Abstract
Following a request from the European Commission, EFSA was asked to deliver a scientific opinion on the safety and efficacy of the product TYFER™ (ferric tyrosine chelate) as zootechnical feed additive for chickens, turkeys and minor poultry species for fattening or reared for laying/breeding. The additive is safe for chickens for fattening at the maximum expected level of 200 mg TYFER™/kg complete feed; this conclusion can be extended to chickens reared for laying/breeding and extrapolated to turkeys and all minor poultry species for fattening or reared for laying/breeding. No concerns for consumer safety are expected from the use of the additive in poultry nutrition. The EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) considers that the additive poses a risk to users by inhalation, and should also be considered as an irritant to skin, eyes and mucous membranes. Due to the presence of nickel, ferric tyrosine chelate should also be considered as a dermal and respiratory sensitiser. The supplementation of feed with the additive is not expected to pose an environmental risk. TYFER™ used at the minimum recommended level (20 mg/kg feed) in chickens diets has the potential to improve zootechnical parameters of birds. The additive at 20 mg/kg feed has the potential to reduce the caecal load Campylobacter spp. by at least 1 log10‐units in chickens for fattening, thus with a potential impact to reduce the risk of human campylobacteriosis; however, the Panel notes that the load of Campylobacter in the chickens caecum is one of the multiple factors that contribute to Campylobacter load in carcases‐meat. The conclusions on the efficacy of the additive can be extended to chickens reared for laying/breeding and extrapolated to turkeys and all minor poultry species for fattening or reared for laying/breeding. The Panel recommends including a specification for maximum lithium content in a potential authorisation of the additive.
Keywords: zootechnical additives, gut flora stabilisers, TYFER™, ferric tyrosine chelate, safety, efficacy, Campylobacter
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Akeso Biomedical2 for authorisation of the product TYFER™3 (ferric tyrosine chelate), when used as a feed additive for chickens for fattening, chickens reared for laying, minor poultry species for fattening, minor poultry species to point of lay and turkeys for fattening and rearing to point of lay4 (category: zootechnical additives; functional groups: gut flora stabiliser and other zootechnical additives).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 18 July 2017.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the TYFER™ (ferric tyrosine chelate), when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
TYFER™ is a ferric tyrosine chelate. The product has not been authorised in the European Union (EU) as a feed additive. For the purpose of this opinion the additive will be referred to as TYFER™.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier5 in support of the authorisation request for the use of TYFER™ as a feed additive. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No 1831/2003, Regulation (EC) No 429/20086 and the applicable EFSA guidance documents.
The EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers and other scientific reports, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the TYFER™ (ferric tyrosine chelate), in animal feed. The Executive Summary of the EURL report can be found in Annex A.7
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of TYFER™ is in line with the principles laid down in Regulation (EC) No 429/2008 and the relevant guidance documents: Guidance on zootechnical additives (EFSA FEEDAP Panel, 2012a), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012b) and Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012c).
3. Assessment
TYFER™, a ferric tyrosine chelate, is proposed for use for chickens, turkeys and minor poultry species for fattening or reared for laying, as a zootechnical feed additive under two functional groups with the effects indicated below:
‘4b gut flora stabiliser’ – improvement of zootechnical performance and gut flora by suppression of enteropathogens;
‘4d other zootechnical additives’ – enhanced food product quality by reducing Campylobacter jejuni carriage in birds, thus reducing the risk of human campylobacteriosis.8
3.1. Characterisation
3.1.1. Manufacturing Process
The manufacturing process of the product is fully described in the technical dossier.9
■■■■■■■■■■
■■■■■
3.1.2. Characterisation of the additive
Ferric tyrosine chelate, [Iron, tris(l‐tyrosinato‐κN,κOα)]; synonyms: Iron(III)‐tyrosine chelate, Fe(III)Tyr3, CAS No 202406‐43‐7, has a molecular weight of 596.39 Da and the molecular formula is C27H30FeN3O9.11 The structural formula is shown in Figure 1.
Figure 1.
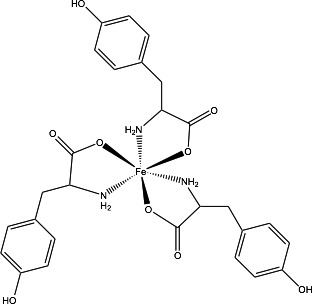
Structural formula of ferric tyrosine chelate
The additive contains by specification a minimum of 82% total tyrosine, 8.0% iron and 6.0% total nitrogen, and a maximum of 3.5% water. The analysis of five batches of the additive confirmed the specifications: total tyrosine was found to be in the range of 84.4–87.9%, iron 9.0–10.3%, nitrogen 6.4–7.0% and water content was between 1.0 and 2.6%.12 The applicant declared that no carriers are added to the ferric tyrosine chelate.
To demonstrate the effective chelation of iron in the additive, three batches of the additive (manufactured in 2016) were analysed by Mössbauer spectroscopy,13 and eight additional batches were analysed for total iron and nitrogen.14 The Mössbauer spectra showed that all the iron in the additive was Fe3+, and it was all chelated to tyrosine in an octahedral geometry with the oxygen of the carboxylate and nitrogen of the amino group bound to the iron. Moreover, the elemental analysis of the total amount of iron and nitrogen of TYFER™ showed that the experimental and theoretical percentages of nitrogen and iron were in good agreement and sustained the molecular formula of TYFER™ with molar ratio 1:3 (Fe3+:Tyrosine).
