Abstract
The European Commission is routinely asking EFSA for scientific and technical support in the epidemiological analysis of animal disease outbreaks (i.e. African swine fever, lumpy skin disease and avian influenza) and to report or assess surveillance data (i.e. Echinococcus multilocularis and avian influenza). For this purpose, EFSA has over the last years carried out several data collections and gathered specific information on outbreaks, surveillance activities and concerned animal populations (i.e. poultry, domestic pigs, cattle and wildlife such as wild boar). EFSA aims to work together closely with Member States in order to (i) reduce the Member States’ manual input of the data to be submitted to EFSA; (ii) avoid double reporting to EFSA; (iii) provide the Member States with tools to produce automatically their own draft national reports on animal health and surveillance in a protected environment to ensure data protection; (iv) increase the quality of the data received from the Member States; and (v) shorten the time to retrieve up‐to‐date data, relevant for risk assessment purposes. With this purpose, EFSA launched a project called SIGMA. It is important to highlight that the SIGMA – Animal Disease Data Model (σ‐ADM) focuses on data which are known to be already collected by several Member States under different legal frameworks and for different purposes. The version presented in this report, will be subject to modifications and updates derived from the feedback during the implementation phase.
Keywords: SIGMA, data model, data collection, standardisation, population, surveillance
1. Background
The European Commission is routinely asking the European Food Safety Authority (EFSA) for scientific and technical support in the epidemiological analysis of animal disease outbreaks (i.e. African swine fever (ASF), lumpy skin disease (LSD) and avian influenza (AI)) and to report or assess surveillance data (i.e. Echinococcus multilocularis (EM) and AI). For this purpose, EFSA has over the last years carried out several data collections and gathered specific information on outbreaks, surveillance activities and concerned animal populations (i.e. poultry, domestic pigs, cattle and wildlife such as wild boar). These mandated undertakings related to specific animal diseases can be considered ‘ad hoc animal health data collections’. Data collections on animal populations are also implemented in relation to the EFSA–ECDC zoonoses summary report (EFSA and ECDC, 2017) and by EUROSTAT, but the resolution of the data is often not sufficiently detailed to be used in analytical epidemiology and risk assessments. In addition, EFSA is currently using ad hoc data models specifically tailored to a single disease, with a consequent lack of harmonisation across the different data collection processes and with the zoonoses data model (EFSA, 2017).
Taking into account the experience gained by EFSA in the field of the animal disease data collection over the last decade and considering: (i) the increasing demand of data‐driven scientific advice to the risk managers to face animal health threatens and, on the other hand, (ii) the steady progress made by information technology, EFSA decided to initiate a process of harmonisation across the different animal health data collection activities, including zoonotic agents, linked to outbreaks and monitoring/surveillance.
EFSA aims to work together closely with Member States on the technical aspects of ‘ad hoc animal health data collections’, in particular to:
reduce the Member States’ manual input of the data to be submitted to EFSA;
avoid double reporting to EFSA;
provide the Member States with tools to produce automatically their own draft national reports on animal health and surveillance in a protected environment to ensure data protection;
increase the quality of the data received from the Member States;
shorten the time to retrieve up‐to‐date data, relevant for risk assessment purposes.
With this purpose, EFSA launched a project called SIGMA. This report provides a brief outline of the entire project and describes the details on a unique and harmonised Animal Disease Data Model (σ‐ADM) and the possible steps leading to its adoption.
It is important to highlight that the σ‐ADM focuses on data which are known to be already collected by several Member States under different legal frameworks and for different purposes. The version presented in this report, will be subject to modifications and updates derived from the feedback during the implementation phase.
2. The SIGMA project in a nutshell
The SIGMA project originates from an internal critical assessment of the current practices to collect data on animal population and animal diseases and has the purpose of optimising the entire process.
As a preliminary step, EFSA performed an internal technical analysis to identify all limitations related to the ongoing data collections. The main problems were: (i) each mandate (i.e. a formal request for support to EFSA by the European Commission) usually entails the design of an ad hoc data model making it unique and only partially compatible with those designed for other mandates; (ii) the ad hoc data models are designed to collect information about ongoing outbreaks, resulting in Member States (MSs) assigning extra resources to fill in these data models, at a time when they should be focusing on disease control focus on disease control; (iii) despite the considerable effort put in the past years to harmonise the definitions across the European Union (EU) MSs, there are still unavoidable differences due to many different factors, which make the data analysis a complicated task and require heavy assumptions.
Nowadays, information technology (IT) offers solutions that were not available a few years ago. In addition, the majority of the data (related to outbreaks, surveillance activities, laboratory analysis and animal populations) that EFSA needs to produce sound scientific outputs are in most cases already stored at country level and shared with EFSA upon request. Therefore, it is a matter of gathering/collating, with suitable agreement, the existing data (properly anonymised), standardise them and safely store them in the EFSA Scientific Data Warehouse (hereinafter also referred as S‐DWH)1 using the tools made available by the current technology.
For illustrative purposes, Figure 1 shows the optimal and final setting of the process of data collection/collation and reporting and how MSs could contribute to and benefit from the SIGMA project. Note that SIGMA will deal with data already submitted to EFSA but using different channels and aggregated in many different ways according to the topic and the purpose.
Figure 1.
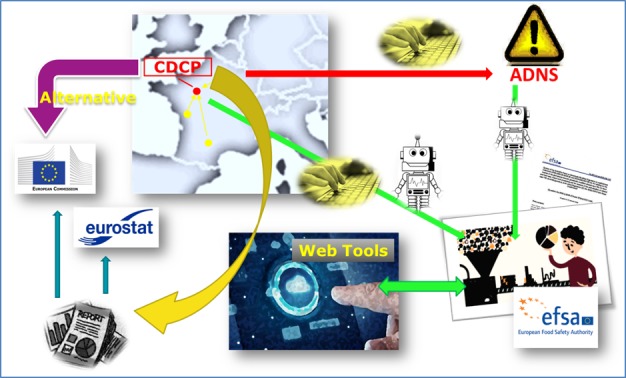
Data flow from the different data providers (public institutions and MSs) to the EFSA Scientific Data Warehouse
- Yellow spots: National Data Sources; CDCP: Country Data Collection Point; ADNS: Animal Disease Notification System; Keyboard icon: manual input; Robot icon: automated process; Web Tools: web applications designed to produce standard reports, including the ones required by the European Commission to the Member States (MSs); Purple arrow ‘Alternative’: transmission of the reports that the European Commission requires from the MSs without using the tools made available by EFSA.
The outbreak/disease notification data will still have to be submitted manually into the Animal Disease Notification System (ADNS), as currently done, following the relevant legislation in force. However, the aim is to connect the ADNS and the S‐DWH, so that the MSs will be requested to provide EFSA solely with the additional epidemiological information. These additional epidemiological data will be collected by means of the EFSA Data Collection Framework (hereinafter, DCF) and its integrated controlled terminology and validation rules. As these epidemiological data are essential to estimate important parameters (e.g. prevalence, occurrence of disease) and to correctly interpret information originating from risk‐based sampling designs (e.g. sampling strategy, geographical allocation of the samples, etc.), the SIGMA project, with the help of the awarded consortium, aims at centralising the data flow towards a unique Country Data Collection Point (CDCP, as an ideal set up and in agreement with the MS). Once all the relevant information is in the CDCP, they can be standardised against the σ‐ADM by means of dedicated ‘Extract‐Transform‐Load’ (ETL) processes (i.e. the data will be automatically converted from the national data model into the σ‐ADM). At the end of the standardisation process, the information will be ready for provisioning to EFSA. The data upload should be automatically handled by the system taking advantage of the data exchange protocol implemented in DCF in compliance to EFSA Guidance on Data Exchange v2.0. The MS can choose to send data to EFSA automatically (e.g. a periodical submission on a monthly basis) or to send them on an ‘ad hoc basis’. EFSA will therefore receive a set of pre‐standardised data from the MSs and will be able to (i) perform risk assessment at European level using harmonised information and producing highly comparable outputs; and (ii) give the MSs the opportunity of using web applications to analyse their own data and produce any type of report, including the ones foreseen by law. Even in this case, this will not be an obligation: each MS will be able to choose between the EFSA tools and any other way to analyse their data.
Considering the complexity of the project and the ambition of creating a framework that could be used by all MSs, EFSA launched a call asking for support in the technical implementation of the project. The awarded consortium is led by the Istituto Zooprofilattico Sperimentale (IZS) Abruzzo e Molise ‘G. Caporale’ and is in partnership with the Friedrich Loeffler Institut (FLI), the Swedish National Veterinary Institute (SVA), the Bulgarian Food Safety Agency (BFSA) and the Institute of Veterinary Medicine and Animal Sciences, Estonian University of Life Sciences.
The SIGMA consortium will provide technical support to interested MSs:
to improve animal health data flows within the country (ideally, from the national data sources to the CDCP);
to improve animal health data flow from the CDCP to EFSA;
to standardise the MS data based on the σ‐ADM;
to connect (preferably existing) tools for data analysis to the S‐DWH to facilitate harmonised reporting by national and European risk assessment bodies.
It is important to specify that there are no legal obligations behind the project and the decision of taking advantage of this framework is entirely on the single country.
SIGMA has been planned as a three‐year project with three main phases as described in the following sections.
2.1. SIGMA Phase 1
In this first phase, the main goals are:
to design a harmonised data model, the SIGMA Animal Disease Data Model (σ‐ADM) able to gather, from the concerned MSs and from the existing data collection systems, those data essential to address the requests related to the ongoing mandates (ASF, AI, LSD, EM);
to produce a ‘country card’, i.e. a comprehensive overview, at MS level, of the authorities responsible for the collection of the data related to animal health and animal population (at this point in time, considering the ongoing mandates received from the European Commission, poultry, bovines and pigs);
to outline the data flows in place, within each MS and from each MS to EFSA, to highlight potential drawbacks and propose technical solutions to optimise the system;
-
to provide a list of online tools for the data analysis and for the reporting of disease outbreaks/surveillance activities with the aim to make them available to the MSs to query the S‐DWH, where provided data are stored. Particular attention will be given to those consolidated tools that are already in use:
2.2. SIGMA Phase 2
The second phase will be mainly dedicated to the concrete implementation of the framework with the MSs that volunteer to take part in the pilot. This phase will be targeted on those diseases that EFSA has been requested to deal with (AI, ASF and LSD). In detail:
Planning and development of solutions to enhance the data flows;
Data mapping (matching between MS naming conventions and σ‐ADM);
Support the volunteering MSs in designing the ETL processes to select, transform and transmit the standardised relevant national data to the EFSA DWH.
These activities will be performed on outbreak and surveillance data on AI, ASF and LSD and related animal populations, based on the specific situation of the volunteering MSs.
2.3. SIGMA Phase 3
In the third phase, based on the outcomes from the previous phases, the implementation of the SIGMA approach will be (i) finalised; and (ii) extended to other MSs and/or other diseases (in case of new mandates from the European Commission) and/or other animal population data that were not included in Phase 2. In addition, the selected web tools will be connected to the DWH and made freely available to the MSs providing the data.
In detail:
ETL processes: implementation of the ETL processes in the volunteering MSs;
Analytical interactive online tools: connection of relevant online tools to EFSA's DWH to support AI, ASF, LSD disease outbreak analysis and reporting;
Analytical interactive online tools: connection of relevant online tools to EFSA's DWH to support Avian Influenza Surveillance and Echinococcus multilocularis surveillance analysis and reporting.
3. The SIGMA Animal Disease Data Model (σ‐ADM)
The first milestone of Phase 1 of the SIGMA project is about the creation of a comprehensive data model that is able to encompass different needs related to animal disease risk assessment. The steps followed to achieve the σ‐ADM are described in the following points (Figure 2).
The first step towards the harmonisation of the data collection was to analyse and summarise the risk assessment requests from the risk managers, i.e. European Commission (see Section 5.1, Appendices D and E);
Once the risk assessment questions were retrieved, they were grouped into categories, each characterised by a common possible statistical approach. The outcome of this step was a set of Envisaged analysis (i.e. a set of hypothetical statistical approaches that could address the identified risk assessment requests), essential to identify the data needs (see point 3 and Section 5.2);
As each statistical approach needs specific input data, the Preliminary Plan of Analysis played a crucial role to identify and define exactly and in a concrete way the type of data needed (see Section 5.3);
The data need was then formalised in a data model: the SIGMA Animal Disease Data Model (σ ‐ADM, see Section 5.4);
The σ ‐ADM was then tested against all the ongoing EFSA data collection to make sure that the experience gained over the last years was well integrated and the σ‐ADM was comprehensive enough to encompass all types of risk assessment needs (see Section 7).
Figure 2.
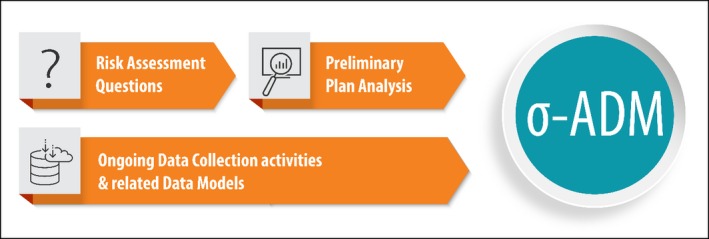
Sources of information in the process generating the SIGMA‐Animal Disease Data Model (σ‐ADM)
The first version of the model was then circulated among the members of the Animal Health Network (see also the EFSA event report ‘SIGMA A comprehensive animal disease data collection approach’5) and of the Animal Health and Welfare Panel to check its compatibility with all possible scenarios. See Section 4 for more information about the features of the σ‐ADM.
The final goal was to have in the EFSA DWH a set of European data fully harmonised and ready to be used:
by EFSA to run the statistical analysis envisaged in the Preliminary Plan of Analysis and address the requests from the Risk Managers
by the MSs to create their draft country reports to be submitted to the relevant institutions as laid down in the relevant legislation in force (e.g. Commission Regulation (EU) No 1152/2011 on Echinococcus multilocularis)
The objective of this scientific report is to describe the five steps of the SIGMA harmonisation process, up to the development of the SIGMA Animal Data Model on animal diseases (σ‐ADM).
It is important to note that the data model resulting from this exercise will be tailored to fit the animal diseases for which EFSA has an ongoing mandate, i.e. ASF, LSD, AI, EM, and will be compatible with the data model used for zoonotic diseases included in the EFSA annual report (EFSA and ECDC, 2017). Diseases, other than those for which EFSA has an ongoing mandate, will only be included in the σ‐ADM if EFSA would receive a specific mandate to provide the risk managers with scientific information on those diseases.
The development of this scientific report is linked to the strategic objectives to widen EFSA's evidence base and optimise access to its data, build the EU's scientific assessment capacity and knowledge community and prepare for future risk assessment challenges (EFSA Strategy, 2020, https://www.efsa.europa.eu/sites/default/files/151008.pdf).
4. The σ‐Animal Disease Data Model
The σ‐ADM will be a compromise between the justifiable ambition of addressing the risk assessment questions using the most sophisticated methodology and the pragmatism on the actual data availability across the different data providers (MSs, International Databases), while ensuring there are no additional resource requirements for the MS. The σ‐ADM was designed to be harmonised, compatible, flexible and fit for purpose (see Figure 4).
Figure 4.
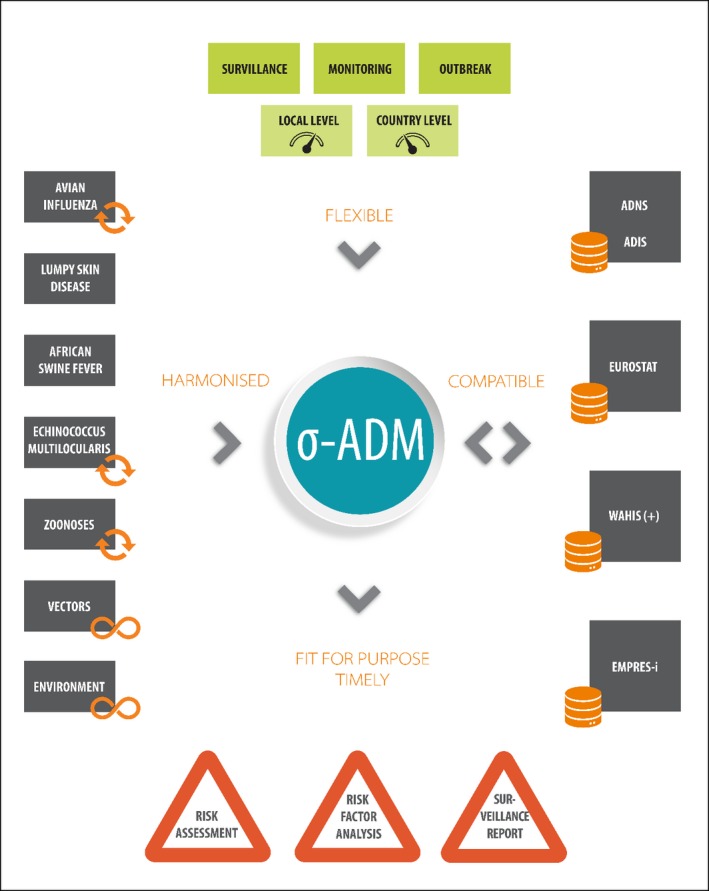
σ‐ADM features in a glance. The σ‐ADM will result from the harmonisation of the existing and ongoing data collection activities; will be compatible with the existing official international databases; will be flexible enough to gather data generated under different programmes and at different level of resolutions; will be fit for different purposes
With the publication of this scientific report, the σ‐ADM can be considered consolidated in its first release (σ‐ADM v.1). From now onwards EFSA, within the framework of the SIGMA project and with the help of the awarded Consortium, will work together with the MSs to improve and automate, where possible, the data flow from the national databases to the EFSA DWH.
It is important to highlight that, for the MSs that will volunteer to be part of the project, the development of the necessary technical steps will be performed by the SIGMA Consortium and financed by EFSA.
