Abstract
The Panel on Food Additives and Flavourings (FAF Panel) of the European Food Safety Authority was requested to evaluate the genotoxic potential of the flavouring substances from subgroup 1.2.1 of FGE.19 in the Flavouring Group Evaluation 204 (FGE.204). In the present revision of this FGE (FGE.204Rev1), the FAF Panel evaluated new data provided by Industry following a request from the former Panel on Food Contact materials, Enzymes, Flavourings and Processing Aids (CEF Panel). This request followed from positive results in an in vitro micronucleus test for clastogenicity and a negative result, but with no proof of bone marrow exposure, in an in vivo micronucleus assay for the representative substance 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177]. Subsequently, the Industry submitted an in vivo comet assay which was considered equivocal in the liver. The study was repeated confirming that 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] did not induce primary DNA damage in the liver and duodenum. Based on the available data, the Panel concluded that the concern for genotoxicity can be ruled out for [FL‐no: 07.177] and the 15 structurally related substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.187, 07.188, 07.244, 07.258] which can be evaluated through the Procedure for flavouring substances.
Keywords: FGE.19; subgroup 1.2.1; aliphatic; mono‐unsaturated; α,β‐unsaturated ketones
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
Background
The use of flavourings is regulated under Regulation (EC) No 1334/20081 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20122. The list contains flavouring substances for which the scientific evaluation should be completed in accordance with Commission Regulation (EC) No 1565/20003.
On 21 November 2012, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids adopted an opinion on Flavouring Group Evaluation 204 (FGE.204): Consideration of genotoxicity data on 18 monounsaturated, aliphatic, α,β‐unsaturated ketones and precursors from chemical subgroup FGE.204 (FGE.19 s.g. 1.2.1).
The Panel concluded that for the representative substance 7‐Methyl‐3‐octen‐2‐one [FL‐no: 07.177] of subgroup 1.2.1 of FGE.19, the Panel's concern with respect to genotoxicity could not be ruled out and consequently additional data are requested.
On 31 September 2014 (Ares(2014)207551) the applicant submitted to the Commission and to EFSA data on the potential presence of the substance FL‐no 07.177 in plasma (analytical data).
On 9 January 2015 (Ares(2015)202297) the applicant submitted additional studies on the representative substance FL‐no: 07.177 in relation to this EFSA evaluation. This additional data examines the systemic exposure of rats following oral administration of this substance, using the same dosing regimen employed in the combined micronucleus and comet test previously submitted. The data on this representative substance is intended to cover the following 16 substances in this group, namely: FL.nos: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.187, 07.188, 07.244, and 07.258.
Terms of reference
The European Commission requests the European Food Safety Authority (EFSA) to evaluate this new information and, depending on the outcome, proceed to the full evaluation of the flavouring substances mentioned above in accordance with Commission Regulation (EC) No 1565/2000.
2. Data and methodologies
2.1. History of the evaluation of FGE.19 substances
Flavouring Group Evaluation 19 (FGE.19) contains 360 flavouring substances from the EU Register being α,β‐unsaturated aldehydes or ketones and precursors which could give rise to such carbonyl substances via hydrolysis and/or oxidation (EFSA, 2008a).
The α,β‐unsaturated aldehyde and ketone structures are structural alerts for genotoxicity (EFSA, 2008a). The Panel noted that there were limited genotoxicity data on these flavouring substances but that positive genotoxicity studies were identified for some substances in the group.
The α,β‐unsaturated carbonyls were subdivided into subgroups on the basis of structural similarity (EFSA, 2008a). In an attempt to decide which of the substances could go through the Procedure, a (quantitative) structure–activity relationship ((Q)SAR) prediction of the genotoxicity of these substances was undertaken considering a number of models that were available at that time (DEREKfW, TOPKAT, DTU‐NFI‐MultiCASE Models and ISS‐Local Models, (Gry et al., 2007)).
The Panel noted that for most of these models internal and external validation has been performed, but considered that the outcome of these validations was not always extensive enough to appreciate the validity of the predictions of these models for these α,β‐unsaturated carbonyls. Therefore, the Panel considered it inappropriate to totally rely on (Q)SAR predictions at this point in time and decided not to take substances through the Procedure based on negative (Q)SAR predictions only.
The Panel took note of the (Q)SAR predictions by using two ISS Local Models (Benigni and Netzeva, 2007a, b) and four DTU‐NFI MultiCASE Models (Gry et al., 2007; Nikolov et al., 2007) and the fact that there are available data on genotoxicity, in vitro and in vivo, as well as data on carcinogenicity for several substances. Based on these data, the Panel decided that 15 subgroups (1.1.1, 1.2.1, 1.2.2, 1.2.3, 2.1, 2.2, 2.3, 2.5, 3.2, 4.3, 4.5, 4.6, 5.1, 5.2 and 5.3) (EFSA, 2008a) could not be evaluated through the Procedure due to concern with respect to genotoxicity. Corresponding to these subgroups, 15 FGEs were established: FGE.200, 204, 205, 206, 207, 208, 209, 211, 215, 219, 221, 222, 223, 224 and 225.
For 11 subgroups, the Panel decided, based on the available genotoxicity data and (Q)SAR predictions, that a further scrutiny of the data should take place before requesting additional data from the Flavouring Industry on genotoxicity. These subgroups were evaluated in FGE.201, 202, 203, 210, 212, 213, 214, 216, 217, 218 and 220. For the substances in FGE.202, 214 and 218, it was concluded that a genotoxic potential could be ruled out and accordingly these substances were evaluated using the Procedure. For all or some of the substances in the remaining FGEs, FGE.201, 203, 210, 212, 213, 216, 217 and 220 the genotoxic potential could not be ruled out.
To ease the data retrieval of the large number of structurally related α,β‐unsaturated substances in the different subgroups for which additional data are requested, EFSA worked out a list of representative substances for each subgroup (EFSA, 2008c). In selecting the representative substances expert judgement was applied. In each subgroup, the representative substances were selected taking into account chain length, chain branching, lipophilicity and additional functional groups. Likewise, an EFSA genotoxicity expert group has worked out a test strategy to be followed in the data retrieval for these substances (EFSA, 2008b).
The Flavouring Industry has been requested to submit additional genotoxicity data according to the list of representative substances and test strategy for each subgroup.
2.2. Presentation of the substances belonging to FGE.204
Flavouring Group Evaluation 204 (FGE.204) initially concerned 18 substances corresponding to subgroup 1.2.1 of FGE.19 (EFSA, 2008b) and presented in Table A.1 in Appendix A.
Table A.1.
Specification Summary of the Substances in the present group (JECFA, 2002a)
| FL‐no JECFA‐no | EU Register name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubilitya Solubility in ethanol b | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. indexd Spec. gravity e | EFSA Comments |
|---|---|---|---|---|---|---|---|---|
|
02.102 1140 |
Oct‐3‐en‐2‐olf |
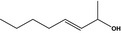
|
3602 76649‐14‐4 |
Liquid C8H16O 128.22 |
Insoluble Miscible |
73–76 (13 hPa) IR NMR MS 98% |
1.422–1.428 0.826–0.836 |
|
|
02.193 1141 |
Oct‐2‐en‐4‐olf |
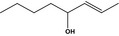
|
3888 4798‐61‐2 |
Liquid C8H16O 128.22 |
Insoluble 50% Soluble in ethanol |
IR NMR MS 95% |
1.438–1.442 0.830–0.838 |
|
|
07.044 1124 |
Pent‐3‐en‐2‐onef |
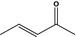
|
3417 666 625‐33‐2 |
Liquid C5H8O 84.12 |
Slightly soluble Miscible |
122 NMR 98% |
1.433–1.437 0.860–0.865 |
|
|
07.048 1125 |
4‐Hexen‐3‐onef |
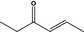
|
3352 718 2497‐21‐4 |
Liquid C6H10O 98.15 |
Slightly soluble Miscible |
93 (195 hPa) NMR 98% |
1.437–1.443 0.855–0.861 |
|
|
07.082 1129 |
Oct‐2‐en‐4‐onef |
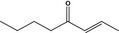
|
3603 2313 4643‐27‐0 |
Liquid C8H14O 126.20 |
Insoluble Miscible |
81 (26–27 hPa) IR NMR 96% |
1.440–1.446 0.835–0.842 |
|
|
07.101 1131 |
4‐Methylpent‐3‐en‐2‐one |
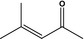
|
3368 11853 141‐79‐7 |
Liquid C6H10O 98.14 |
Slightly soluble Miscible |
126.76 NMR 95% |
1.442–1.447 0.862–0.868 |
|
|
07.104 1126 |
Hept‐2‐en‐4‐onef |
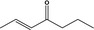
|
3399 11093 4643‐25‐8 |
Liquid C7H12O 112.17 |
Slightly soluble Miscible |
156–157 IR NMR 99% |
1.440–1.445 0.845–0.852 |
|
|
