Abstract
Background:
Inherited retinal dystrophies are a leading cause of irreversible blindness in children in the United States. Topical carbonic anhydrase inhibitors have improved central vision and cystoid macular edema in patients with retinal dystrophies, but few studies have assessed their efficacy in children.
Materials and Methods:
A retrospective chart review was performed with Institutional Review Board approval to identify pediatric patients with inherited retinal dystrophies who received topical brinzolamide at a single university center between 2008 and 2015. Serial visual acuity and central macular thicknesses were compared to assess efficacy of brinzolamide.
Results:
Seven subjects were identified who met inclusion criteria. Four had juvenile X-linked retinoschisis, two had retinitis pigmentosa, and one had Leber congenital amaurosis. All were prescribed brinzolamide thrice daily; however, one patient was completely non-compliant. Four of the six treated patients exhibited a mild decrease in central macular thickness in both eyes during the study with all six treated patients having significantly improved vision at the first endpoint, 33.2 +/− 8.2 months after treatment initiation. For treated patients, average visual acuity (LogMAR) +/− standard error of the mean improved from 0.5 +/− 0.04 pre-treatment to 0.3 +/− 0.1 at the second endpoint, 50.2 +/− 7.3 months after treatment initiation.
Conclusions:
Mild anatomic improvement of macular cysts was seen in pediatric patients using brinzolamide. Visual acuity improvement occurred even without significant reduction in macular cysts. Further studies are needed to determine whether the beneficial effects of carbonic anhydrase inhibitors are sustained in children with inherited retinal degenerations.
Keywords: Brinzolamide/Carbonic Anhydrase Inhibitors, Inherited retinal dystrophy, Leber congenital amaurosis (LCA), Retinitis pigmentosa (RP), X-linked juvenile retinoschisis (XLRS)
Introduction
Inherited retinal degenerations are among the leading causes of irreversible, progressive blindness in children in the United States. These diseases include X-linked retinoschisis (XLRS), Leber congenital amaurosis (LCA), and retinitis pigmentosa (RP), among others. Macular cysts develop in many of these retinal degenerations and can cause acute decrease in central vision. Optical coherence tomography (OCT) demonstrates that these cysts appear very similar to cystoid macular edema (CME) seen in inflammatory and post-surgery conditions. It is unknown whether the long-term presence of cysts in the macula of children with retinal degenerations hastens permanent visual acuity loss. However, it is known that chronic CME from other causes may result in macular atrophy (1). It is, therefore, reasonable to assume that resolution of the cystic lesions in these children may have both short- and long-term beneficial effects.
Acetazolamide, an oral carbonic anhydrase inhibitor (CAI), was the first CAI shown to inhibit carbonic anhydrase activity in the ciliary body and, subsequently, to decrease aqueous humor secretion (2). Acetazolamide therapy has been demonstrated to have a reductive effect on macular cysts in patients with RP and XLRS (3, 4). However, it can produce systemic side effects of the endocrine, gastrointestinal, cardiovascular, and central nervous systems that many patients may find intolerable (5). Dorzolamide hydrochloride (2%), a topical CAI, has fewer systemic side effects but can produce burning and stinging discomfort in the eyes upon administration in up to 40% of patients (6). Despite its discomfort, dorzolamide has been shown to improve macular cysts in adults with X-linked retinoschisis (7, 8). Apushkin and Fishman reported that topical dorzolamide resulted in a significant visual acuity improvement in five of eight adult patients with XLRS (7).
Unique challenges are present in the pediatric patient population, including compliance and reliance on a caregiver to administer therapy. To date, few studies have been conducted on the use of CAIs in children for the treatment of macular cysts. The newer generation of CAIs is better tolerated by children since they do not cause stinging upon application (9). Brinzolamide (1%), a high-affinity and high-specificity carbonic anhydrase II inhibitor, is one such drug and is FDA-approved for topical use in patients with primary open-angle glaucoma (10). It is also used off-label for treatment of macular cysts, and there are case reports demonstrating favorable outcomes in the reduction of macular cysts in XLRS patients (11).
The aim of this study was to investigate the short-term and longer-term efficacy of topical brinzolamide in pediatric patients with XLRS, RP, and LCA in one pediatric ophthalmic genetics clinic as measured by best-corrected visual acuity (BCVA) and OCT at two endpoints over an average total treatment duration of approximately 50 months.
Materials and Methods
This study was conducted at the Institute for Vision Research and Department of Ophthalmology and Visual Sciences at the University of Iowa. Institutional Review Board approval was obtained to complete a retrospective chart review. Records of 14 eyes of seven patients with LCA, XLRS, or RP met inclusion criteria and were identified for data collection. The subjects were all pediatric patients who were prescribed topical brinzolamide thrice daily in both eyes for macular cysts; they were all seen at the University of Iowa Pediatric Ophthalmic Genetics Clinic at least three times between 2008 and 2015. All patients had molecular genetic testing performed by the John and Marcia Carver Nonprofit Genetic Testing Laboratory at the University of Iowa. Due to the varying nature of patients’ individual treatment courses, the short- and long-term intervals were determined according to data collection benchmarks (i.e., defined time points) rather than treatment duration, as described in detail below.
Data were collected from three time points: the visit at which treatment was prescribed (i.e., the baseline); the visit closest to May 2014 when the study was initiated (i.e., the first endpoint); and the visit closest to November 2015 when data collection was concluded (i.e., the second endpoint). Best corrected visual acuity (BCVA) and central macular thickness (CMT) were analyzed for each eye for comparison between baseline and each of the two study endpoints. Given there is some systemic absorption of the topical drops, the visual acuity and macular thickness were also analyzed as an average between the two eyes for each patient at all three study time points.
