Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on l‐histidine monohydrochloride monohydrate produced by fermentation with Escherichia coli (NITE BP‐02526) when used as a nutritional additive or as a feed flavouring compound in feed and water for drinking for all animal species. The product under assessment is l‐histidine HCl H2O produced by fermentation with a genetically modified strain of E. coli (NITE BP‐02526). The production strain and its recombinant DNA were not detected in the final products. l‐Histidine HCl H2O does not give rise to any safety concern to the production strain. The use of l‐histidine HCl H2O is safe for the target species when used to supplement the diet in appropriate amounts. It is safe at the proposed use level of 25 mg/kg when used as a flavouring compound for all animal species. The use of l‐histidine HCl H2O in animal nutrition raises no safety concerns for consumers of animal products. The additive is not irritating to the skin or eyes and is not a skin sensitiser. There is a risk for persons handling the additive from the exposure to endotoxins by inhalation. The use of l‐histidine as a feed additive does not represent a risk to the environment. The additive l‐histidine HCl H2O is regarded as an effective source of the amino acid l‐histidine when used as a nutritional additive. For the supplemental l‐histidine to be as efficacious in ruminants as in non‐ruminant species, it requires protection against degradation in the rumen. It is also considered efficacious as a feed flavouring compound under the proposed conditions of use.
Keywords: nutritional additive, amino acid, l‐Histidine monohydrochloride monohydrate, Escherichia coli (NITE‐BP 02526), feed additive, safety
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Ajinomoto Animal Nutrition Europe2 for authorisation of the product l‐histidine monohydrochloride monohydrate produced by Escherichia coli (NITE BP‐02526), when used as a feed additive for all animal species (category: nutritional additive; functional group: amino acids, their salts and analogues and category: sensory additive; functional group: flavouring).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 22 November 2018.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of l‐histidine monohydrochloride monohydrate produced by E. coli (NITE BP‐02526), when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
l‐Histidine monohydrochloride monohydrate produced by E. coli (NITE BP‐02526) has not been previously authorised as a feed additive in the European Union (EU).
l‐Histidine monohydrochloride monohydrate produced by E. coli (ATCC 9637) is already authorised for its use in salmonids as a nutritional additive (functional group: amino acids, their salts and analogues).
l‐Histidine, produced by chemical synthesis or protein hydrolysis, is an already authorised for its use in all animal species as a sensory additive (functional group: flavouring compounds).
l‐Histidine (FLAVIS No. 17.008, CAS 71‐00‐1) is authorised as food flavouring in all categories of flavoured foods under the Regulation (EU) No 872/2012.
l‐Histidine is authorised for use in food,3 cosmetics4 and as a veterinary medicinal product.5 , 6
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier7 in support of the authorisation request for the use of l‐histidine monohydrochloride monohydrate produced by E. coli (NITE BP‐02526) as a feed additive.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the l‐histidine monohydrochloride monohydrate produced by Escherichia coli (NITE BP‐02526) in animal feed. The Executive Summary of the EURL report can be found in Annex A.8
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of l‐histidine monohydrochloride monohydrate produced by E. coli (NITE BP‐02526) is in line with the principles laid down in Regulation (EC) No 429/20089 and the relevant guidance documents: Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c) and Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012).
3. Assessment
l‐Histidine monohydrochloride monohydrate (≥ 98% on a dry matter (DM) basis) is produced by fermentation with a genetically modified E. coli strain. It is proposed to be used as a nutritional additive (functional group: amino acids, their salts and analogues) and as a sensory additive (functional group: flavouring) for all animal species.
3.1. Characterisation
3.1.1. Characterisation of the production organism
The additive l‐histidine monohydrochloride monohydrate is produced by a genetically modified strain of E. coli K‐12, which is deposited in the National Institute of Technology Evaluation (NITE) of Japan Culture Collection with accession number NITE BP‐02526.
The strain was identified as E. coli K‐12 by molecular serotyping, and multi‐locus sequence typing (MLST) using data obtained by whole genome sequencing (WGS).
Escherichia coli NITE BP‐02526 was tested for antibiotic susceptibility ■■■■■ WGS analysis did not identify any intact antibiotic resistance genes in the genome of the production strain NITE BP‐02526.
The WGS analysis also indicated the absence of known E. coli virulence factors, including genes encoding enterotoxins, Shiga toxins, and adhesion and invasion factors.
3.1.1.1. Information relating to the genetically modified microorganism
Characteristics of the recipient or parental microorganism
■■■■■
■■■■■
■■■■■
Description of the genetic modification process
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
3.1.2. Manufacturing process
l‐Histidine monohydrochloride monohydrate is produced by fermentation process (fed‐batch fermentation) with E. coli (NITE BP‐02526), the cells in the fermentation broth are inactivated ■■■■■.
■■■■■ then the product is crystallised, dried and packaged.
The applicant stated that no antibiotics are used during fermentation process.
3.1.3. Characterisation of active substance/additive
l‐Histidine monohydrochloride monohydrate (International Union of Pure and Applied Chemistry (IUPAC) name: (2S)‐2‐amino‐3‐(1H‐imidazol‐5‐yl)propanoic acid;hydrate;hydrochloride), is a compound identified with the Chemical Abstracts Service (CAS) No 5934‐29‐2, and the European Inventory of Existing Commercial Chemical Substances (EINECS) No 211‐438‐9. It has a molecular weight of 191.62 Da. The chemical formula of l‐histidine monohydrochloride monohydrate is C6H9N3O2.HCl.H2O. The structural formula is given in Figure 1.
Figure 1.
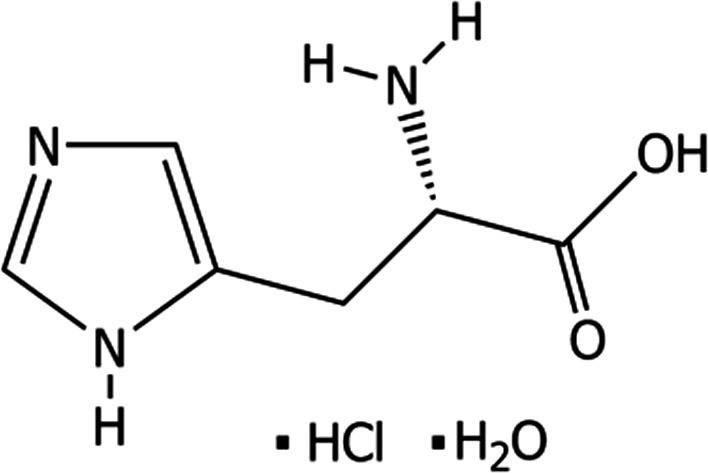
Structural formula of l‐histidine monohydrochloride monohydrate
The product is specified to contain a minimum of 98% l‐histidine HCl H2O on DM basis and a minimum of 72% of l‐histidine on DM basis, other components are residual moisture and other substances (amino acids, organic acids and minerals).
The applicant provided results on compositional analysis of 10 industrial pilot batches. The analysis showed an average value of l‐histidine monohydrochloride monohydrate of 99.5% on DM basis (range 99.1–100.4%). The analysis also showed an average value of l‐histidine of 73.7% on DM basis (range 73.4–74.4%). Moisture average was 0.042% (range 0.04–0.05%) and no quantifiable free amino acids other than l‐histidine could be detected. Quantifiable ammonium, nitrates, nitrites and betaine were analysed in three batches of the product and was on average 0.026% (0.02–0.04%). Sum of quantifiable organic acids was on average 0.066% (0.05–0.09%). Sum of quantifiable inorganic cations and anions was < 0.002% (analysed in three batches).10
Analytical data on specific optical rotation of five batches showed a range + 9.3° to + 9.4°, which is within the range described in the European Pharmacopoeia for this amino acid (+ 9.2° to + 10.6°) and confirms the l‐enantiomer of histidine.10
3.1.3.1. Impurities
Three batches were analysed for the possible presence of heavy metals, metals and metalloids. Arsenic, cadmium, chromium, copper, iron, lead, mercury, nickel, phosphorus, fluorine, melamine and (hydro)‐cyanic acid were tested. The levels found were below the quantification levels.11 , 12
The sum of dioxins (polychlorinated dibenzo‐p‐dioxins and polychlorinated dibenzofurans (PCDD/F)) and dioxin‐like polychlorinated biphenyls (DL‐PCBs) ranged from 0.0877 to 0.107 ng WHO‐PCDD/F‐PCB TEQ/kg. Non‐DL‐PCBs (IECS‐6) ranged from 0.0094 to 0.0111 ng/kg TEQ.11
Residues of organochlorine (including pyrethroids) and organophosphorus pesticides (analysed in three batches) were all below the limit of quantification (LOQ).13 , 11
The microbial analyses of 10 batches of the product showed the absence of Salmonella spp. (25 g samples), total germ count (at 30°C) < 100 CFU/g; coliforms, Staphylococcus coagulase positive, yeast and moulds < 10 CFU/g, and Bacillus cereus (at 30°C) < 100 CFU/g.
