Abstract
According to Article 12 of Regulation (EC) No 396/2005, EFSA has reviewed the maximum residue levels (MRLs) currently established at European level for the pesticide active substance imidacloprid. To assess the occurrence of imidacloprid residues in plants, processed commodities, rotational crops and livestock, EFSA considered the conclusions derived in the framework of Directive 91/414/EEC, the MRLs established by the Codex Alimentarius Commission as well as the import tolerances and/or European authorisations reported by Member States (including the supporting residues data). Based on the assessment of the available data, MRL proposals were derived and a consumer risk assessment was carried out. Some information required by the regulatory framework was missing and a possible chronic/acute risk to consumers was identified. Hence, the consumer risk assessment is considered indicative only, some MRL proposals derived by EFSA still require further consideration by risk managers and measures for reduction of the consumer exposure should also be considered.
Keywords: imidacloprid, MRL review, Regulation (EC) No 396/2005, consumer risk assessment, neonicotinoid/nitroguanidine group, insecticide
Summary
Imidacloprid was included in Annex I to Directive 91/414/EEC on 1 August 2009 by Commission Directive 2008/116/EC, and has been deemed to be approved under Regulation (EC) No 1107/2009, in accordance with Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulation (EU) No 541/2011. As the active substance was approved after the entry into force of Regulation (EC) No 396/2005 on 2 September 2008, the European Food Safety Authority (EFSA) is required to provide a reasoned opinion on the review of the existing maximum residue levels (MRLs) for that active substance in compliance with Article 12(1) of the aforementioned regulation. To collect the relevant pesticide residues data, EFSA asked Germany, as the designated rapporteur Member State (RMS), to complete the Pesticide Residues Overview File (PROFile) and to prepare a supporting evaluation report. The PROFile and evaluation report provided by the RMS were made available to the Member States. A request for additional information was addressed to the Member States in the framework of a completeness check period, which was initiated by EFSA on 2 May 2016 and finalised on 2 July 2016. After having considered all the information provided, EFSA prepared a completeness check report which was made available to Member States on 26 August 2016.
Based on the conclusions derived by EFSA in the framework of Directive 91/414/EEC, the MRLs established by the Codex Alimentarius Commission and the additional information provided by the RMS and Member States, EFSA prepared in October 2018 a draft reasoned opinion, which was circulated to Member States for consultation via a written procedure. Comments received by 14 November 2018 were considered during the finalisation of this reasoned opinion. The following conclusions are derived.
Based on the recent EFSA conclusions on the peer review of the updated pesticide risk assessment for bees, the conditions of approval for imidacloprid were recently restricted to uses in permanent greenhouses or for the treatment of seeds intended to be used only in permanent greenhouses, with crops staying within a permanent greenhouse during its entire life cycle. Member States were required to amend or withdraw their authorisations by 19 September 2018, with a maximum period of grace expiring on the 19 of December, 2018.
As the good agricultural practices (GAPs) and the supporting residue data considered in this MRL review were collected before the new conditions of approval entering into force, the data assessed in the present reasoned opinion are reflecting not only the uses compliant with the new conditions of approval, but also the (former) authorised European Union (EU) outdoor uses. In particular, in order to support risk managers in the decision making process, EFSA considered in this assessment:
Residue data reflecting the EU indoor GAPs and the uses authorised in third countries (import tolerances) only, in line with the new conditions of approval for imidacloprid. This data was used to derive the MRL recommendations for plant and animal commodities as reported in the summary table and in Appendix B.4. These MRLs are also expected to cover the possible carry‐over from the (former) authorised EU outdoor uses.
Residue data reflecting all uses, including the EU outdoor GAPs. This data was used to derive a list of alternative MRLs possibly safe for consumers that could be considered by risk managers to support emergency authorisations. The list of alternative MRLs derived considering all uses and the results of the related risk assessment are reported, respectively, in Appendices G and B.3.2 to this reasoned opinion. Moreover, residue trials supporting the outdoor EU uses were also considered to assess the possible carry‐over of imidacloprid in plant and animal commodities after the entry into force of the new conditions of approval.
The metabolism of imidacloprid was investigated in primary (fruit, root and leafy crops, cereals and pulses and oilseeds) and in rotational crops (root and leafy crops, cereals). Based on the results of the metabolism in primary and rotational crops, the residue definition for enforcement in plant commodities is proposed as imidacloprid only. For risk assessment, the residue definition is confirmed as the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid. The same residue definitions apply to rotational crops and processed commodities.
It is noted that results from the available residue trials suggest that imidacloprid only could not be a sufficient marker in pulses and oilseeds. Nevertheless, the limited residue data available does not allow concluding if a different residue definition for enforcement is required for these crops. Therefore, it is underlined that based on the results of the additional trials on dry beans, peanuts, beans and peas without pods required to support the existing import tolerances, the residue definition for enforcement in pulses and oilseeds may need to be reconsidered.
A sufficiently validated analytical method is available for the enforcement of the proposed residue definition in high water content, high acid content and dry commodities at the limit of quantification (LOQ) of 0.01 mg/kg, in high oil content at the LOQ of 0.02 mg/kg and in hops at 0.2 mg/kg. There are indications that imidacloprid can be enforced in coffee beans with an LOQ of 0.01 mg/kg, however a confirmatory method and an independent laboratory validation (ILV) are still missing. According to the EURLs, during routine analyses an LOQ of 0.01 mg/kg is achievable in the four main matrices.
Regarding the magnitude of residues expected in primary crops from the uses compliant with the new conditions of approval (indoor uses and import tolerances only), the available data were sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for currants, gooseberries, rose hips, mulberries, azaroles, elderberries, granate apples, lettuce and other salad plants where the available data were insufficient to derive even tentative MRLs.
As imidacloprid is a persistent active substance expected to accumulate in soil following multiannual applications and the available studies demonstrated that it can be taken up from the soil by the plant, in the assessment of the magnitude of residues in rotational crops, EFSA considered not only the uses compliant with the new conditions of approval, but also the possible carry‐over from the (former) authorised EU outdoor uses.
When considering only the uses compliant with the new conditions of approval, it is concluded that specific MRLs for rotational crops are not needed, provided that Member States will take adequate risk mitigation measures (e.g. use only on sweet peppers grown with soil‐less growing systems) in order to avoid significant residues to occur in rotational crops.
When considering the possible carry‐over of residues in plant commodities due to (former) authorised EU outdoor uses, it is concluded that specific temporary MRLs for plant commodities are not required to cover the possible carry‐over from (former) outdoor EU uses. On other hand, as significant residues of parent and metabolites can be expected in cereals straw, their impact on the residues in livestock was considered further.
Imidacloprid is authorised for use on several crops (dry pulses, citrus fruits and peanuts) that might be fed to livestock. Livestock dietary burdens were therefore calculated for different groups of livestock according to OECD guidance. As EU outdoor GAPs are expected to be withdrawn according to the new conditions of approval, only indoor uses and import tolerances were considered for the calculation of the livestock exposure. Moreover in order to cover the carry‐over in cereals due to the (former) authorised EU outdoor uses, the results from the available outdoor trials on wheat and barley (grain and straw) were also considered for the calculation of the livestock exposure. Since, the dietary burdens calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM), the behaviour of residues was assessed in all commodities of animal origin.
Metabolism studies in lactating goats and laying hens were submitted and evaluated during the peer review. According to the results of these studies, it is clear that parent compound is almost completely degraded in the liver and kidney of ruminants and in poultry tissues and eggs, with glucuronide conjugates of hydroxy‐metabolites, imidacloprid olefine metabolite (M06) and a glycine‐conjugate of 6‐chloropyridine‐3‐carboxylic acid, representing the main identified compounds. Nevertheless, on the basis of livestock exposure resulting from the uses assessed in this review, no significant residues are expected in animal commodities. Hence, the residue definition for enforcement in all animal commodities is proposed as parent compound only (by default) and MRLs and risk assessment values for the relevant commodities in ruminants and poultry can be established at the LOQ level. These MRLs are expected to cover the possible carry‐over in cereal due to the (former) authorised outdoor EU uses. For risk assessment, it is still proposed to keep the following residue definition as agreed during the peer review: sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid. It is underlined that, if additional uses leading to significant increase in livestock exposure will be granted in the future, the residue definition for animal commodities should be reconsidered.
Analytical methods for the enforcement of the proposed residue definition were evaluated during the peer review and showed that imidacloprid can be enforced in milk at the LOQ of 0.01 mg/kg and in animal tissues and in eggs at an LOQ of 0.03 mg/kg. According to the EURLs, based on the general experience with this compound, although only a screening method is available for animal commodities (except for honey validated down to 0.002 mg/kg), it is expected that imidacloprid residues can be enforced with an LOQ of 0.01 mg/kg in all commodities of animal origin.
Chronic and acute exposure calculations resulting from the authorised indoor uses and import tolerances (in line with the new conditions of approval) reported in the framework of this review were performed using revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo). This calculation is also expected to cover the possible carry‐over in cereals from the former authorised outdoor EU uses. For those commodities where data were insufficient to derive an MRL, EFSA considered the existing EU MRL for an indicative calculation. According to the RMS, MRLs in the EU legislation are currently established for the parent compound only, but are actually based on data according to the so‐called ‘total residue’ which is expected to cover the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety. Therefore, when considering the existing EU MRL, no conversion factor from enforcement to risk assessment was applied. Based on these calculations, a potential risk to consumers was identified for the use of imidacloprid on escaroles and no further refinements of the risk assessment were possible. For the remaining commodities, although uncertainties remain due to the data gaps identified in the assessment, the indicative exposure calculation did not indicate a risk to consumers.
Chronic and acute exposure calculations for all uses (including the former authorised outdoor EU uses) reported in the framework of this review were also performed using revision 2 of the EFSA PRIMo (EFSA, 2007). For those commodities where data were insufficient to derive an MRL in Section 1, EFSA considered the existing EU MRL for an indicative calculation. For the same reasons reported above, when considering the existing MRL, no conversion factor from enforcement to risk assessment was applied. Based on these calculations, a potential risk to consumers was identified for the southern outdoor GAPs on escaroles, sweet peppers and kale. For these commodities, fall‐back GAPs were identified in order to reduce the exposure of consumers. For the remaining commodities, although uncertainties remain due to the data gaps identified in the assessment, the indicative exposure calculation did not indicate a risk to consumers.
Apart from the MRLs evaluated in the framework of this review, internationally recommended CXLs have also been established for imidacloprid. Nevertheless, as the residue definition for enforcement of the CXLs is not compatible with the residue definition for enforcement proposed in the framework of this review, for information purposes, an indicative risk assessment was performed considering the existing CXLs only. These calculations indicate a potential risk to consumers for the existing CXLs on celery and kales. For the remaining CXLs, the indicative exposure calculation did not indicate a risk to consumers. However, considering that CXLs are currently expressed according to a residue definition for enforcement not compatible with the one proposed by EFSA, they are not recommended for inclusion in the EU legislation.
Background
Regulation (EC) No 396/20051 (hereinafter referred to as ‘the Regulation’) establishes the rules governing the setting and the review of pesticide maximum residue levels (MRLs) at European level. Article 12(1) of that Regulation stipulates that the European Food Safety Authority (EFSA) shall provide within 12 months from the date of the inclusion or non‐inclusion of an active substance in Annex I to Directive 91/414/EEC2 a reasoned opinion on the review of the existing MRLs for that active substance. As imidacloprid was included in Annex I to Council Directive 91/414/EEC on 1 August 2009 by means of Commission Directive 2008/116/EC3, and has been deemed to be approved under Regulation (EC) No 1107/20094, in accordance with Commission Implementing Regulation (EU) No 540/20115, as amended by Commission Implementing Regulation (EU) No 541/20116, EFSA initiated the review of all existing MRLs for that active substance.
According to the legal provisions, EFSA shall base its reasoned opinion in particular on the relevant assessment report prepared under Directive 91/414/EEC. It should be noted, however, that, in the framework of Directive 91/414/EEC, only a few representative uses are evaluated, whereas MRLs set out in Regulation (EC) No 396/2005 should accommodate all uses authorised within the European Union (EU), and uses authorised in third countries that have a significant impact on international trade. The information included in the assessment report prepared under Directive 91/414/EEC is therefore insufficient for the assessment of all existing MRLs for a given active substance.
To gain an overview of the pesticide residues data that have been considered for the setting of the existing MRLs, EFSA developed the Pesticide Residues Overview File (PROFile). The PROFile is an inventory of all pesticide residues data relevant to the risk assessment and MRL setting for a given active substance. This includes data on:
the nature and magnitude of residues in primary crops;
the nature and magnitude of residues in processed commodities;
the nature and magnitude of residues in rotational crops;
the nature and magnitude of residues in livestock commodities;
the analytical methods for enforcement of the proposed MRLs.
Germany, the designated rapporteur Member State (RMS) in the framework of Directive 91/414/EEC, was asked to complete the PROFile for imidacloprid and to prepare a supporting evaluation report. The PROFile and the supporting evaluation report (Germany, 2015) were submitted to EFSA on 11 June 2015 and made available to the Member States. A request for additional information was addressed to the Member States in the framework of a completeness check period which was initiated by EFSA on 2 May 2016 and finalised on 2 July 2016. Additional evaluation reports were submitted by Belgium, the Czech Republic, France, Germany, Greece, Hungary, Italy, the Netherlands, Portugal, Spain and the European Union Reference Laboratories for Pesticide Residues (EURLs) (Belgium, 2016; Chech Republic, 2016a,b; France, 2016; Germany, 2016; Greece, 2016; Hungary, 2016; Italy, 2016a,b; Netherlands, 2016; Portugal, 2016; Spain, 2016; EURLs, 2016) and, after having considered all the information provided by RMS and Member States, EFSA prepared a completeness check report which was made available to all Member States on 26 August 2016. Further clarifications were sought from Member States via a written procedure in August‐October 2016.
Based on the conclusions derived by EFSA in the framework of Directive 91/414/EEC, the MRLs established by the Codex Alimentarius Commission (codex maximum residue limit (CXL)) and the additional information provided by the Member States, EFSA prepared in October 2018 a draft reasoned opinion, which was submitted to Member States for commenting via a written procedure. All comments received by 14 November 2018 were considered by EFSA during the finalisation of the reasoned opinion.
The evaluation reports submitted by the RMS (Germany, 2015, 2016) and the evaluation reports submitted by Member States the Czech Republic, France, Germany, Greece, Hungary, Italy, the Netherlands, Portugal, Spain and EURLs (Chech Republic, 2016a,b; France, 2016; Germany, 2016; Greece, 2016; Hungary, 2016; Italy, 2016a,b; Netherlands, 2016; Portugal, 2016; Spain, 2016; EURLs, 2016) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available.
In addition, key supporting documents to this reasoned opinion are the completeness check report (EFSA, 2016) and the Member States consultation report (EFSA, 2018b). These reports are developed to address all issues raised in the course of the review, from the initial completeness check to the reasoned opinion. Also, the chronic and acute exposure calculations for all crops reported in the framework of this review performed using the EFSA Pesticide Residues Intake Model (PRIMo) (excel file) and the PROFile are key supporting documents and made publicly available as background documents to this reasoned opinion. Furthermore, screenshots of the Report sheet of PRIMo (Indoor EU and IT), PRIMo(All uses) and PRIMo(CXL) are presented in Appendix C.
Considering the importance of the completeness check and consultation report, also these documents are considered as background documents to this reasoned opinion and, thus, are made publicly available.
Terms of Reference
According to Article 12 of Regulation (EC) No 396/2005, EFSA shall provide a reasoned opinion on:
the inclusion of the active substance in Annex IV to the Regulation, when appropriate;
the necessity of setting new MRLs for the active substance or deleting/modifying existing MRLs set out in Annex II or III of the Regulation;
the inclusion of the recommended MRLs in Annex II or III to the Regulation;
the setting of specific processing factors as referred to in Article 20(2) of the Regulation.
The active substance and its use pattern
Imidacloprid is the ISO common name for (E)‐1‐(‐(6‐chloro‐3‐pyridylmethyl)‐N‐nitroimidazolidin‐2‐ylideneamine (IUPAC).
Imidacloprid belongs to the group of neonicotinoid/nitroguanidine compounds which are used as insecticides. It is a systemic substance with translaminar activity and with contact and stomach action. It is readily taken up by the plant and further distributed acropetally, with good root‐systemic action. It acts as an antagonist by binding to postsynaptic nicotinic receptors in the insects’ central nervous system. It has broad uses, mainly against aphids, in all crops. In addition, different modes of application are registered for imidacloprid, including foliar sprays from sowing until harvest, granular application in furrow or seedbed, seed dressing and preplanting or post‐harvest dipping of plants.
The chemical structure of the active substance and its main metabolites are reported in Appendix F.
Imidacloprid was evaluated in the framework of Directive 91/414/EEC with Germany designated as rapporteur Member State (RMS). The representative uses supported for the peer review process were seed treatment for sugar beet and foliar application on apples and tomatoes. Following the peer review, which was carried out by EFSA, a decision on inclusion of the active substance in Annex I to Directive 91/414/EEC was published by means of Commission Directive 2008/116/EC, which entered into force on 1 August 2009. According to Regulation (EU) No 540/2011, imidacloprid is deemed to have been approved under Regulation (EC) No 1107/2009. This approval is restricted to uses as insecticide only.
Following a peer review of the pesticides risk assessment for bees the conditions of approval were amended by means of Commission Directive (EU) No 485/20137. Due to risks for bees from treated seeds the use and the placing on the market of seeds treated with plant protection products containing imidacloprid was prohibited for seeds of crops attractive to bees and for seeds of cereals except for winter cereals and seeds used in greenhouses.
Commission Implementing Regulation (EU) No 485/2013 also required the applicants to submit confirmatory data by 31 December 2014, covering all uses that could still be authorised (including certain seed, soil and foliar treatments). Furthermore, on 13 November 2015, EFSA was mandated to provide conclusions concerning an updated risk assessment for bees as regards the uses of imidacloprid applied as seed treatment or granules by organising a peer review and taking into account the data collected in the framework of the specific open call for data and any other new data from studies, research and monitoring activities relevant to the uses under consideration. EFSA conclusions on the confirmatory data and on the peer review of the updated pesticide risk assessment for bees considering the uses as seed treatment and granules were published, respectively, on 11 October 2016 and on 28 February 2018. Taking into account these conclusions, the Standing Committee on Plants, Animals, Food and Feed concluded that the restrictions laid down in Regulation (EU) No 485/2013 needed further modification. Consequently, the conditions of approval were further restricted to uses as insecticide, in permanent greenhouses or for the treatment of seeds intended to be used only in permanent greenhouses, with crops staying within a permanent greenhouse during its entire life cycle (European Commission, 2018). Member States were required to amend or withdraw their authorisations by 19 September 2018, with a maximum period of grace expiring on the 19 December 2018 by means of Commission Implementing Regulation (EU) 2018/7838.
The EU MRLs for imidacloprid are established in Annex IIIA of Regulation (EC) No 396/2005 and CXLs for active substance were also established by the Codex Alimentarius Commission (CAC). An overview of the MRL changes that occurred since the entry into force of the Regulation mentioned above is provided Table 1.
Table 1.
Overview of the MRL changes since the entry into force of Regulation (EC) No 396/2005
| Procedure | Legal implementation | Remarks |
|---|---|---|
| Implementation of CAC 2009 | Commission Regulation (EU) No 459/2010a | CXLs for imidacloprid |
| MRL application | Commission Regulation (EU) No 893/2010b | Modification of the existing MRLs for imidacloprid in rice |
| Implementation of CAC 2013 | Commission Regulation (EU) No 491/2014c | CXLs for imidacloprid |
MRL: maximum residue level; CXL: codex maximum residue limit; CAC: Codex Alimentarius Commission.
Commission Regulation (EU) No 459/2010 of 27 May 2010 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for certain pesticides in or on certain products. OJ L 129, 28.5.2010, p. 3–49.
Commission Regulation (EU) No 893/2010 of 8 October 2010 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acequinocyl, bentazone, carbendazim, cyfluthrin, fenamidone, fenazaquin, flonicamid, flutriafol, imidacloprid, ioxynil, metconazole, prothioconazole, tebufenozide and thiophanate‐methyl in or on certain products. OJ L 260, 9.10.2010, p. 10–38.
Commission Regulation (EU) No 491/2014 of 5 May 2014 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for ametoctradin, azoxystrobin, cycloxydim, cyfluthrin, dinotefuran, fenbuconazole, fenvalerate, fludioxonil, fluopyram, flutriafol, fluxapyroxad, glufosinate‐ammonium, imidacloprid, indoxacarb, MCPA, methoxyfenozide, penthiopyrad, spinetoram and trifloxystrobin in or on certain products. OJ L 146, 16.5.2014, p. 1–91.
For the purpose of this MRL review, the critical uses of imidacloprid authorised within the EU, as well as uses authorised in third countries that might have a significant impact on international trade, have been collected by the RMS and reported in the PROFile. The additional Good Agricultural Practices (GAPs) reported by Member States during the completeness check were also considered. The details of GAPs for imidacloprid received in the framework of this review are given in Appendix A.
It is underlined that, as the GAPs and the supporting residue data considered in this MRL review were collected before the new conditions of approval entering into force, the overall data assessed in the present reasoned opinion is reflecting not only the uses compliant with the new conditions of approval, but also the (former) authorised EU outdoor uses. In particular, in order to support risk managers in the decision making process, EFSA considered in this assessment:
Residue data reflecting the EU indoor GAPs and the uses authorised in third countries (import tolerances) only, in line with the new conditions of approval for imidacloprid. This data was used to derive the MRL recommendations for plant and animal as reported in the summary table and in Appendix B.4. These MRLs are also expected to cover the possible carry‐over from the (former) authorised EU outdoor uses.
Residue data reflecting all uses, including the EU outdoor GAPs. This data was used to derive a list of alternative MRLs possibly safe for consumers that could be considered by risk managers to support emergency authorisations. The list of alternative MRLs derived considering all uses and the results of the related risk assessment are reported, respectively, in Appendices G and B.3.2 to this reasoned opinion. Moreover, residue trials supporting the outdoor EU uses were also considered to assess the possible carry‐over of imidacloprid in plant and animal commodities after the entry into force of the new conditions of approval.
Assessment
EFSA has based its assessment on the PROFile submitted by the RMS, the evaluation report accompanying the PROFile (Germany, 2015), the draft assessment report (DAR) and its addenda prepared under Council Directive 91/414/EEC (Germany, 2005, 2008), the conclusion on the peer review of the pesticide risk assessment of the active substance imidacloprid (EFSA, 2008a), the peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance imidacloprid (EFSA, 2008b), the technical report on the Evaluation of the data on clothianidin, imidacloprid and thiamethoxam for the updated risk assessment to bees for seed treatments and granules in the EU (EFSA, 2018a), the Joint Meeting on Pesticide residues (JMPR) Evaluation report (FAO, 2008, 2015), the previous reasoned opinion on imidacloprid (EFSA, 2010) as well as the evaluation reports submitted during the completeness check (Belgium, 2016; Chech Republic, 2016a,b; France, 2016; Germany, 2016; Greece, 2016; Hungary, 2016; Italy, 2016a,b; Netherlands, 2016; Portugal, 2016; Spain, 2016; EURLs, 2016). The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/20119 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a,b,c,d,e,f,g, 2000, 2010a,b, 2017; OECD, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
Under the peer review of Directive 91/414/EEC, the metabolism of imidacloprid residues in plants was investigated in fruit crops, root crops, leafy crops, cereals, pulses and oilseeds following foliar application (apples, tomatoes, potatoes and tobacco), seed treatment (maize, cotton and rice) and soil granular application (eggplants, potatoes, rice). All available metabolism studies were performed using pyridinyl‐14C‐methylene labelled imidacloprid (Germany, 2005).
After the foliar application, the metabolic pattern in aerial parts of the plants was dominated by the parent compound which represents 70–95% of the extractable residues. Most of the radioactivity remained on the surface of fruits and leaves and could be washed off with methanol. In potato tubers, the total radioactivity was very low (characterisation was not possible) showing that transport from sprayed leaves to tubers was negligible.
Metabolism after soil granular application and seed treatment show active uptake and translocation of the radioactivity to aerial plant parts. Qualitatively, the metabolic routes of degradation suggested by these studies are the same as after foliar treatment, but the residue pattern found after seed and soil treatments reflects a more extensive degradation. In particular, following soil treatment, although parent compound was still present (ranging from 10% total radioactive residue (TRR) in eggplants foliage to 48% TRR in potatoes tubers) the following metabolites were identified above the 10% TRR: imidacloprid‐desnitro (M09, accounting for up to 34% TRR, corresponding to 0.97 mg/kg in eggplants leaves); imidacloprid‐6‐CNA (M14, accounting for up to 13% TRR corresponding to 0.004 mg/kg in eggplants); imidacloprid‐CHMP‐glucoside (M29, accounting for up to 13% TRR corresponding to 0.007 mg/kg in eggplants).
Similarly, following seed treatment, parent compound accounted from 8% TRR (rice straw) to 65% TRR (immature corn) and imidacloprid‐desnitro represented the main metabolite, accounting for up to 36% TRR (0.48 mg/kg) in rice straw. It is noted that in cotton seeds following seed treatment, imidacloprid was not detected and the only measured compound was imidacloprid‐CHMP (M28), accounting for 23% TRR but present at low absolute amounts (0.001 mg eq/kg).
All studies indicate that translocation of the substance in plants occurs by acropetal transport mainly from roots to leaves. In general, it was concluded that metabolism in plants proceeds according to three routes that were observed in almost all plants: (a) hydroxylation of imidazoline ring by forming the mono‐ and dihydroxylated compounds; (b) reduction of nitro group; and (c) oxidative cleavage of the methylene bridge.
1.1.2. Nature of residues in rotational crops
Imidacloprid is authorised on several crops that can be grown in crop rotation. In the framework of the peer review under Directive 91/411/EEC, the rate of degradation of imidacloprid in soil was investigated in field and laboratory studies (Germany, 2005). Since reported field DT90 values largely exceed 100 days (up to 956 days), a special consideration should be given to imidacloprid residues in rotational crops following annual and multiannual applications according to the most critical authorised uses. The metabolism of imidacloprid in rotational crops was investigated in a confined study following the application of pyridinyl‐14C‐methylene‐imidacloprid (Germany, 2005). The study was performed by applying imidacloprid on a bare soil at an application rate of 0.454 kg a.s./ha with Swiss chard, red beet and wheat sown or planted 30, 120 and 271 days after treatment (DAT). The nature of metabolites in rotational crops was observed to be the same as in crops from primary plant metabolism studies. The parent compound (ranging from 0.4% TRR in wheat grain to 47% TRR in wheat forage) was metabolised into several compounds containing 6‐chloropyridinyl moiety, the most abundant being imidacloprid‐desnitro (up to 19% TRR in wheat straw). On the basis of this study it can be concluded that metabolism of imidacloprid in rotational crops proceeds according to a similar pathway as in primary crops.
1.1.3. Nature of residues in processed commodities
Under the peer review of Directive 91/414/EEC, the effects of processing on the nature of imidacloprid residues was investigated in hydrolysis studies by stimulating pasteurisation, baking, brewing, boiling and sterilisation processes (Germany, 2005). The results of a hydrolysis study, performed with radiolabelled methylene‐14C‐imidacloprid, demonstrate that imidacloprid is stable under hydrolytic conditions and does not undergo degradation.
In principle, the effect of processing on the nature of the major metabolites observed in raw plant commodities should also be assessed. However, considering that an extensive degradation of imidacloprid (especially following soil and seed treatment), which proceeds through the 6‐chloropyridinyl moiety, was observed in plant commodities, it is not expected that new metabolites are formed when metabolites including the 6‐chloropyridinyl moiety are subject to standard hydrolysis conditions. Consequently, further studies investigating the degradation of those metabolites through standard hydrolysis are not considered necessary.
1.1.4. Methods of analysis in plants
In the framework of the peer review of Directive 91/414/EEC, an HPLC method and its independent laboratory validation (ILV) were found to be sufficiently validated for the enforcement of imidacloprid in high water content (tomatoes, apples, cabbages), high oil content (cotton seed, rape seed), high acid content (citrus) and dry commodities (wheat grain), as well as in hops. This method allows separate analysis of imidacloprid, metabolite imidacloprid‐5‐hydroxy (M01) and metabolite imidacloprid olefine, with an limit of quantification (LOQ) of 0.02 mg/kg for each compound in the main four matrices and an LOQ of 0.2 mg/kg for each compound in hops (Germany, 2008).
