Abstract
In 2018, no lumpy skin disease (LSD) outbreaks were reported in the Balkan region, after the decline reported in 2017 (385) compared to 2016 (7,483). This confirms the effectiveness of the vaccination campaign based on the LSD homologous vaccine strain which continued throughout 2018 with over 2.5 million animals vaccinated, keeping the mean vaccination coverage above 70%. In 2018, LSD outbreaks were reported in Russia, Turkey and Georgia. In Russia, the LSD epidemics expanded northward and eastward, while in Turkey, the most affected region was in the east. LSD is spreading in Turkey since 2013, despite large vaccination campaigns with heterologous vaccine performed since 2014. This might support the hypothesis that the use of heterologous vaccines results in insufficient protection, and therefore, the use of homologous LSD vaccine in Turkey should be considered to prevent further spread. As the LSD epidemic in Turkey is a risk for reintroduction into the EU, it is recommended to continue the vaccination campaigns in 2019 in the high‐risk areas of Balkan region. Spread rates of LSD within a village were estimated from outbreak data for Albania, which can be used to inform the level of vaccination required to control an outbreak in a village. In terms of vaccine safety, the reports from the field suggest that, compared to the large number of animals vaccinated in the Balkan region since 2015, a very limited number of side effects have been recorded so far, although from published literature, local or even systemic side effects in some animals may occur after vaccination. However, due to inadequate study design in the reviewed studies, there is no consensus on the magnitude of such effects and on their real consequences on production.
Keywords: lumpy skin disease, spread, vaccine, mathematical model, basic reproduction number
Summary
European Food Safety Authority (EFSA) continued the reporting on the lumpy skin disease (LSD) epidemic and the data collection from affected and at‐risk countries in South‐Eastern Europe about LSD outbreaks and vaccination. Monitoring of the LSD epidemics in the region is part of the long‐term objective of the LSD vaccination exit strategy in South‐Eastern, which is to restore the LSD‐free status in the region.
The present report is referred to the situation up to the end of 2018 and provides a description of the epidemiological situation of LSD and the vaccination campaign in place in Europe and in neighbouring countries, such as Turkey and Russian Federation. In addition, the basic reproduction number (R0)1 of the within‐village LSD spread (as a proxy of within‐herd spread) was estimated with the outbreak data for Albania in 2016. The R0 can be used to assess the level of vaccination required to control an outbreak (i.e. to reduce R0 below one).
In 2018, no outbreaks of LSD were reported in the Balkan region, after a decline in the number of outbreaks reported in 2017 (385) compared to 2016 (7,483). This confirms the effectiveness of the vaccination campaign based on the LSD homologous strain and the coordinated control measures put in place in the region. The halt of the LSD epidemic achieved in the Balkans in 2018 is in agreement with the long‐term strategic objective of the LSD vaccination exit strategy in the South‐Eastern Europe, which is to restore the LSD‐free status situation as it was before the occurrence of the LSD epidemics and to stop the LSD vaccination in the region.
In 2018, LSD outbreaks were only reported in Russia (63 outbreaks), Turkey (46 outbreaks) and Georgia (6 outbreaks) in the period between April and November, thus confirming the seasonal pattern of LSD. Compared to 2017, the LSD epidemics in the Russian Federation expanded northward and eastward along the border with Kazakhstan, while in Turkey, most outbreaks were reported in the Eastern regions. The outbreaks in Turkey represent a threat for the neighbouring EU countries, especially Greece and Bulgaria, which can be at risk of new incursion.
Since 2013, the presence of LSD has been constantly reported in Turkey, with active outbreaks reported until the end of 2018. This was despite vaccination campaigns based on heterologous vaccine (SGPV vaccine) performed since 2014 with apparently high levels of vaccination coverage of Turkish cattle population. This might support the hypothesis of insufficient levels of protection conferred by heterologous vaccines, especially when doses less than 10 times those recommended for sheep are used. Therefore, the use of the homologous LSD vaccine in Turkey, at least in the Thrace region, should be encouraged in order to better protect cattle and prevent from further spread of LSD to the EU.
The current epidemiological situation indicates that LSD is still present in some countries neighbouring the EU, thus supporting the recommendations provided by the Standing Group of Experts on LSD in South‐Eastern Europe under the Global Framework for the progressive control of Transboundary Diseases (GF‐TADs) umbrella of continuing the vaccination campaign in 2019, at least in Albania (due to most recent outbreaks reported in autumn 2017), Bulgaria (all or part of), Greece, Kosovo,* Montenegro, Serbia (the southern part), North Macedonia and Turkey.
The vaccination against LSD based on homologous vaccine in South‐Eastern Europe continued throughout 2018 when over 2.5 million cattle were vaccinated, keeping the vaccination coverage above 70% in the whole region in terms of proportion of immunised animals, indicating a very good level of immunity throughout the year and good implementation of the vaccination campaign. At the end of 2018, the lowest value was recorded in Albania (around 22%), then 58% in Kosovo,* 71% in Greece, 80% in Montenegro and over 90% in Bulgaria, Serbia and North Macedonia.
Concerning vaccine safety, given the large size (over two million doses administered yearly) of the vaccination campaign against LSD based on a live‐attenuated homologous strain put in place in the Balkan region since 2015, the number of reports from the field suggests a very limited number of side effects.
Nevertheless, from published literature, it can be concluded that local (at the injection site) or even systemic side effects may occur in some animals after vaccination. However, since the reports are based on case series without proper controls, it is not possible to adequately estimate the production losses (milk yield, mortality, morbidity, etc.) from these studies. Therefore, there is no consensus on the proportion of animals experiencing such effects and on the real consequences of the side effects on production. To quantify production losses due to vaccination, a (retrospective) observational study should be designed using, e.g. milk yield as outcome variable while considering confounding factors and bias. This requires, however, an in‐depth study of a well‐structured database in the naïve cattle population in a country where the disease has not been detected, but vaccination has been implemented such as Croatia and/or Bosnia and Herzegovina.
The transmission rate and basic reproduction number (R0) for spread of LSD virus within a village were estimated from outbreak data for Albania in 2016. There was variation amongst villages in R0 estimates; across all villages, the posterior median for R0 varied from 0.03 to 3.58, with a median of 0.87. In addition, the posterior median for R0 exceeded one (meaning the disease would spread further) in 35.0% of villages, while 9.9% of villages had an estimate for R0 significantly below one (meaning disease would fade out). There was a significant decline in R0 during the epidemic, which is likely to be a consequence of seasonality in the transmission rate, potentially related to changes in vector abundance. Based on the 95th percentile for R0 in each village in Albania and assuming a vaccine effectiveness of 62.5%, the level of vaccine coverage (95% credible interval) required to control an outbreak varies from 7.3% to 91.7%, with a median of 52.5%.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
LSD is a viral disease of cattle that reached continental Europe via Turkey in 2015, affecting North Eastern Greece, between August and December. The disease returned again in the spring of 2016, this time affecting seven countries of South‐Eastern (SE) Europe with numerous outbreaks, namely Greece, Bulgaria, North Macedonia, Serbia, Kosovo,* Albania & Montenegro.
By 2017, thanks to the coordinated vaccination campaigns in all affected countries of SE Europe (annual vaccination of all cattle, with live homologous vaccines, since 2016), the disease was contained. In addition, two non‐affected countries resorted to LSD vaccination too, as a preventive measure, namely Croatia (2016‐2017) and Bosnia and Herzegovina (2017). As a result of this combined effort no new LSD outbreaks were reported, in those countries where sufficient vaccination coverage was achieved.
The regional LSD vaccination strategy will continue in 2018 too, in line with the recommendations of the latest meeting of the Standing Group of Experts on Lumpy Skin Disease in South‐Eastern Europe under the GF‐TADs umbrella (GF TADs SGE LSD5, 19‐20 October 2017, Budva, Montenegro), where EFSA was present too.
Mass vaccination of cattle against LSD using live homologous vaccines figures clearly as the most effective control policy. Nevertheless, there is evidence that LSD virus remains present (e.g. recurrence of LSD in Albania in 2017) and non‐immune cattle remain at risk, even in areas of relatively high vaccine coverage.
In addition, 2018 will be the third consecutive year of mass LSD vaccination across SE Europe and the countries in the region, affected or at risk for LSD, have already expressed their will to collaborate in drafting an LSD exit strategy.
To this end updated scientific information and analysis is needed, to assist the countries of SE Europe in drafting a regional roadmap on an LSD exit strategy from 2018 onwards, as described in the GF TADs SGE LSD5 recommendations.
EFSA, at the request of DG SANTE, has been systematically collecting and analysing LSD epidemiological data since 2016 and has already produced two (2) LSD scientific reports.
1.1.1. Terms of References
In view of the above and in the context of Articles 31 of Regulation (EC) No. 178/2002, EFSA is requested to provide scientific and technical assistance to the Commission on the continuation of the collection and analysis of the lumpy skin disease (LSD) epidemiological data in the context of article 31 of Regulation (EC) No. 178/2002, as detailed in the reference letter Ares(2016) 3953310, of 27 July 2016, for another 2 years. In the framework of this exercise, two reports should be produced, one in January 2019 and the other in January 2020. Given the extent and duration of LSD vaccination in SE Europe, every effort should be taken to include in the analysis as much information as possible, in relation to the performance of the LSD homologous vaccines (effectiveness, safety, etc.), in use since the beginning of the epidemic.
1.2. Introduction and interpretation of the Terms of Reference
The European Commission has requested EFSA to continue the reporting on the LSD epidemic, and the related data collection from affected and at‐risk countries in South‐Eastern Europe both with relation to new outbreaks and vaccination. This is the third report of this kind, which refers to the situation up to the end of 2018.
The monitoring of the LSD epidemics in the region is important because the long‐term objective of the LSD vaccination exit strategy in the SE Europe as foreseen by the Standing Group of Experts on LSD (SGE LSD) in SE Europe under the GF‐TADs umbrella (GF‐TADs2) group is to restore the LSD‐free status, i.e. the situation as it was before the occurrence of the LSD outbreaks and the implementation of LSD vaccination in the region and the ultimate goal being the total elimination of LSD accompanied by cessation of vaccination in the region.