As regards the mycotoxins, analysis of three batches showed values of aflatoxins (B1, B2, G1, G2), zearalenone, deoxynivalenol, ochratoxin A, T‐2 and HT‐2 toxins, fumonisins (B1, B2 and B3) below the limit of quantification.11 , 14
■■■■■■■■■■
The amount of the impurities in the product does not raise a safety concern.
The presence of viable cells of the production strain was tested in three independent batches of the final product from industrial pilot facilities, each batch tested in triplicate.■■■■■■■■■■ Appropriate controls were carried out on the performance of these experiments. No viable cells of the production organism were detected.
The presence of DNA of the production strain was tested in three independent batches of the final product from industrial pilot facilities, each batch tested in triplicate.■■■■■ The test started from 1 g of product, ■■■■■. No DNA was detected.
3.1.3.2. Physical characteristics
The additive is described as greenish crystals or a crystalline powder. Its solubility in water at 25°C is 41.9 g/L; it has a pH ranging from 3.5 to 4.5.18 The packed bulk density measure in 3 batches ranged from 915 to 966 kg/m3.
Three batches of the product were also analysed for dusting potential (Stauber–Haeubach method) and the results ranged from 0.61 to 0.93 g/m3.19
The particle size distribution was analysed in three batches (laser light scattering). Most of the particles size ranges from 100 to 1,000 μm diameter. The fraction of particles with a diameter below 100 is approximately 8% and below 50 μm is around 5%. No particles were detected with a diameter below 17 μm.20
3.1.4. Stability and homogeneity
3.1.4.1. Shelf life
The shelf life of the additive (three batches) was measured when stored at 25°C and 40°C in sealed plastic bags for 12 months. Losses up to 1% of histidine were detected at 25°C. No losses were observed in any of the batches stored at 40°C.21
3.1.4.2. Stability in premixture
The stability of the additive (three batches) was measured in three types of vitamin/mineral premixtures (piglet starter, sow gestation and chickens for fattening premixture, one batch for each) at 25°C or at 40°C in paper bags with plastic in inner layer for 6 months.22 The concentration of choline chloride was 0.8, 1.6 and 2%, respectively. For the piglet premixture, a loss of 2.8% was observed only at 40°C. For sow gestation, a loss of 5.2% was observed at 40°C only. For chickens for fattening, a loss of 3.4% was observed at 25°C and of 10% at 40°C.
3.1.4.3. Stability in feedingstuffs
The stability of the additive (three batches) in three types of pelleted feed (piglet starter, sow gestation and chickens for fattening, one batch each type of feed) was studied when stored at 25°C or at 40°C in sealed plastic bags for 3 months.23 Complete feed for piglets, sows and chickens for fattening was supplemented with histidine at 0.1, 0.15 and 0.2%, respectively.
Feed was preconditioned at 60–65°C (all feeds) and pelleted at 88°C in piglets, 76°C in sows and at 72°C in chickens for fattening. Feed processing represented a loss of 10% in piglets only.
At the end of the storage period, piglets pelleted feed kept at 25°C lost 8%, and 19% at 40°C. Pelleted feed for gestating sows kept at 25°C lost 5% and kept at 40°C lost 8%. Pelleted feed for chickens for fattening kept at 25°C lost 21% and at 40°C lost 11%.
3.1.4.4. Stability in water for drinking
The stability of one batch of the additive in water for drinking was measured at 0.5, 2.5 and 5 g/L when stored at 25°C for 48 h.24 No losses were observed.
3.1.4.5. Homogeneity
The capacity of the additive to distribute homogeneously was studied in the premixtures and feeds described above.25 , 26 Ten subsamples of each premixture were analysed and the coefficients of variation (CV) were 3%, 4% and 3%, respectively. Ten subsamples of each feed (except for chicken for fattening which had 9 samples analysed) were analysed and the CVs were 4, 4 and 5%, respectively.
3.1.4.6. Physico‐chemical incompatibilities in feed
No physico‐chemical incompatibilities in feed are expected with other feed additives, medicinal products or feed materials.
3.1.5. Conditions of use
According to the applicant, the additive can be added directly in compound feed through complementary feed, premixtures and water for drinking and is intended for all animal species.
As a nutritional additive, no proposed inclusion levels are provided, as the optimal daily allowance in quantitative terms depends on the species, the physiological state of the animal, the performance level and the environmental conditions, in particular on the amino acid composition of the unsupplemented diet.
l‐Histidine monochloride monohydrate is proposed to be used as a feed flavouring in feed or in water for drinking at maximum recommended level of inclusion of 25 mg/kg.
3.2. Safety
3.2.1. Safety aspects of the production organism
The genetic modifications performed to obtain the production strain have ■■■■■ l‐histidine ■■■■■.
The recipient organism E. coli K‐12 is considered to be safe. None of the introduced modifications raise a safety concern. The applicant provided sufficient information that neither the production strain nor its recombinant DNA is present in the final product. The final product does not give rise to any safety concern with regard to the genetic modification of the production strain.
3.2.2. Safety for the target species
The absorption, distribution, metabolism and excretion, animal requirements and histidine content in feedingstuffs have been described in a previous opinion (EFSA FEEDAP Panel, 2019).
Safety data in the target species are not normally required for highly purified amino acids. However, the FEEDAP Panel considers that excesses of l‐histidine in the diet are not well tolerated, most probably due to (i) amino acid imbalances, (ii) interactions with trace elements (e.g. Cu, Zn) in the gut, and iii) microbial production of histamine in the gut/rumen (Ahrens, 1967; Aoyama and Kato, 2000; Aoyama et al., 2001; Xu et al., 2003; Glover and Wood, 2008; Golder et al., 2012; Khan and Abidi, 2014). Brain histidine and histamine are involved in the regulation of feed intake. Reduction in feed intake in response to excess dietary histidine intake may be probably triggered by the elevation of brain histidine and histamine levels (Sheiner et al., 1985). High dietary histidine levels have been shown to result in potentially serious adverse effects in both animals (e.g. hyperlipidemia, hypercholesterolemia, enlarged liver) and humans (e.g. increase in urinary zinc, headache, weakness) (Garlick, 2004). In fact, there is evidence from studies in experimental animals and humans that intakes of high levels of histidine can alter copper and zinc metabolism and cause deficiencies of the free forms of these metal ions due to increased excretion (VKM, 2016) (NRC, 2011).
The applicant provided a literature search to address the safety for target animals. PubMed was searched using several search strings.27 Among the papers retrieved, 75 were considered relevant by the applicant. Only the papers that were judged relevant by the Panel for the assessment of the safety for the target species are reported.
A 90‐day study was performed in F344 rats fed diets containing 0, 0.31, 0.62, 1.25, 2.5 and 5% l‐Histidine HCl H2O (Ikezaki et al., 1994). The FEEDAP Panel identified 2.5% in the diet as the maximum tolerable dose of l‐Histidine HCl H2O. A long‐term toxicity and carcinogenicity study (104 weeks) was performed in F344 rats fed diets containing 0, 1.25 and 2.5% l‐Histidine HCl H2O (Ikezaki et al., 1996). There were no significant differences between the control and treated groups of both sexes in overall tumour incidences. In all male groups, tumours of the testes were the most frequent, followed by lesions in the adrenal, haematopoietic organs and pituitary. All testicular tumours were benign interstitial cell tumours, the most frequently encountered spontaneous tumour in F344 rats. Food intake decreased in rats following increased dietary histidine (Kasaoka et al., 2004; Asahi et al., 2016) through its conversion into histamine (Yoshimatsu et al., 2002) which acts on food intake through histamine H1 receptors (Schwartz et al., 1972; Ookuma et al., 1989). Rats fed histidine at levels ranging from 2 g/kg body weight (bw) per day to >4 g/kg bw per day showed growth retardation, hepatomegaly, significant decrease in plasma zinc level and hypercholesterolaemia (Solomon and Geison, 1978; Harvey et al., 1981; Ohmura et al., 1986, 1992). However, supplementation of the rat diet with 5% histidine did not affect zinc (the rate of turnover of 65Zn from 2 to 4 weeks after a single injection of the tracer) whereas the diet supplemented with 8% histidine induced a severe zinc deficiency (50% reduction in the plasma zinc content) (Wensink and Van den Hamer, 1988). This effect of histidine supplementation on zinc status could depend of dietary zinc intake. Indeed when rats were fed a zinc‐adequate diet, histidine supplementation did not cause changes in the zinc status (zinc concentrations, 65Zn tissue distribution and tissue‐specific activities) whereas when zinc intake was low, histidine supplementation led to a lower 65Zn retention, associated with increased faecal excretion and a shorter biological half‐life (Van Wouwe et al., 1990).
