Abstract
The EFSA Panel on Food Additives and Flavourings (FAF) was requested to evaluate 54 flavouring substances attributed to the Flavouring Group Evaluation 05 (FGE.05), using the Procedure as referred to in the Commission Regulation (EC) No 1565/2000. This Revision 3 includes 17 additional substances which have been cleared with respect to genotoxicity in FGE.200Rev1 ([FL‐no: 02.192, 02.231, 05.072, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195, 09.247, 09.400, 09.866, 09.948]) and in FGE.203Rev2 ([FL‐no: 05.081, 05.186, 05.194, 05.196]). The substances were evaluated through a stepwise approach that integrates information on the structure–activity relationships, intake from current uses, toxicological threshold of concern (TTC), and available data on metabolism and toxicity. The Panel concluded that none of the 54 substances gives rise to safety concern at their levels of dietary intake, estimated on the basis of the ‘Maximised Survey‐derived Daily Intake’ (MSDI) approach. Besides the safety assessment of the flavouring substances, the specifications for the materials of commerce have also been considered and found adequate, except for 10 substances ([FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.181, 09.329, 09.335, 09.379 and 09.637]) for which quantitative figures on the composition of stereoisomeric mixtures are missing and for [FL‐no: 09.578] complete specifications should be provided. Normal and maximum use levels were not available for [FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.287, 09.326 and 09.578]. Except for flavouring substances [FL‐no: 05.072, 05.081, 05.186, 05.194, 05.196, 09.934 and 09.942], more reliable intake data should be requested for all the 46 flavouring substances, for which use levels were submitted, as their modified Theoretical Added Maximum Daily Intake (mTAMDI) exposure estimates are above the threshold of concern for structural classes I and II. This would include more reliable intake data and then, if required, additional toxicological data.
Keywords: flavourings; α,β‐unsaturated carbonyls and precursors; FGE.05
1. Introduction
The revision of this flavouring group evaluation concerns the inclusion of 17 α,β‐unsaturated carbonyl substances (or precursors for that) which have been first allocated in FGE.200Rev1 ([FL‐no: 02.192, 02.231, 05.072, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195, 09.247, 09.400, 09.866, 09.948]) and in FGE.203Rev2 ([FL‐no: 05.081, 05.186, 05.194, 05.196]) for their evaluation with respect to genotoxicity. Based on the new genotoxicity data submitted, the Panel concluded that these flavouring substances do not give rise to concern with respect to genotoxicity. According to the Mandates and Terms of Reference of FGE.200Rev1 and FGE.203Rev2, when for a flavouring substance the concern for genotoxicity is ruled out, the European Food Safety Authority (EFSA) proceeds to the full evaluation of these flavouring substances, taking into account the requirements of the Commission Regulation (EC) No 1565/20001 and of Regulation (EU) No 1334/20082.
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background to mandate from FGE.200Rev1 (M‐2018‐0041)
The use flavourings is regulated under Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20123. The list includes a number of flavouring substances for which the safety evaluation should be completed in accordance with Commission Regulation (EC) No 1565/2000.
In February 2011, the EFSA Panel had evaluated a first dossier submitted by Industry in response to the requested data for representative substances in FGE. 200. These data were not considered adequate to alleviate the genotoxicity concern for the substance in subgroup 1.1.1 and the Panel recommended at that time ‘to perform in vivo dietary Comet assays (in drinking water or in feed, not by gavage) for the three linear representatives of subgroup 1.1.1 [FL‐no: 05.073, 05.058 and 05.060]’.
Additional data was submitted in February and June 2013 by Industry related to one representative substance of subgroup 1.1.1, hex‐2(trans)‐enal [FL‐no: 05.073] and two other substances of the group.
On 21 May 2014 the EFSA CEF Panel adopted an opinion on this Flavouring Group Evaluation 200 (FGE.200). The Panel confirmed the need for an in vivo Comet assay performed in duodenum and liver for hex‐2(trans)‐enal [FL‐no: 05.073]. For the two representative substances of subgroup 1.1.1 (nona‐2(trans), 6(cis)‐dienal [FL‐no: 05.058] and oct‐2‐enal [FL‐no: 05.060]), a combined in vivo Comet assay and micronucleus assay would be required and that evidence of bone marrow exposure should be provided.
New data concerning the three representative substances of this group addressing the EFSA opinion have been submitted during 2017. The data also included updated poundage and use levels concerning these substances.
The list of the substances referred to in this letter is included in Annex II.4
1.1.2. Terms of Reference of Mandate from FGE.200Rev1 (M‐2018‐0041)
The European Commission requests the European Food Safety Authority (EFSA) to evaluate the new information submitted and, depending on the outcome, proceed to full evaluation of the substances in this group in accordance with Commission Regulation (EC) No 1565/2000. In accordance with the usual practice by the CEF panel, the first step (assessment of the genotoxicity) should be completed within 9 months. An additional 9 months if necessary is also established for the second step (evaluation through the CEF Procedure).
In case the genotoxic potential cannot be ruled out or the procedure cannot be applied in the first step, EFSA is asked to quantify the exposure.
1.1.3. Background to Mandate from FGE.203Rev2 (M‐2017‐0003)
The use of flavouring is regulated under Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union List of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/2012. The list contains flavouring substances for which the scientific evaluation should be completed taking into account Commission Regulation (EC) No 1565/2000.
The genotoxicity of the twenty substances belonging to the group FGE.203 rev.1; alpha, beta‐unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 were considered in the EFSA opinion of 26 March 2014.
The Authority evaluated the genotoxicity of these substances on the basis of the data on the following two substances selected as representative of the group: the hexa‐2(trans),4(trans)‐dienal (FL‐no: 05.057) and deca‐2(trans),4(trans)‐dienal (FL‐no: 05.140). Overall, the Authority concluded that the safety concern regarding genotoxicity cannot be ruled out for both representative substances of the group and that this conclusion is likewise applicable to the other substances of this FGE.203.
These substances are included in the Union List with no restrictions.
Following this opinion the applicant offered to carry out a number of additional toxicology studies to address the safety concerns raised in the opinion. This set of studies were not requested and not agreed with EFSA or the Commission.
The Commission requested information on poundage and use levels of the substances in order to calculate the exposure and quantify the risks. It also requested information regarding stereoisomerism in particular regarding the substances belonging to this group and not evaluated by JECFA and currently included in the Union List. This information is also attached in the submission.
The studies offered by industry and also the information requested by the Commission were submitted by industry on 22 September 2016.
The Commission submitted for vote at the Standing Committee on Plants, Animals, Food and Feed of the 25 November 2016 a draft Regulation amending the conditions of use of these substances establishing restrictions to the food categories actually in use and also establishing maximum levels for these uses (Ref Doc SANTE 10070/2016). This measure contains the exposure to these substances and also prevents further new uses. The measure was supported by a very substantial qualified majority of the Member States. The measure will continue its usual process of adoption.
1.1.4. Terms of Reference of Mandate from FGE.203Rev2 (M‐2017‐0003)
The European Commission requests the European Food Safety Authority (EFSA) to evaluate the studies in the submission and any new other safety information relevant, and depending on the outcome, proceed to the full evaluation on these flavouring substances, taking into account the requirements of the Commission Regulation (EC) No 1565/2000 and of Regulation (EU) No 1334/2008. The Authority is also asked to characterise the hazards and also quantify the risks also in case its concern on genotoxicity cannot be ruled out and the EFSA CEF panel procedure cannot be applied for any of the substances of the group.
1.2. Additional information
1.2.1. History of the evaluation of the substances in FGE.05
The last Flavouring Group Evaluation 05, Revision 2 (FGE.05Rev2) (EFSA CEF Panel, 2010) involved inclusion of the assessment of eight candidate substances [FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.181, 09.287 and 09.578], additional to the 29 candidate substances which were already considered in FGE.05Rev1 (EFSA AFC Panel, 2008). So, FGE.05Rev2 dealt in total with 37 candidate substances that are branched‐ and straight‐chain unsaturated carboxylic acids and esters of these with aliphatic saturated alcohols from chemical groups 1, 2, 3 and 5.
The revision further involved the reconsideration of a neurotoxicity study on ethyl methacrylate [FL‐no: 09.375], following previously expressed concern by the Panel on the quality of this study. After re‐evaluation of the data available in FGE.05Rev2, the Panel observed that the indications for neurotoxicity after oral exposure to [FL‐no: 09.375] were not confirmed. Thus, the Panel concluded that there is no toxicity data which would preclude the evaluation of ethyl methacrylate [FL‐no: 09.375] (and the other two methacrylate candidate substances [FL‐no: 09.647 and 09.586] in this group) via the A‐side of the Procedure.
Genotoxicity data were available only for a limited number of substances in FGE.05Rev2. However, the data available did not preclude the evaluation of the candidate substances using the Procedure.
All candidate substances in FGE.05Rev2, including the methacrylate esters, in this FGE were anticipated to be metabolised to innocuous products.
It was considered that on the basis of the default maximised survey‐derived daily intake (MSDI) approach none of the 37 candidate substances would give rise to safety concern at the estimated levels of intake arising from their use as flavouring substances. In addition, on the basis of the reported annual production volumes in Europe (MSDI approach), the combined intake of the 37 candidate substances would result in a total intake lower than the thresholds of toxicological concern (TTC) for structural class I and class II (1.800 μg/person per day and 540 μg/person per day, respectively), to which the substances belong to. The total combined intake of candidate and supporting substances in Europe exceeds the TTC I and TTC II. However, for one of the supporting substances [FL‐no: 02.056], a no observed adverse effect level (NOAEL) exists, which provides an adequate margin of safety. Therefore, based on the limited data available, the total combined intake was not considered to be of safety concern.
When the estimated intakes of the 37 candidate substances were based on the modified Theoretical Added Maximum Daily Intake (mTAMDI) approach, except for two substances [FL‐no: 09.934 and 09.942], these intakes were above the threshold of concern for the corresponding structural classes of flavouring substances (I and II). For eight flavouring substances [FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.287, 09.326 and 09.578], use levels were missing. Therefore, for 35 candidate substances, more reliable exposure data were required. On the basis of such additional data, these flavouring substances should be reconsidered using the Procedure. Subsequently, additional data might become necessary.
Adequate specifications including complete purity criteria, information on identity and identity for the materials of commerce were provided for 25 of the 37 candidate substances. Therefore, the final evaluation of the materials of commerce could not be performed for 12 of the 37 flavouring substances ([FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.181, 09.287, 09.329, 09.335, 09.379, 09.578 and 09.637]), pending further information on geometrical isomerism and specifications.
The 37 flavouring substances that have been considered in FGE.05Rev2 will not be readdressed in the current revision, unless additional information is provided or when data gaps are identified (e.g. on production volumes, use levels or specifications).
The present revision of FGE.05 (FGE.05Rev3) concerns the assessment of 17 additional flavouring substances [FL‐no: 02.192, 02.231, 05.072, 05.081, 05.144, 05.184, 05.186, 05.189, 05.190, 05.191, 05.194, 05.195, 05.196, 09.247, 09.400, 09.866 and 09.948] included in this flavouring group. In FGE.19, a concern for genotoxicity for these flavouring substances was identified based on the presence of a structural alert (i.e. α,β‐unsaturated carbonyl or precursor for that), thus preventing their evaluation through the Procedure (Appendix A). Because of this, these 17 substances needed further attention in FGE.200 or FGE.203. Based on the genotoxicity data submitted, these candidate substances were considered of no genotoxic concern in FGE.200Rev1 (EFSA FAF Panel, 2018) and FGE.203Rev2 (EFSA CEF Panel, 2018), and therefore, they can be evaluated in the present revision of this FGE (FGE.05Rev3) using the Procedure.
Taken together with the 37 flavouring substances, which were already considered in FGE.05Rev2, the current revision comprises altogether 54 substances. However, the 25 substances for which the evaluation was finalised in FGE.05Rev2 will not further be discussed. For the sake of completion their information is maintained in the various tables in this FGE. When new information on specifications for the previously considered substances is available, this will be included and reflected in the conclusions of the materials of commerce. Information on the exposure for the 37 substances in FGE.05Rev2 will be taken into account in the assessment of the combined exposure.
| FGE | Adopted by EFSA | Link | No. of Substances |
|---|---|---|---|
| FGE.05 | 23 February 2005 | https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2005.204 | 24 |
| FGE.05Rev1 | 27 September 2007 | https://www.efsa.europa.eu/en/efsajournal/pub/643 | 29 |
| FGE.05Rev2 | 26 November 2009 | https://www.efsa.europa.eu/en/efsajournal/pub/1400 | 37 |
| FGE.05Rev3 | https://www.efsa.europa.eu/en/efsajournal/pub/5761 | 54 |
FGE: Flavouring Group Evaluation.
2. Data and methodologies
2.1. Data
The present revision of the opinion is based on the following data as provided by the applicant:
-
–
Updated specifications and information on isomerism submitted for four flavouring substances [FL‐no: 05.081, 05.186, 05.194, 05.196] in the context of FGE.203Rev2 application (Documentation provided to EFSA n.4).
-
–
Clarification of specifications and isomerism for flavouring substances [FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.181, 09.287, 09.329, 09.335, 09.379, 09.637] submitted by the applicant following EFSA request in FGE.05Rev2 (Documentation provide to EFSA n.7).
-
–
Assay values submitted for flavouring substances [FL‐no: 05.081 and 05.190] (Documentation provided to EFSA n.6).
-
–
Additional information on specifications and/or composition of the stereoisomeric mixtures for flavouring substances [FL‐no: 05.144, 05.189, 05.191, 02.192, 05.081] submitted by the applicant during the assessment process in response to requests from EFSA sent on 02 April 2019 and on 29 May 2019 (Documentation provided to EFSA n. 8 and 21).
-
–
Poundage data and use levels submitted for 13 newly added flavouring substances [FL‐no. 02.192, 02.231, 05.072, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195, 09.247, 09.400, 09.866 and 09.948] in the context of FGE.200Rev1 application (Documentation provided to EFSA n.1 and 2).
-
–
Poundage data and use levels submitted for 4 newly added flavouring substances [FL‐no: 05.081, 05.186, 05.194, 05.196] in the context of FGE.203Rev2 application (Documentation provided to EFSA n.3).
-
–
Absorption, distribution, metabolism and exposure (ADME) data submitted for thirteen flavouring substances [FL‐no. 02.192, 02.231, 05.072, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195, 09.247, 09.400, 09.866 and 09.948] (Documentation provided to EFSA n.5).
-
–
Genotoxicity data evaluated in FGE.200 and FGE.200Rev1, FGE.203, FGE.203Rev1 and FGE.203Rev2 [Refer to documentation provided to EFSA as reported in FGE.200 (EFSA CEF Panel, 2014a), FGE.200Rev1 (EFSA FAF Panel, 2018), FGE.203 (EFSA CEF Panel, 2009), FGE.203Rev1 (EFSA CEF Panel, 2014b) and FGE.203Rev2 (EFSA CEF Panel, 2018)].
The table below summarises all the data provided to EFSA for FGE.05Rev3:
| FL‐no | Chemical name | Data provided for the current revision 3 of FGE.05 | Appendix (Table) and relevant section of the opinion |
|---|---|---|---|
| 02.192 | Oct‐2‐en‐1‐ol | Specifications, EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 02.231 | trans‐2, cis‐6‐Nonadien‐1‐ol | EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 05.072 | trans‐2‐Nonenal | EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 05.081 | 2,4‐Decadienal | Specifications, EU poundage data (MSDI), use levels (mTAMDI) | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5) |
| 05.144 | Dodec‐2(trans)‐enal | Specifications, EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 05.184 | Undec‐2(trans)‐enal | Specifications, EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 05.186 | 2,4‐Octadienal | Specifications, EU poundage data (MSDI), use levels (mTAMDI) | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5) |
| 05.189 | 2‐Hexenal | Specifications, EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 05.190 | trans‐2‐Octenal | Specifications, EU poundage data (MSDI), use levels (mTAMDI); ADME data | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 05.191 | trans‐2‐Decenal | Specifications, EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 05.194 | tr‐2, tr‐4‐Nonadienal | Specifications, EU poundage data (MSDI), use levels (mTAMDI) | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5) |
| 05.195 | trans‐2‐Tridecenal | Specifications, EU poundage data (MSDI), use levels (mTAMDI); ADME data | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 05.196 | tr‐2, tr‐4‐Undecadienal | Specifications, EU poundage data (MSDI), use levels (mTAMDI) | Appendix B (Table B.1); Appendix D (Tables D.2 and D.5) |
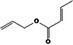
| 09.247 | Allyl crotonate | EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 09.400 | Hex‐2‐enyl phenylacetate | EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 09.866 | Allyl valerate | EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 09.948 | (2E)‐2‐Nonenyl acetate | EU poundage data (MSDI), use levels (mTAMDI), ADME data | Appendix D (Tables D.2 and D.5); Section 3.3.1 |
| 09.329 | Butyl hex‐2‐enoate | Specifications | Appendix B (Table B.1) |
| 08.083 | Hept‐2‐enoic acid | ||
| 08.101 | Non‐2‐enoic acid | ||
| 08.119 | 2‐Hexenoic acid | ||
| 08.120 | 2‐Methyl‐2‐butenoic acid | ||
| 09.181 | Methyl hex‐2‐enoate | ||
| 08.072 | But‐2‐enoic acid (cis and trans) | ||
| 09.287 | Propyl deca‐2,4‐dienoate | ||
| 09.335 | Butyl oct‐2‐enoate | ||
| 09.379 | Ethyl pent‐2‐enoate | ||
| 09.637 | Methyl dec‐2‐enoate |
MSDI: maximised survey‐derived daily intake; mTAMDI: modified Theoretical Added Maximum Daily Intake; ADME: absorption, distribution, metabolism and exposure.
Table B.1.
Specifications summary of the substances in the Flavouring Group Evaluation 5, Revision 3
| Information included in the EU Union List Regulation No. (EU) 1334/2008 as amended | Most recent available specifications dataa | EFSA Comments | |||||
|---|---|---|---|---|---|---|---|
| FL‐no JECFA‐no FEMA no CoE no CAS no | Chemical name | Purity of the named compound | Phys. form Mol. formula Mol. weight | Solubilityc Solubility in ethanold | Boiling point, °Ce Melting point, °C ID test Assay minimum isomers distribution/SCh | Refrac. Indexf Spec. gravityg | |
|
02.192 3887 11804 22104‐78‐5 |
Oct‐2‐en‐1‐ol | b |
Liquid C8H16O 128 |
Insoluble Soluble |
88 (hPa) MS 96% min 95% (2E)‐Oct‐2‐en‐1‐ol 0–3% (2Z)‐ Oct‐2‐en‐1‐ol |
1.4371–1.4571 0.8384–0.8584 |
|
|
02.231 2780 589 28069‐72‐9 |
trans‐2, cis‐6‐Nonadien‐1‐ol | b |
Liquid C9H16O 140.23 |
Insoluble Soluble |
196 MS 95% |
1.463–1.465 0.860–0.880 |
|
|
05.072 3213 733 18829‐56‐6 |
trans‐2‐Nonenal | At least 92%; secondary component 3–4% 2‐nonenoic acid |
Liquid C9H16O 140.22 |
Practically insoluble or insoluble Freely soluble |
90 (1.6 hPa) IR MS 92% |
1.454–1.460 0.855–0.865 |
|
|
05.081 3135 2120 2363‐88‐4 |
2,4‐Decadienal | At least 89%; secondary components: mixture of the (cis, cis)‐; (cis, trans)‐ and (trans, cis)‐ 2,4‐decadienals (sum of all isomers 95%); acetone and isopropanol |
Liquid C10H16O 152.24 |
Insoluble Soluble |
104 MS 89% min. 89% (2E,4E)‐isomer 1–9% (2Z,4E); 0–5% (2E,4Z); 0–2% (2Z,4Z) |
1.512–1.517 0.866–0.876 |
|
|
05.144 2402 20407‐84‐5 |
Dodec‐2(trans)‐enal | At least 93%; secondary component 2–3% 2‐dodecenoic acid |
Liquid C12H22O 182.30 |
Practically insoluble or insoluble Freely Soluble |
272 MS 93% |
1.452–1.458 0.839–0.849 |
|
|
05.184 3423 11827 53448‐07‐0 |
Undec‐2(trans)‐enal | b |
Liquid C11H20O 168.27 |
Insoluble Soluble |
115 (1.3 hPa) MS 98% |
1.452–1.459 0.837–0.847 |
|
|
05.186 3721 11805 5577‐44‐6 |
2,4‐Octadienal | b |
Liquid C8H12O 124.18 |
Insoluble Soluble |
106 (1.1 hPa) MS 95% Up to 85% E,E‐isomer with 10% E,Z‐isomer |
1.519–1.525 0.832–0.839 |
|
|
05.189 748 505‐57‐7 |
2‐Hexenal | At least 92%; secondary component 3–4% 2‐hexenoic acid |
Liquid C6H10O 98.14 |
Very slightly soluble Freely Soluble |
47 (22.7 hPa) MS 92% 70–95% E‐isomer; 5–30% Z‐isomer |
1.443–1.449 0.841–0.848 |
|
|
05.190 3215 2548‐87‐0 |
trans‐2‐Octenal |
At least 92%; secondary components 3–4% 2‐octenoic acid and ethyl octanoate |
Liquid C8H14O 126.2 |
Soluble Soluble |
96 (2.5 hPa) MS 92% SC: 3–4% 2‐octenoic acid and ethyl octanoate |
1.449–1.455 0.835–0.845 |
|
|
05.191 2366 3913‐81‐3 |
trans‐2‐Decenal | At least 92%; secondary component 3‐4% 2‐decenoic acid |
Liquid C10H18O 154.25 |
Practically insoluble or insoluble Freely soluble |
221,95 −8,92 MS 92% SC: 3–4% 2‐decenoic acid |
1.452–1.458 0.836–0.846 |
|
|
05.194 3212 732 5910‐87‐2 |
tr‐2, tr‐4‐Nonadienal | At least 89%; secondary components at least 5% 2,4‐nonadien‐1‐ol and 2‐nonen‐1‐ol and other isomers of 2,4‐nonadienal |
Liquid C9H14O 138.21 |
Insoluble Soluble |
97 (1.3 hPa) MS 89% SC: at least 5% 2,4‐nonadien‐1‐ol and 2‐nonen‐1‐ol and other isomers of 2,4‐nonadienal |
1.522–1.525 0.850–0.870 |
The chemical name should be changed in (2E,4E)‐nonadienal |
