Abstract
In July 2019, the European Commission asked EFSA to provide a statement on the available outcomes of the human health assessment in the context of the pesticides peer review for the renewal of approval of the active substance chlorpyrifos‐methyl conducted in accordance with Commission Implementing Regulation (EC) No 844/2012. The current statement contains a summary of the main findings of the assessment related to human health following the pesticides peer review expert discussions in mammalian toxicology held between 1 and 5 April 2019, as well as EFSA's additional considerations, including whether the active substance can be expected to meet the approval criteria applicable to human health as laid down in Article 4 of Regulation (EC) No 1107/2009. The identified concerns are presented as follows.
Keywords: chlorpyrifos‐methyl, pesticide, insecticide, peer review, human health assessment
Short abstract
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5809/full
Summary
Chlorpyrifos‐methyl is an active substance covered by the third batch of the renewal programme for pesticides (‘AIR3’) in accordance with Commission Implementing Regulation (EU) No 844/2012.
Applications (June 2013) and supplementary dossiers (July 2015) for the renewal of approval of the active substance chlorpyrifos‐methyl were submitted by Dow AgroSciences and by Sapec Agro SA.
An initial evaluation of the dossiers was provided by the rapporteur Member State (RMS) Spain in the Renewal Assessment Report (RAR) which was submitted to European Food Safety Authority (EFSA) in July 2017. Subsequently, EFSA initiated a peer review of the pesticides risk assessment on the RMS evaluation in line with the provisions of Commission Implementing Regulation (EU) No 844/2012.
The commenting period was completed and included a public consultation on the RAR. Following evaluation of the comments received as well as the additional information provided by the applicants in response to a request in accordance with Article 13(3) of Regulation (EU) No 844/2012, a meeting of experts from EFSA and Member States, including relevant experts from the EFSA Panel on Plant Protection Products and their Residues (PPR Panel), took place to discuss certain elements related to mammalian toxicology.
In July 2019, prior to completion of the full peer review process, EFSA was mandated by the European Commission to provide a statement on the available outcomes of the human health assessment in the context of the peer review of chlorpyrifos‐methyl.
The present statement contains a summary of the main findings of the assessment related to mammalian toxicology and human health following the Pesticides Peer Review Expert discussions in mammalian toxicology held between 1 and 5 April 2019, including EFSA's additional considerations including whether the active substance can be expected to meet the approval criteria which are applicable to human health as laid down in Article 4 of Regulation (EC) No 1107/2009.
The hazard assessment of chlorpyrifos‐methyl was discussed in the Pesticides Peer Review Experts’ meeting held between 1 and 5 April 2019 and the approach taken by the experts was largely based on the structural similarity with chlorpyrifos.
The available genotoxicity data set submitted for chlorpyrifos‐methyl did not show any concern. However, the experts considered that it lacked the additional relevant information retrieved e.g. from the literature for chlorpyrifos. Therefore, the experts concluded that the genotoxicity potential of chlorpyrifos‐methyl remains as unclear as that of chlorpyrifos.
As for the developmental neurotoxicity (DNT), a DNT study was available, which did not show relevant effects, however, it had some significant limitations related to the controls, making a reliable statistical analysis impossible. Therefore, all the experts, but one, agreed that, the DNT study on chlorpyrifos‐methyl being inconclusive, a specific DNT no observed adverse effect level (NOAEL) could not be set and the lowest observable adverse effect level (LOAEL) of 0.3 mg/kg body weight (bw) per day derived from the data on chlorpyrifos (study from 1998; Spain, 2019b) could be conservatively applied to chlorpyrifos‐methyl.
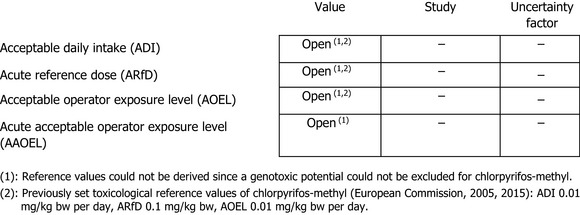
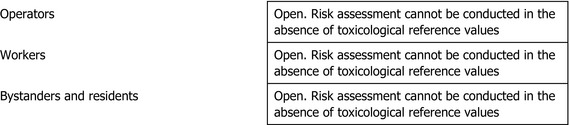
Based on the above, also in the case of chlorpyrifos‐methyl, the experts agreed that no reference values could be set, a fact that made it impossible to perform a risk assessment for consumers, operators, workers, bystanders and residents.
The experts conservatively applied the same approach as for chlorpyrifos, considering that chlorpyrifos‐methyl would also meet the criteria for classification as toxic for reproduction category 1B (regarding developmental toxicity).
Based on the above, it is considered that the approval criteria which are applicable to human health as laid down in Article 4 of Regulation (EC) No 1107/2009, are not met. It is noted that, after the Pesticides Peer Review Experts’ meeting held between 1 and 5 April 2019, EFSA reconsidered the read‐across approach applied for the hazard identification after a full comparison of the available toxicological data: it was agreed to rediscuss this issue in an experts’ meeting. The outcome of the discussions might impact on the assessment of the specific studies, on the possibility to identify a classification as well as on the setting of reference values for chlorpyrifos‐methyl.