The present report provides a description of the epidemiological situation of LSD up to 2018 in Europe and in neighbouring countries, such as Turkey and Russian Federation. A description of LSD occurrence and the control measures applied is provided, and in particular, the progress of the vaccination campaign and the surveillance activities, where these have been put in place, are described.
In addition, by using the kernel‐based spread model, as previously described (EFSA AHAW Panel, 2015, 2016; EFSA, 2017, 2018), the basic reproduction number (R0)1 of the within‐village spread (as a proxy of within‐herd spread) was estimated with the outbreak data from Albania from 2016 (Section 3.4). The R0 can be used to assess the level of vaccination required to control an outbreak (i.e. to reduce R0 to below one).
2. Data and methodologies
The LSD outbreak data were obtained from national authorities and Animal Disease Notification System (ADNS3) for those countries that notify to ADNS and EMPRES Global Animal Disease Information System (Empres‐I4) for the other countries.
The vaccination data at farm and animal level (the latter for Bulgaria and Serbia) related to the vaccination campaign conducted in 2018 were collected from the national authorities of Bulgaria, North Macedonia, Kosovo,* Greece, Serbia. Vaccination data from Albania related to 2018 were only available at district level for which the total number of vaccinated animals was recorded. No data for vaccination were available from Bosnia and Herzegovina. No vaccination data for 2018 were available for Croatia as the last vaccination campaign in Croatia was conducted in 2017.
The data were described in a spatial and temporal context using GIS software.
2.1. Analysis of LSDV spread: within‐village spread
2.1.1. Rationale and data
For this analysis, data from LSD outbreaks in Albania during 2016 were used, as done for previous analysis of transmission of LSD virus (LSDV) between farms (EFSA, 2018), because Albania was the most heavily affected country and did not implement stamping out as a control measure.
In Albania, there are 461,770 cattle in 198,105 herds located in 2,938 villages. This means that herds are mostly small (median herd size: 2 cattle; 99th percentile: 10 cattle) and are clustered in villages (median (range) number of herds per village: 46 (1–939); median (range) number of cattle per village: 103 (1–2051)). In addition, animals from herds in the same village typically share grazing. Consequently, the village rather than the herd was treated as the epidemiological unit when estimating transmission parameters for LSDV.
For the 563 villages in Albania where cases of LSDV were reported in more than one herd (so transmission potentially occurred within the village) during 2016, the time of suspicion for each herd in the village (used as a proxy for the time of infection), the time of vaccination of each herd in the village and the number of cattle in each herd in the village were extracted for each village. Because herds are typically small, all animals in a herd were assumed to be infected at the time of suspicion and then remain infectious for the next 23 days, based on detection of LSDV in skin biopsies, used as a proxy for infectiousness (Tuppurainen et al., 2005; Babiuk et al., 2008). In vaccinated herds, all cattle within a herd were assumed to be vaccinated.
Under the above assumptions, there were time periods in some villages (191 of 563) during which animals became infected despite there being no infectious animals in the village. This could be due to, e.g. the assumptions used to compute the number of infectious cattle, the under‐ascertainment of cases or to multiple introductions of LSDV into a village. However, it is not possible to determine the reasons in most cases and, for consistency, they were all excluded from the study. Therefore, a total of 372 villages were included in the analysis.
2.1.2. Transmission model for LSDV
In the model, the village is treated as the epidemiological unit and cattle are assumed to mix homogeneously within a village (i.e. they are equally likely to contact any other animal in the village, regardless of which herd they belong to). Although LSDV is transmitted by the bites of insects, the dynamics of LSDV in a village can be approximated by a susceptible–exposed–infectious–recovered (SEIR) model with a density‐dependent force of infection. Specifically, the force of infection for cattle in herd h in the village is given by
| (1) |
where I(t) is the number of infectious cattle in the village at time t, α is the transmission rate (in the absence of vaccination) and
| (2) |
is the level of protection from vaccination in the herd. This is assumed to increase linearly from zero at the time of vaccination (tvacc) to the vaccine effectiveness (ε) when full protection is reached (tFP; assumed to be at 21 days post‐vaccination, as per the manufacturer's data sheet) (cf. Gubbins et al., 2019).
The basic reproduction number, R0, for the village is
| (3) |
where H is the number of cattle in the village and 1/rI is the mean duration of the infectious period in cattle (i.e. 23 days). Note that this is for transmission in the absence of vaccination.
2.1.3. Bayesian methods
The transmission rate (α) and vaccine effectiveness (ε) were estimated for each village independently. The log likelihood for each village is given by
| (4) |
where λh(t) is the force of infection for herd h (defined in equation (1)), Nh is the number of cattle in herd h; t0, tI and tend are the times at which the first herd in the village became infected, each herd in the village became infected and when no more animals in the village were infectious, respectively, Iv is a list of herds in the village which became infected and Uv is a list of herds which remained uninfected. An exponential prior (with mean 100) was used for the transmission rate, while a Uniform (0,1) prior was used for the vaccine effectiveness.
Samples from the joint posterior density were generated using an adaptive Metropolis scheme (Haario et al., 2001), modified so that the scaling factor was tuned during burn‐in to ensure an acceptance rate between 20% and 40% for more efficient sampling of the target distribution (Andrieu & Thoms 2008). Two chains of 50,000 iterations were run, with the preceding 50,000 iterations discarded to allow for burn‐in of the chain. The chains were then thinned (taking every 10th sample) to reduce autocorrelation amongst the samples. Convergence of the scheme was assessed visually and by examining the Gelman–Rubin statistic provided in the coda package (Plummer et al., 2006) in R (R Core Team, 2018).
3. Assessment
3.1. Epidemiological situation
3.1.1. Update of the spatial and temporal development of LSD epidemics in Europe
Considering Eurasia, in 2018, LSD outbreaks were reported in Russia (63 outbreaks), Turkey (46 outbreaks) and Georgia (6 outbreaks). Compared to 2017, the LSD epidemic in the Russian Federation expanded northward and eastward along the border with Kazakhstan, while in Turkey, most outbreaks were reported in the Eastern regions. No outbreaks were reported in the Balkan region. Figures 1 and 2 show the yearly temporal and geographical evolution of LSD epidemic in Europe since 2014, while this https://www.youtube.com/watch?v=7WFxZxwPRYI&feature=youtu.be shows an animation of the LSD epidemics evolution since 2012.5
Figure 1.
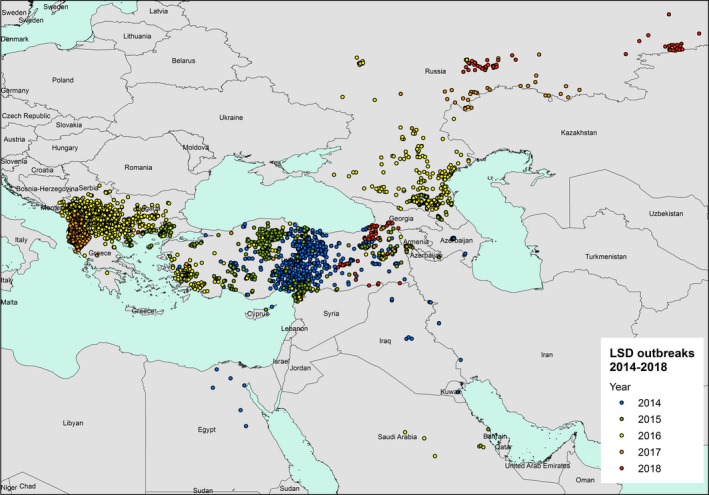
LSD outbreaks notified in Europe and Middle East between 2014 and 2018 (Data source: national authorities and ADNS for those countries that notify to ADNS and Empres‐I for the other countries)
Figure 2.
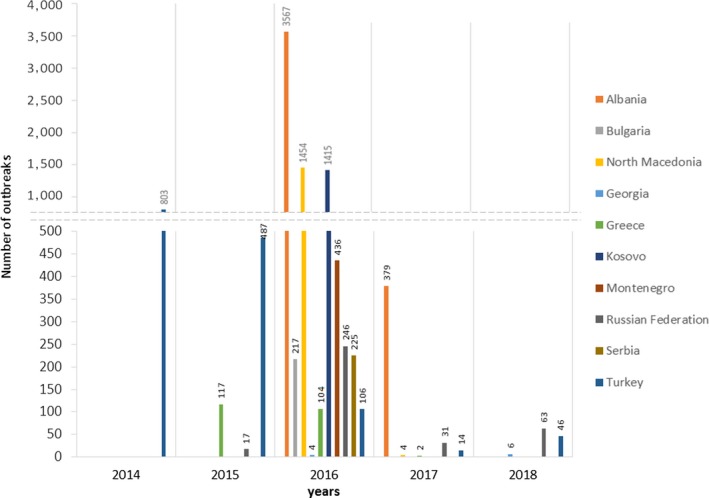
Temporal distribution of LSD outbreaks in the different countries and years since 2014 (Data source: national authorities and ADNS for those countries that notify to ADNS and Empres‐I for the other countries)
From the Figure 3, it appears that outbreaks of LSD in 2018 were concentrated in two areas, (i) Eastern Turkey and southern part of Georgia on the borders with Turkey and (ii) Russian Federation along the border with Kazakhstan, with most outbreaks in September and October 2018 and located mainly in the east.
Figure 3.
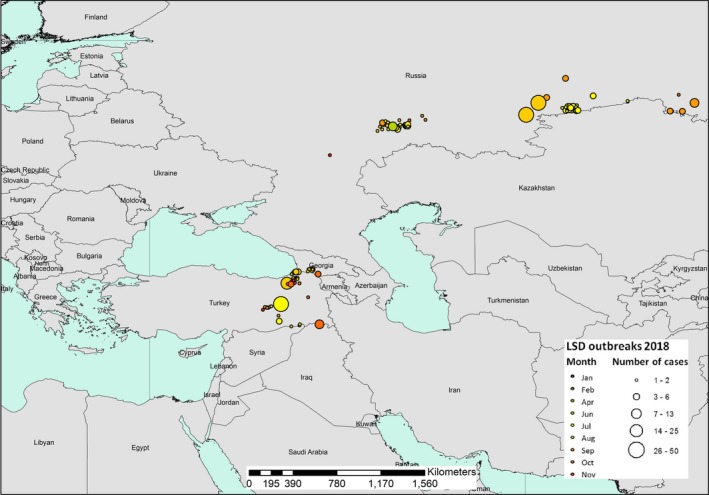
Temporal distribution of the LSD outbreaks by month (coloured bubbles) in 2018. The number of cases reported in each outbreak is proportional to the size of the bubble. (Data source: Empres‐I and ADNS)
Taking into account the limited available information on vaccination activities in place in these countries and possible uncertainties deriving from disease under‐reporting or under‐ascertainment by prudently interpreting the actual picture as depicted by available official data, some considerations can be made about the epidemiological situation in countries where LSD was still active in 2018.
