Abstract
In 2016, the EFSA Panel on Contaminants in the Food Chain (CONTAM) published a scientific opinion on the acute health risks related to the presence of cyanogenic glycosides (CNGs) in raw apricot kernels in which an acute reference dose (ARfD) of 20 μg/kg body weight (bw) was established for cyanide (CN). In the present opinion, the CONTAM Panel concluded that this ARfD is applicable for acute effects of CN regardless the dietary source. To account for differences in cyanide bioavailability after ingestion of certain food items, specific factors were used. Estimated mean acute dietary exposures to cyanide from foods containing CNGs did not exceed the ARfD in any age group. At the 95th percentile, the ARfD was exceeded up to about 2.5‐fold in some surveys for children and adolescent age groups. The main contributors to exposures were biscuits, juice or nectar and pastries and cakes that could potentially contain CNGs. Taking into account the conservatism in the exposure assessment and in derivation of the ARfD, it is unlikely that this estimated exceedance would result in adverse effects. The limited data from animal and human studies do not allow the derivation of a chronic health‐based guidance value (HBGV) for cyanide, and thus, chronic risks could not be assessed.
Keywords: cyanide, cyanogenic glycosides, health‐based guidance values, risk assessment
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1601/full
Summary
Following a request from the European Commission, the European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain (CONTAM Panel) evaluated the risks to human health related to the presence of cyanogenic glycosides (CNGs) in foods other than raw apricot kernels. Previous assessments from the EFSA, in particular the opinion on acute health risks related to the presence of CNGs in raw apricot kernels and products derived from raw apricot kernels (2016), and assessments from other international and national scientific bodies have been used as a starting point for the evaluation together with publications identified in a targeted literature search. EFSA guidance documents and general principles for risk assessment have been applied for hazard and exposure assessment in this opinion.
CNGs contain chemically bound cyanide and are present in foods such as almonds, linseed or cassava. When the plant cells are damaged, by for example grinding or chewing, CNGs and their degrading enzymes are brought into contact and cyanide is released. Cyanide is readily absorbed from the gastrointestinal tract and rapidly distributed to all organs. Peak concentrations of cyanide in blood and tissue depend on the amount of CNGs in the food consumed and the rate of release of cyanide which in turn depends on the presence and activity of the degrading enzymes. Peak blood cyanide concentration (assessed by serial measurements of cyanide in whole‐blood after ingestion) can be used as a reliable biomarker for acute cyanide exposure. In a human bioavailability study, mean peak concentrations of cyanide in blood were different after consumption cassava root, linseed and persipan, indicating a fast and practically complete release of cyanide after chewing of bitter almonds and cassava roots but not with linseed and persipan.
In experimental animals, acute toxicity of cyanide and CNGs is characterised by dyspnoea, ataxia, arrhythmia, convulsions, loss of consciousness, decreased respiration and death. Upon repeated dose exposure to cyanide, histopathological alterations in the thyroid, kidney, liver and central nervous system (CNS), and changes in epididymis cauda weights, sometimes paralleled with clinical signs have been reported, but the findings are not consistent between different studies. With the CNGs linamarin and amygdalin, alterations in haematology and clinical chemistry parameters and histopathological alterations were seen. With gari (a cassava product for direct human consumption) and cassava, behavioural changes have been observed. There are indications of developmental effects in hamsters exposed to CNGs or cassava and in rats exposed to potassium cyanide (KCN), which were often observed in the presence of maternal toxicity. Cyanide is not genotoxic. No information is available on the genotoxicity of CNGs.
The acute lethal oral dose of cyanide in humans is reported to be between 0.5 and 3.5 mg/kg body weight (bw). The toxic threshold value for cyanide in blood is considered to be between 0.5 mg/L (ca. 20 μM) and 1.0 mg/L (ca. 40 μM), the lethal threshold value ranges between 2.5 mg/L (ca. 100 μM) and 3.0 mg/L (ca. 120 μM). Signs of acute cyanide poisoning in humans include headache, vertigo, agitation, respiratory depression, metabolic acidosis, confusion, coma, convulsions and death. Poisoning cases, some fatal, have resulted from ingestion of amygdalin preparations, bitter almonds and cassava. Several neurological disorders and other diseases have been associated with chronic exposure to cyanide in populations where cassava constitutes the main source of calories.
The primary mode of action for acute toxicity of cyanide is the inhibition of oxidative phosphorylation leading to anaerobic energy production. Due to the high oxygen and energy demand, brain and heart are particularly sensitive to cyanide which can result in hypoxia, metabolic acidosis and impairment of vital functions. The role of cyanide in neurological impairment upon long‐term consumption of foods containing CNGs has not been elucidated.
The CONTAM Panel concluded that there are no data indicating that the acute reference dose (ARfD) for cyanide of 20 μg/kg bw, established in 2016, should be revised and that it is applicable for acute effects of cyanide regardless of the dietary source. For exposure to cyanide from foods other than raw apricot kernels, bitter almonds and cassava roots, this ARfD is likely to be over‐conservative because of the lower bioavailability of cyanide from these foods, but establishment of different ARfDs for different types of food is not appropriate. However, to account for the differences in cyanide bioavailability after ingestion of certain food items, for cassava and cassava derived products and for almonds a factor of 1, for linseed a factor of 3 and for marzipan/persipan, a factor of 12 was calculated based on results from a human bioavailability study. Occurrence data on these foods were divided by the respective factors for inclusion in the exposure assessment. For all other food items, no data on bioavailability were available, and a factor of 1 was used as a default worst‐case value assuming complete cyanide bioavailability. The limited data from animal and human studies do not allow the derivation of a chronic health‐based guidance value (HBGV) for cyanide (CN).
A total of 2,586 analytical results on total cyanide in foods were available in the EFSA database (of which about 89% came from Germany and of which 46% were left‐censored) to estimate acute and chronic dietary exposure. Highest occurrence values were reported in bitter almonds (mean concentration 1,437 mg/kg) and in linseed (mean concentration 192.1 mg/kg). No occurrence data were available in the database for cassava and products derived thereof.
Estimated acute exposures to cyanide originating from foods containing CNGs across 43 different dietary surveys and all age groups ranged from 0.0 to 13.5 μg/kg bw per day (mean, minimum lower bound (LB) to mean maximum upper bound (UB)) and 0.0–51.7 μg/kg bw per day (95th percentile (P95), minimum LB to maximum UB). Estimated chronic exposures to cyanide originating from foods containing CNGs across 38 different dietary surveys and all age groups ranged from 0.0 to 13.5 μg/kg bw per day (mean, minimum LB to maximum UB) and from 0.6 to 34.5 μg/kg bw per day (P95, minimum LB to maximum UB). The highest acute and chronic exposures were estimated for ‘Infants’, ‘Toddlers’ and ‘Other children’ and the main contributors to acute and chronic exposure to cyanide in all age groups were ‘Biscuits (cookies)’, ‘Juice or nectar from fruits’ and ‘Pastries and cakes’.
Estimated mean dietary acute exposures did not exceed the ARfD of 20 μg CN/kg bw in any age group. At the P95, the ARfD was exceeded by up to about 2.5‐fold in some consumption surveys for ‘Infants’, ‘Toddlers’, ‘Other children’ and the adolescent age groups. The CONTAM Panel notes that these are likely overestimations, in particular because of the assumptions made regarding full cyanide bioavailability from foods other than bitter almonds, cassava roots, linseed, persipan and marzipan.
A chronic exposure assessment has also been carried out, although there are insufficient data to characterise potential risks of chronic exposure to cyanide in a European population.
In addition, exposure ‘back‐calculations’ have been carried out to estimate the amount of certain food items that can be ingested without exceeding the ARfD. This was done for raw cassava root, gari, cassava flour, ground linseed and bitter almonds as well as for food items for which an EU maximum level (ML) for cyanide has been established. The bioavailability factors applied for the exposure assessment have also been applied for these calculations. Depending on the body weight, consumption of 1.3–14.7 g ground linseed containing a high concentration of 407 mg CN/kg could reach the ARfD, the corresponding values for consumption of raw cassava root containing a high concentration of 235 mg CN/kg, being 0.7–8.5 g. If gari or cassava flour containing the respective Codex Alimentarius Commission (Codex) MLs of 2 mg total CN/kg and 10 mg total CN/kg, respectively, are consumed, the ARfD is reached with consumption of 87–1,000 g gari and with 17–200 g cassava flour. Consumption of 0.1–1.4 g bitter almonds (1,477 mg CN/kg) reaches the ARfD. This corresponds to an amount of less than half a small kernel in ‘Toddlers’ and of 1 large kernel in ‘Adults’. If marzipan or persipan containing the respective EU maximum limit (ML) of 50 mg CN/kg are consumed, the ARfD is reached with 42–480 g. Consumption of 35–400 g canned stone fruits containing the respective EU ML of 5 mg total cyanide/kg leads to an exposure equivalent to the ARfD. If stone fruit marc spirits and stone fruit spirits contain the EU ML of 35 mg total cyanide/kg, the ARfD is reached by consumption of 26–57 g, depending on the body weight of the individual.
The overall uncertainty incurred with the present assessment is considered as high. It is more likely to overestimate than to underestimate the risk.
Validated methods for the quantification of CNGs and total cyanide and investigations on the variation of hydrolytic enzymes are needed in different foods. The variation of hydrolytic enzymes in food crops and the potential to identify cultivars of crops with relatively low content of CNG or of hydrolytic enzymes need to be investigated. More occurrence data for cyanide in raw and processed foods and consumption data for CNG containing foods are also needed. Human toxicokinetics of CNGs and released cyanide after ingestion of food items containing CNGs need to be studied further. More information is needed on the presence of hydrolytic activity in processed foods. More data are needed to evaluate the potential of cyanide and food items that contain CNGs to cause chronic effects.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
On 1 March 2016, the Panel on Contaminants in the Food Chain (CONTAM) adopted the scientific opinion on acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels.1
The CONTAM Panel established an ARfD for cyanide of 0.02 mg/kg bw (20 μg/kg bw) for use in assessing the risks associated with the presence of cyanogenic glycosides in apricot kernels.
Cyanogenic glycosides are also present in other food such as linseed and cassava.
Furthermore, maximum levels for hydrocyanic acid are established in nougat, marzipan or its substitutes or similar products (50 mg/kg) canned stone fruits (5 mg/kg) and alcoholic beverages (35 mg/kg) by Regulation (EC) No 1334/20082 and 7 g of hydrocyanic acid per hectolitre of 100% vol. alcohol in stone fruit spirits and fruit marc spirit, established by Regulation (EC) No 110/20083.
In the scientific literature there is evidence that this acute reference dose is applicable to unprocessed foods with cyanogenic glycosides also containing intact plant β‐glucosidase. It is mentioned that for some foods the approach may be overly conservative due to the delayed and/or incomplete release of cyanide from the cyanogenic glycosides depending on many factors, as was demonstrated for linseed. In case of missing or inactivated β‐glucosidase, the hazard potential would be much lower.4
Furthermore, in the scientific opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on hydrocyanic acid in flavourings and other food ingredients with flavouring properties,5 adopted on 7 October 2004 the following is concluded ‘Cassava flour is used as a staple food mainly outside Europe; a consumption of 200 g/person would lead to an estimated intake level of 30 μg HCN/kg bw for a 60 kg adult. In accordance with the JECFA view such an intake would not be associated with acute toxicity. The highest level of HCN found in retail marzipan paste is 20 mg HCN/kg. Assuming on one sitting a person of 60 kg consumes 100 g marzipan containing such a level, that intake would be equivalent to 2 mg HCN or to 0.03 mg/kg bw’.
It is appropriate to consider the need to take regulatory measures as regards the presence of cyanogenic glycosides in foods which are not yet regulated at EU level and to assess the appropriateness of existing maximum levels for hydrocyanic acid in food to provide a high level of human health protection.
Therefore, it is appropriate that EFSA assesses the applicability of the Acute Reference Dose (ARfD) for cyanogenic glycosides in raw apricot kernels to other food in which cyanogenic glycosides are present. In case it is concluded that the ARfD for cyanogenic glycosides in raw apricot kernels is not applicable to other foods in which cyanogenic glycosides are present, EFSA is requested to assess the human health risks of the presence of cyanogenic glycosides in foods other than raw apricot kernels.
1.1.2. Terms of Reference
In accordance with Art. 29 (1) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority for a scientific opinion on the human health risks related to the presence of hydrocyanic acid in foods other than raw apricot kernels and products derived from apricot kernels (ground, milled, cracked, chopped).
In particular, the scientific opinion should inter alia comprise:
Evaluation of the applicability of the ARfD established for cyanogenic glycosides in raw apricot kernels for other foods in which cyanogenic glycosides are present.
Evaluation of the relevance of chronic effects related to the human dietary exposure to cyanogenic glycosides.
Estimation of acute and (if relevant) chronic dietary exposure of the EU population, including consumption patterns of specific (vulnerable) groups of the population.
1.2. Interpretation of the Terms of Reference
In the Terms of Reference (ToR) as provided by the European Commission, EFSA was requested to address the risks to human health related to the presence of hydrocyanic acid (hydrogen cyanide, HCN) in foods other than raw apricot kernels. The EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) noted that free HCN is actually not present in food at toxicologically relevant concentrations and that any risks are related to the release of HCN from cyanogenic glycosides (CNGs) present in plant‐derived food. CNGs are produced as secondary metabolites by various plant species and probably serve as a defence mechanism against herbivores, because CNGs release highly toxic HCN when hydrolysed. Hydrolytic enzymes are stored separately from CNGs in intact plants. However, when plant material is chewed or otherwise processed, hydrolytic enzymes and CNGs come in contact and HCN is formed.
Because of its weak acidity, HCN always exists as a mixture of non‐dissociated acid (HCN) and its dissociated form (cyanide ions, CN−) in aqueous biological fluids, the proportion of each form in the dissociation equilibrium depending on the pH of the fluid. Therefore, the term ‘cyanide’ (or CN) will be used throughout this opinion to inclusively represent the inorganic forms of cyanide, i.e. the undissociated HCN and the dissociated CN−.
Very low levels of cyanide are also produced in the brain as neuromodulators (Cipollone and Visca, 2007). This source is negligible in terms of toxicity.
The CONTAM Panel limited the assessment to plant‐derived foods as in terms of CNG content, occurrence in foodstuffs and consumption, non‐plant‐derived foods were considered to be a negligible source of dietary cyanide.
1.3. Additional information
1.3.1. Chemistry
Hydrocyanic acid (hydrogen cyanide or HCN) does virtually not occur in plants as free compound but ‘hidden’ in so‐called CNGs, which allow the plant to store HCN without suffering from its toxicity.
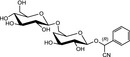
Cyanogenic glycosides
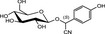
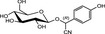
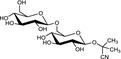
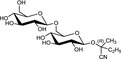
At least 60 different CNGs have been identified in plants (Seigler, 1991). In general, CNGs contain cyanide (CN) in a chemically fixed state as a cyanohydrin (α‐hydroxynitrile) which is stabilised as a β‐glycoside of a monosaccharide like glucose or a disaccharide like gentiobiose (Poulton, 1990; Jones, 1998; Ballhorn, 2011). As an example, the complete chemical structures of the widely occurring glucoside linamarin and its homologous gentiobioside, linustatin are depicted in Figure 1. In intact plant cells, CNGs are stored in vacuoles and thereby separated from β‐glycosidase enzymes (EC 3.2.1.21) located in plant cell walls. When plant cells are physically destroyed, e.g. by chewing or grinding, the CNGs come into contact with the β‐glycosidase enzymes and are degraded with the release of HCN. In aqueous biological fluids, free HCN exists in a pH‐dependent dissociation equilibrium with cyanide ions (CN−). The mixture of non‐dissociated HCN and cyanide ions is termed ‘cyanide’ (see EFSA CONTAM Panel, 2016).
Figure 1.
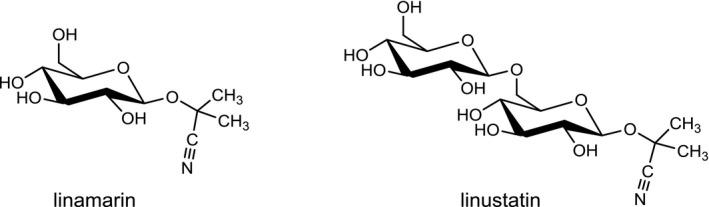
Chemical structures of linamarin and linustatin
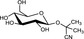
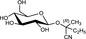
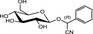
The chemical structures and some of the features of typical CNGs are listed in Table 1. The aglycones of some but not all of the CNGs contain chiral centres, i.e. C‐atoms with four different substituents. Of particular practical importance is the fact that different amounts of CN are released from different CNGs, because of the different molecular masses. For example, 1 g of linamarin, which has a relatively low molecular mass, yields almost twice as much HCN compared to 1 g of amygdalin with a much higher molecular mass. Due to the polar glycoside group, all CNGs are solids with quite high melting points and a similar solubility, which is much higher in polar solvents like water or ethanol than in non‐polar solvents such as chloroform or benzene.
Table 1.
Important cyanogenic glycosides (CNGs) in food plants, arranged according to maximum release of CN (calculated as HCN equivalents)
| Chemical structure | CAS number | Element formula | Molecular mass | CN (mg/g CNG) | Examples for occurrencea |
|---|---|---|---|---|---|
|
554‐35‐8 | C10H17NO6 | 247.3 | 109.2 |
Cassava (Manihot esculenta Crantz) Lima beans (Phaseolus lunatus L.) |
|
534‐67‐8 | C11H19NO6 | 261.3 | 103.3 |
Cassava (Manihot esculenta Crantz) Lima beans (Phaseolus lunatus L.) |
|
99‐18‐3 | C14H17NO6 | 295.3 | 91.4 | Bitter almonds (Prunus amygdalus var. amara Stokes) |
|
499‐20‐7 | C14H17NO7 | 311.3 | 86.7 | Sorghum (Sorghum bicolor (L.) Moench) |
|
21401‐21‐8 | C14H17NO7 | 311.3 | 86.7 | Bamboo (Bambusa vulgaris Schrad. and Bambusa edulis Carriere) |
|
72229‐40‐4 | C16H27NO11 | 409.4 | 66.0 | Linseed (Linum usitatissimum L.) |
|
72229‐42‐6 | C17H29NO11 | 423.4 | 63.8 | Linseed (Linum usitatissimum L.) |
|
29883‐15‐6 | C20H27NO11 | 457.4 | 59.0 |
Apricot kernels (Prunus armeniaca L.) Almond kernels (Prunus amygdalus var. dulcis Stokes) |
Latin names and names on authors according to ‘The PlantList – a working list of all plant species’ (http://www.theplantlist.org). All relevant synonyms may also be found at this list. Chiral Catoms in the aglycones (i.e. C‐atoms carrying four different substituents) are labelled with the stereochemical descriptors R or S according to the Cahn–Ingold–Prelog system.
The biosynthesis of CNGs, which is believed to occur in more than 3,000 plant species, follows a general scheme starting with the cytochrome P450‐mediated hydroxylation of an aliphatic or aromatic amino acid (e.g. valine, isoleucine, phenylalanine, or tyrosine) to an N‐hydroxyl amino acid, which is converted by oxidative decarboxylation to an oxime. Subsequent release of water yields a nitrile. Another hydroxylation then leads to an α‐hydroxynitrile, which is finally stabilised by glycosylation. As an example, the biosynthesis of linamarin is depicted in Figure 2.
Figure 2.
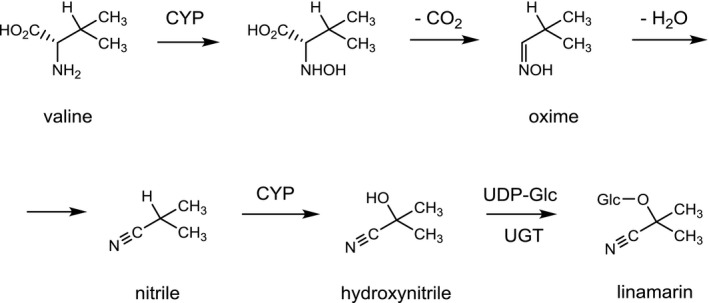
Biosynthesis of linamarin
- CYP: cytochrome P450; Glc: glucose; UDP‐Glc: uridine diphosphoglucose; UGT: uridine diphosphoglucosyltransferase.
Whereas CNGs are chemically quite stable both under acidic and alkaline conditions, the intermediate α‐hydroxynitriles (cyanohydrins) are only stable in acidic media but spontaneously dissociate into the respective carbonyl compound and CN at neutral or alkaline pH (Fomunyam et al., 1985). Thus, if the glycosidic bond is hydrolysed, a process known as cyanogenesis is initiated as shown in Figure 3 for linamarin (McMahon et al., 1995). The hydrolysis of linamarin to acetone cyanohydrin and glucose is mediated by the β‐glucosidase linamarase (EC 3.2.1.21). The subsequent conversion of acetone cyanohydrin to acetone and HCN proceeds spontaneously, but is much faster in the presence of the enzyme hydroxynitrile lyase (EC 4.1.2.37). Complete hydrolysis of 1 g of linamarin generates 109 mg of HCN (see Table 1).
Figure 3.
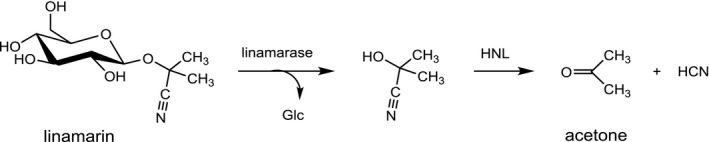
Formation of HCN from linamarin
- Glc: glucose; HNL: hydroxynitrile lyase.
The process of cyanogenesis is sometimes also called the ‘cyanide bomb’ (Morant et al., 2008). CNGs and their catabolic enzymes are stored in separate compartments in intact plant cells, but are brought into contact upon tissue disruption, caused, e.g. by chewing or physical processes such as maceration or freezing during food processing (Gleadow and Woodrow, 2002).
The strategy of handling CNGs and their catabolic enzymes as a binary system endows plants with an effective defence against generalist herbivores. Because CNGs protect plants for herbivore attacks, they are referred to as ‘phytoanticipins’. As an additional role, CNGs are believed to represent a pool of nitrogen to be used by the plant if needed (Gleadow and Møller, 2014).
The hydrolysis of CNGs to release cyanide can involve various enzymes. With regard to the genuine glycosidases of the plant tissue, the activity may vary between cultivars (Iglesias et al., 2002). In addition to the plant enzymes mentioned above, β‐glucosidases located in the mammalian intestinal epithelium and in colonic bacteria appear to play an important role (see Section 3.1.1 on Toxicokinetics).
Hydrocyanic acid is also named hydrogen cyanide, formonitrile, methanenitrile or prussic acid, among others. It has the chemical formula HCN, the molecular mass 27.03 g/mol and the Chemical Abstracts Service (CAS) number 74‐90‐8. In pure form, it is a colourless liquid with a boiling point of 25.6°C and a melting point of −14°C. Its density is 0.687 g/mL and its vapour pressure is 630 mm Hg at 20°C. It is completely miscible with water or ethanol. HCN is a very weak acid with a pKa of 9.2 and a pKb of 4.8, and aqueous solutions of its alkali salts (cyanides) are therefore quite alkaline. HCN vapours have a characteristic odour like bitter almond oil, but one person out of four does not readily smell HCN (Brown and Robinette, 1967).
1.3.2. Analytical methods
This chapter does not provide a full list of potential methods to quantify the concentration of CNGs, cyanohydrins and cyanide (originating from CNGs) in food. Rather, the intention is to identify methods that are used as the standard methods of analysis.
Quantification of cyanogenic glycosides
The extraction step from food samples is one crucial aspect of any analytical procedure due to the potential of CNGs for enzymatic degradation and epimerisation (summarised in FAO/WHO, 2012 and EFSA CONTAM Panel, 2016). High‐performance liquid chromatography with UV detection (HPLC‐UV) or with diode‐array detection (HPLC‐DAD) has been widely applied to quantify CNGs in food samples after extraction. More recently, solid‐phase extraction along with liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) analysis has been applied, improving both sensitivity and selectivity of the analyses. Besides liquid chromatography‐based techniques, less frequently gas chromatography‐mass spectrometry (GC‐MS) as well as enzyme‐linked immunosorbent assays (ELISAs) have been applied to quantify CNGs in food (FAO/WHO, 2012; EFSA CONTAM Panel, 2016). No validated methods are available for the quantification of CNGs in food items.
Quantification of total cyanide
Crucial steps in the analysis of total cyanide (cyanide originating from CNGs and cyanohydrins by complete hydrolysis during sample preparation) in food samples include the sample handling and the complete hydrolysis of the CNGs. Hydrolysis can be achieved by acid catalysis or enzymatic degradation. The enzyme used should be ensured to have the CNG in question as accepted substrate. To ensure that all released CN is retained for analysis, food samples should be incubated with the enzymes or the diluted acid in sealed containers. Methods of quantifying the released cyanide include colorimetry, spectrophotometry and chromatography with subsequent detection (FAO/WHO, 2012; FSANZ, 2014; EFSA CONTAM Panel, 2016). The European Standard EN 16160 of 2012 (EN, 2012) (HPLC‐based measurement) exists for quantification of total cyanide in feed.
1.3.3. Previous risk assessments
In the present section, the term HCN (that corresponds to the term total cyanide used in the present opinion) has been retained for consistency reasons when as used in previous assessments.
In 2004, the EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) has published an opinion on hydrocyanic acid in flavourings and other food ingredients with flavouring properties (EFSA, 2004). In dogs and rats, Median lethal doses (LD50s) were equivalent to 2.13 and 4.0–6.03 mg CN−/kg body weight (bw), respectively. The lowest lethal dose identified in humans was 0.56 mg HCN/kg bw. The lethal oral dose of linamarin in rat was 450 mg/kg bw. Based on the limited data available, the AFC Panel could not establish a safe acute intake level for HCN (i.e. ARfD). The Panel concluded that the epidemiological studies available were not adequate to establish a No observed adverse effect level (NOAEL) for chronic exposure and that adequate long‐term toxicity studies in animals to derive a NOAEL were lacking. Therefore, a Tolerable daily intake (TDI) could not be established either. The Panel furthermore concluded that exposure to cyanide from flavouring ingredients (at the 97.5th percentile 3.6 μg/kg bw per day) was unlikely to cause acute toxicity in humans. Consumption of either 200 g cassava or 100 g marzipan in 1 day by a 60 kg individual would lead to an intake of 30 μg HCN/kg bw and would not be associated with acute toxicity.
In 2012, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) published a risk assessment of CNGs (FAO/WHO, 2012) in which both toxicity data on CNGs and on HCN were evaluated. Acute toxicity symptoms upon administration of CNGs and HCN are metabolic acidosis, decreased cytochrome oxidase activity and respiratory depression. In repeated dose studies with cyanide, histopathological changes in the nervous system and effects on the thyroid and on reproduction and development are seen. In humans, long‐term consumption of cassava is associated with konzo,6 tropical ataxic neuropathy7 and also with goitre. The JECFA selected skeletal defects in hamster foetuses (missing presacral vertebrae, agenesis of 13th rib) seen in a developmental toxicity study with linamarin (Frakes et al., 1985) as the appropriate endpoint for an acute dose–response analysis. A benchmark dose lower confidence limit 10% (BMDL10)8 of 85.26 mg linamarin/kg bw was calculated and by application of an uncertainty factor (UF) of 100 the Committee established an ARfD for linamarin of 0.9 mg/kg bw, equivalent to 0.09 mg CN/kg bw. This cyanide equivalent ARfD applies only to foods containing CNGs as a main source of cyanide. For the chronic dose response analysis, the JECFA selected adverse effects related to male reproduction (decreased cauda epididymis and testis weights and decreased testicular spermatid concentration) observed in a 13‐week study where sodium cyanide was given to rats via drinking water [National toxicology programme (NTP), 1993]. A BMDL1SD 9 of 1.9 mg CN/kg bw per day was calculated to which an UF of 100 was applied resulting in a Preliminary tolerable daily intake (PMTDI) of 20 μg CN/kg bw. The JECFA decided not to apply an additional UF to account for the absence of a long‐term study, taking into account the acute nature of cyanide toxicity and the sensitivity of the effect (i.e. the reduction of absolute cauda epididymis weight).
Using national acute dietary exposure assessments, the ARfD of 0.09 mg/kg body was exceeded threefold with cassava by adults, less than twofold with apple juice by children, between two‐ and fivefold with apricot kernels and up to 10‐fold with ready‐to‐eat cassava chips/crisps depending on the different population groups. Using national chronic dietary exposure assessments, the PMTDI of 0.02 mg/kg bw was exceeded between one‐ and threefold in children and between one‐ and twofold in children and adults, respectively, that consumed cassava as staple food. Chronic dietary exposure from flavouring agents did not lead to exceedances of the PMTDI.
In 2014, the Food Standards Australia New Zealand (FSANZ) published a survey of CNGs in plant‐based foods in Australia and New Zealand 2010–2013 that contained an acute and chronic risk assessment of cyanide (FSANZ, 2014). For the chronic risk characterisation, the JECFA PMTDI of 20 μg cyanide/kg bw (FAO/WHO, 2012) was used. For the acute risk characterisation, FSANZ used an ARfD of 80 μg HCN/kg bw. This ARfD was established in a previous risk assessment of FSANZ (2008) based on the maternal NOAEL of 70 mg/kg bw per day in the developmental study with linamarin in hamsters, in which at the next higher dose of 100 mg/kg bw per day dyspnoea, hyperpnoea, ataxia, tremors, hyperthermia was observed (Frakes et al., 1985). This endpoint differs from that used by JECFA, but the resulting ARfD is similar. Using a consumption size of 32 apricot kernels per day, acute exposure estimates for adults ranged from 724 to 755 μg HCN/kg bw per day exceeding the ARfD of 80 μg HCN/kg bw per day. High consumption of linseed containing bread led to exposure estimates of up to 511 μg HCN/kg bw per day thereby exceeding the ARfD of 80 μg HCN/kg bw per day, whereas high consumption of cassava resulted in exposures at the ARfD. FSANZ concluded that consumption of raw apricot kernels poses a very severe health risk. Although acute exposures with linseed containing bread exceeded the ARfD, FSANZ concluded that linseed and foods containing linseed do not represent an appreciable health risk as there are not reports in the literature of human poisonings upon consumption of linseed and in a study in which human volunteers consumed 100 g of ground linseed no cyanide was detected in the blood (Schilcher et al., 1986). Likewise, although consumption of cassava could lead to exposures reaching the ARfD, FSANZ concluded that, because of the worst‐case assumptions made in the exposure estimates and the absence of adverse effects reported in individuals consuming properly processed cassava, it is not of concern.
In 2016, the EFSA CONTAM Panel published a scientific opinion on the acute health risks related to the presence of CNGs in raw apricot kernels and products derived from raw apricot kernels (EFSA CONTAM Panel, 2016). The Panel concluded that amygdalin is the major CNG present in apricot kernels and is degraded to cyanide by chewing or grinding. The lethal dose of cyanide is reported to be 0.5–3.5 mg/kg bw. An ARfD for cyanide of 20 μg/kg bw was derived from a study where exposure to a dose of 0.105 mg/kg bw was associated with a non‐toxic blood cyanide level of 20 μM (Abraham et al., 2016) and applying an UF of 1.5 to account for toxicokinetic and of 3.16 to account for toxicodynamic interindividual differences. The variations in peak blood levels seen in the study from Abraham et al. (2016) were small (mean ± SD: 20.06 ± 3.35 μM in women, 12.17 ± 3.19 μM in men). Therefore, the CONTAM Panel concluded that a default factor of 3.16 was not required and that a factor of 1.5 was sufficient to cover any additional variability in toxicokinetics.
Since no consumption data were available the Panel used the highest intakes of kernels promoted (10 and 60 kernels/day for the general population and cancer patients, respectively) for assessing exposures which exceeded the ARfD 17–413 and 3–71 times in toddlers and adults, respectively. The quantity of apricot kernels that can be consumed without exceeding the ARfD was estimated to be 0.06 and 0.37 g in toddlers and adults, respectively. The Panel concluded that the ARfD would be exceeded by consumption of one small kernel in toddlers and by more than three small kernels in adults or less than half of a large kernel.
