Abstract
This guidance document is intended to assist the applicant in the preparation and the presentation of an application, as foreseen in Article 7.6 of Regulation (EC) No 1831/2003, for the authorisation of additives used in animal nutrition. It specifically covers the assessment of the safety for the environment.
| Draft endorsed by the FEEDAP Panel | 2 October 2018 |
| Submitted for public consultation | 8 October 2018 |
| End of public consultation | 19 November 2018 |
| Adoption by the FEEDAP Panel | 27 February 2019 |
| Implementation date | 1 September 2019 |
Keywords: guidance, environment, risk assessment, feed additives
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1595/full
Background and Terms of Reference
Regulation (EC) No 1831/2003 establishes the rules governing the Community authorisation of additives for use in animal nutrition. Moreover, Regulation (EC) No 429/2008 provides detailed rules for the implementation of Regulation (EC) No 1831/2003 as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives.
The Panel on Additives and Products or Substances used in Animal Feed (FEEDAP Panel) has adopted a series of guidance documents which aim at complementing Regulation (EC) No 429/2008 to support applicants in the preparation and submission of technical dossiers for the authorisation of additives for use in animal nutrition according to Regulation (EC) No 1831/2003.
The European Food Safety Authority (EFSA) asked its FEEDAP Panel to:
identify from the current guidance documents, those that need to be updated, taking into consideration the most recent scientific developments and the experience gained in the assessment of feed additives;
update the guidance documents in need of revision accordingly; this activity can be conducted in different rounds on the basis of the priorities identified and on the feasibility of the revision according the resources available;
taking into account the sensitivity and the relevance of some of the guidance documents under revision and the entity of the revision itself (e.g. substantial or not), consider initiatives like preparatory info‐sessions or public consultations of the draft guidance documents. The relevant comments received in either step will have to be considered and addressed if appropriate in the final version of the guidance documents.
The first of the terms of reference was addressed by a statement of the FEEDAP Panel (EFSA FEEDAP Panel, 2016), in which it was identified the need to update most of the guidance documents that it produced and set priorities for this update.
This output addresses the second and third terms of reference with regard to the update of the guidance documents dealing with the assessment of the environmental risk of feed additives.
Scope of the guidance
This guidance document is intended to assist the applicant in the preparation and the presentation of its application, as foreseen in Article 7.6 of Regulation (EC) No 1831/2003. This document does not substitute for the obligation of an applicant to comply with the requirements of Regulation (EC) No 1831/2003 and its implementing rules. This guidance document is intended to provide the information necessary to properly assess the environmental impact of a feed additive, in order to demonstrate compliance with the requirements of Article 5.3 of Regulation (EC) No 1831/2003.
Applicants should justify the omission from the dossier of any data or any deviations from the requirements detailed in this guidance.
A feed additive may be a well characterised chemical or agent (e.g. a crystallised amino acid of > 98% active substance); a mixture of active chemicals or agents each of which is clearly definable (qualitatively and quantitatively); or a complex mixture in which not all constituents can be identified (typically plant extracts, containing several different chemically defined and/or undefined compounds). Different risk assessment procedures are considered. When the additive contains one or more clearly definable chemicals or agents, the ERA described in this guidance should be performed for each chemical/agent.
For complex mixtures with unidentified constituents, the FEEDAP Panel notes that developing an environmental risk assessment for such mixtures is not in the scope of the present guidance. The EFSA Scientific Committee is currently developing a guidance to assess mixtures of chemicals. Once the Scientific Committee of EFSA has officially published their guidance on risk assessment for mixtures, the FEEDAP Panel will consider it in a future update of this guidance.
For additives falling under the scope of Regulation (EC) No 1829/20031, the requirements for GMOs should be fulfilled.
When assessing the impact of microorganisms used as active agents as feed additives (i.e. feed additives containing viable microorganisms) to the environment, the following scenarios may apply:
For microorganisms included in the QPS list, any impact on the environment is assessed in the framework of the qualified presumption of safety (QPS) evaluation (EFSA BIOHAZ Panel, 2017). When the identity of such a microorganism included in the QPS list is unequivocally established and any qualification (if existing) is met, safety for the environment is presumed.
Strains carrying acquired genes for antimicrobial resistance are presumed to pose a risk for human and animal health via the environment.
-
For microorganisms not included in the QPS list the following applies:
-
–
For those naturally present in soils, plants or gastrointestinal tract of animals, their use as a feed additive is considered unlikely to introduce disturbances in the microenvironment where they are already prevalent. Consequently, the Panel considers that their use as feed additives would not pose a risk for the environment.
-
–
For those not naturally present in soils, plants or gastrointestinal tract of the animals, a case‐by‐case assessment would be needed. The principles of http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2012)1%26doclanguage=en (SANCO, 2012) or the principles of the EFSA guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use (EFSA GMO Panel, 2011) may be used as a guide. Furthermore, the European Commission is currently developing a guidance document on the risk assessment of metabolites produced by microorganism after application as active substances in plant protection products. Such guidance document can be considered in a future update of this guidance.
-
–
This guidance is divided in four sections. The introduction provides the principles of the environmental risk assessment (ERA) for feed additives. A Phase I decision tree is provided in Section 2, including the predicted environmental concentrations (PECs) for feed additives for terrestrial and aquatic environments. The PEC formulas and related default values were derived from the European Medicines Agency (EMA) guidance for the environmental risk assessment of veterinary medical products. The Phase II assessment, containing information on determination of predicted no effect concentrations (PNECs), on refinement of PECs and refinement of PNECs is given in Section 3. Section 3 includes also the assessment of persistent, bioaccumulative and toxic (PBT) substances and the assessment for secondary poisoning. Section 4 describes how to provide information on studies retrieved from the literature.
1. Introduction
This document provides guidance on how to conduct and report studies concerning the assessment of the safety of feed additives for the environment. It is an update of the previous one (EFSA, 2008a) and supersedes it.
Consideration of the environmental impact of feed additives is important since administration of these substances typically occurs over long periods, often involves large groups of livestock animals and the constitutive active substance(s) may be excreted to a considerable extent either as the parent compound or its metabolites.
Regulation (EC) No 1831/2003 and its implementing rules (Regulation (EC) No 429/2008) describe that an environmental risk assessment (ERA) should be conducted for (1) terrestrial compartment (via spreading of animal manure contaminated with feed additives on agricultural soils), (2) the aquatic compartment (via drainage and run‐off from agricultural fields to surface water, via direct discharge of waste water from land‐based fish farms to surface water, or via excreta from fish farmed in cages to sediment), and (3) the groundwater compartment (via leaching from soil). As referring to the air compartment, according to ECHA (2008b), ‘methods for the determination of effects of chemicals on species arising from atmospheric contamination have not yet been fully developed, except for inhalation studies with mammals. Therefore, the methodology used for hazard assessment (and therefore the risk characterisation) of chemicals in water and soil cannot be applied yet in the same manner to the atmosphere’.
The ERA decision schemes described in this document aim to protect non‐target plant and animal species in the receiving environment at the population level, while the protection level for microbes and protozoans is set at the biological functional group level.2 As default the ‘ecological threshold option’ (see Appendix A) is selected as specific protection goal (SPG). In this option, the magnitude of tolerable effect on key organism groups in the receiving environment is set at small (e.g. < 10% effect relative to controls). The ERA for feed additives (and their metabolites) is based on the precautionary principle meaning that, in the absence of relevant and reliable data, the PEC and PNEC estimates are based on worst‐case assumptions, which could be refined by generating more relevant and reliable data.
To determine the environmental impact of feed additives, a stepwise approach is followed. All feed additives should be assessed through Phase I to identify those feed additives which do not need further testing. For the other feed additives, a second phase (Phase II) assessment is needed. Additional information has to be provided, based upon which further studies may be considered necessary. Some feed additives that might otherwise stop in Phase I may require additional environmental information to address particular concerns associated with their potential risk. These situations are expected to be the exception rather than the rule and some evidence in support of the concern should be available.
The option of post marketing monitoring should be considered in the case that the negative effects of feed additive on the environment could not be undoubtedly excluded.
For the purpose of this guidance, the following definitions apply:
-
–
Active substance: any substance or mixture of substances intended to be used as/in a feed additive that provides the intended effect.3
-
–
Active agent: any microorganism intended to be used as/in a feed additive and that provides the intended effect.
-
–
Feed additive: substances, microorganisms or preparations other than feed materials and premixtures which are intentionally added to feed or water in order to perform one or more functions mentioned in Article 5.2 of Regulation (EC) No 1831/2003.
2. Phase I assessment
The purpose of Phase I assessment is to determine if a significant environmental effect of the additive is likely and whether a Phase II assessment is necessary. Phase I is based on a list of exclusion criteria structured in a decision tree. By using a minimum set of information, it is aimed to screen additives that do not need a Phase II ERA. The ERA of major species can be extrapolated to minor species when the same conditions of use are proposed.
Exemption from Phase II assessment may be made on the following criteria, unless there is scientifically based evidence for concern:
The additive is intended for non‐food producing animals only;
The additive is a natural substance, or made of natural substances, the use of which as a feed additive would not exceed its natural occurring concentrations in feed sources, and/or would not substantially alter the concentration and/or distribution of the substance in the receiving environment;
The additive is extensively metabolised in the target animal;
The feed additive is not a potential persistent, bioaccumulative and toxic (PBT) or/and very persistent and very bioaccumulative (vPvB) substance;
The additive does not trigger concern due to a specific mode of action or due to accumulation in the receiving environment over the years; and
The PEC for each compartment of concern, calculated based on (i) the annual input of the manure, and (ii) the assumption that 100% of the dose ingested is excreted as the parent substance, does not meet the threshold value that triggers a Phase II assessment.
A decision tree is presented below (see Figure 1: Quick check), with explanatory notes for each question in Sections 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.6.1, 2.6.2, 2.7–2.7.
Figure 1.
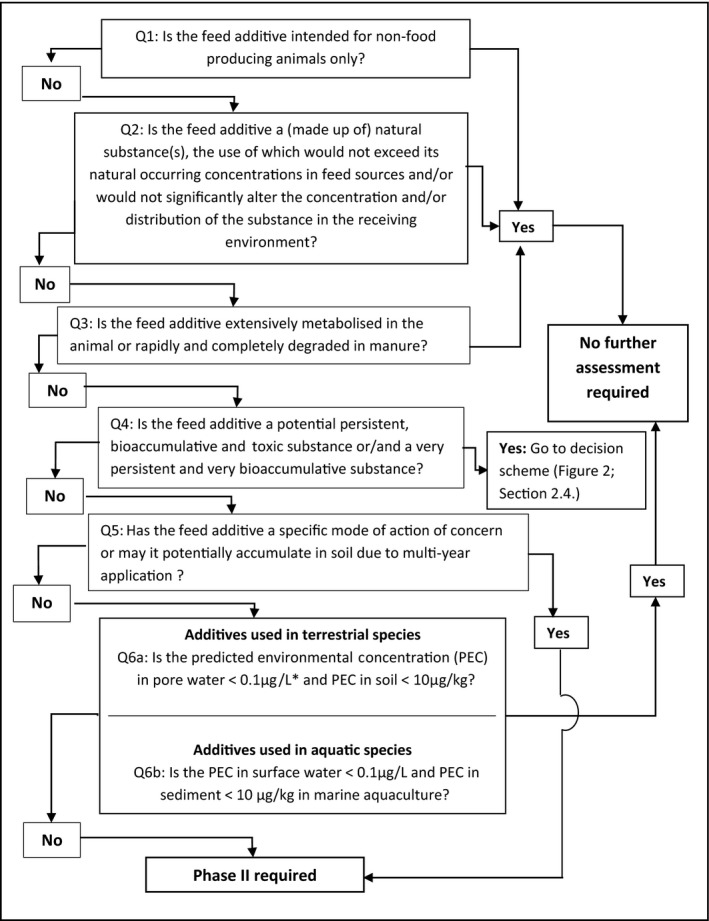
Quick‐check – Environmental Risk Assessment: Phase I
- *PEC in ground water is set equal to PEC in pore water (see Section 2.6.2).
Further clarifications on these questions are given in the following subsections
2.1. Question 1: Is the feed additive intended for non‐food producing animals only?
Generally, non‐food producing animals are not intensively reared and/or their excrements are not spread over agricultural land. Therefore, due to the limited total amount of product used, feed additives for non‐food animals are expected to produce less environmental concern than the feed additives in food‐producing animals. As a consequence, besides exceptional cases (e.g. additives used in intensively reared fur‐producing animals), no further assessment is required (Figure 1). For those exceptional cases, the ERA would proceed through the following questions.
2.2. Question 2: Is the feed additive a (made up of) natural substance(s), the use of which would not exceed its natural occurring concentrations in feed sources and/or would not significantly alter the concentration and/or distribution of the substance in the receiving environment?
Evidence should be provided showing that comparable concentrations of the feed additive can be expected in other plant(s) and/or that the use of the feed additive will not significantly alter the concentration of the additive in the receiving environmental compartments of concern. For this purpose, the excretion rates (as active substance) in target species exposed to the additive at the highest permitted level in the EU or at the highest intended concentration in feed, should be compared with the lower ranges of reported background concentrations in soils, water and plants. If applicable, its degradability in the receiving environment may also be considered. Evidence on which to base such scientific rationale should be provided. This evidence can be based on available information retrieved from structured literature reviews and/or on analytical data (see Section 4).
For instance, if the concentration of a colouring agent used in fish feed is similar to that encountered in the natural diet of the fish species of concern (see EFSA FEEDAP Panel, 2014), or the concentration of a flavouring compound in feed does not exceed its natural concentration in plants (see EFSA FEEDAP Panel, 2016), no adverse impact is expected for the environment.
2.3. Question 3: Is the feed additive extensively metabolised in the target animal or rapidly and completely degraded in manure?
A feed additive is considered to be ‘extensively metabolised’ if converted into metabolites present in the excreta that do not possess a biological activity of environmental concern, like water, CO2 and common salts. A similar approach as in EMA, 2016 is followed: As a part of the Phase I assessment, data (analytical and/or from the scientific literature, see Section 4) on degradation of the active residue in manure may be submitted. If the active residue is rapidly and completely degraded in manure then the assessment may end at Phase I. In order to fully satisfy the requirements and to be in compliance with the definition of extensive metabolism, complete degradation should be demonstrated either by total mineralisation or by the presence of degradation products all representing ≤ 5% of the initial concentration in feed. When the application covers several target species/categories, it is recognised that it may be very demanding to provide studies for all potential target species receiving the feed additive. Therefore, interspecies extrapolation of data can be applied. The applicant is referred to the http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.5022/epdf, in its Section 2.1.1.1, to select the most representative species to be investigated.4
2.4. Question 4: Is the feed additive a potential persistent, bioaccumulative and toxic substance or/and a very persistent and very bioaccumulative substance?
Substances that are PBT or vPvB are of very high concern (REACH Regulation (EC) No. 1907/2006 and subsequent amendments).5 Due to the combination of these intrinsic properties and possible redistribution across environmental compartments, they pose serious hazards to non‐target organisms.
Substances are considered as PBT or vPvB substances when they fulfil the criteria as laid down in Annex XIII of the REACH Regulation (EC) No 1907/2006 (and subsequent amendments),6 for all three inherent properties P, B and T or both of the inherent properties vP and vB, respectively. To ensure a harmonised approach, these criteria together with the methodology in the current REACH guidance on PBT assessment (ECHA, 2017a,b,c,d) and the guideline on the assessment of PBT or vPvB substances in veterinary medicinal products (EMA, 2015), should be considered.
If based on the available information or screening information the active substance is a (potential) PBT and/or vPvB substance, a separate PBT/vPvB assessment in phase II needs to be conducted. Where only screening information is available for one or more endpoints, the first step consists in screening whether the substance may fulfil the criteria. Screening information listed in Appendix E can be used as a help for comparing the screening information with screening thresholds (screening criteria) established for this purpose (for further details, see ECHA Guidance Chapter 11 on PBT/vPvB assessment (ECHA, 2017a) and ECHA Guidance on information requirements and chemical safety assessment Part C (ECHA, 2017e), Section C.4.1). If for one or more endpoints the technical dossier contains only the information as required in Phase I, the applicant (based on screening information and other information available) must:
-
–
either derive an unequivocal conclusion that the substance does not fulfil the criteria; or
-
–
when this is not possible and there are indications that the substance may fulfil the criteria, the applicant must obtain further information needed to fulfil the objective of the PBT and vPvB assessment.
The applicant should explain why the models they have used are appropriate for the substance in question.
A decision scheme for assessing PBT or vPvB properties of the feed additive is presented in Figure 2.
Figure 2.
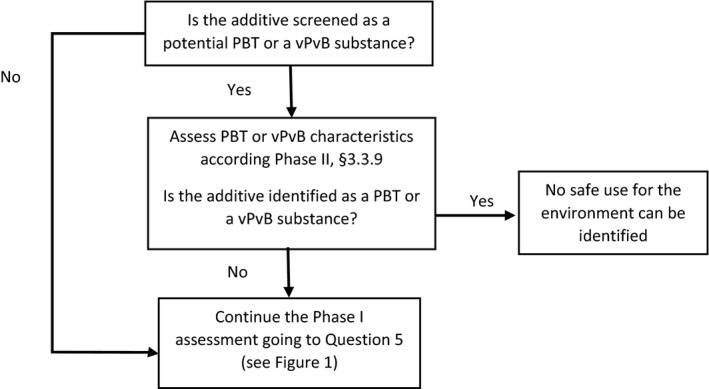
Decision scheme for assessing PBT or vPvB properties of the feed additive
2.5. Question 5: Has the feed additive a specific mode of action of concern or may it potentially accumulate in soil due to multiyear application?
Coccidiostats and histomonostats are chemicals with a specific toxic mode‐of‐action against harmful protozoa. Currently, they are authorised as feed additives in poultry and rabbit feed and, consequently, may be toxic to non‐target organisms in environments that receive poultry/rabbit manure. A Phase II ERA is expected for these feed additives (see Section 3). Other substances, on the basis of toxicological studies on laboratory animals or other evidence, may show toxicological properties in vivo that are of potential concern for environmental biota at sublethal concentrations, e.g. reproductive toxicity. Substances that hardly dissipate in the environment of concern may accumulate in the receiving compartment(s), which can only be properly assessed when information on long‐term fate is available. Therefore, when there is already evidence (either experimental or by screening) that a feed additive is not degradable and hardly dissipates, e.g. metals or other chemical elements that are excreted at amounts that can significantly increase the concentration in environmental compartments (see Question 2), these substances have to be assessed in Phase II.
2.6. Question 6a: Is the predicted environmental concentration of the feed additive used in terrestrial livestock species below a trigger value?
When excreta from livestock are applied on land, the use of feed additives can lead to contamination of soil, ground water and surface water (via drainage and run‐off).
The PECs used in Phase I would arise considering all excreted compounds being spread on land and other specified assumptions (see Sections 2.6.1 and 2.6.2) which reflect in summary worst‐case conditions.
If PEC for soil (PECsoil) (default: 5 cm depth) is less than 10 μg/kg dry weight; and
PEC for pore water (PECpw, surrogate for PECgw) (default: 20 cm soil depth) is less than 0.1 μg/L,
the substance is considered not to pose a risk for the environment, and therefore, no further assessment is necessary, unless there is available scientific evidence that it could represent a risk for human health and/or the environment.
2.6.1. Calculation of PEC in soil (PECsoil)
The amount of manure/slurry containing the feed additives allowed to be spread on land depends on the nitrogen content of the manure and the annual nitrogen load. Based on the data on feed intake and nitrogen content in manure, the maximum amount of parent compound per kg nitrogen excreted can be calculated by multiplying the concentration of the additive in feed with the feed consumption and dividing it by the corresponding nitrogen excretion. In Table 1, the feed intake and corresponding nitrogen excretion is given for the more relevant food‐producing species/categories. Other data can be used if justified.
Table 1.
Default values for feed intake and nitrogen excretion (see in Appendix H the assumptions made in the different calculations)
| Animals | Body weight start‐end (kg) | Productive cycles/yeara | Feed intake (kg/animal place per year)b | Nitrogen excreted (kg/animal place per year) |
|---|---|---|---|---|
| Piglet | 7–30 | 7.4 | 296 | 4 |
| Pig for fattening | 30–115 | 3.2 | 800 | 9 |
| Sow with piglets | 200 | 2.4 | 1,140 | 23 |
| Cattle for fattening | 250–630 | 1.2 | 4050 | 54 |
| Veal calf | 45–250 | 1.5 | 730 | 11 |
| Dairy cowc | 650 | 0.92 | 6,584 | 125 |
| Lamb for fattening | 4–32 | 1.5g | 273 | 5 |
| Sheep for fattening | 15–55 | 1.5g | 267 | 5 |
| Meat sheep | 60 | 1 | 607 | 10 |
| Dairy sheep | 60 | 1 | 580 | 10 |
| Dairy goat | 50 | 1 | 714 | 16.4 |
| Chicken for fattening | 0.045–2.2 | 6.5 | 22 | 0.33 |
| Laying hend | 1.4–2 | 0.84 | 42 | 0.8 |
| Turkey for fatteninge | 0.05–10(f)/16(m) | 2.6 | 70 | 1 |
| Rabbit for fattening | 0.9–3.1 | 4.8 | 30 | 0.5 |
| Horsef | 500 | 1 | 3,650 | 58 |
| Horse for fattening | 270–480 | 1.5g | 2,385 | 43 |
Number of productive cycles per animal place during a year.
Feed containing 88% DM in non‐ruminant species and 100% DM in ruminant species.
Considering a milk production of 8,000 kg/year.
Considering a production of 300 eggs/year.
Considering an average final weight (males (m) and females (f)) of 13 kg at slaughter.
Considering a mature horse in maintenance phase.
Calculated considering the seasonality of the oestrus of this species.
For a worst‐case estimation of the concentration in soil, the following assumptions are made:
The additive is continuously applied at the maximal recommended dose (as proposed by the applicant) to the feed of the target animal;
Total intake of the active substance is considered to be excreted as parent compound;
The current annual nitrogen load standard for slurry/manure spread on farm/livestock unit in nitrogen vulnerable areas is 170 kg N/ha per year (EU nitrate directive 91/676/EEC). The annual nitrogen emission standard is an average value that might be applied on a farm per year. According to the code of good agricultural practices, the emission to particular non‐vulnerable fields with crops/grass could exceed this value. It is recognised that in current agricultural practice in EU this average value could be exceeded and a different value could be considered (See Appendix G – aimed to support the refinement of the ERA at Member States level when a concern exists on use of higher amount of manure on soil);
There is no dissipation of the parent compound during storage and spreading of slurry/manure;
The standard assumption, when slurry/manure is spread on land, is that the additive is mixed in the soil up to 5 cm depth.7
Feed intake and the nitrogen excretion are dependent on the size, production level and age of the animal. Typically, both the intake and the excretion are calculated over a position in a stable (‘animal place’) for 1 year.
If the feed additive is intended for use in a livestock species or animal category that is not listed in Table 1, the proposed value should be motivated by providing scientific evidence to allow EFSA evaluating the proposal.
The following equations should be used to calculate PEC in manure and soil:
where:
| Symbol | Parameter | Default Valuea | Unit |
|---|---|---|---|
| Input | |||
| Cadd | Concentration of the additive in feed | mg/kg complete feed | |
| FItotal | Total feed intake (DM) per animal per year | kg feed/year | |
| Nexcreted | Total N excretion per animal per year | kg N/year | |
| RHOd soil | Bulk density of (dry) soil | 1,500 | kg/m3 |
| DEPTHfield | Mixing depth with soil | 0.05 | m |
| CONVarea field | Conversion factor for the area of the agricultural field | 10,000 | m2/ha |
| Q | Annual nitrogen emission standard | 170 | kg N/ha |
| Intermediate results | |||
| PECmanure | Concentration of the additive (parent compound) in manure expressed per amount nitrogen | mg/kg N | |
| Output | |||
| PECsoil dw | Concentration of the additive (parent compound) in soil (dry weight) | mg/kg soildw | |
The use of the indicated default values in the equations is recommended. Reasons for any deviations from these values should be given by the applicant.
Using these formulas, the concentration of a feed additive (mg/kg feed) that would correspond to a PECsoil below the trigger value for the different species can be calculated back as shown in Appendix F.
2.6.2. Estimation of PEC in groundwater (PECgw)
Several numerical models are available to calculate groundwater concentrations of agrochemicals (mainly for pesticides). These models, however, require a characterisation of the soil to a high level of detail. This makes these models less appropriate for a preliminary assessment. Therefore, as an indication for potential groundwater levels, the concentration in pore water of agricultural soil is taken. PEC in groundwater is set equal to PEC in pore water. It should be noted that this is a worst‐case assumption, neglecting transformation and dilution in deeper soil layers.
The PEC of pore water (PECpw) is calculated using the approach described in REACH guidance R16, (ECHA, 2016).
In this screening model, partitioning depends on equilibrium sorption to solids, no saturation at binding places and steady‐state conditions. This model provides a worst‐case estimate of the pore water concentrations as movement, dilution, desorption, transformation, weather or crops are not considered. Soil is defined through compartment volumes for solids, water and air, dry bulk density and texture (mineral and organic fraction). The soil depth for calculation of the PECsoil used for calculating the PECpw is set at 20 cm.
Where no measured Koc value is available, in the Phase I assessment estimation techniques can be used based on correlation with the Kow or water solubility given in https://archive.epa.gov/scipoly/sap/meetings/web/pdf/106_adsorption_desorption_using.pdf (Soil Adsorption/Desorption) or from a quantitative structure–activity relationships (QSAR) calculation as described in Appendix D. When experimental data is available, explanations on how to select the Koc are given in Section 3.3.1.
The model calculation of the concentration in pore water is as follows:
where:
| Symbol | Parameter | Default Valuea | Unit |
|---|---|---|---|
| Additive properties | |||
| Cadd | Concentration of the additive in feed | mg/kg complete feed | |
| VP | Vapour pressure | Pa | |
| MOLW | Molar mass | g/mol | |
| SOL | Water solubility | mg/L | |
| Koc b | Organic carbon normalised partition coefficient | dm3/kg | |
| Substance independent input | |||
| RHOw soil | Bulk density of (wet) soil | 1,700 | kg/m3 |
| DEPTHfield | Mixing depth with soil | 0.2 | m |
| RHOsolid | Bulk density of soil solids | 2,500 | kg/m3 |
| Fairsoil | Fraction air in fresh field soil | 0.2 | m3/m3 |
| Fwater–soil | Fraction water in fresh field soil | 0.2 | m3/m3 |
| Fsolidsoil | Fraction solids in fresh field soil | 0.6 | m3/m3 |
| Focsoil | Weight fraction organic carbon in dry weight soil | 0.02 | kg/kg1 |
| TEMP | Temperature at air–water interface | 285 | °K |
| R | Gas constant | 8.314 | Pa m3/mol/°K |
| FItotal | Total feed intake (DM) per animal in a year | See Table 1 | kg feed/year |
| Nexcreted | Total N excretion per animal in a year | See Table 1 | kg N/year |
| Q | Annual nitrogen emission to soil | 170 | kg N/ha |
| CONVarea field | Conversion factor for the area of the agricultural field | 10,000 | m2/ha |
| Intermediate results | |||
| Ksoil–water | Partition coefficient solids and water in soil (v/v) | m3/m3 | |
| Kpsoil | Partition coefficient solids and water in soil (v/w) | dm3/kg | |
| Kair–water | Partition coefficient air and water in soil | m3/m3 | |
| Output | |||
| PECmanure | Concentration of the additive (parent compound) in manure expressed per amount nitrogen | mg/kg N | |
| PECsoil ww | Concentration of the additive (parent compound) in soil (wet weight) | mg/kg soilww | |
| PECpw | Concentration of the additive (parent compound) in pore water | mg/L | |
The use of the indicated default values in the equations is recommended. Reasons for any deviations from these values should be given by the applicant.
