Abstract
Following a request from the European Commission, EFSA was asked to deliver a scientific opinion on the safety and efficacy of copper chelates of lysine and glutamic acid (Copper‐LG) as a nutritional feed additive for all animal species. The EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) concludes that, owing to safety considerations, Copper‐LG should not be used in water for drinking. Copper‐LG is safe for chickens for fattening; this conclusion can be extrapolated to all animal species and categories provided that the maximum authorised levels in the EU for total copper in feed are not exceeded. No increases in the copper content of animal tissues/products are expected from the use of Copper‐LG in animal nutrition. There is no indication that the toxicity of Copper‐LG is essentially different from that of inorganic divalent copper. The use of Copper‐LG in animal nutrition is of no concern for consumer safety provided that the maximum authorised total copper in feed is respected. Owing to the copper and nickel content of Copper‐LG, the handling of the additive, poses a risk to users by inhalation. The additive is considered as a skin and respiratory sensitiser; it is corrosive to the eye while it is non‐irritant to skin. The additive is intended to be a substitute for other authorised copper additives and will not further increase the environmental burden of copper; therefore, the FEEDAP Panel considers that the use of the additive in animal nutrition would not pose an additional risk for the environment. Copper‐LG is a bioavailable source of copper, comparable to the standard inorganic copper source, and therefore, the additive is efficacious in meeting the birds copper requirements; this conclusion can be extrapolated to all animal species/categories. The FEEDAP Panel posed a recommendation concerning the description of the additive.
Keywords: nutritional additives, compounds of trace elements, copper chelates of lysine and glutamic acid, ProPath Cu, copper, safety, efficacy
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Zinpro Animal Nutrition (Europe), Inc.2 for authorisation of the product copper chelates of lysine and glutamic acid, when used as a feed additive for all animal species (category: feed additives; functional group: compounds of trace elements).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 28 March 2018.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product copper chelates of lysine and glutamic acid, when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
The additive, copper chelates of lysine and glutamic acid, is intended to be used as a source of copper in all animal species. The additive has not been previously authorised as a feed additive in the European Union (EU).
The applicant holds a patent on the copper chelates of lysine and glutamic acid, with the title ‘Mixed amino acid metal salt complexes’.3
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier4 in support of the authorisation request for the use of copper chelates of lysine and glutamic acid as a feed additive.
The EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers and other scientific reports, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the copper chelates of lysine and glutamic acid in animal feed. The Executive Summary of the EURL report can be found in Annex A.5
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of copper chelates of lysine and glutamic acid is in line with the principles laid down in Regulation (EC) No 429/20086 and the relevant guidance documents: Guidance on nutritional additives (EFSA FEEDAP Panel, 2012a), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008), Guidance for the preparation of dossiers for additives already authorised for use in food (EFSA FEEDAP Panel, 2012b), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012c) and Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012d).
3. Assessment
The additive under assessment is ‘Copper chelates of lysine and glutamic acid’ (trade name: ProPath Cu). The additive corresponds to the active compound. As abbreviation, the short name of Copper‐LG will be used throughout this opinion to refer to the additive under assessment. It is intended to be used in feed (either mashed or pelleted form) and water (via complementary feed) as a nutritional additive (functional group compounds of trace elements) – source of copper, for all animal species and categories.7
3.1. Characterisation
3.1.1. Characterisation of the additive
The active substance is divalent copper in the form of chelates of lysine and glutamic acid in a mixture 1:1.8 The chemical names according to International Union of Pure and Applied Chemistry (IUPAC) are copper‐2,6‐diaminohexanoic acid and copper‐2‐aminopentanedioic acid. The compounds are not identified by the Chemical Abstracts Service (CAS) number. The chemical formulas of the two compounds are C6H15ClCuN2O6S and C5H9CuNNaO8.5S, respectively. The structural formulas are shown in Figure 1 and the corresponding molecular weights are 360.27 g/mol for the copper chelate of lysine, and 337.73 g/mol for copper chelate of glutamic acid. The theoretical content of copper is 17.6% and 18.8%, respectively.9
Figure 1.