07.105 1127 |
Hept‐3‐en‐2‐onef |
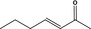
|
3400 11094 1119‐44‐4 |
Liquid C7H12O 112.17 |
Slightly soluble Miscible |
162 NMR 96% |
1.439–1.448 0.841–0.847 |
|
|
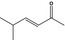
07.106 1132 |
5‐Methylhex‐3‐en‐2‐onef |
|
3409 11149 5166‐53‐0 |
Liquid C7H12O 112.17 |
Insoluble Miscible |
77.5 (65 hPa) NMR 99% |
1.437–1.441 0.838–0.843 |
|
|
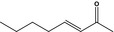
07.107 1128 |
Oct‐3‐en‐2‐onef |
|
3416 11170 1669‐44‐9 |
Liquid C8H14O 126.19 |
Insoluble Miscible |
75‐79 (26 hPa) NMR 94% |
1.445–1.449 0.834–0.839 |
|
|
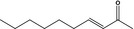
07.121 1130 |
Dec‐3‐en‐2‐onef |
|
3532 11751 10519‐33‐2 |
Liquid C10H18O 154.25 |
Almost insoluble Miscible |
125‐126 NMR 95% |
1.446–1.452 0.809–0.813 |
|
|
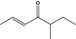
07.139 1133 |
5‐Methylhept‐2‐en‐4‐onef |
|
3761 81925‐81‐7 |
Liquid C8H14O 126.19 |
Slightly soluble Miscible |
86‐87 (78 hPa) NMR 98% |
1.440–1.445 0.845–0.852 |
|
|
07.177 1135 |
7‐Methyl‐3‐octenone‐2 |
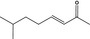
|
3868 33046‐81‐0 |
Liquid C9H16O 140.2 |
Slightly soluble Miscible |
198 IR NMR MS 94% |
1.446–1.451 0.838–0.847 |
Mainly E‐isomer >90%. Secondary components: from 2 to 4% 7‐methyl‐4‐ octen‐2‐one, 5,6‐dimethyl‐3‐hepten‐2one and 3‐nonen‐ 2‐one. Substance name in Union List to be changed into 7‐methyl‐3‐octen‐2‐one. |
| 07.187 | Non‐2‐en‐4‐onef |
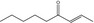
|
11162 32064‐72‐5 |
Liquid C9H16O 140.22 |
Insoluble Freely soluble |
82 (27 hPa) MS 95% |
1.422–1.428 0.823–0.829 |
|
|
07.188 1136 |
Non‐3‐en‐2‐onef |

|
3955 11163 14309‐57‐0 |
Liquid C9H16O 140.22 |
Insoluble Miscible |
198 IR MS 95% |
1.443–1.452 0.843–0.846 |
|
|
07.244 1138 |
trans‐6‐Methyl‐3‐hepten‐2‐one |
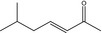
|
4001 20859‐10‐3 |
Liquid C8H14O 126.2 |
Insoluble Miscible |
170‐180 NMR 96% |
1.438–1.447 0.840–0.850 |
|
| 07.258 | 6‐Methyl‐3‐hepten‐2‐onef |
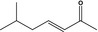
|
2009‐74‐7 |
Liquid C8H14O 126.20 |
Practically insoluble or insoluble Freely soluble |
179 MS 96% |
1.436–1.442 0.842–0.848 |
|
| 07.261 | 4‐Methyl‐3‐hepten‐5‐onef , g |
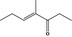
|
22319‐31‐9 |
Liquid C8H14O 126.20 |
Insoluble Freely soluble |
179 MS 96.12% |
1.442–1.462 0.851–0.871 |
Evaluated in FGE.201Rev2 |
FGE: Flavouring Group Evaluation; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FL‐no: FLAVIS number; FLAVIS: Flavour Information System; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; ID: identity; IR: infrared spectroscopy; NMR: nuclear magnetic resonance; MS: mass spectrometry.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
Stereoisomeric composition not specified.
The substance [FL‐no: 07.261] has been evaluated in FGE.201Rev2 (EFSA FAF Panel, 2018).
Fifteen of the flavouring substances have previously been evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 2002a,b). A summary of their current evaluation status by JECFA and the outcome of this consideration is presented in Table B.1 in Appendix B.
Table B.1.
Summary of Safety Evaluation of the JECFA substances in the present group (JECFA, 2002b)
| FL‐no JECFA‐no | EU Register name | Structural formula | EU MSDIa US MSDI (μg/capita per day) | Classb Evaluation procedure pathc | JECFA Outcome on the named compoundd or e | EFSA conclusion on the named compound (genotoxicity) |
|---|---|---|---|---|---|---|
|
02.102 1140 |
Oct‐3‐en‐2‐ol |

|
1.2 ND |
Class I A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.193 1141 |
Oct‐2‐en‐4‐ol |
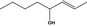
|
1.84 f ND |
Class I A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
07.044 1124 |
Pent‐3‐en‐2‐one |
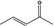
|
0.26 ND |
Class I A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
07.048 1125 |
4‐Hexen‐3‐one |
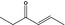
|
13 1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
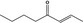
07.082 1129 |
Oct‐2‐en‐4‐one |
|
0.85 3 |
Class II A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
07.101 1131 |
4‐Methylpent‐3‐en‐2‐one |

|
0.34 ND |
Class II A3: Intake below threshold |
d | No safety concern with respect to genotoxicity. Evaluated through the Procedure in FGE.63Rev2 |
|
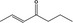
07.104 1126 |
Hept‐2‐en‐4‐one |
|
0.012 ND |
Class I A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
07.105 1127 |
Hept‐3‐en‐2‐one |

|
0.16 0.07 |
Class II A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
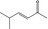
07.106 1132 |
5‐Methylhex‐3‐en‐2‐one |
|
0 0.1 |
Class II A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
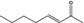
07.107 1128 |
Oct‐3‐en‐2‐one |
|
0.63 1 |
Class II A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
07.121 1130 |
Dec‐3‐en‐2‐one |

|
0.012 ND |
Class II A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
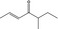
07.139 1133 |
5‐Methylhept‐2‐en‐4‐one |
|
5.8 1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
07.177 1135 |
7‐Methyl‐3‐octenone‐2 |

|
0.04f 2 |
Class II A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 07.187 | Non‐2‐en‐4‐one |
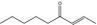
|
0.0012 |
Class II No evaluation |
Not evaluated by JECFA | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
07.188 1136 |
Non‐3‐en‐2‐one |

|
13 13 |
Class II A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
07.244 1138 |
trans‐6‐Methyl‐3‐hepten‐2‐one |

|
3.4 3 |
Class II A3: Intake below threshold |
d | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 07.258 | 6‐Methyl‐3‐hepten‐2‐one |

|
0.061 |
Class II No evaluation |
Not evaluated by JECFA | Evaluated in FGE.204Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 07.261 | 4‐Methyl‐3‐hepten‐5‐one |
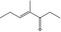
|
0 | No evaluation | Not evaluated by JECFA | Evaluated in FGE.201Rev2 (EFSA FAF Panel, 2018) |
JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FL‐no: FLAVIS number; FLAVIS: Flavour Information System; MSDI: maximised survey‐derived daily intake; TAMDI: Theoretical Added Maximum Daily Intake; ND: not determined.
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita/day.
Thresholds of concern: Class I = 1800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
MSDI value calculated based on EFFA poundage survey covering 2015, submitted by EFFA to European Commission (EFFA, 2019)
The present revision of FGE.204 (FGE.204Rev1), concerns 14 monounsaturated, aliphatic α,β‐unsaturated ketones and two precursors for such ketones.
The α,β‐unsaturated aldehyde and ketone structures are structural alerts for genotoxicity (EFSA, 2008b) and the data on genotoxicity evaluated in FGE.204 (EFSA CEF Panel, 2012a) did not rule out the concern for genotoxicity for these 16 flavouring substances that are evaluated in the present FGE.204Rev1.
2.3. History of the evaluation of the substances in subgroup 1.2.1
In 2008, the CEF Panel identified two substances in subgroup 1.2.1 (EFSA, 2008c) for which genotoxicity data according to the test strategy (EFSA, 2008b) have been requested. These substances are listed in Table 1.
Table 1.
Representative substances for subgroup 1.2.1 of FGE.19 (EFSA, 2008c)
| FL‐no JECFA‐no | Subgroup | EU Register name | Structural formula | FEMA no CoE no CAS no |
|---|---|---|---|---|
|
07.101 1131 |
1.2.1 | 4‐Methylpent‐3‐en‐2‐one |
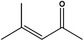
|
3368 11853 141‐79‐7 |
|
07.177 1135 |
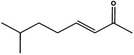
1.2.1 | 7‐Methyl‐3‐octenone‐2 |
|
3868 – 33046‐81‐0 |
FGE: Flavouring Group Evaluation; FL‐no: FLAVIS number; FLAVIS: Flavour Information System; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service.
Substance [FL‐no: 07.101] was considered to be representative for itself only, since it is the only substance with a methyl substituent on the β‐carbon atom of the double bond. The other substance, [FL‐no: 07.177], was considered representative of the remaining substances of this FGE.
Subsequent to the publication of the list of representative substances (EFSA, 2008c), the CEF Panel noted that 4‐methyl‐3‐hepten‐5‐one [FL‐no: 07.261] differs structurally from all other substances in this subgroup owing to the presence of a methyl group in α‐position of the double bond. Therefore, this substance is considered as a ‘stand‐alone’ substance. The Panel noted that the 2‐methyl substituted α,β‐unsaturated aldehydes in FGE.201Rev1 (EFSA CEF Panel, 2012b) can be considered as structurally related to [FL‐no: 07.261]. For the substances in FGE.201Rev1, 2‐methylpent‐2‐enal [FL‐no: 05.090] was selected as representative and further genotoxicity data were required. Thus, the final conclusion on [FL‐no: 07.261] has been drawn based on the outcome of the evaluation of FGE.201Rev2 (EFSA FAF Panel, 2018), where the Panel concluded that the genotoxicity concern is ruled out for the substances in FGE.19 subgroup 1.1.2, including 4‐methyl‐3‐hepten‐5‐one [FL‐no: 07.261].
