Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Syngenta Crop Protection AG submitted a request to the competent national authority in Greece to modify the existing maximum residue levels (MRLs) for lambda‐cyhalothrin in celeries, fennel and rice. The data submitted in support of the request were found to be sufficient to derive tentative MRL proposals for the concerned crops. They are tentative as formally the general data gap identified in the MRL review for further investigations of the toxicological properties of the compounds formed under sterilisation conditions has not yet been addressed. Adequate analytical enforcement methods are available to control the residues of lambda‐cyhalothrin in the commodities under consideration. Based on the risk assessment results, EFSA concluded that the short‐term and long‐term intake of residues resulting from the uses of lambda‐cyhalothrin according to the reported agricultural practices is unlikely to present a risk to consumer health. The consumer risk assessment presented might need to be reconsidered in the light of the confirmatory data requested following the renewal of the approval and the review of the existing MRLs.
Keywords: lambda‐cyhalothrin, celery, fennel, rice, MRL application, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, Syngenta Crop Protection AG submitted an application to the competent national authority in Greece (evaluating Member State (EMS)) to modify the existing maximum residue levels (MRLs) for the active substance lambda‐cyhalothrin. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005 which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 3 October 2016. The EMS proposed to raise the existing MRLs for lambda‐cyhalothrin in various crops.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation. EFSA identified data gaps which needed further clarification, which were requested from the EMS. Since missing information was still identified for specific parts of the application, only the proposed uses on celeries, fennels and rice were taken forward. Request to modify MRLs on tree nuts and pears was not supported any longer in the framework of this MRL application.
Based on the conclusions derived by EFSA in the framework of the original approval and its renewal, the review of the existing MRLs for lambda‐cyhalothrin with its revisions and the additional data provided by the EMS in the framework of this application, the following conclusions were derived.
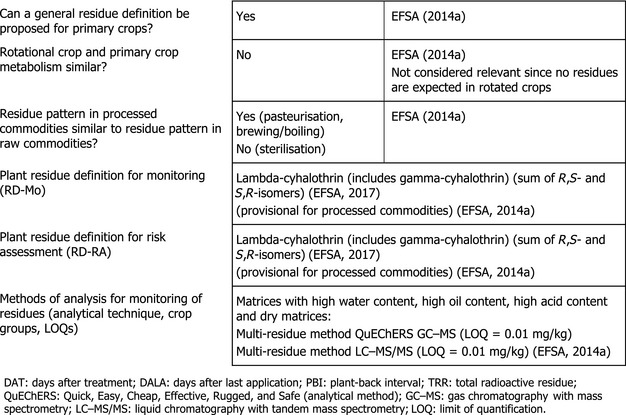
The metabolism of lambda‐cyhalothrin in primary and in rotational crops was sufficiently investigated in different crop category groups. Studies investigating the effect of processing on the nature of lambda‐cyhalothrin (hydrolysis studies) demonstrated that the active substance was stable under pasteurisation and baking/brewing and boiling but extensively degraded under sterilisation conditions, forming degradation products Ia, IV and gamma‐lactone.
In the framework of the MRL review, based on the results of the metabolism studies, the hydrolysis studies, the capability of the currently available enforcement analytical methods and taking into account that analytical methods do not allow to discriminate between lambda‐ and gamma‐cyhalothrin, the residue definitions for enforcement and risk assessment applicable to all plant commodities have been set as ‘lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S‐ and S,R‐isomers)’. The residue definition was set on provisional basis for processed products, pending the assessment of further data investigating the toxicological properties of the compounds formed under conditions simulating sterilisation. These residue definitions are considered appropriate also for the crops assessed under this application.
Sufficiently validated analytical methods are available to quantify residues of lambda‐cyhalothrin in the crops assessed in this application according to the enforcement residue definition. The methods enable quantification of residues at or above 0.01 mg/kg (limit of quantification (LOQ)) in all plants, but do not allow distinguishing the different isomers of lambda‐cyhalothrin.
The available residue trials are sufficient to derive MRL proposals of 0.2 mg/kg for celeries and rice and 0.3 mg/kg for fennel.
Studies investigating the magnitude of lambda‐cyhalothrin residues in processed celeries, fennel and rice were not submitted. Processing factors have been previously derived for washed, cooked and canned beans with pods and canned tomatoes, for possible extrapolation to the two vegetables assessed, and for polished rice and rice bran. A reduction of residues of the active substance and of compound Ia was observed in these studies. The degradation products compound IV and gamma‐lactone were analysed for in canned beans and tomatoes and they were not found (< 0.01 mg/kg). Supposing that the processing studies with beans and tomatoes are representative for the expected degradation in processed celery and fennel, it may be reasonably assumed that quantifiable residues of compounds Ia, IV and gamma‐lactone are not present in processed celeries and fennel. Taking into account that the common processing practice for rice is cooking, and that lambda‐cyhalothrin was concluded to be stable under conditions representative for boiling, studies addressing the magnitude of residues in cooked rice were not requested at this stage. However, the general data gap identified in the framework of the MRL review for investigation of the toxicological properties of compounds Ia, IV and gamma‐lactone still has to be addressed.
Based on the available information on the magnitude of residues, EFSA concluded that significant residue levels are unlikely to occur in rotational crops provided that the active substance is used according to the proposed good agricultural practices (GAPs).
As the rice straw and bran/pollard are used as feed products, a potential carry‐over into food of animal origin was assessed. However, the contribution of lambda‐cyhalothrin residues arising from the proposed use in rice feed items does not trigger a revision of the existing MRLs for commodities of animal origin.
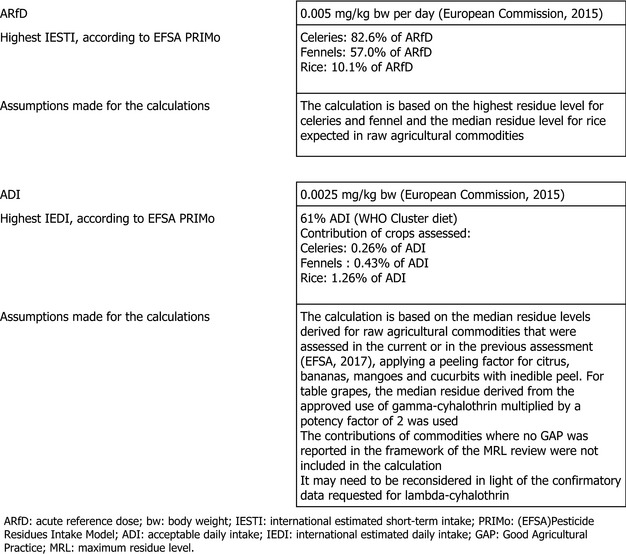
The toxicological profile of lambda‐cyhalothrin was assessed in the framework of the EU pesticides peer review for renewal of the approval and the data were sufficient to derive an acceptable daily intake (ADI) of 0.0025 mg/kg body weight (bw) per day and an acute reference dose (ARfD) of 0.005 mg/kg.
The consumer risk assessment was performed with revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo). The estimated long‐term intake was in the range of 7–61% of the ADI. The estimated short–term exposure conducted according to the currently agreed methodology did not exceed the ARfD for any of the crops under assessment.
EFSA concluded that the proposed use of lambda‐cyhalothrin on celeries, fennel and rice will not result in a consumer exposure exceeding the toxicological reference values and therefore is unlikely to pose a risk to consumers’ health. The consumer risk assessment presented has to be regarded as provisional and might need to be reconsidered in the light of the confirmatory data requested following the renewal of the approval and the review of the existing MRLs for lambda‐cyhalothrin.
