Abstract
Purpose of Review
Burst wave lithotripsy and ultrasonic propulsion of kidney stones are novel, noninvasive emerging technologies to separately or synergistically fragment and reposition stones in an office setting. The purpose of this review is to discuss the latest refinements in technology, to update on testing of safety and efficacy, and to review future applications.
Recent Findings
Burst wave lithotripsy produced consistent, small passable fragments through transcutaneous applications in a porcine model, while producing minimal injury and clinical trials are now underway. A more efficient ultrasonic propulsion design that can also deliver burst wave lithotripsy effectively repositioned 95% of stones in 18 human subjects (18 of 19 kidneys) and clinical trials continue. Acoustic tractor beam technology is an emerging technology with promising clinical applications through the manipulation of macroscopic objects.
Summary
The goal of the reviewed work is an office-based system to image, fragment, and reposition urinary stones to facilitate their natural passage. The review highlights progress in establishing safety, effectiveness, and clinical benefit of these new technologies. The work is also anticipating challenges in clinical trials and developing the next generation of technology to improve on the technology as it is being commercialized today.
Keywords: Burst Wave Lithotripsy, Ultrasonic Propulsion, Nephrolithiasis
1. Introduction
The incidence of kidney stone disease has increased over the past several decades, with now approximately 1 in 11 being affected over the course of his or her lifetime [1]. Despite technological advancements in shock wave lithotripsy (SWL), ureteroscopy (URS), and percutaneous nephrolithotomy (PCNL), reintervention rates are high, ranging from 26–34% depending on the modality of treatment [2]. Although the mainstays of surgical treatment for kidney stone disease have remained largely the same, recent innovations in ultrasound (US) technology are in development to expand its clinical applications to outside of imaging. As previously reported, they now include the treatment of kidney stones through ultrasonic propulsion and burst wave lithotripsy (BWL) [3,4]. These novel, noninvasive applications have the potential to fundamentally change the approach in treatment of kidney stones and this article reports on the latest refinements in technology, provides updates on testing of safety and efficacy, and discusses new applications in development.
2. Ultrasonic Propulsion
2.1. C5–2 probe
Previously, a C5–2 HDI curvilinear commercial probe (Philips Ultrasound, Bothell, WA), was reported in a first in-human clinical feasibility study of ultrasonic propulsion [3]. The probe delivered 50 millisecond burst of 2-MHz pulses at a 73% duty cycle and was developed to reposition kidney stones to relieve acute symptoms and facilitate passage of residual stones through transcutaneous application of focused ultrasound energy [5]. As described, this investigational device repositioned stones in 14 of 15 human study participants, displacing them > 3 mm in more than half of the subjects (8 of 15), and facilitated the passage of > 30 fragments in more than half of the subjects status post lithotripsy (4 of 6) [3]. There was minimal discomfort during exposures, and one patient experienced significant pain relief during treatment of a stone at the ureteropelvic junction (UPJ) [3].
2.2. New SX-C probe
More recently, a custom probe, SC-X (Sonic Concepts, Woodinville, WA), was designed to address several of the technical limitations of the original C5–2 probe. These limitations included 1) short focal length, limiting energy delivery at clinically relevant depths 2) narrow beam width, restricting the targeting of large groups of fragments and 3) limited burst duration and a required pause due to probe surface heating [6]. The re-designed probe delivers a broader beam width a longer focal depths and pulse durations, while relying on active probe surface cooling through a water-coupled interface. Ex vivo investigations demonstrated improved efficacy at expelling stones at greater depths (4.5, 9.5 cm) with lower probe heating, regardless of stone composition [6,7]. In addition, in a preclinical safety study in a porcine model, there was no evidence of injury on histology or any serum, urine, and behavioral changes [6].