In the Phase I assessment, estimation techniques can be used (correlation with Kow or water solubility or QSAR calculation).
2.7. Question 6b: Is the predicted environmental concentration of the feed additive used in aquaculture below a trigger value?
Feed additives used in aquaculture can result in contamination of sediment and water.
The method to calculate the PEC in sediment and water varies for the different European fish production systems: sea cages versus land‐based aquaculture (ponds, tanks and recirculation systems). In aquaculture operations involving the use of sea cages, benthic organisms (living in or on sediments) are considered to be most at risk, whereas both waterborne exposure of both pelagic organisms (living in the water column) and benthic organisms present the main risk from land‐based fish farms that discharge to shallow freshwater ecosystems.
The PECs used in Phase I should be calculated considering all excreted compounds being dispersed to sediment and water and other specified assumptions (see Sections 2.7.1 and 2.7.2) which reflect in summary worst‐case conditions.
The organic carbon content of the sediment may influence the bioavailability and therefore the toxicity of the test substance. Therefore, for comparison of sediment tests, the organic carbon content of the test sediment should be within a certain range. The OECD guideline 218 for the test with Chironomus using spiked sediment recommends an organic carbon content of the test sediment of 2% (± 0.5%) (EMA, 2016).
If PEC for sediment (PECsed) (default: 5 cm depth assuming 2 ± 0.5% organic carbon (OC)) is:
-
–
less than 10 μg/kg dry weight; and
-
–
PEC for surface water (PECsw) is less than 0.1 μg/L
the substance is considered not to pose a risk for the environment, and therefore no further assessment is necessary.
2.7.1. Calculation of PEC in the sediment (PECsed) for sea cages
The calculation of PECsed is considered a realistic worst‐case value that covers the use of feed additives for a wide range of fish species. It should be calculated as follows:
where:
| Symbol | Parameter | Default valuea | Unit |
|---|---|---|---|
| Input | |||
| Cadd | Concentration additive in feed | mg/kg complete feed | |
| CF | Conversion factor (kg feed to kg total carbon in faeces) | 15.1b | kg/kg carbon |
| kdep | Maximum deposition rate of faeces | 0.01c | kg carbon/m2 per day |
| Tproduction | Number of production days | 365 | day |
| RHOsolid | Bulk density of solids | 2,500d | kg/m3 |
| DEPTHsed | Mixing depth in sediment | 0.05 | m |
| Fsolid | Volume fraction of solids in fresh field collected sediment | 0.2 | m3/m3 |
| Output | |||
| PCfaeces | Concentration of the additive (parent compound) in the carbon fraction of faeces | mg/kg carbon | |
| PECsed | Highest initial concentration of additive in dry weight sediment | mg/kg | |
The use of the indicated default values in the equations is recommended. Reasons for any deviations from these values should be given by the applicant.
Concentration of the additive in feed (Cadd) given in mg/kg feed has to be converted in mg/kg C feed (2.06). Subsequently, mg/kg1 C feed is converted to into mg/kg C faeces (7.3), hence the total conversion is 2.06 × 7.3 = 15.1.
According to Hansen et al., 1991; Karakassis et al., 2002; Corner et al., 2006; Holmer et al., 2006; Kutti et al., 2007.
Assumed to be similar for soil and sediment (see Section 2.6.2).
2.7.2. Calculation of PEC in surface water from aquaculture (PECswaq) in raceway/pond/tanks and recirculation systems
In Phase I, it is assumed that the total amount of the additive in feed is released into the aquaculture system (i.e. there is no retention in ‘sludge’ such as water material that is filtered or settles out within the facility).
For feed daily ration and water flow rate, the following default settings are proposed for some fish species commonly farmed in Europe. The information of Table 2 for sea bass, sea bream and turbot refers to their breeding in inland aquaculture systems. For species not listed in Table 2, the applicant may propose other values and provide a justification.
Table 2.
Feed ration and water flow rate in fish farming in Europe
| Fish types | Feed Ration (kg feed/kg fish per day) | Water flow rate (L/kg fish and day) |
|---|---|---|
| Salmon | 0.01a | 865d |
| Rainbow trout | 0.02 | 1400b |
| Sea bass/Sea bream | 0.01c | 400c |
| Turbot | 0.01c | 720c |
The PECswaq can be calculated as follows:
where:
| Symbol | Parameter | Unit |
|---|---|---|
| Input | ||
| Cadd | Concentration of the additive in feed | mg/kg complete feed |
| FR | Feed Ration | kg feed/kg fish per day |
| Flow | Water flow rate through the system | L/kg fish per day |
| DF | Dilution Factor | 10 |
| Output | ||
| PECswaq | Highest initial concentration of additive (parent compound) in surface water | mg/L |
3. Phase II assessment
The aim of Phase II is to assess the potential for additives to affect non‐target species in the environment, including both aquatic and terrestrial species or to reach deeper groundwater at levels above a concentration of 0.1 μg/L. It is not practical to evaluate the effects of additives on every species in the environment that may be exposed to the additive following its administration to the target species. Therefore, certain taxa/endpoints are recommended to be tested and intended to serve as surrogates or indicators for the range of species/functions present in the environment.
The Phase II assessment is based on a risk quotient approach, where the calculated PEC and PNEC values for each compartment of concern should be compared. The PNEC is determined from experimentally determined endpoints divided by an appropriate assessment (safety) factor. The value of the assessment factor (AF) is dependent on the amount of accurate and relevant data available, associated uncertainties and harmonisation requirements between different legislations.
For the effect assessment (e.g. PNEC derivation), the tier 1 usually is based on the basic dossier requirements. Since lower tiers should be more conservative than higher tiers, effect estimates (e.g. PNECs) generated at higher tiers should be higher than those at lower tiers. Consequently, higher tier information can be used to validate/calibrate lower tiers. Ideally, the consistency of the different tiers within an ERA scheme should be evaluated for a number of benchmark feed additives.
If the feed additive is a metal salt and data for the same metal but a different salt is available, these can be used in the PNEC derivation when scientifically justified and properly documented.
The Phase II assessment is based on a tiered approach (Figure 3). The first tier, Phase IIA, makes use of a limited number of fate and effect studies to produce a conservative assessment of risk based on exposure and effects in the environmental compartment of concern. This would also mean that the PECs from Phase I have to be recalculated (PECA) using the information on metabolism in the target animal(s) and experimental fate data, i.e. adsorption and degradation.
Figure 3.
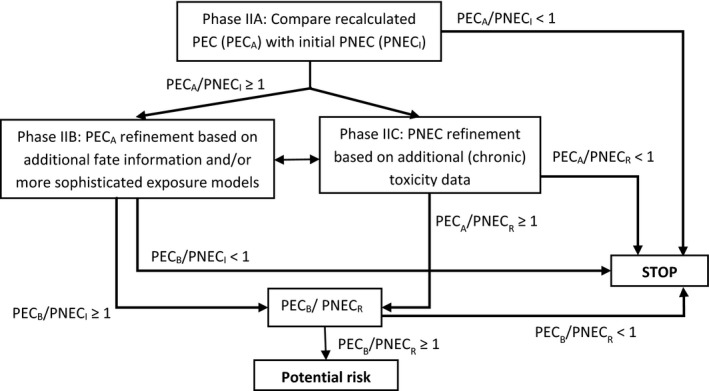
Phase II decision tree for the environmental risk assessment of soil and aquatic compartment for terrestrial animals (PECA and PECB concern PECs for soil, groundwater, surface water and sediment recalculated using procedures described in Sections 3.3.1–3.3.5 and Section 3.4, respectively; PNECI and PNECR are initial and refined PNECs calculated using procedures described in Sections 3.3.6 and 3.5, respectively)
In all tiers (Phases IIA to IIC), a comparison should be made between the PEC and the PNEC (or threshold value for the groundwater):
Phase IIA: If the PECA is lower than the PNECI values and the trigger value for groundwater is not exceeded, no further assessment is required, unless accumulation is expected (for further details see Section 3.3);
Phase IIB: If the PECA/PNECI is ≥ 1, a more refined PEC (= PECB) can be calculated based on additional data not yet considered (for further details, see Section 3.4);
Phase IIC: If the PECA/PNECI or PECB/PNECI ratio predicts a potential risk (ratio ≥ 1), a more refined PNEC (= PNECR) can be derived to better estimate the environmental risks (for further details, see Section 3.5).
The comparison of the PEC to PNEC estimates is based on the following principles (see Sections A.5 and A.7 of Appendix A):
The effect assessment and exposure assessment is based on the same ecotoxicologically relevant type of concentration.
When the PNEC is derived from acute toxicity data, only the predicted environmental peak concentration (PECmax) is used for comparison.
-
When the PNEC is derived from chronic toxicity data, the PECmax can be considered as a precautionary worst‐case approach. Alternatively, the time‐weighted average (PECtwa) may be used if:
Reciprocity of effects is demonstrated/likely.
The chronic toxicity estimates (EC10 or NOECs) on which the PNEC is based are expressed in terms of (geometric) mean concentrations during the exposure period of the test; in case measured concentrations in the course of the experiment are within 20% of nominal, the nominal concentration can be used as a proxy of the mean concentration.
The time frame of the PECtwa estimate should be less than or equal to than the duration of the exposure periods in the chronic toxicity tests that drive the PNEC.
Toxicity data that are expressed in terms of initial exposure concentration and show a decline larger than 20% in the course of the experiment, may be used to derive a PNEC if in the ERA this PNEC is compared with the PECmax and it is likely/plausible that the decline in exposure is not faster in the toxicity tests than that predicted for the environment. To demonstrate this, either validated exposure models or chemical monitoring data are required that enable to characterise the dynamics in exposure concentration of the feed additive for the environmental compartment of concern. If these models/data are not available, a precautionary approach is advocated by expressing the laboratory toxicity estimates in terms of mean (e.g. geometric mean or time‐weighted average) exposure concentration during the test and by selecting the PECmax.
In case of difficult substances, consider http://www.oecd-ilibrary.org/environment/guidance-document-on-aquatic-toxicity-testing-of-difficult-substances-and-mixtures_9789264078406-en (Guidance Document on Aquatic Toxicity Testing of Difficult Substances and Mixtures). If the problem cannot be solved using this guidance, an additional environmentally more realistic study may be requested.
3.1. Physico‐chemical properties studies
In order to evaluate the fate and toxicity of the feed additive, some basic physico‐chemical properties are needed. The studies required are reported in Table 3 (EMA, 2005).
Table 3.
Physico‐chemical properties studies in Phase IIA (EMA, 2005)
Calculation only, though a study is recommended when other physical–chemical properties, e.g. molecular weight, melting temperature, thermogravimetric analysis suggest that the vapour pressure may exceed 10−5 Pa at 20°C.
This parameter is not strictly needed in the assessment. Nevertheless, melting point/melting range together with vapour pressure provide information on the distribution of the substance within and between the environmental media (water, soil and air).
Water solubility provides information on how likely the feed additive will be distributed by the hydrological cycle and gain access to living organisms. It is also important to set up test conditions for a range of fate (e.g. biodegradation, bioaccumulation) and effects studies.
Dissociation constants in water may affect the adsorption of the substance on soils and sediments and absorption into biological cells. It may also be an important factor in deciding which method or conditions should be used to determine the octanol–water partition coefficient and soil adsorption partition coefficient (see Section 3.2).
UV–Visible absorption spectrum gives information on the potential of a substance to photodegrade and/or to be phototoxic under environmental relevant conditions.
The n‐octanol/water partition coefficient (Kow) is used to estimate the environmental partitioning, e.g. adsorption and bioaccumulation. Some precautions must be taken regarding the use of the shake‐flask method ( http://www.oecd-ilibrary.org/environment/test-no-107-partition-coefficient-n-octanol-water-shake-flask-method_9789264069626-en) or the high‐performance liquid chromatography (HPLC) method ( http://www.oecd-ilibrary.org/environment/test-no-117-partition-coefficient-n-octanol-water-hplc-method_9789264069824-en) to determine log Kow for very lipophilic compounds. These are outlined in the http://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html:
‘The shake‐flask method is recommended when the log Kow value falls within the range from –2 to 4. The shake‐flask method applies only to essential pure substances soluble in water and n‐octanol. For highly lipophilic substances, which slowly dissolve in water, data obtained by employing a slow‐stirring method are generally more reliable. Furthermore, the experimental difficulties, associated with the formation of microdroplets during the shake‐flask experiment, can to some degree be overcome by a slow‐stirring method where water, octanol, and test compound are equilibrated in a gently stirred reactor. With the slow‐stirring method (OECD http://miranda.sourceoecd.org/vl=6367206/cl=17/nw=1/rpsv/ij/oecdjournals/1607310x/v1n1/s22/p1) a precise and accurate determination of Kow of compounds with log Kow of up to 8.2 is allowed. As for the shake‐flask method, the slow‐stirring method applies only to essentially pure substances soluble in water and n‐octanol. The HPLC method, which is performed on analytical columns, is recommended when the log Kow value falls within the range 0 to 6. The HPLC method is less sensitive to the presence of impurities in the test compound compared to the shake‐flask method’.
It should also be emphasised that the log Kow for ionisable substances should be measured on the non‐ionised form at environmentally relevant pH values.
3.2. Environmental fate studies
Biodegradation studies should be performed in soil for feed additives intended for use in terrestrial species and in aquatic systems for feed additives intended for aquatic animals. The soil adsorption/desorption test should be used for additives for both terrestrial and aquatic species as long as there is no validated test for sediment. Table 4 describes the studies required for Phase IIA (EMA, 2005).
Table 4.
Environmental fate studies for phase IIA (EMA, 2005)
Recommended only for the terrestrial branch.
Recommended only for additives used in aquaculture.
3.2.1. Soil adsorption/desorption
Adsorption/desorption studies should report both the organic carbon–water partitioning coefficient (Koc) and the distribution constant (Kd) values for a range of soils. http://www.oecd-ilibrary.org/environment/test-no-121-estimation-of-the-adsorption-coefficient-koc-on-soil-and-on-sewage-sludge-using-high-performance-liquid-chromatography-hplc_9789264069909-en guideline to determine the log Koc by means of HPLC should be used with care. For polar compounds especially, this method is not fully validated and may provide unreliable Koc values. Also, log Koc values higher than 5.6 should not be considered to be reliable. For this reason, the http://www.oecd-ilibrary.org/environment/test-no-106-adsorption-desorption-using-a-batch-equilibrium-method_9789264069602-en test method is recommended. As a minimum five different soils or sediments should be selected to investigate the dependency of the Koc value to the different soil properties. Depending on the distribution constant these substances could dissociate into ionic species around environmental pH values, which may have significantly different water solubilities and partition coefficients than the non‐dissociated species. If the acid distribution constant (pKa) value is within the environmentally relevant pH range, the selected soils should cover a wide range of pH, in order to evaluate the adsorption of the substance in its ionised and unionised forms as recommended in the http://www.oecd-ilibrary.org/environment/test-no-106-adsorption-desorption-using-a-batch-equilibrium-method_9789264069602-en.
Other soil components with polar and/or charged surfaces may also act as sorbents, e.g. cations can often sorb to clay particles instead of organic material.
In most cases, the Koc can be used to estimate the sorption of the feed additive (active substance) to soil or sediment, but a direct estimation of the Ksoil‐water can also be useful. Especially for ionophores, it is important to know the main factors that govern the sorption of the molecule to soil or sediment. For compounds that are mainly sorbed to clay, the partition coefficient (Kp) can be calculated for a standard soil or sediment containing 20% clay. When appropriate, models need to be adapted to account for additional sorbents and pH‐dependence of sorption. Further information on the acceptability criteria to be considered for deriving a proper Koc, please make reference to the https://www.efsa.europa.eu/en/supporting/pub/en-1326, (EFSA, 2017)
3.2.2. Soil biodegradation and degradation in aquatic compartment
The soil degradation simulation study ( http://www.oecd-ilibrary.org/environment/test-no-307-aerobic-and-anaerobic-transformation-in-soil_9789264070509-en) is recommended for feed additives used in livestock. For feed additives used in aquaculture, the OECD 307 study should be replaced by a water/sediment degradation simulation study ( http://www.oecd-ilibrary.org/environment/test-no-308-aerobic-and-anaerobic-transformation-in-aquatic-sediment-systems_9789264070523-en). For feed additives used in mariculture, it may be more appropriate to do this study under saltwater conditions.
3.2.3. Photodegradation and hydrolysis
Investigation of photolysis is optional as it is expected that there will be little direct exposure of the feed additive to light in the manure or soil matrix and that therefore photodecomposition does not play a significant role in the overall degradation of feed additives here.
Information on hydrolysis might only be relevant when this process will dominate the degradation of the feed additive in the aquatic environment.
3.3. Phase II A
In Phase IIA, the PECA recalculated as described below is compared with a PNECI based on minimum data requirements for feed additives. The PNECI derivation is largely based on short‐term toxicity tests.
3.3.1. Phase II A PECsoil calculation
In Phase IIA, the PECA is calculated based on the methodology described in Section 2 taking the following into account:
The measured concentration of active substance/metabolites of concern in manure following administration of the additive to livestock animals at the proposed dose level. This calculation should include consideration of dosage rates and amount of excreta produced. Metabolites representing less than 10% of the administered dose can be subtracted from the total dose administered. In addition, the biological activity of metabolites compared to the parent compound should be considered. This procedure will result in the calculation of the fraction of the administered dose still considered to be active.
The adsorption/desorption of the active substance/metabolites of concern onto soil is determined by studies in soil.
Degradation in soil: In accordance to the EFSA guidance (EFSA, 2014), it is recommended to use the geometric mean of the degradation rates as inputs in the exposure models. In case there are indications the degradation rate depends on soil properties such as clay or pH, the https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/gw/NewDocs/focusGWReportOct2014.pdf guidance (FOCUS, 2014) should be followed to determine the appropriate PECs. If a high persistence in soil is anticipated (time to degradation of 50% of original concentration of the compound (DT50 > 60 days at 12°C)), the potential for accumulation should be considered. If data at 12°C are not available, data obtained at 20°C could be extrapolated using the Arrhenius equation (activation energy: 65.4 kJ/mol according to the EFSA guidance for use in FOCUS (EFSA, 2008b)). Consequently, a factor of 2.12 was used to calculate the DT50 at 12°C (DT50 at 12°C = DT50 at 20˚C × 2.12). The single first‐order kinetics, where possible, is the preferred mode for deriving a proper DT50. Criteria for deriving a proper DT50 are described in FOCUS guidance on kinetics (FOCUS, 2006)
Ploughing depth: In some countries, manures are mainly spread on and mixed into arable land used for crop production, e.g. Belgium, Denmark, Finland, France, Germany, Italy and Spain. In other countries, e.g. Greece, Ireland and the UK, it is common practice to distribute manure directly onto grassland (Burton and Turner, 2003). These differences prevent a general refinement of the 5 cm mixing depth used in Phase I (EMA, 2016). Therefore, concentrations in soil should be calculated for application in grassland (PECsoil,grassland; depth of 5 cm) but possible dilution of the feed additive due to ploughing (PECsoil, arable land; 20 cm soil depth) will be taken also into account.
3.3.1.1. Recalculation based on metabolism
When metabolism data are considered, the PECsoil A is calculated based on the methodology described in Phase I and recalculated as shown:
where:
| Symbol | Parameter | Unit |
|---|---|---|
| PECsoil A | Refined concentration of the additive (parent compound) in dry soil | mg/kg |
| PECsoil initial | Concentration of the additive (parent compound) in dry soil in Phase I | mg/kg |
| Faa | Fraction of the dose considered to be active (% of the parent active substance that is excreted) | – |
[value between 0 and 1].
When the application covers several target species/categories, it is recognised that it may be unrealistic to expect studies in all potential target species for which application is made, especially when the application is for all animal species. Therefore, interspecies extrapolation of data can be applied. The applicant is referred to the EFSA FEEDAP Panel (2017) http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.5022/epdf (Section 2.1.1.1) to select the most representative species to be investigated.
3.3.1.2. Recalculation based on degradation in soil
If the feed additive is not expected to degrade within a year (i.e. DT50 > 60 days at 12°C), the potential for residues to accumulate in soil should be considered. In those cases, the PECsoil plateau at steady state should be calculated at the start of Phase IIA as follows:
where:
| Symbol | Parameter | Unit |
|---|---|---|
| Input | ||
| DT50 | Degradation rate of additive (parent compound) in soil at 12°C | day |
| PECsoil initial | Concentration of the additive (parent compound) in dry soil in Phase I | mg/kg |
| Intermediate results | ||
| Fd | Fraction of additive (parent compound) degraded in 1 year | – |
| Output | ||
| PECsoil 1 year | Concentration of the additive (parent compound) 1 year after spreading in dry soil | mg/kg |
| PECsoil A plateau | PECsoil A at plateau concentration in dry soil | mg/kg |
The PEC in soil can be refined based on either information related to the metabolism of the substance in the target animals or degradation in manure or soil. In every case, kinetic results such as the degradation rates and degradation half‐lives should correspond to an environmentally relevant temperature, i.e. by default 12°C (ECHA, 2017c: Guidance on Information Requirements and Chemical Safety Assessment Chapter R.7b: Endpoint specific guidance, Section 7.9.4.1).
3.3.1.3. Recalculation based on degradation in soil under multiple applications
Refinement of PECsoil based on soil degradation data is possible when it is realistic to assume that manure is spread in more than one spreading event. In that case, the concentration calculated after the last spreading event should be taken.
In the case of arable land, manure/slurry is usually applied to fulfil the permissible limit during a single, annual application event. This partly reflects the fact that the presence of a crop will prevent applications of manure/slurry throughout much of the year.
In the case of grassland, it is more typical to make a number of applications of manure/slurry throughout the year. It is up to the applicant to provide information to support the number of spreading events which have been taken to occur on grassland.
As the storage capacity shows a large variation among the different EU Member States, it is recommended to set the storage capacity/time equal to the production period of the target animal up to 3 months, unless the number of cycles is more than four per year. In this case, the storage time is set equal to the period of the cycle. Similar default values on storage time (days) are indicated in the http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004386.pdf (EMA, 2016).
The following formula can be used to calculate the PECsoil after the last spreading event:
Where PECsoil 1 event is given by
where:
| Symbol | Parameter | Unit |
| Input | ||
| PECsoil 1‐event | Concentration of the additive (parent compound) in dry weight soil immediately after spreading | mg/kg |
| PECsoil initial | Concentration of the additive (parent compound) in dry soil in Phase I | |
| Nspreading | Number of spreading events | |
| Tinterval spreading | Time between spreading events | day |
| DT50 | Degradation rate of additive (parent compound) in soil | day |
| K | Rate constant | |
| Intermediate results | ||
| Frs | Fraction remaining in soil after time Tinterval spreading | |
| Output | ||
| PECsoil A | Refined Concentration of the additive (parent compound) in dry weight soil after last spreading event | mg/kg |
3.3.2. Phase II A PECgw calculation
Based on the experimentally determined Koc value, the concentration in groundwater (expressed as porewater) is recalculated using the same methodology as used in Phase I (see Section 2.6.2).
In accordance to the EFSA guidance (EFSA PPR Panel, 2014a), it is recommended to use the geometric mean of the Koc values as inputs in the exposure models. In case there are indications the adsorption depends on soil properties such as clay or pH, the https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/gw/NewDocs/focusGWReportOct2014.pdf guidance (FOCUS, 2014) should be followed to determine the appropriate PECs;
If the feed additive is not expected to degrade within a year (i.e. DT50 > 60 days at 12˚C), the potential for residues to accumulate in soil should be considered by using a PECsoil plateau. This can be calculated by dividing the PECsoil ww by the fraction of additive (parent compound) degraded in 1 year (Fd) as calculated in Section 3.3.1.2.
where:
| Symbol | Parameter | Default Value* | Unit |
|---|---|---|---|
| Input | |||
| RHOw soil | Bulk density of (wet) soil | 1,700 | kg/m3 |
| Intermediate results | |||
| Ksoil‐water | Partition coefficient solids and water in soil (v/v) | See Section 3.3.1.2 | m3/m3 |
| Fd | Fraction of additive (parent compound) degraded in 1 year | See Section 3.3.1.2 | |
| Output | |||
| PECpw plateau | Concentration of the additive (parent compound) in pore water | mg/L | |
3.3.3. Phase II A PEC surface water calculation (PECsw)
As a first estimate of the concentration in surface water resulting from run‐off or drainage, it is assumed that one part run‐off/drainage water will be diluted by two parts receiving water (Montforts, 1997, Montforts, 1999). The concentration in run‐off/drainage water is assumed to be equal to the concentration in pore water as calculated in the previous Section 3.3.2.
where:
| Symbol | Parameter | Unit |
|---|---|---|
| Input | ||
| PECpw A | Concentration of the additive (parent compound) in pore water | mg/L |
| Output | ||
| PECsw A | Concentration of the additive (parent compound) in surface water | mg/L |
If the feed additive is not expected to degrade within a year (i.e. DT50 > 60 days at 12°C), the potential for residues to accumulate in soil should be considered. In that case, the PECpw plateau should be used as calculated in Section 3.3.2.
3.3.4. Phase II A – PEC sediment calculation (PECsed, fresh water)
In Phase IIA, the PECsed A is calculated from PECsw A using the equilibrium partitioning (EqP) concept (Ref) as follows:
where:
| Symbol | Parameter | Default Valuea | Unit |
|---|---|---|---|
| Input | |||
| Ksusp–water | Suspended matterb–water partition coefficient | m3/m3 | |
| RHOsusp | Bulk density of (wet) suspended matterc | 1,150 | kg/m3 |
| RHOsolid | Bulk density of solids | 2,500 | kg/m3 |
| PECsw A | Predicted environmental concentration for surface water | mg/L | |
| CONVsusp | Conversion factor for suspended matter concentrations: wet weight to dry weight | kgww/kgdw | |
| Fwatersusp | Volume fraction of water in suspended matter | 0.9 | m3/m3 |
| 1,000 | Conversion for litre to m3 | L/m3 | |
| Fsolidsusp | Volume Fraction of solids in suspended matter | 0.1 | m3/m3 of water–solid slurry |
| Kpsusp | Partition coefficient solids and water in suspended matter (v/w) | L/kg | |
| Koc | Organic carbon partition coefficient | L/kg1 | |
| Focsusp | Weight fraction organic carbon in suspended solid | 0.1 | kg/kg |
| Output | |||
| PECsed A | Predicted environmental concentration in sediment (fresh water) dry weight | mg/kgd | |
The use of the indicated default values in the equations is recommended. Reasons for any deviations from these values should be given by the applicant.