EFSA derived tentative MRL proposals as reported in the summary table below; they are tentative as formally the general data gap identified in the framework of the MRL review requesting further investigations of the toxicological properties of compounds Ia, IV and gamma‐lactone formed under sterilisation conditions has not yet been addressed. A risk management decision is required whether it is appropriate to take over the tentative MRLs in the MRL legislation. Risk managers should take into account that the MRL application was made after the publication of the EFSA reasoned opinion where this data gap was identified but before the general data gap was taken over in the MRL legislation.
Full details of all end points and the consumer risk assessment can be found in Appendices B–D.
| Codea | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S‐ and S,R‐isomers)F | ||||
| 0270030 | Celeries | 0.03 ft |
0.2 ft Further risk management considerations necessary |
The submitted data are sufficient to derive a MRL proposal for the NEU use. Risk for consumers unlikely A risk management decision is required whether it is appropriate to take over the MRL in the MRL legislation, despite the lack of toxicological data for certain degradation products (compounds Ia, IV and gamma‐lactone) observed in standard hydrolysis studies representative for sterilisation conditions |
| 0270040 | Florence fennels | 0.2 ft | 0.3 ft Further risk management considerations necessary |
The submitted data are sufficient to derive, by extrapolation and proportionally scaling residue data on fennels, a MRL proposal for the SEU use. Risk for consumers unlikely A risk management decision is required whether it is appropriate to take over the MRL in the MRL legislation, despite the lack of toxicological data for certain degradation products (compounds Ia, IV and gamma‐lactone) observed in standard hydrolysis studies representative for sterilisation conditions |
| 0500060 | Rice | 0.01* ft |
0.2 ft Further risk management considerations necessary |
The submitted data are sufficient to derive a MRL proposal for the SEU use. Risk for consumers unlikely A risk management decision is required whether it is appropriate to take over the MRL in the MRL legislation, despite the lack of toxicological data for certain degradation products (compounds Ia, IV and gamma‐lactone) observed in standard hydrolysis studies representative for sterilisation conditions |
MRL: maximum residue level; NEU: northern Europe; SEU: southern Europe.
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
a Commodity code number according to Annex I of Regulation (EC) No 396/2005.
b Existing EU MRL and corresponding footnote on confirmatory data.
F Fat soluble.
ft The European Food Safety Authority identified some information on certain metabolites (compounds Ia, IV and gamma‐lactone) formed under sterilisation conditions as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 6 July 2020, or, if that information is not submitted by that date, the lack of it.
Assessment
The European Food Safety Authority (EFSA) received an application to modify the existing maximum residue levels (MRLs) of lambda‐cyhalothrin for celeries, fennel and rice. The detailed description of the intended uses of lambda‐cyhalothrin, which are the basis for the current MRL application, is reported in Appendix A.
Lambda‐cyhalothrin is the ISO common name for (R)‐α‐cyano‐3‐phenoxybenzyl (1S)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐ α‐cyano‐3‐phenoxy‐benzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate (IUPAC). It represents a 1:1 mixture of two of the four components of the insecticide cyhalothrin: the R,S‐ and the S,R‐isomers. These two isomers are not distinguishable with laboratory analytical methods and the isomer S,R alone is the active substance gamma‐cyhalothrin, which is also approved for use in plant protection products. The chemical structures of the active substances and its main metabolites are reported in Appendix E.
Lambda‐cyhalothrin was included in Annex I to Directive 91/414/EEC on 1 January 2002 by Commission Directive 2000/80/EC1 and is deemed to be approved under Regulation (EC) No 1107/2009 in accordance with Commission Implementing Regulation (EU) No 540/20112. The approval has been renewed by Commission Implementing Regulation (EU) 2016/1463 which entered into force on 1 April 2016. The representative uses evaluated in the peer review for renewal were foliar spraying applications on wheat, potato, plum, peach and tomato. The renewal assessment report (RAR) has been peer reviewed by EFSA (2014b). Sweden acted as the rapporteur member state (RMS) in both the original and renewal approval procedures. Lambda‐cyhalothrin was approved for the use as an insecticide, but the applicant was requested to submit confirmatory information to the Commission, the Member States and EFSA by 1 April 2018.4 The peer review of the confirmatory data assessment has not yet been initiated. Lambda‐cyhalothrin has been included in the list of candidates for substitution.
The European Union (EU) MRLs for lambda‐cyhalothrin are established in Annex II of Regulation (EC) No 396/2005. The review of the existing MRLs for lambda‐cyhalothrin according to Article 12 of Regulation (EC) No 396/2005 was performed in 2014 (EFSA, 2014a), revised in 2015 (EFSA, 2015) taking into account the lower toxicological end points set during the EU pesticides peer review renewal (European Commission, 2015b) and reconsidered in the light of the uses of gamma‐cyhalothrin of potential concerns for the consumers (EFSA, 2017). The MRL modifications recommended by EFSA have been implemented in the MRL legislation by Regulation (EU) 2018/9605. For most of the plant and animal commodities for which MRLs were proposed by EFSA, including those recommended for celeries, fennel and rice, certain information was missing (data gaps). Footnotes were included in the Regulation (EU) 2018/9606, requesting additional information to be provided by 6 July 2020.
In accordance with Article 6 of Regulation (EC) No 396/2005, Syngenta Crop Protection AG submitted an application to the competent national authority in Greece (evaluating Member State (EMS)) to modify the existing MRLs for the active substance lambda‐cyhalothrin. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005 which was submitted to the European Commission and forwarded to EFSA on 3 October 2016. The EMS proposed to raise the existing MRLs for lambda‐cyhalothrin in various crops. EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation. EFSA identified data gaps which needed further clarification, which were requested from the EMS. Since missing information was still identified for specific parts of the application, only proposed uses on celeries, fennels and rice were taken forward. Request to modify MRLs on tree nuts and pears was not supported any longer in the framework of this MRL application.
EFSA has based its assessment on the evaluation report submitted by the EMS and revised in 2018 (Greece, 2016), the RAR and its final addendum prepared under Regulation (EU) No 1141/20107 (Sweden, 2013, 2014), the revised Commission review report on lambda‐cyhalothrin (European Commission, 2015b), the conclusion on the peer review of the pesticide risk assessment of the active substance (EFSA, 2014b) and the reasoned opinions related to the review of the existing MRLs for lambda‐cyhalothrin (EFSA, 2014a, 2015, 2017).
For this application, the data requirements established in Regulation (EU) No 544/2011 and the guidance documents applicable at the date of submission of the application to the EMS are applicable (European Commission, 1997a,b,c,d,e,f,g, 2000, 2010a,b, 2015a; OECD, 2011, 2013). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20118.
A selected list of end points of the studies assessed by EFSA in the framework of the this MRL application, including the end points of relevant studies assessed previously, are presented in Appendix B.
The evaluation report submitted by the EMS as revised in 2018 (Greece, 2016) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of lambda‐cyhalothrin in primary crops after foliar and local applications has been previously investigated in fruit crops, leafy crops, pules/oilseeds and cereals. Lambda‐cyhalothrin was the predominant residue (37–95% total radioactive residue (TRR)) while compound Ia was identified as a significant metabolite in soya bean and cotton leaves only (17–25% TRR).
Based on the chiral analysis on residue trial samples assessed in the framework of the EU pesticides peer review renewal, EFSA concluded that the impact of the change in the ratio of the isomers on the toxicological burden the consumer is exposed to, was of low concern (EFSA, 2014a,b).
For the crops under assessment, belonging to the crop groups of cereals and leafy crops, EFSA concluded that the metabolism of lambda‐cyhalothrin was sufficiently investigated.
