Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) derived dietary reference values (DRVs) for sodium. Evidence from balance studies on sodium and on the relationship between sodium intake and health outcomes, in particular cardiovascular disease (CVD)‐related endpoints and bone health, was reviewed. The data were not sufficient to enable an average requirement (AR) or population reference intake (PRI) to be derived. However, by integrating the available evidence and associated uncertainties, the Panel considers that a sodium intake of 2.0 g/day represents a level of sodium for which there is sufficient confidence in a reduced risk of CVD in the general adult population. In addition, a sodium intake of 2.0 g/day is likely to allow most of the general adult population to maintain sodium balance. Therefore, the Panel considers that 2.0 g sodium/day is a safe and adequate intake for the general EU population of adults. The same value applies to pregnant and lactating women. Sodium intakes that are considered safe and adequate for children are extrapolated from the value for adults, adjusting for their respective energy requirement and including a growth factor, and are as follows: 1.1 g/day for children aged 1–3 years, 1.3 g/day for children aged 4–6 years, 1.7 g/day for children aged 7–10 years and 2.0 g/day for children aged 11–17 years, respectively. For infants aged 7–11 months, an Adequate Intake (AI) of 0.2 g/day is proposed based on upwards extrapolation of the estimated sodium intake in exclusively breast‐fed infants aged 0–6 months.
Keywords: Sodium, Dietary Reference Value
Short abstract
This publication is linked to the following EFSA Supporting Publications articles: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1679/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.e15121/full
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5779/full
Summary
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver a Scientific Opinion on dietary reference values (DRVs) for the European population, including sodium.
Sodium (Na+) is the dominant cation in the extracellular fluid (ECF) of the body. The functions of sodium lie in its participation in the control of the volume and systemic distribution of total body water; enabling the cellular uptake of solutes; and the generation via interactions with potassium of transmembrane electrochemical potentials.
Dietary sodium deficiency is rare in healthy European populations. Sodium chloride and other sodium salts are ubiquitous in the diet, and there are adaptive physiological mechanisms that reduce the losses of sodium in urine, faeces and sweat at low levels of sodium intake. Sodium chloride added during industrial food processing and discretionary use or food preservation is the major source of dietary sodium in Western diets. Other sources of sodium include inherently native sources and sodium‐containing food additives, in which sodium may be associated with anions other than chloride.
In healthy people, almost all dietary sodium is absorbed, even at very high level of intake. Following absorption, sodium ions are distributed by portal and systemic circulations, where their concentrations are maintained within a narrow range. Up to 95% of sodium body content is in the ECF, including a large proportion in bone, skin and muscle. The pool of sodium in bone, muscle and skin has been proposed to be a sodium depot or reserve, but could also have a homeostatic and adaptive role as an extra‐renal clearance depository for handling excessive systemic accumulation of sodium. The excretion and retention (i.e. homeostasis) of sodium is effected by an integrated neurohormonal control from centres located in the hypothalamus. The kidney is the main organ mediating the excretion and retention of sodium. It efficiently excretes sodium in response to high dietary intakes and salvages sodium when dietary intake is low. By contrast, sodium losses in the faeces are relatively stable and typically limited to a few mmol/day. The amount of sodium lost in sweat can vary widely, depending on, for example environmental conditions or the levels of physical activity.
The Panel reviewed the reliability of the methods and biomarkers used to assess sodium intake. Urinary sodium excretion in 24‐h collections is considered the most reliable biomarker of sodium daily intake. However, a single 24‐h urine collection may not reliably reflect an individual's usual intake. Also, incomplete collections of 24‐h urine samples can introduce bias in measuring daily sodium excretion. Multiple collections and quality control procedures are required to estimate an individual's usual sodium intake reliably.
Homeostatic mechanisms maintain the plasma sodium concentration of healthy individuals within a narrow range. Hyponatraemia and hypernatraemia are typically related to disorders affecting water and electrolyte balances. They are seldom due to inappropriate sodium intake. The Panel considers that there is no biomarker of sodium status that can be used for setting DRVs for sodium in the general population.
Evidence from balance studies on sodium and on the relationship between sodium intake and health outcomes, in particular cardiovascular disease (CVD)‐related endpoints and bone health, was reviewed.
Balance studies indicate that adaptation mechanisms enable the maintenance of sodium balance over a wide range of sodium intakes. Recent data from a long‐term study of sodium and other electrolytes metabolism suggest that rhythmical variations in the sodium body pools may occur independently from sodium intake. This complicates the interpretation of balance studies and of 24‐h urine collections. Overall, the Panel considers that balance studies cannot be used to determine sodium requirements.
The literature on the relationship between sodium intake and selected health outcomes, i.e. blood pressure, cardiovascular disease‐related endpoints and bone health, was systematically reviewed. To minimise the risk of bias in the evidence used in the assessment, the review was restricted to randomised controlled trials (RCTs) and prospective studies, studies that excluded participants with pre‐existing medical conditions, and studies that used at least one 24‐h urinary collection to estimate sodium intake. Risk of bias in eligible studies was assessed using the OHAT‐NTP critical appraisal tool. Studies were categorised according to their risk of bias based on a three‐tier system (i.e. at low, moderate or high risk of bias).
Eligible studies on bone health provided limited and inconsistent evidence for an association between sodium intake and bone mineral density and could not be used to set DRVs for sodium.
Meta‐analyses and modelling of the dose–response between 24‐h sodium urinary excretion (UNa) and blood pressure were conducted. Random effects meta‐analyses of the 32 eligible RCTs showed significant effects of sodium reduction on systolic blood pressure (SBP) (−3.9 (95% CI −5.1, −2.8) mm Hg; I2 61.9%, p < 0.001) and diastolic blood pressure (DBP) (−2.0 (−2.8, −1.2) mm Hg; I2 60.6%, p < 0.001). Using mixed‐effects meta‐regression models, mean SBP increased by 5.3 mm Hg (95% CI: 3.6–6.9 mm Hg) and mean DBP increased by 2.6 mm Hg (95% CI: 1.6–3.7 mm Hg) for each 100 mmol (2.3 g)/24 h increase in mean UNa. The Panel considers that there is strong evidence for a positive relationship between UNa and SBP and DBP over the range of mean UNa observed in the studies (between 49 and 209 mmol/24 h (1.3–4.8 g/day)). This is also supported by an eligible prospective observational study that investigated the long‐term relationship between UNa and blood pressure levels and by the eligible studies that assessed the relationship between UNa and risk of hypertension (two RCTs and two prospective observational studies).
A small number of prospective observational studies assessing the relationship between UNa and CVD risk was eligible for the assessment: three cohort studies investigated the association between UNa and risk of stroke or coronary heart disease (CHD); three cohort studies investigated the association between UNa and risk of total CVD. Overall, only limited conclusions can be drawn on the relationship between UNa and risk of CVD. The Panel considers that, over the range of UNa observed in these studies:
There is some evidence for a positive association between UNa and risk of CHD. The positive relationship between UNa and blood pressure levels/incidence of hypertension, which is an established independent risk factor for CHD, supports this association.
There is some evidence for an inverse association between UNa and risk of stroke. However, the number of eligible studies available investigating this outcome is small and the mechanisms by which UNa could be inversely associated with the risk of stroke are unclear, particularly considering the positive relationship between UNa and blood pressure, which is an established risk factor for stroke.
There is some evidence for a positive association between UNa and risk of total CVD, which is consistent with the evidence for a positive association between UNa and risk of CHD and the positive relationship between UNa and blood pressure levels/incidence of hypertension.
Overall, the Panel considers that the available evidence cannot be used to determine the sodium requirement in the population; so, an average requirement (AR) and population reference intake (PRI) for sodium cannot be established. Data on the relationship between sodium intake and blood pressure or CVD risks could inform about the levels of sodium intake associated with a reduced risk of chronic diseases. Balance studies could inform about the levels of sodium intake that are adequate to maintain a null sodium balance. Expert judgement was used to weigh the available evidence and take account of the associated uncertainties by means of a formal expert knowledge elicitation (EKE). The EKE allows a representation of the uncertainty about the quantity (parameter) of interest using a probability distribution.
Integrating the available evidence and associated uncertainties, the Panel considers that a sodium intake of 2.0 g/day represents a level of sodium for which there is sufficient confidence in a reduced risk of CVD in the general adult population. Also, a sodium intake of 2.0 g/day is likely to allow most of the general adult population to maintain physiological sodium balance. Therefore, the Panel considers that 2.0 g sodium/day is a safe and adequate intake for the general EU population of adults.
The requirement for the daily accretion rate of sodium in fetal and maternal tissues can be met by the adaptive changes that maintain sodium homeostasis during pregnancy. There is no evidence that the sodium requirement of lactating women differs from the requirement of non‐lactating women. So, 2.0 g of sodium/day is a safe and adequate intake for pregnant and lactating women.
Sodium intakes that are considered safe and adequate for children are extrapolated from the value for adults, adjusting for their respective energy requirement and including a growth factor, and are as follows: 1.1 g/day for children aged 1–3 years, 1.3 g/day for children aged 4–6 years, 1.7 g/day for children aged 7–10 years and 2.0 g/day for children aged 11–17 years, respectively.
For infants aged 7–11 months, an Adequate Intake (AI) of 0.2 g/day is proposed based on upwards extrapolation of the estimated sodium intake in exclusively breast‐fed infants aged 0–6 months, on the basis of the energy requirements of the respective age groups.
The Panel notes that the mean/median intake of sodium in the European adult populations exceeds the safe and adequate intakes set for sodium. The risk of inadequate (insufficient) intake in European populations is low. Concerns for European populations instead relate to excess intake of sodium. Therefore, in practice, the values proposed can be used to inform the setting of population goals for the reduction in sodium intake.
Background as provided by the European Commission
The scientific advice on nutrient intakes is important as the basis of Community action in the field of nutrition, for example such advice has in the past been used as the basis of nutrition labelling. The Scientific Committee for Food (SCF) report on nutrient and energy intakes for the European Community dates from 1993. There is a need to review and if necessary to update these earlier recommendations to ensure that the Community action in the area of nutrition is underpinned by the latest scientific advice.
In 1993, the SCF adopted an opinion on the nutrient and energy intakes for the European Community.1 The report provided Reference Intakes for energy, certain macronutrients and micronutrients, but it did not include certain substances of physiological importance, for example dietary fibre.
Since then new scientific data have become available for some of the nutrients, and scientific advisory bodies in many European Union Member States and in the United States have reported on recommended dietary intakes. For a number of nutrients these newly established (national) recommendations differ from the reference intakes in the SCF (1993) report. Although there is considerable consensus between these newly derived (national) recommendations, differing opinions remain on some of the recommendations. Therefore, there is a need to review the existing EU Reference Intakes in the light of new scientific evidence, and taking into account the more recently reported national recommendations. There is also a need to include dietary components that were not covered in the SCF opinion of 1993, such as dietary fibre, and to consider whether it might be appropriate to establish reference intakes for other (essential) substances with a physiological effect.
In this context the EFSA is requested to consider the existing Population Reference Intakes for energy, micro‐ and macronutrients and certain other dietary components, to review and complete the SCF recommendations, in the light of new evidence, and in addition advise on a Population Reference Intake for dietary fibre.
For communication of nutrition and healthy eating messages to the public it is generally more appropriate to express recommendations for the intake of individual nutrients or substances in food‐based terms. In this context the EFSA is asked to provide assistance on the translation of nutrient based recommendations for a healthy diet into food based recommendations intended for the population as a whole.
Terms of reference as provided by the European Commission
In accordance with Article 29(1)(a) and Article 31 of Regulation No 178/2002,2 the Commission requests EFSA to review the existing advice of the Scientific Committee for Food on population reference intakes for energy, nutrients and other substances with a nutritional or physiological effect in the context of a balanced diet which, when part of an overall healthy lifestyle, contribute to good health through optimal nutrition.
In the first instance the EFSA is asked to provide advice on energy, macronutrients and dietary fibre. Specifically advice is requested on the following dietary components:
Carbohydrates, including sugars;
Fats, including saturated fatty acids, polyunsaturated fatty acids and monounsaturated fatty acids, trans fatty acids;
Protein;
Dietary fibre.
Following on from the first part of the task, the EFSA is asked to advise on population reference intakes of micronutrients in the diet and, if considered appropriate, other essential substances with a nutritional or physiological effect in the context of a balanced diet which, when part of an overall healthy lifestyle, contribute to good health through optimal nutrition.
Finally, the EFSA is asked to provide guidance on the translation of nutrient based dietary advice into guidance, intended for the European population as a whole, on the contribution of different foods or categories of foods to an overall diet that would help to maintain good health through optimal nutrition (food‐based dietary guidelines).
Data and methodologies
The assessment is conducted in accordance with the NDA Panel's Scientific Opinion on principles for deriving and applying dietary reference values (DRVs), thereafter referred to as ‘the opinion on principles’ (EFSA NDA Panel, 2010).
In addition, some parts of the assessment were undertaken by applying the four‐step approach for evidence use (i.e. plan/carry out/verify/report) described in the EFSA report on principles and process for dealing with data and evidence3 (the ‘PROMETHEUS approach’) (EFSA, 2015).
The opinion is structured as follows:
-
•
Sections 1, 2, 2.1, 2.2, 2.2.1, 2.2.2, 2.2.2.1, 2.2.2.2, 2.3, 2.3.1, 2.3.2, 2.3.3, 2.3.4, 2.3.4.1, 2.3.4.2, 2.3.4.3, 2.3.4.4, 2.4, 2.5, 2.5.1, 2.5.2, 2.5.3, 2.6, 2.6.1, 2.6.1.1, 2.6.1.2, 2.6.2, 2.7, 3, 3.1, 3.2, 3.2.1, 3.2.2–4 include relevant background information on sodium; this encompasses an introduction (Section 1), information on chemistry, function, physiology, metabolism, interaction with other nutrients and biomarkers of intake and status (Section 2), information on dietary sources and intake data (Section 3) and an overview of DRVs and recommendations from other bodies (Section 4).
-
•
Section 5 covers the assessment of the evidence on the criteria (endpoints) on which to base DRVs.
-
•
Section 6 provides the integration of the available evidence and derivation of DRVs.
The data and methodologies used to inform the respective sections are described below.
-
1
Collection of relevant background information
To inform Sections 1, 2, 2.1, 2.2, 2.2.1, 2.2.2, 2.2.2.1, 2.2.2.2, 2.3, 2.3.1, 2.3.2, 2.3.3, 2.3.4, 2.3.4.1, 2.3.4.2, 2.3.4.3, 2.3.4.4, 2.4, 2.5, 2.5.1, 2.5.2, 2.5.3, 2.6, 2.6.1, 2.6.1.1, 2.6.1.2, 2.6.2, 2.7, 3, 3.1, 3.2, 3.2.1, 3.2.2–4 of the Scientific Opinion, a literature search covering sodium physiology and metabolism in healthy adults, biomarkers of intake, and genotypes affecting sodium metabolism was commissioned to the University of Hertfordshire (Lewis et al., 2015).
To complement the information gathered in a previous opinion (SCF, 2003), a comprehensive review of the literature published from January 2000 on the concentration of sodium in breast milk from healthy women living in Europe, North America and Australia was conducted by LASER Analytica (LASER Analytica, 2014).
An ad hoc questionnaire developed by the members of the working group on DRVs for minerals was disseminated to EFSA focal points and the members of the EFSA Food Consumption Network to collect information on the levels of urinary sodium excretion, to ascertain current information on sodium intake by European populations.
Additional background information was gathered by the members of the working group on DRVs for minerals and EFSA staff. Recent textbooks, authoritative reviews and research papers were used as sources of information. They were retrieved through searches in bibliographic databases, and were selected on the basis of their relevance.
-
2
Identification of the criteria on which to base DRVs
In Section 5, the NDA Panel assesses the evidence on possible criteria on which to base DRVs. To that end, the Panel considers:
-
•
biomarkers as indicators of sodium requirement (Section 5.1);
-
•
studies on sodium balance (Section 5.2);
-
•
indicators of sodium requirement in pregnancy and lactation (Section 5.3);
-
•
indicators of sodium requirement in children (Section 5.4);
-
•
sodium intake and health consequences (Section 5.5).
The NDA Panel assessed the suitability of each criterion to set DRVs for the nutrient on the basis of considerations of the available evidence and its inherent uncertainty and the possibility of deriving quantitative estimates.
Sections 5.1, 5.2, 5.2.1, 5.2.2, 5.2.3, 5.2.4, 5.3, 5.4 draw from the background information gathered in Sections 2 and 4 of the Scientific Opinion, expert knowledge from the members of the working group on DRVs for minerals, and targeted searches in bibliographic databases.
In line with the PROMETHEUS approach, a draft protocol (Annex A) was developed for Section 5.5 of the Scientific Opinion. Systematic reviews were conducted on the relationship between sodium intake and selected health outcomes. The protocol describes the steps followed for the collection, selection, appraisal and synthesis of evidence.
-
3
Integration of the available evidence and derivation of DRVs
Section 6 outlines the criteria considered by the NDA Panel as the most appropriate for setting DRVs, and provides DRVs for sodium. To that end, the Panel considered the quantitative relationships between sodium intake and the selected criteria together with the related uncertainties. The principles of the EFSA Scientific Committee guidance documents on uncertainties analysis and on the use of weight of evidence approaches in scientific assessments (EFSA Scientific Committee et al., 2017; EFSA Scientific Committee et al., 2018aa; EFSA Scientific Committee et al., 2018bb) were applied. In view of the limited evidence available and of the associated uncertainties, a formal expert knowledge elicitation (EKE) was undertaken by the members of the working group on DRVs for minerals to integrate the evidence and express related uncertainties. EKE is a systematic, documented and reviewable process to retrieve expert judgements from a group of experts in the form of a probability distribution. Several methods are described in the EFSA guidance to elicit knowledge of the experts (EFSA, 2014). For the sodium mandate, the roulette method was chosen. This is a formal approach that allows the experts to draw their own distribution of uncertainty on the parameter to be estimated by placing different numbers of plastic counters along the range of possible parameter values conveniently split in subintervals. The judgements were elicited following the Sheffield protocol, in which experts first make separate judgements about the distribution, then share and discuss their distributions, and finally develop a consensus distribution and document their reasoning (EFSA, 2014).
-
4
Public consultations
In September 2017, Sections 1, 2, 2.1, 2.2, 2.2.1, 2.2.2, 2.2.2.1, 2.2.2.2, 2.3, 2.3.1, 2.3.2, 2.3.3, 2.3.4, 2.3.4.1, 2.3.4.2, 2.3.4.3, 2.3.4.4, 2.4, 2.5, 2.5.1, 2.5.2, 2.5.3, 2.6, 2.6.1, 2.6.1.1, 2.6.1.2, 2.6.2, 2.7, 3, 3.1, 3.2, 3.2.1, 3.2.2, 4, 4.1, 4.2, 4.3, 5, 5.1, 5.2, 5.2.1, 5.2.2, 5.2.3, 5.2.4, 5.3–5.4 of the draft Scientific Opinion, as well as the draft protocol developed for Sections 5.5 and 6 (Annex A), were published for public consultation.4 This was to receive input from stakeholders on the parts of the opinion that have been used to inform the draft protocol, and on the methodology foreseen to inform the parts covered by the protocol. The draft opinion and draft protocol were revised in the light of the comments received. A technical report which addresses the comments received during the consultation has been published (EFSA, 2017b).
Following the public consultation, the protocol was implemented and the opinion was completed. In April 2019, the draft Scientific Opinion was published for public consultation to collect comments on the new sections that had been integrated (Sections 5.5 and 6, conclusions and recommendation for research).5 The opinion was then finalised considering the comments received, where appropriate. A technical report which addresses the comments received during the consultation has been published (EFSA, 2019).
Assessment
1. Introduction
In 1993, the SCF adopted an opinion on the nutrient and energy intakes for the European Community. For sodium, an acceptable range of intakes (0.575–3.5 g/day, corresponding to 25‐150 mmol/day) was set for adults. For children, pregnant and lactating women, no value was set.
2. Definition/category
2.1. Chemistry
Sodium (Na+) is an alkali metal with an atomic mass of 22.99 Da (Lide, 2009; Wieser et al., 2013). Only one sodium isotope (23Na) is stable in nature. At normal temperature and pressure, sodium is a solid metal; it is highly reactive in both water and air and is not found naturally in its elemental form. In the Earth's crust, there is an abundance of sodium salts such as those with carbonate, nitrate, sulfate, borate, and particularly with halogens, especially chloride (Greenwood and Earnshaw, 1997; Lide, 2009).
Sodium chloride (NaCl) is the main constituent of table salt. One gram of sodium chloride provides 0.4 g of sodium and 0.6 g of chloride (17 mmol sodium and chloride).
2.2. Function of the nutrient
2.2.1. Biochemical functions
Sodium exists as the electrolyte Na+ in body fluids; it is the dominant cation in the extracellular fluid (ECF). Chloride (Cl–) is its accompanying extracellular anion and together they contribute the major component of the extracellular osmolality of 275–295 mOsm/kg of water. The principal elements of the corresponding intracellular osmotic activity are contributed by potassium (K+), chloride and low molecular organic metabolites. The ECF sodium content approximates to 135–145 mmol/L and that of potassium is 3.5–5.5 mmol/L, whereas within cells sodium and potassium contents approximate 15 mmol/L and 150 mmol/L, respectively (Heer et al., 2000; Gropper et al., 2009; Bailey et al., 2014). The homeostasis of water and sodium, and to an extent that of chloride and potassium, are interdependent and integrated to maintain these conditions (Sterns, 2015). The functions of sodium lie in: (i) its participation in the control of the volume and systemic distribution of total body water; (ii) enabling the cellular uptake of solutes; and (iii) the generation via interactions with potassium of transmembrane electrochemical potentials.
The systemic control of ECF volume, and the maintenance of osmotic equilibrium between the ECF and the intracellular fluid depends on the mechanisms and transport systems that control the entry of Na+ into cells and the energy‐dependent extrusion of Na+ out of cells. This function is seen in epithelial polarised cells (e.g. the intestinal and renal tubular epithelia). Such cells have an energy‐dependent sodium pump (Na+/K+‐ATPase) on their basolateral membrane that exchanges three intracellular molecules of Na+ for two extracellular K+ molecules entering the cells. This creates a gradient between intracellular and extracellular sodium ionic concentrations and activities, and the passive flow of Na+ down this gradient is enabled and regulated by specific cellular membrane pores and carriers that couple the flow of Na+ to the entry of water and of solutes (e.g. amino acids, monosaccharides) into the cells (Section 2.3.1). The link between sodium transport and co‐transport systems underpins cellular uptake and transport of water and solutes in all organs; the energy expended in these processes represents around 25% of the metabolic rate of a human at rest (EFSA, 2005a).
The differential transmembrane distribution and activity gradients of sodium and potassium induced by carrier proteins and Na+/K+‐ATPase create a polarised cell membrane potential. This is present in most cells but is particularly evident in muscle (of all types) and in neurones. In these cells, the membranes have ion channels that, in response to stimuli, open to allow the ions to flow across and depolarise the membrane. So, Na+ and K+ in tandem are fundamental for electrical signalling in the nervous system, muscle and heart; the sodium channels involved are classified as voltage gated channels (Campbell and Reece, 2002).
2.2.2. Health consequences of deficiency and excess
The health consequences of both chronic and acute deficiencies and excesses of sodium are related to the distribution of total body water and sodium in the extracellular and intracellular fluid compartments. This is more obvious in acute deficiency and excess (Sections 2.2.2.1 and 2.2.2.2) than it is for long‐term excessive exposure to sodium accompanied by its systemic accumulation in bone, connective tissue, muscle and skin (Titze et al., 2014) (Section 2.3.3).
The features of acute sodium excess and deficiency are predominantly neurological and arise from exceeding the homeostatic systems controlling hydration of the central nervous system. Sodium is generally enabled to pass easily across vascular endothelia and, as a result, the sodium concentrations (i.e. activities) of plasma and ECF, including interstitial fluid, are virtually identical. However, in the central nervous system, the capillaries have tight endothelial junctions that block sodium movements. So, changes in plasma and ECF sodium concentrations can create osmotic gradients causing water to move in or out of the cerebral spinal fluid (Sterns, 2015). This phenomenon affects other tissues and organs. However, organs such as the liver or the kidney can swell, or shrink, to accommodate changes in volume induced by redistribution of water within them, whereas the brain, being encased within the skull, is more susceptible to extreme changes in volume. This is probably why the brain has sensors of plasma osmolality and blood pressure to exercise control of body water, and of intracellular and extracellular tonicity (Section 2.3.4).
2.2.2.1. Deficiency
Dietary sodium deficiency is rare in healthy European populations. Sodium chloride and other sodium salts are ubiquitous in the diet (Sections 3.1 and 3.2), and there are adaptive physiological mechanisms that reduce the losses of sodium in urine, faeces and sweat at low levels of sodium intake (Section 2.3.4).
Hyponatraemia, defined as a serum sodium concentration less than 135 mmol/L (Sterns, 2015), is indicative of systemic sodium imbalance but not necessarily of systemic sodium deficiency (Andersson et al., 1982; Speedy et al., 2000). The threshold of 135 mmol/L is the lower point of the reference range and, as such, is indicating potential sodium depletion and deficiency. However, because of the systemic interaction of sodium with water balance, the serum sodium concentrations at which symptoms of sodium deficiency become apparent are not well characterised. Severe neurological symptoms are generally associated with serum sodium concentrations < 120 mmol/L, at which level cerebral oedema develops. Overall, the symptoms progress from malaise, nausea, vomiting and headache to lethargy, impaired consciousness, seizures and coma (Adrogué and Madias, 2000a; Sterns, 2015).
2.2.2.2. Excess
Rapid onset of sodium excess secondary to dietary sodium intake is also uncommon, but acute toxicity may arise from high exposures to sodium, usually as sodium chloride, from ingestion (e.g. self‐poisoning) or from parenteral administration in clinical care. Hypernatraemia, defined as a serum sodium concentration > 145 mmol/L, is typically a consequence of dehydration rather than of excessive sodium intake. The symptoms of hypernatraemia are similar to those of hyponatraemia and also include non‐specific features such as headache, confusion, fever, nausea and vomiting (Adrogué and Madias, 2000b; Sterns, 2015).
In 2005, the NDA Panel explored the relationship between high exposures to sodium (usually as sodium chloride) and hypertension (EFSA, 2005a, 2005b). For groups of individuals, there was strong evidence for an exposure‐dependent rise in blood pressure. The Panel noted that this is a continuous relationship that embraces the whole range of habitual sodium intakes and considered that it was not possible to determine a threshold of habitual sodium consumption below which adverse effects on blood pressure were unlikely. The Panel also noted that epidemiological studies indicated a positive association between sodium intake and risk of morbidity and mortality from cardiovascular diseases. Evidence for a direct adverse effect of high sodium intake on heart function (i.e. independent of raised blood pressure) was inconclusive. The Panel concluded that sodium itself was not carcinogenic but that high intakes of sodium chloride could increase susceptibility to carcinogens such as nitrosamines, gastric infection with Helicobacter pylori, or give inadequate protection against free radical‐induced damage. Overall, the NDA Panel did not set a tolerable upper intake level (UL) for sodium because of insufficient data. However, the Panel noted that there was strong evidence for the contribution of sodium to high blood pressure in European populations, and that high blood pressure has been directly related to the development of cardiovascular and renal diseases.
2.3. Physiology and metabolism
As sodium is integral for the homeostasis of total body water, its absorption, distribution and excretion are controlled by systems that monitor and regulate ECF osmolality (tonicity) and volume.
2.3.1. Intestinal absorption and secretion
Sodium is absorbed throughout most of the length of the small and large intestine, with the quantity and mechanism of absorption varying with intestinal sites (Chang and Leung, 2014). In the small intestine, epithelial uptake of sodium is facilitated by specific co‐transporters on the apex of the enterocytes. These co‐transporters couple the flow of sodium into the enterocytes to facilitate the uptake of low molecular solutes and micronutrients. The flow of sodium is generated by an electrolyte concentration gradient that is created by the extrusion of sodium by Na+/K+‐ATPase on the basolateral membranes of the cells (Section 2.2.1). This transfers the solutes out of the gut lumen into the enterocytes on the gut villi and into the portal plasma. The vascular structure creates a countercurrent system dependent on the osmotic activity that results from the localised accumulation of solutes and sodium in the villi. This increased osmotic activity, in turn, draws water into and across the epithelium by paracellular pathways, and the flow drags other luminal solutes across the epithelium (solute drag). Sodium is recycled into the small intestinal lumen via the gastric, intestinal, pancreatic and hepatic secretions that accompany digestion and absorption.
The small intestine is estimated to handle 8–10 L of water in the course of a day. This comprises water from endogenous secretions and from the diet (1–1.5 L/day). More than 98% of the fluid load is absorbed in the gut. About 1–2 L daily enter in the distal ileum and colon, which are the regions where net absorption of sodium and water occurs. These distal processes also include adjustments involved with the homeostasis of potassium, chloride and bicarbonate, as well as the uptake and transfer of intraluminal fermentation products from the colon (Sandle, 1998; Kato and Romero, 2011).
In the distal bowel, other carriers are responsible for the uptake of sodium. These include sodium exchange for hydrogen ions (Na+/H+ exchangers) (Pan et al., 2012) and/or absorption of anions, chloride and bicarbonate, to maintain electroneutrality (Fordtran et al., 1968; Turnberg et al., 1970; Chang and Leung, 2014). The sodium secretion that accompanies active chloride secretion is a passive process, driven by the transepithelial potential difference resulting from chloride secretion (Kato and Romero, 2011; Chang and Leung, 2014). In the rectum, active sodium absorption occurs against large electrochemical gradients through electrogenic sodium channels (Levitan et al., 1962; Sandle, 1998; Chang and Leung, 2014) (Table 1).
Table 1.
Regulation of sodium excretion in the kidney and distal bowel
| Site | Na reabsorbed (%) | Mechanism | Water transfer | Regulating factors |
|---|---|---|---|---|
| Proximal renal tubule | 60–70 |
Co‐transporters with solutes. Active Na+/K+ ATP‐ase dependent Some Na/H+ |
Principal site for water reabsorption Highly permeable driven by osmolality |
Angiotensin II SNS catecholamines |
| Loop of Henle | 20–30 | Na+/K+/2Cl– co‐transport | Impermeable to water |
Flow dependent Pressure Natriuresis |
| Distal tubule | 5–10 | Na+‐Cl– co‐transport | Impermeable to water |
Aldosterone Flow dependent |
| Collecting ducts | 5 | Na+ exchange K+ channels | ADH‐mediated permeability via aquaporins |
Aldosterone Atrial natriuretic peptide |
| Distal bowel | Na+ channels | Aquaporins | Aldosterone |
ADH: antidiuretic hormone; Cl: chloride; K: potassium; Na: sodium; SNS: sympathetic nervous system.
Four 7‐day balance studies spaced seasonally over a year in healthy young adults consuming self‐selected diets, with a mean daily intake of 3.4 g (148 mmol) of sodium, indicated that approximately 98.5% of ingested sodium is absorbed (Holbrook et al., 1984) (Section 5.2.1). Shorter balance studies (5–12 days following 2–4 days of adaptation) in Japanese adults with a wider range of intakes showed that the absolute amount of sodium absorbed increases linearly with increasing sodium intake. Mean sodium absorption was 97.8 ± 1.9% for daily intakes of 39–142 mg/kg body weight (bw) or 2.2–6.8 g (95–295 mmol)/day of sodium (Kodama et al., 2005). Mean 24‐h urinary sodium excretion was 33.2 ± 0.8 g (1,443 ± 35) after 72 h in 14 healthy men consuming 34.5 g (1,500 mmol) of sodium/day, indicating that sodium absorption is maintained at approximately 96% even at very high intake (Luft et al., 1979).
2.3.2. Transport in blood
Following absorption, sodium ions are distributed by portal and systemic circulations, where their concentrations are maintained within a narrow range by the mechanisms described below (Section 2.3.4). In healthy adults, serum sodium concentrations are between approximately 135 and 145 mmol/L (Heer et al., 2000; Gropper et al., 2009; Bailey et al., 2014). Reference ranges vary slightly among different laboratories depending on the measurement technique used (Morimatsu et al., 2003).
2.3.3. Distribution to tissues
Typical total body content of sodium is 1.3–1.5 g (55–65 mmol)/kg bw, equivalent to a total of 85–96 g (3,700–4,200 mmol) for a 70‐kg man (Penney, 2008; James and Reid et al., 2011), 95% of which is in the ECF. A large proportion of body sodium is in bone, skin and muscle (Bie, 2018). Within cells (e.g. myocytes), sodium is present at a lower concentration, approximately 3 mmol/L (Bailey et al., 2014), with some variation depending on cell types (Yunos et al., 2010). These pools of sodium have different turnover times, with the most exchangeable pools being ECF and intracellular sodium, and the pool of sodium bound to connective tissue is slower (Titze et al., 2014; Rakova et al., 2017; Bie, 2018).
The pool of sodium in bone, muscle and skin has been proposed to be a sodium depot or reserve, but it could have other roles. Sodium bound to proteoglycans in connective tissue creates a high osmotic force that supports the hydration of these tissues and enables them to withstand high‐pressures. This sodium pool could also have a homeostatic and adaptive role as an extra‐renal clearance depository for handling excessive systemic accumulation of sodium (Titze et al., 2014; Rakova et al., 2017; Selvarajah et al., 2017; Bie, 2018) which is discussed below.
The deposition of sodium in bone, muscle and skin could explain why an increase in sodium intake is not necessarily associated with a change in body weight, an increase in ECF volume, nor with an increase in plasma sodium concentration or its renal loss (Heer et al., 2000; Titze et al., 2014; Sterns, 2015; Rakova et al., 2017). A study by Heer et al. (2000) found that, compared with a sodium intake of 1.2 g (50 mmol)/day, a daily sodium intake of 12.7 g (550 mmol) was associated with an increase in plasma volume of approximately 300 mL, but with no change in body weight. This implies that there was a redistribution of water from the interstitial ECF to plasma. It is proposed that total body sodium ‘fluctuates’ independently of sodium intake (Titze et al., 2014). Long‐term balance studies in men with constant and controlled daily sodium intakes of 2.5 g (110 mmol), 3.6 g (155 mmol) and 4.8 g (210 mmol) demonstrated weekly and half‐weekly cycles of urinary sodium excretion that were inversely related to urine content of aldosterone and its metabolites (as marker of aldosterone production) and directly related to glucocorticoid/cortisol production (Rakova et al., 2013). This pattern of sodium excretion was accompanied by a monthly cycle of changes in total body sodium content of ± 4.6–9.2 g (200–400 mmol), without parallel changes in total body water content. These studies indicated that osmolyte and water balance are regulated by a rhythmical release of mineralocorticoids and glucocorticoids, the accrual of endogenous water (i.e. water released as a product of systemic metabolism) to adjust ECF osmolality, and by weekly and monthly cyclical excretions of sodium (Titze et al., 2014; Rakova et al., 2017).
There is evidence that sodium accumulates in the connective tissue of bone, muscle and skin with age. In a cross‐sectional study, 23Na magnetic resonance of the midcalf showed higher sodium concentrations in the tissues of 57 hypertensive subjects as compared with the 56 normotensive controls. In men (with or without hypertension), but not in women, muscle content of Na increased with age, with no associated increase in muscle water content, whereas water content in the skin increased with that of sodium. Generally, these compositional age‐related changes were larger in men than in women (Kopp et al., 2013; Titze, 2015) and have been associated with increased vascular stiffness (Safar et al., 2009; Olde Engberink et al., 2015).
Collectively, the above observations contribute to the biological mechanistic plausibility of the positive association of sodium intakes/exposure with systemic blood pressure. Also, cyclic hormonal regulatory processes may contribute to the inherent within‐person variability in 24‐h urine sodium excretion, beyond daily variations in intake.
2.3.4. Elimination
The excretion and retention (i.e. homeostasis) of sodium and water are effected by an integrated neurohormonal control from centres located in the hypothalamus (Lowell, 2019). Plasma osmolality and volume are sensed by four interdependent sensor systems. These comprise a group that detects plasma osmolality, and a system of pressure‐sensitive receptors (baroreceptors).
ECF osmolality is sensed by the circumventricular organs (CVOs), which are highly vascularised areas in the hypothalamus. Neuronal osmoreceptors in CVOs have direct exposure to the ECF through gaps in the blood–brain barrier. The osmoreceptors shrink when exposed to increased ECF osmolality, e.g. as would result from a decrease in total body water. This shrinkage is relayed to the hypothalamic supraoptic nucleus, which stimulates thirst and the release of antidiuretic hormone (ADH) by the pituitary gland. ADH enables water retention by increasing the number of water permeable channels (aquaporins) in the luminal cell membranes of the renal collecting ducts. ADH has a half‐life of 20 min and it is continuously secreted to maintain the physiological range of osmolality. So, the supraoptic nucleus reacts to increased osmolality by increasing water intake and by reducing renal water excretion (Sterns, 2015). When serum sodium concentration falls below 135 mmol/L, ADH secretion and thirst are inhibited.
Plasma volume is sensed by two types of pressure‐sensitive receptors (or baroreceptors) that respond to either high or low vascular pressure. ‘High‐pressure’ baroreceptors are located in the cerebral and carotid arteries, aortic arch and the juxta glomerular apparatus of renal glomeruli, whereas ‘low‐pressure’ baroreceptors lie in the thoracic veins, the cardiac atria and right ventricle, and the pulmonary (thoracic) veins.
Collectively, these sensors regulate the neurohumoral control of total body water and ECF. The osmosensors operate by mediating changes in water intake and renal distal tubule retention or loss of water through aquapores. The baroreceptors induce changes in renal retention or loss of sodium to mediate water homeostasis. The mediators include the sympathetic nervous system (SNS) and catecholamines; atrial natriuretic peptide; and the renin–angiotensin–aldosterone system (RAAS).
The renin–angiotensin–aldosterone pathway is active in several different tissues, but most notably the kidney (Penney, 2008; Hamlyn, 2014; Lowell, 2019). Renin is released from the juxtaglomerular apparatus in response to reduced pressure in the afferent renal arterioles, increased sympathetic nerve activity and decreased sodium and chloride concentrations in the distal tubular fluid. Renin hydrolyses angiotensinogen into angiotensin I that is converted by the angiotensin‐converting enzyme into angiotensin II. Angiotensin II stimulates: (i) sodium, and therefore water, reabsorption in the proximal tubule; (ii) constriction of the arterioles; and (iii) the release of aldosterone from the adrenal cortex. Aldosterone stimulates renal recovery of sodium in exchange for excreted potassium in the epithelia of the distal tubule and collecting ducts, and in the distal colon. Aldosterone also facilitates the simultaneous reabsorption of water with that of sodium by stimulating the release of ADH (which increases the number of aquaporins in the distal tubules) and stimulates sodium intake via neuronal control of salt appetite (Lowell, 2019).
As with sodium kinetics (Section 2.3.3), the regulation of electrolyte and water homeostasis exhibits a circadian rhythm affecting blood pressure, glomerular perfusion and filtration rate, which results in a variable rate of urinary excretion of sodium and potassium. This biological clock is mediated by regulation of the expression of genes responsible for the neuroendocrine control of the renal handling of sodium (Gumz et al., 2015; Solocinski and Gumz, 2015).
2.3.4.1. Urine
The kidney is the main organ mediating the excretion and retention of sodium and, so, water homeostasis, as outlined above. It efficiently excretes sodium in response to high dietary intakes, and salvages sodium when dietary intake is low (Section 5.2).
In experiments in which subjects at a steady state were shifted to a lower level of sodium intake, the half‐life for the reduction in renal sodium excretion was about 24 h (Strauss et al., 1958; Epstein and Hollenberg, 1976) and, consequently, a steady state between sodium intake and urinary sodium excretion is considered to be achieved within a few days (Cogswell et al., 2013).
A meta‐analysis investigating urinary sodium excretion relative to sodium intake included 35 studies in which a constant quantity of dietary sodium was provided to participants for a minimum of 3 days (Lucko et al., 2018). This was considered to be the minimum duration to ensure that participants were at a steady state of urine sodium excretion relative to sodium intake. In a subgroup analysis, the length of this stabilisation period (categorised as a minimum 3, 5 or 7 days) was not found to alter the percentage of dietary sodium excreted. On average, 92.8% of daily dietary sodium was excreted in 24‐h urine (95% CI 90.7, 95.0; I2 95.1%, p < 0.001). The average excretion of ingested sodium varied from 76% to 122% across studies. The pooled estimate was similar when the analysis was restricted to studies conducted in healthy people (93.7%, 95% CI 90.5, 96.8; I2 97.1%, p < 0.001). Comparable excretion rates have been reported in studies in which people consumed their usual diet: 86% of the sodium ingested over 4 weeks (1 week per season over a year, to cover variability) was excreted in 24‐h urine samples collected during the same period (Holbrook et al., 1984) and 98% of dietary sodium was excreted in 24‐h urine in another 3‐day study (Schachter et al., 1980).
The nephron, under the neurohormonal control systems described above, is the key organ regulating sodium excretion and maintaining normal ECF and plasma volumes. Glomerular filtration allows a filtrate free of cells and macromolecules (the electrolyte content of which resembles that of plasma) to pass into the proximal tubules. The glomerular filtration rate (measured as creatinine clearance, with a usual value of around 125 mL/min) decreases with age.
The kidney has the capacity to filter large amounts of sodium, more than 99% of which is then reabsorbed in the renal tubules via co‐transporters driven by the basolateral Na+/K+‐ATPase. Approximately 60–70% of sodium reabsorption occurs via co‐transporters in the proximal tubule, along with organic molecules (amino acids, glucose and organic acids), mediated by membrane Na+/H+ exchange (Greger, 2000). Water follows this movement of solutes. In the ascending limb of the loop of Henle, 20–30% of the filtered sodium chloride is absorbed via the Na+/2Cl–/K+ co‐transport system. However, this loop is impermeable to water and the filtrate becomes less concentrated. In the early distal tubule, another 6% of the filtered sodium is recovered, and this further dilutes the fluid. About 5–10% of sodium reabsorption occurs in the distal tubule, through active sodium transport via the Na+‐Cl– co‐transporter (Greger, 2000). In the distal tubule of the nephron, aldosterone enhances sodium reabsorption and potassium excretion.
2.3.4.2. Faeces
The efficiency of distal intestinal absorption of intraluminal sodium is responsive to aldosterone (Sandle, 1998) and has been described in Section 2.3.1.
Balance studies have shown that sodium losses in the faeces were relatively stable and limited to a few mmol/day for sodium intakes over a range between 1.2 and 12.7 g/day (50 and 550 mmol/day) (Holbrook et al., 1984; Heer et al., 2000; Palacios et al., 2004). For example, in the balance study by Holbrook et al. (1984) in which sodium losses were estimated for four periods of 7 days in 28 US men and women consuming their usual diet (Section 5.2), mean (± SD) sodium losses in faeces were 56 ± 26 mg/day (2.4 ± 1.1 mmol/day), representing less than 2% of sodium intake.
2.3.4.3. Dermal losses
Sodium concentration in sweat varies widely. Values between 10 mmol/L (0.23 g/L) and 180 mmol/L (4.20 g/L) have been reported in adults (IOM, 2005; Bates and Miller, 2008; Kaptein et al., 2016). Influencing factors include levels of sodium intake, sweat rate, hydration status and degree of heat acclimation (Allan and Wilson, 1971; Allsopp et al., 1998; Bates and Miller, 2008). Interindividual variability may also be influenced by physiological determinants of sodium reabsorption in the sweat gland (Brown et al., 2011). Sodium concentration in sweat decreases following heat acclimation (Consolazio et al., 1963; Allsopp et al., 1998; Buono et al., 2007; Bates and Miller, 2008), contributing to the maintenance of sodium balance under conditions of high sweat excretion (Consolazio et al., 1963; Allsopp et al., 1998) (Section 5.2).
Studies conducted under conditions of moderate temperature and exercise levels indicate small sodium losses via sweat across a wide range of sodium intake levels (Barr et al., 1991; Heer et al., 2000; Palacios et al., 2004). In the balance study by Palacios et al. (2004) in 36 female adolescents under sedentary conditions (see Section 5.2), sweat losses represented ca. 3% of total sodium losses under the ‘high’ sodium diet (4 g (174 mmol)/day) and 10% under the ‘low’ sodium diet (1.3 g (56 mmol)/day). Sodium losses via sweat can be considerably higher in situations of exercise and heat (Sharp, 2006; Bates and Miller, 2008; Cogswell et al., 2015).
2.3.4.4. Breast milk
Colostrum has higher concentrations of sodium than mature milk (Koo and Gupta, 1982; Atkinson et al., 1995). The sodium content of breast milk decreased rapidly in the first days post‐partum, as the mammary gland undergoes the transition between pregnancy and lactation (i.e. closure of the intercellular junctions) (Atkinson et al., 1995). This is followed by a gradual decline in the sodium concentration of mature milk.
The concentration of electrolytes, including sodium, in human milk is lower than in plasma. It is determined by an electrical potential gradient in the mammary epithelial cells regulated through membrane transport pathways (Wack et al., 1997; Truchet and Honvo‐Houeto, 2017). It is not influenced by maternal sodium intake (Filippi et al., 1981; Keenan et al., 1982; Ereman et al., 1987). Diurnal variations in breast milk sodium concentration, reciprocal to potassium concentration, have been reported (Keenan et al., 1982, 1983). Factors that have been associated with increased sodium concentration in breast milk include pathological processes such as mastitis or localised inflammation of breast tissue (Morton, 1994), premature birth (Gross et al., 1980) and manual compared with mechanical (pump) expression (Lang et al., 1994).
Based on 11 studies on sodium concentration in breast milk from 511 women in the USA, the UK and Canada, Atkinson et al. (1995) reported mean sodium concentrations across studies between 17.1 and 22.3 mmol/L (393 and 513 mg/L) at day 3 (colostrum), 9.4 and 13.1 mmol/L (216 and 301 mg/L) at day 14 (transitional milk), 5.9 and 17.1 mmol/L (136 and 393 mg/L), 4.7 and 8.0 mmol/L (108 and 184 mg/L), and 3.6 and 6.0 mmol/L (83 and 138 mg/L) at days 30, 90 and 180 of lactation (mature milk), respectively.
Appendix A reports data on sodium concentration in breast milk from additional studies that involved mothers of term infants in Western populations. Mean sodium concentrations are between 3.0 and 10.6 mmol/L (70 and 244 mg/L) from eight studies that analysed mature breast milk (Keenan et al., 1982; Koo and Gupta, 1982; Parr et al., 1991; Holt, 1993; Motil et al., 1997; Wack et al., 1997; Fly et al., 1998; Bjorklund et al., 2012) and 11.2 mmol/L (257 mg/L) in one study that used mixed samples (collected between 1 and 8 weeks post‐partum) (Bauer and Gerss, 2011).
Based on the data presented in Appendix A, the Panel considers an approximate midpoint of sodium concentration in mature breast milk of women from Western countries as 150 mg/L (6.5 mmol)/L. Based on a mean milk transfer of 0.8 L/day (Butte et al., 2002; FAO/WHO/UNU, 2004; EFSA NDA Panel, 2009) during the first 6 months of lactation in exclusively breastfeeding women, the Panel estimates the maternal loss of sodium through breast milk to be 120 mg (5.2 mmol)/day.
2.4. Modification of sodium metabolism during pregnancy
During pregnancy, there is an expansion of the ECF, including the plasma volume, starting within 2 weeks of conception. Expansion of the plasma volume is between 1 and 1.6 L. These changes occur irrespective of the mother's size, are usually larger in multigravida, and are accompanied by a fall in both plasma osmolality and plasma sodium concentrations (Davidson and Repke et al., 1998). The expansion of the ECF represents a change in the homeostasis of total body water that is accompanied by increased cardiac output, increased vascular perfusion of organs and tissues, and reduced SBP in the first half of pregnancy. As a consequence, the volume of the kidneys increases by around 30%. The renal blood flow almost doubles, the glomerular filtration rate is increased by 50%, and these changes are accompanied by an increased tubular reabsorption of sodium. Simultaneously, there is an increased renal clearance of low‐molecular‐weight solutes such as proteins, amino acids and glucose. Creatinine clearance is increased by 25% in the fourth week of gestation and by 45% in the ninth week (Cheung and Lafayette, 2013).
Progesterone has a major influence on these changes. This hormone induces smooth muscle relaxation and vasodilation and it reduces the response of the distal tubules to aldosterone, even though aldosterone production is also increased early in pregnancy. However, there are other adaptations, which have been poorly characterised, namely the production of hormones involved in the regulation of body water and a reduced responsiveness of receptors, particularly the RAAS, to these hormones (Brown, 1989; Wintour, 1998; Cheung and Lafayette, 2013). The ECF changes disappear by 1 month after delivery but the reversal of the renal adaptations may take up to 6 months post‐partum.
2.5. Interaction with other nutrients
2.5.1. Potassium
The metabolism of potassium and sodium are strongly interrelated, in part due to Na+/K+‐ATPase exchange mechanisms (Adrogué and Madias, 2014) (Section 2.2.1). Additionally, and importantly, the efficiency of sodium homeostasis, particularly its renal regulation, is related to that of potassium.
In its previous assessment of DRVs for potassium, the Panel concluded that dietary potassium intake modulates the influence of sodium on blood pressure (EFSA NDA Panel, 2016). There is also evidence that the effect of potassium intake on blood pressure may be higher in individuals with high sodium chloride intake compared with those with low sodium chloride intake. In a meta‐analysis of randomised controlled trials (RCTs) on the effect of potassium intake on blood pressure, Aburto et al. (2013) conducted subgroup analyses according to levels of sodium intake, as assessed through baseline urinary sodium excretion. The largest blood pressure‐lowering effect of potassium was associated with the highest category of sodium intake (greater than 4 g (174 mmol)/day) compared with the lower categories (< 2 g (87 mmol)/day and 2–4 g (87–174 mmol)/day).
In its opinion on DRVs for potassium (EFSA NDA Panel, 2016), the Panel considered whether the sodium‐to‐potassium intake ratio could influence blood pressure outcomes more than either potassium or sodium intakes alone. According to the systematic review by Perez and Chang (2014), evidence from RCTs carried out in hypertensive subjects suggests that the sodium‐to‐potassium excretion ratio, on a molar basis, is more strongly associated with blood pressure outcomes than either sodium or potassium alone. Only four RCTs were conducted in normotensive subjects. The Panel notes, however, that none of the RCTs included in the review was designed to assess the effect of a change in the sodium‐to‐potassium ratio vs a change in either nutrient alone on blood pressure outcomes. This systematic review also included one prospective cohort study that reported that the sodium‐to‐potassium ratio, on a weight basis (assessed through 3‐day weighed records), was more strongly associated with hypertension and/or systolic and diastolic blood pressure levels than either sodium or potassium alone (Du et al., 2014). The Panel notes that additional prospective cohort studies have investigated the association between the sodium‐to‐potassium intake or excretion ratio, assessed through variable methods (dietary questionnaire, spot or 24‐h urine excretion), and blood pressure and CVD outcomes with inconsistent results (Chien et al., 2008; Kieneker et al., 2014; Okayama et al., 2016; Tabara et al., 2017; O'Donnell et al., 2019).
A possible moderating effect of potassium intake on the relationship between sodium and blood pressure was explored in the meta‐analyses of RCTs conducted for the present opinion (Section 5.5.1.2 and Appendix I). Stratified analysis by levels of potassium intake/excretion are presented in Tables I.1 and I.2. When building the meta‐regression models, potassium intake was not retained as it did not explain a significant proportion of the heterogeneity. Such analyses were, in addition, limited by the number of studies for which information on potassium intake/excretion was missing (13 out of 35 studies).
Table I.1.
Pooled estimates of the effect of sodium reduction on SBP (mean difference expressed in mm Hg)
| N studies | N particip. | Mean diff. | 95% CI | I2 | p | |||
|---|---|---|---|---|---|---|---|---|
| All | Adults | 35 | 3,407 | −3.9 | −5.1 | −2.8 | 62% | < 0.001 |
| BP | Hypertensive | 17 | 721 | −5.6 | −8.1 | −3.1 | 58% | 0.001 |
| Mixed | 10 | 770 | −3.5 | −5.4 | −1.7 | 67% | 0.001 | |
| Normotensive | 8 | 1,916 | −2.0 | −3.3 | −0.7 | 26% | 0.218 | |
| Age | Adults < 50 years | 16 | 632 | −2.2 | −3.3 | −1.1 | 49% | 0.014 |
| Adults ≥ 50 years | 19 | 2,775 | −6.1 | −8.2 | −4.1 | 50% | 0.007 | |
| Sex | > 55% men | 14 | 2,430 | −4.5 | −6.5 | −2.6 | 74% | < 0.001 |
| 45−55% both genders | 14 | 761 | −4.7 | −7.0 | −2.5 | 55% | 0.007 | |
| > 55% women | 7 | 216 | −1.8 | −3.6 | 0.0 | 20% | 0.277 | |
| BMI | < 25 | 1 | 66 | −5.0 | −10.6 | 0.6 | – | – |
| 25−29 | 10 | 1,132 | −4.6 | −6.3 | −2.8 | 26% | 0.207 | |
| ≥ 30 | 2 | 384 | −5.3 | −8.0 | −2.7 | 52% | 0.151 | |
| NR | 22 | 1,825 | −3.1 | −4.7 | −1.6 | 62% | < 0.001 | |
| Ethnicity | Caucasian | 3 | 65 | −7.6 | −12.9 | −2.3 | 0% | 0.991 |
| African (including AA) | 1 | 40 | −8.0 | −13.7 | −2.3 | – | – | |
| Mixed | 12 | 2,540 | −5.7 | −8.0 | −3.4 | 82% | < 0.001 | |
| NR | 19 | 762 | −1.7 | −2.5 | −0.8 | 0% | 0.578 | |
| Potassium | ≤ 60 mmol/day | 4 | 103 | −1.0 | −2.1 | 0.1 | 9% | 0.346 |
| > 60−≤ 70 mmol/day | 8 | 2,008 | −3.0 | −4.6 | −1.4 | 52% | 0.043 | |
| > 70−≤ 80 mmol/day | 7 | 442 | −4.3 | −6.2 | −2.5 | 0% | 0.743 | |
| > 80 mmol/day | 3 | 174 | −3.8 | −8.7 | 1.2 | 41% | 0.183 | |
| NR | 13 | 680 | −6.4 | −9.5 | −3.3 | 59% | 0.004 | |
| Design | Parallel | 8 | 2,217 | −2.0 | −3.1 | −1.0 | 20% | 0.272 |
| Crossover | 26 | 1,134 | −5.0 | −6.8 | −3.3 | 67% | < 0.001 | |
| Cluster‐randomised | 1 | 56 | −2.2 | −8.4 | 4.0 | – | – | |
| Specific design | Run‐in – normal diet | 7 | 380 | −5.2 | −9.6 | −0.9 | 75% | 0.001 |
| Run‐in – low Na diet | 9 | 386 | −5.8 | −8.6 | −2.9 | 43% | 0.083 | |
| Run‐in – high Na diet | 4 | 504 | −5.3 | −7.0 | −3.6 | 0% | 0.552 | |
| No run‐in | 15 | 2,137 | −1.6 | −2.5 | −0.8 | 18% | 0.254 | |
| Trial duration | 1 month | 20 | 1,005 | −5.1 | −7.0 | −3.1 | 72% | < 0.001 |
| 2–3 months | 12 | 618 | −4.0 | −5.5 | −2.5 | 0% | 0.755 | |
| ≥ 1 year | 3 | 1784 | −1.6 | −2.8 | −0.4 | 40% | 0.191 | |
| Intervention type | Feeding | 28 | 1,309 | −4.0 | −5.3 | −2.7 | 44% | 0.007 |
| Counselling | 7 | 2,098 | −3.7 | −6.2 | −1.1 | 85% | < 0.001 | |
| Position | Supine | 17 | 517 | −5.6 | −7.5 | −3.6 | 0% | 0.762 |
| Seated | 16 | 2,826 | −2.5 | −3.6 | −1.5 | 57% | 0.003 | |
| NR | 2 | 64 | −14.7 | −20.6 | −8.8 | 13% | 0.284 | |
| UNa difference | ≤ 50 mmol | 6 | 2,018 | −2.0 | −3.0 | −0.9 | 23% | 0.26 |
| 51−75 mmol | 14 | 773 | −2.4 | −3.6 | −1.2 | 27% | 0.169 | |
| 76−100 mmol | 11 | 511 | −6.5 | −8.3 | −4.7 | 0% | 0.814 | |
| > 100 mmol | 4 | 105 | −8.6 | −19.0 | 1.7 | 85% | < 0.001 | |
| Tier | Tier 1 | 30 | 3,171 | −3.3 | −4.4 | −2.2 | 50% | 0.001 |
| Tier 2 | 5 | 236 | −6.9 | −12.8 | −1.1 | 75% | 0.003 | |
BP: blood pressure; 95% CI: 95% confidence interval; diff.: difference; N: number; Na: sodium; NR: not reported; particip.: participants; SBP: systolic blood pressure; UNa: sodium urinary excretion.
Table I.2.
Pooled estimates of the effect of sodium reduction on DBP (mean difference expressed in mm Hg)
| N studies | N particip. | Mean diff. | 95% CI | I2 | p | |||
|---|---|---|---|---|---|---|---|---|
| All | Adults | 35 | 3,407 | −2.0 | −2.8 | −1.2 | 61% | < 0.001 |
| BP | Hypertensive | 17 | 721 | −2.9 | −4.2 | −1.6 | 47% | 0.016 |
| Mixed | 10 | 770 | −1.7 | −3.3 | −0.2 | 75% | < 0.001 | |
| Normotensive | 8 | 1,916 | −0.9 | −1.6 | −0.2 | 9% | 0.365 | |
| Age | Adults < 50 years | 16 | 632 | −1.0 | −2.0 | 0.0 | 61% | 0.001 |
| Adults ≥ 50 years | 19 | 2,775 | −2.9 | −4.0 | −1.9 | 40% | 0.036 | |
| Sex | > 55% men | 14 | 2,430 | −2.7 | −3.9 | −1.5 | 70% | < 0.001 |
| 45−55% both genders | 14 | 761 | −1.9 | −3.3 | −0.5 | 53% | 0.01 | |
| > 55% women | 7 | 216 | −0.6 | −2.0 | 0.8 | 33% | 0.173 | |
| BMI | < 25 | 1 | 66 | −3.0 | −7.1 | 1.1 | – | – |
| 25−29 | 10 | 1,132 | −1.9 | −2.6 | −1.2 | 0% | 0.739 | |
| ≥ 30 | 2 | 384 | −2.8 | −4.0 | −1.6 | 16% | 0.276 | |
| NR | 22 | 1,825 | −1.7 | −2.8 | −0.5 | 69% | < 0.001 | |
| Ethnicity | Caucasian | 3 | 65 | −2.2 | −4.9 | 0.5 | 0% | 0.579 |
| African (including AA) | 1 | 40 | −3.0 | −6.5 | 0.5 | – | – | |
| Mixed | 12 | 2,540 | −3.0 | −4.2 | −1.7 | 74% | < 0.001 | |
| NR | 19 | 762 | −1.2 | −2.3 | 0.0 | 54% | 0.003 | |
| Potassium | ≤ 60 mmol/day | 4 | 103 | 0.4 | −1.1 | 1.9 | 51% | 0.105 |
| > 60−≤ 70 mmol/day | 8 | 2,008 | −2.2 | −3.4 | −0.9 | 66% | 0.005 | |
| > 70−≤ 80 mmol/day | 7 | 442 | −2.4 | −3.5 | −1.3 | 0% | 0.742 | |
| > 80 mmol/day | 3 | 174 | −2.5 | −4.8 | −0.2 | 0% | 0.573 | |
| NR | 13 | 680 | −2.9 | −4.5 | −1.2 | 51% | 0.017 | |
| Design | Parallel | 8 | 2,217 | −1.8 | −3.0 | −0.7 | 63% | 0.009 |
| Crossover | 26 | 1,134 | −2.0 | −3.1 | −0.9 | 61% | < 0.001 | |
| Cluster‐randomised | 1 | 56 | −3.9 | −7.6 | −0.2 | – | – | |
| Specific design | Run‐in – normal diet | 7 | 380 | −3.6 | −5.4 | −1.7 | 55% | 0.036 |
| Run‐in – low Na diet | 9 | 386 | −2.7 | −4.1 | −1.2 | 38% | 0.119 | |
| Run‐in – high Na diet | 4 | 504 | −2.7 | −3.7 | −1.7 | 0% | 0.57 | |
| No run‐in | 15 | 2137 | −0.5 | −1.3 | 0.3 | 30% | 0.131 | |
| Duration | 1 month | 20 | 1,005 | −2.1 | −3.4 | −0.8 | 67% | < 0.001 |
| 2–3 months | 12 | 618 | −2.8 | −3.7 | −1.9 | 0% | 0.501 | |
| ≥ 1 year | 3 | 1,784 | −1.1 | −2.3 | 0.1 | 66% | 0.052 | |
| Intervention type | Feeding | 28 | 1,309 | −1.7 | −2.6 | −0.9 | 46% | 0.004 |
| Counselling | 7 | 2,098 | −2.7 | −4.4 | −1.0 | 83% | < 0.001 | |
| Position | Supine | 17 | 517 | −2.7 | −3.8 | −1.5 | 0% | 0.66 |
| Seated | 16 | 2,826 | −1.4 | −2.3 | −0.5 | 70% | < 0.001 | |
| NR | 2 | 64 | −5.9 | −11.7 | −0.1 | 55% | 0.138 | |
| UNa difference | ≤ 50 mmol | 6 | 2,018 | −1.3 | −2.3 | −0.2 | 52% | 0.063 |
| 51−75 mmol | 14 | 773 | −1.5 | −2.8 | −0.2 | 64% | 0.001 | |
| 76−100 mmol | 11 | 511 | −2.9 | −3.9 | −1.9 | 0% | 0.869 | |
| > 100 mmol | 4 | 105 | −4.6 | −10.1 | 0.8 | 76% | 0.006 | |
| Tier | Tier 1 | 30 | 3,171 | −1.7 | −2.4 | −0.9 | 56% | < 0.001 |
| Tier 2 | 5 | 236 | −4.1 | −7.0 | −1.2 | 52% | 0.081 | |
BP: blood pressure; 95% CI: 95% confidence interval; diff.: difference; N: number; Na: sodium; NR: not reported; particip.: participants; DBP: diastolic blood pressure; UNa: sodium urinary excretion.
The Panel concludes that the interrelationship between sodium, potassium and blood pressure or CVD outcomes has not been sufficiently characterised to inform the DRVs for sodium.
2.5.2. Chloride
The interaction between sodium and chloride is biologically crucial in that they, with potassium, diffuse freely in aqueous medium. In biological systems the three ions are compartmentalised by lipid membranes in such a way that their individual physicochemical properties maintain osmotic balance, electroneutrality, and acid–base balance between intracellular compartments and the cytoplasm, and between the cytoplasm and ECF. Regulated changes in the transmembrane balance for these ions in particular are fundamental for the transport of solutes across membranes (e.g. in intestinal absorption), and the generation of electrical signals in the muscle, and in the peripheral and central nervous systems (Berend et al., 2012; Imbrici et al., 2015). So, the functions of sodium depend on the availability of chloride as a counter‐ion (Section 2.2.1).
Chloride is rate limiting for the transport of sodium and chloride in the thin ascending loop of Henle, because of the differences in the affinities of sodium and chloride for the co‐transporters. Therefore, the availability of chloride has a determinant effect on the release of renin (Kotchen et al., 1987). Although chloride has biological functions independent of sodium, any direct role, independent of sodium or potassium, in modulating blood pressure has not been established (McCallum et al., 2015; EFSA NDA Panel, 2019).
Data from studies on hypertensive rats, a limited number of clinical observations, and accumulating reports on putative mechanisms suggest that the full‐expression of sodium chloride‐dependent elevation in blood pressure relies on the concomitant presence of both sodium and chloride (Kurtz et al., 1987; Shore et al., 1988; Luft et al., 1990; Kotchen and Kotchen, 1997; McCallum et al., 2015). It is noteworthy that dietary sodium chloride causes a greater rise of mean blood pressure, in both normotensive and hypertensive subjects, than does sodium combined with other anions (e.g. citrate, phosphate, bicarbonate) (Shore et al., 1988; McCallum et al., 2015; EFSA NDA Panel, 2019).
The Panel notes that there is evidence that chloride can contribute to the effect of sodium chloride on blood pressure.
2.5.3. Calcium
Calcium and sodium share common transport mechanisms in the kidney; the reabsorption of calcium parallels the reabsorption of sodium at the renal tubular level (Yu, 2015; Moor and Bonny, 2016). There is consistent evidence that an increase in sodium intake increases urinary calcium excretion, while a reduction in sodium intake lowers urinary calcium excretion (Afssa, 2001; EFSA, 2005a; IOM, 2005).
In a cross‐sectional study of 484 post‐menopausal women, Nordin and Polley (1987) reported that urinary calcium excretion was positively and independently related to calculated 24‐h urinary sodium excretion. The correlation between urinary calcium and urinary sodium was stronger in those on lower dietary calcium intakes (less than 1,250 mg/day), as assessed by food frequency questionnaires (FFQs).
Subsequently, two RCTs have assessed whether the quantitative relationship between sodium intake and calcium excretion, and the effect of increasing calcium excretion on calcium balance, depend on background calcium intake (Lin et al., 2003; Teucher et al., 2008). In a 2 × 3 factorial design, Lin et al. (2003) randomised 186 adult men and women to a control diet, supplying 450 mg calcium/day, or the Dietary Approaches to Stop Hypertension (DASH) diet, supplying 1,250 mg calcium/day, and to three sodium intake levels of 1.1 g (50 mmol), 2.3 g (100 mmol) and 3.4 g (150 mmol)/day for 30 days. In a crossover design, Teucher et al. (2008) assigned 11 postmenopausal women to dietary interventions characterised by calcium intakes of 518 versus 1,284 mg/day and by sodium chloride intakes of 3.9 g (170 mmol) vs 11.2 g (487 mmol)/day) for four 5‐week periods. The two studies provided consistent evidence that sodium intake affects urinary calcium excretion both at ‘low’ and ‘high’‐calcium intake. Teucher et al. (2008) estimated bone calcium balances using a compartmental model. With the ‘low’‐calcium diets, negative bone calcium balances were estimated at both levels of sodium intakes. A negative bone sodium balance was also estimated on the ‘high’‐calcium/’high’‐sodium chloride diet, while bone calcium balance was positive on the ‘high’‐calcium/’low’‐sodium chloride diet. The only kinetic parameter significantly affected by sodium chloride intake was urinary calcium excretion.
The Panel notes that increasing sodium intake induces an increase in urinary calcium excretion that may negatively affect bone calcium balance, even when dietary calcium intake is above the PRI for calcium (EFSA NDA Panel, 2015).
2.6. Biomarkers
2.6.1. Biomarkers of intake
In healthy people, almost all dietary sodium is absorbed (Section 2.3.1) and urine is the major route of sodium excretion (Section 2.3.4.1). Urinary sodium excretion has traditionally been used as a biomarker of sodium intake, as it is considered to be more reliable than estimates of intake based on dietary assessments (Section 3.2).
2.6.1.1. Measurements in 24‐h urine collection
Twenty‐four‐hour urinary sodium excretion is used as a measure of average sodium intake at the population level (WHO, 2011). In a recent meta‐analysis of 35 studies (Lucko et al., 2018), mean 24‐h urine sodium was a close (93% on average) estimate of mean 24‐h dietary intake of sodium (Section 2.3.4.1). Adjustments to account for sodium excretion through sweat or in stools have been made only in a few studies, and in most cases, 24‐h urinary sodium excretion is used as a marker of daily sodium intake, without correction for other routes of sodium losses (Cogswell et al., 2015).
Incomplete collections of 24‐h urine samples can, however, introduce bias in measuring daily sodium excretion, and investigators need to implement quality control procedures (Cobb et al., 2014; Lucko et al., 2018). Several markers exist to assess and ensure complete collections, including: (i) urinary recovery of ingested para‐aminobenzoic acid (PABA) (usual criterion ≥ 85%), which is considered as the reference method; (ii) 24‐h urinary creatinine excretion; (iii) self‐report of missed voids; (iv) total urine volume (less than a specific threshold); and (v) duration of collection time (typically accepted range between 20 and 28 h); or (vi) combinations of the above (e.g. ratio of urinary to predicted creatinine excretion with total urine volume).
In eight studies using PABA, the percentage of incomplete collections ranged between 6% and 47% (John et al., 2016). Based on 24‐h urinary samples from 507 subjects, Wielgosz et al. (2016) assessed the impact of different methods of assessing completeness of collection on sodium intake estimates. Methods such as exclusion of individuals who collected urine for more than or less than 24 h, time‐adjustment of urine collections that varied from 24 h and creatinine‐based exclusion criteria were assessed. Estimated mean daily sodium intake varied between 3.6 g (156 mmol) and 7.3 g (317 mmol) in the same set of urine samples depending on the method used to exclude or correct incomplete 24‐h urine collections.
John et al. (2016) reviewed the literature to evaluate the validity of various methods using PABA recovery as the referent marker. The indices that were based on creatinine excretions had a moderate sensitivity (6–63% in four studies), but higher specificity (57–99.7%) to identify incomplete collection. Taking PABA recovery as the reference, the most valid method for identifying incomplete collections was the ratio of observed to predicted creatinine excretion (ratio < 0.7). The Panel acknowledges the risk that incomplete collection of 24‐h urine can lead to underestimated urinary sodium excretions. The Panel notes that assessments of the reliability of daily sodium intake estimates based on urinary excretion need to take into account the quality control measures that were applied by the researchers to ensure and assess the completeness of urine collections.
Sodium levels in 24‐h urine collections are inherently variable. This variation is usually assumed to reflect daily variations in intake, although considerable day‐to‐day variability has also been observed in 24‐h sodium excretion of individuals under conditions of well controlled, fixed sodium intakes (Rakova et al., 2013; Weaver et al., 2016; Lucko et al., 2018) (Section 2.3.3).
The number of 24‐h urine collections needed to cover intraindividual variability range between 5 and 10 (Luft et al., 1982; Siani et al., 1989; Lerchl et al., 2015; Weaver et al., 2016). Luft et al. (1982) conducted a study among 43 free‐living individuals to examine the utility of 24‐h urine collections in capturing variations in sodium intake. They reported that nine 24‐h collections were optimal to predict usual intake (r = 0.75). According to two prolonged balance studies (105 and 250 days) that involved 10 healthy young men, Lerchl et al. (2015) concluded that a single, accurately collected, 24‐h urine sample was not able to detect a 3 g difference in the individual sodium chloride intake (corresponding to a difference of 1.2 g of sodium) among men with sodium chloride intakes of 6, 9 or 12 g/day (corresponding to 2.5 (110 mmol), 3.6 (155 mmol) and 4.8 g (210 mmol) of sodium). This resulted in a misclassification of half of the study participants with respect to their usual sodium intake. A collection of three consecutive 24‐h urine samples reduced the number of misclassified individuals to 25%, and a collection of seven samples to 8%. In the study by Weaver et al. (2016), at least 10 repeated 24‐h samples were required on an average sodium intake of about 4 g (175 mmol)/day to reach a level of 75% reliability in the estimation of individual levels of sodium excretion.