4.1. Harmonised
The first level of harmonisation was conducted internally, at EFSA (internal harmonisation). Starting from the different ongoing ad hoc data collections, EFSA harmonised the way the data were collected using a unique way of classification, based on existing standards where possible, e.g. SSD2 (ANSVSA, 2015) or setting new standards when these did not already exist (see Figure 4).
The second level of harmonisation was conducted across the different data providers in the different Member States (external harmonisation). It should be pointed out that in this case, what SIGMA is proposing is a harmonisation sensu stricto rather than a difficult ‘alignment’. It is well known that each country has its way of naming and classifying at least part of the information generated in the field for many reasons, including history and culture (Figure 3). Each country will have a specific name for ‘pear’. An ‘alignment’ would entail a new name for this fruit to be imposed for the purpose of collecting comparable data. On the contrary, a real harmonisation builds on the existing concepts: it is sufficient to know how that specific fruit is called in the different countries and translate it into any meaningful way, according to the purpose. It is also clear that, assuming a reasonable stability over time of the naming convention within each country, this translation can be completely automated. Therefore, the MSs will not have to modify anything of the way they collect the data. Nonetheless, EFSA will have a set of standardised and therefore highly comparable data available for the analysis.
Figure 3.
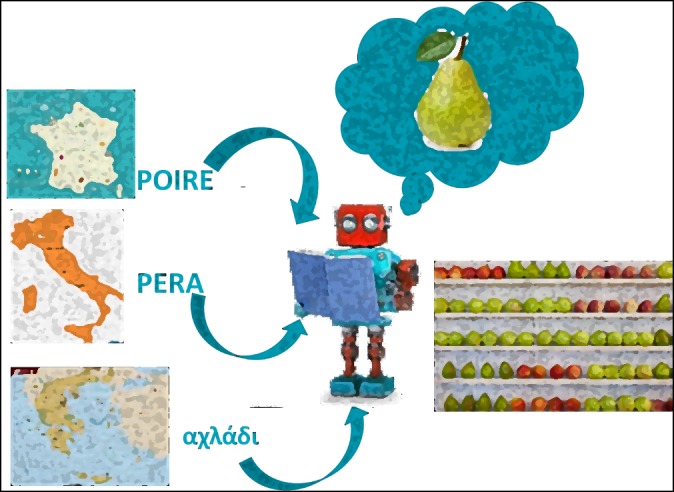
Harmonisation sensu stricto, i.e. building on the existing. There is no need to impose new naming conventions to the data providers. Once it is known how a given object is called (in this case and just for illustrative purposes, a pear) it is possible to translate the information into a standard language
4.2. Flexible
The σ‐ADM is flexible enough to adapt to the different settings in place and/or solutions adopted by the different Member States. It is also able to gather data related to different types of activity in the field, e.g. surveillance, monitoring or outbreak related sampling (see Figure 4).
Particular attention was dedicated to the level of resolution (or scale) of the data. An example is given by the geographical location of the unit of interest, e.g. a farm. The σ‐ADM has been designed to deal with the highest resolution possible, i.e. the geographic coordinates (x–y, latitude–longitude) of an establishment (e.g. a farm, see Table 1). However, a MS can decide not to share the data with ESFA at this level of resolution. In this case, the σ‐ADM offers the option to report the location of the establishment at lower level of geographical resolution (e.g. the NUTS3 regions). From a more technical point of view, in order to fulfil the requirements of the S‐DWH, when a data provider wants to provide data at a lower resolution, the data will be collected in an ‘aggregate‐able’ structure, as shown in Table 2). In this way, it will be possible at least to know the number of farms in each NUTS3 region.
Table 1.
Fictitious sketch of a set of data with two types of variables: anonymised identifier of the farm and HIGH‐RESOLUTION geo‐coordinates (for illustrative purpose)
| Dummy identifier farm | x | y |
|---|---|---|
| 1#uvhbwoh | 45.64 | 7.27 |
| 2#vjenvijnb | 45.71 | 7.29 |
| 3#ijvbirb | 45.34 | 7.62 |
Table 2.
Fictitious sketch of a set of ‘aggregate‐able’ data, with two types of variables: anonymised identifier of the farm and LOW‐RESOLUTION geo‐coordinates (for illustrative purpose)
| Dummy identifier farm | x | y | NUTS3 |
|---|---|---|---|
| 1#uvhbwoh | – | – | Aosta |
| 2#vjenvijnb | – | – | Aosta |
| 3#ijvbirb | – | – | Torino |
The goal of this flexibility is of course to make use of all possible information available at European level. Certainly, the higher the resolution, the more sophisticated the analysis that is possible to run. A spread model is a good example of a technique that would benefit of highly resolved information to be able to identify patterns, potential risk factors, etc. of a given disease, mainly because of the higher quality (accuracy, completeness, uniformity across EU) of the input data.
In terms of flexibility, it is also important to highlight that the σ‐ADM has a modular structure. This means that at any time another set of information is required (e.g. the collection of data that are not recorded in official registers and require manual input, like the data generated from a case–control study in the field) or another source of information is identified (e.g. the CORINE land cover data6), a new component can be designed, inserted in the σ‐ADM and link it with the existing categories of data.
4.3. Compatible
The σ‐ADM should be as compatible as possible with international databases on animal health data, namely: the ADNS from the European Commission, the EUROSTAT database, the Animal Disease Information System (ADIS) – which is under development by the European Commission in collaboration with the World Organisation for Animal Health (OIE) – the OIE's World Animal Health Information System (WAHIS) and the Global Animal Disease Information System EMPRES‐I from FAO (see Figure 4).
This compatibility will allow EFSA to retrieve from these international databases all information of interest, avoiding therefore, as much as possible, any type of duplication in data submission for the MSs (see Section 2 and Figure 1).
4.4. Fit for purpose
The σ‐ADM, will integrate a great variety of heterogeneous data in a harmonised way. Overall, data will be fit for different types of analyses and purposes (see Figure 4). As an example, the standardised collection of domestic and wild animal populations will support not only the epidemiological analysis of animal disease outbreaks and a good variety of risk assessment approaches (including risk factor analysis), but also the analysis related to the EFSA–ECDC zoonoses summary report (EFSA and ECDC, 2017).
The suitability for different type of quantitative analyses will not be the only feature that the σ‐ADM aims to bring to the animal disease risk assessment process. Indeed, having in place a data model developed, discussed and agreed with all the MSs during ‘peace‐time’ will increase EFSA's preparedness as: (i) the data can be submitted by the MSs on a regular basis in order to have at any time an updated situation, e.g. of the affected populations; and (ii) in case an ad hoc submission is required, e.g. upon urgent request to EFSA from the European Commission following an outbreak, it will be much easier for the MSs to provide the data as the data flow will be already in place. As a result, EFSA will have higher quality data in a shorter time at its disposal and will be able to provide timely replies to the European Commission, with benefits to the concerned MSs as the outbreak will be considered in its broader picture.
5. From the critical analysis to the solution: a stepwise approach towards the SIGMA‐ADM
5.1. Risk assessment requests/Terms of reference
As a first step, in line with the EFSA Prometheus7 , 8 approach (EFSA, 2015), the scope of previous risk assessments was reviewed. Fourteen mandates received from the European Commission since 2014 on the diseases under evaluation (ASF, LSD, AI and EM) were considered. Since 2014, EFSA received a total of 14 mandates (4 on AI, 5 on ASF, 3 on EM and 3 on LSD) and issued a total of 34 outputs. The results of the systematic search can be found in Appendix D.
Particular attention was given to the Terms of Reference (ToRs) in the different mandates as they represent the explicit questions to be addressed. The information was collected in an Excel database to facilitate the interpretation (see Appendix E).
Each ToR was then classified in a Category of Epidemiological Question. As an example, one of the ToRs in one of the mandates related to AI was to estimate/assess the probability that the virus ‘could be transmitted from wild birds to domestic bird holdings’. This question focusing on the probability that the animal production industry could be affected by a novel disease was classified under the ‘Probability of Introduction’ category. In Table 3, the identified Categories of Epidemiological Questions are listed together with their frequency of occurrence. It emerged that the most frequent question category to be addressed is the descriptive statistics. This outcome is not surprising as the parameters included in the ‘descriptive statistics’ category (e.g. prevalence, incidence, etc.) constitute the basis for more sophisticated analysis.
Table 3.
Frequency of occurrence of epidemiological question category and per for each of the diseases for which EFSA has a mandate
| Epidemiological question category | AI | ASF | EM | LSD | Total counts |
|---|---|---|---|---|---|
| Descriptive statistics | 3 | 3 | 3 | 3 | 12 |
| Effectiveness of biosecurity measures | 2 | 1 | 3 | ||
| Effectiveness of countermeasures | 3 | 2 | 2 | 7 | |
| Effectiveness of protection measures | 1 | 1 | 2 | ||
| Effectiveness of sampling schemes | 3 | 5 | 1 | 9 | |
| Impact assessment | 1 | 1 | 2 | ||
| Probability of endemicity | 1 | 1 | |||
| Probability of introduction | 2 | 1 | 3 | ||
| Risk factors analysis | 2 | 2 | 3 | 2 | 9 |
| Spread pattern analysis | 1 | 1 | 2 | ||
| Trend analysis | 4 | 2 | 6 |
Next frequent categories refer to the assessment of the effectiveness of the sampling schemes (9) and the risk factor analysis (9). Categories of Epidemiological Questions relating to the effectiveness of countermeasures, biosecurity measures and protection measures together sum up to 12.
5.2. Envisaged Analysis
The second step in the process, in line with the Prometheus approach, was to envisage the type of statistical analysis/approaches and/or the parameters to be estimated to address a given category of epidemiological question. In Table 4, typical statistical analyses/approaches are listed for each Category of Epidemiological Questions. The full list of the proposed analyses and approaches, together with the related description, is reported in Appendix F.
Table 4.
List of the identified Epidemiological Question Categories. For each category, the related possible statistical analysis/approaches and the Category of Input Data have been listed
| Epidemiological question category | Possible statistical analysis/approaches/parameters | Category of input data and parameters |
|---|---|---|
| Descriptive statistics |
Count Proportion Prevalence Rate Relative risk/risk ratio Odds/Odds ratio Incidence Distribution maps Risk mapping |
Time Location Species Population size Population composition Cases |
| Effectiveness of sampling schemes |
Probability of detection Freedom from disease Time to first detection Scenario tree models Simulation techniques |
Relative risk Time Sampling scheme Cases Population size Test diagnostic specificity Test diagnostic sensitivity |
| Risk factors analysis |
Attack rates Secondary attack rates Relative risk Incidence rate ratio Odds ratio (Population) attributable RISK (Population) attributable fraction Regression techniques Risk mapping Spatial regression models |
Population Location Exposure Time Cases |
| Effectiveness of counter measures |
Odds ratio Simulation techniques Relative risk Hazard rate |
Time Exposure Cases Population size Population composition |
| Trend analysis | Regression techniques prevalence |
Time population Cases |
| Effectiveness of biosecurity measures |
Odds ratio Relative risk Simulation techniques |
Time Exposure Cases Population size Population composition |
| Risk of introduction |
Probability of introduction Simulation techniques Time to first detection |
Animal movements Place of origin Prevalence Cases Population size Diagnostic test specificity Diagnostic test sensitivity |
| Effectiveness of protection measures |
Odds ratio Vaccine effectiveness Simulation techniques Relative risk Hazard rate |
Exposure Cases Population size Population composition Vaccination status |
| Impact assessment |
Attack rates Secondary attack rates Case fatality rate Incidence rate Simulation techniques Modelling techniques (e.g. S.I.R) |
Population Time Cases |
| Spread pattern analysis |
Simulation techniques Modelling techniques (e.g. S.I.R) Transmission rate transmission kernel |
Exposure Population composition Population Location Time Cases |
| Probability of endemicity |
Simulations techniques Modelling techniques (e.g. S.I.R) Probability of freedom |
Probability of transmission Population Vaccination |
It should be noted that the list of purposeful analyses is not exhaustive, and the goal is to identify the data worth collecting from the MSs. When the SIGMA project is running, and data flows are working, an ad hoc Plan of Analyses, based on the envisaged analyses, will be tailored to the set of data actually available.
5.3. Definition of the data needs
The third step in the Prometheus approach consists of the identification of the data needs based on the analyses, approaches identified in the previous step as well as associated parameters. The categories of input data required to run the relevant analyses are listed in Table 4. The full list of the input data categories and related definitions is reported in Appendix G.
5.4. Data Model building
Starting from the data needs, i.e. the list of input data categories (see Section 5.3), the σ‐ADM was built following the steps listed below:
-
Identification of Entities and Relationships, where:
-
–
‘Entity’ is a database object capable to represent a thing in the real world, and can be concrete, like ‘animal’ and ‘establishment’, or more abstract like ‘source’ and ‘programme type’;
-
–
‘Relationship’ is an association among two or more entities that are described by one of three ratios (one‐to‐one, one‐to‐many, many‐to‐many), e.g. one establishment can contain many sub‐units.
-
–
-
Identification of the Attributes for each Entity, where an ‘Attribute’ is a characteristic of the Entity (i.e. a column in the table) and can be of different types:
-
–
Primary Key (PK) is an identifier identifying univocally an entity (i.e. a record in a table);
-
–
Foreign Key (FK) is an attribute defining a link to another entity (i.e. a record stored in another table);
-
–
all the other attributes further describe the entity modelled in the table and they can be either unique or non‐unique characteristics of an entity.
-
–
-
Identification, for each Attribute, of the possible Values, i.e.:
-
–
the data type (e.g. text, number);
-
–
the related Enumeration, in case the possible Values can be described with a reference terminology (i.e. each value is taken from a controlled, agreed and predetermined list, mutable over time).
-
–
For each entity, attribute and value identified, a definition was selected to make clear and unequivocal the type of information that need to be collected.
It is important to stress that these definitions can be agreed with the data providers in the different MSs without the need to impose, at MS level, the adoption of the same description or the replacement of all definitions currently in use across EU. This is, in fact, one of the strengths of the σ‐ADM: the descriptions reported in this document aim at having a common understanding, across EU, of the variables of interest and not at finding a new name to be used at EU level in the future. Nevertheless, all definitions were submitted to the members of the Animal Health Network with the intent to check if the σ‐ADM was comprehensive to cover all possible situations in the different MSs. At a later stage, EFSA and the Consortium, will perform a data mapping exercise and the result will be a set of ‘data dictionaries’ enabling EFSA to understand how the variables of interest are named in the different MSs, translate them to make them fit to the σ‐ADM and finally store them in the S‐DWH.
The different definitions were not elaborated from scratch as this would have been a far too theoretical approach. On the contrary, for each item, EFSA collected the existing definitions in the European legislation in force. The extraction of the available legal definitions was the ground on which the SIGMA definitions were built. After a thorough discussion, the EFSA ad hoc WG proposed a set of definitions which are:
The simple adoption of the definition as stands in the legislation and without any modification OR
The synthesis of two or more definitions from two or more legal documents OR
The modification of an existing definition OR
The creation of a new definition (in case no official definition was retrieved).
The following sections (from Sections 5.4.1, 5.4.2, 5.4.3, 5.4.4, 5.4.5, 5.4.6, 5.4.7, 6, 7, 8, 8.1, 8.1.1, 8.2, 8.2.1, H.1., H.2., H.2.1., H.2.2., H.2.3.–5.4.7), one per entity, provide the list of the final version of the definitions that will be used in the σ‐ADM. The full list of the legal references that were consulted can be found in Appendix C, together with the major points of discussion.
As a general rule, the agreed definitions adhere as much as possible to the new Animal Health Law (Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health). Only when the definitions were not present or did not describe exhaustively and unequivocally the item of the σ‐ADM, EFSA took the initiative to modify those definitions, consult other legal references or to create a new definition.
5.4.1. DATA MODEL SPECIFICATION
The following paragraphs describe the different entities and related attributes.
For each attribute, it is specified:
ATTRIBUTE_NAME
DATA TYPE: Attributes relaying on reference terminology contain as data type a reference to an enumeration, further specified in Section 5.4.7.
M/O: M stands for Mandatory and O for Optional
KEY: Constraint type (if any) – it can be PK or FK
DESCRIPTION.
Each entity is described as a single table, and also as a unique flat table in Appendix A.
An Entity–Relationship diagram is presented in Appendix B and it also shows links to the SSD2 data model which will be used to store monitoring/surveillance data mostly generated from laboratory results. SSD2 is currently under revision and it will encompass also changes required by the SIGMA project.
5.4.2. ESTABLISHMENT
SIGMA definition: Any premises, structure, or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for (a) households where pet animals are kept and (b) veterinary practices or clinics [Regulation (EU) 2016/429 (AHL), art. 4(27)].
| ATTRIBUTE NAME | DATA TYPE | M/O | KEY | DESCRIPTION |
|---|---|---|---|---|
| ESTABLISHMENT_ID | xs:string(200) | M | PK | Dummy identifier OR official identifier (according to the MS visibility policy) of the Establishment/Holding. The standards may change according to the national relevant legislation |
| GEO_LOCATION_ID | xs:string(200) | M | FK | Identifier of the record in the GEO_LOCATION table containing the location of the Establishment |
| ESTABLISHMENT_TYPE | EstablishmentTypeEnum | M | Type of Establishment, characterised by a specific aim and by a specific epidemiological role |
MS: Member State; PK: Primary Key; FK: Foreign Key.
5.4.3. SUB_UNIT
SIGMA definition: Management group of animals as part of an establishment.