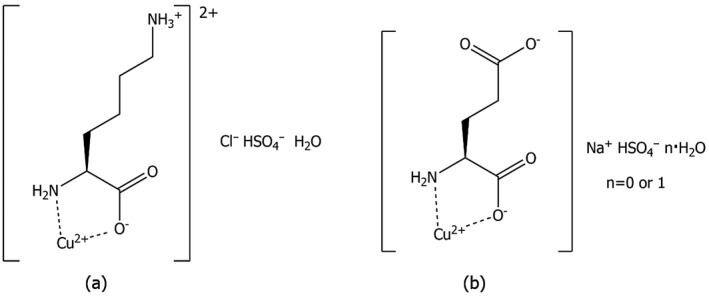
Structural formulas of copper chelate of lysine (a) and copper chelate of glutamic acid (b)
Five batches of the additive were analysed for copper, lysine, glutamic acid, chloride and sulfur. The average content of copper was about 17.7% (17.6–17.8), lysine 19.9% (19.3–20.3), glutamic acid 19.7% (19.1–20.1), chloride 4.7% (4.6–4.7), sulfur 9.8% (9.6–10.0) and water 2.5% (2–3).10 The remaining composition was not identified, but it can be assumed to be represented by the other components of the counter ions (hydrogen and oxygen from HSO4 – and sodium). The FEEDAP Panel notes that about 4% of the additive might be unidentified.
Five batches were analysed for undesirable substances. Levels of heavy metals (cadmium (Cd), lead (Pb) and mercury (Hg)), arsenic (As) and fluorine (F) were provided8 (As: < 0.20 mg/kg, Cd: 0.25–0.29 mg/kg, Pb: 4.7–5.2 mg/kg, Hg: 0.02 mg/kg and F: 5.2 mg/kg).11 The levels of dioxins (polychlorinated dibenzofurans (PCDF) and polychlorinated dibenzo(p)dioxins (PCDD)) and the sum of dioxins and dioxin‐like polychlorinated biphenyls (PCBs) were 0.024–0.026 ng WHO‐PCDD/F‐TEQ/kg and 0.025–0.028 ng WHO‐PCDD/F‐PCB‐TEQ/kg, respectively. The concentrations of the undesirable substances analysed comply with those set in Directive 2002/32/EC for compounds of trace elements or, if not mentioned in the Directive, do not represent a concern.12 Nickel was analysed in three batches of the additive and the values reported were in the range 12–13 mg/kg.7
Analysis of three batches (aged from seven to eight months) for potential microbiological contamination, showed that counts of Enterobacteriaceae and Escherichia coli were below the limit of quantification (LOQ) (< 10 cfu/g) and Salmonella was not detected. Levels of aflatoxin B1 and ochratoxin A analysed in three batches and were below the LOQ (< 0.1 μg/kg).13
The additive is a solid product, soluble in water, with a melting point at 177°C. The bulk density tested in three batches of the additive is 0.724 g/cm3.14
Dusting potential was analysed in three batches by the Stauber–Heubach method (four measurements of each batch). The values reported were in the range of 2.0–3.0 g airborne dust/m3 of air. The same batches were submitted for analysis of the particle size by laser diffraction. The results (v/v) showed that on average 14.9% (range 14.2–15.8), 54.7% (range 53.1–56.6) and 87.2% (range 86.7–88.0) of particles were < 10 μm, < 50 μm and < 100 μm, respectively.14 Copper content in the dust was provided with analysis of three batches of the additive with an average of 17.7% (range 17.5–17.9).7
3.1.2. Manufacturing process
■■■■■
■■■■■■■■■■
3.1.3. Stability and homogeneity
For compounds of trace elements (including chelates), stability studies are generally not required.
Two stability studies to determine the shelf‐life of the additive, each with one batch stored at 25°C/60% relative humidity (RH) and at 40°C/70% RH were performed. After 24 months, the copper recovery was 101.2% and 100%; the total lysine/glutamic acid recovery was 101.8% and 102.9%, under the two conditions, respectively.19
A stability study of the additive in premixtures, mash feed and pellet feed measuring copper content was provided.20 After 6 months of storage, a recovery of 96.4% was reported for mash starter feed, of 96.3% for mash grower feed, of 95.8% for pellet grower feed, of 82.4% for mash starter premixture and a recovery of 81.7% was reported for mash grower premixture. The content of choline chloride in the premixture was of 6.25%.21
The capacity of the additive to homogeneously distribute in premixtures and complete feed (mash and pelleted) for chickens for fattening was investigated, analysing the copper content in ten subsamples each.20 The coefficient of variation (CV) of the copper concentration in the premixture for starter (mean 4,973 mg/kg) and grower vitamin premix (mean 4,643 mg/kg) was 2.2% and 3.6%, respectively. The CV of the mash feed for starter (mean copper content: 28 mg/kg), grower (mean copper content: 27 mg/kg) and grower pelleted feed (mean copper content: 24 mg/kg) was 3.5%, 5.6% and 4.4%, respectively.
3.1.4. Physico‐chemical incompatibilities or interactions
No incompatibilities are expected due to the nature of the product.