1.3.4. Legislation and international standards
Council Regulation (EEC) No 315/9310 stipulates that food containing a contaminant in an amount unacceptable for public health shall not be placed on the market, that contaminant levels should be kept as low as can reasonably be achieved and that, if necessary, the European Commission may establish maximum levels for specific contaminants. These maximum levels are laid down in the Annex of Commission Regulation (EC) No 1881/200611 and may include limits for the same contaminants in different foods, analytical detection limits and reference to the sampling and analysis methods to be used. Commission Regulation (EU) 2017/123712 amending this regulation provides MLs of 20 mg HCN or HCN bound in CNGs/kg in unprocessed whole, ground, milled, cracked or chopped apricot kernels placed on the market for the final consumer. These MLs are based on the outcome of the previous EFSA risk assessment on apricot kernels (EFSA CONTAM Panel, 2016). Regulation (EC) No 1334/200813 governs the use of flavourings and food ingredients with flavouring properties in foods. The regulation also provides maximum levels of certain substances naturally present in flavourings and food ingredients with flavouring properties. A maximum level for HCN of 50 mg/kg has been established for nougat, marzipan or its substitutes or similar products, of 5 mg/kg in canned stone fruits and of 35 mg/kg in alcoholic beverages. Regulation (EC) No 110/200814 governs the definition, description, presentation, labelling and protection of geographical indications of spirit drinks and establishes a maximum content of HCN of 7 g/hL of 100% volume alcohol (70 mg/L) in stone fruit marc spirits and stone fruit spirits.
Directive 2002/32/EC15 provides a maximum content of hydrocyanic acid in feed materials and complete feeding stuffs of 50 mg/kg (relative to a moisture content of 12%). Exceptions are linseed, linseed cakes and manioc products/almond cakes for which maximum contents are 250, 350 and 100 mg hydrocyanic acid/kg, respectively, and complete feeding stuffs for chicks which can contain a maximum of only 10 mg/kg.
The Codex Alimentarius Commission (Codex) has issued several documents regarding the definitions of cassava food commodities and measures to reduce hazards by cassava consumption. The code of practice for the reduction of hydrocyanic acid (HCN) in cassava and cassava products (CAC/RCP 73‐2013)16 gives guidance on how to produce cassava products with safe concentrations of cyanogenic compounds and advice in support of reduction of HCN in cassava and lowering uptake of cassava. There are Codex standards defining gari17 (Codex STAN 151‐1989),18 edible cassava flour (Codex STAN 176‐1989),19 sweet cassava (Codex STAN 238‐2003)20 and bitter cassava (Codex STAN 300‐2010).21 In the general standard for contaminants and toxins in food and feed (Codex STAN 193‐1995),22 MLs of 2 and 10 mg/kg HCN for gari and cassava flour have been set which are based on the risk assessment of CNGs of JECFA (FAO/WHO, 2012).
2. Data and methodologies
2.1. Collection and appraisal of occurrence, toxicokinetics and toxicity data collected from public literature
For the previous EFSA opinion on CNGs in raw apricot kernels (EFSA CONTAM Panel, 2016), a series of previous risk assessments on HCN and CNGs has been collected and evaluated. Any relevant original studies referenced in these previous risk assessments have been retrieved as a first step. Since it contained the latest comprehensive EFSA hazard assessment of CN, the opinion of the AFC Panel on HCN in flavourings and flavouring ingredients (EFSA, 2004) was considered as a starting point for the previous opinion on CNGs in apricot kernels and a literature search was carried out to retrieve all relevant studies published after this assessment, i.e. in the years from 2004 to 2015. During the development of the opinion on CNGs in apricot kernels, additional publications were collected by applying a ‘forward snowballing approach’.23 In total, 171 original publications were retrieved for the previous opinion and, where relevant, have been considered also for the present assessment.
While the previous opinion (EFSA CONTAM Panel, 2016) focussed on acute effects of a single food commodity (i.e. apricot kernels), the present assessment required also collection and evaluation of information on chronic effects of cyanide and consideration of potentially all cyanogenic foods. The CONTAM Panel identified the JECFA assessment on cyanide in food (FAO/WHO, 2012), which contained both an acute and chronic risk evaluation as the most recent comprehensive risk assessment and as a starting point for the present assessment, as it was assumed that it covered comprehensively all information/studies on potentially relevant cyanogenic foods at that time. To cover also any further literature published since then, a literature search on studies on formation, occurrence, processing, exposure, toxicokinetics, acute and chronic toxicity and epidemiology of cyanogenic foods, CNGs and CN in the period from 1 January 2012 until 22 June 2017 (the date of the search) was carried out. The database used was Web of Science24 and references retrieved were managed using Endnote.25 The search terms used and the results obtained are described in detail in Appendix A. In brief, after removing duplicates, in total, 640 publications were obtained. Upon screening of their abstracts using expert judgement, 178 studies were considered as potentially relevant and full text originals were retrieved for further consideration. During the development of the opinion, it was agreed that with regard to acute effects of CN and CNGs, the previous JECFA assessment (FAO/WHO, 2012) could not be used as a starting point for assessing acute effects of CN or CNGs in humans because the ARfD derived by JECFA was based on a study with linamarin and it could not be excluded that effects are specific to this CNG and not related entirely to CN. In addition, the JECFA assessment did not include an extensive evaluation of individual CN or CNG poisoning cases in humans. Therefore, an additional search was carried out for publications in this field without setting a time limit, which yielded a total of 1,206 publications. It was agreed that such an amount of publications could not reasonably be evaluated and also that the older publications might be of lesser relevance as their findings are likely reflected in later studies and reviews. Therefore, only abstracts from publications from 1970 onwards (in total 667) were screened of which 60 were considered as relevant and therefore retrieved (for details on this additional literature search, see Appendix B).
2.2. Occurrence data used for the assessment
The data used for the present scientific report were derived from analytical data submitted by Member States via a continuous annual call for data. All data were submitted to EFSA according to the data model ‘Standard sample description version 1’ (SSD1) (EFSA, 2010a) by different data provider organisations and stored in the EFSA scientific data warehouse (SDWH). The SSD data model contains different data elements (database fields) and several coded standard terminologies for non‐free‐text data elements. The field names and terms mentioned in the present report refer to the SSD1 model.
In the analysis of CN occurrence data, the left‐censored data [results below limit of detection (LOD) or below limit of quantification (LOQ)] were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO, 2009). The same method is indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b) as an option in the treatment of left‐censored data. The guidance suggests that the lower bound (LB) and upper bound (UB) approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). The LB is obtained by assigning a value of zero (minimum possible value) to all samples reported as lower than the LOD (< LOD) or LOQ (< LOQ). The UB is obtained by assigning the numerical value of LOD to values reported as < LOD and LOQ to values reported as < LOQ (maximum possible value), depending on whether LOD or LOQ is reported by the laboratory.
In addition to the occurrence data collected from the Member States within the call for data, analytical data obtained through literature review of CN concentration only in raw cassava sampled in European countries were used for estimating the maximum amount of raw cassava that can be consumed without exceeding the ARfD (see Section 3.5 on Risk characterisation).
2.3. Food consumption data
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. It was first built in 2010 (EFSA, 2011; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011). The latest version of the Comprehensive Database updated in 2018 contains results from a total of 60 different dietary surveys carried out in 25 different Member States covering 119,458 individuals. Within the dietary studies, subjects are classified in different age classes as follows:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Two additional surveys provided information on specific population groups: ‘Pregnant women’ (≥ 15 years to ≤ 45 years old; Latvia) and ‘Lactating women’ (≥ 28 years to ≤ 39 years old; Greece). For chronic exposure assessment, food consumption data were available from 44 different dietary surveys carried out in 22 different European countries. For the acute assessment, recent food consumption data were available for 43 surveys of 25 countries. In Annex A.1, these dietary surveys and the number of subjects available for the acute and chronic exposure assessment are described. The food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls or dietary records covering from 3 to 7 days per subject. Because of the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
2.4. Methodology for exposure assessment
2.4.1. Methodology for acute exposure assessments
Since it was not possible to identify the consumption events of processed products potentially containing cyanide due to ingredients like almonds, marzipan/persipan and stone fruits (e.g. ‘Pastries and cookies’, ‘Biscuits’, ‘Fruit juices’), for each of these categories, the CONTAM Panel selected a list of FoodEx categories that could contain almonds, marzipan/persipan and stone fruits and these foods were used for the assessment of acute exposure.
Acute dietary exposure to CN originating from foods containing CNGs was estimated using a probabilistic approach. For calculating acute dietary exposure CN, originating from food containing CNGs, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Only consumption events related to the lowest (most detailed) FoodEx category levels assumed by the Panel to potentially contain CNGs were used in the assessment of acute exposure. In addition, the different FoodEx categories were grouped within food groups to better present their contribution to the total dietary exposure to CN. The complete list of the selected FoodEx categories and food groups is available in Annex A.2. The acute dietary exposure to CN was calculated for each reporting day, since individual meals are recorded for only a few countries in the consumption database. The preferred option is, therefore, to use individual days of consumption. Days of consumption offer a conservative estimate of the exposure, since it will sum the contribution of all meals during the same day. Acute exposure was assessed for each reporting day by multiplying the total consumption amount for each food category by an occurrence level randomly drawn among individual results available for that food category. Respective intakes of the foods consumed that day were summed and finally divided by the individual's body weight. This process was iterated 500 times for each day of consumption reported by each participant. For the calculations, occurrence data estimated using the UB and LB approach were used. The 95% confidence interval was defined as the 2.5th and 97.5th percentiles obtained from the 500 iterations. All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1® 26), including the modelling of the probabilistic acute exposure.
Due to the lack of occurrence data on cassava and cassava products, the panel decided to perform a backwards calculation to estimate the maximum amount of fresh raw cassava that can be eaten in one eating occasion by each age class without the exceeding the ARfD. The highest value reported in literature for raw cassava purchased in Europe was used for this assessment.
A similar approach was used for linseed, for which the highest occurrence value reported by the member states and stored in the SDWH was used to calculate the maximum amount of linseed that can be eaten in one eating occasion by each age class without exceeding the ARfD.
Additionally, backward calculations were carried out for food items for which maximum limits for HCN exist, such as marzipan or its substitutes or similar products or canned stone fruits (Regulation EC No 1334/2008), spirits (Regulation EC No 110/2008), gari and cassava flour (Codex STAN 193‐1995). Here, the respective MLs were applied to assess the maximum amount of consume the respective food that can be consumed in one eating occasion by each age class without exceeding the ARfD.
2.4.2. Methodology for chronic exposure assessment
Since it was not possible to identify the consumption events of processed products potentially containing cyanide due to ingredients like almonds, marzipan/persipan and stone fruits (e.g. ‘Pastries and cookies’, ‘Biscuits’, ‘Fruit juices’), for each of these categories, the CONTAM Panel selected a list of FoodEx categories that could contain almonds, marzipan/persipan and stone fruits and these foods were used for the assessment of chronic exposure.
As suggested by the EFSA WG on Food Consumption and Exposure (EFSA, 2011), dietary surveys with only one consumption day per subject were not considered for chronic exposure assessments as they are not adequate to assess repeated exposure. Similarly, subjects who participated only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded for the chronic exposure assessment. Not all countries provided consumption information for all age groups, and in some cases, the same country provided more than one consumption survey. For calculating chronic dietary exposure to CN, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Only consumption events related to the lowest (most detailed) FoodEx category levels assumed by the Panel to potentially contain CNGs were used in the assessment of chronic exposure. In addition, the different FoodEx categories were grouped within food groups to better present their contribution to the total dietary exposure to CN. The complete list of the selected FoodEx categories and food groups is available in Annex A.2. The mean and the high (P95) chronic dietary exposures were calculated by combining total CN mean occurrence values for food samples collected in different countries (pooled European occurrence data) with the average daily consumption for each food at individual level in each dietary survey and age class. Consequently, individual average exposures per day and body weight were obtained for all individuals. On the basis of distributions of individual exposures, the mean and P95 exposure were calculated per survey and per age class. Dietary exposure was assessed using overall European LB and UB mean occurrence of total CN. The contribution (%) of each food category to overall mean dietary chronic exposure of total CN was calculated for each age group and dietary survey. All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1).
2.4.3. Methodology for risk characterisation
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by the WHO (2009), which include hazard identification and characterisation, exposure assessment and risk characterisation. Additionally to the principles described by the WHO (2009), EFSA guidance pertaining to risk assessment has been applied for the present assessment. The EFSA guidance covers the procedures currently used within EFSA for the assessment of dietary exposure to different chemical substances and the uncertainties arising from such assessments. EFSA guidance documents applied for the present risk assessment are the guidance on uncertainties in dietary exposure assessment (EFSA, 2007), on transparency in scientific aspects of risk assessments (EFSA, 2009), on standard sample description for food and feed (EFSA, 2010a), on management of left‐censored data in dietary exposure assessments (EFSA, 2010b), on use of the EFSA comprehensive food consumption database in intakes assessment (EFSA, 2011), on genotoxicity testing (EFSA Scientific Committee, 2011), on selected default values to be used in the absence of data (EFSA Scientific Committee, 2012a) and on risk assessment terminology (EFSA Scientific Committee, 2012b).
3. Assessment
3.1. Hazard identification and characterisation
3.1.1. Toxicokinetics
CNGs present in food items pose a health hazard because they can release cyanide. As defined before (EFSA CONTAM Panel, 2016), the term ‘cyanide’ comprises both cyanide ions (CN−) and undissociated hydrogen cyanide (HCN). As described in Section 1.3.1 on Chemistry, CNGs are degraded to cyanide by β‐glycosidase and α‐hydroxynitrile lyase, two families of enzymes stored separately from the CNGs in plant cells. CNGs are typically confined to the vacuoles, whereas β‐glycosidases may be present in the apoplastic space, bound to the cell wall, in the cytoplasm, in small vesicles or in the chloroplast, depending on the plant species (Gleadow and Møller, 2014). The location of the α‐hydroxynitrile lyases is less well known but appears to be cytoplasmic in the cases studied. The degrading enzymes, which are quite specific for the CNGs of the respective plant, are brought into contact with the CNG upon destruction of the intact cells, e.g. by chewing or food processing.
Orally ingested food items derived from cyanogenic plants may contain a mixture of compounds ranging from the original CNGs, the intermediate cyanohydrin, the released cyanide and carbonyl compounds (see, e.g. Figure 3 in Section 1.3.1). The components of the ingested mixture can be absorbed as such or after biotransformation by mammalian or bacterial enzymes present in the gastrointestinal tract.
The toxicokinetics of cyanide have been well studied because it is an important industrial chemical as well as a military and environmental toxin. Very low levels of cyanide are also produced in the brain and are proposed to physiologically act as neuromodulators (Cipollone and Visca, 2007).
The metabolism of CNGs invariably involves their degradation to cyanohydrins and subsequently cyanide, but comparatively little is known about the kinetics (absorption, distribution and excretion) of the parent CNGs (listed in Table 1 in Section 1.3.1 on Chemistry) and their cyanohydrins.
Experimental animals
The toxicokinetics and metabolism of amygdalin and prunasin, which are the predominant CNGs of apricot kernels, have been discussed in detail in a recent EFSA opinion (EFSA CONTAM Panel, 2016). Briefly, in vivo and in vitro studies in various animal species suggest that the gentiobioside amygdalin (see Table 1 in Section 1.3.1) itself is only very poorly absorbed in the gastrointestinal tract, but hydrolysed to the glucoside prunasin in the jejunum, which is then well absorbed and subsequently excreted in the urine without releasing much of its cyanide. The jejunal absorption of prunasin is facilitated by a glucose transporter. The release of cyanide appears to depend on the enzymatic activity of the gut microflora, most convincingly demonstrated by the observation that rats with an intact bacterial flora were much more susceptible to the toxicity of amygdalin than germfree rats, which lack this flora (Carter et al., 1980). Both amygdalin and prunasin were degraded to cyanide by the contents of rat and hamster caecum, as well as by rumen fluid from cattle, with prunasin being a better substrate for bacterial degradation than amygdalin (EFSA CONTAM Panel, 2016).
Very limited toxicokinetic studies in experimental animals have been conducted with linamarin, the major CNG of cassava. When a single dose of 1 mmol of pure linamarin per kg bw was administered by stomach tube to young Wistar rats, no intact linamarin was found in blood or faeces, but about 20% of the dose was excreted unchanged in the urine, together with 12% of the linamarin dose as the cyanide metabolite thiocyanate (Barrett et al., 1977). The failure to detect linamarin in blood may be due to the rather insensitive paper chromatography method used. Maduagwu (1989) administered four single doses ranging from 0.04 to 1.42 mmol/kg bw intragastrically to young male Wistar rats and determined the amounts of unchanged linamarin (measured as glycosidic cyanide), liberated (i.e. non‐glycosidic) cyanide and thiocyanate in the 24‐h urine. The percentage excreted as linamarin was independent of the dose and accounted for only about 2%, whereas the percentage of urinary free cyanide increased from 0.03 to 0.5% and that of thiocyanate from 0.1 to 1% with increasing dose of linamarin. After intravenous injection of doses of 0.04, 0.20 and 0.40 mmol of linamarin per kg bw, elimination of glycosidic cyanide from rat blood was observed to occur with a half‐life of about 90 min for all three dose levels (Maduagwu, 1989).
These few animal studies indicate that unchanged linamarin is partly absorbed from the gastrointestinal tract. As described before (EFSA CONTAM Panel, 2016), partial absorption has also been observed with prunasin, whereas intact amygdalin appears not to be absorbed. In contrast to prunasin and linamarin, which are monoglucosides, amygdalin is a diglucoside containing gentiobiose. For the intestinal absorption of prunasin, involvement of a glucoside carrier has been shown (Wagner and Galey, 2003), but no corresponding studies have been identified for linamarin. No studies on the absorption of the other CNGs listed in Table 1 of Section 1.3.1 on Toxicokinetics nor on their respective cyanohydrins have been identified.
As discussed in more detail in the recent opinion on CNGs in apricot kernels (EFSA CONTAM Panel, 2016), non‐dissociated HCN is a small and non‐polar molecule which is readily absorbed through the gastric and intestinal mucosa. In the blood, most of the cyanide is bound to methaemoglobin and rapidly distributed via the systemic circulation into all tissues. After oral administration of a single dose of 3.0 mg potassium cyanide (KCN)/kg bw, the half‐life of cyanide in blood was 0.64, 0.54 and 1.28 h in rats, pigs and goats, respectively, and the apparent volume of distribution was about 0.35 L/kg (Sousa et al., 2003).
Humans
The previous opinion on CNGs in apricot kernels (EFSA CONTAM Panel, 2016) has addressed the toxicokinetics and metabolism of amygdalin, prunasin and cyanide in humans in detail. For example, Ames et al. (1981) reported that the ingestion of 1.5 g (3.28 mmol) of pure amygdalin per day for 21 days gave rise to only marginal levels of unchanged amygdalin (peak at 1.1 nmol/mL) in blood plasma but much higher levels of cyanide (ca. 80 nmol/mL) in whole‐blood. This finding is in agreement with the animal studies discussed above, indicating that intact amygdalin is virtually not absorbed from the gastrointestinal tract but partially degraded to cyanide, probably by the gut microflora. In vitro studies using simulated human digestive fluids suggest that degradation of amygdalin to prunasin may already start in the upper human gastrointestinal tract (Shim and Kwon, 2010). It should be noted that the studies by Ames et al. (1981) and Shim and Kwon (2010) were conducted with pure amygdalin in the absence of degrading plant enzymes (see Section 1.3.1 on Chemistry). The most recent study on the bioavailability of cyanide after ingestion of amygdalin was conducted by Abraham et al. (2016) in a human volunteer and is also discussed in more detail in EFSA CONTAM Panel (2016). After ingestion of 120 mg isolated amygdalin containing 6.8 mg cyanide, a peak cyanide level of 3.4 μM was reached after 60 min, indicating some minor degradation of amygdalin (by the intestinal flora) occurring in the human body even in the absence of the plant enzymes. A distinct higher level of 10.0 μM was reached after 30 min when sweet almonds (containing the degrading plant enzymes but no amygdalin) were ingested together with the same dose of isolated amygdalin. When 6.8 mg cyanide were ingested as potassium cyanide, a peak cyanide level of 20.1 μM was reached after 15 min, not much higher than the peak levels of 19.5 μM (after 30 min) and 15.4 μM (after 15 min) observed after ingestion of 62 g unprocessed cassava and 2.1 g apricot kernels, respectively, both containing the same dose of 6.8 mg cyanide. These results suggest that the bound cyanide present in cassava and apricot kernels, i.e. in the presence of their plant enzymes, is almost completely released and bioavailable. In contrast, a lower bioavailability (peak level 6.5 μM after 60 min) was observed after ingestion of 30.9 g linseed also containing 6.8 mg cyanide. Higher doses of 60 and 100 g of the same linseed led to an over proportional increase of the peak levels (19.8 μM after 80 min and 42.3 μM after 160 min, respectively) in this volunteer.
In the study by Abraham et al. (2016), the bioavailability of cyanide was also investigated in a group of 12 volunteers who ingested apricot kernels (about 2.1 g), unprocessed cassava root (76–150 g), linseed (30.9 g) and persipan paste27 (100 g), all containing a cyanide amount of 6.8 mg. Furthermore, the double amount of 200 g persipan was ingested. Results of cyanide peak levels are compiled in Table 2.
Table 2.
Evaluation of individual cyanide peak blood levels (C max) and time to C max (t max) of 12 volunteers after consumption of different foods with relatively high levels of cyanogenic glycosides (cyanide dose 6.8 mg, but 13.6 mg in case of 200 g persipan)
| Food consumed | C max (mean ± SD in μM) | Range of C max (μM) | t max median (min) | Range of t max (min) |
|---|---|---|---|---|
| Persipan 100 g | 1.44 ± 0.60 | 0.61–2.72 | 105 | 75–120 |
| Persipan 200 g | 3.40 ± 2.38 | 0.78–9.12 | 150 | 105–260 |
| Linseed | 6.40 ± 3.34 | 1.69–13.85 | 40 | 30–60 |
| Apricot kernels | 15.46 ± 5.12 | 7.48–22.59 | 20 | 5–40 |
| Cassava | 16.95 ± 5.96 | 10.31–31.87 | 30 | 22.5–52.5 |
C max: maximum concentration achieved in the plasma following dose administration; t max: the time at which C max is attained; SD: standard deviation.
The highest blood peak levels of cyanide were again observed for apricot kernels and for cassava, indicating a rapid release of a considerable amount of cyanide. The lower peak blood levels of cyanide observed after linseed as compared to cassava and apricot kernels containing equivalent amounts of bound cyanide can been explained by the lower activity of the degrading enzymes in linseed, in particular of the respective β‐glucosidase (Schneider et al., 2014; Abraham et al., 2016). The slow release of cyanide from linseed has also been reported by Schulz et al. (1982). Even lower peak levels were observed after consumption of 100 g persipan paste, most likely due to heating during the production process leading to a distinctly reduced activity of the plant β‐glucosidase (Abraham et al., 2016, concentration–time curves are displayed in Appendix C). Several reports are available on the fate of CNGs from insufficiently processed cassava in various African populations. Brimer and Rosling (1993) demonstrated for the first time that linamarin is excreted at concentrations of about 200 nmol/mL in the urine of Mozambican subjects, indicating that the major CNG in cassava may be absorbed from the human gastrointestinal tract. Likewise, the mean urinary concentration of linamarin was about 100 nmol/mL and that of the cyanide metabolite thiocyanate was ca. 500 nmol/mL in Tanzanian subjects (Carlsson et al., 1995). Carlsson et al. (1999) concluded from another study conducted in Tanzania that about one quarter of the linamarin ingested with cassava is excreted unchanged, less than one‐half is converted to cyanide and subsequently thiocyanate and one quarter is metabolised to an as yet unknown compound. In contrast to the high levels observed by Carlsson et al. (1995) in Tanzanian subjects eating insufficiently processed cassava, urinary levels of only 14 and 50 nmol/mL of linamarin and thiocyanate, respectively, were observed in farmers in Malawi eating food prepared from bitter cassava roots after appropriate processing for detoxification (Chiwona‐Karltun et al., 2000). Similarly, low urinary concentrations of linamarin and thiocyanate were reported for Cuban subjects eating large amounts of boiled fresh roots of sweet cassava (Hernandez et al., 1995), which has much lower levels of CNGs than the bitter variety (see Section 3.2 on Occurrence data of total cyanide).
Detoxification of cyanide
The mammalian organism has developed several metabolic pathways for the detoxification of cyanide which are depicted in Figure 4 (EFSA CONTAM Panel, 2016).
Figure 4.
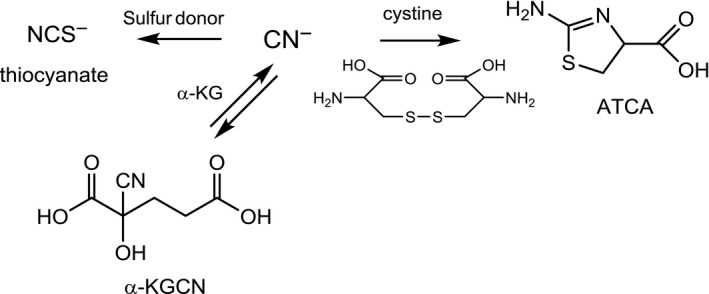
Detoxification of cyanide ions (from EFSA CONTAM Panel, 2016)
- ATCA: 2‐amino‐2‐thiazoline‐4‐carboxylic acid; α‐KG: α‐ketoglutarate; α‐KGCN: α‐ketoglutarate cyanhydrin
In the presence of a sulfur donor, e.g. thiosulfate, and a sulfur transferase, e.g. rhodanese (see below), about 70% of a dose of cyanide is metabolised to thiocyanate. In contrast to cyanide, thiocyanate does not block the electron transport in the mitochondrial respiratory chain. Based on the oral LD50 in rats, the acute toxicity of thiocyanate is about 100‐fold lower than that of cyanide (Bilska‐Wilkosz et al., 2015). Therefore, metabolism to thiocyanate is a detoxification of cyanide. At high doses, however, thiocyanate has been implicated as a possible aetiologic factor in the alteration of thyroid function and development of goitre in humans and rats, particularly if organisms are iodine deficient (Erdogan, 2003; Chandra, 2015). Like several other monovalent anions (e.g. nitrate, bromide and perchlorate), thiocyanate competes with the uptake of iodide into the thyroid follicle cells via the sodium iodide symporter (Eisenbrand and Gelbke, 2016). Thiocyanate is transferred from blood into milk, although levels in human breast milk are only about half of the maternal blood concentrations (Dorea, 2004). Confounding factors contributing to thiocyanate levels in blood and milk are tobacco smoke and the degradation of glucosinolates from certain food items. Thyroid disorders due to CNGs have only been reported in populations eating poorly detoxified cassava in areas of iodine deficiency and under conditions of insufficient protein nutrition (Dorea, 2004).
In another detoxification pathway, cyanide can react with L‐cystine through the putative intermediate β‐thiocyanoalanine to 2‐amino‐2‐thiazoline‐4‐carboxylic acid (ATCA). This pathway accounts for about 15–20% of cyanide metabolism. Thiocyanate and ATCA are chemically stable metabolites which are not further metabolised but excreted with the urine. A further detoxification pathway is the reaction of cyanide with endogenous α‐ketoglutarate to form α‐ketoglutarate cyanohydrin (α‐KGCN). This pathway is assumed to become important when the thiocyanate and ATCA pathways are overwhelmed. Other minor pathways, which are of interest primarily as biomarkers for exposure have also been described, e.g. the reaction with cysteine disulfide groups in serum albumin. In addition to binding to methaemoglobin, cyanide binds to hydroxocobalamin (vitamin B12b). The complex of cyanide with hydroxocobalamin is excreted in the urine.
In contrast to the formation of ATCA and α‐KGCN, the primary detoxification pathway of cyanide, i.e. formation of thiocyanate, involves three enzymes. The first enzyme is thiosulfate: cyanide sulfurtransferase (EC 2.8.1.1), also termed rhodanese, which transfers sulfur from thiosulfate to cyanide. The second enzyme, i.e. 3‐mercaptopyruvate: cyanide sulfurtransferase (EC 2.8.1.2, MPST) catalyses the transfer of sulfur from 3‐mercaptopyruvate to a variety of sulfur acceptors, including sulfite and cyanide. Thereby, MPST not only can provide thiosulfate to rhodanese but also directly convert cyanide to thiocyanate. 3‐Mercaptopyruvate is formed through transamination of cysteine. The third enzyme, i.e. cystathione γ‐lyase (EC 4.4.1.1, cystathionase), converts cystine to thiocysteine and thiocystine, which also serve as sulfane sulfur donor substrates for rhodanese.
Rhodanese is a ubiquitous enzyme present in many tissues of humans and other species, with the highest activities commonly measured in the liver and kidney, but also in the epithelium of rumen, omasum and reticulum of sheep and cattle. Within the cell, rhodanese is located predominantly in the mitochondria. Species differences in rhodanese activity have been reported but cannot be directly correlated with the sensitivity to cyanide because of the participation of other enzymes and pathways in cyanide detoxification. Moreover, the availability of sulfur donors is of paramount importance for the rate of detoxification of cyanide, because both rhodanese and MPST need sulfane sulfur. Indeed, the availability of sulfur appears to represent the rate‐limiting step in the detoxification of cyanide. According to Schulz et al. (1982), the rate of detoxification of cyanide in healthy humans is only about 1 μg/kg bw per min, which corresponds to about 4.2 mg cyanide per h in a 70 kg person. The major sulfur donors are the sulfur‐containing amino acids cysteine and methionine, which provide the sulfur to form thiosulfate from sulfite in the cells. Orally administered thiosulfate is very poorly absorbed from the gastrointestinal tract, and even after intravenous administration remains mostly in the extracellular space. If the availability of cysteine and methionine in humans is very low, e.g. in situations of malnutrition, formation of cyanate from cyanide has been observed (Tor‐Agbidye et al., 1999).
Summary remarks
To date, only a few studies exist on the toxicokinetics of amygdalin, prunasin and linamarin, and none on other CNGs. The limited data available suggest that gastrointestinal absorption of the intact CNG depends on the chemical structure. The release of cyanide depends mostly on the presence and activity of the respective plant enzymes. The CNGs present in apricot kernels and cassava are more rapidly degraded to cyanide than the CNGs in linseed and persipan paste. In the former, this leads to a much faster systemic uptake of cyanide and much higher peak blood and organ levels triggering a possible toxic effect. Some degradation of CNGs to cyanide appears to be mediated by the intestinal microflora.
Cyanide is readily absorbed from the gastrointestinal tract, rapidly distributed in the body and detoxified through several metabolic pathways, predominantly to thiocyanate. Toxic tissue concentrations of cyanide are to be expected if the rate of absorption exceeds the rate of detoxification for which the availability of sulfur donors is a limiting factor. In healthy humans, the rate of detoxification of cyanide is only about 1 μg/kg bw per min, which corresponds to about 4.2 mg cyanide per h in a 70 kg person (Schulz et al., 1982).
3.1.2. Biomarkers of exposure
Exposure to CNGs could, theoretically, be monitored by either measuring the absorbed parent CNGs or their common degradation product cyanide and its metabolites in plasma or tissues. Parent CNGs are only suitable biomarkers if they are absorbed to an appreciable extent, as is the case for linamarin and prunasin but not amygdalin. No data on the gastrointestinal absorption in humans of the other CNGs listed in Table 1 in Section 1.3.1 on Chemistry have been identified.
Cyanide in blood
Despite some limitations, cyanide in whole‐blood is frequently used as an exposure biomarker for CNGs. Quantification of cyanide is based on colorimetric reaction followed by spectrophotometric detection as well as high‐performance liquid chromatography‐mass spectrometry (HPLC‐MS), gas chromatography‐nitrogen phosphorous detection (GC‐NPD), gas chromatography‐electron capture detection (GC‐ECD) and GC‐MS [summarised in Agency for Toxic Substances and Disease Registry (ATSDR), 2007]. In the literature, there are different opinions concerning the biomaterial (whole‐blood, erythrocytes or plasma) to be preferred for this purpose. Since cyanide exists in blood almost entirely as HCN, whose half‐life in blood is less than 1 h, all steps of storage, sample preparation and the analytic process itself have to be carried out with caution to minimise the risk of cyanide loss and falsely low levels. After ingestion of food items containing CNGs, the peak levels of cyanide in whole‐blood, erythrocytes or plasma are used as biomarkers for cyanide‐induced acute toxic effects. Therefore, after ingestion, serial measurements of cyanide in whole‐blood have to be taken in order to identify the cyanide peak levels serving as a surrogate marker for the peak level of cyanide in tissues triggering the acute effect of cyanide (Abraham et al., 2016).