Undesirable substances were analysed in three batches. Levels of heavy metals (lead: 0.73–12 mg/kg, cadmium: < 0.10 mg/kg), fluorine (< 2.5 mg/kg), arsenic (0.76–0.96 mg/kg), aflatoxin B1 (< 0.2 μg/kg), aflatoxin B2 (< 0.2 μg/kg), aflatoxin G1 (< 0.5 μg/kg), aflatoxin G2 (< 0.2 μg/kg)15 dioxins (0.16–0.44 ng WHO‐PCDD/F‐TEQ per kg) and the sum of dioxins and dioxin‐like PCBs (0.30–0.70 ng WHO‐PCDD/F‐PCB‐TEQ per kg)16 complied with the limits set for compounds of trace elements in Directive 2002/32/EC17 or if not specified do not represent a concern.18 The nickel content, measured on seven batches, showed a high variability, with values between 7 and 118 mg/kg.19 The applicant specified a maximum content of lithium of 0.12%; analysis of 10 batches showed values ranging from 11.0 to 729 mg/kg.12 , 20 Microbial impurities, analysed in the same batches tested for heavy metals, showed Salmonella (25 g sample) absent, total coliforms and Escherichia coli plate counts < 10 colony forming units (CFU)/g, mould and yeasts counts < 100 CFU/g.21
3.1.3. Physical properties
The product is a light brown to brown solid fine powder. It is insoluble in organic solvents or water either at 20–30 or 65–70°C; the additive can be dissolved in acidic water (with hydrochloric acid) at a pH of about 2.22 Density,23 measured on three batches of the additive, ranged from 1.554 to 1.559 g/cm3.
Bulk density,24 particle size25 and dusting potential26 were measured in three batches, results showing a high difference of one batch against the two other batches. One batch had a bulk density of 290 kg/m3, a mean particle size of 18 μm and a dusting potential of 2.330 g/m3; the other two batches showed a bulk density of 387 and 360 kg/m3, a mean particle size of 227 and 265 μm and a dusting potential of 0.14 and 0.09 g/m3. Laser diffraction analysis of the dust from the batch showing the highest dusting potential showed that about 88% were particles of respirable size (< 10 μm).27
3.1.4. Stability and homogeneity
The applicant submitted data on the shelf‐life of TYFER™ showing the chelation in the additive manufactured in 2016, demonstrating that the chelate is stable for approximately 2 years (see Section 3.1.2). Stability data of the additive in premixtures and complete feed were not provided.
The capacity of the additive to homogenously distribute in feed was evaluated by analysing 10 subsamples of a mashed chicken feed containing ‘microtraced’ TYFER™ at 22 mg/kg feed (20 mg TYFER™/kg plus 2 mg microtracer (G‐Red)/kg)28; the coefficient of variation was 10%.29 However, the data confirm only that the microtracer was homogenously distributed; no evidence that the additive or its individual components followed the microtracer in its distribution in feed has been provided.
The applicant tried to deduce homogeneity data of TYFER™ Chelate in mash feed from diets without iron supplementation and graded additions of the additive used in an efficacy study presented in the dossier.30 The analytical data referred to total iron, the control diet contained between 80 and 94 mg Fe/kg, the iron addition due to the additive was only 2, 5 and 20 mg Fe/kg, respectively. The data are not suitable to conclude on the distribution of the additive in the feed.
3.1.5. Conditions of use
The product is intended to be used in feed for chickens, turkeys and minor poultry species for fattening or reared for laying at a minimum level of 20 mg/kg complete feed directly in compound feedingstuffs or via vitamin–mineral premixtures.10
The applicant indicated that the expected usage rates would be 20–200 mg TYFER™/kg feed, delivering 2–20 mg Fe/kg complete feed.
3.2. Safety
3.2.1. Safety for the target species
A tolerance study with 180 one‐day‐old Ross 308 chickens (half females and half males) was performed.31 The trial consisted of a complete randomised block design with five dietary treatments (each consisting of six replicate pens with six birds each), TYFER™ was incorporated into the basal wheat–soybean–barley diet at 0, 20, 200, 1,000 and 2,000 mg/kg feed. The intended levels of the product were confirmed by analyses using a microtracer (G‐red Lake) as a marker. Birds were fed ad libitum in both starter (days 1–20) and grower phase (days 20–35) with diet in mash form. Health status and mortality was monitored throughout the study. Body weight and feed intake were recorded on days 1, 20 and 35; average weight gain, average feed intake and mortality adjusted feed to gain ratio were calculated. At the end of the study, two birds per pen were killed and blood samples were taken to determine blood biochemistry32 and haematology33 profiles; this procedure was spread over 3 days (days 35–37) to facilitate laboratory management of samples. Data were analysed with analysis of variance (ANOVA) with pen as the statistical unit for zootechnical parameters and the individual animal for blood parameters.
The birds remained healthy throughout the study. The overall mortality rate was low (1.6%) and not treatment‐related. There were no significant treatment‐related differences (p > 0.05) on zootechnical performance of birds between the control and the TYFER™ groups; the following average values were obtained for all the treatments: 1.94 kg as average total weight gain, 2.93 kg as average feed intake and 1.51 as feed to gain ratio. Similarly, no treatment‐related effects were noted in haematology or blood biochemistry.
As the additive contains nickel as a contaminant up to 118 mg/kg, the FEEDAP Panel assessed the impact of nickel on safety for the target species. According to the National Research Council (NRC), the nickel maximum tolerable level (MTL) for poultry is 250 mg/kg feed (NRC, 2005). At the maximum expected level of 200 mg TYFER™/kg feed, the nickel added to the feed if supplemented with the additive, would amount to about 0.02 mg Ni/kg feed. Considering the background nickel in feed (i.e. 4 or 9 mg/kg DM feed; Nicholson et al., 1999; Van Paemel et al., 2010; EFSA CONTAM Panel, 2015), the contribution from TYFER™ would be negligible. Therefore, the nickel content of the additive does not represent a safety concern for the target species.
The FEEDAP Panel also considered the lithium content of the additive. According to the NRC, the lithium MTL for poultry is about 25 mg/kg feed (NRC, 2005). At the maximum use level indicated by the applicant, 200 mg TYFER™/kg feed, the lithium added to the feed (considering the specification of ≤ 0.12% Li in the additive) would not exceed 0.24 mg/kg feed. The NRC also described the lithium content of various feedingstuffs grown on lithium‐rich and lithium‐poor soils, ranging from 0.5 to 4.1 mg/kg. Therefore, the lithium contribution from the additive, even at the highest expected amount, would not represent a safety concern for the target species.