1.1.2. Nature of residues in rotational crops
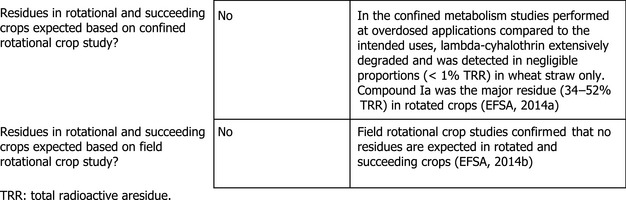
The crops under consideration may be grown in rotation. The metabolism of lambda‐cyhalothrin in rotational crops has been previously investigated (EFSA, 2014a,b). Parent compound extensively degraded in the edible parts of the rotated crops and was only detected in negligible proportions in wheat straw (< 1% TRR). Compound Ia was the major residue (34–52% TRR).
For the crops under assessment, EFSA concluded that the metabolism of lambda‐cyhalothrin in rotational crops was sufficiently investigated.
1.1.3. Nature of residues in processed commodities
Lambda‐cyhalothrin remained stable under hydrolytic conditions representative of pasteurisation and baking, brewing and boiling (82–91% TRR), while a significant degradation occurred under conditions simulating sterilisation. Hydrolytic cleavage of the parent molecule to compound Ia, compound IV and gamma‐lactone was noted. Since the toxicity of these compounds has not been sufficiently addressed, a general data gap was identified in the framework of the MRL review for investigation of the toxicological properties of compounds Ia, IV and gamma‐lactone formed under sterilisation conditions (EFSA, 2014a).
For all crops which may be consumed after processing that were assessed in the MRL review, a general data gap was implemented in the EU legislation by Regulation (EU) 2018/960 published in the Official Journal of the European Union on 6 July 2018 as confirmatory data to be submitted by 6 July 2020. It is noted that this MRL application was submitted to the national competent authority in Greece before Regulation (EU) 2018/960 has been published.
1.1.4. Methods of analysis in plants
The multi‐residue analytical method also known as the QuEChERS method (Quick, Easy, Cheap, Effective, Rugged, and Safe) using gas chromatography–mass spectrometry detection (GC–MS) and the multi‐residue method using liquid chromatography–tandem mass spectrometry (LC–MS/MS) were considered sufficiently validated for monitoring lambda‐cyhalothrin in plant commodities at the limit of quantification (LOQ) of 0.01 mg/kg in high water content, high oil content, high acidic content and dry commodities (EFSA, 2014a,b, 2015). It is noted that the analytical methods available do not allow distinguishing the different isomers of lambda‐cyhalothrin.
EFSA concluded that sufficiently validated analytical methods are available for enforcing the proposed MRLs for lambda‐cyhalothrin in the crops under assessment.
1.1.5. Stability of residues in plants
Studies on the storage stability of lambda‐cyhalothrin under frozen conditions were assessed in the framework of the EU pesticides peer review renewal and the MRL review (EFSA, 2014a,b).9 It was demonstrated that for the crops assessed in the framework of this MRL application, residues are stable for 26 months when stored ≤ –18°C.
1.1.6. Proposed residue definitions
In the framework of the MRL review, based on the results of the metabolism studies, the hydrolysis studies and the capability of the currently available enforcement analytical methods (see Section 1.1.4), the residue definition for enforcement and risk assessment in all plants was set as ‘lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S‐ and S,R‐isomers)’. For processed commodities the same residue definitions as for unprocessed products were proposed on a provisional basis, pending the assessment of further toxicological data investigating the toxicological properties of degradation products formed under conditions simulating sterilisation conditions, i.e. compounds Ia, IV and gamma‐lactone (EFSA, 2014a, 2015).
The residue definition for enforcement in Regulation (EC) No 396/2005 is identical as the above‐mentioned. It is noted that the definition is not specific to lambda‐cyhalothrin and covers also residues arising from the use of gamma‐cyhalothrin (EFSA, 2017).
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
Celeries: Based on the results of four good agricultural practice (GAP)‐compliant residue trials conducted in northern Europe with lambda‐cyhalothrin (trials performed in two seasons), EFSA derived a MRL proposal of 0.2 mg/kg for celeries.
Fennels: Based on the results of four overdosed residue trials on celeries conducted in southern Europe (trials performed in two seasons), and after having proportionally scaled the results to the nominal application rate intended for fennels, EFSA derived a MRL proposal of 0.3 mg/kg for fennels by extrapolation. The proposed extrapolation is in line with the EU guidance document (European Commission, 2015a).
Rice: Based on the results of eight GAP‐compliant residue trials conducted in Italy (trials performed in two seasons), where lambda‐cyhalothrin was applied twice on rice before or after flooding rice fields, EFSA derived a MRL of 0.2 mg/kg for rice (grain).
In addition, the applicant provided residue trials investigating the magnitude of residues in rice straw, which was used for the dietary burden calculation (see Section 2).
According to the EMS, the analytical methods used to analyse the samples of the residue trials have been sufficiently validated and residue data were valid with regard to storage stability (Greece, 2016).
1.2.2. Magnitude of residues in rotational crops
Based on the results of the confined rotational crop metabolism studies, which were conducted at a maximum total application rate significantly higher (about 13N) than the intended rates on the crops under assessment (maximum 34.5 g/ha), significant residue levels (< 0.01 mg/kg) are not expected in the edible parts of the rotated crops, provided that lambda‐cyhalothrin is applied in compliance with the intended GAPs.
This conclusion was confirmed by rotational crop field trials conducted at a total dose rate of 500 g/ha which resulted in residues of lambda‐cyhalothrin and compound Ia below the LOQ in the edible parts at 30 and 60 day plant‐back intervals (EFSA, 2014b).
1.2.3. Magnitude of residues in processed commodities
No new processing studies were submitted in this MRL application. Considering the high toxicity of the active substance and the insufficient information on the toxicological proprieties of the compounds formed during conditions representative of sterilisation, the magnitude of residues in processed products that may undergo heating by processing shall in principle be addressed.
For celeries/fennels, the applicant proposed to consider the processing factors (PFs) derived for washed, cooked and canned beans with pods derived in the framework of the EU pesticides peer review renewal and the MRL review revisions (EFSA, 2014a,b, 2015). In this study, all samples were analysed for the parent compound and the degradation product Ia. The processing studies on beans were conducted at about 8N or 13N the dose rate of the intended GAPs on celeries and fennel, respectively, leading to residues up to 0.26 mg/kg for lambda‐cyhalothrin and 0.04 mg/kg for compound Ia in unprocessed beans.10 Overall, a reduction of residues (parent and compound Ia) was seen in the processed products (including the intermediate processed products like washed and cooked beans) compared with the raw agricultural commodity. In the processed products, compound Ia accounted for up to 0.028 mg/kg in cooked beans and 0.017 mg/kg in sterilised beans. Canned beans were also analysed for IV and gamma‐lactone and residues were not found (< 0.01 mg/kg) following sterilisation (Sweden, 2013).
Additionally, the applicant referred to processing studies on tomatoes that demonstrated that residues of parent lambda‐cyhalothrin and compounds Ia, IV and gamma‐lactone were below the LOQ (< 0.01 mg/kg) in sterilised canned tomatoes, while the concentration of lambda‐cyhalothrin in unprocessed tomatoes accounted for 0.11 mg/kg11 (Sweden, 2013; EFSA, 2014b).
According to OECD guidance document, processing studies in beans are representative for all types of canned vegetables and therefore results on beans can be extrapolated to the other commodities of this type (OECD, 2008). Assuming that these two crops, when not eaten raw, will undergo processing like cooking and canning comparable with the conditions investigated in the processing studies with beans and tomatoes, it may be reasonably assumed that residues of compound Ia, IV and gamma‐lactone are not present in quantifiable concentrations.