There has been a decreasing trend in the number of outbreaks in Turkey, from around 500 outbreaks in 2015 to 14 in 2017; however, in 2018, again 46 outbreaks were reported. According to the information presented during the seventh meeting of the SGE LSD for SE Europe,6 a live‐attenuated vaccine, based on a sheep and goat pox strain (SGPV) isolated in 1975 in Turkey, was extensively used in Turkey to control LSD. Cattle older than 3 months of age are annually vaccinated with a dose three times higher than the SGPV vaccine dose used in small ruminant for the control of sheep and goat pox. The vaccination with the SGPV vaccine conducted in Turkey may have had some effect in reducing the LSD infection, also considering the coverage reported by the Turkish national authorities (66% in 2016, 89% in 2017 and 93% in 2018 all over the country). Nevertheless, the disease has not disappeared in 2018, albeit the outbreaks were concentrated in the eastern part of Turkey, which may be considered an area more difficult to control due to political issues and the effects of the war in Syria. The outbreaks reported in Turkey are of importance for the neighbouring EU countries and may raise doubts about the effectiveness of the SGPV vaccine, which was estimated to be around 50% (data reported by Sciensano7). However, the fluctuating number of outbreaks in Turkey could be linked to the natural dynamics of the disease and not necessarily to the vaccination effects. Fluctuations in morbidity were observed also in Southern Africa despite very low vaccine coverage. Disease quiescence in that region was suggested to be due to unfavourable climatic conditions that reduce vector prevalence, with a concomitant reduction of the host immunity that later results in extensive outbreaks (Hunter and Wallace, 2001).
A similar trend, decrease from 2016 to 2017 and then increase in 2018, can be observed in the Russian Federation, with the greatest number of outbreaks (246) reported in 2016 in Dagestan and Chechnya Republic followed by a lower number of reported outbreaks in 2017 (31 outbreaks) and 2018 (63 outbreaks), with LSD spreading northward and eastward, close to the borders with Kazakhstan. In the Russian Federation, as well as in Turkey, a heterologous strain (SGPV vaccine) has been used, mostly as reactive vaccination (i.e. vaccination implemented after the disease has entered in a region) in the affected areas, achieving a coverage of around 70%.
Georgia has been experiencing the disease since 2016, when two outbreaks were reported in Oni district in the Racha region, close to each other (17 km) and in the border area of the Russian Federation and Georgia. In 2018, six outbreaks were notified in the south‐western part of the country, most likely connected to the ones reported in eastern Turkey, showing the need for further improvements of the LSD control strategies. Georgia has vaccinated cattle against LSDV in the high‐risk regions using a sheep and goat pox strain vaccine produced in Turkey (Poxvac, Vetal8). The vaccine is different from the vaccine used in Russia. Georgia started vaccination of cattle already in 2014, after LSD outbreaks were detected in neighbouring Azerbaijan by creating a buffer zone of at least 10 km along the borders with Azerbaijan. In 2015 and 2016, vaccinations were also carried out along the borders with Armenia and Turkey, except in Adjara Region, because there are mountains that prevent common grazing of animals from both countries, coupled with an awareness campaign. In September 2018, Georgia received a first donation from the EU vaccine bank of 100,000 doses of live homologous vaccines (OBP, South Africa), to be used in the vaccination campaign along with the heterologous vaccines already in use. Later on in 2018, a second donation of 100,000 doses of the same vaccine was received from the EU which is kept as vaccine reserve in Georgia for the vaccination campaign in 2019.
In SE Europe (Balkan region), after the steady decline registered in 2017, no outbreaks at all were reported in 2018, thus confirming once again the effectiveness of the vaccination campaign and of the coordinated control measures put in place in the last years. In the areas where outbreaks were still reported in 2017, mainly in Albania (379 outbreaks in 2017), no outbreaks were confirmed or reported in 2018. Seven suspected cases were detected by clinical passive surveillance and investigated and all tested negative in the laboratory. One case was initially reported to ADNS in the European Turkey in April 2018 (this was also reported at the GF TADs meeting in October 20189), in the Edirne region, close to the Greek border; but later, the Turkish authorities informed that it resulted negative by epidemiological investigation and laboratory analysis, and it was removed from ADNS. More detailed data from Turkey would be needed to clarify the real epidemiological situation in Thrace and to estimate in a more robust way the real risk of LSD reintroduction into the EU.
Figure 4 shows the temporal distribution of LSD outbreaks reported in Russian Federation and Turkey in 2018 compared with the two previous years (2016–2017). A clear seasonal pattern can be observed in both countries, with most of the outbreaks being reported between April and October: in 2018, 2017 and 2016, 97%, 86%, and 91% of all outbreaks were reported during the April–October period, respectively. This is in agreement with patterns observed previously in the Balkan areas (EFSA, 2017, 2018) as well as with a predictive model recently developed which showed that LSD risk is related to cattle density, annual mean temperature and temperature diurnal range (Allepuz et al., 2018).
Figure 4.
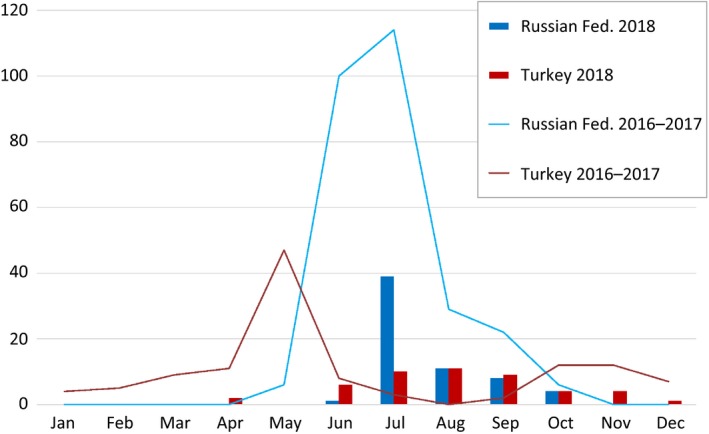
Monthly reported number of LSD outbreaks in Turkey and Russian Federation in 2016–2017 (lines) and 2018 (bars)
A certain difference in the period of peak of outbreaks can be observed for Turkey when the temporal distribution of LSD cases occurred in 2018 is compared with the two previous years. Many factors could have influenced this difference: the time of LSD introduction in the areas involved in 2018, and differences in the climatic and geographical conditions (while the coastal area was mainly involved in 2016 and 2017, in 2018, the eastern part of Turkey, which is mainly mountainous, was the most involved). This hypothesis could be verified looking at the temperatures in 2016–2018 outbreak locations. Finally, different levels of vaccination coverage may also be a confounder.
The marked seasonality of LSD in the temperate zones was previously observed in outbreaks which occurred in Turkey, in Israel and in the Balkans (Sevik et al., 2016; Kahana‐Sutin et al., 2017; Mercier et al., 2017). This seasonality was well explained by the change in the abundance of Stomoxys calcitrans (Kahana‐Sutin et al., 2017; Gubbins et al., 2019).
Conclusions:
In 2018, LSD was reported in Eastern Turkey, Georgia and in the Russian Federation along the border with Kazakhstan. In Turkey, one case was clinically suspected and initially reported in the western part of the country, in Thrace region; however, it resulted negative after epidemiological investigation and laboratory analysis;
Based on the reporting system in place in the Balkan region (passive surveillance, apart from Croatia and north‐eastern Greece where also active clinical surveillance is in place), no outbreaks were reported in SE Europe. In Albania, seven clinically suspected cases were reported, which resulted negative after epidemiological investigation and laboratory tests;
Since 2013, the presence of LSD has been constantly reported in Turkey. Outbreaks have been reported until the end of 2018, despite vaccination campaigns using a heterologous vaccine (SGPV vaccine) since 2014 with apparent high levels of vaccination coverage. This supports the hypothesis that heterologous vaccines do not induce a sufficient protection, especially when doses less than 10 times those recommended for sheep are used. This could be also an argument supporting the decision to use a homologous LSD vaccine in Turkey, at least in the Thrace region, to reach a more solid immunity in Turkish cattle population.
The apparent LSD spread eastwards in the Russian Federation, along the border with Kazakhstan might also be attributable to the use of heterologous vaccines.
Among other countries that may be considered at risk of LSD incursion (Romania and Ukraine), no outbreaks have been reported so far. Concerning Romania, it may have benefitted from the vaccination campaigns carried out in bordering countries, such as Bulgaria and Serbia. In relation to Ukraine, it is noteworthy that the outbreaks in the Russian Federation reported in 2016 occurred at only 120 km far from the Ukrainian border.
The current epidemiological situation indicated that LSD is still present in some countries neighbouring the EU, thus supporting the recommendations by the GF TADs of continuing the vaccination campaign in 2019, at least in Albania (most recent outbreaks reported in autumn 2017), Bulgaria (all or part of), Greece, Kosovo,* Montenegro, Serbia (the southern part), North Macedonia and Turkey.
3.2. Vaccination campaign against LSD in 2018
All countries in SE Europe (Albania, Bosnia and Herzegovina, Bulgaria, Greece, Kosovo,* Montenegro, Serbia, North Macedonia) continued to vaccinate against LSD in 2018, with the exception of Croatia. In all these countries, a live‐attenuated vaccine based on homologous LSD vaccine strain10 was used. Vaccine strains used were from different companies, either based on Neethling strain like LSD Vaccine for Cattle (Onderstepoort Biological Products; OBP, South Africa11) or Bovivax (MCI Santè Animale, Morocco12) or based on SIS Neethling type (Lumpyvax, MSD Animal Health‐Intervet, South Africa13).