Examples: flock, pen, herd, house, shed, etc.
| ATTRIBUTE NAME | DATA TYPE | M/O | KEY | DESCRIPTION |
|---|---|---|---|---|
| SUB_UNIT_ID | xs:string(200) | M | PK | Dummy identifier OR official identifier (according to the MS visibility policy) of the sub‐unit. The standards may change according to the relevant national legislation |
| SUB_UNIT_UPDATEY | xs:int(4) | M | Year – Date at which the information was generated (last update) | |
| SUB_UNIT_UPDATEM | xs:int(2) | M | Month – Date at which the information was generated (last update) | |
| SUB_UNIT_UPDATED | xs:int(2) | M | Day – Date at which the information was generated (last update) | |
| ESTABLISHMENT_ID | xs:string(200) | M | FK | Dummy identifier OR official identifier (according to the MS visibility policy) of the Establishment to which the Sub_unit belongs |
| GEO_LOCATION_ID | xs:string(200) | M | Identifier of the record in the GEO_LOCATION table containing the location of the Sub_unit | |
| SPECIES | SpeciesEnum | M | The common name, the genus, the species and the breed of the sub‐unit of concern. This is particularly relevant in the cases in which the single animals do not have an animal id | |
| PRODUCTION_TYPE | ProductionTypeEnum | M | Type of final product of the Establishment OR aim for which the animals are kept and/or bred | |
| CAPACITY | xs:integer(6) | M | The capacity of the establishment, i.e. the permitted maximum number of animals that the establishment can host. For some species, it can be, as an example, number of cubicles or pen places | |
| ACTUAL_NUMBER | xs:integer(6) | M | Number of animals at the date the information was generated (last update) |
PK: Primary Key; FK: Foreign Key.
5.4.4. KEPT_ANIMAL
SIGMA definition: any terrestrial animal which is kept by humans and registered with a unique ID.
Legal references: Regulation (EU) 2016/429 (AHL), art. 4(2), Regulation (EU) 2016/429 (AHL), art. 4(5).
| ATTRIBUTE NAME | DATA TYPE | M/O | KEY | DESCRIPTION |
|---|---|---|---|---|
| KEPT_ANIMAL_ID | xs:string(200) | M | PK | Dummy identifier OR official identifier (according to the MS visibility policy) of the individual kept animal (for the relevant species). The standards may change according to the relevant legislation. [Commission Implementing Regulation (EU) 2017/949, art. 2] |
| KEPT_ANIMAL_UPDATEY | xs:int(4) | M | Year – Date at which the information was generated (last update) | |
| KEPT_ANIMAL_UPDATEM | xs:int(4) | M | Month – Date at which the information was generated (last update) | |
| KEPT_ANIMAL_UPDATED | xs:int(4) | M | Day – Date at which the information was generated (last update) | |
| ESTABLISHMENT_ID | xs:string(200) | M | FK | Dummy identifier OR official identifier (according to the MS visibility policy) of the Establishment to which the animal belongs |
| SUB_UNIT_ID | xs:string(200) | M | FK | Dummy identifier OR official identifier (according to the MS visibility policy) of the sub‐unit to which the animal belongs |
| SPECIES | SpeciesEnum (only mammals) | M | The common name, the genus, the species and the breed of the sub‐unit of concern. This is particularly relevant in the cases in which the single animals do not have an animal id | |
| PRODUCTION_TYPE | ProductionTypeEnum (only related to mammals) | M | Type of final product of the Establishment OR aim for which the animals are kept and/or bred | |
| SEX | GenderEnum | M | Sex of the kept animal | |
| BIRTH_Y | xs:int(4) | O | Year – Date of birth of the kept animal | |
| BIRTH_M | xs:int(2) | O | Month – Date of birth of the kept animal | |
| BIRTH_D | xs:int(2) | O | Day – Date of birth of the kept animal | |
| BIRTH_ESTABLISHMENT_ID | xs:string(200) | O | Dummy identifier OR official identifier (according to the MS visibility policy) of the Establishment where the kept animal was born. The standards may change according to the relevant legislation | |
| BIRTH_SUB_UNIT_ID | xs:string(200) | O | Dummy identifier OR official identifier (according to the MS visibility policy) of the Sub_unit where the kept animal was born. The standards may change according to the relevant legislation | |
| BIRTH_COUNTRY | xs:string(2) | O | ISO code of the country where the kept animal was born | |
| MOTHER_ANIMAL_ID | xs:string(200) | O | Dummy identifier OR official identifier (according to the MS visibility policy) of the mother of the individual kept animal (for the relevant species). The standards may change according to the relevant legislation. This attribute is logically a link referring the same KEPT_ANIMAL table. As such is should be captured as FK, but since data of the referred animal might not be contained in the same table (i.e. animal died or moved in a different country prior to any data submission) the constraint is not strictly implemented |
MS: Member State; PK: Primary Key; FK: Foreign Key.
5.4.5. GEO_LOCATION
SIGMA definition: positioning on the Earth of the unit of interest, i.e. an establishment or a single animal, at the highest available resolution.
| ATTRIBUTE NAME | DATA TYPE | M/O | KEY | DESCRIPTION |
|---|---|---|---|---|
| GEO_LOCATION_ID | xs:string(200) | M | PK | Identifier of the geographical location |
| COORD_PRECISION | CoordPrecisionEnum | O | Precision of the provided coordinates | |
| X_COORD | xs:decimal | O | Longitude (degrees) E/W | |
| Y_COORD | xs:decimal | O | Latitude (degrees) N/S | |
| ADDRESS | xs:string(200) | O | Address of the located entity | |
| ZIP_CODE | xs:string(10) | O | ZIP code of the located entity | |
| MUNICIPALITY | xs:string(200) | O | Municipality of the located entity | |
| NUTS3 | NutsEnum | M | NUTS code level 3 of the located entity |
PK: Primary Key.
5.4.6. DISEASE_DETECTION
SIGMA definition: string of information related to the reporting of a possible outbreak as recorded in the ADNS (or in the ADIS, when available) or, failing that, from other similar systems (WAHIS, EFSA DCF).
| ATTRIBUTE NAME | DATA TYPE | M/O | KEY | DESCRIPTION |
|---|---|---|---|---|
| DISEASE_DETECTION_ID | xs:string(200) | M | PK | It is the outbreak/disease detection number as registered within the Country and reported to the Commission (e.g. in ADNS) |
| GEO_LOCATION_ID | xs:string(200) | M | Identifier of the record in the GEO_LOCATION table containing the location of the disease detection | |
| DISEASE | DiseaseEnum | M | Disease to be notified | |
| SPECIES | SpeciesEnum | M | The common name, the genus, the species and the breed of the sub‐unit of concern. This is particularly relevant in the cases in which the single animals do not have an animal id | |
| PRODUCTION_TYPE | ProductionTypeEnum | M | Type of final product of the Establishment OR aim for which the animals are kept and/or bred | |
| OUTBREAK_TYPE | OutbreakTypeEnum | M | The type of outbreak (primary or secondary) | |
| SUSPICION_DATEY | xs:int(4) | M | Year – Date of suspicion of outbreak | |
| SUSPICION_DATEM | xs:int(2) | M | Month – Date of suspicion of outbreak | |
| SUSPICION_DATED | xs:int(2) | M | Day – Date of suspicion of outbreak | |
| CONFIRMATION_DATEY | xs:int(4) | M | Year – Date of confirmation of outbreak | |
| CONFIRMATION_DATEM | xs:int(2) | M | Month – Date of confirmation of outbreak | |
| CONFIRMATION_DATED | xs:int(2) | M | Day – Date of confirmation of outbreak | |
| TYPE_SUBTYPE | xs:string(200) | O | Comma separated of list disease type/subtype code | |
| NR_SUSCEPTIBLE | xs:int(4) | O | Number of total susceptible animal present at farm | |
| NR_AFFECTED | xs:int(4) | O | Number of affected animals (clinically affected or positive at diagnostic test) at confirmation date | |
| NR_DEAD | xs:int(4) | O | Number of dead animals at confirmation date | |
| NR_KILLED | xs:int(4) | O | Number of animals killed at confirmation date | |
| NR_DESTROYED | xs:int(4) | O | Number of carcases destroyed at confirmation date |
ADNS: Animal Disease Notification System; PK: Primary Key.
5.4.7. ENUMERATION
All the enumerations will be implemented as catalogues in the reference terminology management system of EFSA, integrated with the DCF and enabling automatic data validation.
Each enumeration might be either created as a new EFSA catalogue or implemented by extending an existing one.
| ENUMERATION | VALUE | DESCRIPTION |
|---|---|---|
| EstablishmentTypeEnum | Quarantine premises | Establishment where the animals are kept in isolation with no direct or indirect contact with animals outside this epidemiological unit, for the purpose of ensuring that there is no spread of one or more specified diseases while the animals in isolation are undergoing observation for a specified length of time and, if appropriate, testing and treatment. [based on Regulation (EU) 2016/429 (AHL), art. 4(38)] |
| EstablishmentTypeEnum | Assembly centre | Establishment, approved by the competent authority, where kept terrestrial animals are assembled from more than one establishment for a period shorter than the required residency period for the species of animals concerned, for NATIONAL and INTERNATIONAL movements |
| EstablishmentTypeEnum | Market | Establishment, registered by the competent authority, where kept terrestrial animals are assembled from more than one establishment for a period shorter than the required residency period for the species of animals concerned, for NATIONAL movements |
| EstablishmentTypeEnum | Exhibition | Permanent establishments where animals of domestic or wild species are kept for exhibition to the public for 7 or more consecutive days a year (e.g. zoos, petting centres), with the exception of circuses and pet shops. |
| EstablishmentTypeEnum | Show | Temporary events where animals of domestic or wild species are brought together for exhibition to the public for less than 7 consecutive days a year |
| EstablishmentTypeEnum | Farm | Establishment where the animals are kept by humans, since birth OR for a rearing/production period OR for the required residency period for the species of animals concerned, for commercial purposes, i.e. to breed and/or rear and/or sell animals and/or products of animal origin. Hatcheries are excluded |
| EstablishmentTypeEnum | Genetic centre | Establishment where the animals (bovines, equines, swine, sheep, goats) are kept by humans, for the collection of germinal products. |
| EstablishmentTypeEnum | Hatchery | An establishment which incubates and hatches eggs and supplies day‐old chicks (art 2, Council Directive 2009/158/EC of 30 November 2009 on animal health conditions governing intra‐community trade in, and imports from third countries of, poultry and hatching eggs) |
| EstablishmentTypeEnum | Slaughtering centre | Establishment used for slaughtering and dressing animals, the meat of which is intended for human consumption OR establishment in which game and game meat obtained after hunting are prepared for placing on the market. Stalls, pens, covered areas or fields associated with or part of slaughterhouse operations are included |
| EstablishmentTypeEnum | Health & Research centres | Any permanent, geographically limited and approved establishment where one or more species of animal are habitually: (i) kept for fundamental or applied scientific research; or (ii) bred for the purposes of such research (iii) kept to undergo veterinary medicine practices. For example, research laboratories, veterinary hospitals, etc. |
| EstablishmentTypeEnum | Pasture/Co‐pasture | 2000/115 refers to land used for (common) grazing which is under the control of a local authority |
| ProductionTypeEnum | Germinal products | ‘Germinal products’ means: (i) semen, oocytes and embryos intended for artificial reproduction; (ii) hatching eggs [Regulation (EU) 2016/429 (AHL), art. 4(27)] |
| ProductionTypeEnum | Breeders | ‘Breeders’ are animals of high genetic value kept for reproduction purposes. For example, grandparents and parent flocks (poultry); pedigree dams and sires; etc. |
| ProductionTypeEnum | Meat/Fattening | Rearing or keeping in captivity animals for the primary purpose of producing meat |
| ProductionTypeEnum | Milk | Rearing or keeping in captivity animals for the primary purpose of producing raw milk, i.e. milk produced by the secretion of the mammary gland of farmed animals that has not been heated to more than 40°C or undergone any treatment that has an equivalent effect. |
| ProductionTypeEnum | Eggs | Rearing or keeping in captivity animals for the primary purpose of producing eggs, where ‘Eggs’ means unfertilised eggs in shell – e.g broken, fresh table or cooked eggs – that are produced by farmed birds and are fit for direct human consumption or for the preparation of egg products AND technical purposes (cosmetics) |
| ProductionTypeEnum | SPF | Animals or eggs which are used for diagnostic procedures in laboratories, for the production and testing of vaccines and for research and pharmaceutical purposes |
| ProductionTypeEnum | Foie‐gras | Rearing or keeping in captivity animals for the production of foie gras, where foie‐gras means the livers of geese, or of ducks of the species Cairina muschata or Cairina muschata x Anas platyrhynchos which have been fed in such a way as to produce hepatic fatty cellular hypertrophy |
| ProductionTypeEnum | Game | Animals kept in captivity for restocking supplies of game animals |
| SpeciesEnum | Mammals | Mammals (and all subcategories) |
| SpeciesEnum | Birds | Birds (and all subcategories) |
| GenderEnum | Female | Female |
| GenderEnum | Male | Male |
| GenderEnum | Mixed females and males | Mixed females and males |
| CoordPrecisionEnum | Centroid admin | Centroid of an administrative area (region or country) |
| CoordPrecisionEnum | Centroid generic | Coordinates indicating the centroid of a non‐administrative area |
| CoordPrecisionEnum | Exact | Exact location XY coordinates of trap or point of sample |
| CoordPrecisionEnum | Estimated | Near location XY coordinates based on village, town or identifiable geographical feature (national park, lake, river, etc.) |
| CoordPrecisionEnum | Unknown | Location Unknown |
| NutsEnum | – Many – | NUTS code, according to EUROSTAT. Information should be provided at least at NUTS level 3 |
| DiseaseEnum | HPAI poultry | HPAI poultry |
| DiseaseEnum | HPAI captive birds | HPAI captive birds |
| DiseaseEnum | HPAI wild birds | HPAI wild birds |
| DiseaseEnum | LPAI poultry | LPAI poultry |
| DiseaseEnum | LPAI captive birds | LPAI captive birds |
| DiseaseEnum | LPAI wild birds | LPAI wild birds |
| DiseaseEnum | LSD | LSD |
| DiseaseEnum | ASF | ASF |
| DiseaseEnum | Echinococcus multilocularis | Echinococcus multilocularis |
| OutbreakTypeEnum | Primary | Primary |
| OutbreakTypeEnum | Secondary | Secondary |
LSD: lumpy skin disease; ASF: African swine fever; HPAI: High Pathogenic Avian Influenza; LPAI: Low Pathogenic Avian Influenza.
6. σ‐ADM (Relational structure)
The outcome of the three steps as outlined in the section dedicated to the data model building (see Section 5.4) was a draft data model, ideally applicable to any type of pathogen and disease and fitted in order to gather data required for different statistical analyses and to ensure a good level of harmonisation between MSs. Appendix B shows the structure of the first draft of the SIGMA Data Model, including the relationships between the different entities. Further details about entities, attributes and values can be found in Section 5.4 and in Appendix C.
7. Testing the fitness of the draft σ‐ADM
As described in Section 4 and Figure 2, the input data categories were checked against the data models underpinning the current and ongoing data collections within the remit of the Animal Health and Welfare team and of the Biological Hazards team.
Once the first version of the σ‐ADM was drafted, this was circulated within EFSA to the relevant project owners, dealing with the European Commission mandates on LSD, ASF, vector‐borne diseases, AI, EM and the zoonoses and zoonotic agents included in the relevant legislation. It confirmed that the type of information and the level of detail that σ‐ADM was designed to collect addressed the needs in the requests of the different mandates received from the European Commission.
Nevertheless, it has to be made clear that the present version of the σ‐ADM will be potentially subject to modifications and adaptations based on the feedback received by the SIGMA Consortium during the implementation phase.
8. User case and benefits
8.1. Animal Health and Welfare: the case of the avian influenza
The whole idea of the SIGMA project originated from a very pragmatic issue related to the mandate in which EFSA was tasked with the provision of technical assistance regarding the ongoing AI outbreak in 2017 (Question Number: http://registerofquestions.efsa.europa.eu/roqFrontend/questionLoader?question=EFSA-Q-2017-00229).
Considering that the AI epidemic in 2016–2017 has been one of the largest in terms of number of poultry outbreaks, geographical spread and number of dead wild birds, the Terms of Reference (ToR) in the mandate were understandably challenging. The European Commission requested EFSA to:
analyse the epidemiological data on HPAI and LPAI, where co‐circulating or linked within the same epidemic, from HPAI disease affected MSs;
analyse the temporal and spatial pattern of HPAI and LPAI as appropriate in poultry, captive birds and wild birds, as well as the risk factors involved in the occurrence, spread and persistence of HPAI viruses in these avian populations;
based on the findings from the points above, describe the effect of prevention and control measures;
provide for regular quarterly reports updating on the AI situation within the Union and worldwide, in particular with a view to describing the evolution of virus spread from certain regions towards the EU. In case of significant changes in the epidemiology of AAI, these reports could be needed more frequently. These reports should, in particular, closely follow the developments of zoonotic AI viruses, such as HPAI A(H5N6) and LPAI A(H7N9), in collaboration with the European Centre for Disease Prevention and Control (ECDC).
In this framework, EFSA explored different European sources of information about poultry demography (holdings, herds, flocks, birds, species, etc.) at a sufficient level of resolution (e.g. NUTS3), but without success. This was expected as the data in the different EU systems (e.g. EUROSTAT) are collected for other purposes than risk assessment.
EFSA was able to retrieve the data inserted by the MSs in the ADNS. Therefore, EFSA decided to ask the MSs to complement those data with additional information on the holdings and or birds affected by the disease. Considering the limited timeframe, it was not possible to put in place a web‐based application to standardise and validate the additional data required, which were finally submitted by means of spreadsheet files. The latter lead to the identification of the following issues:
the spreadsheets were prone to human error; EFSA had to dedicate considerable resources to validate the submitted data for consistency (internal‐validation);
a certain variability across the MSs in the interpretation of the standard terminology was recorded which required an intense communication between EFSA and the MSs to make the data fully comparable (external validation).