According to the RMS, the multi‐residue Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method in combination with high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) is also sufficiently validated for the enforcement of imidacloprid with a LOQ of 0.01 mg/kg in high water content (cucumbers), high acid content (lemons, oranges) and in dry commodities (wheat flour) (Germany, 2015).
An additional HPLC–MS/MS method for the enforcement of imidacloprid validated in cocoa beans (that could be used for the enforcement in coffee beans) was also reported by the RMS in the framework of this review (Germany, 2015). Based on this method, there are indications that imidacloprid can be enforced in specific matrices such as coffee beans with an LOQ of 0.01 mg/kg, however a confirmatory method and an ILV are still missing.
According to the information provided by the EURLs, during routine analyses an LOQ of 0.01 mg/kg is achievable in the four main matrices by using the QuEChERS method (EURLs, 2016).
1.1.5. Stability of residues in plants
The storage stability of imidacloprid residues in various plant matrices was investigated in the framework of the peer review (EFSA, 2008a). Imidacloprid parent compound as well as mixtures of imidacloprid and its main metabolites (when analysed as sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety), were found to be stable under deep frozen conditions for at least 24 months in dry commodities, in commodities with high water, high acid and high oil content. Additional storage stability studies covering the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety were assessed by the JMPR. According to these studies, the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety is stable for up to 53 months in high oil content matrices, for up to 41 months in high water content commodities, for up to 34 months in dry commodities and for up to 25 months in coffee, stored under deep frozen conditions (FAO, 2008).
1.1.6. Proposed residue definitions
Considering that the plant metabolic pattern is covered by the toxicological studies on the active substance itself, and that the produced metabolites have the same toxicological profile as the parent compound, the peer review concluded to set the risk assessment residue definition for plants as the ‘sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, all expressed as imidacloprid’. No final decision on the enforcement residue definition was taken in the peer review. Two options were proposed:
to consider parent imidacloprid as the main residue for enforcement;
to establish enforcement residue definition the same as the risk assessment residue definition.
In the framework of this MRL review, the RMS proposed to consider parent compound only for enforcement. Based on the results of the metabolism in primary and rotational crops and considering that the 6‐chloropyridinyl moiety is not specific to imidacloprid as it is also contained in other neonicotinoid pesticides, EFSA agrees with the RMS considering imidacloprid only a sufficient marker for enforcement. For risk assessment, the residue definition is confirmed as the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid. The same residue definitions apply to rotational crops and processed commodities.
It is noted that results from the available residue trials suggest that imidacloprid only could not be a sufficient marker in pulses and oilseeds. Nevertheless, the limited residue data available does not allow concluding if a different residue definition for enforcement is required for these crops. Therefore, the proposed residue definition for enforcement in pulses and oilseeds should be considered tentative only and may need to be reconsidered based on the results of the additional trials on dry beans, peanuts, beans and peas without pods required to support the existing import tolerances (see Sections 1.2.1 and 1.2.3).
It is underlined that all available metabolism studies were performed using pyridinyl‐14C‐methylene labelled imidacloprid only. During the peer review, the expert meeting on residues estimated that the amount of cleaved metabolites was low in comparison to that of uncleaved metabolites, showing that this route of metabolism is minor in plants. In addition, further degradation of the imidazoline moiety to nitrosimine is not expected to be a preferred pathway. It was nevertheless concluded that the applicant should submit a robust scientific assessment/statement on possible formation of nitrosimines or other degradates of toxicological concern from the cleaved nitroimino‐imidazoline moiety in plants. During the peer review, the applicant submitted a scientific statement on possible formation of nitrosimines or other degradates of toxicological concern from the cleaved nitroimino‐imidazoline moiety in plant metabolism. These comments were however not peer reviewed but included in the peer review report (EFSA, 2008b). In the framework of this MRL review, EFSA considered the statement provided by the applicant sufficient to exclude the formation of nitrosimines or other compounds containing the nitroimino‐imidazoline moiety at significant levels. Therefore, it is concluded that the available studies are considered sufficient to elucidate the metabolism in plant and an additional metabolism study performed with imidacloprid labelled at the imidazolidine ring is not required.
A sufficiently validated analytical method is available for the enforcement of the proposed residue definition in high water content, high acid content and dry commodities at the LOQ of 0.01 mg/kg, in high oil content at the LOQ of 0.02 mg/kg and in hops at the LOQ of 0.2 mg/kg. There are indications that imidacloprid can be enforced in coffee beans with an LOQ of 0.01 mg/kg; however, a confirmatory method and an ILV are still missing and are required.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
To assess the magnitude of imidacloprid residues resulting from the reported GAPs, EFSA considered all residue trials reported by the RMS in its evaluation report (Germany, 2015), including additional data submitted during the completeness check (Chech Republic, 2016a,b; France, 2016; Germany, 2016; Greece, 2016; Hungary, 2016; Italy, 2016a,b; Netherlands, 2016; Portugal, 2016; Spain, 2016; EURLs, 2016). All residue trial samples considered in this framework were stored in compliance with the demonstrated storage conditions. Decline of residues during storage of the trial samples is therefore not expected.
The number of residue trials and extrapolations were evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2017). Although MRLs and risk assessment values were also derived from the EU outdoor GAPs, as these uses are expected to be withdrawn according to the new conditions of approval, only data gaps relevant for the indoor uses and the import tolerances are reported below.
Residue trials are not available to support the indoor authorisations or the import tolerances on currants, gooseberries, rose hips, mulberries, azaroles, elderberries, pomegranate, lettuce and other salad plants. Therefore, MRL or risk assessment values for these crops could not be derived by EFSA and the following data gaps were identified:
currants, gooseberries, rose hips, mulberries, azaroles, elderberries: complete data set compliant with the import tolerance GAP for these crops;
pomegranates: complete data set compliant with the import tolerance GAP for this crop;
lettuce and other salad plants: complete data set compliant with the indoor GAP for these crops.
For all other crops, available residue trials are sufficient to derive (tentative) MRL and risk assessment values, taking note of the following considerations:
Citrus fruits, table and wine grapes and dry beans: only residue trials analysing for the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety are available to support the import tolerance for these crops. Therefore, the derived MRLs are expected to be overestimated and full data sets supporting the import tolerance on these crops are still required;
Pecans: only residue trials analysing for the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety are available to support the import tolerance for this crop. However, the available trials are considered acceptable in this case because all results were below the LOQ and a no residues situation is expected. Further residue trials are therefore not required;
Blueberries and cranberries: although not explicitly mentioned in the current guidance document, the extrapolation from blueberries to cranberries was considered acceptable as both crops belong to the Vaccinium genus. Nevertheless, only residue trials analysing for the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety are available to support the import tolerance for these crops. Therefore, the derived MRL is expected to be overestimated and a full data set supporting the import tolerance on these crops is still required;
Bananas: the number of residue trials supporting the import tolerance is not compliant with the data requirements for this crop. Moreover, residues were only analysed for the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety. However, the available trials are considered acceptable in this case because all results were below the LOQ and a no residues situation is expected. Further residue trials are therefore not required;
Okra: trials supporting the indoor GAP were overdosed. Although tentative MRL and risk assessment values can be derived from the available data, a full data set compliant with the indoor GAP for okra is still required;
Cucurbits with inedible peel: the number of residue trials supporting the indoor GAP is not compliant with the data requirements for these crops. Moreover, the four trials on watermelons were overdosed. Although tentative MRL and risk assessment values can be derived from the available data, two additional trials on melons and 4 additional trials on watermelons, all compliant with the indoor GAP, are still required;
Beans and peas with and without pods, peanuts: only residue trials analysing for the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety are available to support the import tolerance for these crops. Moreover, all trials were performed according to a more critical GAP. Therefore, the derived MRLs are expected to be overestimated and full data sets supporting the import tolerance on these crops are still required;
Coffee beans: the number of residue trials supporting the import tolerance is not compliant with the data requirements for this crop. Moreover, only residue trials performed according to a more critical GAP and analysing for the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety are available. Therefore, the derived MRL is expected to be overestimated and a full data set supporting the import tolerance on this crop is still required.
Hops: the number of residue trials supporting the import tolerance is not compliant with the data requirements for this crop. Moreover, residues were only analysed for the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety and one of the residue trials was overdosed. Therefore, the derived MRL is expected to be overestimated and a full data set supporting the import tolerance on this crop is still required.
Available residue trials also allow deriving conversion factors from enforcement to risk assessment (CFs). Median CFs were derived for each commodity, considering only residues of parent and the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety above the LOQ. A CF of 1 was proposed when in all residue trials both imidacloprid and the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety were below the LOQ and when residues were analysed only according to the residue definition for risk assessment (mainly for the import tolerances).
It is noted that, according to the available residue trials, for some crops very high CFs were calculated. This was the case for beans without pods (derived CF of 10), dry peas (derived CF of 33) and beans without pods (derived CF of 10). In particular, in dry peas, the parent was not present at all, while the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety accounted for up to 0.53 mg/kg. Similarly, a very high CF (320) was calculated in one study on cotton seed processed into meal (see Section 1.2.3). While these results suggest that imidacloprid only could not be a sufficient marker for enforcement in pulses and oilseeds, the limited residue data available does not allow concluding if a different residue definition for enforcement is required for these crops. Therefore, it is underlined that based on the results of the additional trials on dry beans, peanuts, beans and peas without pods required to support the existing import tolerances, the residue definition for enforcement in pulses and oilseeds may need to be reconsidered.
1.2.2. Magnitude of residues in rotational crops
In the available confined rotational crop study, significant TRRs were measured in all rotated crops and at all plant‐back intervals (PBIs). The lowest TRRs were found in wheat grains and red beet roots ranging from 0.03 (PBI of 271 and 408 days) to 0.07 mg/kg (PBI of 120 days). In all other rotated crops, TRRs were higher, accounting for up to 0.26 and 0.24 mg/kg in red beet leaves and Swiss chard and for up to 1.0 and 2.38 mg/kg in wheat forage and straw, respectively. Although residues in rotated crops decreased with soil ageing, TRR after the third rotation (408 days) were still significant, ranging from 0.03 mg/kg in wheat grain to 0.96 mg/kg in wheat straw (Germany, 2005). These results suggests possible soil uptake, even at long plant‐back intervals.
Therefore, a field study was conducted with an application rate of 0.14 kg imidacloprid/ha. Following bare soil application, imidacloprid was incorporated into the soil at a depth of about 5 cm. Barley was used as a primary crop and was either destroyed and incorporated into soil simulating crop failure or grown until normal harvest, simulating normal rotation practice. Lettuce and turnip were sown as succeeding crops 30 days or 112 DAT. Since imidacloprid is used for seed treatment of cereals and several residue trials are available, small grain crops were not tested as rotational crops. At maturity, residues of imidacloprid were below the LOQ of 0.01 mg/kg in both crops and ‘total residues’ according to the risk assessment residue definition were detected in turnip leaves and leaves of immature lettuce at levels below the LOQ of 0.05 mg/kg. The parent compound was also analysed in soil. Initial imidacloprid residues in soil (0–10 cm depth) were 0.08 mg/kg and declined to a minimum of 0.04 mg/kg, 212 DAT (Germany, 2005). Considering that imidacloprid was incorporated at a depth of 5 cm, it is expected that the rotated crops were exposed to a soil concentration two times higher compared to the analysed samples (0.16 and 0.08 mg/kg soil).
As imidacloprid is a persistent active substance expected to accumulate in soil following multiannual applications and the available studies demonstrated that it can be taken up from the soil by the plant, when assessing the magnitude of residues in rotational crops, EFSA considered not only the uses compliant with the new conditions of approval, but also the possible carry‐over from the (former) authorised EU outdoor uses. In particular, in order to conclude if specific MRLs and/or risk mitigation measures should be recommended for rotational crops, imidacloprid concentrations measured in the tested soils detailed above were compared with the imidacloprid concentrations expected in soil following annual and multiannual applications according to the most critical indoor and outdoor EU GAPs, respectively.
1.2.2.1. Magnitude of residue in rotational crops considering the new conditions of approval (indoor uses only)
Considering the degradation rates of imidacloprid (see Section 1.1.2), the maximum application rate of 2 × 0.31 kg/ha per year (indoor soil application by drip irrigation on sweet peppers) assessed in this review, a soil bulk density of 1.5 g/cm3, a soil depth of 20 cm and no crop interception, the soil concentration that would result from a single year use and the plateau concentration in soil taking into account accumulation over the years were calculated as 0.203 mg/kg soil and as 0.348 mg/kg soil, respectively.
On the basis of the same assumptions on soil depth and density with no crop interception, the same calculation was also performed for the following most critical GAP currently authorised on cucurbits with edible peel (indoor soil application by drip irrigation at 2 × 0.15 kg/ha) and expected to cover also the treatment conditions of the other indoor uses. For this GAP, the soil concentration that would result from a single year use and the plateau concentration in soil were calculated as 0.098 mg/kg soil and 0.168 mg/kg soil, respectively.
According to the results of these calculations, imidacloprid concentration tested in the rotational field studies (0.16 mg/kg soil) is not covering the soil concentration expected from annual and multiannual applications according to the most critical indoor GAP currently authorised for sweet peppers (0.203 mg/kg soil and 0.348 mg/kg soil). As a consequence, following both annual and multiannual applications of imidacloprid according to this indoor GAP, a possible uptake by crops grown in rotation cannot be excluded.
Therefore, field rotational crops studies covering the most critical indoor GAP on sweet peppers are still required. In the meanwhile, Member States granting authorisations for imidacloprid should take the appropriate risk mitigation measures (e.g. restricting the use only on sweet peppers grown with soil‐less growing systems) in order to avoid the presence of significant residues in rotational crops.
For all other indoor uses assessed, based on the calculated plateau and the results of the field study, significant residues are not expected in rotational crops provided that imidacloprid is used according to the GAPs reported in this review.
1.2.2.2. Carry‐over of residues in plant commodities due to (former) authorised EU outdoor uses
On the basis of the same assumptions on soil depth and density with no crop interception, the plateau in soil was also calculated for the most critical (former) authorised EU outdoor uses which remain possible uses until December 2018 (e.g. cereal and potato seed treatment).
An annual soil application rate of 0.213 kg/ha was used for the calculation. The value of 0.213 kg/ha represents a rotation of the use on potatoes (highest dose rate 0.34 kg/ha) followed by 3 years of use on winter cereals (highest dose rate 0.17 kg/ha), in line with the approach followed for the recent risk assessment on bees (see EFSA, 2018a for further details). The accumulated plateau concentration in soil resulting from many years of this rotation was calculated as 0.05 mg/kg.
According to the results of these calculations, imidacloprid concentrations tested in the rotational field studies (0.16 mg/kg soil) is covering the soil concentration expected from the multiannual applications according to the most critical EU outdoor GAPs. Therefore, based on the field study performed with rotated lettuce and turnip, a significant carry‐over is not expected in leafy and root crops.
Considering the available metabolism study on cotton showing that, following seed treatment, imidacloprid is not translocated to the mature seeds (see Section 1.1.1), a significant carry‐over can also be excluded for pulses and oilseeds. This is also confirmed by outdoor residue trials performed on rape seed and cotton seed following seed treatment at up to 1.4 kg a.s./100 kg seeds where residues of imidacloprid and ‘total imidacloprid’ in seeds were always below the LOQs of 0.01 and 0.05 mg/kg (Germany, 2015).
Nevertheless, the confined rotational crops study showed a significant soil uptake in cereal straw, grain and forage. Therefore, in order to estimate if specific temporary MRLs are required to cover the possible carry‐over in these crops, EFSA considered the available outdoor residue trials on cereals reported in Appendix B.1.2.1. In cereals (barley and wheat), following seed treatment at 70 g a.s./100 kg seeds corresponding to 0.17 kg/ha (expected to result in a soil concentration of 0.056 mg/kg soil), residues of imidacloprid and ‘total imidacloprid’ in straw ranged from < 0.01 to 0.11 mg/kg and from < 0.02 to 0.28 mg/kg, respectively. In grain, residues of imidacloprid and ‘total imidacloprid’ were always below the LOQs of 0.01 and 0.05 mg/kg.
Based on the overall available data, it is therefore concluded that specific temporary MRLs covering the possible carry‐over from (former) outdoor EU uses are not required for any plant commodity relevant for human consumption. However, as significant residues of parent and metabolites can be expected in cereals straw, their impact on the residues in livestock was considered further in section 2.
1.2.3. Magnitude of residues in processed commodities
Studies investigating the magnitude of residues in processed commodities from apples, citrus fruits, grapes, peaches, tomatoes, cucurbits with inedible peel, beans with pods, cotton seeds, olives, potatoes, head cabbages, peanuts, coffee beans and hops were reported in the framework of this review (Germany, 2015). In all studies, except for coffee beans and peanuts, residues were analysed simultaneously for imidacloprid and for the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, in line with the proposed residue definitions.
Robust processing factors could be derived for citrus fruits (peeled and juice), apples (juice, sauce) and pears (juice), canned peaches, wine grapes (wet pomace, must, red wine and white wine), tomatoes paste, peeled cucurbits with inedible peel, beans with pods (cooked, canned), cotton seeds (crude oil) and olives for oil production (virgin oil, refined oil and press cake).
For all other processed commodities, no robust processing factors could be derived as the number of studies was not sufficient. Nevertheless, further processing studies are not required in this case as they are not expected to affect the outcome of the risk assessment. If more robust processing factors were to be required by risk managers, in particular for enforcement purposes, additional processing studies would be needed.
It is noted that in cotton seeds, parent was below the LOQ in both raw and processed commodities, while imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety were present at up to 2.7 mg/kg in the raw commodities and concentrated up to 3.2 mg/kg in meal. Similarly in beans with pods, while parent compound was present at very low levels in the raw and in the processed commodities, imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety were present at up to 0.39 mg/kg in the raw commodities and concentrated to up to 0.48 mg/kg in canned beans. As underlined in Section 1.2.1, these results suggest that imidacloprid only could not be a sufficient marker in pulses and oilseeds; nevertheless the limited data available does not allow concluding if a different residue definition for enforcement is required for these crops (see also Section 1.2.1).
1.2.4. Proposed MRLs
Consequently, when considering the magnitude of residues expected from the uses compliant with the new conditions of approval (indoor uses and import tolerances only), the available data were sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for currants, gooseberries, rose hips, mulberries, azaroles, elderberries, granate apples, lettuce and other salad plants where the available data were insufficient to derive even tentative MRLs.
Specific MRLs for rotational crops are not needed, provided that Member States will take adequate risk mitigation measures (e.g. use only on sweet peppers grown with soil‐less growing systems) in order to avoid significant residues to occur in rotational crops.
2. Residues in livestock
Imidacloprid is authorised for use on several crops (dry pulses, citrus fruits and peanuts) that might be fed to livestock. Livestock dietary burdens were therefore calculated for different groups of livestock according to OECD guidance (OECD, 2013), which has now also been agreed upon at European level. As EU outdoor GAPs are expected to be withdrawn according to the new conditions of approval, only indoor uses and import tolerances were considered for the calculation of the livestock exposure. Moreover, in order to cover the carry‐over in cereals due to the (former) authorised EU outdoor uses, the results from the available outdoor trials on wheat and barley (grain and straw) were also considered for the calculation of the livestock exposure. The input values for all relevant commodities are summarised in Appendix D.1. The dietary burdens calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM). Behaviour of residues was therefore assessed in all commodities of animal origin.
Metabolism studies in lactating goats and laying hens were submitted and evaluated during the peer review (Germany, 2005).
In lactating goats fed for three consecutive days with imidacloprid at 10 mg/kg body weight (bw) per day, the parent compound dominates the metabolic pattern in milk, fat and muscles, representing up to 74% of TRR. In liver and kidney, a more complex metabolic pattern was observed with imidacloprid almost completely degraded and several different metabolites identified. In particular, glucuronide conjugates of hydroxy‐metabolites, imidacloprid olefine metabolite (M06) and a glycine‐conjugate of 6‐chloropyridine‐3‐carboxylic acid were major constituents of the residue in kidneys accounting for 14%, 18% and 17% of the TRR, respectively. In liver, only imidacloprid‐desnitro metabolite (M09) was identified above 10% of the TRR (16% TRR).
In hens fed with imidacloprid at 10 mg/kg bw per day, parent compound was still present at significant levels only in fat (12% TRR) while imidacloprid olefine metabolite (M06) was identified as the major constituent of the residue in liver, muscle, fat and eggs, representing 15%, 27%, 23% and 29% of the TRR, respectively.
Based on the results of the available metabolism studies, it is clear that the parent compound is almost completely degraded in the liver and kidney of ruminants and in poultry tissues and in eggs. Nevertheless, on the basis of livestock exposure resulting from the uses assessed in this review, no significant residues are expected in any animal commodities. This was also demonstrated by feeding studies performed in dairy goats and laying hens, with the lowest dose being 4.8N the expected critical exposure for ruminants and 15N the expected critical exposure for poultry. Under these conditions, total imidacloprid residues (analysed as the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety) were below the LOQ (0.02 mg/kg) in milk, eggs, muscle and fat. In liver and kidneys, total residues ranged from 0.02 to 0.05 mg/kg. This shows that at the calculated dietary burdens, no significant residues are expected in all animal tissues, in milk and in eggs.
Hence, the residue definition for enforcement in all animal commodities is proposed as parent compound only (by default) and MRLs and risk assessment values for the relevant commodities in ruminants and poultry can be established at the LOQ level. These MRLs are expected to cover the possible carry‐over in cereal (grain and straw) due to the (former) authorised outdoor EU uses. For risk assessment, it is still proposed to keep the following residue definition as agreed during the peer review: sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid. As in the livestock feeding studies, residues were analysed only according to the risk assessment residue definition, a conversion factor of 1 is proposed for risk assessment.
It is underlined that, if additional uses leading to significant increase in livestock exposure will be granted in the future, the residue definition for animal commodities should be reconsidered (and eventually additional feeding studies performed according to the proposed residue definitions should be submitted).
Analytical methods for the enforcement of the proposed residue definition were evaluated during the peer review and showed that imidacloprid can be enforced in milk at the LOQ of 0.01 mg/kg and in animal tissues and in eggs at an LOQ of 0.03 mg/kg (Germany, 2008).
According to the EURL, based on the general experience with this compound, although only a screening method is available for animal commodities (except for honey validated down to 0.002 mg/kg), it is expected that imidacloprid residues can be enforced with an LOQ of 0.01 mg/kg in all commodities of animal origin (EURL, 2016).
3. Consumer risk assessment
In order to support risk managers in the decision making process, in the framework of this MRL review, three separate risk assessments were performed:
A risk assessment reflecting the EU indoor GAPs and the uses authorised in third countries (import tolerances) only, in line with the new conditions of approval for imidacloprid (Section 3.1).
A risk assessment reflecting all uses, including the EU outdoor GAPs that are expected to be withdrawn by Member States (Section 3.2). This calculation was performed to derive a list of alternative MRLs possibly safe for consumers that could be considered by risk managers to support emergency authorisations.
An indicative risk assessment considering the CXLs only (Section 3.3).
Since according to the new conditions of approval, all EU outdoor uses are expected to be withdrawn by Member States and the CXLs are not compatible with the EU MRL (see also Section 3.3 for further details), only the calculations described under Section 3.1 and reflecting the new conditions of approval were considered by EFSA as a basis for the MRL recommendations.
3.1. Consumer risk assessment without consideration of the existing CXLs – Indoor uses and import tolerances
Chronic and acute exposure calculations for the indoor uses and the import tolerances reported in the framework of this review were performed using revision 2 of the EFSA PRIMo (EFSA, 2007). This calculation is also expected to cover the possible carry‐over in cereals from the former authorised outdoor EU uses. Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix D. Hence, for those commodities where a tentative MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies (FAO, 2009). For those commodities where data were insufficient to derive an MRL in Section 1, EFSA considered the existing EU MRL for an indicative calculation. According to the RMS, MRLs in the EU legislation are currently established for the parent compound only, but are actually based on data according to the so‐called ‘total residue’ which is expected to cover the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety (Germany, 2015). Therefore, when considering the existing EU MRL, no conversion factor from enforcement to risk assessment was applied. All input values included in the exposure calculations are summarised in Appendix D.2.
The exposures calculated were compared with the toxicological reference values for imidacloprid, derived by EFSA (2008a) under Directive 91/414/EEC. The highest chronic exposure was calculated for WHO cluster diet B, representing 6% of the acceptable daily intake (ADI). With regard to the acute exposure, however, an exceedance of the acute reference dose (ARfD) was identified for escaroles, representing 109% of the ARfD. A second exposure calculation was therefore performed, excluding this crop. According to the results of this second calculation, the highest chronic exposure remained unchanged; the highest acute exposure was then calculated for cucumbers, representing 76% of the ARfD.
Based on these calculations, a potential risk to consumers was identified for the use of imidacloprid on escaroles and no further refinements of the risk assessment were possible. For the remaining commodities, although uncertainties remain due to the data gaps identified in the previous sections, the indicative exposure calculation did not indicate a risk to consumers.
3.2. Consumer risk assessment without consideration of the existing CXLs – all uses
Chronic and acute exposure calculations for all uses reported in the framework of this review were performed using revision 2 of the EFSA PRIMo (EFSA, 2007). Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix D. Hence, for those commodities where a (tentative) MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies (FAO, 2009). For those commodities where data were insufficient to derive an MRL in Section 1, EFSA considered the existing EU MRL for an indicative calculation. For the same reasons reported above, when considering the existing MRL, no conversion factor from enforcement to risk assessment was applied. All input values included in the exposure calculations are summarised in Appendix D.3.
The exposures calculated were compared with the toxicological reference values for imidacloprid, derived by EFSA (EFSA, 2008a) under Directive 91/414/EEC. The highest chronic exposure was calculated for WHO cluster diet B, representing 7% of the ADI. With regard to the acute exposure, however, an exceedance of the ARfD was identified for escaroles, sweet peppers and kale, representing 270%, 231% and 108% of the ARfD, respectively. A second exposure calculation was therefore performed, considering fall‐back GAPs for these commodities: NEU outdoor GAP for escarole and kale and EU indoor GAP for peppers. According to the results of the second calculation, the highest chronic exposure declined to 6% of the ADI for WHO Cluster diet B; the highest acute exposure was then calculated for cucumbers, representing 76% of the ARfD.
Based on these calculations, a potential risk to consumers was identified for the southern outdoor GAPs on escaroles, sweet peppers and kale. For these commodities, fall‐back GAPs were identified in order to reduce the exposure of consumers. For the remaining commodities, although uncertainties remain due to the data gaps identified in the previous sections, the indicative exposure calculation did not indicate a risk to consumers.
3.3. Indicative consumer risk assessment of the existing CXLs
As the residue definition for enforcement of the CXLs (sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid) is not compatible with the residue definition for enforcement proposed by EFSA (imidacloprid only), for information purposes, EFSA has performed an indicative risk assessment with the existing CXLs only, considering the relevant data from the JMPR evaluations (FAO, 2008, 2015).
As the CXLs and the risk assessment values from JMPR were derived according to the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, the risk assessment input values as derived by the JMPR could be directly considered for an indicative risk assessment, without applying a conversion factor. An overview of the input values used for this exposure calculation is also provided in Appendix D.4.
Chronic and acute exposure calculations were performed using revision 2 of the EFSA PRIMo and the exposures calculated were compared with the toxicological reference values derived for imidacloprid. The highest chronic exposure was calculated for WHO Cluster diet B, representing 8% of the ADI. With regard to the acute exposure, an exceedance of the ARfD was identified for celery and kale, representing 184% and 169% of the ARfD, respectively. As this indicative assessment only aims to the identification of CXLs not safe for consumers, a further refinement of the risk assessment was not performed.
These calculations indicate a potential risk to consumers for the existing CXLs on celery and kales. For the remaining CXLs, although major uncertainties remain due to the data gaps identified in the previous sections, the indicative exposure calculation did not indicate a risk to consumers. However, considering that CXLs are currently expressed according to a residue definition for enforcement not compatible with the one proposed by EFSA, they are not recommended for inclusion in the EU legislation.
Conclusions
Based on the recent EFSA conclusions on the peer review of the updated pesticide risk assessment for bees, the conditions of approval for imidacloprid were recently restricted to uses in permanent greenhouses or for the treatment of seeds intended to be used only in permanent greenhouses, with crops staying within a permanent greenhouse during its entire life cycle. Member States were required to amend or withdraw their authorisations by 19 September 2018, with a maximum period of grace expiring on the 19 December 2018.