Cyanide metabolites and cyanide adducts with serum albumin in serum or plasma
As summarised in EFSA CONTAM Panel (2016), a limited number of papers suggest the cyanide metabolites thiocyanate in serum or plasma (ATSDR, 2006), ATCA in plasma (Lundquist et al., 1995; Logue, 2005, 2009; Vinnakota et al., 2012) and a thiocyanate adduct at Cys567 formed by reaction of cyanide with the C‐terminal Cys558Cys567 disulfide bond of human serum albumin (Fasco et al., 2007, 2011) as potential biomarkers for cyanide exposure. Currently, however, there is not sufficient data to determine if useful correlations exist between these potential biomarkers and the internal exposure to cyanide levels.
CNGs in urine
Several studies have used the urinary excretion of linamarin as a biomarker to assess the exposure of certain populations to cassava. For example, Hernandez et al. (1995) showed that the mean levels of linamarin increased from 2 ± 1 to 68 ± 16 μmol/L in the urine of adult Cuban men and women after consumption of 1–4 kg of boiled fresh roots of sweet cassava. In another study, it was shown that the mean value of urinary linamarin in people from konzo‐affected families in Zaire was significantly higher (632 ± 105 μmol/L in konzo patients and 657 ± 52 μmol/L in their household members) than in members of control households (351 ± 28 μmol/L) and in unaffected villages (147 ± 18 μmol/L) (Banea‐Mayambu et al., 1997).
Thiocyanate in urine
As reviewed in FAO/WHO (2012) for consumers of cassava, higher urinary thiocyanate levels have been reported as compared with individuals who never consumed cassava. Consumption of varieties of cassava with low levels of CN as well as frequent or high consumption of cassava, if processed effectively with reduced levels of CNGs, has been shown to result in low levels of urinary thiocyanate. Both occupational exposure of people working in cassava processing plants and smoking are well known to also increase urinary thiocyanate.
Summary remarks
The acute toxicity of cyanide is determined by its peak levels reached in the body, and thus, the peak cyanide blood concentration (assessed by serial measurements of cyanide in whole‐blood after ingestion) can be used as a reliable biomarker for acute cyanide exposure. The CONTAM Panel concluded that although the determination of linamarin or other partially absorbed CNGs as well as their metabolite thiocyanate in urine is useful for comparing different chronic exposure levels, it cannot provide information on the absolute exposure, because the degree of absorption and the proportion of the CNG degraded to cyanide in the intestine or colon are not known and because urinary thiocyanate might be strongly confounded by other factors including smoking.
3.1.3. Toxicity
Animals
This section summarises all data reported in previous assessments (WHO, 2004; FAO/WHO, 2012; EFSA CONTAM Panel, 2016) relevant for the present opinion in tables and reviews the most recent manuscripts not included in previous assessments.
For studies reporting only concentrations of compounds in the diet, the applied doses have been converted to mg/kg bw per day following the respective EFSA or WHO guidance (IPCS, 2009; EFSA FEEDAP Panel, 2012; EFSA Scientific Committee, 2012a).
Since the potential toxicity of CNGs in food depends on production of cyanide, toxicological data for cyanide were also reviewed.
Acute toxicity of cyanide
Acute toxicity of cyanides (HCN, NaCN, KCN, Ca(CN)2) is characterised by dyspnoea, ataxia, loss of consciousness, convulsions, asphyxiation and death in experimental animals. Acute oral LD50s have been derived from rabbit, rat, mouse and dog and values range from 2.13 to 6 mg CN−/kg bw (for details, see Table 1 of EFSA CONTAM Panel, 2016).
Repeated dose toxicity of cyanide
The identified repeated dose toxicity studies for cyanides are summarised in Tables 3 and 4. Data are organised according to the time of exposure that, among different studies, covers an interval ranging from 14 days to 11 months. Different species such as rats, mice, rabbits, pigs and goats were considered. All of them were orally exposed to KCN or NaCN dissolved in the drinking water, provided with the diet or by gavage. Histopathological alterations have most frequently been observed in the thyroid (rat, pig and goat), kidney (rat, pig and rabbit), liver (rat, pig, rabbit) and central nervous system (CNS) (rat and goat), sometimes paralleled with clinical signs.
Table 3.
Summary on repeated dose toxicity of cyanide salts with dose effect descriptors
| Compound | Animals | Exposure | CN− equivalents | Findings | Dose descriptorsa | Reference |
|---|---|---|---|---|---|---|
| KCN | Wistar rat, male, n = 6–10 per group | Drinking water, 0.0, 0.3, 0.9, 3.0 and 9.0 mg/kg bw per day, 15 days | 0.0, 0.12, 0.36, 1.2 and 3.6 mg/kg bw per day | Histopathology: kidney (congestion and cytoplasmic vacuolisation of the epithelial cells of the proximal tubules); liver: (hepatocytes degeneration); thyroid: (increased number of reabsorption vacuoles); increased plasma thiocyanate |
NOAEL: 0.36 mg CN−/kg bw per day LOAEL: 1.2 mg CN−/kg bw per day based on moderate kidney vacuolisation and congestion |
Sousa et al. (2002) |
| KCN | Pig, Landrace‐Large White, 45 days old, n = 5 or 10 per group | Diet, 0, 2, 4 and 6 mg/kg bw per day, 10 weeks | 0.0, 0.8, 1.6 and 2.4 mg/kg bw per day | Decreased ALT (≥ 0.8 mg/kg bw per day), increased urea and creatinine (1.6 and 2.4 mg/kg bw per day), thyroid weight (2.4 mg/kg bw per day). Dose‐dependent histopathological changesb of thyroid (vacuoles in the colloid of thyroid follicles), liver (karyolysis and pyknosis in hepatocytes) and kidney (degeneration of renal tubular epithelial cells). T3 and T4 were not altered | LOAEL: 2.4 mg CN−/kg bw per day based on increased thyroid weight | Manzano et al. (2007) |
| NaCN | Mouse, male and female, B6C3F1, n = 10 per group and sex |
Drinking water, males: 0.0, 0.5, 1.8, 5.1, 16.2 and 45.9 mg/kg bw per day, 13 weeks Females: 0.0, 0.6, 2.1, 6.2, 19.1 and 54.3 mg/kg bw per day, 13 weeks |
Males: 0.0, 0.27, 0.96, 2.71, 8.60 and 24.37 mg/kg bw per day Females: 0.0, 0.32, 1.11, 3.29, 10.14 and 28.83 mg/kg bw per day |
Decreased relative weight of the epididymis and cauda epididymis |
LOAEL: 8.6 mg CN−/kg bw per day based on decreased relative weight of the epididymis and cauda epididymis in males No treatment‐related effects in females |
NTP (1993) |
| NaCN | Rat, male and female, F344/N, n = 10 per group and sex |
Drinking water, males: 0.0, 0.3, 0.9, 2.7, 8.5 and 23.6 mg/kg bw, 13 weeks Females: 0.0, 0.3, 1.0, 3.2, 9.2 and 23.5 mg/kg bw per day, 13 weeks |
Males: 0.0, 0.16, 0.48, 1.44, 4.51 and 12.5 mg/kg bw per day Females: 0.0, 0.16, 0.0, 0.53, 1.70, 4.88 and 12.5 mg/kg bw per day |
Dose‐dependent reduction in cauda epididymis weight (> 1.44 mg CN−/kg bw per day), reduced number of spermatid heads per testis and spermatid count (at 12.5 mg CN−/kg bw per day), oestrous cycle variation (from 4.9 mg CN−/kg bw per day onwards); increased urine thiocyanate (from 0.48 mg CN−/kg bw per day onwards) |
LOAEL: 1.44 mg CN−/kg bw per day, based on reduction in absolute (set by NTP, 1993) and relative (set by US EPA, 2010) cauda epididymis weight BMDL1SD c: 1.9 mg CN−/kg bw per day based on decreased absolute cauda epididymis weight (as derived in FAO/WHO, 2012) |
NTP (1993) |
| NaCN | Rat, male, Wistar albino, n = 7 per group | Gavage: 0, 0.64, 1.2 and 3.2 mg/kg bw per day, 90 days | 0, 0.34, 0.64 and 1.7 mg/kg bw per day | Decreased body weight gain (1.7 mg CN−/kg bw per day), decreased testis and prostate weight (0.64 mg CN−/kg bw per day), decreased epididymis weight (1.7 mg CN−/kg bw per day), decreased sperm count and motility (0.64 mg CN−/kg bw per day) and increased sperm abnormality (1.7 mg CN−/kg bw per day), decreased follicle‐stimulating hormone (1.7 mg CN−/kg bw per day), decreased luteinising hormone and testosterone (0.64 mg CN−/kg bw per day). Histopathology: Testis (atrophy, degenerated seminiferous tubules, cell debris in the lumina), epididymis (vacuolisation in the laminar cell layer, low sperm density), prostate (decreased secretion, desquamation of the glandular epithelium) |
NOAEL: 0.34 mg CN−/kg bw per day LOAEL: 0.64 mg CN−/kg bw per day based on reduced sperm count and motility |
Shivanoor and David (2014) |
| KCN | Mini‐pig, 5 weeks old, n = 3 per group | Diet, 24 weeks | 0.0, 0.4, 0.7 and 1.2 mg/kg bw per day | Decreased T3 and T4 and behavioural changes (1.2 mg CN−/kg bw) |
NOAEL: 0.7 CN−/kg bw per day LOAEL: 1.2 CN−/kg bw per day Both based on decrease in T3 and T4 and behavioural changes |
Jackson (1988) |
ALT: alanine aminotransferase; BMDL: Benchmark dose lower confidence limit; bw: body weight; LOAEL: lowest observed adverse effect level; n: number; NOAEL: no observed adverse effect level; T3: triiodothyronine; T4: thyroxine.
Dose descriptors (e.g. NOAEL, LOAEL, BMDL as identified by the CONTAM Panel) are the points on a dose–response relationship that can be used in a risk characterisation.
Neither incidence nor statistical analysis of histological lesions was reported.
The BMDL (benchmark dose lower confidence limit) for a BMR (benchmark dose response) of one standard deviation of control mean. Lower end of a BMDL1SD: 1.9–5.6 mg CN−/kg bw per day range.
Table 4.
Summary on repeated dose toxicity of cyanides without dose descriptorsa
| Compound | Animals | Exposure | CN− equivalents | Findings | Reference |
|---|---|---|---|---|---|
| KCN | Rat, male, strain not specified, n = 10–24 per group | Diet, 0.0 or 0.2% (2 g/kg), 14 days | 0 and 800 mg/kg feed, equivalent to 96 mg/kg bw per dayd | Increased thyroid weight and TSH serum levels | Kreutler et al. (1978) |
| KCN | Wistar rat, female, n = 6 to 3 per group | Gavage, 0 or 7 mg/kg bw per day, 14 days | 0.0 and 2.8 mg/kg bw per day | Increased serum thiocyanate and blood glucose, decreased ALT, cytochrome c inhibition, hepatic rhodanese inhibition; histopathology of CNS (demyelination in medulla oblongata and chromatolysis, degeneration of cerebrocortical cells), liver (vacuolar degeneration of hepatocytes), heart (focal myocardia degeneration) and kidney (glomerular congestion, tubular lesions) | Tulsawani et al. (2005) |
| KCN | Sprague‐Dawley rat, male, n = 7 per group | Drinking water, 0 or 200 mg/L, 21 days | 0 or 80 mg/L, equivalent to 24 mg/kg bw per dayd | Increased liver weight | Palmer and Olson (1979) |
| KCN | Boer‐Spanish goat, female, 10 months old, n = 4 per group | Gavage or diet, 0 or 2.5 mg/kg bw KCN equivalent dose per day, 30 days | 0.0 or 2.4 mg/kg bw per day | Convulsion (1/4 animals, given diet, on day 6), histological lesions in the thyroid and in the mesencephalon (spongiosis and spheroids) | Soto‐Blanco et al. (2008) |
| KCN | Wistar rat, male, n = 6–7 per group | Gavage, 0.0, 0.15, 0.3 and 0.6 mg/kg bw per day, 12 weeks | 0.0, 0.06, 0.12 and 0.24 mg/kg bw per dayb | Dose‐related histopathological changes of spinal cord (spheroid bodies on white matter), neuronal loss in the hippocampus and cerebellum (damaged Purkinje cells, loss of white matter) | Soto‐Blanco et al. 2008 |
| KCN | Rat, male | Drinking water, 0, 40, 80 and 160 mg/kg bw per day, 13 weeks | 0, 16, 32 and 64 mg/kg bw per day | Increased proteinuria, dose‐dependent increase relative organ weight, reduced thymus weight (160 mg/kg bw) | Leuschner and Neumann (1989) |
| KCN | Wistar rat, male and female, n = 10 per group | Gavage, 0 or 1.4 mg/kg bw per day, 13 weeks | 0.0 or 0.56 mg/kg bw per day | Decreased motor coordination, oxidative damage (liver and brain), histopathology of liver (microgranuloma, spotty necrosis, moderate portal inflammation) | Mathangi et al. (2011) |
| KCN | Alpine‐Saanen goat, 30–45 days old, n = 6–8 per group | Milk (for 3 months) then drinking water (for 2 months), 0.0, 0.3, 0.6, 1.2 and 3 mg/kg bw per day | 0.0, 0.12, 0.24, 0.48 and 1.2 mg/kg bw per day | Muscular tremors and ataxia (1/8, at highest dose), congestion, haemorrhage and gliosis in cerebellum, pons and spinal cord and spheroids on the grey matter of the spinal cord (at two highest doses)b; Damage and loss of Purkinje cells in the cerebellum, spongiosis in the pons and spheroids, axonal swelling, gliosis, spongiosis and ghost cells in the medulla oblongata (high‐dose group) | Soto‐Blanco et al. (2002) |
| KCN | New Zealand rabbit, male, n = 6 per group | Diet, cyanide control diet 9 ppm, KCN‐enriched diet: 702 mg/kg 10 months | 0.2 and 20 mg/kg bw per day | Decrease body weight and feed efficiency (20 mg/kg bw), increased clinical chemical (serum) parameters, ALP reduced in lung, increased LDH activity in liver and kidney, histopathology of liver (focal areas of hepatic necrosis, congestion), kidney (tubular and glomerular necrosis) and lungs (focal pulmonary oedema and necrosis) | Okolie and Osagie (1999, 2000) |
| KCN | Rat, strain not specified, male weanlings (43 g bw), n = 6 per group | 0 or 1,500 mg/kg feed, 4 and 11 months | 0 or 44 mg/kg day,c equivalent to 75 mg/kg bw per dayd | Decreased bw, reduced plasma thyroxine (only 4 months), 11 months: increased relative thyroid weight, histopathology of spinal cord (vacuolisation of the white matter); reduced cyanide metabolism into thiocyanate | Philbrick et al. (1979) |
ALP: alkaline phosphatase; ALT: alanine aminotransferase; bw: body weight; CNS: central nervous system; LDH: lactate dehydrogenase; n: number; TSH; thyroid‐stimulating hormone.
Dose descriptors (e.g. NOAEL, LOAEL, BMDL as identified by the CONTAM Panel) are the points on a dose–response relationship that can be used in a risk characterisation.
NOAEL and LOAEL cannot be derived due to approximate description of histopathological changes.
Based on the average food intake across rat strain and adjusting for molecular weight ratio of cyanide to potassium cyanide (US EPA, 2010).
No deaths have been reported in these repeated dose toxicity studies, although some of the doses were equal or higher than the respective oral LD50s. The absence of mortality in these studies is possibly due to the lack of exhaustion of the detoxification activity of rhodanese, which is allowed by the slower absorption rate following dietary exposure but not after bolus administration as it occurs in LD50 tests (Hayes, 1967).
For five (Jackson, 1988; NTP, 1993; Sousa et al., 2002; Manzano et al., 2007; Shivanoor and David, 2014) of 15 studies, dose descriptors28 (i.e. NOAEL, Lowest observed adverse effect level (LOAEL) or BMDL) were available (Table 3).
The CONTAM Panel noted poor reporting of the study design and results in the studies of Manzano et al. (2007), Jackson (1988) and Sousa et al. (2002). For the Manzano et al. (2007) study, inconsistencies between methods and results sections regarding the number of animals per experimental group have been identified. Poor reporting on statistics and inconsistencies in the reporting of the number of male and female animals are major limitations in the Jackson (1988) study. Sousa et al. (2002) reported histological lesions in the kidney based on a limited number of animals per each experimental group (n = 3).
In the 13‐week NTP studies (NTP, 1993), left epididymis weight was decreased significantly in male rats and mice treated with the high dose. Left testis weight was also decreased in high‐dose rats. However, the magnitude of these effects was small (≤ 10%) and therefore, not considered to be biologically meaningful. Significantly decreased left cauda epididymis weight was observed in rats (all three dose groups, 13% in the high‐dose group) and in mice (high‐dose group, 18%). Because of the lack of precise boundaries between the epididymal corpus and cauda, the gross anatomical dissection does not allow a precise separation of each epididymal region, and consequently, changes in epididymal cauda weights are associated with a high level of uncertainty and therefore, not considered useful for risk assessment. The number of spermatid heads per testis was significantly decreased in high‐dose rats (14%). However, the assessment of the level of spermatogenesis used in the NTP report is based on a method not well defined nor validated.29 The sperm motility was significantly lower in rats (all three dose groups); however, the magnitudes were small (2.3–3.8%) and not dose dependent and therefore, not considered to be biologically relevant. No histopathological changes were observed in testes or epididymides in rats or mice. The CONTAM Panel concluded that the findings in the NTP studies cannot be used as a basis to set a point of departure for effects on the male reproductive system.
In a more recent rat study (Shivanoor and David, 2014), effects on the male reproductive system were also reported mainly in the high‐dose group, i.e. decreased absolute testis, epididymis and prostate weights; decreased sperm count and motility, sperm abnormality; changes in hormone levels (FSH, LH, testosterone); and histopathological changes in testis, epididymis and prostate. The CONTAM Panel noted several inconsistencies in the reporting. Therefore, the CONTAM Panel was not able to interpret the findings.
Acute toxicity of individual cyanogenic glycosides (CNGs)
Acute toxicity of CNGs depends on the release of cyanide and its subsequent absorption. It is characterised by arrhythmias, ataxia, convulsions, lethargy, decreased respiratory rate and death (Tables 5 and 6). Acute oral LD50s of prunasin, amygdalin and linamarin have been derived from rats and range from 450 to 880 mg/kg bw. LD50s expressed as equivalents of CN− range from 29.6 to 51.0 mg CN−/kg bw (Table 5). The slow and incomplete release of cyanide from CNGs explains the lower acute toxicity as compared to cyanide (EFSA, 2004; EFSA CONTAM Panel, 2016).
Table 5.
Median lethal doses (LD50s) of cyanogenic glycosides
| Compound | Animals | Exposure | CN− equivalents | LD50s | Reference |
|---|---|---|---|---|---|
| Linamarin | Rat, strain and sex not specified, n = not specified | Gavage, variable, single | Not reported |
LD50: 450 mg/kg bw LD50: 47.3 CN− mg/kg bwa |
Oke (1979) |
| Amygdalin | Fischer 344 rat, female, n = 5–20 per group | Gavage, 0 and < 400–1,100 mg/kg bw, single | 0.0 and 22.7–62.4 mg/kg bwa |
LD50: 522 mg/kg bw LD50: 29.6 CN− mg/kg bwa |
Newton et al. (1981) |
| Amygdalin | Wistar rat, n = 20 for LD50 | Gavage, 0 and 600–1,400 mg/kg bw, single | 0.0 and 34–79.4 mg/kg bwa |
LD50: 880 mg/kg bw LD50: 49.9 CN− mg/kg bwa |
Adewusi and Oke (1985) |
| Prulaurasin b (95% pure) | Wistar rat, male, n = 6 per group | Gavage, 0 and 300–1,000 mg/kg bw, single | 0.0 and 27.3–91 mg/kg bwa |
LD50: 560 mg/kg bw LD50: 51.0 CN− mg/kg bwa |
Sakata et al. (1987) |
LD50: (median lethal dose); n: number.
Calculated considering 1,000 mg prunasin equivalent to 87.98 mg CN−; 1,000 mg amygdalin equivalent to 56.7 mg CN−; 1,000 mg linamarin equivalent to 105.2 mg CN− (see FAO/WHO, 2012).
Prulaurasin (D,L‐Mandelonitrile‐b‐d‐glucoside) is a mixture of prunasin and sambunigrin.
Table 6.
Summary on acute toxicity of cyanogenic glycosides
| Compound | Animals | Exposure | CN− equivalents | Findings | Reference |
|---|---|---|---|---|---|
| Linamarin | Wistar rat, male, n = 12 | Gavage, 0 and 500 mg/kg bw, single | 0.0 and 52.6 mg/kg bwa | Cardiac arrhythmias, ataxia, respiratory changes, death | Philbrick et al. (1977) |
| Amygdalin (approx. 99% pure) | Sprague‐Dawley rat, n = 25 | Oral, 600 mg/kg bw, single (no control) | 34 mg/kg bwa | Lethargy, convulsion and death (12 of 25 animals); increased blood concentration of cyanide and thiocyanate | Carter et al. (1980) |
| Linamarin | Wistar rat, male, n = 9 | Gavage, 0, 250 or 500 mg/kg bw, single | 0, 26.3 and 52.6 mg/kg bwa | Metabolic acidosis, decreased cytochrome oxidase activity, atrial fibrillation, decreased respiratory rates, death (500 mg/kg) | Philbrick et al. (1981) |
| Amygdalin (99% pure) | Golden Syrian hamster, female, n = 20 | Gavage, 201 mg/kg bw, single (no control) | 11.4 mg/kg bwa | Symptoms of cyanide poisoning, 20% mortality rate; increased blood cyanide and thiocyanate | Frakes et al. (1986) |
| Linamarin (> 95% pure) | Golden Syrian hamster, female, n = 22 | Gavage, 108 mg/kg bw, single (no control) | 11.36 mg/kg bwa | Symptoms of cyanide poisoning, 18% mortality rate; increased blood cyanide and thiocyanate | Frakes et al. (1986) |
n: number; bw: body weight.
Calculated considering 1,000 mg amygdalin equivalent to 56.7 mg CN−; 1,000 mg linamarin equivalent to 105.2 mg CN− (see FAO/WHO, 2012).
Additional studies reporting acute toxicity and death induced by CNGs, without deriving LD50, are summarised in Table 6.
No studies on acute toxicity of foods containing CNGs were identified by the CONTAM Panel.
Repeated dose toxicity of individual CNGs and foods containing CNGs
For individual CNGs, the only repeated dose toxicity studies identified were carried out with linamarin and amygdalin (Table 7). In all three studies, only one dose level was used and effects on haematology and clinical chemistry parameters were observed.
Table 7.
Summary on repeated dose toxicity of cyanogenic glycosides
| Compound | Animals | Exposure | CN− equivalents | Findings | Reference |
|---|---|---|---|---|---|
| Amygdalin (Laetrile) ≥ 97% a | Duncan‐Hartley Guinea pig, n = not reported | 0 or 10 mg in 10% sucrose solution per day, 24 days | 0.0 or 0.57 mg/kgb equivalent to 0.44 mg/kg bw per dayc | No effect on body weight and liver | Basu (1983) |
| Linamarin | Wistar rat, male, n = 6 | Gavage, 0 or 94 mg/kg bw per day, 5 weeks | 0.0 or 9.89 mg/kg bwb | Reduced blood systolic pressure, cardiac cytochrome oxidase activity, increased LDH/pyruvate ratio | Philbrick et al. (1977) |
| Amygdalin ≥ 97% b | Rat, male, n = 8 | Gavage, 0 or 20 mg/kg bw per day, 14 weeks | 0.0 or 1.13 mg/kg bwb | Increased haemoglobin concentration, packed cell volume and serum lactate, decrease in blood pH | Oyewole and Olayinka (2009) |
LDH: lactate dehydrogenase; bw: body weight; n: number.
Amygdalin was purchased from Sigma‐Aldrich, purity derived from the commercial catalogue. Sigma uses the term laetrile as a synonym for amygdalin which is not correct.
Calculated considering 1,000 mg amygdalin equivalent to 56.7 mg CN−; 1,000 mg linamarin equivalent to 105.2 mg CN−.
Repeated dose toxicity studies with foods containing CNGs have been extensively reviewed in FAO/WHO (2012) and are summarised in Table 8. A study performed on dogs fed a cassava or NaCN‐containing diet (Kamalu, 1993) has been excluded because of the potential impact of the parallel treatment of the animals with ecto‐ and endoparasites. Rivadeneyra‐Domínguez et al. (2013) administered intraoesophageally linamarin in cassava juice (0.075–0.3 mg/kg bw) to male Wistar rats once a day for 28 days and observed dose and time‐dependent increases in locomotor activity and uncoordinated behaviour. In the applied cassava juice, linamarin was quantified by HPLC‐UV, no other CN containing molecules or total CN were assessed. Since the presence of other CNG‐releasing compounds as well as CNG degradation products cannot be excluded, the CONTAM Panel decided that this study cannot be used to for risk assessment.
Table 8.
Summary of repeated dose toxicity of foods containing cyanogenic glycosides
| Food | Animals | Exposure | CN− equivalents | Findings | Reference |
|---|---|---|---|---|---|
| Gari | Dog, male, n = 6 | Diet, control with rice or with cassava, estimated release of HCN 10 mg/kg cooked food; 100 g diet per day, 1.08 mg/kg bw HCN per day,a 14 days | 1.04 mg/kg bw per day | Proteinuria, histopathology of kidney (congestion, vacuolisation, swelling and rupture of proximal tubules epithelial cells), liver (congestion, periportal vacuolation) and myocardium (haemorrhage, pyknotic nuclei, fibre muscle swelling). Increased plasma thiocyanate. | Kamalu (1993) |
| Cassava | Wistar rat, male, n = 10 | Diet, normal rat feed or 75% fresh cassava root, 30 days | 8–10 mg/kg, equivalent to 1.0–1.2 mg/kg bw per dayb | Behavioural changes (open field) and decreased catecholamine in the hypothalamus | Mathangi and Namasivayam (2000) |
| Cassava | Sprague‐Dawley rat, male, n = 6 | Diet, cassava free or 71% boiled cassava, ad libitum, 60 days | 7–9 mg/kg, equivalent to 0.8–1.1 mg/kg bw per dayb | Increased hepatic rhodanese; serum thiocyanate and blood cyanide | Boby and Indira (2004) |
| Cassava | Wistar rat, male and female, n = 10 per group | Diet, normal rat chow, 50% (I) or 75% (II) fresh cassava, 1 year |
Diet I = 0.075 mg per animal per day Diet II = 0.102 mg per animal per day equivalent to approx. 0.0075 and 0.01 mg/kg bw per dayb |
Serum insulin (only diet II), histopathology of pancreas mild atrophy of the acini, minimal focal dilatation of ducts (only diet III) and liver (hyperplasia, microvascular changes in hepatocytes), decreased body weight, motor incoordination | Mathangi et al. (1999); Mathangi and Namasivayam (2000) |
Histopathologic lesions in kidney, liver, pancreas, myocardium and behavioural changes were observed upon repeated dose exposure to CNGs producing foods and products thereof (Table 8).
Developmental toxicity of cyanide, individual CNGs and foods containing CNGs
Tables 9, 10, 11–12 summarise developmental toxicity studies with cyanide, CNGs or foods containing CNGs. Animals were exposed via the diet, drinking water or by gavage either at gestation day (GD) 8 (Willhite, 1982; Frakes et al., 1985; see Table 10) or during GD6‐GD20 according to standardised protocols (de Sousa et al., 2007, see Table 9; Soto‐Blanco and Gorniak, 2004, see Table 11; Frakes et al., 1986, see Table 12) or during gestation to postnatal day (PND) 50 (Malomo et al., 2004, see Table 9; Imosemi et al., 2005, see Table 11). Tewe and Maner (1981) fed rat dams and pups for 49 and 28 days, respectively.
Table 9.
Summary on developmental studies of cyanides providing dose descriptors
| Compound | Animals | Exposure | CN− equivalents | Findings | Dose descriptorsa | Reference |
|---|---|---|---|---|---|---|
| KCN | Wistar rat, n = 10 dams per group; 40 foetuses over 10 L | Drinking water, 0.0 1.0, 3.0 and 30 mg/kg bw per day, GD6 to GD20 | 0.0 0.4, 1.2 and 12 mg/kg bw per day | Dams (GD20): increased glucose; increased serum thiocyanate Dams (GD20) and litter (PND21): histopathology of liver (congestion, microvesicular vacuolisation of hepatocytes) and CNS (focal neuronal necrosis, focal nodular gliosis, mild congestion and white matter vacuolisation in the cerebellum) at 12 mg/kg bw per day |
NOAEL: 1.2 mg/kg bw per day LOAEL: 12 mg/kg bw per day Based on histological alterations both in dams and PND21 litter |
de Sousa et al. (2007) |
| KCN | Wistar rat, dams n = 20; offspring n = 5 | Diet, 0 or 500 mg/kg feed per day, gestation to PND50 | 0 or 20 mg/kg bwb per day | Litter: altered cerebellar development | LOAEL: 20 mg/kg bw(c) per day based on altered maturation of cerebellum | Malomo et al. (2004) |
bw: body weight; CNS: central nervous system; GD: gestation day; LOAEL: lowest observed adverse effect level; n: number; NOAEL: no observed adverse effect level; PND: post‐natal day.
Dose descriptors (e.g. NOAEL, LOAEL, BMDL as identified by the CONTAM Panel) are the points on a dose–response relationship that can be used in a risk characterisation.
Calculated by FAO/WHO (2012).
Table 10.
Summary on developmental studies of cyanogenic glycosides providing dose descriptors
| Compound | Animals | Exposure | CN− equivalents | Findings | Dose descriptorsa | Reference |
|---|---|---|---|---|---|---|
| D,L‐Amygdalin | Golden Syrian hamster, dams n = 5–12 per group, foetuses n = 66–100 | Gavage, 0, 200, 225, 250 and 275 mg/kg bw, single, GD8 | 0.0, 11.3, 12.8, 14.2 and 15.6 mg/kg bwb |
Respiratory effects, ataxia and convulsion in mothers (≥ 14.2 mg CN−/kg bw) Dose‐dependent foetus malformation (≥ 14.2 mg CN−/kg bw) at GD14 |
NOAEL: 225 mg amygdalin/kg bw or 12.8 mg CN−/kg bwb LOAEL: 250 mg amygdalin/kg bw or 14.2 mg CN−/kg bwb based on foetal abnormalities |
Willhite (1982) |
| Linamarin 95% pure | Golden Syrian hamster, dams n = 10–13, foetuses n = 54–67 over 11 to 8 L | Gavage, 0, 70, 100, 120 and 140 mg/kg bw, single, GD8 | 0.0, 7.4, 10.5, 12.6 and 14.7 mg/kg bwb |
Dams: reversible dose‐dependent dyspnoea, ataxia, tremors and hypoxia starting from 10.5 mg CN−/kg bw, death (1/11 at 120 mg/kg bw per day; 2/13 140 mg/kg bw per day) Dose‐dependent increase in foetal skeletal defects at GD15 No differences in litter with prenatal deaths, number of live foetuses per litter and foetal body weight |
NOAEL: 70 mg linamarin/kg bw or 7.4 mg CN−/kg bwb LOAEL: 100 mg linamarin/kg bw per day or 10.5 mg CN−/kg bw per dayb based on foetal skeletal defects BMDL10: 85 mg linamarin/kg bw per day or 8.9 mg CN−/kg bw per dayb based on foetal skeletal defects |
Frakes et al. (1985) |
bw: body weight; BMDL10: 90th percentile benchmark dose lower confidence limit; GD: gestation day; GD: gestation day; NOAEL: No observed adverse effect level; LOAEL: Lowest observed adverse effect level.