For rice, PFs were derived from one processing study, investigating residues in polished rice, hulls and bran (FAO, 2008). The study showed a significant reduction of residues of lambda‐cyhalothrin in polished (white) rice and bran. Studies to investigate the effect of heating on the magnitude of residues in rice are not available. Taking into account that the common processing practice for rice is cooking and that lambda‐cyhalothrin was concluded to be stable under conditions representative for boiling (see Section 1.1.3), studies addressing the magnitude of residues in cooked rice were not requested at this stage.
It should be highlighted that the general data gap identified in the framework of the MRL review for investigation on the toxicological properties of compounds Ia, IV and gamma‐lactone still needs to be addressed. Pending the confirmation of the residue definition for processed products, further investigation on processed commodities may need to be generated.
1.2.4. Proposed MRLs
EFSA concluded that sufficient information was provided to calculate a MRL proposal of 0.2 mg/kg for celeries and rice and 0.3 mg/kg for fennels. The MRL proposals shall be regarded as tentative; the general data gap identified in the framework of the MRL review has not yet been addressed (see Section 1.1.3).
2. Residues in livestock
Rice straw and rice by‐products such as rice bran/pollard can be used as feed items in livestock. The most recent livestock dietary burden calculation was conducted in the framework of the review of MRLs under Article 12 of Regulation (EC) No 396/2005, where EFSA calculated the dietary intake of livestock taking into account the authorised EU uses of lambda‐cyhalothrin (EFSA, 2015); the calculation was performed according to the methodology described in the previously used European guidance document (European Commission, 1996). The maximum dietary burden for dairy and beef cattle was 0.89 and 1.39 mg/kg dry matter (DM), respectively, for poultry 0.17 mg/kg DM and for pigs 0.52 mg/kg DM. The EU MRLs for muscle, fat, liver and kidney were derived from the existing Codex MRLs (CXL), considering the maximum dietary burden of 6.2 mg/kg DM for beef cattle (FAO, 2008).
In the framework of the current application, the dietary burden was re‐calculated in accordance with the OECD guidance document (OECD, 2013) which is the methodology applicable at the date of submission of the MRL application. The input values are summarised in Appendix D.1. The results of the calculations are presented in Appendix B.3. Comparing the results obtained with the new methodology and the results reported in the previous EFSA opinion (EFSA, 2015), it became evident that different methodology had an impact on the result, with a slightly changed dietary exposure for the different animal species included in both models in the calculations using the OECD methodology. The contribution residues in rice straw and bran/pollard are not significantly changing the livestock intakes.
Thus, EFSA concluded that the intended use on rice will not trigger a revision of the existing MRLs for commodities of animal origin.
3. Consumer risk assessment
The consumer risk assessment was performed with revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo). This exposure assessment model contains the relevant European food consumption data for different subgroups of the EU population12 (EFSA, 2007).
The estimated exposure was then compared with the toxicological reference values derived for lambda‐cyhalothrin during the EU pesticides peer‐review renewal process (European Commission, 2015b).
The most recent long‐term exposure assessment performed by EFSA (2017) was updated with the median residue value (STMR) derived for celeries, fennel and rice derived from the residue trials submitted with this application. The acute exposure assessment was performed only with regard to the use of lambda‐cyhalothrin on the commodities under consideration assuming the consumption of a large portion of the food item as reported in the national food surveys and considering the highest residue (HR) level for celeries and fennel and the STMR for rice. The input values used for the dietary exposure calculation are summarised in Appendix D.
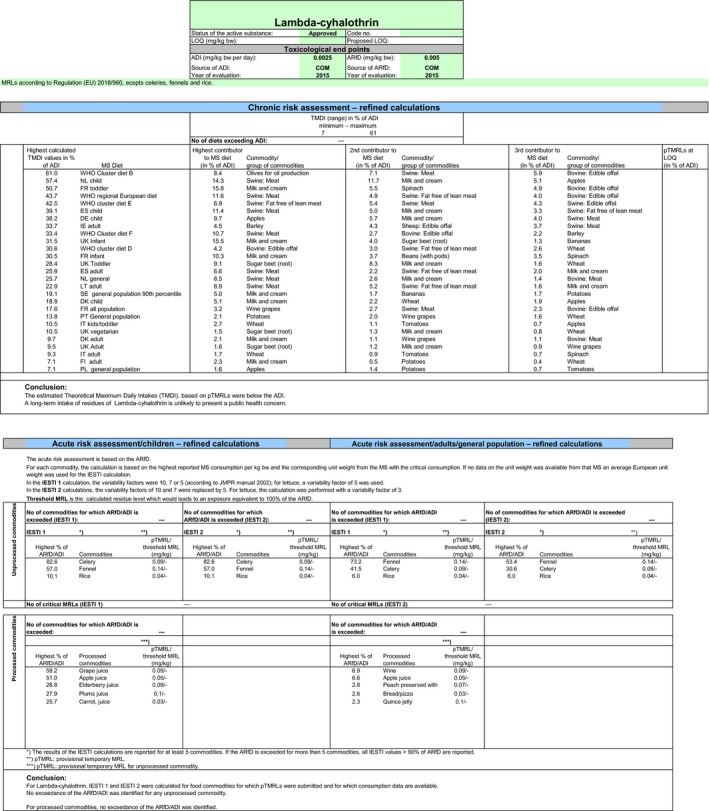
No long‐term and short‐term consumer intake concerns were identified for any of the European diets incorporated in the EFSA PRIMo. The total chronic calculated intake accounted for up to 61% of the acceptable daily intake (ADI) (WHO Cluster diet). The contribution of the residues on the crops under consideration to the total exposure accounted for a maximum of 1.3% of ADI (rice).
The expected short‐term exposure calculated according to the internationally agreed methodology described above did not exceed the toxicological reference value derived for lambda‐cyhalothrin. It should be highlighted that the safety margin to the acute reference dose (ARfD) is narrow for celeries (82.6% of the ARfD). It is expected that the exposure is slightly lower, if celeries are consumed after cooking, leading to a reduction of residues in the processed product.
4. Conclusion and Recommendations
The data submitted in support of the request were found to be sufficient to derive tentative MRL proposals for celeries, fennel and rice; they are tentative as formally the general data gap identified in the framework of the MRL review requesting further investigations of the toxicological properties of compounds Ia, IV and gamma‐lactone formed under sterilisation conditions has not yet been addressed. Based on the risk assessment results, EFSA concluded that the short‐term and long‐term intake of residues resulting from the uses of lambda‐cyhalothrin according to the reported agricultural practices is unlikely to present a risk to consumer health. The consumer risk assessment presented might need to be reconsidered in the light of the confirmatory data requested following the renewal of the approval and the review of the existing MRLs.
A risk management decision is required whether it is appropriate to take over the tentative MRLs in the MRL legislation. Risk managers should take into account that the MRL application was made after the publication of the EFSA reasoned opinion where this data gap was identified (EFSA, 2014a, 2015) but before the general data gap was published in the MRL legislation.
The MRL recommendations are summarised in Appendix B.4.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CF
conversion factor for enforcement to risk assessment residue definition
- CS
capsule suspension
- CXL
Codex maximum residue limit
- DALA
days after last application
- DAT
days after treatment
- DM
dry matter
- EMS
evaluating Member State
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- GC–MS
gas chromatography with mass spectrometry
- GEMS Food
Global Environment Monitoring System/Food Contamination Monitoring and Assessment Programme
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- InChiKey
International Chemical Identifier Key.