As the GAPs and the supporting residue data considered in this MRL review were collected before the new conditions of approval entering into force, the data assessed in the present reasoned opinion are reflecting not only the uses compliant with the new conditions of approval, but also the (former) authorised EU outdoor uses. In particular, in order to support risk managers in the decision making process, EFSA considered in this assessment:
Residue data reflecting the EU indoor GAPs and the uses authorised in third countries (import tolerances) only, in line with the new conditions of approval for imidacloprid. This data was used to derive the MRL recommendations for plant and animal commodities as reported in the summary table and in Appendix B.4. These MRLs are also expected to cover the possible carry‐over from the (former) authorised EU outdoor uses.
Residue data reflecting all uses, including the EU outdoor GAPs. This data was used to derive a list of alternative MRLs possibly safe for consumers that could be considered by risk managers to support emergency authorisations. The list of alternative MRLs derived considering all uses and the results of the related risk assessment are reported respectively in Appendices G and B.3.2 to this reasoned opinion. Moreover, residue trials supporting the outdoor EU uses were also considered to assess the possible carry‐over of imidacloprid in plant and animal commodities after the entry into force of the new conditions of approval.
The metabolism of imidacloprid was investigated in primary (fruit, root and leafy crops, cereals and pulses and oilseeds) and in rotational crops (root and leafy crops, cereals). Based on the results of the metabolism in primary and rotational crops the residue definition for enforcement in plant commodities is proposed as imidacloprid only. For risk assessment, the residue definition is confirmed as the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid. The same residue definitions apply to rotational crops and processed commodities.
It is noted that results from the available residue trials suggest that imidacloprid only could not be a sufficient marker in pulses and oilseeds. Nevertheless, the limited residue data available does not allow concluding if a different residue definition for enforcement is required for these crops. Therefore, the proposed residue definition for enforcement in pulses and oilseeds should be considered tentative only and, based on the results of the additional trials on dry beans, peanuts, beans and peas without pods required to support the existing import tolerances, may need to be reconsidered.
A sufficiently validated analytical method is available for the enforcement of the proposed residue definition in high water content, high acid content and dry commodities at the LOQ of 0.01 mg/kg, high oil content at the LOQ of 0.02 mg/kg and in hops at 0.2 mg/kg. There are indications that imidacloprid can be enforced in coffee beans with an LOQ of 0.01 mg/kg; however, a confirmatory method and an ILV are still missing. According to the EURLs, during routine analyses an LOQ of 0.01 mg/kg is achievable in the four main matrices.
Regarding the magnitude of residues expected in primary crops from the uses compliant with the new conditions of approval (indoor uses and import tolerances only), the available data were sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for currants, gooseberries, rose hips, mulberries, azaroles, elderberries, granate apples, lettuce and other salad plants where the available data were insufficient to derive even tentative MRLs.
As imidacloprid is a persistent active substance expected to accumulate in soil following multiannual applications and the available studies demonstrated that it can be taken up from the soil by the plant, in the assessment of the magnitude of residues in rotational crops, EFSA considered not only the uses compliant with the new conditions of approval, but also the possible carry‐over from the (former) authorised EU outdoor uses.
When considering only the uses compliant with the new conditions of approval, it is concluded that specific MRLs for rotational crops are not needed, provided that Member States will take adequate risk mitigation measures (e.g. use only on sweet peppers grown with soil‐less growing systems) in order to avoid significant residues to occur in rotational crops.
When considering the possible carry‐over of residues in plant commodities due to (former) authorised EU outdoor uses, it is concluded that specific temporary MRLs for plant commodities are not required to cover the possible carry‐over from (former) outdoor EU uses. On other hand, as significant residues of parent and metabolites can be expected in cereals straw, their impact on the residues in livestock was considered further.
Imidacloprid is authorised for use on several crops (dry pulses, citrus fruits and peanuts) that might be fed to livestock. Livestock dietary burdens were therefore calculated for different groups of livestock according to OECD guidance. As EU outdoor GAPs are expected to be withdrawn according to the new conditions of approval, only indoor uses and import tolerances were considered for the calculation of the livestock exposure. Moreover, in order to cover the carry‐over in cereals due to the (former) authorised EU outdoor uses, the results from the available outdoor trials on wheat and barley (grain and straw) were also considered for the calculation of the livestock exposure. Since, the dietary burdens calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg DM, the behaviour of residues was assessed in all commodities of animal origin.
Metabolism studies in lactating goats and laying hens were submitted and evaluated during the peer review. According to the results of these studies is clear that parent compound is almost completely degraded in liver and kidney of ruminants and in poultry tissues and eggs, with glucuronide conjugates of hydroxy‐metabolites, imidacloprid olefine metabolite (M06) and a glycine‐conjugate of 6‐chloropyridine‐3‐carboxylic acid, representing the main identified compounds. Nevertheless, on the basis of livestock exposure resulting from the uses assessed in this review, no significant residues are expected in animal commodities. Hence, the residue definition for enforcement in all animal commodities is proposed as parent compound only (by default) and MRLs and risk assessment values for the relevant commodities in ruminants and poultry can be established at the LOQ level. These MRLs are expected to cover the possible carry‐over in cereal due to the (former) authorised outdoor EU uses. For risk assessment, it is still proposed to keep the following residue definition as agreed during the peer review: sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid. It is underlined that, if additional uses leading to significant increase in livestock exposure will be granted in the future, the residue definition for animal commodities should be reconsidered.
Analytical methods for the enforcement of the proposed residue definition were evaluated during the peer review and showed that imidacloprid can be enforced in milk at the LOQ of 0.01 mg/kg and in animal tissues and in eggs at an LOQ of 0.03 mg/kg. According to the EURLs, based on the general experience with this compound, although only a screening method is available for animal commodities (except for honey validated down to 0.002 mg/kg), it is expected that imidacloprid residues can be enforced with an LOQ of 0.01 mg/kg in all commodities of animal origin.
Chronic and acute exposure calculations resulting from the authorised indoor uses and import tolerances (in line with the new conditions of approval) reported in the framework of this review were performed using revision 2 of the EFSA PRIMo. This calculation is also expected to cover the possible carry‐over in cereals from the former authorised outdoor EU uses. For those commodities where data were insufficient to derive an MRL, EFSA considered the existing EU MRL for an indicative calculation. According to the RMS, MRLs in the EU legislation are currently established for the parent compound only, but are actually based on data according to the so‐called ‘total residue’ which is expected to cover the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety. Therefore, when considering the existing EU MRL, no conversion factor from enforcement to risk assessment was applied. Based on these calculations, a potential risk to consumers was identified for the use of imidacloprid on escaroles and no further refinements of the risk assessment were possible. For the remaining commodities, although uncertainties remain due to the data gaps identified in the assessment, the indicative exposure calculation did not indicate a risk to consumers.
Chronic and acute exposure calculations for all uses (including the former authorised outdoor EU uses) reported in the framework of this review were also performed using revision 2 of the EFSA PRIMo (EFSA, 2007). For those commodities where data were insufficient to derive an MRL in Section 1, EFSA considered the existing EU MRL for an indicative calculation. For the same reasons reported above, when considering the existing MRL, no conversion factor from enforcement to risk assessment was applied. Based on these calculations, a potential risk to consumers was identified for the southern outdoor GAPs on escaroles, sweet peppers and kale. For these commodities fall‐back GAPs were identified in order to reduce the exposure of consumers. For the remaining commodities, although uncertainties remain due to the data gaps identified in the assessment, the indicative exposure calculation did not indicate a risk to consumers.
Apart from the MRLs evaluated in the framework of this review, internationally recommended CXLs have also been established for imidacloprid. Nevertheless, as the residue definition for enforcement of the CXLs is not compatible with the residue definition for enforcement proposed in the framework of this review, for information purposes, an indicative risk assessment was performed considering the existing CXLs only. These calculations indicate a potential risk to consumers for the existing CXLs on celery and kales. For the remaining CXLs, the indicative exposure calculation did not indicate a risk to consumers. However, considering that CXLs are currently expressed according to a residue definition for enforcement not compatible with the one proposed by EFSA, they are not recommended for inclusion in the EU legislation.
Recommendations
MRL recommendations were derived in compliance with the decision tree reported in Appendix E of the reasoned opinion (see Table 2). It is underlined that only the authorised uses reflecting the new conditions of approval were considered by EFSA as a basis for the MRL recommendations. All MRL values listed as ‘Recommended’ in the table are sufficiently supported by data and are therefore proposed for inclusion in Annex II to the Regulation. The remaining MRL values listed in the table are not recommended for inclusion in Annex II because they require further consideration by risk managers (see summary table footnotes for details). In particular, some tentative MRLs and/or existing EU MRLs need to be confirmed by the following data:
Additional residue trials on citrus fruits, table and wine grapes, blueberries, cranberries, currants, gooseberries, rose hips, mulberries, azaroles, elderberries, pomegranate, cucurbits with inedible peel, okra, lettuce and other salad plants, beans and peas with and without pods, dry beans, peanuts, coffee beans and hops.
Confirmatory method and ILV of the analytical method for enforcement in coffee beans.
Table 2.
Summary table (based on GAPs compliant with the new conditions of approval)
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition: imidacloprid | |||||
| 110010 | Grapefruit | 1 | 1 | 0.9 | Further consideration neededa |
| 110020 | Oranges | 1 | 1 | 0.9 | Further consideration neededa |
| 110030 | Lemons | 1 | 1 | 0.9 | Further consideration neededa |
| 110040 | Limes | 1 | 1 | 0.9 | Further consideration neededa |
| 110050 | Mandarins | 1 | 1 | 0.9 | Further consideration neededa |
| 120010 | Almonds | 0.05* | 0.01 | – | Further consideration neededb |
| 120020 | Brazil nuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120030 | Cashew nuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120040 | Chestnuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120050 | Coconuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120060 | Hazelnuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120070 | Macadamia | 0.05* | 0.01 | – | Further consideration neededb |
| 120080 | Pecans | 0.05* | 0.01 | 0.02* | Recommendedc |
| 120090 | Pine nuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120100 | Pistachios | 0.05* | 0.01 | – | Further consideration neededb |
| 120110 | Walnuts | 0.05* | 0.01 | – | Further consideration neededb |
| 130010 | Apples | 0.5 | 0.5 | – | Further consideration neededb |
| 130020 | Pears | 0.5 | 1 | – | Further consideration neededb |
| 140010 | Apricots | 0.5 | 1.5 | – | Further consideration neededb |
| 140020 | Cherries | 0.5 | 4 | – | Further consideration neededb |
| 140030 | Peaches | 0.5 | 1.5 | – | Further consideration neededb |
| 140040 | Plums | 0.3 | 1.5 | – | Further consideration neededb |
| 151010 | Table grapes | 1 | 1 | 0.7 | Further consideration neededa |
| 151020 | Wine grapes | 1 | 1 | 0.7 | Further consideration neededa |
| 152000 | Strawberries | 0.5 | 0.5 | – | Further consideration neededb |
| 153010 | Blackberries | 5 | 5 | – | Further consideration neededb |
| 153020 | Dewberries | 5 | 5 | – | Further consideration neededb |
| 153030 | Raspberries | 5 | 5 | – | Further consideration neededb |
| 154010 | Blueberries | 5 | 5 | 5 | Further consideration neededa |
| 154020 | Cranberries | 0.05* | 0.05* | 5 | Further consideration neededa |
| 154030 | Currants (red, black and white) | 5 | 5 | 5 | Further consideration neededd |
| 154040 | Gooseberries | 5 | 5 | 5 | Further consideration neededd |
| 154050 | Rose hips | 5 | 5 | 5 | Further consideration neededd |
| 154060 | Mulberries | 5 | 5 | 5 | Further consideration neededd |
| 154070 | Azarole (Mediterranean medlar) | 0.05* | 5 | 0.05 | Further consideration neededd |
| 154080 | Elderberries | 5 | 5 | 5 | Further consideration neededd |
| 161030 | Table olives | 0.5 | 2 | – | Further consideration neededb |
| 161040 | Kumquats | 0.05* | 1 | – | Further consideration neededb |
| 163020 | Bananas | 0.05* | 0.05 | 0.01* | Recommendedc |
| 163030 | Mangoes | 0.2 | 0.2 | – | Further consideration neededb |
| 163050 | Pomegranate | 1 | 1 | 1 | Further consideration neededd |
| 211000 | Potatoes | 0.5 | 0.5 | – | Further consideration neededb |
| 212010 | Cassava | 0.5 | 0.5 | – | Further consideration neededb |
| 212020 | Sweet potatoes | 0.5 | 0.5 | – | Further consideration neededb |
| 212030 | Yams | 0.5 | 0.5 | – | Further consideration neededb |
| 212040 | Arrowroot | 0.5 | 0.5 | – | Further consideration neededb |
| 213010 | Beetroot | 0.5 | 0.5 | – | Further consideration neededb |
| 213020 | Carrots | 0.5 | 0.5 | – | Further consideration neededb |
| 213030 | Celeriac | 0.5 | 0.5 | – | Further consideration neededb |
| 213040 | Horseradish | 0.5 | 0.5 | – | Further consideration neededb |
| 213050 | Jerusalem artichokes | 0.5 | 0.5 | – | Further consideration neededb |
| 213060 | Parsnips | 0.5 | 0.5 | – | Further consideration neededb |
| 213070 | Parsley root | 0.5 | 0.5 | – | Further consideration neededb |
| 213080 | Radishes | 0.5 | 0.5 | – | Further consideration neededb |
| 213090 | Salsify | 0.5 | 0.5 | – | Further consideration neededb |
| 213100 | Swedes | 0.5 | 0.5 | – | Further consideration neededb |
| 213110 | Turnips | 0.5 | 0.5 | – | Further consideration neededb |
| 220020 | Onions | 0.1 | 0.1 | – | Further consideration neededb |
| 231010 | Tomatoes | 0.5 | 0.5 | 0.3 | Recommendedc |
| 231020 | Peppers | 1 | 1 | 0.9 | Recommendedc |
| 231030 | Aubergines (egg plants) | 0.5 | 0.2 | 0.3 | Recommendedc |
| 231040 | Okra, lady's fingers | 0.5 | ‐ | 0.5 | Further consideration needede |
| 232010 | Cucumbers | 1 | 1 | 0.5 | Recommendedc |
| 232020 | Gherkins | 0.5 | ‐ | 0.4 | Recommendedf |
| 232030 | Courgettes | 1 | 1 | 0.4 | Recommendedc |
| 233010 | Melons | 0.5 | 0.2 | 0.15 | Further consideration neededa |
| 233020 | Pumpkins | 0.5 | ‐ | 0.15 | Further consideration needede |
| 233030 | Watermelons | 0.2 | 0.2 | 0.15 | Further consideration neededa |
| 234000 | Sweet corn | 0.1 | 0.02* | – | Further consideration neededb |
| 241010 | Broccoli | 0.5 | 0.5 | – | Further consideration neededb |
| 241020 | Cauliflower | 0.5 | 0.5 | – | Further consideration neededb |
| 242010 | Brussels sprouts | 0.5 | 0.5 | – | Further consideration neededb |
| 242020 | Head cabbage | 0.5 | 0.5 | – | Further consideration neededb |
| 243020 | Kale | 0.3 | 5 | – | Further consideration neededb |
| 251010 | Lamb's lettuce | 2 | ‐ | 2 | Further consideration neededg |
| 251020 | Lettuce | 2 | 2 | 2 | Further consideration neededd |
| 251030 | Escarole (broad‐leaf endive) | 1 | ‐ | – | Further consideration neededh |
| 251040 | Cress | 2 | ‐ | 2 | Further consideration neededg |
| 251050 | Land cress | 2 | ‐ | 2 | Further consideration neededg |
| 251070 | Red mustard | 2 | ‐ | 2 | Further consideration neededg |
| 251080 | Leaves and sprouts of Brassica spp. | 2 | ‐ | 2 | Further consideration neededg |
| 256080 | Basil | 2 | 20 | – | Further consideration neededb |
| 260010 | Beans (fresh, with pods) | 2 | 2 | 5 | Further consideration neededa |
| 260020 | Beans (fresh, without pods) | 2 | 2 | 2 | Further consideration neededa |
| 260030 | Peas (fresh, with pods) | 5 | 5 | 5 | Further consideration neededa |
| 260040 | Peas (fresh, without pods) | 2 | 2 | 2 | Further consideration neededa |
| 270030 | Celery | 2 | 6 | – | Further consideration neededb |
| 270060 | Leek | 0.05* | 0.05* | – | Further consideration neededb |
| 300010 | Beans (dry) | 2 | 2 | 2 | Further consideration neededa |
| 300020 | Lentils (dry) | 2 | 2 | – | Further consideration neededb |
| 300030 | Peas (dry) | 2 | 2 | – | Further consideration neededb |
| 300040 | Lupins (dry) | 2 | 2 | – | Further consideration neededb |
| 401020 | Peanuts | 1 | 1 | 0.5 | Further consideration neededa |
| 401050 | Sunflower seed | 0.1 | 0.05* | – | Further consideration neededb |
| 401060 | Rape seed | 0.1 | 0.05* | – | Further consideration neededb |
| 401070 | Soya bean | 0.05* | 3 | – | Further consideration neededb |
| 402010 | Olives for oil production | 1 | 2 | – | Further consideration neededb |
| 500010 | Barley grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500020 | Buckwheat grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500030 | Maize grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500040 | Millet grain | 0.05* | 0.05 | – | Further consideration neededb |
| 500050 | Oats grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500060 | Rice grain | 1.5 | 0.05 | – | Further consideration neededb |
| 500070 | Rye grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500080 | Sorghum grain | 0.05* | 0.05 | – | Further consideration neededb |
| 500090 | Wheat grain | 0.1 | 0.05 | – | Further consideration neededb |
| 610000 | Tea (dried leaves and stalks,fermented or otherwise of Camellia sinensis) | 0.05* | 50 | – | Further consideration neededb |
| 620000 | Coffee beans | 1 | 1 | 1 | Further consideration neededa |
| 700000 | Hops (dried),including hop pellets and unconcentrated powder | 10 | 10 | 15 | Further consideration neededa |
| 900010 | Sugar beet (root) | 0.5 | 0.5 | – | Further consideration neededb |
| 1011010 | Swine muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1011020 | Swine fat (free of lean meat) | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1011030 | Swine liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1011040 | Swine kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1012010 | Bovine muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1012020 | Bovine fat | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1012030 | Bovine liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1012040 | Bovine kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1013010 | Sheep muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1013020 | Sheep fat | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1013030 | Sheep liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1013040 | Sheep kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1014010 | Goat muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1014020 | Goat fat | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1014030 | Goat liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1014040 | Goat kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1015010 | Horse muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1015020 | Horse fat | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1015030 | Horse liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1015040 | Horse kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1016010 | Poultry muscle | 0.05* | 0.02 | 0.03* | Recommendedc |
| 1016020 | Poultry fat | 0.05* | 0.02 | 0.03* | Recommendedc |
| 1016030 | Poultry liver | 0.05* | 0.05 | 0.03* | Recommendedc |
| 1016040 | Poultry kidney | 0.05* | 0.05 | 0.03* | Recommendedc |
| 1020010 | Cattle milk | 0.1 | 0.1 | 0.01* | Recommendedc |
| 1020020 | Sheep milk | 0.1 | 0.1 | 0.01* | Recommendedc |
| 1020030 | Goat milk | 0.1 | 0.1 | 0.01* | Recommendedc |
| 1020040 | Horse milk | 0.1 | 0.1 | 0.01* | Recommendedc |
| 1030000 | Birds’ eggs | 0.05* | 0.02 | 0.03* | Recommendedc |
| – | Other commodities of plant and animal origin | Regulation (EU) No 491/2014 | – | – | Further consideration neededi |
MRL: maximum residue level; GAP: Good Agricultural Practice; CXL: codex maximum residue limit.
* Indicates that the MRL is set at the limit of quantification.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); CXL is not compatible with EU residue definitions (combination E‐II in Appendix E).
There are no relevant INDOOR authorisations or import tolerances reported at EU level; CXL is not compatible with EU residue definitions. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐II in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; CXL is not compatible with EU residue definitions (combination G‐II in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); CXL is not compatible with EU residue definitions (combination C‐II in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination E‐I in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination G‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination C‐I in Appendix E).
GAP evaluated at EU level is not supported by data and a risk to consumers cannot be excluded for the existing EU MRL; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination B‐I in Appendix E).
There are no relevant INDOOR authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Moreover, EFSA identified the following data gap which is not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
Field rotational crops studies covering the most critical indoor GAP on sweet peppers.
Pending the submission of this study, Member States granting authorisations for imidacloprid should take the appropriate risk mitigation measures (e.g. restricting the use only on sweet peppers grown with soil‐less growing systems) in order to avoid the presence of significant residues in rotational crops.
If the above‐reported data gaps are not addressed in the future, Member States are recommended to withdraw or modify the relevant authorisations at national level. Member States are in any case recommended to withdraw the indoor GAP on escaroles currently authorised as a risk for consumers could not be excluded for this use (Table 2).
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CF
conversion factor for enforcement residue definition to risk assessment residue definition
- CXL
codex maximum residue limit
- DAR
draft assessment report
- DAT
days after treatment
- DB
dietary burden
- DM
dry matter
- DT90
period required for 90% dissipation (define method of estimation)
- EC
emulsifiable concentrate
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- EURLs
EU Reference Laboratories (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- HPLC‐MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- MW
molecular weight
- NEU
northern European Union
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- Rber
statistical calculation of the MRL by using a non‐parametric method
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SEU
southern European Union
- SL
soluble concentrate
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TAR
total applied radioactivity
- TRR
total radioactive residue
- WG
water‐dispersible granule
- WHO
World Health Organization
Appendix A – Summary of authorised uses considered for the review of MRLs
1.
