampling and analysis was noted, but no information on the conditions of preservation of these samples was reported in the study reports. In additional separate documents, produced a few months after the comet assays, the peroxide values were reported as 40 μg O2/g and 210 μg O2/g for the ‘fresh’ and the ‘aged’ sample, respectively, with a purity of 95.5%. From these additional documents, it is again not clear whether the peroxide analysis was carried out at the time when the comet assay was done or if they were generated after several months of ageing.
3.1.3.2. Comet assay in liver with ‘fresh’ α‐damascone
The α‐damascone sample termed by the applicant as ‘fresh’ was tested for genotoxicity using the comet assay to determine its potential to induce DNA damage in liver cells of male rats.
In this study report (BioReliance, 2018a), the test article purity is not indicated; however, peroxides levels of 61–78 μg O2/g were given (see Section 3.1.3.1). In a separate document, additional information on the chemical analysis is reported (e.g. purity 95.5%, peroxide value of 40 μg O2/g), but it is not clearly indicated that this analysis is referred to the material tested in this in vivo comet assay. The certificate of analysis included in the study report refers to the original batch, not to the distilled product that was tested in the comet assay.
Corn oil was selected as the vehicle and ethyl methanesulfonate (EMS) as positive control. Test article and control formulations were administered at a dose volume of 10 mL/kg bw by oral gavage with two dose administrations (days 1 and 2); the second dose was administered approximately 21 h after the first dose. All animals were euthanised 3–4 h after the last dose on day 2.
The study was conducted in accordance with OECD TG 489 (OECD, 2016) and GLP principles although deviations were observed (e.g. purity and stability not reported in the study report, analysis to determine the concentration, uniformity and stability of the test article dose formulations were not performed).
In a dose range‐finder assay, groups of three male and three female Sprague‐Dawley (Hsd:SD) rats were given two administrations (at 0 and 21 h) of α‐damascone, at 1,000 or 2,000 mg/kg bw per day. Piloerection, lethargy and hunched position were observed. Based on these results, 500 mg/kg bw per day was estimated to be the MTD.
Only male rats were used in the main study. Groups of six rats per dose group were administered doses by gavage of 0 (corn oil), 125, 250 or 500 mg/kg bw of α‐damascone on two consecutive days (0 and 21 h). A positive control group of three male rats was given doses of EMS 200 mg /kg bw once approximately 3–4 h prior to euthanasia on day 2. No mortality occurred and no appreciable reductions in mean group body weight were observed. The definitive assay was repeated due to technical issues. Only data from the repeat definitive assay were presented in the report.
A statistically significant and dose‐dependent increase in % tail intensity was observed at the highest dose tested (0.91 ± 0.31) compared to the vehicle control (0.17 ± 0.16).
According to the study authors, the increase in % tail DNA observed is in the range of the negative historical control (95% confidence range 0.00–0.98%); therefore, this increase was considered by the authors as not biologically relevant.
However, there is a statistically significant increase at the top dose and a statistically significant dose‐related increase. At the top dose, the value 0.91% tail DNA is close to the upper limit of the 95% confidence range of historical controls (95% confidence range 0.00–0.98%). The slides of the high‐dose group were rescored and the result of this second scoring was 0.98 ± 0.24%, confirming the increase in tail intensity observed in the first scoring. Two animals (out of five) in the top dose group showed tail intensity values (1.22 and 1.17%) that are out of the limits of historical negative controls (95% confidence range 0.00–0.98%), this was confirmed for one animal also in the repeated scoring (tail intensity 1.38%). However, the group mean % tail intensities were within the range of historical negative controls.
In both scorings, only five of six animals treated with 500 mg/kg bw per day were scored, because one animal was considered as an outlier using the ‘Q‐test’. However, individual results for this animal were not presented and the exclusion criteria were not reported.
The Panel considered that the following two criteria for evaluation and interpretation of results as positive (OECD TG 489) were fulfilled:
at least one of the test doses exhibits a statistically significant increase compared with the concurrent negative control;
the increase is dose related when evaluated with an appropriate trend test.
However, the Panel considered that the third criterion (‘any of the results are outside the distribution of the historical negative control data for a given species, vehicle, route, tissue and number of administrations’) is not met because the % tail intensity of both scorings (0.91 + 0.31% and 0.98 ± 0.24%) are inside the range of historical negative controls (0.00–0.98%).
Accordingly, the results observed in the in vivo comet assay in liver with this freshly distilled sample of α‐damascone are considered as equivocal by the Panel.
3.1.3.3. Comet assay in liver with ‘aged’ alpha‐damascone
The α‐damascone sample termed by the applicant as ‘aged’ was evaluated for genotoxicity using the comet assay to determine its potential to induce DNA damage in liver cells of male rats.