In FGE.204 (EFSA CEF Panel, 2012a), the CEF Panel concluded that 4‐methylpent‐3‐en‐2‐one [FL‐no: 07.101] does not present a safety concern with respect to genotoxicity and accordingly this flavouring substance was evaluated through the Procedure in FGE.63Rev2 (EFSA CEF Panel, 2013). Therefore, the present revision of FGE.204 (FGE.204Rev1) includes 17 substances of which 16 are evaluated based on new data submitted (Appendix B, Table B.1).
| FGE | Adopted by the CEF Panel | Link | No. of Substances |
|---|---|---|---|
| FGE.204 | 21 November 2012 | http://www.efsa.europa.eu/en/efsajournal/pub/2992 | 18 |
| FGE.204Rev1 | 5 June 2019 | http://www.efsa.europa.eu/en/efsajournal/pub/5750 | 17 |
FGE: Flavouring Group Evaluation.
Following the request for additional data for the representative substance 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177], indicated by the CEF Panel in FGE.204 (EFSA CEF Panel, 2012a), industry has submitted: plasma analysis from a satellite group of animals dosed in the in vivo bone marrow micronucleus study (Henderson, 2012) that was evaluated in FGE.204 and 3 in vivo comet assay studies. These data are evaluated in the present revision of FGE.204 (FGE.204Rev1).
Section 2.4 of this opinion reports the same information that was presented in FGE.204. Section 3 reports the evaluation of the new data submitted by industry.
2.4. Genotoxicity data evaluated by the Panel in FGE.2044
The Industry has submitted data concerning genotoxicity studies for the two representative substances for this subgroup (EFFA, 2012):
4‐Methylpent‐3‐en‐2‐one [FL‐no: 07.101] in vitro tests in bacteria and mammalian cell systems.
7‐Methyl‐3‐octenone‐2 [FL‐no: 07.177] in vitro tests in bacteria and mammalian cell systems and in vivo micronucleus test in rats.
2.4.1. In vitro data
2.4.1.1. Bacterial Reverse Mutation Assay
Results of the in vitro studies described below are summarised in Table C.1 in Appendix C.
Table C.1.
Summary of in vitro genotoxicity data evaluated in FGE.204
| FL‐no | Chemical Name | Test System in vitro | Test Object | Concentrations of Substance and Test Conditions | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| 07.101 | 4‐Methylpent‐3‐en‐2‐one | Reverse mutation |
Salmonella Typhimurium TA98, TA100, TA102, TA1535 and TA1537 |
1.6–5,000 μg/platea | Negative | Williams (2009) | Valid. Study design complies with current recommendations |
| 156.25–5,000 μg/platea , b | Negative | ||||||
| Micronucleus assay | Human peripheral blood lymphocytes | 600–981.4 μg/mLc | Negative | Stone (2011) |
Valid Complies with OECD Guideline 487 |
||
| 200–981.4 μg/mLd | Negative | ||||||
| 100–500 μg/mLd | Negative | ||||||
| 100–300 μg/mLe | Negative | ||||||
| 07.177 | 7‐methyl‐3‐octenone‐2 | Reverse mutation |
S. Typhimurium TA102 |
1.6–5,000 μg/platea | Negative | Ballantyne (2011) | Valid. Studies combined comply with current recommendations |
| 51.2–5,000 μg/platea , b | Negative | ||||||
|
S. Typhimurium TA98, TA100, TA1535, TA1537, and TA1538 |
15–5,000 μg/platea | Negative | Thompson (1996) | ||||
| Micronucleus assay | Human peripheral Blood lymphocytes | 5–15 μg/mLc | Equivocal | Lloyd (2009) |
Valid Testing strategies including FISH analyses for determination of potential clastogenicity or aneugenicity. The study complies with OECD Guideline 487 |
||
| 30–60 μg/mLd | Equivocal | ||||||
| 2–6 μg/mLe | Positive | ||||||
| 5.5–8 μg/mLe | Positive | Lloyd (2010) |
FGE: Flavouring Group Evaluation; FL‐no: FLAVIS number; FLAVIS: Flavour Information System; OECD: Organisation for Economic Co‐operation and Development; FISH: Fluorescence in situ hybridisation.
With and without S9‐mix metabolic activation.
Assay modified with pre‐incubation in the presence of S9‐mix.
Without metabolic activation, 3 h treatment + 21 h recovery.
With metabolic activation, 3 h treatment + 21 h recovery.
Without metabolic activation, 24 h + 0 h recovery.
Validity of genotoxicity studies:
Valid.
Limited validity (e.g. if certain aspects are not in accordance with OECD Guidelines or current standards and/or limited documentation).
Insufficient validity (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards inappropriate/not validated test system).
Validity cannot be evaluated (e.g. insufficient documentation, short abstract only, too little experimental details provided, text not in a Community language).
4‐Methylpent‐3‐en‐2‐one [FL‐no: 07.101]
An Ames assay was conducted in Salmonella Typhimurium strains TA98, TA100, TA1535, TA1537 and TA102 to assess the mutagenicity of 4‐methylpent‐3‐en‐2‐one [FL‐no: 07.101], both in the absence and presence of rat liver metabolising system (S9‐mix) in two experiments (Williams, 2009). A preliminary range‐finding cytotoxicity experiment using standard plate‐incorporation methodology was conducted in strain TA100 only at concentrations of 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate in the absence and presence of S9‐mix, plus negative (solvent) and positive controls. Evidence of toxicity, in terms of a slight thinning of the background bacterial lawn, was observed only at the top concentration in the presence of S9‐mix. The data from the range‐finding experiment were considered acceptable for mutation assessment, and therefore, to complete the first experiment, the remaining four strains were tested at the same concentrations both in the presence and absence of S9‐mix using the same methodology. No evidence of toxicity was observed in these strains, and no increases in reverse mutants relative to the vehicle control were observed.
In a second experiment, 4‐methylpent‐3‐en‐2‐one was tested in all five S. Typhimurium strains with and without S9‐mix, using a narrowed concentration range of 156.25, 312.5, 625, 1,250, 2,500 and 5,000 μg/plate. A pre‐incubation step was also included when the chemical was tested in the presence of S9‐mix. Following these treatments, evidence of toxicity in the form of a slight thinning of the background bacterial lawn was observed at the highest concentrations (2,500 and 5,000 μg/plate) in all strains in the presence of S9‐mix and in strain TA102 in the absence of S9‐mix. A small increase (1.5‐fold) in TA1535 revertants was seen at the highest concentration in the absence of S9‐mix that was significant at p < 0.05, but this small increase was not seen in the first experiment at similar concentrations and was considered by the study authors to be due to chance. No increases in revertant numbers were observed for the other strains and treatment conditions.
Based on the above results, the Panel concluded that 4‐methylpent‐3‐en‐2‐one [FL‐no: 07.101] did not induce mutations in five strains of S. Typhimurium when tested up to toxic concentrations in the absence and in the presence of metabolic activation (Williams, 2009).
7‐Methyl‐3‐octenone‐2 [FL‐no: 07.177]
Previously, an Ames assay had been performed with 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] in S. Typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538 with and without S9‐mix metabolic activation at concentrations ranging from 15 to 5,000 μg/plate. The test compound showed toxicity in terms of reduction of bacterial lawn at dose levels of 1,500 μg/plate and higher, both in the absence and presence of S9‐mix. No significant increases in the frequencies of revertant colonies were observed in any of the tester strains employed, at any dose‐level assayed with and without metabolic activation (Thompson, 1996). To supplement these results and thus provide data for a battery of test strains consistent with the requirements for current regulatory guidelines (OECD TG 471, 1997), a new Ames study (standard plate incorporation method) using the S. Typhimurium tester strain TA102 at concentrations of 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate with and without S9‐mix was performed with 7‐methyl‐3‐octenone‐2 (Ballantyne, 2011). Evidence of toxicity in the presence and absence of S9‐mix was observed at the highest concentration tested. No increase in reverse mutant counts following treatments with 7‐methyl‐3‐octenone‐2 compared with the vehicle control were observed at any dose‐level assayed. In a second experiment, 7‐methyl‐3‐octenone‐2 was assayed in strain TA102 with and without S9‐mix and a narrowed concentration range of 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate. In the presence of S9‐mix, a pre‐incubation step was also included. Following 7‐methyl‐3‐octenone‐2 treatment, evidence of toxicity was observed in the presence and absence of S9‐mix, on plates treated at 2,000 or 5,000 μg/plate. Treatment of strain TA102 with 7‐methyl‐3‐octenone‐2, with and without S9‐mix, did not induce any statistically significant increase in revertant numbers (data were analysed at the 1% level using Dunnett's test) at the tested concentrations (Ballantyne, 2011).
Taken together with the data of Thompson (1996), the Panel concluded that 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] did not induce mutations in six strains of S. Typhimurium when tested up to toxic concentrations in the absence and in the presence of metabolic activation (Thompson, 1996; Ballantyne, 2011).
2.4.1.2. In vitro micronucleus assays
Results of the studies described below are summarised in Table C.1 in Appendix C.
4‐Methylpent‐3‐en‐2‐one [FL‐no: 07.101]
4‐Methylpent‐3‐en‐2‐one was tested for the induction of chromosome damage and potential aneugenic effects in mammalian cells in vitro by examining the effect on the frequency of micronuclei in cultured human peripheral blood lymphocytes, treated in the absence and presence of rat liver metabolising system (S9‐mix) (Stone, 2011).