2.3. First Human Studies with SX-C Probe
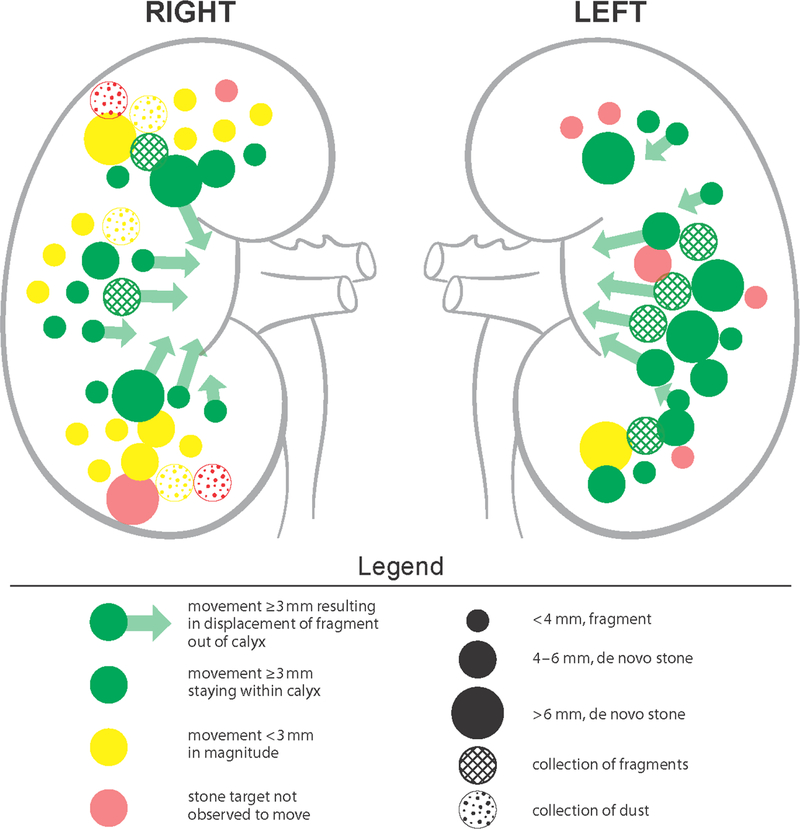
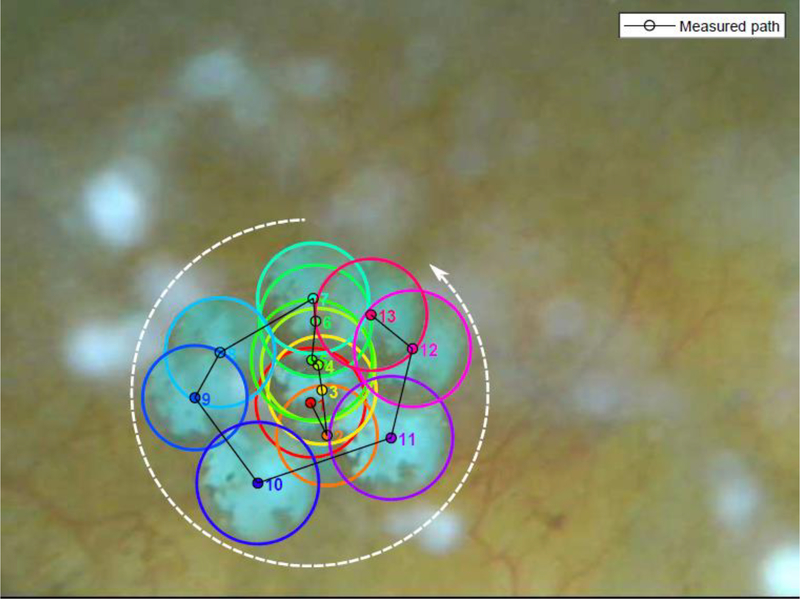
In a prospective clinical feasibility study, human subjects who were undergoing ureteroscopy and laser lithotripsy of at least one renal or ureteral stone were recruited to have fragments and stones ≤ 7 mm targeted and repositioned intraoperatively with the SC-X probe under ultrasound and endoscopic guidance (Figure 1). With exposures of 350 kHz frequency, ≤ 200 W/cm2 focal intensity, and ≤ 3-second bursts per push, stones were moved ≥ 3 mm in 95% of treated kidneys (18 of 19), as scored by three blinded endourologist reviewers from ureteroscopic videos. On recorded ultrasound videos of the same kidneys, the reviewers marked stone movements ≥ 3 mm in only 15 of 19 of kidneys. However, this was often due to stones being propelled out of the imaging plane, rendering it difficult for interpretation (Figure 2). No serious or unanticipated adverse events occurred in the human subjects – there was only transient skin redness in 3 of 14 patients, skin bruising in 1 of 14 patients, and skin irritation in 1 of 14 patients. In two independent scenarios, the exposure therapy actually repositioned stones that would have otherwise required expert manipulation with basket repositioning, highlighting one of the potential applications of this modality [8]. Therefore, by comparison with prior generation of C5–2 probes, the SX-C probe demonstrated a significant improvement over stone propulsion without compromising clinical safety [9]. There were several limitations, including the need to consider angle of therapy, as suboptimal positioning of the probe may cause stone propulsion into a calyceal wall, rather than through a targeted infundibulum since the force generated by the probe is unidirectional. In addition, efficacy of therapy declined with increased elapsed time, due to air being introduced from increased endoscopic and lithotripsy manipulation. Immediately adjacent and adherent stones were also observed to limit propulsion, due to the shielding effect [8].