The characteristics of suspended matter are used in EqP calculations for sediment rather than the characteristics of bulk‐sediment to reflect the concentration in the upper layer of the sediment, which is considered the major part of exposure for sediment dwelling organisms rather than via the deeper sediment layers.
The concentration in freshly deposited sediment is taken as the PEC for sediment. Therefore, the properties of suspended matter are used.
If the PNECsed has to be expressed on a wet weight basis, the expression CONVsusp is omitted from the first equation.
If the feed additive is not expected to degrade within a year (i.e. DT50 > 60 days at 12˚C), the potential for residues to accumulate in sediment should be considered. In that case the PECfw–sed plateau should be used as calculated above.
3.3.5. Phase II A – PEC sediment calculation for marine and fresh water aquaculture
There are no advanced models accepted at the EU level which can be suggested in this guidance for the refinement of the exposure for marine and freshwater aquaculture. In Phase I, it is assumed that there is no retention in the system. In Phase II, for freshwater aquaculture, this could be considered as a further PEC refinement. An applicant could also present further assessment, using other modelling tools, more studies or relevant arguments provided that these models, studies and/or arguments are scientifically underpinned.
3.3.6. PNEC derivation based on minimum data requirements
The initial PNEC (PNECI) derivation is largely based on short‐term toxicity tests. If for the same test species, toxicity data of different quality are available as influenced for the experimental design of the study, those that are in line with OECD criteria for valid studies will be selected. If for the same species, more than one valid and comparable (same endpoint and test duration) toxicity value is available, the geometric mean is used.
3.3.6.1. Terrestrial compartment
One nitrogen transformation test on soil microorganisms (28 days), one acute toxicity test on earthworms and one growth test in six different terrestrial plant species (at least two monocotyledonous and two dicotyledonous species) are required.
Tests required should be conducted according to OECD Guidelines http://titania.sourceoecd.org/vl=1315079/cl=54/nw=1/rpsv/ij/oecdjournals/1607310x/v1n2/s17/p1 (Soil Microorganisms, Nitrogen Transformation Test (28 days)), http://titania.sourceoecd.org/vl=1315079/cl=54/nw=1/rpsv/ij/oecdjournals/1607310x/v1n2/s8/p1 (Earthworm, Acute Toxicity Test) and http://titania.sourceoecd.org/vl=1315079/cl=54/nw=1/rpsv/ij/oecdjournals/1607310x/v1n2/s17/p1 (Terrestrial Plants, Seedling Emergence and Seedling Growth Test).
The Phase IIA PNECI;soil for soil organisms should be derived as described in Table 5, by selecting the lowest value:
Table 5.
Ecotoxicity studies required in Phase IIA to derive PNECI;soil
| Study | Toxicity endpoint | AF | Remark |
|---|---|---|---|
| Nitrogen Transformation (28 days), OECD 216. | ≤ 25% of control | 1 | Exposure 1X and 10X PECmax |
| Terrestrial plants (14–21 days), OECD 208. | EC50 | 100 | The most sensitive endpoint (emergence, biomass or height of sprout) of all plant species tested |
| Earthworm acute (14 days), OECD 207. | LC50 | 1,000 | – |
AF: assessment factor; EC50: concentration of the additive causing effect in 50% of the population the most sensitive OECD endpoint; LC50: concentration of the additive that kills 50% of the population.
When a critical toxicity value (e.g. LC50) concerns a ‘larger than’ value (i.e. LC50 > 5,000 mg/kg), this value is used as a precautionary approach in the risk quotient.
When a sufficient number of appropriate chronic toxicity values (EC10 or NOEC values from long‐term tests) for rooted plants (i.e. six plant species) and soil invertebrates are available, the Phase IIA PNECI (which is assumed to be sufficiently conservative) may be superseded by a Phase IIC PNECR (see Section 3.5.1).
3.3.6.2. Freshwater compartment (including sediment)
For feed additives to be used in terrestrial livestock animals or freshwater aquaculture, as a minimum Phase IIA data set, one L(E)C50 value each for a freshwater alga, a daphnid and a fish are required. For the assessment of the Phase IIA PNECI for pelagic freshwater organisms, the OECD Guidelines http://titania.sourceoecd.org/vl=1315079/cl=54/nw=1/rpsv/ij/oecdjournals/1607310x/v1n2/s2/p1 (Freshwater Alga and Cyanobacteria, Growth Inhibition Test), http://titania.sourceoecd.org/vl=1315079/cl=54/nw=1/rpsv/ij/oecdjournals/1607310x/v1n2/s3/p1 (Daphnia Acute Immobilization test) and http://titania.sourceoecd.org/vl=1315079/cl=54/nw=1/rpsv/ij/oecdjournals/1607310x/v1n2/s4/p1 (Fish Acute Toxicity test) should be followed.
The Phase IIA PNECIsw for pelagic water organisms should be derived as described in Table 6 by selecting the lowest value.
Table 6.
: Ecotoxicity studies required in Phase IIA to derive PNECI;sw
| Study | Toxicity endpoint | AF | Remark |
|---|---|---|---|
| Algal growth inhibitiona, OECD 201. | 72‐h ErC50 b | 1,000 | EyC50 c may be used if ErC50 not reported |
| Daphnia immobilization, OECD 202. | 48‐h EC50 | 1,000 | – |
| Fish acute toxicity, OECD 203. | 96‐h LC50 | 1,000 | – |
In case problems arise with coloured additives, Lemna ( https://www.oecd-ilibrary.org/environment/test-no-221-lemna-sp-growth-inhabition-test_9789264016194-en) can be used.
ErC50: the concentration of test substance which results in a 50 percent reduction in growth rate.
EyC50: the concentration of the test substance with results in a 50% reduction of yield.
When an older test for Algal growth inhibition was performed in 96‐h period, this endpoint may be considered adequate. The assessment of acute toxicity tests considers the following statement of the OECD guidance document on the aquatic toxicity testing of difficult substances and mixtures (OECD, 2002): ‘It is important to note that an absence of acute toxic effects at the saturation concentration cannot be used as the basis for predicting no chronic toxicity at saturation or at lower concentrations’.
A long‐term test has to be carried out for substances showing no toxicity in short‐term tests if the log Kow > 3 (or a bioconcentration factor (BCF) > 100) and if the PECA sw is > 1/100th of the water solubility. The long‐term toxicity test should normally include tests on an invertebrate and algae species (preferred species Daphnia; http://www.oecd-ilibrary.org/environment/test-no-211-daphnia-magna-reproduction-test_9789264185203-en). To avoid unnecessary vertebrate testing, it is sufficient to perform a chronic fish test only if fish is the most sensitive organism group of the acute assessment tier. For more details, please see Section 3.5.2.2.
According to REACH (ECHA, 2008b), a log Koc or log Kow ≥ 3 for an organic chemical is used as a trigger value for sediment effect assessment. If this trigger is met, in Phase IIA the PNECI of an organic feed additive for freshwater sediment‐dwelling organisms will be derived on basis of the Phase IIA PNECI for pelagic water organisms and the EqP concept. The concept of EqP is based on the work of Di Toro et al. (1991).
According to the EqP concept, the PNEC for sediment organisms can be estimated as follows:
where:
| Symbol | Parameter | Default Valuea | Unit |
|---|---|---|---|
| Input | |||
| Ksusp‐water | Suspended matterb‐water partition coefficient | m3/m3 | |
| RHOsusp | Bulk density of (wet) suspended matterc | 1,150 | kg/m3 |
| RHOsolid | Bulk density of solids | 2,500 | kg/m3 |
| PNECsw | Predicted no effect concentration for aquatic organisms | μg/L | |
| CONVsusp | Conversion factor for suspended matter concentrations: wwt to dwt | kgww/kgdw | |
| Fwatersusp | Volume fraction of water in suspended matter | 0.9 | m3/m3 |
| 1000 | Conversion for litre to m3 | l/m3 | |
| Fsolidsusp | Volume Fraction of solids in suspended matter | 0.1 | m3/m3 |
| Kpsusp | Partition coefficient solids and water in suspended matter (v/w) | l/kg | |
| Koc | Organic carbon partition coefficientd | l/kg | |
| Focsusp | Weight fraction organic carbon in suspended solids | 0.1 | kg/kg |
| Output | |||
| PNECsed;EqP | Predicted no effect concentration for sediment dwelling organisms | μg/kgdw e | |
The use of the indicated default values in the equations is recommended. Reasons for any deviations from these values should be given by the applicant.
The characteristics of suspended matter are used in EqP calculations for sediment rather than the characteristics of bulk‐sediment to reflect the concentration in the upper layer of the sediment which is considered the major part of exposure for sediment dwelling organisms rather than via the deeper sediment layers.
The concentration in freshly deposited sediment is taken as the PEC for sediment. Therefore, the properties of suspended matter are used.
For a correct comparison, the Koc value should be the same as used for the PEC calculation
When expressing PNECsed on a wet weight basis, the expression CONVsusp is omitted from the first equation**.
EqP approach neglects sediment ingestion as a relevant uptake pathway, as it only represents transfer occurring through passive partitioning. According to REACH (European Commission, 2003; ECHA, 2008a), for chemicals with a log Kow > 5 an AF of 10 may be required to account for risks due to sediment ingestion.
The Phase IIA PNECI;sed;EqP for sediment‐dwelling organisms should be derived following Table 7.
Table 7.
Procedure to derive Phase IIA PNECsed
| Study | Toxicity endpoint | AF | Remark |
|---|---|---|---|
| Initial PNEC (PNECI) for pelagic water organisms and EqP approach | PNECI;sed;EqP |
1 10 |
If the log Kow ≤ 5 If the log Kow > 5 |
EqP: equilibrium partitioning
When experimental chronic toxicity values (EC10 or NOEC values from long‐term tests that assess sublethal endpoints) for sediment‐dwelling organisms are available, the Phase IIA PNECI;sed;EqP (which is assumed to be sufficiently conservative) may be superseded by a Phase IIC PNECR;sed (see Section 3.5.3).
3.3.6.3. Marine compartment
For feed additives used in mariculture, three marine sediment species have to be tested. At present, no internationally accepted, i.e. ISO or OECD, guidelines are available, except the 10‐day ISO 16712 test for Corophium volutator (ISO, 2005). Several relevant guidelines are available from the American Society for Testing of Materials ( http://webstore.ansi.org/SdoInfo.aspx?sdoid=41%26Acro=ASTM%26DpName=ASTM%20International%26source=googe%26keyword=astm%26gclid=CIWamLjO8ZMCFSgtagodXjJ9VQ) for toxicity in salt water systems which can be considered appropriate.
In the Phase IIA effect assessment, the PNECI;sed can be derived from sediment‐spiked 10‐day toxicity tests with benthic organisms for which test protocols are available by applying an appropriate AF. In Phase IIC (PNECR;sed derivation), chronic tests with these species will be considered.
An overview of the available sediment‐spiked 10‐day toxicity tests with marine/estuarine sediment‐dwelling invertebrates is presented in Table 8. Note that nearly all test species mentioned in Table 8 concern crustaceans. In addition, a standard ASTM Guide for Conducting Renewal Microplate‐Based Life‐Cycle Toxicity Tests with a Marine Meiobenthic Copepod ( http://webstore.ansi.org/RecordDetail.aspx?sku=ASTM+E2317-04) is available. This Copepod test, however, concerns a pore water test and not a sediment‐spiked test.
Table 8.
Overview of marine/estuarine benthic invertebrate test species for which protocols are available to conduct a 10‐day sediment‐spiked toxicity tests
| Test species | Semi‐chronic test guideline | Remark |
|---|---|---|
|
Leptocheirus plumulosus (crustacean) |
10‐day test; ASTM E1706 (ASTM, 2010a) | Occurs in estuarine habitats |
|
Eohaustorius estuarius (crustacean) |
10‐day test; US‐EPA 1996 and ASTM E1367 (ASTM, 2010b) | Occurs in estuarine habitats |
|
Ampelisca abdita (crustacean) |
10‐day test; US EPA 1996 and ASTM E1367 (ASTM, 2010b) | Occurs in marine habitats |
|
Rhepoxynius abronius (crustacean) |
10‐day test; US EPA, 1996 and ASTM E1367 (ASTM, 2010b) | Occurs in marine habitats |
| Corophium volutator (crustacean) | 10‐day test; ISO 16712 (ISO, 2005), OSPAR 2006 Part A, ASTM E1367‐03 (2014) | Occurs in estuarine and marine habitats |
|
Neanthes arenaceodentata (polychaete worm) |
10‐day test; ASTM E1611 (ASTM, 2007) | Occurs in estuarine and marine habitats |
ASTM: American Society for Testing of Materials; US EPA: United States Environmental Protection Agency.
Based on available pesticides toxicity data (EFSA PPR Panel, 2015), there is no reason to assume that fresh water and marine/estuarine benthic invertebrates differ in their species sensitivity distribution for feed additives, although some taxonomic groups predominantly occur in freshwater habitats (e.g. Insecta) or marine/estuarine habitats (e.g. Polychaeta and Echinodermata). Assuming that species sensitivity distributions of benthic species do not differ substantially between freshwater and marine/estuarine habitats, also sediment‐spiked 10‐day protocol toxicity tests with freshwater invertebrates might be used if the AF for extrapolation is high enough. This approach is also adopted by the EFSA scientific opinion on the effect assessment for pesticides on sediment organisms (EFSA PPR Panel, 2015). An overview of the available sediment‐spiked 10‐day toxicity tests with freshwater sediment‐dwelling invertebrates is presented in Table 9.
Table 9.
Overview of freshwater benthic test invertebrates for which protocols are available to conduct a 10‐day sediment‐spiked toxicity tests
| Test species | Semi‐chronic test guideline | Remarks |
|---|---|---|
|
Chironomus spp. (insect) |
10‐day test; ASTM E1706 (ASTM, 2010a) | Insects are rarely found in marine/estuarine environments |
|
Hexagonia spp. (insect) |
10‐day test; ASTM E1706 (ASTM, 2010a) | Insects are rarely found in marine/estuarine environments |
|
Hyalella azteca (crustacean) |
10‐day test; ASTM E1706 (ASTM, 2010a) | Found in freshwater and estuarine environments |
|
Diporeia spp. (crustacean) |
10‐day test; ASTM E1706 (ASTM, 2010a) | – |
|
Tubifex tubifex (oligochaete worm) |
10‐day test; ASTM E1706 (ASTM, 2010a) | – |
ASTM: American Society for Testing of Materials.
The Phase IIA PNECI;sed for sediment invertebrates in the marine environment should be derived following Table 10 by selecting the lowest toxicity value for the three benthic species.
Table 10.
Ecotoxicity studies required in Phase IIA to derive PNECI;sed for invertebrates in marine environment
| Study | Toxicity endpoint | AF | Remark |
|---|---|---|---|
| Corophium volutator (ISO 16712) | 10‐day LC50 | 1,000 | Recommended marine species |
| Second marine/estuarine benthic species (Table 8)* | 10‐day LC50 | 1,000 | At least another taxonomic group than Crustacea is required in the data set |
| Third benthic marine/estuarine or freshwater species (Tables 8 and 9)* | 10‐day LC50 | 1,000 | At least another taxonomic group than Crustacea is required in the data set |
If in the near future ISO and/or OECD guidelines for short‐term toxicity tests with marine/estuarine benthic species become available, these protocol tests are preferred.
In order to allow a correct comparison between the PECsed A (the PECsed as assessed in Section 3.3.4 for sea cages) and initial PNECI;sed, the toxicity tests underlying the PNEC need to be normalised to the OC content of suspended solids used to derive the PEC sediment (i.e. 10% on dry weight basis) using the following equation:
Alternatively, the PEC and PNEC estimates can be expressed in terms of μg/g OC in dry sediment to allow a proper linking of exposure to effects.
When the adsorption is pH dependent, it might also be appropriate to investigate whether the Koc value related to the pH of the sediment used in the toxicity test will significantly deviate from the Koc value used for the PEC calculation. If so, then the PNEC could be further normalised using the following equation.
Note that when a sufficient number of chronic toxicity values (EC10 or NOEC values from long‐term tests that assess sublethal endpoints) for sediment‐dwelling invertebrates are available the Phase IIA PNECI;sed (which is assumed to be sufficiently conservative) may be superseded by a Phase IIC PNECR;sed (see Section 3.5.3).
3.3.7. Phase II A Risk assessment for secondary poisoning
If a substance has a log Kow ≥ 3, the risk for secondary poisoning (food web transfer) has to be assessed. For feed additives, it might be appropriate to first consider if the safety assessment for the target species may also cover the assessment for secondary poisoning in non‐target species or whether a separate assessment is needed. In this case, the methodology outlined in the http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004386.pdf (EMA, 2016) and REACH regulation (ECHA, 2008a,b, 2016, 2017d) and subsequent amendments should be followed.
3.3.8. Phase II A Risk characterisation
For the different compartments, the calculated PECA's are compared with the initial PNEC (PNECI) derived; if the ratio of the PECA to the PNECI is lower than 1, no further assessment is required. Otherwise, proceed with Phase IIB to refine the PECs when possible, or proceed to Phase IIC to refine the PNEC (PNECR) and recalculate the risk quotient (RQ) values. If PECA ground water is > 0.1 μg/L, proceed to Phase IIB.8
3.3.9. Assessment of persistent, bioaccumulative and toxic substances
Feed additives that on the basis of the screening assessment in Phase I are considered to be potential PBT and/or vPvB substances need to be further assessed in Phase II with the PBT and vPvB criteria according to Section 1 of Annex XIII of the REACH Regulation.6 These criteria together with the methodology in the REACH guidance on PBT/vPvB‐assessment ( https://echa.europa.eu/documents/10162/13632/information_requirements_r11_en.pdf and Chapters https://echa.europa.eu/documents/10162/13632/information_requirements_r7a_en.pdf/e4a2a18f-a2bd-4a04-ac6d-0ea425b2567f, https://echa.europa.eu/documents/10162/13632/information_requirements_r7b_en.pdf/1a551efc-bd6a-4d1f-b719-16e0d3a01919, and https://echa.europa.eu/documents/10162/13632/information_requirements_r7c_en.pdf/e2e23a98-adb2-4573-b450-cc0dfa7988e5 on endpoints specific guidance) (ECHA, 2017a,b,c,d,e) and the http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/09/WC500193826.pdf (EMA/CVMP/ERA/52740/2012), should be considered.
Following the strategy outlined in these guidance documents, a definitive assessment of P/vP, including assessment of any newly generated information, should be conducted first. Definitive assessment of P/vP should normally be based on degradation half‐life data collected under adequate conditions for the relevant compartment(s) of exposure. For feed additives used in terrestrial and aquatic animals, the most relevant compartments are soil and water/sediment systems, respectively.
If the substance is considered to fulfil the P and/or vP criterion, the PBT/vPvB assessment is continued by evaluation of the B/vB criterion including assessment of any newly generated additional information. Definitive assessment of B/vB should normally be based on measured data on bioconcentration in aquatic species. If such data is not yet available, it is recommended to conduct a bioaccumulation study in fish according to http://www.oecd-ilibrary.org/environment/test-no-305-bioaccumulation-in-fish-aqueous-and-dietary-exposure_9789264185296-en.
If the substance is not identified as vPvB but considered to fulfil the P and B criteria, the PBT assessment is continued by evaluation of the T criterion based the standard aquatic toxicity studies described in Section 3.3.6.2. Definitive assessment of T should be based on evaluation of the data for classification of the substance for human health hazards and/or on NOEC/EC10 values from long‐term toxicity tests with aquatic organisms, including reproductive cycle tests when appropriate as indicated in Section 3.5.2.2.
3.4. Phase II B to derive refined PEC estimates
Based on data not considered in Phase IIA, a more refined PEC can be calculated for each environmental compartment of concern. In ascertaining the refined PEC, account should be taken of:
The potential degradation of the excreted active substance/metabolites of concern during normal manure processing practice and storage prior to its application to land;
Other factors such as hydrolysis, photolysis, evaporation, etc.
Use of more sophisticated models. The applicant is encouraged to check the Joint Research Centre (European Soil Data Centre) website for FOCUS models.9
3.4.1. PECB refinement for soil
3.4.1.1. Refinement based on degradation in manure
As a part of the Phase II assessment, data on degradation of the additive in manure may be submitted. Studies on degradation in manure should be performed according to the Guideline on determining the fate of veterinary medicinal products in manure (EMA, 2011).
As the storage capacity shows a large variation among the different EU Member States, it is recommended to set the storage capacity/time equal to the production period of the target animal up to three months, unless the number of cycles is more than four per year. In this case, the storage time is set equal to the period of the cycle. Indicative default values of storage time (days) were also published by EMA (2016).
If degradation is to be considered in Phase II, the PECmanure should be calculated for a storage time similar to one animal production cycle and, by doing so, the amount of manure is also set equal to the amount produced in that storage period, which fills the annual nitrogen quota of 170 kg N/ha (EMA, 2016). It is also necessary to consider that the animals could be given a feed additive at a particular period. If animals are given a feed additive at the beginning of the storage period, there will be more time for the active ingredient to degrade than if they were given the additive at the end of the storage period. For this reason, the time for degradation of the active substance is taken to be half the storage time of the manure (EMA, 2016).
To calculate the PECsoil B by taking into account the degradation during storage, the following equations should be used:
where:
| Symbol | Parameter | Default Valuea | Unit |
|---|---|---|---|
| Input | |||
| Cadd | Concentration of the additive in feed | mg/kg complete feed | |
| FItotal | Total feed intake (DM) per year | kg feed | |
| Nexcreted | Total N excretion per year | kg N | |
| RHOd soil | Bulk density of (dry) soil | 1,500 | kg/m3 |
| DEPTHfield | Mixing depth with soil | 0.05 | m |
| CONVarea field | Conversion factor for the area of the agricultural field | 10,000 | m2/ha |
| Q | Annual nitrogen load standard | 170 | kg N/ha |
| DT50 | Degradation rate of the additive in manure | day | |
| K | Rate constant | ||
| Tst | Length of time manure is stored | day | |
| Intermediate results | |||
| PECmanure | Concentration of the additive (parent compound) in manure expressed per amount nitrogen | mg/kg N | |
| Output | |||
| PECsoil B | Highest concentration of the additive (parent compound) in soil dry weight | mg/kg | |
The use of the indicated default values in the equations is recommended. Reasons for any deviations from these values should be given by the applicant.
3.4.2. PEC refinement for groundwater, surface water and sediment and for additives used in livestock animals
The equations used in Phase IIA provide worst‐case estimates of the exposure concentrations of the additive in pore water (see Sections 2.6.2 and 3.3.2) and surface waters (see Sections 3.3.3 and 3.3.4). If Risk Quotient (RQ) values for surface water organisms are ≥ 1 and/or the PECpw is > 0.1 μg/L, more advanced models could be used to predict more realistic concentrations of the additive in deeper groundwater and surface waters.
More sophisticated models have been developed by the http://esdac.jrc.ec.europa.eu/projects/focus-dg-sante (Forum for the Coordination of Pesticide Fate Models and Their Use) group. Justification for using these models is given in the http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2007.529/epdf on the development of an approach for the environmental risk assessment of additives, products and substances used in animal feed.
The applicant could also present further assessment using other modelling tools, more studies or relevant arguments as to why exceeding the trigger value for groundwater or the RQ for aquatic organisms should not be considered a risk, provided that these models, studies and/or arguments are scientifically underpinned.
3.4.2.1. Groundwater
Groundwater calculations developed by FOCUS involve the simulation of the leaching behaviour of agrochemicals using a set of four models (PEARL, PELMO, PRZM and MACRO) in a series of up to nine geographic settings with various combinations of crop, soil and climate. Groundwater concentrations are estimated by determining the annual average concentrations in shallow groundwater (1 m soil depth) for a period of 20 consecutive years, rank ordering the annual average values and then selecting the 80th percentile value (Metcalfe et al., 2006).
When using the FOCUS models, a simple first step of this assessment can be based on a realistic worst‐case FOCUS scenario. For reasons given in the http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2007.529/epdf, it seems most appropriate to base such a leaching assessment on the FOCUS Okehampton scenario using PEARL.
In order to simplify the first step in the refined exposure assessment, calculations were performed with FOCUS_PEARL v3.0 applying a dose of 1 kg/ha on 3 October every year over a 20‐year period. The dose was incorporated into the top 20 cm of soil. The crop was winter cereal. All substance properties except organic‐matter/water distribution coefficient (KOM) and DT50 were equal to the model substance D as defined by FOCUS. Runs were carried out with 90 KOM–DT50 combinations covering FOCUS leaching concentrations ranging from 0.001 to about 100 μg/L. The results were fitted to a metamodel to be able to estimate leaching concentrations without running a FOCUS scenario (EFSA, 2007). Based on this analysis, the following inequalities can be used for the first‐tier leaching assessments of feed additives (see Table 11).
Table 11.
Requirements for the KOM as a function of the FOCUS leaching concentration
| CFOCUS (μg L−1) | Requirement for the KOM |
|---|---|
| < 0.001–0.01 | KOM > −5.9 + 9.1 DT50 |
| 0.01 to < 0.1 | KOM > −5.9 + 6.5 DT50 |
| ≥ 0.1 to 1 | KOM > −5.9 + 3.8 DT50 |
| 1–10 | KOM > −5.9 + 1.2 DT50 |
KOM= Koc/1.7; DT50 : time to degrade half the concentration of the substance.
The inequalities explain the requirement of Koc and soil DT50 to define whether a substance is prone to leaching or not. The first two concentrations (CFOCUS) identify compounds that do not leach to shallow groundwater. The third and fourth ones identify a possible leaching compound. In this last case, FOCUS models are needed to address the issue.
Note that these relationships are based on a dose of 1 kg/ha. In the event that the actual dose is substantially lower or higher, then a less or more stringent relationship should be used in proportion to the dose (e.g. when the dose is < 0.1 kg/ha, the relationship KOM > ‐5.9 + 3.8 DT 50 can be used to ensure that the leaching concentrations are < 0.1 μg/L).
If it is not possible to exclude the likelihood that groundwater concentration is > 0.1 μg/L based on the metamodel, then it is necessary to run the PEARL model using the scenarios recommended in the http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2007.529/epdf. Table 12 indicates which scenarios have to be run for the specific target animals, taking into account the indicated considerations.
Table 12.
Proposed FOCUS GW scenarios for PECB;gw calculation of feed additives
| Target animal | Bovine | Ovine | Swine | Avian |
|---|---|---|---|---|
| FOCUS GW | N: Jokioinen | C: Okehampton | N: Jokioinen | N: Jokioinen |
| S: Sevilla, Piacenza | S: Sevilla, Thiva | S: Piacenza | S: Piacenza |
N: Northern/Scandinavian; C: Central; S: Southern/Mediterranean.