The level of the vaccination coverage (calculated as the immunity level, i.e. proportion of animals vaccinated in the last 12 months14 out of the total animals present) achieved in April and October 2018 (beginning and end of the vector season) in the Balkan region is displayed in Figure 5.
Figure 5.
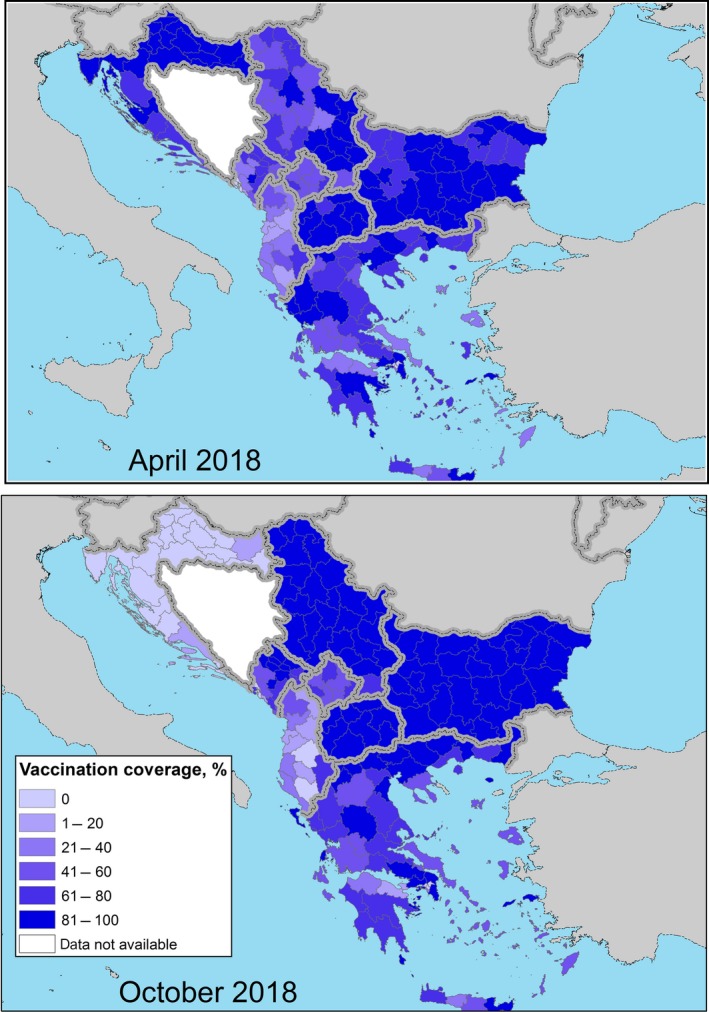
Vaccination coverage achieved in 2018 (proportion of immunised animals out of the total animals present) in the Balkan region at the beginning (April) and at the end (October) of the vector season
The mean vaccination coverage in the region in April and October 2018 was 71% and 78%, respectively (excluding Croatia), thus indicating a very good level of immunity throughout the year and implementation of the vaccination campaign. In October 2018, the lowest value of vaccination coverage was in Albania (22%15), which is low but Albania may benefit of the overall protection of the herd immunity established in the whole region, then 58% in Kosovo,* 71% in Greece, 80% in Montenegro and over 90% in Bulgaria, North Macedonia and Serbia. Nevertheless, the low vaccination coverage in Albania may represent a risk of LSD recurrence for the other countries.
In 2018, over 2.5 million cattle were vaccinated with LSD homologous live‐attenuated vaccine strain (Neethling strain11 , 12, and SIS Neethling type13) in the Balkan regions in Bulgaria, Greece, Montenegro, North Macedonia, Kosovo, Serbia, Bosnia and Herzegovina, with a contribution from EU vaccine bank of 776 thousands doses, the highest supply since the beginning of the campaign in 2015.
Live‐attenuated heterologous strain vaccine (SGPV vaccine) have been continuously used in Turkey (14 million doses in 2018, reaching a coverage of 93%) and in Russian Federation (4 million doses in the affected areas).
For countries where data on exact location (coordinates) of vaccinated farm were available (Bulgaria,16 Serbia, North Macedonia, Kosovo,* Greece17), the timeline of the vaccination campaign along 2018 can be displayed also per each farm, as in Figure 6.
Figure 6.
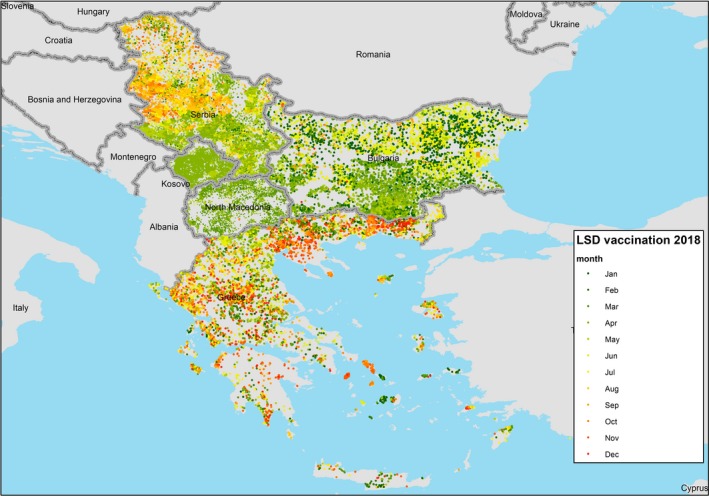
Monthly progress of the vaccination per farm in 2018 in Bulgaria, North Macedonia, Kosovo,* Greece and Serbia
In most parts of the Balkan region, the vaccination in 2018 took place in spring and summer months, because the epidemic started in spring–summer 2016 and the vaccination started in this period. In northern Serbia, the vaccination in 2018 was carried out in autumn, and in this region, the vaccination will be also stopped in 2019. In Greece, 2018 is the fourth year of LSD vaccination campaign; in this country, the vaccination campaign takes place in the farms in different months of the year since the vaccination started in September 2015 in Eastern Greece and progressively was extended to the whole country, so farms in different regional units were revaccinated in different periods of the year.
In Croatia, the last vaccination campaign was implemented in 2017, and in Figure 5, it is visible that some residual immunity is still present in April 2018. Given the favourable epidemiological conditions in the region and the reduced risk of LSD introduction in the country, Croatia stopped the vaccination campaign in 2018. As indicated by the article 11.9.4 of the OIE code18 for the recovery of free status after preventive vaccination is conducted in the absence of LSD cases (8 months after the last vaccination), the lift of the vaccination in Croatia was replaced by a surveillance scheme based on clinical, virological and serological surveillance launched in June 2018 as follows:
640 randomly selected bovines from eight at‐risk counties (mainly bordering with Serbia and Bosnia and Herzegovina) were sampled monthly and tested by quantitative polymerase chain reaction (QPCR) for virological and by enzyme‐linked immunosorbent assay(ELISA) for serological surveillance; in addition, 540 vaccinated bovines were tested by ELISA for immunity control. In total, 4,909 ELISA tests were performed: 1,368 (28%) samples were seropositive and cattle that were vaccinated twice had higher antibody levels compared to animals that were vaccinated only once.
4,378 samples tested negative using QPCR method.
Active surveillance by clinical examinations was conducted monthly targeting a 10% within‐herd prevalence, and including at least examination of mucosae, udder and perineal area and skin palpation; almost 35,000 clinical examinations were performed among 2,000 herds and no clinical signs of LSD were detected.
Passive surveillance is ongoing overall the Croatian territory; a suspicion of LSD was ruled out in three bovine herds.
Unvaccinated bulls from three semen collection centres were tested monthly from January until September by VNT, ELISA, QPCR in blood and QPCR in semen; in total, 32 bulls were tested with negative results.
Pathological examinations were conducted on dead bovines (fallen stock) and no pathological signs of LSD were detected.
Based on the above, it was concluded that there is no LSD virus circulation in Croatia; thus, according to the Commission Implementing decision (EU) 2019/8119 and 2019/82,20 all OIE requirements for disease‐free status for LSD in the absence of the occurrence of LSD cases are fulfilled, since more than 8 months have elapsed since the last LSD vaccination and the restrictions in relation to LSD vaccination in Croatia are lifted.
3.3. Vaccine safety
Compared to the large size (over two million doses administered yearly) of the vaccination campaign against LSD based on a live‐attenuated homologous strain put in place in the Balkan region since 2015, a very limited number of side effects have been recorded so far. Nevertheless, since some authors claim that the vaccine used may have relevant safety issues (see references in the next Section 3.3.1), it is necessary to discuss the appropriate way to conduct a study to explore this aspect. In the following sections, the currently available literature and the experience gained by the national authorities of the countries that have used the vaccine are discussed.
3.3.1. Safety of vaccination against LSD: literature review
In the scientific literature, adverse effects of vaccination with the live‐attenuated homologous vaccine (see footnotes 10, 11, 12, 13 at Section 3.2), such as local reactions at the vaccination site, generalised skin reactions in some vaccinated animals and decrease in milk yield have been reported in South Africa (Weiss, 1968; Hunter and Wallace, 2001). In addition, it has been reported that European dairy cattle may show adverse reactions after vaccination with the Kenyan sheep and goat pox (KSGP O‐240) vaccine in Africa (Yeruham et al., 1995; Kitching, 2003; Ayelet et al., 2013), which was likely associated with a low attenuation level of the vaccine, while the so‐called KSGP strain was later molecularly identified to be in fact a LSDV (Lamien et al., 2011a,b; Tuppurainen et al., 2014). More recently, field veterinarians in Jordan reported a range of clinical signs seen after the administration of different capripoxvirus‐containing vaccines to prevent LSD infection in cattle (Abutarbush et al., 2014).
According to the Israeli experience, LSD vaccine caused only mild adverse effects at a very low incidence (0.38%) (Ben‐Gera et al., 2015). However, the Israeli cattle were vaccinated once with sheep pox strain vaccine before vaccination with Neethling vaccine (see footnote 10 at Section 3.2), which may have reduced the adverse effects.