1. Introduction
Chlorpyrifos‐methyl is an active substance covered by the third batch of the renewal programme for pesticides (‘AIR3’) in accordance with Commission Implementing Regulation (EU) No 844/20121.
Applications (June 2013) and supplementary dossiers (July 2015) for the renewal of approval of the active substance chlorpyrifos‐methyl were submitted by Dow AgroSciences and by Sapec Agro SA. The rapporteur Member State (RMS) is Spain and the co‐rapporteur Member State (co‐RMS) is Poland.
An initial evaluation of the dossiers was provided by the RMS in the Renewal Assessment Report (RAR) which was submitted to European Food Safety Authority (EFSA) on 3 July 2017 (Spain, 2017). On 18 October 2017, EFSA initiated a peer review of the pesticides risk assessment on the RMS evaluation, by dispatching the RAR to the Member States and applicants for consultation and comments in line with the provisions of Commission Implementing Regulation (EU) No 844/2012. In addition, a public consultation was also conducted.
After the completion of the commenting period, and following a comment evaluation phase, on 4 July 2018, EFSA requested the applicants to provide certain additional information related to all areas of the assessment including mammalian toxicology in accordance with Article 13(3) of Regulation (EU) No 844/2012, which was evaluated by the RMS and presented in an updated RAR (Spain, 2019a). Subsequently, in April 2019, a meeting of experts from EFSA and Member States, including relevant experts from the EFSA PPR Panel, took place to discuss certain elements related to mammalian toxicology.
By means of the mandate received on 1 July 2019 from the European Commission, prior to completion of the full peer review process, EFSA was requested to provide a statement with an overview of the available outcomes of the human health assessment in the context of the peer review of chlorpyrifos‐methyl.
The present document is an EFSA statement containing a summary of the outcome of the expert consultation outlining the main findings of the assessment related to mammalian toxicology and human health following the pesticides peer review expert discussions in mammalian toxicology held in April 2019, including EFSA's additional considerations and an indication whether the active substance can be expected to meet the approval criteria which are applicable to human health as laid down in Article 4 of Regulation (EC) No 1107/20092.
The list of endpoints for the active substance and the representative formulations assessed in the context of the peer review with regard to the impact on human health is available in Appendix A.
1.1. Background and Terms of Reference as provided by the requestor
On 1 July 2019 EFSA was mandated by the European Commission to provide a statement with an overview on the available outcomes of the human health assessment in the context of the pesticides peer review for the renewal of approval of the active substance chlorpyrifos‐methyl conducted in accordance with Commission Implementing Regulation (EU) No 844/2012.
In addition, EFSA was requested to indicate, whether the active substance chlorpyrifos‐methyl can be expected to meet the approval criteria which are applicable to human health as laid down in Article 4 of Regulation (EC) No 1107/2009.
2. Assessment
2.1. Mammalian toxicity
The toxicological profile of the active substance chlorpyrifos‐methyl was discussed at the Pesticides Peer Review Experts’ Meeting 01 in April 2019 and assessed based on the following guidance documents: SANCO/10597/2003‐rev. 10.1 (European Commission, 2012), Guidance on dermal absorption (EFSA PPR Panel, 2012), ECHA/EFSA Guidance for the identification of endocrine disruptors (ECHA/EFSA, 2018) and Guidance on the application of the classification, labelling and packaging (CLP) Criteria (ECHA, 2017).
The hazard assessment of chlorpyrifos‐methyl discussed in the peer review experts’ meeting was largely based on the structural similarity with chlorpyrifos. It is noted that, after the Pesticides Peer Review Experts’ meeting in April 2019, EFSA reconsidered the read‐across approach applied for the hazard identification after a full comparison of the available toxicological data: it was agreed to rediscuss this issue in an experts’ meeting, that will take place in September 2019: the outcome of the discussion might impact on the conclusions reached during the April 2019 peer review meeting, as well as on the setting of reference values for chlorpyrifos‐methyl.
Regarding the technical specifications of the substance placed on the market by either of the two applicants, they are not supported by the toxicological assessment since most impurities were not tested at the levels in the technical specification. However, regarding the toxicological relevance of the impurities, considering the toxicological profile of chlorpyrifos‐methyl, as discussed in the April 2019 peer review meeting, it is not expected that the impurities present in the technical specification would have the potential to add additional hazard established for the parent. Two impurities (sulfotemp and sulfotemp ester) have been considered as toxicologically relevant by the European Commission (European Commission, 2012) who established a maximum level of 5 g/kg. Therefore, their maximum levels in the newly proposed technical specification of 5 and 3 g/kg, respectively, are in agreement with these requirements. The analytical methods used in the toxicological studies were not available for most of the studies, representing a concern in particular for the genotoxicity assessment (based on regulatory studies) but not for the critical findings which were retrieved for chlorpyrifos from the published literature (such as the Columbia Center for Children's Environmental Health (CCCEH) study).