Settings of the FOCUS model for groundwater
As explained above, manure application to arable land is most typically carried out in the early autumn. In order to standardise, the exposure assessments timing of application to soil is assumed to coincide with drilling of winter cereals (in the absence of pure grassland scenario) as these crops are typically grown throughout Europe and represent a significant input of manures on a total mass basis across Europe (EMA, 2016). The soil DT50 values should be the geometric mean values from the experimental data. In Section 3.3.1, guidance is given to select the most appropriate soil DT50 and Koc values.
It is assumed that manure will be applied at a rate of 170 kg N/ha in one spreading event. As the input in FOCUS is expressed in kg/ha, the PECsoil dw has to be converted to kg/ha before running the FOCUS model (EMA, 2016). Recommended input parameters on the application of FOCUS model is presented in the Appendix B.
3.4.2.2. Surface water
The surface water and sediment calculations developed by FOCUS include three progressively refined tiers of evaluation, ranging from initial spreadsheet‐based evaluations of potential aquatic concentrations to more detailed mechanistic calculations of drift, runoff, erosion and field drainage loaded into a series of small water bodies (EMA, 2016). Additionally, a final Step 4 allows a detailed site‐specific approach in case all previous Steps fail. The surface water and sediment calculations are performed using an overall calculation shell called SWASH which controls models that simulate runoff and erosion (PRZM), leaching to field drains (MACRO), spray drift (internal in SWASH) and finally aquatic fate in ditches, ponds and streams (TOXSWA). Those simulations provide detailed assessments of potential aquatic concentrations in a range of water body types in up to ten separate geographic and climatic settings.
Detailed explanations of the FOCUS models as well as the modelling scenarios, key assumptions, required modelling inputs and model outputs are provided in the respective FOCUS modelling reports (FOCUS, 2000, 2001) (EFSA, 2007). The FOCUS surface water and groundwater models have been placed on a website ( http://esdac.jrc.ec.europa.eu/projects/focus-dg-sante) where they can be freely downloaded.
Based on the EFSA, 2007 opinion, the runoff and drainage scenarios given in Table 13 were identified as potential ‘base‐set’ scenarios:
Table 13.
Proposed FOCUS SWASH scenarios for PECsw B and PECsed B; calculation of feed additives
| Target animal | Bovine | Ovine | Swine | Avian |
|---|---|---|---|---|
| FOCUS SW scenario (drainage) | D4 | D6 | D4, D3 | D5, D3 |
| FOCUS SW scenario (runoff) | R1, R3 | R4 | R1, R3 | R1, R3 |
This selection covers not only the areas identified by FOCUS but also several areas in the Member States that joined the EU after May 2005 and is supported by a study carried within ERAPharm Project (Schneider et al., 2007).
If, when using FOCUS the OC fraction of the sediment on which the PECsed B is based differs from that of the sediment used in toxicity tests, a normalisation of the PEC to a standard sediment is required (EFSA PPR Panel, 2015; see Section 9.3). Alternatively, the PECsed B and the PNECR;sed should be expressed in terms of μg/g OC in dry sediment to allow a proper linking of exposure to effects.
Settings of the FOCUS model for surface water
As proposed for groundwater, the application of manure to arable and grass land is considered to coincide with the drilling of cereals in autumn (in the absence of a pure grassland scenario) (EMA, 2016). The soil DT50 values should be the geometric mean values from the experimental data. In Section 3.3.1, guidance is given to select the most appropriate soil DT50 and Koc values. In order to select the most appropriate application date, the FOCUS PAT (Pesticide Application Time) tool, part of the software package MACRO and PRZM, should be used. As a realistic worst case, it is assumed that manure will be applied at a rate of 170 kg N/ha in one spreading event. Without information on the degradation in a water/sediment, the degradation rate is set to zero. When needed, the PEC surface water could be further refined based on a water/sediment simulation study according to http://www.oecd-ilibrary.org/environment/test-no-308-aerobic-and-anaerobic-transformation-in-aquatic-sediment-systems_9789264070523-en. As mentioned for groundwater as the input in FOCUS is expressed in kg/ha, the PECsoil dw has to be converted to kg/ha before running the FOCUS model (EMA, 2016). Recommended input parameters on the application of FOCUS model are presented in the Appendix C.
3.4.2.3. Interpretation of results from FOCUS
In FOCUS groundwater models, the 80th percentile annual average recharge concentrations leaving the top 1 m soil layer for a 20‐year period is presented.
The results for surface water are presented as the maximum predicted PECsw and PECsed at the time of occurrence of the peak. The annual exposure profiles are presented graphically and PECtwa concentrations for certain time windows can be derived.
For further guidance to investigate leaching to groundwater under field conditions, the reader is referred to the FOCUS groundwater guidance (2014), and more details on FOCUS Surface Water models can be found in FOCUS (2015).
3.4.3. Phase II B Risk characterisation
For the different compartments the refined PECB's are compared with the initial PNEC (PNECI) derived. If the ratio of the PECB to the PNECI is lower than 1, no further assessment is required. Otherwise, proceed with Phase II C to refine the PNECs when possible.
3.5. Phase II C to estimate refined PNEC (PNECR) values
For those additives where, following Phase IIA or Phase IIB assessment, an environmental risk cannot be excluded, further tests are needed to determine the chronic and more specific effects on appropriate microbial, plant and animal species. This additional information will allow the application of a lower AF.10
Suitable additional ecotoxicological tests are described in a number of publications, e.g. in http://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm guidelines. Careful choice of such tests is necessary to ensure that they are appropriate to the situation in which the additive and/or its metabolites may be released and dispersed in the environment. The refinement of the effect assessment for soil (PNECR;soil) may be based on studies on the chronic effects on terrestrial invertebrates, additional studies on soil microflora and a number of relevant plant species.10 The refinement of the effect assessment for water/sediment may be based on chronic toxicity tests on the most sensitive aquatic/benthic organisms identified in Phase IIA assessment. The refined PNEC (PNECR) derivation is largely based on chronic toxicity tests, including reproduction and/or developmental tests when suggested by previous indications. If for the same test species toxicity data of different quality are available (after normalisation in soil‐ and sediment‐spiked test, see Sections 3.3.6.1 or 3.5.2.1) as influenced by the experimental design of the study, those that are in line with OECD criteria for valid studies will be selected. If for the same species more than one valid and comparable (same test duration and endpoint) toxicity value is available, the geometric mean is used.
The refinement of the risk assessment for secondary poisoning may be based on a bioaccumulation study in fish according to OECD 305.
3.5.1. Toxicity tests and PNECR soil derivation: Terrestrial compartment
When for one or more of the taxonomic groups a risk has been identified, for these taxonomic groups, the PNEC can further be refined by the following chronic studies: the OECD Guidelines http://www.oecd-ilibrary.org/environment/test-no-216-soil-microorganisms-nitrogen-transformation-test_9789264070226-en (Soil Microorganisms, Nitrogen Transformation Test, 100 days), http://www.oecd-ilibrary.org/environment/test-no-208-terrestrial-plant-test-seedling-emergence-and-seedling-growth-test_9789264070066-en (Terrestrial Plants, Growth Test, Additional species) and soil invertebrates (Earthworm Reproduction Test ( http://www.oecd-ilibrary.org/environment/test-no-220-enchytraeid-reproduction-test_9789264070301-en/ http://www.oecd-ilibrary.org/environment/test-no-222-earthworm-reproduction-test-eisenia-fetida-eisenia-andrei_9789264070325-en), springtail Folsomia candida ( http://www.oecd-ilibrary.org/environment/test-no-232-collembolan-reproduction-test-in-soil_9789264076273-en) or the predatory mite Hypoaspis aculeifer ( http://www.oecd-ilibrary.org/environment/test-no-226-predatory-mite-hypoaspis-geolaelaps-aculeifer-reproduction-test-in-soil_9789264067455-en)).
Field‐collected soils used in ecotoxicological tests could differ in characteristics such as organic matter and clay content, soil pH and soil moisture content. The bioavailability of the test compound, and therefore the toxicity observed, could be influenced by those soil properties. This means that results from different test soils cannot be compared directly (van Gestel, 2012). If possible, data should be normalised using relationships that describe the bioavailability of chemicals in soils. If there is evidence that the bioavailability of the compound is related to the organic matrix, results are converted to a standard soil, which is defined as a soil with an organic matter content of 3.4% or an organic carbon content of 2.0 ± 0.5% (since this OC fraction is also considered in calculating the PECsoil A or PECsoil B). Using an OC harmonised (2% on dry weight basis) PNECR estimate allows a proper linking of exposure to effects. Alternatively, toxicity estimates can be expressed in terms of μg/g OC in dry soil. The PNEC derived from such studies should then be compared with a PEC expressed in terms of μg/g OC in dry soil.
For the derivation of the PNECR soil for terrestrial organisms, the same effect assessment is followed as performed for veterinary products (EMA, 2005), which means that separate assessment factors are applied to every taxonomic group. The lowest PNEC determines the PNECR soil for the terrestrial compartment.
3.5.1.1. Terrestrial plants
At Phase IIA, the effect assessment for plants is based on the application of an assessment factor of 100 to the lowest EC50 value of six species (see Section 3.3.6.1). If a risk is identified in this lower tier, at Phase IIC the EC10 values from the most sensitive end point from all tested species should be used by applying an assessment factor of 10.
3.5.1.2. Terrestrial invertebrates
At phase IIA, the effect assessment for earthworms can be based on an acute toxicity study. The PNECI is derived by applying an assessment factor of 1,000 to the LC50 value.
If based on the acute earthworm toxicity test a risk cannot be excluded, at Phase IIC the chronic toxicity on earthworms (OECD guideline http://www.oecd-ilibrary.org/environment/test-no-220-enchytraeid-reproduction-test_9789264070301-en/ http://www.oecd-ilibrary.org/environment/test-no-222-earthworm-reproduction-test-eisenia-fetida-eisenia-andrei_9789264070325-en) and on a second soil invertebrate needs to be investigated (either springtail Folsomia candida ( http://www.oecd-ilibrary.org/environment/test-no-232-collembolan-reproduction-test-in-soil_9789264076273-en) or the predatory mite Hypoaspis aculeifer ( http://www.oecd-ilibrary.org/environment/test-no-226-predatory-mite-hypoaspis-geolaelaps-aculeifer-reproduction-test-in-soil_9789264067455-en). Note that the OECD guideline 222 requires the substance to be mixed into the soil and that clean manure is added to promote the reproduction of the earthworms. The test is not designed to study exposure via manure. The PNECR soil is derived by applying an AF of 10 to the lowest EC10/NOEC value. If there is evidence that the lowest EC10/NOEC of the six terrestrial plants is at least one order of magnitude lower than the chronic EC10/NOEC for earthworms, then no additional chronic toxicity test for a second invertebrate is needed.
3.5.1.3. Microorganisms
The Soil Microorganisms, Nitrogen Transformation Test ( http://www.oecd-ilibrary.org/environment/test-no-216-soil-microorganisms-nitrogen-transformation-test_9789264070226-en) should be conducted at 1× and 10× the PEC. At Phase IIA, this study is conducted during a period of 28 days (see Section 3.3.6.1). If, on day 28, differences between treated and untreated soils are ≥ 25%, at Phase IIC measurements have to be continued to a maximum of 100 days. When the difference in the rates of nitrate formation between the maximum PEC and control is ≤ 25% at any sampling after day 28 (considering sampling intervals of 14 days), the product can be evaluated as having no long‐term influence on nitrogen transformation in soils.
3.5.1.4. PNECR derivation for soil organisms
The Phase IIC PNECR for soil organisms should be derived as indicated in Table 14, by selecting the lowest value
Table 14.
Procedure to derive Phase IIC PNECR for soil organisms
| Study | Toxicity endpoint | AF | Remark |
|---|---|---|---|
| Terrestrial plants | 14‐ to 21‐day EC10 (or NOEC) | 10 |
Most sensitive end point of all tested species Section 3.5.1.1 |
| Earthworm subacute/reproduction, OECD 220/222. | 56‐day EC10 (or NOEC) | 10 | Section 3.5.1.2 |
|
Folsomia candida (OECD 232) or Hypoaspis aculeifer (OECD 226) |
28‐day EC10 (or NOEC) 14‐day EC10 (or NOEC) |
10 | Section 3.5.1.2; not required if the EC10/NOEC of most sensitive plant is at least 10 times lower than that of the earthworm |
| Nitrogen Transformation (100 days) | ≤ 25% of control | 1 |
Exposure 1x and 10x PECmax Section 3.5.1.3 |
EC10: the concentration of test substance which results in a 10 percent reduction of the effect tested; NOEC: no‐observed‐effect‐concentration. It is usually the highest test concentration at which no toxic effects are observed.
If both the PECsoil B and refined PNECR;soil estimates described above still trigger risks a further refinement of the effect assessment may be considered by conducting chronic laboratory toxicity tests with additional species (e.g. to allow the species sensitivity distribution (SSD) approach), by conducting a semi‐field experiment and/or by advanced modelling approaches (e.g. EFSA PPR Panel, 2014a). If at least one of the taxonomic groups mentioned in Table 14 triggers a potential risk, and the most sensitive taxonomic group is at least an order of magnitude more sensitive, then an SSD approach focussing on this taxonomic group only is a logical step forward (see e.g. the EMA and EFSA PPR approach for terrestrial plants; EFSA PPR Panel, 2014b; EMA, 2017). If more taxonomical groups mentioned in Table 14 trigger potential risk, it may be appropriate to include several taxonomic groups in the SSD (see e.g. the REACH procedure in ECHA, 2008b, Chapter R 10).
For plant PNECR derivation on basis of the SSD approach, see the EMA CVMP Guideline on terrestrial plants (EMA, 2017).
3.5.2. Toxicity tests and refined PNEC derivation: Fresh water compartment
3.5.2.1. Freshwater pelagic and sediment‐dwelling organisms
In order to refine the effect assessment for the freshwater compartment in case risks to pelagic organisms are triggered by Phase IIA or Phase IIB assessments, studies based on the OECD Guidelines http://titania.sourceoecd.org/vl=1315079/cl=54/nw=1/rpsv/ij/oecdjournals/1607310x/v1n2/s12/p1 (Daphnia magna Reproduction), http://www.oecd-ilibrary.org/environment/test-no-210-fish-early-life-stage-toxicity-test_9789264203785-en (Fish, Early‐life Stage) and the ErC10 (or NOErC) derived from http://www.oecd-ilibrary.org/environment/test-no-201-alga-growth-inhibition-test_9789264069923-en on algal Growth Inhibition are recommended. The latter study is already required in Phase IIA (for ErC50 derivation). If from this study also a valid ErC10 (or NOErC) can be derived, this value can be used without additional chronic tests (Daphnia and/or fish) to derive the PNECR;sw when the ErC50 for that alga is at least one order of magnitude more sensitive than the acute L(E)C50 values for Daphnia and fish. If not, more standard test species chronic EC10 (or NOEC) values are required for PNECR;sw derivation.
In addition, if risks of sediment exposure to benthic species is triggered in Phase IIA by a PNECI;sed derived on basis of the EqP approach (Section 3.3.4), also the sediment‐spiked Sediment‐Water Chironomid Toxicity Test ( http://www.oecd-ilibrary.org/environment/test-no-218-sediment-water-chironomid-toxicity-using-spiked-sediment_9789264070264-en), the Sediment‐Water Lumbriculus Toxicity Test ( http://www.oecd-ilibrary.org/environment/test-no-225-sediment-water-lumbriculus-toxicity-test-using-spiked-sediment_9789264067356-en) and a chronic EC10/NOEC for a third benthic species are recommended. This latter species can be selected from test species mentioned in Table 19 (see below in Section 3.5.3). Preferably, the third benthic species is a freshwater species, but if an appropriate toxicity estimate is available for a marine/estuarine species this value may be used as well.
Table 19.
Overview of freshwater and estuarine/marine benthic test species for which protocol tests are available for the conduct of chronic sediment‐spiked toxicity tests
| Test species | Long‐term (chronic) test guideline | Remark |
|---|---|---|
|
Chironomus spp. (insect) |
28‐ to 65‐day tests; OECD 218 (OECD, 2004) 44‐ to 100‐day life‐cycle test; OECD 233 (OECD, 2010) |
Freshwater habitats |
|
Hyalella azteca (crustacean) |
(28‐)42‐day test; US EPA, 1996, 2000 and ASTM E1706 (ASTM, 2010a) | Freshwater and estuarine habitats |
|
Lumbriculus variegatus (oligochaete worm) |
28‐day test; OECD 225 (OECD, 2007) | Freshwater habitats |
|
Caenorhabditis elegans (nematode worm) |
4‐day test; ISO 10872 (ISO, 2010b) | Freshwater and soil habitats |
|
Myriophyllum spicatum (vascular plant) |
14‐day test; OECD 239 (OECD, 2014) | Freshwater habitats |
|
Myriophyllum aquaticum (vascular plant) |
7‐day test; ISO 16191(ISO, 2010a) | Freshwater habitats |
|
Leptocheirus plumulosus (crustacean) |
28‐d test; US EPA 2001 and ASTM E1367 (ASTM, 2010b) | Estuarine habitats |
|
Eohaustorius estuaries (crustacean) |
28‐day test; US EPA, 1996 | Estuarine habitats |
|
Ampelisca abdita (crustacean) |
28‐day test; US EPA, 1996 | Marine habitats |
|
Rhepoxynius abronius (crustacean) |
28‐day test; US EPA, 1996 | Marine habitats |
|
Neanthes arenaceodentata (polychaete worm) |
20‐ to 28‐day test; ASTM E1611 (ASTM, 2007) | Estuarine/marine habitats |
The composition of the sediment used for the tests depends on the requirements of the test species and should therefore follow that in the respective test methods. The use of artificial sediment is recommended. However, field collected sediment can also be used for the test as long as the properties of the sediment are described in detail.
The organic carbon content of sediment may influence bioavailability and consequently the toxicity of the test substance. Therefore, for comparison of sediment tests, the organic carbon content of the test sediment should be within a certain range. The http://www.oecd-ilibrary.org/environment/test-no-218-sediment-water-chironomid-toxicity-using-spiked-sediment_9789264070264-en and OECD http://www.oecd-ilibrary.org/environment/test-no-225-sediment-water-lumbriculus-toxicity-test-using-spiked-sediment_9789264067356-en (Sediment‐Water Lumbriculus Toxicity Test Using Spiked Sediment) use sediment with an organic carbon content of 2 ± 0.5%. ASTM tests with benthic invertebrates usually use field‐collected sediments that may vary in OC content. For the risk characterisation, the toxicity estimates that underlie the PNECR;sed should be normalised to the same organic carbon content that is used for the PEC calculation, i.e. 10% in dry sediment, using the equation mentioned in Section 3.3.6.3. If, when using FOCUS the OC fraction of the sediment on which the PECsed B is based differs from that of the sediment used in toxicity tests, a normalisation of the PEC to a standard sediment is required (see Section 9.3 of EFSA PPR Panel, 2015). Alternatively, the PECsed B and the PNECR;sed should be expressed in terms of μg/g OC in dry sediment to allow a proper linking of exposure to effects. When the adsorption is pH dependent it might also be appropriate to investigate whether the Koc value related to the pH of the sediment used in the toxicity test does not deviate too much from the Koc value used for the PEC calculation. If so, than further adjustment could be considered as outlined in Section 3.3.6.3.
3.5.2.2. Refined PNEC derivation for freshwater pelagic (PNECR;sw) and sediment (PNECR;sed) organisms
PNEC sw for pelagic freshwater organisms
The Phase IIC PNECR;sw for pelagic water organisms should be derived as indicated in Tables 15 and 16.
Table 15.
Endpoints to be used to derive the Phase IIC PNECR;sw for pelagic organisms
| Study | Toxicity endpoint | Remark |
|---|---|---|
| Algal growth inhibition, OECD 201 | 72‐ to 96‐h ErC10 or NOErC | EyC10 or NOEyC may be used if ErC10 or NOErC not reported |
| Daphnia reproduction, OECD 211 | 21‐day EC10 or NOEC | |
| Fish early life‐cycle test, OECD 210 | EC10 or NOEC | Duration of test dependent on test species |
ErC10: Concentration that reduces growth in 10%; EyC10: Concentration that reduces the yield in 10%; NOEC: no observed effects concentration.
Table 16.
Assessment factors to apply to derive the Phase IIC PNECR;sw for pelagic organisms based on the available ecotoxicity data set
| Available data | AF | Remark |
|---|---|---|
| One long‐term EC10/NOEC algae | 100 | An AF of 100 to the EC10 (NOEC) of the algae can only be applied if based on acute L(E)C50 data there is evidence that algae are at least one order of magnitude more sensitive than Daphnia and fish |
| Two long‐term EC10/NOECs (algae and Daphnia or fish) | 50 | Species tested should cover the most sensitive from the acute data set (Section 3.3.6.2). The lowest value should be used to derive the PNEC |
| Three long‐term EC10/NOECs | 10 | The lowest value should be used to derive the PNEC |
EC10: Concentration of the additive causing effect on 10% of the population; NOEC: no observed effects concentration.
If both the PECsw B and PNECR;sw estimates described above still trigger risks a further refinement of the effect assessment may be considered by conducting chronic laboratory toxicity tests with additional species (e.g. to allow the SSD approach), by conducting a semi‐field experiment and/or by advanced modelling approaches (e.g. toxicokinetic/toxicodynamic (TK‐TD) and population models; EFSA PPR Panel, 2014a). The methods proposed by the ECHA Guidance (ECHA, 2008b) and http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2013.3290/abstract (EFSA PPR Panel, 2013) may be consulted for further guidance.
PNEC R:sed for freshwater sediment‐dwelling organisms
The Phase IIC PNECR;sed for freshwater sediment‐dwelling organisms should be derived as indicated in Tables 17 and 18. Note that in the Phase IIC PNECR;sed derivation, sediment‐spiked toxicity test are required only if the EqP approach based on the PNEC for freshwater pelagic organisms (either the Phase IIA PNECsw, but preferably the Phase IIC PNECR;sw) trigger a potential risk (see Section 3.3.6.2).
Table 17.
Endpoints to be used to derive the Phase IIC PNECR;sed for freshwater sediment‐dwelling organisms if the EqP approach triggers a potential risk
| Study | Toxicity endpoint | Remark |
|---|---|---|
| Sediment‐Water Chironomid Toxicity Test | 28‐day EC10 or NOEC | http://www.oecd-ilibrary.org/environment/test-no-218-sediment-water-chironomid-toxicity-using-spiked-sediment_9789264070264-en |
| Sediment‐Water Lumbriculus Toxicity Test | 28‐day EC10 or NOEC | OECD 225 |
| Chronic test with other benthic freshwater or marine/estuarine species | EC10 or NOEC | Table 19 |
EC10: Concentration of the additive causing effect on 10% of the population; NOEC: no observed effects concentration.
Table 18.
Assessment factors to apply to derive the Phase IIC PNECR;sed for sediment‐dwelling freshwater organisms based on the available ecotoxicity data set
| Available data | AF | Remark |
|---|---|---|
| One long‐term EC10/NOEC (Chironomus) | 100 | Sediment‐Water Chironomid Toxicity Test currently is a data requirement |
| Two long‐term EC10/NOEC (Chironomus and Lumbriculus) | 50 | – |
| Three long‐term EC10/NOECs (Table 17) | 10 |
EC10: Concentration of the additive causing effect on 10% of the population; NOEC: no observed effects concentration.
If both the PECsed B and refined PNECR;sed estimates for freshwater ecosystems described above still trigger risks a further refinement of the effect assessment may be considered by conducting chronic laboratory toxicity tests with additional sediment‐dwelling species mentioned in Table 19 (e.g. to allow the SSD approach), by conducting a semi‐field experiment and/or by advanced modelling approaches (e.g. TK‐TD and population models). The methods proposed by the ECHA Guidance (ECHA, 2008b), the https://www.efsa.europa.eu/en/efsajournal/pub/4176 (EFSA PPR Panel, 2015) and Diepens et al. (2017) may be consulted for further guidance.
3.5.3. Toxicity tests and PNECRsed derivation: Marine compartment
In order to refine the effect assessment for the marine sediment compartment, long‐term sediment‐spiked tests with benthic invertebrates can be selected (see Table 19) informed by the results of Phase IIA PNECI;sed assessment (Section 3.3.6.3). If in the near future other internationally approved ISO/OECD tests for sediment‐spiked tests with marine/estuarine invertebrates become available these tests should be considered.
The Phase IIC PNECR;sed for sediment invertebrates in the marine environment is derived as indicated in Tables 20 and 21.
Table 20.
Endpoints to be used to derive the Phase IIC PNECR;sed for sediment invertebrates in marine environment mentioned in Table 19
| Study | Toxicity endpoint | Remark |
|---|---|---|
| Marine/estuarine crustacean | EC10 or NOEC | |
| Second marine/estuarine benthic invertebrate | EC10 or NOEC | At least another taxonomic group than Crustacea is required in the data set |
| Third benthic marine/estuarine or freshwater invertebrate | EC10 or NOEC | At least another taxonomic group than Crustacea is required in the data set |
Table 21.
Assessment factors to apply to derive the Phase IIC PNECRsed for invertebrates in marine environment based on the available ecotoxicity data set
| Available data | AF | Remark |
|---|---|---|
| One long‐term EC10/NOEC | 100 | Species tested should cover the most sensitive species from the acute data set (Section 3.3.6.3) |
| Two long‐term EC10/NOEC values (different taxonomic groups) | 50 | Species tested should cover the most sensitive species from the acute data set (Section 3.3.6.3) |
| Three long‐term EC10/NOECs | 10 | Table 20 |
EC10: Concentration of the additive causing effect on 10% of the population; NOEC: no observed effects concentration; AF: assessment factor.
If the full basic chronic data set (three taxa) is not made available, the PNECR;sed for the marine environment, might be derived as indicated in Table 21, under the condition that the full short‐term toxicity data set is available (Section 3.3.6.3)
If both the PECsed B and PNECR;sed estimates for the marine environment described above still trigger risks a further refinement of the effect assessment may be considered by conducting chronic laboratory toxicity tests with additional sediment‐dwelling species mentioned in Table 19 (e.g. to allow the SSD approach), by conducting a semi‐field experiment and/or by advanced modelling approaches (e.g. TK‐TD and population models; EFSA PPR Panel, 2014a). The methods proposed by the Technical Guidance for Deriving Environmental Quality Standards (European Commission, 2011) and Diepens et al. (2017) may be consulted for further guidance.
3.5.4. Phase II C Risk assessment for secondary poisoning
The QSAR estimate of the BCF value can be replaced by an experimental value determined in a study conducted according the http://www.oecd-ilibrary.org/environment/test-no-305-bioaccumulation-in-fish-aqueous-and-dietary-exposure_9789264185296-en to further refine the assessment of secondary poisoning when in phase IIB still a risk has been identified.
3.5.5. Phase II C Risk characterisation
For the different compartments, the refined PNECsC are compared with the PECA/B derived. If the ratio of the PECA/B to the PNECR is lower than 1, no further assessment is required. If not, a risk for the environment cannot be excluded and further mitigation measures should be considered.