| Critical outdoor GAPs for northern Europe | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max. 250 characters) | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Apples | Malus domestica | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Foliar treatment – spraying | 70 | 79 | 1 | 0.07 | 0.11 | kg a.i./ha | 14 | Covers also CZ GAP. More critical GAPs authorised in BE (2 × 0.125 g/ha) and DE (2 × 0.175 g/ha) are not sufficiently supported by data | |||
| Pears | Pyrus communis | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Foliar treatment – spraying | 71 | 79 | 1 | 0.08 | kg a.i./ha | 14 | Post‐flowering uses | ||||
| Quinces | Cydonia oblonga | NEU | Outdoor | AT | Foliar treatment – spraying | 70 | 79 | 1 | 0.07 | 0.11 | kg a.i./ha | 14 | BBCH 54 or 70‐79 or 91‐92 | |||||||
| Apricots | Armeniaca vulgaris, syn: Prunus armeniaca | NEU | Outdoor | DE | Foliar treatment – spraying | 71 | 1 | 0.11 | kg a.i./ha | 21 | Application rate for standard tree of 3 m height | |||||||||
| Peaches | Persica vulgaris, syn: Prunus persica | NEU | Outdoor | DE | Foliar treatment – spraying | 71 | 1 | 0.11 | kg a.i./ha | 21 | Application rate for standard tree of 3 m height | |||||||||
| Table grapes | Vitis vinifera | NEU | Outdoor | AT | Foliar treatment – spraying | 73 | 81 | 1 | 0.11 | kg a.i./ha | 35 | Growth stage 13–59 or 73–81 | ||||||||
| Wine grapes | Vitis vinifera | NEU | Outdoor | AT | Foliar treatment – spraying | 73 | 81 | 1 | 0.11 | kg a.i./ha | 35 | Application rate for standard tree of 3 m height | ||||||||
| Potatoes | Solanum tuberosum subsp. tuberosum | NEU | Outdoor | DE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.01 | kg a.i./100 kg | n.a. |
Sowing rate: max. 28 dt/ha (dt = 0.1 t), dose rate corresponding: 0.34 kg/ha A no residue situation can be anticipated for foliar application, CZ (1 × 0.06 kg/ha; PHI: 14) and HU GAPs (2 × 0.06 kg/ha; PHI: 28) |
||||||||
| Garlic | Allium sativum | NEU | Outdoor | DE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 4.50 | kg a.i./100 kg | n.a. |
Sowing rate: 4 u/ha 1 u = 250,000 seeds Dose rate: 0.045 kg a.i. = 0.075 kg Pdt/u (Pdt: formulated product) corresponding to 0.18 kg/ha |
||||||||
| Onions | Allium cepa Common Onion group | NEU | Outdoor | DE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 4.50 | kg a.i./100 kg | n.a. |
Sowing rate: 9 u/ha 1 u = 250,000 seeds Dose rate: 0.020 kg a.i. = 0.029 kg Pdt/u corresponding to 0.18 kg/ha |
||||||||
| Shallots | Allium cepa Aggregatum group, syn: Allium ascalonicum | NEU | Outdoor | DE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 4.50 | kg a.i./100 kg | n.a. |
Sowing rate: 9 u/ha 1 u = 250,000 seeds Dose rate: 0.020 kg a.i. = 0.029 kg Pdt/u corresponding to 0.18 kg/ha |
||||||||
| Spring onions | Allium cepa Common Onion group; Allium fistulosum | NEU | Outdoor | DE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 4.50 | kg a.i./100 kg | n.a. |
Sowing rate: 9 u/ha 1 u = 250000 seeds Dose rate: 0.020 kg a.i. = 0.029 kg Pdt/u corresponding to 0.18 kg/ha |
||||||||
| Broccoli | Brassica oleracea var. italica | NEU | Outdoor | NL | Soil treatment – general (see also comment field) | 11 | 12 | 1 | 0.18 | kg a.i./ha | n.a. |
Tray treatment before re‐planting Max. 50,000 plts (plants)/ha corresponding to 3.6 mg a.s./plant Nursery up to BBCH 12 can be in glasshouse, culture in field |
||||||||
| Cauliflowers | Brassica oleracea var. botrytis | NEU | Outdoor | NL | Soil treatment – general (see also comment field) | 11 | 12 | 1 | 0.18 | kg a.i./ha | n.a. |
Tray treatment before re‐planting Max. 50,000 plts (plants)/ha (plts: plants) corresponding to 3.6 mg a.s./plant Nursery up to BBCH 12 can be in glasshouse, culture in field |
||||||||
| Brussels sprouts | Brassica oleracea var. gemmifera | NEU | Outdoor | PL | Foliar treatment – spraying | n.a. | n.a. | 1 | 0.10 | kg a.i./ha | 14 | |||||||||
| Head cabbages | Brassica oleracea var. capitata | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Soil treatment – general (see also comment field) | 11 | 12 | 1 | 0.18 | kg a.i./ha | n.a. |
Tray treatment before re‐planting Max. 50,000 plts (plants)/ha corresponding to 3.6 mg a.s./plant Nursery up to BBCH 12 can be in glasshouse, culture in field |
||||
| Chinese cabbages | Brassica rapa subsp. pekinensis | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Soil treatment – general (see also comment field) | 11 | 12 | 1 | 0.18 | kg a.i./ha | n.a. |
Tray treatment before re‐planting Max. 50,000 plts (plants)/ha corresponding to 3.6 mg a.s./plant Nursery up to BBCH 12 can be in glasshouse, culture in field |
||||
| Kales | Brassica oleracea var. sabellica; Brassica oleracea var. viridis | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Soil treatment – general (see also comment field) | 11 | 12 | 1 | 0.18 | kg a.i./ha | n.a. |
Tray treatment before re‐planting Max. 50,000 plts (plants)/ha corresponding to 3.6 mg a.s./plant Nursery up to BBCH 12 can be in glasshouse, culture in field |
||||
| Kohlrabies | Brassica oleracea var. gongylodes | NEU | Outdoor | DE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.16 | kg a.i./unit | n.a. |
Sowing rate: 0.9 seed units/ha Dose rate: 0.164 kg a.i./seed unit corresponding to 0.15 kg/ha Leaves nor suitable for human or animal consumption |
||||||||
| Lettuces | Lactuca sativa | NEU | Outdoor | NL, CZ, BE, DE | Insects | WG | 700.0 | g/kg | Seed treatment – general | 0 | 0 | 1 | 0.12 | kg a.i./ha | n.a. | |||||
| Escaroles | Cichorium endivia var. latifolia | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Seed treatment – general | 0 | 0 | 1 | 0.12 | kg a.i./ha | n.a. | |||||
| Cresses | Lepidium sativum subsp. sativum | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Seed treatment – general | 0 | 0 | 1 | 0.12 | kg a.i./ha | n.a. | |||||
| Land cresses | Barbarea verna | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.12 | kg a.i./ha | n.a. | |||||
| Red mustards | Brassica juncea var. rugosa | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Seed treatment – general | 0 | 0 | 1 | 0.12 | kg a.i./ha | n.a. | |||||
| Baby leaf crops | Not specified | NEU | Outdoor | NL | Insects | WG | 700.0 | g/kg | Seed treatment – general | 0 | 0 | 1 | 0.12 | kg a.i./ha | n.a. | |||||
| Witloofs | Cichorium intybus Foliosum group | NEU | Outdoor | NL | insects | WG | 700.0 | g/kg | Seed treatment – spraying (see also comment field) | 0 | 0 | 1 | 0.18 | kg a.i./ha | n.a. | Treatment on the seed in the furrow | ||||
| Leeks | Allium ampeloprasum ampeloprasum Leek group, syn: Allium porrum | NEU | Outdoor | DE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.05 | kg a.i./unit | n.a. |
Sowing rate: 2 u/ha 1u = 250,000 seeds Dose rate: 0.045 kg a.i. = 0.0643 kg Ptd/u = 0.09 kg a.i./ha |
||||||||
| Barley | Hordeum vulgare | NEU | Outdoor | FR, BE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.07 | kg a.i./100 kg | n.a. |
Sowing rate: 1.8 dt/ha Dose rate: 0.13 kg a.i./ha Winter cereals only |
||||||||
| Oat | Avena sativa | NEU | Outdoor | FR | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.07 | kg a.i./100 kg | n.a. |
Sowing rate: 1.6 dt/ha Dose rate: 0.070 kg a.i./dt = 0.200 L Pdt (product)/dt = 0.11 kg/ha Winter cereals only |
||||||||
| Rye | Secale cereale | NEU | Outdoor | FR | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.07 | kg a.i./100 kg | n.a. |
Sowing rate: 1.8 dt/ha Dose rate: 0.070 kg a.i./dt = 0.200 L Pdt/dt = 0.13 kg/ha Winter cereals only |
||||||||
| Wheat | Triticum aestivum | NEU | Outdoor | FR | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.07 | kg a.i./100 kg | n.a. |
Sowing rate: 2.4 dt/ha Dose rate: 0.070 kg a.i./dt = 0.17 kg/ha Winter cereals only |
||||||||
| Hops | Humulus lupulus | NEU | Outdoor | DE, CZ, BE | Foliar treatment – spraying | 35 | 85 | 1 | 0.12 | kg a.i./ha | 35 | |||||||||
| Sugar beets | Beta vulgaris subsp. vulgaris var. altissima | NEU | Outdoor | DE, FR, CZ, BE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.09 | kg a.i./unit | n.a. |
Sowing rate: 1.3 u/ha 1 u = 100,000 seeds Dose rate: 0.090 kg a.i. = 0.150 kg Pdt (product)/u = 0.118 kg a.i./ha |
||||||||
| Fodder beets | Beta vulgaris subsp. vulgaris var. crassa | NEU | Outdoor | DE, FR, CZ, BE | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.09 | kg a.i./unit | n.a. |
Sowing rate: 1.3 u/ha 1 u = 100,000 seeds Dose rate: 0.090 kg a.i. = 0.118 kg a.i./ha |
||||||||
| Critical outdoor GAPs for southern Europe | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max. 250 characters) | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Grapefruits | Citrus paradisi | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 69 | 1 | 0.18 | 0.30 | kg a.i./ha | 14 | A more critical GAP authorised in PT (2 × 0.3 kg/ha) is not sufficiently supported by data (only 3 trials available) | ||||
| Oranges | Citrus sinensis | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 69 | 1 | 0.18 | 0.30 | kg a.i./ha | 14 | A more critical GAP authorised in PT (2 × 0.3 kg/ha) is not sufficiently supported by data (only 3 trials available) | ||||
| Lemons | Citrus limon | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 69 | 1 | 0.30 | kg a.i./ha | 14 | ||||||
| Limes | Citrus aurantiifolia | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 69 | 1 | 0.30 | kg a.i./ha | 14 | ||||||
| Mandarins | Citrus reticulata, syn: Citrus deliciosa | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 69 | 1 | 0.30 | kg a.i./ha | 14 | ||||||
| Almonds | Amygdalus communis, syn: Prunus dulcis | SEU | Outdoor | IT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 69 | 1 | 0.07 | 0.10 | kg a.i./ha | 14 | |||||
| Apples | Malus domestica | SEU | Outdoor | IT | Aphids, Psylla, leaf miners | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 79 | 1 | 0.07 | 0.15 | kg a.i./ha | 14 | More critical/different GAPs authorised in ES (2 × 0.175 kg/ha) and in PT (2 × 0.1 kg/ha) are not sufficiently supported by data | |||
| Pears | Pyrus communis | SEU | Outdoor | EL | Cacopsylla pyri | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 79 | 1 | 0.15 | kg a.i./ha | 7 | Covers also IT GAP. More critical/different GAPs authorised in ES (2 × 0.175 kg/ha) and in PT (2 × 0.1 kg/ha) are not sufficiently supported by data | ||||
| Apricots | Armeniaca vulgaris, syn: Prunus armeniaca | SEU | Outdoor | EL, PT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 79 | 2 | 0.08 | 0.15 | kg a.i./ha | 14 | ||||
| Cherries | Cerasus avium, syn: Prunus avium | SEU | Outdoor | PT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 2 | 0.07 | 0.10 | kg a.i./ha | 14 | ||||
| Peaches | Persica vulgaris, syn: Prunus persica | SEU | Outdoor | ES, PT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 79 | 2 | 0.10 | 0.15 | kg a.i./ha | 14 | ||||
| Plums | Prunus domestica | SEU | Outdoor | ES | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 79 | 2 | 0.10 | kg a.i./ha | 21 | A different GAP authorised in IT (1 × 0.15 kg/ha; PHI: 14 days) is not sufficiently supported by data | ||||
| Table grapes | Vitis vinifera | SEU | Outdoor | ES, PT, IT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 81 | 1 | 0.10 | kg a.i./ha | 14 | |||||
| Wine grapes | Vitis vinifera | SEU | Outdoor | ES, PT, IT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 81 | 1 | 0.10 | kg a.i./ha | 14 | |||||
| Table olives | Olea europaea | SEU | Outdoor | ES | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 79 | 85 | 1 | 5 | 0.02 | kg a.i./ha | 7 | Covers also IT GAP | |||
| Avocados | Persea americana | SEU | Outdoor | PT | Avocado lace bug | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 2 | 0.20 | kg a.i./ha | 30 | |||||
| Mangoes | Mangifera indica | SEU | Outdoor | PT | APHIDS | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 2 | 0.20 | kg a.i./ha | 30 | |||||
| Potatoes | Solanum tuberosum subsp. tuberosum | SEU | Outdoor | PT | Aphids and Colorado potato beetle | SL | 200.0 | g/L | Foliar treatment – spraying | 45 | 97 | 1 | 2 | 0.10 | 0.13 | kg a.i./ha | 14 | Covers also EL and IT GAPs. A different GAP (in‐furrow application at 2 × 0.175) authorised in ES is not supported by data | ||
| Tomatoes | Lycopersicon esculentum | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.15 | kg a.i./ha | 3 | Covers drip application and EL GAP (2 × 0.1; PHI: 7 days) | |||||
| Sweet peppers | Capsicum annuum | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.15 | kg a.i./ha | 3 | ||||||
| Aubergines | Solanum melongena | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.15 | kg a.i./ha | 3 | Covers drip application and EL GAP (2 × 0.1; PHI: 7 days) | |||||
| Okra | Abelmoschus esculentus | SEU | Outdoor | PT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 0.10 | kg a.i./ha | 3 | ||||||
| Cucumbers | Cucumis sativus | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 0.15 | kg a.i./ha | 7 | ||||||
| Gherkins | Cucumis sativus | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 0.15 | kg a.i./ha | 7 | ||||||
| Courgettes | Cucurbita pepo Zucchini group | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 0.15 | kg a.i./ha | 7 | ||||||
| Melons | Cucumis melo | SEU | Outdoor | IT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 0.15 | kg a.i./ha | 7 | A different GAP authorised in PT (2 × 0.10 kg/ha; PHI: 3 days) is not sufficiently supported by data | |||||
| Pumpkins | Cucurbita maxima | SEU | Outdoor | PT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 2 | 0.10 | kg a.i./ha | 3 | |||||
| Watermelons | Citrullus vulgaris, syn: Citrullus lanatus | SEU | Outdoor | IT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 0.15 | kg a.i./ha | 7 | A different GAP authorised in PT (2 × 0.10 kg/ha; PHI: 3 days) is not sufficiently supported by data | |||||
| Broccoli | Brassica oleracea var. italica | SEU | Outdoor | ES, PT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 13 | 48 | 2 | 0.03 | 0.12 | kg a.i./ha | 14 | ||||
| Cauliflowers | Brassica oleracea var. botrytis | SEU | Outdoor | ES, PT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 13 | 48 | 1 | 2 | 0.03 | 0.12 | kg a.i./ha | 14 | |||
| Brussels sprouts | Brassica oleracea var. gemmifera | SEU | Outdoor | ES | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 13 | 48 | 2 | 0.03 | 0.12 | kg a.i./ha | 15 | ||||
| Head cabbages | Brassica oleracea var. capitata | SEU | Outdoor | IT, PT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 13 | 1 | 0.10 | kg a.i./ha | 14 | ||||||
| Chinese cabbages | Brassica rapa subsp. pekinensis | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 69 | 1 | 0.10 | kg a.i./ha | 14 | ||||||
| Kales | Brassica oleracea var. sabellica; Brassica oleracea var. viridis | SEU | Outdoor | IT, PT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 69 | 1 | 0.10 | kg a.i./ha | 14 | ||||||
| Lamb's lettuces | Valerianella locusta | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Lettuces | Lactuca sativa | SEU | Outdoor | ES | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 13 | 48 | 2 | 0.11 | 0.13 | kg a.i./ha | 7 | ||||
| Escaroles | Cichorium endivia var. latifolia | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Cresses | Lepidium sativum subsp. sativum | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Land cresses | Barbarea verna | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Roman rocket | Eruca sativa | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Red mustards | Brassica juncea var. rugosa | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Baby leaf crops | Not specified | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Chervil | Anthriscus cerefolium | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Chives | Allium schoenoprasum | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Celery leaves | Apium graveolens var. secalinum | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Parsley | Petroselinum crispum | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Sage | Salvia officinalis | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Rosemary | Rosmarinus officinalis | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Thyme | Thymus vulgaris | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Basil | Ocimum basilicum | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Laurel | Laurus nobilis | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Tarragon | Artemisia dracunculus | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 20 | 1 | 0.04 | 0.10 | kg a.i./ha | 3 | |||||
| Beans (with pods) | Phaseolus vulgaris | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 0.10 | kg a.i./ha | 3 | ||||||
| Beans (without pods) | Phaseolus vulgaris | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 0.10 | kg a.i./ha | 3 | ||||||
| Cardoons | Cynara cardunculus Cardoon group | SEU | Outdoor | IT | Aphids | OD | 200.0 | g/L | Foliar treatment – spraying | 41 | 47 | 1 | 0.08 | 0.10 | kg a.i./ha | 3 | ||||
| Globe artichokes | Cynara cardunculus Globe artichoke group | SEU | Outdoor | EL | Foliar treatment – spraying | 13 | 48 | 2 | 0.10 | kg a.i./ha | 3 | |||||||||
| Peas (dry) | Pisum sativum | SEU | Outdoor | IT | Foliar treatment – spraying | 71 | 77 | 1 | 2 | 0.08 | 0.09 | kg a.i./ha | 28 | |||||||
| Cotton seeds | Gossypium barbadense; Gossypium herbaceum | SEU | Outdoor | EL | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 83 | 2 | 14 | 0.10 | kg a.i./ha | 28 | ||||
| Olives for oil production | Olea europaea var. europaea | SEU | Outdoor | IT | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 69 | 1 | 0.13 | kg a.i./ha | 7 | Trials reported expected to cover also the EL GAP (2 × 0.08; PHI: 7 days) | |||||
| Barley | Hordeum vulgare | SEU | Outdoor | FR | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.07 | kg a.i./100 kg | n.a. |
Sowing rate: 1.6 dt/ha Dose rate: 0.070 kg a.i./dt = 0.200 L Pdt (product)/dt = 0.11 kg a.i./ha |
||||||||
| Oat | Avena sativa | SEU | Outdoor | FR | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.07 | kg a.i./100 kg | n.a. |
Sowing rate: 1.6 dt/ha Dose rate: 0.070 kg a.i./dt = 0.200 L Pdt/dt = 0.11 kg a.i./ha |
||||||||
| Wheat | Triticum aestivum | SEU | Outdoor | FR | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.07 | kg a.i./100 kg | n.a. |
Sowing rate: 1.6 dt/ha Dose rate: 0.070 kg a.i./dt = 0.200 L Pdt/dt = 0.11 kg a.i./ha |
||||||||
| Sugar beets | Beta vulgaris subsp. vulgaris var. altissima | SEU | Outdoor | ES, EL | Seed treatment – general (see also comment field) | 0 | 0 | 1 | 0.09 | kg a.i./unit | n.a. |
Sowing rate: 1.5– 1.8 u/ha 1 u = 100,000 seeds Dose rate: 0.091 kg a.i./u = 0.130 kg Pdt/u |
||||||||
| Critical indoor GAPs for northern and southern Europe (including post‐harvest treatments) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Region | Outdoor/Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max. 250 characters) | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Tomatoes | Lycopersicon esculentum | NEU/SEU | Indoor | ES, EL | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 15 | 83 | 1 | 2 | 14 | 0.10 | 0.15 | kg a.i./ha | 3 | Covers the IT and PT GAPs. A more critical GAP authorised in AT and FI (2 × 0.25 kg/ha; PHI: 1 day) is not supported by data | |
| Sweet peppers | Capsicum annuum | NEU/SEU | Indoor | FI | Soil treatment – general (see also comment field) | 10 | 89 | 1 | 2 | 0.31 | kg a.i./ha | 3 | Apply in irrigation water | |||||||
| Aubergines | Solanum melongena | NEU/SEU | Indoor | ES, EL | Aphids, white fly | SL | 200.0 | g/L | Foliar treatment – spraying | 15 | 83 | 1 | 2 | 14 | 0.10 | 0.15 | kg a.i./ha | 3 | Covers treatment by drip irrigation (3 × 0.14; PHI: 1 day) | |
| Okra | Abelmoschus esculentus | NEU/SEU | Indoor | PT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 1 | 0.10 | kg a.i./ha | 3 | ||||||
| Cucumbers | Cucumis sativus | NEU/SEU | Indoor | NL | Aphids | WG | 700.0 | g/kg | Soil treatment – general (see also comment field) | 11 | 81 | 2 | 0.01 | kg a.i./unit | 1 | Unit: 1,000 plants corresponding to 10 mg/plant. Apply in irrigation water | ||||
| Gherkins | Cucumis sativus | NEU/SEU | Indoor | AT | Soil treatment – general (see also comment field) | 10 | 89 | 2 | 0.15 | kg a.i./ha | 1 | Apply in irrigation water max. 15,000 plts/ha | ||||||||
| Courgettes | Cucurbita pepo Zucchini group | NEU/SEU | Indoor | AT | Soil treatment – general (see also comment field) | 10 | 89 | 2 | 0.15 | kg a.i./ha | 1 | Apply in irrigation watermax. 15,000 plts (plants)/ha | ||||||||
| Melons | Cucumis melo | NEU/SEU | Indoor | PT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 2 | 0.10 | kg a.i./ha | 3 | A more critical GAP authorised in EL (2 × 0.15 kg/ha) is not sufficiently supported by data | |||||
| Watermelons | Citrullus vulgaris, syn: Citrullus lanatus | NEU/SEU | Indoor | PT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 2 | 0.10 | kg a.i./ha | 3 | A more critical GAP authorised in EL (2 × 0.15 kg/ha) is not sufficiently supported by data | |||||
| Pumpkins | Cucurbita maxima | NEU/SEU | Indoor | PT | Aphids | SL | 200.0 | g/L | Foliar treatment – spraying | 71 | 2 | 0.10 | kg a.i./ha | 3 | ||||||
| Lamb's lettuces | Valerianella locusta | NEU/SEU | Indoor | IT | Sucking insects | WG | 700.0 | g/kg | Soil treatment – general (see also comment field) | 0 | 18 | 1 | 0.12 | kg a.i./ha | n.a. | Watering, before planting | ||||
| Lettuces | Lactuca sativa | NEU/SEU | Indoor | IT, CZ, DE | Sucking insects | WG | 700.0 | g/kg | Soil treatment – general (see also comment field) | 0 | 18 | 1 | 0.12 | kg a.i./ha | n.a. | Watering, before planting | ||||
| Escaroles | Cichorium endivia var. latifolia | NEU/SEU | Indoor | DE, IT | Sucking insects | SC | 200.0 | g/L | Soil treatment – general (see also comment field) | 0 | 18 | 1 | 0.12 | kg a.i./ha | n.a. | Watering, before planting | ||||
| Cresses | Lepidium sativum subsp. sativum | NEU/SEU | Indoor | DE, IT | Sucking insects | SC | 200.0 | g/L | Soil treatment – general (see also comment field) | 0 | 18 | 1 | 0.12 | kg a.i./ha | n.a. | Watering, before planting | ||||
| Land cresses | Barbarea verna | NEU/SEU | Indoor | DE, IT | Sucking insects | SC | 200.0 | g/L | Soil treatment – general (see also comment field) | 0 | 18 | 1 | 0.12 | kg a.i./ha | n.a. | Watering, before planting | ||||
| Red mustards | Brassica juncea var. rugosa | NEU/SEU | Indoor | DE, IT | Sucking insects | SC | 200.0 | g/L | Soil treatment – general (see also comment field) | 0 | 18 | 1 | 0.12 | kg a.i./ha | n.a. | Watering, before planting | ||||
| Baby leaf crops | Not specified | NEU/SEU | Indoor | DE, IT | Sucking insects | SC | 200.0 | g/L | Soil treatment – general (see also comment field) | 0 | 18 | 1 | 0.12 | kg a.i./ha | n.a. | Watering, before planting | ||||
| Beans (with pods) | Phaseolus vulgaris | NEU/SEU | Indoor | IT | Foliar treatment – spraying | 1 | 0.10 | kg a.i./ha | 3 | |||||||||||
| Critical GAPs for import tolerances (non‐European indoor, outdoor or post‐harvest treatments) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max 250 characters) | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Grapefruits | Citrus paradisi | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 2 | 0.14 | 0.28 | kg a.i./ha | 0 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Oranges | Citrus sinensis | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 2 | 0.14 | 0.28 | kg a.i./ha | 0 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Lemons | Citrus limon | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 2 | 0.14 | 0.28 | kg a.i./ha | 0 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Limes | Citrus aurantiifolia | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 2 | 0.14 | 0.28 | kg a.i./ha | 0 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Mandarins | Citrus reticulata, syn: Citrus deliciosa | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 2 | 0.14 | 0.28 | kg a.i./ha | 0 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Pecans | Carya illinoinensis | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 4 | 0.05 | 0.10 | kg a.i./ha | 7 |
Maximum rate allowed per crop season: 0.40 kg a.i./ha GAP USA: 3 × 0.112 kg a.i./ha |
|||||||||
| Table grapes | Vitis vinifera | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 2 | 0.06 | kg a.i./ha | 0 | It covers also application, e.g. via chemigation into root zone: subsurface side‐dress or hill drench with maximum rate allowed per crop season: 0.56 kg a.i./ha | |||||||||
| Wine grapes | Vitis vinifera | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 2 | 0.06 | kg a.i./ha | 0 | It covers also application, e.g. via chemigation into root zone: subsurface side‐dress or hill drench with maximum rate allowed per crop season: 0.56 kg a.i./ha | |||||||||
| Blueberries | Vaccinium angustifolium; Vaccinium corymbosum; Vaccinium formosum; Vaccinium virgatum | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 5 | 0.04 | 0.11 | kg a.i./ha | 3 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Cranberries | Vaccinium macrocarpon | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 5 | 0.04 | 0.11 | kg a.i./ha | 3 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Currants | Ribes nigrum; Ribes rubrum | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 5 | 0.04 | 0.11 | kg a.i./ha | 3 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Gooseberries | Ribes uva‐crispa | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 5 | 0.04 | 0.11 | kg a.i./ha | 3 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Rose hips | Rosa canina; Rosa majalis; Rosa rugosa | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 5 | 0.04 | 0.11 | kg a.i./ha | 3 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Mulberries | Morus alba; Morus nigra | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 5 | 0.04 | 0.11 | kg a.i./ha | 3 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Azaroles | Crataegus azarolus | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 5 | 0.04 | 0.11 | kg a.i./ha | 3 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Elderberries | Sambucus nigra | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 5 | 0.04 | 0.11 | kg a.i./ha | 3 | Maximum rate allowed per crop season: 0.56 kg a.i./ha | ||||||||
| Bananas | Musa acuminata; Musa balbisiana; Musa acuminata x Musa balbisiana | Non‐EU | Outdoor | Cameroon | Local treatment – drenching | 0.50 | kg a.i./ha | n.a. | Application done at the base of the plant by drenching. The dose rate corresponds to 0.25 g per plant with 2000 plants/ha. | |||||||||||
| Pomegranate (Granate apples) | Punica granatum | Non‐EU | Outdoor | USA | Soil treatment – general (see also comment field) | 1 | 2 | 0.28 | 0.56 | kg a.i./ha | 0 | Maximum rate allowed per crop season: 0.56 kg a.i./ha application via chemigation into the root zone | ||||||||
| Beans (with pods) | Phaseolus vulgaris | Non‐EU | Outdoor | USA | Soil treatment – general (see also comment field) | 1 | 2 | 0.28 | 0.42 | kg a.i./ha | 21 | USA: foliar spray 1–3 × 0.049 kg a.i./ha | ||||||||
| Beans (without pods) | Phaseolus vulgaris | Non‐EU | Outdoor | USA | Soil treatment – general (see also comment field) | 1 | 2 | 0.28 | 0.42 | kg a.i./ha | 21 | USA: foliar spray 1–3 × 0.049 kg a.i./ha | ||||||||
| Peas (with pods) | Pisum sativum | Non‐EU | Outdoor | USA | Soil treatment – general (see also comment field) | 1 | 2 | 0.28 | 0.42 | kg a.i./ha | 21 | Maximum rate allowed per crop season: 0.42 kg a.i./ha; Application e.g. via chemigation into root zone; narrow band or in‐furrow spray or post‐seeding drench | ||||||||
| Peas (without pods) | Pisum sativum | Non‐EU | Outdoor | USA | Soil treatment – general (see also comment field) | 1 | 2 | 0.28 | 0.42 | kg a.i./ha | 21 | Maximum rate allowed per crop season: 0.42 kg a.i./ha; Application e.g. via chemigation into root zone; narrow band or in‐furrow spray or post‐seeding drench | ||||||||
| Beans (dry) | Phaseolus vulgaris | Non‐EU | Outdoor | USA | Foliar treatment – general (see also comment field) | 1 | 2 | 0.28 | 0.52 | kg a.i./ha | 7 | Seed treatment + soil application + foliar spray applications | ||||||||
| Peanuts | Arachis hypogaea | Non‐EU | Outdoor | USA | Soil treatment – general (see also comment field) | 1 | 0.28 | 0.42 | kg a.i./ha | 14 | Maximum rate allowed per crop season: 0.42 kg a.i./haApplication via in‐furrow spray during planting or chemigation into root zone | |||||||||
| Coffee beans | Coffea arabica; Coffea canephora, syn: Coffea robusta; Coffea liberica | Non‐EU | Outdoor | USA | Soil treatment – general (see also comment field) | 1 | 2 | 0.28 | 0.56 | kg a.i./ha | 7 | USA: foliar spray 1–5 × 0.112 kg a.i./ha | ||||||||
| Hops | Humulus lupulus | Non‐EU | Outdoor | USA | Foliar treatment – spraying | 1 | 3 | 0.11 | kg a.i./ha | 28 | Maximum rate allowed per crop season: 0.34 kg a.i./ha | |||||||||
MRL: maximum residue level; GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient; WG: water‐dispersible granule; SL: soluble concentrate; a.s.: active substance; SC: suspension concentrate.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) |
|---|---|---|---|---|
| Fruit crops | Apples | Foliar, 375 g a.s./ha | 0, 14 | |
| Tomatoes | Foliar, 0.25 kg/ha | 4, 7, 14, 21 | ||
| Eggplants | Soil granules, 400 g/ha | 14, 35, 49, 67, 69 | ||
| Root crops | Potatoes | Soil granules, 1250 g/ha | 129 | |
| Foliar, 134 g/ha | 7, 28, 64 | |||
| Leafy crops | Tobacco | 1 Soil and 3 foliar, 740 g/ha (total rate) | 14 | |
| Cereals/grass crops | Rice | Soil granules, 500 g/ha | 79 | |
| Seed (nursery box), 320 and 1260 g/ha with 200 box/ha | 65, 124 | |||
| Maize | Seed, 721 g/100 kg seeds | 33, 61, 134 | ||
| Pulses/oilseeds | Cotton | Seed, 460 g/100 kg seeds | 211 | |
|
Metabolism studies with [Pyridinyl‐14C‐methylene]‐imidacloprid only, considered acceptable Source: Germany (2005) | ||||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) |
|---|---|---|---|---|
| Root/tuber crops | Red beet | Bare soil, 454 g a.s./ha | 30, 120, 271 | |
| Leafy crops | Swiss chards | Bare soil, 454 g a.s./ha | 30, 120, 271 | |
| Cereal (small grain) | Wheat | Bare soil, 454 g a.s./ha | 30, 120, 271 | |
| Source: Germany (2005) | ||||
| Processed commodities (hydrolysis study) | Conditions | Investigated? |
|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | |
| Sterilisation (20 min, 120°C, pH 6) | Yes | |
| Source: Germany (2005) | ||
| Can a general residue definition be proposed for primary crops? | Yes |
| Rotational crop and primary crop metabolism similar? | Yes |
| Residue pattern in processed commodities similar to residue pattern in raw commodities? | Yes |
| Plant residue definition for monitoring (RD‐Mo) |
All plant commodities with exception of pulses and oilseeds: Imidacloprid Pulses and oilseeds: Imidacloprid (tentative, pending submission of trials supporting the import tolerances on dry beans, peanuts, beans and peas without pods) |
| Plant residue definition for risk assessment (RD‐RA) | Sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid |
| Conversion factor (monitoring to risk assessment) | See Appendix B.1.2.1 |
| Methods of analysis for monitoring of residues (analytical technique, crop groups, LOQs) |
High water content, high acid content, dry commodities:
High oil content commodities:
Hops:
Coffee beans (validated in cocoa beans):
According to the EURLs, during routine analyses an LOQ of 0.01 mg/kg is achievable in the four main matrices (EURLs, 2016) |
a.s.: active substance; DAT: days after treatment; PBI: plant‐back interval; QuEChERS: Quick, Easy, Cheap, Effective, Rugged, and Safe; HPLC–MS/MS: high‐performance liquid chromatography with tandem mass spectrometry; LOQ: limit of quantification; ILV: independent laboratory validation.