|
05.195 3082 7069‐41‐2 |
trans‐2‐Tridecenal | At least 92%; secondary components 2–5% 2‐tridecenoic acid and 3‐5% cis‐2‐tridecenal |
Liquid C13H24O 196.33 |
Insoluble Soluble |
117 (1.3 hPa) MS 92% SC: 2–5% 2‐tridecenoic acid and 3–5% cis‐2‐tridecenal |
1.455–1.462 0.842–0.862 |
|
|
05.196 3422 10385 30361‐29‐6 |
tr‐2, tr‐4‐Undecadienal | b |
Liquid C11H18O 166.26 |
Practically insoluble or insoluble Freely soluble |
129 (1.73 hPa) NMR MS 95% |
1.500–1.505 0.896–0.906 |
The chemical name should be changed in (2E,4E)‐undecadienal |
|
08.072 3908 10080 3724‐65‐0 |
But‐2‐enoic acid (cis and trans) | b |
Solid C4H6O2 86.09 |
Slightly soluble Soluble |
189 70–73 MS 99% |
n.a. n.a. |
Composition of stereoisomeric mixture to be specified. EU Union List chemical name to be changed to (E,Z)‐But‐2‐enoic acid |
|
08.083 3277 10102 18999‐28‐5 |
Hept‐2‐enoic acid | b |
Liquid C7H12O2 128.16 |
Soluble Soluble |
228 MS 97% Mixture of (Z)‐ or (E)‐isomers |
1.447–1.457 0.968–0.978 |
Composition of stereoisomeric mixture to be specified. EU Union List chemical name to be changed to (E,Z)‐Hept‐2‐enoic acid |
|
08.101 3954 10153 3760‐11‐0 |
Non‐2‐enoic acid | b |
Liquid C9H16O2 156.22 |
Slightly soluble Soluble |
132 MS 97% Mixture of (Z)‐ or (E)‐isomers |
1.456–1.464 0.930–0.940 |
Composition of stereoisomeric mixture to be specified. EU Union List chemical name to be changed to (E,Z)‐Non‐2‐enoic acid |
|
08.119 3169 11777 1191‐04‐4 |
2‐Hexenoic acid | b |
Solid C6H10O2 114.14 |
Slightly soluble Soluble |
36 MS 97% Mixture of (Z)‐ or (E)‐isomers |
n.a. n.a. |
Composition of stereoisomeric mixture to be specified. EU Union List chemical name to be changed to (E,Z)‐Non‐2‐enoic acid |
|
08.120 3599 10168 13201‐46‐2 |
2‐Methyl‐2‐butenoic acid | b |
Solid C5H8O2 100.11 |
Slightly soluble Soluble |
67 MS 99% Mixture of (Z)‐ or (E)‐isomers |
n.a. n.a. |
Composition of stereoisomeric mixture to be specified. EU Union List chemical name to be changed to (E,Z)‐Methyl‐2‐butenoic acid |
|
09.181 2709 583 2396‐77‐2 |
Methyl hex‐2‐enoate | b |
Liquid C7H12O2 128.17 |
Practically insoluble or insoluble Soluble |
169 NMR 95% Mixture of (Z)‐ or (E)‐isomers |
1.432–1.438 0.911–0.916 |
Composition of stereoisomeric mixture to be specified. EU Union List chemical name to be changed to (E,Z)‐Methyl hex‐2‐enoate |
|
09.247 4072 2222 20474‐93‐5 |
Allyl crotonate | b |
Liquid C7H10O2 126.15 |
Freely soluble |
146 MS 95% |
0.932–0.937 | |
|
09.248 3486 2244 623‐70‐1 |
Ethyl trans‐2‐butenoate | b |
Liquid C6H10O2 114.14 |
Practically insoluble or insoluble Freely soluble |
143 MS 98% |
1.423–1.427 0.914–0.918 |
|
|
09.266 1807 3354 10688 19089‐92‐0 |
Hexyl 2‐butenoate | b |
Liquid C10H18O2 170.25 |
Practically insoluble or insoluble Freely soluble |
97 (2 hPa) NMR 95% only E‐isomer |
1.428–1.449 0.880–0.895 |
|
|
09.287 3648 10889 28316‐62‐3 |
Propyl deca‐2,4‐dienoate | b |
C13H22O2 210.32 |
[FL‐no: 09.287] should be deleted from the UL as this substance is covered by [FL‐no: 09.840] (Documentation provided to EFSA n.7), provided that the compositions of the mixtures of stereoisomers for these two substances are the same | |||
|
09.321 7785‐64‐0 |
Butyl 2‐methylbut‐2(cis)‐enoate | b |
Liquid C9H16O2 156.22 |
Insoluble Freely soluble |
74 (12 hPa) MS 95% |
1.432–1.438 0.906–0.912 |
|
|
09.324 591‐63‐9 |
Butyl but‐(2E)‐enoate | b |
Liquid C8H14O2 142.2 |
Insoluble Freely soluble |
80 (56 hPa) MS 98% |
1.425–1.435 0.901–0.909 |
|
|
09.326 10529 28369‐24‐6 |
Butyl deca‐(2E,4Z)‐dienoate | b |
Liquid C14H24O2 224.34 |
Practically insoluble or insoluble Freely soluble |
69 (0.001 hPa) MS 95% |
1.480–1.486 0.893–0.899 |
|
|
09.329 13416‐74‐5 |
Butyl hex‐2‐enoate | b |
Liquid C10H18O2 170.25 |
Insoluble Freely soluble |
217 MS 95% Mixture of (Z)‐ or (E)‐isomers |
1.439–1.445 0.890–0.895 |
Composition of stereoisomeric mixture to be specified EU Union List chemical name to be changed to (E,Z)‐Butyl hex‐2‐enoate |
|
09.330 118869‐62‐8 |
Butyl hex‐(3E)‐enoate | b |
Liquid C10H18O2 170.25 |
Insoluble Freely soluble |
217 MS 95% |
1.438–1.444 0.890–0.895 |
|
|
09.335 10536 57403‐32‐4 |
Butyl oct‐2‐enoate | b |
Liquid C12H22O2 198.25 |
Insoluble Freely soluble |
253 NMR 95% Mixture of (Z)‐ or (E)‐isomers |
1.450–1.456 0.884–0.889 |
Composition of stereoisomeric mixture to be specified EU Union List chemical name to be changed to (E,Z)‐Butyl oct‐2‐enoate |
|
09.365 10610 638‐10‐8 |
Ethyl 3‐methylcrotonate | b |
Liquid C7H12O2 128.17 |
Insoluble Freely soluble |
150 MS 95% |
1.434–1.441 0.917–0.923 |
|
|
09.370 10579 67233‐91‐4 |
Ethyl dec‐9‐enoate | b |
Liquid C12H22O2 198.31 |
Insoluble Freely soluble |
135 (37 hPa) MS 95% |
1.434–1.440 0.874–0.879 |
|
|
09.372 10584 28290‐90‐6 |
Ethyl dodec‐(2E)‐enoate | b |
Liquid C14H26O2 226.36 |
Insoluble Freely soluble |
144 (20 hPa) MS 95% |
1.436–1.442 0.864–0.870 |
|
|
09.374 54340‐72‐6 |
Ethyl hept‐(2E)‐enoate | b |
Liquid C9H16O2 156.22 |
Insoluble Freely soluble |
80 (27 hPa) MS 95% |
1.435–1.441 0.885–0.891 |
|
|
09.375 97‐63‐2 |
Ethyl methacrylate | b |
Liquid C6H10O2 114.14 |
Insoluble Freely soluble |
117 MS 95% |
1.410–1.416 0.910–0.916 |
|
|
09.379 10623 2445‐93‐4 |
Ethyl pent‐2‐enoate | b |
Liquid C7H12O2 128.17 |
Insoluble Freely soluble |
157 MS 95% Mixture of (Z)‐ or (E)‐isomers. |
1.428–1.434 0.904–0.910 |
Composition of stereoisomeric mixture to be specified. EU Union List chemical name to be changed to (E,Z)‐Ethyl pent‐2‐enoate |
|
09.578 3354 10688 1617‐25‐0 |
Hexyl (E)‐but‐2‐enoate | b |
C10H18O2 170.25 |
MS | Complete specifications should be requested for this flavouring substance | ||
|
09.400 68133‐78‐8 |
Hex‐2‐enyl phenylacetate | b |
Solid C14H18O2 218.29 |
Practically insoluble or insoluble Freely soluble |
336 37 NMR 95% |
n.a. n.a. |
|
|
09.586 97‐86‐9 |
Isobutyl 2‐methylprop‐2‐enoate | b |
Liquid C8H14O2 142.20 |
Insoluble Freely soluble |
155 MS 95% |
1.409–1.415 0.882–0.888 |
|
|
09.596 10482‐55‐0 |
Isopentyl‐(Z)‐but‐2‐enoate | b |
Liquid C10H18O2 170.25 |
Insoluble Freely soluble |
202 MS 95% |
1.437–1.442 0.889–0.894 |
|
|
09.603 10729 6284‐46‐4 |
Isopropyl crotonate | b |
Liquid C7H12O2 128.17 |
Practically insoluble or insoluble Freely soluble |
146 MS 95% |
1.419–1.425 0.889–0.895 |
|
|
09.624 6622‐76‐0 |
Methyl 2‐methylcrotonate | b |
Liquid C6H10O2 114.14 |
Insoluble Freely soluble |
137 MS 95% |
1.430–1.436 0.938–0.944 |
|
|
09.625 33603‐30‐4 |
Methyl 2‐methylpent‐3(E)‐enoate | b |
Liquid C7H12O2 128.17 |
Insoluble Freely soluble |
142 NMR 95% Racemate |
1.415–1.421 0.902–0.907 |
|
|
09.636 623‐43‐8 |
Methyl crotonate | b |
Liquid C5H8O2 100.12 |
Slightly soluble Freely soluble |
119 MS 98% |
1.424–1.427 0.977–0.983 |
|
|
09.637 11799 2482‐39‐5 |
Methyl dec‐2‐enoate | b |
Liquid C11H20O2 184.28 |
Insoluble Freely soluble |
123 (21 hPa) MS 95% Mixture of (Z)‐ or (E)‐isomers. |
1.442–1.448 0.887–0.892 |
Composition of stereoisomeric mixture to be specified. EU Union List chemical name to be changed to (E,Z)‐Methyl dec‐2‐enoate |
|
09.641 10792 6208‐91‐9 |
Methyl dodec‐(2E)‐enoate | b |
Liquid C13H24O2 212.33 |
Insoluble Freely soluble |
151 (20 hPa) MS 95% |
1.445–1.451 0.881–0.886 |
|
|
09.647 1834 4002 80‐62‐6 |
Methyl methacrylate | b |
Liquid C5H8O2 100.12 |
Insoluble Freely soluble |
100 MS 95% |
1.409–1.415 0.933–0.939 |
|
|
09.652 10836 112‐62‐9 |
Methyl oleate | b |
Liquid C19H36O2 296.54 |
Insoluble Freely soluble |
160 (4 hPa) MS 95% |
1.448–1.454 0.876–0.882 |
|
|
09.680 7785‐63‐9 |
Pentyl 2‐methylisocrotonate | b |
Liquid C10H18O2 170.25 |
Insoluble Freely soluble |
213 MS 95% |
1.439–1.445 0.891–0.896 |
|
|
09.699 10352‐87‐1 |
Propyl crotonate | b |
Liquid C7H12O2 128.17 |
Insoluble Freely soluble |
158 MS 98% |
1.425–1.431 0.903–0.909 |
|
|
09.865 20290‐84‐0 |
Hexyl (9Z)‐octadecenoate | b |
Liquid C24H46O2 366.63 |
Insoluble Freely soluble |
207 (7 hPa) MS 95% |
1.454–1.460 0.866‐0.872 |
|
|
09.866 4074 6321‐45‐5 |
Allyl valerate | b |
Liquid C8H14O2 142.20 |
Freely soluble |
58 (16 hPa) MS 95% |
0.999–1.005 | |
|
09.934 1630 4165 41654‐15‐3 |
Methyl (5Z)‐Octenoate | b |
Liquid C9H16O2 156.20 |
Very slightly soluble Freely soluble |
187 (97.5 hPa) IR NMR MS 95.1% |
1.438–1.432 0.921–0.925 |
|
|
09.942 4306 97890‐13‐6 |
2‐Methylbutyl‐3‐methyl‐2‐butenoate | b |
Liquid C10H18O2 170.25 |
Practically insoluble or soluble Freely soluble |
58(4.7 hPa) NMR 98% Racemate |
1.451–1.461 0.881–0.891 |
|
|
09.948 4552 30418‐89‐4 |
(2E)‐2‐Nonenyl acetate | b |
Liquid C11H20O2 184.79 |
Sparingly soluble Very soluble |
228 IR NMR MS 98% |
1.4325–1.4425 0.874–0.894 |
|
FL‐no: FLAVIS number; CAS: Chemical Abstract Service; CoE: Council of Europe; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavor and Extract Manufacturers Association; ID: Identify; MS: mass spectrometry; IR: infrared; NMR: nuclear magnetic resonance.
Documentation provided to EFSA n. 4, 5, 6, 7, 8, 10, 21, 23.
At least 95% unless otherwise specified.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1,013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise state.
SC: secondary components.
Table D.2.
Normal and maximum use levels (mg/kg) of the candidate substances in FGE.05Rev3 in food categories listed in Annex III of Reg. (EC) 1565/200 The ‘normal and maximum use levels’ have been provided by industry for 46 out of the 54 candidate substances in the present revision of FGE.05 (FGE.05Rev3). For eight flavouring substances ([FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.287, 09.326 and 09.578]) use levels are missing
| FL‐no | Food categories | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal use levels (mg/kg)a Maximum use levels (mg/kg) | ||||||||||||||||||
| 01.0 | 02.0 | 03.0 | 04.1 | 04.2 | 05.0 | 06.0 | 07.0 | 08.0 | 09.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.1 | 14.2 | 15.0 | 16.0 | |
| 02.192 | 5.7 | 1.5 | 0.9 | – | 5 | 5.5 | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | 14.46 | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 02.231 | 5.7 | 1.5 | 0.9 | – | 5 | 5.5 | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | 14.46 | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 05.072 | 1.66 | – | – | – | – | 1.39 | 1.66 | 2.45 | 1.7 | – | – | – | 0.1 | – | 1.14 | 0 | – | – |
| 2.23 | – | – | – | – | 2.09 | 2.23 | 3.35 | 3.51 | – | – | – | 0.5 | – | 1.92 | 0 | – | – | |
| 05.081 | 1.5 | 0.5 | 0.5 | – | 1 | 1 | 1 | 1 | 2 | 0.5 | 0.01 | 2.5 | 3 | – | 0.2 | 0.06 | 5 | 0.5 |
| 5 | 1.5 | – | 5 | 5 | 5 | 5 | 10 | 3 | 1 | 7.5 | 10 | – | 1 | 1 | 20 | 1.5 | ||
| 05.144 | 5.7 | 1.5 | 0.9 | – | 5 | 5.5 | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | 14.46 | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 05.184 | 5.7 | 1.5 | 0.9 | – | 5 | 5.5 | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | 14.46 | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 05.186 | 0.01 | – | 0.3 | – | – | 5.5 | 0.2 | 0.5 | 0.5 | – | – | – | 0.5 | – | 0.25 | 0 | 0.051 | 0.5 |
| 1 | – | 1 | – | – | 10 | 5 | 2 | 2 | – | – | – | 2 | – | 3 | 0 | 1 | 2 | |
| 05.189 | 5.7 | 1.5 | 0.9 | – | 5 | 5.5 | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | 14.46 | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 05.190 | 5.7 | 1.5 | 0.9 | – | 5 | 5.5 | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | 14.46 | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 05.191 | 5.7 | 1.5 | 0.9 | – | 5 | – | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | – | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 05.194 | 0.5 | 0.5 | 0.01 | – | 0.01 | 1 | 0.051 | 1 | 3 | 2 | 0.01 | 0.01 | 3 | – | 0.05 | 0.02 | 3 | 0.01 |
| 1.5 | 5 | 1 | – | 1 | 5 | 1 | 5 | 5 | 5 | 1 | 1 | 10 | – | 1 | 1 | 5 | 1 | |
| 05.195 | 5.7 | 1.5 | 0.9 | – | 5 | 5.5 | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | 14.46 | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 05.196 | 0.1 | 0.5 | 0.01 | – | 0.01 | 0.1 | 0.03 | 0.5 | 0.5 | 0.5 | 0.01 | 0.01 | 0.3 | – | 0.01 | 0.02 | 0.5 | 0.1 |
| 1 | 5 | 1 | – | 1 | 1 | 1 | 5 | 3 | 3 | 1 | 1 | 1 | – | 1 | 1 | 3 | 1 | |
| 09.247 | 5.7 | 1.5 | 0.9 | – | 5 | 5.5 | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | 14.46 | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 09.400 | 4.1 | 3.3 | 3.3 | – | 3.3 | 6.17 | 3.3 | 6.8 | 3.3 | 3.3 | 3.3 | 3.3 | 1 | – | 1.75 | 1.32 | 3.3 | 3.3 |
| 7.77 | 5.95 | 5.95 | – | 5.95 | 9.81 | 5.95 | 9.71 | 5.95 | 5.95 | 5.95 | 5.95 | 3 | – | 2.69 | 2.17 | 5.95 | 5.95 | |
| 09.948 | 5.7 | 1.5 | 0.9 | – | 5 | – | 4.8 | 8 | 0.9 | 0.9 | – | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | – | 11.55 | 19.07 | 2.98 | 2.98 | – | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 09.866 | 5.7 | 1.5 | 0.9 | – | 5 | 5.5 | 4.8 | 8 | 0.9 | 0.9 | 0.9 | 0.9 | 2 | – | 2 | 1 | 2.5 | 0.9 |
| 12 | 14.25 | 2.98 | – | 5.03 | 14.46 | 11.55 | 19.07 | 2.98 | 2.98 | 2.98 | 2.98 | 5 | – | 4.43 | 2 | 4.5 | 2.98 | |
| 09.181 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.248 | 6 | 0 | 3 | – | – | 60 | 1.2 | 2.4 | 1 | 1 | – | – | 1 | – | 20 | 30 | 1 | – |
| 8 | 1 | 8 | – | – | 100 | 7 | 6 | 2 | 5 | – | – | 2 | – | 50 | 100 | 10 | – | |
| 09.266 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.321 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.324 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.329 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.330 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.335 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.365 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.370 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.372 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.374 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.375 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.379 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.586 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.596 | 7 | 5 | 10 | 7 | – | 10 | 5 | 1 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.603 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.624 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.625 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.636 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.637 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.641 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.647 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 5 | 5 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 25 | 25 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.652 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 5 | 5 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 25 | 25 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.680 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.699 | 7 | 5 | 1 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | 5 | 10 | 5 | 10 | 20 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | 25 | 50 | 25 | 50 | 100 | 25 | |
| 09.865 | 7 | 5 | 10 | 7 | – | 10 | 5 | 10 | 2 | 2 | – | – | – | – | 5 | 10 | 10 | 5 |
| 35 | 25 | 50 | 35 | – | 50 | 25 | 50 | 10 | 10 | – | – | – | – | 25 | 50 | 50 | 25 | |
| 09.934 | 1 | 2 | 1 | 0.5 | 0.5 | 1 | 2 | 5 | 5 | 5 | – | – | 1 | 1 | 0.2 | 0.2 | 2 | 1 |
| 3 | 5 | 2 | 1 | 1 | 3 | 5 | 10 | 10 | 10 | – | – | 3 | 3 | 2 | 2 | 5 | 5 | |
| 09.942 | 2 | – | 5 | 4 | 4 | 5 | 5 | – | – | – | – | – | 5 | – | 2 | 4 | – | – |
| 5 | – | 10 | 10 | 10 | 10 | 10 | – | – | – | – | – | 10 | – | 8 | 10 | – | – | |
‘Normal use’ is defined as the average of reported usages and ‘maximum use’ is defined as the 95th percentile of reported usages (Documentation provided to EFSA n. 22).
Table D.5.
Estimated intakes based on the MSDI approach and the mTAMDI approach
| FL‐no | EU Union List chemical name | MSDIa (μg/capita per day) | mTAMDIb (μg/person per day) | Structural class | TTC (μg/person per day) |
|---|---|---|---|---|---|
| 08.072 | But‐2‐enoic acid (cis and trans) | 4 | Class I | 1,800 | |
| 08.083 | Hept‐2‐enoic acid | 6.1 | Class I | 1,800 | |
| 08.101 | Non‐2‐enoic acid | 6.1 | Class I | 1,800 | |
| 08.119 | 2‐Hexenoic acid | 240 | Class I | 1,800 | |
| 08.120 | 2‐Methyl‐2‐butenoic acid | 6.1 | Class I | 1,800 | |
| 09.181 | Methyl hex‐2‐enoate | 0.037 | 3,900 | Class I | 1,800 |
| 09.248 | Ethyl trans‐2‐butenoate | 12 | 9,500 | Class I | 1,800 |
| 09.266 | Hexyl 2‐butenoate | 0.12 | 3,900 | Class I | 1,800 |
| 09.287 | Propyl deca‐2,4‐dienoate | 0.61 | Class I | 1,800 | |
| 09.321 | Butyl 2‐methylbut‐2(cis)‐enoate | 1.2 | 3,900 | Class I | 1,800 |
| 09.324 | Butyl but‐(2E)‐enoate | 1.7 | 3,900 | Class I | 1,800 |
| 09.326 | Butyl deca‐(2E,4Z)‐dienoate | 0.0012 | Class I | 1,800 | |
| 09.329 | Butyl hex‐2‐enoate | 1 | 3,900 | Class I | 1,800 |
| 09.330 | Butyl hex‐(3E)‐enoate | 0.12 | 3,900 | Class I | 1,800 |
| 09.335 | Butyl oct‐2‐enoate | 0.66 | 3,900 | Class I | 1,800 |
| 09.365 | Ethyl 3‐methylcrotonate | 0.0012 | 3,900 | Class I | 1,800 |
| 09.370 | Ethyl dec‐9‐enoate | 0.012 | 3,900 | Class I | 1,800 |
| 09.372 | Ethyl dodec‐(2E)‐enoate | 0.34 | 3,900 | Class I | 1,800 |
| 09.374 | Ethyl hept‐(2E)‐enoate | 0.61 | 3,900 | Class I | 1,800 |
| 09.379 | Ethyl pent‐2‐enoate | 0.037 | 3,900 | Class I | 1,800 |
| 09.578 | Hexyl (E)‐but‐2‐enoate | 2.6 | Class I | 1,800 | |
| 09.596 | Isopentyl‐(Z)‐but‐2‐enoate | 0.012 | 3,900 | Class I | 1,800 |
| 09.603 | Isopropyl crotonate | 0.24 | 3,900 | Class I | 1,800 |
| 09.624 | Methyl 2‐methylcrotonate | 0.12 | 3,900 | Class I | 1,800 |
| 09.625 | Methyl 2‐methylpent‐3(E)‐enoate | 0.0012 | 3,900 | Class I | 1,800 |
| 09.636 | Methyl crotonate | 0.12 | 3,900 | Class I | 1,800 |
| 09.637 | Methyl dec‐2‐enoate | 0.37 | 3,900 | Class I | 1,800 |
| 09.641 | Methyl dodec‐(2E)‐enoate | 0.56 | 3,900 | Class I | 1,800 |
| 09.652 | Methyl oleate | 1.2 | 3,900 | Class I | 1,800 |
| 09.680 | Pentyl 2‐methylisocrotonate | 0.74 | 3,900 | Class I | 1,800 |
| 09.699 | Propyl crotonate | 0.085 | 3,900 | Class I | 1,800 |
| 09.865 | Hexyl (9Z)‐octadecenoate | 0.24 | 3,600 | Class I | 1,800 |
| 09.934 | Methyl (5Z)‐Octenoate | 3.7 | 820 | Class I | 1,800 |
| 09.942 | 2‐Methylbutyl‐3‐methyl‐2‐butenoate | 1.2 | 1,600 | Class I | 1,800 |
| 02.192 | Oct‐2‐en‐1‐ol | 7.7 | 2,000 | Class I | 1,800 |
| 02.231 | trans‐2, cis‐6‐Nonadien‐1‐ol | 8.7 | 2,000 | Class I | 1,800 |
| 05.072 | trans‐2‐Nonenal | 1.7 | 740 | Class I | 1,800 |
| 05.081 | 2,4‐Decadienal | 19 | 560 | Class I | 1,800 |
| 05.144 | Dodec‐2(trans)‐enal | 0.75 | 2,000 | Class I | 1,800 |
| 05.184 | Undec‐2(trans)‐enal | 0.84 | 2,000 | Class I | 1,800 |
| 05.186 | 2,4‐Octadienal | 1.4 | 310 | Class I | 1,800 |
| 05.189 | 2‐Hexenal | 1.2 | 2,000 | Class I | 1,800 |
| 05.190 | trans‐2‐Octenal | 0.79 | 2,000 | Class I | 1,800 |
| 05.191 | trans‐2‐Decenal | 8.1 | 1,800 | Class I | 1,800 |
| 05.194 | tr‐2, tr‐4‐Nonadienal | 2.4 | 560 | Class I | 1,800 |
| 05.195 | trans‐2‐Tridecenal | 0.12 | 2,000 | Class I | 1,800 |
| 05.196 | tr‐2, tr‐4‐Undecadienal | 3 | 89 | Class I | 1,800 |
| 09.400 | Hex‐2‐enyl phenylacetate | 0.012 | 1,800 | Class I | 1,800 |
| 09.948 | (2E)‐2‐Nonenyl acetate | 0.012 | 1,800 | Class I | 1,800 |
| 09.375 | Ethyl methacrylate | 0.12 | 3,900 | Class II | 540 |
| 09.586 | Isobutyl 2‐methylprop‐2‐enoate | 0.012 | 3,900 | Class II | 540 |
| 09.647 | Methyl methacrylate | 0.061 | 3,900 | Class II | 540 |
| 09.247 | Allyl crotonate | 0.043 | 2,000 | Class II | 540 |
| 09.866 | Allyl valerate | 0.012 | 2,000 | Class II | 540 |
Based on EU production volumes submitted by industry (Documentation provided to EFSA n. 1, 3, 14, 15, 16, 17, 18, 19, 20).
Based on use levels submitted by industry (Documentation provided to EFSA n. 2, 3, 9, 10, 11, 12, 13, 14, 15, 16).
In addition, the following documentation was used:
-
–
Scientific opinion of the EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on Flavouring Group Evaluation 05: Esters of 23 branched‐ and straight‐chain aliphatic saturated primary alcohols and of one secondary alcohol, and 24 branched‐ and straight‐chain unsaturated carboxylic acids from chemical groups 1, 2, and 5 (EFSA AFC Panel, 2005).
-
–
Scientific opinion of the EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on Flavouring Group Evaluation 05 Revision 1 (FGE.05Rev1): Esters of branched‐ and straight‐chain aliphatic saturated primary alcohols and of one secondary alcohol, and branched‐ and straight‐chain unsaturated carboxylic acids from chemical groups 1, 2, and 5 (EFSA AFC Panel, 2008).
-
–
Scientific opinion of the EFSA Panel Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on Flavouring Group Evaluation 05 Revision 2 (FGE.05Rev2): Branched‐ and straight‐chain unsaturated carboxylic acids and esters of these with aliphatic saturated alcohols from chemical groups 1, 2, 3 and 5 (EFSA CEF Panel, 2010).
-
–
Scientific opinion of the EFSA Panel Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on Flavouring Group Evaluation 14 Revision 1 (FGE.14Rev1): Phenethyl alcohol, aldehyde, acetals, carboxylic acid and related esters from chemical group 15 and 22 (EFSA AFC Panel, 2007).
-
–
Scientific data retrieved from public literature (see reference list).
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA SC, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee. The assessment strategy applied for the evaluation programme of flavouring substances, laid down in Commission Regulation (EC) 1565/2000, is based on the Opinion on a Programme for the Evaluation of Flavouring substances of the Scientific Committee on Food (SCF, 1999).
2.2.1. Procedure for the safety evaluation of flavouring substances
The approach for safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/2000, named the ‘Procedure’, is described in Appendix A.
2.2.2. Approach used for the calculation of exposure
The approach used for the calculation of exposure to flavouring substances, as referred to in Commission Regulation (EC) No 1565/2000, is described in Appendix A (section ‘Intake’) and in Appendix D.
3. Assessment
3.1. Presentation of the substances in Flavouring Group Evaluation 5, Revision 3
The present Flavouring Group Evaluation 5 Revision 3 (FGE.05Rev3) deals with 17 newly included flavouring substances.
-
The 17 candidate substances are α,β‐unsaturated carbonyl compounds, alcohols and esters:
-
–
Seven aliphatic monounsaturated aldehydes [FL‐no: 05.072, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195];
-
–
Four aliphatic diunsaturated aldehydes [FL‐no: 05.081, 05.186, 05.194, 05.196];
-
–
Two esters of straight‐chain aliphatic unsaturated primary alcohols and straight‐chain saturated carboxylic acids [FL‐no: 09.866, 09.948];
-
–
One ester of a straight‐chain aliphatic unsaturated primary alcohol and a straight‐chain unsaturated carboxylic acid [FL‐no: 09.247];
-
–
One ester of a straight‐chain aliphatic unsaturated primary alcohol and straight‐chain saturated carboxylic acid with a phenyl functional group [FL‐no: 09.400];
-
–
One aliphatic monounsaturated primary alcohol [FL‐no: 02.192];
-
–
One aliphatic diunsaturated primary alcohol FL‐no: 02.231].
-
–
| FL‐no | Chemical name | Structural formula | Structural class |
|---|---|---|---|
| 02.192 | Oct‐2‐en‐1‐ol |
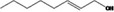
|
I |
| 02.231 | trans‐2, cis‐6‐Nonadien‐1‐ol |
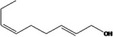
|
I |
| 05.072 | trans‐2‐Nonenal |