All patients were initially prescribed brinzolamide eye drops twice a day in the worse seeing eye, per institutional protocol. This was subsequently increased to both eyes (OU) three times daily if visual acuity was improved in the treated eye at the first follow-up visit and if there were no side effects. Compliance according to the parent/guardian was documented at each visit.
Ophthalmologic Examination
All patients had complete eye examinations upon establishing care at the University of Iowa with special testing as needed for specific signs and symptoms. Measurements evaluated for this study included BCVA and CMT, which were recorded for the visit at which treatment was first prescribed, at the first study endpoint, and at the second study endpoint. Visual acuity was measured using a Snellen chart at a standard distance of 20 feet.
Optical Coherence Tomography (OCT)
Heidelberg Spectralis OCT (Heidelberg Engineering Inc., Heidelberg, Germany) was utilized for imaging of the macula and measuring the CMT. The CMT was measured in microns on the volume scan. OCT data were collected from the visit at which therapy was started, the first study endpoint, and at the second study endpoint.
Statistical Analysis
Decreases in CMT greater than 20% were considered clinically significant as reported by Apushkin, et al (7). BCVA was calculated by converting Snellen acuity to LogMAR for analysis. The averaged BCVA was compared between the three endpoints using one-way analysis of variance (ANOVA), where time was considered a variable. Similarly, the averaged CMT values were compared between the three endpoints using one-way ANOVA with time considered a variable. A post-hoc analysis using the Tukey test was performed for multiple comparisons. The BCVA and CMT data were also analyzed with a mixed effects model fit to OU given that there were three measurements on a subject. Pearson’s correlation coefficient (r) was determined and a linear regression was performed to determine the strength of the association between two variables, CMT and BCVA. For all analyses, p<0.05 was considered statistically significant. All statistical tests and graphs were performed using GraphPad Prism 4.0b for Macintosh (GraphPad Software, San Diego, CA), and all values were reported as mean ± standard error of the mean (SEM).
Results
Seven patients (14 eyes) met inclusion criteria on retrospective chart review. The average age at initial treatment was 6.3 +/− 0.9 (Range: 3–9 years). Among the patients, four had XLRS, two had RP, and one had LCA. All were male patients, and their genetic testing results are found in Table 1. Duration of treatment at first study endpoint ranged from 12–59 months with an average duration of 30.1 +/− 7.6 months. Duration of treatment at second study endpoint, when data were revisited for longer-term evaluation, was an average of 49.3 +/− 6.3 months with a range of 29–75 months.
Table 1:
Best Corrected Visual Acuity and Central Macular Thickness of Pediatric Patients with Inherited Retinal Dystrophies Before and After Topical Brinzolamide Therapy.
| Subject | Diagnosisa | Genetic Mutation |
Age
at Initial Treatment (years) |
Best Corrected Visual Acuity (LogMAR) | Central Macular Thickness (μm) | Duration (months after baseline) |
Complianceb | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Treatment | First Endpoint | Second Endpoint | Pre-Treatment | First Endpoint | Second Endpoint | First Endpoint |
Second Endpoint |
|||||||||||||||||
| OD | OS | OU | OD | OS | OU | OD | OS | OU | OD | OS | OU | OD | OS | OU | OD | OS | OU | |||||||
| 1 | XLRS | RS1 Try89Cys TAT>TGT hemizygous | 3 | 0.4 | 0.3 | 0.35 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 351 | 362 | 356.5 | 369 | 399 | 384 | 406 | 433 | 419.5 | 16 | 29 | I |
| 2 | XLRS | RS1 Try89Cys TAT>TGT hemizygous | 6 | 0.48 | 0.3 | 0.39 | 0 | 0.1 | 0.05 | 0 | 0 | 0 | 228 | 227 | 227.5 | 256 | 241 | 248.5 | 222 | 219 | 220.5 | 59 | 69 | I |
| 3 | XLRS | RS1 Try89Cys TAT>TGT hemizygous | 4 | 0.6 | 0.6 | 0.6 | 0.3 | 0.3 | 0.3 | 0 | 0.1 | 0.05 | 444 | 432 | 438 | 392 | 347 | 369.5 | 442 | 428 | 435 | 59 | 75 | I |
| 4 | LCA | RDH12 GCC>ACC Ala47Thr and TCG>TTG Ser175Leu | 9 | 0.7 | 0.3 | 0.5 | 0.6 | 0.18 | 0.39 | 0.8 | 0.3 | 0.55 | 167 | 191 | 179 | 148 | 187 | 167.5 | 182 | 193 | 187.5 | 24 | 42 | I |
| 5 | XLRP | RPGR Thr801 del2acAG hemizygous | 9 | 0.54 | 0.48 | 0.51 | 0.48 | 0.3 | 0.39 | 0.6 | 0.4 | 0.5 | 432 | 486 | 459 | 485 | 474 | 479.5 | 491 | 505 | 498 | 22 | 46 | I |