A preliminary range‐finding experiment was conducted with and without S9‐mix in order to determine the effect of the test substance upon Replication Index (RI), which was used as a basis for choosing a range of concentrations to be evaluated in the main study. 4‐Methylpent‐3‐en‐2‐one was added to cell cultures after 48 h from culture initiation (stimulation by phytohaemagglutinin (PHA)), either for 3 h in the absence or presence of S9‐mix, or for 24 h in the absence of S9‐mix. Micronuclei were analysed at multiple concentrations for each treatment group. For the 3‐h treatment (3 + 21 h recovery), the concentrations were 0, 600, 800 and 981.4 μg/mL (without S9‐mix) and 0, 200, 400, 800 and 981.4 μg/mL (with S9‐mix). The levels of cytotoxicity (reduction in RI) induced at the top concentrations were 22% and 54% in the absence and presence of S9‐mix, respectively. Although the recommended range of toxicity (50–60%) was not reached in the absence of S9‐mix, the top concentration of 981.4 μg/mL was equivalent to 10 mM, which is the required upper limit for a nontoxic substance. For the 24‐h treatment without S9‐mix, the concentrations were 0, 100, 200, 275 and 300 μg/mL and the level of cytotoxicity (reduction in RI) at the top concentration reached 62%, which exceeded the target (50–60%) range. One thousand binucleate cells per culture from two replicate cultures per concentration were scored for micronuclei.
Treatment of cells with 4‐methylpent‐3‐en‐2‐one for 3 h in the presence of S9‐mix resulted in statistically significant (p ≤ 0.05) increases in micronucleated binucleate cells (MNBN) frequency compared to the concurrent vehicle control at the highest concentration analysed (981.4 μg/mL). However, only one replicate culture in the assay resulted in MNBN cell frequencies outside of the normal range and the authors considered this result as equivocal. Therefore, a confirmatory experiment was performed with 4‐methylpent‐3‐en‐ 2‐one at concentrations of 0, 100, 200, 400 and 500 μg/mL for 3 h with S9‐mix. The lower concentrations chosen in the second experiment were on the basis of an unexplained shift in toxicity, but the concentrations selected for analysis in this experiment gave comparable toxicity to those selected in the prior experiment under this treatment condition, and 58% cytotoxicity (determined as reduction in RI) was achieved at the top concentration. These treatments resulted in frequencies of MNBN cells that were similar to concurrent controls and there were no significant differences.
Considering that the significant increase in MNBN cell frequencies in the first experiment were not reproduced in the second one, the Panel concluded that 4‐methylpent‐3‐en‐2‐one [FL‐no: 07.101] did not induce micronuclei in cultured human peripheral blood lymphocytes when tested up to toxic concentrations in both the absence and presence of S9‐mix metabolism (Stone, 2011).
7‐Methyl‐3‐octenone‐2 [FL‐no: 07.177]
7‐Methyl‐3‐octenone‐2 [FL‐no: 07.177] was tested in an in vitro micronucleus assay in cultured human peripheral blood lymphocytes treated in the absence and presence of rat liver metabolising system (S9‐mix) (Lloyd, 2009). 7‐Methyl‐3‐octenone‐2 [FL‐no: 07.177] was added at 48 h following mitogen stimulation by PHA either for 3 h in the absence or presence of S9‐mix, or for 24 h in the absence of S9‐mix. The test substance concentrations for micronucleus analysis were selected by evaluating the effect of 7‐methyl‐3‐octenone‐2 on the RI. In the main experiment, micronuclei were analysed at multiple concentrations for each treatment group.
For the 3‐h treatment without S9‐mix, the concentrations were 5, 10 and 15 μg/mL; for the 3‐h treatment with S9‐mix, the concentrations were 30, 40 and 60 μg/mL; and for the 24‐h treatment without S9‐mix, the concentrations were 2, 4 and 6 μg/mL. The levels of cytotoxicity (reduction in RI) at the top concentrations reached 45% (in the 3 + 21 h treatment without S9‐mix), 58% (in the 3 + 21 h treatment with S9‐mix) and 59% (in the 24 h treatment without S9‐mix). One thousand binucleate cells per culture from two replicate cultures per concentration were scored for micronuclei.
Treatment of cells with 7‐methyl‐3‐octenone‐2 for 3 + 21 h resulted in frequencies of MNBN cells that were significantly higher (p ≤ 0.05) than those observed in concurrent vehicle controls at the highest concentrations analysed in the absence and presence of S9‐mix (15.00 μg/mL and 60.00 μg/mL, respectively). However, the MNBN cell frequencies in all treated cultures in the absence and presence of S9‐mix fell within historical range values for vehicle control. These observations were not considered biologically relevant by the authors of the study report.
For the 24‐h treatment, at the two highest concentrations (4.0 and 6.0 μg/mL), the frequencies of MNBN cells were significantly higher (p ≤ 0.05, p ≤ 0.001, respectively) than those observed in the concurrent vehicle control (0.2%). The MNBN cell frequencies in those cultures treated with the highest concentration exceeded the normal ranges and therefore this was considered to be a positive result.
Therefore, the Panel concluded that 7‐methyl‐3‐octenone‐2 induced micronuclei in cultured peripheral blood lymphocytes when tested up to toxic concentrations for 24 h in the absence of S9‐mix (Lloyd, 2009).
In order to determine whether 7‐methyl‐3‐octenone‐2 was acting as a clastogen or an aneugen, a follow‐up study was performed using fluorescence in situ hybridisation (FISH) analysis (Lloyd, 2010). Micronuclei were analysed at multiple concentrations separated by narrow intervals, which were based on the toxicity displayed in a preliminary range‐finding experiment for 24 h without S9‐mix treatment. The concentrations analysed were 5.5, 6.0, 7.0 and 8.0 μg/mL. The levels of cytotoxicity (reduction in RI) was concentration‐dependent and, at the top concentration, reached 56%.
All concentrations of 7‐methyl‐3‐octenone‐2 (5.5, 6.0, 7.0 and 8.0 μg/mL) resulted in significant increases of MNBN cells (p ≤ 0.05, p ≤ 0.01, p ≤ 0.01 and p ≤ 0.001, respectively) relative to vehicle controls. The increase of MNBN cells ranged from 2.3 to 4.7 fold greater than the concurrent control, and exceeded the historical control range at the highest concentration tested (1.4% mean MNBN cell frequency vs. 0.1–0.9% historical control range).
The FISH analyses, with a fluorochrome‐labelled pan‐centromeric human DNA probe specific for all human chromosomes, to identify micronuclei containing centromeres, were performed on slides generated from vehicle control, 7‐methyl‐3‐octenone‐2 along with a clastogenic positive control (mitomycin C) and an aneugenic positive control (vinblastine).
Treatment with 7 and 8 μg/mL 7‐methyl‐3‐octenone‐2 resulted in 20% and 18% centromere‐positive micronuclei, respectively compared to 46% centromere‐positive micronuclei for the vehicle control, 9% for the reference clastogen and 84% for the reference aneugen. On this basis, the authors concluded that the induction of increased MNBN cell frequency is primarily a result of chromosome breakage rather than chromosome loss following treatment of cultured peripheral blood lymphocytes with 7‐methyl‐3‐octenone‐2 for 24 h in the absence of S9‐mix (Lloyd, 2010). Based on these results, the Panel agreed that 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] is an in vitro clastogen for human lymphocytes.
2.4.2. In vivo data
Considering the results from the in vitro micronucleus assays, it was concluded that 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] was an in vitro clastogen for human lymphocytes, and therefore, it was decided that it was most appropriate to carry out an in vivo micronucleus assay to determine whether the results obtained in the in vitro micronucleus assays could be confirmed in vivo. Therefore, groups of Han‐Wistar rats were administered 7‐methyl‐3‐octenone‐2 via oral gavage and the induction of micronuclei in the polychromatic erythrocyte (PCE) of the bone marrow was examined.
As no substantial difference in toxicity was observed between males and females in the range‐finding experiment, male rats only were used in the micronucleus experiment. Groups of male (6 animals/group) rats were administered 7‐methyl‐3‐octenone‐2 by oral gavage at 500, 1,000 and 2,000 mg/kg body weight (bw) per day on two occasions 24 h apart (Appendix C, Table C.2). An additional satellite group of animals was dosed at 2,000 mg/kg bw per day to facilitate later blood analysis if desired. Animals were sampled 24 h after the final administration, thus enabling examination of cells exposed to the test article over a period of 24–48 h prior to sampling. No clinical signs of toxicity were observed in any animals in any main treatment groups. In the satellite group, animals showed decreased signs of activity on day 2 at 0.5 and 1.0 h post dose.
Table C.2.
Summary of in vivo genotoxicity data evaluated in FGE.204
| FL‐no | Chemical name | Test system in vivo | Test object/Sex No per group/groups | Route | Concentrations of substance | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|---|
| 07.177 | 7‐methyl‐3‐octenone‐2 | Micronucleus Assay | Male Han Wistar rats/6 animals/group | Gavage on 2 occasions 24 h apart | 0, 500, 1,000 and 2,000 mg/kg bw per day | Negative | Henderson (2012) | Valid. Complies with OECD guideline 474. Although the study was performed at the maximum recommended highest dose‐level (2,000 mg/kg) no clear indication of toxicity was observed indicating that test substance might not have been systemically available. This is also supported by positive findings observed in the in vitro micronucleus assay in the absence of S9 metabolism which indicates a direct reactivity of test compound |
FGE: Flavouring Group Evaluation; FL‐no: FLAVIS number; FLAVIS: Flavour Information System; OECD: Organisation for Economic Co‐operation and Development.
Validity of genotoxicity studies:
Valid.
Limited validity (e.g. if certain aspects are not in accordance with OECD Guidelines or current standards and/or limited documentation).
Insufficient validity (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards inappropriate/not validated test system).
Validity cannot be evaluated (e.g. insufficient documentation, short abstract only, too little experimental details provided, text not in a Community language).