3.1.5. Conditions of use
Copper‐LG is intended to be used in all types of feed (supplementation of feed should be done via premixture) or water (via complementary feed) for all animal species up to a maximum total copper content of 15 mg/kg complete feed (bovines before the start of the rumination and ovines), 30 mg/kg complete feed (other bovines), 35 mg/kg complete feed (caprines), 50 mg/kg complete feed (crustaceans), 150 mg/kg complete feed (piglets suckling and weaned up to 4 weeks after weaning), 100 mg/kg complete feed (piglets from 5th week after weaning up to 8 weeks after weaning) and 25 mg/kg complete feed (other species).22
The applicant proposed half of the levels in feed for use in water for drinking.7
3.2. Safety
The additive is a mixture of copper chelate of lysine and copper chelate of glutamic acid. The additive will introduce only a minor fraction of the amino acids lysine and glutamic acid, which contributes negligibly to the intake of the animals. The sources of amino acids are already authorised and have been assessed by EFSA as feed additives (EFSA FEEDAP Panel, 2014, 2015a). Therefore, no relevance for the safety assessment is foreseen for the amino acids delivered by the additive.
Owing the safety concerns for the use of copper compounds in water for drinking, described and discussed in depth in a previous opinion (EFSA FEEDAP Panel, 2015b), the FEEDAP Panel considers that Copper‐LG should not be used in water for drinking. Thus, the Panel reiterates its previous statement that compounds of trace elements should generally not be used in water for drinking (EFSA FEEDAP Panel, 2010).
3.2.1. Safety for the target species
Where a feed additive application is made as a nutritional additive for all animal species, tolerance data may be limited to one species. The maximum tolerable levels for copper have been reviewed by the FEEDAP Panel in previous opinions (e.g. EFSA FEEDAP Panel, 2015b).
3.2.1.1. Tolerance study
The applicant provided a tolerance study with Copper‐LG in chickens for fattening with a duration of 37 days.23
A total of 504 one‐day‐old male chickens for fattening (Ross 308) were allocated to seven treatments. Birds were housed in 42 pens with 12 birds per pen (six replicate pens per treatment), randomly allocated to treatment in three environmentally controlled rooms. The treatments consisted of a control without added copper (T1), Copper‐LG at three supplementation levels low – 15 (T2), standard – 25 (T3) and tolerance – 200 (T4) mg total copper per kg of feed, and an inorganic copper source (copper sulfate) at three supplementation levels low – 15 (T5), standard – 25 (T6) and tolerance – 200 (T7) mg total copper/kg of feed, see Table 1. All chickens were fed a basal mashed feed (starter and grower). The diets were mainly composed of barley, wheat and soya and contained as starter (1–21 days) 214 g/kg of crude protein and 12.8 MJ/kg of apparent metabolisable energy (AME) and as grower (21–35 days) 195 g/kg of crude protein and 13.1 MJ/kg of AME.
Table 1.
Description of the seven treatment groups
| Treatment | Source | Added copper mg/kg | Total copper mg/kg (Intended) | Total copper mg/kg(Analysed) | |
|---|---|---|---|---|---|
| Starter | Grower | ||||
| T1 | None1 | 0 | 8 | 10 | 8 |
| T2 | Copper‐LG2 | 7 | 15 | 17 | 16 |
| T3 | 17 | 25 | 28 | 29 | |
| T4 | 192 | 200 | 231 | 206 | |
| T5 | Copper Sulfate3 | 7 | 15 | 19 | 14 |
| T6 | 17 | 25 | 27 | 23 | |
| T7 | 192 | 200 | 210 | 181 | |
Since the background copper concentration of the basal diets was 6 mg/kg, 2 mg Cu from copper sulfate/kg diet were added to meet the minimum requirements of copper for chickens for fattening.
Copper‐LG contains 17% copper.
commercial product providing 25% copper.
Mortality and general health were monitored throughout the study. Performance was assessed at day 1 (only body weight), and days 21 and 35 by measuring feed intake and body weight and calculating feed to gain ratio (F/G). At the study termination (days 35, 36 and 37), a total of 28 birds (4 birds/treatment/day: two birds from two pens per treatment on each day (selected on a first caught basis, chosen to be representative for weight and health of the pen to remove bias)) were killed and blood samples were taken for analysis of haematology24 and biochemistry;25 during this procedure also tissue and organ samples were taken for residue study (see Section 3.2.2.1).
Data were statistically analysed by analysis of variance (ANOVA); when a significant difference was detected, the Duncan's test was applied. The pen was considered as the statistical unit for the performance parameters, whilst the individual animal was the statistical unit for haematology and biochemical parameters.