In their review of observational cohort studies evaluating the association between sodium intake and health‐related outcomes, Cobb et al. (2014) noted that the error introduced by the high day‐to‐day variability in sodium intake appears to be random and does not lead to biased estimates of the overall mean intake, when a single 24‐h urine collection is used. It limits, however, the accurate classification of study participants on the basis of their individual usual sodium intakes, which additionally leads to an overestimation of the proportion of individuals being classified in the tails of the intake distribution (Cogswell et al., 2015). In an analysis based on follow‐up data from the US Trials of Hypertension Prevention that included multiple 24‐h urine collections per subject, the use of the single (first) measured 24‐h urinary sodium collection flattened the relationship between sodium intake and overall mortality compared with the average of multiple (three to seven) measured 24‐h collections (He et al., 2018). The Panel notes that a single 24‐h urine collection does not reliably reflect an individual's usual intake, primarily due to within‐person day‐to‐day variability in sodium intake and excretion.
The Panel therefore considers that a single 24‐h collection can be used to estimate average group sodium daily intakes, but a single 24‐h urine collection can lead to random misclassification of study participants in relation to their usual sodium intake. In addition, the Panel notes that incomplete 24‐h urine collections can introduce bias in intake estimates.
2.6.1.2. Casual spot urine collections and timed spot collections
Other methods such as casual spot and timed spot urine collections (i.e. collection during the day, evening, or overnight) have also been used as indicators of sodium intake. Day‐to‐day and diurnal variations in sodium excretion render these measures highly variable at the individual level; hence, these methods are subject to greater within‐person variability in sodium excretion than 24‐h urine collections (Ji et al., 2012; Wang et al., 2013). Predictive equations have been developed to estimate 24‐h urinary sodium excretion from spot urine samples (Kawasaki et al., 1993; Tanaka et al., 2002; Brown et al., 2013). Their validity has been assessed in a number of studies (Kawasaki et al., 1993; Tanaka et al., 2002; Brown et al., 2013; Cogswell et al., 2013; Ji et al., 2014; Mente et al., 2014; Pfister et al., 2014; Polonia et al., 2017; Zhou et al., 2017) (Appendix B). Associations between these estimates and 24‐h urinary excretions have primarily been assessed through correlation coefficients, which ranged between +0.33 (one timed morning urine collection in a sample of 297 white women in the UK and application of the Tanaka formula (Ji et al., 2014)) and + 0.53 (one morning urine collection in a sample of 159 men and women in Japan and application of the Kawasaki formula (Kawasaki et al., 1993)). In more recent studies, agreement between predicted and observed excretions has also been assessed through Bland–Altman plots, which indicate overestimation of predicted 24‐h excretions at lower levels and underestimation at higher levels of observed 24‐h urinary excretions for both men and women (Brown et al., 2013; Cogswell et al., 2013; Ji et al., 2014; Mente et al., 2014; Polonia et al., 2017).
Cogswell et al. (2013) undertook a study to assess the validity of various equations predicting 24‐h urinary sodium excretion based on spot urine concentrations (Appendix B). A sample of 407 adults aged 18–39 years provided one sample of 24‐h urine collection, from which four timed voids (morning, afternoon, evening and overnight) were selected. The published Kawasaki, Tanaka and INTERSALT equations were used to predict 24‐h sodium excretion with spot urine by specimen timing and race–sex subgroups. Bias was assessed through calculating the mean differences between estimated and measured 24‐h sodium excretion and with the use of Bland–Altman plots. The authors concluded that the INTERSALT equation when applied to sodium concentration in morning, afternoon and evening (but not overnight) samples provided the least biased estimates of population mean sodium intakes. The Tanaka equation was more reliable for mean population estimates when applied to overnight samples. However, the authors observed significant overestimation and underestimation among individuals and concluded that none of the equations provided unbiased estimates of individual 24‐h sodium excretion. The use of spot vs 24‐h urine samples for measuring differences in mean sodium excretion between population samples was assessed in the China Salt Substitute and Stroke Study (Huang et al., 2018). The Tanaka, Kawasaki and INTERSALT equations provided substantially underestimated differences between intervention and control groups compared with the values obtained using 24‐h urine samples.
Spot urine collections are, however, considered useful for trend analysis at group/population level (WHO, 2011; Ji et al., 2012; Cogswell et al., 2013).
The Panel notes that the reliability of both overnight and spot urine collections to estimate daily sodium intake is largely affected by circadian variations in individual sodium excretion. The Panel further notes that estimates of individual daily intakes from predictive equations based on spot urine samples can be biased, particularly at the lower and higher ends of the distribution and can therefore substantially misclassify exposure.
2.6.2. Biomarkers of status
Homeostatic mechanisms maintain plasma sodium concentration of healthy individuals within a narrow range (Sterns, 2015) (Section 2.3.2). Red blood cells concentrations are around 11 mmol/L (Cox, 1995; Penney, 2008). Differences by sex have been observed (Beilin et al., 1966). Slightly elevated plasma sodium concentration (by about 1–3 mmol/L) is often observed in hypertensive subjects (de Wardener et al., 2004; Blaustein et al., 2012).
The plasma sodium concentration is closely related to overall sodium balance, as well as potassium and water homeostasis. So, changes in plasma sodium concentration are related to the overall osmolarity of the diet, blood and gastrointestinal fluids, sweat and urine (Sterns, 2015). Hyponatraemia and hypernatraemia are typically related to disorders affecting water and electrolyte balances, and are seldom due to inappropriate sodium intake (Section 2.2.2). Plasma sodium concentration does not accurately reflect sodium body content.
The Panel considers that there is no biomarker of sodium status that can be used for setting DRVs for sodium in the general population.
2.7. Effects of genotype
There is heterogeneity in sodium‐dependent trafficking systems, much of which is not noticed because of the redundancy in many metabolic pathways that enable compensatory systems. Nonetheless, many genotypic variants that affect sodium‐dependent solute carriers for amino acids, monosaccharides, vitamins, potassium, calcium and bile acid, for example, have been reported. Additionally, there are monogenic defects affecting voltage‐gated sodium channels in neurons, and all types of muscle. Depending on the channel affected, the clinical features embrace cardiac conductivity defects, myopathies, increased sensitivity to pain, paroxysmal extreme pain, neuropathies, epilepsy and autonomic dysfunction (OMIM on line6).
Many monogenic changes affecting the renal excretion and salvage of sodium, chloride and water have also been identified, and these constitute a spectrum of defects involving both hypotension and hypertension (Schafer, 2002; Padmanabhan et al., 2015). Inherited defects in sodium reabsorption in the loop of Henle are associated with increased loss of sodium and chloride (salt‐losing tubulopathies) accompanied by hypokalaemia and alkalosis (e.g. Bartter's syndrome, Gitelman's syndrome). These genotypes are usually associated with normal or reduced blood pressure (Schafer, 2002; Padmanabhan et al., 2015). Other genotypes are associated with increased epithelial Na channel (ENaC) function causing syndromes involving excess aldosterone and mineralocorticoid activity leading to volume expansion and hypertension, hypokalaemia and, occasionally, alkalosis (e.g. Liddle's syndrome). In some instances, these syndromes include increased mucus viscosity and bronchiectasis. Other syndromes involve loss of ENaC activity resulting in a syndrome of pseudohypoaldosteronism with reduced ECF volume, sodium loss with hyperkalaemia and hypotension (Schafer, 2002; Padmanabhan et al., 2015).
‘Salt sensitivity’ is defined as a trait present in humans, by which the blood pressure of some members of the population exhibits changes parallel to changes in salt intake (Elijovich et al., 2016). As a response to ‘high’ exposure to sodium or sodium chloride in the population at large is a rise in blood pressure levels, the distinctiveness of a ‘salt‐sensitive’ individual lies in the immediacy of the observed changes in blood pressure levels induced by alterations in sodium chloride intake. ‘Salt sensitivity’ can be regarded as one end of a Gaussian distribution of blood pressure responses to change in dietary sodium chloride, while the other extreme has been termed ‘salt resistant’ (Weinberger, 1996; Strazzullo et al., 2000; He et al., 2009). The range of responses depends on the balance of environmental, dietary, for example potassium intake, and lifestyle factors with individuals’ physiological characteristics and genetic profiles (Lupoli et al., 2013; Luzardo et al., 2015; Padmanabhan et al., 2015; Elijovich et al., 2016). Although there is evidence for a genetic basis of ‘salt sensitivity’, the identification of genetic variants associated with ‘salt sensitivity’ is challenging (Elijovich et al., 2016). The Panel notes that, as yet, there is no consensus on the characterisation of ‘salt sensitivity’ (Luzardo et al., 2015; Elijovich et al., 2016).
The Panel considers that as yet no genotype has been characterised sufficiently to merit consideration about the estimation of DRVs for sodium in the general population.
3. Dietary sources and intake data
3.1. Dietary sources
All unprocessed foods contain sodium, although at low levels. The sodium content of unprocessed, raw meat and fish is typically between 30 and 150 mg (1.3 and 6.5 mmol)/100 g, and fruits and vegetables generally contain less than 50 mg (2.2 mmol)/100 g (UK Food Standards Agency, 2002; Anses, 2016c; National Institute for Health and Welfare, 2016).
Sodium is present in variable amounts in water, with mineral deposits, seawater spray, sewage effluents, and salt used in road de‐icing contributing significant quantities of sodium to water (WHO, 2003). Water treatment chemicals, such as sodium fluoride, sodium bicarbonate and sodium hypochlorite, can also increase the sodium content of water. Median (25–75th) sodium concentrations in tap water sampled in 30 European countries was 0.4 (0.2–0.9) mmol/L (9.5 (4.3–20.0) mg/L) (n = 579 samples) (Banks et al., 2015). High variability was found in European samples of bottled mineral water, from 0.04 mmol (1 mg) to 61.7 mmol (1,419 mg)/L (n = 73) (Azoulay et al., 2001). So, the contribution of drinking water to dietary sodium intake may vary substantially depending on the source and quantity of the water that is consumed.
Sodium is added to food mostly as sodium chloride during processing. In addition, sodium may be added in the form of sodium‐containing food additives, such as sodium bicarbonate in fine bakery wares or sodium nitrate in processed meat. Authorised sodium‐containing food additives include riboflavin 5′‐phosphate sodium, d‐pantothenate sodium, sodium‐l‐ascorbate, ferric sodium diphosphate, ferric sodium ethylenediaminetetraacetate (EDTA), sodium iodide, sodium iodate, sodium bicarbonate, sodium carbonate, sodium citrate, sodium gluconate, sodium lactate, sodium hydroxide, sodium salts of orthophosphoric acid, sodium selenate, sodium selenite, sodium hydrogen selenite, sodium fluoride, sodium molybdate and sodium borate, which can be added to both foods7 and food supplements.8 Sodium sulfate and sodium monofluorophosphate are authorised for use in food supplements only.7 The sodium content of infant and follow‐on formulae9 and processed cereal‐based foods and baby foods for infants and young children10 is regulated.
The sodium content of processed foods can vary substantially between countries, reflecting dietary habits and taste preferences. In addition, large variations have been observed in the sodium content of food items belonging to the same food group. Studies conducted in the Netherlands, Australia and the UK agree on the wide range of sodium content among similar food items. Based on these estimations, food groups with the highest sodium content are sauces (particularly Asian ones), processed meat, cheese and canned fish, whereas food groups with the lowest sodium content are rice, pasta, cereal products (excluding bread) and processed fruits and vegetables (Webster et al., 2010; Ni Mhurchu et al., 2011; Capuano et al., 2013; Eyles et al., 2013). In European populations, the main contributors to sodium intake are bread, meat and meat products, and cheese and dairy products (European Commission, 2012; Kloss et al., 2015).
The relative contributions of different sodium sources (inherently food‐borne, processing‐added, table salt, cooking salt) to intake is variable, as illustrated by studies conducted in the UK (Farrimond et al., 1995; Henderson et al., 2003), Denmark (Andersen et al., 2009) and Italy (Leclercq and Ferro‐Luzzi, 1991). Estimates of the mean contribution of discretionary sodium chloride (i.e. added during the preparation of the meals or at the table) vary from 10% in the study in Denmark to more than one third of the total intake in the study in Italy. The Panel however notes the substantial uncertainty associated with these estimates.
The Panel notes that sodium chloride added during industrial food processing, discretionary use or food preservation is the major source of dietary sodium in Western diets. Other sources of sodium include inherently native sources, and sodium‐containing food additives, in which sodium may be associated with anions other than chloride.
3.2. Dietary intake
3.2.1. Methodological considerations
In dietary surveys, sodium intake is estimated through recalls (of recent or usual diet) or food diaries capturing intakes in real‐time. In addition to the limitations inherent to the use of dietary questionnaires, i.e. the inaccurate reporting of participants and the use of incomplete or outdated food composition tables to determine the sodium content of food, errors in estimating sodium intake can be introduced by the failure to capture the sodium chloride added at the table and/or during cooking. These errors can result in biased estimates of sodium intake, which if they differ by exposure or disease status (i.e. high consumers or hypertensive subjects may misreport more often than individuals with lower intakes or normal blood pressure, respectively), can have an unpredictable impact on the estimated association between sodium intake and disease risk.
In a pooled analysis of data collected in five large validation studies conducted in the USA, Freedman et al. (2015) assessed the relative validity of FFQs and 24‐h dietary recalls (DRs) in capturing sodium intake through comparisons with 24‐h urinary sodium levels. On average, weighted by the inverse of the variance, underreporting was 28% (men) and 39% (women) with a FFQ and 4% (men) and 13% (women) with a single 24‐h DR. Underreporting of sodium was strongly associated with higher body mass index (BMI) for both instruments and also with being black, male, and having a high school education versus a college education or higher for FFQs. Correlation coefficients between self‐reported questionnaires and 24‐h urinary estimates improved when sodium intake was expressed in relation to total energy intake (i.e. as sodium density) and when multiple 24‐h DRs were collected as compared with a single 24‐h DR.
Two recent reviews of validation studies compared estimates of sodium intakes based on dietary questionnaires (24‐h dietary recalls, food diaries or FFQs) with 24‐h urinary sodium excretion (McLean et al., 2017, 2018). Studies were quite heterogeneous in the period covered, i.e. number of days covered by 24‐h DRs or records, of months captured by FFQs and the number of 24‐h urine samples collected; in whether sodium chloride used for preparation or added at the table was taken into consideration; in considering participants’ characteristics in the analysis; and in the way the data were analysed. In most studies, correlation coefficients were estimated and ranged from 0.11 to 0.49 for food diaries (six studies) and from 0.16 to 0.72 for 24‐h DRs (10 studies) (McLean et al., 2018). The results of Bland–Altman analysis performed in two studies generally point to poor agreement between estimations based on 24‐h DRs and urine collections. In particular, one study reported a negative association (increasing urinary sodium excretion was associated with increasing underestimation by dietary method) but the limits of agreement were not reported. The second study reported a mean difference of 0.087 g/day, but with 95% limits of agreement ranging from −3.1 to +3.3 g/day, indicating a wide range of bias in the estimations. The correlation coefficients between intakes assessed through FFQs and 24‐h urines ranged from 0.07 to 0.36 across 16 eligible validation studies (McLean et al., 2017). Concurrent urine collections did not substantially improve the correlations. One eligible study indicated poor agreement between estimates from a FFQ and 24‐h urine collection based on the Bland–Altman method. There was no obvious bias at low or high sodium intakes in that study.
The Panel notes that there is generally poor agreement between sodium intake estimates based on dietary questionnaires and 24‐h urine collections. The Panel considers that estimates of sodium intake based on 24‐h urinary excretion are more accurate than estimates of intake based on dietary questionnaires (Section 2.5.1). Twenty four‐hour urine collection is the recommended method for assessing population mean sodium intake. There are limitations, however, in the use of this method, including potential bias due to inaccurate collection. The Panel additionally considers that when estimates of individual intakes are based on single measurements or the average of a small number of urine collections, they may be prone to random errors due to within‐individual day‐to‐day variability in sodium excretion, which may result in the estimation of inaccurate percentiles of sodium intake.
3.2.2. Sodium urinary excretion in European populations and abroad
In 2016, an overview of sodium intake in European populations was prepared based on data on sodium urinary excretion in European populations collected through EFSA focal points and the members of the EFSA Food Consumption Network.11 Data were collected from 18 countries, and the most recent surveys, conducted between 2002 and 2014, were selected. Appendix C provides urinary sodium excretion data in children in four countries (Austria, Iceland, Italy, Spain). Appendices D and E provide urinary sodium excretion data of adult men and women in 17 countries (Austria, Belgium, Croatia, the Czech Republic, Finland, Germany, Greece, Hungary, Ireland, Italy, Norway, Slovenia, Spain, Sweden, Switzerland, the Netherlands and the United Kingdom). Most countries used 24‐h urine collection, while three countries collected spot or timed urine collection and estimated daily sodium excretion through arithmetic extrapolation. Studies using 24‐h urine collection were heterogeneous with respect to the methods and criteria applied for the assessment and exclusion of incomplete or unreliable urine collections (e.g. PABA recovery, creatinine excretion levels, urinary volume, self‐reporting of incomplete samples). Some studies were designed as national monitoring surveys, while others were conducted as part of broader observational studies. Sample sizes also varied widely, from tens to thousands of people.
Mean sodium urinary excretion levels across countries ranged between 3.2 and 6.1 g/day (141 and 266 mmol/day) in adult men, and between 2.6 and 4.2 g/day (112 and 182 mmol/day) in adult women. Across all countries, mean sodium excretion levels were higher in men than in women. In children, values ranged between 1.7 g/day (72 mmol/day) in 6‐year‐old boys and girls in Iceland and 2.8 and 3.5 g/day (122 and 154 mmol/day) in Austrian boys and girls aged 13–14 years old.
Powles et al. (2013) combined data from national and subnational adult population surveys of 24‐h urinary sodium excretion and dietary sodium intake, conducted in 187 countries (21 regions) between 1980 and 2010. Dietary estimates were converted into urine equivalents based on surveys having data for both measurements from the same individuals, and mean sodium intake was estimated through Bayesian hierarchical modelling. Across European countries, mean (95% uncertainty interval) sodium intake estimates ranged from 3.27 (2.98–3.58) g/day (Denmark) to 4.42 (4.22–4.61) g/day (Italy) for men and women combined. World‐wide, sodium intakes were the highest in East and Central Asia and Eastern Europe (mean > 4.2 g/day), and in Central Europe and Middle East/North Africa sodium intake ranged between 3.9 and 4.2 g/day. Regional mean intakes in North America, Western Europe and Australia/New Zealand ranged between 3.4 and 3.8 g/day. Intakes were lower (< 3.3 g/day) in sub‐Saharan Africa and Latin America, but more uncertain due to the few data sources available.
In the INTERSALT study, an international study undertaken in 1982–1985 in which 52 centres from 32 countries participated, 200 men and women (aged 20–59 years) equally distributed in age and sex groups were recruited in each centre. Participants were asked to provide a 24‐h urine collection, following a standardised protocol. Urine analyses were conducted in the same laboratory. The study pointed to a large variation in the sodium intake of free‐living healthy individuals, with median urinary sodium excretion ranging between 0.005 g (0.2 mmol)/day in Yanomamo Indians in Brazil and 5.6 g (242.1 mmol)/day in China (Intersalt Cooperative Research Group, 1988).
4. Overview of dietary reference values and recommendations
4.1. Adults
For adults aged 19 and older, the US National Academies of Sciences, Engineering, and Medicine (NASEM) set an adequate intake (AI) of 1.5 g/day (NASEM, 2019). The lowest levels of sodium intake evaluated in randomised trials conducted among adults (DASH‐sodium trial and eight other trials) and the balance study from Allsopp et al. (1998) which indicated neutral balance with heat stress at this level of intake were considered in setting the AI. The NASEM concluded that evidence of harmful effects of low sodium intake on type 2 diabetes, glucose tolerance, and insulin sensitivity, blood pressure, plasma lipid concentrations, cardiovascular disease and all‐cause mortality was insufficient and inconsistent. The NASEM also established a Chronic Disease Risk Reduction Intake (CDRR) for sodium, defined as the lowest level of intake for which there was sufficient strength of evidence to characterise a chronic disease risk reduction. In the sodium intake range of 2.3–4.1 g/day (100–178 mmol/day), the strength of evidence was considered high that reducing sodium intake reduces chronic disease risk, based on evidence of reduction in cardiovascular disease incidence, reduction in hypertension incidence, and lowering of systolic and diastolic blood pressure. A sodium CDRR of reducing intakes if above 2.3 g/day (100 mmol/day) was proposed, which is applicable to adults with and without hypertension, irrespective of sex, age or race/ethnicity. The 2015–2020 Dietary Guidelines for Americans recommended that adults limit sodium intake to less than 2.3 g/day (HHS/USDA, 2015).
The German‐speaking countries (D–A–CH, 2016; Strohm et al., 2018) based their DRVs on data from the balance study by Allsopp et al. (1998) in which male subjects achieved a positive sodium balance with sodium intake of 1.5 g/day after 8 days, under conditions of moderate physical activity and heat exposure. In addition, it was noted that the requirements of other nutrients, with the exception of iodine and fluoride, can be achieved with a diet providing 1.5 g sodium/day (Deutsche Gesellschaft für Ernährung, 2015). An AI of 1.5 g/day was set for all adults. In a separate statement, the German Nutrition Society (DGE) emphasised the relationship between sodium chloride intake and blood pressure, and that a high consumption of sodium chloride is associated with an elevated or ‘suboptimal’ blood pressure while a low consumption is associated with blood pressure in the normal or ‘optimal’ range (Strohm et al., 2016). This association was considered a convincing proof of an indirect effect of high sodium chloride intake via hypertension on the risk of CVD, while the evidence for a direct effect of sodium chloride intake on CVD risk was considered inconsistent. A target value for dietary sodium chloride of 6 g/day (2.4 g sodium) for adults was recommended.
The Nordic countries (Nordic Council of Ministers, 2014) acknowledged that trials showed a decrease in blood pressure when sodium intake was reduced, and that blood pressure is a risk factor for CVD (Bibbins‐Domingo et al., 2010). It was stated that recommended intakes for sodium should be based on public health considerations rather than actual requirements. A sodium chloride intake of less than 6 g/day or a sodium intake of less than 2.4 g/day was considered a population goal.
In 2014, the Italian Society of Nutrition (SINU) set an AI of 1.5 g sodium/day for adults aged 18–59 years, in line with IOM's conclusions (IOM, 2005). A suggested dietary target (SDT) of 2 g sodium/day was proposed for this age group for the prevention of cardiovascular and other chronic diseases, consistent with the recommendation from the WHO (2012b). It was stated that the target for the population is a sodium intake below the SDT. For older adults (≥ 60 years), SINU proposed a decrease in the AI and SDT compared with younger adults, in proportion with the requirement for energy. This was taking into consideration the fact that the sensitivity of blood pressure to sodium chloride intake increases with age (Khaw and Barrett‐Connor, 1990; Vollmer et al., 2001), and also accounted for reduced renal and cardiovascular functions in older adults.
The WHO (2012b) has not set DRVs for sodium but recommends a reduction in sodium intake to < 2 g/day sodium (5 g/day sodium chloride) in adults (≥ 16 years of age) (strong recommendation12). This was based on evidence from systematic reviews on the relationship between sodium and blood pressure and risk of cardiovascular disease, stroke and coronary heart disease in adults (WHO, 2012a,c).
The Health Council of the Netherlands concluded that, in a large number of RCTs, lowering sodium intake reduces blood pressure, which is a causal risk factor of cardiovascular diseases (Kromhout et al., 2016). The Committee observed that the protective effect of a low intake of sodium was stronger in hypertensive than in normotensive people (Graudal et al., 2011; Aburto et al., 2013; He et al., 2013). The guideline could not be quantified because of insufficient data from high‐quality cohort studies on sodium intake and cardiovascular risk. Therefore, the Committee decided to maintain its previous guideline to limit salt intake to 6 g/day (2.4 g sodium) (Health Council of the Netherlands, 2006).
Afssa (2001) did not set a PRI for sodium because of a lack of intervention studies (in particular on cardiovascular morbidity and mortality) to define a PRI. A lack of consensus was noted on the relationship between sodium intake and blood pressure (Alderman et al., 1997; Stamler et al., 1997; Weinberger, 1997; McCarron, 1998; Taubes, 1998; MacGregor and de Wardener, 1999; Swales, 1999). Afssa (2001) suggested that healthy adults should not consume more than 12 g/day and not less than 5 g/day of sodium chloride (corresponding to 4.8 and 2.0 g/day of sodium). In 2016, Anses considered recent literature on the relationship between sodium intake and blood pressure (Mente et al., 2014) and cardiovascular risk (IOM, 2013; Adler et al., 2014; Graudal et al., 2014; O'Donnell et al., 2014; Pfister et al., 2014) and noted a lack of consensus; the experts concluded that current data were insufficient to set a UL, a PRI or an AI for sodium (Anses, 2016b). In its update of the food‐based dietary guidelines for the French population, Anses selected the median consumption of sodium as the maximum value not to be exceeded, which amounts to reducing intake in the half of the population with higher intake levels, in agreement with public health policies. The French consumption survey INCA2 reported median daily intakes of sodium of 2,273 mg for women and 2,994 mg for men (excluding sodium from salt added at the table) (Anses, 2016a).
The SCF (1993) did not set a PRI for sodium but an acceptable range of intakes of 0.575–3.5 g sodium/day. The lower intake took into account reports on maintenance of sodium balances at intakes as low as 0.069–0.46 g/day, and observed habitual intakes in some populations of 0.23–0.92 g/day (Glieberman, 1973; INTERSALT Cooperative Research Group, 1988; Law et al., 1991a), allowing for changes in physical activity and ambient temperature. The upper intake was based on evidence that an intake higher than 4.6 g/day may be associated with increased risk of hypertension, especially in older adults (Frost et al., 1991; Law et al., 1991a,b), and the public health consideration that intakes should be lower than this amount to reduce the risk of hypertension and CVD.
The UK COMA (DH, 1991) set DRVs based on the balance of ‘risks and benefits’ of sodium intakes. The COMA was unable to derive an estimated average requirement (EAR) but set a lower reference nutrient intake (LRNI) for sodium at 25 mmol/day (0.575 g/day) and a reference nutrient intake (RNI) at 70 mmol/day (1.6 g/day). It was noted that a reduction in sodium intake decreases blood pressure in people with established hypertension, but this may not be seen in people with normal blood pressure. The COMA was unable to determine a potentially toxic threshold for sodium intake. It was noted that 10% of the population may be affected by a genetic susceptibility to sodium‐related hypertension apparent at sodium intakes of 3.2–4.7 g/day. In 2003, the UK Scientific Advisory Committee on Nutrition (SACN) endorsed the RNI of 70 mmol/day (1.6 g/day) and recommended a target sodium chloride intake of less than 6 g/day (2.4 g (100 mmol) sodium)) for the adult population by multiplying the RNI by a factor of 1.5 (SACN, 2003). The Committee noted that this is higher than the RNI and substantially greater than the sodium chloride intake required to maintain the sodium content of the body. It noted that the target salt intakes set for adults do not represent ideal or optimum consumption levels, but an achievable population goal as part of a public health strategy.
Table 2.
Overview of dietary reference values (adequate intakes) for sodium for adults
| NASEM (2019) | D–A–CH (2016) | SINU (2014) | SCF (1993) | DH (1991); SACN (2003) | |
|---|---|---|---|---|---|
| Age (years) | ≥ 19 | ≥ 19 | ≥ 19 | ≥ 18 | ≥ 19 |
|
AI Men (g/day) Women (g/day) |
1.5 1.5 |
1.5 1.5 |
1.5 1.5 |
0.575–3.5a 0.575–3.5a |
1.6 1.6 |
| Age (years) | ≥ 60 | ||||
|
AI Men (g/day) Women (g/day) |
1.2 1.2 |
AI: adequate intake; D–A–CH: Deutschland–Austria–Confoederatio Helvetica; DH: Department of Health; NASEM: National Academies of Sciences, Engineering, and Medicine; SACN: Scientific Advisory Committee on Nutrition; SCF: Scientific Committee for Food; SINU: Italian Society of Nutrition.
Acceptable range of intakes.
Table 3.
Overview of population goal/target for sodium and sodium chloride intake for adults
| Strohm et al. (2016) (DGE) | Anses (2016a) | HHS/USDA (2015) | Nordic Council of Ministers (2014) | SINU (2014) | WHO (2012b) | Health Council of the Netherlands (2006) | SACN (2003) | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | ≥ 18 | ≥ 19 | ≥ 18 | ≥ 18 | ≥ 16 | ≥ 18 | ≥ 19 | |
| Sodium chloride (g/day) | 6 | – | 5.75 | 6 | 5 | 5 | 6 | 6 |
| Sodium (g/day) | 2.4 |
M: ≤ 2,994 mg/day W: ≤ 2,273 mg/day |
2.3 | 2.4 | 2.0 | 2.0 | 2.4 | 2.4 |
| Age (years) | ≥ 60 | |||||||
| Sodium chloride (g/day) | 4 | |||||||
| Sodium (g/day) | 1.6 |
Anses: French Agency for Food, Environmental and Occupational Health and Safety; DGE: German Society of Nutrition; HHS/USDA: Health and Human Services/United States Department of Agriculture; M: men; SACN: Scientific Advisory Committee on Nutrition; SINU: Italian Society of Nutrition; W: women; WHO: World Health Organization.
4.2. Infants and children
For infants aged 7–12 months, the NASEM (2019) set an AI of 370 mg/day (16.1 mmol/day) based on the sodium intake from breast milk (approximately 70 mg/day (3.0 mmol/day)) and from complementary foods (300 mg/day (13.0 mmol/day)). For children and adolescents 1–18 years of age, the AIs were derived by extrapolating from the sodium AI for adults based on average Estimated Energy Requirements for sedentary children, as compared to an Estimated Energy Requirement for adults. Regarding CDRR intake, the NASEM noted that evidence to assess the relationship between sodium intake and chronic disease in children and adolescents was insufficient and the uncertainties about the long‐term chronic disease benefits of reduced sodium intake beginning in childhood. However, the committee considered that the risk of not setting a CDRR for children outweighed the risk of setting a sodium CDRR intake for children based on evidence of blood pressure tracking to adulthood, the public health importance, and consideration of salt‐taste sensitivity and preferences starting to develop as early as 3–4 months of age. The sodium CDRRs for children were extrapolated from the adult sodium CDRR, based on energy requirements, and were set as follows: 1.2 g/day for children aged 1–3 years, 1.5 g/day for children aged 4–8 years, and 1.8 g/day for children aged 9–13 years and 2.3 g/day for children aged 14–17 years. The 2015–2020 Dietary Guidelines for Americans (HHS/USDA, 2015) recommended that children limit sodium intake to less than the ULs established by the IOM in 2005.
For infants aged 4–11 months, the German‐speaking countries (D–A–CH, 2016) derived an AI of 0.2 g/day. This was based on an estimated sodium intake from breast milk of 0.13 g/day for infants aged 0–4 months (considering a sodium content of breast milk of 0.170 g/L and assuming an average breast milk intake of 0.75 L/day) and upward extrapolation considering differences in body weight. AIs for children were extrapolated down from the AI of adults, based on difference in body weight and applying a growth factor.
For children, Nordic countries stated that data suggest that a reduction in sodium intake at an early age is associated with a lower blood pressure in later life. For children below 2 years of age, it was recommended to limit sodium chloride intake to below 0.5 g/MJ (equivalent to 0.2 g/MJ of sodium), to avoid developing a preference for a diet with a high sodium chloride level. From 2–9 years, it was recommended not to exceed a sodium chloride intake of about 3–4 g/day (Nordic Council of Ministers, 2014).
For infants aged 7–12 months, SINU (2014) set an AI based on the sodium intake from breast milk and from complementary foods, in line with the approach taken by IOM (2005). For children aged 1–10 years, AIs and SDTs were extrapolated down from the AI for adults in proportion of the energy requirement of the respective age groups. For children and adolescents aged 11–18 years, the same AI and SDT as for adults were proposed.
The WHO (2012b) has not set DRVs for sodium but recommends a reduction in sodium intake to control blood pressure in children aged 2–15 years of age (strong recommendation). The recommended maximum level of intake of 2 g/day sodium in adults should be adjusted downward based on the energy requirements of children relative to those of adults.
Afssa (2001) and the SCF (1993) did not set DRVs for sodium in infants and children due to insufficient evidence.
The UK COMA (DH, 1991) set LRNIs and RNIs for infants and children. For infants and children above 6 months, RNIs were derived factorially by calculating the daily increase in total body sodium content allowing for the declining proportion with age of ECF in body mass (Friis‐Hansen, 1961), with an allowance for dermal, faecal and urinary losses. So, LRNIs between 0.2 g sodium/day (7 months to 3 years) and 0.575 g sodium/day (15 to 18 years) were set. In 2003, the UK Scientific Advisory Committee on Nutrition endorsed the RNI proposed by COMA and multiplied them by a factor of 1.5 to set daily target average sodium chloride intake (SACN, 2003). The Committee noted that target sodium chloride intakes do not represent ideal or optimum consumption levels, but an achievable population goal.
Table 4.
Overview of dietary reference values (adequate intakes) for sodium for infants and children
| NASEM (2019) | D–A–CH (2016) | SINU (2014) | DH (1991) | |
|---|---|---|---|---|
| Age (months) | 0–6 | 4–11 | 4–6 | |
| AI (g/day) | 0.11 | 0.2 | 0.28a | |
| Age (months) | 7–12 | 6–12 | 7–9 | |
| AI (g/day) | 0.37 | 0.4 | 0.32a | |
| Age (months) | 10–12 | |||
| AI (g/day) | 0.35a | |||
| Age (years) | 1–3 | 1–3 | 1–3 | 1–3 |
| AI (g/day) | 0.8 | 0.4 | 0.7 | 0.5a |
| Age (years) | 4–8 | 4–6 | 4–6 | 4–6 |
| AI (g/day) | 1.0 | 0.5 | 0.9 | 0.7a |
| Age (years) | 7–9 | 7–10 | 7–10 | |
| AI (g/day) | 0.75 | 1.1 | 1.2a | |
| Age (years) | 9–13 | 10–12 | 11–14 | 11–14 |
| AI (g/day) | 1.2 | 1.1 | 1.5 | 1.6a |
| Age (years) | 14–18 | 13–14 | 15–17 | 15–18 |
| AI (g/day) | 1.5 | 1.4 | 1.5 | 1.6a |
| Age (years) | 15–18 | |||
| AI (g/day) | 1.5 |
AI: adequate intake; D–A–CH: Deutschland–Austria–Confoederatio Helvetica; DH: Department of Health; NASEM: National Academies of Sciences, Engineering, and Medicine; SINU: Italian Society of Nutrition.
Reference nutrient intake (RNI).
Table 5.
Overview of population goal/target for sodium and sodium chloride intake for children
| HHS/USDA (2015) | Nordic Council of Ministers (2014) | SINU (2014) | SACN (2003) | |
|---|---|---|---|---|
| Age (months) | 0–6 | |||
| Sodium chloride (g/day) | 1 | |||
| Sodium (g/day) | 0.4 | |||
| Age (months) | 0–24 | 7–12 | ||
| Sodium chloride (g/day) | 0.5a | 1 | ||
| Sodium (g/day) | 0.2a | 0.4 | ||
| Age (years) | 1–3 | 2–9 | 1–3 | 1–3 |
| Sodium chloride (g/day) | 3.75 | 3–4 | 2.25 | 2 |
| Sodium (g/day) | 1.5 | 1.2–1.6 | 0.9 | 0.8 |
| Age (years) | 4–8 | 10–18 | 4–6 | 4–6 |
| Sodium chloride (g/day) | 4.75 | 6 | 3 | 3 |
| Sodium (g/day) | 1.9 | 2.4 | 1.2 | 1.2 |
| Age (years) | 9–13 | 7–10 | 7–10 | |
| Sodium chloride (g/day) | 5.5 | 3.75 | 5 | |
| Sodium (g/day) | 2.2 | 1.5 | 2.0 | |
| Age (years) | 14–18 | 11–17 | 11–18 | |
| Sodium chloride (g/day) | 5.75 | 5.0 | 6 | |
| Sodium (g/day) | 2.3 | 2.0 | 2.4 |
HHS/USDA: Health and Human Services/ United States Department of Agriculture; SACN: Scientific Advisory Committee on Nutrition; SINU: Italian Society of Nutrition.
Expressed as nutrient density, i.e. g of sodium/MJ of energy intake.
4.3. Pregnancy and lactation
The NASEM considered that there was a lack of evidence to suggest that sodium requirements of pregnant women differ from that of non‐pregnant women and proposed a sodium AI for pregnant women of 1.5 g/day (65 mmol/day) (NASEM, 2019). Regarding lactating women, the NASEM noted that sodium is excreted in breast milk but the concentrations are determined by an electrical potential gradient, rather than by maternal dietary intake. The sodium requirements for lactating women does not appear to differ from that of non‐pregnant, non‐lactating women and the same AI of 1.5 g/day (65 mmol/day) was set for this group. The NASEM considered that there was insufficient evidence that a different sodium CDRR is needed for pregnant or lactating females compared to their non‐pregnant, non‐lactating age group counterparts. The same sodium CDRR of 2.3 g/day was proposed for these groups.
D–A–CH (2016) considered that the extra sodium requirement of 0.07 g/day (3 mmol/day) during pregnancy, due to the expansion of ECF volume, can be covered by homeostatic mechanisms. The same was considered to be true for the additional requirement of 0.13 g/day (6 mmol/day) during lactation due to sodium losses with breast milk.
SINU (2014) considered that the AI for non‐pregnant non‐lactating women was sufficient to cover the increase in sodium requirement during pregnancy and lactation; the same AI as for other adults (1.5 sodium/day) was maintained for these population groups. SINU recommended that the SDT for other adults (2 g sodium/day) also applies to pregnant and lactating women.
The Nordic countries (Nordic Council of Ministers, 2014) concluded that there was a lack of evidence to suggest that the sodium requirement during pregnancy and lactation differs significantly from that of non‐pregnant women; no DRVs for sodium were set for these population groups. Likewise, the SCF (1993) and the UK COMA (DH, 1991) did not set specific DRVs for sodium for pregnant and lactating women.
Table 6.
Overview of dietary reference values (adequate intakes) for sodium for pregnant and lactating women
| NASEM (2019) | D–A–CH (2016) | SINU (2014) | |
|---|---|---|---|
| Age (years) | 14–50 | ||
| AI pregnancy (g/day) | 1.5 | 1.5 | 1.5 |
| AI lactation (g/day) | 1.5 | 1.5 | 1.5 |
AI: adequate intake; D–A–CH: Deutschland–Austria–Confoederatio Helvetica; g: gram; NASEM: National Academies of Sciences, Engineering, and Medicine; SINU: Italian Society of Nutrition.
5. Criteria (endpoints) on which to base dietary reference values
5.1. Biomarkers as indicators of sodium requirement
As stated in Section 2.4, the Panel considers that there are no appropriate biomarkers of sodium status that can be used for deriving DRVs for sodium.
5.2. Balance studies
Balance studies are based on the assumption that a healthy subject on an adequate diet maintains equilibrium or a null balance, between nutrient intakes and nutrient losses. At this null balance, the intake matches the requirement determined by the physiological state of the individual. When intakes exceed losses (positive balance), there is nutrient accretion that may be attributable to growth or weight gain, anabolism, or repletion of stores; when losses exceed intakes (negative balance), nutrient stores are progressively depleted, resulting in clinical symptoms of deficiency in the long term. In addition to numerous methodological concerns about accuracy and precision in the determination of nutrient intakes and losses (Baer et al., 1999), the validity of balance studies to calculate nutrient requirements has been questioned. Differences between intakes and losses of a nutrient may only reflect adaptive changes before a new steady state is reached (Young, 1986), or the conditions for the maintenance of nutrient ‘stores’ in the context of a given diet, whereas the health relevance of the size of body pools still needs to be established for each nutrient (Mertz, 1987).
Although the term ‘balance studies’ is used in this section, the Panel considered studies that describe comparative assessments between sodium intake and sodium excretion provided that their designs allowed for an adaptation period (see Section 2.3.4.1). The key characteristics and results of eligible balance studies are tabulated in Appendix F.1 and summarised below.
5.2.1. Balance studies in adults
Holbrook et al. (1984) aimed to assess sodium ‘balance’ in 28 United States healthy (20–53 years), free‐living men and women consuming self‐selected diets. For 1 week in each season of the year (four 7‐day periods), participants collected duplicate samples of food and beverages consumed and 24‐h urine and faecal samples for sodium content analysis. Sodium ‘balance’ was calculated as the difference between sodium intake (including salt added during preparation and at the table) and sodium excretion through urine and faeces. Dermal losses were not measured. Results are given for the four 7‐day periods combined (28 days). Most participants decreased their energy intake, as assessed by dietary records (mean decrease of about 17% for men and 13% for women), during the collection periods. Mean sodium intake was 3.4 g (148 mmol)/day. Mean ± SD difference between sodium intake and excretion was +0.47 ± 0.32 g (+20 ± 14 mmol)/day. The 95% CI of the mean did not include zero. The mean difference between sodium intake and excretion was more positive during the summer (+0.6 g (26 mmol)/day versus +0.4 g (17 mmol)/day in other periods). The Panel notes the positive difference between sodium intake and excretion in these free‐living individuals with a mean sodium intake of 3.4 g (148 mmol)/day. The Panel also notes that dermal losses were not assessed, which may have contributed to the positive difference.
One study (Allsopp, 1997; Allsopp et al., 1998) investigated sodium balance in 25 men (18–40 years, BMI 20–34 kg/m2, not on regular strenuous physical training), who were confined to an experimental chamber at 25°C for 3 days (days 1–3), followed by 5 days at 40°C from 08:00 to 18:00 h and then at 25°C from 18:00 to 08:00 h (days 4–8). Relative humidity and air velocity were kept constant. Sodium intake was controlled and kept constant throughout the study at doses of 1.5 g (65 mmol)/day (n = 9), 4 g (174 mmol)/day (n = 9) or 8 g (348 mmol)/day (n = 7). Sodium losses were estimated from daily 24‐h urine collections, faecal samples and whole‐body wash‐downs on days 3, 4 and 8. Plasma aldosterone concentration was measured from morning blood samples. On day 8, mean ± SD sodium balance was +0.04 ± 0.35 g/day, +0.79 ± 0.64 g/day and +0.67 ± 1.19 g/day in the groups of men consuming 1.5, 4 and 8 g sodium/day, respectively. On day 8, no two groups were dissimilar with respect to net sodium balance. In the group consuming 1.5 g of sodium/day, mean plasma levels of aldosterone had increased by day 3 (vs no increase in the other two groups). During the heat exposure period, such an increase was observed in all groups, but it was more apparent in the group consuming 1.5 g of sodium/day.
The Panel notes that men with low physical activity and a daily exposure to prolonged heat were on average in balance, partly mediated by changes in aldosterone secretion, after 8 days of a controlled diet that provided 1.5 g (65 mmol) of sodium/day.
A series of studies addressing Na, K, Ca, Mg, P, Fe, Zn, Cu and Mn was conducted in Japan and have been collectively analysed on several occasions (Kodama et al., 2005; Nishimuta et al., 2005, 2006, 2012, 2018). Nishimuta et al. (2012) calculated the ‘estimated equilibrated dietary intakes (EEDI)’ for sodium (the intercept of a linear regression equation between intake and balance) using data from 13 ‘balance’ studies conducted in young Japanese women (n = 131, 18–26 years). Daily sodium intake, assessed through duplicate diet samples, ranged between 2.5 g (107 mmol) and 4.8 g (209 mmol). Each study included an adaptation period of 2–4 days. Sodium concentration in urine and faeces were measured. Sodium losses in sweat during exercise were measured in five studies by collecting sweat from the arm. The duration of the balance periods ranged from 8 to 12 days. Notwithstanding the uncertainty induced by measurements of sweat losses, the Panel notes that, in this collective analysis of primary studies among Japanese women, mean sodium ‘balance’ was ‘positive’ (+6.07 ± 4.06 mg/kg bw) with sodium intakes above 2.5 g (107 mmol).
Other sodium balance studies or experiments assessing sodium excretion in different circumstances are also available in the literature (McCance, 1936; Falconer and Lyall, 1937; Dole et al., 1950; Heer et al., 2000, 2009; Rakova et al., 2013). The Panel considers that these ‘balance studies’ cannot be used for setting DRVs for sodium due to the very small number of study participants (two in McCance (1936); three in Falconer and Lyall (1937)), the inclusion of participants with pre‐existing medical conditions (Dole et al., 1950), the absence of adaptation periods (Heer et al., 2000, 2009), or the lack of measurements of both faecal and dermal losses (Rakova et al., 2013).
5.2.2. Balance studies in children
Using a randomised cross‐over design, Palacios et al. (2004) assessed sodium retention in 14 white and 22 black US girls (11–15 years) during 3 weeks on a ‘low’ (mean ± SEM: 1.31 ± 0.04 g (57 ± 2 mmol)/day) and 3 weeks on a ‘high’ (3.95 ± 0.05 g (172 ± 2 mmol)/day)) sodium intake, separated by a 2‐week wash‐out in which participants consumed self‐selected diets. The first week of each period served as an equilibration period. The study was conducted in summer time. Eight white and 15 black girls completed both periods, while six white and seven black girls completed one of the two periods only. Before the study initiation, subjects completed six 24‐h dietary recalls. Dietary intakes of protein, fat, fibre, potassium, calcium and phosphorus were controlled, at levels representative of usual intakes in this population. Faecal and 24‐h urine samples were collected daily for 20 days during each study period. Corrections for incomplete samples were applied based on the excretion of creatinine in urine and polyethylene glycol in faeces (‘normalised’ 24‐h pools). Whole‐body sweat was collected after 2 weeks of acclimatisation to the diet for 24 h by a whole‐body scrub‐down procedure, taking measures to minimise the contribution of exfoliated skin to the estimate. Mean sodium balance over the last 2 weeks of each period was calculated including sodium losses in sweat. Aldosterone concentration and renin activity were measured in plasma sampled upon awakening (‘resting’ samples) and 2 h later (‘stimulated’ samples).
Mean (± SD) sodium balance was positive during ‘low’ and ‘high’ sodium intakes (+0.4 ± 0.31 g/day among blacks (n = 19) and +0.2 ± 0.14 g/day among whites (n = 12) on ‘low’ intake and +1.0 ± 0.61 g/day among blacks (n = 19) and +0.3 ± 0.28 g/day among whites (n = 10) on ‘high’ intake). Results were similar when urine and faecal samples were not normalised and when only subjects completing both dietary periods were analysed. The Panel notes that this study in adolescent girls reports positive mean sodium balances with a daily sodium intake of 1.31 g (57 mmol). Plasma aldosterone concentrations were elevated at the end of the ‘low’ sodium period compared with the ‘high’ sodium period (Appendix F.1).
5.2.3. Mechanistic considerations
In daily life, the body constantly adapts to maintain sodium (and water) homeostasis in response to changing environmental conditions. Sodium balance is tightly regulated through neural and hormonal mechanisms (including the SNS and the RAAS) affecting its renal reabsorption and intake (‘salt appetite’) (Section 2.3.4). Several meta‐analyses of human intervention studies have investigated the effect of a reduction in sodium intake on these systems (Graudal et al., 2011; WHO, 2012c; He et al., 2013) (Table F.1 in Appendix F.2). In the meta‐analysis from He et al. (2013), which included most of the studies that were also considered in the other two meta‐analyses, a reduction in sodium intake, resulting in 24‐h urinary sodium excretion in the range of 50–100 mmol (1.2–2.3 g)/day, was associated with mean increases in plasma renin activity of 0.26 (95% CI 0.17, 0.36) ng/mL per h, plasma aldosterone concentration of 73.20 (95% CI 44.92, 101.48) pmol/L and plasma noradrenaline concentration of 31.67 (95% CI 6.57, 56.77) pg/mL). The effect estimate on plasma adrenaline concentration was 6.70 (95% CI –0.25, 13.64) pg/mL (He et al., 2013). The trials included in these analyses lasted from 4 to 11 weeks.
Table F.1.
Effect of sodium intake on blood catecholamines and aldosterone concentrations and renin activity – meta‐analyses of trials of at least 4 weeks
| Ref | Inclusion criteria | Included studies | N | Pooled effect (95% CI) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study type | Achieved sodium difference between experimental groups | Intervention duration | Participants | Co‐intervention | MacGregor et al. (1982) | Watt et al. (1983) | Andersson et al. (1984) | Richards et al. (1984) | Grobbee et al. (1987) | MacGregor et al. (1989) | Carney et al. (1991) | Singer et al. (1991) | Benetos et al. (1992) | Fotherby and Potter (1993) | Ruppert et al. (1993) | Schorr et al. (1996) | Ames (2001) | Cappuccio et al. (1997) | Nowson et al. (2003) | Gates et al. (2004) | Swift et al. (2005) | Melander et al. (2007) | He et al. (2009) | ||||
| He et al. (2013) | RCTs allocating to a modestly reduced salt intake or usual salt intake | A reduction in 24‐h urinary sodium within the range of 40–120 mmol | At least 4 weeks |
|
Studies with concomitant interventions (i.e. non‐pharmacological interventions, antihypertensive or other medications) were excluded | RA | X | X | X | X | X | X | X | X | X | X | X | X | X | 13 | 0.26 (0.17, 0.36) ng/mL per h | ||||||
| ALD | X | X | X | X | X | X | X | X | 8 | 73.20 (44.92, 101.48) pmol/L | |||||||||||||||||
| NOR | X | X | X | X | X | X | 6 | 31.67 (6.57, 56.77) pg/mL | |||||||||||||||||||
| ADR | X | X | X | X | 4 | 6.70 (−0.25, 13.64) pg/mL | |||||||||||||||||||||
| Graudal et al. (2011) | RCTs allocating subjects to either a low or a high sodium diet | Any | Any. Subgroup analysis restricted to studies ≥ 4 weeks |
|
Studies with concomitant interventions were included if the co‐intervention was identical during the low and high sodium diet | RAa | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 14 | 0.47 (0.35, 0.60)b | ||||
| ALD | X | X | X | X | X | X | X | X | X | 9 | 0.70 (0.37, 1.04)b | ||||||||||||||||
| NOR | X | X | X | X | X | X | 6 | 0.06 (−0.19, 0.32)b | |||||||||||||||||||
| ADR | X | X | X | X | X | 5 | 0.24 (−0.04, 0.52)b | ||||||||||||||||||||
| WHO (2012c) | RCTs which included an intervention that planned to or achieved a reduced sodium intake | A reduction in 24‐h urinary sodium > 40 mmol/day | At least 4 weeks |
|
Studies with concomitant interventions (e.g. physical activity, medical treatment (e.g. diuretics or beta blockers)) were included if the co‐intervention was identical in the intervention and control groups | RA | Not assessed | ||||||||||||||||||||
| ALD | Not assessed | ||||||||||||||||||||||||||
| NOR | X | X | X | X | X | X | X | 7 | 8.23 (−27.84, 44.29) pg/mL | ||||||||||||||||||
| ADR | X | X | X | X | 4 | 6.90 (−2.17, 15.96) pg/mL | |||||||||||||||||||||
ADR: adrenaline; ALD: aldosterone; BP: blood pressure; NOR: noradrenaline; RA: renin activity; RCT: randomised controlled trial.
Through their systematic review, Graudal et al. (2011) retrieved 15 trials lasting ≥ 4 weeks that reported on RA. However, the paper indicates that the pooled analysis for the subgroup of studies lasting ≥ 4 weeks included 14 trials. The list of references included in the pooled analysis is not provided.
Standardised mean difference, calculated for outcome measures with different units. The difference in effect between two treatments is divided by the standard deviation of the measurements.
Cross‐sectional analyses of data from subjects with different levels of sodium intake indicate curvilinear relationships between sodium intake, plasma renin activity and urinary aldosterone excretion; both increased sharply with 24‐h urinary sodium excretion < 50 to 100 mmol/day (1.2 to 2.3 g/day) (Laragh et al., 1972; Laragh and Sealey, 2011).
In a study of sodium metabolism in young men consuming controlled amounts of sodium for several weeks (Rakova et al., 2013), average daily urinary excretion of aldosterone increased with gradual sodium restriction from 4.9 g/day to 2.5 g/day, each level maintained constant for > 29 days (Table F.2 in Appendix F.2). In contrast, a decrease in urinary levels of glucocorticoids in urine was observed. On average, 95% of dietary sodium was recovered in urine over each dietary phase, which suggests that sodium balance was achieved at each level of sodium intake, through the activation of hormonal regulatory mechanisms. A high day‐to‐day variability in urinary excretion of sodium was also observed, as well as weekly and monthly rhythms of fluctuations in aldosterone and glucocorticoids that correlated with body sodium accumulation (apparent positive balance) and release (apparent negative balance), independent of sodium intake.
Table F.2.
Results from long‐term metabolic study from Rakova et al. (2013)
| Refs | Subjects | Conditions | Duration – NaCl target intake | Na intake (g/day) | Na excretiona (g/day) | Diff = Na intake–UNa (mean ± SD)b | Regulatory hormones (in urine) (mean ± SD)b | Comment | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Aldosterone (μg/day) | Cortisol (μg/day) | Cortisone (μg/day) | ||||||||
| Rakova et al. (2013) | Mars105 c 4 men |
Controlled (18–25°C; no rigorous activities) |
105 days, 3 periods: 35 day – 12 g NaCl 35 day – 9 g NaCl 29 day – 6 g NaCl |
4.9 g/day 3.6 g/day 2.5 g/day |
4.3 ± 0.8 g/day 3.3 ± 0.7 g/day 2.6 ± 0.9 g/day |
+0.5 ± 0.9 +0.3 ± 0.7 –0.1 ± 0.9 |
12.0 ± 4.8 13.5 ± 4.7 15.3 ± 5.5 |
24.8 ± 10.1 21.6 ± 8.4 18.8 ± 6.6 |
83.8 ± 21.9 73.3 ± 20.5 72.3 ± 21.1 |
Weekly and monthly rhythms of body Na accumulation and release correlated with fluctuations in aldosterone, cortisol and cortisone – independent of Na intake |
| Mars520 c 6 men | As above |
205 days, 4 periods: 61 day – 12 g NaCl 60 day – 9 g NaCl 60 day – 6 g NaCl 36 day – 12 g NaCl |
4.5 g/day 3.3 g/day 2.2 g/day 4.4 g/day |
4.2 ± 0.8 g/day 2.9 ± 0.8 g/day 1.9 ± 0.6 g/day 4.1 ± 1.0 g/day |
0.2 ± 0.9 0.4 ± 0.9 0.3 ± 0.8 0.3 ± 1.1 |
11.4 ± 4.8 13.5 ± 4.4 15.4 ± 5.3 9.2 ± 3.6 |
24.0 ± 7.2 20.1 ± 5.0 17.4 ± 5.4 22.8 ± 7.4 |
76.3 ± 16.3 63.7 ± 16.4 68.7 ± 18.9 71.7 ± 19.2 |
||
Na: sodium; NaCl: sodium chloride; SD: standard deviation; UNa: sodium urinary excretion.
Sweat and faecal losses not measured.
Mean over the last 29 days of each period.
The paper reports on two studies named Mars105 and Mars 520.
5.2.4. Conclusions
Mean sodium intake assessed in eligible balance studies ranged between 1.5 g and 4.9 g/day in adults (three studies) and between 1.31 and 3.95 g/day in adolescents (one study). No data are available for sodium intakes below 1.3 g/day (adolescents) and 1.5 g/day (adults).
The Panel notes that adaptation mechanisms triggered by neural and hormonal signals enable the maintenance of sodium balance over a wide range of sodium intakes. The concerns about using balance studies to establish nutrient requirements are particularly relevant for sodium. Recent data from a long‐term study of sodium metabolism suggest that rhythmical variations in the sodium body pools independent of sodium intake may occur. This complicates the interpretation of balance studies. Overall, the Panel considers that balance studies cannot be used to determine sodium requirements, but could be used to inform about the levels of sodium intake that are adequate to maintain a null sodium balance. The Panel also notes the lack of data on the health effects of a sustained activation of the SNS and the RAAS system in the general population.
5.3. Indicators of sodium requirement in pregnancy and lactation
Total body sodium is estimated to increase by 23 g (1,000 mmol) during pregnancy, and has been assumed to represent an additional daily requirement of 70–90 mg (3–4 mmol) of sodium (Pitkin et al., 1972). However, the increase is not evenly distributed throughout pregnancy. The need arises from the expansion of the maternal ECF volume (approximating 3.0 L) that comprises 60% of the extra requirement, and the remaining 40% meets the compositional requirements of the fetus, placenta and the amniotic fluid. The changes in the volume and distribution of body water start early in pregnancy and are associated with the initial endocrine response to pregnancy that, among other things, increases the homeostatic retention of sodium (Section 2.4).
Oliver et al. (1981) undertook a comparative study among women of two South American tribes to understand how hormonal adaptation impacts on sodium balance during pregnancy and lactation. The first group consisted of pregnant (n = 4), non‐pregnant/lactating (n = 16) and non‐pregnant/non‐lactating (n = 16) Yanomamo Indian women living in villages in northern Brazil and southern Venezuela with no access to sodium chloride. The second group included pregnant (n = 7) and non‐pregnant (n = 9) Guaymi Indian women of Panama, who had free access to sodium chloride and used it to cook vegetables and preserve meat. The second (control) group was included to address concerns about genetic susceptibility. In both groups, measurements of arterial blood pressure, blood and spot urine samples were collected between 08:00 and 10:00 h in the morning. Breast milk samples were collected only among the Yanomamo women. Urinary sodium concentrations among Yanomamo women ranged between 0.7 mmol/L (pregnant) and 1.4 mmol/L (non‐pregnant/non‐lactating women) and between 77.8 mmol/L (pregnant) and 119.0 mmol/L (non‐pregnant) among Guaymi women. In breast milk, the concentrations of sodium ranged between 5 and 9 mmol/L (0.1–0.2 g/L) meaning that it was comparable to the sodium concentration in sodium‐affluent regions. The pregnant Yanomamo had high urinary concentrations of aldosterone, associated with higher plasma renin activities and serum aldosterone concentrations than in all other subjects. Pregnant Guaymi also had elevated serum and urinary aldosterone concentrations, but significantly lower (p < 0.001) than in Yanomamo women. Prolonged lactation in the Yanomamo was associated with elevation of plasma renin activity, serum and urinary aldosterone concentration compared with the Guaymi, but was not higher than in non‐lactating Yanomamo women. Based on evidence from two Indian tribes living in South America, the Panel notes that pregnancy in a salt‐poor environment is associated with hormonal adaptation associated with sodium retention, similar to non‐pregnant adults (Allsopp et al., 1998).
The Panel considers that the requirement for the daily accretion rate of sodium in fetal and maternal tissues can be met by the adaptive changes that maintain sodium homeostasis during pregnancy.
Sodium losses in human milk are relatively small (a few mmol/day). The concentration of sodium in human milk is not influenced by maternal sodium intake (Section 2.3.4.4). The Panel notes that there is no evidence that sodium requirement of lactating women differs from the requirement of non‐lactating women.
5.4. Indicators of sodium requirement in infants and children
Sodium is retained with growth of tissues during child development (Section 5.2.2).
The neonate is in a state of relative total body water and ECF excess, and the typical diuresis accompanied by sodium loss, which occurs immediately after birth, is considered physiological. During the first days of postnatal life, fractional excretion of sodium decreases progressively, and a state of positive balance is reached (Ross et al., 1977; Al‐Dahhan et al., 1984). Multiple endocrine systems are integrated with the renal regulatory mechanisms to enhance sodium retention in infants, in part through an activation of the RAAS (Al‐Dahhan et al., 1983; Chevalier, 2001).
As few data were available to calculate reference values for infants, the UK COMA Panel on Dietary Reference Values (1991) used a factorial approach based on intracellular and ECF concentrations of sodium with allowances for growth‐related changes in the volumes of these compartments according to body compositional reference data (Widdowson, 1980). Values for the first 6 months of life were also based on reference values for the composition of breast milk and a presumed intake of 850 mL/day. As there was appreciable uncertainty in these data, the derived values were regarded as LNRIs rather than ARs. The SCF (1993) considered these data as too uncertain to set reference intakes for sodium in infants and young children.
Fomon (1993) also attempted to estimate the physiological requirement for sodium of infants via a factorial approach by adding requirements for growth to obligatory losses. Sodium accretion with growth was estimated from data on sodium content of the whole body and of cellular and extracellular water, taking into account the quantities of these fluids at different ages of reference infants, and from the amount of sodium in osseous mineral at different ages (Pitts, 1974; Forbes, 1975; Fomon et al., 1982; Widdowson, 1982; Boskey, 1988). It resulted in a sodium requirement for growth of 27 mg (1.2 mmol)/day for infants aged 0–4 months and 16 mg (0.7 mmol)/day for infants aged 4–12 months, respectively. Sodium losses via the skin, dependent on the body surface area, were estimated to be 24 mg (1.0 mmol) from 0 to 4 months and 30 mg (1.3 mmol)/day from 4 to 12 months, respectively (Forbes, 1975). Assuming an absorption of 95%, 54 mg (2.3 mmol) of dietary sodium/day were calculated to be needed at the age of 0–4 months, to account for growth demands and replace losses, and 48 mg (2.1 mmol)/day for the age 4–12 months. Fomon (1993) proposed a daily recommended intake of 80 mg (3.5 mmol) of sodium for infants throughout the first year of life in consideration of both the uncertainty created by the limited data available and the need for assumptions to be made, and the necessity to provide for individual variability in requirements. The Panel notes that the quantity of sodium provided by human milk during the first 6 months of life (i.e. 120 mg/day assuming a volume of 0.8 L/day and a sodium concentration of 150 mg/L (see Section 2.3.4.4)) is higher than this calculated physiological requirement.
5.5. Sodium intake and health consequences
Three categories of health outcomes were selected as the most suitable to inform the setting of DRVs: blood pressure, cardiovascular disease‐related endpoints and bone health. They were selected on the basis of their biological relevance for the general healthy population, the biological plausibility of their relationship with sodium intake, and the type of evidence (i.e. RCTs and/or prospective observational studies) (see protocol, Annex A). Systematic reviews of the literature were conducted to characterise the relationship between sodium intake and these outcomes. The subquestions addressed by the systematic reviews are reported in Table 7.
Table 7.
Subquestions addressed by the systematic reviews on sodium
| No | Subquestion |
|---|---|
| 1 | What is the relationship between sodium intake and blood pressure in humans? |
| 2 | What is the relationship between sodium intake and cardiovascular disease‐related outcomes in humans? |
| 3 | What is the relationship in childrena between sodium intake and bone mineral density (BMD) and/or bone mineral content (BMC)? |
| 4 | What is the relationship in adultsb between sodium intake and BMD? |
| 5 | What is the relationship in adultsb between sodium intake and the risk of osteoporotic fractures? |
6 months to < 18 years.
≥ 18 years.
Eligible study design included randomised controlled parallel (RCTs) or crossover trials (with a wash‐out period of any duration) and prospective studies including cohort studies, nested case–control and case–cohort studies. Trials were eligible if the intervention consisted in a change in sodium intake compared with usual diet or placebo.
In relation to blood pressure and CVD‐related outcomes, trials with concomitant interventions deemed to affect the outcome of interest were excluded. For bone‐related outcomes, trials in which the same concomitant intervention was applied to all study groups were included. On study duration, trials on blood pressure with a minimum duration of 4 weeks and trials on CVD outcomes with a minimum duration of 6 months were eligible. Trials on BMD or risk of osteoporotic fractures in adults had to last at least 1 year.
On subject characteristics, eligible studies involved adults (≥ 18 years) and children (6 months to < 18 years) from the general population. Trials including diseased individuals, individuals on a therapeutic diet (including weight loss diet), hypertensive subjects on blood pressure‐lowering medications, trials in pregnant women and trials with specialised exercise (e.g. athletes, militaries) and extreme environmental conditions (e.g. prolonged exposure to unusually high temperature) were excluded. Observational studies that did not explicitly exclude prevalent (i.e. pre‐existing) cases of the outcome of interest at baseline were excluded.
Studies were eligible if sodium intake was assessed based on urinary sodium excretion calculated from single or multiple 24‐h urine collection(s). Other types of sodium intake measurements were excluded.
The literature searches were conducted in three electronic bibliographic databases (Cochrane library, Embase, PubMed) and two resources indexing PhD theses (DART, PQDT Open) in February 2018, and updated in October 2018. Search strings are described in the protocol (Annex A). Two separate searches were performed to address subquestions 1 and 2 (Appendix G.1) and subquestions 3–5 (Appendix G.2). Two independent reviewers screened the literature identified through the searches.
In relation to subquestions 1 and 2 (blood pressure, hypertension and CVD outcomes), 7,141 unique references were identified after removing duplicates (see PRISMA Chart, Appendix G.1.1). The title and abstract screening left 402 relevant articles that underwent a full‐text review. Of those, 357 were excluded (Appendix G.1.2). A total of 45 publications reporting on 36 RCTs and 9 prospective observational studies were included. Screening of the reference lists of these publications (877 references) did not yield additional eligible studies. Also, no additional eligible studies were identified among the studies included in similar systematic reviews (Appendix G.1.3).
In relation to subquestions 3–5 (bone health), 1,732 unique references were identified after removing duplicates (see PRISMA Chart, Appendix G.2.1). The title and abstract screening identified 40 articles that underwent a full‐text review. In total, 38 articles were excluded (Appendix G.2.2). A final two articles were included (Devine et al., 1995; Ilich et al., 2010). No additional eligible study was found from the reference lists of the two eligible articles or from the list of articles included in a systematic review with a similar research question identified through the search (Appendix G.2.3).
Risk of bias (RoB) in eligible studies was appraised using tailored versions of the OHAT‐NTP RoB tool (OHAT/NTP, 2015) and studies were classified as being at low (tier 1), moderate (tier 2) or high (tier 3) RoB (Appendix G.3).
5.5.1. Blood pressure and hypertension
5.5.1.1. Office blood pressure in children
The characteristics of eligible studies and the outcome of the RoB appraisal are presented in Appendices H.1 and H.2, respectively.
Experimental studies
Two RCTs met the eligibility criteria described in the review protocol (Miller et al., 1988; He et al., 2015).
In a 12‐week trial in the UK, school‐age identical pairs of twins and their families received instructions to restrict their sodium intake (Miller et al., 1988). During the middle 4‐week period, one member of each twin pair, chosen at random, received a daily NaCl supplement designed to return sodium intake to baseline levels. In total, 88 twins completed the study. During the supplement period, higher UNa was observed in the sodium‐supplemented group (72.1 mmol/24 h) compared with the control group (44.4 mmol/24 h), while there were no between‐group differences in SBP (mean (SEM) 0.3 (0.6) mm Hg) and DBP (−0.2 (0.7) mm Hg). Overall, this study was judged to be at low RoB (tier 1).
In a cluster‐randomised controlled study, 28 primary schools in China were randomly assigned to an intervention consisting of a ‘low‐salt’ education programme for 3.5 months (n = 141 children, mean (SD) age: 10.2 (0.5) years) or to their usual nutrition education programme with no particular reference to sodium chloride intake (n = 138 children, 10.0 (0.5) years) (He et al., 2015). Baseline mean (SE) UNa were 116.7 (5.2) mmol/day and 124.2 (5.1) mmol/day in the control and intervention group, respectively. A reduction in UNa was observed in the intervention group (−12.1 (95% CI −19.9, −4.2) mmol/24 h), whereas sodium excretion increased in the control group (+20.5 (12.6, 28.4) mmol/24 h). The adjusted difference between the two groups in the change of sodium excretion was −33.3 (−44.2, −22.3) mmol/24 h (p < 0.001). In both groups, SBP and DBP increased between baseline and the end of intervention. The adjusted difference between the two groups in the blood pressure change from baseline (intervention vs control) was −0.8 (−3.0, 1.5) mm Hg for SBP (p = 0.51) and −1.2 (−3.7, 1.2) mm Hg for DBP (p = 0.33). This study was judged to be at moderate RoB (tier 2), in particular due to a potential RoB in relation to the outcome assessment (unblinded outcome assessors).
Observational studies
Two publications from the DONALD prospective cohort study met the eligibility criteria set out in the review protocol (Shi et al., 2014; Krupp et al., 2015). DONALD is an open cohort study implemented in Germany since 1985. Examinations are conducted at ages 3, 6, 9, 12, 18 and 24 months and then annually until young adulthood and comprise anthropometry, a 3‐day weighed dietary record, a 24‐h urine sample (from age 3–4 years onwards), medical examinations and parental interviews.
Shi et al. (2014) analysed data from 435 healthy participants, for whom at least three repeated measurements of blood pressure had been taken and who had provided three parallel 24‐h urine samples. The median age was 6 years at baseline and 16 years during the last assessment. In boys, the median (25–75th percentile) UNa was 67.4 (50.6–89.9) mmol/day and 131 (96.9–176) mmol/day during the first and last assessment, respectively. Corresponding values in girls were 58.7 (45.9–74.5) mmol/day and 108 (81.7–133) mmol/day. In the prepubertal stage, no association between changes in UNa and SBP or DBP was observed. In the pubertal stage, the association (β (95% CI)) between intraindividual changes in UNa and blood pressure was 0.1 (−0.004, 0.2) mm Hg for SBP (p = 0.06) and 0.1 (−0.02, 0.2) mm Hg for DBP (p = 0.09) by 1 mmol/MJ per day increase in UNa. When analysing differences in UNa and mean blood pressure between the subjects, the associations were 0.1 (−0.1, 0.4) mm Hg for SBP (p = 0.3) and 0.2 (−0.4, 0.04) mm Hg for DBP (p = 0.1). This study was judged to be at low RoB (tier 1).
Among the DONALD participants who had already reached adult age, Krupp et al. (2015) selected 206 participants who had three repeated urinary, dietary and blood pressure measurements during adolescence (11–16 years) and one blood pressure measurement in young adulthood (18–25 years). The estimated mean (SD) sodium chloride excretion was 116 (27) and 105 (32) mmol/day in boys and girls, respectively. In multivariable linear regression models, there was a positive association between UNa (per 1 mmol/day increase) and adult SBP in boys (β (95% CI) = 0.10 (0.03, 0.18), p = 0.01) but not in girls (−0.05 (−0.11, 0.02), p = 0.1). No association was found between UNa during adolescence and adult DBP (boys: 0.02 (−0.08, 0.04), p = 0.6; girls: 0.02 (−0.03, 0.08), p = 0.4). This study was judged to be at low RoB (tier 1).