Rats treated with 7‐methyl‐3‐octenone‐2 exhibited a very weak but dose‐related decrease in mean % PCE, which was however comparable with the historical control data for this experiment at the testing laboratory. No statistically significant increase in micronucleus frequency was observed for any of the groups receiving the test substance, compared to the concurrent vehicle control (Appendix C, Table C.3). On this basis, the authors concluded that 7‐methyl‐3‐octenone‐2 did not induce micronuclei in the polychromatic erythrocytes of the bone marrow of male rats treated up to 2,000 mg/kg per day (Henderson, 2012).
Table C.3.
Comparison of reported group mean values of PCE (%) for 7‐methyl‐3‐octenone‐2 treatment groups and historical group mean values of PCE (%) for vehicle control (Henderson, 2012)
| Chemical Name | Test system in vivo | Test object | No. of animals | Route of administration | Dose levels mg/kg bw | Group mean PCE (%) | Historical Group mean range values of (%) PCE for the vehicle control group |
|---|---|---|---|---|---|---|---|
| 7‐methyl‐3‐octenone‐2 | Bone marrow Micronucleus test | Male rats | 6 |
Oral gavage 2 occasions 24 h apart |
0 500 1,000 2,000 |
42.60 39.15 38.38 36.85 |
32.41–56.26 |
| Cyclophosphamide (positive control) | Oral gavage once 24 h before sacrifice | 20 | 46.38 |
PCE: polychromatic erythrocytes; bw: body weight.
Results of the in vivo studies described above are summarised in Appendix C.
2.4.3. Discussion of genotoxicity data
The two representative substances, 4‐methylpent‐3‐en‐2‐one [FL‐no: 07.101] and 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177], are both considered negative in the Ames test with S. Typhimurium tester strains consistent with the requirements for current regulatory guidelines. Statistically significant increase in the number of revertant colonies observed in tester strain TA1535 in the absence of S9‐mix metabolism in one experiment following treatment with 4‐methylpent‐3‐en‐2‐one are judged not biologically relevant, since they were not reproduced in the second experiment (Williams, 2009; Ballantyne, 2011).
Investigations at chromosome and genome levels in mammalian cells in vitro showed that 4‐methylpent‐3‐en‐2‐one induced a small but statistically significant increase in the frequency of MNBN cells only in the presence of S9‐mix metabolism following a 3‐h treatment at the highest concentration tested (981.4 μg/mL). However, only one replicate culture fell outside the historical vehicle control range values. Following additional scoring of 2,000 lymphocytes, the resulting MNBN frequencies, although still significantly higher than concurrent vehicle control, lied within historical control range values. In a second confirmatory experiment (3‐h treatment in the presence of S9‐mix) performed at concentrations lower than concentrations used in the previous experiment, due to an unexplained shift of toxicity (comparable toxicity to those observed in the first experiment, but at lower concentrations), no significant increase in MNBN frequencies was observed. Based on these results, the CEF Panel concluded that 4‐methylpent‐3‐en‐2‐one did not induce micronuclei in human peripheral blood lymphocytes, both in the absence and presence of rat liver S9‐mix metabolism. On the contrary, 7‐methyl‐3‐octenone‐2 induced statistically significant increases in MNBN following the 3‐h treatment both in the absence and presence of S9‐mix at the highest concentrations tested. However, the observed increase of MNBN cell frequencies fell within relevant historical control values. In the 24‐h treatment in the absence of S9‐mix, 7‐methyl‐3‐octenone‐2 induced statistically significant increase in MNBN cell frequencies at the two highest concentrations, selected for scoring and exceeded the historical control range values (Lloyd, 2009). Therefore, the Panel concluded that 7‐methyl‐3‐octenone‐2 induces micronuclei in human peripheral blood lymphocytes in vitro in the absence of S9‐mix metabolism following the 24‐h treatment.
To determine whether 7‐methyl‐3‐octenone‐2 was acting as a clastogen or an aneugen, a follow‐up study was performed, in the absence of S9‐mix, following a treatment of 24 h at similar concentrations used in the previous study (Lloyd 2010). FISH analysis was performed to identify micronuclei containing centromeres. Results obtained confirmed previous findings and clearly indicated that 7‐methyl‐3‐octenone‐2 is acting as a clastogenic compound. In order to determine whether positive results obtained in vitro for 7‐methyl‐3‐octenone‐2 could be confirmed in vivo, a bone marrow micronucleus test in rats treated in vivo was performed.
The test substance 7‐methyl‐3‐octenone‐2 administered by oral gavage at doses of 500, 1,000 and 2,000 mg/kg bw on two occasions with 24 h apart to Han‐Wistar rats did not prove to induce micronuclei in bone marrow PCE. However, this outcome was accompanied by complete absence of clinical signs of toxicity in any animal, in any treatment group. The observed very weak dose‐related decreases in mean % PCE was considered by the Panel not indicative of bone marrow exposure as suggested by the author of the study report, since group mean values of % PCE fall within observed historical range values at 95% confidence interval as reported in the study report (Henderson, 2012) and displayed in Table C.3. These findings do not allow to conclude a bone marrow exposure and therefore absence of induction of micronuclei in bone marrow erythrocytes does not necessarily reflect absence of clastogenicity in vivo of the test substance. Blood was appropriately collected and processed but, as stated in the study report, it was not analysed to measure the plasma level of 7‐methyl‐3‐octenone‐2 due to the lack of a bioanalytical method. The direct reactivity of the test substance evinced by clastogenicity induced in vitro, (in the absence of S9‐mix metabolism) indicates a reduced systemic availability of the test substance.
2.4.4. Conclusion in FGE.204
On the basis of the available data, the CEF Panel noted that no conclusions can be drawn about in vivo genotoxicity of 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] and more appropriate in vivo genotoxicity tests, considering also first site of contact, should be performed. Alternatively, chemical analysis of the already available blood samples demonstrating target tissue exposure would also be suitable to make the results of the available micronucleus assay acceptable for the assessment of the genotoxic potential.
For 4‐methylpent‐3‐en‐2‐one [FL‐no: 07.101], the data available showed that it did not induce mutations in bacteria or micronuclei in human peripheral blood lymphocytes, neither in the presence nor in the absence of rat liver S9‐mix metabolic activation. Based on these findings, the CEF Panel concluded that 4‐methylpent‐3‐en‐2‐one does not present a safety concern with respect to genotoxicity and accordingly the flavouring substance can be evaluated using the Procedure. Since this substance is considered to be representative for itself only (‘stand‐alone’), as it is the only substance with a methyl substituent on the β‐carbon atom of the double bond, this conclusion does not apply to any of the other candidate substances in this FGE.
Subsequent to the publication of the list of representatives (EFSA, 2008c), the CEF Panel noted that 4‐methyl‐3‐hepten‐5‐one [FL‐no: 07.261] differs structurally from all others in this subgroup owing to the presence of a methyl group in α‐position of the double bond. Therefore, this substance is considered as a ‘stand‐alone’ substance. The CEF Panel noted that the 2‐methyl substituted α,β‐unsaturated aldehydes in FGE.201Rev1 (EFSA CEF Panel, 2012b) can be considered as structurally related to [FL‐no: 07.261]. For the substances in FGE.201Rev1, 2‐methylpent‐2‐enal [FL‐no 05.090] has been selected as representative and further genotoxicity data were required. Thus, the final conclusion on [FL‐no: 07.261] will be drawn based on the outcome of the evaluation of FGE.201Rev1.
3. Assessment
3.1. Additional data evaluated by the Panel in FGE.204Rev1
The present revision of FGE.204 (FGE.204Rev1), concerns the evaluation of additional data submitted by Industry for the representative substance 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177]: plasma analysis (Covance, 2015) from a satellite group of animals dosed in the in vivo bone marrow micronucleus study (Henderson, 2012) that was evaluated in FGE.204, three in vivo comet assays in the liver and duodenum (Covance, 2017; BioReliance, 2018). The list of the studies evaluated and the summary of results are reported in Table 2 and in Appendix D, Table D.1.
Table 2.
List of studies evaluated in FGE.204Rev1
| Test substance FL‐no | Test | Reference |
|---|---|---|
|
7‐methyl‐3‐octenone‐2 07.177 |
Plasma analysis | Covance (2015) |
|
2 in vivo comet assays in liver 1 in vivo comet assay in duodenum |
Covance (2017) | |
| In vivo comet assay in liver and duodenum | BioReliance (2018, 2019) |
FGE: Flavouring Group Evaluation.
Table D.1.
Summary of additional data evaluated in FGE.204Rev1
| Chemical Name [FL‐no] | Test system in vivo | Test object route | Dose (mg/kg bw per day) | Result | Reference | Comments |
|---|---|---|---|---|---|---|
| 7‐methyl‐3‐octenone‐2 [07.177] | Plasma concentrations from animals tested in the in vivo micronucleus assaya | Han Wistar rats Oral gavage | 0 and 2,000 | Inconclusive | Covance (2015) | GC‐MSD method validated (recovery, accuracy and precision). Linearity and working range were assessed. The concentration of 7‐methyl‐3‐octenone‐2 detected was below the linearity range. Not a GLP study |
| Comet assay in liver and duodenum | Han Wistar rats Oral gavage | 0, 500, 1,000 and 2,000 |
Liver: equivocal Duodenum: Negative |
Covance (2017) | Reliable without restrictions. Study performed in accordance with OECD TG 489 | |
| Comet assay in liver and duodenum |
Sprague–Dawley rats Oral gavage |
0, 500, 1,000 and 2,000 |
Liver: Negative Duodenum: Negative |
BioReliance (2019) |
Reliable with minor restrictions. Study performed in accordance with OECD TG 489 |
FGE: Flavouring Group Evaluation; FL‐no: FLAVIS number; FLAVIS: Flavour Information System; bw : body weight; GC‐MSD: gas chromatography with mass selective detection; GLP: Good Laboratory Practice; OECD: Organisation for Economic Co‐operation and Development.