|
I |
| 05.081 | 2,4‐Decadienal |

|
I |
| 05.144 | Dodec‐2(trans)‐enal |

|
I |
| 05.184 | Undec‐2(trans)‐enal |

|
I |
| 05.186 | 2,4‐Octadienal |
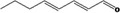
|
I |
| 05.189 | 2‐Hexenal |

|
I |
| 05.190 | trans‐2‐Octenal |

|
I |
| 05.191 | trans‐2‐Decenal |

|
I |
| 05.194 | tr‐2, tr‐4‐Nonadienal |

|
I |
| 05.195 | trans‐2‐Tridecenal |

|
I |
| 05.196 | tr‐2, tr‐4‐Undecadienal |

|
I |
| 09.400 | Hex‐2‐enyl phenylacetate |
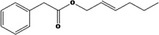
|
I |
| 09.948 | (2E)‐2‐Nonenyl acetate |
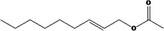
|
I |
| 09.247 | Allyl crotonate |
|
II |
| 09.866 | Allyl valerate |
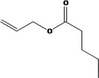
|
II |
FL‐no: FLAVIS number.
The 17 flavouring substances under consideration, with their chemical Register names, FLAVIS‐ (FL‐), Chemical Abstract Service‐ (CAS‐), Council of Europe‐ (CoE‐) and Flavor and Extract Manufacturers Association‐ (FEMA‐) numbers, structure and specifications, are also listed in Appendix B – Table B.1. In this Appendix also the previously evaluated substances are presented.
The hydrolysis products of esters candidate substances are listed in Appendix B – Table B.2.
Table B.2.
Evaluation status of hydrolysis products of candidate esters in FGE.05Rev3
| FL‐no | EU Union List chemical name JECFA no | Structural formula | SCF statusa JECFA status CoE statusb EFSA status | Structural class Procedure path (JECFA)c | Comments |
|---|---|---|---|---|---|
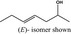
| 4‐Hepten‐2‐ol |
|
Not evaluated as flavouring substance | Not in Union List | ||
| Hexadecanoic acid |
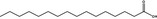
|
Not evaluated as flavouring substance | Not in Union List | ||
| Allyl alcohol | Not evaluated as flavouring substance | Not in Union List; JECFA ADI available (JECFA, 1996) | |||
| 02.001 |
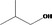
2‐Methylpropan‐1‐ol 251 |
|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999 Category A (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 02.002 |
Propan‐1‐ol 82 |
|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 02.003 |
Isopentanol 52 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 1997) Category A (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 02.004 |
Butan‐1‐ol 85 |
|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 02.005 |
Hexan‐1‐ol 91 |
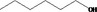
|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 02.040 |
Pentan‐1‐ol 88 |
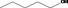
|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 02.076 |
2‐Methylbutan‐1‐ol 1199 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 2004b) Category B (CoE, 1992) FGE.76 |
Class I A3: Intake below threshold |
|
| 02.078 |
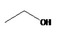
Ethanol 41 |
|
Category 1 (SCF, 1995) No safety concern (JECFA, 1997) |
No evaluation | At the 46th meeting (1996), the Committee concluded that ethanol posed no safety concern at its current level of intake when ethyl esters are used as flavouring agents |
| 02.079 |
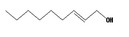
Isopropanol 277 |
|
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 02.112 | Non‐2(cis)‐en‐1‐ol |
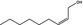
|
No safety concern (JECFA, 2004b) FGE.200Rev1 |
Class I A3: Intake below threshold |
Evaluated in FGE.200Rev1 as of no genotoxicity concern. Pending the finalisation of safety evaluation in FGE.71Rev1 |
| 02.090 |
Non‐(2E)‐en‐1‐ol 1365 |
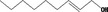
|
No safety concern (JECFA, 2005) FGE.200Rev1 |
Class I A3: Intake below threshold |
Evaluated in FGE.200Rev1 as of no genotoxicity concern. Pending the finalisation of safety evaluation in FGE.71Rev1 |
| 02.156 | Hex‐2(cis)‐en‐1‐ol |
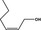
|
No safety concern (JECFA, 2004b) FGE.200Rev1 |
Class I A3: Intake below threshold |
Evaluated in FGE.200Rev1 as of no genotoxicity concern. Pending the finalisation of safety evaluation in FGE.71Rev1 |
| 02.157 | Hex‐(2E)‐en‐1‐ol |

|
Evaluation request withdrawn from FGE.200 | Not in the UL (as no longer supported by Industry) | |
| 08.002 | Acetic acid |
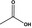
|
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
Class I A3: Intake above threshold, A4: Endogenous |
|
| 08.007 | Valeric acid |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 2000) Category A (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 08.038 | Phenylacetic acid |
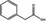
|
No safety concern (JECFA, 2000) Category B (CoE, 1992) FGE.53 |
Class I A3: Intake below threshold |
|
| 08.013 |
Oleic acid 333 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Deleted (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 08.050 |
Hex‐3‐enoic acid 317 |

|
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
Class I A3: Intake below threshold |
|
| 08.058 |
2‐Methylpent‐3‐enoic acid 347 |
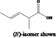
|
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
Class I A3: Intake below threshold |
|
| 08.064 |
(2E)‐Methylcrotonic acid 1205 |
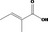
|
No safety concern (JECFA, 2004b) FGE.72 |
Class I A3: Intake below threshold |
|
| 08.065 |
Dec‐9‐enoic acid 328 |
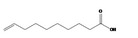
|
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
Class I A3: Intake below threshold |
|
| 08.070 |
3‐Methylcrotonic acid 1204 |

|
No safety concern (JECFA, 2004b) FGE.72 |
Class I A3: Intake below threshold |
|
| 08.072 | But‐2‐enoic acid (cis and trans) |
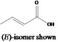
|
FGE.05 |
Class I A3: Intake below threshold |
|
| 08.073 |
Dec‐2‐enoic acid 1372 |
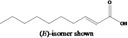
|
No safety concern (JECFA, 2005) FGE.71 |
Class I A3: Intake below threshold |
|
| 08.083 | Hept‐2‐enoic acid |

|
FGE.05 |
Class I A3: Intake below threshold |
|
| 08.107 |
(E)‐Pent‐2‐enoic acid 1804 |
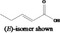
|
No safety concern (JECFA, 2008) FGE.95 |
Class I A3: Intake below threshold |
|
| 08.114 |
2‐Octenoic acid 1805 |