| 6 | XLRS | RS1 TGG>CGG Trp96Arg hemizygous | 7 | 1 | 0.48 | 0.74 | 0.48 | 0.48 | 0.48 | 0.48 | 0.54 | 0.51 | 628 | 449 | 538.5 | 341 | 386 | 363.5 | 321 | 305 | 313 | 19 | 40 | B |
| Average (Treated Eyes) | 6.33 | 0.62 | 0.41 | 0.52 | 0.36 | 0.28 | 0.32 | 0.36 | 0.27 | 0.32 | 375.00 | 357.83 | 366.42 | 331.83 | 339.00 | 335.42 | 344.00 | 347.17 | 345.58 | 33.17 | 50.17 | |||
| ± SEM | 1.02 | 0.09 | 0.05 | 0.04 | 0.09 | 0.05 | 0.04 | 0.13 | 0.08 | 0.07 | 67.69 | 50.07 | 57.11 | 47.64 | 43.51 | 45.03 | 50.57 | 51.89 | 51.12 | 8.24 | 7.32 | |||
| 7c | ADRP | RHO Arg135Leu CGG>CTT heterozygous | 6 | 0.18 | 0.7 | 0.44 | 0.3 | 0.8 | 0.55 | 0.1 | 0.6 | 0.35 | 295 | 296 | 295.5 | 292 | 342 | 317 | 271 | 266 | 270.5 | 12 | 44 | N |
| Average (All Eyes) | 6.29 | 0.56 | 0.45 | 0.50 | 0.35 | 0.35 | 0.35 | 0.33 | 0.32 | 0.32 | 363.57 | 349.00 | 356.29 | 326.14 | 339.43 | 332.79 | 333.57 | 335.57 | 334.86 | 28.05 | 44.72 | |||
| ± SEM | 0.87 | 0.10 | 0.06 | 0.05 | 0.07 | 0.09 | 0.06 | 0.12 | 0.08 | 0.08 | 58.34 | 43.23 | 49.31 | 40.66 | 36.78 | 38.15 | 43.99 | 45.36 | 44.51 | 7.60 | 6.25 | |||
Diagnoses:
XLRS = X-linked Juvenile Retinoschisis
LCA = Leber Congenital Amaurosis
XLRP = X-Linked Retinitis Pigmentosa
ADRP = Autosomal Dominant Retinitis Pigmentosa
Compliance:
I: Intermediate (Prescribed TID but used at least BID)
B: Best (Prescribed TID and used TID)
N: Noncompliant (Prescribed TID and not used at all)
Control subject who receive no topical brinzolamide. He received systemic carbonic anhydrase prior to the second endpoint.
All patients were initially prescribed brinzolamide eye drops twice a day in the worse seeing eye, per institutional protocol, and subsequently increased to both eyes three times daily. No adverse effects related to brinzolamide were noted for any subjects. Compliance according to the parent/guardian was documented at each visit. “Intermediate compliance” was defined as application of drops fewer than three times a day but more than once daily on average; Five patients were in this category (Patients 1–5). “Best compliance” was defined as strict adherence to application three times daily as reported by the parent/guardian; One patient achieved this (Patient 6). One patient with RP (Patient 7) was completely non-compliant with brinzolamide and, thus, served as a control (Table 1); however, this patient received oral acetazolamide for a different disease process prior to the second study endpoint.
Central Macular Thickness (CMT)
Four of the six brinzolamide-treated patients had decreased CMT post-treatment in at least one eye at the first study endpoint, and three patients showed improvement in at least one eye from baseline at the second study endpoint (Table 1). The average reduction in CMT among treated eyes was 8.5% from baseline at the first endpoint and 5.7% from baseline at the second endpoint. Comparing all treated eyes across the study, there was no treatment effect on CMT (p=0.4; F(1.3, 14.1)=0.9). We also analyzed the data with a mixed effects model fit OU given there were three measurements per patient (Supplemental Fig.), and age (i.e., time) had an insignificant effect on CMT (p=0.4). In the control patient (Patient 7), the CMT increased by 7.3% at the first endpoint and decreased by 8.0% at the second study endpoint without use of brinzolamide. Marked improvement in CMT and the appearance of cysts on OCT was seen in Patient 6, who had the best-documented brinzolamide compliance.
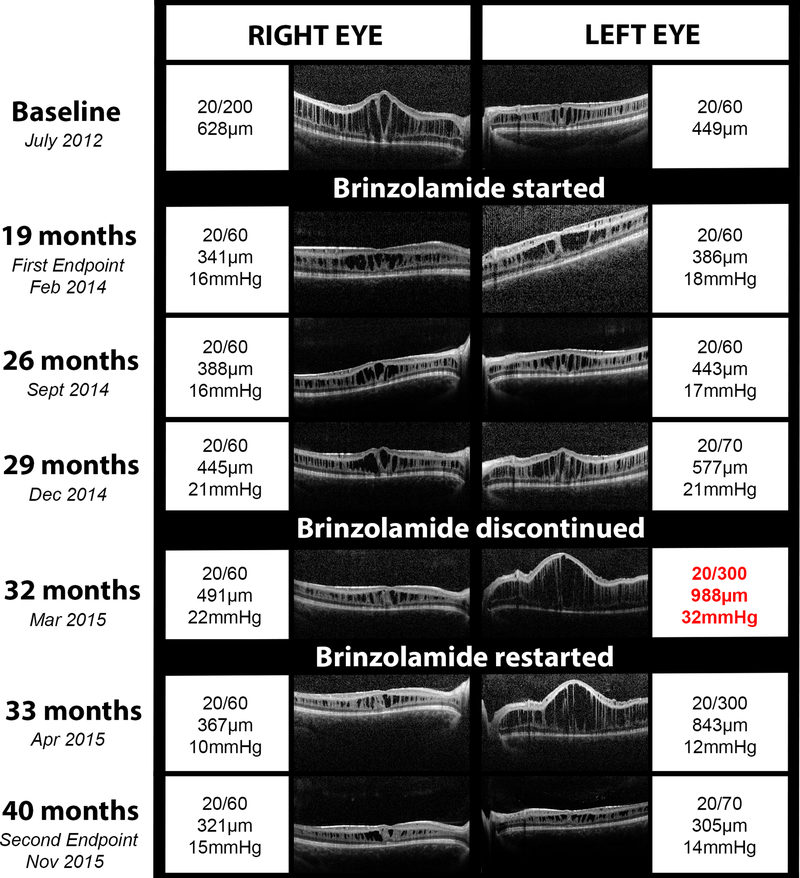
Averaging both eyes, Patient 6 had a 32.5% reduction in CMT at the first endpoint (363.5 microns) and a 41.9% reduction at the second endpoint (313 microns) compared to the baseline measurement (538.5 microns; Figure 4). At the second endpoint, the CMT had decreased by 48.9% in the right eye (OD) and 32.1% in the left eye (OS) from baseline. This was the only patient to achieve significant CMT reduction of greater than 20%. BCVA in this patient improved from 20/200 in the right eye to 20/60 at the first endpoint and then was stable for the duration of the study. The BCVA in the left eye was stable at 20/60 at the first endpoint and slightly decreased to 20/70 at the second endpoint; however, the clinical course of the left eye was atypical and complex as described in detail in the case report below (Fig. 4).