In this study report (BioReliance, 2018b), the test article purity is not indicated; however, peroxides levels of 173 to 219 μg O2/g were given (see Section 3.1.3.1). In a separate document, additional information on the chemical analysis is reported (e.g. purity 95.5%, peroxide value of 210 μg O2/g), but it is not clearly indicated that this analysis is referred to the material tested in this in vivo comet assay. No certificate of analysis has been included in the study report.
The study was conducted in accordance with OECD TG 489 (OECD, 2016) and GLP principles although deviations were observed (e.g. no certificate of analysis included, no batch no., no date of production).
Corn oil was selected as the vehicle and EMS as positive control. Test article and control formulations were administered at a dose volume of 10 mL/kg bw by oral gavage with two dose administrations (days 1 and 2), the second dose was administered approximately 21 h after the first dose. All animals were euthanised 3–4 h after the last dose on day 2.
In a dose range‐finder assay, groups of three male and three female Sprague‐Dawley (Hsd:SD) rats were given two administrations (at 0 and 21 h) of α‐damascone, at 1,000 or 2,000 mg/kg bw per day. Piloerection, lethargy and irregular breathing were observed. Based on these results, 500 mg/kg bw per day was estimated to be the MTD.
Only male rats were used in the main study. Groups of six rats per dose group were administered doses by gavage of 0 (corn oil), 125, 250 or 500 mg/kg bw per day of alpha‐damascone on two consecutive days (0 and 21 h). A positive control group of three male rats were given doses of EMS 200 mg/kg bw once approximately 3–4 h prior to euthanasia on day 2. No mortality occurred and no appreciable reductions in mean group body weight were observed.
None of the test article‐treated animal slides, up to 500 mg/kg bw per day, had significant increases in the % tail intensity compared to the vehicle controls. No dose‐dependent increase in % tail intensity was observed.
The author of the study report indicated that the vehicle control % tail DNA was within the historical vehicle control range for the liver and that the positive control induced a statistically significant increase in %tail DNA compared to the vehicle control. The Panel, however, noted that the mean tail intensity value of the negative control group (3.43 ± 1.46%) is outside the 95% confidence range of the historical negative controls (0.00–0.98%). Consequently, the Panel considered that the validity criteria of this study were not met. Therefore, from this study, no conclusion can be drawn on the genotoxicity of this α‐damascone sample.
4. Discussion
The Panel noted that α‐damascone induced a weak but statistically significant increase of MN frequency in all treatment conditions both in the absence and in the presence of metabolic activation in the new in vitro MN assay (Covance, 2014). Although these effects were concentration‐related, the observed increases of MN frequency were within the 95% range of historical negative controls; therefore, the Panel evaluated this study as equivocal.
α‐Damascone did not induce MN in the in vivo MN assay in bone marrow. A small reduction of PCE/NCE ratio at the highest dose was observed, which was not statistically significant and therefore was not considered as an indication of bone marrow exposure. However, findings in haematology and histopathology from a 90‐day toxicity study in rats indicate that bone marrow was exposed to α‐damascone. Since these findings were observed in the same species, and with the same route of administration in both studies, the Panel considered that bone marrow was exposed also in the in vivo MN assay. Therefore, the Panel concluded that α‐damascone did not induce MN in bone marrow under these testing conditions. However, since genotoxicity was observed in liver (see below), the absence of MN formation in bone marrow is insufficient to rule out the concern that α‐damascone can be genotoxic in vivo.
In a first in vivo comet assay, α‐damascone did not induce DNA damage in duodenum, but it was positive in the liver in the same study, in which a sevenfold increase in tail intensity compared to the control group was observed. The authors of the study report argued that the increase of tail intensity observed in the liver comet assay could have been caused by a high concentration of peroxides in the α‐damascone sample used in this study.
In a repeated study where effort was made to avoid formation of oxidation products, no statistically significant increases in mean % tail intensity were observed in the liver or in the duodenum. This, in the view of the applicant, supported the hypothesis that the positive effects observed in the liver in the first study might have been due to peroxidation products. The Panel noted, however, that a statistically significant linear trend was observed in this repeated comet assay in liver performed with α‐damascone (peroxides < 180 μg O2/g). Therefore, the Panel considered the results of this study as equivocal. No information has been provided on the chemical structures of potential peroxidation products and whether the formation of such peroxidation products can be avoided under the normal use conditions. The Panel noted that, since peroxide molecules are direct mutagens, positive results would have been expected in duodenum in the first experiment which was, however, not the case.