Conclusion
The Panel notes that two RCTs (tiers 1 and 2) did not provide evidence for an effect of sodium reduction on blood pressure in school‐age children. The observational study (tier 1) showed no significant association between UNa and blood pressure in pre‐pubertal and pubertal children and provided weak evidence for a positive association between UNa during adolescence and SBP in adulthood.
5.5.1.2. Office blood pressure in adults
The characteristics of eligible studies and the outcome of the RoB appraisal are presented in Appendices H.1 and H.2, respectively.
Experimental evidence
In line with the protocol (Annex A), eligible RCTs were used to conduct quantitative analyses with the aim to characterise the dose–response relationship between sodium intake and blood pressure. The analysis report is provided in Annex B. The main results are outlined below and Appendices I.3 and I.4.
In total, 32 RCTs, providing 35 comparisons, met the eligibility criteria. Seven were parallel RCTs, including one cluster‐randomised trial and 25 were crossover RCTs. In total, 25 trials modified sodium intake by providing subjects with NaCl or placebo tablets or a controlled diet with various amounts of Na (‘feeding trials’), while seven trials used sodium reduction counselling (‘counselling trials’). The between‐group differences in mean UNa ranged from 13.3 to 285 mmol/day, with a median mean value of 72 mmol/day. The study size ranged from 11 to 1,159 participants and the duration of the intervention lasted from 4 weeks to 36 months. In total, 17 studies involved hypertensive individuals, eight studies involved normotensive individuals and seven studies involved mixed populations. In total, 27 studies were classified in tier 1 and five studies in tier 2.
-
1
Meta‐analyses at study level
The results of the random‐effects meta‐analyses of trials of effects of sodium reduction on blood pressure are presented in Appendix I.3 and Annex B.
A random‐effects meta‐analysis of the 32 eligible RCTs showed significant effects of sodium reduction on SBP (−3.9 (95% CI −5.1, −2.8) mm Hg; I2 61.9%, p < 0.001) and DBP (−2.0 (−2.8, −1.2) mm Hg; I2 60.6%, p < 0.001) (Figures I.1 and I.5).
Figure I.1.
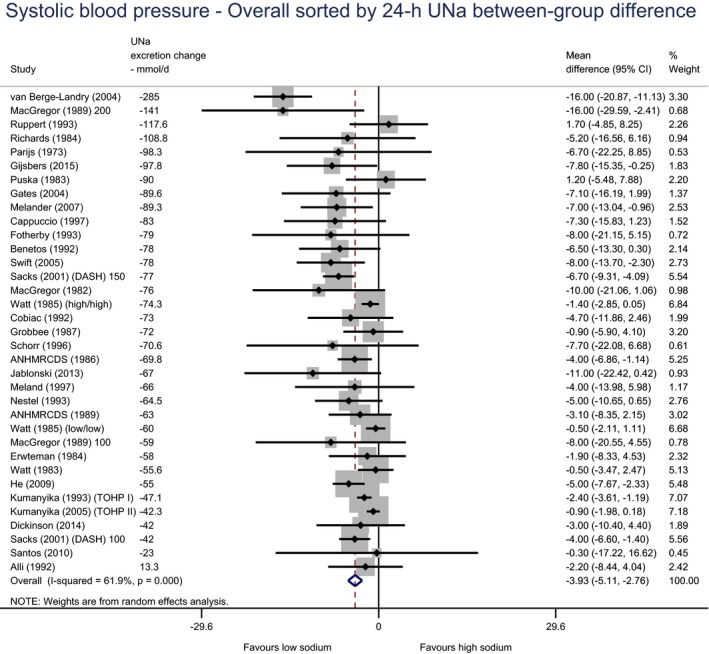
sbp – all adults, sorted by 24‐h UNa between‐group difference
- 95% CI: 95% confidence interval; UNa: sodium urinary excretion.
Figure I.5.
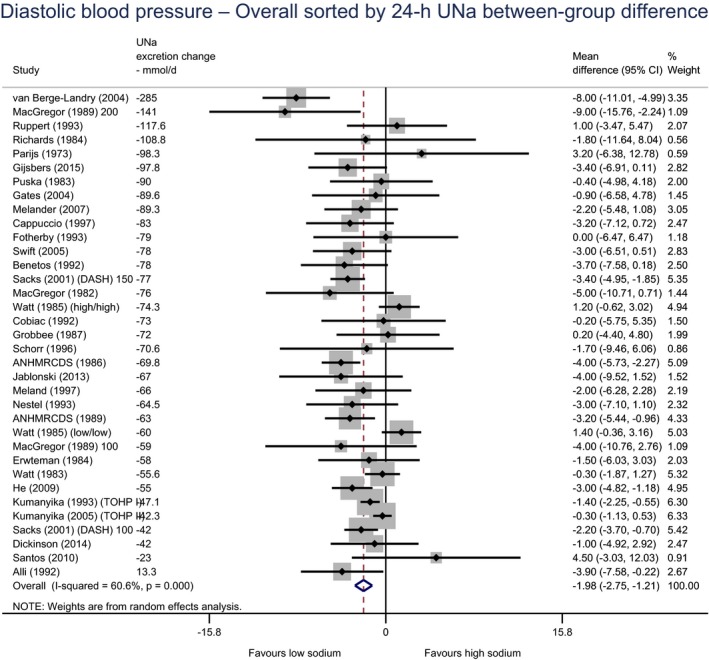
DBP – all adults, sorted by 24‐h UNa between‐group difference
- 95% CI: 95% confidence interval; UNa: sodium urinary excretion.
Contextual sources of heterogeneity were explored in subgroup analyses. A larger effect was found in hypertensive than normotensive individuals, for both SBP (hypertensive: −5.6 (−8.1, −3.1) mm Hg vs normotensive: −2.0 (−3.3, −0.7) mm Hg) and DBP (hypertensive: −2.9 (−4.2, −1.6) mm Hg vs normotensive: −0.9 (−1.6, −0.2) mm Hg) (Figures I.2 and I.6). The effect of reduction in sodium intake was higher among subjects aged 50 years or more (SBP −6.1 (−8.2, −4.1) mm Hg; DBP −2.9 (−4.0, −1.9) mm Hg) than among subjects younger than 50 years (SBP −2.2 (−3.3, −1.1) mm Hg; DBP −1.0 (−2.0, 0.0) (Figures I.3 and I.7). With respect to sex, a higher effect was found in studies which consisted mostly of men (i.e. > 55% of total sample) than in studies which consisted mostly of women. The exploration of the potential moderating effects of ethnicity, BMI or potassium intake was limited by the small number of studies for which information on these factors was available (Tables I.1 and I.2 in Appendix I.3).
Figure I.2.
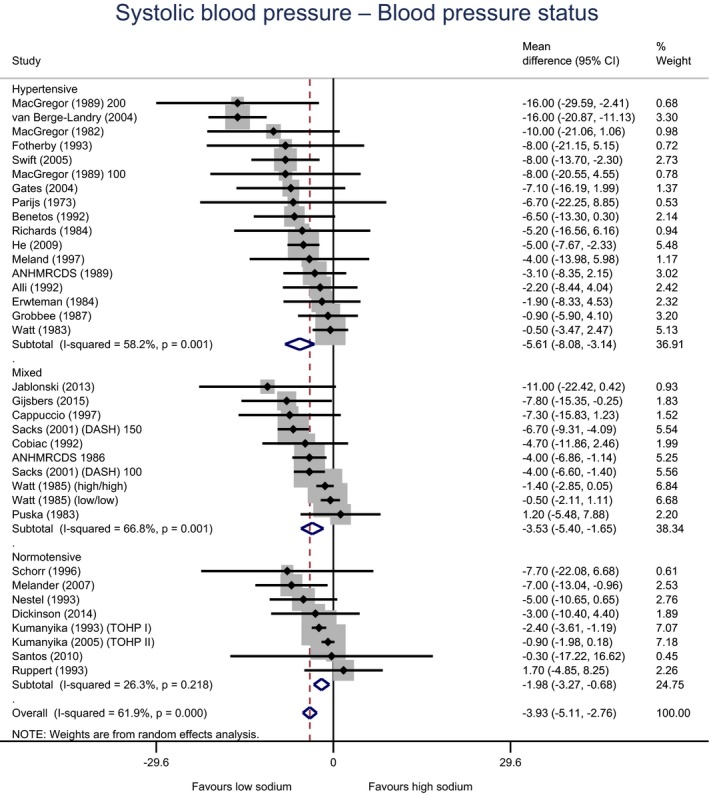
SBP – all adults, subgroups by blood pressure status
- 95% CI: 95% confidence interval.
Figure I.6.
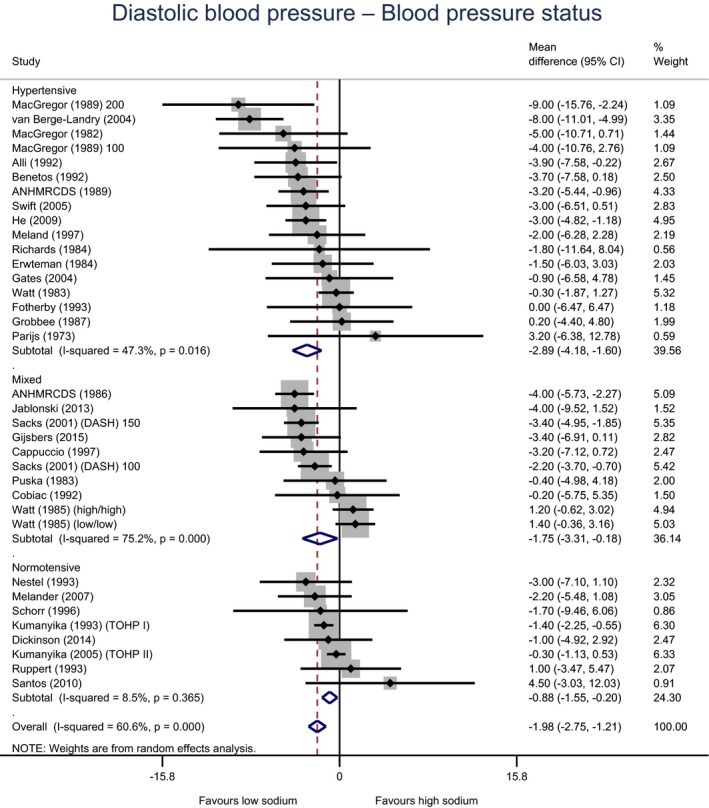
DBP – all adults, subgroups by blood pressure status
- 95% CI: 95% confidence interval.
Figure I.3.
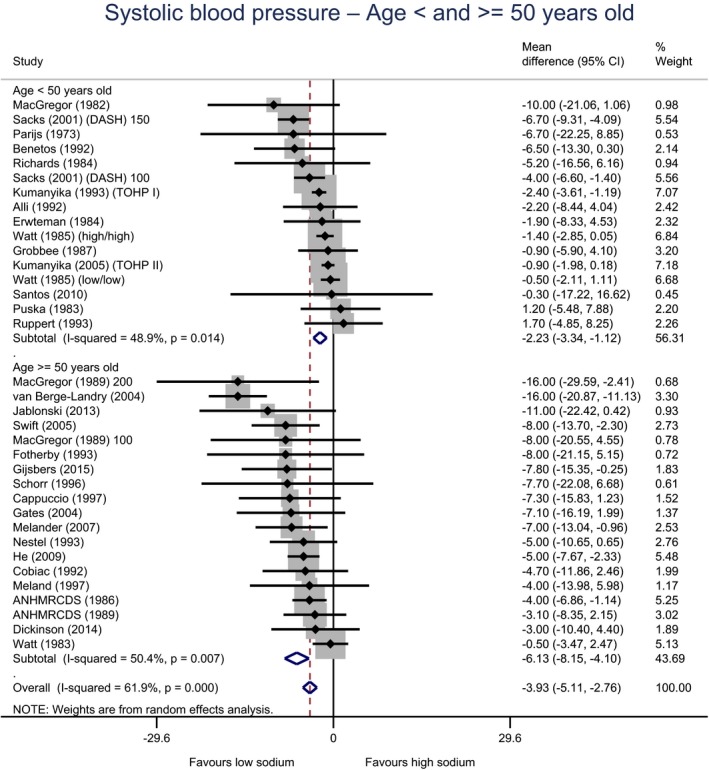
SBP – all adults, subgroups by age < or ≥ 50 years
- 95% CI: 95% confidence interval.
Figure I.7.
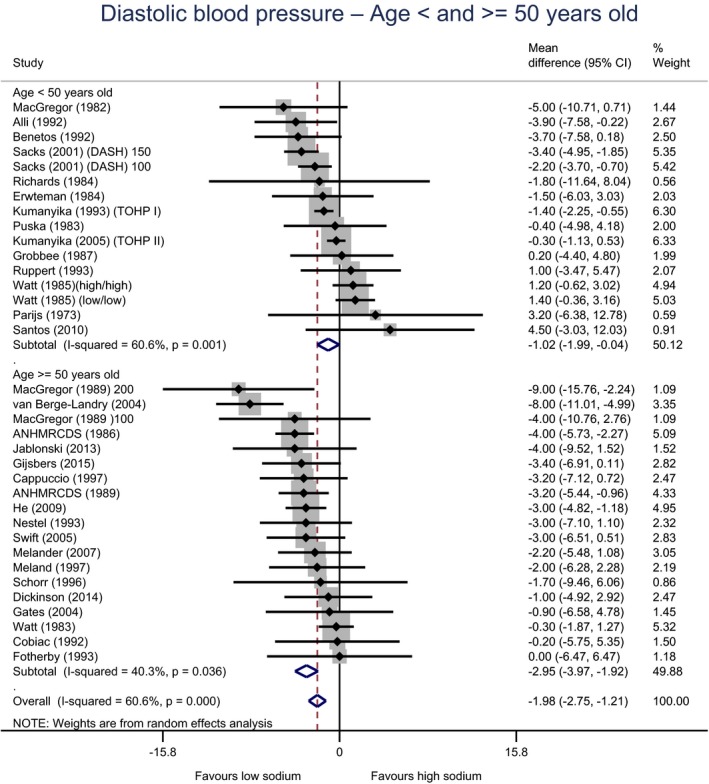
DBP – all adults, subgroups by age < or ≥ 50 years
- 95% CI: 95% confidence interval.
On the identified methodological sources of heterogeneity, larger effects were found in crossover compared with parallel trials, when measuring blood pressure in supine position compared with sitting position, (Tables I.1 and I.2). The effect of sodium reduction was smaller in trials of longer duration (≥ 1 year) compared with trials of shorter duration (4 weeks) (Figures I.4 and I.8). After exclusion of the van Berge‐Landry and James (2004) study as an outlier, the effect of sodium reduction on SBP was −1.8 (−2.9, −0.8) mm Hg in ‘counselling’ trials and −4.0 (−5.3, −2.7) mm Hg in ‘feeding’ trials. The respective values for DBP were −1.7 (−2.6, −0.9) mm Hg and −1.8 (−3.2, −0.5) mm Hg.
Figure I.4.
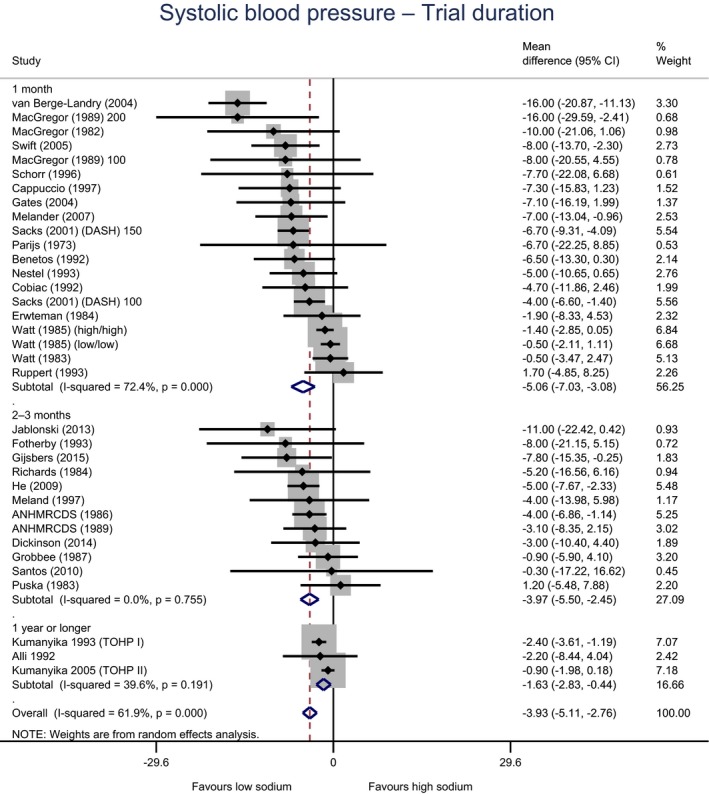
SBP – all adults, subgroups by trial duration
- 95% CI: 95% confidence interval.
Figure I.8.
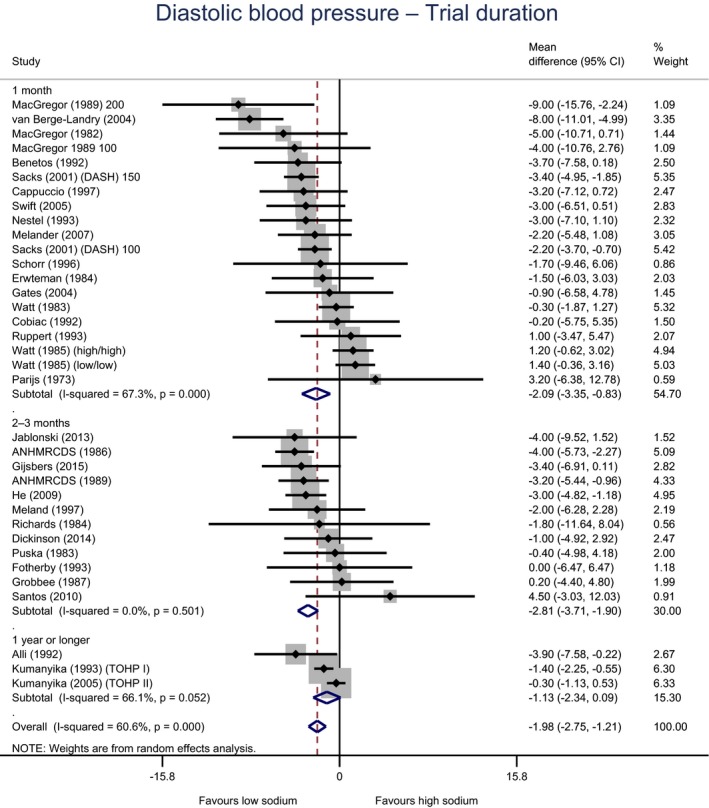
DBP – all adults, subgroups by trial duration
- 95% CI: 95% confidence interval.
-
2
Meta‐analyses, meta‐regression and dose–response modelling at arm level
The results of the mixed‐effects meta‐regression models of the relationship between 24‐h urinary sodium excretion and absolute blood pressure levels are presented in Appendix I.4 and Annex B.
To investigate the dose–response association between mean absolute values of 24‐h urinary sodium excretion and mean absolute values of blood pressure, all arms (68) from the eligible RCTs (32) were subjected to meta‐analysis assuming a random‐effects model.
The pooled mean estimate across arms was 137.1 (134.2–140.1) mm Hg for SBP and 84.0 (82.0–85.9) mm Hg for DBP; subgroup analyses were repeated at the arm level applying the same a priori categorisations of relevant potential modifiers to identify candidate moderators to be included in the multivariable models, producing comparable results to those from the meta‐analyses at study level.
In total, 60 points (arms as unit of analysis) from 28 RCTs were included in the final dose–response models. Two RCTs were excluded from the meta‐analysis pool because of missing information on age (Puska et al., 1983; Richards et al., 1984). One (Alli et al., 1992) was excluded after thorough consideration of some inconsistencies in the design and results of the study. Van Berge‐Landry and James (2004) were selected only for sensitivity analysis, given the fact that it was the only study with UNa values in the control and intervention arms well beyond the range covered by all other trials (achieved urinary excretion: 309 and 24 mmol/day at the end of the ‘high’ and ‘low’ sodium interventions, respectively). Two arms were included from all trials except for MacGregor et al. (1989) (three arms), Sacks et al. (2001) (three arms) and Watt et al. (1985) (four arms). After the exclusion of the eight arms, 14 were from parallel RCTs, 46 were from crossover RCTs and none from cluster‐randomised trials.
Fifty‐two arms were from ‘feeding trials’ and eight arms from ‘counselling trials’. Mean 24‐h sodium excretion, once van Berge‐Landry was excluded, ranged from 49.0 to 202.9 mmol/day, with a median of 126.7 mmol/day. The arm size ranged from 10 to 515 participants, who were hypertensive in 33 out of the 60 arms.
Mixed‐effects meta‐regression models were fitted to account for the multilevel structure in the data, with arms nested within studies; two random effects (intercepts) on arm and study were specified and five fixed effects were included from the list of potential moderators tested in univariate meta‐regressions.
Different functional forms were explored for the shape of the dose–response relationship; non‐linearities as tested by fitting restricted cubic and linear splines were not statistically significant once both random effects were specified in the models.
The final set of moderators included in both SBP and DBP models was: age at baseline (< 40 years old (reference), 40–49, 50–59, ≥ 60 years old); blood pressure status at baseline (normotensive (reference) hypertensive); blood pressure measurement method (supine (reference), sitting); mean urinary sodium excretion at baseline (< 100 mmol/day (reference), 100–149, ≥ 150 mmol/day); and specific trial design (no run‐in (reference), run‐in normal diet, run‐in low‐sodium diet) (Tables I.3 and I.4 in Appendix I.4).
Table I.3.
Multivariable mixed‐effects meta‐regression model on SBP, only fixed effects reported
| Covariate | β coefficient | Std. Err. | p > |z| | 95% CI |
|---|---|---|---|---|
| Mean UNa – per 100 mmol/day | 5.3 | 0.8 | < 0.001 | (3.6 to 6.9) |
| Age at baseline | ||||
| Age < 40 years olda | 0 | |||
| Age 40–49 years old | 18.2 | 4.4 | < 0.001 | (9.6 to 26.9) |
| Age 50–59 years old | 11.6 | 4.7 | 0.013 | (2.4 to 20.7) |
| Age ≥ 60 years old | 12.4 | 4.2 | 0.003 | (4.3 to 20.6) |
| Blood pressure status | ||||
| Normotensivea | 0 | |||
| Hypertensive | 11.4 | 4.5 | 0.011 | (2.6 to 20.1) |
| UNa at baselineb | ||||
| < 100 mmol/daya | 0 | |||
| 100–149 mmol/day | 25.2 | 6.3 | < 0.001 | (12.7 to 37.6) |
| ≥150 mmol/day | 18.7 | 6.4 | 0.004 | (6.0 to 31.3) |
| Not reported | 13.4 | 5.3 | 0.012 | (3.0 to 23.8) |
| BP measurement method | ||||
| Point office, supinea | 0 | |||
| Point office, sitting | −13.2 | 3.2 | < 0.001 | (−19.6 to −6.9) |
| Specific trial design | ||||
| No Run‐ina | 0 | |||
| Run‐in, Normal diet | 11.9 | 3.4 | < 0.001 | (5.3 to 18.5) |
| Run‐in, Low Na diet | 19.7 | 5.1 | < 0.001 | (9.7 to 29.8) |
| Constant | 95.5 | 6.8 | < 0.001 | (82.2 to 108.8) |
95% CI: 95% confidence interval; Std. Err.: standard error; UNa: sodium urinary excretion.
Reference category.
UNa at baseline: corresponds to UNa at the start of the intervention (i.e. after run‐in, where applicable).
Model centred at 49 mmol/day sodium excretion (minimum mean UNa observed in the data set); total heterogeneity (random effects on trial) estimated from null model = 275.2 (95% CI: 161.4–468.9), residual heterogeneity from full model = 33.4 (95% CI: 13.3–53.5).
Table I.4.
Multivariable mixed‐effects meta‐regression model on DBP, only fixed effects reported
| Covariate | β coefficient | Std. Err | p > |z| | 95% CI |
|---|---|---|---|---|
| Mean UNa – per 100 mmol/day | 2.6 | 0.5 | < 0.001 | (1.6 to 3.7) |
| Age at baseline | ||||
| Age < 40 years olda | 0 | |||
| Age 40–49 years old | 18.2 | 3.2 | < 0.001 | (11.9 to 24.6) |
| Age 50–59 years old | 11.8 | 3.5 | 0.001 | (5.1 to 18.6) |
| Age ≥ 60 years old | 7.8 | 3.1 | 0.011 | (1.8 to 13.8) |
| Blood pressure status | ||||
| Normotensivea | 0 | |||
| Hypertensive | 8.7 | 3.3 | 0.008 | (2.3 to 15.1) |
| UNa at baselineb | ||||
| < 100 mmol/daya | 0 | |||
| 100–149 mmol/day | 10.4 | 4.5 | 0.022 | (1.5 to 19.3) |
| ≥ 150 mmol/day | 10.2 | 4.7 | 0.028 | (1.1 to 19.4) |
| Not reported | 10.4 | 3.8 | 0.007 | (2.9 to 17.9) |
| BP measurement method | ||||
| Point office, supinea | 0 | |||
| Point office, sitting | −6.6 | 2.4 | 0.005 | (−11.3 to −2.0) |
| Specific trial design | ||||
| No Run‐ina | 0 | |||
| Run‐in, Normal diet | 7.9 | 2.5 | 0.002 | (3.0 to 12.7) |
| Run‐in, Low Na diet | 7.7 | 3.7 | 0.037 | (0.5 to 15.0) |
| Constant | 55.4 | 4.8 | < 0.001 | (45.9 to 64.8) |
BP: blood pressure; 95% CI: 95% confidence interval; Std. Err: standard error; UNa: sodium urinary excretion.
Reference category.
UNa at baseline: corresponds to UNa at the start of the intervention (i.e. after run‐in, where applicable).
Model centred at 49 mmol/day sodium excretion (minimum mean UNa observed in the data set); total heterogeneity (random effects on trial) estimated from null model = 129.1 (95% CI: 76.1–218.8), residual heterogeneity from full model = 18.8 (95% CI: 8.1–29.4).
Other variables, including potassium intake, BMI and ethnicity, did not explain a significant proportion of heterogeneity in a consistent manner in both SBP and DBP analyses and/or suffered from a high proportion of missing data, so they were not retained in the final models.
For each 100 mmol (2.3 g)/24‐h increase in mean UNa, holding all other covariates constant, mean SBP increased by 5.3 mm Hg (95% CI: 3.6–6.9 mm Hg) and mean DBP increased by 2.6 mm Hg (95% CI: 1.6–3.7 mm Hg) (Figures I.9 and I.10 in Appendix I.4). Similar effects were estimated in crude models (with no other covariates than mean sodium excretion).
Figure I.9.
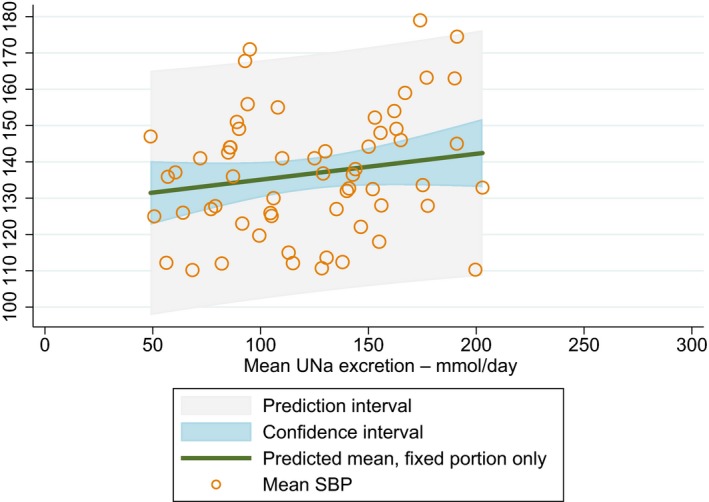
Linear dose–response relationship between mean urinary sodium excretion and mean SBP (mm Hg) from meta‐regression modelling of trials’ arms (crude model)
- Circles represents mean SBP by arm and their size is proportional to weights from the mixed‐effects model. The slope from the full model with moderators did not differ substantially (UNa unadjusted coefficient: 5.2 mm Hg per 100 mmol/day, 95% CI: 3.6–6.9).
Figure I.10.
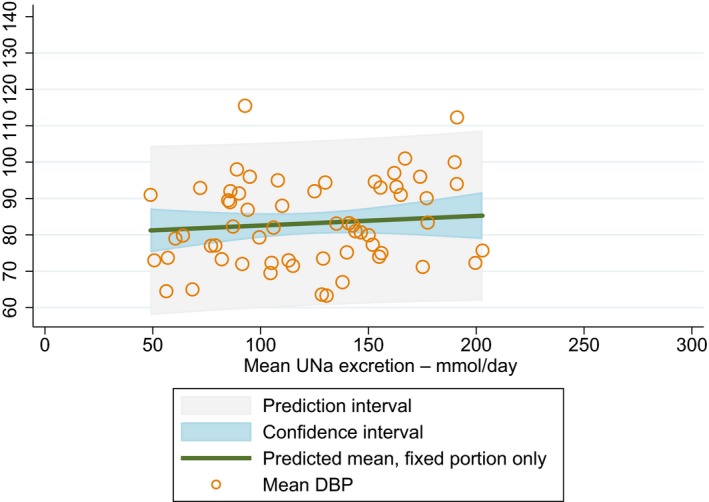
Linear dose–response relationship between mean urinary sodium excretion and mean DBP (mm Hg) from meta‐regression modelling of trials’ arms (crude model)
- Circles represents mean DBP by arm and their size is proportional to weights from the mixed‐effects model. The slope from the full model with moderators did not differ substantially (UNa unadjusted coefficient: 2.6 mm Hg per 100 mmol/day, 95% CI: 1.6–3.7).
Mean sodium excretion explained only 4% of the heterogeneity across trials in the SBP model and 3% in the DBP model. However, in both models the set of moderators explained more than 85% of the between‐study heterogeneity.
Moderating effects of age and hypertensive status were explored in stratified analyses. A larger association was found in hypertensive than normotensive individuals, for both SBP (hypertensive: 6.4 (4.3, 8.6) mm Hg vs normotensive: 4.4 (2.1, 6.6) mm Hg) and DBP (hypertensive: 3.7 (2.5, 5.0) mm Hg vs normotensive: 1.7 (0.1, 3.3) mm Hg) (Annex B). The effect of sodium was higher among subjects aged 50 years or more (SBP 7.1 (5.0, 9.2) mm Hg; DBP 3.8 (2.6, 5.0) mm Hg) than among subjects younger than 50 years (SBP 3.5 (1.5, 7.4) mm Hg; DBP 1.2 (−0.4, 2.9) (Annex B).
-
3
Limitations of the models
Sources of uncertainty specific to the statistical analysis and their potential impact on the final estimates, where possible, were identified and described (see Annex B).
Using arms’ absolute values generated in controlled settings was expected to allow a better characterisation of the dose–response relationship between sodium intake and blood pressure levels than using data from observational studies (potential confounding). Still, relationships described via meta‐regression are observational in nature as they do not have the benefit of randomisation to support a causal interpretation (Thompson and Higgins, 2002). The models are representations of the relationship between mean UNa and mean SBP or DBP at ‘group’ level (potential aggregation bias). Also, some potential moderators could not be explored (e.g. BMI, ethnicity, potassium intake and energy intake13) due to missing information.
Given the influence of the design of the trials on data structure and incompleteness of information there was a strong ‘methodological’ component (covariates that are linked to the experimental setting) specified in the models to reach a good fitting and contributing to explain a large part of the heterogeneity between studies (random effects). This added challenges on the interpretation of the models. Constants (intercepts) and predictions were substantially influenced by experimental factors and were difficult to reconcile with ‘populations’ values. So, the model cannot be used to make predictions at population level, but it provides evidence for a positive linear relationship between mean sodium excretion and mean levels of SBP and DBP.
Observational studies
One prospective cohort study met the inclusion criteria (Stolarz‐Skrzypek et al., 2011). The analysis combined data from 1,499 participants aged ≥ 20 years from Belgium, the Czech Republic, Italy, Poland and the Russian Federation of the European EPOGH and FLEMENGHO cohorts, without antihypertensive treatment and CVD history at baseline. During a follow‐up of 6.1 years, a 100 mmol increase in sodium excretion was associated with a 1.71 mm Hg (95% CI 0.79, 2.64) increase in SBP. The value for DBP was 0.38 mm Hg (95% CI –0.31, 1.07). This study was judged to be at moderate RoB (tier 2), in particular due to the lack of adjustment for significant confounders (energy intake, smoking and physical activity), potential misclassification of participants in the lowest category of sodium excretion related to the apparent undercollection of urine samples compared with the other categories (i.e. lower urinary volume and creatinine excretion) and substantial attrition during follow‐up.
Conclusion
Based on data from RCTs, the Panel considers that there is strong evidence for a positive relationship between UNa and SBP and DBP over the range of mean UNa observed in the studies (between 49 and 209 mmol/24 h (1.3 to 4.8 g/day)). One eligible prospective observational study (tier 2) investigating the long‐term relationship between UNa and blood pressure levels supports such relationship.
5.5.1.3. Hypertension
The characteristics of eligible studies, the outcome of the RoB appraisal and descriptive forest plots are presented in Appendices J.1, J.2 and J.3, respectively.
Experimental studies
Two RCTs assessed the effect of sodium reduction on the incidence of hypertension, namely the Trials of Hypertension Prevention (TOPH), phases I and II (The Trials of Hypertension Collaborative Research Group, 1997; Whelton et al., 1997). Both trials were conducted in the USA and involved individuals aged 35 to 54 years with ‘high‐normal’ blood pressure at baseline (DBP of 80–89 mm Hg in TOPH I; DBP of 83–89 mm Hg and SBP < 140 mm Hg in TOPH II), not taking antihypertensive treatment. TOPH II selected overweight individuals (BMI 110–165% of desirable body weight). Participants were randomised to a dietary sodium reduction counselling programme or a usual care group. TOPH I lasted 18 months, while TOPH II lasted 36–48 months. The incidence of hypertension in the intervention group vs control group during follow‐up was compared. In TOPH I, baseline mean (SD) UNa was 154.6 (77.9) and 156.4 (60.5) mmol in the sodium reduction group (n = 326) and usual care group (n = 417), respectively, while values were 186.1 (80.7) mmol and 188.0 (80.9) mmol/day in the sodium reduction group (n = 581) and usual care group (n = 576) in TOPH II.
In TOPH I (Whelton et al., 1997), UNa decreased to 99.4 (60.0) mmol/day in the intervention group compared with 146.5 (79.2) mmol/day in the control group after 18 months, corresponding to a net (between groups) difference of 47.2 mmol (p < 0.0001). During follow‐up, the incidence of hypertension was 8.6% in the sodium reduction group compared with 11.3% in the usual care group (RR (95% CI) = 0.76 (0.49, 1.18)). This study was judged to be at low RoB (tier 1).
In TOPH II (The Trials of Hypertension Collaborative Research Group, 1997), the estimated net reduction in UNa was 40 mmol/day (p < 0.001) after 36 months. Through 48 months, the incidence of hypertension was 38.1% in the intervention group compared with 44.4% in the usual care group (RR = 0.82, p = 0.05). This study was judged to be at low RoB (tier 1).
Observational studies
Two prospective cohort studies assessed the association between UNa and the incidence of hypertension (Stolarz‐Skrzypek et al., 2011; Forman et al., 2012). The EPOGH/FLEMENGHO ‘hypertension cohort’ followed up individuals aged ≥ 20 years from Belgium, the Czech Republic, Italy, Poland and the Russian Federation (Stolarz‐Skrzypek et al., 2011). The PREVEND cohort investigated the natural course of albuminuria and its relationship with renal and cardiovascular diseases in Dutch subjects aged 25–75 years and was characterised by an oversampling of participants with elevated albumin excretion at baseline (> 10 mg/L) (Forman et al., 2012). At baseline, mean (SD) 24‐h sodium excretion was 174.2 (74.1) mmol in the EPOGH/FLEMENGHO cohort, while median (interquartile range (IQR)) sodium excretion was 137 (106–171) mmol/day in the PREVEND cohort.
In the PREVEND cohort (Forman et al., 2012), hazard ratio (HR) (95% CI) for incident hypertension was 1.05 (1.00–1.10) for each 43 mmol (1 g) higher UNa (5,556 men and women; 878 cases; median follow‐up 6.4 years). The HR was 1.21 (0.98–1.51) comparing the highest (median (IQR) UNa: 271 (242–316) mmol) to the lowest quartile (97 (79–110) mmol) of UNa. The association of UNa with incident hypertension was modified by serum uric acid (SUA) and albumin urine excretion (UAlbumin). The adjusted HRs were 0.98 (0.89–1.08), 1.05 (0.96–1.15) and 1.09 (1.02–1.16) per each 1 g (43 mmol) increase in UNa in the lowest, middle and highest tertiles of SUA, respectively. Corresponding HRs were 0.99 (0.93–1.06), 1.02 (0.92–1.12) and 1.18 (1.07–1.29) per each 1 g (43 mmol) increase in UNa among those with UAlbumin < 10 mg/day, between 10 and 15 mg/day, and > 15 mg/day, respectively. This study was judged to be at low RoB (tier 1).
In the EPOGH/FLEMENGHO cohort (Stolarz‐Skrzypek et al., 2011) HRs (95% CI) for the incidence of hypertension were 1.00 (0.87–1.16), 1.02 (0.89–1.16) and 0.98 (0.86–1.12) in the low (mean (SD) UNa: women 94.4 (21.5) mmol; men 121.3 (27.9) mmol), medium (women 147.4 (14.3) mmol; 185.3 (16.1) mmol) and high (women 222.1 (47.2) mmol; men 282.2 (56.4) mmol) sex‐specific tertiles of sodium excretion, respectively, compared with the whole population (2,096 men and women; 552 events; median follow‐up 6.5 years). This study was judged to be at moderate RoB (tier 2), in particular due to the overadjustment for SBP, potential misclassification of participants in the lowest category of sodium excretion related to the apparent undercollection of urine samples compared with the other categories (i.e. lower urinary volume and creatinine excretion) and substantial attrition during follow‐up.
Conclusion
A small number of studies, two RCTs (tier 1) and two prospective observational studies (tiers 1 and 2) investigating the relationship between UNa and risk of hypertension were eligible for the assessment. Overall, the Panel considers that these studies support a positive relationship between UNa and blood pressure.
5.5.2. Cardiovascular disease
Six publications, pertaining to five different cohort studies, were eligible (Tuomilehto et al., 2001; Stolarz‐Skrzypek et al., 2011; Cook et al., 2014; Joosten et al., 2014; Kieneker et al., 2018; Lelli et al., 2018). Three cohorts were based on random samples of adult populations: (i) the EPOGH/FLEMENGHO ‘outcome cohort’ involved individuals aged ≥ 20 years from Belgium, the Czech Republic, Italy, Poland and the Russian Federation (Stolarz‐Skrzypek et al., 2011); (ii) the ‘Finnish cohort’ involved individuals aged 25–64 years (Tuomilehto et al., 2001); and (iii) the InCHIANTI cohort involved individuals aged ≥ 65 years in Italy (Lelli et al., 2018). Another cohort was constituted of the control groups of the TOPH I and TOPH II sodium reduction trials that involved United States individuals aged 30–54 years with ‘high‐normal’ blood pressure at baseline (post‐trials follow‐up) (Cook et al., 2014). Finally, the PREVEND cohort involved subjects aged 25–75 years and was characterised by an oversampling of participants with elevated albumin excretion at baseline (> 10 mg/L) (Joosten et al., 2014; Kieneker et al., 2018).
These publications addressed the association between sodium intake and risk of stroke (three papers), risk of CHD (three papers) and risk of total CVD (three papers) (Table 8). As per the protocol eligibility criteria, only analyses that excluded prevalent cases at baseline were considered. Two publications reported HRs for sodium excretion expressed as a continuous variable, one publication reported HRs by categories of sodium excretion, and three publications reported both. One publication used the whole study population as reference group to calculate HR in each sodium excretion category, which hindered its inclusion in pooled analyses. Because of the small number of eligible studies available for pooling for each outcome type, meta‐analyses were not conducted.
Table 8.
Outcomes and references of prospective cohort studies on CVD
| Outcome type | No. publications | References (cohort name) | |
|---|---|---|---|
| Stroke | Non‐fatal | 1 | Tuomilehto et al. (2001) (Finnish cohort) |
| Fatal and non‐fatal | 2 |
Stolarz‐Skrzypek et al. (2011) (EPOGH/FLEMENGHO) Kieneker et al. (2018) (PREVEND) |
|
| CHD | Non‐fatal | 1 | Tuomilehto et al. (2001) (Finnish cohort) |
| Fatal and non‐fatal | 2 |
Stolarz‐Skrzypek et al. (2011) (EPOGH/FLEMENGHO) Joosten et al. (2014) (PREVEND) |
|
| CVD (total) | Fatal | 1 | Stolarz‐Skrzypek et al. (2011) (EPOGH/FLEMENGHO) |
| Non‐fatal | 1 | Lelli et al. (2018) (InCHIANTI) | |
| Fatal and non‐fatal | 2 |
Cook et al. (2014) (TOPH I/TOPH II) Stolarz‐Skrzypek et al. (2011) (EPOGH/FLEMENGHO) |
|
CHD: coronary heart disease; CVD: cardiovascular disease; EPOGH/FLEMENGHO: European Project on Genes in Hypertension/Flemish Study on Genes and Health Outcomes; InCHIANTI: Invecchiare in Chianti; PREVEND: Prevention of Renal and Vascular End‐stage Disease; TOPH: Trials of Hypertension Prevention
The study characteristics, the outcome of the RoB appraisal and descriptive forest plots, are presented in Appendices K.1, K.2 and K.3.
In relation to sodium exposure measurement, multiple 24‐h urine samples were collected in the PREVEND and TOPH cohorts, while single 24‐h urine collections were available in the other three cohorts. In four publications, quality measures have been applied to deal with inaccurate urine collections. Two papers excluded samples deemed to be incomplete from their analyses a priori (Stolarz‐Skrzypek et al., 2011), based on sex‐specific creatinine cut‐offs and urine volume; (Tuomilehto et al., 2001), based on self‐reporting); two papers excluded samples in sensitivity analyses (Cook et al., 2014), based on intraindividual variability in creatinine/body weight ratio; (Joosten et al., 2014) based on estimated urine volume vs actual).
The overall RoB was judged to be low for four publications (tier 1), and moderate for three publications (tier 2). For the three papers for which the overall RoB was judged moderate (tier 2), the main concerns related to: (i) overadjustment for blood pressure (Tuomilehto et al., 2001; Lelli et al., 2018); (ii) potential for reverse causality due to the inclusion of hypertensive subjects in the sample (Tuomilehto et al., 2001; Stolarz‐Skrzypek et al., 2011; Lelli et al., 2018); (iii) potential misclassification of participants in the lowest category of sodium intake related to the apparent undercollection of urine samples compared with the other categories (i.e. lower creatinine excretion relative to body weight) (Stolarz‐Skrzypek et al., 2011); and (iv) higher attrition rate in the highest category of sodium intake compared with the other categories (Stolarz‐Skrzypek et al., 2011).
At baseline, median (IQR) UNa was 137 (106–171) mmol (min–max: 50–315 mmol) in the PREVEND cohort, 205 mmol in men (IQR Not Reported (NR); min–max: 25–552) and 154 mmol in women (IQR NR; min–max: 12 to 512 mmol) in the Finnish cohort, 236 (160–306) mmol in the InCHIANTI cohort, and 158 (127–194) mmol in the TOPHI/II cohort (min–max: NR). The mean (SD) UNa was 178.0 (74.8) mmol (min–max: 50–400 mmol) in the EPOGH/FLEMENGHO cohort.
5.5.2.1. Stroke
In the PREVEND cohort (7,330 men and women), 183 fatal and non‐fatal stroke events occurred during a median follow‐up of 12.5 years (Kieneker et al., 2018). The HR (95% CI) was 1.44 (1.14–1.82) per 51 mmol/24 h decrement in UNa. By sex‐specific quintiles of UNa, HRs were 1.45 (0.92–2.29) (57 cases, 16,272 person‐years) in Q1, 1.13 (0.71–1.79) (49 cases, 16,515 person‐years) in Q2, 1.04 (0.64–1.71) (25 cases, 16,720 person‐years) in Q4 and 0.81 (0.46–1.41) in Q5 (19 cases, 16,908 person‐years), with Q3 taken as the reference category (33 cases, 16,774 person‐years). Similar HRs for the risk of fatal and non‐fatal stroke were estimated after adjustment for potential mediators, i.e. SBP and antihypertensive medication (1.44 (1.14–1.82)), plasma renin (1.50 (1.18–1.90)), aldosterone (1.54 (1.21–1.97)), and sodium levels (1.49 (1.17–1.90)), respectively. In sensitivity analyses, the association did not change after exclusion of individuals who were taking antihypertensive drugs at baseline (6,388 subjects (126 events)) and those with malignancies, type 2 diabetes, or chronic kidney disease at baseline (6,054 subjects (112 events)). In a weighted analysis, that accounted for the sampling design of the study, the HR was 1.68 (1.12–2.50) per 51 mmol/24 h decrement in UNa. This study was judged to be at low RoB (tier 1).
In the Finnish cohort (2,420 men and women), 84 non‐fatal stroke events occurred during 8 to 13 years follow‐up (Tuomilehto et al., 2001). The HR (95% CI) was 1.13 (0.84–1.51) per 100 mmol/24 h increase in UNa. This study was judged to be at moderate RoB (tier 2).
In the EPOGH/FLEMENGHO cohort (3,681 men and women), 33 fatal and non‐fatal stroke events occurred during a median follow‐up of 7.9 years (Stolarz‐Skrzypek et al., 2011). The HRs (95% CI) were 1.05 (0.56–1.96) (13 events), 1.28 (0.75–2.17) (13 events) and 0.78 (0.46–1.33) (7 events) in the low, medium and high sex‐specific tertiles of UNa, respectively, compared with the overall risk in the entire cohort. This study was judged to be at moderate RoB (tier 2).
5.5.2.2. Coronary heart disease
In the PREVEND cohort (7,543 men and women), 452 fatal and non‐fatal CHD events occurred during a median follow‐up of 10.5 years (Joosten et al., 2014). The HR was 1.07 (0.98–1.18) for each 1 g/day (43 mmol/24 h) increment in UNa. In stratified analysis, each 1 g/day increment in UNa was associated with an increased risk for CHD in subjects with hypertension (1.14 (1.01–1.28); n = 2,363) and in subjects with plasma N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) concentrations above the sex‐specific median (1.16 (1.03–1.30); 320 events; n = 3,771). This study was judged to be at low RoB (tier 1).
In the Finnish cohort (2,402 men and women), 128 non‐fatal CHD events occurred during 8 to 13 years follow‐up (Tuomilehto et al., 2001). The HR (95% CI) was 1.34 (1.08–1.67) per 100 mmol/24 h increase in UNa. This study was judged to be at moderate RoB (tier 2).
In the EPOGH/FLEMENGHO cohort (3,681 men and women), 98 fatal and non‐fatal CHD events occurred during a median follow‐up of 7.9 years (Stolarz‐Skrzypek et al., 2011). HRs (95% CI) were 1.41 (0.99–2.01) (45 events), 1.15 (0.87–1.52) (34 events) and 0.87 (0.66–1.15) (19 events) in the low, medium and high sex‐specific tertiles of UNa, respectively, compared with the overall risk in the entire cohort. This study was judged to be at moderate RoB (tier 2).
5.5.2.3. Cardiovascular disease (composite outcome)
In a 10–15 years post‐trial follow‐up, the association between UNa and risk of fatal and non‐fatal CVD events was assessed among subjects who had not been assigned to an active sodium reduction intervention group in the TOPH I and TOPH II trials (Cook et al., 2014). In total, 193 events occurred (68 myocardial infarctions, 77 coronary revascularisations, 22 strokes (1 participant reported both myocardial infarction and stroke), and 27 CVD deaths). Four categories of UNa were defined as follows: < 100 mmol (< 2.3 g), 100 to < 157 mmol (2.3 to < 3.6 g), 157 to < 209 mmol (3.6 to < 4.8 g) (reference category), and ≥ 209 mmol (≥ 4.8 g) sodium/24 h. HRs were 0.68 (0.34–1.37; 15 events/189 total) and 0.75 (0.50–1.11; 48/590) for the two lowest categories compared with the reference category (40/427), while it was 1.05 (0.68–1.62; 23/191) for the highest category compared with the reference category (p for trend = 0.13). There was a linear 17% increase in risk per 43 mmol (1 g)/24 h increase in UNa (p = 0.054). Spline curves supported a linear association of UNa with CVD events (p value for linearity = 0.044; p value for nonlinearity = 0.76). This study was judged to be at low RoB (tier 1).
In the InCHIANTI cohort (514 men and women), 169 non‐fatal CVD events (CVD types no reported) occurred during a median follow‐up of 9 years (Lelli et al., 2018). A RR (95% CI) of 0.96 (0.90–1.02) was reported (reference unit of UNa not reported). This study was judged to be at moderate RoB (tier 2).
In the EPOGH/FLEMENGHO cohort, 232 fatal and non‐fatal CVD events occurred during a median follow‐up of 7.9 years (non‐fatal events included: 43 heart failures, 33 coronary revascularisations, 27 myocardial infarctions (MI), 14 pulmonary heart diseases, 13 strokes, 9 pulmonary embolisms, 4 coronary syndrome, 3 ischaemic heart diseases (IHD) and 2 aortic aneurysms; fatal events included: 29 heart failure, 20 stroke, 19 MI, 6 IHD, 6 sudden deaths, 2 pulmonary embolisms, 1 arterial embolism and 1 aortic aneurysm) (Stolarz‐Skrzypek et al., 2011). HRs (95% CI) were 1.12 (0.90–1.41) (100 events), 1.09 (0.88–1.34) (79 events) and 0.92 (0.74–1.13) (53 events) in the low, medium and high sex‐specific tertiles of UNa, respectively, compared with the overall risk in the entire cohort (3,681 men and women). The adjusted HRs for fatal CVD events only were 1.41 (0.94–2.12) (50 events), 0.98 (0.69–1.40) (24 events) and 1.02 (0.71–1.45) (10 events), respectively. This study was judged to be at moderate RoB (tier 2).
5.5.2.4. Mechanistic considerations
There is a positive relationship between sodium intake and blood pressure (Section 5.5.1), which, in turn, is an established independent risk factor for CVD, mostly CHD and stroke.
Other mechanisms have been proposed by which sodium intake may affect CVD risk (reviewed by Farquhar et al. (2015)), including through an effect on endothelial function, arterial stiffness and left ventricular mass. In a meta‐analysis of trials investigating the effect of sodium intake on carotid‐femoral pulse wave velocity (PWV), as a marker of arterial stiffness, D'Elia et al. (2018) reported that an average reduction in sodium intake of 89.3 mmol/day was associated with a pooled effect of 2.84% (95% CI: 0.51–5.08, I2 14%; pooling data of 14 cohorts from 11 studies) reduction in PWV. There is some evidence that the effect of sodium reduction on PWV could, at least in part, be independent of the changes in blood pressure (D'Elia et al., 2018).
There is uncertainty on the health effects of changes in regulatory hormones associated with sodium reduction (Section 5.2.3), including how they might affect the risk of stroke at low UNa, beyond the well‐established effect of blood pressure on stroke risk.
5.5.2.5. Conclusions
A small number of observational prospective studies was eligible for the assessment. Three cohorts (PREVEND, EPOGH/FLEMENGHO and the Finnish cohort) investigated the association between UNa and risk of stroke (1 in tier 1; 2 in tier 2) and risk of CHD (1 in tier 1; 2 in tier 2). Three cohorts (EPOGH/FLEMENGHO, InCHIANTI and TOPHI/II) investigated the association between UNa and risk of CVD (1 in tier 1; 2 in tier 2).
Overall, limited conclusions can be drawn on the relationship between UNa and risk of CVD. The Panel considers that, over the range of UNa observed in these studies:
There is some evidence for a positive association between UNa and risk of CHD. The positive relationship between UNa and blood pressure levels/incidence of hypertension, which is an established independent risk factor for CHD, supports this association.
There is some evidence for an inverse association between UNa and risk of stroke. However, the number of eligible studies available investigating this outcome is small and the mechanisms by which UNa could be inversely associated with the risk of stroke are unclear, particularly considering the positive relationship between UNa and blood pressure, which is an established risk factor for stroke.
There is some evidence for a positive association between UNa and risk of CVD. The Panel notes that CVD as a composite outcome combines different diseases that may be differentially affected by sodium intake. The suggestion of a positive association reported by Cook et al. (2014), in which MI and coronary revascularisation interventions represented most cases, is consistent with the evidence for a positive association between UNa and risk of CHD and the positive relationship between UNa and blood pressure levels/incidence of hypertension.
5.5.3. Bone health
There is consistent evidence that an increase in sodium intake increases urinary calcium excretion, while a reduction in sodium intake lowers urinary calcium excretion (Afssa, 2001; EFSA, 2005a; IOM, 2005). The increase in urinary calcium excretion with increasing sodium intake may negatively affect bone calcium balance, even when dietary calcium intake is above the PRI for calcium (see Section 2.5.3). It is biologically plausible that a long‐term increase in urinary calcium excretion leading to negative bone calcium balance would both lower BMD and increase the risk of osteoporotic bone fractures. However, evidence for a relationship between sodium intake and bone health was considered inconclusive in previous assessments (EFSA, 2005a; IOM, 2005).
Two articles were eligible for the assessment (Devine et al., 1995; Ilich et al., 2010) (Appendix G.2). Study characteristics and results and the outcome of the RoB appraisal are presented in Appendices L.1 and L.2, respectively.
Although the two eligible studies were primarily designed as RCTs, they have been categorised as ‘prospective cohort studies’ because of the type of analyses conducted. Devine et al. (1995) used data from a 2‐year RCT investigating the effect of supplemental calcium and physical activity on BMD (Prince et al., 1995). Sodium intake was observed by means of 24‐h urine collections at baseline and after 1 and 2 years of intervention. Pooled data from all intervention groups were used to explore the association between sodium intake and change in BMD. Ilich et al. (2010) aimed at investigating the effect of dietary sodium intake on BMD and other bone‐related outcomes in a 3‐year RCT in which half of the participants were assigned to a group instructed to reduce sodium intake (target 1.5 g sodium/day), while the others maintained their usual diet (~3 g sodium/day). Sodium intake was estimated by means of 24‐h urinary collection every 6 months. With time, some of the participants in the control group reduced their sodium intake and those in the intervention group did not comply in reducing their sodium intake. Therefore, in the final analyses, all participants were grouped together and data were pooled to be analysed as a longitudinal observational study, regardless of to which group each subject was initially assigned.
The two studies involved postmenopausal women, with mean (SD) baseline sodium excretion of 121 (47) mmol (2,783 (1,081) mg)/day (n = 168, in Australia) (Devine et al., 1995) and 105 (42) mmol (2,404 (963) mg)/day (n = 136, in the USA) (Ilich et al., 2010), respectively. In the study from Devine et al. (1995), physical activity level and calcium intake varied depending on the intervention groups, while in the study from Ilich et al. (2010) participants maintained their usual physical activity and received calcium (~630 mg/day) and vitamin D (~400 IU/day) supplements. BMD was measured by DEXA at different sites Devine et al. (1995): hip, ankle and lumbar spine; Ilich et al. (2010): hip, forearm, lumbar spine and total body.
In multivariate analysis, Devine et al. (1995) reported a negative association between average UNa and 2‐year change in BMD at the hip and ankle. No association was found at the lumbar spine. This study was judged to be at moderate RoB (tier 2) because of concerns about the substantial attrition rate (124 out of 168 women included in the analysis) without clear reporting about the characteristics of the subjects excluded, and the lack of information on the quality measures applied for the 24‐h urine collection.
Ilich et al. (2010) reported a positive association between the cumulative average of urinary Na/creatinine excretion and BMD at 36 months in total body and total forearm (intention‐to‐treat analysis). Using random‐effects regressions accounting for missing data and repeated measurements, individuals with higher UNa were found to have higher forearm and lumbar spine BMD at baseline and subsequent time points. No association was found with total body or femur BMD. This study was judged to be at low RoB (tier 1).
The Panel notes the limited and inconsistent evidence for an association between sodium intake and BMD provided by these two studies.
The Panel concludes that these data cannot be used to set DRVs for sodium.
5.5.4. Scope of the review
The conclusions described above are based on the evidence eligible for the assessment. In defining the inclusion/exclusion criteria of this review, methodological choices were made to minimise the RoB. In particular, the review was restricted to:
RCTs and prospective observational studies;
studies that excluded participants with pre‐existing conditions, to minimise the risk of reverse causality;
studies that used at least one 24‐h urinary collection to estimate sodium intake, to minimise the RoB related to exposure misclassification.
In relation to observational data, the exclusion of studies that relied on dietary questionnaires or spot urine collections to assess sodium intake resulted in a relatively small number of eligible studies. This precluded any quantitative integration of the evidence relating sodium intake and disease risk (e.g. through meta‐analyses) and limited the exploration and characterisation of sources of heterogeneity in the body of evidence.
6. Data on which to base dietary reference values
6.1. Adults
Based on the review of the available evidence, the Panel considers that relevant data to inform the setting of DRVs for sodium is provided by: (i) balance studies on sodium (Section 5.2); and (ii) studies informing the relationship between sodium intake and blood pressure level or CVD risk (Sections 5.5.1 and 5.5.2).
The Panel considers that the available evidence cannot be used to determine the sodium requirement nor its distribution in the population (see Section 5); so, an AR and population reference intake (PRI) for sodium cannot be established.
Data on the relationship between sodium intake and level of blood pressure or CVD risk could inform about the levels of sodium intake associated with a reduced risk of chronic diseases. Balance studies could inform about the levels of sodium intake that are adequate to maintain a null sodium balance.
Because of the limited evidence available and of the associated uncertainties, it is not possible to identify such levels of sodium intake with certainty. The Panel therefore used expert judgement to weigh the available evidence and take account of the associated uncertainties ((a ‘weight of evidence’ approach EFSA Scientific Committee et al., 2017)). This was achieved by means of a formal EKE (EFSA, 2014) undertaken with the members of the working group on DRVs for minerals (Appendix M). The EKE offers several advantages, since:
It supports experts in making evidence‐based judgements about a quantity of interest (typically a parameter) in a structured way, while limiting bias.
It allows a representation of the uncertainty about the quantity of interest using a probability distribution to express judgements about the range of possible values and their relative likelihoods.
As a result, the probability distribution generated through the EKE reflects the uncertainty about the ‘true’ value of the quantity elicited.
The elicitation was carried out using the roulette method and following the Sheffield protocol (see section on Data and Methodologies, part C and Appendix M).
In line with the principles for setting DRVs (EFSA NDA Panel, 2010), the Panel aimed at setting reference values for the general population, excluding diseased populations and subpopulations with extreme and distinct vulnerabilities due to genetic predisposition or other conditions.
Two separate elicitations were conducted and are reported below.
6.1.1. EKE on the relationship between sodium intake and blood pressure and CVD
Evidence on the relationship between sodium intake and blood pressure or CVD risks could inform about the levels of sodium intake associated to a reduced risk of chronic diseases.
From the literature reviewed in Section 5, there is strong evidence for a positive relationship between sodium and blood pressure levels, which is an established risk factor for CVD risk. The meta‐regression dose–response modelling provided evidence for a linear relationship between blood pressure and UNa in the range covered by the eligible studies (ca. 50–200 mmol/day) (Section 5.5.1.2); any increase in blood pressure level is considered adverse. Based on eligible prospective observational studies, there is some evidence for a positive association between UNa and risk of hypertension, CHD and CVD. There is some evidence for an inverse association between UNa and risk of stroke, with substantial uncertainty as the number of eligible studies is small and the mechanisms by which UNa could be inversely associated with the risk of stroke are unclear (Section 5.5.2). So, in view of the available evidence, experts judgement was elicited on the lowest level of sodium associated with a reduced risk of stroke and CHD integrating all the relevant evidence, including evidence on blood pressure.
The following question was used for the elicitation: What is the lowest level of sodium intake at which the risk of chronic disease (i.e. stroke, CHD) is minimised in the majority (≥ 97.5%) of the general population of adults?
Eligible studies in relation to this source of evidence used UNa as marker of sodium intake. So, the scale used for the elicitation referred to UNa and was expressed in mmol/24 h.
The empirical probability distribution obtained after the group achieved a consensus is depicted in Figure 1 and the rationale for the distribution is presented in Table 9. The distribution reflects the uncertainty of the experts about the lowest level of sodium intake at which the risk of chronic disease (i.e. stroke, CHD) is minimised in the general population.
Figure 1.
Group consensus uncertainty probability distribution on the lowest level of sodium intake at which the risk of chronic disease (i.e. stroke, CHD) is minimised in the general population
- UNa: sodium urinary excretion.
- EKE question: ‘What is the lowest level of sodium intake at which the risk of chronic disease (i.e. stroke, CHD) is minimised in the majority (≥ 97.5%) of the general population of adults?’. Experts expressed the probability that each of the proposed ranges of UNa might include the ‘true’ level of interest. Therefore, the probability distribution reflects the uncertainty about the level elicited. It does NOT represent the probability (or risk) of disease associated to a given range of UNa. Ranges that did not receive any probability are not retained in the figure.
Table 9.
Rationale for the group consensus uncertainty probability distribution on the lowest level of sodium intake at which the risk of chronic disease (i.e. stroke, CHD) is minimised in the general population
| UNa range (mmol/24 h) | Probabilitya | Rationale |
|---|---|---|
| 0–40 | 1% |
|
| 40–80 | 44% |
|
| 80–120 | 50% |
|
| 120–200 | 5% |
|
CHD: coronary heart disease; UNa: sodium urinary excretion; TOPH: Trials of Hypertension Prevention.
Consensus judgement of the expert group about the probability that the corresponding range of UNa includes the ‘true’ level of interest.
6.1.2. EKE on balance studies
As discussed in Section 5.2, balance studies are based on the assumption that a healthy subject on an adequate diet maintains equilibrium or a null balance, between nutrient intakes and nutrient losses. At this null balance, the intake matches the requirement determined by the physiological state of the individual. When losses exceed intakes (negative balance), nutrient stores are progressively depleted, resulting in deficiency in the long term; when intakes exceed losses (positive balance), there is nutrient accretion. In the case of sodium, a sustained positive balance, albeit small, may lead to a systemic accumulation overtime with, possibly, adverse implications (Section 2.3.3).
In relation to sodium, the Panel considers that balance studies cannot be used to determine sodium requirements but could be used to inform about the levels of sodium intake that are adequate to maintain a null sodium balance (Section 5.2.4).
The following question was used for the elicitation: What is the lowest level of sodium intake which is adequate (i.e. amount which allows to maintain sodium balance) for the majority (≥ 97.5%) of the general population of adults?
The group consensus probability distribution elicited in response to the question is depicted in Figure 2 and the rationale for the distribution is provided in Table 10. The distribution reflects the uncertainty of the experts about the lowest level of sodium intake which allows to maintain sodium balance for most of the general population.
Figure 2.
Group consensus uncertainty probability distribution on the lowest level of sodium intake which allows to maintain sodium balance for most of the general population
- EKE question: ‘What is the lowest level of sodium intake that is adequate (i.e. amount which allows to maintain sodium balance) for the majority (≥ 97.5%) of the general population of adults?’. Experts expressed the probability that each of the proposed ranges of sodium intake might include the ‘true’ level of interest. Therefore, the probability distribution reflects the uncertainty about the level elicited. It does NOT represent the probability (or risk) of sodium ‘imbalance’ associated to a given range of sodium intake. Ranges that did not receive any probability are not retained in the figure.
Table 10.
Rationale for the group consensus uncertainty probability distribution on the lowest level of sodium intake which to maintain sodium balance for most of the general population
| Sodium intake range (g/day) | Probabilitya | Rationale |
|---|---|---|
| 0.5–1.0 | 5% |
|
| 1.0–1.5 | 32.5% |
|
| 1.5–2.0 | 37.5% | |
| 2.0–2.5 | 20% | |
| 2.5–3.5 | 5% |
|
INTERSALT: International Cooperative Study on Salt, Other Factors and Blood Pressure; RAAS: renin–angiotensin–aldosterone system.
Consensus judgement of the expert group about the probability that the corresponding range of sodium intake includes the ‘true’ level of interest.
6.1.3. Integration of the evidence and conclusions
Parametric distributions were fitted to the group consensus uncertainty empirical probability distributions. At this stage, UNa values expressed in mmol/24 h were converted into g/day and a factor of 1.07 was applied to allow for the percentage of dietary sodium excreted through urine. This conversion factor was chosen based on the meta‐analysis of Lucko et al. (2018) that estimated that, on average, 93% of daily dietary sodium was excreted in 24‐h urine (Sections 2.3.4.1 and 2.6.1.1).
The parametric distributions fitted to the centiles of the two group consensus uncertainty distributions were, respectively: a log‐normal distribution with parameters 0.72 (mean) and 0.23 (standard deviation) for the question on sodium intake at which CVD risk is reduced; a log‐normal distribution with parameters 0.5 (mean) and 0.3 (standard deviation) for the question on sodium balance (Appendix M). Based on the two fitted parametric distributions, cumulative uncertainty density functions were derived (Figure 3).
Figure 3.
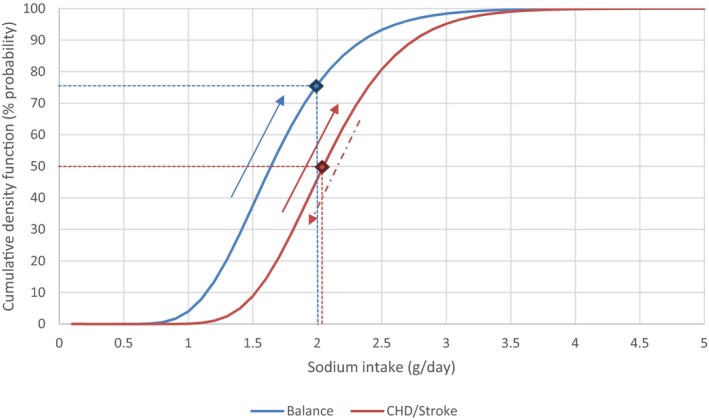
Cumulative uncertainty density functions
- Log‐normal distributions were fitted to the empirical uncertainty probability distributions elicited through the two EKEs and used to derive the two cumulative density functions depicted above.
- The red curve is the cumulative density function corresponding to the elicitation of the lowest level of sodium intake at which the risk of chronic disease (i.e. stroke, CHD) is minimised in the general population. As one goes towards the right of the curve, the uncertainty that the identified range includes the level of sodium intake that ‘minimises’ the risk of CHD increases; similarly, as one moves towards the left, the uncertainty that the identified range includes the level of sodium intake that ‘minimises’ the risk of stroke increases. The curve integrates experts’ considerations about the strength of the evidence for the respective outcomes and associated uncertainties. At the median level of the distribution (red dot), there is equal certainty that the level of sodium that it is associated with a reduction of CVD risk is neither overestimated (50% probability) nor underestimated (50% probability). So, the Panel considers that there is sufficient confidence in the data that a sodium intake of 2 g/day is associated with a reduction of CVD risk.
- The blue curve is the cumulative density function corresponding to the elicitation of the lowest level of sodium intake that allows to maintain sodium balance for most of the general population. The curve represents the probability/certainty that the ‘lowest level of sodium intake that is adequate (i.e. to maintain sodium balance) for the majority (≥ 97.5%) general population of adults’ is any value from the lower bound of the range up to the identified value. The interpretation of the curve is unidirectional: as one goes towards the right, the confidence (certainty) that the identified range includes the level of sodium intake that is adequate increases. The Panel considers that a level of sodium intake of 2 g/day (blue dot) is likely (i.e. with a 75% probability) to be adequate to maintain sodium balance for most of the general adult population in the EU.
- The curves do NOT represent the probability (or risk) of given outcome (i.e. CVD disease, sodium ‘imbalance’) associated with a given level of sodium intake.
The Panel used the cumulative uncertainty density functions to integrate the evidence and associated uncertainties and derive a reference value for sodium (see Figure 3). The Panel considered that:
A sodium intake of 2.0 g/day represents a level of sodium for which there is sufficient confidence that it is associated within a reduced risk of CVD in the general adult population.
A sodium intake of 2.0 g/day is likely to allow most of the general population to maintain sodium balance.
Therefore, the Panel considers that 2.0 g of sodium/day is a safe and adequate intake for the general EU population of adults. BOX‐1 provides an explanation for the terms.
This value provides guidance on a level of sodium intake compatible with good health that can inform population goals for sodium. However, the value has limited utility for assessing and planning the diet of individuals. At the individual level, if the usual intake of sodium exceeds this value, it could be associated with an increased risk of cardiovascular diseases, including concurring risk factors such as primary hypertension.
The Panel recognised several challenges arising from the integration of the available evidence to set any sort of advisory level for sodium intake. One is that, due to the limitations of the evidence, there are substantial uncertainties associated with levels of interest for both sodium balance and stroke/CHD risk. Another challenge arises from the integration of several outcomes that differ in their nature, i.e. physiological requirements versus chronic disease risk. The setting of a single value is a pragmatic choice, which represents a compromise between the different types of evidence and risks.
6.1.3.1.
BOX 1 – Safe and adequate intake: explanation for the terms
Safe: Although the term ‘safe intake’ is not defined in the principles on deriving and applying DRVs (EFSA NDA Panel, 2010), the concept of a safe intake has been used in previous assessments regarding a daily intake of a nutrient which does not give rise to concerns about adverse health effects, in instances when a tolerable upper intake level (UL) could not be established (SCF, 2000; EFSA NDA Panel, 2012b). The reference value for sodium is called ‘safe’ because the value proposed takes account of the evidence describing the relationship between sodium intake and CVD risk in the general population.
Adequate: An adequate intake (AI) is the value estimated when a population reference intake (PRI) cannot be established because an average requirement (AR) cannot be determined (EFSA NDA Panel, 2010). The AI is the level of intake that is assumed to be sufficient based on observations from groups of apparently healthy people. The reference value for sodium is called ‘adequate’ in line with this definition.
6.2. Pregnant and lactating women
The Panel considers that the requirement for the daily accretion rate of sodium in fetal and maternal tissues can be met by adaptive changes that maintain sodium homeostasis during pregnancy (Section 5.3). The Panel also notes that there is no evidence that sodium requirement of lactating women differs from the requirement of non‐lactating women (Section 5.3).
So, the Panel considers that 2.0 g sodium/day is a safe intake and adequate intake for pregnant and lactating women.
6.3. Infants
There is a lack of data from which an AR could be derived for infants (Section 5.4).