|
No safety concern (JECFA, 2008) FGE.95 |
Class I A3: Intake below threshold |
|
| 08.119 | 2‐Hexenoic acid |
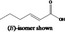
|
FGE.05 |
Class I A3: Intake below threshold |
FL‐no: FLAVIS number; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; SCF: Scientific Committee on Food; CoE: Council of Europe; FGE: Flavouring Group Evaluation.
Category 1: Considered safe in use, Category 2: Temporarily considered safe in use, Category 3: Insufficient data to provide assurance of safety in use, Category 4: Not acceptable due to evidence of toxicity, Category N: Cannot be listed in cat. 1‐4 due to e.g. not used in the EU, not considered to be a flavour.
Category A: Flavouring substance, which may be used in foodstuffs, Category B: Flavouring substance which can be used provisionally in foodstuffs.
3.1.1. Supporting substances
Among the 17 additional substances assessed in the current revision (FGE.05Rev3), 14 candidate substances are structurally related to the JECFA flavouring groups ‘Aliphatic, alicyclic, linear, α,β‐unsaturated di‐ and trienals and related alcohols, acids and esters’ (61st JECFA meeting) and ‘Aliphatic, linear α,β‐unsaturated aldehydes, acids and related alcohols, acetals and esters’ (63rd and 69th JECFA meetings). Within these two JECFA flavouring groups, there are five substances ([FL‐no: 02.231, 05.040, 05.140, 05.071 and 05.108]) which have been evaluated by EFSA in the FGE.70Rev1 (EFSA FAF Panel, 2019) and other nine substances ([FL‐no: 05.171, 05.037, 05.109, 05.073, 05.060, 05.076, 05.078, 02.020 and 02.090] are currently under evaluation in the FGE.71Rev1. These supporting substances are also closely related to the 37 substances that have been evaluated in FGE.05Rev2 (EFSA CEF Panel, 2010).
The remaining three flavouring substances are two allyl esters (allyl crotonate [FL‐no: 09.247] and allyl valerate [FL‐no: 09.866]) and one phenylacetic acid‐related ester (Hex‐2‐enyl phenylacetate [FL‐no: 09.400]). These esters have not been evaluated by JECFA as such. However, JECFA evaluated in its 46th meeting other allyl esters for which a group acceptable daily intake (ADI) is available for allyl alcohol (JECFA, 1997), the corresponding alcohol deriving from the hydrolysis of the two candidate esters [FL‐no: 09.247 and 09.866]. Crotonic acid (i.e. but‐2‐enoic acid, [FL‐no: 08.072]) is the carboxylic acid released from [FL‐no: 09.247] and it has been evaluated in FGE.05Rev2 as of no safety concern (EFSA CEF Panel, 2010). Valeric acid (i.e. pentanoic acid [FL‐no: 08.007]) is the carboxylic acid released from [FL‐no: 09.866] and it has been evaluated by JECFA in its 49th meeting as no safety concern (JECFA, 1999). With respect to the phenylacetic acid‐related ester [FL‐no: 09.400], its corresponding hydrolysis products are hex‐2‐en‐1‐ol and phenylacetic acid. Both compounds are JECFA‐evaluated substances (JECFA‐no: 1354 and 1007, respectively) as of no safety concern. In addition, phenylacetic acid ([FL‐no: 08.083]) had been considered of no safety concern in FGE.53 and hex‐2‐en‐1‐ol ([FL‐no: 02.020]) is currently under consideration in FGE.71Rev1. Substances related to phenyl acetic acid can also be found in FGE.14Rev1, but because [FL‐no: 09.400] is also strongly connected to α,β‐unsaturated carbonyls in the present FGE, the Panel preferred to maintain this substance in FGE.05Rev3. Relevant information for the evaluation of this substance will be taken from FGE.14Rev1 (EFSA AFC Panel, 2007).
The chemical names and structures of the structurally related supporting substances for FGE.05Rev3, together with their evaluation status, are listed in Appendix C – Table C.1.
3.1.2. Specifications
Specifications including complete purity criteria, information on identity and identity for the materials of commerce have been provided for all the 17 candidate flavouring substances, assessed in the present revision FGE.05Rev3. The specifications for these 17 substances are adequate.
For flavouring substance ([FL‐no: 09.578]), considered in the previous revision of this FGE (FGE.05Rev2), no specifications were available.
Stereoisomers
It is recognised that geometrical and optical isomers of substances may have different properties. Their flavour may be different and they may have different chemical properties resulting in possible variation of their absorption, distribution, metabolism, elimination and toxicity. Thus, information must be provided on the configuration of the flavouring substance, i.e. whether it is one of the geometrical/optical isomers, or a defined mixture of stereoisomers. The available specifications of purity will be considered in order to determine whether the safety evaluation carried out for candidate substances for which stereoisomers may exist can be applied to the material of commerce. Flavouring substances with different configurations should have individual chemical names and codes (CAS number, FLAVIS number, etc.).
For 10 of the previously evaluated substances in FGE.05Rev2 ([FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.181, 09.329, 09.335, 09.379 and 09.637]), information on geometrical isomerism has not been specified. The applicant has informed that they exist as a ‘mixture of isomers’ (Documentation provided to EFSA n. 7). However, the Panel did not consider this information sufficient and requested quantitative data on the composition of these stereoisomeric mixtures.
The newly allocated 17 flavouring substances [FL‐no: 02.192, 02.231, 05.072, 05.081, 05.144, 05.184, 05.186, 05.189, 05.190, 05.191, 05.194, 05.195, 05.196, 09.247, 09.400, 09.866 and 09.948] are all α,β‐unsaturated and consequently they can exist as geometrical isomers. The 17 substances have been fully characterised with respect to the stereoisomeric composition.
Industry has informed that flavouring substance [FL‐no: 09.287], evaluated in FGE.05Rev2, is the (2E,4Z)‐isomer which is covered by the [FL‐no: 09.840] from FGE.70 and accordingly [FL‐no: 09.287] can be deleted from the Union List (Documentation provided to EFSA n. 7). However, the Panel noted that in FGE.70 it is indicated that [FL‐no: 09.840] is an unidentified mixture of isomers. Therefore, the stereochemistry of this substance should be further clarified before proceeding to the deletion of the substance [FL‐no: 09.287].
The Panel noted that the information related to the geometrical stereoisomerism (i.e. (E) stereoisomer) for two flavouring substances [FL‐no: 05.194 and 05.196], previously evaluated in FGE.05Rev2, is not correctly reflected in the chemical name reported in the EU Union List of flavouring substances. Therefore, the chemical name of [FL‐no: 05.194 and 05.196] should be changed as reported in Appendix B – Table B.1 (see ‘EFSA comments’).
The detailed specifications for the flavouring substances in FGE.05Rev3 are described in Appendix B – Table B.1.
3.2. Intake data
3.2.1. Natural occurrence in food
Of the 17 newly added candidate flavouring substances in FGE.05Rev3, 15 have been reported to occur naturally. These occurrences include among others: milk and milk products, beef, chicken, lamb, fish, shrimps, tomato, plum, citrus fruits, apples, potato chips, maize, tea, camomile, nuts and wine (the complete data set retrieved on the natural occurrence is presented in Appendix G). The highest quantified occurrences in foods are presented in Table 1.
Table 1.
Flavouring candidate substances reported to occur in food (Triskelion, 2018)
| FL‐no | Name | Quantitative data reported |
|---|---|---|
| 05.072 | trans‐2‐Nonenal | Up to 1,000 mg/kg in citrus fruits |
| 05.081 | 2,4‐Decadienal | Up to 2,000 mg/kg in mentha oils |
| 05.144 | Dodec‐2(trans)‐enal | Up to 27,000 mg/kg in coriander leaf oil |
| 05.184 | Undec‐2(trans)‐enal | Up to 7,000 mg/kg in camomile |
| 05.189 | 2‐Hexenal | Up to 26,000 mg/kg in lemon balm |
| 05.190 | trans‐2‐Octenal | Up to 1,000 mg/kg in camomile |
| 05.191 | trans‐2‐Decenal | Up to 130,000 mg/kg in caraway oil and up to 268,000 mg/kg in coriander leaf oil |
Two of the newly added flavouring substances (allyl valerate [FL‐no: 09.866] and (2E)‐2‐nonenyl acetate [FL‐no: 09.948]) have not been reported to naturally occur in any food items (Triskelion, 2018).
3.2.2. Estimated daily per capita intake (MSDI approach)
The intake estimation is based on the MSDI approach, which involves the acquisition of data on the amounts used in food as flavourings (SCF, 1999). These data are derived from surveys on annual production volumes in Europe. The intake approach does not consider the possible natural occurrence in food. Average per capita intake (MSDI) is estimated on the assumption that the amount added to food is consumed by 10% of the population5 (Eurostat, 1998). This is derived for candidate substances from estimates of annual volume of production provided by industry and incorporates a correction factor of 0.6 to allow for incomplete reporting (60%) in the industry surveys (SCF, 1999 and see also Appendix A, section Intake).
The MSDI values for the 17 newly included flavouring substances in FGE.05Rev3 from FGE.200Rev1 ([FL‐no: 02.192, 02.231, 05.072, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195, 09.247, 09.400, 09.866 and 09.948]) and FGE.203Rev3 ([FL‐no: 05.081, 05.186, 05.194, 05.196]), are derived from surveys on annual production volumes (poundage data) in Europe. European Flavours Association (EFFA) conducted a survey in late 2016 for flavouring substances in FGE.200Rev1, in which flavour manufacturers reported the total amount of each flavouring substance incorporated into food sold in the EU for the calendar year 2015. In the course of 2017, the data were reviewed and the final figures were validated (Documentation provided to EFSA n. 1). The survey for the flavouring substances in FGE.203Rev2 has been conducted for the years 2010 to 2015 (Documentation provided to EFSA n. 3). For consistency with flavouring substances from FGE.200Rev1, the poundage data for year 2015 have been considered in the MSDI calculations for the four substances coming from FGE.203Rev2. The MSDI values for these 17 substances range from 0.012 to 19 μg/capita per day for 15 structural class I substances and from 0.012 and 0.043 μg/capita per day for the two substances allocated to structural class II (see Appendix D – Table D.5).
3.2.3. Intake estimated on the basis of the modified TAMDI (mTAMDI)
For the evaluation of the 54 candidate flavouring substances in the entire FGE.05, information on normal and maximum use levels were submitted for 46 of the substances by the Flavour Industry (Documentation provided to EFSA n. 2, 3, 9, 10, 11, 12, 13, 14, 15, 16). This includes the 17 newly added candidate substances.
The 17 candidate substances are used in flavoured food products divided into the food categories, outlined in Annex III of the Commission Regulation (EC) No 1565/2000, as shown in Appendix D – Table D.2.
For the 15 candidate substances from structural class I for which Industry has submitted use levels, the estimated intakes based on the mTAMDI range from 89 to 2,000 μg/person per day. For flavouring substances [FL‐no: 05.072, 05.081, 05.186, 05.194 and 05.196] the mTAMDI values are below the TTC for their structural class I (i.e. 1,800 μg/person per day). These candidate substances are also expected to be metabolised to innocuous products. For 10 structural class I substances [FL‐no: 02.192, 02.231, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195, 09.400, 09.948] the mTAMDI values are equal or above their corresponding TTC.
The mTAMDI estimated intakes for the two substances [FL‐no: 09.247 and 09.866] assigned to structural class II is 2,000 μg/person per day which is above their corresponding TTC (i.e. 540 μg/person per day).
Therefore, for 12 of the 17 newly included candidate substances [FL‐no: 02.192, 02.231, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195, 09.400, 09.948, 09.247 and 09.866], for which normal and maximum use levels were submitted, further information is required. This would include more reliable intake data and then, if required, additional toxicological data. This also applies to 35 substances evaluated in FGE.05Rev2. Among these 35 substances, there are eight substances ([FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.287, 09.326 and 09.578]) for which normal and maximum levels for their use in food are still missing.
The detailed information on use levels and the comparison of the MSDI and mTAMDI intake estimations are reported in Appendix D – Tables D.2 and D.5, respectively, for all 54 candidate substances in FGE.05. In the case where different normal use levels were reported for different food categories, the highest reported normal use level has been given in Appendix D – Table D.2. This value was used for the mTAMDI calculation.
3.2.4. Considerations of combined intakes from use as flavouring substances
Because of structural similarities of candidate and supporting substances, it can be anticipated that many of the flavourings are metabolised through the same metabolic pathways and that the metabolites may affect the same target organs. Further, in case of combined exposure to structurally related flavourings, the pathways could be overloaded. Therefore, combined intake should be considered. As flavouring substances not included in this FGE may also be metabolised through the same pathways, the combined intake estimates presented here are only preliminary. Currently, the combined intake estimates are only based on MSDI exposure estimates, although it is recognised that this may lead to underestimation of exposure. After completion of all FGEs, this issue should be readdressed. The combined exposure will take into account exposures to the substances evaluated in the previous version, the currently new included 17 substances and their supporting substances.
The total estimated combined daily per capita intake of structurally related flavourings is estimated by summing up the MSDI for individual substances.
On the basis of the reported annual production volumes in Europe, the combined estimated per capita intake as flavouring of the 49 candidate substances assigned to structural class I is 346 μg/person per day, which does not exceed the threshold of concern for the structural class I of 1,800 μg/person per day. For the five candidate substances assigned to structural class II, the combined intake is 0.245 μg/person per day, which does not exceed the threshold of concern for structural class II of 540 μg/person per day (Documentation provided to EFSA n. 1; 3; 14; 15; 16; 17; 18; 19 and 20) (see Appendix D – Table D.5). Therefore, the combined exposure of the candidate substances in this FGE is not exceeding the TTCs for the respective structural classes.
The 54 candidate substances considered in this FGE for combined exposure are structurally related to 61 supporting substances evaluated by JECFA in the 46th, 49th, 51st, 61st, 63rd and 68th meeting (JECFA, 1997, 1999, 2004a, 2005, 2007) (Appendix C – Table C.1). All supporting substances belong to structural class I.
The total estimated combined intake of the 49 candidate and 61 supporting substances, which belong to structural class I, would be 8,873 μg/capita per day (European data were not available for four of the supporting substances) (Appendix D – Table D.5 and Appendix C – Table C.1). This intake is almost five times higher than the threshold of concern for the corresponding structural class I (i.e. 1,800 μg/capita per day). The highest intake contribution of the supporting substances comes from four substances (hex‐3(cis)‐en‐1‐ol FL‐no: 02.056], MSDI: 3,700 μg/capita per day; Hex‐2(trans)‐enal [FL‐no: 05.073], MSDI: 2,800 μg/capita per day; Hex‐2‐en‐1‐ol [FL‐no: 02.020], MSDI: 650 μg/capita per day; phenylacetic acid [FL‐no: 08.038], MSDI: 240 μg/capita per day). These four substances contribute with 7,390 μg/person per day (0.123 mg/kg bw per day) to the total combined MSDI 8,873 μg/person per day. For flavourings [FL‐no: 02.056 and 05.073], there are NOAELs available: 127 mg/kg bw per day (Gaunt et al., 1969) for [FL‐no: 02.056] and 257 mg/kg bw per day for [FL‐no: 05.073] (Gaunt et al., 1983) (Appendix F – Table F.1). By taking the lowest NOAEL value (127 mg/kg bw per day), a margin of safety of 1,030 could be derived for the combined intake of these four supporting substances ([FL‐no: 02.056, 05.073, 02.020 and 08.038]). Since there are no supporting substances in structural class II, there is no need to calculate the total combined exposure for candidate and supporting substances for this structural class.
Table F.1.
Subacute, subchronic and chronic toxicity studies considered in FGE.05Rev3. The supporting substances are listed in brackets
| UL chemical name [FL‐no] | Species; Sex No./Group | Route | Dose levels (mg/kg bw per day) | Duration | NOAEL (mg/kg bw per day) | Reference | Comments |
|---|---|---|---|---|---|---|---|
|
(2‐trans‐6‐cis‐dodecadienal [FL‐no:05.120]) plus (2‐trans‐4‐cis‐7‐cis‐tridecatrienal [FL‐no:05.064]) |
Rats; Male, Female 6/sex per group |
Diet (microencapsulated in maltodextrin) | 0 (maltodextrin) [FL‐no:05.120]: up to 1.93 and 2.06 in males and females, respectively [FL‐no:05.064]: up to 30.9 and 33 in males and females, respectively, as a mixture | 4 weeks |
[FL‐no:05.120]: 2.06 [FL‐no:05.064]: 33 |
Edwards (1973) | Unpublished report cited by JECFA (2004b) not available to EFSA. NOAELs based on the mixture; not from data on individual substances |
| (2,4‐decadienal [FL‐no: 05.140]) |
Rats; Male, Female 10/sex per group |
Gavage | 0, 50, 100, 200, 400 and 800 in corn oil 5 days/week | 14 weeks | 100 | NTP (2011) | Forestomach hyperplasia, probably caused by high local concentration of the irritant compound and/or effects on body weight and lethargy |
|
Mice; Male, Female 10/sex per group |
Gavage | 0, 50, 100, 200, 400 and 800 in corn oil 5 days per week |
100 (males) 200 (females) |
||||
| (Hex‐2(trans)‐enal [FL‐no: 05.073]) |
Rats; Male, Female 15/sex per group |
Diet |
0, 18, 45, 110 and 257 in males 0, 21, 52, 131 and 304 in females |
13 weeks | 257 (males) and 304 (females) | Gaunt et al. (1983) | NOAEL is highest dose level tested |
| (hex‐3(cis)‐en‐1‐ol [FL‐no: 02.056]) |
Rats; Male, Female 30/sex per group |
Drinking water | 0, 30, 127, 410 in males 0, 42 168, 721 in females | 14 weeks |
127 (males 168 (females) |
Gaunt et al. (1969) | Slight effect on relative kidney weight and urine volume/specific gravity in male rats at highest dose |
| (Hexa‐2(trans),4(trans)‐dienal [05.057]) | Rats; Male and Female, 10/sex per group | Gavage | 0, 7.5, 15, 30, 60 and 120 in corn oil 5 days/week | 14 weeks | 60 | NTP (2003) | Based on the magnitude of the observed effect (body weight changes) |
| Mice Male and Female, 10/sex per group | Gavage | 0, 7.5, 15, 30, 60 and 120 in corn oil 5 days/week | 120 | No effects on body weight. Minimal to moderate hyperplasia of forestomach in both rats and mice at 120 mg/kg bw per day, probably due to local irritant effect of test substance | |||
|
Rats; Male, Female 50/sex per /group |
Gavage | 0 (controls), 22.5, 45, or 90 in corn oil 5 days/week | 2 years | Effects on forestomach – not applicable to the use of flavourings | NTP (2003) | Increased incidence of hyperplasia, squamous cell papillomas and squamous cell carcinoma of the forestomach. The Panel considered the effects to be due to local irritant effects of the tested substance | |
|
Mice; Male, Female 50/sex per group |
Gavage | 0 (controls), 30, 60, or 120, in corn oil 5 days/week | 2 years | Effects on forestomach – not applicable to the use of flavourings. Squamous cell carcinoma of the tongue observed in two mice of the high‐dose group | NTP (2003) | Increased incidence of hyperplasia, squamous cell papillomas and squamous cell carcinoma of the forestomach. The Panel considered the effects to be due to local irritant effects of the tested substance |
FGE: Flavouring Group Evaluation; FL‐no: FLAVIS number; bw: body weight; NOAEL: no observed adverse effect level.
The Panel concluded that the total combined exposure (based on MSDI) does not raise safety concern. Moreover, simultaneous exposure to all 115 candidate plus supporting substances on a single day is unlikely and it is even more unlikely that this could occur repeatedly over a life‐time.
3.3. Biological and toxicological data
3.3.1. Absorption, distribution, metabolism and elimination (ADME)
The 17 flavouring substances evaluated in the present revision of FGE.05 (FGE.05Rev3) are α,β‐unsaturated aldehydes, alcohols and their related esters. The Panel considered these candidate substances structurally and metabolically related to the JECFA flavouring groups ‘aliphatic, alicyclic, linear, α,β‐unsaturated di‐ and trienals and related alcohols, acids and esters’ and ‘aliphatic, linear α,β‐unsaturated aldehydes, acids and related alcohols, acetals and esters’, which have either been evaluated in the FGE.70Rev1 or are currently under evaluation in FGE.71Rev1 by EFSA. Therefore, the metabolism of the supporting substances is similar to that of the candidate substances in FGE.05Rev3.
The relevant metabolic pathways are fully described in the corresponding JECFA reports (JECFA, 2004b, 2005, 2008) as summarised in FGE.70Rev1 (EFSA FAF Panel, 2019).
Generally, the esters are hydrolysed by carboxylesterase enzymes in the intestinal mucosa to unsaturated alcohols and carboxylic acids. After the hydrolysis, the resulting components are absorbed into the portal circulation. The unsaturated alcohols are oxidised to their corresponding aldehydes and carboxylic acids, which will be metabolised through the main biochemical pathways, i.e. fatty acid pathway and citric acid cycle. At low intakes, α,β‐unsaturated aldehydes can be metabolically detoxified by β‐oxidation pathway or, to a lesser extent, by glutathione (GSH) conjugation. In addition, humans can biotransform 2‐alkenols and 2‐alkenals by oxidation to the corresponding acids which may undergo β‐oxidative cleavage and then completely be metabolised via the tricarboxylic acid cycle. Another possible minor pathway is the GSH conjugation, followed by excretion as mercapturic acid derivatives. α,β‐unsaturated carbonyls can also react with other cell constituents, such as DNA, through Michael Addition to form adducts with DNA nucleotides. However, the levels of exposure to α,β‐unsaturated aldehydes (and precursors) as flavouring substances are not high enough to result in GSH depletion as well as to lead to oxidative stress. Additionally, the concern for genotoxicity was ruled out in FGE.200Rev1 and FGE.203Rev2 (EFSA CEF Panel, 2018; EFSA FAF Panel, 2018).
The Panel noted that flavouring substance Hex‐2‐enyl phenylacetate [FL‐no: 09.400] is an aromatic ester for which routes of metabolism have been described in FGE.14Rev1. In that FGE, it is indicated that this ester will be hydrolysed in hex‐2‐en‐1‐ol [FL‐no: 02.020] and phenyl acetic acid [FL‐no: 08.038]. For hex‐2‐en‐1‐ol the metabolic routes described above are applicable. The metabolism of the phenyl acetic acid has been described FGE.14Rev1 as follows: ‘Phenylacetic acid is an endogenous end product of phenylalanine metabolism and is present in human urine as a conjugate (Seakins, 1971). The types of conjugates formed from phenylacetic acid are both dose dependent and species‐specific. The major metabolic options available to phenylacetic acid are conjugation with glucuronic acid, glycine, taurine or glutamine, or elimination as the free acid. In humans, phenylacetic acid is mainly excreted in conjugation with glutamine’.
Overall, the Panel concludes that these flavouring substances are metabolised through common metabolic routes. The expected metabolic intermediates can be considered endogenous and based on these aspects the end products can be considered innocuous. Therefore, the Panel agrees to evaluate these flavouring substances along the A‐side of the Procedure (Appendix A).
3.3.2. Genotoxicity data
This revision involves the inclusion of 17 flavouring substances, for which in FGE.19 a concern for genotoxicity had been identified based on the presence of a structural alert (i.e. α,β‐unsaturated carbonyl or precursor for that), preventing their evaluation through the Procedure (see Appendix A). Because of this, these 17 substances needed further attention in FGE.200 or FGE.203. The genotoxicity of flavouring substances [FL‐no: 02.192, 02.231, 05.072, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195, 09.247, 09.400, 09.866 and 09.948] has been assessed in FGE.200 (EFSA CEF Panel, 2014a) and FGE.200Rev1 (EFSA FAF Panel, 2018). Based on the genotoxicity data submitted, the Panel concluded that the concern with respect to genotoxicity could be ruled out for these flavouring substances. The genotoxicity of flavouring substances [FL‐no: 05.081, 05.186, 05.194, 05.196] has been assessed in FGE.203 (EFSA CEF Panel, 2009), FGE.203Rev1 (EFSA CEF Panel, 2014b) and FGE.203Rev2 (EFSA CEF Panel, 2018). Based on the genotoxicity data submitted, the Panel concluded that the concern with respect to genotoxicity could be ruled out for these flavouring substances.
3.3.3. Toxicological data
Subacute, subchronic and chronic toxicity studies are available for six structurally related supporting substances [FL‐no: 02.056, 05.057, 05.064, 05.120, 05.140 and 05.073].
3.4. Application of the Procedure for the Safety Evaluation of Flavouring substances
The application of the Procedure is based on intakes estimated on the basis of the MSDI approach. Where the mTAMDI approach indicates that the intake of a flavouring substance might exceed its corresponding threshold of concern, a formal safety assessment is not carried out using the Procedure. In these cases, the Panel requires more precise data on use and use levels. For comparison of the intake estimations based on the MSDI approach and the mTAMDI approach, see Appendix D – Table D.5. For the safety evaluation of the 17 additional flavouring substances considered in FGE.05Rev3 the Procedure was applied and here below described. The safety evaluations of the 17 substances are summarised in Appendix E – Table E.1.
Table E.1.
Summary of safety evaluations applying the Procedure
| FL‐no | EU Union List chemical name | Structural formula | MSDIa (μg/capita per day) | Classb Evaluation procedure pathc Outcome on the named compound and on the material of commerce | EFSA comments |
|---|---|---|---|---|---|
|
05.072 1362 |
trans‐2‐Nonenal |
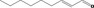
|
1.7 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 05.190 | trans‐2‐Octenal |
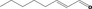
|
0.79 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 08.072 | But‐2‐enoic acid (cis and trans) |
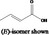
|
4 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Composition of the mixture to be specified |
| 08.083 | Hept‐2‐enoic acid |