In order to clarify the potential impact of peroxides on the obtained results from the genotoxicity testing, the Panel asked for comet assays in the liver in vivo with portions of a newly synthesised batch of α‐damascone stored either under nitrogen or under normal conditions that allow peroxidation. In response, the applicant provided a study with α‐damascone obtained by distillation of a batch already synthesised in 2013. The results with this sample of α‐damascone must be considered as equivocal (two of three criteria for a judgement as positive were fulfilled). In addition, in this study with the freshly distilled material, one animal in the highest exposure group was not included in the statistical analysis, because it was considered an outlier. However, individual data were not provided and a rationale for excluding this animal was not provided.
The applicant also performed a comet assay with an α‐damascone sample prepared by mixing the distilled material with the original batch that was produced in 2013 in unknown proportion. The study authors stated that the study was negative. The Panel, however, considered the study inconclusive, because the negative control group animals showed a comet tail intensity that was outside the 95th percentile of the historical control range.
The Panel noted that the difference in the peroxides values (approximately a factor of 3) of the two α‐damascone samples used in the studies described above were not as pronounced as reported in freshly opened and used samples reported in May 2017 (EFFA, 2017) which differed by a factor up to 50. Overall the Panel considered that, regarding the quality and design, these studies do not allow a conclusion on the role of peroxides and/or other impurities in the obtained results from the genotoxicity testing with α‐damascone.
Some examples of data on α‐damascone occurrence in food and information on exposure are reported in Appendix H.
5. Conclusions
Based on the data available, the concern for genotoxicity cannot be ruled out for the representative substance α‐damascone [FL‐no: 07.134] and for the four structurally related substances δ‐damascone [FL‐no: 07.130], cis‐1‐(2,6,6‐trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one [FL‐no: 07.225], tr‐1‐(2,6,6‐trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one [FL‐no: 07.226] and α‐damascenone [FL‐no: 07.231]. The information available is insufficient to conclude whether the genotoxicity observed for some α‐damascone samples should be attributed to α‐damascone as such or to impurities including possible peroxides.
Documentation provided to EFSA
Ballantyne M, 2011. Reverse mutation in one histidine‐requiring strain of Salmonella typhimurium. Allyl alpha‐ionone. Covance Laboratories LTD. Study no. 8233104. May 2011. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Benigni R and Netzeva T, 2007a. Report on a QSAR model for prediction of genotoxicity of α,β‐unsaturated aldehydes in S. typhimurium TA100 and its application for predictions on α,β‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
Benigni R and Netzeva T, 2007b. Report on a QSAR model for prediction of genotoxicity of α,β‐unsaturated ketones in S. typhimurium TA100 and its application for predictions on α,β‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
BioReliance, 2018a. In vivo mammalian alkaline comet assay. BioReliance study number AF07JZ.423M.BTL. October 2018. Unpublished study report submitted by EFFA.
BioReliance, 2018b. In vivo mammalian alkaline comet assay. BioReliance study number AF07KA.423M.BTL. October 2018. Unpublished study report submitted by EFFA.
Bowen R, 2011. Reverse mutation in five histidine‐requiring strains of Salmonella typhimurium. alpha‐Ionone. Covance Laboratories Ltd. Study no. 8233103. May 2011. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Covance, 2014. alpha‐Damascone: In Vitro Human Lymphocyte Micronucleus Assay. Covance Laboratories Ltd. Study No. 8302483. September 2014. Unpublished final report submitted by EFFA.
Covance, 2016. alpha‐Damascone: Rat Micronucleus and Alkaline Comet Assay. Covance Laboratories Ltd. Study no. 8308845. 19 February 2016. Unpublished final report submitted by EFFA.
EFFA (European Flavour & Fragrance Association), 2002. EFFA letter to the Commission and FLAVIS Secretariat on definitions and data clarifications. 31 October 2002.
EFFA (European Flavour Association), 2017. Further clarification on alpha‐damascone qualities and presence of peroxides: FGE.19 Subgroup 2.4 (FGE.210 Rev3). Additional data submitted by EFFA on 30/05/2017 in reply to EFSA letter dated 9/02/2017.
EFFA (European Flavour Association), 2018. Flavouring Group Evaluation 19 Subgroup 2.4b, alpha‐Damascone: 1 Flavouring Substance of the Chemical Group 3 (Annex I of 1565/2000/EC) Alicyclic α,β‐unsaturated aldehydes, ketones and related substances with the alpha,beta‐conjugation in the ring or in the side chain. Alicyclic ketones (α,β‐unsaturation in sidechain) Used as Flavouring Substances. Prepared by International Organization of the Flavor Industry, 15/10/2018. Additional data submitted by EFFA on 7/12/2018 in reply to EFSA letter dated 29/06/2017, which was followed by a clarification teleconference on 25/07/2017 and on 8/11/2018.
EFFA (European Flavour Association), 2019. EFFA Submission of Poundage information and use levels – FGE.210Rev3 (FGE.19 subgroup 2.4). Additional data submitted by EFFA on 28/02/2019 in reply to EFSA letter dated 8/02/2019.
