Abstract
The latest in this series of annual reports describes in detail the official control activities carried out for pesticide residues by EU Member States, Iceland and Norway in 2017. Under Article 31 of Regulation (EC) No 396/2005, Member States are requested to share the results of their official control activities and other relevant information with the European Commission, EFSA and other Member States. Based on the results provided by the reporting countries, a detailed analysis was performed on the pesticide occurrence data in the relevant food products consumed and the dietary risk related to the exposure of European consumers to pesticide residues was estimated. Overall, 95.9% of the 88,247 samples analysed fell within the legal limits (84,627, samples). In 54.1% of the tested samples, no quantifiable residues were reported (residue levels below the limit of quantification (LOQ)), while 41.8% of the samples analysed contained quantified residues at or below the maximum residue levels (MRLs). The dietary risk assessment indicated that, for the samples analysed, the probability of European citizens being exposed to pesticide residue levels that could lead to negative health outcomes is low. Based on the analysis of the 2017 results, EFSA derived several recommendations to increase the efficiency of the European control systems to ensure a continuing high level of consumer protection.
Keywords: pesticide residues, food control, monitoring, maximum residue levels, consumer risk assessment, Regulation (EC) No. 396/2005
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1666/full
Summary
This report provides an overview of the 2017 official control activities on pesticide residues carried out in the European Union (EU) Member States, Iceland and Norway. It summarises the results of both the 2017 EU‐coordinated control programme (EUCP) and the national control programmes (NP). While the NPs are mostly risk based (so called enforcement samples) focusing on pesticides or products originating from countries where a number of exceedances have been observed in the past, the EUCP aims to present a statistically representative snapshot of the situation of pesticide residues in food products that are mostly consumed in the EU following a random sampling procedure. The report includes the outcome of a dietary risk assessment based on the results of the overall 2017 control programmes.
The comprehensive analysis of the results of all reporting countries provides risk managers with a sound‐based evidence for designing future monitoring programmes, in particular for taking decisions on which pesticides and food products should be targeted in risk‐based national programmes.
EU‐coordinated programme (EUCP)
To allow the assessment of representative consumer exposure to pesticide residues by food commodity, the same pattern of commodities is monitored for the presence of pesticides residues in 3‐year cycles. Regarding the 2017 EUCP, results were compared with the ones of 2014 for those commodities sampled in both years.
In 2017 EUCP, 12 food products were considered: oranges, pears, kiwi fruits, cauliflowers, onions, carrots, potatoes, beans (dried), rye grain, husked rice grain, poultry fat and sheep fat. Kiwi fruits, onions and dried beans were included in the programme for the first time, so no comparison with results of 2014 was possible for these three food products. The samples taken were analysed for 171 pesticides; 149 of those in food of plant origin, 8 in food of animal origin and 14 in both food of plant and animal origin.
Of the 11,158 samples analysed in these food commodities:
7,236 or 64.9% were found to be without quantifiable levels of residues (residues < LOQ).
3,743 or 33.5% contained one or more pesticide residues in concentrations below or equal to the legally permitted maximum residue levels (MRLs).
179 or 1.6% contained residue concentrations exceeding the legally permitted MRLs. Among these, 80 or 0.7% of the total samples were considered non‐compliant, when also considering the measurement uncertainty.
For products of plant origin, the highest MRL exceedance rates were identified for pesticide residues found in rice and pears followed by dried beans, carrots, rye, kiwi fruits, potatoes, oranges, cauliflower and onions.
Of the 28 MRL exceedances reported for pears in 2017, 4 of them were originated from third countries, the rest being of EU origin. Noteworthy MRL exceedances were reported for chlormequat, ethephon, chlorpyrifos and propiconazole.
For rice, MRL exceedances were recorded in 48 samples, 28 of which originated from South‐East Asia, most of them from India (21). Twenty‐three of the MRL exceedances in rice were reported for carbendazim (RD).1 Among the 39 pesticides with residue levels at or above the LOQ, the ones most frequently quantified were isoprothiolane (quantified in 12.1% of the tested samples) and bromide ion (quantified in 10.1% of the tested samples).
Pesticides not approved in the EU should not be found in samples grown in the EU. However, these can be used in third countries as long as they do not exceed the legal limit when entering the EU market. Among commodities of plant origin, the following non‐EU‐approved pesticides were found in samples produced in the EU: dieldrin (RD), parathion‐methyl (RD), and procymidone (RD) in carrots, dicloran in onions, fenthion (RD), methidathion and profenofos in oranges, permethrin in pears, clothianidin in potatoes, biphenyl and carbendazim (RD) in dried beans, carbendazim (RD), permethrin and dichlorvos in rice and permethrin in rye. Whereas, in samples originating from third countries, the following pesticides exceeded the legal limits: methidathion in kiwi fruits, chlorfenapyr, methidathion and profenofos in oranges, carbaryl and diazinon in dried beans, acephate, carbendazim (RD), hexaconazole, methamidophos and triazophos, in rice.
Regarding commodities of animal origin (i.e. poultry fat and sheep fat), the most frequently quantified pesticides were fat‐soluble persistent organic pollutants (dichlorodiphenyltrichloroethane (DDT) (RD) and hexachlorobenzene). Although the persistent organic pollutants (POPs) are prohibited at international level under the Stockholm convention (UNEP, 2001), they are still found in the environment mainly due to their persistence. Apart from an MRL exceedance identified for lindane in one sample of sheep fat, no exceedances were reported in samples of animal origin.
EU‐coordinated and national programmes (EUCP + NP)
The overall EU pesticide monitoring programmes for 2017 incorporate the results of both the EUCP and national programmes, as implemented by the 28 Member States, Iceland and Norway.
The reporting countries analysed 88,247 samples for 801 pesticides. On average, 229 pesticides were analysed per sample. Most of the samples (56,718, 64.3% of the total) originated from the reporting countries (EU, Iceland and Norway); 25,409 samples (28.8%) were from products imported from third countries. The origin of the products was unknown for 6,120 samples (6.9%).
Overall, 95.9% of the samples analysed (EUCP and national programmes) fell within the legal limits (84,627 samples),2 i.e. the measured levels did not exceed the MRLs permitted in EU legislation. In 4.1% of the samples, the residue levels exceeded the MRLs (3,620 samples). Considering the measurement uncertainty, 2.5% of the samples (2,221 samples) exceeded the legal limits (non‐compliance) triggering legal or administrative actions. 54.1% of the samples tested did not contain quantifiable residue levels (residue levels were below the LOQ) and 41.8% contained quantified residues below the MRLs.
In 2017, the MRL exceedance rate was 4.1% vs 3.8% in 2016. This difference between 2016 and 2017 can be explained to a certain extent by the increased number of enforcement samples taken in 2017, which was more than twice the number taken in 2016 (10,677 enforcement samples in 2017, or 12.1% of total samples vs 4,173 samples in 2016, or 4.9% of total samples). This demonstrates the importance and effect the targeted controls can have on detecting MRL exceedances.
Residues in unprocessed food products were not quantified in 51.7% of the samples; 44% of them contained quantified residues within the legal limits and 4.3% exceeded the MRLs. Processed products had a higher rate of samples without quantified residues (71.4%) and a lower occurrence of quantified residues (25.9%) as well as a lower MRL exceedance rate (2.7%).
Samples from third countries had a higher MRL exceedance rate (7.6%) and a higher non‐compliance rate (5.5%) compared to food produced in the EU, which had MRL exceedance rate of 2.6% and non‐compliance rate of 1.3%.
Regulation (EC) No 669/2009 on import controls covered 76,789 consignments of products imported to the EU; 10,089 of these consignments were selected for laboratory analyses of which 304 (3.0%) were considered non‐compliant with the MRLs in place.
Reporting countries analysed 1,546 samples of baby food. In 94.6% of the samples, quantifiable residues were not reported (residues were below the LOQ), whereas 84 samples (5.4%) contained quantifiable residues at or above the LOQ. Twenty‐three of these samples (1.5% of samples) exceeded the MRL of 0.01 mg/kg applicable for baby food.3 Residues of glyphosate and persistent environmental contaminants were not found above the limit of quantification in any of the baby food samples analysed. The most frequently measured residues were chlorates, copper, dodine, mercury and spinosad. However, chlorates, copper and mercury residues may also originate from different sources, so their presence is not necessarily linked to the use of pesticides (e.g. food processing by‐products, natural occurring substances, environmental contaminants, etc.).
Overall, 5,806 samples of organic food (excluding baby food samples) were sampled; 5,010 samples (86.3%) did not contain quantifiable residues, whereas 711 samples (12.2%) contained residues within legal limits; most of these samples contained only residues of substances that do not necessarily come from a pesticide use (e.g. naturally occurring substances and persistent organic pollutants). The MRLs were exceeded in 1.5% of the organic samples analysed (85 samples), of which 0.7% (38 samples) were non‐compliant.
Most of the animal origin products analysed were free of quantifiable residues (8,475 samples out of 9,682, 87.5%) while 1,207 samples (12.5%) were found to contain one or several pesticides in quantified concentrations. MRL exceedances were identified in 102 samples (1.1%) of which 66 samples (0.8%) were non‐compliant considering the measurement uncertainty. In 2016, the MRL exceedance rate was 1.9%, mainly due to chlorate residues in milk.4
Multiple residues (i.e. more than one pesticide in the same sample) were reported in 24,292 samples (27.5%). The frequency of multiple residues in unprocessed products (29%) was higher than in processed products (12.0%). In unprocessed products, the highest frequency of multiple residues was found in currants (black, red and white) (71.7% of samples), blackberries (69.3%), limes (65.2%), lemons (63.3%), sweet cherries (62.5%), strawberries (61.7%) and lamb's lettuce/corn salads (61.0%). These commodities coincide with findings from previous years.
Dietary exposure and dietary risk assessment
Dietary exposure to pesticide residues is estimated by combining EU food consumption information from dietary surveys provided by Member States with occurrence data of pesticide residues per food commodity. Based on current scientific knowledge, when dietary exposure to a substance is found to be lower than or equal to its toxicological reference values, the probability of this substance presenting a health risk to consumers is low. When dietary exposure to a substance exceeds its toxicological reference values, negative health outcomes cannot be excluded.
The short‐term or acute risk assessment compares the short‐term dietary exposure per pesticide residue (mg of residue/kg body weight (bw) per day) to the substance's acute reference dose (ARfD, in mg of residue/kg bw).
The chronic or long‐term risk assessment compares the long‐term dietary exposure per pesticide residue (mg of residue/kg bw per day) to the substance's acceptable daily intake (ADI in mg of residue/kg bw per day).
Short‐term dietary risk assessment
EFSA performed the acute (short‐term) dietary risk assessment for the pesticide/food product combinations covered by the EUCP using the conservative deterministic model Pesticide Residues Intake Model (PRIMo) 3.0. The model is expected to result in an overestimation of the exposure. Samples taken under the EUCP were pooled with those from national programmes matching the EUCP pesticide/crop combinations.
For 147 pesticides of the 171 pesticides analysed in 16,515 samples, the exposure estimates were below their respective ARfDs.
For the following 24 pesticides (197 determinations out of 10,063), the exposure assessment exceeded the acute reference dose: acetamiprid (RD), carbendazim (RD), carbofuran (RD), chlorpropham (RD), chlorpyrifos, deltamethrin, dithiocarbamates (RD), dimethoate (RD), dodine, fenpyroximate (RD), fenthion (RD), flonicamid (RD), fluazifop‐P (RD), fosthiazate, imazalil, imidacloprid, iprodione (RD), lambda‐cyhalothrin (RD), phosmet (RD), pyraclostrobin, tebuconazole (RD), tefluthrin, thiabendazole (RD) and thiacloprid. For most of the above pesticides with exposure estimates higher than their ARfDs, appropriate risk management actions have already been taken.
Based on the above, EFSA concluded that according to current scientific knowledge, short‐term dietary exposure to the 171 pesticide residues of the 2017 EUCP at the assessed levels for the food commodities analysed, is unlikely to pose concerns for consumer health.
Long‐term dietary risk assessment
EFSA estimated long‐term exposure to pesticides for all food products for which a consumption value was provided in PRIMo 3.0 and for which residue concentrations were reported. The assessment was based on results submitted for the 171 pesticides covered by the EUCP and analysed in 79,411 samples covering all unprocessed products from Annex I (part A) of Regulation (EC) No 396/2005. Two scenarios were calculated, i.e. the adjusted upper‐bound scenario and the lower‐bound scenario. The lower‐bound scenario assumes that if not quantified (i.e. samples with residue level < LOQ), the residues are not present in the food product analysed. This scenario may result in an underestimation of the long‐term exposure. The adjusted upper‐bound scenario assumes that even if not quantified (i.e. results < LOQ), residues are present at the level of LOQ. It consists of a conservative approach which is likely to overestimate the long‐term exposure to a pesticide residue. The lower‐ and upper‐bound scenarios were used to frame the boundaries of a more realistic exposure estimate and better address the impact of the analytical uncertainties linked to the presence of residues at levels below the LOQ.
Using the lower‐bound scenario, ADI exceedances from pesticide consumption were not identified. The three highest long‐term exposure estimates were for dimethoate (RD) (47% of the ADI for omethoate) and dithiocarbamates (RD) (34% of the ADI of ziram and 26% of the ADI of propineb).
Using the more conservative adjusted upper‐bound scenario, two ADI exceedances were identified: the intake of dimethoate (RD) that was 108% of the omethoate ADI and dithiocarbamates (RD) that was 120% of the ziram ADI. The other pesticides (169 of the 171 tested) gave intake estimates lower than their corresponding ADIs.
EFSA noted that the high proportion of samples with pesticide residues below the LOQ may result in particularly high upper‐bound exposure values due to the assumption that even if not quantified, residues are present at the level of LOQ. Therefore, there are differences in the exposure estimates between the lower‐bound and the adjusted upper‐bound scenarios. Based on the above, EFSA concluded that according to current scientific knowledge, long‐term dietary exposure to the 171 pesticide residues of the 2017 EUCP at the assessed levels for the food commodities analysed, is unlikely to pose concerns for consumer health.
1. Background
1.1. Legal Basis
Pesticide residues resulting from the use of plant protection products on crops or food products that are used for food or feed production may pose a risk for public health. For this reason, a comprehensive legislative framework has been established in the European Union (EU), which defines rules for the approval of active substances used in plant protection products,5 the use of plant protection products and for pesticide residues in food. In order to ensure a high level of consumer protection, legal limits, so called ‘maximum residue levels’ or briefly ‘MRLs’, are established in Regulation (EC) No 396/20056. EU‐harmonised MRLs are set for more than 500 pesticides covering 370 food products/food groups. A default MRL of 0.01 mg/kg is applicable for pesticides not explicitly mentioned in the MRL legislation. Regulation (EC) No 396/2005 imposes on Member States the obligation to carry out controls to ensure that food placed on the market is compliant with the legal limits. This regulation establishes both EU and national control programmes:
EU‐coordinated programme: this programme defines the food products and pesticides that should be monitored by all Member States. The EU‐coordinated programme (EUCP) relevant for the calendar year 2017 was set up in Commission Implementing Regulation (EU) No 2016/6627 hereafter referred to as ‘2017 monitoring regulation’;
National control programmes: Member States usually define the scope of national control programmes focussing on certain products, which are expected to contain residues in concentrations exceeding the legal limits, or on products that are more likely to pose risks for consumer safety (Article 30 of Regulation (EC) No 396/2005).
According to Article 31 of Regulation (EC) No 396/2005, Member States are requested to share the results of the official controls and other relevant information with the European Commission, the European Food Safety Authority (EFSA) and other Member States. EFSA is responsible for preparing an Annual Report on pesticide residues, analysing the data in view of the MRL compliance of food available in the EU and the exposure of European consumers to pesticide residues. In addition, based on the findings, EFSA should derive recommendations for future monitoring programmes.
Specific MRLs are set in Directives 2006/125/EC8 and 2006/141/EC9 for food intended for infants and young children. Following the precautionary principle, the legal limit for this type of food products was set at a very low level (limit of quantification); in general, a default MRL of 0.01 mg/kg is applicable unless lower legal limits for the residue levels are defined in these Directives. Regulation (EU) No 609/201310 repeals the aforementioned Directives; however, the pesticide MRLs of Directive 2006/125/EC and 2006/141/EC were still applicable in 2017. In the framework of the 2017 EUCP, each Member State had to take at least 5 samples of infant formula and 5 samples of follow‐on formula, according to the 2017 monitoring regulation.
It is noted that some of the active substances for which legal limits are set under Regulation (EC) No 396/2005 are also covered by Commission Regulation (EU) No 37/2010 on pharmacologically active substances.11 For these so‐called dual use substances, Member States perform controls in accordance with Council Directive 96/23/EC12 for veterinary medicinal products; results of the controls for dual use substances13 are also reported in the framework of this report.
It should be highlighted that for organic products no specific MRLs are established. Thus, the MRLs set in Regulation (EC) No 396/2005 apply equally to organic food and to conventional food. However, Article 5 of Regulation (EC) No 889/200814 on organic production of agricultural products defines the restriction of using plant protection products.
Regulation (EC) No 669/200915 lays down rules concerning the increased level of official controls to be carried out on a list of food and feed of non‐animal origin which, based on known or emerging risks, requires an increased level of controls prior to their introduction into the EU. The food products, the country of origin of the products, the frequency of checks to be performed at the point of entry into the EU territories and the hazards (e.g. pesticides residues, not approved food additives, mycotoxins) are specified in Annex I to this regulation which is regularly updated; for the calendar year 2017, three updated versions are relevant.16 , 17 , 18
1.2. Terms of Reference
In accordance with Article 32 of Regulation (EC) No 396/2005, EFSA shall prepare an annual report on pesticide residues concerning the official control activities for food and feed carried out in 2017.
The annual report shall include at least the following information:
an analysis of the results of the controls on pesticide residues provided by EU Member States;
a statement of the possible reasons why the MRLs were exceeded, together with any appropriate observations regarding risk management options;
an analysis of chronic and acute risks to the health of consumers from pesticide residues;
an assessment of consumer exposure to pesticide residues based on the information provided by Member States and any other relevant information available, including reports submitted under Directive 96/23/EC19.
In addition, the report may include an opinion on the pesticides that should be included in future programmes.
2. Introduction
This report provides a detailed insight in the control activities at European level and the most relevant results on the official control activities performed by the EU Member States, including Iceland and Norway that are members of the European Free Trade Association (EFTA) and of the European Economic Area (EEA). The main purpose of the data analysis presented in this report is to give risk managers the necessary information to decide on risk management policy issues. At the same time, the report should also inform citizens who have an interest in food safety on the situation regarding pesticide residues in food. In particular, the following questions should be addressed:
What actions were taken by the national competent authorities responsible for food control to ensure that pesticide residues in food comply with the European food standards?
How frequently were pesticide residues found in food?
Which food products frequently contained pesticide residues?
Which pesticides were found?
Compared with previous years, are there any trends?
In which products were violations of the legal limits identified by the Member States?
Did the residues in food pose a risk to consumer health?
This report, by its use of graphics aims to convey the answers to these questions in a way that can be understood without detailed knowledge on the subject.
Together with this report, EFSA has published an Excel file as a supplement, where detailed results on the determinations/samples exceeding the legal limit can be found. Some of the results presented in this document can also be visualised in an interactive online EFSA Pesticides Dashboard.20
The following terminology is used throughout the report to describe the results for the analysed samples:
The term ‘pesticides’ is used as a synonym for plant protection products (PPP). They consist of or contain active and other substances added to plants and/or their products to ensure, among others, their protection against harmful organisms, influence their life processes (e.g. growth regulators), destroy or prevent growth of undesired plants or parts of them in the fields, etc.
A ‘pesticide residue’ consists of or contain measurable amounts of an active substance and/or related metabolites and degradation products that can be found on harvested crops or in foods of animal origin.
The term ‘residue definition (RD)’ in this report refers to all substances generated from the presence of a pesticide in the crop, food and feed. A residue definition may be a simple (i.e. one substance only) or a complex one (i.e. more than one substance). Considering that the substances used for the estimation of the dietary exposure to a pesticide residue may not coincide with the ones used for setting and enforcing maximum residue limits (MRLs), different residue definitions may be implemented at EU level for risk assessment and enforcement purposes. In this report, dealing with pesticide monitoring, the RD refers to the enforcement RD.
Samples without quantifiable residues or quantifiable pesticide residues were not found: the terms are used to describe results where the analytes were not present in concentrations at or exceeding the limit of quantification (LOQ). The LOQ is the smallest concentration of an analyte that can be quantified. It is commonly defined as the minimum concentration of the analyte in the test sample that can be determined with acceptable precision and accuracy.
Samples with quantified residues within the legal limits (below or at the MRL): these samples contained quantified residues of one or several pesticides in concentrations below or at the MRL.
Samples with quantified residues exceeding the legal limit (above the MRL) for one or several pesticides, as reported by the Member States.
Non‐compliant samples: samples containing residue concentrations clearly exceeding the legal limits, considering the measurement uncertainty. The concept of measurement uncertainties and the impact on the decision of non‐compliance is described in Figure 1 of the 2017 guidance document on reporting data on pesticide residues (EFSA, 2018d). It is required in official controls that the uncertainty of the analytical measurement is considered before legal or administrative sanctions are imposed on food business operators for infringement of the MRL legislation (Codex, 2006; Ellison and Williams, 2012; European Commission, 2018).
Figure 1.
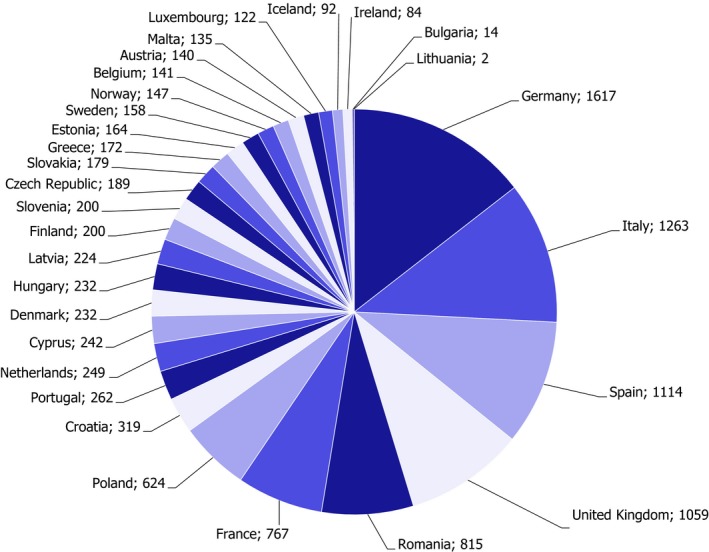
Number of samples taken by reporting country under the EUCP (excluding food for infant formulae and follow‐on formulae)26
It is noted that a separate analysis of samples with residues below the limit of detection (LOD),21 thus, samples free of any detectable residues, could not be performed, since this information is not reported consistently by the reporting countries. The possibility of accredited laboratories reporting the LOD that was achieved for each batch of samples analysed, depends on the policy of each accredited laboratory for having this parameter validated within their quality system. EFSA recommends including this parameter with a clear description of its definition under the validation criteria in the Guidance on Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed (European Commission, 2018). Alternatively, the LOD could be reported as an external parameter from the accredited scope, though an agreed harmonised definition to be used by laboratories would still be important.
In each EU Member State and EFTA country, two control programmes are in place: an EUCP and a national control programme (NP). The results of the 2017 EU‐coordinated programme, as defined in Commission Implementing Regulation (EU) No 2016/662 are summarised in Section 3 of this report. The purpose of this programme is to gather data on the occurrence and possible MRL exceedances of pesticide residues in food placed on the European common market which are statistically representative to estimate the exposure of the EU consumer to these residues.
In contrast to the EUCP, the NPs are mainly risk based and are complementary to the randomised/non‐targeted controls performed in the context of the EU‐coordinated programme; the design and results of the NPs are reported in Section 4. The results of samples taken in the framework of import control required under Regulation (EC) No 669/2009, as well as results for baby food and for organic products, are also reported in this Section 4. Major focus was put on samples that exceeded the legal limit in place.
The results of the dietary exposure assessments for individual pesticides are described in Section 5. This section is intended to characterise the risks to consumers related to pesticide residues in food.
Additional information and more detailed results related to the 2017 monitoring activities can be found on the websites of the national competent authorities (see Appendix A). In addition, EFSA compiled a technical report (EFSA, 2019) containing the national summary reports submitted by the reporting countries, where further details on the pesticide monitoring activities at national level are provided.
3. EU‐coordinated programme
3.1. Design of the EU‐coordinated programme (EUCP)
According to the 2017 EU monitoring Regulation (EU) No 2016/6627, reporting countries sampled and analysed specific pesticide/food product combinations, set out in Annex I of this Regulation. The following 12 food products were sampled: carrots, cauliflowers, kiwi fruits, onions, oranges, pears, potatoes, dried beans, rye grain, husked rice grain, poultry fat and sheep fat. Kiwi, onions and dried beans were included in the programme for the first time.
Furthermore, Annex II of the above‐mentioned Regulation, sets a minimum number of samples per food product and per Member State (depending on their population). These numbers ranged from 12 to 97 samples per food product.
In the framework of the 2017 EUCP, 11,158 samples were analysed in total. These do not include samples of infant formulae and follow‐on formulae which are presented in section 4.2.6 of the report. The number of samples taken by country under the EUCP is reported in Figure 1. It was noted that for Lithuania and Bulgaria the number of samples was lower than the number set in Annex II of the 2017 Regulation. Clarifications should be provided by these countries.
The EUCP Regulation requests the monitoring of 171 pesticides in total; 149 be analysed in food of plant origin, 8 in food of animal origin and 14 in both food of plant and animal origin. Further details on the list of pesticides covered by the 2017 EUCP are presented in Appendix B – Table B.1. Compared with the 2014 EUCP list (n = 213), the 2017 EUCP pesticide list was reduced (n = 171). Thereof, 44 substances22 were no longer considered relevant to be included in the 2017 EUCP Regulation. The reasons behind the removal of these substances are mostly related to the low frequency of quantification in previous years and/or to analytical shortcomings.23 Isoprothiolane was the only novel substance introduced in the 2017 EUCP. The splitting of the triadimenol RD into two RDs, each one containing its own active substance, resulted in the addition of a second new entry, i.e. triadimefon.24
Table B.1.
Description of the 2017 EUCP
| Pesticide | Type of food analyseda | Residue definition according to Regulation (EC) No 396/2005 on EU MRLsb | Analysis mandatory for the following food productsc |
|---|---|---|---|
| 2,4‐D (RD) | P | 2,4‐D (sum of 2,4‐D, its salts, its esters and its conjugates, expressed as 2,4‐D) | Bd, Cu, Or, Ri |
| 2‐phenylphenol | P | 2‐phenylphenol | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Abamectin (RD) | P | Abamectin (sum of avermectin B1a, avermectinB1b and delta‐8,9 isomer of avermectin B1a, expressed as avermectin B1a) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Acephate | P | Acephate | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Acetamiprid (RD) | P | Acetamiprid | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Acrinathrin | P | Acrinathrin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Aldicarb (RD) | P | Aldicarb (sum of aldicarb, its sulfoxide and its sulfone, expressed as aldicarb) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Azinphos‐methyl | P | Azinphos‐methyl | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Azoxystrobin | P | Azoxystrobin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Bifenthrin | PA | Bifenthrin | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Biphenyl | P | Biphenyl | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Bitertanol | P | Bitertanol | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Boscalid (RD) | P | Boscalid | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Bromide ion | P | Bromide ion | Ri |
| Bromopropylate | P | Bromopropylate | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Bupirimate | P | Bupirimate | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Buprofezin | P | Buprofezin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Captan (RD) | P | Sum of captan and THPI, expressed as captan | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Carbaryl | P | Carbaryl | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Carbendazim (RD) | P | Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Carbofuran (RD) | P | Carbofuran (sum of carbofuran (including any carbofuran generated from carbosulfan, benfuracarb or furathiocarb) and 3‐OH carbofuran expressed as carbofuran) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Chlorantraniliprole | P | Chlorantraniliprole (DPX E‐2Y45) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Chlordane (RD) | A | Chlordane (sum of cis‐ and trans‐isomers and oxychlordane expressed as chlordane) | Fp, Fs |
| Chlorfenapyr | P | Chlorfenapyr | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Chlormequat | P | Chlormequat | Ca, Pe, Ri, Ry |
| Chlorothalonil (RD) | P | Chlorothalonil | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Chlorpropham (RD) | P | Chlorpropham | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Chlorpyrifos | PA | Chlorpyrifos | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Chlorpyrifos‐methyl | PA | Chlorpyrifos‐methyl | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Clofentezine (RD) | P | Clofentezine | Bd, Ca, Cu, Ki, On, Or, Pe, Po |
| Clothianidin | P | Clothianidin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Cyfluthrin | P | Cyfluthrin (cyfluthrin including other mixtures of constituent isomers (sum of isomers)) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Cymoxanil | P | Cymoxanil | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Cypermethrin | PA | Cypermethrin (cypermethrin including other mixtures of constituent isomers (sum of isomers)) | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Cyproconazole | P | Cyproconazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Cyprodinil (RD) | P | Cyprodinil | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Cyromazine | P | Cyromazine | Ca, On, Po |
| DDT (RD) | A | DDT (sum of p,p’‐DDT, o,p’‐DDT, p,p’‐DDE and p,p’‐TDE (DDD) expressed as DDT) | Fp, Fs |
| Deltamethrin | PA | Deltamethrin (cis‐deltamethrin) | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Diazinon | PA | Diazinon | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Dichlorvos | P | Dichlorvos | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Dicloran | P | Dicloran | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Dicofol (RD) | P | Dicofol (sum of p,p’ and o,p’ isomers) | Bd, Ca, Cu, Ki, On, Or, Pe, Po |
| Dieldrin (RD) | PA | Aldrin and Dieldrin (Aldrin and dieldrin combined expressed as dieldrin) | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Diethofencarb | P | Diethofencarb | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Difenoconazole | P | Difenoconazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Diflubenzuron (RD) | P | Diflubenzuron | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Dimethoate (RD) | P | Dimethoate (sum of dimethoate and omethoate expressed as dimethoate) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Dimethomorph | P | Dimethomorph | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Diniconazole | P | Diniconazole (sum of isomers) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Diphenylamine | P | Diphenylamine | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Dithianon | P | Dithianon | Pe, Ri |
| Dithiocarbamates (RD) | P | Dithiocarbamates (dithiocarbamates expressed as CS2, including maneb, mancozeb, metiram, propineb, thiram and ziram) | Bd, Ca, Ki, Or, Pe, Po, Ri, Ry |
| Dodine | P | Dodine | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Endosulfan (RD) | PA | Endosulfan (sum of alpha‐ and beta‐isomers and endosulfan‐sulfate expresses as endosulfan) | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| EPN | P | EPN | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Epoxiconazole | P | Epoxiconazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Ethephon | P | Ethephon | Or, Pe |
| Ethion | P | Ethion | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Ethirimol | P | Ethirimol | Bd, Ca, Cu, Ki, On, Or, Pe, Po |
| Etofenprox | P | Etofenprox | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Famoxadone | PA | Famoxadone | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenamidone | P | Fenamidone | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenamiphos (RD) | P | Fenamiphos (sum of fenamiphos and its sulfoxide and sulfone expressed as fenamiphos) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenarimol | P | Fenarimol | Bd, Ca, Cu, Ki, On, Or, Pe, Po |
| Fenazaquin | P | Fenazaquin | Bd, Ca, Cu, Ki, On, Or, Pe, Po |
| Fenbuconazole | P | Fenbuconazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenbutatin oxide | P | Fenbutatin oxide | Or, Pe |
| Fenhexamid | P | Fenhexamid | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenitrothion | P | Fenitrothion | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenoxycarb | P | Fenoxycarb | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenpropathrin | P | Fenpropathrin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenpropidin (RD) | P | Fenpropidin (sum of fenpropidin and its salts, expressed as fenpropidin) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenpropimorph (RD) | P | Fenpropimorph | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenpyroximate (RD) | P | Fenpyroximate | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenthion (RD) | P | Fenthion (fenthion and its oxygen analogue, their sulfoxides and sulfone expressed as parent) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fenvalerate (RD) | PA | Fenvalerate (any ratio of constituent isomers (RR, SS, RS and SR) including esfenvalerate) | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Fipronil (RD) | P | Fipronil (sum fipronil and sulfone metabolite (MB46136) expressed as Fipronil) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Flonicamid (RD) | P | Sum of flonicamid, TFNA and TFNG expressed as flonicamid | Pe, Po, Ri, Ry |
| Fluazifop‐P (RD) | P | Fluazifop‐P‐butyl (fluazifop acid (free and conjugate)) | Bd, Ca, Cu, Po |
| Flubendiamide | P | Flubendiamide | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fludioxonil (RD) | P | Fludioxonil | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Flufenoxuron | P | Flufenoxuron | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fluopyram (RD) | P | Fluopyram | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fluquinconazole | P | Fluquinconazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Flusilazole (RD) | P | Flusilazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Flutriafol | P | Flutriafol | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Folpet (RD) | P | Sum of folpet and phthalimide, expressed as folpet | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Formetanate | P | Formetanate: Sum of formetanate and its salts expressed as formetanate(hydrochloride) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Fosthiazate | P | Fosthiazate | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Glyphosate | P | Glyphosate | Or, Pe, Ry |
| Heptachlor (RD) | A | Heptachlor (sum of heptachlor and heptachlor epoxide expressed as heptachlor) | Fp, Fs |
| Hexachlorobenzene | A | Hexachlorobenzene | Fp, Fs |
| Hexachlorocyclohexane (alpha) | A | Hexachlorocyclohexane (HCH), alpha‐isomer | Fp, Fs |
| Hexachlorocyclohexane (beta) | A | Hexachlorocyclohexane (HCH), beta‐isomer | Fp, Fs |
| Hexaconazole | P | Hexaconazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Hexythiazox | P | Hexythiazox | Bd, Ca, Cu, Ki, On, Or, Pe, Po |
| Imazalil | P | Imazalil | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Imidacloprid | P | Imidacloprid | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Indoxacarb | PA | Indoxacarb (sum of indoxacarb and its R enantiomer) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Iprodione (RD) | P | Iprodione | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Iprovalicarb | P | Iprovalicarb | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Isocarbophos | P | Isocarbophos | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Isoprothiolane | P | Isoprothiolane | Ri |
| Kresoxim‐methyl (RD) | P | Kresoxim‐methyl | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Lambda‐cyhalothrin (RD) | P | Lambda‐Cyhalothrin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Lindane | A | Lindane (gamma‐isomer of hexachlorocyclohexane (HCH)) | Fp, Fs |
| Linuron | P | Linuron | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Lufenuron | P | Lufenuron | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Malathion (RD) | P | Malathion (sum of malathion and malaoxon expressed as malathion) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Mandipropamid | P | Mandipropamid | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Mepanipyrim | P | Mepanipyrim | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Mepiquat | P | Mepiquat | Pe, Ri, Ry |
| Metalaxyl | P | Metalaxyl and metalaxyl‐M (metalaxyl including other mixtures of constituent isomers including metalaxyl‐M (sum of isomers)) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Methamidophos | P | Methamidophos | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Methidathion | P | Methidathion | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Methiocarb (RD) | P | Methiocarb (sum of methiocarb and methiocarb sulfoxide and sulfone, expressed as methiocarb) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Methomyl (RD) | P | Methomyl and Thiodicarb (sum of methomyl and thiodicarb expressed as methomyl) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Methoxychlor | A | Methoxychlor | Fp, Fs |
| Methoxyfenozide | P | Methoxyfenozide | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Monocrotophos | P | Monocrotophos | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Myclobutanil (RD) | P | Myclobutanil | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Oxadixyl | P | Oxadixyl | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Oxamyl | P | Oxamyl | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Oxydemeton‐methyl (RD) | P | Oxydemeton‐methyl (sum of oxydemeton‐methyl and demeton‐S‐methylsulfone expressed as oxydemeton‐methyl) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Paclobutrazol | P | Paclobutrazol | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Parathion | PA | Parathion | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Parathion‐methyl (RD) | P | Parathion‐methyl (sum of Parathion‐methyl and paraoxon‐methyl expressed as Parathion‐methyl) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Penconazole | P | Penconazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Pencycuron | P | Pencycuron | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Pendimethalin | P | Pendimethalin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Permethrin | PA | Permethrin (sum of isomers) | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Phosmet (RD) | P | Phosmet (phosmet and phosmet oxon expressed as phosmet) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Pirimicarb (RD) | P | Pirimicarb | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Pirimiphos‐methyl | PA | Pirimiphos‐methyl | Bd, Ca, Cu, Fp, Fs, Ki, On, Or, Pe, Po, Ri, Ry |
| Procymidone (RD) | P | Procymidone | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Profenofos | P | Profenofos | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Propamocarb (RD) | P | Propamocarb (Sum of propamocarb and its salt expressed as propamocarb) | Ca, Cu, On, Po |
| Propargite | P | Propargite | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Propiconazole | P | Propiconazole (sum of isomers) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Propyzamide (RD) | P | Propyzamide | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Pyraclostrobin | P | Pyraclostrobin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Pyridaben | P | Pyridaben | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Pyrimethanil (RD) | P | Pyrimethanil | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Pyriproxyfen | P | Pyriproxyfen | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Quinoxyfen | P | Quinoxyfen | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Spinosad | P | Spinosad (sum of Spinosyn A and Spinosyn D, expressed as spinosad) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Spirodiclofen | P | Spirodiclofen | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Spiromesifen | P | Spiromesifen | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Spiroxamine (RD) | P | Spiroxamine (sum of isomers) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| tau‐Fluvalinate | P | Tau‐Fluvalinate | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Tebuconazole (RD) | P | Tebuconazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Tebufenozide | P | Tebufenozide | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Tebufenpyrad | P | Tebufenpyrad | Bd, Ca, Cu, Ki, On, Or, Pe, Po |
| Teflubenzuron | P | Teflubenzuron | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Tefluthrin | P | Tefluthrin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Terbuthylazine | P | Terbuthylazine | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Tetraconazole | P | Tetraconazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Tetradifon | P | Tetradifon | Bd, Ca, Cu, Ki, On, Or, Pe, Po |
| Thiabendazole (RD) | P | Thiabendazole | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Thiacloprid | P | Thiacloprid | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Thiamethoxam | P | Thiamethoxam | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Thiophanate‐methyl | P | Thiophanate‐methyl | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Tolclofos‐methyl | P | Tolclofos‐methyl | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Tolylfluanid (RD) | P | Tolylfluanid (Sum of tolylfluanid and dimethylaminosulfotoluidide expressed as tolylfluanid) | Bd, Ca, Cu, Ki, On, Or, Pe, Po |
| Triadimenol (RD) | P | Triadimefon and triadimenol (sum of triadimefon and triadimenol)Triadimenol (any ratio of constituent isomers) | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Triadimefon | P | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry | |
| Triazophos | P | Triazophos | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Trifloxystrobin (RD) | P | Trifloxystrobin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Triflumuron | P | Triflumuron | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
| Vinclozolin | P | Vinclozolin | Bd, Ca, Cu, Ki, On, Or, Pe, Po, Ri, Ry |
P: to be analysed in plant products; A: to be analysed in animal products.
Legal residue definition applicable in 2017 for the relevant food products covered by the EUCP; if not specifically mentioned, the residue definition comprises the parent compound only.
Bd: Beans (dry); Ca: Carrots; Cu: Cauliflowers; Ki: Kiwi fruits (green, red, yellow); On: Onions; Or: Oranges; Pe: Pears; Po: Potatoes; Ri: Rice; Ry: Rye; Fp: Fat (poultry); Fs: Fat (sheep).
For the 169 pesticides and food products monitored in the 2017 and 2014 EUCPs, EFSA performed a comparative assessment of the reported results.
Carrots, oranges, pears, potatoes, rice and poultry fat were analysed in the context of both 201425 and 2017 EUCP. For food commodities sampled only in 2017, i.e. cauliflowers, kiwi fruits, onions, dried beans, rye and sheep fat, a comparison was not possible.
The 2017 EUCP requested sampling at least one sample from organic production for each of the 12 food products reported in Annex I of this Regulation, providing that such samples were available, and their sampling was proportionate to the market share of these commodities in each country. Overall, 965 organic samples were collected in the context of this Regulation.
In addition, the 2017 EUCP requested at least 5 samples of infant formulae and 5 samples of follow‐on formulae to be sampled. Overall, 604 samples of infant formulae and follow‐on formulae were collected in the context of this Regulation. Lithuania, France and Iceland did not report baby food samples. Clarifications should be provided by these countries. A comprehensive analysis of the results for baby food samples under the EUCP Regulation together with the results on other type of baby food products is reported in Section 4.2.6.
3.2. Results by pesticide
Among the 163 pesticides to be analysed in plant products, the following 37 were not quantified in any of the samples analysed27: aldicarb (RD) (8,029), azinphos‐methyl (8,723), bitertanol (8,626), bromopropylate (9,757), bupirimate (9,732), cymoxanil (7,919), dicofol (RD) (6,656), diethofencarb (8,955), endosulfan (RD) (9,052), EPN (9,064), ethion (9,584), ethirimol (6,678), famoxadone (8,002), fenamidone (9,344), fenamiphos (RD) (6,943), fenbuconazole (8,902), fenpropidin (RD) (6,148), flufenoxuron (8,437), flusilazole (RD) (9,606), folpet (RD) (4,188), formetanate (6,092), iprovalicarb (9,482), isocarbophos (7,363), lufenuron (8,233), mepanipyrim (9,109), methiocarb (RD) (8,786), monocrotophos (8,985), oxadixyl (9,203), oxydemeton‐methyl (RD) (7,228), parathion (9,178), propargite (9,459), spiroxamine (RD) (9,128), terbuthylazine (8,929), tetradifon (7,958), tolylfluanid (RD) (6,140), triadimefon28 (4,369) and vinclozolin (8,191).
In plant products, 126 substances were quantified. Residues exceeding legal limits were related to 60 different pesticides. Pesticides which were quantified in at least 1% of plant products, or for which an exceedance was identified in at least 0.05% of the samples, are presented in Figure 2. In this figure, the pesticides’ findings are ordered alphabetically and the figures in brackets next to the name of the pesticide refer to the number of samples without quantified residues (residues below the LOQ), the number of samples with quantified residues within the MRL and the number of samples exceeding the MRLs, respectively.
Figure 2.
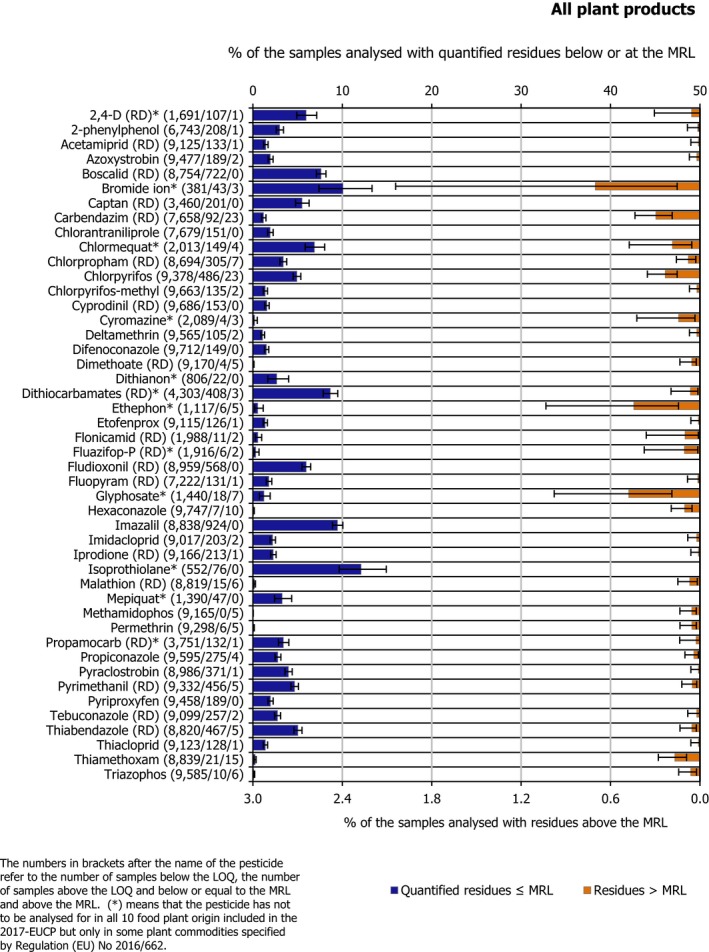
Pesticides quantified in plant products (quantification rate > 1% and/or MRL exceedance rate > 0.05%), sorted alphabetically
Isoprothiolane and bromide ion, were analysed only in rice and found to be by far the most frequently quantified residues in this commodity (isoprothiolane was quantified in 12.1% of the samples in which it was analysed and bromide ion in 10.1% of the samples in which it was analysed). Besides isoprothiolane and bromide ion, the most frequently quantified substances assessed in the food products selected were dithiocarbamates (RD) (8.7%), chlormequat (7.1%) and 2,4‐D (RD) (6.0%).
Among the pesticides analysed in all the plant products, the most frequently quantified (occurrence in more than 5% of the samples analysed) were imazalil (9.5%), boscalid (RD) (7.6%), fludioxonil (RD) (5.9%), captan (RD) (5.5%), chlorpyrifos (5.1%) and thiabendazole (RD) (5.1%). Further details on the pesticides analysed under the EU‐coordinated control are reported in Appendix B and Section 3.3.
Regarding food products of animal origin (poultry fat and sheep fat), 15 of the 22 pesticides covered by the EUCP were not found in quantifiable concentrations in any of the samples tested (the number in brackets refers to the total number of samples analysed), these were: methoxychlor (781), diazinon (775), chlorpyrifos (765), chlorpyrifos‐methyl (763), parathion (750), pirimiphos‐methyl (741), endosulfan (RD) (711), bifenthrin (703), deltamethrin (700), permethrin (677), cypermethrin (634), chlordane (RD) (628), fenvalerate (RD) (600) and famoxadone (441).
The other 7 pesticides were found sporadically. Among them, the banned persistent organic pollutant (POP) dichlorodiphenyltrichloroethane (DDT) (RD) was found to be the most frequently quantified compound (11.5% in sheep fat samples). The reason of its presence in the food chain is the persistence of this substance in the environment. Other POPs such as dieldrin, hexachlorocyclohexane (HCH)‐alpha, HCH‐beta and lindane, were quantified in less than 1% of the samples of animal origin tested for each one of these substances.