Figure 4. Comparison of optical coherence tomography (OCT), best corrected visual acuity (BCVA), and intraocular pressure (IOP) in a single patient with X-linked retinoschisis and excellent drop compliance.
Serial OCT images from different clinic visits are shown for the right and left eyes with documentation of the BCVA, central macular thickness, and IOP (if recorded) for each eye. Brinzolamide was prescribed as thrice daily dosing in the right eye then the left eye in July 2012. Brinzolamide was discontinued in December 2014, approximately 2.5 years after starting the topical therapy. The patient subsequently developed acute angle closure and recurrence of cystoid macular edema in the left eye (red text). Upon restarting brinzolamide, the macular edema improved in both eyes, the IOP normalized, and the vision returned to baseline.
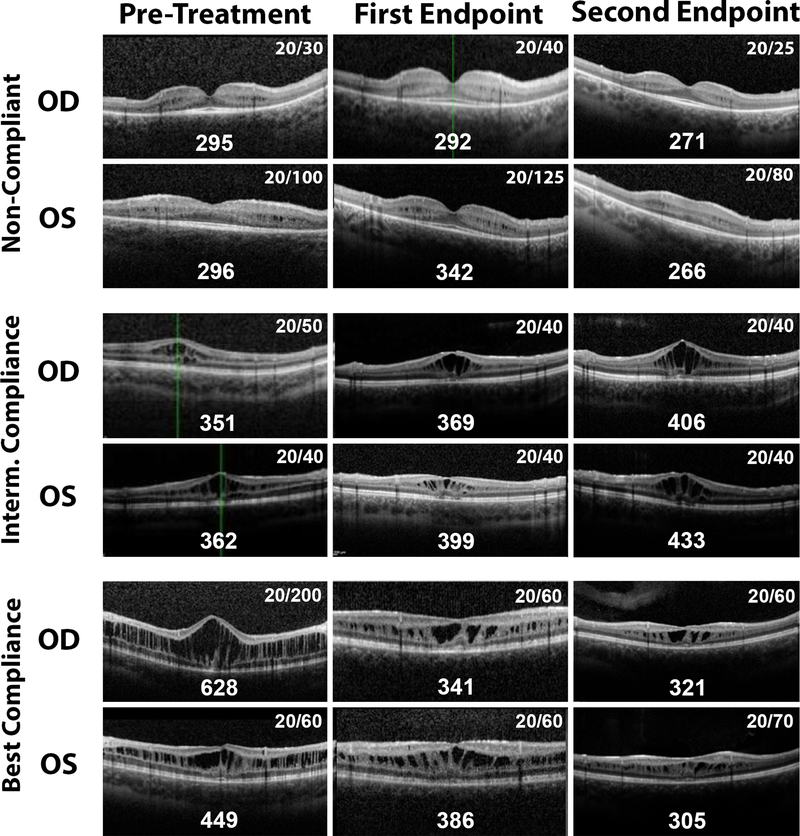
Two other patients (Patients 2 and 3) also exhibited dramatic improvement in BCVA over the study duration. In Patient 2, the averaged CMT between both eyes was actually increased by 9.2% at the first endpoint and then modestly reduced by 3.1% at the second endpoint. In Patient 3, the CMT was decreased 15.6% at the first endpoint, yet the CMT returned to baseline thickness at the second endpoint despite continued BCVA improvement. When comparing all CMT values to the corresponding LogMAR visual acuities over all visits for treated patients, there was no significant correlation between these two variables (R=0.3, p=0.1). OCT images of Patient 1 (Intermediate compliance), Patient 6 (Best Compliance), and Patient 7 (Non-Compliance) are shown in Figure 1.
Figure 1: Optical coherence tomography (OCT) and best corrected visual acuity (BCVA) in the right eye (OD) and the left eye (OS) for three patients with varying medication compliances at baseline and the two study enpoints.
Patient 7’s OCT are shown in the upper panels; he was the only patient who did not use topical brinzolamide in this study. However, he did receive systemic carbonic anhydrase inhibitor therapy prior to the second endpoint. Patient 1 had intermediate compliance with topical drops, and his OCTs are shown in the middle panels. Patient 6 had the best compliance with the treatment regimen, and his OCTs are shown in the bottom panels. The central macular thicknesses in microns are shown along the bottom edge of each OCT image, and the BCVA is listed in the upper right corner of each image.