Flanders L, 2006. Screening L5178Y TK +/‐ mutation assay. beta‐Ionone epoxide. Safepharm Laboratories. Project no. 1543/0154. September 6, 2006. Unpublished report submitted by Flavour Industry to FLAVIS Secretariat.
Gry J, Beltoft V, Benigni R, Binderup M‐L, Carere A, Engel K‐H, Gürtler R, Jensen GE, Hulzebos E, Larsen JC, Mennes W, Netzeva T, Niemelä J, Nikolov N, Nørby KK and Wedebye EB, 2007. Description and validation of QSAR genotoxicity models for use in evaluation of flavouring substances in Flavouring Group Evaluation 19 (FGE.19) on 360 α,β‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
Haddouk H, 2001. Bacterial reverse mutation test. ST05C01. CIT, Evreux, France. Study no. 21664 MMJ. 11 July 2001. Unpublished report submitted by EFFA to FLAVIS Secretariat.
IOFI (International Organization of the Flavor Industry), 2013a. Flavouring Group Evaluation 19 Subgroup 2.4a: 10 Flavouring Substances of the Chemical Group 3 (Annex I of 1565/2000/EC) Alicyclic α,β‐unsaturated aldehydes, ketones and related substances with the alpha, beta‐conjugation in the ring or in the side chain. Alicyclic ketones (alpha, beta‐unsaturation in sidechain) Used as Flavouring Substances. 12/02‐2013. FLAVIS/8.184.
IOFI (International Organization of the Flavor Industry), 2013b. Flavouring Group Evaluation 19 Subgroup 2.4/2.7, alpha‐Damascone and beta‐Damascone: 3 Flavouring Substances of the Chemical Group 3 (Annex I of 1565/2000/EC) Alicyclic α,β‐unsaturated aldehydes, ketones and related substances with the α,β‐conjugation in the ring or in the side chain. Alicyclic ketones (α,β‐unsaturation in side chain) Used as Flavouring Substances. 19/04‐2013. FLAVIS/8.198.
Jones E and Wilson LA, 1988. Ames metabolic activation test to assess the potential mutagenic effect of beta‐jonon epoxide. Huntingdon Research Centre Ltd. Report no. ULR 220A/88986. 13 September 1988. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Kringstad J, 2005. Bacterial mutagenicity test – ames assay. Beta ionone epoxide. AppTec Laboratory Services. Project no. 34051. August 22, 2005. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Krsmanovic L and Huston T, 2006. Mammalian erythrocyte micronucleus test. alpha‐Ionone. BioReliance. Study no. AB23FJ.123.BTL. 20 April 2006. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Lloyd M, 2012. Induction of micronuclei in cultured human peripheral blood lymphocytes. alpha‐Damascone. Covance Laboratories LTD. Study no. 8233102. July 2012. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Lloyd M, 2013a. Induction of micronuclei in cultured human peripheral blood lymphocytes. Allyl alpha‐ionone. Covance Laboratories LTD. Study no. 8272012. February 2013. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Lloyd M, 2013b. Induction of micronuclei in cultured human peripheral blood lymphocytes. alpha‐Ionone. Covance Laboratories LTD. Study no. 8272011. January 2013. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Lloyd M, 2013c. Final summary report. Induction of micronuclei in cultured human peripheral blood lymphocytes. alpha‐Damascone. Covance Laboratories LTD. Study no. 8272016. April 2013. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Lloyd M, 2014. Allyl alpha‐ionone: In Vitro Human Lymphocyte Micronucleus Assay. Covance Laboratories Ltd. Study No. 8302482. September 2014. Unpublished final report submitted by EFFA to EFSA.
Nikolov N, Jensen GE, Wedebye EB and Niemelä J, 2007. Report on QSAR predictions of 222 α,β‐unsaturated aldehydes and ketones from Flavouring Group Evaluation 19 (FGE.19) on 360 α,β‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
Products Safety Labs, 2015. Alpha‐damascone: a 14‐day dietary toxicity/palatability study in rats. Study number 40131, 27 July 2015. Unpublished study report submitted by EFFA.
Products Safety Labs, 2016. Alpha‐damascone: a 90‐day dietary study in rats. Study number 40132, 29 February 2016. Unpublished study report submitted by EFFA.