3.3. Results by food product
In this section, detailed results concerning the 12 food products covered by the 2017 EUCP are reported. For each food product, the following analyses are presented:
Key figures to describe the results for the matrices analysed, such as the number of samples analysed, the percentage of samples free of quantifiable residues (samples with residues below the LOQ), percentage of samples with multiple residues, the number/percentage of samples exceeding the legal limit and number/percentage of samples found to be non‐compliant;
Key characteristics regarding the pesticides found (e.g. number of pesticides quantified, most frequently found pesticides per product and number of pesticides with MRL exceedance);
Pie charts presenting the percentages of samples free of quantifiable residues (residues below the LOQ) and of samples with single and multiple residues (residues ≥ LOQ)29;
Bar charts sorted by frequency of quantification vs MRL exceedances in 2017. The percentages of samples with one or several residues at or above the LOQ but below or equal to the MRL are included on the left part of the chart (blue bars; upper x‐axis scale). On the right part of the chart, (orange bars; lower x‐axis scale) the percentages of samples with one or several residues exceeding the MRLs are included. The figures in brackets next to the name of the pesticide refer to the number of samples without quantifiable residues (samples with residues below the LOQ), the number of samples with quantified residues within the legal permitted concentrations (MRLs) and the number of samples exceeding the MRLs, respectively. The number and percentage of samples exceeding the legal limit are based on the judgement of the reporting country. The light bars in the left and in the right parts, refer to the results of 2014, while the bars in the darker shade refer to the results of 2017. A maximum of 45 pesticides are plotted for each food product. The pesticides not quantified in 2017, but with MRL exceedances observed in 2014, are plotted at the bottom part of the bar chart. The only two pesticides: isoprothiolane and triadimefon, that were to be monitored in 2017 but not in 2014, were marked with an asterisk if they are present in the graphs. Confidence intervals (CI) associated to frequency of quantification and frequency of MRL exceedances are added. CI30 for a proportion (e.g. % of quantified samples) were estimated using the Clopper–Pearson (exact) method that is a common method for calculating binomial CIs (Clopper and Pearson, 1934; Abraham, 2013).
Dot plot figures present the distribution of the measured residue levels, expressed as a percentage of the MRL applicable for the specific pesticide/crop combination. The figures in brackets next to the name of the pesticide refer to the number of samples without quantifiable residues, the number of samples with quantified residues within the legally permitted concentrations and the number of samples exceeding the MRLs, respectively.31 An asterisk (*) is used after the number of MRL exceedance to flag that the MRL changed during the reporting season. Each result at or above the LOQ is depicted as a dot in the respective figure. Results above 300% of the MRL are mentioned on the right side of the chart. The MRL in place at the beginning of the calendar year 2017 was used as a reference value to recalculate the reported residue concentration as percentage of the MRL or the one reported by the MS in case of a change during the year.32 , 33
In a separate Excel file published as a supplement to this report (that can be found in the online version of this output), the full list of samples exceeding the MRLs are presented, including information on the measured residue concentrations and the origin of the samples.
3.3.1. Carrots
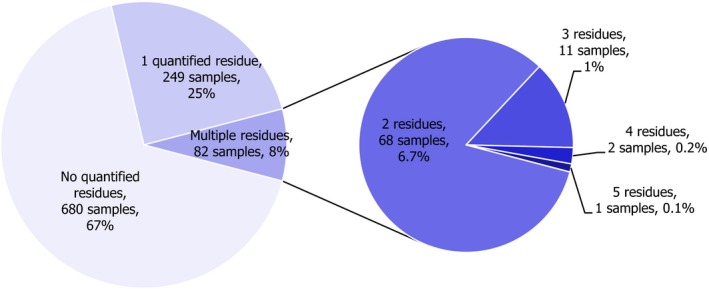
In 2017, 1,182 samples of carrots were analysed. In 697 samples (59.0%), quantifiable pesticide residues were not found, while 485 samples (41.0%) contained one or several pesticides in quantified concentrations. Multiple residues were reported in 217 samples (18.4%); up to eight different pesticides were reported in an individual carrot sample (Figure 3). The overall quantification rate recorded in 2017 is practically the same as in 2014 (42.8% of the 2014 samples contained pesticide residues).
Figure 3.
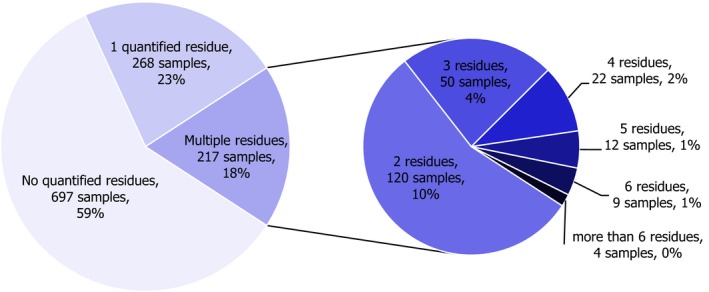
Number of quantified residues in individual carrot samples
In 1.9% of the samples (22 samples), the residue concentrations exceeded the MRLs related to 13 pesticides; 0.8% of the samples (9 samples) were reported as non‐compliant with the MRL, considering the measurement uncertainty. All MRL exceedances were related to carrots produced in the EU (3 samples from Greece, 3 from Italy, 3 from the Netherlands, 2 from Croatia, 2 from Cyprus, 2 from France, 1 from Belgium, 1 from Poland with two non‐compliant results, 1 from Portugal, 1 from Slovakia and 1 from Spain).
In total, 50 different pesticides were quantified (residue levels equal to or greater than the LOQ). The most frequently quantified pesticides were boscalid (RD) (quantified in 25.5% of the tested samples) and azoxystrobin (9.3%). The MRL was exceeded for 13 different pesticides, most frequently for chlorpyrifos (in three samples originating from Greece, one sample from Poland and one other from Portugal), dieldrin (RD) (two samples from the Netherlands and one from Belgium) and dimethomorph (two samples from Italy and one sample from Slovakia). For the rest, there was an exceedance in only one sample for each of the following pesticides: acetamiprid (RD) (originating from the Netherlands), chlorpropham (RD) (originating from France), cyromazine (originating from Cyprus), fenazaquin (originating from Spain), flutriafol (originating from Poland), linuron (originating from Hungary), mandipropamid (originating from Croatia), propamocarb (RD) (originating from Lithuania), propyzamide (RD) (originating from Croatia) and tolclofos‐methyl (originating from Italy).
Figure 4 depicts the 2017 and 2014 results for all pesticides with MRL exceedances and for the most frequently quantified pesticides. The quantification rate in 2017 was in the same range as in 2014. Exceedances which were not identified in 2014 were reported for linuron (1 sample), cyromazine (2 samples), dimethomorph (3 samples), propamocarb (RD) (1 sample), fenazaquin (1 sample), tolclofos‐methyl (1 sample), acetamiprid (RD) (1 sample) and flutriafol (1 sample) in the context of the 2017 EUCP.
Figure 4.
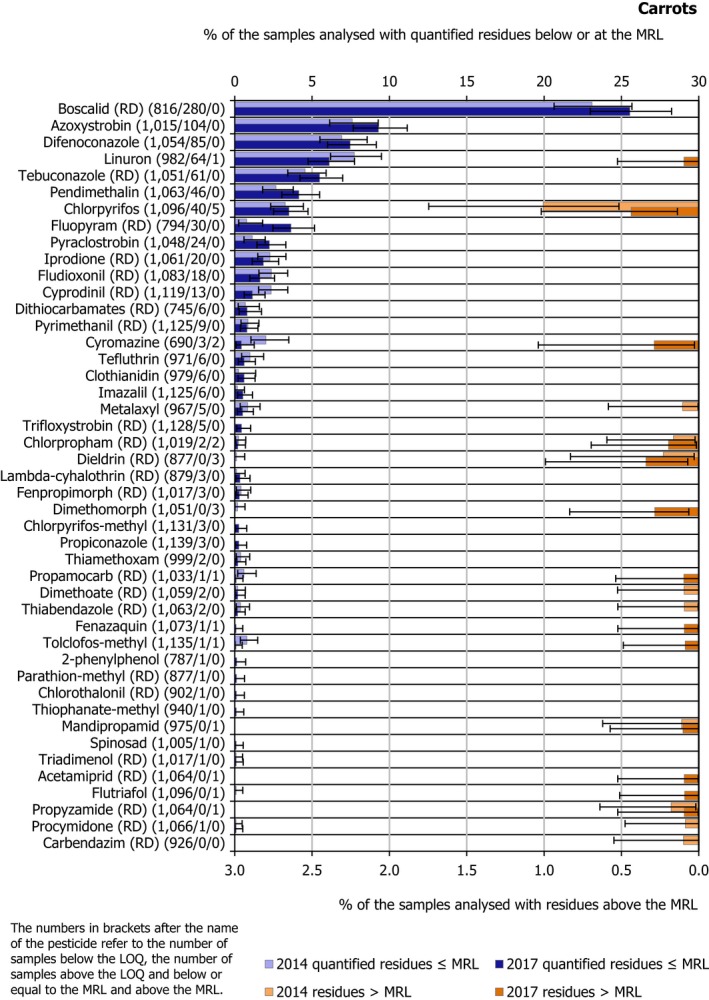
Percentage of carrot samples with quantified residues below or equal to the MRL and with residues above the MRL
The individual residue concentrations expressed as a percentage of the respective MRL per pesticide are plotted in Figure 5. Further information on the pesticide residues most frequently quantified in carrots analysed under the EUCP in 2017 in at least 10% of the samples, is compiled in Table 1.
Figure 5.
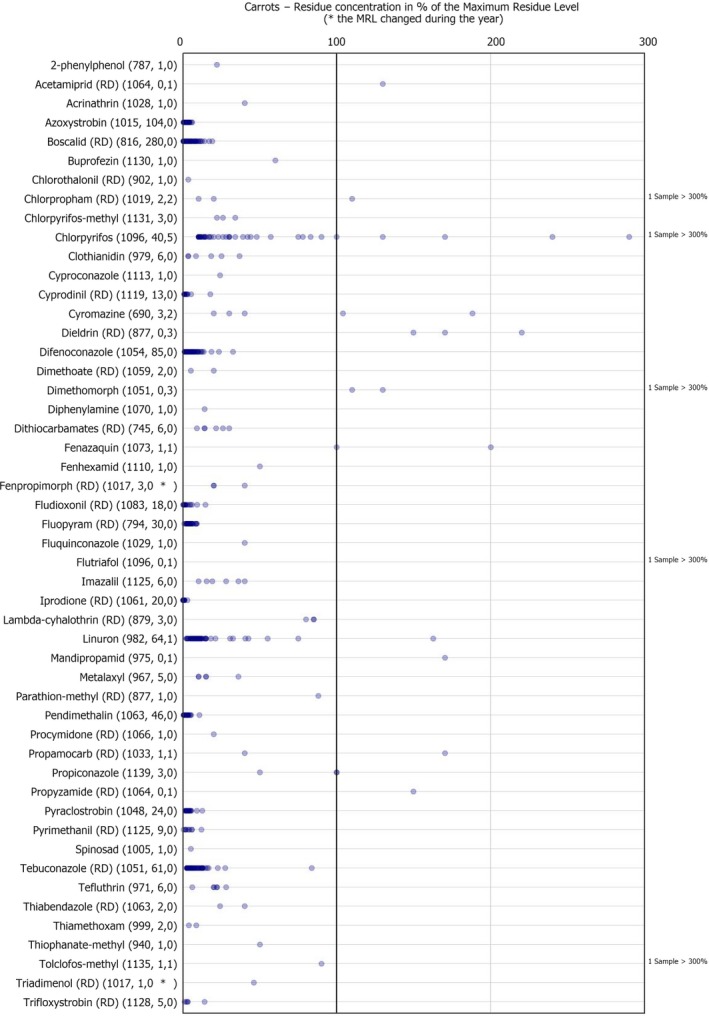
Residue concentrations measured in carrots, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
Table 1.
Pesticides most frequently quantified in carrots in 2017
| ReportName | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Boscalid (RD) | 25.5 | Approved fungicide |
| Azoxystrobin | 9.3 | Approved fungicide |
| Difenoconazole | 7.5 | Approved fungicide |
| Linuron | 6.2 | Approved herbicidea |
| Tebuconazole (RD) | 5.5 | Approved fungicide |
LOQ: limit of quantification; RD: residue definition.
Commission Implementing Regulation (EU) 2017/244 of 10 February 2017 concerns the non‐renewal of the approval of the active substance linuron. The deadline for the withdrawal of the authorisations for plant protection products containing linuron as active substance at Member State level was the 3 June 2017.
3.3.2. Cauliflower
In 2017, 905 samples of cauliflowers were analysed; in 834 samples (92.2%), quantifiable pesticide residues were not found, while 71 samples (7.8%) contained one or several pesticides in quantified concentrations. Multiple residues were reported in 15 samples (1.7%); up to five different pesticides were reported in an individual cauliflower sample (Figure 6). Cauliflower was not part of the EUCP in 2014.
Figure 6.

Number of quantified residues in individual cauliflower samples
In 0.8% of the samples (seven samples), the residue concentrations exceeded the MRLs; three samples were reported as non‐compliant, considering the measurement uncertainty. The MRL exceedances were all related to EU products (1 sample from Germany, 1 sample from the Netherlands, 1 sample from Germany, 2 samples from Cyprus, 1 sample from Spain and 1 from Poland).
Figure 7 presents the 2017 quantification and MRL exceedance rates. In total, 34 different pesticides were quantified (residue levels equal to or greater than the LOQ). The most frequently found pesticides was chlorpyrifos (quantified in 2.3% of the tested samples). The MRL was exceeded for 7 different pesticides in only one occasion, all of them approved at EU level: chlorpropham (RD), dimethoate (RD), etofenprox, methomyl (RD), propiconazole, pyrimethanil (RD) and thiophanate‐methyl.
Figure 7.
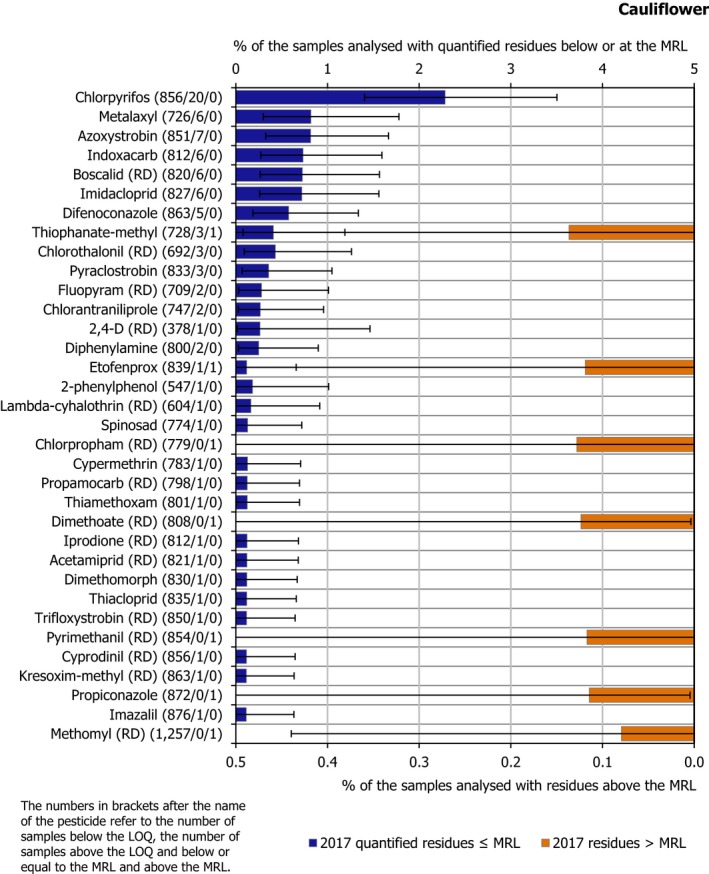
Percentage of cauliflower samples with quantified residues below or equal to the MRL and with residues above the MRL
The individual residue concentrations expressed as a percentage of the respective MRL per pesticide are plotted in Figure 8.
Figure 8.
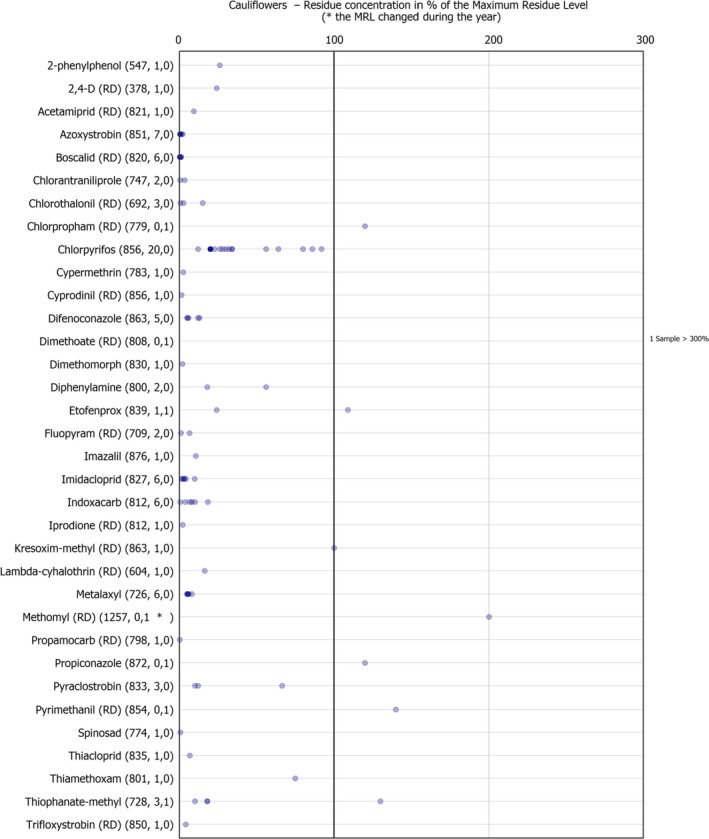
Residue concentrations measured in cauliflower, expressed as a percentage of the MRL (only samples with residues ≥ LOQ) 32 , 33
Further information on the most frequently quantified pesticides found in cauliflowers in 2017 in at least 2% of the samples is compiled in Table 2.
Table 2.
Pesticides most frequently quantified in cauliflowers in 2017
| Pesticide | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Chlorpyrifos | 2.3 | Approved insecticide/acaricide |
LOQ: limit of quantification.
3.3.3. Kiwi fruits
In 2017, 1,011 samples of kiwi fruit were analysed. In 680 samples (67.3%), quantifiable pesticide residues were not found, while 331 samples (32.7%) contained one or several pesticides in quantified concentrations. Multiple residues were reported in 82 samples (8.1%), 20 of them originating from Chile; up to 5 different pesticides were reported in the same kiwi fruit sample (Figure 9). Kiwi fruit samples were not part of the EUCP in 2014.
Figure 9.
Number of quantified residues in individual kiwi fruit samples
In 1.3% of the samples (13 samples), the residue concentrations exceeded the MRLs; 0.4% of the samples (4 samples) were reported as non‐compliant, considering the measurement uncertainty. The origin of the samples that exceeded the MRL was: 6 from Italy, 2 from Chile, 2 from France, 2 from New Zealand and 1 from Greece.
In total, 30 different pesticides were found in concentrations equal to or greater than the LOQ. The most frequently found pesticides were fludioxonil (RD) and iprodione (RD), quantified in 14.9% and 13.5% of the tested samples, respectively (see Table 3). MRL exceedances were reported for 11 different pesticides. Chlorpyrifos (2 samples) and dithiocarbamates (RD) (2 samples) were the ones most frequently found exceeding their corresponding MRLs; methidathion was reported as an exceedance in a sample grown in Chile. This pesticide was not approved in the EU in 2017.
Table 3.
Pesticides most frequently quantified in kiwi fruit in 2017
| Pesticide | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Fludioxonil (RD) | 14.9 | Approved fungicide |
| Iprodione (RD)a | 13.5 | Approved fungicide/nematicide |
| Fenhexamid | 6.3 | Approved fungicide |
LOQ: limit of quantification; RD: residue definition.
Commission Implementing Regulation (EU) 2017/2091 of 14 November 2017 concerning the non‐renewal of approval of the active substance iprodione, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending Commission Implementing Regulation (EU) No 540/2011. OJ L 297, 15.11.2017, p. 25–27.
Figure 10 presents the 2017 quantification and MRL exceedances. Kiwi fruit was introduced for the first time in the 2017 EU‐coordinated programme following a recommendation by EFSA (2015a).
Figure 10.
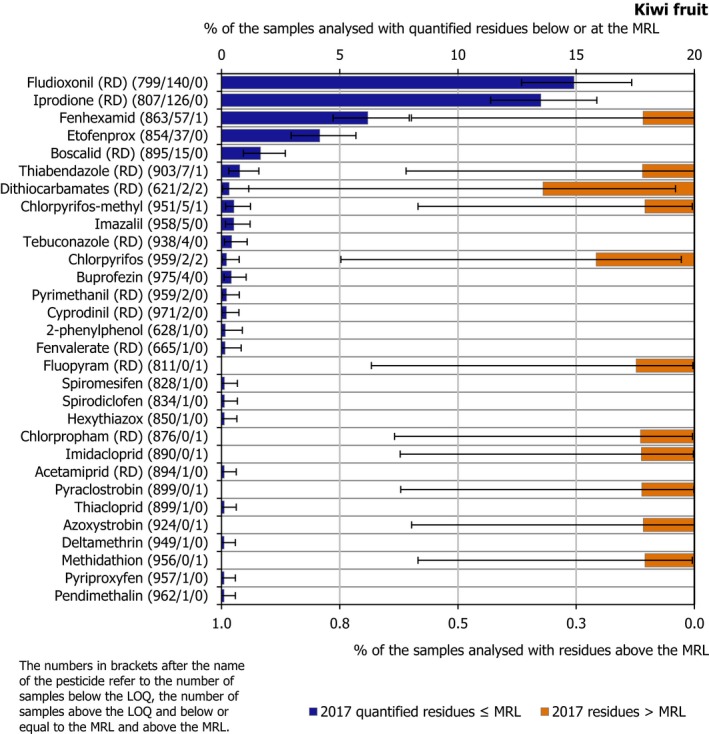
Percentage of kiwi fruit samples with quantified residues below or equal to the MRL and with residues above the MRL
The individual residue concentrations expressed as a percentage of the respective MRL for the pesticide are plotted in Figure 11. Further information on the most frequently quantified pesticides found in kiwi fruit in 2017 in at least 5% of the samples is compiled in Table 3.
Figure 11.
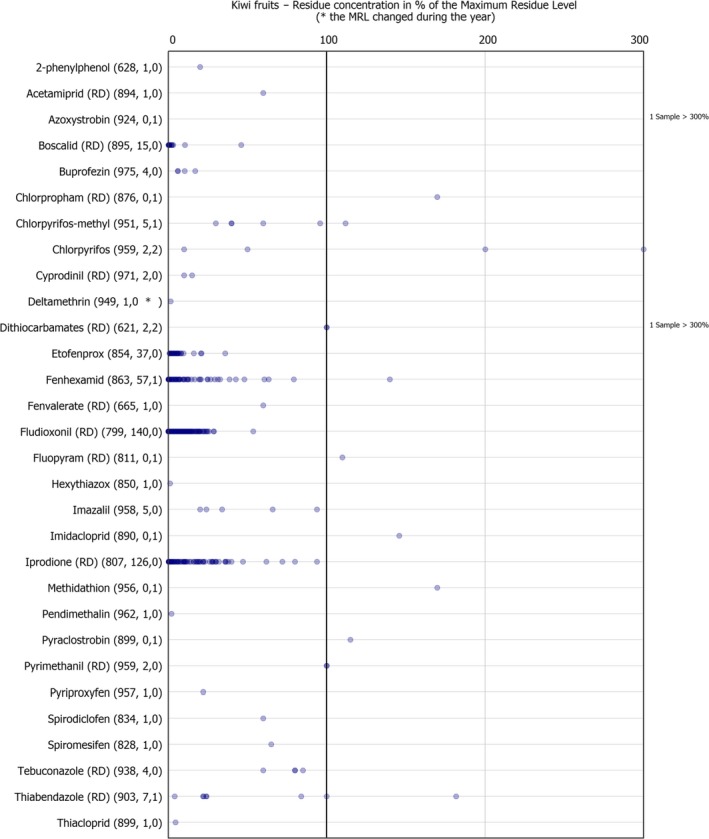
Residue concentrations measured in kiwi fruit, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
3.3.4. Onions
In 2017, 1,013 samples of onion were analysed; in 934 samples (92.2%), quantifiable pesticide residues were not found while 79 samples (7.8%) contained one or several pesticides in quantified concentrations. Multiple residues were reported in 21 samples (2.1%); up to 6 different pesticides were reported in an individual onion sample. Onion samples were not part of the EUCP in 2014 but were introduced in 2017 following a recommendation by EFSA (2015a).
Figure 12.

Number of quantified residues in individual onion samples
In 0.3% of samples (3 samples), the following residues were found to occur in concentrations exceeding their respective MRLs: chlorpropham (RD), cypermethrin and oxamyl. No non‐compliances were identified for any of these samples. The three samples with pesticide residues exceeding the MRLs were originated from the following countries: 1 sample from Romania, 1 from Italy and 1 from Peru.
In total, 25 different pesticides were found in concentrations equal to or greater than the LOQ. The most frequently found pesticides was fluopyram (RD) in 2.9% of the tested samples (22 samples). Fipronil (RD) was found in 1.4% of the samples (10 samples), 9 grown in Germany and 1 in Belgium.
Figure 13 presents the results for all pesticides with MRL exceedances and the most frequently quantified pesticides.
Figure 13.
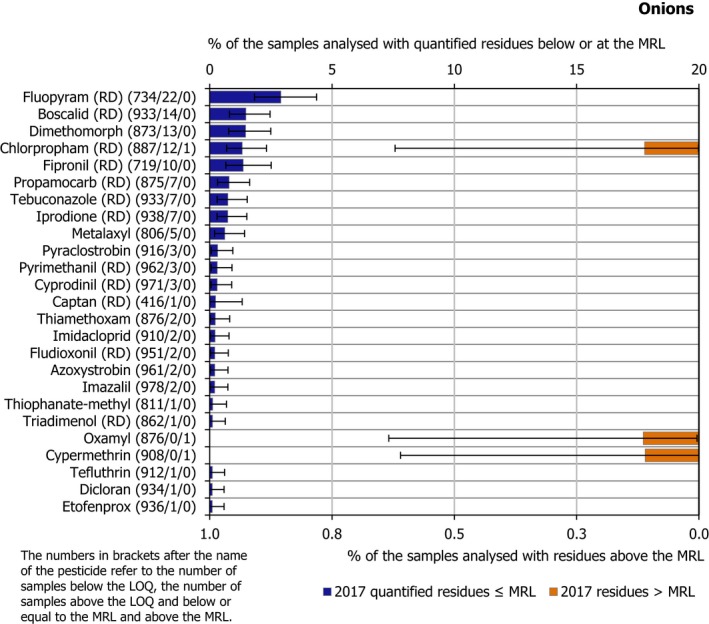
Percentage of onion samples with quantified residues below or equal to the MRL and with residues above the MRL
The individual residue concentrations expressed as a percentage of the respective MRL for the pesticide are plotted in Figure 14. Further information on the most frequently quantified pesticides found in onion in 2017 in at least 2% of the samples is compiled in Table 4.
Figure 14.
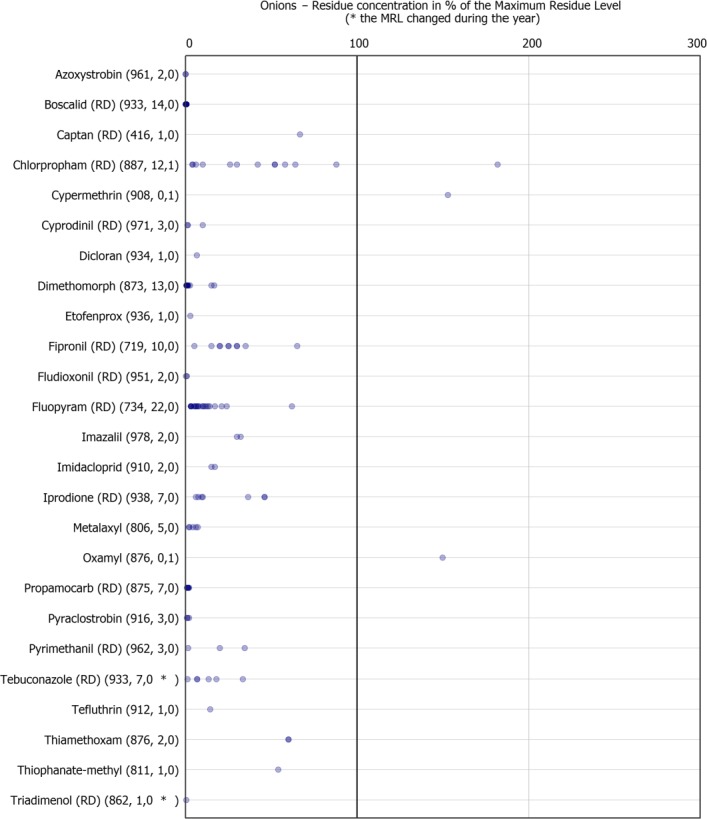
Residue concentrations measured in onion, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
Table 4.
Pesticides most frequently quantified in onion in 2017
| Pesticide | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Fluopyram (RD) | 2.9 | Approved fungicide |
LOQ: limit of quantification; RD: residue definition.
3.3.5. Oranges
In 2017, 1,497 samples of oranges were analysed. In 457 samples (30.5%), quantifiable pesticide residues were not found, while 1,040 samples (69.5%) contained one or several pesticides in quantified concentrations. Multiple residues were reported in 879 samples (58.7%) (Figure 15). In two individual orange samples with third country origin, up to 12 different pesticides were reported. Two other samples with EU origin were found to contain 11 pesticide residues each. When comparing with the results from 2014, the quantification rate decreased from 79.6% in 2014 to 69.5% in 2017.
Figure 15.
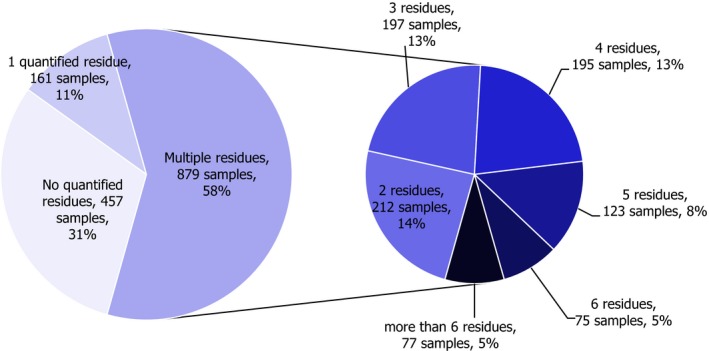
Number of quantified residues in individual oranges samples
In 1.1% of the samples (17 samples), the residues identified were found to exceed the MRL and among them, 0.6% of the samples (9 samples) were reported as non‐compliant, considering the measurement uncertainty. These MRL exceedances were related to EU products (3 samples from Spain, 2 samples from Malta and 1 sample from Portugal) as well as to products from third countries (6 samples from South Africa, 3 from Egypt, 2 from Argentina, 1 from Morocco and 1 from Uruguay).
In 2017, 59 different pesticides were quantified in total. The most frequently quantified pesticides were imazalil (quantified in 59.4% of the samples), thiabendazole (RD) (31.4%), chlorpyrifos (27.8%) and pyrimethanil (RD) (24.5%). As in 2014, imazalil and thiabendazole were the two pesticides mostly used in oranges as post harvest treatment.
The MRL was exceeded for 11 different pesticides: 2,4‐D (RD) (in 1 sample from South Africa), chlorfenapyr (in 1 sample from Uruguay), chlorpyrifos (in 1 sample from Morocco), chlorpyrifos‐methyl (in 1 sample from Spain), deltamethrin (in 1 sample from Portugal), dimethoate (RD) (in 2 samples from Egypt, in 1 sample from Malta and in 1 sample from Spain), fenthion (RD) (in 1 sample from Malta and another sample from Spain), methidathion (in 1 sample from South Africa), profenofos (in 1 sample from Egypt), pyrimethanil (RD) (in 1 sample from Argentina and another one from South Africa), thiabendazole (RD) (in 1 sample from Argentina and 3 samples from South Africa). It is noted that chlorfenapyr, fenthion, methidathion and profenofos were not approved substances in the EU in 2017.
Figure 16 presents the results for all pesticides with MRL exceedances and for the most frequently quantified pesticides. In 2017, lower quantification rates were recorded for imazalil, chlorpyrifos and 2,4‐D (RD) than in 2014 results. MRL exceedances identified for chlorpyrifos, pyrimethanil (RD), 2,4‐D (RD), chlorpyrifos‐methyl, deltamethrin, fenthion (RD) and profenofos in 2017 were not found in the 2014 results. In 2014, exceedances reported for imazalil, malathion (RD), ethephon, fenvalerate (RD), carbendazim (RD) and fenhexamid were not found in the 2017 results.
Figure 16.
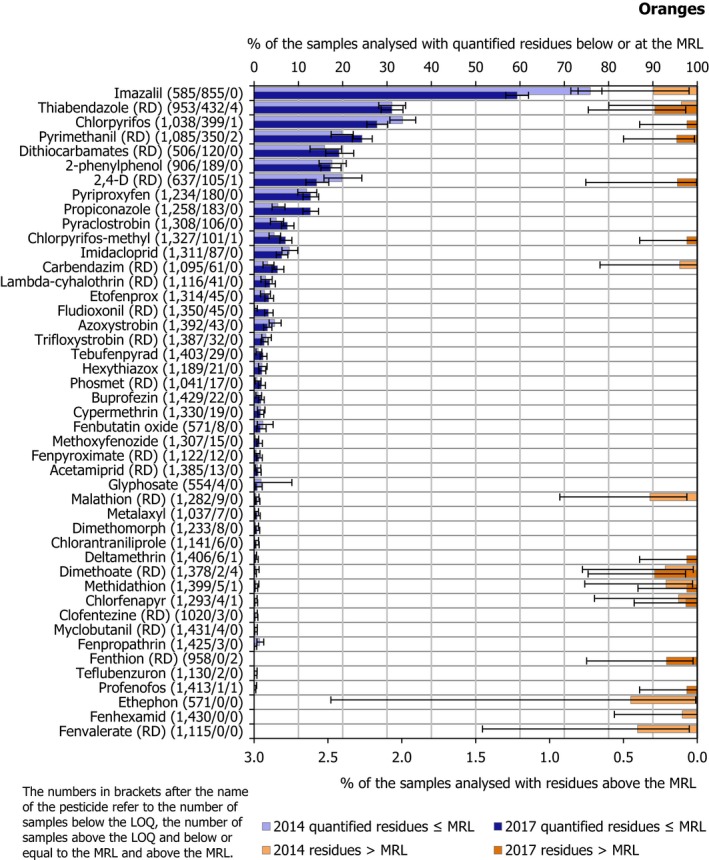
Percentage of orange samples with quantified residues below or equal to the MRL and with residues above the MRL
The individual residue concentrations expressed as a percentage of the respective MRL per pesticide are plotted in Figure 17. Further information on the most frequently quantified pesticides found in oranges in 2017 in at least 10% of the samples is compiled in Table 5.
Figure 17.
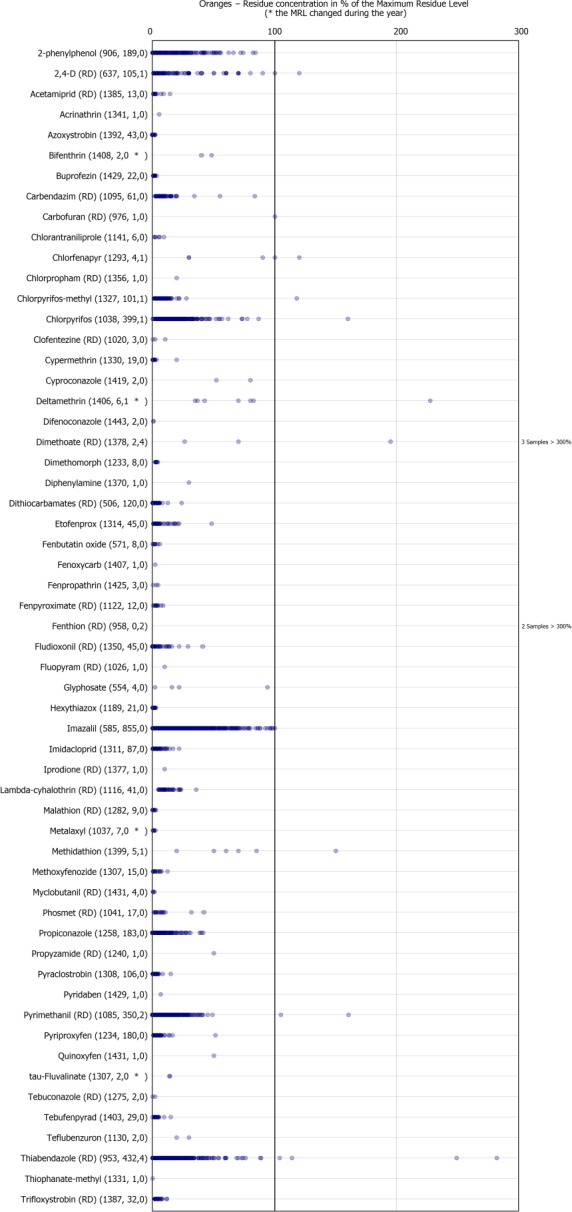
Residue concentrations measured in oranges, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
Table 5.
Pesticides most frequently quantified in oranges in 2017
| Pesticide | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Imazalil | 59.4 | Approved post‐harvest treatment fungicide |
| Thiabendazole (RD) | 31.4 | Approved post‐harvest treatment fungicide |
| Chlorpyrifos | 27.8 | Approved insecticide/acaricide |
| Pyrimethanil (RD) | 24.5 | Approved fungicide |
| Dithiocarbamates (RD) | 19.2 | Approved fungicides: maneb, mancozeb, metiram, ziram, propineb and thiram |
| 2‐phenylphenol | 17.3 | Approved fungicide |
| 2,4‐D (RD) | 14.3 | Approved herbicide and plant growth regulator |
| Pyriproxyfen | 12.7 | Approved insecticide |
| Propiconazole | 12.7 | Approved fungicide |
LOQ: limit of quantification; RD: residue definition.
3.3.6. Pears
In 2017, 1,199 samples of pears were analysed; in 336 samples (28.0%), quantifiable pesticide residues were not found, while 863 samples (72.0%) contained one or several pesticides in quantified concentrations. Multiple residues were reported in 727 samples (60.6%); up to 12 different pesticides were reported in an individual sample (Figure 18) (six of these detections were fungicides, the rest insecticides). The pattern of multiple fungicides and insecticides used in the same sample is repeated in other pear samples with multiple residues. The overall quantification rate is slightly lower in 2017 than in 2014: 72.0% vs 74.9% of samples containing one or more pesticide residues, respectively.
Figure 18.

Number of quantified residues in individual pear samples
In 2.3% of the samples (28 samples), the residues reported were found to exceed the MRL and among them 1.2% of the samples (14 samples) were reported as non‐compliant, considering the measurement uncertainty. MRL exceedances were related to products grown in the EU (8 samples from Italy, 4 from Croatia, 3 from Belgium, 3 from Poland, 2 from the Netherlands, 2 from Spain, 1 from Greece and 1 from Portugal) and from third countries (2 samples from South Africa, 1 from Argentina and 1 from Chile).
In total, 67 different pesticides were quantified. The most frequently found pesticides were captan (RD) (48.2%), dithiocarbamates (RD) (35.5%), boscalid (RD) (35.5%) and fludioxonil (RD) (32.8%). The MRL was exceeded for 11 different pesticides, most frequently for chlorpyrifos (4 samples from Croatia and 3 from Italy), ethephon (2 samples from Italy, 2 from South Africa and 1 from Chile), chlormequat (3 samples from Belgium and 1 from Poland), diphenylamine (1 sample from Portugal and 1 from Spain), propiconazole (2 samples from the Netherlands and 1 from Poland) azoxystrobin (1 sample from Argentina), chlorpropham (RD) (2 samples from Italy), glyphosate (in 1 sample from Poland), imidacloprid (1 sample from Spain), permethrin (1 sample from Greece) and thiacloprid (1 sample from Italy).
Figure 19 presents the results for all pesticides with MRL exceedances and for the most frequently quantified pesticides. It was noted that the quantification rate in 2017 was lower than in 2014 for imidacloprid, imazalil, thiabendazole (RD), diphenylamine,34 chlorpyrifos and 2‐phenylphenol but higher for captan (RD), boscalid (RD), fludioxonil (RD), acetamiprid (RD), fluopyram (RD), phosmet (RD), fenoxycarb and etofenprox. Detection of the not approved at EU level carbendazim (RD) was reported in both years. The presence of carbendazim in the pear samples analysed might be explained to a certain extent by the fact that it is major degradation product of the approved active substance thiophanate‐methyl (EFSA, 2014d). An MRL of 0.2 mg/kg is currently applicable for carbendazim in pears.
Figure 19.
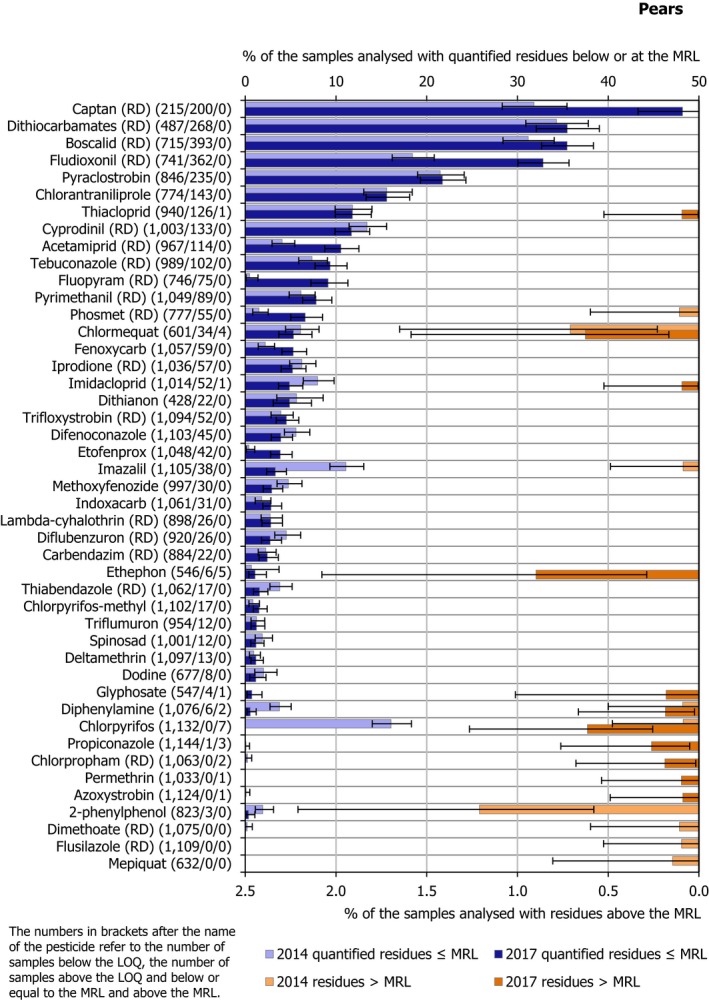
Percentage of pear samples with quantified residues below or equal to the MRL and with residues above the MRL
In terms of MRL exceedances, a lower rate was observed in 2017 for chlormequat and thiophanate‐methyl whereas higher rates were identified for ethephon, chlorpyrifos, propiconazole and chlorpropham (RD).
MRL exceedances identified for thiacloprid (1 exceedance), imidacloprid (1), glyphosate (1), propiconazole (3), chlorpropham (RD) (2), permethrin (1) and azoxystrobin (1) in 2017, were not observed in the 2014 results. On the other hand, no exceedances were observed for phosmet (RD), imazalil, 2‐phenylphenol, dimethoate (RD), flusilazole (RD) and mepiquat in 2017 although reported in samples taken in the context of the 2014 EUCP. Ethephon, was found to exceed the MRL in 5 out of 546 pear samples analysed in 2017 for this parameter but was not reported to exceed its corresponding MRL in pear samples in the 2014 results. On the other hand, 2‐phenylphenol with 10 MRL exceedances reported for 10 out of the 801 samples analysed in 2014 for this parameter did not show MRL exceedance in the 2017 results.
The 7 MRL exceedances reported for chlorpyrifos may be due to the 2014 revision of the toxicological reference value for chlorpyrifos and update of the MRLs for the substance in 201635 (EFSA, 2014a, 2015b).
The presence of chlormequat, might be due to remaining residues from former uses, considering that the pesticide is no longer authorised for use in pears and that lower exceedance rates were recorded for the substance in 2017. The one MRL exceedance identified for glyphosate in pears, is likely linked to contamination, consequent to weed control operations in a pear orchard.
The individual residue concentrations expressed as a percentage of the respective MRL per pesticide are plotted in Figure 19. Further information on the most frequently quantified pesticides found in pears in 2017 in at least 10% of the samples is compiled in Table 6.
Table 6.
Pesticides most frequently quantified in pears in 2017
| ReportName | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Captan (RD) | 48.2 | Approved fungicide |
| Dithiocarbamates (RD) | 35.5 | Approved fungicides: mancozeb, maneb, metiram, propineb, thiram and ziram |
| Boscalid (RD) | 35.5 | Approved fungicide |
| Fludioxonil (RD) | 32.8 | Approved fungicide |
| Pyraclostrobin | 21.7 | Approved fungicide/plant growth regulator |
| Chlorantraniliprole | 15.6 | Approved insecticide |
| Thiacloprid | 11.9 | Approved insecticide |
| Cyprodinil (RD) | 11.7 | Approved fungicide |
| Acetamiprid (RD) | 10.5 | Approved insecticide |
LOQ: limit of quantification; RD: residue definition.
Figure 20.
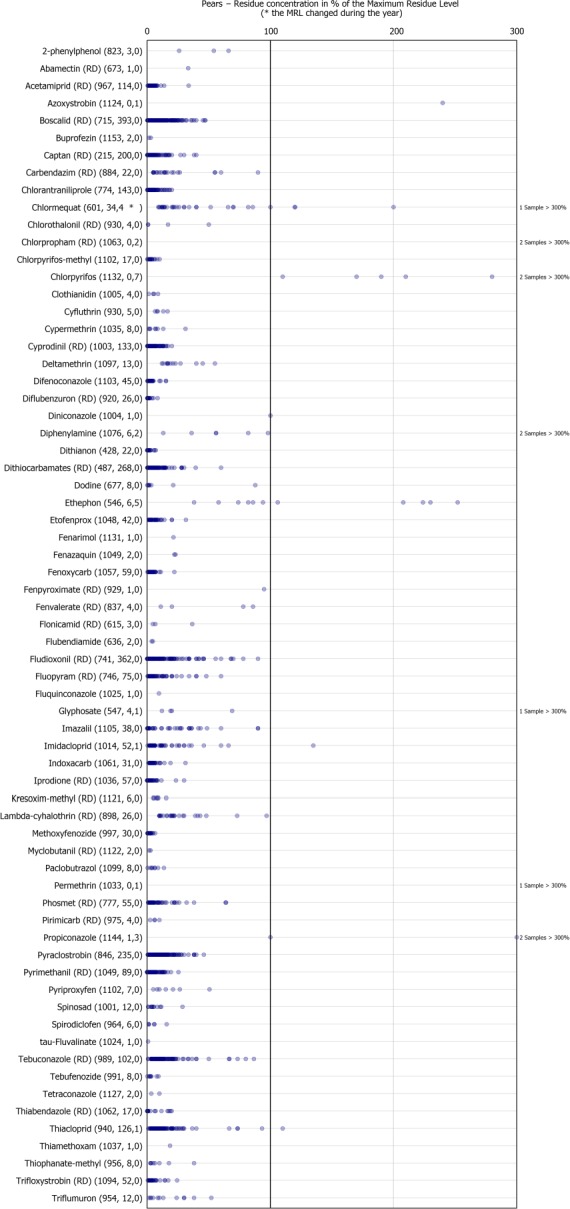
Residue concentrations measured in pears, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
3.3.7. Potatoes
In 2017, 1,389 samples of potatoes were analysed; in 929 samples (66.9%), quantifiable pesticide residues were not found, while 460 samples (33.1%) contained one or several pesticides in quantified concentrations. Multiple residues were reported in 88 samples (6.3%); up to 4 different pesticides were reported in three individual potato samples (Figure 21). The overall quantification rate was 4% higher in 2017 than in 2014 (29.1% of samples contained pesticides).