4. Literature reviews
Reference can be made to published studies to support the safety of the additive under the proposed conditions of use for the environment. An extensive literature search should be performed. The analysis of these data must establish that the active substance(s)/agent(s) in literature studies is (are) identical to that under application or, if not, would still allow conclusions on the additive under application to be made. For additives produced by fermentation, identity includes the production strain. For additives consisting of a mixture, the extensive literature search should cover all the components of the mixture. The concentration of the active substance/agent in feed should preferably exceed or at least cover that proposed in the application. The species covered in the literature search should be relevant to the environmental compartment considered. Application level, replicates, duration and endpoints measured should allow a conclusion on the absence of adverse effects. This may be achieved by the consideration of data from a number of independent studies
Relevant information sources should be searched in a structured manner. The applicant should make reasonable efforts to locate all sources of relevant information and provide reasons for the selection of such sources. Bibliographic databases (including at least environmental, biological, ecological, agricultural/aquacultural and medical/veterinary databases) which record documents such as journals, reports, conference proceedings and books should be searched. In addition, the search should consider sources other than bibliographic databases, such as reference lists of full‐text journal articles (e.g. reviews), websites of conferences or organisations
Applicants should follow the recommendations of the ‘Technical manual for performing electronic literature searches in food and feed safety’ when performing the searches and documenting its outcome. Moreover, applicants are encouraged to refer to Appendix D of the ‘Tools for critically appraising different study designs, systematic review and literature searches’ for assessing the quality of the search.
The search methodology must be documented and reported in detail to ensure transparency and enable the evaluation and replication of the strategy. The following must be reported:
For database searches:
-
–
the name of the database and the service provider used;
-
–
the date of the search and the date range searched;
-
–
any limits placed on the search such as language or publication status;
-
–
the full search strategy (all terms and set combinations) and the number of records retrieved.
For sources other than bibliographic databases:
-
Websites and journal table of contents
-
—
the name of the resource (i.e. website name, the journal name in case of searching in specific tables of contents);
-
—
the URL (uniform resource locator, the internet address);
-
—
the date on which the search was conducted and the date range of the search, or the dates, volumes and issues in the case of table of contents;
-
—
the method of searching, e.g. browsing, using the search engine or scanning tables;
-
—
any limits applied to the search (e.g. publication types);
-
—
the search terms used and the number of relevant summary records or full‐text documents retrieved.
-
—
-
References lists
-
—
the bibliographic details of the documents whose reference lists were scanned;
-
—
the number of relevant bibliographic references retrieved.
-
—
The extensive literature search should cover at least the last 20 years. The inclusion and exclusion criteria that drove the selection of relevant scientific papers shall be described. The list of relevant references included should be compiled in a reference management software and provided in ‘.RIS’ format. Copies of the relevant papers should be provided. The applicant must ensure that terms and conditions asserted by any copyright holder of publications or information submitted to EFSA are fully satisfied. The applicant should consult with copyright licensing authorities (i.e. at national level) for guidance on purchasing copyright licenses to reproduce any publications provided to EFSA. The applicant remains solely responsible and liable for obtaining all necessary authorisations and rights to use, reproduce and share the publications provided to EFSA.
Abbreviations
- AF
assessment factor
- a.i.
active ingredient
- ASTM
American Society for Testing of Materials
- BIOWIN
A wastewater treatment process simulator that ties together biological, chemical, and physical process models
- BCF
bioconcentration factor
- Cadd
concentration of the additive (parent compound) in feed
- Cfocus
FOCUS leaching concentration (μg/L)
- CF
conversion factor (kg feed to kg carbon in faeces)
- CONVarea field
conversion factor for the area of the agricultural field
- CONVsed
conversion factor for sediment concentrations: wwt to dwt
- DEPTHfield
mixing depth with soil
- DEPTHsed
mixing depth in sediment
- DF
dilution factor
- DT50
time to degradation of 50% of original concentration of the compound in the tested soils
- DT90
time to degradation of 90% of original concentration of the compound in the tested soils
- EAG
exposure assessment goals
- EC50
the concentration of a test substance which results in 50% of the test organisms being adversely affected, i.e. both mortality and sublethal effects
- ECOSAR
ecological structure activity relationship
- EMA
European Medicines Agency
- EqP
Equilibrium partitioning
- ERA
environmental risk assessment
- ErC50
the concentration of a test substance which results in a 50% of inhibition of algal growth rate
- ERC
ecologically relevant type of concentration
- EyC50
the concentration of the test substance with results in a 50% reduction of yield
- Fa
fraction of the dose considered to be active
- Fairsoil
fraction air in soil
- Fd
fraction of additive (parent compound) degraded in 1 year
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FItotal
total feed intake (DM) per year
- Flow
water flow rate through the system
- Focsed
weight fraction organic carbon in sediment
- Focsoil
weight fraction organic carbon in soil
- FOCUS
The FOrum for Co‐ordination of pesticide fate models and their USe
- FR
feed ration
- Frs
fraction remaining in soil after time Tinterval spreading
- Fsolid sed
volume Fraction of solids in sediment
- Fsolid soil
fraction solids in soil
- Fwater sed
volume fraction of water in sediment
- Fwater–soil
fraction water in soil
- GLP
Good laboratory practice
- GW
groundwater
- HPLC
high‐performance liquid chromatography
- k
rate constant
- Kair–water
partition coefficient air and water in soil
- Kd
sorption/desorption coefficient
- kdep
maximum deposition rate of faeces
- Koc
organic carbon–water partitioning coefficient
- KOM
organic‐matter/water distribution coefficient (L/kg). It corresponds to Koc/1.724
- Kow
n‐octanol/water partitioning coefficient
- Kpsed
partition coefficient solids and water in sediment (v/w)
- Kpsoil
partition coefficient solids and water in soil (v/w)
- Ksed–water
sediment–water partition coefficient
- Ksoil–water
partition coefficient solids and water in soil (v/v)
- LC50
the concentration of a test substance which results in a 50% mortality of the test species
- MCI
Molecular Connectivity Index
- MOLW
molar mass
- Nexcreted
total N excretion per year
- NOEC
no observed effect concentration, i.e. the test concentration at which no adverse effect occurs
- Nspreading
number of spreading events
- NVZ
nitrate vulnerable zones
- OC
organic carbon
- OECD
Organisation for Economic Co‐operation and Development
- PAT
Pesticide Application Time
- PBT
persistent, bioaccumulative and toxic substance
- PCfaeces
concentration of the additive (parent compound) in faeces
- PEC
predicted environmental concentration
- PECfaeces
predicted concentration of the additive (parent compound) in faeces
- PECfw sed
concentration of the additive (parent compound) in fresh water sediment
- PECmanure
concentration of the additive (parent compound) in manure expressed per amount nitrogen
- PECpw
concentration of the additive (parent compound) in porewater
- PECsed
concentration of additive (parent compound) in sediment
- PECsed refined
refined concentration of the additive (parent compound) in sediment
- PECsoil
concentration of the additive (parent compound) in soil
- PECsoil dw
concentration of the additive (parent compound) in soil (dry weight)
- PECsoil ww
concentration of the additive (parent compound) in soil (wet weight)
- PECsoil 1 year
concentration of the additive (parent compound) 1 year after spreading
- PECsoil initial
concentration of the additive (parent compound) in dry soil in Phase I
- PECsoil plateau
PECsoil at plateau concentration
- PECsoil refined
refined concentration of the additive (parent compound) in soil
- PECsoil1 event
concentration of the additive (parent compound) in soil immediately after spreading
- PECsw
concentration of the additive (parent compound) in surface water
- PECswaq
highest initial concentration of additive (parent compound) in surface water ‐ aquaculture
- PECmaxsw
highest initial concentration of additive (parent compound) in surface water
- PNEC
predicted no effect concentration
- PNECi
initial predicted no effect concentration
- PNECr
refined predicted no effect concentration
- PNECsed
predicted no effect concentration for sediment‐dwelling organisms
- PNECsoil
predicted no effect concentrations in soil
- PNECsw
predicted no effect concentration for aquatic organisms
- PRZM
Pesticide Root Zone Model
- Q
annual nitrogen load standard
- QPS
Qualified Presumption of Safety approach for risk assessment of microbials
- QSAR
quantitative structure–activity relationship
- R
gas constant
- REACH
Regulation of the European Union, adopted to improve the protection of human health and the environment from the risks that can be posed by chemicals, while enhancing the competitiveness of the EU chemicals industry. It also promotes alternative methods for the hazard assessment of substances in order to reduce the number of tests on animals.
- RHOd soil
bulk density of (dry) soil
- RHOsusp
bulk density of (wet) suspended matter
- RHOsoil
bulk density of fresh wet soil
- RHOsolid
bulk density of solids in soil or sediment
- RHOw soil
bulk density of (wet) soil
- RQ
risk quotient
- SMILES
simplified molecular‐input line‐entry system
- SOL
water solubility
- SPG
specific protection goal
- SPU
service‐providing unit
- SSD
species sensitivity distribution approach
- SWASH
Surface Water Scenarios Help
- TEMP
temperature at air–water interface
- Tinterval spreading
Time between spreading events
- TK–TD
toxicokinetic/toxicodynamic models
- TOXSWA
TOXic substances in Surface Waters
- Tproduction
number of production days
- Tst
length of time manure is stored
- USEPA
US Environmental Protection Agency
- VICH
Veterinary international Cooperation on Harmonisation
- VP
vapour pressure
- vPvB
very persistent and very bioaccumulative substance
- wt
weight
Appendix A – Specific protection goal options and associated exposure assessment goal options for environmental risk assessments of feed additives
A.1. General protection goals
A.1.1. Introduction
Feed additives are subject to an environmental risk assessment (ERA) before they can be approved for placing on the market. The first step of an ERA is to establish the context for the assessment by identifying which ecosystems/habitats of the environment potentially become exposed by feed additives, and which components of these ecosystems/habitats (e.g. species, ecosystem services) are valued by civil society and/or protected by relevant laws and policies. In Regulation (EC) No 1831/2003 on additives for use in animal nutrition (European Commission, 2003), the following general statements can be found to protect the environment:
In order to protect….the environment, feed additives should undergo a safety assessment through a Community procedure before being placed on the market…
Action by the Community relating to….the environment should be based on the precautionary principle
It is necessary to introduce….a post‐market monitoring plan in order to trace and identify any direct or indirect, immediate, delayed, or unforeseen effect resulting from the use of feed additives on…..the environment…..
The purpose of this Regulation is to establish a Community procedure for authorising the placing on the market and use of feed additives….in order to provide the basis for the assurance of a high level of protection of…..the environment
The feed additive shall not have an adverse effect on…the environment
Since the current ERA for feed additives aims to harmonise with the ERA procedures for veterinary medicinal products (VMPs), it is important to also consider the general statements on environmental protection in CVMP/VICH (2005). In this document, the following statements on protection goals can be found:
The overall target is the protection of ecosystems
The aim of the guidance is to assess the potential for VMPs to affect non‐target species in the environment, including both aquatic and terrestrial species
The taxonomic levels tested are intended to serve as surrogates or indicators for the range of species in the environment
Impacts of greatest potential concern are usually those at community and ecosystem function levels, with the aim being to protect most of species
There may be a need to distinguish between local and landscape level
Issues associated with cumulative impact of some VMPs may be appropriate at the landscape level
Residues are generally assumed to be uniformly distributed in the environment, even though distribution may be patchy.
A.1.2. Environmental compartments and organisms to be protected
From the information presented in Regulation (EC) No 1831/2003 and its implementing rules, the Technical Guidance for ERA of feed additives (EFSA, 2008a) and discussions with risk managers it is clear that at least an ERA should be conducted for (1) non‐target organisms in agricultural soils that receive animal manure/slurry contaminated with feed additives, (2) non‐target organisms in the water and sediment compartment of surface waters subject to input of feed additives via drainage and run‐off from agricultural fields, or via land‐based fish farms, (3) the non‐target organisms in the sediment compartment under fish cages in the marine environment, and (4) the quality of deeper groundwater as influenced by leaching of feed additives from soil.
Considering the quality of deeper groundwater, it is understood that the trigger value for groundwater concerns the groundwater quality standard for pesticides of 0.1 μg/L. Although not explicitly mentioned in Regulation (EC) No 1831/2003 possible specific protection goals (SPGs) for typical groundwater communities and dung fauna were also explored by the working group although no typical dung fauna for poultry dung/manure could be identified.
While feed additives might have a positive or negative influence on air quality (methane emission, N2O) this is considered beyond the scope of this technical guidance, since ERA on this topic is not addressed in Regulation (EC) No 1831/2003 nor requested by risk managers.
Direct or indirect, immediate, delayed or unforeseen effects of feed additives and their metabolites on non‐target organisms in soil, surface water and sediment need to be identified to ensure a high level of protection. This suggests that also impacts of long‐term exposures should be assessed (need for chronic effect assessment procedure, or an appropriate extrapolation of results of an acute effect assessment procedure).
In the previous Technical Guidance that needs to be updated reference is made to a stepped ERA approach based on Risk Quotients (RQs) = PEC/PNEC values. The use of PNECs in the effects assessment suggests that no adverse effects on plant and animal species or processes performed by microbes are allowed. Although not explicitly mentioned, the protection of non‐target plants and animal species likely concerns the population level and that of microbes the functional group level. In defining SPG options, this should be made more explicit.
According to Regulation (EC) No 1831/2003, the ERA for feed additives and their metabolites should be based on the precautionary principle. This can be interpreted as follows: In the absence of relevant and reliable data the ERA should be based on worst‐case assumptions, while this can be relaxed if these data become available.
A.2. Deriving specific protection goals
Policy protection goals as described in Regulation (EC) No 1831/2003 are too generic and vague to be directly used in ERA schemes for feed additives. Terms like ‘high level of protection’ and ‘risks of adverse effects’ need to be operationalised. EFSA has developed a procedure to operationalise generic protection goals and to define SPG for ERA schemes and regulatory decision making by using the Ecosystem Services Concept (EFSA PPR Panel, 2010; EFSA SC, 2016a). Ecosystem services are the benefits people obtain from ecosystems. They include provisioning services such as food and water; regulating services such as flood and disease control; cultural services such as spiritual, recreational, and cultural benefits; and supporting services such as nutrient cycling that maintain the conditions for life on Earth (Millennium Ecosystem Assessment, 2005).
EFSA's ecosystem service‐based framework to define SPGs follows sequential steps:
Identifying ecosystems/habitats potentially impacted by the regulated product or agent
Identifying relevant ecosystem services potentially impacted by the exposure to the regulated product/agent in these ecosystems/habitats
Identifying service‐providing units (SPUs), the structural and functional components of biodiversity that provide or support these ecosystem services
Specifying the level of protection of these SPUs by using the following dimensions: (a) ecological entity of the SPU to be protected, e.g. individual, population, functional group, (b) the attribute to protect, e.g. survival, abundance, biomass, processes, (c) the maximum tolerable impact, e.g. negligible – < 10%; small – between > 10% and < 30%; medium – between > 30% and < 60%; large > 60%, (d) temporal scale of tolerable effect, e.g. < 1 day; days, weeks, months, (e) spatial scale of tolerable effect, e.g. field, edge‐of‐field, watershed/landscape)
Evaluation whether standard test species and endpoints already adopted, or mentioned as data requirements, in regulatory frameworks can be linked to the SPGs options identified
Linking of SPG options developed for specific SPU groups to vulnerable species within this SPU (or grouped SPUs). This is important for the development of a tiered ERA scheme that overall is protective for all field species within SPU groups covered by the SPG and that are potentially at risk. Vulnerability of a species is determined by (i) the chance to become exposed to the feed additive (and/or its major metabolites), (ii) the intrinsic sensitivity to the chemicals of concern, (iii) the potential for ecological recovery, and (iv) species‐traits that make the species susceptible to indirect effects. If in ERA schemes the aim is to accept negligible population‐level effects only (ecological threshold option), the chance to become exposed and the intrinsic sensitivity are the main drivers for the risk assessment. If in ERA schemes some population‐level effect are locally accepted under the condition that ecological recovery takes place (ecological recovery option), then all aspects of vulnerability should be considered (see also EFSA SC, 2016b)
Identifying the ecotoxicologically relevant type of concentration (ERC) to select as ‘C’ in the effect estimates such as the laboratory toxicity data to derive a PNEC and the field exposure estimates or PECs (e.g. for soil or sediment organisms the total concentration of the substance in dry soil or dry sediment or the freely dissolved fraction in pore water of soil or sediment)
A.3. SPG options for feed additives and aquatic SPUs (including those of groundwater ecosystems)
A.3.1. SPG options for aquatic ecosystems (water and sediment organisms)
Building on the experience of using the EFSA approach in defining SPGs for aquatic organisms and plant protection products (e.g. EFSA PPR Panel, 2010, 2013, 2015) the SPU organism groups mentioned below (Tables A.1) might be useful for ERA of feed additives. In this table relevant SPU organism groups and related standard test species frequently used in aquatic ERA are mentioned, as well as the standard test species required for feed additives in the EFSA FEEDAP 2008 ERA guidance document.
Table A.1.
Overview of relevant aquatic SPU organisms, examples of related standard test species and current (2017) basic data requirements in the EFSA FEEDAP 2008 ERA guidance for feed additives
| SPU‐Organism group | Examples of standard test species/assays | Phase II data requirements Feed additives |
|---|---|---|
| Aquatic microbes | OECD test on inhibition of anaerobic bacteria in sludge or sediment; ISO test on inhibition of nitrification in activated sludge | No |
| Aquatic Protozoa | Currently no official OECD Test Guideline available (the freshwater protozoan Tetrahymena pyriformis and the marine protozoan Uronema marinum may be good candidates for guideline development) | No |
| Algae | OECD tests with algae (e.g. Pseudokirchneriella subcapitata) | Yes |
| Aquatic macrophytes | OECD tests with Lemna sp. and Myriophyllum spicatum | No |
| Aquatic arthropods | OECD tests with Daphnia sp. and Chironomus sp.; ASTM test with Hyalella azteca, Diporeia spp., Leptocheirus plumulosus, Eohaustorius estuarius, Ampelisca abdita, Rhepoxynius abronius and Hexagonis spp.; ISO test with Corophium volutator |
Yes, for freshwater ecosystems Daphnia magna and a sediment‐dwelling organism (e.g. Chironomus) Yes for marine sediment–dwelling taxa (e.g. Leptocheirus, Ampelisca, Rhepoxynius and Corophium) |
| Other invertebrates | OECD test with Lumbriculus variegatus; ISO test with Caenorhabditis elegans; ASTM test with Neanthes arenaceodentata |
Yes, for freshwater ecosystems a sediment‐dwelling organism (e.g. Lumbriculus or Caenorhabditis) Yes for marine sediment‐dwelling taxa (e.g. Neanthes) |
| Aquatic vertebrates | OECD test with Oncorhynchus mykiss; ASTM test with Rana pipiens | Yes for freshwater fish |
Coccidiostats used as feed additives have a specific mode of action that may impact Protozoa. For this reason Protozoa are included as a relevant group of SPU organisms.
For persistent mobile substances, there is a concern that they may affect typical ground water species. These species generally have a longer life‐span than taxonomically related aquatic species that dwell in surface waters. In addition, if they are impacted, the decline in population density will last longer because of their poor ability to recolonize impacted groundwater habitats. In other words, typical groundwater species may be more vulnerable than taxonomically related species in surface water.
According to EMA (2017) and Kolar and Finizio (2017), and literature cited, the largely unrecognised biodiversity in groundwater ecosystems needs more attention in ERA and they propose that the protection of groundwater organisms should be a compulsory part of the overall ERA for contaminants, including pharmaceuticals and feed additives. Important groundwater habitats can be found on hypogean karst (fractures, channels, caves) and alluvial gravel interstitial systems. Since spring habitats (the transition between groundwater and surface water) are fed by groundwater, the typical organisms living there also deserve protection. An important element to be considered for the ERA of groundwater ecosystems is prolonged exposure and the need to conduct chronic assessments. The components of biodiversity of groundwater ecosystems that need special attention are flatworms, annelids, molluscs, arthropods (e.g. Niphargus ssp.) and amphibians (e.g. Proteus anguinus). Currently, no specific standard tests are developed for typical groundwater fauna, so that the OECD tests developed for typical freshwater invertebrates and vertebrates need to be considered as surrogate test species.
Note that in the data requirements underlying the FEEDAP 2008 guidance, standard tests with aquatic microbes, aquatic protozoans and aquatic macrophytes, currently are not mentioned. Standard tests with an alga, Daphnia magna and a sediment organism (aquatic invertebrates) and fish (aquatic vertebrate) are required. It is uncertain, but assumed, that for exposure to feed additives these standard test species sufficiently cover the SPG for microbes, protozoans and aquatic vascular plants.
According to EFSA PPR (2010) and EFSA SC (2016a), overall most non‐target organisms need to be protected at the population‐level, except microbes and vertebrates. The selected ecological entity for microbes is the functional group and the attribute to assess are processes. Also note that it currently is almost impossible to assess chemical effects on microbes at the population‐level. The selected ecological entity for vertebrates is set at the individual (acute toxicity) to population (chronic toxicity) level, since suffering of vertebrates due to exposure to regulated agents generally is not accepted by risk managers and the public at large. All options presented below assume that when protecting the selected SPU‐key organism groups in aquatic habitats nearby the site of application, this also will guarantee a high level of protection in more remote aquatic habitats where the exposure to feed additives (and their major metabolites) most likely will be lower than nearby the site of application.
Three SPG options for feed additives and pelagic and benthic aquatic organisms are presented below (Tables A.2–A.4), viz.: (A) the high margin of safety option, (B) the ecological threshold option, and (C), the ecological recovery option.
Table A.2.
Overview of proposed aquatic SPU organisms and their SPG dimensions for the ‘high margin of safety option’
| SPU‐Organism group | Ecological entity | Attribute | Magnitude of tolerable effect | Temporal scale | Spatial scale |
|---|---|---|---|---|---|
| Aquatic microbes | Functional group | Processes | Negligible + extra AF | < days | (Near) site of application |
| Aquatic Protozoa | Functional group or population? | Processes or abundance? | Negligible + extra AF | < days | (Near) site of application |
| Algae | Population |
Abundance/ biomass |
Negligible + extra AF | < days | (Near) site of application |
| Aquatic macrophytes | Population |
Abundance/ biomass |
Negligible + extra AF |
< days | (Near) site of application |
| Aquatic arthropods | Population |
Abundance/ biomass |
Negligible + extra AF |
< days | (Near) site of application |
| Other invertebrates (e.g. worms and molluscs) | Population |
Abundance/ biomass |
Negligible + extra AF |
< days | (Near) site of application |
| Aquatic vertebrates (e.g. fish and amphibians) | Individual | Survival | Negligible + extra AF | < days | (Near) site of application |
| Population | Abundance/biomass |
Table A.4.
Overview of proposed aquatic SPU organisms and their SPG dimensions for the ‘ecological recovery option’
| SPU‐Organism group | Ecological entity | Attribute | Magnitude of effect | Temporal scale | Spatial scale |
|---|---|---|---|---|---|
| Aquatic microbes | Functional group | Processes | Small | Months | (Near) site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Aquatic Protozoa | Functional group or population? | Processes or abundance? | Small | Months | (Near) site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Algae | Population |
Abundance/ biomass |
Small | Months | (Near) site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Aquatic macrophytes | Population |
Abundance/ biomass |
Small | Months | (Near) site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Aquatic arthropods | Population |
Abundance/ biomass |
Small | Months | (Near) site of application and possibly watershed for mobile species |
| Medium | Weeks | ||||
| Large | Days | ||||
| Other invertebrates (e.g. worms and molluscs) | Population |
Abundance/ biomass |
Small | Months | (Near) site of application and possibly watershed for mobile species |
| Medium | Weeks | ||||
| Large | Days | ||||
| Aquatic vertebrates (e.g. fish and amphibians) | Individual | Survival | Negligible | < days | (Near) site of application |
| Population | Abundance/biomass |
A.3.1.1. The high margin of safety option for pelagic and benthic aquatic organisms
The ‘high margin of safety option’ (see Table A.2) assumes that an extra margin of safety should be used when assessing the risks of individual (types of) feed additives, since aquatic organisms may become exposed simultaneously to different types of feed additives that are assessed separately, or the presence of endangered species in the aquatic habitats of concern may require a precautionary approach (see also EFSA SC, 2016c). The extra margin of safety may be achieved by applying an extra Assessment Factor to the PNEC derived for the substance(s) under evaluation (here provisionally placed under the SPG dimension Magnitude of tolerable effect). Taking into account the vulnerability of groundwater fauna and the lack of standard test protocols for groundwater invertebrates and vertebrates, EMA (2017) and Kolar & Finizio (2017) propose to adopt a precautionary approach by applying an extra AF of 10 to the PNEC derived for typical freshwater test species.
A.3.1.2. The ecological threshold option for pelagic and benthic aquatic organisms
This ‘ecological threshold option’ (Table A.3) assumes that by only allowing negligible effects of exposure to a specific (type of) feed additive, the SPU‐key organism groups will be sufficiently protected also in case of simultaneous exposure to different types of feed additives. Since the magnitude of tolerable effect is set at negligible for this option, the ecological threshold option seems to be the option that up till now is used by calculating the PEC/PNEC ratio on basis of the most sensitive (standard) test species.
Table A.3.
Overview of proposed aquatic SPU organisms and their SPG dimensions for the ‘ecological threshold option’
| SPU‐Organism group | Ecological entity | Attribute | Magnitude of tolerable effect | Temporal scale | Spatial scale |
|---|---|---|---|---|---|
| Aquatic microbes | Functional group | Processes | Negligible | < days | (Near) site of application |
| Aquatic Protozoa | Functional group or population? | Processes or abundance? | Negligible | < days | (Near) site of application |
| Algae | Population |
Abundance/ biomass |
Negligible | < days | (Near) site of application |
| Aquatic macrophytes | Population |
Abundance/ biomass |
Negligible | < days | (Near) site of application |
| Aquatic arthropods | Population |
Abundance/ biomass |
Negligible | < days | (Near) site of application |
| Other invertebrates (e.g. worms and molluscs) | Population |
Abundance/ biomass |
Negligible | < days | (Near) site of application |
| Aquatic vertebrates (e.g. fish and amphibians) | Individual | Survival | Negligible | < days | (Near) site of application |
| Population | Abundance/biomass |
A.3.1.3. The ecological recovery option for pelagic and benthic aquatic organisms
This ‘ecological recovery option’ (Table A.4) allows a local but temporal effect on processes by aquatic microbes, and on population structure of aquatic algae and aquatic invertebrates, as long as the permissible direct effects do not result in unacceptable indirect effects. Note that when selecting this option the ERA scheme should be protective as well for vulnerable field populations within the SPU‐key organism groups. This may not be feasible if organisms at stake are potentially sensitive, have a long and complex life‐cycle and a limited dispersal capacity. In addition, when selecting this option the ERA may need to be conducted at the local and landscape level if external recovery processes and ‘action at a distance’ play a prominent role, which can be assumed for mobile aquatic invertebrates and fish (see EFSA SC, 2016b).