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- MRL
maximum residue level
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- RA
risk assessment
- RAC
raw agricultural commodity
- RAR
renewal assessment report
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern Europe
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TRR
total radioactive residue
- WHO
World Health Organization
Appendix A – Summary of intended GAP triggering the amendment of existing EU MRLs
1.
| Crop and/or situation | NEU, SEU, MS or country | F G or Ia | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc |
Number min–max |
Interval between application (min) |
g a.s./hL min–max |
Water L/ha min–max |
Rate | Unit | ||||||
| Celeries | NEU (BE) | F | Biking and sucking insects | CS | 100 g/kg | Foliar spray | At infestation | 2 | 14 | 12.50 | g/ha | 7 | |||
| Fennels | SEU (IT) | F | Biking and sucking insects | CS | 100 g/kg | Foliar spray | At infestation | 2 | 14 | 500–1000 | 7.50 | g/ha | 7 | ||
| Rice | SEU (IT) | F | Biking and sucking insects | CS | 100 g/kg | Foliar spray | At infestation | 2 | 60 | 300–400 | 17.25 | g/ha | 42 | Application 3–4 days before or after flooding (Greece, 2016) | |
GAP: Good Agricultural Practice; MRL: maximum residue level; NEU: northern European Union; SEU: southern European Union; MS: Member State; a.s.: active substance; CS: capsule suspension.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application (s) | Sampling | Comment/source |
|---|---|---|---|---|---|
| Fruit crops | Apples | Spotting onto fruit, 33 μg/fruit | 0, 7, 14, 28, 56 DAT | [cyclopropyl‐14C]‐cyhalothrin (EFSA, 2014a) | |
| Tomatoes | Foliar, 4 × 100 g/ha | 3 DALA | [cyclopropyl‐14C] and [phenoxy‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) | ||
| Leafy crops | Cabbages | Spotting onto crop, 26 μg/leaf | 2, 4, 5, 6, 7 weeks after application | [cyclopropyl‐14C]‐cyhalothrin (EFSA, 2014a) | |
| Foliar, 4‐8 × 55 g/ha | 7 DALA | [cyclopropyl‐14C]‐cyhalothrin (EFSA, 2014a) | |||
| Cereals/grasses | Wheat | Foliar, 2 × 224 g/ha | 14, 85 DALA | [cyclopropyl‐14C] and [benzyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) | |
| Foliar, 3 × 224 g/ha | 30 DALA | ||||
| Pulses/oilseeds | Soya beans | Foliar, 2 × 20 g/ha | 39, 51 DALA | [cyclopropyl‐14C] and [benzyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) | |
| Cotton | Foliar, 3 × 66 g/ha | 30, 50 DALA | [cyclopropyl‐14C] and [benzyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) |
| Rotational crops (available studies) | Crop groups | Crop(s) | Application (s) | PBI (DAT) | Comment/source |
|---|---|---|---|---|---|
| Root/tuber crops | Carrots | Bare soil, 1 × 470 g/ha | 30, 60, 120 | [cyclopropyl‐14C] and [phenyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) | |
| Bare soil, 1 × 110 g/ha | 30, 120 | [cyclopropyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) | |||
| Leafy crops | Lettuces | Bare soil, 1 × 470 g/ha | 30, 60, 120 | [cyclopropyl‐14C] and [phenyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) | |
| Bare soil, 1 × 110 g/ha | 30, 120 | [cyclopropyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) | |||
| Cereal (small grain) | Wheat | Bare soil, 1 × 470 g/ha | 30, 60, 120 | [cyclopropyl‐14C] and [phenyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) | |
| Bare soil, 1 × 110 g/ha | 30, 120 | [cyclopropyl‐14C]‐lambda‐cyhalothrin (EFSA, 2014a) |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes |
[cyclopropyl‐14C]‐ and [phenyl‐14C]‐lambda‐cyhalothrin Extensive degradation of the parent to form compounds Ia (59% TRR), IV (63% TRR) and gamma‐lactone (15% TRR) (EFSA, 2014a) |
|
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | ||
| Sterilisation (20 min, 120°C, pH 6) | No |
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Apple, peach, sugar beet root, cabbage, potato, peas | −18 | 26 | Months | Parent | EFSA (2014a) | |
| High oil content | Cotton seed, rape seed | −18 | 26 | Months | Parent | EFSA (2014a) | |
| Hops | −18 | 8 | Months | Parent | EFSA (2014a) | ||
| Dry/High starch | Wheat grain | −18 | 26 | Months | Parent | EFSA (2014a) | |
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Commodity | Region/indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) |
|---|---|---|---|---|---|---|
| Celeries | NEU | 0.03; 2 × 0.05; 0.09 | Residue trials on celeries compliant with the GAP | 0.2 | 0.09 | 0.05 |
| Celeries | SEU |
Overdosed: 0.08; 0.20; 0.23; 0.29 Scaled: 0.04; 0.10; 0.11; 0.14 |
Residue trials on celeries overdosed. Scaled down (factor: 0.47–0.50) to nominal application rate of the GAP on fennels Extrapolation to fennel possible |
0.3 | 0.14 | 0.11 |
| Rice | SEU | Grain : 0.01; 0.02; 3 × 0.04; 0.08; 2 × 0.09 | Residue trials on rice compliant with the GAP | 0.2 | 0.09 | 0.04 |
| Straw: 0.06; 2 × 0.07; 2 × 0.08; 0.09; 0.12; 0.16 | Currently, no MRLs are set for feed items, like rice straw | – | 0.16 | 0.08 |
MRL: maximum residue level; GAP: Good Agricultural Practice.
NEU: Outdoor trials conducted in northern Europe; SEU: Outdoor trials conducted in southern Europe, Indoor; indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
B.1.2.2. Residues in rotational crops
B.1.2.3. Processing factors
| Processed commodity | Number of valid studies | Processing factor (PF) | CFP a | Comment/sourceb | |
|---|---|---|---|---|---|
| Individual values | Median PF | ||||
| Beans and peas with pods, washed | 6 | 0.71; 0.91; 0.92; 1.09; 1.13; 1.33 | 1.01 | TBE | Tentative (EFSA, 2014a) |
| Beans and peas with pods, cooked | 4 | 0.76, 0.64, 0.96, 1.22 | 0.86 | TBE | Tentative (EFSA, 2014a) |
| Beans and peas with pods, canned (whole can) | 4 | 0.29; 0.36; 0.43; 0.71 | 0.40 | TBE | Tentative (EFSA, 2014a) |
| Beans with pods, canned (separated beans) | 4 | 0.67; 0.73; 0.91; 1.43 | 0.82 | TBE | Tentative (EFSA, 2014a) |
| Rice, polished | 1 | < 0.01 | TBE | Tentative (FAO, 2008) | |
| Rice bran | 1 | 0.22 | TBE | Tentative (FAO, 2008) | |
Conversion factor (CF) for risk assessment in processed commodities is pending final decision on the residue definition in processed products (TBE, to be established).
A tentative PF is derived based on a limited data set and/or provisional residue definition).
B.2. Residues in livestock
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical sub groupa | Most critical commodityb | Trigger exceeded (Y/N) max burden | |||
|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||||
| Median | Maximum | Median | Maximum | ||||
| Cattle (all) | 0.014 | 0.027 | 0.43 | 0.75 | Dairy cattle | Barley straw | Yes |
| Cattle (dairy only) | 0.014 | 0.027 | 0.37 | 0.71 | Dairy cattle | Barley straw | Yes |
| Sheep (all) | 0.023 | 0.052 | 0.57 | 1.22 | Lamb | Barley straw | Yes |
| Sheep (ewe only) | 0.019 | 0.041 | 0.57 | 1.22 | Ram/Ewe | Barley straw | Yes |
| Swine (all) | 0.006 | 0.010 | 0.27 | 0.41 | Swine breeding | Beet, mangel fodder | Yes |
| Poultry (all) | 0.013 | 0.018 | 0.19 | 0.27 | Poultry layer | Wheat straw | Yes |
| Poultry (layer only) | 0.013 | 0.018 | 0.19 | 0.27 | Poultry layer | Wheat straw | Yes |
bw: body weight; DM: dry matter.