At the end of the process, EFSA had a consistent set of data to perform the analysis. However, these data were limited as only the affected holdings and birds were reported, without any detailed information about the portion of the population of interest that was not affected by the disease, therefore limiting the extent of the analyses that could be performed. The results that EFSA was able to produce and the related discussion on their interpretation is available in the Scientific Report published in collaboration with the ECDC and the European Union Reference Laboratory for Avian Influenza (EFSA and ECDC, 2017, 9), Section 3.1.4 (Characterisation of the HPAI‐affected poultry holdings (from October 2016 to April 2017)).
Another important aspect that has to be considered in relation to AI is the decision of the European Commission, following the exit of the United Kingdom from the EU, to appoint EFSA as the responsible body in charge of the development and the publication of the annual report on the AI surveillance activity at EU level (M‐2017‐0221). The goal in this case is different from the quarterly report. In the latter, the focus is on the evolution of the outbreaks, while in the former it is to actively monitor the situation in each MS for early detection of new cases and/or strains through poultry and wild bird surveillance. However, also in this case, the data submitted pertain only to the poultry premises that were selected and tested in the monitoring programme. Therefore, once more, a detailed set of information about the demography of the poultry population is missing and simple statistics like the intensity of the sampling activity is rather difficult to obtain, unless requested to each involved MS.
8.1.1. Benefits of the σ‐ADM on the EFSA quarterly report and the Avian Influenza surveillance report
In this context, the benefits that the SIGMA project could bring are rather sensitive, both for EFSA and the MSs; in brief:
MSs could decide to automate the process of submitting data (previously transformed to fit the σ‐ADM with the help of the SIGMA Consortium). This would not only provide a validated and consistent set of data but also reduce the effort needed for this recurrent exercise.
These data have to be submitted only once and will serve for reporting on outbreaks, surveillance activities and relevant zoonotic diseases (e.g. Salmonella).
8.2. Zoonotic agents: the case of ‘double reporting’ (see also Appendix H)
In the context of the general zoonoses mandate, EFSA collects MS‐specific data on animal populations. However, certain of these animal population data are also collected from MS by the European Commission (DG Santé: G2 unit ‘Animal health and welfare’ and D4 unit ‘Food safety programme, emergency funding’), in the context of MSs’ control and eradication programmes that are co‐financed by the European Commission. These data are alike, although not always identical because of the different perspective they are collected for, and the compulsory requirement for MS to report these to EFSA and to European Commission is underpinned by EU legislation. This issue is commonly known as ‘double reporting’.
It has been estimated that, on average, the effort required by a MS to collect and to submit to EFSA the relevant animal population data and manage them to fit the requirement of EFSA is of one person for one week. The collection, validation and submission of the complete zoonoses data sets require several months resources for one person (two to three).
Regarding the data submission of data on zoonoses, currently only few MSs (four) transmit their annual animal population in the zoonoses domain to EFSA without manual manipulation and are extracted from national databases and transmitted to EFSA using XML. The majority of the MSs, however, use an EFSA zoonoses Excel‐based mapping tool where data are manually inserted ad managed before sending to EFSA using an XML generating tool. The shaping up of data in the EFSA mapping tool is very resource‐intensive. In addition, the MSs transmit their annual animal population data to the European Commission with manual input in a PDF tool with an embedded XML structure.
Another problem relates to the fact that the national reporters, submitting the relevant data to EFSA and to the European Commission, are staff employed in different units or agencies and institutes which might have slightly different objectives and missions. This may lead to some discrepancies across the different reports: despite the data are the same, a different way of aggregating them may lead to apparent differences, e.g. in counts and proportions. For this reason MSs, EFSA and the European Commission carry out annually a thorough cross‐validation exercise to ensure that no discrepant statistics are published in the MSs’ national zoonoses reports, nor in the EFSA scientific reports, nor in the reports published by the European Commission. This exercise is extremely demanding and requires a lot of resources.
8.2.1. Benefits of the SIGMA project in the field of the zoonotic diseases
The SIGMA Data Model is flexible and can deal with many different types of data, from sample‐based to aggregated data. The SSD2, which defines the standards to describe the information related to the individual sample, is part of the SIGMA Data Model, which fits the requirements of the EFSA DWH.
It is proposed that the MSs submit to EFSA using the relevant standards defined by the SIGMA Data Model all the data needed by EFSA and the European Commission as regards animal population in the zoonoses domain. Once those data are submitted and stored in the DWH, all concerned parties, with different levels of permission, will be able to access the relevant data to generate different types of report. As an example, the MSs will be able to access their own data and generate the national reports to be submitted to the European Commission or other type of reports for internal use; the European Commission will be able to generate summaries and overviews based on specific needs. This possibility will be implemented by means of web‐based tools directly linked to the data stored in the EFSA DWH.
This solution will save a lot of time and resources to the MS which will have to submit only once the data of concern, which will be available to EFSA and the European Commission for more than one purpose. In addition, the data will be standardised at EU level. The only action required to the MSs is to align the data submitted to EFSA with the SIGMA Data Model standards. However, it has to be noted that this work is carried out by the SIGMA Consortium which is financed by EFSA.
Glossary
- Attribute
A characteristic of the Entity which can be of different types: O Primary Key (PK) attributes identify uniquely the entity; O Foreign Key (FK) attributes define relationships between entities; O all the other attributes further describe the entity modelled in a table, and they are non‐unique characteristic of an entity
- Entity
A database object capable to represent a thing in the real world, and can be concrete, like ‘animal’ and ‘establishment’, or more abstract like ‘source’, ‘geographical location’ and ‘programme type’
- Value
A possible manifestation of the Attribute. It can be about: O the data type (e.g. text, number); O the related Enumeration, in case the possible Values can be described with a controlled list (a.k.a. reference terminology)
Abbreviations
- ADIS
Animal Disease Information System
- ADNS
Animal Disease Notification System
- AHL
Animal Health Law
- AI
avian influenza
- ASF
African swine fever
- BFSA
Bulgarian Food Safety Agency
- CDCP
Country Data Collection Point
- CSF
Classical Swine Fever
- DCF
Data Collection Framework
- DWH
Data Warehouse
- ECDC
European Centre for Disease Prevention and Control
- EFSA
European Food Safety Authority
- EM
Echinococcus multilocularis
- EUSR
European Union Summary Report
- ETl
Extract‐Transform‐Load
- FLI
Friedrich Loeffler Institut
- FK
Foreign Key
- HPAI
High Pathogenic Avian Influenza
- IT
information technology
- IZS
Istituto Zooprofilattico Sperimentale
- LPAI
Low Pathogenic Avian Influenza
- LSD
lumpy skin disease
- MS(s)
Member State(s)
- NCP
National Contact Point
- OIE
World Organisation for Animal Health
- OR
Odds ratio
- PK
Primary Key
- RAW
Risk Assessment Workflow
- RR
Relative risk
- S‐DWH
Scientific Data Warehouse
- SVA
Swedish National Veterinary Institute
- WG
Working Group
- σ‐ADM
SIGMA – Animal Data Model
- TOR
Terms of Reference
- WAHIS
World Animal Health Information System
Appendix A – Full data model (flat)
1.
The following table describes how demographic data could be reported by joining all the tables of the relational schema into a single flat table.
The table does not contain Foreign Keys (FKs) attributes since they are not required when data are provided in a de‐normalised way (i.e. by replicating the information of the ESTABLISHMENT and of the SUB_UNIT for each animal).
The entities described in the flat DEMOGRAPHIC_DATA table are:
E: ESTABLISHMENT
EGL: GEOLOCATION of the ESTABLISHMENT
SU: SUB UNIT
SUGL: GEOLOCATION of the SUB UNIT
KA: KEPT ANIMAL (all attributes related to the animal are not mandatory due to the fact that not all the animals are individually registered, e.g. poultry)
For the scope note related to the single value of an enumeration please refer to the description in chapter 5.
| E | ATTRIBUTE NAME | DATA TYPE | M/O | ENUM VALUES | DESCRIPTION |
|---|---|---|---|---|---|
| E | ESTABLISHMENT_ID | xs:string(200) | M | Dummy identifier OR official identifier (according to the MS visibility policy) of the Establishment/Holding. The standards may change according to the national relevant legislation | |
| E | ESTABLISHMENT_TYPE | EstablishmentTypeEnum | M |
Quarantine premises Assembly centre Market Exhibition Show Farm Genetic centre Hatchery Slaughtering Centre Health & Research centres Pasture/Co‐pasture |
Type of Establishment, characterised by a specific aim and by a specific epidemiological role |
| EGL | ESTABLISHMENT_COORD_PRECISION | CoordPrecisionEnum | O |
Centroid admin Centroid generic Exact Estimated Unknown |
Precision of the provided coordinates |
| EGL | ESTABLISHMENT_X_COORD | xs:decimal | O | Longitude (degrees) E/W | |
| EGL | ESTABLISHMENT_Y_COORD | xs:decimal | O | Latitude (degrees) N/S | |
| EGL | ESTABLISHMENT_ADDRESS | xs:string(200) | O | Address of the located entity | |
| EGL | ESTABLISHMENT_ZIP_CODE | xs:string(10) | O | ZIP code of the located entity | |
| EGL | ESTABLISHMENT_MUNICIPALITY | xs:string(200) | O | Municipality of the located entity | |
| EGL | ESTABLISHMENT_NUTS3 | NutsEnum | M | NUTS code, according to EUROSTAT. Information should be provided at least at NUTS level 3 | NUTS code level 3 of the located entity |
| SU | SUB_UNIT_ID | xs:string(200) | M | Dummy identifier OR official identifier (according to the MS visibility policy) of the sub‐unit. The standards may change according to the relevant national legislation | |
| SU | SUB_UNIT_UPDATEY | xs:int(4) | M | Year – Date at which the information was generated (last update) | |
| SU | SUB_UNIT_UPDATEM | xs:int(2) | M | Month – Date at which the information was generated (last update) | |
| SU | SUB_UNIT_UPDATED | xs:int(2) | M | Day – Date at which the information was generated (last update) | |
| SU | SPECIES | SpeciesEnum | M |
Mammals (and all subcategories) Birds (and all subcategories) |
The common name, the genus, the species and the breed of the sub‐unit of concern. This is particularly relevant in the cases in which the single animals do not have an animal id |
| SU | PRODUCTION_TYPE | ProductionTypeEnum | M |
Germinal products Breeders Meat/Fattening Milk Egg SPF Foie‐gras Game |
Type of final product of the Establishment OR aim for which the animals are kept and/or bred |
| SU | CAPACITY | xs:integer(6) | M | The capacity of the establishment, i.e. the permitted maximum number of animals that the establishment can host. For some species, it can be, as an example, number of cubicles or pen places | |
| SU | ACTUAL_NUMBER | xs:integer(6) | M | Number of animals at the date the information was generated (last update) | |
| SUGL | SUB_UNIT_COORD_PRECISION | CoordPrecisionEnum | O |
Centroid admin Centroid generic Exact Estimated Unknown |
Precision of the provided coordinates |
| SUGL | SUB_UNIT_X_COORD | xs:decimal | O | Longitude (degrees) E/W | |
| SUGL | SUB_UNIT_Y_COORD | xs:decimal | O | Latitude (degrees) N/S | |
| SUGL | SUB_UNIT_ADDRESS | xs:string(200) | O | Address of the located entity | |
| SUGL | SUB_UNIT_ZIP_CODE | xs:string(10) | O | ZIP code of the located entity | |
| SUGL | SUB_UNIT_MUNICIPALITY | xs:string(200) | O | Municipality of the located entity | |
| SUGL | SUB_UNIT_NUTS3 | NutsEnum | M | NUTS code, according to EUROSTAT. Information should be provided at least at NUTS level 3 | NUTS code level 3 of the located entity |
| KA | KEPT_ANIMAL_ID | xs:string(200) | O | Dummy identifier OR official identifier (according to the MS visibility policy) of the individual kept animal (for the relevant species). The standards may change according to the relevant legislation. [Commission Implementing Regulation (EU) 2017/949, art. 2] | |
| KA | KEPT_ANIMAL_UPDATEY | xs:int(4) | O | Year – Date at which the information was generated (last update) | |
| KA | KEPT_ANIMAL_UPDATEM | xs:int(4) | O | Month – Date at which the information was generated (last update) | |
| KA | KEPT_ANIMAL_UPDATED | xs:int(4) | O | Day – Date at which the information was generated (last update) | |
| KA | SPECIES | SpeciesEnum | O | Mammals (and all subcategories) | The common name, the genus, the species and the breed of the sub‐unit of concern. This is particularly relevant in the cases in which the single animals do not have an animal id |
| KA | PRODUCTION_TYPE | ProductionTypeEnum | O |
Germinal products Breeders Meat/Fattening Milk SPF |
Type of final product of the Establishment OR aim for which the animals are kept and/or bred |
| KA | SEX | GenderEnum | O |
Female Male Mixed females and males |
Sex of the kept animal |
| KA | BIRTH_Y | xs:int(4) | O | Male | Year – Date of birth of the kept animal |
| KA | BIRTH_M | xs:int(2) | O | Mixed females and males | Month – Date of birth of the kept animal |
| KA | BIRTH_D | xs:int(2) | O | Day – Date of birth of the kept animal | |
| KA | BIRTH_ESTABLISHMENT_ID | xs:string(200) | O | Dummy identifier OR official identifier (according to the MS visibility policy) of the Establishment where the kept animal was born. The standards may change according to the relevant legislation | |
| KA | BIRTH_SUB_UNIT_ID | xs:string(200) | O | Dummy identifier OR official identifier (according to the MS visibility policy) of the Sub_unit where the kept animal was born. The standards may change according to the relevant legislation | |
| KA | BIRTH_COUNTRY | xs:string(2) | O | ISO code of the country where the kept animal was born | |
| KA | MOTHER_ANIMAL_ID | xs:string(200) | O | Dummy identifier OR official identifier (according to the MS visibility policy) of the mother of the individual kept animal (for the relevant species). The standards may change according to the relevant legislation |
Appendix B – Entity Relationship diagram (relational model)
1.
The diagram shows the entities related to the ADM. The monitoring data are only partially described since they are extensively described in the SSD2 guidance.
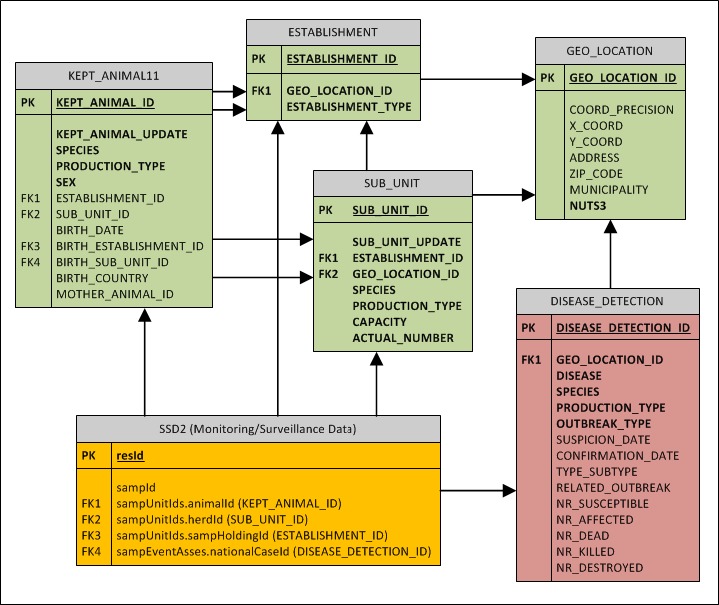
Legend: PK: Primary Key; FK: Foreign Key. Green: demographic data; Orange: monitoring data; Red: Disease notifications.
Appendix C – Legal references, discussions, justifications and reasoning
1.
Appendix C provides a set of definitions found in different types of documents (EU legislation, EU guidelines OIE terrestrial code) that they were considered to be adapted for the entities and attributes of the σ ADM. In some cases, the definitions are controversial not clear enough and not able to fit the purposes of a consistent analysis of the data. The definition adopted in the σ ADM (see Section 5.4) is either:
The simple adoption of the definition as stands in the legislation and without any modification OR
The synthesis of two or more definitions from two or more legal documents OR
The modification of an existing definition OR
The creation of a new definition (in case no official definition was retrieved).
1. ESTABLISHMENT
Initially, one of the most important entities identified to describe the animal population of interest was the Holding. However, it appeared that the definition of ‘Establishment’ in the AHL was much more in line with what the EFSA's epidemiologists need to perform statistical analysis and risk assessment, i.e. that entity, characterised by a single geographical location point, where animals are grouped for a given scope. In fact, a ‘holding’ can be associated to more than one geographical location point, as it may consist of two or more buildings, not necessarily located in the same place.
Commission Regulation (EU) 206/2010 (Def ID1) did not fit the purpose as it includes wildlife and hunting reserves, which, in SIGMA, are kept separated. The other three references (Def ID2, 3 and 4) could actually fit, but considering the point above, instead of choosing among those three definitions, EFSA decided to adhere to the Animal Health Law (AHL) definition of ‘Establishment’.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN, art. 4(27) | ‘establishment’ means any premises, structure, or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for: (a) households where pet animals are kept; (b) veterinary practices or clinics |
| 2 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010R0206%26from=en, art. 2 (ungulates, Equidae) | ‘Holding’ means a farm or other officially supervised agricultural, industrial or commercial undertaking, including zoos, amusement parks and wildlife or hunting reserves where live animals are regularly kept or bred |
| 3 | https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32000R1760%26from=EN, art. 2 (bovines) | ‘Holding’ means any establishment, construction or, in the case of an open‐air farm, any place situated within the territory of the same Member State, in which animals covered by this Regulation are held, kept or handled |
| 4 | http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1992L0065:20040703:EN:PDF, art 2 | Any permanent, geographically limited establishment where one or more species of animal are habitually kept or bred, whether or not for commercial ends, and exclusively for one or more of the following purposes [omissis] |
| 5 | https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31982L0894%26from=EN, art. 2(a) | ‘holding’ means any establishment (agricultural or other) situated in the territory of a Member State, in which animals are reared or kept |
| 6 | Council Directive 92/119/EEC, art 2(1) | Holding: any establishment (agricultural or other), situated in the territory of a Member State, in which animals are kept or bred |
2. ESTABLISHMENT_TYPE (under ESTABLISHMENT)
No legal reference was found for this Attribute of the Establishment.