Best-Corrected Visual Acuity
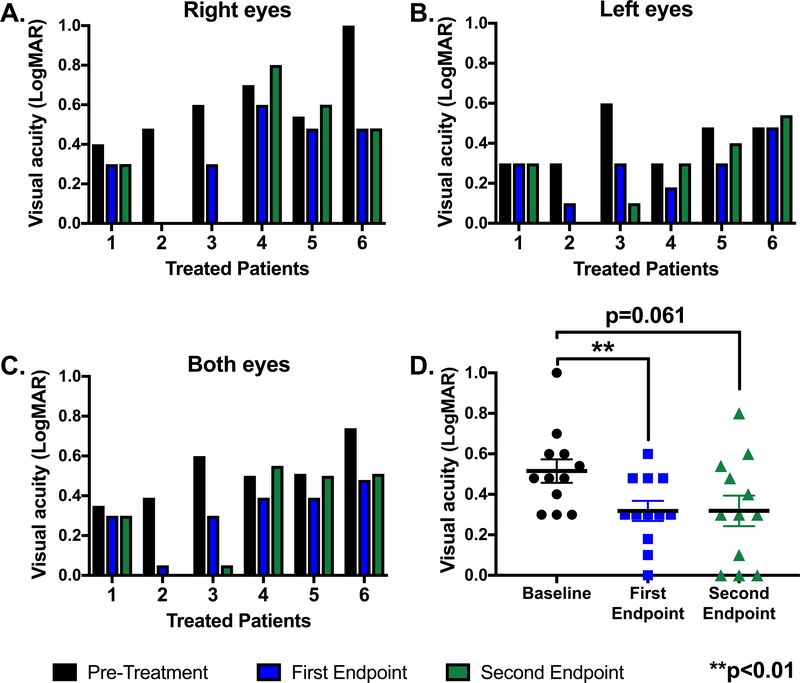
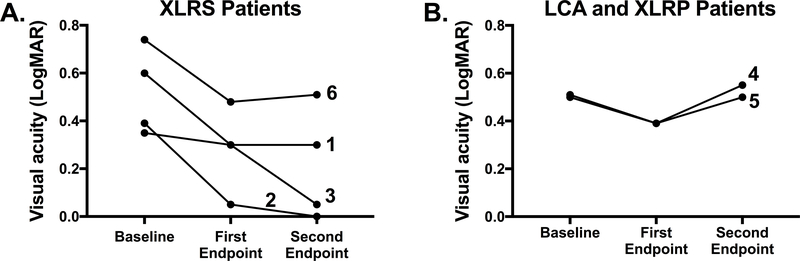
Eleven of the 12 brinzolamide-treated eyes had improved BCVA at the first endpoint (Fig. 2A–B), whereas seven of the treated eyes had improved vision at the second endpoint compared to baseline (Fig. 2A–B). There was a significant treatment effect (p=0.01; F(1.3, 13.9)=7.7) when analyzing the averaged LogMAR scores for brinzolamide-treated eyes over the three study time points (Fig. 2C–D). Averaged BCVA in LogMAR +/− SEM significantly improved from 0.5 +/− 0.04 at baseline to 0.3 +/− 0.04 at the first endpoint (p=0.006) and remained stable at 0.3 +/− 0.07 (p=0.06) at the study’s conclusion. This represented an improvement from 20/66 to 20/41 on the Snellen chart in the treated cohort over the study duration. Significance was lost from the first study endpoint due to a slight increase in variance at the second endpoint. The data were also analyzed with a mixed effects model fit OU given there were three measurements per patient (Supplemental Fig.); there was a significant decrease in LogMAR values (i.e., improvement in BCVA) with increased age of the patients (p=0.002). The BCVA for XLRS patients (Fig. 3A) improved over the study duration more than the patients with LCA or X-linked RP (Fig. 3B).
Figure 2: Best corrected visual acuity of all treated patients at baseline (pre-treatment) and the first and second study endpoints.
The visual acuities for the right eyes (A), left eyes (B) and both eyes averaged (C) for each treated patient are recorded in LogMAR at baseline (black), the first endpoint (blue), and the second endpoint (green). Patients 1, 2, 3 and 6 had XLRS. Patient 4 had LCA, and patient 5 had X-linked RP. D. The individual LogMAR visual acuities per eye for all treated patients at baseline (black), the first endpoint (blue), and the second endpoint (green) are shown as individual points. The black horizontal bars represent the average of all treated eyes at that time point; error bars represent standard error of the mean. A LogMAR acuity of 0.0 indicated 20/20 vision whereas a LogMAR acuity of 1.0 indicated 20/200 on the Snellen chart. Significance was defined as **p<0.01 compared to baseline. Patient 7 was not treated, and, thus, was not included.
Figure 3: Best corrected visual acuity of all treated patients at baseline (pre-treatment) and the first and second study endpoints.
The visual acuities for the patients with X-linked retinoschisis (A) and the patients with LCA or X-linked RP (B) are shown over the three study time points. Each line represents a single treated patient. Patients 1, 2, 3 and 6 had XLRS. Patient 4 had LCA, and patient 5 had X-linked RP. The individual LogMAR visual acuities averaged for both eyes are shown as individual points. A LogMAR acuity of 0.0 indicated 20/20 vision whereas a LogMAR acuity of 1.0 indicated 20/200 on the Snellen chart. Patient 7 was not treated, and, thus, was not included.
In Patient 7, the averaged BCVA in LogMAR worsened from 0.44 (20/55) pre-treatment to 0.55 (20/71) at the first endpoint and then subsequently improved to 0.35 (20/45). Although this patient did not use the prescribed topical brinzolamide, he developed idiopathic intracranial hypertension (IIH) that required oral acetazolamide approximately three months prior to his second endpoint. The subsequent use of a different class of CAI is likely the reason his visual acuity and CMT improved at the second endpoint despite not using brinzolamide. At the visits prior to developing IIH, his BCVA was either stable or slightly worse than at the first endpoint, which was expected of the normal disease progression for autosomal dominant RP.