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability (months/years) |
|---|---|---|---|---|
| High water content | Apples, potatoes, lettuce, cauliflowers, tomatoes, sugar beet | –18 | 24 months | |
| High oil content | Cotton seeds, sunflower seeds | –18 | 24 months | |
| Dry | Corn, wheat, barley | –18 | 24 months | |
| High acid content | Lemons, oranges | –18 | 24 months | |
| Others | Straw, hops (dry cones) | –18 | 24 months | |
|
Source: Germany (2005) The demonstrated storage stability period covers both imidacloprid and the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety Additional storage stability studies covering the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety were assessed by the JMPR. According to these studies, the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety is stable for up to 53 months in high oil content matrices (peanuts), for up to 41 months in high water content commodities (radish roots), for up to 34 months in dry commodities (dry peas) and for up to 25 months in coffee, stored under deep frozen conditions (FAO, 2008) | ||||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) | Recommendations/comments (OECD calculations) | MRL proposals (mg/kg) | HRMo (mg/kg)b | STMRMo (mg/kg)c | CFd |
|---|---|---|---|---|---|---|---|
| Numbers in bold represent MRL and risk assessment values derived from uses compliant with the new conditions of approval in EU | |||||||
| Citrus fruits | SEU |
Oranges Mo: 0.082; 0.17; 0.10; 0.072; 0.067; 0.093; 0.087; 0.21 RA: 0.15; 0.23; 0.12; 0.20; 0.081; 0.11; 0.13; 0.23 Mandarins Mo: 0.10; 0.23; 0.19; 0.17; 0.19; 0.17; 0.21; 0.25 RA: 0.13; 0.35; 0.24; 0.20; 0.26; 0.25; 0.29; 0.34 |
Combined data set on oranges and mandarins compliant with GAP (Germany, 2015). Extrapolation to all citrus fruits possible MRLOECD = 0.45 |
0.5 | 0.25 | 0.17 | 1.4 |
| Import (USA) |
Grapefruits Mo: – RA: 0.30; 0.32; 0.17; 0.17; 0.14; 0.18 Oranges Mo: – RA: 0.61; 0.28; 0.18; 0.26; 0.29; 0.26; 0.34; 0.21; 0.15; 0.36; 0.36; 0.37 Lemons Mo: – RA: 0.31; 0.62; 0.33; 0.19; 0.265 |
Combined data set on grapefruit, oranges and lemons compliant with GAP for citrus fruits. Residues analysed only according to the risk assessment residue definition (Germany, 2015) MRLOECD = 0.87 |
0.9 (tentative)e | 0.62 | 0.28 | 1.0 | |
| Almonds | SEU |
Mo: < 0.01 RA: < 0.05 |
Residue trial performed with 2 applications instead of 1 is acceptable as the first application done at an early growth stage is not expected to have an impact on the final residue level and residues were below the LOQ (Italy, 2016a). Trials performed according to a more critical GAP and used to support the import tolerance on pecans, confirm a no residue situation | 0.02* | 0.02 | 0.02 | 1.0 |
| Pecans | Import (USA) |
Pecans: Mo: – RA: 5 x < 0.01 Almonds: Mo: – RA: 5 x < 0.01 |
Combined data set on pecans and almonds performed at 2 × 0.2 kg/ha. Residues analysed only according to the risk assessment residue definition but trials acceptable since residues were below the LOQ (Germany, 2015) | 0.02 * | 0.02 | 0.02 | 1.0 |
|
Apples Quinces |
NEU |
Mo: 0.04; 0.03; 0.02; 0.02; 0.01; < 0.01; < 0.01; 0.01; 0.013; 0.017; < 0.01; 0.02; 0.03; 0.02 RA: 0.08; 0.06; < 0.05; < 0.05; < 0.05; < 0.05; < 0.05; < 0.05; 0.08; < 0.05; < 0.05; < 0.05; 0.05; 0.06 |
Trials on apples. First 8 trials compliant with GAP or with dose rate within 25% deviation. Other trials performed with 2 applications acceptable since the first application done at an early stage is not expected to impact the final residue level (Germany, 2015). Extrapolation to quinces possible MRLOECD = 0.06 |
0.06 | 0.04 | 0.02 | 2.5 |
| SEU |
Apples Mo: 0.04; 0.02; 0.017; 0.023; 0.023; < 0.01; 0.011; 0.013 RA: 0.06; 0.06; 0.086; 0.046; 0.035; < 0.03; < 0.03; < 0.03 Pears Mo: 0.06; 0.035; 0.018 RA: 0.08; 0.052; 0.081 |
Combined data set on apples (8) and pears (3). First 2 trials on apples and first trial on pears compliant with GAP. Other trials performed with 2 applications acceptable since first application done at early stage is not expected to impact final residue level (Germany, 2015). No authorised for use on quinces in SEU MRLOECD = 0.08 |
0.09 | 0.06 | 0.02 | 2.2 | |
| Pears | NEU |
Mo: 0.04; 0.03; 0.02; 0.02; 0.01; < 0.01; < 0.01; 0.01; 0.013; 0.017; < 0.01; 0.02; 0.03; 0.02 RA: 0.08; 0.06; < 0.05; < 0.05; < 0.05; < 0.05; < 0.05; < 0.05; 0.08; < 0.05; < 0.05; < 0.05; 0.05; 0.06 |
Trials on apples. First 8 trials compliant with GAP or with dose rate within 25% deviation. Other trials performed with 2 applications acceptable since first application done at early stage is not expected to impact final residue level (Germany, 2015). Extrapolation to pears possible MRLOECD = 0.06 |
0.06 | 0.04 | 0.02 | 2.5 |
| SEU |
Apples Mo: 0.04; 0.02; 0.035; 0.015; 0.011; 0.017 RA: 0.06; 0.06; 0.089; < 0.03; < 0.03; < 0.03 Pears Mo: 0.08 RA: 0.10 |
Combined data set on apples and pears. First 2 trials on apples and trial on pears compliant with GAP. Other trials performed with 2 applications acceptable since first application done at early stage is not expected to impact final residue level (Germany, 2015) MRLOECD = 0.13 |
0.15 (tentative)f | 0.08 | 0.02 | 2.0 | |
| Apricots | NEU | – | No residue trials compliant with GAP | – | – | – | – |
| SEU |
Mo: 0.04; 0.03; 0.06; 0.09; 0.09; 0.06; 0.06; 0.01; 0.06; 0.07 RA: 0.06; 0.06; 0.12; 0.19; 0.12; 0.15; 0.10; < 0.05; 0.12; 0.15 |
Trials on peaches compliant with GAP. Extrapolation to apricots tentatively possible pending submission of residue trials on apricots (Germany, 2015) MRLOECD = 0.17 |
0.2 (tentative)f | 0.09 | 0.06 | 2.0 | |
| Cherries (sweet) | SEU |
Mo: 0.11; 0.07; 0.09; 0.05 RA: 0.28; 0.16; 0.22; 0.11 |
Trials on cherries compliant with GAP (Germany, 2015; Portugal, 2016) MRLOECD = 0.24 |
0.3 | 0.11 | 0.08 | 2.4 |
| Peaches | NEU |
Mo: < 0.01 RA: < 0.05 |
Trial on peaches compliant with GAP (Germany, 2016) | – | – | – | – |
| SEU |
Mo: 0.04; 0.03; 0.06; 0.09; 0.09; 0.06; 0.06; 0.01; 0.06; 0.07 RA: 0.06; 0.06; 0.12; 0.19; 0.12; 0.15; 0.10; < 0.05; 0.12; 0.15 |
Trials on peaches compliant with GAP (Germany, 2015) MRLOECD = 0.17 |
0.2 | 0.09 | 0.06 | 2.0 | |
| Plums | SEU |
Mo: < 0.01; < 0.01; 0.01; 0.03 RA: < 0.05; < 0.05; < 0.05; 0.12 |
Trials on plums compliant with GAP (Germany, 2015) Rber = 0.05 MRLOECD = 0.06 |
0.07 (tentative)f | 0.03 | 0.01 | 4.0 |
|
Table grapes Wine grapes |
NEU |
Mo: 0.05; 0.05; 0.04; 0.08; 0.056; 0.06; 0.024; 0.11 RA: 0.08; 0.12; 0.10; 0.15; 0.11; 0.15; 0.074; 0.19 |
Trials on grapes. First 4 trials performed with 2 applications acceptable since first application, done at early growth stage, is not expected to impact final residue level. Other trials overdosed (Germany, 2015) MRLOECD = 0.18 |
0.2 | 0.11 | 0.05 | 2.2 |
| SEU |
Mo: 0.02; 0.03; 0.03; 0.03; 0.01; < 0.01; 0.07; 0.01 RA: 0.06; 0.06; 0.05; 0.07; < 0.05; < 0.05; 0.09; < 0.05 |
Trials on grapes compliant with GAP (Germany, 2015) MRLOECD = 0.11 |
0.15 | 0.07 | 0.03 | 2.0 | |
| Import (USA) |
Mo: – RA: < 0.05; 0.05; 0.06; 0.06; 0.06; 0.11; 0.11; 0.11; 0.12; 0.12; 0.16; 0.17; 0.19; 0.2; 0.21; 0.61 |
Trials on grapes compliant with GAP. Residues analysed only according to the risk assessment residue definition (Germany, 2015) MRLOECD = 0.69 |
0.7 (tentative)e | 0.61 | 0.12 | 1.0 | |
|
Blueberries Cranberries |
Import (USA) |
Mo: – RA: 0.35; 0.39; 0.48; 0.86; 1.1; 1.9; 2.56 |
Trials on blueberries compliant with GAP. Although not explicitly mentioned in the (European Commission, 2017), extrapolation from blueberries to cranberries is acceptable (both crops belong to the Vaccinium genus). Residues analysed only according to the residue definition for risk assessment (Germany, 2015) MRLOECD = 4.47 |
5 (tentative)e | 2.56 | 0.86 | 1.0 |
|
Currants (black, red and white) Gooseberries (green, red and yellow) Rose hips Mulberries (black and white) Azaroles/Mediterranean medlars Elderberries |
Import (USA) | – | No residue trials compliant with GAP | – | – | – | – |
| Table olives | SEU |
Mo: 0.40; 0.40; 0.03; 0.30; 0.05; 0.02; 0.42; 0.11 RA: 0.71; 1.1; 0.14; 0.63; 0.11; < 0.05; 0.49; 0.22 |
Trials on olives compliant with GAP (Germany, 2015) MRLOECD = 0.94 |
1 | 0.42 | 0.21 | 2.2 |
| Avocados | SEU | – | No residue trials available | – | – | – | – |
| Bananas | Import (Cameroon) |
Mo: – RA: 4 × < 0.01 |
Trials on banana compliant with GAP for soil treatment. Residues analysed only according to the residue definition for risk assessment is acceptable since residues always below the LOQ (Germany, 2015) | 0.01 * | 0.01 | 0.01 | 1.0 |
| Mangoes | SEU | – | – | – | – | – | – |
| Pomegranates/Granate apples/ | Import (USA) | – | No residue trials compliant with GAP for soil treatment | – | – | – | – |
| Potatoes | NEU |
Mo: 0.01; 0.01; 0.02; 0.02 RA: < 0.05; < 0.05; < 0.05; < 0.05 |
Trials on potatoes compliant with GAP for seed treatment (Germany, 2015) MRLOECD = 0.04 |
0.05 (tentative)f | 0.02 | 0.02 | 2.5 |
| SEU |
Mo: 7 × < 0.01 RA: 7 × < 0.05 |
Trials on potatoes with dose rate within 25% deviation (Portugal, 2016; Greece, 2016) | 0.01* | 0.01 | 0.01 | 1.0 | |
|
Garlic Onions Shallots Spring onions/green onions and Welsh onions |
NEU |
Mo: < 0.01; < 0.01; < 0.01; < 0.01; < 0.01; < 0.01; < 0.01; 0.03 RA: < 0.05; < 0.05; < 0.05; < 0.05; < 0.05; < 0.05; < 0.05; 0.06 |
Trials on onions compliant with GAP for seed treatment. Extrapolation to bulb vegetables possible (Germany, 2015) MRLOECD = 0.04 |
0.04 | 0.03 | 0.01 | 2.0 |
|
Tomatoes Aubergines/eggplants |
SEU |
Mo: 0.012; 0.013; 0.021; 0.03; 0.021; 0.013; < 0.01; < 0.01 RA: < 0.03; 0.034; 0.059; 0.051; 0.037; < 0.03; < 0.03; < 0.03 |
Trials on tomatoes compliant with GAP (Italy, 2016a). Extrapolation to aubergines possible MRLOECD = 0.04 |
0.05 | 0.03 | 0.01 | 2.4 |
| EU |
Tomatoes Mo: 0.06; 0.09; 0.06; 0.2; 0.14; 0.07; 0.07; 0.06; 0.09; 0.10; 0.06; 0.12; 0.09; 0.11 RA: 0.18; 0.14; 0.10; 0.29; 0.19; 0.09; 0.11; 0.07; 0.14; 0.15; 0.06; 0.17; 0.15; 0.21 Aubergines Mo: 0.08; < 0.01 RA: 0.14; < 0.03 |
Combined data set on tomatoes and aubergines compliant with GAP (Greece, 2016; Italy 2016a). Extrapolation to aubergines possible MRLOECD = 0.26 |
0.3 | 0.20 | 0.09 | 1.6 | |
| Sweet peppers/bell peppers | SEU |
Mo: 1.4; 0.022; 0.04; 0.078 RA: 1.5; 0.047; 0.095; 0.16 |
Trials on peppers compliant with GAP (Germany, 2015) MRLOECD = 3.09 |
4 (tentative)f | 1.40 | 0.06 | 2.1 |
| EU |
Mo: 0.48; 0.14; 0.15; 0.16; 0.48; 0.07; 0.09; 0.31 RA: 0.61; 0.16; 0.19; 0.16; 0.62; 0.08; 0.22; 0.39 |
Trials on peppers compliant with GAP for soil treatment (Germany, 2015) MRLOECD = 0.9 |
0.9 | 0.48 | 0.16 | 1.3 | |
| Okra/lady's fingers | SEU |
Mo: 1.4; 0.022; 0.04; 0.078 RA: 1.5; 0.047; 0.095; 0.16 |
Trials on peppers overdosed (1 × 0.150 instead of 0.1 kg/ha) tentatively extrapolated to okra (Germany, 2015) MRLOECD = 3.09 |
4 (tentative)g | 1.40 | 0.06 | 2.1 |
| EU |
Mo: 0.047; 0.20; 0.32; 0.066; 0.05; 0.051; 0.13; 0.082 RA: 0.063; 0.25; 0.35; 0.17; 0.056; 0.075; 0.20; 0.087 |
Trials on peppers overdosed (1 × 0.150 instead of 0.1 kg/ha) tentatively extrapolated to okra (Germany, 2015) MRLOECD = 0.51 |
0.5 (tentative)g | 0.32 | 0.07 | 1.3 | |
| Cucumbers | SEU |
Mo: 0.025; < 0.01; < 0.01; < 0.01; < 0.01; < 0.01; 0.014; 0.033 RA: 0.031; < 0.03; < 0.03; < 0.03; < 0.03; < 0.03; 0.035; 0.038 |
Trials on courgettes compliant with GAP. Extrapolation to cucumbers possible (Germany, 2015) MRLOECD = 0.05 |
0.05 | 0.03 | 0.01 | 1.2 |
| EU |
Mo: < 0.01; < 0.01; 0.11; 0.03; 0.21; 0.05; 0.17; 0.09; 0.18; 0.06; 0.23 RA: 0.16; 0.06; 0.56; 0.29; 0.93; 0.50; 0.76; 0.39; 0.35; 0.13; 0.25 |
Trials on cucumbers compliant with GAP for soil treatment (Germany, 2015; Italy, 2016a) MRLOECD = 0.43 |
0.5 | 0.23 | 0.09 | 4.5 | |
| Courgettes Gherkins | SEU |
Mo: 0.025; < 0.01; < 0.01; < 0.01; < 0.01; < 0.01; 0.014; 0.033 RA: 0.031; < 0.03; < 0.03; < 0.03; < 0.03; < 0.03; 0.035; 0.038 |
Trials on courgettes compliant with GAP. Extrapolation to gherkins possible (Germany, 2015) MRLOECD = 0.05 |
0.05 | 0.03 | 0.01 | 1.2 |
| EU |
Mo: 0.01; < 0.01; 0.11; 0.03; 0.21; 0.05; 0.17; 0.09; 0.18; 0.06 RA: 0.16; 0.06; 0.56; 0.29; 0.93; 0.50; 0.76; 0.39; 0.35; 0.13 |
Trials on cucumbers compliant with GAP for soil treatment on gherkins and courgettes (Germany, 2015) MRLOECD = 0.38 |
0.4 | 0.21 | 0.08 | 4.8 | |
|
Melons Watermelons |
SEU |
Mo: < 0.01; < 0.01; < 0.01; 0.011; 0.015; 0.018; 0.015; 0.012 RA: < 0.03; < 0.03; < 0.03; 0.033; 0.078; 0.035; 0.039; 0.04 |
Trials on melons compliant with GAP. Extrapolation to watermelons possible (Germany, 2015) MRLOECD = 0.03 |
0.03 | 0.02 | 0.01 | 3.0 |
| EU |
Melons Mo: 0.07; < 0.01 RA: 0.18; 0.12 Watermelons Mo: 0.02; 0.01; 0.01; 0.04 RA: 0.06; < 0.05; < 0.05; 0.11 |
Combined data set of trials on melons compliant with GAP and on watermelons overdosed (Greece, 2016; Portugal, 2016) MRLOECD = 0.12 |
0.15 (tentative)f , g | 0.07 | 0.02 | 2.9 | |
| Pumpkins | SEU |
Mo: < 0.01; < 0.01; < 0.01 RA: 0.08; < 0.05; < 0.05 |
Trials on melons compliant with GAP for pumpkins (Portugal, 2016). CF calculated from indoor data set considered more robust as based on a larger number of positive trials | 0.01* (tentative)f | 0.01 | 0.01 | 2.9 |
| EU |
Melons Mo: 0.07; < 0.01 RA: 0.18; 0.12 Watermelons Mo: 0.02; 0.01; 0.01; 0.04 RA: 0.06; < 0.05; < 0.05; 0.11 |
Combined data set of trials on melons compliant with GAP and on watermelons overdosed (Greece, 2016; Portugal, 2016) tentatively extrapolated to pumpkins MRLOECD = 0.12 |
0.15 (tentative)f , c | 0.07 | 0.02 | 2.9 | |
|
Broccoli Cauliflowers |
NEU |
Mo: 21 × < 0.01 RA: 21 × < 0.05 |
Combined data set on cauliflowers (2), Brussels sprouts (8), Chinese cabbage (2) and head cabbage (9) compliant with GAP for soil treatment on brassicas except Brussels sprouts (Germany, 2015) | 0.01* | 0.01 | 0.01 | 1.0 |
| SEU |
Cauliflowers Mo: < 0.01; 0.01; 0.01; < 0.01; < 0.01; 0.02 RA: 0.07; 0.06; 0.09; < 0.050; < 0.05; 0.05 Broccoli Mo: 0.07; 0.02; 0.03; 0.02; 0.01; 0.01; < 0.01; < 0.01 RA: 0.31; 0.10; 0.29; 0.08; 0.05; 0.09; 0.11; 0.07 |
Combined data set on cauliflowers and broccoli compliant with GAP for soil treatment on flowering brassica (Germany, 2015) MRLOECD = 0.08 |
0.09 | 0.07 | 0.01 | 6.5 | |
| Brussels sprouts | NEU |
Mo: 0.056; 0.038; 0.01; < 0.01 RA: 0.092; 0.061; < 0.03; < 0.03 |
Trials on Brussels sprouts compliant with GAP (Germany, 2015) MRLOECD = 0.12 |
0.15 | 0.06 | 0.02 | 1.6 |
| SEU | – | No residue trials compliant with GAP | – | – | – | – | |
| Head cabbages | NEU |
Mo: 21 × < 0.01 RA: 21 × < 0.05 |
See broccoli and cauliflowers NEU | 0.01* | 0.01 | 0.01 | 1.0 |
| SEU |
Mo: 0.064; < 0.01; < 0.01; 0.015; < 0.01; < 0.01; 0.02; < 0.01; < 0.01; < 0.01; < 0.01; 0.01 RA: 0.10; < 0.03; < 0.03; 0.041; < 0.05; < 0.05; 0.12; < 0.05; < 0.05; < 0.05; 0.08; 0.05 |
Trials on head cabbages. First 4 trials compliant with GAP. Other trials performed with 2 applications instead of 1 tentatively considered (Germany, 2015) MRLOECD = 0.08 |
0.08 (tentative)g | 0.06 | 0.01 | 5.0 | |
|
Chinese cabbages/pe‐tsai Kales |
NEU |
Mo: 21 × < 0.01 RA: 21 × < 0.05 |
See broccoli and cauliflowers NEU. | 0.01* | 0.01 | 0.01 | 1.0 |
| SEU |
Mo: 0.035; 0.034; 0.17; < 0.01 RA: 0.28; 0.24; 0.70; 0.09 |
Trials on kale compliant with GAP (Germany, 2015). Extrapolation to Chinese cabbages possible MRLOECD = 0.35 |
0.5 | 0.17 | 0.03 | 7.5 | |
| Kohlrabies | NEU | – | No residue trials compliant with GAP | – | – | – | – |
|
Lamb's lettuces/corn salads Escaroles/broad‐leaved endives Cresses and other sprouts and shoots Land cresses Roman rocket/rucola Red mustards Baby leaf crops (including Brassica species) Fresh herbs |
NEU |
Mo: < 0.01; 0.01; 0.02; 0.01; 0.02; 0.02; 0.03; < 0.01 RA: < 0.05; < 0.05; 0.07; < 0.05; < 0.05; < 0.05; 0.06; < 0.05 |
Trials on lettuce compliant with GAP for seed treatment. Extrapolation to other salad plants possible (Germany, 2015). Not authorised for use on lamb's lettuce, rockets and fresh herbs in NEU MRLOECD = 0.05 |
0.05 | 0.03 | 0.02 | 2.5 |
| SEU |
Mo: 0.95; 0.12; 0.15; 0.25; 0.14; 0.20; 0.23; 0.80; 0.38 RA: 1.5; 0.66; 0.27; 0.41; 0.38; 0.59; 0.87; 1.2; 0.98 |
Trials on lettuce performed with 2 applications instead of 1. First 7 trials on open leaf varieties. Extrapolation to salads (except lettuce) and fresh herbs possible (Germany, 2015) MRLOECD = 1.58 |
2 | 0.95 | 0.23 | 2.6 | |
| EU | – | No residue trials available. Not authorised for indoor use on rockets and fresh herbs | – | – | – | – | |
| Lettuce | NEU |
Mo: < 0.01; 0.01; 0.02; 0.01; 0.02; 0.02; 0.03; < 0.01 RA: < 0.05; < 0.05; 0.07; < 0.05; < 0.05; < 0.05; 0.06; < 0.05 |
Trials on lettuce compliant with GAP for seed treatment (Germany, 2015) MRLOECD = 0.05 |
0.05 | 0.03 | 0.02 | 2.5 |
| SEU |
Mo: 0.33; 0.018; 0.17; 0.049; 0.14; 0.17; 0.16; 0.07 RA: 0.80; 0.16; 0.28; 0.30; 0.55; 0.80; 0.69; 0.50 |
Trials on lettuce open leaf varieties with dose rate within 25% deviation (Germany, 2015) MRLOECD = 0.53 |
0.6 | 0.33 | 0.15 | 4.5 | |
| EU | – | No residue trials available. | – | – | – | – | |
| Witloofs/Belgian endives | NEU |
Mo: 6 × < 0.01 RA: 6 × < 0.05 |
Trials on witloof compliant with GAP for seed treatment (Germany, 2015) | 0.01* | 0.01 | 0.01 | 1.0 |
|
Beans with pods Peas with pods |
SEU |
Mo: < 0.01; 0.078; 0.015; 0.093; 0.16; 0.025; 0.11; 0.071 RA: < 0.03; 0.18; 0.092; 0.33; 0.32; 0.21; 0.31; 0.22 |
Trials on beans with pods compliant with GAP (Germany, 2015). No authorised for use on peas with pods in SEU MRLOECD = 0.28 |
0.3 | 0.16 | 0.07 | 3.1 |
| EU |
Mo: 0.15; 0.14; 0.085; 0.064; 0.082; 0.19; 0.25; 0.072 RA: 0.42; 0.42; 0.32; 0.16; 0.31; 0.24; 0.31; 0.29 |
Trials on beans with pods compliant with GAP (Germany, 2015). No authorised for indoor use on peas with pods MRLOECD = 0.39 |
0.4 | 0.25 | 0.11 | 2.9 | |
| Import (USA) |
Beans with pods Mo: – RA: 0.61; 0.80; 0.23; 0.45 Peas with pods Mo: – RA: 0.19; 3.13; 0.88; 0.22 |
Combined data set on beans with pods and peas with pods performed according to a more critical GAP (1 seed treatment followed by 1 soil and 3 foliar sprays). Residues analysed only according to the residue definition for risk assessment (Germany, 2015) MRLOECD = 4.71 |
5 (tentative) e , g | 3.13 | 0.53 | 1.0 | |
|
Beans without pods Peas without pods |
SEU |
Mo: < 0.01; < 0.01; < 0.01; < 0.01 RA: 0.11; < 0.03; < 0.03; 0.10 |
Trials on beans without pods compliant with GAP (Germany, 2015). No authorised for use on peas without pods in SEU MRLOECD = 0.01 |
0.01* (tentative)f | 0.01 | 0.01 | 10 |
| Import (USA) |
Beans without pods Mo: – RA: 0.12; 0.25; 0.17; < 0.05; < 0.05 Peas without pods Mo: – RA: 0.51; 0.38; 0.26; 0.83; 0.99 |
Combined data set on beans without pods and peas without pods performed according to a more critical GAP (1 seed treatment followed by 1 soil and 3 foliar sprays). Residues analysed only according to the residue definition for risk assessment (Germany, 2015) MRLOECD = 1.66 |
2 (tentative)e , g | 0.99 | 0.26 | 1.0 | |
| Cardoons | SEU | – | No residue trials available. | – | – | – | – |
| Globe artichokes | SEU |
Mo: 0.09; 0.09; 0.11; 0.12; 0.10; 0.14 RA: 0.15; 0.13; 0.19; 0.18; 0.18; 0.21 |
Trials on globe artichokes compliant with GAP (Germany, 2015) MRLOECD = 0.32 |
0.4 | 0.14 | 0.11 | 1.6 |
| Leeks | NEU |
Mo: 4 × < 0.01 RA: 4 × < 0.05 |
Trials on leeks overdosed (seed treatment performed at 0.06 kg/unit instead of 0.045 kg/unit) acceptable since residues were always below the LOQ (Germany, 2015) | 0.01* | 0.01 | 0.01 | 1.0 |
| Beans (dry) | Import (USA) |
Dry beans Mo: – RA: 0.71; 0.59; 0.69; 0.99; 0.21; 0.13; 0.20; 0.34; 0.79; 0.49; 0.25 Dry peas Mo: – RA: 0.121; 0.181; 0.269; 0.684; 0.811; 0.864 |
Combined data set on dry beans and dry peas compliant with GAP. Residues analysed only according to the risk assessment residue definition (Germany, 2015) MRLOECD = 1.66 |
2 (tentative)e | 0.99 | 0.49 | 1.0 |
| Peas (dry) | SEU |
Mo: < 0.01; < 0.01; < 0.01; < 0.01; < 0.01; < 0.01; < 0.01 RA: 0.41; 0.31; 0.53; 0.22; 0.23; 0.33; 0.35 |
Trials on dry peas compliant with GAP (Germany, 2015) | 0.01* (tentative)f | 0.01 | 0.01 | 33 |
| Peanuts/groundnuts | Import (USA) |
Mo: – RA: < 0.05; < 0.05; < 0.05; 0.18; 0.355; 0.095; 0.10; < 0.05; 0.14; 0.20; 0.21; 0.115 |
Trials on peanuts performed according to a more critical GAP for soil treatment (1 soil application followed by 3 foliar applications) used to derive a tentative MRL. Residues analysed only according to the residue definition for risk assessment (Germany, 2015) MRLOECD = 0.5 |
0.5 (tentative)e , g | 0.36 | 0.11 | 1.0 |
| Cotton seeds | SEU | – | No residue trials available. | – | – | – | – |
| Olives for oil production | SEU |
Mo: 0.16; 0.44; 0.16; 0.20; 0.09; 0.04; 0.11; 0.01 RA: 0.29; 0.79; 0.26; 0.43; 0.26; 0.15; 0.22; 0.08 |
Trials on olives with dose rate within 25% deviation (4) and with 2 applications instead of 1 (4) acceptable as the first application at early growth stage is not expected to have a significant impact on the final residue level (Germany, 2015; Italy, 2016b) MRLOECD = 0.68 |
0.7 | 0.44 | 0.14 | 2.1 |
|
Barley grains Oat grains Rye grains Wheat grains |
NEU |
Barley Mo: 10 × < 0.01 RA: 2 × < 0.02; 3 × < 0.03; 5 × < 0.05 Wheat Mo: 10 × < 0.01 RA: 2 × < 0.02; 2 × < 0.03; 6 × < 0.05 |
Combined data set on barley and wheat compliant with GAP for seed treatment. Extrapolation to oats and rye possible (Germany, 2015) | 0.01* | 0.01 | 0.01 | 1.0 |
| SEU |
Barley Mo: < 0.01 RA: < 0.03 Wheat Mo: 7 × < 0.01 RA: 3 × < 0.03; 4 × < 0.05 |
Combined data set on barley and wheat compliant with GAP for seed treatment (Germany, 2015; France, 2016). Extrapolation to barley, oats and wheat possible. No authorised for use on rye in SEU | 0.01* | 0.01 | 0.01 | 1.0 | |
|
Barley straw Oat straw Rye straw Wheat straw |
NEU |
Barley Mo: < 0.1; < 0.1; < 0.05; < 0.05; < 0.1; < 0.01; < 0.01; < 0.01; < 0.01; < 0.01 RA: < 0.05; < 0.05; 0.11; 0.28; < 0.05; < 0.03; < 0.03; < 0.03; < 0.02; < 0.02 Wheat Mo: < 0.1; 0.05; < 0.05; 0.05; < 0.1; < 0.10; < 0.01; < 0.01; < 0.01; < 0.01 RA: < 0.05; 0.11; 0.08; 0.21; < 0.05; 0.13; < 0.03; < 0.03; < 0.02; < 0.02 |
Combined data set on barley and wheat compliant with GAP for seed treatment. Extrapolation to rye and oats possible (Germany, 2015) MRLOECD = 0.2 |
0.2 (tentative)h | 0.10 | 0.05 | 2.2 |
| SEU |
Barley Mo: < 0.01 RA: < 0.03 Wheat Mo: 6 × < 0.01; 0.015 RA: 2 × < 0.03; 4 × < 0.05; 0.16 |
Combined data set on barley and wheat compliant with GAP for seed treatment (Germany, 2015; France, 2016). Extrapolation to barley, oats and wheat possible. CF calculated from NEU considered more robust as based on a larger number of positive trials. No authorised for use on rye in SEU MRLOECD = 0.02 |
0.02 (tentative)h | 0.02 | 0.01 | 2.2 | |
| Coffee beans | Import (USA) |
Mo: – RA: 0.18; 0.235; 0.285; 0.37; 0.47 |
Trials on coffee beans performed at a more critical GAP (5 foliar spray instead of 2 soil applications). Residues analysed only according to the risk assessment residue definition (Germany, 2015) MRLOECD = 0.92 |
1 (tentative)e , f , h , i | 0.47 | 0.29 | 1.0 |
| Hops | NEU |
Mo: 8 × < 0.2 RA: 0.48; 0.59; 0.73; 0.73; 0.81; 1.2; 1.3; 1.6 |
Trials on hops compliant with GAP (Germany, 2015) MRLOECD = 0.2 |
0.2 | 0.20 | 0.20 | 3.9 |
| Import (USA) |
Mo: – RA: 1.04; 4.58; 4.76 |
Trials on hops. Last trial overdosed (performed with 3 applications at 0.17–0.25 kg/ha) considered on a tentative basis. Residues analysed only according to the risk assessment residue definition (Germany, 2015) MRLOECD = 11.85 |
15 (tentative)e , f , g | 4.76 | 4.58 | 1.0 | |
|
Sugar beet roots Fodder beet roots |
NEU |
Mo: 15 × < 0.01 RA: 15 × < 0.05 |
Trials on sugar beet compliant with GAP for seed treatment (except one trial overdosed but acceptable since residues below the LOQ). Extrapolation to fodder beet possible (Germany, 2015) | 0.01* | 0.01 | 0.01 | 1.0 |
| SEU |
Mo: 3 × < 0.01 RA: 3 ×< 0.05 |
Trials on sugar beet compliant with GAP for seed treatment or with dose rate within the 25% variation (Germany, 2015). No authorised for use on fodder beet in SEU | 0.01* | 0.01 | 0.01 | 1.0 | |
|
Sugar beet tops Fodder beet tops |
NEU |
Mo: 15 × < 0.01 RA: 10 × < 0.05; 0.06; 0.07; 0.11; 0.11; 0.14 |
Trials on sugar beet compliant with GAP for seed treatment (except one trial overdosed but acceptable since residues below the LOQ). Extrapolation to fodder beet possible (Germany, 2015) | 0.01* (tentative)h | 0.01 | 0.01 | 11 |
| SEU |
Mo: < 0.01; < 0.01; < 0.01 RA: < 0.05; 0.063; 0.066 |
Trials on sugar beet compliant with GAP for seed treatment or with dose rate within the 25% variation (Germany, 2015). No authorised for use on fodder beet in SEU | 0.01* (tentative)h | 0.01 | 0.01 | 6.5 | |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level; Mo: monitoring; RA: risk assessment.
* Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, EU: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for monitoring.
Supervised trials median residue according to the residue definition for monitoring.
Conversion factor for risk assessment; median of the individual conversion factors at the supported PHI for each residues trial. CF was calculated considering only residues of parent and the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety above LOQ. A CF of 1 was proposed when in all residue trials both imidacloprid and the sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety were below the LOQ and when residues were analysed only according to the residue definition for risk assessment (mainly for the import tolerances).
Tentative MRL derived from residue trials with samples analysed only according to the risk assessment residue definition.
Tentative MRL is derived based on a reduced number of trials.
Tentative MRL is derived based on trials performed according to a more critical GAP.
Tentative MRL is derived in future view of setting MRLs in livestock feed items.
Tentative MRL is derived as a confirmatory method and an ILV are still required for the enforcement in coffee beans.
B.1.2.2. Residues in succeeding crops
| Confined rotational crop study (quantitative aspect) | In the available confined rotational crop study performed with a bare soil application at 0.454 kg/ha, the lowest total radioactive residues (TRR) were found in wheat grains and red beet roots ranging from 0.03 to 0.07 mg/kg. In all other rotated crops, TRRs were higher, accounting for up to 0.26 and 0.24 mg/kg in red beet leaves and Swiss chard and for up to 1.0 and 2.38 mg/kg in wheat forage and straw, respectively. Although residues in rotated crops decreased with soil ageing, TRR after the third rotation (408 days) were still significant, ranging from 0.03 mg/kg in wheat grain to 0.96 mg/kg in wheat straw. These results suggests possible soil uptake, even at long plant‐back intervals |
| Field rotational crop study | In the available rotational crop field study performed with a bare soil application of imidacloprid at 1 × 0.15 kg/ha, no residues above the LOQ of 0.05 mg/kg were found in rotational crops. Nevertheless, imidacloprid concentration tested in the rotational field studies (0.16 mg/kg soil) is not covering the soil concentration expected from annual and multiannual applications according to the most critical indoor GAP currently authorised for sweet peppers (calculated as 0.203 mg/kg soil and as 0.348 mg/kg soil, respectively). Consequently, following both annual and multiannual applications of imidacloprid according to the most critical GAP currently authorised for sweet peppers, a possible uptake by crops grown in rotation cannot be excluded |
LOQ: limit of quantification; GAP: Good Agricultural Practice.
B.1.2.3. Processing factors
| Processed commodity | Number of studiesa | Processing factor (PF) | CFP b | |
|---|---|---|---|---|
| Individual values | Median PF | |||
| Robust processing factors (sufficiently supported by data) | ||||
| Citrus fruits, peeled | 3 | 0.08; 0.14; 0.18 | 0.14 | 2.0 |
| Citrus fruits, juice | 3 | 0.12; 0.14; 0.17 | 0.14 | 2.5 |
| Apples and pears, juice | 6 | 0.30; 0.67; 0.50; 0.50; 0.50; 0.25 | 0.5 | 2.1 |
| Apples, sauce | 6 | 0.30; 0.67; 0.75; 0.50; 0.50; 0.25 | 0.5 | 2.1 |
| Peaches, canned | 4 | 0.25; 0.50; 0.50; 0.57 | 0.5 | 1.1 |
| Wine grapes, wet pomace | 9 | 3.8; 7.0; 2.13; 3.18; 1.99; 1.48; 2.12; 1.81; 2.94 | 2.13 | 1.7 |
| Wine grapes, must | 11 | 2.0; 1.75; 2.25; 1.4; 1.5; 0.87; 1.12; 0.29; 1.21; 0.92; 0.44 | 1.21 | 1.7 |
| Wine grapes, red wine (unheated) | 4 | 1.0; 1.5; 0.98; 0.81 | 0.99 | 2.1 |
| Wine grapes, white wine | 7 | 2.0; 1.75; 1.2; 1.02; 0.3; 1.07; 0.38 | 1.07 | 1.9 |
| Tomatoes, paste | 4 | 2.5; 1.5; 7.25; 2.0 | 2.25 | 3.2 |
| Melons, pumpkins and watermelons, peeled | 16 | 3.0; 1.04; 0.48; 0.38; 2 × 0.33; 2 × 0.83; 0.48; 2 × 0.91; 0.48; 0.55; 2 × 0.67; 0.56 | 0.61 | 1.0 |
| Beans (with pods), cooked | 3 | < 0.33; < 0.5; 1.0 | < 0.5 | 33 |
| Beans (with pods), canned | 3 | 0.33; < 0.5; 1.0 | 0.5 | 14 |
| Cotton seeds, crude oil | 3 | 1.0; 1.0; 1.0 | 1.0 | 1.0 |
| Olives for oil production, virgin oil after cold press | 5 | 0.11; 0.25; 0.18; 0.50; 0.17 | 0.18 | 1.0 |
| Olives for oil production, refined oil after warm press | 4 | 0.25; 0.09; 0.50; 0.06 | 0.17 | 1.0 |
| Olives for oil production, press cake | 5 | 1.44; 1.0; 1.27; 2.0; 1.0 | 1.27 | 1.6 |
| Indicative processing factors (limited data set) | ||||
| Oranges, marmalade | 2 | 0.71; 0.83 | 0.77 | 1.4 |
| Apples and pears, dry pomace | 2 | 2.5; 3.4 | 2.96 | 2.5 |
| Cherries, jam | 2 | 0.33; 0.50 | 0.42 | 2.2 |
| Table grapes, dried (raisins) | 1 | 5.50 | 5.5 | 2.4 |
| Wine grapes, juice | 1 | 0.13 | 0.13 | 1.0 |
| Potatoes, peeled and boiled | 2 | 0.5; 1.0 | 0.75 | 1.0 |
| Potatoes, fried | 1 | 0.5 | 0.5 | 1.0 |
| Potatoes, dry pulp | 2 | < 2.5; < 5.0 | < 1.75 | 1.0 |
| Tomatoes, ketchup | 2 | 1.5; 2.0 | 1.75 | 2.7 |
| Tomatoes, juice | 1 | 1.5 | 1.5 | 3.3 |
| Head cabbages, cooked | 2 | 0.29; 0.91 | 0.6 | 2.0 |
| Head cabbages, sauerkraut | 2 | 0.29; 1.1 | 0.69 | 1.8 |
| Head cabbages, sauerkraut juice | 2 | 0.29; 1.0 | 0.65 | 1.9 |
| Peanuts, crude oil | 2 | 0.20; 0.31 | 0.26c | 1.0d |
| Peanuts, meal/press cake | 2 | 2.48; 3.06 | 2.77c | 1.0d |
| Cotton seeds, refined oil | 1 | 1.0 | 1.0 | 1.0 |
| Cotton seeds, meal/press cake | 1 | 1.0 | 1.0 | 320 |
| Coffee beans, roasted beans | 1 | 0.43 | 0.43c | 1.0d |
| Coffee beans, instant coffee | 1 | 1.35 | 1.35c | 1.0d |
| Hops, beer | 2 | < 0.01; < 0.01 | < 0.01 | 2.0 |
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Conversion factor for risk assessment in the processed commodity; median of the individual conversion factors for each processing study.
Since residues were not analysed for parent compound, the processing factor is calculated according to the residue definition for risk assessment and is indicative only.
Since residues were analysed only according to the risk assessment residue definition, a conversion factor from enforcement to risk assessment of 1 is proposed.
B.2. Residues in livestock
| Relevant groups | Dietary burden expressed in | Most critical dieta | Most critical commoditya | Trigger exceeded (Y/N) | |||
|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||||
| Med. | Max. | Med. | Max. | ||||
| Cattle (all diets) | 0.029 | 0.031 | 0.76 | 0.80 | Cattle (dairy) | Grapefruits, dried pulp | Yes |
| Cattle (dairy only) | 0.029 | 0.031 | 0.76 | 0.80 | Cattle (dairy) | Grapefruits, dried pulp | Yes |
| Sheep (all diets) | 0.011 | 0.014 | 0.26 | 0.33 | Sheep (lamb) | Bean, seed | Yes |
| Sheep (ewe only) | 0.009 | 0.011 | 0.26 | 0.33 | Sheep (ram/ewe) | Bean, seed | Yes |
| Swine (all diets) | 0.013 | 0.013 | 0.57 | 0.57 | Swine (breeding) | Grapefruits, dried pulp | Yes |
| Poultry (all diets) | 0.011 | 0.012 | 0.16 | 0.17 | Poultry (turkey) | Bean, seed | Yes |
| Poultry (layer only) | 0.011 | 0.012 | 0.16 | 0.17 | Poultry (layer) | Bean, seed | Yes |
bw: body weight; DM: dry matter.
Calculated for the maximum dietary burden.
B.2.1. Nature of residues and methods of analysis in livestock
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock (available studies) | Animal | Dose (mg/kg bw per day) | Duration (days) | N rate/comment |
|---|---|---|---|---|
| Laying hen | 10–50 | 3 | 833–4167N compared to the maximum dietary burden calculated for poultry | |
| Lactating goat | 10 | 3 | 323N compared to the maximum dietary burden calculated for cattle | |
| Source: Germany (2005) | ||||
| Time needed to reach a plateau concentration in milk and eggs (days) | 3 (according to the livestock feeding study) |
| Metabolism in rat and ruminant similar (Yes/No) | Yes |
| Animal residue definition for monitoring (RD‐Mo) | Imidacloprid, by default |
| Animal residue definition for risk assessment (RD‐RA) | Sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid |
| Conversion factor (monitoring to risk assessment) | See Table B.2.2.1 |
| Fat soluble residues (Yes/No) | No |
| Methods of analysis for monitoring of residues (analytical technique, crop groups, LOQs) |
Muscle, fat, liver, kidney and eggs:
Milk:
Honey:
According to the EURLs, based on the general experience with this compound, it is expected that imidacloprid residues can be enforced with an LOQ of 0.01 mg/kg in all commodities of animal origin (EURLs, 2016) |
bw: body weight; HPLC‐MS/MS: high‐performance liquid chromatography with tandem mass spectrometry; LOQ: limit of quantification; LC–MS/MS: liquid chromatography with tandem mass spectrometry; QuEChERS: Quick, Easy, Cheap, Effective, Rugged, and Safe.
B.2.1.2. Stability of residues in livestock
| Animal products (available studies) | Animal | Commodity | T (°C) | Stability (months/years) |
|---|---|---|---|---|
| Poultry | Muscle | –18 | 12 months | |
| Poultry | Liver | –18 | 12 months | |
| Bovine | Kidney | –18 | 12 months | |
| Bovine | Fat | –18 | 12 months | |
| Bovine | Milk | –18 | 12 months | |
| Poultry | Egg | –18 | 12 months | |
|
The demonstrated storage stability period covers imidacloprid and metabolites (M01, M06, M09, M14) Source: Germany (2005) | ||||
B.2.2. Magnitude of residues in livestock
B.2.2.1. Summary of the residue data from livestock feeding studies
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | CFc | ||
|---|---|---|---|---|---|---|
| Mean | Highest | STMRa (mg/kg) | HRb (mg/kg) | |||
|
Cattle (all diets) Closest feeding level (0.15 mg/kg bw; 4.8N rate)d | ||||||
| Muscle | < 0.02 | < 0.02 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Fat | < 0.02 | < 0.02 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Liver | 0.05 | 0.05 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Kidney | 0.03 | 0.03 | < 0.03 | < 0.03 | 0.03* | 1.0 |
|
Cattle (dairy only) Closest feeding level (0.15 mg/kg bw; 4.8N rate)d | ||||||
| Milke | < 0.02 | n.a. | 0.01 | 0.01 | 0.01* | 1.0 |
|
Sheep (all diets) f Closest feeding level (0.15 mg/kg bw; 11N rate)d | ||||||
| Muscle | < 0.02 | < 0.02 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Fat | < 0.02 | < 0.02 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Liver | 0.05 | 0.05 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Kidney | 0.03 | 0.03 | < 0.03 | < 0.03 | 0.03* | 1.0 |
|
Sheep (dairy only) f Closest feeding level (0.15 mg/kg bw; 14N rate)d | ||||||
| Milke | < 0.02 | n.a. | 0.01 | 0.01 | 0.01* | 1.0 |
|
Swine f Closest feeding level (0.15 mg/kg bw; 12N rate)d | ||||||
| Muscle | < 0.02 | < 0.02 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Fat | < 0.02 | < 0.02 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Liver | 0.05 | 0.05 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| kidney | 0.03 | 0.03 | < 0.03 | < 0.03 | 0.03* | 1.0 |
|
Poultry (all diets) Closest feeding level (0.18 mg/kg bw; 15N rate)d | ||||||
| Muscle | < 0.02 | < 0.02 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Fat | < 0.02 | < 0.02 | < 0.03 | < 0.03 | 0.03* | 1.0 |
| Liver | 0.04 | 0.05 | < 0.03 | < 0.03 | 0.03* | 1.0 |
|
Poultry (layer only) Closest feeding level (0.18 mg/kg bw; 15N rate)d | ||||||
| Egg | < 0.02 | < 0.02 | < 0.03 | < 0.03 | 0.03* | 1.0 |
MRL: maximum residue level; CF: conversion factor for enforcement residue definition to risk assessment residue definition; STMR: supervised trials median residue; HR: highest residue; bw: body weight.
* Indicates that the MRL is proposed at the limit of quantification.
n.a.: not applicable.
n.r.: not reported.
The mean residue level for milk and the median residue levels for eggs and tissues were recalculated at the 1N rate for the median dietary burden.
The mean residue level in milk and the highest residue levels in eggs and tissues were recalculated at the 1N rate for the maximum dietary burden.
As reported residue levels were analysed according to the residue definition for risk assessment, a CF of 1 is proposed from enforcement to risk assessment.
Closest feeding level and N dose rate related to the maximum dietary burden.
Highest residue level from day 1 to day 28 (daily mean of 3 cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in sheep and swine.
B.3. Consumer risk assessment
B.3.1. Consumer risk assessment without consideration of the existing CXLs – Indoor uses and import tolerances
| ADI | 0.06 mg/kg bw per day (EFSA, 2008a) |
| Highest IEDI, according to EFSA PRIMo |
Scenario EU1 (without risk mitigation measures): 6% ADI (WHO cluster diet B) Scenario EU2 (with risk mitigation measures): 6% ADI (WHO cluster diet B) |
| Assumptions made for the calculations |
Scenario EU1 (without risk mitigation measures): The calculation is based on the median residue levels in the raw agricultural commodities, except for citrus fruits and cucurbits with inedible peel where the relevant peeling factors were applied For those commodities where data were insufficient to derive an MRL, EFSA considered the existing EU MRL for an indicative calculation The contributions of commodities where no GAP was reported in the framework of this review were not included in the calculation Scenario EU2 (with risk mitigation measures): The EU MRL for escaroles was disregarded (assuming that the authorisation for this crop will be withdrawn) |
| ARfD | 0.08 mg/kg bw (EFSA, 2008a) |
| Highest IESTI, according to EFSA PRIMo |
Scenario EU1 (without risk mitigation measures): 109% ARfD (escarole) Scenario EU2 (with risk mitigation measures): 76% ARfD (cucumbers) |
| Assumptions made for the calculations |
Scenario EU1 (without risk mitigation measures): The calculation is based on the highest residue levels in the raw agricultural commodities, except for citrus fruits and cucurbits with inedible peel where the relevant peeling factors were applied For those commodities where data were insufficient to derive an MRL, EFSA considered the existing EU MRL for an indicative calculation Scenario EU2 (with risk mitigation measures): The EU MRL for escaroles was disregarded (assuming that the authorisation for this crop will be withdrawn) |
ADI: acceptable daily intake; bw: body weight; IEDI: international estimated daily intake; PRIMo: (EFSA) Pesticide Residues Intake Model; WHO: World Health Organization; ARfD: acute reference dose; IESTI: international estimated short‐term intake; CXL: codex maximum residue limit; MRL: maximum residue level.
B.3.2. Consumer risk assessment without consideration of the existing CXLs – all uses
| ADI | 0.06 mg/kg bw per day (EFSA, 2008a) |
| Highest IEDI, according to EFSA PRIMo |
Scenario EU1 (without risk mitigation measures): 7% ADI (WHO cluster diet B) Scenario EU2 (with risk mitigation measures): 6% ADI (WHO cluster diet B) |
| Assumptions made for the calculations |
Scenario EU1 (without risk mitigation measures): The calculation is based on the median residue levels in the raw agricultural commodities, except for citrus fruits and cucurbits with inedible peel where the relevant peeling factors were applied For those commodities where data were insufficient to derive an MRL, EFSA considered the existing EU MRL for an indicative calculation The contributions of commodities where no GAP was reported in the framework of this review were not included in the calculation Scenario EU2 (with risk mitigation measures): The median residue levels for escarole, sweet peppers and kale, resulting from the GAPs of concern (SEU outdoor), are replaced by the median residue levels resulting from the fall‐back GAPs (NEU outdoor for escarole and kale and EU indoor for peppers) |
| ARfD | 0.08 mg/kg bw (EFSA, 2008a) |
| Highest IESTI, according to EFSA PRIMo |
Scenario EU1 (without risk mitigation measures): 270% ARfD (escarole) 231% ARfD (sweet peppers) 108% ARfD (kale) Scenario EU2 (with risk mitigation measures): 76% ARfD (cucumbers) |
| Assumptions made for the calculations |
Scenario EU1 (without risk mitigation measures): The calculation is based on the highest residue levels in the raw agricultural commodities, except for citrus fruits and cucurbits with inedible peel where the relevant peeling factors were applied For those commodities where data were insufficient to derive an MRL, EFSA considered the existing EU MRL for an indicative calculation Scenario EU2 (with risk mitigation measures): The highest residue levels for escarole, sweet peppers and kale, resulting from the GAPs of concern (SEU outdoor), are replaced by the highest residue levels resulting from the fall‐back GAPs (NEU outdoor for escarole and kale and EU indoor for peppers) |
ADI: acceptable daily intake; bw: body weight; IEDI: international estimated daily intake; PRIMo: (EFSA) Pesticide Residues Intake Model; WHO: World Health Organization; ARfD: acute reference dose; IESTI: international estimated short‐term intake; CXL: codex maximum residue limit; MRL: maximum residue level; GAP: Good Agricultural Practice; SEU: southern European Union; NEU: northern European Union.
B.3.3. Consumer risk assessment with consideration of the existing CXLs
| ADI | 0.06 mg/kg bw per day (EFSA, 2008a) |
| Highest IEDI, according to EFSA PRIMo | Indicative results considering CXLs only: 8% ADI (WHO Cluster diet B) |
| Assumptions made for the calculations |
CXLs have been established for imidacloprid (as sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid). However, this residue definition for enforcement is not compatible with the residue for enforcement proposed by EFSA. Therefore, a consumer risk assessment including CXLs values together with EU MRLs could not be performed As CXLs were derived according to residue definition for risk assessment, an indicative risk assessment with the existing CXLs only, was performed. The input values as derived by the JMPR could directly be considered, without applying any conversion factor. The calculation is based on the median residue levels in the raw agricultural commodities, except for citrus fruits where the relevant peeling factor was applied |
| ARfD | 0.08 mg/kg bw (EFSA, 2008a) |
| Highest IESTI, according to EFSA PRIMo |
Indicative results considering CXLs only: 184% ARfD (celery) 169% ARfD (kale) |
| Assumptions made for the calculations |
CXLs have been established for imidacloprid (as sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid). However, this residue definition for enforcement is not compatible with the residue for enforcement proposed by EFSA. Therefore, a consumer risk assessment including CXLs values together with EU MRLs could not be performed As CXLs were derived according to residue definition for risk assessment, an indicative risk assessment with the existing CXLs only, was performed. The input values as derived by the JMPR could directly be considered, without applying any conversion factor. The calculation is based on the highest residue levels in the raw agricultural commodities, except for citrus fruits where the relevant peeling factor was applied |
ADI: acceptable daily intake; bw: body weight; IEDI: international estimated daily intake; PRIMo: (EFSA) Pesticide Residues Intake Model; WHO: World Health Organization; ARfD: acute reference dose; IESTI: international estimated short‐term intake; CXL: codex maximum residue limit; MRL: maximum residue level.