Upwards extrapolation from the estimated sodium intake of fully breast‐fed infants during the first 6 months of life of 120 mg/day (Section 2.3.4.4), based on ARs for energy of infants aged 3 months (2.0 MJ/day for men and women (EFSA NDA Panel, 2013a)) and 9 months (2.8 MJ/day for men and women (EFSA NDA Panel, 2013b)), results in an estimated sodium intake of 168 mg/day.
After rounding to the closest 0.1, an AI of 0.2 g/day is proposed for infants aged 7–11 months.
6.4. Children
There is a lack of data from which an AR could be derived for children (Sections 5.2 and 5.4).
For children, the Panel decided to draw up reference values based on downwards extrapolation from the reference value for adults, based on the AR for energy (EFSA NDA Panel, 2013b) and including a growth factor to take into account requirements for growth, as follows:
Valuechild = Valueadult × (AR for energy of children/AR for energy of adults aged 18–29 years) ×(1 + growth factor)
The AR for energy varies with age and physical activity level (PAL) (EFSA NDA Panel, 2013b). For the calculations, the AR for energy of adults aged 18–29 years was taken as a reference. The values calculated by using the ARs corresponding to the different PAL values were close (< 0.2 g/day difference). Therefore, a single value is proposed that applies to all levels of physical activity.
Growth factors are derived from the proportional increase in protein requirement for growth relative to the maintenance requirement at the different ages (EFSA NDA Panel, 2012a; EFSA, 2017a).
The age categories proposed by the EFSA NDA Panel (2010) were applied. For each age category, the Panel decided to set a single value for boys and girls and the average of the values calculated for both sexes was taken (Table 11).
Table 11.
Safe and adequate intake of sodium for children
| Safe and adequate intakea (g/day) | |
|---|---|
| 1–3 years | 1.1 |
| 4–6 years | 1.3 |
| 7–10 years | 1.7 |
| 11–17 years | 2.0 |
Values for children were derived from the value for adults after adjustment on the basis of differences in average requirement for energy (EFSA NDA Panel, 2013b) and application of a growth factor (EFSA, 2017a). The average requirements for energy of adults aged 18 to 29 years was used in the calculations. The average of the values for boys and girls was calculated for each year of age. For each age category, the proposed value corresponds to the average of the values calculated for each year of age. A single value for the age categories 11–14 years and 15–17 years was selected. Values are rounded to the closest 0.1.
Conclusions
The Panel concludes that the available evidence cannot be used to derive an AR and a PRI for sodium. Evidence on the relationship between sodium intake and level of blood pressure and risk of CVD as well as data from balance studies are used as a basis to determine a safe and adequate intake of sodium of 2.0 g/day for the general population of adults (Table 12). The Panel proposes that the reference value for adults also applies to pregnant and lactating women. Reference values for children are extrapolated from the reference value for adults based on the energy requirements of the age groups and applying a growth factor. For infants over 6 months of age, an AI is derived by extrapolating the estimated sodium intake of fully breast‐fed infants during the first 6 months of life based on the energy requirement of the respective age groups.
Table 12.
Summary of dietary reference values for sodium
| Safe and adequate intakea (g/day) | |
|---|---|
| 7–11 months | 0.2b |
| 1–3 years | 1.1 |
| 4–6 years | 1.3 |
| 7–10 years | 1.7 |
| 11–17 years | 2.0 |
| ≥ 18 years c | 2.0 |
Equivalent to: 9 mmol/day for infants 7–11 months, 48 mmol/day for children aged 1–3 years, 57 mmol/day for children aged 4–6 years, 74 mmol/day for children aged 7–10 years, 87 mmol/day for children aged 11–17 years, 87 mmol for adults, including pregnant and lactating women.
Adequate intake.
Including pregnant and lactating women.
The Panel notes that the mean/median intake of sodium in the European adult populations exceeds the safe and adequate intakes set for sodium (see Appendices C, D and E). The risk of inadequate (insufficient) intake in European populations is low. Concerns for European populations instead relate to ‘excess’ intake of sodium. Therefore, in practice, the values proposed can be used to inform the setting of population goals for the reduction in sodium intake.
The Panel acknowledges that the concept of a safe and adequate intake is not addressed in the EFSA's Scientific Opinion on the principles for deriving and applying DRVs published in 2010 (EFSA NDA Panel, 2010). The principles were established when the review of DRVs for European populations was initiated and was meant as a guidance document. As for other guidance documents in EFSA, it is a living document that could be updated in the future in the light of the experience gained and new scientific and methodological developments in the field.
Recommendations for research
There is a need for studies, using robust assessment methods for sodium intake and the outcome of interest, investigating:
the moderating effect of energy intake on the relationship between sodium intake and blood pressure;
the health effects of sodium and of the Na/K ratio at intakes approximating their respective DRVs;
the life course effects of sodium intake on blood pressure, in particular the effect of sodium intake on neurohormonal control during childhood and adolescence, including programming;
the effect of prolonged exposure to ‘low’ sodium on the effective functioning of its homeostatic regulation (i.e. SNS and RAAS);
the effects of sodium intake on bone health in growing and ageing populations;
the role of sodium intake on the pathogenesis of kidney disease in the general population;
the characterisation of genes involved in determining ‘salt‐sensitive’ phenotypes and of moderating factors of ‘salt sensitivity’.
Abbreviations
- 24‐h DR
24‐hour dietary recall
- AAS
atomic absorption spectrophotometry
- ADH
antidiuretic hormone
- ADR
adrenaline
- Afssa
Agence Française de Sécurité Sanitaire des Aliments
- AI
adequate intake
- ALD
aldosterone
- Anses
French Agency for Food, Environmental and Occupational Health and Safety
- AR
average requirement
- BMC
bone mineral content
- BMD
bone mineral density
- BMI
body mass index
- BP
blood pressure
- bw
body weight
- CDRR
Chronic Disease Risk Reduction
- CHD
coronary heart disease
- 95% CI
95% confidence interval
- COMA
Committee on Medical Aspects of Food Policy
- CVD
cardiovascular disease
- CVOs
circumventricular organs
- D–A–CH
Deutschland–Austria–Confederation Helvetica
- DASH
Dietary approaches to Stop Hypertension
- DBP
diastolic blood pressure
- DCS
Doetinchem Cohort Study
- DEXA
dual‐energy X‐ray absorptiometry
- DGE
German nutrition society
- DH
UK Department of Health
- DONALD
Dortmund Nutritional and Anthropometrical Longitudinally Designed study
- DRs
dietary recalls
- DRV
dietary reference value
- EAR
estimated average requirement
- ECF
extracellular fluid
- EDTA
ethylenediaminetetraacetate
- EEDI
estimated equilibrated dietary intake
- EFCOVAL
European Food Consumption Validation
- EKE
expert knowledge elicitation
- ENaC
epithelial sodium channel
- EPOGH
European Project on Genes in Hypertension
- FAO
Food and Agriculture Organization
- FFM
fat‐free mass
- FFQ
food frequency questionnaire
- FLEMENGHO
Flemish Study on Genes and Health Outcomes
- FVI
fruit and vegetable intake
- GDPS
General Doetinchem Population Sample
- GFR
glomerular filtration rate
- GOOD
Gothenburg Obesity and Osteoporosis Determinants
- HR
hazard ratio
- HT
hypertensive
- ICP‐AES
inductively coupled plasma atomic emission spectroscopy
- ICP‐MS
inductively coupled plasma mass spectrometry
- IHD
ischaemic heart disease
- InCHIANTI
Invecchiare in Chianti
- INTERSALT
International Cooperative Study on Salt, Other Factors and Blood Pressure
- IOM
US Institute of Medicine of the National Academy of Sciences
- IQR
interquartile range
- LRNI
lower reference nutrient intake
- MET
metabolic equivalent activity
- MI
myocardial infarction
- NASEM
National Academies of Sciences, Engineering, and Medicine
- N/A
not available
- Na+/K+ ATPase
sodium/potassium adenosine triphosphatase
- NDA
EFSA Panel on Nutrition, Novel Foods and Food Allergens
- NOR
noradrenaline
- NR
not reported
- NT‐proBNP
N‐terminal pro‐B‐type
- OHAT‐NTP
Office of Health Assessment and Translation – National Toxicology Programme
- OMIM
Online Mendelian Inheritance in Man
- PABA
para‐aminobenzoic acid
- PAL
physical activity level
- PREVEND
Prevention of Renal and Vascular End‐stage Disease
- PRI
population reference intake
- PRISMA
Preferred Reporting items for Systematic reviews and Meta‐Analyses
- PROMETHEUS
Promoting Methods for Evidence Use in Scientific assessments
- PWV
pulse wave velocity
- RA
renin activity
- RAAS
renin–angiotensin–aldosterone system
- RCT
randomised controlled trial
- RNI
reference nutrient intake
- RoB
risk of bias
- RR
relative risk
- SACN
Scientific Advisory Committee on Nutrition
- SBP
systolic blood pressure
- SCF
Scientific Committee for Food
- SD
Standard deviation
- SDT
suggested dietary target
- SE
standard error
- SEM
standard error of the mean
- SING
Salt Intake in northern Greece
- SINU
Italian Society of Nutrition
- SLAN
Survey of Lifestyle, Attitudes and Nutrition
- SNS
sympathetic nervous system
- SRC
standardised regression coefficient
- SUA
serum uric acid
- TEI
total energy intake
- TOPH
Trials of Hypertension Prevention
- UAlbumin
albumin urinary excretion
- UCa
calcium urinary excretion
- UCr
creatinine urinary excretion
- UK
potassium urinary excretion
- UL
tolerable upper intake level
- UNa
sodium urinary excretion
- UNU
United Nations University
- WHO
World Health Organization
Appendix A – Sodium concentration in breast milk from mothers of term infants in Western countries
1.
| Reference | N women (N samples) | Country | Stage of lactation | Sodium concentration mg/L (mmol/L) | Analytical method | |
|---|---|---|---|---|---|---|
| Mean ± SD | Median | |||||
| Koo and Gupta (1982) |
45 (5) (8) (8) (9) (5) (9) (9) (52) (52) |
Australia |
Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Days 8–14 Days 15–28 |
1,630 ± 262 (70.9 ± 11.4) 1,244 ± 200 (54.1 ± 8.7) 501 ± 71 (21.8 ± 3.1) 651 ± 108 (28.3 ± 4.7) 359 ± 64 (15.6 ± 2.8) 435 ± 92 (18.9 ± 4.0) 398 ± 53 (17.3 ± 2.3) 301 ± 14 (13.1 ± 0.6) 244 ± 14 (10.6 ± 0.6) |
Milk expressed manually Flame photometry |
|
| Keenan et al. (1982) |
(14) (14) (12) |
USA |
3.5–6 weeks 8.5–18 weeks 20–32 weeks |
182 ± 69 (7.9 ± 3.0) 108 ± 46 (4.7 ± 2.0) 124 ± 30 (5.4 ± 1.3) |
Milk expressed using an electric breast pump Flame photometry |
|
| Parr et al. (1991) | (71) | Hungary | 3 months | 105 ± 6 (4.6 ± 0.3) |
Milk expressed using breast pump AAS |
|
| (29) | Sweden | 88 ± 17 (3.8 ± 0.7) | ||||
| Holt (1993) | 4 (28) | UK | 5–16 weeks | 107 ± 29 (4.65 ± 1.24) |
Manual expression Flame photometry |
|
| Wack et al. (1997) | 30 (140) | USA |
0–60 days 61–120 days 121–180 days 181–240 days 241–300 days 301–360 days > 360 days |
182 ± 83 (7.9 ± 3.6) 129 ± 61(5.6 ± 2.7) 136 ± 76 (5.9 ± 3.3) 139 ± 142 (6.0 ± 6.2) 124 ± 65 (5.4 ± 2.8) 122 ± 123 (5.3 ± 5.3) 126 ± 49 (5.5 ± 2.1) |
Manual expression or breast pump Plasma emission spectrophotometry |
|
| Motil et al. (1997) | 11 adolescents (16.5 ± 0.6 years) | USA |
6 weeks 12 weeks 18 weeks 24 weeks |
136 ± 29 (5.9 ± 1.3) 127 ± 34 (5.5 ± 1.5) 93 ± 31 (4.0 ± 1.3) 116 ± 51 (5.0 ± 2.2) |
Milk expressed by manual or mechanical pumping AAS |
|
|
11 adults (31.1 ± 3.8 years) |
USA |
6 weeks 12 weeks 18 weeks 24 weeks |
94 ± 27 (4.1 ± 1.2) 71 ± 23 (3.1 ± 1.0) 70 ± 16 (3.0 ± 0.7) 75 ± 23 (3.3 ± 1.0) |
|||
| Fly et al. (1998) | 14 (28) | USA | 2–8 months |
Mean ± SE 115 ± 11 (5.00 ± 0.48) (at rest) 109 ± 5 (4.73 ± 0.22) (after exercise) |
Milk expressed mechanically (electric breast pump) ICP‐AES |
|
| Gulson et al. (2001) | 17 (68) | Australia (Australian and migrant subjects) | Not reported | 167.1 ± 62.4 (7 ± 2) | 170.0 | Not reported |
| Savilahti and Saarinen (2007) |
Exclusive breast‐feeding < 0.5 months: 109 (96) |
Finland | 1–5 days | 597 (26) | Not reported | |
|
Exclusive breast‐feeding > 3.5 months: 119 (106) |
Finland | 1–5 days | 459 (20) | Not reported | ||
| Manganaro et al. (2007) | 208a (208) | Italy | 3 days | 540 ± 25 (23 ± 1) | Mode of expression not reported. Flame photometer. | |
| Bauer and Gerss (2011) | 10 | Germany | Mixed (1–8 weeks; samples collected at weekly intervals from the first to the eighth week post‐partum) | 257 ± 48 (11.2 ± 2.1) |
Milk expressed mechanically (electric breast pump) AAS and colorimetry |
|
| Galipeau et al. (2012) | 151 (151) | Canada | Day 3 | 982 ± 508 (42.7 ± 22.1) | (range: 333–2,758 (14.5–120.0)) |
Milk expressed manually Gasometry apparatus |
| Bjorklund et al. (2012) | 60 (840) | Sweden | 14–21 days (samples collected daily for 7 days) | 217 ± 77 (9.4 ± 3.3) | 192 (range: 136–480) (8.3 (range: 5.9–20.9)) |
Milk sampled using a manual breast milk pump and/or a passive breast milk sampler ICP‐MS |
Studies were identified by a comprehensive literature search for publications from January 2010 to January 2014 (LASER Analytica, 2014) and additional searches of the literature before these dates. If studies did not report whether infants were born at term or not, it was presumed that infants were born at term.
AAS: atomic absorption spectrophotometry; ICP‐AES: inductively coupled plasma atomic emission spectroscopy; ICP‐MS: inductively coupled plasma mass spectrometry; N: number; SD: standard deviation; SE: standard error.
Including five mothers of preterm infants.
Appendix B – Comparisons of measured 24‐h urinary sodium excretions vs 24‐h urinary sodium excretions estimated from equations based on concentrations of sodium in spot urine samples
1.
| Reference | Population | Methods | Estimated 24‐h Na excretion (extrapolation method) Mean ± SD | Measured 24‐h Na excretion (reference) Mean ± SD | Correlation | Comparison | Bland–Altman analysis |
|---|---|---|---|---|---|---|---|
|
Kawasaki et al. (1993) Japan |
159 healthy M and W (20–79 years) (Data set used to derive the equation) |
Collection 3–5 × 2nd morning voiding urine collected within 4 h after the 1st voiding but before breakfast + Kawasaki equation to estimate 24 h Na Reference: 3–5 × 24‐h urine (same days) |
NR | NR | 0.728 | ||
| 91 healthy M and W (40–67 years) |
Collection 1 × 2nd morning voiding urine collected within 4 h after the 1st voiding but before breakfast + Kawasaki equation to estimate 24‐h Na Reference: 1 × 24‐h urine (same day) |
NR | NR | 0.531 |
No statistically significant difference was found between mean estimated 24‐h Na and measured 24‐h Na |
||
| 15 healthy M and W (21–54 years) |
Collection 3 successive × 2nd morning voiding urine collected within 4 h after the 1st voiding but before breakfast + Kawasaki equation to estimate 24‐h Na Reference: 3 successive × 24‐h urine (same days) Procedure repeated 4–6 times |
NR | NR | 0.821 |
No statistically significant difference was found between mean estimated 24‐h Na and measured 24‐h Na |
||
|
Tanaka et al. (2002) Japan |
295 M and 296 W (20–59 years) from INTERSALT (Data set used to derive the equation) |
1 × casual spot urine + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h urine collection (started after the casual collection) |
178.9 ± 36.2 mmol/day | 187.2 ± 65.8 mmol/day | 0.54 |
Difference between measured 24‐h Na and estimated 24‐h Na 8.3 mmol/day |
|
|
336 M and W (20–59 years) (Validation data set) |
1 × casual spot urine + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h urine collection (started after the casual collection) |
154.5 ± 32.7 mmol/day | 178.5 ± 59.5 mmol/day | 0.32 |
Difference between mean estimated 24‐h Na and mean measured 24‐h Na, by quintile of estimated 24‐h Na Q1: 45.4 mmol/day Q2: 27.6 mmol/day Q3: 25.6 mmol/day Q4: 21.4 mmol/day Q5: 0.5 mmol/day |
||
|
Brown et al. (2013) 15 countries from North America and Europe (INTERSALT) |
2,948 M and W (20–59 years), from 29 cities (Data set used to derive the equation) |
1 × casual spot urine + INTERSALT equation to estimate 24‐h Na Reference: 1 × 24‐h urine collection (undertaken after the casual collection) |
NR |
Mean (mix, max) M: Belgium, Charleroi: 147.2 mmol/day Poland, Krakow: 240 mmol/day W: Germany, Cottbus: 117.8 mmol/day Italy, Bassiano: 167.5 mmol/day |
M: 0.51 W: 0.52 |
||
| 2,745 M and W (20–59 years), from 29 cities (Validation data set) |
1 × casual spot urine + INTERSALT equation to estimate 24‐h Na Reference: 1 × 24‐h urine collection (undertaken after the casual collection) |
M: USA, Hawaii: 176.3 mmol/day Poland, Krakow: 223.6 mmol/day W: Iceland, Reykjavik: 126.6 mmol/day Italy, Bassiano: 178.0 mmol/day |
M: USA, Hawaii: 144.2 mmol/day Poland, Krakow: 239.7 mmol/day W: Iceland, Reykjavik: 115.0 mmol/day Italy, Bassiano: 170.6 mmol/day |
M: 0.50 W: 0.51 |
Difference between mean measured 24‐h Na and mean estimated 24‐h Na: M: −1.6 mmol W: +2.3 mmol |
Bland–Altman showed overestimation of excretion at lower levels and underestimation at higher levels for both men and women | |
| Mente et al. (2014) 11 countries | 1,083 M and W (35–70 years) |
1 × fasting morning collection + Kawasaki equation to estimate 24‐h Na Reference: 1 × 24‐h urine collection (previous day) |
4,430 ± 1,253 mg/day | 4,116 ± 1,978 mg/day |
Intraclass correlation coefficient 0.71 (95% CI: 0.65 to 0.76) |
Difference between estimated 24‐h Na and measured 24‐h Na: 313 (95% CI: 182 to 444) mg/day |
Bland–Altman plot evidenced a systematic bias towards overestimation at the lower end and underestimation at the higher end |
|
1 × fasting morning collection + INTERSALT equation to estimate 24‐h Na Reference: 1 × 24‐h urine collection (previous day) |
3,257 ± 860 mg/day | 4,116 ± 1,978 mg/day |
Intraclass correlation coefficient 0.49 (0.29 to 0.62) |
Difference between estimated 24‐h Na and measured 24‐h Na: −872 (−728 to −1016) mg/day |
Bias significantly higher than Kawasaki equation | ||
|
1 × fasting morning collection + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h urine collection (previous day) |
3,569 ± 782 mg/day | 4,116 ± 1,978 mg/day |
Intraclass correlation coefficient 0.54 (0.42 to 0.62) |
Difference between estimated 24‐h Na and measured 24‐h Na: −548 (−408 to −688) mg/day |
Bias significantly higher than Kawasaki equation | ||
| Pfister et al. (2014) UK | 163 M and W (EPIC‐Norfolk study) |
1 × casual spot urine sample + INTERSALT equation to estimate 24‐h Na Reference: Mean of up to 6 × 24 h collections over 1 year |
NR | NR | NR |
Mean of differences between measured and estimated 24‐h Na: −21 mmol/day (95% CI: −32 to −11 mmol/day) |
Variance of the differences reasonably constant, with few outliers and most points lying within the calculated limits of agreement |
| Cogswell et al. (2013) USA | 407 M and W (18–39 y, 52% white) |
1 morning spot urine specimen (first specimen after discarding the first void; 08:30–12:30) +INTERSALT equation to estimate 24‐h Na Reference: 1 × 24‐h collection (same day) |
3,157 ± 891 mg/day | 3,323 ± 1,437 mg/day | 0.47 |
Difference between estimated and measured 24‐h Na: –165 mg (95% CI: −295, −36) |
Overestimation occurred at the low levels of 24‐h Na excretion and underestimation at the high level |
|
1 afternoon spot urine specimen (12:31–17:30) +INTERSALT equation to estimate 24‐h Na Reference: 1 × 24‐h collection (same day) |
3,197 ± 824 mg/day | 3,287 ± 1,408 mg/day | 0.49 | −90 mg (95% CI: −208, 28) | |||
|
1 evening spot urine specimen (17:31–23:59) +INTERSALT equation to estimate 24‐h Na Reference: 1 × 24‐h collection (same day) |
3,178 ± 846 mg/day | 3,298 ± 1,399 mg/day | 0.54 | −120 mg (95% CI: −230, −11) | |||
|
1 overnight specimen (first void collected the next morning after the longest period of sleep (04:00–12:00)) + INTERSALT equation to estimate 24‐h Na Reference: 1 × 24‐h collection (same day) |
3,028 ± 809 mg/day | 3,295 ± 1,400 mg/day | 0.44 | −267 mg (95% CI: −384, −151) | |||
|
Morning as above + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h collection |
3,574 ± 893 mg/day | 3,323 ± 1,437 mg/day | 0.50 | 251 mg (95% CI: NR) | Overestimation occurred at the low levels of 24‐h Na sodium excretion and underestimation at the high levels | ||
|
Afternoon as above + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h collection (same day) |
3,655 ± 870 mg/day | 3,287 ± 1408 mg/day | 0.51 | 368 mg (95% CI: NR) | |||
|
Evening as above + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h collection (same day) |
3,553 ± 820 mg/day | 3,298 ± 1399 mg/day | 0.59 | 255 mg (95% CI: NR) | |||
|
Overnight as above + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h collection (same day) |
3,272 ± 779 mg/day | 3,295 ± 1400 mg/day | 0.47 | −23 mg (95% CI: −141, 95) | |||
|
Morning as above + Kawasaki equation to estimate 24‐h Na Reference: 1 × 24‐h collection (same day) |
4,623 ± 1,471 mg/day | 3,323 ± 1,437 mg/day | 0.52 | 1,300 mg (95% CI: 1,152, 1,300) | Overestimation appeared to occur across low to high levels of 24‐h Na excretion | ||
| Ji et al. (2014) UK/Italy | 915 untreated M and W (297 white, 326 of black African origin and 292 South Asian; 40–59 years old) |
1 × timed morning urine sample + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h urinary collection (previous day) |
NR | NR | From 0.055 in black women to 0.330 in white women | Bland–Altman plots indicated consistent bias with overestimate for low and underestimate for high intakes. The bias was mainly due to the inaccuracy of age, weight and height to predict 24‐h creatinine excretion in the three ethnic groups, particularly in those of African origin | |
|
1 × timed morning urine sample + arithmetic extrapolation to 24‐h scale Reference: 1 × 24‐h urinary collection (previous day) |
NR | NR | From 0.116 in black women to 0.367 in white women | Bias was detected with both Bland–Altman plots and through quintile analyses (underestimate at low levels and overestimate at high levels) | |||
| 148 white M (mean age 58.3 years), in Italy |
1 × timed morning urine sample + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h urinary collection (previous day) |
NR | NR | 0.499 | Bland–Altman plot indicated overestimated values in the low 24‐h urinary Na levels and underestimated values in the high 24‐h urinary Na levels indicating consistent bias | ||
|
1 × timed morning urine sample + arithmetic extrapolation to 24‐h scale Reference: 1 × 24‐h urinary collection |
NR | NR | 0.329 | Arithmetic extrapolation produced underestimated values in the low 24‐h urinary Na levels and overestimated values in the high 24‐h urinary Na levels indicating consistent bias | |||
| Polonia et al. (2017) Portugal | 2,399 M and W (51% W; aged 18–96 years) |
1 × casual urine sample + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h urinary collection (1–7 days before) |
By quintiles of measured 24‐h Na: Q1: 3,755 ± 876 Q2: 3,973 ± 862 Q3: 3,986 ± 803 Q4: 4,150 ± 837 Q5: 4,342 ± 801 |
By quintiles of measured 24‐h Na: Q1: 2,361 ± 280 Q2: 3,121 ± 192 Q3: 3,825 ± 219 Q4: 4,661 ± 279 Q5: 6,298 ± 921 |
0.232 Intraclass correlation coefficient 0.340 (95% CI: 0.285, 0.391) |
Difference between measured and estimated 24‐h Na Overall: 11 mg/day (95% CI: −48.6, 70.6) By quintiles of measured 24‐h Na: Q1:−1,394 ± 905 Q2:−852 ± 875 Q3:−161 ± 832 Q4: 511 ± 867 Q5: 1,956 ± 1,140 |
Bias was detected with both Bland–Altman plots and through quintile analyses (overestimate at low levels and underestimate at high levels). Bland–Altman plots indicated that formula‐based estimates seem to have poorer clarification at higher levels than lower levels |
| 2,399 M and W (51% W; aged 18–96 years) |
1 × casual urine sample + Kawasaki equation to estimate 24‐h Na Reference: 1 × 24‐h urinary collection (1–7 days before) |
By quintiles of measured 24‐h Na: Q1: 4,844 ± 1,402 Q2: 5,171 ± 1,414 Q3: 5,240 ± 1,363 Q4: 5,524 ± 1,398 Q5: 5,864 ± 1,365 |
By quintiles of measured 24‐h Na: Q1: 2,361 ± 280 Q2: 3,121 ± 192 Q3: 3,825 ± 219 Q4: 4,661 ± 279 Q5: 6,298 ± 921 |
0.25 Intraclass correlation coefficient 0.303 (95% CI: 0.050, 0.475) |
Overall: −1,277 (95% CI: −1,346.7, −1,206.4) By quintiles of measured 24‐h Na: Q1: −2,483 ± 1,409 Q2: −2,050 ± 1,413 Q3: −1,416 ± 1,380 Q4: −864 ± 1,408 Q5: 434 ± 1,533 |
Bias was detected with both Bland–Altman plots and through quintile analyses (overestimate at low levels and underestimate at high levels) | |
| 2,399 M and W (51% W; aged 18–96 years) |
1 × casual urine sample + INTERSALT equation to estimate 24‐h Na Reference: 1 × 24‐h urinary collection (1–7 days before) |
By quintiles of measured 24‐h Na: Q1: 3,019 ± 841 Q2: 3,289 ± 848 Q3: 3,467 ± 901 Q4: 3,677 ± 923 Q5: 3,965 ± 894 |
By quintiles of measured 24‐h Na: Q1: 2,361 ± 280 Q2: 3,121 ± 192 Q3: 3,825 ± 219 Q4: 4,661 ± 279 Q5: 6,298 ± 921 |
0.359 Intraclass correlation coefficient 0.457 (95% CI: 0.337, 0.549) |
Overall: 569 (95% CI: 512.4, 624.7) By quintiles of measured 24‐h Na: Q1: −658 ± 852 Q2: −168 ± 857 Q3: 357 ± 914 Q4: 984 ± 930 Q5: 2,333 ± 1,150 |
Bias was detected with both Bland–Altman plots and through quintile analyses (overestimate at low levels and underestimate at high levels) | |
| Zhou et al. (2017) China | 141 M and W (27–64 years) |
1 morning spot urine specimen + Kawasaki equation to estimate 24‐h Na Reference: 1 × 24‐h urinary collection (started after the spot collection) |
246.1 ± 66.8 mmol/day | 220.8 ± 78.5 mmol/day | 0.31 |
Median (95% CI) difference between measured and estimated 24‐h Na 6.4 (−17.5, 36.8) mmol/day The individual absolute difference was > 51.3 mmol/day (3 g salt) in 52.5% of the participants |
|
|
1 morning spot urine specimen + INTERSALT equation to estimate 24‐h Na Reference: 1 × 24‐h urinary collection (started after the spot collection) |
143.6 ± 24.7 mmol/day | 220.8 ± 78.5 mmol/day | 0.25 |
−67.3 (−96.5, −46.9) mmol/day The individual absolute difference was > 51.3 mmol/day (3 g salt) in 63.1% of the participants |
|||
|
1 morning spot urine specimen + Tanaka equation to estimate 24‐h Na Reference: 1 × 24‐h urinary collection (started after the spot collection) |
183.7 ± 39.0 mmol/day | 220.8 ± 78.5 mmol/day | 0.35 |
−42.9 (−59.1, −24.8) mmol/day The individual absolute difference was > 51.3 mmol/day (3 g salt) in 48.3% of the participants |
95% CI: 95% confidence interval; INTERSALT: International Cooperative Study on Salt, Other Factors and Blood Pressure; M: men; Na: Sodium; N/A: not available; NR: not reported; SD: standard deviation; W: women.
Appendix C – Daily sodium urinary excretion in children, boys and girls, in European countries
1.
| Country | Year | Methodology | Age (years) | Participants’ characteristics |
Na excreted in urine (mmol/day) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Median (P50) |
IQR (P25–P75) |
|||||
| Austria (Elmadfa, 2012) | 2010–2012 | Casual spot urine samples; 24‐h excretion estimated by multiplying Na concentration by an average urine volume of 1.1 L/day in children | 7–14 | 392 boys and girls |
Boys: 7–9 years: 153 10–12 years: 165 13–14 years: 154 Girls: 7–9 years: 144 10–12 years: 155 13–14 years: 122 |
N/A |
Boys: 7–9 years: 133–172 10–12 years: 151–178 13–14 years: 122–187 Girls: 7–9 years: 126–163 10–12 years: 143–167 13–14 years: 93–151 |
N/A | N/A |
| Iceland (Kristbjornsdottir et al., 2012) | 2002–2003 |
24‐h urine collection; Completeness of urine collection assessed by PABA recovery (≥ 85%). For samples with a PABA recovery between 50% and 85%, urinary Na excretion was corrected (formula from Johansson et al. (1999)) |
6 |
30 boys and 28 girls. A subsample of a national dietary survey, including 6‐year‐old children living in the greater Reykjavik area |
71 | 23 | N/A | N/A | N/A |
| Italy (Campanozzi et al., 2015) | 2008–2012 | 24‐h urine collection with control for urine volume and creatinine | 6–18 |
766 boys and 658 girls Recruited in participating National Health Service centres in 10 Italian regions |
Boys: 129 Girls: 117 |
N/A | N/A |
Boys: 120 Girls: 107 |
Boys: 84–162 Girls: 77–146 |
| Spain (Aparicio et al., 2017) | 2014 |
24‐h urine collection; Completeness of urine collection assessed on the basis of Cr excretion (samples with Cr excretion rate < 0.1 mmol/kg/day considered incomplete (Remer et al., 2002)) |
7–11 |
Boys: 109 Girls: 96 From five primary schools in various Spanish provinces |
Boys: 142 Girls: 122 |
Boys: 57 Girls: 41 |
N/A |
Boys: 136 Girls: 119 |
Boys: 122–177 Girls: 91–154 |
95% CI: 95% confidence interval; Cr: creatinine; IQR: interquartile range; Na: sodium; N/A: not available; PABA: para‐aminobenzoic acid; SD: standard deviation.
Appendix D – Daily sodium urinary excretion in adult men in European countries
1.
| Country (reference) | Year | Methodology | Age (years) | N and participants’ characteristics | Na excreted in urine (mmol/day)a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Median (P50) |
IQR (P25–P75)d |
|||||
|
Austria (Elmadfa, 2012) |
2010–2012 | Casual spot urine samples; 24‐h excretion estimated by multiplying Na concentration by an average urine volume of 1.75 L/day from a subsample of 19 people from whom 24‐h urine were collected |
18–64 65–80 |
N/A (total sample: 419 men and women aged 18–64 and 196 men and women aged 65–80) Sample stratified by gender, age and geographical areas |
18–24 years: 187 25–50 years: 191 51–64 years: 204 65–80 years: 183 |
N/A |
18–24 years: 152–226 25–50 years: 178–204 51–64 years: 174–235 65–80 years: 139–204 |
N/A | N/A |
|
Belgium (Koppen et al., 2015) |
2015 |
24‐h urine collection; exclusion of samples with Cr excretion levels outside the normal range of 0.177–0.230 mmol/kg per day for men and self‐reported as incomplete |
23–64 |
99 Sampled via an occupational health survey centre (random cluster sampling), spread over the different Belgian provinces |
179 | 63 | N/A | 172 | N/A |
| Belgium | 2007–2008 |
2 × 24‐h urine collection (1 month interval); completeness of urine collection assessed by PABA recovery (≥ 85%). For samples with a PABA recovery between 50% and 85%, urinary Na excretion was corrected (formula from Johansson et al. (1999)) |
45–65 | 63 | 209b | N/A | 195–223 | N/A | N/A |
| Czech Republicc | 58 | 252b | 78 | N/A | 241 |
P5–P95 149–395 |
|||
|
Norway (De Keyzer et al., 2015) |
62 Subsamples of the European EFCOVAL study; subjects recruited by convenience sampling through advertisements |
204b | N/A | 191–218 | N/A | N/A | |||
|
Croatia (Premužić et al., 2010) |
2009 |
24‐h urine collection; Completeness check not reported |
46.3 ± 7.3 |
N/A (total sample of 93 men and women) From two out‐patient clinics (one urban, one rural); ‘salt‐mapping survey’ |
231 | 74 | N/A | N/A | N/A |
|
Croatia (Dika et al., 2009) |
2009 | Morning spot urine samples; 24‐h Na excretion estimated by applying Kawasaki, INTERSALT and Tanaka equations | N/A |
N/A (total sample of 1,669 men and women); Random sample (door‐to‐door method) in the continental rural part of Croatia |
Kawasaki: 229 | 80 | N/A | N/A | N/A |
| INTERSALT: 194 | 40 | N/A | N/A | N/A | |||||
| Tanaka: 186 | 49 | N/A | N/A | N/A | |||||
|
Finland (Laatikainen et al., 2006) |
2002 |
24‐h urine collection; exclusion of incomplete samples with Cr levels ≤ 5.0 mmol/day or Cr levels ≤ 6.0 mmol/day together with a urine volume < 1,000 mL |
25–64 |
423 North Karelia, n = 168 Southwestern Finland, n = 128 Helsinki area, n = 127 Sampled as part of the national FINRISK 2002 study; 10‐year age group and sex‐stratified subsample of the population aged 25–64 years drawn in north Karelia, southwestern Finland and in the Helsinki area |
North Karelia: 163 Southwestern Finland: 170 Helsinki area: 148 |
N/A |
North Karelia: 153–173 Southwestern Finland: 156–183 Helsinki area: 132–164 |
N/A | N/A |
|
Germany (Johner et al., 2015) |
2008–2011 | Casual spot urine samples; 24‐h Na excretion estimated from the Na:Cr ratio by multiplication with age‐ and sex‐stratified Cr excretion reference values (Remer et al., 2002) | 18–79 |
3,340 18–29 years, n = 507 30–39 years, n = 403 40–49 years, n = 586 50–59 years, n = 630 60–69 years, n = 671 70–79 years, n = 543 Random sample, as part of the German National Health Interview and Examination Survey 2008–2011, representative for the German adult population |
N/A | N/A |
Overall: 165–177 18–29 years: 148–173 30–39 years: 163–197 40–49 years: 148–173 50–59 years: 167–189 60–69 years: 165–194 70–79 years: 158–178 |
Overall: 170 18–29 years: 160 30–39 years: 180 40–49 years: 163 50–59 years: 177 60–69 years: 177 70–79 years: 167 |
Overall: 117–252 18–29 years: 113–263 30–39 years: 123–264 40–49 years: 110–231 50–59 years: 115–261 60–69 years: 117–252 70–79 years: 118–245 |
|
Greece (unpublished data) |
2013–2014 | For 7 days, the weight and time of each void was recorded and a sample of urine was collected. For each day, a sample of 10 mL was reconstituted from the individual samples based on the ratio of the volume of each void to the total volume of urinary excretion for the day. Exclusion of samples with Cr levels > 3,500 mg/day or < 350 mg/day | 20–60 | 89 Participants equally divided in each decade of life. | 159 | 51 | N/A | 148 | |
|
Greece (Vasara et al., 2017) |
2015–2016 | 24‐h urine collection; exclusion of incomplete samples on the basis of (a) Urinary volume < 500 mL/24‐h, (b) urinary creatinine less than 2 SD from the mean, (c) timing of collection outside the range 23–25 h, (d) self‐reporting of incomplete collection | 18–74 |
114 Salt intake in northern Greece (SING) Study – Regional study conducted in Thessaloniki greater metropolitan area. Recruitment was carried out at various sites and venues including churches and workplaces |
194.3 | 76.8 | 180.1–208.6 | 181.9 | N/A |
|
Hungary (unpublished data) |
2010 | 24‐h urine collection; completeness of the samples checked on the basis of participants’ compliance with the protocol | Adults (age not specified) |
67 Random sample of the Hungarian adult population recruited through primary care physicians |
190 | 73 | N/A | N/A | N/A |
| Ireland (Morgan et al., 2008) | 2007 | Spot urine samples; daily Na excretion estimated through arithmetic extrapolation (Na concentration (mmol/L) multiplied by 1.97 for men) | ≥ 18 |
484 Random sample of both Irish citizens and non‐Irish national residents sampled as part of the Survey of Lifestyle, Attitudes and Nutrition (SLAN) 2007, representative of the general population in Ireland |
≥ 18 years: 176 45–64 years: 188 ≥ 65 years: 158 |
≥ 18 years: 85 45–64 years: 88 ≥ 65 years: 78 |
N/A | N/A | N/A |
| Ireland (Kearney et al., 2013) | 2010–2011 | Morning urine samples; daily Na excretion estimated through arithmetic extrapolation (Na concentration (mmol/L) multiplied by 1.97 for men) | 45–74 |
999 Registered patients attending the Living Health Clinic of Mitchelstown, Ireland, sampled as part of the Cork and Kerry Diabetes and Heart Disease Study Phase II |
Overall: 211 45–54 years: 218 55–64 years: 211 65–74 years: 201 |
Overall: 89 45–54 years: 92 55–64 years: 87 55–74 years: 89 |
N/A | N/A | N/A |
|
Italye |
2009–2012 | 24‐h urine collection; A sample if 24‐h urine volume < 500 mL or creatinine content referred to body weight < mean minus 2 SD from the population mean | 35–79 |
1,962 Random samples of the Italian adult population from 20 Italian regions, stratified by age and sex (MINISAL‐GIRCSI Programme) |
Overall: 183 35–44 years: 184 45–54 years: 190 55–64 years: 182 65–74 years: 180 75–79 years: 176 |
Overall: 70 35–44 years: 72 45–54 years: 70 55–64 years: 70 65–74 years: 70 75–79 years: 60 |
Overall: 180–187 35–44 years: 178–191 45–54 years: 184–197 55–64 years: 176–188 65–74 years: 173–187 75–79 years: 166–185 |
Overall: 175 35–44 years: 174 45–54 years: 182 55–64 years: 173 65–74 years: 170 75–79 years: 172 |
N/A |
| Slovenia (Ribic et al., 2010) | 2005 | 24‐h urine collection; exclusion of incomplete samples with Cr level < 120 μmol/kg bw per day for men (Osredkar, 1998) | 25–65 |
61 Participants randomly sampled from census data from all regions, representative of the general population in Slovenia |
221 | 86 | N/A | N/A | N/A |
| Spain (Ortega et al., 2011) | 2009 | 24‐h urine collection; completeness of the samples assessed by considering the correlation between urinary Cr and FFM of each subject; FFM estimated from 24‐h Cr excretion and result compared with measured FFM obtained by electrical bioimpedance method (Lopez‐Sobaler and Quintas et al., 2006) | 18–60 |
196 Selected as a representative sample of the Spanish young and middle‐aged adult population (from 15 randomly selected provinces; stratified by age and sex) |
196 | 82 | N/A | 196 | 140–250 |
| Sweden (Hulthen et al., 2010) | 2005 | 24‐h urine collection; completeness of urine collection assessed by PABA recovery (≥ 85%) | 18−20 |
79 Participants recruited in the city of Gothenburg, as part of the Gothenburg Obesity and Osteoporosis Determinants (GOOD) study |
198 | 69 | N/A | N/A | |
| Switzerland (Chappuis et al., 2011) | 2010–2011 | 24‐h urine collection; exclusion of incomplete samples on the basis of: (1) urinary volume < 300 mL/24 h, (2) self‐reporting of incomplete collection, or (3) Cr level ≤ 0.121 mmol/kg bw per day in men | ≥ 15 |
706 Age‐ and sex‐ stratified sample in various cantons of Switzerland, randomly selected. The low participation rate was compensated by recruiting volunteers |
Overall: 185 15–29 years: 171 30–44 years: 190 45–59 years: 194 ≥ 60 years: 180 |
N/A | N/A | N/A | |
| Netherlands (Hendriksen et al., 2014) | 2010 | 24‐h urine collection; exclusion of incomplete samples on the basis of (1) Cr excretion ≤ 5 mmol/day, or ≤ 6 mmol/day together with a urine volume < 1,000 mL or (2) missing or overcollection of more than one urine void | 19–70 |
180 Individuals participating in an ongoing long‐term monitoring study on chronic disease risk factors (the Doetinchem Cohort Study (DCS)) or randomly drawn from the municipal register of Doetinchem (General Doetinchem Population Sample (GDPS)) |
174 | 63 | N/A | 163 | 126–212 |
| Netherlands (Hendriksen et al., 2016) | 2015 |
24‐h urine collection; start and end time of the urine collection were recorded. Participants had to report urine losses Based on this data, the researchers determined whether participants had an incomplete urine collection |
19–70 |
135 Participants aged 50–70 recruited from an ongoing long‐term monitoring study on chronic disease risk factors (the Doetinchem Cohort Study (DCS)); Participants aged 19–49 randomly drawn from the municipal register of Doetinchem (General Doetinchem Population Sample (GDPS)); Exclusion of individuals had participated in the 24‐h urine test in 2006 and 2010 in the study of Hendriksen et al. (2014), kidney patients and pregnant women |
N/A | N/A | N/A |
Overall: 153 19–49 years: 146 50–70 years: 174 |
Overall: 125–202 19–49 years: 110–195 50–70 years: 138–214 |
| United Kingdom (Sadler et al., 2011) | 2011 | 24‐h urine collection; exclusion of samples on the basis of PABA recovery (< 70% (incomplete) or > 104% (unfeasibly high); individuals who elected not to take PABA, or did not take all PABA tablets, but recorded they had completed a 24‐h urine collection were included if recorded collection time was collected between 23 and 25 h | 19–64 |
250 Participants from the England National Diet and Nutrition Survey sample and from a ‘Na boost’ study; stratified sample randomly selected in various regions of England |
19–34 years: 162 35–49 years: 171 50–64 years: 141 |
19–34 years: 69 35–49 years: 66 50–64 years: 82 | N/A |
19–34 years: 159 35–49 years: 165 50–64 years: 133 |
P2.5–P97.5 19–34 years: 72–296 35–49 years: 73–321 50–64 years: 54–308 |
bw: body weight; 95% CI: 95% confidence interval; Cr: creatinine; EFCOVAL: European Food Consumption Validation; FFM: fat‐free mass; INTERSALT: International Cooperative Study on Salt, Other Factors and Blood Pressure; IQR: interquartile range; N: number; Na: sodium; N/A: not available; PABA: para‐aminobenzoic acid; SD: standard deviation.
For comparison purposes, results provided in g NaCl/day were converted back in mmol/day by multiplying by 0.4 and dividing by 23.
Geometric means; based on two 24‐h collection per subject.
The values reported are unpublished data provided by the National Institute of Public Health of the Czech Republic.
Unless indicated otherwise.
The values reported are unpublished data provided by the Italian Instituto Superiore di Sanità.
Appendix E – Daily sodium urinary excretion in adult women in European countries
1.
| Country | Year | Methodology | Age (years) | N and participants’ characteristics | Na excreted in urine (mmol/day)a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Median (P50) |
IQR (P25–P75)b |
|||||
|
Austria (Elmadfa, 2012) |
2010–2012 | Casual spot urine samples; 24‐h excretion estimated by multiplying Na concentration by an average urine volume of 1.75 L/day from a subsample of 19 people from whom 24‐h urine were collected |
18–64 65–80 |
NA (total sample: 419 men and women aged 18–64 and 196 men and women aged 65–80) Sample stratified by gender, age and geographical areas |
18–24 years: 157 25–50 years: 161 51–64 years: 130 65–80 years: 152 |
N/A |
18–24 years: 117–200 25–50 years: 143–178 51–64 years: 104–157 65–80 years: 126–174 |
N/A | N/A |
|
Belgium (Koppen et al., 2015) |
2015 |
24‐h urine collection; exclusion of samples with Cr excretion levels outside the normal range of 0.133–0.177 mmol/kg per day for women and self‐reported as incomplete |
23–64 |
106; Sampled via an occupational health survey centre (random cluster sampling), spread over the different Belgian provinces |
137 | 56 | N/A | 122 | N/A |
| Belgium | 2007–2008 |
2 × 24‐h urine collection (1 month interval); completeness of urine collection assessed by PABA recovery (≥ 85%). For samples with a PABA recovery between 50% and 85%, urinary Na excretion was corrected (formula from Johansson et al. (1999)) |
45–65 | 60 | 173b | N/A | 161–185 | N/A | N/A |
| Czech Republica | 60 | 182b | 56 | 173 |
P5‐P95 101–288 |
||||
|
Norway (De Keyzer et al., 2015) |
62 Subsamples of the European EFCOVAL study; subjects recruited by convenience sampling through advertisements |
151b | N/A | 141–161 | N/A | N/A | |||
|
Croatia (Premužić et al., 2010) |
2009 |
24‐h urine collection; completeness check not reported |
46.3 ± 7.3 |
N/A (total sample of 93 men and women); From two out‐patient clinics (one urban, one rural); ‘salt‐mapping survey’ |
177 | 73 | N/A | N/A | |
|
Croatia (Dika et al., 2009) |
2009 | Morning spot urine samples; 24‐h Na excretion was estimated by applying Kawasaki, INTERSALT and Tanaka equations | N/A |
N/A (total sample of 1,669 men and women); Random sample (door‐to‐door method) in the continental rural part of Croatia |
Kawasaki: 214 | 78 | N/A | N/A | |
| INTERSALT: 222 | 56 | N/A | N/A | ||||||
| Tanaka: 178 | 69 | N/A | N/A | ||||||
| Finland (Laatikainen et al., 2006) | 2002 |
24‐h urine collection; exclusion of incomplete samples with Cr levels ≤ 5.0 mmol/day or Cr levels ≤ 6.0 mmol/day together with a urine volume < 1,000 mL |
25–64 |
486 North Karelia, n = 174 Southwestern Finland, n = 156 Helsinki area, n = 156 Sampled as part of the national FINRISK 2002 study; 10‐year age group and sex‐stratified subsample of the population aged 25–64 years drawn in north Karelia, southwestern Finland and in the Helsinki area |
North Karelia: 128 Southwestern Finland: 127 Helsinki area: 119 |
N/A |
North Karelia: 120–135 Southwestern Finland: 119–135 Helsinki area: 111–127 |
N/A | |
| Germany (Johner et al., 2015) | 2008–2011 | Casual spot urine samples; 24‐h Na excretion estimated from the Na:Cr ratio by multiplication with age‐ and sex‐stratified Cr excretion reference values (Remer et al., 2002) | 18–79 |
3,622 18–29 years, n = 507 30–39 years, n = 403 40–49 years, n = 586 50–59 years, n = 630 60–69 years, n = 671 70–79 years, n = 543 Random sample, as part of the German National Health Interview and Examination Survey 2008–2011, representative for the German adult population |
N/A | N/A |
Overall: 138–148 18–29 years: 116–136 30–39 years: 129–153 40–49 years: 141–165 50–59 years: 146–167 60–69 years: 134–153 70–79 years: 127–144 |
Overall: 143 18–29 years: 126 30–39 years: 139 40–49 years: 155 50–59 years: 156 60–69 years: 146 70–79 years: 134 |
Overall: 92–217 18–29 years: 85–184 30–39 years: 96–205 40–49 years: 103–226 50–59 years: 96–235 60–69 years: 87–212 70–79 years: 90–223 |
|
Greece (unpublished data) |
2013–2014 | For 7 days, the weight and time of each void were recorded and a sample of urine was collected. For each day, a sample of 10 mL was reconstituted from the individual samples based on the ratio of the volume of each void to the total volume of urinary excretion for the day. Exclusion of samples on the basis of Cr excretion (> 3,500 mg/day or < 350 mg/day) | 20–60 |
83 Participants equally divided in each decade of life |
130 | 48 | N/A | 124 | |
|
Greece (Vasara et al., 2017) |
2015–2016 | 24‐h urine collection; exclusion of incomplete samples on the basis of (a) urinary volume < 500 mL/24 h, (b) urinary creatinine less than 2 SD from the mean, (c) timing of collection outside the range 23–25 h and (d) self‐reporting of incomplete collection | 18–75 |
138 Salt intake in northern Greece (SING) Study – regional study conducted in Thessaloniki greater metropolitan area. Recruitment was carried out at various sites and venues including churches and workplaces |
158.5 | 64.1 | 147.7–169.3 | 151.0 | |
|
Hungary (unpublished data) |
2010 | 24‐h urine collection; completeness of the samples checked on the basis of participants’ compliance with the protocol | Adults (age not specified) |
86 Random sample of the Hungarian adult population recruited through primary care physicians |
163 | 71 | N/A | N/A | |
| Ireland (Morgan et al., 2008) | 2007 | Spot urine samples; daily Na excretion estimated through arithmetic extrapolation (Na concentration (mmol/L) multiplied by 1.67 for women) | ≥ 18 |
611 Random sample of both Irish citizens and non‐Irish national residents sampled as part of the Survey of Lifestyle, Attitudes and Nutrition (SLAN) 2007, representative of the general population in Ireland |
≥ 18 years: 128 45–64 years: 132 ≥ 65 years: 116 |
≥ 18 years: 72 45–64 years: 75 ≥ 65 years: 62 |
N/A | N/A | N/A |
| Ireland (Kearney et al., 2013) | 2010–2011 | Morning urine samples; daily Na excretion estimated through arithmetic extrapolation (Na concentration (mmol/L) multiplied by 1.67 for women) | 45–74 |
1,029 Registered patients attending the Living Health Clinic of Mitchelstown, Ireland, sampled as part of the Cork and Kerry Diabetes and Heart Disease Study Phase II |
overall: 129 45–54: 138 55–64: 128 65–74: 125 |
overall: 64 45–54: 72 55–64: 61 65–74: 59 |
N/A | N/A | N/A |
|
Italyc (Donfrancesco et al., 2013; Cappuccio et al., 2015) |
2009–2012 | 24‐h urine collection; A sample if 24‐h urine volume < 500 mL or creatinine content referred to body weight < mean minus 2 SD from the population mean | 35–79 |
1,900 Random samples of the Italian adult population from 20 Italian regions, stratified by age and sex (MINISAL‐GIRCSI Programme) |
Overall: 142 35–44 years: 142 45–54 years: 147 55–64 years: 143 65–74 years: 140 75–79 years: 135 |
Overall: 57 35–44 years: 61 45–54 years: 58 55–64 years: 54 65–74 years: 55 75–79 years: 56 |
Overall: 139–145 35–44 years: 136–148 45–54 years: 141–152 55–64 years: 138–148 65–74 years: 135–145 75–79 years: 126–144 |
Overall: 136 35–44 years: 135 45–54 years: 141 55–64 years: 138 65–74 years: 132 75–79 years: 129 |
|
| Slovenia (Ribic et al., 2010) | 2005 | 24‐h urine collection; exclusion of incomplete samples with Cr level < 120 μmol/kg bw per day for men (Osredkar, 1998) | 25–65 |
82 Participants randomly sampled from census data from all regions, representative of the general population in Slovenia |
170 | 74 | N/A | N/A | N/A |
| Spain (Ortega et al., 2011) | 2009 | 24‐h urine collection; completeness of the samples assessed by considering the correlation between urinary Cr and FFM of each subject; FFM estimated from 24‐h Cr excretion and result compared with measured FFM obtained by electrical bioimpedance method (Lopez‐Sobaler and Quintas et al., 2006) | 18–60 |
222 Selected as a representative sample of the Spanish young and middle‐aged adult population (from 15 randomly selected provinces; stratified by age and sex) |
143 | 66 | N/A | 131 | 97–178 |
| Switzerland (Chappuis et al., 2011) | 2010–2011 | 24‐h urine collection; exclusion of incomplete samples on the basis of: (1) urinary volume < 300 mL/24 h, (2) self‐reporting of incomplete collection, or (3) Cr level ≤ 0.082 mmol/kg bw per day in women | ≥ 15 |
742 Age‐ and sex‐ stratified sample in various cantons of Switzerland, randomly selected. The low participation rate was compensated by recruiting volunteers |
Overall: 137 15–29 years: 135 30–44 years: 140 45–59 years: 144 ≥ 60 years: 125 |
N/A | N/A | N/A | N/A |
| Netherlands (Hendriksen et al., 2014) | 2010 | 24‐h urine collection; exclusion of incomplete samples on the basis of (1) Cr excretion ≤ 5 mmol/day, or ≤ 6 mmol/day together with a urine volume < 1,000 mL or (2) missing or overcollection of more than one urine void | 19–70 |
180 Individuals participating in an ongoing long‐term monitoring study on chronic disease risk factors (the Doetinchem Cohort Study (DCS)) or randomly drawn from the municipal register of Doetinchem (General Doetinchem Population Sample (GDPS)) |
128 | 43 | N/A | 122 | 96–154 |
| Netherlands (Hendriksen et al., 2016) | 2015 |
24‐h urine collection; Start and end time of the urine collection were recorded. Participants had to report urine losses Based on this data, the researchers determined whether participants had an incomplete urine collection |
19–70 |
154 Participants aged 50–70 recruited from an ongoing long‐term monitoring study on chronic disease risk factors (the Doetinchem Cohort Study (DCS)); Participants aged 19–49 randomly drawn from the municipal register of Doetinchem (General Doetinchem Population Sample (GDPS)); Exclusion of individuals had participated in the 24‐h urine test in 2006 and 2010 in the study of Hendriksen et al. (2014), kidney patients and pregnant women |
N/A | N/A | N/A |
Overall: 120 19–49 years: 122 50–70 years: 113 |
Overall: 93–146 19–49 years: 103–148 50–70 years: 85–140 |
| United Kingdom (Sadler et al., 2011) | 2011 |
24‐h urine collection; exclusion of samples on the basis of PABA recovery (< 70% (incomplete) or > 104% (unfeasibly high); individuals who elected not to take PABA, or did not take all PABA tablets, but recorded they had completed a 24‐h urine collection were included if recorded collection time was collected between 23–25 h |
19–64 |
297 Participants from the England National Diet and Nutrition Survey sample and from a ‘sodium boost’ study; stratified sample randomly selected in various regions of England |
19–34 years: 122 35–49 years: 116 50–64 years: 112 |
19–34 years: 55 35–49 years: 52 50–64 years: 59 |
N/A |
19–34 years: 120 35–49 years: 104 50–64 years: 108 |
P2.5–P97.5 19–34 years: 47–261 35–49 years: 57–206 50–64 years: 44–218 |
bw: body weight; 95% CI: 95% confidence interval; Cr: creatinine; EFCOVAL: European Food Consumption Validation; FFM: fat‐free mass; IQR: interquartile range; N: number; Na: sodium; N/A: not available; PABA: para‐aminobenzoic acid, SD: standard deviation.
For comparison purposes, results provided in g NaCl/day were converted back in mmol/day by multiplying by 0.4 and dividing by 23.
Geometric means; based on two 24‐h collection per subject.
The values reported are unpublished data provided by the National Institute of Public Health of the Czech Republic.
Unless indicated otherwise.
The values reported are unpublished data provided by the Italian Instituto Superiore di Sanità.
Appendix F – Balance studies
F.1. Evidence table
| Study | Subjects | Conditions | Duration | Na intake (mean) | Balance (mean ± SD) | Regulatory hormones | Limitations |
|---|---|---|---|---|---|---|---|
| Data in adults | |||||||
| Holbrook et al. (1984) |
12 M/16 F (20–53 years) |
Free living | 7 day × 4 (1 week per season) |
All = 3.4 g (148 mmol)/day M = 4.2 g (183 mmol)/day F = 2.7 g (117 mmol)/day |
All = +0.47 ± 0.32 g (+20 ± 14 mmol)/day M = +0.73 ± 0.83 g (+32 ± 36 mmol)/day F = +0.26 ± 0.48 g (11 ± 21 mmol)/day |
Not measured | Dermal losses not measured |
| Allsopp (1997); Allsopp et al. (1998) |
25 M (18–40 years) |
Controlled 25°C + 40°C |
3 day + 5 day |
Low = 1.5 g (65 mmol)/day Mod = 4 g (174 mmol)/day High = 8 g (348 mmol)/day |
On day 8 +0.04 ± 0.35 g +0.79 ± 0.64 g +0.67 ± 1.19 g |
Aldosterone ↑/↑ Aldosterone –/↑ Aldosterone –/– |
– |
| Nishimuta et al. (2012) |
131 F (18–26 years) (13 studies) |
Controlled | 2–4 day + 8–12 day | 2.5 g (107 mmol)‐4.8 g (209 mmol)/day | +6.07 ± 4.06 mg/kg bw | Not measured | Sweat loss in five studies only |
| Data in children | |||||||
| Palacios et al. (2004) | 36 adolescent girls (14 W, 22 B; 12.4 ± 0.3 years) | Semi‐controlled. Summer time | 3 weeks × 2 (crossover) |
Low intake: 1.31 g/day High intake: 3.95 g/day |
Low intake: +0.4 ± 0.07 g/day among blacks (n = 19) +0.2 ± 0.04 g/day among whites (n = 12) High intake: +1.0 ± 0.14 g/day among blacks (n = 19) +0.3 ± 0.09 g/day among whites (n = 10) |
Aldosterone ↑ Aldosterone ↑ Aldosterone – Aldosterone – |
– |
B: black; bw: body weight; F: female; M: male; Na: sodium; SD: standard deviation; W: white.
F.2. Mechanistic data
Appendix G – Literature screening and RoB appraisal
G.1. Blood pressure, hypertension and CVD outcomes
G.1.1. PRISMA chart
G.1.2. Reference list of studies excluded based on full text screening
n = 98, not eligible because of study design
Abu‐Saad K, Novikov I, Gimpelevitz I, Benderly M, Alpert G, Goldbourt U, Kalter‐Leibovici O, 2017. Micronutrient intake and adherence to DASH diet are associated with incident major adverse cardiovascular events and all‐cause mortality in a bi‐ethnic population. European heart journal. Conference: European society of cardiology, ESC congress 2017. Spain, 38, 1120.
Adamzik M, Frey UH, Bitzer K, Jakob H, Baba HA, Schmieder RE, Schneider MP, Heusch G, Peters J, Siffert W, 2008. A novel‐1364A/C aquaporin 5 gene promoter polymorphism influences the responses to salt loading of the renin‐angiotensin‐aldosterone system and of blood pressure in young healthy men. Basic Research in Cardiology, 103, 598–610.
Alderman MH, Lamport B, 1990. Moderate sodium restriction: do the benefits justify the hazards? American Journal of Hypertension, 3, 499–504.
Alderman M, McCarron D, Graudal N, 2016. Strategy to optimize sodium intake for individual patients. Journal of hypertension. Conference: 26th european meeting on hypertension and cardiovascular protection, ESH 2016. France, 34, e78.
Anonymous, 1989. The INTERSALT study. An international co‐operative study of electrolyte excretion and blood pressure: further results. Journal of human hypertension, 3, 279–407.
Anonymous, 2016. American Society of Hypertension 31st Annual Scientific Meeting. Journal of the American Society of Hypertension, 10.
Anonymous, 2017. Abstracts from the 38th Annual Scientific Meeting of the High Blood Pressure Research Council of Australia. Hypertension, 69.
Asayama K, Stolarz‐Skrzypek K, Persu A, Staessen JA, 2014. Systematic review of health outcomes in relation to salt intake highlights the widening divide between guidelines and the evidence. American Journal of Hypertension, 27, 1138–1142.
Australian national health and Medical Research Council Management Committee, 1987. Australian Dietary Salt Study in mild Hypertension. Study Design, Protocol and Pilot Study. Mild hypertension: from drug trials to practice, V, 165–180.
Baciu A, 2012. Essential arterial hypertension occurring in children and teenagers anthropological correlations. International Journal of Collaborative Research on Internal Medicine and Public Health, 4, 1017–1039.
Bosu WK, 2016. Determinants of Mean Blood Pressure and Hypertension among Workers in West Africa. International Journal of Hypertension, 2016, 3192149.
Brown Jr WJ, Brown FK, Krishan I, 1971. Exchangeable sodium and blood volume in normotensive and hypertensive humans on high and low sodium intake. Circulation, 43, 508–519.
Charllton K, Steyn K, Levitt N, Lombard C, 2013. From research to policy in chronic disease prevention: mandatory salt reduction in South Africa. Annals of Nutrition and Metabolism, 63, 820.
Charlton K, Ware LJ, Menyanu E, Biritwum RB, Naidoo N, Pieterse C, Madurai SL, Baumgartner J, Asare GA, Thiele E, Schutte AE, Kowal P, 2016. Leveraging ongoing research to evaluate the health impacts of South Africa's salt reduction strategy: a prospective nested cohort within the WHO‐SAGE multicountry, longitudinal study. British Medical Journal Open, 6, e013316.
Chen L, He F, Dong Y, Harshfield GA, Zhu H, 2017. Sodium reduction, miRNA profiling and insights into cardiovascular phenotypes in hypertensives. Circulation. Conference: resuscitation science symposium, Press 2017. United states, 136.
Cheung BM, Ho SP, Cheung AH, Lau CP, 2000. Diastolic blood pressure is related to urinary sodium excretion in hypertensive Chinese patients. An International Journal of Medicine, 93, 163–168.
Cooper RS, Rotimi CN, Kauftnan JS, Muna WFT, Mensah GA, 1998. Hypertension treatment and control in sub‐Saharan Africa: The epidemiological basis for policy. British Medical Journal, 316, 614–617.
Cooper R, Soltero I, Liu K, Berkson D, Levinson S, Stamler J, 1980. The association between urinary sodium excretion and blood pressure in children. Circulation, 62, 97–104.
Corcoran AC, Taylor RD, Page IH, 1951. Controlled observations on the effect of low sodium dietotherapy in essential hypertension. Circulation, 3, 1–16.
Cusi D, Barlassina C, Azzani T, Casari G, Citterio L, Devoto M, Glorioso N, Lanzani C, Manunta P, Righetti M, Rivera R, Stella P, Troffa C, Zagato L, Bianchi G, 1997. Polymorphisms of alpha‐adducin and salt sensitivity in patients with essential hypertension. Lancet, 349, 1353–1357.
Dahl LK, 1972. Salt and hypertension. American Journal of Clinical Nutrition, 25, 231–244.
del Negro MC, Oliver M, Arocha I, Arias F, 1992. Arterial hypertension in the adolescent: its possible relation to the salt taste threshold. Revista española de cardiología, 45, 227–231.
DiNicolantonio JJ, Di Pasquale P, Taylor RS, Hackam DG, 2013. Retraction. Low sodium versus normal sodium diets in systolic heart failure: systematic review and meta‐analysis. Heart. Published Online First: 21 August 2012 https://doi.org/10.1136/heartjnl-2012-302337. Heart, 99, 820.
Ducher M, Fauvel JP, Maurin M, Laville M, Maire P, Paultre CZ, Cerutti C, 2003. Sodium intake and blood pressure in healthy individuals. Journal of Hypertension, 21, 289–294.
Fagerberg B, Andersson OK, Persson B, Hedner T, Hedner J, Towle A, 1985. Fluid homeostasis and haemodynamics during sodium restriction in hypertensive men. Journal of hypertension. Suppl, 3, S327–329.
Flottorp S, Farah MG, Thurmer H, Johansen M, Fretheim A, 2008. NIPH Systematic Reviews: Executive Summaries. Non‐Pharmacological Interventions to Reduce the Risk for Cardiovascular Disease: A Summary of Systematic Reviews.
Foroughi M, Akhavanzanjani M, Maghsoudi Z, Ghiasvand R, Khorvash F, Askari G, 2013. Stroke and nutrition: a review of studies. International Journal of Preventive Medicine, 4, S165–179.
Ghahremani L, Ghodrati K, Kojuri J, Fararouei M, 2016. The effect of educational intervention based on the health belief model on hypertensive population in rural areas of Sarvestan city, Iran: a randomized controlled trial. Global journal of health science, 9, 59663.
Graudal N, 2015. The data show a U‐shaped association of sodium intake with cardiovascular disease and mortality. American Journal of Hypertension, 28, 424–425.
Grobbee DE, Hofman A, 1986. Does sodium restriction lower blood pressure? BRITISH MEDICAL JOURNAL, 293, 27–29.
Gudmundsson O, 1984. Sodium and blood pressure. Studies in young and middle‐aged men with a positive family history of hypertension. Acta medica Scandinavica. Supplementum, 688, 1–65.
Gudmundsson O, Andersson OK, Aurell M, Wikstrand JM, Berglund GL, 1984. Calf muscle haemodynamics and the renin‐angiotensin‐aldosterone system in normotensive subjects with a familial predisposition to hypertension: changes during increased salt intake. Journal of Hypertension, 2, 291–296.
Gudmundsson O, Berglund G, Herlitz H, Andersson O, Jonsson O, 1983. Influence of age on the response to increased salt intake: Effects on blood pressure and sodium in erythrocytes. Journal of Hypertension, 1, 15–17.
Harsha DW, Bray G A, 2008. Weight loss and blood pressure control (Pro). Hypertension, 51, 1420–1425.
He FJ, MacGregor GA, 2011. Salt reduction lowers cardiovascular risk: Meta‐analysis of outcome trials. The Lancet, 378, 380–382.
He FJ, Pombo‐Rodrigues S, MacGregor GA, 2014. Salt reduction in England from 2003 to 2011: Its relationship to blood pressure, stroke and ischaemic heart disease mortality. British Medical Journal Open, 4, e004549.
Irwin BL, Schuck C, 1951. Observations of patients on low‐sodium diets. Journal of the American Dietetic Association, 27, 1066–1070.
Joshi S, Gupta S, Tank S, Malik S, Salgaonkar DS, 2003. Essential hypertension: antecedents in children.Indian Pediatrics, 40, 24–29.
Jousilahti P, Harald K, Jula A, Laatikainen T, Mannisto S, Peltonen M, Perola M, Puska P, Salomaa V, Tuomilehto J, et al, 2017. Salt intake and the risk of heart failure. European heart journal. Conference: European society of cardiology, ESC congress 2017. Spain, 38, 240.
Juraschek SP, Miller ER, Weaver CM, Appel LJ, 2017. Effects of sodium reduction and the dash diet by level of baseline blood pressure: pronounced benefits among adults with higher blood pressure. Circulation. Conference: Resuscitation science symposium, Press 2017. United states, 136.
Karppanen H, Mervaala E, 1998. Sodium intake and mortality. Lancet, 351, 1509; author reply 1509–1510.
Karvonen MJ, Punsar S, 1977. Sodium excretion and blood pressure of west and east Finns. Acta Medica Scandinavica, 202, 501–507.
Kerry SM, Cappuccio FP, Emmett L, Plange‐Rhule J, Eastwood JB, 2005. Reducing selection bias in a cluster randomized trial in West African villages. Clinical Trials, 2, 125–129.
Klaus D, Hoyer J, Middeke M, 2010. Salt restriction for the prevention of cardiovascular disease.Deutsches Ärzteblatt international Bundesärztekammer, 107, 457–462.
Konerman MC, Hummel SL, 2016. Does limiting salt intake prevent heart failure? A critical appraisal. Current Cardiovascular Risk Reports, 10.
Koo HS, Kim YC, Ahn SY, Oh SW, Kim S, Chin HJ, 2014. Analysis of correlation between 24‐hour urinary sodium and the degree of blood pressure control in patients with chronic kidney disease and non‐chronic kidney disease. Journal of Korean Medical Science, 29 Suppl 2, S117–122.
Koopman H, Spreeuwenberg C, Westerman RF, Donker AJM, 1990. Dietary treatment of patients with mild to moderate hypertension in a general practice: A pilot intervention study. (2) Beyond three months. Journal of Human Hypertension, 4, 372–374.
Korownyk C, Burgess E, Taylor I, 2013. Does sodium reduction affect mortality?. Canadian Family Physician, 59, 640.
Kotliar C, Kempny P, Gonzalez S, Castellaro C, Forcada P, Obregon S, Cavanagh E, Chiabaut Svane J, Casarini MJ, Rojas M, Inserra F, 2014. Lack of RAAS inhibition by high‐salt intake is associated with arterial stiffness in hypertensive patients. Journal of the Renin‐Angiotensin‐Aldosterone System, 15, 498–504.
Krakoff LR, Wassertheil‐Smoller S, 1995. Defining the patient group for cost‐effective withdrawal of antihypertensive therapy. Pharmacoeconomics, 7, 221–228.
Larsson SC, 2017. Dietary approaches for stroke prevention. Stroke, 48, 2905–2911.
Legris GJ, Dearborn D, Stern RC, Geiss CL, Hopfer U, Douglas JG, Doershuk CF, 1998. Sodium space and intravascular volume: dietary sodium effects in cystic fibrosis and healthy adolescent subjects. Pediatrics, 101, 48–56.
Leyvraz M, Taffe P, Chatelan A, Paradis G, Tabin R, Bovet P, Bochud M, Chiolero A, 2016. Sodium intake and blood pressure in children and adolescents: protocol for a systematic review and meta‐analysis. British Medical Journal Open, 6, e012518.
Lima NK, Tozetto DJ, Lima LG, Nobre F, Moriguti JC, Ferriolli E, Foss MC, 2009. Salt and insulin sensitivity after short and prolonged high salt intake in elderly subjects. Brazilian Journal of Medical and Biological Research, 42, 738–743.
Logan A, Marsden J, Freeman J, Kent B, 2017. Effectiveness of non‐pharmacological interventions in treating orthostatic hypotension in the elderly and people with a neurological condition: A systematic review protocol. JBI Database of Systematic Reviews and Implementation Reports, 15, 948–960.
