Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Du Pont de Nemours GmbH submitted two requests to the competent national authority in Ireland to modify the existing EU maximum residue levels (MRLs) and to set import tolerances for oxathiapiprolin in various plant commodities in order to accommodate the intended EU uses and the authorised uses of this active substance in China, Canada and the United States. The data submitted in support of the request were found to be sufficient to derive MRL proposals for all crops under consideration, except for Brussels sprouts and peas (without pods), for which residue data were either not submitted or were insufficient to support the use. Adequate analytical methods for enforcement are available to control the residues of oxathiapiprolin in commodities under consideration at the validated limit of quantification (LOQ) of 0.01 mg/kg. Based on the risk assessment results, EFSA concluded that, taking into account the existing and the intended uses, the long‐term intake of residues of oxathiapiprolin is unlikely to present a risk to consumer health.
Keywords: oxathiapiprolin, various crops, pesticide, MRL, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, Du Pont de Nemours GmbH submitted an application to the competent national authority in Ireland (evaluating Member State (EMS)) to modify the existing maximum residue levels (MRLs) for the active substance oxathiapiprolin in onions, garlic, pepper, sunflower seeds and hops to accommodate for the intended European uses of oxathiapiprolin. Additionally, a second application was submitted by the same applicant to Ireland in order to set import tolerances for oxathiapiprolin in table‐ and wine grapes, bulb vegetables, leek, fruiting vegetables, cucurbits with edible and inedible peel, flowering brassica, head brassica, lettuces and salad plants, spinaches and similar leaves, peas with and without pods and ginseng.
The EMS drafted two evaluation reports in accordance with Article 8 of Regulation (EC) No 396/2005, which were submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 17 July 2017.
The metabolism of oxathiapiprolin following foliar treatment of primary crops belonging to fruit, leafy and root crop groups has been investigated in the European Union (EU) pesticides peer review. The metabolism of oxathiapiprolin in plants proceeds via the hydroxylation at phenyl ring and the cleavage of the bond between piperidine and pyrazole rings. The main residue in most crops was parent oxathiapiprolin, with exception of mature grapes, where metabolites containing the pyrazole moiety (IN‐E8S72 and IN‐WR791) were major residues. The metabolism of oxathiapiprolin in rotational crops was investigated in the EU pesticides peer review and was found to be different; residues were exclusively composed of metabolites containing pyrazole moiety (IN‐E8S72 and its conjugate IN‐SXS67).
In the framework of the current assessment, new primary crop metabolism studies were submitted, investigating nature of oxathiapiprolin in fruit, leafy and root crops following soil application. The new studies confirm that after soil treatment, the metabolism proceeds in a similar pathway to that in rotational crops, forming metabolites IN‐E8S72, IN‐WR791 and IN‐RZB21/IN‐RZD74. New studies investigating metabolism in rotational crops following soil treatment at higher application rates were submitted under the current assessment. The results were comparable with the previously assessed studies by the peer review.
Studies investigating the effect of processing on the nature of oxathiapiprolin (hydrolysis studies) demonstrated that the active substance is stable.
Although different metabolic pathway in primary and rotational crops was observed, the peer review concluded that residue definitions for enforcement and risk assessment derived for primary crops as parent oxathiapiprolin are also applicable to rotational crops, since main rotational crop metabolites (IN‐E8S72, IN‐SXS67) are of a lower toxicity than oxathiapiprolin and therefore they are not proposed for the inclusion in the plant residue definitions. The toxicological relevance of plant metabolite IN‐WR791 was assessed in the current application. It was concluded that IN‐WR791 is expected to have similar toxicological profile as IN‐E8S72; the available data does not give an indication that this metabolite exhibits genotoxic effects.
Based on the metabolic pattern identified in the metabolism studies, hydrolysis studies and the toxicological significance of metabolites, the residue definitions for plant products were proposed by the peer review as ‘oxathiapiprolin’ for enforcement and risk assessment. The same residue definitions are confirmed for the current assessment and are implemented in the Regulation (EC) No 396/2005.
Some crops from the intended European uses can be grown in a crop rotation and therefore the magnitude of residues in rotational crops was further assessed. Considering the highest residue levels observed in crops from various field studies performed with 2‐ to 8‐fold of the intended EU application rate, it can be concluded that residues of oxathiapiprolin will be below 0.01 mg/kg in food commodities and below 0.05 mg/kg in feed commodities grown in a 30‐day crop rotation. At the shortest plant‐back intervals, residues of metabolites IN‐E8S72 and IN‐SXS67 could occur above 0.01 mg/kg in cereal grain, pulses, strawberries, legumes, lettuces, spinach, mustard greens and oilseeds and the metabolite IN‐WR791 in leafy vegetables. Quantifiable residues may occur in feed commodities. In order to avoid residues in crops that have relatively short vegetation period and are rotated within short plant‐back intervals, Member States granting authorisations of oxathiapiprolin might consider applying risk mitigation measures.
Sufficiently validated analytical methods are available to quantify residues in the crops assessed in this application according to the enforcement residue definition at or above the validated limit of quantification (LOQ) of 0.01 mg/kg.
The available data are considered sufficient to derive MRL proposals as well as risk assessment values for all crops under consideration in support of the intended EU uses and authorised uses in China, the USA and Canada, except for Brussels sprouts and peas (without pods).
In the framework of the current application, the applicant submitted processing studies which demonstrated that the transfer of residues of oxathiapiprolin from hops to beer is low.
The assessment of oxathiapiprolin residues in livestock is not relevant for the import tolerance request. Among the intended EU uses assessed, only sunflower seeds meal is used as a feed item. Therefore, the livestock dietary burden was calculated according to the OECD methodology, considering residue data in sunflower from the intended use and in potatoes from the existing use. The results of the dietary burden calculation demonstrated that expected dietary burdens for all livestock species are below the trigger value of 0.004 mg/kg body weight and therefore the nature and magnitude of oxathiapiprolin residues in livestock was not further investigated.
The toxicological profile of oxathiapiprolin was assessed in the framework of the EU pesticides peer review and the data were sufficient to derive an acceptable daily intake (ADI) of 0.14 mg/kg body weight per day. An acute reference dose (ARfD) was deemed unnecessary. The consumer risk assessment was performed with revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo).
The long‐term exposure assessment was performed taking into account the median residue values derived from the residue trials for the commodities under consideration. For the remaining commodities the existing EU MRLs and, if available, the risk assessment values were used. The estimated long‐term dietary intake accounted for a maximum of 2% of the ADI (FR toddler diet). The contribution of oxathiapiprolin residues expected in the commodities assessed in this application to the overall long‐term exposure is low (maximum 1.7% of ADI for spinaches; 0.5% for lettuce, 0.3% for wine grapes and 0.3% for leeks).
EFSA concluded that the long‐term intake of residues of oxathiapiprolin resulting from the existing and the intended uses is unlikely to present a risk to consumer health. EFSA proposes to amend the existing MRL as reported in the summary table below.
Full details of all endpoints and the consumer risk assessment can be found in Appendices B–D.
| Codea | Commodity |
Existing EU MRL (mg/kg) |
Proposed EU MRL (mg/kg) |
Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Oxathiapiprolin | ||||
|
0151010 0151020 |
Table and wine grapes | 0.7 | No change | The submitted data are sufficient to derive an MRL proposal for the authorised Chinese GAP which confirms the existing EU MRL. Risk to consumers unlikely. The MRL applicable in China is 1 mg/kg |
| 0220010 | Onions | 0.01* | 0.04 |
The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 0.04 mg/kg for onions, garlic, shallots; 2 mg/kg for spring onions and 0.5 mg/kg for tomatoes |
| 0220020 | Garlic | |||
| 0220030 | Shallots | |||
| 0220040 | Spring onions | 0.01* | 2.0 | |
| 0231010 | Tomatoes | 0.2 | 0.4 | |
| 0231020 | Sweet peppers/bell peppers | 0.01* | 0.2 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada and the intended EU indoor GAP. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 0.5 mg/kg |
| 0231030 | Aubergines | 0.2 | 0.4 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 0.5 mg/kg |
| 0231040 | Okra/lady's fingers | 0.01* | 0.2 | |
|
0232010 0232020 |
Cucumbers Gherkins |
0.1 | 0.2 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 0.2 mg/kg |
| 0232030 | Courgettes | 0.1 | 0.15 or 0.2 further risk management considerations needed | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada of 0.15 mg/kg. As alternative option, the setting of a group MRL of 0.2 mg/kg can be considered. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 0.2 mg/kg |
| 0233010 | Melons | 0.15 | 0.2 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 0.2 mg/kg |
| 0233020 | Pumpkins | 0.01* | ||
| 0233030 | Watermelons | 0.01* | ||
|
0241010 0241020 |
Broccoli Cauliflower |
0.01* | 1.5 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 1.5 mg/kg |
| 0242010 | Brussels sprouts | 0.01* | No proposal | The submitted data are not sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada |
| 0242020 | Head cabbage | 0.01* | 0.7 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 1.5 mg/kg |
|
0251010 0251030 0251010 0251010 0251010 0251010 0251010 |
Lamb's lettuce Escaroles Cresses Land cresses Rucola Red mustards Baby leaf crops |
0.01* | 5 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 15 mg/kg |
| 0251020 | Lettuces | 0.3 | 5 | |
|
0252010 0252020 0252030 |
Spinaches Purslanes Chards/beet leaves |
0.01* | 15 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 15 mg/kg |
| 0260030 | Peas (with pods) | 0.01* | 1.0 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 1 mg/kg |
| 0260040 | Peas (without pods) | 0.01* | No proposal | The submitted data are not sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada |
| 0270060 | Leeks | 0.01* | 2 | The submitted data on spring onions are sufficient to derive by extrapolation an MRL proposal for leek for the GAP authorised in the USA and Canada. Risk to consumers unlikely.The MRL applicable in the USA and Canada is 2 mg/kg. |
| 0401050 | Sunflower seeds | 0.01* | No change | The submitted data confirm the existing EU MRL for the intended NEU/SEU use. Risk to consumers unlikely |
| 0633020 | Ginseng | 0.05* | 0.15 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 0.15 mg/kg |
| 0700000 | Hops | 0.05* | 8.0 | The submitted data are sufficient to derive an MRL proposal for the intended NEU use. Risk to consumers unlikely |
MRL: maximum residue level; GAP: Good Agricultural Practice; NEU: northern Europe; SEU: southern Europe.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Assessment
Oxathiapiprolin is the ISO common name for 1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydro‐1,2‐oxazol3‐yl]‐1,3‐thiazol‐2‐yl}‐1‐piperidyl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone (IUPAC). The chemical structures of the active substance and its main metabolites are reported in Appendix F.
The detailed description of the intended European uses of oxathiapiprolin and the authorised uses of oxathiapiprolin in the United States (USA) and Canada, which are the basis for the current maximum residue level (MRL) application, is reported in Appendix A.
Oxathiapiprolin was evaluated in the framework of Regulation (EC) No 1107/20091 with Ireland designated as rapporteur Member State (RMS) for the representative uses as a fungicide on grapes, potatoes, tomatoes and aubergines. The draft assessment report (DAR) prepared by the RMS has been peer reviewed by the European Food Safety Authority (EFSA, 2016). Oxathiapiprolin was approved2 for the use as a fungicide on 3 March 2017.
In the framework of the peer review, MRL proposals were derived for table and wine grapes, tomatoes, aubergines, cucumbers, gherkins, courgettes, melons, lettuce and grape leaves which were implemented in Annex II of Regulation (EC) No 396/20053 by Commission Regulation (EU) 2017/10164. The JMPR has evaluated oxathiapiprolin in 2016 and 2018 (FAO, 2016b, 2018); Codex MRL proposals were derived for a wide range of crops, including those under consideration in this assessment.5 So far, CXLs have not been implemented in the EU MRL legislation.
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Du Pont de Nemours GmbH submitted an application to the competent national authority in Ireland (evaluating Member State, EMS) to modify the existing MRLs for the active substance oxathiapiprolin in onions, garlic, pepper, sunflower seeds and hops, to accommodate the intended EU uses. Additionally, the applicant notified a modification of the European Union Good Agricultural Practice (EU GAP) for wine grapes which was assessed in the framework of the peer review; however, this modification does not have an impact on the current MRL established for wine grapes.
In the second application submitted by the applicant Du Pont de Nemours GmbH to Ireland, the raising of the existing EU MRLs for oxathiapiprolin to accommodate the authorised uses of oxathiapiprolin in the United States, Canada and China was proposed.