Figure 21.
Number of quantified residues in individual potato samples
In 1.2% of the samples (16 samples), the residues identified were found in concentrations exceeding the MRLs; from those, 0.4% of the samples (6 samples) were reported as non‐compliant, considering the measurement uncertainty. The MRL exceedances were only related to products grown in the EU (3 samples from Cyprus, 3 from Italy, 3 from the United Kingdom, 2 from Spain and 1 from each of the following countries: France, Germany, the Netherlands, Poland and Romania).
In total, 25 different pesticides were quantified (concentrations at or above the LOQ) in 2017 (fenazaquin and lufenuron were only quantified in 2014, but not in 2017). The most frequently quantified pesticides were chlorpropham (RD) (24.0%) and propamocarb (RD) (10.5%) (Table 7). The MRL was exceeded for 10 different pesticides, most frequently for chlorpyrifos.
Table 7.
Pesticides most frequently quantified in potatoes in 2017
| Pesticide | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Chlorpropham (RD) | 24.0 | Approved plant growth regulator/herbicide |
| Propamocarb (RD) | 10.5 | Approved fungicide |
LOQ: limit of quantification; RD: residue definition.
Figure 22 depicts the results for all pesticides with MRL exceedances and the most frequently quantified pesticides with residues below or at the MRL. Compared to 2014, the quantification rate was in the same range for most pesticides. Slightly lower quantification rates were recorded for propamocarb (RD), chlorpyrifos and cyromazine, and slightly higher for flonicamid (RD). MRL exceedances reported for flonicamid (RD), pyrimethanil (RD), fluazifop‐P (RD), 2‐phenylphenol and pirimiphos‐methyl in 2017, were not reported in the 2014 results.
Figure 22.
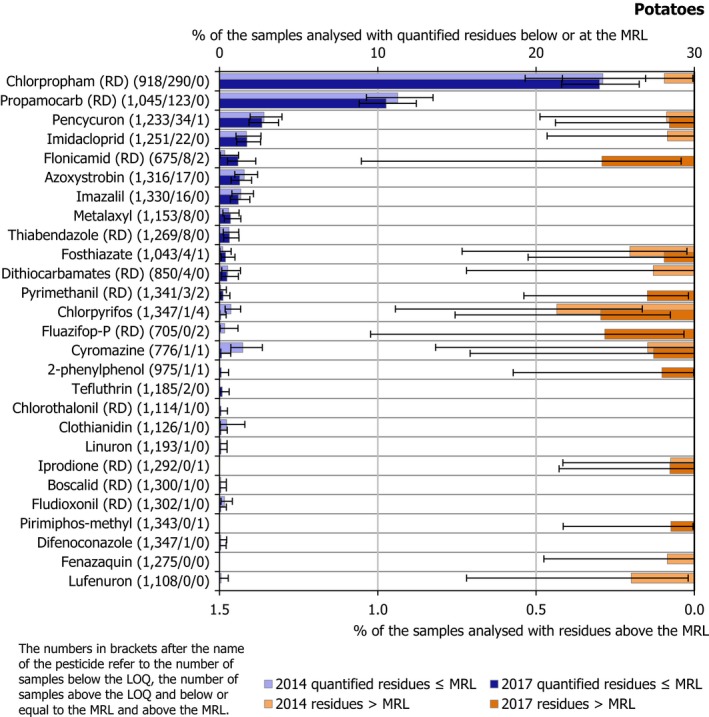
Percentage of potato samples with quantified residues below or equal to the MRL and with residues above the MRL
The individual residue concentrations expressed as a percentage of the respective MRL for the pesticide are plotted in Figure 23. Further information on the most frequently quantified pesticides found in potatoes in 2017 in at least 5% of the samples is compiled in Table 7.
Figure 23.
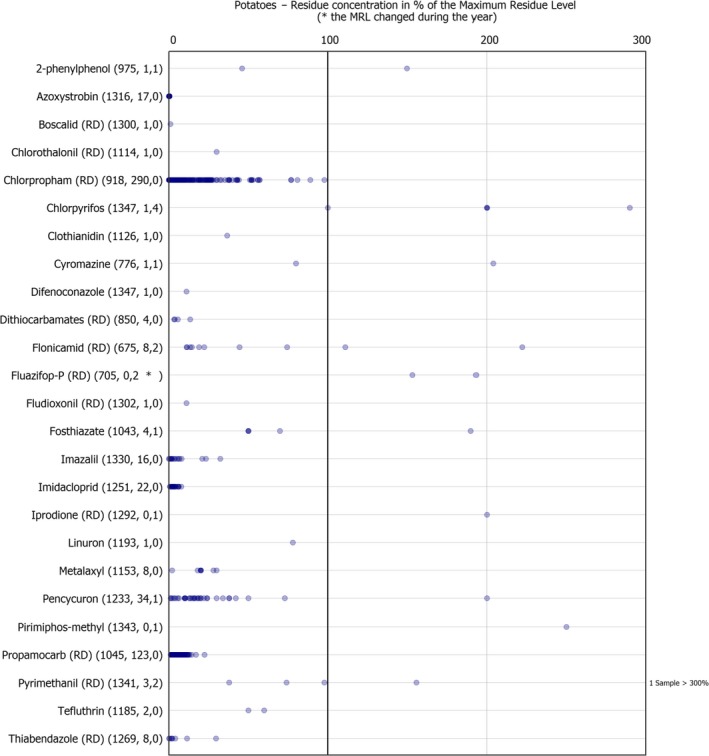
Residue concentrations measured in potatoes, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
3.3.8. Beans (dry)
In 2017, 617 samples of dried beans were analysed; in 561 samples (90.9%), quantifiable pesticide residues were not found, while 56 samples (9.1%) contained one or several pesticides in quantifiable concentrations. Multiple residues were reported in 13 samples (2.1%); in an individual sample grown in Peru four different pesticides were reported (Figure 24). Based on the recommendations highlighted on the reviewed design assessment of the EUCP done by EFSA, this was the first time a commodity belonging to pulses group such as dried beans, was sampled as part of the EU‐coordinated programme (EFSA, 2015a).
Figure 24.
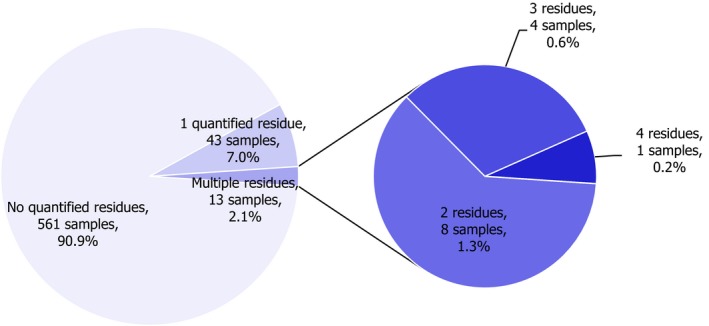
Number of quantified residues in individual samples of dried beans
The residue concentrations exceeded the MRLs in 2.3% of the samples (14 samples) and 1.3% of the samples (8 samples) were reported as non‐compliant, considering the measurement uncertainty. The MRL exceedances were related to products grown in the EU (3 samples from Greece, 1 sample from Romania and 1 sample from Spain), in third countries (4 samples from Madagascar, 3 samples from Ethiopia, 1 sample from Canada and 1 sample from Peru) and 2 samples with origin unknown.
In total, 22 different pesticides were quantified. The most frequently found pesticides were fluazifop‐P (RD) (quantified in 3.0% of the tested samples) and boscalid (RD) (2.3%). The MRL was exceeded for 9 pesticides: biphenyl (1 sample from Spain), carbaryl (1 sample from Madagascar), chlorpyrifos (1 sample from Madagascar and another one from Romania), cypermethrin (1 sample from Peru), diazinon (1 sample from Ethiopia), dithiocarbamates (RD) (1 sample from Greece), malathion (RD) (2 samples from Ethiopia, 1 sample from Greece, 1 sample from Madagascar and 2 samples with origin unknown), methomyl (RD) (1 sample from Greece) and pirimiphos‐methyl (1 sample from Canada).
It was noted that biphenyl, carbaryl, carbendazim (RD) and diazinon, quantified in several samples, were all not approved pesticides at EU level (see Figure 25).
Figure 25.
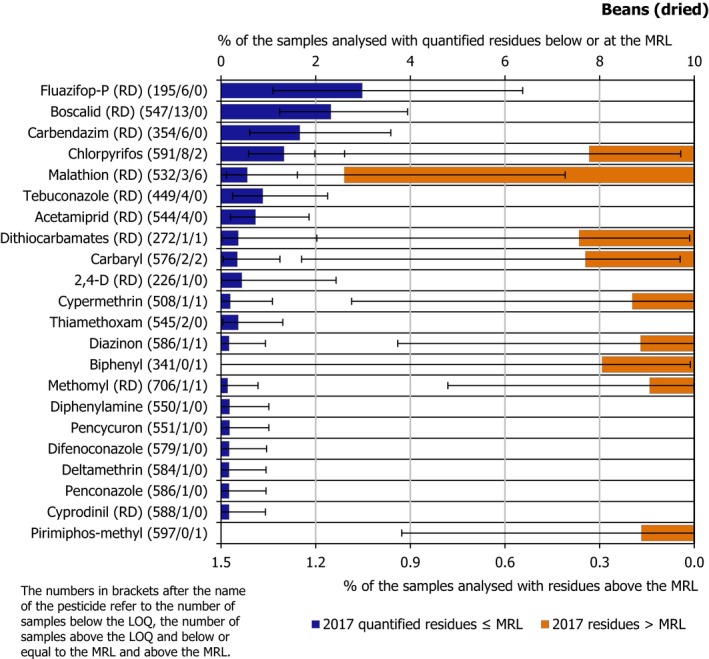
Percentage of samples of dried beans with quantified residues below or equal to the MRL and with residues above the MRL
Figure 25 presents the results for pesticides quantified below and above the MRL. The individual residue concentrations expressed as a percentage of the respective MRL per pesticide are plotted in Figure 26. Further information on the most frequently quantified pesticides found in dried beans in 2017 in at least 2% of the samples is compiled in Table 8.
Figure 26.

Residue concentrations measured in samples of dried beans, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
Table 8.
Pesticides most frequently quantified in dried beans in 2017
| Pesticide | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Fluazifop‐P (RD) | 3.0 | Approved herbicide |
| Boscalid (RD) | 2.3 | Approved fungicide |
LOQ: limit of quantification; RD: residue definition.
3.3.9. Rice
In 2017, 937 samples of rice were analysed of which, 592 were husked rice samples and 346 were polished rice samples. In 628 samples (67.0%), quantifiable pesticide residues were not found while 309 samples (33.0%) contained one or several pesticides in quantifiable concentrations. Multiple residues were reported in 153 samples (16.3%) (Figure 27). In an individual sample, 11 different pesticides were reported. The overall pesticide quantification rate was found to be higher in 2017 than in 2014 (27.4% of the samples with at least one residue).
Figure 27.
Number of quantified residues in individual samples of rice
The residue concentrations exceeded the MRLs in 5.1% of the samples (48 samples), including 26 samples (2.8%) reported as non‐compliant considering the measurement uncertainty. These MRL exceedances were related to products grown and/or potentially treated post harvest in the EU (10 samples from the United Kingdom, 3 from Portugal, 1 from France, 2 from Italy, 1 from Spain, 1 from Germany and 1 with reported origin EEA), as well as products grown in third countries (21 samples from India, 2 from Thailand, 2 from Vietnam, 2 from Cambodia and 1 from Pakistan) and 1 with origin unknown.
In total, 39 different pesticides were quantified. The most frequently found pesticides were isoprothiolane36 (quantified in 12.1% of the tested samples) and bromide ion36 (10.8%). Isoprothiolane was not part of the 2014 EU‐coordinated programme. It was included in the programme because of the repeatedly exceedance of the legal limits observed in the framework of the NPs (EFSA, 2016e).
MRL exceedances were reported for 12 different pesticides: acephate, bromide ion, carbendazim (RD), chlorpyrifos, deltamethrin, hexaconazole, methamidophos, permethrin, profenofos, tebuconazole (RD), thiamethoxam and triazophos. It was noted that acephate, carbendazim (RD), hexaconazole, methamidophos, permethrin, profenofos and triazophos are not approved at EU level. Other non‐EU‐approved pesticides quantified in levels below their corresponding MRLs were carbofuran, dichlorvos, fenitrothion and isoprothiolane.
Figure 28 presents the results for pesticides quantified below and above the MRL. Comparing with the 2014 results, the quantification rates 2014 vs 2017 for rice samples tested for each one of the pesticides below its MRL decreased for pirimiphos‐methyl (8.5% vs 4.2%) whereas increased for bromide ion (8.1% vs 10.1%), propiconazole (6.6% vs 9.7%), deltamethrin (7.4% vs 9.0%), tebuconazole (RD) (5.3% vs 8.6%), buprofezin (4.8% vs 7.4%), imidacloprid (2.4% vs 4.3%), carbendazim (RD) (1.3% vs 3.6%) and thiamethoxam (1.5% vs 3.7%). These results may be linked to the different types of rice sampled in these two different reporting periods, 2017 and 2014 (husked and/or polished rice).
Figure 28.
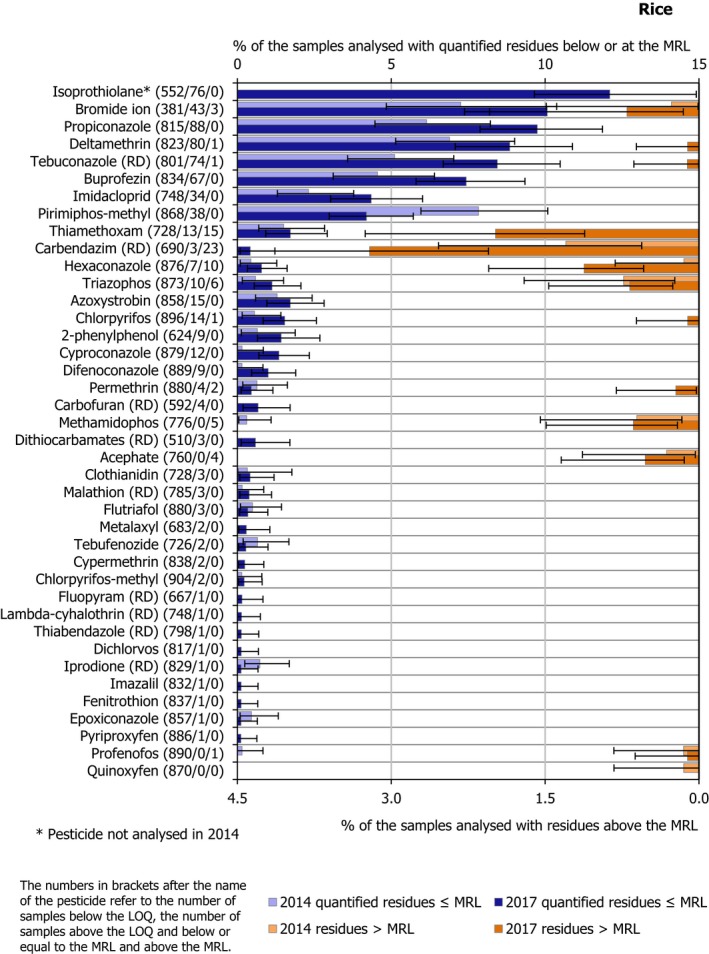
Percentage of samples of rice with quantified residues below or equal to the MRL and with residues above the MRL
It was noted that although 2.0% of the samples tested for thiamethoxam in 2017 exceeded their corresponding MRLs (15 samples), no MRL exceedances were reported for this substance in 2014. This is likely due to the lower MRL set for thiamethoxam in rice in 2016 (from 0.6 mg/kg to 0.01 mg/kg).37 Regarding carbendazim (RD), 3.2% of the samples tested in 2017 exceeded the respective MRL for this residue definition (23 samples). The exceedance rate for carbendazim (RD) in 2014 was 1.3% (8 samples).
The individual residue concentrations expressed as a percentage of the respective MRL per pesticide are plotted in Figure 29. Further information on the most frequently quantified pesticides found in rice in 2017 in at least 5% of the samples is compiled in Table 9.
Figure 29.

Residue concentrations measured in samples of rice, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
Table 9.
Pesticides most frequently quantified in rice in 2017
| Pesticide | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Isoprothiolane | 12.1 | Not approved fungicidea |
| Bromide ion | 10.8 | Naturally occurring |
| Propiconazole | 9.7 | Approved fungicide |
| Deltamethrin | 9.0 | Approved insecticide |
| Tebuconazole (RD) | 8.6 | Approved fungicide |
| Buprofezin | 7.4 | Approved acaricide/insecticide |
3.3.10. Rye
In 2017, 527 samples of rye were analysed. In 353 samples (67.0%), quantifiable pesticide residues were not found, while 174 samples (33.0%) contained one or several pesticides in quantified concentrations. Multiple residues were reported in 68 samples (12.9%); up to 4 different pesticides were reported in four individual rye samples (Figure 30). Although rye was not sampled in the 2014 EU‐coordinated programme, it was sampled in 2016. The quantification rate in 2016 (34.9%) was practically the same as in 2017 and the same detection pattern was observed in both years (EFSA, 2018e).
Figure 30.
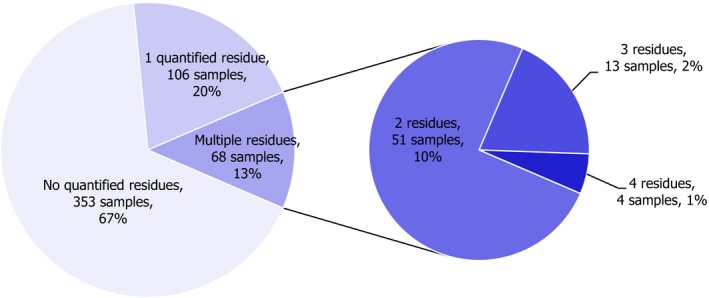
Number of quantified residues in individual rye samples
The residue concentrations exceeded the MRLs in 1.9% of the samples (10 samples), including 1 sample (0.2%) reported as non‐compliant considering the measurement uncertainty. The country of origin reported for this sample was Poland; the sample was found to contain the non‐approved pesticide permethrin.
In total, 20 different pesticides were quantified, most frequently chlormequat (quantified in 34.7% of the samples) and mepiquat (16.4%). The MRLs were exceeded for chlorpyrifos (1 sample from Hungary), glyphosate (1 sample from Italy, 4 from EEA and 1 with origin unknown), permethrin (1 sample from Poland), pirimiphos‐methyl (1 sample from France) and tebuconazole (RD) (1 sample from Hungary).
Figure 31 depicts the results for all pesticides with MRL exceedances and all quantified pesticides with residues below or at the MRL.
Figure 31.
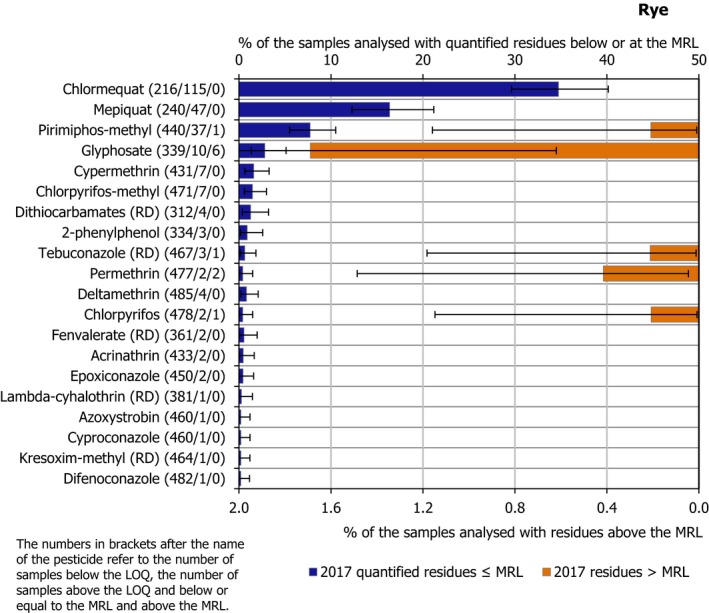
Percentage of rye samples with quantified residues below or equal to the MRL and with residues above the MRL set for rye
The individual residue concentrations expressed as a percentage of the respective MRL per pesticide are plotted in Figure 32. Further information on the most frequently quantified pesticides found in rye in 2017 in at least 5% of the samples is compiled in Table 10.
Figure 32.
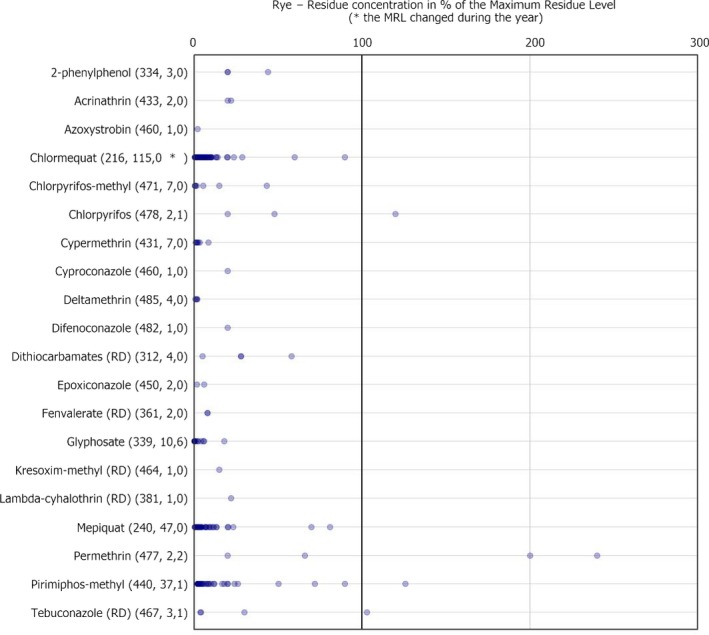
Residue concentrations measured in rye, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
Table 10.
Pesticides most frequently quantified in rye in 2017
| Pesticide | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| Chlormequat | 34.7 | Approved plant growth regulator |
| Mepiquat | 16.4 | Approved plant growth regulator |
| Pirimiphos‐methyl | 7.9 | Approved insecticide |
LOQ: limit of quantification; RD: residue definition.
3.3.11. Poultry fat
In 2017, 483 samples of poultry fat were analysed. In 479 samples (99.2%), quantifiable pesticide residues were not found, while 4 samples (0.8%) contained one or several pesticides in quantifiable concentrations. Multiple residues were reported in 1 sample (0.2%); up to two different pesticides were found in an individual poultry fat sample (Figure 33). When these results are compared with the ones from 2014, a 1.1% decrease in the overall pesticide residue quantification rate is observed in the samples of poultry fat.
Figure 33.

Number of quantified residues in individual poultry fat samples
In total, three different pesticides were quantified at levels at or lower than the MRL in the poultry fat samples analysed. All of them were found to be persistent organic pollutants in levels lower or at the MRL (Figure 34). In the case of DDT (RD), the frequency of detection was lower in 2017 than in 2014. The other two pesticides detected (hexachlorobenzene (HCB) and dieldrin (RD)) were not reported in 2014. Although POPs are prohibited at international level under the Stockholm convention39 (UNEP, 2001), they are still present in the environment due to their persistence.
Figure 34.
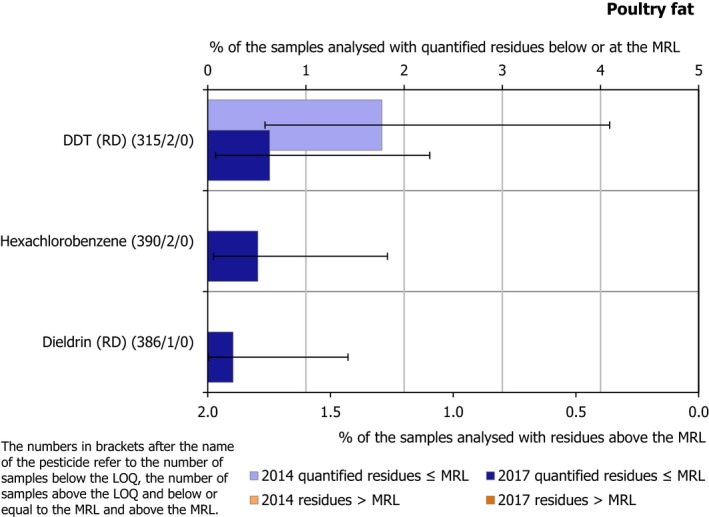
Percentage of poultry fat samples with quantified residues below or equal to the MRL
The individual residue concentrations expressed as a percentage of the respective MRL for the pesticide are plotted in Figure 35, where a significant distance is seen between the dots representing the findings and the 100% MRL. EFSA, recommends considering lowering the MRL in poultry fat for these POP pesticides. Further information all quantified pesticides found in poultry fat in 2017 is compiled in Table 11.
Figure 35.
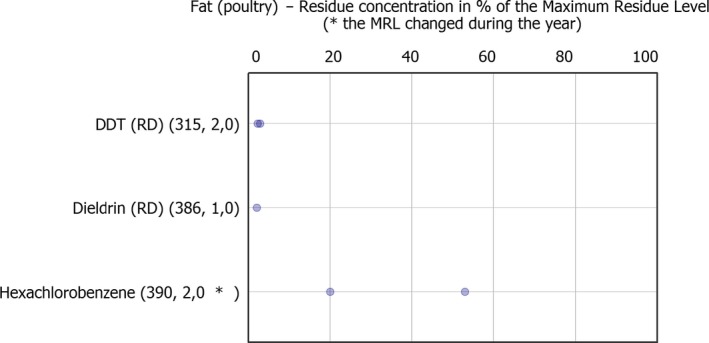
Residue concentrations measured in poultry fat, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
Table 11.
Pesticides most frequently quantified in poultry fat in 2017
| Pesticide | % samples above LOQ | Approval status in 2017 (Reg. (EC) No. 1107/2009) |
|---|---|---|
| DDT (RD) | 0.6 |
Persistent organic pollutants, banned at international level (Stockholm Convention, UNEP, 2001) and Regulation (EC) No 850/2004 |
| Hexachlorobenzene | 0.5 | |
| Dieldrin (RD) | 0.3 |
LOQ: limit of quantification; RD: residue definition.
3.3.12. Sheep fat
This was the first‐time sheep fat was sampled as part of the EU‐coordinated programme. Out of the 398 samples of sheep fat analysed in 2017, 348 samples (87.4%) were free of quantifiable pesticide residues; 50 samples (12.6%) contained one or several pesticides in quantified concentrations (Figure 36). In 20 samples (5.0%), multiple residues were reported; up to four different pesticides were reported in an individual sheep fat sample from Spain (see Figure 36).
Figure 36.
Number of quantified residues in individual sheep fat samples
Figure 37 depicts the results for all pesticides with MRL exceedances and those quantified with residues below or at the MRL in sheep fat. Only one MRL exceedance was reported for lindane in 1 sample from Spain (0.3% of samples) (see Figure 37).
Figure 37.
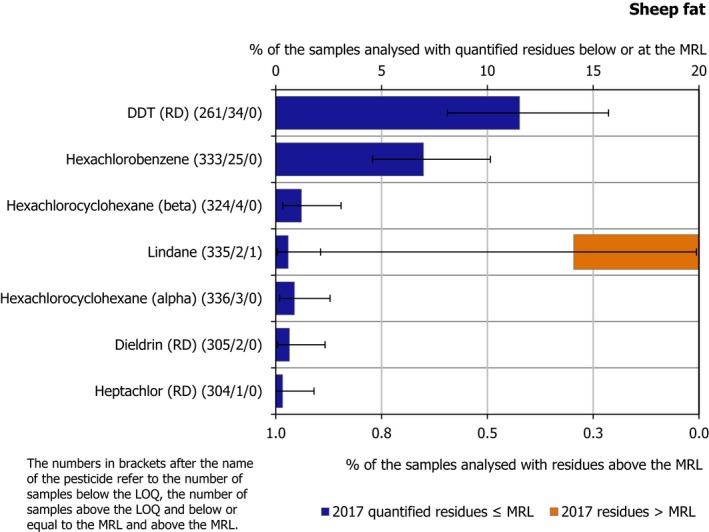
Percentage of sheep fat samples with quantified residues below or equal to the MRL and with residues above the MRL
In total, seven different pesticides were quantified in sheep fat. The most frequently found were the POPs, such as DDT (11.5% of the tested samples), HCB (7.0%), hexachlorocyclohexane (HCH)‐beta (1.2%), lindane (0.9%), HCH‐alpha (0.9%), dieldrin (RD) (0.7%) and heptachlor (RD) (0.3%). Although POPs are prohibited at international level under the Stockholm convention39 (UNEP, 2001), they are still present in the environment due to their persistence.
The individual residue concentrations expressed as a percentage of the respective MRL per pesticide are plotted in Figure 38. Further information on the most frequently quantified (above 5%) pesticides found in sheep fat in 2017 is compiled in Table 12.
Figure 38.

Residue concentrations measured in sheep fat, expressed as a percentage of the MRL (only samples with residues ≥ LOQ)32 , 33
Table 12.
Pesticides most frequently quantified in sheep fat in 2017
| Pesticide | % samples above LOQ | Approval status and comments |
|---|---|---|
| DDT (RD) | 11.5 | Fat soluble persistent organic pollutants, banned at international level (Stockholm Convention (UNEP, 2001); Regulation (EC) No 850/2004a) |
| Hexachlorobenzene | 7.0 |
LOQ: limit of quantification; RD: residue definition.
Regulation (EC) No 850/2004 of the European Parliament and of the Council of 29 April 2004 on persistent organic pollutants and amending Directive 79/117/EEC. OJ L 158, 30.4.2004, p. 7–49.
3.4. Overall results of the EU‐coordinated programme
In the framework of the 2017 EUCP, the following 12 food products were considered: carrots, cauliflowers, kiwi fruits, onions, oranges, pears, potatoes, beans (dried), rye grain, husked rice grain, poultry fat and sheep fat. Kiwi, onions and dried beans were introduced for the first time in the programme.
Overall, for 64.9% of samples (7,236 out of the 11,158 samples analysed) quantifiable levels of residues were not reported (residues were below the LOQ). The number of samples with pesticide residues within the legally permitted levels (at or above the LOQ but below or at the MRL) was 3,743 (33.5%). MRLs were exceeded in 1.6% of the samples (179 samples), 0.7% of which (80 samples) were found to be non‐compliant based on the measurement uncertainty (Figure 39). Comparing the 2017 results with those from 2014 for the common commodities only (see All products* in Figure 39), a decrease in the MRL exceedance rate is observed between these 2 years. For these commodities, the estimated exceedance rate in 2014 was 1.6% vs 1.2% in 2017 (131 samples).
Figure 39.
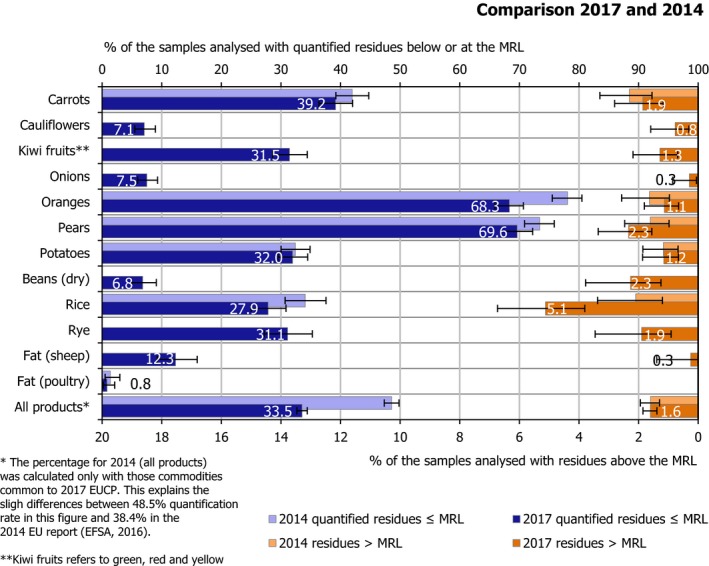
Overall proportion of EUCP samples with residues exceeding the MRL and samples with quantified residues below or at the MRL
Regarding the individual food commodities, the MRL exceedance rate between 2014 and 2017 increased in pears (from 1.6% in 2014 to 2.3% in 2017) and rice (from 2.1% in 2014 to 5.1% in 2017).
Among the commodities of plant origin tested in the framework of the 2017 EU‐coordinated programme, the following non‐EU‐approved pesticides were found in samples produced in the EU, in some cases exceeding the legal limit: dieldrin (RD), parathion‐methyl (RD), and procymidone (RD) in carrots, dicloran in onions, fenthion (RD), methidathion and profenofos in oranges, permethrin in pears, clothianidin in potatoes, biphenyl and carbendazim (RD) in dried beans, carbendazim (RD), permethrin and dichlorvos in rice and permethrin in rye.
Among the EUCP samples with non‐internal market origin, the following non‐EU‐approved pesticides were found to exceed the legal limits: methidathion in kiwi fruits, chlorfenapyr, methidathion and profenofos in oranges, carbaryl and diazinon in dried beans, acephate, carbendazim (RD), hexaconazole, methamidophos, and triazophos, in rice.
Regarding the commodities of animal origin tested in the framework of the 2017 EU‐coordinated programme (i.e. poultry fat and sheep fat), the most frequently quantified pesticides were fat‐soluble POPs: DDT (RD) and HCB. Although the POPs are prohibited at international level under the Stockholm convention (UNEP, 2001), they are still present in the environment due to their persistence. Except for an MRL exceedance identified for lindane in one fat sheep sample, no MRL exceedances were reported in samples of animal origin (sheep fat and poultry fat).
4. Overall monitoring programmes (EUCP and national programmes)
This chapter incorporates both the results of the EUCP and the national programmes, as implemented by the 28 Member States, Iceland and Norway.
Compared with the EUCP, the NPs are rather risk based, focussing on products likely to contain pesticide residues or for which MRL infringements were identified in previous monitoring programmes. These programmes are not designed to provide statistically representative results for residues expected in food placed on the European market. The reporting countries define the priorities for their NPs considering the importance of food products in trade or in the national diets, the products with high residue prevalence or non‐compliance rates in previous years, the use pattern of pesticides and the laboratory capacities. The number of samples and/or the number of pesticides analysed by the participating countries is determined by the capacities of national control laboratories and the available budget resources. Considering the specific needs in the reporting countries and the particularities of NPs, the results of NPs are not directly comparable.
In the framework of the NPs, reporting countries also provide results of import controls performed under Regulation (EC) No. 669/2009. These specific import controls are inter alia based on previously observed high incidences of non‐compliant products imported from certain countries from outside the Union and/or notifications under the Rapid Alert System for Food and Feed of the European Commission.
The first part of this chapter (Section 4.1) gives an overview of the national programmes, highlighting the sample origin (e.g. domestic samples), type (e.g. processed, unprocessed), number of samples and pesticides tested per reporting country. In the second part of the chapter (Section 4.2), the results of the national control activities are analysed and discussed. The findings, in particular the MRL exceedances, are used by risk managers for their considerations and/or to take decisions on designing the risk based national monitoring programmes, e.g. which pesticides should be covered by the analytical methods used to analyse food products, or which types of products should be included in the NPs in order to make the programmes more efficient. The findings are also valuable source of information for food business operators and can be used to enhance the efficiency of self‐control systems.
4.1. Overview of the overall monitoring programmes
In 2017, in total 88,247 samples40 of food products covered by Regulation (EC) No. 396/2005 were analysed for pesticide residues in the 30 reporting countries. The total number of samples analysed in 2017 increased by 3.9% compared to 2016, where results on 84,652 samples were reported.
The number of samples per reporting country and the sampling frequency per 100,000 inhabitants of the reporting country are presented in Figures 40 and 41.
Figure 40.
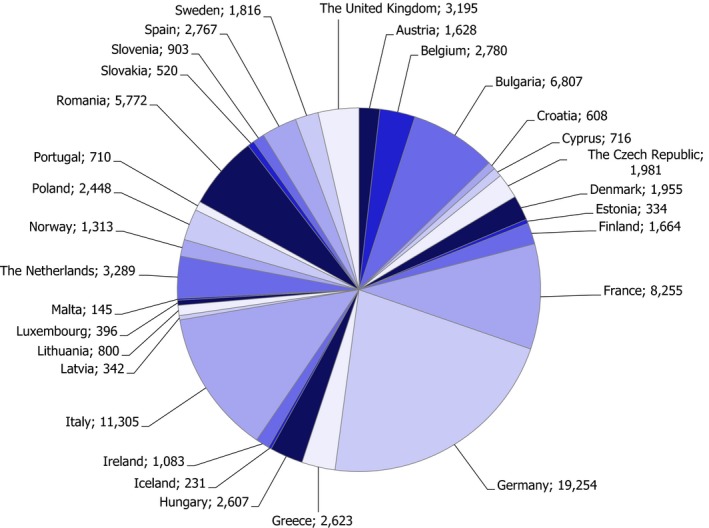
Number of samples analysed per reporting country41
Figure 41.
Number of samples normalised per number of inhabitants
The sampling rate from domestic and other EU/EEA countries decreased from 2016 to 2017, whereas the ratio of samples from third countries increased within the same timeframe. The countries with the highest sampling rates of imported products from third countries were Bulgaria (95.4%), the Netherlands (63.7%), Lithuania (49.1%) and Sweden (46.8%); Greece and Spain mainly focussed on domestic sampling (more than 80% of the samples analysed). Information on the origin of samples included in the 2017 programme, is presented in Figure 42.
Figure 42.
Origin of samples per reporting country
Overall, 56,718 samples (64%) were originated from EU reporting countries (EU MS, Norway and Iceland), 25,409 samples (28.8%) concerned products imported from third countries and for 6,120 samples (6.9%) no food product origin was reported.
A more detailed analysis of the origin of the samples is presented in Figure 43.
Figure 43.
Origin of tested samples (reporting countries and third countries)
As in previous years, a wide scope of pesticides and different food products was analysed. Considering all samples, the reporting countries analysed in total 801 different pesticides. The broadest analytical scopes at country level was noted for Germany (692 pesticides), followed by Belgium (597 pesticides), Luxembourg (564 pesticides), Spain (549 pesticides), France (544 pesticides), Austria (517 pesticides); Croatia, the Netherlands, Hungary, Sweden and Italy analysed at least 400 different pesticides. On average, 229 different pesticides were analysed per sample (230 pesticides in 2016) (Figure 44).
Figure 44.
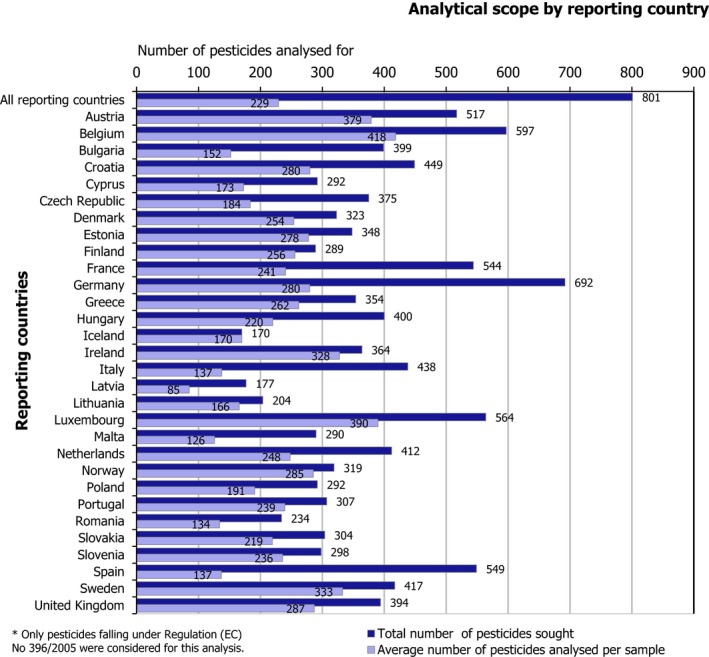
Number of pesticides analysed by reporting country
Reporting countries covered a wide variety of unprocessed and processed food products (e.g. cereal products such as flour, polished rice, wine, vegetable oils, fruit and vegetable juices, canned fruits and vegetables, milk products, dried fruits such as raisins, dried herbs, different types of baby food, etc.) allowing to get a comprehensive picture of the food placed on the EU market.
The heterogeneity of NPs needs to be kept in mind when comparing results of different reporting countries. In the next sections, a detailed analysis of the NPs shows the different scopes of the national MRL enforcement strategies.
More information on the NPs can be found in the separate EFSA technical report that summarises the national results (EFSA, 2019).
4.2. Results of the overall monitoring programmes
Overall, 95.9% of the 88,247 samples analysed in 2017 fell within the legal limits (84,627 samples); 47,759 of these samples (54.1% of the total number of samples tested) did not contain quantifiable residues (results below the LOQ for all pesticides analysed) while 41.8% of the samples analysed contained quantified residues not exceeding the legal limits (36,868 samples). MRLs were exceeded in 4.1% of the samples analysed in 2017 (3,620 samples; Figure 45). Considering the measurement uncertainty, 2.5% of all samples analysed in 2017 (2,221 samples) clearly exceeded the legal limits, triggering legal sanctions or administrative actions; these samples are considered as non‐compliant with the legal limits.
Figure 45.
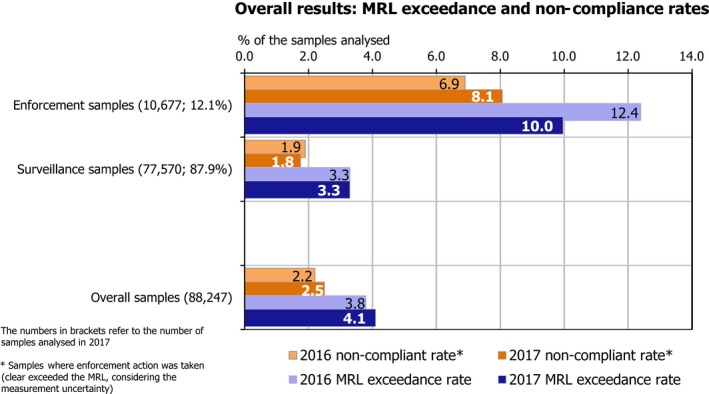
Percentage of samples compliant with the legal limit/exceeding the legal limit (MRL)
Most samples (77,570 samples, 87.9%) were classified as surveillance samples, meaning that the samples were taken without targeting specific growers/producers/importers or consignments likely to be non‐compliant. On the contrary, 12.1% of the cases were enforcement samples (where a suspect sampling strategy was applied). This means that samples were taken after concrete indications that certain food may be of higher risk as regards non‐compliance or consumer safety (e.g. Rapid Alert notifications or follow‐up enforcement samples following MRL violations identified in a first analysis of the product under scrutiny).
Overall, MRL exceedance and non‐compliance rates increased slightly in 2017 in comparison with the previous year. The MRL exceedance rate increased from 3.8% in 2016 to 4.1% in 2017; the non‐compliance rate increased from 2.2% in 2016 to 2.5% in 2017. It was noted that although the rates for surveillance samples regarding MRL exceedances (3.3% in both 2016 and 2017) and non‐compliances (1.9% in 2016 vs 1.8% in 2017) were practically the same, the rate of non‐compliance in the case of enforcement samples increased from 6.9% (2016) to 8.1% (2017). This difference can be explained to a certain extent by the increased number of enforcement samples taken in 2017 (10,677; 12.1%), which was more than twice the number in 2016 (4,173; 4.9%).
The results presented in the following sections refer to complete data sets, comprising results of surveillance and enforcement samples as well as unprocessed and processed food products. In specific cases where the analysis is restricted to a subset of results, this is clearly indicated in the relevant section.
4.2.1. Results by country of food origin
Among the sample originating from one of the reporting countries (i.e. from EU Member States, Iceland and Norway) 56.6% were found to be free of quantifiable residues while 40.8% contained residues at or above the LOQ but below or equal to the MRL; 2.6% of the samples exceeded the MRL and 1.3% were considered non‐compliant with the MRL, based on the measurement uncertainty.
Samples from third countries were found to have a higher MRL exceedance rate (7.6%) and a higher non‐compliance rate (5.5%) compared to food produced in the EU (Figure 46). The percentage of samples from third countries without quantifiable residues was 45.1% while the percentage of samples containing quantifiable residues within the legal limits was 47.3%.
Figure 46.
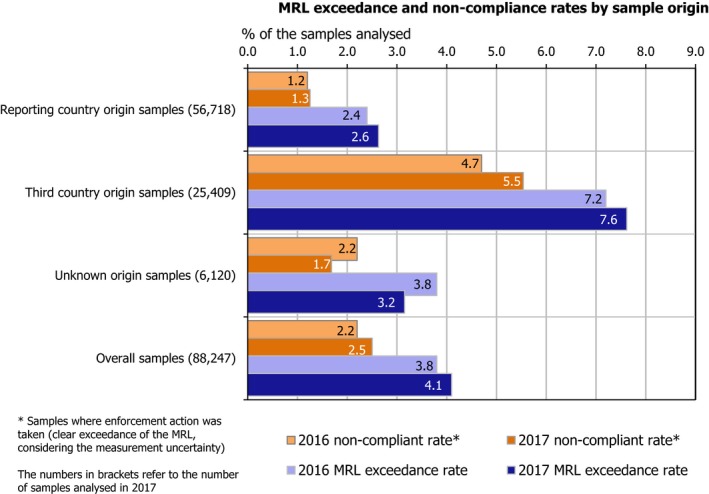
Percentage of samples exceeding the MRL and non‐compliant by origin
In Figures 47 and 48, detailed quantification and MRL exceedance rates are plotted for samples originating from the reporting countries and samples from third countries, respectively. Results from the previous reporting year are plotted in both charts, allowing comparison with the one in 2017. The numbers in these figures need to be interpreted with caution when comparing monitoring results between countries setting different priorities in the design of their national monitoring activities (e.g. more/less risk‐based sampling, different national food trade interests, dietary habits, pattern of pesticides used in crops, etc.). Therefore, the use of national data to derive comparative conclusions could be misleading.