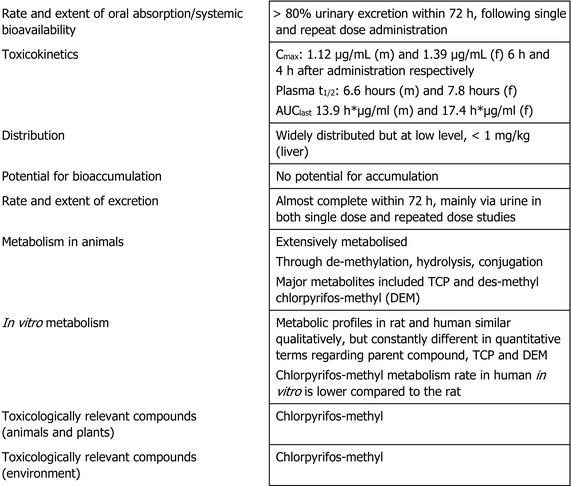
In rats, chlorpyrifos‐methyl is extensively absorbed after oral administration, it is widely distributed, extensively metabolised through de‐methylation, hydrolysis and conjugation, and eliminated mostly through urine within 72 h. An in vitro metabolism study indicates that the metabolic profiles in rat and human are qualitatively similar, but different in quantitative terms. Chlorpyrifos‐methyl metabolism rate in humans is lower compared to that of rats in vitro.
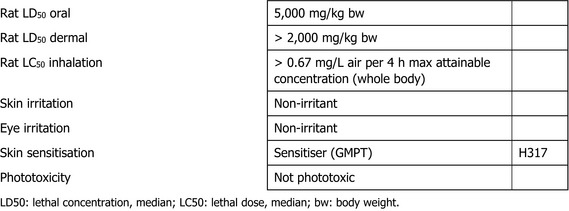
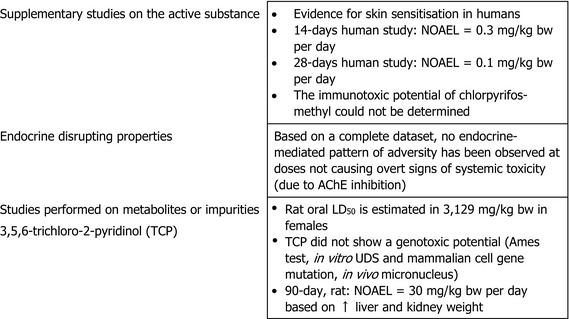
In the acute toxicity studies, chlorpyrifos‐methyl showed low toxicity when administered by the oral, dermal or inhalation routes. The substance did not elicit a potential for skin or eye irritation, or for phototoxicity, but was shown to be a skin sensitiser. Accordingly, chlorpyrifos‐methyl is classified according to the CLP criteria as Skin Sens 1, H317 ‘may cause an allergic skin reaction’, as established in Annex VI of Regulation (EC) No 1272/20083 regarding human health.
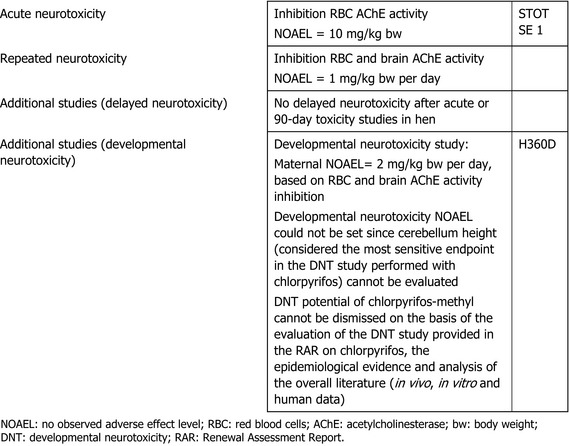
At the April 2019 Peer Review Experts’ meeting, the experts suggested4 that classification of chlorpyrifos‐methyl as acute neurotoxicant STOT SE 1, in accordance with the criteria set out in Regulation (EC) No 1272/2008, would be appropriate based on the available toxicological dataset.
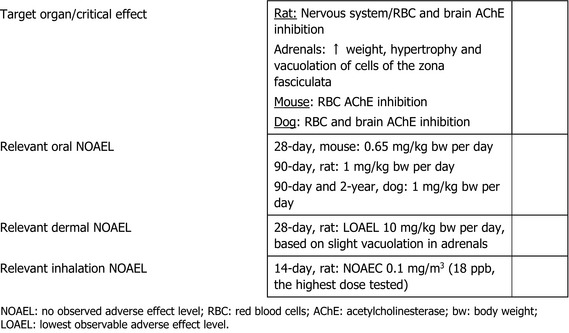
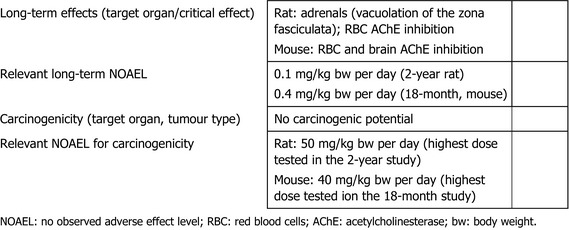
The main effect following short‐ to long‐term repeated oral administration of chlorpyrifos‐methyl was the inhibition of acetylcholinesterase (AChE) activity, which, at high‐dose levels, was leading to endogenous cholinergic overstimulation resulting in typical cholinergic symptoms. Erythrocyte (red blood cell (RBC)) AChE inhibition was the critical effect in all studies conducted with rats, mice and dogs. Additionally, the adrenals (increased weight, hypertrophy and vacuolation of cells of the zona fasciculata) were identified as target organ of chlorpyrifos‐methyl in rats. The relevant no observed adverse effect level (NOAEL) for short‐term toxicity was 0.65 mg/kg body weight (bw) per day from the 28‐day toxicity study in mice and 0.1 mg/kg bw per day for long‐term exposure from the 2‐year study in rats based on significant decrease of RBC AChE activity in both studies and adrenal toxicity upon long‐term exposure in rats only. No evidence for a carcinogenicity potential was found upon chlorpyrifos‐methyl administration in rats or mice.