In Croatia, the country that has vaccinated its cattle with homologous LSDV vaccine but where infection did not occur, adverse reactions were reported in 0.09% of the vaccinated animals (EFSA, 2017). In contrast to other countries, lack of field infection prevents involvement of field LSD strain here. However, the results were based on passive surveillance only and the farmers were compensated if adverse effects were considered plausible. Passive surveillance may have been associated with under‐reporting, whereas compensation may have led to over‐reporting. Consequently, it is not possible to estimate the real incidence and magnitude of adverse effects from this report.
A number of studies reporting adverse effects of vaccination have been published recently (Agianniotaki et al., 2017; Bedekovic et al., 2017; Abutarbush and Tuppurainen, 2018; Katsoulos et al., 2018).
Agianniotaki et al. (2017) carried out sequence comparisons of LSDV found in field samples from three unvaccinated animals originating from different geographical regions in Greece (northern, central and southern Greece), along with 10 additional nodular samples, which were obtained from vaccinated animals (vaccinated with either LSD Vaccine for Cattle, Onderstepoort Biological Products; OBP, South Africa or Lumpyvax, MSD Animal Health‐Intervet, South Africa) presenting LSD clinical signs. The vaccine virus was identified in five of the vaccinated animals. The nodules sampled from these animals were located at various parts of their body (i.e. mainly on the head and neck but also on the trunk), looked like LSD nodules, but were smaller and more superficial, as recorded in the clinical examination reports. No other clinical signs (i.e. fever, salivation, ocular and nasal discharge, lymph node enlargement) were observed in these animals. The lack of controls reduces the value of this study.
Bedekovic et al. monitored 15 cows in each of eight randomly selected farms before and after vaccination with two homologous vaccines, i.e. Lumpyvax (MSD Intervet South Africa (Pty) Ltd., Spartan, RSA, 104 TCID50/mL live‐attenuated SIS Neethling type virus) and LSD vaccine for cattle (Onderstepoort Biological Products SOC Ltd, Onderstepoort, RSA, 103.5 TCID50/mL live attenuated Neethling strain). In total, eight animals from four farms showed a localised reaction at the site of vaccination and 10 animals from five farms had generalised skin nodules. Vaccine virus could be detected in nodules, blood and milk of some of the cows. The authors concluded that the homologous live vaccines do not meet the OIE safety requirements because vaccine virus may become generalised.
Katsoulos et al. (2018) conducted a study in one dairy herd of 215 bovines in Greece immunised with a commercial LSD strain homologous vaccine (LSD Vaccine for Cattle, Onderstepoort Biological Products SOC Ltd., South Africa). Pronounced swelling was observed at injection sites of 12% of the vaccinated animals starting at 6 days post‐vaccination for 2–4 days. In 9% of the vaccinated animals (19/215), small‐sized (< 0.5 cm) lumps were developed in adult animals but not in calves and heifers between days 8 and 18 p.v. and resolved after 10 days.
Abutarbush and Tuppurainen (2018) performed a study comprising 57 Holstein Friesian cattle which were randomly assigned into three experimental groups of 17 cattle according to the RM65 (sheep pox) vaccine dose used (one, five and ten times the dose used for sheep in the field), and a control group of six cattle that did not receive the vaccine. The animals were monitored closely for the development of any abnormality or side effects. The authors reported a decrease in total milk production a week after vaccination, which returned to prevaccination levels by the fifth week of the experiment. However, a major limitation in this study is the lack of any variance measures for milk production as well as incomplete data on the number of animals for which milk production was measured and lack of any detail on the method of milk production measurement. It is, therefore, impossible to deduce from this study the extent of milk production loss and if such loss was significant at all. Clinical side effects were observed in five animals of the group that received 10 times the SPP vaccine dose. Observed side effects included fever, decreased feed intake and milk production as well as skin lesions. Skin nodules appeared between 7 and 17 days post‐vaccination and remained for 11–17 days. Systemic reactions were likely to be associated with higher dosage and all affected cattle recovered.
In Europe, homologous live‐attenuated LSD vaccines have been widely used and there is no evidence for horizontal transmission so far. Recently, Croatian scientists isolated and sequenced a LSD vaccine strain from an animal showing an adverse skin reaction after vaccination. Sequencing data confirmed that, after passaged eight times in Madin‐Darby bovine kidney (MDBK) cells, the genome of the vaccine virus remained stable with 100% similarity to the original vaccine virus (Lojkić et al., 2018). Although these results are based on sequencing only one isolate, this finding suggests that vaccine virus can be isolated from the skin of a vaccinated animal, and after passage, there is no effect on sequence composition and thus on the nature of the vaccine virus itself (e.g. reacquisition of virulence).
In general, it can be concluded from published literature that local (at the injection site) or even systemic side effects in some animals may occur after vaccination. However, there is no consensus on the proportion of animals experiencing such effects and on the real consequences of the side effects on production.
3.3.2. Safety of vaccination against LSD: field experience in Europe
According to the vaccine producers, the development of a protective immunity takes approximately 2–3 weeks and vaccine may cause a local reaction at the vaccination site, with generalised skin lesions and a temporary decrease in milk production sometimes. A warning is included in the vaccine package insert, and the farmers and veterinarians have been informed about possible vaccine side effects. Indeed, in most countries, some farmers complained about clinical signs after vaccination, mainly temporary fever, lump at the vaccination site and reduction of milk production (Tuppurainen et al., 2018).
In SE Europe, Croatia and Bosnia and Herzegovina are probably the best candidates for the study of LSD vaccine side effects since the absence of LSD excluded any possible interference with circulating (field) LSDV.
Detection of clinical signs in vaccinated herds led to complicated situations in the field. Common side effects reported in Greece were a transient decline in milk production, loss of appetite and swelling and/or skin nodules around the injection site. Generalised symptoms after vaccination were officially reported in two herds and the vaccine strain was identified by the laboratory analysis. These herds were considered as suspicious and kept under official supervision by the Veterinary Authorities (VAs). Later on, all the stored samples collected from the previously affected farms were analysed in order to differentiate field from vaccine strain. The vaccine strain alone was identified in the stored samples from three outbreaks (reported when the DIVA (Differentiation of Infected from Vaccinated Animals) test was not yet available) out of the total number of outbreaks reported in Greece (223), whereas in the rest, the field strain was identified.21
In Bulgaria, 21% of the outbreaks were confirmed between 3 and 21 days after vaccination. Although laboratory analysis of collected samples confirmed the presence of LSD virus, no analyses aiming at distinguishing between LSD vaccine and field strain were carried out. All animals that showed ‘skin lumps’ post‐vaccination were treated as LSD field virus‐infected animals.
In Serbia, clinical symptoms of LSD were observed 3–7 days after vaccination. Decline in milk production was observed, and in some individual cases, abortion was reported. Laboratory testing was performed using DIVA PCR (Polymerase Chain Reaction) (Vidanovic et al., 2016). Cases of abortion were investigated, and some differential diagnostic tests were negative. However, no conclusions could be made as to whether abortions were related to the use of live LSD vaccine while numerous other pathogens and factors can cause abortions in cattle.
In Albania, 159 suspected vaccine adverse reactions were reported in 2016. Unfortunately, at that time, no PCR methods were available in the Albanian reference laboratory to investigate if the clinical signs observed in vaccinated cattle were caused by the virulent field virus or the vaccine strain itself. After vaccination in 2017, the number of herds where clinical signs were observed in vaccinated animals decreased to 30 herds.
In Montenegro, adverse reactions were observed in cattle after the administration of the LSDV vaccine only after the first vaccination campaign. A large number of animals showed mild skin reaction 4–5 days post‐vaccination which then disappear in a couple of days. In addition, a drop in milk production, fever, abortion and in some cases, deaths were observed. Nevertheless, in the beginning, it was impossible to differentiate between the vaccine and the field strain, but later, a DIVA test method was set up at the Diagnostic Veterinary Laboratory and is currently being used.
Field experience obtained from endemic countries indicates that when the animals are vaccinated for the second time, they usually do not show adverse reactions any more (Tuppurainen et al., 2018). This was indeed observed in Albania, Montenegro and elsewhere; adverse effects were rarely reported after the second vaccination campaign. Large‐scale preventive vaccinations were carried out in Croatia and Bosnia and Herzegovina in 2016 and 2017. From these countries, it was possible to obtain further information on the side effects of the vaccine without interference by the field strain (Bedekovic et al., 2017). Initial data reported by Croatian VAs through pharmacovigilance system showed post‐vaccination adverse reactions in 0.19% of the vaccinated farms, in 0.09% of the vaccinated animals with 0.02% deaths (EFSA, 2017). The majority of symptoms were reported within 2 weeks after vaccination and included fever, decrease in milk production and oedema at the injection site.
In conclusion, it can be stated that side effects are possible after vaccination, but it is not possible from the available information to properly estimate their incidence or their consequences on production. All available information is based on case series. The lack of proper controls prevents the estimation of production losses (milk yield, mortality, morbidity, etc.) from these studies. Studying production losses is not easy because all cattle in the concerned countries/regions are usually vaccinated in a limited time frame, hampering the availability of contemporary controls. Nevertheless, better studies are possible, because information of previous and following years can be collected and not all farms are vaccinated simultaneously enabling to correct for year effects. This requires, however, an in‐depth study of a well‐structured database in the naïve cattle population in Croatia and/or Bosnia and Herzegovina. Nevertheless, considering the number of vaccine doses administered in the region since 2015, over 2.5 million doses every year, the evidence from the field collected by VAs would suggest that the amount of adverse effects is very limited compared to the advantages brought by the vaccine in term of immune protection.
3.4. Analysis of LSDV spread: within‐village spread
3.4.1. Results
Estimates for the transmission rate, basic reproduction number and vaccine effectiveness for each village are shown in Figure 7. Note that the transmission rate and, hence, R0 are for transmission in the absence of vaccination. The estimates would include the effects of other control measures (e.g. culling of clinically affected animals or use of insecticides to control vectors), but these are assumed to be minimal in Albania.
Figure 7.