This is an overarching category, encompassing all relevant type of activities, each characterised by a specific epidemiological role. It is possible that not all activities are captured under this Attribute. However, it was believed by EFSA that this list should cover the vast majority of the activities in place where there is an interaction between humans and animals. In addition, often, minor activities (e.g. backyard) are not officially recorded in the national databases, making the data retrieval impossible or very difficult. EFSA is still interested in these kind of information, as the role of these minor activities has not been yet clarified in full, but it is outside the scope of this project to include those data which may be retrieved by other means. Should these activities, at a certain point in time, be officially recorded following a new or a modified legislation, the σ‐ADM will be ready to include them.
3. QUARANTINE PREMISES (under ESTABLISHMENT – ESTABLISHMENT_TYPE)
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(38) | ‘Quarantine’ means the keeping of animals in isolation with no direct or indirect contact with animals outside the epidemiological unit, for the purpose of ensuring that there is no spread of one or more specified diseases while the animals in isolation are undergoing observation for a specified length of time and, if appropriate, testing and treatment |
The AHL defines in Art. 4 (38) what is ‘Quarantine’. With the σ‐ADM, beyond the activity itself, EFSA wants to collect information on the recorded establishment dedicated to this activity. For this reason, the definition in the σ‐ADM was slightly modified specifying that the target is, indeed, the premises dedicated to quarantine the animals.
4. ASSEMBLY CENTRE (under ESTABLISHMENT – ESTABLISHMENT_TYPE)
Agreed definition: Establishment, approved by the competent authority, where kept terrestrial animals are assembled from more than one establishment for a period shorter than the required residency period for the species of animals concerned.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(49) | ‘assembly operation’ means the assembling of kept terrestrial animals from more than one establishment for a period shorter than the required residency period for the species of animals concerned |
| 2 | http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1964L0432:20071113:EN:PDF , art. 2(2)(o) (bovines, swine) | Holdings, collection centres and markets, at which bovine animals or swine originating from different holdings are grouped together to form consignments of animals intended for trade. These assembly centres must be approved for trading purposes and meet the requirements laid down in Article 11 |
| 3 | https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003L0050%26from=EN, art. 1(b)10 | Premises on which ovine or caprine animals originating from different holdings are grouped together to form consignments of animals intended for intra‐Community trade; |
| 4 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31991L0068%26from=en , art. 2(9) (ovine, caprine) | Any place, other than the holding, where ovine or caprine animals are sold, bought and/or assembled or loaded, and which complies with Article 3 (7) of Directive 64/432/EEC and Article 5 (1) (b) (i) of Directive 90/425/EEC for approved markets or assembly centres |
The assembly centre was among one of the most debated definitions as the existing legislation was either too broad (e.g. merging assembly centres and markets, which EFSA wanted to keep separated) and/or lacking a sharp definition on the maximum time an animal can be kept in such centres. Last, it was important to distinguish between national and international movements. The definition in the σ‐ADM covers all these aspects.
5. MARKET (under ESTABLISHMENT – ESTABLISHMENT_TYPE)
Agreed definition: Establishment, registered by the competent authority, where kept terrestrial animals are assembled from more than one establishment for a period shorter than the required residency period for the species of animals concerned, for NATIONAL movements.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31991L0068%26from=en , art. 2(9) (ovine, caprine) | Approved market or assembly centre means any place, other than the holding, where ovine or caprine animals are sold, bought and/or assembled or loaded, and which complies with Article 3 (7) of Directive 64/432/EEC and Article 5 (1) (b) (i) of Directive 90/425/EEC for approved markets or assembly centres |
| 2 | http://www.legislation.gov.uk/uksi/1990/2628/made (The Welfare of Animals at Markets Order 1990) | Market place or sale‐yard or any other premises or place to which animals are brought from other places and exposed for sale and includes any lairage adjoining a market and used in connection with it and any place adjoining a market used as a parking area by visitors to the market for parking vehicles |
| 3 | OIE Terrestrial code ( http://www.oie.int/index.php?id=169%26L=0%26htmfile=glossaire.htm ) | MARKET means a place where animals are assembled for the purpose of trade or sale. |
This definition is the outcome of the discussion made on the ‘Assembly centres’. Indeed, the two definitions are basically the same (e.g. regarding the time an animal can spend in such premises), except for the nature of the movements which, in this case, are only within the country of origin. Other definitions in the existing legislation didn't help in having a consistent description of the type of establishments needed in the σ‐ADM, particularly considering their different epidemiological role.
6. EXHIBITION (under ESTABLISHMENT – ESTABLISHMENT_TYPE)
Agreed definition: Permanent establishments where animals of domestic or wild species are kept for exhibition to the public for 7 or more days a year, with the exception of circuses and pet shops (e.g. zoos, petting centres).
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1999:094:0024:0026:EN:PDF , art.2 | ‘zoos’ means all permanent establishments where animals of wild species are kept for exhibition to the public for 7 or more days a year, with the exception of circuses, pet shops and establishments which Member States exempt from the requirements of this Directive on the grounds that they do not exhibit a significant number of animals or species to the public and that the exemption will not jeopardise the objectives of this Directive |
In this case, the existing definition in the relevant directive was considered to be fitting the purpose and was adopted almost without modifications. The second part of the definition (related to the exemption) was not included in the σ‐ADM as implicit in the definition of ‘Establishment’, i.e. officially authorised and recorded in a national database.
7. SHOW (under ESTABLISHMENT – ESTABLISHMENT_TYPE)
Agreed definition: Temporary events where animals of domestic or wild species are kept for exhibition to the public for less than 7 consecutive days a year.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1999:094:0024:0026:EN:PDF , art.2 | ‘zoos’ means all permanent establishments where animals of wild species are kept for exhibition to the public for 7 or more days a year, with the exception of circuses, pet shops and establishments which Member States exempt from the requirements of this Directive on the grounds that they do not exhibit a significant number of animals or species to the public and that the exemption will not jeopardise the objectives of this Directive |
It was important to distinguish between the temporary assemble of animals for a short period from the permanent presence of animals over time. In this case, it was decided to make this distinction upon the duration of the event, i.e. more or less than 7 consecutive days a year.
8. FARM (under ESTABLISHMENT ‐ ESTABLISHMENT_TYPE)
Agreed definition: Establishment where the animals are kept by humans, since birth OR for a rearing/production period OR for the required residency period for the species of animals concerned, for commercial purposes i.e. to breed and/or rear and/or sell animals and/or products of animal origin. Hatcheries are EXCLUDED.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4 |
‘establishment’ means any premises, structure, or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for: (a) households where pet animals are kept; (b) veterinary practices or clinics; |
| 2 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(49) | ‘assembly operation’ means the assembling of kept terrestrial animals from more than one establishment for a period shorter than the required residency period for the species of animals concerned |
| 3 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(5) | ‘kept animals’ means animals which are kept by humans, including, in the case of aquatic animals, aquaculture animals; |
| 4 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(29) |
‘products of animal origin’ means: (a) food of animal origin, including honey and blood; (b) live bivalve molluscs, live echinoderms, live tunicates and live marine gastropods, intended for human consumption; and (c) animals other than those referred to in point (b) intended to be prepared with a view to being supplied live to the final consumer; |
| 5 | Zoonoses catalogue for sampling point | (place of primary production and the basic unit in agriculture) Primary production premises |
The best existing definition was probably the one in the Zoonoses catalogue (Def ID4), but EFSA wanted to give more details on the residency period and on the description of the production type. Note that it was finally decided to keep the hatcheries out of this category.
9. GENETIC CENTRE (under ESTABLISHMENT ‐ ESTABLISHMENT_TYPE)
Agreed definition: Establishment where the animals (bovines, equines, swine, sheep, goats) are kept by humans, for the collection of germinal products.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(5) | ‘kept animals’ means animals which are kept by humans, including, in the case of aquatic animals, aquaculture animals; | |
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(49) | ‘assembly operation’ means the assembling of kept terrestrial animals from more than one establishment for a period shorter than the required residency period for the species of animals concerned | |
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(28) |
‘germinal products’ means: (a) semen, oocytes and embryos intended for artificial reproduction; (b) hatching eggs; |
|
| https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R1012%26from=EN, art. 2(26) | ‘breeding programme’ means a set of systematic actions, including recording, selection, breeding and exchange of breeding animals and their germinal products, designed and implemented to preserve or enhance desired phenotypic and/or genotypic characteristics in the target breeding population | |
| https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R1012%26from=EN, art. 2(2) | ‘breed’ means a population of animals sufficiently uniform to be considered to be distinct from other animals of the same species by one or more groups of breeders which have agreed to enter those animals in breeding books with details of their known ascendants for the purpose of reproducing their inherited characteristics by way of reproduction, exchange and selection within the framework of a breeding programme; |
In this case, the AHL helped in limiting the discussion as the definition of germinal product was very clear. Note that the ‘hatching eggs’ are part of the germinal products, therefore the establishments were these eggs are produced should fall under this category.
10. HATCHERY (ESTABLISHMENT – ESTABLISHMENT_TYPE)
Agreed definition: An establishment where eggs are hatched under artificial conditions. It may be used for ex‐situ conservation purposes, i.e. to breed rare or endangered species under controlled conditions; alternatively, it may be for economic reasons (i.e. to enhance food supplies).
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:343:0074:0113:EN:PDF , art. 2(9d) | An establishment which incubates and hatches eggs and supplies day‐old chicks |
11. SLAUGHTERING CENTRE (under ESTABLISHMENT ‐ ESTABLISHMENT_TYPE)
Agreed definition: Establishment used for slaughtering and dressing animals, the meat of which is intended for human consumption OR establishment in which game and game meat obtained after hunting are prepared for placing on the market. Stalls, pens, covered areas or fields associated with or part of slaughterhouse operations are included.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0853-20070101%26from=HR , APPENDIX I (1.16) | “Slaughterhouse” means an establishment used for slaughtering and dressing animals, the meat of which is intended for human consumption |
| 2 | https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1099%26from=EN , art. 2(c) | “Lairaging” means keeping animals in stalls, pens, covered areas or fields associated with or part of slaughterhouse operations; |
| 3 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0853-20070101%26from=HR – Appendix I (1.18) | “Game‐handling establishment” means any establishment in which game and game meat obtained after hunting are prepared for placing on the market |
| 4 | OIE Animal Health Code (http://www.oie.int/index.php?id=169%26L=0%26htmfile=glossaire.htm) | LAIRAGE means pens, yards and other holding areas used for accommodating animals in order to give them necessary attention (such as water, feed, rest) before they are moved on or used for specific purposes including slaughter |
Bearing in mind that the priority of the σ‐ADM was to identify and locate establishments with a specific epidemiological role, EFSA considered superfluous and artificial to split all the operations related to the production of meat into different categories and also to distinguish between domestic animals and game. For this reason, the final choice was for a more comprehensive definition which could include wild and domestic and all activities related to the production of meat.
12. HEALTH & RESEARCH CENTRE (under ESTABLISHMENT – ESTABLISHMENT_TYPE)
Agreed definition: Any permanent, geographically limited and approved establishment where one or more species of animal are habitually:
kept to perform basic or applied scientific research; or
bred for the purposes of such research
kept to undergo veterinary medicine practices
(e.g. research laboratories, veterinary hospitals, etc.)
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1992L0065:20040703:EN:PDF , art 2(c) |
‘approved body, institute or centre’ means any permanent, geographically limited establishment, approved in accordance with Article 13, where one or more species of animal are habitually kept or bred, whether or not for commercial ends, and exclusively for one or more of the following purposes: — display of the animals and education of the public — conservation of the species; — basic or applied scientific research or breeding of animals for the purposes of such research; |
| 2 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(27) |
‘establishment’ means any premises, structure, or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for: (a) households where pet animals are kept; (b) veterinary practices or clinics; |
In this case, the AHL on its own did not help in defining the required information. In fact, Art. 4(27) excludes the premises where those activities are performed from the definition of ‘Establishment’. It was of course impossible to explore the intentions of the legislator which might have taken into consideration also political aspects, which are outside the scope of the σ‐ADM. For this reason and considering the common epidemiological role, it was agreed to group under this definition the premises where research and veterinary practices are performed.
13. SUB‐UNIT
Agreed definition: Management group of animals as part of an establishment.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| 1 | http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:182:0019:0028:EN:PDF , art. 2(g) | ‘house’ means a building on a holding where a flock of chickens are kept; |
| 2 | http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:182:0019:0028:EN:PDF , art. 2(j) | ‘flock’ means a group of chickens which are placed in a house of a holding and are present in this house at the same time; |
| 3 | [MISSING LEGAL REFERENCE] | ‘pen’ [MISSING DEFINITION] |
| 4 | Zoonoses manual | ‘herd’ means an animal or group of animals kept on a holding as an epidemiological unit (Regulation (EC) No 2160/2003); if more than one herd is kept on a holding, each of these herds shall form a distinct unit and shall have the same health status (Directive 64/432/EEC) |
| 5 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(37) | ‘compartment’ means an animal subpopulation contained in one or more establishments and, in the case of aquatic animals, in one or more aquaculture establishments, under a common biosecurity management system with a distinct health status with respect to a specific disease or specific diseases subject to appropriate surveillance, disease control and biosecurity measures; |
| 6 | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 84 | ‘epidemiological unit’ means a group of animals with the same likelihood of exposure to a disease agent; |
One of the most difficult entities to describe was, indeed, the sub‐unit. The EFSA expert had clear what the required information was about, but the existing definitions did not entirely fit the needs. EFSA were looking for a common term to identify those entities within an Establishment which contain one or more groups of animals, whether or not separated by biosecurity measures. The closest definition is probably the ‘house’ (see Def ID1). However, this definition would be fitting the poultry Establishments only and, in addition, the legislation refers to ‘buildings within a Holding’: by its nature a ‘Holding’ can encompass houses that are located all over a country and this was not in line with what EFSA was looking for (see in this Appendix Definition and Discussion about ‘Establishment’). The definition of ‘Compartment’ and ‘Epidemiological unit’ were found to have a too heavy epidemiological connotation and, in both cases, there could have been the same issue as for the ‘house’. In addition, the term ‘house’ is apparently linked to the avian species and in this sense is too restrictive for the purpose. As a conclusion, EFSA preferred to use a generic term to make clear that the entity of interest, can consist of any type of group of animals within an ‘Establishment’. As a consequence, the ‘Establishment’ and its ‘sub‐units’ share the same geographical location. It is irrelevant if the ‘sub‐units’ have the same type of production, the same level of biosecurity or not.
14. GERMINAL PRODUCTS (under SUB‐UNIT – PRODUCTION TYPE)
Agreed definition: ‘germinal products’ means: (i) semen, oocytes and embryos intended for artificial reproduction; (ii) hatching eggs.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(27) | ‘germinal products’ means: (i) semen, oocytes and embryos intended for artificial reproduction; (ii) hatching eggs; | |
| http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1964L0432:20071113:EN:PDF , art 2(c) | ‘animals for breeding or production’ means bovine animals (including the species Bison bison and Bubalus bubalus) and swine other than those referred to in (b), including those intended for breeding, milk or meat production, or draft purposes, shows or exhibition with the exception of animals taking part in cultural and sporting events; | |
| https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009L0158%26from=it , art. 2(4) | ‘breeding poultry’ means poultry 72 h old or more, intended for the production of hatching eggs; |
No discussion. The decision was to adhere to the Animal Health Law definition.
15. MEAT/FATTENING (under SUB‐UNIT – PRODUCTION TYPE)
Agreed definition: Rearing or keeping in captivity animals for the primary purpose of producing meat.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1964L0432:20071113:EN:PDF , art 2(c) | ‘animals for breeding or production’ means bovine animals (including the species Bison bison and Bubalus bubalus) and swine other than those referred to in (b), including those intended for breeding, milk or meat production, or draft purposes, shows or exhibition with the exception of animals taking part in cultural and sporting events; | |
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(9) |
‘poultry’ means birds that are reared or kept in captivity for: (a) the production of: (i) meat; (ii) eggs for consumption; (iii) other products; |
|
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0853%26from=EN , Annex I, art. 1.1 | “Meat” means edible parts of the animals referred to in points 1.2 to 1.8 (domestic ungulates, poultry, lagomorphs, wild game, farmed game, small wild game, large wild game), including blood | |
| https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2016.EN-991 | Meat production animals (bovines) ‐ bovine animals, other than calves, kept exclusively for the production of meat and including cows, heifers and bulls |
There were no major problems with the definition of this type of production. Note that EFSA decided to insert the term ‘primary purpose’ as there are cases where the establishment may put on the market other categories of products, but in a marginal way. In this case, it is important to record the main activity of the establishment or its sub‐unit.
The definition in Council Directive 64/432/EEC was excluded as the list of products is too inclusive and, at the same time, the list of animals is too restrictive for the purposes of SIGMA.
16. MILK (under SUB‐UNIT – PRODUCTION TYPE)
Agreed definition: Rearing or keeping in captivity animals for the primary purpose of producing raw milk, i.e. milk produced by the secretion of the mammary gland of farmed animals that has not been heated to more than 40°C or undergone any treatment that has an equivalent effect.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0853-20070101%26from=HR , ANNEX I (4.1) | ‘Raw milk’ means milk produced by the secretion of the mammary gland of farmed animals that has not been heated to more than 40°C or undergone any treatment that has an equivalent effect | |
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0853-20070101%26from=HR , ANNEX I (4.2) | Milk production holding” means an establishment where one or more farmed animals are kept to produce milk with a view to placing it on the market as food |
EFSA decided to insert the term ‘primary purpose’ as there are cases where the establishment may put on the market other categories of products, but in a marginal way. In this case, it is important to record the main activity of the establishment or its sub‐unit.