3.2.1.1. Conclusion on the safety for the target species
Based on the results from a tolerance study demonstrating that chickens for fattening tolerated 2,000 mg TYFER™/kg feed without any adverse effects on health, performance or blood parameters, the FEEDAP Panel concludes that the additive is safe for chickens for fattening at the maximum recommended level of 200 mg TYFER™/kg. This conclusion can be extended to chickens reared for laying/breeding and extrapolated to turkeys and all minor poultry species for fattening or reared for laying/breeding.
3.2.2. Safety for the consumer
The applicant did not provide any specific studies to support the safety for the consumers.
The safety for consumers of foods derived from animals whose diets were supplemented with iron compounds, including chelate of amino acids, has been reviewed and assessed by the FEEDAP Panel (EFSA FEEDAP Panel, 2013a, 2014a,b, 2015, 2016a,b,c). It was concluded that there was no evidence that iron from chelate of amino acids used up to maximum authorised levels of iron in feeds for poultry34 would lead to a significantly increased iron concentration in edible tissues and products of animal origin. With respect to the amino acid tyrosine, when supplemented in feedingstuffs, it is incorporated in the body protein of the animal without any change of the protein composition or tissue storage (EFSA FEEDAP Panel, 2013b).
Therefore, the use of TYFER™ in poultry feed is not expected to raise any concern for consumers under the proposed conditions of use.
3.2.3. Safety for the user
No specific studies were provided by the applicant regarding the toxicity of the additive for the users/workers.
3.2.3.1. Effects on the respiratory system
The product under assessment has a significant proportion of particles of respirable size (< 10 μm). The dusting potential showed a wide range of variation between 0.085 and 2.330 g/m3, corresponding to an iron concentration in the dust up to 0.23 g/m3. Inhalation of iron salts may cause serious lung problems (Nemery, 1990). The toxicity of inhaled iron is still under scientific debate (IARC, 1987; Weinberg, 1999; Wild et al., 2009; Ponka et al., 2015). The American Conference of Governmental Industrial Hygienists has set a threshold limit value (TLV) for an 8‐h time‐weighted average (TWA) for iron salts (soluble) of 1 mg/m3 expressed as iron (ACGIH, 2008), which is in agreement with the standards applied in some European countries (Belgium, Finland, Italy, the Netherlands and Switzerland). Handling ferric‐tyrosine chelate leads to an exposure exceeding TLV by more than two orders of magnitude thus indicating a risk by inhalation for users.
The nickel content of the product is up to 118 mg/kg additive. Inhalation of nickel can cause pulmonary toxicity, resulting in bronchitis, fibrosis and lung cancer in humans (Nemery, 1990). The proposed occupational exposure limit (OEL) for the inhalable fraction of water soluble nickel is 0.01 mg Ni/m3 (European Commission, 2011). According to the dusting potential of the product, and assuming equal content as in the additive, the nickel content in the dust would be up to 0.27 mg/m3; therefore, the nickel OEL is exceeded by more than one order of magnitude indicating a risk by inhalation for users.
The FEEDAP Panel recognises that the use of the TLV or OEL as guidance values for user safety of feed additives may result in overly conservative assessments, as the exposure is unlikely to be as continuous and intense as in an industrial scenario, for which TLVs/OELs have been envisaged. Nevertheless, even with the mentioned caveat, a concentration of iron or nickel in the inhalable dust exceeding the guidance values by at least one order of magnitude points to a risk by inhalation for users.
3.2.3.2. Effects on skin and eyes
In the absence of specific studies and considering that several iron compounds are recognised as irritants to skin, eyes and mucous membranes (EFSA FEEDAP Panel, 2013a, 2014a,b, 2015, 2016a,b,c), the product should be considered as a skin, eye and respiratory irritant. Owing to the well‐known sensitisation potential of nickel (European Commission, 2011), the product should be considered as a dermal and respiratory sensitiser.
3.2.3.3. Conclusions on safety for the user
Users may be exposed to iron and nickel from the additive by inhalation at levels exceeding the TLV/OEL values by at least two and one orders of magnitude, respectively. The FEEDAP Panel considers that the compound under assessment poses a risk to users by inhalation. The product should also be considered as an irritant to skin, eyes and mucous membranes. Due to the presence of nickel, TYFER™ should also be considered as a dermal and respiratory sensitiser.
3.2.4. Safety for the environment
The components of the additive, iron and tyrosine, are ubiquitous in the environment. The iron content of soils is typically in the range of 5,000–50,000 mg/kg while tyrosine is a physiological and natural component of animals and plants.
Based on the calculation method provided in the technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008), the highest increase of iron in soil is around 0.12 mg/kg after a 1‐year application of manure from chickens for fattening assuming that 100% of the dose will be excreted. Therefore, any additional load from the use of the product in poultry feed is not expected to pose an environmental risk.
3.3. Efficacy
The applicant claimed two effects from the use of the additive: improvement of zootechnical performance and gut flora by suppression of enteropathogens and enhancing food product quality by reducing C. jejuni carriage in birds.
In order to support the efficacy of the product five in vitro 35 , 36 , 37 , 38 , 39 and six in vivo studies were provided by the applicant. None of the in vitro studies was considered relevant for the assessment, owing to the lack of mimicking to the in vivo conditions.
The long term efficacy studies were performed in chickens for fattening. In all six studies performance was measured and the load of E. coli and Campylobacter in caecum was tested in three and four studies, respectively.
3.3.1. Efficacy studies in chickens for fattening
All six efficacy studies were carried out in a single Member State at two different locations and shared a common design. The details on the study designs are provided in Table 1.
Table 1.
Summary of the design of the efficacy studies performed in chickens for fattening
| Study | Total no of animals Replicates per treatment (animals per replicate) | Composition basal diet (feed form) | Intended TYFER™ supplementation (mg/kg feed) | End points | |
|---|---|---|---|---|---|
| Zootechnical | Microbiological load in caecum | ||||
| 1a |
480 8 (10) |
Barley, wheat, soya (crumbs: starter; pellets: grower/finisher) |
0 10 20 50 100 200 |
✓ | ✓ |
| 2b |
576 12 (12) |
Barley, wheat, soya (mash) |
0 20 50 200 |
✓ | No |
| 3c |
1,568 14 (14) |
Wheat, barley, soybean meal (mash) |
0 10 20 50 100 200 400 600 |
✓ | No |
| 4d |
840 6 (35) |
Wheat, barley, soybean meal (mash) |
0 20 50 200 |
✓ | ✓ |
| 5e |
800 10 (20) |
Barley, wheat, soya (mash) |
0 20 50 200 |
✓ | ✓ |
| 6f |
1,120 8 (35) |
Maize, soybean meal (mash) |
0 20 50 200 |
✓ | ✓ |
Technical dossier/Section IV/Annex IV.3.1.