In the table below, the MRL proposals derived by the EMS are summarised; in addition, the existing EU MRL, the MRLs in place in the country for which the import tolerance was requested and the CXL/Codex MRL proposals are included in the table below.
| Crop/commodity | Existing EU MRL (in mg/kg) | MRL proposal for intended new EU use (in mg/kg) | Proposed import tolerance (EMS proposal) (in mg/kg) | MRL in the country of origin (in mg/kg) | Existing Codex MRL (CXL)/proposed Codex MRL (in mg/kg) |
|---|---|---|---|---|---|
| Table grapes | 0.7 | – | 0.7 | 1 | 0.9 |
| Wine grapes | 0.7 | 0.7 | 0.7 | 1 | 0.9 |
| Onions | 0.01* | – | 0.04 | 0.04 | 0.04 |
| Garlic | 0.01* | – | 0.04 | 0.04 | 0.04 |
| Shallots | 0.01* | – | 0.04 | 0.04 | 0.04 |
| Spring onions | 0.01* | – | 2 | 2 | 2 |
| Tomatoes | 0.2 | – | 0.5 | 0.5 | 0.4 |
| Sweet peppers/bell peppers | 0.01* | – | 0.2 | 0.5 | 0.4 |
| Aubergines | 0.2 | – | 0.5 | 0.5 | 0.4 |
| Okra/lady's fingers | 0.01* | – | 0.2 | 0.5 | 0.4 |
| Cucumbers | 0.1 | – | 0.2 | 0.2 | 0.2 |
| Courgettes | 0.1 | – | 0.2 | 0.2 | 0.2 |
| Gherkins | 0.1 | – | 0.2 | 0.2 | 0.2 |
| Melons | 0.15 | – | 0.2 | 0.2 | 0.2 |
| Pumpkins | 0.01* | – | 0.2 | 0.2 | 0.2 |
| Watermelons | 0.01* | – | 0.2 | 0.2 | 0.2 |
| Broccoli | 0.01* | – | 1.5 | 1.5 | 1.5 |
| Cauliflower | 0.01* | – | 1.5 | 1.5 | 0.3 |
| Brussels sprouts | 0.01* | – | 0.7 | 1.5 | – |
| Head cabbage | 0.01* | – | 0.7 | 1.5 | 0.7 |
| Lettuces and other salad plants (except lettuces) | 0.01* | – | 5 | 15 | – |
| Lettuces | 0.3 | – | 5 | 15 |
3 head lettuce 5 leaf lettuce |
| Spinaches and similar leaves | 0.01* | – | 15 | 15 | 15 |
| Peas (with pods) | 0.01* | – | 1 | 1 | 1 |
| Peas (without pods) | 0.01* | – | 0.05 | 0.05 | 0.05 |
| Leeks | 0.01* | – | 2 | 2 | 2 |
| Sunflower seeds | 0.01* | 0.01* | – | – | 0.01* |
| Ginseng | 0.05* | – | 0.15 | 0.15 | 0.15 |
| Hops | 0.05* | 8 | – | – | – |
MRL: maximum residue level; EMS: evaluating Member State; CXL: Codex maximum residue limit.
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
The EMS drafted two evaluation reports in accordance with Article 8 of Regulation (EC) No 396/2005, which were submitted to the European Commission and forwarded to EFSA on 17 July 2017 (Ireland, 2017a,b). Following requests for clarification and the submission of additional information to address the data gaps, the EMS has updated the evaluation reports; the final versions were submitted in February 2019 and final clarifications were provided in April 2019.
EFSA based its assessment on the updated evaluation reports submitted by the EMS (Ireland, 2017a,b), the DAR (and its addendum) (Ireland 2015, 2016) prepared under Regulation (EC) 1107/2009 and the conclusion on the peer review of the pesticide risk assessment of the active substance oxathiapiprolin (EFSA, 2016).
For this application, the data requirements established in Regulation (EU) No 283/20136 and the guidance documents applicable at the date of submission of the application to the EMS are applicable (European Commission, 1997a, b, c, d, e, f, g, 2000, 2010a, b, 2017; OECD, 2011, 2013, 2018). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20117.
A selected list of end points of the studies assessed by EFSA in the framework of the this MRL application, including the end points of relevant studies assessed previously, are presented in Appendix B.
The evaluation reports submitted by the EMS (Ireland, 2017a,b) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
1. Mammalian toxicology
The toxicological properties of oxathiapiprolin have been assessed in the framework of the peer review; the data were found sufficient to derive an acceptable daily intake (ADI) of 0.14 mg/kg body weight (bw) per day; the setting of an acute reference dose (ARfD) was not considered necessary, due to the low acute toxicity of the active substance (EFSA, 2016).
Toxicological data were also provided for a number of metabolites identified in metabolism studies (primary crop, rotational crop and animal metabolism studies): IN‐RDT31, IN‐SXS67, IN‐WR791 and IN‐E8S72.
For metabolite IN‐E8S72, an ADI of 1.157 mg/kg bw per day was agreed on the basis of a 28‐day rat study (and applying an uncertainty factor of 1,000 to cover extrapolation from subacute to long‐term toxicity and lack of a complete data package).
For metabolite IN‐SXS67, being only a glucoside conjugate of IN‐E8S72, the same ADI was considered applicable. For both compounds, the derivation of an ARfD was not considered necessary.
For metabolite IN‐WR791, no potential for gene mutations (Ames test) or structural chromosomal aberrations (clastogenicity test in vitro) was identified, whereas the potential for the induction of numerical chromosomal aberrations (aneugenic properties) could not be excluded (EFSA, 2016).
In the framework of the current assessment, EFSA requested the applicant to submit further investigations of the aneugenicity potential of the metabolite IN‐WR791 and further assessment of the toxicological profile of the metabolite in comparison with the parent compound. In response to EFSA's request, the applicant performed an in vitro micronucleus assay with human peripheral blood lymphocytes (Ireland, 2017a). The negative results confirmed the absence of aneugenic potential for this metabolite. No further assessment in comparison with the toxicological profile of the parent has been provided by the EMS.
EFSA is of the opinion that additional read‐across considerations should have been given between the metabolite IN‐WR791 and the metabolites IN‐E8S72 and IN‐SXS67. Supported by the absence of genotoxic properties for the three compounds, EFSA would consider that a similar toxicological profile can be expected, demonstrating that IN‐WR791 is of lower toxicity than oxathiapiprolin.
2. Residues in plants
2.1. Nature of residues and methods of analysis in plants
2.1.1. Nature of residues in primary crops
In the framework of the EU pesticides peer review, the metabolism of oxathiapiprolin in primary crops belonging to fruit (grape), leaf (lettuce) and root (potato) crop groups has been investigated following foliar application (3 applications of 70 g/ha; radiolabelling in pyrazole and thiazole moiety) (EFSA, 2016). Due to the low total radioactive residue (TRR) at harvest, identification of the residues was not attempted in potato tubers. In grape, lettuce and potato leaves, oxathiapiprolin was observed as the major component of the TRR, accounting for 25–85%. In contrast, in mature grapes, 2 months after the last application, the main components were identified as metabolites IN‐E8S72 and IN‐WR791, representing 14.4% and 18.6% TRR (0.06 mg/kg), respectively. The peer review concluded that in primary crops the metabolism proceeds by hydroxylation of the molecule at the phenyl ring, the cleavage of the bond between the piperidine and pyrazole rings to form the thiazole‐containing metabolites (IN‐Q9L80 and IN‐QPS10) or the pyrazole metabolites (IN‐E8S72, IN‐KJ552, IN‐R7B20 and IN‐WR791). Further conjugation leads to additional glucoside‐conjugated metabolites (IN‐SXS67) (EFSA, 2016).
Additional studies were submitted for the current assessment where the nature of oxathiapiprolin was investigated after soil application in root (potatoes), leafy (lettuce) and fruit (courgettes) crops (Ireland, 2017b). Oxathiapiprolin, labelled in pyrazole and isoxazoline moiety, was applied preplanting on bare soil at an application rate of 600 g/ha and on the same day crops were sown/planted. The TRR in the crops is summarised in the table below.
| Crop/matrix | Sample/sampling interval | TRR(mg eq./kg) | |
|---|---|---|---|
| Pyrazole‐14C oxathiapiprolin | Isoxazoline‐14C oxathiapiprolin | ||
| Potato | Immature tubers (37‐day PHI; BBCH 65) | 0.023 | 0.013a |
| Immature foliage (37‐day PHI; BBCH 65) | 0.026 | 0.021 | |
| Mature tubers (72‐day PHI; BBCH 91) | 0.013 | 0.006a | |
| Mature foliage (72‐day PHI; BBCH 91) | 0.108 | 0.056 | |
| Lettuce | Immature leaves (44 DAT; BBCH 45) | 0.019 | < 0.008a |
| Mature leaves (57 DAT; BBCH 49) | 0.014 | 0.006a | |
| Courgettes | Immature fruits (44 DAT; BBCH 71) | 0.013 | < 0.006a |
| Immature foliage (44 DAT; BBCH 71) | 0.045 | 0.028 | |
| Mature fruits (79 DAT; BBCH 89) | 0.023 | < 0.006a | |
| Mature foliage (79 DAT; BBCH 89) | 0.17 | 0.008a | |
TRR: total radioactive residue; PHI: preharvest interval; BBCH: growth stages of mono‐ and dicotyledonous plants; DAT: days after treatment.
Not characterised further.
The TRR in potato tubers and lettuce decreased over time, whereas in other matrices an increase of residues was observed. The TRR from isoxazoline study in all matrices were generally lower; in mature edible crops radioactivity was below 0.01 mg eq./kg and thus not further characterised. Parent oxathiapiprolin, if present, did not exceed 10% TRR in mature edible matrices. The main components of the TRR in immature and mature edible matrices (potatoes, lettuce and courgettes) exceeding the trigger value of 10% were metabolites IN‐E8S72, IN‐WR791, IN‐RZB20 and IN‐RZB21/IN‐RZD74. The actual amounts, however, were low, being above 0.01 mg/kg only for metabolite IN‐WR791 in courgettes (0.016 mg/kg). The distribution of TRR in various crop matrices and the summary of identified compounds is summarised in Appendix E, Table E.1.
Table Table E.1.
Nature of residues in primary crops; summary on the characterisation and identification of total radioactive residues (TRR) in primary plants following soil treatment (Ireland, 2017b)
| Soil, 1 × 600 g/ha | Total radioactive residues (TRR % (mg/kg)) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Potato | Lettuce | Courgettes | ||||||||||
| Labelling position | Pyrazole‐14C | Isoxazoline‐14C | Pyrazole‐14C | Isoxazoline‐14C | Pyrazole‐14C | Pyrazole‐14C | Isoxazoline‐14C | ||||||
| Growth stage | 37‐day PHI BBCH 65 | 37‐day PHI BBCH 65 | 37‐day PHI BBCH 65 | 72‐day PHI BBCH 91 | 72‐day PHI BBCH 91 | 72‐day PHI BBCH 91 | 44 DAT BBCH 45 | 57 DAT BBCH 49 | 44 DAT BBCH 71 | 44 DAT BBCH 71 | 79 DAT BBCH 89 | 79 DAT BBCH 89 | 44 DAT BBCH 71 |
| Part analysed | Immature tubers | Immature foliage | Immature foliage | Mature tubers | Mature foliage | Mature foliage | Immature leaves | Mature leaves | Immature foliage | Immature fruit | Mature foliage | Mature fruit | Immature foliage |
| Total TRR (mg/kg) | 0.023 | 0.026 | 0.021 | 0.013 | 0.108 | 0.056 | 0.019 | 0.014 | 0.045 | 0.013 | 0.17 | 0.023 | 0.028 |
| Oxathiapiprolin | 6.9 (0.002) | nd | nd | nd | 4.2 (0.005) | 9.2 (0.005) | nd | nd | < 0.001 (< 0.001) | 0.5 (< 0.001) | 4.6 (0.008) | < 0.001 | 24.4 (0.007) |
| IN‐Q7H09 | nd | nd | nd | nd | nd | 1.7 (0.003) | 18.5 (0.005) | ||||||
| IN‐SXS67 (gluc‐IN‐E8S72) | 3.7 (0.001) | 6.2 (0.002) | nd | 7.1 (0.001) | 4.2 (0.005) | nd | 1.9 (< 0.001) | 3.5 (< 0.001) | 7.2 (0.003) | 4 (0.001) | 6 (0.01) | 1.3 (< 0.001) | nd |
| IN‐E8S72 | 5.8 (0.001) | 11.5 (0.003) | nd | 13.9 (0.002) | 5.1 (0.006) | nd | 18.9 (0.004) | 21.2 (0.003) | 23.5 (0.011) | 4.5 (0.001) | 21.1 (0.036) | 4.3 (0.001) | nd |
| IN‐WR791 | 14.3 (0.003) | 13.3 (0.003) | nd | 25.3 (0.003) | 7.3 (0.008) | nd | 22.7 (0.004) | 29.5 (0.004) | 23.7 (0.011) | 56.7 (0.008) | 27.5 (0.047) | 73.7 (0.016) | nd |
| IN‐KJ552 | 7.3 (0.002) | 4.1 (0.001) | nd | 6.5 (0.001) | 4.4 (0.005) | nd | nd | 3.1 (0.0001 | 3.4 (0.002) | 2.6 (< 0.001) | 1.5 (0.002) | 2 (< 0.001) | nd |
| IN‐RZB20 | 12 (0.003) | 13.1 (0.003) | nd | 12.2 (0.002) | 11.5 (0.012) | nd | 5.1 (0.001) | 6.5 (0.001) | 16.8 (0.008) | 2.2 (< 0.001) | 12.4 (0.021) | 3.3 (0.001) | nd |
| IN‐RZB21/IN‐RZD74 | 2.7 (0.001) | 18.8 (0.005) | nd | 5.5 (0.001) | 13.1 (0.014) | nd | 21.4 (0.004) | 19 (0.003) | 12.7 (0.006) | 4.3 (0.001) | 10.9 (0.018) | 4.3 (0.001) | nd |
| No of unidentified metabolites (% TRR (mg/kg)), of them: | 5 (11.2%; 0.003) | 6 (14%; 0.003) | 10 (40.6%; 0.009) | 2 (4.9%; 0.001) | 13 (26.9%; 0.028) | 18 (66.6%; 0.038) | 0 | 1 | 5 (6.2%; 0.003) | 3 (3.3%; < 0.001) | 4 (6.7%; 0.01) | 2 (5%; 0.002) | 1 |
| ‐highest individual | 2.6 (0.001) | 4.7 (0.001) | 6.9 (0.001) | 4.5 (0.001) | 4.5 (0.005) | 8.5 (0.005) | 0 | 1.2 (< 0.001) | 1.7 (0.001) | 1.3 (< 0.001) | > 2.7 (0.004) | 2.5 (0.001) | 13.2 (0.004) |
| Unextracted | 14.8 (0.003) | 10.8 (0.003) | 20.4 (0.004) | 19.3 (0.003) | 9.2 (0.01) | 15 (0.008) | 9.5 (0.002) | 11.7 (0.002) | 9.3 (0.004) | 6.3 (0.001) | 6 (0.01) | 3.2 (0.001) | 23.1 (0.006) |
PHI: preharvest interval; BBCH: growth stages of mono‐ and dicotyledonous plants; DAT: days after treatment; nd: not identified.