Shinya W, 2006. Mutagenicity test of D.Damascone using microorganisms. Hita Laboratory, Japan. K01‐3612. November 9, 2006. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Whitwell J, 2012. Induction of micronuclei in cultured human peripheral blood lymphocytes. alpha‐Damascone. Covance Laboratories Ltd, England. Study no.8263013. September 2012. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Abbreviations
- BW
body weight
- CA
chromosomal aberrations
- CAS
Chemical Abstract Service
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CHO
Chinese hamster ovary (cells)
- CHL
Chinese hamster Lung (cells)
- CPA
Cyclophosphamide
- CoE
Council of Europe
- DMSO
dimethyl sulfoxide
- EFFA
European Flavour Association
- EMS
ethyl methanesulfonate
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS (FL)
Flavour Information System (database)
- GLP
Good Laboratory Practice
- ID
identity
- IOFI
International Organization of the Flavour Industry
- IR
infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MMC
mitomycin C
- MN
micronuclei
- MNBN
micronucleated binucleate (cells)
- MS
mass spectrometry
- MSDI
maximised survey‐derived daily intake
- mTAMDI
modified Theoretical Added Maximum Daily Intake
- MTD
maximum tolerated dose
- NCE
normochromatic erythrocytes
- No
number
- NOAEL
No observed adverse effect level
- NOS
noscapine
- PCE
polychromatic erythrocytes
- PHA
phytohaemagglutinin
- (Q)SAR
(quantitative) structure–activity relationship
- RI
Replication Index
- tk
thymidine kinase
- WHO
World Health Organization
Appendix A – Specification summary of the substances in the Flavouring Group Evaluation 210Rev3
1.
Appendix B – Summary of safety evaluation applying the procedure (JECFA, 1999, 2006, 2014)
1.
Appendix C – QSAR predictions on mutagenicity in five models for 10 ketones from subgroup 2.4
1.
Appendix D – Genotoxicity data (in vitro/in vivo) considered by the Panel in FGE.210
1.
Appendix E – Genotoxicity data considered by the Panel in FGE.210Rev1
1.
Appendix F – Genotoxicity data considered by the Panel in FGE.210Rev2
1.
Appendix G – Genotoxicity data considered by the Panel in FGE.210Rev3
1.
Appendix H – Exposure
Presence of α‐damascone in food
Damascones are natural aroma components that exhibit fruity, rose‐like odours. δ‐Damascone has been detected as a trace volatile constituent in melon (Chaparro‐Torres et al., 2016). α‐Damascone has been detected as a minor constituent of the volatile fractions in tea (less than 0.5% of the volatile matter) (Kawakami et al., 1995) and tobacco (Schoch et al., 1991), roses (Sarandeses and Luche, 1992) and raw cane sugar (Tokitomo et al., 1980). α‐Damascone has also been reported to occur in whiskey and cognac (Poisson and Schieberle, 2008; Uselmann and Schieberle, 2015). For [FL‐no: 07.225, 07.226, 07.231], no occurrence in food is reported in the database of volatile compounds in food (Triskelion, 2019).
Intended use and use levels as provided by the Flavour Industry
Use levels in the different food categories reported in Annex III of Reg. (EC) 1565/20008 have been submitted by the flavour industry and are reported in Table H.1 (EFFA, 2019, see Documentation provided to EFSA n.12)
Table H.1.
Use levels of α‐damascone [FL‐no: 07.134] and of structurally related substances [FL‐no: 07.130, 07.225, 07.226, 07.231] in food categories listed in Annex III of Reg. (EC) 1565/2000 (EFFA, 2019)
| FL‐no | Food categories | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal use levels (mg/kg)a Maximum use levels (mg/kg) | ||||||||||||||||||
| 01.0 | 02.0 | 03.0 | 04.0 | 05.0 | 05.3b | 06.0 | 07.0 | 08.0 | 09.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.1 | 14.2 | 15.0 | 16 | |
| 07.134 | – | – | – | – | 0.2 | 0.2 | 0.2 | – | – | – | – | – | – | – | 0.2 | 0.2 | – | – |
| – | – | – | – | 0.5 | 0.5 | 0.5 | – | – | – | – | – | – | – | 0.5 | 0.5 | – | – | |
| 07.130 | 0.01 | 0.005 | 0.005 | 0.005 | ||||||||||||||
| 0.02 | 0.1 | 0.02 | 0.02 | |||||||||||||||
| 07.225c | 10.0 | 17.5 | 0.01 | 0.03 | 12.5 | 14 | 9.2 | 12.0 | 5.4 | 5.0 | 2.5 | 0.63 | 1.25 | 2.2 | 2.0 | |||
| 25.0 | 20.0 | 0.10 | 2.5 | 33.0 | 43.0 | 24.0 | 30.0 | 15.0 | 5.0 | 2.5 | 6.1 | 1.25 | 5.2 | 3.9 | ||||
| 07.226 | 3.0 | 2.0 | 3.0 | 2.0 | 5.0 | 2.0 | 5.0 | 1.0 | 1.0 | 2.0 | ||||||||
| 15.0 | 10.0 | 15.0 | 10.0 | 25.0 | 10.0 | 25.0 | 5.0 | 5.0 | 10.0 | |||||||||
| 07.231c | 9.81 | 15.0 | 1.75 | 0.03 | 8.30 | 14.0 | 9.0 | 9.98 | 1.0 | 5.0 | 2.5 | 0.63 | 1.25 | 2.03 | 1.8 | 1.0 | ||
| 25.0 | 20.0 | 3.0 | 1.0 | 25.0 | 40.0 | 25.0 | 30.0 | 10.0 | 5.0 | 2.5 | 5.0 | 2.0 | 5.05 | 4.0 | 1.5 | |||
‘Normal use’ is defined as the average of reported usages and ‘maximum use’ is defined as the 95th percentile of reported usages (EFFA, 2002).