Figure 1.
Summary depicting ultrasonic propulsion of stones and fragments ≤ 7 mm with new SX-C probe intraoperatively as measured by review of ultrasound videos. Various colors represent magnitude of movement, sizes representing size of target, and fills representing groups of stones versus fragments and dust. Adopted with permission from [8].
Figure 2.
Ultrasonic propulsion of stone out of calyx, as visualized on ultrasound and corresponding ureteroscopy. Adopted with permission from [8].
Additional human studies are currently underway to explore various clinical applications. In the emergency department, patients with acute, symptomatic obstructing UPJ or ureterovesical junction (UVJ) stones are being enrolled and treated with ultrasonic propulsion. The goal is to relieve symptomatic obstruction by manipulating obstructing stones either into the bladder (for UVJ stones) or renal pelvis/renal calyx (for UPJ stones. While only 4 of 20 subjects have so far enrolled, preliminary findings have been promising. Out of 2 UPJ stones, 1 was repositioned > 3 mm towards the renal pelvis, and out of 2 UVJ stones, 1 was displaced < 3 mm proximally from a previously obstructing location. The patient with the UVJ stone subsequently passed the calculi within 24 hours and there have been no device-related adverse events thus far [4]. In a concurrent, randomized clinical trial, patients with residual fragments remaining > 1 month after surgery are being enrolled to study the ability of ultrasonic propulsion to facilitate clearance of residual fragments after lithotripsy (either URS with laser lithotripsy or SWL). Initial findings have not yet been reported – so far, 13 of 30 patients have enrolled in the treatment arm, and 8 of 30 patients have enrolled in the control arm [4].
3. Burst Wave Lithotripsy
3.1. Design
Despite advances in lithotripter design, current SWL machines still deliver high peak pressures (30–100 MPa) in single-cycle pulses at slow rate (2 Hz) [10]. By comparison, BWL is a novel technological modality that delivers focused, sinusoidal US pulses in short bursts transcutaneously. This compact hand-held probe, which for some clinical trials is the SC-X probe, relies on US guidance to deliver low peak pressures (<12 MPa) at high rates (<200 Hz) and aims to address several clinical needs 1) minimize cavitation bubble formation to increase fragmentation efficiency 2) deliver exposures that are safe, tolerable, and noninvasive, allowing for applications in the awake patient 3) minimize radiation exposure, an increasing concern in the management of nephrolithiasis, given the nearly 600% increase in per capita radiation over the past 20 years [11,12]. Prior in vitro experiments demonstrated BWL’s efficacy and efficiency in fragmenting human stones (uric acid, struvite, COM, cystine) and artificial stones to small passable fragments, with higher ultrasound frequencies producing finer fragments [13]. More recently, engineering advancements, using the iterative angular spectrum approach and rapid prototyped acoustic lenses, designed a BWL system with larger beam widths to target larger stones. Preliminary studies demonstrated an effective fragmentation of stones, up to 12 mm, 2.8 times more efficiently than the existing BWL system [14].