> 10% TRR highlighted.
All metabolites identified in the new metabolism studies have been also observed in rotational crop and, to a less extent, in primary crop metabolism studies submitted for the EU pesticides peer review.
The metabolic pathway of oxathiapiprolin in primary plants following soil treatment proceeds similarly to that in rotational crops via the cleavage of the bond between piperidine and pyrazole rings. The metabolites containing the pyrazole ring (IN‐E8S72, IN‐KJ552, IN‐RZB20 and IN‐WR791) are preferentially taken up by the plant from soil. For the intended uses, the metabolic behaviour in primary crops is sufficiently addressed.
2.1.2. Nature of residues in rotational crops
Oxathiapiprolin is intended to be used in EU on several crops (onions, garlic, peppers and sunflower) that can be grown in rotation with other crops.
According to the soil degradation studies, the maximum DT90 value of oxathiapiprolin from field studies is 682 days. The maximum DT90 values for relevant soil metabolites of oxathiapiprolin are as follows: DT90lab of 1585 days for metabolite IN‐E8S72, 2266 days for metabolite IN‐QPS10, and 565 days for metabolite IN‐RAB06 (in absence of field data) and DT90 field of 632 days for metabolite IN‐RDT31 (EFSA, 2016). Hence, the nature and magnitude of oxathiapiprolin residues in rotational crops has to be further investigated.
-
a)
Soil treatment at 210 g a.s./ha
The nature of oxathiapiprolin in rotational crops has been investigated in the EU pesticides peer review in studies where bare soil was treated at an application rate of 210 g/ha, sowing wheat, lettuce and turnip as rotational crops 30, 120 and 365 days after the soil treatment (Ireland, 2015; EFSA, 2016). The peer review concluded that in rotational crops the metabolism differs from that in primary crops and it is exclusively composed of metabolites containing the pyrazole moiety (especially metabolite IN‐E8S72 and its glucose‐conjugated IN‐SXS67) accounting for more than 50% of the TRR. Oxathiapiprolin metabolites denoting the structure of the parent compound and metabolites containing the thiazole moiety were almost never detected. The metabolic profile in rotational crops is mostly the result of a preferential uptake from soil of the metabolites containing the pyrazole moiety. Chiral analysis of samples indicated that the enantiomeric ratio (ca 1:1) remained unchanged in plants (EFSA, 2016).
-
b)
Soil treatment at 600 g a.s./ha
In the framework of the current assessment, the applicant submitted new metabolism studies where the nature of [14C]‐oxathiapiprolin was investigated in turnips, lettuce and wheat grown as rotational crops 30, 120 and 365 days following the soil treatment with oxathiapiprolin at a rate of 600 g/ha (Ireland, 2017a). These new studies confirm the conclusions of the peer review. The main metabolites present in rotational crops were IN‐E8S72 (and IN‐SXS67), IN‐WR791, IN‐RZB20 and IN‐RZB21/IN‐RZD74.
In addition, the comparison of both studies indicates that there is no significant difference in the magnitude of residues in crops from the low and the high dose rate studies. The persistent soil metabolites, which have been identified in the soil degradation studies (i.e. IN‐RAB06, IN‐QPS10 and IN‐RDT31) were not identified in the rotational crop metabolism studies.
An overview of all available metabolism studies in rotational crops and the results are provided in Appendix E, Tables E.2–E.4.
Table E.2.
Nature of residues in rotational crops; summary of characterised and identified %TRR (in brackets mg/kg) in wheat
| Wheat | Soil 1 × 210 g/ha (EFSA, 2016) | Soil 1 × 600 g/ha (Ireland, 2017a) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Labelling position | [pyrazole‐14C]oxathiapiprolinb | Isoxazoline‐14C | [pyrazole‐14C]oxathiapiprolinc | |||||||||||||||||
| Part analysed | Forage | Forage | Forage | Straw | Straw | Straw | Grain | Grain | Grain | Straw | Straw | Forage | Forage | Forage | Straw | Straw | Straw | Grain | Grain | Grain |
| PBI | 30 | 120 | 365 | 30 | 120 | 365 | 30 | 120 | 365 | 30 | 120 | 30 | 120 | 365 | 30 | 120 | 365 | 30 | 120 | 365 |
| Total TRRR (mg/kg) | 0.269 | 0.172 | 0.022 | 0.76 | 0.59 | 0.166 | 0.258 | 0.097 | < 0.007 a | 0.024 | 0.04 | 0.066 | 0.168 | 0.234 | 0.697 | 0.668 | 0.477 | 0.135 | 0.191 | 0.117 |
| Oxathiapiprolin | 0.7 (0.002) | nd | nd | 0.7 (0.005) | nd | nd | 1.9 (0.005) | 0.3 (< 0.001) | 12.5 (0.003) | 6.1 (0.004) | nd | 0.9 (0.002) | nd | nd | nd | nd | nd | nd | ||
| IN‐SXS67 (gluc‐IN‐E8S72) | 18 (0.05) | 58.8 (0.1) | 7.3 (0.002) | 19.8 (0.15) | 48.1 (0.28) | 57.2 (0.095) | 4.2 (0.010) | 11.8 (0.011) | 6.1 (0.004) | 36.9 (0.062) | 21.4 (0.05) | 39 (0.27) | 25.4 (0.17) | 26.4 (0.126) | 9.9 (0.013) | 7.9 (0.015) | 9.6 (0.011) | |||
| IN‐E8S72 | 8.8 (0.024) | 11.8 (0.02) | 6.5 (0.001) | 13.1 (0.1) | 13.5 (0.08) | 7.0 (0.011) | 15 (0.039) | 20.1 (0.019) | 13.6 (0.009) | 7.7 (0.013) | 12 (0.028) | 4.5 (0.031) | 8.7 0.058) | 6 (0.029) | 14 (0.019) | 7.4 (0.014) | 8.2 (0.01) | |||
| IN‐WR791 | 42.2 (0.11) | 3.2 (0.006) | nd | 5.7 (0.043) | 2.6 (0.016) | 1.9 (0.003) | 37.7 (0.097) | 21.8 (0.021) | 16.7 (0.011) | 13.1 (0.022) | 31.2 (0.073) | 3.1 (0.022) | 8.3 (0.055) | 5.3 (0.025) | 22.6 (0.03) | 24.7 (0.047) | 36.7 (0.043) | |||
| IN‐KJ552 | 1.4 (0.01) | nd | nd | 4.5 (0.003) | 7.7 (0.013) | 6.8 (0.016) | 2.1 (0.014) | 4.2 (0.028) | < 0.001 | 2.9 (0.004) | 1.7 (0.003) | 0.001 | ||||||||
| IN‐RZB20 | 10.4 (0.028) | 7.5 (0.013) | 55.1 (0.012) | 27 (0.21) | 12.6 (0.075) | 7.8 (0.013) | 8.6 (0.022) | 4.9 (0.005) | 8.3 (0.014) | 5.1 (0.012) | 16.7 (0.116) | 21.8 (0.145) | 25.6 (0.122) | 10.2 (0.014) | 9.2 (0.017) | 13.2 (0.015) | ||||
| IN‐RZB21/IN‐RZD74 | 5.6 (0.015) | 6.6 (0.01) | 9.4 (0.07) | 7.1 (0.042) | 8.3 (0.014) | 4.5 (0.012) | 4.1 (0.004) | 13.7 (0.023) | 10.7 (0.025) | 11.5 (0.08) | 15 (0.10) | 11.8 (0.057) | 8.6 (0.011) | 4.5 (0.008) | ||||||
| IN‐QPS10 | 12 (0.005) | |||||||||||||||||||
| Total unidentified metabolites (% TRR (mg/kg) | 12.3 (0.033) | 4.6 (0.007) | 12.5 (0.002) | 11.6 (0.09) | 5.4 (0.033) | 16.2 (0.026) | 11.5 (0.028) | 8 (0.006) | 23 (0.006) | 14 (0.005) | ||||||||||
| Unextracted | 4 (0.01) | 4 (0.007) | 8 (0.002) | 1 (0.011) | 2 (0.012) | 2 (0.004) | 0 | 10 (0.01) | 37 (0.01) | 34 (0.013) | ||||||||||
PBI: plant‐back interval; TRR: total radioactive residues; nd: not identified.
Not characterised further.
The TRR from [isoxazoline‐14C]‐ and [thizole‐14C]‐ oxathiapiprolin treatments were ≤ 0.01 mg eq./kg (except wheat straw) and thus were not analysed further.
The TRR from [isoxazoline‐14C]‐ oxathiapiprolin treatment were ≤ 0.01 mg eq/kg and thus were not analysed further.
> 10% TRR highlighted.
Table Table E.4.
Nature of residues in rotational crops; summary of characterised and identified %TRR (in brackets mg/kg) in lettuce
| Lettuce | Soil 1 × 210 g/ha (EFSA, 2016) | Soil 1 × 600 g/ha (Ireland, 2017a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [pyrazole‐14C]oxathiapiprolinb | [pyrazole‐14C]oxathiapiprolin c | |||||||||||
| Part analysed | Immature lettuce | Immature lettuce | Immature lettuce | Mature lettuce | Mature lettuce | Mature lettuce | Immature lettuce | Immature lettuce | Immature lettuce | Mature lettuce | Mature lettuce | Mature lettuce |
| PBI | 30 | 120 | 365 | 30 | 120 | 365 | 30 | 120 | 365 | 30 | 120 | 365 |
| Total TRRR (mg/kg) | 0.028 | 0.028 | < 0.01 a | 0.013 | 0.022 | 0.006 a | 0.025 | 0.036 | 0.036 | 0.02 | 0.031 | 0.024 |
| Oxathiapiprolin | nd | nd | 5.8 (0.002) | nd | nd | 1.2 (< 0.001) | nd | nd | nd | |||
| IN‐Q7D41 | 6 (0.002) | |||||||||||
| IN‐SXS67 (gluc‐IN‐E8S72) | nd | 4.7 (0.001) | 2.7 (0.001) | 5 (0.002) | 4 (0.001) | 1.2 (< 0.001) | 4.7 (0.001) | 5.2 (0.001) | ||||
| IN‐E8S72 | 20.8 (0.006) | 76.3 (0.022) | 10.5 (0.001) | 48.1 (0.013) | 19.5 (0.005) | 24 (0.009) | 34.6 (0.012) | 22.1 (0.004) | 21.1 (0.007) | 33.2 (0.008) | ||
| IN‐WR791 | 30.6 (0.009) | 7.5 (0.002) | 12.1 (0.002) | 5.4 (0.001) | 26.8 (0.007) | 25 (0.007) | 23.6 (0.008) | 34.3 (0.007) | 20.2 (0.006) | 27.1 (0.007) | ||
| IN‐KJ552 | 2.2 (0.001) | 4.9 (0.002) | 2.5 (0.001) | 3.5 (0.001) | 4.1 (0.001) | 1.2 (< 0.001) | ||||||
| IN‐RZB20 | 2.9 (0.001) | 13.9 (0.003) | 1.2 (< 0.001) | 3.2 (0.001) | 5 (0.001) | 3.2 (0.001) | 4.7 (0.001) | |||||
| IN‐RZB21/IN‐RZD74 | 3 (0.001) | 4.8 (0.001) | 14.2 (0.005) | 14.4 (0.005) | 20.8 (0.004) | 14.8 (0.005) | 17.7 (0.004) | |||||
| Total unidentified metabolites (% TRR (mg/kg | 27 (0.007) | 17.8 (0.005) | 32 (0.001) | 19 (0.001) | ||||||||
| Unextracted | 7.8 (0.002) | 4.7 (0.001) | 15.7 (0.002) | 4.6 (0.001) | ||||||||
PBI: plant‐back interval; TRR: total radioactive residues; nd: not identified.
not characterised further.
The TRR from [isoxazoline‐14C]‐ and [thizole‐14C]‐ oxathiapiprolin treatments were ≤ 0.01 mg eq./kg and thus were not analysed further.
The TRR from [isoxazoline‐14C]‐ oxathiapiprolin treatment were ≤ 0.01 mg eq./kg and thus were not analysed further.
> 10% TRR highlighted.
2.1.3. Nature of residues in processed commodities
The effect of processing on the nature of parent oxathiapiprolin was investigated in the framework of the EU pesticides peer review (EFSA, 2016). These studies showed that oxathiapiprolin is hydrolytically stable under standard processing conditions.
2.1.4. Methods of analysis in plants
Analytical methods for the determination of oxathiapiprolin residues in food commodities of plant origin were assessed during the EU pesticides peer review (EFSA, 2016). It is concluded that sufficiently validated analytical methods are available for the determination of oxathiapiprolin at the validated limit of quantification (LOQ) of 0.01 mg/kg in matrices under consideration.
Ginseng (dried root) according to EU guidance document (European Commission, 2010b) is considered as a matrix difficult to analyse and therefore for this commodity full validation data shall be presented to prove the suitability of the prosed enforcement method. The residue trial samples of ginseng have been analysed with a method assessed by the peer review as an enforcement method (single residue method). This method has been sufficiently validated in ginseng root and the selectivity of the method was confirmed (Ireland, 2017a). An independent laboratory validation (ILV) has not been performed.
In addition, the applicant submitted validation data (including confirmatory data) and an ILV for the multi residue DFG‐19 method which was proposed as enforcement method by the peer review. Validation data were provided for different matrices that are considered as complex: coffee beans, hops (dried), black tea (leaves) and dried tobacco (Ireland, 2017a). The validation data demonstrate that DFG‐19 method is acceptable to enforce residues of oxathiapiprolin in coffee beans, hops, black tea and dried tobacco at the LOQ of 0.01 mg/kg.