Additional food category 05.3 (chewing gum as per Annex II part D of Reg. (EC) 1333/2008) for which EFFA submitted use levels (EFFA, 2019). These have been considered in the calculation of mTAMDI.
According to the information reported by industry (EFFA, 2019), no ‘surveyed use levels’ were available, but the data from the iterated median use levels for chemical group are representing the use of this substance.
Table H.2.
Distribution of the 18 food categories listed in Commission Regulation (EC) No 1565/2000 into the seven SCF food categories used for TAMDI calculation (SCF, 1995)
| Food categories according to Commission Regulation 1565/2000 | Distribution of the seven SCF food categories | |||
|---|---|---|---|---|
| Key | Food category | Foods | Beverages | Exceptions |
| 01.0 | Dairy products, excluding products of category 02.0 | Foods | ||
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) | Foods | ||
| 03.0 | Edible ices, including sherbet and sorbet | Foods | ||
| 04.1 | Processed fruit | Foods | ||
| 04.2 | Processed vegetables (including mushrooms and fungi, roots and tubers, pulses and legumes), and nuts and seeds | Foods | ||
| 05.0 | Confectionery | Exception a | ||
| 06.0 | Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery | Foods | ||
| 07.0 | Bakery wares | Foods | ||
| 08.0 | Meat and meat products, including poultry and game | Foods | ||
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | Foods | ||
| 10.0 | Eggs and egg products | Foods | ||
| 11.0 | Sweeteners, including honey | Exception a | ||
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. | Exception d | ||
| 13.0 | Foodstuffs intended for particular nutritional uses | Foods | ||
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products | Beverages | ||
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts | Exception c | ||
| 15.0 | Ready‐to‐eat savouries | Exception b | ||
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) – foods that could not be placed in categories 01.0–15.0 | Foods | ||
Intake data from intended use
Annual production volumes of the flavouring substance as surveyed by industry are used to calculate the ‘Maximised Survey‐derived Daily Intake’ (MSDI) assuming that the production figure only represents 60% of the use in food, due to underreporting, and that 10% of the total EU population are consumers (SCF, 1999).
Use levels for α‐damascone [FL‐no: 07.134], δ‐damascone [FL‐no: 07.130], cis‐1‐(2,6,6‐trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one [FL‐no: 07.225], tr‐1‐(2,6,6‐trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one [FL‐no: 07.226] and α‐damascenone [FL‐no: 07.231] provided by industry (EFFA, 2019) are listed in Table H.1. These data have been used to calculate the ‘modified Theoretical Added Maximum Daily Intake’ (mTAMDI).9
The MSDI and mTAMDI exposure estimates are given in Table H.3.
Table H.3.
Exposure to α‐damascone [FL‐no: 07.134] and to the structurally related substances [FL‐no: 07.130, 07.225, 07.226, 07.231]
| FL‐no | Name | EU MSDI μg/capita per day | mTAMDI μg/person per day |
|---|---|---|---|
| 07.130a | δ‐Damascone | 0.31 | 2.57 |
| 07.134b | α‐Damascone | 7.97 | 101.3 |
| 07.225c | cis‐1‐(2,6,6‐Trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one | 1.35 | 3,465.40 |
| 07.226d | trans‐1‐(2,6,6‐Trimethyl‐2‐cyclohexen‐1‐yl)but‐2‐en‐1‐one | 0.27 | 842.0 |
| 07.231e | α‐Damascenone | 0.07 | 2,979.42 |
Based on EU poundage of 2.52 kg (EFFA Poundage Survey covering 2015).
Based on EU poundage of 65.42 kg (EFFA Poundage Survey covering 2015).
Based on EU poundage of 11.05 kg (EFFA Poundage Survey covering 2015).
Based on EU poundage of 2.24 kg (EFFA Poundage Survey covering 2015).
Based on EU poundage of 0.61 kg (EFFA Poundage Survey covering 2015).