|
6.1 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Composition of the mixture to be specified |
| 08.101 | Non‐2‐enoic acid |
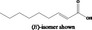
|
6.1 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Composition of the mixture to be specified |
| 08.119 | 2‐Hexenoic acid |

|
240 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Composition of the mixture to be specified |
| 08.120 | 2‐Methyl‐2‐butenoic acid |
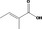
|
6.1 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Composition of the mixture to be specified |
| 09.181 | Methyl hex‐2‐enoate |

|
0.037 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Composition of the mixture to be specified |
| 09.248 | Ethyl trans‐2‐butenoate |
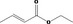
|
12 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
|
09.266 1807 |
Hexyl 2‐butenoate |
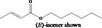
|
0.12 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.287 | Propyl deca‐2,4‐dienoate |
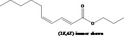
|
0.61 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 [FL‐no: 09.287] should be deleted from the UL as this substance is covered by [FL‐no: 09.840] (FGE.70), provided that the composition of the mixtures of stereoisomers for these two substances is the same |
| 09.321 | Butyl 2‐methylbut‐2(cis)‐enoate |
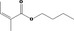
|
1.2 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.324 | Butyl but‐(2E)‐enoate |
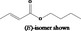
|
1.7 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.326 | Butyl deca‐(2E,4Z)‐dienoate |
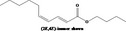
|
0.0012 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.329 | Butyl hex‐2‐enoate |
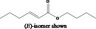
|
1 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Composition of the mixture to be specified |
| 09.330 | Butyl hex‐(3E)‐enoate |
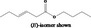
|
0.12 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.335 | Butyl oct‐2‐enoate |

|
0.66 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Composition of the mixture to be specified |
| 09.365 | Ethyl 3‐methylcrotonate |

|
0.0012 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.370 | Ethyl dec‐9‐enoate |

|
0.012 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.372 | Ethyl dodec‐(2E)‐enoate |

|
0.34 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.374 | Ethyl hept‐(2E)‐enoate |

|
0.61 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.379 | Ethyl pent‐2‐enoate |
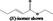
|
0.037 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Composition of the mixture to be specified |
| 09.578 | Hexyl (E)‐but‐2‐enoate |
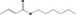
|
2.6 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 Complete specifications should be requested |
| 09.596 | Isopentyl‐(Z)‐but‐2‐enoate |
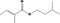
|
0.012 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.603 | Isopropyl crotonate |

|
0.24 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.624 | Methyl 2‐methylcrotonate |
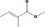
|
0.12 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.625 | Methyl 2‐methylpent‐3(E)‐enoate |
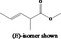
|
0.0012 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.636 | Methyl crotonate |

|
0.12 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.637 | Methyl dec‐2‐enoate |

|
0.37 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.641 | Methyl dodec‐(2E)‐enoate |

|
0.56 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.652 | Methyl oleate |

|
1.2 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.680 | Pentyl 2‐methylisocrotonate |
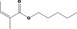
|
0.74 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.699 | Propyl crotonate |
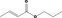
|
0.085 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.865 | Hexyl (9Z)‐octadecenoate |

|
0.24 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
|
09.934 1630 |
Methyl (5Z)‐Octenoate |
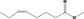
|
3.7 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.942 | 2‐Methylbutyl‐3‐methyl‐2‐butenoate |
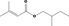
|
1.2 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 02.192 | Oct‐2‐en‐1‐ol |

|
7.7 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 02.231 | trans‐2, cis‐6‐Nonadien‐1‐ol |

|
8.7 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 05.081 | 2,4‐Decadienal |

|
19 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 05.144 | Dodec‐2(trans)‐enal |

|
0.75 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 05.184 | Undec‐2(trans)‐enal |
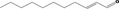
|
0.84 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 05.186 | 2,4‐Octadienal |

|
1.4 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 05.189 | 2‐Hexenal |

|
1.2 409 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 05.191 | trans‐2‐Decenal |

|
8.1 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 05.194 | tr‐2, tr‐4‐Nonadienal |

|
2.4 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 The chemical name should be changed in (2E,4E)‐nonadienal |
| 05.195 | trans‐2‐Tridecenal |

|
0.12 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 05.196 | tr‐2, tr‐4‐Undecadienal |

|
3 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 The chemical name should be changed in (2E,4E)‐undecadienal |
| 09.400 | Hex‐2‐enyl phenylacetate |

|
0.012 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 09.948 | (2E)‐2‐Nonenyl acetate |