Considering the wide range of matrices in which the enforcement of oxathiapiprolin has been sufficiently demonstrated, EFSA concluded that the available analytical enforcement methods are fit for purpose to measure oxathiapiprolin residues also in ginseng root at the LOQ of 0.01 mg/kg.
2.1.5. Stability of residues in plants
The storage stability of oxathiapiprolin in plants stored under frozen conditions was investigated in the framework of the EU pesticides peer review (EFSA, 2016). It is concluded that in the relevant crop matrices under consideration the freezer storage stability of oxathiapiprolin has been addressed for 18 months when stored −20°C.
2.1.6. Proposed residue definitions
Based on the metabolic pattern identified in primary crops following foliar treatment, the results of hydrolysis studies, the toxicological significance of metabolites and the capabilities of enforcement analytical methods, the following residue definitions were proposed by the pesticides peer review (EFSA, 2016):
residue definition for risk assessment: oxathiapiprolin;
residue definition for enforcement: oxathiapiprolin (NB: The residue definition derived by the peer review has been taken over in Regulation (EC) No 396/2005).
The same residue definitions were proposed for processed products.
For rotational crops, although the metabolic pathway was found to be different, the same residue definitions were agreed, considering that main rotational crop metabolites IN‐E8S72 and its conjugate IN‐SXS67 have lower toxicity than parent compound (EFSA, 2016).
The new primary and rotational crop metabolism studies submitted within the current application confirm that after soil applications the metabolism of oxathiapiprolin in primary crops proceeds in a similar pathway as in rotational crops. Among the major identified metabolites, three were confirmed to be present at quantifiable levels in primary crop field trials as well as in rotational crops: IN‐E8S72 (its conjugate IN‐SXS67) and IN‐WR791. The toxicological relevance of plant metabolite IN‐WR791 was discussed under the current assessment (see Section 1) and it was concluded that IN‐WR791 is expected to have similar toxicological profile as IN‐E8S72.
EFSA concludes that the residue definitions proposed by the peer review as parent oxathiapiprolin alone are valid also for the crops assessed in the framework of this application.
2.1.7. Magnitude of residues in plants
2.1.8. Magnitude of residues in primary crops
In support of the MRL application, the applicant submitted residue trials performed on a wide range of crops. The samples were analysed for oxathiapiprolin, metabolites IN‐E8S72, IN‐WR791 and for several crops also for metabolites IN‐Q7H09, IN‐RDG40, IN‐RZB20, IN‐RZD74, IN‐SXS67. According to the assessment of the EMS, the methods used were sufficiently validated and fit for purpose. The samples of these residue trials were stored under conditions for which integrity of the samples had been demonstrated. The detailed residue trials data are reported in the Appendix B.1.2.1.
2.1.8.1. Table and wine grapes
-
a)
EU use: The use on table and wine grapes has been assessed in the framework of the peer review (EFSA, 2016) the GAP for wine grapes (northern Europe (NEU) and southern Europe (SEU)) allowed two applications of 60 g/ha and a preharvest interval (PHI) of 28 days. The SEU GAP for table grapes defined two applications of 60 g/ha and a PHI of 14 days, while the intended NEU GAP has defined two applications of 40 g/ha and a PHI of 14 days. Sufficient residue trials with two applications of 60 g/ha (NEU and SEU) were available to derive a MRL of 0.7 mg/kg. For the less critical GAP with a PHI of 28 days, EFSA noted a data gap. Under the current assessment, the applicant notified a revised GAP for wine grapes, specifying the PHI interval of 14 days instead of 28 days. The revised GAP is fully supported by EU residue trials, leading to a MRL of 0.7 mg/kg.
-
b)
Import tolerance request/table and wine grapes: In support of the authorised use of oxathiapiprolin in China, the applicant submitted eight GAP‐compliant residue trials on grapes, which were performed in various regions of China in 2012, 2013 and 2015. Residue data are sufficient to derive an MRL proposal of 0.7 mg/kg, which confirms the existing EU MRL.
2.1.8.2. Bulb vegetables: onions, garlic, shallots, spring onions, leek
-
a)
EU use/onions, garlic: In support of the intended EU outdoor foliar use on onions and garlic, the applicant submitted eight GAP‐compliant residue trials on onions supporting the NEU use and eight GAP‐compliant residue trials on onions supporting the SEU use. Trials were performed in various EU countries over growing seasons of 2012 and 2013. Trials were performed as bridging trials, using different types of formulation (OD and SE). The proposed extrapolation of calculated MRL proposal of 0.01 mg/kg (LOQ) from onions to garlic is acceptable (European Commission, 2017).
-
b)
Import tolerance request/onions, garlic, shallots: In support of the authorised outdoor foliar use in Canada and the USA on bulb vegetables, the applicant submitted 11 GAP‐compliant residue trials on onions,8 which were performed in the USA and Canada in 2011 and 2012. The submitted data are sufficient to derive an MRL proposal of 0.04 mg/kg for onions; extrapolation to shallots and garlic is acceptable in accordance with the EU guidance document (European Commission, 2017).
-
c)
Import tolerance request/spring onions, leeks: The applicant submitted five GAP‐compliant residue trials on spring onions, which were performed in the USA in 2011. For spring onions sufficient data are submitted to derive an MRL proposal of 2 mg/kg. The applicant and the EMS proposed to extrapolate residue data from spring onions to leek, which, according to EU guidelines, is acceptable.
2.1.8.3. Fruiting vegetables: tomatoes, aubergines, peppers, okra
-
a)
EU use/peppers: In support of the intended EU indoor foliar use on peppers, the applicant submitted nine GAP‐compliant residue trials on peppers, which were performed in 2014 in Spain, Italy, the Netherlands, France and Greece. Five trials were performed on chilli peppers and four trials on bell peppers. The submitted residue data are sufficient to derive an MRL proposal of 0.2 mg/kg in support of the EU indoor use on peppers.
-
b)
Import tolerance request/peppers, okra: In support of the authorised outdoor foliar and soil use in Canada and the USA on peppers and okra, the applicant submitted 16 GAP‐compliant residue trials on peppers performed in the USA and Canada in 2011. Trials were designed in a way that separate plots received either foliar or soil (drip/drench) treatment. Based on the residue trials with foliar application, an MRL proposal of 0.2 mg/kg was derived.
Following soil treatment, oxathiapiprolin residues ranged from < 0.01 to 0.017 mg/kg. The soil application would result in an MRL proposal of 0.02 mg/kg.
In support of the authorised indoor foliar use in Canada and the USA on peppers and okra, the applicant submitted two trials on peppers, which are insufficient to support the use and to derive an MRL proposal.
Based on the foliar outdoor use an MRL of 0.2 mg/kg is proposed for pepper; extrapolation from peppers to okra (lady's fingers) is acceptable according to the EU guidance document.
-
c)
Import tolerance request/tomatoes, aubergines: In support of the authorised indoor foliar use in Canada and the USA on tomatoes and aubergines, the applicant submitted four GAP‐compliant residue trials on tomatoes which have been performed in the USA in 2011. The number of trials is not sufficient to support the authorised use and to derive an MRL proposal.
In support of the authorised outdoor foliar and soil use in Canada and the USA on tomatoes and aubergines, the applicant submitted 19 GAP‐compliant residue trials on tomatoes which were performed in the USA and Canada in 2011. Residue trials were performed with standard size tomatoes as well as cherry tomatoes. Trials were designed in a way that separate plots received either foliar or soil (drip/drench) treatment. Oxathiapiprolin accounted for 0.01–0.31 mg/kg from the foliar use and for < 0.01–0.24 mg/kg from soil treatment.
For the foliar use an MRL of 0.4 mg/kg is derived, while for soil treatment an MRL of 0.3 mg/kg is sufficient. The proposed extrapolation of residue data from tomatoes to aubergines is acceptable according to the EU guidance documents (European Commission, 2017). It is noted that the applicant and the EMS proposed an MRL of 0.5 mg/kg, based on the residue data in cherry tomatoes only which is in line with the tolerance set in CAN and the USA, but not in accordance with currently applicable EU guidelines.
2.1.8.4. Cucurbits with edible peel/cucumbers, gherkins, courgettes
In support of the authorised indoor foliar use in Canada and the USA, the applicant submitted four GAP‐compliant residue trials on cucumbers which have been performed in the USA and Canada in 2011. Two trials were performed on small size cucumbers. The number of trials is not sufficient to derive an MRL proposal for cucumbers and courgettes; however, the trials would be sufficient to derive an MRL proposal of 0.15 mg/kg for gherkins.
In support of the outdoor foliar and soil use in Canada and the USA, the applicant submitted 12 GAP‐compliant residue trials on cucumbers and 10 GAP‐compliant reside trials on courgettes, which were performed in the USA and Canada in 2011. One residue trial on cucumbers from soil treatment was disregarded as it was under dosed. Trials were designed in a way that separate plots received either foliar or soil (drip/drench) treatment. Decline trials indicate the decrease of oxathiapiprolin with longer PHI intervals; metabolites were not detected.
Oxathiapiprolin in cucumbers accounted for < 0.01–0.012 mg/kg from soil treatment and for < 0.01–0.09 mg/kg following foliar treatment. Oxathiapiprolin in courgettes accounted for 0.01–0.12 mg/kg from foliar treatment and for < 0.01–0.026 mg/kg from soil treatment.
Residue data from the foliar use are more critical and were used to derive an MRL proposal of 0.15 mg/kg for cucumbers and 0.2 mg/kg for courgettes. To be in line with the tolerance of 0.2 mg/kg set for cucurbits with edible peel in the USA and Canada, the applicant and the EMS proposes to extrapolate the residue data on courgettes to cucumbers and gherkins. Such proposal is supported by EFSA.
2.1.8.5. Cucurbits with inedible peel/ melons, watermelons, pumpkins
In support of authorised outdoor foliar and soil use in Canada and the USA, the applicant submitted in total 12 GAP‐compliant residue trials on melons which were performed in the USA and Canada in 2011. Trials were designed in a way that separate plots received either foliar treatment or soil (drip/drench) treatment. Decline trials indicate decrease of oxathiapiprolin and no formation of metabolites with PHI intervals above 7 days.
Parent oxathiapiprolin accounted for 0.014‐0.12 mg/kg from foliar use and for < 0.01‐0.034 mg/kg from soil treatment. The foliar use results in a more critical residue situation and was therefore used to derive an MRL proposal of 0.2 mg/kg in melons, which can be extrapolated to watermelons and pumpkins.
In three trials (foliar treatment), the pulp of melon was analysed separately; no quantifiable residues were measured in the edible part of the crop.
In support of the authorised indoor foliar use in Canada and the USA, no residue trials were submitted.
2.1.8.6. Flowering brassica (broccoli, cauliflower)
In support of the authorised outdoor foliar use in Canada and the USA, the applicant submitted 6 GAP‐compliant residue trials on cauliflower and 4 GAP‐compliant residue trials on broccoli. Trials were performed in the USA and Canada in 2011. One trial on broccoli, designed as decline trial, indicates that with longer PHI intervals oxathiapiprolin residues decrease, whereas the levels of metabolite IN‐WR791, although below the limit of detection (LOD), constantly increase, reaching a maximum of 0.01 mg/kg in broccoli at 29 DAT. Other metabolites were not detected at levels above the LOQ.
In three trials (two broccoli and one cauliflower), a washed crop was analysed for residues. The indicative processing factors were in the range of 0.1–1.1; thus, a reliable conclusion cannot be drawn on the effect of washing on the magnitude of residues.
The residue data on broccoli and cauliflower are similar and were therefore combined to derive an MRL proposal of 1.5 mg/kg in support of the authorised foliar use in flowering brassica.
2.1.8.7. Head brassica/head cabbage, Brussels sprouts
In support of the authorised outdoor foliar use in Canada and the USA, the applicant submitted 10 GAP‐compliant residue trials on head cabbage. Trials were performed in the USA and Canada in 2011. One trial on head cabbage, designed as decline trial, indicates that with longer PHI intervals oxathiapiprolin residues decrease, whereas the levels of metabolite IN‐WR791 although below the LOD constantly increase, but did not exceed the level of 0.01 mg/kg. No other metabolites were detected at levels above the LOQ.
In three trials, the effect of washing on the residues level in head cabbage was investigated. The derived processing factors ranged from < 0.06 to 0.8 and give an indication that washing reduces residues.
The residue data on head cabbage are sufficient to derive an MRL proposal of 0.7 mg/kg. The EMS and the applicant proposed to extrapolate residue data to Brussels sprouts, but such an extrapolation is not supported according to EU guidance documents (European Commission, 2017). No MRL proposal can therefore be derived to support the authorised use on Brussels sprouts.
2.1.8.8. Lettuces and salad plants (lamb's lettuces, lettuces, escaroles, cresses, land cresses, Roman rocket, red mustards, baby leaf crops)
In support of the authorised outdoor foliar and soil use in Canada and the USA, the applicant submitted in total 22 GAP‐compliant trials on lettuce (leaf and head forming varieties), representing each use. Trials were performed in the USA and Canada in 2011; on the test sites, separate plots received either foliar or soil (drip/drench) treatment. In none of the trials at none of the PHI intervals, metabolites were present above the LOQ of 0.01 mg/kg.
In addition, lettuce samples from six trials were prepared for consumption, i.e., washed and chopped (two trials), and then analysed for residues. Data indicate that, except for one trial where no reduction of residues was observed, in the remaining trials in lettuce the treatment reduced residues on average for 70%. The results of these trials were not used for the calculation of MRL proposals, since the EU MRLs are established for the unprocessed, raw agricultural commodity, and therefore washing of samples is not appropriate.