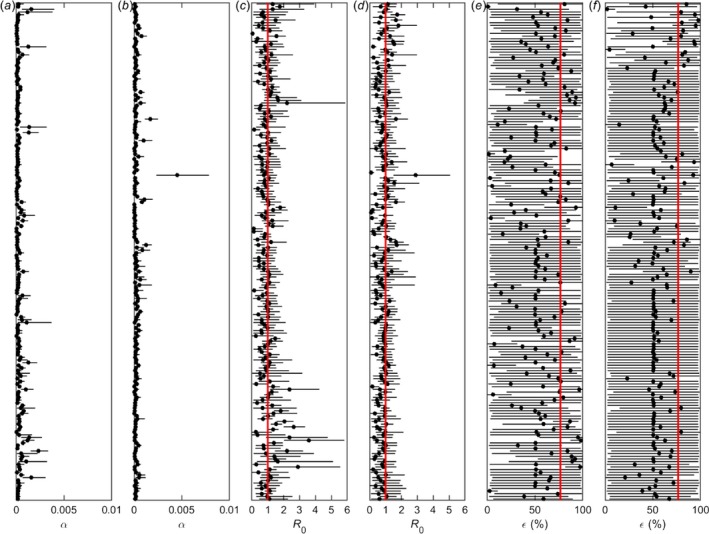
Transmission of lumpy skin disease virus within villages in Albania
- Estimates for the (a,b) transmission rate (α), (c,d) basic reproduction number (R0) and (e,f) vaccine effectiveness (%; ε) for 372 villages which reported cases of lumpy skin disease in 2016. Each panel shows the posterior median (circles) and 95% credible interval (error bars) for the parameter for a village. In panels (c,d), the red line indicates the threshold at R0 = 1, while in panels (e,f), the red line indicates the vaccine effectiveness estimated for Albania as a whole when analysing spread between farms (Gubbins et al., 2019).
There was variation in the estimated transmission rates (α) for villages (Figure 7a,b), which is reflected in variation amongst villages in R0 (Figure 7c,d). Across all villages, the posterior median for R0 varied from 0.03 to 3.58, with a median of 0.87. In addition, the posterior median for R0 exceeded one in 35.0% (130 of 372) of villages. Overall, 9.9% (37 of 372) of villages had an estimate for R0 significantly below one (i.e. the 95th percentile of the posterior distribution was below one), while 7.3% (27 of 372) had an estimate for R0 significantly above one (i.e. the 5th percentile of the posterior distribution was above one). In addition, there was a significant decline in R0 (regression coefficient: b = −0.0029; p < 0.001) during the epidemic (Figure 8). The plot shows the posterior median for R0 for each village (black dots) in relation to the day of the first case in the village (in days since 1 January 2016). The red line shows the fitted trend.
Figure 8.
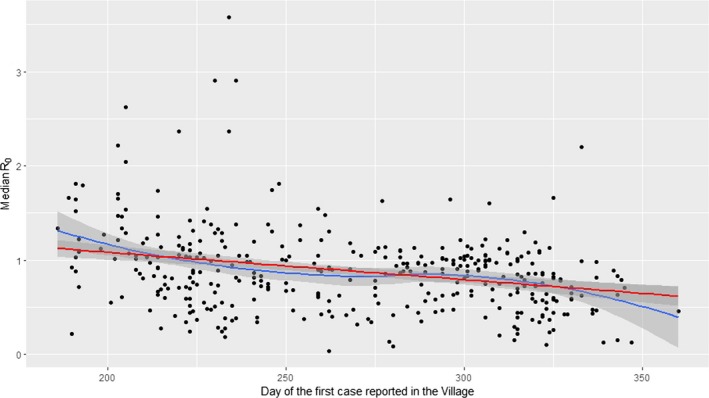
Temporal trends (red and blue lines) in the basic reproduction number (median values of R0, black dots) for lumpy skin disease virus in villages in Albania
Estimates for vaccine effectiveness varied considerably amongst villages (Figure 7e,f). The posterior median for vaccine effectiveness in a village ranged from 0.6% to 97.9%, with a median of 53.4%. In addition, the 95% credible intervals were very wide (covering over 90% of the range of the parameter) for 64.5% (240 of 372) villages. For those villages where the estimated effectiveness was low (posterior median < 25%), a majority of the affected herds within the village were vaccinated or a few large, vaccinated herds became infected. For those villages where the median effectiveness was around 50%, herds had either never been vaccinated or only vaccinated towards the end of the outbreak in the village (i.e. when no more animals in the village were infectious). Finally, for those villages where the estimated effectiveness was high (posterior median > 75%), vaccinated (and protected) herds were exposed to infection, but most animals did not succumb.
3.4.2. Discussion
Two previous studies have estimated the within‐farm (or village) transmission rate for LSDV and used this to infer R0. One study estimated R0 to be around one (median 1.04; range: 0.90–1.15) for outbreaks in eight cattle herds in Ethiopia in 2014–2015 (Molla et al., 2017b). These estimates are similar to those obtained for villages in Albania in the present study, though the analysis of the Ethiopian data assumed a much shorter duration of infectiousness (around 10 days compared with 23 days here). By contrast, the other study estimated R0 to be around 15.7 for an outbreak on a dairy farm in Israel (Magori‐Cohen et al., 2012). Moreover, because cattle on the farm were removed on the day they showed generalised disease, this implicitly assumed an infectious period of around 1 day.
The number of cattle in the farms or villages considered in all three studies are comparable (Albania: 25–2088; Ethiopia: 46–623 (Molla et al., 2017b); Israel: 610 (Magori‐Cohen et al., 2012)), so herd size is unlikely to account for differences in the magnitude of R0. However, the density of cattle could differ amongst the studies, though no estimates of density are available. The differences in estimates of R0 could reflect differences in the vectors involved in the transmission of LSDV in each country. However, the vector species in Ethiopia are not known (Molla et al., 2017a), while the stable fly, Stomoxys calcitrans, is believed to be an important vector in both Albania (Gubbins et al., 2019) and Israel (Kahana‐Sutin et al., 2017). In our analysis of the Albanian data, we have assumed that cattle within a village mix homogeneously (i.e. they are equally likely to contact any other animal in the village, regardless of which herd they belong to). If animals are more likely to contact other animals in the same herd than animals in other herds in the same village, this would lead to underestimation of R0. Finally, if there is under‐ascertainment of cases, this would result in underestimation of R0.
There was evidence for a reduction in R0 over time, with R0 being lower for villages which reported cases later in the epidemic (Figure 8). Analysis of the spread of LSDV between farms also showed a similar pattern of a reduction in the between‐farm force of infection later in the year, which could be ascribed to a reduction in temperature and its potential impact on the relative abundance of biting insects (Gubbins et al., 2019). Consequently, the reduction in R0 is likely a consequence of seasonality in the transmission rate, potentially related to changes in vector abundance. Indeed, for a vector‐borne disease such as LSDV, the basic reproduction number will vary throughout the year (as well as geographically) in response to changes in environmental factors, such as temperature and rainfall, and their influence on vector abundance and activity.
The high level of uncertainty in the estimates of vaccine effectiveness for most villages (Figure 7e,f) makes their interpretation. Although the 95% credible intervals for around 90% of the villages contain previous estimates for vaccine effectiveness in Albania derived from transmission modelling (76.5%; Gubbins et al., 2019) or survival analysis (62.5%; Klement et al., 2019), this reflects the width of the credible intervals rather than consistency of the estimates.
The high level of uncertainty in the estimates of vaccine effectiveness for most villages (Figure 7e,f) makes their interpretation. Although the 95% credible intervals for around 90% of the villages contain previous estimates for vaccine effectiveness in Albania derived from transmission modelling (76.5%; Gubbins et al., 2019) or survival analysis (62.5%; Klement et al., 2019), this reflects the width of the credible intervals rather than consistency of the estimates.
The basic reproduction number can be used to inform the level of vaccination required to control an outbreak in a village (i.e. to reduce R0 to below one). This can be achieved if the proportion of animals protected (i.e. the product of vaccine coverage and vaccine effectiveness) exceeds the critical vaccination proportion, pc = 1−1/R0 (Keeling and Rohani, 2011). Consequently, if the vaccine effectiveness is less than 100%, the critical level of vaccination coverage required to control an outbreak is given by (1/ε)×(1–1/R0) where ε is the vaccine effectiveness and it is assumed to be strictly greater than zero. The graph showing the relation between the critical vaccination coverage and R0 for different level of vaccine effectiveness is shown in Figure 9.
Figure 9.
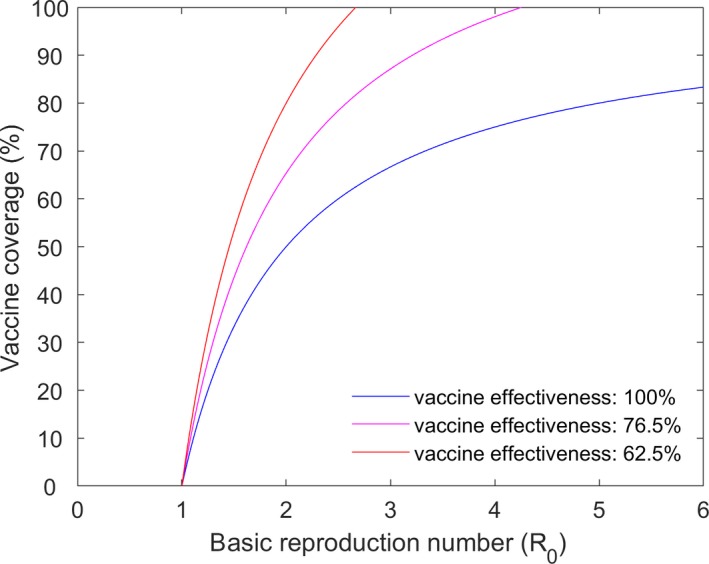
Critical vaccination coverage in relation to different values of R0 and vaccination effectiveness
For example, assuming a vaccine effectiveness of 62.5% (Klement et al., 2019) and using the 95th percentile for each R0, the level of vaccine coverage required to control an outbreak (95% credible interval) varies from 7.3% to 91.7%, with a median of 52.5%. In addition, 37 villages would be protected at any level of coverage (because the 95th percentile for R0 < 1), while 16 villages would not be protected from outbreaks even at 100% coverage (i.e. R0 remains above one because of the vaccine effectiveness).
Figure 10.