Technical dossier/Section IV/Annex IV.3.2.
Technical dossier/Section IV/Annex IV.3.3.
Technical dossier/Section IV/Annex IV.3.4.
Technical dossier/Section IV/Annex IV.3.5.
Technical dossier/Supplementary Information (November 2017)/Annex IV‐3‐8.
In all cases, 1‐day‐old male Ross 308 birds were used in the studies and trials lasted 42 days. The intended content of TYFER™ in diets (ranging from 0 to 600 mg/kg feed) was confirmed analytically by using a microtracer. The health and mortality were monitored throughout the study and the body weight and feed intake were recorded; weight gain and total feed intake per animal and feed to gain ratio (mortality adjusted) were calculated. The data obtained were subjected to analysis of variance using the pen as the experimental unit, followed by a Tukey post hoc test.
Zootechnical parameters were measured in studies 1–6 (main results reported in Table 2). Microbiological parameters, based on Campylobacter spp. and E. coli counts in caecum, were tested in studies 1, 4, 5 and 6 (main results reported in Table 3). Different media (CCDA and Brilliance) were used for the determination of Campylobacter. The applicant argued that the Brilliance media improved specificity (up to 99%) towards C. jejuni and E. coli in broiler caeca, faeces and litter compared to CCDA plates (91% specificity).40 However, Brilliance media was used only in two out of the four studies in which the relevant microbial counts were performed.
Table 2.
Effects of TYFER™ on the zootechnical performance of chickens for fattening. Parameters reported at 42 days for studies 1, 2, 3 and 6, at 41 days for study 4 and at 39 days for study 5
| Study | Intended TYFER™ supplementation (mg/kg feed) | Feed intake (kg) | Body weight (kg) | Weight gain (kg) | Feed to gain ratio | Mortality (%)* |
|---|---|---|---|---|---|---|
| 1 | 0 | 5.36 | 3.34 a | 3.31 b | 1.637 a | 5.0 |
| 10 | 5.30 | 3.48 ab | 3.44 ab | 1.546 b | 7.5 | |
| 20 | 5.31 | 3.47 ab | 3.43 ab | 1.550 b | 2.5 | |
| 50 | 5.21 | 3.40 ab | 3.36 ab | 1.550 b | 6.3 | |
| 100 | 5.35 | 3.53 b | 3.49 a | 1.543 b | 6.3 | |
| 200 | 5.35 | 3.53 b | 3.49 a | 1.536 b | 3.8 | |
| 2 | 0 | 4.82 | 3.13 a | 3.08 b | 1.566 | 3.5 |
| 20 | 4.83 | 3.22 ab | 3.15 ab | 1.522 | 2.8 | |
| 50 | 4.73 | 3.11 a | 3.07 b | 1.549 | 1.3 | |
| 200 | 4.93 | 3.30 b | 3.26 a | 1.523 | 2.8 | |
| 3 | 0 | 4.37 ab | 2.68 ab | 2.64 ab | 1.659 | 2.6 |
| 10 | 4.59 abc | 2.78 ab | 2.73 ab | 1.680 | 1.0 | |
| 20 | 4.40 ab | 2.69 ab | 2.64 ab | 1.667 | 3.1 | |
| 50 | 4.72 c | 2.85 b | 2.80 a | 1.690 | 2.0 | |
| 100 | 4.52 abc | 2.76 ab | 2.71 ab | 1.668 | 3.6 | |
| 200 | 4.28 a | 2.61 a | 2.56 b | 1.672 | 2.6 | |
| 400 | 4.44 abc | 2.71 ab | 2.66 ab | 1.670 | 1.5 | |
| 600 | 4.65 bc | 2.81 b | 2.76 a | 1.683 | 4.6 | |
| 4 | 0 | 3.22 a | 1.87 a | 1.82 b | 1.765 | 2.9 |
| 20 | 3.61 b | 2.08 b | 2.04 a | 1.771 | 1.9 | |
| 50 | 3.61 b | 2.05 b | 2.01 a | 1.798 | 1.9 | |
| 200 | 3.48 b | 2.05 b | 2.01 a | 1.736 | 2.9 | |
| 5 | 0 | 4.03 b | 2.66 a | 2.62 b | 1.544 a | 6.0 |
| 20 | 3.99 ab | 2.73 ab | 2.70 ab | 1.495 b | 6.5 | |
| 50 | 3.89 a | 2.70 a | 2.66 b | 1.471 b | 3.5 | |
| 200 | 4.03 b | 2.79 b | 2.75 a | 1.472 b | 6.5 | |
| 6 | 0 | 3.69 | 2.15 | 2.11 | 1.746 | 3.6 |
| 20 | 3.74 | 2.18 | 2.14 | 1.748 | 2.5 | |
| 50 | 3.80 | 2.21 | 2.18 | 1.747 | 3.9 | |
| 200 | 3.69 | 2.16 | 2.13 | 1.736 | 3.2 |
* Including culling.
a,b,c For a given study and an specific parameter, different superscript within a column indicates significant differences (p ≤ 0.05).
Table 3.