The foliar use results in higher residues and therefore the residue trials representative for the foliar use were considered for calculating the MRL proposal. Residue data populations on leaf and head forming lettuce were found to be different according to U‐tests and were therefore not combined. The foliar treatment of open leaf lettuces results in a MRL proposal of 5 mg/kg. Extrapolation of residue data from open leaf varieties to other crops listed in the group of lettuces and salad plants is acceptable according to the EU guidance document.
2.1.8.9. Spinaches and similar leaves (spinaches, purslanes, chards/beet leaves)
In support of authorised outdoor foliar and soil use in Canada and the USA, the applicant submitted 10 GAP‐compliant residue trials on spinach, representing each use. Trials were performed in the USA and Canada in 2011 and were designed in a way that separate plots received either foliar treatment or soil treatment via drip/drench/shank injection. Residue decline is observed with longer PHI intervals.
In three trials, additional plot received two early soil treatments either at 30–40 or 62–69 days before harvest. In these trials, residues of parent were either not detected or accounted at very low levels; in trial with last treatment at 34‐day PHI only metabolite IN‐E8S72 was present at 0.026 mg/kg.
In addition, spinach samples from three trials were washed in order to estimate the effect of washing on the magnitude of residues. Data indicate that on average the washing reduced residues for 20%.
The foliar use on spinaches results in higher residues and was therefore used to derive an MRL proposal of 15 mg/kg.
The applicant and the EMS proposed to extrapolate the residue data on spinaches to the whole group of spinaches and similar leaves, which, according to EU guidance document (European Commission, 2017) is acceptable.
2.1.8.10. Peas with pods
In support of the authorised outdoor foliar use in Canada and the USA, the applicant submitted six GAP‐compliant residue trials on peas with pods which were performed in Canada and the USA in 2011. Six residue trials provide information on residues in green pods. The residue data are sufficient to derive an MRL proposal of 1 mg/kg for peas (with pods).
2.1.8.11. Peas without pods
For peas (without pods), an insufficient number of residue trials (six) was provided; since peas (without pod) are considered major crop according to EU guidance document, at least eight GAP‐compliant residue trials are required (European Commission, 2017).
2.1.8.12. Sunflower seeds
In support of the intended seed treatment of sunflower in NEU and SEU, the applicant submitted in total 10 residue trials (5 NEU and 5 SEU trails) on sunflower. Trials were performed in 2013 in France, Spain, Italy, Hungary, Slovakia, Austria and the Czech Republic. Trials were compliant with the intended GAP in terms of amount of the active substance per seed. Sunflower seeds were sampled for analysis 104–282 days after the seed treatment. Residues of all compounds in all samples were below the LOQ.
The number of trials is sufficient to derive a MRL proposal of 0.01 mg/kg (at the LOQ), which confirms the existing EU MRL.
2.1.8.13. Ginseng
In support of the authorised outdoor foliar use in Canada and the USA, the applicant submitted four field trials on ginseng which were performed in USA and Canada in 2011. One trial was designed as decline trial; in two trials a second plot was treated at an exaggerated application rate (2N max seasonal application rate). Dried roots of ginseng were analysed for the parent compound and its metabolites. In the trials performed at the application rate compliant with the GAP, only oxathiapiprolin was above the LOQ. The residue data are sufficient to derive an MRL proposal of 0.15 mg/kg.
2.1.8.14. Hops
In support of the intended NEU use, the applicant submitted five GAP‐compliant residue trials on hops, which were performed in Germany, the United Kingdom and the Czech Republic in 2012 and 2013. Three trials were designed as reverse decline trials with hop samples taken at 0, 3, 6, 13–14 and 20–22 days after the last treatment. Samples were analysed for oxathiapiprolin and its metabolites IN‐E8S72 and IN‐WR791. Metabolite IN‐E8S72 was below the LOQ in all hop samples, whereas metabolite IN‐WR791 in 3 trials accounted for 0.014–0.029 mg/kg. Oxathiapiprolin was in the range of 0.69–3.9 mg/kg. The number of residue trials is sufficient to derive an MRL proposal of 8 mg/kg in hops.
2.1.9. Magnitude of residues in rotational crops
The investigation of residues in rotational crops is of no relevance for the import tolerance requests considered under this assessment.
Among the crops for which the requested MRLs were related to intended European uses, onions, garlic, sunflower and pepper can be grown in a crop rotation. The maximum seasonal application for the EU uses is 75 g/ha (use on peppers).
Rotational crop studies (European field trials) performed with 115–210 g/ha (soil treatment or application on cereals) were assessed in the framework of the EU pesticides peer review (EFSA, 2016).
In the framework of the current assessment, the EMS reported a wide range of rotational crop field trials performed in the USA and Canada in 2011/2012 where bare soil was treated with 272–560 g oxathiapiprolin/ha and rotational crops were planted at three plant‐back intervals (PBIs) (Ireland, 2017a). It is noted that North American studies were also taken into account in the EFSA conclusion on the potential residue levels in rotational crops. Since the trials were performed with application rates exceeding the EU representative uses, the peer review decided to scale down the residues observed in rotational crops to the maximum European seasonal application rate of the representative use (90 g/ha). Overall, it was concluded that residues of oxathiapiprolin, IN‐WR791, IN‐E8S72 and IN‐SXS67 are not expected in significant levels in rotational crops (for details see Appendix B.1.2.2.) (EFSA, 2016).
Considering the highest residue levels observed in crops from all available rotational crop field studies (EU and non‐EU) (see Appendix B.1.2.2), which were performed under varying conditions at application rates ranging from 115 to 560 g/ha, it can be concluded that for the PBI of 30 days, residues of oxathiapiprolin will be below 0.01 mg/kg in food commodities and below 0.05 mg/kg in feed commodities grown in a crop rotation. Residues of metabolites IN‐E8S72 and IN‐SXS67 (expressed as IN‐E8S72) might be present in cereal grain and pulses (0.011 mg/kg), immature leafy vegetables (0.19 mg/kg), legumes with/without pods (0.03–0.05 mg/kg), oilseed (0.09 mg/kg) and fruits (0.022 mg/kg). Residues of these metabolites in feed commodities could occur in forage, fodder and hay of cereals (0.20–0.75 mg/kg), forage of legumes/pulses (0.077 mg/kg), fodder of pulses (0.29 mg/kg) and foliage of root crops (0.03 mg/kg). Metabolite IN‐WR791 was present up to 0.012 mg/kg only in leafy vegetables. Based on the available data, only metabolites IN‐E8S72 and IN‐SXS67 may be expected to occur at levels above 0.05 mg/kg in feed commodities.
In order to avoid residues of oxathiapiprolin metabolites in crops that have relatively short vegetation period and are rotated within short PBIs, Member States granting authorisations of oxathiapiprolin should consider to apply risk mitigation measures.
2.1.10. Magnitude of residues in processed commodities
Processing studies with grapes, tomatoes and potatoes have been submitted for the EU pesticides peer review where various processing factors were derived (EFSA, 2016). In the framework of the current application, the applicant submitted processing study for hops where the transfer of residues of oxathiapiprolin and its metabolites IN‐E8S72 and IN‐WR791 into beer was investigated. In three trials, hops were treated according to the intended use pattern and dried cones were used in the beer production. Residues of oxathiapiprolin were present in all hops samples and ranged from 0.4 to 6 mg/kg; metabolite IN‐WR791 accounted for < 0.01–0.02 mg/kg. Metabolite IN‐E8S71 was not detected. In beer, none of the compounds was quantified (< 0.01 mg/kg).
2.1.11. Proposed MRLs
The available data are considered sufficient to derive MRL proposals as well as risk assessment values for all intended EU uses as well as for all authorised uses in the USA and Canada, except in Brussels sprouts and peas (without pods) (see Appendix B.4). In Appendix B.3, EFSA assessed whether residues on these crops resulting from the intended uses and the uses authorised in China, the United States and Canada are likely to pose a consumer health risk.
3. Residues in livestock
The assessment of oxathiapiprolin residues in livestock is not relevant for the import tolerance request. Considering the intended EU uses, only sunflower seeds (meal) is used as a feed item. Thus, it was necessary to update the dietary burden calculation for livestock and to estimate whether the intended use of oxathiapiprolin on sunflower would have an impact on the residues expected in food of animal origin, triggering a revision of the existing EU MRLs for animal products.
The livestock dietary burden was calculated according to the OECD methodology (OECD, 2013), including sunflower seeds/meal and potatoes, the only crop which can be used for feed purpose for which EU MRLs have been assessed previously. The input values for the exposure calculations for livestock are presented in Appendix D.1.
The results of the dietary burden calculation are presented in Appendix B.2; as the calculated dietary burdens for all livestock species were below the trigger value of 0.004 mg/kg bw, there was no need to further investigate the nature and magnitude of oxathiapiprolin residues in livestock.
4. Consumer risk assessment
EFSA performed a dietary risk assessment using revision 2 of the EFSA PRIMo (EFSA, 2007). This exposure assessment model contains food consumption data for different sub‐groups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (FAO, 2016a).
The toxicological reference value for oxathiapiprolin used in the risk assessment (ADI value of 0.14 mg/kg bw per day) was derived in the framework of the EU pesticides peer review (EFSA, 2016). The setting of the ARfD for oxathiapiprolin was considered not necessary.
The peer review also derived the ADI value of 1.15 mg/kg bw per day for the metabolites IN‐E8S72 and IN‐SXS67. In the framework of the current assessment, it was concluded that toxicological reference values derived for IN‐E8S72 could also be applied to IN‐WR791. However, since these metabolites were not included in the residue definition for risk assessment, a risk assessment was not performed for these metabolites.
The long‐term exposure assessment for parent oxathiapiprolin was performed taking into account the STMR values derived for the commodities assessed in this application. For the remaining commodities covered by the EU MRL legislation, the existing EU MRLs and, if available, the STMR values derived in the EU pesticides peer review were selected as input values (EFSA, 2016). The complete list of input values is presented in Appendix D.2.
The estimated long‐term dietary intake accounted for a maximum of 3% of the ADI (FR toddler diet). The contribution of residues expected in the commodities assessed in this application to the overall long‐term exposure is low (maximum 1.7% of ADI for spinaches; 0.5% for lettuce, 0.3% for wine grapes and 0.3% for leeks).
EFSA concluded that the long‐term intake of residues of oxathiapiprolin resulting from the existing and the intended uses is unlikely to present a risk to consumer health.
For further details on the exposure calculations, including a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
5. Conclusion and Recommendations
The data submitted in support of this MRL application were found to be sufficient to derive MRL proposals as well as risk assessment values for all intended EU uses as well as for all uses authorised in China, the United States and Canada, except for the authorised use on Brussels sprouts and peas (without pods), for which residue data were not available or were insufficient.
Based on the risk assessment results, EFSA concluded that the long‐term intake of residues of oxathiapiprolin resulting from the existing and the intended uses is unlikely to present a risk to consumer health.
The MRL recommendations are summarised in Appendix B.4.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CCPR
Codex Committee on Pesticide Residues
- CF
conversion factor for enforcement to risk assessment residue definition
- cGAP
critical GAP
- CXL
Codex maximum residue limit
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DT90
period required for 90% dissipation (define method of estimation)
- EMS
evaluating Member State
- eq.
residue expressed as a.s. equivalent
- FAO
Food and Agriculture Organization of the United Nations
- FS
Flowable concentrate for seed treatment
- GAP
Good Agricultural Practice
- GS
growth stage
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint FAO/WHO Meeting on Pesticide Residues
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- MW
molecular weight
- NEU
northern Europe
- OD
Oil dispersion
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SEU
southern Europe
- SL
soluble concentrate
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TRR
total radioactive residue
- WHO
World Health Organization
Appendix A – Summary of authorised GAP in exporting country triggering the amendment of existing EU MRLs
1.