Figueroa et al. (2003) studied the effects of amino acid supplementation on growing pigs submitted to different concentration of crude protein (CP) (16%, 12% or 11% CP). Effects of histidine were observed on weight gain, daily food intake, fat‐free lean mass gain and feed efficiency.
Supplementation of l‐histidine at 4% reduced weight gain by 48 to 51% in 8‐day‐old crossbred chicks and at 3% caused growth depression of 31% (Edmonds and Baker, 1987). Kopeć et al. (2013) studied the effects of a supplementation with l‐histidine (6.83 g/kg diet), either as pure amino acid or spray‐dried blood cells (SDBC) rich in histidine (6.14 g/kg diet), with or without Zn. Proteins and histidine dipeptide (carnosine and anserine) content in the muscles increased by diet supplemented with histidine.
Schoof et al. (2000) evaluated the effects of continuous duodenal infusion of l‐histidine on the retention of nitrogen and amino acids utilisation in young bulls. No significant differences were observed following histidine infusion compared to the control. The production of milk, protein, casein and lactose were significantly higher following histidine supplementation (2.5 g/L in water for drinking or diet supplemented with 13.6 g/d) compared to control (Doelman et al., 2008; Hadrová et al., 2012). Gao et al. (2015) investigated the effects of leucine and histidine on the mammalian target of rapamycin (mTOR) signalling pathway in milk protein synthesis using CMEC‐H (bovine mammary epithelial cells) and demonstrated that leucine or histidine stimulated the expression of different forms of caseins.
The product is currently authorised for use as a feed additive in salmonids (EFSA, 2005). It is an essential amino acid for fish. N‐Acetyl histidine (NAH) is a prominent biomolecule in ocular lens. Dietary levels of histidine modulate lens NAH concentration and have been found to prevent or slow the progression of cataract development. Different studies have investigated the effect of dietary histidine on cataract severity and prevalence (Trösse et al., 2009; Waagbø et al., 2010; Remø et al., 2011, 2014). The highest level (10.37 μmol/g) of NAH was found in the tissue of Betta splendens (Siamese fighting fish). Moreover, the NAH contents in the tissues of Trichogaster trichopterus (three spot gourami), Kryptopterus bicirrhis (glass catfish), Oreochromis niloticus (Nile tilapia), Mikrogeophagus ramirezi (ram cichlid) and Parachromis managuensis (Guapote tigre) were 3.17–6.16 μmol/g. The skeletal muscle of amphibians (5 species) and reptiles (4 species) had a low level (< 0.25 μmol/g) of NAH (Abe, 2000). In catfish, dietary histidine caused no increase in serum free histidine levels, until the dietary requirement was reached (0.37 ± 0.01%). Carnosine could not be detected in muscle (Wilson et al., 1980). In Nile tilapia juveniles fed diets containing histidine at 4.2, 5.4, 7.1, 8.9, 9.8 and 11.5 g/kg DM in diet, whole‐body protein content was higher in fish fed 7.1 and 9.8 g/kg DM of histidine. Myogenin expression was higher in fish fed histidine at 9.8 and 11.5 g/kg DM to fish fed histidine at 11.5 g/kg DM (Hulata 2015). In grass carp fed histidine supplemented diet for 2 weeks at 2.0‐control, 3.7, 5.9, 7.9, 9.8 and 12.2 g/kg diet, growth performance increased until 7.9 g/kg, above this level growth performance decreased (Jiang et al., 2016).
Although there is limited evidence from the published literature on the effects of supplementing histidine levels above the requirements, the FEEDAP Panel considers that, as with other amino acids, adverse effects might occur with levels of histidine in feeds exceeding the requirements, depending on the balance with other amino acids and the status of some essential trace elements such as copper and zinc.
The FEEDAP Panel, in its previous statement (EFSA FEEDAP Panel, 2010), identified risks of nutritional imbalances and hygienic concerns for amino acids when administered in water for drinking.
Since the levels proposed for the use of l‐histidine monohydrochloride monohydrate as flavouring (up to 25 mg/kg complete feed) are substantially lower than the animal requirements, the FEEDAP Panel considers l‐histidine monohydrochloride monohydrate produced with E. coli (NITE BP‐02526) is safe when used as a flavouring compound.
3.2.2.1. Conclusions on the safety for the target species
The use of l‐histidine monohydrochloride monohydrate produced by fermentation using E. coli (NITE BP‐02526) is safe for the target species when used as a nutritional additive to supplement the diet in appropriate amounts to cover the requirements, depending on the species, the physiological state of the animal, the performance level, the environmental conditions and the background amino acid composition of the unsupplemented diet and the status of some essential trace elements such as copper and zinc. This conclusion would also cover the use as sensory additive.
3.2.3. Safety for the consumer
The product under assessment is produced by fermentation. The production strain has been adequately identified as an E. coli K‐12 derivative. E. coli K‐12 is considered safe. The applicant provided evidence that the production strain does not carry ■■■■■ and that neither the production strain nor its recombinant DNA is present in the final product. Therefore, the FEEDAP Panel considers that no safety concerns would derive from the fermentation process. The additive contains 99.1% l‐histidine HCl monohydrate and the amount of unidentified material is < 1%.
The FEEDAP, however, is aware that the intake of histamine, a metabolic by‐product of histidine, through fish flesh following microbial spoilage is a serious concern for consumers (EFSA BIOHAZ Panel, 2011). Histamine poisoning from fish flesh has been called ‘scombroid’ poisoning because of the edible fish species (e.g. tuna, mackerel) more liable to histamine formation due to the high content of histidine in their flesh. Commission Regulation (EC) No 2073/2005 sets a maximum limit of 200 mg histamine/kg flesh for sea fishery products (raw fish at the point of the first sale) of fish species associated with a high amount of histidine, in particular fish species of the families: Scombridae, Clupeidae, Engraulidae, Coryfenidae, Pomatomidae and Scombresosidae.28
Histamine is a biogenic amine that can be synthesised endogenously from histidine by a l‐histidine decarboxylase. Histamine can be metabolised either extracellularly by a diamino oxidase (DAO) present in the gut mucosa, or intracellularly by a histamine‐N‐methyltransferase (HNMT) (EFSA BIOHAZ Panel, 2011).
Upon EFSA request, the applicant provided a literature search for studies to address the relationship between histidine/histamine concentration in edible tissues/products of food producing animals following histidine addition at recommended use levels. The literature search was performed using first an automatic search in a broad range of databases (LIVIVO and Ovid and sixteen single databases including PubMed and Web of Science), and eight publishers search facilities (including Elsevier, Ingenta, Springer, Wiley), followed by an expert search using the ETH Library Search Portal and Google Scholar.29 Among the papers retrieved, 54 were considered relevant by the applicant. The papers that were judged relevant by the Panel for the assessment of the safety for the target species are described below.
3.2.3.1. Histidine intake and histidine deposition in animal tissues or products
In pigs, the addition of free histidine to the diet results in increased uptakes (Heger et al., 2007), increased histidine plasma levels (Čuperlović, 1973; Izquierdo et al., 1988; Li et al., 2002; Figueroa et al., 2003). No data about free histidine or histamine levels in edible pig tissues are available. Čuperlović (1973) suggested that plasma free levels of histidine are controlled, which would reduce probability of increased tissue levels of histidine in pigs.
In broilers, histidine levels in plasma and breast muscle were increased by dietary histidine. However, levels were low if compared to the increase of the dipeptides carnosine and anserine (Ishibashi et al., 1979; Haug et al., 2008; Kai et al., 2015) or not strongly increased (Kopeć et al., 2013). In turkeys, no increase of histidine level in breast muscle was observed after supplementation (Kopec et al. 2016). Several studies reported increased levels of anserine and carnosine following dietary histidine supplementation. No data on histidine levels in eggs were retrieved.