Dose descriptors (e.g. NOAEL, LOAEL, BMDL as identified by the CONTAM Panel) are the points on a dose–response relationship that can be used in a risk characterisation.
1,000 mg amygdalin is equivalent to 56.7 mg CN−; 1,000 mg linamarin is equivalent to 105.2 mg CN−.
Table 11.
Other developmental studies with cyanide without dose descriptorsa
| Compound | Animals | Exposure | CN− equivalents | Findings | Reference |
|---|---|---|---|---|---|
| KCN | Goat, 1–3 years old, n = 8 to 4 per group | Gavage, 0, 1, 2 and 3 mg/kg bw per day, 24 GD to parturition | 0, 0.4, 0.8 and 1.2 mg/kg bw per dayb | Dams (highest dose): ataxia, convulsions (2/8); abortion (1/8); elevated T3 levels at day 1 returned to control at day 8; vacuoles in thyroid follicular colloid, cerebral spongiosis, cerebellar myelin oedema at 120 day of pregnancy (1/1), no histopathological lesions 3 months after delivery (1/1). Litter: (highest dose): 2 prognata born, 1 prognata aborted; elevated T3 levels at day 1 returned to control at day 8 | Soto‐Blanco and Gorniak (2004) |
| HCN | Rat | Diet, cassava+dietary component to provide HCN 12 mg/kg diet, 49 days dams and 28 days pups | 11.5 mg/kg diet per day, equivalent to 0.115 mg/kg bw per dayc | Pre‐weaning period: increased serum thiocyanate; post‐weaning period: increased serum thiocyanate, reduced feed consumption and daily growth; Dams: gestation and lactation performances not affected | Tewe and Maner (1981) |
| KCN | Wistar rat, dams n = 20, offspring n = 5 | Diet, 0 or 500 mg/kg feed per day, gestation to PND50 | 0 or 20 mg/kg bw per dayb | Dams: aggressive and restless behaviour; Litter (PND1 to 50): reduction of body, brain (PND9 and 14) and cerebellar (PND14, 21, 28) weight, reduced vermal length (PND50) reduced cerebellum (PND28) | Imosemi et al. (2005) |
bw: body weight; GD: Gestation day; n: number; PND: Postnatal day; T3: Triiodothyronine.
Dose descriptors (e.g. NOAEL, LOAEL, BMDL as identified by the CONTAM Panel) are the points on a dose–response relationship that can be used in a risk characterisation.
FAO/WHO could not identify a LOAEL due to the lack of incidence and severity data of histological effects.
Table 12.
Other developmental studies with cyanogenic glycosides or foods containing cyanogenic glycosides
| Compound | Animals | Exposure | Cyanide (CN−) equivalents | Findings | Reference |
|---|---|---|---|---|---|
| Prunasin >90% purity | Golden Syrian hamster, dams n = 8 | Gavage, 0 or 177 mg/kg bw, single, GD8 | 0 or 15.55 mg/kg bwa | Foetus malformation in 15% of living foetuses at GD14 | Willhite (1982) |
| Cassava | Golden Syrian hamster, dams, n = 8–12 per group | Diet, without or with high and low cyanide cassava varieties, GD3–GD15 | 0 or 21 mg/kg bw per day (high), 1.6 mg/kg bw per day (low) per day |
Delayed foetal ossification and dose‐dependent increase of pups with a reduced bw No significant differences in number of implantation, resorptions or live foetuses per litter for both low and high diet Increased blood and urine thiocyanate in mothers, increased foetal thiocyanate |
Frakes et al. (1986) |
bw: body weight; GD: Gestation day; n: number.
Calculated considering 1,000 mg prunasin equivalent to 87.88 mg CN−.
Notably, in five of eight studies, effects in pups have been reported at KCN or CNG doses also toxic to dams (histopathological alterations, ataxia, convulsions and hypoxia), and in consequence, it cannot be excluded that these effects are secondary to maternal toxicity and thus are not specific to development.
Exposed litters mostly display damaged CNS and/or skeletal malformations both after exposure to KCN or CNGs.
The results of four of eight studies allowed derivation of dose descriptors for KCN, amygdalin and linamarin (see Tables 9 and 10). LOAELs in the respective studies range from 8.9 mg CN−/kg bw to 20 mg CN−/kg bw. The JECFA selected skeletal defects in hamster foetuses seen in a developmental toxicity study with linamarin (Frakes et al., 1985) as the appropriate endpoint for an acute dose–response analysis (see Section 1.3.3 on previous risk assessments).
Genotoxicity
Genotoxicity of cyanide
Genotoxicity of cyanide has been summarised in EFSA (2004), WHO (2004), ATSDR (2006), US Environmental Protection Agency (EPA) (2010) and FAO/WHO (1993, 2012).
KCN and/or NaCN did not induce reverse mutations in S. Typhimurium (strains TA79, TA98, TA100, TA1535, TA1537, TA1538) with or without metabolic activation (De Flora, 1981; De Flora et al., 1984; NTP, 1993; Kubo et al., 2002). FAO/WHO (1993) reported that KCN was negative in an Ames test with Salmonella strains TA1537, TA1538 and TA98 with and without metabolic activation, and in a gene mutation assay (HGPRT locus) in cultured Chinese hamster V79 cells with and without metabolic activation up to high, cytotoxic concentrations (Leuschner et al., 1983, 1991, unpublished studies submitted to WHO).
FAO/WHO (1993) also reported that HCN did not induce chromosomal aberrations in vivo in Chinese hamsters treated orally by gavage with a single dose of 0.4 mg HCN/kg bw (Leuschner et al., 1983, 1991, unpublished report submitted to WHO).
KCN induced direct non‐reparable DNA damage in repair‐deficient E. coli strains (WP67, CM871, WP2) (De Flora et al., 1984).
A number of studies have reported that cyanide induces DNA fragmentation at high concentrations in vitro (Bhattacharya and Rao, 1997; Vock et al., 1998), or following intraperitoneal (Mills et al., 1999) and subcutaneous (Yamamoto and Mohanan, 2002) administration to mice. These studies indicate that DNA fragmentation is secondary to the general toxicity of cyanide which results in the release of endonucleases from dying cells. It is notable that KCN has been used as a model cytotoxic non‐genotoxic agent in studies aimed at determining whether the in vitro alkaline elution hepatocyte assay (Storer et al., 1996) and the in vitro Comet assay (Henderson et al., 1998) can discriminate between genotoxic and cytotoxic substances.
The CONTAM Panel concluded that the available data indicate that cyanide is not genotoxic.
Genotoxicity of cyanogenic glycosides
In 2012, JECFA concluded that there was no information available on the genotoxicity of CNGs (FAO/WHO, 2012), and likewise, the CONTAM Panel did not identify studies on genotoxicity of isolated CNGs carried out since then. Application of Quantitative Structure Activity Relationships in the OECD toolbox (version 4.3.130 ) does not indicate a concern for genotoxicity.
3.1.4. Observations in humans
Acute toxicity
The signs and symptoms of cyanide poisoning reflect the extent of cellular hypoxia and occur when the absorption rate of cyanide exceeds its metabolic detoxification. Signs of acute cyanide poisoning include headache, severe hypotension, vertigo, agitation, respiratory depression, metabolic acidosis, confusion, coma, convulsions and death. Definitive laboratory confirmation is generally delayed, but elevated plasma lactate, associated with cardiovascular collapse, and sometimes ‘almond smell’ of the patient's breath, should suggest cyanide intoxication. Furthermore, a low arteriovenous difference of oxygen in blood indicates cyanide intoxication. Cyanide poisoning treatment is based on supportive care with adjunctive antidotal therapy. Multiple antidotes exist and are characterised by different antidotal mechanisms, such as chelation, formation of stable, less toxic complexes, methaemoglobin induction and sulfur supplementation for detoxification by endogenous rhodanese (Borron and Baud, 2012).
Mainly based on the results of Rumack (1983), the toxic threshold value for cyanide in the whole‐blood is considered to be between 0.5 mg/L (ca. 20 μM) and 1.0 mg/L (ca. 40 μM), and the lethal threshold value between 2.5 mg/L (ca. 100 μM) and 3.0 mg/L (ca. 120 μM). A substantial degree of uncertainty is associated to these values due to the fact that the blood samples were collected sometime after the occurrence of the peak blood level (3.1.1). Consequently, these values are to be considered as an underestimation of the cyanide lethal blood level.
The acute lethal oral dose of cyanide in humans is reported to be between 0.5 and 3.5 mg/kg bw. There are a number of reports of fatal and non‐fatal cyanide acute toxicity cases following the ingestion of cyanogenic foods other than apricot kernels. For several of these studies, time of blood sampling is not reported; for others, it is likely that the blood samples were collected sometime after the occurrence of the peak blood level. The reported blood cyanide concentrations among the studies should thereby not be compared to each other and do not necessarily correlate with the severity of the toxic effects after cyanide poisoning.
A 67‐year‐old woman weighing 60 kg with a carcinoma of the large bowel arrived to the emergency room in a comatose state. Blood levels of cyanide were higher than 2 mg/L (ca. 80 μM). The patient fully recovered after treatment. Five months before admission to hospital, the patient self‐administered Laetrile31 by injection (not further specified) for a 2‐month period and subsequently switched to ‘Laetrile tablets’ for a 6‐month period. The day before hospitalisation, the patient had additionally eaten five grounded bitter almonds and started to vomit and having crampy abdominal pains. On the day of admission, she felt well in the morning, and at night, she took another 12 bitter almonds and subsequently collapsed (Shragg et al., 1982). Most likely, the additionally eaten almonds led to additional exposure to cyanide originating from the almonds itself as well as from higher release from Laetrile, due to the almond β‐glucosidase.
A few hours after having eaten ‘gari’ (a cassava based meal), an 18‐year‐old woman started having abdominal pain associated with vomiting and fell into a coma thereafter. She was thus transferred to the emergency room where she died after 24 h from cardiorespiratory arrest. The blood and urine were sampled as soon as the woman was hospitalised and the levels of cyanide were 1.15 mg/L (ca. 46 μM) and 0.67 mg/L, respectively. At the same time as the first patient, an 8‐year‐old boy was brought to the emergency room in a comatose state. It was reported that the boy had been in that state for almost 12 h after sharing the same cassava‐based meal with the first patient. The boy died the same day from cardiorespiratory arrest and his blood and urine levels of cyanide were 0.85 (ca. 34 μM) and 0.56 mg/L, respectively. A 17‐year‐old girl, referred to the hospital with the other two patients after eating the same meal, was conscious on admission, but died after the development of shock and renal failure. Her blood and urine cyanide levels were 1.35 mg/L (ca. 54 μM) and 0.40 mg/L, respectively (Akintonwa and Tunwashe, 1992).
Five male students with a mean age of 24 years, presented with vomiting, abdominal cramps and dizziness 1 h after sharing a cassava‐based meal. All patients recovered fully within 5 h of ingestion and the authors reported that only ‘traces’ of cyanide were detected in blood and urine samples. Similarly, 12 patients presented symptoms of cyanide toxicity after sharing a meal of ‘gari’. All patients fully recovered within 24 h (Akintonwa et al., 1994).
The authors describe also a case of five patients who developed severe signs of cyanide toxicity and finally became comatose 10 h after eating a meal of ‘gari’. Blood cyanide concentration was on average 1.75 mg/L (ca. 70 μM), while the average urine level of cyanide was 0.75 mg/L. All patients died (Akintonwa et al., 1994).
An epidemic of acute intoxication associated with the consumption of bitter cassava was reported in Mozambique when 70 patients were hospitalised with vomiting, abdominal pain, headache, and in more severe cases had altered consciousness and dyspnoea. The clinical picture was consistent with acute cyanide intoxication and all patients reported eating bitter cassava before the onset of the symptoms. Blood cyanide levels were not reported (Cliff and Coutinho, 1995).
A 56‐year‐old woman weighing 60 kg had eaten about 300 g of alcohol‐steeped cherries and developed severe headache followed by nausea, vomiting and sleepiness. On hospital admission, she appeared confused and severely dyspnoeic and was found comatose a few minutes later. Blood analysis revealed severe metabolic acidosis. The patient was intubated and received artificial ventilation. She regained consciousness on the following day, but still was confused and disorientated for the next 14 days, manifesting hallucinations and psychomotor agitation. Twenty‐seven days after admission, she began complaining of blurred vision and distal paraesthesia of the lower limbs. The neurological examination revealed slowed velocity of motor and sensory conduction; there was no spontaneous activity in the muscles. Magnetic resonance imaging showed mild cortical and subcortical atrophy and bilateral high signal intensities in certain brain regions. Fourteen months later, the patient was fully oriented, but she had a masked face, mild rigidity of the upper and lower limbs, a shuffling gait and increased salivation. Her visual acuity was still impaired, but there were no clinical signs of motor‐sensory neuropathy. L‐Dopa treatment did not improve the parkinsonian syndrome. This syndrome is observed in severe cases of intoxications with cyanide salts (e.g. Rosenberg et al., 1989). Laboratory analyses of the spirit and cherries showed cyanide levels ranging from 4.7 to 15 mg/kg in the cherries and from 43 to 45 mg/kg in the spirit. The total cyanide dose was estimated to be between 10 and 20 mg. The case was interpreted as life‐threatening cyanide intoxication with remaining neurological deficits (Pentore et al., 1996). However, cyanide blood levels were not measured, and the estimated dose seems too low to cause such a severe intoxication.
A 30‐month‐old girl suffered severe signs of cyanide toxicity after eating five bitter almonds. Her blood cyanide level was 2.33 mg/L (ca. 93 μM); however, she recovered after treatment with hydroxocobalamin (Nader et al., 2010).
A 58‐year‐old healthy woman developed symptoms of cyanide toxicity 2 h after eating about 50 bitter almonds. Her blood cyanide concentration was 2.77 mg/L (ca. 111 μM) 6 h after coma onset. She recovered following treatment (Sanchez‐Verlaan et al., 2011).
A 5‐year‐old boy ingested 10 bitter almonds and after 3 h developed dizziness, confusion, somnolence and vomiting. He then developed generalised tonic–clonic seizures, and finally became comatose. The child completely recovered after treatment in an intensive care unit. No cyanide levels have been reported (Mouaffak et al., 2013).
After undergoing a routine cystoscopy requiring general anaesthesia, a 67‐year‐old man appeared hypoxic with peripheral pulse oximetric measurement: oxygen levels as measured by pulse oximetry increased slowly to 94%, despite continued administration of 100% oxygen therapy during and after anaesthesia. Doctors confirmed the presence of cyanide in the body in venous blood through a thiocyanate assay, with levels equal to 521 μmol thiocyanate/L, and whole‐blood cyanide levels of 1.6 mg/L (ca. 64 μM). It was then discovered that the patient self‐administered three 2 g tablets of Novodalin (a proprietary amygdalin preparation) and had two teaspoons of home‐made apricot kernel extract per day. Analysis of Novodalin showed cyanide levels of 220 mg/kg and the homemade apricot kernel extract 1,600 mg/kg of cyanide, meaning that the patient ingested daily approximately 17.32 mg of oral cyanide (Konstantatos et al., 2017). While the high blood cyanide level is explained by the ‘medication’ of the patient, it should be noted that cyanide intoxication does not lead to blood hypoxia. The authors discussed a possible functional failure of the peripheral pulse oximetry due to the high cyanide levels.
In the previous opinion (EFSA CONTAM Panel, 2016), all scientific articles concerning human poisoning associated with ingestion of CNGs in herbal preparations or ‘alternative medical treatments’ were described, and since then, no further studies have been published.
Summary remarks on acute toxicity
There are a number of reports of acute cyanide toxicity following the ingestion of amygdalin preparations or cyanogenic foods, primarily apricot kernels, bitter almonds and insufficiently processed cassava. Some of these cases were fatal.
The signs and symptoms of acute cyanide poisoning reflect the extent of cellular hypoxia and occur when the absorption rate of cyanide exceeds its metabolic detoxification. These symptoms may include headache, severe hypotension, vertigo, agitation, respiratory depression, metabolic acidosis, confusion, coma, convulsions and death. The acute lethal oral dose of cyanide in humans is reported to be between 0.5 and 3.5 mg/kg bw. The toxic threshold value for cyanide in the whole‐blood is considered to be between 0.5 mg/L (ca. 20 μM) and 1.0 mg/L (ca. 40 μM), and the lethal threshold value between 2.5 mg/L (ca. 100 μM) and 3.0 mg/L (ca. 120 μM).
Long‐term toxicity
Several neurological disorders, such as spastic paraparesis (konzo), tropical ataxic neuropathy and ankle clonus, have been associated to dietary chronic exposure to cyanide in populations where cassava constitutes the main source of calories. Moreover, in areas with low iodine intake, cyanide chronic exposure from cassava has also been associated to hypothyroidism and goitre. Finally, it has been hypothesised that chronic exposure to cyanide could be associated with type 2 diabetes in malnourished populations, although this hypothesis is not supported by scientific evidence.
In 2012, the JECFA conducted an in‐depth and detailed review of the literature on long‐term health effects of dietary chronic exposure to cyanide in cassava eating populations. The JECFA concluded that the epidemiological association between cassava consumption and konzo was consistent, even though the aetiological mechanism of konzo is still unknown. In particular, konzo has been associated with chronic exposure to cyanogen at sublethal concentrations from cassava or cassava flour in combination, with a low intake of sulfur‐containing amino acids in a very simple and monotonous diet. The main difficulty encountered when further investigating the association between chronic exposure to CNGs/cyanide and spastic paraparesis is thus, that the evidence is based on epidemiological observations confounded by several nutritional deficiencies. Thus, the causal relationship cannot be definitively established. No other cyanogenic foods are known to be ingested over long periods and at comparable doses with regard to the resulting exposure to cyanide.
Similar conclusions have been reached for tropical ataxic neuropathy and ankle clonus: the relationship between intake of cassava foods and dietary cyanide load and these neurological disorders is consistent, although the evidence is based on studies at an aggregate level and conducted in populations with serious nutritional deficiency and low dietary variability.
Finally, health consequences related to iodine deficiency (intake < 100 mg/day) can be considerably aggravated by a chronic dietary exposure to cyanide from insufficiently processed bitter cassava, due to the fact that thiocyanate is similar in size to the iodide ion and interferes with uptake of iodide into the thyroid gland. Since the JECFA evaluation (FAO/WHO, 2012), no further studies have been identified on the long‐term toxicity of cyanide.
Summary remarks on long‐term toxicity
All studies which investigated the long‐term toxicity of cyanide have been conducted in populations characterised by severe malnutrition condition and monotonous diet in which cassava represents the main source of nutrition, which are unlikely to occur in European populations. Consequently, the Panel concluded that these studies did not provide an appropriate basis for dose–response analysis for the present risk assessment.
3.1.5. Mode of action for cyanide toxicity
Cyanide's mode of action for acute toxicity has been described in detail in the previous EFSA opinion (EFSA CONTAM Panel, 2016). Briefly, acute toxicity of cyanide is due to the impairment of oxidative phosphorylation, a process whereby oxygen is used for the production of essential cellular energy sources in the form of adenosine triphosphate (ATP) (Hall and Rumack, 1986; Beasley and Glass, 1998; Guidotti, 2006; Hamel, 2011; Sahin, 2011). Consequently, tissue utilisation of oxygen is inhibited and cells rapidly switch from an aerobic (oxygen‐dependent) metabolism mode that yields ATP, to anaerobic (oxygen‐independent) energy production, which generates by‐products, such as lactate. Consequences of this sudden cessation of aerobic metabolism are hypoxia, metabolic acidosis, and thus impairment of vital functions (Hall and Rumack, 1986; Guidotti, 2006; Hamel, 2011; Sahin, 2011). Organs which require a continuous supply of oxygen and ATP generated from aerobic metabolism, such as the brain and the heart, are particularly prone to cyanide acute toxicity (Guidotti, 2006). All these reactions contribute to the symptoms described during cyanide acute intoxication (WHO, 2004).
Unlike for acute toxicity, the target organ(s) and mode(s) of action for cyanide chronic toxicity have not been identified (Cliff et al., 2015). Long‐term consumption of CNG‐enriched crops or products derived thereof as a main source of nutrition have been associated with neurological impairment (konzo and tropical ataxic neuropathy), which has been hypothesised to result among others from the release of cyanide. Cyanide‐induced neurotoxic effects have been linked to a dietary deficiency of sulfur amino acids that might lead to (i) an impairment of cyanide detoxification processes and an increase of plasma cyanide concentrations directly affecting upper motor neurons (Adamolekun, 2010) or (ii) to a chronic state of neuron glutathione deficiency (Nunn et al., 2011). Neurological damages associated to chronic exposure to CNGs could additionally be due to nitriles, which are cyanide's intermediate metabolites, capable of inducing neuron damage (Llorens et al., 2011). Nevertheless, the observation that (i) spastic paresis has not been associated with cyanide exposure from any other source (FAO/WHO, 2012), (ii) the excretion of thiocyanate does not substantially deviate between cases and controls (FAO/WHO, 2012) and (iii) cyanide‐ load is not proportional to the occurrence of neurologic signs. Onabolu et al. (2001) argue against the primary involvement of cyanide in neurological diseases, observed upon long‐term consumption of food items containing CNGs as main source of nutrition. In conclusion, the mode of action of neurotoxic effects possibly associated with cyanide long‐term exposure is not fully understood.
Continuous exposure to cyanide can aggravate goitre and cretinism due to iodine deficiency. This effect is likely due to thiocyanate, which is similar in size to the iodide ion and interferes with uptake of iodide in the thyroid gland (FAO/WHO, 2012).
3.1.6. Derivation of health‐based guidance values
Acute reference dose (ARfD)
The CONTAM Panel concluded that there are no data indicating that the ARfD for CN of 20 μg/kg bw, established in 2016, should be revised. This ARfD was set in the context of the risk assessment of CNGs in raw apricot kernels. Consumption of raw apricot kernels rapidly releases CN, leading to peak levels of CN in the blood within a short period of time. Consumption of bitter almonds and cassava can result in similarly high peak levels of CN in the blood within a short period of time, whereas consumption of other foods that contain CNGs release CN more slowly, and do not lead to such high blood levels. This is considered by the application of bioavailability factors (see Section 3.4 on Exposure assessment). The modes of action of acute toxicity of CN support the view that the peak blood level is the relevant dose metric in determining whether consumption of CNGs will lead to acute toxicity.
The CONTAM Panel concludes that the ARfD of 20 μg CN/kg bw should be protective for acute effects of CN from CNGs, regardless of the dietary source. However, for foods other than raw apricot kernels, bitter almonds and cassava roots, the ARfD is likely to be over‐conservative. Establishment of different ARfDs for different types of food is not considered appropriate.
Chronic health‐based guidance value
Because foods other than raw apricot kernels, bitter almonds and cassava roots lead to slower and/or less complete release of CN, the CONTAM Panel considered whether a chronic health‐based guidance value (HBGV) should be set in addition to the ARfD.
The CONTAM Panel noted evidence related to long‐term neurological conditions in populations groups with severe malnutrition and a diet in which cassava represents the main source of nutrition. However, a causal relationship cannot be definitively established, and these studies did not provide an appropriate basis for a dose–response analysis and therefore for establishing a chronic HBGV or for a Margin of Exposure approach.
The Panel therefore also considered the data from repeat dose studies in experimental animals treated with NaCN, KCN, amygdalin, prunasin, linamarin and cassava for a period longer than 2 weeks. There was a lack of consistency in the findings of these studies. Many of these studies did not provide biologically plausible responses and/or adequate dose–response information and in some of these severe limitations in study design, statistics and reporting have been identified. The Panel concluded that available evidence from animal studies does not allow identification of a critical effect or reference point that could be used for derivation of a chronic HBGV.
3.2. Occurrence of total cyanide in food
3.2.1. Occurrence data on cyanide in food used for the assessment
The data for the present assessment were provided by national authorities from Italy, Poland, France, Belgium, Lithuania, Spain, Estonia, and from Germany, which alone reported 89% of the analytical results (Figure 5). The sampling dates were from 2000 to 2016. Data were extracted from the SDWH on the 20 April 2018.
Figure 5.
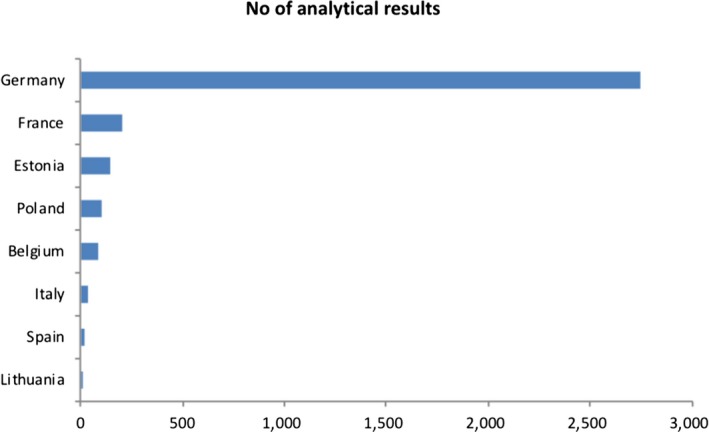
Distribution of analytical results
The initial data set included 3,350 analytical data of which 3,017 were on food for human consumption. The data set was subsequently analysed in order to exclude non‐pertinent data, identify possible issues and prepare the data for occurrence and exposure analysis. Samples were excluded because the food group was not sufficiently specified (10 samples), had an LOQ > 400 mg/kg (21 samples), were apricot kernels (47 samples). One sample of red wine (left‐censored (LC) and water samples (123 samples, 100% LC) were also excluded because the Panel concluded that the presence of cyanide in these food items is not likely. Finally, 2,586 analytical samples were used in the present opinion. Table 13 lists the available data, after the data cleaning. The lowest (most detailed) FoodEx categories associated to each of the food groups reported in Table 13 are listed in Annex A.2.
Table 13.
Distribution of analytical samples for total cyanide used in the present opinion according to food groups
| Food groupsa | No of samples | Left‐censored data (%) | Mean LB (mg/kg) | LB P95 (mg/kg) | Mean UB (mg/kg) | UB P95 (mg/kg) |
|---|---|---|---|---|---|---|
| Grains for human consumption | 2 | 0 | 6.4 | – | 6.4 | – |
| Grain milling products | 1 | 100 | 0.0 | – | 0.3 | – |
| Pastries and cakes | 35 | 91 | 1.2 | – | 3.5 | 15.9 |
| Macaroons and amaretti | 204 | 3 | 12.5 | 26.3 | 12.7 | 26.3 |
| Biscuits (cookies) | 33 | 36 | 3.3 | – | 4.1 | – |
| Other starchy roots and tubers | 7 | 86 | 0.3 | – | 0.6 | – |
| Legumes, beans, dried | 28 | 96 | 0.3 | – | 1.0 | – |
| Almond, sweet (Prunus amygdalus var. dulcis) | 35 | 17 | 4.5 | – | 4.5 | – |
| Almond, bitter (Prunus amygdalus var. amara) | 3 | 0 | 1,437 | – | 1,437 | – |
| Linseed (Linum usitatissimum) | 58 | 0 | 192.1 | – | 192.1 | – |
| Jam, marmalade and other fruit spreads from cherry | 5 | 0 | 2.3 | – | 2.3 | – |
| Fruit products with cherries | 2 | 0 | 4.6 | – | 4.6 | – |
| Pralines | 2 | 0 | 1.0 | – | 1.0 | – |
| Marzipan | 130 | 4 | 8.4 | 30.0 | 8.4 | 30.0 |
| Dragée, sugar coated | 2 | 100 | 0.0 | – | 0.1 | – |
| Nougat | 24 | 100 | 0.0 | – | 0.1 | – |
| Juice or nectar | 10 | 20 | 0.7 | – | 1.0 | – |
| Juices, nectars and soft drinks with cherries | 71 | 1 | 2.8 | 5.8 | 2.8 | 5.8 |
| Wine‐like drinks (e.g. Cider, Perry) | 1 | 100 | 0.0 | – | 1.0 | – |
| Liqueur | 117 | 59 | 2.5 | 19.0 | 3.3 | 19.0 |
| Spirits | 1,815 | 54 | 2.8 | 16.0 | 3.3 | 16.0 |
| Alcoholic mixed drinks | 1 | 100 | 0.0 | – | 2.0 | – |
LB: lower bound; UB: upper bound; P95: 95th percentile.
Only foods that can potentially contain CNGs or cyanide were considered for exposure assessment. Foods or ingredients of foods that can potentially contain CNGs or cyanide are foods (or food ingredients) which have been reported to contain cyanide in publicly available literature or previous risk assessments. The food categories (FoodEx) considered are listed in Annex A.2.
Several data reported in the literature on total cyanide concentration in cassava are listed in Table 14. The highest value measured (235 mg/kg) for raw cassava purchased in Europe was then used for the back‐calculations (see Section 3.5 on Risk characterisation).
Table 14.
Data reported in the literature on total cyanide concentrations in cassava sold as ‘sweet raw’ cassavaa , b , c
| Country of sampling | Country of origin | Year of sampling | LOQ | N samples analysed | mg CN/kg (mean) | Concentration range (mg CN− /kg) | Reference |
|---|---|---|---|---|---|---|---|
| Denmark | Costa Rica | 2008 | 10 mg CN/kg | 25 | 73 | 11–235 | Kolind‐Hansen and Brimer (2010) |
| Australia | Singapore, India, Vietnam, Fiji | 2005 | 1 mg CN/kg | 3 | 68 | 20 (SD)d | Burns et al. (2012) |
| Australia | Singapore, India, Vietnam, Fiji | 2006 | 1 mg CN/kg | 3 | 83 | 5 (SD)d | |
| Australia | Singapore, India, Vietnam, Fiji | 2007 | 1 mg CN/kg | 3 | 84 | 1 (SD)d | |
| Australia | Singapore, India, Vietnam, Fiji | 2008 | 1 mg CN/kg | 3 | 51 | 2 (SD)d | |
| Australia | Singapore, India, Vietnam, Fiji | 2011 | 1 mg CN/kg | 3 | 7 | 4 (SD)d | |
| Ireland | Costa Rica | 2001 | Not reported | 36 | 34.5 | 13–72.6 | O'Brien et al. (2013) |
| Germany | Brazil | Not reported | Not reported | 22 | 125 | 69–215 | Abraham et al. (2016) |
| Australia | Not reported | 2010 | 3–5 mg CN/kg | 15 | 21 | 8.6–43.6 | FSANZ (2014) |
| Australia | Not reported | 2013 | 3–5 mg CN/kg | 3 | 37.3 | 34–40 | |
| Australia | Not reported | 2013 | 3–5 mg CN/kg | 3 | 23.6 | 16–32 | |
| Australia | Not reported | 2013 | 3–5 mg CN/kg | 3 | 50.9 | 31–81 | |
| Fiji | Fiji | Not reported | 0.1 mg CN/kg | 80 | 39 | 33–92 | Dolodolotawake and Aalbersberg (2011) |
| Tonga | Tonga | Not reported | 0.1 mg CN/kg | 48 | 61 | 19–130 | |
| Vanuatu | Vanuatu | Not reported | 0.1 mg CN/kg | 10 | 47 | 26–78 |
LOQ: limit of quantification; SD: standard deviation.
Total cyanide is defined as cyanide originating from CNGs and cyanohydrins by complete hydrolysis during sample preparation.
Note that Codex defines ‘sweet cassava’ as cassava having a cyanide content of less than 50 mg total cyanide/kg (Codex STAN 238‐2003).
Note that ‘sweet cassava’ is usually marketed just as ‘cassava’.
In the paper from Burns et al. (2012), concentration ranges have not been reported, SDs to the mean have been inserted instead.
3.2.2. Occurrence data on food reported in previous assessments
In the opinion on hydrocyanic acid in flavourings and other food ingredients with flavouring properties (EFSA, 2004) and in the risk assessment of JECFA (FAO/WHO, 2012), the total cyanide concentrations of a wide variety of different plant food commodities have been reported and are presented in a summarised form in Table 15 below.
Table 15.