Logan Alexander G, 1986. Sodium manipulation in the management of hypertension. The view against its general use. Canadian Journal of Physiology and Pharmacology, 64, 793–802.
Mattes RD, Westby E, De Cabo R, Falkner B, 1999. Dietary compliance among salt‐sensitive and salt‐insensitive normotensive adults. American Journal of the Medical Sciences, 317, 287–294.
Meland E, Laerum E, Aakvaag A, Ulvik RJ, 1994. Salt restriction and increased insulin production in hypertensive patients. Scandinavian Journal of Clinical and Laboratory Investigation, 54, 405–409.
Meland E, Laerum E, Ulvik RJ, 1994. Salt restriction in hypertension ‐ the effect of dietary advice and self monitoring of chloride concentration in urine. Scandinavian Journal of Clinical and Laboratory Investigation, 54, 399–404.
Meltzer JI, MacGregor G, Alderman MH, Laragh JH, 1996. Low urinary sodium and myocardial infarction. Hypertension, 27, 155–157.
Morgan T, Creed R, Hopper J, 1986. Factors that determine the response of people with mild hypertension to a reduced sodium intake. Clinical and Experimental Hypertension, 8, 941–962.
Morgan T, Nowson C, 1987. Comparative studies of reduced sodium and high potassium diet in hypertension. Nephron, 47 Suppl 1, 21–26.
Morgan T, Nowson C, 1986. The role of sodium restriction in the management of hypertension. Canadian Journal of Physiology and Pharmacology, 64, 786–792.
Nakano M, 2014. UMIN‐CTR Clinical Trial: Effect of salt reduction by aggressive nutritional education on clinic, home, and ambulatory BP levels. UMIN000014935. Available online: https://upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_view.cgi?recptno=R000017378
O'Donnell M, Mann JFE, Schutte AE, Staessen JA, Lopez‐Jaramillo P, Thomas M, Mente A, Saulnier P J, Yusuf S, 2016. Dietary sodium and cardiovascular disease risk. New England Journal of Medicine, 375, 2404–2406.
Otto MC, Afshin A, Micha R, Khatibzadeh S, Fahimi S, Singh G, Danaei G, Sichieri R, Monteiro C A, Louzada ML, Ezzati M, Mozaffarian D, 2016. The Impact of Dietary and Metabolic Risk Factors on Cardiovascular Diseases and Type 2 Diabetes Mortality in Brazil. PLoS One, 11, e0151503.
Pecker MS, James GD, Laragh JH, Difabio B, Sealey J E, Atlas SA, 1988. Effects of changes in sodium (Na) intake on plasma atrial natriuretic factor (ANF) levels in mild hypertension. American Journal of Hypertension, 1.
Polonia J, Monteiro J, Almeida J, Silva J, Bertoquini S, 2015. 5d.03: High Salt Intake Is Independently Associated with a Higher Risk of Cardiovascular Events. A 12 Years Evaluation of a Hypertensive Cohort. Journal of Hypertension, 33 Suppl 1, e71.
Poulter NR, Khaw KT, Mugambi M, Peart WS, Sever PS, 1985. Migration‐induced changes in blood pressure: a controlled longitudinal study. Clinical and Experimental Pharmacology and Physiology, 12, 211–216.
Prineas RJ, Stephens WB, Lovell RR, 1973. Blood pressure and its treatment in a community: the Albury blood pressure study. Medical Journal of Australia, 1, 5–9.
Rhee My, Lee Sy, 2017. Reduction of dietary sodium/potassium ratio is more effective in lowering of nighttime blood pressure. European heart journal. Conference: European society of cardiology, ESC congress 2017. Spain, 38, 1366.
Robare JF, Milas NC, Bayles CM, Williams K, Newman AB, Lovalekar MT, Boudreau R, McTigue K, Albert SM, Kuller LH, 2010. The key to life nutrition program: results from a community‐based dietary sodium reduction trial. Public Health Nutrition, 13, 606–614.
Rodrigues SL, Souza Junior PR, Pimentel EB, Baldo MP, Malta DC, Mill JG, Szwarcwald CL, 2015. Relationship between salt consumption measured by 24‐h urine collection and blood pressure in the adult population of Vitoria (Brazil). Brazilian Journal of Medical and Biological Research, 48, 728–735.
Schaefer EJ, Tani M, 2015. Nutrition and coronary heart disease prevention. 1, 329–341.
Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H, 2017. Impact of different dietary approaches on blood pressure in hypertensive and prehypertensive patients: protocol for a systematic review and network meta‐analysis. British Medical Journal Open, 7, e014736.
Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA, 2001. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. Journal of the American College of Cardiology, 38, 506–513.
Singh RB, Sharma VK, Rastogi SS, Singh NK, 1992. In patients with mild hypertension, does exercise and a gradual rather than abrupt increase in fatty acid and salt intake cause less rise in cardiovascular risk factors? Clinical Nutrition, 11, 309–314.
Smith WC, Crombie IK, Tavendale R, Irving J M, Kenicer MB, Tunstall Pedoe H, 1987. The Scottish Heart Health Study: objectives and development of methods. Health Bulletin (Edinburgh), 45, 211–217.
Stamler J, Chan Q, Daviglus ML, Dyer AR, Van Horn L, Garside DB, Miura K, Wu Y, Ueshima H, Zhao L, Elliott P, 2018. Relation of Dietary Sodium (Salt) to Blood Pressure and Its Possible Modulation by Other Dietary Factors: The INTERMAP Study. Hypertension, 71, 631–637.
Strasser T, 1982. Trials of the Treatment of Mild Hypertension ‐ an Interim Analysis. Lancet, 1, 149–156.
Strazzullo P, Cappuccio F P, Trevisan M, 1983. Association between blood pressure, dietary salt intake and family history of hypertension in a five year follow‐up study. Journal of Hypertension, 1, 159–161.
Strazzullo P, Trevisan M, Farinaro E, Cappuccio FP, Ferrara LA, Decampora E, Mancini M, 1983. Characteristics of the Association between Salt Intake and Blood‐Pressure in a Sample of Male Working Population in Southern Italy. European Heart Journal, 4, 608–613.
Tanskanen A, 1981. Salt intake and blood pressure Kuopio: University of Kuopio. Community Health, Series Original Reports, 2.
Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S, 2013. WITHDRAWN: Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews,, Cd009217.
Tochikubo O, Sasaki O, Umemura S, Kaneko Y, 1986. Management of hypertension in high school students by using new salt titrator tape. Hypertension, 8, 1164–1171.
Tuomilehto J, Puska P, Tanskanen A, Karppanen H, Pietinen P, Nissinen A, Enlund H, Ruotsalainen P, 1981. A community‐based intervention study on the feasibility and effects of the reduction of salt intake in North Karelia, Finland. Acta Cardiologica, 36, 83–104.
US Department of Health and Human Services, 1998. Facts about the DASH diet. National Institutes of Health Publication No. 03‐4082.
Van der Stouwe JG, Carmeli C, Aeschbacher S, Schoen T, Krisai P, Wenger G, Ehret G, Ponte B, Pruijm M, Ackermann D, Guessous I, Paccaud F, Pechere‐Bertschi A, Vogt B, Mohaupt MG, Martin PY, Burnier M, Risch M, Risch L, Bochud M, Conen D, 2018. Association of 24‐Hour Blood Pressure With Urinary Sodium Excretion in Healthy Adults. American Journal of Hypertension, 31, 784–791.
Vijayalakshmi A, Sravya G, Pavithra D, 2018. A prospective study on the effect of sodium intake on renal function in hypertensive patients. Drug Invention Today, 10, 356–360.
Volpe M, Muller FB, Trimarco B, 1985. Transient enhancement of sympathetic nervous system activity by long‐term restriction of sodium intake. Circulation, 72, 47–52.
Watson RL, Langford HG, Abernethy J, Barnes TY, Watson MJ, 1979. Urinary electrolytes, body weight, and blood pressure: Pooled cross‐sectional results among four groups of adolescent females. Hypertension, 1, 287–291.
Watson R, Langford H, 1967. Sodium and socio‐cultural effects on blood pressure of high school students. Circulation, 36, 246.
Whelton PK, 2015. Dietary sodium intake: scientific basis for public policy. Blood Purification, 39, 16–20.
Whelton PK, Applegate WB, Ettinger WH, 1996. Efficacy of weight loss and reduced sodium intake in a trial of nonpharmacological intervention in the elderly (TONE). Circulation, 94, I‐78.
Wilson DK, Klesges LM, Klesges RC, Eck LH, Hackett‐Renner C A, Alpert B S, Dalton E T, 1992. A prospective study of familial aggregation of blood pressure in young children. Journal of Clinical Epidemiology, 45, 959–969.
Wojciechowska W, Stolarz‐Skrzypek K, Olszanecka A, Bednarski A, Barton H, Folta M, Kawecka‐Jaszcz K, Czarnecka D, 2018. Left ventricular diastolic function in relation to sodium dietary intake and renal handling. Journal of hypertension. Conference: 28th scientific meeting of the european society of hypertension, ESH 2018. Spain, 36, e226.
Zhu H, He F, Choi JH, Dong Y, Huang Y, Harshfield GA, 2016. Effect of changing sodium intake on miRNA profiling in hypertensives. Circulation. Conference: american heart association's 2016 scientific sessions and resuscitation science symposium. United states, 134.
Zito A, Cosola C, Maranzano V, Dalfino G, Pertosa GB, Manno C, Carbonara R, Gesualdo L, Ciccone MM, 2017. The effect of a dietary salt restriction with low‐sodium bread on the blood pressure (BP) and the endothelial function. European heart journal. Conference: european society of cardiology, ESC congress 2017. Spain, 38, 1364–1365.
n = 32, study population not of interest
Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH, 1995. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension, 25, 1144–1152.
Alderman M, Sealey J, Cohen H, Madhavan S, Laragh J, 1997. Urinary sodium excretion and myocardial infarction in hypertensive patients: a prospective cohort study. American Journal of Clinical Nutrition, 65, 682s–686s.
Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR, 2001. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE).Archives of Internal Medicine, 161, 685–693.
Beard TC, Cooke HM, Gray WR, Barge R, 1982. Randomised controlled trial of a no‐added‐sodium diet for mild hypertension. Lancet, 2, 455–458.
Beard TC, Gray WR, Cooke HM, Barge R, 1982. Randomized Controlled Trial of a No‐Added‐Sodium Diet for Mild Hypertension. Lancet, 2, 455–458.
Bulpitt CJ, Daymond M, Bulpitt PF, Ferrier G, Harrison R, Lewis PJ, Dollery CT, 1984. Is Low Salt Dietary Advice a Useful Therapy in Hypertensive Patients with Poorly Controlled Blood‐Pressure. Annals of Clinical Research, 16, 143–149.
Cappuccio FP, Kerry SM, Micah FB, Plange‐Rhule J, Eastwood JB, 2006. A community programme to reduce salt intake and blood pressure in Ghana (ISRCTN88789643). BioMed Central Public Health, 6, 13.
Cohen SJ, Weinberger MH, Fineberg NS, Miller JZ, Grim CE, Luft FC, 1991. The effect of a household partner and home urine monitoring on adherence to a sodium restricted diet. Social Science & Medicine, 32, 1057–1061.
Cornelio ME, Godin G, Rodrigues RC, de Freitas Agondi R, Alexandre NM, Gallani MC, 2016. Effect of a behavioral intervention of the SALdável program to reduce salt intake among hypertensive women: A randomized controlled pilot study. European Journal of Cardiovascular Nursing, 15, e85–94.
Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe D L, Brown MD, 2012. Relationship of visit‐to‐visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertension Research, 35, 55–61.
Dubbert PM, Cushman WC, Meydrech EF, Rowland AK, Maury P, 1995. Effects of dietary instruction and sodium excretion feedback in hypertension clinic patients. Behavior Therapy, 26, 721–732.
Geleijnse JM, Hofman A, Witteman JC, Hazebroek AA, Valkenburg HA, Grobbee DE, 1997. Long‐term effects of neonatal sodium restriction on blood pressure. Hypertension, 29, 913–917.
Hofman Albert, 1983. Blood pressure in childhood: epidemiological probes into the aetiology of high blood pressure.
Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR, 2013. Dietary sodium restriction reverses vascular endothelial dysfunction in middle‐aged/older adults with moderately elevated systolic blood pressure. Journal of the American College of Cardiology, 61, 335–343.
Mann KV, Sullivan PL, 1987. Effect of task‐centered instructional programs on hypertensives’ ability to achieve and maintain reduced dietary sodium intake. Patient Education and Counseling, 10, 53–72.
Meland E, Aamland A, 2009. Salt restriction among hypertensive patients: modest blood pressure effect and no adverse effects. Scandinavian Journal of Primary Health Care, 27, 97–103.
Morgan T, Adam W, Gillies A, Wilson M, Morgan G, Carney S, 1978. Hypertension treated by salt restriction. Lancet, 1, 227–230.
Morgan T, Anderson A, 1987. Sodium restriction can delay the return of hypertension in patients previously well‐controlled on drug therapy. Canadian Journal of Physiology and Pharmacology, 65, 1752–1755.
Nowson CA, Morgan TO, Gibbons C, 2003. Decreasing dietary sodium while following a self‐selected potassium‐rich diet reduces blood pressure. Journal of Nutrition, 133, 4118–4123.
Olde Engberink RHG, Van den Hoek TC, Van Noordenne ND, Van den Born BH, Peters‐Sengers H, Vogt L, 2017. Use of a Single Baseline Versus Multiyear 24‐Hour Urine Collection for Estimation of Long‐Term Sodium Intake and Associated Cardiovascular and Renal Risk. Circulation, 136, 917–926.
Sakaki M, Tsuchihashi T, Arakawa K, Fukui H, Kameda W, Tominaga M, 2014. Long‐term variability of urinary salt excretion and blood pressure in hypertensive patients. Hypertension Research, 37, 939–943.
Singer P, Cohen H, Alderman M, 2015. Assessing the associations of sodium intake with long‐term all‐cause and cardiovascular mortality in a hypertensive cohort. American Journal of Hypertension, 28, 335–342.
Staessen J, Bulpitt CJ, Fagard R, Joossens JV, Lijnen P, Amery A, 1988. Salt intake and blood pressure in the general population: A controlled intervention trial in two towns. Journal of Hypertension, 6, 965–973.
Thaler BI, Paulin JM, Phelan EL, Simpson FO, 1982. A pilot study to test the feasibility of salt restriction in a community. New Zealand Medical Journal, 95, 839–842.
Todd AS, Macginley RJ, Schollum JB, Johnson RJ, Williams SM, Sutherland WH, Mann JI, Walker RJ, 2010. Dietary salt loading impairs arterial vascular reactivity. American Journal of Clinical Nutrition, 91, 557–64.
Tunstall‐Pedoe H, 1999. Does dietary potassium lower blood pressure and protect against coronary heart disease and death? Findings from the Scottish Heart Health Study?. Seminars in Nephrology, 19, 500–502.
Tunstall‐Pedoe H, Woodward M, Tavendale R, A'Brook R, McCluskey MK, 1997. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. British Medical Journal, 315, 722–729.
Whitten CF, Stewart RA, 1980. The effect of dietary sodium in infancy on blood pressure and related factors. Studies of infants fed salted and unsalted diets for five months at eight months and eight years of age. Acta paediatrica Scandinavica. Supplement, 279, 1–17.
Williams JS, Chamarthi B, Goodarzi MO, Pojoga LH, Sun B, Garza AE, Raby BA, Adler GK, Hopkins PN, Brown NJ, Jeunemaitre X, Ferri C, Fang R, Leonor T, Cui J, Guo X, Taylor KD, Ida Chen YD, Xiang A, Raffel LJ, Buchanan TA, Rotter JI, Williams GH, Shi Y, 2012. Lysine‐specific demethylase 1: an epigenetic regulator of salt‐sensitive hypertension. American Journal of Hypertension, 25, 812–817.
Williams JS, Hopkins PN, Jeunemaitre X, Brown NJ, 2011. CYP4A11 T8590C polymorphism, salt‐sensitive hypertension, and renal blood flow. Journal of Hypertension, 29, 1913–1918.
Yang GH, Zhou X, Ji WJ, Liu JX, Sun J, Shi R, Jiang TM, Li YM, 2018. Effects of a low salt diet on isolated systolic hypertension. Medicine (united states), 97.
Yasutake K, Miyoshi E, Misumi Y, Kajiyama T, Fukuda T, Ishii T, Moriguchi R, Murata Y, Ohe K, Enjoji M, Tsuchihashi T, 2018. Self‐monitoring of urinary salt excretion as a method of salt‐reduction education: a parallel, randomized trial involving two groups. Public Health Nutrition, 21, 2164–2173.
n = 53, not eligible because of study duration
Baldoncini R, Bellini C, Desideri G, De Angelis C, Ferri C, Santucci A, 1997. Elevated albumin excretion in nonmodulating essential hypertensive patients. Nephron, 76, 264–269.
Campese VM, Romoff MS, Levitan D, Saglikes Y, Friedler RM, Massry SG, 1982. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt‐sensitive patients with essential hypertension. Kidney International, 21, 371–378.
Campese VM, Tawadrous M, Bigazzi R, Bianchi S, Mann AS, Oparil S, Raij L, 1996. Salt intake and plasma atrial natriuretic peptide and nitric oxide in hypertension. Hypertension, 28, 335–340.
Cappuccio FP, Markandu ND, Sagnella GA, Macgregor GA, 1985. Sodium restriction lowers high blood pressure through a decreased response of the renin system — direct evidence using saralasin. Journal of Hypertension, 3, 243–247.
Chiolero A, Maillard M, Nussberger J, Brunner HR, Burnier M, 2000. Proximal sodium reabsorption: An independent determinant of blood pressure response to salt. Hypertension, 36, 631–637.
Dimsdale JE, Ziegler M, Mills P, Berry C, 1990. Prediction of salt sensitivity. American Journal of Hypertension, 3, 429–435.
Draaijer P, de Leeuw P, Maessen J, Van Hooff J, Leunissen K, 1995. Salt‐sensitivity testing in patients with borderline hypertension: reproducibility and potential mechanisms. Journal of Human Hypertension, 9, 263–269.
Ferri C, Bellini C, Desideri G, Giuliani E, De Siati L, Cicogna S, Santucci A, 1998. Clustering of endothelial markers of vascular damage in human salt‐sensitive hypertension: influence of dietary sodium load and depletion. Hypertension, 32, 862–868.
Friberg P, Meredith I, Jennings G, Lambert G, Fazio V, Esler M, 1990. Evidence for increased renal norepinephrine overflow during sodium restriction in humans. Hypertension, 16, 121–130.
Fuchs FD, Wannmacher CMD, Wannmacher L, Guimaraes FS, Rosito GA, Gastaldo G, Hoeffel CP, Wagner EM, 1987. Effect of Sodium‐Intake on Blood‐Pressure, Serum Levels and Renal Excretion of Sodium and Potassium in Normotensives with and without Familial Predisposition to Hypertension. Brazilian Journal of Medical and Biological Research, 20, 25–34.
Gow IF, Dockrell M, Edwards CR, Elder A, Grieve J, Kane G, Padfield PL, Waugh CJ, Williams BC, 1992. The sensitivity of human blood platelets to the aggregating agent ADP during different dietary sodium intakes in healthy men. European Journal of Clinical Pharmacology, 43, 635–638.
Gumieniak O, Perlstein TS, Hopkins PN, Brown NJ, Murphey LJ, Jeunemaitre X, Hollenberg NK, Williams GH, 2004. Thyroid function and blood pressure homeostasis in euthyroid subjects. Journal of Clinical Endocrinology and Metabolism, 89, 3455–3461.
He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA, 2005. Plasma sodium: ignored and underestimated. Hypertension, 45, 98–102.
Heer M, Baisch F, Kropp J, Gerzer R, Drummer C, 2000. High dietary sodium chloride consumption may not induce body fluid retention in humans. American Journal of Physiology‐Renal Physiology, 278, F585–F595.
Helber A, Wambach G, Hummerich W, 1980. Evidence for a subgroup of essential hypertensives with non‐suppressible excretion of aldosterone during sodium loading. Klinische Wochenschrift, 58, 439–447.
Iwaoka T, Umeda T, Ohno M, Inoue J, Naomi S, Sato T, Kawakami I, 1988. The effect of low and high NaCl diets on oral glucose tolerance. Klinische Wochenschrift, 66, 724–728.
Kawasaki T, Delea CS, Bartter FC, Smith H, 1978. The effect of high‐sodium and low‐sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. The American Journal of Medicine, 64, 193–198.
Keane P, Burgess E, Watanabe M, Wong T, 1991. Plasma sodium‐potassium ATPase inhibition activity in low‐ and normal‐renin hypertension. American Journal of Hypertension, 4, 9–13.
Kerstens MN, Van der Kleij FG, Boonstra AH, Sluiter WJ, Koerts J, Navis G, Dullaart RP, 2003. Salt loading affects cortisol metabolism in normotensive subjects: relationships with salt sensitivity. Journal of Clinical Endocrinology and Metabolism, 88, 4180–4185.
Krikken JA, Lely AT, Bakker SJL, Navis G, 2007. The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney International, 71, 260–265.
Laffer CL, Scott RC 3rd, Titze JM, Luft FC, Elijovich F, 2016. Hemodynamics and Salt‐and‐Water Balance Link Sodium Storage and Vascular Dysfunction in Salt‐Sensitive Subjects. Hypertension, 68, 195–203.
Leenen FHH, Boer P, Mees EJD, 1980. Oral contraceptives and responsiveness of plasma renin activity and blood pressure in normotensive women. Clinical and Experimental Hypertension, 2, 197–211.
Luft FC, Rankin LI, Bloch R, Weyman AE, Willis LR, Murray RH, Grim CE, Weinberger MH, 1979. Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation, 60, 697–706.
Luft FC, Rankin LI, Henry DP, Bloch R, Grim CE, Weyman AE, Murray RH, Weinberger MH, 1979. Plasma and urinary norepinephrine values at extremes of sodium intake in normal man. Hypertension, 1, 261–266.
Luft FC, Weinberger MH, Grim CE, 1982. Sodium sensitivity and resistance in normotensive humans. The American Journal of Medicine, 72, 726–736.
Mallamaci F, Leonardis D, Bellizzi V, Zoccali C, 1996. Does high salt intake cause hyperfiltration in patients with essential hypertension? Journal of Human Hypertension, 10, 157–161.
Mark AL, Lawton WJ, Abboud FM, Fitz AE, Connor WE, Heistad DD, 1975. Effects of High and Low Sodium Intake on Arterial‐Pressure and Forearm Vascular‐Resistance in Borderline Hypertension. Circulation Research, 36, 194–198.
Masuo K, Ogihara T, Kumahara Y, Yamatodani A, Wada H, 1983. Plasma norepinephrine and dietary sodium intake in normal subjects and patients with essential hypertension. Hypertension, 5, 767–771.
Mattes RD, Falkner B, 1999. Salt‐sensitivity classification in normotensive adults. Clinical Science, 96, 449–459.
McCallum L, Boal A, Padmanabhan S, 2016. Association between dietary chloride, blood pressure and heart rate‐a randomised cross‐over study. Journal of hypertension. Conference: 26th european meeting on hypertension and cardiovascular protection, ESH 2016. France, 34, e108–e109.
Morgan TO, 1982. The effect of potassium and bicarbonate ions on the rise in blood pressure caused by sodium chloride. Clinical Science, 63, 407s–409s.
Morgan T, Anderson A, 1988. Interaction in Hypertensive Man between Sodium‐Intake, Converting Enzyme‐Inhibitor (Enalapril), Plasma‐Renin and Blood‐Pressure Control. Journal of Human Hypertension, 1, 311–315.
Morgan T, Anderson A, 1988. Interaction of slow‐channel calcium blocking drugs with sodium restriction, diuretics and angiotensin converting enzyme inhibitors. Journal of hypertension. Suppl, 6, S652–S654.
Myers J, Morgan T, 1983. The effect of sodium intake on the blood pressure related to age and sex. Clinical and Experimental Hypertension, 5, 99–118.
Myers J, Morgan T, Waga S, Manley K, 1982. The effect of sodium intake on blood pressure related to the age of the patients. Clinical and Experimental Pharmacology and Physiology, 9, 287–289.
Nicholls MG, Kiowski W, Zweifler AJ, Julius S, Schork MA, Greenhouse J, 1980. Plasma norepinephrine variations with dietary sodium intake. Hypertension, 2, 29–32.
Nielsen LH, Ovesen P, Hansen MR, Brantlov S, Jespersen B, Bie P, Jensen BL, 2016. Changes in the renin‐angiotensin‐aldosterone system in response to dietary salt intake in normal and hypertensive pregnancy. A randomized trial. Journal of the American Society of Hypertension, 10, 881–890.e884.
Poch E, Gonzalez D, De la Sierra A, Giner V, Bragulat E, Botey A, Coca A, Rivera F, 2000. Genetic variation of the gamma subunit of the epithelial Na+ channel and essential hypertension. Relationship with salt sensitivity. American Journal of Hypertension, 13, 648–653.
Rankin LI, Luft FC, Henry DP, Gibbs PS, Weinberger MH, 1981. Sodium intake alters the effects of norepinephrine on blood pressure. Hypertension, 3, 650–656.
Schorr U, Blaschke K, Beige J, Distler A, Sharma AM, 1999. Angiotensinogen M235T variant and salt sensitivity in young normotensive Caucasians. Journal of Hypertension, 17, 475–479.
Sharma AM, Kribben A, Schattenfroh S, Cetto C, Distler A, 1990. Salt sensitivity in humans is associated with abnormal acid‐base regulation. Hypertension, 16, 407–413.
Sharma AM, Schattenfroh S, Kribben A, Distler A, 1989. Reliability of salt‐sensitivity testing in normotensive subjects. Klinische Wochenschrift, 67, 632–634.
Sharma AM, Schorr U, Oelkers W, Distler A, 1993. Effects of sodium salts on plasma renin activity and norepinephrine response to orthostasis in salt‐sensitive normotensive subjects. American Journal of Hypertension, 6, 780–785.
Shore Angela C, Markandu Nirmala D, MacGregor Graham A, 1988. A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. Journal of Hypertension, 6, 613–617.
Skrabal F, Herholz H, Neumayr M, Hamberger L, Ledochowski M, Sporer H, Hortnagl H, Schwarz S, Schonitzer D, 1984. Salt sensitivity in humans is linked to enhanced sympathetic responsiveness and to enhanced proximal tubular reabsorption. Hypertension, 6, 152–158.
Sowers JR, Martin VI, Beck FW, 1983. Effects of dietary sodium on circadian rhythm and physiological responses of 18‐hydroxycorticosterone. Clinical Science (London), 64, 295–301.
Starmans‐Kool MJ, Stanton AV, Xu YY, Mc G Thom SA, Parker KH, Hughes AD, 2011. High dietary salt intake increases carotid blood pressure and wave reflection in normotensive healthy young men. Journal of Applied Physiology (1985), 110, 468–471.
Stein CM, Nelson R, Brown M, He H, Wood M, Wood AJ, 1995. Dietary sodium intake modulates systemic but not forearm norepinephrine release*. Clinical Pharmacology & Therapeutics, 58, 425–433.
Sudhir K, Friberg P, Meredith IT, Woods RL, Esler MD, Jennings GL, 1989. Cardiac secretion and renal clearance of atrial natriuretic peptide in normal man: effect of salt restriction. Clinical Science (London), 77, 605–610.
Sullivan JM, Ratts TE, Taylor JC, Kraus DH, Barton BR, Patrick DR, Reed SW, 1980. Hemodynamic effects of dietary sodium in man: a preliminary report. Hypertension, 2, 506–514.
Toering TJ, Gant CM, Visser FW, Van der Graaf AM, Laverman GD, Danser AHJ, Faas MM, Navis G, Lely AT, 2017. Gender Differences in Renin Angiotensin Aldosterone System Affect Extra Cellular Volume in Healthy Subjects. American Journal of Physiology‐Renal Physiology, ajprenal.00109.02017.
Van der Kleij FG, de Jong PE, Henning RH, de Zeeuw D, Navis G, 2002. Enhanced responses of blood pressure, renal function, and aldosterone to angiotensin I in the DD genotype are blunted by low sodium intake. Journals of the American Society of Nephrology, 13, 1025–1033.
Zemel MB, Gualdoni SM, Sowers JR, 1986. Sodium excretion and plasma renin activity in normotensive and hypertensive black adults as affected by dietary calcium and sodium. Journal of Hypertension, 4, S343–S345.
n = 22, co‐intervention deemed to affect the outcome of interest
Andersson OK, Fagerberg B, Persson B, Aurell M, Hedner T, 1986. Hemodynamic and humoral adaptation to weight stable chronic sodium restriction in comparison with weight reduction in moderately obese hypertensive men. Acta medica Scandinavica. Supplementum, 714, 65–69.
Anonymous, 1993. The treatment of mild hypertension study. Hospital Practice, 28, 96–97.
Appel LJ, Champagne CM, Harsha DW, Prochazka AV, 2003. Lifestyle recommendations reduced blood pressure in patients with above optimal blood pressure. Evidence‐Based Medicine, 8, 174.
Cooper JN, Evans RW, Mori Brooks M, Fried L, Holmes C, Barinas‐Mitchell E, Sutton‐Tyrrell K, 2014. Associations between arterial stiffness and platelet activation in normotensive overweight and obese young adults. Clinical and Experimental Hypertension, 36, 115–122.
Cooper JN, Fried L, Tepper P, Barinas‐Mitchell E, Conroy MB, Evans RW, Mori Brooks M, Woodard GA, Sutton‐Tyrrell K, 2013. Changes in serum aldosterone are associated with changes in obesity‐related factors in normotensive overweight and obese young adults. Hypertension Research, 36, 895–901.
De Freitas Agondi R, Cornelio ME, Rodrigues R C, Gallani MC, 2014. Implementation Intentions on the Effect of Salt Intake among Hypertensive Women: A Pilot Study. Nursing Research and Practice, 2014, 196410.
Ho GY, Blaufox MD, Wassertheil‐Smoller S, Oberman A, Langford H, 1994. Plasma renin predicts success of antihypertensive drug withdrawal. American Journal of Hypertension, 7, 679–684.
Jula AM, Karanko HM, 1994. Effects on left ventricular hypertrophy of long‐term nonpharmacological treatment with sodium restriction in mild‐to‐moderate essential hypertension. Circulation, 89, 1023–1031.
Jula AM, Ronnemaa TE, Piha SJ, Maki JP, 1992. Response of diastolic blood pressure to long‐term sodium restriction is posture related. Scandinavian Journal of Clinical and Laboratory Investigation, 52, 159–167.
Jula A, Ronnemaa T, Tikkanen I, Karanko H, 1992. Responses of atrial natriuretic factor to long‐term sodium restriction in mild to moderate hypertension. Journal of Internal Medicine, 231, 521–529.
Kojuri J, Rahimi R, 2007. Effect of “no added salt diet” on blood pressure control and 24 hour urinary sodium excretion in mild to moderate hypertension. BioMed Central Cardiovascular Disorders, 7, 34.
Koopman H, Spreeuwenberg C, Westerman RF, Donker AJ, 1990. Dietary treatment of patients with mild to moderate hypertension in a general practice: a pilot intervention study (1). The first three months. Journal of Human Hypertension, 4, 368–371.
Kostis JB, Rosen RC, Brondolo E, Taska L, Smith DE, Wilson AC, 1992. Superiority of nonpharmacologic therapy compared to propranolol and placebo in men with mild hypertension: a randomized, prospective trial. American Heart Journal, 123, 466–474.
Langford HG, Blaufox MD, Oberman A, Hawkins CM, Curb JD, Cutter GR, Wassertheil‐Smoller S, Pressel S, Babcock C, Abernethy JD, 1985. Dietary therapy slows the return of hypertension after stopping prolonged medication. Journal of the American Medical Association, 253, 657–664.
Molchanova O, Freeva G, Britov A, 2015. Dynamic of main characteristics of cardiovascular risk in subjects under non‐pharmacological intervention and the natural dynamics in a cohort study. European Journal of Cardiovascular Nursing, 14, S107.
Nana‐Goar PN, Ausheva A, Karpova A, 2014. The effect of lifestyle advice program in hypertensive patients. European Journal of Preventive Cardiology, 21, S53.
Naseem S, Ghazanfar H, Assad S, Ghazanfar A, 2016. Role of sodium‐restricted dietary approaches to control blood pressure in Pakistani hypertensive population. Journal of the Pakistan Medical Association, 66, 837–842.
Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ, 2018. Effect of individualised dietary advice for weight loss supplemented with walnuts on blood pressure: the HealthTrack study. European Journal of Clinical Nutrition, 72, 894–903.
Nowson CA, Wattanapenpaiboon N, Pachett A, 2009. Low‐sodium Dietary Approaches to Stop Hypertension‐type diet including lean red meat lowers blood pressure in postmenopausal women. Nutrition Research, 29, 8–18.
Obarzanek E, Vollmer WM, Lin PH, Cooper LS, Young DR, Ard JD, Stevens VJ, Simons‐Morton DG, Svetkey LP, Harsha DW, Elmer PJ, Appel LJ, 2007. Effects of individual components of multiple behavior changes: the PREMIER trial. American Journal of Health Behavior, 31, 545–560.
Silman AJ, Locke C, Mitchell P, Humpherson P, 1983. Evaluation of the effectiveness of a low sodium diet in the treatment of mild to moderate hypertension. Lancet, 1, 1179–1182.
Wing Lindon MH, Arnolda Leonard F, Harvey Paula J, Upton Jane, Molloy Danielle, Gabb Genevieve M, Bune Alexandra JC, Chalmers John P, 1998. Low‐dose diuretic and/or dietary sodium restriction when blood pressure is resistant to ACE inhibitor. Blood Pressure, 7, 299–307.
n = 6, NaCl replaced by potassium salt
Hunt SC, Geleijnse JM, Wu LL, Witteman JC, Williams RR, Grobbee DE, 1999. Enhanced blood pressure response to mild sodium reduction in subjects with the 235T variant of the angiotensinogen gene. American Journal of Hypertension, 12, 460–466.
Jardine M, Li N, Ninomiya T, Feng X, Zhang J, Shi J, Zhang Y, Zhang R, Perkovic V, Lambers H H, Wu Y, Yan L, Neal B, 2014. Dietary sodium reduction reduced albuminuria in 1,903 rural Chinese: a cluster randomised trial. Nephrology. 19, 28.
Li N, Yan LL, Niu W, Labarthe D, Feng X, Shi J, Zhang J, Zhang R, Zhang Y, Chu H, Neiman A, Engelgau M, Elliott P, Wu Y, Neal B, 2013. A large‐scale cluster randomized trial to determine the effects of community‐based dietary sodium reduction–the China Rural Health Initiative Sodium Reduction Study. American Heart Journal, 166, 815–822.
Little P, Kelly J, Barnett J, Dorward M, Margetts B, Warm D, 2004. Randomised controlled factorial trial of dietary advice for patients with a single high blood pressure reading in primary care. British Medical Journal, 328, 1054.
Puska P, Iacono JM, Nissinen A, Korhonen HJ, Vartianinen E, Pietinen P, Dougherty R, Leino U, Mutanen M, Moisio S, Huttunen J, 1983. Controlled, randomised trial of the effect of dietary fat on blood pressure. Lancet, 1, 1–5.
Zhou X, Liu JX, Shi R, Yang N, Song DL, Pang W, Li YM, 2009. Compound ion salt, a novel low‐sodium salt substitute: From animal study to community‐based population trial. American Journal of Hypertension, 22, 934–942.
n = 19, outcome(s) not of interest
Anderson CA, Cobb LK, Miller ER 3rd, Woodward M, Hottenstein A, Chang AR, Mongraw‐Chaffin M, White K, Charleston J, Tanaka T, Thomas L, Appel LJ, 2015. Effects of a behavioural intervention that emphasizes spices and herbs on adherence to recommended sodium intake: results of the SPICE randomized clinical trial. American Journal of Clinical Nutrition, 102, 671–679.
Blaufox MD, Langford HG, Oberman A, Hawkins CM, Wassertheil‐Smoller SW, Cutter GR, 1984. Effect of dietary change on the return of hypertension after withdrawal of prolonged antihypertensive therapy (DISH). Dietary Intervention Study of Hypertension. Journal of hypertension. Suppl, 2, S179–S181.
Chen L, Zhang Z, Chen W, Whelton PK, Appel LJ, 2016. Lower Sodium Intake and Risk of Headaches: Results From the Trial of Nonpharmacologic Interventions in the Elderly. American Journal of Public Health, 106, 1270–1275.
Cook NR, Appel LJ, Whelton PK, 2016. Sodium Intake and All‐Cause Mortality Over 20 Years in the Trials of Hypertension Prevention. Journal of the American College of Cardiology, 68, 1609–1617.
Cook N, Appel L, Whelton P, 2015. Sodium intake and all‐cause mortality over 20 years of follow‐up. Circulation, 132.
Espeland M A, Kumanyika S, Yunis C, Zheng B, Brown W M, Jackson S, Wilson A C, Bahnson J, 2002. Electrolyte intake and nonpharmacologic blood pressure control. Annals of Epidemiology, 12, 587–595.
Gijsbers L, Dower JI, Schalkwijk CG, Kusters YH, Bakker SJ, Hollman PC, Geleijnse JM, 2015. 5d.06: Effects of Sodium and Potassium Supplementation on Endothelial Function and Inflammation in Untreated (Pre)Hypertensives: A Fully Controlled Dietary Intervention Study. Journal of Hypertension, 33 Suppl 1, e72.
Hu G, Jousilahti P, Peltonen M, Lindstrom J, Tuomilehto J, 2005. Urinary sodium and potassium excretion and the risk of type 2 diabetes: a prospective study in Finland. Diabetologia, 48, 1477–1483.
Ireland DM, Clifton PM, Keogh JB, 2010. Achieving the Salt Intake Target of 6 g/Day in the Current Food Supply in Free‐Living Adults Using Two Dietary Education Strategies. Journal of the American Dietetic Association, 110, 763–767.
Kirkendall AM, Connor WE, Abboud F, Rastogi SP, Anderson TA, Fry M, 1976. The effect of dietary sodium chloride on blood pressure, body fluids, electrolytes, renal function, and serum lipids of normotensive man. Journal of Laboratory and Clinical Medicine, 87, 411–434.
Kostis JB, Wilson AC, Shindler DM, Cosgrove NM, Lacy CR, 2000. Non‐drug therapy for hypertension: do effects on weight and sodium intake persist after discontinuation of intervention?. The American Journal of Medicine, 109, 734–736.
Langford HG, Blaufox MD, Oberman A, Hawkins CM, Curb JD, Cutter GR, Wassertheil‐Smoller S, Pressel S, Babcock C, Abernethy JD, 1984. Return of hypertension after withdrawal of prolonged antihypertensive therapy, effect of weight loss, sodium reduction, and baseline factors. Transactions of the Association of American Physicians, 97, 190–196.
Liu YP, Thijs L, Kuznetsova T, Gu YM, Asayama K, Stolarz‐Skrzypek K, Jin Y, Verhamme P, Struijker‐Boudier HA, Staessen JA, 2013. Central systolic augmentation indexes and urinary sodium in a white population. American Journal of Hypertension, 26, 95–103.
Schmid TL, Jeffery RW, Onstad L, Corrigan SA, 1991. Demographic, knowledge, physiological, and behavioural variables as predictors of compliance with dietary treatment goals in hypertension. Addictive Behaviors, 16, 151–160.
Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O'Donnell MJ, Mann JF, Clase CM, 2014. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney International, 86, 1205–1212.
Takahashi Y, Sasaki S, Okubo S, Hayashi M, Tsugane S, 2006. Maintenance of a low‐sodium, high‐carotene and ‐vitamin C diet after a 1‐year dietary intervention: the Hiraka dietary intervention follow‐up study. Preventive Medicine, 43, 14–19.
Todd AS, Walker RJ, MacGinley RJ, Kelly J, Merriman TR, Major TJ, Johnson RJ, 2017. Dietary Sodium Modifies Serum Uric Acid Concentrations in Humans. American Journal of Hypertension, 30, 1196–1202.
Wassertheil S, Langford HG, Blaufox MD, Oberman A, Hawkins M, 1983. Diuretics and salt restriction in blood pressure control. Current Concepts in Nutrition, 12, 175–189.
Wassertheil‐Smoller S, Langford HG, Blaufox MD, 1985. Effective dietary intervention in hypertensives: Sodium restriction and weight reduction. Journal of the American Dietetic Association, 85, 423–430.
n = 78, 24‐h urine sodium excretion not measured
Alvarez Li FC, Espinosa Brito AD, Ordunez Garcia PO, Silva Aycaguer LC, 1999. Risk markers and high blood pressure. The Cienfuegos global project. Longitudinal study 1992‐1994. Clinical and Translational Investigation, 51, 151–158.
Ambrosioni E, Costa FV, Borghi C, Montebugnoli L, Giordani MF, Magnani B, 1982. Effects of moderate salt restriction on intralymphocytic sodium and pressor response to stress in borderline hypertension. Hypertension, 4, 789–94.
Anonymous, 1990. The Hypertension Prevention Trial: three‐year effects of dietary changes on blood pressure. Hypertension Prevention Trial Research Group. Archives of internal medicine, 150, 153–162.
Ascherio A, Rimm EB, Giovannucci EL, Colditz GA, Rosner B, Willett WC, Sacks F, Stampfer MJ, 1992. A prospective study of nutritional factors and hypertension among US men. Circulation, 86, 1475–1484.
Avolio AP, Clyde KM, Beard TC, 1986. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis, 6, 166–169.
Baguena Gomez JC, Abellan Aleman J, Merino Sanchez J, 1988. Treatment of arterial hypertension in the Murcia region. Analysis of its course from 1981 to 1986. Data from a longitudinal epidemiological study, Murcia HTA/81. Medicina clínica, 91, 764–768.
Bang HO, Bechgaard P, Nielsen AL, 1949. Low‐salt diet in treatment of hypertension and hypertensive heart disease. British Medical Journal, 4638, 1203–1206.
Barba G, Galletti F, Cappuccio FP, Siani A, Venezia A, Versiero M, Della Valle E, Sorrentino P, Tarantino G, Farinaro E, Strazzullo P, 2007. Incidence of hypertension in individuals with different blood pressure salt‐sensitivity: results of a 15‐year follow‐up study. Journal of Hypertension, 25, 1465–1471.
Batis C, Gordon‐Larsen P, Cole SR, Du S, Zhang B, Popkin B, 2013. Sodium intake from various time frames and incident hypertension among Chinese adults. Epidemiology, 24, 410–418.
Blais CA, Pangborn RM, Borhani NO, Ferrell MF, Prineas RJ, Laing B, 1986. Effect of dietary sodium restriction on taste responses to sodium chloride: a longitudinal study. American Journal of Clinical Nutrition, 44, 232–243.
Borah PK, Kalita HC, Paine SK, Khaund P, Bhattacharjee C, Hazarika D, Sharma M, Mahanta J, 2018. An information, education and communication module to reduce dietary salt intake and blood pressure among tea garden workers of Assam. Indian Heart Journal, 70, 252–258.
Brion MJ, Ness AR, Davey Smith G, Emmett P, Rogers I, Whincup P, Lawlor DA, 2008. Sodium intake in infancy and blood pressure at 7 years: findings from the Avon Longitudinal Study of Parents and Children. European Journal of Clinical Nutrition, 62, 1162–1169.
Buendia JR, Bradlee ML, Daniels SR, Singer MR, Moore LL, 2015. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. Journal of the American Medical Association pediatrics, 169, 560–568.
Calabrese EJ, Tuthill RW, 1985. The Massachusetts Blood Pressure Study, Part 3. Experimental reduction of sodium in drinking water: effects on blood pressure. Toxicology and industrial health, 1, 19–34.
Chen ML, Huang TP, Chen TW, Chan HH, Hwang BF, 2018. Interactions of Genes and Sodium Intake on the Development of Hypertension: A Cohort‐Based Case‐Control Study. International Journal of Environmental Research and Public Health, 15.
Chien KL, Hsu HC, Chen PC, Su TC, Chang WT, Chen MF, Lee YT, 2008. Urinary sodium and potassium excretion and risk of hypertension in Chinese: report from a community‐based cohort study in Taiwan. Journal of Hypertension, 26, 1750–1756.
Clark VA, Chapman JM, Coulson AH, 1967. Effects of various factors on systolic and diastolic blood pressure in the Los Angeles heart study. Journal of Chronic Diseases, 20, 571–581.
Cooper R, Van Horn L, Liu K, Trevisan M, Nanas S, Ueshima H, Larbi E, Yu CS, Sempos C, LeGrady D, 1984. A randomized trial on the effect of decreased dietary sodium intake on blood pressure in adolescents. Journal of Hypertension, 2, 361–366.
De Simone G, Devereux RB, Roman MJ, Schlussel Y, Alderman MH, Laragh JH, 1991. Echocardiographic left ventricular mass and electrolyte intake predict arterial hypertension. Annals of Internal Medicine, 114, 202–209.
Ellekjaer EF, Wyller TB, Sverre JM, Holmen J, 1992. Lifestyle factors and risk of cerebral infarction. Stroke, 23, 829–834.
Ellison RC, Capper AL, Stephenson WP, Goldberg RJ, Hosmer DW Jr, Humphrey KF, Ockene JK, Gamble WJ, Witschi JC, Stare FJ, 1989. Effects on blood pressure of a decrease in sodium use in institutional food preparation: the Exeter‐Andover Project. Journal of Clinical Epidemiology, 42, 201–208.
Flack JM, Grimm RH Jr, Staffileno BA, Dnsc, Elmer P, Yunis C, Hedquist L, Dudley A, 2002. New salt‐sensitivity metrics: variability‐adjusted blood pressure change and the urinary sodium‐to‐creatinine ratio. Ethnicity & Disease, 12, 10–19.
Forte JG, Miguel JM, Miguel MJ, de Padua F, Rose G, 1989. Salt and blood pressure: a community trial. Journal of Human Hypertension, 3, 179–184.
Frank GC, Farris RP, Cresanta JL, Webber LS, Berenson GS, 1985. Dietary trends of 10‐ and 13‐year‐old children in a biracial community–the Bogalusa Heart Study. Preventive Medicine, 14, 123–139.
Fujiwara S, Kotani K, Brantley PJ, Tsuzaki K, Matsuoka Y, Domichi M, Sano Y, Kajii E, Sakane N, 2010. Dietary salt reduction in rural patients with albuminurea using family and community support: the Mima study. Asia Pacific Family Medicine, 9, 6.
Gao J, Sun H, Liang X, Gao M, Zhao H, Qi Y, Wang Y, Liu Y, Li J, Zhu Y, Zhao Y, Wang W, Ma L, Wu S, 2015. Ideal cardiovascular health behaviors and factors prevent the development of hypertension in prehypertensive subjects. Clinical and Experimental Hypertension, 37, 650–655.
Geleijnse JM, Witteman JC, Stijnen T, Kloos MW, Hofman A, Grobbee DE, 2007. Sodium and potassium intake and risk of cardiovascular events and all‐cause mortality: the Rotterdam Study. European Journal of Epidemiology, 22, 763–770.
George J, Majeed W, Mackenzie IS, MacDonald TM, Wei L, 2013. Association between cardiovascular events and sodium‐containing effervescent, dispersible, and soluble drugs: Nested case‐control study. British Medical Journal (Online), 347.
Guerra A, Monteiro C, Breitenfeld L, Jardim H, Rego C, Silva D, Prata A, Matos J, Pereira A, Santos NT, Bicho M, 1997. Genetic and environmental factors regulating blood pressure in childhood: prospective study from 0 to 3 years. Journal of Human Hypertension, 11, 233–238.
Hirayama A, Konta T, Hozawa A, Kawasaki R, Watanabe T, Shibata Y, Kayama T, Fukao A, Kubota I, 2015. Slight increase in urinary albumin excretion within the normal range predicts incident hypertension in a community‐based Japanese population: the Takahata study. Hypertension Research, 38, 56–60.
Howe PR, Cobiac L, Smith RM, 1991. Lack of effect of short‐term changes in sodium intake on blood pressure in adolescent schoolchildren. Journal of Hypertension, 9, 181–186.
Irwan AM, Kato M, Kitaoka K, Ueno E, Tsujiguchi H, Shogenji M, 2016. Development of the salt‐reduction and efficacy‐maintenance program in Indonesia. Nursing & Health Sciences, 18, 519–532.
Jones JB, Provost M, Keaver L, Breen C, Ludy MJ, Mattes RD, 2014. A randomized trial on the effects of flavourings on the health benefits of daily peanut consumption. American Journal of Clinical Nutrition, 99, 490–496.
Juraschek SP, Choi HK, Tang O, Appel LJ, Miller ER 3rd, 2016. Opposing effects of sodium intake on uric acid and blood pressure and their causal implication. Journal of the American Society of Hypertension, 10, 939–946.e932.
Juraschek SP, Miller ER 3rd, Weaver CM, Appel LJ, 2017. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. Journal of the American College of Cardiology, 70, 2841–2848.
Juraschek SP, Woodward M, Sacks FM, Carey VJ, Miller ER 3rd, Appel LJ, 2017. Time Course of Change in Blood Pressure From Sodium Reduction and the DASH Diet. Hypertension, 70, 923–929.
Juraschek S, Woodward M, Sacks F, Carey V, Miller E, Appel L, 2017. Time course of change in blood pressure from the dash diet and sodium reduction. Circulation. Conference: American heart association's epidemiology and prevention/lifestyle and cardiometabolic health 2017 scientific sessions. United states, 135.
Kagan A, Popper JS, Rhoads GG, Yano K, 1985. Dietary and other risk factors for stroke in Hawaiian Japanese men. Stroke, 16, 390–396.
Kaplan NM, Simmons M, McPhee C, Carnegie A, Stefanu C, Cade S, 1982. Two techniques to improve adherence to dietary sodium restriction in the treatment of hypertension. Archives of internal medicine, 142, 1638–1641.
Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ, Joosten MM, 2014. Urinary potassium excretion and risk of developing hypertension: the prevention of renal and vascular end‐stage disease study. Hypertension, 64, 769–776.
Kitaoka K, Kitade A, Nagaoka J, Tsuzaki K, Harada K, Aoi W, Wada S, Asano H, Sakane N, Higashi A, 2015. Lifestyle intervention might easily improve blood pressure in hypertensive men with the C genotype of angiotensin II type 2 receptor gene. Nutrition Research and Practice, 9, 385–392.
Kumanyika SK, Espeland MA, Bahnson JL, Bottom JB, Charleston JB, Folmar S, Wilson AC, Whelton PK, 2002. Ethnic comparison of weight loss in the Trial of Nonpharmacologic Interventions in the Elderly. Obesity research, 10, 96–106.
Li Y, Huang Z, Jin C, Xing A, Liu Y, Huangfu C, Lichtenstein AH, Tucker KL, Wu S, Gao X, 2018. Longitudinal Change of Perceived Salt Intake and Stroke Risk in a Chinese Population. Stroke, 49, 1332–1339.
Li Y, Zagato L, Kuznetsova T, Tripodi G, Zerbini G, Richart T, Thijs L, Manunta P, Wang JG, Bianchi G, Staessen JA, 2007. Angiotensin‐converting enzyme I/D and alpha‐adducin Gly460Trp polymorphisms: from angiotensin‐converting enzyme activity to cardiovascular outcome. Hypertension, 49, 1291–1297.
Manze M, Rose AJ, Orner MB, Berlowitz DR, Kressin NR, 2010. Understanding racial disparities in treatment intensification for hypertension management. Journal of General Internal Medicine, 25, 819–825.
Mente A, O'Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez‐Jaramillo P, Lanas F, Li W, Lu Y, Yi S, Rensheng L, Iqbal R, Mony P, Yusuf R, Yusoff K, Szuba A, Oguz A, Rosengren A, Bahonar A, Yusufali A, Schutte AE, Chifamba J, Mann JF, Anand SS, Teo K, Yusuf S, 2016. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet, 388, 465–475.
Micha R, Penalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D, 2017. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. Journal of the American Medical Association, 317, 912–924.
Morgan TO, Adams WR, Hodgson M, Gibberd RW, 1980. Failure of therapy to improve prognosis in elderly males with hypertension. The Medical Journal of Australia, 2, 27–31.
Morikawa N, Yamasue K, Tochikubo O, Mizushima S, 2011. Effect of salt reduction intervention program using an electronic salt sensor and cellular phone on blood pressure among hypertensive workers. Clinical and Experimental Hypertension, 33, 216–222.
Mu J, Liu Z, Liu F, Xu X, Liang Y, Zhu D, 2009. Family‐based randomized trial to detect effects on blood pressure of a salt substitute containing potassium and calcium in hypertensive adolescents. American Journal of Hypertension, 22, 943–947.
Mu J, Zheng S, Lian Q, Liu F, Liu Z, 2012. Evolution of blood pressure from adolescents to youth in salt sensitivies: a 18‐year follow‐up study in Hanzhong children cohort. Nutrition Journal, 11, 70.
Nakamura M, Aoki N, Yamada T, Kubo N, 2003. Feasibility and effect on blood pressure of 6‐week trial of low sodium soy sauce and miso (fermented soybean paste). Circulation Journal, 67, 530–534.
Oikonen M, Tikkanen E, Juhola J, Tuovinen T, Seppala I, Juonala M, Taittonen L, Mikkila V, Kahonen M, Ripatti S, Viikari J, Lehtimaki T, Havulinna AS, Kee F, Newton‐Cheh C, Peltonen L, Schork NJ, Murray SS, Berenson GS, Chen W, Srinivasan SR, Salomaa V, Raitakari OT, 2011. Genetic variants and blood pressure in a population‐based cohort: the Cardiovascular Risk in Young Finns study. Hypertension, 58, 1079–1085.
Okubo Y, Miyamoto T, Suwazono Y, Kobayashi E, Nogawa K, 2000. The effects of job‐related factors and lifestyle on the five‐year cumulative incidence of hypertension in Japanese steelworkers. Journal of Occupational Health, 42, 304–314.
Pazoki R, Dehghan A, Evangelou E, Warren H, Gao H, Caulfield M, Elliott P, Tzoulaki I, 2017. Genetic Predisposition to High Blood Pressure and Lifestyle Factors: Associations with Midlife Blood Pressure Levels and Cardiovascular Events. Circulation.
Riegel G, Moreira LB, Fuchs SC, Gus M, Nunes G, Correa V Jr, Wiehe M, Goncalves CC, Fernandes FS, Fuchs FD, 2012. Long‐term effectiveness of non‐drug recommendations to treat hypertension in a clinical setting. American Journal of Hypertension, 25, 1202–1208.
Russo P, Siani A, Venezia A, Iacone R, Russo O, Barba G, D'Elia L, Cappuccio FP, Strazzullo P, 2002. Interaction between the C(‐344)T polymorphism of CYP11B2 and age in the regulation of blood pressure and plasma aldosterone levels: cross‐sectional and longitudinal findings of the Olivetti Prospective Heart Study. Journal of Hypertension, 20, 1785–1792.
Saptharishi L, Soudarssanane M, Thiruselvakumar D, Navasakthi D, Mathanraj S, Karthigeyan M, Sahai A, 2009. Community‐based Randomized Controlled Trial of Non‐pharmacological Interventions in Prevention and Control of Hypertension among Young Adults. Indian Journal of Community Medicine, 34, 329–334.
Satoh M, Kikuya M, Hosaka M, Asayama K, Inoue R, Metoki H, Tsubota‐Utsugi M, Hara A, Hirose T, Obara T, Mori T, Totsune K, Hoshi H, Mano N, Imai Y, Ohkubo T, 2015. Association of aldosterone‐to‐renin ratio with hypertension differs by sodium intake: the Ohasama study. American Journal of Hypertension, 28, 208–215.
Satoh M, Kikuya M, Ohkubo T, Mori T, Metoki H, Hara A, Utsugi M T, Hashimoto T, Hirose T, Obara T, Inoue R, Asayama K, Kanno A, Totsune K, Hoshi H, Satoh H, Imai Y, 2012. Aldosterone‐to‐renin ratio as a predictor of stroke under conditions of high sodium intake: the Ohasama study. American Journal of Hypertension, 25, 777–783.
Scheelbeek PFD, Chowdhury MAH, Haines A, Alam DS, Hoque MA, Butler AP, Khan AE, Mojumder SK, Blangiardo MAG, Elliott P, Vineis P, 2017. Drinking Water Salinity and Raised Blood Pressure: Evidence from a Cohort Study in Coastal Bangladesh. Environmental Health Perspectives, 125, 057007.
Schoppen S, Pérez‐Granados AM, Carbajal Á, Oubiña P, Sánchez‐Muniz FJ, Gómez‐Gerique JA, Vaquero MP, 2004. A Sodium‐Rich Carbonated Mineral Water Reduces Cardiovascular Risk in Postmenopausal Women. Journal of Nutrition, 134, 1058–1063.
Setayeshgar S, Ekwaru JP, Maximova K, Majumdar SR, Storey KE, McGavock J, Veugelers PJ, 2017. Dietary intake and prospective changes in cardiometabolic risk factors in children and youth. Applied Physiology, Nutrition, and Metabolism, 42, 39–45.
Simic BS, Simic A, Markovic R, Todorovica P, 1963. Longitudinal Study on the Effect of Diets with Different Caloric Values, with Varied Amounts and Contents of Fats and with Diverse Sodium Chloride Levels on the Blood Pressure and on the Incidence of Abnormal Electrocardiographic Pictures in the Aged. Acta medica Iugoslavica, 17, 154–174.
Subramanian H, Soudarssanane MB, Jayalakshmy R, Thiruselvakumar D, Navasakthi D, Sahai A, Saptharishi L, 2011. Non‐pharmacological Interventions in Hypertension: A Community‐based Cross‐over Randomized Controlled Trial. Indian Journal of Community Medicine, 36, 191–196.
Sun Z, Zheng L, Detrano R, Zhang X, Xu C, Li J, Hu D, Sun Y, 2010. Risk of progression to hypertension in a rural Chinese women population with prehypertension and normal blood pressure. American Journal of Hypertension, 23, 627–632.
Sun Z, Zheng L, Detrano R, Zhang X, Xu C, Li J, Hu D, Sun Y, 2010. Incidence and predictors of hypertension among rural Chinese adults: results from Liaoning province. Annals of Family Medicine, 8, 19–24.
Timpka S, Stuart JJ, Tanz LJ, Rimm EB, Franks PW, Rich‐Edwards JW, 2017. Lifestyle in Progression from Hypertensive Disorders of Pregnancy to Chronic Hypertension in Nurses’ Health Study II: Observational Cohort Study. Obstetrical and Gynecological Survey, 72, 701–703.
Todd AS, Macginley RJ, Schollum JB, Williams SM, Sutherland WH, Mann JI, Walker R J, 2012. Dietary sodium loading in normotensive healthy volunteers does not increase arterial vascular reactivity or blood pressure. Nephrology (Carlton), 17, 249–256.
Toxqui L, Vaquero MP, 2016. An Intervention with Mineral Water Decreases Cardiometabolic Risk Biomarkers. A Crossover, Randomised, Controlled Trial with Two Mineral Waters in Moderately Hypercholesterolaemic Adults. Nutrients, 8.
Trevisan M, Cooper R, Stamler R, Gosch F, Allen A, Liu K, Ostrow D, Stamler J, 1983. Dietary salt and blood pressure. Preventive Medicine, 12, 133–137.
Tuthill RW, Calabrese EJ, 1985. The Massachusetts Blood Pressure Study, Part 4. Modest sodium supplementation and blood pressure change in boarding school girls. Toxicology and Industrial Health, 1, 35–43.
Visser MC, Grobbee DE, Hofman A, 1987. Determinants of rise in blood pressure in normotensive children. Journal of Hypertension, 5, 367–370.
Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ, Hu FB, 2011. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Archives of internal medicine, 171, 1183–1191.
Yang X, He J, Gu D, Hixson JE, Huang J, Rao DC, Shimmin LC, Chen J, Rice TK, Li J, Schwander K, Kelly TN, 2014. Associations of epithelial sodium channel genes with blood pressure changes and hypertension incidence: the GenSalt study. American Journal of Hypertension, 27, 1370–1376.
Zhang D, Gu D, He J, Hixson JE, Rao DC, Li C, He H, Chen J, Huang J, Chen J, Rice TK, Chen S, Kelly TN, 2017. Associations of the Serum/Glucocorticoid Regulated Kinase Genes With BP Changes and Hypertension Incidence: The Gensalt Study. American Journal of Hypertension, 30, 95–101.
Zhao X, Yang X, Zhang X, Li Y, Zhao X, Ren L, Wang L, Gu C, Zhu Z, Han Y, 2014. Dietary salt intake and coronary atherosclerosis in patients with prehypertension. Journal of clinical hypertension (Greenwich), 16, 575–580.
Zheng L, Sun Z, Zhang X, Xu C, Li J, Hu D, Sun Y, 2010. Predictors of progression from prehypertension to hypertension among rural Chinese adults: results from Liaoning Province. European Journal of Cardiovascular Prevention and Rehabilitation, 17, 217–222.
n = 49, other reasons
Alam S, Purdie DM, Johnson AG, 1999. Evaluation of the potential interaction between NaCl and prostaglandin inhibition in elderly individuals with isolated systolic hypertension. Journal of Hypertension, 17, 1195–1202.
Allaert FA, 2017. Effect of NaCl+ Chitosan 3% vs. NaCl on high blood pressure parameters of healthy volunteers with prehypertension. Minerva Cardioangiologica, 65, 563–576.
Allaert F, Melero C, 2015. CO‐42: Observational study of the effect of substituting NaCl with NaCl+ chitosan 3% (Symbiosal®) in the diet of elderly subjects on their blood pressure values. Annales de Cardiologie et d'Angeiologie, 64, S19.
Anonymous, 1977. Randomised controlled trial of treatment for mild hypertension: design and pilot trial. Report of Medical Research Council Working Party on Mild to Moderate Hypertension. British Medical Journal, 1, 1437–1440.
Ard JD, Coffman CJ, Lin PH, Svetkey LP, 2004. One‐year follow‐up study of blood pressure and dietary patterns in dietary approaches to stop hypertension (DASH)‐sodium participants. American Journal of Hypertension, 17, 1156–1162.
Beckmann SL, Os I, Kjeldsen SE, Eide IK, Westheim AS, Hjermann I, 1995. Effect of dietary counselling on blood pressure and arterial plasma catecholamines in primary hypertension. American Journal of Hypertension, 8, 704–711.
Beer‐Borst S, Luta X, Hayoz S, Sommerhalder K, Krause CG, Eisenblatter J, Jent S, Siegenthaler S, Aubert R, Haldimann M, Strazzullo P, 2018. Study design and baseline characteristics of a combined educational and environmental intervention trial to lower sodium intake in Swiss employees. BioMed Central Public Health, 18, 421.
Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ, 2004. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: Results of the DASH‐sodium trial. American Journal of Cardiology, 94, 222–227.
Chen L, He FJ, Dong Y, Huang Y, Harshfield GA, Zhu H, 2018. Sodium Reduction, miRNA Profiling and CVD Risk in Untreated Hypertensives: a Randomized, Double‐Blind, Placebo‐Controlled Trial. Scientific Reports, 8, 12729.
Cheng Y, Song H, Pan X, Xue H, Wan Y, Wang T, Tian Z, Hou E, Lanza I R, Liu P, Liu Y, Laud P W, Usa K, He Y, Liang M, 2018. Urinary Metabolites Associated with Blood Pressure on a Low‐ or High‐Sodium Diet. Theranostics, 8, 1468–1480.
Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK, 2007. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow‐up of the trials of hypertension prevention (TOHP). British Medical Journal, 334, 885–888.
Cook NR, Kumanyika SK, Cutler JA, 1998. Effect of change in sodium excretion on change in blood pressure corrected for measurement error. The Trials of Hypertension Prevention, Phase I. American Journal of Epidemiology, 148, 431–444.
Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK, 2009. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow‐up study. Archives of Internal Medicine, 169, 32–40.
Fagerberg B, Isaksson B, Herlitz H, Andersson OK, 1986. Body composition, intraerythrocyte sodium content, volume regulation and blood pressure during moderate sodium restriction in hypertensive men. Acta Medica Scandinavica, 219, 371–379.
Gao M, Ikeda K, Hattori H, Miura A, Nara Y, Yamori Y, 1999. Cardiovascular risk factors emerging in Chinese populations undergoing urbanization. Hypertension Research, 22, 209–215.
Gijsbers L, Dower JI, Schalkwijk CG, Kusters YH, Bakker SJ, Hollman PC, Geleijnse JM, 2015. Effects of sodium and potassium supplementation on endothelial function: a fully controlled dietary intervention study. British Journal of Nutrition, 114, 1419–1426.
Gillum RF, Elmer PJ, Prineas RJ, 1981. Changing sodium intake in children. The Minneapolis Children's Blood Pressure Study. Hypertension, 3, 698–703.
He FJ, Marciniak M, Markandu ND, Antonios TF, MacGregor GA, 2010. Effect of modest salt reduction on skin capillary rarefaction in white, black, and Asian individuals with mild hypertension. Hypertension, 56, 253–259.
He J, Klag MJ, Appel LJ, Charleston J, Whelton PK, 1998. Seven‐year incidence of hypertension in a cohort of middle‐aged African Americans and whites. Hypertension, 31, 1130–1135.
He J, Whelton PK, Appel LJ, Charleston J, Klag MJ, 2000. Long‐term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension, 35, 544–549.
Holme I, Helgeland A, Hjermann I, Leren P, Mogensen SB, 1988. Correlates of blood pressure change in middle‐aged male mild hypertensives: results from the untreated control group in the Oslo hypertension trial. The Oslo Study. American Journal of Epidemiology, 127, 742–752.
Hunt SC, Cook NR, Oberman A, Cutler JA, Hennekens CH, Allender PS, Walker WG, Whelton PK, Williams RR, 1998. Angiotensinogen genotype, sodium reduction, weight loss, and prevention of hypertension: trials of hypertension prevention phase II. Hypertension, 32, 393–401.
James GD, Pecker MS, Pickering TG, 1996. Sex differences in casual and ambulatory blood pressure responses to extreme changes in dietary sodium. Blood Pressure Monitoring, 1, 397–401.
James GD, Pecker MS, Pickering TG, Jackson S, Difabio B, Carroll L, Laragh JH, 1994. Extreme changes in dietary sodium effect daily variability and level of blood pressure in borderline hypertensive patients. American Journal of Human Biology, 6, 283–291.
Kostis JB, Wilson AC, Shindler DM, Cosgrove NM, Lacy CR, 2002. Persistence of normotension after discontinuation of lifestyle intervention in the trial of TONE. Trial of Nonpharmacologic Interventions in the Elderly. American Journal of Hypertension, 15, 732–734.
Kostis John B, Espeland Mark A, Appel Lawrence, Johnson Karen C, Pierce June, Wofford James L, 1998. Does withdrawal of antihypertensive medication increase the risk of cardiovascular events?. The American Journal of Cardiology, 82, 1501–1508.
Krupp D, Shi L, Remer T, 2014. Longitudinal relationships between diet‐dependent renal acid load and blood pressure development in healthy children. Kidney International, 85, 204–210.
Lai JY, Xiao X, You GY, Xu Y, Wang JL, Liao H, Shi D, Zhang X, Wang Y, 2014. Influence of health education by nurses on effects of blood pressure control in hypertensive patients: a clinical controlled trial. Chinese journal of evidence‐based medicine, 14, 655–658.
Lennie T, Biddle M, Chung M, Mudd‐Martin G, Bailey A, Casey B, 2013. An intervention to reduce sodium intake can lower blood pressure in adults with multiple cardiovascular risk factors living in a rural austere environment. Circulation, 128.
Li N, Yan L, Niu W, Yao C, Feng X, Shi J, Zhang Y, Zhang R, Hao Z, Chu H, Zhang J, Li X, Li Z, Sun J, Zhou B, Zhao Y, Yu Y, Labarthe D, Ma J, Delong E, Elliott P, MacMahon S, Wu Y, Neal B, 2013. China rural health initiative ‐ Sodium reduction study: the effects of a community‐based sodium reduction program on 24 hr urinary sodium and blood pressure in rural China. Circulation. 128, 2707.
Liu Li‐sheng, Zhang Kai‐hua, Wang Jing, Zhang Xiu‐e, Wu Hong‐jiang, Lin Mei‐qing, Gui Rui‐lin, Du Jia‐hui, Gu Mei‐ling, 1987. Primary prevention of hypertension by sodium restriction. Chinese Medical Journal, 100, 899–902.
Macgregor GA, Markandu ND, Sagnella GA, 1982. Dietary sodium restriction in normotensive subjects and patients with essential hypertension. Clinical Science, 63, 399s–402s.
Morgan TO, Myers JB, 1981. Hypertension treated by sodium restriction. The Medical Journal of Australia, 2, 396–397.
Murtaugh MA, Beasley JM, Appel LJ, Guenther PM, McFadden M, Greene T, Tooze JA, 2018. Relationship of Sodium Intake and Blood Pressure Varies With Energy Intake: Secondary Analysis of the DASH (Dietary Approaches to Stop Hypertension)‐Sodium Trial. Hypertension, 71, 858–865.
Murtaugh M, Appel L, Beasley J, Guenther P, Greene T, McFadden M, Tooze J, 2017. Higher levels of sodium density (MG/KCAL) are associated with increased blood pressure independent of absolute sodium (MG): the dash sodium trial. Circulation. Conference: american heart association's epidemiology and prevention/lifestyle and cardiometabolic health 2017 scientific sessions. United states, 135.
Niarchos AP, Weinstein DL, Laragh JH, 1984. Comparison of the effects of diuretic therapy and low sodium intake in isolated systolic hypertension. The American Journal of Medicine, 77, 1061–1068.
Obarzanek E, Proschan MA, Vollmer WM, Moore TJ, Sacks FM, Appel LJ, Svetkey LP, Most‐Windhauser MM, Cutler JA, 2003. Individual blood pressure responses to changes in salt intake: Results from the DASH‐sodium trial. Hypertension, 42, 459–467.
Ohta Y, Tsuchihashi T, Kiyohara K, Oniki H, 2013. Increased uric acid promotes decline of the renal function in hypertensive patients: a 10‐year observational study. Internal Medicine, 52, 1467–1472.
Ohta Y, Tsuchihashi T, Kiyohara K, Oniki H, 2013. High salt intake promotes a decline in renal function in hypertensive patients: a 10‐year observational study. Hypertension Research, 36, 172–176.
Omvik P, Lund‐Johansen P, 1993. Long‐term hemodynamic effects at rest and during exercise of newer antihypertensive agents and salt restriction in essential hypertension: review of epanolol, doxazosin, amlodipine, felodipine, diltiazem, lisinopril, dilevalol, carvedilol, and ketanserin. Cardiovascular Drugs and Therapy, 7, 193–206.