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Gürtler R, Husøy T, Moldeus P, Oskarsson A, Shah R, Waalkens‐Berendsen I, Wölfle D, Benigni R, Bolognesi C, Chipman K, Cordelli E, Degen G, Marzin D, Svendsen C, Carfì M, Vianello G and Mennes W, 2019. Scientific Opinion on Flavouring Group Evaluation 210 Revision 3 (FGE.210Rev3): Consideration of genotoxic potential for α,β‐unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19. EFSA Journal 2019;17(5):5676, 42 pp. 10.2903/j.efsa.2019.5676
Requestor: European Commission
Question numbers: EFSA‐Q‐2016‐00308, EFSA‐Q‐2016‐00309, EFSA‐Q‐2016‐00310, EFSA‐Q‐2016‐00311, EFSA‐Q‐2016‐00312.
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus, Agneta Oskarsson, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle and Maged Younes.
Acknowledgements: The FAF Panel wishes to thank the Working Group on Genotoxicity of the former EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) for the preparatory work on this scientific opinion, in particular Mona‐Lise Binderup, Francesca Marcon and Pasquale Mosesso. The Panel wishes to thank the hearing experts: Vibe Beltoft and Karin Nørby for the support provided to this scientific output.
Adopted: 28 March 2019
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
The data presented in Section 2.4 are cited from the first opinion on FGE.210. These data are the basis for the conclusions in FGE.210 requesting additional genotoxicity data.
The data presented in Section 2.5 are cited from the FGE.210Rev1. These data are the basis for the conclusions in FGE.210Rev1 requesting additional genotoxicity data.
The data presented in Section 2.6 are cited from the FGE.210Rev2.
R software, CochranArmitage test for trend https://www.rdocumentation.org/packages/DescTools/versions/0.99.19/topics/CochranArmitageTest
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
mTAMDI estimation is based on an approach used by the SCF up to 1995 (SCF, 1995) and is calculated on the basis of standard portions and normal use levels for flavoured beverages and foods in general, with exceptional levels for particular foods.
References
- Chaparro‐Torres LA, Bueso MC and Fernández‐Trujillo JP, 2016. Aroma volatiles obtained at harvest by HS‐SPME/GC‐MS and INDEX/MS‐E‐nose fingerprint discriminate climacteric behaviour in melon fruit. Journal of the Science of Food and Agriculture, 96, 2352–2365. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Minutes of the 26th Plenary meeting of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food, Held in Parma on 27–29 November 2007. Parma, 7 January 2008.
- EFSA (European Food Safety Authority), 2008b. Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19 – Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). EFSA Journal 2008;6(12):854, 5 pp. 10.2903/j.efsa.2008.854 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Scientific Opinion. List of alpha,beta‐unsaturated aldehydes and ketones representative of FGE.19 substances for genotoxicity testing – Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). EFSA Journal 2008;6(12):910, 5 pp. 10.2903/j.efsa.2008.910 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Scientific Opinion of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Flavouring Group Evaluation 210: alpha,beta‐unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19. EFSA Journal 2009;7(4):1030, 18 pp. 10.2903/j.efsa.2009.1030 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2014. Scientific Opinion on Flavouring Group Evaluation 210 Revision 1 (FGE.210Rev1): Consideration of genotoxic potential for α,β‐unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19. EFSA Journal 2014;12(2):3587, 35 pp. 10.2903/j.efsa.2014.3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2015. Scientific Opinion on Flavouring Group Evaluation 210 Revision 2 (FGE.210Rev2): Consideration of genotoxic potential for α,β‐unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19. EFSA Journal 2015;13(7):4172, 33 pp. 10.2903/j.efsa.2015.4172 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific opinion on Genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Clarification of some aspects related to genotoxicity assessment. EFSA Journal 2017;15(12):5113, 25 pp. 10.2903/j.efsa.2017.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1998. Compendium of food additive specifications. Addendum 6. Joint FAO/WHO Expert Committee of Food Additives 51st session. Geneva, 9–18 June 1998. FAO Food and Nutrition paper 52 Add. 6. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999. Safety evaluation of certain food additives. Fifty‐first Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series: 42. IPCS, WHO, Geneva. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Compendium of food additive specifications. Addendum 8. Joint FAO/WHO Expert Committee of Food Additives 55th session. Geneva, 6–15 June 2000. FAO Food and Nutrition paper 52 Add. 8.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001. Compendium of food additive specifications. Addendum 9. Joint FAO/WHO Expert Committee of Food Additives 57th session. Rome, 5–14 June 2001. FAO Food and Nutrition paper 52 Add. 9.