Mortality was 4.6% in the overall study and it was not linked to any treatment; this mortality rate is in line with industry standards for Ross 308. No significant differences were identified for performance parameters, with the exception of feed intake; however no dose or source trend was identified (see Table 2).
Table 2.
Effect of Copper‐LG on performance parameters in chickens for fattening after 35 days
| Treatment/Source | Copper‐Intended (mg/kg diet) | Body weight (kg) | Feed intake (g/bird/day) | Feed to gain |
|---|---|---|---|---|
| Control | 8 | 2.527 | 102.71 c | 1.464 |
| Copper‐LG | 15 | 2.422 | 96.82 ab | 1.445 |
| 25 | 2.493 | 99.14 bc | 1.432 | |
| 200 | 2.390 | 94.76 a | 1.445 | |
| Copper sulfate | 15 | 2.495 | 99.49 bc | 1.434 |
| 25 | 2.501 | 100.70 bc | 1.444 | |
| 200 | 2.335 | 96.73 ab | 1.484 |
a,b,c Different superscript within a column indicates significant differences p < 0.05.
No significant differences were observed between treatments for the haematology parameters confirming no biological detrimental effect of feeding Copper‐LG at low, standard or tolerance doses. Regarding biochemical parameters, statistically significant differences for total protein, albumin, creatinine, calcium and triglycerides were observed but the values were within the physiological range; moreover, these differences did not indicate a lower tolerance of the additive under assessment compared to the inorganic source in chickens for fattening.
The tolerance study indicates that Copper‐LG is safe up to 200 mg Cu/kg feed; a margin of safety of at least 8 can be derived in chickens for fattening.
The additive will introduce only a minor fraction of the amino acids lysine and glutamic acid, which contributes negligibly to the intake of the animals; no relevance for target animals’ safety is foreseen.
3.2.1.2. Conclusions on safety for the target species
Based on a tolerance study, the FEEDAP Panel concludes that the additive is safe for chickens for fattening. This conclusion can be extrapolated to all animal species and categories provided that the maximum authorised levels in the EU for total copper in feed are not exceeded.
3.2.2. Safety for the consumer
The sources used for both amino acids are authorised in the EU and their safety have been established. Therefore, the FEEDAP Panel retains that only copper is of interest concerning the consumer safety.
3.2.2.1. Deposition study
The applicant submitted a study on tolerance of chickens for fattening (see Section 3.2.1.1). This study provided data on copper deposition in tissues and organs (breast muscle, fat/skin, kidney, liver, and bone (tibia)) of chickens for fattening fed the Copper‐LG. Samples were taken at days 35, 36 and 37 after necropsy, from a total of 28 birds (four birds/treatment).
There were no statistically significant differences between treatments for copper levels of edible tissues and tibia, indicating that no significant deposition of copper occurred at the relevant tested supplementation levels (Table 3).
Table 3.
Analytical results copper content in edible tissues and tibia (results in fresh matter)
| Treatment | Source | Cu in feed mg/kg 1 | Skin & Fat mg/kg | Muscle mg/kg | Liver mg/kg | Kidney mg/kg | Tibia mg/kg |
|---|---|---|---|---|---|---|---|
| T1 | None | 8 | 0.26 | 0.30 | 3.16 | 2.31 | 1.30 ab |
| T2 | Copper‐LG | 15 | 0.23 | 0.27 | 3.36 | 2.31 | 1.34 ab |
| T3 | 25 | 0.25 | 0.26 | 3.15 | 2.27 | 1.30 ab | |
| T5 | Copper Sulfate | 15 | 0.37 | 0.30 | 3.08 | 2.28 | 1.39 b |
| T6 | 25 | 0.24 | 0.28 | 3.08 | 2.24 | 1.25 a |
1 Confirmed by analysis.
a,b Different superscript within a column indicates significant differences.
3.2.2.2. Toxicology of copper
The toxicology of copper has been reviewed by Ellingsen et al. (2015) and by the FEEDAP Panel (EFSA FEEDAP Panel, 2015b). To the knowledge of the FEEDAP Panel, there are no new relevant toxicology studies on copper that could modify the previous review.
Under normal circumstances, copper homoeostasis ensures that copper overload in humans does not occur. An excess of copper has been recorded and shown to cause problems only under certain specific conditions, notably genetic disorders such as Wilson disease (EFSA NDA Panel, 2015). The primary target of copper toxicity is the hepatocyte and copper excess impairs liver function (EC, 2003). The Scientific Committee on Food (SCF), based on adverse effects on liver, set a tolerable upper intake level (UL) of 5 mg Cu/day for adults and 1 mg/day for toddlers (1–3 years of age) (EC, 2003).