Palmer RM, Osterweil D, Loon‐Lustig G, Stern N, 1989. The effect of dietary salt ingestion on blood pressure of old‐old subjects. A double‐blind, placebo‐controlled, crossover trial. Journal of the American Geriatrics Society, 37, 931–936.
Riphagen IJ, Gijsbers L, van Gastel MD, Kema IP, Gansevoort RT, Navis G, Bakker SJ, Geleijnse JM, 2016. Effects of potassium supplementation on markers of osmoregulation and volume regulation: results of a fully controlled dietary intervention study. Journal of Hypertension, 34, 215–220.
Sebba BW, Arantes A, Bernardes RR, Cassia LAY, Cristina DSA, Borges EM, Cristina DSNT, Rezende ML, Veiga JT, Lima SA, Veiga JP, 2017. Central home and office blood pressure measurement to evaluate changes associated with diet salt reduction. Journal of hypertension. Conference: 27th european meeting on hypertension and cardiovascular protection, ESH 2017. Italy, 35, e135.
Sinaiko AR, Gomez‐Marin O, Prineas RJ, 1993. Effect of low sodium diet or potassium supplementation on adolescent blood pressure. Hypertension, 21, 989–994.
Svetkey LP, Simons‐Morton DG, Proschan MA, Sacks FM, Conlin PR, Harsha D, Moore TJ, 2004. Effect of the dietary approaches to stop hypertension diet and reduced sodium intake on blood pressure control. Journal of clinical hypertension (Greenwich), 6, 373–381.
Svetkey LP, Simons‐Morton D, Vollmer WM, Appel LJ, Conlin PR, Ryan DH, Ard J, Kennedy BM, 1999. Effects of dietary patterns on blood pressure: Subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Archives of Internal Medicine, 159, 285–293.
Trevisan M, Cooper R, Ostrow D, Miller W, Sparks S, Leonas Y, Allen A, Steinhauer M, Stamler J, 1981. Dietary sodium, erythrocyte sodium concentration, sodium‐stimulated lithium efflux and blood pressure. Clinical Science (London), 61 Suppl 7, 29s–32s.
Ueda K, Kashiba A, Miyai N, Mure K, Arita M, 2017. Effects of a home blood pressure monitoring by mobile phone‐based self‐management support system in mild hypertension: the Katsuragi study. Journal of hypertension. Conference: 27th European meeting on hypertension and cardiovascular protection, ESH 2017. Italy, 35, e56‐e57.
Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons‐Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, Karanja N, 2001. Effects of diet and sodium intake on blood pressure: Subgroup analysis of the DASH‐sodium trial. Annals of Internal Medicine, 135, 1019–1028.
G.1.3. Systematic reviews screened for eligible references
Graudal N, Jurgens G, Baslund B, Alderman MH, 2014. Compared with usual sodium intake, low‐ and excessive‐sodium diets are associated with increased mortality: a meta‐analysis. American Journal of Hypertension, 27, 1129–1137.
Jayedi A, Ghomashi F, Zargar MS, Shab‐Bidar S, 2018. Dietary sodium, sodium‐to‐potassium ratio, and risk of stroke: A systematic review and nonlinear dose‐response meta‐analysis. Clinical Nutrition, pii: S0261–5614(18)30202‐4. (Epub ahead of print)
Leyvraz M, Chatelan A, da Costa BR, Taffe P, Paradis G, Bovet P, Bochud, M, Chiolero A, 2018. Sodium intake and blood pressure in children and adolescents: a systematic review and meta‐analysis of experimental and observational studies. International Journal of Epidemiology, 47, 1796–1810.
Li XY, Cai, XL, Bian PD, Hu LR, 2012. High salt intake and stroke: meta‐analysis of the epidemiologic evidence. CNS neuroscience & therapeutics, 18, 691–701.
Newberry SJ, Chung M, Anderson CAM, Chen C, Fu Z, Tang A, Zhao N, Booth M, Marks J, Hollands S, Motala A, Larkin J, Shanman R, Hempel S, 2018. Sodium and potassium intake: effects on chronic disease outcomes and risks. AHRQ Comparative Effectiveness Reviews Number 206. AHRQ Publication No. 18‐EHC009‐EF. Rockville, MD, USA. 951 pp. Available online: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/cer-206-report-sodium-potassium-update.pdf
Poggio R, Gutierrez L, Matta MG, Elorriaga N, Irazola V, Rubinstein A, 2015. Daily sodium consumption and CVD mortality in the general population: systematic review and meta‐analysis of prospective studies. Public Health Nutrition, 18, 695–704.
Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP, 2009. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. British Medical Journal, 339, b4567.
Subasinghe AK, Arabshahi S, Busingye D, Evans RG, Walker KZ, Riddell MA, Thrift AG, 2016. Association between salt and hypertension in rural and urban populations of low to middle income countries: a systematic review and meta‐analysis of population based studies. Asia Pacific Journal of Clinical Nutrition, 25, 402–413.
G.2. Bone health
G.2.1. PRISMA chart
G.2.2. Reference list of studies excluded based on full text screening
n = 22, not eligible because of study design
Angelakos K, Hew‐Butler T, 2015. Effects of sodium loading plus walking on‐bone mineral content (BMC). FASEB Journal, 29.
Arcand J, Webster J, Johnson C, Raj TS, Neal B, McLean R, Trieu K, Wong MMY, Leung AA, Campbell NRC, 2016. Announcing “Up to Date in the Science of Sodium”. Journal of Clinical Hypertension, 18, 85–88.
Bass M, Ford MA, Brown B, Mauromoustakos A, Keathley RS, 2006. Variables for the prediction of femoral bone mineral status in American women. Southern Medical Journal, 99, 115–122.
Burger H, Grobbee DE, Drueke T, 2000. Osteoporosis and salt intake. Nutrition, Metabolism & Cardiovascular Diseases, 10, 46–53.
Carbone LD, Bush AJ, Barrow KD, Kang AH, 2003. The relationship of sodium intake to calcium and sodium excretion and bone mineral density of the hip in postmenopausal African‐American and Caucasian women. Journal of Bone and Mineral Metabolism, 21, 415–420.
Chan R, Woo J, Lau W, Leung J, Xu L, Zhao X, Yu W, Lau E, Pocock N, 2009. Effects of lifestyle and diet on bone health in young adult Chinese women living in Hong Kong and Beijing. Food and Nutrition Bulletin, 30, 370–378.
Forbes GB, Tobin RB, Lewis A, 1959. Response of bone sodium to acute changes in extracellular fluid composition (cat). American Journal of Physiology, 196, 69–73.
Greendale GA, Barrett‐Connor E, Edelstein S, 1994. High sodium intake is not an osteoporosis risk factor. Journal of the American Osteopathic Association, 94, 912.
Harrington M, Cashman KD, 2003. High salt intake appears to increase bone resorption in postmenopausal women but high potassium intake ameliorates this adverse effect. Nutrition Reviews, 61, 179–183.
Ilich JZ, Brownbill RA, Tamborini L, 2003. Bone and nutrition in elderly women: Protein, energy, and calcium as main determinants of bone mineral density. European Journal of Clinical Nutrition, 57, 554–565.
Lim H S, Ji SI, Hwang H, Kang J, Park YH, Lee HH, Kim TH, 2018. Relationship between Bone Density, Eating Habit, and Nutritional Intake in College Students. Journal of Bone Metabolism, 25, 181–186.
Lim HS, Park YH, Kim SK, 2016. Relationship between Serum Inflammatory Marker and Bone Mineral Density in Healthy Adults. Journal of Bone Metabolism, 23, 27–33.
Magner P, 1990. Dietary sodium and osteoporosis. New Zealand Medical Journal, 103, 355.
Massey LK, Whiting SJ, 1996. Dietary salt, urinary calcium, and bone loss. Journal of Bone and Mineral Research, 11, 731–736.
Matkovic V, Ilich JZ, Andon MB, Hsieh LC, Tzagournis MA, Lagger BJ, Goel PK, 1995. Urinary calcium, sodium, and bone mass of young females. American Journal of Clinical Nutrition, 62, 417–425.
Palmieri MA, Pitcock JA, 1995. Osteoporosis and hypercalciuria secondary to excessive salt ingestion. Journal of Laboratory and Clinical Medicine, 126, 503.
Park JS, Choi SB, Rhee Y, Chung JW, Choi EY, Kim DW, 2015. Parathyroid hormone, calcium, and sodium bridging between osteoporosis and hypertension in postmenopausal Korean women. Calcified Tissue International, 96, 417–429.
Park SM, Jee J, Joung JY, Cho YY, Sohn SY, Jin SM, Hur KY, Kim JH, Kim SW, Chung JH, Lee MK, Min YK, 2014. High Dietary Sodium Intake Assessed by 24‐hour Urine Specimen Increase Urinary Calcium Excretion and Bone Resorption Marker. Journal of Bone Metabolism, 21, 189–194.
Park SM, Joung JY, Cho YY, Sohn SY, Hur KY, Kim JH, Kim SW, Chung JH, Lee MK, Min YK, 2015. Effect of high dietary sodium on bone turnover markers and urinary calcium excretion in Korean postmenopausal women with low bone mass. European Journal of Clinical Nutrition, 69, 361–366.
Renner RP, Boucher LJ, Kaufman HW, 1984. Osteoporosis in postmenopausal women. Journal of Prosthetic Dentistry, 52, 581–588.
Schaafsma G, van Beresteyn EC, Raymakers JA, Duursma SA, 1987. Nutritional aspects of osteoporosis. World Review of Nutrition and Dietetics, 49, 121–159.
Wong MM, Arcand J, Leung AA, Raj TS, Trieu K, Santos JA, Campbell NR, 2016. The Science of Salt: A Regularly Updated Systematic Review of Salt and Health Outcomes (August to November 2015). Journal of clinical hypertension (Greenwich), 18, 1054–1062.
n = 8, 24‐h urine sodium excretion not measured
Burger H, De Laet CEDH, Van Daele PLA, Weel AEAM, Witteman JCM, Hofman A, Pols HAP, 1998. Risk factors for increased bone loss in an elderly population: The Rotterdam Study. American Journal of Epidemiology, 147, 871–879.
Carbone L, Johnson KC, Huang Y, Pettinger M, Thomas F, Cauley J, Crandall C, Tinker L, LeBoff MS, Wactawski‐Wende J, Bethel M, Li W, Prentice R, 2016. Sodium Intake and Osteoporosis. Findings From the Women's Health Initiative. Journal of Clinical Endocrinology and Metabolism, 101, 1414–1421.
Chan R, Woo J, Leung J, 2011. Effects of food groups and dietary nutrients on bone loss in elderly Chinese population. Journal of Nutrition Health & Aging, 15, 287–294.
Fenton TR, Eliasziw M, Tough SC, Lyon AW, Brown JP, Hanley DA, 2010. Low urine pH and acid excretion do not predict bone fractures or the loss of bone mineral density: a prospective cohort study. BioMed Central Musculoskeletal Disorders, 11, 88.
Gunnes M, Lehmann EH, 1996. Physical activity and dietary constituents as predictors of forearm cortical and trabecular bone gain in healthy children and adolescents: a prospective study. Acta Paediatrica, 85, 19–25.
Houtkooper LB, Ritenbaugh C, Aickin M, Lohman TG, Going SB, Weber JL, Greaves KA, Boyden TW, Pamenter RW, Hall MC, 1995. Nutrients, body composition and exercise are related to change in bone mineral density in premenopausal women. Journal of Nutrition, 125, 1229–1237.
Hubert HB, Fries JF, 1994. Predictors of physical disability after age 50. Six‐year longitudinal study in a runners club and a university population. Annals of Epidemiology, 4, 285–294.
Reid IR, Ames RW, Evans MC, Sharpe SJ, Gamble GD, 1994. Determinants of the rate of bone loss in normal postmenopausal women. Journal of Clinical Endocrinology and Metabolism, 79, 950–954.
n = 4, outcome(s) not of interest
Breslau NA, Sakhaee K, Pak CY, 1985. Impaired adaptation to salt‐induced urinary calcium losses in postmenopausal osteoporosis. Transactions of the Association of American Physicians, 98, 107–115.
Goulding A, Lim PE, 1983. Effects of varying dietary salt intake on the fasting urinary excretion of sodium, calcium and hydroxyproline in young women. New Zealand Medical Journal, 96, 853–854.
McParland BE, Goulding A, Campbell AJ, 1989. Dietary salt affects biochemical markers of resorption and formation of bone in elderly women. British Medical Journal, 299, 834–835.
Wigertz K, Palacios C, Jackman LA, Martin BR, McCabe LD, McCabe GP, Peacock M, Pratt JH, Weaver CM, 2005. Racial differences in calcium retention in response to dietary salt in adolescent girls. American Journal of Clinical Nutrition, 81, 845–850.
n = 1, exposure not of interest
Merrilees MJ, Smart EJ, Gilchrist NL, Frampton C, Turner JG, Hooke E, March RL, Maguire P, 2000. Effects of diary food supplements on bone mineral density in teenage girls. European Journal of Nutrition, 39, 256–262.
n = 1, outcome measurement method (not DEXA)
Nordin BEC, Polley KJ, 1987. Metabolic consequences of the menopause. A cross‐sectional, longitudinal, and intervention study on 557 normal postmenopausal women. Calcified Tissue International, 41, S1–S59.
n = 2, other reasons
Chao D, Espeland MA, Farmer D, Register TC, Lenchik L, Applegate WB, Ettinger WH Jr, 2000. Effect of voluntary weight loss on bone mineral density in older overweight women. Journal of the American Geriatrics Society, 48, 753–759.
Frassetto LA, Hardcastle AC, Sebastian A, Aucott L, Fraser WD, Reid DM, Macdonald HM, 2012. No evidence that the skeletal non‐response to potassium alkali supplements in healthy postmenopausal women depends on blood pressure or sodium chloride intake. European Journal of Clinical Nutrition, 66, 1315–1322.
G.2.3. Systematic review screened for eligible references
Fatahi S, Namazi N, Larijani B and Azadbakht L, 2018. The association of dietary and urinary sodium with bone mineral density and risk of osteoporosis: a systematic review and meta‐analysis. Journal of the American College of Nutrition, 37, 522–532.
G.3. Criteria used to appraise RoB in eligible studies
G.3.1. Randomised controlled trials
| Question | Rating | Explanation for expert judgement |
|---|---|---|
|
1. Was administered dose or exposure level adequately randomised? Key question |
++ | There is direct evidence that subjects (or clusters) were allocated to any study group including controls using a method with a random component. Acceptable methods of randomisation include: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, or drawing of lots (Higgins and Green, 2011). Restricted randomisation (e.g. blocked randomisation) to ensure particular allocation ratios will be considered low risk of bias. Similarly, stratified randomisation and minimisation approaches that attempt to minimise imbalance between groups on significant prognostic factors (e.g. body weight) will be considered acceptable |
| + |
There is indirect evidence that subjects (or clusters) were allocated to study groups using a method with a random component (i.e. authors state that allocation was random, without description of the method used) OR It is deemed that allocation without a clearly random component during the study would not appreciably bias results. For example, approaches such as biased coin or urn randomisation, replacement randomisation, mixed randomisation, and maximal randomisation may require consultation with a statistician to determine risk‐of‐bias rating (Higgins and Green, 2011) |
|
| NR | There is insufficient information provided about how subjects (or clusters) were allocated to study groups. | |
| − |
There is indirect evidence that subjects (or clusters) were allocated to study groups using a method with a non‐random component. NOTE: Non‐random allocation methods may be systematic, but have the potential to allow participants or researchers to anticipate the allocation to study groups. Such ‘quasi‐random’ methods include alternation, assignment based on date of birth, case record number, or date of presentation to study |
|
| − – | There is direct evidence that subjects (or clusters) were allocated to study groups using a non‐random method including judgement of the clinician, preference of the participant, the results of a laboratory test or a series of tests, or availability of the intervention (Higgins and Green, 2011) | |
| 2. Was allocation to study groups adequately concealed? | ++ | There is direct evidence that at the time of recruitment the research personnel and subjects did not know what study group subjects were allocated to, and it is unlikely that they could have broken the blinding of allocation until after assignment was complete and irrevocable. Acceptable methods used to ensure allocation concealment include central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes; or equivalent methods |
| + |
There is indirect evidence that the research personnel and subjects did not know what study group subjects were allocated to and it is unlikely that they could have broken the blinding of allocation until after recruitment was complete and irrevocable OR It is deemed that lack of adequate allocation concealment would not appreciably bias results (e.g. some crossover designs) |
|
| NR | There is insufficient information provided about allocation to study groups | |
| − |
There is indirect evidence that at the time of recruitment it was possible for the research personnel and subjects to know what study group subjects were allocated to, or it is likely that they could have broken the blinding of allocation before assignment was complete and irrevocable NOTE: Inadequate methods include using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or any other explicitly unconcealed procedure. For example, if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed |
|
| − – | There is direct evidence that at the time of recruitment it was possible for the research personnel and subjects to know what study group subjects were allocated to, or it is likely that they could have broken the blinding of allocation before recruitment was complete and irrevocable | |
| 3. Were the research personnel and human subjects blinded to the study group during the study? | ++ | There is direct evidence that the subjects and research personnel were adequately blinded to study group, AND it is unlikely that they could have broken the blinding during the study. Methods used to ensure blinding include central allocation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes; or equivalent methods |
| + |
There is indirect evidence that the subjects and research personnel were adequately blinded to study group, AND it is unlikely that they could have broken the blinding during the study OR There is direct evidence for no blinding during the study (including where it was not possible to implement) AND it is deemed that no blinding would appreciably bias results BUT bias minimising measures have been adequately implemented OR It is deemed that lack of adequate blinding or no blinding during the study would not appreciably bias results (e.g. controls unlikely to behave differently for factors other than sodium intake) (e.g. cross‐over) |
|
| NR | There is insufficient information provided about blinding to study group during the study (including possible breaking and minimising measures) | |
| − | There is indirect evidence that it was possible for research personnel or subjects to infer the study group AND it is deemed that lack of adequate blinding or no blinding during the study would appreciably bias results (e.g. no comparable treatment of controls, including not comparable exposure to factors other than the interventions of interest; differential behaviour) AND no bias minimising measures have been adequately implemented | |
| − – | There is direct evidence for lack of adequate blinding of the study group (including no blinding or incomplete blinding) of research personnel and subjects AND it is deemed that lack of adequate blinding or no blinding during the study would appreciably bias results (e.g. no comparable treatment of controls, including not comparable exposure to factors other than the interventions of interest, differential behaviour) AND no bias minimising measures have been adequately implemented | |
| 4. Were outcome data complete without attrition or exclusion from analysis? | ++ |
There is direct evidence that there was no loss of subjects during the study and outcome data were complete OR Loss of subjects (i.e. incomplete outcome data) was adequately addressed and reasons were documented when human subjects were removed from a study or analyses. Review authors should be confident that the participants included in the analysis are exactly those who were randomised into the trial. Acceptable handling of subject attrition includes: very few missing outcome data (e.g. less than 10% in each group (Genaidy et al., 2007)) AND reasons for missing subjects unlikely to be related to outcome (for survival data, censoring unlikely to be introducing bias) AND missing outcome data balanced in numbers across study groups, with similar reasons for missing data across groups (i.e. unlikely to be related to exposure) OR Analyses (such as intention‐to‐treat analysis) in which missing data have been imputed using appropriate methods (ensuring that the characteristics of subjects lost to follow up or with unavailable records are described in identical way and are not significantly different from those of the study participants) NOTE: Participants randomised but subsequently found not to be eligible need not always be considered as having missing outcome data) (Higgins and Green, 2011 |
| + |
There is indirect evidence that loss of subjects (i.e. incomplete outcome data) was adequately addressed and reasons were documented when human subjects were removed from a study OR It is deemed that the proportion lost to follow‐up would not appreciably bias results (e.g. less than 20% in each group in parallel studies (Genaidy et al., 2007)). This would include reports of no statistical differences in characteristics of subjects lost to follow up or with unavailable records from those of the study participants. Generally, the higher the ratio of participants with missing data to participants with events, the greater potential there is for bias. For studies with a long duration of follow‐up, some withdrawals for such reasons are inevitable NB: For crossover designs, this may be less of an issue |
|
| NR | There is insufficient information provided about numbers of subjects lost to follow‐up | |
| − | There is indirect evidence that loss of subjects (i.e. incomplete outcome data) was unacceptably large (e.g. greater than 20% in each group in parallel studies (Genaidy et al., 2007)) and not adequately addressed | |
| − – | There is direct evidence that loss of subjects (i.e. incomplete outcome data) was unacceptably large and not adequately addressed (e.g. greater than 20% in each group in parallel studies (Genaidy et al., 2007)). Unacceptable handling of subject attrition includes: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across study groups (i.e. likely to be related to the exposure); or potentially inappropriate application of imputation | |
|
5. Can we be confident in the exposure characterisation? Key question |
++ |
There is direct evidence that the exposure (including compliance with the treatment, if applicable) was independently characterised AND that exposure was consistently administered (i.e. with the same method and time‐frame) across treatment groups NOTE: applies to studies that collected single or multiple 24‐h urine samples for each time point AND checked the completeness of the samples (by any kind of method) |
| + |
There is indirect evidence that the exposure (including compliance with the treatment, if applicable) was independently characterised AND there is indirect evidence that exposure was consistently administered (i.e. with the same method and time‐frame) across treatment groups NOTE: applies to studies that collected single or multiple 24‐h urine samples without completeness check of the urine samples (or not reported), but there is no evidence for concern |
|
| NR | There is insufficient information provided to judge the exposure characterisation | |
| − |
There is indirect evidence that the exposure (including compliance with the treatment, if applicable) was assessed using poorly validated methods (e.g. FFQs, spot urine etc.) OR There is indirect evidence that the exposure assessment was probably biased NOTE: applies to studies for which 24‐h urine samples were collected but there is indirect evidence that the proportion of incomplete samples included in the analysis was substantial |
|
| − – |
There is direct evidence that the exposure (including compliance with the treatment, if applicable) was assessed using poorly validated methods (e.g. FFQs, spot urine etc.) OR There is direct evidence that the exposure assessment was biased NOTE: applies to studies for which 24 h urine samples were collected but there is direct evidence that the proportion of incomplete samples included in the analysis was substantial |
|
|
6. Can we be confident in the outcome assessment? Key question |
++ |
There is direct evidence that the outcome was assessed using well‐established methods (e.g. for office BP: according to a clearly described methodology, including e.g. repeated measurements per visit, trained technician, resting period before each measurement) AND There is direct evidence that the outcome assessors were adequately blinded to the study group, and it is unlikely that they could have broken the blinding prior to reporting outcomes |
| + |
There is indirect evidence that the outcome was assessed using acceptable methods (i.e. deemed valid and reliable but not the gold standard) OR it is deemed that the outcome assessment methods used would not appreciably bias results AND There is indirect evidence that the outcome assessors were adequately blinded to the study group, and it is unlikely that they could have broken the blinding before reporting outcomes OR it is deemed that lack of adequate blinding of outcome assessors would not appreciably bias results |
|
| NR | There is insufficient information provided about blinding of outcome assessors or the method of measurement | |
| − |
There is indirect evidence that the outcome assessment method is an unacceptable method OR There is indirect evidence that it was possible for outcome assessors to infer the study group before reporting outcomes |
|
| − – |
There is direct evidence that the outcome assessment method is an unacceptable method OR There is direct evidence for lack of adequate blinding of outcome assessors (including study subjects if home BP is the outcome), including no blinding or incomplete blinding |
|
| 7. Were all measured outcomes reported? | ++ | There is direct evidence that all of the study's measured outcomes (primary and secondary) outlined in the protocol, methods, abstract, and/or introduction (that are relevant for the evaluation) have been reported |
| + |
There is indirect evidence that all of the study's measured outcomes (primary and secondary) outlined in the methods, abstract, and/or introduction (that are relevant for the evaluation) have been reported OR Analyses that had not been planned in advance (i.e. retrospective unplanned subgroup analyses) are clearly indicated as such and it is deemed that the unplanned analyses were appropriate and selective reporting would not appreciably bias results (e.g. appropriate analyses of an unexpected effect). This would include outcomes reported with insufficient detail such as only reporting that results were statistically significant (or not) |
|
| NR | There is insufficient information provided about selective outcome reporting | |
| − |
There is indirect evidence that all of the study's measured outcomes (primary and secondary) outlined in the methods, abstract, and/or introduction (that are relevant for the evaluation) have not been reported OR There is indirect evidence that unplanned analyses were included that may appreciably bias result |
|
| – – | There is direct evidence that all of the study's measured outcomes (primary and secondary) outlined in the methods, abstract, and/or introduction (that are relevant for the evaluation) have not been reported. In addition to not reporting outcomes, this would include reporting outcomes based on composite score without individual outcome components or outcomes reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified or reporting outcomes not pre‐specified, or that unplanned analyses were included that would appreciably bias results | |
|
8. Were there no other potential threats to internal validity? NOTE: Baseline characteristics should be appraised only if Q1 (randomisation) was rated with ++/+ and Q2 (allocation concealment) was rated with ++/+/NR |
++ |
There is evidence that variables, other than the exposure and outcome, did not differ between groups during the course of the intervention in a way that could bias results AND, in case randomisation is rated ‘probably low’/‘definitely low’ RoB and allocation concealment is rated ‘probably low’/‘definitely low’ RoB or ‘not reported’: There is no evidence of differences in baseline characteristics OR There is no information on both BUT no concern |
| + |
1. There is evidence that variables, other than the exposure and outcome, differed between groups during the course of the intervention AND it is deemed that these differences would not appreciably bias results (no concern or adequately addressed by analysis) AND, in case randomisation is rated ‘probably low’/‘definitely low’ RoB and allocation concealment is rated ‘probably low’/‘definitely low’ RoB or ‘not reported’: There is evidence that reported variables differed between groups at baseline ANDIt is deemed that these differences would not appreciably bias results (no concern or adequately addressed by analysis)‐‐‐‐‐‐‐OR2. There is evidence that variables, other than the exposure and outcome, did not differ between groups during the course of the intervention in a way that could bias results AND, in case randomisation is rated ‘probably low’/‘definitely low’ RoB and allocation concealment is rated ‘probably low’/‘definitely low’ RoB or ‘not reported’: There is evidence that reported variables differed between groups at baseline.AND It is deemed that these differences would not appreciably bias results (no concern or adequately addressed by analysis).‐‐‐‐‐‐OR3. There is evidence that variables, other than the exposure and outcome, differed between groups during the course of the intervention.ANDIt is deemed that these differences would not appreciably bias results (no concern or adequately addressed by analysis) AND, in case randomisation is rated ‘probably low’/‘definitely low’ RoB and allocation concealment is rated ‘probably low’/‘definitely low’ RoB or ‘not reported’: There is no evidence of differences in baseline characteristics.ORThere is no information BUT no concern |
|
| − |
There is no information on baseline characteristics AND/OR there is no information about differences between groups during the course of the intervention. AND There is concern |
|
| − – |
There is evidence that variables, other than the exposure and outcome, differed between groups during the course of the intervention.ANDIt is deemed that these differences appreciably biased results (there is concern (e.g. not adequately addressed by analysis)) OR, in case randomisation is rated ‘probably low’/‘definitely low’ RoB and allocation concealment is rated ‘probably low’/‘definitely low’ RoB or ‘not reported’:There is evidence that reported variables differed between groups at baseline.ANDIt is deemed that these differences appreciably biased results (there is concern (e.g. not adequately addressed by analysis)) |
++: Definitely low RoB; +: Probably low RoB; NR: Not Reported; –: Probably high RoB; – −: Definitely high RoB.
G.3.2. Prospective observational studies
| Question | Rating | Explanation for expert judgement |
|---|---|---|
|
Did the study design or analysis account for important confounding? Key question |
++ |
There is direct evidence that appropriate adjustments or explicit considerations were made for the potential confounders in the final analyses through the study design (e.g. matching, restriction) and/or through the use of statistical models to reduce research‐specific bias including standardisation, adjustment in multivariate model, stratification, propensity scoring, or other methods that were appropriately justified NOTE: Acceptable consideration of appropriate adjustment factors includes cases when the factor is not included in the final adjustment model because: i) there was evidence indicating that a factor did not need to be included as a confounders (e.g. the author conducted analyses that indicated it did not need to be included; study restricted to males only) OR ii) it is deemed that not considering the factor would not bias the result ANDThere is direct evidence that confounders were assessed using reliable methods |
| + |
There is indirect evidence that appropriate adjustments were made, OR it is deemed that not considering or only considering a partial list confounders in the final analyses would not substantially bias results AND There is evidence (direct or indirect) that confounders were assessed using reliable methods, OR it is deemed that the methods used would not appreciably bias results (i.e., the authors justified the validity of the methods from previously published research) |
|
| NR |
There is insufficient information provided about the distribution of potential confounders (record ‘NR’ as basis for answer) OR There is insufficient information provided about the methods used to assess confounders (record ‘NR’ as basis for answer) |
|
| − | There is indirect evidence that the distribution of potential confounders differed between the groups and was not appropriately adjusted for in the final analyses | |
| − – |
There is direct evidence that the distribution of confounders differed between the groups, confounding occurred but was not adjusted for in the final analyses OR There is direct evidence that confounders were assessed using non‐reliable methods |
|
| 2. Were outcome data complete without attrition or exclusion from analysis? | ++ |
There is direct evidence that loss of subjects (i.e. incomplete outcome data) was adequately addressed and reasons were documented when human subjects were lost or removed from a study NOTE: Acceptable handling of subject attrition includes: very few missing outcome data AND reasons for missing subjects unlikely to be related to outcome (for survival data, censoring unlikely to be introducing bias) AND missing outcome data balanced in numbers across study groups, with similar reasons for missing data across groups (i.e. unlikely to be related to exposure) |
| + |
There is indirect evidence that loss of subjects (i.e. incomplete outcome data) was adequately addressed and reasons were documented when human subjects were removed from a study OR It is deemed that the proportion lost to follow‐up would not appreciably bias results, due to the similarity between the characteristics of subjects lost to follow‐up and study participants. Generally, the higher the ratio of participants with missing data to participants with events, the greater potential there is for bias. For studies with a long duration of follow‐up, some withdrawals for such reasons are inevitable |
|
| − | There is indirect evidence that loss of subjects (i.e. incomplete outcome data) was unacceptably large and not adequately addressed | |
| − – | There is direct evidence that loss of subjects (i.e. incomplete outcome data) was unacceptably large and not adequately addressed. Unacceptable handling of subject attrition includes: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across study groups (i.e. likely to be related to the exposure) | |
|
3. Can we be confident in the exposure characterisation? Key question |
++ |
Sodium intake was assessed through multiple 24‐h urinary collection (in a ‘reasonably short time‐frame’) AND There is direct evidence that quality assurance measures were in place for the collection of 24‐h urine (e.g. first and last void at the clinic; careful instructions of the participants) OR incomplete collections were excluded on the basis of any method (e.g. PABA, creatinine, self‐reported, volume…) |
| + |
Sodium intake was assessed through a single 24‐h urinary collection AND There is direct evidence that quality assurance measures were in place for the collection of 24‐h urine (e.g. first and last void at the clinic; careful instructions of the participants) OR incomplete collections were excluded on the basis of any method (e.g. PABA, creatinine, self‐reported, volume…) |
|
| NR | There is insufficient information provided about the method of exposure assessment | |
| – |
There is indirect evidence that the exposure (including compliance with the treatment, if applicable) was assessed using poorly validated methods (e.g. FFQs, spot urine etc.) OR There is no evidence that quality assurance measures were in place for the collection of 24‐h urine (single or multiple) AND no measures were taken to exclude incomplete samples |
|
| − – |
There is direct evidence that the exposure (including compliance with the treatment, if applicable) was assessed using poorly validated methods (e.g. FFQs, spot urine etc.) OR There is direct evidence for systematic error in the exposure characterisation (exposure misclassification) |
|
|
4. Can we be confident in the outcome assessment? Key question |
++ | There is direct evidence that the outcome was assessed using well‐established methods |
| + |
There is indirect evidence that the outcome was assessed using acceptable methods (i.e. deemed valid and reliable but not the gold standard) OR It is deemed that the outcome assessment methods used would not appreciably bias results |
|
| NR | There is insufficient information provided about the method of measurement | |
| – | There is indirect evidence that the outcome assessment method is an unacceptable method | |
| − – | There is direct evidence that the outcome assessment method is an unacceptable method | |
| 5. Were all measured outcomes reported? | ++ | There is direct evidence that all of the study's measured outcomes (primary and secondary) outlined in the protocol, methods, abstract, and/or introduction (that are relevant for the evaluation) have been reported. This would include outcomes reported with sufficient detail to be included in meta‐analysis or fully tabulated during data extraction and analyses had been planned in advance |
| + |
There is indirect evidence that all of the study's measured outcomes (primary and secondary) outlined in the methods, abstract, and/or introduction (that are relevant for the evaluation) have been reported ORAnalyses that had not been planned in advance (i.e. retrospective unplanned subgroup analyses) are clearly indicated as such and it is deemed that the unplanned analyses were appropriate and selective reporting would not appreciably bias results (e.g. appropriate analyses of an unexpected effect). This would include outcomes reported with insufficient detail such as only reporting that results were statistically significant (or not) |
|
| NR | There is insufficient information provided about selective outcome reporting | |
| – |
There is indirect evidence that all of the study's measured outcomes (primary and secondary) outlined in the methods, abstract, and/or introduction (that are relevant for the evaluation) have been reported OR There is indirect evidence that unplanned analyses were included that may appreciably bias results |
|
| − – | There is direct evidence that all of the study's measured outcomes (primary and secondary) outlined in the methods, abstract, and/or introduction (that are relevant for the evaluation) have not been reported. In addition to not reporting outcomes, this would include reporting outcomes based on composite score without individual outcome components or outcomes reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified or reporting outcomes not pre‐specified, or that unplanned analyses were included that would appreciably bias results | |
| 6. Were the statistical methods applied appropriate? | ++ | There is direct evidence that the statistical analysis was appropriate |
| + | There is indirect evidence that the statistical analysis was appropriate | |
| NR | There is insufficient information provided about the statistical analysis | |
| – | There is indirect evidence that the statistical analysis was not appropriate | |
| − – | There is direct evidence that the statistical analysis was not appropriate |
++: Definitely low RoB; +: Probably low RoB; NR: Not Reported; –: probably high RoB; – −: Definitely high RoB.
G.3.3. Algorithm applied to allocate studies to the three tiers of RoB
The judgements to the RoB questions were combined into an overall RoB judgement for each individual study (by outcome). As a result, studies were classified as being at low (tier 1), moderate (tier 2) or high (tier 3) RoB. Following the OHAT/NTP guidance (OHAT/NTP, 2015), key questions were identified for each type of design (see Table below) and the following algorithm was applied to allocate the studies to the tiers:
Tier 1: study rated as ‘definitely low’ or ‘probably low’ risk of bias for the key questions AND most other applicable questions answered ‘definitely low’ or ‘probably low’ risk of bias.
Tier 2: study met neither the criteria for tiers.
Tier 3: study rated as ‘definitely high’ or ‘probably high’ risk of bias for the key questions AND most other applicable questions answered ‘definitely high’ or ‘probably high’ risk of bias.
| Bias Domains and Questions | RCT | Prospective observational |
|---|---|---|
| Selection Bias | ||
| Was administered dose or exposure level adequately randomised? | X * | |
| Was allocation to study groups adequately concealed? | X | |
| Confounding Bias | ||
| Did the study design or analysis account for important confounding? | X * | |
| Performance Bias | ||
| Were the research personnel and human subjects blinded to the study group during the study? | X | |
| Attrition/Exclusion Bias | ||
| Were outcome data complete without attrition or exclusion from analysis? | X | X |
| Detection Bias | ||
| Can we be confident in the exposure characterisation? | X * | X * |
| Can we be confident in the outcome assessment? | X * | X * |
| Selective Reporting Bias | ||
| Were all measured outcomes reported? | X | X |
| Other Sources of Bias | ||
| Were there no other potential threats to internal validity? | X | |
| Were the statistical methods applied appropriate? | X | |
* Key question.
Appendix H – Outcome of the systematic review on blood pressure levels in children
H.1. Evidence tables
Experimental studies
|
References (country) |
Design | Subject characteristics at baseline |
UNa (mmol/24 h) |
BP measurement SBP/DBP (mm Hg) |
|---|---|---|---|---|
|
He et al. (2015) (China) School‐EduSalt |
Study design: cluster‐randomised Run‐in type: none Intervention type: counselling N participants randomised/completed: 279/274 Duration: 14 weeks |
% boys G1: 47.5 G2: 48.6 Age (mean ± SD, years) G1: 10.0 ± 0.5 G2: 10.2 ± 0.5 BMI (mean ± SD, kg/m 2 ) G1: 17.1 ± 3.2 G2: 16.7 ± 2.7 Ethnicity: NR BP status: NR 24‐h UK (mean ± SE, mmol) G1: 23.5 ± 0.9 G2: 25.4 ± 0.9 Energy intake: NR |
Type: multiple 24 h Mean ± SE Beginning of intervention G1: 124.2 ± 5.1 G2: 116.7 ± 5.2 End of intervention G1: 112.2 ± 5.1 G2: 137.2 ± 5.2 |
Type: point office, sitting Mean ± SE Beginning of intervention G1: 106.2 ± 1/67.0 ± 1.1 G2: 106.2 ± 1/66.8 ± 1.1 End of intervention G1: 110 ± 1/69.4 ± 1.1 G2: 110.6 ± 1/70.2 ± 1.1 |
|
Miller et al. (1988) (USA) |
Study design: parallel Run‐in type: low sodium diet Intervention type: feeding N participants randomised/ completed: 88/NR Duration: 4 weeks |
% boys: NR Age (years): NR BP status: NT Ethnicity: NR 24‐h UK (mean, mmol) G1: 35.3 G2: 36.7 Energy intake: NR |
Type: multiple 24 h Mean Beginning of intervention NR End of intervention G1: 44.4 G2: 72.1 |
Type: point office, sitting Mean Beginning of intervention NR End of intervention G1: 91.7/53.0 G2: 92/52.9 |
BMI: body mass index; BP: blood pressure; DBP; diastolic blood pressure; G1: group 1 (‘low’ sodium); G2: group 2 (‘high’ sodium); mm Hg: millimetre of mercury; N: number; UNa: sodium urinary excretion; NR: not reported; NT: normotensive; SBP: systolic blood pressure; SD: standard deviation; SE: standard error; UK: potassium urinary excretion.
Observational studies
| References (country) | Design | Baseline characteristics | Na intake assessment method | UNa (mmol/24 h) |
Outcomes assessed |
Confounders adjusted for | Results |
|---|---|---|---|---|---|---|---|
|
Shi et al. (2014) (Germany) DONALD |
Prospective cohort N = 1,107 DONALD initial cohort N = 435 included in the analyses (included: at least 3 BP measurements with three parallel 24‐h UNa and 3‐day weighed dietary records; excluded: preterm children, birth weight and breast‐feeding data missing; taking BP‐lowering treatment; implausible SBP/DBP values) Mean duration: 10 years |
Male (%) 51.0 Age (median (p25–p75), years) b 6 (4–8) g 6 (4–7) BMI (median (p25–p75), kg/m 2 ) b 15.7 (15.0–16.8) g 15.3 (14.7–16.4) SBP (median (p25–p75), mm Hg) b 97.1 (90.8–104.0) g 97.0 (90.0–102.0) DBP (median (p25–p75), mm Hg) b 57.0 (50.0–65.0) g 55.0 (49.6–64.1) 24‐h UK (median (p25–p75), mmol) b 39.1 (29.7–47.8) g 32.4 (26.2–38.9) 24‐h UK UCr (median (p25–p75), mmol/kg bw) b 0.16 (0.14–0.18) g 0.15 (0.13–0.17) Energy intake (median (p25–p75), MJ/day) b 6.1 (5.3–7.2) g 5.3 (4.8–6.3) |
Single 24‐h urine sample at yearly intervals |
Median (p25–p75) First assessment b 67.4 (50.6–89.9) g 58.7 (45.9–74.5) Last assessment b 131.0 (96.9–176.0) g 108.0 (81.7–133.0) |
Longitudinal change in SBP and DBP BP measured every 2 years (2 BP readings averaged at each visit) |
Age, age 2, age 3, sex, pubertal group, intraindividual change in Na excretion × pubertal group, person‐specific mean‐Na excretion × pubertal group, TEI, TEI × pubertal group, BMI‐SDS, height‐SDS, birth weight, full breast‐feeding status, maternal SBP, FVI, FVI × pubertal group |
Change in SBP (mm Hg)/1 mmol/MJ increase in Na excretion (β (95% CI)): Prepubertal group Between person effect −0.2 (−0.4, 0.04) p = 0.1 Within person effect −0.03 (−0.2, 0.09) p = 0.6 Pubertal group Between person effect 0.1 (−0.1, 0.4) p = 0.3 Within person effect 0.1 (−0.004, 0.2) p = 0.06 |
| Age, age 2, age 3, sex, pubertal group, intraindividual change in Na excretion × pubertal group, person‐specific mean‐Na excretion × pubertal group, TEI, TEI × pubertal group, BMI‐SDS, height‐SDS, growth velocity, full breast‐feeding status, maternal SBP, FVI, FVI × pubertal group and Ca intake |
Change in DBP (mm Hg)/1 mmol/MJ increase in Na excretion (β (95% CI)): Prepubertal group Between person effect −0.1 (−0.4,0.07) p = 0.2 Within person effect −0.1 (−0.2, 0.03) p = 0.1 Pubertal group Between person effect −0.2 (−0.4, 0.04) p = 0.1 Within person effect 0.1 (−0.02, 0.2) p = 0.09 |
||||||
|
Krupp et al. (2015) (Germany) DONALD |
Prospective cohort N = NR N = 206 included in the analyses (‘included: reached adult age, had a BP measurement and assessed in parallel anthropometrical data between 18 and 25 years, had ≥ 3 parallel plausible dietary records 24 h UNa and ≥ 3 BP measurements during adolescence (11–16 years); excluded: preterm, missing data on birth weight and gestational age, implausible BP data) Mean duration: 7 years |
Male (%) 52.0 Age (median (p25–p75), years) b 12.4 (12.1–13.0) g 12.3 (12.0–13.0) BMI (mean (SD), kg/m 2 ) b 18.9 (2.3) g 18.7 (2.7) SBP (mean (SD), mm Hg) b 104 (7) g 103 (8) DBP (mean (SD), mm Hg) b 62 (6) g 61 (6) 24‐h UK (mean (SD), mmol) b 56 (14) g 51 (13) 24‐h UK UCr (mean (SD), mmol/kg bw) b 0.18 (0.02) g 0.17 (0.02) Energy intake (mean (SD), MJ/day) b 9.1 (1.5) g 7.9 (0.9) |
Single 24‐h urine sample at yearly intervals Samples with UCr < 0.1 mmol/kg BW excluded from the analysis |
NaCl (mean (SD)) b 116 (27) g 105 (32) calculated as (Na (mmol/day) + Cl (mmol/day))/2 |
Longitudinal change in SBP and DBP BP measured every 2 years (2 blood pressure readings averaged at each examination) |
Mean pubertal SBP SDS, adult age, standardised energy intake, intake of saturated fat, height‐SDS, maternal education, maternal BP, FVI, adult BMI |
Change in SBP (mm Hg)/1 mmol increase in NaCl excretion (ß (95% CI)): b 0.1 (0.03, 0.18) p = 0.01 g ‐0.05 (−0.11, 0.02) p = 0.1 |
| Mean pubertal SBP SDS, adult age, standardised energy intake, calcium intake, birth weight, smoking in the household, maternal BP, FVI, adult BMI |
Change in DBP (mm Hg)/1 mmol increase in NaCl excretion (ß (95% CI)): b 0.02 (−0.08, 0.04) p = 0.6 g 0.02 (−0.03, 0.08) p = 0.4 |
b: boys; BMI: body mass index; BP: blood pressure; bw: body weight; 95% CI: 95% confidence interval; Cl: chloride; DBP: diastolic blood pressure; DONALD: Dortmund Nutritional and Anthropometrical Longitudinally Designed study FVI: fruit and vegetable intake; g: girls; MJ: megajoule; mm Hg: millimetre of mercury; mmol: millimole; N: number; Na: sodium; NR: not reported; SBP: systolic blood pressure; SD: standard deviation; SDS: standard deviation score; TEI: total energy intake; UCr: creatinine urinary excretion; UK: potassium urinary excretion; UNa: sodium urinary excretion.
H.2. Outcome of the RoB appraisal
Experimental studies
| References | Risk of bias domainsa | Tierb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomisation | Allocation concealment | Blinding | Attrition | Exposure | Outcome | Reporting | Other threats to internal validity | ||
| Miller et al. (1988) | + | NR | − | + | ++ | + | + | + | 1 |
| He et al. (2015) | ++ | ++ | + | ++ | ++ | − | ++ | + | 2 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (−−): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
Observational studies
| References | Risk of bias domainsa | Tierb | |||||
|---|---|---|---|---|---|---|---|
| Confounding | Attrition | Exposure | Outcome | Reporting | Statistics | ||
| Krupp et al. (2015) | ++ | − | + | ++ | ++ | ++ | 1 |
| Shi et al. (2014) | ++ | + | + | ++ | ++ | + | 1 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (−−): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
Appendix I – Outcome of the systematic review on blood pressure levels in adults
I.1. Evidence tables
I.1.1. Experimental studies
|
References (country) |
Design | Subject characteristics at baseline |
UNa (mmol/24 h) |
BP measurement SBP/DBP (mm Hg) |
|---|---|---|---|---|
|
Alli et al. (1992) (Italy) |
Study design: cluster‐randomised Run‐in type: normal diet Intervention type: counselling N participants randomised/completed: 77/56 Duration: 12 months |
% men G1: 35 G2: 50 Age (mean ± SD, years) G1: 44.3 ± 10.2 G2: 51.7 ± 11 BMI (mean ± SD, kg/m 2 ) G1: 25 ± 2.5 G2: 24.8 ± 2.8 Ethnicity: NR BP status: HT 24‐h UK (mean ± SD, mmol) G1: 61.2 ± 18.1 G2: 59.8 ± 16.5 Energy intake: NR |
Type: single 24 h Mean ± SD Beginning of intervention G1: 178.7 ± 60.5 G2: 168.8 ± 65 End of intervention G1: 186.5 ± 73 G2: 163.4 ± 60.7 |
Type: point office, supine Mean ± SD Beginning of intervention G1: 148.5 ± 13.5/96.2 ± 4.4 G2: 148.2 ± 10.6/97.9 ± 4.9 End of intervention G1: 144.4 ± 8.1/90.6 ± 8.2 G2: 146.6 ± 15.1/94.5 ± 5.3 |
|
ANHMRCDS (1986) (Australia) |
Study design: parallel Run‐in type: normal diet Intervention type: counselling N participants randomised/completed: 107/100 Duration: 12 weeks |
% men G1: 83 G2: 89 Age (mean ± SE, years) G1: 52.3 ± 0.8 G2: 52.3 ± 0.8 BP status: mixed Ethnicity: NR 24‐h UK (mean, mmol) G1: 66 G2: 71 Energy intake: NR |
Type: single 24 h Mean ± SE Beginning of intervention G1: 142 G2: 157 End of intervention G1: 85.8 ± 7.1 G2: 155.6 ± 8.3 |
Type: point office, sitting Mean Beginning of intervention G1: 150/95 G2: 149/95 End of intervention G1: 144/89 G2: 148/93 |
|
ANHMRCDS (1989) (Australia) Australian National Health h Medical Research Council Dietary Salt Study |
Study design: parallel Run‐in type: normal diet Intervention type: feeding N participants randomised/completed: 111/108 Duration: 8 weeks |
% men G1: 80 G2: 87 Age (years) G1: 18 + G2: 18 + Ethnicity: NR BP status: HT 24‐h UK (mean ± SE, mmol) G1: 73 ± 3 G2: 74 ± 3 Energy intake: NR |
Type: single 24 h Mean ± SE Beginning of intervention G1: 142 ± 6 G2: 134 ± 6 End of intervention G1: 90 ± 6 G2: 153 ± 6 |
Type: point office, sitting Mean ± SE Beginning of intervention G1: 155.2 ± 2.2/95.1 ± 0.6 G2: 152.8 ± 2.1/95.4 ± 0.6 End of intervention G1: 149.1 ± 1.9/91.4 ± 0.7 G2: 152.2 ± 1.9/94.6 ± 0.9 |
|
Benetos et al. (1992) (France) |
Study design: cross‐over Wash out: 1 week Intervention type: feeding N participants randomised/completed: 22/20 Duration: 4 weeks |
% men: 45 Age (mean ± SE, years): 41.5 ± 2.4 Ethnicity: NR BP status: HT 24‐h UK (mean ± SE, mmol): 70 ± 3 Energy intake: NR |
Type: single 24 h Mean ± SE Beginning of intervention 173 ± 13 End of intervention G1: 85 ± 9.6 G2: 163 ± 13.3 |
Type: point office, supine Mean ± SE Beginning of intervention 154 ± 2.2/96.1 ± 1.5 End of intervention G1: 142.6 ± 2.6/89.5 ± 1.5 G2: 149.1 ± 2.3/93.2 ± 1.3 |
|
Cappuccio et al. (1997) (United Kingdom) |
Study design: cross‐over Wash out: none Run‐in type: low sodium diet Intervention type: feeding N participants randomised/completed: 48/47 Duration: 4 weeks |
% men: 51 Age (mean ± SD, years): 66.8 ± 5.3 Ethnicity: mixed BP status: mixed 24‐h UK (mean ± SD, mmol): 66 ± 20 Energy intake: NR |
Type: multiple 24 h Mean ± SD Beginning of intervention 72 ± 40 End of intervention G1: 94 ± 50 G2: 177 ± 49 |
Type: point office, supine Mean ± SD Beginning of intervention 154.9 ± 20.4/87.1 ± 9.5 End of intervention G1: 155.9 ± 21.6/86.9 ± 8.8 G2: 163.2 ± 20.6/90.1 ± 10.5 |
|
Cobiac et al. (1992) (Australia) |
Study design: parallel Run‐in type: low sodium diet + sodium tablets Intervention type: feeding N participants randomised/completed: 57/54 Duration: 4 weeks |
% men G1: 69 G2: 64 Age (mean ± SE, years) G1: 67 ± 1 G2: 67 ± 1 BMI (mean ± SE, kg/m 2 ) G1: 25 ± 1 G2: 25 ± 1 Ethnicity: NR BP status: mixed 24‐h UK (mean ± SE, mmol) G1: 74 ± 4 G2: 68 ± 6 Energy intake: NR |
Type: single 24 h Mean ± SE Beginning of intervention G1: 166 ± 10 G2: 166 ± 9 End of intervention G1: 79 ± 7 G2: 152 ± 10 |
Type: point office, sitting Mean ± SE Beginning of intervention G1: 132 ± 2/77 ± 2 G2: 135 ± 3/78 ± 2 End of intervention G1: 127.8/77.1 G2: 132.5/77.3 |
|
Dickinson et al. (2014) (Australia) |
Study design: cross‐over Wash out: none Intervention type: feeding N participants randomised/completed: 50/25 Duration: 6 weeks |
% men: 32 Age (years): 18+ BMI (range, kg/m 2) : 27–40 Ethnicity: NR BP status: NT 24‐h UK (mean ± SD, mmol): 76 ± 23 Energy intake (mean ± SD, MJ/d): 9.1 ± 2.5 |
Type: multiple 24 h Mean ± SD Beginning of intervention 154 ± 58 End of intervention G1: 113 ± 45 G2: 155 ± 58 |
Type: point office, sitting Mean ± SD Beginning of intervention 120 ± 13/77 ± 7 End of intervention G1: 115 ± 10/73 ± 6 G2: 118 ± 16/74 ± 8 |
|
Erwteman et al. (1984) (Netherlands) |
Study design: Parallel Intervention type: counselling N participants randomised/completed: 107/94 Duration: 4 weeks |
% men G1: 61 G2: 62 Age (mean ± SD, years) G1: 45 ± 11 G2: 46.5 ± 9.5 Ethnicity: mixed BP status: HT Energy intake: NR |
Type: multiple 24 h mean ± SD Beginning of intervention NR End of intervention G1: 72 ± 31 G2: 130 ± 50 |
Type: point office, supine Mean ± SD Beginning of intervention G1: 157 ± 11.4/101.5 ± 5.4 G2: 156.2 ± 11.8/100.5 ± 3.4 End of intervention G1: 141 ± 15.4/92.9 ± 10.4 G2: 142.9 ± 16.4/94.4 ± 12.0 |
|
Fotherby and Potter (1993) (United Kingdom) |
Study design: cross‐over Wash out: none Run‐in type: low sodium diet Intervention type: feeding N participants randomised/completed: 18/17 Duration: 5 weeks |
% men: 22 Age (mean (range), years): 73 (66–79) Ethnicity: Caucasian BP status: HT 24‐h UK (mean ± SD, mmol): 66 ± 25 Energy intake: NR |
Type: multiple 24 h Mean ± SD Beginning of intervention 104 ± 59 End of intervention G1: 95 ± 36 G2: 174 ± 40 |
Type: point office, supine Mean ± Beginning of intervention 176 ± 17/96 ± 11 End of intervention G1: 171 ± 21/96 ± 8 G2: 179 ± 18/96 ± 11 |
|
Gates et al. (2004) (USA) |
Study design: cross‐over Wash out: none Intervention type: feeding N participants randomised/completed: 12/12 Duration: 4 weeks |
% men: 50 Age (mean ± SE, years): 63 ± 1 BMI (mean ± SE, kg/m 2): 25.1 ± 1 Ethnicity: Caucasian BP status: HT 24‐h UK (mean ± SE, mmol): 72 ± 8 Energy intake (mean ± SE, MJ/d): 9.4 ± 0.7 |
Type: single 24 h Mean ± SE Beginning of intervention 134.5 ± 13.4 End of intervention G1: 60.5 ± 6.8 G2: 150.1 ± 17.9 |
Type: point office, supine Mean ± SE Beginning of intervention 148.1 ± 2.3/84.0 ± 2.4 End of intervention G1: 137.1 ± 2.8/79.0 ± 2.1 G2: 144.2 ± 3.7/79.9 ± 2.0 |
|
Gijsbers et al. (2015) (Netherlands) |
Study design: cross‐over Wash out: none Run‐in type: low sodium diet Intervention type: feeding N participants randomised/completed: 37/36 Duration: 4 weeks |
% men: 67 Age (mean (range), years): 65.8 (40–80) BMI (mean, kg/m 2): 27.2 Ethnicity: Caucasian BP status: mixed 24‐h UK (mean, mmol): 81.8 Energy intake (mean, kcal/d): 2,774 |
Type: single 24 h mean ± SD Beginning of intervention 90.8 End of intervention G1: 105.1 ± 39.7 G2: 202.9 ± 54.8 |
Type: point office, supine Mean ± SD Beginning of intervention 133.4/75.7 End of intervention G1: 125.1 ± 15/72.3 ± 7.7 G2: 132.9 ± 17.6/75.7 ± 7.5 |
|
Grobbee et al. (1987) (Netherlands) |
Study design: cross‐over Wash out: none Run‐in type: normal diet Intervention type: feeding N participants randomised/completed: 42/40 Duration: 6 weeks |
% men: 85 Age (mean ± SD, years): 24 ± 3 Ethnicity: NR BP status: HT 24‐h UK (mean ± SD, mmol): 71 ± 22 Energy intake: NR |
Type: multiple 24 h mean ± SE Beginning of intervention 141 ± 7 End of intervention G1: 57 ± 4 G2: 129 ± 5 |
Type: point office, supine Mean ± SE Beginning of intervention 143 ± 2.2/78 ± 1.6 End of intervention G1: 135.9 ± 1.8/73.7 ± 1.5 G2: 136.8 ± 1.8/73.5 ± 1.8 |
|
He et al. (2009) (United Kingdom) |
Study design: cross‐over Wash out: none Run‐in type: low sodium diet Intervention type: feeding N participants randomised/completed: 185/169 Duration: 6 weeks |
% men: 67 Age (mean ± SD, years): 50 ± 11 BMI (mean ± SD, kg/m 2 ) : 29 ± 5 Ethnicity: mixed BP status: HT 24‐h UK (mean ± SD, mmol): 77 ± 26 Energy intake: NR |
Type: multiple 24 h Mean ± SD Beginning of intervention NR End of intervention G1: 110 ± 49 G2: 165 ± 58 |
Type: point office, sitting Mean ± SD Beginning of intervention NR End of intervention G1: 141 ± 12/88 ± 9 G2: 146 ± 13/91 ± 8 |
|
Jablonski et al. (2013) (USA) |
Study design: cross‐over Wash out: none Intervention type: feeding N participants randomised/completed: 11/11 Duration: 5 weeks |
% men: 73 Age (mean ± SE, years): 60 ± 2 BMI (mean ± SE, kg/m 2 ): 27.2 ± 1.3 Ethnicity: mixed BP status: mixed Energy intake: NR |
Type: multiple 24 h Mean ± SE Beginning of intervention 159 ± 13 End of intervention G1: 77 ± 9 G2: 144 ± 7 |
Type: point office, supine mean ± SE Beginning of intervention 139 ± 2/83 ± 2 End of intervention G1: 127 ± 3/77 ± 2 G2: 138 ± 5/81 ± 2 |
|
Kumanyika et al. (2005) (USA) TOHP II |
Study design: parallel Intervention type: counselling N participants randomised/completed: 1159/1159 Duration: 36 months |
% men G1: 65 G2: 68 Age (mean ± SD, years) G1: 44.2 ± 6.1 G2: 43.2 ± 6.1 BMI (range, kg/m 2 ) 24.4–37.4 Ethnicity: mixed BP status: NT 24‐h UK (mean ± SD, mmol) G1: 66.8 ± 28.1 G2: 65.3 ± 26.6 Energy intake: NR |
Type: multiple 24 h Mean ± SD Beginning of intervention G1: 186.1 ± 80.7 G2: 188.0 ± 80.9 End of intervention G1: −50.9 ± 86.3 G2: −10.5 ± 88.5 |
Type: point office, sitting Mean ± SD Beginning of intervention G1: 127.7 ± 6.6/86.1 ± 1.9 G2: 127.3 ± 6.4/85.8 ± 1.9 End of intervention G1: −0.7 ± 9.2/−3.0 ± 6.5 G2: 0.6 ± 8.5/−2.4 ± 7.0 |
|
Kumanyika et al. (1993) (USA) TOPH I |
Study design: parallel Intervention type: counselling N participants randomised/completed: 744/744 Duration: 18 months |
% men G1: 71 G2: 72 Age (mean ± SD, years) G1: 43.4 ± 6.6 G2: 42.6 ± 6.5 BMI (mean ± SD, kg/m 2 ) G1: 27.1 ± 3.8 G2: 27.1 ± 3.6 Ethnicity: mixed BP status: NT 24‐h UK (mean ± SD, mmol) G1: 61.8 ± 23.4 G2: 62.8 ± 23.8 Energy intake: NR |
Type: single 24 h Mean ± SD Beginning of intervention G1: 154.6 ± 59.9 G2: 156.4 ± 60.5 End of intervention G1: 99.4 ± 60.0 G2: 146.5 ± 79.2 |
Type: point office, sitting Mean ± SD Beginning of intervention G1: 124.8 ± 8.5/83.7 ± 2.7 G2: 125.1 ± 8.1/83.9 ± 2.8 End of intervention G1: −5.08 ± 7.94/−4.35 ± 5.65 G2: −3.02 ± 8.31/−3.18 ± 5.8 |
|
MacGregor et al. (1982) (United Kingdom) |
Study design: cross‐over Wash out: none Run‐in type: low sodium diet Intervention type: feeding N participants randomised/completed: 19/19 Duration: 4 weeks |
% men: 74 Age (mean (range), years): 49 (30–66) Ethnicity: mixed BP status: HT 24‐h UK (mean ± SE, mmol): 59 ± 5 Energy intake: NR |
Type: multiple 24 h Mean ± SE Beginning of intervention 83 ± 11 End of intervention G1: 86 ± 9 G2: 162 ± 9 |
Type: point office, supine Mean ± SE Beginning of intervention 142 ± 3/92 ± 1 End of intervention G1: 144 ± 4/92 ± 1.5 G2: 154 ± 4/97 ± 2.5 |
|
MacGregor et al. (1989) (United Kingdom) |
Study design: cross‐over Wash out: none Run‐in type: low sodium diet Intervention type: feeding N participants randomised/completed: 20/20 Duration: 4 weeks |
% men: 55 Age (mean (range), years): 57 (42–72) Ethnicity: mixed BP status: HT Energy intake: NR |
Type: multiple 24 h Mean ± SE Beginning of intervention NR End of intervention G1: 49 ± 8 G2: 108 ± 10 G3: 190 ± 11 |
Type: point office, supine Mean ± SE Beginning of intervention 164 ± 4 / 101 ± 2 End of intervention G1: 147 ± 4/91 ± 2 G2: 155 ± 3/95 ± 2 G3: 163 ± 4/100 ± 2 |
|
Meland et al. (1997) (Norway) |
Study design: cross‐over Wash out: none Run‐in type: normal diet Intervention type: feeding N participants randomised/completed: 16/16 Duration: 8 weeks |
% men: 81 Age (mean (range), years): 50 (20–69) Ethnicity: NR BP status: HT Energy intake: NR |
Type: single 24 h Mean (95% CI) Beginning of intervention 177 (149, 204) End of intervention G1: 125 (104, 146) G2: 191 (159, 223) |
Type: point office, sitting Mean ± (95% CI) Beginning of intervention 146 (140, 153)/95 (92, 98) End of intervention G1: 141 (135, 147)/92 (89, 94) G2: 145 (137, 153)/94 (90, 97) |
|
Melander et al. (2007) (Sweden) |
Study design: cross‐over Wash out: none Intervention type: feeding N participants randomised/completed: 46/39 Duration: 4 weeks |
% men: 51 Age (mean ± SD, years): 53 ± 11 BMI (mean ± SD, kg/m 2 ): 26.3 ± 3.1 Ethnicity: NR BP status: NT 24‐h UK (mean ± SD, mmol): 75 ± 22.9 Energy intake: NR |
Type: single 24 h Mean ± SD Beginning of intervention 165 ± 67.4 End of intervention G1: 50.7 ± 17.3 G2: 140 ± 39.5 |
Type: point office, supine Mean ± SD Beginning of intervention 136 ± 12.6/78.2 ± 7.8 End of intervention G1: 125 ± 12.4/73 ± 7.3 G2: 132 ± 14.7/75.2 ± 7.5 |
|
Nestel et al. (1993) (Australia) |
Study design: parallel Run‐in type: low sodium diet + sodium tablets Intervention type: feeding N participants randomised/completed: 70/66 Duration: 6 weeks |
% men G1: 50 G2: 50 Age (mean ± SD, years) G1: 65.5 ± 4 G2: 65.5 ± 4 BMI (mean ± SD, kg/m 2 ) G1: 24.5 ± 3 G2: 24.5 ± 3 Ethnicity: NR BP status: NT 24‐h UK (mean ± SD, mmol) G1: 87 ± 26 G2: 76.5 ± 20 Energy intake: NR |
Type: single 24 h Mean ± SD Beginning of intervention G1: 182.5 ± 48 G2: 162 ± 47 End of intervention G1: 91.5 ± 41 G2: 156 ± 40 |
Type: point office, sitting Mean ± SD Beginning of intervention G1: 126.5 ± 10/72.5 ± 7 G2: 127.5 ± 13/73.5 ± 9 End of intervention G1: 122.5 ± 9/72 ± 8 G2: 127.5 ± 14/74.5 ± 9 |
|
Parijs et al. (1973) (Belgium) |
Study design: cross‐over Wash out: none Run‐in type: normal diet Intervention type: feeding N participants randomised/completed: 22/17 Duration: 4 weeks |
% men: 46 Age (mean ± SD, years): 41.2 ± 8.21 Ethnicity: NR BP status: HT Energy intake: NR |
Type: single 24 h Mean ± SD Beginning of intervention NR End of intervention G1: 92.8 ± 41.8 G2: 191.1 ± 61.2 |
Type: point office, supine mean ± Beginning of intervention 179.1 ± 17.8/115.2 ± 10.9 End of intervention G1: 167.8 ± 24.3/115.5 ± 12.45 G2: 174.5 ± 20.02/112.3 ± 15.17 |
|
Puska et al. (1983) (Finland) |
Study design: parallel Intervention type: counselling N participants randomised/completed: 76/72 Duration: 6 weeks |
% men: NR Age (range, years): 30–50 Ethnicity: NR BP status: mixed 24‐h UK (mean ± SE, mmol) G1: 81 ± 4 G2: 73 ± 3 Energy intake (mean ± SE, kcal/d) G1: 2,665 ± 120 G2: 2,609 ± 103 |
Type: single 24 h Mean ± SE Beginning of intervention G1: 192 ± 11 G2: 165 ± 9 End of intervention G1: 77 ± 5 G2: 167 ± 8 |
Type: point office, sitting Mean ± SE Beginning of intervention G1: 138.9 ± 2.3/89.6 ± 1.7 G2: 137.8 ± 2/89.3 ± 1.5 End of intervention G1: 137.2 ± 2.7/86.5 ± 1.8 G2: 136 ± 2.1/86.9 ± 1.5 |
|
Richards et al. (1984) (New Zealand) |
Study design: cross‐over Wash out: none Intervention type: feeding N participants randomised/completed: 16/12 Duration: 5 weeks |
% men: 67 Age (range, years): 19–52 Ethnicity: NR BP status: HT Energy intake: NR |
Type: multiple 24 h Mean ± SE Beginning of intervention NR End of intervention G1: 94 ± 7.1 G2: 202.8 ± 14.1 |
Type: point office, supine mean ± SE Beginning of intervention NR End of intervention G1: 144.7 ± 4/90.6 ± 3.6 G2: 149.9 ± 4.2/92.4 ± 3.5 |
|
Ruppert et al. (1993) (Germany) |
Study design: cross‐over Wash out: none Intervention type: feeding N participants randomised/completed: 25/25 Duration: 4 weeks |
% men: 40 Age (mean ± SE, years): 47 ± 5 Ethnicity: NR BP status: NT Energy intake: NR |
Type: single 24 h Mean ± SE Beginning of intervention NR End of intervention G1: 82 ± 3.4 G2: 199.6 ± 5.3 |
Type: point office, sitting Mean ± SE Beginning of intervention NR End of intervention G1: 112 ± 2.1/73.3 ± 1.4 G2: 110.3 ± 2.6/72.3 ± 1.8 |
|
Sacks et al. (2001) (USA) DASH |
Study design: cross‐over Wash out: none Run‐in type: high sodium diet Intervention type: feeding N participants randomised/completed: 204/192 Duration: 4 weeks |
% men: 46 Age (mean ± SD, years): 49 ± 10 BMI (mean ± SD, kg/m 2 ): 30 ± 5 Ethnicity: mixed BP status: mixed (41% HT) Energy intake: NR |
Type: single 24 h Mean ± SD Beginning of intervention 152 ± 72 End of intervention G1: 64 ± 37 G2: 106 ± 44 G3: 141 ± 55 |
Type: point office, sitting mean ± Beginning of intervention 134.8 ± 9.5/85.7 ± 4.5 End of intervention G1: 126 ± 10/79.8 ± 6 G2: 130 ± 11.7/82 ± 6.4 G3: 132.7 ± 11.9/83.2 ± 6.9 |
|
Santos et al. (2010) (Portugal) |
Study design: cross‐over Wash out: 6 weeks Intervention type: feeding N participants randomised/completed: 17/17 Duration: 7 weeks |
% men: 47 Age (range, years): 24–53 Ethnicity: NR BP status: NT Energy intake (mean (range) kcal/d): 2,290 (1,461–3,646) |
Type: single 24 h Median (IQR) Beginning of intervention NR End of intervention G1: 115 (87, 162) G2: 138 (123, 170) |
Type: point office, supine Median (IQR) Beginning of intervention NR End of intervention G1: 118.3 (89, 124.3)/68 (51.7, 97.7) G2: 116.7 (92.3, 125)/68.3 (50.3, 81.3) |
|
Schorr et al. (1996) (Germany) |
Study design: cross‐over Wash out: 2 weeks Intervention type: feeding N participants randomised/completed: 21/16 Duration: 4 weeks |
% men: 44 Age (mean ± SD, years): 64.1 ± 3.6 BMI (mean ± SD, kg/m 2 ): 26.1 ± 3.6 Ethnicity: NR BP status: NT Energy intake: NR |
Type: multiple 24 h Mean ± SD Beginning of intervention 141.8 ± 33.6 End of intervention G1: 104.6 ± 21.7 G2: 175.2 ± 29.6 |
Type: point office, NR mean ± Beginning of intervention 132.5 ± 22.2/73.6 ± 12.5 End of intervention G1: 125.9 ± 17.3/69.5 ± 10.1 G2: 133.6 ± 23.7/71.2 ± 12.2 |
|
Swift et al. (2005) (United Kingdom) |
Study design: cross‐over Wash out: none Run‐in type: low sodium diet Intervention type: feeding N participants randomised/completed: 46/40 Duration: 4 weeks |
% men: 43 Age (mean ± SD, years): 50 ± 10 BMI (mean ± SD, kg/m 2 ): 28 ± 4 Ethnicity: African including African Americans BP status: HT 24‐h UK (mean ± SD, mmol): 63 ± 16 Energy intake: NR |
Type: NR Mean ± SD Beginning of intervention NR End of intervention G1: 89 ± 52 G2: 167 ± 73 |
Type: point office, supine Mean ± SD Beginning of intervention NR End of intervention G1: 151 ± 13/98 ± 8 G2: 159 ± 13/101 ± 8 |
|
van Berge‐Landry and James (2004) (USA) |
Study design: cross‐over Wash out: none Run‐in type: normal diet Intervention type: counselling N participants randomised/completed: 48/48 Duration: 4 weeks |
% men: 79 Age: middle‐aged adults Ethnicity: mixed BP status: HT Energy intake: NR |
Type: single 24 h Mean ± SD Beginning of intervention NR End of intervention G1: 24 ± 13 G2: 309 ± 88 |
Type: point office, NR Mean ± SD Beginning of intervention NR End of intervention G1: 128 ± 10/85 ± 7 G2: 144 ± 14/93 ± 8 |
|
Watt et al. (1983) (United Kingdom) |
Study design: cross‐over Wash out: none Run‐in type: low sodium diet Intervention type: feeding N participants randomised/completed: 20/18 Duration: 4 weeks |
% men: 33 Age (mean, range, years): 52 (31–64) Ethnicity: NR BP status: HT 24‐h UK (mean, mmol): 59.2 Energy intake: NR |
Type: single 24 h Mean Beginning of intervention NR End of intervention G1: 87.2 G2: 142.8 |
Type: point office, sitting Mean Beginning of intervention NR End of intervention G1: 136/82.3 G2: 136.5/82.6 |
|
Watt et al. (1985) (United Kingdom) High/high |
Study design: cross‐over Wash out: none Intervention type: feeding N participants randomised/completed: 75/66 Duration: 4 weeks |
% men: 37 Age (mean, years): 22.3 Ethnicity: NR BP status: mixed 24‐h UK (mean ± SD, mmol): 53.8 ± 18.9 Energy intake: NR |
Type: single 24 h Mean ± SD Beginning of intervention 129.7 ± 55.3 End of intervention G1: 56.3 G2: 130.6 |
Type: point office, sitting mean ± Beginning of intervention 113.1 ± 13.3/65.2 ± 11.2 End of intervention G1: 112.2/64.5 G2: 113.6/63.3 |
|
Watt et al. (1985) (United Kingdom) Low/low |
Study design: cross‐over Wash out: none Intervention type: feeding N participants randomised/completed: 75/66 Duration: 4 weeks |
% men: 45 Age (mean, years): 22.7 Ethnicity: NR BP status: mixed 24‐h UK (mean ± SD, mmol): 60 ± 18.3 Energy intake: NR |
Type: single 24 h Mean ± SD Beginning of intervention 151.1 ± 63.6 End of intervention G1: 68.4 G2: 128.4 |
Type: point office, sitting mean ± SD Beginning of intervention 108.8 ± 11.5/61.9 ± 9.5 End of intervention G1: 110.2/65 G2: 110.7/63.6 |
BMI: body mass index; BP: blood pressure; 95% CI: 95% confidence interval; DBP: diastolic blood pressure; G1: group 1 (‘low’ sodium); G2: group 2 (‘high’ sodium); G3: group 3 (‘highest’ sodium, for studies with three sodium intake levels); HT: hypertensive; IQR: interquartile range; mm Hg: millimetre of mercury; N: number; Na: sodium; NR: not reported; NT: normotensive; SBP: systolic blood pressure; SD: standard deviation; SE: standard error; UK: potassium urinary excretion; UNa: sodium urinary excretion.