3.2. Safety and Effectiveness
Tissue injury during BWL exposure is detectable in real-time and manifests as increased echogenicity on US imaging. The threshold duration of echogenicity for > 20 seconds has demonstrated to correlate 100% with BWL-related renal injury [4,15]. This is a potential imaging feedback system, allowing for immediate adjustments to avoid renal injury during exposure therapy. In porcine kidneys treated with deliberate, sustained doses of BWL (170 kHz – 335 kHz, 5.8–8.1 MPa), histologic analysis demonstrated a pattern of renal hemorrhagic injury similar to previously described shockwave lithotripsy (SWL) mediated injury [16]. Injury was seen in 10 of 21 treated sites, with total renal injury estimated to be <0.1% with 335 kHz transducer and <5.2% with 170 kHz transducer. The mechanism is thought to be from cavitation bubbles rapidly forming and collapsing during BWL pulses, causing mechanical trauma to small vessels [15]. In a porcine 7-day survival safety study, 10 animals (6 treatment, 4 controls) were exposed to 30-minute BWL treatments at 7 MPa, 350 kHz transmit frequency, 10 Hz pulse repetition frequency (PRF), and 24 cycle pulse duration. There were no significant histology changes to the kidney or surrounding tissues, and no changes to blood chemistry, urine values, or animal behaviors [17]. In a follow up porcine acute safety study, 6 – 7 mm human COM stones were surgically implanted in the 3 pigs’ kidneys and exposed to BWL at 6.5 – 7 MPa, 350 kHz, 10 Hz PRF for 30 minutes. Five of 6 stones were treated (1 not targeted due to poor acoustic window) with 87% of stone reduced to fragments < 2 mm (Figure 3). Subsequent MRI, histology, and gross examination demonstrated no injury in the renal parenchyma, although there was evidence of petechial hemorrhage and surface erosion to the immediate urothelium circumscribing the stone (Figure 4) [18]. These results have helped investigators identify BWL parameters for effective stone comminution, while minimizing tissue injury [16,18]. Clinical trials with the BWL in humans have been approved by the FDA and are currently underway.
Figure 3.
Representative stone fragments after 30 mins. BWL treatment. The stones were implanted in pig ureters and corresponding fragments below were subsequently extracted after treatment. Adopted with permission from [16].
Figure 4.
Representative kidney tissue after 30 mins. BWL treatment. No evidence of gross injury, but petechial hemorrhage evident as seen in the immediate tissue circumscribing the stone. White scale bar = 1 cm. Adopted with permission from [16].
3.3. Ultrasonic Propulsion with Burst Wave Lithotripsy
There appears to be a synergistic effect on fragmentation efficacy when ultrasonic propulsion is administered in combination with BWL. In vitro experimental investigations demonstrated increased rates of comminution of both human COM and artificial stone models when BWL was delivered with interleaved ultrasonic propulsion. Under the same BWL settings, fragmentation rates increased in a dose dependent relationship, with higher rates of comminution when ultrasonic propulsion pulses were faster (6 pulses/min versus 60 pulses/min) [19]. There may be several mechanisms to account for the observed effect: 1) ultrasonic propulsion facilitates the release of loose, weakened fragments from stone surface 2) the reorientation of stone redistributes stress and lead to new stress fractures 3) ultrasonic propulsion prevents accumulation of cavitation bubbles and debris that may provide shielding effect 4) ultrasonic propulsion forms stress fractures and help oscillate formed cavitation bubbles, further damaging targeted stone [19,20]. More importantly, these studies reflect how investigations have only just begun to characterize the potential and ultimate effectiveness of BWL and ultrasonic propulsion. There has been sparse effort in studying and optimizing the technologies among a wide range of experimental variables, and only what has been demonstrated to be safe and effective in pre-clinical investigations has been translated to clinical trials. Currently, there are engineering efforts in developing pulses that can accelerate fragmentation even further, while also providing US feedback of real-time comminution or tissue injury – such a system design enables feedback adjustment of outputs, improving efficacy without at risk of injury [20].
4. Acoustic Tractor Beam
As the developments of UP and BWL are being clinically implemented and studied, certain limitations are being recognized. With the current design of the UP probe, the target object (i.e. kidney stone or stone fragment) can only be moved in one direction (away from the probe). However, recent work being done by same team at University of Washington (UW) have focused on manipulating objects in a three-dimensional path with an acoustic tractor beam (Figure 5) [21].
Figure 5.
Representation of acoustic tractor beam technology. A 5-mm glass bead is guided through a path outlined above. Adopted with permission from [21].
The notion of the “acoustic tweezer”—manipulation and movement of small objects with sound waves—is not new. Other groups have described using acoustic waves to surround and control small, light objects in air and water, with utility limited to the cellular scale and microfluidic environments [22,23]. Until recently, there has been a lack of research in manipulating large dense objects within a medium mimicking a living body. The team at UW is working towards filling this void and are specifically investigating the use of acoustic tweezers in the potential non-invasive management of kidney stones [21]. It should be noted that the term “acoustic tractor beam” is used by the UW team, which may be more descriptive of the actual mechanism of this process than “acoustic tweezers”. Unlike other acoustic tweezers, a single source is used to trap and manipulate the objects in 3D space by electronically steering the acoustic beam. A 1.5 MHz 256-element focused ultrasound array was developed that has been configured to produce uniform two-dimensional acoustic beam shapes and create high enough pressure confined to a focal region where large objects (such as kidney stones) can be trapped and moved both along and transverse to the beam axis [21]. First, the vibrations from the multi-element array were characterized and measured to determine the specific force that is needed to generate vortex beams capable of object manipulation [24]. This was the first time lateral acoustic forces on large objects were able to be quantified in this manner and compared with the theoretical model [25]. Three-dimensional traps were synthesized, and millimeter-sized glass spheres were able to be levitated and manipulated in water. Ultimately, a 3-mm spherical glass bead (mimicking a kidney stone) was successfully manipulated in 3D path within the bladder of a living pig under ultrasound imaging and confirmed through endoscopic view (Figure 6) [20].