3.2.2.3. Assessment of consumer safety
The available data on copper intake by the European population (reviewed in EFSA NDA Panel, 2015) indicate that the average intake is below 50% of the UL and most of the intake is not contributed by food of animal origin. In addition, the copper intake of the European population reflects also the current use of copper supplementation of feed. Copper‐LG will be used as a substitute for other copper‐containing additives and considering that it is not expected to increase copper deposition in edible tissues and products compared to other additives, its use in animal nutrition would not have consequences on consumer exposure.
3.2.2.4. Conclusions on safety for the consumer
No increases in the copper content of animal tissues and products are expected from the use of Copper‐LG in animal nutrition. There is no indication that the toxicity of Copper‐LG is essentially different from that described for inorganic divalent copper. The FEEDAP Panel concludes that the use of Copper‐LG in animal nutrition is of no concern for consumer safety provided that the maximum authorised total copper levels in feed are respected.
3.2.3. Safety for the user
3.2.3.1. Effects on the respiratory system
No specific studies were provided by the applicant regarding the toxicity of the additive on the respiratory system.
The highest dusting potential of the additive was 3.0 g/m3 and the copper maximum concentration in the dust was 17.9% (see section 3.1.1). Thus, it can be calculated that a maximum concentration of 537 mg Cu/m3 could be released by the dust when handling the additive. Considering that the respirable and the thoracic fractions amounted up to 15.8% and 56.6%, respectively (see Section 3.1.1), based on a conservative approach, it could be estimated that respirable copper from dust would be about 150 mg/m3, assuming that the dust consists only of particles ≤ 50 μm and its respirable fraction about 28% (15.8 of 56.6). Considering the copper occupational exposure limit (OEL) of 0.01 mg/m3 (EC, 2014), the copper OEL is exceeded by more than four orders of magnitude.
The nickel maximum content of the additive was 13 mg/kg. The dusting potential of the product amounted up to 3.0 g/m3, corresponding to about 0.039 mg Ni/m3. Considering that the OEL for the inhalable fraction of water‐soluble nickel is 0.01 mg Ni/m3 (EC, 2011), the nickel OEL is exceeded by about four times.
The FEEDAP Panel considers that handling the additive, poses a risk to users by inhalation.
Due to its nickel content, the additive should be considered as a respiratory sensitiser.
3.2.3.2. Effects on the eyes and skin
The applicant presented two acute irritation studies according OECD guidelines No 404 and No 405, respectively for skin26 and eye.26 Under the experimental conditions adopted, the additive was found to be corrosive for the eye of the rabbit, while it was non‐irritant for the skin.
The nickel content of the additive is up to 13 mg/kg; given its well‐known sensitisation potential (European Commission, 2011) and in the absence of skin sensitisation studies the additive is classified as a skin sensitiser.6
3.2.3.3. Conclusions on safety for the user
Owing to the copper and nickel content of Copper‐LG, the handling of the additive poses a risk to users by inhalation. The additive is considered as a skin and respiratory sensitiser. It is corrosive to the eye while it is non‐irritant to skin.
3.2.4. Safety for the environment
The additive under assessment, Copper‐LG, is intended to be a substitute for other authorised copper additives and will not further increase the environmental burden of copper. Therefore, the FEEDAP Panel considers that the use of the additive in animal nutrition would not pose an additional risk for the environment.
3.3. Efficacy
For demonstration of the efficacy of nutritional additives, one study in a single animal species or category, including laboratory animals, is considered sufficient (EFSA FEEDAP Panel, 2012a).
3.3.1. Study in chickens for fattening
The applicant provided a combined tolerance/efficacy study in chickens for fattening27 (see Sections 3.2.1.1 and 3.2.2.1). The experimental groups in the study are shown in Table 1. In this trial, copper concentration in edible tissues/organs and tibias was measured (Table 3).
Animals receiving the Copper‐LG supplemented diets showed no differences in copper deposition in liver as well as in other tissues, compared to those in the control group or the copper sulfate at comparable levels of copper supplementation in diets. This lack of difference among all the experimental groups (both Copper‐LG and inorganic copper) with the control group may be explained by the already adequate copper supplementation in the diet of that group (8 mg Cu/kg feed). Only at very high copper concentration in poultry diet (approximately 300 mg Cu/kg), copper concentration in liver or tissues shows a marked increase (see also EFSA FEEDAP Panel, 2016).