Effects of TYFER™ on the microbiological load of caeca of chickens for fattening measured at 42 days
| Study | Intended TYFER™ supplementation (mg/kg feed) | Microbial load (log10 CFU/g) | ||
|---|---|---|---|---|
| Campylobacter spp. | E. coli | |||
| CCDA medium | Brilliance medium | |||
| 1 | 0 | 5.1 a | ||
| 10 | 4.7 ab | |||
| 20 | 4.7 ab | |||
| 50 | 4.4 b | |||
| 100 | 4.6 a | |||
| 200 | 4.5 b | |||
| 4 | 0 | 5.9 a | 4.8 a | 6.4 a |
| 20 | 5.0 ab | 3.6 b | 5.4 b | |
| 50 | 4.1 bc | 2.4 c | 5.7 ab | |
| 200 | 3.4 c | 1.6 c | 5.1 b | |
| 5 | 0 | 5.9 a | 7.9 a | |
| 20 | 5.1 ab | 7.2 ab | ||
| 50 | 4.0 b | 7.1 ab | ||
| 200 | 3.9 b | 6.7 b | ||
| 6 | 0 | 4.9 a | 5.0 a | 6.0 a |
| 20 | 3.7 b | 4.0 b | 5.5 b | |
| 50 | 2.5 c | 3.0 b | 5.2 b | |
| 200 | 2.9 bc | 3.2 b | 5.3 b | |
CFU: colony forming unit.
a,b,c For a given study and an specific parameter, different superscript within a column indicates significant differences (p ≤ 0.05).
The testing of the microbiological load for Campylobacter spp. and E. coli was done as follows:
Study 1: At 20 days of age, litter from a commercial flock that had tested positive for Campylobacter spp. was added (approx. 2 kg/pen) to all pens. At the end of the study, all birds were killed and pooled caecal content per pen were used for Campylobacter spp. enumeration (CCDA medium).
Study 4: At 42 days, 5 birds per pen (total 150 animals) were killed and caeca removed and submitted to analysis of Campylobacter spp. enumeration (CCDA and Brilliance media).
Study 5: At 20 days of age, all birds including control were challenged with C. jejuni through ‘seeded litter tray procedure’.41 At 42 days, 10 birds per pen were killed and caeca removed and submitted to analysis of Campylobacter spp. (CCDA medium) and E. coli counts.
Study 6: At 42 days, 6 birds per pen (total 192 animals) were killed and caeca removed and submitted to analysis of Campylobacter spp. (CCDA and Brilliance media) and E. coli counts.
Feed to gain ratio was significantly improved at 10 mg TYFER™/kg in study 1 and at the minimum recommended dose (20 mg/kg) in another study (study 5), while increased body weight and weight gain was observed in study 4 also at 20 mg/kg and 200 mg in another study (study 2); no significant improvements were shown in performance in the other three studies at the minimum recommended level.
The zootechnical data from the six above described studies were pooled and subjected to an ANOVA.42 The model included TYFER™ supplementation (control vs additive at 20 mg/kg feed) and study as main effects and the interaction between study and treatment; significance was declared at p ≤ 0.05. No significant interaction between TYFER™ supplementation and study was detected for body weight, weight gain or feed to gain ratio. At study end, birds fed the diets supplemented with TYFER™ at the minimum recommended level of 20 mg/kg compared to the control had significantly higher weight gain (2.74 vs 2.66 kg) and better feed to gain ratio (1.623 vs 1.644).
Due to the inherent variability of the microbial analysis, small differences in counts (< 1 log‐unit) have no microbiological significance.
The use of the additive significantly reduced Campylobacter spp. caecal counts by at least 1 log reduction in two of the studies provided at 20 mg/kg feed (the minimum recommended level), and in another study at 50 mg TYFER™/kg feed (Table 3).
Data from trials 1, 4, 5 and 6 were pooled to examine the outcome of the caecal Campylobacter spp. counts.43 The pooled data were subjected to ANOVA. The model included TYFER™ supplementation (control vs additive at 20 mg/kg feed) and study as main effects, and the interaction between study and treatment. Significance was declared at p ≤ 0.05. Data showed that Campylobacter spp. counts (CCDA medium) were significantly reduced in caeca of chickens fed TYFER™ at the minimum recommended level (20 mg/kg feed) compared to chickens receiving the control diet: 4.55 log 10 CFU/g vs. 5.47 log 10 CFU/g.
On the basis of monitoring data in four EU countries and applying a model to the data, the EFSA Panel on Biological Hazards (BIOHAZ Panel), indicated that reducing the numbers of Campylobacter in the intestines of chickens at slaughter by 1 log‐unit, by 2 log‐units, by 3 log‐units and by 6 log10‐units would reduce to at least 65%, 91%, 98% and 100%, respectively, the public health risk – human cases of campylobacteriosis (EFSA BIOHAZ Panel, 2011). In the same opinion, the BIOHAZ Panel reported that, on the basis of published data, reducing the numbers of Campylobacter in the intestines at slaughter by 1, 2, and 3 log10‐units would reduce the public health risk‐human cases of campylobacteriosis by at least 48%, 76% and 90%, respectively, while the 100% would be reached with the 6 log‐units.
The FEEDAP Panel retains that a reduction of at least 1 log‐unit in Campylobacter in caecum is required to support a potential to reduce the carriage in carcases/meat and, consequently, the risk of human campylobacteriosis.
No experimental data, performed with the additive, were provided by the applicant to support the claim of food quality product enhancement consequent to the reduction of C. jejuni in the caecum of the birds. Instead, a literature search was performed by the applicant using the database PubMed and covering the period from the year 1992–2018.11 The following keywords were used: Campylobacter, chicken/broiler, contaminated/contamination, caeca/ceca, carcass/surface. A total of 17 hits were found, of which only four were considered relevant by the applicant: Allen et al. (2007), Reich et al. (2008), Chokboonmongkol et al. (2013) and Seliwiorstow et al. (2015). Only Reich et al. (2008) showed a relationship between Campylobacter colonisation in caecum and carcass Campylobacter load. The BIOHAZ Panel reported further evidence indicating that such a correlation exists; however other factors, apart from initial caecal load, influence the microbial meat contamination, including that of Campylobacter (see EFSA BIOHAZ Panel, 2011).
The use of the additive significantly reduced with > 1 log the load of E. coli in two studies, one at 20 mg/kg feed (the minimum recommended level) and in another at 200 mg TYFER™/kg feed (Table 3).
3.3.1.1. Conclusions on efficacy
The FEEDAP Panel concludes that TYFER™ used at the minimum recommended level (20 mg/kg feed) in diets for chickens has the potential to improve zootechnical parameters of birds.