Case Report
Patient 6 presented with decreased vision at age seven. He had a family history of decreased vision in his maternal grandfather, his grandfather’s brothers, and maternal male cousins of his grandfather. He was found to have bilateral decreased vision, hyperopia, petalloid appearance of both maculas, tortuous vessels, and splitting of the nerve fiber layer in both eyes on OCT. On presentation, the CMT was 628 microns OD and 449 microns OS. BCVA at initial visit was 20/200 OD and 20/60 OS. His findings were consistent with XLRS, which was later confirmed by genetic testing. He was given a new glasses prescription and started on brinzolamide twice daily OD, which was subsequently changed to thrice daily OU, per institution protocol.
At interval visits in the following two years after starting brinzolamide, vision had been stable at 20/60 OD and 20/60 OS. When he presented for routine follow-up in September 2014, he complained of blurred vision in the left eye only; however, vision was stable at 20/60 and CMT had increased only slightly from 386 to 443 microns. The patient’s parents were instructed on strict compliance with drops. In December of 2014, vision in the left eye had decreased to 20/70, and CMT was markedly thicker at 577 microns. Because tachyphylaxis was suspected, a one-month discontinuation of brinzolamide in the left eye was recommended. The patient returned three months later in March 2015 with headaches, markedly increased CMT to 988 microns OS, and increased IOP (22 OD, 32 OS) in the setting of a shallow anterior chamber. The mechanism of angle closure in this child was likely related to forward rotation of the lens-iris diaphragm due to drug-induced ciliary body rotation. Given the patient’s age and discomfort at the time of examination, neither gonioscopy nor ultrasound biometry could not be successfully completed. BCVA was 20/60 OD and 20/300 OS. After reviewing treatment options with the parents, oral acetazolamide was started. Within 24 hours of starting acetazolamide, the patient experienced nausea and vomiting and was switched back to topical brinzolamide three times daily in both eyes without systemic therapy.
Upon returning to clinic six weeks later in April 2015, his visual acuity was stable in the left eye, IOP had normalized, and schisis was slightly improved on OCT. The right eye remained stable during this interval. Visual acuity in the left eye resolved to pre-discontinuation exam levels after five months on brinzolamide thrice daily OU with a follow-up CMT of 305 microns (Fig. 4).
Discussion
This study demonstrates that in pediatric retinal dystrophy patients with cystic macular changes, brinzolamide improves visual acuity and, to a lesser extent, central macular thickness. Our findings corroborate previous reports showing improvement in visual acuity and macular edema after initiating topical CAI therapy for patients with inherited retinal diseases. For example, topical CAIs led to sustained vision improvement in adult patients with RP over a period of 7–15 months in one study (12). Genead, et al., demonstrated that topical dorzolamide improved vision by seven letters or more in 31% of RP patients and decreased the CMT in 67% of the same cohort (13). Similarly, Liew, et al., found that adults with autosomal recessive RP had improvement in CMT after three months of treatment with topical dorzolamide (14). Approximately 40% of eyes receiving dorzolamide showed improvement in intraretinal cysts compared to 28% of eyes in the group treated with oral acetazolamide; however, direct comparisons between the topical and oral treatments cannot be made given the patients were not randomly assigned to treatment groups (14). However, this is, to our knowledge, the first study to document short- and long-term outcomes of topical CAI therapy for patients with inherited retinal diseases.
Unlike prior studies studying CAIs in adult cohorts, this study demonstrates that visual acuity improvement is more robust than, and disproportionate to, macular edema improvement in most children. Visual acuity improvement with topical brinzolamide did not significantly correlate with CMT improvement in children with retinal degenerations. For treated patients in this study, the average CMT modestly decreased by 8.5% at the first study endpoint, whereas the visual acuity had a significant improvement from 0.52 LogMAR to 0.32 LogMAR, a two-line improvement on the Snellen chart. Similarly, Pennesi, et al., recently reported that mean cyst cavity volume determined on OCT had no correlation with visual acuity in 56 XLRS patients (age ≥7 years), yet the patients who started CAI treatment had improved BCVA compared to those who were untreated (15).
These data suggest that CAIs, including brinzolamide, act via multiple pathways to improve vision in these affected children. It is known that brinzolamide inhibits carbonic anhydrase II in the ciliary processes, decreasing aqueous humor secretion and, thereby, decreasing intraocular pressure (16). Iester showed that brinzolamide improved blood flow to the optic nerve in patients with glaucoma as measured by Doppler flowmetry (17, 18). However, in other studies of normal or glaucomatous human eyes, brinzolamide had no significant physiologic effect on vasculature (19, 20). An alternate mechanism of carbonic anhydrase inhibitors includes improving retinal pigment epithelium metabolic pump function, which may contribute to visual acuity improvement without directly decreasing cystoid macular edema appearance on OCT (21). Brinzolamide has been reported to decrease the amplitude of nystagmus in patients with congenital nystagmus syndrome, again suggesting multiple beneficial effects of topical CAIs on the eye and BCVA (22). Interestingly, the patient with the best compliance (Patient 6) was the only patient in this study to have a significant (i.e., greater than 20%) reduction in CMT. Thus, it is possible that at higher doses of brinzolamide the CMT and visual acuity may have a stronger correlation.
All treated patients had a statistically significant improvement in BCVA at the first endpoint after starting topical brinzolamide, whereas the control patient had worsened vision from 0.44 LogMAR (20/55) to 0.55 LogMAR (20/71). By the second endpoint, three treated patients had decreases in vision compared to the first endpoint but were improved or stable compared to baseline. One treated patient remained stable, while two treated patients had continued improvement on long-term follow-up. The two patients who had continued improvement both had XLRS, while the three patients who worsened after initial improvement represented one each of RP, LCA, and XLRS (Figure 3). It is possible that the loss of statistical significance at the second, long-term endpoint was due to worsening of vision in these three patients due to the natural history of the underlying diseases. We suspect that the vision of Patient 6, who had XLRS, was complicated by his clinical course rather than his disease progression (Fig. 4). Both vision and CMT worsened dramatically in the setting of severe schisis and acute angle closure glaucoma secondary to presumed ciliary body rotation in the left eye after a brinzolamide holiday; interestingly, Patient 6 ultimately had improved vision and retinal architecture on OCT compared to baseline after reinstitution and continued use of brinzolamide, despite these clinical challenges.