In bovines, plasma levels of free histidine were found to be positively correlated with histidine supplementation (Hofherr, 2010). Uptake of free histidine from the blood by the udder was increased correspondingly (Bequette et al., 2000; Cant et al., 2001). One study reported no increase of histidine blood levels when feeding rumen‐protected histidine (Robinson et al., 2010). However, histidine uptake by the udder seems to be efficiently controlled by the amount of milk protein that can or shall be produced: as histidine influx and efflux are both controlled by free histidine levels in blood and the mammary gland, an enrichment of free histidine in milk is unlikely and has not been reported (Huhtanen et al. 2002; Korhonen et al., 2000).
In fish, no effect on histidine levels in muscle of Japanese flounder was found (Han et al., 2013), but levels of histidine in blood and tissues (especially muscle) increased significantly with increased histidine feed levels in Atlantic salmon (Breck et al., 2005; Waagbø et al., 2010; Remø et al., 2014), juvenile grass carp (Gao et al. 2016), cod (Førde‐Skjærvik et al., 2006), carp fingerlings (Murai et al., 1989), juvenile yellowtail (Ogata, 2002), and juvenile red drum (Peachey et al., 2018).
3.2.3.2. Histamine concentration in animal tissues or products in relation to dietary histidine
As review by the BIOHAZ Panel (2011), the histamine concentration in raw meat of mammals and birds is considered lower compared to fish flesh. Histamine poisoning is also related to fermented food of animal origin. The threshold for adverse health effects (acute reference dose) is 50 mg histamine per consumption for healthy individuals, and below detectable limits for individuals with histamine intolerance.
The studies retrieved provide little data on the relation of histidine levels in feed and histamine levels in animal products/tissues.
In a study performed in rats (Lee et al. 1981), following histidine supplementation, the concentration of histamine in muscle increased (2‐fold) in the two higher dietary levels compared to the lower one, averaging 4.3 and 4.5 μg/kg vs. 2.5 μg/kg, respectively. Histamine level in muscles was higher (by 1 to 2 magnitude orders) than in other tissues (brain or kidney) at all dietary levels. However, differently from histidine, histamine level in muscle reached a plateau from the intermediate to highest dietary level.
One study reported increase histamine levels in reproductive organs and tissues of laying hens receiving histidine at levels ten times higher as needed for optimal egg production (Singh, 1980). On the contrary, another study reported decrease of histamine in chicken tissues when dietary histidine levels were increased (Ishibashi et al., 1979).
The gut flora may convert histidine to histamine (Golder et al., 2014). However, in ruminants, absorption of histamine was not increased following ruminal histamine increase; dietary supplementation with histidine had no impact on rumen histamine concentrations (Golder et al., 2012).
The histamine concentration in raw meat of mammals and birds is considered lower compared to fish flesh. Histamine poisoning is also related to fermented food of animal origin. The threshold for adverse health effects is 50 mg histamine per service for healthy individuals, and below detectable limits for individuals with histamine intolerance (BIOHAZ Panel, 2011).
The FEEDAP Panel considers that histamine food poisoning is mainly associated with the consumption of fish. Regarding fish species, none of the studies mentioned above analysed histamine content in edible tissues or products (Murai et al., 1989; Ogata, 2002; Breck et al., 2005; Førde‐Skjærvik et al., 2006; Waagbø et al., 2010; Remø et al., 2014; Gao et al. 2016; Peachey et al., 2018).
The scarce evidence seems to suggest that supplementation of fish with histidine increases histidine deposition in muscle. The data reviewed by FAO in 2013 indicate that the levels of histidine in salmonids are comparatively lower than in other wild sea fish species (FAO/WHO, 2013, 2018). Compared with scombroid fish which have free histidine levels ranging from approximately 5,000 mg/kg to 20,000 mg/kg, most species in the Salmonidae family have less than 1,000 mg/kg histidine (FAO/WHO, 2013). The Panel notes that it is unclear if salmonids exposed to dietary concentrations of histidine to prevent cataract were used in these studies. Thus, most members of the Salmonidae family have somewhere between 10 and 200 times less free histidine than scombroid fish. The FEEDAP Panel assumes than the levels of histidine reported in the FAO report would reflect histidine supplementation under current aquaculture conditions.
Although histidine is a precursor of histamine, the main factors influencing histamine formation in fish are storage time, temperature, pH, hygienic conditions (e.g. bacterial contamination) or starter cultures of fermented foods, which have been reviewed in previous publications (BIOHAZ Panel, 2011, FAO, 2013, 2018; Technical report EFSA, 2017).
As pointed out by FAO (2018), ‘the available evidence highlights that under appropriate time×temperature control, and within the sensory shelf‐life of the product, histamine development in Salmonidae to the levels that cause SFP is unlikely to occur’.
Considering the above, the FEEDAP Panel considers that supplementing the diets of salmonids with histidine to cover the requirements is unlikely to result in the increase of histamine formation provided that appropriate handling and storage of fish are ensured. Although there is no evidence from other aquaculture species, the Panel considers that the above conclusions can be extrapolated to other commonly farmed fish. For fish species associated with high levels of histidine in flesh,30 the Panel notes that supplemental histidine may increase histidine concentration in fish flesh and the possibility to have higher levels of histamine in fish flesh following improper storage. However, there are limits established for histamine to protect the consumer, in particular for Scombroid fish species.
In the absence of histamine poisoning records associated with raw mammal or poultry edible tissues and products, the FEEDAP Panel considers it unlikely that supplementation of feed with histidine to cover animal requirements will increase the risk of histamine poisoning upon consumption of such raw edible tissues and products from mammals and birds provided that appropriate handling and storage are ensured.
3.2.3.3. Conclusions on the safety for the consumer
l‐Histidine HCl monohydrate produced using E. coli (NITE BP‐02526) supplemented at levels appropriate for the requirements of the target species is considered safe for the consumer.
3.2.4. Safety for the user
3.2.4.1. Effects on respiratory system
A valid acute inhalation test in laboratory animals, performed according to OECD Guideline 403, showed an LC50 above 5.2 g/m3 . 31
Users can suffer from occupational respiratory disease depending on the level of endotoxins in air and dust (Rylander, 1999; Thorn, 2001). The bacterial endotoxin activity (the three new batches) was up to 14 to 34.9 IU/mg. The dusting potential ranges from 0.61 to 0.93 g/m3.
The scenario used to estimate the exposure of persons handling the additive to endotoxins in the dust, based on the EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012) is described in Appendix A. The health‐based recommended threshold for the quantity of inhaled endotoxins per working day is 900 IU, derived from provisional occupational exposure limits given by the Dutch Expert Committee on Occupational Safety (DECOS) (Health Council of the Netherlands, 2010) and the Health and Safety Executive (HSE, 2013). Based on the calculation of the potential endotoxin content in dust as described in Appendix A, the inhalation exposure could be up to 1,000 endotoxin IU per 8‐h working day, indicating a risk from the exposure to endotoxins for people handling the additive.
3.2.4.2. Effects on skin and eyes
The eye irritation potential of l‐histidine monohydrochloride monohydrate was tested in a valid study performed according to OECD guideline 438, which showed that it is not an eye irritant.32
The skin irritation potential of l‐histidine monohydrochloride monohydrate was tested in a valid study performed according to OECD guideline 439 (human skin model test), which showed that it is not a skin irritant.33
In a valid skin sensitisation study (local lymph‐node assay, LLNA) following OECD guideline 429, l‐histidine monohydrochloride monohydrate did not show any skin sensitisation potential.34
3.2.4.3. Conclusions on safety for the user
l‐Histidine monohydrochloride monohydrate produced by E. coli (NITE BP‐02526) is not irritant to skin or eyes, nor a skin sensitiser. There is a risk for persons handling the additive from the exposure to endotoxins by inhalation.
3.2.5. Safety for the environment
Regarding the production strain, none of the introduced modifications raise a safety concern. ■■■■■ The production strain and its DNA were not detected in the final product. Consequently, no safety concerns for the environment arise regarding the production strain.
l‐Histidine is a physiological and natural component of animals and plants. l‐Histidine present in the additive l‐Histidine monohydrochloride monohydrate will replace l‐histidine from natural sources which would be normally present in diet. l‐Histidine in the additive l‐histidine monohydrochloride monohydrate is absorbed and metabolised as well as l‐histidine present in natural sources. No increased excretion of l‐histidine is expected. Therefore, the addition of l‐histidine monohydrochloride monohydrate to feedingstuffs will not lead to an increase of l‐histidine levels in the excreta of animals and in the environment.
The FEEDAP Panel concludes that the use of the product l‐histidine monohydrochloride monohydrate produced by E. coli NITE BP‐02526 in animal nutrition would not pose a risk to the environment.