Plasma obtained from satellite group of animals in the study by Henderson (2012).
These data are considered to cover also the genotoxicity evaluation for the 15 structurally related substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.187, 07.188, 07.244 and 07.258], reported in Appendix B, Table B.1.
In FGE.204 (EFSA CEF Panel, 2012a), the CEF Panel concluded that 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] is clastogenic in vitro in the absence of metabolic activation. The in vivo micronucleus assay (Henderson, 2012) was not sufficient to investigate the potential clastogenicity of [FL‐no: 07.177], because in this study there was no evidence of bone marrow exposure, therefore in FGE.204 (EFSA CEF Panel, 2012a) the CEF Panel requested more appropriate in vivo genotoxicity tests, considering also first site of contact, or chemical analysis of the already available blood samples demonstrating target tissue exposure.
Therefore, Industry provided plasma analysis (Covance, 2015) from a satellite group of animals dosed during the in vivo micronucleus assay (Henderson, 2012).
Additional information was sought from the applicant during the assessment process in response to a request from EFSA sent on 15/12/2015, 9/8/2017, 11/7/2018 and was consequently provided (see Documentation provided to EFSA n. 4, 5, 7, 9). Information requested is summarised below.
Since data from plasma analysis were not sufficient to demonstrate target tissue exposure, the Panel requested to investigate the potential genotoxicity of 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] through an in vivo comet assay with scoring of duodenum and liver cells (EFSA letter dated 15/12/2015).
The applicant provided the requested study on 30/5/2017 (see documentation provided to EFSA n.7 and Section 3.4).
Due to inconsistent results in the comet assay studies provided, the Panel requested a further comet assay and asked for clarifications on specifications, stability and storage conditions of the substance tested (EFSA letter dated 9/8/2017). These data were submitted on 5/3/2018 (see documentation provided to EFSA n 4, 9 and Sections 3.3 and 3.5).
The Panel requested details of the statistical analysis applied to the comet assay data of both the duodenum and liver (EFSA letter dated 11/7/2018). These data were provided on 18/2/2019 together with an amended study report of the in vivo comet assay (see documentation provided to EFSA n.5 and Section 3.5).
3.2. Plasma analysis
In order to demonstrate bone marrow exposure in animals administered with 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] in the micronucleus assay by Henderson (2012) (see Section 2.4.2), a plasma analysis of a satellite group of animals was provided (Covance, 2015). Six male Han Wistar rats were dosed, by oral gavage, with 2,000 mg/kg bw per day of 7‐methyl‐3‐octenone‐2. A method was developed for the analysis of 7‐methyl‐3‐octenone‐2 using gas chromatography with mass selective detection (GC‐MSD) (Covance, 2015).
According to the applicant, satisfactory linearity, recovery and repeatability were found for [FL‐no: 07.177] when the substance was spiked and analysed in rat plasma samples.
The Panel, however, noted that linearity in plasma extracts was in the range of 10–400 μg/mL, but the highest concentration reported for 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] in rat plasma samples (3.4 μg/mL) was below this range. Moreover, the recovery and accuracy of the method were only determined from 50 μg/mL and above. Therefore, the data of plasma analysis obtained in vivo are unreliable and cannot be considered as demonstration of sufficient bone marrow exposure (Appendix D, Table D.1).
3.3. Stability and decomposition products
The Panel noted that in the in vivo comet assay (Covance, 2017) for 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177], the substance was stored under nitrogen; this, however, does not correspond to the conditions of storage of the flavouring substances expected under normal conditions of use (i.e. storage for 12 months at temperatures < 18°C and out of direct light and air) (EFFA, 2018).
To decide whether the substance subjected to genotoxicity testing can be considered representative of the materials of commerce, the Panel requested information on the stability of 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] under its intended conditions of use. The applicant provided data from capillary gas chromatographic analyses of freshly prepared flavouring substance and of flavouring substance stored close to the end of its shelf‐life. Since old samples were not available, the applicant forced aging storing a sample for 9 days at 60°C in an oven. The purity of the fresh sample was 98.2% (7‐methyl‐oct‐4‐en‐2‐one (as sum of E/Z isomers) was identified < 1.5%). The purity of the ‘forced aging’ sample was 97.5% (7‐methyl‐oct‐4‐en‐2‐one (as sum of E/Z isomers) was identified < 2.0%). Hence, under normal conditions of storage, the purity is expected to decrease from > 98% to 94–96% but it will remain at least 94%.
For 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177], the only changes observed after the forced aging, was related to the formation of 7‐methyl‐oct‐4‐en‐2‐one (sum of E/Z isomers), that was the only secondary component identified. Since the double bond in 7‐methyl‐oct‐4‐en‐2‐one is shifted, this derivative is not an α,β‐unsaturated ketone. Other possible secondary components are: 5,6‐dimethyl‐3‐hepten‐2‐one and 3‐nonen‐2‐one. The material of commerce is mainly as E‐isomer (> 90%), (EFFA, 2018).
Overall, the minimum purity assay for the flavouring substance [FL‐no: 07.177] is 94% and secondary components (7‐methyl‐4‐octen‐2‐one, 5,6‐dimethyl‐3‐hepten‐2‐one and 3‐nonen‐2‐one) can range from 2% to 4% (EFFA, 2018).
The Panel concluded that the materials tested in the genotoxicity studies are representative of the material of commerce.
Specifications, including purity criteria, of the flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.101, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.187, 07.188, 07.244, 07.258 and 07.261] are summarised in Appendix A, Table A.1.
3.4. In vivo comet assays in rats
The Panel requested to investigate the potential clastogenicity of 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] in an in vivo comet assay with scoring of duodenum and liver cells.
7‐Methyl‐3‐octenone‐2 (purity 98.2%, stored at 15–25°C under nitrogen and protected from light) was tested for its potential to induce DNA strand breaks in the liver and duodenum of Han Wistar rats in two independent experiments (Covance, 2017). In experiment 1, only liver data were reported because no valid duodenum data were obtained due to technical problems during tissue processing. In experiment 2, both liver and duodenum data are reported (Covance, 2017). The study was performed in accordance with OECD TG 489 (OECD, 2016) and Good Laboratory Practice (GLP). Doses were selected based on the range‐finding experiment reported by Henderson (2012). 7‐Methyl‐3‐octenone‐2 was dissolved in 0.5% aqueous methylcellulose (used as a vehicle control) and administered to rats (six male animals per group) via gavage at 500, 1,000 and 2,000 mg/kg bw per day in two administrations at 0 (day 1) and 21 h (day 2). Ethyl methanesulfonate (EMS, 150 mg/kg bw per day) was administered at a single administration at 21 h (day 2) to three animals, as a positive control.
Post‐dose observations were limited to experiment 1, where reduced activity and raised body hair were observed prior to necropsy on day 2 in three out of six animals dosed at 2,000 mg/kg bw per day.
In experiment 1, a minor reduction in body weight gain was noted at 2,000 mg/kg bw per day compared to the vehicle control. In experiment 2, a loss of body weight (day 1 to day 2) was apparent at 2,000 mg/kg bw per day.
In both experiments, a small increase of cholesterol and a small decrease in albumin and total protein was observed at 1,000 and 2,000 mg/kg bw per day. In experiment 1 also, a small decrease of albumin/globulin ratio was observed. In experiment 2, a small decrease of globulin and calcium was observed at the two highest doses.
In experiment 1, macroscopically, in the stomach, slight or marked distension was present in animals of all groups treated with 7‐methyl‐3‐octenone‐2, with a dose relationship. The mucosal surface of the cardia region was gelatinous, red and/or thick in animals from all groups given 7‐methyl‐3‐octenone‐2, generally with a dose‐related increase in incidence and/or severity. Microscopically, in the liver, there was an increase in hepatocyte mitosis and a decrease in glycogen in hepatocytes of animals from all 7‐methyl‐3‐octenone‐2 treated groups.
In experiment 2, macroscopically, in the stomach, distension, gelatinous mucosal surface of the cardia region, cysts and/or thick stomach were present in animals from all groups administered 7‐methyl‐3‐octenone‐2. The stomach was red or had dark areas in some animals administered 2,000 mg/kg bw per day. In the duodenum, degeneration of epithelial cells was present in animals administered 1,000 or 2,000 mg/kg bw per day. Microscopically, in the liver, there was an increase in hepatocyte mitosis, with no dose relationship, and a decrease in glycogen in hepatocytes, with a dose relationship, in animals from all groups given 7‐methyl‐3‐octenone‐2.
In experiment 1, for the duodenum, the comet data of the vehicle control group mean fell outside the 95% reference range of the historical control. According to the author of the study, there was a technical error during the processing of several vehicle control animals as well as sporadic treated animals; therefore, they considered data on the duodenum as unreliable for genotoxicity assessment. These data were invalidated and not described in the study report; the study was repeated. In experiment 2, there was no dose‐related increase in the percentage of clouds in duodenum cells following treatment with 7‐methyl‐3‐octenone‐2, thus demonstrating that treatment did not cause toxicity that could have interfered with comet analysis. There was a slight increase in mean % tail intensity values for dose groups 1,000 and 2,000 mg/kg bw (% tail intensity 1.27 ± 0.62 and 2.11 ± 1.19, respectively, compared with 0.72 ± 0.09 in concurrent controls), but this increase was not statistically significant and the results were within the laboratory's historical control data for the duodenum (0.24–5.60, 95% reference range).
In the liver, there was no dose‐related increase in the percentage of % clouds in any of the two experiments, demonstrating that treatment with 7‐methyl‐3‐octenone‐2 did not cause excessive liver toxicity which could interfere with comet analysis.