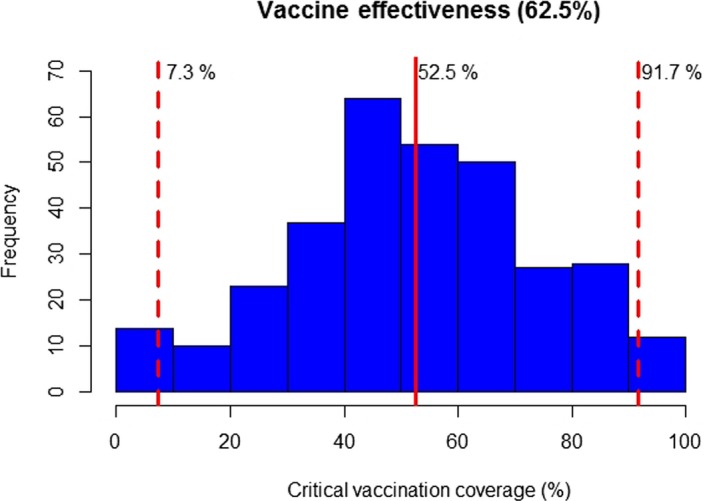
Median and 2.5th and 97.5th percentiles for the critical vaccination coverage based on the 95th percentile for R0 for each village. Villages where R0 < 1 or where R0 is sufficiently high that the critical coverage exceeds 100% are not displayed
Assuming a vaccine effectiveness of 76.5% (Gubbins et al., 2019), the level of vaccine coverage required to control an outbreak (95% credible interval) ranges from 5.9% to 83.8%, with a median of 43.6% (and, for this vaccine effectiveness, six villages would not be protected even at 100% coverage).
4. Conclusions
In 2018, no outbreaks of LSD were reported in the Balkan region, after a decline in the number of outbreaks reported in 2017 (385) compared to 2016 (7,483). This confirms once again the effectiveness of the vaccination campaign based on the LSD homologous strain and the coordinated control measures put in place in the region. The halt of the LSD epidemic achieved in the Balkans in 2018 is in agreement with the long‐term strategic objective of the LSD vaccination exit strategy in the SE Europe, which is to restore the LSD‐free status situation as it was before the occurrence of the LSD epidemics and to stop the LSD vaccination in the region;
In 2018, LSD outbreaks were reported only in Russia (63 outbreaks), Turkey (46 outbreaks) and Georgia (6 outbreaks), all in the period between April and November, thus confirming the seasonal pattern of LSD. Furthermore, seven cases were suspected in Albania and one in Turkish Thrace; however, they resulted negative after epidemiological investigation and laboratory analysis;
Compared to 2017, the LSD epidemics in the Russian Federation expanded northward and eastward along the border with Kazakhstan, while in Turkey, most outbreaks were reported in the Eastern regions, and one outbreak in Thrace close to the border with Greece (18 km).
Since 2013, the presence of LSD disease has been constantly reported in Turkey, with active outbreaks reported until the end of 2018 despite vaccination campaigns based on heterologous vaccine (SGPV vaccine) performed since 2014 with apparent high levels of vaccination coverage of Turkish cattle population. This might support the hypothesis of insufficient levels of protection provided by heterologous vaccines, especially when doses less than 10 times those recommended for sheep are used.
The current epidemiological situation indicated that LSD is still present in some countries neighbouring the EU, thus supporting the recommendations provided by the GF TADs of continuing the vaccination campaign in 2019, at least in Albania (due to the most recent outbreaks reported in autumn 2017), Bulgaria (all or part of), Greece, Kosovo*, Montenegro, Serbia (the southern part), North Macedonia and Turkey.
The vaccination against LSD based on homologous vaccine in SE Europe continued throughout 2018 when over 2.5 million cattle were vaccinated, keeping the mean vaccination coverage above 70% in the whole region throughout 2018 in terms of proportion of immunised animals, indicating a very good level of herd immunity throughout the year and good implementation of the vaccination campaign. At the end of 2018, the vaccination coverage was 22% in Albania, 58% in Kosovo,* 71% in Greece, 80% in Montenegro and over 90% in Bulgaria, Serbia and North Macedonia;
From published literature and from field evidence, it can be concluded that local (at the injection site) or even systemic side effects in some animals may occur after vaccination. However, due to the fact that the reports are based on case series without proper controls, it is not possible to properly estimate the production losses (milk yield, mortality, morbidity, etc.) from these studies. Therefore, there is no consensus on the proportion of animals experiencing such effects and on the real consequences of the side effects on production.
Compared to the large size (over two million doses administered yearly) of the vaccination campaign against LSD based on a live‐attenuated homologous strain put in place in the Balkan region since 2015, a very limited number of side effects have been recorded so far.
The transmission rate and basic reproduction number ( R0) for spread of LSDV within a village were estimated from outbreak data for Albania in 2016. There was a variation amongst villages in R0; across all villages, the posterior median for R0 varied from 0.03 to 3.58, with a median of 0.87. In addition, the posterior median for R0 exceeded one (meaning the disease would spread further) in 35.0% of villages, while 9.9% of villages had an estimate for R0 significantly below one (meaning disease will fade out). There was a significant decline in R0 during the epidemic, which is likely to be a consequence of seasonality in the transmission rate, potentially related to changes in vector abundance.
Based on the 95th percentile for R0 in each village in Albania and assuming a vaccine effectiveness of 62.5% (as estimated previously), the level of vaccine coverage (95% credible intervals) required to control an outbreak varies from 7.3% to 91.7%, with a median of 52.5%.
5. Recommendations
Although, in the Balkan region, a steady decline of outbreaks was already registered in 2017 and no outbreaks at all were reported in 2018, LSD is still present in some countries neighbouring the EU, such as Turkey, thus the recommendation by the GF TADs to continue the vaccination campaign in 2019 in Albania, Bulgaria (all or part of), Kosovo,* Greece, Montenegro, Serbia (southern part), North Macedonia and Turkey is valid.
Due to the outbreak reported in Turkish Thrace in 2018, the use of the homologous LSD vaccine in Turkey, at least in the Thrace region, should be encouraged in order to better protect cattle and prevent from further spread to EU.
As considered in the OIE's TAHC, the lift of the vaccination from a country should be replaced by a surveillance scheme based on clinical, virological and serological surveillance as, for example, implemented in Croatia in 2018, where it could be demonstrated that there is no LSD virus circulation, and thus, the freedom status can be reacquired and the restrictions in relation to LSD vaccination can be lifted.
To quantify production losses due to vaccination, a (retrospective) observational study should be designed using milk yield as outcome variable while taking into account confounding factors and bias. This requires, however, an in‐depth study of a well‐structured database in the naïve cattle population in country where the disease has not been detected, but vaccination has been implemented such as Croatia and/or Bosnia and Herzegovina.
Abbreviations
- ADNS
Animal Disease Notification System
- DIVA
Differentiation of Infected from Vaccinated Animals
- ELISA
enzyme‐linked immunosorbent assay
- GF‐TADs
Global Framework for the progressive control of Transboundary Diseases
- LSD
lumpy skin disease
- MDBK
Madin‐Darby bovine kidney
- PCR
Polymerase Chain Reaction
- QPCR
quantitative polymerase chain reaction
- SEIR
susceptible–exposed–infectious–recovered
- SGE LSD
Standing Group of Experts on Lumpy Skin Disease
- SGPV
sheep and goat pox strain
Suggested citation: EFSA (European Food Safety Authority) , Calistri P, DeClercq K, Gubbins S, Klement E, Stegeman A, Cortiñas Abrahantes J, Antoniou S‐E, Broglia A and Gogin A, 2019. Scientific report on lumpy skin disease: III. Data collection and analysis. EFSA Journal 2019;17(3):5638, 26 pp. 10.2903/j.efsa.2019.5638
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00290
Acknowledgements: EFSA wishes to thank the Animal Health and Welfare Panel members Julio Alvarez, Dominique Bicout, Klaus Depner, Julian Ashley Drewe, Bruno Garin‐Bastuji, Virginie Michel, Søren Nielsen Saxmose, Helen Roberts, Liisa Sihvonen, Karl Ståhl, Jose Luis Gonzales Rojas, Christian Gortázar Schmidt, Miguel Angel Miranda, Hans Spoolder, Arvo Viltrop and Christoph Winckler for reviewing the document. A special thanks is due to Directorate of Deregulation, Permits, Licences and Monitoring, Ministry of Agriculture and Rural Development, Albania; Aleksandra Miteva from Food Safety Agency, Bulgaria; Žaklin Acinger‐Rogić from Ministry of Agriculture, Croatia; Ilektra Fragkou and Chrysoula Dile of the Animal Health Directorate of the Greek Ministry of Rural Development and Food, Greece; Bafti Murati from the Food and Veterinary Agency, Kosovo*; Aleksandar Nemet and Zorana Mehmedbašić, Animal Health and Welfare Department, Veterinary Office of Bosnia Herzegovina; Marko Nikolic and Vesna Dakovic from the Ministry of Agriculture and Rural Development, Montenegro; Vanja Kondratenko and Zoran Atanasov, Food and Veterinary Agency, North Macedonia; Tatjana Labus and Budimir Plavsić from the Ministry of Agriculture and Environmental Protection, Republic of Serbia, for the precious collaboration and timely provision of the epidemiological data.
Approved: 27 February 2019
This designation is without prejudice to positions on status and is in line with UNSCR 1244 and the ICJ Opinion on the Kosovo Declaration of Independence. This footnote applies all the time Kosovo is mentioned in this document.
Notes
The animated map can be downloaded from this http://doi.org/10.5281/zenodo.2583168.
The Seventh meeting (SGE LSD7) of the Standing Group of Experts on Lumpy Skin Disease (SGE LSD) for South‐East Europe under the GF‐TADs umbrella was held in Ohrid, North Macedonia, on 18–19 October 2018. More details at: http://web.oie.int/RR-Europe/eng/Regprog/en_GF_TADS%20-%20Standing%20Group%20LSD.htm
Live‐attenuated vaccines are commercially available against LSD. Homologous vaccine is based on lumpy skin disease virus strain; the most used strain is the Neethling strain from South Africa. Heterologous vaccines are based on other capripox virus strains, mainly sheep and goat pox virus (SGPV strains such as Kenyan SGP O‐240, Yugoslavian RM‐65 and Romanian SPPV strains), which are supposed to confer a certain level of cross protection in cattle, although this is not satisfactory. An increased effectiveness can be achieved when the vaccine is used at 10‐fold dosis than the one used in small ruminants for the control of sheep and goat pox. One new inactivated vaccine has been developed by MCI Sante’ Animale in Morocco, but its efficacy and safety are currently under testing.