| Crop and/or situation | NEU, SEU, MS or country |
F G or Ia |
Pests or Group of pests controlled |
Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb |
Conc. a.s. |
Method kind | Range of growth stages & seasonc |
Number min–max |
Interval between application (min) |
g a.s./hL min–max |
Water L/ha min–max |
Rate (g/ha) | ||||||
| Wine grapes | NEU/SEU | F | Plasmopara viticola | OD | 100 g/L | Hydraulic sprayer with/without air assistance/atomiser/backpack | BBCH 13–89 (spring/summer) | 1–2 | 10 | 4 | 400/1,600 | 60 | 14 |
Revised GAP Evaluation report (Ireland, 2017b): Do not use more than 60 g a.s./ha (0.6 L product/ha) Target rate (1 N rate): 4 g a.s./hL = 40 mL product/hL Basic appl. rate: 0.16 L/ha in max. 400 L water BBCH 61: 0.32 L/ha in max. 800 L water BBCH 71: 0.48 L/ha in max. 1200 L water BBCH > 75: 0.6 L/ha in max. 1600 L water water volumes are theoretical volumes for rate/ha calculation |
|
Table grapes Wine grapes |
China | F | Downy mildew | OD | 100 g/L | Hydraulic sprayer with/without air assistance/atomiser/backpack | Before disease infection | 2 | 10 | 4–5 | 750 |
a) 37.5 b) 75 |
21 |
Evaluation report (Ireland, 2017b): Use rate for grapes is specified as 33.3–50 mg/kg which indicates a max application rate of 50 mg a.s./kg application solution Assuming an application solution density of 1 kg/L, the application rate is 50 mg a.s./L × (1 g/1,000 mg) × (100 L/1 hL) = 5 g a.s./hL Application volume is not specified on the label but in practice is ~ 750 L/ha leading to an application rate of 37.5 g a.s./h |
| Flowering brassica: Broccoli, cauliflower | CAN/USA | F | Peronospora parasitica | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 5 | – | Min 2 (aerial)‐ min 10 (ground) | 8.8–35 | 0 | Maximum 140 g/ha year |
|
Head brassica: head cabbage, Brussels sprouts |
CAN/USA | F | Peronospora parasitica | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 5 | – | Min 2 (aerial)‐ min 10 (ground) | 8.8–35 | 0 | Maximum 140 g/ha year |
|
Bulb vegetables: onions, garlic, shallots, spring onions Leek |
CAN/USA | F | Peronospora destructor | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 5 | – | Min 2 (aerial)‐min 10 (ground) | 8.8–35 | 0 | Maximum 140 g/ha year |
| Onions, garlic | NEU | F | Peronospora destructor | OD | 100 g/L | Hydraulic sprayer | BBCH 13–PHI (spring/summer) | 1–3 | 7 | 2.5–10 | 200–800 | 20 | 7 | |
| SEU | 2–10 | 200–1,000 | ||||||||||||
|
Cucurbit vegetables with edible peel: cucumbers, courgettes, gherkins |
CAN/USA | F/G | Pseudoperonospora cubensis, Phytophthora capsici | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 3 (5 for P. capsici) | – | Min 2 (aerial)–min 10 (ground) | 4.4–35 | 0 | Max 4 applications by any method |
| CAN/USA | F | Phytophthora capsici | OD/SC | 100 g/L 200 g/L | Dip, transplant water, surface band or directed, in‐furrow | Full season | 1–4 | 7 | – | Min volume needed to move product to target zone | 35–280 | 0 | Maximum 560 g/ha year. Max 4 applications by any method | |
|
Cucurbit vegetables with inedible peel: Melons, Pumpkins, Water‐melons |
CAN/USA | F/G | Pseudoperonospora cubensis, Phytophthora capsici | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 3 (5 for P. capsici) | – | Min 2 (aerial)–min 10 (ground) | 4.4–35 | 0 | Max 4 applications by any method |
| CAN/USA | F | Phytophthora capsici |
OD/ SC |
100 g/L 200 g/L | Dip, transplant water, surface band or directed, in‐furrow | Full season | 1–4 | 7 | – | Min volume needed to move product to target zone | 35–280 | 0 | Maximum 560 g/ha year. Max 4 applications by any method | |
| Solanaceae: tomatoes, peppers, aubergines, okra | CAN/USA | F/G | Pseudoperonospora cubensis, Phytophthora capsici | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 5 | – | Min 2 (aerial)‐ min 10 (ground) | 4.4–35 | 0 | Max 4 applications by any method |
| CAN/USA | F | Phytophthora capsici | OD/SC | 100 g/L 200 g/L | Dip, transplant water, surface band or directed, in‐furrow | Full season | 1–4 | 7 | – | Min volume needed to move product to target zone | 35–280 | 0 | Maximum 560 g/ha year. Max 4 applications by any method | |
| Peppers | EU | G | Phytophthora capsici | OD | 100 g/L | Hydraulic sprayer ±air assistance, atomiser | BBCH 15‐89 | 1–3 | 7 | 2.5–5 | 500–1,000 | 25 | 3 | |
| Ginseng | CAN/USA | F | Phytophthora cactorum | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 14 | – | Min 2 (aerial)‐ min 10 (ground) | 35–280 | 14 | Maximum 560 g/ha year |
|
Lettuces and salad plants: lamb's lettuce, lettuces, escaroles, cress and other sprouts and shoots, land cress, rucola, red mustards, baby leaf crops |
USA | F | Bremia lactucae, Peronospora farinosa | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 3 | – | Min 2 (aerial)‐ min 10 (ground) | 4.4–35 | 0 | Max 4 applications by any method |
| CAN/USA | F | Bremia lactucae | OD/SC | 100 g/L 200 g/L | Dip, transplant water, surface band or directed, in‐furrow | Full season | 1–4 | 7 | – | Min volume needed to move product to target zone | 70–280 | 0 | Maximum 560 g/ha year. Max 4 applications by any method | |
|
Spinaches and similar leaves: spinaches, purslanes, chards/beet leaves |
USA | F | Bremia lactucae, Peronospora farinosa | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 3 | – | Min 2 (aerial)‐ min 10 (ground) | 4.4–35 | 0 | Max 4 applications by any method |
| CAN/USA | F | Bremia lactucae | OD/SC | 100 g/L 200 g/L | Dip, transplant water, surface band or directed, in‐furrow | Full season | 1–4 | 7 | – | Min volume needed to move product to target zone | 70–280 | 0 | Maximum 560 g/ha year. Max 4 applications by any method | |
| Peas with pods | CAN/USA | F | Peronospora viciae, Phytophthora phaseoli | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 5 | – | Min 2 (aerial)‐ min 10 (ground) | 18–35 | 0 | Maximum 140 g/ha year |
| Peas without pods | CAN/USA | F | Peronospora viciae, Phytophthora phaseoli | OD | 100 g/L | Foliar broadcast | Full season | 1–4 | 5 | – | Min 2 (aerial)‐ min 10 (ground) | 18–35 | 0 | Maximum 140 g/ha year |
| Sunflower | NEU/SEU | F | Plasmopora halstedii | FS | 200 g/L | Seed treatment | BBCH 00 | 1 | – | – | – | 1.69 | n.a. | Application rate is based on seeding rate of 90,000 seeds/ha and 18.75 μg/seed |
| Hops | NEU | F | Peronospora humuli | OD | 100 g/L | Hydraulic sprayer with air assistance | BBCH 37–85 | 1–2 | 10 | 7.14 | 70–2,800 | 50 | 14 |
BBCH 37 = 25 g/ha (0.25 L product/ha) BBCH 55 = 35 g (0.35 L product/ha) BBCH > 55 = 50 g (0.5 L product/ha) |
GAP: Good Agricultural Practice; MRL: maximum residue level; NEU: northern European Union; SEU: southern European Union; MS: Member State; a.s.: active substance; OD: Oil dispersion; SC: suspension concentrate; FS: Flowable concentrate for seed treatment.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT, DATx a, DALA) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | Grapes |
Foliar: 3 × 70 g/ha (BBCH 63‐95; BBCH 73 and 77; 14‐day interval) |
Foliage: 0 DAT1,2,3, 14 DAT2,3, 76 DALA Berries: 14 DAT2,3, 0 DAT3, 76 DALA |
Radiolabelled active substance: pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (Ireland, 2015) | |
| Courgette | Soil: 1 × 600 g/ha (preplanting) | 44 DAT, 79 DAT (maturity) | Radiolabelled active substance: pyrazole‐14C‐ and isoxazoline‐14C‐oxathiapiprolin (Ireland, 2017b) | ||
| Root crops | Potatoes | Foliar: 3 × 70 g/ha (BBCH 53; BBCH 59 and 69; 14‐day interval |
Foliage, tubers: 0 DAT2 (foliage only), 14 DAT1,2,3, 28 DAT3 |
Radiolabelled active substance: pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (Ireland, 2015) | |
| Soil: 1 × 600 g/ha (preplanting) | Foliage, tubers: 37 DAT, 72 DAT (maturity) | Radiolabelled active substance: pyrazole‐14C‐ and isoxazoline‐14C‐oxathiapiprolin (Ireland, 2017b) | |||
| Leafy crops | Lettuce | Foliar: 3 × 70 g/ha (BBCH 15; BBCH 17 and 19; 10‐day interval) | 0 DAT1,2,3, 10 DAT1,2, 0 DAT3, 3, 7, 14 DALA | Radiolabelled active substance: pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (Ireland, 2015) | |
| Soil: 1 × 600 g/ha (preplanting) | 30, 44, 57 DAT | Radiolabelled active substance: pyrazole‐14C‐ and isoxazoline‐14C‐oxathiapiprolin (Ireland, 2017b) | |||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
| Root/tuber crops | Turnip | Soil: 1 × 210 g/ha | 30, 120 and 365 DAT | Radiolabelled active substance: pyrazole‐14C‐, thiazole‐14C‐ and isoxazoline‐14C oxathiapiprolin (Ireland, 2015) | |
| Soil: 1 × 600 g/ha | Radiolabelled active substance: pyrazole‐14C and isoxazoline‐14C oxathiapiprolin (Ireland, 2017a) | ||||
| Leafy crops | Lettuce | Soil: 1 × 210 g/ha | 30, 120 and 365 DAT | Radiolabelled active substance: pyrazole‐14C‐, thiazole‐14C‐ and isoxazoline‐14C oxathiapiprolin. (Ireland, 2015) | |
| Soil: 1 × 600 g/ha | Radiolabelled active substance: pyrazole‐14C and isoxazoline‐14C oxathiapiprolin (Ireland, 2017a) | ||||
| Cereal (small grain) | Wheat | Soil: 1 × 210 g/ha | 30, 120 and 365 DAT | Radiolabelled active substance: pyrazole‐14C‐, thiazole‐14C‐ and isoxazoline‐14C oxathiapiprolin (Ireland, 2015) | |
| Soil: 1 × 600 g/ha | Radiolabelled active substance: pyrazole‐14C and isoxazoline‐14C oxathiapiprolin (Ireland, 2017a) | ||||
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source | ||
| Pasteurisation (20 min, 90°C, pH 4) | Yes | Studies performed with pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (Ireland, 2015) | |||
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | ||||
| Sterilisation (20 min, 120°C, pH 6) | Yes | ||||
| Other processing conditions | – | ||||
DALA: days after last application; BBCH: growth stages of mono‐ and dicotyledonous plants; PBI: plant‐back interval.
DATx: days after x treatment.
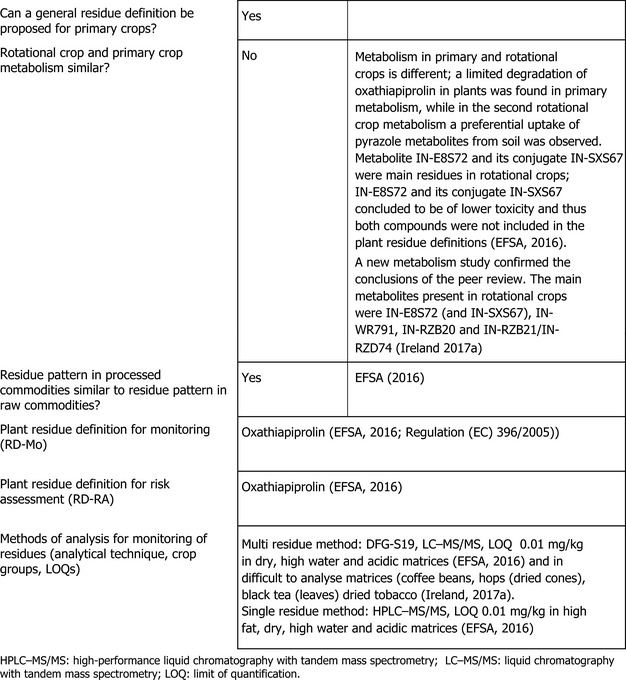
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Tomatoes | −20 | 18 | Months | Oxathiapiprolin, IN‐Q7H09, IN‐RDG40, IN‐E8S72, IN‐RZB20, IN‐RZD74, IN‐SXS67 and IN‐WR791 | EFSA (2016) | |
| High oil content | Soya bean seed | ||||||
| High protein content | Dried bean seed | ||||||
| Dry/High starch | Potatoes, wheat | ||||||
| High acid content | Grapes | ||||||
| Others | Wheat forage | ||||||
| Rape dry pomace | |||||||
| Wheat straw | |||||||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Commodity | Region/Indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) |
|---|---|---|---|---|---|---|
|
Enforcement residue definition: Oxathiapiprolin Risk assessment residue definition: Oxathiapiprolin | ||||||
| Table and wine grapes | China (foliar) | 0.038; 0.053; 0.072; 0.103; 0.133; 0.243; 0.31; 0.331 | Residue trials on grapes compliant with the GAP. Residue trials confirm the existing EU MRL of 0.7 mg/kg | 0.7 | 0.33 | 0.12 |
| Onions, shallots, garlic | NEU/SEU (foliar) | Onions: 16 × < 0.01 | Residue trials on onions compliant with the GAP. Extrapolation to shallots and garlic possible | 0.01* | 0.01 | 0.01 |
| USA/CAN (outdoor foliar) | Onions: 4 × < 0.01; 0.01; 0.011; 0.012; 0.014; 2 × 0.02; 0.026 | Residue trials on onions compliant with the GAP. Extrapolation to shallots and garlic | 0.04 | 0.03 | 0.01 | |
| Spring onions leeks | USA/CAN (outdoor foliar) | 0.57; 0.40; 0.85; 0.45; 0.63 | Residue trials on spring onions compliant with the GAP. Extrapolation to leeks | 2.0 | 0.85 | 0.57 |
| Tomatoes, aubergines | USA/CAN (indoor/foliar) | 2 × < 0.01; 0.031; 0.079g | Residue trials on tomatoes (including cherry tomatoes) compliant with the GAP. Insufficient number of trials provided to derive a MRL proposal | – | – | – |
| USA/CAN (outdoor/foliar) |
Cherry tomatoes: 0.022; 0.032; 0.035; 0.047g; 0.078; 0.10; 0.12e; 0.145e; 0.31 Standard size tomatoes: < 0.01; 0.023; 0.024; 2 × 0.032; 0.034; 0.039; 0.042; 0.048; 0.075 |