3.3. Efficacy
Efficacy studies are not required for amino acids naturally occurring in proteins of plants and animals. The nutritional role of the amino acid l‐histidine monochloride monohydrate is well established in the scientific literature.
In general, the product l‐histidine monochloride monohydrate is considered as an efficacious source of the amino acid l‐histidine for non‐ruminant animal species. For the supplemental l‐histidine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
Since l‐histidine [17.008] is used in food as a flavouring compound, and their function in feed is essentially the same as that in food no further demonstration of efficacy is necessary.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation35 and Good Manufacturing Practice.
4. Conclusions
The production strain and its recombinant DNA were not detected in the final products. l‐Histidine HCl monohydrate manufactured by fermentation using E. coli (NITE BP‐02526) does not give rise to any safety concern regarding the production strain and its genetic modification.
The use of l‐histidine monohydrochloride monohydrate produced by fermentation using E. coli (NITE BP‐02526) is safe for the target species when used as a nutritional additive to supplement the diet in appropriate amounts to cover the requirements, depending on the species, the physiological state of the animal, the performance level, the environmental conditions and the background amino acid composition of the unsupplemented diet and the status of some essential trace elements such as copper and zinc. This conclusion would also cover the use as a sensory additive.
l‐Histidine HCl monohydrate produced using E. coli NITE (BP‐02526) supplemented at levels appropriate for the requirements of target species is considered safe for the consumer.
l‐Histidine HCl monohydrate produced by E. coli NITE (BP‐02526) is not irritant to skin or eyes, nor a skin sensitiser. There is a risk for persons handling the additive from the exposure to endotoxins by inhalation.
The use of l‐histidine HCl monohydrate produced by E. coli NITE (BP‐02526) in animal nutrition is not expected to represent a risk to the environment.
l‐Histidine HCl monohydrate is considered an efficacious source of the essential amino acid l‐histidine for non‐ruminant animal species. For the supplemental l‐histidine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen. It is also considered efficacious as a feed flavouring compound under the proposed conditions of use.
Documentation provided to EFSA/Chronology
| Date | Event |
|---|---|
| 03/11/2018 | Dossier received by EFSA |
| 09/10/2018 | Reception mandate from the European Commission |
| 22/11/2018 | Application validated by EFSA – Start of the scientific assessment |
| 04/03/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation and safety for the consumer. |
| 22/02/2019 | Comments received from Member States |
| 22/03/2019 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 30/04/2019 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 19/06/2019 | Spontaneous submission of information by the applicant. Issues: safety for the consumer |
| 02/07/2019 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- bw
body weight
- CAS
Chemical Abstracts Service
- CFU
colony forming unit
- CP
crude protein
- CV
coefficient of variation
- DECOS
Dutch Expert Committee on Occupational Safety
- DL‐PCB
dioxin‐like polychlorinated biphenyl
- DM
dry matter
- EINECS
European Inventory of Existing Commercial Chemical Substances
- EURL
European Union Reference Laboratory
- FAO
Food Agricultural Organization
- FEEDAP
EFSA Panel on additives and products or substances used in Animal feed
- FLAVIS
The EU Flavour Information System
- GC–MS
gas chromatography–mass spectrometry
- HNMT
histamine‐N‐methyltransferase
- HSE
Health and Safety Executive
- IEC‐VIS/FLD
ion exchange chromatography coupled to visible or fluorescence detection
- IUPAC
International Union of Pure and Applied Chemistry
- LC50
lethal dose, 50%
- LLNA
local lymph‐node assay
- LOQ
limit of quantification
- MLST
multi‐locus sequence typing
- mTOR
mammalian target of rapamycin
- MW
molecular weight
- NAH
N‐acetyl histidine
- NITE
National Institute of Technology Evaluation
- OECD
Organisation for Economic Co‐operation and Development
- PCDD/F
polychlorinated dibenzo‐p‐dioxin and polychlorinated dibenzofuran
- RH
relative humidity
- RSDip
relative standard deviation for intermediate precision
- RSDr
relative standard deviation for repeatability
- SDBC
spray‐dried blood cells
- TEQ
toxic equivalent
- WGS
whole genome sequencing
- WHO
World Health Organization
Appendix A – Safety for the user
1.
The effects of the endotoxin inhalation and the exposure limits have been described in a previous opinion (EFSA FEEDAP Panel, 2015).
Calculation of maximum acceptable levels of exposure from feed additives
The likely exposure time according to EFSA guidance (EFSA FEEDAP Panel, 2012) for additives added in premixtures assumes a maximum of 40 periods of exposure per day, each comprising 20 s, equal to = 800 s/day. With an uncertainty factor of 2, maximum inhalation exposure would occur for 2 × 800 = 1,600 s (0.444 h/day). Again, assuming a respiration volume of 1.25 m3/h, the inhalation volume providing exposure to potentially endotoxin‐containing dust would be 0.444 × 1.25 = 0.556 m3 per day. This volume should contain no more than 900 IU endotoxin, so the dust formed from the product should contain no more than 900/0.556 = 1,619 IU/m 3.
Calculation of endotoxin content of dust
Two key measurements are required to evaluate the potential respiratory hazard associated with endotoxin content of the product (the dusting potential of the product, expressed in g/m3; the endotoxin activity of the dust, determined by the Limulus amoebocyte lysate assay (expressed in IU/g)). If data for the dust are not available, the content of endotoxins of the product can be used instead. If the content of endotoxins of the relevant additive is IU/g and the dusting potential is b g/m3, then the content of endotoxins of the dust, c IU/m3, is obtained by the simple multiplication a × b. This resulting value is further used for calculation of potential inhalatory exposure by users to endotoxin from the additive under assessment (Table A.1) (EFSA FEEDAP Panel, 2012).
Table A.1.
Estimation of user exposure to endotoxins from the additive l‐histidine produced by Escherichia coli K‐12 NITE BP‐02526 including consideration of using filter half mask (FF P2 or FF P3)36 as a preventative measure
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | Endotoxin content IU/g product | 34,900 | Technical dossier | |
| b | Dusting potential (g/m3) | 0.93 | Technical dossier | |
| a × b | c | Endotoxin content in the air (IU/m3) | 32,457 | |
| d | No of premixture batches made/working day | 40 | EFSA FEEDAP Panel (2012) | |
| e | Time of exposure (s)/production of one batch | 20 | EFSA FEEDAP Panel (2012) | |
| d × e | f | Total duration of daily exposure/worker (s) | 800 | |
| g | Uncertainty factor | 2 | EFSA FEEDAP Panel (2012) | |
| f × g | h | Refined total duration of daily exposure (s) | 1,600 | |
| h/3 600 | i | Refined total duration of daily exposure (h) | 0.44 | |
| j | Inhaled air (m3)/eight‐hour working day | 10 | EFSA FEEDAP Panel (2012) | |
| j/8 × i | k | Inhaled air during exposure (m3) | 0.56 | |
| c × k | l | Endotoxin inhaled (IU) during exposure/eight‐hour working day | 18,031 | |
| m | Health‐based recommended exposure limit of endotoxin (IU/m3)/eight‐hour working day | 90 | Health Council of the Netherlands (2010) | |
| m × j | n | Health‐based recommended exposure limit of total endotoxin exposure (IU)/eight‐hour working day | 900 | |
| l/10 | Endotoxins inhaled (IU)/eight‐hour working day reduced by filter half mask FF P2 (reduction factor 10) | 1,803 | ||
| l/20 | Endotoxins inhaled (IU)/eight‐hour working day reduced by filter half mask FF P3 (reduction factor 20) | 902 |
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for l‐histidine monohydrochloride monohydrate produced by Escherichia coli (NITE BP‐02526)
1.
In the current application, authorisation is sought under Article 4(1) for l‐histidine monohydrochloride monohydrate using the bacteria strain NITE BP‐02526, under the category/functional groups 3(c) ‘nutritional additives’/’amino acids, their salts and analogues’ and 2(b) ‘sensory additives/flavouring compounds’ according to Annex I of Regulation (EC) No 1831/2003. Authorisation is sought for all animal species.
According to the Applicant, l‐histidine monohydrochloride monohydrate has a minimum purity (mass fraction) of 98%. As a nutritional feed additive, the amino acid is intended to be added directly into feedingstuffs or through premixtures and water for drinking. As sensory feed additive, l‐histidine monohydrochloride monohydrate is intended to be added into feedingstuffs and water for drinking through flavouring premixtures. However, the Applicant did not propose any minimum or maximum content of l‐histidine monohydrochloride monohydrate in feedingstuffs.