Figure 47.
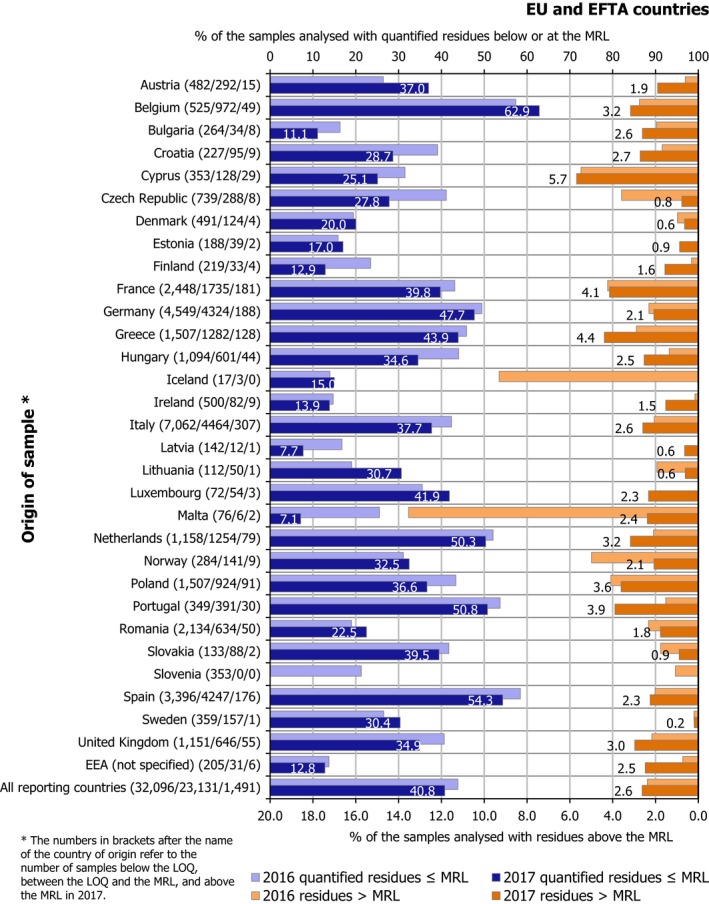
MRL exceedance and quantification rates by country of origin (reporting countries)
Figure 48.
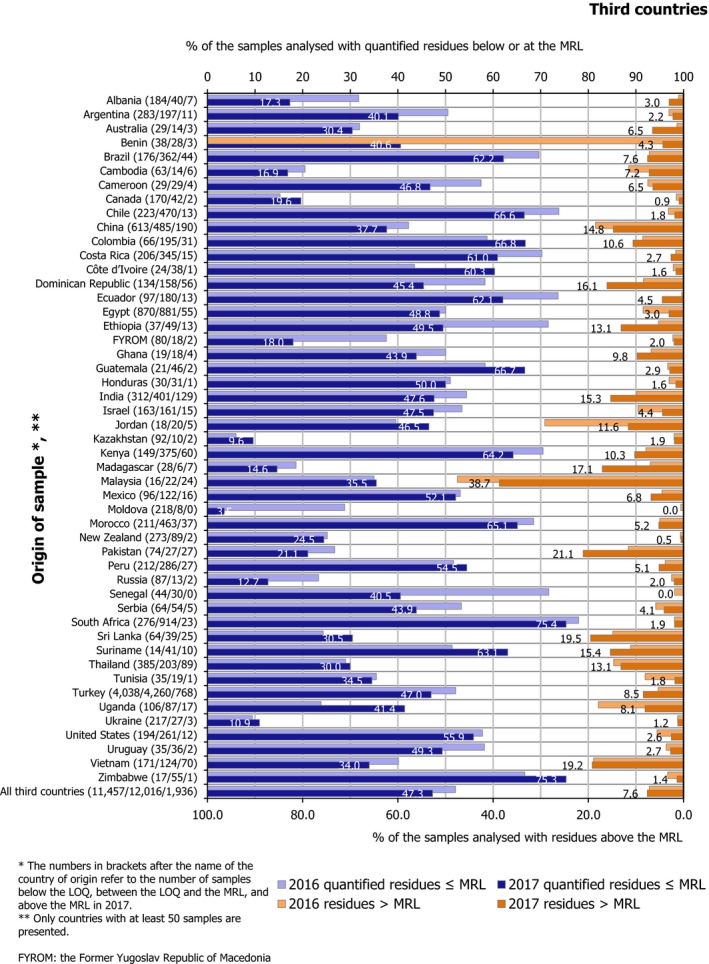
MRL exceedance and quantification rates by country of origin (third countries)
Among the reporting countries (Figure 47), the highest MRL exceedance rates were reported for products from Cyprus, Greece and France (more than 4% of the samples exceeded the MRL). Compared with 2016, decrease of non‐compliant samples was observed for Malta (from 13.5% in 2016 to 2.4% in 2017).
Regarding samples originating from third countries (countries with more than 40 samples analysed), the highest MRL exceedance rates (more than 10% of the samples) were reported for Malaysia, Pakistan, Sri Lanka, Vietnam, Madagascar, the Dominican Republic, Suriname, India, China, Thailand, Ethiopia, Jordan, Colombia and Kenya.
4.2.2. Results by food product
Among unprocessed food products,42 4.3% of the samples analysed in 2017 contained residues exceeding their corresponding MRLs. This percentage is higher than the one reported in the 2016 results (3.9% in 2016). The percentage of samples containing quantified residues within the legal limits was 44% in 2017 vs 47.9% in 2016, whereas samples without quantifiable residues were 51.7% in 2017 vs 48.2% in 2016 (Figure 49).
Figure 49.
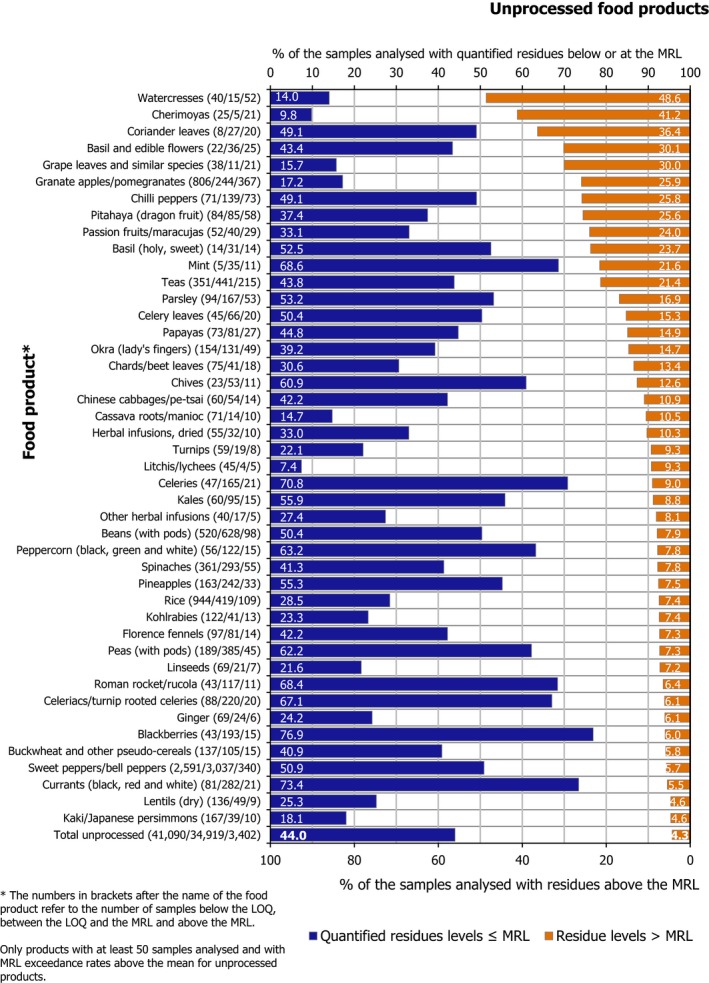
MRL exceedance rate and quantification rate for unprocessed food products in 2017, sorted by decreasing MRL exceedance rate
No MRL exceedances (products with at least 60 samples analysed) were reported for unprocessed oat, seeds from sesame and sunflower and for a number of products of animal origin, such as bovine (liver), poultry (muscle), swine (muscle, kidney, fat and liver), sheep (muscle and kidney) and goats (milk).
Among the unprocessed products with at least 50 samples analysed, the highest MRL exceedance rates (greater than 10%) were identified for watercresses, cherimoyas, coriander leaves, basil and edible flowers, grape leaves and similar species, granate apples/pomegranates, chilli peppers, pitahaya (dragon fruit), passion fruits/maracujas, basil (holy, sweet), mint, teas, parsley, celery leaves, papayas, okra (lady's fingers), chards/beet leaves, chives, chinese cabbages/pe‐tsai, cassava roots/manioc and dried herbal infusions.
Some of the products particulary exceeding the MRL were risk‐based samples subject to increased import controls (i.e. coriander leaves, basil, grape leaves, pomegranates, chilli peppers, pitahaya, basil, mint, teas, parsley, celery leaves and okra) falling within the 2017 amendments of Regulation (EC) No. 669/2009. Although the number of exceedances identified for these risk‐based samples does not represent the average pesticide levels expected to be found in these commodities, the monitoring and reporting of these results is a call for action at Member State level in line with Article 50 of Regulation (EC) No. 178/2002. Generally, Member States reply with appropriate measures to those MRL exceedances resulting in non‐compliant samples (e.g. administrative fines, Rapid Alert System for Food and Feed (RASFF) notifications43 and follow up actions, etc.). Based on the Commission's 2017 RASFF annual report,43 132 out of the 186 notifications on pesticide residues concerned rejections at the EEA border. More details on results for this specific sampling programme can be found in Section 4.2.4.
Regarding processed food products, the overall MRL exceedance rate was lower (2.7%) (Figure 50) than the one of unprocessed products (4.3%) (Figure 49). Like in 2016, frequent MRL exceedances were reported for pesticide residues in processed: grape leaves (and similar species), fruits and tree nuts, tomatoes, wild fungi, sweet peppers and rice. MRLs exceedances were also identified in milk (cattle), pumpkin seeds and table grapes (more than 4% of the samples). Therefore, it is suggested to continue monitoring the above listed food items in the national control plans, especially those food items not covered by the 3‐year EUCP rolling programme (e.g. grape leaves and wild fungi).
Figure 50.
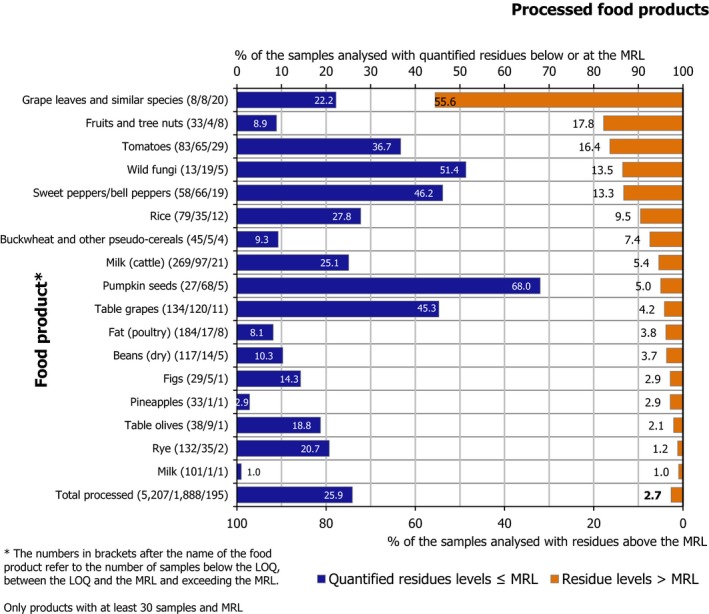
MRL exceedance rate and quantification rate for processed food products (excluding baby foods), sorted by decreasing MRL exceedance rate
4.2.3. Results by pesticide
In 2017, more than 20 million analytical determinations (individual results) were submitted to EFSA and used for the analysis presented in this report. The number of single determinations for which the residue levels were quantified at or above the LOQ amounted to 104,880 (0.52% of the total determinations; 0.56% in 2016) in relation to 40,326 total samples (41,722 in 2016) and 353 different pesticides (350 in 2016).
As in 2016, the pesticides mostly quantified (in terms of absolute numbers of positive analysis at or above the LOQ) were boscalid (6,597 determinations), imazalil (4,511 determinations), fludioxonil (4,290 determinations), acetamiprid (3,312 determinations), azoxystrobin (3,312 determinations) and chlorpyrifos (3,257 determinations) (Table C.1, Appendix C).
Table C.1.
Scope of the 2017 pesticide analyses in alphabetical order by pesticide name
| Pesticide | No. of analysis | No. of quantifications (levels > LOQ) | Quantification rate (%) | No of countries analysing | Pesticide covered by 2017 EUCP |
|---|---|---|---|---|---|
| 1,1‐dichloro‐2,2‐bis(4‐ethylphenyl)ethane | 5,503 | 0 | 5 | ||
| 1,2‐Dibromo‐3‐chloropropane | 2,318 | 0 | 4 | ||
| 1,4‐Dimethylnaphthalene | 337 | 0 | 1 | ||
| 1‐Naphthylacetamide | 22,213 | 31 | 0.14 | 10 | |
| 1‐Naphthylacetic acid | 5,080 | 5 | 0.10 | 3 | |
| 2,3,4,5‐TCNB (2,3,4,5‐Tetrachloronitrobenzene) | 1,519 | 0 | 1 | ||
| 2,3,5‐Trimethacarb | 5,703 | 0 | 2 | ||
| 2,4,5‐T (RD) | 3,805 | 0 | 9 | ||
| 2,4‐D (RD) | 19,409 | 242 | 1.25 | 23 | Yes |
| 2,4‐DB (RD) | 13,279 | 1 | 0.01 | 14 | |
| 2,4‐Dichlorobenzamide | 341 | 0 | 1 | ||
| 2‐Naphthyloxyacetic acid | 12,221 | 1 | 0.01 | 6 | |
| 2‐phenylphenol | 52,595 | 1,060 | 2.02 | 28 | Yes |
| 3,4,5‐Trimethacarb | 2,469 | 0 | 4 | ||
| 4‐CPA | 13,203 | 7 | 0.05 | 7 | |
| 6‐Benzyladenine | 10,171 | 0 | 8 | ||
| 8‐hydroxyquinoline | 23 | 0 | 1 | ||
| Abamectin (RD) | 39,249 | 65 | 0.17 | 24 | Yes |
| Acephate | 71,250 | 73 | 0.10 | 30 | Yes |
| Acequinocyl | 2,806 | 3 | 0.11 | 3 | |
| Acetamiprid (RD) | 69,348 | 3,312 | 4.78 | 29 | Yes |
| Acetochlor | 19,293 | 1 | 0.01 | 17 | |
| Acibenzolar‐S‐methyl (RD) | 10,480 | 0 | 10 | ||
| Acifluorfen | 2,441 | 0 | 1 | ||
| Aclonifen | 40,649 | 41 | 0.10 | 24 | |
| Acrinathrin | 70,666 | 123 | 0.17 | 30 | Yes |
| Alachlor | 30,882 | 3 | 0.01 | 20 | |
| Alanycarb | 5,354 | 0 | 2 | ||
| Aldicarb (RD) | 60,041 | 0 | 28 | Yes | |
| Aldimorph | 56 | 0 | 1 | ||
| Allethrin | 10,892 | 0 | 12 | ||
| Allidochlor | 4,253 | 0 | 2 | ||
| Alloxydim | 115 | 0 | 1 | ||
| Ametoctradin (RD) | 37,445 | 177 | 0.47 | 19 | |
| Ametryn | 26,490 | 4 | 0.02 | 14 | |
| Amidithion | 2,010 | 0 | 2 | ||
| Amidosulfuron (RD) | 17,541 | 0 | 12 | ||
| Aminocarb | 16,522 | 0 | 10 | ||
| Aminopyralid | 3,780 | 2 | 0.05 | 4 | |
| Amisulbrom | 10,625 | 0 | 10 | ||
| Amitraz (RD) | 34,849 | 40 | 0.11 | 26 | |
| Amitrole | 4,414 | 0 | 6 | ||
| Ancymidol | 8,335 | 0 | 3 | ||
| Anilazine | 2,711 | 0 | 3 | ||
| Anilofos | 5,307 | 0 | 5 | ||
| Anthraquinone | 30,124 | 241 | 0.80 | 15 | |
| Aramite | 122 | 0 | 3 | ||
| Aspon | 6,909 | 0 | 4 | ||
| Asulam | 15,589 | 0 | 11 | ||
| Atraton | 4,078 | 0 | 3 | ||
| Atrazine | 50,559 | 7 | 0.01 | 25 | |
| Azaconazole | 23,690 | 0 | 14 | ||
| Azadirachtin | 17,682 | 16 | 0.09 | 9 | |
| Azamethiphos | 11,802 | 0 | 14 | ||
| Azimsulfuron | 9,068 | 0 | 7 | ||
| Azinphos‐ethyl | 59,522 | 2 | 0.003 | 28 | |
| Azinphos‐methyl | 69,041 | 1 | 0.001 | 30 | Yes |
| Aziprotryne | 6,328 | 0 | 4 | ||
| Azoxybenzene | 1,696 | 0 | 1 | ||
| Azoxystrobin | 75,237 | 3,312 | 4.40 | 30 | Yes |
| BAC (RD) | 11,102 | 233 | 2.10 | 11 | |
| Barban | 2,546 | 0 | 2 | ||
| Beflubutamid | 17,016 | 0 | 11 | ||
| Benalaxyl | 51,299 | 14 | 0.03 | 24 | |
| Benazolin | 2,451 | 0 | 2 | ||
| Bendiocarb | 31,745 | 6 | 0.02 | 17 | |
| Benfluralin | 32,714 | 5 | 0.02 | 14 | |
| Benfuresate | 2,484 | 0 | 2 | ||
| Benodanil | 3,921 | 0 | 4 | ||
| Bensulfuron | 29 | 0 | 1 | ||
| Bensulfuron‐methyl | 8,912 | 0 | 8 | ||
| Bensulide | 4,231 | 0 | 2 | ||
| Bensultap | 2,441 | 0 | 1 | ||
| Bentazone (RD) | 11,925 | 4 | 0.03 | 17 | |
| Benthiavalicarb | 8,804 | 0 | 10 | ||
| Benzobicyclon | 1,530 | 0 | 1 | ||
| Benzovindiflupyr | 6,541 | 0 | 8 | ||
| Benzoximate | 6,463 | 0 | 6 | ||
| Benzoylprop | 466 | 0 | 1 | ||
| Benzoylprop‐Ethyl | 6,759 | 0 | 6 | ||
| Benzthiazuron | 1 | 0 | 1 | ||
| Bifenazate (RD) | 11,885 | 65 | 0.55 | 11 | |
| Bifenox | 25,230 | 2 | 0.01 | 16 | |
| Bifenthrin | 77,269 | 744 | 0.96 | 30 | Yes |
| Bioallethrin | 3,372 | 1 | 0.03 | 5 | |
| Bioresmethrin | 3,684 | 0 | 6 | ||
| Biphenyl | 50,956 | 39 | 0.08 | 28 | Yes |
| Bis(tributyltin) oxide | 24 | 0 | 1 | ||
| Bispyribac | 6,994 | 0 | 5 | ||
| Bitertanol | 67,846 | 5 | 0.01 | 29 | Yes |
| Bixafen (RD) | 37,325 | 2 | 0.01 | 20 | |
| Boscalid (RD) | 73,583 | 6,597 | 8.97 | 29 | Yes |
| Bromacil | 26,304 | 1 | 0.004 | 14 | |
| Bromadiolone | 398 | 0 | 2 | ||
| Bromfenvinfos | 3,543 | 0 | 7 | ||
| Bromfenvinfos‐methyl | 1,053 | 0 | 4 | ||
| Bromide ion | 3,896 | 269 | 6.90 | 23 | Yes |
| Bromobutide | 1,181 | 0 | 2 | ||
| Bromocyclen | 8,097 | 0 | 5 | ||
| Bromofenoxim | 1 | 0 | 1 | ||
| Bromophos | 38,765 | 0 | 23 | ||
| Bromophos‐ethyl | 52,919 | 0 | 25 | ||
| Bromopropylate | 73,608 | 9 | 0.01 | 30 | Yes |
| Bromoxynil | 16,821 | 0 | 15 | ||
| Bromuconazole | 62,878 | 12 | 0.02 | 29 | |
| Bupirimate | 74,577 | 179 | 0.24 | 30 | Yes |
| Buprofezin | 74,200 | 755 | 1.02 | 30 | Yes |
| Butachlor | 7,239 | 0 | 9 | ||
| Butafenacil | 12,746 | 0 | 10 | ||
| Butamifos | 5,324 | 0 | 3 | ||
| Butocarboxim | 15,700 | 0 | 11 | ||
| Butoxycarboxim | 13,698 | 0 | 11 | ||
| Butralin | 19,291 | 0 | 12 | ||
| Buturon | 4,857 | 0 | 6 | ||
| Butylate | 10,993 | 0 | 8 | ||
| Cadusafos | 62,301 | 1 | 0.002 | 29 | |
| Cafenstrole | 3,314 | 0 | 2 | ||
| Camphechlor (RD) | 60 | 0 | 1 | ||
| Captafol | 19,223 | 0 | 16 | ||
| Captan (RD) | 22,590 | 1,117 | 4.94 | 21 | Yes |
| Carbaryl | 74,160 | 19 | 0.03 | 30 | Yes |
| Carbendazim (RD) | 60,330 | 1,491 | 2.47 | 27 | Yes |
| Carbetamide | 27,070 | 0 | 16 | ||
| Carbofuran (RD) | 50,250 | 50 | 0.10 | 26 | Yes |
| Carbophenothion | 28,275 | 1 | 0.004 | 17 | |
| Carboxin | 50,574 | 2 | 0.004 | 26 | |
| Carfentrazone‐ethyl | 8,347 | 0 | 11 | ||
| Carpropamid | 3,022 | 0 | 5 | ||
| Chinomethionat | 32,371 | 0 | 20 | ||
| Chloramben | 10 | 0 | 1 | ||
| Chlorantraniliprole | 61,989 | 1,549 | 2.50 | 27 | Yes |
| Chlorates | 5,750 | 713 | 12.40 | 8 | |
| Chlorbenside | 9,410 | 0 | 12 | ||
| Chlorbromuron | 22,355 | 0 | 14 | ||
| Chlorbufam | 20,506 | 0 | 15 | ||
| Chlordane (RD) | 39,064 | 5 | 0.01 | 27 | Yes |
| Chlordecone | 2,411 | 212 | 8.79 | 4 | |
| Chlordimeform | 6,094 | 0 | 6 | ||
| Chlorfenapyr | 67,710 | 265 | 0.39 | 28 | Yes |
| Chlorfenethol | 1,696 | 0 | 1 | ||
| Chlorfenprop‐Methyl | 8,220 | 0 | 6 | ||
| Chlorfenson | 28,773 | 1 | 0.003 | 21 | |
| Chlorfenvinphos | 66,381 | 4 | 0.01 | 29 | |
| Chlorfluazuron | 26,743 | 6 | 0.02 | 15 | |
| Chlorflurenol | 161 | 0 | 2 | ||
| Chlorflurenol‐Methyl | 158 | 0 | 1 | ||
| Chloridazon (RD) | 28,038 | 18 | 0.06 | 17 | |
| Chlorimuron | 10 | 0 | 1 | ||
| Chlormephos | 21,625 | 0 | 15 | ||
| Chlormequat | 9,638 | 755 | 7.83 | 26 | Yes |
| Chlornitrofen | 2,248 | 0 | 1 | ||
| Chlorobenzilate | 46,128 | 1 | 0.002 | 27 | |
| Chloroneb | 7,873 | 0 | 10 | ||
| Chloropropylate | 14,521 | 1 | 0.01 | 9 | |
| Chlorothalonil (RD) | 53,933 | 356 | 0.66 | 28 | Yes |
| Chlorotoluron | 32,173 | 0 | 18 | ||
| Chloroxuron | 22,074 | 0 | 14 | ||
| Chlorpropham (RD) | 67,776 | 614 | 0.91 | 28 | Yes |
| Chlorpyrifos | 79,575 | 3,257 | 4.09 | 30 | Yes |
| Chlorpyrifos‐methyl | 79,181 | 929 | 1.17 | 30 | Yes |
| Chlorsulfuron | 8,828 | 0 | 12 | ||
| Chlorthal‐dimethyl | 38,377 | 0 | 19 | ||
| Chlorthiamid | 4,341 | 0 | 5 | ||
| Chlorthion | 1,606 | 0 | 4 | ||
| Chlorthiophos | 11,034 | 0 | 10 | ||
| Chlozolinate | 42,686 | 0 | 24 | ||
| Chromafenozide | 14,062 | 1 | 0.01 | 6 | |
| Cinidon‐ethyl | 7,703 | 0 | 8 | ||
| Cinosulfuron | 10,141 | 0 | 7 | ||
| Clethodim (RD) | 23,054 | 13 | 0.06 | 13 | |
| Climbazole | 1,845 | 0 | 3 | ||
| Clodinafop | 5,790 | 0 | 9 | ||
| Clofentezine (RD) | 53,331 | 79 | 0.15 | 26 | Yes |
| Clomazone | 47,707 | 22 | 0.05 | 24 | |
| Clopyralid | 20,181 | 15 | 0.07 | 13 | |
| Cloransulam‐Methyl | 10 | 0 | 1 | ||
| Clothianidin | 66,673 | 343 | 0.51 | 30 | Yes |
| Copper | 2,830 | 2,287 | 80.81 | 3 | |
| Coumachlor | 4,138 | 0 | 1 | ||
| Coumaphos | 38,736 | 5 | 0.01 | 26 | |
| Coumatetralyl | 4,903 | 0 | 1 | ||
| Crimidine | 4,103 | 0 | 5 | ||
| Crotoxyphos | 2,528 | 0 | 3 | ||
| Crufomate | 1,775 | 0 | 3 | ||
| Cyanamide | 101 | 0 | 1 | ||
| Cyanazine | 31,114 | 0 | 18 | ||
| Cyanofenphos | 12,662 | 0 | 15 | ||
| Cyanophos | 13,466 | 0 | 11 | ||
| Cyantraniliprole | 8,339 | 74 | 0.89 | 9 | |
| Cyazofamid | 49,950 | 121 | 0.24 | 25 | |
| Cyclanilide | 6,652 | 0 | 6 | ||
| Cycloate | 12,768 | 0 | 12 | ||
| Cyclosulfamuron | 10 | 0 | 1 | ||
| Cycloxydim (RD) | 15,600 | 0 | 16 | ||
| Cycluron | 8,805 | 0 | 4 | ||
| Cyenopyrafen | 2,552 | 1 | 0.04 | 3 | |
| Cyflufenamid | 37,339 | 103 | 0.28 | 19 | |
| Cyflumetofen | 11,069 | 2 | 0.02 | 8 | |
| Cyfluthrin | 57,068 | 113 | 0.20 | 28 | Yes |
| Cyhalofop‐butyl (RD) | 6,129 | 0 | 9 | ||
| Cyhalothrin | 1,094 | 3 | 0.27 | 5 | |
| Cyhalothrin, gamma‐ | 628 | 0 | 3 | ||
| Cyhexatin (RD) | 729 | 0 | 5 | ||
| Cymiazole | 6,361 | 0 | 12 | ||
| Cymoxanil | 61,096 | 27 | 0.04 | 28 | Yes |
| Cypermethrin | 73,581 | 1,323 | 1.80 | 29 | Yes |
| Cyphenothrin | 4,436 | 0 | 5 | ||
| Cyprazin | 4,221 | 0 | 1 | ||
| Cyproconazole | 73,744 | 109 | 0.15 | 30 | Yes |
| Cyprodinil (RD) | 71,335 | 3,142 | 4.40 | 29 | Yes |
| Cyprofuram | 1,855 | 0 | 2 | ||
| Cyromazine | 33,643 | 47 | 0.14 | 21 | Yes |
| Cythioate | 1,503 | 0 | 1 | ||
| Daimuron | 2,441 | 0 | 1 | ||
| Dalapon | 2,441 | 0 | 1 | ||
| Daminozide (RD) | 1,855 | 0 | 4 | ||
| Dazomet | 1,216 | 0 | 5 | ||
| DDAC | 9,296 | 71 | 0.76 | 9 | |
| DDT (RD) | 53,981 | 229 | 0.42 | 28 | Yes |
| Deltamethrin | 75,968 | 870 | 1.15 | 30 | Yes |
| Demeton | 102 | 0 | 1 | ||
| Demeton‐O‐methyl | 38 | 0 | 1 | ||
| Demeton‐S | 4,658 | 0 | 7 | ||
| Demeton‐S‐Methyl | 34,364 | 0 | 26 | ||
| Desmedipham | 25,945 | 0 | 17 | ||
| Desmetryn | 11,716 | 0 | 13 | ||
| Diafenthiuron | 32,558 | 14 | 0.04 | 19 | |
| Dialifos | 15,861 | 0 | 11 | ||
| Di‐allate | 3,863 | 0 | 9 | ||
| Diazinon | 78,291 | 61 | 0.08 | 30 | Yes |
| Dicamba | 18,520 | 3 | 0.02 | 15 | |
| Dichlobenil | 34,182 | 0 | 21 | ||
| Dichlofenthion | 20,535 | 0 | 14 | ||
| Dichlofluanid | 56,414 | 0 | 27 | ||
| Dichlone | 21 | 0 | 2 | ||
| Dichlorophen | 1,414 | 0 | 2 | ||
| Dichlorprop (RD) | 16,172 | 4 | 0.02 | 16 | |
| Dichlorvos | 72,760 | 6 | 0.01 | 30 | Yes |
| Diclobutrazol | 25,762 | 0 | 12 | ||
| Diclofop (RD) | 13,438 | 0 | 9 | ||
| Dicloran | 70,569 | 3 | 0.004 | 30 | Yes |
| Diclosulam | 10 | 0 | 1 | ||
| Dicofol (RD) | 58,672 | 14 | 0.02 | 28 | Yes |
| Dicrotophos | 54,435 | 0 | 29 | ||
| Dicyclanil | 10 | 0 | 1 | ||
| Dieldrin (RD) | 61,081 | 48 | 0.08 | 29 | Yes |
| Dienochlor | 10 | 0 | 1 | ||
| Diethatyl | 403 | 0 | 1 | ||
| Diethofencarb | 68,660 | 2 | 0.003 | 30 | Yes |
| Difenoconazole | 73,706 | 2,110 | 2.86 | 30 | Yes |
| Difenoxuron | 3,090 | 0 | 5 | ||
| Difenzoquat | 2,628 | 0 | 4 | ||
| Diflubenzuron (RD) | 59,615 | 106 | 0.18 | 28 | Yes |
| Diflufenican | 45,138 | 5 | 0.01 | 22 | |
| Diflufenzopyr | 5,093 | 0 | 3 | ||
| Dikegulac | 2,540 | 1 | 0.04 | 3 | |
| Dimefox | 4,315 | 0 | 8 | ||
| Dimefuron | 13,238 | 0 | 6 | ||
| Dimepiperate | 2,965 | 0 | 3 | ||
| Dimethachlor | 19,751 | 3 | 0.02 | 16 | |
| Dimethenamid–p | 16,425 | 2 | 0.01 | 15 | |
| Dimethipin | 974 | 0 | 1 | ||
| Dimethirimol | 1,513 | 0 | 2 | ||
| Dimethoate (RD) | 70,206 | 264 | 0.38 | 30 | Yes |
| Dimethomorph | 68,755 | 1,335 | 1.94 | 30 | Yes |
| Dimethylvinphos | 4,105 | 0 | 4 | ||
| Dimetilan | 2,185 | 0 | 3 | ||
| Dimoxystrobin (RD) | 38,447 | 26 | 0.07 | 26 | |
| Diniconazole | 68,179 | 9 | 0.01 | 29 | Yes |
| Dinitramine | 4,884 | 0 | 7 | ||
| Dinobuton | 2,619 | 0 | 3 | ||
| Dinocap (RD) | 5,776 | 0 | 10 | ||
| Dinoseb (RD) | 5,040 | 0 | 5 | ||
| Dinotefuran | 37,676 | 25 | 0.07 | 21 | |
| Dinoterb (RD) | 3,014 | 0 | 4 | ||
| Dioxabenzofos | 1,439 | 0 | 4 | ||
| Dioxacarb | 14,342 | 0 | 10 | ||
| Dioxathion | 11,453 | 0 | 13 | ||
| Diphenamid | 14,881 | 0 | 11 | ||
| Diphenylamine | 70,837 | 86 | 0.12 | 30 | Yes |
| Dipropetryn | 3,513 | 0 | 5 | ||
| Diquat | 669 | 6 | 0.90 | 7 | |
| Disulfoton (RD) | 37,221 | 0 | 26 | ||
| Ditalimfos | 22,249 | 0 | 17 | ||
| Dithianon | 14,822 | 288 | 1.94 | 19 | Yes |
| Dithiocarbamates (RD) | 15,461 | 1,350 | 8.73 | 28 | Yes |
| Dithiopyr | 5,383 | 0 | 3 | ||
| Diuron | 42,566 | 23 | 0.05 | 22 | |
| DNOC | 3,563 | 0 | 5 | ||
| Dodemorph | 13,257 | 0 | 12 | ||
| Dodine | 50,043 | 313 | 0.63 | 24 | Yes |
| Edifenphos | 10,137 | 0 | 10 | ||
| Emamectin | 18,013 | 26 | 0.14 | 15 | |
| Empenthrin | 1,229 | 0 | 2 | ||
| Endosulfan (RD) | 70,960 | 37 | 0.05 | 29 | Yes |
| Endrin | 51,310 | 3 | 0.01 | 29 | |
| EPN | 69,208 | 0 | 30 | Yes | |
| Epoxiconazole | 74,674 | 48 | 0.06 | 30 | Yes |
| EPTC | 13,097 | 0 | 14 | ||
| Esprocarb | 5,748 | 0 | 4 | ||
| Etaconazole | 14,885 | 0 | 8 | ||
| Ethalfluralin | 10,016 | 0 | 9 | ||
| Ethametsulfuron‐methyl | 3,425 | 0 | 6 | ||
| Ethephon | 8,993 | 354 | 3.94 | 25 | Yes |
| Ethidimuron | 3,919 | 0 | 4 | ||
| Ethiofencarb | 40,346 | 3 | 0.01 | 20 | |
| Ethion | 74,493 | 40 | 0.05 | 30 | Yes |
| Ethiprole | 9,749 | 1 | 0.01 | 9 | |
| Ethirimol | 62,159 | 94 | 0.15 | 29 | Yes |
| Ethofumesate (RD) | 11,936 | 0 | 17 | ||
| Ethoprophos | 67,034 | 6 | 0.01 | 30 | |
| Ethoxyquin | 23,491 | 3 | 0.01 | 16 | |
| Ethoxysulfuron | 5,764 | 0 | 5 | ||
| Ethylene oxide (RD) | 29 | 7 | 24.14 | 2 | |
| Etobenzanid | 10 | 0 | 1 | ||
| Etofenprox | 71,521 | 819 | 1.15 | 30 | Yes |
| Etoxazole | 45,186 | 92 | 0.20 | 22 | |
| Etridiazole | 29,268 | 2 | 0.01 | 17 | |
| Etrimfos | 38,857 | 0 | 26 | ||
| Famoxadone | 64,412 | 111 | 0.17 | 30 | Yes |
| Famphur | 8,741 | 0 | 6 | ||
| Fenamidone | 70,738 | 48 | 0.07 | 30 | Yes |
| Fenamiphos (RD) | 52,186 | 12 | 0.02 | 28 | Yes |
| Fenarimol | 74,477 | 3 | 0.004 | 30 | Yes |
| Fenazaflor | 1,537 | 0 | 1 | ||
| Fenazaquin | 69,181 | 33 | 0.05 | 30 | Yes |
| Fenbuconazole | 69,014 | 318 | 0.46 | 30 | Yes |
| Fenbutatin oxide | 20,792 | 52 | 0.25 | 23 | Yes |
| Fenchlorphos (RD) | 20,644 | 0 | 19 | ||
| Fenfluthrin | 1,443 | 0 | 3 | ||
| Fenfuram | 6,614 | 0 | 2 | ||
| Fenhexamid | 73,175 | 1,494 | 2.04 | 30 | Yes |
| Fenitrothion | 73,562 | 9 | 0.01 | 30 | Yes |
| Fenobucarb | 21,604 | 11 | 0.05 | 13 | |
| Fenothiocarb | 11,451 | 0 | 9 | ||
| Fenoxanil | 10 | 0 | 1 | ||
| Fenoxaprop | 13,429 | 0 | 5 | ||
| Fenoxaprop‐ethyl | 1,876 | 0 | 3 | ||
| Fenoxaprop‐P | 4,549 | 0 | 8 | ||
| Fenoxaprop‐P‐Ethyl | 6,985 | 0 | 12 | ||
| Fenoxycarb | 72,330 | 102 | 0.14 | 29 | Yes |
| Fenpiclonil | 18,478 | 0 | 10 | ||
| Fenpropathrin | 74,066 | 99 | 0.13 | 29 | Yes |
| Fenpropidin (RD) | 46,306 | 9 | 0.02 | 27 | Yes |
| Fenpropimorph (RD) | 69,274 | 64 | 0.09 | 28 | Yes |
| Fenpyrazamine | 28,048 | 62 | 0.22 | 16 | |
| Fenpyroximate (RD) | 60,618 | 156 | 0.26 | 27 | Yes |
| Fenson | 24,027 | 0 | 12 | ||
| Fensulfothion | 32,274 | 0 | 27 | ||
| Fenthion (RD) | 59,213 | 3 | 0.01 | 29 | Yes |
| Fentin | 2,678 | 0 | 13 | ||
| Fentrazamide | 10 | 0 | 1 | ||
| Fenuron | 11,737 | 0 | 12 | ||
| Fenvalerate (RD) | 53,944 | 112 | 0.21 | 28 | Yes |
| Fipronil (RD) | 50,394 | 164 | 0.33 | 29 | Yes |
| Flamprop | 1,216 | 0 | 3 | ||
| Flamprop‐isopropyl | 2,880 | 0 | 3 | ||
| Flamprop‐methyl | 4,647 | 0 | 5 | ||
| Flamprop‐M‐Isopropyl | 360 | 0 | 3 | ||
| Flamprop‐M‐Methyl | 10 | 0 | 1 | ||
| Flazasulfuron | 10,134 | 0 | 12 | ||
| Flocoumafen | 265 | 0 | 1 | ||
| Flonicamid (RD) | 27,103 | 312 | 1.15 | 22 | Yes |
| Florasulam | 18,702 | 0 | 17 | ||
| Fluacrypyrim | 5,199 | 0 | 4 | ||
| Fluazifop‐P (RD) | 32,518 | 54 | 0.17 | 20 | Yes |
| Fluazinam | 32,245 | 5 | 0.02 | 21 | |
| Fluazolate | 10 | 0 | 1 | ||
| Fluazuron | 5,077 | 0 | 2 | ||
| Flubendiamide | 39,856 | 50 | 0.13 | 27 | Yes |
| Flubenzimine | 2,154 | 0 | 2 | ||
| Fluchloralin | 8,004 | 0 | 7 | ||
| Flucycloxuron | 8,934 | 0 | 3 | ||
| Flucythrinate | 24,564 | 0 | 21 | ||
| Fludioxonil (RD) | 70,277 | 4,290 | 6.10 | 29 | Yes |
| Flufenacet | 27,421 | 1 | 0.004 | 21 | |
| Flufenoxuron | 65,719 | 18 | 0.03 | 30 | Yes |
| Flufenzin | 715 | 0 | 4 | ||
| Flumethrin | 2,438 | 0 | 3 | ||
| Flumetralin | 13,554 | 0 | 10 | ||
| Flumetsulam | 1,176 | 0 | 1 | ||
| Flumiclorac‐Pentyl | 10 | 0 | 1 | ||
| Flumioxazine | 11,413 | 0 | 9 | ||
| Fluometuron | 13,945 | 0 | 12 | ||
| Fluopicolide | 61,389 | 376 | 0.61 | 27 | |
| Fluopyram (RD) | 58,177 | 2,899 | 4.98 | 28 | Yes |
| Fluorodifen | 3,980 | 0 | 3 | ||
| Fluoroglycofene | 10 | 0 | 1 | ||
| Fluoroimide | 10 | 0 | 1 | ||
| Fluotrimazole | 11,101 | 0 | 5 | ||
| Fluoxastrobin (RD) | 30,495 | 2 | 0.01 | 15 | |
| Flupyradifurone | 5,483 | 1 | 0.02 | 5 | |
| Flupyrsulfuron‐methyl | 6,281 | 0 | 6 | ||
| Fluquinconazole | 68,468 | 4 | 0.01 | 30 | Yes |
| Fluridone | 4,743 | 0 | 5 | ||
| Flurochloridone | 22,300 | 4 | 0.02 | 15 | |
| Fluroxypyr (RD) | 15,765 | 0 | 18 | ||
| Flurprimidole | 8,775 | 0 | 6 | ||
| Flurtamone | 19,722 | 0 | 10 | ||
| Flusilazole (RD) | 71,659 | 30 | 0.04 | 29 | Yes |
| Flusulfamide | 4,263 | 0 | 3 | ||
| Fluthiacet‐Methyl | 2,652 | 0 | 3 | ||
| Flutolanil (RD) | 58,301 | 26 | 0.04 | 26 | |
| Flutriafol | 71,778 | 216 | 0.30 | 30 | Yes |
| Fluvalinate | 5,347 | 0 | 10 | ||
| Fluxapyroxad | 42,056 | 49 | 0.12 | 26 | |
| Folpet (RD) | 24,634 | 74 | 0.30 | 20 | Yes |
| Fomesafen | 10,209 | 1 | 0.01 | 6 | |
| Fonofos | 35,809 | 0 | 22 | ||
| Foramsulfuron | 8,715 | 0 | 10 | ||
| Forchlorfenuron | 26,893 | 15 | 0.06 | 16 | |
| Formetanate | 41,588 | 39 | 0.09 | 26 | Yes |
| Formothion | 40,773 | 0 | 27 | ||
| Fosetyl‐Al (RD) | 6,119 | 1,385 | 22.63 | 8 | |
| Fosthiazate | 60,814 | 42 | 0.07 | 29 | Yes |
| Fosthietan | 10 | 0 | 1 | ||
| Fuberidazole | 21,363 | 0 | 16 | ||
| Furalaxyl | 25,050 | 1 | 0.004 | 13 | |
| Furfural | 4 | 0 | 1 | ||
| Furmecyclox | 2,373 | 0 | 3 | ||
| Genite | 1,864 | 0 | 3 | ||
| Gibberellic acid | 2,790 | 20 | 0.72 | 3 | |
| Glufosinate (RD) | 4,056 | 22 | 0.54 | 7 | |
| Glyphosate | 8,672 | 212 | 2.44 | 25 | Yes |
| Griseofulvin | 42 | 0 | 1 | ||
| Halauxifen‐methyl (RD) | 1,289 | 0 | 1 | ||
| Halfenprox | 6,001 | 0 | 7 | ||
| Halofenozide | 13,461 | 1 | 0.01 | 5 | |
| Halosulfuron‐methyl | 8,390 | 0 | 4 | ||
| Haloxyfop (RD) | 25,822 | 44 | 0.17 | 25 | |
| Heptachlor (RD) | 34,026 | 2 | 0.01 | 27 | Yes |
| Heptenophos | 40,629 | 0 | 27 | ||
| Hexachlorobenzene | 53,538 | 282 | 0.53 | 29 | Yes |
| Hexachlorobutadiene | 302 | 0 | 3 | ||
| Hexachlorocyclohexane (alpha) | 39,902 | 12 | 0.03 | 26 | Yes |
| Hexachlorocyclohexane (beta) | 39,837 | 29 | 0.07 | 26 | Yes |
| Hexachlorocyclohexane (RD) | 34,962 | 1 | 0.003 | 23 | |
| Hexaconazole | 73,608 | 79 | 0.11 | 30 | Yes |
| Hexaflumuron | 37,315 | 2 | 0.01 | 21 | |
| Hexazinone | 29,622 | 1 | 0.003 | 15 | |
| Hexythiazox | 67,359 | 433 | 0.64 | 30 | Yes |
| Hydramethylnon | 924 | 0 | 3 | ||
| Hydrogen phosphide | 96 | 9 | 9.38 | 3 | |
| Hymexazol | 4,164 | 0 | 3 | ||
| Imazalil | 73,698 | 4,511 | 6.12 | 30 | Yes |
| Imazamethabenz | 5,158 | 0 | 6 | ||
| Imazamox | 16,239 | 13 | 0.08 | 12 | |
| Imazapic | 1,291 | 0 | 2 | ||
| Imazapyr | 17,227 | 5 | 0.03 | 13 | |
| Imazaquin | 15,153 | 0 | 9 | ||
| Imazethapyr | 11,101 | 3 | 0.03 | 9 | |
| Imazosulfuron | 8,027 | 0 | 6 | ||
| Imibenconazole | 7,976 | 0 | 5 | ||
| Imidacloprid | 71,730 | 2,771 | 3.86 | 30 | Yes |
| Inabenfide | 2,806 | 0 | 3 | ||
| Indolylbutyric acid | 2,441 | 0 | 1 | ||
| Indoxacarb | 73,267 | 797 | 1.09 | 30 | Yes |
| Iodofenphos | 16,108 | 0 | 12 | ||
| Iodosulfuron‐methyl | 13,300 | 0 | 14 | ||
| Ioxynil (RD) | 18,204 | 0 | 18 | ||
| Ipconazole | 13,833 | 0 | 13 | ||
| Iprobenfos | 15,055 | 0 | 11 | ||
| Iprodione (RD) | 69,690 | 1,710 | 2.45 | 29 | Yes |
| Iprovalicarb | 72,064 | 110 | 0.15 | 30 | Yes |
| Isazofos | 12,932 | 0 | 11 | ||
| Isobenzan | 3,208 | 0 | 3 | ||
| Isocarbamid | 1,696 | 0 | 1 | ||
| Isocarbophos | 57,147 | 7 | 0.01 | 30 | Yes |
| Isodrin | 10,543 | 0 | 10 | ||
| Isofenphos | 41,102 | 0 | 25 | ||
| Isofenphos‐methyl | 59,105 | 0 | 28 | ||
| Isomethiozin | 3,644 | 0 | 3 | ||
| Isonoruron | 1,796 | 0 | 3 | ||
| Isoprocarb | 49,761 | 1 | 0.002 | 26 | |
| Isopropalin | 9,371 | 0 | 8 | ||
| Isoprothiolane | 58,526 | 140 | 0.24 | 28 | Yes |
| Isoproturon | 49,007 | 1 | 0.002 | 27 | |
| Isopyrazam | 22,741 | 26 | 0.11 | 15 | |
| Isouron | 1,242 | 0 | 2 | ||
| Isoxaben | 23,330 | 3 | 0.01 | 12 | |
| Isoxaflutole (RD) | 11,661 | 0 | 14 | ||
| Isoxathion | 10,632 | 0 | 8 | ||
| Ivermectin | 1,008 | 0 | 5 | ||
| Karbutilate | 1,172 | 0 | 1 | ||
| Kresoxim‐methyl (RD) | 71,089 | 206 | 0.29 | 29 | Yes |
| Lactofen | 6,521 | 0 | 4 | ||
| Lambda‐cyhalothrin (RD) | 56,905 | 1,159 | 2.04 | 28 | Yes |
| Lenacil | 35,030 | 16 | 0.05 | 20 | |
| Leptophos | 9,335 | 0 | 10 | ||
| Lindane | 62,219 | 36 | 0.06 | 30 | Yes |
| Linuron | 68,646 | 478 | 0.70 | 30 | Yes |
| Lufenuron | 63,273 | 84 | 0.13 | 29 | Yes |
| Malathion (RD) | 68,057 | 168 | 0.25 | 29 | Yes |
| Maleic hydrazide (RD) | 5,543 | 175 | 3.16 | 9 | |
| Mandestrobin | 1,148 | 0 | 2 | ||
| Mandipropamid | 65,786 | 328 | 0.50 | 29 | Yes |
| MCPA (RD) | 18,357 | 5 | 0.03 | 19 | |
| Mecarbam | 50,475 | 3 | 0.01 | 28 | |
| Mecoprop | 12,436 | 2 | 0.02 | 17 | |
| Mefenacet | 8,895 | 0 | 7 | ||
| Mefluidide | 4,573 | 0 | 2 | ||
| Mepanipyrim | 70,419 | 140 | 0.20 | 29 | Yes |
| Mephosfolan | 11,172 | 0 | 9 | ||
| Mepiquat | 13,215 | 241 | 1.82 | 26 | Yes |
| Mepronil | 36,794 | 2 | 0.01 | 20 | |
| Meptyldinocap (RD) | 11,661 | 7 | 0.06 | 8 | |
| Mercury | 1,294 | 91 | 7.03 | 1 | |
| Merphos | 10 | 0 | 1 | ||
| Mesosulfuron | 11,229 | 0 | 9 | ||
| Mesotrione | 7,280 | 0 | 9 | ||
| Metaflumizone | 46,865 | 49 | 0.10 | 24 | |
| Metalaxyl | 60,771 | 1,003 | 1.65 | 30 | Yes |
| Metaldehyde | 5,836 | 13 | 0.22 | 4 | |
| Metamitron | 44,575 | 13 | 0.03 | 22 | |
| Metazachlor (RD) | 16,156 | 0 | 15 | ||
| Metconazole | 63,188 | 9 | 0.01 | 28 | |
| Methabenzthiazuron | 27,186 | 1 | 0.004 | 14 | |
| Methacrifos | 45,330 | 0 | 27 | ||
| Methamidophos | 70,655 | 61 | 0.09 | 30 | Yes |
| Methidathion | 76,995 | 19 | 0.02 | 30 | Yes |
| Methiocarb (RD) | 66,090 | 38 | 0.06 | 30 | Yes |
| Methomyl (RD) | 81,876 | 57 | 0.06 | 30 | Yes |
| Methoprene | 2,987 | 0 | 6 | ||
| Methoprotryne | 9,609 | 0 | 9 | ||
| Methothrin | 13 | 0 | 1 | ||
| Methoxychlor | 57,491 | 2 | 0.003 | 30 | Yes |
| Methoxyfenozide | 69,485 | 595 | 0.86 | 30 | Yes |
| Metobromuron | 50,637 | 10 | 0.02 | 26 | |
| Metolachlor | 20,019 | 4 | 0.02 | 18 | |
| Metolcarb | 12,486 | 0 | 9 | ||
| Metominostrobin | 5,248 | 0 | 2 | ||
| Metosulam | 19,125 | 0 | 12 | ||
| Metoxuron | 22,999 | 0 | 17 | ||
| Metrafenone | 52,643 | 474 | 0.90 | 27 | |
| Metribuzin | 58,959 | 14 | 0.02 | 29 | |
| Metsulfuron‐methyl | 23,230 | 0 | 15 | ||
| Mevinphos (RD) | 56,232 | 0 | 28 | ||
| Milbemectin (RD) | 7,174 | 0 | 2 | ||
| Mirex | 16,523 | 0 | 14 | ||
| Molinate | 26,363 | 0 | 17 | ||
| Monalide | 6,840 | 0 | 2 | ||
| Monocrotophos | 70,165 | 11 | 0.02 | 29 | Yes |
| Monolinuron | 30,149 | 0 | 21 | ||
| Monuron | 14,160 | 0 | 9 | ||
| Myclobutanil (RD) | 71,877 | 788 | 1.10 | 29 | Yes |
| Naled | 12,553 | 0 | 10 | ||
| Napropamide | 38,000 | 9 | 0.02 | 19 | |
| Naptalam | 5,227 | 0 | 3 | ||
| Neburon | 9,166 | 0 | 10 | ||
| Nicosulfuron | 17,005 | 0 | 13 | ||
| Nicotine | 3,364 | 102 | 3.03 | 5 | |
| Nitenpyram | 50,285 | 1 | 0.002 | 26 | |
| Nitralin | 6,958 | 0 | 8 | ||
| Nitrapyrin | 5,330 | 0 | 4 | ||
| Nitrofen | 45,620 | 0 | 29 | ||
| Nitrothal‐Isopropyl | 15,555 | 0 | 11 | ||
| Norflurazon | 7,056 | 0 | 9 | ||
| Novaluron | 29,236 | 21 | 0.07 | 15 | |
| Noviflumuron | 2,441 | 0 | 1 | ||
| Nuarimol | 37,755 | 0 | 22 | ||
| Ofurace | 23,361 | 0 | 13 | ||
| Orbencarb | 3,659 | 0 | 3 | ||
| Orthosulfamuron | 1,616 | 0 | 2 | ||
| Oryzalin | 7,591 | 0 | 3 | ||
| Oxadiargyl | 15,331 | 0 | 9 | ||
| Oxadiazon | 37,755 | 9 | 0.02 | 19 | |
| Oxadixyl | 70,064 | 3 | 0.004 | 30 | Yes |
| Oxamyl | 68,568 | 11 | 0.02 | 28 | Yes |