No information has been provided on the immunotoxic potential of chlorpyrifos‐methyl, therefore a data gap was identified.
2.2. Genotoxicity
During the Pesticides Peer Review 01 Experts’ meeting, the experts discussed the in vitro and in vivo regulatory studies provided in the RAR:
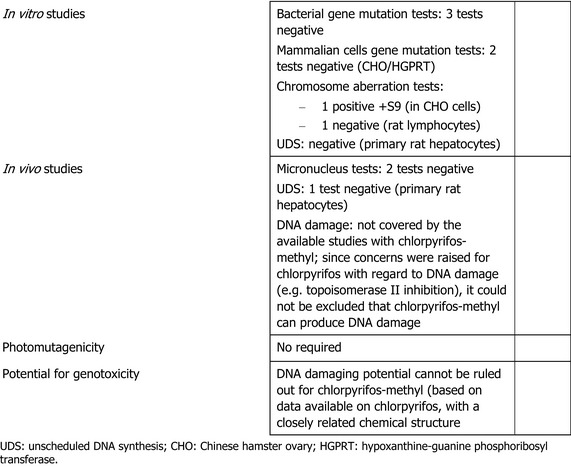
gene mutation: the experts considered that the results from the three bacterial and the two mammalian gene mutations assays overall showed that chlorpyrifos‐methyl does not induce gene mutations in vitro.
chromosome aberration in vitro: the results of two different assays were discussed and chlorpyrifos‐methyl was considered positive in the presence of rat liver metabolic activation system (S9) in Chinese hamster ovary (CHO) cells but negative in rat lymphocytes both in the absence and in the presence of S9.
unscheduled DNA synthesis (UDS): one in vitro study was submitted and produced negative results.
in vivo studies in somatic cells (mouse bone marrow micronucleus test): the two studies available in the dossier and evaluated in the RAR showed negative findings.
in vivo rat liver DNA repair test (UDS): chlorpyrifos‐methyl did not damage DNA in rat liver.
The regulatory data package showed positive findings just in one in vitro chromosome aberration study in CHO cells in the presence of S9. Overall, the data package did not show any concern and the experts discussed whether DNA damage was considered covered by the available studies. It was also noted that there is no public literature available for chlorpyrifos‐methyl with regard to the genotoxic potential, while several publications were available for chlorpyrifos instead. The experts discussed the structural similarity between chlorpyrifos and chlorpyrifos‐methyl and the similar toxicokinetics of the two molecules and agreed to read across between chlorpyrifos and chlorpyrifos‐methyl. Since concerns were raised for chlorpyrifos with regard to chromosome aberration, DNA damage (oxidative stress and topoisomerase II inhibition), the experts concluded that a data gap is present for chlorpyrifos‐methyl with regard to DNA damage. All the experts agreed that these uncertainties should be considered in the risk assessment of chlorpyrifos‐methyl as well, i.e. it cannot be excluded that chlorpyrifos‐methyl may have DNA damaging potential.
The database submitted for chlorpyrifos‐methyl did not show any specific concern; however, it lacked the additional relevant information retrieved e.g. from the literature. Therefore, the experts concluded that also the genotoxicity potential of chlorpyrifos‐methyl remains unclarified as that of chlorpyrifos. It is noted however that, after the experts’ meeting, EFSA reconsidered the read‐across approach applied by the experts and topic will be reconsidered in an experts’ meeting in September 2019.