For the quantification of l‐histidine monohydrochloride monohydrate in the feed additive and premixtures, the Applicant submitted the ring‐trial validated method EN ISO 17180:2013 specifically designed for lysine, methionine and threonine in products containing more than 10% of amino acid. This standard method is based on ion exchange chromatography coupled to visible or fluorescence detection (IEC‐VIS/FLD). It does not distinguish between the salts of amino acids and cannot differentiate between enantiomers. The Applicant presented results from validation and verification studies demonstrating the extension of the scope of the above mentioned ISO method for the determination of l‐histidine monohydrochloride monohydrate in the feed additive and premixtures (containing more than 10% histidine). The following performance characteristics are reported: a relative standard deviation for repeatability (RSDr) ranging from 0.6 to 4.3%, a relative standard deviation for intermediate precision (RSDip) ranging from 1.1 to 4.8% and a recovery rate from 91 to 103%.
For the quantification of l‐histidine monohydrochloride monohydrate in feedingstuffs, the Applicant submitted the ring‐trial validated Community method (Commission Regulation (EC) No 152/2009) based on IEC coupled with photometric detection (VIS). The method, designed only for the analysis of amino acids in premixtures and feedingstuffs, does not distinguish between the salts and the amino acid enantiomers. This method was further ring‐trial validated by 23 laboratories, resulting in the EN ISO 13903:2005 method. The following performance characteristics were reported for the quantification of total histidine: RSDr ranging from 2.4 to 7.0% and RSDR ranging from 13 to 23%. In the frame of the stability studies, the Applicant presented experimental data obtained analysing the feed additive in water according to EN ISO 13903:2005 thus demonstrating its applicability for the determination of l‐histidine monohydrochloride monohydrate in water.
In the frame of this authorisation, the EURL recommends for official control (i) the ring‐trial validated method EN ISO 17180:2013 based on IEC‐VIS/FLD to quantify free l‐histidine monohydrochloride monohydrate in the feed additive and premixtures (containing more than 10% histidine); (ii) the ring‐trial validated Community method based on IEC‐VIS for the quantification of l‐histidine monohydrochloride monohydrate in premixtures and feedingstuffs; and (iii) the ring‐trial validated EN ISO 13903:2005 method based on IEC‐VIS for the quantification of l‐histidine monohydrochloride monohydrate in water.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005), as last amended by Regulation (EU) 2015/1761) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Glandorf B, Herman L, Maradona Prieto M, Saarela M, Tosti L, Anguita M, Galobart J, Holczknecht O, Manini P, Tarres‐Call J, Pettenati E and Pizzo F, 2019. Scientific Opinion on the safety and efficacy of l‐histidine monohydrochloride monohydrate produced by fermentation with Escherichia coli (NITE BP‐02526) for all animal species. EFSA Journal 2019;17(8):5785, 22 pp. 10.2903/j.efsa.2019.5785
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00782
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Maryline Kouba, Mojca Kos Durjava, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Acknowledgements: The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Adopted: 2 July 2019
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Ajinomoto Animal Nutrition Europe, Rue Guersant, 32 Paris (France).
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009, OJ L 181, 29.6.2013, p. 35.
Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. OJ L 97, 5.4.2006, pp. 1–528.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.
Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. OL L 152, 16.6.2009, p. 11.
FEED dossier reference: FAD‐2018‐0070.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep_fad-2018-0070_l-histidine.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II/Annex II_4.
Technical dossier/Section II/Annex II_5.
Technical dossier/Section II/Annex II_16. LOQ: arsenic 0.05 mg/kg; cadmium 0.005 mg/kg; chromium 0.10 mg/kg; copper 0.1 mg/kg; iron 1 mg/kg; lead 0.02 mg/kg; mercury 0.005 mg/kg; nickel 0.10 mg/kg; phosphorus 3.0 mg/kg; fluorine 20 mg/kg; melamine 0.05 mg/kg; (hydro)cyanic acid 1.5 mg/kg.
Technical dossier/Section II/Annex II_16. LOQ: organochlorine pesticides 0.005‐0.1 mg/kg; organophosphorus pesticides 0.01–0.03 mg/kg.
Technical dossier/Section II/Annex II_16. LOQ: aflatoxins 0.1‐0.2 µg/Kg, zearalenone 20 µg/Kg; deoxynivalenol 50 µg/Kg; ochratoxin µg/Kg; toxin T‐2, HT2 20 µg/Kg; fumonisins: 20 µg/kg.
■■■■■
■■■■■
■■■■■
Technical dossier/Section II/Annex Merck_HISHCl_15th_2013.
Technical dossier/Section II/Annex_32.
Technical dossier/Section II/Annex_6.
Technical dossier/Section II/Annex_53.
Technical dossier/Section II/Annex_55.
Technical dossier/Section II/Annex_57.
Technical dossier/Section II/Annex_II_61.
Technical dossier/Section II/Annex_An_II_55.
Technical dossier/Section II/Annex_An_II_57.
Technical dossier/Section II/Annex_III_1.
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ 22.12.2005, L 338/1 26 pp.
Technical dossier/Section II/Supplementary information/Annex_III.
According to the Regulation, Scombroid species are Scombridae, Clupeidae, Engraulidae, Coryfenidae, Pomatomidae and Scombresosidae.
Technical dossier/Section III/Annex_21.
Technical dossier/Section III/Annex_30.
Technical dossier/Section III/Annex_31.
Technical dossier/Section III/Annex_32.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
Filtering face piece or filtering half mask according to European standard EN 149. They are graded from 1 to 3 depending on their filtering capacity.
References
- Abe H, 2000. Role of Histidine‐Related Compounds as Intracellular Proton Buffering Constituents in Vertebrate Muscle. Biochemistry (Moscow), 65, 757–765. Translated from Biokhimiya, pp. 891–900 [PubMed] [Google Scholar]
- Ahrens FA, 1967. Histamine, lactic acid and hypertonicity as factors in the development of rumenitis in cattle. American Journal of Veterinary Research, 28, 1335–1342. [PubMed] [Google Scholar]
- Aoyama Y and Kato C, 2000. Suppressive effect of excess dietary histidine on the expression of hepatic metallothionein‐1 in rats. Bioscience Biotechnology Biochemistry, 64, 588–591. [DOI] [PubMed] [Google Scholar]
- Aoyama Y, Kato C and Sakakibara S, 2001. Expression of metallothionein in the liver and kidney of rats is influenced by excess dietary histidine. Comparative Biochemistry and Pathology Part C, 128, 339–347. [DOI] [PubMed] [Google Scholar]
- Asahi R, Tanaka K, Fujimi TJ, Kanzawa N and Nakajima S, 2016. Proline Decreases the Suppressive Effect of Histidine on Food Intake and Fat Accumulation. Journal of Nutritional Science and Vitaminology, 62, 277–280. 10.3177/jnsv.62.277 [DOI] [PubMed] [Google Scholar]
- Bequette BJ, Hanigan MD, Calder AG, Reynolds CK, Lobley GE and Macrae JC, 2000. Amino Acid Exchange by the Mammary Gland of Lactating Goats when Histidine Limits Milk Production. Journal of Dairy Science, 83, 765–775. [DOI] [PubMed] [Google Scholar]
- Breck O, Bjerkås E, Campbell P, Rhodes JD, Sanderson J and Waagbø R, 2005. Histidine nutrition and genotype affect cataract development in Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 28, 357–371. [DOI] [PubMed] [Google Scholar]
- Cant JP, Trout DR, Qiao F and McBride BW, 2001. Milk Composition Responses to Unilateral Arterial Infusion of Complete and Histidine‐Lacking Amino Acid Mixtures to the Mammary Glands of Cows. Journal of Dairy Science, 84, 1192–1200. [DOI] [PubMed] [Google Scholar]
- Čuperlović M, 1973. Intestinal loads of lysine and histidine and their concentration in systemic blood plasma of pigs after feeding. Research in Veterinary Science, 14, 398–400. [PubMed] [Google Scholar]
- Doelman J, Purdie NG, Osborne VR and Cant JP, 2008. Short Communication: The Effects of Histidine‐Supplemented Drinking Water on the Performance of Lactating Dairy Cows. Journal of Dairy Science, 91, 3998–4001. 10.3168/jds.2008-1131 [DOI] [PubMed] [Google Scholar]
- Edmonds MS and Baker DH, 1987. Comparative Effects of Individual Amino Acid Excesses When Added to a Corn‐Soybean Meal Diet: Effects on Growth and Dietary Choice in the Chick. Journal of Animal Science, 65, 699–705. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the safety and the bioavailability of product l‐Histidine monohydrochloride monohydrate for salmonids. EFSA Journal 2005; 3(4):195, 10 pp. 10.2903/j.efsa.2005.195 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazard), 2011. Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA Journal 2011; 9(10):2393, 93 pp. 10.2903/j.efsa.2011.2393 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2010. Scientific opinion on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12):1956, 9 pp. 10.2903/j.efsa.2010.1956. Available online: http://www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015. Scientific Opinion on the safety and efficacy ofL‐tryptophan produced byEscherichia coliCGMCC 7.59 for all animalspecies based on a dossier submitted by HELM AG on behalf of Meihua Holdings Co. Ltd. EFSA Journal 2015;13(2):4015, 17 pp. 10.2903/j.efsa.2015.4015 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on additives and products or substances used in animal feed), 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on additives and products or substances used in Animal Feed), 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Products or Substances used in Animal Feed), 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2018. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2019 Scientifc Opinion on the safety and efficacy of l‐histidine monohydrochloride monohydrate produced using Corynebacterium glutamicum KCCM 80172 for all animal species. EFSA Journal 2019;17(7):5783, 256 pp. 10.2903/j.efsa.2019.5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization), 2013. Joint FAO/WHO Expert Meeting on the Public Health Risks of Histamine and Other Biogenic Amines from Fish and Fishery Products. Meeting Report.
- FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization), 2018. Histamine in Salmonids. ISBN 978‐92‐5‐130888‐2 (FAO), ISBN 978‐92‐4‐151443‐9 (WHO)
- Figueroa JL, Lewis AJ, Miller PS, Fischer RL and Diedrichsen RM, 2003. Growth, Carcass Traits, and Plasma Amino Acid Concentrations of Gilts Fed Low‐Protein Diets Supplemented with Amino Acids Including Histidine, Isoleucine, and Valine. Journal of Animal Science, 81, 1529–1537. 10.2527/2003.8161529x [DOI] [PubMed] [Google Scholar]
- Førde‐Skjærvik O, Skjærvik O, Mørkøre T, Thomassen MS and Rørvik K‐ A, 2006. Dietary influence on quality of farmed Atlantic cod (Gadus morhua): Effect on glycolysis and buffering capacity in white muscle. Aquaculture, 252, 409–420. [Google Scholar]
- Gao Hai‐na, Han Hu, Zheng Nan and Wang J‐Q, 2015. Leucine and Histidine Independently Regulate Milk Protein Synthesis in Bovine Mammary Epithelial Cells via MTOR Signaling Pathway. Journal of Zhejiang University. Science. B, 16, 560–572. 10.1631/jzus.B1400337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Liu YJ, Chen XQ, Yang HJ, Li XF and Tian LX, 2016. Effects of graded levels of histidine on growth performance, digested enzymes activities, erythrocyte osmotic fragility and hypoxia‐tolerance of juvenile grass carp Ctenopharyngodon idella. Aquaculture, 452, 388–394. [Google Scholar]
- Garlick P, 2004. The nature of human hazards associated with excessive intake of amino acids. Journal of Nutrition, 134, 1633S–1639S. [DOI] [PubMed] [Google Scholar]
- Glover C and Wood C, 2008. Absorption of copper and copper‐histidine complexes across the apical surface of freshwater rainbow trout intestine. Journal of Comparative Physiology B, 178, 101–109. [DOI] [PubMed] [Google Scholar]
- Golder HM, Celi P, Rabiee AR, Heuer C, Bramley E, Miller DW, King R, Lean IJ, 2012. Effects of grain, fructose, and histidine on ruminal pH and fermentation products during an induced subacute acidosis protocol. Journal of Dairy Science, 95, 1971–1982. [DOI] [PubMed] [Google Scholar]
- Golder HM, Denman SE, McSweeney C, Celi P and Lean IJ, 2014. Ruminal bacterial community shifts in grain‐, sugar‐, and histidine‐challenged dairy heifers. Journal of Dairy Science, 97, 5131–5150. 10.3168/jds.2014-8003 [DOI] [PubMed] [Google Scholar]
- Hadrová S, Krizova L, Richter M, Třináctý J and Dračková M, 2012. The Effect of Duodenal Infusion of Histidine on Milk Yield, Milk Composition, and Plasma Amino Acids in Dairy Cows. Journal of Animal and Feed Sciences, 21, 555–565. 10.22358/jafs/66129/2012 [DOI] [Google Scholar]
- Han Y, Koshio S, Ishikawa M and Yokoyama S, 2013. Interactive effects of dietary arginine and histidine on the performances of Japanese flounder Paralichthys olivaceus juveniles. Aquaculture, 414–415, 173–182. [Google Scholar]
- Harvey PW, Hunsaker HA and Allen KGD, 1981. Dietary l‐Histidine‐Induced Hypercholesterolemia and Hypocupremia in the Rat. The Journal of Nutrition, 111, 639–647. [DOI] [PubMed] [Google Scholar]
- Haug A, Rødbotten R, Mydland LT and Christophersen OA, 2008. Increased broiler muscle carnosine and anserine following histidine supplementation of commercial broiler feed concentrate. In: Acta Agriculturae Scandinavica, Section A – Animal Science, 58, 71–77.
- Health Council of the Netherlands , 2010. Endotoxins. Health‐based recommended occupational exposure limit. Publication no 2010/04OSH, 100 pp.
- Heger J, Patráš P, Nitrayová S, Dolešová P and Sommer A, 2007. Histidine maintenance requirement and efficiency of its utilization in young pigs. Archives of Animal Nutrition, 61, 179–188. [DOI] [PubMed] [Google Scholar]
- Hofherr M, 2010. The Effect Of Abomasal Infusion Of Histidine And Proline On Mil Composition And Mammary Amino Acid Utilization In High Producing Lactating Dairy Cows.
- HSE (Health and Safety Executive), 2013. Occupational hygiene implications of processing waste at materials recycling facilities (MRFs). RR977 Research Report, HSE, London, UK. 41 pp
- Huhtanen P, Vanhatalo A and Varvikko T, 2002. Effects of Abomasal Infusions of Histidine, Glucose, and Leucine on Milk Production and Plasma Metabolites of Dairy Cows Fed Grass Silage Diets. Journal of Dairy Science, 85, 204–216. [DOI] [PubMed] [Google Scholar]
- Hulata . 2015. ‘Aquaculture | Cutting Edge Science in Aquaculture 2015 | ScienceDirect.Com’. 2015. https://www.sciencedirect.com/journal/aquaculture/vol/467
- Ikezaki S, Nishikawa A, Furukawa F, Imazawa T, Enami T, Mitsui M and Takahashi M, 1994. ‘13‐week subchronic toxicity study of l‐histidine monohydrochloride in F344 rats’. Eisei Shikenjo Hokoku. Bulletin of National Institute of Hygienic Sciences,, 112, 57–63. [PubMed] [Google Scholar]
- Ikezaki S, Nishikawa A, Furukawa F, Enami T, Mitsui M, Tanakamaru Z, Kim HC, Lee IS, Imazawa T and Takahashi M, 1996. Long‐Term Toxicity/Carcinogenicity Study of l‐Histidine Monohydrochloride in F344 Rats. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 34, 687–691. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Donis O, Fitzpatrick D, Lee NS, Turetsky O and Fisher H, 1979. Effect of age and dietary histidine on histamine metabolism of the growing chick. Agents and Actions, 9, 435–444. 10.1007/bf01968107 [DOI] [PubMed] [Google Scholar]
- Izquierdo OA, Wedekind KJ and Baker DH, 1988. Histidine requirement of the young pig. Journal of Animal Science, 66, 2886–2892. [DOI] [PubMed] [Google Scholar]
- Jiang W‐D, Qu B, Feng L, Jiang J, Kuang S‐Y, Wu P, Tang L, Tang WN, Zhang YA, Zhou XQ, Liu Y, 2016. Histidine prevents Cu‐induced oxidative stress and the associated decreases in MRNA from encoding tight junction proteins in the intestine of grass carp (Ctenopharyngodon idella). PLoS ONE, 11, e0157001 10.1371/journal.pone.0157001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai S, Watanabe G, Kubota M, Kadowaki M and Fujimura S, 2015. Effect of dietary histidine on contents of carnosine and anserine in muscles of broilers. Animal Science Journal = Nihon Chikusan Gakkaiho, 86, 541–546. [DOI] [PubMed] [Google Scholar]
- Kasaoka S, Tsuboyama‐Kasaoka N, Kawahara Y, Inoue S, Tsuji M, Ezaki O, Kato H, Tsuchiya T, Okuda H and Nakajima S, 2004. Histidine Supplementation Suppresses Food Intake and Fat Accumulation in Rats. Nutrition (Burbank, Los Angeles County, Calif.), 20, 991–996. 10.1016/j.nut.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Khan M and Abidi S, 2014. Dietary histidine requirement of Singhi Heteropneustes fossilis fry (Bloch). Aquaculture, 45, 1341–1354. [Google Scholar]