Total cyanide contents in food commodities containing cyanogenic glycosidesa
| Food commodity | HCN concentration (mg/kg)b , c | Reference | |
|---|---|---|---|
| Almonds | Ground | 1.4 | Anonymous (1975) |
| Paste | 3 | Schmidt (1972) | |
| Kernel, bitter | 300–3,400 | Sturm and Hansen (1967), Lindner (1974); FAO/WHO (1993) | |
| Oil, bitter | 800–4,000 | Rosling (1987); Gupta (1987) | |
| Kernel, bitter | 4,690 (single value) | Shragg et al. (1982) | |
| Amaretti | – | 44 (single value) | Corradi and Micheli (1982) |
| Apricot | Kernel | 120–4,000 | Gupta (1987), Holzbecher et al. (1984) |
| Juice | 0.3–7.8 | Stadelmann (1976) | |
| > 0.1d | Schmidt (1972) | ||
| Kernel | 89–2,170 | IPCS (2004) | |
| Bamboo | Shoot | 114–1,460 | Haque and Bradbury (2002) |
| 171–1,164 | Haorongbam et al. (2009) | ||
| Immature shoot tip | 7,700 (single value) | IPCS (2004) | |
| Cassava, sweet | Root | 10–20 | Ogunsua (1989) |
| Root | 10–20 | FAO/WHO (2008) | |
| 1–132 | Chiwona‐Karltun et al. (2004) | ||
| 15–93 | Mkumbira et al. (2003) | ||
| 8–1,064e | Oluwole et al. (2007) | ||
| Cassava, bitter | Root | 60–200 | Ogunsua (1989) |
| 55 | Lindner (1974) | ||
| 15–1,120 | Rosling (1987) | ||
| Root | 15–1,120 | FAO/WHO (2008) | |
| 22–661 | Chiwona‐Karltun et al. (2004) | ||
| 43–251 | Mkumbira et al. (2003) | ||
| 27–543 | Oluwole et al. (2007) | ||
| Cassava | Flour | 26–57 | Ernesto et al. (2000) |
| Flour (gari) | 20–30 | Adindu et al. (2003) | |
| Chips | < 10–145 | FSANZ (2009) | |
| Cherry | Juice | 0.5–12b | Stadelmann (1976), Schmidt (1972), Eid and Schmidt (1978) |
| Garden bean | Seed | 20 | Lindner (1974) |
| Lima bean | Seed | 144–167 | Gupta (1987), Holzbecher et al. (1984) |
| Seed | 265‐530 | Ologhobo et al. (1984) | |
| Linseed | Seed | >500 | Honig et al. (1983) |
| Seed | 238–373 | Oomah et al. (1992) | |
| 17–6,500 | Kobaisy et al. (1996) | ||
| Ground seed (meal) | 140–370 | Haque and Bradbury (2002) | |
| Marzipan | – | 15–50 | Schmidt (1972) |
| Pea | Seed | 20 | Lindner (1974) |
| Peach | Kernel | 470 | Lindner (1974) |
| Peach | Juice | 2.3–5.9d | Stadelmann (1976) |
| Plum | Juice | 0.33–1d | Stadelmann (1976), Schmidt (1972) |
| Soya bean | Protein | 0.03–0.07 | Honig et al. (1983) |
| Stone fruit | Canned | ≤ 4 | Voldrich and Kyzlink (1992) |
| < 0.01–0.02 | Von Misselhorn and Adams (1976) | ||
| Stone fruit brandies | – | < 3d | Schmidt (1972) |
FSANZ published a survey of CNGs in plant‐based foods in Australia and New Zealand 2010–2013 (FSANZ, 2014). In the survey, CNGs (measured as total cyanide) were detected in a wide range of plant‐based foods collected from retailers in Australia and New Zealand which were either consistent with or lower than levels reported in the literature and which are presented in a summarised form in Table 16 below.
Table 16.
| Food commodity | No of samples containing HCN/No of samples taken | HCN concentration (mg/kg)b , c | LOD (mg/kg) |
|---|---|---|---|
| Almonds, whole, flaked, ground, butter | 3/6 | 4.8–12.4 | 4 |
| Amaretti biscuits | 1/1 | 34 | NR |
| Apple juice | 5/116 | 1.6–5.4 | 0.06d |
| Apple puree for infants | 0/8 | ND | 0.01d |
| Apple sauce | 2/3 | 3.6–4.1 | 4 |
| Apricots, canned | 0/4 | ND | 4 |
| Apricot jam | 0/1 | ND | 4 |
| Apricot kernels with skin | 18/18 | 1,240–2,820 | NR |
| Apricot kernels without skin | 10/10 | 49–440 | NR |
| Apricot nectar | 1/4 | 6.5 | 5 |
| Bamboo shoots, canned | 7/7 | 3.7–24.5 | NR |
| Bamboo shoots, raw | 3/3 | 24–550 | NR |
| Bamboo shoots, pickled | 3/3 | 9.6–44 | NR |
| Bamboo shoots, boiled | 3/3 | 28–73 | NA |
| Cassava, frozen root, raw | 3/3 | 34–40 | NA |
| Cassava, steamed | 3/3 | 9.0–26 | 4e |
| Cassava, frozen root, raw | 3/3 | 16–32 | NR |
| Cassava, boiled | 3/3 | 7.3–23 | NR |
| Cassava, frozen root, raw | 3/3 | 31–81 | NR |
| Cassava, fried | 3/3 | 5.4–37 | NR |
| Cherry juice | 0/3 | ND | NR |
| Cherry liqueur | 0/3 | ND | 4 |
| Lima beans, raw | 1/3 | 32 | 4 |
| Linseed, whole or meal | 5/5 | 91–178 | 4 |
| Linseed containing bread | 6/6 | 5.4–49 | NR |
| Marzipan | 1/4 | 5.3 | 4 |
| Passion fruit | 2/5 | 4.7–6.6 | 4 |
| Prune juice | 0/3 | ND | 4 |
| Pumpkin seed | 0/4 | ND | 4 |
| Spinach | 0/2 | ND | 4 |
| Sunflower seed | 0/2 | ND | 4 |
| Taro leaves | 0/2 | ND | 4 |
| Vine leaves, canned | 0/1 | ND | 4 |
ND: not detected; NR: not reported.
Adapted from FSANZ (2014).
Corresponds to total cyanide concentration (originating from CNGs and cyanohydrins by complete hydrolysis during sample preparation) and analysed with acid hydrolysis (following Haque and Bradbury, 2002) unless otherwise specified.
Concentrations refer to fresh weight.
Analysed with EU HPLC method (European Committee for Standardisation, 2012).
Value reported for cassava starch.
3.3. Food processing and impact on release of cyanide
Introduction
Food items produced from cyanogenic plants may pose a health risk for consumers if the levels of CNGs are high. Therefore, the major aim of processing such crops is to decrease their potential for releasing cyanide upon ingestion. As described in detail in Section 1.3.1 on Chemistry, CNGs are water‐soluble compounds, which are chemically quite stable but undergo degradation to cyanide when they get in contact with certain enzymes (β‐glycosidases and hydroxynitrile lyases) present in the plant cells at different locations. Most food detoxification processes of cyanogenic crops utilise the water solubility and degradability of CNGs by endogenous plant enzymes. In general, mechanical destruction of the plant cells is achieved by peeling, chipping, grating or pounding the raw crops, followed by soaking in water to solubilise the CNGs for extraction and enzymatic degradation. The degradation by endogenous enzymes is enhanced by microorganisms associated with the raw crop or added intentionally during the fermentation process. Occasionally, additional enzymes for the destruction of plant cells are added, e.g. pectinase. Drying by sun or oven heat is frequently used to help to evaporate the released cyanide as volatile hydrocyanic acid.
Only few cyanogenic plants are of practical importance as raw materials for food and feed (Brimer, 2010). However, some of them, e.g. cassava and lima beans, serve as the staple food for large numbers of people in some regions of the world. Others, such as almond or apricot kernels, are used for the production of marzipan and persipan, respectively. The current detoxification processes used for such economically important plant materials are described in some detail below.
Processing of major cyanogenic crops
Cassava
Cassava (Manihot esculenta Crantz) is a staple crop for over 500 million people, mostly in sub‐Saharan Africa and parts of South America and Asia (Gnonlonfin et al., 2012). Although consumption in Europe is low, it is on the rise due to high numbers of immigrants from Africa (Kolind‐Hansen and Brimer, 2010). The root tubers of cassava are a rich source of starch but contain only little protein (less than 5% of the dry weight), whereas the leaves contain valuable proteins, minerals and vitamins (Montagnac et al., 2009). However, both roots and leaves of the bitter cultivars of cassava contain high levels of CNGs (linamarin and lotaustralin in a 20:1 ratio), together with some antinutrients (phytate, certain polyphenols, oxalate and saponins). The concentration of CNGs in leaves is about tenfold higher than in the root tubers.
The various processing techniques used for reducing the cyanide content of cassava roots have been reviewed by Montagnac et al. (2009), Gnonlonfin and Brimer (2013) and Brimer (2015). In general, boiling, steaming, baking or frying the whole fresh roots or pieces (chips) of fresh roots are not very effective, usually resulting in cyanide retention of 50% or more. The poor reduction of CNGs is believed to be due to the heat‐induced inactivation of the degrading enzymes, in particular linamarase, which is needed to hydrolyse the heat‐stable linamarin to glucose and acetone cyanohydrin (see also Figure 3 in Section 1.3.1 on Chemistry). In addition, the contact of CNGs with linamarase is poor under these conditions because the plant cells are still mostly intact. For the same reasons, drying of fresh roots or root chips by the sun or in an oven usually does not lead to a cyanide reduction of 50% or more, although sun drying is more effective than oven drying because of the lower drying temperature.
The efficacy of reducing cyanogens and cyanide in cassava roots can be increased to > 90% by mechanical disruption of the plant cells (by crushing, repeated pounding, or grating) or by soaking in water for several days (believed to cause a slow disintegration of the cells), followed by allowing time for fermentation and finally by a roasting step. During fermentation of grated roots, linamarin is efficiently degraded to its cyanohydrin, which is quite stable at the acidic pH of the fermentation but decomposes to hydrocyanic acid and acetone during roasting. Prolonged soaking in water leads to partial extraction of the linamarin from the roots. Over time, different combinations of detoxification techniques have been developed in different geographical regions in order to convert raw cassava to edible products, which usually contain only about 2% of the cyanide present in the raw material. These combinations are described in more detail by Montagnac et al. (2009). A few examples are listed in Table 17 for African food items.
Table 17.
Efficacy of combined processing techniques for lowering the cyanide content of bitter cassava in various African food items (taken from the review of Montagnac et al., 2009)
| Food item | Processing techniques | Used in e.g. | CN− retention |
|---|---|---|---|
| Fufu | Soaking of fresh roots for 3 days, followed by sun drying for 3 days | Ghana, Nigeria | 2.2% |
| Gari | Soaking, fermentation, roasting | Nigeria | 1.8% |
| Akyeke | Grating, fermenting for 5 days, washing and drying, steaming | Ghana | 2% |
In addition to detoxifying raw cassava roots, the processing of cassava flour as obtained on the market appears as a useful option. Bradbury (2006) developed a simple ‘wetting method’, which can be carried out to further reduce the total cyanide content of flour three‐ to sixfold at home just prior to use. In principle, a thin layer of wet cassava flour is kept in the shade for several hours in order to allow the residual linamarase to degrade the residual CNGs. Using this wetting method is hoped to reduce the incidences of cyanide poisoning and konzo in African countries (Bradbury et al., 2011).
Because of their content of proteins, minerals and vitamins, detoxified Cassava leaves often supplement meals made from Cassava flour (Latif and Müller, 2015). As for roots, several traditional detoxification techniques have been developed for cassava leaves in various countries, but each method has some limitations. One common practice is pounding the leaves for 15 min, followed by boiling in water for 10–120 min. Pounding lowers cyanogen content by 60–70% and subsequent boiling provides a product containing only about 3% of the original cyanogens (Montagnac et al., 2009). However, this method leads to a loss of more than half of the proteins and water‐soluble vitamins. Therefore, milder methods for removing cyanogens from cassava leaves have been proposed, e.g. pounding the leaves (step 1), followed by 2 h in the sun or 5 h in the shade (step 2), and finally three times washing with water (step 3). The residual content of cyanogens after steps 1, 2 and 3 was 28, 12 and 1%, respectively (Bradbury and Denton, 2014).
Lima beans
Lima beans (Phaseolus lunatus L.) constitute one of the most widely cultivated pulse crops in temperate and subtropic regions (Adeparusi, 2001). In addition to containing antinutrients such as inhibitors of trypsin and amylase, some cultivars have high levels of CNGs, in particular linamarin (Brimer, 2010). Adeparusi (2001) compared the effects of soaking (for 3, 6 and 9 h at 2°C), autoclaving (at 121°C and 0.01 MPa for 10, 15 or 20 min) and toasting (at 204°C for 10, 15 or 20 min) on the cyanogen content of lima beans. While soaking caused only a moderate decline of cyanogens (30% reduction after 9 h), a more rapid decline was achieved by autoclaving and by toasting, leading to a non‐detectable level after 20 min with both methods.
Bamboo shoots
Young, immature culms emerging from the rhizome of various bamboo species have long been used for edible purpose in South East Asian countries. Bamboo shoots are low in fat and calories but rich in proteins, vitamins and minerals. However, they also contain cyanogens, primarily taxiphyllin (Brimer, 2010) at levels which vary considerably between bamboo species growing in different agroclimatic regions. Pandey and Ojha (2014) studied the decrease of cyanogens in shoots from four bamboo species when boiled in water with different concentrations of NaCl (0, 1, 5 and 10%) for various length of time (10, 15, 20, 25 min). A reduction of about 80–95% of the cyanogens was achieved in most cases, which was, however, accompanied by significant losses of proteins and micronutrients. The authors state that there is no single specific treatment for all bamboo species which removes cyanogens with minimum loss of nutrients. Although earlier studies had suggested that addition of NaCl to the boiling water could speed up the decrease of cyanogens, a clear effect of NaCl was not observed in the study by Pandey and Ojha (2014).
Linseed
Linseed (Linum usitatissimum L., also named flaxseed) has been cultivated for more than 8,000 years in Europe and Asia for its fibre and oil, and more lately for its beneficial micronutrients, in particular highly unsaturated fatty acids and hormonally active lignans. However, linseed also contains considerable amounts of CNGs, primarily linustatin and neolinustatin together with small amounts of linamarin (Brimer, 2010). The detoxification of cyanogens in linseed has been tried by various conventional methods, e.g. boiling, roasting, autoclaving, microwave, extrusion and solvent extraction. These methods have the disadvantage of incomplete degradation of CNGs and partial removal of beneficial constituents (Feng et al., 2003; Barthlet and Bacala, 2010). Yamashita et al. (2007) developed a method to detoxify CNGs in linseed meal on a commercial scale by enzymatic release of HCN and its subsequent removal by steam evaporation. Freshly ground linseed was superior as a source of degrading enzyme compared to β‐glycosidase from sweet almonds or butter beans, because of the higher activity of the β‐glycosidases of linseed for the linseed CNGs. Steam evaporation was more effective than heating or lyophilisation to evaporate the HCN. This method lowered the residual cyanide content below the detection limit without affecting the protein, fat, fibre and lignan content of the linseed.
Almonds, kernels of apricots and peaches and products derived thereof
Almonds, apricot kernels and peach kernels are of importance for the production of marzipan and persipan, which consist of about 40% ground kernels and 60% sugar. All these seeds contain the CNGs amygdalin and prunasin. While the level of cyanogens is rather low in the sweet variety of almonds (about 25 mg CN/kg), bitter almonds and apricots contain cyanogens at levels ranging from about 500 to more than 1,000 mg cyanide/kg (Chaouali et al., 2013). Marzipan is exclusively produced from the kernels of sweet almonds which do not need detoxification due to their low cyanogen content, which is further decreased by the manufacturing process (blanching, chopping and grinding with sugar into almond flour). In contrast, bitter almonds and the kernels of apricots and peaches are detoxified during the production of persipan in order to comply with the EU ML of 50 mg/kg (see Section 2 on Legislation). Tuncel et al. (1995) have studied the effects of grinding, soaking and cooking on the level of cyanogens in apricot kernels with a high content of CNGs. Although considerable reductions were observed, these treatments were not sufficient, and substantial addition of an external β‐glucosidase from almonds was required to achieve full degradation of the cyanogens in raw or blanched apricot kernels (Tuncel et al., 1998). The addition of pectinase, which was hoped to improve the contact between CNGs and endogenous β‐glycosidases, did not increase the degradation of cyanogens.
Products of apples, cherries and plums
Seeds of numerous fruits contain amygdalin and prunasin (Donald, 2009). Although fruit kernels are commonly not ingested, they are present during the production of fruit juices and stone fruit spirits, and cyanogens may seep into such products. Whereas plum seeds have relatively high levels of amygdalin (10–17 mg/g), seeds from apples and cherries are in the range of 1–4 mg/g (Bolarinwa et al., 2014, 2015). When a number of apple juices and apple purees commercially available in Great Britain was analysed for their amygdalin content, values ranging from 0.001 to 0.039 mg/mL were observed, which is in the order of 1% or less of the concentration in apple seeds. A concentration of 0.039 mg amygdalin/mL corresponds to a maximum content of 0.0023 mg cyanide/mL. When commercially available apple juices in Australia were analysed for their total cyanide content, i.e. the sum of CNGs, their cyanohydrins, and free cyanide, similar values were detected (FSANZ, 2014). Thus, it appears that the levels of cyanogens in products from apples are too low to require detoxification measures.
Sorghum malt for beer production and sorghum beer
Beer produced by the fermentation of sorghum sprouts is widely consumed in various African countries, e.g. Benin, Togo, Cameroon, Ghana and Nigeria (Tokpohozin et al., 2016). Sorghum malt from sprouted grains of Sorghum bicolor contains a high level (up to 1,400 mg/kg) of the CNG dhurrin, which is not sufficiently degraded by the aryl‐β‐d‐glucosidase dhurrinase during traditional sorghum malting and mashing. Therefore, traditional African sorghum beers have a cyanide content of around 11 mg/kg. It has been proposed to reduce dhurrin during mashing prior to alcoholic fermentation by using lactic acid bacteria which exhibit aryl‐β‐d‐glucosidase activity. This may also generate good precursors for beer flavouring (Tokpohozin et al., 2016).
Summary remarks
The aim of processing of cyanogenic food plants or their derived food items is to decrease their potential for releasing cyanide upon ingestion. Methods of such detoxification are based on the water solubility of CNGs and their enzymatic degradability to cyanide, followed by evaporation of the liberated cyanide as hydrocyanic acid. Using a multistep approach, an effective detoxification to very low residual levels of cyanide (in the low percentage range of the original levels) can be achieved for cassava, lima beans, linseed, almonds, kernels of apricots and peaches and their products and sorghum.
3.4. Exposure assessment
Current exposure assessment for humans
Availability of cyanide from the intake of CNGs from particular foods
As observed in the study of Abraham et al. (2016), mean peak levels of cyanide in blood are different after consumption of apricot kernels (15.46 μM), cassava root (16.95 μM), linseed (6.40 μM) and persipan (1.44 μM), all containing the same dose of total cyanide (see Table 2). Peak levels are the relevant dose metric determining cyanide acute toxicity, and those of apricot kernels (and bitter almonds) and cassava root reflect the fast and more or less complete release of cyanide after chewing. In contrast, the velocity and/or the completeness of the release are lower in the cases of ground linseed and persipan. For exposure to cyanide from foods other than raw apricot kernels, bitter almonds and cassava roots, this ARfD is likely to be over‐conservative because of the lower bioavailability of cyanide from those foods. To consider this quantitatively in case of exposure assessment of ground linseed, a factor of 3 was calculated from the relation of the mean peak levels (cassava peak to ground linseed). Accordingly, a factor of 12 was calculated for persipan which is also applicable for marzipan. Data on the acute exposure of ground linseed and persipan/marzipan, respectively, were divided by these factors in order to consider the lower bioavailability of cyanide after consumption of these foods. For all other food items, no data on bioavailability are available, and a factor of 1 was used as a default value to consider the worst case. Table 18 provides an overview of the correction factors for release/bioavailability as applied in the exposure assessments.
Table 18.
Correction factors (rounded) applied for certain food groups to consider different CN bioavailability
| Food item | Correction factor | Remarks |
|---|---|---|
| Almonds | 1 | Bioavailability considered not to be different from that of apricot kernels |
| Cassava | 1 | Calculated from peak levels observed from Abraham et al. (2016) |
| Linseed a | 3 | Calculated from peak levels observed in the study of Abraham et al. (2016) |
| Persipan/Marzipan | 12 | Calculated from peak levels observed in the study of Abraham et al. (2016) |
| All other food items | 1 | As default factor (no specific data available) |
The factor of 3, measured for ground linseed, has also used for linseed in general, as this is the worst case. Intact linseed is expected to have a very low bioavailability of cyanide after consumption.
Current acute exposure to cyanide originating from foods containing CNGs using EFSA consumption and occurrence data
The summary statistics (mean and P95) of the probabilistic dietary exposure assessment to cyanide originating from foods containing CNGs across European dietary surveys and different age classes obtained by running 500 iterations among the occurrence data used in this opinion is presented in Table 19.
Table 19.
Summary statistics of the probabilistic dietary acute exposure assessment to cyanide originating from foods containing CNGs across European dietary surveys and different age classes obtained by running 500 iterationsa
| Lower bound | ||||||
|---|---|---|---|---|---|---|
| Age group | No of surveys | Mean dietary exposure (μg/kg bw per day) | No of surveys | P95 dietary exposure (μg/kg bw per day) | ||
| Min (95% CI) | Max (95% CI) | Min (95% CI) | Max (95% CI) | |||
| Infants | 11 | 0.0 (0.0–0.9) | 4.4 (3.9–4.9) | 10 | 0.0 (0.0–0.0) | 21.9 (19.0–24.8) |
| Toddlers | 15 | 0.8 (0.7–1.0) | 8.3 (5.7–13.0) | 15 | 5.3 (4.2–6.2) | 40.5 (32.7–47.7) |
| Other children | 21 | 1.4 (1.3–1.6) | 6.9 (5.9–8.1) | 21 | 6.5 (6.0–7.1) | 37.5 (30.2–47.0) |
| Adolescents | 21 | 0.4 (0.3–0.5) | 3.6 (3.2–4.0) | 21 | 2.3 (2.0–2.7) | 18.5 (14.3–23.3) |
| Adults | 23 | 0.6 (0.5–0.7) | 2.6 (2.5–2.7) | 23 | 2.1 (1.8–2.4) | 13.5 (13.0–14.0) |
| Elderly | 20 | 0.5 (0.3–0.7) | 2.2 (2.0–2.4) | 20 | 1.3 (0.6–2.6) | 11.0 (10.0–12.2) |
| Very elderly | 17 | 0.8 (0.6–1.0) | 1.9 (1.5–2.4) | 15 | 2.8 (1.2–5.2) | 11.3 (7.3–17.0) |
| Pregnant women | 2 | 1.1 (1.0–1.3) | 1.4 (1.0–2.0) | 2 | 6.0 (4.0–8.6) | 6.7 (5.5–8.0) |
| Lactating women | 2 | 1.1 (0.9–1.4) | 1.4 (0.9–2.1) | 2 | 6.3 (4.9–7.7) | 7.2 (5.0–11.4) |
| Upper bound | ||||||
|---|---|---|---|---|---|---|
| Age group | No of surveys | Mean dietary exposure (μg/kg bw per day) | No of surveys | P95 dietary exposure (μg/kg bw per day) | ||
| Min (95% CI) | Max (95% CI) | Min (95% CI) | Max (95% CI) | |||
| Infants | 11 | 0.1 (0.0–0.9) | 6.1 (5.7–6.5) | 10 | 0.2 (0.1–0.4) | 27.8 (25.5–30.5) |
| Toddlers | 15 | 1.1 (0.9–1.2) | 13.5 (12.5–14.5) | 15 | 6.3 (5.6–7.3) | 51.7 (36.4–79.0) |
| Other children | 21 | 2.0 (1.9–2.2) | 12.6 (11.7–13.7) | 21 | 10.2 (9.5–11.0) | 46.4 (39.3–54.5) |
| Adolescents | 21 | 0.5 (0.5–0.7) | 5.7 (5.4–6.1) | 21 | 3.3 (3.0–3.7) | 23.7 (21.9–25.5) |
| Adults | 23 | 0.8 (0.8–0.9) | 4.4 (4.3–4.5) | 23 | 3.9 (3.7–4.1) | 18.8 (18.3–19.3) |
| Elderly | 20 | 0.8 (0.6–1.0) | 3.9 (3.8–4.1) | 20 | 3.0 (2.2–3.8) | 15.8 (14.7–17.0) |
| Very elderly | 16 | 1.2 (1.0–1.4) | 3.6 (3.3–4.0) | 15 | 5.7 (4.9–6.7) | 14.3 (12.8–16.7) |
| Pregnant women | 2 | 1.6 (1.4–1.7) | 2.4 (2.0–2.9) | 2 | 7.7 (6.8–8.8) | 8.2 (6.5–10.3) |
| Lactating women | 2 | 1.6 (1.4–1.9) | 2.7 (2.2–3.3) | 2 | 8.0 (6.9–9.1) | 11.0 (8.6–13.5) |
bw: body weight; CI: confidence interval; Min: minimum; Max: maximum; P95: 95th percentile.
Exposure calculated including the respective factors for the specific food items as given in Table 18.
Table 19 shows the mean and P95 of acute exposure estimates at the UB and LB to cyanide originated from foods containing CNGs obtained for different age groups. The range represents the minimum (Min) to the maximum (Max) from the different countries and the number in the brackets are the 95% confidence intervals. Detailed mean and P95 dietary exposure estimates calculated for each dietary survey under the LB and UB assumption can be found in Annex B.1 and B.2, respectively.
The mean dietary exposure ranged from 0.0 to 13.5 μg/kg bw per day (minimum LB to maximum UB) across different age classes. The highest mean exposures were found in toddlers (range from 0.9 to 13.5 μg/kg bw per day, minimum LB to maximum UB) other children (range from 1.4 to 12.6 μg/kg bw per day, minimum LB to maximum UB) and for infants (range from 0.0 to 6.1 μg/kg bw per day, minimum LB to maximum UB). The P95 of acute dietary exposure to cyanide originating from foods containing CNGs ranged from 0.0 and 51.7 μg/kg bw per day (minimum LB to maximum UB) across different age classes. The highest P95 exposures were found for toddlers (range from 5.3 to 51.7 μg/kg bw per day, minimum LB to maximum UB), other children (range from 6.5 to 46.4 μg/kg bw per day, minimum LB to maximum UB), infants (range from 0.0 to 27.8 μg/kg bw per day, minimum LB to maximum UB) and adolescents (range from 2.3 to 23.7 μg/kg bw per day, minimum LB to maximum UB). Annexes B.1 and B.2 show in detail the estimated mean and P95 of exposure (expressed in μg/kg bw per day, under the UB assumption) across all dietary surveys and age classes.
It is worth noticing that consumption of ‘almond, bitter’ was reported in 12 different eating occasions, each of them from different subjects from Austria (1 ‘Other child’, 8 g), Germany (9 ‘Other children’, up to 0.5 g) and Slovenia (2 ‘Adults’, up to 15 g). Due to the very high levels of total cyanide in ‘almond, bitter’, average exposure in consumers only was estimated as equal to 369.4 μg/kg bw per day (CI: 361.1–379.6) for the Austrian ‘Other child’, 10.3 μg/kg bw per day (CI: 10.2–10.5) for the German ‘Other children’ and 295.1 μg/kg bw per day (CI: 288.6–303.4) in the Slovenian ‘Adults’.
Current chronic exposure to cyanide originating from foods containing CNGs using EFSA consumption and occurrence data
Table 20 shows the summary statistics of the chronic dietary exposure assessment to cyanide originating from foods containing CNGs across European dietary surveys and different age classes. Detailed mean and P95 dietary exposure estimates calculated for each dietary survey under the LB and UB assumption can be found in Annex C.1.
Table 20.
Summary statistics of the chronic dietary exposure assessment to cyanide originating from foods containing CNGs across European dietary surveys and different age classes
| Lower bound | ||||||
|---|---|---|---|---|---|---|
| Age group | No of surveys | Mean dietary exposure (μg/kg bw per day) | No of surveys | P95 dietary exposure (μg/kg bw per day) | ||
| Min | Max | Min | Max | |||
| Infants | 11 | 0.0 | 4.4 | 10 | 0.6 | 17.5 |
| Toddlers | 14 | 0.8 | 8.2 | 12 | 4.5 | 22.8 |
| Other children | 19 | 1.4 | 6.9 | 19 | 4.3 | 20.0 |
| Adolescents | 18 | 0.4 | 3.6 | 17 | 1.8 | 12.9 |
| Adults | 19 | 0.6 | 2.6 | 19 | 2.1 | 10.0 |
| Elderly | 18 | 0.5 | 2.2 | 18 | 1.5 | 12.6 |
| Very elderly | 15 | 0.0 | 1.9 | 10 | 2.5 | 7.4 |
| Pregnant women | 2 | 1.1 | 1.4 | 2 | 3.6 | 4.1 |
| Lactating women | 2 | 1.1 | 1.3 | 2 | 4.0 | 4.4 |
| Upper bound | ||||||
|---|---|---|---|---|---|---|
| Age group | No of surveys | Mean dietary exposure (μg/kg bw per day) | No of surveys | P95 dietary exposure (μg/kg bw per day) | ||
| Min | Max | Min | Max | |||
| Infants | 11 | 0.1 | 6.1 | 10 | 0.9 | 24.7 |
| Toddlers | 14 | 1.1 | 13.5 | 12 | 5.6 | 34.5 |
| Other children | 19 | 2.0 | 12.6 | 19 | 7.3 | 32.9 |
| Adolescents | 18 | 0.5 | 5.7 | 17 | 2.7 | 19.7 |
| Adults | 19 | 0.8 | 4.4 | 19 | 3.5 | 15.6 |
| Elderly | 18 | 0.8 | 3.9 | 18 | 2.0 | 12.9 |
| Very elderly | 15 | 0.0 | 3.6 | 10 | 4.4 | 12.8 |
| Pregnant women | 2 | 1.6 | 2.4 | 2 | 5.6 | 7.2 |
| Lactating women | 2 | 1.6 | 2.5 | 2 | 6.0 | 6.1 |
bw: body weight; CI: confidence interval; Min: minimum; Max: maximum; P95: 95th percentile.
The mean chronic dietary exposure ranged from 0.0 to 13.5 μg/kg bw per day (minimum LB to maximum UB) across different age classes. The highest mean exposures were found in toddlers (range from 0.9 to 13.5 μg/kg bw per day, minimum LB to maximum UB), in other children (range from 1.4 to 12.6 μg/kg bw per day, minimum LB to maximum UB) and in infants (range from 0.0 to 6.1 μg/kg bw per day, minimum LB to maximum UB).
The P95 of chronic dietary exposure to HCN ranged from 0.6 to 34.5 μg/kg bw per day (minimum LB to maximum UB) across different age classes. The highest P95 exposures were found for toddlers (range from 4.5 to 34.5 μg/kg bw per day, minimum LB to maximum UB) followed by other children (range from 4.3 to 32.9 μg/kg bw per day, minimum LB to maximum UB) and infants (range from 0.6 to 24.7 μg/kg bw per day, minimum LB to maximum UB).
Contribution of individual foods to acute and chronic exposure to cyanide via the diet
The contribution to acute and chronic dietary exposure to cyanide for the individual food groups was assessed under the LB and UB assumptions separately for each survey and age group. For all age groups, in most surveys, the food groups that contributed the most to the acute exposure to cyanide were ‘Biscuits (cookies)’, ‘Juice or nectar’ and ‘Pastries and cakes’. Linseed contributed up to 40% to the overall acute exposure under the LB assumption with the highest values (> 30%) found in ‘Very elderly’ (Sweden), ‘Elderly’ (Ireland, Portugal and Sweden) ‘Adults’ (Sweden) and ‘Other children’ (Finland). Marzipan contributed up to 4% and 3% to the overall acute exposure (highest value in ‘Very elderly’ in Denmark) under the LB and UB assumptions, respectively, across all countries and population groups. Bitter almonds contributed to the overall acute exposure only in ‘Other children’ in Austria (LB 23% and UB 13%) and Germany (LB 1% and UB 0.5%) and in ‘Adults’ in Slovenia (LB 60% and UB 40%). The sources of exposure are reported in Annexes B.3 and B.4 for all countries and population groups and under the LB and UB assumption, respectively.