Figure 6.
Still shot series of images of the locations of the 3-mm glass bead inside the pig bladder filmed by a borescope inserted into the bladder through the urethra. The centroid of each location is marked and has the same color as the border of the bead when at that location. The white dashed line shows the general direction of the desired circular motion only. Adopted with permission from [21].
Acoustic trapping has much potential for kidney stone disease as well as for broader applications. Combined with BWL, acoustic tractor beams can be the missing link in the complete non-invasive treatment of kidney stones with the added benefit of using no ionizing radiation. Furthermore, it can theoretically be used for other non-invasive medical applications (e.g. removal of foreign objects, treatment of blood clots, or targeted drug delivery), however the system is not yet ready for commercialization or clinical application.
5. Conclusions and Future Directions
Ultrasonic propulsion and BWL are safe, effective technologies that transcutaneously treat stones within the genitourinary system. The goal is for an office-based system that images, repositions, breaks, and expels targeted stones. The technologies are well into clinical trials in an effort to bring them to clinical use and they continue to yield promising results. The UW team continues to improve the technologies with future directions including new treatment applications.
Key Points.
A novel, updated SC-X probe addresses several technical limitations of prior probes and has superior efficacy in ultrasonic propulsion, but remains noninvasive, safe, and well tolerated in human clinical trials
Burst wave lithotripsy is a novel stone comminution modality and applications in combination with ultrasonic propulsion may help drive a paradigm shift in future kidney stone management
Acoustic tractor beam is an evolving technology that may enable the manipulation and full entrapment of macroscopic objects such as stones, serving as a potential future adjunct to conventional treatment modalities.
The ultimate goal is for an office-based system that can image, reposition, break, and expel targeted stones.
Acknowledgements
The work and review are part of a large collaborative effort, and we appreciate the help of our many collaborators at the University of Washington Center for Industrial and Medical Ultrasound in the Applied Physics Laboratory and the Department of Urology. The authors thank Jonathan D. Harper (Department of Urology, University of Washington) and Adam D. Maxwell (Department of Urology, University of Washington) for their expertise and help in reviewing the article.
Financial support and sponsorship
This work was supported through grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK043881 and DK092197), and NASA. This material was the result of work supported by resources from the Veterans Affairs Puget Sound Health Care System, Seattle, Washington.
Footnotes
Conflicts of interest
M.D. Sorensen and M.R. Bailey have equity in and consult for SonoMotion which has licensed this technology from the University of Washington for commercialization. T.T. Chen and P.C. Samson have nothing to disclose.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
❖= of special interest
- [1].Scales CDJ, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur. Urol 2012;62(1):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Feinstein L, Matlaga B. Kidney Stones in 2017 Urologic Diseases in America NIH Publication No.12–7865. Washington, DC: US Government Printing Office [Google Scholar]
- [3].May PC, Bailey MR, Harper JD. Ultrasonic propulsion of kidney stones: Curr. Opin. Urol 2016;26(3):264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ❖ [4].Bailey MR, Wang Y-N, Kreider W, et al. Update on clinical trials of kidney stone repositioning and preclinical results of stone breaking with one system. In: Victoria, Canada; 2018:020004. [DOI] [PMC free article] [PubMed] [Google Scholar]; We report work toward an office-based, handheld ultrasound device to target, detach, break, and reposition stones and stone fragments in the urinary space to facilitate natural clearance. This publication summarizes latest results of preclinical and ongoing human studies.