Residue trials on tomatoes compliant with the GAP. Extrapolation to aubergines The outdoor foliar application results in a more critical residue situations, based on which the MRL proposal is derived |
0.4 | 0.31 | 0.04 | |
|
USA/CAN (outdoor soil) |
Cherry tomatoes: 7 × < 0.01; 0.028; 0.24 Standard size tomatoes: 10 × < 0.01 |
0.3 | 0.24 | 0.01 | ||
| Peppers, okra | EU (indoor) |
Chilli peppers: 0.088f; 0.091; 0.052; 0.04; 0.083 |
Residue trials on peppers compliant with the GAP | 0.20 | 0.09 | 0.04 |
| USA/CAN (outdoor foliar) | 2 × 0.016; 0.02; 0.027; 0.029; 0.029e; 0.034; 0.037; 0.044e; 0.048; 0.05; 0.055; 0.059; 0.084; 0.12; 0.125 | Residue trials on peppers compliant with the GAP. Extrapolation to okra (lady's fingers) | 0.20 | 0.13 | 0.04 | |
| USA/CAN (outdoor soil) | 15 × < 0.01; 0.017 | Residue trials on peppers compliant with the GAP | 0.02 | 0.01 | 0.017 | |
| USA/CAN (indoor foliar) | 0.061e; 0.12 | Insufficient number of residue trials | – | – | – | |
| Cucumbers, courgettes, gherkins | USA/CAN (indoor/foliar) | 0.044; 0.041; 0.039; 0.022 | Residue trials on cucumbers compliant with the GAP. Insufficient number of residue trials submitted | – | – | – |
| USA/CAN (outdoor/foliar) | Cucumbers: 3 × < 0.01; 0.012; 0.013; 0.023; 0.029; 2 × 0.03; 0.041; 0.067; 0.09 | Residue trials on cucumbers compliant with the GAP | 0.15 | 0.09 | 0.03 | |
| Courgettes: 0.01; 0.02; 0.023; 0.03; 0.031; 0.033; 2 × 0.039; 0.083; 0.12 | Residue trials on courgettes compliant with the GAP. Extrapolation to cucumbers and gherkins | 0.2 | 0.12 | 0.03 | ||
| USA/CAN (outdoor soil) | Cucumbers: 10 × < 0.01; 0.012 Courgettes: 8 × < 0.01; 0.017d; 0.026 | Residue trials on cucumbers and courgettes compliant with the GAP | 0.03 | 0.03 | 0.01 | |
| Melons, watermelons, pumpkins | USA/CAN (outdoor/foliar) |
0.014; 0.015; 0.033; 0.033e; 0.036; 0.042d; 0.052; 0.059d; 0.068; 0.085d; 0.10; 0.12 Pulp: 3 × < 0.01 |
Residue trials on melons compliant with the GAP. Extrapolation to pumpkins and watermelon | 0.2 | 0.12 | 0.05 |
| USA/CAN (outdoor soil) | 8 × < 0.01; 0.019; 0.034d; 0.017e; 0.015d | Residue trials on melons compliant with the GAP | 0.05 | 0.03 | 0.01 | |
|
USA/CAN (indoor/foliar) |
No residue trials submitted | – | – | – | – | |
| Broccoli, cauliflower |
USA/CAN (outdoor/foliar) |
Broccoli: 0.07; 0.17; 0.23; 0.81; Cauliflower: 0.077; 0.08; 0.082; 0.091; 0.14; 0.22e |
Residue trials on cauliflower and broccoli compliant with the GAP. Residue data populations similar and therefore combined to derive MRL proposal | 1.5 | 0.81 | 0.12 |
| Head cabbage | USA/CAN (outdoor/foliar) | Head cabbage: 0.04; 2 × 0.06; 0.12; 0.12e; 0.16; 0.22; 0.29; 0.32; 0.42 | Residue trials on head cabbage compliant with the GAP | 0.7 | 0.42 | 0.14 |
| Brussels sprouts | USA/CAN (outdoor/foliar) | No trials | Applicant proposed extrapolation from head cabbage; this extrapolation is not in line with the EU extrapolation practices | – | – | – |
| Lettuce, lamb's lettuce, escaroles, cresses, land cress, rucola, red mustards, baby leaf crops | USA/CAN (outdoor/foliar) |
Open leaf lettuce: 1.2; 0.80e; 3.0; 1.9; 2.0; 1.30; 1.9; 1.9; 0.54; 0.81; 0.55d; 1.80 Head forming lettuce: 1.40; 0.82; 0.30; 0.83; 0.70; 0.28; 0.23; 0.57; 0.38; 0.50 |
Residue trials on head forming and open leaf lettuce varieties compliant with the GAP. Residue data on open leaf lettuce basis for the MRL proposal, extrapolated to the whole group of lettuces and salad plants (code 0251000) | 5 | 3.0 | 1.3 |
| USA/CAN (outdoor/drip irrigation) |
Open leaf lettuce: 7 × < 0.01; 0.014; 0.016; 0.073; 0.091; 0.37 Head forming lettuce: 8 × < 0.01; 0.37; 0.43 |
Residue trials on head forming and open leaf lettuce varieties compliant with the GAP | 0.6 | 0.43 | 0.01 | |
| Spinaches | USA/CAN (outdoor/foliar) | 1.4; 1.6; 2.2; 2.3; 3.2; 3.5; 4.0; 5.7; 6.4; 6.5 | Residue trials on spinach compliant with the GAP. Extrapolation to the whole group of spinaches and similar leaves | 15 | 6.5 | 3.35 |
|
USA/CAN (outdoor/drip irrigation) |
3 × < 0.01; 0.013d; 0.11; 0.12; 1.6; 1.8d; 1.95d; 2.2d | Residue trials on spinach compliant with the GAP | 5 | 2.20 | 0.12 | |
| Peas without pods | USA/CAN (oudoor/foliar) | 2 × < 0.01; 2 × 0.01; 0.025; 0.026 | Residue trials on peas compliant with the GAP. Insufficient number of trials to derive a MRL proposal | – | – | – |
| Peas with pods | USA/CAN (oudoor/foliar) | 0.2; 2 × 0.3; 0.26; 0.28; 0.55 | Residue trials on peas compliant with the GAP | 1.0 | 0.55 | 0.29 |
| Sunflower seeds | NEU | 5 × < 0.01 | Residue trials on sunflower compliant with the GAP. Reduced number of trials sufficient as residues in all samples below the LOQ. Residue trials confirm the existing EU MRL of 0.01 mg/kg (LOQ) | 0.01 * | 0.01 | 0.01 |
| SEU | 5 × < 0.01 | |||||
| Ginseng | CAN/USA (soil) | 0.043; 0.044; 0.049; 0.061h | Residue trials on ginseng compliant with the GAP | 0.15 | 0.06 | 0.05 |
| Hops | NEU (foliar) | 0.69; 1.3; 1.6; 3.1; 3.9 | Residue trials on hops compliant with the GAP | 8.0 | 3.9 | 1.6 |
MRL: maximum residue level; GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; LOQ: limit of quantification.
Values in bold are the MRL proposals derived for the cGAP.
Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
Higher residues at a longer PHI interval of 2–3 days.
Higher residues at a longer PHI interval of 4–5 days.
Higher residues at a longer PHI interval of 7 days.
Higher residues at a longer PHI interval of 10 days.
Higher residues at a longer PHI interval of 20 days.
B.1.2.2. Residues in rotational crops

B.1.2.3. Processing factors
| Processed commodity | Number of valid studiesa | Processing factor (PF) | Comment/Source | |
|---|---|---|---|---|
| Individual values | Median PF | |||
| Hops, beer | 3 | < 0.01; < 0.01; < 0.03 | 0.01 | Ireland (2017b) |
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
B.2. Residues in livestock
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical subgroupa | Most critical commodityb | Trigger exceeded (Y/N) | |||
|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||||
| Median | Maximum | Median | Maximum | ||||
| Cattle (all) | 0.002 | 0.002 | 0.05(c) | 0.05(c) | Dairy cattle | Potato process waste | No |
| Cattle (dairy only) | 0.002 | 0.002 | 0.04 | 0.04 | Dairy cattle | Potato process waste | No |
| Sheep (all) | 0.002 | 0.002 | 0.05 | 0.05 | Ram/Ewe | Potato process waste | No |
| Sheep (ewe only) | 0.002 | 0.002 | 0.05 | 0.05 | Ram/Ewe | Potato process waste | No |
| Swine (all) | 0.001 | 0.001 | 0.04 | 0.04 | Swine (breeding) | Potato process waste | No |
| Poultry (all) | 0.001 | 0.001 | 0.01 | 0.01 | Turkey | Potato culls | No |
| Poultry (layer only) | 0.000 | 0.000 | 0.01 | 0.01 | Poultry layer | Potato culls | No |
| Fish | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
bw: body weight; DM: dry matter.
When one group of livestock includes several subgroups (e.g. poultry ‘all’ including broiler, layer and turkey), the result of the most critical subgroup is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
B.3. Consumer risk assessment
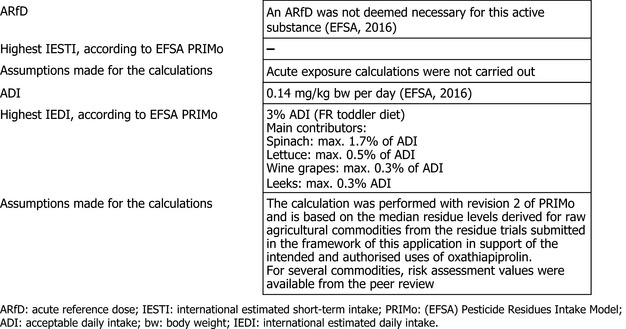
B.4. Recommended MRLs
| Codea | Commodity |
Existing EU MRL (mg/kg) |
Proposed EU MRL (mg/kg) |
Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Oxathiapiprolin | ||||
|
0151010 0151020 |
Table and wine grapes | 0.7 | No change | The submitted data are sufficient to derive an MRL proposal for the authorised Chinese GAP which confirms the existing EU MRL. Risk to consumers unlikely. The MRL applicable in China is 1 mg/kg |
| 0220010 | Onions | 0.01* | 0.04 |
The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely The MRLs applicable in the USA and Canada are 0.04 mg/kg for onions, garlic, shallots; 2 mg/kg for spring onions and 0.5 mg/kg for tomatoes |
| 0220020 | Garlic | |||
| 0220030 | Shallots | |||
| 0220040 | Spring onions | 0.01* | 2.0 | |
| 0231010 | Tomatoes | 0.2 | 0.4 | |
| 0231020 | Sweet peppers/bell peppers | 0.01* | 0.2 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada and the intended EU indoor GAP. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 0.5 mg/kg |
| 0231030 | Aubergines | 0.2 | 0.4 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 0.5 mg/kg |
| 0231040 | Okra/lady's fingers | 0.01* | 0.2 | |
|
0232010 0232020 |
Cucumbers Gherkins |
0.1 | 0.2 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 0.2 mg/kg |
| 0232030 | Courgettes | 0.1 | 0.15 or 0.2 further risk management considerations needed | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada of 0.15 mg/kg. As alternative option, the setting of a group MRL of 0.2 mg/kg can be considered. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 0.2 mg/kg |
| 0233010 | Melons | 0.15 | 0.2 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 0.2 mg/kg |
| 0233020 | Pumpkins | 0.01* | ||
| 0233030 | Watermelons | 0.01* | ||
|
0241010 0241020 |
Broccoli Cauliflower |
0.01* | 1.5 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 1.5 mg/kg |
| 0242010 | Brussels sprouts | 0.01* | No proposal | The submitted data are not sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada |
| 0242020 | Head cabbage | 0.01* | 0.7 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 1.5 mg/kg |
|
0251010 0251030 0251010 0251010 0251010 0251010 0251010 |
Lamb's lettuce Escaroles Cresses Land cresses Rucola Red mustards Baby leaf crops |
0.01* | 5 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 15 mg/kg |
| 0251020 | Lettuces | 0.3 | 5 | |
|
0252010 0252020 0252030 |
Spinaches Purslanes Chards/beet leaves |
0.01* | 15 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRLs applicable in the USA and Canada are 15 mg/kg |
| 0260030 | Peas (with pods) | 0.01* | 1.0 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 1 mg/kg |
| 0260040 | Peas (without pods) | 0.01* | No proposal | The submitted data are not sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada |
| 0270060 | Leeks | 0.01* | 2 | The submitted data on spring onions are sufficient to derive by extrapolation an MRL proposal for leek for the GAP authorised in the USA and Canada. Risk to consumers unlikely.The MRL applicable in the USA and Canada is 2 mg/kg |
| 0401050 | Sunflower seeds | 0.01* | No change | The submitted data confirm the existing EU MRL for the intended NEU/SEU use. Risk to consumers unlikely |
| 0633020 | Ginseng | 0.05* | 0.15 | The submitted data are sufficient to derive an MRL proposal for the GAP authorised in the USA and Canada. Risk to consumers unlikely. The MRL applicable in the USA and Canada is 0.15 mg/kg |
| 0700000 | Hops | 0.05* | 8.0 | The submitted data are sufficient to derive an MRL proposal for the intended NEU use. Risk to consumers unlikely |
MRL: maximum residue level; GAP: Good Agricultural Practice; NEU: northern Europe; SEU: southern Europe.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: oxathiapiprolin | ||||
| Sunflower seeds meal | 0.01 | STMRa | 0.01 | STMRa |
| Potato culls | 0.01 | STMR (EFSA, 2016) | 0.01 | STMR (EFSA, 2016) |
| Potato process waste | 0.01 | STMRb | 0.01 | STMRb |
| Potato dried pulp | 0.01 | STMRb | 0.01 | STMRb |
STMR: supervised trials median residue.
For sunflower seeds meal no default processing factor was applied because oxathiapiprolin is applied early in the growing season and residues are expected to be below the LOQ. Concentration of residues in these commodities is therefore not expected.
For potato process waste and potato dried pulp the default processing factors were not applied as residues in RAC were below the LOQ and residue concentration in processed fractions are not expected.