3.3.2. Conclusions on efficacy
Based on the deposition of copper in liver as well as in other tissues in chickens for fattening, the FEEDAP Panel concludes that the additive is a bioavailable source of copper, comparable to the standard inorganic copper source, and therefore the additive is efficacious in meeting the birds copper requirements. This conclusion can be extrapolated to all animal species and categories.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation28 and Good Manufacturing Practice.
4. Conclusions
The FEEDAP Panel concludes that, owing to safety considerations, Copper‐LG should not be used in water for drinking.
Based on the results of a tolerance study, the FEEDAP Panel concludes that the additive is safe for chickens for fattening. This conclusion can be extrapolated to all animal species and categories provided that the maximum authorised levels in the EU for total copper in feed are not exceeded.
No increases in the copper content of animal tissues and products are expected from the use of Copper‐LG in animal nutrition. There is no indication that the toxicity of Copper‐LG is essentially different from that described for inorganic divalent copper. The FEEDAP Panel concludes that the use of Copper‐LG in animal nutrition is of no concern for consumer safety provided that the maximum authorised total copper levels in feed are respected.
Owing to the copper and nickel content of Copper‐LG, the handling of the additive, poses a risk to users by inhalation. The additive is considered as a skin and respiratory sensitiser. It is corrosive to the eye while it is non‐irritant to skin.
The additive under assessment, Copper‐LG, is intended to be a substitute for other authorised copper additives and will not further increase the environmental burden of copper. Therefore, the FEEDAP Panel considers that the use of the additive in animal nutrition would not pose an additional risk for the environment.
Based on the deposition of copper in liver as well as in other tissues in chickens for fattening, the FEEDAP Panel concludes that the additive is a bioavailable source of copper, comparable to the standard inorganic copper source, and therefore the additive is efficacious in meeting the birds copper requirements. This conclusion can be extrapolated to all animal species and categories.
5. Recommendations
The FEEDAP Panel recommends to include the sources of lysine and glutamic acid (including the production strain(s), where applicable) as proposed by the applicant in the description of the additive. The content of both amino acids in the additive should be also indicated in the description of the product.
Documentation provided to EFSA/Chronology
| Date | Event |
|---|---|
| 21/12/2017 | Dossier received by EFSA. Copper chelates of lysine and glutamic acid for all animal species. Submitted by Zinpro Animal Nutrition (Europe), Inc. |
| 11/01/2018 | Reception mandate from the European Commission |
| 28/03/2018 | Application validated by EFSA – Start of the scientific assessment |
| 03/05/2018 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation, safety for target species and safety for the user |
| 18/06/2018 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 21/06/2018 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 (Addendum) – Scientific assessment suspended. Issue: characterisation |
| 28/06/2016 | Comments received from Member States |
| 22/08/2018 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 17/12/2018 | Spontaneous submission of information by the applicant. Issue: characterisation |
| 24/01/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issue: characterisation |
| 07/03/2019 | Clarification teleconference during risk assessment with the applicant according to the “EFSA's Catalogue of support initiatives during the life‐cycle of applications for regulated products” |
| 14/03/2019 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 14/05/2019 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- AME
apparent metabolisable energy
- ANOVA
analysis of variance
- CAS
Chemical Abstracts Service
- CV
coefficient of variation
- cfu
colony forming unit
- EURL
European Union Reference Laboratory
- F/G
feed to gain ratio
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FSA
UK Food Standards Agency
- HPLC
High Performance Liquid Chromatography
- ICP‐AES
inductively coupled plasma atomic emission spectrometry
- ICP‐MS
inductively coupled plasma mass spectrometry
- IEC‐VIS
ion exchange chromatography coupled with post‐column derivatisation and photometric detection
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- OECD
Organisation for Economic Co‐operation and Development
- OEL
occupational exposure limit
- PCB
polychlorinated biphenyl
- PCDD
polychlorinated dibenzo(p)dioxins
- PCDF
polychlorinated dibenzofurans
- RH
relative humidity
- RSDr
relative standard deviation for repeatability
- SCF
Scientific Committee on Food
- TEQ
toxic equivalent
- UL
tolerable upper intake level
- WHO
World Health Organization
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for copper chelates of lysine and glutamic acid
1.
In the current application authorisation is sought under Article 4(1) for copper chelates of lysine and glutamic acid under the category/functional group (3b) “nutritional additives”/“compounds of trace elements”, according to the classification system of Annex I of Regulation (EC) No 1831/2003. Specifically, authorisation is sought for the use of the feed additive for all categories and species.
Copper chelates of lysine and glutamic acid is a solid preparation with a minimum content of 17% (w/w) of copper, 19% (w/w) of lysine and 19% (w/w) of glutamic acid.