In the first in vivo comet assay in the liver, statistically significant increases of tail intensity were observed at the two highest doses 1,000 and 2,000 mg/kg bw (% tail intensity 0.32 ± 0.07 and 0.25 ± 0.08, respectively, compared with 0.08 ± 0.02 in concurrent control) and the trend test for the evaluation of dose response was statistically significant (p ≤ 0.001).
In the second in vivo comet assay in the liver, no statistically significant increases of tail intensity were observed compared to the concurrent negative control. However, the tail intensity results for the two highest doses are similar to results observed in the first experiment and the tail intensity of the concurrent negative control (0.27 ± 0.04) is more than threefold higher than in the first experiment (0.08 ± 0.02). In addition, the range of historical control values for tail intensity is very broad (0.04–5.50, 95% reference range). The Panel considered such a broad range of two orders of magnitude not appropriate for the use of historical controls in the evaluation of the data. The Panel considered that these inconsistent comet assay results require clarification and requested to repeat the comet assay. A new study was provided, which is described below.
3.5. In vivo comet assay in rats ‐ repeated study
7‐Methyl‐3‐octenone‐2 (purity 99%) was tested in an in vivo comet assay in the duodenum and liver of male Sprague–Dawley rats (BioReliance, 2018). 7‐Methyl‐3‐octenone‐2 was dissolved in 0.5% aqueous methylcellulose (used as a vehicle control) and administered to rats (six male animals per group) via gavage at 500, 1,000 and 2,000 mg/kg bw per day in two administrations at 0 (day 1) and 21 h (day 2). EMS (200 mg/kg bw per day) was administered at a single administration at 21 h (day 2) to three animals, as a positive control. The study was performed in accordance with OECD TG 489 (OECD, 2016) and GLP.
Clinical signs of toxicity were observed at 1,000 and 2,000 mg/kg bw per day, i.e. piloerection, hunched posture, lethargy and prostration. In the highest dose group, one animal moribund was sacrificed. No appreciable reductions in mean group body weights were observed. In the first study report submitted, the author indicated that 7‐methyl‐3‐octenone‐2 did not induce a statistically significant increase in % tail intensity in both liver and duodenum.
The Panel noted that in the high‐dose group, the % tail intensity value observed for one animal in the liver (8.12%) and for one animal in the duodenum (9.03%) were markedly higher than the mean values obtained from the animals of the same group (1.99 ± 3.43, 2.18 ± 3.83 mean % tail intensity for the liver and duodenum, respectively). Moreover, the Panel noted that concerning the statistical analysis, the Anova test was applied to analyse the data, but no test was reported to assess the normal distribution of data. Therefore, the Panel requested to clarify whether data from the animals identified as outliers were included or excluded in the statistical evaluation performed and to provide full details of the statistical analysis conducted on the comet assay data for both liver and duodenum.
The applicant provided a revised statistical analysis of the comet assay and amended the study report accordingly.
In the revised statistical analysis, Barlett's test was used to assess normality. The assumption of normality was satisfied only after log transformation of data and the Anova test (followed by Dunnett's post‐hoc analysis) was applied in the analysis of comet assay data for both liver and duodenum. The study report was amended accordingly (BioReliance, 2019).
Liver
There was a statistically significant increase of mean % tail intensity in the liver (1.99 ± 3.43) at the highest dose tested. This increase of mean % tail intensity is not dose‐related, but is outside the range of historical negative control for the liver (0.00 to 0.63, 95% reference range). The author of the study considered the statistically significant increase in tail intensity as not biologically relevant because one animal in the 2,000 mg/kg bw dose group gave a deviant study result.5 This animal was considered as outlier, not only through statistical analysis,5 but also on the basis of the clinical observations (hunched position, lethargy and prostration) and on the basis of comet data: the % tail intensity value (8.12%), which was outside the range normally observed for this organ, was associated with high percentage of clouds in this liver sample (cloud value at 8% vs an average < 0.7% in vehicle control). Therefore, the statistical analysis was repeated excluding comet assay data from this outlying animal. The new analysis was based on data from four animals instead of at least five, as recommended by OECD TG 489, because one animal was sacrificed and one was excluded being an outlier. The outcome of this new analysis, showing mean % tail intensity of 0.45 ± 0.21 in the highest dose group, resulted in a lack of statistically significant increase in % tail intensity (mean % tail intensity of the vehicle control group is 0.10 + 0.05%). In order to evaluate whether the reduction of the observations considered in the analysis could have affected the robustness of the statistic, a ‘simulating % Tail DNA value’ (the highest % tail intensity from the remaining animals) was used to replace the data of the outlying animal and the analysis was performed again. No statistically significant response was observed in the new analysis. Therefore, the study author concluded that 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] is negative in the in vivo comet assay in the liver.
The Panel considered that the approach adopted by the applicant (i.e. re‐running the analysis by replacing just only the missing observation) was not fully adequate to judge the robustness of the test. Indeed, multiple values should be generated to simulate the inclusion of the missing information. EFSA checked the robustness of the test by multiple imputation6 to replace the missing observation. The outcome of the multiple imputation showed that the test is still robust even excluding one animal and confirmed the non‐statistically significant increase in % tail intensity in liver cells.
The Panel concluded that 7‐methyl‐3‐octenone‐2 did not induce statistically significant increase of % tail intensity in the liver.
Duodenum
The comet assay in the duodenum did not result in statistically significant increase in % tail intensity, according to the amended study report. However, the Panel noted that also in duodenal cells one animal in the 2,000 mg/kg bw dose group showed a markedly higher % tail intensity (9.03%) than the values obtained for the other animals of the same group (0.29–0.59%). Additionally, the Panel noted that this % tail intensity value was similar to the values observed for animals treated with the positive control (EMS). This result was not commented by the study author and it was included also in the revised statistical analysis, which displayed no statistically significant increase in % tail intensity in any dose group (0.40 ± 0.19, 0.80 ± 0.64, 2.18 ± 3.83, in the low‐, mid‐ and high‐dose group, respectively, compared to the vehicle control 0.69 + 0.32).
The Panel concluded that 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] did not induce statistically significant increase in % tail intensity in duodenal cells.
Despite the limitations of the amended statistical analysis performed by the applicant (i.e. not fully appropriate method used to simulate the inclusion of five animals instead of four, due to the exclusion of one outlier in the liver; and the presence of an outlier in the highest dose group in the duodenum that was not investigated in the study report), the Panel considered the analysis of comet assay data still valid. Therefore, the Panel concluded that the flavouring substance 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] does not induce primary DNA damaging in the liver and duodenum.
4. Discussion
7‐Methyl‐3‐octenone‐2 [FL‐no: 07.177] did not induce mutations in six strains of S. Typhimurium when tested up to toxic concentrations in the absence and in the presence of metabolic activation.
7‐Methyl‐3‐octenone‐2 induced micronuclei in vitro in the absence of S9‐mix following the 24‐h treatment, through a clastogenic mechanism.
As in vivo follow‐up, a rat bone marrow micronucleus study was performed by gavage. The negative outcome of this study is considered of limited relevance, because no clear indication of bone marrow exposure was demonstrated, neither through plasma analysis.
To further investigate the potential clastogenicity, an in vivo comet assay in the duodenum and liver of rats was performed. In the duodenum, no statistically significant increase of tail intensity was observed. In the liver, statistically significant increases of tail intensity at the two highest doses and a positive test for trend were observed in a first experiment, but these results were not reproduced in a second experiment. Due to these equivocal results a new comet assay was performed in rats at the same doses. The analysis of liver and duodenum did not show DNA damage.
The Panel concluded that for the representative substance 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] the clastogenicity observed in vitro was not reproduced in vivo.
5. Conclusions
Overall, the Panel concluded that the concern for genotoxicity is ruled out for the representative substance 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] and for the 15 structurally related substances in this subgroup. The flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244] can accordingly be evaluated through the Procedure in FGE.63. The flavouring substances [FL‐no: 07.187 and 07.258] can be evaluated through the Procedure in FGE.07.
Previously, in FGE.204 (EFSA CEF Panel, 2012a), the CEF Panel concluded that 4‐methylpent‐3‐en‐2‐one [FL‐no: 07.101] does not present a safety concern with respect to genotoxicity and accordingly this flavouring substance was evaluated through the Procedure in FGE.63Rev2.
6. Recommendations
The name of the substance 7‐methyl‐3‐octenone‐2 [FL‐no: 07.177] in the Union List1 should be changed into 7‐methyl‐3‐octen‐2‐one.
Documentation provided to EFSA
Ballantyne M, 2011. Reverse mutation in one histidine‐requiring strain of Salmonella typhimurium. 7‐Methyl‐3‐octen‐2‐one. Covance Laboratories Ltd. Study no. 8250468. October 2011. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Benigni R and Netzeva T, 2007a. Report on a QSAR model for prediction of genotoxicity of alpha,beta‐unsaturated aldehydes in S. typhimurium TA100 and its application for predictions on alpha,beta‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
Benigni R and Netzeva T, 2007b. Report on a QSAR model for prediction of genotoxicity of alpha,beta‐unsaturated ketones in S. typhimurium TA100 and its application for predictions on alpha,beta‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
BioReliance, 2018. 7‐Methyl‐3‐octen‐2‐one, in vivo mammalian alkaline comet assay. BioReliance Study Number AF08XB.423M.BTL. Unpublished final report submitted by EFFA.
BioReliance, 2019. 7‐Methyl‐3‐octen‐2‐one, in vivo mammalian alkaline comet assay. BioReliance Study Number AF08XB.423M.BTL. Unpublished amended final report submitted by EFFA. Additional data submitted by EFFA on 18/2/2019 in reply to EFSA letter dated 11/7/2018, which was followed by a clarification teleconference on 5/10/2018.