- Kopeć W, Jamroz D, Wiliczkiewicz A, Biazik E, Pudlo A, Hikawczuk T, Skiba T and Korzeniowska M, 2013. Influence of Different Histidine Sources and Zinc Supplementation of Broiler Diets on Dipeptide Content and Antioxidant Status of Blood and Meat. British Poultry Science, 54, 454–465. 10.1080/00071668.2013.793295 [DOI] [PubMed] [Google Scholar]
- Kopec W, Wiliczkiewicz A, Jamroz D, Biazik E, Pudlo A, Hikawczuk T, Skiba T and Korzeniowska M, 2016. Antioxidant status of turkey breast meat and blood after feeding a diet enriched with histidine. Poultry Science, 95, 53–61. [DOI] [PubMed] [Google Scholar]
- Korhonen M, Vanhatalo A, Varvikko T and Huhtanen P, 2000. Responses to Graded Postruminal Doses of Histidine in Dairy Cows Fed Grass Silage Diets. Journal of Dairy Science, 83, 2596–2608. [DOI] [PubMed] [Google Scholar]
- Lee NS, Fitzpatrick D, Meier E and Fisher H, 1981. Influence of dietary histidine on tissue histamine concentration, histidine decarboxylase and histamine methyltransferase activity in the rat. Agents and Actions, 11, 307–311. [DOI] [PubMed] [Google Scholar]
- Li DF, Zhang JH and Gong LM, 2002. Optimum Ratio of Histidine in the Piglet Ideal Protein Model and its Effects on the Body Metabolism. Archiv für Tierernaehrung, 56, 199–212. [DOI] [PubMed] [Google Scholar]
- Murai T, Daozun W and Ogata H, 1989. Supplementation of methionine to soy flour diets for fingerling carp, Cyprinus carpio . Aquaculture, 77, 373–385. [Google Scholar]
- NRC (National Research Council), 2011. Nutrient requirements of fish and shrimp. The National Academies Press, Washington, DC, USA: p. 376. [Google Scholar]
- Ogata HY, 2002. Muscle buffering capacity of yellowtail fed diets supplemented with crystalline histidine. Journal of Fish Biology, 61, 1504–1512. [Google Scholar]
- Ohmura E, Aoyama Y and Yoshida A, 1986. Changes in lipids in liver and serum of rats fed a histidine‐excess diet or cholesterol‐supplemented diets. Lipids, 21, 748–753. [DOI] [PubMed] [Google Scholar]
- Ohmura E, Amano N, Aoyama Y and Yoshida A, 1992. The effect of a histidine‐excess diet on cholesterol synthesis and degradation in rats. Lipids, 27, 755–760. [DOI] [PubMed] [Google Scholar]
- Ookuma K, Yoshimatsu H, Sakata T, Fujimoto K and Fukagawa F, 1989. Hypothalamic Sites of Neuronal Histamine Action on Food Intake by Rats. Brain Research, 490, 268–275. [DOI] [PubMed] [Google Scholar]
- Peachey BL, Scott EM and Gatlin DM, 2018. Dietary histidine requirement and physiological effects of dietary histidine deficiency in juvenile red drum Sciaenop ocellatus . Aquaculture, 483, 244–251. [Google Scholar]
- Remø SC, Olsvik PA, Torstensen BE, Amlund H, Breck O and Waagbø R, 2011. Susceptibility of Atlantic Salmon Lenses to Hydrogen Peroxide Oxidation Ex Vivo after Being Fed Diets with Vegetable Oil and Methylmercury. Experimental Eye Research, 92, 414–424. 10.1016/j.exer.2011.02.018 [DOI] [PubMed] [Google Scholar]
- Remø SC, Hevrøy EM, Olsvik PA, Fontanillas R, Breck O and Waagbø R, 2014. Dietary Histidine Requirement to Reduce the Risk and Severity of Cataracts Is Higher than the Requirement for Growth in Atlantic Salmon Smolts, Independently of the Dietary Lipid Source. The British Journal of Nutrition, 111, 1759–1772. 10.1017/S0007114513004418 [DOI] [PubMed] [Google Scholar]
- Robinson PH, Swanepoel N and Evans E, 2010. Effects of feeding a ruminally protected lysine product, with or without isoleucine, valine and histidine, to lactating dairy cows on their productive performance and plasma amino acid profiles. Animal Feed Science and Technology, 161, 75–84. [Google Scholar]
- Rylander R, 1999. Health effects among workers in sewage treatment plants. Occupational and Environmental Medicine, 56, 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof A, Gabel M, Voigt J, Schönhusen U and Kluth H, 2000. Investigations on the Influence of Duodenal Histidine Infusion on Nitrogen and Amino Acid Turnover of Growing German Holstein Bulls. Archiv Fur Tierernahrung, 53, 303–321. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Lampart C and Rose C, 1972. Histamine Formation in Rat Brain in Vivo: Effects of Histidine Loads. Journal of Neurochemistry, 19, 801–810. [DOI] [PubMed] [Google Scholar]
- Sheiner JB, Morris P and Anderson GH, 1985. Food Intake Suppression by Histidine. Pharmacology, Biochemistry, and Behavior, 23, 721–726. [DOI] [PubMed] [Google Scholar]
- Singh TJ, 1980. Effect of histidine on egg production in White Leghorn layers. Indian Journal of Poultry Science, 15, 222–226. [Google Scholar]
- Solomon JK and Geison RL, 1978. Effect of Excess Dietary l‐Histidine on Plasma Cholesterol Levels in Weanling Rats. The Journal of Nutrition, 108, 936–943. [DOI] [PubMed] [Google Scholar]
- Thorn J, 2001. The inflammatory response in humans after inhalation of bacterial endotoxin: a review. Inflammatory Response, 50, 254–261. [DOI] [PubMed] [Google Scholar]
- Trösse C, Waagbø R, Breck O, Stavrum A‐K, Petersen K and Olsvik PA, 2009. Genome‐wide transcription analysis of histidine‐related cataract in Atlantic salmon (Salmo salar L). Molecular Vision, 15, 1332–1350. [PMC free article] [PubMed] [Google Scholar]
- Van Wouwe JP, Hoogenkamp S and Van den Hamer CJ, 1990. A Histidine Supplement and Regulation of the Zinc Status in Swiss Random Mice. Biological Trace Element Research, 24, 207–216. [DOI] [PubMed] [Google Scholar]
- VKM , 2016. Report from the Norwegian Scientific Committee for Food Safety (VKM). Risk assessment of “other substances” – L‐histidine.
- Waagbø R, Trösse C, Koppe W, Fontanillas R and Breck O, 2010. Dietary Histidine Supplementation Prevents Cataract Development in Adult Atlantic Salmon, Salmo salar L., in Seawater. The British Journal of Nutrition, 104, 1460–1470. 10.1017/S0007114510002485 [DOI] [PubMed] [Google Scholar]
- Wensink J and Van den Hamer CJ, 1988. Effect of Excess Dietary Histidine on Rate of Turnover of 65Zn in Brain of Rat. Biological Trace Element Research, 16, 137–150. 10.1007/BF02797098 [DOI] [PubMed] [Google Scholar]
- Wilson RP, Poe WE and Robinson EH, 1980. Leucine, Isoleucine, Valine and Histidine Requirements of Fingerling Channel Catfish. The Journal of Nutrition, 110, 627–633. [DOI] [PubMed] [Google Scholar]
- Xu H, Sakakibara S, Morifuji M, Salamatulla Q and Aoyama Y, 2003. Excess of dietary histidine decreases the liver copper level and serum alanine aminotransferase activity in Long‐Evans Cinnamon rats. British Journal of Nutrition, 90, 573–579. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu H, Chiba S, Tajima D, Akehi Y and Sakata T, 2002. Histidine Suppresses Food Intake through Its Conversion into Neuronal Histamine. Experimental Biology and Medicine (Maywood, N.J.), 227, 63–68. [DOI] [PubMed] [Google Scholar]