I.1.2. Observational studies
| References (country) | Design | Baseline characteristics | Na intake assessment method | UNa (mmol/24 h) |
Outcomes assessed |
Confounders adjusted for | Results |
|---|---|---|---|---|---|---|---|
|
(Stolarz‐Skrzypek et al., 2011) (Belgium, Czech Republic, Italy, Poland, Russian Federation) FLEMENGHO/EPOGH |
Prospective cohort N = 3,360 FLEMENGHO initial cohort; N = 1,187 EPOGH initial cohort N = 1,499 included in the analyses (‘blood pressure cohort’) (excluded: participants with treated hypertension, history of CVD, missing/inaccurate 24‐h UNa at baseline) Median duration: 6.1 years |
Male (%) 47.6 Hypertensive (untreated) (%) 9.9 Diabetes mellitus (%) 1.9 Age (mean (SD), years) 38.3 (14.2) BMI (mean (SD), kg/m2) 24.6 (4.0) Smokers (%) 30.4 SBP (mean (SD), mm Hg) 120.9 (12.8) DBP (mean (SD), mm Hg) 74.6 (8.9) 24‐h UK (mean (SD), mmol) 66.3 (22.4) 24‐h UCr (mean (SD), mmol) 11.9 (3.7) |
Single 24‐h urine sample at baseline and at last follow‐up examination Inaccurate urine collections were defined as a volume < 300 mL/24‐h, a 24‐h UCr < 4 mmol or > 25 mmol in women and < 6 mmol or > 30 mmol in men |
Mean (SD) 172.7 (62.5) |
Longitudinal change in SBP and DBP BP measured at baseline and follow‐up examinations (5 blood pressure readings averaged at each examination) |
Study population, sex, baseline values of and changes in: age, BMI, and alcohol intake, 24‐h UK, use of female sex steroids, and use of non‐steroidal anti‐inflammatory drugs |
Change in SBP (mm Hg)/ 100 mmol increase in Na excretion (mean (95% CI)): FLEMENGHO: 2.373 (1.154 to 3.392), p < 0.001 EPOGH: 0.196 (−1.409 to 1.801), p = 0.81 All: 1.711 (0.786 to 2.637), p < 0.001 Change in DBP (mm Hg)/ 100 mmol increase in Na excretion (mean (95% CI)): FLEMENGHO: 0.576 (−0.246 to 1.398) p = 0.17 EPOGH: −0.052 (−1.317 to 1.212), p = 0.94 All: 0.379 (−0.313 to 1.070), p = 0.28 |
BMI: body mass index; BP: blood pressure; 95% CI: 95% confidence interval; CVD: cardiovascular disease; DBP: diastolic blood pressure; EPOGH: European Project on Genes in Hypertension; FLEMENGHO: Flemish Study on Genes and Health Outcomes; m: men; mm Hg: millimetre of mercury; N: number; Na: sodium; SBP: systolic blood pressure; SD: standard deviation; UCr: creatinine urinary excretion; UK: potassium urinary excretion; UNa: sodium urinary excretion; w: women.
I.2. Outcome of the RoB appraisal
I.2.1. Experimental studies
| References | Risk of bias domainsa | Tierb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomisation | Allocation concealment | Blinding | Attrition | Exposure | Outcome | Reporting | Other threats to internal validity | ||
| Alli et al. (1992) | + | − − | − − | − − | ++ | − − | ++ | − | 2 |
| ANHMRCDS (1989) | + | NR | + + | + | + | + + | + + | + | 1 |
| Benetos et al. (1992) | + | NR | + | + + | + | + | ++ | + | 1 |
| Cappuccio et al. (1997) | ++ | NR | + + | ++ | + | ++ | ++ | ++ | 1 |
| ANHMRCDS (1986) | + | NR | − | + + | + | + | + | + | 1 |
| Cobiac et al. (1992) | NR | NR | ++ | + | ++ | + | ++ | + | 2 |
| Dickinson et al. (2014) | ++ | NR | + | − | + | + | ++ | ++ | 1 |
| Erwteman et al. (1984) | + | NR | + | + | + | + + | + + | − | 1 |
| Fotherby and Potter (1993) | + | NR | + | + + | + | + | ++ | ++ | 1 |
| Gates et al. (2004) | + | + | + + | + + | + | ++ | + + | ++ | 1 |
| Gijsbers et al. (2015) | + + | + + | + + | + + | + | + + | + + | + + | 1 |
| Grobbee et al. (1987) | + | NR | + | ++ | + | ++ | − | ++ | 1 |
| He et al. (2009) | ++ | + | ++ | + | + | ++ | ++ | ++ | 1 |
| Jablonski et al. (2013) | ++ | + | + + | + | + | + + | + + | ++ | 1 |
| Kumanyika et al. (2005) | + | NR | + + | + + | + | + + | + + | ++ | 1 |
| Kumanyika et al. (1993) | ++ | ++ | + | ++ | + | ++ | ++ | + | 1 |
| MacGregor et al. (1982) | ++ | ++ | + | ++ | + | ++ | ++ | + | 1 |
| MacGregor et al. (1989) | + | NR | + | + + | + | + + | + + | + + | 1 |
| Meland et al. (1997) | + | NR | + | + + | + | + + | + + | + + | 1 |
| Melander et al. (2007) | + | NR | + | + + | + | + | + + | ++ | 1 |
| Nestel et al. (1993) | + | NR | + + | + | + | + + | + + | + + | 1 |
| Parijs et al. (1973) | NR | NR | + | + + | + + | + | + + | + | 2 |
| Puska et al. (1983) | + | NR | − | + | + | − | + + | + | 1 |
| Richards et al. (1984) | + | NR | + | + + | + + | + | + + | − | 1 |
| Ruppert et al. (1993) | − | − | − | + | + | + | + | + | 2 |
| Sacks et al. (2001) | + | NR | + | + | + | + | ++ | ++ | 1 |
| Santos et al. (2010) | + | + | + | + + | + | + + | + + | ++ | 1 |
| Schorr et al. (1996) | + | NR | − | + + | + | + | + + | + | 1 |
| Swift et al. (2005) | + | NR | + | + | + | + | ++ | + | 1 |
| van Berge‐Landry and James (2004) | + | NR | + + | + + | + | + | ++ | + + | 1 |
| Watt et al. (1983) | + | NR | − | + + | + | NR | + + | + | 2 |
| Watt et al. (1985) | + | NR | + | + + | + | + | + + | + + | 1 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (−−): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
I.2.2. Observational studies
| References | Risk of bias domainsa | Tierb | |||||
|---|---|---|---|---|---|---|---|
| Confounding(a) | Attrition | Exposure | Outcome | Reporting | Statistics | ||
| Stolarz‐Skrzypek et al. (2011) | − | − | − | ++ | ++ | ++ | 2 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (−−): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
I.3. Random‐effects meta‐analyses of trials of effects of sodium reduction on SBP and DBP
I.4. Dose–response modelling
Appendix J – Outcome of the systematic review on incidence of hypertension
J.1. Evidence tables
J.1.1. Experimental studies
| References (country) | Design | Subjects characteristics at baseline | Na intake assessment method | UNa (mmol/24 h) |
Outcomes assessed |
Results |
|---|---|---|---|---|---|---|
|
Whelton et al. (1997) (USA) TOPH I |
RCT (parallel) G1: sodium reduction counselling (goal < 80 mmol/24 h) (N = 326) G2: usual care (N = 417) Recruitment criteria: aged 35–54 years, with high‐normal DBP Duration: 18 months |
Sex (male,%) G1: 70.9 G2: 71.7 Age (years, mean ± SD) G1: 43.4 ± 6.6 G2: 42.6 ± 6.0 Ethnicity (White,%) G1: 78.0 G2: 76.5 bw (kg, mean ± SD) G1: 82.7 ± 14.3 G2: 82.8 ± 14.0 SBP (mm Hg, mean ± SD) G1: 124.8 ± 8.5 G2: 125.1 ± 8.1 DBP (mm Hg, mean ± SD) G1: 83.7 ± 2.7 G2: 83.9 ± 2.8 |
Average of two 24‐h urine collection at baseline single 24‐h urine collection at the 6‐, 12‐ and 18‐month follow‐up visits |
Mean ± SD Baseline G1: 154.6 ± 77.9 G2: 156.4 ± 60.5 Diff: –1.8 At 18 month G1: 99.4 ± 60.0 G2: 146.5 ± 79.2 Diff: –47.2 |
Incident hypertension Hypertension diagnosed when mean of 9 DBP measurements ≥ 90 mm Hg or antihypertensive drug therapy prescribed |
Incidence of hypertension during follow‐up G1: 8.6% G2: 11.3% RR = 0.76 (95% CI 0.49, 1.18) |
|
The Trials of Hypertension Collaborative Research Group (1997) (USA) TOPH II |
RCT (parallel) G1: sodium reduction counselling (goal < 80 mmol/24 h) (N = 594) G2: usual care (N = 596) Recruitment criteria: aged 30–54 years, with high‐normal DBP, overweight Duration: 36 to 48 months |
Sex (male,%) G1: 64.8 G2: 68.3 Age (years, mean ± SD) G1: 44.2 ± 6.1 G2: 43.2 ± 6.1 Ethnicity (White, %) G1: 81.1 G2: 79.5 bw (kg, mean ± SD) G1: 94.0 ± 14.3 G2: 93.6 ± 13.5 SBP (mm Hg, mean ± SD) G1: 127.7 ± 6.6 G2: 127.3 ± 6.4 DBP (mm Hg, mean ± SD) G1: 86.1 ± 1.9 G2: 85.8 ± 1.9 |
Average of two 24‐h urine collection at baseline single 24‐h urine collection at the 18‐, 36‐month follow‐up visits |
Mean ± SD Baseline G1: 186.1 ± 80.7 G2: 188.0 ± 80.9 Diff: –1.9 At 36 month (change from baseline) G1: −50.9 ± 86.3 G2: −10.5 ± 88.5 Diff: −40.4 |
Incident hypertension Hypertension diagnosed when mean of 9 DBP measurements ≥ 90 mm Hg or mean of 9 SBP measurements ≥ 140 mm Hg or antihypertensive drug therapy prescribed |
Incidence of hypertension At 18 month (cases, %) G1: 108 (18.6) G2: 124 (21.1) RR = 0.88 (p = 0.28) At 36 month (cases, %) G1: 198 (34.4) G2: 229 (39.2) RR = 0.88 (p = 0.09) At 48 month (cases, %) G1: 211 (38.1) G2: 248 (44.4) RR = 0.86 (p = 0.04) |
bw: body weight; 95% CI: 95% confidence interval; DBP: diastolic blood pressure; Diff: difference; G1: group 1 (‘low’ sodium); G2: group 2 (‘high’ sodium); mm Hg: millimetre of mercury; N: number; Na: sodium; RCT: randomised controlled trial; RR: relative risk; SBP: systolic blood pressure; SD: standard deviation; TOPH: Trials of Hypertension Prevention; UNa: sodium urinary excretion.
J.1.2. Observational studies
| References (country) | Design | Baseline characteristics | Na intake assessment method | UNa (mmol/24 h) | Outcomes assessed | Confounders adjusted for | Results |
|---|---|---|---|---|---|---|---|
|
Forman et al. (2012) (Netherlands) PREVEND |
Prospective cohort N = 8,592 initial cohort; IMDM and pregnant women excluded; oversampling of subjects with elevated albumin excretion (> 10 mg/L) N = 5,556 included (excluded: participants with hypertension, missing 24‐h UNa at baseline) Median duration: 6.4 years |
Male (%) Q1 32.5 Q2 43.5 Q3 48.1 Q4 58.4 Age (median (IQR), years) Q1 43 (36–52) Q2 43 (36–52) Q3 43 (36–51) Q4 44 (37–52) BMI (median (IQR), kg/m 2 ) Q1 23.7 (21.7–26.2) Q2 24.2 (22.2–26.7) Q3 24.9 (22.6–27.3) Q4 25.7 (23.5–28.4) Smoking status (never, %) Q1 31.5 Q2 30.5 Q3 31.5 Q4 29.4 SBP (median (IQR), mm Hg) Q1 116 (108–126) Q2 118 (110–127) Q3 119 (111–128) Q4 121 (112–129) DBP (median (IQR), mm Hg) Q1 69 (64–74) Q2 70 (65–75) Q3 70 (65–75) Q4 71 (66–76) UK (median (IQR), mmol/24 h) Q1 56 (43–72) Q2 73 (59–91) Q3 87 (71–109) Q4 114 (87–144) 24‐h UCr (median (IQR), g) Q1 1.1 (0.9–1.3) Q2 1.3 (1.1–1.5) Q3 1.4 (1.2–1.7) Q4 1.6 (1.3–1.9) 24‐h UAlbumin (median (IQR), mg) Q1 7.3 (5.3–11.7) Q2 7.9 (5.8–12.5) Q3 8.4 (6.1–13.6) Q4 8.7 (6.4–13.9) |
Two 24‐h urine specimens collected at baseline (1997–1998), two 24‐h urine specimens collected during follow‐up first examination (2001–2003) and two 24‐h urine specimens collected during follow‐up second examination (2003–2006) |
Median (IQR) Overall 137 (106–171) Q1 97 (79–110) (n = 1,389) Q2 142 (132–153) (n = 1,389) Q3 188 (176–203) (n = 1,389) Q4 271 (242–316) (n = 1,389) |
Incident hypertension BP measured at baseline and at follow‐up Hypertension was defined as a SBP > 140 mm Hg, a DBP > 90 mm Hg, or both or the use of antihypertensive medications |
Age, BMI, sex, alcohol intake, smoking status, family history of hypertension, estimated GFR, serum levels of glucose ad cholesterol, and 24‐h UK, 24‐h UCa and 24‐h UCr |
HR for incident hypertension By 1 g (43 mmol) increase in UNa HR (95% CI) = 1.05 (1.00–1.10) (878 cases) By quartile of UNa: Q1 (reference) (incidence 13.9%) Q4 HR (95% CI) = 1.21 (0.98–1.51) (incidence 19.7%) Effect modification by serum uric acid and UAlbumin excretion |
|
Stolarz‐Skrzypek et al. (2011) (Belgium, Czech Republic, Italy, Poland, Russian Federation) FLEMENGHO/EPOGH |
Prospective cohort N = 3,360 FLEMENGHO initial cohort; N = 1,187 initial EPOGH initial cohort N = 2,096 included in the analyses (‘hypertension cohort’) (excluded: participants with treated/untreated hypertension at baseline, history of CVD, missing/inaccurate 24‐h UNa at baseline) Median duration: 6.5 years |
Male (%) T1 45.8 T2 46.0 T3 45.9 Hypertensive (%) none Diabetes mellitus w T1 1.9 T2 1.6 T3 1.9 m T1 1.9 T2 2.4 T3 1.9 Age (mean (SD), years) w T1 38.9 (15.3) T2 38.7 (14.1) T3 36.7 (13.1) m T1 39.3 (16.0) T2 40.1 (15.4) T3 38.2 (13.4) BMI (mean (SD), kg/m 2 ) w T1 23.6 (4.2) T2 24.1 (4.1) T3 25.0 (4.6) m T1 24.6 (3.4) T2 24.9 (3.3) T3 25.2 (3.6) Smokers (%) w T1 32.3 T2 23.6 T3 23.9 m T1 37.7 T2 39.0 T3 32.8 Alcohol intake (5 g/day,%) w T1 8.8 T2 9.9 T3 12.9 m T1 35.2 T2 32.9 T3 39.8 Higher education (%) w T1 10.7 T2 14.3 T3 16.9 m T1 13.8 T2 12.5 T3 17.7 SBP (mean (SD), mm Hg) w T1 115.6 (11.2) T2 115.6 (10.7) T3 116.8 (10.0) m T1 121.8 (9.7) T2 121.8 (8.7) T3 122.0 (9.3) DBP (mean (SD), mm Hg) w T1 71.3 (8.0) T2 72.1 (7.6) T3 73.0 (8.0) m T1 74.8 (8.2) T2 74.0 (7.9) T3 75.5 (7.7) 24‐h UK (mean (SD), mmol) w T1 51.2 (17.2) T2 62.9 (21.1) T3 69.4 (23.4) m T1 62.5 (26.3) T2 73.5 (23.4) T3 84.1 (28.8) 24‐h UCr (mean (SD), mmol) w T1 8.6 (2.2) T2 9.6 (2.0) T3 10.8 (2.5) m T1 12.2 (3.1) T2 14.1 (3.2) T3 16.1 (3.4) |
Single 24‐h urine sample at baseline and at last follow‐up examination Inaccurate urine collections were defined as a volume < 300 mL/24‐h, a 24‐h UCr < 4 mmol or > 25 mmol in women and < 6 mmol or > 30 mmol in men |
Mean (SD) Overall 174.2 (74.1) Women T1 94.4 (21.5) (n = 375) T2 147.4 (14.3) (n = 385) T3 222.1 (47.2) (n = 373) Men T1 121.3 (27.9) (n = 318) T2 185.3 (16.1) (n = 328) T3 282.2 (56.4) (n = 317) |
Incident hypertension BP measured at baseline and follow‐up Hypertension was defined as SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg use of antihypertensive drugs. |
Study population, sex, age, BMI, SBP, 24‐h UK, drinking alcohol and educational attainment |
HR for incident hypertension By tertile of UNa (whole population as reference) T1 HR (95% CI) = 1.00 (0.87, 1.16) (incidence 27.0%, 187 cases) T2 HR (95% CI) = 1.02 (0.89, 1.16) (incidence 26.6%, 190 cases) T3 HR (95% CI) = 0.98 (0.86, 1.12) (incidence 25.4%, 175 cases) |
BMI: body mass index; BP: blood pressure; 95% CI: 95% confidence interval; CVD: cardiovascular disease; DBP: diastolic blood pressure; EPOGH: European Project on Genes in Hypertension; FLEMENGHO: Flemish Study on Genes and Health Outcomes; GFR: glomerular filtration rate; HR: hazard ratio; IMDM: insulin‐mediated diabetes mellitus; IQR: interquartile range; m: men; mm Hg: millimetre of mercury; N: number; Na: sodium; SBP: systolic blood pressure; PREVEND: Prevention of Renal and Vascular End‐stage Disease; SD: standard deviation; UAlbumin: albumin urinary excretion; UCa: calcium urinary excretion; UCr: creatinine urinary excretion; UK: potassium urinary excretion; UNa: sodium urinary excretion; w: women.
J.2. Outcome of the RoB appraisal
J.2.1. Experimental studies
| References | Risk of bias domainsa | Tierb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomisation | Allocation concealment | Blinding | Attrition | Exposure | Outcome | Reporting | Other threats to internal validity | ||
| Whelton et al. (1997) (TOPH I) | ++ | ++ | + | ++ | + | ++ | ++ | + | 1 |
| The Trials of Hypertension Collaborative Research Group (1997) (TOPH II) | ++ | ++ | + | ++ | + | ++ | ++ | + | 1 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (−−): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
J.2.2. Observational studies
| References | Risk of bias domainsa | Tierb | |||||
|---|---|---|---|---|---|---|---|
| Confounding | Attrition | Exposure | Outcome | Reporting | Statistics | ||
| Forman et al. (2012) | + | − | ++ | ++ | + | ++ | 1 |
| Stolarz‐Skrzypek et al. (2011) | − | − | − | ++ | ++ | + | 2 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (−−): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
J.3. Descriptive forest plot
Figure J.1.
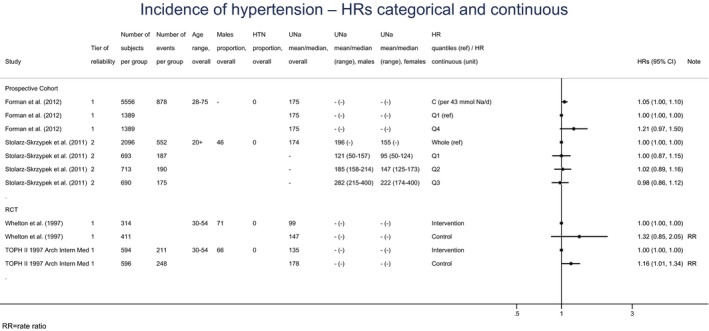
Descriptive forest plot of the eligible observational and experimental studies on the incidence of hypertension
- 95% CI: 95% confidence interval; HR: hazard ratio.
- A hazard ratio > 1 indicates an increased risk of outcome with higher sodium intake.
Appendix K – Outcome of the systematic review on risk of CVD
K.1. Evidence tables
| References (country) | Design | Baseline characteristics | Na intake assessment method | UNa (mmol/24 h) |
Outcomes assessed |
Confounders adjusted for | Results |
|---|---|---|---|---|---|---|---|
|
Cook et al. (2014) (USA) TOPH I/TOPH II |
Prospective cohort N = 2,974 TOPH I/TOPH II participants not in active Na intervention eligible for follow‐up N = 2,275 included in the analyses (excluded: CVD event during trial period, missing 24‐h UNa) Duration: 10 years (TOPH I)/15 years (TOPH II) |
Men (%) TOPH I 71, TOPH II 67 Hypertensive (treated/untreated): none Age (mean, years) TOPH I w Q1 44.6 Q2 44.7 Q3 43.0 Q4 42.7 m Q1 42.1 Q2 42.8 Q3 43.3 Q4 42.7 TOPH II w Q1 44.4 Q2 43.8 Q3 43.1 Q4 44.0 m Q1 42.7 Q2 43.6 Q3 43.6 Q4 42.5 bw (mean, lb) TOPH I w Q1 151.3 Q2 162.4 Q3 174.1 Q4 183.7 m Q1 175.8 Q2 185.4 Q3 198.1 Q4 211.4 TOPH II w Q1 172.7 Q2 181.4 Q3 192.0 Q4 203.1 m Q1 202.4 Q2 207.5 Q3 213.8 Q4 229.2 Ethnicity (%, black) w Q1 19.8 Q2 22.6 Q3 28.8 Q4 30.4 m Q1 14.8 Q2 6.5 Q3 8.8 Q4 7.7 TOPH II w Q1 34.5 Q2 30.4 Q3 28.1 Q4 18.2 m Q1 11.1 Q2 10.6 Q3 9.5 Q4 7.9 Smokers (%, current) TOPH I w Q1 14.8 Q2 10.6 Q3 11.0 Q4 21.7 m Q1 10.2 Q2 10.7 Q3 10.5 Q4 10.1 TOPH II w Q1 10.3 Q2 8.7 Q3 13.5 Q4 9.1 m Q1 22.2 Q2 7.0 Q3 8.3 Q4 8.9 Alcohol intake (≥ 1 drink/wk,%) TOPH I w Q1 41.6 Q2 28.8 Q3 23.3 Q4 39.1 m Q1 63.6 Q2 55.2 Q3 52.5 Q4 42.9 TOPH II w Q1 13.8 Q2 19.9 Q3 14.6 Q4 27.3 m Q1 55.6 Q2 42.2 Q3 44.0 Q4 40.1 College degree (%) TOPH I w Q1 46.5 Q2 46.2 Q3 42.5 Q4 39.1 m Q1 84.1 Q2 71.2 Q3 68.8 Q4 66.7 TOPH II w Q1 62.1 Q2 50.3 Q3 44.9 Q4 45.4 m Q1 100.0 Q2 72.5 Q3 67.1 Q4 62.9 SBP (mm Hg, mean) TOPHI W Q1 125.2 Q2 125.7 Q3 125.3 Q4 123.9 M Q1 124.3 Q2 124.2 Q3 125.2 Q4 125.3 TOPH II W Q1 128.2 Q2 128.4 Q3 128.2 Q4 130.0 M Q1 127.7 Q2 127.5 Q3 126.7 Q4 127.6 DBP (mm Hg, mean) TOPH I W Q1 83.7 Q2 83.7 Q3 84.5 Q4 83.6 M Q1 84.0 Q2 83.7 Q3 84.0 Q4 83.7 TOPH II W Q1 85.7 Q2 85.7 Q3 85.8 Q4 86.2 M Q1 85.8 Q2 86.1 Q3 85.9 Q4 86.2 24‐h UK (mean, mmol) TOPH I w Q1 42 Q2 49 Q3 58 Q4 67 m Q1 51 Q2 60 Q3 68 Q4 78 TOPH II w Q1 39 Q2 50 Q3 56 Q4 68 m Q1 56 Q2 60 Q3 69 Q4 75 24‐h UCr (mean, g) TOPH I W Q1 0.9 Q2 1.1 Q3 1.3 Q4 1.5 M Q1 1.3 Q2 1.5 Q3 1.7 Q4 2.0 TOPH II W Q1 1.0 Q2 1.2 Q3 1.4 Q4 1.5 M Q1 1.5 Q2 1.7 Q3 1.9 Q4 2.2 |
3 to 7 24‐h collection scheduled over 18 months in TOPH I and over 3 to 4 years in TOPH II To adjust for potential inaccuracy in collection, controlled for UCr/bw in multivariate model or excluded subjects with CVs of UCr/bw ≥ 20% or 30% (sensitivity analyses) |
Men and women Q1 < 100 Q2 100–156 Q3 157–208 Q4 ≥ 209 |
CVD death or event, including MI, stroke, coronary artery bypass graft, percutaneous transluminal coronary angioplasty. Standardised questionnaires sent at 2‐year intervals; medical records reviewed by study physician (non‐fatal events); search for National Death Index (fatal events) |
Age, sex, race/ethnicity, clinic and treatment assignment, education status, baseline weight, alcohol se, smoking, exercise, UK, family history of CVD, change in bw, change in smoking and change in exercise during trial periods |
HR (95% CI) for fatal and non‐fatal CVD events By continuous UNa (by 1 g (43‐mmol)/day increase): 1.17 (1.00–1.36) (193 cases, n = 2,275) By quantiles of UNa: Q1 0.68 (0.34–1.37) (TOPH I 15 events/189 total, TOPH II 2 events/47 total) Q2 0.75 (0.50–1.11) (TOPH I 48 events/590 total, TOPH II 13 events/303 total) Q3 1.00 (reference) (TOPH I 40 events/427 total, TOPH II 34 events/341 total) Q4 1.05 (0.68–1.62) (TOPH I 23 events/191 total, TOPH II 18 events/224 total) No significant deviation from linearity (restricted cubic spline). Sensitivity analyses: exclusion of subjects based on the CV for UCr/bw: little change in estimated coefficients |
|
Stolarz‐Skrzypek et al. (2011) (Belgium, Czech Republic, Italy, Poland, Russian Federation) FLEMENGHO/EPOGH |
Prospective cohort N = 3,360 FLEMENGHO initial cohort; N = 1,187 initial EPOGH initial cohort N = 3,681 included in the analyses (‘outcome cohort’) (excluded: participants with history of CVD, missing/inaccurate 24‐h UNa at baseline) Median duration: 7.9 years |
Men (%) T1 47.1 T2 47.4 T3 47.3 Hypertensive (treated) (%) w T1 14.9 T2 12.5 T3 16.6 m T1 11.1 T2 7.4 T3 8.9 Diabetes mellitus w T1 3.3 T2 2.9 T3 4.9 m T1 5.0 T2 4.4 T3 4.5 Age (mean (SD), years) w T1 42.5 (17.6) T2 41.0 (16.0) T3 39.2 (14.7) m T1 41.8 (18.1) T2 41.3 (16.4) T3 39.5 (14.4) BMI (mean (SD), kg/m 2 ) w T1 24.6 (5.1) T2 24.9 (4.6) T3 25.9 (5.3) m T1 24.7 (3.8) T2 25.2 (3.8) T3 26.1 (4.3) Smokers (%) w T1 25.9 T2 22.2 T3 22.1 m T1 35.1 T2 37.3 T3 29.1 Alcohol intake (5 g/day,%) w T1 10.7 T2 10.3 T3 14.0 m T1 34.3 T2 35.6 T3 44.0 Higher education (%) w T1 11.6 T2 15.1 T3 19.3 m T1 15.3 T2 13.0 T3 19.7 SBP (mean (SD), mm Hg) w T1 123.3 (19.4) T2 121.0 (16.8) T3 121.7 (15.8) m T1 128.7 (17.9) T2 126.6 (14.9) T3 128.1 (15.3) DBP (mean (SD), mm Hg) w T1 74.7 (11.0) T2 74.6 (9.7) T3 75.4 (10.2) m T1 77.8 (11.0) T2 76.6 (10.4) T3 79.0 (10.9) 24‐h UK (mean (SD), mmol) w T1 51.6 (17.8) T2 61.0 (20.5) T3 69.1 (24.0) m T1 61.6 (26.3) T2 71.6 (27.1) T3 84.5 (29.2) 24‐h UCr (mean (SD), mmol) w T1 8.4 (2.2) T2 9.5 (2.0) T3 10.6 (2.5) m T1 12.1 (3.2) T2 13.9 (3.4) T3 16.1 (3.8) |
Single 24‐h urine sample at baseline and at last follow‐up examination Inaccurate urine collections were defined as a volume < 300 mL/24‐h, a 24‐h UCr < 4 mmol or > 25 mmol in women and < 6 mmol or > 30 mmol in men |
Mean (SD) Women T1 95.1 (22.0) (n = 645) T2 150.2 (15.0) (n = 658) T3 231.7 (50.9) (n = 638) Men T1 120.1 (28.4) (n = 575) T2 188.8 (17.6) (n = 592) T3 290.5 (56.2) (n = 573) |
CHD events included fatal and non‐fatal MI and coronary revascularisation. Fatal and non‐fatal CVD events comprised CHD events, stroke, fatal and non‐fatal left ventricular HF, aortic aneurysm, cor pulmonale and pulmonary or arterial embolism. Hospitalisations for unstable angina coded as IHD. Standardised questionnaire at follow‐up visits. Physicians ascertained the diseases reported on the death certificates or by the questionnaires against medical records |
Study population, sex, and baseline variables: age, BMI, 24‐h UK, antihypertensive drug treatment, smoking and drinking alcohol, diabetes, total cholesterol and educational attainment |
HRs expressed the risk in each tertile of UNa compared with the overall risk in whole group HR (95% CI) for fatal CVD events T1 1.41 (0.94, 2.12) (50 events, n = 1,220) T2 0.98 (0.69, 1.40) (24 events, n = 1,250) T3 1.02 (0.71, 1.45) (10 events, n = 1,211) |
|
HR (95% CI) for non‐fatal CVD events T1 1.12 (0.90, 1.41) (100 events, n = 1,220) T2 1.09 (0.89, 1.34) (79 events, n = 1,250) T3 0.92 (0.74, 1.13) (53 events, n = 1,211) | |||||||
|
HR (95% CI) for non‐fatal CHD events T1 1.41 (0.99, 2.01) (45 events, n = 1,220) T2 1.15 (0.87, 1.52) (34 events, n = 1,250) T3 0.87 (0.66, 1.15) (19 events, n = 1,211) | |||||||
|
HR (95% CI) for non‐fatal stroke events T1 1.05 (0.56, 1.96) (13 events, n = 1,220) T2 1.28 (0.75, 2.17) (13 events, n = 1,250) T3 0.78 (0.46, 1.33) (7 events, n = 1,211) | |||||||
|
Joosten et al. (2014) (Netherlands) PREVEND |
Prospective cohort N = 8,592 initial cohort; IMDM and pregnant women excluded; oversampling of subjects with elevated albumin excretion (> 10 mg/L) N = 7,543 included (excluded: participants with CVD, renal disease requiring dialysis, malignancies, missing values of covariates at baseline) Median duration: 10.5 years (IQR 9.9–10.8) |
Men (%) Q1 48.7 Q2 48.7 Q3 48.7 Q4 48.7 Age (mean ± SD, years) Q1 50 ± 13 Q2 49 ± 13 Q3 48 ± 12 Q4 47 ± 11 BMI (mean ± SD, kg/m 2 ) Q1 25.0 ± 3.7 Q2 25.5 ± 3.7 Q3 26.1 ± 4.1 Q4 27.5 ± 4.8 Smoking status (never,%) Q1 30.1 Q2 32.1 Q3 28.6 Q4 29.9 SBP (mean ± SD, mm Hg) Q1 129 ± 22 Q2 128 ± 20 Q3 128 ± 20 Q4 129 ± 20 DBP (mean ± SD, mm Hg) Q1 74 ± 10 Q2 74 ± 10 Q3 74 ± 10 Q4 74 ± 9 Antihypertensive drugs (%) Q1 14.3 Q2 12.6 Q3 12.4 Q4 12.7 24‐h UK (median (IQR), mmol) Q1 59 (47–73) Q2 67 (56–81) Q3 73 (61–86) Q4 80 (66–95) 24‐h UCr (median (IQR), mmol) Q1 10.3 (8.4–12.7) Q2 11.5 (9.5–13.8) Q3 12.3 (10.1–15.1) Q4 13.6 (11.1–16.8) 24‐h UAlbumin (median (IQR), mmol) Q1 8.1 (5.5–14.7) Q2 9.1 (6.3–16.4) Q3 9.4 (6.4–17.1) Q4 10.3 (7.0–19.3) |
Two 24‐h urine specimens collected at baseline (1997–1998) Sensitivity analyses excluding 24‐h urine samples with possible over‐ or undercollections (i.e. samples upper and lower 2.5% of the difference between the estimated and measured volume of a subject's 24‐h urine sample.) |
Men Q1 < 122 Q2 122–154 Q3 155–190 Q4 > 190 Women Q1 < 95 Q2 95–121 Q3 122–151 Q4 > 151 |
CHD defined as MI, acute and subacute IHD and coronary artery bypass grafting or percutaneous transluminal coronary angioplasty. Data from Dutch bureau of statistics (fatal events) and Dutch national registry of hospital discharge diagnoses (non‐fatal events) |
Age, sex, body mass index, smoking status, alcohol intake, parental history of coronary heart disease, type 2 diabetes mellitus, total to HDL cholesterol ratio, and 24‐h UK, magnesium and creatinine excretion |
HR (95% CI) for fatal and non‐fatal CHD By continuous UNa (by 1 g (43‐mmol)/day increase): 1.07 (0.98–1.18) p = 0.15 (452 cases; 71,491 person‐years) By sex‐specific quintiles of UNa: Q1 (reference) (123 cases, 17,738 person‐years) Q2 0.99 (0.76–1.29) (111 cases, 17,975 person‐years) Q3 1.09 (0.83–1.44) (112 cases, 17,878 person‐years) Q4 1.19 (0.88–1.62) (106 cases, 18,000 person‐years) No significant deviation from linearity (restricted cubic spline). Evidence for effect modification by mean arterial pressure (Pinteraction = 0.08) and plasma NT‐proBNP concentration (Pinteraction = 0.002). Sensitivity analyses: exclusion of subjects with potential under‐ or overcollections in 24‐h urine samples: results unchanged restricted to subjects with UAlbumin > 10 mg/L: results unchanged |
|
Kieneker et al. (2018) (Netherlands) PREVEND |
Prospective cohort N = 8,592 initial cohort N = 7,330 included (excluded: participants with CVD, renal disease requiring dialysis, missing values of covariates at baseline) Oversampling of subjects with elevated albumin excretion (> 10 mg/L) Median duration: 12.5 years (IQR 11.9–12.9) |
Men (%) Q1 48.6 Q2 48.6 Q3 48.6 Q4 48.6 Q5 48.6 Age (mean ± SD, years) Q1 50.5 ± 13.0 Q2 50.1 ± 12.9 Q3 49.2 ± 12.5 Q4 48.2 ± 12.0 Q5 47.0 ± 11.1 BMI (mean ± SD, kg/m 2 ) Q1 25.0 ± 3.8 Q2 25.3 ± 3.6 Q3 25.7 ± 4.0 Q4 26.3 ± 4.0 Q5 27.8 ± 4.9 Ethnicity (% white) Q1 93.9 Q2 95.2 Q3 96.5 Q4 96.1 Q5 96.0 Smoking status (current, %) Q1 39.0 Q2 33.1 Q3 33.9 Q4 31.6 Q5 32.3 Education (higher vocational education or university, %) Q1 31.1 Q2 32.9 Q3 32.2 Q4 31.4 Q5 26.5 SBP (mean ± SD, mm Hg) Q1 129 ± 21 Q2 129 ± 20 Q3 128 ± 20 Q4 128 ± 20 Q5 129 ± 18 DBP (mean ± SD, mm Hg) Q1 74 ± 10 Q2 74 ± 10 Q3 74 ± 10 Q4 73 ± 10 Q5 74 ± 9 Antihypertensive drugs (%) Q1 14.7% Q2 12.0% Q3 12.2% Q4 12.3% Q5 13.0% 24‐h UK (median (IQR), mmol) Q1 57 (46–72) Q2 66 (55–79) Q3 70 (58–83) Q4 74 (62–88) Q5 81 (68–96) 24‐h UCr (median (IQR), mmol) Q1 10.0 (8.3–12.6) Q2 11.2 (9.3–13.6) Q3 11.8 (9.8–14.3) Q4 12.5 (10.3–15.6) Q5 13.8 (11.3–16.8) 24‐h UAlbumin (median (IQR), mmol) Q1 8.0 (5.5–14.4) Q2 8.9 (6.1–16.0) Q3 9.1 (6.3–16.2) Q4 9.5 (6.5–18.1) Q5 10.5 (7.0–19.9) |
Two 24‐h urine specimens collected at baseline (1997–1998) and two 24‐h urine specimens collected during follow‐up (2001–2003) |
Men Q1 < 116 (712) Q2 116–142 (713) Q3 143–167 (713) Q4 168–201 (713) Q5 > 201 (713) Women Q1 < 89 (753) Q2 89–110 (754) Q3 111–132 (753) Q4 133–160 (753) Q5 > 160 (753) |
Haemorrhagic, ischaemic, unspecified stroke, fatal and non‐fatal events Data from municipal registers (fatal events) and Dutch national registry of hospital discharge diagnoses (non‐fatal events) |
Age, sex, height, weight, race/ethnicity, smoking status, alcohol consumption, education, type 2 diabetes, and total to HDL cholesterol ratio, 24‐h UK, magnesium, creatinine and albumin and estimated GFR |
HR (95% CI) for fatal and non‐fatal stroke By continuous UNa (by 51 mmol/24 h decrease): 1.44 (1.14–1.82) (183 cases; 83,189 person‐years) By sex‐specific quintiles of UNa: Q1 1.45 (0.92–2.29) (57 cases, 16,272 person‐years) Q2 1.13 (0.71–1.79) (49 cases, 16,515 person‐years) Q3 (reference) (33 cases, 16,774 person‐years) Q4 1.04 (0.64–1.71) (25 cases, 16,720 person‐years) Q5 0.81 (0.46–1.41) (19 cases, 16,908 person‐years) Adjustment for potential mediators (SBP and antihypertensive medication, plasma renin, aldosterone, and sodium levels) did not change the result. No evidence for effect modification by age, sex, BMI, hypertension, and UK (all Pinteraction > 0.10). Sensitivity analyses: excluding individuals who were taking antihypertensive drugs at baseline (n = 6,388, 126 cases): results unchanged. excluding individuals with malignancies, type 2 diabetes, or chronic kidney disease at baseline (n = 6,054, 112 cases): results unchanged. |
|
Lelli et al. (2018) (Italy) InCHIANTI |
Prospective cohort N = 1,170 initial cohort N = 514 included (excluded: younger than 65 years, participants with CVD at baseline) Median duration: 9 years |
NR for the subcohort |
Single 24‐h urine specimen collected at baseline (1998–2000) |
NR for the subcohort |
Non‐fatal CVD event, including angina pectoris, MI, heart failure, stroke Standardised questionnaire at follow‐up visits; clinical documentation reviewed |
Age, sex, education, CKD‐EPI, SBP, pack/years, hypertension, diabetes, BMI, caloric intake/bw, and antihypertensive drugs and diuretics) |
RR (95% CI) for non‐fatal CVD events By continuous UNa (By NR mmol/day increase): 0.96 (0.90–1.02) (169 cases, n = 514) |
|
Tuomilehto et al. (2001) (Finland) |
Prospective cohort N = 3,607 initial cohort Non‐fatal CHD analysis N = 2,402 (prevalent cases of CHD also excluded) Non‐fatal stroke analysis N = 2,420 (prevalent cases of stroke also excluded) Duration: up to 14 years |
Age (mean ± SD, years) w Q1 45.7 ± 11.6 Q2 45.4 ± 11.8 Q3 44.8 ± 11.1 Q4 45.6 ± 11.3 m Q1 45.4 ± 11.6 Q2 45.3 ± 11.0 Q3 46.2 ± 10.4 Q4 45.4 ± 10.6 BMI (mean ± SD, kg/m 2 ) w Q1 24.6 ± 4.2 Q2 25.1 ± 4.0 Q3 26.3 ± 4.6 Q4 27.8 ± 5.4 m Q1 25.5 ± 2.4 Q2 26.4 ± 3.3 Q3 26.9 ± 3.3 Q4 28.1 ± 4.2 Smoking (current,%) w Q1 15% Q2 20% Q3 18% Q4 16% m Q1 31% Q2 40% Q3 33% Q4 44% SBP (mean ± SD, mm Hg) w Q1 141 ± 22 Q2 140 ± 22 Q3 141 ± 22 Q4 142 ± 22 m Q1 144 ± 22 Q2 145 ± 19 Q3 148 ± 20 Q4 147 ± 19 DBP (mean ± SD, mm Hg) w Q1 83 ± 12 Q2 83 ± 12 Q3 83 ± 12 Q4 85 ± 22 m Q1 86 ± 11 Q2 86 ± 12 Q3 89 ± 13 Q4 90 ± 13 |
Single 24‐h urine specimens collected at baseline Participants reporting incomplete collection excluded from the analysis |
Median (min–max) Male 205 (25–552) Female 154 (12–512) |
Coronary deaths, non‐fatal coronary events, stroke events, cardiovascular deaths Statistics Finland (fatal events) and national hospital discharge register (non‐fatal events) |
Age, study year, smoking, serum total and HDL cholesterol, SBP and BMI |
HR (95% CI) for non‐fatal CHD events By 100 mmol/24‐h increase in UNa Men 1.34 (1.06–1.70) (98 cases, n = 1145) Women 1.35 (0.77–2.35) (30 cases, n = 1257) Men + Women 1.34 (1.08–1.67) (128 cases, n = 2,402) |
|
HR for non‐fatal stroke events By 100 mmol/24‐h increase in UNa Men 1.00 (0.68–1.47) (43 cases, n = 1161) Women 1.34 (0.87–2.07) (41 cases, n = 1259) Men + Women 1.13 (0.84–1.51) (84 cases, n = 2,420) |
BMI: body mass index; BP: blood pressure; bw: body weight; 95% CI: 95% confidence interval; CKD‐EPI: estimated creatinine clearance; CHD: coronary heart disease; CVD: cardiovascular disease; DBP: diastolic blood pressure; EPOGH: European Project on Genes in Hypertension; FLEMENGHO: Flemish Study on Genes and Health Outcomes; GFR: glomerular filtration rate; HDL: high density lipoprotein; HF: heart failure; HR: hazard ratio; IHD: ischaemic heart disease; IMDM: insulin‐mediated diabetes mellitus; IQR: interquartile range; m: men; mm Hg: millimetre of mercury; MI: myocardial infarction; N: number; Na: sodium; NR: not reported; NT‐proBNP: N‐terminal pro‐B‐type natriuretic peptide; RR: relative risk; SBP: systolic blood pressure; SD: standard deviation; TOPH: Trials of Hypertension Prevention; UAlbumin: urinary albumin; UCr: creatinine urinary excretion; UK: potassium urinary excretion; UNa: sodium urinary excretion; w: women.
K.2. Outcome of the RoB appraisal
K.2.1. Observational studies
| References | Outcomes | Risk of bias domainsa | Tierb | |||||
|---|---|---|---|---|---|---|---|---|
| Confounding | Attrition | Exposure | Outcome | Reporting | Statistics | |||
| Lelli et al. (2018) | Non‐fatal CVD (any) | − | ++ | + | − | ++ | − | 2 |
| Joosten et al. (2014) | Fatal and non‐fatal CHD | + | NR | ++ | ++ | ++ | ++ | 1 |
| Kieneker et al. (2018) | Fatal and non‐fatal stroke | + | NR | + | + | ++ | + | 1 |
| Stolarz‐Skrzypek et al. (2011) |
Fatal CVD Fatal and non‐fatal CVD Fatal and non‐fatal CHD Fatal and non‐fatal stroke |
+ | − | − | + | ++ | − | 2 |
| Cook et al. (2014) | Fatal and non‐fatal CVD (any) | ++ | + | ++ | ++ | ++ | ++ | 1 |
| Tuomilehto et al. (2001) |
Non‐fatal stroke Non‐fatal CHD |
− | ++ | + | + | ++ | − | 2 |
CHD: coronary heart disease; CVD: cardiovascular disease
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (−−): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
K.3. Descriptive forest plots
Figure K.1.
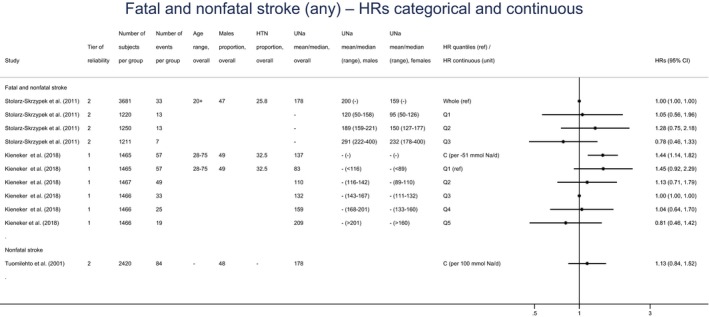
Descriptive forest plot of the eligible observational studies on stroke
- 95% CI: 95% confidence interval; HR: hazard ratio; HTN: hypertensive; UNa: sodium urinary excretion.
- A hazard ratio > 1 indicates an increased risk of outcome with higher sodium intake.
Figure K.2.
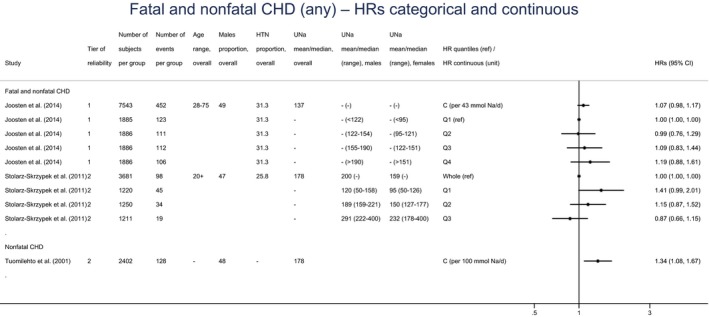
Descriptive forest plot of the eligible observational studies on coronary heart disease
- CHD: coronary heart disease; 95% CI: 95% confidence interval; HR: hazard ratio; HTN: hypertensive; UNa: sodium urinary excretion.
- A hazard ratio > 1 indicates an increased risk of outcome with higher sodium intake.
Appendix L – Outcome of the systematic review on bone health
L.1. Evidence table
| References (country) | Design | Baseline characteristics mean ± SD (N) | Na intake assessment method | UNa (mg/day) Mean ± SD (N) |
Outcomes assessed |
Confounders adjusted for | Key findings |
|---|---|---|---|---|---|---|---|
|
Devine et al. (1995); Prince et al. (1995) (Australia) |
RCT in postmenopausal women (≥ 10 years after menopause) Group 1: Ca suppl. (1 g/day) Group 2: Ca suppl. (1 g/day) + exercise programme Group 3: Placebo Group 4: ~200 mL milk/day (1 g Ca/day) N = 196 screened N = 168 randomised (42/group) Duration: 2 years |
Age: 63 (168) bw: 66 ± 10 (191) Nutrient intakes based on 4‐day weighed diet records (190): Protein: 76 ± 16 g/day Ca (food only): 805 ± 320 mg/day P: 1,269 ± 311 mg/day Energy: 1,632 ± 348 kcal/day |
Single 24‐h urine samples collected at baseline, year 1 and year 2; excretions averaged over the 2‐year period. No information on instructions to participants or on measures to check completeness of collections. |
Baseline UNa: 2,783 ± 1,081 (196) Average year 1 and 2 UNa: 3,049 ± 808 (127) |
BMD (total hip, intertrochanter, femoral neck, ultradistal ankle, lumbar spine) By DEXA at baseline, 1 year and 2 years |
Body weight, change in METs/day over the 2‐year period, average daily Ca intake |
Multiple regression analysis: Negative associations between average UNa and 2‐year change in BMD for total hip (SRC = −0.20, p = 0.002) and ultradistal ankle (SRC = −0.18, p = 0.016) |
|
Ilich et al. (2010) (USA) |
RCT in postmenopausal women (≥ 5 years after menopause); all supplemented with Ca (630 mg/day) and vitamin D (~ 400 IU/day) Group 1: instructed to reduce Na intake to 1,500 mg/day Group 2: usual Na intake (~ 3,000 mg/day) N = 136 randomised (68/group) N = 97 completed Duration: 3 years |
Age: 68.6 ± 7.1 (136) bw: 68.0 ± 11.3 (136) BMI: 26.0 ± 3.8 (136) Nutrient intakes based on 4‐day weighed diet records (136): Protein: 70.6 ± 18.6 g/day Ca (food only): 872 ± 365 mg/day P: 1,077 ± 351 mg/day Energy: 1,691 ± 382 kcal/day |
Single 24‐h urine samples collected every 6 months. Cumulative Na/Cr used as variable. Careful instructions to participants and use of Cr to screen for errors or incomplete collection |
Baseline UNa: 2,404 ± 963 (136) Baseline UNa/g Cr: 2,465 ± 903 (136) |
BMD (composite femur, forearm, lumbar spine, total body) By DEXA at baseline and every 6 months |
Age, height, cumulative lean and/or fat tissue, cumulative total calcium intake and cumulative modes of physical activity |
Multiple regression analysis (ITT): Positive associations between cumulative UNa/Cr and BMD at 36 months at LS (Coeff. = 5.85e−5, p = 0.056), at forearm (Coeff. = 8.12e−5, p = 0.076) and total body BMD (Coeff. = 2.81e−5, p = 0.041) |
|
Random‐effects regression analysis (accounting for repeated measures and missing data): Main effect of UNa in the forearm: higher UNa associated with higher BMD at baseline and subsequent time points (t = 2.63, p = 0.0089). Similar observation in the spine. No effect on total body or femoral BMD |
BMD: bone mineral density; BMI: body mass index; bw: body weight; Ca: calcium; Cr: creatinine; DEXA: dual‐energy X‐ray technology; MET: metabolic equivalent activity; IU: international unit; N: number; Na: sodium; NR: not reported; P: phosphorus; RCT: randomised controlled trial; SD: standard deviation; SRC: standardised regression coefficient; UNa: urinary sodium excretion.
L.2. Outcome of the RoB
L.2.1. Observational evidence
| References | Risk of bias domainsa | Tierb | |||||
|---|---|---|---|---|---|---|---|
| Confounding(a) | Attrition | Exposure | Outcome | Reporting | Statistics | ||
| Devine et al. (1995) | + | − | − | ++ | ++ | ++ | 2 |
| Ilich et al. (2010) | ++ | + | + | ++ | + | + | 1 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (−−): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
Appendix M – Expert knowledge elicitation
1.
In 2014, EFSA published a guidance on expert knowledge elicitation (EKE) in food and feed safety risk assessment (EFSA, 2014). The reader is referred to the guidance for general information about the objectives, procedures and methods of EKE methods. Appendix M details the specific process used for the two EKEs that were conducted to inform the setting of DRVs for sodium.
M.1. Principle
EKE refers to the drawing out of knowledge from one or more experts, following a transparent, structured and comprehensive method based on rigorous probabilistic judgement techniques (EFSA, 2014).
Through the EKE, experts can be asked for judgements about an uncertain quantity of interest. The method aims at obtaining from the experts not only their ‘best judgement’ of the quantity of interest based on the available evidence, but also a representation of the uncertainty surrounding it.
M.2. The Sheffield protocol
The Sheffield protocol is an EKE method that is based on behavioural aggregation, i.e. judgements of several experts are combined via a moderated discussion (EFSA, 2014).14
The main steps of the protocol are illustrated in Figure M.1. It is characterised by two rounds of judgements from the experts. In the first round, experts answer the elicitation question privately, by deriving their individual uncertainty probability distribution. The individual judgements, together with their rationale (see Sections M.3 and M.4 below), are then discussed by the group. In the second round, the group agrees on a consensus judgement through a moderated group discussion. To that end, the group is requested to express the reasonable judgement of an impartial expert informed by the range of judgements and rationales expressed in the first round. The final outcome of the EKE is an uncertainty probability distribution that represents the experts aggregated judgement about the quantity of interest.
Figure M.1.

Main steps of the EKE process following the Sheffield method
- The evidence dossier documents all the evidence and related uncertainties relevant to the formulation of the expert judgement (see Section M.3 below).
- In this step, each expert answered the EKE questions individually. To that end, experts had to express the probability that each of the proposed ranges of sodium intake/excretion would include the ‘true’ level of interest (roulette method, see Section M.4 below). As a result, each expert provides his/her own uncertainty probability distribution.
- Following the collective discussion, a consensus uncertainty probability distribution is elicited from the group of experts, which reflect the aggregated view of the experts on the parameter of interest and related uncertainty.
The protocol requires an experienced facilitator to manage the experts, and to address possible sources of bias in group interactions.
M.3. Preparatory phase: elicitation questions and evidence dossier
Based on the review of the available evidence, it was considered that (i) data on the relationship between sodium intake and level of blood pressure or CVD risk could inform about the levels of sodium intake associated to a reduced risk of chronic diseases; and (ii) balance studies could inform about the levels of sodium intake that are adequate to maintain a null sodium balance. Because of the limited evidence available and of the associated uncertainties, it was not possible to identify such levels of sodium intake with certainty. Two questions were formulated to elicit evidence‐based judgement of the experts about the levels of interest:
EKE question 1: What is the lowest level of sodium intake at which the risk of chronic disease (i.e. stroke, CHD) is minimised in the majority (≥ 97.5%) of the general population of adults?
EKE question 2: What is the lowest level of sodium intake which is adequate (i.e. amount which allows to maintain sodium balance) for the majority (≥ 97.5%) of the general population of adults?
Through the EKE, experts are required to formulate evidence‐based judgements, providing a rationale. An evidence dossier was prepared before the EKE meeting, which assembled all the relevant evidence into a single document, in the form of structured tables and graphical representations (BOX 2). During the process, the experts are requested to provide their rationales behind their probability assessments by referring to the dossier.
The two EKEs were conducted with the seven experts of the EFSA working group on DRVs for minerals,15 who had been in charge of selecting, reviewing and appraising the available evidence. All experts received a training on the method before starting the elicitation process.
M.3..1.
BOX 2 – Content of the evidence dossier
EKE question 1:
-
All relevant evidence on the relationship between sodium and blood pressure, including:
The outcome of the meta‐regression modelling of sodium and blood pressure
The data from the eligible randomised studies on the incidence of hypertension
The data from the eligible prospective cohort study on blood pressure
The data from the eligible prospective cohort studies on the incidence of hypertension
Considerations about the reliability, external validity and consistency of each source of evidence
-
All relevant evidence on the relationship between sodium and risk of CVD, including:
The data from the eligible prospective cohort studies on risk of stroke
The data from the eligible prospective cohort studies on risk of coronary heart disease
The data from the eligible prospective cohort studies on risk of CVD
Considerations about the reliability, external validity and consistency of each source of evidence
The outcome of the risk of bias assessment for each individual study
Graphical representations of the dose‐responses from the eligible observational studies on coronary heart disease and stroke
A summary of relevant mechanistic data
EKE question 2:
The data from the eligible balance studies
Considerations about the reliability, external validity and consistency of the available evidence
A summary of relevant mechanistic data
M.4. Elicitation of the uncertainty probability distributions: the roulette method
For illustrative purposes, the uncertain quantity of interest is denoted X. The objective of the EKE is to elicit a probability distribution for X from the experts. The roulette method was used for the EKEs on sodium (Johnson et al., 2010; EFSA, 2014). The range of possible values for X was divided into fixed intervals (bins) (Figure M.2). In answering the elicitation questions, experts were asked to distribute 20 chips into the bins, with the probability of X lying in a particular bin interpreted as the proportion of chips allocated into that bin. As the total number of chips was 20, each chip represented 5% probability.
Figure M.2.
Illustration of the roulette method. In this example, a range of possible values for X has been set between 5 and 13. Each orange rectangle represents one chip. For instance, by placing four of their 20 chips in the bin (6,7) the expert expresses a probability of 4/20 (20%) that the ‘true value’ of X lies in that bin. The probability that the range 6 to 12 includes the ‘true value’ of X is 100%.
In the first round of judgements, one histogram was obtained from each expert, representing his/her uncertainty probability distribution. In the second round of judgements, the experts built a group histogram, i.e. consensus uncertainty probability distribution, through discussion. The group consensus uncertainty probability distributions elicited in response to the two EKE questions are provided in Figures 1 and 2. The rationales for the distributions were documented and are reported in Tables 9 and 10.
Figure K.3.
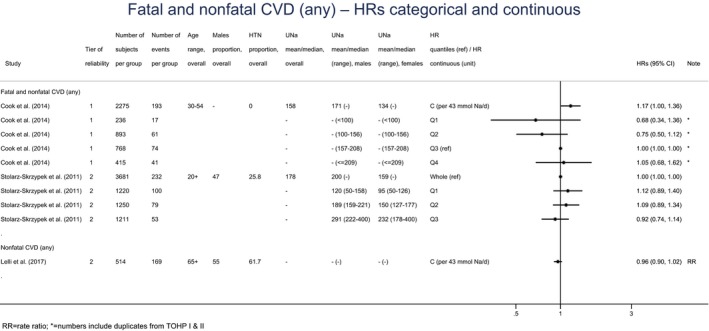
Descriptive forest plot of the eligible observational studies on cardiovascular disease
- CVD: cardiovascular disease; 95% CI: 95% confidence interval; HR: hazard ratio; HTN: hypertensive; UNa: sodium urinary excretion.
- A hazard ratio > 1 indicates an increased risk of outcome with higher sodium intake.
M.5. Derivation of parametric distributions from the group consensus uncertainty probability distributions
Parametric distributions were fitted to the group consensus uncertainty probability distributions to derive probability density functions. This allows the derivation of probabilities for values within the range of each bin used for the elicitation. Several parametric distributions (e.g. normal, log‐normal, truncated normal, gamma, Weibull and logistic) have been considered as possible candidates to estimate the probability density functions that better fit the centiles obtained through the EKE process. Log‐normal distributions were finally chosen based on indicators of goodness of fit (Akaike Information Criterion and Bayesian Information Criterion), with the following parameters: 0.72 (mean) and 0.23 (standard deviation) for the question on sodium intake at which CVD risk is reduced; 0.5 (mean) and 0.3 (standard deviation) for the question on sodium balance.
Based on the two probability density functions, cumulative uncertainty density functions were derived (Figure 3) and served as the basis to inform the setting of the reference value for sodium.
Annex A – Protocol for sections 5.5 and 6 of the scientific opinion on DRVs for sodium: Assessment of the relationship between sodium intake and pre‐specified health outcomes, including dose‐response relationships, and integration of different lines of evidence for setting DRVs for sodium
1.
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2019.5778
Annex B – Analysis of evidence from published scientific literature as preparatory work for the setting of Dietary Reference Values for Sodium
1.
Annex B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2019.5778
Supporting information
Protocol for sections 5.5 and 6 of the scientific opinion on DRVs for sodium: Assessment of the relationship between sodium intake and pre‐specified health outcomes, including dose‐response relationships, and integration of different lines of evidence for setting DRVs for sodium
Analysis of evidence from published scientific literature as preparatory work for the setting of Dietary Reference Values for Sodium
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Castenmiller J, de Henauw S, Hirsch‐Ernst K‐I, Kearney J, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Aggett P, Fairweather‐Tait S, Martin A, Przyrembel H, Ciccolallo L, de Sesmaisons‐Lecarré A, Martinez SV, Martino L and Naska A, 2019. Scientific Opinion on the dietary reference values for sodium. EFSA Journal 2019;17(9):5778, 191 pp. 10.2903/j.efsa.2019.5778
Requestor: European Commission
Question number: EFSA‐Q‐2011–01224
Panel members: Dominique Turck, Jacqueline Castenmiller, Stefaan de Henauw, Karen‐Ildico Hirsch‐Ernst, John Kearney, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Andrew Hart for his contribution as hearing expert and as facilitator for the Expert Knowledge Elicitation process and EFSA staff Elisa Aiassa, Irene Munoz Guajardo and Daniela Tomcikova for their contribution to the implementation of the systematic reviews.
Adopted: 3 July 2019
This publication is linked to the following EFSA Supporting Publications articles: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1679/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.e15121/full
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5779/full
Notes
Scientific Committee for Food, 1993. Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food, 31st series. Food – Science and Technique, European Commission, Luxembourg, 248 pp.
Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
Deliverable 1 of PROMETHEUS project – PROMoting METHods for Evidence Use in Scientific assessments.
Available online: https://www.omim.org/
Regulation (EC) No. 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJ L 404, 30.12.2006, p. 26.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJ L 183, 12.7.2002, p. 51.
Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow‐on formulae and amending Directive 1999/21/EC. OJ L 401, 30.12.2006, p. 1.
Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children. OJ L 339, 6.12.2006, p. 20.
In addition, data were received during the public consultation on the intermediate draft of the Opinion. EFSA (European Food Safety Authority), 2017. Outcome of a public consultation on the Scientific Opinion of EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) on Dietary Reference Values for sodium (intermediate draft) and related protocol. EFSA supporting publication 2017:1356.
A strong recommendation is one for which the guideline development group is confident that the desirable effects of adherence outweigh the undesirable effects.
A secondary analysis of the DASH‐sodium trial indicates that the relationship between absolute sodium intake and blood pressure levels varies with energy intake (Murtaugh et al., 2018). A higher increase in SBP and DBP with increasing sodium intake was reported at lower energy intakes than at higher ones (p interaction < 0.001). This suggests that sodium density may reflect the relationship with blood pressure better than does absolute sodium intake.
The Sheffield Elicitation Framework was created by Tony O‘Hagan and Jeremy Oakley at the University of Sheffield, UK (http://www.tonyohagan.co.uk/shelf/)
References
- Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP and Meerpohl JJ, 2013. Effect of lower sodium intake on health: systematic review and meta‐analyses. British Medical Journal, 346, f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler AJ, Taylor F, Martin N, Gottlieb S, Taylor RS and Ebrahim S, 2014. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews, 12, CD009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrogué HJ and Madias NE, 2000a. Hyponatremia. New England Journal of Medicine, 342, 1581–1589. [DOI] [PubMed] [Google Scholar]
- Adrogué HJ and Madias NE, 2000b. Hypernatremia. New England Journal of Medicine, 342, 1493–1499. [DOI] [PubMed] [Google Scholar]
- Adrogué HJ and Madias NE, 2014. The impact of sodium and potassium on hypertension risk. Seminars in Nephrology, 34, 257–272. [DOI] [PubMed] [Google Scholar]
- Afssa (Agence française de sécurité sanitaire des aliments), 2001. Apports nutritionnels conseillés pour la population française. Editions Tec & Doc, Paris, France, 605 pp.
- Al‐Dahhan J, Haycock GB, Chantler C and Stimmler L, 1983. Sodium homeostasis in term and preterm neonates. I. Renal aspects. Archives of Diseases in Childhood, 58, 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Dahhan J, Haycock GB, Nichol B, Chantler C and Stimmler L, 1984. Sodium homeostasis in term and preterm neonates. III. Effect of salt supplementation. Archives of Diseases in Childhood, 59, 945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderman MH, Anderson S, Bennett WM, Drueke TB, Haynes RB, Kincaid‐Smith PS, Kurtz TW, Laragh JH, Linas SL, Logan AG, Luft FC, Mancia G, McCarron DA, Oparil S, Staessen JAH and Stern JS, 1997. Scientists’ statement regarding data on the sodium‐hypertension relationship and sodium health claims on food labeling. Nutrition Reviews, 55, 172–175. [DOI] [PubMed] [Google Scholar]
- Allan JR and Wilson CG, 1971. Influence of acclimatization on sweat sodium concentration. Journal of Applied Physiology, 30, 708–712. [DOI] [PubMed] [Google Scholar]
- Alli C, Avanzini F, Bettelli G, Bonati M, Colombo F, Corso R, Di Tullio M, Gentile MG, Sangalli L, Taioli E, Tognoni G and the participating doctors 1992. Feasibility of a long‐term low‐sodium diet in mild hypertension. Journal of Human Hypertension, 6, 281–286. [PubMed] [Google Scholar]
- Allsopp AJ (Institute of Naval Medicine), 1997. The effects of altered salt ingestion on acclimation to heat. INM Report No. 97020 July 1997. 31 pp.
- Allsopp AJ, Sutherland R, Wood P and Wootton SA, 1998. The effect of sodium balance on sweat sodium secretion and plasma aldosterone concentration. European Journal of Applied Physiology, 78, 516–521. [DOI] [PubMed] [Google Scholar]
- Ames RP, 2001. The effect of sodium supplementation on glucose tolerance and insulin concentrations in patients with hypertension and diabetes mellitus. American Journal of Hypertension, 14, 653–659. [DOI] [PubMed] [Google Scholar]
- Andersen L, Rasmussen LB, Larsen EH and Jakobsen J, 2009. Intake of household salt in a Danish population. European Journal of Clinical Nutrition, 63, 598–604. [DOI] [PubMed] [Google Scholar]
- Andersson B, Leksell LG and Rundgren M, 1982. Regulation of water intake. Annual Review of Nutrition, 2, 73–89. [DOI] [PubMed] [Google Scholar]
- Andersson OK, Fagerberg B and Hedner T, 1984. Importance of dietary salt in the hemodynamic adjustment to weight reduction in obese hypertensive men. Hypertension, 6, 814–819. [DOI] [PubMed] [Google Scholar]
- ANHMRCDS , 1986. Australian National Health and Medical Research Council dietary salt study in mild hypertension. Journal of Hypertension Supplement, 4, S629–S637. [PubMed] [Google Scholar]
- ANHMRCDS , 1989. Effects of replacing sodium intake in subjects on a low sodium diet: a crossover study. Australian National Health & Medical Research Council Dietary Salt Study Management Committee. Clinical and Experimental Hypertension A, 11, 1011–1024. [DOI] [PubMed] [Google Scholar]
- Anses (Agence nationale de sécurité sanitaire, alimentation, environnement, travail), 2016a. Actualisation des repères du PNNS: révision des repères de consommations alimentaires. Avis de l'Anses. Rapports d'expertise collective. 280 pp.
- Anses (Agence nationale de sécurité sanitaire, alimentation, environnement, travail), 2016b. Actualisation des repères du PNNS: élaboration des références nutritionnelles. Avis de l'Anses. Rapports d'expertise collective. 196 pp.
- Anses (Agence nationale de sécurité sanitaire, alimentation, environnement, travail), 2016c. French food composition table. Table Ciqual version 2016. Available online: https://pro.anses.fr/tableciqual/
- Aparicio A, Rodriguez‐Rodriguez E, Cuadrado‐Soto E, Navia B, Lopez‐Sobaler AM and Ortega RM, 2017. Estimation of salt intake assessed by urinary excretion of sodium over 24 h in Spanish subjects aged 7–11 years. European Journal of Nutrition, 56, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson S, Alston‐Mills B, Lonnerdal B and Neville MC, 1995. Major minerals and ionic constituents of human and bovine milks In: Jensen RJ. (ed.). Handbook of Milk Composition. Academic Press, California, USA: pp. 593–619. [Google Scholar]
- Azoulay A, Garzon P and Eisenberg MJ, 2001. Comparison of the mineral content of tap water and bottled waters. Journal of General Internal Medicine, 16, 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JD, Fong AKH, Novotny JA and Oexmann MJ, 1999. Compartmental modeling, stable isotopes, and balance studies. In: Dennis BH (ed.). Well‐controlled Diet studies in Humans: A Practical Guide to Design and Management. American Dietetic Association. pp. 238–254.