| Oxasulfuron | 9,343 | 0 | 6 | ||
| Oxaziclomefone | 10 | 0 | 1 | ||
| Oxycarboxin | 13,396 | 0 | 12 | ||
| Oxydemeton‐methyl (RD) | 54,609 | 0 | 26 | Yes | |
| Oxyfluorfen | 37,119 | 20 | 0.05 | 20 | |
| Paclobutrazol | 69,502 | 25 | 0.04 | 30 | Yes |
| Paraquat | 607 | 0 | 7 | ||
| Parathion | 76,327 | 1 | 0.001 | 29 | Yes |
| Parathion‐methyl (RD) | 58,377 | 3 | 0.01 | 29 | Yes |
| Pebulate | 7,350 | 0 | 8 | ||
| Penconazole | 74,112 | 440 | 0.59 | 30 | Yes |
| Pencycuron | 71,484 | 77 | 0.11 | 30 | Yes |
| Pendimethalin | 75,635 | 410 | 0.54 | 30 | Yes |
| Penflufen | 19,338 | 0 | 15 | ||
| Penfluron | 4,221 | 0 | 1 | ||
| Penoxsulam | 10,817 | 0 | 6 | ||
| Pentachlorophenol | 6,984 | 0 | 6 | ||
| Pentanochlor | 12,136 | 0 | 6 | ||
| Penthiopyrad | 25,840 | 17 | 0.07 | 17 | |
| Permethrin | 71,979 | 68 | 0.09 | 30 | Yes |
| Pethoxamid | 19,634 | 2 | 0.01 | 13 | |
| Phenkapton | 4,290 | 0 | 5 | ||
| Phenmedipham | 39,386 | 27 | 0.07 | 22 | |
| Phenothrin | 11,802 | 1 | 0.01 | 11 | |
| Phenthoate | 63,075 | 5 | 0.01 | 29 | |
| Phorate (RD) | 33,666 | 63 | 0.19 | 23 | |
| Phosalone | 73,809 | 8 | 0.01 | 29 | |
| Phosfolan | 3,248 | 0 | 6 | ||
| Phosmet (RD) | 56,083 | 426 | 0.76 | 30 | Yes |
| Phosphamidon | 46,007 | 0 | 27 | ||
| Phosphane and phosphide salts | 69 | 1 | 1.45 | 2 | |
| Phoxim | 53,762 | 5 | 0.01 | 28 | |
| Picloram | 5,723 | 0 | 10 | ||
| Picolinafen | 27,139 | 0 | 19 | ||
| Picoxystrobin | 46,533 | 5 | 0.01 | 23 | |
| Pinoxaden | 9,416 | 0 | 12 | ||
| Piperophos | 1,442 | 0 | 5 | ||
| Pirimicarb (RD) | 59,461 | 492 | 0.83 | 27 | Yes |
| Pirimiphos‐ethyl | 41,729 | 1 | 0.002 | 24 | |
| Pirimiphos‐methyl | 78,384 | 601 | 0.77 | 30 | Yes |
| Prallethrin | 1,027 | 0 | 4 | ||
| Pretilachlor | 6,080 | 0 | 7 | ||
| Primisulfuron | 1,368 | 0 | 1 | ||
| Primisulfuron‐Methyl | 3,865 | 0 | 6 | ||
| Probenazole | 4,137 | 0 | 1 | ||
| Prochloraz (RD) | 36,810 | 376 | 1.02 | 25 | |
| Procymidone (RD) | 69,454 | 28 | 0.04 | 29 | Yes |
| Profenofos | 76,628 | 109 | 0.14 | 30 | Yes |
| Profluralin | 15,705 | 0 | 11 | ||
| Profoxydim | 7,170 | 0 | 4 | ||
| Prohexadione | 4,024 | 2 | 0.05 | 3 | |
| Promecarb | 32,341 | 0 | 15 | ||
| Prometon | 7,915 | 0 | 8 | ||
| Prometryn | 44,470 | 2 | 0.004 | 24 | |
| Propachlor | 24,443 | 4 | 0.02 | 19 | |
| Propamocarb (RD) | 66,913 | 1,489 | 2.23 | 29 | Yes |
| Propanil | 21,442 | 2 | 0.01 | 16 | |
| Propaphos | 2,460 | 0 | 3 | ||
| Propaquizafop | 29,840 | 0 | 19 | ||
| Propargite | 72,012 | 122 | 0.17 | 30 | Yes |
| Propazine | 23,006 | 0 | 14 | ||
| Propetamphos | 22,701 | 0 | 15 | ||
| Propham | 43,257 | 0 | 25 | ||
| Propiconazole | 74,987 | 1,264 | 1.69 | 30 | Yes |
| Propineb | 83 | 0 | 1 | ||
| Propisochlor | 2,634 | 0 | 3 | ||
| Propoxur | 57,411 | 38 | 0.07 | 29 | |
| Propoxycarbazone (RD) | 6,252 | 0 | 8 | ||
| Propyzamide (RD) | 66,427 | 100 | 0.15 | 29 | Yes |
| Proquinazid | 45,786 | 99 | 0.22 | 24 | |
| Prosulfocarb | 45,917 | 230 | 0.50 | 24 | |
| Prosulfuron | 16,052 | 0 | 11 | ||
| Prothiocarb | 1,503 | 0 | 1 | ||
| Prothioconazole (RD) | 56,402 | 83 | 0.15 | 27 | |
| Prothiofos | 59,446 | 3 | 0.01 | 29 | |
| Prothoate | 4,147 | 0 | 2 | ||
| Pymetrozine (RD) | 62,672 | 236 | 0.38 | 27 | |
| Pyracarbolid | 3,843 | 0 | 3 | ||
| Pyraclofos | 6,654 | 0 | 9 | ||
| Pyraclostrobin | 71,361 | 2,951 | 4.14 | 30 | Yes |
| Pyraflufen‐ethyl (RD) | 5,078 | 0 | 7 | ||
| Pyrasulfotole | 44 | 0 | 1 | ||
| Pyrazophos | 61,307 | 0 | 28 | ||
| Pyrazoxyfen | 431 | 0 | 2 | ||
| Pyrethrins | 29,311 | 24 | 0.08 | 25 | |
| Pyribencarb | 10 | 0 | 1 | ||
| Pyributicarb | 5,406 | 0 | 3 | ||
| Pyridaben | 73,893 | 409 | 0.55 | 30 | Yes |
| Pyridafol | 3,112 | 0 | 2 | ||
| Pyridalyl | 24,374 | 43 | 0.18 | 13 | |
| Pyridaphenthion | 43,111 | 0 | 23 | ||
| Pyridate (RD) | 12,337 | 0 | 13 | ||
| Pyrifenox | 43,427 | 0 | 22 | ||
| Pyriftalid | 10 | 0 | 1 | ||
| Pyrimethanil (RD) | 72,466 | 2,887 | 3.98 | 29 | Yes |
| Pyrimidifen | 11,063 | 1 | 0.01 | 5 | |
| Pyriminobac‐Methyl | 10 | 0 | 1 | ||
| Pyriofenone | 5,132 | 2 | 0.04 | 6 | |
| Pyriproxyfen | 73,116 | 1,087 | 1.49 | 30 | Yes |
| Pyroquilon | 5,925 | 0 | 7 | ||
| Pyroxsulam | 8,389 | 0 | 10 | ||
| Quassia | 2,397 | 0 | 1 | ||
| Quinalphos | 58,758 | 4 | 0.01 | 28 | |
| Quinclorac | 14,597 | 12 | 0.08 | 14 | |
| Quinmerac | 16,852 | 1 | 0.01 | 11 | |
| Quinoclamine | 13,305 | 0 | 12 | ||
| Quinoxyfen | 73,112 | 199 | 0.27 | 30 | Yes |
| Quintozene (RD) | 46,095 | 5 | 0.01 | 25 | |
| Quizalofop | 13,231 | 2 | 0.02 | 15 | |
| Rabenzazole | 2,443 | 0 | 1 | ||
| Resmethrin | 21,527 | 0 | 20 | ||
| Rimsulfuron | 24,164 | 1 | 0.004 | 17 | |
| Rotenone | 45,476 | 1 | 0.002 | 26 | |
| Schradan | 2,440 | 1 | 0.04 | 1 | |
| Sebuthylazine | 7,391 | 0 | 7 | ||
| Secbumeton | 2,647 | 0 | 5 | ||
| Sedaxane | 2,134 | 0 | 4 | ||
| Siduron | 6,930 | 0 | 4 | ||
| Silafluofen | 7,740 | 0 | 6 | ||
| Silthiofam | 16,834 | 0 | 8 | ||
| Simazine | 43,875 | 2 | 0.005 | 25 | |
| Simeconazole | 10 | 0 | 1 | ||
| Simetryn | 4,940 | 0 | 8 | ||
| Spinetoram | 27,121 | 45 | 0.17 | 13 | |
| Spinosad | 67,379 | 1,044 | 1.55 | 30 | Yes |
| Spirodiclofen | 66,998 | 158 | 0.24 | 29 | Yes |
| Spiromesifen | 62,728 | 476 | 0.76 | 29 | Yes |
| Spirotetramat (RD) | 28,065 | 476 | 1.70 | 17 | |
| Spiroxamine (RD) | 69,608 | 91 | 0.13 | 28 | Yes |
| Streptomycin | 17 | 0 | 2 | ||
| Sulcotrione | 12,459 | 0 | 7 | ||
| Sulfentrazone | 5,115 | 0 | 8 | ||
| Sulfometuron‐Methyl | 10 | 0 | 1 | ||
| Sulfosulfuron | 5,250 | 0 | 9 | ||
| Sulfotep | 40,832 | 1 | 0.002 | 22 | |
| Sulfoxaflor | 11,713 | 22 | 0.19 | 11 | |
| Sulfur | 78 | 7 | 8.97 | 1 | |
| Sulprofos | 10,953 | 0 | 12 | ||
| tau‐Fluvalinate | 68,910 | 53 | 0.08 | 30 | Yes |
| TCMTB | 2,777 | 0 | 4 | ||
| Tebuconazole (RD) | 71,087 | 2,951 | 4.15 | 29 | Yes |
| Tebufenozide | 69,699 | 86 | 0.12 | 30 | Yes |
| Tebufenpyrad | 73,362 | 326 | 0.44 | 30 | Yes |
| Tebupirimphos | 2,084 | 0 | 5 | ||
| Tebutam | 3,824 | 0 | 5 | ||
| Tebuthiuron | 4,682 | 0 | 4 | ||
| Tecloftalam | 4,182 | 0 | 1 | ||
| Tecnazene | 51,090 | 0 | 28 | ||
| Teflubenzuron | 60,242 | 24 | 0.04 | 28 | Yes |
| Tefluthrin | 69,119 | 20 | 0.03 | 29 | Yes |
| Tembotrione (RD) | 8,865 | 0 | 5 | ||
| Temephos | 4,906 | 0 | 5 | ||
| TEPP | 4,383 | 0 | 6 | ||
| Tepraloxydim | 15,278 | 0 | 10 | ||
| Terbacil | 14,390 | 2 | 0.01 | 11 | |
| Terbucarb | 1,368 | 0 | 2 | ||
| Terbufos | 40,457 | 1 | 0.002 | 27 | |
| Terbumeton | 13,311 | 0 | 13 | ||
| Terbuthylazine | 68,956 | 17 | 0.02 | 29 | Yes |
| Terbutryn | 42,668 | 3 | 0.01 | 22 | |
| Tetrachlorvinphos | 30,589 | 0 | 20 | ||
| Tetraconazole | 74,869 | 156 | 0.21 | 30 | Yes |
| Tetradifon | 70,402 | 4 | 0.01 | 30 | Yes |
| Tetramethrin | 50,807 | 11 | 0.02 | 27 | |
| Tetrasul | 11,511 | 0 | 7 | ||
| Thenylchlor | 3,613 | 0 | 2 | ||
| Thiabendazole (RD) | 69,784 | 2,759 | 3.95 | 29 | Yes |
| Thiacloprid | 72,889 | 1,734 | 2.38 | 30 | Yes |
| Thiamethoxam | 66,216 | 828 | 1.25 | 29 | Yes |
| Thiazopyr | 2,484 | 0 | 2 | ||
| Thidiazuron | 6,750 | 1 | 0.01 | 6 | |
| Thiencarbazone | 2,310 | 0 | 5 | ||
| Thifensulfuron | 329 | 0 | 2 | ||
| Thifensulfuron‐methyl | 20,121 | 0 | 13 | ||
| Thiobencarb | 18,696 | 0 | 10 | ||
| Thiocyclam | 5,937 | 0 | 5 | ||
| Thiofanox | 5,957 | 0 | 8 | ||
| Thiometon | 24,795 | 1 | 0.004 | 16 | |
| Thionazin | 9,435 | 0 | 11 | ||
| Thiophanate‐ethyl | 2,553 | 0 | 3 | ||
| Thiophanate‐methyl | 62,774 | 427 | 0.68 | 28 | Yes |
| Thiosultap sodium | 2,440 | 0 | 1 | ||
| Thiram | 461 | 0 | 3 | ||
| Tiocarbazil | 5,910 | 0 | 5 | ||
| Tolclofos‐methyl | 74,203 | 33 | 0.04 | 30 | Yes |
| Tolfenpyrad | 15,581 | 27 | 0.17 | 11 | |
| Tolylfluanid (RD) | 54,818 | 3 | 0.01 | 28 | Yes |
| Topramezone | 2,963 | 0 | 5 | ||
| Tralkoxydim | 15,777 | 0 | 10 | ||
| Tralomethrin | 2,758 | 0 | 6 | ||
| Transfluthrin | 8,301 | 0 | 11 | ||
| Triadimefon | 39,183 | 26 | 0.07 | 18 | Yes |
| Triadimenol (RD) | 71,192 | 401 | 0.56 | 30 | Yes |
| Tri‐allate | 31,716 | 3 | 0.01 | 19 | |
| Triamiphos | 1,697 | 0 | 4 | ||
| Triapenthenol | 1,545 | 0 | 1 | ||
| Triasulfuron | 13,216 | 0 | 15 | ||
| Triazamate | 13,083 | 0 | 9 | ||
| Triazophos | 76,454 | 50 | 0.07 | 30 | Yes |
| Triazoxide | 4,470 | 0 | 5 | ||
| Tribenuron‐methyl | 6,735 | 0 | 11 | ||
| Tribufos | 3,432 | 0 | 4 | ||
| Trichlamide | 2,440 | 0 | 1 | ||
| Trichlorfon | 60,176 | 3 | 0.005 | 28 | |
| Trichloronat | 17,934 | 0 | 10 | ||
| Triclopyr | 24,449 | 11 | 0.04 | 18 | |
| Tricyclazole | 52,831 | 269 | 0.51 | 27 | |
| Tridemorph | 6,739 | 0 | 7 | ||
| Tridiphane | 1,695 | 0 | 1 | ||
| Trietazine | 2,597 | 0 | 2 | ||
| Trifloxystrobin (RD) | 72,058 | 1,335 | 1.85 | 29 | Yes |
| Trifloxysulfuron | 4,230 | 1 | 0.02 | 2 | |
| Triflumizole (RD) | 20,178 | 28 | 0.14 | 15 | |
| Triflumuron | 62,495 | 64 | 0.10 | 30 | Yes |
| Trifluralin | 64,406 | 6 | 0.01 | 29 | |
| Triflusulfuron | 1,459 | 0 | 2 | ||
| Triflusulfuron‐Methyl | 11,008 | 0 | 7 | ||
| Triforine | 30,541 | 1 | 0.003 | 20 | |
| Trimethacarb | 3,800 | 0 | 4 | ||
| Trimethyl‐sulfonium cation | 3,596 | 96 | 2.67 | 4 | |
| Trinexapac | 8,151 | 41 | 0.50 | 8 | |
| Trinexapac‐Ethyl | 8,578 | 1 | 0.01 | 8 | |
| Triticonazole | 66,572 | 2 | 0.003 | 29 | |
| Tritosulfuron | 8,654 | 0 | 8 | ||
| Uniconazole | 5,166 | 1 | 0.02 | 7 | |
| Valifenalate | 14,775 | 0 | 9 | ||
| Vamidothion | 31,364 | 0 | 25 | ||
| Vernolate | 2,440 | 0 | 1 | ||
| Vinclozolin | 54,046 | 1 | 0.002 | 26 | Yes |
| Warfarin | 423 | 0 | 2 | ||
| XMC | 2,786 | 0 | 2 | ||
| Ziram | 408 | 0 | 2 | ||
| Zoxamide | 63,495 | 54 | 0.09 | 29 |
Of the 650 cultivated fungi samples analysed, 9 were found to contain nicotine, 5 of them above the MRL. The pesticides with the highest quantification rates in cultivated fungi were mepiquat (quantified in 103 samples) and chlormequat (quantified in 65 samples). No MRL exceedance was observed for these commodities; the presence of both residues is likely to be due to carry‐over from their use in cereals, e.g. cultivated fungi grown on substrate composed of cereals straw treated with chlormequat (EFSA, 2016c).
MRL exceedances were found in 4,681 analytical determinations. The pesticides most frequently exceeding their corresponding MRLs are presented in Figure 51 (only pesticides with more than 0.05% of MRL exceedances and with at least 2,000 samples analysed). The pesticide with the highest MRL exceedance rate was chlorate4 (6.4% of the samples exceeding the default MRL of 0.01 mg/kg)). This result, being out of scale, is not appearing in Figure 51. Chlorates and copper, contributing to the 2.8% of all exceedances are not necessarily associated with pesticide uses as they may also originate from other uses (see Section 4.3). Anthraquinone (found in tea and infusions from third countries), carbendazim (RD) (found in third‐country originating lemons, teas, pitahaya, sweet peppers, pomegranates and rice), propargite (found in tea samples from China and India), tolfenpyrad (found in tea), acephate (found in beans (with pods), okra and rice) and profenofos (found in chilli peppers, basil and edible flowers and peas (with pods)) are among the EU non‐approved pesticides the most frequently found to exceed the MRLs. They were mainly identified in samples coming from third countries.
Figure 51.

Frequency of MRL exceedances per pesticide and sample origin
The findings of the non‐approved substance carbofuran (RD) in goji berries from China were considered and included in the 2018 amendments of Regulation (EC) No 669/2009.
Information on the number of analyses/determinations, the number of positive quantifications per pesticide, the quantification rate and the number of countries analysing for the single pesticides is available in Appendix C, Table C.1.
4.2.4. Results of glyphosate residues in food
Glyphosate was analysed in 2017 by 25 reporting countries. Overall, 8,672 samples of different food products (including processed products) were analysed for glyphosate residues; of these, 71 were baby food samples44 and 306 were food samples of animal origin (including honey). The results showed that in 97.5% of the samples glyphosate was not quantified. In 2.2% of the samples (191 samples), glyphosate was quantified at levels above the LOQ but below the MRL and in 21 samples (0.2%), the residue levels exceeded the MRL. Glyphosate residues were not quantified in baby food samples.44
MRL exceedances were identified in samples from Germany (7 samples of honey), Italy (1 samples of asparagus and 1 sample of rye), Poland (1 sample of buckwheat and 1 sample of pears), Austria (1 sample of honey), France (1 sample of rice) and 8 of unknown origin (5 samples of rye and 3 samples of buckwheat).
In Figure 52, detailed quantification and MRL exceedance rates for glyphosate are plotted by food product where at least 10 samples were reported. The highest occurrence rate was reported for dry lentils.
Figure 52.

Glyphosate quantification and MRL exceedances rates
The use of plant protection products containing glyphosate trimesium, a variant of glyphosate, may lead not only to residues of glyphosate, but also to residues of trimethyl‐sulfonium cation, a compound for which specific MRLs have been established.
Trimethyl‐sulfonium cation was analysed in 3,596 samples, of which 97.3% were free of quantifiable residues. In 2.2% of the samples (78 samples), residues were above the LOQ but below the MRL and in 0.2% of the samples (18 samples) the MRL of trimethyl‐sulfonium cation was exceeded.
In Figure 53, detailed quantification and MRL exceedance rates for trimethyl‐sulfonium cation are plotted by food product where at least 10 samples were reported. The highest quantification rate was in cultivated fungi, followed by grapefruit.
Figure 53.
Trimethyl‐sulfonium cation quantification and MRL exceedances rates
MRL exceedances were reported in samples from China (4 samples of tea), Japan (4 samples of tea), India (3 samples of tea), Germany (2 samples of cultivated fungi), Nepal (1 sample of tea), Vietnam (1 sample of tea) and unknown origin (2 samples of tea and 1 of dried herbal infusions).
4.2.5. Results on import controls under Regulation (EC) No 669/2009
According to the provisions of Regulation (EC) No. 669/200945 on import controls, in 2017 certain food products from Benin, Cambodia, China, the Dominican Republic, Egypt, Kenya, Thailand, Turkey, and Vietnam were subject to an increased level of official controls for certain pesticides at the point of entry into the EU territory. A description of the required controls (type of products, countries of origin and the type of hazard) relevant for the calendar year 2017 can be found in Appendix C, Table C.2.
Table C.2.
Food to be analysed in 2017 according to Regulation (EC) No 669/2009 on import controls
| Country of origin | Food | Food name (code) in food classification under Reg. 396/2005a |
|---|---|---|
| Cambodia | Aubergines | |
| Chinese celery (Apium graveolens) | Celery leaves (0256030) | |
| Yardlong beans (Vigna unguiculata spp. sesquipedalis) | Beans with pods (0260010) | |
| China | Broccoli | |
| Tea leaves, whether or not flavoured | ||
| Dominican Republic | Aubergines | |
| Bitter melon (Mormodica charantia) | Courgettes (0232030) | |
| Peppers (Capsicum spp.) | ||
| Yardlong beans (Vigna unguiculata spp. sesquipedalis) | Beans with pods (0260010) | |
| Egypt | Peppers (Capsicum spp.) | |
| Strawberries | ||
| Kenya | Peas with pods | |
| Thailand | Aubergines | |
| Peppers (Capsicum spp.) | ||
| Yardlong beans (Vigna unguiculata spp. sesquipedalis) | Beans with pods (0260010) | |
| Turkey | Lemons | |
| Peppers (Capsicum spp.) | ||
| Vine leaves | ||
| Vietnam | Basil (holy, sweet) | |
| Coriander leaves | Celery leaves (0256030) | |
| Dragon fruit (Pitahaya) | Prickly pears/cactus fruits (0162040) | |
| Mint | Basil (0256080) | |
| Okra | Okra/lady's finger | |
| Parsley | ||
| Peppers (Capsicum spp.) |
Corresponding name in the food classification under Regulation (EC) No 396/2005 (only if the food product to be analysed under Regulation 669/2005 is not listed in Annex I, Part A of Regulation 212/2013).
As for the 2016 EU report on pesticide residues, the information presented in this paragraph is based on the 2017 results as provided by the European Commission, i.e. summary statistics on the exceedance rate with no detailed information on the pesticides analysed and quantified.
In 2017, 76,789 consignments of products covered by Regulation (EC) No. 669/2009 on increased level of official controls on imports of certain feed and food of non‐animal origin were imported to the European Union; 10,089 of these consignments were selected for laboratory analyses. 304 of these consignments (3.0%) were considered as non‐compliant with EU legislation on pesticide residue MRLs, when the measurement uncertainty was considered.
Among food commodities analysed in 2017, the highest exceedance rates were reported: vine leaves/Turkey (23.5%), peppers/the Dominican Republic (13.3%), peppers/Egypt (12.3%), pitahaya (dragon fruit)/Vietnam (11.8%), yardlong beans/Dominican Republic (10%), tea/China (9.7%), peppers (other than sweet)/Thailand (8.4%), peas (with pods)/Kenya (5.2%), pomegranates/Turkey (3.1%), broccoli/China (2.3%), aubergines/Uganda (2%), Ethiopian eggplant/Uganda (2%), lemons/Turkey (1.7%), pineapples/Benin (1.5%), sweet peppers/Turkey (1.3%), strawberries/Egypt (1.3%), aubergines/Thailand (1.2%), table grapes/Egypt (0.3%), aubergines/Cambodia (0%), peppers (other than sweet)/Vietnam (0%), yardlong beans/Thailand (0%), herbs (coriander leaves, basil, mint, parsley)/Vietnam (0%). These results are reported in Figure 54. For Ethiopian eggplants (Solanum aethiopicum in Part B of Annex I of Reg. (EC) No. 396/2005) the MRLs set for aubergines (Solanum melongena in Part A of Annex I of Reg. (EC) No. 396/2005) are applicable and used.
Figure 54.
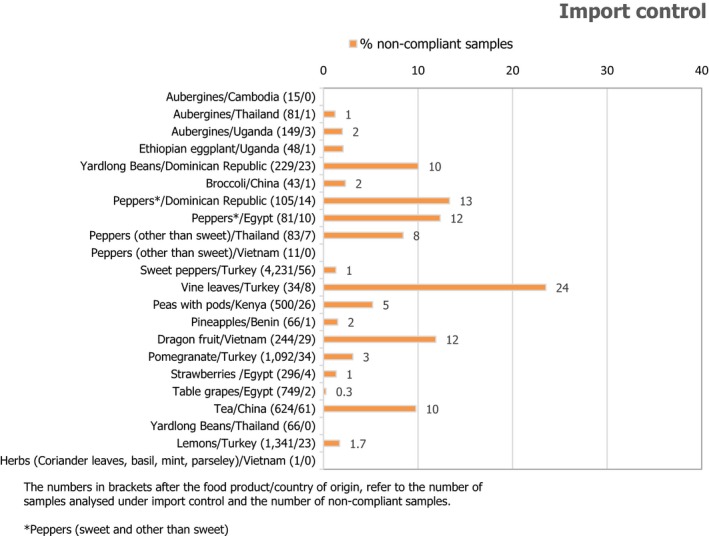
Frequency of exceedance analysed in the framework of the reinforced import controls under Regulation (EC) No 669/2009
4.2.6. Results on food for infants and young children
Reporting countries analysed 1,546 samples of foods for infants and young children as defined in Regulation (EU) No. 609/201310 and covered by Directives 2006/125/EC and 2006/141/EC (herein referred as baby food). More specifically, 384 samples of infant formulae, 298 follow‐on formulae, 231 processed cereal‐based baby foods and 633 other baby foods were analysed. Out of those, 604 samples were taken in the framework of the EUCP. No baby food samples were reported by France, Lithuania and Iceland. From the overall number of baby food samples analysed, 393 samples were flagged as organic samples.
Quantified residues (at or above the LOQ) were found in 84 samples (5.4%), while most samples did not contain quantifiable residues (94.6%). In six samples, more than one residue was quantified in the same sample (Figure 55). One sample from the Czech Republic contained seven pesticide residues, none of them exceeding the legal limits. MRL exceedances46 were reported in 1.5% of the samples (23 samples); 0.6% of the samples (9 samples) were considered non‐compliant with the legal limits, based on the measurement uncertainty. Compared with 2016, the percentage of samples free of quantifiable residues is higher than it was in 2016 (89.8%) and the exceedance rate is reduced (from 1.9% in 2016 to 1.5% in 2017).
Figure 55.
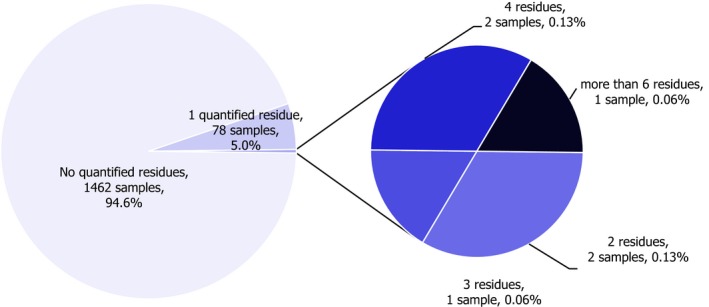
Number of quantified residues per individual baby food samples
Regarding the analytical determinations, 738 different pesticides were analysed, of which 25 were quantified in concentrations at or above the LOQ. Like in the previous reporting years, the most frequently quantified compounds in baby food were chlorates (quantified in 45 samples), followed by copper (20 samples). Pesticides found to occur in three samples were dodine, mercury and spinosad; those found in two samples were difenoconazole, ethephon, etofenprox, fosetyl‐Al (RD), myclobutanil (RD) and tebufenpyrad; the following ones were found in one sample: acetamiprid (RD), benzalkonium chloride (BAC) (RD), boscalid (RD), bromide ion, carbendazim (RD), cyprodinil (RD), fludioxonil (RD), iprodione (RD), methoxyfenozide, pyrimethanil (RD), teflubenzuron, thiacloprid, tricyclazole and trifloxystrobin (RD).
The frequency of occurrence of chlorates can be explained by the fact that they are by‐products of chlorine solutions (chlorine dioxide, chlorite and hypochlorite salts) used as sanitising and disinfection agents in the food industry and as biocides. These uses, being necessary to ensure a good hygiene of food products, lead to detectable residues of chlorate in food most probably not linked to their use as pesticides. The findings of copper can be explained by the fact that is approved as a baby food nutrient. Copper compounds may also result from other sources (natural occurrence of copper in plant or animal products or from feed additive use). The results for fosetyl‐Al may include the presence of phosphonic acid residues coming from potassium phosphonates (which can be used as a foliar feed fertiliser but is also approved as a fungicide) and disodium phosphonate which is also approved for use as a fungicide. BAC and didecyldimethylammonium chloride (DDAC) belong to a group of quaternary ammonium compounds that are widely used in biocides (disinfectants); since these substances have been used as pesticides in the past, they fall under the remit of the pesticide MRL regulation.
No residues of glyphosate and no persistent environmental contaminants were quantified in baby food samples in 2017.
4.2.7. Results on organic food
In 2017, 5,806 samples of organic food (excluding baby food)47 were analysed in total (6.6% of total number of samples vs 6.5% in 2016); the 965 samples of organic products taken in the framework of the EUCP were also included in the total number of samples.
Overall, 5,010 samples did not contain quantifiable residues (86.3% of the analysed samples vs 83.1% in 2016); 711 samples contained quantified residues below or at the MRL level (12.2% vs 16.9% in 2016) and 85 samples were reported with residue levels above their corresponding MRLs (1.5% vs 1.3% in 2016), of which 0.7% (38 samples) were non‐compliant in 2017.
Compared to conventionally produced food (non‐organic), the MRL exceedance and quantification rates were significantly lower in organic food. In 2017, the MRL exceedance rate was 1.5% in organic food, while 4.3% for conventional food;48 the same pattern was observed for the quantification rates, which were 12.2%49 in organic food and 44.6% in conventional food.50 A comparison between organic and conventional foods is presented in Figure 56. Major differences were identified, in particular for fruits and nuts, vegetables and cereals.
Figure 56.
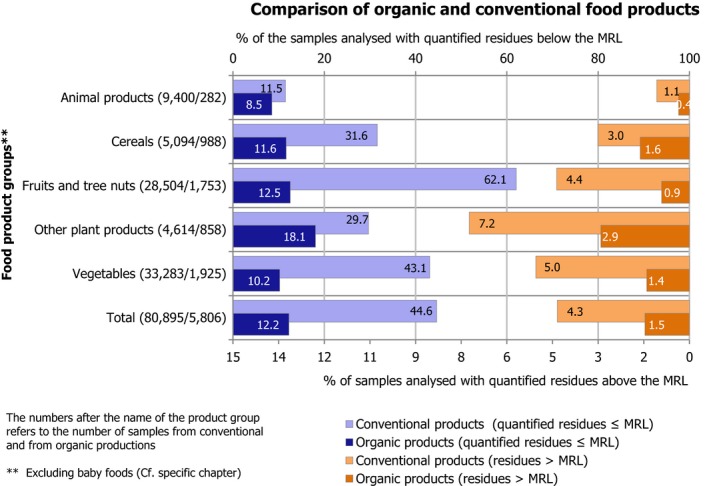
Comparison of organic and conventional foods (excluding baby food): quantification and MRL exceedance rates for main food product groups (including all pesticides)
In 2017, 134 different pesticides (151 pesticides in 2016) were quantified in concentrations at or above the LOQ. The pesticides measured most frequently (quantified in at least five samples) are presented in Figure 57. The pesticides permitted in organic farming, naturally occurring compounds and substances resulting from environmental contamination (persistent pesticides no longer used in the EU) are specifically labelled with an asterisk.
Figure 57.
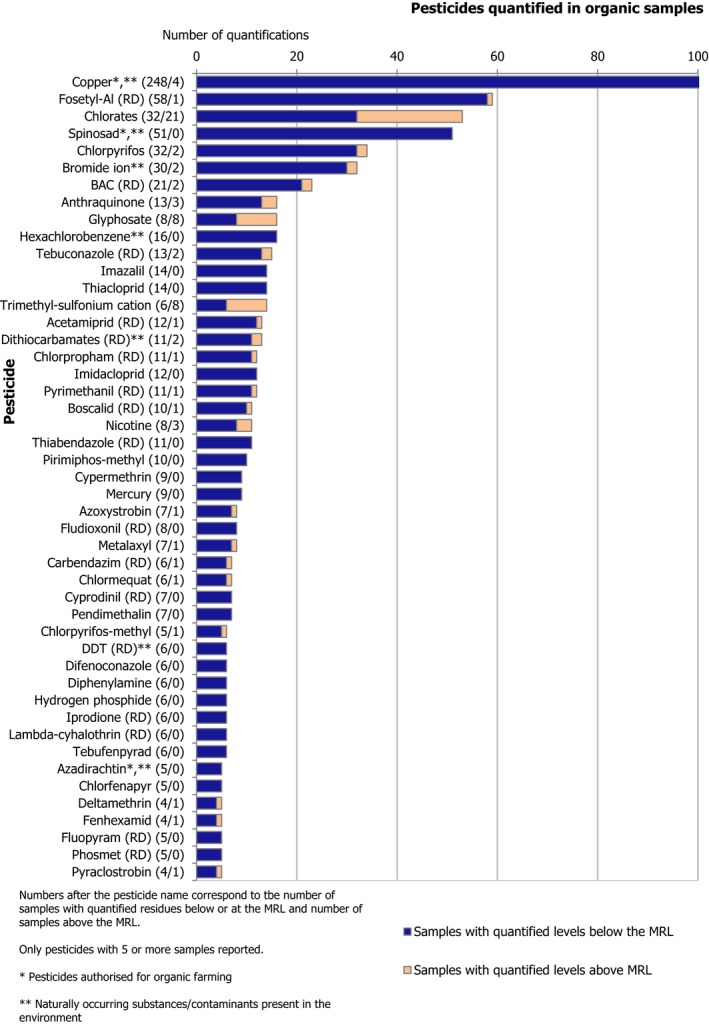
Pesticides most frequently quantified in organic samples (pesticides with at least five positive quantifications reported)
Similar to the previous reporting years, the most frequently quantified residue in organic food was copper, found in 252 samples (in 33 different food items, mostly buckwheat, sesame seeds and carrots), followed by fosetyl‐Al in 59 samples (22 commodities, mostly in wine grapes), chlorates in 53 samples (26 food items, mostly in linseeds), spinosad in 51 samples (18 commodities, mainly in tomatoes), chlorpyrifos in 34 samples (22 commodities, mainly in oranges), bromide ion in 32 samples (10 commodities, mainly in rye), BAC (RD) in 23 samples (15 commodities, mainly in rye), anthraquinone in 16 samples (3 commodities, mainly in teas), glyphosate in 16 samples (7 commodities, mainly in dry lentils) and HCB in 16 samples (5 commodities, mainly in pumpkin seeds). Other pesticides found in less than 16 samples are reported together with the ones above‐mentioned in Figure 57.
MRL exceedances51 in organic products were reported mainly for chlorate (21 samples), followed by 39 other pesticides. The details on samples of organic products exceeding a legal limit can also be found from the Excel file published as a supplement to this report.
It was noted that copper, spinosad, azadirachtin and pyrethrins can be used in organic farming as far as their use is covered by the general agricultural policy in the Member State concerned. Since the presence of residues of these compounds is linked to agricultural practices permitted in organic farming in the Union, the positive measurements of these substances in organic food is not unexpected.
Residues of HCB, DDT and dieldrin result from environmental contaminations (mainly from the soil) due to the use of these persistent in the environment compounds as pesticides in the past. Quantifications of copper, bromide ion, chlorate and dithiocarbamates in certain commodities may also result from other sources, e.g. CS2 measured as a residue from dithiocarbamates also occurs naturally in some plants, particularly in Brassicaceae and Alliaceae.
Fosetyl‐Al residues were among the top three most frequently quantified residues in organic food. Considering that the current residue definition for fosetyl‐Al is ‘sum of fosetyl‐Al and phosphonic acid and their salts expressed as fosetyl’, the results for fosetyl‐Al may include the presence of phosphonic acid residues coming from potassium phosphonates (which can be used as a foliar feed fertiliser but is also approved as a fungicide) and disodium phosphonate which is also approved for use as a fungicide. These findings, therefore, do not necessarily indicate that there was just a use of fosetyl‐Al in the field. This has been explicitly communicated to food business operators in 2014 through a note on the DG SANTE webpage and through the relevant trade associations.
The occurrence of other pesticides not authorised in organic farming can – as for conventional products – be the result of spray drift, environmental contaminations or contaminations during handling, packaging, storage or processing of organic products. This occurrence could also be linked to wrong labelling of conventionally produced food labelled as organic food. Therefore, Member States should try to elucidate the reasons of the presence of pesticides found occasionally in organic food and are not permitted in this type of products (e.g. chlorpyrifos, anthraquinone, glyphosate).
4.2.8. Results on animal products
In total, 9,682 samples of products of animal origin were analysed. Figure 58 shows the total number of samples taken is broken‐down by food group.
Figure 58.
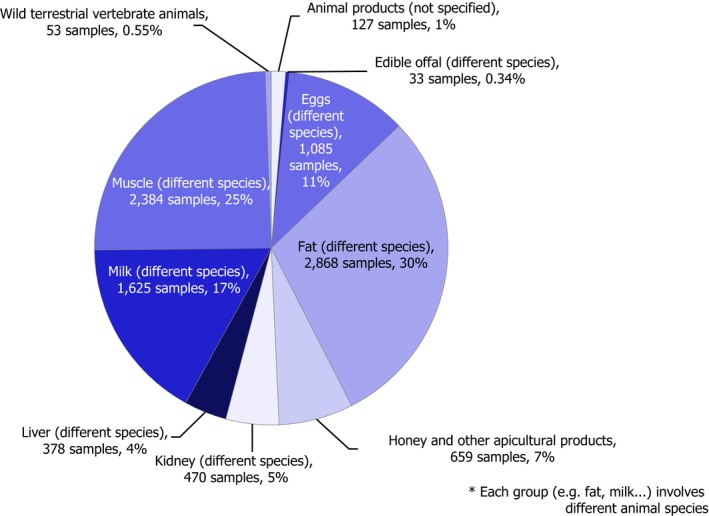
Number of samples of animal products tested, broken‐down by food group
The results showed that 8,475 samples were free of quantifiable residues (87.5% vs 83% in 2016) while 1,207 samples (12.5% vs 17% in 2016) contained one or several pesticides in quantified concentrations. MRL exceedances were identified in 102 samples (1.1% vs 1.9% in 2016, exceedances mainly due to chlorates) of which, 66 samples (0.8%) were non‐compliant considering the measurement uncertainty.
The MRL exceedances identified concern the following products: chicken eggs (39 samples), cattle milk (23 samples), poultry fat (18 samples) and honey and other apicultural products (12 samples). Multiple residues were reported in 192 samples (2.0%%); up to four different pesticides were reported in the same sample (Figure 59).
Figure 59.
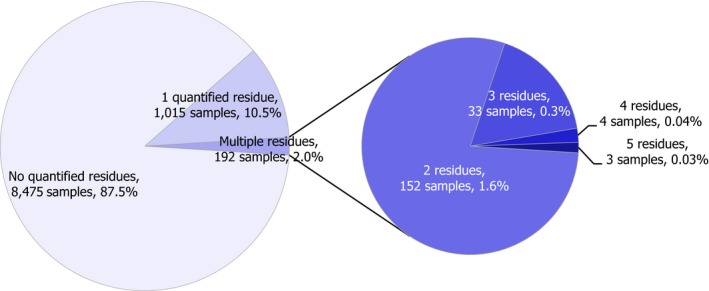
Number of quantified residues per individual sample of animal origin
In Figure 60, the 48 pesticides found in animal products at levels at or above the LOQ are presented. The most frequently quantified substances were copper, HCB, DDT, chlordecone, thiacloprid, fipronil and BAC. HCB, DDT and chlordecone, are still found in the food chain due to their persistence in the environment. These persistent compounds were found homogenously distributed among all the animal products analysed.
Figure 60.