When one group of livestock includes several subgroups (e.g. poultry ‘all’ including broiler, layer and turkey), the result of the most critical subgroup is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
B.2.1. Nature of residues and methods of analysis in livestock
Not relevant.
B.3. Consumer risk assessment
B.4. Recommended MRLs
| Codea | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Lambda‐cyhalothrin (includes gamma‐cyhalothrin) (sum of R,S‐ and S,R‐isomers)F | ||||
| 0270030 | Celeries | 0.03 ft |
0.2 ft Further risk management considerations necessary |
The submitted data are sufficient to derive a MRL proposal for the NEU use. Risk for consumers unlikely A risk management decision is required whether it is appropriate to take over the MRL in the MRL legislation, despite the lack of toxicological data for certain degradation products (compounds Ia, IV and gamma‐lactone) observed in standard hydrolysis studies representative for sterilisation conditions |
| 0270040 | Florence fennels | 0.2 ft |
0.3 ft Further risk management considerations necessary |
The submitted data are sufficient to derive, by extrapolation and proportionally scaling residue data on fennels, a MRL proposal for the SEU use. Risk for consumers unlikely A risk management decision is required whether it is appropriate to take over the MRL in the MRL legislation, despite the lack of toxicological data for certain degradation products (compounds Ia, IV and gamma‐lactone) observed in standard hydrolysis studies representative for sterilisation conditions |
| 0500060 | Rice | 0.01* ft |
0.2 ft Further risk management considerations necessary |
The submitted data are sufficient to derive a MRL proposal for the SEU use. Risk for consumers unlikely A risk management decision is required whether it is appropriate to take over the MRL in the MRL legislation, despite the lack of toxicological data for certain degradation products (compounds Ia, IV and gamma‐lactone) observed in standard hydrolysis studies representative for sterilisation conditions |
MRL: maximum residue level; NEU: northern Europe; SEU: southern Europe.
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
a Commodity code number according to Annex I of Regulation (EC) No 396/2005.
b Existing EU MRL and corresponding footnote on confirmatory data.
F Fat soluble.
ft The European Food Safety Authority identified some information on certain metabolites (compounds Ia, IV and gamma‐lactone) formed under sterilisation conditions as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 6 July 2020, or, if that information is not submitted by that date, the lack of it.
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Authorised uses | ||||
| Barley & oats straw | 0.69 | STMR (EFSA, 2015) | 1.62 | HR (EFSA, 2015) |
| Beet, mangel fodder | 0.15 | STMR (EFSA, 2015) | 0.21 | HR (EFSA, 2015) |
| Head cabbage | 0.03 | STMR (EFSA, 2015) | 0.09 | HR (EFSA, 2015) |
| Kale leaves | 0.08 | STMR (EFSA, 2015) | 0.11 | HR (EFSA, 2015) |
| Wheat & rye straw | 0.64 | STMR (EFSA, 2015) | 1.2 | HR (EFSA, 2015) |
| Carrot culls | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Cassava/tapioca roots | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Potato culls | 0.01 | STMR (EFSA, 2015) | 0.01 | HR (EFSA, 2015) |
| Swede & turnip roots | 0.01 | STMR (EFSA, 2015) | 0.03 | HR (EFSA, 2015) |
| Barley & oat grain | 0.09 | STMR (EFSA, 2015) | 0.09 | STMR (EFSA, 2015) |
| Bean & pea seed (dry) | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Corn cop, grain | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Cotton, undelinted seed | 0.05 | STMR (EFSA, 2015) | 0.05 | STMR (EFSA, 2015) |
| Lupin seed | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Wheat & rye grain | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Sorghum grain | 0.01 | STMR (EFSA, 2015) | 0.01 | STMR (EFSA, 2015) |
| Apple, pomace wet | 0.15 | STMR × PF (EFSA, 2015) | 0.15 | STMR × PF (EFSA, 2015) |
| Beet sugar, dried pulp | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Beet sugar, ensiled pulp | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Beet sugar, molasses | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Brewer's grain, dried | 0.30 | STMR (EFSA, 2015) × PFb | 0.30 | STMR (EFSA, 2015) × PFb |
| Canola (Rape seed), meal | 0.02 | STMR × PF (EFSA, 2015) | 0.02 | STMR × PF (EFSA, 2015) |
| Citrus, dried pulp | 0.31 | STMR × PF (EFSA, 2015) | 0.31 | STMR × PF (EFSA, 2015) |
| Corn field milled by‐prod | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Corn field hominy meal | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Corn field gluten feed & meal | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Cotton seed meal | 0.005 | STMR × PF (EFSA, 2015) | 0.005 | STMR × PF (EFSA, 2015) |
| Distiller's grain, dried | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Linseed meal | 0.02 | STMR × PF (EFSA, 2015) | 0.02 | STMR × PF (EFSA, 2015) |
| Lupin seed meal | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Potato, processed waste | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Potato, dried pulp | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Rapeseed meal | 0.02 | STMR × PF (EFSA, 2015) | 0.02 | STMR × PF (EFSA, 2015) |
| Wheat gluten meal | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Wheat milled by‐prod | 0.01 | STMR (EFSA, 2015)a | 0.01 | STMR (EFSA, 2015)a |
| Intended use under assessment | ||||
| Rice straw | 0.08 | STMR | 0.16 | HR |
| Rice bran/pollard | 0.01 | STMR × PF (0.22) | 0.01 | STMR × PF (0.22) |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
For these by‐products no processing factor (PF) was included in the calculation because residues in the RAC were below the LOQ and concentration of residues in these commodities is not expected.
For dried brewer's grain, in the absence of processing factors supported by data, the default processing factor of 3.3 was applied to consider the potential concentration of residues in this product.