‘Biscuits (cookies)’, ‘Juice or nectar’ and ‘Pastries and cakes’ were also the main contributors to chronic exposure in most countries and age groups (Annex C.2).
The graph in Annex D.1 presents the sources of mean acute exposure considering all subjects for the surveys available for the children age groups (i.e. ‘Infants’, ‘Toddlers’ and ‘Other children’) while the graph in Annex D.2 shows the sources of mean acute exposure to cyanide considering only those children for which exposures exceeded the ARfD. Likewise, in these children, ‘Biscuits (cookies)’, ‘Juice or nectar’ and ‘Pastries and cakes’ were the most important contributors to acute exposure.
The CONTAM Panel noted that, for some of the food items, the number of occurrence values is very limited.
Human exposure to cyanide originating from foods containing CNGs as reported from previous assessments
In the present section, the term HCN (that corresponds to the term ‘total cyanide’ used in the present opinion) has been retained for consistency reasons when used in previous assessments.
In their opinion on hydrocyanic acid in flavourings and other food ingredients with flavouring properties (EFSA, 2004), the EFSA AFC Panel noted that data from the UK showed that average and 97.5th percentile exposures from HCN in flavourings corresponded to about 0.8 and 3.6 μg HCN/kg bw per day, respectively, and that a Norwegian survey showed that average and 97.5th percentile exposures in consumers to HCN was 1.4 and 5.4 μg HCN/kg bw per day, respectively. Consuming 200 g of cassava would lead to an intake of 30 μg HCN/kg bw in a 60‐kg adult which would not cause acute toxicity following previous conclusions from JECFA (FAO/WHO, 1993). Assuming consumption of retail marzipan paste containing the highest amount of 20 mg HCN per kg found in this commodity and assuming that 100 g of such marzipan would be consumed in a single sitting by a 60 kg person, this would result in an acute exposure of 30 μg HCN/kg bw.
The JECFA (FAO/WHO, 2012) concluded that the occurrence and the consumption data available were not sufficient to carry out international estimates for acute or chronic dietary exposure. However, national estimates were reported. In the UK, the highest acute exposures were estimated with consumption of apricot kernels (up to 440 μg HCN/kg bw). For cassava, the highest estimate in adults was 300 μg HCN/kg bw in New Zealand. For cassava chips, estimates were up to 1,044 μg HCN/kg bw for children and 370 μg HCN/kg bw for adults in Australia and New Zealand, respectively. National acute exposure assessments (97.5th percentile) for HCN from apple juice in New Zealand and Australia ranged between 2 and 110 μg HCN/kg bw. Estimated chronic dietary exposure to HCN from national exposure assessments, based either on individual or a range of different foods, ranged between 1 and 60 μg HCN/kg bw per day for average consumers and between 2 and 150 μg HCN/kg bw per day for high consumers.
In the survey of CNGs in plant‐based foods in Australia and New Zealand 2010–2013 (FSANZ, 2014), chronic dietary exposure was assessed using a semi‐probabilistic method where different consumption values for foods from national surveys were combined with a single value for HCN concentration. Raw apricot kernels were not included as no consumption had been recorded in any of the national nutrition surveys. The major contributor to chronic dietary HCN exposure in the adult population (Australia ≥ 17 years, New Zealand ≥ 15 years) in both countries was linseed‐containing bread (75%). Corresponding values were 8% and < 5% for almonds, < 5% and 13% for cooked cassava and 15% and 5% for cassava chips in Australia and New Zealand, respectively. In the non‐adult groups (in Australia 2–16 years, in New Zealand 5–14 years), linseed‐containing bread contributed 37% and 24% to chronic exposure in Australia and New Zeeland, respectively. Corresponding values were 26% and 32% for linseed, < 5% and 7% for cooked cassava, 15% and 22% for cassava chips, 6% and < 5% for each apple juice and passion fruit. Overall, the 90th percentile UB total exposures ranged from 3 to 5 μg HCN/kg bw per day in adult and non‐adult populations, respectively. Acute dietary exposure was assessed deterministically combining a single 97.5th percentile consumption value with the maximum HCN concentration in this food. Using a consumption size of 32 apricot kernels per day, acute exposures in adults ranged from 724 to 755 μg HCN/kg bw per day. High consumption of linseed‐containing bread led to an acute exposure of up to 511 μg HCN/kg bw per day.
3.5. Risk characterisation
The CONTAM Panel concludes that the ARfD of 20 μg CN/kg bw should be protective for acute effects of CN from CNGs, regardless of the dietary source. For exposure to cyanide from foods other than raw apricot kernels, bitter almonds and cassava roots, the release of cyanide is slower and the resultant blood levels are lower and the ARfD is likely to be over‐conservative. Establishment of different ARfDs for different types of food is not considered appropriate, and therefore, the CONTAM Panel applied factors to adjust the cyanide exposure from linseed, persipan and marzipan to allow for the lower bioavailability (see Table 18). No data were available to determine adjustment factors for cyanide exposure from other foods that contain CNGs, and therefore, a factor of 1 relative to raw apricot kernels, bitter almonds and cassava roots was applied as a worst‐case assumption, which is likely to be over‐conservative.
Acute dietary exposure to cyanide from foods containing CNGs was estimated applying these factors. Mean dietary exposure did not exceed the ARfD of 20 μg CN/kg bw for any age group. At the P95, the ARfD was exceeded by up to about 2.5‐fold (up to 51.7 μg/kg bw) in some consumption surveys for ‘Infants’, ‘Toddlers’, ‘Other children’ and ‘Adolescents’. It is likely that these exposures are overestimated, especially due to the assumptions about full cyanide bioavailability from foods other than bitter almonds, cassava roots, linseed, persipan and marzipan. Additionally, the acute exposure assessment estimates acute exposures over or within 1 day, whereas for acute toxicity of cyanide, the amount of the respective consumed food in one eating occasion is more relevant. Taking into account the conservatism in the exposure assessment and in derivation of the ARfD, it is unlikely that this estimated exceedance would result in adverse effects.
The available data on chronic toxicity of cyanide are not sufficient to determine if there are potential risks to consumers in EU populations.
3.6. Estimation of the amount of certain foods that can contain CNGs that could be consumed without exceeding the ARfD
In order to provide information that might be useful for risk managers, the Panel performed an ‘exposure back‐calculation’ estimating the maximum quantity of raw cassava root, gari, cassava flour, ground linseed and bitter almonds as well as for food items for which an EU maximum level (ML) for cyanide has been established that can be ingested without exceeding the ARfD of 20 μg/kg bw for the different age groups. For these back‐calculations, the same factors to account for different bioavailability as those for exposure assessment have been applied.
For raw cassava root, this calculation was performed using the highest concentration value of cyanide in cassava sold as ‘sweet raw’ cassava, usually marketed just as ‘cassava’ reported in the literature (235 mg total cyanide/kg, see Table 14 in Section 3.2) as a worst‐case approach. For raw cassava root, containing 235 mg total cyanide/kg, the ARfD is reached by consumption of 0.7–8.5 g depending on the body weight of the individual (Table 21).
Table 21.
Estimated consumption of raw cassava root (g/eating occasion) that can be consumed without exceeding the ARfD of 20 μg/kg bw using the highest CN levelsa reported in the literature for cassava sold as ‘sweet raw’ cassava as an occurrence value
| Age group | Body weight (kg) | Total CN (mg/kg) | Correction factor | ARfD (mg/kg bw) | Maximum consumption (g/eating occasion) | ||||
|---|---|---|---|---|---|---|---|---|---|
| P5 | Mean | P95 | P5 | Mean | P95 | ||||
| Raw cassava root | |||||||||
| Toddlers | 8.7 | 11.9 | 15.9 | 235b | 1 | 0.02 | 0.7 | 1.0 | 1.4 |
| Other children | 14 | 23.1 | 37 | 235b | 1 | 0.02 | 1.2 | 2.0 | 3.2 |
| Young adolescents | 29.4 | 43.4 | 62 | 235b | 1 | 0.02 | 2.5 | 3.7 | 5.3 |
| Adolescents | 45 | 61.3 | 83 | 235b | 1 | 0.02 | 3.8 | 5.2 | 7.1 |
| Adults | 52 | 73.9 | 100 | 235b | 1 | 0.02 | 4.4 | 6.3 | 8.5 |
ArfD: acute reference dose; bw: body weight; P5: 5th percentile; P95: 95th percentile; ML: maximum level.
Originating from CNGs and cyanohydrins by complete hydrolysis during sample preparation. The term ‘HCN’ used in Codex standards corresponds to the term ‘total cyanide’ used in the present opinion.
The highest concentration (mg total CN/kg) reported in the literature.
Table 22 shows the estimated maximum amount of gari and cassava flour that can be consumed without exceeding the ARfD using the respective Codex MLs of 2 and 10 mg total CN/kg as occurrence values. Depending on the body weight, for gari, consumption of 87–1,000 g can reach the ARfD for cyanide. If reported maximum (230 g) and mean (90 g) portion sizes, as reported from a consumption survey in Nigerian adults (Sanusi and Olurin, 2012) were consumed, these exposures to cyanide would not reach the ARfD. When applying the Codex ML of 10 mg cyanide/kg for cassava flour back‐calculations show that, depending on body weight, consumption of 17–200 g leads to an exposure equivalent to the ARfD. Reported portion sizes for cassava flour in Nigerian adults (Sanusi and Olurin, 2012) with a mean of 380 g and a maximum of 750 g appear very high, but it needs to be pointed out that cassava flour is not consumed as such but is further processed likely leading to significant decreases of the total cyanide concentrations.
Table 22.
Estimated consumption of gari or cassava flour (g/eating occasion) that can be consumed without exceeding the ARfD of 20 μg/kg bw using Codex maximum levels for total cyanidea as an occurrence value
| Age group | Body weight (kg) | ML (mg/kg) | Correction factor | ARfD (mg/kg bw) | Maximum consumption (g/eating occasion) | ||||
|---|---|---|---|---|---|---|---|---|---|
| P5 | Mean | P95 | P5 | Mean | P95 | ||||
| Gari | |||||||||
| Toddlers | 8.7 | 11.9 | 15.9 | 2b | 1 | 0.02 | 87 | 119 | 159 |
| Other children | 14 | 23.1 | 37 | 2b | 1 | 0.02 | 140 | 231 | 370 |
| Young adolescents | 29.4 | 43.4 | 62 | 2b | 1 | 0.02 | 294 | 434 | 620 |
| Adolescents | 45 | 61.3 | 83 | 2b | 1 | 0.02 | 450 | 613 | 830 |
| Adults | 52 | 73.9 | 100 | 2b | 1 | 0.02 | 520 | 739 | 1,000 |
| Cassava flour | |||||||||
| Toddlers | 8.7 | 11.9 | 15.9 | 10c | 1 | 0.02 | 17 | 24 | 32 |
| Other children | 14 | 23.1 | 37 | 10c | 1 | 0.02 | 28 | 46 | 74 |
| Young adolescents | 29.4 | 43.4 | 62 | 10c | 1 | 0.02 | 59 | 87 | 124 |
| Adolescents | 45 | 61.3 | 83 | 10c | 1 | 0.02 | 90 | 122 | 166 |
| Adults | 52 | 73.9 | 100 | 10c | 1 | 0.02 | 104 | 148 | 200 |
ArfD: acute reference dose; bw: body weight; P5: 5th percentile; P95: 95th percentile; ML: maximum level.
Originating from CNGs and cyanohydrins by complete hydrolysis during sample preparation. The term ‘HCN’ used in Codex standards corresponds to the term ‘total cyanide’ used in the present opinion.
Codex ML for gari (Codex STAN 193‐1995) is 2 mg total CN/kg.
Codex ML for cassava flour (Codex STAN 193‐1995) is 10 mg total CN/kg.
Table 23 shows the estimated maximum amount of ground linseed that can be consumed without exceeding the ARfD of 20 μg/kg bw using the highest value reported in the EFSA database (407 mg CN/kg) as a worst‐case approach. Depending on the body weight of the individual, consumption of 1.3–14.7 g of ground, linseed containing CN at this level, reaches the ARfD (see Table 23). It can be expected that consumption of intact linseed (i.e. not freshly ground) would lead to much lower cyanide exposures. The European Medicines Agency (EMA, 2006) recommends consumption of 10–15 g of linseed (whole or ‘broken’ linseed) three times a day to treat constipation in adults and adolescents aged over 12 years. For ground linseed containing the highest level of measured total CN levels (407 mg/kg) as a worst‐case scenario, the ARfD would be exceeded by a toddler when consuming about 4 g of ground linseed (roughly a tea spoon). Taking into account all uncertainties, a risk for adolescents cannot be excluded if ground linseed (e.g. when put in a blender) is consumed at the amount recommended by the EMA.
Table 23.
Estimated amount of ground linseed (g/eating occasion) that can be consumed without exceeding the ARfD of 20 μg/kg bw using the highest CN level reported in the EFSA database as an occurrence value
| Linseed | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Body weight (kg) | Total CN (mg/kg) | Correction factor | ARfD (mg/kg bw) | Maximum consumption (g/eating occasion) | ||||
| P5 | Mean | P95 | P5 | Mean | P95 | ||||
| Toddlers | 8.7 | 11.9 | 15.9 | 407a | 3 | 0.02 | 1.3 | 1.7 | 2.3 |
| Other children | 14 | 23.1 | 37 | 407a | 3 | 0.02 | 2.1 | 3.4 | 5.5 |
| Young adolescents | 29.4 | 43.4 | 62 | 407a | 3 | 0.02 | 4.3 | 6.4 | 9.4 |
| Adolescents | 45 | 61.3 | 83 | 407a | 3 | 0.02 | 6.6 | 9.0 | 12.2 |
| Adults | 52 | 73.9 | 100 | 407a | 3 | 0.02 | 7.7 | 10.9 | 14.7 |
bw: body weight; P5: 5th percentile; P95: 95th percentile; ARfD: acute reference dose.
Highest concentration reported in the EFSA data base.
Table 24 shows the estimated maximum amount of bitter almonds (Prunus amygdalus var. amara) that can be consumed without exceeding the ARfD of 20 μg/kg bw using the highest value reported in the EFSA database (1,477 mg/kg). Depending on the body weight of the individual, consumption of 0.1–1.4 g bitter almonds, containing this level, reaches the ARfD. Considering that the weight of bitter almonds varies between 0.4 and 1 g (Sturm and Hansen, 1967), the ARfD would already be exceeded by consumption of less than half a small kernel in ‘Toddlers’ and by consumption of 1 big kernel in ‘Adults’.
Table 24.
Estimated amount of bitter almonds (Prunus amygdalus var. amara) (g/eating occasion) that can be consumed without exceeding the ARfD of 20 μg/kg bw using the highest CN level reported in the EFSA database as an occurrence value
| Bitter almonds | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Body weight (kg) | Total CN (mg/kg) | Correction factor | ARfD (mg/kg bw) | Maximum consumption (g/eating occasion) | ||||
| P5 | Mean | P95 | P5 | Mean | P95 | ||||
| Toddlers | 8.7 | 11.9 | 15.9 | 1,477a | 1 | 0.02 | 0.1 | 0.2 | 0.2 |
| Other children | 14 | 23.1 | 37 | 1,477a | 1 | 0.02 | 0.2 | 0.3 | 0.5 |
| Young Adolescents | 29.4 | 43.4 | 62 | 1,477a | 1 | 0.02 | 0.4 | 0.6 | 0.8 |
| Adolescents | 45 | 61.3 | 83 | 1,477a | 1 | 0.02 | 0.6 | 0.8 | 1.1 |
| Adults | 52 | 73.9 | 100 | 1,477a | 1 | 0.02 | 0.7 | 1.0 | 1.4 |
bw: body weight; P5: 5th percentile; P95: 95th percentile; ARfD: Acute reference dose.
Highest concentration reported in the EFSA database.
Finally, back‐calculations were carried out for food items for which maximum limits for total cyanide exist, i.e. for marzipan or its substitutes or similar products, canned stone fruits (Regulation EC No 1334/2008) and spirits (Regulation EC No 220/2008). Here, the respective MLs were applied to assess the maximum amount of the respective food that can be consumed in one eating occasion by each age class without exceeding the ARfD (Table 25). Assuming that marzipan or persipan contains the respective maximum limit of 50 mg CN/kg and depending on the body weight, consumption of 42–480 g could reach the ARfD (Table 25). For canned stone fruits, and stone fruit marc spirits and stone fruit spirits, a default correction factor of 1 and the respective MLs were applied in a worst‐case scenario for the back‐calculations (see Table 24). Depending on the body weight of the individual, consumption of 35–400 g canned stone fruits, containing the respective ML of 5 mg CN/kg, leads to an exposure equivalent to ARfD. For stone fruit marc spirits and stone fruit spirits, containing 35 mg total CN/kg, the ARfD is reached by consumption of 26–57 g, depending on the body weight of the individual.
Table 25.
Estimated amount of foods (g/eating occasion) for which EU maximum level for total cyanidea has been established that can be consumed without exceeding the ARfD of 20 μg/kg bw, using the maximum level as occurrence value
| Age group | Body weight (kg) | ML (mg/kg) | Correction factor | ARfD (mg/kg bw) | Maximum consumption (g/eating occasion) | ||||
|---|---|---|---|---|---|---|---|---|---|
| P5 | Mean | P95 | P5 | Mean | P95 | ||||
| Marzipan or its substitutes and similar products (persipan) b | |||||||||
| Toddlers | 8.7 | 11.9 | 15.9 | 50b | 12 | 0.02 | 42 | 57 | 76 |
| Other children | 14 | 23.1 | 37 | 50b | 12 | 0.02 | 67 | 111 | 178 |
| Young adolescents | 29.4 | 43.4 | 62 | 50b | 12 | 0.02 | 141 | 208 | 298 |
| Adolescents | 45 | 61.3 | 83 | 50b | 12 | 0.02 | 216 | 294 | 398 |
| Adults | 52 | 73.9 | 100 | 50b | 12 | 0.02 | 250 | 355 | 480 |
| Canned stone fruits | |||||||||
| Toddlers | 8.7 | 11.9 | 15.9 | 5c | 1 | 0.02 | 35 | 48 | 64 |
| Other children | 14 | 23.1 | 37 | 5c | 1 | 0.02 | 56 | 92 | 148 |
| Young adolescents | 29.4 | 43.4 | 62 | 5c | 1 | 0.02 | 118 | 174 | 248 |
| Adolescents | 45 | 61.3 | 83 | 5c | 1 | 0.02 | 180 | 245 | 332 |
| Adults | 52 | 73.9 | 100 | 5c | 1 | 0.02 | 208 | 296 | 400 |
| Stone fruit marc spirits and stone fruit spirits d, e | |||||||||
| Adolescents | 45 | 61.3 | 83 | 35e | 1 | 0.02 | 26 | 35 | 47 |
| Adults | 52 | 73.9 | 100 | 35e | 1 | 0.02 | 30 | 42 | 57 |
bw: body weight; P5: 5th percentile; P95: 95th percentile; ML: maximum level; ARfD: Acute reference dose.
Originating from CNGs and cyanohydrins by complete hydrolysis during sample preparation. The term ‘HCN’ used in EU legislation corresponds to the term ‘total cyanide’ used in this opinion.
EU ML (as laid down in Reg. 1334/2008) for marzipan or its substitutes or similar products is 50 mg total cyanide/kg.
EU ML (as laid down in Reg. 1334/2008) for canned stone fruits is 5 mg total cyanide/kg.
‘Toddlers’, ‘Other children’ and ‘Adolescents’ were not considered in the calculations as these age groups were considered not relevant for these food items.
EU ML (as laid down in Reg. 110/2008) for stone fruit marc spirits and stone fruit spirits is 7 g HCN/hL of 100% volume alcohol (70 mg/L). Assuming an alcohol content of 50%, this corresponds to approximately 35 mg/kg.
For nougat, no human bioavailability studies were available to establish a correction factor. The CONTAM Panel concluded that a correction factor of 1 (as used for all other food items with no human bioavailability data available) would be over‐conservative in this highly processed food item, which does not necessarily contain a natural source of CNGs. The available occurrence data in the EFSA data base indicate that CN is only present in very low amounts in nougat which are far below the ML.
3.7. Uncertainties
The evaluation of the inherent uncertainties in the present assessment was performed following the guidance of the opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment (EFSA, 2007). The CONTAM Panel took note of the new guidance on uncertainties of the Scientific committee (EFSA Scientific Committee, 2018), but it was not implemented in this opinion.
Assessment objectives
The objectives of the assessment are clarified in Section 1.2 Interpretation of the terms of reference.
Occurrence data/consumption data/exposure assessment
The occurrence data used in the present assessment were not representative of European countries since Germany provided about 89% of the data. Limited occurrence and consumption data were available to EFSA for relevant food commodities such as cassava and cassava‐derived products. Also, from the published literature, only few occurrence data on cyanide in cassava and cassava products were available, with limited information concerning the cassava varieties. It can be assumed that the exposure to CNGs differs between ethnic groups as a consequence of specific dietary habits. Based on the lack of information of dietary habits of such groups within European countries, these potential differences could not be considered in the present assessment and constitute an uncertainty. There is uncertainty in the detection of total cyanide in different food commodities, because the amount of cyanide released from the CNG might be influenced by and depend on the hydrolysation method applied. There is a lack of information on consumption of cassava and products thereof in the European population.
It is not possible to identify the consumption events of processed products potentially containing cyanide because of their ingredients like almonds, marzipan/persipan and stone fruits (e.g. ‘Pastries and cookies’, ‘Biscuits’, ‘Fruit juices’). For each of these categories, the CONTAM Panel selected a list of FoodEx categories that could contain almonds, marzipan/persipan and stone fruits and these foods were used for the assessment of chronic and acute exposure (see Annex A.2). These assumptions are likely to lead to an overestimation of exposure at population level.
Cyanide exposure from processed foods containing CNGs might as well be overestimated when based on their total cyanide content only because food processing (such as heating) will not only reduce the amount of total cyanide which is determined in routine analysis but also the activity of degrading enzyme, which is usually not determined in routine analyses. Factors of 2.65 and 11.8 were calculated from the relation of the mean peak levels i.e. the cassava peak to linseed/persipan peak (which also was applied for marzipan), rounded to 3 and 12, respectively, and the occurrence values were divided by these factors to reflect differential bioavailability of cyanide. However, these factors were derived from limited data. For all other food commodities, a default factor of 1 was applied because of the lack of human cyanide bioavailability data, thereby assuming 100% bioavailability. This is likely to be over‐conservative.
Other uncertainties
All uncertainties associated with the derivation of the ARfD in the opinion on acute health risks related to the presence of CNGs in raw apricot kernels (EFSA CONTAM Panel, 2016), and which are described there more in detail, also apply for the present risk assessment.
Furthermore, the maximum bioavailability of CN from CNG is reached only if the chewing process is fast and effective, if the stomach is empty and if no other foods are eaten simultaneously. Bioavailability under more realistic eating patterns is uncertain.
Relatively little is known about the absorption, distribution and excretion of CNGs and their cyanohydrins in laboratory animals or in humans.
For CNGs other than amygdalin and linamarin, no acute and repeated dose toxicity studies have been identified. There was no information available on the genotoxicity of CNGs.
The potential chronic toxicity of cyanide released from foods containing CNGs could not be characterised and it is unclear whether chronic dietary exposure to cyanide could represent a risk to the health of European consumers. In particular, based on the limited data, it was not possible to conclude on the relevance of observations on male reproduction. This constitutes a major uncertainty.
Using EU or Codex MLs for estimating the maximum tolerable amount of foods in the back‐calculations constitutes an uncertainty as it is not known if these levels can be found in foods on the European market.
Summary of uncertainties
In Table 26, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the source of uncertainty leads to over/underestimation of the resulting risk.
Table 26.
Summary of major uncertainties in the risk assessment of cyanogenic glycosides in foods except raw apricot kernels and products thereof
| Sources of uncertainty | Directiona |
|---|---|
| Limited occurrence data for food commodities such as cassava and cassava‐derived products | +/− |
| Assumption that processed products are potentially containing cyanide due to ingredients like almonds and stone fruits | + |
| Quantification of total CN in different food items | +/− |
| Lack of consumption data on relevant food items and specific dietary habits of ethnic groups in European populations | +/− |
| Use of the correction factors of 3 and 12 to calculate CN exposures due to consumption of linseed and marzipan/persipan | +/− |
| Use of a default factor of 1 for all foods containing CNGs in the absence of appropriate human cyanide bioavailability data | + |
| Limited data on the impact of food processing on CN content in foods | + |
| Assumption that 20 μM CN in blood are a threshold for toxicity in humans, including sensitive subgroups (see EFSA CONTAM Panel, 2016) | +/− |
| Selection of an uncertainty subfactor of 1.5 for toxicokinetic variability (see EFSA CONTAM Panel, 2016) | +/− |
| Application of the default uncertainty subfactor of 3.16 for toxicodynamic variability (see EFSA CONTAM Panel, 2016) | + |
| Maximum bioavailability of CN from CNG under realistic eating patterns | + |
| For CNGs other than amygdalin and linamarin, no acute or repeated dose toxicity studies have been identified | − |
| Lack of information on chronic effects of CN | − |
CN: cyanide; CNG: cyanogenic glycoside.
+ = uncertainty with potential to cause overestimation of exposure/risk; − = uncertainty with potential to cause underestimation of exposure/risk. Extent of potential over/underestimation might differ in direction.
The overall uncertainty incurred with the present assessment is considered as high. The assessment is more likely to overestimate than to underestimate the risk.
4. Conclusions
4.1. Introduction
Cyanogenic glycosides (CNGs) contain chemically bound cyanide groups and are present in numerous plants that are consumed as food such as almonds, linseed, lima beans and cassava. CNGs are stable in the intact plants because their degrading enzymes are stored in different cellular compartments. When the plant cells are damaged, e.g. by grinding or chewing, CNGs and enzymes are brought in contact and cyanide is released, which in an aqueous environment always exists as a mixture of non‐dissociated acid (hydrogen cyanide, HCN) and its dissociated form (cyanide ion, CN−). Depending on their chemical structure, different CNGs release different amounts of cyanide (e.g. linamarin 109 mg HCN/g CNG, amygdalin 59 mg HCN/g CNG). No validated methods are available for the measurement of CNGs as well as total cyanide in food items.
4.2. Toxicokinetics
In general, absorption of intact CNGs from the gastrointestinal appears to depend on the carbohydrate moiety. Absorbed intact CNGs are rapidly excreted unchanged in the urine.
Cyanide released by plant enzymes or gut microbial enzymes is readily absorbed from the gastrointestinal tract and rapidly distributed to all organs of the body. In blood, cyanide is mostly found in erythrocytes bound to methaemoglobin.
Absorbed cyanide is biotransformed to thiocyanate and several other metabolites, which are detoxification products and excreted with the urine. The rate of detoxification is low in humans (about 1 μg/kg bw per min) and depends on the availability of sulfur‐containing amino acids.
Toxic tissue concentrations of cyanide are to be expected if the rate of absorption exceeds the rate of detoxification, and if the availability of sulfur donors is low.
Because of the low rate of detoxification of cyanide, the peak blood and tissue levels of cyanide strongly depend on the amount of CNGs in the food and the rate of release of cyanide which in turn depends on the presence and activity of the degrading plant enzymes.
The peak cyanide blood concentration (assessed by serial measurements of cyanide in whole‐blood after ingestion) can be used as a reliable biomarker for acute cyanide exposure.
Although the determination of absorbed CNGs as well as their metabolite thiocyanate in urine is useful for comparing different chronic exposure levels, it cannot provide information on the absolute exposure. This is because the degree of absorption and the proportion of the CNG degraded to cyanide in the intestine or colon are not known and because urinary thiocyanate can be strongly influenced by other factors including smoking or diet.
In a study on cyanide bioavailability in healthy adults, mean peak levels of cyanide in blood were found to be different after consumption of apricot kernels, cassava root, linseed and persipan, indicating a fast and virtually complete release of cyanide after chewing of apricot kernels, bitter almonds and cassava roots only.
4.3. Toxicity in experimental animals
Acute toxicity of cyanides (HCN, NaCN, KCN, Ca(CN)2) is characterised by dyspnoea, ataxia, loss of consciousness, convulsions, asphyxiation and death.
Acute toxicity of CNGs depends on the release of cyanide and its subsequent absorption. It is characterised by arrhythmias, ataxia, convulsions, lethargy, decreased respiratory rate and death.
Histopathological alterations in the thyroid, kidney, liver and CNS, sometimes paralleled with clinical signs, and decreased cauda epididymis weights, sperm count and motility in rats and cauda epididymis weight in mice have been observed after repeated dose exposure to CN− in some but not all studies. This lack of consistency in the findings of the different studies generates uncertainty regarding the findings in animal studies.
A limited number of repeated dose toxicity studies for both individual CNGs and foods containing CNG were identified. With the CNGs linamarin and amygdalin, alterations in haematology and clinical chemistry parameters and histopathological alterations were seen. With gari and cassava, behavioural changes have been observed. None of these observations allow for the derivation of a dose descriptor.
There are indications of teratogenicity in offspring of hamsters exposed to CNGs or cassava and developmental toxicity in rats exposed to KCN which were often observed in the presence of maternal toxicity.
The available data do not indicate that cyanide is genotoxic. No information is available on the genotoxicity of CNGs.
4.4. Observations in humans
The acute lethal oral dose of cyanide in humans is reported to be between 0.5 and 3.5 mg/kg bw. The toxic threshold value for cyanide in blood is considered to be between 0.5 mg/L (ca. 20 μM) and 1.0 mg/L (ca. 40 μM), and the lethal threshold value between 2.5 mg/L (ca. 100 μM) and 3.0 mg/L (ca. 120 μM).
The rate of detoxification of cyanide in healthy adults is about 1 μg/kg bw per min only, which corresponds to about 4.2 mg cyanide/h in a 70 kg individual.
Signs of acute cyanide poisoning include headache, vertigo, agitation, respiratory depression, metabolic acidosis, confusion, coma, convulsions and death.
Cases of acute cyanide toxicity have resulted from ingestion of amygdalin preparations and of apricot kernels, bitter almonds and cassava. Some of these cases were fatal.
Several neurological disorders and other diseases have been associated to dietary chronic exposure to cyanide in cassava‐eating populations where cassava constitutes the main source of calories. However, a causal relationship cannot definitively be established, and these studies did not provide an appropriate basis for a dose–response analysis.
4.5. Mode of action
The primary mode of action for acute toxicity of cyanide is the inhibition of oxidative phosphorylation leading to anaerobic energy production.
Cessation of aerobic metabolism results in hypoxia, metabolic acidosis and impairment of vital functions.
Due to the high oxygen and energy demand, brain and heart are particularly sensitive to acute cyanide toxicity.
The role of cyanide in neurologic impairment observed upon long‐term consumption of foods containing CNGs has not been elucidated.
Continuous exposure to cyanide can aggravate goitre and cretinism due to iodine deficiency. This effect is likely due to thiocyanate, which is similar in size to the iodide ion and interferes with uptake of iodide in the thyroid gland.
4.6. Health‐based guidance values
The CONTAM Panel concluded that there are no data indicating that the ARfD for CN of 20 μg/kg bw, established in 2016, should be revised.
The ARfD of 20 μg CN/kg bw should be protective for acute effects of CN released from foods containing CNGs, regardless of the dietary source.
For exposure to cyanide from foods other than raw apricot kernels, bitter almonds and cassava roots, this ARfD is likely to be over‐conservative because of the lower bioavailability of cyanide from these foods. To account for these differences, factors were applied for food items where bioavailability data were available. For food items where such data were not available, a factor of 1 was used assuming complete cyanide bioavailability. Establishment of different ARfDs for different types of food is considered not appropriate.
The Panel concluded that available evidence from animal and human studies does not allow the derivation of a chronic HBGV.
4.7. Occurrence
A total of 2,586 analytical results corresponding to the requested criteria were extracted from the EFSA database and analysed to estimate the human acute and chronic dietary exposure to CN originating from foods containing CNGs.
Germany provided about 89% of the occurrence data. Among the occurrence data used, 46% were left‐censored.
The foods with the highest occurrence values were bitter almonds (Prunus amygdalus var. amara) and in linseed (Linum usitatissimum).
No occurrence data were available in the EFSA database for cassava root and products derived thereof.