The additive, used at 20 mg/kg feed, has the potential to reduce the caecal load Campylobacter spp. by at least 1 log10‐units in chickens for fattening, thus with a potential impact to reduce the risk of human campylobacteriosis. However, the FEEDAP Panel notes that the load of Campylobacter in the caecum of chickens for fattening is one of the multiple factors that contribute to the Campylobacter load in carcases‐meat.
These conclusions can be extended to chickens reared for laying/breeding and extrapolated to turkeys and all minor poultry species for fattening or reared for laying/breeding.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation44 and Good Manufacturing Practice.
4. Conclusions
The additive is safe for chickens for fattening at the maximum expected level of 200 mg TYFER™/kg complete feed. This conclusion can be extended to chickens reared for laying and extrapolated to turkeys and all minor poultry species for fattening or reared for laying or reared for breeding.
No concerns for consumer safety are expected from the use of the additive in poultry nutrition.
The FEEDAP Panel considers that the compound under assessment poses a risk to users by inhalation. The product should also be considered as an irritant to skin, eyes and mucous membranes. Due to the presence of nickel, ferric tyrosine chelate should also be considered as a dermal and respiratory sensitiser.
The supplementation of feed with the additive is not expected to pose an environmental risk.
The FEEDAP Panel concludes that TYFER™ used at the minimum recommended level (20 mg/kg feed) in diets for chickens has the potential to improve zootechnical parameters of birds. The additive used at 20 mg/kg feed has the potential to reduce the caecal load Campylobacter spp. by at least 1 log10‐units in chickens for fattening, with a potential impact on the reduction of the risk of human campylobacteriosis. These conclusions can be extended to chickens reared for laying/breeding and extrapolated to turkeys and all minor poultry species for fattening or reared for laying/breeding.
5. Recommendation
The FEEDAP Panel recommends a potential authorisation of the additive to include the specification for a maximum lithium content of 0.12%.
Documentation provided to EFSA
TYFER™, Ferric tyrosine chelate. April 2017. Submitted by AKESO BIOMEDICAL, INC.
TYFER™, Ferric tyrosine chelate. Supplementary information. November 2017. Submitted by AKESO BIOMEDICAL, INC.
TYFER™, Ferric tyrosine chelate. Supplementary information. January 2018. Submitted by AKESO BIOMEDICAL, INC.
TYFER™, Ferric tyrosine chelate. Supplementary information. July 2018. Submitted by AKESO BIOMEDICAL, INC.
TYFER™, Ferric tyrosine chelate. Spontaneous information. September 2018. Submitted by e‐mail by AKESO BIOMEDICAL, INC.
TYFER™, Ferric tyrosine chelate. Supplementary information. December 2018. Submitted by AKESO BIOMEDICAL, INC.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the Methods(s) of Analysis for Ferric tyrosine chelate (TYPLEX®).
Comments from Member States.
Chronology
| Date | Event |
|---|---|
| 15/5/2017 | Dossier received by EFSA |
| 6/6/2017 | Reception mandate from the European Commission |
| 18/7/2017 | Application validated by EFSA – Start of the scientific assessment |
| 12/10/2017 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation, safety for target species and efficacy |
| 7/9/2017 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 18/10/2017 | Comments received from Member States |
| 8/11/2017 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 19/1/2018 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issues: Characterisation |
| 30/1/2018 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 1/6/2018 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issues: Characterisation |
| 19/7/2018 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 25/9/2018 | Reception of spontaneous information from the applicant |
| 9/11/2018 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issues: Characterisation, Efficacy |
| 20/12/2018 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 23/1/2019 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
| 29/1/2019 | Request of the applicant to change brand name of the additive |
Abbreviations
- AAS
atomic absorption spectrometry
- ANOVA
analysis of variance
- CAS
Chemical Abstracts Service
- CFU
colony forming units
- DM
dry matter
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- ICP‐AES
inductively coupled plasma atomic emission spectrometry
- ICP‐MS
inductively coupled plasma mass spectrometry
- MTL
maximum tolerable level
- NRC
National Research Council
- OEL
occupational exposure limit
- PCB
polychlorinated biphenyls
- PCDD/F
polychlorinated dibenzo‐p‐dioxins and dibenzofurans
- Rrec
recovery rate
- TEQ
toxic equivalent
- TLV
threshold limit value
- TWA
time‐weighted average
- WHO
World Health Organization
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for Ferric tyrosine chelate (TYPLEX®)
1.
In the current application authorisation is sought under article 4(1) for ferric tyrosine chelate under the category/functional group (4 b, d) “zootechnical additives”/“gut flora stabilisers”, “other zootechnical additives”, according to the classification system of Annex I of Regulation (EC) No 1831/2003. Specifically, authorisation is sought for the use of the feed additive for all poultry species and categories. The feed additive (ferric tyrosine chelate) is a light brown powder containing a minimum of 8.4% iron (w/w) and 75 to 79% (w/w) tyrosine. The feed additive is intended to be incorporated into feedingstuffs through premixtures with a proposed minimum ferric tyrosine chelate content of 20 mg/kg feedingstuffs.
For the quantification of total iron content in the ferric tyrosine chelate (feed additive) the Applicant submitted a single‐laboratory validated method derived from the official method AOAC 993.14, based on inductively coupled plasma mass spectrometry (ICP‐MS) after pressure digestion. In the frame of the iron group dossiers the EURL evaluated and recommended for official control three CEN ring‐trial validated methods for the quantification of total iron in feed additives: method EN 15510 based on inductively coupled plasma atomic emission spectrometry (ICP‐AES); method EN 15621 based on ICP‐AES after pressure digestion; and method EN ISO 6869 based on atomic absorption spectrometry (AAS).
For the quantification of tyrosine in the feed additive the Applicant submitted the ring‐trial validated method EN ISO 13903, based on ion exchange chromatography coupled with postcolumn derivatisation and photometric detection. This method is derived from the ring‐trial validated Community method, for the determination of free (synthetic and natural) and of total (peptide‐bound and free) amino acids, including L‐tyrosine. Based on the performance characteristics available, the EURL recommends for official control the ring‐trial validated Community method, to quantify tyrosine in the feed additive.