2.3. Reproductive/developmental toxicity and endocrine disruption
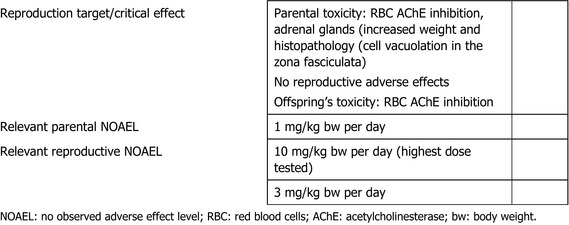
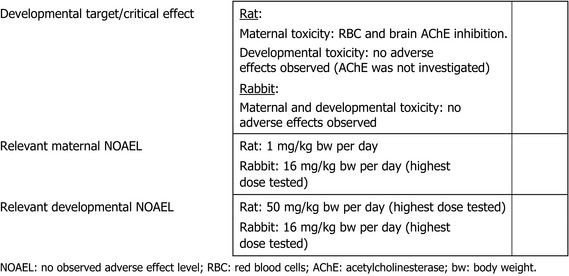
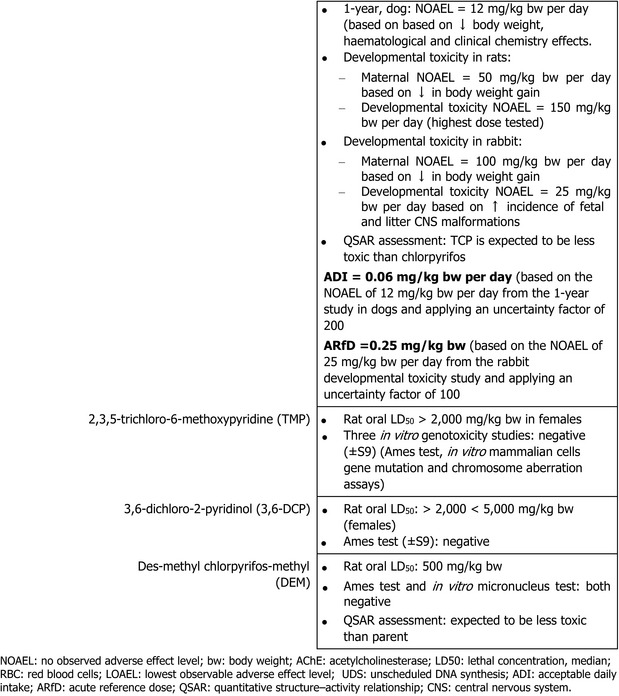
In a two‐generation reproductive toxicity study in rats, chlorpyrifos‐methyl did not affect the reproductive performance up to the highest dose of 10 mg/kg bw per day tested, while RBC AChE inhibition and adrenal toxicity were the critical effects related to parental toxicity with a NOAEL of 1 mg/kg bw per day; in this study, RBC AChE inhibition was the critical effect in pups with a NOAEL of 3 mg/kg bw per day. Developmental toxicity was investigated in rats and rabbits. Erythrocyte AChE and brain AChE inhibition was the critical effect identified regarding maternal toxicity in rats, while no adverse effect was observed in rabbit. No developmental adverse effects were observed in either rats or rabbits.
The availability of a multigenerational study conducted according to the most recent test guideline showed no evidence for endocrine‐mediated adversity at dose levels not producing signs of overt toxicity (AChE inhibition). On this basis, it was concluded that mechanistic studies are not required to assess the endocrine disruption potential of chlorpyrifos‐methyl following the guidance for identification of endocrine disruptors (ECHA/EFSA, 2018). On this basis, all experts agreed that chlorpyrifos‐methyl is not an endocrine disruptor in humans.
2.4. Developmental neurotoxicity (DNT)
During the Pesticides Peer Review 01 Experts’ meeting in April 2019, Member State experts and two experts from EFSA's Panel on Plant Protection Products and their Residues (PPR Panel), discussed the available data regarding developmental neurotoxicity (DNT) of chlorpyrifos‐methyl. They took into consideration and discussed in detail: (a) the DNT study in rats from 2015 (Spain, 2019a); (b) public literature presented in the systematic review provided by the applicants; (c) additional literature provided by the experts or during the commenting period.
In the DNT study in rats, pregnant rats were exposed to different levels of chlorpyrifos‐methyl (0, 2, 10 and 50 mg/kg bw per day) from day 6 of gestation until lactation day 21. The only effects observed were test substance‐related and statistically significant lower RBC AChE and brain AChE activity values compared to the control group in maternal generation at 10 and 50 mg/kg bw per day. Regarding offspring toxicity, pup growth, survival and clinical conditions were unaffected; according to the RMS, no test substance‐related effects were observed on body weights, body weight gains, attainment of developmental landmarks, detailed clinical observations, motor activity, auditory startle, learning and memory, macroscopic examinations and measurements, neuropathology or brain morphometry at any dietary concentration at any age. However, it should be noted that a significant decrease in the height of cerebral hemisphere on post‐natal day (PND) 72 was observed in males at the top dose. In addition, a statistically significant inhibition of RBC AChE was observed in males at 50 mg/kg bw per day on PND 21. At the experts’ meeting in April 2019, all the experts agreed to set a maternal NOAEL at 2 mg/kg bw per day based on decreased RBC AChE and brain AChE activity. The experts noted that, despite the study was performed according to current OECD 426 guideline (OECD, 2007), the cerebellum height in pups (considered the most sensitive endpoint in the DNT study performed with chlorpyrifos) could not be evaluated since just three control samples in females were available on PND 72. Therefore, considering the low statistical power, no reliable analysis could be performed, representing a major deviation from the study protocol. No changes in cerebellum height were reported for males and females at PND 21 and for males at PND 72, but the measurement was only available at the highest dose. In addition, it should be noted that cerebellum height was not corrected by brain weight and reanalysis of the data corrected for brain weight would be useful to compare also the results presented by Mie et al. (2018) in the case of chlorpyrifos, although recognising that statistical analysis could not be performed in the absence of sufficient control samples in females.
All the experts, but one, agreed that, the DNT study on chlorpyrifos‐methyl being inconclusive, a DNT NOAEL could not be set and the LOAEL of 0.3 mg/kg bw per day derived from the data on chlorpyrifos (study from 1998; Spain 2019b) could be conservatively applied to chlorpyrifos‐methyl. The overall approach will be reconsidered in the September 2019 experts’ meeting.