There have been case studies of adult patients with peripapillary retinoschisis who have been reported to have narrow angles associated with schisis extending into the macula (23), and there have been two recent reports of children with XLRS with narrow angles. The presence of angle-closure glaucoma may be genetically linked to RS1 mutations (24), and one case of refractory angle closure glaucoma in a patient with XLRS that was treated by lens extraction, suggesting pupillary block may contribute (25). Angle closure in pediatric patients and young adults is rare and associated with a variety of congenital diseases and structural etiologies, unlike angle closure in older patients (26). A rebound increase in intraocular pressure due to angle closure has not been reported in glaucoma patients using carbonic anhydrase inhibitors. Thus, it is possible that our patient’s sudden increase in intraocular pressure and macular thickness was unrelated to the discontinuation of brinzolamide, but the transient nature of the increase with subsequent decrease in intraocular pressure and cysts after strict compliance with thrice daily dosing makes this unlikely.
Limitations of this study included the retrospective study design and the fact that compliance is a relatively subjective measurement. We defined compliance as using the medication with strict adherence as prescribed, which, for our purpose, was both eyes three times per day. Although compliance was documented at every clinic visit, the actual use may vary by day and depending on the child’s behaviors. Pediatric patients also rely on parents and guardians to administer eye drops and provide information at clinic visits, which triangulates care. It is also unclear if starting patients on brinzolamide drops in the worse seeing eye prior to the fellow eye, per institutional protocol, limits efficacy in the fellow eye. Given the genetically heterogenous nature of the included patients and the small sample size, one limitation is the inability for these data to be directly extrapolated to all pediatric retinal dystrophies. Gonioscopy was not routinely performed given the young age of the patients in this study; thus, the etiology of the acute angle closure in one patient remains unclear. Finally, brinzolamide is more costly than other CAIs, including dorzolamide, and the cost alone may limit the use of this medication for some families.
In conclusion, brinzolamide is well tolerated in pediatric patients with intraretinal cysts associated with inherited retinal disorders. Improvement in vision may be attained even without improvement on OCT imaging. Resolution of cysts may be dose dependent with best effect seen at three doses per day, but fewer doses may still improve visual acuity as shown by significantly improved vision with intermediate compliance. The benefit of brinzolamide on pediatric patients with retinal degenerations may be assessed by clinical improvement rather than an anatomical change in the macula. If there is worsening of vision and/or thickening of CMT while on brinzolamide, cessation of the medication to give a drug holiday may carry a risk of increased IOP and sudden increase in retinal cysts with shallowing of the angle and decrease in vision. Therefore, starting brinzolamide may commit a patient to long-term treatment, and clinicians should be wary of stopping the drug suddenly.
Supplementary Material
A. The visual acuities for the right eyes, left eyes, and both eyes averaged for each treated patient were recorded in LogMAR at baseline, the first endpoint, and the second endpoint and plotted based on the patient’s age at the time of vision measurement. Patients 1, 2, 3 and 6 had XLRS. Patient 4 had LCA, and patient 5 had X-linked RP. B. The central macular thickness for the right eyes, left eyes and both eyes averaged for each treated patient are recorded at baseline, the first endpoint, and the second endpoint and plotted based on the patient’s age at the time of CMT measurement. A LogMAR acuity of 0.0 indicated 20/20 vision, whereas a LogMAR acuity of 1.0 indicated 20/200 on the Snellen chart. A mixed effects model fit to both eyes demonstrated a significant improvement in (A) BCVA (p=0.0021) but not (B) CMT (p=0.421) as the patients aged. Patient 7 was not treated, and, thus, was not included.
Acknowledgements
The John and Marcia Carver Nonprofit Genetic Testing Laboratory, The Chakraborty Family Foundation, and Research to Prevent Blindness provided support for this study. This project was made possible by funding through the NIH Training Grant (T35 HL 7485–35) at the University of Iowa Carver College of Medicine and the University of Iowa Institute for Vision Research.
Grants and Funding: This work was supported by the University of Iowa Institute for Vision Research Endowment; the Chakraborty Family Foundation; the Ronald Keech Professorship; the Research to Prevent Blindness; and the National Institutes of Health under Training Grant T35 HL 7485–35 (Chen). The Carver Laboratory, University of Iowa, performed molecular genetic testing.
Footnotes
Declaration of interest
The authors alone are responsible for the content and writing of this article.
The authors report no conflicts of interest.