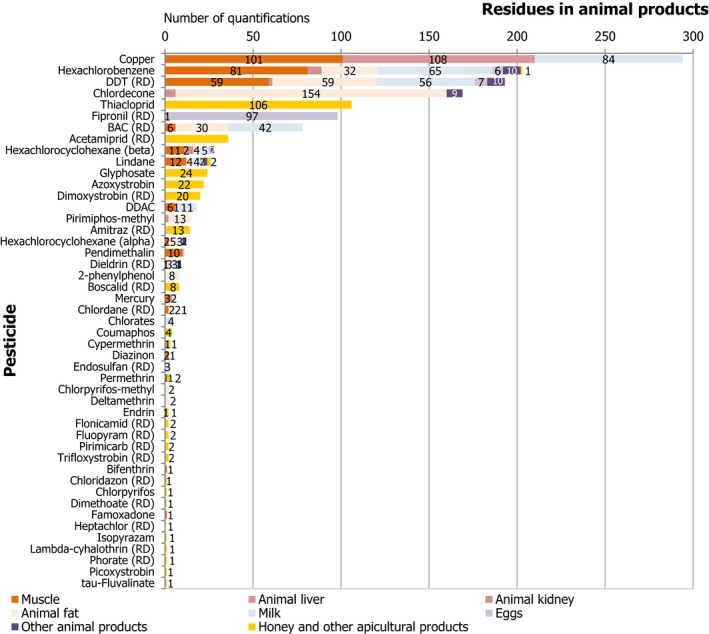
Pesticides most frequently quantified in animal products (in absolute numbers of detections at or above the LOQ)
It is noted that copper residues in animal products are not necessarily linked to the use of copper as pesticide, but may result from the use of feed supplements, which contain copper compounds. Thiacloprid was only reported in honey and other apicultural products, whereas fipronil was mainly found in eggs.
Fipronil, is a veterinary medicinal product or biocide and its presence in eggs is the result of illegal use. Because of the fipronil incident in chicken eggs in summer 2017, pesticides were ranked in the top 10 hazards52 in products originating from member countries in the Commission's 2017 RASFF annual report.43 Following the contamination due to illegal use, EFSA was mandated to open a specific data collection for fipronil in chicken eggs and poultry muscle/fat. A report with analysis and recommendations was published by EFSA on this topic (EFSA, 2018c). The results presented in the 2018 EFSA output on fipronil are complementary to those described here. EFSA recommends Member States to continue analysing for acaricides in animal products.
As in previous reports and due to the importance of beekeeping, EFSA gave specific attention on the pesticide occurrence in honey and other apicultural products. In 2017, 659 samples of honey and other apicultural products were analysed. In 464 samples (70.4%) quantifiable pesticide residues were not found. In 183 samples (27.8%) residues at or above the LOQ but below or at the MRL were identified. MRL exceedances were reported in 12 samples (1.8%), at least for one of the residues analysed for. The number of pesticides sought in honey varies from one reporting country to another. Overall, 589 different pesticides were analysed for. The MRLs were exceeded for the following substances: glyphosate, acetamiprid (RD), thiacloprid and dimethoate (RD).
In the Excel file published as a supplement to this report, further detailed data on the pesticide/food combinations found to exceed the legal limits in animal products is presented.
4.2.9. Multiple residues in the same sample
Multiple residues in one single sample may result from the application of different types of pesticides (e.g. application of herbicides, fungicides or insecticides against different pests or diseases) or use of different active substances avoiding the development of resistant pests or diseases and or uptake of persistent residues from soil from previous seasons treatments or spray/dust drift to fields adjacent to treated fields. Besides multiple residues resulting from agricultural practice, multiple residues may also occur due to mixing or blending of products with different treatment histories at different stages in the supply chain, including contamination during food processing. According to the EU legislation, the presence of multiple residues in a sample is not a non‐compliance, as long as each individual residue level does not exceed the individual MRL set for each active substance.
In 2017, of the 88,247 samples analysed, 40,326 samples (45.7%) contained one or several pesticides in quantified concentrations. Multiple residues were reported in 24,292 samples (27.5% vs 30.1% in 2016); in an individual sweet peppers/bell peppers sample, up to 30 different pesticides were reported (Figure 61).
Figure 61.
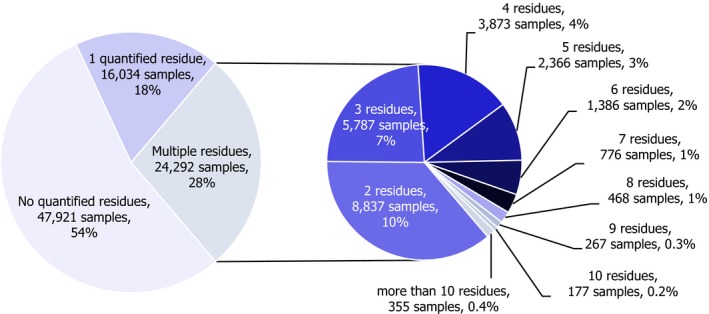
Percentage of samples with single and multiple quantified residues
The frequency of multiple residues was found to be slightly higher in unprocessed products (29%) compared to the processed products (12%) for samples containing more than one pesticide in concentrations higher or equal to the LOQ. Among the 355 samples with more than 10 pesticides, 78 were corresponding to processed products and 277 to unprocessed products.
In Figure 62, the results for the top‐ranked unprocessed food products with multiple residues are presented, broken down by the number of residues found in quantified concentrations; only food products with at least 100 samples analysed are included. The highest frequency of multiple residues in unprocessed products was found in currants (black, red and white) (71.7% of the total unprocessed samples analysed), blackberries (69.3%), limes (65.2%), lemons (63.3%), sweet cherries (62.5%), strawberries (61.7%) and lamb's lettuces/corn salads (61.0%). These findings for these commodities are comparable to those from previous years. Oranges, table grapes, pears, peaches, mandarins, bananas, apricots, celeriacs/turnip rooted celeries, parsley, chilli peppers, celeries and Roman rocket/rucola were found to contain multiple residues in more than 50% of the samples analysed.
Figure 62.
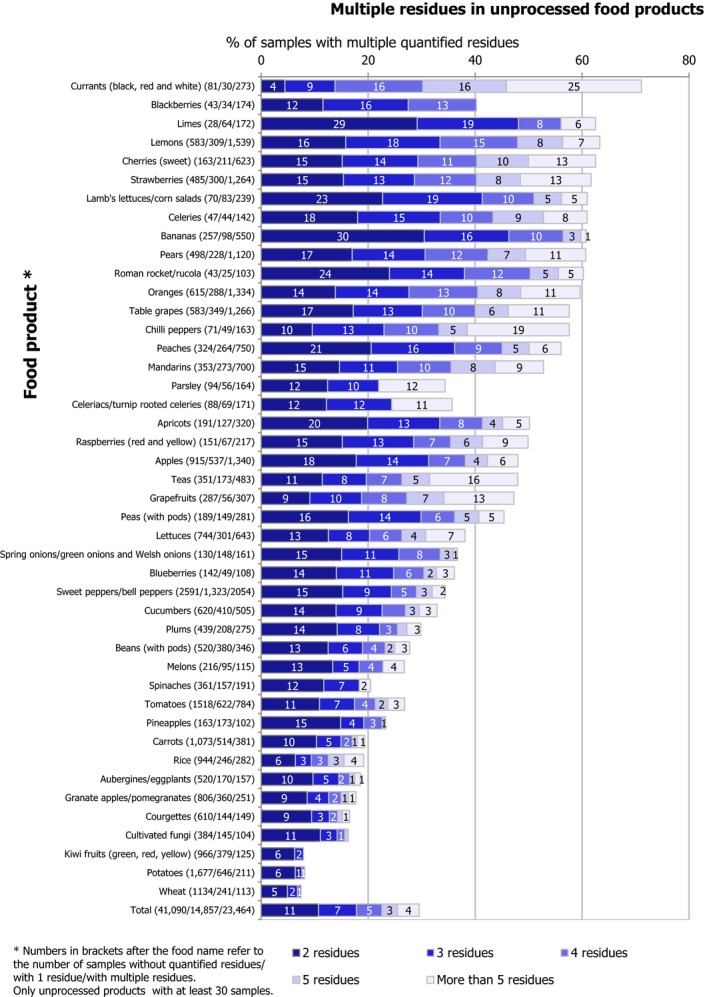
Unprocessed food products most frequently containing multiple quantified residues
A similar analysis was performed for processed food products with multiple residues. In Figure 63, the results for the top‐ranked processed food products with multiple residues are broken down by the number of residues found in quantified concentrations; only food products with at least 10 samples analysed are included. The highest frequency of multiple residues was found for processed sheep milk (54% of the total processed samples analysed), grape leaves and similar species (50%), table grapes (44%), sweet peppers/bell peppers (43%), tomatoes (37%), apricots (36%) and wild fungi (30%).
Figure 63.
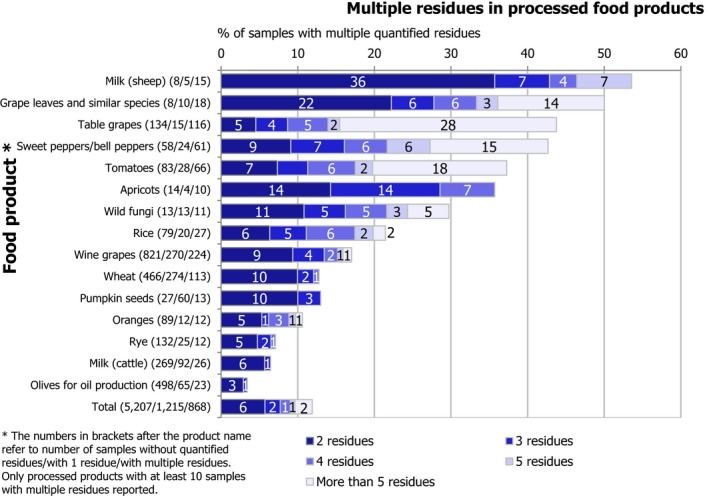
Processed food products most frequently containing multiple quantified residues
4.3. Reasons for MRL exceedances
The legal limits (MRLs) are established based on supervised residue trials that reflect the residue levels expected under field conditions or, for animal products, animal feeding studies based on appropriate dietary requirements of different food producing animals. The MRL value is estimated using statistical methods and is usually established to cover at least the upper CI of the 95th percentile of the expected residue distribution. Therefore, a percentage of approximately 1% MRL exceedances are expected even if the Good Agricultural Practices (GAPs) are fully respected. However, in these cases, the residue levels would be expected to exceed the MRLs only marginally.
In 2017, 4.1% of samples analysed contained pesticide residues exceeding their respective MRLs (3,620 samples). The MRL exceedance rate for 2016 was 3.8% (3,175 samples in total). Multiple MRL exceedances per sample were reported for 2,596 samples (954 from EU/EEA origin, 1,504 from third countries, and 138 for samples of unknown origin).
The possible reasons for MRL exceedances are summarised below:
-
For samples coming from third countries:
-
–
Use of non‐EU‐approved pesticides on crops for which no import tolerances are requested by the importers, as foreseen in Article 6 of Regulation (EC) No 396/2005;
-
–
Use of EU‐approved pesticides on crops for which no import tolerances have been requested by the importers;
-
–
Presence of contaminants with unclear origin in concentrations exceeding the legal limit (e.g. anthraquinone in tea).
-
–
-
For samples originating from the internal market (reporting countries):
-
–
GAP not respected: i.e. different to the ones set as the GAP application rates, preharvest intervals, number or method of applications of the pesticide product (e.g. thiabendazole in oranges). This may also concern drift‐contamination resulting from inappropriate application during adverse weather conditions or unauthorised use of EU‐approved pesticides in crops where MRLs have not been set;
-
–
Use of non‐EU‐approved pesticides (e.g. fenthion in oranges);
-
–
Natural presence in the field (e.g. residues included in the definition for dithiocarbamates in brassica and allium vegetables or bromide ion in rice);
-
–
Changes in the MRL due to amendments in toxicological reference values (e.g. iprodione in kiwi fruits53) and/or changes in MRLs for other reasons (e.g. deltamethrin in rice), while transitional measures apply;
-
–
Presence of biocide residues used as pesticides in the past and continuing to be monitored under the pesticide legislation (Regulation (EU) No 528/201354) (e.g. BAC and DDAC in baby food);
-
–
The use of chlorine solutions (chlorine dioxide, chlorite and hypochlorite salts) used as sanitising and disinfection agents in the food industry generate chlorate salts that exceed the default MRL of 0.01 mg/kg.
-
–
Environmental contamination: POP included in the Stockholm Convention of prohibited substances (UNEP, 2001). These substances are no longer used as pesticides but are very persistent in the environment and found in the food chain (e.g. HCB in fat sheep and poultry and HCH‐alpha/‐beta in fat sheep).
-
–
Among the 6,120 samples of unknown origin analysed in 2017, maximum residue levels were exceeded for 193 samples (138 of these samples had multiple MRL exceedances).
More details on the pesticide/crop combinations exceeding the legal limits are compiled in the Excel file published as a supplement to this report (see ‘Supporting Information’).
5. Dietary exposure and dietary risk assessment
To estimate the dietary exposure to pesticide residues, EU food consumption information originating from dietary surveys is combined with occurrence data provided by reporting countries per food commodity.
The exposure assessment methodology used by EFSA relies on a conservative deterministic model and is expected to result in an overestimation of the exposure to a given substance. The Pesticide Residues Intake Model (PRIMo) implements the principles of the WHO methodologies for short‐term and long‐term risk assessment (FAO, 2016) adjusted to the food consumed by the EU population. In this report the dietary exposure assessment was performed with version 3.0 of the PRIMo model (EFSA, 2018a). The file including the exposure assessment is a supplement to this report, published separately.
Two types of dietary exposure estimates are performed:
The acute or short‐term exposure assessment which is based on the consumption of ‘large portions’ of a specific commodity in a short timeframe (one day or one meal). There have not been any changes in this approach (except for updates in commodity consumption data included in PRIMo v. 3) compared to previous EFSA dietary risk assessments (EFSA, 2013, 2014c,e, 2016c).
The chronic or long‐term exposure assessment which estimates the dietary exposure to pesticides from all food sources over a long time period with the aim of predicting the lifetime dietary exposure to these substances. As for the 2016 report, the long‐term dietary exposure to pesticides was estimated for all food items for which consumption data were available (EFSA, 2018d).
To do the risk assessment, i.e. to estimate the likelihood that a pesticide residue represents a safety concern when present/consumed in the EU diet, EFSA compares the exposure to this residue (i.e. amount of residue consumed) with its corresponding toxicological reference value (i.e. residue concentration above which possible negative health effects cannot be excluded).
For the short‐term risk assessment, the short‐term dietary exposure per pesticide residue (mg of residue/kg body weight (bw) per day) is compared to the substance's acute reference dose (ARfD, in mg of residue/kg bw per day).
For the long‐term risk assessment, the long‐term dietary exposure per pesticide residue is compared to the substance's acceptable daily intake (ADI, in mg of residue/kg bw per day).
Based on the current scientific knowledge, when the dietary exposure to a substance is found to be lower than or equal to its toxicological reference values the probability for this substance to present a health risk for the consumer is low. When the dietary exposure to a given substance exceeds its toxicological reference values, possible negative health outcomes cannot be excluded.
Results associated with the simultaneous exposure to multiple residues (cumulative risk assessment) are not presented in this report. Two pilot assessments on the risks to humans by multiple pesticide residues in food are currently in progress.55 EFSA launched public consultations for the draft outputs dealing with the establishment of cumulative assessment groups of pesticides for their effects on the nervous system56 and the establishment of cumulative assessment groups of pesticides for their effects on the thyroid.57
5.1. Short‐term (acute) risk assessment
The short‐term or acute risk assessment was estimated for all pesticide/crop combinations covered by the 2017 EU‐coordinated programme. Samples flagged as EUCP were pooled with those from national programmes matching the EUCP pesticide/crop combinations. The ARfD values for the active substances covered by the 2017 EU‐coordinated programme are reported in Appendix D – Table D.1.
Table D.1.
Toxicological reference values for compounds included in the 2017 EUCP
| Pesticide | ADI (mg/kg bw per day) | Year | Source | ARfD (mg/kg bw) | Year | Source |
|---|---|---|---|---|---|---|
| 2,4‐D (RD) | 0.02 | 2017 | EFSA | 0.3 | 2017 | EFSA |
| 2‐phenylphenol | 0.4 | 2008 | EFSA | n.n. | 2008 | EFSA |
| Abamectin (RD) | 0.0025 | 2008 | EFSA | 0.005 | 2008 | COM |
| Acephate | 0.03 | 2005 | JMPR | 0.1 | 2005 | JMPR |
| Acetamiprid (RD) | 0.025 | 2013 | EFSA | 0.025 | 2013 | EFSA |
| Acrinathrin | 0.01 | 2013 | EFSA | 0.01 | 2013 | EFSA |
| Aldicarb (RD) | 0.003 | 2001 | JMPR | 0.003 | 2001 | JMPR |
| Azinphos‐methyl | 0.005 | 2006 | COM | 0.01 | 2006 | COM |
| Azoxystrobin | 0.2 | 2011 | COM | n.n. | 2011 | COM |
| Bifenthrin | 0.015 | 2011 | EFSA | 0.03 | 2011 | EFSA |
| Biphenyl | 0.038 | 1999 | WHO | n.n. | 2010 | EFSA |
| Bitertanol | 0.003 | 2011 | COM | 0.01 | 2011 | COM |
| Boscalid (RD) | 0.04 | 2008 | COM | n.n. | 2008 | COM |
| Bromide ion* | 0.1 | 1988 | JMPR | n.n | 2013 | EFSA |
| Bromopropylate | 0.03 | 1993 | JMPR | 0.03 | ||
| Bupirimate | 0.05 | 2011 | COM | n.n. | 2011 | COM |
| Buprofezin | 0.01 | 2010 | COM | 0.5 | 2010 | COM |
| Captan (RD) | 0.1 | 2007 | COM | 0.3 | 2008 | COM |
| Carbaryl | 0.0075 | 2006 | EFSA | 0.01 | 2006 | EFSA |
| Carbendazim (RD) | 0.02 | 2010 | COM | 0.02 | 2010 | COM |
| Carbofuran (RD) | 0.00015 | 2009 | EFSA | 0.00015 | 2009 | EFSA |
| Chlorantraniliprole | 1.56 | 2013 | EFSA | 2013 | EFSA | |
| Chlordane (RD) | 0.0005 | 1994 | JMPR | 0.0005 | ||
| Chlorfenapyr | 0.015 | 1999 | ECCO | 0.015 | 2006 | EFSA |
| Chlormequat | 0.04 | 2008 | EFSA | 0.09 | 2008 | EFSA |
| Chlorothalonil (RD) | 0.015 | 2006 | COM | 0.6 | 2006 | COM |
| Chlorpropham (RD) | 0.05 | 2004 | COM | 0.5 | 2004 | COM |
| Chlorpyrifos | 0.001 | 2014 | EFSA | 0.005 | 2014 | EFSA |
| Chlorpyrifos‐methyl | 0.01 | 2005 | COM | 0.1 | 2005 | COM |
| Clofentezine (RD) | 0.02 | 2010 | COM | 2010 | COM | |
| Clothianidin | 0.097 | 2006 | COM | 0.1 | 2006 | COM |
| Cyfluthrin | 0.003 | 2003 | COM | 0.02 | 2003 | COM |
| Cymoxanil | 0.013 | 2008 | EFSA | 0.08 | 2008 | EFSA |
| Cypermethrin | 0.05 | 2005 | COM | 0.2 | 2005 | COM |
| Cyproconazole | 0.02 | 2011 | COM | 0.02 | 2011 | COM |
| Cyprodinil (RD) | 0.03 | 2006 | COM | 2006 | COM | |
| Cyromazine | 0.06 | 2006 | JMPR | 0.1 | 2006 | JMPR |
| DDT (RD) | 0.01 | 2000 | JMPR | 2000 | JMPR | |
| Deltamethrin | 0.01 | 2003 | COM | 0.01 | 2003 | COM |
| Diazinon | 0.0002 | 2006 | EFSA | 0.025 | 2006 | EFSA |
| Dichlorvos | 0.00008 | 2006 | EFSA | 0.002 | 2006 | EFSA |
| Dicloran | 0.005 | 2010 | EFSA | 0.025 | 2010 | EFSA |
| Dicofol (RD) | 0.002 | 1992 | JMPR | 0.2 | 2011 | JMPR |
| Dieldrin (RD) | 0.0001 | 1994 | JMPR | 0.003 | 2007 | EFSA |
| Diethofencarb | 0.43 | 2010 | EFSA | 2010 | EFSA | |
| Difenoconazole | 0.01 | 2008 | COM | 0.16 | 2008 | COM |
| Diflubenzuron (RD) | 0.1 | 2009 | EFSA | 2009 | EFSA | |
| Dimethoate (RD) | 0.001 | 2013 | EFSA | 0.01 | 2013 | EFSA |
| Dimethoate (RD) ‐ dimethoate | 0.001 | 2007 | COM | 0.01 | 2013 | EFSA |
| Dimethoate (RD) ‐ omethoate | 0.0003 | 2013 | EFSA | 0.002 | 2013 | EFSA |
| Dimethomorph | 0.05 | 2007 | COM | 0.6 | 2007 | COM |
| Diniconazole | 0.02 | 2007 | France | 0.02 | 2007 | France |
| Diphenylamine | 0.075 | 2008 | EFSA | 2008 | EFSA | |
| Dithianon | 0.01 | 2011 | COM | 0.12 | 2011 | COM |
| Dithiocarbamates (RD) | ||||||
| Dithiocarbamates (RD) ‐ mancozeb scenario | 0.028 | 2005 | COM | 0.337 | 2005 | COM |
| Dithiocarbamates (RD) ‐ maneb scenario | 0.029 | 2005 | COM | 0.11 | 2005 | COM |
| Dithiocarbamates (RD) ‐ metiram scenario | 0.004 | 2005 | COM | 2005 | COM | |
| Dithiocarbamates (RD) ‐ propineb scenario | 0.004 | 2003 | COM | 0.053 | 2003 | COM |
| Dithiocarbamates (RD) ‐ thiram scenario | 0.01 | 2003 | COM | 0.025 | 2003 | COM |
| Dithiocarbamates (RD) ‐ ziram scenario | 0.003 | 2004 | COM | 0.04 | 2004 | COM |
| Dodine | 0.1 | 2010 | EFSA | 0.1 | 2010 | EFSA |
| Endosulfan (RD) | 0.006 | 2006 | JMPR | 0.02 | 2006 | JMPR |
| EPN | ||||||
| Epoxiconazole | 0.008 | 2008 | COM | 0.023 | 2008 | COM |
| Ethephon | 0.03 | 2006 | COM | 0.05 | 2008 | COM |
| Ethion | 0.002 | 1990 | JMPR | 0.015 | 1999 | UK ACP |
| Ethirimol | 0.035 | 2010 | EFSA | 2010 | EFSA | |
| Etofenprox | 0.03 | 2009 | COM | 1 | 2009 | COM |
| Famoxadone | 0.006 | 2014 | EFSA | 0.1 | 2014 | EFSA |
| Fenamidone | 2016 | EFSA | 2016 | EFSA | ||
| Fenamiphos (RD) | 0.0008 | 2006 | COM | 0.0025 | 2006 | COM |
| Fenarimol | 0.01 | 2006 | COM | 0.02 | 2006 | COM |
| Fenazaquin | 0.005 | 2013 | EFSA | 0.1 | 2013 | EFSA |
| Fenbuconazole | 0.006 | 2010 | COM | 0.3 | 2010 | COM |
| Fenbutatin oxide | 0.05 | 2011 | COM | 0.1 | 2011 | COM |
| Fenhexamid | 0.2 | 2014 | EFSA | 2014 | EFSA | |
| Fenitrothion | 0.005 | 2006 | EFSA | 0.013 | 2006 | EFSA |
| Fenoxycarb | 0.053 | 2011 | COM | 2 | 2011 | COM |
| Fenpropathrin | 0.03 | 1993 | JMPR | 0.03 | 2012 | JMPR |
| Fenpropidin (RD) | 0.02 | 2012 | COM | 0.02 | 2012 | COM |
| Fenpropimorph (RD) | 0.003 | 2008 | COM | 0.03 | 2008 | COM |
| Fenpyroximate (RD) | 0.01 | 2013 | EFSA | 0.02 | 2013 | EFSA |
| Fenthion (RD) | 0.007 | 2000 | JMPR | 0.01 | 2000 | JMPR |
| Fenvalerate (RD) | 0.0175 | 2014 | EFSA | 0.0175 | 2014 | EFSA |
| Fipronil (RD) | 0.0002 | 2007 | COM | 0.009 | 2007 | COM |
| Flonicamid (RD) | 0.025 | 2010 | COM | 0.025 | 2010 | COM |
| Fluazifop‐P (RD) | 0.01 | 2010 | EFSA | 0.017 | 2010 | EFSA |
| Flubendiamide | 0.017 | 2013 | EFSA | 0.1 | 2013 | EFSA |
| Fludioxonil (RD) | 0.37 | 2007 | COM | 2007 | COM | |
| Flufenoxuron | 0.01 | 2011 | EFSA | 2011 | EFSA | |
| Fluopyram (RD) | 0.012 | 2013 | EFSA | 0.5 | 2013 | EFSA |
| Fluquinconazole | 0.002 | 2011 | COM | 0.02 | 2011 | COM |
| Flusilazole (RD) | 0.002 | 2007 | COM | 0.005 | 2007 | COM |
| Flutriafol | 0.01 | 2011 | COM | 0.05 | 2011 | COM |
| Folpet (RD) | 0.1 | 2013 | EFSA | 0.2 | 2013 | EFSA |
| Formetanate | 0.004 | 2007 | COM | 0.005 | 2007 | COM |
| Fosthiazate | 0.004 | 2003 | COM | 0.005 | 2003 | COM |
| Glyphosate | 0.5 | 2015 | EFSA | 0.5 | 2015 | EFSA |
| Heptachlor (RD) | 0.0001 | 1994 | JMPR | 0.0001 | ||
| Hexachlorobenzene | ||||||
| Hexachlorocyclohexane (alpha) | ||||||
| Hexachlorocyclohexane (beta) | ||||||
| Hexaconazole | 0.005 | 1990 | JMPR | 0.005 | ||
| Hexythiazox | 0.03 | 2011 | COM | 2011 | COM | |
| Imazalil | 0.025 | 2011 | COM | 0.05 | 2011 | COM |
| Imidacloprid | 0.06 | 2013 | EFSA | 0.08 | 2013 | EFSA |
| Indoxacarb | 0.006 | 2005 | COM | 0.125 | 2005 | COM |
| Iprodione (RD) | 0.02 | 2017 | EFSA | 0.06 | 2017 | EFSA |
| Iprovalicarb | 0.015 | 2014 | EFSA | 2014 | EFSA | |
| Isocarbophos | ||||||
| Isoprothiolane | 0.1 | 2012 | EFSA | 0.12 | 2012 | EFSA |
| Kresoxim‐methyl (RD) | 0.4 | 2011 | COM | 2011 | COM | |
| Lambda‐cyhalothrin (RD) | 0.0025 | 2014 | EFSA | 0.005 | 2014 | EFSA |
| Lindane | 0.005 | 2000 | COM | 0.06 | 2000 | COM |
| Linuron | 0.003 | 2002 | COM | 0.03 | 2002 | COM |
| Lufenuron | 0.015 | 2009 | COM | 2009 | COM | |
| Malathion (RD) | 0.03 | 2010 | COM | 0.3 | 2010 | COM |
| Mandipropamid | 0.15 | 2012 | EFSA | 2012 | EFSA | |
| Mepanipyrim | 0.012 | 2017 | EFSA | 0.1 | 2017 | EFSA |
| Mepiquat | 0.2 | 2008 | COM | 0.3 | 2008 | COM |
| Metalaxyl | 0.08 | 2014 | EFSA | 0.5 | 2014 | EFSA |
| Methamidophos | 0.001 | 2007 | COM | 0.003 | 2007 | COM |
| Methidathion | 0.001 | 1997 | JMPR | 0.01 | 1997 | JMPR |
| Methiocarb (RD) | 0.013 | 2007 | COM | 0.013 | 2007 | COM |
| Methomyl (RD) | 0.0025 | 2009 | COM | 0.0025 | 2009 | COM |
| Methoxychlor | 0.005 | 2011 | ATSDR | 0.005 | ||
| Methoxyfenozide | 0.1 | 2017 | EFSA | 0.1 | 2017 | EFSA |
| Monocrotophos | 0.0006 | 1995 | JMPR | 0.002 | 1995 | JMPR |
| Myclobutanil (RD) | 0.025 | 2010 | COM | 0.31 | 2010 | COM |
| Oxadixyl | 0.01 | 1984 | FR | 0.01 | 1984 | FR |
| Oxamyl | 0.001 | 2006 | COM | 0.001 | 2006 | COM |
| Oxydemeton‐methyl (RD) | 0.0003 | 2006 | COM | 0.0015 | 2006 | COM |
| Paclobutrazol | 0.022 | 2011 | COM | 0.1 | 2011 | COM |
| Parathion | 0.0006 | 2001 | ECCO 100 | 0.005 | 2001 | ECCO 100 |
| Parathion‐methyl (RD) | 0.003 | 2002 | COM | 0.03 | 2001 | COM |
| Penconazole | 0.03 | 2009 | COM | 0.5 | 2009 | COM |
| Pencycuron | 0.2 | 2011 | COM | 2011 | COM | |
| Pendimethalin | 0.125 | 2015 | EFSA | 0.3 | 2015 | EFSA |
| Permethrin | 0.05 | 2000 | COM | 1.5 | 2000 | COM |
| Phosmet (RD) | 0.01 | 2007 | COM | 0.045 | 2007 | COM |
| Pirimicarb (RD) | 0.035 | 2006 | COM | 0.1 | 2006 | COM |
| Pirimiphos‐methyl | 0.004 | 2007 | COM | 0.15 | 2007 | COM |
| Procymidone (RD) | 0.0028 | 2007 | DAR FR | 0.012 | 2007 | DAR FR |
| Profenofos | 0.03 | 2007 | JMPR | 1 | 2007 | JMPR |
| Propamocarb (RD) | 0.29 | 2007 | COM | 1 | 2007 | COM |
| Propargitea | 0.03 | 2018 | EFSA | 0.06 | 2018 | EFSA |
| Propiconazole | 0.04 | 2017 | EFSA | 0.1 | 2017 | EFSA |
| Propyzamide (RD) | 0.05 | 2016 | EFSA | 0.13 | 2016 | EFSA |
| Pyraclostrobin | 0.03 | 2004 | COM | 0.03 | 2004 | COM |
| Pyridaben | 0.01 | 2010 | COM | 0.05 | 2010 | COM |
| Pyrimethanil (RD) | 0.17 | 2006 | COM | 2006 | EFSA | |
| Pyriproxyfen | 0.1 | 2008 | COM | 2008 | COM | |
| Quinoxyfen | 0.2 | 2004 | COM | 2003 | COM | |
| Spinosad | 0.024 | 2007 | COM | 2006 | COM | |
| Spirodiclofen | 0.015 | 2009 | EFSA | 2009 | EFSA | |
| Spiromesifen | 0.03 | 2007 | EFSA | 2 | 2007 | EFSA |
| Spiroxamine (RD) | 0.025 | 1999 | COM | 0.1 | 2011 | COM |
| tau‐Fluvalinate | 0.005 | 2010 | COM | 0.05 | 2010 | COM |
| Tebuconazole (RD) | 0.03 | 2013 | EFSA | 0.03 | 2013 | EFSA |
| Tebufenozide | 0.02 | 2011 | COM | 2011 | COM | |
| Tebufenpyrad | 0.01 | 2009 | COM | 0.02 | 2009 | COM |
| Teflubenzuron | 0.01 | 2008 | COM | 2008 | COM | |
| Tefluthrin | 0.005 | 2010 | COM | 0.005 | 2010 | COM |
| Terbuthylazine | 0.004 | 2017 | EFSA | 0.008 | 2017 | EFSA |
| Tetraconazole | 0.004 | 2008 | COM | 0.05 | 2008 | COM |
| Tetradifon | 0.015 | 2001 | DE | 2002 | DE | |
| Thiabendazole (RD) | 0.1 | 2014 | EFSA | 0.1 | 2014 | EFSA |
| Thiacloprid | 0.01 | 2004 | COM | 0.03 | 2004 | COM |
| Thiamethoxam | 0.026 | 2007 | COM | 0.5 | 2007 | COM |
| Thiophanate‐methyl | 0.08 | 2005 | COM | 0.2 | 2005 | COM |
| Tolclofos‐methyl | 0.064 | 2006 | COM | 2006 | COM | |
| Tolylfluanid (RD) | 0.1 | 2006 | COM | 0.25 | 2006 | COM |
| Triadimenol (RD) | 0.05 | 2008 | COM | 0.05 | 2008 | COM |
| Triadimefon | 0.03 | 2004 | JMPR | 0.08 | 2004 | JMPR |
| Triazophos | 0.001 | 2002 | JMPR | 0.001 | 2002 | JMPR |
| Trifloxystrobin (RD) | 0.1 | 2017 | EFSA | 0.5 | 2017 | EFSA |
| Triflumuron | 0.014 | 2011 | COM | 2011 | COM | |
| Vinclozolin | 0.005 | 2006 | COM | 0.06 | 2006 | COM |
ADI: acceptable daily intake; ARfD: acute reference dose; bw: body weight; n.n.: ARfD not necessary.
For tentative risk assessment only.
(EFSA, 2018b).
Overall, this assessment considers results submitted for 171 pesticides covering the 12 food products in the 2017 EUCP: carrots, cauliflower, kiwi fruits, onions, orange, pears, potatoes, dried beans, rice, rye, sheep fat and poultry fat for 16,515 samples. The approximately 30% of samples (5,357 samples) taken in the framework of the national programmes for the above‐mentioned crop/pesticides combinations also concern more targeted (risk‐based) sampling strategies.
5.1.1. Methodology for the estimation of short‐term exposure
The acute dietary exposure per pesticide was calculated using the international estimation of short‐term intake (IESTI) equation, following a methodology described by the experts of the Joint Meeting on Pesticide Residues (JMPR) (FAO, 2016). However, the methodology was modified by EFSA as follows:
Each food item contains the highest measured residue concentration reported to EFSA and a large portion58 per item is consumed. For this, the highest residue level measured at or above the LOQ was identified for each single pesticide/crop combination and used in the acute exposure estimate. This is also applicable for bulked samples, (e.g. dried beans, rice, rye). To retrieve the highest residue concentration for rye, results from raw rye grains and rye whole grain flour59 were pooled. To retrieve the highest residue concentration for rice, results from husked rice and polished rice grain were pooled. In both cases, the unprocessed commodities were found to have the highest levels of residues;
The analysis of samples refers to the unprocessed raw commodity which has not undergone any treatment. Considering that some food items may undergo treatment before consumption (e.g. washing, peeling, cooking, etc.), processing factors were introduced in the estimation of the exposure for specific pesticide/crop combinations when available (e.g. use of peeling factors for the estimation of the exposure to imazalil in oranges). It should be stressed that only a limited number of reliable processing factors are currently available and for most assessed commodities it is assumed that before and after treatment, the same residual levels are present and consumed. Appendix D – Table D.2 contains a list of the processing factors for pesticide/crop combinations used in the context of this report;
The residue concentration in the consumed products is five to seven times higher than the one measured in the samples analysed. The approach followed uses the so‐called unit variability factor which has the aim of covering the inhomogeneous residual distribution among the individual units. For food commodities with a unit weight of more than 250 g (e.g. cauliflower), a variability factor of 5 is applied. For mid‐sized products like carrots, kiwi fruits, onions, oranges, pears and potatoes with a unit size from 25 to 250 g, a variability factor of 7 is applied; no variability factor is used for commodities with unit weights less than 25 g (e.g. dried beans, rice or rye).60 The latter also applies to sheep fat and poultry fat. When validated studies on specific pesticide/crop combinations are available, variability factors different from those indicated above can be derived and used in the EFSA assessments. This was the case in the assessment of thiabendazole (RD) (EFSA, 2016d) and captan (RD) (EFSA, 2014b) in pears where instead of 7, variability factors of 1.6 and 3 were applied, respectively. Appendix D – Table D.3 contains a list of the default and revised variability factors for pesticide/crop combinations evaluated in the context of this report;
The exposure calculations were carried out separately for each pesticide/crop combination as it is considered unlikely that a consumer would eat two or more different food products in large portions within a short period of time and that all these food products would contain residues of the same pesticide at the highest level observed during the reporting year;
Results for commodities with residue concentrations below the LOQ were not considered in the acute exposure assessment, assuming a no residue/no exposure situation;
In PRIMo revision 3, no robust consumption data were available for sheep fat. To estimate the large portion for this commodity, it was assumed that the large portion of sheep meat contains 20% fat;
The estimation of the exposure to pesticides was based on the residue definition for enforcement (in accordance with the EU MRL legislation) and not the residue definition for risk assessment. This was because the monitoring residue/commodity results refer to the residue definition for enforcement and currently a comprehensive list of conversion factors between the enforcement definition and the definitions set for risk assessment is not available;
Table D.2.
Processing factors by pesticide/crop combination used in the context of this report
| Pesticide | Food commodity | Processing factor used in the risk assessment | Reference |
|---|---|---|---|
| Chlorpyrifos | Oranges, peeled | 0.03 | Scholz (2018) |
| Deltamethrin | Rice | 0.5 | Reg. (EU) No. 2016/6625 |
| Imazalil | Oranges, peeled | 0.07 | EFSA (2018f) |
| Imazalil | Potatoes, unpeeled and boiled | 0.22 | EFSA (2018f) |
| Lambda‐cyhalothrin (RD) | Oranges, peeled | 0.25 | EFSA (2015c) |
| Phosmet (RD) | Oranges, peeled | 0.04 | Scholz (2018) |
| Propiconazole | Oranges, peeled | 0.01 | Scholz (2018) |
| Pyraclostrobin | Oranges, peeled | 0.11 | Scholz (2018) |
| Thiabendazole (RD) | Oranges, peeled | 0.17 | Scholz (2018) |
Table D.3.
Revised variability factors for pesticide/crop combination used in the context of this report
| Pesticide | Crop | Default variability factora | Revised variability factorb | Reference |
|---|---|---|---|---|
| Thiabendazole (RD) | Pears | 7 | 1.6 | EFSA (2016d) |
| Captan (RD) | Pears | 7 | 3 | EFSA (2014b) |
Variability factor still used in PRIMo revision 3 for premarketing purposes.
Variability factor used in the context of this report to estimate acute exposure based on studies provided under Art.12 assessments.
The above assumptions for the estimation of the acute exposure to pesticides would be expected to result in an overestimation of the exposure for each pesticide/food combination.
5.1.2. Results
For the acute risk assessment of the 2017 results, EFSA considered the following:
For bromopropylate, chlordane (RD), heptachlor (RD), hexaconazole and methoxychlor where currently only ADIs are set (ARfDs are not currently available), the short‐term risk assessment was performed with the available ADIs (Figure 64). The use of the ADI instead of the ARfD is an additional conservative element to consider in the risk assessment for these substances.
For 2,4‐D, iprodione, mepanipyrim, methoxyfenozide, propiconazole, propargite, terbuthylazine and trifloxystrobin, the ARfD values were amended between 2017 and the date of this report (see Appendix D – Table D.1). In the risk assessment of the above‐mentioned pesticides, the updated ARfD values were used. EFSA is aware that different conclusions might be drawn when different ARfDs apply for the evaluation of the same pesticide and analytical results not relevant for action in 2017 might be relevant to be flagged with respect to future monitoring.
For the legal residue definition of fenvalerate containing esfenvalerate (a compound with a different toxicological profile) the acute risk assessment was based on the ARfD of the authorised active substance esfenvalerate.
The residue definition of dimethoate (sum of dimethoate and omethoate, expressed as dimethoate) contains compounds with significantly different toxicological potencies (i.e. dimethoate and omethoate).61 In order to estimate the actual risk for consumers, two different scenarios were used. Scenario 1 (the ‘optimistic dimethoate scenario’) assumes that the quantified residues are related to the less toxic compound, dimethoate; scenario 2 (the ‘pessimistic omethoate scenario’), assumes that the total quantified residues are related to the more toxic compound, omethoate;
In most cases, dithiocarbamates were analysed using a common moiety method measuring the generation of CS2. This method, however, has a lack of specificity towards the individual active substances applied in the field. Despite the fact that an unambiguous risk assessment for dithiocarbamates was not possible, a conservative approach involving five different scenarios was used by EFSA. This approach assumed that the CS2 concentrations measured referred exclusively to each dithiocarbamates, i.e. either mancozeb, maneb, propineb, thiram or ziram,62 as each one of them has a different toxicological profile.
Figure 64.

Results of short‐term (acute) dietary risk assessment for the highest residues reported by pesticide/crop combination (expressed as a percentage of the toxicological reference value)
For EPN, fenamidone,63 HCB, HCH‐alpha, HCH‐beta and isocarbophos no toxicological reference values (ARfD/ADI) are available (Figure 64).
Among the 171 pesticides analysed in 16,515 food samples:
Thirty‐three64 pesticides were not considered for their risk from acute exposure since they would not be expected to present acute adverse effects to the consumer (the setting of an ARfD for these pesticides was not relevant): 2‐phenylphenol, azoxystrobin, biphenyl, boscalid (RD), bromide ion, bupirimate, chlorantraniliprole, clofentezin (RD), cyprodinil (RD), DDT (RD), diethofencarb, diflubenzuron (RD), diphenylamine, ethirimol, fenhexamid, fludioxonil (RD), flufenoxuron, hexythiazox, iprovalicarb, kresoxim‐methyl (RD), lufenuron, mandipropamid, pencycuron, pyrimethanil (RD), pyriproxyfen, quinoxyfen, spinosad, spirodiclofen, tebufenozide, teflubenzuron, tetradifon, tolclofos‐methyl and triflumuron. These pesticides are marked with footnote b) in Figure 64.
Twenty‐four pesticides were quantified in levels below their corresponding LOQs in all samples: aldicarb, bitertanol, bromopropylate, chlordane, cymoxanil, dicofol (RD), ethion, famoxadone, fenamiphos (RD), fenpropidin (RD), flusilazole (RD), formetanate, mepanipyrim, methiocarb (RD), methoxychlor, monocrotophos, oxadixyl, oxydemeton‐methyl (RD), parathion, spiroxamine (RD), terbuthylazine, tolylfluanid (RD), triadimefon and vinclozolin. Therefore, the short‐term dietary exposure to these pesticides, would not be expected to pose a concern to consumer health. EFSA noted that methoxychlor, for which a proposal to be listed in Annex A to the Stockholm Convention on Persistent Organic Pollutants was recently submitted by the EU Council,65 was among the above‐mentioned 24 non‐quantifiable pesticides.
Eighty‐four quantifiable pesticides were found in levels resulting in an exposure below their corresponding toxicological reference values in all food products analysed: 2,4‐D (RD), abamectin (RD), acephate, acrinathrin, azinphos‐methyl, bifenthrin, buprofezin, captan (RD),66 carbaryl, chlorfenapyr, chlormequat, chlorothalonil (RD), chlorpyrifos‐methyl, clothianidin, cyfluthrin, cypermethrin, cyproconazole, cyromazine, diazinon, dichlorvos, dicloran, dieldrin (RD), difenoconazole, dimethomorph, diniconazole, dithianon, endosulfan (RD), epoxiconazole, ethephon, etofenprox, fenarimol, fenazaquin, fenbuconazole, fenbutatin oxide, fenitrothion, fenoxycarb, fenpropathrin, fenpropimorph (RD), fenvalerate (RD), fipronil (RD), flubendiamide, fluopyram (RD), fluquinconazole, flutriafol, folpet (RD), glyphosate, heptachlor (RD), hexaconazole, indoxacarb, isoprothiolane, lindane, linuron, malathion (RD), mepiquat, metalaxyl, methamidophos, methidathion, methomyl (RD), methoxyfenozide, myclobutanil (RD), oxamyl, paclobutrazol, parathion‐methyl (RD), penconazole, pendimethalin, permethrin, pirimicarb (RD), pirimiphos‐methyl, procymidone (RD), profenofos, propamocarb (RD), propargite, propiconazole, propyzamide (RD), pyridaben, spiromesifen, tau‐fluvalinate, tebufenpyrad, tetraconazole, thiamethoxam, thiophanate‐methyl, triadimenol (RD), triazophos and trifloxystrobin (RD). Therefore, the short‐term dietary exposure to these pesticides, would not be expected to be of concern to consumer health.
Twenty‐four (24) pesticides were quantified in one or more food commodities in levels exceeding their corresponding toxicological reference values (197 determinations in total): acetamiprid (RD), carbendazim (RD), carbofuran (RD), chlorpropham (RD), chlorpyrifos, deltamethrin, dithiocarbamates (RD),67 dimethoate (RD),68 dodine, fenpyroximate (RD), fenthion (RD), flonicamid (RD), fluazifop‐P (RD), fosthiazate, imazalil, imidacloprid, iprodione (RD), lambda‐cyhalothrin (RD), phosmet (RD), pyraclostrobin, tebuconazole (RD), tefluthrin, thiabendazole (RD) and thiacloprid.
ARfD exceedances were identified in pears (63 determinations), potatoes (61 determinations), kiwis (36 determinations), oranges (21 determinations), carrots (12 determinations), rice (3 determinations) and dried beans (1 determination). No results exceeding the available toxicological reference values for acute exposure were observed in cauliflower, onions, rye and animal commodities (sheep fat and poultry fat).
The results per pesticide where the acute exposure was exceeded were the following:
Chlorpyrifos
For chlorpyrifos, 23 samples exceeded the ARfD: 12 samples of carrots (12/1,888), 5 samples of potatoes (5/2,514), 5 samples of pears (5/1,808) and 1 sample of dried beans (1/886). Eight out of the 12 chlorpyrifos ARfD exceedances were reported in samples exceeding their corresponding MRL/commodity. The reasons behind these exceedances could be linked to poor agricultural practices but also to the lowering of the MRLs that recently were applied in pears and potatoes35 (EFSA, 2015b).
The levels of chlorpyrifos in the four remaining carrot samples resulted in exposure estimates above the ARfD in all cases, despite these were lower than the MRL of 0.1 mg/kg, set for the substance in carrots. This is due to the current risk assessment methodology applicable in the contest of an MRL setting and has been identified in other cases (e.g. chlorpropham, lambda‐cyhalothrin (RD), etc.) This issue was identified by EFSA in the past and is currently under discussion at international level (EFSA and RIVM, 2015).
It was noted that high levels of chlorpyrifos were found in several orange samples (274/2,191). Nevertheless, these residues are primarily present in the peel and not in the flesh (endocarp) of the fruit most usually consumed, as was recently shown in a study (Scholz, 2018). This study demonstrated that consumers are exposed to about 3% of the chlorpyrifos quantified in whole oranges when the fruit is eaten peeled. Therefore, based on the evidence of this study, a peeling factor of 0.03 was applied for the estimation of the dietary exposure in this case. The highest estimated exposure to chlorpyrifos from oranges was found equal to 45% of the ARfD.