The experts discussed the epidemiological evidence showing associations between chlorpyrifos and chlorpyrifos‐methyl exposure during neurodevelopment and adverse health effects (attention deficit/hyperactivity disorders, decrease in intelligent quotient and working memory, etc.). In particular, three main birth cohort studies were considered: the Columbia Center for Children's Environmental Health (CCCEH) study (US EPA, 2016), the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) (Castorina et al., 2010; Marks et al., 2010) and Mt. Sinai study (Sebe et al., 2005). Using different biomarkers of exposure, these studies show that prenatal exposure to organophosphates (OPs) produces a consistent pattern of early cognitive and behavioural deficits (Rauh et al., 2012). The experts discussed also other epidemiological evidence from the public literature. The majority of the experts considered that the results from some of these studies (mainly from CCCEH study, Rauh et al., 2012; Engel et al., 2011; Silver et al., 2017) contribute to the evidence of DNT effects in humans due to the exposure to chlorpyrifos and chlorpyrifos‐methyl and occurring at doses lower than that causing 20% inhibition of AChE. Therefore, this would represent a concern to be taken into consideration for the risk assessment. In addition, it should be noted that in the CHAMACOS study measurement of trichloro‐pyridinol (TCP) in urine,5 common metabolite of both chlorpyrifos and chlorpyrifos‐methyl, contributed to the evidence of DNT effects in humans due to the exposure to chlorpyrifos and chlorpyrifos‐methyl.
Taking into consideration the DNT study outcome (reduction in cerebellum height for chlorpyrifos – that could not be explained by the maternal AChE inhibition), the epidemiological evidence showing an association between chlorpyrifos/chlorpyrifos‐methyl exposure during development and neurodevelopmental outcomes, and the overall analysis of the published literature (in vivo, in vitro and human data), the experts suggested6 that classification of chlorpyrifos‐methyl as toxic for the reproduction, REPRO 1B, H360D ‘May damage the unborn child’ in accordance with the criteria set out in Regulation (EC) No 1272/2008 would be appropriate based on the available toxicological dataset.
3. Conclusions
During the Pesticides Peer Review 01 Experts’ meeting in April 2019, all the experts, but one, agreed that the Point of Departure (PoD) for setting the reference values for chlorpyrifos‐methyl, in the absence of data on cerebellum height corrected by brain weight in the DNT study with chlorpyrifos‐methyl (2015; Spain, 2019a), should be, as a conservative assumption, the DNT LOAEL of 0.3 mg/kg bw per day from the DNT study on chlorpyrifos (1988; Spain, 2019b), based on the severity of the effects, until there is no evidence for the contrary. The subject will be rediscussed in an experts’ meeting in September 2019.
In the peer review meeting in April 2019, the experts concluded that:
the concerns raised for chlorpyrifos with regard to chromosome aberration and DNA damage (oxidative stress and topoisomerase II inhibition) may apply to chlorpyrifos‐methyl, resulting in an unclear genotoxicity potential
the DNT effects observed at the lowest dose tested in the DNT study with chlorpyrifos (decrease in cerebellum height corrected by brain weight), indicating a health concern, would be conservatively applied to chlorpyrifos‐methyl;
the epidemiological evidence supports the developmental neurological outcomes in children for both chlorpyrifos and chlorpyrifos methyl.
Overall, considering the unclear genotoxicity effects reported with chlorpyrifos and the bridging with chlorpyrifos‐methyl, the experts agreed that no toxicological reference values could be established for chlorpyrifos‐methyl. Furthermore, additional significant uncertainties were linked to the concerns identified in the DNT study with chlorpyrifos, which was considered applicable to chlorpyrifos‐methyl, supported by the available epidemiological evidence related to developmental neurological outcomes in children. Due to the lack of toxicological reference values, a risk assessment for consumers, operators, workers, bystanders and residents cannot be conducted. This issue represents a critical area of concern for chlorpyrifos‐methyl.
Based on the above and also considering the recorded toxicological effects meeting the criteria for classification as toxic for reproduction category 1B (regarding developmental toxicity), it is considered that the approval criteria which are applicable to human health as laid down in Article 4 of Regulation (EC) No 1107/2009, are not met. The hazard assessment of chlorpyrifos‐methyl discussed in the Pesticides Peer Review Experts’ meeting in April 2019 was largely based on the structural similarity with chlorpyrifos. It is noted that, after the experts’ meeting, EFSA reconsidered the read‐across approach applied for the hazard identification after a full comparison of the available toxicological data: it was agreed to rediscuss this issue in an experts’ meeting (that will take place in September 2019). The outcome of the discussions might impact on the current conclusions as well as on the setting of reference values for chlorpyrifos‐methyl.