The indication of vaccine producers is to revaccinate animals every year, so it is assumed that the immunity duration is 1 year.
Data provided at the last GF TADs meeting held in October 2018, compared to total cattle population as recorded in 2017.
Coordinates were available for around 85% of farms in Bulgaria.
Coordinates available as unique locations is available for 60% of farms, 40% are displayed with coordinates of the centroid of the municipality.
References
- Abutarbush SM, 2014. Efficacy of vaccination against lumpy skin disease in Jordanian cattle. Veterinary Record, 175, 302. [DOI] [PubMed] [Google Scholar]
- Abutarbush SM and Tuppurainen ES, 2018. Serological and clinical evaluation of the Yugoslavian RM 65 sheep pox strain vaccine use in cattle against lumpy skin disease. Transboundary and Emerging Diseases, 65, 1657–1663. [DOI] [PubMed] [Google Scholar]
- Agianniotaki EI, Mathijs E, Vandenbussche F, Tasioudi KE, Haegeman A, Iliadou P, Chaintoutis SC, Dovas CI, Van Borm S, Chondrokouki ED and De Clercq K, 2017. Complete genome sequence of the lumpy skin disease virus isolated from the first reported case in Greece in 2015. Genome Announcements, 5, e00550–00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allepuz A, Casal J and Beltrán‐Alcrudo D, 2018. Spatial analysis of lumpy skin disease in Eurasia—Predicting areas at risk for further spread within the region. Transboundary and Emerging Diseases, 1–10. [DOI] [PubMed] [Google Scholar]
- Andrieu C and Thoms J, 2008. A tutorial on adaptive MCMC. Statistics and Computing, 18, 343–373. [Google Scholar]
- Ayelet G, Abate Y, Sisay T, Nigussie H, Gelaye E, Jemberie S and Asmare K, 2013. Lumpy skin disease: preliminary vaccine efficacy assessment and overview on outbreak impact in dairy cattle at Debre Zeit, central Ethiopia. Antiviral Research, 98, 261–265. [DOI] [PubMed] [Google Scholar]
- Babiuk S, Bowden T, Parkyn G, Dalman B, Manning L, Neufeld J, Embury‐Hyatt C, Copps J and Boyle D, 2008. Quantification of lumpy skin disease virus following experimental infection in cattle. Transboundary and Emerging Diseases, 55, 299–307. [DOI] [PubMed] [Google Scholar]
- Bedekovic T, Simic I, Kresic N and Lojkic I, 2017. Detection of lumpy skin disease virus in skin lesions, blood, nasal swabs and milk following preventive vaccination. Transbound Emerging Disease, 65, 491–496. [DOI] [PubMed] [Google Scholar]
- Ben‐Gera J, Klement E, Khinich E, Stram Y and Shpigel NY, 2015. Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep‐pox live attenuated vaccines for the prevention of lumpy skin disease ‐ The results of a randomized controlled field study. Vaccine, 33, 4837–4842. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Lumpy skin disease: I. Data collection and analysis. EFSA Journal 2017;15(4):4773, 54 pp. 10.2903/j.efsa.2017.4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Scientific report on lumpy skin disease II. Data collection and analysis. EFSA Journal 2018;16(2):5176, 33 pp. 10.2903/j.efsa.2018.5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2015. Scientific Opinion on lumpy skin disease. EFSA Journal 2015;13(1):3986, 73 pp. 10.2903/j.efsa.2015.3986 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2016. Statement: Urgent advice on lumpy skin disease. EFSA Journal 2016;14(8):4573, 27 pp. 10.2903/j.efsa.2016.4573 [DOI] [Google Scholar]
- Gubbins S, Stegeman A, Klement E, Pite L, Broglia A and Cortiñas Abrahantes J, 2019. Inferences about the transmission of lumpy skin disease virus between herds from outbreaks in Albania in 2016. Preventive Veterinary Medicine, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haario H, Saksman E and Tamminen J, 2001. An adaptive Metropolis algorithm. Bernoulli, 7, 223–242. [Google Scholar]
- Hunter P and Wallace D, 2001. Lumpy skin disease in southern Africa: a review of the disease and aspects of control. Journal of the South African Veterinary Association, 72, 68–71. [DOI] [PubMed] [Google Scholar]
- Kahana‐Sutin E, Klement E, Lensky I and Gottlieb Y, 2017. High relative abundance of the stable fly Stomoxys calcitrans is associated with lumpy skin disease outbreaks in Israeli dairy farms. Medical and Veterinary Entomology, 31, 150–160. [DOI] [PubMed] [Google Scholar]
- Katsoulos PD, Chaintoutis SC, Dovas CI, Polizopoulou ZS, Brellou GD, Agianniotaki EI, Tasioudi KE, Chondrokouki E, Papadopoulos O, Karatzias H and Boscos C, 2018. Investigation on the incidence of adverse reactions, viraemia and haematological changes following field immunization of cattle using a live attenuated vaccine against lumpy skin disease. Transboundary and Emerging Diseases, 65, 174–185. [DOI] [PubMed] [Google Scholar]
- Keeling MJ and Rohani P, 2011. Modeling infectious diseases in humans and animals. Princeton University Press, Princeton, USA. [Google Scholar]
- Kitching R, 2003. Vaccines for lumpy skin disease, sheep pox and goat pox. Developments in Biologicals, 114, 161–167. [PubMed] [Google Scholar]
- Klement E, Broglia A, Antoniou S‐E, Tsiamadis V, Plevraki E, Petrović T, Polaček V, Debeljak Z, Miteva A and Alexandrov T, 2019. Neethling vaccine proved highly effective in controlling lumpy skin disease epidemics in the Balkans. Preventive Veterinary Medicine, in press. [DOI] [PubMed] [Google Scholar]
- Lamien CE, Le Goff C, Silber R, Wallace DB, Gulyaz V, Tuppurainen E, Madani H, Caufour P, Adam T and El Harrak M, 2011a. Use of the Capripoxvirus homologue of Vaccinia virus 30 kDa RNA polymerase subunit (RPO30) gene as a novel diagnostic and genotyping target: development of a classical PCR method to differentiate Goat poxvirus from Sheep poxvirus. Veterinary Microbiology, 149, 30–39. [DOI] [PubMed] [Google Scholar]
- Lamien CE, Lelenta M, Goger W, Silber R, Tuppurainen E, Matijevic M, Luckins AG and Diallo A, 2011b. Real time PCR method for simultaneous detection, quantitation and differentiation of capripoxviruses. Journal of Virological Methods, 171, 134–140. [DOI] [PubMed] [Google Scholar]
- Lojkić I, Šimić I, Krešić N and Bedeković T, 2018. Complete Genome Sequence of a Lumpy Skin Disease Virus Strain Isolated from the Skin of a Vaccinated Animal. Genome Announcements, 6, e00482–00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magori‐Cohen R, Louzoun Y, Herziger Y, Oron E, Arazi A, Tuppurainen E, Shpigel NY and Klement E, 2012. Mathematical modelling and evaluation of the different routes of transmission of lumpy skin disease virus. Veterinary Research, 43, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier A, Arsevska E, Bournez L, Bronner A, Calavas D, Cauchard J, Falala S, Caufour P, Tisseuil C and Lefrançois T, 2017. Spread rate of lumpy skin disease in the Balkans, 2015–2016. Transboundary and Emerging Diseases, 65, 240–243. [DOI] [PubMed] [Google Scholar]
- Molla W, de Jong MCM and Frankena K, 2017a. Temporal and spatial distribution of lumpy skin disease outbreaks in Ethiopia in the period 2000 to 2015. BMC Veterinary Research, 13, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla W, Frankena K and De Jong MCM, 2017b. Transmission dynamics of lumpy skin disease in Ethiopia. Epidemiology and Infection, 145, 2856–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M, Best N, Cowles K and Vines K, 2006. CODA: convergence diagnosis and output analysis for MCMC. R News, 6, 7–11. [Google Scholar]
- R Core Team , 2018. R: A language and environment for statistical computing.
- Sevik M, Avci O, Dogan M and Ince OB, 2016. Serum Biochemistry of Lumpy Skin Disease Virus‐Infected Cattle. Biomed Research International, 2016, 6257984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuppurainen ES, Venter E and Coetzer J, 2005. The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort Journal of Veterinary Research, 72, 153–164. [DOI] [PubMed] [Google Scholar]
- Tuppurainen ES, Pearson CR, Bachanek‐Bankowska K, Knowles NJ, Amareen S, Frost L, Henstock MR, Lamien CE, Diallo A and Mertens PP, 2014. Characterization of sheep pox virus vaccine for cattle against lumpy skin disease virus. Antiviral Research, 109, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuppurainen E, Antoniou S, Tsiamadis E, Topkaridou M, Labus T, Debeljak Z, Plavšić B, Miteva A, Alexandrov T and Pite L, 2018. Field observations and experiences gained from the implementation of control measures against lumpy skin disease in South‐East Europe between 2015 and 2017. Preventive Veterinary Medicine, in press. [DOI] [PubMed] [Google Scholar]
- Vidanovic D, Sekler M, Petrovic T, Debeljak Z, Vaskovic N, Matovic K and Hoffmann B, 2016. Real‐time PCR assays for the specific detection of field Balkan strains of lumpy skin disease virus. Acta Veterinaria, 66, 444–454. [Google Scholar]
- Weiss K, 1968. Lumpy skin disease virus. In: Cytomegaloviruses. Rinderpest Virus. Lumpy Skin Disease Virus. Springer, 111–131.
- Yeruham I, Nir O, Braverman Y, Davidson M, Grinstein H, Haymovitch M and Zamir O, 1995. Spread of lumpy skin disease in Israeli dairy herds. Veterinary Record, 137, 91–91. [DOI] [PubMed] [Google Scholar]