|
0.012 |
Class I A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 09.375 | Ethyl methacrylate |
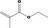
|
0.12 |
Class II A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.586 | Isobutyl 2‐methylprop‐2‐enoate |
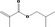
|
0.012 |
Class II A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
|
09.647 1834 |
Methyl methacrylate |
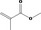
|
0.061 |
Class II A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev2 |
| 09.247 | Allyl crotonate |
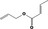
|
0.043 |
Class II A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
| 09.866 | Allyl valerate |
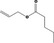
|
0.012 |
Class II A3: Intake below threshold No safety concern based on intake calculated by the MSDI approach |
Concluded in FGE.05Rev3 |
EU MSDI: Amount added to food as flavour in (kg/year) × 109/(0.1 × population in Europe (= 375 × 106) × 0.6 × 365) = μg/capita per day.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
Based on the conclusions on the 17 candidate substances in FGE.200Rev1 and FGE.203Rev2 that these substances can be considered as non‐genotoxic, they can be evaluated through the Procedure (Appendix A – Figure A.1).
Figure A.1.
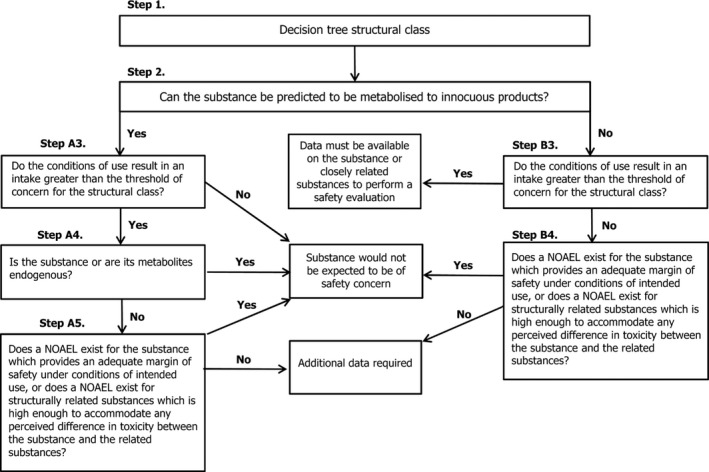
Procedure for safety evaluation of chemically defined flavouring substances
Step 1:
Of the 17 candidate flavouring substances, 15 are classified into structural class I according to the decision tree approach presented by Cramer et al. (1978). The remaining two candidate substances [FL‐no: 09.247 and 09.866], which are allyl esters, are classified as structural class II substances.
Step 2:
All 17 candidate substances were considered to be metabolised to innocuous products. They would not be expected to saturate available detoxification metabolic pathways at the estimated levels of the intake, based on the MSDI approach, from use as flavouring substances. Therefore, the evaluation of these 17 candidate substances proceeds along the A‐side of the Procedure scheme.
Step A3:
The 15 flavouring substances [FL‐no: 02.192, 02.231, 05.072, 05.081, 05.144, 05.184, 05.186, 05.189, 05.190, 05.191, 05.194, 05.195, 05.196, 09.400 and 09.948] assigned to structural class I, have estimated European daily capita intakes (MSDI) ranging from 0.012 to 19 μg/person per day (Appendix D – Table D.5). These intakes are below the threshold of concern of 1,800 μg/person per day for structural class I. The two allyl esters [FL‐no: 09.247 and 09.866], which have been assigned to structural class II, have estimated European daily capita intakes of 0.012 and 0.043 μg/person per day, respectively (Appendix D – Table D.5). These intakes are below the threshold of concern of 540 μg/person per day for structural class II. Based on results of the safety evaluation sequence these 17 candidate substances, proceeding via the A‐side of the Procedure, do not pose a safety concern when used as flavouring substances at the estimated levels of intake based on the MSDI approach.
According to the Procedure scheme (see Appendix A), no toxicological data are required. The toxicological information available for the supporting substances (see Section 3.3.3) does not conflict with the application of the Procedure or with the outcome of the evaluation.
4. Discussion
Following a request from the European Commission, the EFSA Panel on Food Additives and Flavourings (FAF) was asked to deliver a scientific opinion on the implications for human health of chemically defined flavouring substances used in or on foodstuffs in the Member States. In particular, the Panel was requested to evaluate a group of 17 flavouring substances allocated to FGE.05Rev3 using the Procedure as referred to in the Commission Regulation (EC) No 1565/2000. These flavouring substances are listed in the Union List, which was adopted by Commission Regulation (EU) No 872/20122 and its consecutive amendments. In total, FGE.05 consists of 54 substances, 37 of which have already been evaluated in FGE.05Rev2. These 37 substances have only been considered in this FGE with respect to updates on specifications and combined exposure. The present Revision of FGE.05, FGE.05Rev3, therefore, deals with the assessment of 17 additional candidate substances [FL‐no: 02.192, 02.231, 05.072, 05.081, 05.144, 05.184, 05.186, 05.189, 05.190, 05.191, 05.194, 05.195, 05.196, 09.247, 09.400, 09.866 and 09.948]. These substances possess an α,β‐unsaturated carbonyl structure, or precursor for that, which is considered a structural alert for genotoxicity. They have been evaluated by EFSA in FGE.200Rev1 ([FL‐no: 02.192, 02.231, 05.072, 05.144, 05.184, 05.189, 05.190, 05.191, 05.195, 09.247, 09.400, 09.866, 09.948]) and in FGE.203Rev2 ([FL‐no: 05.081, 05.186, 05.194, 05.196]) in which FGEs the concern for genotoxicity concern could be ruled out. Accordingly, the Panel concluded that these flavouring substances can be evaluated through the Procedure.
Of the 17 newly included flavouring substances in FGE.05Rev3, 15 belong to structural class I. The remaining two substances [FL‐no: 09.247 and 09.866], have been assigned to structural class II.
Of the 17 candidate flavouring substances, 15 have been reported to occur naturally in a wide range of food items (Appendix G).
Based on the assessment of the available in vitro and in vivo genotoxicity tests issued in FGE.200Rev1 and FGE.203Rev2, no concern is raised with respect to genotoxicity for the 17 candidate substances under evaluation in the present revision of FGE.05. All the 17 additional candidate substances in FGE.05Rev3 would be expected to be metabolised to innocuous substances at the estimated levels of intake as flavouring substances. Therefore, their evaluation proceeds along the A‐side of the Procedure. According to the default MSDI approach, the 17 flavouring substances have European daily per capita intakes (MSDI) ranging from 0.012 and 19 μg/person per day, which are below the threshold of concern for structural class I and class II substances (i.e. 1,800 μg/person per day and 540 μg/person per day, respectively). Based on results of the safety evaluation sequence, none of these 17 additional candidate substances in FGE.05Rev3 would give rise to safety concerns at the estimated levels of intake arising from their use as flavouring substances, based on the MSDI approach.
The total estimated combined intake of the 54 candidate substances in FGE.05Rev3 and their 61 supporting substances (in Europe) would be 8,872 μg/capita per day. This latter value does exceed the threshold of concern for the structural class I and II (i.e. 1,800 μg/capita per day and 540 μg/capita per day, respectively). However, more than 80% of the combined exposure estimate is represented by four supporting substances [FL‐no: 02.056, 05.073, 02.020 and 08.038]. For Hex‐2(trans)‐enal [FL‐no: 05.073] and hex‐3(cis)‐en‐1‐ol [FL‐no: 02.056], NOAELs have been reported and they provide adequate margins of safety.
The estimated intakes based on the mTAMDI for 12 of the 17 newly included candidate substances for which normal and maximum use levels were submitted, i.e. [FL‐no: 02.192, 02.231, 05.144, 05184,05,189, 05.190, 05.191, 05.195, 09.400, 09.948, 09.247 and 09.866], are above or equal to the TTC for structural class I (i.e. 1,800 μg/person per day) or II (i.e. 540 μg/person per day) (see Appendix D – Table D.5). Therefore, for these substances further information is required. This would include more reliable intake data and then, if required, additional toxicological data. This also applies to 27 substances evaluated in FGE.05Rev2. In addition, for 8 substances ([FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.287, 09.326 and 09.578]) evaluated in FGE.05Rev2, normal and maximum levels for their use in food are still missing.
In order to determine whether the conclusion for these 17 candidate substances evaluated through the Procedure can be applied to the materials of commerce, the Panel considered the available specifications. Adequate specifications, including purity and identity for the materials of commerce, have been provided for the 17 newly added candidate substances. From FGE.05Rev2, for 11 substances [FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.181, 09.329, 09.335, 09.379, 09.578 and 09.637], the information on specifications is incomplete. For ten of these [FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.181, 09.329, 09.335, 09.379 and 09.637] quantitative figures on the composition of stereoisomeric mixtures are missing. For [FL‐no: 09.578], complete specifications should be provided.
5. Conclusions
Overall, the Panel concluded that the 17 flavouring substances [FL‐no: 02.192, 02.231, 05.072, 05.081, 05.144, 05.184, 05.186, 05.189, 05.190, 05.191, 05.194, 05.195, 05.196, 09.247, 09.400, 09.866, 09.948], cleared for genotoxicity in FGE.200Rev1 and FGE.203Rev2, and evaluated through the Procedure in this FGE would not be expected to present a safety concern at their estimated levels of intake based on the MSDI approach.
6. Recommendations
Normal and maximum use levels should be requested for [FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.287, 09.326 and 09.578].
Except for flavouring substances [FL‐no: 05.072, 05.081, 05.186, 05.194, 05.196, 09.934 and 09.942], more reliable intake data should be requested for the 39 remaining flavouring substances, for which use levels were submitted, as their mTAMDI exposure estimates are above the threshold of concern for structural class I or II.
For flavouring substances [FL‐no: 08.072, 08.083, 08.101, 08.119, 08.120, 09.181, 09.329, 09.335, 09.379 and 09.637], quantitative data on the composition of stereoisomeric mixtures should be requested.
Complete specifications should be requested for flavouring substances [FL‐no. 09.578].
Flavouring substance [FL‐no: 09.287] should be deleted from the UL as this substance is covered by [FL‐no: 09.840], provided that the compositions of the stereoisomers mixtures for these two substances are the same. In FGE.70Rev1, it is recommended that this information is requested to the applicant.
In the Union list, the chemical names of flavouring substances [FL‐no: 05.194 and 05.196] should be changed as indicated in Appendix B – Table B.1.
Documentation provided to EFSA
EFFA (European Flavour Association), 2018a. EFFA 2015 poundage information for 74 substances from FGE.19 subgroup 1.1.1 corresponding to FGE.200. Unpublished data submitted from EFFA to EFSA. Dated August 2018.
EFFA (European Flavour Association), 2017a. Use levels survey for 84 substances from FGE.200. Unpublished data submitted from EFFA to EFSA. Dated 31/7/17.
EFFA (European Flavour Association), 2016h. Aggregated Poundage (Volume of use) for 16 substances from FGE.203 for 2010 to 2015. Unpublished data submitted from EFFA to EFSA. Dated 21/9/16.
EFFA (European Flavour Association), 2016i. Identification, characterisation and isomerism of 21 substances from FGE.203. Unpublished data submitted from EFFA to EFSA. Dated 21/9/16.
EFFA (European Flavour Association), 2010. Submission by the European Flavour Association to the European Food Safety Authority. Flavouring Group Evaluation 19 Subgroup 1.1.1(corresponding to FGE.200): Submission of additional data related to FGE.19 subgroup1.1.1. 25 Flavouring Substances (Flavouring Substances) of the Chemical Group 3 (Annex I of 1565/2000/EC) Structurally Related to Straight‐Chain Aliphatic Acyclic alpha,beta‐Unsaturated Aldehydes, with or without non‐Conjugated Double Bonds, Used as Flavouring Substances. 14 April 2010.
EFFA (European Flavour Association), 2011h. Assay values for 42 Register substances submitted by EFFA to FLAVIS Secretariat. September 2011. FLAVIS/8.126.
EFFA (European Flavour Association), 2010a. EFFA Letters to EFSA on clarification of specifications and isomerism for which data were requested in published FGEs.
EFFA (European Flavour Association), 2019. EFFA Submission of additional information on isomeric composition of substances within FGE.200Rev1 (FGE.19 Subgroup 1.1.1) (FGE.05 Rev3).
EFFA (European Flavour Association), 2004e. Intake ‐ Collection and collation of usage data for flavouring substances. Letter from Dan Dils, EFFA to Torben Hallas‐Møller, EFSA. May 31, 2004.
EFFA (European Flavour Association), 2006z. Transfer files for FGE.05Rev2 concerning [FL‐no: 09.181]. Unpublished data from EFFA to FLAVIS Secretariat.
EFFA (European Flavour Association), 2007a. E‐mail from Jan Demyttenaere, EFFA to Flavis Secretariat, National Food Institute, Technical University of Denmark. Dated 8 February 2007. RE: FLAVIS submissions ‐ use levels for Category 14.2 – Alcoholic beverages FLAVIS/8.70.
EFFA (European Flavour Association), 2007i. Submission 2007‐03. Safety evaluation of aliphatic branched‐chain, saturated and unsaturated alcohols, aldehydes, acids and related esters used as flavouring agents (S23‐J40). Submission 2007_03_EFSA S23‐J40. Unpublished report submitted by EFFA to FLAVIS Secretariat. FLAVIS/8.100.
EFFA (European Flavour Association), 2007j. Submission 2007‐01. Safety evaluation of aliphatic, linear alpha, beta‐unsaturated aldehydes, acids and related alcohols, acetals and esters used as flavouring agents (S27‐J47). Submission 2007_03_EFSA S27‐J47. Unpublished report submitted by EFFA to FLAVIS Secretariat. FLAVIS/8.101.
Flavour Industry, 2004g. Unpublished information submitted by Flavour Industry to DG SANCO and forwarded to EFSA. A‐05rev2.
Flavour Industry, 2006a. Unpublished information submitted by Flavour Industry to DG SANCO and forwarded to EFSA. A‐05.
Flavour Industry, 2007k. Unpublished information submitted by Flavour Industry to DG SANCO and forwarded to EFSA. A‐05.
EFFA (European Flavour Association), 2001e. Submission 2000‐3. Flavouring group evaluation of 24 flavouring substances (candidate chemicals) of the chemical groups 1 and 2 (Annex I of 1565/2000/EC), structurally related to linear and branched‐chain aliphatic, unsaturated, unconjugated alcohols, aldehydes, acids, and related esters from FAO/WHO JECFA 42/51. November 20, 2001. SCOOP/FLAV/8.7. European inquiry on volume of use. IOFI, International Organization of the Flavor Industry, 1995. Private communication to FEMA. Unpublished report submitted by EFFA to SCF.
EFFA (European Flavour Association), 2006z. Transfer files for FGE.05Rev2 concerning [FL‐no: 09.181]. Unpublished data from EFFA to FLAVIS Secretariat.
EFFA (European Flavour Association), 2007i. Submission 2007‐03. Safety evaluation of aliphatic branched‐chain, saturated and unsaturated alcohols, aldehydes, acids and related esters used as flavouring agents (S23‐J40). Submission 2007_03_EFSA S23‐J40. Unpublished report s EFFA 2007j. Submission 2007‐01. Safety evaluation of aliphatic, linear alpha, beta‐unsaturated aldehydes, acids and related alcohols, acetals and esters used as flavouring agents (S27‐J47). Submission 2007_03_EFSA S27‐J47. Unpublished report submitted by EFFA to FLAVIS Secretariat. FLAVIS/8.101. Submitted by EFFA to FLAVIS Secretariat. FLAVIS/8.100.
EFFA (European Flavour Association), 2007j. Submission 2007‐01. Safety evaluation of aliphatic, linear alpha, beta‐unsaturated aldehydes, acids and related alcohols, acetals and esters used as flavouring agents (S27‐J47). Submission 2007_03_EFSA S27‐J47. Unpublished report submitted by EFFA to FLAVIS Secretariat. FLAVIS/8.101.
EFFA (European Flavour Association), 2019. Submission of additional information on isomeric composition of substances within FGE.05 Rev3 (FGE.19 Subgroup 1.1.1 & 1.1.4).
EFFA (European Flavour Association), 2002i. Letter from EFFA to Dr. Joern Gry, Danish Veterinary and Food Administration. Dated 31 October 2002. Re.: Second group of questions. FLAVIS/8.26.
EFFA (European Flavour Association), 2010. Letter from EFFA to EFSA: clarifications on EFSA questions on FGE.05Rev1 (The EFSA Journal (2008); 643, 1–80) & FGE.05Rev2.
Abbreviations
- (Q)SAR
(quantitative) structure–activity relationship
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism and exposure
- AFC EFSA
Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food
- bw
body weight
- CAS
Chemical Abstract Service
- CEF
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CoE
Council of Europe
- EFFA
European Flavour and Fragrance Association
- FAF
EFSA Panel on Food Additives and Flavourings
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS (FL)
Flavour Information System (database)
- GSH
glutathione
- ID
identity
- IOFI
International Organization of the Flavour Industry
- IR
infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MSDI
maximised survey‐derived daily intake
- MS
mass spectrometry
- mTAMDI
modified Theoretical Added Maximum Daily Intake
- NMR
nuclear magnetic resonance
- NOAEL
No observed adverse effect level
- No
number
- SCF
Scientific Committee on Food
- TTC
toxicological thresholds of concern
- WHO
World Health Organization
Appendix A – Procedure of the safety evaluation
1.
The approach for a safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/2000, named the ‘Procedure’, is shown in schematic form in Figure A1. The Procedure is based on the Opinion of the Scientific Committee on Food expressed on 2 December 1999 (SCF, 1999), which is derived from the evaluation Procedure developed by the Joint FAO/WHO Expert Committee on Food Additives at its 44th, 46th and 49th meetings (JECFA, 1995, 1996, 1997, 1999).6
The Procedure is a stepwise approach that integrates information on intake from current uses, structure‐activity relationships, metabolism and, when needed, toxicity. One of the key elements in the Procedure is the subdivision of flavourings into three structural classes (I, II and III) for which toxicological thresholds of concern (TTCs) (human exposure thresholds) have been specified. Exposures below these TTCs are not considered to present a safety concern.
Class I contains flavourings that have simple chemical structures and efficient modes of metabolism, which would suggest a low order of oral toxicity. Class II contains flavourings that have structural features that are less innocuous but are not suggestive of toxicity. Class III comprises flavourings that have structural features that permit no strong initial presumption of safety, or may even suggest significant toxicity (Cramer et al., 1978). The TTCs for these structural classes of 1,800, 540 or 90 μg/person per day, respectively, are derived from a large database containing data on subchronic and chronic animal studies (JECFA, 1996).
In step 1 of the Procedure, the flavourings are assigned to one of the structural classes. The further steps address the following questions:
Can the flavourings be predicted to be metabolised to innocuous products7 (Step 2)?
Do their exposures exceed the TTC for the structural class (Steps A3 and B3)?
Are the flavourings or their metabolites endogenous8 (Step A4)?
Does a NOAEL exist on the flavourings or on structurally related substances (Steps A5 and B4)?
In addition to the data provided for the flavouring substances to be evaluated (candidate substances), toxicological background information available for compounds structurally related to the candidate substances is considered (supporting substances), in order to assure that these data are consistent with the results obtained after application of the Procedure.
The Procedure is not to be applied to flavourings with existing unresolved problems of toxicity. Therefore, the right is reserved to use alternative approaches if data on specific flavourings warranted such actions.
Intake
Annual production volumes of the flavouring substances as surveyed by the Industry can be used to calculate the ‘Maximised Survey‐derived Daily Intake’ (MSDI) by assuming that the production figure only represents 60% of the use in food due to underreporting and that 10% of the total EU population are consumers (SCF, 1999).
However, the Panel noted that due to year‐to‐year variability in production volumes, to uncertainties in the underreporting correction factor and to uncertainties in the percentage of consumers, the reliability of intake estimates on the basis of the MSDI approach is difficult to assess.
The Panel also noted that in contrast to the generally low per capita intake figures estimated on the basis of this MSDI approach, in some cases the regular consumption of products flavoured at use levels reported by the Flavour Industry in the submissions would result in much higher intakes. In such cases, the human exposure thresholds below which exposures are not considered to present a safety concern might be exceeded.
Considering that the MSDI model may underestimate the intake of flavouring substances by certain groups of consumers, the SCF recommended also taking into account the results of other intake assessments (SCF, 1999).
One of the alternatives is the ‘Theoretical Added Maximum Daily Intake’ (TAMDI) approach, which is calculated on the basis of standard portions and upper use levels (SCF, 1995) for flavourable beverages and foods in general, with exceptional levels for particular foods. This method is regarded as a conservative estimate of the actual intake by most consumers because it is based on the assumption that the consumer regularly eats and drinks several food products containing the same flavouring substance at the upper use level.
One option to modify the TAMDI approach is to base the calculation on normal rather than upper use levels of the flavouring substances. This modified approach is less conservative (e.g., it may underestimate the intake of consumers being loyal to products flavoured at the maximum use levels reported). However, it is considered as a suitable tool to screen and prioritise the flavouring substances according to the need for refined intake data (EFSA, 2004).
The method for the modified TAMDI (mTAMDI) calculations is described in Appendix D – Table D.3.
Table D.3.
Estimated amount of flavourable foods, beverages, and exceptions assumed to be consumed per person per day (SCF, 1995)
| Class of product category | Intake estimate (g/day) |
|---|---|
| Beverages (non‐alcoholic) | 324.0 |
| Food | 133.4 |
| Exception a: Candy, confectionery | 27.0 |
| Exception b: Condiments, seasonings | 20.0 |
| Exception c: Alcoholic beverages | 20.0 |
| Exception d: Soups, savouries | 20.0 |
| Exception e: Others, e.g. chewing gum | e.g. 2.0 (chewing gum) |
To gather information on the occurrence and levels of a flavouring substance in natural sources, the Triskelion database is used (available on the following link http://www.vcf-online.nl/VcfHome.cfm/ sign in IP address/search/compounds).
Appendix B – Specifications
1.
Appendix C – Supporting substances for FGE.05Rev3
1.
Table C.1.
Summary of supporting substances for FGE.05Rev3
| FL‐no | EU Union List chemical name | Structural formula | FEMA no CoE no CAS no | JECFA no Specification available | MSDI (EU)a (μg/capita per day) | SCF statusb JECFA status CoE statusc | Comments |
|---|---|---|---|---|---|---|---|
| 02.020 | Hex‐2‐en‐1‐ol |

|
2562 69 2305‐21‐7 |
1354 JECFA specification (JECFA, 2005a) |
650 |
No safety concern (JECFA, 2005b) Category B (CoE, 1992) |
|
| 02.049 | Nona‐2,6‐dien‐1‐ol |
|
2780 589 7786‐44‐9 |
1184 JECFA specification (JECFA, 2003b) |
9.1 |
No safety concern (JECFA, 2004b) Category B (CoE, 1992) |
JECFA evaluated 2,6‐Nonadien‐1‐ol (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CAS rn in Union List |
| 02.056 | Hex‐3(cis)‐en‐1‐ol |

|
2563 750c 928‐96‐1 |
315 JECFA specification (JECFA, 1998) |
3,700 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 02.074 | Hex‐4‐en‐1‐ol |

|
3430 2295 6126‐50‐7 |
318 JECFA specification (JECFA, 1998) |
2.4 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
JECFA evaluated 4‐hexen‐1‐ol (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 02.090 | Non‐2(trans)‐en‐1‐ol |
|
3379 10292 31502‐14‐4 |
1365 JECFA specification (JECFA, 2005a) |
0.016 | No safety concern (JECFA, 2005b) | |
| 02.093 | (Z)‐Non‐6‐en‐1‐ol |

|
3465 10294 35854‐86‐5 |
324 JECFA specification (JECFA, 2000b) |
2.2 | No safety concern (JECFA, 2000a) | JECFA evaluated cis‐6‐nonen‐1‐ol (CASrn as in Union List). CASrn in Union List refers to (Z)‐isomer |
| 02.094 | Oct‐3‐en‐1‐ol |

|
3467 10296 20125‐84‐2 |
321 JECFA specification (JECFA, 1998) |
4.7 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated cis‐3‐octen‐1‐ol (CASrn as in Union List). CASrn in Union List refers to the (Z)‐isomer. Union List name to be changed to Oct‐3Z‐en‐1‐ol |
| 02.110 | 2,6‐Dimethylhept‐6‐en‐1‐ol |

|
3663 36806‐46‐9 |
348 JECFA specification (JECFA, 2003b) |
ND |
Category 3 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 2,6‐dimethyl‐6‐hepten‐1‐ol (CASrn as in Union List). (R)‐ or (S)‐enantiomer not specified by CASrn in Union List |
| 02.113 | Oct‐5(cis)‐en‐1‐ol |

|
3722 64275‐73‐6 |
322 JECFA specification (JECFA, 2003b) |
0.4 |
Category 2 (SCF, 1995) No safety concern (JECFA, 2000a) |
|
| 05.035 | Undec‐10‐enal |

|
3095 122 112‐45‐8 |
330 JECFA specification (JECFA, 2001b) |
0.32 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 05.036 | Undec‐9‐enal |

|
3094 123 143‐14‐6 |
329 JECFA specification (JECFA, 2003b) |
0.97 |
No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
JECFA evaluated 9‐undecenal (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 05.037 | 2‐Dodecenal |

|
2402 124 4826‐62‐4 |
1350 JECFA specification (JECFA, 2005a) |
1.2 |
No safety concern (JECFA, 2005b) Category A (CoE, 1992) |
JECFA evaluated 2‐Dodecenal (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 05.040 | alpha‐Pentylcinnamaldehyde |
|
2061 128 122‐40‐7 |
685 JECFA specification (JECFA, 2000b) |
22 |
No safety concern (JECFA, 2001a) Category A (CoE, 1992) |
JECFA evaluated alpha‐Amylcinnamaldehyde (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 05.059 | Non‐6(cis)‐enal |
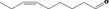
|
3580 661 2277‐19‐2 |
325 JECFA specification (JECFA, 2003b) |
1.7 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 05.060 | Oct‐2‐enal |
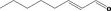
|
3215 663 2363‐89‐5 |
1363 JECFA specification (JECFA, 2005a) |
0.84 |
No safety concern (JECFA, 2005b) Category A (CoE, 1992) |
JECFA evaluated 2‐Octenal (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 05.071 | Nona‐2,4‐dienal |

|
3212 732 6750‐03‐4 |
1185 JECFA specification (JECFA, 2003b); JECFA 1185 evaluated (2E,4E)‐Nona‐2.4‐dienal with CASrn 1771‐49‐0 |
0.94 |
No safety concern (JECFA, 2004b) Category B (CoE, 1992) |
JECFA 1185 evaluated (2E,4E)‐Nona‐2.4‐dienal with CASrn 1771‐49‐0 which is not a valid no. CASrn 5910‐87‐2 refers to (2E,4E)‐2,4‐Nonadienal |
| 05.073 | Hex‐2(trans)‐enal |

|
2560 748 6728‐26‐3 |
1353 | 2,800 |
No safety concern (JECFA, 2005b) Category A (CoE, 1992) |
JECFA evaluated 2‐Hexenal but with CASrn for the trans‐isomer (as in Union List) |
| 05.074 | 2,6‐Dimethylhept‐5‐enal |

|
2389 2006 106‐72‐9 |
349 JECFA specification (JECFA, 2003b) |
27 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
JECFA evaluated 2,6‐dimethyl‐5‐heptenal (CASrn as in Union List). (R)‐ or (S)‐enantiomer not specified by CASrn in Union List |
| 05.075 | Hex‐3(cis)‐enal |

|
2561 2008 6789‐80‐6 |
316 JECFA specification (JECFA, 2000b) |
4.1 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 05.076 | Dec‐2‐enal |

|
2366 2009 3913‐71‐1 |
1349 JECFA specification (JECFA, 2005a) |
13 |
No safety concern (JECFA, 2005b) Category A (CoE, 1992) |
JECFA evaluated 2‐Decenal (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 05.078 | Tridec‐2‐enal |

|
3082 2011 7774‐82‐5 |
1359 JECFA specification (JECFA, 2005a) |
0.97 |
No safety concern (JECFA, 2005b) Category A (CoE, 1992) |
JECFA evaluated 2‐Tridecenal (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 05.085 | (Z)‐Hept‐4‐enal |
|
3289 2124 6728‐31‐0 |
320 JECFA specification (JECFA, 2000b) |
1.6 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
JECFA evaluated 4‐heptenal (CASrn as in Union List). CASrn in Union List refers to the (Z)‐isomer |
| 05.096 | 4‐Decenal |

|
3264 2297 30390‐50‐2 |
326 JECFA specification (JECFA, 2001b) |
0.97 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
JECFA evaluated 4‐decenal (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 05.108 | Undeca‐2,4‐dienal |

|
3422 10385 13162‐46‐4 |
1195 JECFA specification (JECFA, 2003b) |
2.8 | No safety concern (JECFA, 2004b) | JECFA evaluated 2,4‐Undecadienal (CAS rn as in Union List). (Z)‐ or (E)‐isomer not specified by CAS rn in Union List |
| 05.109 | 2‐Undecenal |

|
3423 11827 2463‐77‐6 |
1366 JECFA specification (JECFA, 2005a) |
0.65 | No safety concern (JECFA, 2005b) | JECFA evaluated 2‐Undecenal (CAS rn as in Union List). (Z)‐ or (E)‐isomer not specified by CAS rn in Union List |
| 05.113 | Hex‐4‐enal |