17. EGGS (under SUB‐UNIT – PRODUCTION TYPE)
Agreed definition: Rearing or keeping in captivity animals for the primary purpose of producing eggs, where ‘Eggs’ means eggs in shell — other than broken, incubated or cooked eggs — that are produced by farmed birds and are fit for direct human consumption or for the preparation of egg products (also called ‘Table Eggs’).
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0853-20070101%26from=HR, ANNEX I (5.1) | ‘Eggs’ means eggs in shell — other than broken, incubated or cooked eggs — that are produced by farmed birds and are fit for direct human consumption or for the preparation of egg products | |
| https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2014.3782 |
EFSA decided to insert the term ‘primary purpose’ as there are cases where the establishment may put on the market other categories of products, but in a marginal way. In this case, it is important to record the main activity of the establishment or its sub‐unit. The term ‘Table Eggs’ was also introduced as broadly used in the field.
18. Specific Pathogen Free (SPF) (under SUB‐UNIT – PRODUCTION TYPE)
Agreed definition: Animals or eggs which are used for diagnostic procedures in laboratories, for the production and testing of vaccines and for research and pharmaceutical purposes.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32001D0393%26from=EN | ‘Specified pathogen free (SPF)eggs are hatching eggs, which are used for diagnostic procedures in laboratories, for the production and testing of vaccines and for research and pharmaceutical purposes and have to be marked with a stamp |
19. FOIE GRAS (under SUB‐UNIT – PRODUCTION TYPE)
Agreed definition: Rearing or keeping in captivity animals for the production of foie gras, where foie‐gras means the livers of geese, or of ducks of the species Cairina muschata or Cairina muschata x Anas platyrhynchos which have been fed in such a way as to produce hepatic fatty cellular hypertrophy.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:157:0046:0087:EN:PDF, art. 1(3) |
The livers of geese, or of ducks of the species Cairina muschata or Cairina muschata x Anas platyrhynchos which have been fed in such a way as to produce hepatic fatty cellular hypertrophy. The birds from which such livers are removed shall have been completely bled, and the livers shall be of a uniform colour. The livers shall be of the following weight: — duck livers shall weigh at least 300 g net, — goose livers shall weigh at least 400 g net. |
20. GAME (under SUB‐UNIT – PRODUCTION TYPE)
Agreed definition: Animals kept in captivity for restocking supplies of game animals.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0853-20070101%26from=HR, ANNEX I (1.6) | “Farmed game” means farmed ratites and farmed land mammals other than those referred to in point 1.2.(i.e. Domestic ungulates) | |
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(9) |
‘poultry’ means birds that are reared or kept in captivity for: [omitted] (b) restocking supplies of game birds; |
The definition in the σ‐ADM is the result of two definitions in the available legal acts listed. EFSA agreed to focus the attention to the scope of the farming activity (restocking supplies) rather than on the animal species. It appeared obvious that a dairy farm or a dairy cow cannot fall under this category.
21. CAPACITY (under SUB‐UNIT)
Agreed definition: The capacity of the establishment, i.e. the number of animals approved by the authority that the establishment can host. For some species it can be, as an example, number of cubicles or pen places.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 84(1) |
Operators of establishments keeping terrestrial animals or collecting, producing, processing or storing germinal products shall, in order for their establishments to be registered in accordance with Article 93, before they commence such activities: [omitted] (b) provide the competent authority with the following information: [omitted] (iii) the categories, species and numbers or quantities of kept terrestrial animals or germinal products which they intend to keep on the establishment, and the capacity of the establishment |
It was decided to include this parameter as it could be, in some cases, one of the few harmonised parameter on the consistency of the production activity. The Animal Health Law foresees the recording of this information. It has to be verified what is the level of implementation at EU level.
22. ACTUAL NUMBER (under SUB‐UNIT)
Agreed definition: The number of animals that the operators of an establishment keep or breed or rear in the establishment at a specific point in time.
23. KEPT ANIMAL
Agreed definition: any terrestrial animal which is kept by humans and registered with a unique ID.
| Def ID# | LEGAL REFERENCE | DEFINITION |
|---|---|---|
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(2) | ‘terrestrial animals’ means birds, terrestrial mammals, bees and bumble bees; | |
| http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:084:FULL%26from=EN , art. 4(5) | ‘kept animals’ means animals which are kept by humans, including, in the case of aquatic animals, aquaculture animals; |
At this point in time the species available among the enumeration values are limited to the ones relevant for the mandates received which are on ASF, LSD, AI and EM. However, at any time EFSA shall assist on other diseases, the σ‐ADM will be readily integrated with the required values.
Appendix D – Results of the systematic search in the Risk Assessment Workflow (RAW)
1.
Table D.1.
Summary table of the question number and related mandate grouped by disease
| Disease | Mandate | Output number | Question number |
|---|---|---|---|
| AI | M‐2014‐0316 | ON‐3941 | EFSA‐Q‐2014‐00838 |
| M‐2015‐0079 | EN‐1142 | EFSA‐Q‐2016‐00792 | |
| EN‐1282 | EFSA‐Q‐2017‐00573 | ||
| EN‐1283 | EFSA‐Q‐2017‐00574 | ||
| EN‐1284 | EFSA‐Q‐2017‐00575 | ||
| EN‐1285 | EFSA‐Q‐2017‐00576 | ||
| EN‐1286 | EFSA‐Q‐2017‐00577 | ||
| EN‐1287 | EFSA‐Q‐2017‐00578 | ||
| ON‐4687 | EFSA‐Q‐2016‐00777 | ||
| ON‐4991 | EFSA‐Q‐2015‐00214 | ||
| EFSA‐Q‐2016‐00348 | |||
| M‐2015‐0240 | ON‐4891 | EFSA‐Q‐2016‐00599 | |
| M‐2017‐0062 | ON‐5018 | EFSA‐Q‐2017‐00229 | |
| ON‐5141 | EFSA‐Q‐2017‐00649 | ||
| ASF | M‐2014‐0057 | ON‐3616 | EFSA‐Q‐2014‐00149 |
| M‐2014‐0079 | ON‐4795 | EFSA‐Q‐2017‐00158 | |
| M‐2014‐0323 | EN‐843 | EFSA‐Q‐2015‐00401 | |
| ON‐4163 | EFSA‐Q‐2014‐00897 | ||
| M‐2016‐0048 | EN‐1312 | EFSA‐Q‐2017‐00155 | |
| ON‐4732 | EFSA‐Q‐2016‐00152 | ||
| ON‐5068 | EFSA‐Q‐2017‐00154 | ||
| EM | M‐2012‐0200 | EN‐366 | EFSA‐Q‐2012‐00746 |
| ON‐2973 | EFSA‐Q‐2012‐00640 | ||
| ON‐3465 | EFSA‐Q‐2013‐00535 | ||
| ON‐3875 | EFSA‐Q‐2013‐00536 | ||
| ON‐4310 | EFSA‐Q‐2013‐00537 | ||
| ON‐4649 | EFSA‐Q‐2014‐00734 | ||
| ON‐5051 | EFSA‐Q‐2017‐00403 | ||
| M‐2014‐0287 | ON‐4035 | EFSA‐Q‐2014‐00727 | |
| ON‐5051 | EFSA‐Q‐2017‐00697 | ||
| M‐2014‐0288 | ON‐4373 | EFSA‐Q‐2014‐00728 | |
| LSD | M‐2013‐0332 | ON‐3986 | EFSA‐Q‐2013‐00917 |
| M‐2016‐0122 | ON‐4573 | EFSA‐Q‐2016‐00384 | |
| M‐2016‐0170 | ON‐4773 | EFSA‐Q‐2016‐00542 | |
| ON‐5176 | EFSA‐Q‐2016‐00625 |
Table D.2.
Detailed table of the question number and related mandate and output per disease
| Disease | Keywords | Time span | Mandate | Question number | Subject | Output number |
|---|---|---|---|---|---|---|
| AI | HPAI | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2017‐00576 | Procurement: Data analysis and predictive modelling of HPAI H5 and H7 outbreaks in the EU 2005–2015 | EN‐1285 |
| AI | HPAI | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2017‐00575 | Procurement: Report about HPAI introduction into Europe, HPAI detection in wild birds and HPAI spread between European holdings in the period 2005–2015 | EN‐1284 |
| AI | HPAI | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2016‐00792 | Procurement: Effect of biosecurity measures and early detection systems, mitigation measures and surveillance strategies on the spread of HPAI and LPAI between farms | EN‐1142 |
| AI | HPAI | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2016‐00348 | Art. 29: Scientific opinion on additional issues in relation to EFSA's ongoing mandate on avian influenza (M‐2015‐0079) | ON‐4991 |
| AI | HPAI | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2015‐00214 | Art. 29: Scientific opinion on avian influenza (HPAI) | ON‐4991 |
| AI | LPAI | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2017‐00577 | Procurement: LPAI detection in wild birds and LPAI spread between European holdings in the period 2005–2015 | EN‐1286 |
| AI | LPAI | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2016‐00792 | Procurement: Effect of biosecurity measures and early detection systems, mitigation measures and surveillance strategies on the spread of HPAI and LPAI between farms | EN‐1142 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2017‐0221 | EFSA‐Q‐2017‐00829 | Scientific and technical assistance on avian influenza surveillance ‐ Art 31. | |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2017‐0062 | EFSA‐Q‐2017‐00825 | Art. 31: Scientific and technical assistance on avian influenza monitoring (Dec–Jan–Feb 2017/2018) | |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2017‐0062 | EFSA‐Q‐2017‐00649 | Art. 31: Scientific and technical assistance on avian influenza monitoring (September–October–November 2017) | ON‐5141 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2017‐00578 | Procurement: Mechanisms and risk factors for mutation from low to highly pathogenic avian influenza virus | EN‐1287 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2017‐00574 | Procurement: Narrative overview on wild bird migration in the context of highly pathogenic avian influenza incursion into the European Union | EN‐1283 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2017‐00573 | Risk factors of primary introduction of highly pathogenic and low pathogenic avian influenza virus into European poultry holdings, considering at least material contaminated by wild birds and contact with wild birds | EN‐1282 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2017‐0062 | EFSA‐Q‐2017‐00229 | Art. 31: Scientific and technical assistance on avian influenza monitoring (October 2016–August 2017) | ON‐5018 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2016‐00777 | Urgent reply to and clarifications of terms of reference (ToR)2 of the avian influenza mandate | ON‐4687 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0240 | EFSA‐Q‐2016‐00616 | Procurement: Data collection on INFECTION WITH LOW PATHOGENIC AVIAN INFLUENZA VIRUS | |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0240 | EFSA‐Q‐2016‐00599 | Art. 29: Scientific Opinion on INFECTION WITH LOW PATHOGENIC AVIAN INFLUENZA VIRUS for the listing and categorisation of animal diseases in the framework of the Animal Health Law | ON‐4891 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2016‐00372 | Workshop on Avian Influenza | EN‐1052 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2016‐00348 | Art. 29: Scientific opinion on additional issues in relation to EFSA's ongoing mandate on avian influenza (M‐2015‐0079) | ON‐4991 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2015‐00686 | Procurement linked to scientific opinion on avian influenza: data collection and spatial models for virus spread in preparation to the mandate on avian influenza (OC/EFSA/ALPHA/2015/01) | |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2015‐0079 | EFSA‐Q‐2015‐00214 | Art. 29: Scientific opinion on avian influenza (HPAI) | ON‐4991 |
| AI | Avian influenza | 1/1/2014–31/12/2017 | M‐2014‐0316 | EFSA‐Q‐2014‐00838 | Scientific report on Highly pathogenic avian influenza A subtype H5N8 | ON‐3941 |
| ASF | ASF | 1/1/2014–31/12/2017 | M‐2016‐0048 | EFSA‐Q‐2017‐00155 | Procurement: Simulation‐based investigation of ASF spread and control in wildlife without consideration of human non‐compliance to biosecurity (NP/EFSA/ALPHA/2017/11) | EN‐1312 |
| ASF | ASF | 1/1/2014–31/12/2017 | M‐2014‐0323 | EFSA‐Q‐2015‐00401 | Procurement: Alternative control strategies against ASF in wild boar populations (NP‐EFSA‐ALPHA‐2015‐15‐ASF) | EN‐843 |
| ASF | ASF | 1/1/2014–31/12/2017 | M‐2014‐0323 | EFSA‐Q‐2014‐00897 | Scientific opinion on African swine fever (ASF) | ON‐4163 |
| ASF | African Swine Fever | 1/1/2014–31/12/2017 | M‐2017‐0217 | EFSA‐Q‐2018‐00053 | Art. 31: Scientific and technical assistance on African swine fever | |
| ASF | African Swine Fever | 1/1/2014–31/12/2017 | M‐2017‐0217 | EFSA‐Q‐2017‐00823 | Art. 31: Scientific and technical assistance on African swine fever | |
| ASF | African Swine Fever | 1/1/2014–31/12/2017 | M‐2016‐0048 | EFSA‐Q‐2017‐00725 | Event report for African Swine Fever workshop | EN‐1342 |
| ASF | African Swine Fever | 1/1/2014–31/12/2017 | M‐2014‐0079 | EFSA‐Q‐2017‐00158 | Characterisation of African swine fever virus for scientific opinion on vector‐borne diseases | ON‐4795 |
| ASF | African Swine Fever | 1/1/2014–31/12/2017 | M‐2016‐0048 | EFSA‐Q‐2017‐00154 | Art. 31 Technical and scientific assistance on African swine fever (2017) | ON‐5068 |
| ASF | African Swine Fever | 1/1/2014–31/12/2017 | M‐2016‐0048 | EFSA‐Q‐2016‐00152 | Art. 31 Technical and scientific assistance on African swine fever (2016) | ON‐4732 |
| ASF | African Swine Fever | 1/1/2014–31/12/2017 | M‐2014‐0323 | EFSA‐Q‐2014‐00897 | Scientific opinion on African swine fever (ASF) | ON‐4163 |
| ASF | African Swine Fever | 1/1/2014–31/12/2017 | M‐2014‐0057 | EFSA‐Q‐2014‐00149 | Request for urgent scientific and technical assistance on the evaluation of hunting wild boar as a mitigation measure to prevent the introduction of African swine fever from area infected by the disease. | ON‐3616 |
| LSD | LSD | 1/1/2014–31/12/2017 | M‐2014‐0330 | EFSA‐Q‐2016‐00412 | Workshop on strengthening regional cooperation in South East Europe and Middle East for prevention and control of Lumpy Skin Disease (LSD) | EN‐1059 |
| LSD | Lumpy skin disease | 1/1/2014–31/12/2017 | M‐2016‐0170 | EFSA‐Q‐2016‐00625 | Art. 31 Scientific and technical assistance on Lumpy Skin Disease (report 2018) | ON‐5176 |
| LSD | Lumpy skin disease | 1/1/2014–31/12/2017 | M‐2016‐0170 | EFSA‐Q‐2016‐00542 | Art. 31 Scientific and technical assistance on Lumpy Skin Disease (report 2017) | ON‐4773 |
| LSD | Lumpy skin disease | 1/1/2014–31/12/2017 | M‐2014‐0330 | EFSA‐Q‐2016‐00412 | Workshop on strengthening regional cooperation in South East Europe and Middle East for prevention and control of Lumpy Skin Disease (LSD) | EN‐1059 |
| LSD | Lumpy skin disease | 1/1/2014 ‐ 31/12/2017 | M‐2016‐0122 | EFSA‐Q‐2016‐00384 | Art. 29: Urgent advice on the update of the scientific opinion on lumpy skin disease | ON‐4573 |
| LSD | Lumpy Skin Disease | 1/1/2014–31/12/2017 | M‐2013‐0332 | EFSA‐Q‐2013‐00917 | Scientific opinion on lumpy skin disease | ON‐3986 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2014‐0287 | EFSA‐Q‐2017‐00697 | Art. 31: Scientific and technical assistance on Echinococcus multilocularis infection in animals(2017) | ON‐5051 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2012‐0200 | EFSA‐Q‐2017‐00403 | Art. 31: Scientific and technical assistance on Echinococcus multilocularis infection in animals(2017) | ON‐5051 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2012‐0200 | EFSA‐Q‐2014‐00734 | Art. 31: Scientific and technical assistance on Echinococcus multilocularis infection in animals(2016) | ON‐4649 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2014‐0288 | EFSA‐Q‐2014‐00728 | Scientific opinion Echinococcus multilocularis infection in animals | ON‐4373 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2014‐0287 | EFSA‐Q‐2014‐00727 | Scientific and technical assistance concerning the 2013 Report on Surveillance of Echinococcus Multilocularis in Norway | ON‐4035 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2012‐0200 | EFSA‐Q‐2013‐00537 | Art. 31: Scientific and technical assistance on Echinococcus multilocularis infection in animals (2015) | ON‐4310 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2012‐0200 | EFSA‐Q‐2013‐00536 | Scientific and technical assistance on Echinococcus multilocularis infection in animals (2014) | ON‐3875 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2012‐0200 | EFSA‐Q‐2013‐00535 | Scientific and technical assistance on Echinococcus multilocularis infection in animals (2013) | ON‐3465 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2012‐0200 | EFSA‐Q‐2012‐00640 | Request for scientific and technical assistance on Echinococcus multilocularis infection in animals | ON‐2973 |
| EM | Echinococcus | 1/1/2012–31/12/2017 | M‐2012‐0200 | EFSA‐Q‐2012‐00746 | Request for scientific and technical assistance on Echinococcus multilocularis infection in animals | EN‐366 |
Appendix E – List of terms of reference and related epidemiological question category
1.