With respect to potatoes, pears and dried beans where ARfD exceedances were identified, no processing factors were used for the estimation of the acute exposure to chlorpyrifos. Although the use of processing factors is not relevant for pears which can be consumed without cooking or pealing, it is of relevance for potatoes and dried beans. Evidence‐based studies delivering reliable processing factors for chlorpyrifos with respect to potatoes and dried beans were unavailable at the date of the completion of this report.
Dithiocarbamates (RD)
In all the different dithiocarbamate scenarios, exposure exceeded the ARfD for pears, oranges and dry beans except for mancozeb where the ARfD was exceeded only in pears (Figure 64). For all scenarios, pears were the major commodity where dithiocarbamate ARfD exceedances were reported, followed by oranges and dried beans. In the case of dried beans, the exceedances of ARfD correspond to an exceedance of the MRL of 0.1 mg/kg, set for this commodity. This is not the case for oranges and pears where the ARfD exceedances are related to samples with residual levels lower than the respective MRLs for dithiocarbamates in these commodities. Additionally, studies for the establishment of peeling factors for dithiocarbamates in oranges would be desirable.
EFSA is aware that the ARfD exceedances identified may be due to the shortcomings of the currently available analytical methods which lack specificity with respect to the active substance used in the field. Although this may have led to an ambiguous overestimated exposure to mancozeb, maneb, propineb, thiram or ziram, no solid scenario for the refinement of the exposure to these substances can be introduced as long as analytical methodologies specific to each dithiocarbamate remain unavailable. The evaluation of the applications for renewal of approval of the active substances incorporated in this RD and or the associated decision‐making regarding approval is in progress.
Dimethoate (RD)
When the dimethoate–dimethoate scenario35 was used, 7 orange samples were found to exceed the ARfD for dimethoate (7/2,064). They were all non‐compliant samples exceeding the MRL for this RD. Three of them were originated from the EU market (Italy, Malta and Spain), two from Egypt and two from Lebanon and were all notified through the RASFF. When the dimethoate–omethoate scenario35 was used, an additional ARfD exceedance was reported for a cauliflower sample from the Netherlands.
Imazalil
It was noted that imazalil was quantified in more than 50% of orange samples (1,150/2,175), despite the fact that only one MRL exceedance was reported. The highest imazalil concentration, however, occurs within the peel and not in the flesh (endocarp) of the fruit that is most usually consumed. As was shown in a recent EFSA reasoned opinion on imazalil, consumers are exposed to 7% of the imazalil quantified in oranges (EFSA, 2018f) when only eating the flesh after peeling. Therefore, a peeling factor of 0.07 was applied for the estimation of the dietary exposure in this case. Based on the above, only one orange sample was found to result in a calculated intake exceeding the imazalil ARfD (238% of ARfD); this was related to a non‐compliant sample containing 12.8 mg/kg of the substance (MRL = 5 mg/kg). All the other orange samples (2,174) analysed for imazalil were found to contain the substance in levels resulting in dietary exposure estimates below the ARfD.
A wide variety of processing treatments can be applied for the refinement of exposure to pesticide residues in potatoes (Scholz, 2018). A processing factor of 0.22 (EFSA, 2018f) integrated the effects of boiling of unpeeled potatoes to the overall imazalil exposure from consumption of this commodity. The highest estimated imazalil intake from potatoes was found to be lower than the ARfD in all cases.
ARfD exceedances for imazalil were reported in 17 samples of pears. In this case, the estimation of the exposure refers to the consumption of all fruit, including the peel, since the fruit is also consumed without peeling. The highest concentration reported for imazalil in pears (1.8 mg/kg) resulted in an exposure exceeding its ARfD (499 % of ARfD). Considering that this ARfD exceedance is associated with lower imazalil levels than the MRL set for this substance in pears (MRL = 2 mg/kg), appropriate action is recommended for this pesticide/crop combination. Recommendations on the follow up with respect to the use of imazalil in pears have already been proposed by EFSA in its recent reasoned opinion on imazalil (EFSA, 2018f); regulatory measures are under consideration by the risk managers at the time of finalising this report.
Thiabendazole
For thiabendazole (RD), the acute toxicological threshold was exceeded in 13 samples (6 samples of oranges, 5 samples of pears and 2 samples of potatoes). In the case of orange samples, a peeling factor of 0.17 was used (Scholz, 2018). The highest estimated exposure was found to be 318% of ARfD and was associated with a measured residual level of 14.1 mg/kg, which is above the MRL of 5 mg/kg. EFSA noted that all ARfD exceedances identified for thiabendazole in oranges were on samples exceeding the MRL except for one with a content of thiabendazole close to the MRL.
In potatoes, the ARfD of thiabendazole was exceeded for residue levels below the MRL. The MRLs for thiabendazole in potatoes were revised together with other crops under Commission Regulation (EU) No. 2017/1164.69 The Regulation, applicable from January 2018, implements a lower MRL for potatoes (reduced from 15 mg/kg to 0.04 mg/kg). The updated MRL was based on the 2016 EFSA recommendations, as set out in the review of the existing MRLs for thiabendazole (EFSA, 2016d).
ARfD exceedances were identified for thiabendazole in pears (5 samples). In the EFSA MRL review for thiabendazole (RD), a median variability factor of 1.6 was used, originating from four residue trials investigating unit‐to‐unit variability in pears (EFSA, 2016d). EFSA noted that when the revised variability factor of 1.6 is used based on these valid studies for thiabendazole in pears, the exposure to the highest residue measured does not exceed the active substance's ARfD.70
Chlorpropham (RD)
Forty‐six ARfD exceedances were reported for chlorpropham (RD) in potatoes. All ARfD exceedances identified correspond to residue levels below the MRL of 10 mg/kg for this pesticide/crop combination. EFSA noted that potatoes are generally consumed after high temperature treatment (e.g. boiling or frying). Therefore, a more realistic estimation of the exposure to chlorpropham (RD) from potatoes could be done. In the recently published compendium of processing factors (Scholz, 2018), different factors were provided for different treatments ranging from 0.05 for deep fried peeled potatoes to 0.91 for deep fried unpeeled potatoes (crisps). All these factors are marked as ‘indicative’ by Scholz R. et al. Additionally, for boiling unpeeled potatoes, expected to have a worst‐case reduction factor, no processing factors are available; studies for the establishment of processing factors for chlorpropham (RD) in boiling unpeeled potatoes would be desirable in this case.
Deltamethrin
In rice, deltamethrin was found to exceed the ARfD in three samples. A processing factor of 0.5 5 was applied for the estimation of the exposure to this substance, resulting in 107% of the ARfD for the highest measured concentration of 1.7 mg/kg, despite the fact that an MRL exceedance was not observed. Based on the above and that the MRL of 2 mg/kg is higher than the highest measured residue level of 1.7 mg/kg in rice, revision of the MRL for deltamethrin in rice would need to be recommended. However, EFSA noted that the MRL for deltamethrin in rice was already revised down to 1 mg/kg by risk managers in Commission Regulation (EU) No. 2016/1822.71
It was noted that the substance's ARfD was also exceeded in one orange sample. This exceedance was associated with an MRL exceedance in this case.
Iprodione (RD)
For iprodione (RD), the acute toxicological threshold was exceeded in 45 samples in total (9 samples of pears and 36 samples of kiwi fruits). All ARfD exceedances identified corresponded to residual levels below the respective MRLs for this pesticide/crop combination. Considering that this ARfD exceedance is associated with lower iprodione levels than the MRL set for the substance in pears, appropriate action would need to be recommended on this pesticide/crop combination. However, EFSA noted that the MRLs for iprodione were recently set at the level of LOQ for all commodities,72 following the non‐approval of the active substance after the assessment of the application for renewal.
Lambda‐cyhalothrin (RD)
For lambda‐cyhalothrin (RD), the acute toxicological threshold was exceeded in 7 samples in total (1 sample of oranges and 6 samples of pears). Although the one ARfD exceedance in oranges is linked to an exceedance of the MRL, the ARfD exceedances identified in pears corresponded to residue levels below the MRL for this pesticide/crop combination. The MRL for lambda‐cyhalothrin in pears was revised under Commission Regulation (EU) No. 2018/960.73 The Regulation, applicable from January 2019, implements a lower MRL for lambda‐cyhalothrin (RD) in pears (from 0.1 mg/kg to 0.08 mg/kg). This updated MRL was based on the 2015 EFSA recommendations, expressed in the review of the existing MRLs for lambda‐cyhalothrin (RD) (EFSA, 2015c, 2017a).
Other ARfD exceedances
The ARfD exceedances identified for the following pesticides are associated with exceedance of the MRL in the listed crops for these substances: imidacloprid (1/1,695 pear samples), fluazifop‐P (RD) (3/1,191 potato samples), fosthiazate (3/2,049 potato samples), flonicamid (RD) (1/1,088 potato samples) and tefluthrin (1/2,226 potato samples).
On the other hand, the ARfD exceedances identified for the following substances correspond to samples with residue levels lower than the respective MRLs for each pesticide: acetamiprid (1/1,651 samples), dodine (1/1,059 samples), fenpyroximate (1/1,447 samples), phosmet (1/1,323 samples), pyraclostrobin (2/1,697 samples), tebuconazole (3/1,732 samples) and thiacloprid (4/1,695 samples).
For acetamiprid (1/1,651 samples), appropriate measures were taken by risk managers in this respect, and the MRL for acetamiprid in pears has been lowered from 0.8 mg/kg to 0.4 mg/kg.74
In the EFSA reasoned opinions for the setting of MRLs, the exposures to dodine (1/1,059 samples), phosmet (RD) (1/1,323 samples), pyraclostrobin (2/1,697 samples), (3/1,732 samples) and thiacloprid (4/1,695 samples) in pears were estimated with previous versions of the PRIMo model while a more recent version of PRIMo (rev. 3) which contains updated consumption statistics, has been used in this 2017 report. This explains the differences in acute exposure estimated for these substances compared to previous assessments. According to appropriate risk management practice, it is recommended that updates should be implemented via a review of the MRLs for the substances concerned.
Regarding fenpyroximate (1/1,447 samples), in the EFSA review of the existing MRLs for fenpyroximate studies were provided and demonstrated that the unit to unit variability for pomefruits (apples) is 2.2 (EFSA, 2016a). The use of this factor in the refined exposure to pears, results in an acute exposure which does not exceed the ARfD for fenpyroximate.75
The following not approved pesticides found in orange and pear samples were also present in concentrations leading to exceedance of the ARfDs per active substance: carbendazim (RD) (2/1674 orange samples from Argentina and 2/1413 pear samples from Greece), carbofuran (RD) (1/1419 orange sample from Spain) and fenthion (RD) (2/1499 orange samples from Malta and Spain). The reasons behind these exceedances are associated with unauthorised use of pesticides within the EU or use of products which contain pesticides authorised for use in their country of origin and for which no import tolerances have been requested by the importers. It was noted that the presence of carbendazim may be also due to the metabolism and/or degradation of thiophanate‐methyl, an authorised active substance (EFSA, 2014d). EFSA recommends reporting countries to integrate in their national programmes, intensified controls of non‐EU‐approved substances reported to be used in levels which might raise concerns to consumers.
For six pesticides, no toxicological reference values were available: EPN, fenamidone,76 HCB,77 HCH‐alpha,78 HCH‐beta66 and isocarbophos. These pesticides are marked with footnote d) in Figure 64.
For EPN, fenamidone and isocarbophos quantifiable levels were not reported for any of the samples tested (levels in food < LOQ).
HCB, HCH‐alpha and HCH‐beta are POPs banned for agricultural use in the EU but still present in the environment due to their persistence. The acute risk assessment of these substances marked with an asterisk in Figure 64, could not be based on ARfDs, since neither ARfDs nor ADIs have been set for any of them. The estimated short‐term exposure to HCB, HCH‐alpha and HCH‐beta using the food consumption data of EFSA PRIMo revision 3, is presented in Table 13.
Table 13.
Estimated short‐term exposure to active substances without ARfD/ADI values
| Pesticide | Food product | Short‐term exposure (in mg/kg bw per day) |
|---|---|---|
| Hexachlorobenzene |
Fat sheep Fat poultry |
7.2 × 10−6 5.3 × 10−7 |
| Hexachlorocyclohexane (alpha) | Fat sheep | 1.5 × 10−4 |
| Hexachlorocyclohexane (beta) | Fat sheep | 4.5 × 10−5 |
bw: body weight.
Tentative risk assessments were carried out for HCB, HCH‐alpha and HCH‐beta. For HCB, a health‐based guidance value of 0.00017 mg/kg bw per day was set by IPCS in 1997 and used by the EFSA Scientific Panel on Contaminants in 2006 (EFSA, 2006). The acute exposure to HCB from sheep and poultry fat was found to be lower than the health‐based guidance value and therefore, the short‐term dietary exposure to HCB would not be expected to pose a concern to human health (see Table 13).
Based on the acute exposure estimates for HCH‐alpha and HCH‐beta reported in Table 13, adequate margins of exposure (MOE) can be estimated for both substances in sheep fat when a NOAEL of 0.1 mg/kg bw per day is used for both the alpha‐and beta‐isomers (EFSA, 2005): MOE for HCH‐alpha > 600 and MOE for HCH‐beta > 2,000. Based on these results, the short‐term dietary exposure to HCH‐alpha and HCH‐beta would not be expected to pose concerns to human health.
The results of the short‐term (acute) risk assessment are summarised in Figure 64. The numbers in the cells are read/interpreted based on the following information:
Numbers in the cells express the exposure to a specific pesticide per commodity as a percentage of the residue's ARfD (or ADI, if ARfD not available). Each result corresponds to the sample containing the highest residue concentration for a given pesticide/food combination (most conservative estimate).
When no numbers are reported in the cells, one of the following occurs: (i) no residues were quantified for a specific pesticide/food combination (i.e. residue concentration < LOQ, see white cells), (ii) the acute risk assessment is not relevant and therefore not calculated (see green cells) and (iii) the acute risk assessment is relevant but not calculated due to the absence of toxicological reference values (i.e. missing ARfD/ADI, see Figure 64, footnote d).
The colour of the cells is read/interpreted as follows:
White cells in the grid refer to pesticide/crop combinations for which none of the samples analysed for the given food item contained quantified residues (i.e. residue concentration < LOQ).
Green cells refer to pesticides for which an ARfD was not necessary or not available (footnotes b and d) in Figure 64.
Yellow cells refer to pesticide/crop combinations where the exposure was lower than the residue's ARfD.
Red cells refer to pesticide/crop combinations where the calculated dietary exposure was higher than the residue's ARfD; light red cells correspond to acute exposure estimates ranging between 100% and 1,000% of the ARfD, and dark red cells correspond to acute exposure estimates above 1,000% of the ARfD.
Grey cells refer to pesticide/crop combinations not covered by the 2017 EUCP.
Residues marked with an asterisk refer to pesticide/crop combinations with quantified residues for which the toxicological reference values are missing (ADI/ARfD not available).
The detailed results of the short‐term dietary exposure assessment for the pesticide residues found in the 12 food products covered by the 2017 EUCP are presented in Appendix D – Figures D.1, D.2, D.3, D.4, D.5, D.6, D.7, D.8, D.9, D.10, D.11, D.12–D.12. In these charts, the results for the samples containing residues at or above the LOQ are presented individually, expressing the exposure as percentage of the ARfD. The blue dots refer to results reported under the EU‐coordinated programme, whereas the orange dots refer to findings in samples that were analysed in the framework of the NPs. The figures in brackets next to the name of the pesticides represent the number of samples with residues below the LOQ, number of samples with quantified residues below or at the MRL, and the number of samples with residues above the MRL (the asterisk in the graphs’ labels indicates that the MRL changed during the 2017 monitoring year). The different dithiocarbamate and omethoate scenarios have not been represented in Appendix D.
Figure D.1.
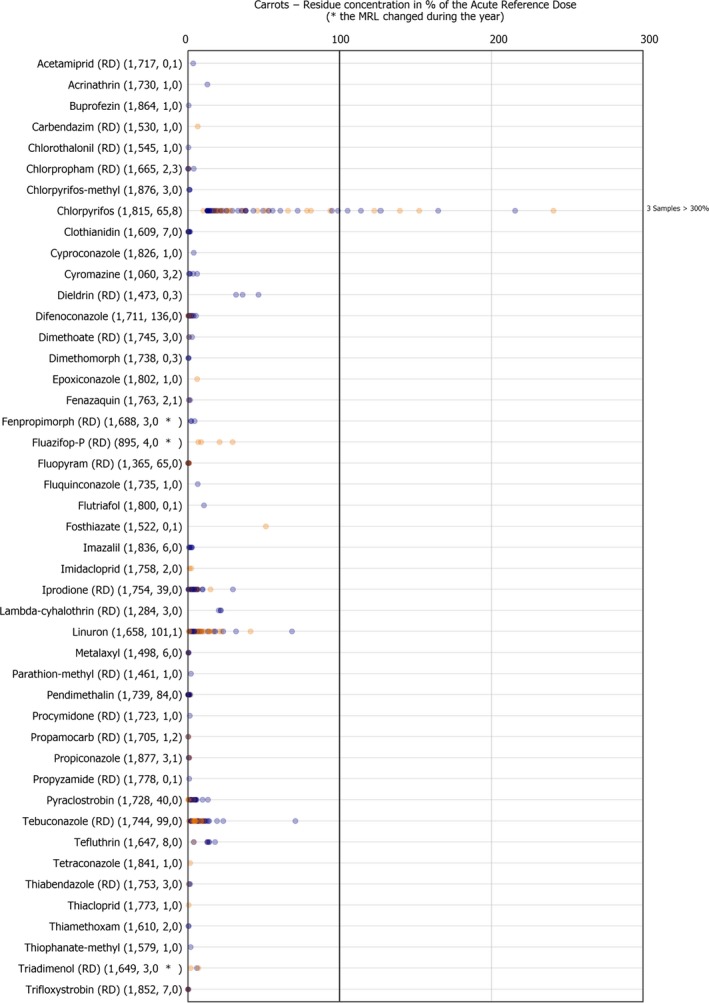
Short‐term dietary exposure assessment – carrots
Figure D.2.
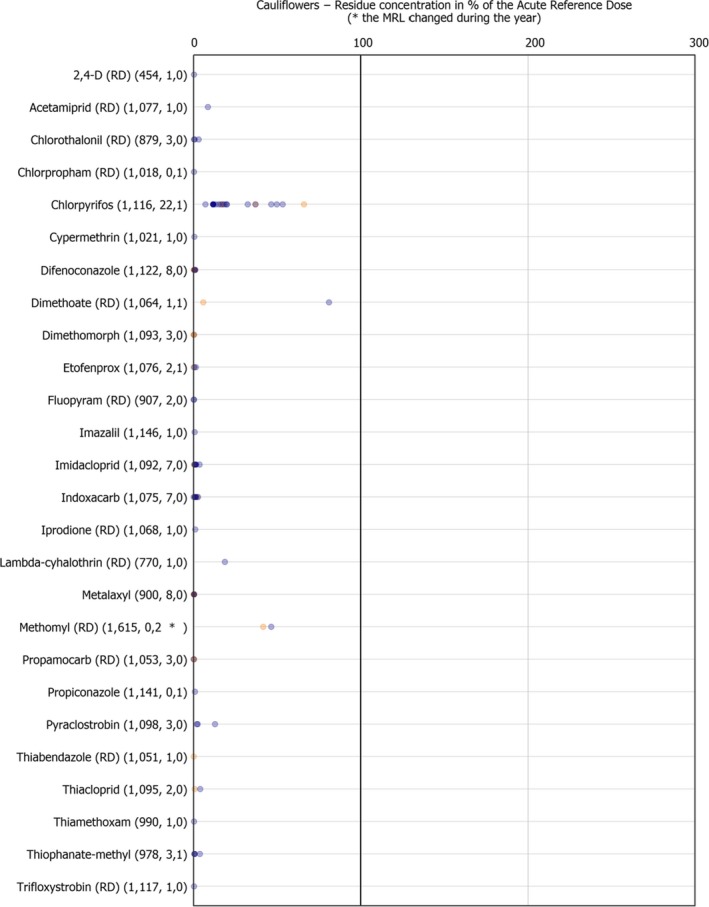
Short‐term dietary exposure assessment – cauliflowers
Figure D.3.

Short‐term dietary exposure assessment – kiwi fruits
Figure D.4.

Short‐term dietary exposure assessment – onions
Figure D.5.
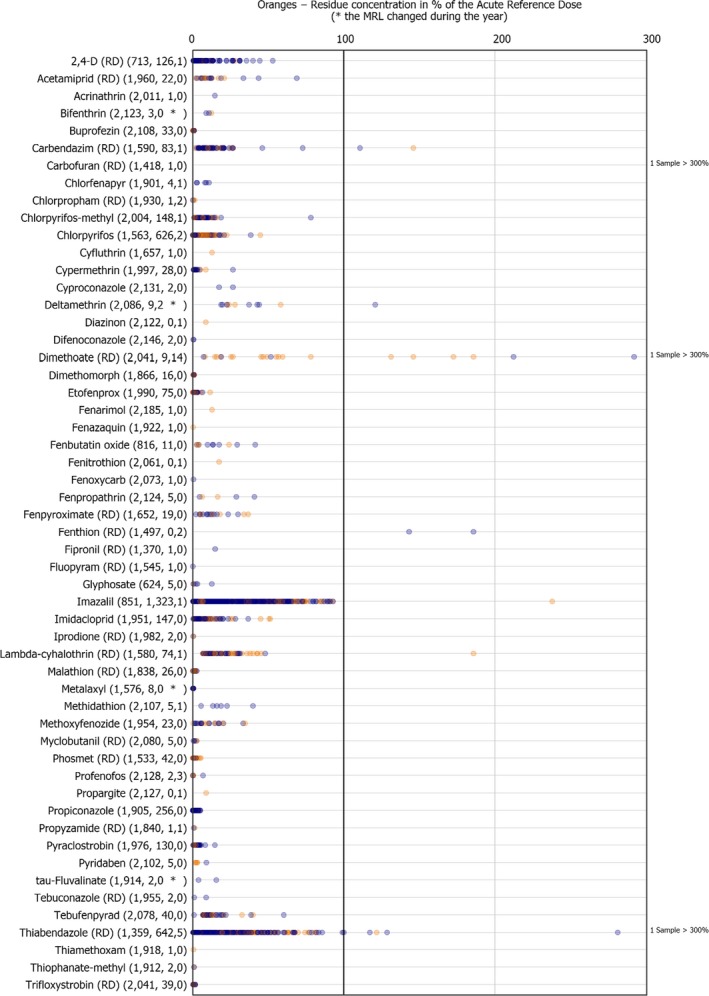
Short‐term dietary exposure assessment – oranges
Figure D.6.
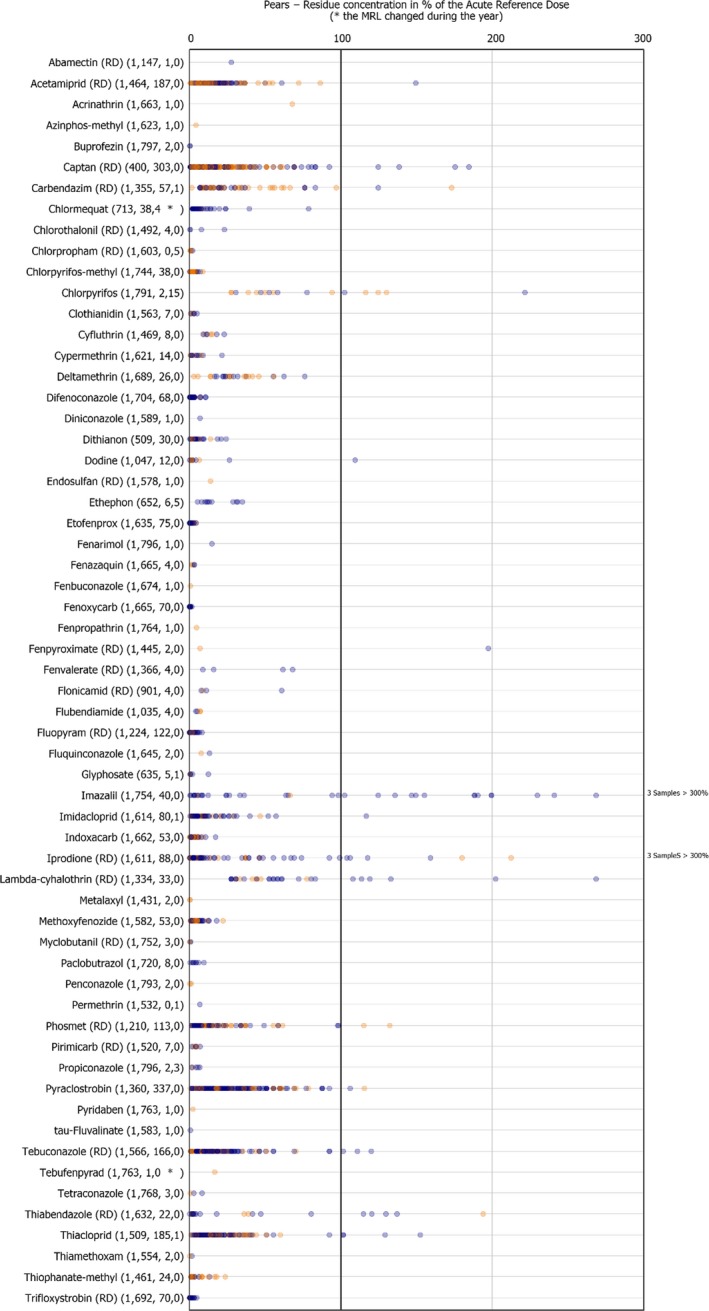
Short‐term dietary exposure assessment – pears82
Figure D.7.

Short‐term dietary exposure assessment – potatoes
Figure D.8.

Short‐term dietary exposure assessment – beans (dried)
Figure D.9.
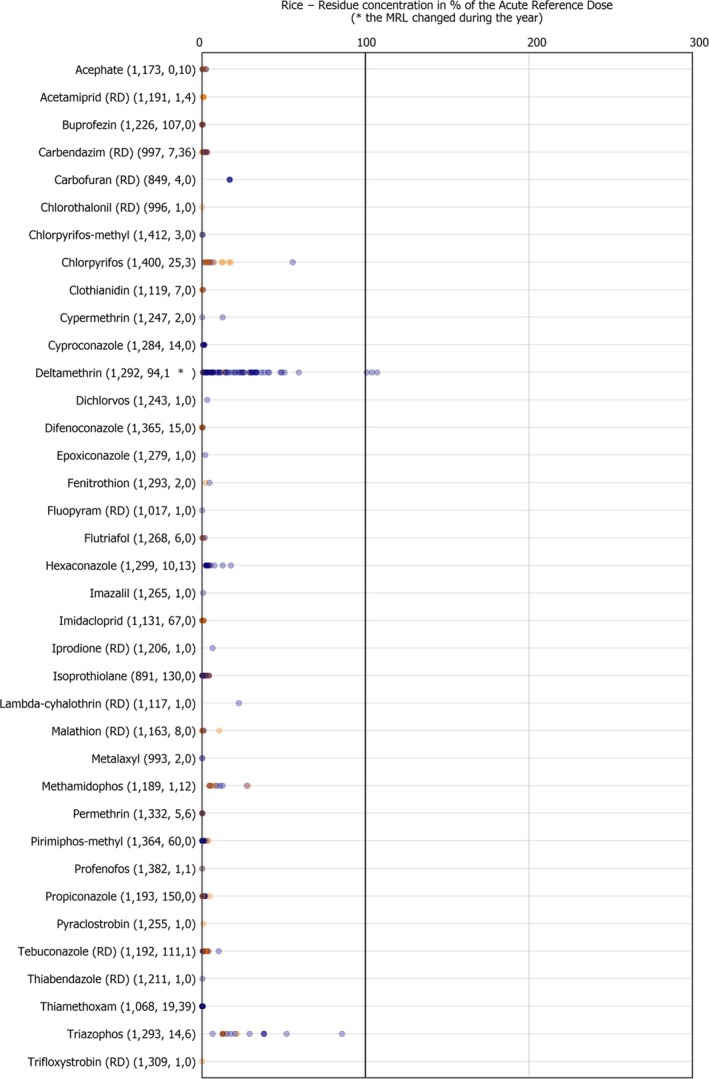
Short‐term dietary exposure assessment – rice
Figure D.10.
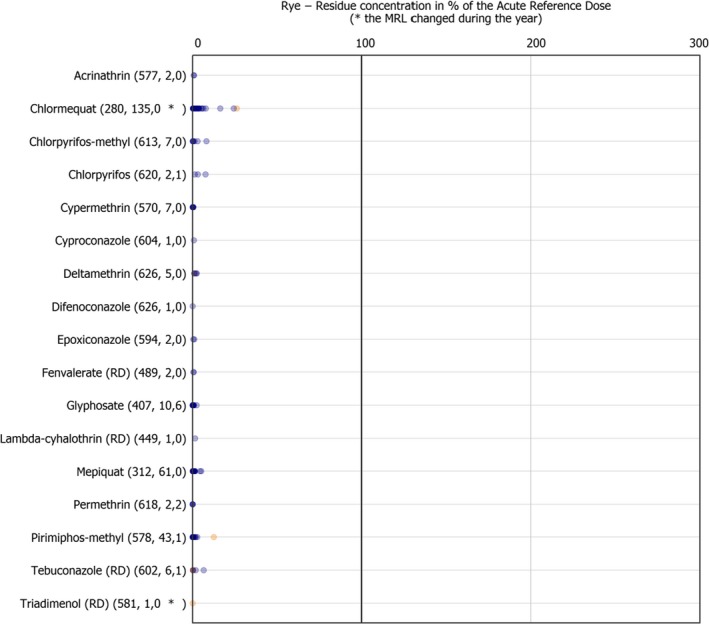
Short‐term dietary exposure assessment – rye
Figure D.11.
Short‐term dietary exposure assessment – poultry fat
Figure D.12.
Short‐term dietary exposure assessment – sheep fat
It should be stressed that the results of the acute exposure assessment reflect the outcome of a conservative screening for risks. In most cases, the exposure calculations were performed without considering that the residues expected in the food consumed after peeling, processing or washing might be significantly lower. For many pesticides, usual consumer practices like washing reduce the residue concentrations significantly. Other practices, like peeling, removal of outer peel, cooking, frying and baking further reduce the residue concentrations in the consumed food. Currently, evidence‐based processing factors were used for some pesticide/crop combinations (e.g. imazalil in oranges), allowing a more realistic acute risk assessment for these substances.
5.2. Long‐term (chronic) risk assessment
The chronic or long‐term risk assessment compares the long‐term dietary exposure per pesticide residue (mg of residue/kg bw per day) to the substance's ADI (in mg of residue/kg bw per day). The ADI values for the active substances are reported in Appendix D – Table D.1.
The assessment deals with results submitted on the 171 pesticides covered by the EUCP and analysed by the reporting countries in 79,411 samples covering all unprocessed products from Annex I (part A) of Reg. (EC) Νο 396/2005.
5.2.1. Methodology for the estimation of long‐term exposure
The chronic or long‐term dietary exposure assessment estimates the dietary exposure to pesticides from all food sources over a long time period, aiming to predict the lifetime dietary exposure to these substances. Its calculation is based on a deterministic approach developed by JMPR (FAO, 2016). It consists of multiplying the mean measured concentration of the pesticide of interest per commodity by the commodity's average daily per capita consumption and summing up the results for all commodities.
EFSA estimated the long‐term exposure for all food products for which a consumption value was provided in EFSA PRIMo revision 3 and for which residue concentrations were reported. In total, 79,411 samples and the 171 pesticides covered by the 2017 EU‐coordinated programme were considered.
Based on 2017 pesticide monitoring results, EFSA calculated two scenarios for long‐term exposure assessment and risk assessment: the adjusted upper‐bound scenario and the lower‐bound scenario.
The adjusted upper‐bound scenario assumes that non‐quantified residues (i.e. results < LOQ) are present in the sample at the level of LOQ.79 It results in a conservative screening which is likely to overestimate the long‐term exposure to a pesticide residue.
The adjusted lower‐bound scenario assumes that if not quantified (i.e. samples with residue level < LOQ), the residues are not present in the food product analysed. This scenario is therefore less conservative, and it may result in an underestimation of the long‐term exposure.
The upper‐ and lower‐bound assessments are used by EFSA to frame the boundaries of a more realistic exposure estimate to pesticide residues and better address the impact of the analytical uncertainties linked to the presence of residues at levels below the LOQ.
For both the upper‐bound and lower‐bound scenarios, the following assumptions were considered:
The mean residue concentration issued from all analytical results per pesticide and crop combination was used.
Only results for unprocessed products from Annex I (part A) of Regulation (EC) No 396/2005 were considered having consumption data available. These include polished rice and rye whole flour, recoded as unprocessed. Results on commodities from Annex I (Table B) of Regulation (EC) No 396/2005 such as basil (holy, sweet), chilli peppers, coriander leaves, mint and pitahaya (dragon fruit) were not included in the risk assessment since no specific information on their consumption is currently available in PRIMo. PRIMo further revisions should allocate these consumption figures (EFSA, 2018a)
Only data on the 171 pesticides of the 2017 EUCP and for which the analysis covered their full RD (i.e. paramTypes P004A and P005A) were used. Results of individual components of a residue definition (i.e. reported as P002A) were not taken into consideration.
Results concerning samples analysed with analytical methods for which the LOQ was greater than the corresponding MRL were disregarded.
If all results reported for a given pesticide/crop combination are below the LOQ for all samples analysed the exposure to the residue from these crops was considered numerically equal to zero in both upper‐ and lower‐bound scenarios.
Both surveillance and enforcement samples (EFSA, 2018d) (i.e. sample strategies ST10A, ST20A and ST30A) were used in the estimation of the exposure considering that enforcement samples are also placed on the market and consumed by the EU citizens.
For fat soluble pesticides in milk and eggs samples for which results were expressed on a fat basis, the residue levels have been recalculated for the whole product assuming a default fat content of 4% in milk and a default fat content of 10% in eggs. This approach was implemented only in case of positive quantifications (results ≥ LOQ).
The estimation of the exposure is based on the residue definition for enforcement (in accordance with the EU MRL legislation) and not the residue definition for risk assessment. This was because the monitoring residue/commodity results refer to the residue definition for enforcement and currently a comprehensive list of conversion factors between the enforcement definition and the definitions set for risk assessment is not available.
5.2.2. Results
The long‐term dietary exposure assessment for each pesticide (adjusted upper‐bound and lower‐bound scenarios) are reported in Table 14. The estimated long‐term exposure for both adjusted upper‐bound and lower‐bound scenarios are expressed as percentage of the ADI.
Table 14.
Results of long‐term dietary exposure assessment
| Pesticide | Long‐term exposure (in % of ADI) | |
|---|---|---|
| Ad. upper‐bound | Lower‐bound | |
| 2,4‐D (RD) | 0.99 | 0.63 |
| 2‐phenylphenol | 0.33 | 0.22 |
| Abamectin (RD) | 6.7 | 0.03 |
| Acephate | 0.12 | 0.01 |
| Acetamiprid (RD) | 1.7 | 0.29 |
| Acrinathrin | 3.8 | 0.24 |
| Aldicarb (RD) | n.r. | |
| Azinphos‐methyl | 1.1 | 0.0002 |
| Azoxystrobin | 0.38 | 0.17 |
| Bifenthrin | 1.8 | 0.18 |
| Biphenyl | 0.04 | 0.02 |
| Bitertanol | 0.54 | 0.01 |
| Boscalid (RD) | 2.4 | 1.4 |
| Bromide ion** | 8.3 | 0.93 |
| Bromopropylate | 0.06 | 0.0002 |
| Bupirimate | 0.40 | 0.01 |
| Buprofezin | 3.6 | 0.45 |
| Captan (RD) | 2.9 | 2.7 |
| Carbaryl | 0.13 | 0.11 |
| Carbendazim (RD) | 1.9 | 0.32 |
| Carbofuran (RD) | 39.5 | 0.43 |
| Chlorantraniliprole | 0.02 | 0.01 |
| Chlordane (RD) | 0.25 | 0.01 |
| Chlorfenapyr | 0.66 | 0.08 |
| Chlormequat | 2.3 | 2.0 |
| Chlorothalonil (RD) | 3.7 | 0.73 |
| Chlorpropham (RD) | 2.7 | 2.4 |
| Chlorpyrifos | 47.1 | 9.4 |
| Chlorpyrifos‐methyl | 4.9 | 0.58 |
| Clofentezine (RD) | 0.98 | 0.04 |
| Clothianidin | 0.24 | 0.01 |
| Cyfluthrin | 10.3 | 0.08 |
| Cymoxanil | 0.41 | 0.002 |
| Cypermethrin | 1.3 | 0.13 |
| Cyproconazole | 1.2 | 0.01 |
| Cyprodinil (RD) | 1.5 | 1.0 |
| Cyromazine | 0.20 | 0.02 |
| DDT (RD) | 6.8 | 0.02 |
| Deltamethrin | 6.0 | 1.0 |
| Diazinon | 24.9 | 0.66 |
| Dichlorvos | 32.0 | 0.22 |
| Dicloran | 0.23 | 0.0002 |
| Dicofol (RD) | 0.56 | 0.01 |
| Dieldrin (RD) | 41.3 | 0.44 |
| Diethofencarb | 0.01 | 0.00003 |
| Difenoconazole | 4.6 | 0.50 |
| Diflubenzuron (RD) | 0.20 | 0.01 |
| Dimethoate (RD) – dimethoate sc. | 35.0 | 15.1 |
| Dimethoate (RD) – omethoate sc. | 108 | 46.8 |
| Dimethomorph | 0.66 | 0.13 |
| Diniconazole | 0.24 | 0.0003 |
| Diphenylamine | 0.43 | 0.01 |
| Dithianon | 4.7 | 3.0 |
| Dithiocarbamates (RD) – mancozeb sc. | 12.9 | 3.6 |
| Dithiocarbamates (RD) – maneb sc. | 12.4 | 3.5 |
| Dithiocarbamates (RD) – metiram sc. | 86.1 | 24.3 |
| Dithiocarbamates (RD) – propineb sc. | 90.3 | 25.5 |
| Dithiocarbamates (RD) – thiram sc. | 36.1 | 10.2 |
| Dithiocarbamates (RD) – ziram sc. | 120 | 34.0 |
| Dodine | 0.25 | 0.09 |
| Endosulfan (RD) | 1.6 | 0.003 |
| EPN | n.r. | |
| Epoxiconazole | 2.2 | 0.01 |
| Ethephon | 3.4 | 0.70 |
| Ethion | 0.54 | 0.08 |
| Ethirimol | 0.45 | 0.01 |
| Etofenprox | 1.1 | 0.18 |
| Famoxadone | 1.5 | 0.73 |
| Fenamidone* | Quantifies residues in one or several commodities | |
| Fenamiphos (RD) | 1.9 | 0.01 |
| Fenarimol | 0.75 | 0.001 |
| Fenazaquin | 4.1 | 0.02 |
| Fenbuconazole | 0.87 | 0.07 |
| Fenbutatin oxide | 0.91 | 0.03 |
| Fenhexamid | 0.23 | 0.16 |
| Fenitrothion | 3.6 | 0.01 |
| Fenoxycarb | 0.42 | 0.02 |
| Fenpropathrin | 0.30 | 0.01 |
| Fenpropidin (RD) | 0.28 | 0.004 |
| Fenpropimorph (RD) | 2.3 | 0.25 |
| Fenpyroximate (RD) | 2.3 | 0.05 |
| Fenthion (RD) | 1.0 | 0.01 |
| Fenvalerate (RD) | 1.4 | 0.05 |
| Fipronil (RD) | 38.4 | 4.5 |
| Flonicamid (RD) | 2.0 | 0.16 |
| Fluazifop‐P (RD) | 0.91 | 0.11 |
| Flubendiamide | 1.1 | 0.01 |
| Fludioxonil (RD) | 0.36 | 0.32 |
| Flufenoxuron | 0.10 | 0.01 |
| Fluopyram (RD) | 3.2 | 0.65 |
| Fluquinconazole | 3.1 | 0.005 |
| Flusilazole (RD) | 1.0 | 0.03 |
| Flutriafol | 2.5 | 0.06 |
| Folpet (RD) | 1.2 | 0.97 |
| Formetanate | 1.7 | 0.08 |
| Fosthiazate | 1.6 | 0.02 |
| Glyphosate | 0.24 | 0.15 |
| Heptachlor (RD) | 4.9 | 0.03 |
| Hexachlorobenzene* | Quantifies residues in one or several commodities | |
| Hexachlorocyclohexane (alpha)* | Quantifies residues in one or several commodities | |
| Hexachlorocyclohexane (beta)* | Quantifies residues in one or several commodities | |
| Hexaconazole | 0.58 | 0.03 |
| Hexythiazox | 0.83 | 0.01 |
| Imazalil | 16.5 | 15.3 |
| Imidacloprid | 0.81 | 0.08 |
| Indoxacarb | 5.9 | 0.37 |
| Iprodione (RD) | 3.4 | 1.4 |
| Iprovalicarb | 0.35 | 0.13 |
| Isocarbophos* | Quantifies residues in one or several commodities | |
| Isoprothiolane | 0.03 | 0.01 |
| Kresoxim‐methyl (RD) | 0.07 | 0.01 |
| Lambda‐cyhalothrin (RD) | 13.0 | 0.89 |
| Lindane | 4.5 | 0.002 |
| Linuron | 2.7 | 0.26 |
| Lufenuron | 1.0 | 0.01 |
| Malathion (RD) | 0.47 | 0.02 |
| Mandipropamid | 0.06 | 0.02 |
| Mepanipyrim | 0.41 | 0.03 |
| Mepiquat | 0.17 | 0.10 |
| Metalaxyl | 0.29 | 0.03 |
| Methamidophos | 2.5 | 0.08 |
| Methidathion | 19.0 | 0.04 |
| Methiocarb (RD) | 0.71 | 0.01 |
| Methomyl (RD) | 1.0 | 0.03 |
| Methoxychlor | 0.12 | 0.0002 |
| Methoxyfenozide | 0.24 | 0.03 |
| Monocrotophos | 0.95 | 0.01 |
| Myclobutanil (RD) | 1.5 | 0.36 |
| Oxadixyl | 0.68 | 0.01 |
| Oxamyl | 5.9 | 0.04 |
| Oxydemeton‐methyl (RD) | n.r. | |
| Paclobutrazol | 0.69 | 0.004 |
| Parathion | 1.5 | 0.01 |
| Parathion‐methyl (RD) | 0.57 | 0.0003 |
| Penconazole | 0.73 | 0.02 |
| Pencycuron | 0.05 | 0.002 |
| Pendimethalin | 0.21 | 0.001 |
| Permethrin | 4.2 | 4.1 |
| Phosmet (RD) | 2.8 | 0.54 |
| Pirimicarb (RD) | 0.68 | 0.11 |
| Pirimiphos‐methyl | 14.5 | 10.2 |
| Procymidone (RD) | 5.7 | 0.06 |
| Profenofos | 0.33 | 0.15 |
| Propamocarb (RD) | 0.17 | 0.08 |
| Propargite | 0.78 | 0.01 |
| Propiconazole | 1.5 | 0.87 |
| Propyzamide (RD) | 0.15 | 0.01 |
| Pyraclostrobin | 1.2 | 0.39 |
| Pyridaben | 3.0 | 0.03 |
| Pyrimethanil (RD) | 1.2 | 0.98 |
| Pyriproxyfen | 0.24 | 0.02 |
| Quinoxyfen | 0.04 | 0.003 |
| Spinosad | 1.4 | 0.13 |
| Spirodiclofen | 1.6 | 0.06 |
| Spiromesifen | 0.59 | 0.06 |
| Spiroxamine (RD) | 0.23 | 0.02 |
| tau‐Fluvalinate | 4.6 | 0.02 |
| Tebuconazole (RD) | 1.6 | 0.25 |
| Tebufenozide | 1.0 | 0.03 |
| Tebufenpyrad | 2.3 | 0.06 |
| Teflubenzuron | 1.0 | 0.005 |
| Tefluthrin | 3.2 | 0.01 |
| Terbuthylazine | 0.28 | 0.003 |
| Tetraconazole | 21.2 | 0.09 |
| Tetradifon | 0.04 | 0.0001 |
| Thiabendazole (RD) | 1.8 | 1.4 |
| Thiacloprid | 3.1 | 0.55 |
| Thiamethoxam | 1.3 | 0.09 |
| Thiophanate‐methyl | 0.48 | 0.03 |
| Tolclofos‐methyl | 0.05 | 0.001 |
| Tolylfluanid (RD) | 0.0003 | 0.00001 |
| Triadimenol (RD) | 0.04 | 0.01 |
| Triadimefon | 0.95 | 0.07 |
| Triazophos | 1.7 | 0.05 |
| Trifloxystrobin (RD) | 0.37 | 0.07 |
| Triflumuron | 1.2 | 0.06 |
| Vinclozolin | n.d. but quantified residues in one or more samples analysed | |
n.r.: No quantified residues in any of the samples analysed; n.d.: No consumption on a specific diet; sc: scenario; ADI: acceptable daily intake.
Active substance for which no ADI was established.
Tentative risk assessment based on ADI of 1 mg/kg bw per day set by JMPR (FAO, 1988).
For the legal residue of fenvalerate containing esfenvalerate, a compound with different toxicological profile, the chronic risk assessment was based on the authorised active substance esfenvalerate.
For dimethoate, two scenarios were used by EFSA.74 Scenario 1 (the ‘optimistic dimethoate scenario’) assumes that the quantified residues are related to the less toxic compound, dimethoate; scenario 2 (the ‘pessimistic omethoate scenario’), assumes that the total quantified residues are related to the more toxic compound, omethoate.
For dithiocarbamates, six scenarios were calculated, considering that the measured CS2 concentrations originated exclusively from maneb, mancozeb, metiram, propineb, thiram or ziram as each one of them has a different toxicological profile and consequently ADI.
When the long‐term risk assessment is based on the lower‐bound scenario, ADI exceedances from pesticide consumption were not identified. The top three highest long‐term exposure estimates correspond to the dimethoate (RD) (47% of the ADI for omethoate) and dithiocarbamates (RD) (34% of the ADI of ziram and 26% of the ADI of propineb).
When the long‐term risk assessment is based on the more conservative adjusted upper‐bound scenario, two ADI exceedances were identified corresponding to consumption of dimethoate (RD) (108% of the omethoate ADI; to apply the pessimistic omethoate scenario, all quantified dimethoate concentrations were converted into omethoate) and dithiocarbamates (RD) (120% of the ziram ADI).
Dimethoate (RD) was found in 257 out of the 64,949 samples analysed for this parameter (0.4%). The major food contributors to the total long‐term exposure to dimethoate were apples (46.6%), oranges (15.3%) and wheat (15.3%).
Dithiocarbamates were found in 1,346 out of the 14,868 samples analysed for this parameter (9.1%). The major contributors to the total long‐term exposure to dithiocarbamates were apples (35.1%), pears (17.6%) and peas (without pods) (12.4%).