|
3496 10337 4634‐89‐3 |
319 JECFA specification (JECFA, 2000b) |
0.024 | No safety concern (JECFA, 2000a) | JECFA evaluated cis‐4‐hexenal (CASrn as in Union List). CASrn in Union List refers to the (Z)‐isomer. Union List name to be changed to Hex‐4Z‐enal |
| 05.128 | Oct‐5(cis)‐enal |

|
3749 41547‐22‐2 |
323 JECFA specification (JECFA, 2003b). |
0.0012 | No safety concern (JECFA, 2000a) | |
| 05.140 | Deca‐2(trans),4(trans)‐dienal |

|
3135 2120 25152‐84‐5 |
1190 JECFA specification (JECFA, 2003b) |
62 | No safety concern (JECFA, 2004b) | |
| 05.171 | Non‐2‐enal |
|
3213 733 2463‐53‐8 |
1362 JECFA specification (JECFA, 2005a) |
9.9 |
No safety concern (JECFA, 2005b) Category A (CoE, 1992) |
JECFA evaluated 2‐Nonenal (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 08.002 | Acetic acid |
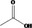
|
2006 2 64‐19‐7 |
81 JECFA specification (JECFA, 2000b) |
ND |
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
|
| 08.007 | Valeric acid |
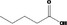
|
3101 7 109‐52‐4 |
90 JECFA specification (JECFA, 1997) |
120 |
Category 1 (SCF, 1995) No safety concern (JECFA, 1999) Category A (CoE, 1992) |
|
| 08.013 | Oleic acid |

|
2815 13 112‐80‐1 |
333 JECFA specification (JECFA, 2000b) |
830 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Deleted (CoE, 1992) |
|
| 08.038 | Phenylacetic acid |
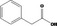
|
2878 672 103‐82‐2 |
1007 JECFA specification (JECFA, 2002b) |
240 |
No safety concern (JECFA, 2002a) Category B (CoE, 1992) |
|
| 08.039 | Undec‐10‐enoic acid |

|
3247 689 112‐38‐9 |
331 JECFA specification (JECFA, 1998) |
26 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 08.041 | Octadeca‐9,12‐dienoic acid |

|
3380 694 60‐33‐3 |
332 JECFA specification (JECFA, 2003b) |
110 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Deleted (CoE, 1992) |
Union List name to be changed to Linoleic acid |
| 08.048 | Pent‐4‐enoic acid |
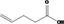
|
2843 2004 591‐80‐0 |
314 JECFA specification (JECFA, 1998) |
3.9 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 08.050 | Hex‐3‐enoic acid |
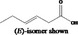
|
3170 2256 4219‐24‐3 |
317 JECFA specification (JECFA, 2000b) |
9.4 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
JECFA evaluated 3‐hexenoic acid (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 08.058 | 2‐Methylpent‐3‐enoic acid |
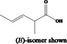
|
3464 10147 37674‐63‐8 |
347 JECFA specification (JECFA, 2001b) |
1.2 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 2‐methyl‐3‐pentenoic‐acid (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 08.059 | 2‐Methylpent‐4‐enoic acid |
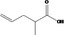
|
3511 10148 1575‐74‐2 |
355 JECFA specification (JECFA, 1998) |
ND |
Category N (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 2‐methyl‐4‐pentenoic‐acid (CASrn as in Union List). (R)‐ or (S)‐enantiomer not specified by CASrn in Union List |
| 08.065 | Dec‐9‐enoic acid |

|
3660 10090 14436‐32‐9 |
328 JECFA specification (JECFA, 2001b) |
0.097 |
Category 1 (SCF, 1995) No safety concern (JECFA, 2000a) |
|
| 08.068 | Dec‐(5‐ and 6)‐enoic acid |
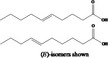
|
3742 72881‐27‐7 |
327 JECFA specification (JECFA, 2000b) |
3.4 |
Category N (SCF, 1995) No safety concern (JECFA, 2000a) |
JECFA evaluated 5 & 6‐decenoic acid (mixture) (CASrn as in Union List). CASrn in Union List refers to incompletely defined substance |
| 09.191 | Ethyl hex‐3‐enoate |
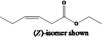
|
3342 2396‐83‐0 |
335 JECFA specification (JECFA, 1998) |
3.2 | No safety concern (JECFA, 2000a) | JECFA evaluated ethyl‐3‐hexenoate (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 09.192 | Ethyl oleate |
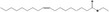
|
2450 633 111‐62‐6 |
345 JECFA specification (JECFA, 1998). |
60 |
No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
|
| 09.236 | Methyl undec‐9‐enoate |
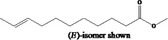
|
2750 2101 5760‐50‐9 |
342 JECFA specification (JECFA, 2000b) |
34 |
No safety concern (JECFA, 2000a) Deleted (CoE, 1992) |
JECFA evaluated methyl 9‐undecanoate (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 09.237 | Ethyl undec‐10‐enoate |
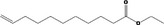
|
2461 10634 692‐86‐4 |
343 JECFA specification (JECFA, 1998) |
1.5 |
No safety concern (JECFA, 2000a) Deleted (CoE, 1992) |
|
| 09.238 | Butyl undec‐10‐enoate |
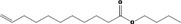
|
2216 2103 109‐42‐2 |
344 JECFA specification (JECFA, 2001b) |
0.037 |
No safety concern (JECFA, 2000a) Category B (CoE, 1992) |
|
| 09.265 | Ethyl oct‐4‐enoate |
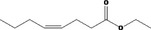
|
3344 10619 34495‐71‐1 |
338 JECFA specification (JECFA, 2003b) |
1.2 | No safety concern (JECFA, 2000a) | JECFA evaluated ethyl cis‐4‐octenoate (CASrn as in Union List). CASrn in Union List refers to (Z)‐isomer. Union List name to be changed to Ethyl oct‐4Z‐enoate |
| 09.267 | Methyl hex‐3‐enoate |
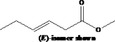
|
3364 10801 2396‐78‐3 |
334 JECFA specification (JECFA, 2001b) |
0.56 | No safety concern (JECFA, 2000a) | Z‐ or E‐isomer not specified by name and CASrn in Union List |
| 09.268 | Methyl oct‐4(cis)‐enoate |
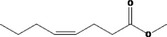
|
3367 10834 21063‐71‐8 |
337 JECFA specification (JECFA, 2003b) |
0.37 | No safety concern (JECFA, 2000a) | |
| 09.284 | Ethyl dec‐4‐enoate |

|
3642 10578 76649‐16‐6 |
341 JECFA specification (JECFA, 2000b) |
1.8 | No safety concern (JECFA, 2000a) | JECFA evaluated ethyl trans‐4‐decenoate (CASrn as in Union List). CASrn refers to (E)‐isomer. Union List name to be changed to E‐Ethyl dec‐4‐enoate |
| 09.290 | Ethyl octa‐4,7‐dienoate |

|
3682 69925‐33‐3 |
339 JECFA specification (JECFA, 2000b) |
1.8 | No safety concern (JECFA, 2000a) | JECFA evaluated ethyl cis‐4,7‐octadienoate (CASrn as in Union List). CASrn in Union List refers to the (Z)‐isomer. Union List name to be changed to Ethyl octa‐4Z,7‐dienoate |
| 09.291 | Hex‐3‐enyl hex‐3‐enoate |

|
3689 61444‐38‐0 |
336 JECFA specification (JECFA, 1998) |
3.2 | No safety concern (JECFA, 2000a) | JECFA evaluated cis‐3‐hexenyl cis‐3‐hexenoate (CASrn as in Union List). CASrn in Union List refers to the (Z)/(Z)‐isomer. Union List name to be changed to Hex‐3Z‐enyl hex‐3Z‐enoate |
| 09.298 | Methyl non‐3‐enoate |