| Mandate number | Terms of reference | Epidemiological question category |
|---|---|---|
| M‐2014‐0316 | Epidemiological analysis of the current situation regarding HPAI subtype H5N8 in Europe in order to assess possible entry routes and in particular the role played by wild birds | Descriptive statistics |
| M‐2014‐0316 | Epidemiological analysis of the current situation regarding HPAI subtype H5N8 in Europe in order to assess possible entry routes and in particular the role played by wild birds | Risk factors analysis |
| M‐2014‐0316 | Review of the epidemiological situation of HP AI subtype H5N8 in the world | Descriptive statistics |
| M‐2015‐0079 | Risk of introduction of HPAI H5N8 and possibly other HPAI viruses considering the possible entry routes in EU | Probability of introduction |
| M‐2015‐0079 | The risk posed by HPAI H5N8 and possibly other HPAI viruses for public and animal health, and specifically with a view to assess the suitability of the provisions on BIOSECURITY and early detection to reduce the risk of its introduction into poultry holdings laid down in Decision 2005/734/EC | Effectiveness of biosecurity measures |
| M‐2015‐0079 | The risk posed by HPAI H5N8 and possibly other HPAI viruses for public and animal health, and specifically with a view to assess the suitability of the provisions on biosecurity and EARLY DETECTION to reduce the risk of its introduction into poultry holdings laid down in Decision 2005/734/EC | Effectiveness of sampling schemes |
| M‐2015‐0079 | The risk posed by HPAI H5N8 and possibly other HPAI viruses for public and animal health, and specifically with a view to assess the suitability of the provisions on PROTECTION MEASURES in poultry in case of its occurrence in wild birds laid down in Decision 2006/563/EC | Effectiveness of protection measures |
| M‐2015‐0079 | The risk posed by HPAI H5N8 and possibly other HPAI viruses for public and animal health, and specifically with a view to assess the suitability of the provisions on THE SURVEILLANCE STRATEGY, in particular objectives and methodology, laid down in Decision 2010/367/EC | Effectiveness of sampling schemes |
| M‐2015‐0079 | The current situation in EU and elsewhere as regards the risk of possible introduction of HPAI H5N8 virus and possibly other HPAI viruses to EU poultry holdings | Descriptive statistics |
| M‐2015‐0079 | The continuous risk posed by LPAI (H5 & H7) for the introduction from the wild bird reservoir into poultry holdings taking into account risks for holdings where poultry is kept in open air runs […] | Probability of introduction |
| M‐2015‐0079 | The continuous risk posed by LPAI (H5 & H7) for the introduction from the wild bird reservoir into poultry holdings taking into account risks for holdings where poultry is kept in open air runs […] | Risk factors analysis |
| M‐2015‐0079 | […] and the suitability of SURVEILLANCE […] | Effectiveness of sampling schemes |
| M‐2015‐0079 | […] and BIOSECURITY MEASURES aimed at protection of poultry against LPAI infection | Effectiveness of biosecurity measures |
| M‐2014‐0057 | Is it feasible to drastically reduce the wild boar population by hunting or by the use of traps? In case of positive reply, for how long that strategy would need to be put in place in order to prevent a new increase of the density of the population? | Trend analysis |
| M‐2014‐0057 | Could increased hunting pressure be a proper disease management tool in disease free areas adjacent to area(s) where the occurrence of virus has been confirmed in the wild boar, to minimise the risk of ASF introduction? | Effectiveness of protection measures |
| M‐2014‐0057 | Would hunting significantly reduce the risk of ASF introduction and its spread? | Effectiveness of countermeasures |
| M‐2014‐0057 | Would prevention of movement of wild boars by feeding or artificial physical barriers reduce the risk of spread of ASF? | Effectiveness of countermeasures |
| M‐2014‐0323 | In view of controlling ASF, assess the best management options for wild boar both in infected areas and in the bordering risk areas, taking into account the local climatic conditions and wild boar ecology. Assess in particular the suitability, effectiveness and the practical aspects of implementation of the main measures, in particular different tailor‐made feed ban(s) for wild boar, selective well‐described hunting practices, taking into account the local situations and giving quantitative baseline indications on these measures as well as spatial and temporal parameters. | Risk factors analysis |
| M‐2014‐0323 | In view of controlling ASF, assess the best management options for wild boar both in infected areas and in the bordering risk areas, taking into account the local climatic conditions and wild boar ecology. Assess in particular the suitability, effectiveness and the practical aspects of implementation of the main measures, in particular different tailor‐made feed ban(s) for wild boar, selective well‐described hunting practices, taking into account the local situations and giving quantitative baseline indications on these measures as well as spatial and temporal parameters | Effectiveness of countermeasures |
| M‐2014‐0323 | Evaluate the epidemiological data on ASF from Lithuania, Poland, Latvia and Estonia in order to obtain indications on the local behaviour of ASF in the wild boar population and its interaction with domestic pigs | Descriptive statistics |
| M‐2014‐0323 | Assess the possible risk of spread of ASF‐Genotype II strains/isolates currently or recently circulating in Europe, and especially in Russia or the Baltic States, by pigs or wild boar becoming “carrier” that might play a role in virus transmission while remaining non‐symptomatic | Spread pattern analysis |
| M‐2014‐0323 | Where new data is available, provide an update of previous Scientific Opinions on ASF, in particular: i) describe identifiable relevant trends in wild boar population dynamics in the EU and its Eastern neighbouring territories; and ii) provide an updated distribution of ASF competent vectors (soft ticks) and its possible role on ASF epidemiology specially in Russia or the Baltic States | Trend analysis |
| M‐2014‐0323 | Where new data is available, provide an update of previous Scientific Opinions on ASF, in particular: i) describe identifiable relevant trends in wild boar population dynamics in the EU and its Eastern neighbouring territories; and ii) provide an updated distribution of ASF competent vectors (soft ticks) and its possible role on ASF epidemiology specially in Russia or the Baltic States | Descriptive statistics |
| M‐2016‐0048 | Analyse the epidemiological data on ASF in Estonia, Latvia, Lithuania, Poland and any other Member States at the eastern borders of the EU that might be affected by ASF | Descriptive statistics |
| M‐2016‐0048 | Include an analysis of the TEMPORAL pattern of ASF in wild boar and domestic pigs | Trend analysis |
| M‐2016‐0048 | Include an analysis of the SPATIAL pattern of ASF in wild boar and domestic pigs | Trend analysis |
| M‐2016‐0048 | Include an analysis of the RISK FACTORS involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic wildlife interface | Risk factors analysis |
| M‐2013‐0332 | Characterise the disease and provide an update on the global occurrence of LSD and changes in the distribution during the last 10 years | Descriptive statistics |
| M‐2013‐0332 | Characterise the disease and provide an update on the global occurrence of LSD and changes in the distribution during the last 10 years | Trend analysis |
| M‐2013‐0332 | Provide a mapping of the region of concern and other countries of the Mediterranean Basin and Black Sea, displaying identified, or likely, major live animal trade routes | Descriptive statistics |
| M‐2013‐0332 | Evaluate all possible pathways of introduction of LSD into the EU, ranking them on the basis of their level of risk, with a view to enhance preparedness and prevention | Risk factors analysis |
| M‐2013‐0332 | Assess the risk and speed of propagation of LSD into the EU and neighbouring countries | Spread pattern analysis |
| M‐2013‐0332 | Assess the risk of LSD becoming endemic in animal population in the EU and neighboring countries | Probability of endemicity |
| M‐2013‐0332 | Assess the impact of LSD if it were to enter the EU considering different scenarios as regards the effectiveness of surveillance and control measures | Impact assessment |
| M‐2013‐0332 | Briefly review the feasibility, availability, and effectiveness of the main disease prevention and control measures (diagnostic tools, biosecurity measures, restrictions on the movements, culling) | Effectiveness of sampling schemes |
| M‐2013‐0332 | Briefly review the feasibility, availability, and effectiveness of the main disease prevention and control measures (diagnostic tools, biosecurity measures, restrictions on the movements, culling) | Effectiveness of biosecurity measures |
| M‐2013‐0332 | Briefly review the feasibility, availability, and effectiveness of the main disease prevention and control measures (diagnostic tools, biosecurity measures, restrictions on the movements, culling) | Effectiveness of countermeasures |
| M‐2016‐0122 | Assess the implications in disease spread and persistence from the implementation of a partial stamping‐out policy (killing and destruction of clinically affected animals only) in holdings where the presence of LSD has been confirmed, against the current EFSA's advice and policy in place for total stamping‐out of infected herds coupled with vaccination | Effectiveness of countermeasures |
| M‐2016‐0170 | Analyse the epidemiological data on LSD from Cyprus, Greece, Bulgaria and any other Member States or non‐EU countries that might be affected by LSD | Descriptive statistics |
| M‐2016‐0170 | Include an analysis of the temporal and spatial patterns of LSD | Trend analysis |
| M‐2016‐0170 | Include an analysis of the risk factors involved in the occurrence, spread and persistence of the LSD virus among the cattle population | Risk factors analysis |
| M‐2012‐0200 | Regular follow‐up of the literature regarding EM infection in animals in the European Union and adjacent countries, including its geographical distribution and prevalence; | Descriptive statistics |
| M‐2012‐0200 | Analysis and critical assessment, in the context of Regulation (EU) No 1152/2011, of (i) the sampling strategy considered for the programmes of the Member States concerned; (ii) the data collected in the framework of these programmes; (iii) the detection methods used | Effectiveness of sampling schemes |
| M‐2012‐0200 | Analysis and critical assessment, in the context of Regulation (EU) No 1152/2011, of (i) the sampling strategy considered for the programmes of the Member States concerned; (ii) the data collected in the framework of these programmes; (iii) the detection methods used | Descriptive statistics |
| M‐2012‐0200 | Analysis and critical assessment, in the context of Regulation (EU) No 1152/2011, of (i) the sampling strategy considered for the programmes of the Member States concerned; (ii) the data collected in the framework of these programmes; (iii) the detection methods used | Effectiveness of sampling schemes |
| M‐2014‐0288 |
1. To describe Echinococcus multilocularis infection in animals in the European Union and adjacent countries and in particular: a) the geographical distribution and prevalence of Echinococcus multilocularis infection in the main infected domestic and wildlife species involved in the Echinococcus multilocularis lifecycle; b) the importance and role of the different host species in the life cycle of the parasite; c) the risk factors for and the probability of introduction and establishment of Echinococcus multilocularis in areas where it has never been recorded, through the movement of infected domestic and wildlife species involved in the Echinococcus multilocularis lifecycle; |
Descriptive statistics |
| M‐2014‐0288 |
1. To describe Echinococcus multilocularis infection in animals in the European Union and adjacent countries and in particular: a) the geographical distribution and prevalence of Echinococcus multilocularis infection in the main infected domestic and wildlife species involved in the Echinococcus multilocularis lifecycle; b) the importance and role of the different host species in the life cycle of the parasite; c) the risk factors for and the probability of introduction and establishment of Echinococcus multilocularis in areas where it has never been recorded, through the movement of infected domestic and wildlife species involved in the Echinococcus multilocularis lifecycle; |
Risk factors analysis |
| M‐2014‐0288 |
1. To describe Echinococcus multilocularis infection in animals in the European Union and adjacent countries and in particular: a) the geographical distribution and prevalence of Echinococcus multilocularis infection in the main infected domestic and wildlife species involved in the Echinococcus multilocularis lifecycle; b) the importance and role of the different host species in the life cycle of the parasite; c) the risk factors for and the probability of introduction and establishment of Echinococcus multilocularis in areas where it has never been recorded, through the movement of infected domestic and wildlife species involved in the Echinococcus multilocularis lifecycle; |
Risk factors analysis |
| M‐2014‐0288 |
1. To describe Echinococcus multilocularis infection in animals in the European Union and adjacent countries and in particular: a) the geographical distribution and prevalence of Echinococcus multilocularis infection in the main infected domestic and wildlife species involved in the Echinococcus multilocularis lifecycle; b) the importance and role of the different host species in the life cycle of the parasite; c) the risk factors for and the probability of introduction and establishment of Echinococcus multilocularis in areas where it has never been recorded, through the movement of infected domestic and wildlife species involved in the Echinococcus multilocularis lifecycle; |
Probability of introduction |
| M‐2014‐0288 | 2. To assess the current situation in the European Union and adjacent countries regarding: a) the monitoring and surveillance programmes of Echinococcus multilocularis infection in definitive and intermediate hosts, and the probability of detection if Echinococcus multilocularis is introduced into areas where it is has never been recorded; b) the programmes for the eradication of Echinococcus multilocularis in wildlife host species; | Effectiveness of sampling schemes |
| M‐2014‐0288 | 2. To assess the current situation in the European Union and adjacent countries regarding: a) the monitoring and surveillance programmes of Echinococcus multilocularis infection in definitive and intermediate hosts, and the probability of detection if Echinococcus multilocularis is introduced into areas where it is has never been recorded; b) the programmes for the eradication of Echinococcus multilocularis in wildlife host species; | Effectiveness of sampling schemes |
| M‐2014‐0288 | 2. To assess the current situation in the European Union and adjacent countries regarding: a) the monitoring and surveillance programmes of Echinococcus multilocularis infection in definitive and intermediate hosts, and the probability of detection if Echinococcus multilocularis is introduced into areas where it is has never been recorded; b) the programmes for the eradication of Echinococcus multilocularis in wildlife host species; | Effectiveness of countermeasures |
| M‐2014‐0288 | 3. To describe the current situation in the European Union and adjacent countries regarding: a) the risk factors associated with human alveolar echinococcosis; b) the impact of Echinococcus multilocularis infection in animals on public health; | Risk factors analysis |
| M‐2014‐0288 | 3. To describe the current situation in the European Union and adjacent countries regarding: a) the risk factors associated with human alveolar echinococcosis; b) the impact of Echinococcus multilocularis infection in animals on public health; | Impact assessment |
| M‐2014‐0288 | 4. To describe the efficacy of available Echinococcus multilocularis drugs and the effectiveness of the current species‐specific treatment protocols to protect domestic species against the parasite; | Effectiveness of countermeasures |
| M‐2014‐0288 | 5. To assess the laboratory techniques for the detection of Echinococcus multilocularis in live and dead animals, in terms of sensitivity, specificity, predictive values and practicability (i.e. rapidity, large scale use, ease of use) | Effectiveness of sampling schemes |
Appendix F – List and definitions of possible statistical analysis and approaches and/or parameters as per the Preliminary Plan of Analysis
1.
| Analysis and approaches | Number of times required | Definition |
|---|---|---|
| (Incidence) rate | 1 |
Ratio in which the denominator is the number of animal‐time units at riska Also, a ratio in which the denominator is time. E.g. cases/day, cases/week, etc. |
| Attack rates | 2 | It is the risk of becoming infected (case) during a specific period of time, such as the duration of an outbreak. It is calculated by dividing the total number of new cases by the total number of individuals at risk (total population exposed) |
| Attributable fraction | 1 | Proportion of disease in exposed individuals that is due to the exposure OR proportion of disease in the exposed group that would be avoided if the exposure were removeda |
| Attributable risk | 1 | Risk of disease in the exposed group MINUS the risk of disease in the non‐exposed group (i.e. increased or decreased probability of disease in the exposed groupa |
| Case fatality rate | 1 |
Proportion of animals with a specific disease that die from it (within a specific time period) It's a risk measure. Used to describe the impact |
| Count | 1 | Simple enumeration of the number of cases of disease or number of animals affected with a condition in a given population. |
| Descriptive risk mapping | 1 | For aggregated data. Crude risk or risk ratios can be calculated and reported in maps. |
| Distribution maps | 1 | Simple geographical representation of a given parameter (e.g. prevalence) or event (e.g. disease notification)a |
| Freedom from disease | 2 | It is an approach based on the Binomial distribution, used in areas where the disease or a pathogen is not circulating, for different purposes, e.g. estimation of the confidence on the free status of a given disease, the calculation of the required sample size to demonstrate freedom, etc |
| Hazard ratio | 2 | In survival analysis, the hazard ratio is the ratio of the hazard rates corresponding to the conditions described by two levels of an explanatory variable. Hazard ratios have similar interpretations to odds ratios and risk ratiosa |
| Incidence count | 1 | Enumeration of new cases in a population during certain timea |
| Incidence rate | 2 | Number of new cases of disease in a population per unit of animal‐time during a given time period. Eg. No. cases/No. animal‐daysa |
| Incidence rate ratio/Incidence density | 1 | Ratio of the disease frequency (measured as incidence rate) in an exposed group to the Incidence Rate in a non‐expose groupa |
| Incidence risk/Cumulative incidence | 1 | Probability that an individual animal will contract or develop a disease in a defined time period. Also referred to as ‘cumulative incidence’a |
| Incidence times | 1 | Times at which incident cases occur. Usually measured as the elapsed time since a reference event (e.g. calving)a |
| Modelling techniques | 8 | The adaptation of a parametric model to empirical distributions in order to estimate the parameters and operate probabilistic calculations |
| Odds | 1 | Ratio in which the numerator is not a subset of the denominator. It can be interpreted as the likelihood that a case would take place. This is the ratio of cases to non‐cases or p/(1 − p)a |
| Odds ratio | 4 | Odds of the disease in the exposed group divided by the disease odds in the non‐expose groupa |
| Population attributable fraction | 1 | Analogous to Attributable Fraction BUT reflects the effect of the disease in the entire population (rather than in the exposed group)a |
| Population attributable risk | 1 | Difference in risk between two groups BUT focus on increase in risk of disease, in the entire population, attributable to the exposurea |
| Prevalence | 1 | Proportion or percentage of cases in the population at a specific point in time. Eg. cases/populationa |
| Proportion | 1 | Ratio in which the numerator is a subset of the denominatora |
| R 0 (Basic reproductive ratio) | 1 | Average number of secondary cases produced by one infectious individual in completely susceptible population |
| Regression techniques | 1 | E.g. Generalised linear models |
| Relative risk/risk ratio | 4 | Ratio of the risk of disease in the exposed group to the risk of disease in the non‐expose group. Also referred to as ‘Risk Ratio’a |
| Secondary attack rates | 2 |
Number of cases MINUS the initial case(s)a It describes the “infectiousness” (or ease of spread) of living agents |
| Simulation techniques | 8 | The implementation of virtual (computer) sampling from a parametric or empirical distribution in order to estimate the parameter of interest (a probability or a quantity) and its uncertainty/variability e.g. SIR models. These techniques are normally used for predictive purposes |
| Smoothing (kernel) methods | 1 | Techniques used in spatial analysis which convert large sets of points into a density surface |
| Transmission kernel | 1 | The transmission kernel describes the risk of transmission as a function of distance between an infectious and a susceptible holding |
| Transmission rate | 1 | The number of new cases produced by one infectious individual per unit of time |
Dohoo, Martin, Stryhn; 2010. Veterinary epidemiological Research – 2nd edition.