Covance, 2015. Development and limited validation of a method for the analysis of plasma samples which may contain 7‐methyl‐3‐octen‐2‐one. Covance laboratory Ltd, study number 8302‐749. Unpublished report submitted by EFFA.
Covance, 2017. Dimethylheptenone: Rat Alkaline Comet assay. Covance Laboratories Ltd. Study no. 8352715. 25 May 2017. Unpublished final report submitted by EFFA.
EFFA (European Flavour Association), 2012. Submission by the European Flavour Association to the European Food Safety Authority. Flavouring Group Evaluation 19 Subgroup 1.2.1 (corresponding to FGE.204): Submission of additional data related to FGE.19 subgroup 1.2.1. 22 March 2012. FLAVIS/8.150.
EFFA (European Flavour Association), 2018. Submission of additional information on substances within FGE.204 (FGE.19 Subgroup 1.2.1). Additional data submitted by EFFA on 5/3/2018 in reply to EFSA letter dated 9/8/2017, which was followed by a clarification teleconference on 22/09/2017.
EFFA (European Flavour Association), 2019. Submission of updated poundage data and use levels for certain flavouring substances. Data submitted to the European Commission and subsequently provided to EFSA.
Gry J, Beltoft V, Benigni R, Binderup M‐L, Carere A, Engel K‐H, Gürtler R, Jensen GE, Hulzebos E, Larsen JC, Mennes W, Netzeva T, Niemelä J, Nikolov N, Nørby KK and Wedebye EB, 2007. Description and validation of QSAR genotoxicity models for use in evaluation of flavouring substances in Flavouring Group Evaluation 19 (FGE.19) on 360 alpha,beta‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
Henderson D, 2012. Induction of micronuclei in the bone marrow of treated rats. 7‐Methyl‐3‐octen‐2‐one. Covance Laboratories Ltd. Study no. 8250467. March 2012. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Lloyd M, 2009. Induction of micronuclei in cultured human peripheral blood lymphocytes. 7‐Methyl‐3‐octen‐2‐one. Covance Laboratories Ltd. Study no. 8203172. October 28, 2009. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Lloyd M, 2010. Induction of micronuclei in cultured human peripheral blood lymphocytes. 7‐Methyl‐3‐octen‐2‐one. Covance Laboratories Ltd. Study no. 8221347. August 19, 2010. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Nikolov N, Jensen GE, Wedebye EB and Niemelä J, 2007. Report on QSAR predictions of 222 alpha,beta‐unsaturated aldehydes and ketones from Flavouring Group Evaluation 19 (FGE.19) on 360 alpha,beta‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
Stone V, 2011. Induction of micronuclei in cultured human peripheral blood lymphocytes. 4‐Methylpent‐3‐en‐2‐one. Covance Laboratories Ltd. Study no. 8241438. August 23, 2011. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Thompson PW, 1996. Reverse mutation assay “Ames Test” using Salmonella typhimurium. 7‐Methyl‐3‐octen‐2‐one. Safepharm Laboratories Limited. SPL Project no. 161/102. November 20, 1996. Unpublished data submitted by EFFA to Scientific Committee on Food.
Williams L, 2009. Reverse mutation in five histidine‐requiring strains of Salmonella typhimurium. 4‐Methylpent‐3‐en‐2‐one. Covance Laboratories Ltd, England. Study no. 8200433. July 29, 2009. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Abbreviations
- bw
body weight
- CAS
Chemical Abstract Service
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CoE
Council of Europe
- EMS
ethyl methanesulfonate
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FISH
fluorescence in situ hybridization
- FLAVIS (FL)
Flavour Information System (database)
- GC‐MSD
gas chromatography with mass selective detection
- GLP
Good Laboratory Practice
- ID
Identity
- IR
infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MNBN
micronucleated binucleate cells
- MS
mass spectra
- MSDI
Maximised Survey‐derived Daily Intake
- NMR
nuclear magnetic resonance
- No
Number
- OECD
Organisation for Economic Co‐operation and Development
- PCE
polychromatic erythrocytes
- PHA
phytohaemagglutinin
- (Q)SAR
(Quantitative) Structure–Activity Relationship
- RI
Replication Index
- TAMDI
Theoretical Added Maximum Daily Intake
- WHO
World Health Organization
Appendix A – Specification Summary of the Substances in the Flavouring Group Evaluation 204 (JECFA, 2002a)
1.
Appendix B – Summary of safety evaluation applying the procedure (Based on Intakes Calculated by the MSDI Approach) (JECFA, 2002b)
1.
Appendix C – Genotoxicity data evaluated in FGE.204
1.
Appendix D – Genotoxicity data evaluated in FGE.204Rev1
1.
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Gürtler R, Husøy T, Moldeus P, Oskarsson A, Shah R, Waalkens‐Berendsen I, Wölfle D, Benigni R, Bolognesi C, Chipman K, Cordelli E, Degen G, Marzin D, Svendsen C, Carfì M, Vianello G and Mennes W, 2019. Scientific Opinion on Flavouring Group Evaluation 204 Revision 1 (FGE.204Rev1): consideration of genotoxicity data on representatives for 17 monounsaturated, aliphatic, α,β‐unsaturated ketones and precursors from chemical subgroup 1.2.1 of FGE.19. EFSA Journal 2019;17(7):5750, 26 pp. 10.2903/j.efsa.2019.5750
Requestor: European Commission
Question numbers: EFSA‐Q‐2015‐00297, EFSA‐Q‐2015‐00298, EFSA‐Q‐2015‐00299, EFSA‐Q‐2015‐00300, EFSA‐Q‐2015‐00301, EFSA‐Q‐2015‐00302, EFSA‐Q‐2015‐00303, EFSA‐Q‐2015‐00304, EFSA‐Q‐2015‐00305, EFSA‐Q‐2015‐00306, EFSA‐Q‐2015‐00307, EFSA‐Q‐2015‐00308, EFSA‐Q‐2015‐00309, EFSA‐Q‐2015‐00310, EFSA‐Q‐2015‐00311, EFSA‐Q‐2015‐00312
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus, Agneta Oskarsson, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle and Maged Younes.
Acknowledgements: The FAF Panel wishes to thank the Working Group on Genotoxicity of the former EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) for the preparatory work on this scientific opinion, in particular Mona‐Lise Binderup, Francesca Marcon and Pasquale Mosesso. The Panel wishes to thank the hearing experts: Vibe Beltoft and Karin Nørby and EFSA staff: José Cortinas Abrahantes for the support provided to this scientific output.
Adopted: 5 June 2019
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
The data presented in Section 2.4 are cited from FGE.204. These data are the basis for the conclusions in FGE.204 requesting additional genotoxicity data.
Alternate Dixon Q test was applied to determine the presence of outliers in the dataset.
R software: VIM and MICE libraries.
References
- EFSA (European Food Safety Authority), 2008a. Minutes of the 26th Plenary meeting of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food, Held in Parma on 27–29 November 2007. Parma, 7 January 2008. Available online: https://www.efsa.europa.eu/sites/default/files/event/afc071127-m.pdf
- EFSA (European Food Safety Authority), 2008b. Genotoxicity test strategy for substances belonging to subgroups of FGE.19 – statement of the panel on food contact materials, enzymes, flavourings and processing aids (CEF). EFSA Journal 2008;6(12):854, 5 pp. 10.2903/j.efsa.2008.854 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Scientific Opinion. List of α,ß‐unsaturated aldehydes and ketones representative of FGE.19 substances for genotoxicity testing – Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). EFSA Journal 2008;6(12):910, 7 pp. 10.2903/j.efsa.2008.910 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2012a. Scientific Opinion on Flavouring Group Evaluation 204 (FGE.204): Consideration of genotoxicity data on representatives for 18 mono‐unsaturated, aliphatic, α,β‐unsaturated ketones and precursors from chemical subgroup 1.2.1 of FGE.19 by EFSA. EFSA Journal 2012;10(12):2992, 25 pp. 10.2903/j.efsa.2012.2992 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2012b. Scientific Opinion on Flavouring Group Evaluation 201 Revision 1 (FGE.201Rev1): 2‐Alkylated aliphatic, acyclic alpha,beta‐unsaturated aldehydes and precursors with or without additional double bonds from chemical subgroup 1.1.2 of FGE.19. EFSA Journal 2012;10(5):2749, 27 pp. 10.2903/j.efsa.2012.2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2013. Scientific Opinion on Flavouring Group Evaluation 63, Revision 2 (FGE.63Rev2): Consideration of aliphatic secondary alcohols, ketones and related esters evaluated by JECFA (59th and 69th meetings) structurally related to saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched‐chain carboxylic acids evaluated by EFSA in FGE.07Rev4. EFSA Journal 2013;11(4):3188, 45 pp. 10.2903/j.efsa.2013.3188 [DOI] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), 2018. Scientific Opinion on Flavouring Group Evaluation 201 Revision 2 (FGE.201Rev2): 2‐alkylated, aliphatic, acyclic alpha,beta‐unsaturated aldehydes and precursors, with or without additional double‐bonds, from chemical subgroup 1.1.2 of FGE.19. EFSA Journal 2018;16(10):5423, 33 pp. 10.2903/j.efsa.2018.5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002a. Compendium of food additive specifications. Addendum 10. Joint FAO/WHO Expert Committee of Food Additives 59th session. Geneva, 4‐13 June 2002. FAO Food and Nutrition paper 52 Add.10.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002b. Evaluation of certain food additives. Fifty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 913. Geneva, 4‐13 June 2002.
- OECD (Organisation for Economic Co‐operation and Development), 1997. Test No. 471: Bacterial reverse mutation test. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2016. Test No. 489: In Vivo Mammalian Alkaline Comet Assay. OECD Guidelines for the Testing of Chemicals, Section 4.