- Bailey JL, Sands JM and Franch HA, 2014. Water, electrolytes and acid–base metabolism In: Ross AC, Caballero B, Cousins RJ, Tucker KL. and Ziegler TR. (eds.). Modern Nutrition in Health and Disease, 11th edition Lippincott Williams & Wilkins, Philadelphia, USA: pp. 102–132. [Google Scholar]
- Banks D, Birke M, Flem B and Reimann C, 2015. Inorganic chemical quality of European tap‐water: 1. Distribution of parameters and regulatory compliance. Applied Geochemistry, 59, 200–210. [Google Scholar]
- Barr SI, Costill DL and Fink WJ, 1991. Fluid replacement during prolonged exercise: effects of water, saline, or no fluid. Medicine & Sci in Sports & Exercise, 23, 811–817. [PubMed] [Google Scholar]
- Bates GP and Miller VS, 2008. Sweat rate and sodium loss during work in the heat. Journal of Occupational Medicine and Toxicology, 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J and Gerss J, 2011. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clinical Nutrition, 30, 215–220. [DOI] [PubMed] [Google Scholar]
- Beilin LJ, Knight GJ, Munro‐Faure AD and Anderson J, 1966. The sodium, potassium, and water contents of red blood cells of healthy human adults. Journal of Clinical Investigation, 45, 1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A, Xiao YY, Cuche JL, Hannaert P and Safar M, 1992. Arterial effects of salt restriction in hypertensive patients. A 9‐week, randomized, double‐blind, crossover study. Journal of Hypertension, 10, 355–360. [DOI] [PubMed] [Google Scholar]
- Berend K, van Hulsteijn LH and Gans ROB, 2012. Chloride: the queen of electrolytes? European Journal of Internal Medicine, 23, 203–211. [DOI] [PubMed] [Google Scholar]
- Bibbins‐Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ and Goldman L, 2010. Projected effect of dietary salt reductions on future cardiovascular disease. New England Journal of Medicine, 362, 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie P, 2018. Mechanisms of sodium balance: total body sodium, surrogate variables, and renal sodium excretion. American Journal of Physiology – Regulatory. Integrative and Comparative Physiology, 315, R945–R962. [DOI] [PubMed] [Google Scholar]
- Bjorklund KL, Vahter M, Palm B, Grander M, Lignell S and Berglund M, 2012. Metals and trace element concentrations in breast milk of first time healthy mothers: a biological monitoring study. Environmental Health, 11, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J and Wier WG, 2012. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt‐dependent hypertension. American Journal of Physiology – Heart and Circulatory. Physiology, 302, H1031–H1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey A, 1988. Calcified tissues; chemistry and biochemistry In: Nordin B. (ed.). Calcium in human biology. Springer‐Verlag, London, UK: pp. 171–186. [Google Scholar]
- Brown MA, 1989. Sodium balance in pregnancy. Fetal and Maternal Medicine Review, 1, 193–212. [Google Scholar]
- Brown MB, Haack KKV, Pollack BP, Millard‐Stafford M and McCarty NA, 2011. Low abundance of sweat duct Cl− channel CFTR in both healthy and cystic fibrosis athletes with exceptionally salty sweat during exercise. American Journal of Physiology – Regulatory. Integrative and Comparative Physiology, 300, R605–R615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J and Elliott P and Group IC‐OR , 2013. Estimating 24‐hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. American Journal of Epidemiology, 177, 1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono MJ, Ball KD and Kolkhorst FW, 2007. Sodium ion concentration vs. sweat rate relationship in humans. Journal of Applied Physiology, 103, 990. [DOI] [PubMed] [Google Scholar]
- Butte NF, Lopez‐Alarcon MG and Garza C, 2002. Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. World Health. Organization., 57, pp. [Google Scholar]
- Campanozzi A, Avallone S, Barbato A, Iacone R, Russo O, De Filippo G, D'Angelo G, Pensabene L, Malamisura B, Cecere G, Micillo M, Francavilla R, Tetro A, Lombardi G, Tonelli L, Castellucci G, Ferraro L, Di Biase R, Lezo A, Salvatore S, Paoletti S, Siani A, Galeone D and Strazzullo P and Group M‐GPS , 2015. High sodium and low potassium intake among Italian children: relationship with age, body mass and blood pressure. PLoS ONE, 10, e0121183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NA and Reece JB, 2002. Biology. Pearson Education Incorporated, Benjamin Cummings, San Francisco, USA. [Google Scholar]
- Cappuccio FP, Markandu ND, Carney C, Sagnella GA and MacGregor GA, 1997. Double‐blind randomised trial of modest salt restriction in older people. Lancet, 350, 850–854. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Ji C, Donfrancesco C, Palmieri L, Ippolito R, Vanuzzo D, Giampaoli S and Strazzullo P, 2015. Geographic and socioeconomic variation of sodium and potassium intake in Italy: results from the MINISAL‐GIRCSI programme. British Medical Journal Open, 5, e007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano E, van der Veer G, Verheijen PJJ, Heenan SP, van de Laak LFJ, Koopmans HBM and van Ruth SM, 2013. Comparison of a sodium‐based and a chloride‐based approach for the determination of sodium chloride content of processed foods in the Netherlands. Journal of Food Composition and Analysis, 31, 129–136. [Google Scholar]
- Carney SL, Gillies AH, Smith AJ and Smitham S, 1991. Increased dietary sodium chloride in patients treated with antihypertensive drugs. Clinical and Experimental Hypertension A, 13, 401–407. [DOI] [PubMed] [Google Scholar]
- Chang EB and Leung PS, 2014. Intestinal Water and Electrolyte Transport. Springer, Dordrecht. [Google Scholar]
- Chappuis A, Bochud M, Glatz N, Vuistiner P, Paccaud F and Burnier M (Service de Néphrologie et Institut Universitaire de Médecine Sociale et Préventive Centre Hospitalier Universitaire Vaudois (CHUV)), 2011. Swiss Survey on Salt Intake: Main Results. 32 pp. Available online: https://serval.unil.ch/resource/serval:BIB_16AEF897B618.P001/REF
- Cheung KL and Lafayette RA, 2013. Renal physiology of pregnancy. Advances in Chronic Kidney Disease, 20, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier RL, 2001. The moth and the aspen tree: sodium in early postnatal development. Kidney International, 59, 1617–1625. [DOI] [PubMed] [Google Scholar]
- Chien KL, Hsu HC, Chen PC, Su TC, Chang WT, Chen MF and Lee YT, 2008. Urinary sodium and potassium excretion and risk of hypertension in Chinese: report from a community‐based cohort study in Taiwan. Journal of Hypertension, 26, 1750–1756. [DOI] [PubMed] [Google Scholar]
- Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M and Appel LJ, American Heart Association Council on Lifestyle and Metabolic Health , 2014. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation, 129, 1173–1186. [DOI] [PubMed] [Google Scholar]
- Cobiac L, Nestel PJ, Wing LM and Howe PR, 1992. A low‐sodium diet supplemented with fish oil lowers blood pressure in the elderly. Journal of Hypertension, 10, 87–92. [DOI] [PubMed] [Google Scholar]
- Cogswell ME, Wang CY, Chen TC, Pfeiffer CM, Elliott P, Gillespie CD, Carriquiry AL, Sempos CT, Liu K, Perrine CG, Swanson CA, Caldwell KL and Loria CM, 2013. Validity of predictive equations for 24‐h urinary sodium excretion in adults aged 18–39 y. American Journal of Clinical Nutrition, 98, 1502–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell ME, Maalouf J, Elliott P, Loria CM, Patel S and Bowman BA, 2015. Use of urine biomarkers to assess sodium intake: challenges and opportunities. Annual Reviews of Nutrition, 35, 349–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consolazio CF, Matoush LO, Nelson RA, Harding RS and Canham JE, 1963. Excretion of sodium, potassium, magnesium and iron in human sweat and the relation of each to balance and requirements. Journal of Nutrition, 79, 407–415. [DOI] [PubMed] [Google Scholar]
- Cook NR, Appel LJ and Whelton PK, 2014. Lower levels of sodium intake and reduced cardiovascular risk. Circulation, 129, 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK, and Trials of Hypertension Prevention Collaborative Research G , 2009. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow‐up study. Archives of Internal Medicine, 169, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PA, 1995. The Elements on Earth: Inorganic Chemistry in the Environment. Oxford University Press, Oxford. [Google Scholar]
- D'Elia L, Galletti F, La Fata E, Sabino P and Strazzullo P, 2018. Effect of dietary sodium restriction on arterial stiffness: systematic review and meta‐analysis of the randomized controlled trials. Journal of Hypertension, 36, 734–743. [DOI] [PubMed] [Google Scholar]
- D–A–CH , 2016. Natrium. In: Referenzwerte für die Nährstoffzufuhr. 2. aktualisierte Ausgabe 2016. Deutsche Gesellschaft für Ernährung – Österreichische Gesellschaft für Ernährung – Schweizerische Gesellschaft für Ernährungsforschung – Schweizerische Vereinigung für Ernährung. 9 pp.
- Davidson K and Repke J, 1998. Mineral metabolism in pregnancy In: Cowett R. (ed.). Principles of Perinatal‐Neonatal Metabolism. Springer‐Verlag, New York, USA: pp. 281–308. [Google Scholar]
- De Keyzer W, Dofkova M, Lillegaard IT, De Maeyer M, Andersen LF, Ruprich J, Rehurkova I, Geelen A, van ‘t Veer P, De Henauw S, Crispim SP, de Boer E, Ocke M, Slimani N and Huybrechts I, 2015. Reporting accuracy of population dietary sodium intake using duplicate 24 h dietary recalls and a salt questionnaire. British Journal of Nutrition, 113, 488–497. [DOI] [PubMed] [Google Scholar]
- de Wardener HE, He FJ and MacGregor GA, 2004. Plasma sodium and hypertension. Kidney International, 66, 2454–2466. [DOI] [PubMed] [Google Scholar]
- Deutsche Gesellschaft für Ernährung , 2015. DGExpert, Version 1.7.6 (BLS 3.02). Bonn, Germany. [Google Scholar]
- Devine A, Criddle RA, Dick IM, Kerr DA and Prince RL, 1995. A longitudinal study of the effect of sodium and calcium intakes on regional bone density in postmenopausal women. American Journal of Clinical Nutrition, 62, 740–745. [DOI] [PubMed] [Google Scholar]
- DH (Department of Health), 1991. Dietary Reference Values for food energy and nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. HMSO, London, UK. 212 pp. [PubMed]
- Dickinson KM, Clifton PM, Burrell LM, Barrett PH and Keogh JB, 2014. Postprandial effects of a high salt meal on serum sodium, arterial stiffness, markers of nitric oxide production and markers of endothelial function. Atherosclerosis, 232, 211–216. [DOI] [PubMed] [Google Scholar]
- Dika Ž, Pećin I, Čvorišćec D, Fištrek M, Fuček M, Karlović K, Kos J, Luketić P, Miletić‐Medved M, Mišić M, Muldini M, Premužić V, Sertić J, Vuković I and Jelaković B, 2009. Salt intake in a continental rural part of Croatia – estimated population 24‐h urinary sodium excretion using spot urine sample. Kidney and Blood Pressure Research, 32, 323. [Google Scholar]
- Dole VP, Dahl LK, Cotzias GC, Eder HA and Krebs ME, 1950. Dietary treatment of hypertension; clinical and metabolic studies of patients on the rice‐fruit diet. Journal of Clinical Investigation, 29, 1189–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donfrancesco C, Ippolito R, Lo Noce C, Palmieri L, Iacone R, Russo O, Vanuzzo D, Galletti F, Galeone D, Giampaoli S and Strazzullo P, 2013. Excess dietary sodium and inadequate potassium intake in Italy: results of the MINISAL study. Nutrition Metabolism & Cardiovascular Diseases, 23, 850–856. [DOI] [PubMed] [Google Scholar]
- Du S, Batis C, Wang H, Zhang B, Zhang J and Popkin BM, 2014. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. American Journal of Clinical Nutrition, 99, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2005a. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the tolerable upper intake level of sodium. EFSA Journal 2005:3(6);209, 26 pp. 10.2903/j.efsa.2005.209 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005b. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the tolerable upper intake level of chloride. EFSA Journal 2005:3(6);210, 9 pp. 10.2903/j.efsa.2005.210 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Guidance on Expert Knowledge Elicitation in food and feed safety risk assessment. EFSA Journal 2014;12(6):3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Scientific report on Principles and process for dealing with data and evidence in scientific assessments. EFSA Journal 2015;13(5):4121, 35 pp. 10.2903/j.efsa.2015.4121 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Dietary Reference Values for nutrients. Summary Report. EFSA supporting publication 2017:e15121. 10.2903/sp.efsa.2017.e15121 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. Outcome of a public consultation on the Scientific Opinion of EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) on Dietary Reference Values for sodium (intermediate draft) and related protocol. EFSA supporting publication 2017:EN‐1356, 52 pp. 10.2903/sp.efsa.2017.en-1356 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2019. Outcome of public consultations on the Scientific Opinions of the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) on Dietary Reference Values for sodium and chloride. EFSA supporting publication 2019:EN‐1679. 39 pp. 10.2903/sp.efsa.2019.en-1679 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2009. Scientific Opinion on the appropriate age for introduction of complementary feeding of infants. EFSA Journal 2009;7(12):1423, 38 pp. 10.2903/j.efsa.2009.1423 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on principles for deriving and applying Dietary Reference Values. EFSA Journal 2010;8(3):1458, 30 pp. 10.2903/j.efsa.2010.1458 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2012a. Scientific Opinion on Dietary Reference Values for protein. EFSA Journal 2012;10(2):2557, 66 pp. 10.2903/j.efsa.2012.2557 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2012b. Scientific Opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA Journal 2012;10(7):2815, 48 pp. 10.2903/j.efsa.2012.2815 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013a. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Journal 2013;11(10):3408, 103 pp. 10.2903/j.efsa.2013.3408 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013b. Scientific Opinion on dietary reference values for energy. EFSA Journal 2013;11(1):3005, 112 pp. 10.2903/j.efsa.2013.3005 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific opinion on dietary reference values for calcium. EFSA Journal 2015;13(5):4101, 82 pp. 10.2903/j.efsa.2015.4101 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Scientific opinion on dietary reference values for potassium. EFSA Journal 2016;14(10):4592, 56 pp. 10.2903/j.efsa.2016.4592 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens), Turck D, Castenmiller J, de Henauw S, Hirsch‐Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Aggett P, Fairweather‐Tait S, Martin A, Przyrembel H, de Sesmaisons‐Lecarré A and Naska A, 2019. Scientific opinion on Dietary Reference Values for chloride. EFSA Journal 2019;17(9):5779, 24 pp. 10.2903/j.efsa.2019.5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger M, Knutsen H, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter J, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry Q, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtuena Martinez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger M, Knutsen H, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter J, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Germini A, Martino L, Merten C, Mosbach‐Schulz O, Smith A and Hardy A, 2018a. The principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger M, Knutsen H, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter J, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018b. Guidance on uncertainty analysis in scientific assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton‐Cheh CH, Sacks FM and Laffer CL, American Heart Association P, Public Education Committee of the Council on H, Council on Functional G, Translational B and Stroke C , 2016. Salt sensitivity of blood pressure: A Scientific Statement From the American Heart Association. Hypertension, 68, e7–e46. [DOI] [PubMed] [Google Scholar]
- Elmadfa I, 2012. Österreichischer Ernährungsbericht 2012. 413 pp.
- Epstein M and Hollenberg NK, 1976. Age as a determinant of renal sodium conservation in normal man. Journal of Laboratory and Clinical Medicine, 87, 411–417. [PubMed] [Google Scholar]
- Ereman RR, Lonnerdal B and Dewey KG, 1987. Maternal sodium intake does not affect postprandial sodium concentrations in human milk. Journal of Nutrition, 117, 1154–1157. [DOI] [PubMed] [Google Scholar]
- Erwteman TM, Nagelkerke N, Lubsen J, Koster M and Dunning AJ, 1984. Beta blockade, diuretics, and salt restriction for the management of mild hypertension: a randomised double blind trial. British Medical Journal (Clinical Research ed.), 289, 406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2012. Survey on Members States’ implementation of the EU salt reduction framework. 26 pp. Available online: https://ec.europa.eu/health//sites/health/files/nutrition_physical_activity/docs/salt_report1_en.pdf
- Eyles H, Webster J, Jebb S, Capelin C, Neal B and Ni Mhurchu C, 2013. Impact of the UK voluntary sodium reduction targets on the sodium content of processed foods from 2006 to 2011: Analysis of household consumer panel data. Preventive Medicine, 57, 555–560. [DOI] [PubMed] [Google Scholar]
- Falconer MA and Lyall A, 1937. The requirements of sodium chloride. The British Medical Journal, 2, 1116–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO/UNU (Food and Agriculture Organization of the United Nations/World Health Organization/United Nations University), 2004. Human energy requirements. Report of a Joint FAO/WHO/UNU Expert Consultation, Rome 17–24 October 2001. FAO Food and Nutrition Technical Report Series, 103 pp.
- Farquhar WB, Edwards DG, Jurkovitz CT and Weintraub WS, 2015. Dietary sodium and health: more than just blood pressure. Journal of the American College of Cardiology, 65, 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrimond S, Ainsworth P and Piper B, 1995. The contribution of discretionary salt to total salt intake. Journal of Consumer Studies & Home Economics, 19, 135–143. [Google Scholar]
- Filippi JPD, Kaanders H and Hoftnan A, 1981. Sodium in diet and milk of breastfeeding women. Acta Pædiatrica, 70, 417–418. [DOI] [PubMed] [Google Scholar]
- Fly AD, Uhlin KL and Wallace JP, 1998. Major mineral concentrations in human milk do not change after maximal exercise testing. American Journal of Clinical Nutrition, 68, 345–349. [DOI] [PubMed] [Google Scholar]
- Fomon S, Haschke F, Ziegler E and Nelson S, 1982. Body composition of reference children from birth to age 10 years. American Journal of Clinical Nutrition, 35, 1169–1175. [DOI] [PubMed] [Google Scholar]
- Fomon S, 1993. Nutrition of Normal Infants. Mosby, St Louis, USA: p. 475. [Google Scholar]
- Forbes G, 1975. Disturbances of water and electrolytes In: Farmer T. (ed.). Pediatric Neurology, 2nd edition Harper and Row, Hagerstown, MD, USA: pp. 75–89. [Google Scholar]
- Fordtran JS, Rector FC Jr and Carter NW, 1968. The mechanisms of sodium absorption in the human small intestine. Journal of Clinical Investigation, 47, 884–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman JP, Scheven L, de Jong PE, Bakker SJ, Curhan GC and Gansevoort RT, 2012. Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation, 125, 3108–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotherby MD and Potter JF, 1993. Effects of moderate sodium restriction on clinic and twenty‐four‐hour ambulatory blood pressure in elderly hypertensive subjects. Journal of Hypertension, 11, 657–663. [DOI] [PubMed] [Google Scholar]
- Freedman LS, Commins JM, Moler JE, Willett W, Tinker LF, Subar AF, Spiegelman D, Rhodes D, Potischman N, Neuhouser ML, Moshfegh AJ, Kipnis V, Arab L and Prentice RL, 2015. Pooled results from 5 validation studies of dietary self‐report instruments using recovery biomarkers for potassium and sodium intake. American Journal of Epidemiology, 181, 473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis‐Hansen B, 1961. Body water compartments in children: changes during growth and related changes in body composition. Pediatrics, 28, 169–181. [PubMed] [Google Scholar]
- Frost CD, Law MR and Wald NJ, 1991. By how much does dietary salt reduction lower blood pressure? II – Analysis of observational data within populations. British Medical Journal, 302, 815–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipeau R, Goulet C and Chagnon M, 2012. Infant and maternal factors influencing breastmilk sodium among primiparous mothers. Breastfeed Medicine, 7, 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates PE, Tanaka H, Hiatt WR and Seals DR, 2004. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension, 44, 35. [DOI] [PubMed] [Google Scholar]
- Genaidy AM, Lemasters GK, Lockey J, Succop P, Deddens J, Sobeih T and Dunning K, 2007. An epidemiological appraisal instrument ‐ a tool for evaluation of epidemiological studies. Ergonomics, 50, 920–960. [DOI] [PubMed] [Google Scholar]
- Gijsbers L, Dower JI, Mensink M, Siebelink E, Bakker SJ and Geleijnse JM, 2015. Effects of sodium and potassium supplementation on blood pressure and arterial stiffness: a fully controlled dietary intervention study. Journal of Human Hypertension, 29, 592–598. [DOI] [PubMed] [Google Scholar]
- Glieberman L, 1973. Blood pressure and dietary salt in human populations. Ecology of Food and Nutrition, 2, 319–328. [Google Scholar]
- Graudal NA, Hubeck‐Graudal T and Jurgens G, 2011. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database of Systematic Reviews, 11, CD004022. [DOI] [PubMed] [Google Scholar]
- Graudal N, Jurgens G, Baslund B and Alderman MH, 2014. Compared with usual sodium intake, low‐ and excessive‐sodium diets are associated with increased mortality year: a meta‐analysis. American Journal of Hypertension, 27, 1129–1137. [DOI] [PubMed] [Google Scholar]
- Greenwood NN and Earnshaw A, 1997. Chemistry of the Elements. Butterworth‐Heinemann, Oxford. [Google Scholar]
- Greger R, 2000. Physiology of renal sodium transport. American Journal of Medical Science, 319, 51–62. [DOI] [PubMed] [Google Scholar]
- Grobbee DE, Hofman A, Roelandt JT, Boomsma F, Schalekamp MA and Valkenburg HA, 1987. Sodium restriction and potassium supplementation in young people with mildly elevated blood pressure. Journal of Hypertension, 5, 115–119. [DOI] [PubMed] [Google Scholar]
- Gropper SS, Smith JL and Groff JL, 2009. Macrominerals In: Gropper SS, Smith JL. and Groff JL. (eds.). Advanced Nutrition and Human Metabolism. Wadsworth Cengage Learning, Belmont, CA, USA: pp. 429–468. [Google Scholar]
- Gross SJ, David RJ, Bauman L and Tomarelli RM, 1980. Nutritional composition of milk produced by mothers delivering preterm. Journal of Pediatrics, 96, 641–644. [DOI] [PubMed] [Google Scholar]
- Gulson BL, Mizon KJ, Korsch MJ, Mahaffey KR and Taylor AJ, 2001. Dietary intakes of selected elements from longitudinal 6‐day duplicate diets for pregnant and nonpregnant subjects and elemental concentrations of breast milk and infant formula. Environmental Research, 87, 160–174. [DOI] [PubMed] [Google Scholar]
- Gumz ML, Rabinowitz L and Wingo CS, 2015. An integrated view of potassium homeostasis. New England Journal of Medicine, 373, 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn JM, 2014. Natriuretic hormones, endogenous ouabain, and related sodium transport inhibitors. Frontiers in Endocrinology, 5, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN and MacGregor GA, 2009. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension, 54, 482–488. [DOI] [PubMed] [Google Scholar]
- He FJ, Li J and MacGregor GA, 2013. Effect of longer‐term modest salt reduction on blood pressure. Cochrane Database of Systematic Reviews, 4, CD004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He FJ, Wu Y, Feng XX, Ma J, Ma Y, Wang H, Zhang J, Yuan J, Lin CP, Nowson C and MacGregor GA, 2015. School based education programme to reduce salt intake in children and their families (School‐EduSalt): cluster randomised controlled trial. British Medical Journal, 350, h770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He FJ, Campbell NRC, Ma Y, MacGregor GA, Cogswell ME and Cook NR, 2018. Errors in estimating usual sodium intake by the Kawasaki formula alter its relationship with mortality: implications for public health. International Journal of Epidemiology, 47, 1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Council of the Netherlands , 2006. Guidelines for a Healthy Diet 2006. 110 pp. Available online: https://www.healthcouncil.nl/documents/advisory-reports/2006/12/18/guidelines-for-a-healthy-diet-2006
- Heer M, Baisch F, Kropp J, Gerzer R and Drummer C, 2000. High dietary sodium chloride consumption may not induce body fluid retention in humans. American Journal of Physiology –. Renal Physiology, 278, F585–F595. [DOI] [PubMed] [Google Scholar]
- Heer M, Frings‐Meuthen P, Titze J, Boschmann M, Frisch S, Baecker N and Beck L, 2009. Increasing sodium intake from a previous low or high intake affects water, electrolyte and acid–base balance differently. British Journal of Nutrition, 101, 1286–1294. [DOI] [PubMed] [Google Scholar]
- Henderson L, Irving K, Gregory J, Bates CJ, Prentice A, Perks J, Swan G and Farron M, 2003. National Diet and Nutrition Survey: adults aged 19‐64 years. Volume 3: vitamin and mineral intake and urinary analytes. London: The Stationery Office.
- Hendriksen MA, van Raaij JM, Geleijnse JM, Wilson‐van den Hooven C, Ocke MC and van der AD, 2014. Monitoring salt and iodine intakes in Dutch adults between 2006 and 2010 using 24 h urinary sodium and iodine excretions. Public Health Nutrition, 17, 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen M, Etemad Z, van den Bogaard C and van der AD, 2016. Zout‐, jodium‐ en kaliuminname 2015 Voedingsstatusonderzoek bij volwassenen uit Doetinchem. RIVM Briefrapport 2016‐0081, 40 pp.
- HHS/USDA (US Department of Health and Human Services and US Department of Agriculture), 2015. 2015–2020 Dietary Guidelines for Americans. 8th edition. 144 pp.
- Higgins J and Green S, 2011. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. Available online: http://www.cochrane-handbook.org [Google Scholar]
- Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W and Smith JC Jr, 1984. Sodium and potassium intake and balance in adults consuming self‐selected diets. American Journal of Clinical Nutrition, 40, 786–793. [DOI] [PubMed] [Google Scholar]
- Holt C, 1993. Interrelationships of the concentrations of some ionic constituents of human milk and comparison with cow and goat milks. Comparative Biochemistry and Physiology: Part A Physiology, 104, 35–41. [DOI] [PubMed] [Google Scholar]
- Huang L, Woodward M, Stepien S, Tian M, Yin X, Hao Z, Li Z, Sun J, Yu Y, Zhou B, Zhao Y, Wu Y and Neal B, 2018. Spot urine samples compared with 24‐h urine samples for estimating changes in urinary sodium and potassium excretion in the China Salt Substitute and Stroke Study. International Journal of Epidemiology, 47, 1811–1820. [DOI] [PubMed] [Google Scholar]
- Hulthen L, Aurell M, Klingberg S, Hallenberg E, Lorentzon M and Ohlsson C, 2010. Salt intake in young Swedish men. Public Health Nutrition, 13, 601–605. [DOI] [PubMed] [Google Scholar]
- Ilich JZ, Brownbill RA and Coster DC, 2010. Higher habitual sodium intake is not detrimental for bones in older women with adequate calcium intake. European Journal of Applied Physiology, 109, 745–755. [DOI] [PubMed] [Google Scholar]
- Imbrici P, Altamura C, Pessia M, Mantegazza R, Desaphy JF and Camerino DC, 2015. ClC‐1 chloride channels: state‐of‐the‐art research and future challenges. Front Cell Neurosci, 9, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INTERSALT Cooperative Research Group , 1988. INTERSALT: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. INTERSALT Cooperative Research Group. British Medical Journal, 297, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine), 2005Dietary reference intakes for water, potassium, sodium, chloride and sulfate. Food and Nutrition Board. National Academies Press, Washington, DC, USA. 618 pp. [Google Scholar]
- IOM (Institute of Medicine), 2013. Sodium Intake in Populations: Assessment of Evidence. National Academies Press, Washington, DC, USA. 224 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, Fleenor BS, Lakatta EG, Bagrov AY and Seals DR, 2013. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clinical Journal of the American Society of Nephrology, 8, 1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T and Reid W, 2011. Fluid and electrolyte disorders In: Ahmed N. (ed.). Clinical Biochemistry. Oxford University Press, Oxford, UK: pp. 115–137. [Google Scholar]
- Ji C, Sykes L, Paul C, Dary O, Legetic B, Campbell NR and Cappuccio FP, Sub‐Group for R and Surveillance of the P‐WHOREGfCDPTP‐wDSR , 2012. Systematic review of studies comparing 24‐hour and spot urine collections for estimating population salt intake. Revista Panamericana de Salud Pública, 32, 307–315. [DOI] [PubMed]
- Ji C, Miller MA, Venezia A, Strazzullo P and Cappuccio FP, 2014. Comparisons of spot vs 24‐h urine samples for estimating population salt intake: Validation study in two independent samples of adults in Britain and Italy. Nutrition, Metabolism and Cardiovascular Diseases, 24, 140–147. [DOI] [PubMed] [Google Scholar]
- Johansson G, Bingham S and Vahter M, 1999. A method to compensate for incomplete 24‐hour urine collections in nutritional epidemiology studies. Public Health Nutrition, 2, 587–591. [DOI] [PubMed] [Google Scholar]
- John KA, Cogswell ME, Campbell NR, Nowson CA, Legetic B, Hennis AJ and Patel SM, 2016. Accuracy and usefulness of select methods for assessing complete collection of 24‐hour urine: a systematic review. Journal of Clinical Hypertension (Greenwich), 18, 456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johner SA, Thamm M, Schmitz R and Remer T, 2015. Current daily salt intake in German year: biomarker‐based analysis of the representative DEGS study. European Journal of Nutrition, 54, 1109–1115. [DOI] [PubMed] [Google Scholar]
- Johnson SR, Tomlinson GA, Hawker GA, Granton JT, Grosbein HA and Feldman B, 2010. A valid and reliable belief elicitation method for Bayesian priors. Journal of Clinical Epidemiology, 63, 370–383. [DOI] [PubMed] [Google Scholar]
- Joosten MM, Gansevoort RT, Mukamal KJ, Lambers Heerspink HJ, Geleijnse JM, Feskens EJ, Navis G and Bakker SJ and Group PS , 2014. Sodium excretion and risk of developing coronary heart disease. Circulation, 129, 1121–1128. [DOI] [PubMed] [Google Scholar]
- Kaptein EM, Sreeramoju D, Kaptein JM and Kaptein MJ, 2016. A systematic literature search and review of sodium concentrations of body fluids. Clinical Nephrology, 86, 203–228. [DOI] [PubMed] [Google Scholar]
- Kato A and Romero MF, 2011. Regulation of electroneutral NaCl absorption by the small intestine. Annual Review of Physiology, 73, 261–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Itoh K, Uezono K and Sasaki H, 1993. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clinical and Experimental Pharmacology and Physiology, 20, 7–14. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Harrington JM, Mc Carthy VJ, Fitzgerald AP and Perry IJ, 2013. Cohort profile: The Cork and Kerry Diabetes and Heart Disease Study. International Journal of Epidemiology, 42, 1253–1262. [DOI] [PubMed] [Google Scholar]
- Keenan BS, Buzek SW, Garza C, Potts E and Nichols BL, 1982. Diurnal and longitudinal variations in human milk sodium and potassium: implication for nutrition and physiology. American Journal of Clinical Nutrition, 35, 527–534. [DOI] [PubMed] [Google Scholar]
- Keenan BS, Buzek SW and Garza C, 1983. Cortisol and its possible role in regulation of sodium and potassium in human milk. American Journal of Physiology, 244, E253–E261. [DOI] [PubMed] [Google Scholar]
- Khaw KT and Barrett‐Connor E, 1990. Increasing sensitivity of blood pressure to dietary sodium and potassium with increasing age. A population study using casual urine specimens. American Journal of Hypertension, 3, 505–511. [DOI] [PubMed] [Google Scholar]
- Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ and Joosten MM, 2014. Urinary potassium excretion and risk of developing hypertension: the prevention of renal and vascular end‐stage disease study. Hypertension, 64, 769–776. [DOI] [PubMed] [Google Scholar]
- Kieneker LM, Eisenga MF, Gansevoort RT, de Boer RA, Navis G, Dullaart RPF, Joosten MM and Bakker SJL, 2018. Association of low urinary sodium excretion with increased risk of stroke. Mayo Clin Proceedings, 93, 1803–1809. [DOI] [PubMed] [Google Scholar]
- Kloss L, Meyer JD, Graeve L and Vetter W, 2015. Sodium intake and its reduction by food reformulation in the European Union — a review. NFS Journal, 1, 9–19. [Google Scholar]
- Kodama N, Morikuni E, Matsuzaki N, Yoshioka YH, Takeyama H, Yamada H, Kitajima H and Nishimuta M, 2005. Sodium and potassium balances in Japanese young adults. Journal of Nutritional Sciences and Vitaminology, 51, 161–168. [DOI] [PubMed] [Google Scholar]
- Koo WW and Gupta JM, 1982. Breast milk sodium. Archives of Disease in Childhood, 57, 500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC and Titze J, 2013. 23Na magnetic resonance imaging‐determined tissue sodium in healthy subjects and hypertensive patients. Hypertension, 61, 635–640. [DOI] [PubMed] [Google Scholar]
- Koppen G, Paulussen M, Van de Mieroop E, De Wolf M‐C, Godderis L, Uytterhoeven M and Stalpaert M, 2015. Estimation of salt intake by the Belgian population through analysis of sodium in 24‐hour urine. Report Number, 2015/MRG/R/0208. [Google Scholar]
- Kotchen TA, Welch WJ, Lorenz JN and Ott CE, 1987. Renal tubular chloride and renin release. Journal of Laboratory and Clinical Medicine, 110, 533–540. [PubMed] [Google Scholar]
- Kotchen TA and Kotchen JM, 1997. Dietary sodium and blood pressure: interactions with other nutrients. American Journal of Clinical Nutrition, 65, 708S–711S. [DOI] [PubMed] [Google Scholar]
- Kristbjornsdottir OK, Halldorsson TI, Thorsdottir I and Gunnarsdottir I, 2012. Association between 24‐hour urine sodium and potassium excretion and diet quality in six‐year‐old children: a cross‐sectional study. Nutrition Journal, 11, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout D, Spaaij CJ, de Goede J and Weggemans RM, 2016. The 2015 Dutch food‐based dietary guidelines. European Journal of Clinical Nutrition, 70, 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp D, Shi L, Egert S, Wudy SA and Remer T, 2015. Prospective relevance of fruit and vegetable consumption and salt intake during adolescence for blood pressure in young adulthood. European Journal of Nutrition, 54, 1269–1279. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK, Hebert PR, Cutler JA, Lasser VI, Sugars CP, Steffen‐Batey L, Brewer AA, Cameron M, Shepek LD, Cook NR, et al., 1993. Feasibility and efficacy of sodium reduction in the Trials of Hypertension Prevention, Phase I. Trials of Hypertension Prevention Collaborative Research Group. Hypertension, 22, 502–512. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK, Cook NR, Cutler JA, Belden L, Brewer A, Cohen JD, Hebert PR, Lasser VI, Raines J, Raczynski J, Shepek L, Diller L, Whelton PK and Yamamoto M and Trials of Hypertension Prevention Collaborative Research G , 2005. Sodium reduction for hypertension prevention in overweight adults: further results from the Trials of Hypertension Prevention Phase II. Journal of Human Hypertension, 19, 33–45. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Al‐Bander HA and Morris RC Jr, 1987. “Salt‐sensitive” essential hypertension in men. Is the sodium ion alone important? New England Journal of Medicine, 317, 1043–1048. [DOI] [PubMed] [Google Scholar]
- Laatikainen T, Pietinen P, Valsta L, Sundvall J, Reinivuo H and Tuomilehto J, 2006. Sodium in the Finnish diet: 20–year trends in urinary sodium excretion among the adult population. European Journal of Clinical Nutrition, 60, 965–970. [DOI] [PubMed] [Google Scholar]
- Lang S, Lawrence CJ and Orme RL, 1994. Sodium in hand and pump expressed human breast milk. Early Human Development, 38, 131–138. [DOI] [PubMed] [Google Scholar]
- Laragh JH, Sealey J and Brunner HR, 1972. The control of aldosterone secretion in normal and hypertensive man: abnormal renin‐aldosterone patterns in low renin hypertension. American Journal of Medicine, 53, 649–663. [DOI] [PubMed] [Google Scholar]
- Laragh JH and Sealey JE, 2011. The plasma renin test reveals the contribution of body sodium‐volume content (V) and renin–angiotensin (R) vasoconstriction to long‐term blood pressure. American Journal of Hypertension, 24, 1164–1180. [DOI] [PubMed] [Google Scholar]
- LASER Analytica , 2014. Comprehensive literature search and review of breast milk composition as preparatory work for the setting of dietary reference values for vitamins and minerals. EFSA supporting publication 2014:EN‐629, 154 pp. Available online: http://www.efsa.europa.eu/en/supporting/pub/en-629 [Google Scholar]
- Law MR, Frost CD and Wald NJ, 1991a. By how much does dietary salt reduction lower blood pressure? I – Analysis of observational data among populations. British Medical Journal, 302, 811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MR, Frost CD and Wald NJ, 1991b. By how much does dietary salt reduction lower blood pressure? III – Analysis of data from trials of salt reduction. British Medical Journal, 302, 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq C and Ferro‐Luzzi A, 1991. Total and domestic consumption of salt and their determinants in three regions of Italy. European Journal of Clinical Nutrition, 45, 151–159. [PubMed] [Google Scholar]
- Lelli D, Antonelli‐Incalzi R, Bandinelli S, Ferrucci L and Pedone C, 2018. Association between sodium excretion and cardiovascular disease and mortality in the elderly year: a cohort study. Journal of the American Medical Directors Association, 19, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchl K, Rakova N, Dahlmann A, Rauh M, Goller U, Basner M, Dinges DF, Beck L, Agureev A, Larina I, Baranov V, Morukov B, Eckardt KU, Vassilieva G, Wabel P, Vienken J, Kirsch K, Johannes B, Krannich A, Luft FC and Titze J, 2015. Agreement between 24‐hour salt ingestion and sodium excretion in a controlled environment. Hypertension, 66, 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan R, Fordtran JS, Burrows BA and Ingelfinger FJ, 1962. Water and salt absorption in the human colon. Journal of Clinical Investigation, 41, 1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KA, Madden A, Tammam J, Tzilivakis J and Vafeiadou K (University of Hertfordshire, UK), 2015Final evidence report as part of preparatory work for the setting of dietary reference values for sodium and chloride. EFSA supporting publication 2015:EN‐69, 88 pp. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2015.EN-691 [Google Scholar]
- Lide DR, 2009. CRC Handbook of Chemistry and Physics. CRC Press, Boca Raton, USA. [Google Scholar]
- Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, Barclay D and Svetkey LP, 2003. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. Journal of Nutrition, 133, 3130–3136. [DOI] [PubMed] [Google Scholar]
- Lopez‐Sobaler A and Quintas E, 2006. Anthropometric survey In: Requejo A. and Ortega R. (eds.). Nutriguia. Manual of Clinical Nutrition in Primary Care, Complutense, Madrid, Spain: pp. 346–352. [Google Scholar]
- Lowell BB, 2019. New Neuroscience of Homeostasis and Drives for Food, Water, and Salt. New England Journal of Medicine, 380, 459–471. [DOI] [PubMed] [Google Scholar]
- Lucko AM, Doktorchik C, Woodward M, Cogswell M, Neal B, Rabi D, Anderson C, He FJ, MacGregor GA, L'Abbe M, Arcand J, Whelton PK, McLean R and Campbell NRC and Consortium T, 2018. Percentage of ingested sodium excreted in 24‐hour urine collections: a systematic review and meta‐analysis. Journal of Clinical Hypertension (Greenwich), 20, 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft FC, Rankin LI, Bloch R, Grim CE, Weyman AE, Murray RH and Weinberger MH, 1979. Plasma and urinary norepinephrine values at extremes of sodium intake in normal man. Hypertension, 1, 261–266. [DOI] [PubMed] [Google Scholar]
- Luft FC, Fineberg NS and Sloan RS, 1982. Estimating dietary sodium intake in individuals receiving a randomly fluctuating intake. Hypertension, 4, 805–808. [DOI] [PubMed] [Google Scholar]
- Luft FC, Zemel MB, Sowers JA, Fineberg NS and Weinberger MH, 1990. Sodium bicarbonate and sodium chloride: effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. Journal of Hypertension, 8, 663–670. [DOI] [PubMed] [Google Scholar]
- Lupoli S, Salvi E and Barlassina C, 2013. Dietary salt intake, blood pressure and genes. Current Nutrition Reports, 2, 134–141. [Google Scholar]
- Luzardo L, Noboa O and Boggia J, 2015. Mechanisms of salt‐sensitive hypertension. Current Hypertension Reviews, 11, 14–21. [DOI] [PubMed] [Google Scholar]
- MacGregor GA, Markandu ND, Best FE, Elder DM, Cam JM, Sagnella GA and Squires M, 1982. Double‐blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet, 1, 351–355. [DOI] [PubMed] [Google Scholar]
- MacGregor GA, Markandu ND, Sagnella GA, Singer DR and Cappuccio FP, 1989. Double‐blind study of three sodium intakes and long‐term effects of sodium restriction in essential hypertension. Lancet, 2, 1244–1247. [DOI] [PubMed] [Google Scholar]
- MacGregor GA and de Wardener HE, 1999. Salt, diet, and health. Lancet, 353, 1709–1710.10335816 [Google Scholar]
- Manganaro R, Marseglia L, Mami C, Palmara A, Paolata A, Loddo S, Gargano R, Mondello M and Gemelli M, 2007. Breast milk sodium concentration, sodium intake and weight loss in breast‐feeding newborn infants. British Journal of Nutrition, 97, 344–348. [DOI] [PubMed] [Google Scholar]
- McCallum L, Lip S and Padmanabhan S, 2015. The hidden hand of chloride in hypertension. Pflügers Archiv: European. Journal of Physiology, 467, 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance RA, 1936. Experimental sodium chloride deficiency in man. Proceedings of the Royal Society of London, 814, 245–268. [DOI] [PubMed] [Google Scholar]
- McCarron DA, 1998. Diet and blood pressure–the paradigm shift. Science, 281, 933–934. [DOI] [PubMed] [Google Scholar]
- McLean RM, Farmer VL, Nettleton A, Cameron CM, Cook NR and Campbell NRC and Consortium T , 2017. Assessment of dietary sodium intake using a food frequency questionnaire and 24‐hour urinary sodium excretion: a systematic literature review. Journal of Clinical Hypertension (Greenwich), 19, 1214–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean RM, Farmer VL, Nettleton A, Cameron CM, Cook NR, Woodward M and Campbell NRC and Consortium T , 2018. Twenty‐four‐hour diet recall and diet records compared with 24‐hour urinary excretion to predict an individual's sodium consumption: a systematic review. Journal of Clinical Hypertension (Greenwich), 20, 1360–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meland E, Laerum E, Aakvaag A, Ulvik RJ and Hostmark AT, 1997. Salt restriction: effects on lipids and insulin production in hypertensive patients. Scandinavian Journal of Clinical Laboratory Investigation, 57, 501–505. [DOI] [PubMed] [Google Scholar]
- Melander O, von Wowern F, Frandsen E, Burri P, Willsteen G, Aurell M and Hulthen UL, 2007. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N‐terminal atrial natriuretic peptide in plasma. Journal of Hypertension, 25, 619–627. [DOI] [PubMed] [Google Scholar]
- Mente A, O'Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez‐Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S and Investigators P, 2014. Association of urinary sodium and potassium excretion with blood pressure. New England Journal of Medicine, 371, 601–611. [DOI] [PubMed] [Google Scholar]
- Mertz W, 1987. Use and misuse of balance studies. Journal of Nutrition, 117, 1811–1813. [DOI] [PubMed] [Google Scholar]
- Miller JZ, Weinberger MH, Daugherty SA, Fineberg NS, Christian JC and Grim CE, 1988. Blood pressure response to dietary sodium restriction in healthy normotensive children. American Journal of Clinical Nutrition, 47, 113–119. [DOI] [PubMed] [Google Scholar]
- Moor MB and Bonny O, 2016. Ways of calcium reabsorption in the kidney. American Journal of Physiology. Renal Physiology, 310, F1337–F1350. [DOI] [PubMed] [Google Scholar]
- Morgan K, McGee H, Watson DG, Perry IJ and Barry M (Department of Health and Children), 2008. SLAN 2007: Survey of Lifestyle, Attitudes & Nutrition in Ireland: Main Report. 159 pp.
- Morimatsu H, Rocktaschel J, Bellomo R, Uchino S, Goldsmith D and Gutteridge G, 2003. Comparison of point‐of‐care versus central laboratory measurement of electrolyte concentrations on calculations of the anion gap and the strong ion difference. Anesthesiology, 98, 1077–1084. [DOI] [PubMed] [Google Scholar]
- Morton JA, 1994. The clinical usefulness of breast milk sodium in the assessment of lactogenesis. Pediatrics, 93, 802–806. [PubMed] [Google Scholar]
- Motil KJ, Kertz B and Thotathuchery M, 1997. Lactational performance of adolescent mothers shows preliminary differences from that of adult women. Journal of Adolescent Health, 20, 442–449. [DOI] [PubMed] [Google Scholar]
- Murtaugh MA, Beasley JM, Appel LJ, Guenther PM, McFadden M, Greene T and Tooze JA, 2018. Relationship of sodium intake and blood pressure varies with energy intake: secondary analysis of the DASH (Dietary Approaches to Stop Hypertension) – sodium trial. Hypertension, 71, 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASEM (National Academies of Sciences, Engineering, and Medicine), 2019. Dietary Reference Intakes for Sodium and Potassium. The National Academies Press, Washington, DC, USA: 498 pp. Available online: https://www.nap.edu/read/25353/chapter/1 [PubMed] [Google Scholar]
- National Institute for Health and Welfare , 2016. Fineli. National Food Composition Database in Finland. Available online: https://fineli.fi/fineli/en/index
- Nestel PJ, Clifton PM, Noakes M, McArthur R and Howe PR, 1993. Enhanced blood pressure response to dietary salt in elderly women, especially those with small waist:hip ratio. Journal of Hypertension, 11, 1387–1394. [DOI] [PubMed] [Google Scholar]
- Ni Mhurchu C, Capelin C, Dunford EK, Webster JL, Neal BC and Jebb SA, 2011. Sodium content of processed foods in the United Kingdom: analysis of 44,000 foods purchased by 21,000 households. American Journal of Clinical Nutrition, 93, 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimuta M, Kodama N, Morikuni E, Yoshioka YH, Matsuzaki N, Takeyama H, Yamada H and Kitajima H, 2005. Positive correlation between dietary intake of sodium and balances of calcium and magnesium in young Japanese adults – low sodium intake is a risk factor for loss of calcium and magnesium. Journal of Nutritional Science and Vitaminology (Tokyo), 51, 265–270. [DOI] [PubMed] [Google Scholar]
- Nishimuta M, Inoue N, Kodama N, Moriknni E, Yoshioka YH, Matsuzaki N, Shimada M, Sato N, Iwamoto T, Ohki K, Takeyama H and Nishimuta H, 2006. Moisture and mineral content of human feces—high fecal moisture is associated with increased sodium and decreased potassium content. Journal of Nutritional Science and Vitaminology (Tokyo), 52, 121–126. [DOI] [PubMed] [Google Scholar]
- Nishimuta M, Kodama N, Shimada M, Yoshitake Y, Matsuzaki N and Morikuni E, 2012. Estimated equilibrated dietary intakes for nine minerals (Na, K, Ca, Mg, P, Fe, Zn, Cu, and Mn) adjusted by mineral balance medians in young Japanese females. Journal of Nutritional Science and Vitaminology (Tokyo), 58, 118–128. [DOI] [PubMed] [Google Scholar]
- Nishimuta M, Kodama N, Yoshitake Y, Shimada M and Serizawa N, 2018. Dietary salt (sodium chloride) requirement and adverse effects of salt restriction in humans. Journal of Nutritional Science and Vitaminology (Tokyo), 64, 83–89. [DOI] [PubMed] [Google Scholar]
- Nordic Council of Ministers , 2014Nordic Nutrition Recommendations 2012. Integrating Nutrition and Physical Activity. 5th edition. 627 pp.
- Nordin BE and Polley KJ, 1987. Metabolic consequences of the menopause. A cross‐sectional, longitudinal, and intervention study on 557 normal postmenopausal women. Calcified Tissue International, 41 Suppl 1, S1–S59. [PubMed] [Google Scholar]
- Nowson CA, Morgan TO and Gibbons C, 2003. Decreasing dietary sodium while following a self‐selected potassium‐rich diet reduces blood pressure. Journal of Nutrition, 133, 4118–4123. [DOI] [PubMed] [Google Scholar]
- O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez‐Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S and Investigators PURE, 2014. Urinary sodium and potassium excretion, mortality, and cardiovascular events. New England Journal of Medicine, 371, 612–623. [DOI] [PubMed] [Google Scholar]
- O'Donnell M, Mente A, Rangarajan S, McQueen MJ, O'Leary N, Yin L, Liu X, Swaminathan S, Khatib R, Rosengren A, Ferguson J, Smyth A, Lopez‐Jaramillo P, Diaz R, Avezum A, Lanas F, Ismail N, Yusoff K, Dans A, Iqbal R, Szuba A, Mohammadifard N, Oguz A, Yusufali AH, Alhabib KF, Kruger IM, Yusuf R, Chifamba J, Yeates K, Dagenais G, Wielgosz A, Lear SA, Teo K, Yusuf S and Investigators P, 2019. Joint association of urinary sodium and potassium excretion with cardiovascular events and mortality: prospective cohort study. British Medical Journal, 364, l772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHAT/NTP (Office of Health Assessment and Translation, Division of the National Toxicology Program), 2015. OHAT Risk of Bias Rating Tool for Human and Animal Studies. 37 pp.
- Okayama A, Okuda N, Miura K, Okamura T, Hayakawa T, Akasaka H, Ohnishi H, Saitoh S, Arai Y, Kiyohara Y, Takashima N, Yoshita K, Fujiyoshi A, Zaid M, Ohkubo T and Ueshima H and the NIPPON DATA80 Research Group , 2016. Dietary sodium‐to‐potassium ratio as a risk factor for stroke, cardiovascular disease and all‐cause mortality in Japan: the NIPPON DATA80 cohort study. British Medical Journal Open, 6, e011632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde Engberink RH, Rorije NM, Homan van der Heide JJ, van den Born BJ and Vogt L, 2015. Role of the vascular wall in sodium homeostasis and salt sensitivity. Journal of the American Society of Nephrology, 26, 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver WJ, Neel JV, Grekin RJ and Cohen EL, 1981. Hormonal adaptation to the stresses imposed upon sodium balance by pregnancy and lactation in the Yanomama Indians, a culture without salt. Circulation, 63, 110–116. [DOI] [PubMed] [Google Scholar]
- Ortega RM, Lopez‐Sobaler AM, Ballesteros JM, Perez‐Farinos N, Rodriguez‐Rodriguez E, Aparicio A, Perea JM and Andres P, 2011. Estimation of salt intake by 24 h urinary sodium excretion in a representative sample of Spanish adults. British Journal of Nutrition, 105, 787–794. [DOI] [PubMed] [Google Scholar]
- Osredkar J, 1998. Laboratorijske preiskave – Biokemične preiskave urina In: Kocijancic A. and Mrevlje F. (eds.). Interna Medicina, 2nd edition Ljubljana, Slovenia: pp. 1228–1230. [Google Scholar]
- Padmanabhan S, Caulfield M and Dominiczak AF, 2015. Genetic and molecular aspects of hypertension. Circulation Research, 116, 937–959. [DOI] [PubMed] [Google Scholar]
- Palacios C, Wigertz K, Martin BR, Jackman L, Pratt JH, Peacock M, McCabe G and Weaver CM, 2004. Sodium retention in black and white female adolescents in response to salt intake. The Journal of Clinical Endocrinology and Metabolism, 89, 1858–1863. [DOI] [PubMed] [Google Scholar]
- Pan W, Borovac J, Spicer Z, Hoenderop JG, Bindels RJ, Shull GE, Doschak MR, Cordat E and Alexander RT, 2012. The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re)absorption. American Journal of Physiology, 312, F943–F956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parijs J, Joossens JV, Van der Linden L, Verstreken G and Amery AK, 1973. Moderate sodium restriction and diuretics in the treatment of hypertension. American Heart Journal, 85, 22–34. [DOI] [PubMed] [Google Scholar]
- Parr RM, DeMaeyer EM, Iyengar VG, Byrne AR, Kirkbright GF, Schoch G, Niinisto L, Pineda O, Vis HL and Hofvander Y, 1991. Minor and trace elements in human milk from Guatemala, Hungary, Nigeria, Philippines, Sweden, and Zaire. Results from a WHO/IAEA joint project. Biological Trace Element Research, 29, 51–75. [DOI] [PubMed] [Google Scholar]
- Penney MD, 2008. Sodium. Water and Potassium, Elsevier Health Sciences. [Google Scholar]
- Perez V and Chang ET, 2014. Sodium‐to‐potassium ratio and blood pressure, hypertension, and related factors. Advances in Nutrition, 5, 712–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ and Khaw KT, 2014. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC‐Norfolk study. European Journal of Heart Failure, 16, 394–402. [DOI] [PubMed] [Google Scholar]
- Pitkin RM, Kaminetzky HA, Newton M and Pritchard JA, 1972. Maternal nutrition. A selective review of clinical topics. Obstetrics and Gynecology, 40, 773–785. [PubMed] [Google Scholar]
- Pitts R, 1974. Volume and composition of body fluids. In: Pitts R (ed.). Physiology of the Kidney and Body Fluids. An Introductory Text. 3rd edition, Mosby, Chicago, USA. pp. 11–35.
- Polonia J, Lobo MF, Martins L, Pinto F and Nazare J, 2017. Estimation of populational 24‐h urinary sodium and potassium excretion from spot urine samples: evaluation of four formulas in a large national representative population. Journal of Hypertension, 35, 477–486. [DOI] [PubMed] [Google Scholar]
- Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G and Mozaffarian D and Global Burden of Diseases Nutrition and Chronic Diseases Expert Group , 2013. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. British Medical Journal Open, 3, e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premužić V, Erceg I, Jovanović A, Reiner Ž and Jelaković B, 2010. Unos soli u odrasloj populaciji Second International Symposium on Hypertension, Translational Medicine in Hypertension (18–21 November 2010 Osijek, Croatia) and Croatian‐Hungarian Young Investigator Conference (17–18 November 2010 Pécs. Hungary/Osijek, Croatia) pp. 109–110. [Google Scholar]
- Prince R, Devine A, Dick I, Criddle A, Kerr D, Kent N, Price R and Randell A, 1995. The effects of calcium supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. Journal of Bone and Mineral Research, 10, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Puska P, Nissinen A, Vartiainen E, Dougherty R, Mutanen M, Iacono J, Korhonen H, Pietinen P, Leino U, Moisio S and Huttunen J, 1983. Controlled, randomised trial of the effect of dietary fat on blood pressure. The Lancet, 321, 1–5. [DOI] [PubMed] [Google Scholar]
- Rakova N, Juttner K, Dahlmann A, Schroder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, Vassilieva G, Lenkova L, Johannes B, Wabel P, Moissl U, Vienken J, Gerzer R, Eckardt KU, Müller DN, Kirsch K, Morukov B, Luft FC and Titze J, 2013. Long‐term space flight simulation reveals infradian rhythmicity in human Na+ balance. Cell Metabolism, 17, 125–131. [DOI] [PubMed] [Google Scholar]
- Rakova N, Kitada K, Lerchl K, Dahlmann A, Birukov A, Daub S, Kopp C, Pedchenko T, Zhang Y, Beck L, Johannes B, Marton A, Müller DN, Rauh M, Luft FC and Titze J, 2017. Increased salt consumption induces body water conservation and decreases fluid intake. Journal of Clinical Investigation, 127, 1932–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer T, Neubert A and Maser‐Gluth C, 2002. Anthropometry‐based reference values for 24‐h urinary creatinine excretion during growth and their use in endocrine and nutritional research. American Journal of Clinical Nutrition, 75, 561–569. [DOI] [PubMed] [Google Scholar]
- Ribic CH, Zakotnik JM, Vertnik L, Vegnuti M and Cappuccio FP, 2010. Salt intake of the Slovene population assessed by 24 h urinary sodium excretion. Public Health Nutrition, 13, 1803–1809. [DOI] [PubMed] [Google Scholar]
- Richards AM, Espiner E, Nicholls MG, Ikram H, Maslowski A, Hamilton E and Wells JE, 1984. Blood‐pressure response to moderate sodium restriction and to potassium supplementation in mild essential hypertension. Lancet, 323, 757–761. [DOI] [PubMed] [Google Scholar]
- Ross B, Cowett RM and Oh W, 1977. Renal functions of low birth weight infants during the first two months of life. Pediatric Research, 11, 1162–1164. [DOI] [PubMed] [Google Scholar]
- Ruppert M, Overlack A, Kolloch R, Kraft K, Göbel B and Stumpe KO, 1993. Neurohormonal and metabolic effects of severe and moderate salt restriction in non‐obese normotensive adults. Journal of Hypertension, 11, 743–749. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons‐Morton DG, Karanja N and Lin PH and Group DA‐SCR , 2001. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. New England Journal of Medicine, 344, 3–10. [DOI] [PubMed] [Google Scholar]
- SACN (Scientific Advisory Committee on Nutrition), 2003. Salt and Health Report. 134 pp.
- Sadler K, Nicholson S, Steer T, Gill V, Bates B, Tipping S, Cox L, Lennox A and Prentice A (UK Department of Health), 2011. National Diet and Nutrition Survey – Assessment of dietary sodium in adults (aged 19 to 64 years) in England, 2011. 32 pp.
- Safar ME, Temmar M, Kakou A, Lacolley P and Thornton SN, 2009. Sodium intake and vascular stiffness in hypertension. Hypertension, 54, 203–209. [DOI] [PubMed] [Google Scholar]
- Sandle GI, 1998. Salt and water absorption in the human colon: a modern appraisal. Gut, 43, 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A, Martins MJ, Guimaraes JT, Severo M and Azevedo I, 2010. Sodium‐rich carbonated natural mineral water ingestion and blood pressure. Revista Portuguesa de Cardiologia, 29, 159–172. [PubMed] [Google Scholar]
- Savilahti E and Saarinen KM, 2007. Colostrum TGF‐beta‐1 associates with the duration of breast‐feeding. European Journal of Nutrition, 46, 238–242. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1993. Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food, 31st Series. Food – Science and Technique, European Commission, Luxembourg. 255 pp. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out89.pdf
- SCF (Scientific Committee on Food), 2000. Guidelines of the Scientific Committee on Food for the Development of Tolerable Upper Intake Levels for Vitamins and Minerals. 11 pp. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out80a_en.pdf
- SCF (Scientific Committee on Food), 2003. Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow‐on Formulae. SCF/CS/NUT/IF/65 Final, 213 pp. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/labelling_nutrition-special_groups_food-children-out199_en.pdf
- Schachter J, Harper PH, Radin ME, Caggiula AW, McDonald RH and Diven WF, 1980. Comparison of sodium and potassium intake with excretion. Hypertension, 2, 695–699. [DOI] [PubMed] [Google Scholar]
- Schafer JA, 2002. Abnormal regulation of ENaC: syndromes of salt retention and salt wasting by the collecting duct. American Journal of Physiology. Renal Physiology, 283, F221–F235. [DOI] [PubMed] [Google Scholar]
- Schorr U, Distler A and Sharma AM, 1996. Effect of sodium chloride‐ and sodium bicarbonate‐rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: a randomized double‐blind crossover trial. Journal of Hypertension, 14, 131–135. [PubMed] [Google Scholar]
- Selvarajah V, Maki‐Petaja KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, Brown MJ, McEniery CM and Wilkinson IB, 2017. Novel mechanism for buffering dietary salt in humans: effects of salt loading on skin sodium, vascular endothelial growth factor C, and blood pressure. Hypertension, 70, 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RL, 2006. Role of sodium in fluid homeostasis with exercise. Journal of the American College of Nutrition, 25, 231S–239S. [DOI] [PubMed] [Google Scholar]
- Shi L, Krupp D and Remer T, 2014. Salt, fruit and vegetable consumption and blood pressure development: a longitudinal investigation in healthy children. British Journal of Nutrition, 111, 662–671. [DOI] [PubMed] [Google Scholar]
- Shore AC, Markandu ND and MacGregor GA, 1988. A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. Journal of Hypertension, 6, 613–617. [DOI] [PubMed] [Google Scholar]
- Siani A, Iacoviello L, Giorgione N, Iacone R and Strazzullo P, 1989. Comparison of variability of urinary sodium, potassium, and calcium in free‐living men. Hypertension, 13, 38–42. [DOI] [PubMed] [Google Scholar]
- Singer DR, Markandu ND, Sugden AL, Miller MA and MacGregor GA, 1991. Sodium restriction in hypertensive patients treated with a converting enzyme inhibitor and a thiazide. Hypertension, 17, 798–803. [DOI] [PubMed] [Google Scholar]
- SINU (Società Italiana di Nutrizione Umana), 2014. LARN Livelli di Assunzione di Riferimento di Nutrienti ed energia per la popolazione italiana IV Revisione. 656 pp.
- Solocinski K and Gumz ML, 2015. The circadian clock in the regulation of renal rhythms. Journal of Biological Rhythms, 30, 470–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speedy DB, Rogers IR, Noakes TD, Thompson JM, Guirey J, Safih S and Boswell DR, 2000. Diagnosis and prevention of hyponatremia at an ultradistance triathlon. Clinical Journal of Sport Medicine, 10, 52–58. [DOI] [PubMed] [Google Scholar]
- Stamler J, Applegate WB, Cohen JD, Cutler JA and Whelton PK, 1997. More on dietary sodium and blood pressure. JAMA –. Journal of the American Medical Association, 277, 1594–1595. [PubMed] [Google Scholar]
- Sterns RH, 2015. Disorders of plasma sodium – causes, consequences, and correction. New England Journal of Medicine, 372, 55–65. [DOI] [PubMed] [Google Scholar]
- Stolarz‐Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovsky J, Kawecka‐Jaszcz K, Nikitin Y and Staessen JA and the European Project on Genes in Hypertension I , 2011. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. Journal of the American Medical Association, 305, 1777–1785. [DOI] [PubMed] [Google Scholar]
- Strauss MB, Lamdin E, Smith WP and Bleifer DJ, 1958. Surfeit and deficit of sodium; a kinetic concept of sodium excretion. AMA Archives of Internal Medicine, 102, 527–536. [DOI] [PubMed] [Google Scholar]
- Strazzullo P, Siani A and Russo P, 2000. Salt‐sensitivity of blood pressure: a paradigm of gene–environment interaction. Italian Heart Journal, 1(Suppl 3), S15–S19. [PubMed] [Google Scholar]
- Strohm D, Boeing H, Leschik‐onnet E, Heseker H, Arens‐Azevêdo U, Bechthold A, Knorpp L and Kroke A, 2016. Salt intake in Germany, health consequences, and resulting recommendations for action. A scientific statement from the German Nutrition Society (DGE). Ernahrungs Umschau, 63, 62–70. [Google Scholar]
- Strohm D, Bechthold A, Ellinger S, Leschik‐Bonnet E, Stehle P and Heseker H and the German Nutrition Society , 2018. Revised reference values for the intake of sodium and chloride. Annals of Nutrition and Metabolism, 72, 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swales J, 1999. Salt, diet, and health – reply. Lancet, 353, 1710–1710.10335817 [Google Scholar]
- Swift PA, Markandu ND, Sagnella GA, He FJ and MacGregor GA, 2005. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension, 46, 308–312. [DOI] [PubMed] [Google Scholar]
- Tabara Y, Takahashi Y, Setoh K, Kawaguchi T, Kosugi S, Nakayama T and Matsuda F and the Nagahama Study Group , 2017. Prognostic significance of spot urine Na/K for longitudinal changes in blood pressure and renal function: the Nagahama Study. American Journal of Hypertension, 30, 899–906. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H and Hashimoto T, 2002. A simple method to estimate populational 24‐h urinary sodium and potassium excretion using a casual urine specimen. Journal of Human Hypertension, 16, 97–103. [DOI] [PubMed] [Google Scholar]
- Taubes C, 1998. The (Political) Science of Salt. Science, 281, 898–907. [DOI] [PubMed] [Google Scholar]
- Teucher B, Dainty JR, Spinks CA, Majsak‐Newman G, Berry DJ, Hoogewerff JA, Foxall RJ, Jakobsen J, Cashman KD, Flynn A and Fairweather‐Tait SJ, 2008. Sodium and bone health: impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. Journal of Bone and Mineral Research, 23, 1477–1485. [DOI] [PubMed] [Google Scholar]
- The Trials of Hypertension Collaborative Research Group , 1997. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure: The Trials of Hypertension Prevention, Phase II. Archives of Internal Medicine, 157, 657–667. [PubMed] [Google Scholar]
- Thompson SG and Higgins JP, 2002. How should meta‐regression analyses be undertaken and interpreted? Statistics in Medicine, 21, 1559–1573. [DOI] [PubMed] [Google Scholar]
- Titze J, 2015. A different view on sodium balance. Current Opinion in Nephrology and Hypertension, 24, 14–20. [DOI] [PubMed] [Google Scholar]
- Titze J, Dahlmann A, Lerchl K, Kopp C, Rakova N, Schroder A and Luft FC, 2014. Spooky sodium balance. Kidney International, 85, 759–767. [DOI] [PubMed] [Google Scholar]
- Truchet S and Honvo‐Houeto E, 2017. Physiology of milk secretion. Best Practice & Research: Clinical Endocrinology & Metabolism, 31, 367–384. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P and Nissinen A, 2001. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet, 357, 848–851. [DOI] [PubMed] [Google Scholar]
- Turnberg LA, Bieberdorf FA, Morawski SG and Fordtran JS, 1970. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. Journal of Clinical Investigation, 49, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Food Standards Agency , 2002. McCance and Widdowson's The Composition of Foods. Book series.
- van Berge‐Landry H and James GD, 2004. Serum electrolyte, serum protein, serum fat and renal responses to a dietary sodium challenge: allostasis and allostatic load. Annals of Human Biology, 31, 477–487. [DOI] [PubMed] [Google Scholar]
- Vasara E, Marakis G, Breda J, Skepastianos P, Hassapidou M, Kafatos A, Rodopaios N, Koulouri AA and Cappuccio FP, 2017. Sodium and potassium intake in healthy adults in Thessaloniki greater metropolitan area – the salt intake in northern Greece. (SING) Study. Nutrients, 9, pii, E417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons‐Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ and Karanja N and Group DA‐STCR , 2001. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH‐sodium trial. Annals of Internal Medicine, 135, 1019–1028. [DOI] [PubMed] [Google Scholar]
- Wack RP, Lien EL, Taft D and Roscelli JD, 1997. Electrolyte composition of human breast milk beyond the early postpartum period. Nutrition, 13, 774–777. [DOI] [PubMed] [Google Scholar]
- Wang CY, Cogswell ME, Loria CM, Chen TC, Pfeiffer CM, Swanson CA, Caldwell KL, Perrine CG, Carriquiry AL, Liu K, Sempos CT, Gillespie CD and Burt VL, 2013. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24‐hour calibration study. Journal of Nutrition, 143, 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt GC, Edwards C, Hart JT, Hart M, Walton P and Foy CJ, 1983. Dietary sodium restriction for mild hypertension in general practice. British Medical Journal (Clinical Research Edition), 286, 432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt GC, Foy CJ, Hart JT, Bingham G, Edwards C, Hart M, Thomas E and Walton P, 1985. Dietary sodium and arterial blood pressure: evidence against genetic susceptibility. British Medical Journal (Clinical Research Edition), 291, 1525–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CM, Martin BR, McCabe GP, McCabe LD, Woodward M, Anderson CA and Appel LJ, 2016. Individual variation in urinary sodium excretion among adolescent girls on a fixed intake. Journal of Hypertension, 34, 1290–1297. [DOI] [PubMed] [Google Scholar]
- Webster JL, Dunford EK and Neal BC, 2010. A systematic survey of the sodium contents of processed foods. American Journal of Clinical Nutrition, 91, 413–420. [DOI] [PubMed] [Google Scholar]
- Weinberger MH, 1996. Salt sensitivity of blood pressure in humans. Hypertension, 27, 481–490. [DOI] [PubMed] [Google Scholar]
- Weinberger MH, 1997. Sodium, potassium, and blood pressure. American Journal of Hypertension, 10, S46–S48. [PubMed] [Google Scholar]
- Whelton PK, Kumanyika SK, Cook NR, Cutler JA, Borhani NO, Hennekens CH, Kuller LH, Langford H, Jones DW, Satterfield S, Lasser NL and Cohen JD, 1997. Efficacy of nonpharmacologic interventions in adults with high‐normal blood pressure: results from phase 1 of the Trials of Hypertension Prevention. Trials of Hypertension Prevention Collaborative Research Group. American Journal of Clinical Nutrition, 65, 652S–660S. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2003. Sodium drinking‐water. Background document for development WHO Guidelines for Drinking‐water. Quality., WHO/SDE/WSH/03.04/15, 6 pp. [Google Scholar]
- WHO (World Health Organization), 2011. Strategies to monitor and evaluate population sodium consumption and sources of sodium in the diet. Report of a joint technical meeting convened by WHO and the Government of Canada. Canada, October 2010. 40 pp.
- WHO (World Health Organization), 2012a. Effect of reduced sodium intake on cardiovascular disease, coronary heart disease and stroke. 77 pp.
- WHO (World Health Organization), 2012b. Guideline: sodium intake for adults and children. 46 pp. [PubMed]
- WHO (World Health Organization), 2012c. Effect of reduced sodium intake on blood pressure, renal functions, blood lipids and other potential adverse effects. p. 150.
- Widdowson EM, 1980. Adventures in nutrition over half a century. Proceedings of the Nutrition Society, 39, 293–306. [DOI] [PubMed] [Google Scholar]
- Widdowson EM, 1982. Importance of nutrition in development, with special reference to feeding low‐birth‐weight infants In: Sauls H, Bachhuber W. and Lewis L. (eds.). Meeting Nutritional Goals for Low‐Birth‐Weight Infants. Columbus, OH: pp. 4–11. [Google Scholar]
- Wielgosz A, Robinson C, Mao Y, Jiang Y, Campbell NR, Muthuri S and Morrison H, 2016. The impact of using different methods to assess completeness of 24‐hour urine collection on estimating dietary sodium. Journal of Clinical Hypertension (Greenwich), 18, 581–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser ME, Holden N, Coplen TB, Böhlke JK, Berglund M, Brand WA, De Bièvre P, Gröning M, Loss RD, Meija J, Hirata T, Prohaska T, Schoenberg R, O'Connor G, Walczyk T, Yoneda S and Zhu X‐K, 2013. Atomic weights of the elements 2011 (IUPAC Technical Report). Pure and Applied Chemistry, 85, 1047–1078. [Google Scholar]
- Wintour E, 1998. Water and electrolyte metabolism in the fetal placental unit In: Cowett R. (ed.). Principles of Perinatal‐Neonatal Metabolism. Springer‐Verlag, New York, USA: pp. 511–534. [Google Scholar]
- Young VR, 1986. Nutritional balance studies: indicators of human requirements or of adaptive mechanisms? Journal of Nutrition, 116, 700–703. [DOI] [PubMed] [Google Scholar]
- Yu ASL, 2015. Claudins and the kidney. Journal of the American Society of Nephrology: JASN, 26, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunos NM, Bellomo R, Story D and Kellum J, 2010. Bench‐to‐bedside review: chloride in critical illness. Critical Care, 14, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Tian Y, Fu JJ, Jiang YY, Bai YM, Zhang ZH, Hu XH, Lian HW, Guo M, Yang ZX and Zhao LC, 2017. Validation of spot urine in predicting 24‐h sodium excretion at the individual level. American Journal of Clinical Nutrition, 105, 1291–1296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for sections 5.5 and 6 of the scientific opinion on DRVs for sodium: Assessment of the relationship between sodium intake and pre‐specified health outcomes, including dose‐response relationships, and integration of different lines of evidence for setting DRVs for sodium
Analysis of evidence from published scientific literature as preparatory work for the setting of Dietary Reference Values for Sodium