Glossary and abbreviations
- AAOEL
acute acceptable operator exposure level
- AChE
acetylcholinesterase
- ADI
acceptable daily intake
- AOEL
acceptable operator exposure level
- ARfD
acute reference dose
- AUC
area under the blood concentration/time curve
- bw
body weight
- CaMKII
calcium/calmodulin‐dependent protein kinase type II
- CCCEH
Columbia Center for Children's Environmental Health
- CHAMACOS
Center for the Health Assessment of Mothers and Children of Salinas
- CHO
Chinese hamster ovary
- CLP
classification, labelling and packaging
- Cmax
concentration achieved at peak blood level
- CNS
central nervous system
- co‐RMS
co‐rapporteur Member State
- DNT
developmental neurotoxicity
- ECHA
European Chemicals Agency
- HGPRT
hypoxanthine‐guanine phosphoribosyl transferase
- LC50
lethal concentration, median
- LD50
lethal dose, median; dosis letalis media
- LOAEL
lowest observable adverse effect level
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- OP
organophosphate
- PND
post‐natal day
- PoD
point of departure
- ppb
parts‐per‐billion (10−9)
- PPR panel
EFSA's Panel on Plant Protection Products and their Residues
- QSAR
quantitative structure–activity relationship
- RAR
Renewal Assessment Report
- RBC
red blood cells
- RMS
rapporteur Member State
- S9
rat liver metabolic activation system
- t1/2
half‐life (define method of estimation)
- UDS
unscheduled DNA synthesis
- US EPA
United States Environmental Agency
Appendix A – List of endpoints for the active substance and the representative formulations with regard to impact on human health
1.
Impact on Human and Animal Health
Absorption, distribution, metabolism and excretion (toxicokinetics) (Regulation (EU) No 283/2013, Annex Part A, point 5.1)
Acute toxicity (Regulation (EU) No 283/2013, Annex Part A, point 5.2)
Short‐term toxicity (Regulation (EU) No 283/2013, Annex Part A, point 5.3)
Genotoxicity (Regulation (EU) No 283/2013, Annex Part A, point 5.4)
Long‐term toxicity and carcinogenicity (Regulation (EU) No 283/2013, Annex Part A, point 5.5)
Reproductive toxicity (Regulation (EU) No 283/2013, Annex Part A, point 5.6)
Reproduction toxicity
Developmental toxicity
Neurotoxicity (Regulation (EU) No 283/2013, Annex Part A, point 5.7)
Other toxicological studies (Regulation (EU) No 283/2013, Annex Part A, point 5.8)
Medical data (Regulation (EU) No 283/2013, Annex Part A, point 5.9)

Summary
7
(Regulation (EU) No 1107/2009, Annex II, point 3.1 and 3.6)
Dermal absorption (Regulation (EU) No 284/2013, Annex Part A, point 7.3)
Exposure scenarios (Regulation (EU) No 284/2013, Annex Part A, point 7.2)
Classification with regard to toxicological data (Regulation (EU) No 283/2013, Annex Part A, Section 10)

Appendix B – Used compound codes
1.
| Code/trivial name | IUPAC name/SMILES notation/InChIKeya | Structural formulab |
|---|---|---|
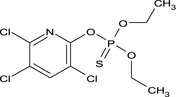
| chlorpyrifos |
O,O‐diethyl O‐3,5,6‐trichloro‐2‐pyridyl phosphorothioate Clc1cc(Cl)c(Cl)nc1OP(=S)(OCC)OCC SBPBAQFWLVIOKP‐UHFFFAOYSA‐N |
|
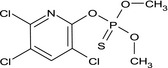
| chlorpyrifos‐methyl |
O,O‐dimethyl O‐3,5,6‐trichloro‐2‐pyridyl phosphorothioate Clc1cc(Cl)c(Cl)nc1OP(=S)(OC)OC HRBKVYFZANMGRE‐UHFFFAOYSA‐N |
|
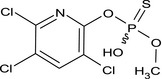
| des‐methyl chlorpyrifos‐methyl (DEM) |
O‐methyl O‐(3,5,6‐trichloro‐2‐pyridyl) hydrogen phosphorothioate Clc1cc(Cl)c(Cl)nc1OP(O)(=S)OC DYESOQMZDNCQNZ‐UHFFFAOYSA‐N |
|
| sulfotemp |
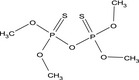
O,O,O′,O′‐tetramethyl dithiopyrophosphate COP(=S)(OC)OP(=S)(OC)OC XKBNJDRCYDBEAH‐UHFFFAOYSA‐N |
|
| sulfotemp ester |
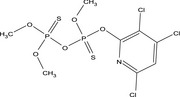
O,O,O′‐trimethyl O′‐(3,4,6‐trichloro‐2‐pyridyl) dithiopyrophosphate Clc1c(OP(=S)(OC)OP(=S)(OC)OC)nc(Cl)cc1Cl WDHGBTACZJLMHA‐UHFFFAOYSA‐N |
|
| TCP |
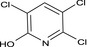
3,5,6‐trichloro‐2‐pyridinol Clc1cc(Cl)c(Cl)nc1O WCYYAQFQZQEUEN‐UHFFFAOYSA‐N |
|
| TMP |
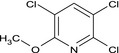
2,3,5‐trichloro‐6‐methoxypyridine Clc1cc(Cl)c(Cl)nc1OC RLIVUWLXZBDMBL‐UHFFFAOYSA‐N |
|
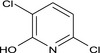
| 3,6‐DCP |
3,6‐dichloro‐2‐pyridinol Oc1nc(Cl)ccc1Cl UGPDKBDRRLFGFD‐UHFFFAOYSA‐N |
|
ACD/Name 2018.2.2 ACD/Labs 2018 Release (File version N50E41, Build 103230, 21 July 2018).