The Applicant is aware that due to the presence of endogenous iron in feedingstuffs the direct determination of the ferric tyrosine chelate content added to premixtures or feedingstuffs is not achievable by analysis. The Applicant proposed instead an indirect single‐laboratory validated and verified method, based on the enumeration of the colour coated graphite marker particles added to the feed additive for the quantification of added ferric tyrosine chelate content in premixtures and feedingstuffs . Precision ranging from 8.1% to 17.4% and a recovery rate (Rrec) ranging from 97% to 113% were reported. Based on these performance characteristics, the EURL recommends for official control this indirect method for the quantification of the added content of ferric tyrosine chelate in premixtures and feedingstuffs only if the marker added to the feed additive is clearly characterised and the inclusion content to the additive, expressed as number of graphite particles per mass of the feed additive, is specified.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005, as last amended by Regulation (EU) 2015/1761) is not considered necessary.
Suggested citation: EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Mantovani A, Chesson A, Dierick N, Gropp J, Martelli G, Renshaw D, López‐Gálvez G and Kouba M, 2019. Scientific Opinion on the safety and efficacy of TYFER™ (ferric tyrosine chelate) as a zootechnical feed additive for chickens, turkeys and minor poultry species for fattening or reared for laying/breeding. EFSA Journal 2019;17(2):5608, 18 pp. 10.2903/j.efsa.2019.5608
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00481
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Maryline Kouba, Mojca Kos Durjava, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Legal Note: Relevant information or parts of this scientific output have been blackened in accordance with the European Commission decision on the confidentiality requests formulated by the applicant. The full output has been shared with the European Commission, EU Member States and the applicant.
Acknowledgements: The FEEDAP Panel wishes to thank the following for the support provided to this scientific output (in alphabetical order of the last name): Montserrat Anguita, Agnese Balzani, Jaume Galobart, Orsolya Holczknecht and Jordi Tarrés.
Adopted: 23 January 2019
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Akeso Biomedical, Inc. USA, represented in the EU by Pen & Tec Consulting S.L.U. Pl. Ausias March 1, 4th Floor, D01,Mirasol, ES‐08195, Sant Cugat del Vallès, Catalunya, Spain.
The applicant notified EFSA a request to change the brand name from TYPLEX® to TYFER™.
The initial application was for All Poultry Species; during the course of the assessment the applicant changed the target species, limiting to only some poultry species/categories.
FEED dossier reference: FAD‐2017‐0027.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep-fad-2017-0027-ferric_tyrosine_chelate.pdf
The initial application included an additional effect under this functional group: Improvement of health/welfare.
■■■■■
Technical Dossier/Supplementary Information (January 2018).
Technical Dossier/Supplementary Information (December 2018).
Technical Dossier/Supplementary Information (July 2018)/Annex I.
Technical Dossier/Supplementary Information (December 2018)/Annex I.
Technical Dossier/Supplementary Information (December 2018)/Annex II.
Technical Dossier/Section II/Annex II.1.4.1.2.
Technical Dossier/Supplementary Information (January 2018)/Annex II.1.4.1.4.
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 140, 30.5.2002, p. 10.
Figures preceded by the sign ‘<’ means the limit of quantification. Technical Dossier/Supplementary Information (May 2018).
Technical Dossier/Supplementary Information (November 2017).
Technical Dossier/Section II/Annex.II.1.3.2.
Technical Dossier/Section II/Annex II.1.4.
Technical Dossier/Section II/Annex II.2.1.
Technical Dossier/Section II/Annex II.1.5.4.
Technical Dossier/Section II/Annex II.1.5.3.
Technical Dossier/Section II/Annex II.1.5.1. On the same three batches used for bulk/solid density.
Technical Dossier/Section II/Annex II.1.5.2. On the same three batches used for bulk/solid density.
Technical Dossier/Section II/Annex II.1.5.5.
Technical dossier/Section II/Annex II.1.5.2.
Technical Dossier/Section II/Annex II.4.2.
Technical Dossier/Section II/Annex IV.3.7.
Technical Dossier/Section III/Annex III.1.1.1.
Alkaline phosphatase, aspartate amino transferase, alanine amino transferase, gamma‐glutamyl transferase, lactate dehydrogenase, total protein, albumin, globulin, amylase and glucose.
Red blood cell count, haemoglobin, haematocrit, white blood cell count, heterophils, lymphocytes, monocytes, eosinophils, and basophils.
At the time of the reported FEEDAP assessments, the maximum iron authorised in feed for poultry was 750 mg/kg. This limit has been decreased to 450 mg/kg poultry feed (Commission Implementing Regulation (EU) 2017/2330 of 14 December 2017 concerning the authorisation of Iron(II) carbonate, Iron(III) chloride hexahydrate, Iron(II) sulphate monohydrate, Iron(II) sulphate heptahydrate, Iron(II) fumarate, Iron(II) chelate of amino acids hydrate, Iron(II) chelate of protein hydrolysates and Iron(II) chelate of glycine hydrate as feed additives for all animal species and of Iron dextran as feed additive for piglets and amending Regulations (EC) No 1334/2003 and (EC) No 479/2006).
Technical Dossier/Section II/Annex III.1.2.1.
Technical Dossier/Section II/Annex IV.1.3.
Technical Dossier/Section II/Annex IV.1.1.
Technical Dossier/Section II/Annex IV.1.2.
Technical Dossier/Section II/Annex IV.1.4.
Technical Dossier/Supplementary Information (May 2018).
Each tray was produced by mixing 400 g of dry litter with 1,000 mL of deionized water. A suspension containing 10 g of dry and grounded poultry excreta and 10 mL of Campylobacter jejuni culture (4.5 × 105 cfu/mL Campylobacter per tray) suspended in 20 mL of Mueller–Hinton broth was then sprinkled over the wet litter in the tray. The tray produced was embedded in already existing litter in the pen. The seeded litter tray stayed in the pen until the study was terminated at day 42.
Technical dossier/Supplementary Information (November 2017)/Annex IV‐3‐10.