The feed additive is intended to be incorporated into premixtures and feedingstuffs. The Applicant proposed maximum levels of total copper in feedingstuffs ranging from 15 to 170 mg/kg – depending of the animal species/category – and thus complying with the limits set in the Regulations (EC) No 1334/2003 and (EC) No 479/2006.
For the quantification of total copper in the feed additive, premixtures and feedingstuffs the Applicant submitted two internationally recognised ring‐trial validated CEN methods based on inductively coupled plasma atomic emission spectrometry (ICP‐AES): EN 15510 and EN 15621. These two methods together with the Community method based on atomic absorption spectrometry, which was further ring‐trial validated by the UK Food Standards Agency (FSA), were previously evaluated and recommended by the EURL in the frame of the Copper group dossier.
In addition, during the review process the EURL identified two ring‐trial validated methods, namely: ISO 6869 based on atomic absorption spectrometry and EN 17053 based on inductively coupled plasma mass spectrometry (ICP‐MS).
Based on the acceptable method performance characteristics available, the EURL recommends for official control the five ring‐trial validated methods: i) EN 15621 and ISO 6869 for the quantification of total copper in the feed additive, premixtures and feedingstuffs; ii) EN 15510 and EN 17053 for the quantification of total copper in premixtures and feedingstuffs; and iii) the Community method (Commission Regulation (EC) No 152/2009 – Annex IV‐C) for the quantification of total copper in feedingstuffs.
For the quantification of lysine and glutamic acid in the feed additive the Applicant submitted the ring‐trial validated EN ISO 13903 method based on ion exchange chromatography coupled with post‐column derivatisation and photometric detection (IEC‐VIS). This standard method is equivalent to the experimental protocol described in the Community method designed for the determination of free (synthetic and natural) and total (peptide‐bound and free) amino acids including lysine and glutamic acid, using an amino acid analyser or a High Performance Liquid Chromatography (HPLC) equipment. This method does not distinguish between the salts and the amino acid enantiomers.
The Applicant applied the above mentioned IEC‐VIS method for analysis of five batches of the feed additive with an average content of 20% (w/w) for lysine and glutamic acid and obtained a relative standard deviation for repeatability (RSDr) ranging from 1.8% to 2.0%. This is in agreement with the precision values reported in the frame of the two ring‐trial validation studies.
Based on the performance characteristics available, the EURL recommends for official control the method based on IEC‐VIS to quantify lysine and glutamic acid in the feed additive.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005 as last amended by Regulation (EU) 2015/1761) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis, V , Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Sanz Y, Villa RE, Woutersen R, Cubadda F, Flachowsky G, Mantovani A, López‐Gálvez G and Ramos F, 2019. Scientific Opinion on the safety and efficacy of copper chelates of lysine and glutamic acid as a feed additive for all animal species. EFSA Journal 2019;17(6):5728, 14 pp. 10.2903/j.efsa.2019.5728
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00011
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Maryline Kouba, Mojca Kos Durjava, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Acknowledgements: The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) wishes to thank the following for the support provided to this scientific output (in alphabetical order of the last name): Jaume Galobart, Orsolya Holczknecht, Niovi Kordali and Paola Manini.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the European Commission decision on the confidentiality requests formulated by the applicant. A previous, provisional version of this output which had been made publicly available pending the adoption of the decision has been replaced by this version. The full output was shared with the European Commission, EU Member States and the applicant.
Adopted: 15 May 2019
Amended: 16 January 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Zinpro Animal Nutrition (Europe), Inc. Akkerdistel 2E. 5831 PJ. Boxmeer. The Netherlands.
Technical Dossier/Section II/Annex II‐37. Patent Number: US20120315372A1. Available online: https://patents.google.com/patent/US20120315372
FEED dossier reference: FAD‐2017‐0071.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep-fad-2017-0071-cu-lys-glu.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical Dossier/Supplementary Information/August 2018.
Technical Dossier Supplementary Information/August 2018.
Technical Dossier/Spontaneous Information/August 2018.
Technical Dossier/Section II/Annex II‐1 to II‐15.
Values preceded by the sign ‘<’ mean the LOQ.
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 140, 30.5.2002, p. 10.
Technical Dossier/Section II/2.1.4.
Technical Dossier/Section II/Annex II‐36.
■■■■■
■■■■■
■■■■■
■■■■■
Technical Dossier/Section II/Annex II‐41 and Annex II‐42.
Technical Dossier/Section II/Annex II‐43.
Technical Dossier/Supplementary Information/August 2018 and Spontaneous information.