Appendix G – List and definitions of the identified categories of data input
1.
| Input data category | Number of times required | Definition |
|---|---|---|
| Case | 10 | An individual animal infected by a pathogenic agent, with or without clinical signs. (OIE terrestrial code) |
| Time | 8 | It's the point in time (usually a date) linked to a specific event or on which a given information is recorded |
| Population | 4 | As a very broad definition, the population can be described as any group of units sharing a common defined characteristic (OIE). From a statistical point of view, this can be either the target population (i.e. the population to which it might be possible to extrapolate results from a study) or the source population (i.e. the population from which the study subjects are drawn). It is at source population level that a risk assessor is asked to infer |
| Population size | 6 | Total number of units in a population |
| Population composition | 5 | Description of the population according to defined characteristics e.g. Sex, age, production type |
| Exposure | 5 |
Epidemiological term applied to any factor (risk factor) that may be associated with an outcome of interest. If the outcome of interest is occurrence of disease, then exposure is any characteristic (e.g. existence of infected animals, vaccination status, etc.) that may affect the health outcome (diseased/not‐diseased) In relation to a given risk factor, an individual animal has come into contact with, or is predisposed to becoming infected with a pathogen which would result in an increased likelihood of becoming infected |
| Geographical Location | 3 | The most detailed information on the positioning of an entity on Earth |
| Test diagnostic sensitivity | 2 | The diagnostic sensitivity of the analytical test (according to the OIE Manual) |
| Test diagnostic specificity | 2 | The diagnostic specificity of the analytical test (according to the OIE Manual) |
| Animal movements | 1 | The list of all movements of an animal since its birth until its death, with details on the establishments in which it is kept |
| Sampling scheme | 1 | The underpinning rationale/statistical approach used to perform all relevant steps in a sampling exercise, i.e. definition of the goal, estimation of the sample size, selection of the sampling units, etc. |
| Species/Breed | 1 | Genus, species and, when relevant, breed of the concerned animals |
| Vaccination status | 1 | The status of the animal in relation to the vaccine intervention |
Appendix H – A Zoonoses perspective: the case of double reporting of animal populations
Introduction
H.1. Issue
For more than 10 years, the European Food Safety Authority (EFSA) has been tasked with the European Union (EU)‐wide data collection on zoonoses, zoonotic agents, antimicrobial resistance and food‐borne outbreaks. Annually EFSA produces, jointly with the European Centre for Disease Prevention and Control, EU Summary Reports that integrate all information along the food chain. Member States (MSs) have a legal obligation to monitor trends and sources of zoonoses, zoonotic agents and antimicrobial resistance and food‐borne outbreaks and to transmit the results of monitoring programmes to the European Commission, which should be subsequently be forwarded to EFSA. In 2004, the European Commission entrusted EFSA with the task of setting up an electronic reporting system and a database concerning monitoring of zoonoses (EFSA mandate No 2004‐017810). Thus, in practice, data are sent directly from the MS data providers to EFSA (Gilsenan MB, 201511). A short review summarising the achievements made with this integrated food‐chain data collection and information system, identifying lessons learnt and describing challenges for improving the quality and the use of the collected data on food, animals and feed can be found elsewhere.12
Table H.1.
Comparison between animal zoonoses data provided to EFSA and to the European Commission
| EFSA | European Commission | |
|---|---|---|
| Tasks and purpose |
|
|
| Data collected |
Food animal data, i.e.:
Information on data models is in following of the annual zoonoses monitoring data reporting on zoonoses, food‐borne outbreaks and antimicrobial resistance (https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2018.EN-1368) |
Food animal data, i.e.:
Financial data (Commission Decision 2014/288/EU):
Epidemiological data:
|
It is in this context of its general zoonoses mandate that EFSA collects MS‐specific data on animal populations. However, certain of these animal population data are also collected from MS by the European Commission (DG Santé: G2 unit ‘Animal health and welfare’ and D4 unit ‘Food safety programme, emergency funding’), in the context of MS’ control and eradication programmes that are co‐financed by the European Commission.
These data are alike, although not always identical because of the different perspective they are collected for, and the compulsory requirement for MS to report these to EFSA and to the European Commission is underpinned by EU legislation. This issue is called ‘double reporting’.
It has been estimated that, on average, the effort required by a MS to collect and to submit to EFSA the relevant animal population data and manage them to fit the requirement of EFSA is of one person for one week. The collection, validation and submission of the complete zoonoses datasets require several months resources for one person (two to three).
H.2. Legislation
H.2.1. Animal population data submitted by MS to EFSA
Several pieces of legislation prescribe animal population data that MS ought to submit to EFSA. The animal population data are submitted by MS to EFSA using the Data Collection Framework (DCF) using XML or Excel or CSV formats. Animal population data can be submitted at level of animal species (e.g. Gallus gallus) or subcategories/subpopulations (e.g. broilers).
‘Susceptible animal population data’
According to the Zoonoses Directive 2003/99/EC13, EFSA is responsible for examining the data on zoonoses, antimicrobial resistance and food‐borne outbreaks collected from the MSs and for preparing the EU Summary Report (EUSR) (on trends of zoonoses and sources of zoonoses/food‐borne outbreaks) from the results.
These data, submitted to EFSA using the Animal population data model, include, as mentioned in the Appendix IV, Figure H1
— number of herds or flocks,
— total number of animals, and
— where relevant, methods of production involved,
and also the date these data (figures) relate to.
Animal population data in the context of Salmonella National Control Programmes in poultry
According to EU Regulation (EC) No 2160/200314, MS have to set up Salmonella National Control Programmes (NCP) aimed at reducing the prevalence of Salmonella serovars, which are considered relevant for public health, in certain animal populations. Currently, prevalence targets have been defined for breeding flocks of Gallus gallus (Regulation (EC) No 200/201015), laying hens (Regulation (EU) No 517/201116), broilers (Regulation (EC) No 200/201217) and breeding and fattening turkeys (Regulation (EU) No 1190/201218). The National Contact Points (NCP) are set up in individual MS to achieve the EU prevalence targets in these animal populations at the primary production level. NCP have to be approved by the European Commission, which evaluates the compliance of the programmes with the relevant EU legislation. The results of the programmes have to be reported to the European Commission for reimbursement and to EFSA as part of the annual EU zoonoses monitoring.
These data submitted to EFSA using the Prevalence data model, include;
-
–
Total number of adult flocks under the NCPs
-
–
Total number of adult flocks checked (tested).
Animal population data in the context of National Control and Eradication Programmes for bovine tuberculosis in cattle and in brucellosis in cattle and sheep and goats
Other Community legislation provides for the monitoring and control of certain zoonoses in animal populations. In particular, Council Directive 64/432/EEC of 26 June 1964 on animal health problems affecting intra‐community trade in bovine animals and swine deals with bovine tuberculosis and bovine brucellosis. Council Directive 91/68/EEC of 28 January 1991 on animal health conditions governing intra‐Community trade in ovine and caprine animals deals with ovine and caprine brucellosis. Since directive 2003/99/EC may not create any unnecessary duplication of existing reporting requirements, the EFSA zoonoses reporting system integrated the reporting tables specified in the two aforementioned directives (64/432/EEC and 91/68/EEC). These data, submitted to EFSA using the Disease Status data model, include;
-
–
Number of existing herds/animals
-
–
Number of herds/animals under the control programme
-
–
Number of herds/animals checked (tested).
H.2.2. Animal population data provided to the European Commission
According to Regulation (EU) No 652/201419, grants may be awarded to MSs’ annual or multiannual national programmes for the eradication, control and surveillance of a list of animal diseases and zoonoses (listed in Annex II of Regulation (EU) No 652/2014). These or called ‘EC co‐financed’ programmes. More information on National Veterinary Programmes, in EU can be found at https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en
After execution MSs submit – for their co‐financed programmes – reports to the European Commission on the obtained results and these reports serve also the purpose of underpinning their request for reimbursement. Those reports are also published on the mentioned website.
What the European Commission requests the MS to provide are the measures performed during an implementing year (from 1/1 to 31/12) for which MSs incurred a cost (i.e. the state budget paid totally or partially the cost of the measure); this cost should have been paid by the time the MS send their reimbursement claim, i.e. by 30/4 of the next year at the latest. For sampling (if the eligible co‐financed measure is the act of taking samples), the samples shall have been taken between 1/1 and 31/12 and the MS shall have paid its financial part before they send their reimbursement claim to the European Commission. It results that animal population data sent by MS to the European Commission in this context may partly be different from those sent to EFSA, which receives data in principal from 1/1 to 31/12 (see Table H.2).
Table H.2.
Comparison between annual animal population data provided to EFSA and to the European Commission
| EFSA | European Commission | |
|---|---|---|
| Type of data | Aggregated | Aggregated |
| Scope | Epidemiological analysis |
Claiming reimbursement (EU‐approved and veterinary programmes co‐funded ONLY) Need direct data transmission for financial accountability |
| Variables |
Information on the animal population data model is on p. 42 and on the disease status data model is on p. 45 and following of the annual zoonoses monitoring data reporting on zoonoses, food‐borne outbreaks and antimicrobial resistance (https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2018.EN-1368) |
Different data elements (only partial overlap) and text forms Data model seems comparable with EFSA but more detailed analysis needed |
| Submission date | 31 May | 30 April |
H.2.3. Creating a WIN‐WIN situation for MSs, EFSA and the European Commission with SIGMA and avoid double reporting of animal population in the zoonoses domain
The different data streams regarding animal population in the zoonoses domain from MS to EFSA and from MS to the European Commission are detailed above.
Data transmission
Currently, only few MSs (four) transmit their annual animal population in the zoonoses domain to EFSA without manual manipulation and are extracted from national databases and transmitted to EFSA using XML. The majority of the MSs, however, use an EFSA zoonoses Excel‐based mapping tool where data are manually inserted ad managed before sending to EFSA using an XML generating tool. The shaping up of data in the EFSA mapping tool is very resource‐intensive.
In addition, the MSs transmit their annual animal population data to the European Commission with manual input in a PDF tool with an embedded XML structure.
Validation
It may be that the national reporters submitting the abovementioned data to EFSA and to the European Commission are staff of different units or agencies (institutes) with different missions. This may lead to some discrepancies across the different reports: despite the data are the same, a different way of aggregating them may lead to apparent differences in counts and proportions.
For this reason MSs, EFSA and the European Commission carry out annually a thorough cross‐validation exercise to ensure that no discrepant statistics are published in the MSs’ national zoonoses reports, nor in the EFSA scientific reports, nor in the reports published by the European Commission. This exercise is extremely demanding and requires a lot of resources.
Leaning
The SIGMA Data Model is flexible and can deal with many different types of data, from sample‐based to aggregated data. The SSD2, which defines the standards to describe the information related to the individual sample, is part of the SIGMA Data Model, which fits the requirements of the EFSA Data Warehouse.
It is proposed that the MSs submit to EFSA using the relevant standards defined by the SIGMA Data Model all the data needed by EFSA and the European Commission as regards animal population in the zoonoses domain. Once those data are submitted and stored in the Data Warehouse, all concerned parties, with different levels of permission, will be able to access the relevant data to generate different types of report. As an example, the MSs will be able to access their own data and generate the national reports to be submitted to the European Commission or other type of reports for internal use; the European Commission will be able to generate summaries and overviews based on specific needs. This possibility will be implemented by means of web‐based tools directly linked to the data stored in the EFSA data warehouse.
This solution will save a lot of time and resources to the MS which will have to submit only once the data of concern, which will be available to EFSA and the European Commission for more than one purpose. In addition, the data will be standardised at EU level. The only action required to the MSs is to align the data submitted to EFSA with the SIGMA Data Model standards. However, it has to be noted that this work is carried out by the SIGMA Consortium which is financed by EFSA.
Suggested citation: EFSA (European Food Safety Authority) , Zancanaro G, Antoniou SE, Bedriova M, Boelaert F, Gonzales Rojas J, Monguidi M, Roberts H, Saxmose Nielsen S and Thulke H‐H, 2019. Scientific report on the SIGMA Animal Disease Data Model: A comprehensive approach for the collection of standardised data on animal diseases. EFSA Journal 2019;17(1):5556, 60 pp. 10.2903/j.efsa.2019.5556
Requestor: EFSA
Question number: EFSA‐Q‐2018‐00080
Acknowledgements: EFSA wishes to thank the members of the EFSA Scientific Network on Animal Health and Welfare: Robert Wolf, Kirstine Ceulemans, Lilyana Polihronova, Drazen Knezevic, Georgios Krasias, Richard Wallo, Anette Boklund, Ave Ly Toomvap, Pascal Hendrikx, Aik Plevraki, Melinda Kocsis, Ronan O'Neill, Fabrizio De Massis, Edvins Olsevkis, Olaf Stenvers, Dean Bas, Przemyslaw Cwynar, Yolanda Vaz, Anna Ondrejkova, Luis Romero, Cecilia Hulten for their input and feedback; the Animal Health and Welfare Panel members for their contribution; and EFSA staff members: Frank Verdonck, Francesca Baldinelli, Inmaculada Aznar, Andrey Gogin, Alessandro Broglia, Sofie Dhollander, Stefano Cappè, Yves Van Der Stede, José Cortiñas Abrahantes and Federica Barrucci for the input and the support provided to this scientific output.
Approved: 12 December 2018
Notes
European Food Safety Authority, European Centre for Disease Prevention and Control, European Union Reference Laboratory for Avian influenza, Brown I, Mulatti P, Smietanka K,Staubach C, Willeberg P, Adlhoch C, Candiani D, Fabris C, Zancanaro G, Morgado J and Verdonck F,2017. Scientific report on the avian influenza overview October 2016–August 2017. EFSA Journal 2017;15(10):5018, 101 pp. https://doi.org/10.2903/j.efsa.2017.5018
EFSA Register of Questions: (entre the mandate number from this page).
Gilsenan M, 2015. Data handling: observatories/databases/data/storage/legal framework EFSA data collection. Options Méditerranéennes, A111, 75–82.
Boelaert F, Amore G, Van der Stede Y, Hugas M, 2016. EU‐wide monitoring of biological hazards along the food chain: achievements, challenges and EFSA vision for the future. Current Opinion in Food Science, 12, 52–62.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, p. 31–40.
Regulation (EC) No 2160/2003 of the European Parliament and of the Council and Regulation of 17 November 2003 on the control of Salmonella and other specified food‐borne zoonotic agents. OJ L 325, 12.12.2003, p. 1–15.
Commission Regulation (EU) No 200/2010 of 10 March 2010 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of Salmonella serotypes in adult breeding flocks of Gallus gallus. OJ L 61, 11.3.2010, p. 1–9.
Commission Regulation (EU) No. 517/2011 of 25 May 2011 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of certain Salmonella serotypes in laying hens of Gallus gallus and amending Regulation (EC) No. 2160/2003 and Commission Regulation (EU) No 200/2010. OJ L 138, 26.5.2011, p. 45–51.
Commission Regulation (EC) No. 200/2012 of 8 March 2012 on a Union target for the reduction of Salmonella Enteritidis and Salmonella Typhimurium in flocks of broilers, as provided for in Regulation (EC) No. 2160/2003 of the European Parliament and of the Council. OJ L 71, 9.3.2012, p. 31–36.
Commission Regulation (EU) No. 1190/2012 of 12 December 2012 on a Union target for the reduction of Salmonella Enteritidis and Salmonella Typhimurium in flocks of turkeys, as provided for in Regulation (EC) No. 2160/2003 of the European Parliament and of the Council. OJ L 340, 13.12.2012, p. 29–34.
Regulation (EU) No 652/2014 of the European Parliament and of the Council of 15 May 2014 laying down provisions for the management of expenditure relating to the food chain, animal health and animal welfare, and relating to plant health and plant reproductive material, amending Council Directives 98/56/EC, 2000/29/EC and 2008/90/EC, Regulations (EC) No 178/2002, (EC) No 882/2004 and (EC) No 396/2005 of the European Parliament and of the Council, Directive 2009/128/EC of the European Parliament and of the Council and Regulation (EC) No 1107/2009 of the European Parliament and of the Council and repealing Council Decisions 66/399/EEC, 76/894/EEC and 2009/470/EC.
References
- ANSVSA (National sanitary Veterinary and for Food Safety Authority of Romania), 2015. Use of the EFSA Standard Sample Description ver. 2.0 (SSD2) for the reporting of data on the control of pesticide residues in food and feed according to Regulation (EC) No 396/2005. EFSA Supporting publication 2015:EN‐918, 80 pp. https://www.efsa.europa.eu/it/supporting/pub/918e [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Scientific report on Principles and process for dealing with data and evidence in scientific assessments. EFSA Journal 2015;13(5):4121, 35 pp. 10.2903/j.efsa.2015.4121 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Data dictionaries—guidelines for reporting data on zoonoses, antimicrobial resistance and food‐borne outbreaks using the EFSA data models for the Data Collection Framework (DCF) to be used in 2017, for 2016 data. EFSA supporting publication 2017:EN‐1178, 106 pp. 10.2903/sp.efsa.2017.en-1178 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal 2017;15(12):5077, 228 pp. 10.2903/j.efsa.2017.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]