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002. Compendium of food additive specifications. Addendum 10. Joint FAO/WHO Expert Committee of Food Additives 59th session. Geneva, 4–13 June 2002. FAO Food and Nutrition paper 52 Add. 10.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2003. Compendium of food additive specifications. Addendum 11. Joint FAO/WHO Expert Committee of Food Additives 61st session. Rome, 10–19 June 2003. FAO Food and Nutrition paper 52 Add. 11.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2005. Compendium of food additive specifications. Addendum 13. Joint FAO/WHO Expert Committee of Food Additives 65th session. Geneva, 7–16 June 2005. FAO Food and Nutrition paper 52 Add. 13.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Safety evaluation of certain food additives and contaminants. Sixty‐fifth meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 56. IPCS, WHO, Geneva. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2014. Evaluation of certain food additives. Seventy‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives, WHO technical report series 990. WHO, Geneva. [Google Scholar]
- Kasamaki A, Takahashi H, Tsumura N, Niwa J, Fujita T and Urasawa S, 1982. Genotoxicity of flavoring agents. Mutation Research, 105, 387–392. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Ganguly SN, Banerjee J and Kobayashi A, 1995. Aroma composition of Oolong tea and black tea by brewed extraction method and characterizing compounds of Darjeeling tea aroma. Journal of Agricultural and Food Chemistry, 43, 200–207. [Google Scholar]
- Oda Y, Hamono Y, Inoue K, Yamamoto H, Niihara T and Kunita N, 1978. Mutagenicity of food flavors in bacteria. Osaka‐Furitsu Koshu Eisei Kenkyu Hokoku Shokuhin Eisei Hen, 9, 177–181. (In Japanese) [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 1997a. Test No. 471: Bacterial reverse mutation test. OECD Guideline for testing of chemicals. Section 4. Available online: http://www.oecd.org/dataoecd/18/31/1948418.pdf
- OECD (Organisation for Economic Co‐operation and Development), 1997b. Test No. 474: Mammalian erythrocyte micronucleus test. OECD Guideline for testing of chemicals. Section 4. Available online: http://www.oecd.org/dataoecd/18/34/1948442.pdf
- OECD (Organisation for Economic Co‐operation and Development), 1997c. Test No. 476: In vitro mammalian cell gene mutation test. OECD Guidelines for the Testing of Chemicals. Section 4. Available online: http://www.oecd.org/chemicalsafety/risk-assessment/1948426.pdf
- OECD (Organisation for Economic Co‐operation and Development), 2010. Test No. 487: In vitro mammalian cell micronucleus test. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2014. Test Guideline No. 489: In vivo mammalian alkaline comet assay. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2016. Test Guideline No. 489: In vivo mammalian alkaline comet assay. OECD Guidelines for the Testing of Chemicals, Section 4.
- Poisson L and Schieberle P, 2008. Characterization of the most odor‐active compounds in an American Bourbon whisky by application of the aroma extract dilution analysis. Journal of Agricultural and Food Chemistry, 56, 5813–5819. [DOI] [PubMed] [Google Scholar]
- Sarandeses LA and Luche J‐L, 1992. A new synthesis of α‐ and β‐damascones from the ionones. Journal of Organic Chemistry, 57, 2757–2760. [Google Scholar]
- SCF (Scientific Committee for Food), 1995. Scientific Committee for Food. First annual report on chemically defined flavouring substances. May 1995, 2nd draft prepared by the SCF Working Group on Flavouring Substances (Submitted by the SCF Secretariat, 17 May 1995). CS/FLAV/FL/140‐Rev2. Annex 6 to Document III/5611/95, European Commission, Directorate‐General III, Industry.
- SCF (Scientific Committee for Food), 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). Scientific Committee on Food. SCF/CS/FLAV/TASK/11 Final 6/12/1999. Annex I to the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- Schoch E, Benda I and Schreier P, 1991. Bioconversion of α‐damascone by Botrytis cinerea . Applied and Environmental Microbiology, 57, 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokitomo Y, Kobayashi A, Yamanishi T and Muraki S, 1980. Studies on the “Sugary Flavor” of raw cane sugar. Proceedings of the Japan Academy, Ser. B, 56, 452–456. [Google Scholar]
- Triskelion , 2019. VCF online. Volatile compounds in food In: Nijssen B, van Ingen‐Visscher K. and Donders J. (eds.). Database version 16.6.1. Triskelion, Zeist, The Netherlands: pp. 1992–2019. [Google Scholar]
- Uselmann V and Schieberle P, 2015. Decoding the combinatorial aroma code of a commercial cognac by application of the sensomics concept and first insights into differences from a German brandy. Journal of Agricultural and Food Chemistry, 63, 1948–1956. [DOI] [PubMed] [Google Scholar]
- Wild D, King MT, Gocke E and Eckhard K, 1983. Study of artificial flavouring substances for mutagenicity in the Salmonella/microsome, BASC and micronucleus tests. Food and Chemical Toxicology, 21, 707–719. [DOI] [PubMed] [Google Scholar]