D.2. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Citrus fruits | 0.003 | STMR × PF (EFSA, 2015) | Acute risk assessment performed only for the crops under consideration | |
| Tree nuts | 0.01 | STMR (EFSA, 2015) | ||
| Apples | 0.02 | STMR (EFSA, 2015) | ||
| Pears | 0.02 | STMR (EFSA, 2015) | ||
| Medlar | 0.08 | STMR (EFSA, 2015) | ||
| Loquat | 0.08 | STMR (EFSA, 2015) | ||
| Quinces | 0.08 | STMR (EFSA, 2015) | ||
| Apricots | 0.03 | STMR (EFSA, 2015) | ||
| Cherries | 0.13 | STMR (EFSA, 2015) | ||
| Peaches | 0.03 | STMR (EFSA, 2015) | ||
| Plums | 0.02 | STMR (EFSA, 2015) | ||
| Table grapes | 0.02 | STMR (EFSA, 2017)a | ||
| Wine grapes | 0.02 | STMR (EFSA, 2015) | ||
| Cane fruits | 0.02 | STMR (EFSA, 2015) | ||
| Blueberries, Cranberries | 0.02 | STMR (EFSA, 2015) | ||
| Currants | 0.06 | STMR (EFSA, 2015) | ||
| Gooseberries, Rose hips | 0.02 | STMR (EFSA, 2015) | ||
| Mulberries | 0.02 | STMR (EFSA, 2015) | ||
| Azaroles, Elderberries | 0.02 | STMR (EFSA, 2015) | ||
| Table olives | 0.13 | STMR (EFSA, 2015) | ||
| Kaki/Japanese persimmons | 0.02 | STMR (EFSA, 2015) | ||
| Kiwi | 0.01 | STMR (EFSA, 2015) | ||
| Bananas | 0.02 | STMR × PF (EFSA, 2015) | ||
| Mangoes | 0.01 | STMR × PF (EFSA, 2015) | ||
| Potatoes | 0.01 | STMR (EFSA, 2015) | ||
| Tropical roots and tuber vegetables | 0.01 | STMR (EFSA, 2015) | ||
| Beetroot | 0.01 | STMR (EFSA, 2015) | ||
| Carrots | 0.01 | STMR (EFSA, 2015) | ||
| Celeriac | 0.03 | STMR (EFSA, 2015) | ||
|
Horseradish Jerusalem artichokes Parsnips Parsley root Salsify Swedes Turnips |
0.01 | STMR (EFSA, 2015) | ||
| Radishes | 0.02 | STMR (EFSA, 2015) | ||
| Bulb vegetables | 0.05 | STMR (EFSA, 2015) | ||
| Tomatoes | 0.02 | STMR (EFSA, 2015) | ||
| Peppers | 0.02 | STMR (EFSA, 2015) | ||
| Aubergines | 0.03 | STMR (EFSA, 2015) | ||
| Okra | 0.03 | STMR (EFSA, 2015) | ||
| Cucumbers | 0.01 | STMR (EFSA, 2015) | ||
| Gherkins | 0.04 | STMR (EFSA, 2015) | ||
| Courgettes | 0.04 | STMR (EFSA, 2015) | ||
| Cucurbits with inedible peel | 0.01 | STMR × PF (EFSA, 2015) | ||
| Sweet corn | 0.01 | STMR (EFSA, 2015) | ||
| Flowering brassica | 0.02 | STMR (EFSA, 2015) | ||
| Brussels sprouts | 0.02 | STMR (EFSA, 2015) | ||
| Head cabbages | 0.03 | STMR (EFSA, 2015) | ||
| Chinese cabbages | 0.08 | STMR (EFSA, 2015) | ||
| Kohlrabi | 0.01 | STMR (EFSA, 2015) | ||
| Lamb's lettuces | 0.34 | STMR (EFSA, 2015) | ||
| Lettuces | 0.03 | STMR (EFSA, 2015) | ||
| Escarole | 0.02 | STMR (EFSA, 2015) | ||
| Cresses, Land cresses | 0.23 | STMR (EFSA, 2015) | ||
| Roman rocket | 0.23 | STMR (EFSA, 2015) | ||
| Baby leaf crops | 0.23 | STMR (EFSA, 2015) | ||
| Spinach | 0.20 | STMR (EFSA, 2015) | ||
| Chards/Beet leaves | 0.05 | STMR (EFSA, 2015) | ||
| Herbs and edible flowers | 0.23 | STMR (EFSA, 2015) | ||
| Beans with pods | 0.11 | STMR (EFSA, 2015) | ||
| Beans without pods | 0.02 | STMR (EFSA, 2015) | ||
| Peas with pods | 0.02 | STMR (EFSA, 2015) | ||
| Peas without pods | 0.02 | STMR (EFSA, 2015) | ||
| Lentils | 0.02 | STMR (EFSA, 2015) | ||
| Asparagus | 0.01 | STMR (EFSA, 2015) | ||
| Celeries | 0.05 | STMR | 0.09 | HR |
| Fennel | 0.11 | STMR | 0.14 | HR |
| Globe artichokes | 0.04 | STMR (EFSA, 2015) | ||
| Leeks | 0.02 | STMR (EFSA, 2015 ) | ||
| Wild fungi | 0.17 | STMR (EFSA, 2015) | ||
| Pulses | 0.01 | STMR (EFSA, 2015) | ||
| Oilseeds | 0.01 | STMR (EFSA, 2015) | ||
| Olives for oil production | 0.11 | STMR (EFSA, 2015) | ||
| Barley | 0.09 | STMR (EFSA, 2015) | ||
| Maize/corn | 0.01 | STMR (EFSA, 2015) | ||
| Oats | 0.09 | STMR (EFSA, 2015) | ||
| Rice | 0.04 | STMR | 0.04 | STMR |
| Sorghum | 0.01 | STMR (EFSA, 2015) | ||
| Wheat, Rye | 0.01 | STMR (EFSA, 2015) | ||
| Coffee | 0.01 | STMR (FAO, 2015) | ||
| Hops | 3.30 | STMR (EFSA, 2015) | ||
| Fruits spices, except cardamom | 0.03 | STMR (EFSA, 2015) | ||
| Cardamom | 0.28 | STMR (EFSA, 2017) | ||
| Root and rhizome spices | 0.05 | STMR (EFSA, 2015 | ||
| Sugar beet roots | 0.01 | STMR (EFSA, 2015) | ||
| Sugar canes | 0.02 | STMR (EFSA, 2015) | ||
| Chicory roots | 0.01 | STMR (EFSA, 2015) | ||
| Swine meat, goat meat | 0.23 | STMR (EFSA, 2015) | ||
| Swine fat | 1.00 | STMR (EFSA, 2015) | ||
| Swine liver | 0.01 | STMR (EFSA, 2015) | ||
| Swine kidney | 0.03 | STMR (EFSA, 2015) | ||
| Swine edible offal | 1.00 | STMR | ||
| Bovine, sheep meat | 0.05 | STMR (EFSA, 2015) | ||
| Ruminant fat | 1.00 | STMR (EFSA, 2015) | ||
| Ruminant liver | 0.01 | STMR (EFSA, 2015) | ||
| Ruminant kidney | 0.03 | STMR (EFSA, 2015) | ||
| Ruminant edible offal | 1.00 | STMR | ||
| Poultry meat | 0.01 | STMR (EFSA, 2015) | ||
| Poultry fat | 0.01 | STMR (EFSA, 2015) | ||
| Poultry liver | 0.01 | STMR (EFSA, 2015) | ||
| Ruminant milk | 0.01 | STMR (EFSA, 2015) | ||
| Bird's eggs | 0.01 | STMR (EFSA, 2015) | ||
STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
STMR derived from the approved use of gamma‐cyhalothrin multiplied by a potency factor of 2 to take into account the hazard contribution of gamma‐cyhalothrin to lambda‐cyhalothrin (EFSA, 2017).
Appendix E – Used compound codes
1.