B.4. Proposed MRLs (based on GAPs compliant with the new conditions of approval)
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition: imidacloprid | |||||
| 110010 | Grapefruit | 1 | 1 | 0.9 | Further consideration neededa |
| 110020 | Oranges | 1 | 1 | 0.9 | Further consideration neededa |
| 110030 | Lemons | 1 | 1 | 0.9 | Further consideration neededa |
| 110040 | Limes | 1 | 1 | 0.9 | Further consideration neededa |
| 110050 | Mandarins | 1 | 1 | 0.9 | Further consideration neededa |
| 120010 | Almonds | 0.05* | 0.01 | – | Further consideration neededb |
| 120020 | Brazil nuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120030 | Cashew nuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120040 | Chestnuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120050 | Coconuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120060 | Hazelnuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120070 | Macadamia | 0.05* | 0.01 | – | Further consideration neededb |
| 120080 | Pecans | 0.05* | 0.01 | 0.02* | Recommendedc |
| 120090 | Pine nuts | 0.05* | 0.01 | – | Further consideration neededb |
| 120100 | Pistachios | 0.05* | 0.01 | – | Further consideration neededb |
| 120110 | Walnuts | 0.05* | 0.01 | – | Further consideration neededb |
| 130010 | Apples | 0.5 | 0.5 | – | Further consideration neededb |
| 130020 | Pears | 0.5 | 1 | – | Further consideration neededb |
| 140010 | Apricots | 0.5 | 1.5 | – | Further consideration neededb |
| 140020 | Cherries | 0.5 | 4 | – | Further consideration neededb |
| 140030 | Peaches | 0.5 | 1.5 | – | Further consideration neededb |
| 140040 | Plums | 0.3 | 1.5 | – | Further consideration neededb |
| 151010 | Table grapes | 1 | 1 | 0.7 | Further consideration neededa |
| 151020 | Wine grapes | 1 | 1 | 0.7 | Further consideration neededa |
| 152000 | Strawberries | 0.5 | 0.5 | – | Further consideration neededb |
| 153010 | Blackberries | 5 | 5 | – | Further consideration neededb |
| 153020 | Dewberries | 5 | 5 | – | Further consideration neededb |
| 153030 | Raspberries | 5 | 5 | – | Further consideration neededb |
| 154010 | Blueberries | 5 | 5 | 5 | Further consideration neededa |
| 154020 | Cranberries | 0.05* | 0.05* | 5 | Further consideration neededa |
| 154030 | Currants (red, black and white) | 5 | 5 | 5 | Further consideration neededd |
| 154040 | Gooseberries | 5 | 5 | 5 | Further consideration neededd |
| 154050 | Rose hips | 5 | 5 | 5 | Further consideration neededd |
| 154060 | Mulberries | 5 | 5 | 5 | Further consideration neededd |
| 154070 | Azarole (Mediterranean medlar) | 0.05* | 5 | 0.05 | Further consideration neededd |
| 154080 | Elderberries | 5 | 5 | 5 | Further consideration neededd |
| 161030 | Table olives | 0.5 | 2 | – | Further consideration neededb |
| 161040 | Kumquats | 0.05* | 1 | – | Further consideration neededb |
| 163020 | Bananas | 0.05* | 0.05 | 0.01* | Recommendedc |
| 163030 | Mangoes | 0.2 | 0.2 | – | Further consideration neededb |
| 163050 | Pomegranate | 1 | 1 | 1 | Further consideration neededd |
| 211000 | Potatoes | 0.5 | 0.5 | – | Further consideration neededb |
| 212010 | Cassava | 0.5 | 0.5 | – | Further consideration neededb |
| 212020 | Sweet potatoes | 0.5 | 0.5 | – | Further consideration neededb |
| 212030 | Yams | 0.5 | 0.5 | – | Further consideration neededb |
| 212040 | Arrowroot | 0.5 | 0.5 | – | Further consideration neededb |
| 213010 | Beetroot | 0.5 | 0.5 | – | Further consideration neededb |
| 213020 | Carrots | 0.5 | 0.5 | – | Further consideration neededb |
| 213030 | Celeriac | 0.5 | 0.5 | – | Further consideration neededb |
| 213040 | Horseradish | 0.5 | 0.5 | – | Further consideration neededb |
| 213050 | Jerusalem artichokes | 0.5 | 0.5 | – | Further consideration neededb |
| 213060 | Parsnips | 0.5 | 0.5 | – | Further consideration neededb |
| 213070 | Parsley root | 0.5 | 0.5 | – | Further consideration neededb |
| 213080 | Radishes | 0.5 | 0.5 | – | Further consideration neededb |
| 213090 | Salsify | 0.5 | 0.5 | – | Further consideration neededb |
| 213100 | Swedes | 0.5 | 0.5 | – | Further consideration neededb |
| 213110 | Turnips | 0.5 | 0.5 | – | Further consideration neededb |
| 220020 | Onions | 0.1 | 0.1 | – | Further consideration neededb |
| 231010 | Tomatoes | 0.5 | 0.5 | 0.3 | Recommendedc |
| 231020 | Peppers | 1 | 1 | 0.9 | Recommendedc |
| 231030 | Aubergines (egg plants) | 0.5 | 0.2 | 0.3 | Recommendedc |
| 231040 | Okra, lady's fingers | 0.5 | – | 0.5 | Further consideration needede |
| 232010 | Cucumbers | 1 | 1 | 0.5 | Recommendedc |
| 232020 | Gherkins | 0.5 | – | 0.4 | Recommendedf |
| 232030 | Courgettes | 1 | 1 | 0.4 | Recommendedc |
| 233010 | Melons | 0.5 | 0.2 | 0.15 | Further consideration neededa |
| 233020 | Pumpkins | 0.5 | – | 0.15 | Further consideration needede |
| 233030 | Watermelons | 0.2 | 0.2 | 0.15 | Further consideration neededa |
| 234000 | Sweet corn | 0.1 | 0.02* | – | Further consideration neededb |
| 241010 | Broccoli | 0.5 | 0.5 | – | Further consideration neededb |
| 241020 | Cauliflower | 0.5 | 0.5 | – | Further consideration neededb |
| 242010 | Brussels sprouts | 0.5 | 0.5 | – | Further consideration neededb |
| 242020 | Head cabbage | 0.5 | 0.5 | – | Further consideration neededb |
| 243020 | Kale | 0.3 | 5 | – | Further consideration neededb |
| 251010 | Lamb's lettuce | 2 | – | 2 | Further consideration neededg |
| 251020 | Lettuce | 2 | 2 | 2 | Further consideration neededd |
| 251030 | Escarole (broad‐leaf endive) | 1 | – | – | Further consideration neededh |
| 251040 | Cress | 2 | – | 2 | Further consideration neededg |
| 251050 | Land cress | 2 | – | 2 | Further consideration neededg |
| 251070 | Red mustard | 2 | – | 2 | Further consideration neededg |
| 251080 | Leaves and sprouts of Brassica spp. | 2 | – | 2 | Further consideration neededg |
| 256080 | Basil | 2 | 20 | – | Further consideration neededb |
| 260010 | Beans (fresh, with pods) | 2 | 2 | 5 | Further consideration neededa |
| 260020 | Beans (fresh, without pods) | 2 | 2 | 2 | Further consideration neededa |
| 260030 | Peas (fresh, with pods) | 5 | 5 | 5 | Further consideration neededa |
| 260040 | Peas (fresh, without pods) | 2 | 2 | 2 | Further consideration neededa |
| 270030 | Celery | 2 | 6 | – | Further consideration neededb |
| 270060 | Leek | 0.05* | 0.05* | – | Further consideration neededb |
| 300010 | Beans (dry) | 2 | 2 | 2 | Further consideration neededa |
| 300020 | Lentils (dry) | 2 | 2 | – | Further consideration neededb |
| 300030 | Peas (dry) | 2 | 2 | – | Further consideration neededb |
| 300040 | Lupins (dry) | 2 | 2 | – | Further consideration neededb |
| 401020 | Peanuts | 1 | 1 | 0.5 | Further consideration neededa |
| 401050 | Sunflower seed | 0.1 | 0.05* | – | Further consideration neededb |
| 401060 | Rape seed | 0.1 | 0.05* | – | Further consideration neededb |
| 401070 | Soya bean | 0.05* | 3 | – | Further consideration neededb |
| 402010 | Olives for oil production | 1 | 2 | – | Further consideration neededb |
| 500010 | Barley grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500020 | Buckwheat grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500030 | Maize grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500040 | Millet grain | 0.05* | 0.05 | – | Further consideration neededb |
| 500050 | Oats grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500060 | Rice grain | 1.5 | 0.05 | – | Further consideration neededb |
| 500070 | Rye grain | 0.1 | 0.05 | – | Further consideration neededb |
| 500080 | Sorghum grain | 0.05* | 0.05 | – | Further consideration neededb |
| 500090 | Wheat grain | 0.1 | 0.05 | – | Further consideration neededb |
| 610000 | Tea (dried leaves and stalks, fermented or otherwise of Camellia sinensis) | 0.05* | 50 | – | Further consideration neededb |
| 620000 | Coffee beans | 1 | 1 | 1 | Further consideration neededa |
| 700000 | Hops (dried), including hop pellets and unconcentrated powder | 10 | 10 | 15 | Further consideration neededa |
| 900010 | Sugar beet (root) | 0.5 | 0.5 | – | Further consideration neededb |
| 1011010 | Swine muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1011020 | Swine fat (free of lean meat) | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1011030 | Swine liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1011040 | Swine kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1012010 | Bovine muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1012020 | Bovine fat | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1012030 | Bovine liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1012040 | Bovine kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1013010 | Sheep muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1013020 | Sheep fat | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1013030 | Sheep liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1013040 | Sheep kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1014010 | Goat muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1014020 | Goat fat | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1014030 | Goat liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1014040 | Goat kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1015010 | Horse muscle | 0.1 | 0.1 | 0.03* | Recommendedc |
| 1015020 | Horse fat | 0.05* | 0.1 | 0.03* | Recommendedc |
| 1015030 | Horse liver | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1015040 | Horse kidney | 0.3 | 0.3 | 0.03* | Recommendedc |
| 1016010 | Poultry muscle | 0.05* | 0.02 | 0.03* | Recommendedc |
| 1016020 | Poultry fat | 0.05* | 0.02 | 0.03* | Recommendedc |
| 1016030 | Poultry liver | 0.05* | 0.05 | 0.03* | Recommendedc |
| 1016040 | Poultry kidney | 0.05* | 0.05 | 0.03* | Recommendedc |
| 1020010 | Cattle milk | 0.1 | 0.1 | 0.01* | Recommendedc |
| 1020020 | Sheep milk | 0.1 | 0.1 | 0.01* | Recommendedc |
| 1020030 | Goat milk | 0.1 | 0.1 | 0.01* | Recommendedc |
| 1020040 | Horse milk | 0.1 | 0.1 | 0.01* | Recommendedc |
| 1030000 | Birds’ eggs | 0.05* | 0.02 | 0.03* | Recommendedc |
| – | Other commodities of plant and animal origin | Regulation (EU) No 491/2014 | – | – | Further consideration neededi |
MRL: maximum residue level; GAP: Good Agricultural Practice; CXL: codex maximum residue limit.
* Indicates that the MRL is set at the limit of quantification.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); CXL is not compatible with EU residue definitions (combination E‐II in Appendix E).
There are no relevant INDOOR authorisations or import tolerances reported at EU level; CXL is not compatible with EU residue definitions. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐II in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; CXL is not compatible with EU residue definitions (combination G‐II in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); CXL is not compatible with EU residue definitions (combination C‐II in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination E‐I in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination G‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination C‐I in Appendix E).
GAP evaluated at EU level is not supported by data and a risk to consumers cannot be excluded for the existing EU MRL; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination B‐I in Appendix E).
There are no relevant INDOOR authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
PRIMo(Indoor EU.1 and IT)
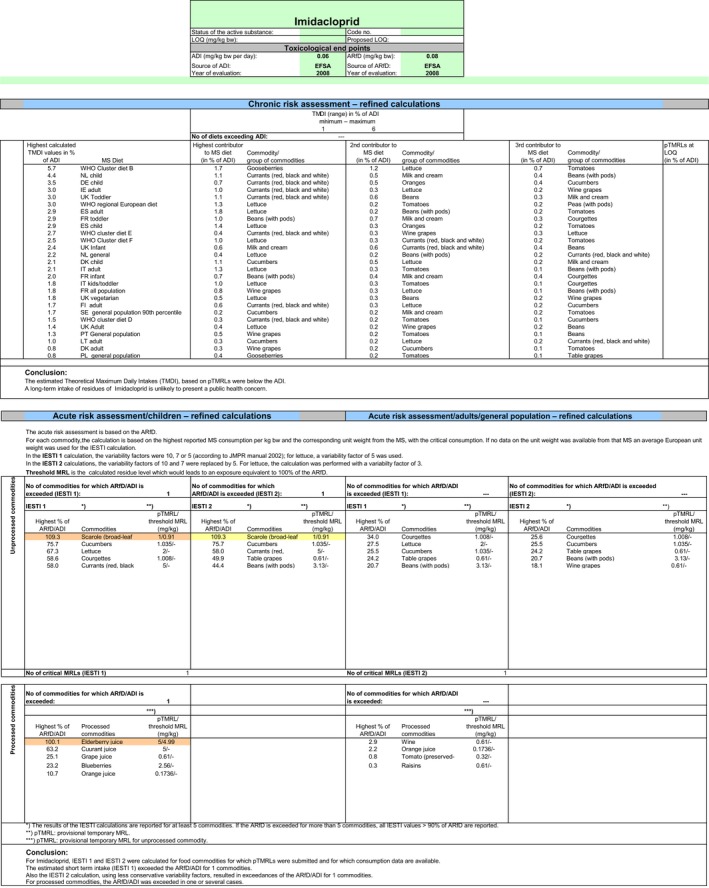
PRIMo(Indoor EU.2 and IT)
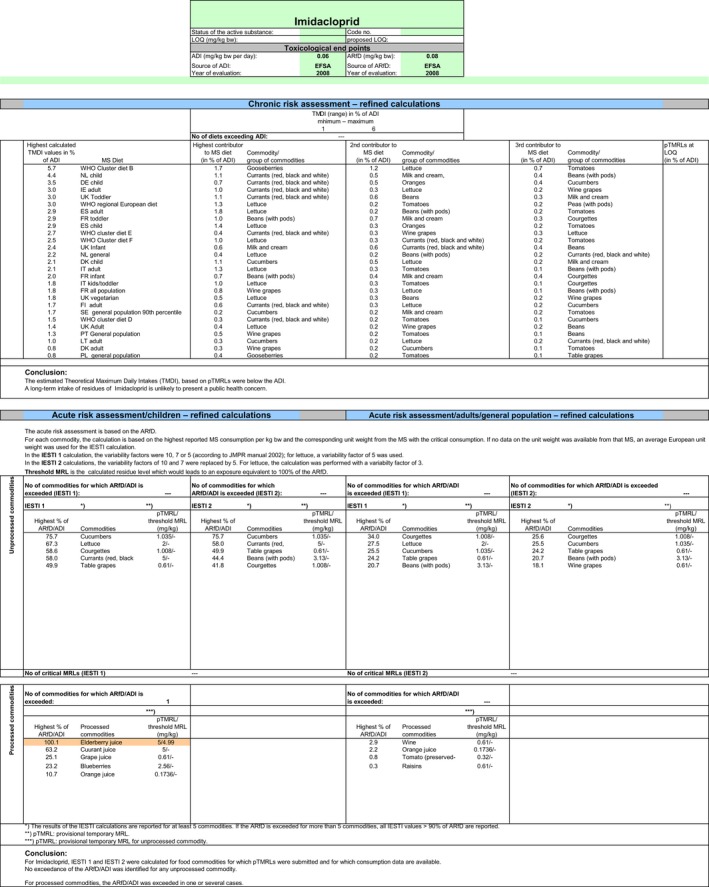
PRIMo(EU.1, All uses)
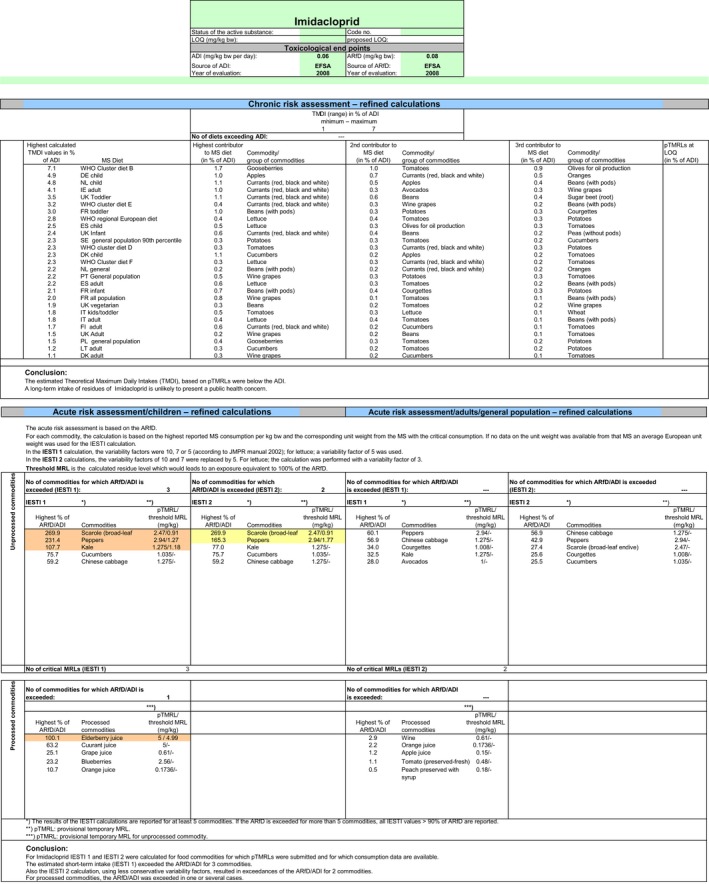
PRIMo(EU.2, All uses)
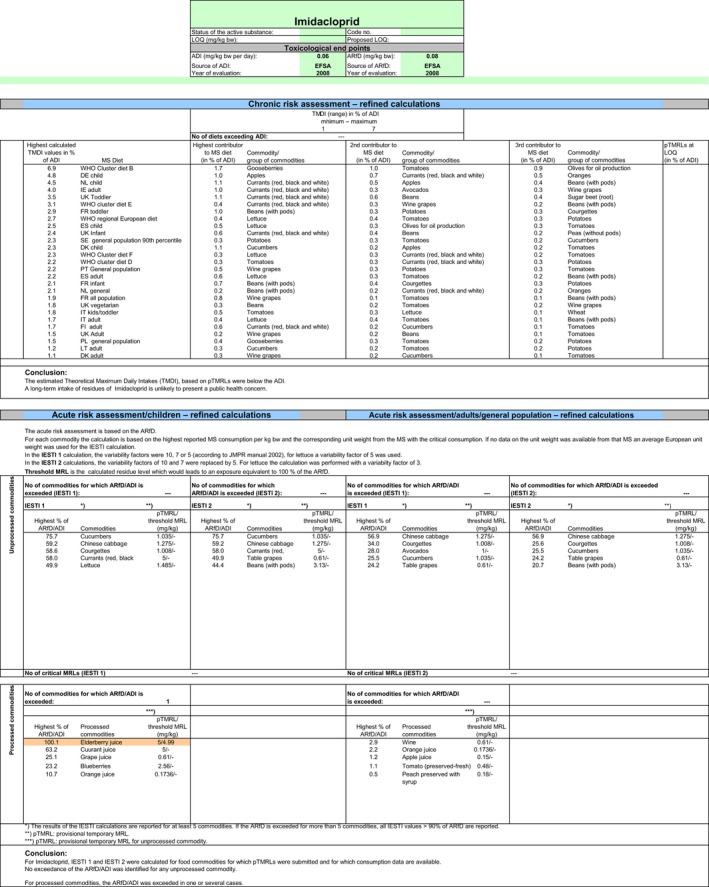
PRIMo(CXL)
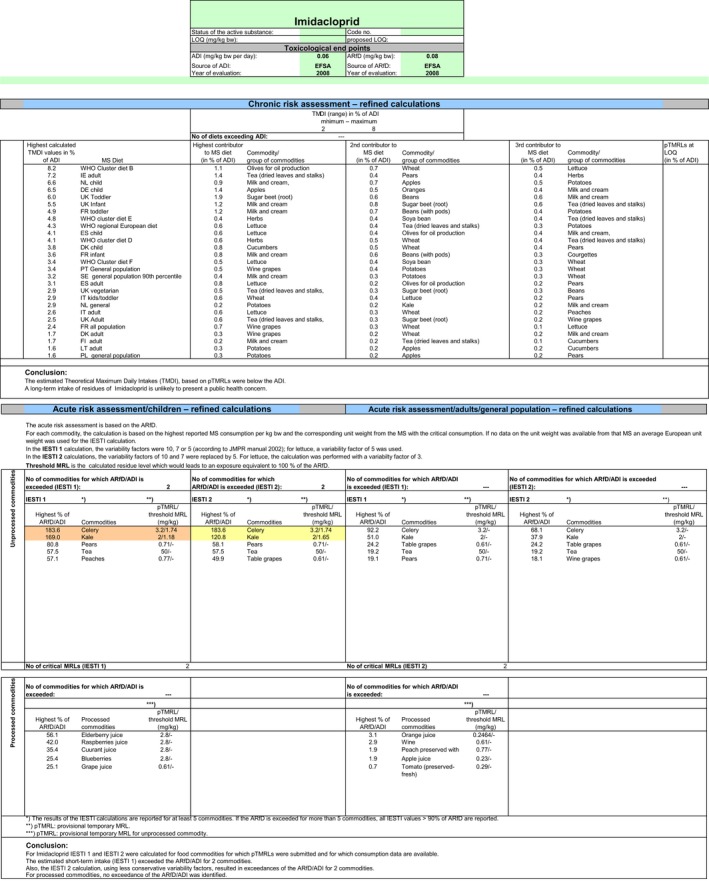
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid | ||||
| Reflecting the new conditions of approval | ||||
| Citrus fruits, dried pulp | 2.80 | STMRMo × CF × PFa (tentative) | 2.80 | STMRMo × CF × PFa (tentative) |
| Bean, seed (dry) | 0.49 | STMRMo × CF (tentative) | 0.49 | STMRMo × CF (tentative) |
| Cowpea, seed | 0.49 | STMRMo × CF (tentative) | 0.49 | STMRMo × CF (tentative) |
| Peanut, meal | 0.30 | STMRMo × CF × PF (tentative) | 0.30 | STMRMo × CF × PF (tentative) |
| Covering the possible carry‐over due to (former) authorised EU outdoor uses | ||||
| Barley, oat, rye, triticale and wheat grain | 0.01* | STMRMo × CF | 0.01* | STMRMo × CF |
| Brewer's grain, dried | 0.01* | STMRMo b × CF | 0.01* | STMRMo b × CF |
| Wheat, distiller's grain (dry) | 0.01* | STMRMo b × CF | 0.01* | STMRMo b × CF |
| Wheat gluten, meal | 0.01* | STMRMo b × CF | 0.01* | STMRMo b × CF |
| Wheat, milled by‐products | 0.01* | STMRMo b × CF | 0.01* | STMRMo b × CF |
| Barley, oat, rye, triticale and wheat, straw | 0.11 | STMRMo × CF | 0.22 | HRMo × CF |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; CF: conversion factor for enforcement residue definition to risk assessment residue definition; Mo: monitoring.
* Indicates that the input value is proposed at the limit of quantification.
For dried pulp of citrus fruits, in the absence of processing factors supported by data, a default processing factor of 10 was included in the calculation to consider the potential concentration of residues in this commodity.
For processed commodities from cereals, no default processing factor was applied because imidacloprid residues in the raw commodities are below the LOQ and concentration of residues in these commodities is not expected.
D.2. Consumer risk assessment without consideration of the existing CXLs – Indoor uses and Import tolerances
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid | ||||
| Commodities for which a risk for consumers was identified are reported in bold | ||||
| Citrus fruits | 0.08 | STMRMo × PF × CFP (tentative) | 0.17 | HRMo × PF × CFP (tentative) |
| Pecans | 0.02* | STMRMo × CF | 0.02* | HRMo × CF |
| Table grapes | 0.12 | STMRMo × CF (tentative) | 0.61 | HRMo × CF (tentative) |
| Wine grapes | 0.12 | STMRMo × CF (tentative) | 0.61 | HRMo × CF (tentative) |
| Blueberries | 0.86 | STMRMo × CF (tentative) | 2.56 | HRMo × CF (tentative) |
| Cranberries | 0.86 | STMRMo × CF (tentative) | 2.56 | HRMo × CF (tentative) |
| Currants (black, red and white) | 5 | EU MRL | 5 | EU MRL |
| Gooseberries (green, red and yellow) | 5 | EU MRL | 5 | EU MRL |
| Rose hips | 5 | EU MRL | 5 | EU MRL |
| Mulberries (black and white) | 5 | EU MRL | 5 | EU MRL |
| Azaroles/Mediterranean medlars | 0.05 | EU MRL | 0.05 | EU MRL |
| Elderberries | 5 | EU MRL | 5 | EU MRL |
| Bananas | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Granate apples/pomegranates | 1 | EU MRL | 1 | EU MRL |
| Tomatoes | 0.14 | STMRMo × CF | 0.32 | HRMo × CF |
| Sweet peppers/bell peppers | 0.20 | STMRMo × CF | 0.62 | HRMo × CF |
| Aubergines/eggplants | 0.14 | STMRMo × CF | 0.32 | HRMo × CF |
| Okra/lady's fingers | 0.10 | STMRMo × CF (tentative) | 0.41 | HRMo × CF (tentative) |
| Cucumbers | 0.41 | STMRMo × CF | 1.04 | HRMo × CF |
| Gherkins Courgettes | 0.36 | STMRMo × CF | 1.01 | HRMo × CF |
| Cucurbits with inedible peel | 0.01 | STMRMo × PF × CF (tentative) | 0.04 | STMRMo × PF × CF (tentative) |
| Lamb's lettuces/corn salads | 2 | EU MRL | 2 | EU MRL |
| Lettuces | 2 | EU MRL | 2 | EU MRL |
| Escaroles/broad‐leaved endives | 1 | EU MRL | 1 | EU MRL |
| Cresses and other sprouts and shoots | 2 | EU MRL | 2 | EU MRL |
| Land cresses | 2 | EU MRL | 2 | EU MRL |
| Red mustards | 2 | EU MRL | 2 | EU MRL |
| Baby leaf crops (including brassica species) | 2 | EU MRL | 2 | EU MRL |
| Beans (with pods) | 0.53 | STMRMo × CF (tentative) | 3.13 | HRMo × CF (tentative) |
| Beans (without pods) | 0.26 | STMRMo × CF (tentative) | 0.99 | HRMo × CF (tentative) |
| Peas (with pods) | 0.53 | STMRMo × CF (tentative) | 3.13 | HRMo × CF (tentative) |
| Peas (without pods) | 0.26 | STMRMo × CF (tentative) | 0.99 | HRMo × CF (tentative) |
| Beans (dry) | 0.49 | STMRMo × CF (tentative) | 0.99 | HRMo × CF (tentative) |
| Peanuts/groundnuts | 0.11 | STMRMo × CF (tentative) | 0.36 | HRMo × CF (tentative) |
| Coffee beans | 0.29 | STMRMo × CF (tentative) | 0.47 | HRMo × CF (tentative) |
| Hops | 4.58 | STMRMo × CF (tentative) | 4.76 | HRMo × CF (tentative) |
| Swine meat | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Swine fat | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Swine liver | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Swine kidney | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Swine muscle | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Ruminant meat | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Ruminant fat | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Ruminant liver | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Ruminant kidney | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Poultry meat | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Poultry fat | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Poultry liver | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
| Ruminant milk | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Bird's eggs | 0.03* | STMRMo × CF | 0.03* | HRMo × CF |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; CF: conversion factor for enforcement residue definition to risk assessment residue definition; Mo: monitoring; MRL: maximum residue level.
* Indicates that the input value is proposed at the limit of quantification.
D.3. Consumer risk assessment without consideration of the existing CXLs – All uses
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid | ||||
| Commodities for which a risk for consumers was identified are reported in bold | ||||
| Citrus fruits | 0.08 | STMRMo × PF × CFp (tentative) | 0.17 | HRMo × PF × CFp (tentative) |
| Almonds | 0.02* | STMRMo × CF | 0.02* | HRMo × CF |
| Pecans | 0.02* | STMRMo × CF | 0.02* | HRMo × CF |
| Apples | 0.05 | STMRMo × CF | 0.15 | HRMo × CF |
| Pears | 0.05 | STMRMo × CF | 0.20 | HRMo × CF |
| Quinces | 0.05 | STMRMo × CF | 0.10 | HRMo × CF |
| Apricots | 0.12 | STMRMo × CF (tentative) | 0.18 | HRMo × CF (tentative) |
| Cherries (sweet) | 0.19 | STMRMo × CF | 0.26 | HRMo × CF |
| Peaches | 0.12 | STMRMo × CF | 0.18 | HRMo × CF |
| Plums | 0.04 | STMRMo × CF (tentative) | 0.12 | HRMo × CF (tentative) |
| Table grapes | 0.12 | STMRMo × CF (tentative) | 0.61 | HRMo × CF (tentative) |
| Wine grapes | 0.12 | STMRMo × CF (tentative) | 0.61 | HRMo × CF (tentative) |
| Blueberries | 0.86 | STMRMo × CF (tentative) | 2.56 | HRMo × CF (tentative) |
| Cranberries | 0.86 | STMRMo × CF (tentative) | 2.56 | HRMo × CF (tentative) |
| Currants (black, red and white) | 5 | EU MRL | 5 | EU MRL |
| Gooseberries (green, red and yellow) | 5 | EU MRL | 5 | EU MRL |
| Rose hips | 5 | EU MRL | 5 | EU MRL |
| Mulberries (black and white) | 5 | EU MRL | 5 | EU MRL |
| Azaroles/Mediterranean medlars | 0.05 | EU MRL | 0.05 | EU MRL |
| Elderberries | 5 | EU MRL | 5 | EU MRL |
| Table olives | 0.45 | STMRMo × CF | 0.92 | HRMo × CF |
| Avocados | 1 | EU MRL | 1 | EU MRL |
| Bananas | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Mangoes | 0.2 | EU MRL | 0.2 | EU MRL |
| Granate apples/pomegranates | 1 | EU MRL | 1 | EU MRL |
| Potatoes | 0.04 | STMRMo × CF (tentative) | 0.05 | HRMo × CF (tentative) |
|
Garlic Onions Shallots |
0.02 | STMRMo × CF | 0.06 | HRMo × CF |
| Spring onions/green onions and Welsh onions | 0.02 | STMRMo × CF | 0.06 | HRMo × CF |
| Tomatoes Aubergines/eggplants | 0.20 | STMRMo × CF | 0.48 | HRMo × CF |
| Sweet peppers/bell peppers | 0.33 | STMRMo × CF (tentative) | 2.94 | HRMo × CF (tentative) |
| 0.20 | STMRMo × CF (fall‐back) | 0.62 | HRMo × CF (fall‐back) | |
| Okra/lady's fingers | 0.16 | STMRMo × CF (tentative) | 2.94 | HRMo × CF (tentative) |
| Cucumbers | 0.41 | STMRMo × CF | 1.04 | HRMo × CF |
|
Gherkins Courgettes |
0.36 | STMRMo × CF | 1.01 | HRMo × CF |
| Cucurbits with inedible peel | 0.01 | STMRMo × PF × CF (tentative) | 0.04 | HRMo × PF × CF (tentative) |
|
Broccoli Cauliflowers |
0.07 | STMRMo × CF | 0.46 | HRMo × CF |
| Brussels sprouts | 0.04 | STMRMo × CF | 0.09 | HRMo × CF |
| Head cabbages | 0.05 | STMRMo × CF | 0.32 | HRMo × CF |
| Chinese cabbages/pe‐tsai | 0.26 | STMRMo × CF | 1.28 | HRMo × CF |
| Kales | 0.26 | STMRMo × CF | 1.28 | HRMo × CF |
| 0.01* | STMRMo × CF (fall‐back) | 0.01* | STMRMo × CF (fall‐back) | |
| Kohlrabies | 0.3 | EU MRL | 0.3 | EU MRL |
|
Lamb's lettuces/corn salads Cresses and other sprouts and shoots Land cresses Roman rocket/rucola Red mustards Baby leaf crops (including brassica species) |
0.60 | STMRMo × CF | 2.47 | HRMo × CF |
| Escaroles/broad‐leaved endives | 0.60 | STMRMo × CF | 2.47 | HRMo × CF |
| 0.04 | STMRMo × CF (fall‐back) | 0.08 | STMRMo × CF (fall‐back) | |
| Lettuces | 0.68 | STMRMo × CF | 1.49 | HRMo × CF |
| Witloofs/Belgian endives | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Fresh herbs | 0.60 | STMRMo × CF | 2.47 | HRMo × CF |
|
Beans (with pods) Peas (with pods) |
0.53 | STMRMo × CF (tentative) | 3.13 | HRMo × CF (tentative) |
|
Beans (without pods) Peas (without pods) |
0.26 | STMRMo × CF (tentative) | 0.99 | HRMo × CF (tentative) |
| Cardoons | 0.5 | EU MRL | 0.5 | EU MRL |
| Globe artichokes | 0.17 | STMRMo × CF | 0.22 | HRMo × CF |
| Leeks | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Beans (dry) | 0.49 | STMRMo × CF (tentative) | 0.99 | HRMo × CF (tentative) |
| Peas (dry) | 0.33 | STMRMo × CF (tentative) | 0.33 | HRMo × CF (tentative) |
| Peanuts/groundnuts | 0.11 | STMRMo × CF (tentative) | 0.36 | HRMo × CF (tentative) |
| Cotton seeds | 1 | EU MRL | 1 | EU MRL |
| Olives for oil production | 0.28 | STMRMo × CF | 0.92 | HRMo × CF |
|
Barley grains Oat grains Rye grains Wheat grains |
0.01* | STMRMo × CF | 0.01* | HRMo × CF |
| Coffee beans | 0.29 | STMRMo × CF (tentative) | 0.47 | HRMo × CF (tentative) |
| Hops | 4.58 | STMRMo × CF (tentative) | 4.76 | HRMo × CF (tentative) |
| Sugar beet roots | 0.01* | STMRMo × CF | 0.01* | HRMo × CF |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; CF: conversion factor for enforcement residue definition to risk assessment residue definition; Mo: monitoring; MRL: maximum residue level.