Technical dossier/Supplementary Information (November 2017)/Annex IV‐3‐9.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- ACGIH (American Conference of Governmental Industrial Hygienists), 2008. TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. Cincinnati, OH, USA. [Google Scholar]
- Allen VM, Bullb SA, Corrya JEL, Dominguec G, Jørgensenb F, Frostd JA, Whytee R, Gonzalez A, Elvissb N and Humphrey TJ, 2007. Campylobacter spp. contamination of chicken carcasses during processing in relation to flock colonisation. International Journal of Food Microbiology, 113, 54–61. [DOI] [PubMed] [Google Scholar]
- Chokboonmongkol C, Patchanee P, Gölz G, Zessin K‐H and Alter T, 2013. Prevalence, quantitative load, and antimicrobial resistance of Campylobacter spp. from broiler ceca and broiler skin samples in Thailand. Poultry Science, 92, 462–467. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel Biological Hazards), 2011. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA Journal 2011;9(4):2105, 141 pp. 10.2903/j.efsa.2011.2105 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2015. Scientific Opinion on the risks to animal and public health and the environment related to the presence of nickel in feed. EFSA Journal 2015;13(4):4074, 76 pp. 10.2903/j.efsa.2015.4074 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Technical guidance: tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for zootechnical additives. EFSA Journal 2012;10(1):2536, 19 pp. 10.2903/j.efsa.2012.2536 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2013a. Scientific Opinion on the safety and efficacy of iron compounds (E1) as feed additives for all species: iron chelate of amino acids, hydrate, based on a dossier submitted by Zinpro Animal Nutrition Inc. EFSA Journal 2013;11(7):3287, 28 pp. 10.2903/j.efsa.2013.3287 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2013b. Scientific opinion on the safety and efficacy of l‐tyrosine for all animal species. EFSA Journal 2013;11(7): 3310, 18 pp. 10.2903/j.efsa.2013.3310 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014a. Scientific Opinion on the safety and efficacy of iron compounds (E1) as feed additives for all species: ferrous sulphate heptahydrate based on a dossier submitted by Kronos International, Inc. EFSA Journal 2014;12(2):3566, 24 pp. 10.2903/j.efsa.2014.3566 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014b. Scientific Opinion on the safety and efficacy of iron compounds (E1) as feed additives for all species: ferrous sulphate monohydrate based on a dossier submitted by Kronos International, Inc. EFSA Journal 2014;12(3):3607, 25 pp. 10.2903/j.efsa.2014.3607 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015. Scientific Opinion on the safety and efficacy of iron compounds (E1) as feed additives for all animal species: ferrous carbonate based on a dossier submitted by Ankerpoort N.V.1. EFSA Journal 2015;13(5):4109, 31 pp. 10.2903/j.efsa.2015.4109 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016a. Scientific opinion on the safety and efficacy of iron compounds (E1) as feed additives for all species: ferric oxide based on a dossier submitted by Poortershaven Industri eleMineralen B.V. EFSA Journal 2016;14(6):4508, 26 pp. 10.2903/j.efsa.2016.4508 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016b. Scientific opinion on the safety and efficacy of iron compounds (E1) as feed additives for all animal species: ferrous carbonate; ferric chloride, hexahydrate; ferrous fumarate; ferrous sulphate, heptahydrate; ferrous sulphate, monohydrate; ferrous chelate of amino acids, hydrate; ferrous chelate of glycine, hydrate, based on a dossier submitted by FEFANA asbl. EFSA Journal 2016;14(2):4396, 47 pp. 10.2903/j.efsa.2016.4396 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016c. Scientific opinion on the safety and efficacy of iron oxide black, red and yellow for all animal species. EFSA Journal 2016;14(6):4482, 16 pp. 10.2903/j.efsa.2016.4482 [DOI] [Google Scholar]
- European Commission , 2011. Recommendation from the Scientific Committee on Occupational Exposure Limits (SCOEL) for nickel and inorganic nickel compounds. Employment, Social Affairs and Inclusion. SCOEL/SUM/85, June 2011. Available online: http://ec.europa.eu/social/BlobServlet?docId=3803&langId=en
- IARC (International Agency for Research on Cancer), 1987. IARC monographs on the evaluation of the carcinogenic risks to humans—overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1 to 42, suppl. 7, IARC, Lyon, France, p. 61. [PubMed]
- Nemery N, 1990. Metal toxicity and the respiratory tract. European Respiratory Journal, 3, 202–219. [PubMed] [Google Scholar]
- Nicholson FA, Chambers BJ, Williams JR and Unwin RJ, 1999. Heavy metal contents of livestock feeds and animal manures in England and Wales. Bioresource Technology, 70, 2331. [Google Scholar]
- NRC (National Research Council). 2005. Mineral Tolerance of Animals. Second Revised Edition. The National Academies Press, Washington, DC, USA. [Google Scholar]
- Ponka P, Tenenbein M and Eaton JW, 2015. Iron. In: Nordberg GF, Fowler BA and Nordberg M (eds.). Handbook on the toxicology of metals. Elsevier and Academic Press, London, UK. pp. 879–902. [Google Scholar]
- Reich F, Atanassova V, Haunhorst E and Klein G, 2008. The effects of Campylobacter numbers in caeca on the contamination of broiler carcasses with Campylobacter . International Journal of Food Microbiology, 127, 116–120. [DOI] [PubMed] [Google Scholar]
- Seliwiorstow T, Baré J, Van Damme I, Uyttendaele M and De Zutter L, 2015. Campylobacter carcass contamination throughout the slaughter process of Campylobacter‐positive broiler batches. International Journal of Food Microbiology, 194, 25–31. [DOI] [PubMed] [Google Scholar]
- Van Paemel M, Dierick N, Janssens G, Fievez V and De Smet S, 2010. Selected trace and ultratrace elements: Biological role, content in feed and requirements in animal nutrition – Elements for risk assessment. Technical Report submitted to EFSA. Available online: http://www.efsa.europa.eu/en/supporting/pub/68e.htm
- Weinberg ED, 1999. The development of awareness of the carcinogenic hazard of inhaled iron. Oncology Research, 11, 109–113. [PubMed] [Google Scholar]
- Wild P, Bourgkard E and Paris C, 2009. Lung cancer and exposure to metals: the epidemiological evidence. Methods in Molecular Biology, 472, 139–167. [DOI] [PubMed] [Google Scholar]