Some plants used for food production, in particular bitter cassava, require detoxification of CNGs by extraction and enzymatic degradation, followed by evaporation of the liberated hydrocyanic acid. The CNGs in apricot and bitter almond kernels are reduced to acceptable levels during the process of manufacturing persipan.
4.8. Exposure assessment
The CONTAM Panel concluded that factors should be applied to assess cyanide exposure, because of the differences in cyanide availability from particular foods.
Since it is not possible to identify the consumption events of processed products potentially containing cyanide due to ingredients like almonds, marzipan/persipan and stone fruits (e.g. ‘Pastries and cookies’, ‘Biscuits’, ‘Fruit juices’, for each of these categories, the CONTAM Panel selected a list of FoodEx categories that could contain almonds, marzipan/persipan and stone fruits and these foods were used for the assessment of chronic/acute exposure.
For cassava and cassava‐derived products and almonds, a factor of 1; for linseed, a factor of 3; and for marzipan/persipan, a factor of 12 were calculated based on a human bioavailability study. Occurrence data on these foods were divided by the respective factors for inclusion in exposure assessment. For all other food items, no data on bioavailability were available, and a factor of 1 was used as a default worst‐case value.
The estimates of mean acute exposure to cyanide originating from foods containing CN across 43 different dietary surveys and all age groups ranged from 0.0 to 13.5 μg/kg bw per day (minimum LB to maximum UB). The estimates of P95 acute exposure ranged from 0.0 to 51.7 μg/kg bw per day (minimum LB to maximum UB). The highest acute dietary exposures were estimated for ‘Infants’, ‘Toddlers’ and ‘Other children’.
The estimates of mean chronic exposure to cyanide across 38 different dietary surveys and all age groups ranged from 0.0 to 13.5 μg/kg bw per day (minimum LB to maximum UB). The estimates of P95 chronic exposure ranged from 0.6 to 34.5 μg/kg bw per day (minimum LB to maximum UB). The highest chronic dietary exposures were estimated for ‘Infants’, ‘Toddlers’ and ‘Other children’.
The main contributors to acute and chronic dietary exposure to cyanide in all age groups were ‘Biscuits (cookies)’, ‘Juice or nectar’ and ‘Pastries and cakes’.
4.9. Risk characterisation
The CONTAM Panel concludes that the ARfD of 20 μg CN/kg bw should be protective for acute effects of CN from CNGs, regardless of the dietary source. Mean dietary exposure did not exceed the ARfD of 20 μg CN/kg bw for any age group.
At the P95, the ARfD was exceeded by up to about 2.5‐fold in some consumption surveys used in the exposure assessment for ‘Infants’, ‘Toddlers’, ‘Other children’ and ‘Adolescents’. It is likely that these exposures are overestimated in particular because of the assumption that cyanide is fully bioavailable from foods other than bitter almonds, cassava roots, linseed, persipan and marzipan.
Taking into account the conservatism in the exposure assessment and in derivation of the ARfD, it is unlikely that this estimated exceedance would result in adverse effects.
The data on chronic toxicity of cyanide are not sufficient to determine if there are potential risks to consumers in EU populations.
4.10. Estimation of the amount of certain foods that can contain CNGs that could be consumed without exceeding the ARfD
Exposure ‘back‐calculations’ have been carried out to estimate the amount of certain food items that can be ingested without exceeding the ARfD of 20 μg/kg bw for the different age groups. This was carried out for raw cassava root, gari, cassava flour, ground linseed and bitter almonds (Prunus amygdalus var. amara) as well as for food items for which EU maximum levels (MLs) for cyanide have been established. For these back‐calculations, the same factors to account for different bioavailability as those for exposure assessment have been applied.
Depending on the body weight, consumption of 1.3–14.7 g ground linseed containing a high concentration of 407 mg CN/kg could reach the ARfD. Taking into account all uncertainties, a risk for younger age groups cannot be excluded if grounded linseed (e.g. when put in a blender) is consumed. Consumption of 0.1–1.4 g bitter almonds (1,477 mg CN/kg) reaches the ARfD, which corresponds to an amount of less than half a small kernel in ‘Toddlers’ and of 1 large kernel in ‘Adults’.
The corresponding values for consumption of raw cassava root containing a high concentration of 235 mg CN/kg are 0.7–8.5 g. If gari or cassava flour containing the respective Codex MLs of 2 mg total CN/kg and 10 mg total CN/kg, respectively, are consumed, the ARfD is reached with 87–1,000 g gari and 17–200 g cassava flour.
If marzipan or persipan containing the respective maximum limit (ML) of 50 mg CN/kg is consumed, the ARfD is reached with 42–480 g. Consumption of 35–400 g canned stone fruits containing the respective EU ML of 5 mg total cyanide/kg leads to an exposure equivalent to the ARfD. If stone fruit marc spirits and stone fruit spirits contain the EU ML of 35 mg total cyanide/kg, the ARfD is reached by consumption of 26–57 g, depending on the body weight of the individual.
5. Recommendations
Validated methods are needed for the quantification of CNGs and total cyanide in different food items.
The variation of hydrolytic enzymes in food crops needs to be investigated. The potential to identify cultivars of crops with relatively low content of CNG or of hydrolytic enzymes need to be investigated.
Additional occurrence data for cyanide and CNGs are needed for raw and processed food commodities.
Consumption data are needed for a number of foods that can contain CNGs (such as cassava root and leaf products) and are present on the European market. Consumption data reflecting specific dietary habits of ethnic groups are also needed.
Human toxicokinetics of CNGs and released cyanide after ingestion of food items containing CNGs need to be studied further.
More information is needed on the presence of hydrolytic activity in processed foods.
More data are needed to evaluate the potential of cyanide and food items that contain CNGs to cause chronic effects.
More information is needed on the long‐term effect of cyanide on male reproductive system.
Abbreviations
- AFC Panel
EFSA Panel on food additives, flavourings, processing aids and materials in contact with food
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- ARfD
Acute reference dose
- ATCA
2‐amino‐2‐thiazoline‐4‐carboxylic acid
- ATP
Adenosine triphosphate
- ATSDR
Agency for Toxic Substances and Disease Registry
- BMD
Benchmark dose
- BMDL5
The 95th percentile benchmark dose lower confidence limit
- BMDL10
The 90th percentile benchmark dose lower confidence limit
- BMR
Benchmark response
- Bw
Body weight
- CAS
Chemical Abstracts Service
- Cmax
Maximum concentration achieved in the plasma following dose administration
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- CN
Cyanide
- CN−
Cyanide ion
- CNG
Cyanogenic glycoside
- CNS
Central nervous system
- Code
Codex Alimentarius Commission
- CYP
Cytochrome P450
- EC50
Half maximal effective concentration
- ELISA
Enzyme‐linked immunosorbent assay
- EMA
European Medicines Agency
- EN
European Standard
- EPA
Environmental Protection Agency
- FAO/WHO
Food and Agriculture Organization of the United Nations/World Health Organization
- FEEDAP Panel
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FSANZ
Food Standards Australia New Zealand
- GC‐MS
Gas Chromatography‐Mass Spectrometry
- GC‐ECD
Gas chromatography‐Electron capture detection
- GC‐NPD
Gas chromatography‐Nitrogen phosphorous detection
- GD
Gestation day
- Glc
Glucose
- HBGV
Health based guidance value
- HCN
Hydrocyanic acid
- HNL
Hydroxynitrile lyase
- HPLC
High‐performance liquid chromatography
- HPLC‐DAD
High performance liquid chromatography high performance liquid chromatographic method with diode‐array detection
- HPLC‐MS
High performance liquid chromatography‐mass spectrometry
- HPLC‐UV
High performance liquid chromatography with UV detection
- IPCS
International programme on chemical safety
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- KCN
potassium cyanide
- α‐KGCN
α‐ketoglutarat cyanhydrin
- LB
Lower bound
- LC
Left‐censored
- LC‐MS/MS
Liquid chromatography‐tandem mass spectrometry
- LD50
Median lethal dose
- LDH
Lactate dehydrogenase
- LOAEL
Lowest observed adverse effect level
- LOD
Limit of detection
- LOQ
Limit of quantification
- ML
Maximum level
- MS
Mass spectrometry
- MS/MS
Tandem mass spectrometry
- MPST
3‐mercaptopyruvate:cyanide sulfurtransferase
- NOAEL
No observed adverse effect level
- NTP
National toxicology programme
- P5
5th percentile
- P95
95th percentile
- PMTDI
Preliminary tolerable daily intake
- PND
Postnatal day
- SD
Standard deviation
- SDWH
Scientific data warehouse
- SSD1
Standard sample description version 1
- T3
Triiodothyronine
- T4
Thyroxine
- TDI
Tolerable daily intake
- tmax
The time at which C max is attained
- ToR
Terms of reference
- TSH
Thyroid‐stimulating hormone
- UB
Upper bound
- UF
Uncertainty factor
- UDP‐Glc
Uridine diphosphoglucose
- UGT
Uridine diphosphoglucossyl transferase
- UV
Ultraviolet
- WHO
World Health Organization
Appendix A – Identification and selection of relevant scientific literature and reports
1.
| Formation, Occurrence, Exposure | |
| Search terms | TOPIC: (“hydrocyanic acid” OR “cyanogenic glycosides” OR cyanide OR amygdalin OR prunasin OR “prussic acid” dhurrin OR linamarin or lotaustralin OR linustatin OR taxiphyllin OR triglochinin OR) AND TOPIC: (occurrence OR exposure OR levels OR concentrate* OR formation |
| Numbers of papers retrieved | 183 |
| Papers selected as relevant | 68 |
| Toxicokinetics | |
| Search terms | TOPIC: (“hydrocyanic acid” OR “cyanogenic glycosides” OR cyanide OR amygdalin OR prunasin OR “prussic acid” dhurrin OR linamarin or lotaustralin OR linustatin OR taxiphyllin OR triglochinin OR) AND TOPIC: (toxicokinetic* OR metabolism OR distribution OR excretion OR absorption OR distribution OR biomarker OR mode of action OR biotransformation OR elimination OR reduction OR detoxification OR extraction) |
| Numbers of papers retrieved | 109 |
| Papers selected as relevant | 5 |
| Food, Processing | |
| Search terms | TOPIC: (“hydrocyanic acid” OR “cyanogenic glycosides” OR cyanide OR amygdalin OR prunasin OR “prussic acid” dhurrin OR linamarin or lotaustralin OR linustatin OR taxiphyllin OR triglochinin OR) AND TOPIC: (apricot OR sorghum OR cassava OR flax OR linseed OR apple OR peach OR plum OR nectarine OR bamboo OR almond OR lima bean OR cherry OR marzipan OR stone fruit liquor OR amarett* OR persipan OR soy* OR fruit marc spirit OR nougat |
| Numbers of papers retrieved | 162 |
| Papers selected as relevant | 50 |
| Toxicity | |
| Search terms | TOPIC: (“hydrocyanic acid” OR “cyanogenic glycosides” OR cyanide OR amygdalin OR prunasin OR “prussic acid” dhurrin OR linamarin or lotaustralin OR linustatin OR taxiphyllin OR triglochinin AND TOPIC: (toxicity OR toxi* OR acute OR subacute OR subchronic OR chronic OR mutagen* OR carcino* OR genotox* OR reprotox* OR nephrotox* OR neurotox* OR hepatotox* OR immunotox* OR haemotox* OR hematotox* OR cytotox* OR develop* toxicity OR thyroid OR endocri* OR poisoning OR incidental poisoning OR rat OR mouse OR lab animal OR animal* OR case studies) |
| Numbers of papers retrieved | 152 |
| Papers selected as relevant | 40 |
| Human observations | |
| Search terms | TOPIC: (“hydrocyanic acid” OR “cyanogenic glycosides” OR cyanide OR amygdalin OR prunasin OR “prussic acid” dhurrin OR linamarin or lotaustralin OR linustatin OR taxiphyllin OR triglochinin AND TOPIC: (biomarker OR biological marker OR case studies OR incidental poisoning OR poisoning OR human poisoning) |
| Numbers of papers retrieved | 34 |
| Papers selected as relevant | 15 |
| Database used | Web of Science |
| Time limit | 2012–2017 |
| Date of search | 22 June 2017 |
| Total numbers retrieved (after removal of duplicates) | 604 |
| Number considered potentially relevant | 178 |
Appendix B – Identification and selection of relevant scientific literature and reports in the field of acute effects in humans
1.
| Acute effects in humans | |
| Search terms | hydrocyanic acid OR cyanogenic glycosides OR amygdalin OR prunasin OR prussic acid OR dhurrin OR linamarin or lotaustralin OR linustatin OR taxiphyllin OR triglochinin AND TOPIC: human case studies OR incidental human poisoning OR poisoning OR human poisoning OR acute poisoning |
| Numbers of papers retrieved | 667 |
| Papers selected as potentially relevant | 60 |
| Database used | Web of Science |
| Time limit | 1970–2017 |
| Date of search | 12 June 2017 |
Appendix C – Individual and mean (in bold) concentration–time curves observed after ingestion of the four foods (persipan paste, apricot kernels, linseed, cassava)
1.
Figure C.1.
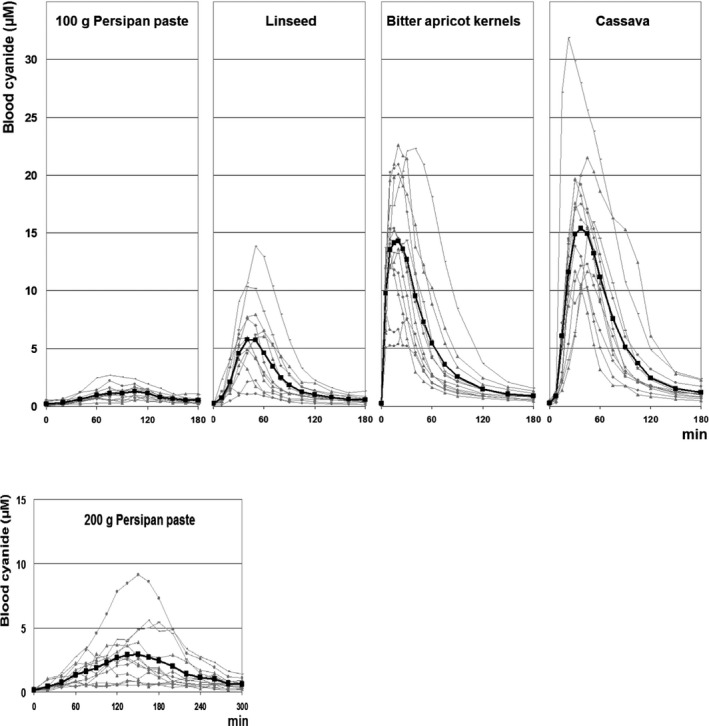
Individual and mean (in bold) concentration–time curves observed after ingestion of the four foods (persipan paste, apricot kernels, linseed, cassava) (taken from Abraham et al., 2016)
Annex A – Dietary surveys and FoodEx categories used for exposure assessment
1.
Annex A can be found in the online version of this output (‘Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5662
Description: The annex is an excel file which presents tables on dietary surveys and FoodEx categories used for exposure assessments.
Annex B – Results of probabilistic acute dietary exposure assessment to cyanide
1.
Annex B can be found in the online version of this output (‘Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5662
Description: The annex is an excel file which presents tables on the results of probabilistic acute dietary exposure assessment to cyanide.
Annex C – Results of chronic dietary exposure assessment to cyanide
1.
Annex C can be found in the online version of this output (‘Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5662
Description: The annex is an excel file which presents tables on the results of chronic dietary exposure assessment to cyanide.
Annex D – Average acute exposure per food category in children
1.
Annex D can be found in the online version of this output (‘Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5662
Description: The annex is an excel file which presents tables on the average acute exposure per food category in children.
Supporting information
Dietary surveys and FoodEx categories used for exposure assessment
Results of probabilistic acute dietary exposure assessment to cyanide
Results of chronic dietary exposure assessment to cyanide
Average acute exposure per food category in children
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc J‐C, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Vleminckx C, Wallace H, Benford D, Brimer L, Mancini FR, Metzler M, Viviani B, Altieri A, Arcella D, Steinkellner H and Schwerdtle T, 2019. Scientific opinion on the evaluation of the health risks related to the presence of cyanogenic glycosides in foods other than raw apricot kernels. EFSA Journal 2019;17(4):5662, 78 pp. 10.2903/j.efsa.2019.5662
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00802
Panel members: Margherita Bignami, Laurent Bodin, James Kevin Chipman, Jesús del Mazo, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Jean‐Charles Leblanc, Carlo Stefano Nebbia, Elsa Nielsen, Evangelia Ntzani, Annette Petersen, Salomon Sand, Dieter Schrenk, Tanja Schwerdtle, Christiane Vleminckx and Heather Wallace.
Acknowledgements: The Panel wishes to thank the hearing expert Klaus Abraham for the support provided to this scientific output.
Adopted: 18 March 2019
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure C1: © Abraham K, Buhrke T, Lampen A, 2015.
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1601/full
Notes
EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2016. Scientific opinion on the acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels. EFSA Journal 2016;14(4):4424, 47 pp. https://doi.org/10.2903/j.efsa.2016.4424 http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/4424.pdf
Regulation (EC) No 1334/2008 of the European Parliament and of Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34.
Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the definition, description, presentation, labelling and the protection of geographical indications of spirit drinks and repealing Council Regulation (EEC) No 1576/89. OJ L 39, 13.2.2008, p. 16.
Abraham K., Buhrke T., Lampen A. (2016) Bioavailability of cyanide after consumption of a single meal of foods containing high levels of cyanogenic glycosides: a crossover study in humans. Arch. Toxicol (2016) 90: 559–574.
An upper motor neuron disease manifested principally as spastic paraplegia, seen in Africa.
A syndrome characterized by sensory polyneuropathy, sensory ataxia, bilateral optic atrophy and bilateral sensorineural deafness.
The benchmark dose (BMD) is a dose level, estimated from the fitted dose–response curve, associated with a specific change in response, the benchmark response (BMR). The BMDL is the BMD's lower confidence bound. In the case of a BMDL10, it is the lower confidence bound of a specific change in response of > 10%.
The BMDL1SD is the lower confidence bound of a specific change in response of > 1 standard deviation (SD).
Council Regulation (EEC) No 315/93 of February 1993 laying down Community procedures for contaminants in food. OJ L 37, 13.2.1993, p. 1–5.
Regulation (EC) No 1881/2006 of the European Parliament and the Council of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJ L 364, 20.12.2006, p. 5–24.
Commission Regulation (EU) 2017/1237 of 7 July 2017 amending Regulation (EC) No 1881/2006 as regards a maximum level of hydrocyanic acid in unprocessed whole, ground, milled, cracked, chopped apricot kernels placed on the market for the final consumer. OJ L177, 8.7.2017, p. 36–38.
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 160/1, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the definition, description, presentation, labelling and the protection of geographical indications of spirit drinks and repealing Council Regulation (EEC) No 1576/89. OJ L 39, 13.2.2008, p. 16.
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 140, 30.5.2002, p. 10–21.
Cassava root, dried and ground.
Identifying articles that have been cited in articles found in a search (see Jalali and Wohlin, 2012).
Web of Science (WoS), formally ISI Web of Knowledge, Thomson Reuters. http://thomsonreuters.com/thomson-reuters-web-of-science/
EndNote X5, Thomson Reuters. http://endnote.com/
Persipan paste is produced from apricot kernels, sugar and water.
Dose descriptors (e.g. NOAEL, LOAEL, BMDL) are the points on a dose–response relationship that can be used in a risk characterisation.
Homogenisation of the whole testis (after removing tunica albuginea) in phosphate‐buffered saline solution and 10% DMSO followed by the count (with a hematocytometer) of the nuclei ‘resistant’ to a homogenisation step that was not described in detail. These nuclei cannot be considered as exclusively coming from spermatid cells as the method is neither sufficiently standardized nor sufficiently supported by cytology to allow discrimination of the stage of the spermatogenesis of the cellular nuclei detected.
Available from: http://www.oecd.org/chemicalsafety/risk-assessment/oecd-qsar-toolbox.htm
Amygdalin is also sometimes referred to as laetrile. This designation is wrong, as the real laetrile is a semisynthetic cyanogenic glucuronide promoted as an alternative anticancer agent with a chemical structure different from that of amygdalin (see EFSA CONTAM Panel, 2016).
References
- Abraham K, Buhrke T and Lampen A, 2016. Bioavailability of cyanide after consumption of a single meal of foods containing high levels of cyanogenic glycosides: a crossover study in humans. Archives of Toxicology, 90, 559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamolekun B, 2010. Etiology of Konzo, epidemic spastic paraparesis associated with cyanogenic glycosides in cassava: role of thiamine deficiency? Journal of Neurological Sciences, 296, 30–33. 10.1016/j.jns.2010.06.016 [DOI] [PubMed] [Google Scholar]
- Adeparusi EO, 2001. Effect of processing on the nutrients and anti‐nutrients of lima bean (Phaseolus lunatus L.). Nahrung/Food, 45, 94–96. [DOI] [PubMed] [Google Scholar]
- Adewusi SR and Oke OL, 1985. On the metabolism of amygdalin. 2. The distribution of beta‐glucosidase activity and orally administered amygdalin in rats. Canadian Journal of Physiology and Pharmacology, 63, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Adindu MN, Olayemi FF and Nze‐Dike OU, 2003. Cyanogenic potential of some cassava products in Port Harcourt markets in Nigeria. Journal of Food Composition and Analysis, 16, 21–24. [Google Scholar]
- Akintonwa A and Tunwashe OL, 1992. Fatal cyanide poisoning from cassava‐based meal. Human and Experimental Toxicology, 11, 47–49. [DOI] [PubMed] [Google Scholar]
- Akintonwa A, Tunwashe O and Onifade A, 1994. Fatal and non‐fatal acute poisoning attributed to cassava‐based meal. Acta Horticulturae, 375, 285–288. [Google Scholar]
- Ames MM, Moyer TP, Kovach JS, Moertel CG and Rubin J, 1981. Pharmacology of amygdalin (laetrile) in cancer patients. Cancer Chemotherapy and Pharmacology, 6, 51–57. [DOI] [PubMed] [Google Scholar]
- Anonymous , 1975. Annual report of Tokyo Metropolitan Research Laboratory of Public Health, 26, 183–186.
- ATSDR (Agency for Toxic Substances and Disease Registry), 2006. Toxicological Profile for Cyanide. U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA, USA: Available online: http://www.atsdr.cdc.gov/ToxProfiles/tp8.pdf [Google Scholar]
- Ballhorn DJ, 2011. Cyanogenic glycosides in nuts and seeds In: Preedy VR, Watson RR. and Patel VB. (eds.). Nuts and Seeds in Health and Disease Prevention. 1st Edition, Academic Press, London: pp. 129–136. 10.1016/b978-0-12-375688-6.10014-3 [DOI] [Google Scholar]
- Banea‐Mayambu JP, Tylleskar T, Gitebo N, Matadi N, Gebre‐Medhin M and Rosling H, 1997. Geographical and seasonal association between linamarin and cyanide exposure from cassava and the upper motor neurone disease konzo in former Zaire. Tropical Medicine and International Health, 2, 1143–1151. [DOI] [PubMed] [Google Scholar]
- Barrett MD, Hill DC, Alexander JC and Zitnak A, 1977. Fate of orally administered linamarin in the rat. Canadian Journal of Physiology and Pharmacology, 55, 134–136. [DOI] [PubMed] [Google Scholar]
- Barthlet VJ and Bacala R, 2010. Development of optimized extraction methodology for cyanogenic glycosides from flaxseed (Linum usitatissimum). Journal of AOAC International, 93, 478–484. [PubMed] [Google Scholar]
- Basu TK, 1983. High‐dose ascorbic acid decreases detoxification of cyanide derived from amygdalin (laetrile): studies in guinea pigs. Canadian Journal of Physiology and Pharmacology, 61, 1426–1430. [DOI] [PubMed] [Google Scholar]
- Beasley DM and Glass WI, 1998. Cyanide poisoning: pathophysiology and treatment recommendations. Occupational Medicine, 48, 427–431. [DOI] [PubMed] [Google Scholar]
- Bhattacharya R and Rao PVL, 1997. Cyanide induced DNA fragmentation in mammalian cell cultures. Toxicology, 123, 207–215. [DOI] [PubMed] [Google Scholar]
- Bilska‐Wilkosz A, Dudek M, Knutelska J and Wlodek L, 2015. The effect of lipoic acid administration on the urinary excretion of thiocyanate in rats exposed to potassium cyanide. Acta Poloniae Pharmaceutica, 72, 49–52. [PubMed] [Google Scholar]
- Boby RG and Indira M, 2004. Effect of co‐administration of cassava (Manihot esculenta Crantz) rich diet and alcohol in rats. Indian Journal of Physiology and Pharmacology, 48, 41–50. [PubMed] [Google Scholar]
- Bolarinwa IF, Orfila C and Morgan MRA, 2014. Amygdalin content of seeds, kernels and food products commercially available in the UK. Food Chemistry, 152, 133–139. [DOI] [PubMed] [Google Scholar]
- Bolarinwa IF, Orfila C and Morgan MRA, 2015. Determination of amygdalin in apple seeds, fresh apples and processed apple juice. Food Chemistry, 170, 437–442. [DOI] [PubMed] [Google Scholar]
- Borron SW and Baud FJ, 2012. Antidotes for acute cyanide poisoning. Current Pharmaceutical Biotechnology, 13, 1940–1948. [DOI] [PubMed] [Google Scholar]
- Bradbury JH, 2006. Simple wetting method to reduce cyanogen content of cassava flour. Journal of Food Composition Analysis, 19, 388–393. [Google Scholar]
- Bradbury JH and Denton IC, 2014. Mild method for removal of cyanogens from cassava leaves with retention of vitamins and protein. Food Chemistry, 158, 417–420. [DOI] [PubMed] [Google Scholar]
- Bradbury JH, Cliff J and Denton IC, 2011. Uptake of wetting method in Africa to reduce cyanide poisoning and konzo from cassava. Food and Chemical Toxicology, 49, 539–542. [DOI] [PubMed] [Google Scholar]
- Brimer L, 2010. Cyanogenic glycosides in food, feeding stuffs and green medicine In: Bernhoft A. (ed.). Bioactive Compounds in Plants – Benefits and Risks for Man and Animals. The Norwegian Academy of Science and Letters, Oslo: pp. 125–143. ISBN 978‐82‐7099‐583‐7. [Google Scholar]
- Brimer L, 2015. Cassava production and processing and impact on biological compounds In: Preedy VR. (ed.). Processing and Impact on Active Components in Food. Academic Press, London/Waltham/San Diego: pp. 81–86. [Google Scholar]
- Brimer L and Rosling H, 1993. Microdiffusion method with solid state detection of cyanogenic glycosides from cassava in human urine. Food and Chemical Toxicology, 31, 599–603. [DOI] [PubMed] [Google Scholar]
- Brown KS and Robinette RR, 1967. No simple pattern of inheritance in ability to smell solutions of cyanide. Nature, 215, 406–408. [DOI] [PubMed] [Google Scholar]
- Burns AE, Bradbury JH, Cavagnaro TR and Gleadow RM, 2012. Total cyanide content of cassava food products in Australia. Journal of Food Composition and Analysis, 25, 79–82. 10.1016/j.jfca.2011.06.005 [DOI] [Google Scholar]
- Carlsson L, Mlingi N, Ronquist G and Rosling H, 1995. A specific and sensitive method for the determination of linamarin in urine. Natural Toxins, 3, 378–382. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Mlingi N, Juma A, Ronquist G and Rosling H, 1999. Metabolic fates in humans of linamarin in cassava flour ingested as stiff porridge. Food and Chemical Toxicology, 37, 307–312. [DOI] [PubMed] [Google Scholar]
- Carter JH, McLafferty MA and Goldman P, 1980. Role of the gastrointestinal microflora in amygdalin (laetrile)‐induced cyanide toxicity. Biochemical Pharmacology, 29, 301–304. [DOI] [PubMed] [Google Scholar]
- Chandra AK, 2015. Iodine, thiocyanate and the thyroid. Biochemistry and Pharmacology, 4, 171 10.4172/2167-0501.1000171 [DOI] [Google Scholar]
- Chaouali N, Gana I, Dorra A, Khelifi F, Nouioui A, Masri W, Belwaer I, Ghorbel H and Hedhili A, 2013. Potential toxic levels of cyanide in almonds (Prunus amygdalus), apricot kernels (Prunus armeniaca), and almond syrup. ISRN Toxicology, 2013, 610648 10.1155/2013/610648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiwona‐Karltun L, Tylleskär T, Mkumbira J, Gebre‐Medhin M and Rosling H, 2000. Low dietary cyanogen exposure from frequent consumption of potentially toxic cassava in Malawi. International Journal of Food Science and Nutrition, 51, 33–43. [DOI] [PubMed] [Google Scholar]
- Chiwona‐Karltun L, Brimer L, Kalenga Saka JD, Mhone AR, Mkumbira J, Johansson L, Bokanga M, Mahungu NM and Rosling H, 2004. Bitter taste in cassava roots correlates with cyanogenic glucoside levels. Journal of the Science of Food and Agriculture, 84, 581–590. 10.1002/jsfa.1699 [DOI] [Google Scholar]
- Cipollone R and Visca P, 2007. Is there evidence that cyanide can act as a neuromodulator? IMBMB Life, 59, 187–189. [DOI] [PubMed] [Google Scholar]
- Cliff J and Coutinho J, 1995. Acute intoxication from newly‐introduced cassava during drought in Mozambique. Tropical Doctor, 25, 193 10.1177/004947559502500424 [DOI] [PubMed] [Google Scholar]
- Cliff J, Nzwalo H and Muquingue H, 2015. Cyanide in the production of long‐term adverse health effects in humans In: Hall AH, Isom GE. and Rockwood GA. (eds.). Toxicology of Cyanides and Cyanogens. Experimental, Applied and Clinical Aspects, Chapter 7, Wiley‐Blackwell, New York: pp. 98–107. [Google Scholar]
- Corradi C and Micheli G, 1982. Sul contenuto di acido cianidrico totale degli amaretti. (About the total amount of cyanhydric acid in amaretti). Industrie Alimentari, 21, 459–465. [Google Scholar]
- De Flora S, 1981. Study of 106 organic and inorganic compounds in Salmonella/microsome test. Carcinogenesis, 2, 283–298. [DOI] [PubMed] [Google Scholar]
- De Flora S, Camoirano A, Zanacchi P and Bennicelli C, 1984. Mutagenicity testing with TA97 and TA102 of 30 DNA‐damaging compounds, negative with other Salmonella strains. Mutation Research, 134, 159–165. [DOI] [PubMed] [Google Scholar]
- Dolodolotawake U and Aalbersberg WGL, 2011. Cyanide content of cassava and some of its products in some South Pacific Island countries. Professional and Technical Reports, University of South Pacific (USP) Electronic Research Repository. http://repository.usp.ac.fj/id/eprint/4852
- Donald GB, 2009. Cyanogenic foods (cassava, fruit kernels, and cycad seeds). Medical Toxicology of Natural Substances, 55, 336–352. [DOI] [PubMed] [Google Scholar]
- Dorea JG, 2004. Maternal thiocyanate and thyroid status during breast‐feeding. Journal of the American College of Nutrition, 23, 97–101. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on hydrocyanic acid in processing and other food ingredients with ingredient properties. EFSA Journal 2004;2(11):105, 28 pp. 10.2903/j.efsa.2004.105 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Guidance of the Scientific Committee on a request from EFSA related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;4(12):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(5):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Use of the EFSA Comprehensive European Food Consumption Database in Intakes Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2016. Scientific opinion on the acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels. EFSA Journal 2016;14(4):4424, 47 pp. 10.2903/j.efsa.2016.4424 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Scientific Opinion on risk assessment terminology. EFSA Journal 2012;10(5):2664, 43 pp. 10.2903/j.efsa.2012.2664 [DOI] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on uncertainty analysis in scientific assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid K and Schmidt K, 1978. Cyanide content of stone fruit products. I. Effect of damaged stones and of enzyme treatment on the free HCN in morello cherry juice. Flüssiges‐Obst, 45, 43–44. [Google Scholar]
- Eisenbrand G and Gelbke HP, 2016. Assessing the potential impact on the thyroid axis of environmentally relevant food constituents/contaminants in humans. Archives of Toxicology, 90, 1841–1857. 10.1007/s00204-016-1735-6 [DOI] [PubMed] [Google Scholar]
- EMA (European Medicines Agency), 2006. Assessment report on Linum usitatissimum L., Semen. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2010/01/WC500059156.pdf
- EN (European Standard), 2012. EN 16160:2012. Animal feeding stuffs. Determination of Hydrocyanic acid by HPLC. 9780580669958
- Erdogan MF, 2003. Thiocyanate overload and thyroid disease. BioFactors, 19, 107–111. [DOI] [PubMed] [Google Scholar]
- Ernesto M, Cardoso AP, Cliff J and Bradbury JH, 2000. Cyanogens in Cassava Flour and Roots and Urinary Thiocyanate Concentration in Mozambique. Journal of Food Composition and Analysis, 13, 1–12. [Google Scholar]
- European Committee for Standardization (CEN), 2012. Animal feeding stuffs –Determination of Hydrocyanic acid by HPLC, EN16160:2012 (E).