Commission Implementing Regulation (EU) 2018/1039 of 23 July 2018 concerning the authorisation of Copper(II) diacetate monohydrate, Copper(II) carbonate dihydroxy monohydrate, Copper(II) chloride dihydrate, Copper(II) oxide, Copper(II) sulphate pentahydrate, Copper(II) chelate of amino acids hydrate, Copper(II) chelate of protein hydrolysates, Copper(II) chelate of glycine hydrate (solid) and Copper(II) chelate of glycine hydrate (liquid) as feed additives for all animal species and amending Regulations (EC) No 1334/2003, (EC) No 479/2006 and (EU) No 349/2010 and Implementing Regulations (EU) No 269/2012, (EU) No 1230/2014 and (EU) 2016/2261.
Technical Dossier/Section III/Annex III_3_1.
RBC, PVC, haemoglobin, WBC, eosinophils, lymphocytes, monocytes, heterophils/neutrophils, platelets, MCH, MCHC and MCV.
Serum albumin, albumin:globulin (A:G) ratio, total protein serum, ALT, AST, ALP, amylase, serum gamma glutamyl transferase (gGT), glutamate dehydrogenase (GLDH), lactate dehydrogenase (LDH), serum bilirubin, triglycerides, glucose, serum uric acid, cholesterol, copper, serum Na, serum K, serum Cl, serum Ca, phosphate, creatine phosphokinase (CPK), creatinine and bile acid.
Technical dossier/Section III/Annex_III_3_2.
Technical Dossier/Section IV/Annex_IV_4_1.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- EC (European Commission), 2003. Opinion of the Scientific Committee on Food (SCF) on the Upper Intake Level of Copper (27 March 2003). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out176_en.pdf
- EC (European Commission), 2011. Recommendation from the Scientific Committee on Occupational Exposure Limits (SCOEL) for nickel and inorganic nickel compounds. Employment, Social Affairs and Inclusion. SCOEL/SUM/85, June 2011. Available online: http://ec.europa.eu/social/BlobServlet?docId=3803&langId=en
- EC (European Commission), 2014. Recommendation from the Scientific Committee on Occupational Exposure Limits for Copper and its inorganic compounds. SCOEL/SUM/171, March 2014. Available online: http://ec.europa.eu/social/BlobServlet?docId=11815&langId=en
- EFSA (European Food Safety Authority), 2008. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008,6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances Used in Animal Feed), 2010. Scientific Opinion on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12):1956, 9 pp. 10.2903/j.efsa.2010.1956 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Technical guidance: Tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for nutritional additives. EFSA Journal 2012;10(1):2535, 14 pp. 10.2903/j.efsa.2012.2535 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for the preparation of dossiers for additives already authorised for use in food. EFSA Journal 2012;10(1):2538, 4 pp. 10.2903/j.efsa.2012.2538 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012d. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of the use of amino acids (chemical group 34) when used as flavourings for all animal species. EFSA Journal 2014;12(5):3670, 19 pp. 10.2903/j.efsa.2014.3670 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015a. Scientific Opinion on the safety and efficacy of L‐lysine monohydrochloride, technically pure, produced with Escherichia coli CGMCC 3705 and L‐lysine sulphate produced with Corynebacterium glutamicum CGMCC 3704 for all animal species, based on a dossier submitted by HELM AG. EFSA Journal 2015;13(7):4156, 25 pp. 10.2903/j.efsa.2015.4156 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015b. Scientific Opinion on the safety and efficacy of copper compounds (E4) as feed additives for all animal species (cupric acetate, monohydrate; basic cupric carbonate, monohydrate; cupric chloride, dihydrate; cupric oxide; cupric sulphate, pentahydrate; cupric chelate of amino acids, hydrate; cupric chelate of glycine, hydrate), based on a dossier submitted by FEFANA asbl. EFSA Journal 2015;13(4):4057, 52 pp. 10.2903/j.efsa.2015.4057 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016. Scientific opinion on the revision of the currently authorised maximum copper content in complete feed. EFSA Journal 2016;14(8):4563, 100 pp. 10.2903/j.efsa.2016.4563 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific Opinion on Dietary Reference Values for copper. EFSA Journal 2015;13(10):4253, 51 pp. 10.2903/j.efsa.2015.4253 [DOI] [Google Scholar]
- Ellingsen DG, Moller LB and Aaseth J, 2015. Copper In: Nordberg GF, Fowler BA, Nordberg M. (eds.). Handbook on the Toxicology of Metals. Chapter 35, Vol. II, 4th Edition. Elsevier and Academic Press, Amsterdam: pp. 765–786. [Google Scholar]