| Code/trivial name | Chemical name/SMILES notation/InChiKeya | Structural formulab |
|---|---|---|
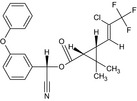
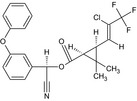
| Lambda‐cyhalothrin |
A 1:1 mixture of: (R)‐α‐cyano‐3‐phenoxybenzyl (1S,3S)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate or a 1:1 mixture of: (R)‐α‐cyano‐3‐phenoxybenzyl (1S)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate Cl\C(=C/[C@@H]3[C@H](C(=O)O[C@@H](C#N)c2cccc(Oc1ccccc1)c2)C3 (C)C)C(F)(F)F.N#C[C@@H](OC(=O)[C@@H]1[C@H](/C=C(\Cl)C(F)(F)F)C1(C)C)c3cccc(Oc2ccccc2)c3 BFPGVJIMBRLFIR‐GUCBCRIZSA‐N |
|
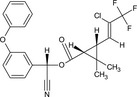
| Gamma‐cyhalothrin |
(S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate or (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate CC1([C@H]([C@H]1C(=O)O[C@H](C#N)c2cccc(c2)Oc3ccccc3)/C=C(/C(F)(F)F)\Cl)C BFPGVJIMBRLFIR‐GUCBCRIZSA‐N |
|
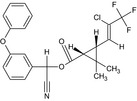
| Cyhalothrin |
(RS)‐α‐cyano‐3‐phenoxybenzyl (1RS,3RS)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate or (RS)‐α‐cyano‐3‐phenoxybenzyl (1RS)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate Cl\C(=C/[C@H]3[C@@H](C(=O)OC(C#N)c2cccc(Oc1ccccc1)c2)C3(C)C)C(F)(F)F.FC(F)(F)C(/Cl)=C/[C@@H]3[C@H](C(=O)OC(C#N)c2cccc(Oc1ccccc1)c2)C3(C)C OOAOVGPMANECPJ‐RWEUCVCFSA‐N |
|
| Compound Ia |
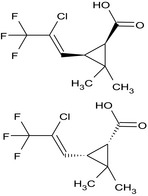
(1RS,3RS)‐3‐[(1Z)‐2‐chloro‐3,3,3‐ trifluoro‐1‐propen‐1‐yl]‐2,2‐dimethylcyclopropanecarboxylic acid Cl\C(=C/[C@H]1[C@@H](C(=O)O)C1(C)C)C(F)(F)F.FC(F)(F)C(/Cl)=C/[C@@H]1[C@H](C(=O)O)C1(C)C DPUIEEBDWOJPHB‐OBDQHKNMSA‐N |
|
| Compound IV |
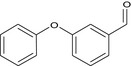
3‐phenoxybenzaldehyde O=Cc2cc(Oc1ccccc1)ccc2 MRLGCTNJRREZHZ‐UHFFFAOYSA‐N |
|
| Gamma‐lactone (R947650) |
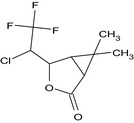
(1RS,4RS,5SR)‐4‐[(1RS)‐1‐chloro‐2,2,2‐trifluoroethyl]‐6,6‐dimethyl‐3‐oxabicyclo[3.1.0]hexan‐2‐one (Unstated stereochemistry) CC2(C)C1C(=O)OC(C(Cl)C(F)(F)F)C12 ZSSZFVGRINYCPY‐UHFFFAOYSA‐N |
|
| Metabolite V (PBA) |
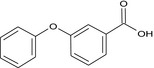
3‐phenoxybenzoic acid O=C(O)c2cc(Oc1ccccc1)ccc2 NXTDJHZGHOFSQG‐UHFFFAOYSA‐N |
|
| Metabolite XXIII (PBA(OH) |
3‐(4‐hydroxyphenoxy)benzoic acid O=C(O)c2cc(Oc1ccc(O)cc1)ccc2 OSGCDVKVZWMYBG‐UHFFFAOYSA‐N |
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2017.2.1 ACD/Labs 2017 Release (File version N40E41, Build 96719, 6 September 2017).
ACD/ChemSketch 2017.2.1 ACD/Labs 2017 Release (File version C40H41, Build 99535, 14 February 2018).
Suggested citation: EFSA (European Food Safety Authority) , Abdourahime H, Anastassiadou M, Brancato A, Brocca D, Carrasco Cabrera L, De Lentdecker C, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Medina P, Miron I, Molnar T, Nave S, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2019. Reasoned Opinion on the modification of the existing maximum residue levels for lambda‐cyhalothrin in celeries, fennel and rice. EFSA Journal 2019;17(1):5546, 28 pp. 10.2903/j.efsa.2019.5546
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00634
Approved: 3 December 2018
Notes
Commission Directive 2000/80/EC of 4 December 2000 amending Annex I to Council Directive 91/414/EEC concerning the placing of plant protection products on the market, so as to consolidate that Annex and include a further active substance. OJ L 309, 9.12.2000, p. 14–23.
Commission Implementing Regulation (EU) No 540/2011 of 23 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) 2016/146 of 4 February 2016 renewing the approval of the active substance lambda‐cyhalothrin, as a candidate for substitution, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Implementing Regulation (EU) No 540/2011. OJ L 30, 5.2.2016, p. 7–11.
The applicants shall submit confirmatory information as regards: 1. A systematic review to assess the evidence available as regards potential sperm effects linked to exposure to lambda‐cyhalothrin using guidance available (e.g. EFSA GD on Systematic Review methodology, 2010); 2. Toxicological information to assess the toxicological profile of the metabolites V (PBA) and XXIII (PBA(OH)). The applicants shall submit that information to the Commission, the Member States and the Authority by 1 April 2018.
Commission Regulation (EU) 2018/960 of 5 July 2018 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for lambda‐cyhalothrin in or on certain products. OJ L 169, 6.7.2018, p. 27–50.
The general request for information on three compounds (i.e. Ia, IV and gamma‐lactone) that were found in standard hydrolysis studies representative for sterilization conditions was introduced for all crops assessed in the framework of the MRL review.
Commission Regulation (EU) No 1141/2010 of 7 December 2010 laying down the procedure for the renewal of the inclusion of a second group of active substances in Annex I to Council Directive 91/414/EEC and establishing the list of those substances. OJ L 322, 8.12.2010, p. 10–19.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
The EMS provided the results of frozen storage stability studies on high acid (lemon) and high protein (lentil) matrices. Since not relevant for the current MRL application, EFSA did not assess these data.
Values corrected for recovery (Sweden, 2013).
Value corrected for recovery (Sweden, 2013).
The calculation of the long‐term exposure (chronic exposure) is based on the mean consumption data representative for 22national diets collected from MS surveys plus one regional and four cluster diets from the WHO GEMS Food database; for the acute exposure assessment the most critical large portion consumption data from 19 national diets collected from Member States surveys is used. The complete list of diets incorporated in EFSA PRIMo is given in its reference section (EFSA, 2007).
References
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers’ health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for lambda‐cyhalothrin according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(1):3546, 117 pp. 10.2903/j.efsa.2014.3546 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Conclusion on the peer review of the pesticide risk assessment of the active substance lambda‐cyhalothrin. EFSA Journal 2014;12(5):3677, 170 pp. 10.2903/j.efsa.2014.3677 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Revision of the review of the existing maximum residue levels for the active substance lambda‐cyhalothrin. EFSA Journal 2015;13(12):4324, 119 pp. 10.2903/j.efsa.2015.4324 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Reasoned Opinion on the focussed review of the existing maximum residue levels for lambda‐cyhalothrin in light of the unspecific residue definition and the existing good agricultural practices for the substance gamma‐cyhalothrin. EFSA Journal 2017;15(7):4930, 29 pp. 10.2903/j.efsa.2017.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 1996. Appendix G. Livestock Feeding Studies. 7031/VI/95‐rev.4.
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2015a. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.1, 2 01 December 2015.
- European Commission , 2015b. Review report for the active substance lambda‐cyhalothrin. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 11 December 2015 in view of the inclusion of lambda‐cyhalothrin in Annex I of Council Directive 91/414/EEC. SANCO/12282/2014 Rev 4, 11 December 2015, 18 pp.
- FAO (Food and Agriculture Organization of the United Nations), 2008. Lambda‐cyhalothrin. In: Pesticide residues in food –2008. Evaluations, Part I, Residues. FAO Plant Production and Protection Paper 194.
- FAO (Food and Agriculture Organization of the United Nations), 2015. Lambda‐cyhalothrin. In: Pesticide residues in food –2015. Evaluations, Part I, Residues. FAO Plant Production and Protection Paper 226.
- Greece , 2016. Evaluation report on the modification of MRLs for lambda‐cyhalothrin in various crops prepared by the evaluating Member State Greece under Article 8 of Regulation (EC) No 396/2005. 20 September 2016, Updated version (02/08/2018), 53 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2008. Guidance document on the magnitude of pesticide residues in processed commodities. In: Series of Testing and Assessment No 96. ENV/JM/MONO(2008)23, 29 July 2008.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues.
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- Sweden , 2013. Renewal Assessment Report (RAR) on the active substance lambda‐cyhalothrin prepared by the rapporteur Member State Sweden in the framework of Commission Regulation (EU) No 1141/2010. February 2013. Available online: http://www.efsa.europa.eu
- Sweden , 2014. Final Addendum to Renewal Assessment Report on lambda‐cyhalothrin, compiled by EFSA, February 2014. Available online: http://www.efsa.europa.eu