EFSA noted that the upper‐bound exposure estimates for dimethoate (RD) and dithiocarbamates, are biaised by a number of uncertainties linked to the scenario used, e.g. ‘omethoate’ and ‘ziram’ scenario or to the background sources of CS2 in onions, cabbages, etc.
ADI exceedances were not identified for all other pesticides based on the upper‐bound risk assessment. For 147 pesticides, the estimated long‐term exposure was less than 10% of the ADI, for 75 thereof the result was lower than 1% of the ADI. For aldicarb, EPN, oxydemeton‐methyl (RD) and parathion‐methyl, covered by the 2017 EUCP, quantifiable residues were not reported for all the food items tested. Aldicarb, EPN and parathion‐methyl were also not quantified as residues in any of the 2016 food commodity samples (EFSA, 2018e).
EFSA noted that fenamidone, HCB, HCH‐alpha, HCH‐beta and isocarbophos were quantified in different food commodities, however, no internationally agreed toxicological reference values are currently set for these pesticides. The estimated exposure to these pesticides, using the food consumption data of EFSA PRIMo revision 3, is reported in Table 15.
Table 15.
Results of long‐term exposure assessment for active substances without ADI values
| Pesticide | Long‐term exposure (in mg/kg bw per day) | |
|---|---|---|
| Adjusted upper‐bound approach | Lower‐bound approach | |
| Bromide ion | 0.083 | 0.0093 |
| Fenamidonea | 0.0001 | 0.000001 |
| Hexachlorobenzene | 0.0002 | 0.0000004 |
| Hexachlorocyclohexane (alpha) | 0.0002 | 0.0000002 |
| Hexachlorocyclohexane (beta) | 0.00004 | 0.00000003 |
| Isocarbophos | 0.00001 | 0.0000002 |
ADI: acceptable daily intake; bw: body weight.
In the framework of the Pesticides Peer Review Experts’ Meeting 134 on fenamidone (EFSA, 2016b), no toxicological reference values were set because of the lack of conclusive data on the potential genotoxicity. During the renewal procedure most of the experts considered that the setting of reference values of fenamidone cannot be supported because of no conclusion on the genotoxic potential of fenamidone could be drawn leading to a critical area of concern. That is why the reference values set in 2003 were not used in the exposure assessment.
For bromide ion, a tentative risk assessment was carried out based on an ADI of 1 mg/kg bw per day set by JMPR (FAO, 1988). In both lower‐ and upper‐bound scenarios the exposure to the naturally occurring bromide ion was below this ADI.
Tentative risk assessments were also carried out for HCB, HBH‐alpha and HBH‐beta. On the basis of the health‐based value set by IPCS for HCB (0.00017 mg/kg bw per day) (EFSA, 2006), the long‐term exposure to HCB would be 0.23% of the health‐based value based on the lower‐bound scenario and 146% of the health‐based value based on the upper‐bound scenario. Although HCB and other environmental compounds would be expected to occur in animal commodities, the overall frequency of quantification of the substance was low. Therefore, the probability of the upper‐bound result occurring for an individual can be considered low.
Based on a NOAEL of 0.1 mg/kg bw per day from subchronic toxicity studies in rodents (EFSA, 2005), no safety concern would be expected from the long‐term exposure to HCH‐alpha and HCH‐beta.
In general, the estimated exposure was significantly lower in the lower‐bound scenario compared to the adjusted upper‐bound approach. EFSA noted that the high proportion of samples with pesticide residues below the LOQ may result in particularly high upper‐bound exposure values due to the assumption that even if not quantified, residues are present in all samples at the level of LOQ. This indicates the high conservatism of the exposure assessment methodology when it comes to the use of LOQ values and explains the differences in the exposure estimates between the lower‐bound and upper‐bound scenarios.
Taking into consideration all food items for which consumption data are provided in PRIMo revision 3, the higher contributors to the overall EU pesticide dietary exposure are those covered by the 3‐year cycle of the EU‐coordinated programme. Overall, EFSA concludes that based on the results of the 2017 pesticide monitoring programmes (EUCP and NP), the long‐term dietary exposure to the pesticides covered by the 2017 EUCP and for which toxicological data are available, would be unlikely to pose a health risk to consumers.
6. Conclusions and recommendations
In 2017, the number of samples analysed by reporting countries for pesticide residues has slightly increased compared to 2016. In the context of the national programmes (including the EUCP), the MRL exceedance rate increased from 3.8% in 2016 to 4.1% in 2017. This 0.3% increase in the MRL exceedance rate compared with the previous year can be explained to a certain extent by the increased number of enforcement samples taken in 2017 which was more than twice the number of enforcement samples taken in 2016 (10,677 in 2017 vs 4,173 in 2016).This demonstrates the importance and effectiveness of the targeted controls can have on detecting MRL exceedances. At the same time, in the frame of the EUCP the percentage of samples with residues below the limit of quantification increased by 4.8% in the EUCP (from 60.1% in 2014 to 64.9% in 2017), demonstrating that the overall situation slightly improved for the commodities and pesticides analysed in a random manner compared to previous years. In the EUCP, an increased number of MRL exceedances was observed in rice and pear samples. Overall, several MRL exceedances were reported for non‐approved substances in domestically produced samples in the EU.
The results of the monitoring programmes are a valuable source of information to estimate the dietary exposure of EU consumers to pesticide residues. As in previous years, EFSA performed an acute (short‐term) dietary risk assessment for the pesticide/food product combinations covered by the EUCP. With the deterministic models currently used for this purpose, exceedances of the acute reference dose have been identified for several food/pesticide combinations. In the future, the use of probabilistic models for acute dietary exposure assessment along with the use of food processing factors would allow more realistic exposure estimates.
A deterministic approach expected to result in an overestimation of the acute exposure was used to assess 171 pesticides in the 12 food products covered by the 2017 EUCP. EFSA concluded that the probability of these pesticides presenting a health risk to consumer is low.
The long‐term (chronic) exposure was calculated, considering all unprocessed food products for which residue data on the pesticides covered by the EUCP were reported. Overall, the estimated exposure was well below the ADI for each active substance in practically all the calculated scenarios. Therefore, EFSA concluded that according to current scientific knowledge, the long‐term dietary exposure to pesticides covered by the 2017 EUCP is unlikely to pose a health risk to consumers.
Based on the 2017 pesticide monitoring findings, EFSA recommends the following:
In the framework of the EUCP, the number of samples recorded by Lithuania and Bulgaria is lower than the minimum number of samples set in Annex II of the 2017 EUCP Regulation. Additionally, Lithuania, France and Iceland did not provide data on pesticide occurrence in baby food.
The 2017 EUCP results indicated an increase in the MRL exceedance rate for rice from 2014 to 2017. Although this increase may be related to the type of samples taken (husked and/or polished rice), Member States are recommended to consider this commodity when designing their respective national control activities, as rice is not covered by the 2018 and 2019 EUCP.
Based on the high quantification rates identified for isoprothiolane in rice and the increase in quantification rates observed for specific residues between 2014 and 2017, it would be relevant to continue monitoring the following pesticides in rice in the context of national programmes: isoprothiolane, bromide ion, propiconazole, deltamethrin, tebuconazole, buprofezin, imidacloprid, carbendazim and thiamethoxam.
The high quantification rates identified for some pesticides in pears suggest that monitoring of the following pesticides for this commodity should continue in the context of national programmes: carbendazim (RD), thiacloprid, imidacloprid, ethephon, glyphosate, diphenylamine, chlorpyrifos, propiconazole, chlorpropham (RD), permethrin and azoxystrobin.
-
Several non‐EU‐approved pesticides were found repeatedly in samples from food produced in the EU, in some cases exceeding the legal limit, e.g.:
-
o
carrots: dieldrin (RD), parathion‐methyl (RD), and procymidone (RD);
-
o
onions: dicloran;
-
o
oranges: fenthion, methidathion and profenofos;
-
o
pears: permethrin;
-
o
potatoes: clothianidin;
-
o
dried beans: biphenyl, carbaryl and carbendazim;
-
o
rice: carbendazim, and dichlorvos;
-
o
rye: permethrin
-
o
Since these results give an indication of possible misuses of non‐approved active substances, it is recommended that Member States follow‐up on these findings, investigating the reasons and taking corrective measures where appropriate.
Monitoring data in pears showed that the quantification rate for diphenylamine fell from 2014 to 2017. Nevertheless, the substance was still present in these matrices at low levels in 2017. For this reason, the continuous monitoring of diphenylamine in pears and apples through the integration of the substance in the national control activities would be relevant.
-
Several non‐EU‐approved pesticides were found in samples from food of non‐EU origin, in concentrations exceeding the legal limit:
-
o
Kiwi fruits: methidathion;
-
o
oranges: chlorfenapyr, methidathion and profenofos;
-
o
dried beans: carbaryl and diazinon;
-
o
rice: acephate, carbendazim, hexaconazole, methamidophos and triazophos.
-
o
A follow‐up on these pesticides/crop combinations is recommended.
Multiple pesticide residues were reported for a number of samples of non‐EU origin (e.g. kiwi fruit samples from Chile, oranges from South Africa). Additional screening analysis of different commodities searching for multiple residues in the context of national programmes is recommended.
The following pesticides were quantified in one or more food commodities at levels exceeding their corresponding ARfDs: carbendazim (RD), carbofuran (RD), chlorpropham (RD), chlorpyrifos, dithiocarbamates (RD), dimethoate (RD), fenthion (RD), flonicamid (RD), fluazifop‐P (RD), fosthiazate, imidacloprid, iprodione (RD) and tefluthrin. Despite appropriate actions having already been put in place or being under discussion at EU and international level with respect to these exceedances, continuous monitoring of the above‐mentioned substances in the context of both EUCP and national programmes is recommended.
Following the revision of the toxicological reference values for chlorpyrifos, the EU MRLs for this substance were lowered to the limit of quantification in several commodities in 2016. Nevertheless, a high number of samples originating in the EU and third countries still contain residues of chlorpyrifos at levels exceeding the new lower legal limits. Continuous monitoring and investigation of the reasons behind these results is recommended.
Among the 659 honey samples and other apicultural products analysed in 2017, 27.8% contained quantifiable residues at levels at or below their respective MRLs and 1.8% contained residues exceeding the legal limits. Since honey is a minor contributor to exposure, EFSA recommends the analysis of honey samples by Member States be done under their national programmes, keeping the analytical scope as wide as possible.
EFSA noted that the following substances exceeded their respective MRLs in honey and other apicultural products and recommends including them in national pesticide monitoring activities: glyphosate, acetamiprid (RD), thiacloprid and dimethoate (RD).
Following the fipronil80 contamination incidents in summer 2017, EFSA recommends Member States should continue analysing acaricides in animal products.
The following pesticides are some of the unexpected pesticides occasionally found in organic crops: chlorpyrifos, anthraquinone, glyphosate, tebuconazole (RD), etc. (Figure 57). Member States should try to elucidate the reasons for these findings.
The ARfD exceedances identified for substances found compliant with the MRL legal requirements for some pesticide/crop combinations are estimated on the basis of a conservative deterministic model which is expected to overestimate exposure to these residues (see Section 5.1.1). EFSA noted that a more realistic estimate of exposure to chlorpyrifos in potatoes and dried beans, dithiocarbamates in oranges and chlorpropham in boiled unpeeled potatoes would be possible if appropriate, evidence‐based processing factors for each pesticide/crop combination were derived. For this reason, it would be relevant to prioritise studies on the establishment of relevant processing factors for these pesticide/crop combinations.
When the long‐term risk assessment is based on the upper‐bound scenario, indications of significant exposure to dimethoate (RD) and dithiocarbamates (RD) were observed. However, these figures are biased by a number of uncertainties linked to the scenarios used e.g. ‘omethoate’ and ‘ziram’ scenario or to the background sources of CS2 in onions or cabbages, etc., the continuous monitoring of both dithiocarbamate and dimethoate residues in the context of EUCP and national programmes is recommended.
Measures taken at Member State level for pesticide/crop combinations exceeding MRLs are generally described as follow‐up actions and administrative actions. Only limited information with respect to these measures is reported to EFSA. Gathering such data in a centralised way may help to establish a more refined and complete risk assessment estimate. Discussions between EFSA, the Commission and the Member States on how to better update the way this information is disseminated between stakeholders would be relevant.
Considering that the residues of three pesticides (aldicarb, EPN and parathion‐methyl), which are currently included in the EUCPs were not quantified in any of the samples analysed during two consecutive years, risk managers may consider taking them out of the mandatory testing in the framework of the EU‐coordinated programme. Instead, it might be sufficient to test samples taken under the national programmes.
Animal fat product commodities were found to present background levels of POPs (e.g. sheep and poultry fat samples in this year's EUCP). EFSA noted decreasing levels of POPs in poultry fat throughout the years and currently recommends revision of the MRLs for DDT, dieldrin (RD) and HCB in this commodity. Samples of sheep fat were included for the first time in the EUCP, so continuous monitoring is recommended to assess the evolution of POP levels within a reasonable timeframe.
As in 2016, MRL exceedances for anthraquinone in tea were identified. Measures in tea producing countries are needed to fully investigate the causes and to implement best practices in tea production.
EFSA reiterates its previous recommendation to develop analytical methods specific to identify the active substance belonging to the class of dithiocarbamates used in the field. This information would allow EFSA to perform a more accurate dietary risk assessment for pesticides belonging to the group of dithiocarbamates in food.
Information on the percentage of samples free of residues (i.e. residues below the LOD) would contribute to a more realistic dietary risk assessment. At the moment, reporting countries are not systematically providing information on whether residues are still visible below the LOQ level for a given analyte. Discussions between EFSA, the Commission and the Member States on how to report this information to EFSA, even if this parameter would be out of the scope of the accreditation, would be relevant.
This report is intended to provide information to the interested public and all partners who have responsibilities in the food chain, in particular food supply chain operators. It gives information on how to enhance the efficiency of self‐control systems. The report should be consulted to identify which pesticides and food products are to be controlled with high priority, considering the findings of the official controls performed by the competent Member State authorities. Efficient strategies to identify at an early stage food products that potentially violate EU food safety standards can contribute to the reduction of non‐compliant food being placed on the market which will improve the quality of food and ensure that dietary exposure of European consumers to pesticide residues is unlikely to pose a health risk to consumers.
Abbreviations
EU/EEA country codes
- AT
Austria
- BE
Belgium
- BG
Bulgaria
- CY
Cyprus
- CZ
Czech Republic
- DE
Germany
- DK
Denmark
- EE
Estonia
- EL
Greece
- ES
Spain
- FI
Finland
- FR
France
- HR
Croatia
- HU
Hungary
- IE
Ireland
- IS
Iceland
- IT
Italy
- LT
Lithuania
- LU
Luxembourg
- LV
Latvia
- MT
Malta
- NL
Netherlands
- PL
Poland
- PT
Portugal
- RO
Romania
- SE
Sweden
- SI
Slovenia
- SK
Slovak Republic
- UK
United Kingdom
Other abbreviations
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BAC
benzalkonium chloride
- bw
body weight
- CAG
Cumulative Assessment Group
- CI
confidence interval
- CS2
carbon disulfide
- DDAC
didecyldimethylammonium chloride
- DCF
Data Collection Framework
- DDT
dichlorodiphenyltrichloroethane
- DWH
Data Warehouse
- EEA
European Economic Area
- EFTA
European Free Trade Association
- EUCP
EU‐coordinated programme
- EURL
European Union Reference Laboratory
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- HCB
hexachlorobenzene
- HCH
hexachlorocyclohexane
- IESTI
International Estimation of Short‐Term Intake
- LOD
limit of detection
- LOQ
limit of quantification
- MOE
margins of exposure
- MRL
maximum residue level
- NOAEL
no‐observed‐adverse‐effect‐level
- NP
national control programme
- POP
persistent organic pollutants
- PPP
plant protection products
- PRIMo
Pesticide Residue Intake Model
- RASFF
Rapid Alert System for Food and Feed
- RD
residue definition
- WHO
World Health Organization
Appendix A – Authorities responsible in the reporting countries for pesticide residue monitoring
1.
| Country | National competent authority | Web address for published national monitoring reports |
|---|---|---|
| Austria | Federal Ministry Labour, Social Affairs, Health and Consumer Protection | https://www.verbrauchergesundheit.gv.at/lebensmittel/lebensmittelkontrolle/monitoring/pestizid.html |
| Austrian Agency for Health and Food Safety | http://www.ages.at/themen/rueckstaende-kontaminanten/pflanzenschutzmittel-rueckstaende/pestizidmonitoringberichte/ | |
| Belgium | Federal Agency for the Safety of the food Chain (FASFC) | http://www.favv-afsca.fgov.be/publicationsthematiques/pesticide-residue-monitoring-food-plant-origin.asp |
| Bulgaria | Risk Assessment Centre on Food Chain | http://www.babh.government.bg/en/ |
| Croatia | Ministry of Agriculture | http://www.mps.hr/ |
| Cyprus | Pesticides Residues Laboratory of the State General Laboratory of Ministry of Health | http://www.moh.gov.cy/sgl |
| Czech Republic | Czech Agriculture and Food Inspection Authority | http://www.szpi.gov.cz |
| State Veterinary Administration | http://www.svscr.cz | |
| Denmark | Danish Veterinary and Food Administration | https://www.foedevarestyrelsen.dk/Kontrol/Kontrolresultater/Sider/Pesticidrester.aspx |
| National Food Institute, Technical University of Denmark | http://www.food.dtu.dk/publikationer/kemikaliepaavirkninger/pesticider-i-kosten | |
| Estonia | Veterinary and Food Board | http://www.vet.agri.ee |
| Finland | Finnish Food Safety Authority Evira and Finnish Customs | https://www.ruokavirasto.fi/en/companies/food-sector/production/common-requirements-for-composition/residues-of-plant-protection-products/control-of-plant-protection-product-residues-in-food/ |
| France | Ministère de l’économie et des finances/Direction générale de la concurrence, de la consommation et de la répression des fraudes (DGCCRF) | http://www.economie.gouv.fr/dgccrf/securite/produits-alimentaires |
| Ministère de l'Agriculture et de l'Alimentation, Direction générale de l'alimentation (DGAL) | http://agriculture.gouv.fr/plans-de-surveillance-et-de-controle | |
| Germany | Federal Office of Consumer Protection and Food Safety (BVL) | http://www.bvl.bund.de/berichtpsm |
| Greece | Ministry of Rural Development and Food | http://www.minagric.gr/index.php/en/citizen-menu/foodsafety-menu |
| http://www.minagric.gr/index.php/el/for-farmer-2/crop-production/fytoprostasiamenu/ypoleimatafyto | ||
| Hungary | National Food Chain Safety Office | https://www.nebih.gov.hu |
| Iceland | MAST – The Icelandic Food and Veterinary Authority | http://www.mast.is |
| Ireland | Department of Agriculture Food and the Marine | http://www.pcs.agriculture.gov.ie |
| Italy | Ministero della Salute – Direzione Generale per l'Igiene e la Sicurezza degli Alimenti e la Nutrizione – Ufficio 7 | http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=1105&area=fitosanitari&menu=vegetali |
| Latvia | Ministry of AgricultureFood and Veterinary Service of Latvia | http://www.zm.gov.lv |
| Lithuania | National Food and Veterinary Risk Assessment Institute | http://www.nmvrvi.lt |
| Luxembourg | Ministry of Health, Directorate for public health, Division of Food Safety (Secualim) | http://www.securite-alimentaire.public.lu |
| Ministry of Health, Administration of Veterinary Services (ASV) | ||
| Malta | Malta Competition and Consumer Affairs Authority | http://www.mccaa.org.mt |
| Netherlands | Netherlands Food and Consumer Product Safety Authority (NVWA) | http://www.nvwa.nl |
| Norway | Norwegian Food Safety Authority | http://www.mattilsynet.no https://www.mattilsynet.no/mat_og_vann/uonskede_stofferimaten/rester_av_plantevernmidler_i_mat/rester_av_plantevernmidler_i_naeringsmidler_2017.31315 |
| Poland | The State Sanitary Inspection | http://www.gis.gov.pl |
| Portugal | Direção‐Geral de Alimentação e Veterinária (DGAV) | http://www.dgv.min-agricultura.pt/portal/page/portal/DGV/genericos?generico=4217393&cboui=4217393t |
| Romania | National Sanitary Veterinary and Food Safety Authority | http://www.ansvsa.ro |
| Ministry of Agriculture and Rural Development | http://www.madr.ro | |
| Ministry of Health | ||
| Slovakia | State Veterinary and Food Administration of the Slovakian Republic | http://www.svps.sk/ |
| Public Health Authority of the Slovakian Republic | ||
| Slovenia | Administration of the Republic of Slovenia for Food Safety, Veterinary Sector and Plant Protection | http://www.uvhvvr.gov.si/si/delovna_podrocja/ostanki_pesticidov |
| Spain | Spanish Agency for Food Safety and Nutrition (AESAN) | http://www.aecosan.msssi.gob.es/AECOSAN/web/seguridad_alimentaria/subseccion/programa_control_residuos.htm |
| Sweden | National Food Agency | http://www.livsmedelsverket.se |
| United Kingdom | Health and Safety Executive, Chemicals Regulation Division | https://www.gov.uk/government/publications/expert-committee-on-pesticide-residues-in-food-prif-annual-report |
Appendix B – Background information on the EU‐coordinated programme
1.
Appendix C – Background information and detailed results on the overall control programmes
1.
Appendix D – Background information and detailed results on dietary risk assessment
1.
Results of short‐term dietary risk assessment for food products in focus of the EUCP, expressed as percentage of the ARfD
In the following figures,81 the short‐term exposure calculated for each sample with residues above the LOQ was presented individually, expressing the result as percentage of the ARfD. The blue dots refer to results reported under the EUCP, whereas the orange dots refer to findings in samples that were analysed in the framework of the national control programmes. The figures in brackets next to the name of the pesticides represent the number of samples with residues below the LOQ, number of samples with quantified residues below the MRL, and the number of samples with residues above the MRL.
Supporting information
Supplement MRL 2017 exceedances
PRIMo 2017monitoring results
Suggested citation: EFSA (European Food Safety Authority) , 2019. Scientific report on the 2017 European Union report on pesticide residues in food. EFSA Journal 2019;17(6):5743, 152 pp. 10.2903/j.efsa.2019.5743
Requestor: European Commission
Question number: EFSA‐Q‐2014‐00104
Acknowledgements: The Pesticide Residues Unit wishes to thank Anneli Widenfalk (National Food Agency, Sweden) for her valuable proposals and the independent scientific review of this report.
A summary in MicroStrategy of the data used in this report is available at: :https://www.efsa.europa.eu/en/microstrategy/pesticides-dc-2017 The raw datasets used in the analyses are available on the EFSA Knowledge Junction at https://zenodo.org/communities/efsa-kj. To search for individual Member State data, search using the following syntax: “[country name] results from the monitoring of pesticide residues in food”
Adopted: 26 May 2019
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2019.EN-1666/full
Notes
The term ‘(RD)’ added after the name of an active substance indicates that the full residue definition of the active substance is considered.
Throughout the report, results describing percentage of samples above the legal limit, within the legal limit and samples without quantifiable residues are provided with one decimal. Due to the rounding to one decimal place, the added results for these three categories may slightly differ from 100%.
The legal limit for food intended for infants and young children is set at a level equal or close to the limit of quantification; in general, a default MRL of 0.01 mg/kg is applicable unless lower legal limits for the residue levels are defined in Directives 2006/125/EC and 2006/141/EC.
Chlorates are by‐products of chlorine solutions, used as sanitizing and disinfection agents in the food industry and their presence is not necessarily related to the use as plant protection product. Discussions on the revision/setting of more realistic MRLs for chlorates by the risk managers are currently on‐going. In 2017, detections might not have been reported in the absence of a revised and agreed MRL.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC Text with EEA relevance. OJ L 70, 16.3.2005, p. 1–16.
Commission implementing Regulation (EU) 2016/662 of 1 April 2016 concerning a coordinated multiannual control programme of the Union for 2017, 2018 and 2019 to ensure compliance with maximum residue levels of pesticides and to assess the consumer exposure to pesticide residues in and on food of plant and animal origin. OJ L 115, 29.4.2016, p. 2‐15.
Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children. OJ L 339, 6.12.2006, p. 16–35.
Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow‐on formulae and amending Directive 1999/21/EC. OJ L 401, 30.12.2006, p. 1–33.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. OJ L 181, 29.6.2013, p. 35–56.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 015 20.1.2010, p. 1.
Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. OJ L 125, 23.5.1996, p. 10.
The comprehensive results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products are published in a separate report (EFSA, 2017b).
Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control. OJ L 250, 18.9.2008, p. 1–84.
Commission Regulation (EC) No 669/2009 of 24 July 2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non‐animal origin and amending Decision 2006/504/EC. OJ L 194, 25.7.2009, p. 11–21.
Commission Implementing Regulation (EU) 2016/2107 of 1 December 2016 amending Annex I to Regulation (EC) No 669/2009 as regards the list of feed and food of non‐animal origin subject to an increased level of official controls on imports. OJ L 327, 2.12.2016, p. 50–56.
Commission Implementing Regulation (EU) 2017/186 of 2 February 2017 laying down specific conditions applicable to the introduction into the Union of consignments from certain third countries due to microbiological contamination and amending Regulation (EC) No 669/2009 OJ L 29, 3.2.2017, p. 24‐–34.
Commission Implementing Regulation (EU) 2017/1142 of 27 June 2017 amending Annex I to Regulation (EC) No 669/2009 as regards the list of feed and food of non‐animal origin subject to an increased level of official controls on imports. OJ L 165, 28.6.2017, p. 29‐35.
Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. OJ L 125, 23.5.1996, p. 10–32.
Possible discrepancies on the results presented in this report compared to those present in the 2017 EFSA Pesticides Dashboard can be noticed. This is due to the permanently possibility of updating the data stored in the Data Warehouse (DWH) and used to create the Dashboard in comparison with the data reported by the closing of the 2017 data collection, used to create this report.
The LOD is the lowest concentration of a pesticide residue that can be identified in a sample with an acceptable degree of certainty. However, the amount of the analyte cannot be quantified reliably.
Amitraz (RD), amitrole, azinphos‐ethyl, benfuracarb, bromuconazole, carbosulfan, chlorfenvinphos, chlorobenzilate, dichlofluanid, dichlorprop (RD), dicrotophos, endrin, ethoprophos, formothion, glufosinate (RD), haloxyfop‐R (RD), ioxynil (RD), isofenphos‐methyl, isoprocarb, maleic hydrazide (RD), meptyldinocap (RD), metaflumizone, metazachlor, metconazole, metobromuron, nitenpyram, phenthoate, phosalone, phoxim, prochloraz (RD), propoxur, prothioconazole (RD), prothiofos, pymetrozine, pyrazophos, pyrethrins, resmethrin, rotenone, tetramethrin, topramezone, trichlorfon, trifluralin, triticonazole, zoxamide.
Many of the pesticides removed from the EUCP were included in the ‘Working document on pesticides to be considered for inclusion in the national control programmes to ensure compliance with maximum residue levels of pesticides residues in and on food of plant and animal origin’. Detailed updates of the different document versions can be consulted in the EURL pesticide residues web site: http://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=629&LabID=100&Lang=EN. For some of these substances, improvements of analytical methods were considered necessary, while for others it needs to be verified whether residues are found in a significant percentage of the samples. After an evaluation period under the national programmes, the inclusion of these substances in the EUCP will be reconsidered.
Commission Regulation (EU) No. 2017/627 of 3 April 2017 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for fenpyroximate, triadimenol and triadimefon in or on certain products. OJ L 96, 7.4.2017, p. 44–70.
For 2014, EUCP Regulation (EU) No. 788/2012 was applicable together with Regulation (EU) No 480/2013 on the number of samples to be monitored by Croatia.
Ireland and Spain reported more samples than the number indicated in this report. This concerns the EU‐coordinated programme and is related to the differences in the number of results validated and later used in this report. Member States should be aware that despite all valid data received through the Data Collection Framework (DCF) are included in the EFSA DWH, only part of it is used in this report. Excluded results were those for feed, fish and food not specified, results reported with paramtype=P002A or with a missing reportname, and all results not compliant with any of the Matrix Tool tables.
The numbers in the brackets refer to the total number of samples analysed for the given pesticide, e.g. 8,029 samples were analysed for aldicarb, 8,723 samples were analysed for azinphos‐methyl, etc.
Triadimefon became a separate residue definition (RD) on 27 October 2017 under Regulation (EU) No 627/2017. Before this date, results for this active substance were reported together with results for triadimenol. The no finding situation can be explained since is a non‐approved substance in the EU and that the stand‐alone residue definition was applicable in 2017 only for the two last months.
Due to the rounding of the numerical results, the total calculated sum may slightly differ from 100%.
The estimation is based on the total number of samples analysed (as indicated in each specific section and figure) and the number of samples quantified (e.g. number of samples with at least one quantification). No CI is included if no samples were quantified.
The number of samples within and exceeding the legal limit, is based on the judgement of the reporting country.
The MRLs applicable at the beginning of the reference period (January 2017) as reported in the MRL database of the European Commission were used for the calculation. Whenever a MRL changed during the calendar year, the MRL reported by the MS to be in place at the time of sampling, was taken. The reason for this approach is that the exact date of sampling to decide which MRL is applicable is not reported to EFSA.
The fact that the MRL used to express the residue concentration as percentage of the MRL (i.e. MRL in place at the beginning of the calendar year) and the MRL used by reporting country to decide on an MRL exceedance may be different, explains some inconsistencies between the bar charts and the dot plot figures and the results presented on the online Pesticides Dashboard.
Temporary MRLs were set to address an unavoidable cross‐contamination that affected untreated apples and pears, due to the presence of residues of diphenylamine in storage facilities. The timeline for application of these temporary MRLs was extended until 22 January 2018. Monitoring data from the 2016 and 2017 EUCP showed that residues of diphenylamine in apples (2016 EUCP) and pears (2017 EUCP) decreased but are still present. Regulation (EU) No 2018/1515, sets the MRL of diphenylamine for these commodities at the level of LOQ (0.05* mg/kg) and applies from 1 May 2019.
Commission Regulation (EU) 2016/60 of 19 January 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for chlorpyrifos in or on certain products. OJ L 14, 21.1.2016, p. 1–17.
Isoprothiolane and bromide ion in the 2017 EUCP were analysed only in rice.
Commission Regulation (EU) 2016/156 of 18 January 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for boscalid, clothianidin, thiamethoxam, folpet and tolclofos‐methyl in or on certain products. OJ L 31, 6.2.2016.
Commission Regulation (EU) No 592/2012 of 4 July 2012 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for bifenazate, captan, cyprodinil, fluopicolide, hexythiazox, isoprothiolane, metaldehyde, oxadixyl and phosmet in or on certain products in or on certain products. OJ L 176, 6.7.2012, p. 1–37.
Council Decision 2006/507/EC of 14 October 2004 concerning the conclusion, on behalf of the European Community, of the Stockholm Convention on Persistent Organic Pollutants. OJ L 209, 31.7.2006, p. 1–2.
In addition to these 88,247 samples, results on 627 samples of feed and fish were also reported to EFSA. However, with no legal limits currently set for feed and fish under Regulation (EC) No 396/2005, these samples were not considered in the analysis of the 2017 overall monitoring results.
France, Slovenia and Spain reported to have sent more samples than the number indicated in this report. This concerns both the EU‐coordinated and national programmes and is related to the differences in the number of results validated and later used in this report. Member‐States should be aware that despite all valid data received through the Data Collection Framework (DCF) are included in the EFSA DWH, only part of it is used in this report. Excluded results were those for feed, fish and food not specified, results reported with paramtype=P002A or with a missing reportname, and all results not compliant with any of the Matrix Tool tables.
Food products compliant with the description in Annex I of Regulation (EC) No 396/2005 are considered as unprocessed products. It should be noted that this food classification comprises mainly unprocessed raw agricultural products, but also some processed products such as fermented tea, dried spices, dried herbal infusions etc., are considered ‘unprocessed’ products in the framework of this report.
Baby food samples are referring to: ‘baby foods other than processed cereal‐based foods, processed cereal‐based foods for infants and young children and infant formulae’.
Commission Regulation (EC) No 669/2009 of 24 July 2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non‐animal origin and amending Decision 2006/504/EC, OJ L 194, 25.7.2009, p. 11–21.
In general, a default MRL of 0.01 mg/kg is applicable for food covered by Directive 2006/125/EC and 2006/141/EC unless lower legal limits for the residue levels are defined in the Directives. Thus, the provisions are more restrictive than for other food falling under the provisions of Regulation (EC) No 396/2005.
The baby food samples (1,546 baby food samples of which 393 were specifically flagged as organic samples) were not included in this analysis since the results for this food group are presented in detail in the previous chapter.
The overall MRL exceedance rate for the 88,247 samples is 4.1%. In this section, baby food samples are excluded.
For this comparison, all pesticides were considered; the naturally occurring substances covered by the MRL legislation were not excluded since they are also present in conventional food and are therefore also covered in the calculation of the quantification rate for conventional food.
The overall quantification rate for the 88,247 samples is 41.8%. In this section, baby food samples are excluded.
For conventional and organic products, the MRLs established in Regulation (EC) No 396/2005 are applicable.
As defined in Article 3(14) of Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Regulation (EU) No. 2017/2091 no longer approves the use of iprodione in the EU.
Regulation (EU) No 528/2012 of the European Parliament and of the council of 22 May 2012 concerning the making available on the market and use of biocidal products, OJ L 167, 27.6.2012, p. 1–123
Further explanations on the cumulative dietary exposure ongoing can be found in EFSA web site: http://www.efsa.europa.eu/en/press/news/181205
Public consultation on the draft Scientific report of EFSA on the establishment of cumulative assessment groups of pesticides for their effects on the nervous system: deadline 13 June 2018. http://www.efsa.europa.eu/en/consultations/call/180508-0
Public consultation on the draft Scientific report of EFSA on the Establishment of cumulative assessment groups of pesticides for their effects on the thyroid: deadline 22 March 2019 http://www.efsa.europa.eu/en/consultations/call/190213
Normally 97.5th percentile of the daily food consumption reported in food surveys, considering only persons who have consumed the pertinent food item during the reference period.
According to the 2017 EUCP control programme, samples of rye whole grain flour could have been taken in case rye grains’ samples were not available for monitoring porpoises. The same applies to polished rice grain in case no husked rice samples were available.
In 2016, JMPR recommended using a variability factor of 3 (which is the rounded mean of 2.8) for all commodities (FAO, 2016). At EU level, the choice of the most appropriate variability factor to be used for the acute risk assessment is still under discussion. So far, Member States did not agree to reduce the variability factor.
Regulation (EU) No. 1135/2017, setting two different RDs for the two active substances dimethoate and omethoate, entered into force on 17 January 2018 and is not considered in the risk assessment of the 2017 results. Individual concentrations reported of each of the two active substances not being consistent with the RD applying for the 2017 results were not taken into consideration.
For metiram, no ARfD was considered necessary. Thus, no metiram scenario was calculated.
In the framework of the Pesticides Peer Review Experts’ Meeting 134 on fenamidone (EFSA, 2016b), no toxicological reference values were set because of the lack of conclusive data on the potential genotoxicity. During the renewal procedure most of the experts considered that the setting of reference values of fenamidone cannot be supported because no conclusion on the genotoxic potential of fenamidone could be drawn leading to a critical area of concern. That is why the reference values set in 2003 were not used in the exposure assessment.
Metiram ‐ an active substance with low acute toxicity falling within the dithiocarbamates (RD) ‐ is not counted among the above‐mentioned substances, otherwise their number should be 33. In this case however, the total number of EUCP pesticides would not be 171 but 177 considering the six dithiocarbamates and two dimethoate scenarios.
Council Decision (EU) 2019/448 of 18 March 2019 on the submission, on behalf of the European Union, of a proposal for the listing of methoxychlor in Annex A to the Stockholm Convention on Persistent Organic Pollutants. OJ L 77, 20.3.2019, p. 74–75.
In the EFSA MRL review for captan (RD) (EFSA, 2014b) a variability factor of 3 (instead of 7) was used, issued from GAP compliant residues trials on apples and pears. EFSA noted that when the revised and based on valid studies variability factor of 3 is used, the exposure to the highest residue measured for captan (RD) (i.e. 4 mg/kg) does not exceed the active substance's ARfD.
This involves the five dithiocarbamates scenarios for which the acute risk assessment is relevant: mancozeb, maneb, propineb, thiram and ziram scenarios.
This involves the two dimethoate scenarios: optimistic dimethoate and pessimistic omethoate scenarios.
Commission Regulation (EU) 2017/1164 of 22 June 2017 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acrinathrin, metalaxyl and thiabendazole in or on certain products. OJ L 170, 1.7.2017, p. 3–30.
This refinement has been included in PRIMo supplement published along this report but not in the dot plots for pears in Appendix D –Figure D.6.
Commission Regulation (EU) No. 2016/1822 of 13 October 2016 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for aclonifen, deltamethrin, fluazinam, methomyl, sulcotrione and thiodicarb in or on certain products. OJ L 281, 18.10.2016, p. 1–44.
Commission Regulation (EU) No. 2019/38 of 10 January 2019 amending Annexes II and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for iprodione in or on certain products. OJ L 9, 11.1.2019, p. 94–105.
Commission Regulation (EU) No. 2018/960 of 5 July 2018 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for lambda‐cyhalothrin in or on certain products. OJ L 169, 6.7.2018, p. 27–50.
Commission Regulation (EU) No. 2019/88 of 18 January 2019 amending Annex II to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acetamiprid in certain products. OJ L 22, 24.1.2019, p. 1–12.
The use of a lower VF for fenpyroximate in pears was not taken in PRIMo tool published along with this report.
In the framework of the Pesticides Peer Review Experts’ Meeting 134 on fenamidone (EFSA, 2016b), no toxicological reference values were set because of the lack of conclusive data on the potential genotoxicity. During the renewal procedure, most of the experts considered that the setting of reference values of fenamidone cannot be supported because of no conclusion on the genotoxic potential of fenamidone could be drawn leading to a critical area of concern. That is why the reference values set in 2003 were not used in the exposure assessment.
The MRL for hexachlorobenzene in sheep and poultry fat, was amended from 0.2 mg/kg to 0.01 mg/kg with Commission Regulation (EU) 2016/1866 of 17 October 2016 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for 3‐decen‐one, acibenzolar‐S‐methyl and hexachlorobenzene in or on certain products. OJ L 286, 21.10.2016, p. 4‐31.
The MRLs for hexachlorocyclohexane (alpha) and hexachlorocyclohexane (alpha) in sheep and poultry fat were lowered from 0.2 mg/kg to 0.01 mg/kg with Commission Regulation (EU) 2017/978 of 9 June amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for fluopyram; hexachlorocyclohexane (HCH), alpha‐isomer; hexachlorocyclohexane (HCH), beta‐isomer; hexachlorocyclohexane (HCH), sum of isomers, except the gamma isomer; lindane (hexachlorocyclohexane (HCH), gamma‐isomer); nicotine and profenofosn in or on certain products. OJ L 151, 14.06.2017, p.1‐37.
SANCO/12574/2014 (rev. 5) (European Commission, 2017) entered into force on 1 January 2017. The provision relates the reporting of the LOQ to multicomponent residue definitions (RD). Individual LOQ for each component of a multicomponent RD, needed to be reported to EFSA. Additionally, the calculated sum LOQ of the RD or a default ‘99999’ must have been reported. If ‘99999’ was reported, EFSA calculated the sum LOQ based on the individual LOQ and the molecular weight factor. This recalculation was used when calculating the mean upper‐bound scenario. In case no individual LOQs were reported, EFSA replaced the value of ‘99999’ by 0.01 mg/kg and included in the calculation.
Fipronil is a veterinary medicinal product or biocide and its presence in eggs was the result of illegal use.
In the following figures, there are some cases where the ARfD was exceeded, while the samples were still within the MRL. Some of these cases are related to ARfD values recently amended. However, for the other cases, risk managers should be aware that it is caused of the difference of HR/MRL.
For thiabendazole (RD), under Art12 (EFSA, 2016d) the VF = 2 was used instead of 7. This would drop the acute exposure to 55.4% for the highest residue.
References
- Abraham J, 2013. Computation of Cis for Binomial proportions in SAS and its practical difficulties. Kreara Solutions Pvt. Ltd. PhUSE 2013, Paper SP05, Thiruvananthapuram, India.
- CAG/GL 59‐2006 Guidelines on Estimation of Uncertainty of Results . 2006Codex Alimentarius Comimission, Rome Italy, 2006. Available online: http://www.codexalimentarius.net/download/report/701/al31_24e.pdf
- Clopper CJ and Pearson ES, 1934. The Use of Confidence or Fiducal Limits illustrated in the case of the binomial. Biometrika, 26, 404–413. 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to gamma‐HCH and other hexachlorocyclohexanes as undesirable substances in animal feed. EFSA Journal 2005;3(7):250, 39 pp. 10.2903/j.efsa.2005.250 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to hexachlorobenzene. EFSA Journal 2006;4(10):402, 49 pp. 10.2903/j.efsa.2006.402 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Reasoned opinion on the setting of a new MRL for isoprothiolane in rice. EFSA Journal 2012;10(3):2607, 29 pp. 10.2903/j.efsa.2012.2607 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. The 2010 European Union report on pesticide residues in food. EFSA Journal 2013;11(3):3130, 808 pp. 10.2903/j.efsa.2013.3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Conclusion on the peer review of the pesticide human health risk assessment of the active substance chlorpyrifos. EFSA Journal 2014;12(4):3640, 34 pp. 10.2903/j.efsa.2014.3640 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for captan according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(4):3663, 55 pp. 10.2903/j.efsa.2014.3663 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014c. The 2011 European Union report on pesticide residues in food. EFSA Journal 2014;12(5):3694, 511 pp. 10.2903/j.efsa.2014.3694 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014d. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for thiophanate‐methyl and carbendazim according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(12):3919, 118 pp. 10.2903/j.efsa.2014.3919 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014e. The 2012 European Union report on pesticide residues in food. EFSA Journal 2014;12(12):3942, 156 pp. 10.2903/j.efsa.2014.3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2015a. Pesticide Monitoring Program: Design Assessment. EFSA Journal 2015;13(2):4005, 52 pp. 10.2903/j.efsa.2015.4005 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015b. Reasoned opinion on the refined risk assessment regarding certain maximum residue levels (MRLs) of concern for the active substance chlorpyrifos. EFSA Journal 2015;13(6):4142, 41 pp. 10.2903/j.efsa.2015.4142 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015c. Reasoned opinion on the revision of the review of the existing maximum residue levels for lambda‐cyhalothrin. EFSA Journal 2015;13(12):4324, 119 pp. 10.2903/j.efsa.2015.4324 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016a. Reasoned opinion on the review of the existing maximum residue levels for fenpyroximate according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2016;14(1):4382, 48 pp. 10.2903/j.efsa.2016.4382 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016b. Conclusion on the peer review of the pesticide risk assessment of the active substance fenamidone. EFSA Journal 2016;14(2):4406, 173 pp. 10.2903/j.efsa.2016.4406 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016c. Reasoned opinion on the review of the existing maximum residue levels for chlormequat according to Article 12 of Regulation (EC) No 396/2005.EFSA Journal 2016;14(3):4422, 47 pp. 10.2903/j.efsa.2016.4422 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016d. Reasoned opinion on the revision of the review of the existing maximum residue levels for thiabendazole. EFSA Journal 2016;14(6):4516, 45 pp. 10.2903/j.efsa.2016.4516 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016e. The 2014 European Union report on pesticide residues in food. EFSA Journal 2016;14(10):4611, 139 pp. 10.2903/j.efsa.2016.4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Reasoned Opinion on the focussed review of the existing maximum residue levels for lambda‐cyhalothrin in light of the unspecific residue definition and the existing good agricultural practices for the substance gamma‐cyhalothrin. EFSA Journal 2017;15(7):4930, 29 pp. 10.2903/j.efsa.2017.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. Report for 2015 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Supporting publication 2017;14(11):EN‐1150, 69pp. 10.2903/sp.efsa.2017.en-1150 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2018a. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018b. Reasoned opinion on the setting of maximum residue limits for propargite in citrus fruits and tea. EFSA Journal 2018;16(2):5193, 25 pp. 10.2903/j.efsa.2018.5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018c. Scientific report on the occurrence of residues of fipronil and other acaricides in chicken eggs and poultry muscle/fat. EFSA Journal 2018;16(5):5164, 30 pp. 10.2903/j.efsa.2018.5164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018d. Guidance for reporting data on pesticide residues in food and feed according to Regulation (EC) No 396/2005 (2017 data collection). EFSA Journal 2018;16(6):5285, 63 pp. 10.2903/j.efsa.2018.5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018e. The 2016 European Union report on pesticide residues in food. EFSA Journal 2018;16(7):5348, 139 pp. 10.2903/j.efsa.2018.5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018f. Reasoned Opinion on the updated review of the existing maximum residue levels for imazalil according to Article 12 of Regulation (EC) No 396/2005 following new toxicological information. EFSA Journal 2018;16(10):5453, 52 pp. 10.2903/j.efsa.2018.5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2019. National summary reports on pesticide residue analysis performed in 2017. EFSA supporting publication 2019:EN‐1666, 147 pp. 10.2903/sp.efsa.2019.en-1666 [DOI] [Google Scholar]
- EFSA and RIVM (European Food Safety Authority and the Dutch National Institute for Public health and the Environment), 2015. EFSA Scientific Workshop, co‐sponsored by FAO and WHO: Revisiting the International Estimate of Short‐Term Intake (IESTI equations) used to estimate the acute exposure to pesticide residues via food. EFSA supporting publication 2015;12(2):EN‐907, 81 pp. 10.2903/sp.efsa.2015.en-907 [DOI] [Google Scholar]
- Ellison SLR and Williams A (eds.), 2012. Eurachem/CITAC guide: Quantifying Uncertainty in Analytical Measurement, Third edition, (2012), ISBN 978‐0‐948926‐30‐3.
- European Commission , 2017. Working document on the summing up of LOQs in case of complex residue definitions. SANCO/12574/2014. rev. 5(1). Application date: 1 January 2017. 30/11‐01/12 2015.
- European Commission , 2018. Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed. SANTE/11813/2017 (implemented from 01.01.2018).
- FAO (Food and Agriculture Organisation of the United Nations), 1988. Bromide ion in pesticide residues in food – 1988. Evaluations. Part II. Toxicology. FAO Plant Production and Protection Paper 93.
- FAO (Food and Agriculture Organization of the United Nations), 2016. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 3rd Ed. FAO Plant Production and Protection Paper 225, 286 pp.
- Scholz R, 2018. European database of processing factors for pesticides. EFSA supporting publication 2018;15(11):EN‐1510, 50 pp. 10.2903/sp.efsa.2018.en-1510 [DOI] [Google Scholar]
- UNEP , 2001. Stockholm Convention on Persistent Organic Pollutants, United Nation Environment Program, Stockholm Convention Secretariat. Available online: http://chm.pops.int/TheConvention/Overview/tabid/3351/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement MRL 2017 exceedances
PRIMo 2017monitoring results