D.2. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Table and wine grapes | 0.12 | STMR | Not performed, not considered necessary, since no ARfD was established | |
| Onions, garlic, shallots | 0.01 | STMR | ||
| Spring onions | 0.57 | STMR | ||
| Tomatoes, aubergines, peppers, okra | 0.04 | STMR | ||
| Cucumbers, gherkins, courgettes | 0.03 | STMR | ||
| Melons, pumpkins, watermelons | 0.05 | STMR | ||
| Cauliflower, broccoli | 0.12 | STMR | ||
| Head cabbage | 0.14 | STMR | ||
| Lettuces and other salad plants | 1.3 | STMR | ||
| Spinaches and similar leaves | 3.35 | STMR | ||
| Peas with pods | 0.29 | STMR | ||
| Leeks | 0.57 | STMR | ||
| Sunflower seeds | 0.01 | STMR | ||
| Ginseng | 0.05 | STMR | ||
| Hops | 1.6 | STMR | ||
| Potatoes | 0.01 | STMR (EFSA, 2016) | ||
| Wine leaves | 8.80 | STMR (EFSA, 2016) | ||
| Other commodities of plant and animal origin | MRL | Commission Regulation (EU) 2017/1016 | ||
STMR: supervised trials median residue; ARfD: acute reference dose; MRL: maximum residue level.
Appendix E – Summaries of metabolism studies submitted under the current applications
1.
Table E.3.
Nature of residues in rotational crops; summary of characterised and identified %TRR, (in brackets mg/kg) in turnip
| Turnip | Soil 1 × 210 g/ha (EFSA, 2016) | Soil 1 × 600 g/ha (Ireland, 2017a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Labelling position | [pyrazole‐14C]oxathiapiprolinb | [pyrazole‐14C]oxathiapiprolinc | ||||||||||
| Part analysed | Root | Root | Root | Mature foliage | Mature foliage | Mature foliage | Root | Root | Root | Mature foliage | Mature foliage | Mature foliage |
| PBI | 30 | 120 | 365 | 30 | 120 | 365 | 30 | 120 | 365 | 30 | 120 | 365 |
| Total TRRR (mg/kg) | 0.014 | 0.023 | 0.008 a | 0.122 | 0.174 | 0.016 | 0.02 | 0.011 a | 0.016 | 0.086 | 0.031 | 0.043 |
| Oxathiapiprolin | nd | 15.2 (0.003) | na | nd | nd | nd | nd | nd | ||||
| IN‐SXS67 (gluc‐IN‐E8S72) | 9.9 (0.012) | 6 (0.01) | nd | 5.6 (0.001) | 7.2 (0.001) | 8.1 (0.007) | 9.7 (0.003) | 4.7 (0.002) | ||||
| IN‐E8S72 | 18.6 (0.003) | 18.1 (0.004) | 20.2 (0.025) | 47.7 (0.083) | 12.3 (0.002) | 9 (0.002) | 3.8 (0.001) | 18.6 (0.016) | 12.9 (0.004) | 41.9 (0.018) | ||
| IN‐WR791 | 48.8 (0.007) | 9.4 (0.002) | 45 (0.055) | 18.6 (0.032) | 45.5 (0.007) | 4.3 (0.001) | 16.5 (0.003) | 26.7 (0.023) | 19.4 (0.006) | 30.2 (0.013) | ||
| IN‐KJ552 | 9.3 (0.002) | 9.9 (0.002) | 1.2 (0.001) | 2.3 (0.001) | ||||||||
| IN‐RZB20 | 10.3 (0.013) | 6.2 (0.011) | 10.6 (0.002) | 4 (0.001) | 2.4 (< 0.001) | 18.6 (0.016) | 25.8 (0.008) | 4.7 (0.002) | ||||
| IN‐RZB21/IN‐RZD74 | 4.8 (0.006) | 4.6 (0.008) | 32 (0.005) | 13.7 (0.003) | 4.4 (0.001) | 15.1 (0.013) | 9.7 (0.003) | 16.3 (0.007) | ||||
| Total unidentified metabolites (% TRR (mg/kg | 13.6 (< 0.001) | 12.1 (0.002) | 7.5 (0.009) | 0.3 (0.001) | 5.4 (0.001) | |||||||
| Unextracted | 12 (0.002) | 26 (0.006) | 2.6 (0.003) | 4.4 (0.008) | 6.9 (0.001) | |||||||
PBI: plant‐back interval; TRR: total radioactive residues; nd: not identified..
not characterised further.
The TRR from [isoxazoline‐14C]‐ and [thizole‐14C]‐oxathiapiprolin treatments were ≤ 0.01 mg eq./kg and thus were not analysed further.
The TRR from [isoxazoline‐14C]‐ oxathiapiprolin treatment were ≤ 0.01 mg eq./kg and thus were not analysed further.
> 10% TRR highlighted.
Appendix F – Used compound codes
1.
| Code/trivial name | Chemical name/SMILES notation8 | Structural formula[Link] |
|---|---|---|
| Oxathiapiprolin |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydroisoxazol‐3‐yl]thiazol‐2‐yl}‐1‐piperidyl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone FC(F)(F)c1cc(C)n(n1)CC(=O)N1CCC(CC1)c1nc(cs1)C=1CC(ON=1)c1c(F)cccc1F IAQLCKZJGNTRDO‐UHFFFAOYSA‐N |
|
| IN‐Q7H09 |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluoro‐4‐hydroxyphenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}piperidin‐1‐yl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone FC(F)(F)c1cc(C)n(n1)CC(=O)N2CCC(CC2)c3nc(cs3)C=4CC(ON=4)c5c(F)cc(O)cc5F XYJWPIOIQYWLNP‐UHFFFAOYSA‐N |
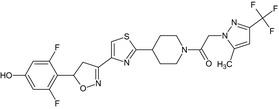
|
| IN‐RAB06 |
1‐[2‐(4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}piperidin‐1‐yl)‐2‐oxoethyl]‐3‐(trifluoromethyl)‐1H‐pyrazole‐5‐carboxylic acid O=C(O)c5cc(nn5CC(=O)N1CCC(CC1)c2nc(cs2)C=3CC(ON=3)c4c(F)cccc4F)C(F)(F)F QALOLIVQRGAZRP‐UHFFFAOYSA‐N |
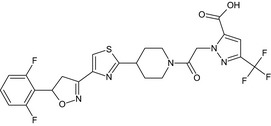
|
| IN‐RDT31 |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}‐4‐hydroxypiperidin‐1‐yl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone FC(F)(F)c1cc(C)n(n1)CC(=O)N2CCC(O)(CC2)c3nc(cs3)C=4CC(ON=4)c5c(F)cccc5F WNMKBALSHJAXGE‐UHFFFAOYSA‐N |
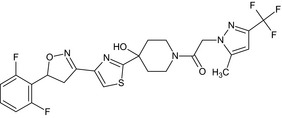
|
| IN‐RDG40 |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluoro‐3‐hydroxyphenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}piperidin‐1‐yl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone FC(F)(F)c1cc(C)n(n1)CC(=O)N2CCC(CC2)c3nc(cs3)C=4CC(ON=4)c5c(F)ccc(O)c5F MCUWVCQCPFWXQQ‐UHFFFAOYSA‐N |
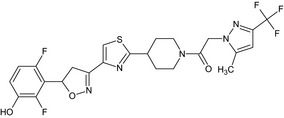
|
| IN‐QPS10 |
4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}piperidine Fc1cccc(F)c1C2CC(=NO2)c3csc(n3)C4CCNCC4 HZZFIEJFXTXVHO‐UHFFFAOYSA‐N |
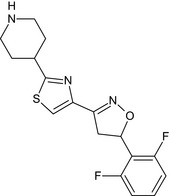
|
| IN‐E8S72 |
3‐(trifluoromethyl)‐1H‐pyrazole‐5‐carboxylic acid FC(F)(F)c1cc(nn1)C(O)=O CIVNBJPTGRMGRS‐UHFFFAOYSA‐N |
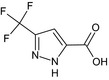
|
| IN‐SXS67 |
1‐β‐d‐glucopyranosyl‐3‐(trifluoromethyl)‐1H‐pyrazole‐5‐carboxylic acid O=C(O)c2cc(nn2[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C(F)(F)F IYVPJWXJEGAHCP‐DDIGBBAMSA‐N |
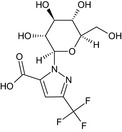
|
| IN‐WR791 |
[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]acetic acid OC(=O)Cn1nc(cc1C)C(F)(F)F RBHQAIFXLJIFFM‐UHFFFAOYSA‐N |
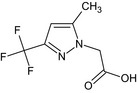
|
| IN‐RZB20 |
[5‐(hydroxymethyl)‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]acetic acid OC(=O)Cn1nc(cc1CO)C(F)(F)F LGHWWTCDTBCQQI‐UHFFFAOYSA‐N |
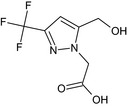
|
| IN‐RZB21 |
5‐(Hydroxymethyl)‐3‐(trifluoromethyl)‐1Hpyrazole‐1‐acetamide O=C(N)Cn1nc(cc1CO)C(F)(F)F LDXIZNIPWOQNPY‐UHFFFAOYSA‐N |
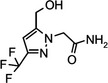
|
| IN‐KJ552 |
5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazole FC(F)(F)c1cc(C)[NH]n1 DLCHCAYDSKIFIN‐UHFFFAOYSA‐N |
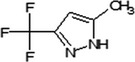
|
| IN‐RZD74 |
[3‐(trifluoromethyl)‐1H‐pyrazol‐5‐yl]methanol FC(F)(F)c1cc(CO)nn1 KUVPCLYQVMRTPU‐UHFFFAOYSA‐N |
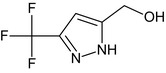
|
| IN‐Q9L80 |
(4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}piperidin‐1‐yl)(oxo)acetic acid O=C(O)C(=O)N1CCC(CC1)c2nc(cs2)C=3CC(ON=3)c4c(F)cccc4F SPPNZGUAGRWQIX‐UHFFFAOYSA‐N |
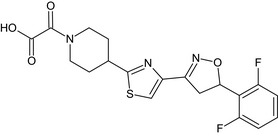
|
SMILES: simplified molecular‐input line‐entry system.
ACD/Name 2015 ACD/Labs 2015 Release (File version N20E41, Build 75170, 19 Dec 2014).
ACD/ChemSketch 2015 ACD/Labs 2015 Release (File version C10H41, Build 75059, 17 Dec 2014).
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2019. Reasoned opinion on the modification of the existing maximum residue levels and setting of import tolerances for oxathiapiprolin in various commodities. EFSA Journal 2019;17(7):5759, 49 pp. 10.2903/j.efsa.2019.5759
Requestor: European Commission
Question numbers: EFSA‐Q‐2017‐00581; EFSA‐Q‐2017‐00582
Approved: 21 June 2019
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) 2017/239 of 10 February 2017 approving the active substance oxathiapiprolin in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. C/2017/0694. OJ L 36, 11.2.2017, p. 39–42.
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.03.2005, p. 1–16.
Commission Regulation (EU) 2017/1016 of 14 June 2017 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for benzovindiflupyr, chlorantraniliprole, deltamethrin, ethofumesate, haloxyfop, Mild Pepino Mosaic Virus isolate VC1, Mild Pepino Mosaic Virus isolate VX1, oxathiapiprolin, penthiopyrad, pyraclostrobin, spirotetramat, sunflower oil, tolclofos‐methyl and trinexapac in or on certain products OJ L 159, 21.6.2017, p. 1–47
In 2017, a number of CXLs for plant and animal commodities have been adopted by the Codex Alimentarius Commission (CAC). The EU made a reservation in the meeting of the CAC, since toxicological information on metabolites were missing. The Codex MRL proposals derived in 2018 will be discussed in 2019 Codex Alimentarius Commission. The EU made a reservation in the CCPR meeting in April 2019, due to the ongoing assessment of the current MRL application, which was expected to provide clarity on the toxicological properties of metabolites identified in primary and rotational crop metabolism studies.
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Two trials were considered not independent and therefore the higher result of the two trials was selected to be used for calculating the MRL proposal.
References
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers’ health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Conclusion on the peer review of the pesticide risk assessment of the active substance oxathiapiprolin. EFSA Journal 2016;14(7):4504, 19 pp. 10.2903/j.efsa.2016.4504 [DOI] [Google Scholar]
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.3, 13 June 2017.
- FAO (Food and Agriculture Organization of the United Nations), 2016a. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 3rd Ed. FAO Plant Production and Protection Paper 225, 298 pp.
- FAO (Food and Agriculture Organization of the United Nations), 2016b. Pesticide residues in food‐2016. Oxathiapiprolin in – Report on the Joint FAO/WHO Meeting on pesticide residues, FAO Plant Production and Protection Paper 229, p.257‐282
- FAO (Food and Agriculture Organization of the United Nations), 2018. Pesticide residues in food 2018. Oxathiapiprolin in – Report on the Joint FAO/WHO Meeting on pesticide residues, FAO Plant Production and Protection Paper 234, p.271‐283
- Ireland , 2015. Draft Assessment Report (DAR) on the active substance oxathiapiprolin prepared by the rapporteur Member State Ireland in the framework of Regulation (EC) No 1107/2009, February 2015. Available online: http://www.efsa.europa.eu
- Ireland , 2016. Revised Draft Assessment Report (DAR) on oxathiapiprolin prepared by the rapporteur Member State Ireland in the framework of Regulation (EC) No 1107/2009, March 2016. Available online: http://www.efsa.europa.eu
- Ireland , 2017a. Evaluation report on the setting of an Import Tolerance for oxathiapiprolin in multiple crop commodities. June 2017. 253 pp., updated on April 2018 and February 2019.
- Ireland , 2017b. Evaluation report on the modification of MRLs for oxathiapiprolin in wine grapes, bulb onions, peppers, sunflowers and hops. June 2017, updated on April 2018 and February 2019, 253 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- OECD (Organisation for Economic Co‐operation and Development), 2018. Guidance document on residues in rotational crops. In: Series on Pesticides No 97. ENV/JM/MONO(2018)9, 22 May 2018.