A.3.2. Selected SPG option for aquatic ecosystems (water and sediment organisms)
After the description of the SPG options by the working group, they were presented to the FEEDAP Panel and risk managers of the European Commission. Risk managers indicated that they require more time to evaluate the proposed SPGs and the procedure to derive them, as well as the possible consequences (cost‐benefit analysis) for placing feed additives on the European market. Based on the oral comments received, it was decided to select the ‘Ecological Threshold Option’ as SPG for water and sediment organisms. This option is most in line with the ERA schemes developed for feed additives in the old Technical Guidance. Since risk managers did not (yet) request developing ERA decision schemes for exposure of typical groundwater organisms to feed additives, the protection goal of deeper groundwater remains for the time being the ground water quality standard of 0.1 μg/L (Directive 2006/118/EC).11
A.4. Example of SPG options for feed additives and terrestrial SPUs
A.4.1. Soil organisms
Similar SPG options as presented for ERA of feed additives and aquatic sediment‐inhabiting organisms can be used for soil organisms exposed to feed additives in agricultural fields. Again, the three SPG options mentioned below for soil organisms have the same SPU‐key group organisms. In Table A.5, relevant soil SPU organism groups and related standard test species frequently used in ERA for soil organisms are mentioned, as well as the current standard test species required for feed additives. Since coccidiostats are an important group of feed additives, Protozoa are included as a relevant group of SPU organisms.
Table A.5.
Overview of relevant soil SPU organisms, examples of related standard test species and basic data requirements in the EFSA FEEDAP 2008 ERA guidance document
| SPU‐Organism group | Examples of standard test species/assays | Phase II data requirements Feed additives |
|---|---|---|
| Soil microbes | OECD nitrogen transformation test; ISO test on spore germination of mycorrhizal fungi | Yes |
| Soil Protozoa | ? | No |
| Terrestrial plants | OECD tests on terrestrial plants (seedling emergence and growth; vegetative vigour) | Yes, studies with three plant species |
| Earthworms | OECD/ISO earthworm tests (Eisenia fetida/Eisenia andrei) | Yes |
| Soil arthropods | OECD/ISO predatory mite (Hypoaspis aculeifer) and collembolan (Folsomia) test | No |
| Other soil invertebrates | ISO test with Caenorhabditis elegans | No |
| Soil vertebrates | ? | No |
Note that for feed additives the basic data requirements underlying the EFSA FEEDAP 2008 ERA guidance document comprise studies with three plant species. It therefore seems that for the agricultural soil compartment the provisioning services of (crop) plants have a high priority.
Furthermore, in these data requirements, standard tests with soil arthropods (e.g. predatory mites and collembolans), other soil invertebrates (e.g. nematodes, molluscs and enchytraeids) and soil vertebrates (e.g. mole) were not mentioned. It apparently was assumed that the required standard tests for microbes, terrestrial plants and earthworms sufficiently cover the SPG for these SPU groups (Table A.5). It may be argued that potential risks of feed additives to typical soil vertebrates (e.g. mole) is already covered by the risk assessment of livestock animals.
A.4.1.1. The high margin of safety option for soil organisms
The ‘high margin of safety option’ (see Table A.6) assumes that an extra AF should be used when assessing the magnitude of tolerable effects for individual (types of) feed additives, since soil organisms may become exposed simultaneously to different types of feed additives that are assessed separately or the presence of endangered species in the soil habitats of concern may require a precautionary approach (see also EFSA SC, 2016c).
Table A.6.
Overview of proposed soil SPU organisms and their SPG dimensions for the ‘high margin of safety option’
| SPU‐Organism group | Ecological entity | Attribute | Magnitude of tolerable effect | Temporal scale | Spatial scale |
|---|---|---|---|---|---|
| Soil microbes | Functional group | Processes | Negligible + extra AF | < days | Site of application |
| Soil Protozoa | Functional group or population? | Processes or abundance? | Negligible + extra AF | < days | Site of application |
| Terrestrial plants | Population |
Abundance/ biomass |
Negligible + extra AF | < days | Site of application |
| Earthworms | Population |
Abundance/ biomass |
Negligible + extra AF |
< days | Site of application |
| Soil arthropods | Population |
Abundance/ biomass |
Negligible + extra AF |
< days | Site of application |
| Other soil invertebrates (e.g. enchytraeids, molluscs, nematodes) | Population |
Abundance/ biomass |
Negligible + extra AF |
< days | Site of application |
| Soil vertebrates (e.g. mole) | Individual | Survival | Negligible + extra AF | < days | Site of application |
| Population | Abundance/biomass |
A.4.1.2. The ecological threshold option for soil organisms
This ‘ecological threshold option’ (Table A.7) assumes that by allowing negligible effects of exposure to a specific (type of) feed additive, the SPU‐key organism groups will be sufficiently protected also in case of simultaneous exposure to different types of feed additives. Since the magnitude of tolerable effect is set at negligible for this option, the ecological threshold option seems to be the option that up till now is used by calculating the PEC/PNEC ratio on basis of the most sensitive (standard) test species.
Table A.7.
Overview of proposed soil SPU organisms and their SPG dimensions for the ‘ecological threshold option’
| SPU‐Organism group | Ecological entity | Attribute | Magnitude of tolerable effect | Temporal scale | Spatial scale |
|---|---|---|---|---|---|
| Soil microbes | Functional group | Processes | Negligible | < days | Site of application |
| Soil Protozoa | Functional group or population? | Processes or abundance? | Negligible | < days | Site of application |
| Terrestrial plants | Population |
Abundance/ biomass |
Negligible | < days | Site of application |
| Earthworms | Population |
Abundance/ biomass |
Negligible | < days | Site of application |
| Soil arthropods | Population |
Abundance/ biomass |
Negligible | < days | Site of application |
| Other invertebrates (e.g. enchytraeids, molluscs, nematodes) | Population |
Abundance/ biomass |
Negligible | < days | Site of application |
| Soil vertebrates (e.g. mole) | Individual | Survival | Negligible | < days | Site of application |
| Population | Abundance/biomass |
A.4.1.3. The ecological recovery option for soil organisms
This ‘ecological recovery option’ (Table A.8) allows a local but temporal effect on processes by terrestrial microbes and population structure of terrestrial plants and soil invertebrates, as long as the permissible direct effects do not result in unacceptable indirect effects. Temporal effects on vertebrates are not permissible. Note that when selecting this option the ERA scheme should be protective as well for vulnerable field populations within the SPU‐key organism groups. This may not be feasible if organisms at stake are potentially sensitive, have a long and complex life‐cycle and a limited dispersal capacity.
Table A.8.
Overview of proposed soil SPU organisms and their SPG dimensions for the ‘ecological recovery option’
| SPU‐Organism group | Ecological entity | Attribute | Magnitude of effect | Temporal scale | Spatial scale |
|---|---|---|---|---|---|
| Soil microbes | Functional group | Processes | Small | Months | Site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Soil Protozoa | Functional group or population? | Processes or abundance? | Small | Months | Site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Terrestrial plants | Population |
Abundance/ biomass |
Small | Months | Site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Earthworms | Population |
Abundance/ biomass |
Small | Months | Site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Soil arthropods | Population |
Abundance/ biomass |
Small | Months | Site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Other soil invertebrates (e.g. enchytraeids, molluscs, nematodes) | Population |
Abundance/ biomass |
Small | Months | Site of application |
| Medium | Weeks | ||||
| Large | Days | ||||
| Soil vertebrates (e.g. mole) | Individual | Survival | Negligible | < days | Site of application |
| Population | Abundance/biomass |
A.4.2. Dung dwelling fauna
Dung, especially from free‐roaming larger mammals but potentially also chicken dung spread on the top‐soil (Giner‐Santonja et al., 2017), makes up a complex and highly dynamic ecosystem. The organisms involved in dung decomposition provide four vital ecosystem services, viz. (1) the removal of dung as a source of pathogens, parasites and pests, (2) the mineralisation of dung and the supply of nutrients to plants, (3) dung fauna as food source for birds and other insectivorous animals, and (4) dung as habitat for endangered dung fauna. In a guideline on the higher tier testing of veterinary medicinal products to dung fauna, the European Medicines Agency (EMA) particularly mentions dung dwelling beetles (among which several endangered species) and flies as taxa to protect (EMA, 2016). In developing SPGs for feed additives, these taxa of dung fauna might be taken into consideration as well.
In Table A.9, relevant dung fauna SPU organism groups and related standard test species are mentioned. Note that these standard test species at the time of writing this guidance are not a basic data requirement for feed additives.
Table A.9.
Overview of dung SPU organisms (in dung of free‐roaming grazers like cattle), examples of related standard test species and basic data requirements in the EMA 2016 guideline
| SPU‐Organism group | Examples of standard test species/assays | Possible data requirements Feed additives |
|---|---|---|
| Dung flies | OECD dung fly larvae test (OECD 228) | No |
| Dung beetles | OECD dung beetle larvae test (OECD 122) | No |
A.4.2.1. The high margin of safety option for dung fauna
The ‘high margin of safety option’ (see Table A.10) assumes that an extra AF should be used when assessing the magnitude of tolerable effects for individual (types of) feed additives, since dung fauna may become exposed simultaneously to different types of feed additives that are assessed separately, or the presence of endangered species in dung pads of concern may require a precautionary approach (see also EFSA SC, 2016c). Since for dung flies no endangered species are mentioned by EMA (2016), their ecological entity to consider is either the functional group or population. Protecting dung flies at the functional group level probably secures the ecosystem services that concern the removal of dung as a source of pathogens, parasites and pests, the mineralisation of dung and the supply of nutrients to plants, and dung fauna as food source for birds and other insectivorous animals. For dung beetles, EMA (2016) reports a list of endangered species.
Table A.10.
Overview of proposed dung fauna SPU organisms and their SPG dimensions for the ‘high margin of safety option’
| SPU‐Organism group | Ecological entity | Attribute | Magnitude of tolerable effect | Temporal scale | Spatial scale |
|---|---|---|---|---|---|
| Dung flies | Functional group/Population | Abundance/biomass | Negligible + extra AF | < days | Dung pads in individual agricultural fields or meadows |
| Dung beetles | Population | Abundance/biomass | Negligible + extra AF | < days | Dung pads in individual agricultural fields or meadows |
A.4.2.2. The ecological threshold option for dung fauna
This ‘ecological threshold option’ (Table A.11) only differs from the previous option in the ‘Magnitude of tolerable effect’ dimension and assumes that by allowing negligible effects of exposure to a specific (type of) feed additive, the SPU‐key organism groups will be sufficiently protected also in case of simultaneous exposure to different types of feed additives. The effect assessment scheme described in EMA (2016) is more or less in line with this option.
Table A.11.
Overview of proposed dung fauna SPU organisms and their SPG dimensions for the ‘ecological threshold option’
| SPU‐Organism group | Ecological entity | Attribute | Magnitude of tolerable effect | Temporal scale | Spatial scale |
|---|---|---|---|---|---|
| Dung flies | Functional group/Population | Abundance/biomass | Negligible | < days | Dung pads in individual agricultural fields or meadows |
| Dung beetles | Population | Abundance/biomass | Negligible | < days | Dung pads in individual agricultural fields or meadows |
A.4.2.3. The ecological recovery option for dung fauna
This ‘ecological recovery option’ (Table A.12) allows a local but temporal effect on the abundance of dung flies and dung beetles, as long as the permissible local direct effects do not result in unacceptable indirect effects at a larger spatial scale (e.g. limited food for insectivorous birds) and the protection of vulnerable populations is guaranteed at a relevant spatial scale of the landscape. Considering the fact that the life‐span of dung pads is relatively short (weeks to months) the ecological recovery option for dung fauna needs to be assessed for a larger population of dung pads in the relevant patch of landscape.
Table A.12.
Overview of proposed dung fauna SPU organisms and their SPG dimensions for the ‘ecological threshold option’
| SPU‐Organism group | Ecological entity | Attribute | Magnitude of tolerable effect | Temporal scale | Spatial scale |
|---|---|---|---|---|---|
| Dung flies | Functional group/Population | Abundance/biomass | Medium | Life‐span of dung pad | Dung pads in individual agricultural fields or meadows |
| Negligible to small | Life‐span of dung pad | Population of dung pads in landscape | |||
| Dung beetles | Population | Abundance/biomass | Medium | Life‐span of dung pad | Individual dung pad |
| Negligible to small | Life‐span of dung pad | Population of dung pads in landscape |
A.4.3. Selected SPG option for terrestrial ecosystems (soil organisms and dung fauna)
After the description of the SPG options by the working group, they were presented to the FEEDAP Panel and risk managers of the European Commission. It was argued that feed additives, with the exception of coccidiostats, do not possess endo‐ or ectoparasiticidal activities and that therefore in most cases the risks to typical dung fauna need not to be assessed. Furthermore, coccidiostats predominantly will occur in chicken manure that either is worked into the soil or spread on grassland. The members of the working group and the FEEDAP Panel are not aware of typical dung fauna associated with chicken manure applied on the top‐soil. Consequently, it was decided not to develop specific ERA schemes for dung fauna exposed to feed additives. It was considered that the ERA schemes for feed additives and soil fauna sufficiently cover the possible risks to typical dung fauna.
With respect to the SPG options for soil organisms, risk managers indicated that they require more time to evaluate the proposed SPGs and the procedure to derive them, as well as the possible consequences (cost–benefit analysis) for placing feed additives on the European market. Based on the oral comments received, it was decided to select the ‘Ecological Threshold Option’ as SPG for soil organisms. This option is most in line with the ERA schemes developed for feed additives in the old Technical Guidance. In addition, it is assumed that the environmental risk of typical soil vertebrates (e.g. mole) is sufficiently covered with the risk assessment of livestock animals.
A.5. Development of relevant exposure assessment goals (EAGs)
The SPGs developed and selected are mainly defined in eco(toxico)logical terms, and, consequently inform the development of effect assessment schemes (e.g. to derive PNECs) in particular. The overall protection of the environment, however, is determined by the combination of effect and exposure assessment. Just like SPGs are fundamental to inform tiered effect assessment schemes, EAGs are fundamental to inform tiered exposure assessment schemes. Further explanation on EAGs can e.g. be found at http://pfmodels.org/downloads/EMW7_options_groundwater_protection_goals.pdf.
EAGs can be defined by posing the following questions:
What is the ERC to select as ‘C’ in the PEC? (e.g. the total concentration of the substance in dry soil or the freely dissolved fraction in pore water of soil); this ERC should not be in conflict with that selected for the effect assessment (the ERC for the PNEC and PEC estimates should be the same – e.g. wet weight or dry weight)
What is the temporal dimension of the ERC to select as ‘C’ in the PEC? (e.g. the maximum peak concentration per year or the highest time‐weighted average concentration over an ecologically relevant time frame, e.g. a 28‐day TWA concentration)
What is the spatial dimension of the ERC to select as ‘C’ in the PEC (e.g. the concentration in the upper 5 cm or 20 cm of soil),
What are the spatial units for the statistical population of concentrations to consider (e.g. the concentrations in the top‐soil of all treated agricultural fields in a specific area, e.g. a region, a Member State, a regulatory zone in the EU)
What are the multiyear temporal units for the statistical population of concentrations to consider (e.g. the past 5, 10, 15 climatic years)
Which percentile from the statistical spatial‐temporal population of concentrations should be selected for the final PEC? (e.g. the overall 90th percentile PECmax or PECtwa in soil)
A.6. Dialogue with risk managers
The SPG options and related EAG options derived for ERA schemes of feed additives (and their major metabolites) are needed for the dialogue with risk managers. The responsibility of risk assessors is (i) to acknowledge existing general protection goals and regulatory data requirements, (ii) to propose possible SPG options and related EAG options, and (iii) to describe the possible environmental consequences of each option. What is a tolerable level of risk, and thus whether a regulated product can be commercialised, is decided by risk managers (EFSA SC, 2016a). This means that they may request to adapt the options presented and/or that they select a preferred option. It may also be possible that risk managers prefer that ERA schemes are developed for more than one SPG‐EAG option.
As discussed above, risk managers indicated that they require more time to evaluate the proposed SPGs and EAG options, as well as the possible consequences (cost–benefit analysis) for placing feed additives on the European market. Nevertheless, based on the oral input the SPG and EAG options selected for the updated Technical Guidance are in line with the ERA schemes developed for feed additives in the old Technical Guidance. This, however, is made more transparent by the procedure described in this Appendix.
A.7. Developing an ERA scheme for each SPG‐EAG combination
Key is that a tiered ERA scheme should be internally consistent. This means that lower tiers require less effort but are more conservative than higher tiers. Higher tiers aim at being more realistic than lower tiers. In each tier all available relevant scientific information is used. All effect assessment tiers within a scheme aim to address the same SPG and all exposure assessment tiers within that scheme aim to address the same related EAG. Lower tiers can be calibrated/validated by higher tiers (see Figure A.1).
Figure A.1.
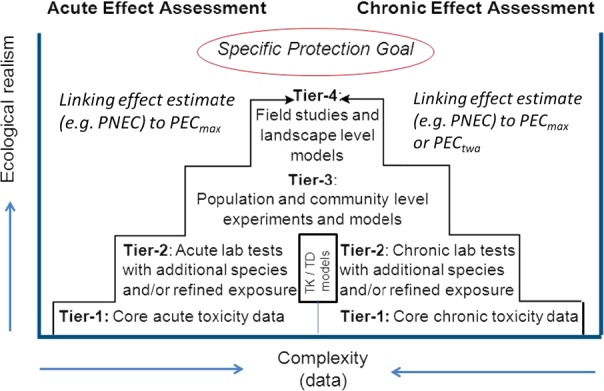
Tiers in an effect assessment scheme, showing the refinement of the process through the acquisition of additional data (redrafted after EFSA PPR Panel, 2013)
For the effect assessment (e.g. PNEC derivation), the tier 1 usually is based on the basic dossier requirements. Since lower tiers should be more conservative than higher tiers, effect estimates (e.g. PNECs) generated at higher tiers should be higher than those at lower tiers. Consequently, higher tier information can be used to validate/calibrate lower tiers. Ideally, the consistency of the different tiers within an ERA scheme should be evaluated for a number of benchmark feed additives.
In a realistic worst‐case ERA, the linking of exposure (PEC estimates) and effects (e.g. PNEC estimates) is not in conflict by acknowledging the following principles:
The effect assessment and exposure assessment is based on the same ERC (e.g. wet weight or dry weight)
In both acute and chronic risk assessments the PECmax can be used. Use of the PECmax in chronic ERA can be considered a precautionary worst‐case approach
-
In chronic risk assessments under certain conditions the PECtwa may be used
Reciprocity of effects demonstrated/likely
Toxicity estimates on which the PNEC is based are expressed in terms of (geometric) mean concentrations during the exposure period of the test
Time frame of the PECtwa estimate should be shorter that the duration of the exposure periods in the toxicity tests that drive the PNEC
Toxicity data that are expressed in terms of initial exposure concentration may be used to derive a PNEC if in the ERA this PNEC is compared with the PECmax and it is likely/plausible that the decline in exposure is not faster in the toxicity tests than that predicted for the field.
A.8. References
CVMP/VICH, 2005. Topic GL38. Guideline on Environmental impact assessment for veterinary medicinal products Phase II, CVMP/VICH/790/03‐Final, London, October 2005
European Commission, 2003. Regulation (EC) No 1831/2003 of the European Parliament and the council of 22 September 2003 on additives for use in animal nutrition. Official Journal of the European Union L 268/29
EFSA, 2008a. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008,6(10):842, 28 pp. https://doi.org/10.2903/j.efsa.2008.842
EFSA PPR Panel, 2010. Scientific Opinion on the development of specific protection goal options for environmental risk assessment of pesticides, in particular in relation to the revision of the Guidance Documents on Aquatic and Terrestrial Ecotoxicology (SANCO/3268/2001 and SANCO/10329/2002). EFSA Journal 2010;8(10):1821, 55 pp. https://doi.org/10.2903/j.efsa.2010.1821
EFSA PPR Panel, 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290
EFSA PPR Panel, 2015. Scientific Opinion on the effect assessment for pesticides on sediment organisms in edge‐of‐field surface water. EFSA Journal 2015;13(7):4176
EFSA SC, 2016a. Guidance to develop specific protection goal options for environmental risk assessment at EFSA, in relation to biodiversity and ecosystem services. EFSA Journal 2016; 14(6) :4499
EFSA SC, 2016b. Recovery in environmental risk assessments at EFSA. EFSA Journal 2016; 14(2):4313
EFSA SC, 2016c. Coverage of endangered species in environmental risk assessment at EFSA. EFSA Journal 2016; 14(2):4312
EMA, 2016. Guideline on the higher tier testing of veterinary medicinal products to dung fauna. EMA/CVMP/ERA/409350/2010, 18 pp
EMA, 2017. Guideline on assessing the toxicological risk to human health and groundwater communities from veterinary pharmaceuticals in groundwater. AMA/CVMP/ERA/103555/2015, 11 pp
Giner‐Santonja G, Georgitzikis K, Scalet BM, Montobbio P, Roudier S and Delgado‐Sancho L, 2017. Best Available Techniques (BAT) Reference Document for the Intensive Rearing of Poultry or Pigs. EUR 20674 EN. Available on line: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/best-available-techniques-bat-reference-document-intensive-rearing-poultry-or-pigs
Kolar B and Finizio A, 2017. Assessment of environmental risks to groundwater ecosystems related to use of veterinary medicinal products. Regulatory Toxicology and Pharmacology, 88, 303‐309.
Appendix B – Application of FOCUS models in Ground water
1.
Input parameters PEARL
1. Scenario:
Location: → pick one
Crop Calendar: → WCEREALS
Irrigation: → irrigation scenarios are considered for Chateaudun, Piacenza, Sevilla, Thiva; No irrigation in the other cases.
Tillage: → No tillage
Repeat interval for application events (a): → 1
2. Simulation Control:
Start date: → 01/01/1901
Stop date: → 31/12/1926
Stop criterion (kg ha‐1): → default zero
Repeat hydrology: → no tick
Although the total time is 26 years, the protocol on the reactive tracer will be for only 20 years.
3. Output Control:
Summary report: → pick FOCUS report
No additional changes.
4. Swap Hydrological Method:
Option Hydrology: → Run SWAP and then PEARL only
No additional changes.
5. Substance:
General
Molar mass (g/mol): → enter value
Saturated vapour pressure (Pa): → enter value
Molar enthalpy of vapourisation (kJ/mol): → 95 (default pesticides)
Solubility in water (mg/L): → enter value
Molar enthalpy of dissolution (kJ/mol): → 27 (default pesticides)
Freundlich sorption
KOM: → enter value (KOM = KOC/1.724)
No additional changes.
Transformation
Half‐life (d): → enter value
No additional changes.
Diffusion
No changes, use default settings from pesticides.
Crop
Wash‐off factor (m−1): → ≥ 10−6, even if there is no wash‐off.
Coefficient for uptake by plant: → no uptake
6. Application
Advice should be given, which application form is most appropriate for feed additives. Since for feed additives, either arable land or grassland without harvest are considered, absolute application seems more appropriate than relative application.
As the input in FOCUS is expressed in kg/ha, the PEC soil has to be converted using the following equation (see also Section 2.6.1):
Where:
| Symbol | Parameter | Default Value | Unit |
|---|---|---|---|
| Input | |||
| PECsoil | Concentration of the additive (parent compound) in soil (dry weight) | mg/kg soildw | |
| RHOsoil | Bulk density of (dry) soil | 1,500 | kg/m3 |
| DEPTHfield | Mixing depth with soil | 0.05 | m |
| Conversion factor | 100 | mg/kg × ha/m2 | |
| Output | |||
| ApplRate | Application rate | kg/ha | |
Absolute applications
Application type: → either incorporation or application to the soil surface
Date: → enter date of application (pre‐emergence)
Dosage (kg/ha): → enter value
Depth (m): → default 5 cm for PECsoil;tot 20 cm for refinement
7. Deposition
No deposition
Appendix C – Application of FOCUS models in surface water
1.
SWASH
1 Actions/Create view and edit substances
General:
Enter information on chemical properties (molar mass, vapour pressure, solubility in water, metabolism).
For molar enthalpy of vaporisation and dissolution and diffusion coefficients in water and air the default values from pesticides may be used.
Maybe a short comment regarding the applicability of the default values especially to macromolecules should be inserted, since these properties are generally assumed to be substance specific.
Sorption:
Enter either KOM or KOC, the other value will be calculated internally.
Enter Freundlich exponent. (The corresponding Freundlich exponent for soil or sediment is internally calculated from the given KOM or KOC value and the fraction of organic matter in the soil of the chosen scenario.)
Reference concentration in the liquid phase [g/m3]: This refers to the concentration at which the sorption parameters were determined. If it was at 1 g/m3, then the default value of 1 is correct. In case the concentration was significantly different from 1 g/m3, the appropriate value should be inserted. This is then used for internal correction of the Freundlich parameters.
Uptake and wash‐off:
Do not assume any plant/root uptake or wash off. Hence, set all parameters zero.
Transformation:
Enter DT 50 in water, soil and sediment and the respective temperatures.
If you assume no transformation in the crop (or no data are available), set a large DT 50 in crop (e.g. 103).
Effect of temperature: Use default value from pesticides if no data are available.
Specifications on transformation in soil: Use default values from pesticides for the dependence of transformation on soil moisture/water content.
2. Focus wizard
Use Wcereals for crops selection. Although more realistic, a pure grassland scenario is not available. Root uptake zero has to be set to zero (in the window ‘uptake and wash off’).
3. User defined wizard
Selected crop according to the chosen crop above.
Accept selected water body types.
Accept appropriate scenarios.
4. View projects and define applications
View and edit application: Enter number of applications, as well as the application mode (granular application is the closest scenario to manure spreading). For run‐off scenarios, the depth of incorporation is also required.
Appendix D – Quantitative structure–activity relationships calculations
1.
Data requirements and quantitative structure–activity relationships calculations in Phase I
In the absence of experimental data, the physical–chemical and fate properties needed as screening information in phase I can be estimated using non‐testing approaches, such as QSARs or read‐across procedures (ECHA, 2008a). The development and application of non‐testing methods is based on the similarity principle, i.e. hypothesis that similar compounds should have similar biological activities (ECHA, 2008a).
Read‐across uses relevant information from analogous (‘source’) substances to predict the properties of ‘target’ substances, providing a major alternative approach for filling data gaps. In the context of this Guidance, read‐across is expected to support the assessment of the ecotoxicological activities of metabolites (see Section 3.3.1). In quantitative read‐across, the known value(s) of a property for one or more source chemicals is used to estimate the unknown value of the same property for the target chemical (ECHA, 2008a). SARs and QSARs, collectively referred to as (Q)SARs, are theoretical models that can be used to predict in a qualitative or quantitative manner the physico‐chemical, biological (e.g. toxicological) and environmental fate properties of compounds from a knowledge of their chemical structure (ECHA, 2008a). In the ideal situation, (Q)SAR results can be used on their own provided they are relevant, reliable and adequate for the purpose, and if they are documented in an appropriate manner. These aspects are further discussed in the http://www.oecd.org/chemicalsafety/risk-assessment/validationofqsarmodels.htm, the http://www.oecd.org/chemicalsafety/risk-assessment/groupingofchemicalschemicalcategoriesandread-across.htm and the http://www.oecd.org/chemicalsafety/risk-assessment/groupingofchemicalschemicalcategoriesandread-across.htm. The careful use of expert judgement to define the boundaries of a chemical category is crucial to the reliable application of QSAR models or other methods to estimate values for untested chemicals. For instance for ionisable active substances, the proper QSAR should be used when the active substance can be ionised between pH 3 and 9 (common soil pH values).