|
3710 13481‐87‐3 |
340 JECFA specification (JECFA, 2000b) |
1.6 | No safety concern (JECFA, 2000a) | JECFA evaluated methyl 3‐nonenoate (CASrn as in Union List). (Z)‐ or (E)‐isomer not specified by CASrn in Union List |
| 09.524 | Ethyl 2‐methylpent‐3‐enoate |
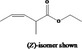
|
3456 10612 1617‐23‐8 |
350 JECFA specification (JECFA, 2001b) |
4.9 | No safety concern (JECFA, 2000a) | JECFA evaluated ethyl 2‐methyl‐3‐pentenoate (CASrn as in Union List). (Z)‐ or (E)‐isomer nor (R) or (S) enantiomer not specified by Union List CASrn |
| 09.527 | Ethyl 2‐methylpent‐4‐enoate |
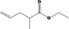
|
3489 10613 53399‐81‐8 |
351 JECFA specification (JECFA, 1998) |
0.024 | No safety concern (JECFA, 2000a) | (R) or (S) enantiomer not specified by Union List CASrn |
| 09.540 | Ethyl 2‐methylpenta‐3,4‐dienoate |
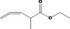
|
3678 60523‐21‐9 |
353 JECFA specification (JECFA, 2000b) |
0.012 | No safety concern (JECFA, 2007) | (R) or (S) enantiomer not specified by Union List CASrn. |
| 09.546 | Hexyl‐2‐methylpent‐(3 and 4)‐enoate |
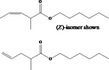
|
3693 58625‐95‐9 |
352 JECFA specification (JECFA, 2001b) |
0.024 | No safety concern (JECFA, 2000a) | JECFA evaluated hexyl 2‐methyl‐3&4‐pentenoate (mixture) (CASrn as in Union List). Union List CASrn refers to the (E)‐isomer. (R) or (S) enantiomer not specified by Union List CASrn |
| 09.559 | Hex‐3(cis)‐enyl 2‐methylcrotonate |
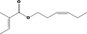
|
3931 67883‐79‐8 |
1277 JECFA specification (JECFA, 2003b) |
0.024 | No safety concern (JECFA, 2004b) | |
| 09.566 | (3Z)‐Hexenyl (E)‐but‐2‐enoate |
|
3982 65405‐80‐3 |
1276 JECFA specification (JECFA, 2003b) |
0.24 | No safety concern (JECFA, 2004b) | |
| 09.568 | (3Z)‐Hexenyl (E)‐hexenoate |
|
3928 53398‐87‐1 |
1279 JECFA specification (JECFA, 2003b) |
0.12 | No safety concern (JECFA, 2004b) | JECFA evaluated 3‐hexenyl 2‐hexenoate (CASrn as in Union List). Union List CASrn refers to the (3Z,2E)‐isomer. |
| 09.646 | Methyl linolenate |
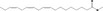
|
3411 714 301‐00‐8 |
346 JECFA specification (JECFA, 2003b) |
ND |
No safety concern (JECFA, 2000a) Category A (CoE, 1992) |
JECFA evaluated a mixture of methyl linoleate and methyl linolenate (CASrn as in Union List). Union List CASrn refers to the (Z)/(Z)/(Z)‐isomer (i.e. methyl linolenate) |
FGE: Flavouring Group Evaluation; FL‐no: FLAVIS number; FEMA: Flavor and Extract Manufacturers Association; CAS: Chemical Abstract Service; CoE: Council of Europe; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; MSDI: maximised survey‐derived daily intake; ND: No intake data reported; SCF: Scientific Committee on Food.
EU MSDI: Amount added to food as flavouring substance in (kg/year) × 109/(0.1 × population in Europe (= 375 × 106) × 0.6 × 365) = μg/capita per day.
Category 1: Considered safe in use, Category 2: Temporarily considered safe in use, Category 3: Insufficient data to provide assurance of safety in use, Category 4: Not acceptable due to evidence of toxicity, Category N: Cannot be listed in cat. 1‐4 due to e.g. not used in the EU, not considered to be a flavour.
Category A: Flavouring substance, which may be used in foodstuffs, Category B: Flavouring substance which can be used provisionally in foodstuffs.
Appendix D – Exposure estimates
D.1. Normal and maximum use levels
Table D.1.
Food categories according to Commission Regulation 1565/2000 (Annex III)
| For each of the 18 Food categories in which the candidate substances are used, Flavour Industry reports a ‘normal use level’ and a ‘maximum use level’. According to the industry, the ‘normal use’ is defined as the average of reported usages and ‘maximum use’ is defined as the 95th percentile of reported usages (Documentation provided to EFSA n. 22). The normal and maximum use levels in different food categories have been obtained from surveyed data or extrapolated from figures derived from 12 model flavouring substances (iterated use levels) (Documentation provided to EFSA n. 9) | |
| Food category | Food category |
| 01.0 | Dairy products, excluding products of category 02.0 |
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) |
| 03.0 | Edible ices, including sherbet and sorbet |
| 04.1 | Processed fruit |
| 04.2 | Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds |
| 05.0 | Confectionery |
| 06.0 | Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery |
| 07.0 | Bakery wares |
| 08.0 | Meat and meat products, including poultry and game |
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms |
| 10.0 | Eggs and egg products |
| 11.0 | Sweeteners, including honey |
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. |
| 13.0 | Foodstuffs intended for particular nutritional uses |
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products |
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts |
| 15.0 | Ready‐to‐eat savouries |
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) – foods that could not be placed in categories 01.0–15.0 |
D.2. mTAMDI calculations
The method for calculation of modified Theoretical Added Maximum Daily Intake (mTAMDI) values is based on the approach used by SCF (SCF, 1995). The assumption is that a person may consume the amount of flavourable foods and beverages listed in Table D.3. These consumption estimates are then multiplied by the reported use levels in the different food categories and summed up.
The mTAMDI calculations are based on the normal use levels reported by Industry. The seven food categories used in the SCF TAMDI approach (SCF, 1995) correspond to the 18 food categories, as outlined in Commission Regulation (EC) No 1565/2000, and reported by the Flavour Industry in the following way (see Table II.2.2):
Beverages (SCF, 1995) correspond to food category 14.1.
Foods (SCF, 1995) correspond to the food categories 1, 2, 3, 4.1, 4.2, 6, 7, 8, 9, 10, 13, and/or 16.
Exception a (SCF, 1995) corresponds to food category 5 and 11.
Exception b (SCF, 1995) corresponds to food category 15.
Exception c (SCF, 1995) corresponds to food category 14.2.
Exception d (SCF, 1995) corresponds to food category 12.
Exception e (SCF, 1995) corresponds to others, e.g. chewing gum.
Table D.4.
Distribution of the 18 food categories listed in Commission Regulation (EC) No 1565/2000 into the seven SCF food categories used for TAMDI calculation (SCF, 1995)
| Food categories according to Commission Regulation 1565/2000 | Distribution of the seven SCF food categories | |||
|---|---|---|---|---|
| Key | Food category | Foods | Beverages | Exceptions |
| 01.0 | Dairy products, excluding products of category 02.0 | Foods | ||
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) | Foods | ||
| 03.0 | Edible ices, including sherbet and sorbet | Foods | ||
| 04.1 | Processed fruit | Foods | ||
| 04.2 | Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds | Foods | ||
| 05.0 | Confectionery | Exception a | ||
| 06.0 | Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery | Foods | ||
| 07.0 | Bakery wares | Foods | ||
| 08.0 | Meat and meat products, including poultry and game | Foods | ||
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | Foods | ||
| 10.0 | Eggs and egg products | Foods | ||
| 11.0 | Sweeteners, including honey | Exception a | ||
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. | Exception d | ||
| 13.0 | Foodstuffs intended for particular nutritional uses | Foods | ||
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products | Beverages | ||
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts | Exception c | ||
| 15.0 | Ready‐to‐eat savouries | Exception b | ||
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) ‐ foods that could not be placed in categories 01.0–15.0 | Foods | ||
The MSDI and mTAMDI intake estimates (when use levels available) for flavouring substances in FGE.05Rev3, are reported in the table below.
Appendix E – Summary of safety evaluations
1.
Appendix F – Toxicity studies
1.
Appendix G – Natural food occurrence
1.
| FL‐no | EU Union List name | CAS no | VCF online (Triskelion, 2018) |
|---|---|---|---|
| 02.192 | Oct‐2‐en‐1‐ol | 22104‐78‐5 | Quantified in apple, asparagus, beef, desert truffle, fish, malt, oysters, potato, Vaccinium species from 0.0007 up to 4.36 mg/kg and up to 7.5 mg/kg in mushroom. Identified in black currants, capers, date, olive, soybean, tea, truffle and wine |
| 02.231 | tr‐2, cis‐6‐Nonadien‐1‐ol | 28069‐72‐9 | Quantified in brown algae, melon, milk products, prickly pear from 0.0002 up to 1.87 mg/kg and identified in cucumber, fish, malt, pepino fruit, rapeseed, spinach and tea |
|
05.072 1362 |
trans‐2‐Nonenal | 18829‐56‐6 | Quantified in more than 50 food items with up to 46 mg/kg in vanilla and up to 1,000 mg/kg in citrus fruits and identified in more than 40 other food items, e.g. nuts, shrimps, tomatoes and rice |
| 05.081 | 2,4‐Decadienal | 2363‐88‐4 | Quantified in beef, cabbage, chicken, citrus fruits, guinea hen, lamb, malt, Mangifera species, peanut, potato chips, tea, tomato and Vaccinium species from 0.0001 up to 1,000 mg/kg in maize and up to 2,000 mg/kg in mentha oils. Identified in more than 30 food items, e.g. beans, cassava and eggs |
| 05.144 | Dodec‐2(trans)‐enal | 20407‐84‐5 | Quantified in chicken, citrus fruits, coriander seed, milk products and olive from 0.002 up to 4,400 mg/kg and up to 27,000 mg/kg in coriander leaf. Identified in allium species, ginger, mushrooms and peanut |
| 05.184 | Undec‐2(trans)‐enal | 53448‐07‐0 | Quantified in black currant, camomile, chicken, citrus fruits, fig, fish, guinea hen, maize, milk products, pecan and tea from 0.002 up to 1,300 mg/kg and up to 7,000 mg/kg in camomile. Identified in beef, cashew apple, Chinese liquor, coriander leaf and seed, katsuobushi, mate, mushroom, olive, peanut, potato chips, rambutan, rice, rooibos tea and sesame seed (roasted) |
| 05.186 | 2,4‐Octadienal | 5577‐44‐6 | Quantified in peanut up to 0.001 mg/kg and identified in banana, buckwheat, caviar, cheese, fish, katsuobushi, oats, prickly pear, rice, rooibos tea, sesame seed, soybean and wheaten bread |
| 05.189 | 2‐Hexenal | 505‐57‐7 | Quantified in beer, cabbage, cauliflower and broccoli, chicken, citrus fruits, loganberry juice, malt, olive, origanum, peanut, raspberry, blackberry and boysenberry and tea from trace up to 160 mg/kg and up to 26,000 mg/kg in lemon balm. Identified in nearly 40 food items e.g. beans, beef, caviar, cucumber and kiwifruit |
| 05.190 | trans‐2‐Octenal | 2548‐87‐0 | Quantified in processed apples, apricot, beef, beer, black currant, brown algae, camomile, chicken, citrus fruits, truffle, fig, fish, ginger, guava and feyoa, guinea hen, maize, Mangifera species, melon, mentha oils, milk, mushroom, plum, pork, potato, potato chips, rice, rice cake, sherry, tomato, Vaccinium species, vanilla and wine. Identified in more than 40 other food items e.g. allium species, avocado, capsicum species, cocoa, date, egg, honey, olive and peanut |
| 05.191 | trans‐2‐Decenal | 3913‐81‐3 | Quantified in more than 30 food items with up to 2,000 mg/kg in camomile and citrus fruits, up to 130,000 mg/kg in caraway and up to 268,000 mg/kg in coriander leaf. Identified in more than 20 other food items, e.g. gin, buckwheat and ginger |
| 05.194 | tr‐2, tr‐4‐Nonadienal | 5910‐87‐2 | Quantified in 30 food items e.g. milk and milk products, oats and chicken from trace amounts up to 7.41 mg/kg in capers. Identified in more than 20 other food items, e.g. beef, cheese, raspeberry and sesame seed. |
| 05.195 | trans‐2‐Tridecenal | 7069‐41‐2 | Quantified in citrus fruits up to 100 mg/kg and milk and milk products, 2.4 mg/kg, and identified in chicken, coriander leaf or tea |
| 05.196 | tr‐2, tr‐4‐Undecadienal | 30361‐29‐6 | Quantified in beer, chicken and potato chips from 0.0003 up to 0.1 mg/kg |
| 08.072 | But‐2‐enoic acid (cis and trans) | 3724‐65‐0 | Quantified in mussels, 0.16 mg/kg and identified in banana, beer, bread and bread preferment, cherimoya, cocoa, coffee, fish, honey, papaya, passion fruit, shoyu, soybean and tea |
| 08.083 | Hept‐2‐enoic acid | 18999‐28‐5 | Quantified in beer, < 0.01 mg/kg and identified in chicken |
| 08.101 | Non‐2‐enoic acid | 3760‐11‐0 | Quantified in beer, < 0.01 mg/kg and identified in chicken and lamb and mutton |
| 08.119 | 2‐Hexenoic acid | 1191‐04‐4 | Quantified in black choke berry juice, 0.14 mg/kg and lamb and mutton, 0.04 mg/kg and identified in processed apple, beer, chicken, gabiroba, hop, kumazasa, papaya, raspberry, blackberry and boysenberry, rice and strawberry |
| 08.120 | 2‐Methyl‐2‐butenoic acid | 13201‐46‐2 | Identified in coffee, honey, katsuobushi, shoyu and strawberry |
| 09.181 | Methyl hex‐2‐enoate | 2396‐77‐2 | Quantified in mountain papaya < 0.01 mg/kg and papaya 0.0001 mg/kg. Identified in peas and soursop |
| 09.247 | Allyl crotonate | 20474‐93‐5 | Identified in hazelnut (filbert) |
| 09.248 | Ethyl trans‐2‐butenoate | 623‐70‐1 | Quantified in guava and feyoa from 0.25 to 1.25 mg/kg, licorice, 0.11 mg/kg, melon up to 0.0006 mg/kg, mussels, 0.99 mg/kg, passion fruit, 0.08 mg/kg and pawpaw, 0.095 mg/kg. Identified in apple, babaco fruit, cashew apple wine, citrus fruits, durian, guava wine, Mangifera species, mountain papaya, naranjilla fruit, pineapple, rambutan and wine |
|
09.266 1807 |
Hexyl 2‐butenoate | 19089‐92‐0 | Quantified in mountain papaya, < 0.01 mg/kg and identified in apple, cherimoya and plum |
| 09.287 | Propyl deca‐2,4‐dienoate | 28316‐62‐3 | Identified in pear and pear brandy |
| 09.321 | Butyl 2‐methylbut‐2(cis)‐enoate | 7785‐64‐0 | Quantified in camomile from 7,600 up to 9,000 mg/kg |
| 09.324 | Butyl but‐(2E)‐enoate | 591‐63‐9 | Quantified in passion fruit, < 0.01 mg/kg and pawpaw, 0.024 mg/kg. Identified in babaco fruit, citrus fruits, Mangifera species, mountain papaya and caja fruit (tapereba) |
| 09.326 | Butyl deca‐(2E,4Z)‐dienoate | 28369‐24‐6 | Quantified in apple from 0.2 to 0.3 mg/kg and identified in pear and pear brandy |
| 09.329 | Butyl hex‐2‐enoate | 13416‐74‐5 | No natural occurrence data |
| 09.330 | Butyl hex‐(3E)‐enoate | 118869‐62‐8 | Quantified in passion fruit < 0.01 mg/kg |
| 09.335 | Butyl oct‐2‐enoate | 57403‐32‐4 | Identified in mountain papaya |
| 09.365 | Ethyl 3‐methylcrotonate | 638‐10‐8 | Identified in cashew apple, cocoa, Mangifera species and Vaccinium species |
| 09.370 | Ethyl dec‐9‐enoate | 67233‐91‐4 | Quantified in cheese, Chinese quince, grape brandy, guava and feyoa, litchi wine, olive and wine from 0 up to 0.9 mg/kg in whisky. Identified in apple brandy, beer, bilberry wine, cider, guava wine, sherry and strawberry wine |
| 09.372 | Ethyl dodec‐(2E)‐enoate | 28290‐90‐6 | Identified in pear and pear brandy and in quince (marmelo) |
| 09.374 | Ethyl hept‐(2E)‐enoate | 54340‐72‐6 | Identified in wine |
| 09.375 | Ethyl methacrylate | 97‐63‐2 | Identified in litchi, Mangifera species, quince (marmelo) and starfruit |
| 09.379 | Ethyl pent‐2‐enoate | 2445‐93‐4 | Identified in grape |
| 09.400 | Hex‐2‐enyl phenylacetate | 68133‐78‐8 | Identified in mentha oils |
| 09.578 | Hexyl (E)‐but‐2‐enoate | 1617‐25‐0 | Quantified in passion fruit, < 0.01 mg/kg and identified in babaco fruit |
| 09.586 | Isobutyl 2‐methylprop‐2‐enoate | 97‐86‐9 | Quantified in camomile, 16,200 mg/kg |
| 09.596 | Isopentyl‐(Z)‐but‐2‐enoate | 10482‐55‐0 | Quantified in camomile, 43,400 mg/kg |
| 09.603 | Isopropyl crotonate | 6284‐46‐4 | No natural occurrence data |
| 09.624 | Methyl 2‐methylcrotonate | 6622‐76‐0 | Identified in camomile, Mangifera species, naranjilla fruit, pineapple and starfruit |
| 09.625 | Methyl 2‐methylpent‐3(E)‐enoate | 33603‐30‐4 | No natural occurrence data |
| 09.636 | Methyl crotonate | 623‐43‐8 | Quantified in strawberry from 0.0019 to 0.0072 mg/kg and identified in chestnut, Mangifera species, naranjilla fruit, pineapple and soursop |
| 09.637 | Methyl dec‐2‐enoate | 2482‐39‐5 | No product occurrence data |
| 09.641 | Methyl dodec‐(2E)‐enoate | 6208‐91‐9 | Identified in pear |
|
09.647 1834 |
Methyl methacrylate | 80‐62‐6 | Identified in beef, Mangifera species, wheaten bread and wine |
| 09.652 | Methyl oleate | 112‐62‐9 | Quantified in allium species, cocoa and noni from 0.08 up to 25 mg/kg in vanilla. identified in babaco fruit, buckwheat, cape gooseberry, melon, pear, pineapple and walnut |
| 09.680 | Pentyl 2‐methylisocrotonate | 7785‐63‐9 | Quantified in camomile |
| 09.699 | Propyl crotonate | 10352‐87‐1 | Identified in camomile |
| 09.865 | Hexyl (9Z)‐octadecenoate | 20290‐84‐0 | No natural occurrence data |
| 09.866 | Allyl valerate | 6321‐45‐5 | No natural occurrence data |
|
09.934 1630 |
Methyl (5Z)‐Octenoate | 41654‐15‐3 | Identified in pineapple |
| 09.942 | 2‐Methylbutyl‐3‐methyl‐2‐butenoate | 97890‐13‐6 | No natural occurrence data |
| 09.948 | (2E)‐2‐Nonenyl acetate | 30418‐89‐4 | No natural occurrence data |
FL‐no: FLAVIS number; CAS: Chemical Abstract Service.
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Gürtler R, Husøy T, Moldeus P, Oskarsson A, Shah R, Waalkens‐Berendsen I, Wölfle D, Benigni R, Bolognesi C, Chipman K, Cordelli E, Degen G, Marzin D, Svendsen C, Carfì M, Martino C, Vianello G and Mennes W, 2019. Scientific Opinion on Flavouring Group Evaluation 5, Revision 3 (FGE.05Rev3): Branched‐ and straight‐chain unsaturated aldehydes, dienals, unsaturated and saturated carboxylic acids and related esters with saturated and unsaturated aliphatic alcohols and a phenylacetic acid related ester from chemical groups 1, 2, 3, 5 and 15. EFSA Journal 2019;17(8):5761, 69 pp. 10.2903/j.efsa.2019.5761
Requestor: European Commission
Question numbers: EFSA‐Q‐2018‐00862, EFSA‐Q‐2018‐00861, EFSA‐Q‐2018‐00860, EFSA‐Q‐2018‐00859, EFSA‐Q‐2018‐00858, EFSA‐Q‐2018‐00857, EFSA‐Q‐2018‐00856, EFSA‐Q‐2018‐00855, EFSA‐Q‐2018‐00854, EFSA‐Q‐2018‐00853, EFSA‐Q‐2018‐00852, EFSA‐Q‐2018‐00851, EFSA‐Q‐2018‐00850, EFSA‐Q‐2018‐00667, EFSA‐Q‐2018‐00651, EFSA‐Q‐2018‐00650, EFSA‐Q‐2018‐00649.
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus, Agneta Oskarsson, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle and Maged Younes.
Acknowledgements: The Panel wishes to thank the hearing experts: Vibe Beltoft and Karin Nørby for the support provided to this scientific output.
Adopted: 26 June 2019
Notes
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Annex II refers here to the annex of the mandate letter from the EC to EFSA related to FGE.200Rev1.
EU figure: 375 million. This figure relates to EU population at the time for which production data are available and is consistent (comparable) with evaluations conducted prior to the enlargement of the EU. No production data are available for the enlarged EU (Eurostat, 1998).
The FAF Panel is aware that a Revised Procedure for the Safety Evaluation of Flavouring agents has been agreed by JECFA (JECFA, 2016). The EFSA Scientific Committee has also recently developed a modified procedure for evaluation of substances based on the TTC approach (EFSA SC, 2019). However, these developments have no impact on the present evaluation, which should follow the requirements as set out in Commission Regulation (EC) No 1565/2000.
‘Innocuous metabolic products’: Products that are known or readily predicted to be harmless to humans at the estimated intakes of the flavouring agent (JECFA, 1997).
‘Endogenous substances’: Intermediary metabolites normally present in human tissues and fluids, whether free or conjugated; hormones and other substances with biochemical or physiological regulatory functions are not included (JECFA, 1997).
References
- CoE (Council of Europe), 1992. Flavouring substances and natural sources of flavourings. 4th Ed. vol. I. Chemically defined flavouring substances. Partial agreement in the social and public health field. Strasbourg.
- Cramer GM, Ford RA and Hall RL, 1978. Estimation of toxic hazard – a decision tree approach. Food and Cosmetics Toxicology, 16, 255–276. [DOI] [PubMed] [Google Scholar]
- Edwards KB, 1973. Biological evaluation of 2,6‐dodecadienal and 2,4,7‐tridecatrienal. 4‐week feeding study in rats. Unpublished report. Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA. [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Minutes of the 7th Plenary meeting of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food, Held in Brussels on 12‐13 July 2004. Brussels, 28 September 2004. Available online: http://www.efsa.europa.eu/cs/BlobServer/Event_Meeting/afc_minutes_07_en1.pdf?ssbinary=true
- EFSA AFC Panel (EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food), 2005. Scientific opinion on Flavouring Group Evaluation 5: Esters of 23 branched‐ and straight‐chain aliphatic saturated primary alcohols and of one secondary alcohol, and 24 branched‐ and straight‐chain unsaturated carboxylic acids from chemical groups 1, 2, and 5 (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA Journal 2005;3(7):204, 74 pp. 10.2903/j.efsa.2005.204 [DOI] [Google Scholar]
- EFSA AFC Panel (EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food), 2007. Scientific opinion on Flavouring Group Evaluation 14, Revision 1 (FGE.14Rev1): Phenethyl alcohol, aldehyde, acetals, carboxylic acid and related esters from chemical group 15 and 22. EFSA Journal 2009;7(1):930, 53 pp. 10.2903/j.efsa.2009.930 [DOI] [Google Scholar]
- EFSA AFC Panel (EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food), 2008. Scientific opinion on Flavouring Group Evaluation 5, Revision 1 (FGE.05Rev1): Esters of branched‐ and straight‐chain aliphatic saturated primary alcohols and of one secondary alcohol, and branched‐ and straight‐chain unsaturated carboxylic acids from chemical groups 1, 2, and 5 (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA Journal 2008;6(3):643, 81 pp. 10.2903/j.efsa.2008.643 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2009. Scientific Opinion on Flavouring Group Evaluation 203: alpha,beta‐Unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 with two or more conjugated double bonds and with or without additional non‐conjugated double bonds (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA Journal 2009;7(4):877, 23 pp. 10.2903/j.efsa.2009.877 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2010. Scientific opinion on Flavouring Group Evaluation 5, Revision 2 (FGE.05Rev2): Branched‐ and straight‐chain unsaturated carboxylic acids and esters of these with aliphatic saturated alcohols from chemical groups 1, 2, 3 and 5. EFSA Journal 2010;8(10):1400, 84 pp. 10.2903/j.efsa.2010.1400. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2014a. Scientific Opinion on Flavouring Group Evaluation 200 (FGE.200): 74 α,β‐unsaturated aldehydes and precursors from subgroup 1.1.1 of FGE.19. EFSA Journal 2014;12(6):3709, 57 pp. 10.2903/j.efsa.2014.3709 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2014b. Scientific Opinion on Flavouring Group Evaluation 203 Revision 1 (FGE.203Rev1): α,β‐Unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 with two or more conjugated double‐bonds and with or without additional non‐conjugated double‐bonds. EFSA Journal 2014;12(4):3626, 31 pp. 10.2903/j.efsa.2014.3626 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2018. Scientific Opinion on Flavouring Group Evaluation 203, Revision 2 (FGE.203Rev2): α,β‐unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 with two or more conjugated double‐bonds and with or without additional non‐conjugated double‐bonds. EFSA Journal 2018;16(7):5322, 39 pp. 10.2903/j.efsa.2018.5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), 2018. Scientific Opinion on Flavouring Group Evaluation 200, Revision 1 (FGE.200 Rev.1): 74 α,β‐unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.1 of FGE.19. EFSA Journal 2018;16(10):5422, 60 pp. 10.2903/j.efsa.2018.5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), 2019. Scientific Opinion on Flavouring Group Evaluation 70, Revision 1 (FGE.70 Rev1): consideration of aliphatic, linear, α,β‐unsaturated, di‐ and trienals and related alcohols, acids and esters evaluated by JECFA (61st‐68th‐69th meeting). EFSA Journal 2019;17(7):5749, 41 pp. 10.2903/j.efsa.2019.5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA SC (EFSA Scientific Committee), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA SC (EFSA Scientific Committee), 2019. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA Journal 2019;17(6):5708, 17 pp. 10.2903/j.efsa.2019.5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eurostat , 1998. Total population. Cited in Eurostat, 2004. The EU population, Total population. Available online: http://epp.eurostat.ec.europa.eu/portal/page?_pageid=1090,30070682,1090_33076576%26_dad=portal%26_schema=PORTAL, Population and social conditions, Population, Demography, Main demographic indicators, Total population. December 2008.
- Gaunt IF, Colley J, Grasso P, Lansdown ABG and Gangolli SD, 1969. Acute (rat and mouse) and short term (rat) toxicity studies on cis‐3‐hexen‐1‐ol. Food and Chemical Toxicology, 7, 451–459. [DOI] [PubMed] [Google Scholar]
- Gaunt IF, Wright MG, Cottrell R and Gangolli SD, 1983. Short‐term toxicity of 2,6‐dimethylhept‐5‐en‐1‐al in rats. Food and Chemical Toxicology, 21, 543–549. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1995. Evaluation of certain food additives and contaminants. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 859. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996. Toxicological evaluation of certain food additives. The forty‐fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives and contaminants. WHO Food Additives Series: 35. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Evaluation of certain food additives and contaminants. Forty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 6–15 February 1996. WHO Technical Report Series, no. 868. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1998. Compendium of food additive specifications. Addendum 6. Joint FAO/WHO Expert Committee of Food Additives 51st session. Geneva, 9‐18 June 1998. FAO Food and Nutrition paper 52 Add. 6.* [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999. Evaluation of certain food additives and contaminants. Forty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Rome, 17–26 June 1997. WHO Technical Report Series, no. 884. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000a. Evaluation of certain food additives. Fifty‐first Meeting of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 9‐18 June 1998. WHO Technical Report Series, no. 891. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000b. Compendium of food additive specifications. Addendum 8. Joint FAO/WHO Expert Committee of Food Additives. Fifty‐fifth Meeting. Geneva, 6‐15 June 2000. FAO Food and Nutrition paper 52 Add. 8.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001b. Compendium of food additive specifications. Addendum 9. Joint FAO/WHO Expert Committee of Food Additives 57th session. Rome, 5‐14 June 2001. FAO Food and Nutrition paper 52 Add. 9.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002a. Evaluation of certain food additives. Fifty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 913. Geneva, 4‐13 June 2002.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002b. Compendium of food additive specifications. Addendum 10. Joint FAO/WHO Expert Committee of Food Additives 59th session. Geneva, 4‐13 June 2002. FAO Food and Nutrition paper 52 Addition 10
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2004a. Evaluation of certain food additives and contaminants, Sixty‐first report of the Joint FAO/WHO Expert committee on Food Additives, WHO Technical Report Series, no. 922, 2004, Rome, Italy.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2004b. Safety evaluation of certain food additives and contaminants. Sixty‐first meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 52. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2005. Evaluation of certain food additives, Sixty‐third meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series, WHO Technical Report Series, no. 928. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2007. Evaluation of certain food additives and contaminants, Sixty‐eighth report of the Joint FAO/WHO Expert committee on Food Additives, WHO Technical Report Series, no. 947, 2007, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2008. Evaluation of certain food additives, sixty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives, WHO technical report series; no. 952, 2008, Rome.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016. Evaluation of certain food additives, eighty‐second report of the Joint FAO/WHO Expert Committee on Food Additives, WHO technical report series; no. 1000, 2016, Geneva, Switzerland.
- NTP (National Toxicology Program), 2003. Toxicology and carcinogenesis studies of 2,4‐hexadienal in F344/N rats and B6C3F1 mice (gavage studies). NTP TRS 509, NIH Publication No. 04–4443. Available online: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr509.pdf
- NTP (National Toxicology Program), 2011. Technical Report on the Toxicity Studies of 2,4‐Decadienal (CAS No. 25152‐84‐5) Administered by Gavage to F344/N Rats and B6C3F1 Mice. NTP TRS 76. NIH Publication No. 11‐5969. Available online: https://ntp.niehs.nih.gov/ntp/htdocs/ST_rpts/Tox076.pdf
- SCF (Scientific Committee for Food), 1995. First annual report on chemically defined flavouring substances. May 1995, 2nd draft prepared by the SCF Working Group on Flavouring Substances (Submitted by the SCF Secretariat, 17 May 1995). CS/FLAV/FL/140‐Rev2. Annex 6 to Document III/5611/95, European Commission, Directorate‐General III, Industry.
- SCF (Scientific Committee on Food), 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). SCF/CS/FLAV/TASK/11 Final 6/12/1999. Annex I the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- Seakins JWT, 1971. The determination of urinary phenylacetylglutamine as phenylacetic acid. Studies on its origin in normal subjects and children with cystic fibrosis. Clinica Chimica Acta, 35, 121–131. [DOI] [PubMed] [Google Scholar]
- Triskelion , 2018. VCF online. Volatile compounds in food In: Nijssen B, van Ingen‐Visscher K. and Donders J. (eds.). Database Version 16.6.1. Triskelion, Zeist, Netherland. [Google Scholar]