* Indicates that the input value is proposed at the limit of quantification.
D.4. Indicative consumer risk assessment of the existing CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of imidacloprid and its metabolites containing the 6‐chloropyridinyl moiety, expressed as imidacloprid | ||||
| Commodities for which a risk for consumers was identified are reported in bold | ||||
| Citrus fruits | 0.07 | STMR × PF (CXL) | 0.25 | HR × PF (CXL) |
| Tree nuts | 0.01 | STMR (CXL) | 0.01 | HR (CXL) |
| Apples | 0.07 | STMR (CXL) | 0.23 | HR (CXL) |
| Pears | 0.38 | STMR (CXL) | 0.71 | HR (CXL) |
| Apricots | 0.36 | STMR (CXL) | 0.77 | HR (CXL) |
| Cherries | 0.55 | STMR (CXL) | 2.50 | HR (CXL) |
| Peaches | 0.36 | STMR (CXL) | 0.77 | HR (CXL) |
| Plums | 0.28 | STMR (CXL) | 0.70 | HR (CXL) |
| Table grapes | 0.11 | STMR (CXL) | 0.61 | HR (CXL) |
| Wine grapes | 0.11 | STMR (CXL) | 0.61 | HR (CXL) |
| Strawberries | 0.17 | STMR (CXL) | 0.35 | HR (CXL) |
| Blackberries | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Dewberries | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Raspberries | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Blueberries | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Cranberries | 0.05* | STMR (CXL) | 0.05* | HR (CXL) |
| Currants (red, black and white) | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Gooseberries | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Rose hips | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Mulberries | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Azarole (Mediterranean medlar) | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Elderberries | 0.89 | STMR (CXL) | 2.80 | HR (CXL) |
| Table olives | 0.36 | STMR (CXL) | 1.10 | HR (CXL) |
| Kumquats | 0.26 | STMR (CXL) | 0.88 | HR (CXL) |
| Bananas | 0.01 | STMR (CXL) | 0.05 | HR (CXL) |
| Mangoes | 0.05 | STMR (CXL) | 0.15 | HR (CXL) |
| Pomegranate | 0.43 | STMR (CXL) | 0.55 | HR (CXL) |
| Potatoes | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Cassava | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Sweet potatoes | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Yams | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Arrowroot | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Beetroot | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Carrots | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Celeriac | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Horseradish | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Jerusalem artichokes | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Parsnips | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Parsley root | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Radishes | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Salsify | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Swedes | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Turnips | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Onions | 0.05 | STMR (CXL) | 0.06 | HR (CXL) |
| Tomatoes | 0.08 | STMR (CXL) | 0.29 | HR (CXL) |
| Peppers | 0.15 | STMR (CXL) | 0.48 | HR (CXL) |
| Aubergines (egg plants) | 0.05 | STMR (CXL) | 0.14 | HR (CXL) |
| Cucumbers | 0.31 | STMR (CXL) | 0.39 | HR (CXL) |
| Courgettes | 0.31 | STMR (CXL) | 0.39 | HR (CXL) |
| Melons | 0.05 | STMR (CXL) | 0.15 | HR (CXL) |
| Watermelons | 0.05 | STMR (CXL) | 0.10 | HR (CXL) |
| Sweet corn | 0.01 | STMR (CXL) | 0.02 | HR (CXL) |
| Broccoli | 0.08 | STMR (CXL) | 0.32 | HR (CXL) |
| Cauliflower | 0.08 | STMR (CXL) | 0.32 | HR (CXL) |
| Brussels sprouts | 0.08 | STMR (CXL) | 0.32 | HR (CXL) |
| Head cabbage | 0.08 | STMR (CXL) | 0.32 | HR (CXL) |
| Kale | 1.30 | STMR (CXL) | 2.00 | HR (CXL) |
| Lettuce | 0.90 | STMR (CXL) | 1.20 | HR (CXL) |
| Basil | 5.00 | STMR (CXL) | 7.30 | HR (CXL) |
| Beans (fresh, with pods) | 0.40 | STMR (CXL) | 0.88 | HR (CXL) |
| Beans (fresh, without pods) | 0.40 | STMR (CXL) | 0.88 | HR (CXL) |
| Peas (fresh, with pods) | 0.60 | STMR (CXL) | 3.80 | HR (CXL) |
| Peas (fresh, without pods) | 0.58 | STMR (CXL) | 1.10 | HR (CXL) |
| Celery | 0.37 | STMR (CXL) | 3.20 | HR (CXL) |
| Leek | 0.05* | STMR (CXL) | 0.05* | HR (CXL) |
| Beans (dry) | 0.50 | STMR (CXL) | 1.00 | HR (CXL) |
| Lentils (dry) | 0.50 | STMR (CXL) | 1.00 | HR (CXL) |
| Peas (dry) | 0.50 | STMR (CXL) | 1.00 | HR (CXL) |
| Lupins (dry) | 0.50 | STMR (CXL) | 1.00 | HR (CXL) |
| Peanuts | 0.12 | STMR (CXL) | 0.40 | HR (CXL) |
| Sunflower seed | 0.05* | STMR (CXL) | 0.05* | HR (CXL) |
| Rape seed | 0.05* | STMR (CXL) | 0.05* | HR (CXL) |
| Soya bean | 0.38 | STMR (CXL) | 1.50 | HR (CXL) |
| Olives for oil production | 0.36 | STMR (CXL) | 1.10 | HR (CXL) |
| Barley grain | 0.05 | STMR (CXL) | 0.05 | HR (CXL) |
| Buckwheat grain | 0.05 | STMR (CXL) | 0.05 | HR (CXL) |
| Maize grain | 0.05 | STMR (CXL) | 0.05 | HR (CXL) |
| Millet grain | 0.05 | STMR (CXL) | 0.05 | HR (CXL) |
| Oats grain | 0.05 | STMR (CXL) | 0.05 | HR (CXL) |
| Rice grain | 0.05 | STMR (CXL) | 0.05 | HR (CXL) |
| Rye grain | 0.05 | STMR (CXL) | 0.05 | HR (CXL) |
| Sorghum grain | 0.05 | STMR (CXL) | 0.05 | HR (CXL) |
| Wheat grain | 0.05 | STMR (CXL) | 0.05 | HR (CXL) |
| Tea (dried leaves and stalks, fermented or otherwise of Camellia sinensis) | 6.40 | STMR (CXL) | 28 | HR (CXL) |
| Coffee beans | 0.35 | STMR (CXL) | 0.48 | HR (CXL) |
| Hops (dried), including hop pellets and unconcentrated powder | 0.70 | STMR (CXL) | 5.80 | HR (CXL) |
| Sugar beet (root) | 0.05 | STMR (CXL) | 0.28 | HR (CXL) |
| Swine meat | 0.01 | STMR (CXL) | 0.04 | HR (CXL) |
| Swine fat tissue | 0.01 | STMR (CXL) | 0.02 | HR (CXL) |
| Swine liver | 0.06 | STMR (CXL) | 0.18 | HR (CXL) |
| Swine kidney | 0.06 | STMR (CXL) | 0.18 | HR (CXL) |
| Ruminant meat | 0.01 | STMR (CXL) | 0.04 | HR (CXL) |
| Ruminant fat tissue | 0.01 | STMR (CXL) | 0.02 | HR (CXL) |
| Ruminant liver | 0.06 | STMR (CXL) | 0.18 | HR (CXL) |
| Ruminant kidney | 0.06 | STMR (CXL) | 0.18 | HR (CXL) |
| Poultry meat | 0.01 | STMR (CXL) | 0.01 | HR (CXL) |
| Poultry fat tissue | 0.01 | STMR (CXL) | 0.01 | HR (CXL) |
| Poultry liver | 0.01 | STMR (CXL) | 0.02 | HR (CXL) |
| Ruminant milk | 0.02 | STMR (CXL) | 0.02 | HR (CXL) |
| Birds eggs | 0.01 | STMR (CXL) | 0.01 | HR (CXL) |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; CXL: codex maximum residue limit.
* Indicates that the input value is proposed at the limit of quantification.
Appendix E – Decision tree for deriving MRL recommendations
1.
Appendix F – Used compound codes
1.
| Code/trivial namea | Chemical name/SMILES notationb | Structural formulac |
|---|---|---|
| Imidacloprid |
(E)‐1‐[(6‐chloro‐3‐pyridyl)methyl]‐N‐nitroimidazolidin‐2‐ylideneamine [O‐][N+](=O)/N=C1\NCCN1Cc1cnc(Cl)cc1 YWTYJOPNNQFBPC‐DLSJENCCNA‐N |
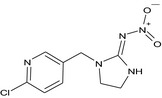
|
| imidacloprid‐5‐hydroxy (M01) |
(5RS)‐1‐[(6‐chloropyridin‐3‐yl)methyl]‐2‐(nitroamino)‐4,5‐dihydro‐1H‐imidazol‐5‐ol [O‐][N+](=O)NC1=NCC(O)N1Cc1cnc(Cl)cc1 MATMQDMQFSFQHB‐UHFFFAOYSA‐N |
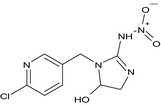
|
| imidacloprid olefin (M06) |
1‐[(6‐chloropyridin‐3‐yl)methyl]‐N‐nitro‐1H‐imidazol‐2‐amine [O‐][N+](=O)Nc1nccn1Cc1cnc(Cl)cc1 TYLCDJYHUVCRBH‐UHFFFAOYSA‐N |
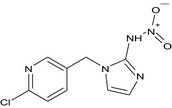
|
| imidacloprid‐desnitro (M09) |
1‐[(6‐chloropyridin‐3‐yl)methyl]‐4,5‐dihydro‐1H‐imidazol‐2‐amine Clc1ncc(CN2CCNC2=N)cc1 UEQZFAGVRGWPDK‐UHFFFAOYSA‐N |
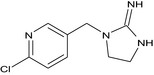
|
| imidacloprid‐6‐CNA (M14) |
6‐chloronicotinic acid OC(=O)c1cnc(Cl)cc1 UAWMVMPAYRWUFX‐UHFFFAOYSA‐N |
|
| imidacloprid‐CHMP (M28) |
(6‐chloropyridin‐3‐yl)methanol OCc1cnc(Cl)cc1 GOXYBEXWMJZLJB‐UHFFFAOYSA‐N |
|
| imidacloprid‐CHMP‐glucoside (M29) |
(6‐chloropyridin‐3‐yl)methyl D‐glucopyranoside Clc1ccc(COC2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cn1 ZRRXFGLNJBNGQI‐AZMJIDJFSA‐N |
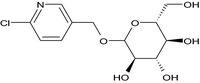
|
| glycine‐conjugate of 6‐chloropyridine‐3‐carboxylic acid |
N‐[(6‐chloropyridin‐3‐yl)carbonyl]glycine O=C(NCC(=O)O)c1cnc(Cl)cc1 VGSLNHSCEKVAIM‐UHFFFAOYSA‐N |
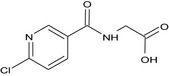
|
SMILES: simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2017.2.1 ACD/Labs 2017 Release (File version N40E41, Build 96719, 6 September 2017).
ACD/ChemSketch 2017.2.1 ACD/Labs 2017 Release (File version C40H41, Build 99535, 14 February 2018).
Appendix G – Alternative MRLs derived considering also the (former) authorised outdoor EU uses
1.
| Code number | Commodity | MRL (mg/kg) | Comment |
|---|---|---|---|
| Enforcement residue definition: imidacloprid | |||
| 110000 | Citrus fruits | 0.9 | Tentative MRL derived from the current import tolerance. Covers also the critical outdoor use for southern Europe |
| 120010 | Almonds | 0.02* | MRL derived from the critical outdoor use for southern Europe |
| 120080 | Pecans | 0.02* | MRL derived from the current import tolerance. No critical outdoor uses were notified for northern and southern Europe |
| 130010 | Apples | 0.09 | MRL derived from the critical outdoor use for southern Europe |
| 130020 | Pears | 0.15 | Tentative MRL derived from the critical outdoor use for southern Europe |
| 130030 | Quinces | 0.06 | MRL derived from the critical outdoor use for northern Europe |
| 140010 | Apricots | 0.2 | Tentative MRL derived from the critical outdoor use for southern Europe |
| 140020 | Cherries (sweet) | 0.3 | MRL derived from the critical outdoor use for southern Europe |
| 140030 | Peaches | 0.2 | MRL derived from the critical outdoor use for southern Europe |
| 140040 | Plums | 0.07 | Tentative MRL derived from the critical outdoor use for southern Europe |
| 151010 | Table grapes | 0.7 | Tentative MRL derived from the current import tolerance. Covers also the critical outdoor uses for northern and southern Europe |
| 151020 | Wine grapes | 0.7 | Tentative MRL derived from the current import tolerance. Covers also the critical outdoor uses for northern and southern Europe |
| 154010 | Blueberries | 5 | Tentative MRL derived from the current import tolerance. No critical outdoor uses were notified for northern and southern Europe |
| 154020 | Cranberries | 5 | Tentative MRL derived from the current import tolerance. No critical outdoor uses were notified for northern and southern Europe |
| 154030 | Currants (black, red and white) | 5 | MRL based on the existing EU MRL (current import tolerance is not supported by residue trials). No critical outdoor uses were notified for northern and southern Europe |
| 154040 | Gooseberries (green, red and yellow) | 5 | MRL based on the existing EU MRL (current import tolerance is not supported by residue trials). No critical outdoor uses were notified for northern and southern Europe |
| 154050 | Rose hips | 5 | MRL based on the existing EU MRL (current import tolerance is not supported by residue trials). No critical outdoor uses were notified for northern and southern Europe |
| 154060 | Mulberries (black and white) | 5 | MRL based on the existing EU MRL (current import tolerance is not supported by residue trials). No critical outdoor uses were notified for northern and southern Europe |
| 154070 | Azaroles/Mediterranean medlars | 0.05 | MRL based on the existing EU MRL (current import tolerance is not supported by residue trials). No critical outdoor uses were notified for northern and southern Europe |
| 154080 | Elderberries | 5 | MRL based on the existing EU MRL (current import tolerance is not supported by residue trials). No critical outdoor uses were notified for northern and southern Europe |
| 161030 | Table olives | 1 | MRL derived from the critical outdoor use for southern Europe |
| 163010 | Avocados | 1 | MRL based on the existing EU MRL (the critical outdoor use for southern Europe is not supported by residue trials) |
| 163020 | Bananas | 0.01* | MRL derived from the current import tolerance. No critical outdoor uses were notified for northern and southern Europe |
| 163030 | Mangoes | 0.2 | MRL based on the existing EU MRL (the critical outdoor use for southern Europe is not supported by residue trials) |
| 163050 | Granate apples/pomegranates | 1 | MRL based on the existing EU MRL (current import tolerance is not supported by residue trials). No critical outdoor uses were notified for northern and southern Europe |
| 211000 | Potatoes | 0.05 | Tentative MRL derived from the critical outdoor use for northern Europe |
| 220010 | Garlic | 0.04 | MRL derived from the critical outdoor use for northern Europe. |
| 220020 | Onions | 0.04 | MRL derived from the critical outdoor use for northern Europe |
| 220030 | Shallots | 0.04 | MRL derived from the critical outdoor use for northern Europe |
| 220040 | Spring onions/green onions and Welsh onions | 0.04 | MRL derived from the critical outdoor use for northern Europe |
| 231010 | Tomatoes | 0.3 | MRL derived from the critical indoor use for northern and southern Europe. Covers also the critical outdoor use for southern Europe |
| 231020 | Sweet peppers/bell peppers | 0.9 | MRL derived from the critical indoor use for northern and southern Europe. An exceedance of the ARfD has been identified for the critical outdoor use for southern Europe |
| 231030 | Aubergines/eggplants | 0.3 | MRL derived from the critical indoor use for northern and southern Europe. Covers also the critical outdoor use for southern Europe |
| 231040 | Okra/lady's fingers | 4 | Tentative MRL derived from the critical outdoor use for southern Europe |
| 232010 | Cucumbers | 0.5 | MRL derived from the critical indoor use for northern and southern Europe. Covers also the critical outdoor use for southern Europe |
| 232020 | Gherkins | 0.4 | MRL derived from the critical indoor use for northern and southern Europe. Covers also the critical outdoor use for southern Europe |
| 232030 | Courgettes | 0.4 | MRL derived from the critical indoor use for northern and southern Europe. Covers also the critical outdoor use for southern Europe |
| 233010 | Melons | 0.15 | Tentative MRL derived from the critical indoor use for northern and southern Europe. Covers also the critical outdoor use for southern Europe |
| 233020 | Pumpkins | 0.15 | Tentative MRL derived from the critical indoor use for northern and southern Europe use. Covers also the critical outdoor use for southern Europe |
| 233030 | Watermelons | 0.15 | Tentative MRL derived from the critical indoor use for northern and southern Europe. Covers also the critical outdoor use for southern Europe |
| 241010 | Broccoli | 0.09 | MRL derived from the critical outdoor use for southern Europe |
| 241020 | Cauliflowers | 0.09 | MRL derived from the critical outdoor use for southern Europe |
| 242010 | Brussels sprouts | 0.15 | MRL derived from the critical outdoor use for northern Europe |
| 242020 | Head cabbages | 0.08 | Tentative MRL derived from the critical outdoor use for southern Europe |
| 243010 | Chinese cabbages/pe‐tsai | 0.5 | MRL derived from the critical outdoor use for southern Europe |
| 243020 | Kales | 0.01* | MRL derived from the critical outdoor use for northern Europe. An exceedance of the ARfD has been identified for the critical outdoor use for southern Europe |
| 244000 | Kohlrabies | 0.3 | MRL based on the existing EU MRL (the critical outdoor use for northern Europe is not supported by residue trials) |
| 251010 | Lamb's lettuces/corn salads | 2 | MRL derived from the critical outdoor use for southern Europe |
| 251020 | Lettuces | 0.6 | MRL derived from the critical outdoor use for southern Europe |
| 251030 | Escaroles/broad‐leaved endives | 0.05 | MRL derived from the critical outdoor use for northern Europe. An exceedance of the ARfD has been identified for the critical outdoor use for southern Europe. |
| 251040 | Cresses and other sprouts and shoots | 2 | MRL derived from the critical outdoor use for southern Europe |
| 251050 | Land cresses | 2 | MRL derived from the critical outdoor use for southern Europe |
| 251060 | Roman rocket/rucola | 2 | MRL derived from the critical outdoor use for southern Europe |
| 251070 | Red mustards | 2 | MRL derived from the critical outdoor use for southern Europe |
| 251080 | Baby leaf crops (including Brassica species) | 2 | MRL derived from the critical outdoor use for southern Europe |
| 255000 | Witloofs/Belgian endives | 0.01* | MRL derived from the critical outdoor use for northern Europe |
| 256000 | Fresh herbs | 2 | MRL derived from the critical outdoor use for southern Europe |
| 260010 | Beans (with pods) | 5 | Tentative MRL derived from the current import tolerance. Covers also the critical outdoor use for southern Europe and the critical indoor use for northern and southern Europe |
| 260020 | Beans (without pods) | 2 | Tentative MRL derived from the current import tolerance. Covers also the critical outdoor use for southern Europe |
| 260030 | Peas (with pods) | 5 | Tentative MRL derived from the current import tolerance. Covers also the critical outdoor use for southern Europe and the critical indoor use for northern and southern Europe |
| 260040 | Peas (without pods) | 2 | Tentative MRL derived from the current import tolerance. Covers also the critical outdoor use for southern Europe |
| 270020 | Cardoons | 0.5 | MRL based on the existing EU MRL (the critical outdoor use for southern Europe is not supported by residue trials) |
| 270050 | Globe artichokes | 0.4 | MRL derived from the critical outdoor use for southern Europe |
| 270060 | Leeks | 0.01* | MRL derived from the critical outdoor use for northern Europe |
| 300010 | Beans (dry) | 2 | Tentative MRL derived from the current import tolerance. No critical outdoor uses were notified for northern and southern Europe |
| 300030 | Peas (dry) | 0.01* | Tentative MRL derived from the critical outdoor use for southern Europe |
| 401020 | Peanuts/groundnuts | 0.5 | Tentative MRL derived from the current import tolerance. No critical outdoor uses were notified for northern and southern Europe |
| 401090 | Cotton seeds | 1 | MRL based on the existing EU MRL (the critical outdoor use for southern Europe is not supported by residue trials) |
| 402010 | Olives for oil production | 0.7 | MRL derived from the critical outdoor use for southern Europe |
| 500010 | Barley grains | 0.01* | MRL derived from the critical outdoor uses for northern and southern Europe |
| 500050 | Oat grains | 0.01* | MRL derived from the critical outdoor uses for northern and southern Europe |
| 500070 | Rye grains | 0.01* | MRL derived from the critical outdoor uses for northern and southern Europe |
| 500090 | Wheat grains | 0.01* | MRL derived from the critical outdoor uses for northern and southern Europe |
| 620000 | Coffee beans | 1 | Tentative MRL derived from the current import tolerance. No critical outdoor uses were notified for northern and southern Europe |
| 700000 | Hops | 15 | Tentative MRL derived from the current import tolerance. Covers also the critical outdoor use for northern Europe |
| 900010 | Sugar beet roots | 0.01* | MRL derived from the critical outdoor uses for northern and southern Europe |
MRL:: maximum residue level; ARfD: acute reference dose.
* Indicates that the MRL is set at the limit of quantification.
Suggested citation: EFSA (European Food Safety Authority) , Abdourahime H, Anastassiadou M, Brancato A, Brocca D, Carrasco Cabrera L, De Lentdecker C, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Medina P, Miron I, Molnar T, Nave S, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2019. Reasoned Opinion on the review of the existing maximum residue levels for imidacloprid according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2019;17(1):5570, 108 pp. 10.2903/j.efsa.2019.5570
Requestor: European Commission
Question number: EFSA‐Q‐2009‐00143
Acknowledgement: EFSA wishes to thank the rapporteur Member State Germany for the preparatory work on this scientific output.
Approved: 12 December 2018
Notes
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Commission Directive 2008/116/EC of 15 December 2008 amending Council Directive 91/414/EEC to include aclonifen, imidacloprid and metazachlor as active substances. OJ No L 337, 15.12.2008, p. 86–91.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187–188.
Regulation (EU) 485/2013 amending Regulation (EU) 540/2011, as regards the conditions of approval of certain active substances, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. OJ L 139, 25.5.2013, p. 12–26.
Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance imidacloprid. OJ L 132, 30.5.2013, p. 31–34.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Belgium , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, September 2016. Available online: http://www.efsa.europa.eu
- Chech Republic , 2016a. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, June 2016. Available online: http://www.efsa.europa.eu
- Chech Republic , 2016b. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, September 2016. Available online: http://www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers’ health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Conclusion on the peer review of the pesticide risk assessment of the active substance imidacloprid. EFSA Journal 2008;6(7):148, 120 pp. 10.2903/j.efsa.2008.148r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance imidacloprid. Available online: http://www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority), 2010. Reasoned opinion on the modification of the existing MRLs for imidacloprid in rice. EFSA Journal 2010;8(4):1589, 24 pp. 10.2903/j.efsa.2010.1589 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Completeness check report on the review of the existing MRLs of active substance prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 17 August 2016. Available online: http://www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2018a. Evaluation of the data on clothianidin, imidacloprid and thiamethoxam for the updated risk assessment to bees for seed treatments and granules in the EU. EFSA supporting publication 2018:EN‐1378. EFSA Journal 2008;6(7):148, 120 pp. 10.2903/j.efsa.2008.148r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2018b. Member States consultation report on the review of the existing MRLs of active substance prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 7 December 2018. Available online: http://www.efsa.europa.eu
- EURLs (European Union Reference Laboratories for Pesticide Residues), 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Analytical methods validated by the EURLs and overall capability of official laboratories to be considered for the review of the existing MRLs for imidacloprid, July 2016. Available online: http://www.efsa.europa.eu
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev.10.3, June 2017.
- European Commission , 2018. Addendum to the Review report for the active substance imidacloprid Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 15 March 2013 in view of the review of imidacloprid as regards the risk to bees in accordance with Article 21 of Regulation (EC) No 1107/20092. Imidacloprid SANCO/10590/2013 rev 8, 27 April 2018.
- FAO (Food and Agriculture Organization of the United Nations), 2008. Imidacloprid. In: Pesticide residues in food – 2008. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. FAO Plant Production and Protection Paper 193.
- FAO (Food and Agriculture Organization of the United Nations), 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Ed. FAO Plant Production and Protection Paper 197, 264 pp. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2015. Imidacloprid. In: Pesticide residues in food – 2015. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. FAO Plant Production and Protection Paper 223.
- France , 2016. Evaluation report prepared under Article 12.1 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing MRLs for imidacloprid, June 2016. Available online: http://www.efsa.europa.eu
- Germany , 2005. Draft assessment report on the active substance imidacloprid prepared by the rapporteur Member State Germany in the framework of Council Directive 91/414/EEC, December 2005. Available online: http://www.efsa.europa.eu
- Germany , 2008. Final addendum to the draft assessment report on the active substance imidacloprid, compiled by EFSA, March 2008. Available online: http://www.efsa.europa.eu
- Germany , 2015. Evaluation report prepared under Article 12.1 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing MRLs for imidacloprid, June 2015. Available online: http://www.efsa.europa.eu
- Germany , 2016. Evaluation report prepared under Article 12.1 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing MRLs for imidacloprid, June 2016. Available online: http://www.efsa.europa.eu
- Greece , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, July 2016. Available online: http://www.efsa.europa.eu.
- Hungary , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, June 2016. Available online: http://www.efsa.europa.eu
- Italy , 2016a. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, July 2016. Available online: http://www.efsa.europa.eu
- Italy , 2016b. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, September 2016. Available online: http://www.efsa.europa.eu
- Netherlands , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, July 2016. Available online: http://www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- Portugal , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, July 2016, amended on September 2016. Available online: http://www.efsa.europa.eu
- Spain , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imidacloprid, October 2016. Available online: http://www.efsa.europa.eu