One of the QSAR tools that can be used is the https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface of the US Environmental Protection Agency (USEPA), which uses as input a simplified molecular‐input line‐entry system (SMILES) notation to run different programs to estimate the physical–chemical and fate properties. In the EPI Suite™, the organic carbon partitioning coefficient (Koc) can be estimated using the Molecular Connectivity Index (MCI) method or the octanol–water partition coefficient (log Kow) methodology. The MCI method is overall somewhat more accurate than the log Kow method. As a first worst‐case estimate for the leaching of compounds to groundwater the lowest Koc should be selected.
The Biowin models can be used to screen whether a chemical potentially meets the P criterion in the PBT assessment, as outlined in Appendix E. To determine whether a chemical could accumulate over multiple year application, a first rough estimate of the aerobic degradation rate (DT50 soil) at room temperature can be made using the rating number provided by BioWin3 (Ultimate Survey Model) in the formula developed by Arnot et al. (2005):
Although this DT50 soil is considered a rough estimate of the ultimate degradation of an active substance to minerals and carbon dioxide, it can be used to calculate a refined PEC for persistent active substances. In case that r < 2.5, corresponding with a degradation rate of more than 28 days at room temperature, further experimental data on the biodegradability of the compound is needed and the assessment should go to phase II. For more rapidly degradable compounds the degradation rate does not play an important role in the calculation of the initial PECpw in Phase I since no degradation is assumed.
QSAR calculations to estimate ecotoxicity in Phase II
Generally, experimental data from Good laboratory practice (GLP)‐accredited toxicity studies should be available for Phase II. In some specific circumstances, the FEEDAP Panel might allow the use of QSAR derived data.
The ecological structure activity relationship (ECOSAR) program within the EPI Suite™ developed by the US EPA is one of the tools that can be used to estimate the half‐maximal effective concentration (EC50) or lethal concentration (LC50) for earthworms, fish, green algae and daphnids. Like for the other QSARs, it should be checked whether the QSAR model selected by ECOSAR is appropriate. The default QSAR for ‘neutral organic’ active substances should only be used for active substances where minimal toxicity can be expected based on the chemical structure.
To cover the uncertainty on the QSAR prediction the PNEC for the aquatic compartment (PNECsw) can be derived by selecting the lowest predicted toxicity value (obtained from the QSAR data set of short term studies for daphnids, green algae and fish) for aquatic organisms and by applying an extra AF of 10 to the AF of 1,000 that is applied to experimental data. This additional AF can be lowered when additional information compensates for uncertainties resulting from the uncertainty on the (Q)SAR.
In the absence of experimental terrestrial toxicity data, the equilibrium partitioning method can be applied to calculate the PNECsoil from the PNECsw. The method assumes that toxicity in the soil, expressed as the concentration in pore water, is the same as toxicity measured in water‐only exposure. Consequently, soil organisms show similar species sensitivity distributions (EC50 or LC50 expressed in μg/L pore water) than aquatic organisms (EC50 or LC50 expressed in μg/L surface water). When a PNECsw is estimated from the aquatic toxicity tests, this value can be used to calculate a PNECsoil.
The RHO dry soil of 1,500 kg/m3 can be used. In addition, the PNECsoil;total can be derived from a QSAR from earthworms which is also available in ECOSAR for a number of active substances. The LC50 for earthworms is divided by 1,000 and should only be used when it is lower than the PNECsoil;total derived from the equilibrium partitioning method.
An environmental risk assessment based on QSAR data can only be used for screening purposes to decide for which compounds more data should become available.
Example of risk assessment using QSARs
The following example aims to illustrate how the output of EPI Suite™ for myrcene is used. It is assumed that the concentration in feed is 5 mg a.i./kg. From the CAS number, the program derives the SMILE notation based on which the physico‐chemical properties are estimated (see Table D.1). The EPI Suite™ database had an experimental solubility of 4 mg/L available which is preferred over the calculated solubility. Note that EPI Suite™ estimated a log Koc of 3.031 using the MCI method and a log Koc of 3.758 using the Kow method. The lowest Koc calculated via the MCI method and the Kow method is selected to calculate the concentration in pore water and surface water. The BioWin3 model gives an outcome of 2.8981, based on which the estimated DT50 in soil is 10 days at room temperature which gives a DT50 of 22 days at 12 °C, indicating that the active substance does not accumulate over the years and that the initial PEC can be used as a reasonable worst case. The PECs are calculated as described above in this guideline. The calculations are performed for pigs for fattening (see Appendix F) because these give the highest PECsoil at a given feed dose, compared to the other animal categories.
Table D.1.
The physico‐chemical properties predicted by EPISUITE 4.1 for myrcene
| EU Register name | CAS No. | Predicted by EPIWEB 4.1 | ||||
|---|---|---|---|---|---|---|
| DT50 a (days) | Molecular weight (g/mol) | Vapour pressure (Pa) | Solubility (mg/L) | K oc b (L/kg) | ||
| Myrcene | 123‐35‐3 | 10 | 136.24 | 320 | 4 | 1074 |
CAS No: Chemical Abstracts Service.
DT50: degradation rate of the additive at room temperature (EPI 4.1.BioWin3).
K oc: organic carbon sorption constant (EPI 4.1.KocWin2.0).
When the toxicity data is based on QSARs, the PNEC for the aquatic compartment (PNECsw) is derived from the lowest toxicity value for freshwater organisms by applying a AF of 10,000. To derive the PNECsoil there are two options: The LC50 for earthworms divided by a AF of 10,000 or the equilibrium partitioning method using the PNECsw. The PNECsoil from the equilibrium partitioning method is much lower than the PNECsoil from the earthworm QSAR. Generally the approach should be over conservative to invite applicants to provide real data.
Table D.2.
Phase II environmental risk assessment of myrcene in soil and aquatic compartments (Exposure and effect data were modelled using EPISUITE 4.1 and ECOSAR 2.0)
| Soil | LC50 a Earthworm (mg/kg) | PNECsoil (μg/kg) | PECsoil (μg/kg) | PEC/PNEC | ||
|---|---|---|---|---|---|---|
| Myrcene | 119 | 11.9 | 101 | 8 | ||
| Aquatic |
LC 50 Fish (mg/L) |
LC 50 Daphnids (mg/L) |
EC 50 b Algae (mg/L) |
PNEC c aquatic (μg/L) |
PEC sw d (μg/L) |
PEC/PNEC |
| Myrcene | 0.292 | 0.216 | 0.483 | 0.0216 | 0.4 | 18 |
| Soil using PNECaquatic | PNEC aquatic | K soil water (L/kg) |
PNEC soil, EP (μg/kg) |
PEC soil (μg/kg) |
PEC/PNEC | |
| 0.0216 | 21 | 0.45 | 101 | 223 | ||
PNEC: predicted no effect concentration.
LC50: the concentration of a test substance which results in a 50% mortality of the test species.
EC50: the concentration of a test substance which results in 50% of the test animals being adversely affected (i.e. both mortality and sublethal effects).
Experimental data selected in preference to modelled data for derivation of the PNEC
PECsw: predicted environmental concentration in surface water.
This example shows that a concentration of 5 mg a.i./kg feed will pose a risk for both the aquatic and terrestrial environment. Table D.3 shows the concentrations in feed that result in PECs not exceeding the PNEC for the terrestrial and aquatic environment and the groundwater trigger of 0.1 μg/L. Based on this first screening a dose of 0.02 mg/kg could be considered safe for all compartments, which can be refined when experimental data becomes available.
Table D.3.
Doses of example substance safe for different compartments
| Dose mg/kg feed | PECsoil (μg/kg) | PECpore water (μg/L) | PECsw (μg/L) | Safe for Compartment |
|---|---|---|---|---|
| 0.02 | 0.45 | 0.005 | 0.002 | Terrestrial EP |
| 0.29 | 6 | 0.07 | 0.0216 | Aquatic |
| 0.59 | 11.9 | 0.13 | 0.045 | Terrestrial |
EP: equilibrium partitioning.
Appendix E – Screening information for Persistence, Bioaccumulation and Toxicity
1.
1. Screening information for Persistence, Bioaccumulation, and Toxicity
Table E.1.
Screening criteria according to ECHA (2017e) Part C: PBT/vPvB assessment (Section C.4.1)
| Type of screening information | Screening criterion | Conclusion |
|---|---|---|
| Persistence | ||
|
Biowin 2 (non‐linear model prediction) and Biowin 3 (ultimate biodegradation time) or Biowin 6 (MITI non‐linear model prediction) and Biowin 3 (ultimate biodegradation time) or other modelsa |
Does not biodegrade fast (p < 0.5)a and ultimate biodegradation timeframe prediction: ≥ months (value < 2.25 (to 2.75)b) or Does not biodegrade fast (p < 0.5)a and ultimate biodegradation timeframe prediction: ≥ months (value < 2.25 (to 2.75)b) or Model specific values |
Potentially P or vP Potentially P or vP Potentially P or vP |
| Ready biodegradability test (including modifications allowed in the respective TGs) |
≥ 70% biodegradation measured as DOC removal (OECD TGs 301A, 301E and 306) or ≥ 60% biodegradation measured as ThCo2 (OECD TG 301B) or ThOD (OECD TGs 301C, 301D, 301F, 306 and 310)c < 70% biodegradation measured as DOC removal (OECD TGs 301A, 301E and 306) or < 60% biodegradation measured as ThCo2 (OECD TG 301 B) or ThOD (OECD TGs 301C, 301D, 301F, 306 and 310) |
Not P and not vP Potentially P or vP |
| Enhanced screening testsd | biodegradable not biodegradabled |
Not P and not vP Potentially P or vP |
|
Specified tests on inherent biodegradability:
|
≥ 70 % mineralisation (DOC removal) within 7d; log phase no longer than 3d; removal before degradation occurs below 15%; no preadapted inoculum Any other resulte ≥ 70% mineralisation (O2 uptake) within 14 days; log phase no longer than 3d; no preadapted inoculum Any other resulte |
Not P and not vP Potentially P or vP Not P and not vP Potentially P or vP |
| Bioaccumulation | ||
|
Octanol‐water partitioning coefficient (experimentally determined or estimated by QSAR) Combination of the Octanol water partitioning coefficient with the octanol air partitioning coefficient (both experimentally determined or estimated by QSAR) |
Log Kow ≤ 4.5 Log Kow > 4.5 Log Kow > 2 and Log Koa > 5 |
not B and not vB (f) (in aquatic organisms) Potentially B or vB (in aquatic organisms) Potentially B (in airbreathing organisms) |
| Toxicity | ||
| Toxicity Short‐term aquatic toxicity (algae, daphnia, fish) | EC50 or LC50 < 0.01 mg/Lf | T criterion considered to be definitely fulfilled |
| Short‐term aquatic toxicity (algae, daphnia, fish) | EC50 or LC50 < 0.1 mg/Lf | Potentially T |
The probability is low that it biodegrades fast (see Section R.7.9.4.1 in Chapter R.7b of the Guidance on IR&CSA). Other models are described in Section R.7.9.3.1 in Chapter R.7b of the Guidance on IR&CSA and in this section below.
For substances fulfilling this but BIOWIN 3 indicates a value between 2.25 and 2.75 more degradation relevant information is generally warranted.
These pass levels have to be reached within the 28‐day period of the test. The conclusions on the P or vP properties can be based on these pass levels only (not necessarily achieved within the 10‐day window) for monoconstituent substances. For multiconstituents substances and UVCBs, these data have to be used with care as detailed in Section R.11.4.2.2 of Chapter R.11 of the Guidance on IR&CSA.
See Sections R.7.9.4 and R.7.9.5 in Chapter R.7b of the Guidance on IR&CSA. Expert judgement and/or use of Weight of Evidence also employing other information may be required to reach a conclusion (i.e. concerning « biodegradable/not biodegradable »).
See section below for concluding ultimately on persistence in particular cases (in particular ‘Tests on inherent biodegradation’).
Care must be taken and a case‐by‐case assessment made if a substance is known to bioaccumulate by a mechanism other than passive diffusion driven by hydrophobicity. For example, specific binding to proteins instead of lipids might result in an erroneously low bioaccumulation potential if it is estimated from log Kow.
Care must also be taken for substances classified as polar non‐volatiles (with low log Kow and high log Koa). This group of substances has a low bioaccumulation potential in aquatic organisms but a high bioaccumulation potential in air‐breathing organisms (unless they are rapidly metabolised).
These threshold values only apply for the aquatic compartment.
2. PBT and vPvB criteria according to Annex XIII to REACH Property
Table E.2.
PBT and vPvB criteria according to ECHA (2017e) Guidance on information requirements and chemical safety assessment, Part C: PBT/vPvB assessment
| Property | PBT‐criteria | vPvB‐criteria |
|---|---|---|
| Persistence |
A substance fulfils the persistence criterion (P) in any of the following situations:
|
A substance fulfils the “very persistent” criterion (vP) in any of the following situations:
|
| Bioaccumulation | A substance fulfils the bioaccumulation criterion (B) when: BCF > 2000 | A substance fulfils the “very bioaccumulative” criterion (vB) when: BCF > 5000 |
| Toxicity |
A substance fulfils the toxicity criterion (T) in any of the following situations:
|
– |
Appendix F – Concentration of a feed additive (mg/kg feed) that would correspond to a PEC below the trigger value for the different species
1.
The ratio between the feed intake and the nitrogen excretion determines the PEC manure. A dose of 1 mg feed additive/kg feed results in different manure concentrations in the different species/categories expressed in mg/kg nitrogen in manure. Note that the animal categories in Table F.1 are ordered for a decreasing PEC manure resulting from 1 mg/feed additive/kg feed. This indicates that a dose that causes no environmental concern for pig for fattening will not cause an environmental concern for the other animal categories. This is based on the assumption in Phase 1 of the risk assessment that there is no metabolism of the feed additive.
Table F.1.
Feed intake and nitrogen excretion cause different manure concentrations for the animal categories
| Animal categorya | Feed intake (kg/animal place and year)a | N excretion (kg/animal place and year) | Concentration in mg/kg feed resulting in a PEC of 10 μg/kg soil | PEC manure in mg/kg Nitrogen from 1 mg/kg feed |
|---|---|---|---|---|
| Pig for fattening | 800 | 9 | 0.5 | 89 |
| Cattle for fattening | 4,050 | 54 | 0.6 | 75 |
| Piglet | 296 | 4 | 0.6 | 74 |
| Turkey for fattening | 70 | 1 | 0.6 | 70 |
| Chicken for fattening | 22 | 0.33 | 0.7 | 67 |
| Veal calf | 730 | 11 | 0.7 | 66 |
| Horse | 3,650 | 58 | 0.7 | 63 |
| Meat sheep | 607 | 10 | 0.7 | 61 |
| Rabbit for fattening | 30 | 0.5 | 0.7 | 60 |
| Dairy sheep | 580 | 10 | 0.8 | 58 |
| Horse for fattening | 2,385 | 43 | 0.8 | 55 |
| Lamb for fattening | 273 | 5 | 0.8 | 55 |
| Sheep for fattening | 267 | 5 | 0.8 | 53 |
| Dairy cow | 6,584 | 125 | 0.8 | 53 |
| Laying hen | 42 | 0.8 | 0.8 | 53 |
| Sow with piglets | 1,140 | 23 | 0.9 | 50 |
| Dairy goat | 714 | 16.4 | 1.0 | 44 |
For the characteristics of these animal categories refer to Table 1 of the guidance document.
The feed concentrations in fourth column of Table F.1 all result in a PEC manure of 44 mg/kg nitrogen and therefore in a PEC soil of 10 μg/kg soil.
Appendix G – Nitrogen load to agricultural land from manure application
1.
The FEEDAP panel reconsidered the nitrogen load to agricultural land from manure application which was set as a standard value for the calculation of the PECsoil according to the Technical Guidance for assessing the safety of feed additives for the environment from 2008 (EFSA, 2008b). In the guidance, it was stated that: ‘The amount of manure/slurry containing the feed additives allowed to be spread on land depends on the nitrogen content of the manure and the nitrogen load standard’. The standard load of 170 kg N/ha was set according to the Nitrate Directive12 to the maximum allowed annual amount of nitrogen originating from animal manure on a farm within nitrate vulnerable zones (NVZ).
In order to prevent underestimation of the exposure of feed additives to the primary receiving terrestrial compartment, the FEEDAP panel notes that predicted environmental concentrations in soil would be more realistic if instead of the nitrogen standard load (170 kg N/ha per year) a value of about 250 kg N/ha per year is used due to following reasons:
In the accordance with the Nitrate Directive (European Commission, 1991), NVZ are designated in order to protect the groundwater against the pollution with nitrates. Member states designated different portions of their territory as NVZ (see Table G.1). According to the data from Eurostat (EUROSTAT, 2009) some Member states such as Denmark, Germany, Austria, Ireland, Latvia, Luxemburg, Malta, the Netherlands, Austria, Slovenia and Finland designated all their territory as NVZ. On the other hand, in several member states NVZ covers around or less than 10% of the total state territory (Poland 1.5%). In average, the NVZ covers less than 41% of total area of the territory of the EU Member states. In addition, some member states applied for derogation (the Netherlands, Denmark, Germany, the UK and parts of Belgium and Italy) allowing to use 230–250 kg N/ha per year. Consequently, it is difficult to justify the value of 170 kg N/ha per year as a standard load value for all of arable land and grassland in EU Member states as it applies to less than 30% of total area of the territory of the EU Member states.
To ensure the protection of the water bodies, the Nitrate directive set the maximum nitrogen load value of 170 kg N/ha per year for each farm or livestock unit per year. However, within the farm/livestock unit, the amount of applied manure on a field with a particular crop can be substantially higher. Namely, the value of 170 kg N/ha per year is an average load that applies to the entire farm, while some crops need for their growth and development substantially more nitrogen. According to the good agricultural practice for the use of manure on the NVZ, it is possible to spread more than 170 kg N/ha per year, however, the all‐over sum for nitrogen on the farm should not exceed that nitrogen standard. For example, the most important fodder plants in EU, the maize for grains and the green maize, require for normal development and growth from 230 to 250 kg N/ha per year, while the application to the grassland can reach up to 300 kg N/ha per year (Kristensen, 2015; Bundesministerium für Land‐ und Forstwirtschaft Umwelt und Wasserwirtschaft (Oesterreich), 2012; Sušin, Jože, & Helena, 2016). The terrestrial compartment is exposed to a dose of a feed additive that is applied to the field with the certain crop, not to an average dose for the whole farm. Therefore, soil microbial communities, soil fauna and plants on the field with maize or grassland are exposed to the manure corresponding to the nitrogen load of 230–300 kg N/kg per year.
The farm/livestock unit is not an environmental entity, while the size of a farm in EU Member states varies substantially. The species and communities on the fields with nitrogen high demand crops can be exposed to the higher annual load of manure than average farm load of 170 kg N/ha per year. The value of 250 kg N/ha per year for N‐load on the field with corn was therefore considered as realistic worth case scenario for the potential exposure to feed additives applied with the manure.
The FOCUS emission scenarios used to refine the PECGW/SW values are refereeing to the field with the crop, not to the whole farm/livestock unit. Consequently, the nitrogen load to the field with crop would be a more scientifically sound way of calculation of exposure of terrestrial compartment than the use of an average value of 170 kgN/ha per year that applies to farm/livestock unit.
Table G.1.
Nitrate vulnerable zones in 27 Member States (Eurostat, 2009)
| Total Area 1 | Area nitrogen‐vulnerable zones (3) | ||
|---|---|---|---|
| (1,000 km3) | (1,000 km3) | % | |
| EU‐27 | 4,325 | 1,771 | 40.9 |
| BE | 31 | 21 | 67.8 |
| BG | 111 | 59 | 53.1 |
| CZ | 79 | 31 | 39.8 |
| DK 3 | 43 | 43 | 100.0 |
| DE 3 | 357 | 357 | 100.0 |
| EE | 45 | 3 | 7.5 |
| IE 3 | 70 | 70 | 100.0 |
| EL | 132 | 32 | 24.2 |
| ES | 505 | 64 | 12.6 |
| FR | 549 | 250 | 45.6 |
| IT | 301 | 38 | 12.6 |
| CY 4 | 9 | 1 | 6.8 |
| LV | 65 | 8 | 12.7 |
| LT 3 | 65 | 65 | 100.0 |
| LU 3 | 3 | 3 | 100.0 |
| HU | 93 | 43 | 45.8 |
| MT 3 | 0 | 0 | 100.0 |
| NL 3 | 37 | 37 | 100.0 |
| AT 3 | 84 | 84 | 100.0 |
| PL | 313 | 5 | 1.5 |
| PT | 92 | 3 | 3.7 |
| RO | 238 | 16 | 6.7 |
| SI 3 | 20 | 20 | 100.0 |
| SK | 49 | 16 | 33.5 |
| FI 3 | 338 | 338 | 100.0 |
| SE | 450 | 68 | 15.0 |
| UK | 244 | 94 | 38.7 |
Eurostat, LUCAS, 2009.
Implementation of an Action Programme on the whole territory in accordance with Art 3(5) of the Nitrates Directive; this does not necessarily mean that the whole territory is nitrate vulnerable according to Art 3(2) of the Nitrates Directive.
Based on Information made available to the Commission in digital form. The estimate of designated area does not include some designations communicated in paper form only.
According to Protocol 10 of Accession the application of the acquis Communautaire is suspended in the areas of the Republic of Cyprus not under the effective control of the Government of the Republic.
Special values: 0 means less than half the final digit shown and greater than real zero.
However, since there are several worst‐case assumptions in the model, increasing this default value to higher nitrogen loads would need to include further refinements on storage and application of the manure (e.g. frequency of application).
REFERENCES
Bundesministerium für Land‐ und Forstwirtschaft Umwelt und Wasserwirtschaft (Oesterreich). Verordnung des Bundesministers für Land‐ und Forstwirtschaft, Umwelt und Wasserwirtschaft über das Aktionsprogramm 2012 zum Schutz der Gewässer vor Verunreinigung durch Nitrat aus landwirtschaftlichen Quellen, CELEX Nr. 391L0676 14 (2012). Retrieved from https://www.bmlfuw.gv.at/wasser/wasser-oesterreich/wasserrecht_national/recht_gewaesserschutz/APNitrat2012.html
EFSA. (2008). Technical Guidance Studies concerning the safety of use of the additive for users/workers Prepared by the Panel on Additives and Products or Substances used in Animal Feed Adopted on 17 September 2008. The EFSA Journal (2008), 842, 1–28.
EUROSTAT (2009). http://ec.europa.eu/eurostat/statistics-explained/index.php/Agri-environmental_indicator_-_manure_storage.
Kristensen, S. (2015). Rules and key values related to fertiliser and animal manure in general and requirements related to extension of the animal production, updated to year 2010/2011. Supplemental Literature for the Agro‐Ecosystems Master Course: “Agro‐Ecosystem Analysis and Management at Farm Scale (2016),” (Kristensen 2016), 1–16.
Sušin, J., Jože, V., & Helena, M. (2016). Smernice za izvajanje zahtev varstva voda pred onesnaže vanjem z nitrati iz kmetijskih virov. Republika Slovenija, Ministrstvo Za Okolje in Prostor
Appendix H – Calculations and assumptions made to update the values of feed intake and nitrogen excretion of different animal species/categories
1.
The default values for the calculation of PECmanure and PECsoil of Table 1 were reviewed by:
-
–
characterising the animal species/category in terms of body weight and production cycle;
-
–
calculating the corresponding feed intake, protein input via feed, the fraction of nitrogen ingested (nitrogen = protein × 0.16) that is retained by the animal, and the nitrogen excreted.
The calculations are based in a series of assumptions that are described for the different animal species/categories. It is recognised that is difficult to set a single default value for FI and N excretion given the variety of diets, animal breads, production systems… The aim was to set a single value for FI and N excretion that covers a realistic worst‐case scenario.
The following acronyms were used:
-
–
BW: body weight
-
–
FI: feed intake
-
–
Run: production cycle
-
–
CP: crude protein
-
–
N : nitrogen
1) Piglet

Assumptions:
-
−
N retained from N ingested is 60% (Ju et al., 2008).
2) Pig for fattening

Assumptions:
-
−
N retained from N ingested is 58% (Lee et al., 2016).
3) Chicken for fattening

Assumptions:
-
−
http://cobb-vantress.com/docs/default-source/cobb-500-guides/Cobb500_Broiler_Performance_And_Nutrition_Supplement.pdf breed, males and females
-
−
2.2 kg weight gain during a production cycle that lasts for 35 days
-
−
Cleaning period between production cycles established at 21 days
-
−
N retained from ingested is 60% (Moss et al., 2017).
4) Turkey for fattening

Assumptions:
-
−
http://www.aviagenturkeys.com/uploads/2016/09/06/POCLLB6_V1_BUT%206_Commercial%20Live%20Goals_UK.pdf breed, males and females.
-
−
13 kg average weight (10 kg females and 16 kg males) in a production cycle of 17 weeks
-
−
Cleaning period between production cycles established at 21 days
-
−
Feed to gain in males is 1.98 and in females 2.17 kg feed/kg body weight.
-
−
N retained from ingested is 60% (Jankowski et al., 2013).
5) Rabbit for fattening

Assumptions:
-
−
Production cycle of 72 days (from day 28 – weaning – to day 90 – slaughter) http://www.fao.org/docrep/t1690e/t1690e09.htm
-
−
Cleaning period established at 5 days
-
−
Body weight gain of 1.9 kg
-
−
Feed to gain ratio 3.3 kg feed/kg body weight (Guidenne et al., 2017)
-
−
N retained from ingested is 39% (Birolo et al., 2016).
6) Cattle for fattening

Assumptions:
-
−
Production cycle of 10 months (from 250 kg to 630 kg body weight)
-
−
Feed to gain ration of 8.9
-
−
N retained from ingested is 45% (Van Dung et al., 2013).
7) Veal calf
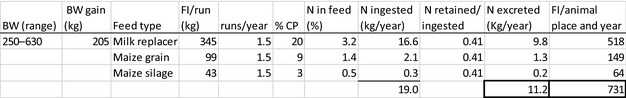
Assumptions:
-
−
Italian production system (Dell'Orto et al., 2009), based on a study using 6,700 veal calves Holstein Friesland males
-
−
Production cycle of 8 months
-
−
Main diet consisting in milk replacer containing 20% crude protein
-
−
Solid diet representing 142 kg/production cycle, consisting in maize grain (70%) and maize silage (30%)
-
−
N retained from ingested is 41% (Gorrill and Nicholson, 1969).