ACD/ChemSketch 2018.2.2 ACD/Labs 2018 Release (File version C60H41, Build 106041, 07 December 2018).
Suggested citation: EFSA (European Food Safety Authority) , 2019. Statement on the available outcomes of the human health assessment in the context of the pesticides peer review of the active substance chlorpyrifos‐methyl. EFSA Journal 2019;17(8):5810, 18 pp. 10.2903/j.efsa.2019.5810
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00415
Acknowledgements: EFSA wishes to thank the following for the support provided to this scientific output: Antonio Hernandez and Susanne Hougaard Bennekou.
Approved: 31 July 2019
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5809/full
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
It should be noted that classification is formally proposed and decided in accordance with Regulation (EC) No 1272/2008.
Post‐meeting note: it is also possible that a significant portion of TCP present in urine samples can result from direct intake of TCP preformed in the environment and not as a result of chlorpyrifos or chlorpyrifos‐methyl ingestion (Eaton et al., 2008).
It should be noted that classification is formally proposed and decided in accordance with Regulation (EC) No 1272/2008.
For metabolites, refer to section: Studies performed on metabolites or impurities.
References
- Castorina R, Bradman A, Fenster L, Barr DB, Bravo R, Vedar MG, Harnly ME, McKone TE, Eisen EA and Eskenazi B, 2010. Comparison of current‐use pesticide and other toxicant urinary metabolite levels among pregnant women in the CHAMACOS cohort and NHANES. Environmental Health Perspectives, 118, 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte‐Hermann R and Spencer PS, 2008. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Critical Reviews in Toxicology, 38(Suppl 2), 1–125. [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency), 2017. Guidance on the Application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0, July 2017. Reference: ECHA‐17‐G‐21‐EN; ISBN: 978‐92‐9020‐050‐5. Available online: https://echa.europa.eu/guidance-documents/guidance-on-clp
- ECHA (European Chemicals Agency) and EFSA (European Food Safety Authority) with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311 ECHA‐18‐G‐01‐EN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL and Wolff MS, 2011. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environmental Health Perspectives, 119, 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2005. Review report for the active substance chlorpyrifos‐methyl finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 3 June 2005 in view of the inclusion of chlorpyrifos‐methyl in Annex I of Directive 91/414/EEC. SANCO/3061/99 – rev. 1.6, 3 June 2005.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2015. Review report for the active substance chlorpyrifos‐methyl finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 3 June 2005 in view of the inclusion of chlorpyrifos‐methyl in Annex I of Directive 91/414/EEC. SANCO/3061/99 – rev. 2, 20 March 2015.
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N and Eskenazi B, 2010. Organophosphate pesticide exposure and attention in young Mexican‐American children: the CHAMACOS Study. Environmental Health Perspectives, 118, 1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mie A, Rudén C and Grandjean P, 2018. Safety of safety evaluation of pesticides: developmental neurotoxicity of chlorpyrifos and chlorpyrifos‐methyl. Environmental Health, 17, 77 10.1186/s12940-018-0421-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2007. Test No. 426: Developmental Neurotoxicity Study, OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris: 10.1787/9789264067394-en [DOI] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Ravi Bansal R, Hao X, Liu J, Barr DB, Slotkin TA and Peterson BS, 2012. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences of the United States of America, 109, 7871–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe A, Satar S, Alpay R, Kozaci N and Hilal A, 2005. Organophosphate poisoning associated with fetal death: a case study. The Mount Sinai Journal of Medicine, 72, 354–356. [PubMed] [Google Scholar]
- Silver MK, Shao J, Zhu B, Chen M, Xia Y, Kaciroti N, Lozoff B and Meeker JD, 2017. Prenatal naled and chlorpyrifos exposure is associated with deficits in infant motor function in a cohort of Chinese infants. Environment International, 106, 248–256. 10.1016/j.envint.2017.05.015 [Epub 2017 Jun 8]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain , 2017. Renewal Assessment Report (RAR) on the active substance chlorpyrifos‐methyl prepared by the rapporteur Member State Spain in the framework of Commission Implementing Regulation (EU) No 844/2012, April 2017. Available online: http://www.efsa.europa.eu
- Spain , 2019a. Revised Renewal Assessment Report (RAR) on the active substance chlorpyrifos‐methyl, volumes relevant for mammalian toxicology, prepared by the rapporteur Member State Spain in the framework of Commission Implementing Regulation (EU) No 844/2012, February 2019.
- Spain , 2019b. Revised Renewal Assessment Report (RAR) on the active substance chlorpyrifos, volumes relevant for mammalian toxicology, prepared by the rapporteur Member State Spain in the framework of Commission Implementing Regulation (EU) No 844/2012, February 2019.
- US EPA (United States Environmental Protection Agency), 2016. US EPA, Office of Chemical Safety and Pollution prevention. Memorandum on Chlorpyrifos: Revised Human Health Risk Assessment for Registration Review. November 3, 2016