References
- 1.Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23(1):1–7; quiz 137–8. [DOI] [PubMed] [Google Scholar]
- 2.KINSEY VE, REDDY DV Turnover of total carbon dioxide in the aqueous humors and the effect thereon of acetazolamide. AMA Arch Ophthalmol. 1959;62(1):78–83. [DOI] [PubMed] [Google Scholar]
- 3.Fishman GA, Gilbert LD, Fiscella RG, Kimura AE, Jampol LM. Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol. 1989;107(10):1445–52. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Reyes R, Lee W, Chen CL, Chan L, Sujirakul T, et al. Rapid resolution of retinoschisis with acetazolamide. Doc Ophthalmol. 2015;131(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust. 2019;210(4):180–7. [DOI] [PubMed] [Google Scholar]
- 6.Whitson JT, Henry C, Hughes B, Lee DA, Terry S, Fechtner RD. Comparison of the safety and efficacy of dorzolamide 2% and brimonidine 0.2% in patients with glaucoma or ocular hypertension. J Glaucoma. 2004;13(2):168–73. [DOI] [PubMed] [Google Scholar]
- 7.Apushkin MA, Fishman GA. Use of dorzolamide for patients with X-linked retinoschisis. Retina. 2006;26(7):741–5. [DOI] [PubMed] [Google Scholar]
- 8.Collison FT, Genead MA, Fishman GA, Stone EM. Resolution of mid-peripheral schisis in x-linked retinoschisis with the use of dorzolamide. Ophthalmic Genet. 2014;35(2):125–7. [DOI] [PubMed] [Google Scholar]
- 9.Michaud JE, Friren B, Group IBAS. Comparison of topical brinzolamide 1% and dorzolamide 2% eye drops given twice daily in addition to timolol 0.5% in patients with primary open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132(2):235–43. [DOI] [PubMed] [Google Scholar]
- 10.DeSantis L Preclinical overview of brinzolamide. Surv Ophthalmol. 2000;44 Suppl 2:S119–29. [DOI] [PubMed] [Google Scholar]
- 11.Yang FP, Willyasti K, Leo SW. Topical brinzolamide for foveal schisis in juvenile retinoschisis. J AAPOS. 2013;17(2):225–7. [DOI] [PubMed] [Google Scholar]
- 12.Fishman GA, Apushkin MA. Continued use of dorzolamide for the treatment of cystoid macular oedema in patients with retinitis pigmentosa. Br J Ophthalmol. 2007;91(6):743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genead MA, Fishman GA, Walia S. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with X-linked retinoschisis. Arch Ophthalmol. 2010;128(2):190–7. [DOI] [PubMed] [Google Scholar]
- 14.Liew G, Moore AT, Webster AR, Michaelides M. Efficacy and prognostic factors of response to carbonic anhydrase inhibitors in management of cystoid macular edema in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2015;56(3):1531–6. [DOI] [PubMed] [Google Scholar]
- 15.Pennesi ME, Birch DG, Jayasundera KT, Parker M, Tan O, Gurses-Ozden R, et al. Prospective Evaluation of Patients With X-Linked Retinoschisis During 18 Months. Invest Ophthalmol Vis Sci. 2018;59(15):5941–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mincione F, Scozzafava A, Supuran CT. The development of topically acting carbonic anhydrase inhibitors as antiglaucoma agents. Curr Pharm Des. 2008;14(7):649–54. [DOI] [PubMed] [Google Scholar]
- 17.Iester M, Altieri M, Michelson G, Vittone P, Traverso CE, Calabria G. Retinal peripapillary blood flow before and after topical brinzolamide. Ophthalmologica. 2004;218(6):390–6. [DOI] [PubMed] [Google Scholar]
- 18.Iester M Brinzolamide ophthalmic suspension: a review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin Ophthalmol. 2008;2(3):517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaup M, Plange N, Niegel M, Remky A, Arend O. Effects of brinzolamide on ocular haemodynamics in healthy volunteers. Br J Ophthalmol. 2004;88(2):257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siesky B, Harris A, Sines D, Rechtman E, Malinovsky VE, McCranor L, et al. A comparative analysis of the effects of the fixed combination of timolol and dorzolamide versus latanoprost plus timolol on ocular hemodynamics and visual function in patients with primary open-angle glaucoma. J Ocul Pharmacol Ther. 2006;22(5):353–61. [DOI] [PubMed] [Google Scholar]
- 21.Coussa RG, Kapusta MA. Treatment of cystic cavities in X-linked juvenile retinoschisis: The first sequential cross-over treatment regimen with dorzolamide. Am J Ophthalmol Case Rep. 2017;8:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurtell MJ, Leigh RJ. Treatment of nystagmus. Curr Treat Options Neurol. 2012;14(1):60–72. [DOI] [PubMed] [Google Scholar]
- 23.Kahook MY, Noecker RJ, Ishikawa H, Wollstein G, Kagemann L, Wojtkowski M, et al. Peripapillary schisis in glaucoma patients with narrow angles and increased intraocular pressure. Am J Ophthalmol. 2007;143(4):697–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvan H, Sharma A, Birla S, Gupta S, Somarajan BI, Gupta V. Molecular characterization of a rare phenotype of X-linked retinoschisis with angle-closure glaucoma. Indian J Ophthalmol. 2019;67(7):1226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low S, Mohamed R, Ting M, Webster AR, Garway-Heath DF. The treatment of refractory angle-closure glaucoma in a patient with X-linked juvenile retinoschisis. Ophthalmic Genet. 2018;39(5):625–7. [DOI] [PubMed] [Google Scholar]
- 26.Ritch R, Chang BM, Liebmann JM. Angle closure in younger patients. Ophthalmology. 2003;110(10):1880–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. The visual acuities for the right eyes, left eyes, and both eyes averaged for each treated patient were recorded in LogMAR at baseline, the first endpoint, and the second endpoint and plotted based on the patient’s age at the time of vision measurement. Patients 1, 2, 3 and 6 had XLRS. Patient 4 had LCA, and patient 5 had X-linked RP. B. The central macular thickness for the right eyes, left eyes and both eyes averaged for each treated patient are recorded at baseline, the first endpoint, and the second endpoint and plotted based on the patient’s age at the time of CMT measurement. A LogMAR acuity of 0.0 indicated 20/20 vision, whereas a LogMAR acuity of 1.0 indicated 20/200 on the Snellen chart. A mixed effects model fit to both eyes demonstrated a significant improvement in (A) BCVA (p=0.0021) but not (B) CMT (p=0.421) as the patients aged. Patient 7 was not treated, and, thus, was not included.