8) Lamb for fattening
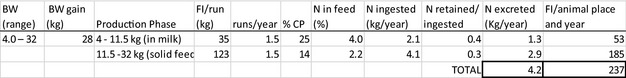
Assumptions:
-
−
Production cycle of 4.5 months divided in two phases: milk feeding (1 month) and solid feed feeding (last 3.5 months)
-
−
Milk feeding: milk replacer (35 kg/lamb) containing 25% CP ( http://www.merckvetmanual.com/management-and-nutrition/nutrition-sheep/feeding-practices-in-sheep). Body weight gain is 0.25 kg/day in the first month (7.5 kg) ( http://www.eblex.org.uk/wp/wp-content/uploads/2014/07/brp-manual-5-Growing-and-finishing-lambs290714.pdf, Agriculture and Horticulture development board ‐UK, 2014). Nitrogen retained from ingested is 40%.
-
−
Solid feeding: concentrate containing 14% crude protein, considering a feed to gain ratio of 6 kg concentrate/kg body weight gain. Nitrogen retained from ingested is 30% (Tripathi et al., 2006) to 25% (Neville et al., 2010).
9) Sheep for fattening

Assumptions:
-
−
Mean daily feed intake of 1.2 kg
-
−
Daily weight gain of 0.27 kg
-
−
N retained from ingested is 30%.
10) Sow
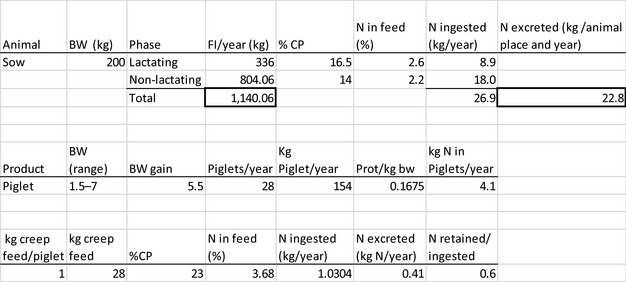
Assumptions:
-
−
French production system ( https://www.ifip.asso.fr/fr/resultats-economiques-gttt-graphique.html)
-
−
Lactation lasting 28 days/production cycle
-
−
2.4 production cycles/year, resulting in 67 lactation days, 278 pregnancy days and 19 days weaning to conception period. 28 piglets/year
-
−
Daily feed intake of non‐lactating period is 2.7 kg.
11) Dairy cow
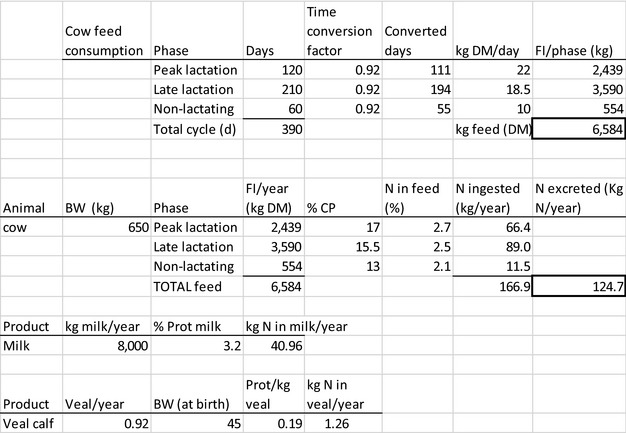
Assumptions:
-
−
Production cycle of 13 months
-
−
0.92 Veals produced per year (one every 13 months)
-
−
Pick lactation phase of 120 days with a feed consumption of 22 kg DM/day
-
−
Late lactation phase of 210 days with a feed consumption of 18.5 kg DM/day
-
−
Non‐lactating (dry phase) of 60 days with a feed consumption of 14 kg DM/day
-
−
Veal body weight at birth is 45 kg and contains 19% protein.
12) Meat sheep (sheep producing lambs for meat)
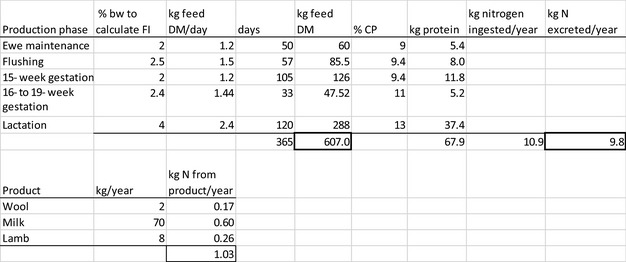
Assumptions,
-
−
Suffolk ewe of 60 kg body weight
-
−
Yearly milk production of 70 kg
-
−
Milk containing 5.4% protein
-
−
2 Lambs per year
-
−
Body weight of lamb at birth is 4 kg
-
−
Yearly wool production is 2 kg, containing 33% keratin and 25% N in keratin.
13) Dairy sheep (milk/cheese production)
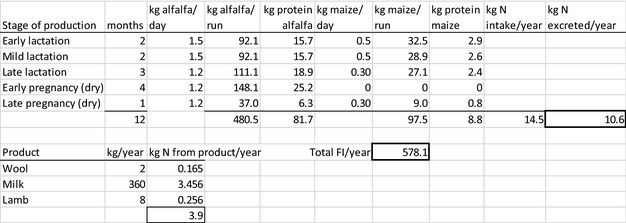
Assumptions:
-
−
Ewe of 60 kg body weight
-
−
2 lambs produced per year, each with a body weight of 4 kg
-
−
Lambs feed only 2 days from the ewe's milk
-
−
360 kg of milk produced per year in a 7‐month lactation period (210 days)
-
−
Milk contains 6% protein
-
−
Yearly wool production of 2 kg, containing 33% keratin and 25% N in keratin.
-
−
Maize containing 9% protein
-
−
Alfalfa hay containing 17% protein.
14) Dairy goat
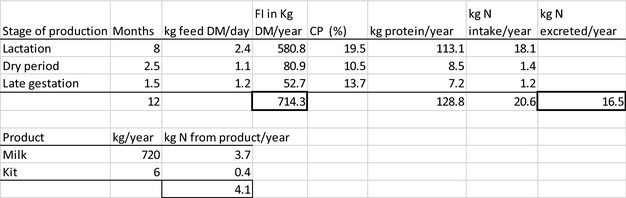
Assumptions:
-
−
Dairy goat of 60 kg body weight
-
−
Production cycle consisting on a lactation period of 8 m, a maintenance (dry) period of 2.5 m and a late gestation period of 1.5 m.
-
−
Yearly milk production of 720 kg in 240 days
-
−
1.5 Kits produced per year
15) Laying hen
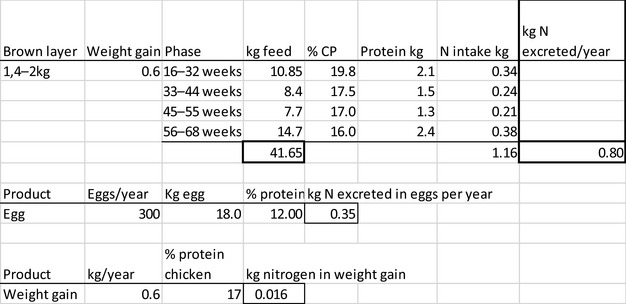
Assumptions:
-
−
Brown layer hen
-
−
Hen's body weight at start (16 weeks of age) is 1.4 kg and increases to 2 kg at the end (68 weeks of age)
-
−
Feed consumption is 0.08 kg in weeks 16 and 17; 0.095 kg in weeks 18–23; and 0.1 kg from week 24 onwards.
-
−
Yearly egg production of 321 eggs (ITAVI, 2014)
-
−
An egg weights 0.06 kg in average
-
−
Chicken have a protein content of 20% body weight.
-
−
N excreted in feathers is assumed to end up in the manure and in the environment.
16) Horse (adult, maintenance)

Assumptions:
-
−
Average mature horse of 500 kg body weight, in maintenance.
-
−
Daily feed intake (DM) of 2% of body weight
-
−
10% of the daily feed intake (DM) is protein.
17) Horse for fattening (to produce horse meat)
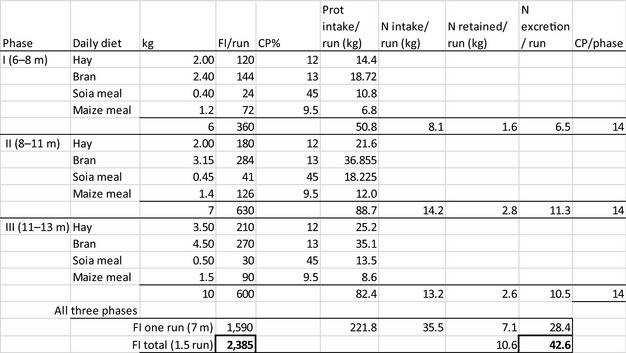
Assumptions:
-
−
Heavy (draft) horse that will reach an adult weight of 700‐800 kg (e.g. Belgian Ardennes, Breton, Comtois breeds)
-
−
Production cycle of 7 months, starting at weaning (6 m of age) with a body weight of 270 kg and finishing at 13 m of age with a body weight of 480 kg.
-
−
Daily weight gain is 1 kg
-
−
Feed to gain ratio is 7.5 kg feed/kg body weight
-
−
Number of production cycles/year is 1.5 (limited by the seasonality of the oestrus)
-
−
Feed contains 14% crude protein along the whole production cycle
-
−
N retained from ingested is 20%.
Suggested citation: The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Bastos M, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, de Knecht J, Kolar B, van Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648
Requestor: European Food Safety Authority
Question number: EFSA‐Q‐2016‐00400
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria Bastos, Henrik Christensen, Birgit Dusemund, Maryline Kouba, Mojca Kos Durjava, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Acknowledgements The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) wishes to thank the following for the support provided to this scientific output: Gerhard Flachowsky, Boet Glandorf, Jürgen Groop, Lieve Herman, Guido Rychen, Maria Saarela, Maria Arena, Antonio Luís González Sánchez, Lucilla Gregoretti, Paola Manini.
Adopted: 27 February 2019
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1595/full
Notes
Regulation (EC) No 1829/2003 of the European Parliament and the Council of 22 September 2003 on genetically modified food and feed.
According to the ‘Guidance to develop specific protection goals options for the environmental risk assessment at EFSA, in relation to biodiversity and ecosystem services’ (EFSA Journal 2016:14(6):4499), a functional group is a collection of organisms with similar functional trait attributes and that are likely to be similar in their response to environmental changes and effects on ecosystem functioning.
Mixtures of substances means mixture of chemicals and/or agents.
EFSA Journal 2017;15(10):5022.
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. OJ L 396, 30.12.2006, p. 1.
OJ L 396, 30.12.2006, p. 1.
The former guidance of environmental risk assessment of feed additives (EFSA, 2008b) considered a soil depth default value of 20 cm in PECsoil calculations only for poultry manure when applied on arable land. There is evidence than poultry manure is spread on grass land in Europe and it is not incorporated in to the soil by ploughing (Giner‐Santonja et al., 2017). For this reason, it was decided to take a default value of 5 cm for all animal species.
If in the near future the specific protection goal for ground water organisms is adopted, the PECA ground water needs to be compared with the proper PNEC for ground water organisms.
FOCUS DG Sante, available online: https://esdac.jrc.ec.europa.eu/projects/focus-dg-sante
Regulation (EC) No 429/2008, OJ L 133 22.5.2008, p. 1.
Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration. OJ 27.12.06, L 372/20.
Council Directive of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources (91/676/EEC). OJ L 375 31.12.91, 8 pp.
References
- Arnot J, Gouin T and Mackay D, 2005. Practical Methods for Estimating Environmental Biodegradation Rates, Report to Environment Canada. CEMN Report No 200503. Canadian Environmental Modelling Network, Trent University, Peterborough, ON, Canada.
- ASTM (American Society for Testing and Materials), 2007. E1611. Standard Guide for Conducting Sediment Toxicity Tests with Polychaetous Annelids. ASTM International, West Conshohocken, PA, USA.
- ASTM (American Society for Testing and Materials), 2010a. E1706‐05. Standard Test Method for Measuring the Toxicity of Sediment‐Associated Contaminants with Freshwater Invertebrates. ASTM International, West Conshohocken, PA, USA
- ASTM (American Society for Testing and Materials), 2010b. E1367‐03. Standard Test Method for Measuring the Toxicity of Sediment‐Associated Contaminants with Estuarine and Marine Invertebrates. ASTM International, West Conshohocken, PA, USA
- ASTM (American Society for Testing and Materials), 2014. E1367‐03. Standard Test Method for Measuring the Toxicity of Sediment‐Associated Contaminants with Estuarine and Marine Invertebrates, ASTM International, West Conshohocken, PA, 2014, http://www.astm.org 10.1520/e1367-03r14 [DOI]
- Bailey J, 2003. Energy requirements and feeding behaviour of salmonids in culture. Doctoral diss. Dept. of Aquaculture, SLU. Acta Universitatis agriculturae Sueciae. Silvestria, 264, 28.
- Burton CH and Turner C, 2003. Manure Management. Treatment strategies for sustainable agriculture. Silsoe Research Institute, Wrest Park, Silsoe, Bedford, UK. ISBN: 0 9531282 6 1.
- Corner RA, Brooker AJ, Telfer TC and Ross LG, 2006. A fully integrated GIS‐based model of particulate waste distribution from marine fish‐cage sites. Aquaculture, 258, 299–311. [Google Scholar]
- CVMP/VICH (Committee for medicinal products for veterinary use/International cooperation on harmonisation of technical rquirements for registratin of veterinary medicinal products), 2005. Environmental impact assessment for Veterinary Medicinal products, Phase II guidance.
- Di Toro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE, Pavlou SP, Allen HE, Thomas NA and Paquin PR, 1991. Technical basis for establishing sediment quality criteria for non‐ionic organic chemicals using equilibrium partitioning. Environmental Toxicology and Chemistry, 10, 1541–1583. [Google Scholar]
- Diepens NJ, Koelmans AA, Baveco H, van den Brink PJ, van den Heuvel‐Greve MJ and Brock TCM, 2017. Prospective environmental risk assessment for sediment‐bound organic chemicals: A proposal for tiered effect assessment. Reviews of Environmental Contamination and Toxicology, 239, 1–77. 10.1007/398_2015_5004 [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency), 2008a. Guidance on information requirements and chemical safety assessment Chapter R.6: QSAR and grouping of chemicals. Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r6_en.pdf/77f49f81-b76d-40ab-8513-4f3a533b6ac9
- ECHA (European Chemicals Agency), 2008b. Guidance on information requirements and chemical safety assessment Chapter R.10: Characterisation of dose [concentration]‐response for environment. https://echa.europa.eu/documents/10162/13632/information_requirements_r10_en.pdf
- ECHA (European Chemicals Agency), 2016. Guidance on Information Requirements and Chemical Safety Assessment Chapter R.16: Environmental exposure assessment. Available on line: https://echa.europa.eu/documents/10162/13632/information_requirements_r16_en.pdf
- ECHA (European Chemicals Agency), 2017a. Guidance on information requirements and chemical safety assessment.Chapter R.11: PBT/vPvB assessment. Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r11_en.pdf
- ECHA (European Chemicals Agency), 2017b. Guidance on information requirements and chemical safety assessment. Chapter R7.a: Endpoint specific guidance. Version 6.0. Available on line: https://echa.europa.eu/documents/10162/13632/information_requirements_r7a_en.pdf/e4a2a18f-a2bd-4a04-ac6d-0ea425b2567f
- ECHA (European Chemicals Agency), 2017c. Guidance on information requirements and chemical safety assessment. Chapter R.7b: Endpoint specific guidance. Version 4.0. Available on line: https://echa.europa.eu/documents/10162/13632/information_requirements_r7b_en.pdf/1a551efc-bd6a-4d1f-b719-16e0d3a01919
- ECHA (European Chemicals Agency), 2017d. Guidance on information requirements and chemical safety assessment. Chapter R7.c: Endpoint specific guidance. Version 3.0. Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r7c_en.pdf/e2e23a98-adb2-4573-b450-cc0dfa7988e5
- ECHA (European Chemicals Agency), 2017e. Guidance on information requirements and chemical safety assessment. Part C: PBT/vPvB assessment. Available online: https://echa.europa.eu/documents/10162/13643/information_requirements_part_c_en.pdf
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed on the development of an approach for the environmental risk assessment of additives, products and substances used in animal feed. EFSA Journal 2007;5(8):529, 73 pp. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2007.529/epdf [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Technical report on the outcome of the pesticides peer review meeting on the OECD 106 evaluators checklist. EFSA Supporting Publication 2017;14(11):EN‐1326, 17 pp. 10.2903/sp.efsa.2017.en-1326 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A,Bolton D, Bolton D, Chemaly M, Davies R, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B,Robertson L, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J,Wahlstr€om H, Wahlstr€om H, Cocconcelli PS, Klein G (deceased), Prieto Maradona M, Querol A, Peixe L, Suarez JE,Sundh I, Vlak JM, Aguilera‐Gomez M, Barizzone F, Brozzi R, Correia S, Heng L, Istace F, Lythgo C and Fernandez Escamez PS, 2017. Scientific Opinion on the update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA Journal 2017;15(3):4664, 177 pp. 10.2903/j.efsa.2017.4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of synthetic astaxanthin as feed additive for salmon and trout, other fish, ornamental fish, crustaceans and ornamental birds. EFSA Journal 2014;12(6):3724, 35 pp. 10.2903/j.efsa.2014.3724 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016. Scientific opinion on the safety and efficacy of pyridine, pyrrole, and quinoline derivatives belonging to chemical group 28 when used as flavourings for all animal species. EFSA Journal 2016;14(2):4390, 19 pp. 10.2903/j.efsa.2016.4390 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA GMO Panel (Panel on Genetically Modified Organisms), 2011. Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use. EFSA Journal 2011;9(6):2193, 54 pp. 10.2903/j.efsa.2011.2193 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 268 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2014a. Good modelling practice in the context of mechanistic effect models for risk assessment of plant protection products. EFSA Journal 2014;12(3):3589, 92 pp. 10.2903/j.efsa.2014.3589 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2014b. Scientific opinion addressing the state of the science on risk assessment of plant protection risk for non target plants. EFSA Journal 2014;12(7):3800, 163 pp. 10.2903/j.efsa.2014.3800 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2015. Scientific Opinion on the effect assessment for pesticides on sediment organisms in edge‐of‐field surface water. EFSA Journal 2015;13(7):4176, 145 pp. 10.2903/j.efsa.2015.4176 [DOI] [Google Scholar]
- EMA (European Medicines Agency), 2005. Guideline on environmental impact assessment for veterinary medicinal products phase II. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004393.pdf
- EMA (European Medicines Agency), 2011. Guideline on determining the fate of veterinary medicinal products in manure (EMA/CVMP/ERA/430327/2009). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/03/WC500104495.pdf
- EMA (European Medicines Agency), 2015. Guideline on the assessment of persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB) substances in veterinary medicinal products. EMA/CVMP/ERA/52740/2012. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/09/WC500193826.pdf
- EMA (European Medicines Agency), 2016. Revised guideline on environmental impact assessment for veterinary medicinal products in support of the VICH guidelines GL6 and GL38, Rev. 1 (EMA/CVMP/ERA/418282/2005‐Rev.1). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004386.pdf
- EMA (European Medicines Agency), 2017. Guideline on the plat testing strategy for veterinary medical products. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/03/WC500224304.pdf
- European Commission , 2003. Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances; Commission Regulation (EC) No 1488/94 on Risk Assessment for existing substances; and Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market. Part II. Available online: https://echa.europa.eu/documents/10162/16960216/tgdpart2_2ed_en.pdf
- European Commission , 2011. Common Implementation Strategy for the Water Framework Directive (2000/60/EC), Technical Guidance Document For deriving Environmental Quality Standards, Technical Report‐2011‐055, 203 pp, 10.2779/43816 [DOI]
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2000. FOCUS groundwater scenarios in the EU review of active substances. Report of the FOCUS Groundwater Scenarios Workgroup, EC Document Reference Sanco/321/2000 rev.2, 202 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.1, March 2012.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2006. Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration. Report of the FOCUS Work Group on Degradation Kinetics, EC Document Reference Sanco/10058/2005 version 2.0. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/dk/docs/FOCUSkineticsvc1.1Dec2014.pdf
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2014. “Assessing Potential for Movements of Active Substances and their Metabolites to Ground Waters in the EU” ‐ Report of the FOCUS Ground Water Work Group, Version 3 of 10 October 2014. EC Document Reference Sanco/13144/2010 version 3, 613 pp, as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2, May 2014. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/gw/NewDocs/focusGWReportOct2014.pdf
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their use), 2015. Generic guidance for FOCUS surface water scenarios. 367pp. Available online https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/sw/docs/Generic%20FOCUS_SWS_vc1.4.pdf
- van Gestel CAM, 2012. Soil ecotoxicology: state of the art and future directions. ZooKeys, 176, 275–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner‐Santonja G, Georgitzikis K, Scalet BM, Montobbio P, Roudier S and Delgado‐Sancho L, 2017. Best Available Techniques (BAT) Reference Document for the Intensive Rearing of Poultry or Pigs. EUR 20674 EN. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/best-available-techniques-bat-reference-document-intensive-rearing-poultry-or-pigs
- Hansen PK, Pittman K and Ervik A, 1991. Organic waste from marine fish farms‐effects on the sea bed. In: Marine Aquaculture and Environment. Ed.: T. Makinen. Copenhagen, Denmark: Nordic Council of Ministers. pp. 105‐119.
- Holmer M, Diaz‐Almela E, Duarte CM, Tsepakis M and Danovaro R, 2006. Sedimentation of organic matter from fish farms in oligotrophic Mediterranean assessed through bula and stable isotope analysis. Aquaculture, 26, 268–280. [Google Scholar]
- Hussenot J, Lefebvre S and Brossard N, 1998. Open‐air treatment of wastewater from land‐based marine fish farms in extensive and intensive systems: current technology and future perspectives. Aquatic Living Resources, 11, 297–304. [Google Scholar]
- ISO (International Organization for Standardization), 2005. Water quality – Determination of acute toxicity of marine or estuarine sediment to amphipods. SO/CD16712:2005(E). First Edition. TC 147/SC5/WG2, 16 pp.
- ISO (International Organization for Standardization), 2010a. ISO/DIS 16191: Water quality – Determination of the toxic effect of sediment and soil on the growth behaviour of Myriophyllum aquaticum. ISO, Geneva, Switzerland.
- ISO (International Organization for Standardization), 2010b. 10872:2010 Water quality – Determination of the toxic effect of sediment and soil samples on growth, fertility and reproduction of Caenorhabditis elegans (Nematoda). ISO, Geneva, Switzerland.
- Karakassis I, Tsapakis M, Smith CJ and Rumohr H, 2002. Fish farming impact in the Mediterranean studied through sediment profiling imagery. Marine Ecology Progress Series, 227, 125–133. [Google Scholar]
- Kutti T, Kupka Hansen P, Ervik A, Høisæter T and Johannessen P, 2007. Effects of organic effluents from a salmon farm on a fjord system II. Temporal and spatial patterns in infauna community composition. Aquaculture, 262, 355–366. [Google Scholar]
- Mattilsynet (Norwegian Food Safety Authority), 2014. Vannkwalitet og dyrevelferd (water quality and animal welfare, In Norwegian language). Available online: https://www.mattilsynet.no/fisk_og_akvakultur/fiskevelferd/mattilsynet__rapport_om_vannkvalitet_og_fiskevelferd_2004.5943/binary/Mattilsynet%20-%20Rapport%20om%20vannkvalitet%20og%20fiskevelferd%202004
- Metcalfe C, Boxal A, Fenner K, Kolpin D, Servos M, Silberhorn E and Staveley J, 2006. Exposure assessment of veterinary medicines in aquatic systems. In Veterinary medicines in the environment (Crane M, Boxal ABA and Barret K Editors)
- Millennium Ecosystem Assessment , 2005. Ecosystems and Human Well‐being: Synthesis. Island Press, Washington, DC: Available online https://www.millenniumassessment.org/documents/document.356.aspx.pdf [Google Scholar]
- Montforts MHMM, 1997. Environmental risk assessment for veterinary medicinal products. 1. Other than GMO‐containing and immunological products. RIVM, Report 613310001. Bilthoven, The Netherlands. [Google Scholar]
- Montforts M, 1999. Environmental risk assessment for veterinary medicinal products Part I. Other than GMO‐containing and immunological products. First Update. Report of the National Institute of Public Health and the Environment, Centre for Substances and Risk Assessment. Available online: http://www.rivm.nl/dsresource?objectid=17b7a462-ee59-4fba-a9ab-a5f4dbe3b81a&type=org&disposition=inline
- Norwegian Food Safety Authority , 2004. Water quality and animal welfare. 85 pp. (in Norwegian language). available online https://www.mattilsynet.no/fisk_og_akvakultur/fiskevelferd/mattilsynet__rapport_om_vannkvalitet_og_fiskevelferd_2004.5943/binary/Mattilsynet%20-%20Rapport%20om%20vannkvalitet%20og%20fiskevelferd%202004
- OECD (Organisation for economic co‐operation and development), 2004. Test No. 218: Sediment‐Water Chironomid Toxicity Using Spiked Sediment, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, 10.1787/9789264070264-en [DOI] [Google Scholar]
- OECD (Organisation for economic co‐operation and development), 2007. Test No. 225: Sediment Water Lumbriculus Toxicity Test Using Spiked Sediment, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, https://doi.org/10.1787%2f9789264067356-en
- OECD (Organisation for economic co‐operation and development), 2010. Test No. 233: Sediment‐Water Chironomid Life‐Cycle Toxicity Test Using Spiked Water or Spiked Sediment, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, 10.1787/9789264090910-en [DOI] [Google Scholar]
- OECD (Organisation for economic co‐operation and development), 2014. Test No. 239: Water‐Sediment Myriophyllum spicatum Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, https://doi.org/10.1787%2f9789264224155-en
- SANCO (Health and Consumer Protection Directorate‐General of the European Commission), 2012. Working Document to the Environmental Safety Evaluation of Microbial Biocontrol Agents. SANCO/12117/2012 –rev. 0. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_ppp_app-proc_guide_fate_wd-env-sft-eval-mbca.pdf
- Schneider MK, Stamm C and Fenner K, 2007. Selecting scenarios to assess exposure of surface waters to veterinary medicines in Europe. Environmental Science and Technology, 41, 4669–4676. [DOI] [PubMed] [Google Scholar]
- US EPA (United States Environmental Protection Agency, 1996. Ecological Effect Test Guidelines OPPTS 850.1740. Whole Sediment Acute Toxicity Invertebrates, Marine. Office of Prevention, Pesticides and Toxic Substances, Washington DC, USA.
- US EPA (United States Environmental Protection Agency), 2000. Methods for measuring the toxicity and bioaccumulation of sediment‐associated contaminants with freshwater invertebrates, second edition. E.600/R‐99/064. Available online: http://www.epa.gov/waterscience/cs/library/freshmanual.pdf
- US EPA (United States Environmental Protection Agency), 2001. Methods for assessing the chronic toxicity of marine and estuarine sediment‐associated contaminants with the amphipod Leptocheirus plumulosus. First Edition. EPA/600/R‐01/020, 130 pp.