- [5].Cunitz BW, Dunmire B, Bailey MR. Characterizing the Acoustic Output of an Ultrasonic Propulsion Device for Urinary Stones. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017;64(12):1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Janssen KM, Brand TC, Cunitz BW, et al. Safety and Effectiveness of a Longer Focal Beam and Burst Duration in Ultrasonic Propulsion for Repositioning Urinary Stones and Fragments. J. Endourol 2017;31(8):793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Janssen KM, Brand TC, Bailey MR, et al. Effect of Stone Size and Composition on Ultrasonic Propulsion Ex Vivo. Urology 2018;111:225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ❖ [8].Dai JC, Sorensen MD, Chang HC, et al. Quantitative assessment of effectiveness of ultrasonic propulsion of kidney stones. J. Endourol 2019. doi: 10.1089/end.2019.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]; We report first results of ultrasonic propulsion in humans with a newly redesigned probe and ureteroscopic observation to quantify stone repositioning.
- [9].Harper JD, Cunitz BW, Dunmire B, et al. First in Human Clinical Trial of Ultrasonic Propulsion of Kidney Stones. J. Urol 2016;195(4 Part 1):956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xing Y, Chen TT, Simmons WN, et al. Comparison of Broad vs Narrow Focal Width Lithotripter Fields. J. Endourol 2017;31(5):502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen TT, Wang C, Ferrandino MN, et al. Radiation Exposure during the Evaluation and Management of Nephrolithiasis. J. Urol 2015;194(4):878–885. [DOI] [PubMed] [Google Scholar]
- [12].Hunter C, Maxwell AD, Cunitz B, et al. Impact of stone characteristics on cavitation in burst wave lithotripsy. J. Acoust. Soc. Am 2018;144(3):1779–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maxwell AD, Cunitz BW, Kreider W, et al. Fragmentation of Urinary Calculi In Vitro by Burst Wave Lithotripsy. J. Urol 2015;193(1):338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Randad AP, Ghanem MA, Bailey MR, Maxwell AD. Design of a transducer for fragmenting large kidney stones using burst wave lithotripsy. 2018;35:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].May PC, Kreider W, Maxwell AD, et al. Detection and Evaluation of Renal Injury in Burst Wave Lithotripsy Using Ultrasound and Magnetic Resonance Imaging. J. Endourol 2017;31(8):786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maxwell AD, Wang Y-N, Kreider W, et al. Evaluation of Renal Stone Comminution and Injury by Burst Wave Lithotripsy in a Pig Model. J. Endourol 2019:end.2018.0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sorensen MD, Wang Y-N, Kreider W, et al. Preclinical safety and effectiveness of burst wave lithotripsy. World Congr. Endourol 2018. [Google Scholar]
- [18].Wang Y-N, Kreider W, Hunter C, et al. An in vivo demonstration of efficacy and acute safety of burst wave lithotripsy using a porcine model. In: Victoria, Canada; 2018:020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ❖ [19].Zwaschka TA, Ahn JS, Cunitz BW, et al. Combined Burst Wave Lithotripsy and Ultrasonic Propulsion for Improved Urinary Stone Fragmentation. J. Endourol 2018;32(4):344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]; We report a new synergistic effect on urinary stone fragmentation efficacy when ultrasonic propulsion is administered in combination with BWL in vitro.
- [20].Maeda K, Maxwell AD, Colonius T, et al. Energy shielding by cavitation bubble clouds in burst wave lithotripsy. J. Acoust. Soc. Am 2018;144(5):2952–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ❖ [21].Ghanem MA. Acoustic Manipulation of Macroscopic Objects. Thesis PhD--Univ. Wash 2018:121. [Google Scholar]; We report acoustic manipulation of macroscopic objects, kidney stones, specifically for non-invasive medical treatments.
- [22].Marzo A, Seah SA, Drinkwater BW, et al. Holographic acoustic elements for manipulation of levitated objects. Nat. Commun 2015;6:8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Baresch D, Thomas J-L, Marchiano R. Observation of a Single-Beam Gradient Force Acoustical Trap for Elastic Particles: Acoustical Tweezers. Phys. Rev. Lett 2016;116(2):024301. [DOI] [PubMed] [Google Scholar]
- [24].Ghanem MA, Maxwell AD, Kreider W, et al. Field Characterization and Compensation of Vibrational Nonuniformity for a. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018;65(9):1618–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ghanem MA, Maxwell AD, Sapozhnikov OA, et al. Quantification of acoustic radiation forces on solid objects in fluid. Phys Rev Appl In Press. Available at: https://journals.aps.org/prapplied/accepted/ad07dK59S5a1460763ab84a840b44d323cdb236b6. [DOI] [PMC free article] [PubMed] [Google Scholar]