- FAO/WHO (Food and Agricultural Organization/World Health Organization), 1993. Toxicological evaluation of certain food additives and natural occurring toxicants. Report of the 39th meeting of the Joint FAO/WHO Experts Committee on Food Additives (JECFA). Food Additives Series No. 30. World Health Organization, Geneva: pp. 299–337. [Google Scholar]
- FAO/WHO (Food and Agricultural Organization/World Health Organization), 2008. Discussion paper on cyanogenic glycosides. Rome, Italy, Food and Agriculture Organization of the United Nations and World Health Organization, Joint FAO/WHO Food Standards Programme, Codex Alimentarius Commission, Codex Committee on Contaminants in Foods (CX/CF 09/3/11)
- FAO/WHO (Food and Agricultural Organization/World Health Organization), 2012. Safety evaluation of certain food additives and contaminants prepared by the seventy‐fourth meeting of the joint FAO/WHO expert committee on food additives. WHO Food Additives Series, 65, 1–833. [Google Scholar]
- Fasco MJ, Hauer CR 3rd, Stack RF, O'Hehir C, Barr JR and Eadon GA, 2007. Cyanide adducts with human plasma proteins: albumin as a potential exposure surrogate. Chemical Research in Toxicology, 20, 677–684. [DOI] [PubMed] [Google Scholar]
- Fasco MJ, Stack F, Lu S, Hauer CR 3rd, Schneider E, Dailey M and Aldous KM, 2011. Unique cyanide adduct in human serum albumin – potential as a surrogate exposure marker. Chemical Research in Toxicology, 24, 505–514. 10.1021/tx100344e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Shen Y and Chavez ER, 2003. Effectiveness of different processing methods in reducing hydrogen cyanide context of flaxseeds. Science of Food and Agriculture, 83, 836–841. 10.1002/jsfa.1412 [DOI] [Google Scholar]
- Fomunyam RT, Adegbola AA and Oke OL, 1985. The stability of cyanohydrins. Food Chemistry, 17, 221–225. [Google Scholar]
- Frakes RA, Sharma RP and Willhite CC, 1985. Developmental toxicity of the cyanogenic glycoside linamarin in the golden hamster. Teratology, 31, 241–246. [DOI] [PubMed] [Google Scholar]
- Frakes RA, Sharma RP and Willhite CC, 1986. Comparative metabolism of linamarin and amygdalin in hamsters. Food and Chemical Toxicology, 24, 417–420. [DOI] [PubMed] [Google Scholar]
- FSANZ (Food Standards Australia New Zealand), 2008. Proposal P1002 – Hydrocyanic acid in ready‐to‐eat cassava chips. Assessment Report. 6 March 2008. FSANZ, Canberra: Available online: http://www.foodstandards.gov.au/code/proposals/Pages/proposalp1002hydrocy3848.aspx [Google Scholar]
- FSANZ (Food Standards Australia New Zealand), 2009. Hydrocyanic acid in ready‐to‐eat cassava chips. First review report. Food Standards Australia New Zealand, Canberra, Australia: (Report No. 2‐09). [Google Scholar]
- FSANZ (Food Standards Australia New Zealand), 2014. Survey of cyanogenic glycosides in plant‐based foods in Australia and New Zealand 2010–13. 1–78.
- Gleadow RM and Møller BL, 2014. Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annual Review of Plant Biology, 65, 155–185. 10.1146/annurev-arplant-050213-040027 [DOI] [PubMed] [Google Scholar]
- Gleadow RM and Woodrow IE, 2002. Constraints on effectiveness of cyanogenic glycosides in herbivore defense. Journal of Chemical Ecology, 28, 1301–1313. [DOI] [PubMed] [Google Scholar]
- Gnonlonfin GJB and Brimer L, 2013. Cassava (Manihot esculenta Crantz) as a source of chemically safe food: a critical review In: Gagne CM. and Jones DB. (eds.). Processed Foods: Quality, Safety Characteristics and Health Implications. Nova Science Publisher Inc, New York: pp. 59–81. [Google Scholar]
- Gnonlonfin GJB, Sanni A and Brimer L, 2012. Preservation of cassava (Manihot esculenta Crantz): a major crop to nourish people worldwide In: Bhat R, Alias AK. and Paliyath G. (eds.). Progress in Food Preservation. John Wiley & Sons, Ltd, Chichester: pp. 331–342. [Google Scholar]
- Guidotti T, 2006. Acute cyanide poisoning in prehospital care: new challenges, new tools for intervention. Prehospital and Disaster Medicine, 21, s40–s48. [DOI] [PubMed] [Google Scholar]
- Gupta YP, 1987. Anti‐nutritional and toxic factors in food legumes: a review. Plant Foods for Human Nutrition, 37, 201–228. [DOI] [PubMed] [Google Scholar]
- Hall AH and Rumack BH, 1986. Clinical toxicology of cyanide. Annals of Emergency Medicine, 15, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Hamel J, 2011. A review of acute cyanide poisoning with a treatment update. Critical Care Nurse, 31, 72–81. [DOI] [PubMed] [Google Scholar]
- Haorongbam S, Elangbam D and Nirmala C, 2009. Cyanogenic glucosides in juvenile edible shoots of some Indian bamboos In: Proceedings of the VIII World Bamboo Conference, Bangkok, Thailand. Vol. 6. pp. 22–30. Avaiable online: http://www.bambusc.org.br/wp-content/gallery/WBC2009/WBCVIII-Vol_06.pdf
- Haque RM and Bradbury HJ, 2002. Total cyanide determination of plants and foods using the picrate and acid hydrolysis methods. Food Chemistry, 77, 107–114. [Google Scholar]
- Hayes WJ, 1967. The 90 day LD50 and chronicity factors as a measure of toxicity. Toxicology and Applied Pharmacology, 11, 327–335. [Google Scholar]
- Henderson L, Wolfreys A, Fedyk J, Bourner C and Windebank S, 1998. The ability of the Comet assay to discriminate between genotoxins and cytotoxins. Mutagenesis, 13, 89–94. [DOI] [PubMed] [Google Scholar]
- Hernandez T, Lundquist P, Oliveira L, Perez Cristia R, Rodriguez E and Rosling H, 1995. Fate in humans of dietary intake of cyanogenic glycosides from roots of sweet cassava consumed in Cuba. Natural Toxins, 3, 114–117. [DOI] [PubMed] [Google Scholar]
- Holzbecher MD, Moss MA and Ellenberger HA, 1984. The cyanide content of laetrile preparations, apricot, peach and apple seeds. Chemical Toxicology, 22, 341–347. [DOI] [PubMed] [Google Scholar]
- Honig DH, Hockridge ME, Gould RM and Rackis JJ, 1983. Determination of cyanide in soybeans and soybean products. Journal of Agricultural and Food Chemistry, 31, 272–275. [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐Majem L, Volatier J‐L, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren JD, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias CA, Sanchez T and Yeoh H‐H, 2002. Cyanogens and linamarase activities in storage roots of cassava plants from breeding program. Journal of Food Composition and Analysis, 15, 379–387. [Google Scholar]
- Imosemi IO, Malomo AO, Oladejo OW, Osuagwu FC, Ekpo OE, Akang EE and Shokunbi MT, 2005. Gross morphological studies on the effect of cyanide on the developing cerebellum of Wistar rat (Rattus novegicus). African Journal of Medical and Health Sciences, 34, 59–63. [PubMed] [Google Scholar]
- IPCS (International Programme on Chemical Safety), 2004. Hydrogen Cyanide and Cyanides: Human Health Aspects. World Health Organization, International Programme on Chemical Safety; (Concise International Chemical Assessment Document 61), Geneva, Switzerland. [Google Scholar]
- IPCS (International Programme on Chemical Safety), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. Environmental Health Criteria 240. Available online: http://www.inchem.org/documents/ehc/ehc/ehc240_index.htm
- Jackson LC, 1988. Behavioral effects of chronic sublethal dietary cyanide in an animal model: implications for humans consuming cassava (Manihot esculenta). Human Biology, 60, 597–614. [PubMed] [Google Scholar]
- Jalali S and Wohlin C, 2012. Systematic literature studies: Database searches vs. backward snowballing. 6th ACM‐IEEE International Symposium on Empirical Software Engineering and Measurement. Lund. Available online: http://www.diva-portal.org/smash/get/diva2:834640/FULLTEXT01.pdf
- Jones DA, 1998. Why are so many food plants cyanogenic? Phytochemistry, 47, 155–162. [DOI] [PubMed] [Google Scholar]
- Kamalu BP, 1993. Pathological changes in growing dogs fed on a balanced cassava (Manihot esculenta Crantz) diet. British Journal of Nutrition, 69, 921–934. [DOI] [PubMed] [Google Scholar]
- Kobaisy M, Oomah BD and Mazza G, 1996. Determination of cyanogenic glycosides in flaxseed by barbituric acid−pyridine, pyridine−pyrazolone, and high‐performance liquid chromatography methods. Journal of Agricultural and Food Chemistry, 44, 3178–3181. [Google Scholar]
- Kolind‐Hansen L and Brimer L, 2010. The retail market for fresh cassava root tubers in the European Union (EU): the case of Copenhagen, Denmark – a chemical food safety issue? Journal of the Science of Food and Agriculture, 90, 252–256. [DOI] [PubMed] [Google Scholar]
- Konstantatos A, Kumar MS, Burrell A and Smith J, 2017. An unusual presentation of chronic cyanide toxicity from self‐prescribed apricot kernel extract. BMJ Case Reports, bcr–2017–220814 10.1136/bcr-2017-220814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutler PA, Varbanov V, Goodman W, Olaya G and Stanbury JB, 1978. Interactions of protein deficiency, cyanide, and thiocyanate on thyroid function in neonatal and adult rats. American Journal of Clinical Nutrition, 31, 282–289. [DOI] [PubMed] [Google Scholar]
- Kubo T and Urano K. and Utsumi H, 2002. Mutagenicity characteristics of 255 environmental chemicals. Journal of Health Sciences, 48, 545–554. [Google Scholar]
- Latif S and Müller J, 2015. Potential of cassava leaves in human nutrition: a review. Trends in Food Science and Technology, 44, 147–158. [Google Scholar]
- Leuschner F and Neumann BW, 1989. 13‐Week toxicity study of potassium cyanide administered to Sprague‐Dawley rats in the drinking water. Unpublished manuscript.
- Leuschner F, Neumann BW and Liebsch N, 1983. Mutagenicity study of Hydrocyanic acid in the Ames Salmonella/microsome plate test (in vitro) Unpublished study, Laboratory of Pharmacology and Toxicology, Hamburg, August 1983, submitted to the WHO by Detia Freyberg GmbH.
- Leuschner J, Winkler A and Leuschner F, 1991. Toxicokinetic aspects of chronic cyanide exposure in the rat. Toxicology Letters, 57, 195–201. [DOI] [PubMed] [Google Scholar]
- Lindner E, 1974. Toxikologie der Nahrungsmittel. Georg Thieme Verlag, Stuttgart: pp. 15–20. [Google Scholar]
- Llorens J, Soler‐Martín C, Saldaña‐Ruíz S, Cutillas B, Ambrosio S and Boadas‐Vaello P, 2011. A new unifying hypothesis for lathyrism, konzo and tropical ataxic neuropathy: nitriles are the causative agents. Food and Chemical Toxicology, 49, 563–570. 10.1016/j.fct.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Logue BA, Kirschten NP, Petrikovic I, Moser MA, Rockwood GA and Baskin SI, 2005. Determination of cyanide metabolites 2‐aminothiazoline‐4‐carboxylic acid in urine and plasma by gas chromatography–mass spectrometry. Journal of Chromatography B, 819, 237–244. [DOI] [PubMed] [Google Scholar]
- Logue BA, Maserek WK, Rockwood GA, Keebaugh MW and Baskin SI, 2009. The analysis of 2‐amino‐2‐thiazoline4‐carboxylic acid in the plasma of smokers and non‐smokers. Toxicology Mechanisms and Methods, 19, 202–208. 10.1080/15376510802488165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist P, Kagedal B, Nilsson L and Rosling H, 1995. Analysis of the cyanide metabolite 2‐aminothiazoline4‐carboxylic acid in urine by high‐performance liquid chromatography. Analytical Biochemistry, 228, 27–34. [DOI] [PubMed] [Google Scholar]
- Maduagwu EN, 1989. Metabolism of linamarin in rats. Food and Chemical Toxicology, 27, 451–454. [DOI] [PubMed] [Google Scholar]
- Malomo AO, Imosemi IO, Osuagwu FC, Oladejo OW, Akang EE and Shokunbi MT, 2004. Histomorphometric studies on the effect of cyanide consumption of the developing cerebellum of Wistar rat (Rattus novegicus). West African Journal of Medicine, 23, 323–328. [DOI] [PubMed] [Google Scholar]
- Manzano H, de Sousa AB, Soto‐Blanco B, Guerra JL, Maiorka PC and Górniak SL, 2007. Effects of long‐term cyanide ingestion by pigs. Veterinary Research Communications, 31, 93–104. [DOI] [PubMed] [Google Scholar]
- Mathangi DC and Namasivayam A, 2000. Effect of cassava consumption on open‐field behavior and brain neurotransmitters in albino rats. Physiology and Behavior, 70, 89–93. [DOI] [PubMed] [Google Scholar]
- Mathangi DC, Mohan V and Namasivayam A, 1999. Effect of Cassava on motor co‐ordination and neurotransmitter level in the albino rat. Food and Chemical Toxicology, 37, 57–60. [DOI] [PubMed] [Google Scholar]
- Mathangi DC, Shyamala R, Vijayashree R, Rao KR, Ruckmani A, Vijayaraghavan R and Bhattacharya R, 2011. Effect of alpha‐ketoglutarate on neurobehavioral, neurochemical and oxidative changes caused by sub‐chronic cyanide poisoning in rats. Neurochemical Research, 36, 540–548. 10.1007/s11064-010-0376-z [DOI] [PubMed] [Google Scholar]
- McMahon JM, White WLB and Sayre RT, 1995. Cyanogenesis in cassava (Manihot esculenta Crantz). Journal of Experimental Botany, 46, 731–741. [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives and Contaminants. Part A, 28, 975–995. 10.1080/19440049.2011.576440 [DOI] [PubMed] [Google Scholar]
- Mills EM, Gunasekar PG, Li L, Borowitz JL and Isom GE, 1999. Differential susceptibility of brain areas to cyanide involves different modes of cell death. Toxicology and Applied Pharmacology, 156, 6–16. [DOI] [PubMed] [Google Scholar]
- Mkumbira J, Chiwona‐Karltun L, Lagercrantz U, Mahungu NM, Saka J, Mhone A, Bokanga M, Brimer L, Gullberg U and Rosling H, 2003. Classification of cassava into ‘bitter’ and ‘cool’ in Malawi: from farmers’ perception to characterisation by molecular markers. Euphytica, 132, 7–22. [Google Scholar]
- Montagnac JA, Davis CR and Tanumihardjo SA, 2009. Processing techniques to reduce toxicity and antinutrients of cassava for use as a stable food. Comprehensive Reviews in Food Science and Food Safety, 8, 17–27. [DOI] [PubMed] [Google Scholar]
- Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez‐Pérez R, Møller BL and Bak S, 2008. beta‐Glucosidases as detonators of plant chemical defense. Phytochemistry, 69, 1795–1813. 10.1016/j.phytochem.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Mouaffak Y, Zegzouti F, Boutbaoucht M, Najib M, El Adib AG, Sbihi M and Younous S, 2013. Cyanide poisoning after bitter almond ingestion. Annals of Tropical Medicine and Public Health, 6, 679–680. [Google Scholar]
- Nader R, Mathieu‐Daudé JC, Deveaux M, Faure K, Hayek‐Lanthois M and de Haro L, 2010. Child cyanide poisoning after ingestion of bitter almonds. Clinical Toxicology, 48, 574–575. 10.3109/15563650.2010.492351 [DOI] [PubMed] [Google Scholar]
- Newton GW, Schmidt ES, Lewis JP, Conn E and Lawrence R, 1981. Amygdalin toxicity studies in rats predict chronic cyanide poisoning in humans. Western Journal of Medicine, 134, 97–103. [PMC free article] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 1993. NTP technical report on toxicity studies of sodium cyanide (CAS No. 143‐33‐9) administered in drinking water to F344/N rats and B6C3F1 mice. Public Health Service, U.S. Department of Health and Human Services; NTP TR 37; NIH Publication 94‐3386. Available online: http://ntp.niehs.nih.gov/ntp/htdocs/ST_rpts/tox037.pdf
- Nunn PB, Lyddiard JR and Christopher Perera KP, 2011. Brain glutathione as a target for aetiological factors in neurolathyrism and konzo. Food and Chemical Toxicology, 49, 662–667. 10.1016/j.fct.2010.08.037 [DOI] [PubMed] [Google Scholar]
- O'Brien GM, Weir RR, Moody K and Liu PWS, 2013. Cyanogenic potential of fresh and frozen cassava on retail sale in three Irish cities: a snapshot survey. International Journal of Food Science and Technology, 48, 1815–1821. [Google Scholar]
- Ogunsua A, 1989. Total cyanide levels in bread made from wheat/cassava composite flours. International Journal of Food Science and Technology, 24, 361–365. [Google Scholar]
- Oke OL, 1979. Some aspects of the role of cyanogenic glycosides in nutrition. World Review of Nutrition and Dietetics, 33, 70–103. [DOI] [PubMed] [Google Scholar]
- Okolie NP and Osagie AU, 1999. Liver and kidney lesions and associated enzyme changes induced in rabbits by chronic cyanide exposure. Food and Chemical Toxicology, 37, 745–750. [DOI] [PubMed] [Google Scholar]
- Okolie NP and Osagie AU, 2000. Differential effects of chronic cyanide intoxication on heart, lung and pancreatic tissues. Food and Chemical Toxicology, 38, 543–548. [DOI] [PubMed] [Google Scholar]
- Ologhobo AD, Fetuga BL and Tewe OO, 1984. The cyanogenic glycoside contents of raw and processed lima bean varieties. Food Chemistry, 13, 117–128. [Google Scholar]
- Oluwole OSA, Onabolu AO, Mtunda K and Mlingi N, 2007. Characterization of cassava (Manihot esculenta Crantz) varieties in Nigeria and Tanzania, and farmers’ perception of toxicity of cassava. Journal of Food Composition and Analysis, 20, 7559–7567. [Google Scholar]
- Onabolu AO, Oluwole OS, Bokanga M and Rosling H, 2001. Ecological variation of intake of cassava food and dietary cyanide load in Nigerian communities. Public Health Nutrition, 4, 871–876. [DOI] [PubMed] [Google Scholar]
- Oomah BD, Mazza G and Naschuk EO, 1992. Cyanogenic compounds in flaxseed. Journal of Agricultural and Food Chemistry, 40, 1346–1348. [Google Scholar]
- Oyewole OI and Olayinka ET, 2009. Hydroxocobalamin (vit b12a) effectively reduced extent of cyanide poisoning arising from oral amygdalin ingestion in rats. Journal of Toxicology Environmental Health Sciences, 1, 8–11. [Google Scholar]
- Palmer IS and Olson OE, 1979. Partial prevention by cyanide of selenium poisoning in rats. Biochemical and Biophysical Research Communications, 90, 1379–1386. [DOI] [PubMed] [Google Scholar]
- Pandey AK and Ojha V, 2014. Precooking processing of bamboo shoots for removal of anti‐nutrients. Journal of Food Science and Technology, 51, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentore R, Venneri A and Nichelli P, 1996. Accidental choke cherry poisoning: early symptoms and neurological sequelae of an unusual case of cyanide intoxication. The Italian Journal of Neurological Sciences, 17, 233–235. [DOI] [PubMed] [Google Scholar]
- Philbrick DJ, Hill DC and Alexander JC, 1977. Physiological and biochemical changes associated with linamarin administration to rats. Toxicology and Applied Pharmacology, 42, 539–551. [DOI] [PubMed] [Google Scholar]
- Philbrick DJ, Hopkins JB, Holl DC, Alexander JC and Thompson RG, 1979. Effects of prolonged cyanide and thyocyanate feeding in rats. Journal of Toxicology and Environmental Health, 5, 579–592. [DOI] [PubMed] [Google Scholar]
- Philbrick DJ, Hill DC and Alexander JC, 1981. Influence of dose level and methionine intake on the effects of linamarin administration to rats. Journal of Toxicology and Environmental Health, 8, 159–168. [DOI] [PubMed] [Google Scholar]
- Poulton JE, 1990. Cyanogenesis in plants. Plant Physiology, 94, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivadeneyra‐Domínguez E, Vázquez‐Luna A, Rodríguez‐Landa JF and Díaz‐Sobac R, 2013. Neurotoxic effect of linamarin in rats associated with cassava (Manihot esculenta Crantz) consumption. Food and Chemical Toxicology, 59, 230–235. 10.1016/j.fct.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Rosenberg NL, Myers JA and Martin WR, 1989. Cyanide‐induced parkinsonism: clinical, MRI, and 6‐fluorodopa PET studies. Neurology, 39, 142–144. [DOI] [PubMed] [Google Scholar]
- Rosling H, 1987. Cassava Toxicity and Food Security. In: Rosling H. (ed.). Tryck Kontakt, Uppsala, Sweden: pp. 3–40. [Google Scholar]
- Rumack BH, 1983. Cyanide poisoning In: Newball HH. (ed.). Respiratory Care of Chemical Casualties, Proceedings of the Symposium on Respiratory Care of Chemical Casualties (McLean, Virginia, 28–30 November 1983). US Army Medical Research and Development Command, Fredrick, MD: 186 pp. [Google Scholar]
- Sahin S, 2011. Cyanide poisoning in a children caused by apricot seeds. Journal Health and Medical Informatics, 2, 1. [Google Scholar]
- Sakata M, Yoshida A, Yuasa C, Sakata K and Haga M, 1987. Toxicity of D, L‐mandelonitrile‐beta‐D‐glucoside, “prulaurasin” in rat. Journal of Toxicological Sciences, 12, 47–55. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Verlaan P, Geeraerts T, Buys S, Riu‐Poulenc B, Cabot C, Fourcade O, Mégarbane B and Genestal M, 2011. An unusual cause of severe lactic acidosis: cyanide poisoning after bitter almond ingestion. Intensive Care Medicine, 37, 168–169. 10.1007/s00134-010-2029-8 [DOI] [PubMed] [Google Scholar]
- Sanusi RA and Olurin A, 2012. Portion and serving sizes of commonly consumed foods, in Ibadan, Southwestern Nigeria. African Journal of Biomedical Research, 15, 149–158. [Google Scholar]
- Schilcher H, Schulz V and Nissler A, 1986. Zur Wirksamkeit und Toxikologie von Semen Lini. Zeitschrift für Phytotherapie, 7, 113–117. [Google Scholar]
- Schmidt K, 1972. Bestimmung von Blausaure aus Steinobst mit Hilfe der Gaschromatographie unter Verwendung eines stickstoffselektiven Detektors (TID). Lebenmittelchemie und gerichtliche Chemie, 31, 110–112. [Google Scholar]
- Schneider L, Buhrke T, Lampen A and Abraham K, 2014. Activity of ß‐glucosidase in plants with high content of cyanogenic glycosides as important factor determining their toxicity. Toxicology Letters, 229S, S170 10.1016/j.toxlet.2014.06.582 [DOI] [Google Scholar]
- Schulz V, Gross R, Pasch T, Busse J and Loeschcke G, 1982. Cyanide toxicity of sodium nitroprusside in therapeutic use with and without sodium thiosulfate. Klinische Wochenschrift, 60, 1393–1400. [DOI] [PubMed] [Google Scholar]
- Seigler DS, 1991. Cyanide and cyanogenic glycosides In: Rosenthal GA. and Berenbaum MR. (eds.). Herbivores. Their Interaction with Secondary Plant Metabolites, vol. 1. Academic Press, San Diego, CA: pp. 35–77. [Google Scholar]
- Shim SM and Kwon H, 2010. Metabolites of amygdalin under simulated human digestive fluids. International Journal of Food Science and Nutrition, 61, 770–779. [DOI] [PubMed] [Google Scholar]
- Shivanoor SM and David M, 2014. Subchronic cyanide toxicity on male reproductive system of albino rat. Toxicological Research, 4, 57–64. [Google Scholar]
- Shragg TA, Albertson TE and Fisher CJ Jr, 1982. Cyanide poisoning after bitter almond ingestion. Western Journal of Medicine, 136, 65–69. [PMC free article] [PubMed] [Google Scholar]
- Soto‐Blanco B and Gorniak SL, 2004. Prenatal toxicity of cyanide in goats‐a model for teratological studies in ruminants. Theriogenology, 62, 1012–1026. [DOI] [PubMed] [Google Scholar]
- Soto‐Blanco B, Marioka PC and Górniak SL, 2002. Effects of long‐term low‐dose cyanide administration to rats. Ecotoxicology and Environmental Safety, 53, 37–41. [DOI] [PubMed] [Google Scholar]
- Soto‐Blanco B, Stegelmeier BL, Pfister JA, Gardner DR and Panter KE, 2008. Comparative effects of prolonged administration of cyanide, thiocyanate and chokecherry (Prunus virginiana) to goats. Journal of Applied Toxicology, 28, 356–363. [DOI] [PubMed] [Google Scholar]
- Sousa AB, Soto‐Blanco B, Guerra JL, Kimura ET and Górniak SL, 2002. Does prolonged oral exposure to cyanide promote hepatotoxicity and nephrotoxicity? Toxicology, 174, 87–95. [DOI] [PubMed] [Google Scholar]
- Sousa AB, Manzano H, Soto‐Blanco B and Górniak SL, 2003. Toxicokinetics of cyanide in rats, pigs and goats after oral dosing with potassium cyanide. Archives of Toxicology, 77, 330–334. [DOI] [PubMed] [Google Scholar]
- de Sousa AB, Maiorka PC, Gonçalves ID, Marques de Sá LR and Górniak SL, 2007. Evaluation of effects of prenatal exposure to the cyanide and thiocyanate in Wistar rats. Reproductive Toxicology, 23, 568–577. [DOI] [PubMed] [Google Scholar]
- Stadelmann W, 1976. Blausäuregehalt von Steinobstsäften. Flüssiges Obst, 43, 45–47. [Google Scholar]
- Storer RD, McKelvey TW, Kraynak AR, Elia MC, Barnum JE, Harmon LS, Nichols WW and DeLuca JG, 1996. Revalidation of the in vitro alkaline elution/rat hepatocyte assay for DNA damage: improved criteria for assessment of cytotoxicity and genotoxicity and results for 81 compounds. Mutation Research, 368, 59–101. [DOI] [PubMed] [Google Scholar]
- Sturm W and Hansen E, 1967. Über Cyanwasserstoff in Prunoideensamen und einigen anderen Lebensmitteln. Zeitschrift für Lebensmittel‐Untersuchung und Forschung, 135, 249–259. [Google Scholar]
- Tewe OO and Maner JH, 1981. Long‐term and carry‐over effect of dietary inorganic cyanide (KCN) in the life cycle performance and metabolism of rats. Toxicologi and Applied Pharmacology, 58, 1–7. [DOI] [PubMed] [Google Scholar]
- Tokpohozin SE, Fischer S, Sacher B and Becker T, 2016. β‐D‐Glucosidase as “key enzyme” for sorghum cyanogenic glucoside (dhurrin) removal and beer bioflavouring. Food and Chemical Toxicology, 97, 217–223. [DOI] [PubMed] [Google Scholar]
- Tor‐Agbidye J, Palmer VS, Lasarev MR, Craig AM, Blythe LL, Sabri MI and Spencer PS, 1999. Bioactivation of cyanide to cyanate in sulfur amino acid deficiency: relevance to neurological disease in humans subsisting on cassava. Toxicological Sciences, 50, 228–235. [DOI] [PubMed] [Google Scholar]
- Tulsawani RK, Debnath M, Pant SC, Kumar O, Prakash AO, Vijayaraghavan R and Bhattacharya R, 2005. Effect of sub‐acute oral cyanide administration in rats: protective efficacy of alpha‐ketoglutarate and sodium thiosulfate. Chemico‐Biological Interactions, 156, 1–12. [DOI] [PubMed] [Google Scholar]
- Tuncel G, Nout MJR and Brimer L, 1995. The effects of grinding, soaking and cooking on the degradation of amygdalin of bitter apricot seeds. Food Chemistry, 53, 447–451. [DOI] [PubMed] [Google Scholar]
- Tuncel G, Nout MJR and Brimer L, 1998. Degradation of cyanogenic glycosides of bitter apricot seeds (Prunus armeniaca) by endogenous and added enzymes as affected by heat treatments and particle size. Food Chemistry, 63, 65–69. [Google Scholar]
- US EPA (US Environmental Protection Agency), 2010. Toxicological review of hydrogen cyanide and cyanide salts (CAS No. various) in support of summary information on the Integrated Risk Information System (IRIS). Environmental Protection Agency, Washington, DC, USA: Available online: http://www.epa.gov/iris/toxreviews/0060tr.pdf [Google Scholar]
- Vinnakota CV, Peetha NS, Perrizo MG, Ferris DG, Oda RP, Rockwood GA and Logue BA, 2012. Comparison of cyanide exposure markers in the biofluids of smokers and non‐smokers. Biomarkers, 17, 625–633. 10.3109/1354750X.2012.709880 [DOI] [PubMed] [Google Scholar]
- Vock EH, Lutz WK, Hormes P, Hoffmann HD and Vamvakass A, 1998. Discrimination between genotoxicity and cytotoxicity in the induction of DNA double‐strand breaks in cells treated with etoposide, melphalan, cisplatin, potassium cyanide, Triton X100, and gamma‐irradiation. Mutation Research, 413, 83–94. [DOI] [PubMed] [Google Scholar]
- Voldrich M and Kyzlink V, 1992. Cyanogenesis in canned stone fruits. Journal of Food Chemistry, 57, 161–162, 189. [Google Scholar]
- Von Misselhorn K and Adams R, 1976. Über Cyanidgehalte in Steinobstprodukten. Die Branntweinwirtschaft, 4, 49–50. [Google Scholar]
- Wagner B and Galey WR, 2003. Kinetic analysis of hexose transport to determine the mechanism of amygdalin and prunasin absorption in the intestine. Applied Toxicology, 23, 371–375. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2004. Hydrogen cyanide and cyanides: human health aspects. Concise International Chemical Assessment Document 61. Available online: http://www.inchem.org/documents/cicads/cicads/cicad61.htm
- WHO (World Health Organization), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. WHO Library Cataloguing‐in‐Publication Data. Environmental Health Criteria 240. ISBN 978 92 4 157240 8.
- Willhite CC, 1982. Congenital malformations induced by laetrile. Science, 215, 1513–1515. [DOI] [PubMed] [Google Scholar]
- Yamamoto H and Mohanan PV, 2002. Melatonin attenuates brain mitochondria DNA damage induced by potassium cyanide in vivo and in vitro . Toxicology, 179, 29–36. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Sano T, Hashimoto T and Kanzawa K, 2007. Development of a method to remove cyanogenic glycosides from flaxseed meal. International Journal of Food Science and Technology, 42, 70–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dietary surveys and FoodEx categories used for exposure assessment
Results of probabilistic acute dietary exposure assessment to cyanide
Results of chronic dietary exposure assessment to cyanide
Average acute exposure per food category in children


